Abstract
Significance: Fibrinogen-related proteins (FRePs) comprise an intriguing collection of extracellular molecules, each containing a conserved fibrinogen-like globe (FBG). This group includes the eponymous fibrinogen as well as the tenascin, angiopoietin, and ficolin families. Many of these proteins are upregulated during tissue repair and exhibit diverse roles during wound healing.
Recent Advances: An increasing body of evidence highlights the specific expression of a number of FRePs following tissue injury and infection. Upon induction, each FReP uses its FBG domain to mediate quite distinct effects that contribute to different stages of tissue repair, such as driving coagulation, pathogen detection, inflammation, angiogenesis, and tissue remodeling.
Critical Issues: Despite a high degree of homology among FRePs, each contains unique sequences that enable their diversification of function. Comparative analysis of the structure and function of FRePs and precise mapping of regions that interact with a variety of ligands has started to reveal the underlying molecular mechanisms by which these proteins play very different roles using their common domain.
Future Directions: Fibrinogen has long been used in the clinic as a synthetic matrix serving as a scaffold or a delivery system to aid tissue repair. Novel therapeutic strategies are now emerging that harness the use of other FRePs to improve wound healing outcomes. As we learn more about the underlying mechanisms by which each FReP contributes to the repair response, specific blockade, or indeed potentiation, of their function offers real potential to enable regulation of distinct processes during pathological wound healing.

Kim S. Midwood, PhD
Scope and Significance
Many extracellular matrix (ECM) proteins are expressed during wound healing and are essential for the correct progression of tissue repair. In this study, we discuss the contribution of one particular subset of these molecules to a successful healing response. Fibrinogen-related proteins (FRePs) contain a common and ancient domain that has evolved to play key roles in tissue repair. We highlight the different and overlapping roles of these proteins, focus on how these domains interact with and signal to cells, and summarize the potential of targeting these molecules in pathological wound healing.
Translational Relevance
FRePs provide a temporary scaffold to support cell adhesion and infiltration, upon which to lay new tissue during rebuilding, but they also actively modulate cell behavior, including adhesion, migration, differentiation, proliferation, and survival. Expression of these molecules during tissue injury is extraordinarily orchestrated and their induction, downregulation, and clearance from the tissue once repair is completed are very tightly regulated. This creates a dynamic microenvironment at the wound site where fluctuating levels of extracellular proteins direct the cell phenotype to mediate tissue repair.
Clinical Relevance
Defects in wound healing, such as excessive scarring or fibrosis and delayed wound repair, pose significant clinical challenges. The pivotal role of FRePs in controlling wound healing and their restricted expression in healthy tissues implicates them as eminently tractable targets for the improvement of repair-related pathological conditions. Some are already well established in clinical use, while the potential of others is only just becoming apparent.
Discussion
Definition of FRePs
Fibrinogen is a large glycoprotein comprising pairs of three nonidentical polypeptide chains (α β γ)2, which radiate out from a central nodule containing the N-terminus of each peptide. The C-terminal regions of the β and γ chains, each forms a 30 kDa globular domain that is absent in the α chain and exhibits a unique fold and structure containing three distinct subdomains, A, B, and P (Fig. 1).1 Domains with high homology to this globular region are also found in other proteins; this structure has become defined as a fibrinogen-like globe (FBG) and FRePs as proteins that contain an FBG domain.2–4
Figure 1.
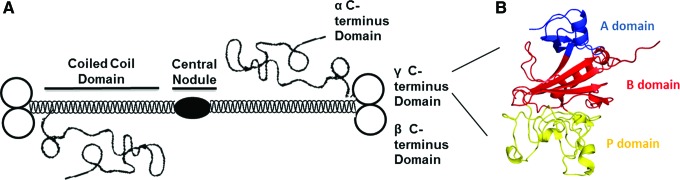
Fibrinogen and its C-terminal globular domain. (A) Schematic structure of fibrinogen showing the central nodule, coiled-coil domain, and α, β, and γ C-terminus domains. (B) Three-dimensional structure of the γ C-terminus globule showing its constituent three subdomains: A, B, and P. The A subdomain is at the N-terminus of the globule and consists of ∼50 amino acids. The B subdomain is in the middle of the structure and comprises ∼100 residues. Finally, at the C-terminus is the P subdomain, which contains ∼100 amino acids. This image was modified from the Yee et al.1 protein data bank number 1FID and colored using the PyMOL Molecular Graphics System, Version 1.5.0.4 Schrödinger, LLC. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
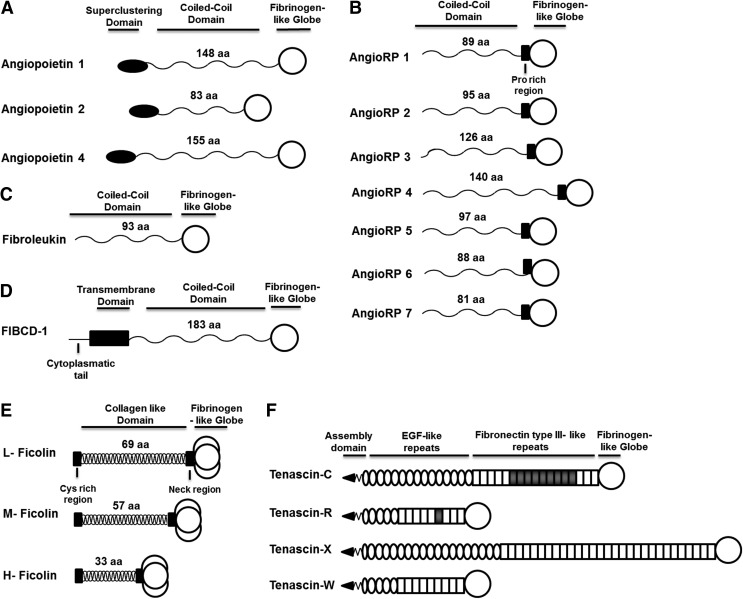
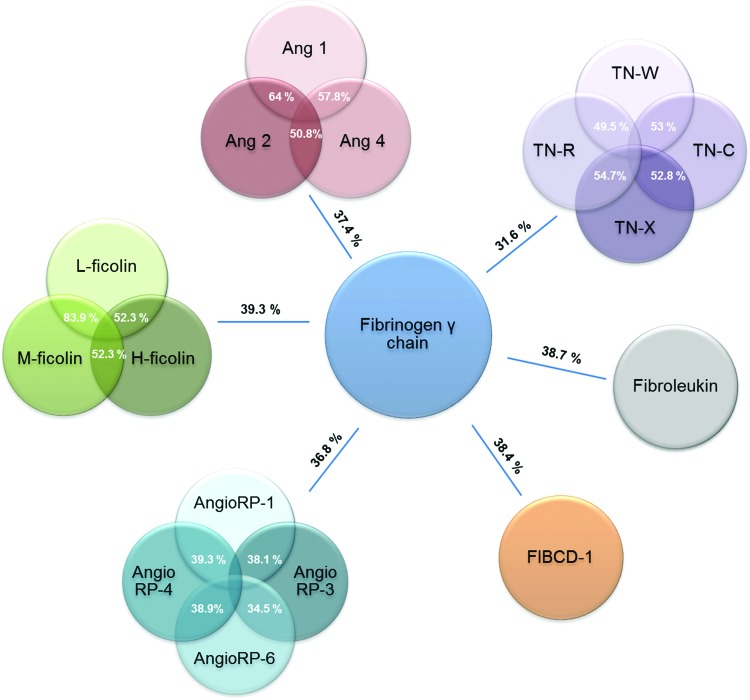
Although the FBG domain was first described in mammalian fibrinogen, it is an ancient domain. There are 2,515 FRePs in eukaryotes, all in multicellular organisms. Some molecules exist that are homologous to the A subdomain in choanoflagellates5; however, the sea sponge (Phylum Porifera) contains the first complete FBG domain with all three subdomains present. In invertebrates, FRePs are essential for protection against infection with additional functions in development and allorecognition (reviewed in Hanington and Zhang4). In mammals, there are 541 FRePs with 21 different human FRePs, including fibrinogen, tenascins, ficolins, angiopoietins, angiopoietin-related proteins, fibroleukin, and fibrinogen C domain-containing protein-1 (FIBCD-1; Table 1). Typically, FRePs are multidomain proteins where the FBG domain is at the C-terminus of the molecule and this is true for all human FRePs (Fig. 2). There is ∼30–40% sequence homology among the FBG domains of these proteins, indicating a high level of conservation (Fig. 3), and crystal structures of the FBG domains of human ficolin-H, -L, and -M, angiopoietin-2, FIBCD-1, and crab thachylectin-5A exhibit the same subdomain organization and structural folds.6–10
Table 1.
