Abstract
The term “matricellular proteins” describes a family of structurally unrelated extracellular macromolecules that, unlike structural matrix proteins, do not play a primary role in tissue architecture, but are induced following injury and modulate cell:cell and cell:matrix interactions. When released to the matrix, matricellular proteins associate with growth factors, cytokines and other bioactive effectors and bind to cell surface receptors transducing signaling cascades. Matricellular proteins are upregulated in the injured and remodeling heart and play an important role in regulation of inflammatory, reparative, fibrotic and angiogenic pathways. Thrombospondins (TSP)-1, -2 and -4, tenascin-C and –X, secreted protein acidic and rich in cysteine (SPARC), osteopontin, periostin and members of the CCN family (including CCN1 and CCN2/Connective Tissue Growth Factor) are involved in a variety of cardiac pathophysiologic conditions, including myocardial infarction, cardiac hypertrophy and fibrosis, aging-associated myocardial remodeling, myocarditis, diabetic cardiomyopathy and valvular disease. This review manuscript discusses the properties and characteristics of the matricellular proteins and presents our current knowledge on their role in cardiac adaptation and disease. Understanding the role of matricellular proteins in myocardial pathophysiology and identification of the functional domains responsible for their actions may lead to design of peptides with therapeutic potential for patients with heart disease.
I. INTRODUCTION
The extracellular matrix is a key component of multicellular organisms forming an intricate proteinaceous network that fills the extracellular spaces and provides structural support and tissue organization (342). In addition to their role in providing mechanical support, the extracellular matrix proteins and structures are important regulators and integrators of molecular signals, and critically modulate cellular responses (188). Collagen-based matrix is a characteristic of all multicellular organisms. Emergence of the vertebrates was associated with a marked expansion of the diversity of the extracellular matrices due to appearance of new members in existing gene families, increased number of spliced variants and the evolution of new glycoproteins such as fibronectin and the tenascins (187), (36). The increased complexity of extracellular matrix proteins in vertebrates not only resulted in formation of new structural components, such as bones and teeth, but also contributed to the emergence of complex and tightly regulated responses to tissue injury. Most matrix proteins in vertebrates are large molecules that include multiple functional domains, capable of binding cellular receptors. Cell:matrix interactions mediate adhesion, but also transduce signals that modulate cell survival, proliferation, differentiation, phenotype and function. Many matrix proteins bind growth factors regulating their availability, activation and presentation to cells. Matrix-bound growth factors may be released following tissue injury or may act as solid-phase ligands. Moreover, matrix fragments generated following injury may directly bind growth factor receptors and activate signaling cascades. In the complex environment of vertebrate tissues, the versatility of cell:matrix interactions permits generation of tightly regulated adaptive and reparative responses, linking modulation of the cellular phenotype with alterations in matrix proteins that serve as sensors of the extracellular milieu.
A. The fundamental properties of the matricellular proteins
Paul Bornstein coined the term “matricellular proteins” to describe a family of structurally unrelated extracellular macromolecules that interact with cell surface receptors, growth factors, proteases and other bioactive effectors, as well as with structural matrix proteins, without subserving a direct structural role (45), (46). Thus, matricellular proteins play a limited role in tissue architecture, but serve as links between cells and the matrix, acting as dynamic integrators of microenvironmental signals that modulate cellular behavior in response to external stimuli. Identification of this subclass of secreted proteins highlighted the dynamic reciprocal relation between cells and matrix, emphasizing that altered composition of the matrix network directly modulates cellular phenotype. The “founding members” of the matricellular family were thrombospondin (TSP)-1, SPARC (secreted protein acidic and rich in cysteine), and tenascin-C; however the rapid expansion in our understanding of cell:matrix interactions resulted in inclusion of several additional proteins, such as TSP-2 and -4, tenascin-X, osteopontin (OPN), periostin, and the members of the CCN family (Table 1). Matricellular proteins exhibit remarkable functional complexity in vivo, reflecting the contextual nature of their effects that depend on the various structural proteins, cytokines, and growth factors they associate with, and the cell types with which they interact in different tissues. Although matricellular proteins have distinct functional properties, several general characteristics have been identified (Table 1):
Table 1.
The Main Characteristics of the Matricellular Proteins
| Matricellular Proteins | General properties of the matricellular proteins |
|---|---|
|
| |
|
Proteins with established credentials as matricellular proteins: TSP-1, -2, -4 Tenascin-C, Tenascin-X SPARC Hevin Osteopontin Periostin CCN1, CCN2, CCN3, CCN4, CCN5 |
Absence of a direct role in tissue structure. Binding to extracellular matrix proteins, cell surface receptors, cytokines, growth factors and proteases modulates cell function and integrates signaling cascades. Expression is generally low in most adult tissues, but is upregulated following injury. |
|
Proteins exhibiting some matricellular functions Small leucine rich proteoglycans Syndecans Galectins Plasminogen Activator Inhibitor type I (PAI-1) Fibulin-5 Autotaxin |
Targeted disruption of most matricelllular genes results in relatively subtle abnormalities, suggesting a limited role in homeostasis. De-adhesive or counteradhesive properties. |
matricellular proteins bind to various structural extracellular matrix proteins and to cell surface receptors, while associating with cytokines, growth factors and proteases. These interactions allow them to serve as key integrators of signaling cascades.
in contrast to the adhesivity of most extracellular matrix proteins, matricellular proteins often promote cellular “de-adhesion” (304), promoting an intermediate adhesive state that activates survival signals, and inducing expression of genes associated with adaptation and repair.
expression of matricellular proteins is generally low in normal adult tissues, but is upregulated during development and in response to injury.
because most matricellular proteins are not involved in tissue homeostasis, mice with targeted disruption in matricellular genes have only subtle abnormalities in the absence of injury. In contrast, loss of matricellular proteins is associated with a wide range of alterations in injured and remodeling tissues.
B. Matricellular proteins in the heart
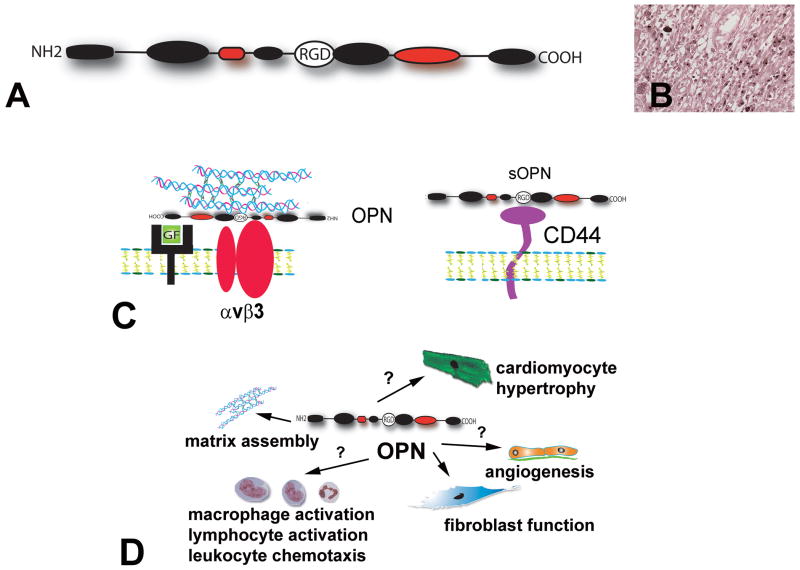
Most matricellular proteins are minimally expressed in normal young adult hearts, but are markedly upregulated following cardiac injury. A growing body of evidence suggests an important role for several members of the matricellular family in a variety of cardiac pathophysiologic conditions (392), (73), (120), (393), (167), (339); these actions are mediated through effects on cardiomyocytes and interstitial cells and through modulation of matrix organization and metabolism. The cardiac muscle is uniquely susceptible to injurious processes triggered by ischemia, inflammation, pressure or volume overload. Because the heart has negligible regenerative capacity, extensive cardiomyocyte loss following infarction results in formation of a collagen-based scar that provides structural support to the ventricle while altering its mechanical properties. Matricellular proteins induced in the infarcted heart appear to serve as transducers of key molecular signals in cardiac repair and act as modulators of cell migration, proliferation and adhesion. In the pressure-overloaded myocardium matricellular proteins deposited in the interstitium may modulate cytokine and growth factor signaling, affecting the susceptibility of cardiomyocytes to apoptosis and hypertrophic growth, regulating matrix assembly and metabolism and modulating the fibrogenic potential of inflammatory cells and fibroblasts. During cardiac senescence, upregulation of certain members of the matricellular family may play a role in preservation of the structural integrity of the heart, while other matricellular proteins may be involved in the pathogenesis of age-associated fibrosis. Because cardiac function and geometry are intricately dependent on the interactions between myocardial cells and the matrix, the effects of matricellular proteins in cardiac pathophysiology have profound consequences on systolic and diastolic performance of the ventricle. Considering the rapid growth in understanding the involvement of matricellular proteins in cardiac adaptation and disease, the current manuscript will try to fulfill several goals: First, to review the extensive and rapidly growing literature on the role of members of the matricellular family in cardiac pathophysiology. Second, to identify specific cellular events and molecular pathways modulated by the matricellular proteins in the infarcted and remodeling heart. Third, to provide a clinically relevant conceptual paradigm on the role of the non-structural components of the matrix network in myocardial disease.
II. CELL:MATRIX INTERACTIONS IN CARDIAC ADAPTATION AND DISEASE
A. Cell:matrix interactions in the normal heart
The mammalian heart is comprised of cardiomyocytes, non-cardiomyocytes and an extensive network of extracellular matrix (Figure 1). Although cardiac myocytes constitute the bulk of the volume of the adult cardiac muscle, non-cardiomyocytes are more numerous than myocytes in the heart. Based on morphological criteria only 30% of the cells in the adult rat heart were identified as cardiac myocytes, the remaining 70% were non-cardiomyocytes (313). Endothelial cells, fibroblasts and pericytes are abundant in the myocardium, smaller numbers of macrophages and mast cells are also noted in the perivascular and interstitial space (160). The cellular elements are enmeshed in a complex network of extracellular matrix (33) that is primarily composed of type I collagen with smaller amounts of type III, type V collagen, fibronectin, proteoglycans and basement membrane components (such as laminin and type IV collagen). In the normal heart the matrix not only serves as a scaffold for muscle fibers and vessels, but also plays an important role in transducing cell survival signals, in shielding fibroblasts from mechanical stress promoting a quiescent phenotype and in maintaining normal chamber geometry and ventricular function (37), (453). The homeostatic effects of the matrix on myocardial cells are mediated through interactions between matrix proteins and cellular receptors (such as dystroglycans and integrins); these actions are required for contractile synchrony and cardiomyocyte function.
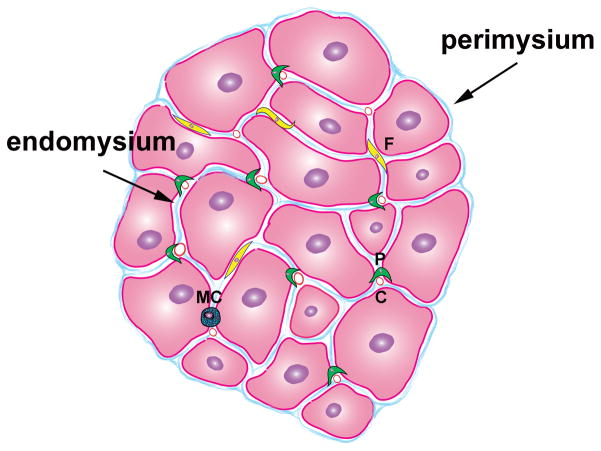
Figure 1. Morphology of the normal mammalian heart.
The adult heart contains cardiomyocytes, non-cardiomyocytes and a complex network of extracellular matrix. Each myocyte is surrounded by collagen (endomysium); individual fibers are also enmeshed in connective tissue (perimysium). Interactions between the matrix and cardiomyocytes are essential for their survival and function. Non-myocytes outnumber cardiomyocytes in the normal adult heart. The heart contains a rich vascular network comprised of capillary (c), venous and arteriolar endothelial cells, pericytes (P) and smooth muscle cells. A large number of resident fibroblasts is also noted (F). Normal mammalian hearts also contain small numbers of macrophages, mast cells (MC), lymphocytes and dendritic cells.
B. The concept of cardiac remodeling
In biology, “remodeling” describes alterations that result in rearrangement of existing structures (437). Although tissue remodeling is not necessarily linked with pathological conditions, the term “cardiac remodeling” is used almost exclusively to describe the consequences of disease states on the myocardium. Although initially coined to define the geometric and structural alterations of the myocardium following infarction (366), (348), cardiac remodeling is widely used to describe changes occurring in a wide variety of cardiac conditions. Thus, pressure (438) and volume overload, various inherited cardiomyopathic conditions, metabolic disease (2), toxic insults are capable of inducing remodeling of the ventricle. Although in all cases the extent of geometric remodeling is a determinant of adverse prognosis, the characteristics of the alterations observed in the ventricle are dependent on the initial cause of injury.
C. Cell:matrix interactions in post-infarction remodeling
The most dramatic changes in the composition of the cardiac extracellular matrix occur in the setting of acute myocardial infarction. The dynamic alterations in composition of the matrix in the infarcted heart are critical determinants of outcome. Excessive early degradation of the cardiac matrix network and defective or delayed formation of newly-synthesized matrix proteins may play an important role in the pathogenesis of cardiac rupture, a dramatic and fatal complication of acute myocardial infarction. In the later stages of healing, defects in extracellular matrix composition alter the mechanical properties of the heart resulting in enhanced ventricular dilation and increased sphericity of the ventricle. These geometric changes, termed post-infarction remodeling, are associated with increased mortality and a higher incidence of arrhythmias, and are intertwined with the development of chronic heart failure (341).
Infarct healing can be divided in three distinct, but overlapping phases: the inflammatory phase, the proliferative phase, and the maturation phase (141). Dying cells release subcellular constituents that activate the complement cascade while matrix fragments activate Toll-like receptor (TLR) signaling and tissue ischemia generates reactive oxygen species in the infarcted myocardium. These pathways activate Nuclear Factor (NF)-κB-dependent cytokine and chemokine upregulation (146), (61), (121) in resident myocardial cells triggering the inflammatory cascade (142), (60). Abundant inflammatory leukocytes infiltrate the infarcted area. Macrophages phagocytose dead cells and matrix debris, and produce growth factors, inducing fibroblast migration, proliferation, and activation. During the proliferative phase of healing, repression of pro-inflammatory signals is noted, as fibroblasts undergo phenotypic modulation and produce large amounts of extracellular matrix proteins (147), (483), (99). At the same time there is active angiogenesis and an extensive vascular network evolves. Maturation of the scar follows: inflammatory cells, fibroblasts and vascular cells undergo apoptosis and a collagen-based scar is formed. During all phases of infarct healing, the composition of the extracellular matrix plays a critical role in regulating cell behavior (120).
The extracellular matrix in the healing infarct undergoes dynamic changes that dramatically alter the microenvironment. During the inflammatory phase early activation of latent collagenases induces degradation of matrix proteins in the infarcted heart. Matrix metalloproteinase (MMP) activation is noted in the cardiac interstitium within 10 minutes after coronary occlusion (129). After the latent pool of collagenases has been depleted, new synthesis of MMPs promotes collagenolytic activity in the infarcted area (99). Collagenases cleave collagens at unique sites generating fragments.. Collagen fragments are further degraded into amino acids and oligopeptides by the gelatinases MMP-2 and MMP-9 (467) and by serine proteases (423), (98), (11). Fragmentation of extracellular matrix constituents during the early stages following infarction is not limited to fibrillar collagen; glycosaminoglycans, such as hyaluronan, may also undergo degradation leading to generation of low molecular weight fragments with pro-inflammatory properties (118), (185). As the original cardiac matrix network is degraded, a fibrin-based provisional matrix is formed (145), (118). Extravasation of plasma proteins through the hyperpermeable vasculature results in generation of a complex and dynamic matrix network based on fibrin and plasma fibronectin. In addition to its hemostatic role, the plasma-derived provisional matrix promotes leukocyte infiltration and supports migration and proliferation of mesenchymal cells facilitating the reparative response. Migrating cells use integrin receptors to interact with the extracellular matrix molecules, which also provide signals that modulate cellular phenotype and gene expression (103). Subsequently, the initial plasma-derived provisional matrix is lysed by proteolytic enzymes produced by granulation tissue cells and is quickly replaced by an organized cell-derived “second order” provisional matrix containing cellular fibronectin and hyaluronan (479). During this highly dynamic phase of cardiac repair, matricellular proteins are released into the infarct and activate signaling pathways essential for the reparative process. As the wound matures, matricellular proteins are degraded and the deposited collagen is cross-linked, leading to formation of a stable scar.
D. Cardiac hypertrophy and fibrosis
While cardiac growth occurs primarily through cardiomyocyte proliferation during embryonic development, after birth, cardiac myocytes are resistant to cell cycle reentry (412). Cardiac growth continues during the postnatal period and is mediated through hypertrophy of individual cardiomyocytes; a three-fold increase in the diameter of cardiac myocytes is noted in humans during development from infants to adults (184). Postnatal cardiac growth and the hypertrophy observed in athletes as a response to exercise are physiological responses, associated with normal cardiac structure and function. In contrast, the hypertrophic response caused by an increased mechanical load is maladaptive and is associated with increased morbidity and mortality due to heart failure. Although initially cardiac hypertrophy serves to sustain cardiac output and normalize the increased wall stress in the presence of an external load, a persistent hypertrophic response ultimately evolves into cardiac dysfunction as the hypertrophied ventricle dilates. Several excellent reviews have discussed the signaling pathways regulating the maladaptive alterations in cardiomyocytes in the hypertrophied heart (297), (152), (276).
Although often neglected in studies of cardiac hypertrophy, non-cardiomyocytes and the matrix network play an important role in the pathogenesis of cardiac dysfunction in the chronically overloaded ventricle (478). Fibroblasts respond to alterations in mechanical loading by enhancing their matrix-synthetic capacity and by transdifferentiating into myofibroblasts, activated cells that express contractile proteins (157). Thus, the development of fibrosis is a hallmark of cardiac hypertrophy and heart failure and a major determinant of cardiac function. In animal models fibrotic remodeling of the cardiac interstitium is accompanied by increased stiffness and diastolic dysfunction. In contrast, degradation of the collagen fibers in the endomysium and perimysium is associated with impaired systolic function and chamber dilation. Three distinct mechanisms are responsible for hypocontractility upon disruption of the myocardial collagen network. First, loss of critical matrix-cardiomyocyte interactions may result in decreased survival and impaired contractile function of cardiomyocytes. Second, the sliding displacement (“slippage”) observed after loss of the collagen scaffold results in a decrease in the number of cardiomyocyte layers in the ventricular wall leading to dilation of the chamber. Third, a degraded collagen network disrupts the coordinated contraction of cardiomyocytes (37). The balance between matrix-preserving and matrix-degrading pathways, regulated by MMPs and their inhibitors, plays an essential role in the structural characteristics of the matrix and profoundly affects cardiac function.
E. Aging-related cardiac remodeling
Aging is associated with an increase in the prevalence of left ventricular hypertrophy accompanied by a decline in diastolic function (108), (84). Both vascular and myocardial alterations are implicated in the pathogenesis of cardiac dysfunction in aging subjects. Age-associated remodeling of the vascular wall results in luminal dilation and vascular stiffening increasing vascular load and contributing to the development of cardiac hypertrophy. On the other hand, senescence also directly influences cardiac structure. Increased cardiomyocyte necrosis and apoptosis is noted in senescent rat hearts (14), (88) while surviving cardiomyocytes undergo hypertrophy. Beyond its effects on cardiomyocytes, aging also affects the phenotype and function of cardiac fibroblasts leading to expansion of the myocardial interstitial space. Deposition and cross-linking of extracellular matrix proteins in the cardiac interstitium play an important role in the pathogenesis of diastolic heart failure in aging hearts. TGF-β appears to be implicated in the pathogenesis of fibrotic cardiomyopathy in aging subjects. Loss of one TGF-β1 allele in TGF-β1 heterozygous mice reduces age-associated myocardial fibrosis and improves left ventricular compliance (58).
F. Obesity and diabetes as causes of cardiac remodeling
Metabolic diseases, such as obesity and diabetes are also associated with cardiac remodeling (2), (17). Cardiac hypertrophy and fibrosis are often observed in animal models of diabetes and obesity (2), (17), (449) and may be responsible for the development of diastolic dysfunction in obese diabetic patients. Because of the pathophysiologic heterogeneity of obesity and diabetes and the common co-existence with other conditions that may profoundly affect cardiac morphology and function (such as hypertension and ischemic heart disease), dissection of the contribution of metabolic disease in remodeling of the heart is challenging.
III. THE MATRICELLULAR PROTEINS. KEY MODULATORS OF CELL:CELL and CELL:MATRIX INTERACTIONS IN CARDIAC ADAPTATION AND DISEASE
A. The thrombospondins
1. Structure
In vertebrates the five known TSPs are divided in two subgroups according to their oligomerization status and molecular architecture (Figure 2). TSP-1 and -2 (group A) form trimers, whereas TSP-3, -4 and -5 (group B) are assembled as pentameric proteins (6), (68). The carboxyterminal regions of the TSP subunits are highly conserved: all TSPs contain a variable number of EGF-like repeats (type 2) that are contiguous with seven TSP type 3 repeats and a globular C-terminal region (CTD). The aminoterminal regions are more varied between individual TSPs. Group A TSPs contain a globular N-terminal domain (NTD), a coiled-coil oligomerization region, and a pro-collagen (or von Willebrand factor) homology domain (vWF-C). A distinctive characteristic of group A TSPs is the presence of three properdin-like repeats, the so-called type 1 domains (thrombospondin repeats, TSRs). Type 1 domains in TSP-1 and -2 have important functions in mediating inhibition of angiogenesis and in supporting cell attachment. In contrast, the pentameric thrombospondins (TSP -3, -4 and -5) lack the procollagen homology domain and the type 1 repeats, and contain four (instead of three) copies of the type 2 repeat. Extensive evidence is available on the expression patterns of TSP-1 and -2 in vitro and in vivo; in contrast, information on pentameric TSPs remains limited.
Figure 2. Structure of the TSPs.
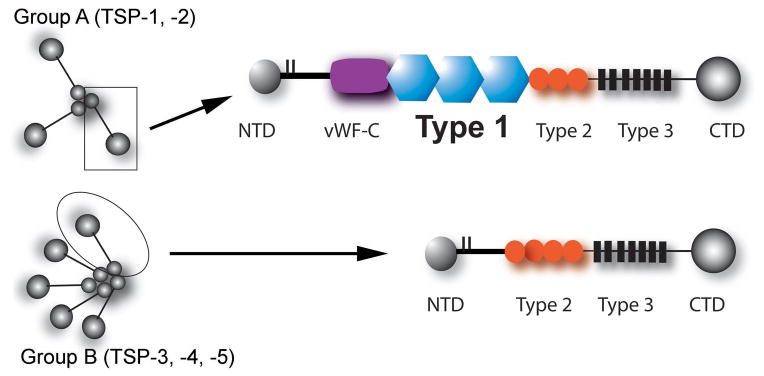
On the basis of their oligomerization status and architecture, TSPs are divided into trimeric (Group A) and pentameric (Group B) TSPs (see text). Abbreviations: NTD, N-terminal domain; vWF-C von Willebrand Factor homology domain; CTD, C-terminal domain.
2. Expression, synthesis and degradation of the TSPs
Each TSP exhibits a distinct pattern of expression in developing and adult tissues (5). In the developing mouse TSP-1 is the predominant from embryonic days 10–13 and is transiently expressed in the neural tube, head mesenchyme and in the cardiac cushions (195). Persistently high levels of TSP-1 expression are observed in megacaryocytes. In contrast, TSP-2 expression is primarily confined to the connective tissue of many organs and peaks after TSP-1 levels have decreased. Distribution of the pentameric TSPs in embryonic tissues seems to be more limited: TSP-3 expression is restricted to the brain, cartilage and lung (195), whereas TSP-4 is expressed in the nervous system (15), cornea and skeletal muscle (5) and TSP-5 is confined to the articular cartilage (5). As prototypical matricellular proteins, TSPs are expressed at low levels in most adult tissues and are not part of the normal extracellular matrix; however, marked upregulation of TSP expression has been observed in response to injury.
TSP-1 is a major constituent of platelet α-granules, but can also be synthesized by many other cell types including endothelial cells, vascular smooth muscle cells, fibroblasts, keratinocytes and macrophages (356). In vitro, TSP-1 expression is highest in proliferating cells and is upregulated by stimulation with growth factors, such as TGF-β1 (315), Platelet Derived Growth Factor (PDGF) and Fibroblast Growth Factor (FGF)-2 (186) and by angiotensin II (314). In contrast, the pro-inflammatory cytokines Tumor Necrosis Factor (TNF)-α and Interleukin (IL)-1β suppress TSP-1 synthesis (298). In vivo, intense upregulation of TSP-1 message and protein is observed following tissue injury (241). In human cutaneous wounds, TSP-1 expression is markedly increased at the wound margins (357). Degranulation of α-granules from platelets and new expression by macrophages and vascular cells are the main sources of TSP-1 in wound healing (241), (357), (116). TSP-2 and TSP-4 are also upregulated in healing and remodeling tissues (241), (311).
TSP upregulation following injury is transient. After an early peak, TSP synthesis in healing tissues is suppressed and the protein may be degraded. TSP-1 is cleaved by cathepsins, leukocyte elastases, plasmin and by ADAMTS1 (a disintegrin and metalloproteinase with thrombospondin motifs-1) (194). While cleavage by elastase and plasmin results in TSP-1 degradation, other enzymes release specific fragments with distinct properties (194). Information on the in vivo role of these interactions in modulating and diversifying the effects of TSP-1 is lacking.
3. Molecular interactions of the TSPs
As typical matricellular proteins, TSPs bind to structural components of the matrix network (including matrix proteins and proteoglycans) (446), interact with cytokines, growth factors and proteases in the microenvironment, and modulate cellular phenotype through activation of specific receptors. The ability of TSPs to interact with collagen and the binding sequences are conserved in most members of the family, involving interactions with the TSP C-terminal domain. (370), (320), (36). TSP-1 is known to bind type V collagen (303) and fibrinogen (258), accelerating formation of fibrin fibrils (27). In addition, TSP-1 may be incorporated into the matrix through interactions with fibronectin (258). TSPs are known to bind a large number of calcium ions; these interactions induce conformational changes in the type 3 repeats of the TSP molecule modulating its sensitivity to proteolysis and its cell attachment activity. TSPs (in particular TSP-1) also interact with a variety of cytokines, growth factors and proteases modulating activity of their binding partner. Thus, TSP-1 binding reduces the catalytic activity of thrombin, cathepsin G and plasmin. Moreover, both TSP-1 and-2 bind to MMP-2, inhibiting its activity (34). The interaction between TSP-1 and TGF-β1 is particularly important in vivo and plays a crucial role in TGF-β activation (discussed in more detail in the next section).
Secreted TSPs alter cellular phenotype through binding to many different ligands, including adhesion proteins and surface receptors. Specific interactions between various functional domains of the TSP-1 molecule and specific receptors have been characterized (80); however, the pathways involved in cellular signaling through other TSPs are less well understood. Many biological actions of TSP-1 are mediated through CD36, a major scavenger receptor that binds and internalizes oxidized LDL and fatty acids, but also acts as an adhesive molecule. Extensive evidence suggests that CD36 on the surface of platelets, monocytes and endothelial cells serves as a TSP-1 receptor (80). The interaction with CD36 has been implicated as a key molecular pathway mediating plasmin-induced activation of TGF-β1 in rat alveolar macrophages (499), as an important mechanism in macrophage uptake of apoptotic cells (387), (388) and as a crucial mediator in the angiostatic effects of TSP-1. TSP-2 also mediates its angiostatic effects through CD36 (415).
TSPs also signal through binding to integrins. Interactions with β3 (αvβ3 and αIIbβ3), and β1 integrins (α3β1, α4β1, α5β1) appear to mediate several effects of TSP-1. β3 integrins are implicated in TSP-1-mediated accentuation of growth factor responses in smooth muscle cells (510) and in binding of TSP-1 to the platelet surface (251). β1 integrins mediate the antimigratory effects of type 1 repeats in endothelial cells (413) and may be implicated in the angiostatic actions of TSP-1. Both β3 and β1 interactions may be involved in TSP-1-mediated effects on the inflammatory response. Although evidence on interactions of other TSPs with integrins is scarce, TSP-5/integrin-mediated actions have been implicated in supporting chondrocyte attachment (79). In addition to these pathways, TSP-1 also signals through binding to CD47/Integrin-associated protein (IAP). Extensive evidence suggests that TSP-1/CD47 interactions affect integrin activity in a variety of cell types, modulating their adhesive potential (80).
4. Cellular effects of the TSPs
Most of the evidence on the cellular actions of the TSPs refers to TSP-1, the first member of the TSP family to be identified. Studies on the cell biological effects of TSP-1 have revealed a wide range of cell-specific actions; the focus of this discussion will be limited to effects with an established role in mediating in vivo functions of the molecule.
i. Effects of TSPs on cell adhesion and motility
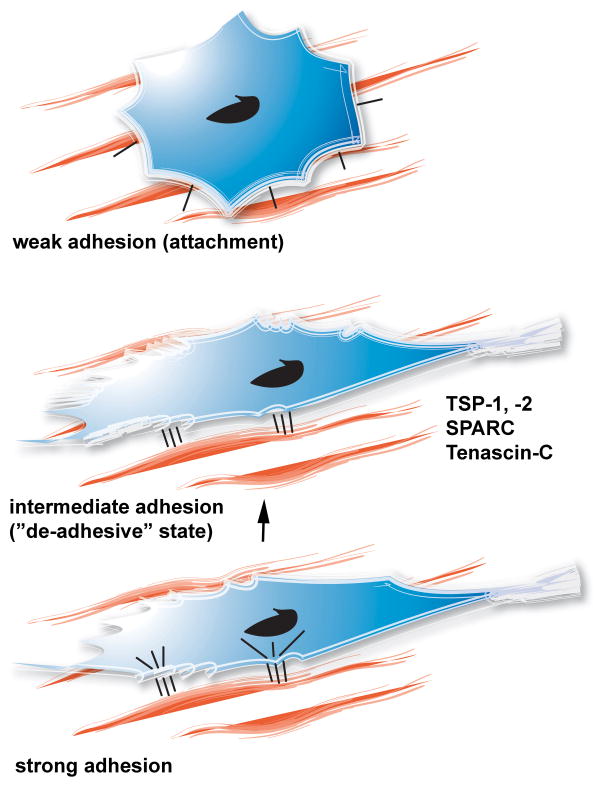
The process of cell adhesion to the matrix consists of three phases: attachment (weak adhesion), spreading (intermediate adhesion) and formation of focal adhesions and stress fibers (strong adhesion) (Figure 3) (304). During attachment, cellular integrins interact with their corresponding matrix ligands. The initial cell:matrix interactions increase the cell surface contact area with the extracellular matrix and result in cell spreading and formation of actin microfilaments (intermediate adhesion). In the presence of appropriate matrix-derived signals, the state of “intermediate adhesion” is followed by organization of the cellular cytoskeleton and formation of focal adhesions and stress fibers (strong adhesion). The reverse sequence of cellular events, where cells transition from a state of strong adhesion to weaker adhesive interactions, may be highly relevant in cytokinesis and in tissue remodeling. TSP-1 and other matricellular proteins (including SPARC and tenascin-C) stimulate the loss of focal adhesions and stress fibers in endothelial cells, fibroblasts and smooth muscle cells, inducing the intermediate adhesive state, a process termed “de-adhesion” (305), (304). De-adhesion occurs primarily through rapid disassembly of focal adhesions. The de-adhesive properties of TSP-1 may be important in cell motility.
Figure 3. The concept of “de-adhesion” in tissue remodeling.
In remodeling tissues, induction of the prototypical matricellular proteins (TSP-1, tenascin-C, SPARC) may stimulate disassembly of focal adhesions and stress fibers in strongly adherent cells, inducing a state of intermediate cell adhesion. This process, called “de-adhesion” and may be important in promoting cell motility while preventing cell anoikis.
ii. Proliferation and apoptosis
TSP-1 modulates cell proliferation and apoptosis in a cell type-specific manner. TSP-1 enhances growth factor-mediated proliferation in vascular smooth muscle cells (277) and induces clonal expansion of T cells (459). The pro-apoptotic effects of TSP-1 on endothelial cells are particularly important in mediating its angiostatic actions (209). TSP-1-induced endothelial apoptosis is dependent on CD36 and involves MAPK and caspase-3-dependent pathways. The pro-apoptotic actions of TSP-1 may be specific to endothelial cells; TSP-1/CD36 activation does not induce apoptosis in macrophages (484).
5. In vivo functions of the TSPs
The diverse and sometimes contradictory in vitro effects of the TSPs reported in the literature can be explained by their multiple functional domains, by the extensive repertoire of their cellular and molecular effects and by the contextual nature of their functions. The biological significance of some of these effects in vivo remains unclear. The generation and characterization of TSP null mice provided extensive information on the functional role of these intriguing molecules in tissue homeostasis and in various disease states. The relatively subtle abnormalities observed in mouse gene knockout studies clearly demonstrated that none of the TSPs is essential for survival (252), (243), (352), (153). However, the defective and altered responses exhibited by various TSP knockouts after injury revealed significant pathophysiological functions of the TSPs in many organ systems.
i. TSP-1 activates TGF-β
Activation of TGF-β is a crucial event in inflammatory, reparative and fibrotic processes (62), (119), (38). TGF-β is secreted as a complex, containing the C-terminal mature TGF-β and LAP. (226). Upon secretion it is covalently bound to one of the four Latent TGF-β binding proteins (LTBPs) forming the large latent complex. LTBP is covalently associated with the extracellular matrix and contributes to localization of the complex in specific areas. After proteolytic cleavage of TGF-β from its propeptide, the LAP propeptide dimer remains associated with the TGF-β dimer by noncovalent interactions forming the small latent complex. The LAP:TGF-β interaction inhibits TGF-β bioactivity. Thus, in order for bioactive TGF-β to be released, several sequential events need to occur (13):
-
Step 1)
The large latent complex needs to be assembled and localized in the extracellular matrix. This requires formation of a covalent link between LTBP and the matrix that is mediated by tissue Transglutaminase (tTG).
-
Step 2)
TGF-β needs to be proteolytically cleaved and separated from LAP. This step involves processing of the proTGF-β complex by a plasma membrane bound furin, or another extracellular protease, such as plasmin (273). Once processing has occurred the complex is competent and can be activated.
-
Step 3)
Active TGF-β needs to be released from the activation competent LAP:TGF-β complex.
TSP-1 appears to play a key role in TGF-β activation through a cell and protease-independent mechanism (Figure 4A) (309), (402); in contrast the other TSPs do not activate TGF-β. The mechanism of activation involves direct binding of TSP-1 to the sequence LSKL in the LAP; this interaction alters the conformation of TGF-β making it accessible to its receptor (308). The second TSR and the RFK sequence of the TSP-1 molecule have been reported to be essential (504) for TGF-β activation.
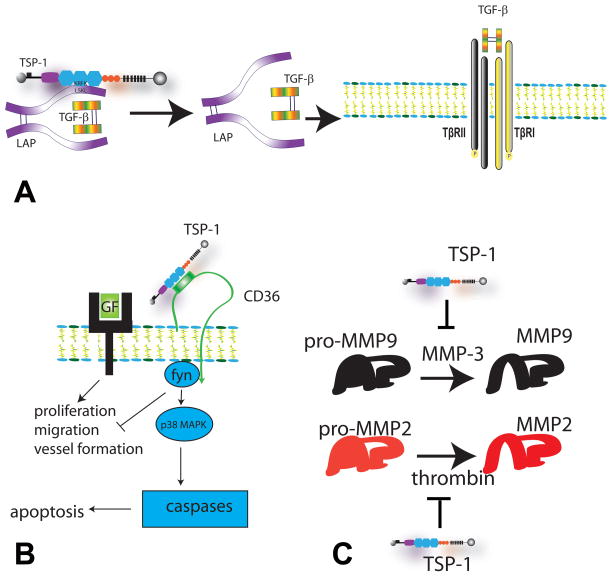
Figure 4. Main in vivo actions of TSP-1.
A. TSP-1 is an important activator of TGF-β. After proteolytic cleavage of TGF-β from its propeptide, the TGF-β dimer remains bound to the Latency-Associated Peptide (LAP) by non-covalent interactions forming the small latent complex. TSP-1 binds to the sequence LSKL in the LAP and alters the conformation of TGF-β making it accessible to its receptors, TβRII and TβRI. Other TSPs do not exert TGF-β-activating effects. B. TSP-1 is a potent angiostatic agent through actions involving CD36. TSP-1 inhibits angiogenesis by modulating angiogenic growth factor (GF) signaling and by inducing endothelial cell apoptosis through a CD36/fyn/p38 mediated cascade. C. TSP-1 inhibits protease activity. TSP-1 inhibits MMP-3-dependent MMP-9 activation and attenuates thrombin-induced MMP-2 activation.
