Abstract
Since their discovery in the late 1970’s, protein kinase C (PKC) isozymes represent one of the most extensively studied signaling kinases. PKCs signal through multiple pathways and control the expression of genes relevant for cell cycle progression, tumorigenesis and metastatic dissemination. Despite the vast amount of information concerning the mechanisms that control PKC activation and function in cellular models, the relevance of individual PKC isozymes in the progression of human cancer is still a matter of controversy. Although the expression of PKC isozymes is altered in multiple cancer types, the causal relationship between such changes and the initiation and progression of the disease remains poorly defined. Animal models developed in the last years helped to better understand the involvement of individual PKCs in various cancer types and in the context of specific oncogenic alterations. Unraveling the enormous complexity in the mechanisms by which PKC isozymes impact on tumorigenesis and metastasis is key for reassessing their potential as pharmacological targets for cancer treatment.
Keywords: Protein kinase C (PKC), mitogenesis, apoptosis, survival, tumorigenesis, metastasis, animal models
Introduction
Protein kinase C (PKC), a prototypical class of serine/threonine kinases, exemplifies specific signaling molecules that link multiple cellular processes to cancer. Originally identified as a cellular receptor for the phorbol ester tumor promoters more than 30 years ago [1–2], PKC became the subject of intense studies by academic laboratories and pharmaceutical companies (>50,000 citations in PubMed, which is even more than other ABC kinases such as PKA or PKB/Akt). Extensive work established these kinases as pleiotropic regulators of cell function, including proliferation, differentiation, survival and motility [3]. To date, it is clear that PKCs are associated with a number of diseases, including cancer, cardiovascular dysfunctions and metabolic disorders. The complexity in PKC signaling arises from the fact that PKC is a multifamily of structurally related kinases with diverse biological functions. Indeed, mammalian PKCs encompass 10 members that represent the products of 9 different genes located in different chromosomes. PKC isozymes have been classified into three groups: “conventional” or “classical” PKCs (cPKCs) that are composed of PKCα, two splice variants of PKCβ (PKCβI and PKCβII) and PKCγ; “novel” PKCs (nPKCs), a group that includes PKCδ, PKCε, PKCη, and PKCθ; and “atypical” PKCs (aPKCs) ζ and ι (λ). cPKCs and nPKCs are activated by diacylglycerol (DAG), a lipid second messenger transiently generated upon stimulation of membrane receptors such as tyrosine-kinase and G-protein-coupled receptors. DAG mimics the action of phorbol esters, as they bind to the C1 domains in the regulatory region. Only the cPKCs are calcium-sensitive, as they have a calcium-binding C2 domain (the C2 domain in nPKCs is calcium-insensitive). aPKCs display unique regulatory properties: they are unable to bind DAG or calcium and rather depend on protein-protein interactions and phosphorylation for their activation [3] (Fig. 1).
Figure 1.
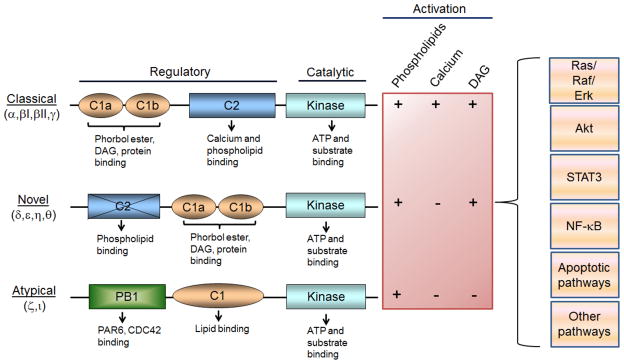
Structure of PKC isozymes. PKCs are multidomain proteins that are regulated by lipids and protein-protein interactions. Diacyclycerol (DAG) generated upon activation of receptors causes the activation of cPKCs and nPKCs, and its actions are mimicked by phorbol esters. aPKCs do not respond to DAG or phorbol esters. PKCs activate a number of signal transduction pathways that regulate tumorigenesis and metastasis.
In the last years we have witnessed major advances in our understanding of the roles of PKCs in tumor development and progression, including in late stages of the disease and metastasis. This review summarizes the knowledge on PKC isozymes in cancer progression and highlights the most recent advances in the field, particularly using genetically modified mouse models in the context of specific oncogenic alterations.
