Background: Both AlaRS editing domains and their free-standing editing forms, AlaX, are thought to exclusively deacylate mischarged tRNAAla.
Results: Contrary to this belief, we find that AlaX-S deacylates Ser-tRNAAla and Ser-tRNAThr.
Conclusion: AlaX-S is likely to be the ancestor of AlaX, AlaRS editing, and bacterial-like ThrRS editing domains.
Significance: The biochemical activities of AlaX-S link, for the first time, the activities of the AlaRS and ThrRS editing domains.
Keywords: Aminoacyl tRNA Synthetase, phylogenetics, Protein Evolution, RNA Editing, tRNA, AlaX, Genetic Code, Mistranslation, Proofreading
Abstract
Accurate protein synthesis requires the hydrolytic editing of tRNAs incorrectly aminoacylated by aminoacyl-tRNA synthetases (ARSs). Recognition of cognate tRNAs by ARS is less error-prone than amino acid recognition, and, consequently, editing domains are generally believed to act only on the tRNAs cognate to their related ARSs. For example, the AlaX family of editing domains, including the editing domain of alanyl-tRNA synthetase and the related free-standing trans-editing AlaX enzymes, are thought to specifically act on tRNAAla, whereas the editing domains of threonyl-tRNA synthetases are specific for tRNAThr. Here we show that, contrary to this belief, AlaX-S, the smallest of the extant AlaX enzymes, deacylates Ser-tRNAThr in addition to Ser-tRNAAla and that a single residue is important to determine this behavior. Our data indicate that promiscuous forms of AlaX are ancestral to tRNA-specific AlaXs. We propose that former AlaX domains were used to maintain translational fidelity in earlier stages of genetic code evolution when mis-serylation of several tRNAs was possible.
Introduction
Mistranslation is a biological phenomenon caused by the misacylation of tRNAs by aminoacyl-tRNA synthetases (ARSs).3 Bona fide translation errors are often deleterious, but mistranslation can also be an adaptive strategy to correct other kinds of insults (1–3). The reported cases of toxic mistranslation are invariably due to errors in amino acid recognition. In contrast, adaptive mistranslation is mostly due to tRNAs that are charged promiscuously or by ARSs that acylate different tRNAs (4–8).
Toxic mistranslation is prevented by cells through proofreading mechanisms (9). Error-prone ARSs are toxic to both bacterial and mammalian cells, and, to avoid mischarging, they have evolved editing domains to recognize mischarged tRNAs (10–15). In some instances, editing domains display tRNA selectivity, and, in other cases, the domains per se are not tRNA-specific and rely on the specificity of other domains for their activity (16–19). For example, YbaK is not tRNA-specific in vitro and will deacylate any tRNA charged with Cys (20, 21). In vivo, YbaK tRNA specificity requires interactions with ProRS (20). In contrast, AlaXs and ProXp-ala (a YbaK homolog) are tRNA-specific and recognize the same conserved tRNA acceptor stem identity elements (16, 22, 23).
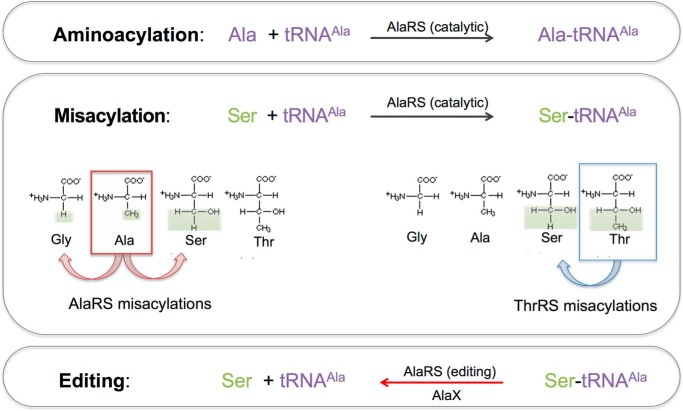
Single domain AlaX editing proteins are widely distributed phylogenetically, and clear tRNAAla mischarged with Ser or Gly by AlaRS (Fig. 1) (24). They are homologous to the editing domains of alanyl- and threonyl-tRNA synthetases (AlaRS and ThrRS) (10, 22, 25–28). There are three types of freestanding AlaXs: AlaX-S, AlaX-M, and AlaX-L, which stand for small, medium, and large, respectively (Fig. 2A). AlaX-S is homologous to the C-terminal portion of the AlaRS editing domain (29, 30). AlaX-M contains an additional Gly-rich motif in its N-terminal region (31, 32). Finally, AlaX-L closely resembles the entire editing domain of AlaRS and contains an additional C-terminal domain (C-Ala) (27, 33).
FIGURE 1.
Aminoacylation, misacylation, and deacylation reactions catalyzed by AlaRS and AlaX.
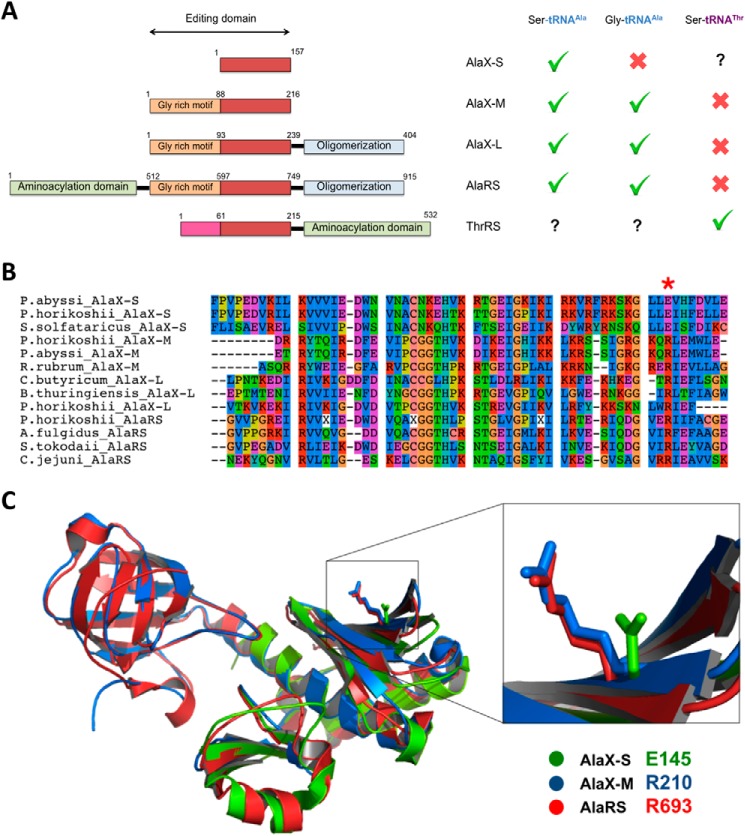
FIGURE 2.
