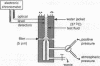
Abstract
We have examined the filterability of sickle erythrocytes, using an initial-flow-rate method, to determine whether sufficient hemoglobin S polymer forms at arterial oxygen saturation to adversely affect erythrocyte deformability. The amount of intracellular polymer was calculated as a function of oxygen saturation to estimate the polymerization tendency for each of eight patients with sickle cell anemia (SCA). Progressive reduction of oxygen tension within the arterial range caused a sudden loss of filterability of SCA erythrocytes through 5-micron-diam pores at a critical PO2 between 110 and 190 mmHg. This loss of filterability occurred at a higher PO2 than did morphological sickling, and the critical PO2 correlated significantly (r = 0.844-0.881, P less than 0.01) with the polymerization tendency for each patient. Study of density-gradient fractionated cells from four SCA patients indicated that the critical PO2 of dense cells was reached when only a small amount of polymer had formed, indicating the influence of this subpopulation on the results obtained for unfractionated cells. Impairment of erythrocyte filterability at high oxygen saturation (greater than 90%) suggests that small changes in oxygen saturation within the arterial circulation cause rheological impairment of sickle cells.
Full text
PDF
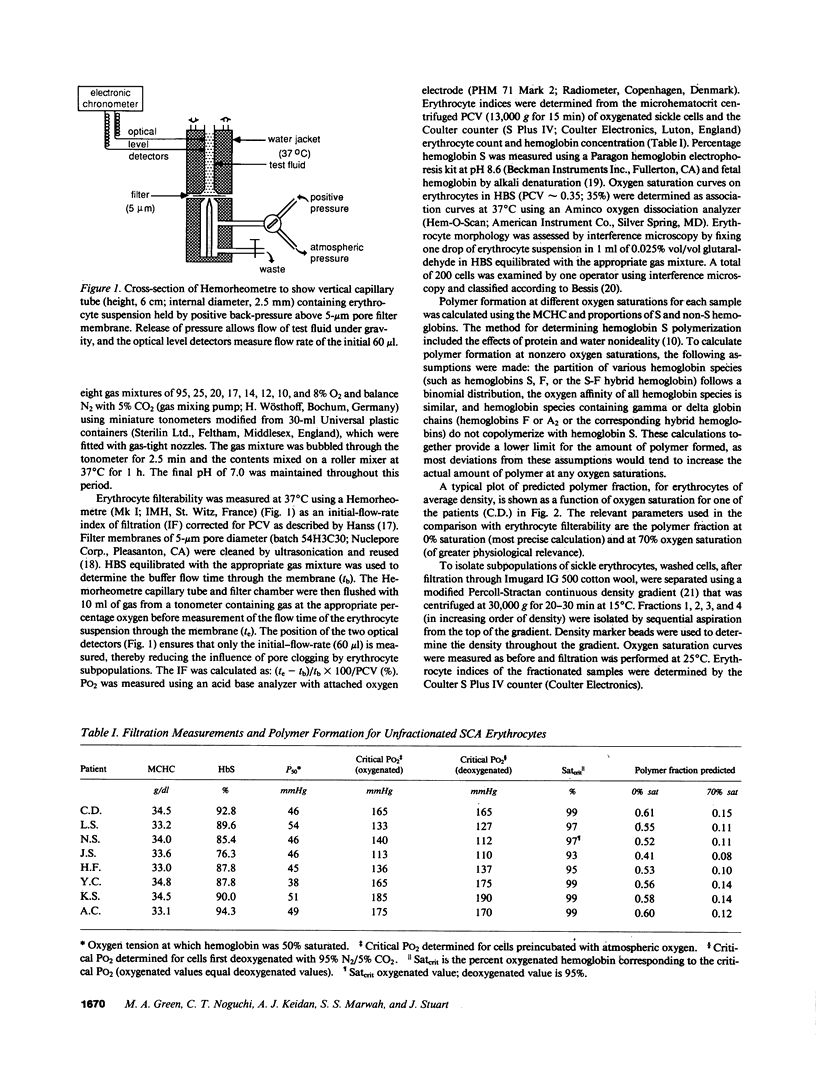
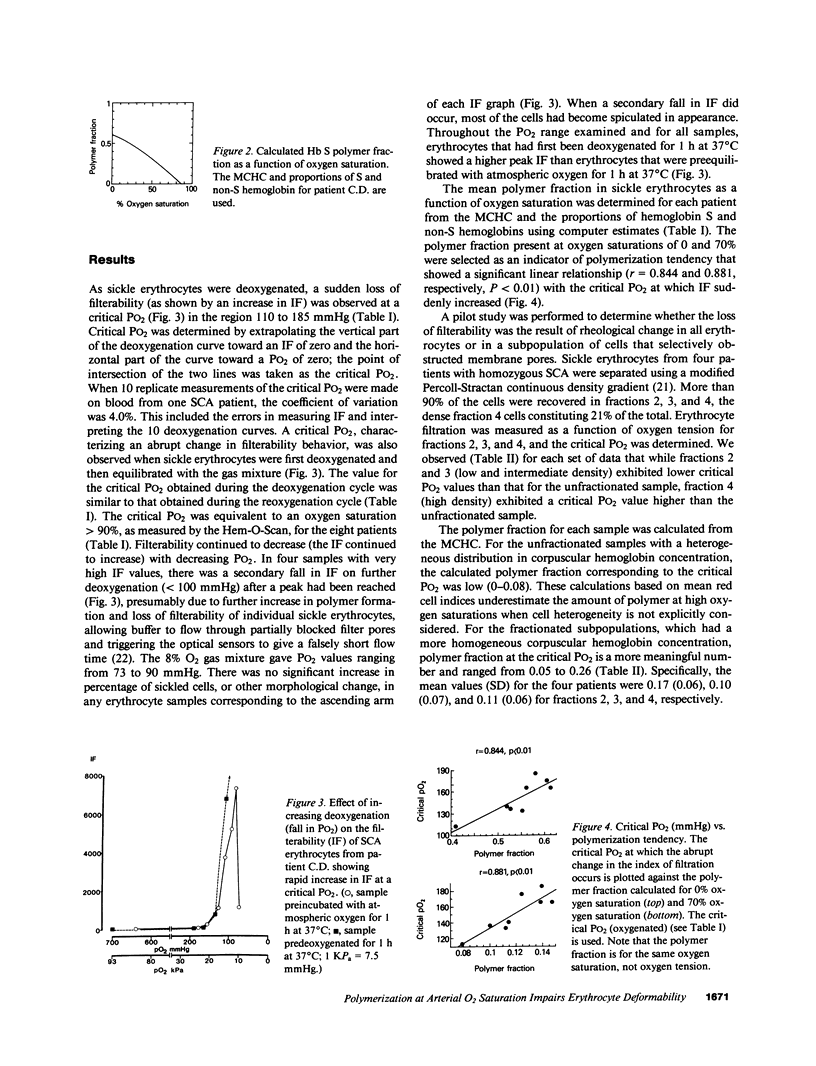
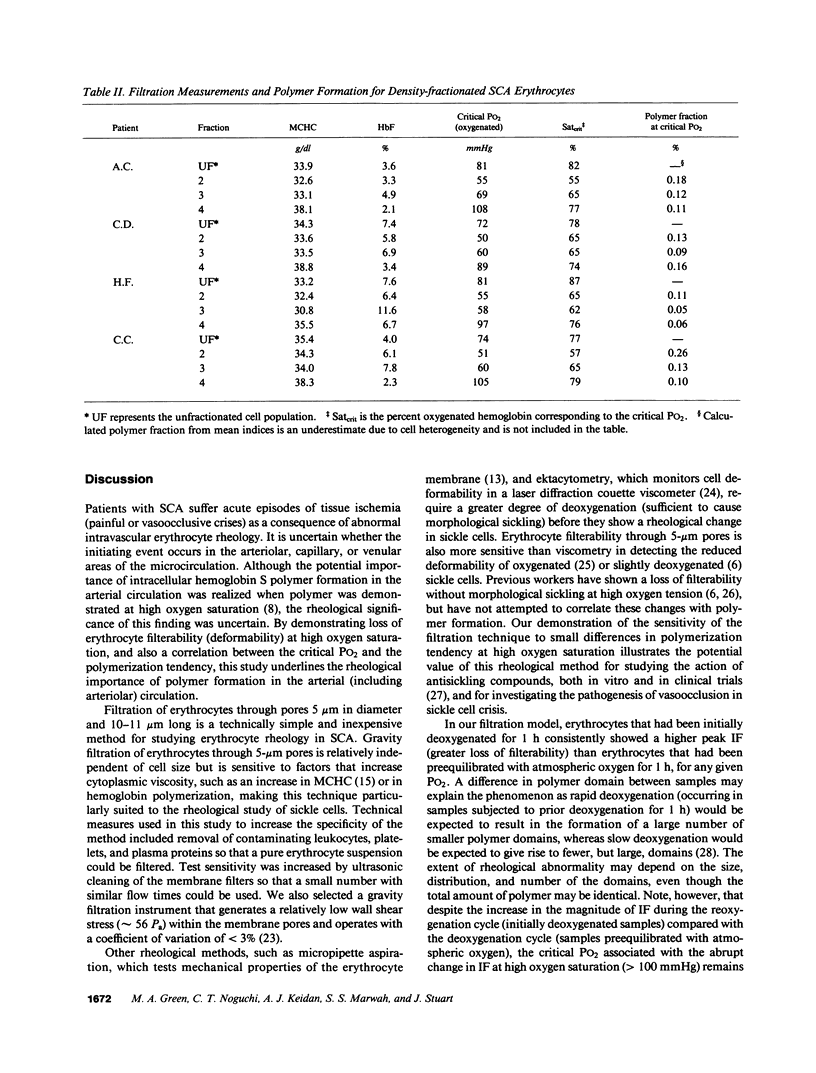


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertles J. F., Döbler J. Reversible and irreversible sickling: a distinction by electron microscopy. Blood. 1969 Jun;33(6):884–898. [PubMed] [Google Scholar]
- Bessis M., Feo C., Jones E., Nossal M. Adaptation of the ektacytometer to automated continuous pO2 changes: determination of erythrocyte deformability in sickling disorders. Cytometry. 1983 Jan;3(4):296–299. doi: 10.1002/cyto.990030412. [DOI] [PubMed] [Google Scholar]
