Abstract
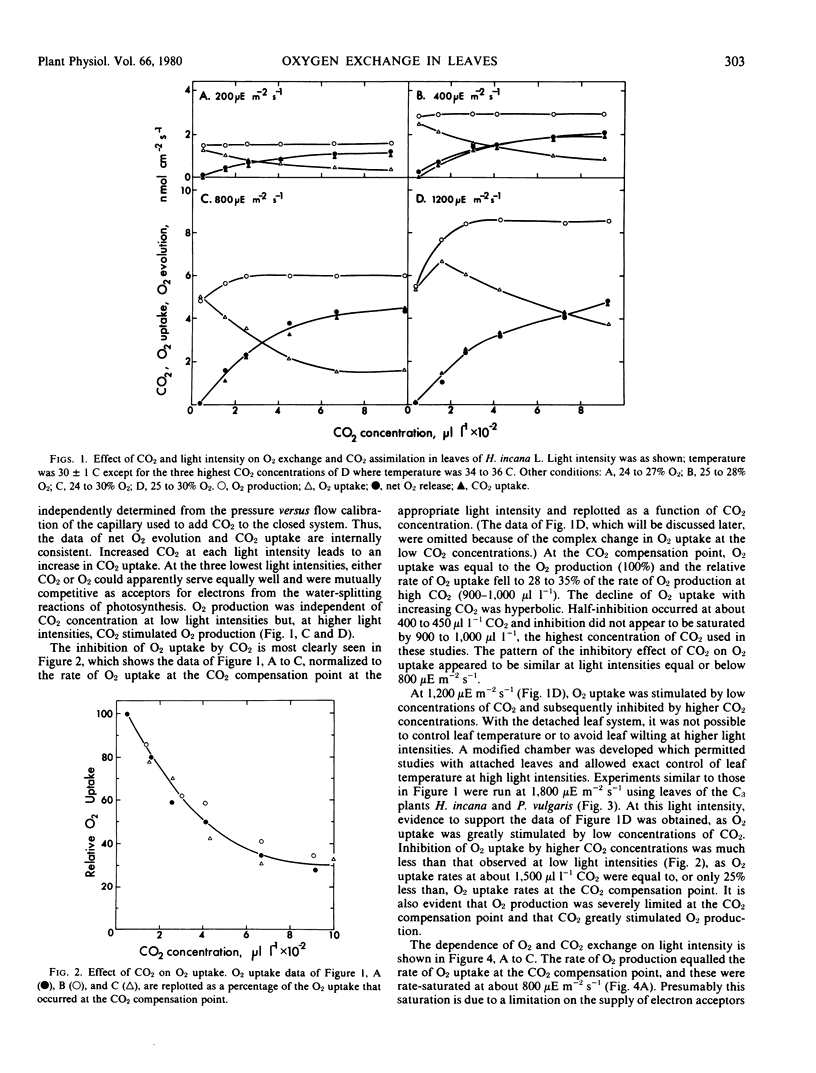
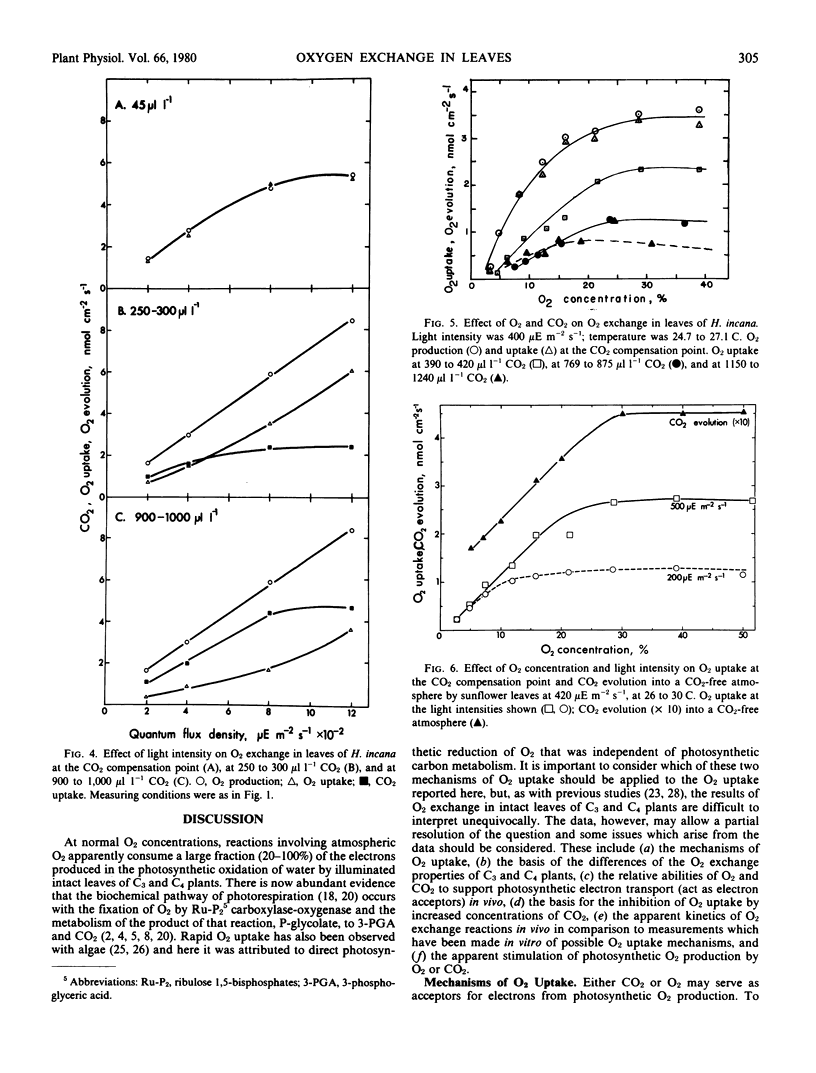
Photosynthetic O2 production and photorespiratory O2 uptake were measured using isotopic techniques, in the C3 species Hirschfeldia incana Lowe., Helianthus annuus L., and Phaseolus vulgaris L. At high CO2 and normal O2, O2 production increased linearly with light intensity. At low O2 or low CO2, O2 production was suppressed, indicating that increased concentrations of both O2 and CO2 can stimulate O2 production. At the CO2 compensation point, O2 uptake equaled O2 production over a wide range of O2 concentrations. O2 uptake increased with light intensity and O2 concentration. At low light intensities, O2 uptake was suppressed by increased CO2 concentrations so that O2 uptake at 1,000 microliters per liter CO2 was 28 to 35% of the uptake at the CO2 compensation point. At high light intensities, O2 uptake was stimulated by low concentrations of CO2 and suppressed by higher concentrations of CO2. O2 uptake at high light intensity and 1000 microliters per liter CO2 was 75% or more of the rate of O2 uptake at the compensation point. The response of O2 uptake to light intensity extrapolated to zero in darkness, suggesting that O2 uptake via dark respiration may be suppressed in the light. The response of O2 uptake to O2 concentration saturated at about 30% O2 in high light and at a lower O2 concentration in low light. O2 uptake was also observed with the C4 plant Amaranthus edulis; the rate of uptake at the CO2 compensation point was 20% of that observed at the same light intensity with the C3 species, and this rate was not influenced by the CO2 concentration. The results are discussed and interpreted in terms of the ribulose-1,5-bisphosphate oxygenase reaction, the associated metabolism of the photorespiratory pathway, and direct photosynthetic reduction of O2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Effects of CO2, O2 and temperature on a high-affinity form of ribulose diphosphate carboxylase-oxygenase from spinach. Biochem Biophys Res Commun. 1974 Sep 9;60(1):204–210. doi: 10.1016/0006-291x(74)90192-2. [DOI] [PubMed] [Google Scholar]
- Berry J. A., Osmond C. B., Lorimer G. H. Fixation of O(2) during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C(3) Plants. Plant Physiol. 1978 Dec;62(6):954–967. doi: 10.1104/pp.62.6.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes G., Ogren W. L. Oxygen inhibition and other properties of soybean ribulose 1,5-diphosphate carboxylase. J Biol Chem. 1972 Apr 10;247(7):2171–2176. [PubMed] [Google Scholar]
