Abstract
Using eutherian comparative genomic analysis protocol and public genomic sequence data sets, the present work attempted to update and revise two gene data sets. The most comprehensive third party annotation gene data sets of eutherian adenohypophysis cystine-knot genes (128 complete coding sequences), and d-dopachrome tautomerases and macrophage migration inhibitory factor genes (30 complete coding sequences) were annotated. For example, the present study first described primate-specific cystine-knot Prometheus genes, as well as differential gene expansions of D-dopachrome tautomerase genes. Furthermore, new frameworks of future experiments of two eutherian gene data sets were proposed.
Keywords: Comparative genomic analysis, Gene annotations, Molecular evolution, Phylogenetic analysis
Highlights
-
•
Revision of eutherian adenohypophysis cystine-knot genes
-
•
Initial description of primate-specific Prometheus genes
-
•
Revision of eutherian D-dopachrome tautomerase genes
-
•
Revision of eutherian macrophage migration inhibitory factor genes
-
•
Initial description of primate-specific D-dopachrome tautomerase differential gene expansions
Introduction
The free availability of public eutherian genomic sequence data sets ushered in new era in field of eutherian comparative genomics (Flicek et al., 2014, Margulies et al., 2005, Murphy et al., 2001, O'Leary et al., 2013, Wilson and Reeder, 2005). Indeed, the public eutherian genomic sequences were suitable in computational analyses (Blakesley et al., 2004, Lindblad-Toh et al., 2011). For example, new human gene annotations were expected to revise gene data sets (Harrow et al., 2012, International Human Genome Sequencing Consortium, 2001). In biomedical research, such new human gene annotations were expected to uncover potential new drugs and drug targets, as well as to contribute to better understanding of both physiological and pathological processes. For example, the comprehensive eutherian adenohypophysis cystine-knot gene data sets included major protein hormone genes of both clinical and physiological importance, such as thyroid stimulating hormone beta subunit genes, follicle stimulating hormone beta subunit genes, luteinizing hormone beta subunit genes and chorionic gonadotropin genes (Alvarez et al., 2009, Dos Santos et al., 2011, Jiang et al., 2014, Li and Ford, 1998, Roch and Sherwood, 2014). Likewise, the comprehensive eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor gene data sets included key regulatory immune response genes (Esumi et al., 1998, Merk et al., 2011). Yet, because of the incompleteness of eutherian genomic sequence assemblies (Harrow et al., 2012) and potential sequence errors (International Human Genome Sequencing Consortium, 2004, Mouse Genome Sequencing Consortium, 2009) eutherian gene data sets were subject to future updates. For example, the eutherian comparative genomic analysis protocol was proposed as guidance in protection against sequence errors in public eutherian genomic sequence assemblies (Premzl, 2014a, Premzl, 2014b, Premzl, 2014c). The protocol included new test of reliability of public eutherian genomic sequences that used genomic sequence redundancies, as well as protein molecular evolution test that used relative synonymous codon usage statistics. Thus, using public eutherian genomic sequence data sets and new genomics and protein molecular evolution tests, the present work made attempts to update and revise gene data sets of eutherian adenohypophysis cystine-knot genes, and eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor genes respectively.
Materials and methods
Gene annotations
The gene annotations included identification of genes in eutherian genomic sequence assemblies, analysis of gene features, tests of reliability of eutherian public genomic sequences and alignments of genomic sequences. The protocol made use of free available genomic sequence data sets in public databases and software. The BioEdit 7.0.5.3 program was used in nucleotide and protein sequence analyses (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The Ensembl genome browser, and its BLAST or BLAT tools were used in identification of genes in genomic sequence assemblies (http://www.ensembl.org/index.html) (Flicek et al., 2014). The analysis of gene features used direct evidence of eutherian gene annotations in NCBI's nr, est_human, est_mouse, and est_others databases (http://www.ncbi.nlm.nih.gov). The protocol first annotated potential coding sequences that were tested using tests of reliability of eutherian public genomic sequences. The tests made use of genomic sequence redundancies and primary experimental sequence data in NCBI's Trace Archive database (http://www.ncbi.nlm.nih.gov/Traces/trace.cgi). The first test step included analysis of nucleotide sequence coverage of each potential coding sequence using primary experimental sequence data and NCBI's program Netblast (ftp://ftp.ncbi.nlm.nih.gov/blast/documents/netblast.html). The second test step included classification of potential coding sequences. The potential coding sequences were designated as complete coding sequences if consensus trace sequence coverage was available for every nucleotide. Alternatively, they were described as putative coding sequences. The complete coding sequences were used in phylogenetic and protein molecular evolution analyses. The guidelines of human and mouse gene nomenclature were used in gene descriptions (http://www.genenames.org/guidelines.html and http://www.informatics.jax.org/mgihome/nomen/gene.shtml). The complete coding sequence data sets were reviewed by EBI as third party annotation gene data sets (http://www.ebi.ac.uk/embl/Documentation/third_party_annotation_dataset.html). The alignments of genomic sequences first included identification and masking of transposable elements in genomic sequences. The RepeatMasker program version open-3.3.0 was used, using default settings except simple repeats and low complexity elements were not masked (sensitive mode, cross_match version 1.080812, RepBase Update 20110920, RM database version 20110920 (http://www.repeatmasker.org/). However, the PREA1-3 and PREB genomic sequences were not masked in alignments. Then the mVISTA web tool was used in alignments of genomic sequences (http://genome.lbl.gov/vista/index.shtml). The default settings and AVID algorithm were used in alignments. The cutoffs of detection of common genomic sequence regions in each pairwise genomic sequence alignment were determined empirically (Supplementary data files Supplementary data file 3, Supplementary data file 9). The potential regulatory genomic sequence regions were aligned using ClustalW implemented in BioEdit 7.0.5.3, and nucleotide sequence alignments were corrected manually. Using BioEdit 7.0.5.3, the pairwise nucleotide sequence identities of common predicted promoter genomic sequence regions were calculated and used in statistical analysis (Microsoft Office Excel).
Phylogenetic analysis
The phylogenetic analysis included alignments of protein sequences, alignments of nucleotide sequences, calculations of phylogenetic trees and calculations of nucleotide sequence identities. The complete coding sequences were first aligned at amino acid level using ClustalW implemented in BioEdit 7.0.5.3. Then the protein sequence alignments and nucleotide sequence alignments were corrected manually. The MEGA5 program was used in calculations of phylogenetic trees (http://www.megasoftware.net). The phylogenetic trees were calculated using neighbour-joining method (default settings, except gaps/missing data = pairwise deletion) (not shown), minimum evolution method (default settings, except gaps/missing data = pairwise deletion) and maximum parsimony method (default settings, except gaps/missing data = use all sites) (not shown). However, because their homogeneity and stationarity assumptions were not satisfied, the maximum likelihood methods were not used in phylogenetic analysis (data not shown). The pairwise nucleotide sequence identities of complete coding sequences were calculated using BioEdit 7.0.5.3. The calculations were used in statistical analysis (Microsoft Office Excel).
Protein molecular evolution analysis
The protein molecular evolution analysis included new tests of protein molecular evolution. The tests integrated patterns of nucleotide sequence similarities of aligned complete coding sequences with protein tertiary structures. The relative synonymous codon usage statistic R was calculated using MEGA5 as ratio between observed and expected amino acid codon counts. The amino acid codons with R ≤ 0.7 were designated as not preferable amino acid codons. The not preferable amino acid codons in analysis of eutherian GPB5, TSHB, FSHB and LHB-CGB close gene homologues were: TTT (0.69), TTA (0.03), TTG (0.38), CTA (0.17), ATT (0.46), ATA (0.52), GTT (0.37), GTA (0.38), TCA (0.27), TCG (0.12), CCG (0.44), ACA (0.59), ACG (0.33), GCG (0.18), CAA (0.46), AAT (0.63), AAA (0.66), GAT (0.59), GAA (0.62), CGT (0.26), CGA (0.57), AGT (0.61), GGT (0.36) and GGA (0.64). In analysis of GPA1 and GPA2 close gene homologues, they were: TTA (0.06), TTG (0.52), CTA (0.23), ATT (0.53), ATA (0.52), GTT (0.37), GTA (0.39), TCA (0.34), TCG (0.16), CCG (0.38), ACG (0.35), GCG (0.25), CAA (0.41), AAT (0.63), GAT (0.7), GAA (0.61), CGT (0.28), CGA (0.53), GGT (0.51) and GGA (0.68). In analysis of DDT and MIF close gene homologues, the not preferable amino acid codons were: TTT (0.62), TTA (0.2), TTG (0.61), CTT (0.17), CTA (0.37), ATT (0.5), ATA (0.46), GTT (0.28), GTA (0.44), TCT (0.22), TCA (0.13), TCG (0.2), CCT (0.53), CCA (0.26), ACT (0.68), ACA (0.44), GCT = (0.4), GCA (0.29), TAT (0.42), CAT (0.18), CAA (0.09), AAT (0.23), AAA (0.64), GAT (0.19), GAA (0.1), TGT (0.35), CGT (0.14), (AGT (0.29), AGA (0.14), AGG (0.55), GGT (0.37) and GGA (0.15). In protein molecular evolution analyses, the reference protein sequence residues were designated as invariant amino acid sites (invariant alignment positions), forward amino acid sites (variant alignment positions that did not include amino acid codons with R ≤ 0.7) or compensatory amino acid sites (variant alignment positions including amino acid codons with R ≤ 0.7). Thus, the presence of preferable amino acid codons and absence of not preferable amino acid codons indicated that forward amino acid sites could have major influence on protein function. Conversely, the presence of not preferable amino acid codons indicated that compensatory amino acid sites could have minor influence on protein function. The DeepView/Swiss-PdbViever 4.0.1 program was used for analyses of protein tertiary structures (http://spdbv.vital-it.ch/). The prediction of N-terminal signal peptide presence was undertaken using SignalP-4.0 (http://www.cbs.dtu.dk/services/SignalP/).
