Abstract
ERBB3, a member of the Epidermal Growth Factor Receptor (EGFR) family of receptor tyrosine kinases, has been implicated in activation of the phosphatidyl-inositol 3-kinase (PI3K) pathway in human lung adenocarcinomas driven by EGFR mutations. We investigated the contribution of ERBB3 to the initiation, progression and therapeutic response of EGFR-induced lung adenocarcinomas using tetracycline- and tamoxifen- inducible transgenic mouse models. Deletion of Erbb3 at the time of induction of mutant EGFR had no effect on tumorigenesis, demonstrating that ERBB3 is not required to initiate tumorigenesis. Tumors that developed in the absence of ERBB3 remained sensitive to EGFR TKIs and retained activation of the PI3K/AKT pathway. Interestingly, acute loss of Erbb3 suppressed further growth of established EGFRL858R-mediated lung tumors. Four weeks after deletion of Erbb3, the tumors exhibited phosphorylation of EGFR, of the adaptor proteins GAB1 and GAB2 and, of the downstream signaling molecules AKT and ERK suggesting that alternative signaling pathways could compensate for loss of Erbb3. Similar to our observations with mouse tumors, we found that GAB adaptor proteins play a role in ERBB3 independent activation of the PI3K pathway by mutant EGFR in EGFR mutant human cell lines. Finally, in such cell lines, increased levels of phosphorylation of ERBB2 or MET were associated with reduced sensitivity to acute loss of ERBB3, suggesting remarkable plasticity in the signaling pathways regulated by mutant EGFR with important therapeutic implications.
Introduction
The epidermal growth factor receptor (EGFR) is the prototypical member of a family of four receptor tyrosine kinases: EGFR, ERBB2, ERBB3 and ERBB4. EGFR-induced signaling is initiated upon ligand binding, with the formation of EGFR homodimers or heterodimers with other members of the EGFR family (1). This leads to phosphorylation of residues on the cytoplasmic tail of the receptor that are then recognized and bound by intracellular signaling molecules. The four members of the EGFR family have distinctive properties. For example, ERBB2 is unable to bind any known ligands for this family, and ERBB3 lacks intrinsic tyrosine kinase activity (2). Further, the receptors contain different combinations of protein docking sites in their cytoplasmic domains. These features greatly increase the diversity of signals that can be transduced from specific homo- and hetero- dimers.
EGFR can form heterodimers with all three of the other EGFR family members (1). These heterodimers may have distinct and important roles in EGFR-mediated signaling in both normal cellular processes and during carcinogenesis. Evidence for these roles is especially provocative for the EGFR-ERBB3 heterodimer that is the focus of this report.
Mutations in exons encoding the tyrosine kinase domain of EGFR are found in approximately 10–15% of lung adenocarcinomas in the United States and over 40% in Asia (3–6). Two types of mutations account for 90% of all lung adenocarcinoma-associated EGFR mutations and are associated with sensitivity to treatment with the tyrosine kinase inhibitors (TKIs) gefitinib and erlotinib: i) small in-frame deletions in exon 19 that lead to elimination of an LREA motif in the protein (DEL) and ii) a point mutation in exon 21 that substitutes an arginine for a leucine at position 858 in the protein (L858R). As a result of these changes, both the EGFRDEL and EGFRL858R mutants have the ability to transform cells and initiate tumorigenesis when overexpressed in transgenic mouse models (7–9). Human lung tumors bearing EGFR mutations show radiographic responses to TKIs that meet RECIST (“response and evaluation criteria in solid tumors”) criteria in about 70% of cases (10). Eventually, resistance to these TKIs emerges and is most frequently associated with the presence of a secondary mutation in EGFR (T790M) (11).
Studies with human lung cancer cell lines carrying EGFR mutations indicate that the phosphoinositide 3-kinase (PI3K) and signal transducer and activator of transcription (STAT) signaling pathways are important downstream mediators of cell survival (12). Although EGFR itself can activate the PI3K pathway through the adaptor protein GAB1 (GRB2-associated binding protein 1) (13), several lines of evidence indicate that ERBB3 might be the major activator of PI3K/AKT signaling induced by EGFR. First, ERBB3 has seven Tyr-X-X-Met motifs in ERBB3 that upon phosphorylation are recognized by the PI3K regulatory subunit p85, but these motifs are not found in EGFR and ERBB2 (14). Second, gefitinib-sensitive lung cancer cell lines have been shown to use ERBB3 to activate the PI3K pathway (15). Third, in a subset of EGFR mutant TKI-resistant lung cancers with MET amplification, MET dimerizes with ERBB3 to activate the PI3K pathway and thus confers resistance to gefitinib (16). Reduced expression of ERBB3 with siRNAs in these cells reduces the activity of Akt (15,16). Finally, combined treatment of erlotinib-resistant EGFRL858R+T790M-induced tumors with the EGFR antibody cetuximab and an anti-ERBB3 antibody MM-121 causes tumor regression (17). Together these studies have led to the hypothesis that therapeutically targeting of ERBB3 and EGFR together, a strategy currently in clinical trials, may be superior to inhibition of EGFR alone.
To formally test whether the EGFR-ERBB3 heterodimer is the functional oncogenic element in mutant EGFR-driven lung cancer, we investigated the requirement for ERBB3 for lung adenocarcinoma formation in a previously generated transgenic mouse model of EGFR mutant lung adenocarcinoma (9). In this model, human EGFR mutants are regulated by a tetracycline-responsive element (TetO), and the reverse tetracycline transactivator (rtTA) is controlled by the CCSP (clara cell specific protein) promoter. (rtTA is expressed in type II pneumocytes in this transgenic strain.) Delivery of doxycycline activates expression of mutant EGFR transgenes. These mice subsequently develop lung adenocarcinomas that are responsive to EGFR TKI treatment (9).
We used these mouse models to delete mouse Erbb3 in the same cells that express mutant EGFR prior to tumor formation and in established tumors to test its role in tumor initiation and maintenance, respectively. Our findings show that Erbb3 is not required to initiate mutant EGFR-driven lung tumorigenesis when over-expressed. Tumors that formed in the presence or absence of Erbb3 had similar histology and did not differ in their response to the TKI erlotinib. Interestingly, ERBB3 loss in established tumors restrained tumor growth. We also explored the significance of these findings with respect to intracellular signaling as presented below.
Materials and Methods
Mouse husbandry
All animals were kept in pathogen-free housing under guidelines approved by the Yale and Memorial Sloan-Kettering Cancer Center Animal Care and Use Committees. Doxycycline was administered by feeding mice with doxycycline-impregnated food pellets after mice were weaned (625 ppm; Harlan-Teklad). The tetracycline-inducible EGFR transgenic models (9), Erbb3 floxed mice (18), Nkx2.1-CreERT2 mice (19) and TetO-Cre (20) mice were all previously described.
Cell culture and transient transfections
PC9, H3255, H1975, and HCC827 cells have been maintained in the Politi laboratory since 2010 and were obtained from the Varmus Laboratory. PC9BRc1 cells were obtained from William Pao (21). All cells were grown in RPMI 1640 medium containing 10% FBS and penicillin/streptomycin in a 37°C incubator with 5% CO2. Cells were examined regularly for mycoplasma contamination and authenticated by STR profiling (GenePrint® 10 System) at the Yale University DNA Analysis Facility in December 2014.
Drug treatment
Mice were treated with 25mg/kg/day of erlotinib for either 5 days or for 4–6 weeks (9). Tamoxifen was administered 5mg/day in 250 μl corn oil for two days to induce deletion of Erbb3. Tumor regression was monitored using MRI as described in the Supplementary Materials and Methods.
