Background: The RNase A ribonucleases are enzymatically active secretory proteins that can promote innate immunity.
Results: Mouse eosinophil-associated RNase (mEar) 11 is expressed in response to IL-33 and promotes TLR2-independent macrophage activation.
Conclusion: Mouse Ear 11 is an RNase A ribonuclease with unique expression, targets, and functions.
Significance: This work elucidates the versatility of RNase A ribonucleases in promoting innate immunity.
Keywords: Eosinophil, Evolution, Macrophage, Ribonuclease, Toll-like receptor (TLR)
Abstract
RNase A is the prototype of an extensive family of divergent proteins whose members share a unique disulfide-bonded tertiary structure, conserved catalytic motifs, and the ability to hydrolyze polymeric RNA. Several members of this family maintain independent roles as ribonucleases and modulators of innate immunity. Here we characterize mouse eosinophil-associated RNase (Ear) 11, a divergent member of the eosinophil ribonuclease cluster, and the only known RNase A ribonuclease expressed specifically in response to Th2 cytokine stimulation. Mouse Ear 11 is differentially expressed in somatic tissues at baseline (brain ≪ liver < lung < spleen); systemic stimulation with IL-33 results in 10–5000-fold increased expression in lung and spleen, respectively. Ear 11 is also expressed in response to protective priming of the respiratory mucosa with Lactobacillus plantarum; transcripts are detected both locally in lung as well as systemically in bone marrow and spleen. Mouse Ear 11 is enzymatically active, although substantially less so than mEar 1 and mEar 2; the relative catalytic efficiency (kcat/Km) of mEar 11 is diminished ∼1000–1500-fold. However, in contrast to RNase 2/EDN and mEar 2, which have been characterized as selective chemoattractants for CD11c+ dendritic cells, mEar 11 has prominent chemoattractant activity for F4/80+CD11c− tissue macrophages. Chemoattractant activity is not dependent on full enzymatic activity, and requires no interaction with the pattern recognition receptor, Toll-like receptor 2 (TLR2). Taken together, this work characterizes a divergent RNase A ribonuclease with a unique expression pattern and function, and highlights the versatility of this family in promoting innate immunity.
Introduction
The RNase A ribonucleases are a family of vertebrate-specific secretory proteins with multiple, distinct lineages that have undergone extensive structural and functional diversification (reviewed in Refs. 1 and 2). Specifically, these proteins as a family share distinct structural and catalytic elements and all have some degree of enzymatic activity against single-stranded RNA substrates; however, their primary sequences have diverged significantly from one another, and in many cases there is evidence suggesting that diversification has resulted in novel function. In several specific RNase A family lineages (e.g. RNases 2/3, RNases 7/8, the rodent RNases 5, avian leukocyte RNases A1/A2), there is strong evidence suggesting that sequence diversification may relate to constraints promoting innate immunity, also known as host defense (3–8).
The eosinophil-derived neurotoxin and the eosinophil cationic protein, also known as RNases 2 and 3, respectively, were first identified as cationic secretory mediators stored in the large specific granules of human eosinophilic leukocytes (9–11). Among the founding members of the larger RNase A family, RNase 2/EDN and RNase 3/ECP emerged as a gene pair from a relatively recent duplication event, which was followed by rapid diversification (12, 13). Both RNase 2/EDN and RNase 3/ECP have prominent roles in promoting host defense via cytotoxic interactions with bacterial and helminthic pathogens as well as via antiviral activity, albeit characterized to date in experiments carried out primarily in vitro (reviewed in Refs. 14–16).
Larson and colleagues (17) identified the first murine orthologs in the RNase 2/RNase 3 lineage, and created the term “eosinophil-associated RNases” (Ears).5 Mouse Ears form species-limited clusters that are highly divergent from their human counterparts (only ∼50% amino acid sequence homology); Zhang and colleagues (18) identified the constraints that generated these clusters as rapid gene-sorting followed by positive selection, an unusual diversification pattern that had been reported previously for T cell receptor, immunoglobulin, and major histocompatibility complex genes. Mouse Ears, notably mEar 1 and mEar 2, were detected in secretory granules of mouse eosinophils (17, 19, 20). Although they have maintained the name “eosinophil-associated” because they are orthologous to human RNase 2/EDN and RNase 3/ECP, mouse Ears are also expressed in cells and tissues other than eosinophilic leukocytes. For example, Moreau and colleagues (21) detected mEar 2 in lung tissue of BALB/c mice and O'Reilly and colleagues (22) found that ozone exposure resulted in diminished expression of mEar 1 in airway epithelial cells in vivo.
Among the 12 characterized members of the Mus musculus mEar cluster, mouse eosinophil-associated RNase 11 (mEar 11) displays a particularly unusual expression pattern. Specifically, Cormier and colleagues (23) found that mEar 11 was expressed in alveolar macrophages in response to acute stimulation with Th2 cytokines IL-4 and IL-13. We have also detected expression of mEar 11 in lung tissue, likewise in settings of Th2 predominance, in conjunction with elevated levels of the Th2-cell chemoattractants CCL17 and CCL22 in virus-infected mice devoid of type I interferon receptor-mediated signaling (24).
Given our larger interests in the RNase A family and its role in promoting host defense, here we examine the biology of mEar 11 and reveal the larger extent of its differential expression together with its interactions with other innate immune cells.
EXPERIMENTAL PROCEDURES
Mice
The mice utilized in this study include wild-type BALB/c and C57BL/6 mice from the Division of Cancer Therapeutics, National Cancer Institute, Frederick, MD, NJ.1638 IL-5 transgenic mice (25), and Toll-like receptor-2 gene-deleted (TLR2−/−) mice (Jackson Laboratories, stock 004650). All protocols were evaluated and approved as per the National Institutes of Allergy and Infectious Diseases and carried out in accordance with the Institute's Animal Care and Use Committee Guidelines.
Generation of Bacterial (Escherichia coli) Expression Constructs
cDNAs for mEars 1, 2, and 11 were generated from mRNA from splenocytes from IL-5 transgenic mice (cDNA synthesis kit, Roche Diagnostics, Basel, Switzerland), amplified with sequence-specific primers that included 5′ restriction sites to facilitate cloning (see below). The bacterial expression vector, pET-24a(+), and amplification products were subjected to restriction digestion with enzymes NdeI and XhoI and purified by gel electrophoresis. The amino terminus identified for mEar 11 was based on homology with those defined experimentally for mEars 1 and 2 (17). We have shown previously that a short carboxyl tag does not interfere with post-translational folding or enzymatic activity of RNase A ribonucleases (26). Amplified mEar coding sequences and the pET-24a(+) vector were ligated at a 1:1 molar ratio (T4 DNA ligase; Invitrogen), transformed into One Shot® TOP10 chemically competent E. coli (Invitrogen) and selected on kanamycin-agar plates. Selected clones were confirmed by sequencing. To generate mEar11K35R, lysine 35 (codon AAA) was mutated to arginine (codon AGA) by site-directed mutagenesis (QuikChange Lightning Site-directed Mutagenesis Kit; Agilent Technologies) as per the manufacturer's instructions. Primers used to generate, mutagenize, and sequence pET-24a(+) constructs included: generation of mEar 1, 5′-CACCACCACCATATGCAAACCCCTTCCCAGAAGTTTGCCA-3′ and 5′-GTGGTGGTGCTCGAGAAATGTCCCATCCAAGTGAACTGGAACCACT-3′; generation of mEar 2, 5′-CACCACCACCATATGCAAACCCCTTCCCAGTGGTTTGCCA-3′ and 5′-GTGGTGGTGCTCGAGAAATGTCCCATCCAAGTGAACTGGAACCACT-3′; generation of mEar 11, 5′-CTCCTCCTCCATATGTTGACCCCCTCCCGGTGGTT-3′ and 5′-CTCCTCCTCCTCGAGAATATCCCATCCAAGTGAA-3′; sequencing of pET-24(+) constructs, 5′-GCTAGTTATTGCTCAGCGGT-3′ and 5′-GGGGAATTGTGAGCGGATAA-3′; introduction K35R mutation, 5′-GCGGGCCGTTAACAGTTACACAGGAGTGTGTAGAGACATAAATACTTTTCTTC-3′ and 5′-GAAGAAAAGTATTTATGTCTCTACACACTCCTGTGTAACTGTTAACGGCCCGC-3′.