- Bessis M. Red cell shapes. An illustrated classification and its rationale. Nouv Rev Fr Hematol. 1972 Nov-Dec;12(6):721–745. [PubMed] [Google Scholar]
- Brittenham G. M., Schechter A. N., Noguchi C. T. Hemoglobin S polymerization: primary determinant of the hemolytic and clinical severity of the sickling syndromes. Blood. 1985 Jan;65(1):183–189. [PubMed] [Google Scholar]
- Clark M. R., Guatelli J. C., Mohandas N., Shohet S. B. Influence of red cell water content on the morphology of sickling. Blood. 1980 May;55(5):823–830. [PubMed] [Google Scholar]
- Ferrone F. A., Hofrichter J., Eaton W. A. Kinetics of sickle hemoglobin polymerization. I. Studies using temperature-jump and laser photolysis techniques. J Mol Biol. 1985 Jun 25;183(4):591–610. doi: 10.1016/0022-2836(85)90174-3. [DOI] [PubMed] [Google Scholar]
- Glader B. E., Nathan D. G. Cation permeability alterations during sickling: relationship to cation composition and cellular hydration of irreversibly sickled cells. Blood. 1978 May;51(5):983–989. [PubMed] [Google Scholar]
- Hanss M. Erythrocyte filtrability measurement by the initial flow rate method. Biorheology. 1983;20(2):199–211. doi: 10.3233/bir-1983-20209. [DOI] [PubMed] [Google Scholar]
- Keidan A. J., Franklin I. M., White R. D., Joy M., Huehns E. R., Stuart J. Effect of BW12C on oxygen affinity of haemoglobin in sickle-cell disease. Lancet. 1986 Apr 12;1(8485):831–834. doi: 10.1016/s0140-6736(86)90941-4. [DOI] [PubMed] [Google Scholar]
- Martin-Caburi J., Hermann T., Garel M. C., Domenget C., Galacteros F., Healy J. C., Hanss M., Beuzard Y. Utilisation d'un nouveau rhéomètre pour étudier la filtrabilité d'une suspension de globules rouges drepanocytaires en fonction de la PO2. C R Seances Acad Sci III. 1982 Oct 11;295(5):355–358. [PubMed] [Google Scholar]
- Molden D. P., Alexander N. M., Neeley W. E. Fetal hemoglobin: optimum conditions for its estimation by alkali denaturation. Am J Clin Pathol. 1982 May;77(5):568–572. doi: 10.1093/ajcp/77.5.568. [DOI] [PubMed] [Google Scholar]
