Abstract
Vitamin D deficiency and adipocytokines have been implicated in the etiology of aging-related diseases such as cancer, osteoporosis, and diseases of the cardiovascular system. The association between elevated parathyroid hormone (PTH) and low 25-hydroxyvitamin D(25-OH-VitD) in plasma is used to define vitamin D deficiency, yet their associated mechanistic pathways are unclear. Utilizing plasma samples from women in a previous intervention study, we measured plasma 25-OH-VitD, leptin, adiponectin, PTH, and lipid levels. We observed strong positive associations for leptin with PTH, γ-tocopherol, and body mass index (BMI) and inverse associations with 25-OH-VitD and adiponectin. Although commonly accepted that vitamin D deficiency causes hyperparathyroidism, we observed this association primarily in individuals with elevated leptin levels, suggesting that leptin may be an important modifier of this effect consistent with 25-OH-VitD-mediated inhibition of leptin. Leptin was highly correlated with the BMI/25-OH-VitD ratio (r = 0.80; P < 0.0001), consistent with a model in which BMI (adiposity) and 25-OH-VitD are the primary determinants of circulating leptin and PTH levels. This model may explain the failure of some studies to observe elevated PTH in vitamin D deficient adolescents and provides important insight into epidemiological studies exploring the associations of these individual biomarkers with chronic disease risk and mortality.
INTRODUCTION
Epidemiological studies have suggested a relation for vitamin D with reduced risk for cancer (1–3), cardiovascular disease (4), and all-cause mortality (5), whereas obesity is associated with increased heart disease (6), mortality (7), and cancer risk, particularly for breast, colon, and prostate cancers (8–10). Obesity in turn is associated with the production of adipocytokines, such as leptin, a hormone secreted by adipose tissue (11,12) that is positively associated with cancer risk (13,14) and adiponectin, which is secreted by adipocytes and is associated with reduced risk of developing cancer (15,16).
Vitamin D, derived from a pro-vitamin produced in human skin or obtained from supplements is best known for its ability to prevent osteoporosis and in extreme cases, rickets, through its effects on bone metabolism [reviewed in (17)]. The bioactive forms of vitamin D, 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3 (1,25(OH)2VitD), are characterized as regulators of calcium and bone metabolism (18) and stimulate absorption of calcium and increase bone mineral density (BMD) without causing hypercalcemia (19). Circulating levels of their precursors, 25-hydroxyvitamin D2 and D3 (25-OH-VitD) are believed to be the best biomarker for vitamin D status in humans. Because low 25-OH-VitD levels are associated with elevated parathyroid hormone (PTH) in humans (4,20), this association has been used to define vitamin D deficiency as levels of 25-OH-VitD less than 30 ng/ml (80 nM). Below this concentration, PTH levels are observed to rise (4,20) with the effect of regulating plasma calcium and phosphorus levels and stimulating bone growth, thereby partially compensating for the effects of vitamin D deficiency on bone metabolism. In recent years, in addition to its clear association with osteoporosis, vitamin D deficiency has been increasingly linked to many other diseases (21) including cardiovascular disease (4,22), chronic liver disease (23), cancer (24), and decreased immune function (25, 26), suggesting that its regulation and interactions with other micronutrients and proteins may have significant implications for understanding the etiology of many aging-related diseases.
Since the discovery of leptin in 1994 (27), there has been growing interest in its possible role in the etiology of cancer and other obesity-related diseases. Leptin exhibits both mitogenic and antiapoptotic properties, which may explain its ability to stimulate tumor growth (28–30). The function of leptin in human osteoporosis is less clear, and it remains an open question as to whether leptin enhances or inhibits bone formation (31,32). Although leptin has been shown to lower bone formation in mice and sheep (33,34), Matsunuma et al. (35) found that leptin administration in mice increases circulating levels of PTH. Although it is generally accepted that BMI is inversely associated with osteoporosis (36), the molecular mechanism for this relationship has not been established. With an in vitro tissue culture model, Menendez et al. (37) found that leptin secretion by human adipose tissue is negatively and powerfully controlled by 25-OH-VitD. Leptin, therefore, may be crucial in elucidating the relationships between 25-OH-VitD, PTH, and obesity with aging-related diseases such as cancer, cardiovascular disease, decreased immune function, and osteoporosis.
The implications for a leptin 25-OH-VitD displacement in human plasma, if any, could suggest that despite prolonged exposure to UV radiation, obese individuals could be at a disadvantage in producing active vitamin D and/or that high vitamin D levels could in turn suppress leptin formation in an individual with relatively high body mass, thereby ameliorating the deleterious consequences associated with higher BMI. Obesity-induced leptin production could conceivably lead to a corresponding decrease in conversion of vitamin D to 25-OH-VitD as well as decreased conversion of 25-OH-VitD to 1,25(OH)2VitD; whereas vitamin D deficient individuals with low BMI and corresponding lower leptin production may not exhibit the classic pattern of enhanced PTH secretion and therefore be at higher risk for bone loss due to lower formation of 1,25-(OH)2-VitD. Bone mineral density is positively correlated with obesity, giving weight gain a strong protective effect against osteoporosis (36). Although it is commonly hypothesized that this association is related to increased weight-bearing effects on bone mass, definitive evidence is lacking, and other mechanisms may play a role. Recently, it was reported that nearly 50% of young study subjects living in Hawaii and exposed to significant levels of UV were deficient in 25-OH-VitD, suggesting confounding regulatory mechanisms independent of sun exposure (38). This study also curiously failed to observe a significant association between 25-OH-VitD deficiency and elevated PTH.
Lipid levels, including triglycerides, cholesterol, and lipid-soluble antioxidants such as the tocopherols, carotenoids, retinoids, and coenzyme Q10, have also been commonly associated, both positively and negatively, as markers of risk for many aging-related diseases. Few studies, however, have considered the interactions of these molecules with one another, or the lipid-soluble vitamin D, in relation to regulation of their plasma levels and function. Suzuki et al. (39) did, however, note an inverse relationship between plasma α-tocopherol and β-carotene with BMI. Because of their potential interactive effects as mediated by signaling pathways such as the retinoid, deltanoid, and pregnane receptors (40,41), it is possible that plasma levels of one lipid may affect levels of another. Observations of seasonal changes in plasma retinol that vary inversely to seasonal changes vitamin D levels have been postulated to occur as partial compensation for seasonal deficiencies in the latter vitamin (42). Seasonal variations in mammographic breast density, a marker of risk for breast cancer, have also been linked to seasonal changes in 25-OH-VitD (43).
