Abstract
Signal transducer and activator of transcription 3 (STAT3) integrates key signals of cell surface immune receptors, yet its precise role in cluster of differentiation (CD)4+ T cells is not well-established. Current research has indicated T-helper cell 17–inducing roles but also tolerogenic roles. To address this issue, human T cells were transduced with the constitutively active STAT3 mutant STAT3C. Following stimulation, STAT3C+ T cells up-regulated IL-10 (4.1 ± 0.5-fold; P < 0.001) and granzyme B (2.5 ± 1.2, P < 0.05) secretion, combined with significantly reduced IFN-γ (35 ± 5%), IL-2 (57 ± 4%), TNF-α (64 ± 8%), and IL-13 (89 ± 3%) secretion (P < 0.001). CD3/CD2- or CD3/CD28-activated STAT3C+ T cells revealed reduced proliferation (53.4 ± 23.5% and 70.5 ± 10.4%, respectively), which was independent of IL-10 production and significantly suppressed effector T cell proliferation by 68.7 ± 10.6% and 65.9 ± 2.6%, respectively (P < 0.001). Phenotypically, STAT3C-transgenic CD4+ T cells resembled effector T cells regarding expression of T regulatory cell markers, but up-regulated granzyme B expression levels by 2.4-fold (P < 0.05). Suppression was cell contact dependent and mediated by granzyme B-induced cell death, but was independent of IL-10 and TGF-β. Notably, peripheral blood CD4+CD45RA−lymphocyte activation gene-3+CD49+ type 1 regulatory T cells revealed activation-induced hyperphosphorylation of STAT3. In agreement, pharmacological inhibition of STAT3 activation partially reverted hyporesponsiveness of peripheral type 1 regulatory T cells (increasing their division index from 0.46 ± 0.11 to 0.89 ± 0.04; P < 0.01). These observations indicate a clear-cut relation between activation of STAT3 and the acquisition of a tolerogenic program, which is also used by peripheral blood type 1 regulatory T cells.—Schmetterer, K. G., Neunkirchner, A., Wojta-Stremayr, D., Leitner, J., Steinberger, P., Pickl, W. F. STAT3 governs hyporesponsiveness and granzyme B-dependent suppressive capacity in human CD4+ T cells.
Keywords: regulatory T cells, Tr1 cells
The signal transducer and activator of transcription 3 (STAT3) is a key molecule in the integration of signals derived from receptor tyrosine kinases and nonreceptor tyrosine kinases. Similar to other STAT proteins, phosphorylation of STAT3 leads to dimerization of the cytosolic protein followed by translocation to the nucleus, where STAT3 dimers act as transcription factors. Because of its highly pleiotropic role in different tissues, STAT3 is involved in a multitude of physiologic and pathophysiologic processes ranging from embryogenesis and immunoregulation to carcinogenesis.
In myeloid immune cells, both loss-of-function (1–4) and overexpression studies (3, 5) and studies in human dendritic cells (6) have confirmed that activation of STAT3 mainly governs anti-inflammatory and tolerogenic processes. In contrast, the role of STAT3 in lymphocytes appears to be more ambiguous. Conditional knockout of Stat3 in cluster of differentiation (CD)4+ T cells completely abrogates their ability to differentiate into T-helper (Th)17 cells. Reversely, overexpression of a constitutively active form of STAT3, termed STAT3C, was shown to strongly induce Th17 polarization in murine T cells (7–9), which is governed by the upstream activity of PKC-θ (10). Interestingly, the Th17-inducing capacity of STAT3C was not consistently found but only observed in the absence of IFN-γ, identifying IFN-γ as a potential antagonist of Th17 polarization (8).
Conversely, potential tolerogenic aspects in CD4+ T cells have been highlighted as the second major function of STAT3 signaling in recent reports (11–13). Remarkably, deletion of Stat3 in CD4+CD25+ naturally occurring T regulatory cells (nTreg) impaired their ability to suppress Th17 responses (11), which could subsequently be defined as an IL-10-dependent process (12). Similarly, pharmacological or siRNA-mediated inhibition of STAT3 decreased conversion of CD4+CD25− T cells into regulatory T cells (13). Previous reports also suggested that tolerogenic aspects of STAT3 might play an important role in the induction and function of IL-10-secreting type 1 regulatory T cells (Tr1). These cells are marked by a typical cytokine secretion profile including high levels of IL-10, intermediate levels of IFN-γ, and low levels of IL-2 and IL-4 (14). Tr1 cells can be induced in vitro by different protocols including stimulation with immature dendritic cells (15), IL-10 and/or IFN-α (16), and IL-27 (17–20), all inducing STAT3 signaling in target T cells [reviewed by Gregori et al. (21)]. The recent identification of CD4+CD45RA−lymphocyte activation gene-3 (LAG3)+CD49b+ phenotype as a specific cell surface marker combination for human peripheral blood (PB) Tr1 cells (22) offers the possibility to separate these cells from PB and to study their biology in a more unbiased way without the need for prior induction from nonregulatory T cells. However, to the best of our knowledge, the activation status of STAT3 in these cells has thus far not been examined.
The 2 roles of STAT3 are probably best reflected by the pathophysiology caused by autosomal-dominant STAT3 mutations in hyper IgE syndrome. In this disease, patients are deficient for Th17 cells but also display typical signs and symptoms of immune dysregulation, such as IgE hyperproduction and eczema, both of which are typically associated with other well-described loss-of-tolerance diseases such as immunodeficiency, polyendocrinopathy, enteropathy, X-linked syndrome [forkhead box protein 3 (FOXP3) mutations], autoimmune, polyendocrinopathy, candidiasis, ectodermal dystrophy syndrome (autoimmune regulator mutations), and Omenn’s syndrome (recombination-activating gene mutations) (23).
To elucidate the functional role of STAT3 in human CD4+ T cells, we ectopically expressed a constitutively active form of STAT3, designated STAT3C (24), in PB CD4+ T cells of healthy human individuals. STAT3C-transduced T cells were analyzed for their phenotype in regard to nTreg and Tr1 markers. We also assessed their proliferation and cytokine production potential following T cell receptor–mediated activation in association with different costimulatory signals. Furthermore, their suppressive capacity was investigated in cocultures with effector T cells in response to various forms of antigen-presenting cells (APCs) and compared with FOXP3-transduced T cells, which represent in functional terms bona fide nTreg cells (25–27). Finally, we correlated the results obtained in overexpression studies with the activation status of STAT3 in resting and activated human PB Tr1 cells in comparison with effector PB T cells and assessed the influence of STAT3 activation on the proliferative capacity of Tr1 cells.
MATERIALS AND METHODS
Molecular cloning and generation of multicistronic vectors
The STAT3 cDNA was amplified from a human T cell cDNA library (28) with the following primers: STAT3 forward, 5′-CCCGCGAAGCTTGCCACCATGGCCCAATGGAATCAGCTACAGC-3′; STAT3 reverse, 5′-CCCGCGGCGGCCGCTTTACATGGGGGAGGTAGCGCACTC-3′; STAT3Cint forward, 5′-ATGGGCTATAAGATCATGGATTGCACCTGCATCCTGGTGTCTCCACTG-3′; STAT3Cint reverse, 5′-CAGTGGAGACACCAGGATGCAGGTGCAATCCATGATCTTATAGCCCAT-3′ (bold sequences mark restriction enzyme sites). The STAT3(p.A661C, p.N663C) (in the following referred to as STAT3C) cDNA was generated from the wild-type STAT3 construct via PCR mutagenesis. Both constructs were digested with HindIII and NotI and ligated into the retroviral pMMP-internal ribosomal entry site (IRES)-green fluorescent protein (GFP) vector. The pMMP-FOXP3-IRES-GFP and empty control pMMP-IRES-GFP vector were described elsewhere (27).
Cell lines and primary cells
The HEK-293 cell line (human embryonic kidney cells) was cultured as described previously (29). Peripheral blood mononuclear cells were isolated from healthy volunteers in compliance with the ethics committee of the Medical University of Vienna. CD4+ T cells were isolated using the Human CD4+ T cell Isolation Kit II (Miltenyi Biotech, Bergisch Gladbach, Germany). Human CD14+ monocytes were isolated using CD14 MicroBeads (Miltenyi Biotech). For generation of monocyte-derived dendritic cells (mdDCs), CD14+ monocytes were cultured for 7 d with GM-CSF and IL-4. Some cultures were additionally supplemented with 100 ng/ml LPS in the final 48 h of culture to generate mature mdDCs. The mouse thymoma cell line BW5417 expressing a membrane-bound OKT3 single-chain variable fragment (scFv) (BW 3/2) in concert with the costimulatory molecule CD58 was cultured and maintained as described previously (30).
Transfection of HEK-293 cells
HEK-293 cells were transfected using Ca2PO4 precipitation method as described previously (29). For the production of amphotropic T cell–transducing retrovirus supernatants, MoMLV gag-pol, the envelope protein GalV and transgene DNA were transfected in a 2:1:3 ratio.
Retroviral transduction of peripheral blood T cells
CD4+ PB T cells (5 × 106/well) were stimulated in 6-well flat bottom plates with 5 × 106 anti-CD3/CD28 coated microbeads (Dynabeads, Invitrogen, Carlsbad, CA, USA) and 300 U/ml IL-2 (Peprotech, London, United Kingdom) for 48 h. Retroviral transduction was performed by addition of cell-free retroviral supernatant in the presence of 8 µg/ml polyprene (Sigma-Aldrich, St. Louis, MO, USA) followed by centrifugation at 600 g for 2 h. Twenty-four hours after transduction, cells were transferred to fresh medium containing 100 U/ml IL-2 and cultured for another 6–7 d (31).
Flow cytometric analyses
Cells were stained as described previously (32) using the mAbs indicated in Supplemental Table 1 (20 µg/ml). For intracellular staining of the transcription factors FOXP3 and STAT3, the FOXP3 Fix/Perm Buffer Set (BioLegend, San Diego, CA, USA) was used according to the manufacturers’ recommendations. For intracellular staining of granzyme A, granzyme B, and cytotoxic T-lymphocyte antigen (CTLA)-4, the Fix and Perm Kit from ADG (An der Grub, Kaumberg, Austria) was used. For intracellular staining of phosphorylated STAT3, the BD Phosflow Fixation Buffer I, Permeabilization Buffer III system (BD Biosciences, Franklin Lakes, NJ, USA) was used according to the manufacturer’s instructions. Analyses were performed on a BD LSRII Fortessa flow cytometer using the FlowJo software (BD Biosciences).