The human fibrinogen-related proteins
| FBG domain | Accession number | Amino acids position | Molecular weight (kDa) | Number of amino acids | pI |
|---|---|---|---|---|---|
| Angiopoietin 1 | Q15389 | 277–497 | 27.1 | 237 | 8.40 |
| Angiopoietin 2 | O15123 | 275–495 | 28.2 | 248 | 5.78 |
| Angiopoietin 4 | Q9Y264 | 282–502 | 30.1 | 221 | 8.89 |
| Angiopoietin-related protein 1 | O95841 | 271–491 | 25.7 | 221 | 6.18 |
| Angiopoietin-related protein 2 | Q9UKU9 | 269–493 | 25.9 | 223 | 7.83 |
| Angiopoietin-related protein 3 | Q9Y5C1 | 237–460 | 26.1 | 224 | 6.89 |
| Angiopoietin-related protein 4 | Q9BY76 | 179–406 | 25.6 | 228 | 8.71 |
| Angiopoietin-related protein 5 | Q86XS5 | 141–390 | 28.2 | 248 | 7.17 |
| Angiopoietin-related protein 6 | Q8NI99 | 251–270 | 25.2 | 220 | 6.64 |
| Angiopoietin-related protein 7 | O43827 | 122–343 | 26.2 | 225 | 7.18 |
| Fibrinogen β | P02675 | 232–491 | 29.8 | 260 | 7.06 |
| Fibrinogen γ | P02679 | 170–453 | 32.1 | 284 | 5.35 |
| Fibroleukin | Q14314 | 204–437 | 27.2 | 234 | 8.58 |
| FIBCD-1 | Q8N539 | 235–461 | 25.7 | 227 | 5.44 |
| Ficolin-H | 075636 | 84–299 | 24.8 | 219 | 6.31 |
| Ficolin-L | Q15485 | 96–313 | 24.0 | 221 | 6.07 |
| Ficolin-M | O00602 | 109–326 | 25.6 | 231 | 5.87 |
| Tenascin-C | P24821 | 1,974–2,202 | 26.1 | 229 | 8.78 |
| Tenascin-R | Q92752 | 1,128–1,359 | 27.0 | 232 | 8.63 |
| Tenascin-W | Q9UQP3 | 1,060–1,300 | 27.6 | 240 | 9.18 |
| Tenascin-X | P22105 | 4,013–4,243 | 26.1 | 231 | 5.54 |
FBG, fibrinogen-like globe; FIBCD, fibrinogen C domain-containing protein-1.
Figure 2.
Domain organization of human FRePs. (A) Angiopoietins 1, 2, and 4 are found in humans; angiopoietin 3 exists only in mice. Angiopoietins contain an N-terminal superclustering domain, followed by a coiled-coil domain, in which the number of amino acids varies between family members, and the C-terminus is capped by the FBG domain. (B) Angiopoietin-related proteins are structurally similar to angiopoietins. Seven have been reported in humans. They lack a superclustering domain, instead containing a proline-rich region before the FBG domain. (C) Fibroleukin contains a coiled-coil domain and a C-terminal FBG domain. (D) FIBCD-1 is the only FReP that is not secreted; it contains a transmembrane domain, followed by coiled-coil domain and FBG domain. (E) Three ficolins are found in humans: L-, M-, and H-ficolin. They contain an N-terminal collagen-like domain, which includes a cysteine-rich region and a neck region that connects with the FBG domain. These proteins have a unique mode of oligomerization; three coil-coiled chains interlace forming a collagen-like helix that brings three FBG domains together to create a trimer at the C-terminus, and (F) Tenascins are a family of four extracellular matrix glycoproteins, tenascin-C, -R, -W, and -X. Tenascins have an N-terminal region that contains a coiled-coil structure and disulfide bonds necessary for oligomer formation. Next are the EGF-like repeats, followed by FNIII, whose number varies among the tenascins, and then the FBG domain. EGF, epidermal growth factor; FBG, fibrinogen-like globe; FIBCD-1, fibrinogen C domain-containing protein-1; FNIII, fibronectin type III-like domain; FRePs, fibrinogen-related proteins.
Figure 3.
Degree of identity among human FRePs. In white is the % of identity among FReP family members, and in black is the average of identity of this family with the fibrinogen γ chain. The % of identity was calculated using ClustalW pairwise alignment. There is 40% identity between the C-terminus globular domains of fibrinogen γ and β chains. Ang, angiopoietins; AngioRP, angiopoietin-related proteins; TN, tenascin. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Human FRePs and wound healing
A large body of literature reveals that FRePs play key roles during tissue repair. Each domain within these large multimodular molecules can interact with a wide variety of binding partners to mediate diverse effects on cell behavior. For example, the epidermal growth factor-like (EGF-L) repeats of tenascin-C promote mesenchymal stem cell (MSC) survival in wound healing by activating the EGF receptor,11 protecting them from Fas ligand-induced nuclear DNA damage.12 Likewise, serine 89, located outside the FBG domain of fibroleukin, has prothrombinase activity that drives blood coagulation.13 In this study, however, we focus solely on the functions of the FBG domains of FRePs. Known ligands for the FBG domains of human FRePs are listed in Table 2, and the roles of these interactions during the different stages of wound healing are discussed below.
Table 2.
Binding sites of the ligands for the fibrinogen-like globe domains of human fibrinogen-related proteins
| FBG domain | Ligand | Binding amino acids | Subdomain |
|---|---|---|---|
| Angiopoietin 1 | Tie2 | D218, A219, P222, K238, K243, Y246 | P 103 |
| Angiopoietin 2 | Tie 2 | D218, A219, P222, N237-F239, Y245, Y246, S250 | P 103 |
| Integrin α5β1 | E352-Q366 | P 66 | |
| Angiopoietin-like 3 | Integrin αvβ3 | Not mapped | |
| Angiopoietin-like 4 | Integrin β1/β5 | Not mapped | |
| Fibrinogen γC | Integrin αIIbβ3 | H271-V279 | P 20 |
| Integrin αMβ2 | G48-V60/Y248-G266 | A, P 104 | |
| Integrin αvβ3 | G217-S229 | P 80 | |
| Integrin α5β1 | N236-T254 | P 21 | |
| “a” peptide GPRP | Q245, D246, K254-H256, Y279-G282, R291 | P 14 | |
| Fibrinogen γ'C | Thrombin | Y418, Y422, P413, A414 | P 105 |
| Factor XIII | V408-L427 | P 24 | |
| FIBCD-1 | Chitin | Y250, H260, Y283, A284 | P 49 |
| GlcNAc, ManNAc, acetylated amino acids | Not mapped | ||
| H-ficolin | d-fucose, galactose | C189, Y190, Y208, V218 | P 6 |
| LPS | Not mapped | ||
| L-ficolin | Acetylated sugars | F180, C189, H190, G204, F206, Y218, N219 | P 6 |
| 1,3-β-d-glucan | E226, D53, D55, S34, S86 | A, B 6 | |
| DNA | Not mapped | ||
| M-ficolin | Acetylated sugars | F180, C189, H190, Y206, A207, Y218 | P 7 |
| Tenascin-C | Integrin αvβ3 | W251-K289 | P 80 |
| TLR4 | Not mapped | ||
| Tenascin-X | α11β1 | Not mapped | |
| Small latent complex of TGF-β | Not mapped | ||
| Tropoelastin | Not mapped |
Numbers in superscripts indicate cited references.