Because of the importance of defining the potential interactive relationships between these important dietary and physiologic molecules, we conducted a secondary analysis of previously collected blood samples from a nutritional intervention study among women. Circulating plasma levels of 25-OH-VitD, leptin, adiponectin, PTH, lipids, and lipid-soluble antioxidants were analyzed in order to better characterize their interactions in normal women and increase their utility as potential biomarkers of risk for cancer and osteoporosis as well as other aging-related diseases.
MATERIALS AND METHODS
Blood Collection
Stored samples (kept at −80°C) from a nutritional intervention study as described previously (44) were used in the present study. In brief, 29 women were randomized to a diet of 9 daily servings of fruits and vegetables or a control group who maintained their regular diet. A total of 57 samples drawn after 3 and 6 mo were available for study from 29 women; all baseline samples had been expended. The study population consisted of healthy women who were at least 35 yr of age and who were not taking high-dose vitamin supplements. Participants donated 10 ml blood samples in Li-heparin vacutainers that were immediately stored on ice and centrifuged within 1 h after collection. Plasma was aliquoted under yellow light into 2-ml aliquots and stored at −80°C. Each sample was assayed in duplicate and averaged. Samples from the same woman taken at different time points were averaged, and the mean value for each of the 29 women used in the statistical analyses. Body mass index (BMI) was determined at the time of entry into the study for each subject with measurements of height and weight and then calculated as the weight in kg/height in meters2.
Plasma Analyses
Enzyme-linked immunosorbent assays
Plasma 25-hydroxyvitamin D (as the sum of 25-hydroxyvitamin D2+ 25-hydroxyvitamin D3) was measured according to the manufacturer’s directions utilizing an immunoassay kit purchased from Immunodiagnostic Systems, Ltd. (Fountain Hills, AZ; enzymatic kit AA-35F1). Human leptin and human adiponectin (adipocyte complement-related protein of 30 kDa) were measured according to the manufacturer’s directions utilizing immunoassay kits purchased from R & D Systems, Minneapolis, Minnesota (Catalogue #DLP00 and #DRP300 for leptin and adiponectin, respectively).
PTH was measured utilizing a two-site enzyme-linked immunosorbent assay (ELISA) specific for the biologically intact 84 amino acid chain of PTH. A kit from MD Biosciences Inc. (St. Paul, MN; Catalogue #PTH.96) was utilized in the analysis, which employs two different purified goat polyclonal antibodies, one biotinylated antibody specific for the mid region and C-terminal end of PTH (amino acids 39 to 84) and a second antibody bound with horseradish peroxidase targeting the N terminal region of PTH (1 to 34).
In all assays, color development was stopped with the addition of acid as specified by the manufacturer and the ELISA assay microplate then read at 450 nm and also at 590 nm as a background control. Plots of concentration vs. absorbance for standards were prepared using a 4 parameter fit and concentrations of unknown samples extrapolated from the standard curve and adjusted for any dilution of plasma.
Lipid-soluble antioxidants
Plasma levels of lipid-soluble antioxidants tocopherols and carotenoids were quantified from hexane extracts by HPLC/PDA analysis as described previously (44) by high pressure liquid chromatography with diode array detection.
Statistical Analysis
Although the values for within to between variance were low, indicating a single sample is sufficient to characterize an individual with respect to the group, values for the two time points for each subject were combined for statistical analysis. Means and standard deviations were calculated for control and intervention subjects separately; the difference between groups was assessed with a 2-sample Student’s t-test. To examine the association of BMI, adiponectin, lipids, PTH, and carotenoids with leptin, we created tertiles for leptin, applied ANOVA, estimated means per tertile, and used the Tukey test to find out if the means differed significantly. In addition, we computed Spearman correlation coefficients between leptin and all other variables. Circulating levels of PTH and leptin were computed after stratification by quintile of plasma 25-OH-VitD level for all 57 samples and plotted against the median 25-OH vitamin D level for each quintile.
RESULTS
Mean values for micronutrients in the control and intervention group did not vary significantly between the two groups (Table 1), with the exception of nonprovitamin A carotenoids and overall carotenoid levels, which were significantly increased in the intervention group, as described previously (44). Notably, 25-OH-VitD, leptin, and PTH levels between groups were not significantly different, indicating no apparent effect of increased fruit and vegetable consumption on the parameters of interest in the current study. Significant positive associations for leptin were observed with body mass index (BMI), triglycerides, cholesterol, PTH, and γ-tocopherol (Table 2). Significant inverse associations were observed for leptin with 25-OH vitamin D, adiponectin, and the ratio of α- to γ-tocopherol. No statistically significant associations for carotenoids with leptin, PTH, BMI, or 25-OH-vitD were observed.
TABLE 1.
Mean plasma levels of selected analytes for control and intervention groups based on the mean value for 2 samples taken 3 mo aparta
| Micronutrient | Controlsb Mean ± SEM |
Interventionc Mean ± SEM |
P Value for Difference Between Groups |
W/Bd |
|---|---|---|---|---|
| 25-OH vitamin D (nM) | 87 ± 7 | 90 ± 6 | 0.74 | 0.28 |
| Leptin (ng/ml) | 12.0 ± 2.0 | 11.7 ± 2.0 | 0.91 | 0.14 |
| PTH (pg/ml) | 58 ± 7 | 59 ± 7 | 0.90 | 0.18 |
| Adiponectin (µg/ml) | 14.8 ± 5.6 | 13.3 ± 3.6 | 0.82 | 0.32 |
| α/γ -tocopherol ratio | 21 ± 6 | 14 ± 3 | 0.28 | 0.05 |
| β-carotene (ng/ml) | 302 ± 47 | 460 ± 100 | 0.15 | 0.11 |
| Total carotenoids (ng/ml) | 1,262 ± 89 | 1,852 ± 189 | 0.002 | 0.11 |
| BMI (kg/m2) | 22.2 ± 0.7 | 22.8 ± 1.3 | 0.91 | — |
Abbreviations are as follows: PTH, parathyroid hormone; BMI, body mass index.
n = 16.
n = 13.
