Abstract
Cigarette smoking is the leading preventable cause of death, disease, and disability worldwide. It is well established that cigarette smoke provokes inflammatory activation and impairs antimicrobial functions of human immune cells. Here we explore whether cigarette smoke likewise affects the virulence properties of an important human pathogen, Staphylococcus aureus, and in particular methicillin-resistant S. aureus (MRSA), one of the leading causes of invasive bacterial infections. MRSA colonizes the nasopharynx and is thus exposed to inhalants, including cigarette smoke. MRSA exposed to cigarette smoke extract (CSE-MRSA) was more resistant to macrophage killing (4-fold higher survival; P < 0.0001). CSE-MRSA demonstrated reduced susceptibility to cell lysis (1.78-fold; P = 0.032) and antimicrobial peptide (AMP) (LL-37) killing (MIC, 8 μM versus 4 μM). CSE modified the surface charge of MRSA in a dose-dependent fashion, impairing the binding of particles with charge similar to that of AMPs by 90% (P < 0.0001). These changes persisted for 24 h postexposure, suggesting heritable modifications. CSE exposure increased hydrophobicity by 55% (P < 0.0001), which complemented findings of increased MRSA adherence and invasion of epithelial cells. CSE induced upregulation of mprF, consistent with increased MRSA AMP resistance. S. aureus without mprF had no change in surface charge upon exposure to CSE. In vivo, CSE-MRSA pneumonia induced higher mouse mortality (40% versus 10%) and increased bacterial burden at 8 and 20 h postinfection compared to control MRSA-infected mice (P < 0.01). We conclude that cigarette smoke-induced immune resistance phenotypes in MRSA may be an additional factor contributing to susceptibility to infectious disease in cigarette smokers.
INTRODUCTION
Cigarette smoking is the leading preventable cause of death, disease, and disability worldwide. In 2010, approximately 6 million deaths worldwide were attributed to cigarette smoking and second-hand exposure (1). It is estimated that by 2020 and 2030, 7.5 and 8 million people, respectively, will be dying from cigarette smoke annually (2). Worldwide, the prevalence of smoking ranges from 19 to 51%. In the United States alone, the prevalence of smoking is 20% and costs an estimated $96 billion in direct health care and $97 billion in lost productivity annually (3). Forty percent of children worldwide are regularly exposed to secondhand cigarette smoke, and it is estimated that 600,000 people die from secondhand smoke exposure every year (4).
Both direct and second-hand cigarette smoke exposures increase the risk and severity of developing respiratory tract and other invasive infections. Invasive pneumococcal disease is 2- to 4-fold more common in cigarette smokers, and increased susceptibility to influenza and tuberculosis has also been documented (5, 6). The mechanistic relationship between increased susceptibility to infection and cigarettes has yet to be elucidated; however, prior studies have focused on changes induced in the host.
Cigarette smoking increases mucus production, impairs epithelial elastic properties, decreases IgA production, and affects phagocyte activities (6). These changes facilitate bacterial colonization and exacerbate inflammatory responses leading to epithelial damage, further impairing host immunity and promoting bacterial colonization of the respiratory tract. This continuous loop ultimately leads to chronic inflammation and increased bacterial colonization of the lungs. Lastly, direct and second-hand cigarette smoke exposures alter the normal composition of the nasopharyngeal microflora, opening the door to opportunistic pathogens such as Staphylococcus aureus, Haemophilus influenzae, and Streptococcus pneumoniae (5, 6).
S. aureus is a Gram-positive coccus that causes a broad range of infections, including invasive disease such as pneumonia, bacteremia, and endocarditis (7). Methicillin-resistant S. aureus (MRSA) is associated with a higher mortality rate in invasive infections than methicillin-sensitive S. aureus (MSSA) (8–10). Also, in severe staphylococcal pneumonia, MRSA outnumbers MSSA as the pathogen, accounting for 57% of cases (11). There were >75,000 cases of invasive MRSA infection, leading to nearly 10,000 deaths, in the United States in 2012 (3).
Smokers have higher rates of MRSA colonization than nonsmokers, thus increasing their risk of serious and difficult-to-treat infections (12). Smokers with acute and chronic sinusitis have a higher incidence of S. aureus and MRSA as the pathogen than nonsmokers (13). Children with cystic fibrosis exposed to secondhand smoke more frequently have MRSA grow from respiratory cultures (14). Smokers who are nasal swab positive for MRSA are at high risk for postoperative MRSA infections (15). These clinical data are further supported by in vitro findings by Kulkarni et al. (16), who found that S. aureus exposed to cigarette smoke had increased binding to human cells and biofilm formation, demonstrating that cigarette smoke increases the ability of MRSA to colonize and persist in the human host.
Colonization by MRSA is as common in the community as in the hospital setting (17, 18). All skin and mucosal surfaces can be colonized by MRSA, with nasal carriage being the most common (19–21). It is estimated that 20% of the general population is persistently colonized in the nasopharynx by S. aureus, and an additional 30% are transiently colonized (22). MRSA from the nasopharynx can travel to the lungs to cause pneumonia. In addition, MRSA may be transferred onto other epithelial surfaces, where upon epithelial compromise (cuts or breaks) the MRSA may invade deeper tissues, leading to necrotizing fasciitis or sepsis.
As an alternative hypothesis to the current paradigm that cigarette smoke causes disease primarily via changes to the host, we turned to the other side of the host-pathogen dynamic to determine whether cigarette smoke promotes bacterial virulence. Because MRSA causes severe pneumonia more often than MSSA and because MRSA commonly colonizes the nasopharynx and airways and thus is exposed to inhaled substances, we examined the effect of cigarette smoke on MRSA resistance to host innate immune killing mechanisms and the ability to cause pneumonia in mice.
MATERIALS AND METHODS
CSE preparation.
We conducted a literature review of in vitro cigarette smoke studies and based our design on the most accepted methods of cigarette smoke extract (CSE) preparation. We added 20% Todd-Hewitt broth (THB) to the base mammalian medium, RPMI plus 10% fetal bovine serum (FBS), as bacterial growth kinetics are stunted in its absence. A 60-ml syringe (BD) containing 10 ml base medium was connected to a research cigarette (University of Kentucky, 3R4F), and 50 ml smoke was aspirated, agitated for 15 s, and expelled. This process was repeated until 1 cm remained. The 10 ml of medium exposed to 1 cigarette is referred to as 100% CSE medium, in keeping with prior literature. Absorbance (at 320 nm) and pH were recorded to document consistency between batches, and medium was filtered (0.45 μm) and used immediately. The solid portion (CSE sediment) was acquired by spinning at 4,000 rpm for 30 min and resuspending the pellet in 10 ml. For 24-h-old CSE, CSE was stored at 4°C for 24 h to allow gases to escape.
Cultures.
Overnight cultures of MRSA USA300 were grown in THB at 37°C with shaking. Same-day subcultures were prepared in concentrations of CSE from 0 to 100% and grown to mid-log phase (optical density at 600 nm [OD600], 0.6 to 0.8) before centrifugation (3,000 rpm, 10 min) and resuspension at 1 × 108 CFU/ml (OD600, 0.4) prior to dilution in assay medium to the final CFU/ml. MRSA growth was halted by 100% CSE; therefore, 75% CSE was utilized as the maximum concentration. This concentration is similar to those used in mammalian cell studies, which led to carcinogenic and immune response findings that were confirmed by human subject studies. For enumeration, MRSA was serially diluted and plated on Todd-Hewitt agar (THA). For MH-S (ATCC CRL-2019) phagocytosis, MRSA USA300 TCH 1516 pcm29 (MRSA-green fluorescent protein [GFP]) was utilized. Wild-type (WT) SA113 and SA113 ΔmprF, the knockout of mprF, were used in some surface charge studies. MH-S cells were cultured in RPMI plus 10% FBS plus 0.05 mM beta-mercaptoethanol. HaCaT (CLS) and A549 (ATCC CCL-185) cells were cultured in RPMI plus 10% FBS.