Determination of cytokine secretion
Transduced T cells (5 × 104) were stimulated in 96-well plates with either the BW 3/2 CD58 cell line serving as T cell stimulator cells (2 × 104 irradiated with 60 Gy) or anti-CD3/CD28 coated microbeads (5 × 104). After 24, 48, 72, or 96 h, supernatants were harvested, and cytokine concentrations were determined by multiplex analysis (Luminex 100IS; Biomedica, Vienna, Austria). For cytokine analyses of mixed lymphocyte reaction coculture experiments, cytokines were harvested after 120 h and processed as above.
Determination of granzyme B secretion
Transduced T cells (1 × 105) were stimulated in 96-well plates with either with BW 3/2 CD58 cells (4 × 104 irradiated with 60 Gy) or anti-CD3/CD28 coated microbeads (1 × 105). After 6 h, supernatants were harvested, and granzyme B concentrations were determined using the granzyme B Platinum ELISA (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions.
Proliferation and suppression assays
Transduced T cells (5 × 104) were stimulated in 96-well plates with BW 3/2 CD58 artificial APCs (aAPCs; 2 × 104 irradiated with 60 Gy) or anti-CD3/anti-CD28 coated microbeads at the indicated cell to bead ratios for 72 h, pulsed with [methyl-3H] thymidine (1 µCi per well; Perkin Elmer, Boston, MA, USA) for an additional 18 h, and processed as described previously (29). To test the suppressive capability of transgenic T cells, 5 × 104 effector T cells were cocultured with flow cytometrically sorted STAT3C+, FOXP3+, or control-transduced T cells and stimulated with aAPCs or anti-CD3/anti-CD28 coated microbeads and processed as above. Similarly, CD4+ effector T cells were labeled with the far-red cell proliferation dye eFluor 670 (eBioscience; 1.5 µM), cocultured with transduced T cells, and analyzed by flow cytometry after 96 h. In some experiments, blocking antibodies to IL-10 (3C12C12; Santa Cruz Biotechnology, Heidelberg, Germany), TGF-β1,2,3 (1D11; R&D Systems, Minneapolis, MN, USA), or control mouse IgG1 (11711; R&D Systems) were added at a final concentration of 20 µg/ml in accordance with established methods (33–35). Similarly, in some experiments, the granzyme B-specific inhibitor benzyloxycarbonyl-ala-ala-asp(OMe) chloromethyl ketone (Z-AAD-CMK; granzyme B inhibitor I; Merck Millipore, Darmstadt, Germany) was added to the cocultures at a final concentration of 30 µM (36).
For allogeneic mixed lymphocyte reactions, 5 × 104 CD4+ T cells were cocultured with fluorescence-activated cell sorted (FACS) STAT3C-, FOXP3-, or control-transduced T cells and stimulated with allogeneic immature and LPS-matured mdDCs (5 × 103) in 96-well round-bottom plates for 120 h. Cultures were pulsed with [methyl-3H] thymidine for an additional 18 h and processed as above.
For transwell analyses, 2 × 105 control-transduced or STAT3C+ T cells were seeded into the lower bucket of a transwell system (pore size, 3 µm; Corning Costar, Corning, NY, USA) and stimulated with 1 × 105 BW 3/2 CD58 aAPCs or 1 × 105 anti-CD3/anti-CD28 coated microbeads; 5 × 104 eFluor670 CPD-labeled CD4+ T cells were seeded into the upper well and stimulated with 2 × 104 BW 3/2 CD58 aAPCs. After 96 h, proliferation of the CD4+ responder T cells from the upper well was assessed by flow cytometry as above.
For assessment of the influence of STAT3 activation on Tr1 cell proliferation, magnetic cell isolation and cell separation CD4+ T cells were labeled with the eFluor450 cell proliferation dye (eBioscience; 1 µM), rested overnight in medium before FACS sorting for CD4+CD45RA−CD49b+LAG-3+ Tr1 and CD4+CD45RA−CD49b−LAG-3− effector T cell (TEFF) cells. T cell populations (5 × 104) were activated in a 96-well flat-bottom plates using 5 × 104 anti-CD3/anti-CD28 coated microbeads in the presence or absence of 100 µM STAT3 inhibitor V (STAT Three Inhibitory Compound; Merck, Whitehouse Station, NJ, USA) (37). After a 96 h dilution of eFluor450, Cell Proliferation Dye was determined as a measure for cellular proliferation by flow cytometry.
Determination of cell death
CD4+ T cells (5 × 104) were cocultured in the presence or absence of 5 × 104 control vector- or STAT3C-transduced T cells in 96-well flat-bottom plates and activated using 2 × 104 BW 3/2 CD58 aAPCs. After 72 h, cells were harvested and washed in annexin staining buffer (1 mM [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid], 140 mM NaCl, and 25 µM CaCl2; all Sigma-Aldrich). After addition of 5 µl annexin V APC (eBioscience), cells were incubated at room temperature for 10 min. Immediately prior to analysis, propidium iodide (50 ng/ml; Sigma-Aldrich) was added. Cell death of GFP-negative TEFF was determined by flow cytometry by quantification of the cumulative percentage of cells positive for annexin V and/or propidium iodide. Numbers were then normalized to the degree of cell death observed in TEFF activated in plain medium, which amounted to 45.1 ± 1.5%.
Statistical analyses
For comparison of proliferation data from CPD-labeled effector T cells, a division index was calculated according to the formula division index = [log(mean fluorescence nonstimulated cells/mean fluorescence responder cells)/log2] (38). For multiple group comparisons, 1-way ANOVA followed by Bonferroni correction was performed using GraphPad Prism version 5.0 for Windows (GraphPad Software, San Diego CA, USA). Two-group comparisons were performed using the Student’s t test. Data represent mean values ± sd. Statistically significant values are denoted as follows: *P < 0.05; **P < 0.01; and ***P < 0.001.
RESULTS
Generation and phenotyping of human STAT3+ and STAT3C+ CD4+ T cells
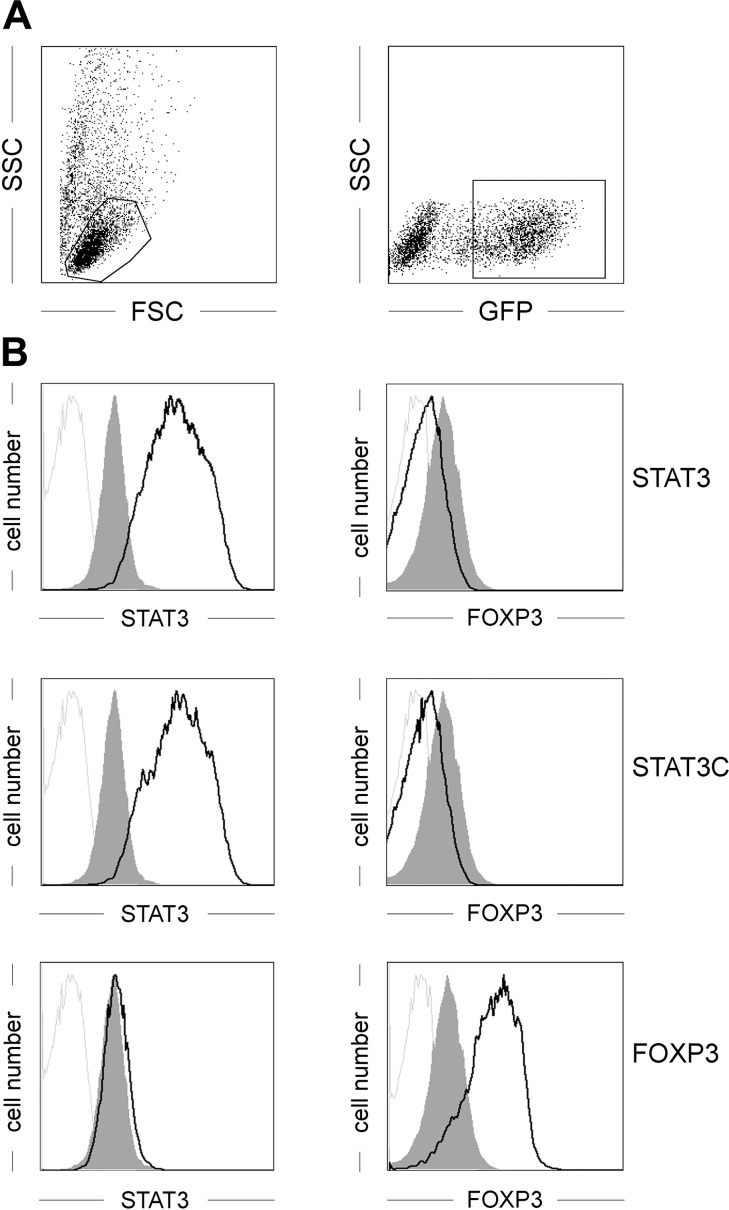
Human STAT3 was cloned from a human dendritic cell cDNA library (39) and modified by PCR mutagenesis to generate the STAT3 (p.A661C, p.N663C) mutant (termed STAT3C), which has been described to act as a constitutively active form of STAT3 (40). Both constructs were cloned into the retroviral pMMP-IRES-GFP vector (27), which enables efficient transduction and monitoring of transgene expression (Fig. 1A). Following retroviral transduction, STAT3-transduced and STAT3C-transduced, but not FOXP3-transduced, CD4+ T cells showed a clear-cut overexpression of STAT3 compared with endogenous expression levels (Fig. 1B). Concomitant analysis for intracellular expression of the transcription factor FOXP3, which is the key mediator of nTreg development and tolerogenic function (41, 42), showed no up-regulation in STAT3-transduced or STAT3C-transduced T cells (Fig. 1B), but was found clearly expressed in FOXP3-transduced T cells.
Figure 1.
Intracellular expression pattern in CD4+ T cells of transcription factors. Primary human CD4+ T cells were retrovirally transduced either with human STAT3, STAT3C, FOXP3, or an empty control vector and analyzed for expression of the indicated transcription factors. A) Gating strategy for positively transduced T cells. B) Intracellular expression levels of the indicated transcription factors in control vector-transduced cells (gray histograms) or transcription factor-transduced cells (black lines) using directly labeled mAbs. Gray lines depict staining with isotype-matched control mAb. One representative experiment of 8 is depicted.