W/B = within to between variance.
TABLE 2.
Mean plasma levels of selected analytes and their association with leptina
| Analyte | Lowest Tertile Mean ± SEMb |
Middle Tertile Mean ± SEMc |
Highest Tertile Mean ± SEMc |
ANOVA F Value (P Value) |
Spearman Correlation Coefficient (P Value) |
|---|---|---|---|---|---|
| Leptin (ng/ml) | 4.8 ± 0.8e | 10.8 ± 0.5f | 19.4 ± 2.4g | 21.79 (<0.0001)d | — |
| 25-OHVitamin D (nM) | 98.3 ± 8.6 | 89.4 ± 9.2 | 78.3 ± 6.6 | 1.49 (0.24)d | −0.40 (0.034) |
| BMI (kg/m2) | 20.7 ± 0.6e | 20.8 ± 0.9e | 25.6 ± 1.1f | 9.69 (0.0007)d | 0.58 (0.001) |
| Adiponectin (µg/ml) | 11.8 ± 2.2 | 21.1 ± 9.3 | 6.9 ± 1.0 | 1.58 (0.23)e | −0.40 (0.030) |
| Triglycerides (mg/dl) | 73.7 ± 10.1 | 88.0 ± 12.1 | 125.8 ± 26.5 | 2.05 (0.15)e | 0.53 (0.003) |
| Cholesterol (mg/dl) | 177.9 ± 13.3 | 174.7 ± 9.7 | 195.1 ± 5.4 | 1.40 (0.27)e | 0.39 (0.034) |
| PTH (pg/ml) | 41.6 ± 2.6e | 55.2 ± 9.0 | 75.9 ± 7.8f | 5.60 (0.010)d | 0.58 (0.001) |
| α-tocopherol (µg/ml) | 11.7 ± 1.9 | 12.8 ± 1.6 | 10.5 ± 0.9 | 0.68 (0.51)e | −0.11 (0.567) |
| γ -tocopherol (µg/ml) | 1.0 ± 0.15e | 0.9 ± 0.16e | 1.6 ± 0.15f | 7.26 (0.003)e | 0.50 (0.006) |
| α/γ-tocopherol ratio | 18.8 ± 7.7 | 22.3 ± 6.2 | 6.8 ± 0.8 | 2.39 (0.11)e | −0.41 (0.027) |
| β-carotene (ng/ml) | 436 ± 156 | 381 ± 61.4 | 268 ± 49.8 | 0.87 (0.43)e | −0.21 (0.267) |
| Total carotenoid (ng/ml) | 1,677 ± 301 | 1,507 ± 107 | 1,316 ± 151 | 0.90 (0.42)e | −0.28 (0.148) |
Abbreviations are as follows: Data for 29 women in the study were stratified by analyte of plasma leptin and mean values ± SEM for each tertile determined. Different subscripts indicate statistically different means.
n = 9.
n = 10.
df = (2, 26).
df = (2,25).
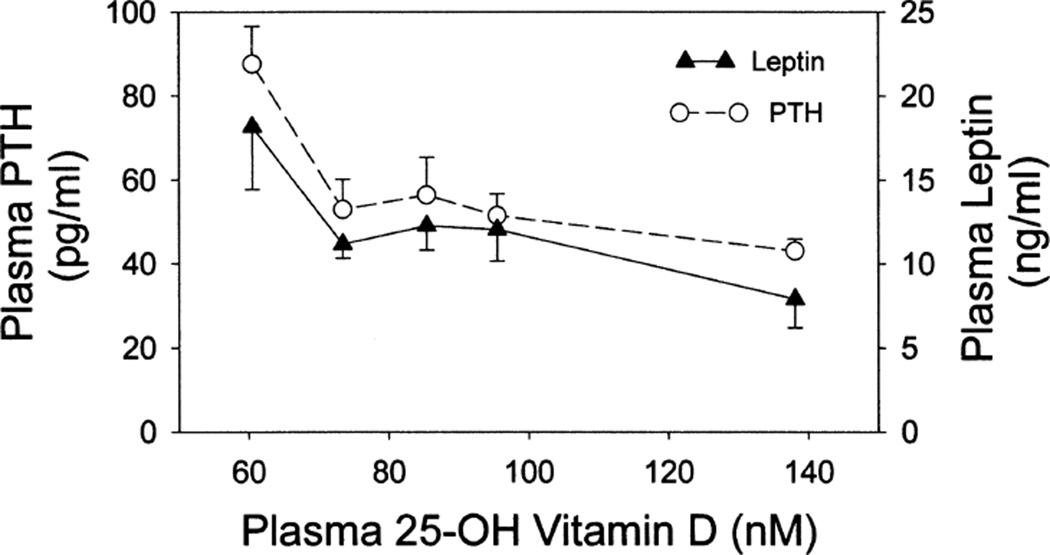
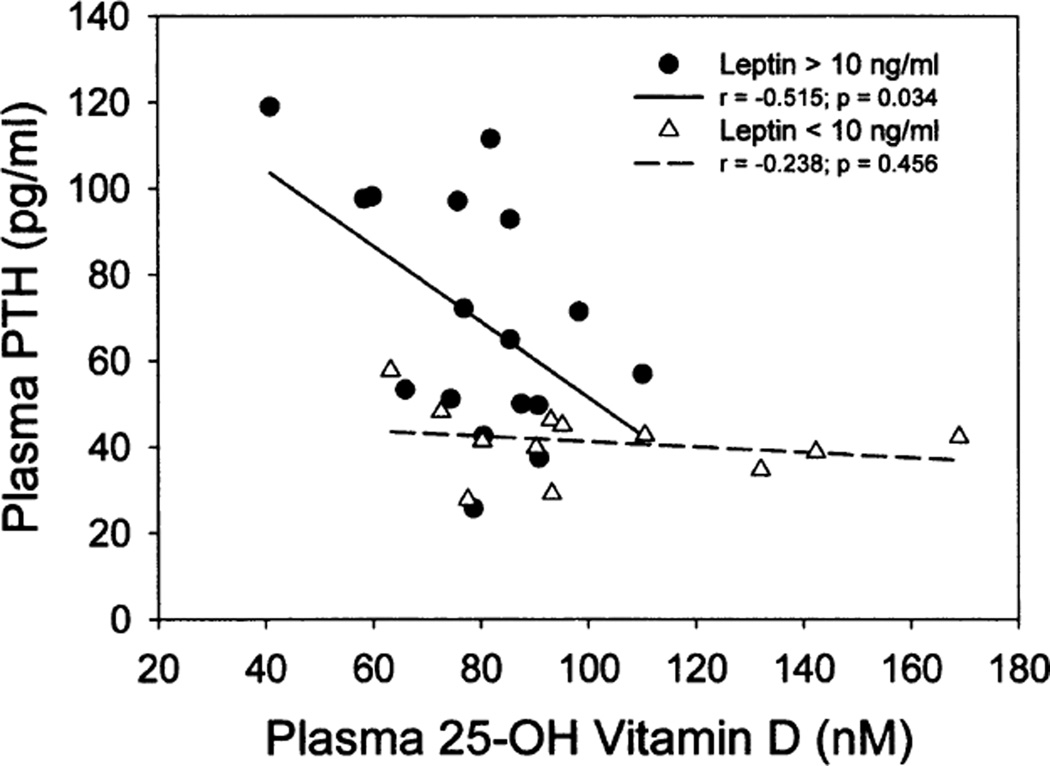
As shown in Fig. 1, plasma PTH and leptin exhibited similar profiles, with each showing elevated levels in association with 25-OH-VitD deficiency (<80 nM 25-OH-VitD). When values were stratified by plasma leptin concentration (> vs.<10 ng/ml) and data plotted for plasma PTH as a function of plasma 25-OH-VitD utilizing the mean values of the 29 subjects (Fig. 2), the elevation in PTH associated with 25-OH-VitD deficiency was predominantly found in individuals in the high leptin group (r = −0.515, P = 0.034). Individuals with low leptin levels showed similar PTH levels across a broad range of plasma 25-OH-VitD (slope not significantly different from zero). Multiple regression analysis indicated that approximately 44% of the variance in plasma PTH could be explained by a model involving plasma leptin and 25-OH-VitD, with leptin providing the most significant contribution.
FIG. 1.
Association of 25-OH-VitD with PTH and leptin in plasma. Data represent means ± SEM of the indicated analyte in plasma relative to median 25-OH-VitD stratified in quintiles (n = 57).
FIG. 2.