Production and Purification of Recombinant mEARs
The pET-24a(+) mEar expression constructs described above were used to transfect One Shot® BL21(DE3) chemically competent E. coli (Invitrogen). Kanamycin-resistant clones were screened for effective isopropyl β-d-1-thiogalactopyranoside induction of recombinant protein, as indicated by Western blotting with rabbit polyclonal anti-His6 antibody (AbCam). Selected clones were then grown overnight to stationary phase, and then to A600 = 0.8 in 1 liter in Terrific Broth (Quality Biologicals); protein synthesis was induced with 1 mm isopropyl β-d-1-thiogalactopyranoside for 4 h. Cells were harvested and lysed with BugBuster Protein Extraction Reagent containing Lysonase (Novagen). The insoluble fraction, which contained recombinant mEars within inclusion bodies, was collected by centrifugation and dissolved overnight in 3.0 ml of reducing buffer (6 m guanidine hydrochloride with 10 mm Tris base, 30 mm acetic acid, 2 mm EDTA, 80 mm reduced glutathione, and 3 mm phenylmethylsulfonyl fluoride, adjusted to pH 8.5). Denatured proteins were refolded over the course of 48 h by addition of 200 ml of oxidizing buffer (10 mm Tris base, 40 mm acetic acid, 0.6 m l-arginine, and 0.3 mm oxidized glutathione, adjusted to pH 8.5) in small aliquots, all maintained at 4 °C. Renatured protein solutions were dialyzed at 4 °C against multiple changes of dialysis buffer (20 mm sodium phosphate, pH 7.4, with 0.5 m NaCl) in 6–8-kDa molecular weight cut-off (MWCO) dialysis tubing. Protein solutions were clarified of all insoluble debris and loaded onto pre-equilibrated 5-ml HisTrap HP columns (GE Healthcare). Columns were washed with cold 40 mm imidazole containing dialysis buffer, and eluted with 15 ml of elution buffer (20 mm sodium phosphate, pH 7.4, and 0.5 m NaCl, and 2 m imidazole, pH 8.5) in 1.0-ml fractions. Fractions with eluted protein were combined and dialyzed against cold dialysis buffer. Protein concentrations were determined by BCA assay (Pierce) against serial dilutions of bovine serum albumin standards.
Generation of Polyclonal Rabbit Anti-mEars Antibody
Rabbit polyclonal antibody was generated using bacterial-derived mEar11-His6 as antigen in a standard protocol (Spring Valley Laboratories). Antibody was purified from the antiserum using Hi-Trap protein A columns as per the manufacturer's instructions (GE Healthcare).
Generation of Pichia pastoris (Yeast) Expression Constructs
The mEar 11 and mEar 11K35R His6-tagged constructs with terminal stop codon were amplified from their respective pET-24a(+) constructs with amplification primers as noted above. Amplification products and the pPinkα-HC yeast expression vector were subjected to restriction digestion with enzymes StuI and KpnI; vector and coding sequences were ligated, downstream of a α-mating factor signal sequence containing Ascomycota Kozak consensus sequence, with T4 DNA ligase (Invitrogen) and used to transfect One Shot® TOP10 chemically competent E. coli (Invitrogen). Clones were isolated and selected for by sequencing with pPinkα-HC-specific primers. Bacterial clones were selected by ampicillin resistance. Ten μg of SpeI-linearized plasmid DNA was used to transfect chemically competent P. pastoris, which was then grown on Pichia Adenine Dropout (PAD) agar (Teknova). Large white colonies were selected and screened by PCR for the presence of the appropriate insert. Positive colonies were used to inoculate 10 ml of yeast extract-peptone-dextrose (YPD) media, which were shaken at 300 rpm for 36 h at 29 °C in aerated tubes. Yeast clones were collected, re-suspended in 1 ml of YPD, 25% glycerol, and frozen as stocks at −80 °C. Primers used to generate P. pastoris mEar 11 constructs include: 5′-CACCACCACGAGTCCACACTTGACCCCCTCCCGGTGG-3′ and 5′-GTGGTGGTGGGTACCGTGTCAGTGGTGGTGGTGGTGGTG-3′; primers for sequencing include: F1 5′-GCGACTGGTTCCAATTGACAAGC-3′, F2 5′-CTACTATTGCCAGCATTGCTGCTAAAGAA-3′, and R 5′-GGCGTGAATTAAGCGGTGAC-3′.
Production and Purification of Recombinant mEar 11 and mEar 11K35R from Yeast (P. pastoris)
Frozen stocks were plated on YPD agar; single colonies were used to inoculate primary YPD cultures and shaken at 300 rpm for 36 h at 29 °C in aerated Falcon tubes. Primary cultures were used to inoculate 1 liter of buffered glycerol-complex medium with 1 ml of antifoam 204 (Sigma) in baffled flasks, shaken at 300 rpm for 20 h at 29 °C. Cells were harvested by centrifugation at 1500 × g for 5 min and re-suspended in 50 ml of 1% methanol-buffered complex medium and shaken at 300 rpm for 6 days at 29 °C, replacing evaporated methanol every 24 h. Methanol-induced cultures were centrifuged at 1000 × g for 10 min. Cell-free supernatants were dialyzed (4 °C) against 20 mm sodium phosphate, pH 8.0, with 375 mm NaCl using 6–8 kDa MWCO tubing for 36 h. 20 mm Imidazole and 1.0 ml of nickel-nitrilotriacetic acid-agarose beads (Qiagen) were added to dialyzed solution. Slurry was rotated end-over-end at 4 °C for 60 min and collected on chromatography columns (Bio-Rad), washed with 20 ml of wash buffer (25 mm sodium phosphate, 375 mm NaCl, 20 mm imidazole, pH 8.0), and eluted into 1.0-ml fractions with 10 ml of elution buffer (25 mm sodium phosphate, 375 mm NaCl, 1.5 m imidazole, pH 8.0. Protein-containing fractions were combined, and dialyzed (4 °C) against 20 mm sodium phosphate, pH 8.0, and 375 mm NaCl in Slide-A-Lyzer Dialysis Cassette 3,500 MWCO (Thermo Scientific, Rockford, IL) and concentrated with Centrifugal Filter Units 3,500 MWCO (Millipore). Protein concentrations were determined by BCA assay (Pierce) as above. Purified recombinant yeast mEars were tested for endotoxin contamination using E-Toxate Kit (Sigma). Serial dilutions of purified P. pastoris-derived mEar 11 were evaluated in parallel with endotoxin standards of 0.015–400 EU/ml and samples containing an internal control of 4 EU/ml; as anticipated, all samples evaluated were below detectable limits.
Ribonuclease Assay
Assays were performed in 400 μl of 40 mm sodium phosphate solution, pH 7.4, in triplicate as previously described (26). Briefly, bacterial-derived recombinant mEars were added to assay solutions at 1.9 (mEar 1), 5.1 (mEar 2), and 160 (mEar 11) nm, respectively, and P. pastoris-derived recombinant mEar 11 and mEar 11K35R, each at 230 ng/ml. Acid-soluble ribonucleotides generated from acid-insoluble tRNA were measured spectrophotometrically at A = 260 nm.
Tissue-specific Gene Expression
Total RNA was extracted from various mouse tissues from both BALB/c and C57BL/6 mice using the RNAzol B reagent as per the manufacturer's instructions. Isolated RNA samples were purified with the RNeasy mini kit with on-column DNase I digestion (Qiagen), then reverse-transcribed to cDNA (Roche Diagnostics) using poly(A) primers. Quantitative PCR was performed using 2× TaqMan Reagent (Life Technologies) with validated sequence-specific primer probes for mEar11 and GAPDH (Life Technologies, catalog numbers 4331182-Mm00519056_s1 and 4308313, respectively). Values for absolute copy numbers for both mEar 11 and GAPDH were interpolated from plasmid standards via methods described previously (27). In specific experiments, mice were treated with 1 μg of recombinant mouse IL-33 (R&D Systems) in 50 μl of PBS by intraperitoneal injection daily for 3 days prior to isolation of lungs and spleen for evaluation of mEar 11 expression and serum Th2 cytokines (28). Control mice received 0.1% bovine serum albumin in phosphate-buffered saline on the same injection schedule.
Expression of mEar 11 by Isolated Alveolar Macrophages
Bronchoalveolar lavage fluid was collected from mice euthanized under isoflurane anesthesia and subjected to repeated instillation and withdrawal of 0.1% BSA in PBS (total 1.4 ml). Cells were isolated by centrifugation (500 × g for 10 min) and re-suspended in culture medium (RPMI 1640, 10% FBS, 2 mm l-glutamine, 100 units/ml of penicillin, 100 μg/ml of streptomycin) at a density of 3 × 105/ml. One ml of cell suspension was plated into a 12-well plate and allowed to adhere for 3 h at 37 °C in 5% CO2. Non-adherent cells were removed, and adherent cells were washed with sterile PBS. Adherent cells were re-fed and incubated overnight at 37 °C in 5% CO2. At 24 h, cells were provided with 1.0 ml of fresh medium alone or with IL-4 (10 ng/ml) or IL-13 (10 ng/ml). At various time points, cells were lysed directly in wells with RNAzol B, and RNA was isolated using RNeasy mini kit with on-column DNase I digestion (Qiagen). Gene expression was evaluated as described.
Expression of mEar 11 in Response to Priming with Lactobacillus plantarum
This protocol was described in detail in Ref. 29. Briefly, 8-week-old BALB/c mice were anesthetized briefly with isoflurane and inoculated intranasally with of 109 colony forming units (cfu) of L. plantarum (BAA-793) in a 50-μl volume of sterile phosphate-buffered saline with 0.1% bovine serum albumin (PBS/BSA) at day 0. Control mice received diluent (PBS/BSA) alone. This inoculation (= priming) was repeated on day 7. Mice were sacrificed by cervical dislocation under isoflurane anesthesia at day 28; total RNA was prepared from bone marrow, spleen, and lung tissue using RNAzol B, and gene expression (copies mEar 11/copies GAPDH) was evaluated as described above.
