Abstract
Constant oxygen supply is essential for proper tissue development, homeostasis and function of all eukaryotic organisms. Cellular response to reduced oxygen levels is mediated by the transcriptional regulator hypoxia-inducible factor-1 (HIF-1). It is a heterodimeric complex protein consisting of an oxygen dependent subunit (HIF-1α) and a constitutively expressed nuclear subunit (HIF-1β). In normoxic conditions, de novo synthesized cytoplasmic HIF-1α is degraded by 26S proteasome. Under hypoxic conditions, HIF-1α is stabilized, binds with HIF-1β and activates transcription of various target genes. These genes play a key role in regulating angiogenesis, cell survival, proliferation, chemotherapy, radiation resistance, invasion, metastasis, genetic instability, immortalization, immune evasion, metabolism and stem cell maintenance. This review highlights the importance of hypoxia signaling in development and progression of various vision threatening pathologies such as diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration and glaucoma. Further, various inhibitors of HIF-1 pathway that may have a viable potential in the treatment of oxygen-dependent ocular diseases are also discussed.
Keywords: Age-related macular degeneration, diabetic retinopathy, hypoxia signaling, hypoxia-inducible factor-1 (HIF-1), ocular neovascularization, retinopathy of prematurity
Hypoxia and the Discovery of HIF-1
A constant oxygen supply is essential for proper tissue development, homeostasis and function of all eukaryotic organisms. Cells require oxygen as an electron acceptor during oxidative phosphorylation for efficient ATP production. Oxidative phosphorylation produces higher energy (∼18 fold) than glycolysis [1, 2]. Oxygen serves as a major element in regulating membrane transport, intracellular signaling, expression of many genes, and cell survival [3, 4]. Hypoxia (∼1% O2) occurs when tissue oxygenation demand exceeds the vascular supply. Response to reduced oxygen levels is mediated by the transcriptional regulator hypoxia-inducible factor 1 (HIF-1). HIF-1 was first discovered by its ability to induce expression of erythropoietin (EPO) in kidney and liver. Such production of EPO is inversely related to tissue oxygen concentration. In response to hypoxia, EPO is stimulated which in turn promotes red blood cell production and oxygen carrying capacity. This information led to the identification of a hypoxia response element (HRE; 5′-RCGTG-3′) in the 3′-enhancer region of EPO [5, 6].
Structure of HIF-1
A functional HIF-1 system is expressed in all metazoan species including the simplest animal Trichoplax adhaerens [7]. The HIF1A gene was mapped on 14q21-q24 human chromosome. HIF-1 is a heterodimeric complex consisting of an oxygen dependent subunit (HIF-1α) and a constitutively expressed nuclear subunit (HIF-1β) [8]. HIF-1β is also known as the aryl hydrocarbon receptor nuclear translocator (ARNT). It was first identified as structural binding component of aryl hydrocarbon receptor (AHR), which induces the transcription of Cyp1a1 metabolizing enzyme [9]. Both subunits are members of basic helix-loop-helix-PER-ARNT-SIM (bHLH-PAS) protein family. In human, HIF1A, EPAS1 and HIF3A genes encode three different isoforms of HIF-α (HIF-1α, HIF-2α and HIF-3α), respectively [10].
Structurally, HIF-1α exhibits bHLH and PAS domains at the N-terminal. The bHLH domain and N-terminal of PAS (PAS-A) (amino acids/aa 1-166) facilitate DNA dimerization while complete bHLH and PAS domains (aa 1-390) facilitate DNA binding [11, 12]. HIF-1α also exhibits an oxygen-dependent degradation (ODD) domain, two transactivating domains (TADs) and two nuclear localizing signals (NLS). The ODD domain (aa 401-575) located within central region plays a predominant role in regulating stability of HIF-1α with respect to oxygen concentration [13]. Two TADs (N-TAD; aa 531-575 and C-TAD; 786-826) help in recruiting various coactivators required for transcription of target genes. Bridged between them is an inhibitory domain (ID; aa 576-785) capable of repressing their transcriptional activity under normoxic conditions (∼20% O2) [14, 15]. N-NLS (aa 17-30) and C-NLS (aa 718-721) promote nuclear translocation of HIF-1α. However, studies have demonstrated that nuclear import is highly dependent on C-NLS [16]. HIF-1α is ubiquitously expressed in all human tissues, while the other related protein HIF-2α is primarily expressed in lung, endothelium and carotid artery [17-19]. HIF-2α shares 48% structural identity with HIF-1α. A third protein, HIF-3α, is also expressed in many tissues including adult thymus, lung, brain, heart, and kidney. This protein lacks C-TAD. However, the N-terminus of this protein shares 57% and 53% structural homology with HIF-1α and HIF-2α, respectively [20, 21]. A splice variant of HIF-3α is the inhibitory PAS domain (IPAS) protein, primarily expressed in Purkinje cells and corneal epithelium. This variant acts like a negative regulator of HIF-1 by binding to amino terminal region of HIF-1α, preventing transactivation. Further, this protein is also induced under hypoxia in heart and lung, suggesting a negative feedback mechanism for HIF-1 activity [22, 23]. The domain organization of both the subunits (HIF-1α and HIF-1β) is depicted in Fig. 1.
Fig. (1).
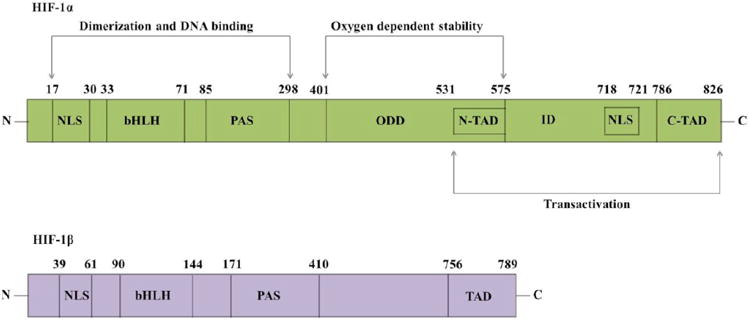
Structure of HIF-1 subunits depicting various domains. (NLS - nuclear localizing signal; bHLH - basic helix-loop-helix; PAS -PER-ARNT-SIM; ODD - oxygen-dependent degradation; TAD - transactivating domain; ID - inhibitory domain).
Regulation of HIF-1
Functional activity of HIF-1 is regulated by levels of oxygen dependent HIF-1α subunit. Although transcription of HIF-1α mRNA occurs at normoxic conditions, the protein is rapidly degraded via the ubiquitin proteasome pathway [24, 25]. HIF-1α protein has a very short half-life (t1/2∼5 minutes) and its stability is highly regulated by posttranscriptional modifications including hydroxylation, ubiquitination, acetylation, phosphorylation and nitrosation [26, 27].
Prolyl Hydroxylation
Hydroxylation of proline residues led to the identification of oxygen sensing mechanism of HIF-1α [28, 29]. It was considered a major breakthrough in delineating the signal transduction of HIF-1. Mutagenic studies substituting proline stabilized HIF-1α even under normal oxygen tension, demonstrate its importance in regulating transcriptional responses. Two proline residues (Pro/P 402 and 564) located within ODD domain are rapidly hydroxylated by 2-oxoglutarate (2-OG) dependent dioxygenases [30-32]. These dioxygenases recognize a conserved amino acid sequence ‘LXXLAP’, where X represents any amino acid. Human dioxygenases have been coined as prolyl hydroxylases (PHDs) or HIF- prolyl hydroxylases (HPHs) [33]. PHDs require oxygen for hydroxylation as well as ferrous ion (Fe2+) and ascorbate as cofactors [34]. During hydroxylation an oxygen molecule is split so that one oxygen atom is transferred on to proline while the other reacts with 2-OG to produce succinate and CO2 [30, 35]. Absolute requirement for Fe2+ ion stems from the observation that iron chelators or transition metal ions can suppress hydroxylation either by reducing the availability of Fe2+ or substituting Fe2+ at the active binding site [36, 37]. Ascorbate plays a very important role in regulating the activity of PHDs and maintaining the Fe2+ state of iron [35].
Molecular cloning studies have identified three isoforms of PHDs (PHD 1, 2 and 3) [38]. All the three isoforms can hydroxylate HIF-1α, with the highest activity exhibited by PHD2. The relative in vitro hydroxylation activity can be demonstrated as PHD2 ≫ PHD3 > PHD1 [33, 39]. Subcellular localization of these isoforms varies. PHD1 is exclusively localized in the nucleus; while PHD2 is localized in the cytoplasm and PHD3 is found in both compartments. However, PHD2 is able to shuttle between cytoplasmic and nuclear components facilitating HIF-1α degradation in both compartments. Further studies have suggested that PHD2 and PHD3 mRNA expression is hypoxia inducible, while PHD1 mRNA expression is not altered by hypoxia [40].
Polyubiquitination
Post hydroxylation, von Hippel-Lindau protein (pVHL) binds HIF-1α. X-ray crystallographic studies have demonstrated that hydroxyproline fits accurately into a pocket in pVHL hydrophobic core and this binding is highly specific [41, 42]. Moreover, pVHL associates with elongin C and this interaction is stabilized by elongin B. Cullin-2 and Rbx1 proteins are also recruited to form the VCB-Cul2 E3 ligase complex which facilitates polyubiquitination and degradation by the 26S proteasome [43, 44]. Although pVHL-E3 ligase complex is predominantly expressed in the cytoplasm, cytoplasmic-nuclear trafficking of the complex facilitates HIF-1α degradation in both compartments [45, 46]. pVHL thus plays a predominant role in the degradation of HIF-1α. Loss of activity or mutation of pVHL has been implicated in the development of many disease processes due to induction of hypoxia regulated genes [47-49].
Lysine Acetylation
Jeong et al., have identified a key lysine residue (Lys/K 532) that plays a crucial role in determining proteasomal degradation of HIF-1α. An acetyl group of acetyl-coA is transferred onto K532, located within ODD domain, by acetyltransferase ARD1. This modification further promotes interaction of HIF-1α with pVHL, in concert with proline hydroxylation. ARD1 is present in all the human tissues and its activity is not dependent on oxygen levels. However, the transcriptional and translational levels of ARD1 are reduced under hypoxia, causing decreased acetylation [50]. Replacing lysine with arginine enhances stability of HIF-1α while increasing acetylation promoted its degradation [51, 52].
Asparagine Hydroxylation
A third hydroxylation site on asparagine 803 (Asn/N 803) was identified on C-TAD of HIF-1α. This asparagyl residue is conserved on HIF-2α isoform (N851) [53, 54]. Unlike other posttranslational modifications already discussed, asparagine hydroxylation may not affect the stabilization of HIF. Rather, it promotes HIF activity via modulation of TADs. Under normoxic conditions, N803 is hydroxylated by a factor inhibiting HIF-1 (FIH-1), an oxygen dependent 2-OG dioxygenase requiring Fe2+ and ascorbate as cofactors [55-57]. It is considered as a second oxygen sensor and is localized in cytoplasm. Transcription of FIH-1 is not dependent on oxygen concentration [40]. Hydroxylation on N803 prevents interaction of HIF-1α with its coactivators CREB binding protein (CBP)/p300 due to steric inhibition. This coactivator recruitment is essential for transactivation of HIF-1α [58, 59].
Phosphorylation
Phosphorylation of HIF-1α by mitogen-activated protein kinase (MAPK) pathway appears to play a crucial role in regulating its activity and function. HIF-1α is highly phosphorylated in vitro by p42/p44 and p38 kinases [60-62]. Such activation promotes transcriptional activity of HIF-1. It is hypothesized that HIF-1β exhibits preferential binding to the phosphorylated HIF-1α protein [63]. Inhibitors of p42/44 protein kinases diminished hypoxia induced transcriptions of target genes, while their stimulation accelerates their translational activity [64]. Threonine (Thr/T) at residues 796 and 844 appear to be the potential phosphorylation sites in HIF-1α and HIF-2α, respectively [65].
Transactivation and Target Genes of HIF-1
In normoxic conditions, de novo synthesized cytoplasmic HIF-1α is rapidly hydroxylated (P402 and P564) and acetylated (L532). Later, HIF-1α is captured by pVHL and degraded by 26S proteasome [28, 42, 44]. However, in hypoxic conditions hydroxylation is inhibited. It becomes stabilized and then translocates into nucleus via NLS. The protein heterodimerizes with constitutively expressed HIF-1β, binds to the pentacore DNA binding sequence, recruits coactivators and activates transcription of various target genes (Fig. 2) [66-68]. To date, more than hundred target genes of HIF-1 have been identified. The target genes play a key role in regulating angiogenesis, cell survival and proliferation, chemotherapy and radiation resistance, invasion and metastasis, genetic instability, immortalization, immune evasion, metabolism and stem cell maintenance [69-76]. Some important target genes have been listed in Table 1.
Fig. (2).