Results and discussion
Initial description of primate-specific cystine-knot Prometheus genes
Gene annotations
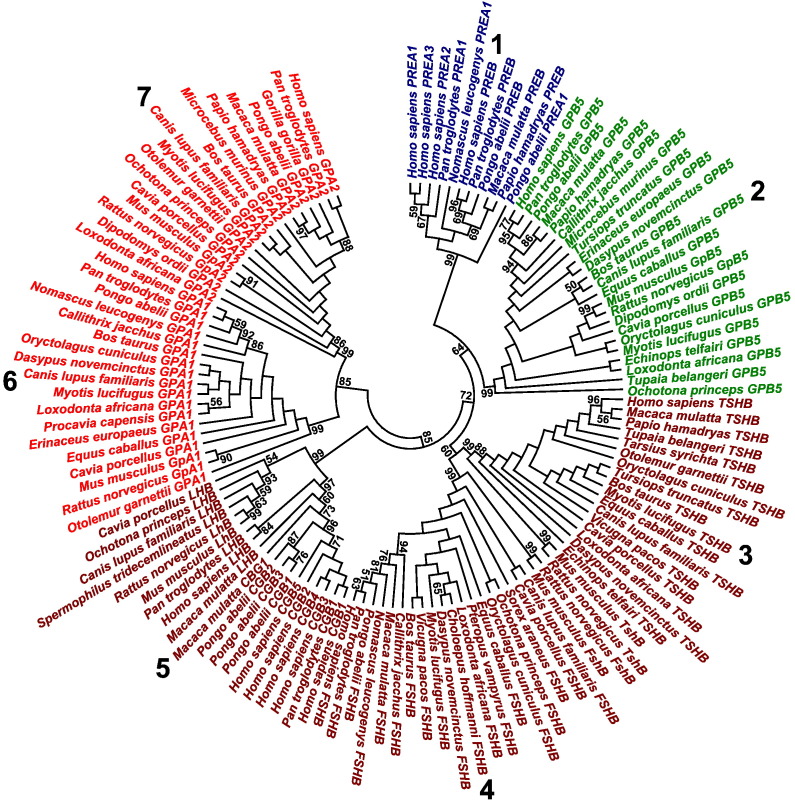
The present analysis annotated most comprehensive data set of eutherian adenohypophysis cystine-knot genes. Among 183 potential coding sequences, the comparative genomic analysis protocol annotated 128 complete coding sequences encoding 11 Prometheus proteins (PREA1-3 and PREB), 23 glycoprotein-B5 proteins (GPB5), 19 thyroid stimulating hormone beta subunits (TSHB), 21 follicle stimulating hormone beta subunits (FSHB), 19 luteinizing hormone beta subunits and chorionic gonadotropins (LHB and CGB), 18 glycoprotein hormone alpha subunits (GPA1), and 17 glycoprotein-A2 proteins (GPA2) (Fig. 1). The gene data set was made available in public databases as one third party annotation gene data set (http://www.ebi.ac.uk/ena/data/view/HF564658-HF564785) (Supplementary data file 1). The present work integrated gene annotations, phylogenetic analysis, and protein molecular evolution analysis and first described primate-specific cystine-knot genes that were named Prometheus genes (Fig. 1, Fig. 2). Although the PREA1-3 genes were annotated in Hominidae genomic sequence assemblies, PREB gene was annotated in Hominidae and Cercopithecidae genomic sequence assemblies. There were both direct and indirect evidence of PREA1-3 and PREB gene annotations (Clamp et al., 2007). The direct evidence included gene transcripts (Supplementary data file 2). For example, the present PREA1-3, and PREB transcript data set annotated seven human PREA2 gene exons, eight human PREA3 gene exons and nine human PREB gene exons. However, the annotated primate-specific PREA1-3 and PREB ORFs were encoded by single translated exons (Supplementary data file 3A). Next, in present primate genomic sequence assemblies, the PREA1-3 and PREB genes were positioned within segmental duplications on chromosome 17 along minimally ~ 31–35 kb that showed > 90% nucleotide sequence identities (Supplementary data file 3A). For example, the human PREA2 and PREA3 genes were positioned within segmental duplications along > 243 kb. Likewise, the primate-specific PREA1-3 and PREB ORFs showed nucleotide sequence similarities to transposable elements. For example, within human PREA1 ORF there were nucleotide sequence similarities to transposable elements MIR3 (151–294 bp) and L2c (359–447 bp). Finally, the computational gene annotations of new adenohypophysis cystine-knot genes were known in human GPB5 and GPA2 genes (Hsu et al., 2002, Macdonald et al., 2005).
Fig. 1.
Phylogenetic analysis of eutherian adenohypophysis cystine-knot genes. The minimum evolution tree of eutherian adenohypophysis cystine-knot genes was calculated using maximum composite likelihood method. The estimates > 50% were shown, after 1000 bootstrap replicates. The major gene clusters were indicated using numbers (1–7).
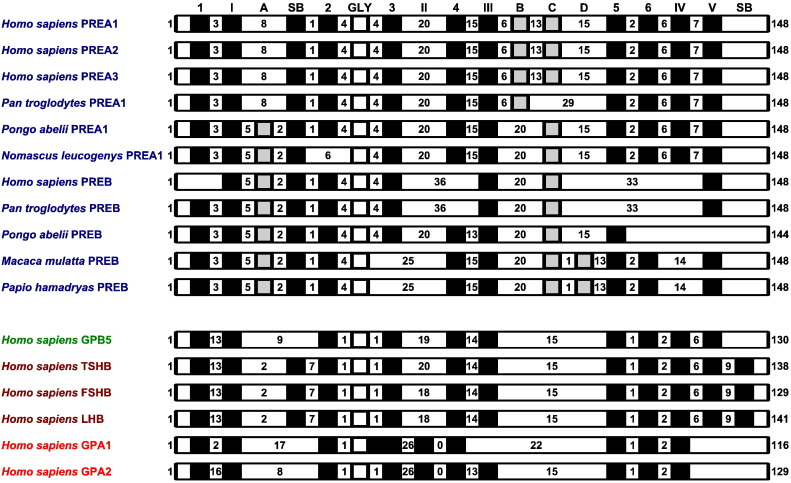
Fig. 2.
Distribution of cysteines in cystine-knot domains of human adenohypophysis cystine-knot proteins. The black rectangles indicated common cysteine residues. The grey rectangles indicated cysteines in primate-specific PREA1-3 and PREB proteins. The white rectangles indicated glycine residues in amino acid motifs Cys-4x-Gly-4x-Cys or Cys-1x-Gly-1x-Cys. The numbers between rectangles indicated numbers of amino acids. Whereas the common cysteine residues were labelled according to Alvarez et al. (2009) and present analysis, PREA1-3- and PREB-specific cysteine residues were labelled A–D.