Splenocyte Isolation and Chemotaxis in Response to mEar 11
Single cell suspensions were generated by chopping spleens into Hanks' buffered saline solution (Life Technologies) with 1% fetal bovine serum (FBS; Lonza) and 10 mm HEPES. Large pieces were pressed through a 40-μm strainer, red blood cells were lysed with dH2O followed by re-equilibration with ×10 PBS. Cells were re-suspended in Hanks' balanced salt solution with 1% FBS and 10 mm HEPES and enumerated on a hemocytometer. Approximately 4 × 107 cells were harvested from each spleen. Isolated splenic cells were re-suspended in RPMI 1640, 1% FBS with 10 mm HEPES to 106 cells/ml. Mouse Ear 11 or mEar 11K35R was re-suspended in assay medium immediately prior to assay at concentrations ranging from 0 to 3.4 μm. RNase inhibitor (Roche Diagnostics) was added to specific experiments as indicated to a final concentration of 7.5 units/μl. Control chemoattractant CCL24 was included at 5 ng/μl (600 nm). One hundred μl (105 cells) were added to the top wells inserts and 100 μl of chemoattractant-containing medium added to the bottom wells of transwell plates. Cells were incubated for 150 min at 37 °C in a humidified 5% CO2 incubator. Migrated cells were collected from the bottom wells and diluted with 150 μl of 0.1% PBS/BSA. Migrated cells were counted for 30 s at high flow rate on an LSRII (BD Bioscience, East Rutherford, NJ). Chemotactic index was calculated by dividing the number of high-side scatter/high forward scatter (SSChi/FSChi) cells (myeloid gate) migrated in response to chemoattractant divided by the number of SSChi/FSChi cells migrated in response to vehicle control.
Characterization of Migrating Splenocytes
Experiments were carried out as above, save for the following: isolated splenocytes from both C57BL/6 wild-type and C57BL/6 TLR2−/− mice were first depleted of CD90.2+ T cells and B220+ B cells via immunomagnetic methods as per the manufacturer's instructions (Miltenyi), then re-suspended at 3 × 105 cells/100 μl and loaded into the upper chamber of the transwell plate. After chemotaxis assay, cells were stained with Ly6G-Brilliant Violet 421 (1:40 dilution), CD11c-FITC (1:25 dilution), and F4/80-APC780 (1:40 dilution) and evaluated for expression of these cell surface antigens within the high side/high forward scatter (SSChi/FSChi) gate as noted above. Expression of TLR2 on C57BL/6 splenocytes was determined using monoclonal anti-TLR2/CD282 clone T2.5 (eBioscience) versus isotype (IgG1κ) control, both at 1:50 dilution.
Statistical Analyses
Data points were analyzed for statistical significance using Mann-Whitney U test, Student's t test, or two-way analysis of variance as appropriate. Phylogenetic analysis was performed utilizing algorithms in MEGA 6.0 (30) with sequences aligned via CLUSTALW including those with the following GenBankTM accession numbers: house mouse (M. musculus), mEar 1, NM_007894.2; mEar 2, NM_007895.2; mEar 3, AF017258.1; mEar 4, NM_017389.2; mEar 5, NM_019398.2; mEar 6, NM_053111.2; mEar 7, AY015176.1; mEar 8, AF171650.1; mEar 9, AF171651.1; mEar 10, NM_053112.1; mEar 11, NM_053113.2; mEar 12, AF408691.1; Mongolian gerbil (Meriones unguiculatus) MuEar 11, AF238393.1; MuEar 24, AF238389.1; MuEar 25, AF238390.1; MuEar 34, AF238391.1; MuEar 36, AF238392.1; MuEar 44, AF238394.1; Chinese hamster (Cricetulus griseus), CgEar 3, AF238389.1; CgEar 7, NM001244518.1; CgEar 10, AF238386.1; CgEar 11, NM_001244516.1; CgEar 15, NM_001244517.1; CgEar 30, AF238445.1; and European wild rabbit (Oryctolagus cuniculus) Oc-RNase 2/3A, Oc-RNase 2/3B, and Oc-RNase 2/3C (31).
RESULTS
Mouse Eosinophil-associated RNase 11 Is a Divergent Sequence within a Multigene Cluster
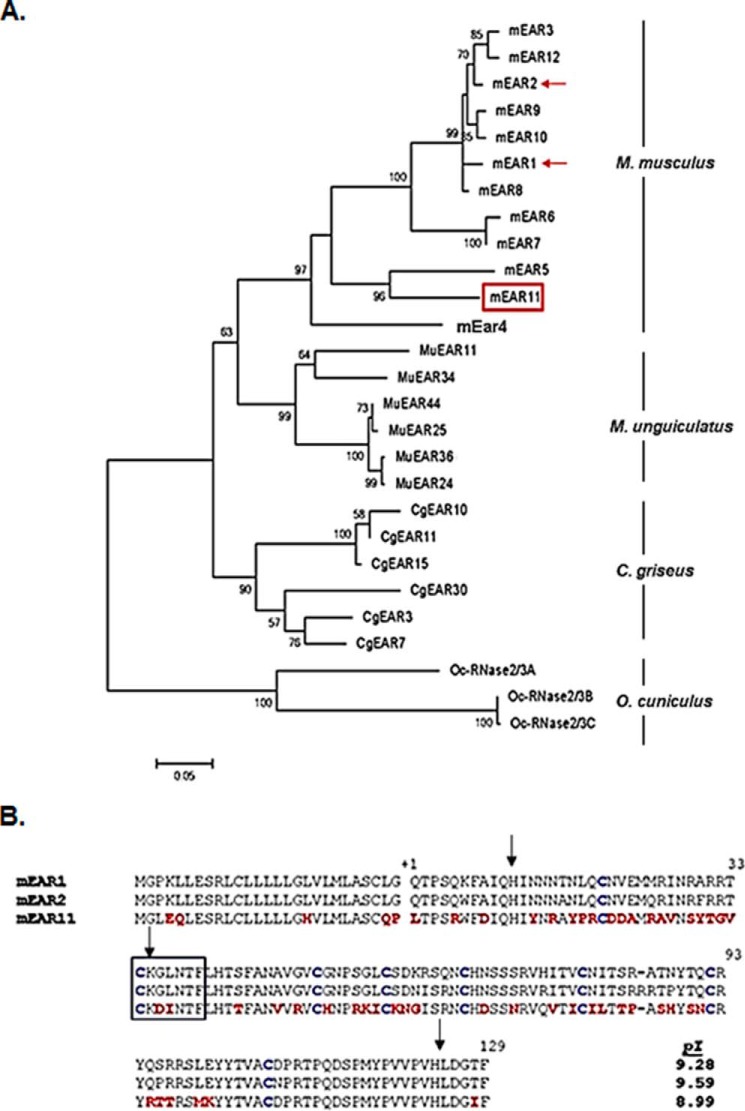
Shown in Fig. 1A is an unrooted neighbor-joining tree featuring rodent eosinophil-associated RNases from the house mouse (M. musculus), Mongolian gerbil (M. unguiculatus), and Chinese hamster (C. griseus); three non-rodent Ear sequences recently identified from the European wild rabbit (Oryctolagus cuniculus (31)) serve as an outgroup. Accession numbers for all sequences are included under “Experimental Procedures.” Of note, the eosinophil-associated RNases form independent species-limited subclusters, suggesting that these sequences underwent multiple duplication events after speciation. A similar pattern was observed when comparing the eosinophil-associated RNases of mice and rats (18, 32), two rodent species estimated to have diverged ∼33 million years ago (33). In contrast, this pattern no longer holds when comparing different species within the Mus genus (18). As shown, mEar 11 has diverged substantially from the major mEar cluster, which includes both mEars 1 and 2 (21 and 20% nucleotide sequence divergence from mEar 11, respectively), although calculations of dN versus dS yielded no evidence of positive or purifying selection (data not shown).
FIGURE 1.
mEar 11 and the rodent Ear gene cluster. A, Neighbor-joining tree documenting phylogenetic relationships among various rodent Ears. Included are nucleotide sequences from house mouse (M. musculus; M), Mongolian gerbil (M. unguiculatus; Mu), and Chinese hamster (C. griseus; Cg); the recently reported sequences of Ears from the European wild rabbit, O. cuniculus; Oc serve as an outgroup (27). Sequences were aligned using ClustalW; the unrooted tree was created with MEGA 6.0 (30) with bootstrap values (5000 replicates) above 50 as shown. The GenBankTM accession numbers for the sequences used to create the tree are listed under “Experimental Procedures.” The featured sequence, mEar 11, is shown within the red box; the prototypical mEars 1 and 2 are denoted with red arrows. B, alignment of the amino acid sequences of mEar 1, mEar 2, and mEar 11. As shown, all three include amino-terminal signal sequences (sequence preceding the amino termini of the secreted proteins, indicated as +1); eight cysteines (in blue), which are cross-linked in the secreted, ribonucleolytically active protein; catalytic histidines (His-24 and His-124 at arrows); and the CKXXNTF signature motif (within box) that includes the conserved lysine (Lys-35; arrow). Amino acids that distinguish the sequence of mEar 11 from those of mEar 1 and 2 are shown in red. Calculated isoelectric points, based on amino acids +1 to +129 are as shown in the right-hand column.
An alignment of the encoded amino acid sequences of mEars 1, 2, and 11 is shown in Fig. 1B. Most mEars characterized to date maintain features characteristic of the RNase A family, including amino-terminal signal sequences, catalytic histidines, and conserved lysine, the latter within a signature “CKXXNTF” motif, and 8 cysteines that form disulfide bonds in the secreted ribonuclease proteins. As anticipated from the aforementioned nucleotide sequences, the amino acid sequence of mEar 11 likewise diverges significantly from those of mEars 1 and 2 (42 and 38% amino acid sequence divergence, respectively), although the carboxyl-terminal region (amino acids 100–129), which includes the catalytic histidine (His-124) and several of the amino acids identified as “chain folding initiation sites” that stabilize protein conformation (34) is comparatively conserved. The calculated isoelectric point of mEar 11 (8.99) is somewhat lower than that calculated for mEar 1 (9.28) or mEar 2 (9.59).