Immunoblotting
Whole lung lysates were made as described for the RTK arrays in the Supplementary Materials and Methods. Protein concentrations were quantified using the Bio-Rad Dc protein assay reagent. For each sample, 50 μg of protein lysate were loaded onto 4–20% gradient gels (Bio-Rad precast) and electrophoresis was performed followed by transfer to nitrocellulose. Membranes were blocked in 0.1 % Tween-20 with 5% w/v nonfat dry milk then incubated with specific antibody to each target protein at 4°C overnight followed by 2 hours of incubation with a secondary antibody and visualization using ECL.
Results
EGFR mutant tumors form at normal rate in the absence of Erbb3
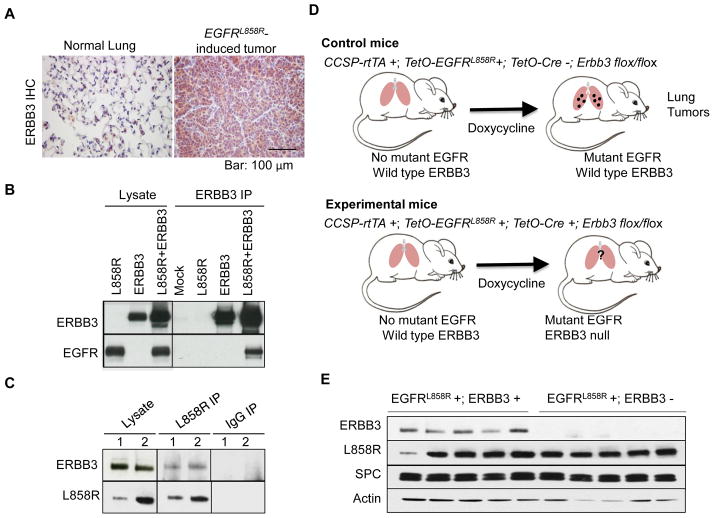
As a first step in establishing whether ERBB3 plays a role in lung tumorigenesis driven by mutant EGFR in mice, we examined whether ERBB3 is produced in tumors induced by mutant EGFR. Immunohistochemical staining of lung tumors obtained from CCSP-rtTA +; TetO-EGFRL858R + mice (9) revealed strong cytoplasmic staining for ERBB3 protein in tumor tissue and minimal staining in normal lung (Fig. 1A).
Figure 1. ERBB3 is present in murine EGFR mutant lung adenocarcinomas and interacts with mutant EGFR.
(A) Immunohistochemical staining for ERBB3 is strong in EGFRL858R-induced lung tumors (right) but weak or non-existent in normal lung from control mice (left). (B) EGFRL858R interacts with ERBB3 in transfected cells. Plasmids encoding human EGFRL858R, mouse ERBB3 or both were transfected into 293T cells. Two days after transfection, an antibody to ERBB3 was used to co-precipitate ERBB3 and proteins associated with it. Immunoprecipitates were blotted with antibodies to EGFR and ERBB3. Results from mock-transfected cells are shown as indicated. (C) Mutant EGFRL858R interacts with ERBB3 in mutant EGFR-induced lung tumors. EGFRL858R or IgG immunoprecipitates from two CCSP-rtTA+;TetO-EGFRL858R+ mice (1 and 2) were immunoblotted with antibodies specific to mutant EGFRL858R and ERBB3. (D) Strategy used to conditionally delete Erbb3 in type II pneumocytes in tetracycline-inducible mouse models of EGFR mutant lung cancer. Control mice (top) express mutant EGFR and wild-type Erbb3 in the lung epithelium upon induction of doxycycline. Experimental mice (bottom) express mutant EGFR but not ERBB3 due to the presence of Cre recombinase, which deletes floxed sequences in the Erbb3 locus. (E) ERBB3 protein is not detected in lungs of mice upon deletion of Erbb3. Immunoblots of lung protein extracts with antibodies to EGFRL858R, ERBB3, Surfactant Protein C (SPC) and Actin are shown. Protein levels were normalized for numbers of pneumocytes as judged by levels of surfactant protein C. EGFRL858R+; Erbb3+ (control mice) and EGFRL858R+; Erbb3− (experimental mice). IP, immunoprecipitation.
Since the transgenic mice express a human EGFR cDNA, we also examined whether transgenic human mutant EGFR interacts with mouse ERBB3 in both cultured cells and mutant EGFR-driven mouse lung tumors that contain both proteins. We first confirmed that human EGFRL858R can bind to murine ERBB3 by overexpressing human EGFRL858R and murine ERBB3, individually or together, in 293T cells via transient transfection. Antibodies that specifically recognize ERBB3, EGFR or only EGFRL858R (L858R) were used for co-immunoprecipitation and immunoblotting. EGFR was detected after immunoprecipitation with the ERBB3 antibody only when both proteins were present (Fig. 1B). In reciprocal experiments, ERBB3 was detected in precipitates formed with an antibody against EGFRL858R (Supplementary Fig. 1). Furthermore, we found that ERBB3 co-precipitated with mutant EGFR from lysates of mouse lung tumors (Fig. 1C). These data indicate that mutant human EGFR is able to interact with mouse ERBB3, that these heterodimers form in mouse tumors induced by mutant human EGFR, and that mutant human EGFR could, in principle, signal through ERBB3 during lung tumorigenesis.
Given these results, we set out to determine whether ERBB3 is functionally important during tumorigenesis initiated by mutant EGFR in mice by deleting Erbb3 in the same cells in which mutant EGFR is expressed. To do this, we crossed CCSP-rtTA +; TetO-EGFRL858R+ mice with mice that carry floxed alleles of Erbb3 (18) and a TetO-Cre transgene (20), creating mice that carry one copy of each of the three transgenes and are homozygous for the floxed allele of Erbb3. In the absence of the tetracycline analog, doxycycline, mutant EGFR is not expressed and the Erbb3 gene remains intact (Fig. 1D). Feeding doxycycline to experimental mice leads to expression of mutant EGFR and Cre from the tetracycline-inducible promoters in type II pneumocytes, with subsequent excision of the floxed portion of the Erbb3 gene (Fig. 1D). Western blotting of whole lung extracts demonstrated that Cre-mediated deletion of floxed Erbb3 alleles causes the loss of ERBB3 protein but production of mutant EGFR is unaffected (Fig. 1E).
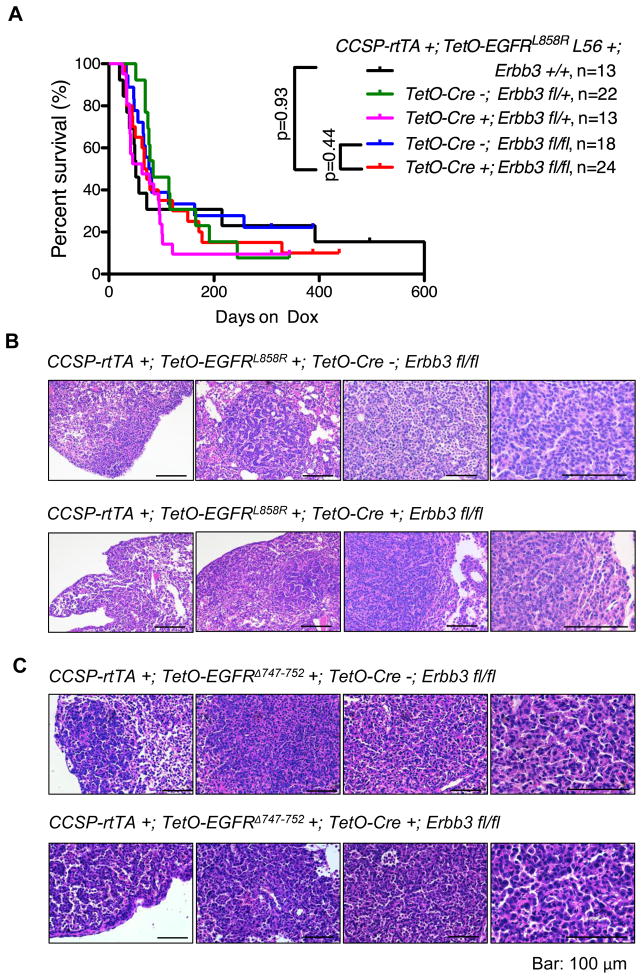
To assess whether loss of Erbb3 had an effect on EGFR-induced lung carcinogenesis, we monitored a cohort of CCSP-rtTA +; TetO-EGFRL858R +; TetO-Cre +; Erbb3 fl/fl mice and littermates of control genotypes for signs of advanced cancer. All mice that carried the CCSP-rtTA and TetO-EGFRL858R transgenes became cachectic, showed decreased activity and had to be sacrificed, regardless of whether they also carried intact Erbb3 alleles. Survival curves derived from mice of the different genotypes did not differ significantly in two different TetO-EGFRL858R transgenic lines (Fig. 2A and Supplementary Fig. 2), implying that deletion of Erbb3 had little or no effect on survival. At necropsy we observed enlarged lungs with macroscopically visible tumor nodules. Histological sections showed adenocarcinomas irrespective of genotype (Fig. 2B). Similar results were observed with mice carrying a transgene with the EGFR exon19 deletion (CCSP-rtTA +; TetO-EGFRΔ747-752 +; TetO-Cre +; Erbb3 fl/fl) (Fig. 2C).