Structural comparisons of the AlaX-S, AlaX-M, AlaX-L, AlaRS, and ThrRS editing domains. A, domain organization of AlaRS, AlaXs, and ThrRS. The residue numbers shown correspond to P. horikoshii proteins, except for ThrRS, which corresponds to the E. coli enzyme. The core editing domain is common in all five proteins (red). AlaX-M, AlaX-L, and AlaRS contain the Gly-rich motif (orange) appended to the editing domain. The deacylation activities described to date are summarized in the right panel: AlaX-S (29), AlaX-M (25, 32), AlaX-L (25, 27), AlaRS (22, 25), and ThrRS (22). B, structure-guided multiple sequence alignment of AlaX-S, AlaX-M, AlaX-L, and AlaRS. The conserved Arg residue that confers specificity for tRNAAla is marked with an asterisk. C, superimposition of P. horikoshii AlaX-S, P. horikoshii AlaX-M, and E. coli AlaRS editing domain crystal structures. Residue Arg-693 of E. coli AlaRS corresponds to Glu-145 in P. horikoshii AlaX-S.
The deacylation activities of the AlaXs vary. Although AlaX-M, AlaX-L, and AlaRS hydrolyze both Ser-tRNAAla and Gly-tRNAAla (22, 25, 27, 32), AlaX-S only hydrolyzes mischarged Ser-tRNAAla (29), possibly because of the lack of the Gly-rich domain (Fig. 2A), which results in an enlarged amino acid binding pocket and a much lower binding affinity toward Gly (29). In contrast, bacterial ThrRS editing domains hydrolyze Ser-tRNAThr (26, 34). AlaX freestanding domains are generally thought to be specific for tRNAAla, and their specificity has been linked to a highly conserved arginine residue (22) that is believed to interact with the unique G3:U70 pair in the tRNAAla acceptor stem. A single R693K mutation in the editing domain of Escherichia coli AlaRS allows this enzyme to hydrolyze Ser-tRNAThr (22).
We observed that, in archaeal AlaX-S, this arginine is substituted by glutamic acid (Fig. 2B and 2C). Intrigued by the lack of conservation of such a critical residue, we investigated the tRNA specificity of AlaX-S. We found that, although AlaX-S retains specificity for serylated tRNAs, it is not tRNA-specific and deacylates Ser mischarged onto tRNAAla, tRNAThr, and tRNAPro. These features set AlaX-S apart from the rest of the AlaX family of enzymes. A phylogenetic analysis of AlaX sequences shows that AlaX-S constitutes an ancient clade that separates from other AlaXs and that is more closely related to bacterial ThrRS editing domains than any other AlaX member. Therefore, on the basis of our biochemical and phylogenetic analyses, we propose a novel evolutionary model for the AlaX family in which the ancestral form of the AlaX family of editing enzymes likely was a tRNA-promiscuous enzyme whose function was to correct widespread mis-serylation of tRNAs by ancient primitive ARSs. This ancestral form would afterward have evolved to deacylate either tRNAAla (AlaX-M, AlaX-L, AlaRS editing domain) or tRNAThr (ThrRS editing domain), fulfilling the need for higher levels of discrimination before being incorporated as the editing domains now characteristic of contemporary synthetases.
EXPERIMENTAL PROCEDURES
Phylogenetic Analysis of AlaX Domains
Hidden Markov Model profiles were built for each protein containing an AlaX domain (AlaX-S, AlaX-M, AlaX-L, and AlaRS editing domain and ThrRS editing domain) using the HMMER package (35), which were used as query to find homologues in the Uniprot database. The resulting sequences were aligned using T-Coffee (36) with the structure-guided alignment mode, and phylogeny was built using the maximum likelihood method with bootstrapping using PHYLIP 3.68 (37). The final tree was visualized with TreeView (38). To build figures that illustrate the structure-guided multiple-sequence alignment (MSA) results, a representative subset of species was selected for each AlaX clade from the full multiple-sequence alignment. Structure-guided multiple-sequence alignment results were visualized with Seaview (39).
Protein Expression and Purification
Pyrococcus horikoshii AlaX-S and AlaRS were a gift from Prof. Sokabe (University of California, Davis). The Pyrococcus abyssi ThrRS N-terminal editing domain (PaThrRS-NTD) was a gift from Prof. Sankaranarayanan (Centre for Cellular and Molecular Biology, India). Expression was induced in E. coli RosettaTM BL21 (Novagen) at A600 = 0.6 with 1 mm isopropyl β-d-thiogalactoside for 6 h at 37 °C. Enzymes were prepared as described previously (29, 40).