FIBCD-1, fibrinogen C domain-containing protein-1; GlcNAc, N-acetylglucosamine; LPS, lipopolysaccharide; ManNAc, N-acetyl-d-mannosamine; TGF-β, transforming growth factor beta; TLR4, toll-like receptor 4.
Blood clotting
Upon injury, blood pervades the wounded space and thrombin cleaves soluble fibrinogen and platelet-associated protease-activated receptors, leading to thrombus formation and deposition of a fibrin matrix creating the hemostatic blood clot that also serves as a scaffold for tissue repair.
Fibrinogen is essential to coagulation, and the FBG domains of the β and γ chains play vital roles in these initial stages of wound healing. Fibrinogen produced by hepatocytes circulates in plasma until tissue injury, whereupon thrombin catalyzes it into fibrin. In this reaction, N-fibrinopeptides from the α chain of fibrinogen are released exposing an N-terminus motif (GPR) that binds to the FBG domain of the γ chain of an adjacent molecule. Using short peptide mimics of the α chain N-terminus sequences, a complementary “a” binding pocket was mapped in the P domain of the γ chain (Table 2).14,15 Blocking this pocket with the GPRP peptide or point mutations of residues in this region of the FBG domain results in impaired fibrinogen polymerization.16,17 The β chain N-terminus is also cleaved, but at a slower rate, and interaction of this region with the FBG domain at the C-terminus of the β chain has been speculated to contribute to lateral fibril association; for a detailed explanation of fibrinogen polymerization see review.18 Fibrin molecules are further cross-linked by factor XXIIa, a transglutaminase, which creates covalent bonds between 405Lys of one γ chain and 398/399Gln of another.19 Calcium is pivotal in accelerating fibrin formation, protecting the molecule against plasmin degradation, and stabilization against heat denaturation, and the calcium-binding site in fibrinogen is located in the FBG domain of the γ chain proximal to the “a” pocket.14 Fibrinogen also plays a key role in aggregating activated platelets; residues H271-V279 in the P subdomain of the γ chain FBG domain bind to platelet αIIbβ3 integrins20 and N236-T254 in this domain bind to α5β1 integrin to mediate platelet adhesion and clot retraction.21
Fibrinogen is subject to alternative splicing. One common variant of FBG domain of the γ chain (γ′) is found in 8–15% of plasma. This variant lacks four amino acids at the end of the γA chain, which are replaced by an additional 20 amino acids.22 This modification creates new binding sites for thrombin and factor XIII (Table 2)23,24; however, this variant cannot bind to platelets due to loss of αIIbβ3 integrin-binding sites.25 γ′ exhibits slower polymerization, thinner fibers, smaller pores, and a stiffer matrix that is more resistant to lysis compared with nonspliced γ chains.26,27 Plasma levels of γ′ are a risk factor for the development of cardiovascular diseases.28 During the healing of full-thickness wounds in rats, those covered with γ fibrin showed higher dermal vasodilator responses and increased breaking strength, while those treated with γ′ fibrin exhibited modest increases in macrophage infiltration. In vitro, the higher fibrinolysis of γ fibrin aided endothelial cell sprouting and vascularization,29 although further analysis is required to understand the effects of these variants on angiogenesis in vivo.
Inflammation
Infection after tissue injury is sensed by pattern recognition receptors (PRRs) of the immune system, which detect pathogen-associated molecular patterns and rapidly activate inflammation. PRRs also mount an inflammatory response upon recognition of endogenous signals of tissue damage (damage-associated molecular patterns [DAMPS]). Invasion of the blood clot by immune cells, including neutrophils and macrophages, enables pathogen and tissue debris removal.
Ficolins are secreted soluble oligomeric proteins that act as PRRs and activate complement-mediated inflammatory pathways (reviewed in Ren et al.30). L-ficolin is made in the liver and secreted into serum; M-ficolin is produced by monocytes, neutrophils, and type II alveolar epithelial cells and is found in serum and lungs. The FBG domain of both these proteins binds to different types of sugar epitopes, such as N-acetylglucosamine (GlcNAc), galactose, 1,3-β-d-glucan, N-acetylgalactosamine, N-acetyl-d-mannosamine (ManNAc), N-acetyl-d-glucosamine, N-acetylcysteine, and lipoteichoic acid,6,31,32 from gram-negative and gram-positive bacteria, such as Salmonella typhimurium, Pseudomonas aeruginosa,33 and type III group B Streptococci and Staphylococcus aureus.34–36 Sugar-binding sites are predominantly located in a series of residues conserved in both ficolins in the P-subdomain (Table 2). L-ficolin can activate Jun kinase phosphorylation and induce interferon-γ, interleukin (IL)-17, IL-6, tumor necrosis factor alpha, and nitric oxide expression in mouse peritoneal macrophages upon acute Mycobacterium tuberculosis infection,37 but little is known about how L-ficolin does this. High levels of L-ficolin are also found in the serum of patients with influenza A and hepatitis C, where it is associated with favorable antiviral responses and clearance.38,39 Interestingly, in addition to binding pathogenic moieties, the FBG domain of L-ficolin can also bind to DNA derived from late apoptotic cells and necrotic cells in a calcium-dependent manner, aiding clearance of dead cells by increasing opsonization and macrophage uptake. This binding was not inhibited by addition of GlcNAc, indicating a distinct binding site for nucleic acids in the FBG domain.40
Polymorphisms in the FBG domain of M-ficolin dictate expression levels and binding ability. Ser268Pro reduces M-ficolin levels in heterozygote individuals and completely abolishes expression in homozygotes. Ser268 is an essential part of the calcium-binding site in the FBG domain of M-ficolin, suggesting that calcium plays an important role in its stability. Other mutations, Ala218Thr and Asn289Ser, also alter M-ficolin levels, and recombinant proteins with these mutations showed impaired binding to the sialic acid residues in the polysaccharide capsule of group B Streptococcus. These residues are proximal to bacterial-binding sites in the P domain and likely give stability to the protein structure and exposure of interaction sites.41
The third member of the ficolin family, H-ficolin is expressed in the liver and the lung and is also found in serum. Its FBG domain binds to galactose and d-fucose through the same residues that L-ficolin and M-ficolin use to bind acetylated sugars (Table 2).6 It also binds to the gram-positive bacterium Aerococcus viridans.42 H-ficolin is specifically elevated in the airways during influenza A virus infection, where it interacts with viral surface glycoproteins inhibiting infection in vivo.43 More recently, H-ficolin has been shown to agglutinate Hafnia alvei and induce bacterial phagocytosis, possibly by the activation of the lectin pathway of complement. The FBG domain of H-ficolin recognizes lipopolysaccharide (LPS) O-specific polysaccharide, and this binding is calcium and magnesium dependent.44 Interaction of H-ficolin with LPS of H. alvei inhibits toll-like receptor 4 (TLR4)/MD-2/cluster of differentiation 4 activation of the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) transcription factor.45 Like L-ficolin, the FBG domain of H-ficolin binds to apoptotic cells and necrotic cells and activates opsonization; however, H-ficolin does not bind to nucleic acids and it is unknown what the ligand is in this context.46 An H-ficolin variant exists where a frameshift in exon 5 (Leu117fs) leads to complete deletion of the FBG domain of this protein. In heterozygous individuals this reduces serum ficolin levels. Levels are abolished in homozygous patients who suffer from severe recurrence of bacterial and lung infection, lung fibrosis, bronchiectasis, and obstructive lung disease as a result,47 indicating the essential role of the FBG domain in this protein against infection.