Figure 2. Development of lung adenocarcinomas in mice lacking ERBB3.
(A) Kaplan-Meier survival curves comparing CCSP-rtTA+;TetO-EGFRL858R+ mice with (Erbb3 +/+, Erbb3fl/fl; Cre−, Erbb3 fl/+; Cre−), without (Erbb3 fl/fl; Cre+) or heterozygous for (Erbb3 fl/+; Cre+) Erbb3. L56, transgenic line 56. (B) Hematoxylin and Eosin staining of EGFRL858R-induced tumors that developed in the presence and absence of ERBB3. Representative images of mouse lungs from mice on doxycycline for approximately two months are shown. Bar, 100μm. (C) Hematoxylin and Eosin staining of EGFRΔ747-752-induced tumors that developed in the presence and absence of ERBB3. Representative images of mouse lungs from mice on doxycycline for approximately four months are shown. Bar, 100μm.
EGFR mutant tumors lacking Erbb3 retain sensitivity to erlotinib
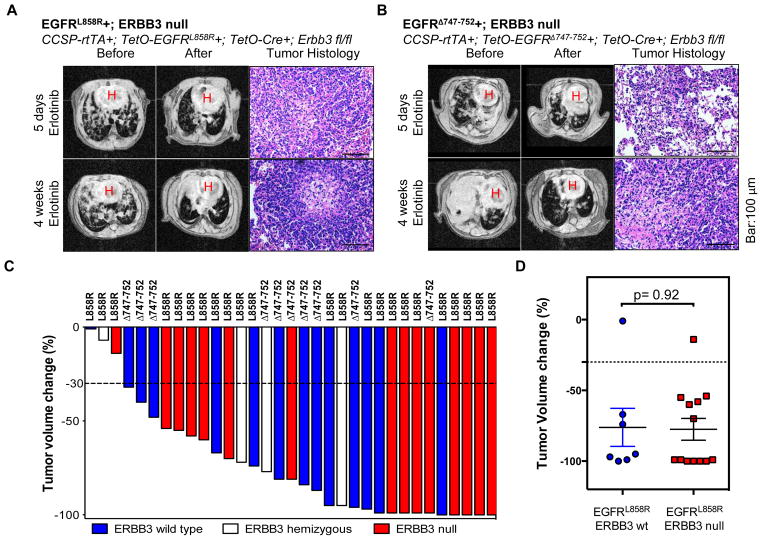
EGFR TKIs block activation of the ERBB3-mediated PI3K/AKT pathway in TKI-sensitive, human lung adenocarcinoma cell lines carrying either wild type or mutant EGFR (15). This implies that a potentially important aspect of therapeutic response is dependent on EGFR-mediated signaling via ERBB3. Therefore, we asked whether mouse lung tumors that arose without ERBB3 responded differently to TKI treatment than those arising with ERBB3. Mice with EGFRL858R- or EGFRΔ747-752-induced lung tumors, both with and without intact Erbb3 genes, were treated with erlotinib for 5 days or 4 weeks, and monitored with magnetic resonance imaging (MRI) before and during treatment. Tumor volumes for each mouse were quantified to evaluate the drug response. After 5 days of erlotinib treatment, tumors regressed in two of three CCSP-rtTA +; TetO-EGFRL858R +; TetO-Cre +; Erbb3 fl/fl mice (Fig. 3A, Supplementary Table 1) and in twelve out of thirteen CCSP-rtTA +; TetO-EGFRL858R +; TetO-Cre +; Erbb3 fl/fl mice after four weeks (Figs. 3A, 3C, Supplementary Table 1). These findings were not significantly different from results with control mice that retained intact Erbb3 alleles (CCSP-rtTA +; TetO-EGFRL858R +; TetO-Cre −; Erbb3 fl/fl): tumors regressed in two of two mice after 5 days treatment (P-value= 0.83) and six of seven mice after four weeks treatment (P-value=0.92, Fig. 3D and Supplementary Table 1). EGFRΔ747-752-induced lung tumors also showed a radiographic response to erlotinib, regardless of Erbb3 status (Figs. 3B, 3C, Supplementary Table 1). In addition, all animals showed histological evidence of tumor regression, such as scarring, after 4 weeks of drug treatment, irrespective of genotype (Fig. 3A, 3B). Thus deletion of Erbb3 does not impair the response of EGFR mutant tumors to TKIs.
Figure 3. Lung tumors that develop in the absence of ERBB3 are sensitive to erlotinib.
(A) CCSP-rtTA +; TetO-EGFRL858R+; TetO-Cre +; Erbb3fl/fl mice respond to short- and long-term erlotinib treatment. Sick mice were treated with erlotinib for 5 days or 4 weeks before they were sacrificed. Mice were imaged prior to (before) and towards the end (after) of treatment. Representative MR images and H&E stained sections are shown. Images of residual tumors observed by histopathology are shown. Bar, 100μm. (B) CCSP-rtTA +; TetO-EGFRΔ747-752+; TetO-Cre +; Erbb3fl/fl mice respond to short- and long-term erlotinib treatment. Sick mice were treated with erlotinib for 5 days or 4 weeks before they were sacrificed. Mice were imaged prior to (before) and towards the end (after) of treatment. Representative MR images and H&E stained sections are shown. Images of residual tumors observed by histopathology are shown. Bar, 100μm. (C) Waterfall plot showing the change in tumor volume from baseline in mice with mutant EGFR-induced lung tumors (EGFRL858R or EGFRΔ747-752) upon treatment with erlotinib for 4 weeks. The Erbb3 genotypes of individual mice are indicated. (D) Lung tumor volume change in mice with EGFRL858R-induced lung tumors upon treatment with erlotinib for 4 weeks.