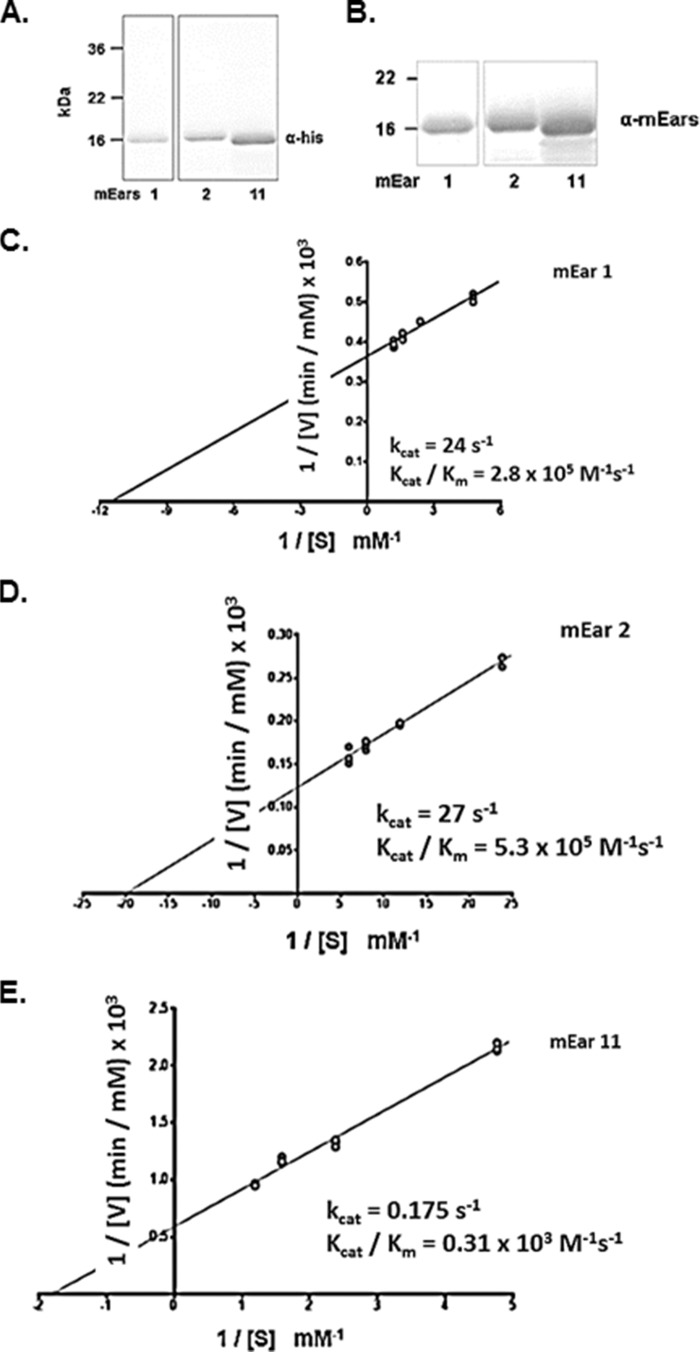
Recombinant mEars 1, 2, and 11 were generated using the pET24(+) bacterial expression system; recombinant protein was isolated from inclusion bodies, denatured, refolded, and purified as shown (Fig. 2, A and B). All refolded proteins were stable in solution and were detected with both monoclonal anti-His tag and polyclonal anti-mEars antibody; mEar 11 displays slightly greater electrophoretic mobility than mEars 1 and 2.
FIGURE 2.
Enzymatic activity of mEars 1, 2, and 11. A, detection of bacterial-derived purified recombinant mEar 1, mEar 2, and mEar 11 on Western blots probed with monoclonal anti-His tag antibody. B, detection of proteins in A on blots probed with polyclonal anti-mEars antibody. C–E, double reciprocal plots of initial rate (1/Vi) versus substrate tRNA concentration (1/[S]) at fixed enzyme concentrations (see “Experimental Procedures”); catalytic constants generated for each enzyme are as shown in the insets.
Enzymatic Activity of mEar 11
Double-reciprocal plots were generated to compare the catalytic properties of the three mEars (Fig. 2, C–E). As anticipated, the catalytic constants determined for mEars 1 and 2 are similar to one another, with Michaelis constants (Km values) of 0.087 and 0.050 mm, and catalytic efficiencies (kcat/Km) at 2.8 and 5.3 × 105 m−1 s−1, respectively. The constant determined for mEar 2 is somewhat lower than that determined for this protein generated by Sf9 cells via the baculovirus expression system (35); this may relate to glycosylation of the latter protein and/or the fact that baculovirus-derived recombinant proteins are purified from cell supernatants already active without the need for chemical re-folding. In contrast, the Km determined for mEar 11 is ∼6–11 fold higher, and the catalytic efficiency, kcat/Km, ∼900–1700 times lower than that calculated for mEar 1 or mEar 2, at the latter at 0.31 × 103 m−1 s−1. Thus, although mEar 11 has retained all the structural features that are critical to support RNase activity, the sequence divergence has resulted in a substantial reduction in catalytic activity. There are several examples within the RNase A family in which gene duplication leads to sequence divergence and substantial loss of enzymatic activity by one member of the resulting gene pair; among these examples, the structural and functional divergence of primate RNase 2/ECP from RNase 3/EDN (3), the paired avian leukocytes RNases A1 and A2 (8), and divergence of RNases 1 and 1b in the leaf-eating primate, douc langur (36). In the first two cases, loss of enzymatic activity is associated with acquisition of host defense function.
Expression of mEar 11 in Response to Th2 Cytokines
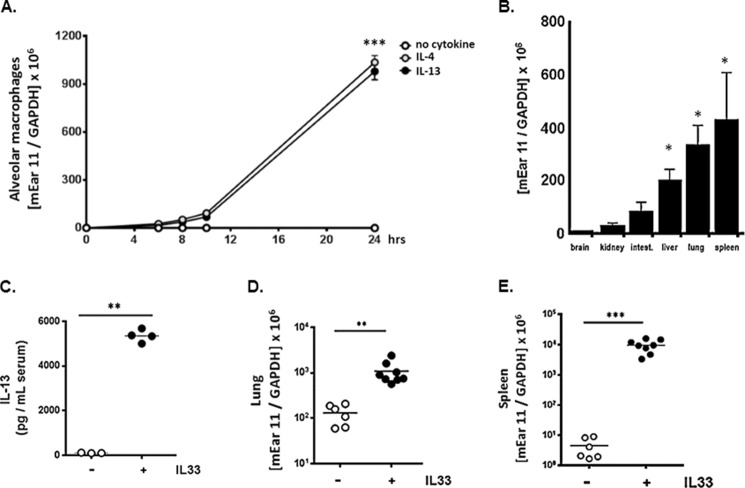
Similar to findings initially reported by Cormier and colleagues (23), we found that isolated alveolar macrophages express mEar 11 in response to direct stimulation with IL-4 or IL-13 (Fig. 3A). Although human RNase 7, mouse RNase 6, and mouse eosinophil-associated RNase 6 (mEar 6) are expressed in response to inflammatory stress (4, 37, 38), to the best of our knowledge, mEar 11 is the only RNase A ribonuclease expressed directly in response to Th2-type cytokine stimulation. To extend this finding, we evaluated the expression of mEar 11 at baseline in various mouse tissues. We detected transcript encoding mEar 11 in macrophage-enriched tissues, including liver, lung, and spleen (Fig. 3B), and, to examine the impact of systemic Th2 stimulation, we inoculated mice with IL-33 via a regimen shown previously to result in elevated serum levels of both IL-4 and IL-13 (28) (Fig. 3C). We detected a 10-fold increase in expression of mEar 11 in lung, and a 5000-fold increase in spleen in response to this treatment protocol (Fig. 3, D and E).
FIGURE 3.
Differential expression of mEar 11 in vivo. A, expression of mEar 11 in primary alveolar macrophage culture in response to IL-4 and IL-13 (each at 10 ng/ml); ***, p < 0.005 versus no cytokine control. B, differential expression of mEar 11 (copies mEar 11/copies GAPDH) in brain, kidney, large intestines, liver, lung, and spleen from BALB/c mice; *, p < 0.05 versus expression in brain tissue. C, serum levels of IL-13 detected in response to intraperitoneal inoculations of IL-33 (1 μg/mouse/day × 3); **, p < 0.01 versus no IL-33 control. D and E, expression of transcripts encoding mEar 11 (copies mEar 11/copies GAPDH) in lung and spleen of C57BL/6 mice in response to systemic administration of IL-33; **, p < 0.01; ***, p < 0.005 versus no IL-33 control.
