Abstract
Direct intravital microscopic examinations were made in nailfold capillaries in subjects with homozygous sickle cell disease (HbSS red cells). In the resting state, capillary red cell (rbc) flux exhibited greater intermittence compared with normal subjects, which increased with painful crisis. In crisis-free HbSS subjects, capillary occlusion and red cell sequestration occurred in only 8.2% of all capillaries and diminished to 5.8% during crisis, possibly due to sequestration of less deformable rbcs in other organs. Velocities of rbc's (Vrbc) were measured by video techniques under resting conditions and during postocclusive reactive hyperemia (PORH) induced by a pressure cuff around the finger. Resting Vrbc was normal in crisis-free HbSS subjects, averaging 0.7 mm/s. In contrast, Vrbc was significantly elevated during crisis, to 0.98 mm/s, apparently due to compensatory arteriolar dilation. Crisis subjects exhibited a significantly depressed PORH with the ratio of peak red cell velocity to resting values reduced by 15% due to a loss of vasodilatory reserve, whereas crisis-free subjects exhibited a normal response. A 55% increase in the time to attain peak Vrbc was attributed to resistance increases, possibly resulting from red cell and leukocyte-to-endothelium adhesion during the induced ischemia.
Full text
PDF
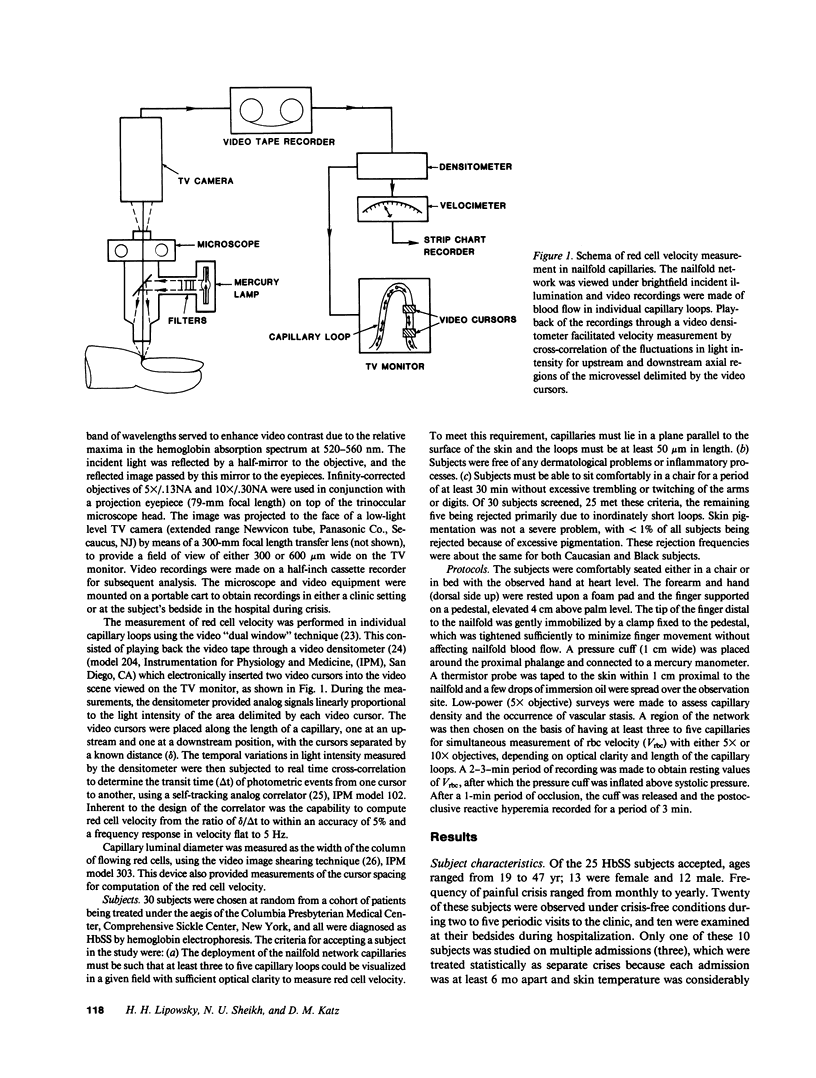
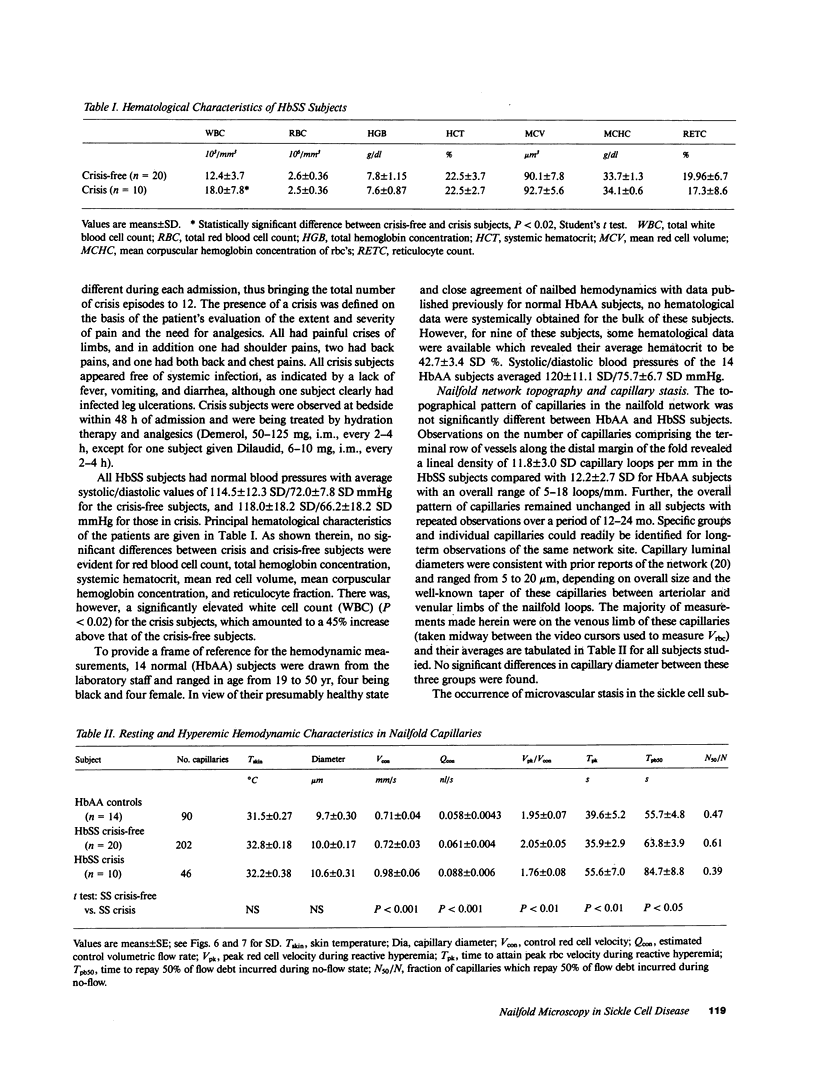
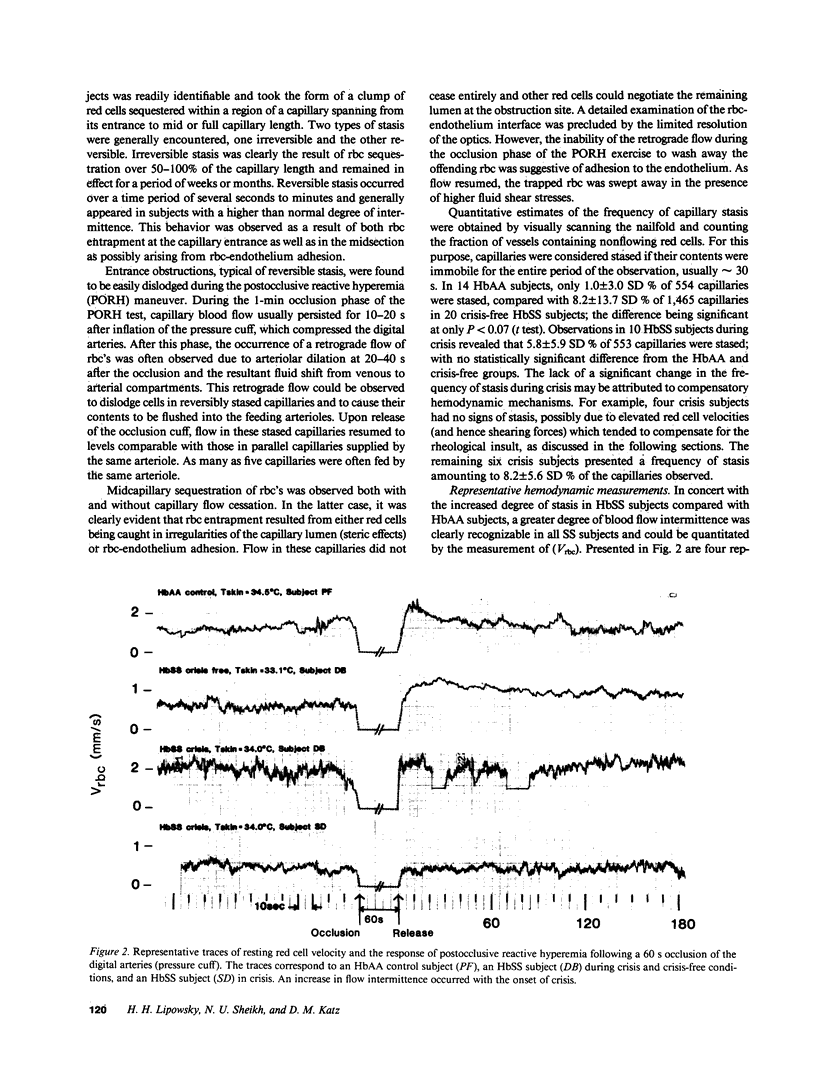
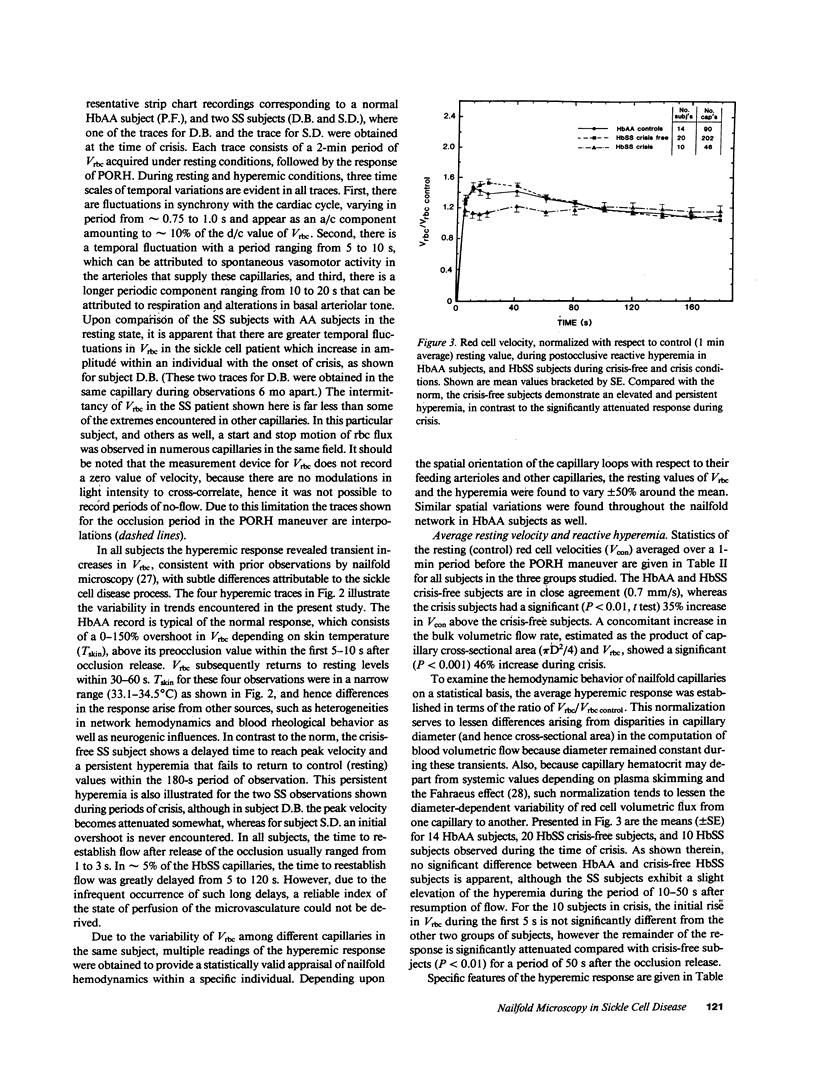
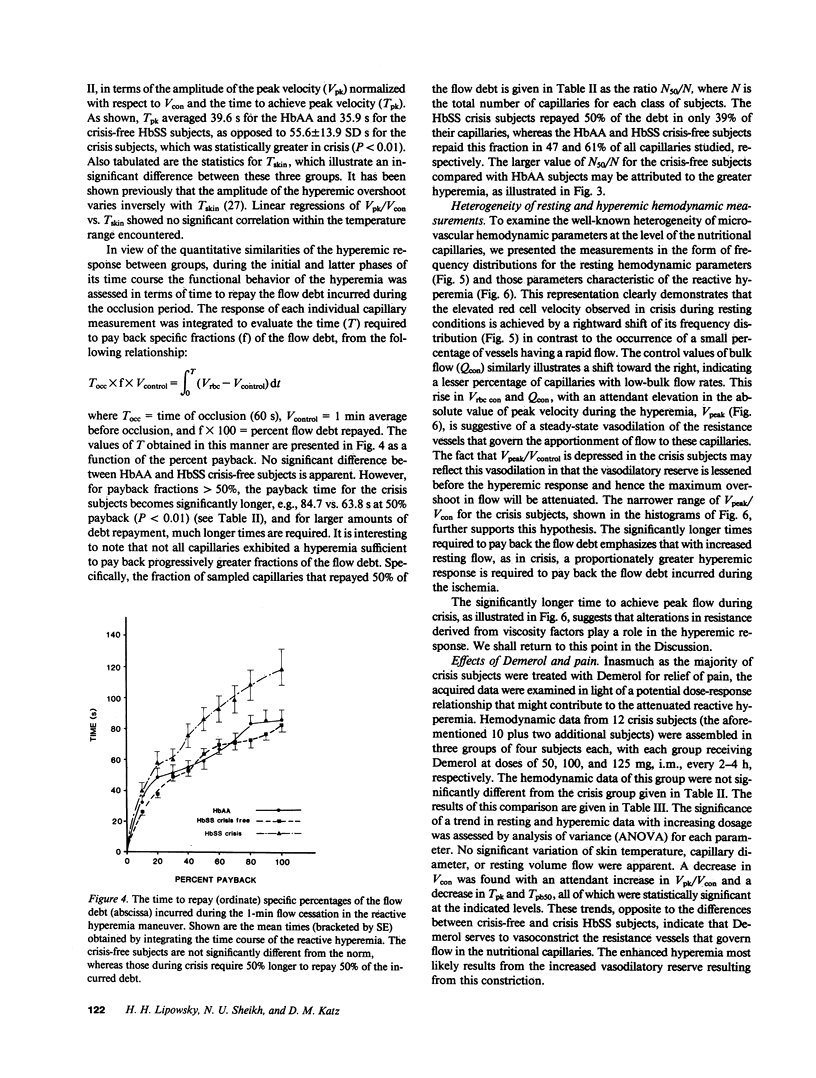
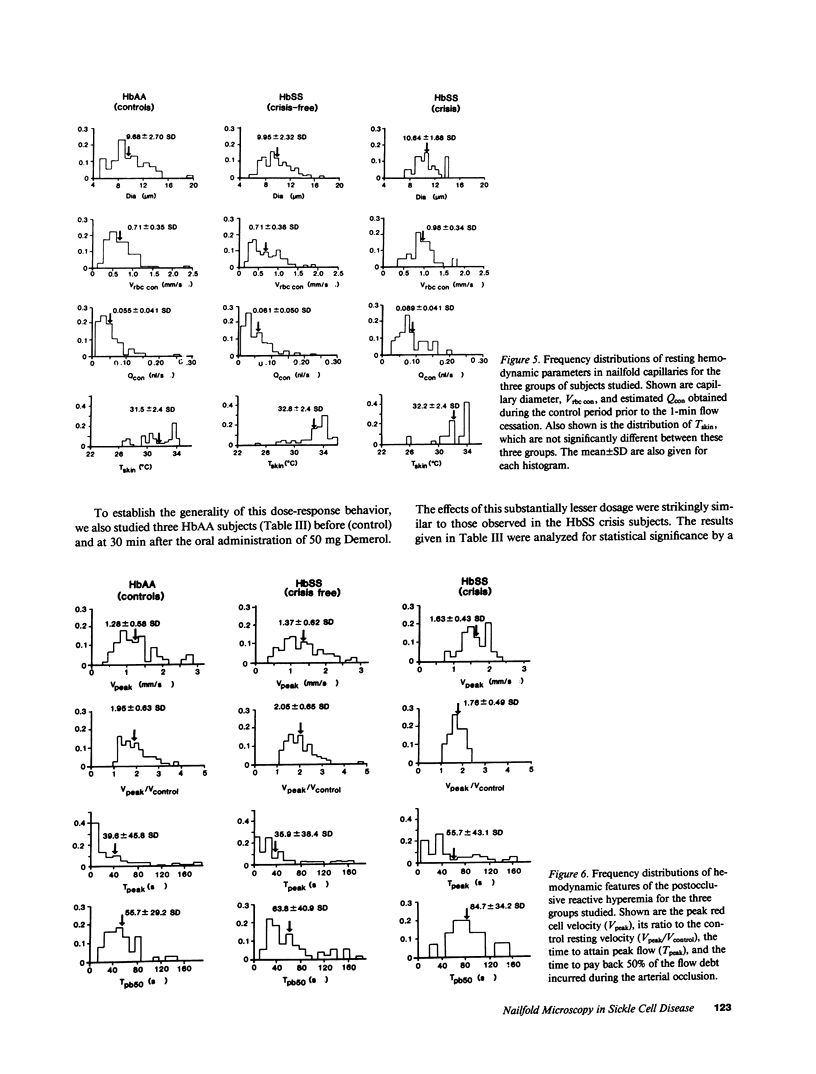
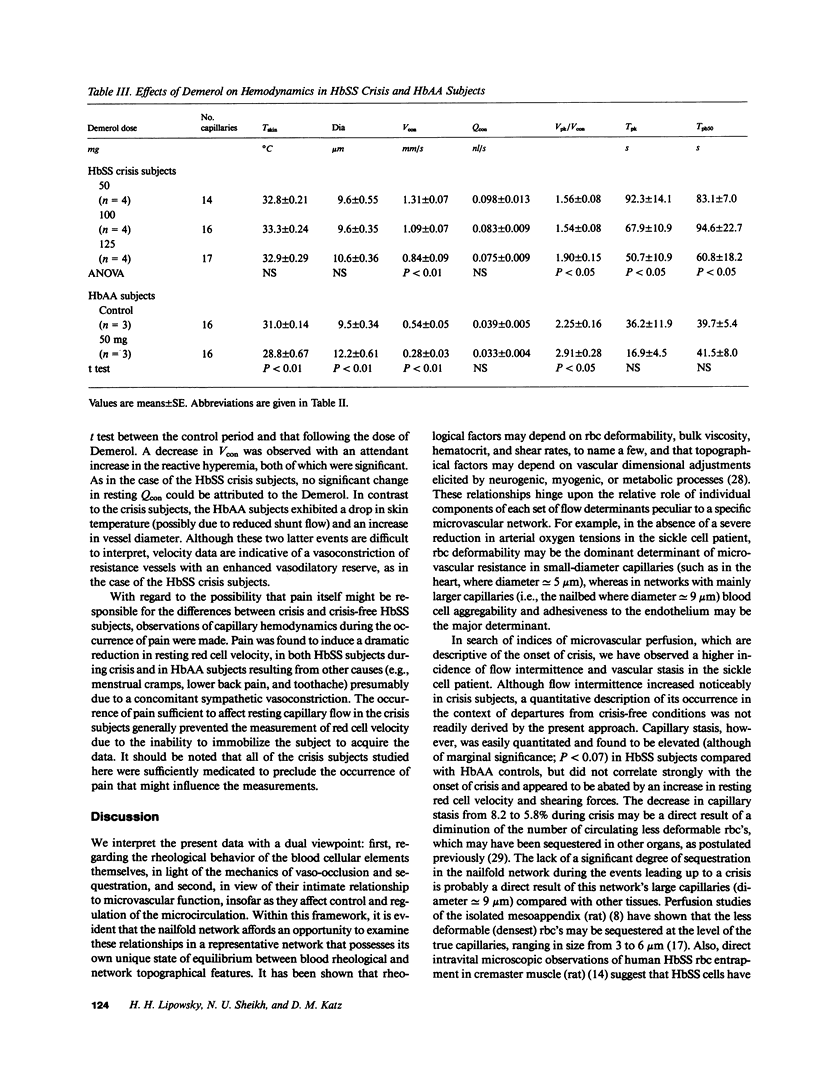



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baez S., Kaul D. K., Nagel R. L. Microvascular determinants of blood flow behavior and HbSS erythrocyte plugging in microcirculation. Blood Cells. 1982;8(1):127–137. [PubMed] [Google Scholar]
- Bollinger A., Butti P., Barras J. P., Trachsler H., Siegenthaler W. Red blood cell velocity in nailfold capillaries of man measured by a television microscopy technique. Microvasc Res. 1974 Jan;7(1):61–72. doi: 10.1016/0026-2862(74)90037-5. [DOI] [PubMed] [Google Scholar]