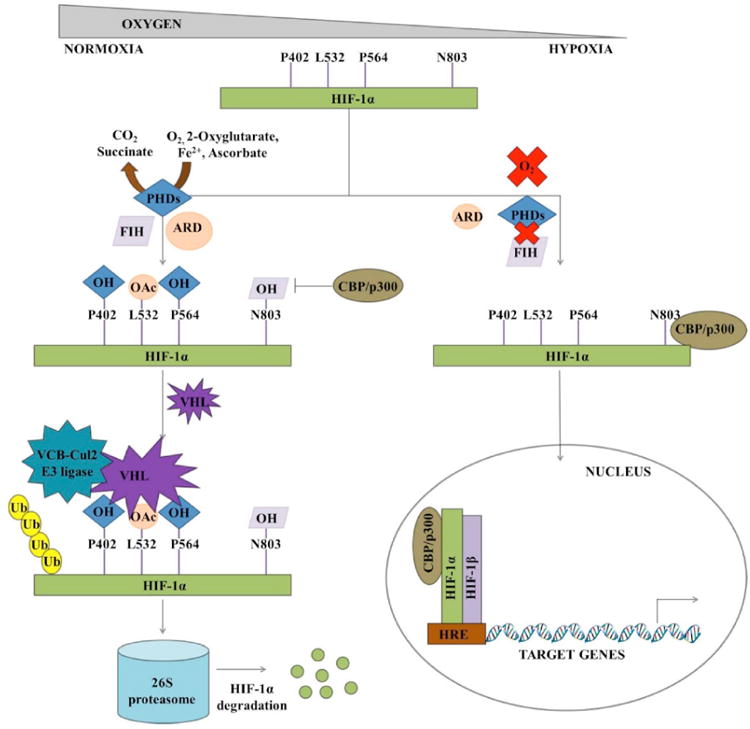
Schematic representation of oxygen dependent HIF-1 stability and transactivation. (PHD - prolyl hydroxylase; FIH - factor inhibiting HIF; ARD - acetyltransferase; CBP - CREB binding protein; VHL - von Hippel-Lindau protein; HRE - hypoxia response element).
Table 1. Target Genes of HIF-1 Pathway.
| Function | Oxygen Regulated Gene | Reference |
|---|---|---|
| Angiogenesis | Endocrine gland derived VEGF (EG-VEGF) | [77] |
| Transforming growth factor-β3 (TGF- β3) | [78] | |
| Vascular endothelial growth factor (VEGF) | [79] | |
| VEGF receptor (VEGFR1/Flt-1) | [80] | |
| Apoptosis | Nip3 | [81] |
| Cell proliferation and survival | Insulin-like growth factor (IGF) 2 | [82] |
| IGF-binding protein (IGFBP) -1 | [83] | |
| IGFBP-2 | [82] | |
| IGFBP-3 | [82] | |
| Transforming growth factor- α (TGF-α) | [84] | |
| Dedifferentiation | Inhibitor of differentiation protein-2 (ID2) | [85] |
| Drug resistance | Breast cancer resistance protein (BCRP) | [86] |
| P-glycoprotein (P-gp/MDR1) | [87] | |
| Energy metabolism | Leptin | [88] |
| Erythropoiesis | Erythropoietin (EPO) | [5] |
| Genetic instability | MutSalpha (MSH2 and MSH6 complex) | [89] |
| Glucose metabolism | Aldolase-A | [90] |
| Aldolase-C | [90] | |
| Enolase-1 | [90] | |
| Glucose transporter-1 (GLUT-1) | [91] | |
| GLUT-3 | [92] | |
| Hexokinase-1 | [90] | |
| Hexokinase-2 | [90] | |
| Lactate dehydrogenase A (LDHA) | [93] | |
| Phosphofructokinase L (PFKL) | [93] | |
| Phosphoglycerate kinase 1 (PGK1) | [93] | |
| Histone modifiers | JMJD2B | [94] |
| Iron metabolism | Ceruloplasmin | [95] |
| Transferrin | [96] | |
| Transferrin receptor | [97] | |
| Matrix metabolism | Collagen prolyl hydroxylase | [98] |
| Matrix metalloproteinases (MMPs) | [99] | |
| Plasminogen activator inhibitor -1 (PAI-1) | [100] | |
| Migration/Invasion | αvβ3 integrin | [101] |
| Chemokine receptor (CXCR4) | [102] | |
| Nucleotide metabolism | Adenylate kinase-3 | [91] |
| pH regulation | Carbonic anhydrase-9 | [103] |
| Transcriptional factor | ETS-1 | [104] |
| Vascular tone | α1B-Adrenergic receptor | [105] |
| Adrenomedulin (ADM) | [106] | |
| Endothelin-1 (ET1) | [107] | |
| Heme oxygenase-1 (HO-1) | [108] | |
| Inducible nitric oxide synthase (iNOS) | [109] |
Angiogenesis is a complex signaling process involving multiple gene products [110]. Many of these genes are upregulated due to hypoxic insult [79, 111-114]. Hypoxia is an important regulatory factor directing angiogenic switch, with HIF-1 playing a predominant role in “flipping the switch” via direct transcriptional upregulation of vascular endothelial cell growth factor (VEGF). VEGF is a potent endothelial-specific mitogen. It interacts with its receptor (VEGFR) localized on endothelial cells and stimulates endothelial cell proliferation [112, 115-117]. Apart from VEGF induction, many complex mechanisms are also involved in HIF-1 mediated angiogenic control. The expression of α1B-adrenergic receptor, adrenomedulin (ADM), angiopoietin 2, endothelin-1 (ET1), heme oxygenease-1 (HO-1), nitric oxide synthase, placental growth factor (PGF), platelet derived growth factor-B (PDGF-B) and stromal derived growth factor-1 (SDF-1) is regulated by hypoxia [105, 107-109, 118-122]. Also, expression of collagen prolyl hydroxylase, matrix metalloproteinases (MMPs) and plasminogen activator receptors and inhibitors (PAIs) under hypoxic control regulates matrix metabolism and vessel maturation (Fig. 3) [92, 99-100].
Fig. (3).
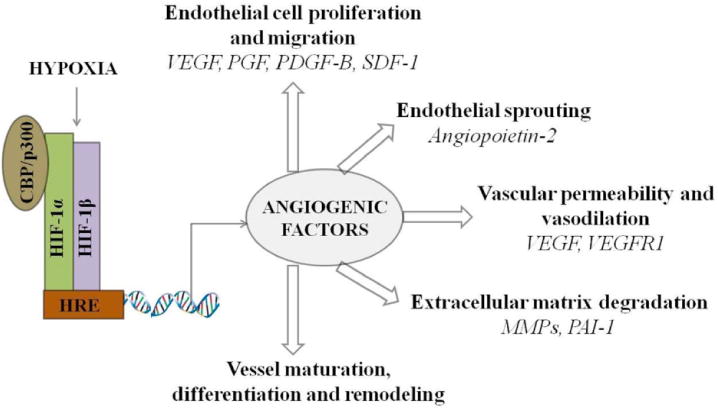
Schematic representation of angiogenic regulation by HIF-1. (VEGF - vascular endothelial cell growth factor; PGF - placental growth factor; PDGF-B - platelet derived growth factor-B; SDF-1 - stromal derived growth factor-1; VEGFR1 - VEGF receptor 1; MMPs -matrix metalloproteinases; PAI-1 - plasminogen activator inhibitor-1).
Role of HIF-1 in Ocular Diseases
Retina, a light sensitive tissue, forms the inner lining of posterior ocular segment and is metabolically one of the most active tissues in human body [123]. Continuous oxygen supply to retina facilitates high energy demand for sensitive and efficient transduction of images to readable neuronal signals [124, 125]. This neuronal function is executed by five different cell types including photoreceptors, bipolar cells, amacrine cells, horizontal cells and ganglion cells. Photoreceptors (cones and rods) play a vital role in phototransduction process. Cones mediate vision in bright light while rods mediate in dim light [126, 127]. Number of rods outweighs the number of cones by ∼20 fold. Under dark conditions, a single rod cell requires 108 ATPs/second for ion homeostasis and signal transduction machinery. However under light exposure due to reduction in ion influx, energy requirement falls by 75% [128]. The energy requirement is met by oxidative phosphorylation process occurring in mitochondria, located within inner segment of photoreceptors [129]. Thus, oxygen concentration tightly controls retinal function.
In human a constant supply of oxygen is regulated via choroidal and retinal circulation. Since human retina is thick, these two separate and distinct systems act to facilitate diffusion. Choroidal vasculature nourishes the outer retina including retinal pigment epithelium (RPE) and photoreceptors while retinal vasculature perfuses the inner retinal layers. Choroidal circulation is highly vascularized. It is under low autoregulation and requires strong sympathetic control. Retinal circulation is relatively sparse, controlled by autoregulation and lacks sympathetic control. Arteriovenous oxygen gradient is also different between the two vasculatures [130, 131]. Further, the choroidal vessels are fenestrated while the retinal vessels lack fenestrations and express tight junctions [132]. Both vasculatures play an important role in regulating retinal physiology. Lack of oxygen supply can lead to vision threatening pathologies such as diabetic retinopathy, retinopathy of prematurity, age-related macular degeneration and glaucoma. Despite the fact that initiating events are different, hypoxia with subsequent neovascularization is a characteristic phenomenon noticed with all these vascular diseases.
Diabetic Retinopathy
Diabetic retinopathy (DR) is a frequent secondary microvascular complication in patients with diabetes mellitus. It is one of the four major causes of visual impairment often leading to blindness [133, 134]. Almost 25-50% of diabetic patients exhibit retinopathy symptoms within the first 10-15 years and this number approaches nearly 100% within 30 years of diabetic onset [135, 136]. DR is characterized by biphasic progression with an initial non-proliferative (vaso-obliterative) phase followed by a proliferative (vaso-proliferative) phase. During initial stages, a persistent rise in blood glucose levels leads to a loss of intramural pericyte function. As a result small saccular capillary outpouchings, known as microaneurysms appear [137]. Intraretinal microvascular abnormalities, hemorrhages, edematous thickening of basement membrane, soft exudates and cotton wool spots are also observed [138-141]. Changes in vasculature and perturbations in oxygen tension lead to development of hypoxia, elevating the expression of angiogenic factors and subsequent neovascularization (proliferative phase) (Fig. 4A) [142]. The newly formed blood vessels are often fragile and permeable. Such vessels grow through the surface of retina into the vitreous and subsequent bleeding may lead to obstructed vision. Further, contraction of associated fibrovascular component may result in retinal detachment, vision loss and blindness [143-146].
Fig. (4).
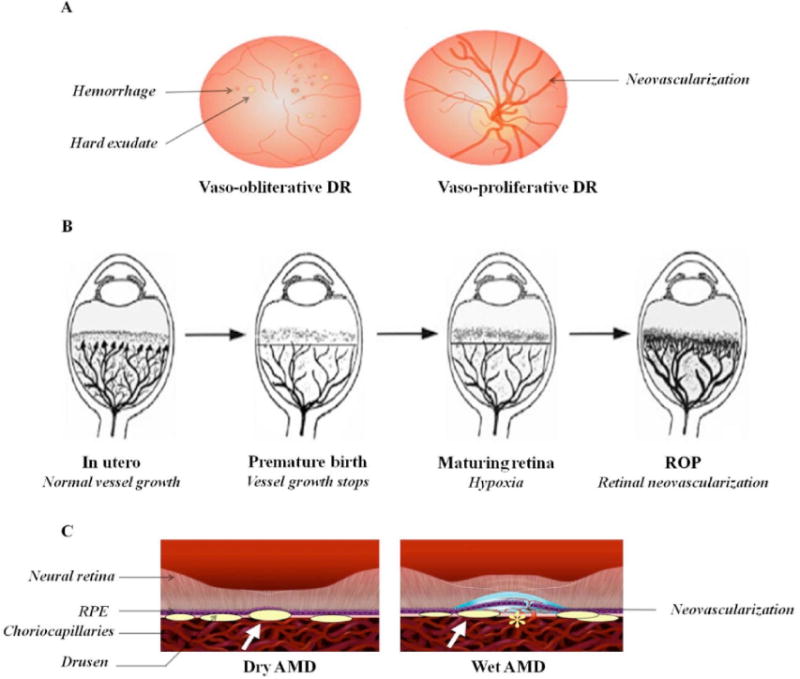
A) Stages of diabetic retinopathy (DR). B) Development of retinopathy of prematurity (ROP). Reproduced with permission from reference [152]. C) Schematic representation of dry and wet age-related macular degeneration (AMD). Reproduced with permission from reference [176].
The role of HIF-1 in the proliferative stage of DR has been clearly established. Mean oxygen tension is significantly lower in diabetics relative to non-diabetic patients in both lens (8.4 ± 0.7 mm Hg vs 10.7 ± 0.8 mm Hg) and vitreous cavity (5.7 ± 0.7 mm Hg vs 8.5 ± 0.6 mm Hg) [147]. Expression levels of HIF-1α and VEGF are elevated in diabetic preretinal membranes compared to non-diabetic idiopathic epiretinal membranes [148, 149]. Moreover, the production of VEGF and intercellular adhesion molecule (ICAM)-1 are diminished in a diabetic mice model lacking Hif-1α expression. It leads to much reduced vascular leakage and neovascularization in Hif-1α knockout mice relative to wild type mice [150]. These findings clearly suggest that alteration in HIF-1α pathway may be an attractive strategy for the treatment of DR.
