Abstract
Objective
The mechanisms by which histamine increases microvascular permeability remain poorly understood. We tested the hypothesis that H1 receptor activation disrupts the endothelial barrier and investigated potential downstream signals.
Methods
We used confluent endothelial cell (EC) monolayers, assessing transendothelial electrical resistance (TER) as an index of barrier function. Human umbilical vein EC (HUVEC), cardiac microvascular EC (HCMEC), and dermal microvascular EC (HDMEC) were compared. Receptor expression was investigated using Western blotting, immunofluorescence (IF) confocal microscopy and RT-PCR. Receptor function and downstream signaling pathways were tested using pharmacologic antagonists and inhibitors, respectively.
Results
We identified H1-H4 receptors on all three EC types. H1 antagonists did not affect basal TER but prevented the histamine-induced decrease in TER. Blockade of H2 or H3 attenuated the histamine response only in HDMEC, while inhibition of H4 attenuated the response only in HUVEC. Combined inhibition of both PKC and PI3K caused exaggerated histamine-induced barrier dysfunction in HDMEC, whereas inhibition of p38 MAP kinase attenuated the histamine response in all three EC types. Inhibition of RhoA, ROCK, or MLCK also prevented the histamine-induced decrease in TER in HDMEC.
Conclusion
The data suggest that multiple signaling pathways contribute to histamine-induced endothelial barrier dysfunction via the H1 receptor.
Keywords: Histamine, endothelial cells, barrier dysfunction, permeability
Introduction
Blood-tissue exchange occurs primarily in capillaries and postcapillary venules. Inflammation increases the permeability of these microvessels, which when excessive can lead to tissue dysfunction as seen in a variety of pathological conditions. Although elevated microvascular leakage is commonly associated with injury, inflammation, hypertension, heart disease and diabetes, there are still no specific curative treatments available [14,17].
Histamine, a principal mediator of inflammation, causes endothelium-dependent vasodilation, elevated microvascular permeability of postcapillary venules, and other cardiovascular effects [3,24,38,42,62]. Several studies have identified molecular signals that contribute to histamine-induced microvascular hyperpermeability using EC monolayer and intact microvessel models. These signals include elevation of intracellular free Ca2+ levels, activation of PKC and Akt, as well as pathways involving eNOS-cGMP-PKG and MEK-1/2 [13,26,40,59]. Histamine also causes a rapid, short-lived increase in phosphorylation of myosin light chains (MLC) by MLC kinase (MLCK) in EC, disrupting peripheral actin and causing cell retraction [45,53]. Involvement of the RhoA/ROCK pathway, which also promotes MLC phosphorylation through inhibition of the MLC phosphatase, has also been reported [58]. Other studies have described histamine-induced tyrosine phosphorylation of VE-cadherin, its dissociation from β-catenin and its sequestration from junctions in cultured EC [1,18,51]. Likewise, electron microscopy studies described formation of gaps between EC in postcapillary venules in response to histamine [37,60]. Despite these advancements, several questions remain about how histamine elicits increased endothelial permeability.
One key question that remains incompletely answered is which histamine receptors contribute to the increase in permeability. There are four known histamine receptors (H1-H4), which are all G protein-coupled receptors (GCPRs), [22]. These receptors differ in location, histamine binding affinity and second messengers activated. Previous studies of histamine receptors on EC revealed differential effects on intracellular free Ca2+ as well as endothelial permeability [25,41,54] that may be due to variable expression of these receptors, resulting in diverse intracellular responses [12,21,27]. H1 receptors reportedly mediate a host of intracellular events that cause elevation of intracellular free Ca2+ whereas H2 activation elicits an elevation of cAMP [20]. The H1 receptor has been reported to be coupled to Gq and is thought to increase endothelial permeability [56]. In this scheme, H1 activation leads to elevation in PLC activity, formation of IP3 and DAG, mobilization of Ca2+ from intracellular stores and downstream activation of PKC [56,57]. The H2 receptor is reported to be Gs-coupled and increases cAMP, leading to activation of PKA and enhanced barrier function [54]. The H3 and H4 receptors may both be coupled to Gi and can signal to decrease net cAMP levels [23,34]. It is important to note that several of the aforementioned studies utilized non-microvascular endothelial cell models. Thus, it is not entirely clear whether these reported couplings between second messengers and particular histamine receptors reflect the true signaling paradigms of the microvascular endothelium.
In this study we investigated the roles of the H1, H2, H3 and H4 receptors in histamine-induced endothelial hyperpermeability. Cultured EC models allow the study of specific endothelial signaling mechanisms [14]. A problem with these cultured EC models has been the relatively weak histamine-induced increases in permeability compared to intact tissue models [62]. However, our recent finding that cultured dermal lymphatic EC produce a stronger response than human umbilical vein EC [7] prompted us to test whether microvascular EC monolayer models may more appropriately reflect intact venules. In addition, we investigated whether the H1, H2, H3 and H4 histamine receptors have differential involvement between EC from different tissue sources. Lastly, we examined downstream intracellular signaling pathways that might mediate histamine-induced endothelial barrier dysfunction in these models.
Methods
Materials
Clonetics microvascular endothelial growth medium (EGM), endothelial basal medium (EBM), and cryopreserved adult human dermal and human cardiac EC (HDMEC and HCMEC, respectively), human umbilical vein EC (HUVEC), all pooled from multiple donors, were purchased from Lonza (Basel, Switzerland). Rat brain EC (RBMEC) was purchased from ScienCell (Carlsbad, CA). Histamine dihydrochloride (catalog no. H7250) was purchased from Sigma (St. Louis, MO). Mepyramine, Cimetidine, Ciproxifan, Cetirizine dihydrochloride, H1152, ML-7, GFX109203X (GFX), PI828 (PI) and Immepip dihydrobromide were purchased from Tocris (Bristol, UK). SB 203580 (SB) and Y16 were purchased from EMD-Millipore (Billerica, MA). JNJ 7777120 was purchased from Johnson & Johnson (New Brunswick, NJ).
Cell culture and determination of barrier function
HDMEC, HCMEC and HUVEC were seeded on gelatin-coated culture plates and maintained in EGM at 37°C, 5% CO2 humidified incubator. An Electrical Cell-Substrate Impedance Sensor (ECIS) ΖΘ System (Applied Biophysics, Troy, NY) was used for barrier function measurements as previously described [7]. Briefly, the cells were seeded at a density of 1.5 × 105 cells per well in gelatin-coated ECIS 8W1E or 96W1E arrays. A single small gold electrode (250 μm diameter) and a large counter electrode are in each well. The cells were allowed to attach overnight and form a confluent monolayer. A 1 V, AC signal at 4 kHz was applied from an approximate constant current source (<1 μA), and the ECIS instrument monitored the voltage across the electrodes and its phase relative to the applied current, providing a report of total impedance. Treating the cell-electrode system as a series RC circuit, the ECIS system converted the impedance data to resistance and capacitance of the cell monolayer, which represent barrier function and membrane capacitance, respectively. Transendothelial resistance (TER) is presented as an index of endothelial barrier function.
Experimental protocols
Confluent HDMEC, HCMEC or HUVEC monolayers displaying a baseline TER of greater than 5000 Ω were included in the study. The medium was changed to Clonetics endothelial basal medium (EBM) at least 2 h prior to the experimental protocols. After measuring a steady baseline TER, histamine was applied at 10 μM, which typically causes a brief yet significant and consistent decrease in TER in HUVEC [7,18,45]. To test roles of different histamine receptors, cells were pretreated with the H1 antagonist mepyramine, H2 antagonist cimetidine, H3 antagonist ciproxifan, or H4 antagonist JNJ 7777120 for 30 min prior to the addition of histamine. In other experiments, cells were pretreated with SB, GFX or PI for 30 min prior to histamine treatment to test the involvement of p38 MAP kinase, PKC, or PI3K, respectively. Additionally, cells were pretreated for 30 min with H1152, Y16, or ML-7 prior to histamine treatment to test the involvement of ROCK, RhoA or MLCK, respectively. The concentrations of the inhibitors used here were selective for the protein of interest and close to the IC50 for each drug.