- Braide M., Amundson B., Chien S., Bagge U. Quantitative studies on the influence of leukocytes on the vascular resistance in a skeletal muscle preparation. Microvasc Res. 1984 May;27(3):331–352. doi: 10.1016/0026-2862(84)90064-5. [DOI] [PubMed] [Google Scholar]
- Buchanan G. R., Glader B. E. Leukocyte counts in children with sickle cell disease. Comparative values in the steady state, vaso-occlusive crisis, and bacterial infection. Am J Dis Child. 1978 Apr;132(4):396–398. doi: 10.1001/archpedi.1978.02120290068013. [DOI] [PubMed] [Google Scholar]
- Butti P., Intaglietta M., Reimann H., Holliger C., Bollinger A., Anliker M. Capillary red blood cell velocity measurements in human nailfold by videodensitometric method. Microvasc Res. 1975 Sep;10(2):220–227. doi: 10.1016/0026-2862(75)90010-2. [DOI] [PubMed] [Google Scholar]
- Chien S., Usami S., Bertles J. F. Abnormal rheology of oxygenated blood in sickle cell anemia. J Clin Invest. 1970 Apr;49(4):623–634. doi: 10.1172/JCI106273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINTENFASS L. RHEOLOGY OF PACKED RED BLOOD CELLS CONTAINING HEMOGLOBINS A-A, S-A, AND S-S. J Lab Clin Med. 1964 Oct;64:594–600. [PubMed] [Google Scholar]