Effect of plasma leptin on the observed association between 25-OH-VitD and PTH. Mean values for the 29 subjects were stratified by plasma leptin into those with levels higher or lower than 10 ng/ml and mean PTH concentrations plotted as a function of median plasma 25-OH-VitD for each quintile. Regression lines and correlation coefficients are shown for each subset of data.
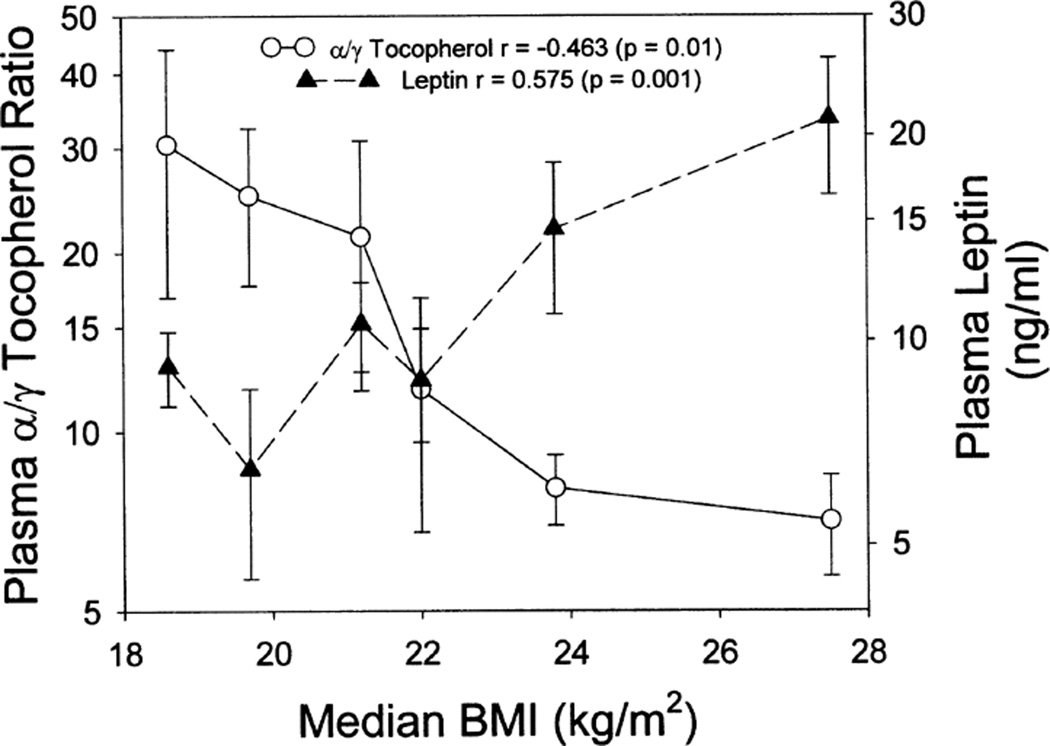
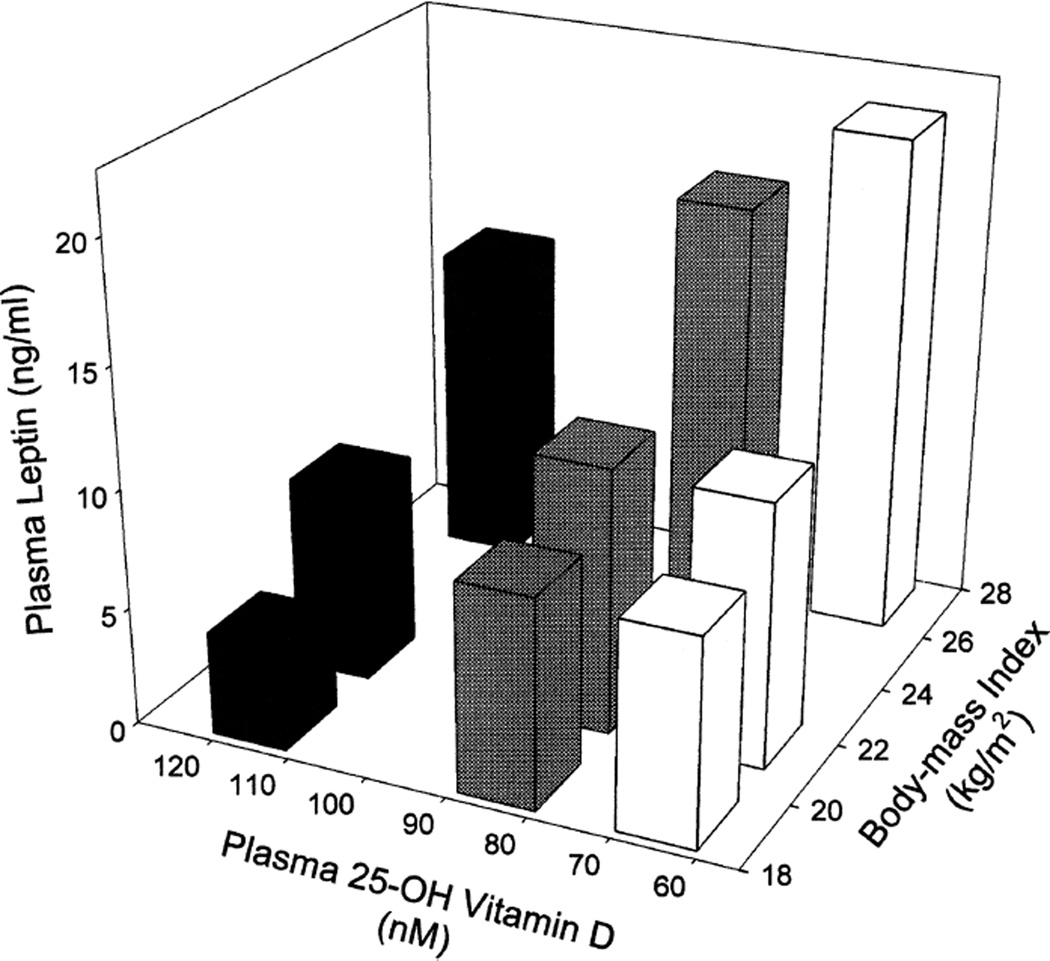
As seen in Fig. 3, BMI (kg/m2) was found to be strongly positively correlated with plasma leptin level (Spearman r = 0.58, P = 0.001); however, no significant association between BMI and 25-OH-VitD was observed. The ratio of α-tocopherol to γ-tocopherol was, however, inversely associated with BMI (Spearman r = 0.463, P = 0.01). The primary factor in this latter association appears to be related to γ-tocopherol, which was significantly positively associated with BMI (Spearman r = 0.443, P = 0.016), whereas α-tocopherol was weakly inversely associated with BMI (r = −0.281, P = 0.14). When plasma leptin was plotted three-dimensionally as a function of tertile of BMI and tertile of plasma 25-OH-VitD (Fig. 4), a strong additive interaction was observed, indicating that plasma leptin may be largely determined by these 2 factors, with BMI positively and 25-OH-VitD negatively associated (Pearson correlation coefficient for linear association between leptin and the ratio of BMI/25-OH-VitD: r = 0.80, P < 0.0001). Multiple regression analysis indicated that a linear model comprising BMI and plasma 25-OH-VitD levels could explain 53.2% of the variance observed for plasma leptin. Although both components significantly contributed to the observed variance, BMI was the most important factor in this model.
FIG. 3.
Association of BMI with leptin and with the ratio of α-tocopherol to γ-tocopherol. Subjects (n = 29) were stratified by their BMI into hexiles and the corresponding mean values for leptin and the ratios of α-tocopherol to γ-tocopherol ± SEM plotted.
FIG. 4.
Interactive association 25-OH-VitD and BMI with leptin levels in plasma. Samples were stratified by tertile of 25-OH-VitD and tertile of BMI. Plasma leptin was then plotted three-dimensionally as a function of 25-OH-VitD and BMI.
DISCUSSION
In agreement with past studies, we observed that plasma PTH is elevated in association with inadequate 25-OH-VitD.We also observe a strong positive association between plasma leptin and elevated PTH. Classification of vitamin D sufficiency and insufficiency is typically based on the observance of elevated PTH (20,45–47), at or around circulating 25-OH-VitD concentrations of less than 80 nM (30 ng/ml). The higher PTH levels observed with increasing deficiency of 25-OH-VitD in the current study are consistent with previous studies (45,48,49). Interestingly, circulating levels of leptin exhibited a similar pattern, increasing