Western Blot Analysis
Protein was obtained by lysis of cells in ice-cold RIPA buffer (EMD-Millipore) containing 1X HALT protease and phosphatase inhibitor cocktail (Pierce, Rockford, IL) for 15 min. Protein concentrations were determined with the Bradford assay for equilibration prior to loading for SDS-PAGE. Proteins were transferred to nitrocellulose for immunoblotting. The following dilutions of primary antibodies were used: 1:500 rabbit anti-H1 (GeneTex, Irvine, TX, GTX102924), 1:500 rabbit anti-H2 (GeneTex, Irvine, TX, GTX108152), 1:500 rabbit anti-H3 (GeneTex, GTX100187) and 1:500 rabbit anti-H4 (GeneTex, GTX100387). After application of 1:1000 donkey anti-rabbit-HRP secondary antibodies (Abcam, Cambridge, MA, cat. no. ab97064), bands were visualized by chemiluminescence (WestPico Supersignal kit, Thermo-Fisher, Waltham, MA) alongside a Bionexus BNPM50 prestained protein marker (Bionexus, Oakland, CA) to determine relative mobility, using a Bio-Rad Chemi Doc XRS+ System with Quantity 1-D Analysis Software (Bio-Rad, Hercules, CA).
Immunofluorescence Labeling and Microscopy
Cells were grown on gelatin-coated coverslips (VWR, Radnor, PA) and allowed to reach confluence. The cells were then fixed at 25°C in 4% paraformaldehyde in PBS. Next, the cells were incubated for 1 h at 25°C in a 5% BSA blocking solution followed by overnight incubation at 4°C with primary antibody: 1:500 anti-H1 (GeneTex, Irvine, TX, cat. no. GTX102924), 1:500 anti-H2 (GeneTex, GTX108152), 1:500 anti-H3 (GeneTex, GTX100187), 1:500 anti-H4 (GeneTex, GTX100387) or rabbit polyclonal IgG isotype control 1:500 (Abcam, Cambridge, MA, ab27478). After washing 3X with TBST, cells were incubated for 1 h at 25°C with the appropriate AlexaFluor488-conjugated secondary antibodies at 1:500 (Life Technologies, Grand Island, NY, cat no. A21206 or A11055), and washed again 3X with TBST followed by one TBS wash. The coverslips were mounted on glass slides with ProLong Gold antifade reagent with DAPI (Life Technologies, cat. no. P36931) labeling the nuclei. Images were obtained with an Olympus FV1000 MPE confocal microscope system (Olympus America Inc., Center Valley, PA). Images were acquired with a 60X UPLAN APO 1.42NA oil objective and collected fluorescence signal using appropriate dichroic mirrors and spectral windows. Confocal z-stacks (0.2-μm interval) were captured with FV10-ASW version 3.0 software.
RT-PCR
Total RNA was isolated from HUVEC, HDMEC and HCMEC using the RNA Mini Kit (Ambion, Grand Island, NY) according to the manufacturer's specifications. RNA concentration and purity was determined using NanoVue (GE Healthcare, Pittsburgh, PA). Total RNA was reverse-transcribed into cDNA with 500 ng of total RNA. RT-PCR was performed using TaqMan Master Mix (Applied Biosystems, Carlsbad, CA). Reactions were performed using a 10 min hold at 95°C, then 95°C for 15 s and at 60°C for 1 min repeated for 40 cycles using RealTime PCR System (Applied Biosystems, Carlsbad, CA). The primer sequences used were NCBI Reference Sequence: NM_021624.3 (H4R), NCBI Reference Sequence: NM_007232.2 (H3R), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Expression of target genes was normalized to that of GAPDH and the mean value set to 1.0. The PCR reaction was run on a 1% agarose gel with SYBR green (Invitrogen, Waltham, MA) and visualized under UVI Safe HD2, transilluminator (UVITECH Limited, Cambridge, UK).
Data analysis
The maximum changes in TER in response to various treatments were averaged and are presented as means ± S.E. T-tests were used to test the significance between two groups. For three or more groups, one-way ANOVA followed by Tukey's multiple comparisons test was used. Significance was accepted at P < 0.05.
Results
Histamine decreases endothelial cell barrier function
We addressed whether histamine causes barrier dysfunction of cultured microvascular EC in a similar manner as previously observed in HUVEC [7]. We compared the histamine-induced changes in TER of confluent HCMEC, HDMEC and HUVEC (Fig. 1A-C). Histamine (10 μM) caused a significant decrease in TER in all three EC types (Fig. 1D). However the duration of the response, i.e. the time for the cell monolayers to recover to baseline TER, was significantly longer in HDMEC monolayers than for HCMEC or HUVEC (Fig. 1E).
Fig. 1. Comparison of Histamine induced changes in TER.
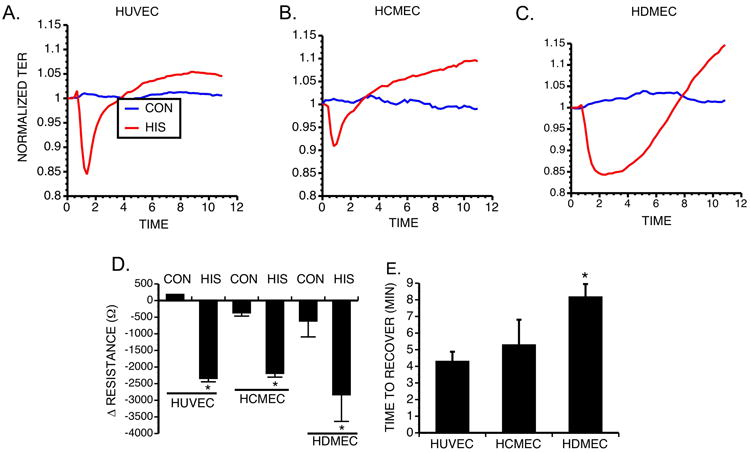
Representative tracing of TER for HUVEC (A), HCMEC (B) and HDMEC (C). (D) Maximum change in resistance of HUVEC, HCMEC and HDMEC upon treatment with 10 μM histamine (n=4 for each group). *P<0.01 compared to respective control. (E) Comparison of the recovery time in HUVEC, HCMEC and HDMEC (n=4 for each group).
Histamine receptor expression
Previous investigations indicate that H1 and H2 histamine receptors are present on ECs [50,54]. However, given that the differences in the time courses of histamine-induced decreases in TER of HUVEC, HDMEC and HCMEC, we investigated whether differential expression of histamine receptors in these EC types may account for the variability. Using Western blot analysis, we identified the presence of H1, H2, H3 and H4 on HUVEC, HDMEC and HCMEC (Fig. 2). Rat brain endothelial cells served as a positive control [29]. HUVEC consistently displayed a relatively weak band for the H1 receptor, and somewhat stronger band for the H3 receptor than the other cell types.
Fig. 2. Western Analysis identifying the Histamine receptors on EC.
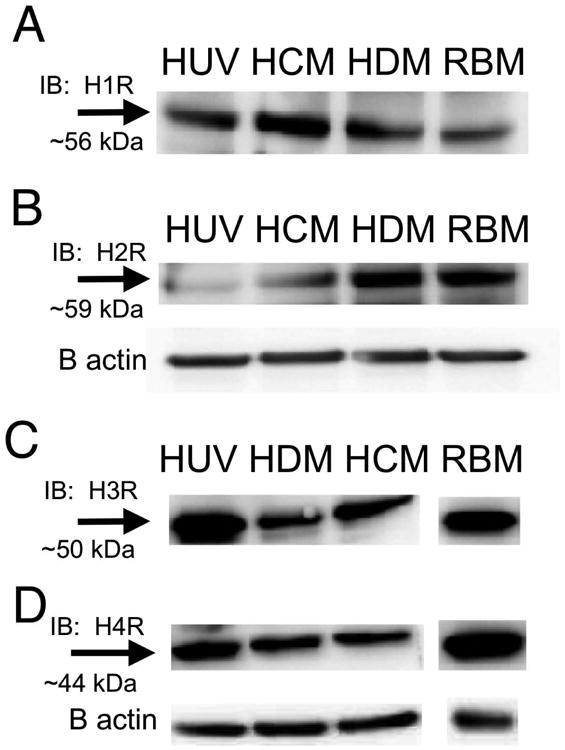
(A) Blots are shown for the H1R (A) H2R (B) H3R (C) and H4R (D). All blots shown are representative of three experiments. Rat brain endothelial cells were used as a positive control.