STAT3C+ T cells are hyporesponsive to TCR-mediated stimulation
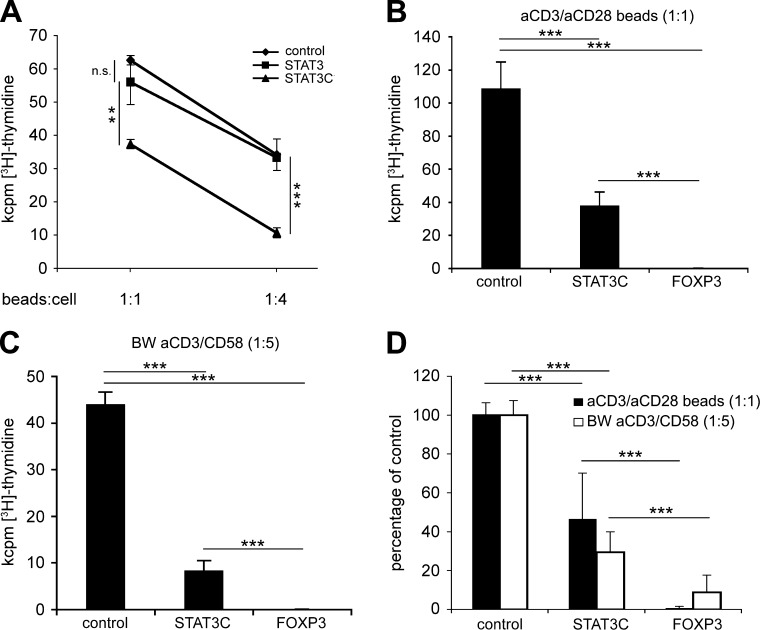
To assess the functional consequences of STAT3 or STAT3C ectopic expression, flow cytometrically sorted STAT3-transduced or STAT3C-transduced T cells were stimulated with anti-CD3/anti-CD28 coated microbeads at different bead to cell ratios (1:1 or 1:4). Under these conditions, STAT3-transduced T cells showed similar proliferative capacity as control-transduced T cells. In contrast, overexpression of constitutively active STAT3C significantly reduced the proliferative response of transgenic T cells following both strong and weak anti-CD3/anti-CD28 mediated stimulation (P < 0.01 and P < 0.001, respectively; Fig. 2A). Hyporesponsiveness of STAT3C+ T cells was also compared with FOXP3+ T cells, which are highly anergic even in response to strong stimulatory signals (25–27). Accordingly, FOXP3+ T cells showed >90% reduction of proliferation compared with control-transduced T cells after stimulation with anti-CD3/anti-CD28 coated microbeads (bead to cell ratio 1:1; Fig. 2B, D). Similar results were obtained on stimulation of transduced T cells with mouse thymoma BW5147 cells expressing a membrane-bound OKT3scFv and CD58 as costimulatory molecule (BW 3/2 CD58). BW 3/2 CD58 cells served as aAPCs to deliver a qualitatively different costimulatory signal than anti-CD28 (Fig. 2C, D). Overall, hyporesponsiveness by STAT3C+ T cells was statistically highly significant compared with control-transduced T cells; however, it was not as pronounced as with FOXP3-transduced T cells, leading to an average reduction of proliferation by 53.4 ± 23.5% (n = 6, P < 0.001) in response to anti-CD3/anti-CD28 stimulation (Fig. 2B, D) and 70.5 ± 10.4% (n = 5, P < 0.001) in response to anti-CD3/CD58 stimulation by aAPCs, respectively (Fig. 2C, D). STAT3C-mediated hyporesponsiveness was independent of STAT3C-induced IL-10 (see below), because neutralization of IL-10 by blocking antibodies did not alter proliferation of STAT3C-transgenic T cells after anti-CD3/anti-CD28 stimulation (n = 3, P < 0.001; Supplemental Fig. S1).
Figure 2.
STAT3C-transduced CD4+ T cells are hyporesponsive. A) Proliferation of STAT3+, STAT3C+, or control-transduced T cells in response to anti-CD3/anti-CD28 coated microbeads at the indicated bead to cell ratio. B, C) Proliferation of control vector–transduced, STAT3C+, or FOXP3+ T cells in response to anti-CD3/anti-CD28 coated microbeads (B) or aAPC expressing a membrane-bound OKT3scFv plus CD58 at the indicated stimulator to T cell ratios (C). Mean values ± sd from triplicate cultures from 1 representative experiment are depicted. D) Percent values normalized for control-transduced T cells from 6 experiments. n.s., not significant; **P < 0.01; ***P < 0.001.
STAT3C+ T cells display a Tr1-like cytokine secretion pattern
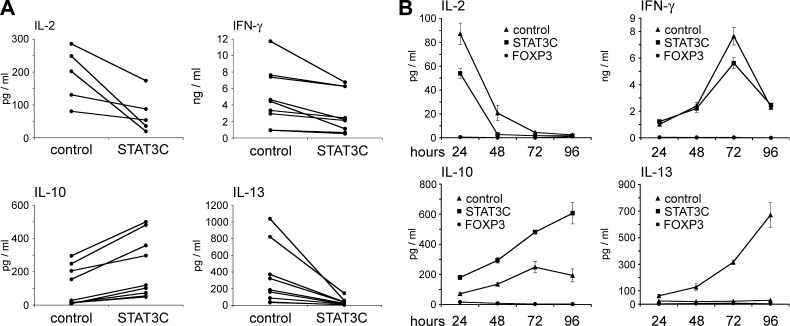
In a next step, we assessed whether the hyporesponsivenss observed in proliferation assays was also reflected at the level of cytokine secretion. Indeed, IL-2 secretion after 24 h was reduced by 57 ± 4% (n = 5, P < 0.001) in STAT3C+ T cells (Fig. 3A). This effect was confirmed at the level of IL-2 transcription in Jurkat T cells expressing a luciferase reporter gene under control of the IL-2 promoter (data not shown). Secretion of IFN-γ (Fig. 3A) and TNF-α (data not shown) by STAT3C+ T cells was reduced by 35 ± 5% and 64 ± 8% (n = 8, P < 0.001), respectively, compared with control-transduced T cells, and IL-13 production was almost abrogated (reduction by 89 ± 3% compared with control-transduced T cells; n = 8, P < 0.001) after 72 h (Fig. 3A). Under these conditions, IL-10 production was strongly and highly significantly induced (4.1 ± 0.5-fold; n = 8, P < 0.001; Fig. 3A). No IL-4 and IL-17 secretion was observed (data not shown) in these experiments. Significantly, these changes in the cytokine secretion pattern of STAT3C+ T cells were detected over the whole course of a T cell response (Fig. 3B). These effects were strictly dependent on the constitutively activated form of STAT3, because wild-type STAT3-transduced T cells displayed a cytokine secretion profile similar to control-transduced T cells (data not shown).
Figure 3.
Cytokine secretion pattern of CD4+ T cells transduced with STAT3C and FOXP3. A) Control vector-transduced (left) and STAT3C+ (right) human CD4+ T cells from healthy donors (n ≥ 5) were stimulated with anti-CD3/anti-CD28 coated microbeads at a 1:1 ratio. Cytokine levels were assessed after 24 (IL-2) or 72 h (IFN-γ, IL-10, and IL-13). Each dataset is representative for mean values from triplicate cultures from individual healthy donors. B) Time course of cytokine secretion of STAT3C+ (squares), FOXP3+ (circles), and control-transduced (triangles) T cells from one representative donor. Mean values ± sd from triplicate values are shown. ***P < 0.001.
STAT3C+ T cells show regulatory capacity
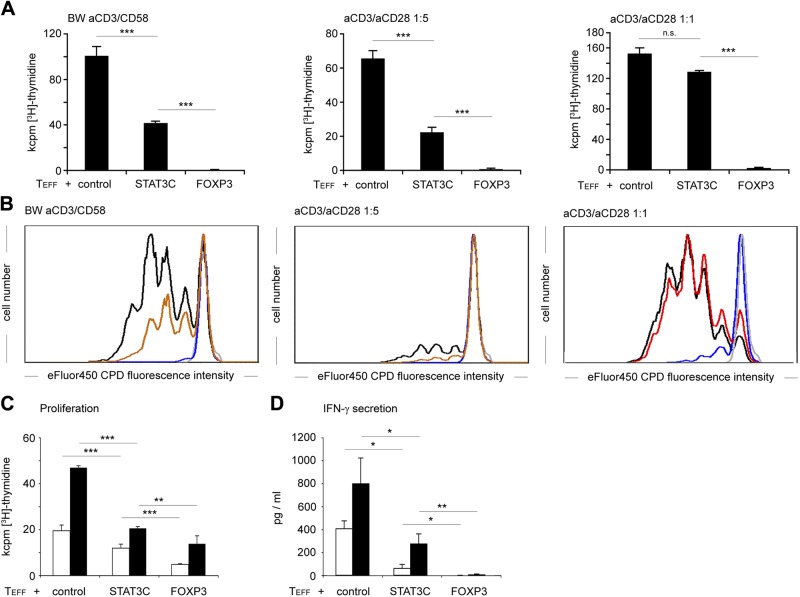
Given that the cytokine and granzyme expression profile of STAT3C+ T cells bears resemblance to Tr1 cells, and stimulation of T cells via the CD58/CD2 axis enforces a Tr1 phenotype (35, 43), we hypothesized that STAT3C+ T cells might also exert regulatory function in this context. Indeed, STAT3C+ T cells activated via the CD58/CD2 axis by BW 3/2 CD58 aAPCs significantly suppressed TEFF proliferation (reduction by 68.7 ± 10.6%, n = 4, P < 0.001; Fig. 4A, B). Consistently, similar suppression was evident following anti-CD3/anti-CD28 mediated stimulation (bead:cell = 1:5), which still resulted in robust proliferation, i.e., 40–70 kcpm. Under these conditions, STAT3C+ T cells significantly suppressed the proliferation of effector T cells by 65.9 ± 2.6% (n = 4, P < 0.001), whereas FOXP3+ T cells completely abrogated effector T cell proliferation (99.8 ± 0.1%, n = 4, P < 0,001; Fig. 4A, B). However, this suppression was overcome in response to a strong anti-CD3/anti-CD28 mediated signal (bead:cell = 1:1) where STAT3C+ T cells were almost unable to suppress the proliferation of cocultured CD4+ effector T cells (Fig. 4A, B), whereas FOXP3+ T cells potently suppressed effector T cell proliferation (98.6 ± 0.5%, n = 4, P < 0.001; Fig. 4A, B). To assess whether STAT3C+ T cells are also able to inhibit alloreactive T cell responses, we performed allogeneic mixed leukocyte reactions using immature and LPS-matured monocyte-derived dendritic cells from HLA-mismatched donors as stimulator cells. We found that coculture with STAT3C+ T cells significantly reduced TEFF proliferation compared with cocultures with control-transduced T cells. Overall, proliferation in cocultures with STAT3C+ T cells was reduced by 34.2 ± 13.1% (n = 4; P < 0.001) in response to stimulation with immature dendritic cells and by 38.8 ± 16.8% (n = 4; P < 0.001) in response to LPS-matured dendritic cells (Fig. 4C). Under these stimulatory conditions, FOXP3-transgenic T cells showed a stronger suppressive capacity with a reduction of proliferation by 74.5 ± 10.6% (n = 4; P < 0.001) for immature DC and 70.7 ± 9.2% (n = 4; P < 0.001) for LPS-matured DC (Fig. 4C). This suppressive capacity of STAT3C+ T cells was also mirrored by significantly decreased IFN-γ levels on coculture after stimulation with both immature mdDCs (52.9 ± 33.4%; n = 4; P < 0.05) and LPS-matured mdDCs (53.1 ± 28.3%; n = 4; P < 0.05; Fig. 4D).