The phenotype of TSP-1 null mice demonstrated the in vivo significance of TSP-1 actions in TGF-β activation. TSP-1 −/− mice exhibit inflammatory changes in the lung and pancreas showing some of the histological features of the TGF-β1 null animals, albeit in much milder form (252). TSP-1 −/− pups treated with KRFK, a TSP-1-derived peptide that activates TGF-β1, demonstrated a partial reversion of the lung and pancreatic abnormalities toward wild type (104). These findings indicated that one of the main functions of TSP-1 is activation of TGF-β in the pulmonary epithelium. In the absence of TSP-1, impaired TGF-β activation results in an accentuated immune response leading to infiltration of the lung parenchyma with inflammatory leukocytes. The significance of TSP-1-mediated TGF-β activation has been further supported by findings in a wide range of biological processes including tissue repair, fibrosis and neoplasia (446).
ii. The angiostatic actions of the TSPs
Both TSP-1 and TSP-2 are potent inhibitors of angiogenesis (Figure 4B) (168), (468). The generation of knockout mice demonstrated that neither TSP-1 nor TSP-2 has a major impact on vascular development. Modest increases in capillary density have been reported in some tissues of TSP-1 null animals (278), whereas TSP-2 absence is associated with a twofold increase in vascular density in the dermis and adipose tissue (243). Despite the relatively subtle effects of the TSPs on embryonic vascular development; extensive evidence implicates both group A TSPs in regulation of angiogenesis in healing wounds, in ischemic tissues and in tumors. Excisional cutaneous wounds in TSP-2 null mice exhibit accelerated healing associated with formation of hypervascular granulation tissue (242). Moreover, platelet-derived TSPs appear to mediate angiostatic actions in the ischemic hindlimb (228). Somewhat surprisingly, TSP-1 null animals had delayed healing of excisional wounds in the absence of altered vascular content; the findings may reflect the more complex biology of TSP-1 that also serves as a TGF-β activator (7). TSP-1, or TSP-2, overexpression suppresses vascular density in tumors (432), (433) and in healing tissues (434). CD36 is critically involved in TSP-1 and TSP-2-mediated angioinhibition. Antibodies to CD36 block the inhibitory effects of TSP-1 on endothelial cell migration (109) and both TSP-1 and TSP-2 inhibit bFGF-mediated angiogenesis in wildtype, but not in CD36 null mice (209), (415).
iii. Modulation of NO signaling
Isenberg and co-workers identified TSP-1 as a potent inhibitor of angiogenic endothelial responses to NO (200). Attenuation of NO/cGMP signaling by TSP-1 is not limited to endothelial cells; TSP-1 potently inhibits NO responses in vascular smooth muscle cells (202) and platelets (201). CD47, but not CD36, is required for the inhibitory effects of TSP-1 on the NO/cGMP pathway (199). Although TSP-2 and TSP-4 appear to exert weak inhibitory actions on NO-stimulated cGMP synthesis in vascular cells (196), potent antagonism of NO signaling is restricted to TSP-1. Because NO plays an important role in a wide range of pathophysiologic responses including, cardiovascular disease and cancer, the effects of TSP-1 on NO signaling may have significant clinical relevance (197).
iv. Regulation of protease activity
TSPs may stabilize the extracellular matrix in remodeling tissues through protease inhibition. TSP-1 is capable of inhibiting a broad spectrum of proteases including plasmin, urokinase plasminogen activator (uPA), neutrophil elastases and MMPs (367), (180). TSP-1 and TSP-2 bind to the gelatinases MMP-2 and MMP-9. TSP-1 inhibits MMP-3-dependent activation of pro-MMP-9 and thrombin-induced activation of pro-MMP2 through interactions involving the type 1 repeats (Figure 4C) (34). TSP-1-mediated regulation of MMP activity may contribute to its angiostatic and tumor inhibitory properties (367), (446) Beyond its direct effects on MMP activity, TSP-1 may modulate protease activity in vivo through its TGF-β-activating properties. TSP-2 absence is associated with enhanced MMP-2 expression in fibroblasts (497) and gelatinase activity is increased in TSP-2 null cells and tissues (233). In injury models TSP-2 deficiency is associated with enhanced MMP-2 and/or MMP-9 expression (233).
v. Effects of the TSPs on the inflammatory response
Group A TSPs play a role in controlling inflammation through several distinct pathways. First, TSP-1/CD47 pathways have been implicated in generation of regulatory T cells (169). In vivo, TSP-1, TSP-2 and CD47 null mice exhibit sustained oxazolone-induced inflammation (246) associated with enhanced T cell activation. Second, TSP-1/CD36 interactions mediate recognition and phagocytosis of neutrophils undergoing apoptosis (388). Third, TSP-1 (but not TSP-2) activates TGF-β, a molecular signal involved in suppression of pro-inflammatory pathways. Recent evidence implicates pentameric TSPs in regulation of inflammatory pathways. TSP-4 null animals exhibited accentuated vascular inflammation and increased atherosclerosis in an ApoE −/− background. The anti-inflammatory effects of TSP-4 appear to be mediated through β2- and β3- integrin-dependent deactivating effects on endothelial cells (153).
vi. The role of TSPs in maintenance of matrix integrity
Although TSPs do not have a primary structural role, they may participate in maintenance of the architectural integrity of tissues through both direct and indirect actions. As a potent activator of TGF-β, TSP-1 contributes to matrix deposition and preservation; this function may be important in reparative and fibrotic processes. Although TSP-2 lacks TGF-β-activating properties, it appears to play a direct role in matrix homeostasis. Although mice lacking TSP-2 appear overtly normal and are fertile, they exhibit significant defects in matrix assembly. Collagen fibers in the skin of TSP-2 null animals were disordered and the presence of unusually large fibrils with an irregular contour was noted in the tendons. These abnormalities resulted in increased fragility and reduced tensile strength of the skin (243).
6. The role of TSPs in cardiac adaptation and disease (Table 2)
Table 2.
Role of the Thrombospondins in cardiac homeostasis and disease
| TSP-1 | TSP-2 | TSP-3, TSP-4, TSP-5 | |
|---|---|---|---|
| Cardiac homeostasis | Very low expression in normal hearts and no major role in cardiac homeostasis. TSP-1 −/− mouse hearts exhibit preserved systolic function and normal wall thickness (278). An association between TSP-1 deficiency and modest increases in vascular density (by 10–15%) and chamber dimensions (by 8%) has been reported (278). TSP-1 has a limited role in blood pressure regulation: TSP-1 loss results in a modest increase in diastolic and mean blood pressure during activity (198). | Very low expression in normal adult hearts (436). No known role in cardiac homeostasis in young animals. | TSP-4 is highly expressed in normal hearts (250); however, its role in cardiac homeostasis is unknown. |
| Cardiac aging | No known role in cardiac aging. | TSP-2 plays an essential protective role in the aging myocardium (436). Aging TSP-2 −/− mice have markedly increased mortality, associated with severe dilated cardiomyopathy, impaired systolic function and fibrosis. TSP-2-induced protection in the aging myocardium is due to activation of pro-survival Akt signalling in cardiomyocytes and to inhibition of MMPs. | No known role in cardiac aging. |
| Myocardial infarction | TSP-1 is markedly upregulated in the infarct border zone and may serve as a “barrier” protecting the non-infarcted myocardium from extension of inflammation and matrix degradation, thus preventing adverse remodelling (148). TSP-1 null mice exhibit enhanced adverse remodelling following infarction associated with extension of the inflammatory reaction into the non-infarcted myocardium and with impaired TGF-beta activation. Protective effects of TSP-1 may also be mediated through inhibition of MMP activity. | A protective role of TSP-2 in the infarcted heart has been suggested. TSP-2 loss was associated with a higher incidence of cardiac rupture suggesting a role in maintaining the structural integrity of the remodelling matrix network. | No known role in myocardial infarction. |
| Cardiac hypertrophy and fibrosis | TSP-1 protects the pressure-overloaded myocardium from dilative remodelling favouring matrix preservation (490). TSP-1 null mice exhibited attenuated dilation in a model of transverse aortic constriction. The protective effects of TSP-1 appear to be due to better matrix preservation mediated through inhibitory effects on MMP activity and through TGF-β activation. In diabetic animals with pressure overload a peptide antagonist of TSP-1-mediated TGF-β activation prevented the progression of cardiac fibrosis (35). | TSP-2 protects the pressure overloaded myocardium my maintaining matrix integrity. Following angiotensin infusion TSP-2 null mice exhibit increased mortality due to cardiac rupture, reflecting defective collagen fibril assembly (401). | Expression of pentameric TSPs is upregulated in remodeling hearts, particularly during the transition to heart failure. Myocardial TSP-3 mRNA expression is upregulated in hypertensive renin-overexpressing rats showing evidence of decompensation (401). TSP-4 expression is increased in pressure-overloaded hearts (373) and in the myocardium of animals undergoing angiotensin or arginine-vasopressin infusion (311).. |
| Toxic cardiomyopathies | No known role. | TSP-2 protects the myocardium from doxorubicin-induced cardiomyopathy by promoting cardiomyocyte survival and by inhibiting matrix degradation (460). | |
| TSPs in human heart disease | TSP-1 expression is increased in chronically ischemic myocardium from patients undergoing bypass surgery (158). TSP-1 in blood cells predicted functional deterioration in patients with acute myocardial infarction (113). The Ser-700 TSP-1 variant is associated with enhanced platelet aggregation and premature coronary disease (454). | TSP-2 expression is increased in hypertrophied (401) and chronically ischemic human myocardium (158). A TSP-2 variant has been associated with protection from myocardial infarction (454). | TSP-4 expression is increased in hypertrophied, failing and chronically ischemic human myocardium (445), (158). A TSP-4 missense variant (A387P) is strongly associated with myocardial infarction (454). |
i. TSPs and cardiac homeostasis
Group A TSPs are expressed at low levels in the myocardium, Moreover, studies using TSP knockout mice suggest a very limited role for the TSPs in cardiac homeostasis. In the absence of injury, TSP-1 −/− animals have normal cardiac morphology (252). A recent study demonstrated a modest increase (12%) in capillary density in the TSP-1 −/− myocardium when compared to the wildtype heart, associated with an 8% increase in left ventricular dimensions (278). Wall thickness and systolic function were comparable between WT and TSP-1 null animals.
Telemetric monitoring studies have demonstrated subtle effects of TSP-1 deficiency on blood pressure regulation (198). During the inactive light period of the day blood pressure was comparable between WT and TSP-1 null animals. However, during the active dark cycles, TSP-1 −/− mice exhibited modest, but statistically significant, increases in diastolic and mean arterial pressure. Any alterations in homeostatic blood pressure regulation in TSP-1 null animals have no significant long-term consequences on left ventricular mass; our observations showed comparable left ventricular hypertrophy in senescent TSP-1 −/− and wildtype mice (C Gonzalez-Quesada and NG Frangogiannis, unpublished data). No significant alterations in cardiac function and morphology have been reported in young TSP-2 null mice (436). Limited information is available on the expression and role of the pentameric TSPs in the normal heart. Lawler and colleagues demonstrated that TSP-4 is highly expressed in the adult human heart (250); however its role in cardiac homeostasis remains unknown.
ii. TSPs and the aging heart
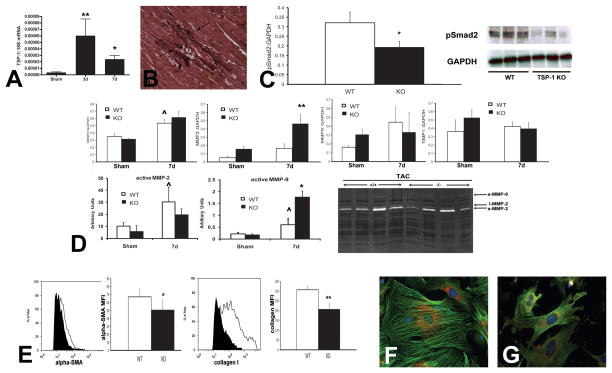
Recent evidence suggested a crucial role for TSP-2 in protecting the aging heart from cardiac dilation and dysfunction (436). Aging TSP-2 null mice had markedly reduced survival rates when compared to wildtype animals. More than 55% of the TSP-2 null mice died between 24 and 60 weeks of age (in comparison, only 10% of WT mice died at the same time interval). TSP-2 absence was associated with severe dilated cardiomyopathy, markedly impaired systolic function and fibrosis. Adverse cardiac remodeling and dysfunction in aging TSP-2 −/− hearts were associated with progressive cardiomyocyte death and increased MMP activation. In contrast, effects of TSP-2 loss on vascular density were not observed. Thus, TSP-2–mediated protection of the aging heart appears to be related to activation of pro-survival Akt-dependent signals in cardiomyocytes and to inhibition of MMP activity. In contrast to the impressive effects of TSP-2 absence on the aging heart, direct evidence implicating TSP-1 in cardiac aging is lacking. In heart failure-prone mice (C57BL6 x 129Sv), age-associated heart failure was linked with increased TSP-1 levels (461). However, TSP-1 null mice in a C57BL/6J background had normal cardiac systolic function and preserved chamber dimensions after at least 100 weeks of follow-up (C Gonzalez-Quesada and NG Frangogiannis, unpublished observations).
iii. TSPs in injury, repair and remodeling following myocardial infarction
TSP-1 mRNA and protein were markedly induced in canine and rodent models of myocardial infarction (148), (406). After 7 days of reperfusion, dead cardiomyocytes in the canine infarct were replaced with granulation tissue and an organized temporary matrix was formed. Although this matrix network was prominent in both the center and the border of the healing infarct, TSP-1 protein showed a strikingly selective pattern of deposition in the infarct border zone after 7–28 days of reperfusion, clearly demarcating the infarcted area from the non-infarcted myocardium (Figure 5A).. The molecular signals responsible for TSP-1 upregulation in the infarcted myocardium have not been identified; however its selective presence in the border zone may reflect the spatial localization of TGF-β/Smad2/3 signaling that is activated predominantly in the margins of the infarct (63).
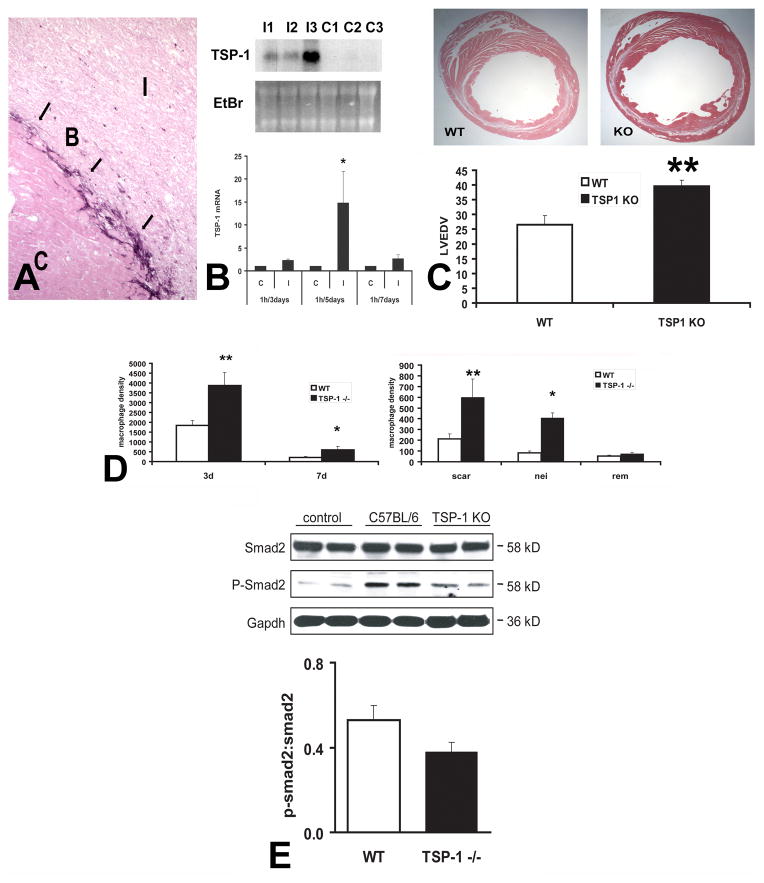
Figure 5. The role of TSP-1 in myocardial infarction.
In the infarcted heart selective upregulation of TSP-1 in the infarct border zone may prevent expansion of the inflammatory infiltrate into the non-infarcted area A. Immunohistochemical staining of the infarcted canine heart demonstrates selective incorporation of TSP-1 (arrows) into the matrix of the infarct border zone (B). C, control non-infarcted myocardium; I, infarct B. Northern blotting shows marked TSP-1 upregulation in the infarcted canine myocardium. C. TSP-1 −/− mice exhibited accentuated dilative remodeling following myocardial infarction. D. Adverse remodeling in TSP-1 −/− mice was associated with expansion of the inflammatory infiltrate into the non-infarcted myocardium indicating failure of the protective “barrier” mechanism preventing expansion of the inflammatory infiltrate into the non-infarcted area. E. TSP-1 absence was associated with decreased Smad2 phosphorylation in the infarcted heart, suggesting impaired TGF-β signaling. TSP-1 deposition in the infarct border zone may protect the infarcted myocardium by inhibiting MMP activity, by exerting direct anti-inflammatory actions, by locally activating TGF-β (thus reducing macrophage inflammatory activity) or through inhibition of uncontrolled angiogenesis. The TSP-1 “barrier” may be responsible for containment of the inflammatory and angiogenic response within the infarct, thus preventing expansion of granulation tissue formation in the viable myocardium (Data reproduced with permission from Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. Critical role of endogenous thrombospondin-1 in preventing expansion of healing myocardial infarcts Circulation 2005;111:2935–42. Copyright 2005, American Heart Association).
In order to explore the functional role of TSP-1 in infarct healing we compared the reparative response between TSP-1 null and WT mice (148). TSP-1 null mice had worse adverse remodeling following myocardial infarction than WT mice (Figure 5). Accentuated dilative remodeling in TSP-1 null animals was associated with prolongation and expansion of the post-infarction inflammatory reaction and extension of granulation tissue formation into the non-infarcted heart. In order to examine whether defective regulation of the post-infarction inflammatory response was due to impaired TGF-β activation in the absence of TSP-1, we assessed activation of the canonical TGF-β/Smad2/3 pathway. After 24h of reperfusion there was a trend for reduced Smad2 phosphorylation in TSP-1 null infarcts. In contrast, histological assessment of the density of vascular profiles demonstrated that infarct angiogenesis was not affected by the absence of TSP-1.
What are the mechanisms responsible for the protective effects of TSP-1 in the remodeling infarcted heart? We suggest that the strikingly localized expression of TSP-1 in the infarct border zone may locally activate anti-inflammatory, angiostatic and matrix-preserving signals preventing expansion of leukocyte infiltration and granulation tissue formation into the non-infarcted area. Thus, selective induction of TSP-1 in the infarct border zone may result in formation of a “barrier” preventing expansion of the inflammatory infiltrate in the non-infarcted area. Several TSP-1-mediated actions may contribute to this functional barrier. First, local activation of TGF-β may suppress inflammation while promoting matrix-preserving pathways. Second, TSP-1-induced angiostatic effects may inhibit formation of inflammatory neovessels preventing expansion of granulation tissue. Third, TSP-1-mediated MMP inhibition may prevent excessive degradation of the matrix in the infarct border zone. Fourth, direct TSP-1-induced anti-inflammatory actions mediated through CD47 may contribute to containment of the post-infarction inflammatory response.
The role of TSP-2 in cardiac remodeling has been studied primarily in models of pressure overload hypertrophy (discussed in more detail below). However, evidence suggests important effects of TSP-2 in the infarcted heart. Myocardial infarction in TSP-2 −/− mice resulted in a high incidence of cardiac rupture (392), suggesting a crucial role for TSP-2 in formation and structural integrity of the remodeling matrix. The mechanisms responsible for these effects remain poorly understood. The role of the pentameric TSPs in infarct healing has not been investigated.
iv. TSPs in cardiac hypertrophy and fibrosis
Both animal model experiments and clinical studies have suggested that cardiac hypertrophy and fibrosis are associated with TSP upregulation. Schroen and co-workers first demonstrated upregulation of both group A and group B TSPs in homozygous renin-overexpressing (Ren-2) rats, a model of cardiac hypertrophy due to severe hypertension. Ren-2 rats have cardiac hypertrophy at 10 weeks of age; almost half of the animals decompensate a few weeks later developing overt heart failure. Myocardial biopsies obtained at 10 weeks of age were used to identify differentially expressed genes in animals that later decompensated. Myocardial TSP-1, -2 and -3 transcripts were significantly higher in animals with decompensation (401). Moreover, increased TSP-1 expression was observed in a mouse model of pressure overload due to transverse aortic constriction (470). TSP-4 expression is also markedly increased in the pressure overloaded myocardium. Transition from left ventricular hypertrophy to hypertensive heart failure in spontaneously hypertensive rats was associated with TSP-4 upregulation (373). Angiotensin II and arginine-vasopressine (AVP) infusion induced rapid upregulation of TSP-4 transcripts in the myocardium; protein expression was primarily localized in vascular endothelial cells (311).
The role of TSPs in the hypertrophied and fibrotic heart is an area of active investigation. In a pathophysiologically complex model of cardiac remodeling induced by abdominal aortic constriction in rats with type I diabetes, a peptide antagonist of TSP-1-mediated TGF-β activation prevented the progression of cardiac fibrosis (35). These findings highlight the important role of the TGF-β-activating actions of TSP-1 in modulating matrix remodeling in the myocardium. Whether TGF-β activation is the predominant function of TSP-1 in cardiac hypertrophy and fibrosis remains unknown. Recent experiments suggested that genetic TSP-1 disruption is associated with adverse remodeling in a mouse model of pressure overload hypertrophy due to transverse aortic constriction (490). The detrimental effects of TSP-1 deficiency are associated with enhanced cardiomyocyte injury, increased MMP activation, and replacement of dead cells with defective fibroblasts, expressing less collagen and exhibiting impaired myofibroblast transdifferentiation. Thus, in the absence of TSP-1, impaired matrix preservation due to defective TGF-β activation and loss of TSP-1-mediated protease inhibition may result in accentuated dilative remodeling (Figure 6).
Figure 6. The role of TSP-1 in cardiac fibrosis due to pressure overload.
TSP-1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. A. qPCR shows marked TSP-1 upregulation in the pressure-overloaded myocardium in a mouse model of transverse aortic constriction. B. TSP-1 in the pressure-overloaded myocardium is localized in the cardiac interstitium. C. TSP-1 null mice exhibit worse dilative remodeling of the pressure-overloaded myocardium. Increased chamber dilation is associated with impaired TGF-β signaling (evidenced by reduced Smad2 phosphorylation). D. TSP-1 null animals exhibit increased MMP-9 activity in the pressure overloaded heart associated with accentuated MMP-3 levels. EG. Cardiac fibroblasts isolated from TSP-1 null pressure overloaded hearts are functionally impaired exhibiting reduced collagen expression and defective myofibroblast transdifferentiation. TSP-1 protects the pressure overloaded heart from chamber dilation by promoting TGF-β-induced myofibroblast transdifferentiation and activation and by inhibiting MMP activity. (Data reproduced with permission from Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li, N, Lee DW, Frangogiannis NG. Endogenous thrombospondin-1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension 2011;58: 902–911: Copyright 2011, American Heart Association)
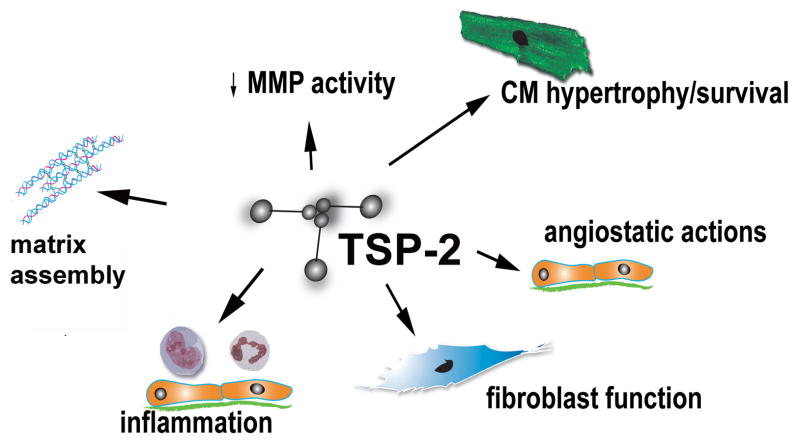
The role of TSP-2 in the pressure-overloaded myocardium was investigated in a model of angiotensin II infusion (401). Seventy percent of TSP-2 null mice died within 4 days following angiotensin infusion due to cardiac rupture; surviving TSP-2 null mice developed heart failure. In contrast, all wildtype mice were followed-up for 4 weeks and survived the infusion. Pronounced mitochondrial swelling, interstitial edema and cardiomyocyte damage was observed in TSP-2 null mice following angiotensin II treatment; these alterations were associated with increased MMP-2 and MMP-9 activity. Thus, the protective effects of TSP-2 on the remodeling heart appear to be due to preservation of matrix integrity, either through direct effects on collagen fibril assembly, or to inhibition of MMP activity (Figure 7). Direct pro-survival effects of TSPs on cardiomyocytes, possibly mediated through integrins, may also contribute to their protective actions.
Figure 7. Actions of TSP-2 in the remodeling myocardium.
Experimental evidence using loss-of-function approaches suggests an important role for TSP-2 in protection of the aging, infarcted and pressure-overloaded heart. TSP-2 null mice develop dilative cardiomyopathy; this may be due to loss of CD47/integrin-mediated pro-survival signals in cardiomyocytes. TSP-2 absence is also associated with cardiac rupture and heart failure in models of myocardial infarction and angiotensin-II-mediated hypertrophy. TSP-2 may protect the remodeling heart by mediated essential actions on assembly and organization of the cardiac matrix, by inhibiting MMP activity, by activating pro-survival signals on cardiomyocytes, or by suppressing inflammation.
v. TSPs in toxic cardiomyopathies
Recent evidence suggests that TSP-2 plays a protective role in doxorubicin-induced cardiomyopathy (460). TSP-2 −/− mice had significantly increased mortality after treatment with doxorubicin; surviving animals exhibited depressed cardiac function in comparison to corresponding WT mice and had increased cardiomyocyte apoptosis and accentuated matrix degradation. The protective effects of TSP-2 were mediated through activation of Akt-dependent pro-survival signaling in cardiomyocytes and through inhibition of MMP-2.
vi. TSPs in human heart disease
The relevance of the animal model experiments in the human pathobiologic process is supported by studies demonstrating increased TSP expression in patients with cardiac hypertrophy. Patients with aortic stenosis exhibited increased myocardial TSP-2 expression; levels were particularly elevated in individuals with depressed systolic function (401). Moreover, failing human hearts from patients with end-stage dilated cardiomyopathy exhibited a 3.5-fold increase in TSP-4 expression when compared with non-failing hearts (445). Evidence also suggests that TSPs are upregulated in human myocardial ischemia. Affymetrix microarray analysis showed elevation of TSP-1, TSP-2 and TSP-4 mRNA expression in “chronically ischemic” myocardium (identified as myocardial segments supplied by a totally or partially occluded coronary artery) biopsied from patients with ischemic cardiomyopathy undergoing aortocoronary bypass surgery (158). Moreover, in an attempt to identify new predictive biomarkers for patients with myocardial infarction Devaux and colleagues found that the levels of TSP-1 mRNA expression in whole blood cells from patients with acute myocardial infarction predicted functional deterioration (113). In contrast, in end-stage heart failure patients, myocardial TSP-1 levels were decreased (32), perhaps reflecting the transient nature of TSP upregulation following cardiac injury. Intense upregulation of TSP-1 has also been reported in human cardiac allograft vasculopathy (508).
Perhaps the strongest evidence for a role of TSPs in human cardiac pathophysiology is derived from studies suggesting an association between single nucleotide polymorphisms (SNP) in TSPs and premature coronary atherothrombotic disease (430). A serine (Ser-700) amino acid rather than the usual asparagine (Asn-700) at residue 700 of TSP-1 has been linked to an increased risk of familial premature myocardial infarction (454), (429). In addition, a missense variant of TSP-4 where a proline substitutes alanine at position 387 (A387P) was strongly associated with myocardial infarction. In contrast, a variant in the 3′ untranslated region of TSP-2 seemed to have a protective effect against myocardial infarction in homozygous individuals (454). The basis for these associations remains poorly understood. A recent investigation demonstrated that the Ser-700 TSP-1 variant increased the rate and extent of platelet aggregation and showed increased surface expression on platelets compared with the Asn-700 variant (319). Furthermore, the A387 TSP-4 variant induces enhanced activation of adherent neutrophils (350). Thus, these SNPs may impart a gain-of function, inducing a prothrombotic and atherogenic phenotype (514).
B. Tenascins
The tenascins are a highly conserved family of oligomeric glycoproteins built from a common set of structural motifs (183) (90). Four tenascin paralogues have been identified in mammals, each designated with a letter derived from earlier eponyms: C, R, X and W. Only tenascins C and X are known to modulate cell adhesion, migration and growth and are considered matricellular proteins.
1. Structure
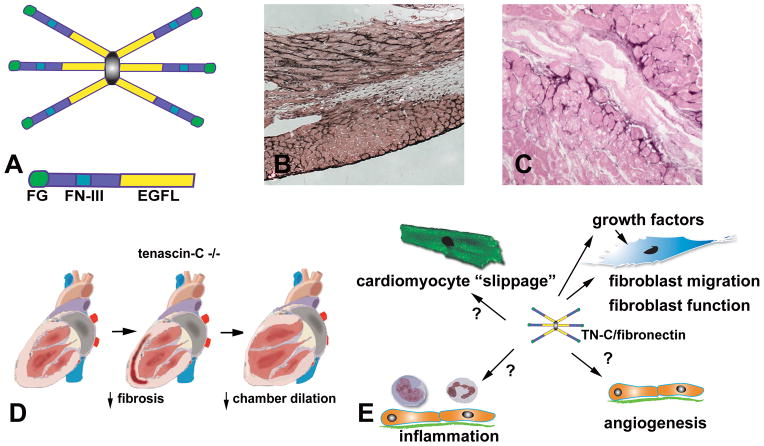
Each tenascin has N-terminal heptad repeats, one or more Epidermal Growth Factor (EGF)-like repeats, a series of fibronectin type III modules and a C-terminal region containing a globular fibrinogen-like domain (211) At the N-terminus each tenascin has an oligomerization domain allowing subunits to assemble, usually into trimers. Tenascin-C, the founding member of the family, assembles into a disulfide-linked hexamer (Figure 8). Isoform variants of tenascin-C, produced through alternative splicing within the fibronectin type III repeats, have been described (183); these variants may exhibit distinct functional properties. Tenascin-C is cleaved by MMP-2 and other proteases potentially revealing cryptic adhesive sites (291); the significance of these interactions in vivo remains unclear.
Figure 8. The role of tenascin-C in cardiac remodeling.
A. tenascin-C assembles into a hexamer; each subunit contains EGF-like repeats (EGFL), a series of fibronectin type III modules (FN-III) and a C-terninal globular fibrinogen-like region (FG). B. Immunohistochemical staining for tenascin-C in reperfused mouse myocardial infarction illustrates that tenascin-C is selectively localized in the infarct border zone. C. In patients with ischemic cardiomyopathy, interstitial tenascin-C expression marks areas exhibiting active remodeling. D. Tenascin-C absence is associated with reduced fibrosis and attenuated chamber dilation following myocardial infarction. E. The effects of tenascin-C on the remodeling heart appear to be related to its profibrotic actions. Furthermore, tenascin-C may modulate the inflammatory and angiogenic response and may facilitate cardiomyocyte slippage.
2. Expression and synthesis of the tenascins
Expression of tenascins-C and -X is regulated by microenvironmental factors; in contrast, tenascins–R and –W exhibit more stable and restricted expression patterns (458). Tenascin-C is highly expressed during embryonic development and organogenesis near migrating cells, at sites of epithelial-mesenchymal interactions and in developing connective tissue. Tenascin-C expression virtually disappears in most adult tissues; however its expression remains abundant in normal tendons and at the osteotendinous and myotendinous junctions (208). Tenascin-C is induced by a variety of growth factors, such as PDGF, FGF2 and TGF-βs, and its expression is markedly upregulated in injured and remodeling tissues, during neovascularization and tumorigenesis. In addition, mechanical stress is an important mechanism inducing tenascin-C synthesis; this explains the persistently high tenascin-C levels in tendons. In vitro, increased mechanical strain markedly upregulates tenascin-C expression in fibroblasts, smooth muscle cells and neonatal rat cardiomyocytes (92), (495).
Tenascin-X is expressed in loose connective tissue in the dermis, epimysium and blood vessels, both during development and in adult organisms (458). In contrast, expression of tenascins-R and –W is more restricted. Tenascin-R is exclusively expressed in the central nervous system, where it is mainly synthesized by oligodendrocytes. Tenascin-W, the most recently described member of the family (398). is found primarily in bone, but is also upregulated in the stroma of certain tumors.
3. Cellular effects of tenascin-C
i. Modulation of cell adhesion
Tenascin-C binds promiscuously to a variety of matrix molecules, including fibronectin (93) and proteoglycans, and serves an adhesion-modulating role. Much like TSP-1, tenascin-C promotes weak cell attachment and induces cellular deadhesion. Extensive evidence suggests that tenascin-C interacts with fibronectin, inhibiting fibroblast attachment (91). The effects of tenascin-C in regulating cellular responses to a fibrin-fibronectin matrix are mediated through modulation of focal adhesion kinase (FAK) and RhoA activation (292). Tenascin-C suppresses Rho activation in fibroblasts leading to loss of stress fibers and dramatically alters actin organization when added to a three-dimensional fibrin-fibronectin matrix (481). Moreover, cells surrounded by a matrix containing tenascin-C, fibrin and fibronectin do not assemble fibronectin fibrils and are unable to induce matrix contraction (292).
ii. The role of tenascin-C in cellular migration and proliferation
In vitro studies examining the effects of tenascin-C on cell migration have produced conflicting results, depending on the context and the type of the cells studied. Tenascin-C supports lymphocyte migration and rolling (97), but inhibits monocyte chemotaxis in vitro (271). Consistent with its modulatory effects on fibroblast adhesion, tenascin-C induces a migratory phenotype in mouse NIH3T3 fibroblasts (292). Effects of tenascin-C on cellular proliferation also appear to be context and cell type-dependent. Thus, tenascin-C enhanced proliferation in endothelial and smooth muscle cells promoting growth factor-mediated responses (210), (95), but inhibited proliferative activity of stimulated fibroblasts (105).
4. In vivo actions of tenascin-C
i. Role of tenascin-C in development, homeostasis and matrix assembly
The development of tenascin-C null mice provided an important new tool to study the role of tenascin-C in various pathophysiologic processes. Two independent groups demonstrated that mice with disruption of the tenascin-C gene develop normally, exhibiting no gross anatomic abnormalities (275), (137), (374). As with other matricellular protein knockouts, however, the absence of gross abnormalities in tenascin-C null mice does not exclude a significant role in tissue homeostasis. More detailed analysis of the matrix architecture in tenascin-C null animals may demonstrate subtle, but important, defects. Moreover, studies using knockouts in several backgrounds may reveal phenotypic alterations that were not apparent in early studies due to variable penetrance. Most importantly, studies examining the response of the knockouts in various disease models have suggested important roles of tenascin-C in pathophysiologic contexts.
ii. Effects of tenascin-C in inflammation and wound healing
Tenascin-C is strongly induced in inflamed and remodeling tissues (130). (509). Tenascin-C upregulation in inflammatory and fibrotic processes is likely due to release and activation of growth factors, capable of stimulating its expression, such as TGF-β (346). Although tenascin-C is dramatically induced in inflammatory processes, its role in regulating inflammation and tissue repair remains poorly understood. Studies using tenascin-C null mice gave contradictory findings in various models of inflammatory injury. Tenascin-C null mice had significantly attenuated airway inflammation and hyperreactivity in a model of allergen-induced bronchial asthma (316); these effects were presumed due to loss of activating actions on T lymphocytes. In contrast, tenascin-C loss was associated with prolonged and accentuated inflammation in a model of chemically-induced dermatitis (232). A recent investigation has suggested that tenascin-C may drive the innate immune response in synovial inflammation. Tenascin-C null mice show rapid resolution of acute zymosan-induced joint inflammation; the pro-inflammatory effects of tenascin-C were mediated through activation of TLR4 signaling (290). Loss-of-function studies have also implicated tenascin-C in tissue repair. In a model of cutaneous injury, tenascin-C null mice had no obvious impairment in the quality of healing (137). Proliferation, migration and apoptosis of epidermal keratinocytes, fibroblasts and macrophages in the healing wounds appeared to be normal; however, deposition of fibronectin in the granulation tissue of tenascin-C knockout mice was significantly lower. This may indicate that the absence of tenascin-C results in a significant defect in matrix organization in the wounds. In addition, tenascin-C null mice had defective healing in a model of corneal suture wounding demonstrating decreased deposition of fibronectin (283). Recent evidence suggested that tenascin-C is important in repair of mechanical skeletal muscle injury (136).
iii. Role of tenascin-C in tissue fibrosis
Tenascin-C expression is associated with the development of fibrosis in both experimental models (491) and in patients with fibrotic conditions (343), (149). Beyond these associative findings, studies using knockout mice suggested a crucial role for tenascin-C in mediating the fibrotic response. Tenascin-C loss attenuated hepatic fibrosis in a model of immune-mediated hepatitis (126) and prevented fibrous tissue deposition in the lung in a model of bleomycin-induced injury (67). Attenuation of fibrosis in the absence of tenascin-C is associated with decreased TGF-β signaling.
iv. Effects of tenascin-C on the vasculature
Extensive evidence suggests a role for tenascin-C in pathological angiogenesis. Tenascin-C expression in tumors correlates with angiogenesis (506). In vitro, tenascin-C promotes endothelial cell migration and migrating endothelial cells express higher amounts of tenascin-C than non-migrating cells (507). Moreover, the fibrinogen globe of tenascin-C is capable of switching bFGF-stimulated endothelial cells into a sprouting phenotype; these actions are related to the de-adhesive properties of the matricellular protein (396). In addition to the significance of these findings in tumor progression, the angiogenic actions of tenascin-C may also be relevant in cardiac neovascularization (28).
5. Tenascin-C and the heart (Table 3)
Table 3.