In Vitro tRNA Transcription and Purification
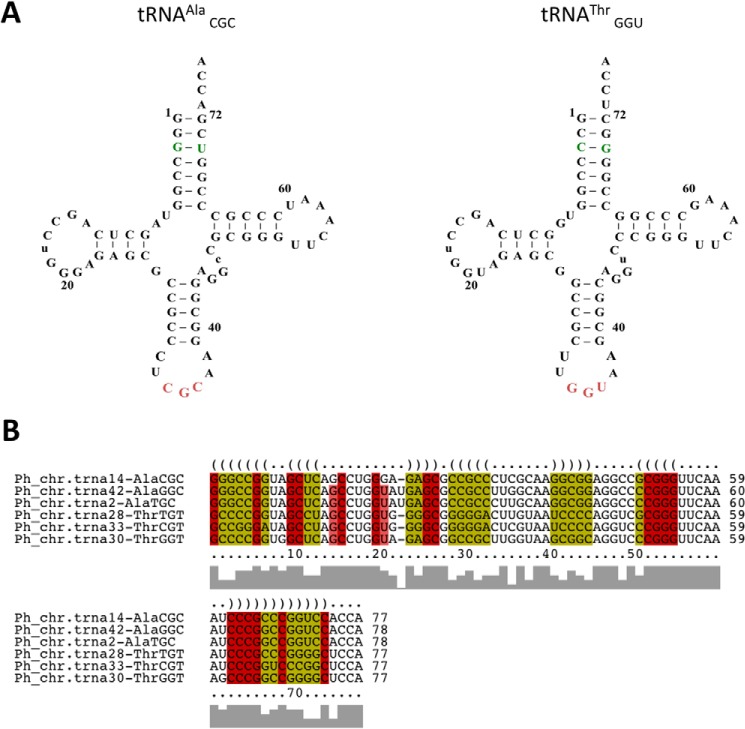
Sequences for P. horikoshii tRNAAla and tRNAThr were taken from gtRNAdb (41) (Fig. 3). In vitro-transcribed P. horikoshii tRNAAla and tRNAThr were prepared as described previously (42). E. coli tRNAPro was prepared following protocols published previously (43). Briefly, each tRNA was transcribed from an NspI-digested pUC19 plasmid template using T7 RNA polymerase. The sample was then loaded into a denaturating 10% polyacrylamide gel, and the resulting tRNA band was cut out, electroeluted, and ethanol-precipitated.
FIGURE 3.
A, cloverleaf representation of P. horikoshii tRNAAla and tRNAThr used in the deacylation assays. B, structural alignment of all annotated P. horikoshii tRNAAla and tRNAThr sequences.
Preparation of Aminoacyl-tRNA Substrates
In vitro-transcribed E. coli tRNAPro, P. horikoshii tRNAAla and tRNAThr were 3′ 32P-labeled using E. coli tRNA nucleotidyltransferase and [α-32P]ATP (PerkinElmer Life Sciences) as described previously (44). Aminoacylation of tRNAs was carried out using biotinylated dFx (Thermo Scientific) using published conditions with minor modifications (45). Briefly, tRNA and dFx (42 μm each, 18-μl volume) and trace amounts of 3′ 32P-labeled tRNA were heated to 95 °C for 1 min, followed by addition of 25 mm MgCl2 and 5 mm of serine-3,5-dinitrobenzyl ester. dFx-catalyzed aminoacylation was carried out for 6 h on ice. Reactions were quenched with 120 μl of 0.3 m NaOAc (pH 5). Biotinylated dFx was removed by incubating the reaction mixture with 150 μl of streptavidin-agarose resin (Novagen) for ∼15 min at room temperature, followed by centrifugation in a tabletop centrifuge at 4 °C for 2 min at 2000 × g. The supernatant containing the aminoacylated tRNA was removed, phenol-chloroform-extracted, and ethanol-precipitated. The pellet was dissolved in diethylpyrocarbonate-treated water and stored at −80 °C.
Deacylation Assays
Deacylation assays were performed as described previously (23). Briefly, reactions containing 50 mm HEPES (pH 7.5), 0.1 mg/ml BSA, 10 mm MgCl2, 5 mm 2-mercaptoethanol, 20 mm KCl, and ∼1.5 μm aminoacyl-tRNA were initiated by addition of enzyme (2 μm P. horikoshii AlaX-S, 2 μm P. horikoshii AlaRS, or 0.5 μm P. abyssi ThrRS-NTD). At the indicated time points, the reactions were quenched into 6 μl of a solution containing 0.4 unit/μl P1 nuclease in 200 mm NaOAc (pH 5). The quenched solution (1 μl) was spotted onto PEI-cellulose thin-layer chromatography plates (prewashed in water) to separate aminoacyl-[32P]AMP and [32P]AMP. The data were analyzed as described previously (44), and the graphs were prepared using SigmaPlot (Systat Software, San Jose, CA), with error bars representing the standard deviation of triplicate data.
RESULTS
Sequence and Structural Analysis of the AlaX Domain Superfamily
Previous work showed that the G3:U70 base pair is critical for aminoacylation of tRNAAla by AlaRS (46, 47). This base pair interacts with a conserved Arg in the AlaRS editing domain (Arg-693 in E. coli), and the substitution of this residue with Lys results in loss of tRNA specificity (22). Although this residue was initially predicted to be universally conserved, our analysis here shows that AlaX-S enzymes contain a Glu residue in the equivalent position (Glu-145 in P. horikoshii AlaX-S) (Fig. 2B). Structural superimposition of E. coli AlaRS, P. horikoshii AlaX-M, and P. horikoshii AlaX-S crystal structures demonstrates that Glu-145 is in a similar orientation as Arg-693 and Arg-210 of E. coli AlaRS and P. horikoshii AlaX-M, respectively (Fig. 2C). On the basis of these observations, we hypothesized that the presence of Glu-145 in AlaX-S alters its substrate specificity, allowing hydrolysis of Ser from different tRNAs.
AlaX-S Is a Nonspecific Ser-tRNA Deacylase
We next examined the tRNA specificity of AlaX-S in vitro. We purified recombinant P. horikoshii AlaX-S, P. horikoshii AlaRS-N752 (PhAlaRS lacking the C-terminal C-Ala domain), and the isolated N-terminal editing domain of P. abyssi ThrRS (ThrRS-NTD). We also prepared P. horikoshii Ser-tRNAAla and Ser-tRNAThr and E. coli Ser-tRNAPro using the Flexizyme (dFx) system (48). It should be noted that the P. abyssi ThrRS editing domain is not related to either the bacterial/eukaryotic ThrRS editing domains or the AlaX family (49–51).