Macrophage killing of bacteria.
Alveolar macrophages (MH-S) were plated at 1 × 105 cells/well in RPMI plus 2% FBS the day prior to the assay and were prestimulated for 45 min with 16 μM phorbol myristate acetate (PMA). Prior to macrophage infection with bacteria, 1 × 108 CFU MRSA were opsonized via incubation at 37°C with gentle shaking in 90% mouse serum (Applied Biosystems) for 30 min. MH-S cells were infected with preopsonized MRSA at a multiplicity of infection (MOI) of 1 and placed back at 37°C with 5% CO2. Starting at 100 min postinfection, total surviving bacteria were harvested from each well at 40-min intervals. In parallel, MH-S cells were harvested hourly and evaluated for eukaryotic cell death via trypan blue exclusion.
Macrophage phagocytosis.
MH-S cells were plated at 1 × 106 cells/well in a 24-well plate (Corning). MRSA-GFP was added at an MOI of 10 and cells incubated at 37°C with 5% CO2 for 1 h. Macrophages were washed with 1 ml phosphate-buffered saline (PBS) three times to remove adherent bacteria. Cells were trypsinized and run on a fluorescence-activated cell sorter (FACS) (BD) to quantify internalized MRSA-GFP.
Bacterial growth curves and H2O2 sensitivity.
Overnight MRSA cultures were diluted 1:100 in 0%, 25%, 50%, 75%, and 100% CSE and incubated at 37°C with shaking. The OD600 was recorded at 30-min intervals until the control reached the plateau phase. For H2O2 sensitivity testing, MRSA was grown in 0% and 75% CSE to an OD600 of 0.6 to 0.8 and then treated with 0.04% H2O2 for 30 min at room temperature, with surviving bacteria plated for enumeration.
Bacterial cell lysis.
Bacteria were grown in medium plus 1 M NaCl to an OD600 of 1.7 to 1.9 (late log phase). MRSA cells were spun at 3,200 rpm for 10 min at 4°C, washed twice with 4°C water, and resuspended to an OD600 of 2.0 in 50 mM Tris-HCl (pH 7.2) with 0.05% Triton X-100. Absorbance at OD580 was recorded every 30 min throughout incubation at 30°C with shaking.
Antimicrobial peptide (AMP) (LL-37) resistance assays.
MRSA was added to plates at 2 × 106 CFU per ml of RPMI plus 5% tryptic soy broth. MIC plates were incubated at 37°C overnight with shaking, and then 675 μg resazurin (Sigma) was added and left for 8 h to evaluate metabolic activity. Bacteria were plated on agar to determine the minimum bactericidal concentration (MBC). Killing kinetic plates were incubated with shaking at 37°C, with samples taken every hour to monitor bacterial growth.
Surface charge of MRSA.
MRSA was grown in 0%, 25%, 50%, and 75% CSE, as well as 24-h-old CSE, CSE precipitate, and 3 mg and 6 mg nicotine, to an OD600 of 0.6 to 0.8. For hereditary studies, MRSA were exposed to THB or 75% CSE daily for 4 days, 3 days, or 24 h prior to evaluation. After each exposure, bacteria were spun down and resuspended in regular THB for incubation at 37°C overnight with shaking. On the day of evaluation, bacteria were not reexposed to CSE. SA113 and SA113 ΔmprF strains were grown in 0% and 75% CSE to an OD600 of 0.6 to 0.8, with ampicillin in the medium for the ΔmprF strain to select for mprF knockouts. Bacteria were washed three times in 0.02 M HEPES (pH 7.5) and resuspended to an OD600 of 0.3, and 2 μM poly-l-lysine (PLL)-fluorescein isothiocyanate (FITC) (Sigma) added. Tubes were vortexed every 5 min for 15 min in the dark. Cells were pelleted and resuspended, and PLL-FITC binding was quantified via FACS.
Bacterial hydrophobicity.
MRSA was resuspended in PBS to an OD600 of 0.7. In 1.5-ml tubes, cells were vortexed for 2 min with 25% n-hexadecane. After 30 min of incubation, a sample was taken from the lower aqueous layer to enumerate the bacteria.
Adherence and invasion of human skin cells.
HaCaT (human keratinocyte) cells were plated at 2 × 105 cells/well in RPMI plus 2% FBS in 24-well plates. MRSA was grown to stationary phase and added at an MOI of 10. For adherence, cells were incubated for 30 min at 37°C with 5% CO2. Cells were washed three times, harvested with trypsin-EDTA, and lysed by pipetting up and down in the presence of 0.025% Triton X-100. For invasion, infected cells were incubated for 2 h at 37°C with 5% CO2. Supernatants were aspirated, and 100 U/ml penicillin, 100 μg/ml streptomycin, and 10 μg/ml gentamicin were added to kill extracellular bacteria for 2 h. Cells were washed five times with PBS, harvested with trypsin-EDTA, and lysed with 0.025% Triton X-100. In parallel, at both time points, additional wells were harvested and evaluated for eukaryotic cell death via trypan blue exclusion.
qRT-PCR of MRSA.
Total RNA was extracted from MRSA grown to an OD600 of 0.7. cDNA was made using SuperScript III first-strand synthesis SuperMix (Life Technologies). Primers for the atl, lytM, walK/R, mprF, and 16S rRNA genes (23, 24) were used for reverse transcription-quantitative PCR (qRT-PCR) with SYBR Fast (KAPA Biosystems). Relative quantification (RQ) scores were calculated through comparative threshold cycle (ΔΔCT) analysis with control MRSA expression set at 1.
Murine pneumonia infection model.
Six- to 10-week-old female CD-1 mice (Charles River) were sedated with ketamine-xylazine and infected intranasally with 1 × 108 CFU MRSA in 100 μl. Mice were kept upright for 1 min and recovered with heads elevated at 30°. Mice were weighed at 0, 8, and 24 h. For bacterial burden studies, 5 mice per group were sacrificed at 8 and 20 h via CO2 asphyxiation. Left lungs were inflated with 10% formalin for histology. Right lungs were weighed and then homogenized using 1.0-mm zirconia-silica beads (Bio Spec Products) and MagNA Lyser (F. Hoffmann-La Roche). Lung homogenates were serially diluted and plated on THA to enumerate surviving bacteria. Experiments were repeated twice. All methods were approved by the VA IACUC, protocol 11-017, and all efforts were made to minimize animal suffering.
Statistical analyses.
All in vitro experiments were done in triplicate and repeated three times. Phagocytosis, hydrophobicity, and PLL binding were analyzed via one-way analysis of variance (ANOVA) with Dunnett's multiple comparisons. MH-S killing and PLL binding of 24-h-old CSE, CSE sediment, and nicotine, as well as 24-h exposure and SA113 strain treatments, were analyzed via two-way ANOVA with Bonferroni's multiple comparisons. LL-37 killing kinetics were analyzed via two-way ANOVA with Sidak's test for multiple comparisons. Growth curves were analyzed with the Friedman test. Resistance to H2O2 and CFU recovered from mouse lungs were analyzed with the Mann-Whitney test. For adherence and invasion assays, unpaired two-tailed t tests with equal standard deviations were used. Mouse pneumonia (n = 20 per group) survival analyses was done with the Mantel-Cox log rank test.