Role of the tenascins in normal and diseased hearts
| Tenascin-C | Tenascin-X | |
|---|---|---|
| Role in cardiac homeostasis | In the normal adult myocardium tenascin-C expression is found only at the chordate tendinae of the papillary muscles (383). There is no known role for tenascin-C in cardiac homeostasis. | Tenascin-X is abundantly expressed in the normal adult heart (287). However, tenascin-X absence did not result in any gross cardiac abnormalities (279). Systematic studies of cardiac function and geometry in tenascin-X null mice have not been reported. |
| Role in cardiac aging | No known role. | Not known. |
| Role in myocardial infarction | Tenascin-C is markedly upregulated in the infarcted myocardium and is predominantly localized in the border zone and in remodelling areas (190). Tenascin-C −/− mice are protected from adverse post-infarction remodeling and have reduced fibrosis in the non-infarcted areas (323). The detrimental effects of tenascin-C in the healing infarct may be mediated through accentuation of pro-fibrotic growth factor signalling. | Not known. |
| Role in cardiac hypertrophy and fibrosis | Tenascin-C is upregulated in the pressure-overloaded myocardium (491). However, its role in hypertrophy and fibrosis is unknown. | Not known. |
| Role in myocarditis, cardiomyopathies and cardiac allograft | Tenascin-C upregulation is a hallmark of cardiac remodelling regardless of etiology. Tenascin-C induction was reported in autoimmune myocarditis (191). In a model of cardiac transplantation, tenascin-C null mice had impaired allograft vascularisation (28). | Not known. |
| Tenascins in human heart disease | Tenascin-C upregulation is consistently found in human cardiomyopathic hearts and is a marker of active remodeling (149). Tenascin-C has potential as a marker of disease activity (reflecting inflammation, fibrosis and remodeling) in human myocarditis and cardiomyopathy (300), (382). | Occasional cases of valvular disease have been reported in human patients with tenascin-X deficiency, a condition that causes a distinct form of the Ehlers-Danlos syndrome (347). |
i. Tenascin-C in cardiac homeostasis
Tenascin-C is highly expressed by precardial mesodermal cells in the embryonic heart when they differentiate into cardiomyocytes; these cells stop producing tenascin when they express sarcomeric proteins (192), (193). In the normal adult heart, tenascin-C is not found in the myocardium except at the chordae tendinae of papillary muscles (383). In the absence of injury, tenascin-C null mice have normal cardiac function and morphology (323) suggesting that this matricellular protein plays no role in maintenance of the structural integrity and homeostasis of the heart.
ii. Tenascin-C in myocardial infarction
In healing myocardial infarcts, tenascin-C is transiently expressed during the proliferative phase of healing in both mammals (190) and fish (69), is predominantly produced by fibroblasts (190) and is localized in the border zone between infarcted and viable remodeling myocardium (Figure 8B). Several growth factors released in healing infarcts (such as TGF-β, bFGF, and PDGF), are capable of upregulating fibroblast tenascin-C synthesis. Smad3 loss results in significantly decreased tenascin-C expression in the infarct border zone and in TGF-β-stimulated fibroblasts suggesting that the TGF-β/Smad2/3 pathway plays an important role in tenascin synthesis following infarction (62). In addition, angiotensin II, an important regulator of cardiac remodeling and fibrous tissue deposition, is also known to stimulate tenascin-C expression (274). Tenascin-C expression virtually disappears in the mature scar (482).
iii. The role of tenascin-C in infarct healing and post-infarction remodelling
Recent experiments using tenascin-C null mice revealed detrimental effects of tenascin-C signaling in non-reperfused myocardial infarction. Tenascin-C −/− and WT animals had comparable survival rates and scar size following infarction. However, tenascin-C loss significantly attenuated dilative remodelling and diastolic dysfunction in the infarcted heart; these protective effects were associated with less pronounced fibrosis of the non-infarcted remodelling myocardium (323). In vitro, tenascin-C accelerated cardiac fibroblast migration, enhanced myofibroblast transdifferentiation upregulating α-smooth muscle actin synthesis and induced collagen gel contraction, without affecting fibroblast proliferation (442). Thus, the detrimental effects of tenascin-C on the remodelling infarcted heart may be due to accentuation of growth factor-induced profibrotic actions. Selective upregulation of tenascin-C in the infarct border zone may facilitate fibroblast migration into the remodelling areas, inducing local fibrosis and worsening diastolic dysfunction. Whether tenascin-C also modulates the matrix-degrading properties of fibroblasts in the infarct has not been investigated. Additional detrimental effects may be due to tenascin-C-mediated weakening of adhesive interactions involving cardiomyocytes, thus leading to cardiomyocyte “slippage” and adverse remodelling (190).
iv. The role of tenascin-C in myocarditis, cardiomyopathy and heart failure
Tenascin-C is markedly upregulated in a variety of pathological processes associated with inflammation and remodeling of the cardiac tissue. Intense, but transient tenascin-C upregulation was noted in mouse models of viral myocarditis (191), pressure overload hypertrophy (491), angiotensin II-induced cardiac fibrosis (324) and ischemic fibrotic cardiomyopathy due to brief repetitive ischemia and reperfusion (114). Moreover, increased tenascin-C levels were found in mice with a chronic adrenergic state due to cardiac overexpression of the α1A-adrenergic receptor (74). In a mouse model of myosin-induced autoimmune myocarditis tenascin-C expression was upregulated at a very early stage (before cell infiltration and myocytolysis became histologically apparent), remained elevated during the active phase and disappeared with scar formation (191). Tenascin-C was predominantly synthesized by interstitial fibroblasts and correlated with disease activity (191). Although tenascin-C upregulation is consistently found in animal models of cardiomyopathy; its role in the pathogenesis of non-infarctive remodelling has not been investigated. Recent evidence suggested that tenascin-C may be involved in neovessel formation in the myocardium. Experiments in a model of cardiac transplantation demonstrated that tenascin-C null mice exhibit impaired vascularisation of cardiac allografts, suggesting an important role in postnatal cardiac angiogenesis (28). Whether tenascin-C plays a role in ischemia-induced angiogenesis remains unknown.
v. Tenascin-C in human heart disease
Increased cardiac expression of tenascin-C is consistently found in patients with a variety of cardiomyopathic conditions. Tenascin-C upregulation has been reported in human myocardial infarcts (482) and in patients with myocarditis (457), dilative (443) and ischemic cardiomyopathy (149). Moreover, tenascin-C expression was found in remodeling valves in patients with heart failure (397); progression of aortic stenosis was associated with increased tenascin-C levels in the valve tissue (385) Several characteristics of its expression pattern in the remodelling heart suggest that tenascin-C has outstanding potential as a marker of disease activity. First, tenascin-C expression is negligible in the normal heart. Second, it is rapidly upregulated following cardiac injury and is deposited in the remodeling interstitial space. Third, tenascin-C expression correlates with inflammatory and fibrogenic activity and disappears in mature scars. Fourth, it is selectively localized in remodeling myocardial segments. In patients with ischemic cardiomyopathy undergoing aortocoronary bypass surgery, high tenascin-C expression was found in segments with reversible recovery of function following revascularization (Figure 8C); levels in segments with irreversible dysfunction was significantly lower (149). These findings reflect the increased expression of tenascin in actively remodeling myocardium and its absence in segments containing mature scar tissue (150), (143). Furthermore, in patients with acute myocarditis, tenascin-C expression may be a clinically useful marker of disease activity. In patients with acute myocarditis undergoing endomyocardial biopsy, tenascin-C expression was noted in patients with active stage inflammation and disappeared in healed lesions (300). Animal experiments have suggested that nuclear imaging using anti-tenascin antibodies is feasible and may be useful for diagnosis of myocarditis (384). Assessment of serum levels of tenascin-C has been suggested as a potentially useful biomarker in patients with post-infarction remodelling and heart failure. In patients with acute myocardial infarction, peak serum tenascin-C levels predicted the development of dilative remodeling (382). Moreover, assessment of serum tenascin-C prior to discharge in patients hospitalized for decompensated heart failure had an incremental prognostic value, when used for risk prediction along with BNP levels (154).
6. Tenascin-X
In humans tenascin-X deficiency causes a distinct, recessive form of the Ehlers-Danlos syndrome, associated with hypermobile joints, hyperelastic skin and easy bruising (391). Occasional cases of valvular disease (in particular mitral valve prolapse) have been reported in tenascin-X-deficient individuals (347); however, the low prevalence of the condition precludes any conclusions regarding the role of tenascin-X in valve morphology and function. In mice, targeted inactivation of tenascin-X mimicked the human condition resulting in progressive skin hyperxtensibility associated with reduced density of collagen fibrils in cutaneous tissues. Although tenascin-X is abundantly expressed in the normal adult heart (287), its absence in genetically targeted mice did not result in any gross cardiac abnormalities (279). However, systolic and diastolic ventricular function, valvular morphology and competence have not been systematically studied in tenascin-X null mice. Thus, important functions of tenascin-X in cardiac homeostasis and potential effects in cardiac pathophysiologic conditions cannot be excluded.
C. SPARC
SPARC (osteonectin/BM-40) is a highly-conserved, multifunctional glycoprotein, expressed in both vertebrates and invertebrates, that was first described as the main non-collagenous constituent of bone (448), (56). A typical matricellular protein, SPARC, regulates cell function and tissue remodeling by exerting counterhadhesive actions, by modulating growth factor signaling and by serving as a cell cycle inhibitor (94).
1. Structure
The mature SPARC protein consists of three distinct regions. The N-terminal acidic module contains a low-affinity, high-capacity Ca2+-binding domain. The central portion of the SPARC molecule is composed of a follistatin-like domain and a protease-like inhibitor region, and contains bioactive peptides that exert different effects on endothelial cells. The C-terminal region constitutes the extracellular Ca2+(EC)- binding module and contains a peptide sequence that inhibits endothelial cell proliferation (238) and a collagen-binding region (380).
2. Synthesis, expression and cleavage
During embryonic development SPARC is expressed in many tissues; its levels are particularly high in areas of chondrogenesis, osteogenesis and somitogenesis (377). SPARC expression is significantly decreased in adult organs with the exception of tissues that exhibit high rates of turnover (such as the bone, gastrointestinal epithelium, skin and glandular tissue) (377). SPARC expression is very low in parenchymal organs, but is markedly upregulated during active tissue remodeling. A wide range of pathophysiologic conditions, such as wound healing, neoplasia, arthritis, fibrotic conditions, obesity and diabetes are associated with SPARC induction (360).
Growth factor-mediated effects appear to be implicated in regulation of SPARC in injured tissues. Most of the in vitro evidence on regulation of SPARC expression is derived from experiments on cells involved in bone metabolism (487); synthesis by other cell types has been less well studied. Extensive evidence suggests that members of the TGF-β family are capable of inducing SPARC in various cell populations. TGF-β1 induces SPARC synthesis in dermal (361) and corneal fibroblasts (1), in periodontal ligament cells (156) and in chondrocytes (70). Bone morphogenetic protein (BMP)-2, another member of the TGF-β family, also increased SPARC expression in chondrocytes (317). Effects of other growth factors are less consistent: VEGF stimulation induced SPARC in endothelial cells (216), whereas PDGF and IGF-1 enhanced SPARC expression in articular chondrocytes (70); however, bFGF and PDGF attenuated SPARC synthesis in periodontal ligament cells (156). Pro-inflammatory cytokines (such as IL-1β and TNF-α) attenuate SPARC synthesis in chondrocytes (70), (317).
Proteolytic processing of SPARC modulates its activity and function; these interactions may be important in vivo. In the protease-rich environment of injured tissues, SPARC may be cleaved to generate smaller fragments through interactions involving MMPs. Several MMPs (including MMP-2, -3, -7 and -13) are capable of inducing proteolytic cleavage of a single peptide bond in the central module of the SPARC molecule, markedly increasing its affinity for collagen (379). Moreover, proteolysis of SPARC by MMP-3 generates several fragments that influence angiogenesis (376).
3. Cellular effects of SPARC
i. Effects on cell adhesion
Much like tenascin-C and TSP-1, SPARC acts as a typical matricellular protein promoting a de-adhesive state through disassembly of focal adhesions (306), (307) in endothelial and in lens epithelial cells (477). Although the effects of SPARC on cell adhesion are likely to modulate cell migration and survival, the significance of SPARC-mediated de-adhesive actions in vivo is unclear.
ii. Growth factor signaling
SPARC modulates the activity of several growth factors critically involved in tissue repair, fibrosis and angiogenesis. SPARC inhibits PDGF-stimulated smooth muscle cell proliferation (302) and binds with PDGF-BB and PDGF-AB, blocking their interaction with their receptor (354). SPARC also regulates VEGF function. VEGF-mediated endothelial cell mitogenesis is markedly inhibited by SPARC; this effect is mediated in part by binding of the matricellular protein to the growth factor (238). Effects of SPARC on FGF-2 signaling have also been reported. SPARC inhibits FGF-2-stimulated proliferation of endothelial cells; however, these effects are not mediated through direct binding, but involve selective regulation of the MAPK cascade, downstream of FGFR1. IGF-1 signaling in mesangial cells is also inhibited by SPARC despite the absence of a direct interaction between the growth factor and the matricellular protein (140). TGF-β and SPARC exhibit mutual regulatory effects: TGF-β induces SPARC synthesis in many cell types, while SPARC increases TGF-β expression (138). SPARC does not bind TGF-β, or its receptors; however, it can enhance TGF-β-mediated Smad2 signaling in mesangial cells (139), epithelial cells (399) and fibroblasts (394).
4. In vivo actions actions of SPARC
In vivo SPARC regulates the assembly and organization of the extracellular matrix and also modulates cellular phenotype and function by regulating growth factor signaling, by attenuating adhesive interactions and by controlling proliferative activity.
i. SPARC and matrix remodeling
SPARC regulates matrix assembly and remodeling both through direct actions on matrix deposition and by modulating protease activity (49). SPARC binds to multiple structural components of the matrix (94), including collagens I, III, IV and V and vitronectin. The generation of SPARC null mice by two independent groups provided extensive information on the role of SPARC in matrix assembly and metabolism. Despite the wide expression of SPARC in murine embryonic tissues, and the evidence suggesting a crucial role of SPARC in development of invertebrates (135), SPARC −/− mice were viable and fertile and had no developmental abnormalities. However, the absence of SPARC resulted in significant morphological defects of the fibrillar collagen and in reduced collagen deposition in many tissues. In SPARC null mice the skin has half the amount of collagen in comparison with wildtype skin (53). Decreased interstitial collagen content was also noted in SPARC −/− myocardium and adipose tissue (50), (52). Collagen fibrils in the dermis of SPARC null mice are smaller and more uniform in diameter than in WT animals (362). The basis for this defect remains unclear. It has been suggested that SPARC may not directly mediate fusion of collagen fibrils, but its absence may result in generation of collagen fibrils that lack the capacity to fuse (49).
Studies in invertebrates suggested that SPARC plays a role in assembly of the basal lamina interacting with collagen IV. In C elegans SPARC is localized in the basement membrane (135) and SPARC mutant flies have no collagen IV in basal laminae and display defects similar to those of collagen IV mutants (281). The contribution of SPARC in basal laminal assembly appears to be important in mammals: both strains of SPARC knockout mice exhibit early onset cataractogenesis (165), (325) associated with abnormal type IV collagen deposition in the lens capsule (496). Beyond its direct role in deposition and assembly of matrix proteins, SPARC may also modulate matrix metabolism by increasing the production and activity of MMPs (455), (164). However, the in vivo significance of these interactions remains unclear.
ii. SPARC in wound healing and fibrosis
Through its effects on matrix deposition and growth factor signaling, SPARC may modulate the reparative response. Although SPARC expression is markedly and consistently upregulated in healing tissues (360), (359), loss-of-function studies have provided conflicting results on its role. In large excisional cutaneous wounds, Basu and co-workers demonstrated that SPARC absence is associated with impaired and delayed healing; the defect was restored by administration of purified SPARC (31). In contrast, Bradshaw and colleagues demonstrated accelerated skin wound closure in SPARC null animals resulting from enhanced contractibility of the collagen-poor scar (55). The basis for the conflicting findings is unclear.
Increased SPARC expression is also found in chronic fibrotic conditions (431). The bulk of the evidence suggests that SPARC exerts pro-fibrotic effects. In a model of bleomycin-induced lung fibrosis Strandjord and co-workers reported attenuated pulmonary collagen deposition in the lungs of SPARC null mice (431). Moreover, SPARC deficiency reduced renal fibrosis in a model of angiotensin-II-mediated hypertension (420) and SPARC inhibition attenuated fibrous tissue deposition in the liver (65) and in the lung (471). The fibrogenic actions of SPARC may be related to its role in matrix deposition and assembly, or to its effects in potentiating TGF-β/Smad2 signaling. Moreover, SPARC suppresses apoptosis in fibroblasts from patients with idiopathic pulmonary fibrosis; defective clearance of these cells may be important in the pathogenesis of the disease (71). In contrast to these findings, Savani and co-workers (386) suggested SPARC-mediated antifibrotic actions demonstrating that SPARC null mice had increased pulmonary fibrosis in a model of bleomycin-induced injury. The basis for this surprising observation was not explored.
iii. SPARC and angiogenesis
SPARC may modulate the angiogenic process through actions on growth factor signaling and through effects on MMP synthesis and activation. SPARC signaling is capable of activating both angiogenic and angiostatic pathways, thus, its role in conditions associated with angiogenesis may be dependent on the context. SPARC expression is upregulated in injured vessels (349). Mice lacking SPARC had increased fibrovascular invasion of subcutaneous sponges, suggesting angiostatic effects of SPARC that may be due to its VEGF-binding properties (54).
iv. SPARC in adipogenesis
Inflammation and fibrosis of the adipose tissue contribute to the metabolic dysfunction associated with obesity. SPARC is linked to human obesity and may enhance matrix deposition in the adipose tissue fibrosis its pro-fibrotic actions (229), (230). Loss-of-function experiments suggested that SPARC modulates the composition adipose tissue matrix and alters the phenotype of adipocytes. SPARC inactivation is associated with increased adiposity in mice fed a standard diet (52) and enhances weight gain in a model of obesity due to a high fat diet (321). In vitro, SPARC inhibited adipocyte differentiation through enhancement of beta-catenin signaling (322) and attenuated mitotic clonal expansion of preadipocytes (321).
5. SPARC in cardiac homeostasis and disease (Table 4)
Table 4.
SPARC in cardiac homeostasis and pathophysiology
| Expression | Role | |
|---|---|---|
| Cardiac homeostasis | Highly expressed in the embryonic heart; expression levels are significantly decreased in the adult myocardium (377) | SPARC contributes to the formation of the collagen network in the adult heart. SPARC null mice have thinner collagen struts. Effects of SPARC deficiency on cardiac function are relatively subtle: SPARC −/− hearts have normal systolic function but exhibit decreased passive stiffness (50), (51). |
| Cardiac aging | Aging hearts have increased expression of SPARC (51). | Aging SPARC −/− mice have decreased collagen deposition and reduced collagen cross-linking resulting in protection from age-associated diastolic stiffness (51). |
| Myocardial infarction | SPARC is abundantly expressed in the infarcted myocardium, primarily localized in myofibroblasts and macrophages infiltrating the myocardium (227), (118). | Findings on the role of SPARC in cardiac repair and post-infarction remodelling are somewhat contradictory. Schellings et al found that SPARC upregulation plays an important role in maintaining matrix integrity following myocardial infarction (394). SPARC loss was associated with increased incidence of cardiac rupture due to formation of disorganized granulation tissue and deposition of immature collagen fibers. SPARC overexpression, on the other hand, improved the quality of the scar preventing adverse remodeling. The actions of SPARC were mediated at least in part through enhancement of TGF-β signaling. In contrast, McCurdy et al reported that SPARC null mice had attenuated systolic dysfunction during the early post-infarction phase (289). |
| Cardiac hypertrophy and fibrosis | SPARC expression is markedly increased in models of cardiac hypertrophy and fibrosis (50). | Much like in the infarct, in the pressure-overloaded myocardium, SPARC regulates matrix remodeling. In a model of pressure overload cardiac fibrosis SPARC null mice have reduced collagen content and attenuated diastolic dysfunction (50). |
| SPARC in human heart disease | Because of its binding to matrix proteins and growth factors, SPARC appears to have a limited role as a circulating biomarker (404), despite its marked upregulation in the myocardium following cardiac injury. | |
i. SPARC in the normal heart
SPARC is highly expressed in the embryonic heart; however, levels of expression are significantly decreased in the adult organ (377). Whether small amounts of SPARC are present in the normal adult cardiac interstitium is unclear. Using immunohistochemistry with highly-specific polyclonal antibodies, Sage and co-workers found no SPARC expression in the normal adult murine cardiac interstitium (377). In contrast, using immunogold staining, Sasaki et al. observed SPARC deposition in the adult mouse heart, localized in the cardiac interstitial matrix (381). As in many other tissues, SPARC contributes to the formation of the collagen network in the adult heart through effects on post-synthetic processing. SPARC null animals have reduced myocardial collagen content when compared to WT mice; the relative proportion of insoluble collagen is also decreased in the absence of SPARC (51), (50). Scanning electron microscopy confirmed the altered formation of collagen in the cardiac interstitium demonstrating thinner fibrillar collagen struts and reduced weave in SPARC null hearts. SPARC regulates the myocardial collagen network by modulating procollagen processing and interactions with fibroblast cell surfaces (175). The alterations in collagen morphology had no effects on systolic ventricular function, blood pressure or effective arterial elastance. However, experiments using isolated papillary muscles demonstrated that SPARC absence was associated with decreased passive stiffness of the cardiac muscle (50).
ii. SPARC in aging-associated cardiomyopathy
Experiments in aging mouse hearts demonstrated increased expression of SPARC protein in the myocardium (51), associated with fibrotic remodeling of the ventricle. In the absence of SPARC, senescent mice had attenuated collagen deposition with lower levels of mature cross-linked collagen. Scanning electron microscopy showed that SPARC deficiency prevented the increase in collagen fibril thickness observed in the senescent myocardium. These alterations in collagen deposition and morphology had significant functional consequences. SPARC absence prevented the increase in diastolic stiffness observed in aged papillary muscle (51), without affecting systolic ventricular function.
iii. SPARC in myocardial infarction
In experimental canine, rat and mouse models of myocardial infarction, SPARC is abundantly expressed in the infarcted heart (427), (227), (118), (488), (213), (394). In both canine and mouse models SPARC shows a prolonged time course of expression, peaking 7–14 days after the acute event, and is primarily localized in myofibroblasts and macrophages infiltrating the myocardium (118), (394). Loss-of-function studies demonstrated that SPARC upregulation plays a critical role in maintaining the integrity of the cardiac matrix and in mediating repair following myocardial infarction. In non-reperfused myocardial infarcts, SPARC absence was associated with a four-fold increase in mortality, resulting from a higher incidence of cardiac rupture and heart failure (Figure 9). SPARC null mice showed formation of disorganized granulation tissue in the infarcted heart, filled with immature collagen fibers. On the other hand, adenoviral overexpression of SPARC in WT mice improved the quality of the matrix in the infarct and protected against cardiac dilatation and dysfunction (394). What are the mechanisms responsible for the protective effects of SPARC in post-infarction cardiac repair? Through its multifunctional actions, SPARC may modulate several pathways essential for cardiac repair. First, in the healing infarct SPARC may be important for assembly of newly-formed collagen promoting generation of a supportive matrix in the healing scar to prevent adverse dilative remodeling. Second, SPARC may regulate growth factor signaling playing an important role in recruitment and activation of reparative cells. Schellings and co-workers demonstrated that SPARC enhances TGF-β signaling in cardiac fibroblasts and that TGF-β infusion rescued SPARC null hearts from rupture (394). These findings suggested that the protective actions of SPARC on the infarcted heart may be mediated, at least in part, through enhancement of TGF-β actions necessary for formation of a supportive scar. Third, SPARC-mediated actions in regulation of infarct angiogenesis may play an important role in granulation tissue formation. However, due to the complex and context-dependent actions of SPARC in the angiogenic process, the significance of these interactions in cardiac repair and remodeling remains unknown. In contrast to these protective actions of SPARC in cardiac repair, a recent study suggested that SPARC absence is associated with attenuated early systolic dysfunction following infarction, accompanied by alterations in expression of fibroblast-derived genes (289).
Figure 9. The role of SPARC in cardiac remodeling.
A. SPARC upregulation in the infarcted mouse myocardium. B-D. SPARC null mice have increased incidence of cardiac rupture (C, arrow), exhibiting intramural hemorrhages in the infarcted myocardium (D, arrows). Data reproduced with permission from: Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d’Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med 2009;206:113–123: Copyright 2009, Rockefeller University Press. E. The SPARC molecule contains an acidic region, a follistatin-like domain and an extracellular Ca2+-binding region (EC-module). F. The mechanisms involved in SPARC-mediated cardiac fibrosis are likely due to enhanced growth factor signaling and to effects on matrix assembly, MMP activity and fibroblast function.
iv. SPARC in cardiac hypertrophy and fibrosis
SPARC expression is markedly increased in experimental models of cardiac hypertrophy and fibrosis. Pressure overload, induced through transverse aortic constriction in mice (50), or through abdominal aortic banding in rats (128), significantly increased myocardial SPARC synthesis. Moreover, in a rat model of cardiac hypertrophy due to β-adrenergic stimulation through administration of isoproterenol, myocardial SPARC expression was markedly increased (282). In the pressure-overloaded heart, SPARC absence attenuates cardiac fibrosis without affecting development of hypertrophy (50). SPARC null mice had less myocardial collagen than wildtype animals after transverse aortic constriction; the proportion of insoluble collagen was also significantly lower. Reduced collagen deposition in pressure-overloaded SPARC null hearts was associated with attenuated diastolic stiffness. Thus, much like in the infarcted myocardium, SPARC upregulation in the hypertrophied heart promotes fibrogenic signaling enhancing collagen deposition. However, the functional outcome of SPARC signaling is very different: SPARC protects from cardiac rupture and adverse remodeling in the infarct, but increases diastolic dysfunction in the pressure-overloaded heart. In contrast to the infarcted heart, where the collagen-based scar provides mechanical support to the ventricle preventing adverse remodeling, in the pressure-overloaded myocardium a SPARC-mediated increase in matrix deposition promotes diastolic dysfunction.
v. SPARC in human heart disease
Because SPARC acts locally by binding to matrix proteins and growth factor its role as a biomarker in heart disease appears to be limited (404). Prior to thrombolysis patients with ST elevation myocardial infarction (STEMI) and healthy controls had comparable serum SPARC levels. Three hours after thrombolysis, a modest but significant increase in SPARC levels was observed in STEMI patients (403). Thus, despite the marked induction of this matricellular protein in the infarcted myocardium, serum SPARC does not hold promise as a biomarker in myocardial infarction. In patients with degenerative aortic stenosis, SPARC expression was localized in valvular blood vessels and appeared to be a marker of lesions with less extensive calcification (72).
D. Osteopontin
Osteopontin (OPN, Eta-1) is a phosphorylated acidic glycoprotein that was originally identified as a bone matrix protein, then recognized as a cytokine that is secreted in body fluids (112). OPN is expressed by many immune cells and is markedly and consistently upregulated in response to tissue injury (472).
1. Structure
OPN is expressed as a 33kDa nascent protein, but due to extensive post-translational modifications its molecular weight increases to about 44kDa. The functional domains of the OPN molecule provide clues to its binding interactions with adhesion molecules. Two major domains are responsible for interactions between OPN and integrins. The central RGD (Arg-Gly-Asp) sequence, a motif common to many extracellular matrix proteins, is responsible for binding with the αvβ1, αvβ3, αvβ5, αvβ6 and α5β1 integrins (500), (30). A second, cryptic integrin binding site is exposed upon cleavage of OPN by thrombin and is important for adherence of leukocytes expressing α4 and α9 integrins (203). OPN interactions with the transmembrane adhesion molecule CD44 appear to be RGD-independent and may involve a C-terminal region of the molecule. OPN is a substrate for several MMPs, including MMP-2, -3, -7, -9 and -12 (390). MMP-mediated cleavage of the OPN molecule may result in formation of fragments with distinct integrin binding properties.
2. Synthesis, expression, and regulation
OPN can be expressed by many different cell types, (including osteoblasts, osteocytes, epithelial cells, fibroblasts, endothelial cells, vascular smooth muscle cells and hematopoietic cells) and is secreted into body fluids. Monocytes express low amounts of OPN; however, monocyte to macrophage differentiation is associated with a marked increase in OPN synthesis (234). Thus, macrophages exhibit constitutive expression of OPN that can be further increased with activation of AP-1 and NF-κB signaling (335). On the other hand, OPN expression in T cells is highly inducible upon activation of TCR signaling and is dependent on T-bet (411), a transcription factor that controls CD4+ Th1 cell lineage commitment. OPN is also highly expressed in immature dendritic cells (218). Angiotensin II, pro-inflammatory cytokines, and growth factors are capable of stimulating OPN expression in fibroblasts and vascular smooth muscle cells. Angiotensin II is a potent inducer of OPN in smooth muscle cells (162) and fibroblasts (492). IL-1β induces OPN in pulmonary fibroblasts (405) and enhances OPN synthesis in Angiotensin-II-stimulated cardiac fibroblasts (492). PDGF-BB, PDGF-AB, FGF-1, FGF-2, and TGF-β potently enhance OPN expression in vascular smooth muscle cells (261), (162), (474). The stimulatory effects of TGF-β on OPN synthesis are mediated through Smad3 and Smad4; Smad3 binds directly to the OPN promoter, whereas Smad4 displaces the transcription repressor Hoxa-9 (25).
The constitutive expression of OPN in macrophages and its inducible synthesis by a wide variety of mediators in many cell types result in marked OPN upregulation in injured tissues. Thus, increased OPN expression has been observed in inflammatory, angiogenic and fibrotic processes, in healing wounds, in calcified lesions, in atherosclerosis and neoplasia (472).
3. Cellular actions of OPN
OPN is a multifunctional protein that signals through integrins- or CD44-mediated pathways to modulate cell adhesion, survival and gene expression. OPN acts as a cytokine, when secreted in a soluble form, and as a matricellular protein when immobilized to the matrix.
i. OPN in cell survival
Extensive evidence suggests that OPN serves as a survival signal; its protective effects are, at least in part, mediated through inhibition of apoptosis (472). Both soluble OPN (which acts as a cytokine), and OPN that is bound to the matrix acting as a matricellular protein, are capable of transducing anti-apoptotic signals (425). Endothelial cells plated on OPN-coated surfaces were protected from apoptosis due to serum deprivation (389). The pro-survival effects of immobilized OPN are mediated through integrin signaling and are dependent on NF-κB activation (389). Moreover, soluble OPN inhibited apoptosis of adherent endothelial cells deprived of essential growth factors (221). The pro-survival actions of OPN may be important in the pathogenesis of neoplasia. OPN expression is increased in many tumors and may prevent apoptotic death of malignant cells (332).
ii. OPN in cell adhesion and migration
Many cell types adhere to OPN through interactions involving integrins or CD44. In vitro, OPN is a chemoattractant for a variety of cells, including monocytes, T cells, endothelial cells, smooth muscle cells and epithelial cells. Malignant cells are often more responsive to the effects of OPN than normal cells; this is due to aberrant expression of integrins by neoplastic cells (450).
iii. OPN regulates function of inflammatory cells
Extensive evidence demonstrates that OPN activates macrophages, indicating that these cells are both a major source and a target of OPN. OPN null macrophages exhibit attenuated basal and chemokine-mediated migration (59). Moreover, OPN induces macrophage-derived IL-12 synthesis, suppressing IL-10 expression, contributing to the development of a Th1 response (19). Effects of OPN on survival, proliferation and differentiation of T lymphocytes have also been reported. These actions are important in mediating the in vivo functions of OPN (390).
4. In vivo functions of OPN
Studies using OPN null mice provided information on the role of OPN in tissue homeostasis and disease. Despite its broad distribution in the collagenous matrix of the bone, OPN is not essential for osteogenesis. OPN −/− mice initially appeared phenotypically normal, were fertile and had no dental or skeletal defects (365), (266). However, further studies revealed a critical role for OPN in mediating a wide range of in vivo functions, including critical effects in immune and inflammatory responses, modulatory actions in bone remodeling and important effects in regulation of wound repair, fibrosis and angiogenesis.
i. OPN and bone remodeling
Although OPN does not play a role in normal bone development, in pathological conditions, OPN modulates bone turnover by inhibiting mineralization, by promoting osteoclast differentiation, and by enhancing osteoclast activity. OPN −/− bones are hypermineralized and more fragile that those from WT mice (47). OPN null mice are resistant to ovariectomy-induced bone resorption (503) and, in vitro, OPN null osteoclasts are dysfunctional exhibiting decreased capacity for bone resorption. The effects of OPN on osteoclast function are mediated through pathways involving CD44 and αvβ3 integrin (75), (12).
ii. OPN and the immune system
Experiments using knockout mice have revealed an important role for OPN in inflammation and immunity. As discussed previously, in vitro experiments demonstrated that OPN is a potent chemoattractant for T cells and macrophages, suggesting that it may regulate recruitment of mononuclear cells in injured tissues. Injection of purified OPN in the rat dermis induced marked macrophage infiltration (163). However, experiments using knockout mice suggested that the role of endogenous OPN in recruitment of macrophages at sites of inflammation may be dependent on the type and tissue of injury. OPN absence was associated with attenuated macrophage accumulation in an experimental model of renal injury (340), but not in cutaneous wounds (266).
Extensive evidence suggests that OPN modulates cell-mediated immunity by promoting the Th1, while inhibiting the Th2, response (334). Because early expression of Th1 cytokines is essential to mount a protective host response against intracellular pathogens, OPN loss results in impaired cellular immunity against viruses (HSV1) and bacteria (Listeria monocytogenes) (19), (472). Moreover, OPN is a key contributor to the mucosal defense after viral infections; OPN null mice exhibited protracted disease after intestinal infection with rotavirus (369).
iii. OPN in wound repair and fibrosis
Cutaneous incisional wounds in OPN null mice exhibited disorganized matrix architecture characterized by altered collagen fibrillogenesis, leading to small diameter collagen fibrils (266). OPN loss was not associated with reduced macrophage density in the wound; however, debridement was impaired suggesting defects in macrophage function and reduced phagocytic activity. Whether defective collagen assembly in OPN −/− wounds is due to direct effects of OPN on collagen fibril formation remains unclear. OPN has been shown to bind to type I collagen in vitro (86); however, the in vivo significance of this interaction is unknown. The function of OPN in collagen fibrillogenesis may also be due to interactions with the SLRPs involved in collagen assembly, such as lumican, decorin and fibromodulin.
In vivo studies have also suggested an important role for OPN in tissue fibrosis. In dystrophic mice OPN loss resulted in attenuated skeletal muscle fibrosis; these findings were associated with alterations in the profile of immune cell infiltration and with reduced TGF-β expression (466). OPN null mice also exhibited decreased matrix deposition in a model of renal fibrosis (340).
iv. The role of OPN in atherosclerosis
OPN is highly upregulated in atherosclerotic lesions, where it is predominantly expressed in macrophages, smooth muscle cells and angiogenic endothelial cells (162). OPN overexpression was associated with increased plaque burden in mice fed a high-fat diet (89). Moreover, loss-of-function studies demonstrated that OPN null mice bred in an ApoE −/− background had attenuated atherosclerosis (286). These studies implicate OPN as an important mediator in the pathogenesis of plaque formation. OPN also appears to be an important regulator of vascular calcification (162).
5. OPN and the heart (Table 5)
Table 5.
Expression and role of osteopontin (OPN) in cardiac homeostasis and disease
| Expression | Role | |
|---|---|---|
| Cardiac homeostasis | Low level expression of OPN is observed in the normal adult myocardium. | No significant role in cardiac homeostasis. OPN null hearts have normal structure and function. However, OPN −/− mice exhibit a modest reduction in systolic blood pressure (312). |
| Cardiac aging | Aging induces no significant changes in myocardial OPN expression. An age-associated increase in aortic OPN levels has been reported (293). | Not known. |
| Myocardial infarction | Marked and consistent upregulation of OPN expression in the infarcted myocardium, primarily localized in macrophages (310), (118). | OPN upregulation in the infarcted myocardium protects the heart from adverse remodeling. OPN −/− mice have exaggerated chamber dilation following infarction associated with marked reductions in collagen deposition in the healing scar (456). The protective effects of OPN may be mediated through facilitated matrix assembly, activation of growth factor signaling and actions on fibroblast function and wound angiogenesis. |
| Cardiac hypertrophy and fibrosis | OPN expression is markedly upregulated in models of cardiac hypertrophy and fibrosis due to pressure overload or angiotensin II infusion (285), (416). | OPN mediates both fibrogenic and hypertrophic responses. In a model of angiotensin II-induced fibrosis, OPN −/− mice had markedly reduced fibrosis (285), (100). Excessive disruption of the matrix network in angiotensin II-treated OPN −/− mice resulted in systolic dysfunction. In a model of pressure overload hypertrophy, OPN loss resulted in reduced cardiomyocyte hypertrophy (493). |
| Valvular disease | OPN is markedly induced in stenotic valvular lesions, primarily associated with calcific changes (333). | OPN may inhibit valvular calcification. In a model of ectopic valvular calcification, OPN loss resulted in accelerated and enhanced calcification (428). |
| Human heart disease | Peak plasma OPN levels after myocardial infarction correlate with left ventricular dilation (435). Plasma OPN levels are elevated in patients with heart failure, reflect the severity of disease and predict mortality (371). OPN levels are elevated in patients with stenotic valvular lesions (505). | |
i. OPN and cardiovascular homeostasis
OPN expression in normal hearts and vessels is low (417). Surprisingly, OPN −/− animals had a modest, but significant reduction in systolic blood pressure when compared with wildtype animals (312) accompanied by an increased heart rate. These alterations were associated with subtle changes in arterial physiology and morphology: blood flow was reduced in the carotid arteries of OPN null mice and the vessels showed increased compliance. Histologically, no differences in the aortic elastic network was noted; however, the adventitial collagen in OPN −/− arteries appeared looser compared with wildtype vessels (312). The subtle hemodynamic changes observed in OPN null mice did not appear to affect cardiac structure and function (285), (100).
ii. OPN and the aging cardiovascular system
Using immunohistochemical techniques Miller and co-workers demonstrated increased OPN expression in senescent rat aortas, predominantly localized in the outer media (293). In contrast, changes in myocardial OPN expression have not been observed (418). Whether OPN absence affects the development of aging-associated vascular and cardiac dysfunction has not been studied.
iii. OPN in myocardial infarction
OPN upregulation is consistently found in experimental models of myocardial infarction. Using a model of cardiac cryoinjury in the rat, Murry and co-workers demonstrated marked upregulation of OPN in the infarcted myocardium (310). OPN protein was localized in a subset of macrophages infiltrating the infarct. OPN upregulation peaked 1–2 days after injury, but was transient; OPN levels were markedly downregulated one week after acute cryoinfarction. Suppression of OPN expression occurred despite the presence of abundant macrophages, suggesting that OPN upregulation in the infarcted heart does not simply reflect macrophage infiltration, but may be due to activation, or recruitment, of distinct macrophage subsets. Marked induction of OPN has been consistently reported in canine (151), (212) porcine (231), rat (227), and mouse (456) models of ischemic infarction. The time course of OPN upregulation in the infarcted myocardium is dependent on the species studied, and on the presence or absence of reperfusion (118). In a mouse model of non-reperfused infarction (456) myocardial OPN mRNA levels peaked 3 days after the acute event, then started to decline after 7 days, but remained above sham levels for at least 28 days after infarction. Increased OPN expression was also noted in samples from human myocardial infarcts (310).
The pathways involved in OPN upregulation in the infarcted heart are poorly understood. Genetic disruption of the CC chemokine CCL2/Monocyte Chemoattractant Protein (MCP)-1 was associated with a marked attenuation in OPN expression in the infarct, disproportionate to the reduction in macrophage density (115). Thus, OPN expression in infarct macrophages may reflect recruitment, activation and maturation of specific macrophage subpopulations. Angiotensin II signaling also appears to play a role in mediating OPN upregulation in the infarcted heart (239). The signals responsible for suppression of OPN synthesis by infarct macrophages in the late stages of healing are unknown.
Loss-of-function experiments suggested an important role for OPN in post-infarction cardiac repair and remodeling (Figure 10). When compared to wildtype animals, OPN null mice had markedly accentuated left ventricular dilation following myocardial infarction (456). Worse dilative remodeling in the absence of OPN was not due to increased cardiomyocyte loss; infarct size and cardiomyocyte apoptosis were comparable between WT and OPN −/− mice. However, OPN null mice had markedly reduced collagen deposition in the healing scar. Electron microscopy demonstrated that OPN loss was associated with a reduction in thin collagen filaments and absence of large collagen fibers in the healing scar (456). Defective collagen synthesis and assembly, in the absence of OPN, may be responsible for enhanced dilation of the infarcted ventricle due a decrease in the tensile strength of the scar. Thus, OPN protects the infarcted ventricle from adverse remodeling; this function is not associated with activation of pro-survival signals to prevent cardiomyocyte apoptosis, but, much like in cutaneous wounds (266), appears to be related to actions on matrix formation. The basis for the essential role of OPN in assembly of the collagenous matrix in the infarcted heart remains unknown. Several mechanisms may be implicated. First, OPN may regulate collagen assembly either through direct actions, or by modulating synthesis of the proteoglycans involved in fibrillogenesis. Second, OPN may modulate expression and signaling of growth factors necessary for fibrous tissue deposition. Although direct effects of OPN on growth factor activity have not been reported, in vivo studies have suggested that OPN absence is associated with reduced growth factor levels in experimental models of repair and fibrosis. Loss of OPN resulted in attenuated TGF-β signaling in ocular fibroblasts and reduced corneal neovascularization by decreasing VEGF expression levels (155). Third, OPN may promote matrix deposition by enhancing activity of the macrophages infiltrating the infarct, or by modulating the profile and functional properties of lymphocyte subpopulations (466) recruited in the infarcted heart. Fourth, OPN may be important in matrix deposition by modulating fibroblast phenotype and function. In vitro experiments demonstrated that OPN mediates the proliferative effects of angiotensin II in cardiac fibroblasts (18). Moreover, OPN null cardiac fibroblasts were more susceptible to oxidant-induced apoptosis than WT cells, suggesting that OPN may protect cardiac fibroblasts from death in the hostile environment of the infarct (513). Finally the effects of OPN in cardiac repair may be mediated through activation of angiogenic pathways. The downstream signals responsible for the effects of OPN on infarct healing have not been investigated. Whether interactions with specific integrins mediate distinct effects of OPN is unknown. On the other hand, animals with targeted disruption of CD44, another important OPN ligand, exhibit reparative defects comparable to OPN null animals when subjected to reperfused infarction. Much like in OPN −/− mice, CD44 knockouts had accentuated dilative remodeling, associated with markedly reduced collagen deposition in the infarct (185). CD44 null fibroblasts had reduced capacity to synthesize matrix proteins without exhibiting a defect in TGF-β/Smad2/3 signaling. Whether the effects of OPN on fibroblasts are mediated, at least in part, through CD44 signaling has not been investigated.
Figure 10. The role of OPN in cardiac remodeling.