- Evans E., Mohandas N., Leung A. Static and dynamic rigidities of normal and sickle erythrocytes. Major influence of cell hemoglobin concentration. J Clin Invest. 1984 Feb;73(2):477–488. doi: 10.1172/JCI111234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabry M. E., Benjamin L., Lawrence C., Nagel R. L. An objective sign in painful crisis in sickle cell anemia: the concomitant reduction of high density red cells. Blood. 1984 Aug;64(2):559–563. [PubMed] [Google Scholar]
- Fabry M. E., Nagel R. L. Heterogeneity of red cells in the sickler: a characteristic with practical clinical and pathophysiological implications. Blood Cells. 1982;8(1):9–15. [PubMed] [Google Scholar]
- Fagrell B., Fronek A., Intaglietta M. A microscope-television system for studying flow velocity in human skin capillaries. Am J Physiol. 1977 Aug;233(2):H318–H321. doi: 10.1152/ajpheart.1977.233.2.H318. [DOI] [PubMed] [Google Scholar]
- Fagrell B., Intaglietta M., Ostergren J. Relative hematocrit in human skin capillaries and its relation to capillary blood flow velocity. Microvasc Res. 1980 Nov;20(3):327–335. doi: 10.1016/0026-2862(80)90033-3. [DOI] [PubMed] [Google Scholar]
- HARRIS J. W., BREWSTER H. H., HAM T. H., CASTLE W. B. Studies on the destruction of red blood cells. X. The biophysics and biology of sickle-cell disease. AMA Arch Intern Med. 1956 Feb;97(2):145–168. doi: 10.1001/archinte.1956.00250200021002. [DOI] [PubMed] [Google Scholar]
- Hebbel R. P., Eaton J. W., Steinberg M. H., White J. G. Erythrocyte/endothelial interactions in the pathogenesis of sickle-cell disease: a "real logical" assessment. Blood Cells. 1982;8(1):163–173. [PubMed] [Google Scholar]
- Hebbel R. P., Yamada O., Moldow C. F., Jacob H. S., White J. G., Eaton J. W. Abnormal adherence of sickle erythrocytes to cultured vascular endothelium: possible mechanism for microvascular occlusion in sickle cell disease. J Clin Invest. 1980 Jan;65(1):154–160. doi: 10.1172/JCI109646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intaglietta M., Silverman N. R., Tompkins W. R. Capillary flow velocity measurements in vivo and in situ by television methods. Microvasc Res. 1975 Sep;10(2):165–179. doi: 10.1016/0026-2862(75)90004-7. [DOI] [PubMed] [Google Scholar]