The PI3K signaling pathway is activated despite deletion of Erbb3
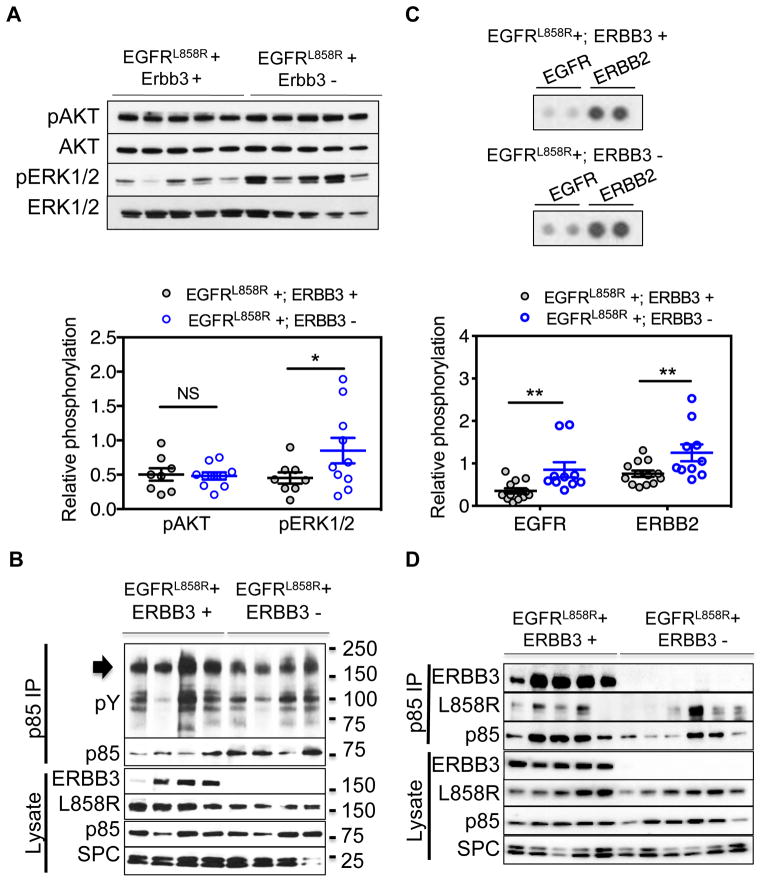
ERBB3 is a strong activator of the PI3K signaling pathway. Therefore we examined whether activation of this pathway is altered in tumors arising in the absence of ERBB3. We used phosphorylation of Akt as measured by Western blotting of lung tumor lysates from two groups of mice (CCSP-rtTA +; TetO-EGFRL858R +; TetO-Cre +; Erbb3 fl/fl and CCSP-rtTA +; TetO-EGFRL858R +; TetO-Cre −; Erbb3 fl/fl mice) (Fig. 4A, top) as an indicator of signaling through PI3K. Tumors arising with and without ERBB3 showed similar amounts of Akt and phospho-Akt, indicating that PI3K signaling does not depend on formation of EGFR-ERBB3 heterodimers (Fig. 4A, bottom p-value=0.9). Immunoblotting of these lysates to measure phosphorylation of ERK1/2, an indicator of signaling through RAS and RAF proteins, revealed a significant increase of phospho-ERK in Erbb3 deficient tumors (Fig. 4A, p-value=0.03). In addition, these experiments did not show differences in phospho-STAT3 levels, suggesting that ERBB3 is not required for activation of the Jak/Stat pathway (Supplementary Fig. 3A).
Figure 4. Intact PI3K and MAPK Signaling in Lung Tumors Deficient for ERBB3.
(A) Immunoblots of protein lysates from lungs of mice with EGFRL858R-induced lung tumors arising in the presence (EGFRL858R+; ERBB3+) or absence (EGFRL858R+; ERBB3−) of ERBB3 and probed with antibodies to pAKT, total AKT, pERK and total ERK (top). The data are presented as the mean ± the standard error (bottom). (B) Immunoprecipitation of p85 from EGFRL858R-induced lung tumors. The immunoprecipitates were blotted with anti-phosphotyrosine and p85 antibodies. The arrow indicates bands the size of EGFR family receptors in the phosphotyrosine immunoblot. Immunoblots of lysates using antibodies to ERBB3, EGFR, p85 and SPC are shown. (C) Phospho-RTK arrays reveal increased phosphorylation of EGFR and ERBB2 upon deletion of ERBB3. Whole lung tumor lysates from eight ERBB3 wild type mice and ten ERBB3 null mice were hybridized to phospho-RTK arrays (top). Signals for EGFR family members on representative arrays are shown. Data are presented as the mean ± standard error (bottom). (D) Immunoprecipitation of p85 from lung tumor lysates that develop in the presence (EGFRL858R+; ERBB3+) and absence (EGFRL858R+; ERBB3−) of Erbb3. Immunoprecipitates were immunoblotted with antibodies to ERBB3, EGFRL858R and p85. IP, immunoprecipitation. *indicates p-value ≤0.05; ** indicates p-value < 0.01; NS, not significant.
Since tumors arising in the absence of ERBB3 continue to phosphorylate AKT, it appears that either (i) activation of the PI3K pathway by mutant EGFR does not normally occur through ERBB3 or (ii) activation of the PI3K pathway by mutant EGFR normally occurs through ERBB3 but that alternative pathways can lead to activation of PI3K/AKT when Erbb3 is lost. To investigate these possibilities, we examined the proteins associated with the regulatory subunit of Class IA PI3K in the presence and absence of Erbb3. Class IA PI3Ks are composed of a regulatory subunit (p85) and a catalytic subunit (p110) (22–24). In the absence of activating signals, p85 stabilizes p110 in an inactive state, inhibiting its catalytic activity. Upon growth factor stimulation, p85 binds to activated receptors or to protein adaptors at the phosphorylated YXXM motif, releasing the catalytic subunit from inhibition (22–24).
We used anti-p85 sera to identify phosphorylated proteins that interact with p85 in the presence and absence of ERBB3. Anti-p85 immunoprecipitates from lysates of mouse lung tumors were immunoblotted with an antibody against phosphotyrosine. Several phosphotyrosine-containing proteins in the size range of EGFR family members (arrow, 170–190 kDa) co-precipitated with p85 in the presence and absence of ERBB3 (Fig. 4B), indicating that proteins other than ERBB3 interact with the regulatory subunit of PI3K in these lung tumors.
We used mouse phospho-receptor tyrosine kinase arrays to identify proteins in the relevant size range that were tyrosine-phosphorylated in mouse lung tumors. EGFR and ERBB2 were among the most prominent phosphotyrosine-containing proteins in these assays, and both were more abundant in ERBB3-deficient tumor lysates (phosphorylated EGFR with a two-fold increase [p-value <0.0001] and phosphorylated ERBB2 with a 1.5-fold increase [p-value <0.0001], Fig. 4C). These findings were confirmed by immunoprecipitation (Supplementary Fig. 3C and 3D). Most other phosphotyrosine-containing receptors measurable with this assay were not activated in lung tumors driven by mutant EGFR, regardless of the presence or absence of Erbb3. Notably, there was a very low level of phosphotyrosine-containing ERBB3, which was only detectable upon long exposure of the array (Supplementary Fig. 3B). Importantly, there was no evidence differential activation of additional receptors on the RTK array upon ERBB3 deletion (Supplementary Fig. 3B).
These results raised the possibility that EGFR may interact with p85 in the absence of ERBB3. We used antisera specific to EGFR and ERBB3 to detect these EGFR family members in anti-p85 immunoprecipitates from extracts of tumors induced in the presence and absence of ERBB3 (Fig. 4D). Both EGFR and ERBB3 were detected in the p85 immunoprecipates from lysates of ERBB3-containing tumors. In reciprocal experiments, p85 was detected in EGFRL858R immunoprecipitates in ERBB3-containing and ERBB3-depleted mouse tumors (Supplementary Fig. 4A). Similar results showing that EGFRL858R co-precipitates with p85 from extracts of cultured 293T cells that lack endogenous ERBB3 (Supplementary Fig. 1). In summary, in the absence of ERBB3, the PI3K signaling pathway is active; this activation could be mediated by a direct or indirect association of mutant EGFR with p85. ERBB2 may also participate in PI3K activation in this setting, however we did not find conclusive evidence in this regard.
Gab Family Proteins Couple the PI3K Pathway to Mutant EGFR
Since EGFR (and ERBB2) lacks a canonical p85-binding site, adaptor proteins are likely to bridge the association between EGFR and p85. Docking proteins---including the GRB2-associated binder protein (GAB) and insulin receptor substrate (IRS) protein families---have been implicated as activators of the PI3K signaling pathway after EGF stimulation (13,25,26)(27)(28).