sharply below the deficiency level for 25-OH-VitD. Although leptin was reported to be positively associated with PTH in mice (35), our data suggest that vitamin D deficiency may be an important component, affecting both circulating leptin and PTH levels. Given the previously identified links between leptin and PTH (28, 50, 51), it is conceivable that leptin mediates, in part, the increase in PTH associated with low levels of 25-OH-VitD. Consistent with this hypothesis, we observe that after stratification for leptin, the elevation of PTH associated with vitamin D deficiency was significantly greater in individuals with leptin levels greater than 10 ng/ml (Fig. 3). Individuals with leptin concentrations less than 10 ng/ml did not show a significant association between plasma 25-OH-VitD level and PTH. Leptin is secreted by adipose tissue; consequently, body fat content would be predicted to be a major determinant of circulating leptin levels. The observation by Donahue et al. (52) that in bears, PTH is responsible for maintaining bone integrity during long periods of inactivity associated with hibernation, further supports the hypothesis that fat/leptin-induced increases in PTH are physiologically important for bone health and may explain the association between increased BMI and decreased incidence of osteoporosis. This would imply that the increased risk for osteoporosis is greater for vitamin D deficient individuals that are thin and therefore have lower levels of leptin and PTH. Although others have reported weak associations between BMI and 25-OH-VitD, we did not observe a significant relationship in the current study, suggesting that any relationship may be indirect and secondary. A more likely hypothesis, supported by in vitro data with human adipose tissue, suggests that 25-OH-VitD acts as a negative regulator of leptin secretion (37). This is consistent with our observations that individuals with levels of 25-OHVitD > 100 nM showed relatively lower leptin levels across a broad range of BMI (Fig. 4). The strongest correlation predicting plasma leptin was found for the ratio of BMI to 25-OHVitD (Pearson r = 0.80, P < 0.0001), and three-dimensional analysis shows an additive interaction between BMI and decreasing 25-OH-VitD levels on leptin concentration in plasma. The model suggested by our data proposes that increased adipose tissue associated with higher BMI leads to increased leptin secretion, which is antagonized by plasma 25-OH-VitD levels. Leptin in turn mediates increased PTH secretion, an effect that may also be antagonized in part by 25-OH-VitD. Leptin may also affect vitamin D metabolism because leptin attenuates gene expression for two hydroxylases that are critical in converting vitamin D to its bioactive form 1,25-(OH)2-VitD in mice models (53), whereas PTH activates the 25-OH-VitD–1α-hydroxylase promoter (50,51).