PKC isozyme expression in cancer: chance or causality?
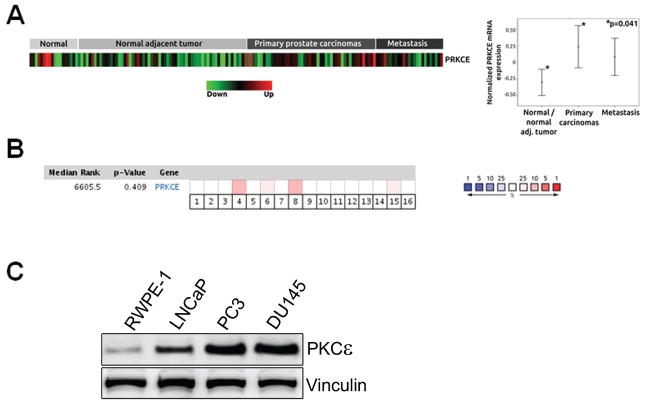
Expression levels of PKC isozymes change in neoplastic diseases. The overall picture is however confusing, partly due to potential issues of antibody specificity in immunohistochemical studies and lack of appropriate validation controls in many reports. The standing question is whether those changes in expression have any causal relationship with disease progression. An additional complication is that, in an era when microarray mRNA databases are routinely used, there are significant discrepancies between the available information on PKC expression at the mRNA and protein levels. This can be epitomized for PKCε, an isozyme that is markedly up-regulated in most epithelial cancers at the protein level [4–7], but shows only marginal or no changes in mRNA databases (Fig. 2). Whereas high expression of PKCε in tumors may involve changes at a transcriptional level, expression underestimation by databases may relate to post-translational events that ultimately modify protein stability. Modeling expression patterns from mRNA expression databases, which in most cases have not been generated from microdissected tissues, can distort the actual profile of PKC protein expression in tumors and ultimately mislead our efforts to correlate those changes with clinicopathological outcomes.
Figure 2.
Expression of PKCε in prostate cancer. (a) In silico PKCε mRNA expression profiling in 81 normal/normal adjacent prostate tumors, 48 primary prostate carcinomas and 25 prostate cancer metastasis obtained from a publicly available dataset (GSE6919). PRKCE, PKCε gene. (b) Meta-analysis of PRKCE mRNA expression across 16 prostate microarray studies from the Oncomine database. This meta-analysis shows non-statistically significant differences in PRKCE mRNA expression (combined p-value=0.41) between normal and prostate cancer groups. Red intensity is a representative of the statistical significance in mean difference between normal and protate cancer for each study. (c) Expression of PKCε in “normal” immortalized prostate epithelial RWPE-1 cells vs. prostate cancer cells. This figure was originally published by Garg R, Blando J, Perez CJ, Wang H, Benavides FJ, and Kazanietz MG. in J Biol Chem. 2012 287:37570–37582, © The American Society for Biochemistry and Molecular Biology.
Another important issue that received little attention is the activation status of PKC isozymes in cancer. There is little experimental evidence supporting either hyperactivation or hypoactivation of PKCs in human tumors. Unlike other important kinases implicated in cancer progression, such as Erk, JNK, or Akt, phosphorylation of PKCs does not necessarily correlate with activation status. One impediment to address this important matter is the lack of reliable readouts associated with the activated status of individual PKCs, in particular PKC isozyme-specific substrates that could be detected in human tumors. Genetically encoded reporters for PKC isozymes reliably detect enzyme activation and substrate phosphorylation in cellular models in culture [8–12]; however, we still lack tools to detect activated PKCs or their specific substrates in tumors by immunohistochemistry. The association of PKCs to membranes is a requisite for the activation of DAG/phorbol ester-regulated PKCs [3]. Whereas some exceptions have been reported, such as the activation by proteolytic cleavage [13], cPKCs and nPKCs translocate to the plasma membrane in response to stimuli such as growth factor receptor activation. Although less understood at a mechanistic level, cPKCs and nPKCs can also redistribute to a number of intracellular compartments, including the translocation to mitochondria, Golgi, endoplasmic reticulum and nuclear membrane. Constitutive association of PKCs to internal membranes has also been reported [14–17]. At present, we do not understand well the significance of such compartmentalization and whether PKCs are fully activated in discrete intracellular locations due to differential membrane compositions, DAG availability, and/or the presence of isozyme-specific protein partners that cooperate for the transition to an activated status. All these factors conspire against a full appreciation on how PKC activation contributes to disease progression.