Retinopathy of Prematurity
Retinopathy of prematurity (ROP), formerly known as retrolental fibroplasias, is the leading cause of visual impairment and blindness in children [151]. It was first described in early 1940s. This condition is associated with low gestational period, low birth weight and hyperoxia [152]. Human retinal vasculature begins to develop during 16th week of gestation and concludes at 40th week. Hence, premature infant's exhibit incompletely developed retinal vasculature and peripheral avascular zone. Oxygen supplementation (hyperoxia) is often needed in premature infants to overcome respiratory insufficiency. Such acute rise in oxygen tension can stimulate apoptosis of vascular endothelial cells and may cause vaso-obliteration via generation of reactive oxygen species (ROS) (Phase I). Further, high perinatal levels of prostaglandins (PGD2 and PGE2) and nitric oxide (NO) accelerates oxidative metabolism. Reduced levels of antioxidants may induce complexity in disease pathology [153-156]. In a subsequent phase, the infant's vaso-obliterated retina undergoes hypoxic/ischemic stress. It triggers a series of events such as stabilization of HIF-1α and production of various proangiogenic factors resulting in neovascularization (Phase II) (Fig. 4B). In contrast to normal developmental vasculature, this pathological vasculature displays excessive, uncontrolled and misdirected growth towards vitreous and lens. It can cause fibrous scarring, retinal detachment and blindness [157-160].
Given the regulatory role of oxygen, it is evident that HIF plays a predominant role in the development and progression of ROP. In a mouse oxygen-induced retinopathy (OIR) model, the expression levels of HIF-1α and HIF-2α proteins peak after two hours of hypoxic exposure. However, HIF-1α is stabilized in neuronal cells and inner retinal layers whereas HIF-2α was upregulated in Muller glia and astrocytes [161]. Indeed, inhibition of Hif-1α and Vegf by gene therapy in mice ischemic retinopathy model inhibited neovascular tufts and nuclei compared to control hypoxia group [162]. Hence, altering the HIF-1α pathway may be beneficial than targeting other downstream factors.
Age-Related Macular Degeneration
Age-related macular degeneration (AMD) is the leading cause of blindness in patients over the age of 65. World Health Organization (WHO) global eye disease survey has revealed that more than 50 million people are affected with AMD and atleast one-third of them are blind or severely visually impaired [163]. Development of AMD is multifactorial including aging, smoking, genetic factors, obesity, hypertension and hypercholesterolemia [164-168]. The disease is characterized by degeneration of central retina leading to disturbed fine and color vision. AMD is classified into two clinical forms, non-exudative/dry AMD and exudative/wet AMD [169]. The dry phase accounts for 85 to 90% of the total cases. It is characterized by the presence of acellular polymorphous debris, termed as drusen, between the basal lamina of RPE and inner collagenous layers of Bruch's membrane (Fig. 4C). As AMD progresses geographical atrophy of photoreceptors and central retina are noticed [170, 171]. Although wet AMD represents 10 to 15% of total cases, it accounts for approximately 90% of vision loss. In certain cases, dry AMD progresses to wet AMD characterized by choroidal neovascularization (CNV). These abnormal blood vessels penetrate Bruch's membrane; grow into RPE and neural retina. This may lead to blurred vision, retinal detachment, fibrosis and complete vision loss [172, 173].
Cell and molecular biology studies have demonstrated that hypoxia, chronic oxidative stress and inflammation play a key role in AMD [174-176]. Hypoxia may result from higher oxygen consumption, resulting from increased metabolic activity of the inflamed retina or due to poor circulation in central macula, resulting from vessel stenosis and microthrombosis [177-179]. Also, thickening of Bruch's membrane and drusen formation further stabilizes HIF [180]. ROS also causes elevation in HIF protein expression and results in increased transcriptional activity of hypoxia regulated genes [181, 182]. Such hypoxic milieu contributes to progression of exudative AMD and development of CNV. Infact, HIF-1α and HIF-2α expression were identified in endothelial cells and macrophages of choroidal neovascular membranes [183, 184].
Glaucoma
Glaucoma represents a multifactorial optic neuropathic disease [185]. It is classified into open and closed-angle depending on the anterior chamber angle. Open-angle glaucoma exhibits unobstructed and normal iridocorneal angle while the closed-angle glaucoma exhibits occlusion of the angle by the peripheral iris [186, 187]. Glaucoma is characterized by increased intraocular pressure, degeneration of retinal ganglion cells and axons. However, the exact role of hypoxia in the development of glaucoma is still unknown. Clinical observations have demonstrated retinal vascular abnormalities and impaired blood flow at the optic nerve head which may result in stabilization of hypoxic factors leading to retinal ganglion cell death [188, 189]. The expression of HIF-1α in control and glaucomatous human donor eyes was studied. Indeed, higher expression of the protein was noticed in retina and optic nerve head of glaucomatous eyes [190]. These findings clearly suggest the importance of hypoxia signaling mechanism in the pathogenesis of glaucoma.
Existing Anti-VEGF Therapeutics
Overproduction of VEGF plays an important role in pathogenesis of DR, ROP, AMD, neovascular glaucoma, central and branch retinal vein occlusion [191-194]. The human VEGF-A gene localized in chromosome 6p21.3 exhibits eight exons and four principal isoforms (121, 165, 189 and 206). The shorter isoform, VEGF121, is an acidic polypeptide and lacks heparin binding domain. The larger isoforms, VEGF189 and VEGF206, are highly basic and exhibit high affinity binding to heparin. The shorter isoform is freely diffusible while the larger isoforms are completely sequestered in the extracellular matrix. VEGF165 exhibits intermediate properties, existing in both diffusible and bound forms [195]. The primary sources of VEGF in retina are RPE cells, Muller cells and ganglion cells. VEGF binds to two types of protein kinase activating receptors, VEGFR1 and VEGFR2. These high affinity receptors have been localized on retinal endothelial cells and pericytes [196-199]. Currently, anti-VEGF therapeutics are indicated in the treatment of ocular neovascular diseases. These include Pegaptanib sodium (Macugen; Eyetech Pharmaceuticals/Pfizer, NY), Ranibizumab (Leucentis; Genentech, CA), Bevacizumab (Avastin; Genentech, CA) and Aflibercept (VEGF Trap-Eye; Regeneron, NY).
Pegaptanib (50 kD) is the first anti-VEGF agent approved by US Food and Drug Administration (FDA) in 2001 for the treatment of exudative AMD. It is a 28-base ribonucleic acid aptamer, covalently linked to two branched polyethylene glycol (PEG- 20kD) moieties. It binds to extracellular VEGF165 with high affinity and prevents the interaction of VEGF with its receptor. Since pegaptanib binds specifically to only one isoform, it exhibits limited efficacy. Approval of this drug molecule began a new era in anti-VEGF therapy. Bevacizumab (149 kD) is a humanized recombinant full-length monoclonal antibody. It binds to all VEGF isoforms. Although not approved for specific intraocular use, bevacizumab has been indicated as an off-label therapeutic in the treatment of ocular diseases. Ranibizumab (48 kD) is the Fab fragment of the former, approved by US FDA in 2010 for treatment of macular edema and vein occlusion. Both these molecules bind all forms of VEGF. Compared to bevacizumab, ranibizumab demonstrates 5-20 fold greater potency due to higher affinity and lack of immunogenicity. Aflibercept (115 kD) was approved by US FDA in 2011 for treatment of AMD. It is a recombinant fusion protein consisting of the VEGF binding domains of human VEGFR1 and VEGFR2 fused to the Fc domain of human immunoglobulin-G1. It acts as a decoy receptor binding free VEGF [200-203].
Although large molecule therapeutics appear to be promising, their long term usage must be considered with caution. A recent investigation by Kurihara et al., reported the deleterious effects following deletion of Vegfa gene in adult mice. Choriocapillaris are completely attenuated following three days of RPE-specific Vegf inactivation. Further, cone photoreceptors were damaged and cone dysfunction was noticed. These dramatic secondary “off-target” effects of Vegf antagonism were not observed when Hif1a, Epas1, and Hif1a/Epas1 were genetically ablated. The transcriptional mutants did not exhibit any morphological, functional, or transcriptional differences relative to control adult mice. Further, deletion of transcription factors reduced pathological angiogenesis in laser photocoagulation model of CNV. These studies clearly reinforce the strategy that molecules aiming at HIF pathway may be an alternative, safer and effective mode of treatment than attenuating VEGF levels alone [204, 205].
Development of HIF-1 Inhibitors
Significant research has been conducted in recent years to identify inhibitors of HIF-1 pathway. Based on their putative mechanism of action, HIF inhibitors may modulate eithe i) HIF-1α mRNA expression, ii) HIF-1α protein translation, iii) HIF-1α protein degradation, iv) HIF-1α DNA binding activity and v) HIF-1α transcriptional activity. Examples of HIF-1 inhibitors are summarized in Table 2 while few of them are described below.
Table 2. Inhibitors of HIF-1 Pathway.
| Target Pathway / Mechanism of Action | Small Molecules | Reference |
|---|---|---|
| HIF-1α mRNA expression | ||
| Transcription | Aminoflavone | [206] |
| EZN-2968 | [207] | |
| RNA interference | [208] | |
| HIF-1α protein expression | ||
| Translation | Digoxin | [209] |
| PX-478 | [210] | |
| Topotecan | [211] | |
| Receptor tyrosine kinases | Erotinib | [212] |
| Gefitinib | [212] | |
| Genistein | [213] | |
| PI3K-AKT pathway | LY294002 | [214] |
| Nelfinavir | [215] | |
| Wortmannin | [214] | |
| ERK-AKT pathway | Resveratrol | [216] |
| mTOR pathway | Everolimus | [217] |
| Rapamycin | [218] | |
| Silibinin | [219] | |
| Temsirolimus | [220] | |
| Ras-MAPK pathway | PD98059 | [64] |
| Sorafenib | [221] | |
| HIF-1α protein degradation | ||
| Hsp90 inhibitor | Apigenin | [222] |
| Deguelin | [223] | |
| Geldanamycin | [224] | |
| HDAC inhibitor | FK228 | [225] |
| SAHA | [226] | |
| Trichostatin A | [227] | |
| Others | ||
| Cyclin-dependent kinase | Flavopiridol | [228] |
| DNA binding | Cisplatin | [229] |
| Doxorubicin | [229] | |
| Echinomycin | [230] | |
| Pyrrole - imidazole polyamide | [231] | |
| Microtubules | 2-methoxyestradiol (2ME2) | [232] |
| Others | ||
| Curcumin | [233] | |
| Mitochondria | Antimycin A1 | [234] |
| p300 interaction | Bortezomib | [235] |
| Chetomin | [236] | |
| RNA polymerase | ECyd | [237] |
| Soluble guanylyl cyclase stimulator | YC-1 | [238] |
| Thioredoxin redox system | Pleurotin | [239] |
(PI3K - Phosphatidylinositide 3-Kinases; AKT - Protein Kinase B; ERK - Extracellular Signal-Regulated Kinases; mTOR - Mammalian Target of Rapamycin; Hsp - Heat Shock Protein; HDAC - Histone Deacetylase).
HIF-1α mRNA Expression
It has been hypothesized that transcriptional level of HIF-1α is the rate limiting factor of HIF-1 activity under hypoxic conditions [240]. Hence, inhibitors that effect HIF-1α mRNA expression can lower the rate of HIF-1 translation. Chen et al., have studied the role of HIF-1α inhibition by RNA interference (RNAi) with shRNA in BALB/C mouse model of corneal neovascularization. The effect of shRNA treatment was assessed by measuring mean neovascularization score. The mean score values were as follows: normal (0), control neovascular eyes (3.59 ± 1.1), saline treated (4.05 ± 0.75), vehicle-treated (3.64 ± 1.02) and RNAi- treated (1.13 ± 0.96). HIF-1α shRNA reduced neovascularization by more than 3 fold compared to control eyes. Further, the expression of angiogenic factors (VEGF and MMPs) and inflammatory mediator (IL-1β) was also diminished. In summary, this study confirmed the role of HIF-1α transcriptional inhibition in reducing corneal neovascularization and associated inflammation [241]. A similar study with RNAi of HIF-1α was performed by Jiang et al., in C57BL/6J mice of ischemic retinopathy. The researchers have counted the number of neovascular nuclei on the vitreal side of inner limiting membrane. The number of nuclei per cross section were as follows: normoxia (0.05 ± 0.29), hypoxia (41 ± 2.8), vehicle-treated (41 ± 2.6) and siRNA-treated (28 ± 2.8). The number of neovessels significantly decreased in transfected group relative to hypoxic group (p< 0.01). This report clearly demonstrates the application of HIF-1α RNAi as a novel therapeutic for the treatment of neovascular eye diseases [162].