The present analysis annotated both new and known potential regulatory genomic sequence regions of eutherian adenohypophysis cystine-knot genes, as guidelines of future experiments. In eutherian GPB5 promoters, there were two common genomic sequence regions that showed nucleotide sequence identity patterns that exceeded criteria of detection of potential regulatory genomic sequence regions (Supplementary data file 3B). In eutherian TSHB promoters, there was one common potential regulatory genomic sequence region (Supplementary data file 3C, Supplementary data file 4A). For example, the common potential regulatory genomic sequence region included functional PIT1 and GATA2 cis-elements, suppressor region, and TATA cis-element (Kashiwabara et al., 2009). The average pairwise nucleotide sequence identity of common potential regulatory genomic sequence region was ā = 0.847 (amax = 0.996, amin = 0.745, āad = 0.034). There was one common potential regulatory genomic sequence region in eutherian FSHB promoters (Supplementary data file 3D, Supplementary data file 4B). For example, the common potential regulatory genomic sequence region included functional LHX3 cis-elements A-C and TATA cis-element (West et al., 2004). The average pairwise nucleotide sequence identity of common potential regulatory genomic sequence region was ā = 0.787 (amax = 0.992, amin = 0.622, āad = 0.073). In eutherian LHB-CGB promoters, there were four common potential regulatory genomic sequence regions (Supplementary data file 3E, Supplementary data file 4C). For example, the common potential regulatory genomic sequence region 4 included functional SF-1 and Egr-1 cis-elements and TATA cis-element (Horton and Halvorson, 2004). The average pairwise nucleotide sequence identity of common potential regulatory genomic sequence region 4 was ā = 0.77 (amax = 0.986, amin = 0.461, āad = 0.095). There were four common potential regulatory genomic sequence regions in eutherian GPA1 promoters (Supplementary data file 3F, Supplementary data file 4D). For example, the common potential regulatory genomic sequence region 4 included functional GSE cis-element, CRE cis-element, Hominidae-specific CRE cis-element, and TATA cis-element (Fowkes et al., 2003). The average pairwise nucleotide sequence identity of common potential regulatory genomic sequence region 4 was ā = 0.766 (amax = 0.983, amin = 0.602, āad = 0.06). Finally, in eutherian GPA2 promoters, there were two common potential regulatory genomic sequence regions (Supplementary data file 3G).
Phylogenetic analysis
The present analysis described seven major gene clusters of eutherian adenohypophysis cystine-knot genes (Fig. 1). The major gene cluster 1 included primate-specific PREA1-3 and PREB genes. The eutherian GPB5 genes comprised major gene cluster 2. The eutherian TSHB genes, FSHB genes, and LHB-CGB genes were grouped in major gene clusters 3, 4, and 5, respectively (Li and Ford, 1998). Although the eutherian GPA1 genes were included in major gene cluster 6, major cluster 7 was comprised of eutherian GPA2 genes. The identical major tree branching patterns were calculated using minimum evolution (Fig. 1), neighbor-joining, and maximum parsimony methods. The present eutherian adenohypophysis cystine-knot gene classification was confirmed by calculations of nucleotide sequence identity patterns (Supplementary data file 5). The major gene cluster 1 paralogues showed high nucleotide sequence identities that were typical in primate-specific gene expansions. In comparisons with major gene cluster 2 genes, the major gene cluster 1 genes showed nucleotide sequence identity patterns of typical homologues, but in comparisons with other major gene clusters, major gene cluster 1 genes showed nucleotide sequence identity patterns of distant homologues. The eutherian major gene cluster 2 to cluster 7 genes, respectively, showed nucleotide sequence identities that were typical in comparisons between eutherian orthologues. In comparisons between major gene cluster 2 to cluster 5 genes, there were nucleotide sequence identity patterns of close homologues, as well as in comparisons between major gene cluster 6 and cluster 7 genes. Finally, in comparisons of major gene cluster 2 to cluster 5 genes with major gene cluster 6 and cluster 7 genes, there were nucleotide sequence identity patterns of typical homologues. The exceptions were nucleotide sequence identity patterns of distant homologues between major gene cluster 3 and cluster 6 genes. The present grouping of eutherian LHB-CGB genes into one major gene cluster (Lapthorn et al., 1994, Li and Ford, 1998) was different to analysis of Hsu et al. (Hsu et al., 2002) that grouped LHB and CGB genes in two groups. Indeed, the eutherian LHB genes and primate-specific CGB genes showed nucleotide sequence identity patterns that were typical in comparisons of eutherian orthologues and paralogues. Finally, the eutherian LHB genes and primate-specific CGB genes included common potential regulatory genomic sequence regions (Supplementary data file 3E, Supplementary data file 4C).
Protein molecular evolution analysis
In cystine-knot domains, the PREA1-3 and PREB proteins included 8–13 common cysteines and PREA1-3- and PREB-specific cysteines, GPB5 proteins included 10 common cysteines, TSHB, FSHB and LHB and CGB proteins included 11 common cysteines and GPA1 and GPA2 proteins included 10 common cysteines (Fig. 2). The primate-specific PREA1-3 and PREB proteins included new cystine-knot domain cysteine patterns. For example, the common Cys(2)-1x-Gly-1x-Cys(3) amino acid sequence motif (Alvarez et al., 2009, Lapthorn et al., 1994) was replaced by Cys(2)-4x-Gly-4x-Cys(3) amino acid sequence motif. Likewise, the common Cys(5)-1x-Cys(6) amino acid sequence motif (Alvarez et al., 2009, Lapthorn et al., 1994) was replaced by Cys(5)-2x-Cys(6) amino acid sequence motif. However, one major difference between PREA1-3 and PREB proteins and adenohypophysis cystine-knot homologues was no prediction of N-terminal signal peptides in primate-specific PREA1-3 and PREB proteins by SignalP analysis. In addition, they did not include potential N-glycosylation signal sites.
The new tests of protein molecular evolution were used in molecular evolution analysis of eutherian GPB5, TSHB, FSHB, and LHB-CGB close protein homologues, including 82 complete coding sequences (Supplementary data file 5, Supplementary data file S6). The human FSHB was used as reference protein amino acid sequence in analysis of FSHB crystal structure 1XWD (Fan and Hendrickson, 2005, Lapthorn et al., 1994). In human FSHB protein amino acid sequence, there were 16 invariant amino acid sites and 7 forward amino acid sites (Supplementary data file 7A, Supplementary data file 7C–D). The present analysis described two amino acid clusters with overrepresented invariant and/or forward amino acid sites that were positioned between amino acid positions W45-R53 and A97-C105. The amino acid clusters included common amino acid sequence motifs Cys(2)-1x-Gly-1x-Cys(3) (Cluster 1) and Cys(5)-1x-Cys(6) (Cluster 2) (Fan and Hendrickson, 2005, Lapthorn et al., 1994). The new tests of protein molecular evolution were used in molecular evolution analysis of eutherian GPA1 and GPA2 close protein homologues including 35 complete coding sequences (Supplementary data file 5, Supplementary data file S6). Using human GPA1 as reference protein amino acid sequence and crystal structure 1XWD (Fan and Hendrickson, 2005, Lapthorn et al., 1994), the present analysis described 27 invariant amino acid sites and 26 forward amino acid sites (Supplementary data file 7B, Supplementary data file 7E–F). There were five amino acid clusters with overrepresented invariant and/or forward amino acid sites that were positioned between amino acid positions: C31-Q37, F42-T63, K75-S79, C83-G96, and E101-K115. The amino acid cluster 1 included amino acid site Cys(I), amino acid cluster 2 included common amino acid sequence motif Cys(2)-1x-Gly-1x-Cys(3), amino acid cluster 4 included amino acid site Cys(4), and amino acid cluster 5 included common amino acid sequence motif Cys(5)-1x-Cys(6) (Fan and Hendrickson, 2005, Lapthorn et al., 1994).