A. Structure of OPN. The RGD sequence is involved in several integrin-mediated actions of the OPN molecule. Ca2+-binding domains are indicated in red. B. Immunohistochemical staining for OPN in the infarcted canine myocardium. In the healing infarct, OPN is predominantly localized in macrophages and may be an indicator of their maturation and differentiation. C. OPN acts a) as a matricellular protein that binds to the matrix and modulates growth factor signaling and integrin-mediated actions and b) as a cytokine (soluble OPN) that signals through CD44. D. The role of OPN in cardiac remodeling has been investigated using loss-of-function models. OPN absence is associated with impaired formation of the collagenous scar following myocardial infarction leading to accentuated dilative remodeling. In models of cardiac pressure overload, OPN null animals exhibit attenuation of fibrosis and hypertrophy. Thus, the effects of OPN in cardiac remodeling appear to be mediated primarily through actions on matrix organization, fibroblast function and cardiomyocyte hypertrophy. The contribution of myocardial OPN upregulation in modulating the inflammatory and angiogenic response following cardiac injury remains unknown.
iv. OPN in cardiac hypertrophy and fibrosis
OPN synthesis is markedly upregulated in experimental models of cardiac hypertrophy and fibrosis; its expression is associated with the development of heart failure. In spontaneously hypertensive rats (SHR) a marked (10-fold) increase in myocardial OPN mRNA levels was found in animals with decompensated heart failure, but not in animals with compensated hypertrophy (416). OPN in the failing myocardium of SHR was predominantly expressed in interstitial cells (416).
Angiotensin II appears to play an important role in mediating OPN upregulation in the hypertrophied myocardium. ACE inhibition attenuated myocardial OPN expression in SHR (416). Moreover, angiotensin II infusion induces markedly increased OPN expression in the myocardium, associated with fibrosis and cardiomyocyte hypertrophy (100), (285). Two independent investigations have examined the role of OPN in mediating the in vivo actions of angiotensin in the myocardium using a model of angiotensin II infusion that induces marked cardiac hypertrophy accompanied by interstitial and perivascular fibrosis. Matsui and co-workers demonstrated that OPN absence attenuated the hypertensive and pro-fibrotic effects of angiotensin II, but had no effect on cardiomyocyte hypertrophy (285). Attenuated collagen deposition in angiotensin II-treated OPN null hearts was associated with impaired systolic dysfunction and ventricular dilation, presumably due to cardiomyocyte slippage (285). Independently, Collins and co-workers made similar observations (100): OPN null mice had a blunted hypertensive response and markedly reduced cardiac fibrosis after three weeks of Angiotensin II infusion. The antifibrotic effects of OPN deficiency were not associated with a reduction in TGF-β mRNA levels; however, OPN −/− cardiac fibroblasts had impaired adhesion to matrix substrates and diminished proliferative capacity (100). Studies in a model of pressure overload due to aortic banding revealed a role of OPN in hypertrophic growth. OPN null mice were protected from the development of hypertrophy (493) in banded animals. In contrast, development of fibrosis, measured through quantitative morphometry in trichrome-stained sections, was not affected.
Thus, in the cardiac response to pressure overload, OPN appears to mediate fibrogenic, and hypertrophic actions. The basis for OPN-induced fibrogenesis may be due to increased macrophage chemotaxis and activation (285), to direct effects on fibroblast adhesion and proliferation (100), or to facilitation of collagen fibrillogenesis. The hypertrophic actions of OPN may be due to integrin-mediated MAPK activation (493).
OPN also activates a pro-fibrotic program in experimental models of genetic heart failure. Mice lacking the muscle-specific intermediate protein desmin develop pronounced cardiomyocyte degeneration and fibrosis associated with impressive upregulation of OPN (288). Desmin −/− OPN −/− double knockouts had attenuated cardiac dysfunction and reduced fibrosis in comparison to desmin null animals. The protective effects of OPN loss were attributed, at least in part, to a dramatic reduction of galectin-3 secretion by infiltrating OPN-null macrophages (353). Moreover, OPN contributed to the cardiomyopathic process in dystrophin-deficient mdx mice through stimulation of MMP-9 expression (106).
v. OPN and valvular disease
Extensive evidence has demonstrated that OPN is markedly induced in valvular lesions suggesting that it may regulate ectopic calcification. In human aortic lesions, levels of OPN expression were associated with the degree of calcification (333), (296), (355), (351). OPN protein in diseased valves was localized in a subset of macrophages. Increased OPN levels associated with calcium deposition and macrophage infiltration were also noted in calcified mitral valves from patients with mitral stenosis (66). Moreover, in human bioprosthetic valves, increased OPN expression correlated with cell accumulation and calcium deposition (424). In contrast, OPN was not found in non-calcified aortic regurgitant lesions (296). Although OPN induction is consistently linked with valve calcification, its role in the pathogenesis and progression of valvular disease remains poorly understood. Consistent with its role as an inhibitor of mineralization in the bone, OPN may have similar effects in inhibiting valvular ectopic calcification in stenotic lesions. In a model of ectopic calcification using subcutaneous implantation of porcine aortic valves in mice, valve leaflets implanted in OPN null mice showed accelerated and accentuated calcification when compared to leaflets implanted in WT animals (428). In vitro and in vivo experiments suggested that OPN not only inhibits mineral deposition, but also promotes resorption of calcium deposits (428), (337).
vi. OPN as a biomarker in patients with heart disease
In addition to its matricellular properties, OPN is secreted in the serum and in body fluids. Recent clinical studies have suggested that plasma OPN levels may serve as a marker of left ventricular dilatation (16). Plasma OPN may be of particular value as an indicator of adverse remodeling and as a predictor of mortality in patients with heart failure or ischemic heart disease. In patients with anterior myocardial infarction plasma OPN levels significantly increased 2 days after the acute event, peaked after 3 days, remained elevated for 7 days, then decreased 14 days after infarction (435). Peak plasma OPN levels correlated positively with indicators of ventricular dilation and negatively with systolic function (435). The relation between plasma OPN and dilative remodeling following infarction was supported by findings in patients recovering from a previous myocardial infarction. More than 3 months after the acute event, plasma OPN levels were significantly higher in the coronary sinus than in the aortic root suggesting release of myocardial OPN in the coronary venous system. The transcardiac gradient of OPN correlated negatively with systolic function and positively with indicators of dilative remodeling (444). Plasma OPN levels were elevated in patients with heart failure irrespective of the etiology (ischemic vs. non-ischemic cardiomyopathy) (371) and reflected the severity of the disease (421), (371). In patients with dilated cardiomyopathy, OPN levels correlated with biomarkers indicating activation of the renin/angiotensin system (111). These clinical observations highlight the role of angiotensin II in OPN upregulation. The predictive value of plasma OPN levels has also been suggested in patients with heart failure and in individuals with stable ischemic heart disease. In a multivariable model that included clinical, demographic and biochemical parameters, plasma OPN levels emerged as an independent predictor of mortality for patients with heart failure (371). In contrast, in patients with heart failure and preserved ejection fraction OPN levels predicted death or heart failure events only in single variable analysis, but not when introduced into a multivariable model (235). Moreover, unloading the failing ventricle through left ventricular assist device support did not consistently reduce OPN levels in heart failure patients (400). A recent study suggested that in patients with stable ischemic heart disease, OPN levels are an independent predictor of adverse outcome (159).
Patients with stenotic aortic (505), (133), or mitral (21) valves also had elevated plasma OPN levels; increased circulating OPN correlates with valvular calcification (21). Whether OPN levels in patients with valvular disease provide independent prognostic information has not been investigated.
E. Periostin
Periostin, first identified as a protein secreted by murine osteoblasts, was originally named osteoblast-specific factor-2 (OSF-2) (441) and was suggested to play a role in bone metabolism. To avoid confusion with a transcription factor with the same name (123), its name was changed to “periostin” reflecting its intense expression in the periosteum and periodontal ligament (182). In recent years along with an explosion in our knowledge on periostin came the appreciation of its role in a wide variety of pathophysiologic conditions, including neoplasia, tissue repair and cardiac injury (174), (329). Understanding of the biological functions of periostin suggested that it should be considered a matricellular protein: periostin is upregulated in injured and remodeling tissues, where it binds to extracellular matrix proteins, and does not play a direct structural role, but modulates cell phenotype and function through integrin-mediated interactions (326).
1. Structure
Periostin is structurally homologous to the insect protein fasciclin-1 (441), which is involved in axon guidance and cell adhesion. The periostin protein has a signal sequence, four repeated domains related to those found in fasciclin-1 (fasciclin-1-like repeats), an aminoterminal cysteine-rich region and heparin-binding domains in the C-terminal end. The function of the periostin domains remains unknown. Periostin is one of four mammalian genes containing fasciclin domains: TGF-β-induced gene-human clone 3 (βigH3) is a secreted protein that shares 49% aminoacid homology with periostin, whereas the stabilins -1 and -2 are significantly more divergent transmembrane molecules, and are expressed in a broad range of tissues (269).
2. Synthesis, expression, and regulation
Although early studies suggested that periostin expression was negligible in most adult tissues (with the exception of the bone and lung), more recent investigations have revealed significant baseline levels of periostin in many other sites. In normal human tissues, very high expression of periostin was found in the skin (207) and breast; several other organs, including the pancreas, liver, lung, colon and lymph nodes showed lower levels of expression (451). Periostin expression is particularly high in collagen-rich connective tissue subjected to mechanical stress in vivo, such as the periosteum and periodontal ligaments (182), the tendons (327), the cornea, the mature cardiac valves and the endocardial cushions in the developing heart. Tissue injury, repair and remodeling are associated with upregulation of periostin expression; moreover, large amounts of periostin are found in many tumors. Increased periostin expression in tissue repair, remodeling, and fibrosis may be due to local activation of TGF-β and BMP signaling. In vitro, TGF-β and BMP-2 are potent inducers of periostin in a variety of cell types, including fibroblasts (480), smooth muscle cells (262), (268), and osteoblasts (182). FGFs, PDGF-BB and angiotensin II are also capable of inducing upregulation of periostin expression in smooth muscle cells through a PI-3K-dependent pathway (262). A variety of fibrogenic mediators induce periostin synthesis in fibroblasts. Angiotensin II increases fibroblast periostin expression by activating Erk1/2/TGF-β1 and Ras/p38 MAPK/CREB pathways (265). Moreover, the fibrogenic Th2 cytokines IL-4 and IL-13 also upregulate periostin synthesis in fibroblasts (439) and in epithelial cells (414).
3. Cellular actions
As a matricellular protein, periostin binds to the matrix and transduces signals to cells through engagement of integrins. Interactions with αvβ3 and αvβ5 signaling are involved in periostin-mediated smooth muscle cell migration (260). αvβ3 and β1 integrin pathways were involved in invasion of mesenchymal cushion cells through 3D collagen gels (64). Integrin engagement by periostin triggers activation of PI-3K, Rho-kinase and FAK-mediated pathways (329) and enhances cell motility. Furthermore, in malignant cells, acquired expression of periostin promotes cell survival by inhibiting apoptosis through αvβ3/Akt/PKB signaling (29)
4. In vivo functions of periostin
i. The phenotype of periostin null mice
Studies in periostin null mice have provided extensive information on the role of periostin in vivo. Three independent groups have generated periostin −/− animals using different approaches; these mice exhibited similar phenotypic characteristics (363), (222), (327), (338). Rios and co-workers generated periostin knockouts through replacement of the first exon with a lacZ reporter gene (PostnlacZ/lacZ mice) (363), whereas Oka et al. induced a deletion of exons 4 through 10 encoding three of the four fasciclin domains (Postn −/− mice) (338); a third group also used a deletion strategy to generate periostin null animals (222). Despite the robust expression of periostin in the embryonic periosteum, periodontal ligaments, cardiac valves and placenta, PostnlacZ/lacZ and Postn −/− pups appeared grossly normal. However after 3–4 weeks of age, periostin knockouts exhibited significant growth retardation and were consistently smaller than WT littermates. About 15% of PostnlacZ/lacZ mutants died within 2–3 weeks after birth (363). Moreover, while PostnlacZ/lacZ males were fertile, female PostnlacZ/lacZ mice were unable to become pregnant (363). PostnlacZ/lacZ mice also had characteristic dental defects exhibiting ameloblast alterations and a marked impairment in the structural organization of enamel. Interventions that reduced mechanical strain on the periodontal ligament, such as feeding null animals with a soft diet, reduced growth retardation and improved female fertility, but had no effect on postnatal lethality. Thus, impaired function of the periodontal ligaments had profound consequences on growth and phenotype in periostin null animals, but did not explain their increased mortality. Later studies demonstrated that early death in a minority of periostin null animals was due to cardiac defects.
ii. Periostin and collagen fibrillogenesis
Periostin binds directly to matrix proteins (including type I and type V collagen, fibronectin and tenascin-C) (439) and regulates assembly of collagen fibrils modulating the biomechanical properties of connective tissues (327). Periostin null mice (PostnlacZ/lacZ) had significantly reduced collagen fibril diameters in the dermis associated with reduced levels of cross-linking (327). Impaired matrix assembly in periostin null animals resulted in lower tensile strength of the skin. Moreover, the alterations in collagen fibrillogenesis observed in the absence of periostin significantly affect the mechanical properties of the valves.
iii. Periostin in tissue repair and fibrosis
Periostin upregulation is observed in wound repair and in fibrotic conditions (511). A TGF-β-inducible gene, periostin exerts potent fibrogenic actions in vivo. In the subepithelial fibrosis associated with asthma, periostin appears to play an important role by enhancing pro-fibrotic TGF-β signaling (414). The effects of periostin on fibrous tissue deposition are of particular importance in cardiac homeostasis and in the pathogenesis of fibrotic cardiomyopathies.
5. Periostin and the heart (Table 6)
Table 6.
Expression and role of periostin in normal and diseased hearts
| Expression | Role | |
|---|---|---|
| Cardiac development | Highly expressed in the embryonic myocardium. Although absent from cells of cardiomyocyte lineage, periostin is a marker for mesenchymal cells. In adult hearts periostin expression decreases and is primarily localized in the tendinous supporting structures of the valves (236), (220), (328). | In contrast to most other matricellular proteins, periostin plays an important role in cardiac development. Periostin null mice exhibit increased early postnatal mortality due to valvular defects and had atrial septal defects (419). During development periostin promotes mesenchymal to fibroblast differentiation while inhibiting cardiomyocyte differentiation; because of the critical role of fibroblast-like cells in valve formation, periostin null mice have defective valves (330), (331). |
| Neonatal cardiac remodeling | Periostin expression is high in neonatal hearts and is primarily expressed in fibroblasts. | Adaptive changes of the myocardium occurring during the neonatal period include a 2-fold increase in fibroblast numbers and maturation of the endomysial collagenous network. Periostin is critically involved in modulation of fibroblast phenotype in neonatal hearts (338), (326). However, these effects have limited functional consequences: systolic function is preserved in adult periostin null mice, whereas chamber dimensions are slightly reduced. |
| Myocardial infarction | Marked and prolonged upregulation of periostin is noted in infarcted hearts and is primarily localized in infarct and border zone fibroblasts (427), (338), (410). | Both loss-of-function and gain-of-function studies demonstrated that periostin protects the infarcted heart from cardiac rupture by promoting migration and activation of myofibroblasts (427), (338). Increased incidence of rupture in periostin null mice is associated with reduced fibroblast recruitment and impaired matrix deposition. However, prolonged expression of periostin in the infarct may be detrimental. Periostin −/− mice surviving the acute phase had significantly reduced fibrosis and attenuated systolic dysfunction 8 weeks after the acute infarct. |
| Cardiac hypertrophy and fibrosis | Periostin expression is consistently elevated in experimental models of cardiac hypertrophy and fibrosis (426). In contrast, physiologic adaptive hypertrophy due to strenuous exercise does not increase myocardial periostin expression (338) | Periostin upregulation in the myocardium activates cardiac fibroblasts, inducing their migration and transdifferentiation, but also induces cardiomyocyte hypertrophy that may be mediated through integrin signaling. As a result periostin loss is associated with reduced myocardial fibrosis and attenuated hypertrophy in models of pressure overload (338). |
| Valvular disease | Valve thickening in mice fed a high-fat diet is associated with periostin upregulation (173). | Emerging evidence suggests a role for periostin in acquired valvular conditions. Periostin absence attenuates valve thickening in mice fed a high fat diet reducing fibrosis and decreasing MMP expression (173). |
| Periostin in human heart disease | Limited evidence is available on the expression and role of periostin in human heart disease. High levels of myocardial periostin were found in patients with advanced heart failure (426) and in a patient with acute myocardial infarction (410). Periostin expression in diseased human valves seems to be dependent on the underlying pathology. | |
i. Periostin expression in the heart
Periostin is highly expressed in the embryonic mouse and chicken heart (236), (328), (220) throughout cardiovascular development. Strong expression of periostin was observed in E10.5 embryonic mouse hearts and was predominantly localized within the enlarging outflow tract and in the atrioventricular endocardial cushions (236). Periostin is absent from cells of the cardiomyocyte lineage, but is a marker of mesenchymal cells that have undergone epithelial to mesenchymal transformation and is transiently expressed in the epicardial mesenchyme of the embryonic heart. Thus, in the developing myocardium periostin is expressed in cardiac fibroblasts, valvular attachment apparatus, chordate tendinae and epicardial/pericardial structures (419), (102).
ii. Role of periostin in development and functional integrity of the cardiac valves
Loss-of-function experiments demonstrated that periostin plays an important role in maturation of non-cardiomyocyte lineages and in stabilization of the extracellular matrix in the mouse heart, and is essential for integrity of the cardiac valves (419). Adult periostin null (PostnlacZ/lacZ) mice had mitral and tricuspid leaflets that were significantly shortened and thickened when compared with wildtype mice. Importantly, the early postnatal mortality in a minority (15%) of PostnlacZ/lacZ mice was due to valvular disease: neonatal hearts in mice dying before weaning had abnormal geometry, while the valves were shorter than in surviving animals and exhibited areas of discontinuity. The truncated valve leaflets found in PostnlacZ/lacZ valves contained islets of cardiomyocytes and smooth muscle cells and exhibited disorganized matrix stratification and impaired TGF-β signaling. An independent investigation produced similar findings: all periostin null mice had large primary atrial septal defects, and exhibited defective valve leaflets and chordae tendinae (330). In the absence of periostin, organization of the valvular matrix was markedly perturbed. Experiments in chicken embryos using adenoviral-mediated knockdown of periostin in the atrioventricular mesenchyme suggested that periostin promotes mesenchymal to fibroblast differentiation while blocking cardiomyocyte differentiation (331). Because formation of a functionally mature valve requires differentiation of valvular mesenchymal cells into fibroblasts, periostin is a critical mediator in valve formation and may be involved in human valvular pathologies (81). A recent study suggested that the absence of periostin significantly attenuates valve thickening, annular fibrosis and MMP expression in thickened valves of mice fed a high-fat diet (173). Thus, beyond its role in congenital valve defects, periostin may also be important in the pathogenesis of acquired valvular conditions.
iii. The role of periostin in physiologic neonatal cardiac remodeling
Physiological remodeling of the heart during the neonatal period is an adaptive process characterized by a twofold increase in the number of fibroblasts and by the formation, alignment and maturation of an endomysial collagenous network (326), (43), (42), (166). Periostin expression in normal myocardium is high during the neonatal period, but subsequently falls to low levels. Because of its known function in fibroblast differentiation and its high levels of expression in the neonatal myocardium, periostin may play an important role in maturation and differentiation of fibroblasts in the developing neonatal heart. This concept was supported by the identification of a large population of undifferentiated mesenchymal-like cells in 3-month old periostin null hearts (326). Moreover, Affymetrix microarray analysis in adult periostin −/− hearts demonstrated significant alterations in a large number of genes associated with fibrosis, matrix remodeling and adhesion (338) suggesting an altered cardiac fibroblast gene program. However, these alterations appear to have limited consequences on function and geometry of the adult heart: 2–6 month-old periostin −/− mice have slightly reduced chamber dimensions while exhibiting normal systolic function (338). Diastolic function in periostin −/− animals has not been systematically studied.
iv. Periostin in myocardial infarction
Myocardial injury triggers a marked upregulation of periostin in the cardiac interstitium. Stanton and co-workers first demonstrated marked periostin induction in a mouse model of myocardial infarction (427). Increased periostin protein expression was observed 4 days after acute infarction in mice and appeared to persist for several weeks after the acute event (338). Periostin was exclusively localized in fibroblasts infiltrating the infarct and the border zone (Figure 11) (338), (410). Loss- and gain-of-function approaches were used to study the role of periostin in the infarcted heart. Two independent studies demonstrated that periostin −/− mice had an increased incidence of cardiac rupture in the first 10 days after myocardial infarction (Figure 11) (338), (410); adenovirus-mediated gene transfer of a spliced form of periostin protected knockout mice from rupture (410). Defective repair in these animals was associated with impaired collagen fibrillogenesis and reduced myofibroblast accumulation (410). However surviving animals had better preserved systolic function 8 weeks after the acute event (338). Protection from dysfunction in periostin null mice during the remodeling phase was associated with attenuation of the inflammatory response and significantly reduced fibrosis of the infarcted heart. Periostin overexpression, on the other hand, did not increase fibrosis, but protected the infarcted mice from cardiac rupture (338).
Figure 11. Role of periostin in myocardial infarction.
A–B. Periostin expression if upregulated in the infarcted myocardium and is primarily localized in the infarct border zone (A) and in the remodeling myocardium (B). The dashed red line shows the infarct border zone. C. Periostin null mice exhibit a high incidence of cardiac rupture. D. However, surviving periostin −/− mice had attenuated dilative post-infarction remodeling. E. Reduced remodeling in periostin null mice was associated with attenuated fibroblast infiltration and smaller and less abundant collagen fibers in the infarct border zone. Data reproduced Data reproduced with permission from: Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 2008;205:295:303: Copyright 2008, Rockefeller University Press.
The findings suggest that periostin upregulation plays an important role in early scar formation, promoting migration and activation of myofibroblasts and assembly of a matrix network. In its absence, impaired early repair results in cardiac rupture. However, the prolonged expression of periostin in the infarcted heart may have deleterious effects inducing expansion of fibrosis and increasing dysfunction.
In a recent investigation Kuhn and co-workers demonstrated that extracellular periostin induced cycle re-entry in differentiated cardiomyocytes (237) through activation of αv, β1, β3 and β5 integrins in the cardiomyocyte surface. In vivo, injection of periostin in mice undergoing infarction protocols improved cardiac function and stimulated cardiomyocyte proliferation reducing cardiac fibrosis (237). These surprising findings indicated that periostin may trigger cardiac regeneration without inducing fibrogenic actions challenging established concepts in the field (122). However, experiments in periostin null and periostin overexpressing mice and in vitro studies examining the effects of periostin on cardiomyocytes failed to support these observations suggesting that periostin does not affect cardiomyocyte content and cell cycle activity (272).
v. Periostin in cardiac hypertrophy and fibrosis
Increased myocardial expression of periostin is consistently found in experimental models of cardiac hypertrophy and fibrosis (426), (470) and in the dilated cardiomyopathy due to isolated volume overload (87). Hypertrophy due to pressure overload induced by transverse aortic constriction (426), (470) and the hypertrophic cardiomyopathy associated with mutations of the α cardiac myosin heavy chain gene were associated with cardiac periostin upregulation (447). In contrast, physiologic adaptive hypertrophy in mice due to strenuous exercise did not increase myocardial periostin expression. In vivo investigations suggested that periostin is an important mediator in the pathogenesis of cardiac hypertrophy and fibrosis. In the pressure-overloaded heart periostin loss results in preservation of function, less fibrosis and attenuated hypertrophy (338). Periostin deficiency also attenuated fibrosis in mice with genetically-induced hypertrophic cardiomyopathy (447). Gain-of function studies have been somewhat less consistent. Periostin overexpressing animals had accentuated hypertrophy after pressure overload without exhibiting functional decompensation and fibrosis (338). Moreover, injection of adenovirus encoding an alternatively spliced form of periostin in the rat myocardium induced a modest hypertrophic response (270). In contrast, rats transfected with the periostin gene exhibited cardiomyocyte loss, cardiac dysfunction, chamber dilation and fibrosis (217). The findings suggest that periostin upregulation in the heart exerts potent actions on cardiac fibroblasts, inducing their migration, differentiation and activation, and modulating their adhesive properties. Moreover, periostin may also trigger cardiomyocyte hypertrophy through activation of integrin-mediated pathways.
vi. Periostin in human heart disease
Knowledge on the expression of periostin in human cardiac pathophysiologic conditions remains limited and fragmented. Some preliminary evidence suggests that the observations made in animal models on the upregulation of periostin in the remodeling heart may be extended in human patients. High expression of periostin was noted in the myocardium of a patient with acute myocardial infarction (410). Moreover, high levels of myocardial periostin were found in patients with advanced heart failure; unloading of the ventricle after implantation of a LVAD significantly reduced periostin expression (426). Evidence on the expression of periostin in valve disease is also very limited and the findings appear to be dependent on the underlying pathology. In adult patients with atherosclerotic and rheumatic valve disease periostin expression was markedly elevated in the subendothelial layer of the valve (173). In contrast to these findings, periostin expression was reported to be markedly reduced in the valves of infants with congenital bicuspid aortic valve stenosis (419).
F. The CCN family of matricellular proteins
The CCN family (76), (254) (Table 7) was named after its first three prototypical members: cysteine-rich protein 61 (Cyr61, CCN1), connective tissue growth factor (CTGF, CCN2) and nephroblastoma overexpressed protein (Nov, CCN3). Three additional CCNs (CCN4, CCN5, CCN6) were identified later as Wnt-inducible secreted proteins. CCNs were initially considered polypeptide growth factors that exert the full range of their functions when bound to cells (48). However, the collective work of several laboratories over the last 15 years, and the development of genetically targeted mice supported the role of the CCNs as matricellular proteins that bind to the matrix and modulate cellular functions through interactions with cell adhesion receptors (76), (254), (249), (498). It is now widely accepted that, although CCN proteins have some independent activity, they act primarily by modifying signaling of other molecules in a context-dependent manner.
Table 7.
Role of the CCN family of matricellular proteins in heart disease
| Role in cardiac homeostasis | Role in heart disease | |
|---|---|---|
| CCN1 | CCN1 −/− mice die during the embryonic period exhibiting defective vessel formation and large AVSDs. 20% of CCN1 +/− have ostium primum ASDs (294), (295). | CCN1 is rapidly upregulated in experimental models of myocardial infarction and pressure overload hypertrophy. Although in vitro experiments suggest potential effects on inflammatory leukocytes, fibroblasts and cardiomyocytes, the role of CCN1 in cardiac remodeling remains unknown. In a model of autoimmune myocarditis CCN1 overexpression attenuated cardiac inflammation (179), (485), (502), (372). |
| CCN2 | CCN2 −/− mice die minutes after birth due to respiratory failure induced by skeletal abnormalities (204). |
Myocardial infarction: CCN2 is markedly upregulated in the infarcted myocardium; its induction is mediated through angiotensin II and TGF-β/Smad3 signaling. In vitro studies and CCN2 overexpression experiments suggest functions in potentiation of TGF—β signaling, profibrotic and angiogenic actions and pro-survival effects on cardiomyocytes (82), (9), (110), (117), (336), (8). Cardiac hypertrophy and fibrosis: CCN2 is consistently upregulated in models of cardiac hypertrophy and fibrosis. CCN2 may mediate hypertrophic and pro-fibrotic actions potentiating TGF-β-mediated effects. CCN2 overexpression studies suggest that by itself CCN2 is not sufficient to induce fibrosis (205), (134), (476), (473), (10), (501), (344), (41), (475). |
| CCN3 | CCN3 −/− mice exhibit septal hypertrophy, ventricular dilation and ectopic septal calcifications (178). | Not known |
| CCN4 | Not known | Myocardial infarction: CCN4 is upregulated in the infarcted heart. Although in vitro studies suggest that CCN4 stimulates fibroblast proliferation and transduces hypertrophic and pro-survival signals in cardiomyocytes, the in vivo significance of these observations is unclear (101), (464), (465). |
| CCN5 | Not known | Cardiac hypertrophy and fibrosis: CCN5 overexpression inhibits hypertrophy and fibrosis induced by cardiac pressure overload attenuating TGF-β/Smad3 signaling (501). Due to the absence of the CT domain, CCN5 appears to act as a dominant negative form of CCN2. |
| CCN6 | No effects | Not known |
1. Structure
CCN proteins share significant structural homology, including an N-terminal secretory signal peptide, followed by four modules: four insulin-like growth factor binding protein (IGFBP) domains (module I), a von Willebrand factor type C repeat (vWC, module II), a TSP type I repeat (module III) and a C-terminal domain that contains a cysteine knot motif (module IV) (44). Binding sites for various integrins have been identified in modules II, III and IV. Modules II and III are linked by a proteolysis-sensitive hinge region.
2. Synthesis, expression, and regulation
The mechanisms of regulation have been characterized in detail for CCN2. CCN2 expression is markedly upregulated by TGF-β stimulation (255) in a variety of cell types, including fibroblasts (181), (189) osteoblasts (345), endothelial cells (489) and smooth muscle cells (368). TGF-β1-mediated CCN2 expression requires the action of Smad proteins (181); a functional Smad element is present within the CCN2 promoter. Smad3 and Smad4 signaling enhance CTGF promoter activity inducing its upregulation, whereas Smad7, an inhibitory Smad, suppresses TGF-β-stimulated CCN2 synthesis (181). Angiotensin II also induces rapid upregulation of CCN2 synthesis in fibroblasts through activation of MAPK signaling (206). Endothelin-1 is another potent inducer of CCN2 in fibroblasts (494), smooth muscle cells (368) and cardiomyocytes (219).
Information on gene regulation for other members of the CCN family is limited. TGF-β appears to be an important regulator of CCN synthesis acting as an inducer of CCN1, CCN4 and CCN5 expression (378), (345), but as a suppressor of CCN3 synthesis (364), (244).
3. Cellular actions of the CCNs
i. CCNs act through binding to integrins and heparan sulfate proteoglycans (HSPGs)
As typical matricellular proteins, CCNs are capable of regulating cell survival, adhesion, differentiation, proliferation and phenotype through binding to cell adhesion receptors, including integrins and HSPGs. Kireeva and co-workers first provided evidence for direct binding of a CCN family member (CCN1) and an integrin (αvβ3); this interaction mediated endothelial cell adhesion (223). Since this report, several other integrin/CCN interactions have been identified as mediators of CCN functions. Moreover, in certain situations CCN binding to HSPGs is important for transduction of integrin signaling; in particular the HSPG syndecan-4 is involved in CCN-mediated functions in fibroblasts (85).
ii. Effects of the CCN proteins on cell adhesion, survival and migration
CCN proteins consistently promote cell adhesion through interactions involving integrins and HSPGs. CCN1 and CCN2 transduce adhesive signaling in human dermal fibroblasts through α6β1/HSPG and activate FAK, paxillin and Rac leading to reorganization of the cytoskeleton, formation of filopodia and lamellipodia and induction of MMP-1 and MMP-3 (83). Thus, in addition to their adhesive effects CCNs also modulate the matrix-degrading capacity of fibroblasts regulating matrix metabolism.
The effects of CCNs on cell migration are also well-documented. Extensive evidence suggests that CCN1, CCN2 and CCN3 stimulate cell migration of endothelial cells (24), (267) and fibroblasts (171); in contrast, CCN4 and CCN5 inhibit cell migration (422), (245). CCN proteins also modulate mitogenesis in a cell-type specific manner. The effects of CCN2 on fibroblast proliferation are somewhat controversial: early investigations have suggested proliferative effects (48), whereas other studies showed that CCN1, CCN2 and CCN3 do not exert direct mitogenic actions, but may enhance the proliferative effects of other mediators (171), (170). The proliferative actions of CCNs may be mediated through the carboxyterminal domain; CCN5, which lacks this domain attenuates cell proliferation (245).
The effects of CCNs on cell survival are context- and cell type-dependent. Although adhesion of cells to the matrix generally promotes survival, some members of the CCN family are capable of inducing apoptosis in certain cell types while supporting adhesive signaling. CCN1 induces apoptosis in fibroblasts through an α6β1/syndecan-4-mediated interaction, but promotes survival of endothelial cells (452).
iii. CCNs interact with cytokines and growth factors modulating their biological actions
Extensive evidence suggests that CCN2 binds to TGF-β through the vWC domain (module II) (4) and potentiates its effects. Accentuation of TGF-β actions by CCN2 is due, not only to an increase in its effective concentration, but also to modification of TGF-β signaling. In vitro experiments using embryonic fibroblasts demonstrated that CCN2 is required for a subset of the responses to TGF-β (409). About 1/3 of TGF-β-inducible genes were not upregulated in CCN2 null fibroblasts, including genes involved in adhesion and matrix remodeling (409). In contrast to enhancing TGF-β-mediated functions, CCNs inhibit BMP actions (4). CCN2 also interacts with VEGF inhibiting its binding to VEGFR2; proteolysis of the CCN2/VEGF complex through MMP activation releases active VEGF. Thus, CCNs regulate bioavailable growth factors in response to microenvironmental cues (176). Another example of CCN-mediated modulation of cytokine actions is the interaction between CCN1 and TNF-α. CCN1 can unmask the cytotoxic effects of TNF-α leading to rapid apoptosis of otherwise resistant primary human fibroblasts (78).
4. In vivo effects of the CCNs
i. CCNs and embryonic development
Generation of CCN1, CCN2 and CCN3 null mice resulted in an explosion in our knowledge of their in vivo functions; in contrast, much less is known about the other three members of the CCN family. In contrast to other matricellular proteins, CCN1 plays an essential role in embryonic development. CCN1 null mice suffer embryonic death; approximately one third succumb to failure in chorioallantoic fusion, while the reminder die due to placental vascular insufficiency and impaired vascular integrity (295). CCN1 knockouts also had defective cardiac development exhibiting severe atrioventricular septal defects (AVSD). CCN1 +/− mice were viable, but 20% of them had an ostium primum atrial septal defect (294).
CCN2 null mice, on the other hand, were recovered among neonates in the expected Mendelian ratio, but died within minutes of birth (204) due to respiratory failure caused by skeletal defects. CCN2 deficiency resulted in severe chondrodysplasia due to impaired chondrocyte proliferation, defective matrix production in the cartilage and reduced growth plate angiogenesis (204). CCN3 null mice have also been generated; these animals produced no detectable full-length CCN3, but expressed low amounts of a mutant CCN3 that lacked the vWC domain (178). More than 50% of CCN3 −/− mice died during the embryonic or perinatal period. CCN3 −/− mice surviving into adulthood were viable and fertile, but exhibited severe skeletal abnormalities and joint malformations. Cardiac development was also affected; CCN3 mutants had septal defects and developed hypertrophic cardiomyopathy associated with chamber dilation and calcifications in the septum (178). Although loss-of-function mutations in CCN6 cause progressive pseudorheumatoid dysplasia, a degenerative condition of the joints in human patients, surprisingly CCN6 null mice (240) and CCN6 overexpressing animals (318) are normal. Thus, it appears that CCN6 has very distinct roles in mice and humans and is not required for murine skeletal growth. In addition to their role in embryonic development, CCNs serve as key regulators in several pathophysiologic conditions.
ii. CCNs regulate tissue repair and fibrosis
Expression of CCN1, CCN2 and CCN3 is upregulated in healing wounds (189), (77) (267). CCN1 and CCN2 may mediate adhesion of infiltrating inflammatory cells and may exert modulatory effects on many cell types involved in tissue repair. CCN1 may induce pro-inflammatory genes in macrophages (26), while activating a genetic program for wound healing in fibroblasts (77), upregulating synthesis of genes involved in adhesion, angiogenesis and matrix metabolism. Recent experiments suggested that CCN1 may induce fibroblast senescence, limiting fibrosis in cutaneous wound healing (214). Through its role in modulating growth factor signaling, its effects on angiogenesis and on fibroblast function, CCN2 may be a key mediator in wound healing. However, relatively little is known regarding the in vivo significance of CCN2-mediated interactions in healing wounds. A recent study suggested an important role for CCN2 in connective tissue repair demonstrating that in a model of calvarial healing in the rat, CCN2 directed fibroblast differentiation rather than ectopic mineralization (257).
Several lines of evidence suggest that CCN2 is strongly associated with tissue fibrosis (57). A TGF-β-inducible gene, CCN2 is markedly upregulated in experimental models of fibrosis regardless of the etiology and the affected organ (172), (248), (247). CCN2 upregulation is also prominent in human fibrotic conditions, such as scleroderma (3). In vivo studies have suggested that CCN2 potentiates TGF-β-mediated fibrogenic actions. When injected into the subcutaneous tissue of newborn mice, TGF-β or CCN2 alone induced only transient granulation tissue formation (299). Application of both CCN2 and TGF-β was required for sustained fibrotic response (299). Moreover, “fibrosis-resistant” BALB/c mice do not show induction of CCN2 upon stimulation with bleomycin, but can be rendered “fibrosis-sensitive” by overexpression of CCN2 (40). CCN2 knockdown by siRNA inhibition attenuated fibrotic remodeling in a rat model of carbon tetrachloride-induced hepatic fibrosis (263) and CCN2 blockade attenuated fibrosis in a model of crescentic glomerulonephritis (215).
Much less is known regarding the role of other CCNs in the fibrotic process. In contrast to the profibrotic actions of CCN2, CCN1 may limit fibrosis by inducing fibroblast senescence (214). CCN3 may also exert anti-fibrotic actions by reducing CCN2 synthesis and by negatively regulating matrix deposition (364).
iii. CCN proteins and angiogenesis
CCN proteins also regulate the angiogenic response in a dose- and context-dependent manner. Recombinant CCN1, CCN2 and CCN3 promote angiogenesis in various in vivo assays (23), (24), (267). The significance of these effects is illustrated by the phenotype of CCN null animals: CCN1 null mice die during the embryonal period exhibiting either a complete failure in chorioallantoic membrane fusion, or placental vascular insufficiency and impaired vessel integrity (295). CCN2 knockouts also exhibit angiogenic defects in the growth plates during bone formation (204). The role of CCNs in pathophysiologic conditions associated with angiogenesis in adults is less well understood. However, evidence suggests that angiogenesis regulation by members of the CCN family may play an important role in cancer (39).
5. CCNs in cardiac homeostasis and disease (Table 7)
i. CCN1
The phenotypic abnormalities observed in mice with targeted disruption of CCN1 demonstrated its essential role in cardiac development. Nullizygosity in CCN1 resulted in severe vascular defects, large atrioventricular septal defects and embryonic lethality (294), (295). Although heterozygous CCN1 +/− mice were viable and fertile, 20% of them had ostium primum ASDs (294). CCN1 deficiency resulted in precocious apoptosis in the cushion tissue proximal to the atrial septum, a site of CCN1 expression. Thus, CCN1 absence deprived cushion tissue cells from essential pro-survival signaling. Moreover, reduced gelatinase activity was observed in CCN1 null hearts. Because CCN1 induces MMP-2 in cardiomyocytes, deficient gelatinase activity in CCN1 −/− hearts may compromise tissue remodeling required for fusion of the septum and endocardial cushion tissue (294). The role of CCN1 in cardiac development is supported by the identification of a human AVSD susceptibility gene in the same region where CCN1 has been mapped (1p21-p31) (408).