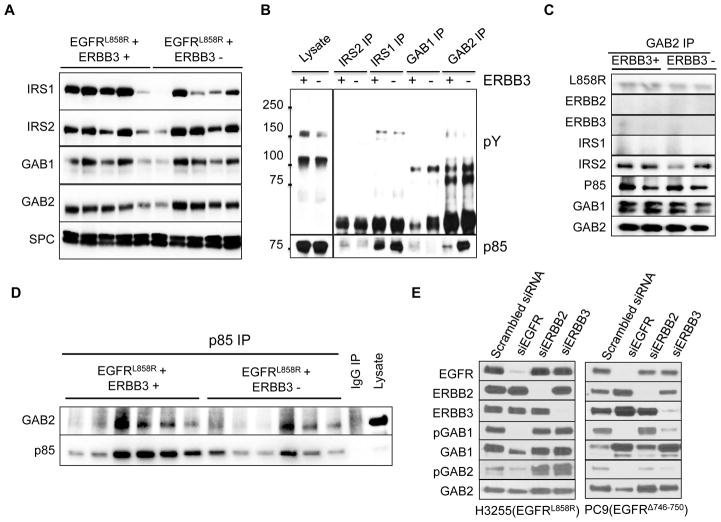
To examine whether mutant EGFR activates the PI3K pathway via these mediators, we first determined the level of these proteins in mutant EGFR-driven lung tumors by Western blotting. Lysates of EGFR-mutant mouse lung tumors contain GAB1, GAB2, IRS1 and IRS2, and the levels of these proteins are unaffected by the absence of ERBB3 (Fig. 5A). To identify which of these proteins might activate PI3K in this context, we determined which adapter proteins and receptors are associated with p85 (Fig. 5B). Interestingly, p85 co-precipitated with IRS1 and GAB2 in the lysates from tumors independent of ERBB3 status (Fig. 5B, 5C, Supplementary Fig. 4B). In the reciprocal experiment, p85 precipitated with GAB2 in mouse tumors (Fig. 5D, Supplementary Fig. 4C). Moreover, EGFR co-precipitated, albeit weakly, with GAB2 in EGFR mutant lung tumors regardless of the presence of ERBB3 (Fig. 5C) while co-precipitation of IRS1 and EGFR was barely detectable (Supplementary Fig. 4B).
Figure 5. Adapter proteins bind p85 in vivo in EGFR mutant lung tumors.
(A) Immunoblots of lung protein lysates from mice with EGFRL858R-induced tumors arising in the presence (EGFRL858R+; ERBB3+) and absence (EGFRL858R+; ERBB3−) of ERBB3 using antibodies to proteins that can directly bind p85 (IRS1, IRS2, GAB1 and GAB2). The same lysates were blotted with an antibody to SPC as a control for the fraction of pneumocytes present. (B) Co-immunoprecipitation of the adapter proteins IRS1, IRS2, GAB1 and GAB2 from lung protein lysates from mice with EGFRL858R-induced tumors arising in the presence (+) and absence (−) of ERBB3. The immunoprecipitates were blotted with antibodies to phosphotyrosine (pY, top) and p85 (bottom). (C) Co-immunoprecipitation of EGFR, GAB1, GAB2, IRS1, IRS2 and p85, with GAB2 from lung protein lysates from mice with EGFRL858R-induced tumors arising in the presence (+) and absence (−) of Erbb3. (D) Co-immunoprecipitation of GAB2 with p85 from lung protein lysates from mice with EGFRL858R-induced tumors arising in the presence (+) and absence (−) of Erbb3. IP, immunoprecipitation. (E) GAB1 and GAB2 are expressed in EGFR mutant lung cancer cell lines and phosphorylated by EGFR. H3255 and PC9 cells were transfected with either scrambled siRNA or siRNA of EGFR, ERBB2 or ERBB3. Total and phospho- protein levels were analyzed by Western blotting.
Taken together, these results indicate that both p85 and EGFR co-precipitate in a GAB1/GAB2-containing complex, which might promote activation of PI3K by EGFR. Consistent with this hypothesis, GAB1 and GAB2 are expressed and phosphorylated in several human lung cancer cell lines (Fig. 5E, 7A) and GAB2 co-precipitates with p85 in these lines regardless of the presence of ERBB3 (Supplementary Figure 4D). Interestingly, HCC827 cells (insensitive to ERBB3 loss) exhibited higher levels of GAB2 and IRS1 than PC-9 cells (partially sensitive to ERBB3 loss; Supplementary Figure 4D). Furthermore, siRNA knock-down of EGFR greatly diminished phosphorylation of GAB1 and GAB2 indicating that phosphorylation of GAB1 and GAB2 is regulated by EGFR in human cancer cells as well (Fig. 5E and Supplementary Fig. 5A). In summary, it seems likely that mutant EGFR can activate the PI3K pathway through at least two different mechanisms: 1) via ERBB3, which binds p85 directly and 2) by binding the adaptor proteins GAB1 or GAB2 to recruit p85.
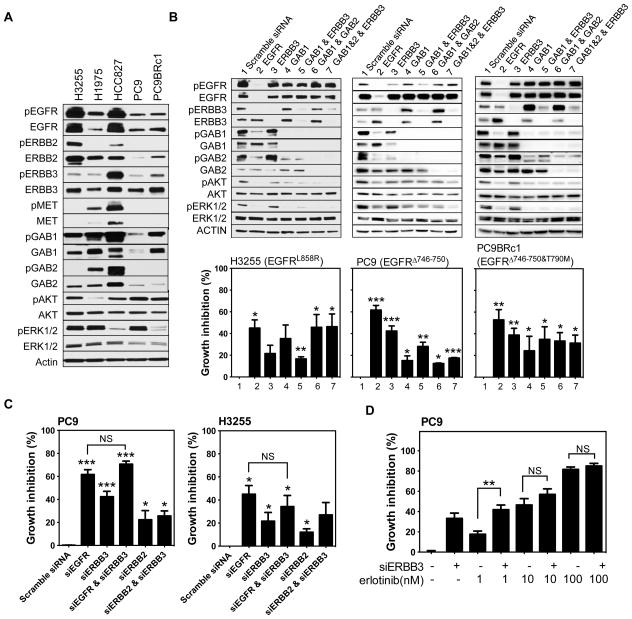
Figure 7. ERBB3 knockdown partially affects the viability of EGFR mutant, human lung cancer cell lines.
(A) Western blots of protein lysates derived from EGFR mutant lung cancer cell lines probed with antibodies as indicated. (B) H3255, PC9 and PC9BRc1 cells were transiently transfected with pools of siRNAs against EGFR, ERBB3, GAB1, GAB1+ERBB3, GAB1+GAB2, GAB1+GAB2+ERBB3 or a scrambled siRNA control as indicated. Three days after transfection, cells were lysed in RIPA buffer and proteins were analyzed by Western blotting and cell viability was examined using the CellTiter blue assay. Experiments were performed at least three times for each cell line. (C) Viability of PC9 and H3255 cells after transfection with pools of siRNAs against EGFR, ERBB3, EGFR+ERBB3, ERBB2 and ERBB2+ERBB3. Cell viability was measured using the Cell Titer Blue Assay. (D) Growth inhibition in PC9 cells upon ERBB3 knockdown treated with varying concentrations of erlotinib as indicated. *indicates p-value ≤ 0.05, *** indicates p-value ≤ 0.01. NS: not significant.