Another FReP that can bind acetyl groups is FIBCD-1. This transmembrane PRR is expressed by epithelial cells in large and small intestinal walls and in salivary glands.48 The FBG domain of FIBCD-1 forms a tetrameric structure through noncovalent interactions of adjacent molecules within the cell membrane and this form binds specifically to chitin (an N-acetyl homopolymer) through residues in the P-subdomain (Table 2), as well as interacting with GlcNAc, ManNAc, and acetylated amino acids.48,49 After ligand binding, FIBCD-1 can endocytose acetylated compounds48 and potential phosphorylated sites in the cytoplasmic region of this protein infer its role as a signaling molecule; however, still little is known about the events downstream of endocytosis.
Fibroleukin (fibrinogen-like protein 2) is a membrane-bound protein expressed by macrophages and endothelial cells, but which is also found circulating in serum.50 Increased soluble fibroleukin is observed in alveolar macrophages from patients with peripheral lungs of chronic obstructive pulmonary disease,51 chronic hepatitis C,52 and myocardial ischemia/reperfusion injury.53 Inhibition of fibroleukin has shown beneficial effects in a model of hepatic reperfusion, decreasing liver injury by lowering the apoptotic levels of mice hepatocytes and sinusoidal endothelial cells.54 In addition to the procoagulatory role of regions outside the FBG domain, the FBG domain of fibroleukin has shown some immune-suppressive effects. It can abolish LPS-induced maturation of bone marrow-derived dendritic cells, resulting in inhibition of T cell proliferation, and this phenomenon could be rescued by adding antibodies against the FBG domain of fibroleukin. Soluble fibroleukin favors the Th2 cytokine profile by increasing levels of anti-inflammatory cytokines, IL-4 and IL-10,55 although it is not known how this change in cytokine profile is mediated.
Indeed, other FRePs can induce cytokine synthesis. The FBG domain of the ECM glycoprotein tenascin-C activates TLR4-mediated inflammation in primary human cells and murine models of inflammation in vivo, driving a persistent innate immune response. Tenascin-C is upregulated in acute inflammation and tissue injury, where it is thought to act as a DAMP in cases of sterile inflammation.56 However, it is not yet known whether this is caused by a direct interaction of the FBG domain with TLR4. At sites of inflammation, increased vascular permeability allows extravasation of intact fibrinogen.57 Fibrinogen can also activate TLR4 stimulating the synthesis of cytokines and chemokines that attract T-cells, neutrophils, and macrophages; however, it is not known if this function is mediated by the FBG domain of the γ or β chain.58 Given the activation of TLR4 by the FBG domain of tenascin-C, it is tempting to speculate that either the FBG domains of fibrinogen or fibroleukin can also activate TLR4. Finally, both fibrinogen and fibrin promote leukocyte adhesion during the inflammatory response through binding of residues G48-V60/Y248-G266 of the γ chain FBG domain to αMβ2 integrins.59
Angiogenesis
Wound neovascularization is mediated by endothelial cells infiltrating into the provisional matrix and proliferating, forming new blood vessels. Angiopoietins are one of the major angiogenic players in wound healing. These secreted proteins expressed by endothelial cells control blood vessel development and stability, promoting or inhibiting angiogenesis, through activation of tyrosine kinase (Tie) receptors by the FBG domain.60
Angiopoietin-2 is expressed from day 3 of dermal wound healing61 and is present during reepithelialization, granulation tissue formation, and remodeling.62,63 Binding of the angiopoietin-2 FBG domain to Tie2 receptor mediates the destabilization of existing vessels promoting vascular remodeling in the presence of vascular endothelial growth factor (VEGF).64 In addition, it can promote endothelial cell survival.65 A recent study also found that the FBG region of angiopoietin-2 binds directly to α5β1 integrin (Table 2) and Gln362 in the P-subdomain is essential for this interaction. This binding site does not overlap with the binding motif of Tie-2 receptor. Interaction with α5β1 integrins plays a role in the adhesion, migration, and invasion of human umbilical vein endothelial cells.66 Angiopoietin-2 null mice are born normally, but die in the first 14 days due to vascular defects and impaired inflammatory responses,67 while overexpression of angiopoietin-2 in endothelial cells or in the dermis results in embryonic death.64 Diabetic wound healing is characterized by a decrease in angiogenesis and granulation tissue formation.68,69 In genetically diabetic db/db mice, angiopoietin-2 and VEGF expression increased in dermal wounds associated with vessel destabilization and neovessel formation. However, VEGF induction was transient and, without sustained elevated levels of this factor, angiopoietin-2 promoted further vessel destabilization and regression, leading to a marked vascular death. In addition, in db/db mice wound healing, there is reduced expression and activation of Tie2 in the skin.61
Angiopoietin-1 is expressed later in dermal wound healing during granulation tissue formation and remodeling.63 In contrast to angiopoietin-2, it enhances the stabilization and maturation of blood vessels through the activation of Tie -2 by its FBG domain64,70,71 and promotes adhesion and migration of endothelial cells.72 Angiopoeitin-1 knockout mice are not viable, but overexpression of angiopoietin-1 in the skin results in an enlargement of vessels that contain increased numbers of endothelial cells and pericytes.70 New therapeutic approaches have focused on restoring Tie2 activation in diabetic wound healing and enhancing levels of angiopoietin-1. Adeno-associated virus (recombinant adeno-associated virus [rAAV]) mediated transfection of the angiopoietin-1 gene into diabetic mice and improved healing of incisional wounds with complete reepithelialization, well-formed granulation tissue, increased matrix maturation, organization, and breaking strength. Angiopoietin-1 rAAV mice also showed a significant increase in the formation of new blood vessels, but no changes in VEGF mRNA and protein expression,73 indicating that angiopoietin-1 can improve wound healing independently of VEGF expression. More recently, MSCs modified to continuously release angiopoietin-1 were implanted in excisional full-thickness wounds in rats and improved wound healing, increasing angiogenesis, and epidermal regeneration were observed.74 Other approaches use Tie receptor agonists, for example, vasculotide, a peptide mimic of angiopoietin-1 can bind Tie2 promoting receptor phosphorylation and induction of mitogen-activated protein kinase, protein kinase B, and endothelial nitric oxide synthase. In vitro, vasculotide protects endothelial cells from serum withdrawal death and promotes cell migration and MMP2 expression. In vivo, delivery of vasculotide in a matrigel plug to excisional wounds of db/db mice enhanced angiogenesis and stabilization of blood, as well as increased granulation tissue and accelerated wound closure.75 Vasculotide also improved murine acute radiation skin damage, reducing inflammation and increasing endothelial cell density and survival.76 Thus, studies focused on restoring the balance in the Tie2 signaling axis show beneficial effects for the treatment of diabetic wound healing and beyond.
Angiopoietin-related proteins or angiopoietin-like proteins are a family of secreted glycoproteins that do not bind to Tie receptors. However, some members of this family participate in the regulation of angiogenesis.77 For example, angiopoietin-like 3, which is found in the liver and serum, induces angiogenesis through its FBG domain, stimulating endothelial cell adhesion and migration through binding to αvβ3 integrin.78 Other FRePs also drive angiogenic processes through ligation of endothelial cell αvβ3 integrin; G217-S229 within the FBG domain of the fibrin γ chain interacts with αvβ3 to induce clot retraction during wound healing and increase angiogenesis and neovascularization by promoting cell adhesion, proliferation, and migration.79,80 The FBG domain of tenascin-C, also modulates endothelial cell adhesiveness and migration, enhancing endothelial sprouting through binding of W251-K289 to αvβ3 integrin.80–82
Tissue remodeling
In the latter stages of wound healing, fibroblasts invade and deposit new ECM initiating granulation tissue formation. Keratinocytes migrate above the provisional matrix reepithelializing dermal wounds. Finally, fibroblasts differentiate into myofibroblasts and initiate wound contraction and further collagen deposition. The provisional matrix is degraded as the wound contracts and collagen fibers are rearranged and aligned as new scar tissue.