Figure 4.
STAT3C-transduced CD4+ T cells have regulatory capacity. A) Purified human CD4+ T cells were cocultured with either STAT3C+, FOXP3+ or control vector-transduced T cells at a 1:1 ratio and stimulated with the indicated amounts of aAPC expressing a membrane-bound OKT3scFv plus CD58 or anti-CD3/anti-CD28 coated microbeads. A) Thymidine incorporation (72 + 16 h [3H]-thymidine pulse). Mean values + sd from triplicate cultures from 1 representative experiment of 4 are depicted. B) The graphs represent the proliferation of eFluor670 CPD-labeled CD4+ T cells cocultured with STAT3C+ (red), FOXP3+ (blue), or control vector-transduced (black) T cells and stimulated as indicated. One representative experiment from duplicate cultures of 4 individual donors is depicted. C, D) Purified human CD4+ T cells were cocultured with the indicated transgenic T cells (control vector-transduced, STAT3C+, or FOXP3+) and stimulated with HLA-mismatched immature (white) or LPS-matured (black) monocyte-derived dendritic cells at a 10:1 ratio. C) Proliferation after 120 + 16 h [3H]-thymidine pulse. D) IFN-γ secretion after 120-h coculture. Mean values ± sd from triplicate values from 1 representative donor of 4 are shown. n.s. not significant; *P < 0.05; **P < 0.01; ***P < 0.001.
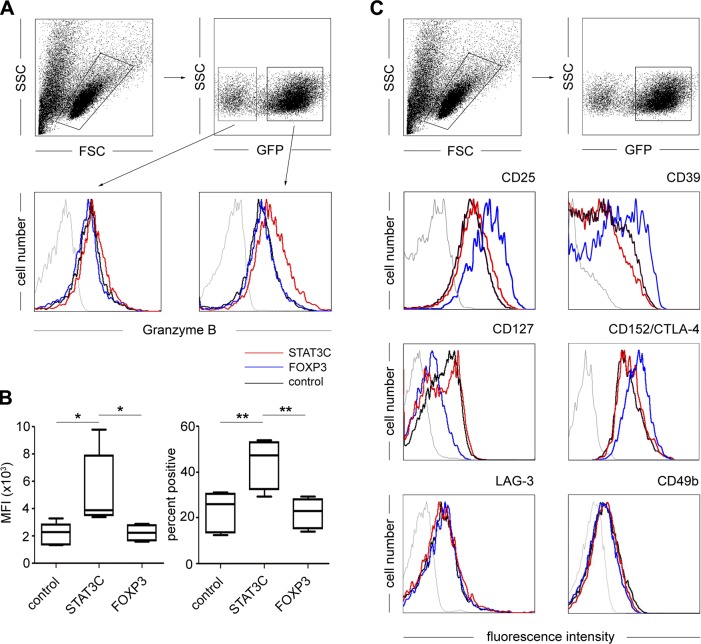
STAT3C+ T cells display increased expression of granzyme B
Because ectopic expression of STAT3C dramatically altered the functional response of CD4+ T cells, we also assessed its influence on cellular phenotype. Intracellular expression of granzyme B, which has been implied as an effector molecule of Tr1 cells (44, 45), was significantly up-regulated in STAT3C-transgenic T cells compared with both control-transduced and FOXP3-transgenic T cells (mean fluorescence intensity: control, 2157 ± 727; STAT3C, 5357 ± 2422; FOXP3, 2231 ± 530; n = 5, P < 0.05; percentage of highly positive cells: control, 22.8 ± 7.9%; STAT3C, 43.9 ± 9.7%; FOXP3, 22.1 ± 5.9%; n = 5, P < 0.01; Fig. 5A, right, and B). This up-regulation was highly specific, because GFP-negative, nontransduced T cells within the same STAT3C bulk transduction experiments did not show elevated granzyme B expression levels (Fig. 5A, left). Under no conditions could the expression of granzyme A be detected (data not shown). Apart from these phenotypic changes, STAT3C+ T cells expressed a TEFF phenotype similar to control-transduced T cells, which was marked by low expression of CD25, CD39, and CTLA-4 and high expression levels of CD127. This was in contrast to FOXP3-transduced T cells, which displayed the nTreg-specific CD25highCD39highCTLA-4highCD127low phenotype (Fig. 5C) as specified recently (27). Similarly, assessment of the Tr1 markers LAG-3 and CD49b, as well as Tr1-associated molecules CD226, A2A-R, 4-1BBL, and CD86 (22), showed no difference in the expression among the 3 groups (Fig. 5C and Supplemental Fig. S2).
Figure 5.
STAT3C-transduced T cells express an effector phenotype. Primary human CD4+ T cells were retrovirally transduced either with human STAT3C (red), FOXP3 (blue), or an empty control vector (black). Transduced (GFP+) T cells were analyzed for (A) intracellular expression of granzyme B. Gating strategies are shown in the upper panels. Nontransduced (left) and transduced T cells (right) are depicted. B) Intracellular expression of granzyme B. Left panel shows box plots of mean fluorescence intensity; right panel shows box plots of percent positive cells of CD4+ T cells transduced with the indicated transgenes (resting phase) and stained for intracellular granzyme B expression. Data are representative of 5 healthy donors. *P < 0.05; **P < 0.01. C) Staining for surface expression of the regulatory T cell markers CD25, CD39, CD127, LAG3, and CD49b and intracellular expression of CD152/CTLA-4. One representative experiment of 5 is shown. Thin gray lines represent staining with isotype-matched control mAb.
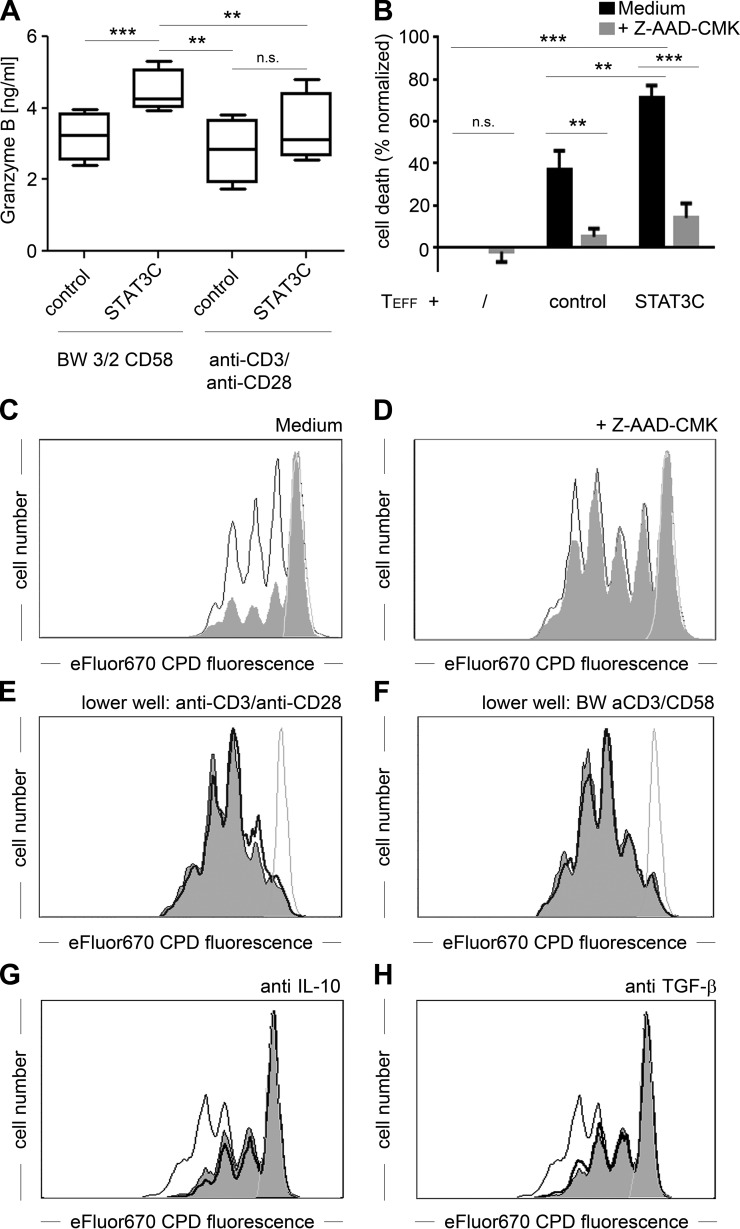
Suppression by STAT3C+ T cells is a granzyme B-dependent process
In accordance with the phenotypic observations, STAT3C+ T cells also showed significantly increased secretion of granzyme B following costimulation via the CD58/CD2 axis with BW 3/2 CD58 aAPCs compared with control-transduced cells (Fig. 6A; 4455 ± 603 vs. 3220 ± 664 pg/ml; n = 4; P < 0.001). A similar tendency was found in response to anti-CD3/anti-CD28 mediated stimulation, which did, however, not reach statistical significance (3449 ± 1073 vs. 2895 ± 1020 pg/ml; n = 4; P = 0.17). To test whether this increase in granzyme B secretion was also involved in the mechanism of suppression by STAT3C+ T cells, we performed coculture experiments in the presence or absence of the granzyme B inhibitor Z-AAD-CMK (final concentration, 30 µM). Viability of TEFF was determined by combined annexin V and propidium iodide staining 72 h after activation with BW 3/2 CD58 cells. These experiments clearly demonstrated that coculture with STAT3C+ T cells led to massive induction of cell death in TEFF (70.5 ± 5.7%, P < 0.001, n = 6), which could be almost completely overcome by the addition of the specific granzyme B inhibitor Z-AAD-CMK (14.5 ± 6.7%; Fig. 6B and Supplemental Fig. S3) (36). This was in contrast to coculture of TEFF with control vector-transduced T cells, where induction of cell death was significantly lower (36.9 ± 8.9%; P < 0.01, n = 6).
Figure 6.