Mouse Ear 11 Is Expressed in Response to Protective Priming with L. plantarum
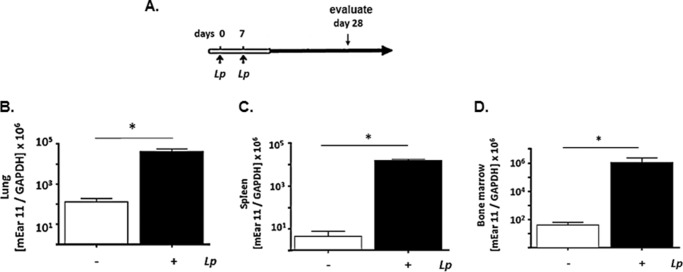
In previous work, we demonstrated that priming of respiratory mucosa with live Lactobacillus species promotes robust and sustained survival in response to an otherwise lethal respiratory virus infection, a property known as heterologous immunity (29, 39, 40). Priming and sustained protection (several months) is associated with profound suppression of proinflammatory cytokines (29, 40) and increased expression of transcripts indicative of M2 (alternatively activated) macrophage polarization in situ in lung tissue.6 Mice were primed with two intranasal inoculations of live L. plantarum on days 0 and 7, after which bacteria are cleared within 24 h of inoculation (39); expression of mEar 11 was assessed both locally in lung tissue and systemically in bone marrow and spleen on day 28 (Fig. 4A). As shown in Fig. 4, B–D, priming alone results in a 1000-fold increase in local expression of mEar 11 in lung tissue, and a 104-fold increased expression systemically in bone marrow and spleen.
FIGURE 4.
Local and systemic expression of mEar 11 in response to protective priming of the respiratory tract with L. plantarum. A, protocol. BALB/c mice received either live L. plantarum (Lp; 109 cfu in 50 μl) at day 0 and day 7 or diluent (PBS/BSA) control prior to evaluation 3 weeks later on day 28. We have shown previously that administration of L. plantarum directly to the respiratory mucosa results in robust and sustained protection against the lethal sequelae of subsequent respiratory virus infection (29). B–D, expression of transcripts encoding mEar 11 (copies mEar 11/copies GAPDH) in lung, spleen, and bone marrow, respectively; *, p < 0.05 versus diluent control, n = 3–6 mice per group.
Mouse Ear 11 Is a Leukocyte Chemoattractant
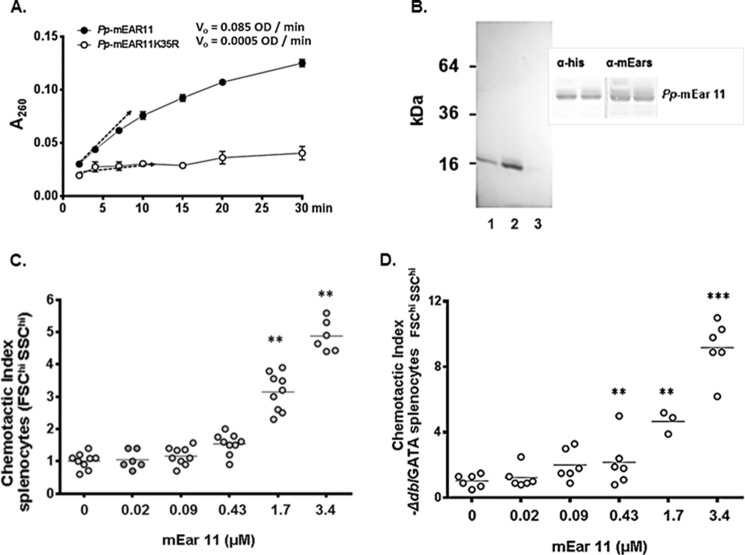
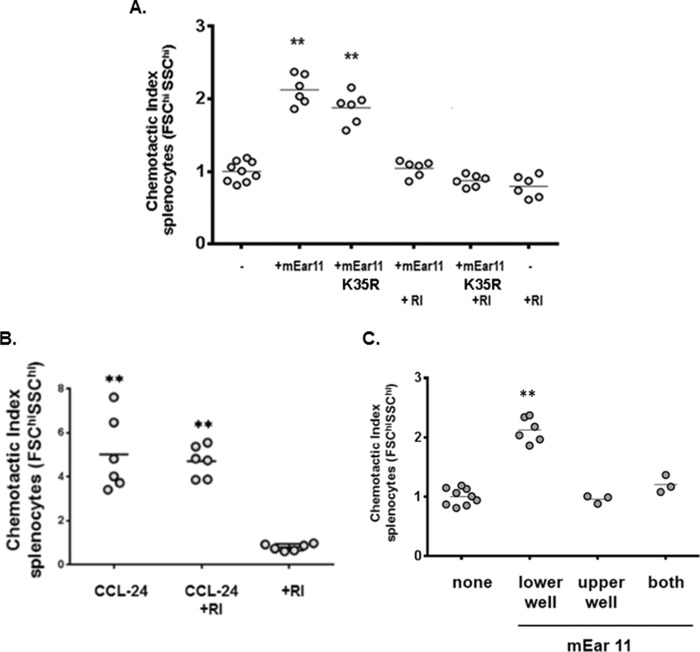
For functional studies of mEar 11, we generated recombinant mEar 11 in the yeast, P. pastoris, so as to avoid confounding issues related to endotoxin contamination found in bacterial preparations (41) (endotoxin/limulus test confirmed, see “Experimental Procedures”). P. pastoris-derived mEar 11 is ribonucleolytically active (Fig. 5A) and has appropriate electrophoretic mobility (Fig. 5B). In earlier studies, Yang and colleagues (43) reported that mouse eosinophil-associated RNase 2 (mEar 2) and human RNase 2/EDN were both chemoattractants for mouse CD11c+ dendritic cells. As mEar 11 has diverged significantly from mEar 2 as well as from RNase 2/EDN, and as such may or may not have similar properties, we set out to determine whether or not mEar 11 was also a leukocyte chemoattractant. In Fig. 5C, we show that mEar 11 is a chemoattractant for splenocytes (FSChi/SSChi gate) from wild-type BALB/c mice; this dose-dependent pattern is reproduced with splenocytes from eosinophil-deficient ΔdblGATA mice (Fig. 5D). In Fig. 6A, we show that enzymatic activity is not essential for chemoattractant activity. Specifically, we generated mEar 11K35R, in which the conserved lysine 35 (see Fig. 1B) was converted to arginine; this protein will be appropriately folded but without the conserved lysine at this position, enzymatic activity is substantially reduced (34) (Fig. 5A). As shown here, mEar 11K35R is equally effective as mEar 11 at eliciting splenocyte migration. In contrast, adding cytoplasmic ribonuclease inhibitor (RI) which binds tightly and specifically to all mammalian RNase A ribonucleases, and masks the exterior surface as well as the catalytic site of the RNase molecule (42), inhibits chemotaxis elicited by both mEar 11 and mEar 11K35R. This result confirms that mEar 11 and mEar 11K35R are the active agents, and that chemotaxis is not elicited by a co-purifying contaminant. Addition of RI alone has no impact on splenocyte chemotaxis. RI also has no impact on chemotaxis elicited by control chemoattractant, CCL24 (Fig. 6B). Standard chemotaxis/chemokinesis controls are included in Fig. 6C.
FIGURE 5.
Recombinant P. pastoris-derived mEar 11 is a chemoattractant for mouse splenocytes. A, initial rates (Vi, generating soluble ribonucleotides measured as OD/min) of P. pastoris-derived (Pp) mEar 11 and Pp-mEar 11K35R, both evaluated at 230 ng/ml. B, purified Pp-mEar 11 (column fractions 1, 2, and 3) evaluated on polyacrylamide gel and stained with Coomassie Blue; inset, detection of P. pastoris-derived mEar 11 on Western blots probed with monoclonal anti-His tag and polyclonal anti-mEars antibodies. C, chemotactic index (cells migrating in response to mEar 11/cells migrating in response to medium alone) for FSChi/SSChi splenocytes from BALB/c mice; **, p < 0.01 versus 0 (no mEar 11) control. D as in C, splenocytes from the eosinophil-deficient ΔdblGATA mice migrating in response to mEar 11; **, p < 0.01 versus no mEar 11 control.
FIGURE 6.
Chemotactic activity is not dependent on enzymatic activity. A, loss of enzymatic activity due to conversion of the conserved lysine to arginine (mEar 11K35R) as in A has no impact on splenocyte chemotaxis; in contrast, addition of cytoplasmic RI, which binds tightly and specifically to the molecular surface of RNase A family ribonucleases, inhibits chemotaxis elicited by mEar 11 and mEar 11K35R. Addition of RI alone has no impact on splenocyte chemotaxis; **, p < 0.01 versus no mEar 11 control. B, CCL-24 (0.6 μm) is a chemoattractant for FSChi/SSChi mouse splenocytes; RI has no impact on chemotaxis elicited by this agent; **, p < 0.01 versus RI only control; C, P. pastoris-derived mEar 11 introduced at 1.7 μm to lower, upper, or both wells of a transwell plate. Results document that mEar 11 elicits chemoattraction, not simply chemokinetic activity; **, p < 0.01 versus no mEar 11 control.
Mouse Ear 11 Is a Chemoattractant for F4/80+CD11c− Tissue Macrophages
As noted above, Yang and colleagues (43) reported earlier that both mEar 2 and RNase 2/EDN were chemoattractants for CD11c+ dendritic cells. Although the chemoattractant receptor on these cells has not been identified, in subsequent work, EDN/RNase 2 was characterized as an alarmin, and found to activate CD11c+ dendritic cells via interactions with the pattern recognition receptor, TLR2 (44).