As expected, deacylation assays confirmed robust editing activity of Ser-tRNAAla by AlaX-S and PhAlaRS. In contrast, ThrRS-NTD does not possess significant deacylation activity for this substrate (Fig. 4A) but efficiently hydrolyzes Ser-tRNAThr (50). PhAlaRS exhibited only weak deacylation activity for Ser-tRNAThr, whereas AlaX-S robustly edited this substrate, in support of our hypothesis (Fig. 4B). The lack of tRNA specificity of AlaX-S was further confirmed by showing that Ser-tRNAPro, which is not known to occur in nature, is also a substrate for the enzyme (Fig. 4C).
FIGURE 4.
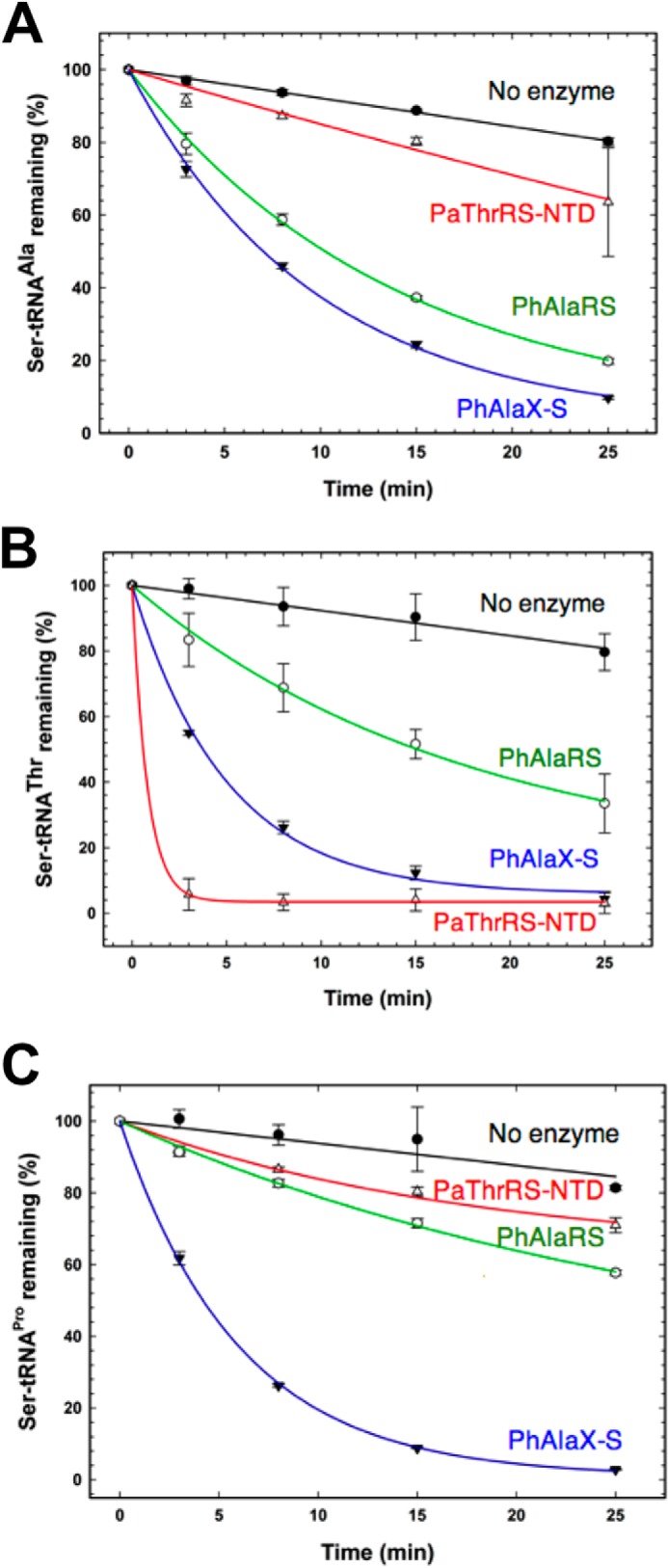
Deacylation activity of AlaX-S. A, deacylation of Ser-tRNAAla by the P. horikoshii AlaX-S (▾), P. horikoshii AlaRS-N752 (○), and P. abyssi ThrRS editing domain (▵) or a no-enzyme control (●). B, Ser-tRNAThr deacylation by the P. horikoshii AlaX-S (▾), P. horikoshii AlaRS-N752 (○), or P. abyssi ThrRS editing domain (▴) or a no-enzyme control (●). C, Ser-tRNAPro deacylation by the P. horikoshii AlaX-S (▾), P. horikoshii AlaRS-N752 (○), or P. abyssi ThrRS editing domain (▵) or a no-enzyme control (●).
We then investigated whether the lack of tRNA specificity observed in AlaX-S is also accompanied by a lack of amino acid specificity. Gly-tRNAAla had already been shown to not be a substrate of AlaX-S (29). We also tested the deacylation activity of AlaX-S upon Pro-tRNAPro, finding that AlaX-S is not capable of deacylating this substrate either (data not shown).
Taken together, these results support the idea that AlaX-S retains characteristics of an ancestral freestanding editing protein, able to deacylate multiple mischarged Ser-tRNAs. In contrast, the editing domain of archaeal P. abyssi ThrRS is capable of discriminating Ser-tRNAs and appears to be highly specific for Ser-tRNAThr.
Phylogenetic Analysis of the AlaX Superfamily
Several phylogenetic trees that include bacterial ThrRS, AlaRS, and freestanding AlaXs have been reported (10, 27). These studies did not distinguish AlaX-S from AlaX-M, which were previously classified together as AlaXp-I (27). For this reason, we performed a new phylogenetic analysis to try to resolve all three types of freestanding AlaX sequences (Fig. 5). We found that, although AlaX sequences are represented in the three domains of life, AlaX-S sequences are only found in Archaea. Previous studies have included several bacterial and eukaryal sequences within the set of AlaX-S sequences (27). However, we believe that these sequences may reflect misannotations because of the shorter sequence length of some bacterial and eukaryal AlaX-M proteins. To confirm the archaeal distribution of AlaX-S, we verified that shorter AlaX sequences from bacteria and eukarya had an Arg residue in the homologous position to Arg-693 of E. coli AlaRS and were evolutionary closer to AlaX-M sequences, indicating that AlaX-S members are only found in Archaea.