The regulatory capacity of STAT3C+ T cells is a granzyme B-dependent process. A) Release of granzyme B from control vector transduced or STAT3C-transduced T cells 6 h after stimulation with BW 3/2 CD58 aAPC (left) or anti-CD3/anti-CD28 coated microbeads (right). Cumulative data from triplicate values from 4 individual donors are depicted. B) Relative cell death of TEFF induced by control vector- or STAT3C-transduced T cells in the presence or absence of 30 µM Z-AAD-CMK as determined by combined annexin V/propidium iodide staining. C, D) CPD-labeled CD4+ T cells were cocultured with control vector-transduced T cells (thin black line) or STAT3C+ T cells (gray filled area) in the absence (C; medium) or presence (D) of 30 µM Z-AAD-CMK. Nonstimulated cells are depicted as a fine gray line. One representative experiment from 4 individual donors is depicted. E, F) Transwell assay: CPD-labeled CD4+ T cells were stimulated with BW 3/2 CD58 aAPC in the upper well of a transwell plate (3-µm pore size). Control-transduced (bold black line) or STAT3C+ T cells (gray filled area) were stimulated in the lower well with either (E) anti-CD3/anti-CD28 coated microbeads or (F) BW 3/2 CD58 aAPC. Nonstimulated cells are depicted as a fine gray line. G, H) CPD-labeled CD4+ T cells were cocultured with control-transduced T cells (fine black line) or STAT3C+ T cells in the presence of 20 µg/ml control antibody (gray filled area) or blocking antibody (bold black line) against (G) IL-10 or (H) TGF-β. One representative experiment of 3 is depicted.
Additionally, Z-AAD-CMK completely abrogated the suppression of TEFF proliferation by STAT3C+ T cells (division index, 2.10 ± 0.07 for cocultures with STAT3C+ T cells vs. 2.19 ± 0.13 for control-transduced T cells; n = 4, P = 0.09), which in the absence of the inhibitor amounted to 40.0 ± 4.7% (division index, 1.50 ± 0.07 vs. 0.90 ± 0.07; n = 4, P < 0.001; Fig. 6B). Remarkably, addition of Z-AAD-CMK also led to an increase of TEFF proliferation by 29.0 ± 3.3% in cocultures with control-transduced T cells. This is compatible with the constitutively up-regulated granzyme B levels observed in these cells. The finding that suppression by STAT3C+ T cells is mediated by granzyme B is in line with transwell experiments showing that their suppressive capacity is contact dependent (Fig. 6C, D), which is a classic feature of granzyme B-mediated cytotoxicity (46). We also assessed the contribution of IL-10 and TGF-β to the suppressive capacity of STAT3C+ T cells, because these cytokines have been reported to be key mediators of Tr1 cell regulatory function (14, 45, 47–49). For that purpose, cocultures of CD4+ TEFF with STAT3C-transduced or control-transduced T cells were stimulated with BW 3/2 CD58 aAPCs and supplemented with either a nonbinding control mAb or blocking antibodies to IL-10 or TGF-β. Neutralization of neither IL-10 (Fig. 6E) nor TGF-β (Fig. 6F) led to the abrogation of the suppressive capacity of STAT3C+ T cells, indicating that these cytokines do not constitute the main T cell regulatory mechanisms of STAT3C+ T cells.
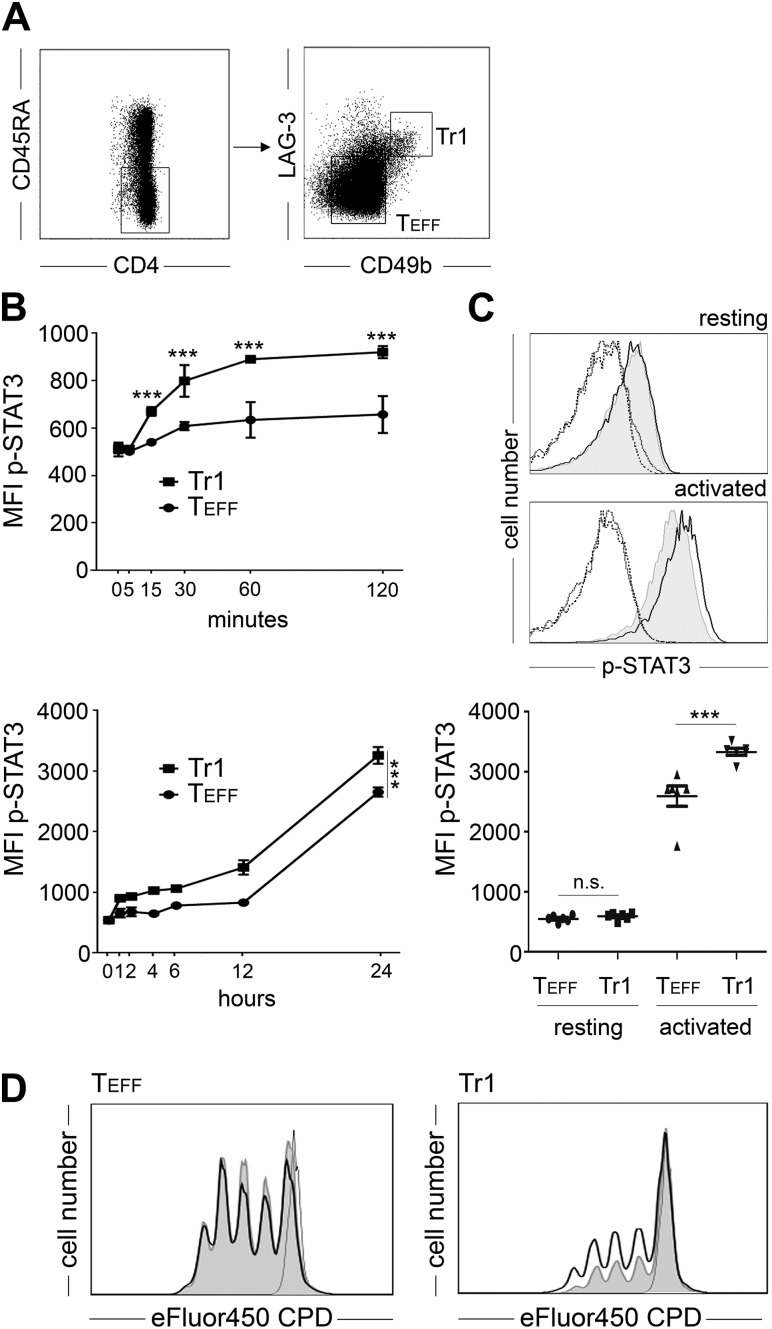
Tr1 cells from peripheral blood show increased STAT3 phosphorylation on activation
Overexpression of STAT3C confers combined functional properties, such as hyporesponsiveness and secretion of IL-10 and granzyme B, which are hallmarks of Tr1 cells. Accordingly, we assessed whether activation, i.e., phosphorylation of STAT3, was also apparent in bona fide Tr1 cells isolated from PB. For that purpose, we relied on the recently described CD4+CD45RA−CD49b+LAG-3+ phenotype (22) to purify Tr1 cells from PB of healthy individuals (Fig. 7A). Using phosphoprotein-specific flow cytometry, we compared STAT3 phosphorylation in FACS-sorted Tr1 cells with CD4+CD45RA−CD49b−LAG-3− TEFF of unrelated healthy individuals. Directly after isolation from PB no difference in STAT3 phosphorylation was observed between Tr1 and TEFF (mean fluorescence intensity, 591 ± 53 vs. 550 ± 51; n = 6, P = 0.32; Fig 7B, C). After activation, a marked up-regulation of STAT3 phosphorylation was observed. In fact, as early as 15 min after activation, Tr1 cells showed significantly higher levels of STAT3 phosphorylation than TEFF (Fig. 7B), which was consistently found during the course of early cellular activation. Of note, the initial increase in STAT3 phosphorylation underwent a further boost between 12 and 24 h, which is consistent with a bimodal course of STAT3 activation. After 24 h, a pronounced difference in STAT3 phosphorylation was evident between Tr1 and TEFF specimens of all donors (mean fluorescence intensity, 3330 ± 193 vs. 2591 ± 417; n = 6; P < 0.001; Fig. 7C). To confirm the functional relevance of this observation, we stimulated FACS-sorted Tr1 and TEFF cells in the presence or absence of STATTIC, which is a small-molecule inhibitor of STAT3 activation and dimerization (37). Addition of STATTIC partially reversed hyporesponsiveness of Tr1 cells (division index, 0.46 ± 0.11 vs. 0.89 ± 0.04; n = 3, P < 0.01; Fig. 7D). As expected, CD49b+LAG-3+ Tr1 cells showed significantly decreased proliferation in comparison to CD49b−LAG-3− TEFF (division index, 0.46 ± 0.11 vs. 2.27 ± 0.25; n = 3, P < 0.001). Although, CD49b−LAG-3− TEFF also presented with an activation-dependent phosphorylation of STAT3, their proliferation was unaffected by the blockade of STAT3 activation (division index, 2.27 ± 0.25 vs. 2.30 ± 0.18; n = 3, not significant).
Figure 7.
Activation-induced hyperphosphorylation and functional potential of STAT3 in Tr1 cells. A) Magnetic-activated cell-sorted CD4+ T cells were stained for surface expression of CD4, CD45RA, and the Tr1 markers CD49b, and LAG-3 and CD4+CD45RA− cells were further FACS sorted into TEFF and Tr1 cells according to the indicated gates. B) FACS-sorted Tr1 cells (black squares) or TEFF (black circles) was analyzed for STAT3 phosphorylation, indicated as mean fluorescence intensity (MFI p-STAT3, mean ± sd, n = 3) after anti-CD3/anti-CD28 mediated stimulation at the indicated early (upper graph) and late (lower graph) time points. C) FACS-sorted Tr1 cells (bold black line) or TEFF (gray histogram) was analyzed for phosphorylation of STAT3 directly after sorting (resting, upper panel) or 24 h after anti-CD3/anti-CD28 mediated stimulation (activated, lower panel). Isotype matched antibodies (dotted line for Tr1, dashed line for TEFF) served as controls. Data from 1 representative donor are depicted. Cumulative data for STAT3 phosphorylation from 6 individual donors are depicted in the lower diagram. D) eFluor450 CPD-labeled CD4+ T cells were sorted according to A and then stimulated with anti-CD3/anti-CD28 coated microbeads for 96 h in the absence (gray histogram) or presence (bold black line) of 100 µM STATTIC. Unstimulated cells (fine black line) served as controls. Left, LAG3-CD49b− TEFF; right, LAG3+CD49b+ Tr1 cells. Data from 1 representative experiment are depicted (n = 4). n.s., not significant; ***P < 0.001.