We also observed localization of these receptors by immunofluorescence confocal microscopy (Fig. 3). These experiments utilized non-permeabilized cells in order to determine surface labeling on the plasma membrane. Control experiments were performed using no primary antibody or an isotype-matched primary antibody control (Supplemental Fig. 1). Each receptor presented distinct localization on the EC surface. Fig. 3 shows projections of the confocal z-stacks for each histamine receptor, and Movie 1 provides an example of 3D reconstruction. H1R was observed throughout the cell surface in all the cell types. Strong labeling of the H2R appeared mainly in perinuclear clusters. The H3R labeling had a punctate distribution on the surface of HUVEC with varying degrees of intensity between different cells, with a similar yet more intense pattern on HCMEC. In HDMEC and HCMEC, H3R labeling was more restricted to the surface above and near the nucleus, with more perinuclear labeling evident in HUVEC. A punctate labeling of the H4R was detected in all three cell types (Fig. 3A). We also performed these experiments using permeabilized cells and wide-field microscopy, and the distributions of the various histamine receptors appeared largely the same as with non-permeabilized cells (data not shown). Because the presence of the H3R and H4R to our knowledge, has not been previously shown in any of the three endothelial cell types in the current study, we verified that the mRNA for the H3R and H4R were present using RT-PCR, indicating active transcription of both of these receptors and supporting the protein detection data (Supplemental Fig 2). GAPDH was used as an internal control for these studies.
Fig. 3. Identification the Histamine receptor on EC using immunocytochemistry.
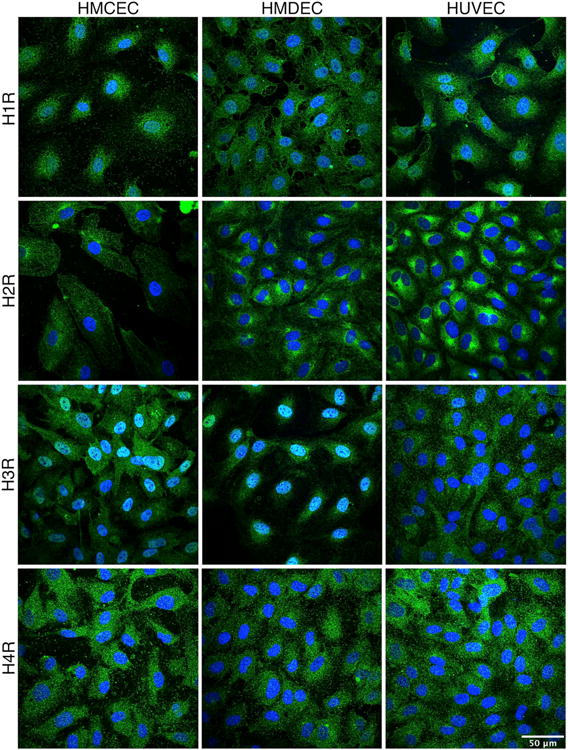
HUVEC, HCMEC, and HDMEC were immunolabeled to visualize localization of the H1R (A), H2R (B), H3R (C) and H4R (D), all labeled in green. Nuclei are labeled in blue. Images are projections of confocal z-stacks, and are representative of three separate experiments for each EC type. Images of the negative controls for labeling are provided in the supplemental data (Suppl. Fig. 1).
Differential inhibition of histamine-induced endothelial barrier dysfunction depending on receptor and tissue source
We tested the roles of different histamine receptor subtypes using specific pharmacological receptor antagonists. The inhibitors alone at the concentrations used did not significantly alter baseline TER prior to addition of histamine. Mepyramine (10 μM), an H1 receptor antagonist with inverse agonist properties, or the more potent H1 receptor antagonist Cetirizine dihydrochloride (100 nM), significantly inhibited the response to histamine in all three endothelial cell types (Fig 4). Cimetidine (10 μM), an H2 receptor-specific antagonist, did not block histamine-induced decreases in TER in HCMEC or HUVEC, but caused a significant inhibition of this response in HDMEC (Fig 5). Similarly, Ciproxifan (1 μM), an H3 receptor-specific antagonist, did not significantly affect histamine-induced decreases in TER in HCMEC or HUVEC but caused a partial, significant inhibition in HDMEC (Fig 6). In contrast, the H4 antagonist, JNJ 7777120 (10 μM) attenuated the response to histamine in HUVEC but had no effect on the histamine-induced decrease in TER in the other cell types (Fig 7). Interestingly, application of immepip, which activates both H3 and H4, caused a brief decrease in TER in both HUVEC and HDMEC (Supplemental Fig. 3), in support of the H3 and H4 antagonist findings. The results indicate differential involvement of the various histamine receptors in endothelial cells, depending upon tissue origin.
Fig. 4. Blockade of H1 and Histamine-Induced Changes in TER.
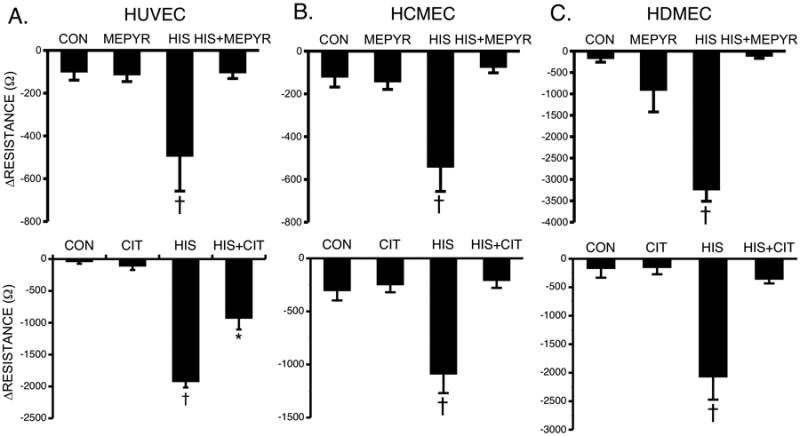
(A) Mean maximal changes in TER of HUVEC pretreated with 10 μM mepyramine (top, n=12 for all groups) or 100 nM cetirizine (bottom, n=8 for all groups) for 30 minutes prior to stimulation with 10 μM histamine for 10 minutes (B) Mean maximal changes in TER of HCMEC pretreated with 10 μM mepyramine (top, n=14 for all groups) or 100 nM cetirizine (bottom, n=7 for all groups) for 30 minutes prior to stimulation with 10 μM histamine for 10 minutes. (C) Mean maximal changes in TER of HDMEC pretreated with 10 μM mepyramine (top, n=8) or 100 nM cetirizine (bottom, n=4) for 30 minutes prior to stimulation with 10 μM histamine for 10 minutes. *P<0.01 versus control group. †P<0.01 versus all other groups.
Fig. 5. Blockade of H2 and Histamine-Induced Changes in TER.
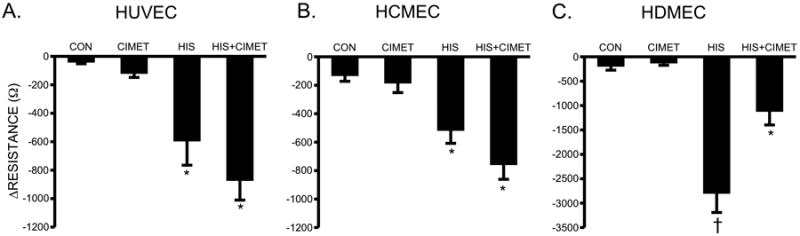
Mean changes in TER of HUVEC (A), HCMEC (B), and HDMEC (C) pretreated with 10 μM cimetidine for 30 minutes prior to stimulation with 10 μM histamine for 10 minutes. †P<0.01 versus all other groups. *P<0.05 versus control. For all HUVEC groups, n=16; for all HCMEC groups, n=12; for all HDMEC groups, n=10.