- Nash G. B., Johnson C. S., Meiselman H. J. Influence of oxygen tension on the viscoelastic behavior of red blood cells in sickle cell disease. Blood. 1986 Jan;67(1):110–118. [PubMed] [Google Scholar]
- Noguchi C. T. Polymerization in erythrocytes containing S and non-S hemoglobins. Biophys J. 1984 Jun;45(6):1153–1158. doi: 10.1016/S0006-3495(84)84263-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. Sickle hemoglobin polymerization in solution and in cells. Annu Rev Biophys Biophys Chem. 1985;14:239–263. doi: 10.1146/annurev.bb.14.060185.001323. [DOI] [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. The intracellular polymerization of sickle hemoglobin and its relevance to sickle cell disease. Blood. 1981 Dec;58(6):1057–1068. [PubMed] [Google Scholar]
- Noguchi C. T., Torchia D. A., Schechter A. N. Determination of deoxyhemoglobin S polymer in sickle erythrocytes upon deoxygenation. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5487–5491. doi: 10.1073/pnas.77.9.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi C. T., Torchia D. A., Schechter A. N. Intracellular polymerization of sickle hemoglobin. Effects of cell heterogeneity. J Clin Invest. 1983 Sep;72(3):846–852. doi: 10.1172/JCI111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi S. T. Viscosity and filtrability measurements of sickle-cell suspensions in the development of anti-sickling drugs. Blood Cells. 1982;8(1):79–87. [PubMed] [Google Scholar]
- Reinhart W. H., Chien S. Roles of cell geometry and cellular viscosity in red cell passage through narrow pores. Am J Physiol. 1985 May;248(5 Pt 1):C473–C479. doi: 10.1152/ajpcell.1985.248.5.C473. [DOI] [PubMed] [Google Scholar]
- Sorette M. P., Lavenant M. G., Clark M. R. Ektacytometric measurement of sickle cell deformability as a continuous function of oxygen tension. Blood. 1987 Jan;69(1):316–323. [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Ferrone F. A., Eaton W. A. Oxygen binding by sickle cell hemoglobin polymers. J Mol Biol. 1982 Jun 25;158(2):251–273. doi: 10.1016/0022-2836(82)90432-6. [DOI] [PubMed] [Google Scholar]