Discussion
To analyze the role of STAT3 in CD4+ T cells, we resorted to ectopic expression of a constitutively active form of STAT3, i.e., STAT3C. A similar approach has previously been used to address STAT3 function in monocytes and dendritic cells (1–3, 5). Activation of STAT3 in CD4+ T cells dramatically altered their functional response to qualitatively and quantitatively different stimuli and induced a specific tolerogenic program. This included hyporesponsiveness to T cell receptor–mediated stimulation, the adoption of a Tr1-like cytokine and granzyme B secretion profile, and the acquisition of suppressive capacity. Furthermore, we demonstrate that suppression by STAT3C+ T cells is granzyme B and contact dependent but IL-10 and TGF-β independent. Although we thus observed a dramatic change in the functional features of STAT3C transgenic T cells, this was not reflected in their surface phenotype. In fact, phenotypic traits of regulatory T cells such as expression patterns of the nTreg markers CD25, CD39, CD127, CTLA-4, and FOXP3 and the Tr1 markers LAG-3, CD49b, CD226, A2A-R, 4-1BBL, and CD86 were unaffected, Finally, we provide the first evidence that activation-induced hyperphosphorylation of STAT3 is a typical feature of PB Tr1 cells, which are involved in the maintenance of their hypoproliferative state.
The tolerogenic effects of STAT3C were compared with those induced by ectopic expression of FOXP3, which mimics the phenotype and function of CD4+CD25+ nTregs to a great extent (25–27). These comparative analyses indeed revealed that the STAT3C-mediated tolerogenic program is distinct from the FOXP3-mediated, nTreg-like tolerogenic program. In natural Tregs or FOXP3-transgenic Tregs (25–27), FOXP3 completely abrogates proliferation and cytokine production including IL-10. In contrast, STAT3C+ T cells retain some proliferative capacity, however, at a significantly reduced level, compared with control-transgenic cells. Moreover, they display a distinct cytokine profile, which includes a >4-fold up-regulation of IL-10 paralleled by the down-regulation of IFN-γ, TNF-α, IL-2, and IL-13. This cytokine profile strongly resembles the IL-10highIFN-γ+IL-2lowIL4−IL13− phenotype, which has been described as a hallmark of Tr1 cells (14, 21). Our results comply with recent data that the activation of STAT3 induces the modulation of cytokine production in CD4+ T cells, in particular the induction of IL-10 production (19, 20) and the down-regulation of IL-2 production (50). Furthermore, this cytokine secretion profile is combined with significantly increased intracellular expression and secretion levels of granzyme B, a feature that has also been attributed to in vitro-induced Tr1 cells (44, 45). In this respect, our observations derived from ectopic expression studies are concordant with recent reports describing STAT3 as a crucial signaling molecule in the regulation of IL-10 expression (51) and the induction of Tr1 cells in response to IL-27 stimulation (3, 16–19).
Mechanistically, the effects observed in STAT3C+ T cells cannot be reduced to the mere induction of IL-10 secretion, because neutralization of IL-10 was not able to abrogate their hypoproliferative state. Thus, it seems that the observed functional features of STAT3C-transgenic T cells are directly governed by the transcription factor activity of STAT3, and IL-10 induction represents 1 feature of the STAT3 transcription signature in T cells.
However, in the present study, STAT3C transgenic T cells did not produce IL-17, indicating that the sole activation of STAT3 is not per se a Th17-polarizing signal in human T cells. This is in marked contrast to a multitude of reports studying the function of murine T cells, which have linked the IL-6-STAT3 axis to Th17 induction and subsequently to Th17-mediated pathologies such as inflammatory bowel disease (7–9). However, in some of these studies, IFN-γ was described to potently inhibit Th17 cytokine production in CD4+ T cells (8). Along those lines, it seems conceivable that the residual production of IFN-γ by STAT3C+ T cells as observed in this study (Fig. 3A, B) might be sufficient to suppress IL-17 production in a negative feedback loop. The makeup of the STAT3C construct (40), which most likely does not allow association with other STAT members, might also influence the further polarization of STAT3C-transduced cells. In this respect, the results observed in this study may certainly reflect the effects of STAT3 homodimers; they might, however, miss concomitant activation steps because of the engagement of other members of the STAT family and subsequent STAT3 heterodimerization effects.
Given that overexpression of STAT3C in CD4+ T cells reconstituted several functional features of Tr1 cells, we hypothesized that these cells might also feature regulatory capacity. Tr1 cells use multiple mechanisms to exert their regulatory capacity, including the secretion of inhibitory cytokines such as IL-10 and TGF-β, as well as killing of target cells via release of perforin and granzyme B (44, 45). These latter mechanisms have been further defined for the killing of myeloid APCs and are dependent on interaction of Tr1 cell-expressed CD2, CD226, and LFA-1 with their respective ligands on APCs (44). In this study, clear-cut suppressive effects of STAT3C-transduced T cells could only be observed when the costimulatory signal was provided via the CD58/CD2 axis or at suboptimal levels of CD28 costimulation. This is of special interest, because CD58-CD2 interactions, although among of the most potent T cell costimulatory pathways (30, 52, 53), seem to serve an important role in Tr1-mediated tolerance. First reports by Wakkach et al. indicated that costimulation via CD2 but not via CD28 induced a Tr1-like cytokine profile marked by secretion of high levels of IL-10 and decreased levels of IL-2 and IFN-γ (35), a feature that could be reproduced by us previously by applying a virus-like particle-based stimulatory platform (43). Along those lines, direct suppression of T cell activation by IL-10 is mainly achieved when T cells are costimulated via CD2 but not via CD28 (54). Similarly, suppressive effects of Tr1 cells via granzyme B and perforin are dependent on CD2-CD58 interaction (44). In our system, both control-transduced and STAT3C-transduced T cells express and secrete robust amounts of granzyme B. This finding is most likely explained by the preactivation needed for transduction, which is in line with reports that preactivation of CD4+ T cells leads to strong up-regulation of granzyme B (45). However, transduction with STAT3C further enhanced granzyme B expression above the activation-induced levels, indicating that STAT3 might be involved in the up-regulation of granzyme B expression in CD4+ T cells. Elevated expression of granzyme B in STAT3C+ T cells was also reflected by increased granzyme B secretion levels following activation, an effect that was more strongly favored by CD58/CD2 costimulation than by CD28 costimulation. In line with the observations described by Magnani et al. (44), this may indicate that costimulation via the CD58/CD2 axis is a prerequisite for the secretion of granzyme B from tolerogenic CD4+ T cells. Indeed, the mechanism of action constituted by STAT3C+ T cells was confirmed to be caused by granzyme B-dependent induction of TEFF cell death. Along those lines, we observed increased levels of cell death on coculture of TEFF with STAT3C+ T cells, which could be overcome by the addition of the specific granzyme B inhibitor Z-AAD-CMK (36), a widely used peptide chloromethyl ketone with selective blocking activity for granzyme B but not granzyme A or K (36, 55–57). Consequently, addition of Z-AAD-CMK also significantly improved TEFF proliferation when cocultured with STAT3C+ T cells. Taken together, these findings provide a plausible explanation that suppression is granzyme B dependent and therefore mainly observed following costimulation of TEFF via the CD58/CD2 axis.
In a final step, we aimed to assess whether the overexpression system applied in this study is of physiologic relevance, i.e., whether activation of STAT3 might be part of the transcriptional signature of human PB Tr1 cells. Although a possible link between IL-10 signaling, STAT3 phosphorylation, and the induction of Tr1 cells has been suggested by several studies, no formal proof for this hypothesis has been provided as of today. Recently, Gagliani et al. identified the combined expression of CD49b and LAG3 as unique markers of CD4+CD45RA− PB Tr1 cells (22). On the basis of these observations, we compared STAT3 phosphorylation in Tr1 cells with effector T cells from PB. Although basal levels of STAT3 phosphorylation were similar in both populations, Tr1 cells showed more pronounced up-regulation of STAT3 phosphorylation following activation. Detailed time course analyses revealed a quick first wave of neo-phosphorylation of STAT3 in Tr1 cells, which was almost undetectable in TEFF cells. Between 12 and 24 h after activation, a second wave of STAT3 phosphorylation became evident, which was also regularly detectable in TEFF, although at a significantly lower magnitude. It is tempting to speculate that a putative feedback mechanism driven by IL-10 secretion could account for this effect. Consistently, pharmacologic blockade of STAT3 activation with the selective inhibitor STATTIC (37) partially reversed hypoproliferation of Tr1 cells, indicating that STAT3 hyperphosphorylation might be involved in the functional features of Tr1 cells. STATTIC has a high potency at 37°C and does not significantly inhibit other dimeric transcription factors such as c-myc/Max, Jun/Jun, and STAT1 or other important signaling molecules such as Src, JAK1, JAK2, Akt, JNK, and ERK1/2 (37). To our knowledge, these data provide the first evidence for an important role of STAT3 in Tr1 cells isolated from PB, because most studies have thus far relied on in vitro-induced Tr1 cells.
In concert with the data obtained in STAT3C overexpression experiments, it seems conceivable that Tr1 cells maintain increased STAT3 phosphorylation following activation to stabilize hyporesponsiveness and secretion of IL-10 and granzyme B, as well as down-regulation of other effector cytokines. Although many functional and phenotypic traits of Tr1 cells can be recapitulated by ectopic expression of STAT3C, surface expression of typical markers designating Tr1 cells, such as CD49b, LAG-3, CD226, A2A-R, 4-1BBL, and CD86, remain unaffected. Several reasons might account for this discrepancy.
One possiblility is that the manifestation of phenotypic traits of PB Tr1 cells are independent of STAT3 activation or require additional signals. Our findings that resting Tr1 cells do not show increased levels of phosphorylation of STAT3 support this hypothesis. Taken together with our results that blockade of STAT3 was not fully sufficient to revert hypoproliferation by Tr1 cells, it seems highly likely that the Tr1 transcriptional signature consists of >1 transcription factor necessary for the full manifestation of their phenotype and function. Along those lines, it has been shown that p38 signaling is a crucial cofactor for the induction of LAG-3 expression (58). Furthermore, transcription factors, such as c-Maf (59) and the aryl hydrocarbon receptor (60), might represent additional elements. It will be interesting to assess individual contributions of these factors in future studies by applying multicistronic overexpression approaches (27, 61). The findings here also might have potential practical implications, providing a general strategy of how to engineer Tr1-like cells for therapeutic application by genetic manipulation, similar to the recently described IL-10 overexpression approaches from Andolfi et al. (62).