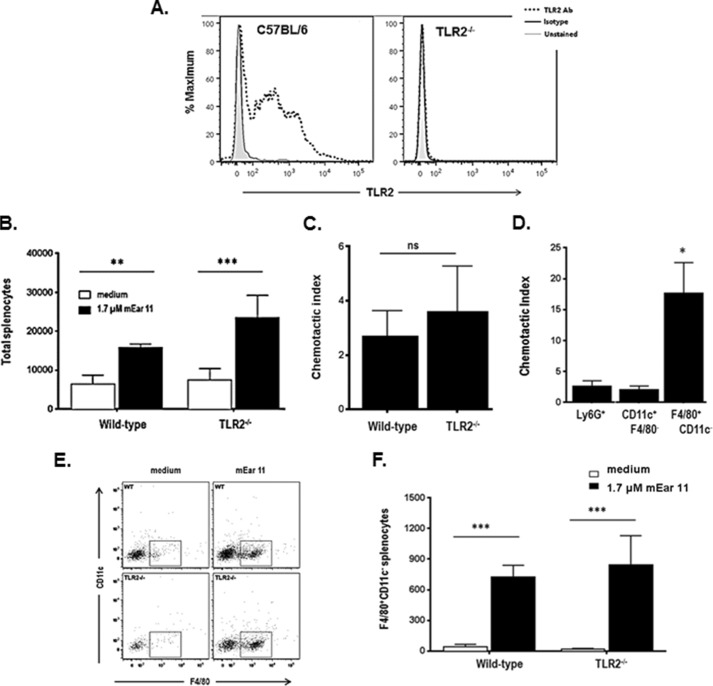
Here, we characterize further the cells migrating in response to mEar 11. First, we have confirmed that TLR2 is expressed prominently on splenocytes from wild-type C57BL/6, but not TLR2-gene deleted mice (Fig. 7A). As shown in Fig. 7, B and C, whereas mEar 11 elicits chemotaxis of splenocytes from C57BL/6 mice as it does splenocytes from BALB/c mice (see Fig. 5), we find that the chemotactic response to mEar 11 is not directly dependent on interactions with TLR2. Furthermore, whereas mEar 11 does have some effect on neutrophils (Ly6G+) and dendritic cells (F4/80−CD11c+), its greatest impact on a per cell basis is on F4/80+CD11c− tissue macrophages (Fig. 7D). Tissue F4/80+CD11c− macrophages undergo prominent migration to mEar 11 likewise with no significant differences between the responses of cells derived from wild-type versus TLR2−/− mice (Fig. 7, E and F).
FIGURE 7.
Recombinant P. pastoris-derived mEar 11 is chemoattractant for F4/80+CD11c− macrophages. A, splenocytes from wild-type C57BL/6 mice and C57BL/6 TLR2−/− mice evaluated for expression of TLR2. B, total splenocytes (of 3 × 105 initial cells) from wild-type C57BL/6 and C57BL/6 TLR2−/− mice migrating in response to 1.7 μm mEar 11 or medium alone; **, p < 0.01; ***, p < 0.001 two-way analysis of variance. C, chemotactic indices (cells migrating to mEar 11/cells migrating to medium alone) calculated from data in B; ns, not significant. D, chemotactic indices calculated for leukocyte subsets, including Ly6G+ (neutrophils), CD11c+F4/80− (dendritic cells), and F4/80+CD11c− (macrophages); *, p < 0.05 Mann-Whitney U test. F, representative flow plot and G, total F4/80+CD11c− cells (of 3 × 105 initial cells) from wild-type C57BL/6 and C57BL/6 TLR2−/− mice migrating in response to 1.7 μm mEar 11 and to medium alone; ***, p < 0.001 two-way analysis of variance.
DISCUSSION
In this study, we have characterized mEar 11, the only RNase A ribonuclease expressed in response to Th2 cytokines, and we have revealed a role for this protein as a macrophage chemoattractant.
The existence of a multilineage RNase A family was first revealed in the late 1980s upon identification of, among others, gene sequences encoding human angiogenin (RNase 5 (45)), eosinophil cationic protein (RNase 3/ECP (46, 47)), and eosinophil-derived neurotoxin (RNase 2/EDN (48, 49)). Upon completion of the sequence of the human genome, eight RNase A ribonucleases that had the potential to be fully functional were identified (numbered RNases 1 through 8) together with five more distantly related sequences (RNases 9–13). Host defense functions have been attributed to three specific lineages of the mammalian branch of the RNase A superfamily. The human eosinophil secretory ribonucleases (RNases 2 and 3) are rapidly diverging proteins with prominent antimicrobial, antiviral, and signaling activities (14–16); RNase 7, is a cationic antimicrobial protein originally isolated from human skin (4), and mouse angiogenin-4, one member of a cluster of proteins originally characterized as promoting blood vessel growth (7) also has anti-pathogen activity. Antimicrobial activities have also been attributed to several non-mammalian RNase A ribonucleases, including the highly cationic chicken leukocyte RNase A2 (8), and Dr-RNases 1, 2, and 3 of the zebrafish, Danio rerio (50, 51).
Very little is known regarding the specific function of any of the individual mouse eosinophil-associated RNases. The mEar cluster was first identified by Larson and colleagues (17), and its evolutionary diversity was characterized by Zhang and colleagues (18). As noted earlier, mouse Ears are prominent components of eosinophilic leukocytes, but individual mEars have been detected in other tissues in response to individual proinflammatory stimuli (22, 37). Mouse Ear 11 is the only known RNase A ribonuclease expressed in response to Th2 cytokines, and specifically in alveolar macrophages (i.e. M2 or alternative activation) (23). Alternatively activated, as distinguished from classically activated macrophages, are generally viewed as promoting immunoregulation and inflammatory suppression (52, 53). In this light, it is intriguing to consider a role of mEar 11 as a prominent local and systemic response to priming with L. plantarum. As we have shown previously, priming of the respiratory mucosa with this immunobiotic organism results in robust and sustained heterologous immunity against subsequent respiratory virus infection in association with profound suppression of the virus-induced cytokine storm (29, 39, 40). Heterologous immunity (also known in other contexts as innate imprinting and trained immunity (54–57)), is a general term that describes nonspecific cross-protection to unrelated microbes, and has been attributed in other experimental settings to activation of circulating monocytes. Among these findings is the recent report of Kleinnijenhuis et al. (58) who reported that cross-protection elicited by Bacille Calmette-Guerin (BCG) vaccination was associated with pattern recognition receptor NOD-2-dependent responses of circulating monocytes. Although protection elicited by L. plantarum priming is not directly dependent on NOD-2 alone,7 the systemic responses elucidated by mEar 11 expression provide insight into the impact and persistence of protection elicited by L. plantarum priming.
Equally intriguing, although human RNase 4 is expressed in adherent monocyte/macrophages (59) we could find no documentation of a human RNase A ribonuclease expressed specifically in alternatively activated macrophages (i.e. in response to Th2 cytokines (60, 61)); this includes the most recent study by Martinez and colleagues (61), which features both microarray and proteomic analysis of human macrophages challenged with IL-4. Furthermore, the 5′ putative promoter region of mEar 11 includes no consensus binding sites for Stat6 (62), as one would anticipate if this transcript was regulated directly (as opposed to indirectly) in response to IL-4 and/or IL-13. It will be interesting to explore this response further among the rodent and other species with Ear clusters (31, 32) to define clearly the nature and molecular basis of this unusual response.
We have found that mEar 11 is a prominent chemoattractant for F4/80+CD11c− macrophages (63), an action that does not require endogenous enzymatic activity, and that is also not directly dependent on interactions between mEar 11 and the pattern recognition receptor, TLR2. This is intriguing, given the findings of Yang and colleagues (43, 44) in which the related ribonucleases, mEar 2 and RNase 2/EDN, were both identified as pertussis toxin-dependent chemoattractants for CD11c+ dendritic cells, and that RNase 2/EDN specifically activates dendritic cells via interactions with TLR2. Botos and colleagues (64) were among the first to note the remarkable structural similarities between TLRs and the ubiquitously expressed cytoplasmic RI, notably the ring array pattern of coiled leucine-rich repeats. As such, it is reasonable to consider the possibility that mEar 11 might interact with TLR2 and/or other TLRs on innate immune cells via these leucine-rich repeats and elicit responses other than what has been evaluated thus far. There are several studies that have implicated TLRs in promoting leukocyte migration (65–67), however, it remains uncertain as to whether the migrating cells were responding directly or indirectly to TLR ligands, as these studies were carried out in complex in vivo environments. Given the abundant expression of TLRs on macrophages (68), it would be intriguing to examine this possibility further, and to determine whether mEar 11 might interact directly with one or more TLRs, and to determine what the structural basis and the outcomes of these interactions might be.
In summary, we have characterized mEar 11, a unique RNase A ribonuclease and the only member of this extensive family known to be expressed in direct response to Th2 stimuli. Transcripts encoding mEar 11 are differentially expressed in somatic tissues at baseline; Th2 cytokine stimulation and local priming with L. plantarum results in dramatic up-regulation in absolute expression in both lung and spleen tissues. Furthermore, mouse Ear 11 is a prominent chemoattractant for F4/80+ macrophages and, despite previous studies indicating that its ortholog, RNase 2/EDN can interact with and signal via TLR2 (44), this specific interaction between mEar 11 and mouse macrophages is TLR2-independent. Further study will define and expand the scope of the interactions between mEar 11 and mouse macrophages both ex and in vivo.