HIF-1α Protein Translation
Although the precise mechanism of HIF-1α protein translation in hypoxic conditions is not clear, several translational inhibitors have been identified. These molecules can directly inhibit translation or inhibit various signaling pathways (receptor tyrosine kinases, PI3K/AKT/mTOR and Ras-MAPK pathway). These signaling pathways play a predominant role in upregulating HIF-1α translation and thus inhibition of these growth factors can alter hypoxic regulation. Cardiac glycosides can inhibit HIF-1α protein translation and digoxin has been identified as a potent inhibitor in a cell-based reporter assay [242]. The effect of digoxin on ocular neovascularization was demonstrated by Yoshida et al., in C57BL/6 mice with ischemic retinopathy. Intraocular injection of digoxin lowered retinal neovascularization by almost 75% compared to saline group. Also, the area of CNV at Bruch's membrane was significantly lowered in presence of digoxin. Apart from inhibiting HIF-1α protein expression, digoxin also inhibited the expression of several angiogenic factors including VEGF, PDGF-B, SDF-1, VEGFR2, chemokine receptor (CXCR4) and Tie2 receptor in ischemic retina. This observation suggests that digoxin may offer advantages over VEGF antagonists in the treatment of neovascular diseases due to inhibition of several proangiogenic pathways. This study signifies that digoxin, a potent HIF-1α inhibitor, can possibly provide a better therapeutic intervention [243].
Genistein, a naturally occurring isoflavonoid, exhibits strong antiangiogenic activity. The underlying mechanism is hypothesized as inhibition of HIF-1α translation caused by inhibition of tyrosine kinases [213]. Wang et al., examined the effects of genistein on retinal neovascularization in C57BL/6 OIR mouse model. Number of vascular nuclei anterior to inner limiting membrane was quantified and data obtained was represented as: normoxia (0.76 ± 0.81), hypoxia (23.9 ± 4.4), genistein (50mg/kg) (20.9 ± 4.7), genistein (100mg/kg) (17.2 ± 4.0) and genistein (200mg/kg) (14.2 ± 3.2). The nuclei numbers were diminished by 87, 72 and 59% respectively, as the dose of genistein was increased. Further, dose-dependent reduction in HIF-1α and VEGF levels were also observed. This report suggests possible pharmacological application of genistein in ocular neovascularization [244].
HIF-1α Degradation
The molecular chaperone, heat shock protein 90 (Hsp90) is required for activity of various signaling proteins [245]. The interaction of Hsp90 with HIF-1α is required for proper conformational stability. Inhibitors of Hsp90 can promote degradation of HIF-1α via oxygen-independent proteasomal degradation [246]. Geldanamycin and its analogs (17-N-allylamino-17-demethoxygeldanamycin (17-AAG) and 17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG/Deguelin)) may interfere with Hsp90/HIF-1α interaction by competing with the ATP binding site [247]. Kim et al. evaluated the potential of deguelin in the treatment of vaso-proliferative retinopathies. In an OIR mouse model, deguelin treated mice exhibited lower neovascularization as assessed by fluorescein angiography (Fig. 5). Further, the number of vascular lumens between posterior lens and anterior inner limiting membrane were estimated. Compared to control group the deguelin injected group showed lower number of vascular lumens (19 ± 3.4 vs 4 ± 2.1). Moreover, deguelin treatment did not alter normal retinal morphology as evident by normal retinal thickness and lack of any inflammation in vitreous, retina or choroid. This data clearly implies that modulation of HIF pathway reduces retinal neovascularization without any retinal toxicity [248].
Fig. (5).
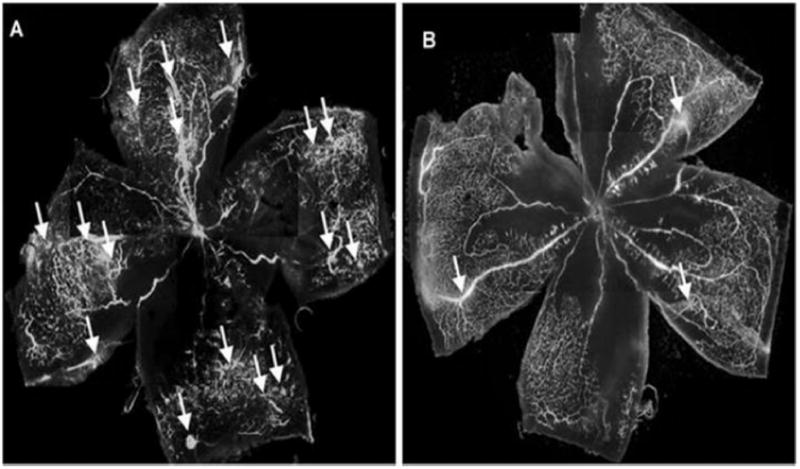
Estimation of retinal neovascularization in (A) control and (B) deguelin treated oxygen-induced retinopathy (OIR) mouse model. Reproduced with permission from reference [248].
HIF-1α DNA Binding and Transcriptional Activity
Binding of active heterodimeric HIF-1 to the consensus -RCGTG- enhancer element of target genes is another crucial step necessary for transcription of hypoxia inducible target genes. Also, the interaction of coactivator p300 with HIF-1α is another potential mechanism required for transcriptional activity of HIF-1. These molecular pathways can also be targeted in the treatment of various neovascular diseases [236, 249, 250].
Conclusions and Future Perspectives
In summary, high levels of energy is required for proper retinal function. Any perturbations in oxygenation may lead to progression of several retinal degenerative diseases including DR, ROP and AMD. Mechanistic studies of cellular and molecular components of hypoxia signaling have opened a new era in the treatment of retinopathies. Given the role of HIF-1 in the etiology of these diseases, it is evident that manipulation of this pathway at various stages can lead to more effective treatment of oxygen-dependent ocular diseases. As discussed in this article, many inhibitors have been identified and evaluated both in in vitro cell culture and animal models. However, many HIF inhibitors exhibit significant side effects and toxicities due to lack of specificity. Hence development of HIF specific inhibitors and further work validating the pharmacological intervention of these inhibitors in retinal diseases and their translation from bench to bedside is necessary. Nonetheless, there is significant optimism that modulation of HIF pathway can provide new treatments for ocular neovascular diseases.
Acknowledgments
This study has been supported by NIH grants R01EY09171 and R01EY010659.
Footnotes
Conflict of Interest: The authors confirm that this article content has no conflicts of interest.
References
- 1.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2(3):336–61. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 2.Semenza GL. Life with oxygen. Science. 2007;318(5847):62–4. doi: 10.1126/science.1147949. [DOI] [PubMed] [Google Scholar]
- 3.Giaccia AJ, Simon MC, Johnson R. The biology of hypoxia: the role of oxygen sensing in development, normal function, and disease. Genes Dev. 2004;18(18):2183–94. doi: 10.1101/gad.1243304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–87. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci USA. 1991;88(13):5680–4. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg MA, Dunning SP, Bunn HF. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988;242(4884):1412–5. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- 7.Loenarz C, Coleman ML, Boleininger A, et al. The hypoxia-inducible transcription factor pathway regulates oxygen sensing in the simplest animal, Trichoplax adhaerens. EMBO Rep. 2011;12(1):63–70. doi: 10.1038/embor.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92(12):5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256(5060):1193–5. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- 10.Greer SN, Metcalf JL, Wang Y, Ohh M. The updated biology of hypoxia-inducible factor. EMBO J. 2012;31(11):2448–60. doi: 10.1038/emboj.2012.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y, Ye D. Cancer therapy by targeting hypoxia-inducible factor-1. Curr Cancer Drug Targets. 2010;10(7):782–96. doi: 10.2174/156800910793605857. [DOI] [PubMed] [Google Scholar]
- 12.Jiang BH, Rue E, Wang GL, Roe R, Semenza GL. Dimerization, DNA binding, and transactivation properties of hypoxia-inducible factor 1. J Biol Chem. 1996;271(30):17771–8. doi: 10.1074/jbc.271.30.17771. [DOI] [PubMed] [Google Scholar]
- 13.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95(14):7987–92. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruas JL, Poellinger L, Pereira T. Functional analysis of hypoxia-inducible factor-1 alpha-mediated transactivation. Identification of amino acid residues critical for transcriptional activation and/or interaction with CREB-binding protein. J Biol Chem. 2002;277(41):38723–30. doi: 10.1074/jbc.M205051200. [DOI] [PubMed] [Google Scholar]
- 15.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem. 1997;272(31):19253–60. doi: 10.1074/jbc.272.31.19253. [DOI] [PubMed] [Google Scholar]
- 16.Kallio PJ, Okamoto K, O'Brien S, et al. Signal transduction in hypoxic cells: inducible nuclear translocation and recruitment of the CBP/p300 coactivator by the hypoxia-inducible factor-1alpha. EMBO J. 1998;17(22):6573–86. doi: 10.1093/emboj/17.22.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12(21):3320–4. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ema M, Taya S, Yokotani N, Sogawa K, Matsuda Y, Fujii-Kuriyama Y. A novel bHLH-PAS factor with close sequence similarity to hypoxia-inducible factor 1alpha regulates the VEGF expression and is potentially involved in lung and vascular development. Proc Natl Acad Sci USA. 1997;94(9):4273–8. doi: 10.1073/pnas.94.9.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11(1):72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- 20.Hara S, Hamada J, Kobayashi C, Kondo Y, Imura N. Expression and characterization of hypoxia-inducible factor (HIF)-3alpha in human kidney: suppression of HIF-mediated gene expression by HIF-3alpha. Biochem Biophys Res Commun. 2001;287(4):808–13. doi: 10.1006/bbrc.2001.5659. [DOI] [PubMed] [Google Scholar]
- 21.Gu YZ, Moran SM, Hogenesch JB, Wartman L, Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 1998;7(3):205–13. [PMC free article] [PubMed] [Google Scholar]
- 22.Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem. 2002;277(36):32405–8. doi: 10.1074/jbc.C200328200. [DOI] [PubMed] [Google Scholar]
- 23.Makino Y, Cao R, Svensson K, et al. Inhibitory PAS domain protein is a negative regulator of hypoxia-inducible gene expression. Nature. 2001;414(6863):550–4. doi: 10.1038/35107085. [DOI] [PubMed] [Google Scholar]
- 24.Wiesener MS, Turley H, Allen WE, et al. Induction of endothelial PAS domain protein-1 by hypoxia: characterization and comparison with hypoxia-inducible factor-1alpha. Blood. 1998;92(7):2260–8. [PubMed] [Google Scholar]
- 25.Kallio PJ, Pongratz I, Gradin K, McGuire J, Poellinger L. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA. 1997;94(11):5667–72. doi: 10.1073/pnas.94.11.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahimi-Horn C, Mazure N, Pouyssegur J. Signalling via the hypoxia-inducible factor-1alpha requires multiple posttranslational modifications. Cell Signal. 2005;17(1):1–9. doi: 10.1016/j.cellsig.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Welsh SJ, Powis G. Hypoxia inducible factor as a cancer drug target. Curr Cancer Drug Targets. 2003;3(6):391–405. doi: 10.2174/1568009033481732. [DOI] [PubMed] [Google Scholar]
- 28.Ivan M, Kondo K, Yang H, et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–8. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 29.Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–72. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 30.Masson N, Ratcliffe PJ. HIF prolyl and asparaginyl hydroxylases in the biological response to intracellular O(2) levels. J Cell Sci. 2003;116(Pt 15):3041–9. doi: 10.1242/jcs.00655. [DOI] [PubMed] [Google Scholar]
- 31.Masson N, Willam C, Maxwell PH, Pugh CW, Ratcliffe PJ. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20(18):5197–206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srinivas V, Zhang LP, Zhu XH, Caro J. Characterization of an oxygen/redox-dependent degradation domain of hypoxia-inducible factor alpha (HIF-alpha) proteins. Biochem Biophys Res Commun. 1999;260(2):557–61. doi: 10.1006/bbrc.1999.0878. [DOI] [PubMed] [Google Scholar]
- 33.Huang J, Zhao Q, Mooney SM, Lee FS. Sequence determinants in hypoxia-inducible factor-1alpha for hydroxylation by the prolyl hydroxylases PHD1, PHD2, and PHD3. J Biol Chem. 2002;277(42):39792–800. doi: 10.1074/jbc.M206955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schofield CJ, Zhang Z. Structural and mechanistic studies on 2-oxoglutarate-dependent oxygenases and related enzymes. Curr Opin Struct Biol. 1999;9(6):722–31. doi: 10.1016/s0959-440x(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 35.Bruick RK, McKnight SL. A conserved family of prolyl-4-hydroxylases that modify HIF. Science. 2001;294(5545):1337–40. doi: 10.1126/science.1066373. [DOI] [PubMed] [Google Scholar]