FIGURE 5.
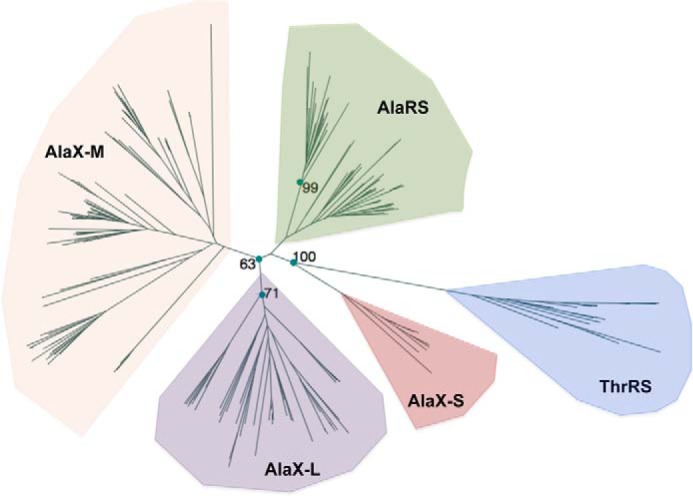
Phylogenetic analysis of the AlaX family. The phylogeny includes the editing domains of AlaRS (green), bacterial-type ThrRS (blue), and the three types of freestanding AlaXps: AlaX-S (red), AlaX-M (orange), and AlaX-L (purple). Maximum likelihood bootstrap values are shown.
Our phylogenetic analysis shows that the editing domain of ThrRS is closest to AlaX-S, which positions AlaX-S as an intermediate evolutionary step between ThrRS and the group of AlaRS, AlaX-M, and AlaX-L, suggesting an early separation that split the original enzyme into two different specificities, one for tRNAThr and the other for tRNAAla. Therefore, our results suggest that all forms of AlaX evolved from a common ancestor and shows that both AlaX-M and AlaX-L are derived from AlaX-S.
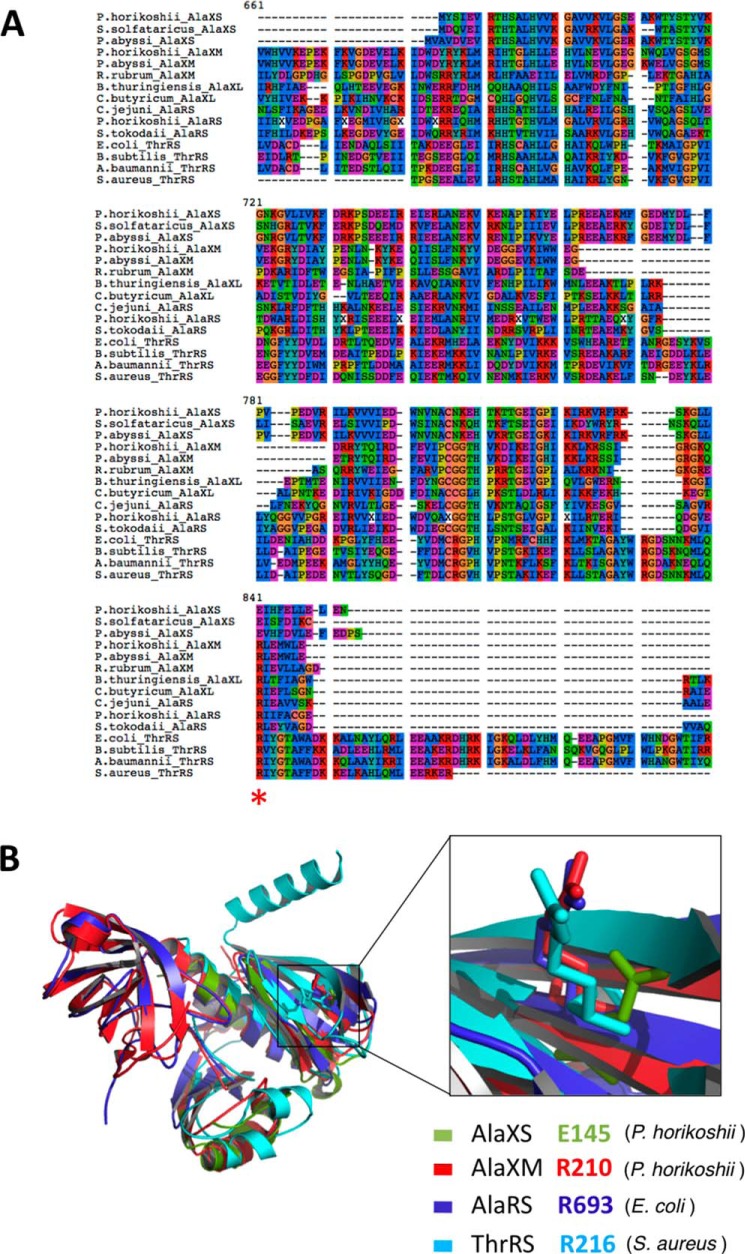
Surprisingly, a structural superimposition of all the homologous AlaX-type editing domains shows that an Arg residue is also found in bacterial ThrRS editing domains at the equivalent position to E. coli AlaRS Arg-693 (Fig. 6). This implies that the Arg residue by itself does not confer tRNAAla specificity, given that bacterial ThrRS has been shown to specifically deacylate Ser-tRNAThr (26). Additional sequence and structural elements are likely to contribute to specific G3:U70 recognition (52).
FIGURE 6.
Multiple sequence alignment of the AlaX-S, AlaX-M, AlaX-L, AlaRS, and ThrRS editing domains. A, structure-guided multiple sequence alignment of AlaX-S, AlaX-M, AlaX-L, AlaRS, and ThrRS. The conserved Arg residue that confers specificity for tRNAAla is marked with an asterisk. B, superimposition of the P. horikoshii AlaX-S, P. horikoshii AlaX-M, E. coli AlaRS, and Staphylococcus aureus ThrRS editing domain crystal structures. Residue Arg-693 of E. coli AlaRS and its corresponding residues in P. horikoshii AlaX-S, P. horikoshii AlaX-M, and S. aureus ThrRS are displayed using stick representations.