Acknowledgment
We gratefully acknowledge the helpful suggestions of Dr. Dragana Jankovic, Laboratory of Parasitic Diseases, NIAID, National Institutes of Health, regarding TLR structure.
This work was supported, in whole or in part, by National Institutes of Health Grants AI000942 from the NIAID Division of Intramural Research (to H. F. R.) and AI000746 (to K. M. D.).
K. J. Yamada, C. M. Percopo, and H. F. Rosenberg, unpublished observations.
C. M. Percopo, T. A. Rice et al., manuscript in preparation.
- Ear
- eosinophil-associated ribonuclease
- TLR
- Toll-like receptor
- EDN
- eosinophil-derived neurotoxin
- ECP
- eosinophil cationic protein
- SSC
- side scatter
- FSC
- forward scatter
- RI
- ribonuclease inhibitor
- MWCO
- molecular weight cutoff.
REFERENCES
- 1. Dyer K. D., Rosenberg H. F. (2006) The RNase A superfamily: generation of diversity and innate host defense. Mol. Divers. 10, 585–597 [DOI] [PubMed] [Google Scholar]
- 2. Rosenberg H. F. (2008) RNase A ribonucleases and host defense: an evolving story. J. Leukoc. Biol. 83, 1079–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosenberg H. F., Dyer K. D. (1995) Eosinophil cationic protein and eosinophil-derived neurotoxin. Evolution of novel function in a primate ribonuclease gene family. J. Biol. Chem. 270, 21539–21544 [DOI] [PubMed] [Google Scholar]
- 4. Harder J., Schröder J. M. (2002) RNase 7, a novel innate immune defense antimicrobial protein of healthy human skin. J. Biol. Chem. 277, 46779–46784 [DOI] [PubMed] [Google Scholar]
- 5. Zhang J., Dyer K. D., Rosenberg H. F. (2003) Human RNase 7: a new cationic ribonuclease of the RNase A superfamily. Nucleic Acids Res. 31, 602–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang J., Dyer K. D., Rosenberg H. F. (2002) RNase 8, a novel RNase A superfamily ribonuclease expressed uniquely in placenta. Nucleic Acids Res. 30, 1169–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hooper L. V., Stappenbeck T. S., Hong C. V., Gordon J. I. (2003) Angiogenins: a new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 4, 269–273 [DOI] [PubMed] [Google Scholar]
- 8. Nitto T., Dyer K. D., Czapiga M., Rosenberg H. F. (2006) Evolution and function of leukocyte RNase A ribonucleases of the avian species, Gallus gallus. J. Biol. Chem. 281, 25622–25634 [DOI] [PubMed] [Google Scholar]
- 9. Durack D. T., Ackerman S. J., Loegering D. A., Gleich G. J. (1981) Purification of human eosinophil-derived neurotoxin. Proc. Natl. Acad. Sci. U.S.A. 78, 5165–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Olsson I., Venge P., Spitznagel J. K., Lehrer R. I. (1977) Arginine-rich cationic proteins of human eosinophil granules: comparison of the constituents of eosinophilic and neutrophilic leukocytes. Lab. Invest. 36, 493–500 [PubMed] [Google Scholar]
- 11. Ackerman S. J., Loegering D. A., Venge P., Olsson I., Harley J. B., Fauci A. S., Gleich G. J. (1983) Distinctive cationic proteins of the human eosinophil granule: major basic protein, eosinophil cationic protein, and eosinophil-derived neurotoxin. J. Immunol. 131, 2977–2982 [PubMed] [Google Scholar]
- 12. Rosenberg H. F., Dyer K. D., Tiffany H. L., Gonzalez M. (1995) Rapid evolution of a unique family of primate ribonuclease genes. Nat. Genet. 10, 219–223 [DOI] [PubMed] [Google Scholar]
- 13. Zhang J., Rosenberg H. F., Nei M. (1998) Positive Darwinian selection after gene duplication in primate ribonuclease genes. Proc. Natl. Acad. Sci. U.S.A. 95, 3708–3713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rosenberg H. F., Domachowske J. B. (1999) Eosinophils, ribonucleases and host defense: solving the puzzle. Immunol. Res. 20, 261–274 [DOI] [PubMed] [Google Scholar]
- 15. Rosenberg H. F., Domachowske J. B. (2001) Eosinophils, eosinophil ribonucleases and their role in host defense against respiratory virus pathogens. J. Leukoc. Biol. 70, 691–698 [PubMed] [Google Scholar]
- 16. Rosenberg H. F., Dyer K. D., Domachowske J. B. (2012) Interactions of Eosinophils with Respiratory Virus Pathogens in Eosinophils in Health and Disease (Lee J. J., Rosenberg H. F., eds) Chapter 9.3, pp. 281–290, Elsevier Publishers, Amsterdam [Google Scholar]
- 17. Larson K. A., Olson E. V., Madden B. J., Gleich G. J., Lee N. A., Lee J. J. (1996) Two highly homologous ribonuclease genes expressed in mouse eosinophils identify a larger subgroup of the mammalian ribonuclease superfamily. Proc. Natl. Acad. Sci. U.S.A. 93, 12370–12375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhang J., Dyer K. D., Rosenberg H. F. (2000) Evolution of the rodent eosinophil-associated RNase gene family by rapid gene sorting and positive selection. Proc. Natl. Acad. Sci. U.S.A. 97, 4701–4706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shamri R., Melo R. C., Young K. M., Bivas-Benita M., Xenakis J. J., Spencer L. A., Weller P. F. (2012) CCL11 elicits secretion of RNases from mouse eosinophils and their cell-free granules. FASEB J. 26, 2084–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shamri R., Young K. M., Weller P. F. (2013) PI3K, ERK, p38 MAPK and integrins regulate CCR3-mediated secretion of mouse and human eosinophil-associated RNases. Allergy 68, 880–889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moreau J. M., Dyer K. D., Bonville C. A., Nitto T., Vasquez N. L., Easton A. J., Domachowske J. B., Rosenberg H. F. (2003) Diminished expression of an antiviral ribonuclease in response to pneumovirus infection in vivo. Antiviral Res. 59, 181–191 [DOI] [PubMed] [Google Scholar]
- 22. O'Reilly M. A., Yee M., Buczynski B. W., Vitiello P. F., Keng P. C., Welle S. L., Finkelstein J. N., Dean D. A., Lawrence B. P. (2012) Neonatal oxygen increases sensitivity to influenza A virus infection in adult mice by suppressing epithelial expression of Ear 1. Am. J. Pathol. 181, 441–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cormier S. A., Yuan S., Crosby J. R., Protheroe C. A., Dimina D. M., Hines E. M., Lee N. A., Lee J. J. (2002) Th2-mediated pulmonary inflammation leads to the differential expression of ribonuclease genes by alveolar macrophages. Am. J. Respir. Cell Mol. Biol. 27, 678–687 [DOI] [PubMed] [Google Scholar]
- 24. Garvey T. L., Dyer K. D., Ellis J. A., Bonville C. A., Foster B., Prussin C., Easton A. J., Domachowske J. B., Rosenberg H. F. (2005) Inflammatory responses to pneumovirus infection in IFN-αβ receptor gene-deleted mice. J. Immunol. 175, 4735–4744 [DOI] [PubMed] [Google Scholar]
- 25. Lee N. A., McGarry M. P., Larson K. A., Horton M. A., Kristensen A. B., Lee J. J. (1997) Expression of IL-5 in thymocytes/T cells leads to the development of a massive eosinophilia, extramedullary eosinophilopoiesis, and unique histopathologies. J. Immunol. 158, 1332–1344 [PubMed] [Google Scholar]
- 26. Rosenberg H. F., Domachowske J. B. (2001) Eosinophil-derived neurotoxin. Methods Enzymol. 341, 273–286 [DOI] [PubMed] [Google Scholar]
- 27. Percopo C. M., Dyer K. D., Karpe K. A., Domachowske J. B., Rosenberg H. F. (2014) Eosinophils and respiratory virus infection: a dual-standard curve qRT-PCR-based method for determining virus recovery from mouse lung tissue. Methods Mol. Biol. 1178, 257–266 [DOI] [PubMed] [Google Scholar]
- 28. Dyer K. D., Percopo C. M., Rosenberg H. F. (2013) IL-33 promotes eosinophilia in vivo and antagonizes IL-5-dependent eosinophil hematopoiesis ex vivo. Immunol. Lett. 150, 41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gabryszewski S. J., Bachar O., Dyer K. D., Percopo C. M., Killoran K. E., Domachowske J. B., Rosenberg H. F. (2011) Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J. Immunol. 186, 1151–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30, 2725–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goo S. M., Cho S. (2013) The expansion and functional diversification of the mammalian ribonuclease superfamily epitomizes the efficiency of multigene families at generating biological novelty. Genome Biol. Evol. 5, 2124–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Singhania N. A., Dyer K. D., Zhang J., Deming M. S., Bonville C. A., Domachowske J. B., Rosenberg H. F. (1999) Rapid evolution of the ribonuclease A superfamily: adaptive expansion of independent gene clusters in rats and mice. J. Mol. Evol. 49, 721–728 [DOI] [PubMed] [Google Scholar]