- 36.Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003;270(5):781–90. doi: 10.1046/j.1432-1033.2003.03445.x. [DOI] [PubMed] [Google Scholar]
- 37.Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93(25):14765–70. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epstein AC, Gleadle JM, McNeill LA, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107(1):43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 39.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22(16):4082–90. doi: 10.1093/emboj/cdg392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metzen E, Berchner-Pfannschmidt U, Stengel P, et al. Intracellular localisation of human HIF-1 alpha hydroxylases: implications for oxygen sensing. J Cell Sci. 2003;116(Pt 7):1319–26. doi: 10.1242/jcs.00318. [DOI] [PubMed] [Google Scholar]
- 41.Hon WC, Wilson MI, Harlos K, et al. Structural basis for the recognition of hydroxyproline in HIF-1 alpha by pVHL. Nature. 2002;417(6892):975–8. doi: 10.1038/nature00767. [DOI] [PubMed] [Google Scholar]
- 42.Min JH, Yang H, Ivan M, Gertler F, Kaelin WG, Jr, Pavletich NP. Structure of an HIF-1alpha -pVHL complex: hydroxyproline recognition in signaling. Science. 2002;296(5574):1886–9. doi: 10.1126/science.1073440. [DOI] [PubMed] [Google Scholar]
- 43.Ivan M, Kaelin WG., Jr The von Hippel-Lindau tumor suppressor protein. Curr Opin Genet Dev. 2001;11(1):27–34. doi: 10.1016/s0959-437x(00)00152-0. [DOI] [PubMed] [Google Scholar]
- 44.Kamura T, Sato S, Iwai K, Czyzyk-Krzeska M, Conaway RC, Conaway JW. Activation of HIF1alpha ubiquitination by a reconstituted von Hippel-Lindau (VHL) tumor suppressor complex. Proc Natl Acad Sci USA. 2000;97(19):10430–5. doi: 10.1073/pnas.190332597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Groulx I, Lee S. Oxygen-dependent ubiquitination and degradation of hypoxia-inducible factor requires nuclear-cytoplasmic trafficking of the von Hippel-Lindau tumor suppressor protein. Mol Cell Biol. 2002;22(15):5319–36. doi: 10.1128/MCB.22.15.5319-5336.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berra E, Roux D, Richard DE, Pouyssegur J. Hypoxia-inducible factor-1 alpha (HIF-1 alpha) escapes O(2)-driven proteasomal degradation irrespective of its subcellular localization: nucleus or cytoplasm. EMBO Rep. 2001;2(7):615–20. doi: 10.1093/embo-reports/kve130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yu F, White SB, Zhao Q, Lee FS. Dynamic, site-specific interaction of hypoxia-inducible factor-1alpha with the von Hippel-Lindau tumor suppressor protein. Cancer Res. 2001;61(10):4136–42. [PubMed] [Google Scholar]
- 48.Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19(48):5435–43. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 49.Cockman ME, Masson N, Mole DR, et al. Hypoxia inducible factor-alpha binding and ubiquitylation by the von Hippel-Lindau tumor suppressor protein. J Biol Chem. 2000;275(33):25733–41. doi: 10.1074/jbc.M002740200. [DOI] [PubMed] [Google Scholar]
- 50.Jeong JW, Bae MK, Ahn MY, et al. Regulation and destabilization of HIF-1alpha by ARD1-mediated acetylation. Cell. 2002;111(5):709–20. doi: 10.1016/s0092-8674(02)01085-1. [DOI] [PubMed] [Google Scholar]
- 51.Kim MS, Kwon HJ, Lee YM, et al. Histone deacetylases induce angiogenesis by negative regulation of tumor suppressor genes. Nat Med. 2001;7(4):437–43. doi: 10.1038/86507. [DOI] [PubMed] [Google Scholar]
- 52.Tanimoto K, Makino Y, Pereira T, Poellinger L. Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J. 2000;19(16):4298–309. doi: 10.1093/emboj/19.16.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sang N, Fang J, Srinivas V, Leshchinsky I, Caro J. Carboxyl-terminal transactivation activity of hypoxia-inducible factor 1 alpha is governed by a von Hippel-Lindau protein-independent, hydroxylation-regulated association with p300/CBP. Mol Cell Biol. 2002;22(9):2984–92. doi: 10.1128/MCB.22.9.2984-2992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lando D, Peet DJ, Whelan DA, Gorman JJ, Whitelaw ML. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science. 2002;295(5556):858–61. doi: 10.1126/science.1068592. [DOI] [PubMed] [Google Scholar]
- 55.Lando D, Peet DJ, Gorman JJ, Whelan DA, Whitelaw ML, Bruick RK. FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 2002;16(12):1466–71. doi: 10.1101/gad.991402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hewitson KS, McNeill LA, Riordan MV, et al. Hypoxia-inducible factor (HIF) asparagine hydroxylase is identical to factor inhibiting HIF (FIH) and is related to the cupin structural family. J Biol Chem. 2002;277(29):26351–5. doi: 10.1074/jbc.C200273200. [DOI] [PubMed] [Google Scholar]
- 57.Mahon PC, Hirota K, Semenza GL. FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001;15(20):2675–86. doi: 10.1101/gad.924501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freedman SJ, Sun ZY, Poy F, Kung AL, Livingston DM, Wagner G, et al. Structural basis for recruitment of CBP/p300 by hypoxia-inducible factor-1 alpha. Proc Natl Acad Sci USA. 2002;99(8):5367–72. doi: 10.1073/pnas.082117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dames SA, Martinez-Yamout M, De Guzman RN, Dyson HJ, Wright PE. Structural basis for Hif-1 alpha/CBP recognition in the cellular hypoxic response. Proc Natl Acad Sci USA. 2002;99(8):5271–6. doi: 10.1073/pnas.082121399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minet E, Michel G, Mottet D, Raes M, Michiels C. Transduction pathways involved in Hypoxia-Inducible Factor-1 phosphorylation and activation. Free Radic Biol Med. 2001;31(7):847–55. doi: 10.1016/s0891-5849(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 61.Sodhi A, Montaner S, Patel V, et al. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60(17):4873–80. [PubMed] [Google Scholar]
- 62.Richard DE, Berra E, Gothie E, Roux D, Pouyssegur J. p42/p44 mitogen-activated protein kinases phosphorylate hypoxia-inducible factor 1alpha (HIF-1alpha) and enhance the transcriptional activity of HIF-1. J Biol Chem. 1999;274(46):32631–7. doi: 10.1074/jbc.274.46.32631. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki H, Tomida A, Tsuruo T. Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia. Oncogene. 2001;20(41):5779–88. doi: 10.1038/sj.onc.1204742. [DOI] [PubMed] [Google Scholar]
- 64.Hur E, Chang KY, Lee E, Lee SK, Park H. Mitogen-activated protein kinase kinase inhibitor PD98059 blocks the trans-activation but not the stabilization or DNA binding ability of hypoxia-inducible factor-1alpha. Mol Pharmacol. 2001;59(5):1216–24. doi: 10.1124/mol.59.5.1216. [DOI] [PubMed] [Google Scholar]
- 65.Gradin K, Takasaki C, Fujii-Kuriyama Y, Sogawa K. The transcriptional activation function of the HIF-like factor requires phosphorylation at a conserved threonine. J Biol Chem. 2002;277(26):23508–14. doi: 10.1074/jbc.M201307200. [DOI] [PubMed] [Google Scholar]
- 66.Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005(306):re12. doi: 10.1126/stke.3062005re12. [DOI] [PubMed] [Google Scholar]
- 67.Huang LE, Arany Z, Livingston DM, Bunn HF. Activation of hypoxia-inducible transcription factor depends primarily upon redox-sensitive stabilization of its alpha subunit. J Biol Chem. 1996;271(50):32253–9. doi: 10.1074/jbc.271.50.32253. [DOI] [PubMed] [Google Scholar]
- 68.Pugh CW, Tan CC, Jones RW, Ratcliffe PJ. Functional analysis of an oxygen-regulated transcriptional enhancer lying 3′ to the mouse erythropoietin gene. Proc Natl Acad Sci USA. 1991;88(23):10553–7. doi: 10.1073/pnas.88.23.10553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barnhart BC, Simon MC. Metastasis and stem cell pathways. Cancer Metastasis Rev. 2007;26(2):261–71. doi: 10.1007/s10555-007-9053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sullivan R, Graham CH. Hypoxia-driven selection of the metastatic phenotype. Cancer Metastasis Rev. 2007;26(2):319–31. doi: 10.1007/s10555-007-9062-2. [DOI] [PubMed] [Google Scholar]
- 71.Chan DA, Giaccia AJ. Hypoxia, gene expression, and metastasis. Cancer Metastasis Rev. 2007;26(2):333–9. doi: 10.1007/s10555-007-9063-1. [DOI] [PubMed] [Google Scholar]
- 72.Moeller BJ, Richardson RA, Dewhirst MW. Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev. 2007;26(2):241–8. doi: 10.1007/s10555-007-9056-0. [DOI] [PubMed] [Google Scholar]
- 73.Bindra RS, Crosby ME, Glazer PM. Regulation of DNA repair in hypoxic cancer cells. Cancer Metastasis Rev. 2007;26(2):249–60. doi: 10.1007/s10555-007-9061-3. [DOI] [PubMed] [Google Scholar]
- 74.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26(2):299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 75.Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer Metastasis Rev. 2007;26(2):273–9. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- 76.Liao D, Corle C, Seagroves TN, Johnson RS. Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res. 2007;67(2):563–72. doi: 10.1158/0008-5472.CAN-06-2701. [DOI] [PubMed] [Google Scholar]
- 77.LeCouter J, Kowalski J, Foster J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412(6850):877–84. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- 78.Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3) J Clin Invest. 2000;105(5):577–87. doi: 10.1172/JCI8316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Levy AP, Levy NS, Wegner S, Goldberg MA. Transcriptional regulation of the rat vascular endothelial growth factor gene by hypoxia. J Biol Chem. 1995;270(22):13333–40. doi: 10.1074/jbc.270.22.13333. [DOI] [PubMed] [Google Scholar]
- 80.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272(38):23659–67. doi: 10.1074/jbc.272.38.23659. [DOI] [PubMed] [Google Scholar]
- 81.Bruick RK. Expression of the gene encoding the proapoptotic Nip3 protein is induced by hypoxia. Proc Natl Acad Sci USA. 2000;97(16):9082–7. doi: 10.1073/pnas.97.16.9082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59(16):3915–8. [PubMed] [Google Scholar]
- 83.Tazuke SI, Mazure NM, Sugawara J, et al. Hypoxia stimulates insulin-like growth factor binding protein 1 (IGFBP-1) gene expression in HepG2 cells: a possible model for IGFBP-1 expression in fetal hypoxia. Proc Natl Acad Sci USA. 1998;95(17):10188–93. doi: 10.1073/pnas.95.17.10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krishnamachary B, Berg-Dixon S, Kelly B, et al. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63(5):1138–43. [PubMed] [Google Scholar]
- 85.Lofstedt T, Jogi A, Sigvardsson M, et al. Induction of ID2 expression by hypoxia-inducible factor-1: a role in dedifferentiation of hypoxic neuroblastoma cells. J Biol Chem. 2004;279(38):39223–31. doi: 10.1074/jbc.M402904200. [DOI] [PubMed] [Google Scholar]
- 86.Krishnamurthy P, Ross DD, Nakanishi T, et al. The stem cell marker Bcrp/ABCG2 enhances hypoxic cell survival through interactions with heme. J Biol Chem. 2004;279(23):24218–25. doi: 10.1074/jbc.M313599200. [DOI] [PubMed] [Google Scholar]
- 87.Comerford KM, Wallace TJ, Karhausen J, Louis NA, Montalto MC, Colgan SP. Hypoxia-inducible factor-1-dependent regulation of the multidrug resistance (MDR1) gene. Cancer Res. 2002;62(12):3387–94. [PubMed] [Google Scholar]
- 88.Grosfeld A, Andre J, Hauguel-De Mouzon S, Berra E, Pouyssegur J, Guerre-Millo M. Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J Biol Chem. 2002;277(45):42953–7. doi: 10.1074/jbc.M206775200. [DOI] [PubMed] [Google Scholar]
- 89.Koshiji M, To KK, Hammer S, et al. HIF-1alpha induces genetic instability by transcriptionally downregulating MutSalpha expression. Mol Cell. 2005;17(6):793–803. doi: 10.1016/j.molcel.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 90.Iyer NV, Kotch LE, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 1998;12(2):149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wood SM, Wiesener MS, Yeates KM, et al. Selection and analysis of a mutant cell line defective in the hypoxia-inducible factor-1 alpha-subunit (HIF-1alpha). Characterization of hif-1alpha-dependent and -independent hypoxia-inducible gene expression. J Biol Chem. 1998;273(14):8360–8. doi: 10.1074/jbc.273.14.8360. [DOI] [PubMed] [Google Scholar]