- Intaglietta M., Tompkins W. R. Microvascular measurements by video image shearing and splitting. Microvasc Res. 1973 May;5(3):309–312. doi: 10.1016/0026-2862(73)90042-3. [DOI] [PubMed] [Google Scholar]
- Kaul D. K., Fabry M. E., Windisch P., Baez S., Nagel R. L. Erythrocytes in sickle cell anemia are heterogeneous in their rheological and hemodynamic characteristics. J Clin Invest. 1983 Jul;72(1):22–31. doi: 10.1172/JCI110960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul D. K., Nagel R. L., Baez S. Pressure effects on the flow behavior of sickle (HbSS) red cells in isolated (ex-vivo) microvascular system. Microvasc Res. 1983 Sep;26(2):170–181. doi: 10.1016/0026-2862(83)90068-7. [DOI] [PubMed] [Google Scholar]
- Lipowsky H. H., Usami S., Chien S. Human SS red cell rheological behavior in the microcirculation of cremaster muscle. Blood Cells. 1982;8(1):113–126. [PubMed] [Google Scholar]
- Nash G. B., Johnson C. S., Meiselman H. J. Mechanical properties of oxygenated red blood cells in sickle cell (HbSS) disease. Blood. 1984 Jan;63(1):73–82. [PubMed] [Google Scholar]
- Rodgers G. P., Schechter A. N., Noguchi C. T., Klein H. G., Nienhuis A. W., Bonner R. F. Periodic microcirculatory flow in patients with sickle-cell disease. N Engl J Med. 1984 Dec 13;311(24):1534–1538. doi: 10.1056/NEJM198412133112403. [DOI] [PubMed] [Google Scholar]
- Tooke J. E., Lins P. E., Ostergren J., Fagrell B. Skin microvascular autoregulatory responses in type I diabetes: the influence of duration and control. Int J Microcirc Clin Exp. 1985;4(3):249–256. [PubMed] [Google Scholar]
- Usami S., Chien S., Scholtz P. M., Bertles J. F. Effect of deoxygenation on blood rheology in sickle cell disease. Microvasc Res. 1975 May;9(3):324–334. doi: 10.1016/0026-2862(75)90069-2. [DOI] [PubMed] [Google Scholar]
- Vayo M. M., Lipowsky H. H., Karp N., Schmalzer E., Chein S. A model of microvascular oxygen transport in sickle cell disease. Microvasc Res. 1985 Sep;30(2):195–206. doi: 10.1016/0026-2862(85)90050-0. [DOI] [PubMed] [Google Scholar]