In adult animals, CCN1 is markedly upregulated in the remodeling heart. Mechanical stretch and pressure overload induce CCN1 synthesis in cardiomyocytes both in vitro and in vivo; CCN1 upregulation is dependent on AT1 signaling. (179). Rapid CCN1 induction is also observed in a mouse model of myocardial infarction, peaking between one and six hours after coronary occlusion (179). The relevance of these findings in human disease were supported by markedly increased CCN1 expression in cardiomyocytes from hearts with end-stage ischemic cardiomyopathy (179) and in patients with dilated cardiomyopathy (485). Although CCN1 expression is increased in response to injury, its role in cardiac repair and remodeling remains unknown. In vitro experiments demonstrated that CCN1 protects cardiomyocytes from apoptosis due to oxidative stress (502). A recent investigation demonstrated that CCN1 modulates cardiac inflammatory responses. Adenovirus-mediated CCN1 overexpression in a model of murine autoimmune myocarditis attenuated cardiac inflammation; in vitro CCN1 diminished transwell migration of monocytes to chemokine gradients (372). In addition, the anti-fibrotic and angiogenic actions of CCN1 may be important in regulation of the reparative response. However, the significance of these interactions in cardiac injury has not been studied.
ii. CCN2
CCN2 is the best-studied member of the CCN family in the cardiovascular system. The generation of genetically targeted mice did not reveal an essential role for CCN2 in cardiac development. However, the marked and consistent upregulation of CCN2 in models of cardiac injury, hypertrophy and fibrosis and its profound effects on cardiomyocytes, fibroblasts and endothelial cells suggest that it may play an important role in cardiac remodeling (253), (107).
a. CCN2 is upregulated following myocardial infarction
Postnatally cardiac CCN2 expression is restricted to the atrium (96). However, CCN2 synthesis in the ventricular myocardium is markedly upregulated in rat and mouse models of myocardial infarction (82), (9), (110), (117) and is localized in myofibroblasts and cardiomyocytes in the viable border zone (336). TGF-β/Smad3 signaling, angiotensin II and endothelin-1 may be important in mediating CCN2 upregulation in the infarcted heart. Experiments in Smad3 −/− mice suggested that late, but not early, CCN2 upregulation was dependent on Smad3 signaling (117). The role of angiotensin II in mediating CCN2 upregulation in infarcts is less established: Ahmed and co-workers demonstrated that AT1 blockade prevented CCN2 upregulation in the infarcted myocardium (9); however, in another study ACE inhibition did not affect CCN2 synthesis in the infarct (110). The role of endothelin-1 is suggested mostly by in vitro studies demonstrating its effects in upregulating CCN2 synthesis by neonatal rat cardiomyocytes (219) and by cultured adult mouse atrial-muscle HL-1 cells (358). The function of endogenous CCN2 in myocardial infarction remains poorly understood. Potential effects of CCN2 in the infarct may include potentiation of TGF-β signaling to promote fibrous tissue deposition, angiogenic actions and direct effects on fibroblast phenotype and function. When subjected to ischemia/reperfusion, mice with cardiac-specific CCN2 overexpression had a marked reduction in infarct size. CCN2 treatment or overexpression activated the Akt/p70 S6 kinase/GSK-3beta salvage kinase pathway in vitro and in vivo and induced cardioprotective genes. These findings suggested that, in addition to its effects on growth factor signalling, fibrosis and angiogenesis, CCN2 may also exert protective actions on cardiomyocytes (8).
b. CCN2 in cardiac hypertrophy and fibrosis
CCN2 upregulation has been demonstrated in many different models of fibrosis, heart failure and cardiac hypertrophy. Marked and consistent CCN2 induction is found in cardiac hypertrophy due to pressure overload, or angiotensin II infusion (205), (134), in models of diabetic cardiomyopathy (476), in a porcine model of pacing-induced cardiomyopathy (10), in aging-associated murine cardiac fibrosis (473), in the mdx mouse model of dystrophic cardiomyopathy (22) and in a model of cardiac fibrosis due to viral myocarditis (247). Much like in the infarcted heart, TGF-β, angiotensin II and endothelin-1 may be involved in mediating CCN2 upregulation in the hypertrophied ventricle. Angiotensin II-mediated cardiac hypertrophy and fibrosis is associated with CCN2 upregulation (134); CCN2 expression colocalizes with TGF-β1 and collagen in areas exhibiting leukocyte infiltration, interstitial and perivascular fibrosis. Angiotensin-II-mediated CCN2 upregulation in vivo is dependent on AT1 signaling and on PKC-delta activation (177). CCN2 expression is also upregulated in models of pressure overload hypertrophy and fibrosis; its expression is induced through activation of angiotensin II/AT1 signaling (205). Recent studies have identified pathways responsible for suppression of CCN2 synthesis that may play a role in limiting pro-fibrotic actions. CCN2 upregulation in left ventricular hypertrophy is regulated by two major cardiac microRNAs (miRNA), miR-133 and miR-30. In cultured cardiomyocytes and fibroblasts; knockdown of these miRNAs increased CCN2 expression; in vivo, decreased expression of the miRNAs was associated with enhanced CCN2 synthesis, hypertrophy and fibrosis (124). Kruppel-like Factor (KLF15) also may play a role in negative regulation of CCN2, mediating repression of the CCN2 promoter in fibroblasts and inhibiting basal and TGF-β-induced CCN2 expression (469).
Despite extensive associative evidence documenting CCN2 upregulation in areas of cardiac hypertrophy and fibrosis, its role in mediating cardiac remodeling remains poorly understood. Three independent groups have developed transgenic mice with cardiomyocyte-specific CCN2 overexpression. Yoon and co-workers found that CCN2-overexpressing mice have normal left ventricular mass and cardiac morphology at baseline (501), but exhibit an accentuated hypertrophic and fibrotic response in the pressure overloaded ventricle. Panek and co-workers also found that cardiac CCN2 overexpression did not induce significant fibrosis; however, their animals developed dilated cardiomyopathy and cardiac dysfunction at the age of 7 months (344). Independently Ahmed and co-workers also generated mice with cardiac restricted CCN2 overexpression (8). These animals had an inconspicuous increase in collagen, but no evidence of dysfunction. The absence of baseline cardiac fibrosis and hypertrophy upon CCN2 overexpression suggests that CCN2 by itself does not induce fibrosis. However, in the cardiac response to stress or injury, induction of CCN2 may potentiate TGF-β-mediated actions accentuating fibrosis and hypertrophy. Unfortunately, systematic loss-of-function investigations testing this hypothesis are lacking. Support for the role of CCN2 in fibrotic cardiac remodelling was provided by a study demonstrating that CCN2 neutralization attenuated graft fibrosis in a mouse model of cardiac allograft rejection (41).
Recent investigations suggested a role for CCN2 in diabetic cardiomyopathy. CCN2 upregulation in the diabetic heart is associated with increased TGF-β expression and with the development of fibrosis (476). In vitro, the hypertrophic effects of high glucose and palmitate on neonatal rat cardiomyocytes were attenuated by CCN2 knockdown (475). However, whether CCN2 is involved in the fibrotic and hypertrophic changes observed in diabetic cardiomyopathy remains unknown.
c. CCN2 as a biomarker in human heart disease
Because of its marked induction in cardiac fibrosis and hypertrophy, CCN2 could serve as a biomarker for patients with conditions associated with cardiac remodeling (256). In biopsies from patients with heart failure due to diastolic dysfunction intense CCN2 immunoreactivity was noted in areas of fibrosis; CCN2 expression correlated with the extent of fibrotic remodeling (224). Plasma CCN2 levels were elevated in patients with chronic heart failure, correlated with plasma BNP levels and TGF-β levels and served as indicators of matrix remodeling (225).
iii. CCN3
Little is known about the role of CCN3 in cardiac homeostasis and disease. CCN3 null mice had enlargement and abnormal modeling of the endocardial cushions associated with atrial septal defects (178). Morphologic assessment of the adult CCN3 null heart suggested the development of septal hypertrophy, ventricular dilation and ectopic septal calcifications; functional analysis has not been performed. The basis for these developmental defects remains unclear.
iv. CCN4
CCN4 expression is increased following myocardial infarction and is primarily localized in the infarct border zone and in the remote remodeling myocardium (101), (464). TNF-α is a potent inducer of CCN4 and may be responsible for its upregulation in the infarcted myocardium (464). Although the role of CCN4 in vivo has not been investigated, in vitro studies have suggested important actions on cardiomyocytes and cardiac fibroblasts. CCN4 stimulates cardiomyocyte hypertrophy (101) and enhances cardiac fibroblast proliferation (101). Moreover, CCN4 appears to play an important role in regulation of TNF-α actions, mediating the profibrotic effects of the cytokine, but inhibiting TNF-α-induced cardiomyocyte death (464). Recent experiments demonstrating protective effects of CCN4 against doxorubicin-mediated cardiomyocyte apoptosis further supported its pro-survival functions (465).
v. CCN5
In contrast to the hypertrophic actions of CCN2, CCN5 inhibits phenylephrine-induced cardiomyocyte hypertrophy in vitro. In vivo, transgenic mice overexpressing CCN5 had significant inhibition of the hypertrophic and fibrotic response triggered by pressure overload; these effects were associated with attenuated TGF-β/Smad signaling (501). The effects of CCN5 are due to the absence of the CT domain, which is responsible for the hypertrophic actions of CCN2. Thus, CCN5 appears to act as a naturally occurring dominant negative molecule (501).
G. Proteins exhibiting some matricellular functions
In addition to the prototypical members of the matricellular protein family discussed above, several other proteins exhibit matricellular functions (Table 1). Some of these molecules also modulate biological functions acting as soluble mediators or as intracellular signals; thus, the in vivo significance of their matricellular effects is unclear. Of these proteins, the galectins, syndecans and PAI-1 play important roles in cardiac pathophysiology.
1. Galectins
Several members of the galectin family have been identified as matricellular proteins (127). For example, the matricellular actions of galectin-8 are well-characterized (259), (512): when immobilized to the matrix galectin-8 promotes cell adhesion activating integrin-mediated cascades. In contrast, as a soluble ligand galectin-8 has deadhesive effects. Galectin-3 is the best-studied member of the family in cardiovascular disease. Using microarray analysis Sharma and co-workers identified galectin-3 as the most robustly overexpressed gene in failing versus compensated hearts from transgenic hypertensive Ren-2 rats (407). Galectin-3 was primarily expressed by activated infiltrating macrophages. Infusion of low-dose galectin-3 into the pericardial sac of healthy rats caused ventricular dysfunction, inducing fibroblast proliferation and matrix deposition (407). A growing body of evidence suggests that galectin-3 may be a promising biomarker in patients with heart failure. Elevated plasma galectin-3 levels improved the prognostic ability of aminoterminal pro-brain natriuretic peptide (NT-proBNP) in patients with acute heart failure (462). Plasma galectin-3 levels were also elevated in ambulatory patients with heart failure and predicted prognosis in unadjusted analysis, but not in multivariable modeling that included NT-proBNP levels (132).
2. Syndecans
The syndecans are widely expressed transmembrane heparan sulfate proteoglycans that modulate biological responses by regulating integrin-mediated adhesive interactions and by presenting growth factors to their primary receptors (486). Syndecans are composed of an extracellular ectodomain, a conserved transmembrane domain and a short cytoplasmic domain (131). Ectodomains can be shed from cells and often bind to the matrix where they may sequester growth factors or compete for cell surface binding; this property of syndecan fragments explains why syndecans have been occasionally considered matricellular proteins (375). There has been a recent surge of interest in the role of syndecans in heart disease. Increased expression of syndecan-1 in the infarcted heart exerted protective effects against dilative remodeling, attenuating inflammation and preventing excessive matrix degradation (463). In the angiotensin II-treated heart syndecan-1 activates a pro-fibrotic program (144) enhancing matrix deposition and CCN2 expression (395). Syndecan-4 is also upregulated in the infarcted heart (264). Two independent studies have suggested protective effects of syndecan-4 during the early phase after acute myocardial infarction (284), (125). The mechanism for syndecan-4-mediated protection remains unclear; however, actions enhancing myofibroblast transdifferentiation and facilitating growth factor-dependent endothelial cell proliferation have been implicated (284).
3. PAI-1
PAI-1, a serine protease inhibitor, critically regulates matrix metabolism. Extensive evidence suggests that PAI-1 is involved in cardiac repair and fibrosis. PAI-1 −/− mice exhibited a high incidence of early cardiac rupture following infarction, related to accentuated proteolytic activity (20), and were protected from the development of fibrosis during the late reparative phase (440). Defective regulation of uPA activity in PAI-1 null mice is associated with spontaneous age-associated fibrosis in the myocardium, but not in other organs (301). Fibrotic remodeling of the PAI-1 null heart may involve spontaneous activation of TGF-β signaling and enhanced endothelial-to-mesenchymal transition (161). In addition to the established role of soluble PAI-1 as an inhibitor of proteolytic pathways, matricellular functions of matrix-bound PAI-1 have also been described (280). When bound to matrix proteins PAI-1 acts as part of a transitory anchoring complex regulating cell adhesion and migration (280). The contribution of the matricellular actions of PAI-1 in mediating its effects on the myocardium remains unknown.
IV. Conclusions
Discoveries made over the last 20 years have transformed our understanding on the role of the extracellular matrix in heart disease. Experimental studies have demonstrated that cardiac injury not only causes alterations of the structural matrix proteins, but also induces upregulation of matricellular proteins that play an essential role in regulating cellular responses to injury. Our knowledge, mostly derived through loss-of-function and overexpression studies, remains largely limited to the effects of specific matricellular proteins on cardiac function and geometry in various pathophysiologic conditions. The mechanisms of action of matricellular proteins in cardiac injury have not been dissected. However, our growing understanding of the functional properties of matricellular proteins allows some general observations regarding their function in the remodeling myocardium:
First, matricellular protein expression in the remodeling heart is tightly regulated. Growth factors, such as TGF-β, angiotensin II and FGFs induce expression of several members of the matricellular family. Matricellular proteins are then incorporated into the matrix where they potentiate growth factor-mediated effects. Thus, the increased expression of growth factors in the remodeling myocardium triggers synthesis of the mediators necessary to facilitate their actions and to transduce their effects into the various cell types involved in the reparative process. Upregulation of matricellular proteins in uncomplicated cardiac repair is transient, but may be prolonged in the presence of a persistent insult. Disappearance of matricellular proteins from the myocardium marks the end of the dynamic phase of tissue remodeling, indicating a state of relative quiescence in the cardiac interstitium.
Second, through direct interactions with structural extracellular matrix proteins, certain members of the matricellular family (such as TSP-2, SPARC and OPN) may play an important role in assembly and organization of the matrix in the injured myocardium. Most matricellular proteins also modulate the structure of the extracellular matrix through indirect effects on growth factor and protease activity.
Third, binding of the matricellular proteins to matrix components, growth factors, and cellular integrins may serve to localize transduction of specific signaling pathways in the areas of injury. Although direct evidence for the role of these interactions in cardiac remodeling is lacking, extensive associative data support this model. Certain matricellular proteins (such as TSP-1 and tenascin-C) show a strikingly selective localization in the matrix of the infarct border zone, a site of active tissue remodeling, characterized by intense activation of TGF-β signaling and marked accumulation of myofibroblasts. The unique spatial distribution of matricellular proteins highlights their potential impact on the reparative process.
Fourth, the de-adhesive actions of some members of the matricellular family (such as the TSPs, tenascin-C and SPARC) may play an important role in regulating motility of reparative cells while promoting an intermediate state of adhesion that prevents their apoptotic death. In the dynamic environment of the infarcted and remodeling myocardium these actions may be essential for cell migration into the healing wound.
Clearly, a lot remains to be learned on the role of the matricellular proteins in the heart. Although loss-of-function studies have suggested that several members of the matricellular family are important regulators of cardiac repair and remodeling, in most cases the specific cellular actions responsible for these effects have not been identified. Because all matricellular proteins are capable of exerting multiple effects through several distinct functional domains, dissection of the role of specific molecular interactions and identification of the cellular targets will be laborious and challenging. However, this knowledge could provide a unique opportunity to identify specific peptides derived from matricellular proteins that mediate important actions in vivo, and can be used therapeutically to optimize repair and to prevent adverse remodeling. Furthermore, the list of matricellular proteins with an important role in cardiac diseases is likely to be expanded as our knowledge on the basic biology of cell:matrix interactions continues to increase.
Perhaps the most challenging goal in advancing the field of matricellular proteins is to translate knowledge obtained from experimental studies into the human cardiac pathobiology. Because matricellular proteins bind to the cardiac extracellular matrix where they exert most of their actions, assessment of their expression in the human heart requires tissue sampling. Due to their negligible expression in the normal heart and intense upregulation in areas of injury and repair, certain members of the matricellular protein family may prove clinically relevant markers of cardiac remodeling. In particular tenascin-C is an excellent indicator of interstitial remodeling in pathologic samples from cardiomyopathic hearts (149) and a marker of activity in patients with acute myocarditis (300). Due to their selective localization in areas of injury, matricellular proteins are generally not useful as biomarkers of cardiac injury in the serum, with the possible exception of OPN, tenascin-C and CCN2 which are also secreted in the bloodstream.
The biology of matricellular proteins is complex; however, understanding their role and mechanism of action may eventually provide important new treatment modalities for patients with heart disease. Members of the matricellular family (including TSP-1, TSP-2, SPARC, and OPN) exert protective actions on the infarcted myocardium by regulating the reparative response; on the other hand in models of chronic pressure overload several matricellular proteins contribute to the development of fibrosis. Identification of the functional domains and pathways responsible for the effects of matricellular proteins will allow design of peptides that selectively reproduce specific protective actions, or inhibit detrimental profibrotic effects. Because of their localized and context-dependent function, matricellular protein-derived peptides may be effective and safe approaches to reduce cardiac remodeling.
Acknowledgments
FUNDING SOURCES: Dr Frangogiannis laboratory is funded by NIH grants R01 HL-76246 and R01 HL-85440, by the Wilf Family Cardiovascular Research Institute and by the Edmond J Safra/Republic National Bank of New York chair in cardiovascular medicine.
References
- 1.Abe K, Hibino T, Mishima H, Shimomura Y. The cytokine regulation of SPARC production by rabbit corneal epithelial cells and fibroblasts in vitro. Cornea. 2004;23:172–179. doi: 10.1097/00003226-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abraham D. Connective tissue growth factor: growth factor, matricellular organizer, fibrotic biomarker or molecular target for anti-fibrotic therapy in SSc? Rheumatology (Oxford) 2008;47 (Suppl 5):v8–9. doi: 10.1093/rheumatology/ken278. [DOI] [PubMed] [Google Scholar]
- 4.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adams JC. Thrombospondins: multifunctional regulators of cell interactions. Annu Rev Cell Dev Biol. 2001;17:25–51. doi: 10.1146/annurev.cellbio.17.1.25. [DOI] [PubMed] [Google Scholar]
- 6.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agah A, Kyriakides TR, Lawler J, Bornstein P. The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol. 2002;161:831–839. doi: 10.1016/S0002-9440(10)64243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahmed MS, Gravning J, Martinov VN, von Lueder TG, Edvardsen T, Czibik G, Moe IT, Vinge LE, Oie E, Valen G, Attramadal H. Mechanisms of Novel Cardioprotective Functions of CCN2/CTGF in Myocardial Ischemia/Reperfusion Injury. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00604.2010. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed MS, Oie E, Vinge LE, Yndestad A, Oystein Andersen G, Andersson Y, Attramadal T, Attramadal H. Connective tissue growth factor--a novel mediator of angiotensin II-stimulated cardiac fibroblast activation in heart failure in rats. J Mol Cell Cardiol. 2004;36:393–404. doi: 10.1016/j.yjmcc.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed MS, von Lueder TG, Oie E, Kjekshus H, Attramadal H. Induction of myocardial connective tissue growth factor in pacing-induced heart failure in pigs. Acta Physiol Scand. 2005;184:27–36. doi: 10.1111/j.1365-201X.2005.01416.x. [DOI] [PubMed] [Google Scholar]
- 11.Aimes RT, Quigley JP. Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J Biol Chem. 1995;270:5872–5876. doi: 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- 12.Alford AI, Hankenson KD. Matricellular proteins: Extracellular modulators of bone development, remodeling, and regeneration. Bone. 2006;38:749–757. doi: 10.1016/j.bone.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 14.Anversa P, Palackal T, Sonnenblick EH, Olivetti G, Meggs LG, Capasso JM. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ Res. 1990;67:871–885. doi: 10.1161/01.res.67.4.871. [DOI] [PubMed] [Google Scholar]
- 15.Arber S, Caroni P. Thrombospondin-4, an extracellular matrix protein expressed in the developing and adult nervous system promotes neurite outgrowth. J Cell Biol. 1995;131:1083–1094. doi: 10.1083/jcb.131.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnlov J, Evans JC, Benjamin EJ, Larson MG, Levy D, Sutherland P, Siwik DA, Wang TJ, Colucci WS, Vasan RS. Clinical and echocardiographic correlates of plasma osteopontin in the community: the Framingham Heart Study. Heart. 2006;92:1514–1515. doi: 10.1136/hrt.2005.081406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 18.Ashizawa N, Graf K, Do YS, Nunohiro T, Giachelli CM, Meehan WP, Tuan TL, Hsueh WA. Osteopontin is produced by rat cardiac fibroblasts and mediates A(II)-induced DNA synthesis and collagen gel contraction. J Clin Invest. 1996;98:2218–2227. doi: 10.1172/JCI119031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ashkar S, Weber GF, Panoutsakopoulou V, Sanchirico ME, Jansson M, Zawaideh S, Rittling SR, Denhardt DT, Glimcher MJ, Cantor H. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 20.Askari AT, Brennan ML, Zhou X, Drinko J, Morehead A, Thomas JD, Topol EJ, Hazen SL, Penn MS. Myeloperoxidase and plasminogen activator inhibitor 1 play a central role in ventricular remodeling after myocardial infarction. J Exp Med. 2003;197:615–624. doi: 10.1084/jem.20021426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atalar E, Ozturk E, Ozer N, Haznedaroglu IC, Kepez A, Coskun S, Aksoyek S, Ovunc K, Kes S, Kirazli S, Ozmen F. Plasma soluble osteopontin concentrations are increased in patients with rheumatic mitral stenosis and associated with the severity of mitral valve calcium. Am J Cardiol. 2006;98:817–820. doi: 10.1016/j.amjcard.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 22.Au CG, Butler TL, Sherwood MC, Egan JR, North KN, Winlaw DS. Increased connective tissue growth factor associated with cardiac fibrosis in the mdx mouse model of dystrophic cardiomyopathy. Int J Exp Pathol. 2011;92:57–65. doi: 10.1111/j.1365-2613.2010.00750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin alphavbeta3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bai S, Shi X, Yang X, Cao X. Smad6 as a transcriptional corepressor. J Biol Chem. 2000;275:8267–8270. doi: 10.1074/jbc.275.12.8267. [DOI] [PubMed] [Google Scholar]
- 26.Bai T, Chen CC, Lau LF. Matricellular protein CCN1 activates a proinflammatory genetic program in murine macrophages. J Immunol. 2010;184:3223–3232. doi: 10.4049/jimmunol.0902792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bale MD, Mosher DF. Effects of thrombospondin on fibrin polymerization and structure. J Biol Chem. 1986;261:862–868. [PubMed] [Google Scholar]
- 28.Ballard VL, Sharma A, Duignan I, Holm JM, Chin A, Choi R, Hajjar KA, Wong SC, Edelberg JM. Vascular tenascin-C regulates cardiac endothelial phenotype and neovascularization. Faseb J. 2006;20:717–719. doi: 10.1096/fj.05-5131fje. [DOI] [PubMed] [Google Scholar]
- 29.Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu M, Shao R, Anderson RM, Rich JN, Wang XF. Periostin potently promotes metastatic growth of colon cancer by augmenting cell survival via the Akt/PKB pathway. Cancer Cell. 2004;5:329–339. doi: 10.1016/s1535-6108(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 30.Barry ST, Ludbrook SB, Murrison E, Horgan CM. A regulated interaction between alpha5beta1 integrin and osteopontin. Biochem Biophys Res Commun. 2000;267:764–769. doi: 10.1006/bbrc.1999.2032. [DOI] [PubMed] [Google Scholar]
- 31.Basu A, Kligman LH, Samulewicz SJ, Howe CC. Impaired wound healing in mice deficient in a matricellular protein SPARC (osteonectin, BM-40) BMC Cell Biol. 2001;2:15. doi: 10.1186/1471-2121-2-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Batlle M, Perez-Villa F, Lazaro A, Garcia-Pras E, Vallejos I, Sionis A, Castel MA, Roig E. Decreased expression of thrombospondin-1 in failing hearts may favor ventricular remodeling. Transplant Proc. 2009;41:2231–2233. doi: 10.1016/j.transproceed.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: friend or foe? Am J Physiol Heart Circ Physiol. 2006;291:H1015–1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 34.Bein K, Simons M. Thrombospondin type 1 repeats interact with matrix metalloproteinase 2. Regulation of metalloproteinase activity. J Biol Chem. 2000;275:32167–32173. doi: 10.1074/jbc.M003834200. [DOI] [PubMed] [Google Scholar]
- 35.Belmadani S, Bernal J, Wei CC, Pallero MA, Dell’italia L, Murphy-Ullrich JE, Berecek KH. A Thrombospondin-1 Antagonist of Transforming Growth Factor-{beta} Activation Blocks Cardiomyopathy in Rats with Diabetes and Elevated Angiotensin II. Am J Pathol. 2007 doi: 10.2353/ajpath.2007.070056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bentley AA, Adams JC. The evolution of thrombospondins and their ligand-binding activities. Mol Biol Evol. 2010;27:2187–2197. doi: 10.1093/molbev/msq107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berk BC, Fujiwara K, Lehoux S. ECM remodeling in hypertensive heart disease. J Clin Invest. 2007;117:568–575. doi: 10.1172/JCI31044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bleau AM, Planque N, Perbal B. CCN proteins and cancer: two to tango. Front Biosci. 2005;10:998–1009. doi: 10.2741/1594. [DOI] [PubMed] [Google Scholar]
- 40.Bonniaud P, Martin G, Margetts PJ, Ask K, Robertson J, Gauldie J, Kolb M. Connective tissue growth factor is crucial to inducing a profibrotic environment in “fibrosis-resistant” BALB/c mouse lungs. Am J Respir Cell Mol Biol. 2004;31:510–516. doi: 10.1165/rcmb.2004-0158OC. [DOI] [PubMed] [Google Scholar]
- 41.Booth AJ, Csencsits-Smith K, Wood SC, Lu G, Lipson KE, Bishop DK. Connective tissue growth factor promotes fibrosis downstream of TGFbeta and IL-6 in chronic cardiac allograft rejection. Am J Transplant. 2010;10:220–230. doi: 10.1111/j.1600-6143.2009.02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Borg TK, Ranson WF, Moslehy FA, Caulfield JB. Structural basis of ventricular stiffness. Lab Invest. 1981;44:49–54. [PubMed] [Google Scholar]
- 43.Borg TK, Rubin K, Lundgren E, Borg K, Obrink B. Recognition of extracellular matrix components by neonatal and adult cardiac myocytes. Dev Biol. 1984;104:86–96. doi: 10.1016/0012-1606(84)90038-1. [DOI] [PubMed] [Google Scholar]
- 44.Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125–130. doi: 10.1016/0014-5793(93)80155-n. [DOI] [PubMed] [Google Scholar]
- 45.Bornstein P. Diversity of function is inherent in matricellular proteins: an appraisal of thrombospondin 1. J Cell Biol. 1995;130:503–506. doi: 10.1083/jcb.130.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal. 2009;3:163–165. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- 48.Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bradshaw AD. The role of SPARC in extracellular matrix assembly. J Cell Commun Signal. 2009;3:239–246. doi: 10.1007/s12079-009-0062-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Boggs J, Lacy JM, Zile MR. Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing. Circulation. 2009;119:269–280. doi: 10.1161/CIRCULATIONAHA.108.773424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradshaw AD, Baicu CF, Rentz TJ, Van Laer AO, Bonnema DD, Zile MR. Age-dependent alterations in fibrillar collagen content and myocardial diastolic function: role of SPARC in post-synthetic procollagen processing. Am J Physiol Heart Circ Physiol. 2010;298:H614–622. doi: 10.1152/ajpheart.00474.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bradshaw AD, Graves DC, Motamed K, Sage EH. SPARC-null mice exhibit increased adiposity without significant differences in overall body weight. Proc Natl Acad Sci U S A. 2003;100:6045–6050. doi: 10.1073/pnas.1030790100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bradshaw AD, Puolakkainen P, Dasgupta J, Davidson JM, Wight TN, Helene Sage E. SPARC-null mice display abnormalities in the dermis characterized by decreased collagen fibril diameter and reduced tensile strength. J Invest Dermatol. 2003;120:949–955. doi: 10.1046/j.1523-1747.2003.12241.x. [DOI] [PubMed] [Google Scholar]
- 54.Bradshaw AD, Reed MJ, Carbon JG, Pinney E, Brekken RA, Sage EH. Increased fibrovascular invasion of subcutaneous polyvinyl alcohol sponges in SPARC-null mice. Wound Repair Regen. 2001;9:522–530. doi: 10.1046/j.1524-475x.2001.00522.x. [DOI] [PubMed] [Google Scholar]
- 55.Bradshaw AD, Reed MJ, Sage EH. SPARC-null mice exhibit accelerated cutaneous wound closure. J Histochem Cytochem. 2002;50:1–10. doi: 10.1177/002215540205000101. [DOI] [PubMed] [Google Scholar]
- 56.Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. doi: 10.1016/s0945-053x(00)00105-0. [DOI] [PubMed] [Google Scholar]
- 57.Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal. 2010;4:1–4. doi: 10.1007/s12079-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor beta(1)heterozygous mice. J Mol Cell Cardiol. 2000;32:187–195. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- 59.Bruemmer D, Collins AR, Noh G, Wang W, Territo M, Arias-Magallona S, Fishbein MC, Blaschke F, Kintscher U, Graf K, Law RE, Hsueh WA. Angiotensin II-accelerated atherosclerosis and aneurysm formation is attenuated in osteopontin-deficient mice. J Clin Invest. 2003;112:1318–1331. doi: 10.1172/JCI18141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bujak M, Dobaczewski M, Chatila K, Mendoza LH, Li N, Reddy A, Frangogiannis NG. Interleukin-1 receptor type I signaling critically regulates infarct healing and cardiac remodeling. Am J Pathol. 2008;173:57–67. doi: 10.2353/ajpath.2008.070974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bujak M, Dobaczewski M, Gonzalez-Quesada C, Xia Y, Leucker T, Zymek P, Veeranna V, Tager AM, Luster AD, Frangogiannis NG. Induction of the CXC chemokine interferon-gamma-inducible protein 10 regulates the reparative response following myocardial infarction. Circ Res. 2009;105:973–983. doi: 10.1161/CIRCRESAHA.109.199471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bujak M, Frangogiannis NG. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential Role of Smad3 in Infarct Healing and in the Pathogenesis of Cardiac Remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 64.Butcher JT, Norris RA, Hoffman S, Mjaatvedt CH, Markwald RR. Periostin promotes atrioventricular mesenchyme matrix invasion and remodeling mediated by integrin signaling through Rho/PI 3-kinase. Dev Biol. 2007;302:256–266. doi: 10.1016/j.ydbio.2006.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Camino AM, Atorrasagasti C, Maccio D, Prada F, Salvatierra E, Rizzo M, Alaniz L, Aquino JB, Podhajcer OL, Silva M, Mazzolini G. Adenovirus-mediated inhibition of SPARC attenuates liver fibrosis in rats. J Gene Med. 2008;10:993–1004. doi: 10.1002/jgm.1228. [DOI] [PubMed] [Google Scholar]
- 66.Canver CC, Gregory RD, Cooler SD, Voytovich MC. Association of osteopontin with calcification in human mitral valves. J Cardiovasc Surg (Torino) 2000;41:171–174. [PubMed] [Google Scholar]
- 67.Carey WA, Taylor GD, Dean WB, Bristow JD. Tenascin-C deficiency attenuates TGF-ss-mediated fibrosis following murine lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299:L785–793. doi: 10.1152/ajplung.00385.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Carlson CB, Lawler J, Mosher DF. Structures of thrombospondins. Cell Mol Life Sci. 2008;65:672–686. doi: 10.1007/s00018-007-7484-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chablais F, Veit J, Rainer G, Jazwinska A. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol. 2011;11:21. doi: 10.1186/1471-213X-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chandrasekhar S, Harvey AK, Johnson MG, Becker GW. Osteonectin/SPARC is a product of articular chondrocytes/cartilage and is regulated by cytokines and growth factors. Biochim Biophys Acta. 1994;1221:7–14. doi: 10.1016/0167-4889(94)90209-7. [DOI] [PubMed] [Google Scholar]
- 71.Chang W, Wei K, Jacobs SS, Upadhyay D, Weill D, Rosen GD. SPARC suppresses apoptosis of idiopathic pulmonary fibrosis fibroblasts through constitutive activation of beta-catenin. J Biol Chem. 2010;285:8196–8206. doi: 10.1074/jbc.M109.025684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Charest A, Pepin A, Shetty R, Cote C, Voisine P, Dagenais F, Pibarot P, Mathieu P. Distribution of SPARC during neovascularisation of degenerative aortic stenosis. Heart. 2006;92:1844–1849. doi: 10.1136/hrt.2005.086595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chatila K, Ren G, Xia Y, Huebener P, Bujak M, Frangogiannis NG. The role of the thrombospondins in healing myocardial infarcts. Cardiovasc Hematol Agents Med Chem. 2007;5:21–27. doi: 10.2174/187152507779315813. [DOI] [PubMed] [Google Scholar]
- 74.Chaulet H, Lin F, Guo J, Owens WA, Michalicek J, Kesteven SH, Guan Z, Prall OW, Mearns BM, Feneley MP, Steinberg SF, Graham RM. Sustained augmentation of cardiac alpha1A-adrenergic drive results in pathological remodeling with contractile dysfunction, progressive fibrosis and reactivation of matricellular protein genes. J Mol Cell Cardiol. 2006;40:540–552. doi: 10.1016/j.yjmcc.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 75.Chellaiah MA, Hruska KA. The integrin alpha(v)beta(3) and CD44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int. 2003;72:197–205. doi: 10.1007/s00223-002-1025-6. [DOI] [PubMed] [Google Scholar]