RESULTS
Cigarette smoke increases MRSA resistance to killing by alveolar macrophages.
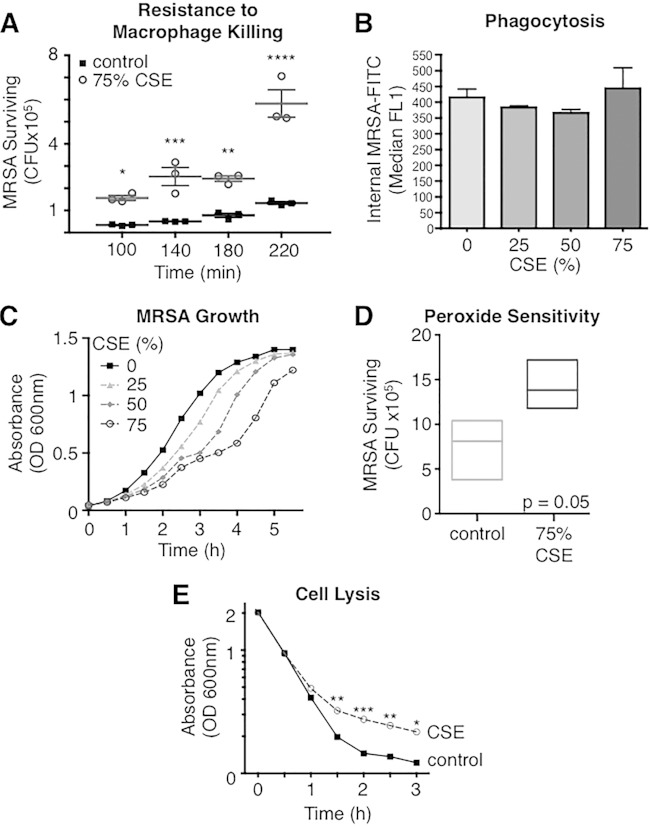
Alveolar macrophages are the most common cell type within airways and are the front line of defense in pulmonary bacterial infections (25). These innate immune cells have multiple mechanisms by which they clear bacteria from airways: phagocytosis, respiratory burst (26), AMP production (27), and extracellular trap formation (28). MRSA is a frequent colonizer of nasal and lower respiratory tract passages and is thus exposed to cigarette smoke when a patient smokes. In vitro exposure of MRSA to CSE decreased its overall susceptibility to killing by murine alveolar macrophages (MH-S cells) relative to the control. Starting from an MOI of 1 × 105, control MRSA was killed over the first 100 min, while MRSA exposed to CSE (CSE-MRSA) resisted macrophage killing and grew, leading to a 4-fold higher level of total CSE-MRSA surviving versus control MRSA at 220 min (P < 0.0001) (Fig. 1A). Macrophage cell death was evaluated during the assays, and no difference was found between macrophages infected with control MRSA and CSE-exposed MRSA (5.5% versus 5.9%, respectively). To evaluate whether the increase in MRSA resistance to macrophage killing from CSE exposure was mediated by decreased phagocytosis, we infected MH-S cells with MRSA-GFP. Phagocytosis of CSE-exposed MRSA-GFP by MH-S cells was no different than that of control MRSA-GFP (P = 0.5) (Fig. 1B). These data suggest that although CSE-MRSA is phagocytosed at the same rate as control MRSA, the CSE-MRSA bacteria are less susceptible to intracellular killing.
FIG 1.
Cigarette smoke exposure reduces MRSA susceptibility to macrophage killing and lysis while suppressing bacterial growth. (A) Starting with an MOI of 1 × 105, control MRSA was killed by alveolar macrophages over 100 min, while MRSA exposed to 75% CSE resisted killing and overgrew. By 220 min, CFU of control MRSA were 4-fold lower than those of CSE-MRSA. (B) CSE exposure did not change the rate of MRSA-GFP phagocytosis by macrophages. (C) The increased numbers of MRSA during killing assays was not due to increased growth, as CSE decreased the rate of MRSA growth in a dose-dependent manner. (D) CSE exposure tended to increase MRSA resistance to killing by H2O2 (oxygen radicals). (E) CSE induced resistance to MRSA cell lysis in the presence of detergent. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Cigarette smoke dose-dependently inhibits growth of MRSA.
Increased survival of CSE-treated MRSA in the face of macrophage killing could reflect increased growth of the pathogen following smoke exposure. To exclude this possibility, we grew MRSA in control bacteriologic medium as well as increasing concentrations of CSE. CSE inhibits MRSA growth in a dose-dependent manner compared to the case for MRSA grown in control medium (P < 0.0001) (Fig. 1C). Thus, increased resistance of CSE-MRSA to macrophage killing is not due to accelerated bacterial growth. In fact, the suppression of bacterial growth by cigarette smoke may be protective, as multiple antimicrobial mechanisms require bacterial cell division (29). In particular, Kristian et al. demonstrated that Staphylococcus aureus becomes more resistant to AMPs when growth is suppressed via antibiotic treatment (30).
Cigarette smoke exposure may reduce MRSA susceptibility to oxygen species.
Another mechanism by which macrophages kill bacteria is via oxygen radical exposure in the phagolysosome. We evaluated resistance to oxygen species by incubating MRSA with H2O2 for 30 min. MRSA exposed to 75% CSE trended toward increased resistance to killing compared to control MRSA (P = 0.05) (Fig. 1D).
MRSA exposed to cigarette smoke is resistant to cell lysis.
Bacteria that are harder to destroy, i.e., those that have cell walls that are resistant to penetration by detergents, may be better at persisting/colonizing and thus more likely to successfully cause invasive infection. We exposed control and CSE-MRSA to a common detergent (Triton X-100) and found that there were similar rates of death for the two until 1.5 h, when the death rate of CSE-MRSA leveled off with higher numbers of surviving bacteria than for the control (P < 0.01) (Fig. 1E). This supports the hypothesis that cigarette smoke induces stress on bacteria, leading to cell wall changes, which make it less amenable to insertion of detergents and thus cell lysis.
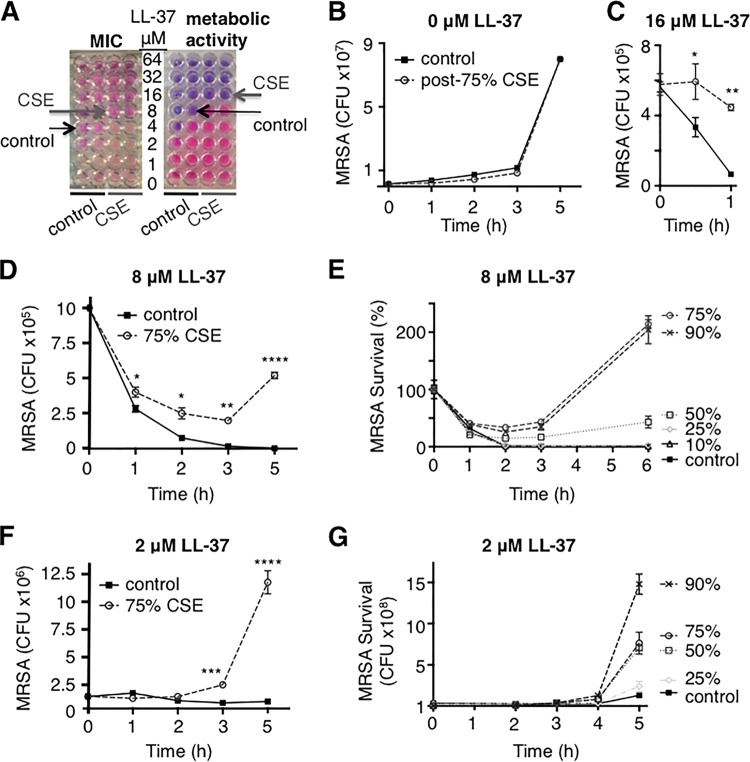
Cigarette smoke exposure increases resistance of MRSA to AMPs.