DISCUSSION
Editing domains are universal features of some ARSs (10, 26, 53). Originally, however, their function might have been unrelated to protein synthesis quality control and linked to other roles in the RNA world (54). Structural data indicate that the incorporation of editing domains to the ARS ancient catalytic core happened first in class I enzymes (55). However, the existence of free forms of these domains raises the question of whether they originated as freestanding hydrolytic enzymes or as ARS-attached domains.
It is generally accepted that early genetic codes were prone to errors that were gradually minimized as organisms required more faithful genetic translation (56). Here we studied the tRNA specificity of a putative ancestral editing domain, AlaX-S. Our goal was to gain a better understanding of the original function of AlaX-type editing enzymes and the origin of tRNA specificity within AlaX-type editing enzymes. Our data show that AlaX-S is capable of deacylating Ser from multiple tRNA acceptors (Fig. 4), whereas AlaRS, other AlaXs, and the archaeal ThrRS editing domain (ThrRS-NTD) are tRNA-specific.
Our phylogenetic analysis shows that AlaX-S forms a distinct clade that separates AlaXs and AlaRS from ThrRS sequences (Fig. 5). One possibility is that an editing domain with relaxed tRNA specificity (AlaX-S) would have evolved in modern archaeal cells. However, this possibility would not explain the phylogenetic position of AlaX-S between ThrRS and other AlaX-type domains. The correct clustering of the different editing enzymes on the basis of its biochemical activities, and the robust bootstrap support, reinforce the possibility that AlaX-S is the closest relative of the ancestor of all AlaX-type editing domains. This ancestral nature of AlaX-S is further supported by the distribution of the Gly-rich motif responsible for the recognition of Gly in addition to Ser- (29), which is found in AlaX-M, AlaX-L, and AlaRS editing domain but not in the AlaX-S or ThrRS editing domains (Fig. 2A). Therefore, AlaX-S is likely an evolutionary intermediate between the AlaRS/AlaX-M/AlaX-L editing domains and bacterial ThrRS editing domains.
On the basis of both our biochemical and phylogenetic results, we propose that AlaX-S is a direct descendant of an ancestral deacylation enzyme that lacked tRNA specificity and is the functional ancestor of the AlaRS and ThrRS editing domains, including their freestanding forms (Fig. 7). This ancestral enzyme might have been in charge of deacylating mis-serylated tRNAs generated by synthetases with poor amino acid selectivity or by seryl-tRNA synthetases with poor tRNA specificity. This activity would be required in an earlier stage of genetic code development where mis-serylation of tRNAs was particularly frequent. Future work reconstructing ancestral sequences and testing their tRNA specificities will provide additional evidence on the evolution of this family.
FIGURE 7.
Evolutionary model of AlaX editing enzymes. AlaX-S has dual tRNAAla/Thr specificity, deacylating both Ser-tRNAAla (blue) and Ser-tRNAThr (red). During evolution, duplications would have occurred, and specific tRNAAla and tRNAThr editing proteins emerged. The Gly-rich motif (orange) was appended to the common AlaX-S homolog region (red), allowing deacylation of both Ser-tRNAAla and Gly-tRNAAla, whereas a ThrRS-specific domain (TSD) was fused to the AlaX-S homolog region, providing specificity toward tRNAThr. In a final step, the freestanding specific editing domains were appended through fusion to their corresponding aminoacylation domains (AD, green).
The precise order by which amino acids entered the genetic code remains unknown (55, 57, 58), but it is generally accepted that similar amino acids were added through the reassignment of similar codons. The extant codons for Ala, Thr, Pro, and Ser occupy a common space in the genetic code and, possibly, initially coded for a single amino acid. Their diversification during the growth of the code might have generated a fidelity problem that was solved by AlaX-S, which can deacylate serylated tRNAAla, tRNAThr, and tRNAPro. By extension, it is plausible that other editing proteins or domains might have retained their ancestral tRNA specificities and, therefore, may provide additional information on the evolution and growth of the genetic code.
Acknowledgments
We thank Dr. Olivier Jaillon for discussions regarding the evolution of protein domains.
This work was supported, in whole or in part, by National Institutes of Health Grant R01 GM049928 (to K. M. F.). This work was also supported by Grant BIO2009-09776 (to L. R. d. P.) from the Spanish Ministry of Education and Science.
- ARS
- aminoacyl-tRNA synthetase
- NTD
- N-terminal domain.