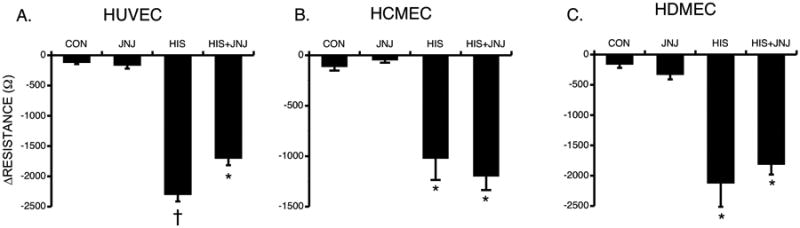
Fig. 6. Blockade of H3 and Histamine-Induced Changes in TER.
(A) Mean changes in TER of HUVEC (A), HCMEC (B), and HDMEC (C) pretreated with 100 nM ciproxifan or vehicle for 30 minutes prior to stimulation with 10 μM histamine for 10 minutes. *P<0.001 versus control. †P<0.01 versus all other groups. For all HUVEC and HCMEC groups, n=8; for all HDMEC groups, n=12.
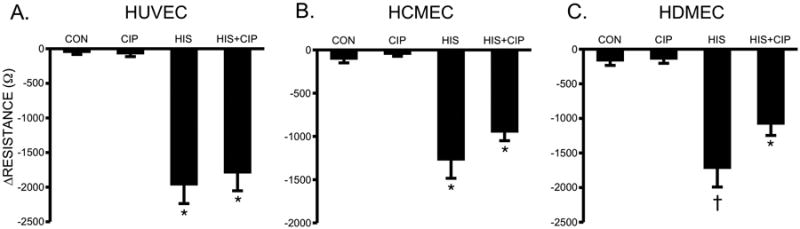
Fig. 7. Blockade of H4 and Histamine-Induced Changes in TER.
(A) Mean changes in TER of HUVEC (A), HCMEC (B), and HDMEC (C) pretreated with 10 μM JNJ or vehicle for 30 minutes prior to stimulation with 10 μM histamine for 10 minutes. Values are means ± SE. *P<0.001 versus control. †P<0.001 versus all other groups. For all HUVEC groups, n=8; for all HCMEC groups, n=4; for all HDMEC groups, n=16.
Effect of PI3K or PKC inhibitors on TER upon histamine treatment
Since activation of both PI3K and PKC by histamine has been reported in the endothelium, we tested their roles in barrier function in HDMEC monolayers. Pretreatment with an inhibitor of either PI3K (PI828, 100 nM) or PKC (GF109203X, 100 nM) did not affect the histamine-induced decrease in TER (Fig. 8). To our surprise, the combination of both PI828 and GF109203X significantly augmented the decrease in TER compared to histamine alone in HDMEC (Fig 8). No effect was observed in HCMEC or HUVEC with inhibition of PI3K, PKC, or their combination (data not shown).
Fig. 8. Effect of inhibitors of PKC and PI3K on TER of HDMEC treated with histamine.
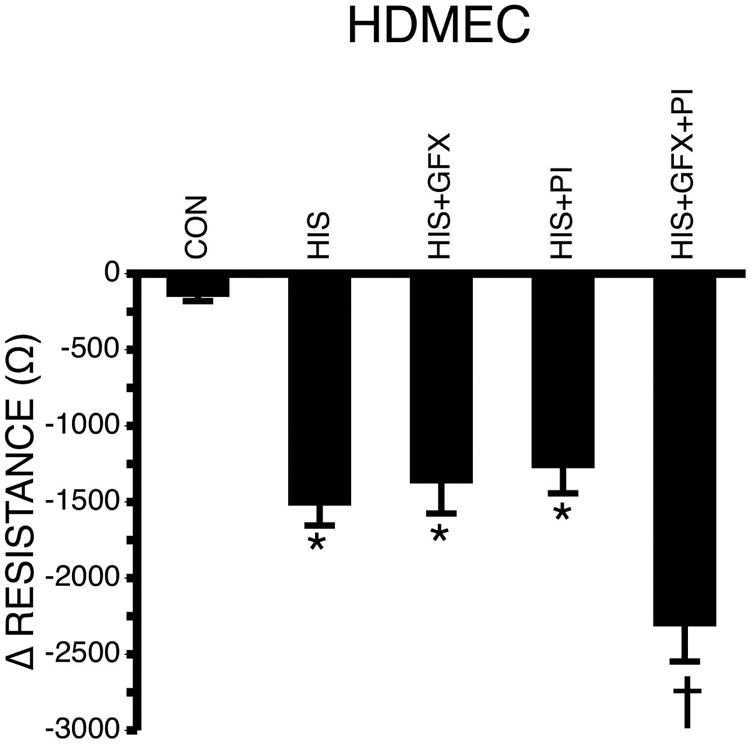
HDMEC were pretreated with 100 nM GFX and/or 100 nM PI 828 in the presence of 10 μM histamine for 30 minutes (n=28 each group). Values are means ± SE. *P<0.001 versus control. †P<0.05 versus all other groups.
Role of p38 MAP kinase in histamine-induced endothelial barrier dysfunction
We evaluated the role of the p38 MAP kinase pathway, which has been reported to be activated by the H1R [49]. Pretreatment with the specific p38 MAP kinase inhibitor SB203580 (10 μM) significantly attenuated the histamine-induced change in TER in all three EC types (Fig 9). SB203580 alone at this concentration had no effect on baseline TER.
Fig. 9. Blockade of p38 MAP kinase and Histamine-Induced Changes in TER.
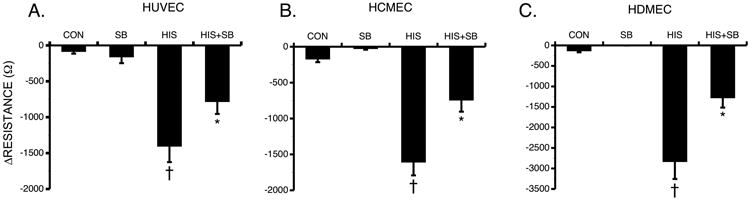
Mean changes in TER of HUVEC (A), HCMEC (B), and HDMEC (C) pretreated with 10 μM SB203580 or vehicle followed by 10 μM histamine for 30 minutes *P<0.01 versus control. †P0.05 compared to all other groups. For all HUVEC groups, n=8; for all HCMEC groups, n=24; for all HDMEC groups, n=8.
Role of MLCK, RhoA, and ROCK in histamine-induced endothelial barrier dysfunction
We also investigated signals that control the phosphorylation of MLC in endothelial cells, which has previously been linked to changes in permeability. Myosin light chain kinase (MLCK) was identified to have a role in histamine-induced increases in permeability of coronary venules [63] and cultured endothelial cell monolayers [61]. The selective inhibitor of MLCK (ML-7, 1 μM) attenuated the decrease in TER induced by histamine in HDMEC monolayers (Fig 10A).
Fig. 10. Inhibition of MLCK, RhoA and ROCK and Histamine-Induced Changes in TER.
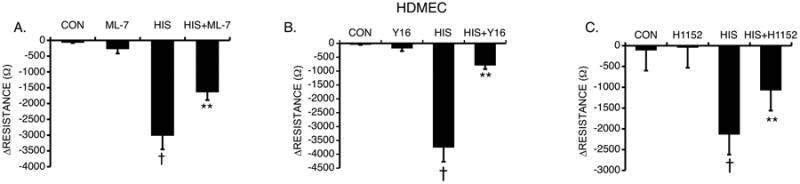
HDMEC were pretreated with either (A) 1 μM ML-7 (n=16), (B) 10 μM Y16 (n=8), (C) 100 nM H1152 (n=8) or vehicle followed by histamine (10 μM) for 30 minutes. **P<0.001 compared to control. †P<0.01 compared to all other groups.