Angiopoietin-like 4 is expressed by keratinocytes in dermal wound healing during reepithelialization83 after activation of peroxisome proliferator-activated receptors β/δ, which regulate epidermal differentiation.84 This FReP promotes keratinocyte migration83 and interacts with other ECM proteins such as fibronectin and vitronectin to delay their proteolytic degradation, although the role of the FBG domain in this process is not clear. The FBG domain does, however, interact with β1/β5 integrins85 activating focal adhesion kinase (FAK)-Src, signaling pathways that enhance keratinocyte migration and accelerate wound healing; indeed, angiopoietin-like 4 knockout mice present delayed wound reepithelialization and impaired keratinocyte migration.85
Angiopoietin-like 4 is considered a potential therapeutic target in impaired wound healing in diabetic mice where levels of this FReP remain low. Topical application of the recombinant FBG domain of angiopoietin-like 4 to full-thickness excisional wounds in db/db mice improved wound closure and accelerated reepithelialization compared with saline-treated wounds. Further study of the mechanisms underlining enhanced wound healing by angiopoietin-like 4, found that FBG domain binding to β1 integrin activates janus kinase 1/signal transducer and activator of transcription 3 signaling pathways, which in turn, upregulate the expression of inducible nitric oxide synthase in keratinocytes and enhance angiogenesis. Thus, cross talk between keratinocytes and epithelial cells may prove to be a unique therapeutic target in the improvement of angiogenesis in diabetic wounds.86
Tenascin-C is expressed by keratinocytes87 and fibroblasts in dermal wound healing during the phases of reepithelialization and granulation tissue formation.88 However, tenascin-C knockout mice show very minor defects in wound healing exhibiting temporarily reduced fibronectin in the granulation tissue.89 Impaired tissue repair was observed in these mice after corneal incision injury90 and articular cartilage damage,91 implying a possible role for tenascin-C in remodeling during repair. Tamaoki et al.92 showed in a model of myocardial injury that the tenascin-C FBG domain enhanced the migration of myocardial fibroblasts and promoted differentiation into myofibroblasts, in addition to increasing wound contraction.
Another family member, tenascin-X, is predominantly expressed during the late phases of matrix maturation after wound remodeling. Tenascin-X deficiency results in the Ehlers–Danlos Syndrome, characterized by skin fragility and hyperextensible skin. No major differences have been found in the wound healing of these patients, although tenascin-X knockout mice exhibit lower wound strength than wild-type mice.93 The FBG domain of tenascin-X can bind to tropoelastin, an interaction which is likely to mediate stability and maturation of elastic skin fibers.94 Tenascin-X also influences epithelial-to-mesenchymal transition, through the interaction of its FBG domain with the small latent complex of transforming growth factor beta (TGF-β), producing a conformational change that exposes active TGF-β. This mechanism is dependent on the binding of the FBG domain to α11β1 integrin in the cell surface.95
Summary
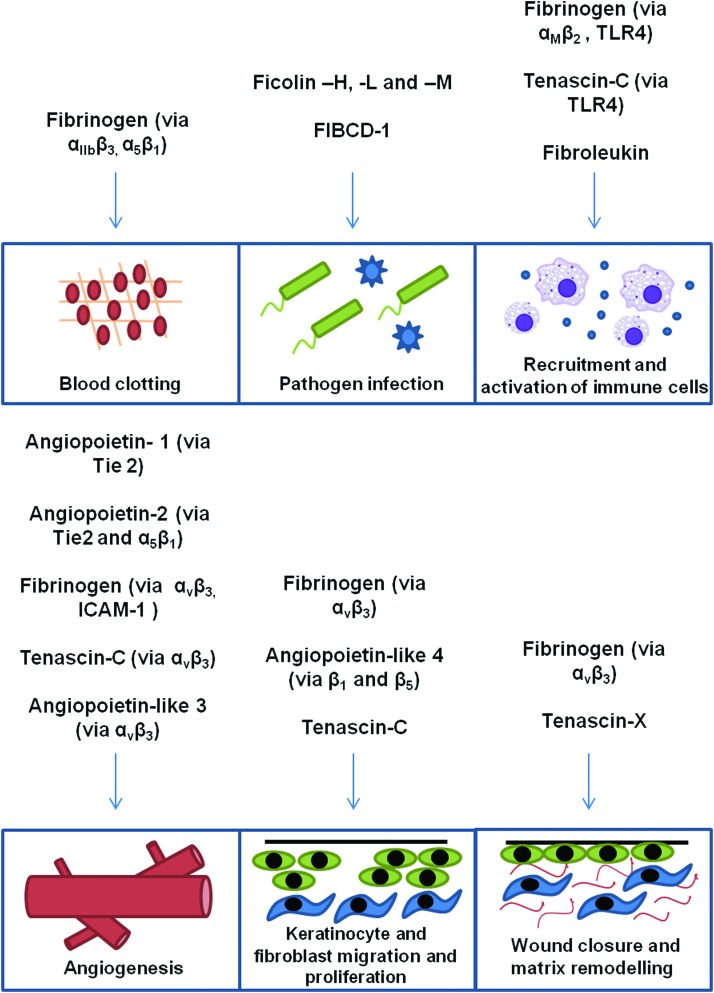
The FBG domains of human FRePs interact with multiple diverse binding partners. A large degree of homology exists among FBG domains of FRePs from the same family and also among members of different families. However, each also contains unique sequences. This is reflected in the overlapping functions of some FRePs and in the unique functions of others during different stages of wound healing (Fig. 4). For example, while the Tie2 receptor-binding site is only present in angiopoietins, the ficolin-P domain sugar-binding sites are conserved not only in all ficolins, but in each human FReP. This raises the question of ficolin redundancy and specificity; it may be that their distinct expression pattern confers tissue-specific functions; moreover, it appears that additional binding sites in the A and B domains confer ligand specificity, for example, L-ficolin binding to elongated carbohydrates, including 1,3-β-d-glucan.6 These data also raise the intriguing possibility that all human FRePs may act as PRRs; however, it is not clear if other FRePs can bind pathogenic ligands. There are data to suggest that this may not be the case; the GPRP peptide mimic of the fibrin α chain that binds to the “a” pocket of fibrinogen during polymerization also prevents the binding of the FBG domain of M-ficolin to GlcNAc,96 suggesting that nonconserved residues surrounding this region allow each FReP to use the same binding sites differently. Many more details remain to be uncovered before we fully understand FRePs; for example, mapping the binding sites for TLR4 and refining the precise amino acids that form integrin-binding sites. Together, these data will enable us to discern whether universal binding sites exist in all FRePs and how these are differentially ligated.
Figure 4.