AMPs are important elements of innate immunity and are produced by a variety of cells, including macrophages, neutrophils, and epithelial cells. Without AMPs, macrophages are less able to kill bacteria, and when bacterial growth is suppressed, bacteria are more resistant to AMP killing (30). Thus, we hypothesized that our slower-growing CSE-MRSA would have decreased susceptibility to AMPs and that this would be the mechanism by which macrophages are less able to kill CSE-MRSA. Utilizing the human AMP LL-37, we found a 2-fold increase in LL-37 MIC upon exposure of MRSA to CSE (8 μM versus 4 μM) (Fig. 2A). Metabolic activity was also inhibited at a 2-fold-higher level of LL-37 (Fig. 2A), which corresponded with an elevated MBC for CSE-exposed MRSA (see Fig. S1 and Table S2 in the supplemental material). These changes in susceptibility to AMPs were not due to differences in growth, as MRSA grew at the same rate as the control once CSE was removed from the medium (Fig. 2B). We evaluated the killing kinetics of LL-37 at the MBC (16 μM), and control MRSA was rapidly killed while CSE-MRSA was resistant (Fig. 2C). When bacteria were exposed to both the MIC (8 μM) and a sub-MIC (2 μM), CSE-MRSA was able to escape LL-37 killing while control MRSA was not (P < 0.0001) (Fig. 2D and F). This resistance was CSE dose dependent (Fig. 2E and G). Thus, cigarette smoke induced changes to MRSA in a dose-dependent fashion, which led to protection from AMP killing.
FIG 2.
Cigarette smoke exposure increases resistance of MRSA to human AMP LL-37. (A) CSE exposure increased the MIC of MRSA to LL-37 from 4 to 8 μM and increased the concentration needed to inhibit metabolic activity from 8 to 16 μM. (B) Growth of control MRSA and MRSA after exposure to 75% CSE was identical during the AMP growth kinetics assays, which were run without CSE present. (C, D, and F) Prior exposure to 75% CSE allowed MRSA to escape killing by LL-37 at 16 μM (C), 8 μM (D), and 2 μM (F). (E and G) These effects were CSE dose dependent, with exposure to 50% through 90% CSE inducing resistance to AMP killing at both 8 μM (E) and 2 μM (G). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
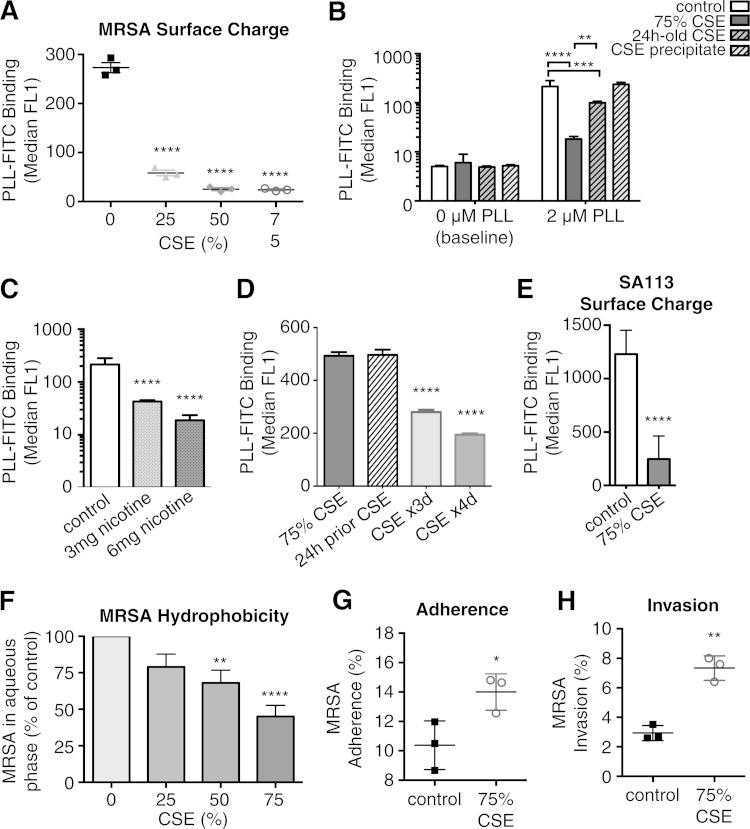
Cigarette smoke exposure alters MRSA surface charge.
AMP binding is strongly influenced by surface charge (31, 32). In general, bacteria have negatively charged surfaces, which are targeted by positively charged AMPs. Thus, one virulence mechanism is to make the surface charge more positive to decrease the attraction and attachment of AMPs (33, 34). The binding of cationic PLL to the surfaces of MRSA and CSE-MRSA was utilized as a measurement of surface charge change. A dose-dependent (5- to 11-fold) decrease in PLL-FITC binding was observed in CSE-MRSA, demonstrating that the surface charge of MRSA became more positive upon smoke exposure (P < 0.0001) (Fig. 3A). Background fluorescence, with no PLL-FITC added, was equivalent among groups (Fig. 3B).
FIG 3.
Cigarette smoke exposure induces MRSA surface changes, including a less negative surface charge and increased hydrophobicity, leading to increased adherence and invasion of epithelial cells. (A) CSE-MRSA binds less of cationic PLL-FITC, consistent with alteration in surface charge (less negative = more positive surface charge) in response to CSE exposure, in a dose-dependent manner. (B) Background fluorescence (no PLL added) was equal among groups. CSE precipitate alone had no impact on surface charge, with equivalent surface binding of 2 μM PLL as control MRSA. However, 24-h-old CSE induced a 2-fold shift in surface charge, but not the 12-fold shift induced by freshly made CSE. (C) Nicotine alone induced shifts in MRSA surface charge leading to less PLL-FITC binding (5-fold by 3 mg and 11-fold by 6 mg), consistent with nicotine contributing to CSE-induced surface charge changes. (D) Surface charge changes induced by CSE exposure persisted after passage into THB two times, for 24 h after exposure, and repetitive daily exposures for 3 and 4 days further enhanced surface charge changes. (E) S. aureus strain SA113 had similar changes in PLL-FITC binding upon exposure to CSE. (F) Exposure to CSE induced increasing hydrophobicity in a dose-dependent manner, as evidenced by fewer bacteria recovered from the aqueous phase as the CSE concentration was increased. (G and H) CSE exposure increased adherence of MRSA to epithelial cells (G) and increased MRSA invasion of and persistence within epithelial cells (H). *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
To evaluate which components of CSE induced the surface charge change, MRSA was exposed to CSE precipitate alone, which had no effect on surface charge (P = nonsignificant). This suggested that larger, less-soluble CSE components did not play a role. We stored CSE for 24 h at 4°C to allow gases to escape and found that exposure to 24-h-old CSE made the surface of MRSA more positive (P < 0.001) (Fig. 3B). However, 24-h-old CSE did not induce as potent of a surface charge change as fresh CSE (P < 0.01) (Fig. 3B). This suggests that of the >4,000 components of cigarette smoke, gases such as carbon monoxide and oxygen radicals may be contributing to surface charge changes. Upon incubation with nicotine alone, a soluble component that would be present in 24-h-old CSE medium, the MRSA surface charge became 5-fold and 11-fold less negative (3 mg and 6 mg nicotine, respectively), suggesting that nicotine also contributes to surface charge change (P < 0.01) (Fig. 3C).