In conclusion, it seems conceivable that sustained phosphorylation of STAT3 in human T cells, potentially maintained by elevated levels of IL-10 and IL-27, induces a tolerogenic program. This includes the adoption of hyporesponsiveness, an anti-inflammatory cytokine secretion pattern, as well as increased expression and secretion of granzyme B, which executes direct suppressive function on bystander effector T cells in a contact-dependent manner. This mechanism is dependent on costimulation via the CD58-CD2 axis. Our observations are further substantiated by the finding that activated PB Tr1 cells reveal increased STAT3 phosphorylation that helps to maintain their hypoproliferative state, indicating that the STAT3-mediated tolerogenic program among possible other cell types described herein might be involved in the tolerogenic functions of bona fide PB Tr1 cells.
Supplementary Material
Acknowledgments
The authors thank Ms. Doris Trapin (Institute of Immunology, Vienna, Austria) for expert technical assistance and Dr. Richard Mulligan (Children’s Hospital, Boston, MA, USA) for sharing the pMD.gagpol expression construct with us. This work was supported by Austrian Science Fund grant SFB-F4609, the Christian Doppler Society, and Biomay AG.
Glossary
- aAPC
artificial antigen-presenting cell
- CD
cluster of differentiation
- CTLA
cytotoxic T-lymphocyte antigen
- FACS
fluorescence-activated cell sorting
- FOXP3
forkhead box protein 3
- GFP
green fluorescent protein
- IRES
internal ribosomal entry site
- LAG-3
lymphocyte activation gene-3
- mdDC
monocyte-derived dendritic cells
- nTreg
naturally occurring regulatory T cells
- PB
peripheral blood
- scFv
single-chain variable fragment
- STAT
signal transducer and activator of transcription
- TEFF
effector T cell
- Th
T-helper cell
- Treg
regulatory T cells
- Tr1
type 1 regulatory T cells
- Z-AAD-CMK
benzyloxycarbonyl-ala-ala-asp(OMe) chloromethyl ketone
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Cheng F., Wang H. W., Cuenca A., Huang M., Ghansah T., Brayer J., Kerr W. G., Takeda K., Akira S., Schoenberger S. P., Yu H., Jove R., Sotomayor E. M. (2003) A critical role for Stat3 signaling in immune tolerance. Immunity 19, 425–436 [DOI] [PubMed] [Google Scholar]
- 2.Herrmann A., Kortylewski M., Kujawski M., Zhang C., Reckamp K., Armstrong B., Wang L., Kowolik C., Deng J., Figlin R., Yu H. (2010) Targeting Stat3 in the myeloid compartment drastically improves the in vivo antitumor functions of adoptively transferred T cells. Cancer Res. 70, 7455–7464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang T., Niu G., Kortylewski M., Burdelya L., Shain K., Zhang S., Bhattacharya R., Gabrilovich D., Heller R., Coppola D., Dalton W. Jove R. Pardoll D. Yu H. (2004) Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat. Med. 10, 48–54 [DOI] [PubMed] [Google Scholar]
- 4.Matta B. M., Raimondi G., Rosborough B. R., Sumpter T. L., Thomson A. W. (2012) IL-27 production and STAT3-dependent upregulation of B7-H1 mediate immune regulatory functions of liver plasmacytoid dendritic cells. J. Immunol. 188, 5227–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams L. M., Sarma U., Willets K., Smallie T., Brennan F., Foxwell B. M. (2007) Expression of constitutively active STAT3 can replicate the cytokine-suppressive activity of interleukin-10 in human primary macrophages. J. Biol. Chem. 282, 6965–6975 [DOI] [PubMed] [Google Scholar]
- 6.Kaebisch R., Mejías-Luque R., Prinz C., Gerhard M. (2014) Helicobacter pylori cytotoxin-associated gene A impairs human dendritic cell maturation and function through IL-10-mediated activation of STAT3. J. Immunol. 192, 316–323 [DOI] [PubMed] [Google Scholar]
- 7.Mathur A. N., Chang H. C., Zisoulis D. G., Stritesky G. L., Yu Q., O’Malley J. T., Kapur R., Levy D. E., Kansas G. S., Kaplan M. H. (2007) Stat3 and Stat4 direct development of IL-17-secreting Th cells. J. Immunol. 178, 4901–4907 [DOI] [PubMed] [Google Scholar]
- 8.Yang X. O., Panopoulos A. D., Nurieva R., Chang S. H., Wang D., Watowich S. S., Dong C. (2007) STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 282, 9358–9363 [DOI] [PubMed] [Google Scholar]
- 9.Zhou L., Ivanov I. I., Spolski R., Min R., Shenderov K., Egawa T., Levy D. E., Leonard W. J., Littman D. R. (2007) IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat. Immunol. 8, 967–974 [DOI] [PubMed] [Google Scholar]
- 10.Kwon M. J., Ma J., Ding Y., Wang R., Sun Z. (2012) Protein kinase C-θ promotes Th17 differentiation via upregulation of Stat3. J. Immunol. 188, 5887–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaudhry A., Rudra D., Treuting P., Samstein R. M., Liang Y., Kas A., Rudensky A. Y. (2009) CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science 326, 986–991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhry A., Samstein R. M., Treuting P., Liang Y., Pils M. C., Heinrich J. M., Jack R. S., Wunderlich F. T., Brüning J. C., Müller W., Rudensky A. Y. (2011) Interleukin-10 signaling in regulatory T cells is required for suppression of Th17 cell-mediated inflammation. Immunity 34, 566–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pallandre J. R., Brillard E., Créhange G., Radlovic A., Remy-Martin J. P., Saas P., Rohrlich P. S., Pivot X., Ling X., Tiberghien P., Borg C. (2007) Role of STAT3 in CD4+CD25+FOXP3+ regulatory lymphocyte generation: implications in graft-versus-host disease and antitumor immunity. J. Immunol. 179, 7593–7604 [DOI] [PubMed] [Google Scholar]
- 14.Groux H., O’Garra A., Bigler M., Rouleau M., Antonenko S., de Vries J. E., Roncarolo M. G. (1997) A CD4+ T-cell subset inhibits antigen-specific T-cell responses and prevents colitis. Nature 389, 737–742 [DOI] [PubMed] [Google Scholar]
- 15.Levings M. K., Gregori S., Tresoldi E., Cazzaniga S., Bonini C., Roncarolo M. G. (2005) Differentiation of Tr1 cells by immature dendritic cells requires IL-10 but not CD25+CD4+ Tr cells. Blood 105, 1162–1169 [DOI] [PubMed] [Google Scholar]
- 16.Levings M. K., Sangregorio R., Galbiati F., Squadrone S., de Waal Malefyt R., Roncarolo M. G. (2001) IFN-alpha and IL-10 induce the differentiation of human type 1 T regulatory cells. J. Immunol. 166, 5530–5539 [DOI] [PubMed] [Google Scholar]
- 17.Awasthi A., Carrier Y., Peron J. P., Bettelli E., Kamanaka M., Flavell R. A., Kuchroo V. K., Oukka M., Weiner H. L. (2007) A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat. Immunol. 8, 1380–1389 [DOI] [PubMed] [Google Scholar]
- 18.Fitzgerald D. C., Zhang G. X., El-Behi M., Fonseca-Kelly Z., Li H., Yu S., Saris C. J., Gran B., Ciric B., Rostami A. (2007) Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat. Immunol. 8, 1372–1379 [DOI] [PubMed] [Google Scholar]
- 19.Stumhofer J. S., Silver J. S., Laurence A., Porrett P. M., Harris T. H., Turka L. A., Ernst M., Saris C. J., O’Shea J. J., Hunter C. A. (2007) Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat. Immunol. 8, 1363–1371 [DOI] [PubMed] [Google Scholar]
- 20.Wang H., Meng R., Li Z., Yang B., Liu Y., Huang F., Zhang J., Chen H., Wu C. (2011) IL-27 induces the differentiation of Tr1-like cells from human naive CD4+ T cells via the phosphorylation of STAT1 and STAT3. Immunol. Lett. 136, 21–28 [DOI] [PubMed] [Google Scholar]
- 21.Gregori S., Goudy K. S., Roncarolo M. G. (2012) The cellular and molecular mechanisms of immuno-suppression by human type 1 regulatory T cells. Front. Immunol. 3, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gagliani N., Magnani C. F., Huber S., Gianolini M. E., Pala M., Licona-Limon P., Guo B., Herbert D. R., Bulfone A., Trentini F., Di Serio C., Bacchetta R., Andreani M., Brockmann L., Gregori S., Flavell R. A., Roncarolo M. G. (2013) Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19, 739–746 [DOI] [PubMed] [Google Scholar]
- 23.Ozcan E., Notarangelo L. D., Geha R. S. (2008) Primary immune deficiencies with aberrant IgE production. J. Allergy Clin. Immunol. 122, 1054–1062 [DOI] [PubMed] [Google Scholar]
- 24.Haga S., Terui K., Zhang H. Q., Enosawa S., Ogawa W., Inoue H., Okuyama T., Takeda K., Akira S., Ogino T., Irani K., Ozaki M. (2003) Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J. Clin. Invest. 112, 989–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aarts-Riemens T., Emmelot M. E., Verdonck L. F., Mutis T. (2008) Forced overexpression of either of the two common human Foxp3 isoforms can induce regulatory T cells from CD4(+)CD25(-) cells. Eur. J. Immunol. 38, 1381–1390 [DOI] [PubMed] [Google Scholar]
- 26.Allan S. E., Alstad A. N., Merindol N., Crellin N. K., Amendola M., Bacchetta R., Naldini L., Roncarolo M. G., Soudeyns H., Levings M. K. (2008) Generation of potent and stable human CD4+ T regulatory cells by activation-independent expression of FOXP3. Mol. Ther. 16, 194–202 [DOI] [PubMed] [Google Scholar]
- 27.Schmetterer K. G., Haiderer D., Leb-Reichl V. M., Neunkirchner A., Jahn-Schmid B., Kung H. J., Schuch K., Steinberger P., Bohle B., Pickl W. F. (2011) Bet v 1-specific T-cell receptor/forkhead box protein 3 transgenic T cells suppress Bet v 1-specific T-cell effector function in an activation-dependent manner. J. Allergy Clin. Immunol. 127, 238–245 [DOI] [PubMed] [Google Scholar]
- 28.Popow I., Leitner J., Grabmeier-Pfistershammer K., Majdic O., Zlabinger G. J., Kundi M., Steinberger P. (2013) A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am. J. Transplant. 13, 3103–3113 [DOI] [PubMed] [Google Scholar]