Acute inactivation of Erbb3 restrains the growth of EGFR mutant lung tumors
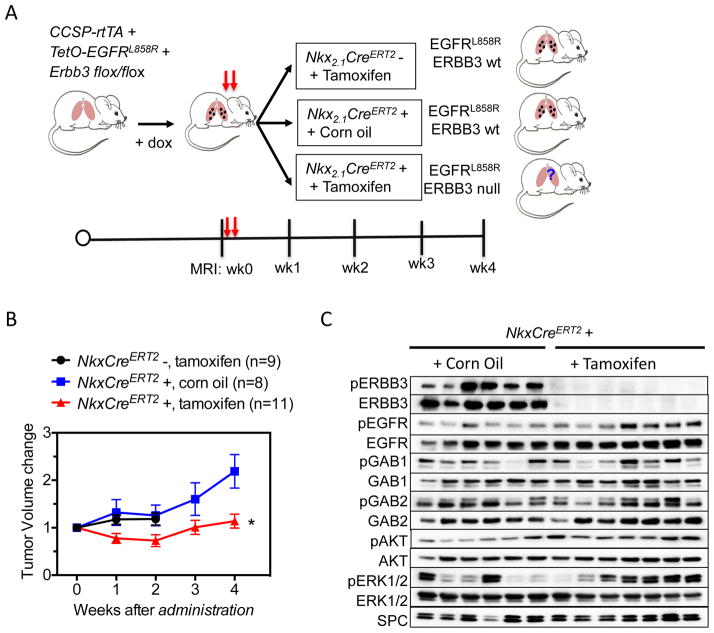
To assess the therapeutic potential of targeting ERBB3 in lung cancer, we tested whether it is required for the survival of established mouse lung tumors. We crossed CCSP-rtTA +; TetO-EGFRL858R + mice to mouse lines with Nkx2.1-CreERT2 (19) and Erbb3 flox/flox alleles (Fig. 6A) to generate CCSP-rtTA +; TetO-EGFRL858R +; Nkx2.1-CreERT2; Erbb3 flox/flox mice and appropriate controls. Tumors were induced using doxycycline to activate transcription of the mutant EGFR transgenes. To restore enzymatic activity to CreERT2 and delete Erbb3 from both normal and transformed mouse lung epithelial cells, tamoxifen was administered to tumor-bearing mice by oral gavage. As controls, mice of the same genotype were treated with corn oil and mice without the Nkx2.1-CreERT2 allele were treated with tamoxifen. The former control is especially important because Nkx2.1 heterozygosity has been shown affect the formation of mutant EGFR-induced tumors (29). Lung tumor volume was assessed weekly for 4 weeks following tamoxifen administration. Overall during the 4-week period of observation following acute Erbb3 loss, tumor size was stable (Fig. 6B), By contrast, in mice with wild type ERBB3, the tumors continued to grow. These data indicate that Erbb3 signaling contributes to the overall growth of established EGFR mutant tumors.
Figure 6. Partial dependence of EGFRL858R-induced lung adenocarcinomas on Erbb3.
(A) Model for tamoxifen induced deletion of Erbb3. Mutant EGFRL858R tetra transgenic mice were fed doxycycline to induce tumors. When the tumor size reached a minimum volume of 100 mm3, experimental mice (Nkx2.1-CreERT2+) were administered tamoxifen by oral gavage at the doses of 250 mg/day for two days. Control animals were Nkx2.1-CreERT2 negative and treated with tamoxifen or Nkx2.1-CreERT2 positive treated with corn oil. After tamoxifen treatment, mice were imaged using MRI weekly for 4 weeks. The tumor burden for each group is shown in Fig. 6B. Mouse lungs were collected at the end of 4 weeks or when moribund and tumor extracts were analyzed by Western blot for the indicated proteins (Fig. 6C).
To explore the molecular changes in mouse lung tumors after loss of Erbb3, we compared signaling pathway activation in mouse lung tissue 4 weeks after corn oil or tamoxifen treatment. As expected, Erbb3 was effectively deleted in mouse lung tissue (Fig. 6C). Upon Erbb3 deletion, the levels of EGFR, GAB1, GAB2, AKT and ERK phosphorylation were maintained and in some cases increased (Fig. 6C), consistent with our observations in tumors initiated in the absence of Erbb3 (Fig. 4C). In summary, acute loss of Erbb3 was associated with sustained EGFR pathway signaling, consistent with the notion that mutant EGFR can use GAB adaptor proteins to activate the PI3 kinase pathway and transduce an oncogenic signal.
ERBB2 and MET phosphorylation levels correlate with the sensitivity of EGFR-mutant human lung adenocarcinomas to loss of ERBB3
The studies described above demonstrate that acute loss of ERBB3 can, at least partially, restrict tumor growth. We used a panel of established EGFR mutant human lung cancer cell lines to further investigate factors associated with the sensitivity of EGFR mutant tumors to the loss of ERBB3. Western blots of lysates from five such lines revealed that all contain ERBB3 and that it is phosphorylated in each cell line, albeit at varying levels (Fig. 7A). We studied the effect of acute loss of ERBB3 in the cell lines by transiently knocking down expression of ERBB3 with siRNAs. Western blotting showed that ERBB3 protein levels were efficiently reduced by siRNA in all cell lines analyzed (Fig. 7B and Supplementary Fig. 5). Similar reduction in EGFR resulted from introduction of EGFR-specific siRNAs (Fig. 7B). However, while loss of EGFR protein reduced cell viability by 50–75%, loss of ERBB3 had a more limited effect, with 0–40% loss of viability, indicating that tumor cell survival depends more heavily on mutant EGFR than on ERBB3, findings consistent with our data in mice. HCC827, H3255 and H1975 cells were the least affected by reduction of ERBB3 levels, and they demonstrated the highest levels of phosphorylation of MET or ERBB2 and of total EGFR (Fig. 7A). Furthermore, knockdown of ERBB3 had the strongest effect on phosphorylation of AKT and ERK in human lung cancer cell lines with relatively low levels of MET and ERBB2 phosphorylation (Fig. 7B, Supplementary Fig. 5). These data suggest that there is intrinsic variability in EGFR mutant tumors with regard to their dependence on ERBB3 for survival and that a normal level of signaling through ERBB3 is not a prerequisite for survival of cells transformed by mutant EGFR. This may be especially so in cells that can sustain signaling through other trans-membrane receptors, such as MET and ERBB2. Of note, our data in HCC827 cells contrast published results in which ERBB3 knock-down was shown to have an effect on the viability of these cells (16). Technical differences in our approaches (siRNA vs. shRNA knock-down and timing of the assays) as well as divergence in our cell lines could account for the discrepancy in these results.
We also noticed that the adapter proteins GAB1 and GAB2 remain phosphorylated in cells after knockdown of ERBB3 with inhibitory RNAs (Fig. 7B and Supplementary Fig. 5). To explore the role of these proteins more directly as activators of the PI3K pathway in EGFR-mutant, human lung cancer cell lines, we knocked down GAB1 alone or both GAB1 and GAB2 in several cell lines. Phosphorylation of Akt and Erk declined in H3255, PC9, and PC9BRc1 cells after GAB1 knock down (Fig. 7B). In H3255 and H1975 cells, which were amongst the least affected by ERBB3 knockdown, combined knockdown of GAB1 and GAB2 reduced viability by 40–50% (Fig. 7B, Supplementary Fig. 5). In contrast, in PC9 and PC9BRc1 cells, which were most susceptible to ERBB3 inhibition, knockdown of GAB1 or GAB1+GAB2 reduced viability by only 20–30%. Combined knockdown of ERBB3, GAB1, and GAB2 did not reduce viability further in any of the cell lines tested except in H3255 cells, suggesting that multiple mechanisms may be available to sustain viability, perhaps through the PI3K pathway.
To test whether inhibition of EGFR and ERBB3 had a synergistic effect on the viability of EGFR mutant lung cancer cells, we knocked down both EGFR and ERBB3 in PC9 and H3255 cells. However, the double knock-down did not affect viability more than knockdown of EGFR alone (Fig. 7C and Supplementary Fig. 6A and 6B). Knockdown of ERBB2 only slightly reduced the viability of these EGFR mutant cell lines, regardless of whether ERBB3 was present (Fig. 7C and Supplementary Fig. 6A and 6B), suggesting that ERBB2/ERBB3 heterodimers do not have an important role in activating survival signaling, presumably through PI3K, in these cells.