REFERENCES
- 1. Pan T. (2013) Adaptive translation as a mechanism of stress response and adaptation. Annu. Rev. Genet. 47, 121–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ribas de Pouplana L., Santos M. A., Zhu J. H., Farabaugh P. J., Javid B. (2014) Protein mistranslation: friend or foe? Trends Biochem. Sci. 39, 355–362 [DOI] [PubMed] [Google Scholar]
- 3. Park S. G., Schimmel P., Kim S. (2008) Aminoacyl tRNA synthetases and their connections to disease. Proc. Natl. Acad. Sci. U.S.A. 105, 11043–11049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gomes A. C., Miranda I., Silva R. M., Moura G. R., Thomas B., Akoulitchev A., Santos M. A. (2007) A genetic code alteration generates a proteome of high diversity in the human pathogen Candida albicans. Genome Biol. 8, R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones T. E., Alexander R. W., Pan T. (2011) Misacylation of specific nonmethionyl tRNAs by a bacterial methionyl-tRNA synthetase. Proc. Natl. Acad. Sci. U.S.A. 108, 6933–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Netzer N., Goodenbour J. M., David A., Dittmar K. A., Jones R. B., Schneider J. R., Boone D., Eves E. M., Rosner M. R., Gibbs J. S., Embry A., Dolan B., Das S., Hickman H. D., Berglund P., Bennink J. R., Yewdell J. W., Pan T. (2009) Innate immune and chemically triggered oxidative stress modifies translational fidelity. Nature 462, 522–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santos M. A., Tuite M. F. (1995) The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res. 23, 1481–1486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wiltrout E., Goodenbour J. M., Fréchin M., Pan T. (2012) Misacylation of tRNA with methionine in Saccharomyces cerevisiae. Nucleic Acids Res. 40, 10494–10506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ibba M., Soll D. (2000) Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 69, 617–650 [DOI] [PubMed] [Google Scholar]
- 10. Beebe K., Ribas De Pouplana L., Schimmel P. (2003) Elucidation of tRNA-dependent editing by a class II tRNA synthetase and significance for cell viability. EMBO J. 22, 668–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cvetesic N., Palencia A., Halasz I., Cusack S., Gruic-Sovulj I. (2014) The physiological target for LeuRS translational quality control is norvaline. EMBO J. 33, 1639–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Döring V., Mootz H. D., Nangle L. A., Hendrickson T. L., de Crécy-Lagard V., Schimmel P., Marlière P. (2001) Enlarging the amino acid set of Escherichia coli by infiltration of the valine coding pathway. Science 292, 501–504 [DOI] [PubMed] [Google Scholar]
- 13. Karkhanis V. A., Mascarenhas A. P., Martinis S. A. (2007) Amino acid toxicities of Escherichia coli that are prevented by leucyl-tRNA synthetase amino acid editing. J. Bacteriol. 189, 8765–8768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee J. W., Beebe K., Nangle L. A., Jang J., Longo-Guess C. M., Cook S. A., Davisson M. T., Sundberg J. P., Schimmel P., Ackerman S. L. (2006) Editing-defective tRNA synthetase causes protein misfolding and neurodegeneration. Nature 443, 50–55 [DOI] [PubMed] [Google Scholar]
- 15. Nangle L. A., Motta C. M., Schimmel P. (2006) Global effects of mistranslation from an editing defect in mammalian cells. Chem. Biol. 13, 1091–1100 [DOI] [PubMed] [Google Scholar]
- 16. Das M., Vargas-Rodriguez O., Goto Y., Suga H., Musier-Forsyth K. (2014) Distinct tRNA recognition strategies used by a homologous family of editing domains prevent mistranslation. Nucleic Acids Res. 42, 3943–3953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang Q., Yao P., Eriani G., Wang E. D. (2012) In vivo identification of essential nucleotides in tRNALeu to its functions by using a constructed yeast tRNALeu knockout strain. Nucleic Acids Res. 40, 10463–10477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ling J., Reynolds N., Ibba M. (2009) Aminoacyl-tRNA synthesis and translational quality control. Annu. Rev. Microbiol. 63, 61–78 [DOI] [PubMed] [Google Scholar]
- 19. Yao P., Zhu B., Jaeger S., Eriani G., Wang E. D. (2008) Recognition of tRNALeu by Aquifex aeolicus leucyl-tRNA synthetase during the aminoacylation and editing steps. Nucleic Acids Res. 36, 2728–2738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. An S., Musier-Forsyth K. (2005) Cys-tRNA(Pro) editing by Haemophilus influenzae YbaK via a novel synthetase·YbaK·tRNA ternary complex. J. Biol. Chem. 280, 34465–34472 [DOI] [PubMed] [Google Scholar]
- 21. Ruan B., Söll D. (2005) The bacterial YbaK protein is a Cys-tRNAPro and Cys-tRNA Cys deacylase. J. Biol. Chem. 280, 25887–25891 [DOI] [PubMed] [Google Scholar]
- 22. Beebe K., Mock M., Merriman E., Schimmel P. (2008) Distinct domains of tRNA synthetase recognize the same base pair. Nature 451, 90–93 [DOI] [PubMed] [Google Scholar]
- 23. Vargas-Rodriguez O., Musier-Forsyth K. (2013) Exclusive use of trans-editing domains prevents proline mistranslation. J. Biol. Chem. 288, 14391–14399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schimmel P., Ribas De Pouplana L. (2000) Footprints of aminoacyl-tRNA synthetases are everywhere. Trends Biochem. Sci. 25, 207–209 [DOI] [PubMed] [Google Scholar]
- 25. Ahel I., Korencic D., Ibba M., Söll D. (2003) Trans-editing of mischarged tRNAs. Proc. Natl. Acad. Sci. U.S.A. 100, 15422–15427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dock-Bregeon A., Sankaranarayanan R., Romby P., Caillet J., Springer M., Rees B., Francklyn C. S., Ehresmann C., Moras D. (2000) Transfer RNA-mediated editing in threonyl-tRNA synthetase: the class II solution to the double discrimination problem. Cell 103, 877–884 [DOI] [PubMed] [Google Scholar]
- 27. Guo M., Chong Y. E., Beebe K., Shapiro R., Yang X. L., Schimmel P. (2009) The C-Ala domain brings together editing and aminoacylation functions on one tRNA. Science 325, 744–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guo M., Chong Y. E., Shapiro R., Beebe K., Yang X. L., Schimmel P. (2009) Paradox of mistranslation of serine for alanine caused by AlaRS recognition dilemma. Nature 462, 808–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sokabe M., Okada A., Yao M., Nakashima T., Tanaka I. (2005) Molecular basis of alanine discrimination in editing site. Proc. Natl. Acad. Sci. U.S.A. 102, 11669–11674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ishijima J., Uchida Y., Kuroishi C., Tuzuki C., Takahashi N., Okazaki N., Yutani K., Miyano M. (2006) Crystal structure of alanyl-tRNA synthetase editing-domain homolog (PH0574) from a hyperthermophile, Pyrococcus horikoshii OT3 at 1.45 A resolution. Proteins 62, 1133–1137 [DOI] [PubMed] [Google Scholar]