Inhibitors of Rho and ROCK have been shown to prevent the decreases in TER in HUVEC and human lymphatic endothelial cells [7]. Rho increases the phosphorylation of MLC via ROCK, which inhibits the activity of MLC phosphatase via phosphorylation of its regulatory/targeting subunit MYPT-1 [58]. To investigate the role of these proteins in HDMEC, the cells were pretreated with selective inhibitors of RhoA (Y16, 10 μM) or ROCK (H1152, 100 nM) prior to treatment with histamine (Fig 10B, C). Both inhibitors significantly reduced the histamine-induced decrease in TER in HDMEC. We also tested the combined inhibition of p38 MAPK and either RhoA or ROCK, but found no additional effect compared to the inhibitors alone (Suppl. Fig 4).
Discussion
In the current study, we observed differences in the time course of histamine-induced endothelial barrier dysfunction in monolayers of human EC derived from different tissues. We detected expression of all four known histamine receptors in all three EC types. We observed some minor differences in the expression of the H1, H2, H3 and H4 histamine receptors among the different EC, and each receptor had its own distinct distribution on EC. Notably, there were functional differences in the histamine receptor subtypes and downstream signaling pathways involved in histamine-induced barrier dysfunction. Understanding these characteristics is important for linking the findings obtained in cell culture models to observations of histamine-induced microvascular permeability seen in vivo or with intact venule models. Furthermore, the variation in responses of the EC from different tissues may reflect important tissue-specific responses to histamine by the microcirculation.
Histamine has a well-established role as a prominent inflammatory mediator and stimulator of endothelium-dependent vasodilation and increased microvascular permeability [24,37]. However, many questions have remained about how histamine causes elevated permeability of the endothelium. In many pathological conditions associated with inflammation, the endothelium becomes locally hyperpermeable for a period of time due to formation of microscopic gaps or pores in the endothelium. Previous cell culture model studies showed that histamine induces a rapid and transient decrease in barrier function evidenced by decreased TER in HUVEC [7,18,44,57]. In the current study, a similar response was observed with HUVEC and HCMEC, however histamine produced a longer lasting change in TER with HDMEC (Fig. 1D). The time for recovery was the quickest in HUVEC with longest recovery times in HDMEC (Fig 1E), better reflecting observations in postcapillary venules in vivo [60].
The immunolabeling of H1 and H2 receptors in all three EC types was remarkably similar to labeling we have observed in the EC of intact rat mesenteric lymphatic vessels [31]. Our detection of the H3 and H4 receptors on all three EC types was an unexpected finding, as to our knowledge expression of H3 and H4 mRNA has only been shown in rat brain endothelial cells [29]. Our Western blots for H3 and H4 produced bands (Fig 3A) with the predicted mobility for these receptors and we further confirmed our findings with the presence of mRNA for H3 and H4 in EC (Supplemental Fig 2). Therefore, our results from these experiments strongly support that these receptors are present in EC.
The impact of histamine on endothelial barrier function seems to be mediated primarily via the H1R because selective antagonists of this receptor significantly inhibited the effects of histamine in all three EC types (Fig 4). Pharmacological blockade of the H2R (Fig 5) or H3R (Fig 6) did not inhibit histamine-induced decreases in TER of HUVEC or HCMEC monolayers but attenuated the drop in TER in HDMEC. On the other hand, inhibition of the H4R (Fig 7) caused a small, yet statistically significant attenuation of the histamine response in HUVEC but did not affect the response in HDMEC or HCMEC. While the reason for this remains unknown, this may reflect that a higher sensitivity of HDMEC to histamine due to their role in the histaminergic effect in skin inflammatory and allergic reactions. Our data leads us to speculate that HDMEC may have a functional state that allows histamine receptors to be more responsive to histamine, or alternatively that the downstream signaling pathways associated with the different histamine receptors favor a stronger response in the HDMEC compared to HCMEC and HUVEC. We think this possibility is likely because histamine is known to activate multiple receptors and we observed that inhibition of H1, H2, or H3 can significantly attenuate the overall response in HDMEC. On the other hand activation of the H3 and H4 using immepip caused a decrease in TER in HUVEC and HDMEC with very little effect on HCMEC (Supp. Fig 3). This time courses of the immepip-induced decreases in TER were slightly different between HUVEC and HDMEC. This finding warrants a detailed future study because all three endothelial cell types had unique TER changes when immepip was applied, which could be used to reveal more insight into the overall mechanism.
Previous studies have reported that the H1R is coupled to Gq/11, H2R to Gs whereas the H3 and H4R are coupled to Gi/o [2,12,22]. The increase in intracellular free Ca2+ due to activation of H1R has been linked to elevated endothelial permeability, whereas activation of the H2R has been reported to elevate intracellular cAMP, decreasing endothelial permeability [54,57]. The H3R can signal via both Ca2+ and cAMP upon activation, however it decreases cAMP levels [36]. The H4R is the most recently discovered of all the receptors and its function is mostly demonstrated in cells of the immune system [55]. H1 and H2 receptors are widely distributed whereas H3 was previously identified mainly in brain and was shown to have an effect on skin circulation post burn injury [25,29,48]. The H4R was shown previously to be largely expressed in hemopoietic cells and has chemotactic properties indicating a role in the immune system [64]. A surprising novel finding of the current study was the identification of all four receptors on EC from multiple human tissues (Fig 2), each with their own unique cellular distribution (Fig 3). H1, H2 and H4 appear to be widely distributed on the cells with H2 having clusters near the nucleus. On the other hand, the H3 receptor seems to be located largely in close proximity to the nucleus. The cellular location of the receptors could be an important factor determining the effect each has on barrier function, and represents a future area of study.
The signal transduction mechanisms we observed to be involved provide additional clues about the mechanism of action in histamine-induced changes in endothelial permeability. The coupling of Gq to the H1R can lead to activation of PLC, PKC and PI3K via an increase in intracellular free Ca2+ [61]. The histamine-induced increase in intracellular free Ca2+ has been described in many cell types [8,30]. This elevation in calcium has been thought to promote signals to the cytoskeleton and endothelial junctions that result in an increase in endothelial permeability [9,16,51]. Others have shown that inhibitors of PI3K can improve endothelial barrier function, which could be selective to isoform function [10,32]. In contrast to some of the reports above, in the current study inhibition of either PKC or PI3K did not have an effect on the TER in response to histamine. This finding indicates that signals independent of the PI3K and PKC pathways must be responsible for the histamine-induced drops in TER. Interestingly, combined inhibition of both PI3K and PKC augmented the barrier dysfunction caused by histamine (Fig 8) in HDMEC. These data suggest the possibility that the combination of these signals is important for regulating the overall response to histamine in dermal endothelium, i.e. preventing an exaggerated response, a potentially interesting topic for future study
The H1R coupling to Gq/11 can activate RhoA independently of the PLC pathway [35]. Activation of RhoA and its downstream effector ROCK leads to accumulation of dually phosphorylated MLC on its regulatory domain, permitting actin-myosin-mediated contraction of the cells, thereby increasing permeability [52,61]. Our findings that inhibition of RhoA or ROCK attenuates histamine-induced decreases in TER are in agreement with these previous reports. In addition, we also studied MLCK, which is directly responsible for phosphorylating the MLC regulatory domain. Inhibition of MLCK almost completely blocked the histamine-induced decrease in TER. These results suggest that phosphorylation of MLC has a central role in histamine-induced barrier dysfunction of HDMEC. This finding supports observations in previous reports using HUVEC monolayers and isolated venules [57,63].