To model combinatorial treatments designed to block both EGFR and ERBB3 signaling, we evaluated the effect of the EGFR TKI erlotinib and ERBB3 knock-down in PC9 cells, which were the most sensitive to loss of ERBB3 (Fig. 7D). We found that reducing ERBB3 levels did not enhance the effects of erlotinib at concentrations well below physiological drug concentrations (30).
Discussion
ERBB3 is not required for mutant EGFR-induced tumorigenesis but its loss can slow the growth of established tumors
Most lung adenocarcinomas with sensitizing EGFR mutations are exquisitely sensitive to tyrosine kinase inhibitor (TKI) treatment (3–5). In such tumors, mutant EGFR activates critical pathways, such as the PI3K/AKT, MEK/ERK and JAK/STAT pathways to promote transformation and tumor cell growth and survival. EGFR activates its downstream signaling pathways by homo- and/or heterodimerizing with other EGFR family receptors including ERBB3. However, prior to this study, very little was known about which dimers of mutant EGFR are the essential oncogenic units during cell transformation. Our data demonstrate that in mice, ERBB3 is not required to initiate tumorigenesis driven by mutant EGFR in a transgenic mouse model. Established tumors, in contrast, partially depend on ERBB3: loss of Erbb3 stabilizes tumor growth. We also show that in human tumor cell lines and in adenocarcinomas in mouse models, response of EGFR-driven tumor cells to the tyrosine kinase inhibitor, erlotinib, is independent of the presence or absence of ERBB3. In contrast to our results with lung cancer, deletion of ERBB3 in the ApcMin mouse model of colon cancer decreased PI3K signaling, promoted apoptosis, and reduced tumor size (18). These results suggest that the requirement for ERBB3 may depend upon both the driver mutations and the tumor cell lineage.
In our mouse models of mutant EGFR-driven lung cancer, few receptors other than EGFR, ERBB2 and ERBB3 (at low levels) are tyrosine phosphorylated. This indicates that mutant EGFR does not require activation of other RTKs for lung tumorigenesis. Both EGFR and ERBB2 were highly phosphorylated in mutant EGFR-driven lung tumors, and phosphorylation increased in tumors when Erbb3 was deleted. It is likely that loss of ERBB3 leads to the increased formation of EGFR homodimers or EGFR/ERBB2 heterodimers, which cause increased phosphorylation of EGFR and ERBB2. The functional consequences of this increase in EGFR and ERBB2 phosphorylation, however, remain to be determined.
These observations raise the possibility that mutant EGFR might cooperate with ERBB2 in certain settings to transform cells. This is supported by the presence of high levels of phosphorylation of ERBB2 in EGFR mutant lung tumors. However, knockdown of ERBB2 or ERBB2/ERBB3 in PC9 and H3255 cells did not decrease cell viability (Fig. 7C and Supplementary Fig. 6). Currently we are performing studies to establish the role of ERBB2 in tumorigenesis driven by mutant EGFR in vivo.
ERBB3-independent activation of the PI3K pathway
It has been shown that activation of the PI3K pathway is coupled to ERBB3 in EGFR mutant lung adenocarcinoma cell lines and that this interaction is sensitive to TKI treatment (15). Our results with mouse models, however, demonstrate that mutant EGFR does not depend upon ERBB3 to transduce its oncogenic signal and suggest that early studies in cell lines may have overestimated the contribution of ERBB3 on PI3K pathway activation in EGFR mutant tumors. Most strikingly, activation of the PI3K signaling pathway was unperturbed in lung tumors arising in the absence of ERBB3, and active in established tumors upon loss of ERBB3. This latter result is especially surprising since these tumors showed stable growth: it is possible that at the 4 week time-point analyzed the tumors were beginning to regrow (as evidenced by a slight increase in the tumor volume between 2–4 weeks following tamoxifen treatment, Fig. 6B) and thus show phosphorylation of Akt. Together, these data strongly suggest that compensatory mechanisms capable of PI3K activation occur in the absence of functional ERBB3.
There are two ways that EGFR can activate the PI3K/AKT pathway: 1) by heterodimerizing with ERBB3 (14) or 2) by directly recruiting adapters (e.g. GAB1, GAB2) that couple to p85 (26). We observed a physical interaction between EGFR and ERBB3 in EGFR mutant mouse lung tumors and also detected the association of EGFR with GAB1/GAB2 in the presence and absence of Erbb3. These results suggest that mutant EGFR uses both ERBB3 and GAB1/GAB2 to activate the PI3K pathway. Based on these results, we propose that mutant EGFR activates the PI3K/AKT pathway through both ERBB3-dependent and ERBB3-independent mechanisms. When ERBB3 is not present, mutant EGFR uses GAB1/GAB2 mediated signaling to activate the PI3K pathway. We cannot exclude that other signaling mechanisms that also engage adapter proteins (including GAB1/GAB2 and IRS1) may also contribute to activation of the PI3K pathway in these tumors. Moreover, increased Ras activation could also contribute to PI3K signaling. In a similar study, Erbb3-mediated PI3K signaling was shown to be dispensable for Erbb2-induced mammary tumorigenesis (31).
The observation that EGFR co-precipitates with p85, GAB1 and GAB2, even in the presence of ERBB3, suggests that activation of PI3K signaling can also occur via the mutant receptor. It is unclear how the proportion of ERBB3-dependent and ERBB3-independent mechanisms of activation of PI3K is determined for receptors like EGFR that have multiple ways to activate the pathway. Published evidence suggests that the mechanism used by EGFR to activate the PI3K/AKT pathway is directly affected by the cellular levels of adapter proteins like GAB1 and ERBB3 (32). In the mutant EGFR-mediated lung tumors and cancer cell lines we tested, ERBB3, GAB1 and GAB2 were all highly expressed and phosphorylated (Fig. 6). Our data point to a prominent role for GAB proteins in AKT activation, since knock-down of GAB proteins in EGFR mutant cell lines reduced phosphorylation of AKT.
The results of our studies have important implications for the treatment of EGFR mutant lung cancer. Our results predict that blocking ERBB3 function alone might stabilize tumors (at least transiently), but not lead to regressions in lung adenocarcinomas with EGFR mutations. Further, it appears that such tumors can adapt to the loss of ERBB3. We also note that the effect of blocking ERBB3 function in human EGFR mutant lung tumors may be predicted from the activation status of MET and ERBB2, since highly phosphorylated MET and ERBB2 proteins are associated with independence from ERBB3 (Fig. 7).
Moreover, we found that EGFR mutant lung tumors exhibit similar sensitivity to erlotinib regardless of the presence or absence of ERBB3 and there is a lack of synergy of ERBB3 knock-down with erlotinib (Fig. 7D) suggesting that combined blockade of ERBB3 with an EGFR TKI like erlotinib may not be beneficial in the TKI-naïve setting. Experiments to test this conclusively are planned. Studies with mouse models have shown that combined treatment with the EGFR antibody cetuximab and the ERBB3 antibody MM-121, leads to tumor regression in erlotinib-resistant L858R+T790M-induced tumors (17). This could indicate that ERBB3 plays a more prominent role in transformation when the T790M mutation is present. Future studies aimed at understanding the role of ERBB3 in tumors that are resistant to TKIs are underway. Indeed, ERBB3 has been suggested to play both direct and indirect roles in resistance to TKIs. In a subset of EGFR mutant TKI-resistant lung cancers with MET amplification, MET dimerizes with ERBB3 to activate the PI3K pathway and in this way confers resistance to gefitinib (16). ERBB3, however, does not always mediate PI3K activation: resistance to EGFR TKIs in EGFR mutant lung cancer cells resulting from Hepatocyte Growth Factor (HGF)-mediated MET activation led to PI3K activation via GAB1 (33).