- 92.Hogenesch JB, Gu YZ, Moran SM et al. The basic helix-loop-helix-PAS protein MOP9 is a brain-specific heterodimeric partner of circadian and hypoxia factors. J Neurosci. 2000;20(13):RC83. doi: 10.1523/JNEUROSCI.20-13-j0002.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Semenza GL, Roth PH, Fang HM, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269(38):23757–63. [PubMed] [Google Scholar]
- 94.Fu L, Chen L, Yang J, Ye T, Chen Y, Fang J. HIF-1alpha-induced histone demethylase JMJD2B contributes to the malignant phenotype of colorectal cancer cells via an epigenetic mechanism. Carcinogenesis. 2012;33(9):1664–73. doi: 10.1093/carcin/bgs217. [DOI] [PubMed] [Google Scholar]
- 95.Mukhopadhyay CK, Mazumder B, Fox PL. Role of hypoxia-inducible factor-1 in transcriptional activation of ceruloplasmin by iron deficiency. J Biol Chem. 2000;275(28):21048–54. doi: 10.1074/jbc.M000636200. [DOI] [PubMed] [Google Scholar]
- 96.Rolfs A, Kvietikova I, Gassmann M, Wenger RH. Oxygen-regulated transferrin expression is mediated by hypoxia-inducible factor-1. J Biol Chem. 1997;272(32):20055–62. doi: 10.1074/jbc.272.32.20055. [DOI] [PubMed] [Google Scholar]
- 97.Lok CN, Ponka P. Identification of a hypoxia response element in the transferrin receptor gene. J Biol Chem. 1999;274(34):24147–52. doi: 10.1074/jbc.274.34.24147. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi Y, Takahashi S, Shiga Y, Yoshimi T, Miura T. Hypoxic induction of prolyl 4-hydroxylase alpha (I) in cultured cells. J Biol Chem. 2000;275(19):14139–46. doi: 10.1074/jbc.275.19.14139. [DOI] [PubMed] [Google Scholar]
- 99.Ben-Yosef Y, Lahat N, Shapiro S, Bitterman H, Miller A. Regulation of endothelial matrix metalloproteinase-2 by hypoxia/reoxygenation. Circ Res. 2002;90(7):784–91. doi: 10.1161/01.res.0000015588.70132.dc. [DOI] [PubMed] [Google Scholar]
- 100.Kietzmann T, Roth U, Jungermann K. Induction of the plasminogen activator inhibitor-1 gene expression by mild hypoxia via a hypoxia response element binding the hypoxia-inducible factor-1 in rat hepatocytes. Blood. 1999;94(12):4177–85. [PubMed] [Google Scholar]
- 101.Cowden Dahl KD, Robertson SE, Weaver VM, Simon MC. Hypoxia-inducible factor regulates alphavbeta3 integrin cell surface expression. Mol Biol Cell. 2005;16(4):1901–12. doi: 10.1091/mbc.E04-12-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zagzag D, Lukyanov Y, Lan L, et al. Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Invest. 2006;86(12):1221–32. doi: 10.1038/labinvest.3700482. [DOI] [PubMed] [Google Scholar]
- 103.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60(24):7075–83. [PubMed] [Google Scholar]
- 104.Oikawa M, Abe M, Kurosawa H, Hida W, Shirato K, Sato Y. Hypoxia induces transcription factor ETS-1 via the activity of hypoxia-inducible factor-1. Biochem Biophys Res Commun. 2001;289(1):39–43. doi: 10.1006/bbrc.2001.5927. [DOI] [PubMed] [Google Scholar]
- 105.Eckhart AD, Yang N, Xin X, Faber JE. Characterization of the alpha1B-adrenergic receptor gene promoter region and hypoxia regulatory elements in vascular smooth muscle. Proc Natl Acad Sci USA. 1997;94(17):9487–92. doi: 10.1073/pnas.94.17.9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cormier-Regard S, Nguyen SV, Claycomb WC. Adrenomedullin gene expression is developmentally regulated and induced by hypoxia in rat ventricular cardiac myocytes. J Biol Chem. 1998;273(28):17787–92. doi: 10.1074/jbc.273.28.17787. [DOI] [PubMed] [Google Scholar]
- 107.Hu J, Discher DJ, Bishopric NH, Webster KA. Hypoxia regulates expression of the endothelin-1 gene through a proximal hypoxia-inducible factor-1 binding site on the antisense strand. Biochem Biophys Res Commun. 1998;245(3):894–9. doi: 10.1006/bbrc.1998.8543. [DOI] [PubMed] [Google Scholar]
- 108.Lee PJ, Jiang BH, Chin BY, et al. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem. 1997;272(9):5375–81. [PubMed] [Google Scholar]
- 109.Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995;182(6):1683–93. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Conway EM, Collen D, Carmeliet P. Molecular mechanisms of blood vessel growth. Cardiovasc Res. 2001;49(3):507–21. doi: 10.1016/s0008-6363(00)00281-9. [DOI] [PubMed] [Google Scholar]
- 111.Giordano FJ, Johnson RS. Angiogenesis: the role of the microenvironment in flipping the switch. Curr Opin Genet Dev. 2001;11(1):35–40. doi: 10.1016/s0959-437x(00)00153-2. [DOI] [PubMed] [Google Scholar]
- 112.Berra E, Milanini J, Richard DE, et al. Signaling angiogenesis via p42/p44 MAP kinase and hypoxia. Biochem Pharmacol. 2000;60(8):1171–8. doi: 10.1016/s0006-2952(00)00423-8. [DOI] [PubMed] [Google Scholar]
- 113.Forsythe JA, Jiang BH, Iyer NV, et al. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16(9):4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bunn HF, Poyton RO. Oxygen sensing and molecular adaptation to hypoxia. Physiol Rev. 1996;76(3):839–85. doi: 10.1152/physrev.1996.76.3.839. [DOI] [PubMed] [Google Scholar]
- 115.Josko J, Gwozdz B, Jedrzejowska-Szypulka H, Hendryk S. Vascular endothelial growth factor (VEGF) and its effect on angiogenesis. Med Sci Monit. 2000;6(5):1047–52. [PubMed] [Google Scholar]
- 116.Harris AL. von Hippel-Lindau syndrome: target for anti-vascular endothelial growth factor (VEGF) receptor therapy. Oncologist. 2000;5(Suppl 1):32–6. doi: 10.1634/theoncologist.5-suppl_1-32. [DOI] [PubMed] [Google Scholar]
- 117.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13(1):9–22. [PubMed] [Google Scholar]
- 118.Nguyen SV, Claycomb WC. Hypoxia regulates the expression of the adrenomedullin and HIF-1 genes in cultured HL-1 cardiomyocytes. Biochem Biophys Res Commun. 1999;265(2):382–6. doi: 10.1006/bbrc.1999.1674. [DOI] [PubMed] [Google Scholar]
- 119.Lima e Silva R, Shen J, Hackett SF, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21(12):3219–30. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 120.Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(7):3186–93. doi: 10.1167/iovs.02-1092. [DOI] [PubMed] [Google Scholar]
- 121.Seo MS, Okamoto N, Vinores MA, et al. Photoreceptor-specific expression of platelet-derived growth factor-B results in traction retinal detachment. Am J Pathol. 2000;157(3):995–1005. doi: 10.1016/S0002-9440(10)64612-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hackett SF, Ozaki H, Strauss RW, et al. Angiopoietin 2 expression in the retina: upregulation during physiologic and pathologic neovascularization. J Cell Physiol. 2000;184(3):275–84. doi: 10.1002/1097-4652(200009)184:3<275::AID-JCP1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 123.Ames A., 3rd Energy requirements of CNS cells as related to their function and to their vulnerability to ischemia: a commentary based on studies on retina. Can J Physiol Pharmacol. 1992;70(Suppl 1):S158–64. doi: 10.1139/y92-257. [DOI] [PubMed] [Google Scholar]
- 124.Campochiaro PA. Ocular neovascularization. J Mol Med (Berl) 2013;91(3):311–21. doi: 10.1007/s00109-013-0993-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Caprara C, Grimm C. From oxygen to erythropoietin: relevance of hypoxia for retinal development, health and disease. Prog Retin Eye Res. 2012;31(1):89–119. doi: 10.1016/j.preteyeres.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 126.Sung CH, Chuang JZ. The cell biology of vision. J Cell Biol. 2010;190(6):953–63. doi: 10.1083/jcb.201006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baccus SA. Timing and computation in inner retinal circuitry. Annu Rev Physiol. 2007;69:271–90. doi: 10.1146/annurev.physiol.69.120205.124451. [DOI] [PubMed] [Google Scholar]
- 128.Okawa H, Sampath AP, Laughlin SB, Fain GL. ATP consumption by mammalian rod photoreceptors in darkness and in light. Curr Biol. 2008;18(24):1917–21. doi: 10.1016/j.cub.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Stone J, van Driel D, Valter K, Rees S, Provis J. The locations of mitochondria in mammalian photoreceptors: relation to retinal vasculature. Brain Res. 2008;1189:58–69. doi: 10.1016/j.brainres.2007.10.083. [DOI] [PubMed] [Google Scholar]
- 130.Flammer J, Mozaffarieh M. Autoregulation, a balancing act between supply and demand. Can J Ophthalmol. 2008;43(3):317–21. doi: 10.3129/i08-056. [DOI] [PubMed] [Google Scholar]
- 131.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27(3):284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 132.Saint-Geniez M, D'Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48(8-9):1045–58. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 133.Kocur I, Resnikoff S. Visual impairment and blindness in Europe and their prevention. Br J Ophthalmol. 2002;86(7):716–22. doi: 10.1136/bjo.86.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ferris FL, 3rd, Davis MD, Aiello LM. Treatment of diabetic retinopathy. N Engl J Med. 1999;341(9):667–78. doi: 10.1056/NEJM199908263410907. [DOI] [PubMed] [Google Scholar]
- 135.Crawford TN, Alfaro DV, 3rd, Kerrison JB, Jablon EP. Diabetic retinopathy and angiogenesis. Curr Diabetes Rev. 2009;5(1):8–13. doi: 10.2174/157339909787314149. [DOI] [PubMed] [Google Scholar]
- 136.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 137.Bhavsar AR. Diabetic retinopathy: the latest in current management. Retina. 2006;26(6 Suppl):S71–9. doi: 10.1097/01.iae.0000236466.23640.c9. [DOI] [PubMed] [Google Scholar]
- 138.Arden GB, Sivaprasad S. Hypoxia and oxidative stress in the causation of diabetic retinopathy. Curr Diabetes Rev. 2011;7(5):291–304. doi: 10.2174/157339911797415620. [DOI] [PubMed] [Google Scholar]
- 139.Wilkinson-Berka JL. Angiotensin and diabetic retinopathy. Int J Biochem Cell Biol. 2006;38(5-6):752–65. doi: 10.1016/j.biocel.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 140.Engerman RL, Kern TS. Retinopathy in animal models of diabetes. Diabetes Metab Rev. 1995;11(2):109–20. doi: 10.1002/dmr.5610110203. [DOI] [PubMed] [Google Scholar]
- 141.Stitt AW, Anderson HR, Gardiner TA, Archer DB. Diabetic retinopathy: quantitative variation in capillary basement membrane thickening in arterial or venous environments. Br J Ophthalmol. 1994;78(2):133–7. doi: 10.1136/bjo.78.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bursell SE, Clermont AC, Kinsley BT, Simonson DC, Aiello LM, Wolpert HA. Retinal blood flow changes in patients with insulin-dependent diabetes mellitus and no diabetic retinopathy. Invest Ophthalmol Vis Sci. 1996;37(5):886–97. [PubMed] [Google Scholar]
- 143.Josifova T, Plestina-Borjan I, Henrich PB. Proliferative diabetic retinopathy: predictive and preventive measures at hypoxia induced retinal changes. The EPMA journal. 2010;1(1):73–7. doi: 10.1007/s13167-010-0003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Morello CM. Etiology and natural history of diabetic retinopathy: an overview. Am J Health Syst Pharm. 2007;64(17 Suppl 12):S3–7. doi: 10.2146/ajhp070330. [DOI] [PubMed] [Google Scholar]
- 145.Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298(8):902–16. doi: 10.1001/jama.298.8.902. [DOI] [PubMed] [Google Scholar]