- 76.Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771–783. doi: 10.1016/j.biocel.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen CC, Mo FE, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001;276:47329–47337. doi: 10.1074/jbc.M107666200. [DOI] [PubMed] [Google Scholar]
- 78.Chen CC, Young JL, Monzon RI, Chen N, Todorovic V, Lau LF. Cytotoxicity of TNFalpha is regulated by integrin-mediated matrix signaling. Embo J. 2007;26:1257–1267. doi: 10.1038/sj.emboj.7601596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen FH, Thomas AO, Hecht JT, Goldring MB, Lawler J. Cartilage oligomeric matrix protein/thrombospondin 5 supports chondrocyte attachment through interaction with integrins. J Biol Chem. 2005;280:32655–32661. doi: 10.1074/jbc.M504778200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen H, Herndon ME, Lawler J. The cell biology of thrombospondin-1. Matrix Biol. 2000;19:597–614. doi: 10.1016/s0945-053x(00)00107-4. [DOI] [PubMed] [Google Scholar]
- 81.Chen JH, Simmons CA. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: critical roles for matricellular, matricrine, and matrix mechanics cues. Circ Res. 2011;108:1510–1524. doi: 10.1161/CIRCRESAHA.110.234237. [DOI] [PubMed] [Google Scholar]
- 82.Chen MM, Lam A, Abraham JA, Schreiner GF, Joly AH. CTGF expression is induced by TGF- beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 83.Chen N, Chen CC, Lau LF. Adhesion of human skin fibroblasts to Cyr61 is mediated through integrin alpha 6beta 1 and cell surface heparan sulfate proteoglycans. J Biol Chem. 2000;275:24953–24961. doi: 10.1074/jbc.M003040200. [DOI] [PubMed] [Google Scholar]
- 84.Chen W, Frangogiannis NG. The role of inflammatory and fibrogenic pathways in heart failure associated with aging. Heart Fail Rev. 2010;15:415–422. doi: 10.1007/s10741-010-9161-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Y, Abraham DJ, Shi-Wen X, Pearson JD, Black CM, Lyons KM, Leask A. CCN2 (connective tissue growth factor) promotes fibroblast adhesion to fibronectin. Mol Biol Cell. 2004;15:5635–5646. doi: 10.1091/mbc.E04-06-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen Y, Bal BS, Gorski JP. Calcium and collagen binding properties of osteopontin, bone sialoprotein, and bone acidic glycoprotein-75 from bone. J Biol Chem. 1992;267:24871–24878. [PubMed] [Google Scholar]
- 87.Chen YW, Pat B, Gladden JD, Zheng J, Powell P, Wei CC, Cui X, Husain A, Dell’italia LJ. Dynamic molecular and histopathological changes in the extracellular matrix and inflammation in the transition to heart failure in isolated volume overload. Am J Physiol Heart Circ Physiol. 2011;300:H2251–2260. doi: 10.1152/ajpheart.01104.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng W, Reiss K, Li P, Chun MJ, Kajstura J, Olivetti G, Anversa P. Aging does not affect the activation of the myocyte insulin-like growth factor-1 autocrine system after infarction and ventricular failure in Fischer 344 rats. Circ Res. 1996;78:536–546. doi: 10.1161/01.res.78.4.536. [DOI] [PubMed] [Google Scholar]
- 89.Chiba S, Okamoto H, Kon S, Kimura C, Murakami M, Inobe M, Matsui Y, Sugawara T, Shimizu T, Uede T, Kitabatake A. Development of atherosclerosis in osteopontin transgenic mice. Heart Vessels. 2002;16:111–117. doi: 10.1007/s003800200005. [DOI] [PubMed] [Google Scholar]
- 90.Chiquet-Ehrismann R, Chiquet M. Tenascins: regulation and putative functions during pathological stress. J Pathol. 2003;200:488–499. doi: 10.1002/path.1415. [DOI] [PubMed] [Google Scholar]
- 91.Chiquet-Ehrismann R, Kalla P, Pearson CA, Beck K, Chiquet M. Tenascin interferes with fibronectin action. Cell. 1988;53:383–390. doi: 10.1016/0092-8674(88)90158-4. [DOI] [PubMed] [Google Scholar]
- 92.Chiquet-Ehrismann R, Tannheimer M, Koch M, Brunner A, Spring J, Martin D, Baumgartner S, Chiquet M. Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J Cell Biol. 1994;127:2093–2101. doi: 10.1083/jcb.127.6.2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chiquet M, Fambrough DM. Chick myotendinous antigen. I. A monoclonal antibody as a marker for tendon and muscle morphogenesis. J Cell Biol. 1984;98:1926–1936. doi: 10.1083/jcb.98.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chlenski A, Cohn SL. Modulation of matrix remodeling by SPARC in neoplastic progression. Semin Cell Dev Biol. 2010;21:55–65. doi: 10.1016/j.semcdb.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 95.Chung CY, Murphy-Ullrich JE, Erickson HP. Mitogenesis, cell migration, and loss of focal adhesions induced by tenascin-C interacting with its cell surface receptor, annexin II. Mol Biol Cell. 1996;7:883–892. doi: 10.1091/mbc.7.6.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chuva de Sousa Lopes SM, Feijen A, Korving J, Korchynskyi O, Larsson J, Karlsson S, ten Dijke P, Lyons KM, Goldschmeding R, Doevendans P, Mummery CL. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn. 2004;231:542–550. doi: 10.1002/dvdy.20162. [DOI] [PubMed] [Google Scholar]
- 97.Clark RA, Erickson HP, Springer TA. Tenascin supports lymphocyte rolling. J Cell Biol. 1997;137:755–765. doi: 10.1083/jcb.137.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. Regulation of collagen degradation in the rat myocardium after infarction. J Mol Cell Cardiol. 1995;27:1281–1292. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 99.Cleutjens JP, Verluyten MJ, Smiths JF, Daemen MJ. Collagen remodeling after myocardial infarction in the rat heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 100.Collins AR, Schnee J, Wang W, Kim S, Fishbein MC, Bruemmer D, Law RE, Nicholas S, Ross RS, Hsueh WA. Osteopontin modulates angiotensin II-induced fibrosis in the intact murine heart. J Am Coll Cardiol. 2004;43:1698–1705. doi: 10.1016/j.jacc.2003.11.058. [DOI] [PubMed] [Google Scholar]
- 101.Colston JT, de la Rosa SD, Koehler M, Gonzales K, Mestril R, Freeman GL, Bailey SR, Chandrasekar B. Wnt-induced secreted protein-1 is a prohypertrophic and profibrotic growth factor. Am J Physiol Heart Circ Physiol. 2007;293:H1839–1846. doi: 10.1152/ajpheart.00428.2007. [DOI] [PubMed] [Google Scholar]
- 102.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008;9:548–555. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Corbett SA, Schwarzbauer JE. Fibronectin-fibrin cross-linking: a regulator of cell behavior. Trends Cardiovasc Med. 1998;8:357–362. doi: 10.1016/s1050-1738(98)00028-0. [DOI] [PubMed] [Google Scholar]
- 104.Crawford SE, Stellmach V, Murphy-Ullrich JE, Ribeiro SM, Lawler J, Hynes RO, Boivin GP, Bouck N. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93:1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 105.Crossin KL. Cytotactin binding: inhibition of stimulated proliferation and intracellular alkalinization in fibroblasts. Proc Natl Acad Sci U S A. 1991;88:11403–11407. doi: 10.1073/pnas.88.24.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dahiya S, Givvimani S, Bhatnagar S, Qipshidze N, Tyagi SC, Kumar A. Osteopontin-stimulated expression of matrix metalloproteinase-9 causes cardiomyopathy in the mdx model of Duchenne muscular dystrophy. J Immunol. 2011;187:2723–2731. doi: 10.4049/jimmunol.1101342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Daniels A, van Bilsen M, Goldschmeding R, van der Vusse GJ, van Nieuwenhoven FA. Connective tissue growth factor and cardiac fibrosis. Acta Physiol (Oxf) 2009;195:321–338. doi: 10.1111/j.1748-1716.2008.01936.x. [DOI] [PubMed] [Google Scholar]
- 108.Dannenberg AL, Levy D, Garrison RJ. Impact of age on echocardiographic left ventricular mass in a healthy population (the Framingham Study) Am J Cardiol. 1989;64:1066–1068. doi: 10.1016/0002-9149(89)90816-3. [DOI] [PubMed] [Google Scholar]
- 109.Dawson DW, Pearce SF, Zhong R, Silverstein RL, Frazier WA, Bouck NP. CD36 mediates the In vitro inhibitory effects of thrombospondin-1 on endothelial cells. J Cell Biol. 1997;138:707–717. doi: 10.1083/jcb.138.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dean RG, Balding LC, Candido R, Burns WC, Cao Z, Twigg SM, Burrell LM. Connective tissue growth factor and cardiac fibrosis after myocardial infarction. J Histochem Cytochem. 2005;53:1245–1256. doi: 10.1369/jhc.4A6560.2005. [DOI] [PubMed] [Google Scholar]
- 111.Del Ry S, Giannessi D, Maltinti M, Cabiati M, Prontera C, Iervasi A, Caselli C, Mazzone AM, Neglia D. Increased plasma levels of osteopontin are associated with activation of the renin-aldosterone system and with myocardial and coronary microvascular damage in dilated cardiomyopathy. Cytokine. 2010;49:325–330. doi: 10.1016/j.cyto.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 112.Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. Osteopontin as a means to cope with environmental insults: regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 2001;107:1055–1061. doi: 10.1172/JCI12980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Devaux Y, Azuaje F, Vausort M, Yvorra C, Wagner DR. Integrated protein network and microarray analysis to identify potential biomarkers after myocardial infarction. Funct Integr Genomics. 2010;10:329–337. doi: 10.1007/s10142-010-0169-0. [DOI] [PubMed] [Google Scholar]
- 114.Dewald O, Frangogiannis NG, Zoerlein M, Duerr GD, Klemm C, Knuefermann P, Taffet G, Michael LH, Crapo JD, Welz A, Entman ML. Development of murine ischemic cardiomyopathy is associated with a transient inflammatory reaction and depends on reactive oxygen species. Proc Natl Acad Sci U S A. 2003;100:2700–2705. doi: 10.1073/pnas.0438035100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dewald O, Zymek P, Winkelmann K, Koerting A, Ren G, Abou-Khamis T, Michael LH, Rollins BJ, Entman ML, Frangogiannis NG. CCL2/Monocyte Chemoattractant Protein-1 regulates inflammatory responses critical to healing myocardial infarcts. Circ Res. 2005;96:881–889. doi: 10.1161/01.RES.0000163017.13772.3a. [DOI] [PubMed] [Google Scholar]
- 116.DiPietro LA, Nissen NN, Gamelli RL, Koch AE, Pyle JM, Polverini PJ. Thrombospondin 1 synthesis and function in wound repair. Am J Pathol. 1996;148:1851–1860. [PMC free article] [PubMed] [Google Scholar]
- 117.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324:475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- 119.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. 2010;48:504–511. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dobaczewski M, Xia Y, Bujak M, Gonzalez-Quesada C, Frangogiannis NG. CCR5 signaling suppresses inflammation and reduces adverse remodeling of the infarcted heart, mediating recruitment of regulatory T cells. Am J Pathol. 2010;176:2177–2187. doi: 10.2353/ajpath.2010.090759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Dorn GW., 2nd Periostin and myocardial repair, regeneration, and recovery. N Engl J Med. 2007;357:1552–1554. doi: 10.1056/NEJMcibr074816. [DOI] [PubMed] [Google Scholar]
- 123.Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- 124.Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–178. 176p. doi: 10.1161/CIRCRESAHA.108.182535. following 178. [DOI] [PubMed] [Google Scholar]
- 125.Echtermeyer F, Harendza T, Hubrich S, Lorenz A, Herzog C, Mueller M, Schmitz M, Grund A, Larmann J, Stypmann J, Schieffer B, Lichtinghagen R, Hilfiker-Kleiner D, Wollert KC, Heineke J, Theilmeier G. Syndecan-4 signalling inhibits apoptosis and controls NFAT activity during myocardial damage and remodelling. Cardiovasc Res. 2011;92:123–131. doi: 10.1093/cvr/cvr149. [DOI] [PubMed] [Google Scholar]
- 126.El-Karef A, Yoshida T, Gabazza EC, Nishioka T, Inada H, Sakakura T, Imanaka-Yoshida K. Deficiency of tenascin-C attenuates liver fibrosis in immune-mediated chronic hepatitis in mice. J Pathol. 2007;211:86–94. doi: 10.1002/path.2099. [DOI] [PubMed] [Google Scholar]
- 127.Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA. Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci. 2007;64:1679–1700. doi: 10.1007/s00018-007-7044-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Engelmann GL, Campbell SE, Rakusan K. Immediate postnatal rat heart development modified by abdominal aortic banding: analysis of gene expression. Mol Cell Biochem. 1996;163–164:47–56. doi: 10.1007/BF00408640. [DOI] [PubMed] [Google Scholar]
- 129.Etoh T, Joffs C, Deschamps AM, Davis J, Dowdy K, Hendrick J, Baicu S, Mukherjee R, Manhaini M, Spinale FG. Myocardial and interstitial matrix metalloproteinase activity after acute myocardial infarction in pigs. Am J Physiol Heart Circ Physiol. 2001;281:H987–994. doi: 10.1152/ajpheart.2001.281.3.H987. [DOI] [PubMed] [Google Scholar]
- 130.Fassler R, Sasaki T, Timpl R, Chu ML, Werner S. Differential regulation of fibulin, tenascin-C, and nidogen expression during wound healing of normal and glucocorticoid-treated mice. Exp Cell Res. 1996;222:111–116. doi: 10.1006/excr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 131.Fears CY, Woods A. The role of syndecans in disease and wound healing. Matrix Biol. 2006;25:443–456. doi: 10.1016/j.matbio.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 132.Felker GM, Fiuzat M, Shaw LK, Clare R, Whellan DJ, Bettari L, Shirolkar SC, Donahue M, Kitzman DW, Zannad F, Pina IL, O’Connor CM. Galectin-3 in Ambulatory Patients with Heart Failure: Results from the HF-ACTION Study. Circ Heart Fail. 2011 doi: 10.1161/CIRCHEARTFAILURE.111.963637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ferrari G, Sainger R, Beckmann E, Keller G, Yu PJ, Monti MC, Galloway AC, Weiss RL, Vernick W, Grau JB. Validation of plasma biomarkers in degenerative calcific aortic stenosis. J Surg Res. 2010;163:12–17. doi: 10.1016/j.jss.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Finckenberg P, Inkinen K, Ahonen J, Merasto S, Louhelainen M, Vapaatalo H, Muller D, Ganten D, Luft F, Mervaala E. Angiotensin II induces connective tissue growth factor gene expression via calcineurin-dependent pathways. Am J Pathol. 2003;163:355–366. doi: 10.1016/S0002-9440(10)63659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Fitzgerald MC, Schwarzbauer JE. Importance of the basement membrane protein SPARC for viability and fertility in Caenorhabditis elegans. Curr Biol. 1998;8:1285–1288. doi: 10.1016/s0960-9822(07)00540-4. [DOI] [PubMed] [Google Scholar]
- 136.Fluck M, Mund SI, Schittny JC, Klossner S, Durieux AC, Giraud MN. Mechano-regulated tenascin-C orchestrates muscle repair. Proc Natl Acad Sci U S A. 2008;105:13662–13667. doi: 10.1073/pnas.0805365105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forsberg E, Hirsch E, Frohlich L, Meyer M, Ekblom P, Aszodi A, Werner S, Fassler R. Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci U S A. 1996;93:6594–6599. doi: 10.1073/pnas.93.13.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Francki A, Bradshaw AD, Bassuk JA, Howe CC, Couser WG, Sage EH. SPARC regulates the expression of collagen type I and transforming growth factor-beta1 in mesangial cells. J Biol Chem. 1999;274:32145–32152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- 139.Francki A, McClure TD, Brekken RA, Motamed K, Murri C, Wang T, Sage EH. SPARC regulates TGF-beta1-dependent signaling in primary glomerular mesangial cells. J Cell Biochem. 2004;91:915–925. doi: 10.1002/jcb.20008. [DOI] [PubMed] [Google Scholar]
- 140.Francki A, Motamed K, McClure TD, Kaya M, Murri C, Blake DJ, Carbon JG, Sage EH. SPARC regulates cell cycle progression in mesangial cells via its inhibition of IGF-dependent signaling. J Cell Biochem. 2003;88:802–811. doi: 10.1002/jcb.10424. [DOI] [PubMed] [Google Scholar]
- 141.Frangogiannis NG. Chemokines in the ischemic myocardium: from inflammation to fibrosis. Inflamm Res. 2004;53:585–595. doi: 10.1007/s00011-004-1298-5. [DOI] [PubMed] [Google Scholar]
- 142.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frangogiannis NG. The pathological basis of myocardial hibernation. Histol Histopathol. 2003;18:647–655. doi: 10.14670/HH-18.647. [DOI] [PubMed] [Google Scholar]
- 144.Frangogiannis NG. Syndecan-1: a critical mediator in cardiac fibrosis. Hypertension. 2010;55:233–235. doi: 10.1161/HYPERTENSIONAHA.109.147256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frangogiannis NG, Mendoza LH, Lewallen M, Michael LH, Smith CW, Entman ML. Induction and suppression of interferon-inducible protein 10 in reperfused myocardial infarcts may regulate angiogenesis. FASEB J. 2001;15:1428–1430. doi: 10.1096/fj.00-0745fje. [DOI] [PubMed] [Google Scholar]
- 146.Frangogiannis NG, Mendoza LH, Ren G, Akrivakis S, Jackson PL, Michael LH, Smith CW, Entman ML. MCSF expression is induced in healing myocardial infarcts and may regulate monocyte and endothelial cell phenotype. Am J Physiol Heart Circ Physiol. 2003;285:H483–492. doi: 10.1152/ajpheart.01016.2002. [DOI] [PubMed] [Google Scholar]
- 147.Frangogiannis NG, Michael LH, Entman ML. Myofibroblasts in reperfused myocardial infarcts express the embryonic form of smooth muscle myosin heavy chain (SMemb) Cardiovasc Res. 2000;48:89–100. doi: 10.1016/s0008-6363(00)00158-9. [DOI] [PubMed] [Google Scholar]
- 148.Frangogiannis NG, Ren G, Dewald O, Zymek P, Haudek S, Koerting A, Winkelmann K, Michael LH, Lawler J, Entman ML. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 149.Frangogiannis NG, Shimoni S, Chang SM, Ren G, Dewald O, Gersch C, Shan K, Aggeli C, Reardon M, Letsou GV, Espada R, Ramchandani M, Entman ML, Zoghbi WA. Active interstitial remodeling: an important process in the hibernating human myocardium. J Am Coll Cardiol. 2002;39:1468–1474. doi: 10.1016/s0735-1097(02)01792-8. [DOI] [PubMed] [Google Scholar]
- 150.Frangogiannis NG, Shimoni S, Chang SM, Ren G, Shan K, Aggeli C, Reardon MJ, Letsou GV, Espada R, Ramchandani M, Entman ML, Zoghbi WA. Evidence for an active inflammatory process in the hibernating human myocardium. Am J Pathol. 2002;160:1425–1433. doi: 10.1016/S0002-9440(10)62568-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frangogiannis NG, Youker KA, Rossen RD, Gwechenberger M, Lindsey MH, Mendoza LH, Michael LH, Ballantyne CM, Smith CW, Entman ML. Cytokines and the microcirculation in ischemia and reperfusion. J Mol Cell Cardiol. 1998;30:2567–2576. doi: 10.1006/jmcc.1998.0829. [DOI] [PubMed] [Google Scholar]
- 152.Frey N, Olson EN. Cardiac hypertrophy: the good, the bad, and the ugly. Annu Rev Physiol. 2003;65:45–79. doi: 10.1146/annurev.physiol.65.092101.142243. [DOI] [PubMed] [Google Scholar]
- 153.Frolova EG, Pluskota E, Krukovets I, Burke T, Drumm C, Smith JD, Blech L, Febbraio M, Bornstein P, Plow EF, Stenina OI. Thrombospondin-4 regulates vascular inflammation and atherogenesis. Circ Res. 2010;107:1313–1325. doi: 10.1161/CIRCRESAHA.110.232371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Fujimoto N, Onishi K, Sato A, Terasaki F, Tsukada B, Nozato T, Yamada T, Imanaka-Yoshida K, Yoshida T, Ito M, Hiroe M. Incremental prognostic values of serum tenascin-C levels with blood B-type natriuretic peptide testing at discharge in patients with dilated cardiomyopathy and decompensated heart failure. J Card Fail. 2009;15:898–905. doi: 10.1016/j.cardfail.2009.06.443. [DOI] [PubMed] [Google Scholar]
- 155.Fujita N, Fujita S, Okada Y, Fujita K, Kitano A, Yamanaka O, Miyamoto T, Kon S, Uede T, Rittling SR, Denhardt DT, Saika S. Impaired angiogenic response in the corneas of mice lacking osteopontin. Invest Ophthalmol Vis Sci. 2010;51:790–794. doi: 10.1167/iovs.09-3420. [DOI] [PubMed] [Google Scholar]
- 156.Fujita T, Shiba H, Van Dyke TE, Kurihara H. Differential effects of growth factors and cytokines on the synthesis of SPARC, DNA, fibronectin and alkaline phosphatase activity in human periodontal ligament cells. Cell Biol Int. 2004;28:281–286. doi: 10.1016/j.cellbi.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 157.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 158.Gabrielsen A, Lawler PR, Yongzhong W, Steinbruchel D, Blagoja D, Paulsson-Berne G, Kastrup J, Hansson GK. Gene expression signals involved in ischemic injury, extracellular matrix composition and fibrosis defined by global mRNA profiling of the human left ventricular myocardium. J Mol Cell Cardiol. 2007;42:870–883. doi: 10.1016/j.yjmcc.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 159.Georgiadou P, Iliodromitis EK, Kolokathis F, Varounis C, Gizas V, Mavroidis M, Capetanaki Y, Boudoulas H, Kremastinos DT. Osteopontin as a novel prognostic marker in stable ischaemic heart disease: a 3-year follow-up study. Eur J Clin Invest. 2010;40:288–293. doi: 10.1111/j.1365-2362.2010.02257.x. [DOI] [PubMed] [Google Scholar]
- 160.Gersch C, Dewald O, Zoerlein M, Michael LH, Entman ML, Frangogiannis NG. Mast cells and macrophages in normal C57/BL/6 mice. Histochem Cell Biol. 2002;118:41–49. doi: 10.1007/s00418-002-0425-z. [DOI] [PubMed] [Google Scholar]
- 161.Ghosh AK, Bradham WS, Gleaves LA, De Taeye B, Murphy SB, Covington JW, Vaughan DE. Genetic deficiency of plasminogen activator inhibitor-1 promotes cardiac fibrosis in aged mice: involvement of constitutive transforming growth factor-beta signaling and endothelial-to-mesenchymal transition. Circulation. 2010;122:1200–1209. doi: 10.1161/CIRCULATIONAHA.110.955245. [DOI] [PubMed] [Google Scholar]
- 162.Giachelli CM, Bae N, Almeida M, Denhardt DT, Alpers CE, Schwartz SM. Osteopontin is elevated during neointima formation in rat arteries and is a novel component of human atherosclerotic plaques. J Clin Invest. 1993;92:1686–1696. doi: 10.1172/JCI116755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am J Pathol. 1998;152:353–358. [PMC free article] [PubMed] [Google Scholar]
- 164.Gilles C, Bassuk JA, Pulyaeva H, Sage EH, Foidart JM, Thompson EW. SPARC/osteonectin induces matrix metalloproteinase 2 activation in human breast cancer cell lines. Cancer Res. 1998;58:5529–5536. [PubMed] [Google Scholar]
- 165.Gilmour DT, Lyon GJ, Carlton MB, Sanes JR, Cunningham JM, Anderson JR, Hogan BL, Evans MJ, Colledge WH. Mice deficient for the secreted glycoprotein SPARC/osteonectin/BM40 develop normally but show severe age-onset cataract formation and disruption of the lens. Embo J. 1998;17:1860–1870. doi: 10.1093/emboj/17.7.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Goldsmith EC, Hoffman A, Morales MO, Potts JD, Price RL, McFadden A, Rice M, Borg TK. Organization of fibroblasts in the heart. Dev Dyn. 2004;230:787–794. doi: 10.1002/dvdy.20095. [DOI] [PubMed] [Google Scholar]
- 167.Golledge J, Clancy P, Maguire J, Lincz L, Koblar S. The role of tenascin C in cardiovascular disease. Cardiovasc Res. 2011;92:19–28. doi: 10.1093/cvr/cvr183. [DOI] [PubMed] [Google Scholar]
- 168.Good DJ, Polverini PJ, Rastinejad F, Le Beau MM, Lemons RS, Frazier WA, Bouck NP. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990;87:6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Grimbert P, Bouguermouh S, Baba N, Nakajima T, Allakhverdi Z, Braun D, Saito H, Rubio M, Delespesse G, Sarfati M. Thrombospondin/CD47 interaction: a pathway to generate regulatory T cells from human CD4+ CD25- T cells in response to inflammation. J Immunol. 2006;177:3534–3541. doi: 10.4049/jimmunol.177.6.3534. [DOI] [PubMed] [Google Scholar]
- 170.Grotendorst GR, Duncan MR. Individual domains of connective tissue growth factor regulate fibroblast proliferation and myofibroblast differentiation. Faseb J. 2005;19:729–738. doi: 10.1096/fj.04-3217com. [DOI] [PubMed] [Google Scholar]
- 171.Grzeszkiewicz TM, Kirschling DJ, Chen N, Lau LF. CYR61 stimulates human skin fibroblast migration through Integrin alpha vbeta 5 and enhances mitogenesis through integrin alpha vbeta 3, independent of its carboxyl-terminal domain. J Biol Chem. 2001;276:21943–21950. doi: 10.1074/jbc.M100978200. [DOI] [PubMed] [Google Scholar]
- 172.Gupta S, Clarkson MR, Duggan J, Brady HR. Connective tissue growth factor: potential role in glomerulosclerosis and tubulointerstitial fibrosis. Kidney Int. 2000;58:1389–1399. doi: 10.1046/j.1523-1755.2000.00301.x. [DOI] [PubMed] [Google Scholar]
- 173.Hakuno D, Kimura N, Yoshioka M, Mukai M, Kimura T, Okada Y, Yozu R, Shukunami C, Hiraki Y, Kudo A, Ogawa S, Fukuda K. Periostin advances atherosclerotic and rheumatic cardiac valve degeneration by inducing angiogenesis and MMP production in humans and rodents. J Clin Invest. 2010;120:2292–2306. doi: 10.1172/JCI40973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hamilton DW. Functional role of periostin in development and wound repair: implications for connective tissue disease. J Cell Commun Signal. 2008;2:9–17. doi: 10.1007/s12079-008-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Harris BS, Zhang Y, Card L, Rivera LB, Brekken RA, Bradshaw AD. SPARC regulates collagen interaction with cardiac fibroblast cell surfaces. Am J Physiol Heart Circ Physiol. 2011;301:H841–847. doi: 10.1152/ajpheart.01247.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hashimoto G, Inoki I, Fujii Y, Aoki T, Ikeda E, Okada Y. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 177.He Z, Way KJ, Arikawa E, Chou E, Opland DM, Clermont A, Isshiki K, Ma RC, Scott JA, Schoen FJ, Feener EP, King GL. Differential regulation of angiotensin II-induced expression of connective tissue growth factor by protein kinase C isoforms in the myocardium. J Biol Chem. 2005;280:15719–15726. doi: 10.1074/jbc.M413493200. [DOI] [PubMed] [Google Scholar]
- 178.Heath E, Tahri D, Andermarcher E, Schofield P, Fleming S, Boulter CA. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Dev Biol. 2008;8:18. doi: 10.1186/1471-213X-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Hilfiker-Kleiner D, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC, Hilfiker A, Drexler H. Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation. 2004;109:2227–2233. doi: 10.1161/01.CIR.0000127952.90508.9D. [DOI] [PubMed] [Google Scholar]
- 180.Hogg PJ. Thrombospondin 1 as an enzyme inhibitor. Thromb Haemost. 1994;72:787–792. [PubMed] [Google Scholar]
- 181.Holmes A, Abraham DJ, Sa S, Shiwen X, Black CM, Leask A. CTGF and SMADs, maintenance of scleroderma phenotype is independent of SMAD signaling. J Biol Chem. 2001;276:10594–10601. doi: 10.1074/jbc.M010149200. [DOI] [PubMed] [Google Scholar]
- 182.Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 183.Hsia HC, Schwarzbauer JE. Meet the tenascins: multifunctional and mysterious. J Biol Chem. 2005;280:26641–26644. doi: 10.1074/jbc.R500005200. [DOI] [PubMed] [Google Scholar]
- 184.Hudlicka O, Brown MD. Postnatal growth of the heart and its blood vessels. J Vasc Res. 1996;33:266–287. doi: 10.1159/000159155. [DOI] [PubMed] [Google Scholar]
- 185.Huebener P, Abou-Khamis T, Zymek P, Bujak M, Ying X, Chatila K, Haudek S, Thakker G, Frangogiannis NG. CD44 Is Critically Involved in Infarct Healing by Regulating the Inflammatory and Fibrotic Response. J Immunol. 2008;180:2625–2633. doi: 10.4049/jimmunol.180.4.2625. [DOI] [PubMed] [Google Scholar]
- 186.Hugo C, Pichler R, Meek R, Gordon K, Kyriakides T, Floege J, Bornstein P, Couser WG, Johnson RJ. Thrombospondin 1 is expressed by proliferating mesangial cells and is up-regulated by PDGF and bFGF in vivo. Kidney Int. 1995;48:1846–1856. doi: 10.1038/ki.1995.483. [DOI] [PubMed] [Google Scholar]
- 187.Huxley-Jones J, Pinney JW, Archer J, Robertson DL, Boot-Handford RP. Back to basics--how the evolution of the extracellular matrix underpinned vertebrate evolution. Int J Exp Pathol. 2009;90:95–100. doi: 10.1111/j.1365-2613.2008.00637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Igarashi A, Okochi H, Bradham DM, Grotendorst GR. Regulation of connective tissue growth factor gene expression in human skin fibroblasts and during wound repair. Mol Biol Cell. 1993;4:637–645. doi: 10.1091/mbc.4.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Imanaka-Yoshida K, Hiroe M, Nishikawa T, Ishiyama S, Shimojo T, Ohta Y, Sakakura T, Yoshida T. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab Invest. 2001;81:1015–1024. doi: 10.1038/labinvest.3780313. [DOI] [PubMed] [Google Scholar]
- 191.Imanaka-Yoshida K, Hiroe M, Yasutomi Y, Toyozaki T, Tsuchiya T, Noda N, Maki T, Nishikawa T, Sakakura T, Yoshida T. Tenascin-C is a useful marker for disease activity in myocarditis. J Pathol. 2002;197:388–394. doi: 10.1002/path.1131. [DOI] [PubMed] [Google Scholar]
- 192.Imanaka-Yoshida K, Hiroe M, Yoshida T. Interaction between cell and extracellular matrix in heart disease: multiple roles of tenascin-C in tissue remodeling. Histol Histopathol. 2004;19:517–525. doi: 10.14670/HH-19.517. [DOI] [PubMed] [Google Scholar]
- 193.Imanaka-Yoshida K, Matsumoto K, Hara M, Sakakura T, Yoshida T. The dynamic expression of tenascin-C and tenascin-X during early heart development in the mouse. Differentiation. 2003;71:291–298. doi: 10.1046/j.1432-0436.2003.7104506.x. [DOI] [PubMed] [Google Scholar]
- 194.Iruela-Arispe ML. Regulation of thrombospondin1 by extracellular proteases. Curr Drug Targets. 2008;9:863–868. doi: 10.2174/138945008785909365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Iruela-Arispe ML, Liska DJ, Sage EH, Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn. 1993;197:40–56. doi: 10.1002/aja.1001970105. [DOI] [PubMed] [Google Scholar]
- 196.Isenberg JS, Annis DS, Pendrak ML, Ptaszynska M, Frazier WA, Mosher DF, Roberts DD. Differential interactions of thrombospondin-1, -2, and -4 with CD47 and effects on cGMP signaling and ischemic injury responses. J Biol Chem. 2009;284:1116–1125. doi: 10.1074/jbc.M804860200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Isenberg JS, Martin-Manso G, Maxhimer JB, Roberts DD. Regulation of nitric oxide signalling by thrombospondin 1: implications for anti-angiogenic therapies. Nat Rev Cancer. 2009;9:182–194. doi: 10.1038/nrc2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Isenberg JS, Qin Y, Maxhimer JB, Sipes JM, Despres D, Schnermann J, Frazier WA, Roberts DD. Thrombospondin-1 and CD47 regulate blood pressure and cardiac responses to vasoactive stress. Matrix Biol. 2009;28:110–119. doi: 10.1016/j.matbio.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Isenberg JS, Ridnour LA, Dimitry J, Frazier WA, Wink DA, Roberts DD. CD47 is necessary for inhibition of nitric oxide-stimulated vascular cell responses by thrombospondin-1. J Biol Chem. 2006;281:26069–26080. doi: 10.1074/jbc.M605040200. [DOI] [PubMed] [Google Scholar]
- 200.Isenberg JS, Ridnour LA, Perruccio EM, Espey MG, Wink DA, Roberts DD. Thrombospondin-1 inhibits endothelial cell responses to nitric oxide in a cGMP-dependent manner. Proc Natl Acad Sci U S A. 2005;102:13141–13146. doi: 10.1073/pnas.0502977102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Isenberg JS, Romeo MJ, Yu C, Yu CK, Nghiem K, Monsale J, Rick ME, Wink DA, Frazier WA, Roberts DD. Thrombospondin-1 stimulates platelet aggregation by blocking the antithrombotic activity of nitric oxide/cGMP signaling. Blood. 2008;111:613–623. doi: 10.1182/blood-2007-06-098392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Isenberg JS, Wink DA, Roberts DD. Thrombospondin-1 antagonizes nitric oxide-stimulated vascular smooth muscle cell responses. Cardiovasc Res. 2006;71:785–793. doi: 10.1016/j.cardiores.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 203.Ito K, Kon S, Nakayama Y, Kurotaki D, Saito Y, Kanayama M, Kimura C, Diao H, Morimoto J, Matsui Y, Uede T. The differential amino acid requirement within osteopontin in alpha4 and alpha9 integrin-mediated cell binding and migration. Matrix Biol. 2009;28:11–19. doi: 10.1016/j.matbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 204.Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Iwamoto M, Hirohata S, Ogawa H, Ohtsuki T, Shinohata R, Miyoshi T, Hatipoglu FO, Kusachi S, Yamamoto K, Ninomiya Y. Connective tissue growth factor induction in a pressure-overloaded heart ameliorated by the angiotensin II type 1 receptor blocker olmesartan. Hypertens Res. 2010;33:1305–1311. doi: 10.1038/hr.2010.189. [DOI] [PubMed] [Google Scholar]
- 206.Iwanciw D, Rehm M, Porst M, Goppelt-Struebe M. Induction of connective tissue growth factor by angiotensin II: integration of signaling pathways. Arterioscler Thromb Vasc Biol. 2003;23:1782–1787. doi: 10.1161/01.ATV.0000092913.60428.E6. [DOI] [PubMed] [Google Scholar]
- 207.Jackson-Boeters L, Wen W, Hamilton DW. Periostin localizes to cells in normal skin, but is associated with the extracellular matrix during wound repair. J Cell Commun Signal. 2009;3:125–133. doi: 10.1007/s12079-009-0057-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Jarvinen TA, Jozsa L, Kannus P, Jarvinen TL, Hurme T, Kvist M, Pelto-Huikko M, Kalimo H, Jarvinen M. Mechanical loading regulates the expression of tenascin-C in the myotendinous junction and tendon but does not induce de novo synthesis in the skeletal muscle. J Cell Sci. 2003;116:857–866. doi: 10.1242/jcs.00303. [DOI] [PubMed] [Google Scholar]
- 209.Jimenez B, Volpert OV, Crawford SE, Febbraio M, Silverstein RL, Bouck N. Signals leading to apoptosis-dependent inhibition of neovascularization by thrombospondin-1. Nat Med. 2000;6:41–48. doi: 10.1038/71517. [DOI] [PubMed] [Google Scholar]
- 210.Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol. 1997;139:279–293. doi: 10.1083/jcb.139.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jones PL, Jones FS. Tenascin-C in development and disease: gene regulation and cell function. Matrix Biol. 2000;19:581–596. doi: 10.1016/s0945-053x(00)00106-2. [DOI] [PubMed] [Google Scholar]
- 212.Jugdutt BI, Jelani A, Palaniyappan A, Idikio H, Uweira RE, Menon V, Jugdutt CE. Aging-related early changes in markers of ventricular and matrix remodeling after reperfused ST-segment elevation myocardial infarction in the canine model: effect of early therapy with an angiotensin II type 1 receptor blocker. Circulation. 2010;122:341–351. doi: 10.1161/CIRCULATIONAHA.110.948190. [DOI] [PubMed] [Google Scholar]
- 213.Jugdutt BI, Palaniyappan A, Uwiera RR, Idikio H. Role of healing-specific-matricellular proteins and matrix metalloproteinases in age-related enhanced early remodeling after reperfused STEMI in dogs. Mol Cell Biochem. 2009;322:25–36. doi: 10.1007/s11010-008-9936-9. [DOI] [PubMed] [Google Scholar]
- 214.Jun JI, Lau LF. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol. 12:676–685. doi: 10.1038/ncb2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kanemoto K, Usui J, Tomari S, Yokoi H, Mukoyama M, Aten J, Weening JJ, Nagata M. Connective tissue growth factor participates in scar formation of crescentic glomerulonephritis. Lab Invest. 2003;83:1615–1625. doi: 10.1097/01.lab.0000096711.58115.46. [DOI] [PubMed] [Google Scholar]
- 216.Kato Y, Lewalle JM, Baba Y, Tsukuda M, Sakai N, Baba M, Kobayashi K, Koshika S, Nagashima Y, Frankenne F, Noel A, Foidart JM, Hata RI. Induction of SPARC by VEGF in human vascular endothelial cells. Biochem Biophys Res Commun. 2001;287:422–426. doi: 10.1006/bbrc.2001.5622. [DOI] [PubMed] [Google Scholar]
- 217.Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T, Sugimura K. Periostin as a novel factor responsible for ventricular dilation. Circulation. 2004;110:1806–1813. doi: 10.1161/01.CIR.0000142607.33398.54. [DOI] [PubMed] [Google Scholar]
- 218.Kawamura K, Iyonaga K, Ichiyasu H, Nagano J, Suga M, Sasaki Y. Differentiation, maturation, and survival of dendritic cells by osteopontin regulation. Clin Diagn Lab Immunol. 2005;12:206–212. doi: 10.1128/CDLI.12.1.206-212.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Kemp TJ, Aggeli IK, Sugden PH, Clerk A. Phenylephrine and endothelin-1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2004;37:603–606. doi: 10.1016/j.yjmcc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 220.Kern CB, Hoffman S, Moreno R, Damon BJ, Norris RA, Krug EL, Markwald RR, Mjaatvedt CH. Immunolocalization of chick periostin protein in the developing heart. Anat Rec A Discov Mol Cell Evol Biol. 2005;284:415–423. doi: 10.1002/ar.a.20193. [DOI] [PubMed] [Google Scholar]
- 221.Khan SA, Lopez-Chua CA, Zhang J, Fisher LW, Sorensen ES, Denhardt DT. Soluble osteopontin inhibits apoptosis of adherent endothelial cells deprived of growth factors. J Cell Biochem. 2002;85:728–736. doi: 10.1002/jcb.10170. [DOI] [PubMed] [Google Scholar]
- 222.Kii I, Amizuka N, Minqi L, Kitajima S, Saga Y, Kudo A. Periostin is an extracellular matrix protein required for eruption of incisors in mice. Biochem Biophys Res Commun. 2006;342:766–772. doi: 10.1016/j.bbrc.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 223.Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem. 1998;273:3090–3096. doi: 10.1074/jbc.273.5.3090. [DOI] [PubMed] [Google Scholar]
- 224.Koitabashi N, Arai M, Kogure S, Niwano K, Watanabe A, Aoki Y, Maeno T, Nishida T, Kubota S, Takigawa M, Kurabayashi M. Increased connective tissue growth factor relative to brain natriuretic peptide as a determinant of myocardial fibrosis. Hypertension. 2007;49:1120–1127. doi: 10.1161/HYPERTENSIONAHA.106.077537. [DOI] [PubMed] [Google Scholar]