Initial description of primate-specific differential gene expansions of D-dopachrome tautomerase genes
Gene annotations
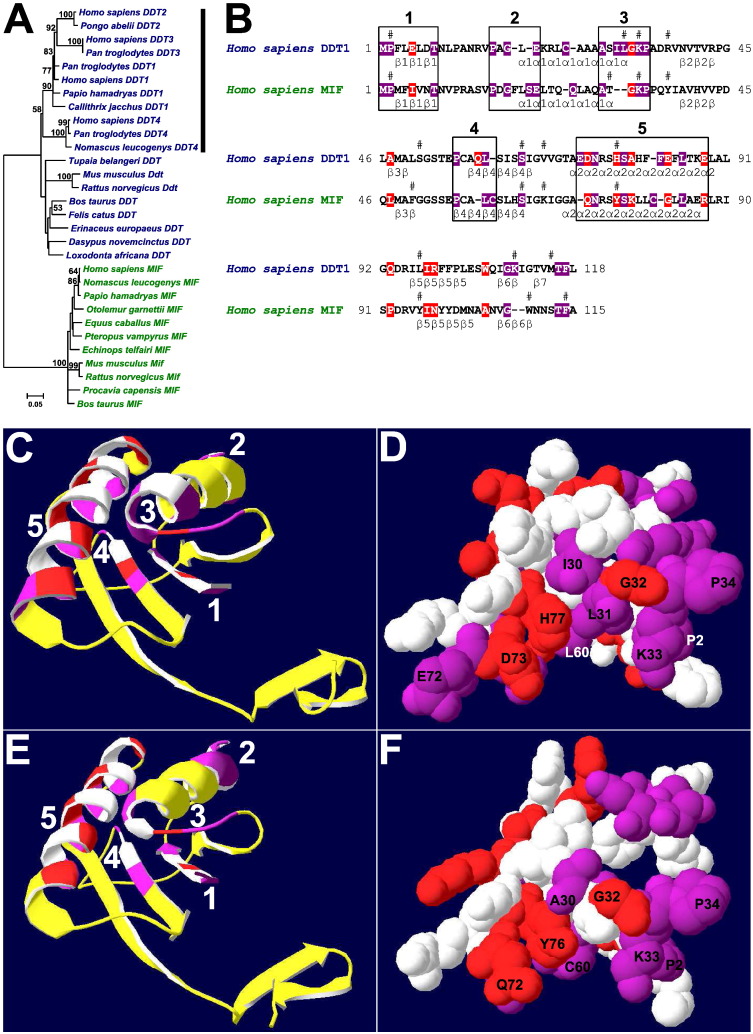
Among 49 potential coding sequences, the eutherian comparative genomic analysis protocol annotated 30 complete coding sequences of 19 eutherian d-dopachrome tautomerases (DDT) and 11 eutherian migration inhibitory factors (MIF) (Fig. 3A). The present most comprehensive eutherian DDT and MIF gene data set was made available in public databases as one third party annotation gene data set (http://www.ebi.ac.uk/ena/data/view/HF564786-HF564815) (Supplementary data file 8). The present work integrated gene annotations, phylogenetic analysis, and protein molecular evolution analysis and first described primate-specific differential gene expansions DDT1-4 (Fig. 3A). For example, the human DDT1-4 genes were present in Hominidae genomic sequence assemblies. There were direct and indirect evidence of gene annotations of primate-specific differential gene expansions DDT1-4 (Clamp et al., 2007). The direct evidence included gene transcripts (Supplementary data file 2). The indirect evidence included nucleotide sequence identity patterns of complete coding sequences, untranslated genomic sequence regions, promoters, and introns (Supplementary data file 5, Supplementary data file 9). For example, the human DDT1 exon 1 translated genomic sequence and exon 2 each showed 100% nucleotide sequence identities in comparisons with human DDT2 exon 1 translated genomic sequence and exon 2. In addition, the primate-specific DDT2 and DDT3 genes showed nucleotide sequence similarities along human DDT1 promoter and introns. The primate-specific DDT4 genes showed nucleotide sequence similarities along human DDT1 exon 3. The alignments of DDT genomic sequences indicated that there was one common potential regulatory genomic sequence region in DDT and MIF promoters, respectively (Supplementary data file 9).
Fig. 3.
Analysis of eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor genes. (A) Minimum evolution tree of eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor genes. The tree was calculated using maximum composite likelihood method. The estimates > 50% were shown, after 1000 bootstrap replicates. The vertical bar labelled primate-specific differential gene expansions DDT1-4. (B–F) Protein molecular evolution analysis. (B) Reference human DDT1 and MIF protein amino acid sequences. The invariant amino acid sites were shown using white letters on violet backgrounds. The forward amino acid sites were shown using white letters on red backgrounds. The amino acid clusters 1–5 with overrepresented invariant or/and forward amino acid sites were labelled using rectangles. The secondary structure elements were designated according to Sugimoto et al. (1999) for DDT1 crystal structure 1DPT, and according to Sun et al. (1996) for MIF crystal structure 1MIF. The amino acid residues implicated in putative DDT1 and MIF active sites (Sugimoto et al., 1999) were labelled by #s. (C–D) Analysis of human DDT1 crystal structure 1DPT. (C) Ribbon representation of human DDT1 crystal structure 1DPT. (D) van-der-Waals representations of amino acid cluster 1-5 amino acids in view identical to that in C. (E–F) Analysis of human MIF crystal structure 1MIF. (E) Ribbon representation of human MIF crystal structure 1MIF. (F) van-der-Waals representations of amino acid cluster 1-5 amino acids in view identical to that in E. (C–F) The amino acid cluster 1-5 invariant amino acid sites were labelled violet, forward amino acid sites were labelled red and compensatory amino acid sites were labelled white.
Phylogenetic analysis
The minimum evolution (Fig. 3A), neighbor-joining and maximum parsimony phylogenetic trees of eutherian DDT and MIF genes showed similar topologies with identical major branching patterns that clustered DDT and MIF genes in two major gene clusters. Indeed, the primate-specific DDT1-4 genes were grouped separately. The present calculations of nucleotide sequence identity patterns between major gene clusters described typical eutherian DDT and MIF genes as close homologues (Supplementary data file 5).
Protein molecular evolution analysis
The new tests of protein molecular evolution were used in molecular evolution analysis of eutherian DDT and MIF close protein homologues including 30 complete coding sequences (Fig. 3B-F, Supplementary data file 10). The present analysis determined 24 invariant amino acid sites and 13 forward amino acid sites in reference human DDT1 protein amino acid sequence, and 22 invariant amino acid sites and 12 forward amino acid sites in reference human MIF protein amino acid sequence. Although the amino acids implicated in putative DDT1 active site (Sugimoto et al., 1999) included five invariant amino acid sites and one forward amino acid site, amino acids implicated in putative MIF active site (Sugimoto et al., 1999) included four invariant amino acid sites and one forward amino acid site. There were five amino acid clusters with overrepresented invariant or/and forward amino acid sites determined in reference protein amino acid sequences: cluster 1 (M1-T8 in both DDT1 and MIF), cluster 2 (P16-E20 in DDT1 and P16-E22 in MIF), cluster 3 (A28-P34 in DDT1 and A30-P34 in MIF), cluster 4 (P56-L60 in DDT1 and P56-C60 in MIF), and cluster 5 (E72-E88 in DDT1 and Q72-R87 in MIF). The present analysis of human DDT1 and MIF crystal structures 1DPT and 1MIF indicated that amino acids in amino acid clusters 1 and 3, C-terminal part of amino acid cluster 4 and N-terminal part of amino acid cluster 5 delineated potential human DDT1 and MIF active sites (Sugimoto et al., 1999).
Conclusions
The present analysis updated and revised gene data sets of eutherian adenohypophysis cystine-knot genes, and DDT and MIF genes respectively. The most comprehensive third party annotation gene data sets were annotated. Indeed, the present study first described primate-specific cystine-knot PREA1-3 and PREB genes, as well as differential gene expansions DDT1-4. The eutherian comparative genomic analysis protocol proposed new frameworks of future experiments of two eutherian gene data sets.
The following are the supplementary related to this article.
Gene data set of eutherian adenohypophysis cystine-knot genes.
Direct evidence of new human gene annotations.