- 146.Watkins PJ. Retinopathy. BMJ. 2003;326(7395):924–6. doi: 10.1136/bmj.326.7395.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Holekamp NM, Shui YB, Beebe D. Lower intraocular oxygen tension in diabetic patients: possible contribution to decreased incidence of nuclear sclerotic cataract. Am J Ophthalmol. 2006;141(6):1027–32. doi: 10.1016/j.ajo.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 148.Lim JI, Spee C, Hinton DR. A comparison of hypoxia-inducible factor-alpha in surgically excised neovascular membranes of patients with diabetes compared with idiopathic epiretinal membranes in nondiabetic patients. Retina. 2010;30(9):1472–8. doi: 10.1097/IAE.0b013e3181d6df09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Abu El-Asrar AM, Missotten L, Geboes K. Expression of hypoxia-inducible factor-1alpha and the protein products of its target genes in diabetic fibrovascular epiretinal membranes. Br J Ophthalmol. 2007;91(6):822–6. doi: 10.1136/bjo.2006.109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lin M, Chen Y, Jin J, et al. Ischaemia-induced retinal neovascularisation and diabetic retinopathy in mice with conditional knockout of hypoxia-inducible factor-1 in retinal Muller cells. Diabetologia. 2011;54(6):1554–66. doi: 10.1007/s00125-011-2081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gilbert C. Retinopathy of prematurity: a global perspective of the epidemics, population of babies at risk and implications for control. Early Hum Dev. 2008;84(2):77–82. doi: 10.1016/j.earlhumdev.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 152.Smith LE. Pathogenesis of retinopathy of prematurity. Semin Neonatol. 2003;8(6):469–73. doi: 10.1016/S1084-2756(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 153.Kuriyama H, Waki M, Nakagawa M, Tsuda M. Involvement of oxygen free radicals in experimental retinal ischemia and the selective vulnerability of retinal damage. Ophthalmic Res. 2001;33(4):196–202. doi: 10.1159/000055670. [DOI] [PubMed] [Google Scholar]
- 154.Hardy P, Dumont I, Bhattacharya M, et al. Oxidants, nitric oxide and prostanoids in the developing ocular vasculature: a basis for ischemic retinopathy. Cardiovasc Res. 2000;47(3):489–509. doi: 10.1016/s0008-6363(00)00084-5. [DOI] [PubMed] [Google Scholar]
- 155.Hardy P, Abran D, Li DY, Fernandez H, Varma DR, Chemtob S. Free radicals in retinal and choroidal blood flow autoregulation in the piglet: interaction with prostaglandins. Invest Ophthalmol Vis Sci. 1994;35(2):580–91. [PubMed] [Google Scholar]
- 156.Flynn JT, Bancalari E, Snyder ES, et al. A cohort study of transcutaneous oxygen tension and the incidence and severity of retinopathy of prematurity. N Engl J Med. 1992;326(16):1050–4. doi: 10.1056/NEJM199204163261603. [DOI] [PubMed] [Google Scholar]
- 157.Sapieha P, Joyal JS, Rivera JC, et al. Retinopathy of prematurity: understanding ischemic retinal vasculopathies at an extreme of life. J Clin Invest. 2010;120(9):3022–32. doi: 10.1172/JCI42142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kermorvant-Duchemin E, Sapieha P, Sirinyan M, et al. Understanding ischemic retinopathies: emerging concepts from oxygen-induced retinopathy. Doc Ophthalmol. 2010;120(1):51–60. doi: 10.1007/s10633-009-9201-x. [DOI] [PubMed] [Google Scholar]
- 159.Smith LE. Through the eyes of a child: understanding retinopathy through ROP the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2008;49(12):5177–82. doi: 10.1167/iovs.08-2584. [DOI] [PubMed] [Google Scholar]
- 160.Chen J, Smith LE. Retinopathy of prematurity. Angiogenesis. 2007;10(2):133–40. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 161.Mowat FM, Luhmann UF, Smith AJ, et al. HIF-1alpha and HIF-2alpha are differentially activated in distinct cell populations in retinal ischaemia. PloS one. 2010;5(6):e11103. doi: 10.1371/journal.pone.0011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jiang J, Xia XB, Xu HZ, et al. Inhibition of retinal neovascularization by gene transfer of small interfering RNA targeting HIF-1alpha and VEGF. J Cell Physiol. 2009;218(1):66–74. doi: 10.1002/jcp.21566. [DOI] [PubMed] [Google Scholar]
- 163.Gehrs KM, Anderson DH, Johnson LV, Hageman GS. Age-related macular degeneration--emerging pathogenetic and therapeutic concepts. Ann Med. 2006;38(7):450–71. doi: 10.1080/07853890600946724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Adams MK, Simpson JA, Aung KZ, Makeyeva GA, Giles GG, English DR, et al. Abdominal obesity and age-related macular degeneration. Am J Epidemiol. 2011;173(11):1246–55. doi: 10.1093/aje/kwr005. [DOI] [PubMed] [Google Scholar]
- 165.Reynolds R, Rosner B, Seddon JM. Serum lipid biomarkers and hepatic lipase gene associations with age-related macular degeneration. Ophthalmology. 2010;117(10):1989–95. doi: 10.1016/j.ophtha.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Klein R, Cruickshanks KJ, Nash SD, et al. The prevalence of age-related macular degeneration and associated risk factors. Arch Ophthalmol. 2010;128(6):750–8. doi: 10.1001/archophthalmol.2010.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Tolppanen AM, Nevalainen T, Kolehmainen M, et al. Single nucleotide polymorphisms of the tenomodulin gene (TNMD) in age-related macular degeneration. Mol Vis. 2009;15:762–70. [PMC free article] [PubMed] [Google Scholar]
- 168.van Leeuwen R, Ikram MK, Vingerling JR, Witteman JC, Hofman A, de Jong PT. Blood pressure, atherosclerosis, and the incidence of age-related maculopathy: the Rotterdam Study. Invest Ophthalmol Vis Sci. 2003;44(9):3771–7. doi: 10.1167/iovs.03-0121. [DOI] [PubMed] [Google Scholar]
- 169.Cook HL, Patel PJ, Tufail A. Age-related macular degeneration: diagnosis and management. Br Med Bull. 2008;85:127–49. doi: 10.1093/bmb/ldn012. [DOI] [PubMed] [Google Scholar]
- 170.Algvere PV, Seregard S. Drusen maculopathy: a risk factor for AMD. Can we prevent visual loss? Acta Ophthalmol Scand. 2003;81(5):427–9. doi: 10.1034/j.1600-0420.2003.00157.x. [DOI] [PubMed] [Google Scholar]
- 171.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257–93. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 172.Bhutto I, Lutty G. Understanding age-related macular degeneration (AMD): relationships between the photoreceptor/retinal pigment epithelium/Bruch's membrane/choriocapillaris complex. Mol Aspects Med. 2012;33(4):295–317. doi: 10.1016/j.mam.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ayoub T, Patel N. Age-related macular degeneration. J R Soc Med. 2009;102(2):56–61. doi: 10.1258/jrsm.2009.080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Donoso LA, Kim D, Frost A, Callahan A, Hageman G. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51(2):137–52. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Beatty S, Koh H, Phil M, Henson D, Boulton M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45(2):115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 176.Arjamaa O, Nikinmaa M, Salminen A, Kaarniranta K. Regulatory role of HIF-1alpha in the pathogenesis of age-related macular degeneration (AMD) Ageing research reviews. 2009;8(4):349–58. doi: 10.1016/j.arr.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 177.Taylor CT. Interdependent roles for hypoxia inducible factor and nuclear factor-kappaB in hypoxic inflammation. J Physiol. 2008;586(Pt 17):4055–9. doi: 10.1113/jphysiol.2008.157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Metelitsina TI, Grunwald JE, DuPont JC, Ying GS, Brucker AJ, Dunaief JL. Foveolar choroidal circulation and choroidal neovascularization in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49(1):358–63. doi: 10.1167/iovs.07-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Grunwald JE, Metelitsina TI, Dupont JC, Ying GS, Maguire MG. Reduced foveolar choroidal blood flow in eyes with increasing AMD severity. Invest Ophthalmol Vis Sci. 2005;46(3):1033–8. doi: 10.1167/iovs.04-1050. [DOI] [PubMed] [Google Scholar]
- 180.Schlingemann RO. Role of growth factors and the wound healing response in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):91–101. doi: 10.1007/s00417-003-0828-0. [DOI] [PubMed] [Google Scholar]
- 181.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217(3):674–85. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Frede S, Berchner-Pfannschmidt U, Fandrey J. Regulation of hypoxia-inducible factors during inflammation. Methods Enzymol. 2007;435:405–19. doi: 10.1016/S0076-6879(07)35021-0. [DOI] [PubMed] [Google Scholar]
- 183.Sheridan CM, Pate S, Hiscott P, Wong D, Pattwell DM, Kent D. Expression of hypoxia-inducible factor-1alpha and -2alpha in human choroidal neovascular membranes. Graefes Arch Clin Exp Ophthalmol. 2009;247(10):1361–7. doi: 10.1007/s00417-009-1133-3. [DOI] [PubMed] [Google Scholar]
- 184.Inoue Y, Yanagi Y, Matsuura K, Takahashi H, Tamaki Y, Araie M. Expression of hypoxia-inducible factor 1alpha and 2alpha in choroidal neovascular membranes associated with age-related macular degeneration. Br J Ophthalmol. 2007;91(12):1720–1. doi: 10.1136/bjo.2006.111583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liu Y, Allingham RR. Molecular genetics in glaucoma. Exp Eye Res. 2011;93(4):331–9. doi: 10.1016/j.exer.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Quigley HA. Glaucoma. Lancet. 2011;377(9774):1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 188.Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Retin Eye Res. 2002;21(4):359–93. doi: 10.1016/s1350-9462(02)00008-3. [DOI] [PubMed] [Google Scholar]
- 189.Osborne NN, Melena J, Chidlow G, Wood JP. A hypothesis to explain ganglion cell death caused by vascular insults at the optic nerve head: possible implication for the treatment of glaucoma. Br J Ophthalmol. 2001;85(10):1252–9. doi: 10.1136/bjo.85.10.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Tezel G, Wax MB. Hypoxia-inducible factor 1alpha in the glaucomatous retina and optic nerve head. Arch Ophthalmol. 2004;122(9):1348–56. doi: 10.1001/archopht.122.9.1348. [DOI] [PubMed] [Google Scholar]
- 191.Ng EW, Adamis AP. Targeting angiogenesis, the underlying disorder in neovascular age-related macular degeneration. Can J Ophthalmol. 2005;40(3):352–68. doi: 10.1016/S0008-4182(05)80078-X. [DOI] [PubMed] [Google Scholar]
- 192.Lashkari K, Hirose T, Yazdany J, McMeel JW, Kazlauskas A, Rahimi N. Vascular endothelial growth factor and hepatocyte growth factor levels are differentially elevated in patients with advanced retinopathy of prematurity. Am J Pathol. 2000;156(4):1337–44. doi: 10.1016/S0002-9440(10)65004-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tripathi RC, Li J, Tripathi BJ, Chalam KV, Adamis AP. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology. 1998;105(2):232–7. doi: 10.1016/s0161-6420(98)92782-8. [DOI] [PubMed] [Google Scholar]
- 194.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–7. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 195.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev. 2004;25(4):581–611. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 196.Famiglietti EV, Stopa EG, McGookin ED, Song P, LeBlanc V, Streeten BW. Immunocytochemical localization of vascular endothelial growth factor in neurons and glial cells of human retina. Brain Res. 2003;969(1-2):195–204. doi: 10.1016/s0006-8993(02)03766-6. [DOI] [PubMed] [Google Scholar]
- 197.Takagi H, King GL, Aiello LP. Identification and characterization of vascular endothelial growth factor receptor (Flt) in bovine retinal pericytes. Diabetes. 1996;45(8):1016–23. doi: 10.2337/diab.45.8.1016. [DOI] [PubMed] [Google Scholar]
- 198.Aiello LP, Northrup JM, Keyt BA, Takagi H, Iwamoto MA. Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol. 1995;113(12):1538–44. doi: 10.1001/archopht.1995.01100120068012. [DOI] [PubMed] [Google Scholar]
- 199.Thieme H, Aiello LP, Takagi H, Ferrara N, King GL. Comparative analysis of vascular endothelial growth factor receptors on retinal and aortic vascular endothelial cells. Diabetes. 1995;44(1):98–103. doi: 10.2337/diab.44.1.98. [DOI] [PubMed] [Google Scholar]
- 200.Kimoto K, Kubota T. Anti-VEGF Agents for Ocular Angiogenesis and Vascular Permeability. J Ophthalmol. 2012;2012:852183. doi: 10.1155/2012/852183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tolentino M. Systemic and ocular safety of intravitreal anti-VEGF therapies for ocular neovascular disease. Surv Ophthalmol. 2011;56(2):95–113. doi: 10.1016/j.survophthal.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 202.Campa C, Harding SP. Anti-VEGF compounds in the treatment of neovascular age related macular degeneration. Curr Drug Targets. 2011;12(2):173–81. doi: 10.2174/138945011794182674. [DOI] [PubMed] [Google Scholar]