Functions of FREPs during wound healing. Schematic representation of the roles of FRePs in the stages of wound healing. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Precise mapping of FBG-binding sites will also enable the development of specific inhibitors that might be useful in controlling coagulation, inflammation, angiogenesis, and tissue rebuilding during wound repair. Fibrinogen is already used extensively in dermal wound repair, its abundance, ease of isolation, and natural properties as a coagulant and provisional matrix making it ideal to aid wound healing (reviewed in Ahmed et al.97 and Janmey et al.98). Preclinical studies described above exemplify how modulation of angiopoietin activity may improve angiogenesis in diabetic and other nonhealing wounds, as well as the potential of angiopoietin-like 4 in controlling the keratinocyte phenotype. Antagonists of other FRePs may also prove useful, for example, blockade of ficolin activity in infectious diseases and of TLR4 activating FRePs to reduce excessive inflammation. Clues may also be gained from studying scar-free tissue regeneration. For example, high levels of tenascin-C are associated with wounds that heal in a scar-free manner in axolotls,99 in cells derived from ear wounds of MRL/MpJ mice, a strain that can regenerate scar-free ear wounds,100 and in fetal wounds where tenascin-C expression increases rapidly before reepithelialization, enhancing cell migration and increasing wound epithelialization rates.101 In contrast, high levels of tenascin-C are found in fibrotic chronic wounds and keloids102 indicating that regulation of tenascin-C expression during wound healing may provide future avenues to improve tissue repair and reduce fibrosis and abnormal scarring.
TAKE-HOME MESSAGES.
• FRePs each contain a globular highly homologous FBG domain.
• Human FRePs include fibrinogen, tenascins, ficolins, angiopoietins, angiopoietin-related proteins, fibroleukin, and FIBCD-1.
• FRePs are specifically upregulated upon tissue injury and infection.
• The FBG domain of each FReP has diverse functions playing distinct roles during tissue repair.
• These diverse functions are mediated by nonconserved residues within the FBG domain, unique to each family.
• Targeting these proteins has the potential to aid pathological wound healing.
Abbreviations and Acronyms
- Ang
angiopoietin
- AngioRP
angiopoietin-related protein
- DAMPS
damage-associated molecular patterns
- ECM
extracellular matrix
- EGF
epidermal growth factor
- FAK
focal adhesion kinase
- FBG
fibrinogen-like globe
- FIBCD-1
fibrinogen C domain-containing protein-1
- FNIII
fibronectin type III-like domain
- FRePs
fibrinogen-related proteins
- GlcNAc
N-acetylglucosamine
- ICAM-1
intracellular adhesion molecule 1
- IL
interleukin
- JAK1
janus kinase 1
- LPS
lipopolysaccharide
- ManNAc
N-acetyl-d-mannosamine
- MSCs
mesenchymal stem cells/multipotent stromal cells
- PRR
pattern recognition receptor
- rAAV
recombinant adeno-associated virus
- TGF-β
transforming growth factor beta
- Tie
tyrosine kinase receptors
- TLR4
toll-like receptor 4
- TN
tenascin
- VEGF
vascular endothelial growth factor
Acknowledgment and Funding Sources
The authors are funded by the Kennedy Trust for Rheumatology Research and Arthritis Research UK.
Author Disclosure and Ghostwriting
No competing financial interests exist. No ghostwriters were used to write this article. The content of this article was written by the authors listed.
About the Authors
Lorena Zuliani-Alvarez, DPhil, is a student in the Kennedy Institute of Rheumatology at Oxford University. Kim S. Midwood, PhD, is a Professor of Matrix Biology in the Kennedy Institute of Rheumatology at Oxford University.
References
- 1.Yee VC, et al. . Crystal structure of a 30 kDa C-terminal fragment from the gamma chain of human fibrinogen. Structure 1997;5:125–138 [DOI] [PubMed] [Google Scholar]
- 2.Doolittle RF. A detailed consideration of a principal domain of vertebrate fibrinogen and its relatives. Protein Sci 1992;1:1563–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doolittle RF, McNamara K, Lin K. Correlating structure and function during the evolution of fibrinogen-related domains. Protein Sci 2012;21:1808–1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanington PC, Zhang SM. The primary role of fibrinogen-related proteins in invertebrates is defense, not coagulation. J Innate Immun 2011;3:17–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King N, et al. . The genome of the choanoflagellate Monosiga brevicollis and the origin of metazoans. Nature 2008;451:783–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garlatti V, et al. . Structural insights into the innate immune recognition specificities of L- and H-ficolins. EMBO J 2007;26:623–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garlatti V, et al. . Structural basis for innate immune sensing by M-ficolin and its control by a pH-dependent conformational switch. J Biol Chem 2007;282:35814–35820 [DOI] [PubMed] [Google Scholar]
- 8.Barton WA, Tzvetkova D, Nikolov DB. Structure of the angiopoietin-2 receptor binding domain and identification of surfaces involved in Tie2 recognition. Structure 2005;13:825–832 [DOI] [PubMed] [Google Scholar]
- 9.Shrive AK, et al. . Crystal structure of the tetrameric fibrinogen-like recognition domain of fibrinogen C domain containing 1 (FIBCD1) protein. J Biol Chem 2014;289:2880–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kairies N, et al. . The 2.0-A crystal structure of tachylectin 5A provides evidence for the common origin of the innate immunity and the blood coagulation systems. Proc Natl Acad Sci U S A 2001;98:13519–13524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swindle CS, et al. . Epidermal growth factor (EGF)-like repeats of human tenascin-C as ligands for EGF receptor. J Cell Biol 2001;154:459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodrigues M, et al. . The matrikine tenascin-C protects multipotential stromal cells/mesenchymal stem cells from death cytokines such as FasL. Tissue Eng Part A 2013;19:1972–1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan CW, et al. . Kinetic analysis of a unique direct prothrombinase, fgl2, and identification of a serine residue critical for the prothrombinase activity. J Immunol 2002;168:5170–5177 [DOI] [PubMed] [Google Scholar]
- 14.Pratt KP, et al. . The primary fibrin polymerization pocket: three-dimensional structure of a 30-kDa C-terminal gamma chain fragment complexed with the peptide Gly-Pro-Arg-Pro. Proc Natl Acad Sci U S A 1997;94:7176–7181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litvinov RI, et al. . Polymerization of fibrin: specificity, strength, and stability of knob-hole interactions studied at the single-molecule level. Blood 2005;106:2944–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Everse SJ, et al. . Crystal structure of fragment double-D from human fibrin with two different bound ligands. Biochemistry 1998;37:8637–8642 [DOI] [PubMed] [Google Scholar]
- 17.Okumura N, Gorkun OV, Lord ST. Severely impaired polymerization of recombinant fibrinogen gamma-364 Asp—>His, the substitution discovered in a heterozygous individual. J Biol Chem 1997;272:29596–29601 [DOI] [PubMed] [Google Scholar]
- 18.Weisel JW, Litvinov RI. Mechanisms of fibrin polymerization and clinical implications. Blood 2013;121:1712–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen R, Doolittle RF. Cross-linking sites in human and bovine fibrin. Biochemistry 1971;10:4487–4491 [DOI] [PubMed] [Google Scholar]
- 20.Farrell DH, Thiagarajan P. Binding of recombinant fibrinogen mutants to platelets. J Biol Chem 1994;269:226–231 [PubMed] [Google Scholar]
- 21.Podolnikova NP, et al. . Identification of a novel binding site for platelet integrins alpha IIb beta 3 (GPIIbIIIa) and alpha 5 beta 1 in the gamma C-domain of fibrinogen. J Biol Chem 2003;278:32251–32258 [DOI] [PubMed] [Google Scholar]