PKCα: tumor promoter or tumor suppressor?
PKCα has been long recognized as a regulator of multiple aspects of tumor growth, including proliferation, survival, differentiation and motility. As several studies linked PKCα to enhanced proliferation and anti-apoptotic signals [18–22], there has been significant interest in this kinase as a potential target for cancer therapy. However, PKCα had limited success as a drug target for cancer. Indeed, due to its very complex and highly tissue-specific functions, PKCα acts as a tumor promoter or a tumor suppressor depending on the context. To add another level of complexity, PKCα is up-regulated in some cancers (such as bladder, endometrial, and breast cancer) and down-regulated in others (such as colorectal tumors and malignant renal cell carcinomas [23–24]). There is little information on substrates specifically phosphorylated by PKCα or genetic programs controlled by PKCα, thus rendering our comprehension of the molecular basis of this functional diversity incomplete.
Early studies in glioma cellular models established that PKCα is up-regulated relative to astrocytes. Antisense oligonucleotides against PKCα inhibit the proliferation of glioma cells [25]. Consistent with these results, overexpression of PKCα in U87 glioblastoma cells enhances proliferation. Although overexpression of PKCα did not protect U87 cells from apoptosis by etoposide, other studies documented that PKCα renders enhanced resistance to apoptosis in response to radiation and chemotherapy [18, 26–27]. In U1242 glioblastoma cells, PKCα regulates the activation of NF-κB, which results in a pro-survival phenotype [28]. Aprinocarsen (Ly900003, ISIS 3521), an antisense oligonucleotide directed against the 3′-untranslated region of PKCα, arrests A172 glioma cells and induces p53. Aprinocarsen also impairs tumor growth in xenograft models. In combination with other chemotherapeutic agents, aprinocarsen was shown to have additive or super-additive antitumor effects [29–31]. Despite encouraging responses in early clinical trials, this anti-PKCα agent failed to make it into phase III clinical trials either alone or in combination with other agents [29]. Another PKCα inhibitor, the staurosporine analogue UCN-01 [32] proved to be a potent anti-tumor agent in preclinical models, but its effect cannot be explained simply by PKCα inhibition [33]. It is also intriguing that PKCα RNAi depletion but not PKCα inhibition impairs the growth of glioma cell lines, suggesting that the effect is independent of the catalytic phosphotransferase activity of the enzyme [34].
Another interesting link between PKCα and cancer progression has been established in breast. Ways et al. showed that ectopic overexpression PKCα in MCF-7 breast cancer cells (which express low levels of PKCα) enhances proliferative rate, confers anchorage-independent growth and tumorigenic potential in nude mice, and drastically alters cell shape by inducing loss of an epithelioid morphology. PKCα overexpressing MCF-7 cells have reduced estrogen receptor (ER) levels, suggesting that PKCα contributes to the switch from ER-positive to ER-negative status [35]. Likewise, Tonetti et al. found that stable overexpression of PKCα in T47-D breast cancer cells is accompanied by down-regulation of ER function [36] and confers hormone-independent tumor growth that cannot be inhibited by tamoxifen [37]. A recent study suggests that this effect may be mediated by Notch-4 [38]. Elevated PKCα expression was suggested to be a predictor of tamoxifen treatment failure, which fits with the observation that patient tumor samples with elevated PKCα levels are generally negative for estrogen receptor (ER) expression, and these patients respond less to endocrine therapy [39–40]. A recent study by Larsson and coworkers demonstrated that PKCα levels correlates with estrogen receptor (ER) and progesterone receptor negativity, proliferative activity, and tumor grade [41]. Thus, altogether it seems that PKCα is a biomarker for poor prognosis and endocrine therapy resistance in breast cancer. PKCα is also an effector of ErbB2 in breast cancer cells, and ErbB2 siRNA depletion decreases PKCα protein levels. Moreover, ErbB2 overexpression correlates with membrane-associated staining of PKCα in human breast cancer specimens, suggesting that ErbB2 drives the constitutive activation of PKCα [42]. Gö6976, a pharmacological inhibitor of cPKCs [43], abrogates ErbB2-mediated up-regulation of urokinase-type plasminogen activator (uPA) and cell invasion [44]. Whereas PKCα expression is higher in triple-negative breast cancers than to other subtypes [45], we still need to underscore meaningful associations of this PKC with genetic alterations specific for each breast cancer subtype.