Despite studies (33, 34) that have shown that leptin reduces bone mineral density in mice and sheep models, the effects of leptin administration in humans are unclear. Various studies seem to have disagreed as to whether leptin increases or decreases bone mineral density in humans (54, 55), whereas Ghazali et al. (56) reported that leptin administration experimentally has a positive effect on bone mass intravenously but a negative effect on bone mass after intercerebroventricular administration. The observations by Matsunuma et al. (35, 53) that leptin attenuates gene expression for two hydroxylases that are critical for converting vitamin D to its bioactive form in mice models would suggest that leptin might enhance vitamin D deficiency in tissue by restricting its conversion to 1,25-(OH)2-VitD. However, Menendez et al.’s (37) observation that leptin secretion by human adipose tissue is negatively and powerfully controlled by vitamin D suggests a vitamin D-controlled change in circulating leptin levels, potentially leading to reduced PTH at higher 25-OH-VitD concentrations, consistent with the observations reported here. The conflicting evidence between human and mice models provides support for Cunningham’s (57) explanation of species differences, and stresses the need for a more critical approach in drawing human-based conclusions from animal studies. Our data suggests that some of the paradox and conflict in the literature may be the result of studying PTH, leptin, and 25-OH-VitD as independent agents when in fact, their plasma levels may be interrelated. Clearly the strong contribution of leptin to elevated PTH levels that we observe in association with 25-OH-VitD inadequacy raises significant questions with respect to the mechanism of PTH regulation in vivo and its relation to vitamin D deficiency.
Increased fat intake associated with higher BMI also causes increased subclinical inflammation (58), which may explain the positive association with γ-tocopherol. γ-tocopherol increases in response to inflammation (59,60) and acts as an anti-inflammatory agent (61). As a consequence of its positive association with risk factors such as BMI, leptin, and inflammation, epidemiological studies of γ-tocopherol may be analytically complex in that γ-tocopherol is thought to be an important modifier of risk for cancer [reviewed in (62)], yet its elevation in response to inflammation might predict a positive association with risk. It is likely, therefore, that the association between γ-tocopherol and leptin may be the result of fat-mediated induction of inflammation resulting in increased plasma γ-tocopherol levels and does not, in and of itself, cause elevated leptin.
Future studies addressing the causal relationships between these essential molecules is needed to better understand their use as biomarkers of risk for cancer and other chronic diseases in humans. The identification of optimal levels of 25-OH-VitD, leptin, and PTH for prevention of chronic diseases such as cancer and osteoporosis will depend on a better understanding of the interactions between these molecules and their precise function in vivo in humans. There is also the need to recognize that U-shaped responses may be the norm in human physiology and that dose-linear responses are the exception. Indeed, in humans, BMI exhibits a U-shaped response with respect to mortality (7), and the data presented here suggest that leptin may be an important mediator of some effects related to obesity as modulated by plasma 25-OH-VitD. Future research elucidating the molecular mechanisms of their interactions and their optimal levels is crucial for understanding the role of vitamin D, adipocytokines, and antioxidant lipids in human health and disease prevention. Furthermore, determining the optimal concentrations of micronutrients and/or biomarkers of risk requires consideration of multiple health outcomes (63) and physiological parameters as well as interactions between related molecules if we are to effectively define their roles in physiology and provide meaningful public health recommendations.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.informaworld.com/terms-and-conditions-of-access.pdf
This article may be used for research, teaching and private study purposes. Any substantial or systematic reproduction, re-distribution, re-selling, loan or sub-licensing, systematic supply or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
REFERENCES
- 1.Schwartz GG, Skinner HG. Vitamin D status and cancer: new insights. Curr Opin Clin Nutr Metabolic Care. 2007;10:6–11. doi: 10.1097/MCO.0b013e328011aa60. [DOI] [PubMed] [Google Scholar]
- 2.Spina CS, Tangpricha V, Uskokovic M, Adorinic L, Maehr H, et al. Vitamin D and cancer. Anticancer Res. 2006;26:2515–2524. [PubMed] [Google Scholar]
- 3.Wu K, Feskanich D, Fuchs CS, Willett WC, Hollis BW, et al. A nested case control study of plasma 25-hydroxyvitamin D concentrations and risk of colorectal cancer. J Natl Cancer Inst. 2007;99:1120–1129. doi: 10.1093/jnci/djm038. [DOI] [PubMed] [Google Scholar]
- 4.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92:39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Autier P, Gandini S. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med. 2007;167:1730–1737. doi: 10.1001/archinte.167.16.1730. [DOI] [PubMed] [Google Scholar]
- 6.Dong F, Ren J. Fitness or fatness—the debate continues for the role of leptin in obesity-associated heart dysfunction. Curr Diabetes Rev. 2007;3:159–164. doi: 10.2174/157339907781368959. [DOI] [PubMed] [Google Scholar]