In addition, we investigated the p38 MAPK kinase because of its involvement in inflammation, response to stress and microvascular hyperpermeability in the lung [5,6,11]. Blockade of p38 MAPK activity has previously been shown to inhibit increases in endothelial permeability in response to VEGF, TNF, pertussis toxin, and H2O2 [15,28,46,47]. In the current study, blocking p38 MAPK partially inhibited the histamine-induced decrease in TER in all three EC cell types (Fig 9). These data suggest a universal role for p38 MAPK in the endothelial response to histamine. However, because only a partial inhibition was achieved, there must also be other parallel pathway(s) independent of p38 MAPK that mediate histamine-induced endothelial barrier dysfunction. We tested whether p38 MAPK may act in parallel with RhoA/ROCK signaling. However, combining p38 MAPK inhibition with blockade of either RhoA or ROCK did not produce additional inhibition of histamine-induced decreases in TER beyond that of RhoA or ROCK blockade alone (Supplemental Fig. 4). Additional studies will be needed to provide better detail as to where these signaling molecules fit in the overall signaling pathway. Another future direction is to determine whether the p38 MAPK is activated via the H1 receptor associated G protein (Gq/11) or a different pathway likely via Gi signaling [33,49]. Additionally, examining the βγ subunits represents another potential area of investigation because they have been identified in the signaling response activated by H1R [39].
Based on our data we are of the opinion that histamine increases endothelial permeability primarily by acting on H1R, while other receptors may have a supportive role in certain tissues like the skin. MLCK, the RhoA/ROCK pathway, and p38 MAP kinase signaling both appear to act as signals, and blockade of each only accounts partially for the ultimate response, since the response was partially attenuated when either was blocked. We are of the opinion that the activation MLC is likely a focal point in the signaling pathway leading to the increase in permeability.
There is a wide use of histamine receptor antagonists for treating allergies and gastric ulcers. In addition, there are investigations into their potential cardiovascular protective effects, and involvement in growth of certain tumors [4,17,43]. Moreover, the H4R has also been shown to have therapeutic potential in atopic dermatitis [19], because of its expression in CD4+T cells and TH2 cells. Despite all of this, the molecular regulation of histamine receptors, particularly in the vascular endothelium, remains incompletely understood. This study implicates the importance of the H1 receptor in EC from different tissues in mediating histamine-induced endothelial hyperpermeability. In addition, the data highlight the importance of multiple histamine receptors in HDMEC, representative of the skin microcirculation in histamine-induced endothelial hyperpermeability. Lastly, this study provides novel evidence for the presence of H3 and H4 receptors on endothelial cells and a role of p38 MAPK in histamine-induced endothelial hyperpermeability.
Perspectives
This data increases our understanding of how histamine elicits increased microvascular permeability. In addition, it provides general clues about the functional states of endothelial cells in different microvascular beds. Such knowledge would be useful for developing new selective therapeutic targets in addition to providing novel perspectives for the prevention and treatment of systemic inflammation
Supplementary Material
Fig S1. Images of the negative controls for labeling. HUVEC, HCMEC, and HDMEC were immunolabeled to visualize localization of the H1R (A), H2R (B), H3R (C) and H4R (D), all labeled in green. Nuclei are labeled in blue. Images are projections of confocal z-stacks, and are representative of three separate experiments for each EC type.
Fig S2. mRNA identifying H3R, H4R and GAPDH in HCMEC, HUVEC, and HDMEC.
Fig S3. Effect of Immepip on endothelial cell resistance. (A) HUVEC, (B) HCMEC, and (C) HDMEC were treated with 0.1-10μM immepip until the cells recovered (n=4).
Fig S4. Effect of inhibition of RhoA, ROCK and p38 on Histamine-Induced Changes in TER (A) HDMEC were pretreated with either Y16 (10 μM) or SB (10 μM) alone or in combination (B) HDMEC were pretreated with either H1152 (100 nM) or SB (10 μM) alone or in combination in the presence of histamine (10 μM) for 30 minutes. Values are means ± SE. †P<0.01 versus all other groups (p < 0.01). N=8 for all groups.
Movie 1. 3D Reconstruction of confocal imaging showing the location of the receptors on endothelial cells. The confocal z-stacks of HUVEC labeling of H1, H2, H3, and H4 (green), counterlabeled with nuclei (blue) are all shown.
Acknowledgments
The authors thank Ms. Sandhya Sarangan for her assistance with performing Western blots and immunofluorescence labeling. In addition the authors thank Dr. Hana Totary-Jain with her assistance with the RT-PCR and Dr. Byeong Jake Cha for his assistance with confocal imaging.
Grants: Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R01HL098215. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Andriopoulou P, Navarro P, Zanetti A, Lampugnani MG, Dejana E. Histamine induces tyrosine phosphorylation of endothelial cell-to-cell adherens junctions. Arterioscler Thromb Vasc Biol. 1999;19:2286–2297. doi: 10.1161/01.atv.19.10.2286. [DOI] [PubMed] [Google Scholar]
- 2.Baker JG. Antagonist affinity measurements at the Gi-coupled human histamine H3 receptor expressed in CHO cells. BMC pharmacology. 2008;8:9. doi: 10.1186/1471-2210-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baxter LT, Jain RK, Svensjo E. Vascular permeability and interstitial diffusion of macromolecules in the hamster cheek pouch: effects of vasoactive drugs. Microvasc Res. 1987;34:336–348. doi: 10.1016/0026-2862(87)90066-5. [DOI] [PubMed] [Google Scholar]
- 4.Blaya B, Nicolau-Galmes F, Jangi SM, Ortega-Martinez I, Alonso-Tejerina E, Burgos-Bretones J, Perez-Yarza G, Asumendi A, Boyano MD. Histamine and histamine receptor antagonists in cancer biology. Inflammation & allergy drug targets. 2010;9:146–157. doi: 10.2174/187152810792231869. [DOI] [PubMed] [Google Scholar]
- 5.Bogatcheva NV, Adyshev D, Mambetsariev B, Moldobaeva N, Verin AD. Involvement of microtubules, p38, and Rho kinases pathway in 2-methoxyestradiol-induced lung vascular barrier dysfunction. American journal of physiology Lung cellular and molecular physiology. 2007;292:L487–499. doi: 10.1152/ajplung.00217.2006. [DOI] [PubMed] [Google Scholar]
- 6.Borbiev T, Birukova A, Liu F, Nurmukhambetova S, Gerthoffer WT, Garcia JG, Verin AD. p38 MAP kinase-dependent regulation of endothelial cell permeability. American journal of physiology Lung cellular and molecular physiology. 2004;287:L911–918. doi: 10.1152/ajplung.00372.2003. [DOI] [PubMed] [Google Scholar]
- 7.Breslin JW. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphatic research and biology. 2011;9:3–11. doi: 10.1089/lrb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock TA, Capasso EA. Thrombin and histamine activate phospholipase C in human endothelial cells via a phorbol ester-sensitive pathway. Journal of cellular physiology. 1988;136:54–62. doi: 10.1002/jcp.1041360107. [DOI] [PubMed] [Google Scholar]
- 9.Budworth RA, Anderson M, Clothier RH, Leach L. Histamine-induced Changes in the Actin Cytoskeleton of the Human Microvascular Endothelial Cell line HMEC-1. Toxicology in vitro : an international journal published in association with BIBRA. 1999;13:789–795. doi: 10.1016/s0887-2333(99)00052-1. [DOI] [PubMed] [Google Scholar]