To evaluate whether changes induced by cigarette smoke exposure are persistent and potentially heritable, MRSA was exposed to CSE once, transferred to THB overnight, and grown again in THB on the day of surface charge evaluation. MRSA exposed to CSE 24 h prior had decreased surface charge, similar to the case for MRSA exposed to CSE for only 3 h immediately prior to surface charge evaluation (Fig. 3D). These findings demonstrate persistence of changes induced by the stress of cigarette exposure. When bacteria were exposed to CSE daily (to model exposure of colonizing MRSA of the nasopharynxes of cigarette smokers), surface charge changes became more pronounced (Fig. 3D). These data suggest that changes in MRSA induced by cigarette smoke may be heritable and that multiple smoke exposures may induce greater changes.
MRSA USA300 is one of many strains of S. aureus. To evaluate whether the effects of cigarette smoke are limited to this strain or are more broadly applicable, we exposed S. aureus strain SA113 to CSE. After CSE exposure, SA113 had surface charge modifications similar to those found in MRSA USA300 (Fig. 3E).
Cigarette smoke exposure increases MRSA hydrophobicity while increasing MRSA adherence and invasion.
Another mechanism by which S. aureus avoids AMP killing is by expressing proteins that decrease cell surface hydrophobicity (35). However, we observed that CSE increased hydrophobicity in a dose-dependent manner, with fewer MRSA bacteria remaining in the aqueous layer (P < 0.0001) (Fig. 3F). Interestingly, increased hydrophobicity has been linked to bacterial interactions with epithelial cells (36–38). Bacterial adherence to epithelial cells leads to colonization and is also the first step in developing invasive infections. MRSA commonly colonizes keratinocytes in the nasopharynx, axilla, and inguinal regions (39). Human keratinocyte cell line HaCaT is a well-established model for adherence and invasion by bacteria (40, 41). Therefore, we infected HaCaT cells and found increased adherence by CSE-MRSA (52% of CSE-MRSA adhered versus 28% of control cells; P < 0.05) (Fig. 3G). By infecting for longer periods of time, we determined that CSE-MRSA better invaded and persisted within HaCaT cells (7.2% CSE-MRSA versus 2.9% control; P < 0.01) (Fig. 3H). There were no differences in cell death between control- and CSE-MRSA-infected cells. Thus, one pathway by which cigarette smoke increases MRSA pathogenicity may be via increasing the ability to adhere to, colonize, and initiate infection of epithelial cells.
Cigarette smoke increases MRSA expression of genes linked to cell surface changes.
The mprF gene encodes a membrane protein that mediates the change of staphylococcal surface charge from negative to positive. mprF was upregulated in MRSA during exposure to CSE (2 h). CSE exposure led to 1.8-fold-increased expression of mprF compared to control results. Increased expression of mprF may be one mechanism by which CSE exposure leads to the surface charge of MRSA becoming cationic and to the corresponding increase in MIC and MBC to LL-37. Interestingly, dlt expression was unchanged (0.7 mRNA copy compared to control), suggesting that mprF-driven changes in surface charge may act through another pathway. Confirmation of mprF involvement in CSE-induced changes in S. aureus was obtained via experiments in the SA113 strain lacking mprF (SA113 ΔmprF). The surface charge of CSE-exposed SA113 ΔmprF was unchanged compared to that of nonexposed SA113 ΔmprF (see Fig. S3 in the supplemental material). These data suggest that mprF plays a significant role in the CSE induction of changes in S. aureus.
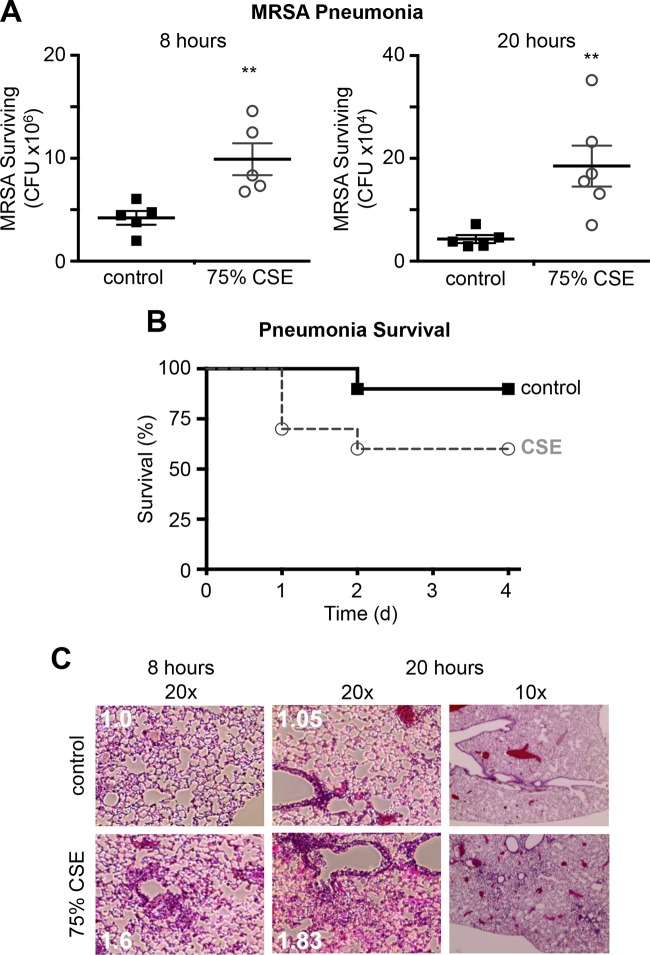
Cigarette smoke exposure increases virulence in a mouse model of pneumonia.
To evaluate the effect of cigarette smoke exposure on MRSA virulence in a physiologic model, we infected CD-1 mice intranasally with control MRSA and CSE-MRSA in a mouse model of pneumonia. The initial inoculum of bacteria was identical for control MRSA- and CSE-MRSA-infected mice; therefore, bacterial counts in the lungs at time zero were equal between groups. CSE-MRSA-infected mice had CFU counts 2-fold higher than those in controls at 8 h (9.9 × 106 versus 4.2 × 106; P = 0.0079) and 4-fold higher at 20 h (18.5 × 104 versus 4.3 × 104; P = 0.0087) (Fig. 4A). These differences in CFU at 8 and 20 h demonstrate that CSE-exposed MRSA was better able to survive in mouse lungs. These data are consistent with our in vitro findings in which CSE exposure increased MRSA resistance to macrophage killing and antimicrobial killing, thus conferring a significant advantage to CSE-MRSA in vivo. When we increased the initial inoculum, 40% of CSE-MRSA-infected mice died within 48 h of infection, while only 10% of control MRSA-infected mice died (n = 20 per group; P = 0.023) (Fig. 4B). The difference in mortality is consistent with CSE-MRSA being more resistant to killing and ultimately able to overwhelm the mouse immune system, while control MRSA is successfully cleared by the alveolar macrophages and other host defenses. Reduced clearance of CSE-MRSA compared to control MRSA led to higher bacterial counts and increased inflammation in the lungs of CSE-MRSA-infected mice histologically (Fig. 4C). Higher numbers of CSE-MRSA led to a greater influx of immune cells, increased inflammation, and finally death. The host inflammatory response does contribute to lung damage and risk of mortality in the clinical setting of pneumonia, but in our model we saw no evidence of an out-of-proportion increase in immune responses to CSE-MRSA. The inflammatory infiltrate increased in proportion with bacterial numbers. Therefore, we conclude that the exposure of MRSA to CSE increases the ability of this organism to survive in the host, via enhanced resistance to killing by innate immune mechanisms, and ultimately leads to increased disease pathology.