- 203.Pieramici DJ, Rabena MD. Anti-VEGF therapy: comparison of current and future agents. Eye. 2008;22(10):1330–6. doi: 10.1038/eye.2008.88. [DOI] [PubMed] [Google Scholar]
- 204.Quaggin SE. Turning a blind eye to anti-VEGF toxicities. J Clin Invest. 2012;122(11):3849–51. doi: 10.1172/JCI65509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kurihara T, Westenskow PD, Bravo S, Aguilar E, Friedlander M. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012;122(11):4213–7. doi: 10.1172/JCI65157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Terzuoli E, Puppo M, Rapisarda A, et al. Aminoflavone, a ligand of the aryl hydrocarbon receptor, inhibits HIF-1alpha expression in an AhR-independent fashion. Cancer Res. 2010;70(17):6837–48. doi: 10.1158/0008-5472.CAN-10-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Greenberger LM, Horak ID, Filpula D, et al. A RNA antagonist of hypoxia-inducible factor-1alpha, EZN-2968, inhibits tumor cell growth. Mol Cancer Ther. 2008;7(11):3598–608. doi: 10.1158/1535-7163.MCT-08-0510. [DOI] [PubMed] [Google Scholar]
- 208.Lin M, Hu Y, Chen Y, et al. Impacts of hypoxia-inducible factor-1 knockout in the retinal pigment epithelium on choroidal neovascularization. Invest Ophthalmol Vis Sci. 2012;53(10):6197–206. doi: 10.1167/iovs.11-8936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhang H, Qian DZ, Tan YS, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105(50):19579–86. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Koh MY, Spivak-Kroizman T, Venturini S, Welsh S, Williams RR, Kirkpatrick DL, et al. Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1alpha. Mol Cancer Ther. 2008;7(1):90–100. doi: 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- 211.Rapisarda A, Uranchimeg B, Scudiero DA, et al. Identification of small molecule inhibitors of hypoxia-inducible factor 1 transcriptional activation pathway. Cancer Res. 2002;62(15):4316–24. [PubMed] [Google Scholar]
- 212.Pore N, Jiang Z, Gupta A, Cerniglia G, Kao GD, Maity A. EGFR tyrosine kinase inhibitors decrease VEGF expression by both hypoxia-inducible factor (HIF)-1-independent and HIF-1-dependent mechanisms. Cancer Res. 2006;66(6):3197–204. doi: 10.1158/0008-5472.CAN-05-3090. [DOI] [PubMed] [Google Scholar]
- 213.Buchler P, Reber HA, Buchler MW, Friess H, Lavey RS, Hines OJ. Antiangiogenic activity of genistein in pancreatic carcinoma cells is mediated by the inhibition of hypoxia-inducible factor-1 and the down-regulation of VEGF gene expression. Cancer. 2004;100(1):201–10. doi: 10.1002/cncr.11873. [DOI] [PubMed] [Google Scholar]
- 214.Jiang BH, Jiang G, Zheng JZ, Lu Z, Hunter T, Vogt PK. Phosphatidylinositol 3-kinase signaling controls levels of hypoxia-inducible factor 1. Cell Growth Differ. 2001;12(7):363–9. [PubMed] [Google Scholar]
- 215.Pore N, Gupta AK, Cerniglia GJ, et al. Nelfinavir down-regulates hypoxia-inducible factor 1alpha and VEGF expression and increases tumor oxygenation: implications for radiotherapy. Cancer Res. 2006;66(18):9252–9. doi: 10.1158/0008-5472.CAN-06-1239. [DOI] [PubMed] [Google Scholar]
- 216.Zhang Q, Tang X, Lu QY, Zhang ZF, Brown J, Le AD. Resveratrol inhibits hypoxia-induced accumulation of hypoxia-inducible factor-1alpha and VEGF expression in human tongue squamous cell carcinoma and hepatoma cells. Mol Cancer Ther. 2005;4(10):1465–74. doi: 10.1158/1535-7163.MCT-05-0198. [DOI] [PubMed] [Google Scholar]
- 217.Mabuchi S, Altomare DA, Cheung M, et al. RAD001 inhibits human ovarian cancer cell proliferation, enhances cisplatin-induced apoptosis, and prolongs survival in an ovarian cancer model. Clin Cancer Res. 2007;13(14):4261–70. doi: 10.1158/1078-0432.CCR-06-2770. [DOI] [PubMed] [Google Scholar]
- 218.Wang W, Jia WD, Xu GL, et al. Antitumoral activity of rapamycin mediated through inhibition of HIF-1alpha and VEGF in hepatocellular carcinoma. Dig Dis Sci. 2009;54(10):2128–36. doi: 10.1007/s10620-008-0605-3. [DOI] [PubMed] [Google Scholar]
- 219.Garcia-Maceira P, Mateo J. Silibinin inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1 signalling pathway in human cervical and hepatoma cancer cells: implications for anticancer therapy. Oncogene. 2009;28(3):313–24. doi: 10.1038/onc.2008.398. [DOI] [PubMed] [Google Scholar]
- 220.Wan X, Shen N, Mendoza A, Khanna C, Helman LJ. CCI-779 inhibits rhabdomyosarcoma xenograft growth by an antiangiogenic mechanism linked to the targeting of mTOR/Hif-1alpha/VEGF signaling. Neoplasia. 2006;8(5):394–401. doi: 10.1593/neo.05820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kumar SM, Yu H, Edwards R, et al. Mutant V600E BRAF increases hypoxia inducible factor-1alpha expression in melanoma. Cancer Res. 2007;67(7):3177–84. doi: 10.1158/0008-5472.CAN-06-3312. [DOI] [PubMed] [Google Scholar]
- 222.Fang J, Zhou Q, Liu LZ, et al. Apigenin inhibits tumor angiogenesis through decreasing HIF-1alpha and VEGF expression. Carcinogenesis. 2007;28(4):858–64. doi: 10.1093/carcin/bgl205. [DOI] [PubMed] [Google Scholar]
- 223.Kim WY, Oh SH, Woo JK, Hong WK, Lee HY. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1alpha. Cancer Res. 2009;69(4):1624–32. doi: 10.1158/0008-5472.CAN-08-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Alqawi O, Moghaddas M, Singh G. Effects of geldanamycin on HIF-1alpha mediated angiogenesis and invasion in prostate cancer cells. Prostate Cancer Prostatic Dis. 2006;9(2):126–35. doi: 10.1038/sj.pcan.4500852. [DOI] [PubMed] [Google Scholar]
- 225.Mie Lee Y, Kim SH, Kim HS, et al. Inhibition of hypoxia-induced angiogenesis by FK228, a specific histone deacetylase inhibitor, via suppression of HIF-1alpha activity. Biochem Biophys Res Commun. 2003;300(1):241–6. doi: 10.1016/s0006-291x(02)02787-0. [DOI] [PubMed] [Google Scholar]
- 226.Shankar S, Davis R, Singh KP, Kurzrock R, Ross DD, Srivastava RK. Suberoylanilide hydroxamic acid (Zolinza/vorinostat) sensitizes TRAIL-resistant breast cancer cells orthotopically implanted in BALB/c nude mice. Mol Cancer Ther. 2009;8(6):1596–605. doi: 10.1158/1535-7163.MCT-08-1004. [DOI] [PubMed] [Google Scholar]
- 227.Yang QC, Zeng BF, Shi ZM, et al. Inhibition of hypoxia-induced angiogenesis by trichostatin A via suppression of HIF-1a activity in human osteosarcoma. J Exp Clin Cancer Res. 2006;25(4):593–9. [PubMed] [Google Scholar]
- 228.Newcomb EW, Ali MA, Schnee T, et al. Flavopiridol downregulates hypoxia-mediated hypoxia-inducible factor-1alpha expression in human glioma cells by a proteasome-independent pathway: implications for in vivo therapy. Neuro Oncol. 2005;7(3):225–35. doi: 10.1215/S1152851704000997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Duyndam MC, van Berkel MP, Dorsman JC, Rockx DA, Pinedo HM, Boven E. Cisplatin and doxorubicin repress Vascular Endothelial Growth Factor expression and differentially down-regulate Hypoxia-inducible Factor I activity in human ovarian cancer cells. Biochem Pharmacol. 2007;74(2):191–201. doi: 10.1016/j.bcp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 230.Kong D, Park EJ, Stephen AG, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65(19):9047–55. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- 231.Olenyuk BZ, Zhang GJ, Klco JM, Nickols NG, Kaelin WG, Jr, Dervan PB. Inhibition of vascular endothelial growth factor with a sequence-specific hypoxia response element antagonist. Proc Natl Acad Sci USA. 2004;101(48):16768–73. doi: 10.1073/pnas.0407617101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mabjeesh NJ, Escuin D, LaVallee TM, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer cell. 2003;3(4):363–75. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 233.Bae MK, Kim SH, Jeong JW, et al. Curcumin inhibits hypoxia-induced angiogenesis via down-regulation of HIF-1. Oncol Rep. 2006;15(6):1557–62. [PubMed] [Google Scholar]
- 234.Maeda M, Hasebe Y, Egawa K, Shibanuma M, Nose K. Inhibition of angiogenesis and HIF-1alpha activity by antimycin A1. Biol Pharm Bull. 2006;29(7):1344–8. doi: 10.1248/bpb.29.1344. [DOI] [PubMed] [Google Scholar]
- 235.Shin DH, Chun YS, Lee DS, Huang LE, Park JW. Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxia-inducible factor-1. Blood. 2008;111(6):3131–6. doi: 10.1182/blood-2007-11-120576. [DOI] [PubMed] [Google Scholar]
- 236.Kung AL, Zabludoff SD, France DS, et al. Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway. Cancer cell. 2004;6(1):33–43. doi: 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 237.Yasui H, Ogura A, Asanuma T, et al. Inhibition of HIF-1alpha by the anticancer drug TAS106 enhances X-ray-induced apoptosis in vitro and in vivo. Br J Cancer. 2008;99(9):1442–52. doi: 10.1038/sj.bjc.6604720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Zhao Q, Du J, Gu H, et al. Effects of YC-1 on hypoxia-inducible factor 1-driven transcription activity, cell proliferative vitality, and apoptosis in hypoxic human pancreatic cancer cells. Pancreas. 2007;34(2):242–7. doi: 10.1097/01.mpa.0000250135.95144.b6. [DOI] [PubMed] [Google Scholar]
- 239.Welsh SJ, Williams RR, Birmingham A, Newman DJ, Kirkpatrick DL, Powis G. The thioredoxin redox inhibitors 1-methylpropyl 2-imidazolyl disulfide and pleurotin inhibit hypoxia-induced factor 1alpha and vascular endothelial growth factor formation. Mol Cancer Ther. 2003;2(3):235–43. [PubMed] [Google Scholar]
- 240.Young RM, Wang SJ, Gordan JD, Ji X, Liebhaber SA, Simon MC. Hypoxia-mediated selective mRNA translation by an internal ribosome entry site-independent mechanism. J Biol Chem. 2008;283(24):16309–19. doi: 10.1074/jbc.M710079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Chen P, Yin H, Wang Y, Xie L. Inhibition of VEGF expression and corneal neovascularization by shRNA targeting HIF-1alpha in a mouse model of closed eye contact lens wear. Molecular vision. 2012;18:864–73. [PMC free article] [PubMed] [Google Scholar]
- 242.Zhang H, Qian DZ, Tan YS, et al. Digoxin and other cardiac glycosides inhibit HIF-1alpha synthesis and block tumor growth. Proc Natl Acad Sci USA. 2008;105(50):19579–86. doi: 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yoshida T, Zhang H, Iwase T, Shen J, Semenza GL, Campochiaro PA. Digoxin inhibits retinal ischemia-induced HIF-1alpha expression and ocular neovascularization. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2010;24(6):1759–67. doi: 10.1096/fj.09-145664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Wang B, Zou Y, Li H, Yan H, Pan JS, Yuan ZL. Genistein inhibited retinal neovascularization and expression of vascular endothelial growth factor and hypoxia inducible factor 1alpha in a mouse model of oxygen-induced retinopathy. J Ocul Pharmacol Ther. 2005;21(2):107–13. doi: 10.1089/jop.2005.21.107. [DOI] [PubMed] [Google Scholar]
- 245.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32(3):517–30. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 246.Isaacs JS, Jung YJ, Mimnaugh EG, Martinez A, Cuttitta F, Neckers LM. Hsp90 regulates a von Hippel Lindau-independent hypoxia-inducible factor-1 alpha-degradative pathway. J Biol Chem. 2002;277(33):29936–44. doi: 10.1074/jbc.M204733200. [DOI] [PubMed] [Google Scholar]
- 247.Kim WY, Oh SH, Woo JK, Hong WK, Lee HY. Targeting heat shock protein 90 overrides the resistance of lung cancer cells by blocking radiation-induced stabilization of hypoxia-inducible factor-1alpha. Cancer research. 2009;69(4):1624–32. doi: 10.1158/0008-5472.CAN-08-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kim JH, Yu YS, Shin JY, Lee HY, Kim KW. Deguelin inhibits retinal neovascularization by down-regulation of HIF-1alpha in oxygen-induced retinopathy. Journal of cellular and molecular medicine. 2008;12(6A):2407–15. doi: 10.1111/j.1582-4934.2008.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci USA. 2009;106(7):2353–8. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 250.Kong D, Park EJ, Stephen AG, et al. Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity. Cancer Res. 2005;65(19):9047–55. doi: 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]