- 7.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW. Body-mass index and mortality in a prospective cohort of U.S. adults. New Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 8.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta analysis of 31 studies with 70,000 events. Cancer Epidem Biomarkers Prev. 2007;16:2533–2547. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 9.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 10.Buschemeyer WC, III, Freedland SJ. Obesity and prostate cancer: epidemiology and clinical implications. Eur Urol. 2007;52:331–343. doi: 10.1016/j.eururo.2007.04.069. [DOI] [PubMed] [Google Scholar]
- 11.Kempf AM, Strother ML, Li C, Kaur H, Huang TT. Leptin as a marker of body fat and hyperinsulinemia in college students. J Am Coll Nutr. 2006;55:175–180. doi: 10.3200/JACH.55.3.175-180. [DOI] [PubMed] [Google Scholar]
- 12.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Brit J Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 13.Mistry T, Digby JE, Desai KM, Randeva HS. Obesity and prostate cancer: a role for adipokines. Eur Urol. 2007;52:46–53. doi: 10.1016/j.eururo.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 14.Housa D, Housova J, Vernerova Z, Haluzik M. Adipocytokines and cancer. Physiol Res. 2006;55:233–244. doi: 10.33549/physiolres.930848. [DOI] [PubMed] [Google Scholar]
- 15.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86:858S–866S. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 16.Wei EK, Giovannucci E, Fuchs CS, Willett WC. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J Natl Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 17.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 18.Lanske, Razzaque S. Vitamin D and aging: old concepts and new insights. J Nutr Biochem. 2007;18:771–777. doi: 10.1016/j.jnutbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sutton AL, MacDonald PN. Vitamin D: more than a “bone-a-fide” hormone. Molec Endocrin. 2003;17:777–791. doi: 10.1210/me.2002-0363. [DOI] [PubMed] [Google Scholar]
- 20.Hollis BW. Circulating 25-hydroxyvitamin D levels indicative of vitamin D sufficiency: implications for establishing a new effective dietary intake recommendation for vitamin D. J Nutr. 2005;135:317–322. doi: 10.1093/jn/135.2.317. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 22.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fisher L, Fisher A. Vitamin D and parathyroid hormone in outpatients with noncholestatic chronic liver disease. Clin Gastroenterol Hepatol. 2007;5:513–520. doi: 10.1016/j.cgh.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Garland CF, Garland FC, Gorham ED, Lipkin M, Newmark H, et al. The role of vitamin D in cancer prevention. Am J Pub Health. 2006;96:252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mathieu C, Adorini L. The coming of age of 1,25-dihydroxyvitamin D(3) analogs as immunomodulatory agents. Trends Molec Med. 2002;8:174–179. doi: 10.1016/s1471-4914(02)02294-3. [DOI] [PubMed] [Google Scholar]
- 26.Adams JS, Liu PT, Chun R, Modlin RL, Hewison M. Vitamin D in defense of the human immune response. Ann NY Acad Sci. 2007;1117:94–105. doi: 10.1196/annals.1402.036. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Proenca R, Maffei M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Chang YC, Liu CL, Liu TP, Chang KJ, et al. Leptin induces proliferation and anti-apoptosis in human hepatocarcinoma cells by upregulating cyclin D1 and down-regulating Bax via a Janus kinase 2-linked pathway. Endocr Relat Cancer. 2007;14:513–529. doi: 10.1677/ERC-06-0027. [DOI] [PubMed] [Google Scholar]
- 29.Hoda MR, Keely SJ, Bertelsen LS, Junger WG, Dharmasena D, et al. Leptin acts as a mitogenic and antiapoptotic factor for colonic cancer cells. Brit J Surg. 2007;94:346–354. doi: 10.1002/bjs.5530. [DOI] [PubMed] [Google Scholar]
- 30.Mauro L, Catalano S, Bossi G, Pellegrino M, Barone I, et al. Evidences that leptin up-regulates E-cadherin expression in breast cancer: effects on tumor growth and progression. Cancer Res. 2007;67:3412–3421. doi: 10.1158/0008-5472.CAN-06-2890. [DOI] [PubMed] [Google Scholar]
- 31.Di Carlo C, Tommaselli GA, Di Spiezio Sardo A, Sammartino A, Attianese W, et al. Longitudinal evaluation of serum leptin and bone mineral density in early post-menopausal women. Menopause. 2007;14:450–454. doi: 10.1097/01.gme.0000236936.28454.6a. [DOI] [PubMed] [Google Scholar]
- 32.Raisz LG. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest. 2005;115:3318–3325. doi: 10.1172/JCI27071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 34.Pogoda P, Egermann M, Schnell JC, Priemel M, Schilling AF, et al. Leptin inhibits bone formation not only in rodents, but also in sheep. J Bone Miner Res. 2006;21:1591–1599. doi: 10.1359/jbmr.060709. [DOI] [PubMed] [Google Scholar]
- 35.Matsunuma A, Kawane T, Maeda T, Hamada S, Horiuchi N. Leptin corrects increased gene expression of renal 25-hydroxyvitamin D-1 alpha-hydroxylase and-24-hydroxylase in leptin-deficient, ob/ob mice. Endocrinology. 2004;145:1367–1375. doi: 10.1210/en.2003-1010. [DOI] [PubMed] [Google Scholar]
- 36.Glauber HS, Vollmer WM, Nevitt MC, Ensrud KE, Orwoll ES. Body weight versus body fat distribution, adiposity, and frame size as predictors of bone density. J Clin Endocrinol Metab. 1995;80:1118–1123. doi: 10.1210/jcem.80.4.7714079. [DOI] [PubMed] [Google Scholar]
- 37.Menendez C, Lage M, Peino R, Baldelli R, Concheiro P, et al. Retinoic acid and vitamin D(3) powerfully inhibit in vitro leptin secretion by human adipose tissue. J Endocrinol. 2001;170:425–431. doi: 10.1677/joe.0.1700425. [DOI] [PubMed] [Google Scholar]