Pairwise genomic sequence alignments. The translated base sequence regions were displayed as indigo rectangles. The untranslated base sequence regions were displayed as cyan rectangles. The aligned genomic sequence regions that showed conservation levels above empirically determined cut-offs of detection of common genomic sequence regions were shown accordingly. (A) Pairwise genomic sequence alignments of primate-specific PREA1-3 and PREB genes. The cut-offs of detection of common genomic sequence regions in pairwise alignments with Homo sapiens PREA2 were 95% per 100 bp (Homo sapiens PREA1 and PREA3) or 90% per 100 bp in other pairwise alignments. The Homo sapiens PREA2 exons were annotated using transcript BC040601.1. (B) Pairwise genomic sequence alignments of eutherian GPB5 genes. The potential regulatory genomic sequence regions 1 and 2 were shown using rectangles. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens GPB5 were: 99% per 100 bp (Pan troglodytes), 95% per 100 bp (Pongo abelii, Macaca mulatta and Papio hamadryas), 90% per 100 bp (Callithrix jacchus), 85% per 100 bp (Microcebus murinus and Tupaia belangeri) or 80% per 100 bp in other pairwise alignments. The Homo sapiens GPB5 exons were annotated using transcript BM709443.1. (C) Pairwise genomic sequence alignments of eutherian TSHB genes. The potential regulatory genomic sequence region 1 was shown using rectangle. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens TSHB were: 95% per 100 bp (Macaca mulatta and Papio hamadryas), 90% per 100 bp (Tarsius syrichta, Otolemur garnettii and Tupaia belangeri), 80% per 100 bp (Cavia porcellus and Oryctolagus cuniculus), 75% per 100 bp (Mus musculus and Rattus norvegicus) or 85% per 100 bp in other pairwise alignments. (D) Pairwise genomic sequence alignments of eutherian FSHB genes. The potential regulatory genomic sequence region 1 was shown using rectangle. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens FSHB were: 95% per 100 bp (Pan troglodytes, Pongo abelii and Nomascus leucogenys), 90% per 100 bp (Macaca mulatta and Callithrix jacchus), 80% per 100 bp (Cavia porcellus, Oryctolagus cuniculus, Ochotona princeps, Canis lupus familiaris and Sorex araneus), 75% per 100 bp (Mus musculus and Rattus norvegicus) or 85% per 100 bp in other pairwise alignments. The Homo sapiens FSHB exons were annotated using transcript BI033915.1. (E) Pairwise genomic sequence alignments of eutherian LHB-CGB genes. The potential regulatory genomic sequence regions 1–4 were shown using rectangles. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens LHB were: 95% per 100 bp (Pan troglodytes), 90% per 100 bp (Macaca mulatta), 80% per 100 bp (Cavia porcellus, Spermophilus tridecemlineatus and Ochotona princeps), 75% per 100 bp (Mus musculus, Rattus norvegicus and Canis lupus familiaris) or 90% per 100 bp in pairwise alignments with CGB genes. The Homo sapiens LHB exons were annotated using transcript CD107666.1. (F) Pairwise genomic sequence alignments of eutherian GPA1 genes. The potential regulatory genomic sequence regions 1–4 were shown using rectangles. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens GPA1 were: 95% per 100 bp (Pan troglodytes and Pongo abelii), 90% per 100 bp (Nomascus leucogenys), 85% per 100 bp (Callithrix jacchus), 80% per 100 bp (Bos taurus, Equus caballus, Canis lupus familiaris, Myotis lucifugus and Erinaceus europaeus), 70% per 100 bp (Mus musculus and Rattus norvegicus) or 75% per 100 bp in other pairwise alignments. The Homo sapiens GPA1 exons were annotated using transcript CR599056.1. (G) Pairwise genomic sequence alignments of eutherian GPA2 genes. The potential regulatory genomic sequence regions 1–2 were shown using rectangles. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens GPA2 were: 99% per 100 bp (Pan troglodytes), 95% per 100 bp (Gorilla gorilla), 90% per 100 bp (Pongo abelii, Macaca mulatta and Papio hamadryas), 85% per 100 bp (Microcebus murinus and Otolemur garnettii), 75% per 100 bp (Mus musculus, Rattus norvegicus, Dipodomys ordii, and Ochotona princeps) or 80% per 100 bp in other pairwise alignments. The Homo sapiens GPA2 exons were annotated using transcript AF260739.1.
Nucleotide sequence alignments of common predicted promoter genomic sequence regions. Using Homo sapiens transcripts as in Supplementary data file 3, the untranslated exons were annotated. The numbers in brackets indicated positions of 3'-terminal nucleotides relative to translated exons. The nucleotide positions were labeled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 85% conservation and black letters on grey background depicted ≥ 70% conservation. (A) Alignments of TSHB common predicted promoter genomic sequence regions. The PIT1 cis-elements (A), GATA2 cis-elements (B), suppressor regions (C) and TATA cis-elements (D) were indicated. (B) Alignments of FSHB common predicted promoter genomic sequence regions. The LHX3 cis-elements (A–C) and TATA cis-elements (D) were indicated, as well as partial first exons (E). (C) Alignments of LHB-CGB common predicted promoter genomic sequence regions (potential regulatory genomic sequence region 4). The composite SF-1 and Egr-1 cis-elements (A) and TATA cis-elements (B) were indicated. (D) Alignments of GPA1 common predicted promoter genomic sequence regions. The GSE cis-elements (A), CRE cis-elements (B), Hominidae-specific CRE cis-elements (C) and TATA cis-elements (D) were indicated, as well as partial first exons (E).
Nucleotide sequence alignments of common predicted promoter genomic sequence regions. Using Homo sapiens transcripts as in Supplementary data file 3, the untranslated exons were annotated. The numbers in brackets indicated positions of 3'-terminal nucleotides relative to translated exons. The nucleotide positions were labelled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 85% conservation and black letters on grey background depicted ≥ 70% conservation. (A) Alignments of TSHB common predicted promoter genomic sequence regions. The PIT1 cis-elements (A), GATA2 cis-elements (B), suppressor regions (C) and TATA cis-elements (D) were indicated. (B) Alignments of FSHB common predicted promoter genomic sequence regions. The LHX3 cis-elements (A–C) and TATA cis-elements (D) were indicated, as well as partial first exons (E). (C) Alignments of LHB-CGB common predicted promoter genomic sequence regions (potential regulatory genomic sequence region 4). The composite SF-1 and Egr-1 cis-elements (A) and TATA cis-elements (B) were indicated. (D) Alignments of GPA1 common predicted promoter genomic sequence regions (potential regulatory genomic sequence region 4). The GSE cis-elements (A), CRE cis-elements (B), Hominidae-specific CRE cis-elements (C) and TATA cis-elements (D) were indicated, as well as partial first exons (E).
Pairwise nucleotide sequence identities of eutherian adenohypophysis cystine-knot genes, and D-dopachrome tautomerase and macrophage migration inhibitory factor genes.
Alignments of eutherian adenohypophysis cystine-knot proteins. The reference human FSHB and GPA1 protein sequence invariant amino acid sites were shown using white letters on violet backgrounds and forward amino acid sites were shown using white letters on red backgrounds. The amino acid positions of GPB5s, TSHBs, FSHBs, LHs and CGBs were labelled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 75% conservation and black letters on grey background depicted ≥ 50% conservation. The GPA1 and GPA2 amino acid positions were labelled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 80% conservation and black letters on grey background depicted ≥ 60% conservation. The stop codon positions were labelled using &s.
Protein molecular evolution analysis. (A) Reference human FSHB protein amino acid sequence. The amino acid clusters 1 and 2 with overrepresented invariant or/and forward amino acid sites were labelled using rectangles. (B) Reference human GPA1 protein amino acid sequence. The amino acid clusters 1–5 with overrepresented invariant or/and forward amino acid sites were labelled using rectangles. (A–B) The invariant amino acid sites were shown using white letters on violet backgrounds. The forward amino acid sites were shown using white letters on red backgrounds. The secondary structure elements were designated according to Fan and Hendrickson (Fan and Hendrickson, 2005) for FSH crystal structure 1XWD. The common cysteine residues were labelled according to Alvarez et al. (2009) and present analysis (Fig. 2). Black triangles labelled predicted signal peptide cleavage sites. (C–D) Analysis of human FSHB crystal structure 1XWD. The amino acid cluster 1 and 2 invariant amino acid sites were labelled violet, forward amino acid sites were labelled red and compensatory amino acid sites were labelled white. (C) Ribbon representation of human FSHB crystal structure 1XWD. (D) van-der-Waals representations of amino acid cluster 1 and 2 amino acids in view identical to that in C. (E–F) Analysis of human GPA1 crystal structure 1XWD. The amino acid cluster 1-5 invariant amino acid sites were labelled violet, forward amino acid sites were labelled red and compensatory amino acid sites were labelled white. (E) Ribbon representation of human GPA1 crystal structure 1XWD. (F) van-der-Waals representations of amino acid cluster 1-5 amino acids in view identical to that in E.
Gene data set of eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor genes.
Eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor genes. (A) Distribution of DDT genes and MIF genes in human, mouse and domestic cattle genomic sequence assemblies. The relative gene orders and orientations in genomic sequences were shown approximately. Genes other than DDTs and MIFs were not shown. BTA, domestic cattle chromosome; HSA, human chromosome; MMU, mouse chromosome. (B–C) Pairwise genomic sequence alignments. The translated base sequence regions were displayed as indigo rectangles. The untranslated base sequence regions were displayed as cyan rectangles. The aligned genomic sequence regions that showed conservation levels above empirically determined cut-offs of detection of common genomic sequence regions were shown accordingly. (B) Pairwise genomic sequence alignments of eutherian DDT genes. The potential regulatory genomic sequence region 1 was shown using rectangle. The cut-offs of detection of common genomic sequence regions in pairwise alignments with Homo sapiens DDT1 were: 99% per 100 bp (Pan troglodytes DDT1 and Homo sapiens DDT2), 95% per 100 bp (Pongo abelii DDT2), 90% per 100 bp (Papio hamadryas DDT1, Callithrix jacchus DDT1, Homo sapiens DDT3 and Pan troglodytes DDT3), 70% per 100 bp (Tupaia belangeri, Bos taurus and Felis catus), 60% per 100 bp (Homo sapiens DDT4 and Pan troglodytes DDT4), 55% per 100 bp (Erinaceus europaeus, Dasypus novemcinctus and Loxodonta africana), 55% per 90 bp (Mus musculus and Rattus norvegicus) and 50% per 100 bp (Nomascus leucogenys DDT4). The Homo sapiens DDT1 exons were annotated using transcript BC005971.1. (C) Pairwise genomic sequence alignments of eutherian MIF genes. The potential regulatory genomic sequence region 1 was shown using rectangle. The cut-offs of detection of common genomic sequence regions in pairwise alignments with Homo sapiens MIF were: 95% per 100 bp (Nomascus leucogenys and Papio hamadryas), 70% per 100 bp (Otolemur garnetii), 65% per 100 bp (Bos taurus, Equus caballus, Pteropus vampyrus, Echinops telfairi and Procavia capensis), and 60% per 100 bp (Mus musculus and Rattus norvegicus). The Homo sapiens MIF exons were annotated using transcript BC013976.2.