- 31. Chong Y. E., Yang X. L., Schimmel P. (2008) Natural homolog of tRNA synthetase editing domain rescues conditional lethality caused by mistranslation. J. Biol. Chem. 283, 30073–30078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fukunaga R., Yokoyama S. (2007) Structure of the AlaX-M trans-editing enzyme from Pyrococcus horikoshii. Acta Crystallogr. D. Biol. Crystallogr. 63, 390–400 [DOI] [PubMed] [Google Scholar]
- 33. Schimmel P. (2011) Mistranslation and its control by tRNA synthetases. Philos Trans. R. Soc. Lond. B. Biol. Sci. 366, 2965–2971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Torres-Larios A., Dock-Bregeon A. C., Romby P., Rees B., Sankaranarayanan R., Caillet J., Springer M., Ehresmann C., Ehresmann B., Moras D. (2002) Structural basis of translational control by Escherichia coli threonyl tRNA synthetase. Nat. Struct. Biol. 9, 343–347 [DOI] [PubMed] [Google Scholar]
- 35. Eddy S. R. (2009) A new generation of homology search tools based on probabilistic inference. Genome Inform. 23, 205–211 [PubMed] [Google Scholar]
- 36. Di Tommaso P., Moretti S., Xenarios I., Orobitg M., Montanyola A., Chang J. M., Taly J. F., Notredame C. (2011) T-Coffee: a web server for the multiple sequence alignment of protein and RNA sequences using structural information and homology extension. Nucleic Acids Res. 39, W13–W17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Felsenstein J. (1988) Phylogenies from molecular sequences: inference and reliability. Annu. Rev. Genet. 22, 521–565 [DOI] [PubMed] [Google Scholar]
- 38. Page R. D. (2002) Visualizing phylogenetic trees using TreeView. Curr. Protoc. Bioinformatics Chapter 6, Unit 6 2 10.1002/0471250953.bi0602s01 [DOI] [PubMed] [Google Scholar]
- 39. Gouy M., Guindon S., Gascuel O. (2010) SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Mol. Biol. Evol. 27, 221–224 [DOI] [PubMed] [Google Scholar]
- 40. Nordin B. E., Schimmel P. (2002) Plasticity of recognition of the 3′-end of mischarged tRNA by class I aminoacyl-tRNA synthetases. J. Biol. Chem. 277, 20510–20517 [DOI] [PubMed] [Google Scholar]
- 41. Chan P. P., Lowe T. M. (2009) GtRNAdb: a database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 37, D93–D97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Geslain R., Aeby E., Guitart T., Jones T. E., Castro de Moura M., Charrière F., Schneider A., Ribas de Pouplana L. (2006) Trypanosoma seryl-tRNA synthetase is a metazoan-like enzyme with high affinity for tRNASec. J. Biol. Chem. 281, 38217–38225 [DOI] [PubMed] [Google Scholar]
- 43. Beuning P. J., Musier-Forsyth K. (2000) Hydrolytic editing by a class II aminoacyl-tRNA synthetase. Proc. Natl. Acad. Sci. U.S.A. 97, 8916–8920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ledoux S., Uhlenbeck O. C. (2008) [3′-32P]-labeling tRNA with nucleotidyltransferase for assaying aminoacylation and peptide bond formation. Methods 44, 74–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goto Y., Katoh T., Suga H. (2011) Flexizymes for genetic code reprogramming. Nat. Protoc 6, 779–790 [DOI] [PubMed] [Google Scholar]
- 46. Hou Y. M., Schimmel P. (1988) A simple structural feature is a major determinant of the identity of a transfer RNA. Nature 333, 140–145 [DOI] [PubMed] [Google Scholar]
- 47. McClain W. H., Foss K. (1988) Changing the identity of a tRNA by introducing a G-U wobble pair near the 3′ acceptor end. Science 240, 793–796 [DOI] [PubMed] [Google Scholar]
- 48. Murakami H., Ohta A., Goto Y., Sako Y., Suga H. (2006) Flexizyme as a versatile tRNA acylation catalyst and the application for translation. Nucleic Acids Symp. Ser. 35–36 [DOI] [PubMed] [Google Scholar]
- 49. Beebe K., Merriman E., Ribas De Pouplana L., Schimmel P. (2004) A domain for editing by an archaebacterial tRNA synthetase. Proc. Natl. Acad. Sci. U.S.A. 101, 5958–5963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hussain T., Kruparani S. P., Pal B., Dock-Bregeon A. C., Dwivedi S., Shekar M. R., Sureshbabu K., Sankaranarayanan R. (2006) Post-transfer editing mechanism of a d-aminoacyl-tRNA deacylase-like domain in threonyl-tRNA synthetase from archaea. EMBO J. 25, 4152–4162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Korencic D., Ahel I., Schelert J., Sacher M., Ruan B., Stathopoulos C., Blum P., Ibba M., Söll D. (2004) A freestanding proofreading domain is required for protein synthesis quality control in Archaea. Proc. Natl. Acad. Sci. U.S.A. 101, 10260–10265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Naganuma M., Sekine S., Chong Y. E., Guo M., Yang X. L., Gamper H., Hou Y. M., Schimmel P., Yokoyama S. (2014) The selective tRNA aminoacylation mechanism based on a single G*U pair. Nature 510, 507–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ahel I., Stathopoulos C., Ambrogelly A., Sauerwald A., Toogood H., Hartsch T., Söll D. (2002) Cysteine activation is an inherent in vitro property of prolyl-tRNA synthetases. J. Biol. Chem. 277, 34743–34748 [DOI] [PubMed] [Google Scholar]
- 54. Geslain R., Ribas de Pouplana L. (2004) Regulation of RNA function by aminoacylation and editing? Trends Genet. 20, 604–610 [DOI] [PubMed] [Google Scholar]
- 55. Schimmel P., Ribas de Pouplana L. (2001) Formation of two classes of tRNA synthetases in relation to editing functions and genetic code. Cold Spring Harb. Symp. Quant. Biol. 66, 161–166 [DOI] [PubMed] [Google Scholar]
- 56. Woese C. R. (1965) On the evolution of the genetic code. Proc. Natl. Acad. Sci. U.S.A. 54, 1546–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Amirnovin R. (1997) An analysis of the metabolic theory of the origin of the genetic code. J. Mol. Evol. 44, 473–476 [DOI] [PubMed] [Google Scholar]
- 58. Ronneberg T. A., Landweber L. F., Freeland S. J. (2000) Testing a biosynthetic theory of the genetic code: fact or artifact? Proc. Natl. Acad. Sci. U.S.A. 97, 13690–13695 [DOI] [PMC free article] [PubMed] [Google Scholar]