- 29.Derdak S. V., Kueng H. J., Leb V. M., Neunkirchner A., Schmetterer K. G., Bielek E., Majdic O., Knapp W., Seed B., Pickl W. F. (2006) Direct stimulation of T lymphocytes by immunosomes: virus-like particles decorated with T cell receptor/CD3 ligands plus costimulatory molecules. Proc. Natl. Acad. Sci. USA 103, 13144–13149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leitner J., Kuschei W., Grabmeier-Pfistershammer K., Woitek R., Kriehuber E., Majdic O., Zlabinger G., Pickl W. F., Steinberger P. (2010) T cell stimulator cells, an efficient and versatile cellular system to assess the role of costimulatory ligands in the activation of human T cells. J. Immunol. Methods 362, 131–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leb V. M., Jahn-Schmid B., Schmetterer K. G., Kueng H. J., Haiderer D., Neunkirchner A., Fischer G. F., Nissler K., Hartl A., Thalhamer J., Bohle B., Seed B., Pickl W. F. (2008) Molecular and functional analysis of the antigen receptor of Art v 1-specific helper T lymphocytes. J. Allergy Clin. Immunol. 121, 64–71 [DOI] [PubMed] [Google Scholar]
- 32.Neunkirchner A., Leb-Reichl V. M., Schmetterer K. G., Mutschlechner S., Kueng H. J., Haiderer D., Schuch K., Wallner M., Jahn-Schmid B., Bohle B., Pickl W. F. (2011) Human TCR transgenic Bet v 1-specific Th1 cells suppress the effector function of Bet v 1-specific Th2 cells. J. Immunol. 187, 4077–4087 [DOI] [PubMed] [Google Scholar]
- 33.Groux H., Bigler M., de Vries J. E., Roncarolo M. G. (1998) Inhibitory and stimulatory effects of IL-10 on human CD8+ T cells. J. Immunol. 160, 3188–3193 [PubMed] [Google Scholar]
- 34.Murugaiyan G., Mittal A., Lopez-Diego R., Maier L. M., Anderson D. E., Weiner H. L. (2009) IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J. Immunol. 183, 2435–2443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakkach A., Cottrez F., Groux H. (2001) Differentiation of regulatory T cells 1 is induced by CD2 costimulation. J. Immunol. 167, 3107–3113 [DOI] [PubMed] [Google Scholar]
- 36.Kam C. M., Hudig D., Powers J. C. (2000) Granzymes (lymphocyte serine proteases): characterization with natural and synthetic substrates and inhibitors. Biochim. Biophys. Acta 1477, 307–323 [DOI] [PubMed] [Google Scholar]
- 37.Schust J., Sperl B., Hollis A., Mayer T. U., Berg T. (2006) Stattic: a small-molecule inhibitor of STAT3 activation and dimerization. Chem. Biol. 13, 1235–1242 [DOI] [PubMed] [Google Scholar]
- 38.Asquith B., Debacq C., Florins A., Gillet N., Sanchez-Alcaraz T., Mosley A., Willems L. (2006) Quantifying lymphocyte kinetics in vivo using carboxyfluorescein diacetate succinimidyl ester (CFSE). Proc. Biol. Sci. 273, 1165–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberger P., Majdic O., Derdak S. V., Pfistershammer K., Kirchberger S., Klauser C., Zlabinger G., Pickl W. F., Stöckl J., Knapp W. (2004) Molecular characterization of human 4Ig-B7-H3, a member of the B7 family with four Ig-like domains. J. Immunol. 172, 2352–2359 [DOI] [PubMed] [Google Scholar]
- 40.Bromberg J. F., Wrzeszczynska M. H., Devgan G., Zhao Y., Pestell R. G., Albanese C., Darnell J. E. Jr (1999) Stat3 as an oncogene. Cell 98, 295–303 [DOI] [PubMed] [Google Scholar]
- 41.Hori S., Nomura T., Sakaguchi S. (2003) Control of regulatory T cell development by the transcription factor Foxp3. Science 299, 1057–1061 [DOI] [PubMed] [Google Scholar]
- 42.Yagi H., Nomura T., Nakamura K., Yamazaki S., Kitawaki T., Hori S., Maeda M., Onodera M., Uchiyama T., Fujii S., Sakaguchi S. (2004) Crucial role of FOXP3 in the development and function of human CD25+CD4+ regulatory T cells. Int. Immunol. 16, 1643–1656 [DOI] [PubMed] [Google Scholar]
- 43.Leb V. M., Jahn-Schmid B., Kueng H. J., Schmetterer K. G., Haiderer D., Neunkirchner A., Fischer G. F., Hartl A., Thalhamer J., Steinberger P., Bohle B., Seed B., Pickl W. F. (2009) Modulation of allergen-specific T-lymphocyte function by virus-like particles decorated with HLA class II molecules. J. Allergy Clin. Immunol. 124, 121–128 [DOI] [PubMed] [Google Scholar]
- 44.Magnani C. F., Alberigo G., Bacchetta R., Serafini G., Andreani M., Roncarolo M. G., Gregori S. (2011) Killing of myeloid APCs via HLA class I, CD2 and CD226 defines a novel mechanism of suppression by human Tr1 cells. Eur. J. Immunol. 41, 1652–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grossman W. J., Verbsky J. W., Tollefsen B. L., Kemper C., Atkinson J. P., Ley T. J. (2004) Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood 104, 2840–2848 [DOI] [PubMed] [Google Scholar]
- 46.Gondek D. C., Lu L. F., Quezada S. A., Sakaguchi S., Noelle R. J. (2005) Cutting edge: contact-mediated suppression by CD4+CD25+ regulatory cells involves a granzyme B-dependent, perforin-independent mechanism. J. Immunol. 174, 1783–1786 [DOI] [PubMed] [Google Scholar]
- 47.Bacchetta R., Bigler M., Touraine J. L., Parkman R., Tovo P. A., Abrams J., de Waal Malefyt R., de Vries J. E., Roncarolo M. G. (1994) High levels of interleukin 10 production in vivo are associated with tolerance in SCID patients transplanted with HLA mismatched hematopoietic stem cells. J. Exp. Med. 179, 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barrat F. J., Cua D. J., Boonstra A., Richards D. F., Crain C., Savelkoul H. F., de Waal-Malefyt R., Coffman R. L., Hawrylowicz C. M., O’Garra A. (2002) In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J. Exp. Med. 195, 603–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Veldman C., Höhne A., Dieckmann D., Schuler G., Hertl M. (2004) Type I regulatory T cells specific for desmoglein 3 are more frequently detected in healthy individuals than in patients with pemphigus vulgaris. J. Immunol. 172, 6468–6475 [DOI] [PubMed] [Google Scholar]
- 50.Oh H. M., Yu C. R., Golestaneh N., Amadi-Obi A., Lee Y. S., Eseonu A., Mahdi R. M., Egwuagu C. E. (2011) STAT3 protein promotes T-cell survival and inhibits interleukin-2 production through up-regulation of Class O Forkhead transcription factors. J. Biol. Chem. 286, 30888–30897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwasaki Y., Fujio K., Okamura T., Yanai A., Sumitomo S., Shoda H., Tamura T., Yoshida H., Charnay P., Yamamoto K. (2013) Egr-2 transcription factor is required for Blimp-1-mediated IL-10 production in IL-27-stimulated CD4+ T cells. Eur. J. Immunol. 43, 1063–1073 [DOI] [PubMed] [Google Scholar]
- 52.Holter W., Fischer G. F., Majdic O., Stockinger H., Knapp W. (1986) T cell stimulation via the erythrocyte receptor. Synergism between monoclonal antibodies and phorbol myristate acetate without changes of free cytoplasmic Ca++ levels. J. Exp. Med. 163, 654–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Meuer S. C., Hussey R. E., Fabbi M., Fox D., Acuto O., Fitzgerald K. A., Hodgdon J. C., Protentis J. P., Schlossman S. F., Reinherz E. L. (1984) An alternative pathway of T-cell activation: a functional role for the 50 kd T11 sheep erythrocyte receptor protein. Cell 36, 897–906 [DOI] [PubMed] [Google Scholar]
- 54.Taylor A., Verhagen J., Akkoç T., Wenig R., Flory E., Blaser K., Akdis M., Akdis C. A. (2009) IL-10 suppresses CD2-mediated T cell activation via SHP-1. Mol. Immunol. 46, 622–629 [DOI] [PubMed] [Google Scholar]
- 55.Bidère N., Briet M., Dürrbach A., Dumont C., Feldmann J., Charpentier B., de Saint-Basile G., Senik A. (2002) Selective inhibition of dipeptidyl peptidase I, not caspases, prevents the partial processing of procaspase-3 in CD3-activated human CD8(+) T lymphocytes. J. Biol. Chem. 277, 32339–32347 [DOI] [PubMed] [Google Scholar]
- 56.Nguyen K. D., Vanichsarn C., Nadeau K. C. (2008) Increased cytotoxicity of CD4+ invariant NKT cells against CD4+CD25hiCD127lo/- regulatory T cells in allergic asthma. Eur. J. Immunol. 38, 2034–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma V., Delgado M., Ganea D. (2006) Granzyme B, a new player in activation-induced cell death, is down-regulated by vasoactive intestinal peptide in Th2 but not Th1 effectors. J. Immunol. 176, 97–110 [DOI] [PubMed] [Google Scholar]
- 58.Che K. F., Shankar E. M., Muthu S., Zandi S., Sigvardsson M., Hinkula J., Messmer D., Larsson M. (2012) p38 Mitogen-activated protein kinase/signal transducer and activator of transcription-3 pathway signaling regulates expression of inhibitory molecules in T cells activated by HIV-1-exposed dendritic cells. Mol. Med. 18, 1169–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pot C., Jin H., Awasthi A., Liu S. M., Lai C. Y., Madan R., Sharpe A. H., Karp C. L., Miaw S. C., Ho I. C., Kuchroo V. K. (2009) Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J. Immunol. 183, 797–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Apetoh L., Quintana F. J., Pot C., Joller N., Xiao S., Kumar D., Burns E. J., Sherr D. H., Weiner H. L., Kuchroo V. K. (2010) The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat. Immunol. 11, 854–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Szymczak A. L., Workman C. J., Wang Y., Vignali K. M., Dilioglou S., Vanin E. F., Vignali D. A. (2004) Correction of multi-gene deficiency in vivo using a single ‘self-cleaving’ 2A peptide-based retroviral vector. Nat. Biotechnol. 22, 589–594 [DOI] [PubMed] [Google Scholar]
- 62.Andolfi G., Fousteri G., Rossetti M., Magnani C. F., Jofra T., Locafaro G., Bondanza A., Gregori S., Roncarolo M. G. (2012) Enforced IL-10 expression confers type 1 regulatory T cell (Tr1) phenotype and function to human CD4(+) T cells. Mol. Ther. 20, 1778–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.