Alignments of eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor proteins. The reference human DDT1 and MIF protein sequence invariant amino acid sites were shown using white letters on violet backgrounds. The forward amino acid sites were shown using white letters on red backgrounds. The amino acid positions were labelled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 85% conservation and black letters on grey background depicted ≥ 70% conservation. The DDT2 and DDT3 C-terminal regions were not used in calculations. The stop codon positions were labelled using &s.
References
- Alvarez E., Cahoreau C., Combarnous Y. Comparative structure analyses of cystine knot-containing molecules with eight aminoacyl ring including glycoprotein hormones (GPH) alpha and beta subunits and GPH-related A2 (GPA2) and B5 (GPB5) molecules. Reprod. Biol. Endocrinol. 2009;7:90. doi: 10.1186/1477-7827-7-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakesley R.W., Hansen N.F., Mullikin J.C., Thomas P.J., McDowell J.C., Maskeri B., Young A.C., Benjamin B., Brooks S.Y., Coleman B.I., Gupta J., Ho S.L., Karlins E.M., Maduro Q.L., Stantripop S., Tsurgeon C., Vogt J.L., Walker M.A., Masiello C.A., Guan X., Comparative Sequencing Program N.I.S.C., Bouffard G.G., Green E.D. An intermediate grade of finished genomic sequence suitable for comparative analyses. Genome Res. 2004;14:2235–2244. doi: 10.1101/gr.2648404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clamp M., Fry B., Kamal M., Xie X., Cuff J., Lin M.F., Kellis M., Lindblad-Toh K., Lander E.S. Distinguishing protein-coding and noncoding genes in the human genome. Proc. Natl. Acad. Sci. U. S. A. 2007;104:19428–19433. doi: 10.1073/pnas.0709013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dos Santos S., Mazan S., Venkatesh B., Cohen-Tannoudji J., Quérat B. Emergence and evolution of the glycoprotein hormone and neurotrophin gene families in vertebrates. BMC Evol. Biol. 2011;11:332. doi: 10.1186/1471-2148-11-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esumi N., Budarf M., Ciccarelli L., Sellinger B., Kozak C.A., Wistow G. Conserved gene structure and genomic linkage for D-dopachrome tautomerase (DDT) and MIF. Mamm. Genome. 1998;9:753–757. doi: 10.1007/s003359900858. [DOI] [PubMed] [Google Scholar]
- Fan Q.R., Hendrickson W.A. Structure of human follicle-stimulating hormone in complex with its receptor. Nature. 2005;433:269–277. doi: 10.1038/nature03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flicek P., Amode M.R., Barrell D., Beal K., Billis K., Brent S., Carvalho-Silva D., Clapham P., Coates G., Fitzgerald S., Gil L., Girón C.G., Gordon L., Hourlier T., Hunt S., Johnson N., Juettemann T., Kähäri A.K., Keenan S., Kulesha E., Martin F.J., Maurel T., McLaren W.M., Murphy D.N., Nag R., Overduin B., Pignatelli M., Pritchard B., Pritchard E., Riat H.S., Ruffier M., Sheppard D., Taylor K., Thormann A., Trevanion S.J., Vullo A., Wilder S.P., Wilson M., Zadissa A., Aken B.L., Birney E., Cunningham F., Harrow J., Herrero J., Hubbard T.J., Kinsella R., Muffato M., Parker A., Spudich G., Yates A., Zerbino D.R., Searle S.M. Ensembl 2014. Nucleic Acids Res. 2014;42:D749–D755. doi: 10.1093/nar/gkt1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowkes R.C., Desclozeaux M., Patel M.V., Aylwin S.J., King P., Ingraham H.A., Burrin J.M. Steroidogenic factor-1 and the gonadotrope-specific element enhance basal and pituitary adenylate cyclase-activating polypeptide-stimulated transcription of the human glycoprotein hormone alpha-subunit gene in gonadotropes. Mol. Endocrinol. 2003;17:2177–2188. doi: 10.1210/me.2002-0393. [DOI] [PubMed] [Google Scholar]
- Harrow J., Frankish A., Gonzalez J.M., Tapanari E., Diekhans M., Kokocinski F., Aken B.L., Barrell D., Zadissa A., Searle S., Barnes I., Bignell A., Boychenko V., Hunt T., Kay M., Mukherjee G., Rajan J., Despacio-Reyes G., Saunders G., Steward C., Harte R., Lin M., Howald C., Tanzer A., Derrien T., Chrast J., Walters N., Balasubramanian S., Pei B., Tress M., Rodriguez J.M., Ezkurdia I., van Baren J., Brent M., Haussler D., Kellis M., Valencia A., Reymond A., Gerstein M., Guigó R., Hubbard T.J. GENCODE: The reference human genome annotation for The ENCODE Project. Genome Res. 2012;22:1760–1774. doi: 10.1101/gr.135350.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton C.D., Halvorson L.M. The cAMP signaling system regulates LHbeta gene expression: roles of early growth response protein-1, SP1 and steroidogenic factor-1. J. Mol. Endocrinol. 2004;32:291–306. doi: 10.1677/jme.0.0320291. [DOI] [PubMed] [Google Scholar]
- Hsu S.Y., Nakabayashi K., Bhalla A. Evolution of glycoprotein hormone subunit genes in bilateral metazoa: identification of two novel human glycoprotein hormone subunit family genes, GPA2 and GPB5. Mol. Endocrinol. 2002;16:1538–1551. doi: 10.1210/mend.16.7.0871. [DOI] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- International Human Genome Sequencing Consortium Finishing the euchromatic sequence of the human genome. Nature. 2004;431:931–945. doi: 10.1038/nature03001. [DOI] [PubMed] [Google Scholar]
- Jiang Xuliang, Dias James A., He Xiaolin. Structural biology of glycoprotein hormones and their receptors: Insights to signaling. Mol. Cell. Endocrinol. 2014;382:424–451. doi: 10.1016/j.mce.2013.08.021. [DOI] [PubMed] [Google Scholar]
- Kashiwabara Y., Sasaki S., Matsushita A., Nagayama K., Ohba K., Iwaki H., Matsunaga H., Suzuki S., Misawa H., Ishizuka K., Oki Y., Nakamura H. Functions of PIT1 in GATA2-dependent transactivation of the thyrotropin beta promoter. J. Mol. Endocrinol. 2009;42:225–237. doi: 10.1677/JME-08-0099. [DOI] [PubMed] [Google Scholar]
- Lapthorn A.J., Harris D.C., Littlejohn A., Lustbader J.W., Canfield R.E., Machin K.J., Morgan F.J., Isaacs N.W. Crystal structure of human chorionic gonadotropin. Nature. 1994;369:455–461. doi: 10.1038/369455a0. [DOI] [PubMed] [Google Scholar]
- Li M.D., Ford J.J. A comprehensive evolutionary analysis based on nucleotide and amino acid sequences of the alpha- and beta-subunits of glycoprotein hormone gene family. J. Endocrinol. 1998;156:529–542. doi: 10.1677/joe.0.1560529. [DOI] [PubMed] [Google Scholar]
- Lindblad-Toh K., Garber M., Zuk O., Lin M.F., Parker B.J., Washietl S., Kheradpour P., Ernst J., Jordan G., Mauceli E., Ward L.D., Lowe C.B., Holloway A.K., Clamp M., Gnerre S., Alföldi J., Beal K., Chang J., Clawson H., Cuff J., Di Palma F., Fitzgerald S., Flicek P., Guttman M., Hubisz M.J., Jaffe D.B., Jungreis I., Kent W.J., Kostka D., Lara M., Martins A.L., Massingham T., Moltke I., Raney B.J., Rasmussen M.D., Robinson J., Stark A., Vilella A.J., Wen J., Xie X., Zody M.C., Broad Institute Sequencing Platform and Whole Genome Assembly Team, Baldwin J., Bloom T., Chin C.W., Heiman D., Nicol R., Nusbaum C., Young S., Wilkinson J., Worley K.C., Kovar C.L., Muzny D.M., Gibbs R.A., Baylor College of Medicine Human Genome Sequencing Center Sequencing Team, Cree A., Dihn H.H., Fowler G., Jhangiani S., Joshi V., Lee S., Lewis L.R., Nazareth L.V., Okwuonu G., Santibanez J., Warren W.C., Mardis E.R., Weinstock G.M., Wilson R.K., Genome Institute at Washington University, Delehaunty K., Dooling D., Fronik C., Fulton L., Fulton B., Graves T., Minx P., Sodergren E., Birney E., Margulies E.H., Herrero J., Green E.D., Haussler D., Siepel A., Goldman N., Pollard K.S., Pedersen J.S., Lander E.S., Kellis M. A high-resolution map of human evolutionary constraint using 29 mammals. Nature. 