- 33. Nei M., Xu P., Glazko G. (2001) Estimation of divergence times from multi-protein sequences for a few mammalian species and several distantly related organisms. Proc. Natl. Acad. Sci. U.S.A. 98, 2497–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chatani E., Hayashi R. (2001) Functional and structural roles of constituent amino acid residues of bovine pancreatic ribonuclease A. J. Biosci. Bioeng. 92, 98–107 [DOI] [PubMed] [Google Scholar]
- 35. McDevitt A. L., Deming M. S., Rosenberg H. F., Dyer K. D. (2001) Gene structure and enzymatic activity of mouse eosinophil-associated ribonuclease 2.Gene 267, 23–30 [DOI] [PubMed] [Google Scholar]
- 36. Zhang J., Zhang Y. P., Rosenberg H. F. (2002) Adaptive evolution of a duplicated pancreatic ribonuclease gene in a leaf-eating monkey. Nat. Genet. 30, 411–415 [DOI] [PubMed] [Google Scholar]
- 37. Nitto T., Dyer K. D., Mejia R. A., Byström J., Wynn T. A., Rosenberg H. F. (2004) Characterization of the divergent eosinophil ribonuclease, mEar 6, and its expression in response to Schistoma mansoni infection in vivo. Genes Immun. 5, 668–674 [DOI] [PubMed] [Google Scholar]
- 38. Becknell B., Eichler T. E., Beceiro S., Li B., Easterling R. S., Carpenter A. R., James C. L., McHugh K. M., Hains D. S., Partida-Sanchez S., Spencer J. D. (2015) Ribonucleases 6 and 7 have antimicrobial function in the human and murine urinary tract. Kidney Int. 87, 151–161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Garcia-Crespo K. E., Chan C. C., Gabryszewski S. J., Percopo C. M., Rigaux P., Dyer K. D., Domachowske J. B., Rosenberg H. F. (2013) Lactobacillus-priming of the respiratory tract: heterologous immunity and protection against lethal respiratory virus infection. Antiviral Res. 97, 270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Percopo C. M., Dyer K. D., Garcia-Crespo K. E., Gabryszewski S. J., Shaffer A. L., 3rd, Domachowske J. B., Rosenberg H. F. (2014) B cells are not essential for Lactobacillus-mediated protection against lethal pneumovirus infection. J. Immunol. 192, 5265–5272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cregg J. M., Tolstorukov I., Kusari A., Sunga J., Madden K., Chappell T. (2009) Expression in the yeast, Pichia pastoris. Methods Enzymol. 463, 169–189 [DOI] [PubMed] [Google Scholar]
- 42. Dickson K. A., Haigis M. C., Raines R. T. (2005) Ribonuclease inhibitor: structure and function. Prog. Nucleic Acids Res. Mol. Biol. 80, 349–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang D., Rosenberg H. F., Chen Q., Dyer K. D., Kurosaka K., Oppenheim J. J. (2003) Eosinophl-derived neurotoxin (EDN) and antimicrobial protein with chemotactic activities for dendritic cells. Blood 102, 3396–3403 [DOI] [PubMed] [Google Scholar]
- 44. Yang D., Chen Q., Su S. B., Zhang P., Kurosaka K., Caspi R. R., Michalek S. M., Rosenberg H. F., Zhang N., Oppenheim J. J. (2008) Eosinophil-derived neurotoxin acts as an alarmin to activate the TLR2-MyD88 signal pathway in dendritic cells and enhances Th2 immune responses. J. Exp. Med. 205, 79–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kurachi K., Davie E. W., Strydom D. J., Riordan J. F., Vallee B. L. (1985) Sequence of the cDNA and gene for angiogenin, a human angiogenesis factor. Biochemistry 24, 5494–5499 [DOI] [PubMed] [Google Scholar]
- 46. Rosenberg H. F., Ackerman S. J., Tenen D. G. (1989) Human eosinophil cationic protein: molecular cloning of a cytotoxin and helminthotoxin with ribonuclease activity. J. Exp. Med. 170, 163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barker R. L., Loegering D. A., Ten R. M., Hamann K. J., Pease L. R., Gleich G. J. (1989) Eosinophil cationic protein cDNA: comparison with other toxic cationic proteins and ribonucleases. J. Immunol. 143, 952–955 [PubMed] [Google Scholar]
- 48. Rosenberg H. F., Tenen D. G., Ackerman S. J. (1989) Molecular cloning of the human eosinophil-derived neurotoxin: a member of the ribonuclease gene family. Proc. Natl. Acad. Sci. U.S.A. 86, 4460–4464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hamann K. J., Barker R. L., Loegering D. A., Pease L. R., Gleich G. J. (1989) Sequence of human eosinophil-derived neurotoxin cDNA: identitiy of deduced amino acid sequence with human nonsecretory ribonucleases. Gene 83, 161–167 [DOI] [PubMed] [Google Scholar]
- 50. Cho S., Zhang J. (2007) Zebrafish ribonucleases are bactericidal: implications for the origin of the vertebrate RNase superfamily. Mol. Biol. Evol. 24, 1259–1268 [DOI] [PubMed] [Google Scholar]
- 51. Zanfardino A., Pizzo E., Di Maro A., Varcamonti M., D'Alessio G. (2010) The bactericidal action on Escherichia coli of ZF-RNase-3 is triggered by the suicidal action of the bacterium OmpT protease. FEBS J. 277, 1921–1928 [DOI] [PubMed] [Google Scholar]
- 52. Mosser D. M. (2003) The many faces of macrophage activation. J. Leukocyte Biol. 73, 209–212 [DOI] [PubMed] [Google Scholar]
- 53. Gordon S., Martinez F. O. (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32, 593–604 [DOI] [PubMed] [Google Scholar]
- 54. Levy O., Netea M. G. (2014) Innate immune memory: implications for development of pediatric immunomodulatory agents and adjuvanted vaccines. Pediatr. Res. 75, 184–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Netea M. G., Quintin J., van der Meer J. W. (2011) Trained immunity: a memory for innate host defense. Cell Host Microbe 9, 355–361 [DOI] [PubMed] [Google Scholar]
- 56. Didierlaurent A., Goulding J., Hussell T. (2007) The impact of successive infections on the lung microenvironment. Immunology 122, 457–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Locati M., Mantovani A., Sica A. (2013) Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 120, 163–184 [DOI] [PubMed] [Google Scholar]
- 58. Kleinnijenhuis J., Quintin J., Preijers F., Joosten A. B., Ifrim D. C., Saeed S., Jacobs C., van Loenhout J., de Jong D., Stunnenberg H. G., Xavier R. J., van der Meer J. W., van Crevel R., Netea M. G. (2012) Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. U.S.A. 109, 17537–17542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Egesten A., Dyer K. D., Batten D., Domachowske J. B., Rosenberg H. F. (1997) Ribonucleases and host defense: identification, localization and gene expression in adherent monocytes in vitro. Biochim. Biophys. Acta 1358, 255–260 [DOI] [PubMed] [Google Scholar]
- 60. Martinez F. O., Gordon S., Locati M., Mantovani A. (2006) Transcriptonal profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J. Immunol. 177, 7303–7311 [DOI] [PubMed] [Google Scholar]
- 61. Martinez F. O., Helming L., Milde R., Varin A., Melgert B. N., Draijer C., Thomas B., Fabbri M., Crawshaw A., Ho L. P., Ten Hacken N. H., Cobos Jiménez V., Kootstra N. A., Hamann J., Greaves D. R., Locati M., Mantovani A., Gordon S. (2013) Genetic programs expressed in resting and IL-4 alternatively activated mouse and human macrophages: similarities and differences. Blood 121, e57–e69 [DOI] [PubMed] [Google Scholar]
- 62. Ehret G. B., Reichenbach P., Schindler U., Horvath C. M., Fritz S., Nabholz M., Bucher P. (2001) DNA binding specificity of different STAT proteins: comparison of in vitro specificity with natural target sites. J. Biol. Chem. 276, 6675–6688 [DOI] [PubMed] [Google Scholar]
- 63. Davies L. C., Jenkins S. J., Allen J. E., Taylor P. R. (2013) Tissue-resident macrophages. Nat. Immunol. 14, 986–99524048120 [Google Scholar]
- 64. Botos I., Segal D. M., Davies D. R. (2011) The structural biology of Toll-like receptors. Structure 19, 447–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Oliveira A. C., Peixoto J. R., de Arruda L. B., Campos M. A., Gazzinelli R. T., Golenbock D. T., Akira S., Previato J. O., Mendonça-Previato L., Nobrega A., Bellio M. (2004) Expression of functional TLR4 confers proinflammatory responsiveness to Trypanosoma cruzi glycoinsoitolphopholipids and higher resistance to infection with T. cruzi. J. Immunol. 173, 5688–5696 [DOI] [PubMed] [Google Scholar]
- 66. Moles A., Murphy L., Wilson C. L., Chakraborty J. B., Fox C., Park E. J., Mann J., Oakley F., Howarth R., Brain J., Masson S., Karin M., Seki E., Mann D. A. (2014) A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. J. Hepatol. 60, 782–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Andrews K., Abdelsamed H., Yi A. K., Miller M. A., Fitzpatrick E. A. (2013) TLR2 regulates neutrophil recruitment and cytokine production with minor contributions from TLR9 during hypersensitivity pneumonitis. PLoS One 8, e73143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kaisho T., Akira S. (2000) Critical roles of Toll-like receptors in host defense. Crit. Rev. Immunol. 20, 393–405 [PubMed] [Google Scholar]