- 10.Cain RJ, Vanhaesebroeck B, Ridley AJ. The PI3K p110alpha isoform regulates endothelial adherens junctions via Pyk2 and Rac1. J Cell Biol. 2010;188:863–876. doi: 10.1083/jcb.200907135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Damarla M, Hasan E, Boueiz A, Le A, Pae HH, Montouchet C, Kolb T, Simms T, Myers A, Kayyali US, Gaestel M, Peng X, Reddy SP, Damico R, Hassoun PM. Mitogen activated protein kinase activated protein kinase 2 regulates actin polymerization and vascular leak in ventilator associated lung injury. PloS one. 2009;4:e4600. doi: 10.1371/journal.pone.0004600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Del Valle J, Gantz I. Novel insights into histamine H2 receptor biology. The American journal of physiology. 1997;273:G987–996. doi: 10.1152/ajpgi.1997.273.5.G987. [DOI] [PubMed] [Google Scholar]
- 13.Di Lorenzo A, Fernandez-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc Natl Acad Sci U S A. 2009;106:14552–14557. doi: 10.1073/pnas.0904073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duran WN, Sanchez FA, Breslin JW. Microcirculatory Exchange Function. In: Tuma RF, Duran WN, Ley K, editors. Handbook of Physiology: Microcirculation. 2nd. San Diego, CA: Academic Press - Elsevier; 2008. pp. 81–124. [Google Scholar]
- 15.Garcia JG, Wang P, Schaphorst KL, Becker PM, Borbiev T, Liu F, Birukova A, Jacobs K, Bogatcheva N, Verin AD. Critical involvement of p38 MAP kinase in pertussis toxin-induced cytoskeletal reorganization and lung permeability. Faseb J. 2002;16:1064–1076. doi: 10.1096/fj.01-0895com. [DOI] [PubMed] [Google Scholar]
- 16.Gardner TW, Lesher T, Khin S, Vu C, Barber AJ, Brennan WA., Jr Histamine reduces ZO-1 tight-junction protein expression in cultured retinal microvascular endothelial cells. The Biochemical journal. 1996;320(Pt 3):717–721. doi: 10.1042/bj3200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Genovese A, Spadaro G. Highlights in cardiovascular effects of histamine and H1-receptor antagonists. Allergy. 1997;52:67–78. doi: 10.1111/j.1398-9995.1997.tb04813.x. [DOI] [PubMed] [Google Scholar]
- 18.Guo M, Breslin JW, Wu MH, Gottardi CJ, Yuan SY. VE-cadherin and beta-catenin binding dynamics during histamine-induced endothelial hyperpermeability. American journal of physiology Cell physiology. 2008;294:C977–984. doi: 10.1152/ajpcell.90607.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gutzmer R, Mommert S, Gschwandtner M, Zwingmann K, Stark H, Werfel T. The histamine H4 receptor is functionally expressed on T(H)2 cells. The Journal of allergy and clinical immunology. 2009;123:619–625. doi: 10.1016/j.jaci.2008.12.1110. [DOI] [PubMed] [Google Scholar]
- 20.Hekimian G, Cote S, Van Sande J, Boeynaems JM. H2 receptor-mediated responses of aortic endothelial cells to histamine. The American journal of physiology. 1992;262:H220–224. doi: 10.1152/ajpheart.1992.262.1.H220. [DOI] [PubMed] [Google Scholar]
- 21.Heltianu C, Simionescu M, Simionescu N. Histamine receptors of the microvascular endothelium revealed in situ with a histamine-ferritin conjugate: characteristic high-affinity binding sites in venules. The Journal of cell biology. 1982;93:357–364. doi: 10.1083/jcb.93.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill SJ, Ganellin CR, Timmerman H, Schwartz JC, Shankley NP, Young JM, Schunack W, Levi R, Haas HL. International Union of Pharmacology. XIII. Classification of histamine receptors. Pharmacological reviews. 1997;49:253–278. [PubMed] [Google Scholar]
- 23.Hofstra CL, Desai PJ, Thurmond RL, Fung-Leung WP. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. The Journal of pharmacology and experimental therapeutics. 2003;305:1212–1221. doi: 10.1124/jpet.102.046581. [DOI] [PubMed] [Google Scholar]
- 24.Horan KL, Adamski SW, Ayele W, Langone JJ, Grega GJ. Evidence that prolonged histamine suffusions produce transient increases in vascular permeability subsequent to the formation of venular macromolecular leakage sites. Proof of the Majno-Palade hypothesis. Am J Pathol. 1986;123:570–576. [PMC free article] [PubMed] [Google Scholar]
- 25.Hossen MA, Fujii Y, Sugimoto Y, Kayasuga R, Kamei C. Histamine H3 receptors regulate vascular permeability changes in the skin of mast cell-deficient mice. Int Immunopharmacol. 2003;3:1563–1568. doi: 10.1016/S1567-5769(03)00009-2. [DOI] [PubMed] [Google Scholar]
- 26.Huang Q, Yuan Y. Interaction of PKC and NOS in signal transduction of microvascular hyperpermeability. Am J Physiol. 1997;273:H2442–2451. doi: 10.1152/ajpheart.1997.273.5.H2442. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda K, Utoguchi N, Makimoto H, Mizuguchi H, Nakagawa S, Mayumi T. Different reactions of aortic and venular endothelial cell monolayers to histamine on macromolecular permeability: role of cAMP, cytosolic Ca2+ and F-actin. Inflammation. 1999;23:87–97. doi: 10.1023/a:1020295718728. [DOI] [PubMed] [Google Scholar]
- 28.Issbrucker K, Marti HH, Hippenstiel S, Springmann G, Voswinckel R, Gaumann A, Breier G, Drexler HC, Suttorp N, Clauss M. p38 MAP kinase--a molecular switch between VEGF-induced angiogenesis and vascular hyperpermeability. Faseb J. 2003;17:262–264. doi: 10.1096/fj.02-0329fje. [DOI] [PubMed] [Google Scholar]
- 29.Karlstedt K, Jin C, Panula P. Expression of histamine receptor genes Hrh3 and Hrh4 in rat brain endothelial cells. British journal of pharmacology. 2013;170:58–66. doi: 10.1111/bph.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim JH, Park SH, Moon YW, Hwang S, Kim D, Jo SH, Oh SB, Kim JS, Jahng JW, Lee JH, Lee SJ, Choi SY, Park K. Histamine H1 receptor induces cytosolic calcium increase and aquaporin translocation in human salivary gland cells. The Journal of pharmacology and experimental therapeutics. 2009;330:403–412. doi: 10.1124/jpet.109.153023. [DOI] [PubMed] [Google Scholar]
- 31.Kurtz KH, Moor AN, Souza-Smith FM, Breslin JW. Involvement of H1 and H2 receptors and soluble guanylate cyclase in histamine-induced relaxation of rat mesenteric collecting lymphatics. Microcirculation. 2014 doi: 10.1111/micc.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee KS, Park SJ, Kim SR, Min KH, Jin SM, Puri KD, Lee YC. Phosphoinositide 3-kinase-delta inhibitor reduces vascular permeability in a murine model of asthma. The Journal of allergy and clinical immunology. 2006;118:403–409. doi: 10.1016/j.jaci.2006.04.041. [DOI] [PubMed] [Google Scholar]
- 33.Leurs R, Traiffort E, Arrang JM, Tardivel-Lacombe J, Ruat M, Schwartz JC. Guinea pig histamine H1 receptor. II. Stable expression in Chinese hamster ovary cells reveals the interaction with three major signal transduction pathways. Journal of neurochemistry. 1994;62:519–527. doi: 10.1046/j.1471-4159.1994.62020519.x. [DOI] [PubMed] [Google Scholar]
- 34.Lovenberg TW, Roland BL, Wilson SJ, Jiang X, Pyati J, Huvar A, Jackson MR, Erlander MG. Cloning and functional expression of the human histamine H3 receptor. Molecular pharmacology. 1999;55:1101–1107. [PubMed] [Google Scholar]
- 35.Lutz S, Freichel-Blomquist A, Yang Y, Rumenapp U, Jakobs KH, Schmidt M, Wieland T. The guanine nucleotide exchange factor p63RhoGEF, a specific link between Gq/11-coupled receptor signaling and RhoA. The Journal of biological chemistry. 2005;280:11134–11139. doi: 10.1074/jbc.M411322200. [DOI] [PubMed] [Google Scholar]
- 36.MacGlashan D., Jr Histamine: A mediator of inflammation. The Journal of allergy and clinical immunology. 2003;112:S53–59. doi: 10.1016/s0091-6749(03)01877-3. [DOI] [PubMed] [Google Scholar]
- 37.Majno G, Palade G. Studies on inflammation I. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Cytol. 1961;11:597–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Majno G, Palade GE, Schoefl GI. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maruko T, Nakahara T, Sakamoto K, Saito M, Sugimoto N, Takuwa Y, Ishii K. Involvement of the betagamma subunits of G proteins in the cAMP response induced by stimulation of the histamine H1 receptor. Naunyn-Schmiedeberg's archives of pharmacology. 2005;372:153–159. doi: 10.1007/s00210-005-0001-x. [DOI] [PubMed] [Google Scholar]