- 225.Koitabashi N, Arai M, Niwano K, Watanabe A, Endoh M, Suguta M, Yokoyama T, Tada H, Toyama T, Adachi H, Naito S, Oshima S, Nishida T, Kubota S, Takigawa M, Kurabayashi M. Plasma connective tissue growth factor is a novel potential biomarker of cardiac dysfunction in patients with chronic heart failure. Eur J Heart Fail. 2008;10:373–379. doi: 10.1016/j.ejheart.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 226.Koli K, Saharinen J, Hyytiainen M, Penttinen C, Keski-Oja J. Latency, activation, and binding proteins of TGF-beta. Microsc Res Tech. 2001;52:354–362. doi: 10.1002/1097-0029(20010215)52:4<354::AID-JEMT1020>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 227.Komatsubara I, Murakami T, Kusachi S, Nakamura K, Hirohata S, Hayashi J, Takemoto S, Suezawa C, Ninomiya Y, Shiratori Y. Spatially and temporally different expression of osteonectin and osteopontin in the infarct zone of experimentally induced myocardial infarction in rats. Cardiovasc Pathol. 2003;12:186–194. doi: 10.1016/s1054-8807(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 228.Kopp HG, Hooper AT, Broekman MJ, Avecilla ST, Petit I, Luo M, Milde T, Ramos CA, Zhang F, Kopp T, Bornstein P, Jin DK, Marcus AJ, Rafii S. Thrombospondins deployed by thrombopoietic cells determine angiogenic switch and extent of revascularization. J Clin Invest. 2006;116:3277–3291. doi: 10.1172/JCI29314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Kos K, Wilding JP. SPARC: a key player in the pathologies associated with obesity and diabetes. Nat Rev Endocrinol. 2010;6:225–235. doi: 10.1038/nrendo.2010.18. [DOI] [PubMed] [Google Scholar]
- 230.Kos K, Wong S, Tan B, Gummesson A, Jernas M, Franck N, Kerrigan D, Nystrom FH, Carlsson LM, Randeva HS, Pinkney JH, Wilding JP. Regulation of the fibrosis and angiogenesis promoter SPARC/osteonectin in human adipose tissue by weight change, leptin, insulin, and glucose. Diabetes. 2009;58:1780–1788. doi: 10.2337/db09-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Kossmehl P, Schonberger J, Shakibaei M, Faramarzi S, Kurth E, Habighorst B, von Bauer R, Wehland M, Kreutz R, Infanger M, Schulze-Tanzil G, Paul M, Grimm D. Increase of fibronectin and osteopontin in porcine hearts following ischemia and reperfusion. J Mol Med. 2005;83:626–637. doi: 10.1007/s00109-005-0642-8. [DOI] [PubMed] [Google Scholar]
- 232.Koyama Y, Kusubata M, Yoshiki A, Hiraiwa N, Ohashi T, Irie S, Kusakabe M. Effect of tenascin-C deficiency on chemically induced dermatitis in the mouse. J Invest Dermatol. 1998;111:930–935. doi: 10.1046/j.1523-1747.1998.00401.x. [DOI] [PubMed] [Google Scholar]
- 233.Krady MM, Zeng J, Yu J, MacLauchlan S, Skokos EA, Tian W, Bornstein P, Sessa WC, Kyriakides TR. Thrombospondin-2 modulates extracellular matrix remodeling during physiological angiogenesis. Am J Pathol. 2008;173:879–891. doi: 10.2353/ajpath.2008.080128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Krause SW, Rehli M, Kreutz M, Schwarzfischer L, Paulauskis JD, Andreesen R. Differential screening identifies genetic markers of monocyte to macrophage maturation. J Leukoc Biol. 1996;60:540–545. doi: 10.1002/jlb.60.4.540. [DOI] [PubMed] [Google Scholar]
- 235.Krum H, Elsik M, Schneider HG, Ptaszynska A, Black M, Carson PE, Komajda M, Massie BM, McKelvie RS, McMurray JJ, Zile MR, Anand IS. Relation of peripheral collagen markers to death and hospitalization in patients with heart failure and preserved ejection fraction: results of the I-PRESERVE collagen substudy. Circ Heart Fail. 2011;4:561–568. doi: 10.1161/CIRCHEARTFAILURE.110.960716. [DOI] [PubMed] [Google Scholar]
- 236.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Dev. 2001;103:183–188. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 237.Kuhn B, del Monte F, Hajjar RJ, Chang YS, Lebeche D, Arab S, Keating MT. Periostin induces proliferation of differentiated cardiomyocytes and promotes cardiac repair. Nat Med. 2007;13:962–969. doi: 10.1038/nm1619. [DOI] [PubMed] [Google Scholar]
- 238.Kupprion C, Motamed K, Sage EH. SPARC (BM-40, osteonectin) inhibits the mitogenic effect of vascular endothelial growth factor on microvascular endothelial cells. J Biol Chem. 1998;273:29635–29640. doi: 10.1074/jbc.273.45.29635. [DOI] [PubMed] [Google Scholar]
- 239.Kusuyama T, Yoshiyama M, Omura T, Nishiya D, Enomoto S, Matsumoto R, Izumi Y, Akioka K, Takeuchi K, Iwao H, Yoshikawa J. Angiotensin blockade inhibits osteopontin expression in non-infarcted myocardium after myocardial infarction. J Pharmacol Sci. 2005;98:283–289. doi: 10.1254/jphs.fp0050056. [DOI] [PubMed] [Google Scholar]
- 240.Kutz WE, Gong Y, Warman ML. WISP3, the gene responsible for the human skeletal disease progressive pseudorheumatoid dysplasia, is not essential for skeletal function in mice. Mol Cell Biol. 2005;25:414–421. doi: 10.1128/MCB.25.1.414-421.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009;3:215–225. doi: 10.1007/s12079-009-0077-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Kyriakides TR, Tam JW, Bornstein P. Accelerated wound healing in mice with a disruption of the thrombospondin 2 gene. J Invest Dermatol. 1999;113:782–787. doi: 10.1046/j.1523-1747.1999.00755.x. [DOI] [PubMed] [Google Scholar]
- 243.Kyriakides TR, Zhu YH, Smith LT, Bain SD, Yang Z, Lin MT, Danielson KG, Iozzo RV, LaMarca M, McKinney CE, Ginns EI, Bornstein P. Mice that lack thrombospondin 2 display connective tissue abnormalities that are associated with disordered collagen fibrillogenesis, an increased vascular density, and a bleeding diathesis. J Cell Biol. 1998;140:419–430. doi: 10.1083/jcb.140.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lafont J, Laurent M, Thibout H, Lallemand F, Le Bouc Y, Atfi A, Martinerie C. The expression of novH in adrenocortical cells is down-regulated by TGFbeta 1 through c-Jun in a Smad-independent manner. J Biol Chem. 2002;277:41220–41229. doi: 10.1074/jbc.M204405200. [DOI] [PubMed] [Google Scholar]
- 245.Lake AC, Bialik A, Walsh K, Castellot JJ., Jr CCN5 is a growth arrest-specific gene that regulates smooth muscle cell proliferation and motility. Am J Pathol. 2003;162:219–231. doi: 10.1016/S0002-9440(10)63813-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Lamy L, Foussat A, Brown EJ, Bornstein P, Ticchioni M, Bernard A. Interactions between CD47 and thrombospondin reduce inflammation. J Immunol. 2007;178:5930–5939. doi: 10.4049/jimmunol.178.9.5930. [DOI] [PubMed] [Google Scholar]
- 247.Lang C, Sauter M, Szalay G, Racchi G, Grassi G, Rainaldi G, Mercatanti A, Lang F, Kandolf R, Klingel K. Connective tissue growth factor: a crucial cytokine-mediating cardiac fibrosis in ongoing enterovirus myocarditis. J Mol Med. 2008;86:49–60. doi: 10.1007/s00109-007-0249-3. [DOI] [PubMed] [Google Scholar]
- 248.Lasky JA, Ortiz LA, Tonthat B, Hoyle GW, Corti M, Athas G, Lungarella G, Brody A, Friedman M. Connective tissue growth factor mRNA expression is upregulated in bleomycin-induced lung fibrosis. Am J Physiol. 1998;275:L365–371. doi: 10.1152/ajplung.1998.275.2.L365. [DOI] [PubMed] [Google Scholar]
- 249.Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- 250.Lawler J, Duquette M, Whittaker CA, Adams JC, McHenry K, DeSimone DW. Identification and characterization of thrombospondin-4, a new member of the thrombospondin gene family. J Cell Biol. 1993;120:1059–1067. doi: 10.1083/jcb.120.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Lawler J, Hynes RO. An integrin receptor on normal and thrombasthenic platelets that binds thrombospondin. Blood. 1989;74:2022–2027. [PubMed] [Google Scholar]
- 252.Lawler J, Sunday M, Thibert V, Duquette M, George EL, Rayburn H, Hynes RO. Thrombospondin-1 is required for normal murine pulmonary homeostasis and its absence causes pneumonia. J Clin Invest. 1998;101:982–992. doi: 10.1172/JCI1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 254.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 255.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 256.Leask A, Parapuram SK, Shi-Wen X, Abraham DJ. Connective tissue growth factor (CTGF, CCN2) gene regulation: a potent clinical bio-marker of fibroproliferative disease? J Cell Commun Signal. 2009;3:89–94. doi: 10.1007/s12079-009-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lee CH, Shah B, Moioli EK, Mao JJ. CTGF directs fibroblast differentiation from human mesenchymal stem/stromal cells and defines connective tissue healing in a rodent injury model. J Clin Invest. 2010;120:3340–3349. doi: 10.1172/JCI43230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Leung LL, Nachman RL. Complex formation of platelet thrombospondin with fibrinogen. J Clin Invest. 1982;70:542–549. doi: 10.1172/JCI110646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Levy Y, Arbel-Goren R, Hadari YR, Eshhar S, Ronen D, Elhanany E, Geiger B, Zick Y. Galectin-8 functions as a matricellular modulator of cell adhesion. J Biol Chem. 2001;276:31285–31295. doi: 10.1074/jbc.M100340200. [DOI] [PubMed] [Google Scholar]
- 260.Li G, Jin R, Norris RA, Zhang L, Yu S, Wu F, Markwald RR, Nanda A, Conway SJ, Smyth SS, Granger DN. Periostin mediates vascular smooth muscle cell migration through the integrins alphavbeta3 and alphavbeta5 and focal adhesion kinase (FAK) pathway. Atherosclerosis. 2010;208:358–365. doi: 10.1016/j.atherosclerosis.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Li G, Oparil S, Kelpke SS, Chen YF, Thompson JA. Fibroblast growth factor receptor-1 signaling induces osteopontin expression and vascular smooth muscle cell-dependent adventitial fibroblast migration in vitro. Circulation. 2002;106:854–859. doi: 10.1161/01.cir.0000024113.26985.cc. [DOI] [PubMed] [Google Scholar]
- 262.Li G, Oparil S, Sanders JM, Zhang L, Dai M, Chen LB, Conway SJ, McNamara CA, Sarembock IJ. Phosphatidylinositol-3-kinase signaling mediates vascular smooth muscle cell expression of periostin in vivo and in vitro. Atherosclerosis. 2006;188:292–300. doi: 10.1016/j.atherosclerosis.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Li G, Xie Q, Shi Y, Li D, Zhang M, Jiang S, Zhou H, Lu H, Jin Y. Inhibition of connective tissue growth factor by siRNA prevents liver fibrosis in rats. J Gene Med. 2006;8:889–900. doi: 10.1002/jgm.894. [DOI] [PubMed] [Google Scholar]
- 264.Li J, Brown LF, Laham RJ, Volk R, Simons M. Macrophage-dependent regulation of syndecan gene expression. Circ Res. 1997;81:785–796. doi: 10.1161/01.res.81.5.785. [DOI] [PubMed] [Google Scholar]
- 265.Li L, Fan D, Wang C, Wang JY, Cui XB, Wu D, Zhou Y, Wu LL. Angiotensin II increases periostin expression via Ras/p38 MAPK/CREB and ERK1/2/TGF-beta1 pathways in cardiac fibroblasts. Cardiovasc Res. 2011;91:80–89. doi: 10.1093/cvr/cvr067. [DOI] [PubMed] [Google Scholar]
- 266.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Lin CG, Leu SJ, Chen N, Tebeau CM, Lin SX, Yeung CY, Lau LF. CCN3 (NOV) is a novel angiogenic regulator of the CCN protein family. J Biol Chem. 2003;278:24200–24208. doi: 10.1074/jbc.M302028200. [DOI] [PubMed] [Google Scholar]
- 268.Lindner V, Wang Q, Conley BA, Friesel RE, Vary CP. Vascular injury induces expression of periostin: implications for vascular cell differentiation and migration. Arterioscler Thromb Vasc Biol. 2005;25:77–83. doi: 10.1161/01.ATV.0000149141.81230.c6. [DOI] [PubMed] [Google Scholar]
- 269.Lindsley A, Li W, Wang J, Maeda N, Rogers R, Conway SJ. Comparison of the four mouse fasciclin-containing genes expression patterns during valvuloseptal morphogenesis. Gene Expr Patterns. 2005;5:593–600. doi: 10.1016/j.modgep.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 270.Litvin J, Blagg A, Mu A, Matiwala S, Montgomery M, Berretta R, Houser S, Margulies K. Periostin and periostin-like factor in the human heart: possible therapeutic targets. Cardiovasc Pathol. 2006;15:24–32. doi: 10.1016/j.carpath.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 271.Loike JD, Cao L, Budhu S, Hoffman S, Silverstein SC. Blockade of alpha 5 beta 1 integrins reverses the inhibitory effect of tenascin on chemotaxis of human monocytes and polymorphonuclear leukocytes through three-dimensional gels of extracellular matrix proteins. J Immunol. 2001;166:7534–7542. doi: 10.4049/jimmunol.166.12.7534. [DOI] [PubMed] [Google Scholar]
- 272.Lorts A, Schwanekamp JA, Elrod JW, Sargent MA, Molkentin JD. Genetic manipulation of periostin expression in the heart does not affect myocyte content, cell cycle activity, or cardiac repair. Circ Res. 2009;104:e1–7. doi: 10.1161/CIRCRESAHA.108.188649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lyons RM, Keski-Oja J, Moses HL. Proteolytic activation of latent transforming growth factor-beta from fibroblast-conditioned medium. J Cell Biol. 1988;106:1659–1665. doi: 10.1083/jcb.106.5.1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Mackie EJ, Scott-Burden T, Hahn AW, Kern F, Bernhardt J, Regenass S, Weller A, Buhler FR. Expression of tenascin by vascular smooth muscle cells. Alterations in hypertensive rats and stimulation by angiotensin II. Am J Pathol. 1992;141:377–388. [PMC free article] [PubMed] [Google Scholar]
- 275.Mackie EJ, Tucker RP. The tenascin-C knockout revisited. J Cell Sci. 1999;112 (Pt 22):3847–3853. doi: 10.1242/jcs.112.22.3847. [DOI] [PubMed] [Google Scholar]
- 276.MacLellan WR, Schneider MD. Genetic dissection of cardiac growth control pathways. Annu Rev Physiol. 2000;62:289–319. doi: 10.1146/annurev.physiol.62.1.289. [DOI] [PubMed] [Google Scholar]
- 277.Majack RA, Goodman LV, Dixit VM. Cell surface thrombospondin is functionally essential for vascular smooth muscle cell proliferation. J Cell Biol. 1988;106:415–422. doi: 10.1083/jcb.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Malek MH, Olfert IM. Global deletion of thrombospondin-1 increases cardiac and skeletal muscle capillarity and exercise capacity in mice. Exp Physiol. 2009;94:749–760. doi: 10.1113/expphysiol.2008.045989. [DOI] [PubMed] [Google Scholar]
- 279.Mao JR, Taylor G, Dean WB, Wagner DR, Afzal V, Lotz JC, Rubin EM, Bristow J. Tenascin-X deficiency mimics Ehlers-Danlos syndrome in mice through alteration of collagen deposition. Nat Genet. 2002;30:421–425. doi: 10.1038/ng850. [DOI] [PubMed] [Google Scholar]
- 280.Maquerlot F, Galiacy S, Malo M, Guignabert C, Lawrence DA, d’Ortho MP, Barlovatz-Meimon G. Dual role for plasminogen activator inhibitor type 1 as soluble and as matricellular regulator of epithelial alveolar cell wound healing. Am J Pathol. 2006;169:1624–1632. doi: 10.2353/ajpath.2006.051053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Martinek N, Shahab J, Saathoff M, Ringuette M. Haemocyte-derived SPARC is required for collagen-IV-dependent stability of basal laminae in Drosophila embryos. J Cell Sci. 2008;121:1671–1680. doi: 10.1242/jcs.021931. [DOI] [PubMed] [Google Scholar]
- 282.Masson S, Arosio B, Luvara G, Gagliano N, Fiordaliso F, Santambrogio D, Vergani C, Latini R, Annoni G. Remodelling of cardiac extracellular matrix during beta-adrenergic stimulation: upregulation of SPARC in the myocardium of adult rats. J Mol Cell Cardiol. 1998;30:1505–1514. doi: 10.1006/jmcc.1998.0714. [DOI] [PubMed] [Google Scholar]
- 283.Matsuda A, Yoshiki A, Tagawa Y, Matsuda H, Kusakabe M. Corneal wound healing in tenascin knockout mouse. Invest Ophthalmol Vis Sci. 1999;40:1071–1080. [PubMed] [Google Scholar]
- 284.Matsui Y, Ikesue M, Danzaki K, Morimoto J, Sato M, Tanaka S, Kojima T, Tsutsui H, Uede T. Syndecan-4 prevents cardiac rupture and dysfunction after myocardial infarction. Circ Res. 2011;108:1328–1339. doi: 10.1161/CIRCRESAHA.110.235689. [DOI] [PubMed] [Google Scholar]
- 285.Matsui Y, Jia N, Okamoto H, Kon S, Onozuka H, Akino M, Liu L, Morimoto J, Rittling SR, Denhardt D, Kitabatake A, Uede T. Role of osteopontin in cardiac fibrosis and remodeling in angiotensin II-induced cardiac hypertrophy. Hypertension. 2004;43:1195–1201. doi: 10.1161/01.HYP.0000128621.68160.dd. [DOI] [PubMed] [Google Scholar]
- 286.Matsui Y, Rittling SR, Okamoto H, Inobe M, Jia N, Shimizu T, Akino M, Sugawara T, Morimoto J, Kimura C, Kon S, Denhardt D, Kitabatake A, Uede T. Osteopontin deficiency attenuates atherosclerosis in female apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:1029–1034. doi: 10.1161/01.ATV.0000074878.29805.D0. [DOI] [PubMed] [Google Scholar]
- 287.Matsumoto K, Saga Y, Ikemura T, Sakakura T, Chiquet-Ehrismann R. The distribution of tenascin-X is distinct and often reciprocal to that of tenascin-C. J Cell Biol. 1994;125:483–493. doi: 10.1083/jcb.125.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Mavroidis M, Capetanaki Y. Extensive induction of important mediators of fibrosis and dystrophic calcification in desmin-deficient cardiomyopathy. Am J Pathol. 2002;160:943–952. doi: 10.1016/S0002-9440(10)64916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.McCurdy SM, Dai Q, Zhang J, Zamilpa R, Ramirez TA, Dayah T, Nguyen N, Jin YF, Bradshaw AD, Lindsey ML. SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;301:H497–505. doi: 10.1152/ajpheart.01070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Midwood K, Sacre S, Piccinini AM, Inglis J, Trebaul A, Chan E, Drexler S, Sofat N, Kashiwagi M, Orend G, Brennan F, Foxwell B. Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med. 2009;15:774–780. doi: 10.1038/nm.1987. [DOI] [PubMed] [Google Scholar]
- 291.Midwood KS, Orend G. The role of tenascin-C in tissue injury and tumorigenesis. J Cell Commun Signal. 2009;3:287–310. doi: 10.1007/s12079-009-0075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Midwood KS, Schwarzbauer JE. Tenascin-C modulates matrix contraction via focal adhesion kinase- and Rho-mediated signaling pathways. Mol Biol Cell. 2002;13:3601–3613. doi: 10.1091/mbc.E02-05-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Miller SJ, Watson WC, Kerr KA, Labarrere CA, Chen NX, Deeg MA, Unthank JL. Development of progressive aortic vasculopathy in a rat model of aging. Am J Physiol Heart Circ Physiol. 2007;293:H2634–2643. doi: 10.1152/ajpheart.00397.2007. [DOI] [PubMed] [Google Scholar]
- 294.Mo FE, Lau LF. The matricellular protein CCN1 is essential for cardiac development. Circ Res. 2006;99:961–969. doi: 10.1161/01.RES.0000248426.35019.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709–8720. doi: 10.1128/MCB.22.24.8709-8720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Mohler ER, 3rd, Adam LP, McClelland P, Graham L, Hathaway DR. Detection of osteopontin in calcified human aortic valves. Arterioscler Thromb Vasc Biol. 1997;17:547–552. doi: 10.1161/01.atv.17.3.547. [DOI] [PubMed] [Google Scholar]
- 297.Molkentin JD, Dorn GW., 2nd Cytoplasmic signaling pathways that regulate cardiac hypertrophy. Annu Rev Physiol. 2001;63:391–426. doi: 10.1146/annurev.physiol.63.1.391. [DOI] [PubMed] [Google Scholar]
- 298.Morandi V, Cherradi SE, Lambert S, Fauvel-Lafeve F, Legrand YJ, Legrand C. Proinflammatory cytokines (interleukin-1 beta and tumor necrosis factor-alpha) down regulate synthesis and secretion of thrombospondin by human endothelial cells. J Cell Physiol. 1994;160:367–377. doi: 10.1002/jcp.1041600218. [DOI] [PubMed] [Google Scholar]
- 299.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. J Cell Physiol. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 300.Morimoto S, Imanaka-Yoshida K, Hiramitsu S, Kato S, Ohtsuki M, Uemura A, Kato Y, Nishikawa T, Toyozaki T, Hishida H, Yoshida T, Hiroe M. Diagnostic utility of tenascin-C for evaluation of the activity of human acute myocarditis. J Pathol. 2005;205:460–467. doi: 10.1002/path.1730. [DOI] [PubMed] [Google Scholar]
- 301.Moriwaki H, Stempien-Otero A, Kremen M, Cozen AE, Dichek DA. Overexpression of urokinase by macrophages or deficiency of plasminogen activator inhibitor type 1 causes cardiac fibrosis in mice. Circ Res. 2004;95:637–644. doi: 10.1161/01.RES.0000141427.61023.f4. [DOI] [PubMed] [Google Scholar]
- 302.Motamed K, Funk SE, Koyama H, Ross R, Raines EW, Sage EH. Inhibition of PDGF-stimulated and matrix-mediated proliferation of human vascular smooth muscle cells by SPARC is independent of changes in cell shape or cyclin-dependent kinase inhibitors. J Cell Biochem. 2002;84:759–771. doi: 10.1002/jcb.10095. [DOI] [PubMed] [Google Scholar]
- 303.Mumby SM, Raugi GJ, Bornstein P. Interactions of thrombospondin with extracellular matrix proteins: selective binding to type V collagen. J Cell Biol. 1984;98:646–652. doi: 10.1083/jcb.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Murphy-Ullrich JE. The de-adhesive activity of matricellular proteins: is intermediate cell adhesion an adaptive state? J Clin Invest. 2001;107:785–790. doi: 10.1172/JCI12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Murphy-Ullrich JE, Hook M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989;109:1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Murphy-Ullrich JE, Lane TF, Pallero MA, Sage EH. SPARC mediates focal adhesion disassembly in endothelial cells through a follistatin-like region and the Ca(2+)-binding EF-hand. J Cell Biochem. 1995;57:341–350. doi: 10.1002/jcb.240570218. [DOI] [PubMed] [Google Scholar]
- 307.Murphy-Ullrich JE, Lightner VA, Aukhil I, Yan YZ, Erickson HP, Hook M. Focal adhesion integrity is downregulated by the alternatively spliced domain of human tenascin. J Cell Biol. 1991;115:1127–1136. doi: 10.1083/jcb.115.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 309.Murphy-Ullrich JE, Schultz-Cherry S, Hook M. Transforming growth factor-beta complexes with thrombospondin. Mol Biol Cell. 1992;3:181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Murry CE, Giachelli CM, Schwartz SM, Vracko R. Macrophages express osteopontin during repair of myocardial necrosis. Am J Pathol. 1994;145:1450–1462. [PMC free article] [PubMed] [Google Scholar]
- 311.Mustonen E, Aro J, Puhakka J, Ilves M, Soini Y, Leskinen H, Ruskoaho H, Rysa J. Thrombospondin-4 expression is rapidly upregulated by cardiac overload. Biochem Biophys Res Commun. 2008;373:186–191. doi: 10.1016/j.bbrc.2008.05.164. [DOI] [PubMed] [Google Scholar]
- 312.Myers DL, Harmon KJ, Lindner V, Liaw L. Alterations of arterial physiology in osteopontin-null mice. Arterioscler Thromb Vasc Biol. 2003;23:1021–1028. doi: 10.1161/01.ATV.0000073312.34450.16. [DOI] [PubMed] [Google Scholar]
- 313.Nag AC. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. [PubMed] [Google Scholar]
- 314.Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yorioka N, Kohno N. Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-beta1. Am J Physiol Renal Physiol. 2004;286:F278–287. doi: 10.1152/ajprenal.00139.2003. [DOI] [PubMed] [Google Scholar]
- 315.Nakagawa T, Li JH, Garcia G, Mu W, Piek E, Bottinger EP, Chen Y, Zhu HJ, Kang DH, Schreiner GF, Lan HY, Johnson RJ. TGF-beta induces proangiogenic and antiangiogenic factors via parallel but distinct Smad pathways. Kidney Int. 2004;66:605–613. doi: 10.1111/j.1523-1755.2004.00780.x. [DOI] [PubMed] [Google Scholar]
- 316.Nakahara H, Gabazza EC, Fujimoto H, Nishii Y, D’Alessandro-Gabazza CN, Bruno NE, Takagi T, Hayashi T, Maruyama J, Maruyama K, Imanaka-Yoshida K, Suzuki K, Yoshida T, Adachi Y, Taguchi O. Deficiency of tenascin C attenuates allergen-induced bronchial asthma in the mouse. Eur J Immunol. 2006;36:3334–3345. doi: 10.1002/eji.200636271. [DOI] [PubMed] [Google Scholar]
- 317.Nakamura S, Kamihagi K, Satakeda H, Katayama M, Pan H, Okamoto H, Noshiro M, Takahashi K, Yoshihara Y, Shimmei M, Okada Y, Kato Y. Enhancement of SPARC (osteonectin) synthesis in arthritic cartilage. Increased levels in synovial fluids from patients with rheumatoid arthritis and regulation by growth factors and cytokines in chondrocyte cultures. Arthritis Rheum. 1996;39:539–551. doi: 10.1002/art.1780390402. [DOI] [PubMed] [Google Scholar]
- 318.Nakamura Y, Cui Y, Fernando C, Kutz WE, Warman ML. Normal growth and development in mice over-expressing the CCN family member WISP3. J Cell Commun Signal. 2009;3:105–113. doi: 10.1007/s12079-009-0040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Narizhneva NV, Byers-Ward VJ, Quinn MJ, Zidar FJ, Plow EF, Topol EJ, Byzova TV. Molecular and functional differences induced in thrombospondin-1 by the single nucleotide polymorphism associated with the risk of premature, familial myocardial infarction. J Biol Chem. 2004;279:21651–21657. doi: 10.1074/jbc.M311090200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Narouz-Ott L, Maurer P, Nitsche DP, Smyth N, Paulsson M. Thrombospondin-4 binds specifically to both collagenous and non-collagenous extracellular matrix proteins via its C-terminal domains. J Biol Chem. 2000;275:37110–37117. doi: 10.1074/jbc.M007223200. [DOI] [PubMed] [Google Scholar]
- 321.Nie J, Bradshaw AD, Delany AM, Sage EH. Inactivation of SPARC enhances high-fat diet-induced obesity in mice. Connect Tissue Res. doi: 10.3109/03008207.2010.483747. [DOI] [PubMed] [Google Scholar]
- 322.Nie J, Sage EH. SPARC inhibits adipogenesis by its enhancement of beta-catenin signaling. J Biol Chem. 2009;284:1279–1290. doi: 10.1074/jbc.M808285200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Nishioka T, Onishi K, Shimojo N, Nagano Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, Imanaka-Yoshida K. Tenascin-C may aggravate left ventricular remodeling and function after myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2010;298:H1072–1078. doi: 10.1152/ajpheart.00255.2009. [DOI] [PubMed] [Google Scholar]
- 324.Nishioka T, Suzuki M, Onishi K, Takakura N, Inada H, Yoshida T, Hiroe M, Imanaka-Yoshida K. Eplerenone attenuates myocardial fibrosis in the angiotensin II-induced hypertensive mouse: involvement of tenascin-C induced by aldosterone-mediated inflammation. J Cardiovasc Pharmacol. 2007;49:261–268. doi: 10.1097/FJC.0b013e318033dfd4. [DOI] [PubMed] [Google Scholar]
- 325.Norose K, Clark JI, Syed NA, Basu A, Heber-Katz E, Sage EH, Howe CC. SPARC deficiency leads to early-onset cataractogenesis. Invest Ophthalmol Vis Sci. 1998;39:2674–2680. [PubMed] [Google Scholar]
- 326.Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci. 2008;1123:30–40. doi: 10.1196/annals.1420.005. [DOI] [PubMed] [Google Scholar]
- 327.Norris RA, Damon B, Mironov V, Kasyanov V, Ramamurthi A, Moreno-Rodriguez R, Trusk T, Potts JD, Goodwin RL, Davis J, Hoffman S, Wen X, Sugi Y, Kern CB, Mjaatvedt CH, Turner DK, Oka T, Conway SJ, Molkentin JD, Forgacs G, Markwald RR. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2007;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Norris RA, Kern CB, Wessels A, Moralez EI, Markwald RR, Mjaatvedt CH. Identification and detection of the periostin gene in cardiac development. Anat Rec A Discov Mol Cell Evol Biol. 2004;281:1227–1233. doi: 10.1002/ar.a.20135. [DOI] [PubMed] [Google Scholar]
- 329.Norris RA, Moreno-Rodriguez R, Hoffman S, Markwald RR. The many facets of the matricelluar protein periostin during cardiac development, remodeling, and pathophysiology. J Cell Commun Signal. 2009;3:275–286. doi: 10.1007/s12079-009-0063-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Norris RA, Moreno-Rodriguez RA, Sugi Y, Hoffman S, Amos J, Hart MM, Potts JD, Goodwin RL, Markwald RR. Periostin regulates atrioventricular valve maturation. Dev Biol. 2008;316:200–213. doi: 10.1016/j.ydbio.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Norris RA, Potts JD, Yost MJ, Junor L, Brooks T, Tan H, Hoffman S, Hart MM, Kern MJ, Damon B, Markwald RR, Goodwin RL. Periostin promotes a fibroblastic lineage pathway in atrioventricular valve progenitor cells. Dev Dyn. 2009;238:1052–1063. doi: 10.1002/dvdy.21933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Noti JD. Adherence to osteopontin via alphavbeta3 suppresses phorbol ester-mediated apoptosis in MCF-7 breast cancer cells that overexpress protein kinase C-alpha. Int J Oncol. 2000;17:1237–1243. doi: 10.3892/ijo.17.6.1237. [DOI] [PubMed] [Google Scholar]
- 333.O’Brien KD, Kuusisto J, Reichenbach DD, Ferguson M, Giachelli C, Alpers CE, Otto CM. Osteopontin is expressed in human aortic valvular lesions. Circulation. 1995;92:2163–2168. doi: 10.1161/01.cir.92.8.2163. [DOI] [PubMed] [Google Scholar]
- 334.O’Regan AW, Nau GJ, Chupp GL, Berman JS. Osteopontin (Eta-1) in cell-mediated immunity: teaching an old dog new tricks. Immunol Today. 2000;21:475–478. doi: 10.1016/s0167-5699(00)01715-1. [DOI] [PubMed] [Google Scholar]
- 335.Ogawa D, Stone JF, Takata Y, Blaschke F, Chu VH, Towler DA, Law RE, Hsueh WA, Bruemmer D. Liver x receptor agonists inhibit cytokine-induced osteopontin expression in macrophages through interference with activator protein-1 signaling pathways. Circ Res. 2005;96:e59–67. doi: 10.1161/01.RES.0000163630.86796.17. [DOI] [PubMed] [Google Scholar]
- 336.Ohnishi H, Oka T, Kusachi S, Nakanishi T, Takeda K, Nakahama M, Doi M, Murakami T, Ninomiya Y, Takigawa M, Tsuji T. Increased expression of connective tissue growth factor in the infarct zone of experimentally induced myocardial infarction in rats. J Mol Cell Cardiol. 1998;30:2411–2422. doi: 10.1006/jmcc.1998.0799. [DOI] [PubMed] [Google Scholar]
- 337.Ohri R, Tung E, Rajachar R, Giachelli CM. Mitigation of ectopic calcification in osteopontin-deficient mice by exogenous osteopontin. Calcif Tissue Int. 2005;76:307–315. doi: 10.1007/s00223-004-0071-7. [DOI] [PubMed] [Google Scholar]
- 338.Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Okamoto H, Imanaka-Yoshida K. Matricellular Proteins: New Molecular Targets To Prevent Heart Failure. Cardiovasc Ther. 2011 doi: 10.1111/j.1755-5922.2011.00276.x. [DOI] [PubMed] [Google Scholar]
- 340.Ophascharoensuk V, Giachelli CM, Gordon K, Hughes J, Pichler R, Brown P, Liaw L, Schmidt R, Shankland SJ, Alpers CE, Couser WG, Johnson RJ. Obstructive uropathy in the mouse: role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int. 1999;56:571–580. doi: 10.1046/j.1523-1755.1999.00580.x. [DOI] [PubMed] [Google Scholar]
- 341.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 342.Ozbek S, Balasubramanian PG, Chiquet-Ehrismann R, Tucker RP, Adams JC. The evolution of extracellular matrix. Mol Biol Cell. 2010;21:4300–4305. doi: 10.1091/mbc.E10-03-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Paivaniemi OE, Maasilta PK, Alho HS, Vainikka TL, Salminen US. Epithelial tenascin predicts obliterative airway disease. J Heart Lung Transplant. 2008;27:400–407. doi: 10.1016/j.healun.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 344.Panek AN, Posch MG, Alenina N, Ghadge SK, Erdmann B, Popova E, Perrot A, Geier C, Dietz R, Morano I, Bader M, Ozcelik C. Connective tissue growth factor overexpression in cardiomyocytes promotes cardiac hypertrophy and protection against pressure overload. PLoS One. 2009;4:e6743. doi: 10.1371/journal.pone.0006743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Parisi MS, Gazzerro E, Rydziel S, Canalis E. Expression and regulation of CCN genes in murine osteoblasts. Bone. 2006;38:671–677. doi: 10.1016/j.bone.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 346.Pearson CA, Pearson D, Shibahara S, Hofsteenge J, Chiquet-Ehrismann R. Tenascin: cDNA cloning and induction by TGF-beta. Embo J. 1988;7:2977–2982. doi: 10.1002/j.1460-2075.1988.tb03160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Peeters AC, Kucharekova M, Timmermans J, van den Berkmortel FW, Boers GH, Novakova IR, Egging D, den Heijer M, Schalkwijk J. A clinical and cardiovascular survey of Ehlers-Danlos syndrome patients with complete deficiency of tenascin-X. Neth J Med. 2004;62:160–162. [PubMed] [Google Scholar]
- 348.Pfeffer MA, Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 349.Pichler RH, Hugo C, Shankland SJ, Reed MJ, Bassuk JA, Andoh TF, Lombardi DM, Schwartz SM, Bennett WM, Alpers CE, Sage EH, Johnson RJ, Couser WG. SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney Int. 1996;50:1978–1989. doi: 10.1038/ki.1996.520. [DOI] [PubMed] [Google Scholar]
- 350.Pluskota E, Stenina OI, Krukovets I, Szpak D, Topol EJ, Plow EF. Mechanism and effect of thrombospondin-4 polymorphisms on neutrophil function. Blood. 2005;106:3970–3978. doi: 10.1182/blood-2005-03-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Pohjolainen V, Taskinen P, Soini Y, Rysa J, Ilves M, Juvonen T, Ruskoaho H, Leskinen H, Satta J. Noncollagenous bone matrix proteins as a part of calcific aortic valve disease regulation. Hum Pathol. 2008;39:1695–1701. doi: 10.1016/j.humpath.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 352.Posey KL, Hankenson K, Veerisetty AC, Bornstein P, Lawler J, Hecht JT. Skeletal abnormalities in mice lacking extracellular matrix proteins, thrombospondin-1, thrombospondin-3, thrombospondin-5, and type IX collagen. Am J Pathol. 2008;172:1664–1674. doi: 10.2353/ajpath.2008.071094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Psarras S, Mavroidis M, Sanoudou D, Davos CH, Xanthou G, Varela AE, Panoutsakopoulou V, Capetanaki Y. Regulation of adverse remodelling by osteopontin in a genetic heart failure model. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr119. [DOI] [PubMed] [Google Scholar]
- 354.Raines EW, Lane TF, Iruela-Arispe ML, Ross R, Sage EH. The extracellular glycoprotein SPARC interacts with platelet-derived growth factor (PDGF)-AB and -BB and inhibits the binding of PDGF to its receptors. Proc Natl Acad Sci U S A. 1992;89:1281–1285. doi: 10.1073/pnas.89.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Raugi GJ, Mumby SM, Abbott-Brown D, Bornstein P. Thrombospondin: synthesis and secretion by cells in culture. J Cell Biol. 1982;95:351–354. doi: 10.1083/jcb.95.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Raugi GJ, Olerud JE, Gown AM. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987;89:551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- 358.Recchia AG, Filice E, Pellegrino D, Dobrina A, Cerra MC, Maggiolini M. Endothelin-1 induces connective tissue growth factor expression in cardiomyocytes. J Mol Cell Cardiol. 2009;46:352–359. doi: 10.1016/j.yjmcc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 359.Reed MJ, Puolakkainen P, Lane TF, Dickerson D, Bornstein P, Sage EH. Differential expression of SPARC and thrombospondin 1 in wound repair: immunolocalization and in situ hybridization. J Histochem Cytochem. 1993;41:1467–1477. doi: 10.1177/41.10.8245406. [DOI] [PubMed] [Google Scholar]
- 360.Reed MJ, Sage EH. SPARC and the extracellular matrix: implications for cancer and wound repair. Curr Top Microbiol Immunol. 1996;213 (Pt 1):81–94. doi: 10.1007/978-3-642-61107-0_6. [DOI] [PubMed] [Google Scholar]
- 361.Reed MJ, Vernon RB, Abrass IB, Sage EH. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J Cell Physiol. 1994;158:169–179. doi: 10.1002/jcp.1041580121. [DOI] [PubMed] [Google Scholar]
- 362.Rentz TJ, Poobalarahi F, Bornstein P, Sage EH, Bradshaw AD. SPARC regulates processing of procollagen I and collagen fibrillogenesis in dermal fibroblasts. J Biol Chem. 2007;282:22062–22071. doi: 10.1074/jbc.M700167200. [DOI] [PubMed] [Google Scholar]
- 363.Rios H, Koushik SV, Wang H, Wang J, Zhou HM, Lindsley A, Rogers R, Chen Z, Maeda M, Kruzynska-Frejtag A, Feng JQ, Conway SJ. periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Riser BL, Najmabadi F, Perbal B, Peterson DR, Rambow JA, Riser ML, Sukowski E, Yeger H, Riser SC. CCN3 (NOV) is a negative regulator of CCN2 (CTGF) and a novel endogenous inhibitor of the fibrotic pathway in an in vitro model of renal disease. Am J Pathol. 2009;174:1725–1734. doi: 10.2353/ajpath.2009.080241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Rittling SR, Matsumoto HN, McKee MD, Nanci A, An XR, Novick KE, Kowalski AJ, Noda M, Denhardt DT. Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res. 1998;13:1101–1111. doi: 10.1359/jbmr.1998.13.7.1101. [DOI] [PubMed] [Google Scholar]
- 366.Roberts CS, Maclean D, Maroko P, Kloner RA. Early and late remodeling of the left ventricle after acute myocardial infarction. Am J Cardiol. 1984;54:407–410. doi: 10.1016/0002-9149(84)90206-6. [DOI] [PubMed] [Google Scholar]
- 367.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–12490. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Rodriguez-Vita J, Ruiz-Ortega M, Ruperez M, Esteban V, Sanchez-Lopez E, Plaza JJ, Egido J. Endothelin-1, via ETA receptor and independently of transforming growth factor-beta, increases the connective tissue growth factor in vascular smooth muscle cells. Circ Res. 2005;97:125–134. doi: 10.1161/01.RES.0000174614.74469.83. [DOI] [PubMed] [Google Scholar]
- 369.Rollo EE, Hempson SJ, Bansal A, Tsao E, Habib I, Rittling SR, Denhardt DT, Mackow ER, Shaw RD. The cytokine osteopontin modulates the severity of rotavirus diarrhea. J Virol. 2005;79:3509–3516. doi: 10.1128/JVI.79.6.3509-3516.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Rosenberg K, Olsson H, Morgelin M, Heinegard D. Cartilage oligomeric matrix protein shows high affinity zinc-dependent interaction with triple helical collagen. J Biol Chem. 1998;273:20397–20403. doi: 10.1074/jbc.273.32.20397. [DOI] [PubMed] [Google Scholar]
- 371.Rosenberg M, Zugck C, Nelles M, Juenger C, Frank D, Remppis A, Giannitsis E, Katus HA, Frey N. Osteopontin, a new prognostic biomarker in patients with chronic heart failure. Circ Heart Fail. 2008;1:43–49. doi: 10.1161/CIRCHEARTFAILURE.107.746172. [DOI] [PubMed] [Google Scholar]
- 372.Rother M, Krohn S, Kania G, Vanhoutte D, Eisenreich A, Wang X, Westermann D, Savvatis K, Dannemann N, Skurk C, Hilfiker-Kleiner D, Cathomen T, Fechner H, Rauch U, Schultheiss HP, Heymans S, Eriksson U, Scheibenbogen C, Poller W. Matricellular signaling molecule CCN1 attenuates experimental autoimmune myocarditis by acting as a novel immune cell migration modulator. Circulation. 2010;122:2688–2698. doi: 10.1161/CIRCULATIONAHA.110.945261. [DOI] [PubMed] [Google Scholar]