- 22.Fornace AJ Jr., et al. . Structure of the human gamma-fibrinogen gene. Alternate mRNA splicing near the 3′ end of the gene produces gamma A and gamma B forms of gamma-fibrinogen. J Biol Chem 1984;259:12826–12830 [PubMed] [Google Scholar]
- 23.Meh DA, et al. . The amino acid sequence in fibrin responsible for high affinity thrombin binding. Thromb Haemost 2001;85:470–474 [PubMed] [Google Scholar]
- 24.Siebenlist KR, Meh DA, Mosesson MW. Plasma factor XIII binds specifically to fibrinogen molecules containing gamma chains. Biochemistry 1996;35:10448–10453 [DOI] [PubMed] [Google Scholar]
- 25.Farrell DH, et al. . Role of fibrinogen alpha and gamma chain sites in platelet aggregation. Proc Natl Acad Sci U S A 1992;89:10729–10732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooper AV, Standeven KF, Ariens RA. Fibrinogen gamma-chain splice variant gamma’ alters fibrin formation and structure. Blood 2003;102:535–540 [DOI] [PubMed] [Google Scholar]
- 27.Collet JP, et al. . Influence of gamma’ fibrinogen splice variant on fibrin physical properties and fibrinolysis rate. Arterioscler Thromb Vasc Biol 2004;24:382–386 [DOI] [PubMed] [Google Scholar]
- 28.Lovely RS, et al. . Assessment of genetic determinants of the association of gamma’ fibrinogen in relation to cardiovascular disease. Arterioscler Thromb Vasc Biol 2011;31:2345–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheung EY, et al. . Specific effects of fibrinogen and the gammaA and gamma’-chain fibrinogen variants on angiogenesis and wound healing. Tissue Eng Part A 2014. [Epub ahead of print]; DOI: 10.1089/ten.tea.2014.0020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren Y, Ding Q, Zhang X. Ficolins and infectious diseases. Virol Sin 2014;29:25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le Y, et al. . Human L-ficolin: plasma levels, sugar specificity, and assignment of its lectin activity to the fibrinogen-like (FBG) domain. FEBS Lett 1998;425:367–370 [DOI] [PubMed] [Google Scholar]
- 32.Lynch NJ, et al. . L-ficolin specifically binds to lipoteichoic acid, a cell wall constituent of Gram-positive bacteria, and activates the lectin pathway of complement. J Immunol 2004;172:1198–1202 [DOI] [PubMed] [Google Scholar]
- 33.Kilpatrick DC, et al. . Stable bronchiectasis is associated with low serum L-ficolin concentrations. Clin Respir J 2009;3:29–33 [DOI] [PubMed] [Google Scholar]
- 34.Aoyagi Y, et al. . Role of L-ficolin/mannose-binding lectin-associated serine protease complexes in the opsonophagocytosis of type III group B streptococci. J Immunol 2005;174:418–425 [DOI] [PubMed] [Google Scholar]
- 35.Frederiksen PD, et al. . M-ficolin, an innate immune defence molecule, binds patterns of acetyl groups and activates complement. Scand J Immunol 2005;62:462–473 [DOI] [PubMed] [Google Scholar]
- 36.Teh C, et al. . M-ficolin is expressed on monocytes and is a lectin binding to N-acetyl-D-glucosamine and mediates monocyte adhesion and phagocytosis of Escherichia coli. Immunology 2000;101:225–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luo F, et al. . Ficolin-2 defends against virulent Mycobacteria tuberculosis infection in vivo, and its insufficiency is associated with infection in humans. PLoS One 2013;8:e73859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu YL, et al. . Early increased ficolin-2 concentrations are associated with severity of liver inflammation and efficacy of anti-viral therapy in chronic hepatitis C patients. Scand J Immunol 2013;77:144–150 [DOI] [PubMed] [Google Scholar]
- 39.Pan Q, et al. . L-ficolin binds to the glycoproteins hemagglutinin and neuraminidase and inhibits influenza A virus infection both in vitro and in vivo. J Innate Immun 2012;4:312–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jensen ML, et al. . Ficolin-2 recognizes DNA and participates in the clearance of dying host cells. Mol Immunol 2007;44:856–865 [DOI] [PubMed] [Google Scholar]
- 41.Ammitzboll CG, et al. . Non-synonymous polymorphisms in the FCN1 gene determine ligand-binding ability and serum levels of M-ficolin. PLoS One 2012;7:e50585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tsujimura M, et al. . Detection of serum thermolabile beta-2 macroglycoprotein (Hakata antigen) by enzyme-linked immunosorbent assay using polysaccharide produced by Aerococcus viridans. Clin Diagn Lab Immunol 2001;8:454–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verma A, et al. . Human H-ficolin inhibits replication of seasonal and pandemic influenza A viruses. J Immunol 2012;189:2478–2487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swierzko A, et al. . New functional ligands for ficolin-3 among lipopolysaccharides of Hafnia alvei. Glycobiology 2012;22:267–280 [DOI] [PubMed] [Google Scholar]
- 45.Michalski M, et al. . Ficolin-3 activity towards the opportunistic pathogen, Hafnia alvei. Immunobiology 2014. DOI: 10.1016/j.imbio.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 46.Honore C, et al. . The innate immune component ficolin 3 (Hakata antigen) mediates the clearance of late apoptotic cells. Arthritis Rheum 2007;56:1598–1607 [DOI] [PubMed] [Google Scholar]
- 47.Munthe-Fog L, et al. . Immunodeficiency associated with FCN3 mutation and ficolin-3 deficiency. N Engl J Med 2009;360:2637–2644 [DOI] [PubMed] [Google Scholar]
- 48.Schlosser A, et al. . Characterization of FIBCD1 as an acetyl group-binding receptor that binds chitin. J Immunol 2009;183:3800–3809 [DOI] [PubMed] [Google Scholar]
- 49.Thomsen T, et al. . The recognition unit of FIBCD1 organizes into a noncovalently linked tetrameric structure and uses a hydrophobic funnel (S1) for acetyl group recognition. J Biol Chem 2010;285:1229–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuwaraj S, et al. . Genomic characterization, localization, and functional expression of FGL2, the human gene encoding fibroleukin: a novel human procoagulant. Genomics 2001;71:330–338 [DOI] [PubMed] [Google Scholar]
- 51.Liu Y, et al. . The FGL2/fibroleukin prothrombinase is involved in alveolar macrophage activation in COPD through the MAPK pathway. Biochem Biophys Res Commun 2010;396:555–561 [DOI] [PubMed] [Google Scholar]
- 52.Foerster K, et al. . The novel immunoregulatory molecule FGL2: a potential biomarker for severity of chronic hepatitis C virus infection. J Hepatol 2010;53:608–615 [DOI] [PubMed] [Google Scholar]
- 53.Jia P, et al. . TNF-alpha upregulates Fgl2 expression in rat myocardial ischemia/reperfusion injury. Microcirculation 2013;20:524–533 [DOI] [PubMed] [Google Scholar]
- 54.Selzner N, et al. . FGL2/fibroleukin mediates hepatic reperfusion injury by induction of sinusoidal endothelial cell and hepatocyte apoptosis in mice. J Hepatol 2012;56:153–159 [DOI] [PubMed] [Google Scholar]
- 55.Chan CW, et al. . Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow-derived dendritic cells. J Immunol 2003;170:4036–4044 [DOI] [PubMed] [Google Scholar]
- 56.Midwood K, et al. . Tenascin-C is an endogenous activator of Toll-like receptor 4 that is essential for maintaining inflammation in arthritic joint disease. Nat Med 2009;15:774–780 [DOI] [PubMed] [Google Scholar]
- 57.Laurens N, Koolwijk P, de Maat MP. Fibrin structure and wound healing. J Thromb Haemost 2006;4:932–939 [DOI] [PubMed] [Google Scholar]
- 58.Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol 2001;167:2887–2894 [DOI] [PubMed] [Google Scholar]
- 59.Yakovlev S, et al. . Interaction of fibrin(ogen) with leukocyte receptor alpha M beta 2 (Mac-1): further characterization and identification of a novel binding region within the central domain of the fibrinogen gamma-module. Biochemistry 2005;44:617–626 [DOI] [PubMed] [Google Scholar]
- 60.Fagiani E, Christofori G. Angiopoietins in angiogenesis. Cancer Lett 2013;328:18–26 [DOI] [PubMed] [Google Scholar]