- 38.Binkley N, Novotny R, Krueger D, Kawahara T, Daida YG, et al. Low vitamin D status despite abundant sun exposure. J Clin Endocrinol Metab. 2007;92:2130–2135. doi: 10.1210/jc.2006-2250. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki K, Ito SK, Ochiai J, Kusuhara Y, Hashimoto S, et al. Relationship between obesity and serum markers of oxidative stress and inflammation in Japanese. Asian Pac J Cancer Prev. 2003;4:259–266. [PubMed] [Google Scholar]
- 40.Carlberg C. Lipid-soluble vitamins in gene regulation. Biofactors. 1999;10:91–97. doi: 10.1002/biof.5520100202. [DOI] [PubMed] [Google Scholar]
- 41.Rowe A. Retinoid X receptors. Int J Biochem Cell Biol. 1997;29:275–278. doi: 10.1016/s1357-2725(96)00101-x. [DOI] [PubMed] [Google Scholar]
- 42.Cooney RV, Franke AA, Hankin JH, Custer LJ, Wilkens LR, et al. Seasonal variations in plasma micronutrients and antioxidants. Cancer Epidemiol Biomarkers Prev. 1995;4:207–215. [PubMed] [Google Scholar]
- 43.Brisson J, Berube S, Diorio C, Sinotte M, Pollack M, et al. Synchronized seasonal variations of mammographic breast density and plasma 25-hydroxyvitamin D. Cancer Epidemiol Biomarkers Prev. 2007;16:929–933. doi: 10.1158/1055-9965.EPI-06-0746. [DOI] [PubMed] [Google Scholar]
- 44.Maskarinec G, Chan CL, Meng L, Franke AA, Cooney RV. Exploring the feasibility and effects of a high-fruit and -vegetable diet in healthy women. Cancer Epidemiol Biomarkers Prev. 1999;8:919–924. [PubMed] [Google Scholar]
- 45.Elder GJ, Mackun K. 25-hydroxyvitamin D deficiency and diabetes predict reduced BMD in patients with chronic kidney disease. J Bone Miner Res. 2006;21:1778–1784. doi: 10.1359/jbmr.060803. [DOI] [PubMed] [Google Scholar]
- 46.Bischoff-Ferrari HA. The 25-hydroxyvitamin D threshold for better health. J Steroid Biochem Molec Biol. 2007;103:614–619. doi: 10.1016/j.jsbmb.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 47.Papapetrou PD, Triantaphyllopoulou M, Karga H, Zagarelos P, Aloumanis K, et al. Vitamin D deficiency in the elderly in Athens, Greece. J Bone Mineral Metab. 2007;25:198–203. doi: 10.1007/s00774-006-0746-4. [DOI] [PubMed] [Google Scholar]
- 48.Lips P, Duong T, Oleksik A, Black D, Cummings S, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2001;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura K, Nashimoto M, Tsuchiya Y, Saito T, Nishiwaki T, et al. Threshold value of serum 25-hydroxyvitamin D concentration in relation to elevated serum parathyroid hormone concentrations in elderly Japanese women. J Bone Mineral Metab. 2006;24:395–400. doi: 10.1007/s00774-006-0699-7. [DOI] [PubMed] [Google Scholar]
- 50.Brenza HL, Kimmel-Jehan C, Jehan F, Shinki T, Wakino S, et al. Parathyroid hormone activation of the 25-hydroxyvitamin D-1α-hydroxylase gene promoter. Proc Natl Acad Sci USA. 1998;95:1387–1391. doi: 10.1073/pnas.95.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenza HL, DeLuca HF. Regulation of 25-hydroxyvitamin D 1-hydroxylase gene expression by parathyroid hormone and 1,25-dihydroxyvitamin D. Arch Biochem Biophys. 2000;381:143–152. doi: 10.1006/abbi.2000.1970. [DOI] [PubMed] [Google Scholar]
- 52.Donahue SW, Galley SA, Vaughan MR, Patterson-Buckendahl P, Demers LM, et al. Parathyroid hormone may maintain bone formation in hibernating black bears (Ursus americanus) to prevent disuse osteoporosis. J Exp Biol. 2006;209:1630–1638. doi: 10.1242/jeb.02185. [DOI] [PubMed] [Google Scholar]
- 53.Matsunuma A, Horiuchi N. Leptin attenuates gene expression for renal 25-hydroxyvitamin D(3)-1 alpha-hydroxylase in mice via the long form of the leptin receptor. Arch Biochem Biophys. 2007;463:118–127. doi: 10.1016/j.abb.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 54.Whitfield JF. Parathyroid hormone and leptin–new peptides, expanding clinical prospects. Exp Opinion Invest Drugs. 2005;14:251–264. doi: 10.1517/13543784.14.3.251. [DOI] [PubMed] [Google Scholar]
- 55.Coen G. Leptin and bone metabolism. J Nephrol. 2004;17:187–189. [PubMed] [Google Scholar]
- 56.Ghazali A, Grados F, Oprisiu R, Bunea D, Morinière P, et al. Bone mineral density directly correlates with elevated serum leptin in haemodialysis patients. Nephrol Dial Transplant. 2003;18:1882–1890. doi: 10.1093/ndt/gfg268. [DOI] [PubMed] [Google Scholar]
- 57.Cunningham M. A mouse is not a rat is not a human: species differences exist. Toxicol Sci. 2002;70:158–159. doi: 10.1093/toxsci/70.2.157. [DOI] [PubMed] [Google Scholar]
- 58.Aeberli I, Molinari L, Spinas G, Lehmann R, l’Allemand D, et al. Dietary intakes of fat and antioxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. Am J Clin Nutr. 2006;84:748–755. doi: 10.1093/ajcn/84.4.748. [DOI] [PubMed] [Google Scholar]
- 59.Jiang Q, Lykkesfeldt J, Shigenaga MK, Shigeno ET, Christen S, et al. Gamma-tocopherol supplementation inhibits protein nitration and ascorbate oxidation in rats with inflammation. Free Radic Biol Med. 2002;33:1534–1542. doi: 10.1016/s0891-5849(02)01091-2. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka Y, Wood LA, Cooney RV. Enhancement of intracellular gammatocopherol levels in cytokine-stimulated C3H 10T1/2 fibroblasts: relation to NO synthesis, isoprostane formation, and tocopherol oxidation. BMC Chem Biol. 2007;3:7. doi: 10.1186/1472-6769-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jiang Q, Ames BN. γ-Tocopherol, but not α-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 62.Tanaka Y, Cooney RV. Chemical and biological properties of tocopherols and their relation to cancer incidence and progression. In: In: Preedy VR, Watson RR, editors. The Encyclopedia of Vitamin E. Wallingford, UK: CABI Publishing; 2007. pp. 853–863. [Google Scholar]
- 63.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25- hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]