- 373.Rysa J, Leskinen H, Ilves M, Ruskoaho H. Distinct upregulation of extracellular matrix genes in transition from hypertrophy to hypertensive heart failure. Hypertension. 2005;45:927–933. doi: 10.1161/01.HYP.0000161873.27088.4c. [DOI] [PubMed] [Google Scholar]
- 374.Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- 375.Sage EH. Regulation of interactions between cells and extracellular matrix: a command performance on several stages. J Clin Invest. 2001;107:781–783. doi: 10.1172/JCI12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Sage EH, Reed M, Funk SE, Truong T, Steadele M, Puolakkainen P, Maurice DH, Bassuk JA. Cleavage of the matricellular protein SPARC by matrix metalloproteinase 3 produces polypeptides that influence angiogenesis. J Biol Chem. 2003;278:37849–37857. doi: 10.1074/jbc.M302946200. [DOI] [PubMed] [Google Scholar]
- 377.Sage H, Vernon RB, Decker J, Funk S, Iruela-Arispe ML. Distribution of the calcium-binding protein SPARC in tissues of embryonic and adult mice. J Histochem Cytochem. 1989;37:819–829. doi: 10.1177/37.6.2723400. [DOI] [PubMed] [Google Scholar]
- 378.Sakamoto S, Yokoyama M, Aoki M, Suzuki K, Kakehi Y, Saito Y. Induction and function of CYR61 (CCN1) in prostatic stromal and epithelial cells: CYR61 is required for prostatic cell proliferation. Prostate. 2004;61:305–317. doi: 10.1002/pros.20098. [DOI] [PubMed] [Google Scholar]
- 379.Sasaki T, Gohring W, Mann K, Maurer P, Hohenester E, Knauper V, Murphy G, Timpl R. Limited cleavage of extracellular matrix protein BM-40 by matrix metalloproteinases increases its affinity for collagens. J Biol Chem. 1997;272:9237–9243. doi: 10.1074/jbc.272.14.9237. [DOI] [PubMed] [Google Scholar]
- 380.Sasaki T, Hohenester E, Gohring W, Timpl R. Crystal structure and mapping by site-directed mutagenesis of the collagen-binding epitope of an activated form of BM-40/SPARC/osteonectin. Embo J. 1998;17:1625–1634. doi: 10.1093/emboj/17.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Sasaki T, Miosge N, Timpl R. Immunochemical and tissue analysis of protease generated neoepitopes of BM-40 (osteonectin, SPARC) which are correlated to a higher affinity binding to collagens. Matrix Biol. 1999;18:499–508. doi: 10.1016/s0945-053x(99)00041-4. [DOI] [PubMed] [Google Scholar]
- 382.Sato A, Aonuma K, Imanaka-Yoshida K, Yoshida T, Isobe M, Kawase D, Kinoshita N, Yazaki Y, Hiroe M. Serum tenascin-C might be a novel predictor of left ventricular remodeling and prognosis after acute myocardial infarction. J Am Coll Cardiol. 2006;47:2319–2325. doi: 10.1016/j.jacc.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 383.Sato I, Shimada K. Quantitative analysis of tenascin in chordae tendineae of human left ventricular papillary muscle with aging. Ann Anat. 2001;183:443–448. doi: 10.1016/S0940-9602(01)80202-8. [DOI] [PubMed] [Google Scholar]
- 384.Sato M, Toyozaki T, Odaka K, Uehara T, Arano Y, Hasegawa H, Yoshida K, Imanaka-Yoshida K, Yoshida T, Hiroe M, Tadokoro H, Irie T, Tanada S, Komuro I. Detection of experimental autoimmune myocarditis in rats by 111In monoclonal antibody specific for tenascin-C. Circulation. 2002;106:1397–1402. doi: 10.1161/01.cir.0000027823.07104.86. [DOI] [PubMed] [Google Scholar]
- 385.Satta J, Melkko J, Pollanen R, Tuukkanen J, Paakko P, Ohtonen P, Mennander A, Soini Y. Progression of human aortic valve stenosis is associated with tenascin-C expression. J Am Coll Cardiol. 2002;39:96–101. doi: 10.1016/s0735-1097(01)01705-3. [DOI] [PubMed] [Google Scholar]
- 386.Savani RC, Zhou Z, Arguiri E, Wang S, Vu D, Howe CC, DeLisser HM. Bleomycin-induced pulmonary injury in mice deficient in SPARC. Am J Physiol Lung Cell Mol Physiol. 2000;279:L743–750. doi: 10.1152/ajplung.2000.279.4.L743. [DOI] [PubMed] [Google Scholar]
- 387.Savill J, Dransfield I, Gregory C, Haslett C. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol. 2002;2:965–975. doi: 10.1038/nri957. [DOI] [PubMed] [Google Scholar]
- 388.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Scatena M, Almeida M, Chaisson ML, Fausto N, Nicosia RF, Giachelli CM. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Scatena M, Liaw L, Giachelli CM. Osteopontin: a multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 391.Schalkwijk J, Zweers MC, Steijlen PM, Dean WB, Taylor G, van Vlijmen IM, van Haren B, Miller WL, Bristow J. A recessive form of the Ehlers-Danlos syndrome caused by tenascin-X deficiency. N Engl J Med. 2001;345:1167–1175. doi: 10.1056/NEJMoa002939. [DOI] [PubMed] [Google Scholar]
- 392.Schellings MW, Pinto YM, Heymans S. Matricellular proteins in the heart: possible role during stress and remodeling. Cardiovasc Res. 2004;64:24–31. doi: 10.1016/j.cardiores.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 393.Schellings MW, van Almen GC, Sage EH, Heymans S. Thrombospondins in the heart: potential functions in cardiac remodeling. J Cell Commun Signal. 2009;3:201–213. doi: 10.1007/s12079-009-0070-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Schellings MW, Vanhoutte D, Swinnen M, Cleutjens JP, Debets J, van Leeuwen RE, d’Hooge J, Van de Werf F, Carmeliet P, Pinto YM, Sage EH, Heymans S. Absence of SPARC results in increased cardiac rupture and dysfunction after acute myocardial infarction. J Exp Med. 2009;206:113–123. doi: 10.1084/jem.20081244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.Schellings MW, Vanhoutte D, van Almen GC, Swinnen M, Leenders JJ, Kubben N, van Leeuwen RE, Hofstra L, Heymans S, Pinto YM. Syndecan-1 amplifies angiotensin II-induced cardiac fibrosis. Hypertension. 2010;55:249–256. doi: 10.1161/HYPERTENSIONAHA.109.137885. [DOI] [PubMed] [Google Scholar]
- 396.Schenk S, Chiquet-Ehrismann R, Battegay EJ. The fibrinogen globe of tenascin-C promotes basic fibroblast growth factor-induced endothelial cell elongation. Mol Biol Cell. 1999;10:2933–2943. doi: 10.1091/mbc.10.9.2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397.Schenke-Layland K, Stock UA, Nsair A, Xie J, Angelis E, Fonseca CG, Larbig R, Mahajan A, Shivkumar K, Fishbein MC, MacLellan WR. Cardiomyopathy is associated with structural remodelling of heart valve extracellular matrix. Eur Heart J. 2009;30:2254–2265. doi: 10.1093/eurheartj/ehp267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Scherberich A, Tucker RP, Samandari E, Brown-Luedi M, Martin D, Chiquet-Ehrismann R. Murine tenascin-W: a novel mammalian tenascin expressed in kidney and at sites of bone and smooth muscle development. J Cell Sci. 2004;117:571–581. doi: 10.1242/jcs.00867. [DOI] [PubMed] [Google Scholar]
- 399.Schiemann BJ, Neil JR, Schiemann WP. SPARC inhibits epithelial cell proliferation in part through stimulation of the transforming growth factor-beta-signaling system. Mol Biol Cell. 2003;14:3977–3988. doi: 10.1091/mbc.E03-01-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Schipper ME, Scheenstra MR, van Kuik J, van Wichen DF, van der Weide P, Dullens HF, Lahpor J, de Jonge N, De Weger RA. Osteopontin: a potential biomarker for heart failure and reverse remodeling after left ventricular assist device support. J Heart Lung Transplant. 2011;30:805–810. doi: 10.1016/j.healun.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 401.Schroen B, Heymans S, Sharma U, Blankesteijn WM, Pokharel S, Cleutjens JP, Porter JG, Evelo CT, Duisters R, van Leeuwen RE, Janssen BJ, Debets JJ, Smits JF, Daemen MJ, Crijns HJ, Bornstein P, Pinto YM. Thrombospondin-2 is essential for myocardial matrix integrity: increased expression identifies failure-prone cardiac hypertrophy. Circ Res. 2004;95:515–522. doi: 10.1161/01.RES.0000141019.20332.3e. [DOI] [PubMed] [Google Scholar]
- 402.Schultz-Cherry S, Murphy-Ullrich JE. Thrombospondin causes activation of latent transforming growth factor-beta secreted by endothelial cells by a novel mechanism. J Cell Biol. 1993;122:923–932. doi: 10.1083/jcb.122.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Serebruany VL, Atar D, Murugesan SR, Jerome S, Semaan H, Gurbel PA. Effect of coronary thrombolysis on the plasma concentration of osteonectin (SPARC, BM40) in patients with acute myocardial infarction. J Thromb Thrombolysis. 2000;10:197–202. doi: 10.1023/a:1018774812613. [DOI] [PubMed] [Google Scholar]
- 404.Serebruany VL, Murugesan SR, Pothula A, Semaan H, Gurbel PA. Soluble PECAM-1, but not P-selectin, nor osteonectin identify acute myocardial infarction in patients presenting with chest pain. Cardiology. 1999;91:50–55. doi: 10.1159/000006876. [DOI] [PubMed] [Google Scholar]
- 405.Serlin DM, Kuang PP, Subramanian M, O’Regan A, Li X, Berman JS, Goldstein RH. Interleukin-1beta induces osteopontin expression in pulmonary fibroblasts. J Cell Biochem. 2006;97:519–529. doi: 10.1002/jcb.20661. [DOI] [PubMed] [Google Scholar]
- 406.Sezaki S, Hirohata S, Iwabu A, Nakamura K, Toeda K, Miyoshi T, Yamawaki H, Demircan K, Kusachi S, Shiratori Y, Ninomiya Y. Thrombospondin-1 is induced in rat myocardial infarction and its induction is accelerated by ischemia/reperfusion. Exp Biol Med (Maywood) 2005;230:621–630. doi: 10.1177/153537020523000904. [DOI] [PubMed] [Google Scholar]
- 407.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JP, Schroen B, Andre S, Crijns HJ, Gabius HJ, Maessen J, Pinto YM. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–3128. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 408.Sheffield VC, Pierpont ME, Nishimura D, Beck JS, Burns TL, Berg MA, Stone EM, Patil SR, Lauer RM. Identification of a complex congenital heart defect susceptibility locus by using DNA pooling and shared segment analysis. Hum Mol Genet. 1997;6:117–121. doi: 10.1093/hmg/6.1.117. [DOI] [PubMed] [Google Scholar]
- 409.Shi-wen X, Stanton LA, Kennedy L, Pala D, Chen Y, Howat SL, Renzoni EA, Carter DE, Bou-Gharios G, Stratton RJ, Pearson JD, Beier F, Lyons KM, Black CM, Abraham DJ, Leask A. CCN2 is necessary for adhesive responses to transforming growth factor-beta1 in embryonic fibroblasts. J Biol Chem. 2006;281:10715–10726. doi: 10.1074/jbc.M511343200. [DOI] [PubMed] [Google Scholar]
- 410.Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A. Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med. 2008;205:295–303. doi: 10.1084/jem.20071297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Shinohara ML, Jansson M, Hwang ES, Werneck MB, Glimcher LH, Cantor H. T-bet-dependent expression of osteopontin contributes to T cell polarization. Proc Natl Acad Sci U S A. 2005;102:17101–17106. doi: 10.1073/pnas.0508666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 412.Shiojima I, Walsh K. Regulation of cardiac growth and coronary angiogenesis by the Akt/PKB signaling pathway. Genes Dev. 2006;20:3347–3365. doi: 10.1101/gad.1492806. [DOI] [PubMed] [Google Scholar]
- 413.Short SM, Derrien A, Narsimhan RP, Lawler J, Ingber DE, Zetter BR. Inhibition of endothelial cell migration by thrombospondin-1 type-1 repeats is mediated by beta1 integrins. J Cell Biol. 2005;168:643–653. doi: 10.1083/jcb.200407060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Simantov R, Febbraio M, Silverstein RL. The antiangiogenic effect of thrombospondin-2 is mediated by CD36 and modulated by histidine-rich glycoprotein. Matrix Biol. 2005;24:27–34. doi: 10.1016/j.matbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 416.Singh K, Sirokman G, Communal C, Robinson KG, Conrad CH, Brooks WW, Bing OH, Colucci WS. Myocardial osteopontin expression coincides with the development of heart failure. Hypertension. 1999;33:663–670. doi: 10.1161/01.hyp.33.2.663. [DOI] [PubMed] [Google Scholar]
- 417.Singh M, Foster CR, Dalal S, Singh K. Osteopontin: role in extracellular matrix deposition and myocardial remodeling post-MI. J Mol Cell Cardiol. 2010;48:538–543. doi: 10.1016/j.yjmcc.2009.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Singh M, Foster CR, Dalal S, Singh K. Role of osteopontin in heart failure associated with aging. Heart Fail Rev. 2010;15:487–494. doi: 10.1007/s10741-010-9158-6. [DOI] [PubMed] [Google Scholar]
- 419.Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Socha MJ, Manhiani M, Said N, Imig JD, Motamed K. Secreted protein acidic and rich in cysteine deficiency ameliorates renal inflammation and fibrosis in angiotensin hypertension. Am J Pathol. 2007;171:1104–1112. doi: 10.2353/ajpath.2007.061273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Soejima H, Irie A, Fukunaga T, Oe Y, Kojima S, Kaikita K, Kawano H, Sugiyama S, Yoshimura M, Kishikawa H, Nishimura Y, Ogawa H. Osteopontin expression of circulating T cells and plasma osteopontin levels are increased in relation to severity of heart failure. Circ J. 2007;71:1879–1884. doi: 10.1253/circj.71.1879. [DOI] [PubMed] [Google Scholar]
- 422.Soon LL, Yie TA, Shvarts A, Levine AJ, Su F, Tchou-Wong KM. Overexpression of WISP-1 down-regulated motility and invasion of lung cancer cells through inhibition of Rac activation. J Biol Chem. 2003;278:11465–11470. doi: 10.1074/jbc.M210945200. [DOI] [PubMed] [Google Scholar]
- 423.Spinale FG. Myocardial matrix remodeling and the matrix metalloproteinases: influence on cardiac form and function. Physiol Rev. 2007;87:1285–1342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 424.Srivatsa SS, Harrity PJ, Maercklein PB, Kleppe L, Veinot J, Edwards WD, Johnson CM, Fitzpatrick LA. Increased cellular expression of matrix proteins that regulate mineralization is associated with calcification of native human and porcine xenograft bioprosthetic heart valves. J Clin Invest. 1997;99:996–1009. doi: 10.1172/JCI119265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179–184. [PubMed] [Google Scholar]
- 426.Stansfield WE, Andersen NM, Tang RH, Selzman CH. Periostin is a novel factor in cardiac remodeling after experimental and clinical unloading of the failing heart. Ann Thorac Surg. 2009;88:1916–1921. doi: 10.1016/j.athoracsur.2009.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF, White RT. Altered patterns of gene expression in response to myocardial infarction. Circ Res. 2000;86:939–945. doi: 10.1161/01.res.86.9.939. [DOI] [PubMed] [Google Scholar]
- 428.Steitz SA, Speer MY, McKee MD, Liaw L, Almeida M, Yang H, Giachelli CM. Osteopontin inhibits mineral deposition and promotes regression of ectopic calcification. Am J Pathol. 2002;161:2035–2046. doi: 10.1016/S0002-9440(10)64482-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 429.Stenina OI, Byzova TV, Adams JC, McCarthy JJ, Topol EJ, Plow EF. Coronary artery disease and the thrombospondin single nucleotide polymorphisms. Int J Biochem Cell Biol. 2004;36:1013–1030. doi: 10.1016/j.biocel.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 430.Stenina OI, Topol EJ, Plow EF. Thrombospondins, their polymorphisms, and cardiovascular disease. Arterioscler Thromb Vasc Biol. 2007;27:1886–1894. doi: 10.1161/ATVBAHA.107.141713. [DOI] [PubMed] [Google Scholar]
- 431.Strandjord TP, Madtes DK, Weiss DJ, Sage EH. Collagen accumulation is decreased in SPARC-null mice with bleomycin-induced pulmonary fibrosis. Am J Physiol. 1999;277:L628–635. doi: 10.1152/ajplung.1999.277.3.L628. [DOI] [PubMed] [Google Scholar]
- 432.Streit M, Riccardi L, Velasco P, Brown LF, Hawighorst T, Bornstein P, Detmar M. Thrombospondin-2: a potent endogenous inhibitor of tumor growth and angiogenesis. Proc Natl Acad Sci U S A. 1999;96:14888–14893. doi: 10.1073/pnas.96.26.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Streit M, Velasco P, Brown LF, Skobe M, Richard L, Riccardi L, Lawler J, Detmar M. Overexpression of thrombospondin-1 decreases angiogenesis and inhibits the growth of human cutaneous squamous cell carcinomas. Am J Pathol. 1999;155:441–452. doi: 10.1016/S0002-9440(10)65140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Streit M, Velasco P, Riccardi L, Spencer L, Brown LF, Janes L, Lange-Asschenfeldt B, Yano K, Hawighorst T, Iruela-Arispe L, Detmar M. Thrombospondin-1 suppresses wound healing and granulation tissue formation in the skin of transgenic mice. EMBO J. 2000;19:3272–3282. doi: 10.1093/emboj/19.13.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 435.Suezawa C, Kusachi S, Murakami T, Toeda K, Hirohata S, Nakamura K, Yamamoto K, Koten K, Miyoshi T, Shiratori Y. Time-dependent changes in plasma osteopontin levels in patients with anterior-wall acute myocardial infarction after successful reperfusion: correlation with left-ventricular volume and function. J Lab Clin Med. 2005;145:33–40. doi: 10.1016/j.lab.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 436.Swinnen M, Vanhoutte D, Van Almen GC, Hamdani N, Schellings MW, D’Hooge J, Van der Velden J, Weaver MS, Sage EH, Bornstein P, Verheyen FK, VandenDriessche T, Chuah MK, Westermann D, Paulus WJ, Van de Werf F, Schroen B, Carmeliet P, Pinto YM, Heymans S. Absence of thrombospondin-2 causes age-related dilated cardiomyopathy. Circulation. 2009;120:1585–1597. doi: 10.1161/CIRCULATIONAHA.109.863266. [DOI] [PubMed] [Google Scholar]
- 437.Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- 438.Swynghedauw B. Remodeling of the heart in chronic pressure overload. Basic Res Cardiol. 1991;86 (Suppl 1):99–105. [PubMed] [Google Scholar]
- 439.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 440.Takeshita K, Hayashi M, Iino S, Kondo T, Inden Y, Iwase M, Kojima T, Hirai M, Ito M, Loskutoff DJ, Saito H, Murohara T, Yamamoto K. Increased expression of plasminogen activator inhibitor-1 in cardiomyocytes contributes to cardiac fibrosis after myocardial infarction. Am J Pathol. 2004;164:449–456. doi: 10.1016/S0002-9440(10)63135-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Takeshita S, Kikuno R, Tezuka K, Amann E. Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J. 1993;294 (Pt 1):271–278. doi: 10.1042/bj2940271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Tamaoki M, Imanaka-Yoshida K, Yokoyama K, Nishioka T, Inada H, Hiroe M, Sakakura T, Yoshida T. Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol. 2005;167:71–80. doi: 10.1016/S0002-9440(10)62954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 443.Tamura A, Kusachi S, Nogami K, Yamanishi A, Kajikawa Y, Hirohata S, Tsuji T. Tenascin expression in endomyocardial biopsy specimens in patients with dilated cardiomyopathy: distribution along margin of fibrotic lesions. Heart. 1996;75:291–294. doi: 10.1136/hrt.75.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Tamura A, Shingai M, Aso N, Hazuku T, Nasu M. Osteopontin is released from the heart into the coronary circulation in patients with a previous anterior wall myocardial infarction. Circ J. 2003;67:742–744. doi: 10.1253/circj.67.742. [DOI] [PubMed] [Google Scholar]
- 445.Tan FL, Moravec CS, Li J, Apperson-Hansen C, McCarthy PM, Young JB, Bond M. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci U S A. 2002;99:11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Tan K, Lawler J. The interaction of Thrombospondins with extracellular matrix proteins. J Cell Commun Signal. 2009;3:177–187. doi: 10.1007/s12079-009-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-beta. J Clin Invest. 2010;120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 448.Termine JD, Kleinman HK, Whitson SW, Conn KM, McGarvey ML, Martin GR. Osteonectin, a bone-specific protein linking mineral to collagen. Cell. 1981;26:99–105. doi: 10.1016/0092-8674(81)90037-4. [DOI] [PubMed] [Google Scholar]
- 449.Thakker GD, Frangogiannis NG, Bujak M, Zymek P, Gaubatz JW, Reddy AK, Taffet G, Michael LH, Entman ML, Ballantyne CM. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H2504–2514. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 450.Thomas GJ, Speight PM. Cell adhesion molecules and oral cancer. Crit Rev Oral Biol Med. 2001;12:479–498. doi: 10.1177/10454411010120060301. [DOI] [PubMed] [Google Scholar]
- 451.Tilman G, Mattiussi M, Brasseur F, van Baren N, Decottignies A. Human periostin gene expression in normal tissues, tumors and melanoma: evidences for periostin production by both stromal and melanoma cells. Mol Cancer. 2007;6:80. doi: 10.1186/1476-4598-6-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Todorovic V, Chen CC, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J Cell Biol. 2005;171:559–568. doi: 10.1083/jcb.200504015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 454.Topol EJ, McCarthy J, Gabriel S, Moliterno DJ, Rogers WJ, Newby LK, Freedman M, Metivier J, Cannata R, O’Donnell CJ, Kottke-Marchant K, Murugesan G, Plow EF, Stenina O, Daley GQ. Single nucleotide polymorphisms in multiple novel thrombospondin genes may be associated with familial premature myocardial infarction. Circulation. 2001;104:2641–2644. doi: 10.1161/hc4701.100910. [DOI] [PubMed] [Google Scholar]
- 455.Tremble PM, Lane TF, Sage EH, Werb Z. SPARC, a secreted protein associated with morphogenesis and tissue remodeling, induces expression of metalloproteinases in fibroblasts through a novel extracellular matrix-dependent pathway. J Cell Biol. 1993;121:1433–1444. doi: 10.1083/jcb.121.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Trueblood NA, Xie Z, Communal C, Sam F, Ngoy S, Liaw L, Jenkins AW, Wang J, Sawyer DB, Bing OH, Apstein CS, Colucci WS, Singh K. Exaggerated left ventricular dilation and reduced collagen deposition after myocardial infarction in mice lacking osteopontin. Circ Res. 2001;88:1080–1087. doi: 10.1161/hh1001.090842. [DOI] [PubMed] [Google Scholar]
- 457.Tsukada B, Terasaki F, Shimomura H, Otsuka K, Otsuka K, Katashima T, Fujita S, Imanaka-Yoshida K, Yoshida T, Hiroe M, Kitaura Y. High prevalence of chronic myocarditis in dilated cardiomyopathy referred for left ventriculoplasty: expression of tenascin C as a possible marker for inflammation. Hum Pathol. 2009;40:1015–1022. doi: 10.1016/j.humpath.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 458.Tucker RP, Chiquet-Ehrismann R. The regulation of tenascin expression by tissue microenvironments. Biochim Biophys Acta. 2009;1793:888–892. doi: 10.1016/j.bbamcr.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 459.Vallejo AN, Mugge LO, Klimiuk PA, Weyand CM, Goronzy JJ. Central role of thrombospondin-1 in the activation and clonal expansion of inflammatory T cells. J Immunol. 2000;164:2947–2954. doi: 10.4049/jimmunol.164.6.2947. [DOI] [PubMed] [Google Scholar]
- 460.van Almen GC, Swinnen M, Carai P, Verhesen W, Cleutjens JP, D’Hooge J, Verheyen FK, Pinto YM, Schroen B, Carmeliet P, Heymans S. Absence of thrombospondin-2 increases cardiomyocyte damage and matrix disruption in doxorubicin-induced cardiomyopathy. J Mol Cell Cardiol. 2011;51:318–328. doi: 10.1016/j.yjmcc.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 461.van Almen GC, Verhesen W, van Leeuwen RE, van de Vrie M, Eurlings C, Schellings MW, Swinnen M, Cleutjens JP, van Zandvoort MA, Heymans S, Schroen B. MicroRNA-18 and microRNA-19 regulate CTGF and TSP-1 expression in age-related heart failure. Aging Cell. 2011;10:769–779. doi: 10.1111/j.1474-9726.2011.00714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 462.van Kimmenade RR, Januzzi JL, Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol. 2006;48:1217–1224. doi: 10.1016/j.jacc.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 463.Vanhoutte D, Schellings MW, Gotte M, Swinnen M, Herias V, Wild MK, Vestweber D, Chorianopoulos E, Cortes V, Rigotti A, Stepp MA, Van de Werf F, Carmeliet P, Pinto YM, Heymans S. Increased expression of syndecan-1 protects against cardiac dilatation and dysfunction after myocardial infarction. Circulation. 2007;115:475–482. doi: 10.1161/CIRCULATIONAHA.106.644609. [DOI] [PubMed] [Google Scholar]
- 464.Venkatachalam K, Venkatesan B, Valente AJ, Melby PC, Nandish S, Reusch JE, Clark RA, Chandrasekar B. WISP1, a pro-mitogenic, pro-survival factor, mediates tumor necrosis factor-alpha (TNF-alpha)-stimulated cardiac fibroblast proliferation but inhibits TNF-alpha-induced cardiomyocyte death. J Biol Chem. 2009;284:14414–14427. doi: 10.1074/jbc.M809757200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Venkatesan B, Prabhu SD, Venkatachalam K, Mummidi S, Valente AJ, Clark RA, Delafontaine P, Chandrasekar B. WNT1-inducible signaling pathway protein-1 activates diverse cell survival pathways and blocks doxorubicin-induced cardiomyocyte death. Cell Signal. 2010;22:809–820. doi: 10.1016/j.cellsig.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 466.Vetrone SA, Montecino-Rodriguez E, Kudryashova E, Kramerova I, Hoffman EP, Liu SD, Miceli MC, Spencer MJ. Osteopontin promotes fibrosis in dystrophic mouse muscle by modulating immune cell subsets and intramuscular TGF-beta. J Clin Invest. 2009;119:1583–1594. doi: 10.1172/JCI37662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Villarreal FJ, Griffin M, Omens J, Dillmann W, Nguyen J, Covell J. Early short-term treatment with doxycycline modulates postinfarction left ventricular remodeling. Circulation. 2003;108:1487–1492. doi: 10.1161/01.CIR.0000089090.05757.34. [DOI] [PubMed] [Google Scholar]
- 468.Volpert OV, Tolsma SS, Pellerin S, Feige JJ, Chen H, Mosher DF, Bouck N. Inhibition of angiogenesis by thrombospondin-2. Biochem Biophys Res Commun. 1995;217:326–332. doi: 10.1006/bbrc.1995.2780. [DOI] [PubMed] [Google Scholar]
- 469.Wang B, Haldar SM, Lu Y, Ibrahim OA, Fisch S, Gray S, Leask A, Jain MK. The Kruppel-like factor KLF15 inhibits connective tissue growth factor (CTGF) expression in cardiac fibroblasts. J Mol Cell Cardiol. 2008;45:193–197. doi: 10.1016/j.yjmcc.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Wang D, Oparil S, Feng JA, Li P, Perry G, Chen LB, Dai M, John SW, Chen YF. Effects of pressure overload on extracellular matrix expression in the heart of the atrial natriuretic peptide-null mouse. Hypertension. 2003;42:88–95. doi: 10.1161/01.HYP.0000074905.22908.A6. [DOI] [PubMed] [Google Scholar]
- 471.Wang JC, Lai S, Guo X, Zhang X, de Crombrugghe B, Sonnylal S, Arnett FC, Zhou X. Attenuation of fibrosis in vitro and in vivo with SPARC siRNA. Arthritis Res Ther. 12:R60. doi: 10.1186/ar2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 472.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 473.Wang M, Zhang J, Walker SJ, Dworakowski R, Lakatta EG, Shah AM. Involvement of NADPH oxidase in age-associated cardiac remodeling. J Mol Cell Cardiol. 48:765–772. doi: 10.1016/j.yjmcc.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 474.Wang X, Louden C, Ohlstein EH, Stadel JM, Gu JL, Yue TL. Osteopontin expression in platelet-derived growth factor-stimulated vascular smooth muscle cells and carotid artery after balloon angioplasty. Arterioscler Thromb Vasc Biol. 1996;16:1365–1372. doi: 10.1161/01.atv.16.11.1365. [DOI] [PubMed] [Google Scholar]
- 475.Wang X, McLennan SV, Allen TJ, Tsoutsman T, Semsarian C, Twigg SM. Adverse effects of high glucose and free fatty acid on cardiomyocytes are mediated by connective tissue growth factor. Am J Physiol Cell Physiol. 2009;297:C1490–1500. doi: 10.1152/ajpcell.00049.2009. [DOI] [PubMed] [Google Scholar]
- 476.Way KJ, Isshiki K, Suzuma K, Yokota T, Zvagelsky D, Schoen FJ, Sandusky GE, Pechous PA, Vlahos CJ, Wakasaki H, King GL. Expression of connective tissue growth factor is increased in injured myocardium associated with protein kinase C beta2 activation and diabetes. Diabetes. 2002;51:2709–2718. doi: 10.2337/diabetes.51.9.2709. [DOI] [PubMed] [Google Scholar]
- 477.Weaver MS, Sage EH, Yan Q. Absence of SPARC in lens epithelial cells results in altered adhesion and extracellular matrix production in vitro. J Cell Biochem. 2006;97:423–432. doi: 10.1002/jcb.20654. [DOI] [PubMed] [Google Scholar]
- 478.Weber KT, Janicki JS, Pick R, Abrahams C, Shroff SG, Bashey RI, Chen RM. Collagen in the hypertrophied, pressure-overloaded myocardium. Circulation. 1987;75:I40–47. [PubMed] [Google Scholar]
- 479.Welch MP, Odland GF, Clark RA. Temporal relationships of F-actin bundle formation, collagen and fibronectin matrix assembly, and fibronectin receptor expression to wound contraction. J Cell Biol. 1990;110:133–145. doi: 10.1083/jcb.110.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Wen W, Chau E, Jackson-Boeters L, Elliott C, Daley TD, Hamilton DW. TGF-ss1 and FAK regulate periostin expression in PDL fibroblasts. J Dent Res. 2010;89:1439–1443. doi: 10.1177/0022034510378684. [DOI] [PubMed] [Google Scholar]
- 481.Wenk MB, Midwood KS, Schwarzbauer JE. Tenascin-C suppresses Rho activation. J Cell Biol. 2000;150:913–920. doi: 10.1083/jcb.150.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 482.Willems IE, Arends JW, Daemen MJ. Tenascin and fibronectin expression in healing human myocardial scars. J Pathol. 1996;179:321–325. doi: 10.1002/(SICI)1096-9896(199607)179:3<321::AID-PATH555>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 483.Willems IE, Havenith MG, De Mey JG, Daemen MJ. The alpha-smooth muscle actin-positive cells in healing human myocardial scars. Am J Pathol. 1994;145:868–875. [PMC free article] [PubMed] [Google Scholar]
- 484.Wintergerst ES, Jelk J, Rahner C, Asmis R. Apoptosis induced by oxidized low density lipoprotein in human monocyte-derived macrophages involves CD36 and activation of caspase-3. Eur J Biochem. 2000;267:6050–6059. doi: 10.1046/j.1432-1327.2000.01682.x. [DOI] [PubMed] [Google Scholar]
- 485.Wittchen F, Suckau L, Witt H, Skurk C, Lassner D, Fechner H, Sipo I, Ungethum U, Ruiz P, Pauschinger M, Tschope C, Rauch U, Kuhl U, Schultheiss HP, Poller W. Genomic expression profiling of human inflammatory cardiomyopathy (DCMi) suggests novel therapeutic targets. J Mol Med. 2007;85:257–271. doi: 10.1007/s00109-006-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Woods A. Syndecans: transmembrane modulators of adhesion and matrix assembly. J Clin Invest. 2001;107:935–941. doi: 10.1172/JCI12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Wrana JL, Maeno M, Hawrylyshyn B, Yao KL, Domenicucci C, Sodek J. Differential effects of transforming growth factor-beta on the synthesis of extracellular matrix proteins by normal fetal rat calvarial bone cell populations. J Cell Biol. 1988;106:915–924. doi: 10.1083/jcb.106.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 488.Wu RX, Laser M, Han H, Varadarajulu J, Schuh K, Hallhuber M, Hu K, Ertl G, Hauck CR, Ritter O. Fibroblast migration after myocardial infarction is regulated by transient SPARC expression. J Mol Med. 2006;84:241–252. doi: 10.1007/s00109-005-0026-0. [DOI] [PubMed] [Google Scholar]
- 489.Wunderlich K, Senn BC, Todesco L, Flammer J, Meyer P. Regulation of connective tissue growth factor gene expression in retinal vascular endothelial cells by angiogenic growth factors. Graefes Arch Clin Exp Ophthalmol. 2000;238:910–915. doi: 10.1007/s004170000199. [DOI] [PubMed] [Google Scholar]
- 490.Xia Y, Dobaczewski M, Gonzalez-Quesada C, Chen W, Biernacka A, Li N, Lee DW, Frangogiannis NG. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–911. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–481. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Xie Z, Singh M, Singh K. ERK1/2 and JNKs, but not p38 kinase, are involved in reactive oxygen species-mediated induction of osteopontin gene expression by angiotensin II and interleukin-1beta in adult rat cardiac fibroblasts. J Cell Physiol. 2004;198:399–407. doi: 10.1002/jcp.10419. [DOI] [PubMed] [Google Scholar]
- 493.Xie Z, Singh M, Singh K. Osteopontin modulates myocardial hypertrophy in response to chronic pressure overload in mice. Hypertension. 2004;44:826–831. doi: 10.1161/01.HYP.0000148458.03202.48. [DOI] [PubMed] [Google Scholar]
- 494.Xu SW, Howat SL, Renzoni EA, Holmes A, Pearson JD, Dashwood MR, Bou-Gharios G, Denton CP, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 induces expression of matrix-associated genes in lung fibroblasts through MEK/ERK. J Biol Chem. 2004;279:23098–23103. doi: 10.1074/jbc.M311430200. [DOI] [PubMed] [Google Scholar]
- 495.Yamamoto K, Dang QN, Kennedy SP, Osathanondh R, Kelly RA, Lee RT. Induction of tenascin-C in cardiac myocytes by mechanical deformation. Role of reactive oxygen species. J Biol Chem. 1999;274:21840–21846. doi: 10.1074/jbc.274.31.21840. [DOI] [PubMed] [Google Scholar]
- 496.Yan Q, Clark JI, Wight TN, Sage EH. Alterations in the lens capsule contribute to cataractogenesis in SPARC-null mice. J Cell Sci. 2002;115:2747–2756. doi: 10.1242/jcs.115.13.2747. [DOI] [PubMed] [Google Scholar]
- 497.Yang Z, Kyriakides TR, Bornstein P. Matricellular proteins as modulators of cell-matrix interactions: adhesive defect in thrombospondin 2-null fibroblasts is a consequence of increased levels of matrix metalloproteinase-2. Mol Biol Cell. 2000;11:3353–3364. doi: 10.1091/mbc.11.10.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 498.Yeger H, Perbal B. The CCN family of genes: a perspective on CCN biology and therapeutic potential. J Cell Commun Signal. 2007;1:159–164. doi: 10.1007/s12079-008-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Yehualaeshet T, O’Connor R, Green-Johnson J, Mai S, Silverstein R, Murphy-Ullrich JE, Khalil N. Activation of rat alveolar macrophage-derived latent transforming growth factor beta-1 by plasmin requires interaction with thrombospondin-1 and its cell surface receptor, CD36. Am J Pathol. 1999;155:841–851. doi: 10.1016/s0002-9440(10)65183-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Yokosaki Y, Tanaka K, Higashikawa F, Yamashita K, Eboshida A. Distinct structural requirements for binding of the integrins alphavbeta6, alphavbeta3, alphavbeta5, alpha5beta1 and alpha9beta1 to osteopontin. Matrix Biol. 2005;24:418–427. doi: 10.1016/j.matbio.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 501.Yoon PO, Lee MA, Cha H, Jeong MH, Kim J, Jang SP, Choi BY, Jeong D, Yang DK, Hajjar RJ, Park WJ. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol. 2010;49:294–303. doi: 10.1016/j.yjmcc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 502.Yoshida Y, Togi K, Matsumae H, Nakashima Y, Kojima Y, Yamamoto H, Ono K, Nakamura T, Kita T, Tanaka M. CCN1 protects cardiac myocytes from oxidative stress via beta1 integrin-Akt pathway. Biochem Biophys Res Commun. 2007;355:611–618. doi: 10.1016/j.bbrc.2007.01.195. [DOI] [PubMed] [Google Scholar]
- 503.Yoshitake H, Rittling SR, Denhardt DT, Noda M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc Natl Acad Sci U S A. 1999;96:8156–8160. doi: 10.1073/pnas.96.14.8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Young GD, Murphy-Ullrich JE. Molecular interactions that confer latency to transforming growth factor-beta. J Biol Chem. 2004;279:38032–38039. doi: 10.1074/jbc.M405658200. [DOI] [PubMed] [Google Scholar]
- 505.Yu PJ, Skolnick A, Ferrari G, Heretis K, Mignatti P, Pintucci G, Rosenzweig B, Diaz-Cartelle J, Kronzon I, Perk G, Pass HI, Galloway AC, Grossi EA, Grau JB. Correlation between plasma osteopontin levels and aortic valve calcification: potential insights into the pathogenesis of aortic valve calcification and stenosis. J Thorac Cardiovasc Surg. 2009;138:196–199. doi: 10.1016/j.jtcvs.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 506.Zagzag D, Friedlander DR, Miller DC, Dosik J, Cangiarella J, Kostianovsky M, Cohen H, Grumet M, Greco MA. Tenascin expression in astrocytomas correlates with angiogenesis. Cancer Res. 1995;55:907–914. [PubMed] [Google Scholar]
- 507.Zagzag D, Shiff B, Jallo GI, Greco MA, Blanco C, Cohen H, Hukin J, Allen JC, Friedlander DR. Tenascin-C promotes microvascular cell migration and phosphorylation of focal adhesion kinase. Cancer Res. 2002;62:2660–2668. [PubMed] [Google Scholar]
- 508.Zhao XM, Hu Y, Miller GG, Mitchell RN, Libby P. Association of thrombospondin-1 and cardiac allograft vasculopathy in human cardiac allografts. Circulation. 2001;103:525–531. doi: 10.1161/01.cir.103.4.525. [DOI] [PubMed] [Google Scholar]
- 509.Zhao Y, Young SL, McIntosh JC. Induction of tenascin in rat lungs undergoing bleomycin-induced pulmonary fibrosis. Am J Physiol. 1998;274:L1049–1057. doi: 10.1152/ajplung.1998.274.6.L1049. [DOI] [PubMed] [Google Scholar]
- 510.Zheng B, Duan C, Clemmons DR. The effect of extracellular matrix proteins on porcine smooth muscle cell insulin-like growth factor (IGF) binding protein-5 synthesis and responsiveness to IGF-I. J Biol Chem. 1998;273:8994–9000. doi: 10.1074/jbc.273.15.8994. [DOI] [PubMed] [Google Scholar]
- 511.Zhou HM, Wang J, Elliott C, Wen W, Hamilton DW, Conway SJ. Spatiotemporal expression of periostin during skin development and incisional wound healing: lessons for human fibrotic scar formation. J Cell Commun Signal. 2010;4:99–107. doi: 10.1007/s12079-010-0090-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 512.Zick Y, Eisenstein M, Goren RA, Hadari YR, Levy Y, Ronen D. Role of galectin-8 as a modulator of cell adhesion and cell growth. Glycoconj J. 2004;19:517–526. doi: 10.1023/B:GLYC.0000014081.55445.af. [DOI] [PubMed] [Google Scholar]
- 513.Zohar R, Zhu B, Liu P, Sodek J, McCulloch CA. Increased cell death in osteopontin-deficient cardiac fibroblasts occurs by a caspase-3-independent pathway. Am J Physiol Heart Circ Physiol. 2004;287:H1730–1739. doi: 10.1152/ajpheart.00098.2004. [DOI] [PubMed] [Google Scholar]
- 514.Zwicker JI, Pevandi F, Palla R, Lombardi R, Canciani MT, Cairo A, Ardissino D, Bernardinelli L, Bauer KA, Lawler J, Mannucci PM. The thrombospondin-1 N700S polymorphism is associated with early myocardial infarction without altering von Willebrand Factor multimer size. Blood. 2006 doi: 10.1182/blood-2006-04-015701. [DOI] [PMC free article] [PubMed] [Google Scholar]