- 61.Kampfer H, Pfeilschifter J, Frank S. Expressional regulation of angiopoietin-1 and -2 and the tie-1 and -2 receptor tyrosine kinases during cutaneous wound healing: a comparative study of normal and impaired repair. Lab Invest 2001;81:361–373 [DOI] [PubMed] [Google Scholar]
- 62.Fiedler U, et al. . The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood 2004;103:4150–4156 [DOI] [PubMed] [Google Scholar]
- 63.Staton CA, et al. . Angiopoietin-1, angiopoietin-2 and Tie-2 receptor expression in human dermal wound repair and scarring. Br J Dermatol 2010;163:920–927 [DOI] [PubMed] [Google Scholar]
- 64.Maisonpierre PC, et al. . Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science 1997;27755–60 [DOI] [PubMed] [Google Scholar]
- 65.Harfouche R, Hussain SN. Signaling and regulation of endothelial cell survival by angiopoietin-2. Am J Physiol Heart Circ Physiol 2006;291:H1635–H1645 [DOI] [PubMed] [Google Scholar]
- 66.Lee HS, et al. . Gln362 of angiopoietin-2 mediates migration of tumor and endothelial cells through association with alpha5beta1 integrin. J Biol Chem 2014;289:31330–31340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gale NW, et al. . Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell 2002;3:411–423 [DOI] [PubMed] [Google Scholar]
- 68.Goodson WH, 3rd, Hung TK. Studies of wound healing in experimental diabetes mellitus. J Surg Res 1977;22:221–227 [DOI] [PubMed] [Google Scholar]
- 69.Bohlen HG, Niggl BA. Adult microvascular disturbances as a result of juvenile onset diabetes in Db/Db mice. Blood Vessels 1979;16:269–276 [DOI] [PubMed] [Google Scholar]
- 70.Thurston G, et al. . Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science 1999;286:2511–2514 [DOI] [PubMed] [Google Scholar]
- 71.Jeansson M, et al. . Angiopoietin-1 is essential in mouse vasculature during development and in response to injury. J Clin Invest 2011;121:2278–2289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carlson TR, et al. . Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem 2001;276:26516–26525 [DOI] [PubMed] [Google Scholar]
- 73.Bitto A, et al. . Angiopoietin-1 gene transfer improves impaired wound healing in genetically diabetic mice without increasing VEGF expression. Clin Sci (Lond) 2008;114:707–718 [DOI] [PubMed] [Google Scholar]
- 74.Li Y, et al. . Mesenchymal stem cells modified with angiopoietin-1 gene promote wound healing. Stem Cell Res Ther 2013;4:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Van Slyke P, et al. . Acceleration of diabetic wound healing by an angiopoietin peptide mimetic. Tissue Eng Part A 2009;15:1269–1280 [DOI] [PubMed] [Google Scholar]
- 76.Korpela E, et al. . Vasculotide, an Angiopoietin-1 mimetic, reduces acute skin ionizing radiation damage in a preclinical mouse model. BMC Cancer 2014;14: 614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hato T, Tabata M, Oike Y. The role of angiopoietin-like proteins in angiogenesis and metabolism. Trends Cardiovasc Med 2008;18:6–14 [DOI] [PubMed] [Google Scholar]
- 78.Camenisch G, et al. . ANGPTL3 stimulates endothelial cell adhesion and migration via integrin alpha vbeta 3 and induces blood vessel formation in vivo. J Biol Chem 2002;277:17281–17290 [DOI] [PubMed] [Google Scholar]
- 79.Sahni A, et al. . FGF-2 binding to fibrin(ogen) is required for augmented angiogenesis. Blood 2006;107:126–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yokoyama K, et al. . Identification of amino acid sequences in fibrinogen gamma-chain and tenascin C C-terminal domains critical for binding to integrin alpha vbeta 3. J Biol Chem 2000;275:16891–16898 [DOI] [PubMed] [Google Scholar]
- 81.Canfield AE, Schor AM. Evidence that tenascin and thrombospondin-1 modulate sprouting of endothelial cells. J Cell Sci 1995;108(Pt 2): 797–809 [DOI] [PubMed] [Google Scholar]
- 82.Schenk S, Chiquet-Ehrismann R, Battegay EJ. The fibrinogen globe of tenascin-C promotes basic fibroblast growth factor-induced endothelial cell elongation. Mol Biol Cell 1999;10:2933–2943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goh YY, et al. . Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration. Am J Pathol 2010;177:2791–2803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pal M, et al. . Angiopoietin-like 4 regulates epidermal differentiation. PLoS One 2011;6:e25377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goh YY, et al. . Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem 2010;285:32999–33009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chong HC, et al. . Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice. Mol Ther 2014;22:1593–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Latijnhouwers M, et al. . Human epidermal keratinocytes are a source of tenascin-C during wound healing. J Invest Dermatol 1997;108:776–783 [DOI] [PubMed] [Google Scholar]
- 88.Chiquet-Ehrismann R, et al. . Tenascin-C expression by fibroblasts is elevated in stressed collagen gels. J Cell Biol 1994;127(6 Pt 2): 2093–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Forsberg E, et al. . Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci U S A 1996;93:6594–6599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sumioka T, et al. . Impaired cornea wound healing in a tenascin C-deficient mouse model. Lab Invest 2013;93:207–217 [DOI] [PubMed] [Google Scholar]
- 91.Okamura N, et al. . Deficiency of tenascin-C delays articular cartilage repair in mice. Osteoarthritis Cartilage 2010;18:839–848 [DOI] [PubMed] [Google Scholar]
- 92.Tamaoki M, et al. . Tenascin-C regulates recruitment of myofibroblasts during tissue repair after myocardial injury. Am J Pathol 2005;167:71–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Egging D., et al. . Wound healing in tenascin-X deficient mice suggests that tenascin-X is involved in matrix maturation rather than matrix deposition. Connect Tissue Res 2007;48:93–98 [DOI] [PubMed] [Google Scholar]
- 94.Egging D, et al. . Interactions of human tenascin-X domains with dermal extracellular matrix molecules. Arch Dermatol Res 2007;298:389–396 [DOI] [PubMed] [Google Scholar]
- 95.Alcaraz LB, et al. . Tenascin-X promotes epithelial-to-mesenchymal transition by activating latent TGF-beta. J Cell Biol 2014;205:409–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tanio M, et al. . Trivalent recognition unit of innate immunity system: crystal structure of trimeric human M-ficolin fibrinogen-like domain. J Biol Chem 2007;282:3889–3895 [DOI] [PubMed] [Google Scholar]
- 97.Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng Part B Rev 2008;14:199–215 [DOI] [PubMed] [Google Scholar]
- 98.Janmey PA, Winer JP, Weisel JW. Fibrin gels and their clinical and bioengineering applications. J R Soc Interface 2009;6:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seifert AW, et al. . Skin regeneration in adult axolotls: a blueprint for scar-free healing in vertebrates. PLoS One 2012;7:e32875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vorotnikova E, et al. . Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol 2010;29:690–700 [DOI] [PubMed] [Google Scholar]
- 101.Whitby DJ, et al. . Rapid epithelialisation of fetal wounds is associated with the early deposition of tenascin. J Cell Sci 1991;99(Pt 3):583–586 [DOI] [PubMed] [Google Scholar]
- 102.Dalkowski A, et al. . Increased expression of tenascin C by keloids in vivo and in vitro. Br J Dermatol 1999;141:50–56 [DOI] [PubMed] [Google Scholar]
- 103.Barton WA, et al. . Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat Struct Mol Biol 2006;13:524–532 [DOI] [PubMed] [Google Scholar]
- 104.Ugarova TP, et al. . Identification of a novel recognition sequence for integrin alphaM beta2 within the gamma-chain of fibrinogen. J Biol Chem 1998;273:22519–22527 [DOI] [PubMed] [Google Scholar]
- 105.Pineda AO, et al. . Crystal structure of thrombin in complex with fibrinogen gamma’ peptide. Biophys Chem 2007;125:556–559 [DOI] [PubMed] [Google Scholar]