FIG 4.
MRSA exposed to CSE has increased virulence in a mouse model of pneumonia. (A) Lungs harvested at 8 and 20 h postinfection had higher numbers of CSE-MRSA (**, P < 0.01). (B) In a mortality model of pneumonia, 40% of mice infected with CSE-MRSA died, whereas 10% of controls survived (P = 0.021; n = 20 mice per group). (C) Histology of lungs from mice with MRSA pneumonia demonstrated more bacteria and inflammation in CSE-MRSA-infected mice at both the 8- and 20-h time points (n = 5 per group at each time point) (inflammation scores shown in white).
DISCUSSION
Cigarette smoke extract induces a general stress response in MRSA, leading to increased resistance to killing by macrophages and AMPs, as well as reduced lysis, culminating in increased virulence in vivo. CSE-induced MRSA resistance to killing may be due to surface charge changes in which the surface of MRSA becomes more positive in the presence of cigarette smoke, thus decreasing the affinity of cationic AMPs for their surface. Although in vitro cigarette smoke exposure reduced bacterial growth, when smoke was removed the growth returned to normal. Thus, suppressed bacterial growth as a mechanism by which MRSA avoids LL-37 killing does not play a role in our models. LL-37 is expressed in the airways, digestive tract, genitourinary system, circulating blood and, skin and thus acts at both the front line of defense (epithelial surfaces) and centrally for invasive infections. LL-37 has both direct and indirect antimicrobial properties (42, 43). S. aureus strains, including MRSA and SA113, are effectively killed by LL-37. The murine homologue of LL-37, cathelicidin-related AMP (CRAMP), requires higher MICs to kill S. aureus due to the positive-charge modifications to S. aureus cell walls (44). Even with this mild resistance to CRAMP at baseline, cigarette smoke exposure leads to further positive-charge modifications to the cell walls of MRSA, induced partially by nicotine content and partially by gases produced by cigarette smoke. These modifications ultimately make MRSA more aggressive and pathogenic when introduced into the airways of mice, leading to increased mortality and increased bacterial burden in our murine model of pneumonia.
The stress induced on MRSA by cigarette smoke increased hydrophobicity, leading to increased adherence (consistent with studies by Kulkarni et al. [16]) and subsequent invasion of epithelial cells. MRSA also became resistant to lysis and partially resistant to killing by oxygen radicals. These changes, i.e., hydrophobicity and resistance to lysis and oxygen radicals, are consistent with multiple changes induced in MRSA via the stress of being exposed to some of the >4,000 chemicals present in cigarette smoke, which are transferred to medium via our in vitro model. Our base medium was designed to take up cigarette smoke components to a similar degree as the layer of mucus which coats epithelial cells along the upper and lower airways. We found that the changes in MRSA induced by cigarette smoke exposure persisted even after the stress was removed, suggesting that cigarette smoke exposure induces heritable changes in MRSA. It has been shown that cigarette smoke and secondhand smoke exposures cause heritable changes in mice and rats (45–48). The data in humans are less vigorous, but one study demonstrated higher rates of asthma in children and grandchildren of women who smoked during pregnancy (49). Knowing that tobacco smoke causes long-standing changes to mammalian cells, it is consistent that smoke exposure could also lead to heritable, persistent changes in bacterial cells.
Previous work has shown that cigarette smoke modulates MRSA expression of genes involved in surface adhesion and biofilm formation (16). In our qRT-PCR studies, we found that CSE exposure induced mild upregulation of MRSA genes atl, lytM, walK/R, and mprF. The atl, lytM, and walK/R genes are directly involved in autolysis of the staphylococcal cell wall; however, our studies showed CSE-MRSA to have less autolysis (Fig. 1E). Atl and LytM are two peptidoglycan hydrolases that are regulated by the two-component regulatory system WalK/R. The predominant autolysin Atl plays an essential role in cell division (50), biofilm formation (50–52), and excretion of cytoplasmic proteins (53). Therefore, the mild increases in atl and lytM expression in CSE-MRSA either do not play a significant role in this system or are involved in nonautolysis functions in CSE-MRSA. These data also suggest that another, unknown, pathway may reduce autolysis in CSE-MRSA. MprF mediates an introduction of positive charges to MRSA phospholipids, membrane lipids which are normally anionic. Through MprF-mediated lysinylation, MRSA can become more resistant to human AMPs (54). Knocking out mprF in S. aureus removed all effects of CSE on surface charge, consistent with CSE inducing increased mprF expression in WT USA300, leading to surface charge modifications and, most likely, the resistance to LL-37.
Iron acquisition via binding of hemoglobin is necessary for S. aureus virulence. Smokers are known to have higher levels of carboxyhemoglobin (6.1 to 6.8%) than nonsmokers (1.3%) (55). S. aureus binds free hemoglobin (at nanomolar concentrations in the blood) via the surface-exposed iron-regulated surface determinant (Isd) system. Heme is extracted, brought inside the cell, and degraded within the cytoplasm to access the iron (56). IsdB in particular is one member of the Isd family that binds hemoglobin. Changes in the conformation of hemoglobin may change the affinity of IsdB for the protein. If IsdB is more efficient at extracting and transferring heme from carboxyhemoglobin, this may increase the virulence of S. aureus in the setting of the host smoking cigarettes and prove detrimental to the host. Therefore, the process of increased virulence in MRSA USA300 induced by cigarette smoke exposure may not be limited to the mucosal interactions that we have studied here.
Cigarette smoke changes the surface charge and hydrophobicity of the staphylococcal cell wall, which confers resistance to killing by AMPs. These changes may be a contributing factor to increased MRSA virulence in vivo and the increased risk of invasive MRSA infections in cigarette smokers. This increased risk is not isolated to lung infections, as MRSA exposed to cigarette smoke in the nasopharynx can be spread to any part of the body, and since we have demonstrated that the changes induced by cigarette smoke persist for 24 h and may be heritable, the specter of more virulent MRSA from the nasopharynx spreading to other parts of the body to cause disease is greater. We hope that these data act as a further impetus to cigarette smokers to quit; as the knowledge that cigarette smoke causes cancer, heart disease, and stroke has not been enough, possibly the concept of driving this antimicrobial resistant “superbug” to be more aggressive will be.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by VA Career Development Award 2, award 1IK2BX001313 (principal investigator, L. E. Crotty Alexander), from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Program.
We thank Tom Alexander for statistical analysis review and guidance, Timothy Bigby for technical help, and members of Victor Nizet's lab, in particular Ross Corriden and Simon Döhrmann, for technical support.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00303-15.