2011;478:476–482. doi: 10.1038/nature10530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald L.E., Wortley K.E., Gowen L.C., Anderson K.D., Murray J.D., Poueymirou W.T., Simmons M.V., Barber D., Valenzuela D.M., Economides A.N., Wiegand S.J., Yancopoulos G.D., Sleeman M.W., Murphy A.J. Resistance to diet-induced obesity in mice globally overexpressing OGH/GPB5. Proc. Natl. Acad. Sci. U. S. A. 2005;102:2496–2501. doi: 10.1073/pnas.0409849102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies E.H., Vinson J.P., NISC Comparative Sequencing Program, Miller W., Jaffe D.B., Lindblad-Toh K., Chang J.L., Green E.D., Lander E.S., Mullikin J.C., Clamp M. An initial strategy for the systematic identification of functional elements in the human genome by low-redundancy comparative sequencing. Proc. Natl. Acad. Sci. U. S. A. 2005;102:4795–4800. doi: 10.1073/pnas.0409882102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merk M., Zierow S., Leng L., Das R., Du X., Schulte W., Fan J., Lue H., Chen Y., Xiong H., Chagnon F., Bernhagen J., Lolis E., Mor G., Lesur O., Bucala R. The D-dopachrome tautomerase (DDT) gene product is a cytokine and functional homolog of macrophage migration inhibitory factor (MIF) Proc. Natl. Acad. Sci. U. S. A. 2011;108:E577–E585. doi: 10.1073/pnas.1102941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouse Genome Sequencing Consortium Lineage-specific biology revealed by a finished genome assembly of the mouse. PLoS Biol. 2009;7 doi: 10.1371/journal.pbio.1000112. e1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy W.J., Eizirik E., Johnson W.E., Zhang Y.P., Ryder O.A., O'Brien S.J. Molecular phylogenetics and the origins of placental mammals. Nature. 2001;409:614–618. doi: 10.1038/35054550. [DOI] [PubMed] [Google Scholar]
- O'Leary M.A., Bloch J.I., Flynn J.J., Gaudin T.J., Giallombardo A., Giannini N.P., Goldberg S.L., Kraatz B.P., Luo Z.X., Meng J., Ni X., Novacek M.J., Perini F.A., Randall Z.S., Rougier G.W., Sargis E.J., Silcox M.T., Simmons N.B., Spaulding M., Velazco P.M., Weksler M., Wible J.R., Cirranello A.L. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science. 2013;339:662–667. doi: 10.1126/science.1229237. [DOI] [PubMed] [Google Scholar]
- Premzl M. Comparative genomic analysis of eutherian Mas-related G protein-coupled receptor genes. Gene. 2014;540:16–19. doi: 10.1016/j.gene.2014.02.049. [DOI] [PubMed] [Google Scholar]
- Premzl M. Comparative genomic analysis of eutherian ribonuclease A genes. Mol. Genet. Genomics. 2014;289:161–167. doi: 10.1007/s00438-013-0801-5. [DOI] [PubMed] [Google Scholar]
- Premzl M. Third party annotation gene data set of eutherian lysozyme genes. Genomics Data. 2014;2:258–260. doi: 10.1016/j.gdata.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch G.J., Sherwood N.M. Glycoprotein hormones and their receptors emerged at the origin of metazoans. Genome Biol. Evol. 2014;6:1466–1479. doi: 10.1093/gbe/evu118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H., Taniguchi M., Nakagawa A., Tanaka I., Suzuki M., Nishihira J. Crystal structure of human D-dopachrome tautomerase, a homologue of macrophage migration inhibitory factor, at 1.54 A resolution. Biochemistry. 1999;38:3268–3279. doi: 10.1021/bi982184o. [DOI] [PubMed] [Google Scholar]
- Sun H.W., Bernhagen J., Bucala R., Lolis E. Crystal structure at 2.6-A resolution of human macrophage migration inhibitory factor. Proc. Natl. Acad. Sci. U. S. A. 1996;93:5191–5196. doi: 10.1073/pnas.93.11.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West B.E., Parker G.E., Savage J.J., Kiratipranon P., Toomey K.S., Beach L.R., Colvin S.C., Sloop K.W., Rhodes S.J. Regulation of the follicle-stimulating hormone beta gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology. 2004;145:4866–4879. doi: 10.1210/en.2004-0598. [DOI] [PubMed] [Google Scholar]
- Wilson D.E., Reeder D.M. 3rd edn. The Johns Hopkins University Press; Baltimore: 2005. Mammal Species of the World: A Taxonomic and Geographic Reference. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene data set of eutherian adenohypophysis cystine-knot genes.
Direct evidence of new human gene annotations.
Pairwise genomic sequence alignments. The translated base sequence regions were displayed as indigo rectangles. The untranslated base sequence regions were displayed as cyan rectangles. The aligned genomic sequence regions that showed conservation levels above empirically determined cut-offs of detection of common genomic sequence regions were shown accordingly. (A) Pairwise genomic sequence alignments of primate-specific PREA1-3 and PREB genes. The cut-offs of detection of common genomic sequence regions in pairwise alignments with Homo sapiens PREA2 were 95% per 100 bp (Homo sapiens PREA1 and PREA3) or 90% per 100 bp in other pairwise alignments. The Homo sapiens PREA2 exons were annotated using transcript BC040601.1. (B) Pairwise genomic sequence alignments of eutherian GPB5 genes. The potential regulatory genomic sequence regions 1 and 2 were shown using rectangles. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens GPB5 were: 99% per 100 bp (Pan troglodytes), 95% per 100 bp (Pongo abelii, Macaca mulatta and Papio hamadryas), 90% per 100 bp (Callithrix jacchus), 85% per 100 bp (Microcebus murinus and Tupaia belangeri) or 80% per 100 bp in other pairwise alignments. The Homo sapiens GPB5 exons were annotated using transcript BM709443.1. (C) Pairwise genomic sequence alignments of eutherian TSHB genes. The potential regulatory genomic sequence region 1 was shown using rectangle. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens TSHB were: 95% per 100 bp (Macaca mulatta and Papio hamadryas), 90% per 100 bp (Tarsius syrichta, Otolemur garnettii and Tupaia belangeri), 80% per 100 bp (Cavia porcellus and Oryctolagus cuniculus), 75% per 100 bp (Mus musculus and Rattus norvegicus) or 85% per 100 bp in other pairwise alignments. (D) Pairwise genomic sequence alignments of eutherian FSHB genes. The potential regulatory genomic sequence region 1 was shown using rectangle. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens FSHB were: 95% per 100 bp (Pan troglodytes, Pongo abelii and Nomascus leucogenys), 90% per 100 bp (Macaca mulatta and Callithrix jacchus), 80% per 100 bp (Cavia porcellus, Oryctolagus cuniculus, Ochotona princeps, Canis lupus familiaris and Sorex araneus), 75% per 100 bp (Mus musculus and Rattus norvegicus) or 85% per 100 bp in other pairwise alignments. The Homo sapiens FSHB exons were annotated using transcript BI033915.1. (E) Pairwise genomic sequence alignments of eutherian LHB-CGB genes. The potential regulatory genomic sequence regions 1–4 were shown using rectangles. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens LHB were: 95% per 100 bp (Pan troglodytes), 90% per 100 bp (Macaca mulatta), 80% per 100 bp (Cavia porcellus, Spermophilus tridecemlineatus and Ochotona princeps), 75% per 100 bp (Mus musculus, Rattus norvegicus and Canis lupus familiaris) or 90% per 100 bp in pairwise alignments with CGB genes. The Homo sapiens LHB exons were annotated using transcript CD107666.1. (F) Pairwise genomic sequence alignments of eutherian GPA1 genes. The potential regulatory genomic sequence regions 1–4 were shown using rectangles. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens GPA1 were: 95% per 100 bp (Pan troglodytes and Pongo abelii), 90% per 100 bp (Nomascus leucogenys), 85% per 100 bp (Callithrix jacchus), 80% per 100 bp (Bos taurus, Equus caballus, Canis lupus familiaris, Myotis lucifugus and Erinaceus europaeus), 70% per 100 bp (Mus musculus and Rattus norvegicus) or 75% per 100 bp in other pairwise alignments. The Homo sapiens GPA1 exons were annotated using transcript CR599056.1. (G) Pairwise genomic sequence alignments of eutherian GPA2 genes. The potential regulatory genomic sequence regions 1–2 were shown using rectangles. The cut-offs of detection of potential regulatory genomic sequence regions in pairwise alignments with Homo sapiens GPA2 were: 99% per 100 bp (Pan troglodytes), 95% per 100 bp (Gorilla gorilla), 90% per 100 bp (Pongo abelii, Macaca mulatta and Papio hamadryas), 85% per 100 bp (Microcebus murinus and Otolemur garnettii), 75% per 100 bp (Mus musculus, Rattus norvegicus, Dipodomys ordii, and Ochotona princeps) or 80% per 100 bp in other pairwise alignments. The Homo sapiens GPA2 exons were annotated using transcript AF260739.1.