In summary our data point to remarkable resiliency and redundancy in activation of the PI3K signaling pathway in lung cancer and highlight the need for treatment strategies that are effective at blocking this pathway, regardless of how it is activated, to produce sustained tumor regression in patients. Moreover, more precise knowledge of the effects of the EGFR heterodimerization partner ERBB3 on mutant EGFR signaling in lung cancer may guide the therapeutic application of EGFR family antagonists.
Supplementary Material
Acknowledgments
Financial Support: This work was funded by R00CA131488 (KP), R01CA120247 (HV and KP), R01CA092479 (DWT), Yale University and by grants R24 CA83084 (MSKCC) and P30 CA08748 (MSKCC), which provided partial support for the core facilities used in conducting this investigation. XS received financial support from the Yale Cancer Center and Uniting Against Lung Cancer.
The authors thank Don Nguyen for critical reading of the manuscript and Mary Ann Melnick for expert technical assistance.
Footnotes
Conflicts of Interest: Rights to a patent application for EGFRT790M testing were licensed on behalf of KP and HV by Memorial Sloan-Kettering Cancer Center to MolecularMD. KP has served as a consultant for Takeda and her lab receives research support from AstraZeneca.
References
- 1.Riese DJ, 2nd, Stern DF. Specificity within the EGF family/ErbB receptor family signaling network. BioEssays : news and reviews in molecular, cellular and developmental biology. 1998;20(1):41–8. doi: 10.1002/(SICI)1521-1878(199801)20:1<41::AID-BIES7>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 2.Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., 3rd Insect cell-expressed p180erbB3 possesses an impaired tyrosine kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(17):8132–6. doi: 10.1073/pnas.91.17.8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350(21):2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 4.Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science (New York, NY. 2004;304(5676):1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 5.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, et al. EGF receptor gene mutations are common in lung cancers from “never smokers” and are associated with sensitivity of tumors to gefitinib and erlotinib. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13306–11. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang CH. EGFR tyrosine kinase inhibitors for the treatment of NSCLC in East Asia: present and future. Lung cancer (Amsterdam, Netherlands) 2008;60 (Suppl 2):S23–30. doi: 10.1016/S0169-5002(08)70102-8. [DOI] [PubMed] [Google Scholar]
- 7.Greulich H, Chen TH, Feng W, Janne PA, Alvarez JV, Zappaterra M, et al. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS medicine. 2005;2(11):e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer cell. 2006;9(6):485–95. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Politi K, Zakowski MF, Fan PD, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes & development. 2006;20(11):1496–510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Uramoto H, Mitsudomi T. Which biomarker predicts benefit from EGFR-TKI treatment for patients with lung cancer? British journal of cancer. 2007;96(6):857–63. doi: 10.1038/sj.bjc.6603665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2(3):e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sordella R, Bell DW, Haber DA, Settleman J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science (New York, NY. 2004;305(5687):1163–7. doi: 10.1126/science.1101637. [DOI] [PubMed] [Google Scholar]
- 13.Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J. A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling. Molecular and cellular biology. 2000;20(4):1448–59. doi: 10.1128/mcb.20.4.1448-1459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soltoff SP, Carraway KL, 3rd, Prigent SA, Gullick WG, Cantley LC. ErbB3 is involved in activation of phosphatidylinositol 3-kinase by epidermal growth factor. Molecular and cellular biology. 1994;14(6):3550–8. doi: 10.1128/mcb.14.6.3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Engelman JA, Janne PA, Mermel C, Pearlberg J, Mukohara T, Fleet C, et al. ErbB-3 mediates phosphoinositide 3-kinase activity in gefitinib-sensitive non-small cell lung cancer cell lines. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(10):3788–93. doi: 10.1073/pnas.0409773102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science (New York, NY. 2007;316(5827):1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 17.Schoeberl B, Faber AC, Li D, Liang MC, Crosby K, Onsum M, et al. An ErbB3 antibody, MM-121, is active in cancers with ligand-dependent activation. Cancer research. 2010;70(6):2485–94. doi: 10.1158/0008-5472.CAN-09-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee D, Yu M, Lee E, Kim H, Yang Y, Kim K, et al. Tumor-specific apoptosis caused by deletion of the ERBB3 pseudo-kinase in mouse intestinal epithelium. The Journal of clinical investigation. 2009;119(9):2702–13. doi: 10.1172/JCI36435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71(6):995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(16):10482–7. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chmielecki J, Foo J, Oxnard GR, Hutchinson K, Ohashi K, Somwar R, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Science translational medicine. 2011;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72(5):767–78. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 23.Cuevas BD, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K, et al. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. The Journal of biological chemistry. 2001;276(29):27455–61. doi: 10.1074/jbc.M100556200. [DOI] [PubMed] [Google Scholar]
- 24.Rordorf-Nikolic T, Van Horn DJ, Chen D, White MF, Backer JM. Regulation of phosphatidylinositol 3′-kinase by tyrosyl phosphoproteins. Full activation requires occupancy of both SH2 domains in the 85-kDa regulatory subunit. The Journal of biological chemistry. 1995;270(8):3662–6. doi: 10.1074/jbc.270.8.3662. [DOI] [PubMed] [Google Scholar]
- 25.Okutani T, Okabayashi Y, Kido Y, Sugimoto Y, Sakaguchi K, Matuoka K, et al. Grb2/Ash binds directly to tyrosines 1068 and 1086 and indirectly to tyrosine 1148 of activated human epidermal growth factor receptors in intact cells. The Journal of biological chemistry. 1994;269(49):31310–4. [PubMed] [Google Scholar]
- 26.Mattoon DR, Lamothe B, Lax I, Schlessinger J. The docking protein Gab1 is the primary mediator of EGF-stimulated activation of the PI-3K/Akt cell survival pathway. BMC biology. 2004;2:24. doi: 10.1186/1741-7007-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong M, Mounier C, Wu J, Posner BI. Epidermal growth factor-induced phosphatidylinositol 3-kinase activation and DNA synthesis. Identification of Grb2-associated binder 2 as the major mediator in rat hepatocytes. The Journal of biological chemistry. 2000;275(46):36035–42. doi: 10.1074/jbc.M005621200. [DOI] [PubMed] [Google Scholar]
- 28.Fujioka T, Kim JH, Adachi H, Saito K, Tsujimoto M, Yokoyama S, et al. Further evidence for the involvement of insulin receptor substrates in epidermal growth factor-induced activation of phosphatidylinositol 3-kinase. European journal of biochemistry/FEBS. 2001;268(15):4158–68. doi: 10.1046/j.1432-1327.2001.02327.x. [DOI] [PubMed] [Google Scholar]
- 29.Maeda Y, Tsuchiya T, Hao H, Tompkins DH, Xu Y, Mucenski ML, et al. Kras(G12D) and Nkx2-1 haploinsufficiency induce mucinous adenocarcinoma of the lung. The Journal of clinical investigation. 2012;122(12):4388–400. doi: 10.1172/JCI64048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rudin CM, Liu W, Desai A, Karrison T, Jiang X, Janisch L, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26(7):1119–27. doi: 10.1200/JCO.2007.13.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lahlou H, Muller T, Sanguin-Gendreau V, Birchmeier C, Muller WJ. Uncoupling of PI3K from ErbB3 impairs mammary gland development but does not impact on ErbB2-induced mammary tumorigenesis. Cancer research. 2012;72(12):3080–90. doi: 10.1158/0008-5472.CAN-11-3513. [DOI] [PubMed] [Google Scholar]
- 32.Sithanandam G, Smith GT, Fields JR, Fornwald LW, Anderson LM. Alternate paths from epidermal growth factor receptor to Akt in malignant versus nontransformed lung epithelial cells: ErbB3 versus Gab1. American journal of respiratory cell and molecular biology. 2005;33(5):490–9. doi: 10.1165/rcmb.2005-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turke AB, Zejnullahu K, Wu YL, Song Y, Dias-Santagata D, Lifshits E, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer cell. 2010;17(1):77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.