REFERENCES
- 1.Anonymous. 2014. The health consequences of smoking—50 years of progress: a report of the surgeon general. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, Atlanta, GA. [PubMed] [Google Scholar]
- 2.WHO. 2011. Report on the global tobacco epidemic. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 3.CDC. 2012. Emerging Infections Program Network. Methicillin-resistant Staphylococcus aureus. Active Bacterial Core Surveillance (ABC) report. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 4.Oberg M, Jaakkola MS, Woodward A, Peruga A, Pruss-Ustun A. 2011. Worldwide burden of disease from exposure to second-hand smoke: a retrospective analysis of data from 192 countries. Lancet 377:139–146. doi: 10.1016/S0140-6736(10)61388-8. [DOI] [PubMed] [Google Scholar]
- 5.Arcavi L, Benowitz NL. 2004. Cigarette smoking and infection. Arch Intern Med 164:2206–2216. doi: 10.1001/archinte.164.20.2206. [DOI] [PubMed] [Google Scholar]
- 6.Garmendia J, Morey P, Bengoechea JA. 2012. Impact of cigarette smoke exposure on host-bacterial pathogen interactions. Eur Respir J 39:467–477. doi: 10.1183/09031936.00061911. [DOI] [PubMed] [Google Scholar]
- 7.Durai R, Ng PC, Hoque H. 2010. Methicillin-resistant Staphylococcus aureus: an update. AORN J 91:599–606. (Quiz, 91: 607–599.) doi: 10.1016/j.aorn.2009.11.065. [DOI] [PubMed] [Google Scholar]
- 8.Porto JP, Santos RO, Gontijo Filho PP, Ribas RM. 2013. Active surveillance to determine the impact of methicillin resistance on mortality in patients with bacteremia and influences of the use of antibiotics on the development of MRSA infection. Rev Soc Brasil Med Trop 46:713–718. doi: 10.1590/0037-8682-0199-2013. [DOI] [PubMed] [Google Scholar]
- 9.Park DA, Lee SM, Peck KR, Joo EJ, Oh EG. 2013. Impact of methicillin-resistance on mortality in children and neonates with Staphylococcus aureus bacteremia: a meta-analysis. Infect Chemother 45:202–210. doi: 10.3947/ic.2013.45.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 11.Rodriguez-Collado W, Flores Anticona E, Farber A, Fang Y, Ghamande S, Arroliga AC. 2014. Severe health care associated MRSA pneumonia has the worst outcomes among all staphylococcal pneumonias. Chest 146:216A. doi: 10.1378/chest.1988948. [DOI] [Google Scholar]
- 12.Durmaz R, Tekerekoglu MS, Kalcioglu T, Ozturan O. 2001. Nasal carriage of methicillin-resistant Staphylococcus aureus among smokers and cigarette factory workers. New Microbiol 24:143–147. [PubMed] [Google Scholar]
- 13.Brook I, Hausfeld JN. 2011. Microbiology of acute and chronic maxillary sinusitis in smokers and nonsmokers. Ann Otol Rhinol Laryngol 120:707–712. doi: 10.1177/000348941112001103. [DOI] [PubMed] [Google Scholar]
- 14.Kopp BT, Sarzynski L, Khalfoun S, Hayes D Jr, Thompson R, Nicholson L, Long F, Castile R, Groner J. 2015. Detrimental effects of secondhand smoke exposure on infants with cystic fibrosis. Pediatr Pulmonol 50:25–34. doi: 10.1002/ppul.23016. [DOI] [PubMed] [Google Scholar]
- 15.Reish RG, Damjanovic B, Austen WG Jr, Winograd J, Liao EC, Cetrulo CL, Balkin DM, Colwell AS. 2013. Infection following implant-based reconstruction in 1952 consecutive breast reconstructions: salvage rates and predictors of success. Plastic Reconstruct Surg 131:1223–1230. doi: 10.1097/PRS.0b013e31828bd377. [DOI] [PubMed] [Google Scholar]
- 16.Kulkarni R, Antala S, Wang A, Amaral FE, Rampersaud R, Larussa SJ, Planet PJ, Ratner AJ. 2012. Cigarette smoke increases Staphylococcus aureus biofilm formation via oxidative stress. Infect Immun 80:3804–3811. doi: 10.1128/IAI.00689-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frazee BW, Lynn J, Charlebois ED, Lambert L, Lowery D, Perdreau-Remington F. 2005. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann Emerg Med 45:311–320. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 18.King MD, Humphrey BJ, Wang YF, Kourbatova EV, Ray SM, Blumberg HM. 2006. Emergence of community-acquired methicillin-resistant Staphylococcus aureus USA 300 clone as the predominant cause of skin and soft-tissue infections. Ann Intern Med 144:309–317. doi: 10.7326/0003-4819-144-5-200603070-00005. [DOI] [PubMed] [Google Scholar]
- 19.Baker SE, Brecher SM, Robillard E, Strymish J, Lawler E, Gupta K. 2010. Extranasal methicillin-resistant Staphylococcus aureus colonization at admission to an acute care Veterans Affairs hospital. Infect Control Hosp Epidemiol 31:42–46. doi: 10.1086/649222. [DOI] [PubMed] [Google Scholar]
- 20.May L, McCann C, Brooks G, Rothman R, Miller L, Jordan J. 2014. Dual-site sampling improved detection rates for MRSA colonization in patients with cutaneous abscesses. Diagn Microbiol Infect Dis 80:79–82. doi: 10.1016/j.diagmicrobio.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Hal SJ, Stark D, Lockwood B, Marriott D, Harkness J. 2007. Methicillin-resistant Staphylococcus aureus (MRSA) detection: comparison of two molecular methods (IDI-MRSA PCR assay and GenoType MRSA Direct PCR assay) with three selective MRSA agars (MRSA ID, MRSASelect, and CHROMagar MRSA) for use with infection-control swabs. J Clin Microbiol 45:2486–2490. doi: 10.1128/JCM.00139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu GY. 2009. Molecular pathogenesis of Staphylococcus aureus infection. Pediatric Res 65:71R–77R. doi: 10.1203/PDR.0b013e31819dc44d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cafiso V, Bertuccio T, Spina D, Purrello S, Campanile F, Di Pietro C, Purrello M, Stefani S. 2012. Modulating activity of vancomycin and daptomycin on the expression of autolysis cell-wall turnover and membrane charge genes in hVISA and VISA strains. PLoS One 7:e29573. doi: 10.1371/journal.pone.0029573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eleaume H, Jabbouri S. 2004. Comparison of two standardisation methods in real-time quantitative RT-PCR to follow Staphylococcus aureus genes expression during in vitro growth. J Microbiol Methods 59:363–370. doi: 10.1016/j.mimet.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 25.Heron M, Grutters JC, ten Dam-Molenkamp KM, Hijdra D, van Heugten-Roeling A, Claessen AM, Ruven HJ, van den Bosch JM, van Velzen-Blad H. 2012. Bronchoalveolar lavage cell pattern from healthy human lung. Clin Exp Immunol 167:523–531. doi: 10.1111/j.1365-2249.2011.04529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang FC. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol 2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- 27.Garin J, Diez R, Kieffer S, Dermine JF, Duclos S, Gagnon E, Sadoul R, Rondeau C, Desjardins M. 2001. The phagosome proteome: insight into phagosome functions. J Cell Biol 152:165–180. doi: 10.1083/jcb.152.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aulik NA, Hellenbrand KM, Czuprynski CJ. 2012. Mannheimia haemolytica and its leukotoxin cause macrophage extracellular trap formation by bovine macrophages. Infect Immun 80:1923–1933. doi: 10.1128/IAI.06120-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hett EC, Rubin EJ. 2008. Bacterial growth and cell division: a mycobacterial perspective. Microbiol Mol Biol Rev 72:126–156. doi: 10.1128/MMBR.00028-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kristian SA, Timmer AM, Liu GY, Lauth X, Sal-Man N, Rosenfeld Y, Shai Y, Gallo RL, Nizet V. 2007. Impairment of innate immune killing mechanisms by bacteriostatic antibiotics. FASEB J 21:1107–1116. doi: 10.1096/fj.06-6802com. [DOI] [PubMed] [Google Scholar]