Nucleotide sequence alignments of common predicted promoter genomic sequence regions. Using Homo sapiens transcripts as in Supplementary data file 3, the untranslated exons were annotated. The numbers in brackets indicated positions of 3'-terminal nucleotides relative to translated exons. The nucleotide positions were labeled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 85% conservation and black letters on grey background depicted ≥ 70% conservation. (A) Alignments of TSHB common predicted promoter genomic sequence regions. The PIT1 cis-elements (A), GATA2 cis-elements (B), suppressor regions (C) and TATA cis-elements (D) were indicated. (B) Alignments of FSHB common predicted promoter genomic sequence regions. The LHX3 cis-elements (A–C) and TATA cis-elements (D) were indicated, as well as partial first exons (E). (C) Alignments of LHB-CGB common predicted promoter genomic sequence regions (potential regulatory genomic sequence region 4). The composite SF-1 and Egr-1 cis-elements (A) and TATA cis-elements (B) were indicated. (D) Alignments of GPA1 common predicted promoter genomic sequence regions. The GSE cis-elements (A), CRE cis-elements (B), Hominidae-specific CRE cis-elements (C) and TATA cis-elements (D) were indicated, as well as partial first exons (E).
Nucleotide sequence alignments of common predicted promoter genomic sequence regions. Using Homo sapiens transcripts as in Supplementary data file 3, the untranslated exons were annotated. The numbers in brackets indicated positions of 3'-terminal nucleotides relative to translated exons. The nucleotide positions were labelled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 85% conservation and black letters on grey background depicted ≥ 70% conservation. (A) Alignments of TSHB common predicted promoter genomic sequence regions. The PIT1 cis-elements (A), GATA2 cis-elements (B), suppressor regions (C) and TATA cis-elements (D) were indicated. (B) Alignments of FSHB common predicted promoter genomic sequence regions. The LHX3 cis-elements (A–C) and TATA cis-elements (D) were indicated, as well as partial first exons (E). (C) Alignments of LHB-CGB common predicted promoter genomic sequence regions (potential regulatory genomic sequence region 4). The composite SF-1 and Egr-1 cis-elements (A) and TATA cis-elements (B) were indicated. (D) Alignments of GPA1 common predicted promoter genomic sequence regions (potential regulatory genomic sequence region 4). The GSE cis-elements (A), CRE cis-elements (B), Hominidae-specific CRE cis-elements (C) and TATA cis-elements (D) were indicated, as well as partial first exons (E).
Pairwise nucleotide sequence identities of eutherian adenohypophysis cystine-knot genes, and D-dopachrome tautomerase and macrophage migration inhibitory factor genes.
Alignments of eutherian adenohypophysis cystine-knot proteins. The reference human FSHB and GPA1 protein sequence invariant amino acid sites were shown using white letters on violet backgrounds and forward amino acid sites were shown using white letters on red backgrounds. The amino acid positions of GPB5s, TSHBs, FSHBs, LHs and CGBs were labelled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 75% conservation and black letters on grey background depicted ≥ 50% conservation. The GPA1 and GPA2 amino acid positions were labelled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 80% conservation and black letters on grey background depicted ≥ 60% conservation. The stop codon positions were labelled using &s.
Protein molecular evolution analysis. (A) Reference human FSHB protein amino acid sequence. The amino acid clusters 1 and 2 with overrepresented invariant or/and forward amino acid sites were labelled using rectangles. (B) Reference human GPA1 protein amino acid sequence. The amino acid clusters 1–5 with overrepresented invariant or/and forward amino acid sites were labelled using rectangles. (A–B) The invariant amino acid sites were shown using white letters on violet backgrounds. The forward amino acid sites were shown using white letters on red backgrounds. The secondary structure elements were designated according to Fan and Hendrickson (Fan and Hendrickson, 2005) for FSH crystal structure 1XWD. The common cysteine residues were labelled according to Alvarez et al. (2009) and present analysis (Fig. 2). Black triangles labelled predicted signal peptide cleavage sites. (C–D) Analysis of human FSHB crystal structure 1XWD. The amino acid cluster 1 and 2 invariant amino acid sites were labelled violet, forward amino acid sites were labelled red and compensatory amino acid sites were labelled white. (C) Ribbon representation of human FSHB crystal structure 1XWD. (D) van-der-Waals representations of amino acid cluster 1 and 2 amino acids in view identical to that in C. (E–F) Analysis of human GPA1 crystal structure 1XWD. The amino acid cluster 1-5 invariant amino acid sites were labelled violet, forward amino acid sites were labelled red and compensatory amino acid sites were labelled white. (E) Ribbon representation of human GPA1 crystal structure 1XWD. (F) van-der-Waals representations of amino acid cluster 1-5 amino acids in view identical to that in E.
Gene data set of eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor genes.
Eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor genes. (A) Distribution of DDT genes and MIF genes in human, mouse and domestic cattle genomic sequence assemblies. The relative gene orders and orientations in genomic sequences were shown approximately. Genes other than DDTs and MIFs were not shown. BTA, domestic cattle chromosome; HSA, human chromosome; MMU, mouse chromosome. (B–C) Pairwise genomic sequence alignments. The translated base sequence regions were displayed as indigo rectangles. The untranslated base sequence regions were displayed as cyan rectangles. The aligned genomic sequence regions that showed conservation levels above empirically determined cut-offs of detection of common genomic sequence regions were shown accordingly. (B) Pairwise genomic sequence alignments of eutherian DDT genes. The potential regulatory genomic sequence region 1 was shown using rectangle. The cut-offs of detection of common genomic sequence regions in pairwise alignments with Homo sapiens DDT1 were: 99% per 100 bp (Pan troglodytes DDT1 and Homo sapiens DDT2), 95% per 100 bp (Pongo abelii DDT2), 90% per 100 bp (Papio hamadryas DDT1, Callithrix jacchus DDT1, Homo sapiens DDT3 and Pan troglodytes DDT3), 70% per 100 bp (Tupaia belangeri, Bos taurus and Felis catus), 60% per 100 bp (Homo sapiens DDT4 and Pan troglodytes DDT4), 55% per 100 bp (Erinaceus europaeus, Dasypus novemcinctus and Loxodonta africana), 55% per 90 bp (Mus musculus and Rattus norvegicus) and 50% per 100 bp (Nomascus leucogenys DDT4). The Homo sapiens DDT1 exons were annotated using transcript BC005971.1. (C) Pairwise genomic sequence alignments of eutherian MIF genes. The potential regulatory genomic sequence region 1 was shown using rectangle. The cut-offs of detection of common genomic sequence regions in pairwise alignments with Homo sapiens MIF were: 95% per 100 bp (Nomascus leucogenys and Papio hamadryas), 70% per 100 bp (Otolemur garnetii), 65% per 100 bp (Bos taurus, Equus caballus, Pteropus vampyrus, Echinops telfairi and Procavia capensis), and 60% per 100 bp (Mus musculus and Rattus norvegicus). The Homo sapiens MIF exons were annotated using transcript BC013976.2.
Alignments of eutherian D-dopachrome tautomerase and macrophage migration inhibitory factor proteins. The reference human DDT1 and MIF protein sequence invariant amino acid sites were shown using white letters on violet backgrounds. The forward amino acid sites were shown using white letters on red backgrounds. The amino acid positions were labelled according to conservation levels: white letters on black background depicted 100% conservation, white letters on dark grey background depicted ≥ 85% conservation and black letters on grey background depicted ≥ 70% conservation. The DDT2 and DDT3 C-terminal regions were not used in calculations. The stop codon positions were labelled using &s.