- Bravdo B. A. Effect of carbon dioxide on photorespiration. Plant Physiol. 1979 Feb;63(2):399–401. doi: 10.1104/pp.63.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egneus H., Heber U., Matthiesen U., Kirk M. Reduction of oxygen by the electron transport chain of chloroplasts during assimilation of carbon dioxide. Biochim Biophys Acta. 1975 Dec 11;408(3):252–268. doi: 10.1016/0005-2728(75)90128-0. [DOI] [PubMed] [Google Scholar]
- Gerbaud A., Andre M. Photosynthesis and photorespiration in whole plants of wheat. Plant Physiol. 1979 Nov;64(5):735–738. doi: 10.1104/pp.64.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindjee, van Rensen J. J. Bicarbonate effects on the electron flow in isolated broken chloroplasts. Biochim Biophys Acta. 1978 Oct 23;505(2):183–213. doi: 10.1016/0304-4173(78)90012-5. [DOI] [PubMed] [Google Scholar]
- Jackson W. A., Volk R. J. Oxygen uptake by illuminated maize leaves. Nature. 1969 Apr 19;222(5190):269–271. doi: 10.1038/222269a0. [DOI] [PubMed] [Google Scholar]
- MEHLER A. H. Studies on reactions of illuminated chloroplasts. II. Stimulation and inhibition of the reaction with molecular oxygen. Arch Biochem Biophys. 1951 Dec;34(2):339–351. doi: 10.1016/0003-9861(51)90012-4. [DOI] [PubMed] [Google Scholar]
- Marsho T. V., Behrens P. W. Photosynthetic oxygen reduction in isolated intact chloroplasts and cells in spinach. Plant Physiol. 1979 Oct;64(4):656–659. doi: 10.1104/pp.64.4.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozbun J. L., Volk R. J., Jackson W. A. Effects of Light and Darkness on Gaseous Exchange of Bean Leaves. Plant Physiol. 1964 Jul;39(4):523–527. doi: 10.1104/pp.39.4.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R. J., Kok B. Photoreduction of O(2) Primes and Replaces CO(2) Assimilation. Plant Physiol. 1976 Sep;58(3):336–340. doi: 10.1104/pp.58.3.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radmer R., Kok B., Ollinger O. Kinetics and Apparent K(m) of Oxygen Cycle under Conditions of Limiting Carbon Dioxide Fixation. Plant Physiol. 1978 Jun;61(6):915–917. doi: 10.1104/pp.61.6.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk R. J., Jackson W. A. Photorespiratory phenomena in maize: oxygen uptake, isotope discrimination, and carbon dioxide efflux. Plant Physiol. 1972 Feb;49(2):218–223. doi: 10.1104/pp.49.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]