- 31.Peschel A, Otto M, Jack RW, Kalbacher H, Jung G, Gotz F. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem 274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 32.Collins LV, Kristian SA, Weidenmaier C, Faigle M, Van Kessel KP, Van Strijp JA, Gotz F, Neumeister B, Peschel A. 2002. Staphylococcus aureus strains lacking d-alanine modifications of teichoic acids are highly susceptible to human neutrophil killing and are virulence attenuated in mice. J Infect Dis 186:214–219. doi: 10.1086/341454. [DOI] [PubMed] [Google Scholar]
- 33.Koprivnjak T, Peschel A. 2011. Bacterial resistance mechanisms against host defense peptides. Cell Mol Life Sci 68:2243–2254. doi: 10.1007/s00018-011-0716-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kraus D, Peschel A. 2008. Staphylococcus aureus evasion of innate antimicrobial defense. Future Microbiol 3:437–451. doi: 10.2217/17460913.3.4.437. [DOI] [PubMed] [Google Scholar]
- 35.Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, Mond JJ, Tarkowski A, Foster SJ. 2007. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe 1:199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Magnusson KE. 1982. Hydrophobic interaction–a mechanism of bacterial binding. Scand J Infect Dis Suppl 33:32–36. [PubMed] [Google Scholar]
- 37.Rosenberg M, Kjelleberg S. 1987. Hydrophobic interactions: role in bacterial adhesion. In Marshall KC. (ed), Advances in microbiology ecology, vol 9 Plenum, New York, NY. [Google Scholar]
- 38.Dahlback B, Hermansson M, Kjelleberg S, Norkrans B. 1981. The hydrophobicity of bacteria—an important factor in their initial adhesion at the air-water interface. Arch Microbiol 128:267–270. doi: 10.1007/BF00422527. [DOI] [PubMed] [Google Scholar]
- 39.Yang ES, Tan J, Eells S, Rieg G, Tagudar G, Miller LG. 2010. Body site colonization in patients with community-associated methicillin-resistant Staphylococcus aureus and other types of S. aureus skin infections. Clin Microbiol Infect 16:425–431. doi: 10.1111/j.1469-0691.2009.02836.x. [DOI] [PubMed] [Google Scholar]
- 40.Abbot EL, Smith WD, Siou GP, Chiriboga C, Smith RJ, Wilson JA, Hirst BH, Kehoe MA. 2007. Pili mediate specific adhesion of Streptococcus pyogenes to human tonsil and skin. Cell Microbiol 9:1822–1833. doi: 10.1111/j.1462-5822.2007.00918.x. [DOI] [PubMed] [Google Scholar]
- 41.Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. 1988. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eisele NA, Anderson DM. 2011. Host defense and the airway epithelium: frontline responses that protect against bacterial invasion and pneumonia. J Pathog 2011:249802. doi: 10.4061/2011/249802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Byfield FJ, Kowalski M, Cruz K, Leszczynska K, Namiot A, Savage PB, Bucki R, Janmey PA. 2011. Cathelicidin LL-37 increases lung epithelial cell stiffness, decreases transepithelial permeability, and prevents epithelial invasion by Pseudomonas aeruginosa. J Immunol 187:6402–6409. doi: 10.4049/jimmunol.1102185. [DOI] [PubMed] [Google Scholar]
- 44.Jann NJ, Schmaler M, Kristian SA, Radek KA, Gallo RL, Nizet V, Peschel A, Landmann R. 2009. Neutrophil antimicrobial defense against Staphylococcus aureus is mediated by phagolysosomal but not extracellular trap-associated cathelicidin. J Leukoc Biol 86:1159–1169. doi: 10.1189/jlb.0209053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maritz GS, Mutemwa M. 2014. The effect of grand maternal nicotine exposure during gestation and lactation on lung integrity of the F2 generation. Pediatr Pulmonol 49:67–75. doi: 10.1002/ppul.22783. [DOI] [PubMed] [Google Scholar]
- 46.Rehan VK, Liu J, Naeem E, Tian J, Sakurai R, Kwong K, Akbari O, Torday JS. 2012. Perinatal nicotine exposure induces asthma in second generation offspring. BMC Med 10:129. doi: 10.1186/1741-7015-10-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yauk CL, Berndt ML, Williams A, Rowan-Carroll A, Douglas GR, Stampfli MR. 2007. Mainstream tobacco smoke causes paternal germ-line DNA mutation. Cancer Res 67:5103–5106. doi: 10.1158/0008-5472.CAN-07-0279. [DOI] [PubMed] [Google Scholar]
- 48.Marchetti F, Rowan-Carroll A, Williams A, Polyzos A, Berndt-Weis ML, Yauk CL. 2011. Sidestream tobacco smoke is a male germ cell mutagen. Proc Natl Acad Sci U S A 108:12811–12814. doi: 10.1073/pnas.1106896108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li YF, Langholz B, Salam MT, Gilliland FD. 2005. Maternal and grandmaternal smoking patterns are associated with early childhood asthma. Chest 127:1232–1241. doi: 10.1378/chest.127.4.1232. [DOI] [PubMed] [Google Scholar]
- 50.Bose JL, Lehman MK, Fey PD, Bayles KW. 2012. Contribution of the Staphylococcus aureus Atl AM and GL murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One 7:e42244. doi: 10.1371/journal.pone.0042244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Biswas R, Voggu L, Simon UK, Hentschel P, Thumm G, Gotz F. 2006. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol Lett 259:260–268. doi: 10.1111/j.1574-6968.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- 52.Singh VK. 2014. High level expression and purification of Atl, the major autolytic protein of Staphylococcus aureus. Int J Microbiol 2014:615965. doi: 10.1155/2014/615965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pasztor L, Ziebandt AK, Nega M, Schlag M, Haase S, Franz-Wachtel M, Madlung J, Nordheim A, Heinrichs DE, Gotz F. 2010. Staphylococcal major autolysin (Atl) is involved in excretion of cytoplasmic proteins. J Biol Chem 285:36794–36803. doi: 10.1074/jbc.M110.167312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kristian SA, Durr M, Van Strijp JA, Neumeister B, Peschel A. 2003. MprF-mediated lysinylation of phospholipids in Staphylococcus aureus leads to protection against oxygen-independent neutrophil killing. Infect Immun 71:546–549. doi: 10.1128/IAI.71.1.546-549.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Castleden CM, Cole PV. 1975. Carboxyhaemoglobin levels of smokers and non-smokers working in the City of London. Br J Ind Med 32:115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pishchany G, Sheldon JR, Dickson CF, Alam MT, Read TD, Gell DA, Heinrichs DE, Skaar EP. 2014. IsdB-dependent hemoglobin binding is required for acquisition of heme by Staphylococcus aureus. J Infect Dis 209:1764–1772. doi: 10.1093/infdis/jit817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.