- 40.Mayhan WG. Nitric oxide accounts for histamine-induced increases in macromolecular extravasation. Am J Physiol. 1994;266:H2369–2373. doi: 10.1152/ajpheart.1994.266.6.H2369. [DOI] [PubMed] [Google Scholar]
- 41.McLeod RL, Mingo GG, Kreutner W, Hey JA. Effect of combined histamine H1 and H3 receptor blockade on cutaneous microvascular permeability elicited by compound 48/80. Life sciences. 2005;76:1787–1794. doi: 10.1016/j.lfs.2004.08.033. [DOI] [PubMed] [Google Scholar]
- 42.Miles AA, Miles EM. Vascular reactions to histamine, histamine-liberator and leukotaxine in the skin of guinea-pigs. J Physiol. 1952;118:228–257. doi: 10.1113/jphysiol.1952.sp004789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morrey C, Estephan R, Abbott GW, Levi R. Cardioprotective effect of histamine H3-receptor activation: pivotal role of G beta gamma-dependent inhibition of voltage-operated Ca2+ channels. The Journal of pharmacology and experimental therapeutics. 2008;326:871–878. doi: 10.1124/jpet.108.137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moy AB, Blackwell K, Kamath A. Differential effects of histamine and thrombin on endothelial barrier function through actin-myosin tension. American journal of physiology Heart and circulatory physiology. 2002;282:H21–29. doi: 10.1152/ajpheart.2002.282.1.H21. [DOI] [PubMed] [Google Scholar]
- 45.Moy AB, Van Engelenhoven J, Bodmer J, Kamath J, Keese C, Giaever I, Shasby S, Shasby DM. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. The Journal of clinical investigation. 1996;97:1020–1027. doi: 10.1172/JCI118493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niwa K, Inanami O, Ohta T, Ito S, Karino T, Kuwabara M. p38 MAPK and Ca2+ contribute to hydrogen peroxide-induced increase of permeability in vascular endothelial cells but ERK does not. Free Radic Res. 2001;35:519–527. doi: 10.1080/10715760100301531. [DOI] [PubMed] [Google Scholar]
- 47.Nwariaku FE, Chang J, Zhu X, Liu Z, Duffy SL, Halaihel NH, Terada L, Turnage RH. The role of p38 map kinase in tumor necrosis factor-induced redistribution of vascular endothelial cadherin and increased endothelial permeability. Shock. 2002;18:82–85. doi: 10.1097/00024382-200207000-00015. [DOI] [PubMed] [Google Scholar]
- 48.Rantfors J, Cassuto J. Role of histamine receptors in the regulation of edema and circulation postburn. Burns : journal of the International Society for Burn Injuries. 2003;29:769–777. doi: 10.1016/s0305-4179(03)00203-1. [DOI] [PubMed] [Google Scholar]
- 49.Robinson AJ, Dickenson JM. Activation of the p38 and p42/p44 mitogen-activated protein kinase families by the histamine H(1) receptor in DDT(1)MF-2 cells. British journal of pharmacology. 2001;133:1378–1386. doi: 10.1038/sj.bjp.0704200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rotrosen D, Gallin JI. Histamine type I receptor occupancy increases endothelial cytosolic calcium, reduces F-actin, and promotes albumin diffusion across cultured endothelial monolayers. J Cell Biol. 1986;103:2379–2387. doi: 10.1083/jcb.103.6.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shasby DM, Ries DR, Shasby SS, Winter MC. Histamine stimulates phosphorylation of adherens junction proteins and alters their link to vimentin. American journal of physiology Lung cellular and molecular physiology. 2002;282:L1330–1338. doi: 10.1152/ajplung.00329.2001. [DOI] [PubMed] [Google Scholar]
- 52.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovascular research. 2010;87:243–253. doi: 10.1093/cvr/cvq086. [DOI] [PubMed] [Google Scholar]
- 53.Srinivas SP, Satpathy M, Guo Y, Anandan V. Histamine-induced phosphorylation of the regulatory light chain of myosin II disrupts the barrier integrity of corneal endothelial cells. Investigative ophthalmology & visual science. 2006;47:4011–4018. doi: 10.1167/iovs.05-1127. [DOI] [PubMed] [Google Scholar]
- 54.Takeda T, Yamashita Y, Shimazaki S, Mitsui Y. Histamine decreases the permeability of an endothelial cell monolayer by stimulating cyclic AMP production through the H2-receptor. J Cell Sci. 1992;101(Pt 4):745–750. doi: 10.1242/jcs.101.4.745. [DOI] [PubMed] [Google Scholar]
- 55.Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nature reviews Drug discovery. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 56.van Hinsbergh VW, van Nieuw Amerongen GP. Intracellular signalling involved in modulating human endothelial barrier function. Journal of anatomy. 2002;200:549–560. doi: 10.1046/j.1469-7580.2002.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Nieuw Amerongen GP, Draijer R, Vermeer MA, van Hinsbergh VW. Transient and prolonged increase in endothelial permeability induced by histamine and thrombin: role of protein kinases, calcium, and RhoA. Circulation research. 1998;83:1115–1123. doi: 10.1161/01.res.83.11.1115. [DOI] [PubMed] [Google Scholar]
- 58.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. Journal of cell science. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 59.Wu MH, Yuan SY, Granger HJ. The protein kinase MEK1/2 mediate vascular endothelial growth factor- and histamine-induced hyperpermeability in porcine coronary venules. The Journal of physiology. 2005;563:95–104. doi: 10.1113/jphysiol.2004.076075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu NZ, Baldwin AL. Transient venular permeability increase and endothelial gap formation induced by histamine. The American journal of physiology. 1992;262:H1238–1247. doi: 10.1152/ajpheart.1992.262.4.H1238. [DOI] [PubMed] [Google Scholar]
- 61.Yuan SY. Signal transduction pathways in enhanced microvascular permeability. Microcirculation. 2000;7:395–403. [PubMed] [Google Scholar]
- 62.Yuan Y, Granger HJ, Zawieja DC, DeFily DV, Chilian WM. Histamine increases venular permeability via a phospholipase C-NO synthase-guanylate cyclase cascade. The American journal of physiology. 1993;264:H1734–1739. doi: 10.1152/ajpheart.1993.264.5.H1734. [DOI] [PubMed] [Google Scholar]
- 63.Yuan Y, Huang Q, Wu HM. Myosin light chain phosphorylation: modulation of basal and agonist-stimulated venular permeability. The American journal of physiology. 1997;272:H1437–1443. doi: 10.1152/ajpheart.1997.272.3.H1437. [DOI] [PubMed] [Google Scholar]
- 64.Zampeli E, Tiligada E. The role of histamine H4 receptor in immune and inflammatory disorders. British journal of pharmacology. 2009;157:24–33. doi: 10.1111/j.1476-5381.2009.00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Images of the negative controls for labeling. HUVEC, HCMEC, and HDMEC were immunolabeled to visualize localization of the H1R (A), H2R (B), H3R (C) and H4R (D), all labeled in green. Nuclei are labeled in blue. Images are projections of confocal z-stacks, and are representative of three separate experiments for each EC type.
Fig S2. mRNA identifying H3R, H4R and GAPDH in HCMEC, HUVEC, and HDMEC.
Fig S3. Effect of Immepip on endothelial cell resistance. (A) HUVEC, (B) HCMEC, and (C) HDMEC were treated with 0.1-10μM immepip until the cells recovered (n=4).
Fig S4. Effect of inhibition of RhoA, ROCK and p38 on Histamine-Induced Changes in TER (A) HDMEC were pretreated with either Y16 (10 μM) or SB (10 μM) alone or in combination (B) HDMEC were pretreated with either H1152 (100 nM) or SB (10 μM) alone or in combination in the presence of histamine (10 μM) for 30 minutes. Values are means ± SE. †P<0.01 versus all other groups (p < 0.01). N=8 for all groups.
Movie 1. 3D Reconstruction of confocal imaging showing the location of the receptors on endothelial cells. The confocal z-stacks of HUVEC labeling of H1, H2, H3, and H4 (green), counterlabeled with nuclei (blue) are all shown.