In a very recent study by the Weinberg laboratory, PKCα was found to be enriched in epithelial-to-mesenchymal (EMT)-induced mammary cells [46]. Interestingly, inhibitors targeting PKCα preferentially kill mesenchymal cells relative to epithelial cell lines, and likewise, depletion of PKCα using shRNA results in a substantial loss of mesenchymal cells. A PKCα signaling network is activated preferentially in cancer stem cells by PDGF and involves the transcription factor FRA1. The activation of the PDGFR-PKCα-FRA1 pathway in breast cancer stem cells makes them particularly susceptible to pharmacological inhibition of PKCα. Whereas the relevance of this pathway has to be established in other cancer types, this study certainly shed light into the potential therapeutic value of targeting PKCα in epithelial cancers.
Despite the reported pro-tumorigenic effects of PKCα, it has been also described as a growth inhibitory kinase in several cell types. For example, activation of PKCα in non-small cell lung cancer (NSCLC) cells leads to p21Cip1 up-regulation, inhibition of cell growth and senescence [47]. Not surprisingly, aprinocarsen showed no significant benefit for NSCLC patients, either alone or in combination with other chemotherapeutic agents [29].
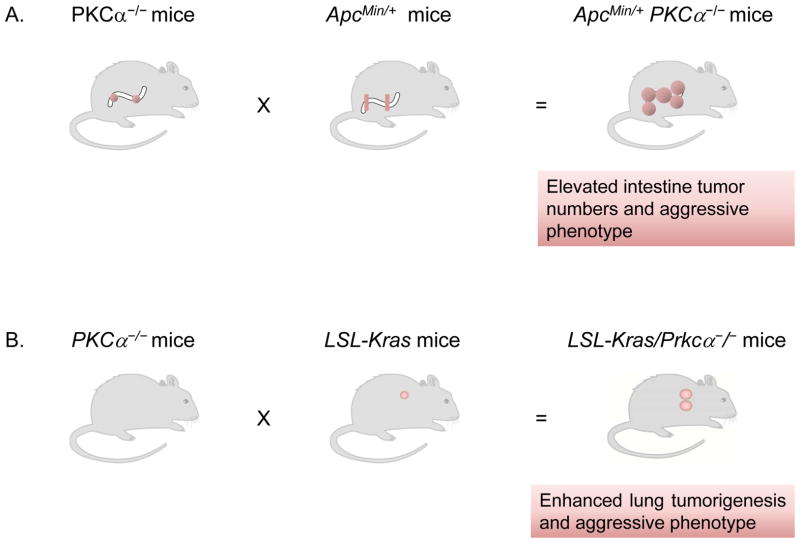
Years ago, Black and coworkers reported that PKCα activation triggers a program of cell cycle exit-specific events in intestinal crypts through the repression of cyclin D1 translation [48–49]. This effect implicates the activation of the translational repressor 4E-BP1 through a phosphatase 2A-dependent mechanism and in a PI3K/Akt-independent manner [50]. PKCα also inhibits the Wnt/β-catenin pathway in colon cancer cells and represses the expression of β-catenin target genes such as c-Myc [51], a mechanism that may involve receptor-related orphan receptor alpha RORα [52]. Interestingly, a small molecule screening identified a compound (CGK062) that promotes PKCα-mediated phosphorylation of β-catenin, leading to its proteasomal degradation. This compound has anti-tumor effects in nude mice [53]. The expression of PKCα in proliferating intestinal epithelial cells is repressed both in vitro and in vivo by the SOX9 transcription factor [54]. All neoplasm arising in APC−/+ mice, which develop multiple intestinal neoplasia, express low levels of PKCα. Remarkably, loss of PKCα directly correlates with aggressiveness of intestinal tumors. Furthermore, tumor formation and aggressiveness are enhanced in double transgenic APCMin/+; PKCα−/− mice (Fig. 3A). Interestingly, spontaneous intestinal tumors develop in PKCα−/− mice [55–56]. PKCα also suppresses skin tumor formation induced by DMBA. However, PKCα deficiency does not alter the size or malignancy of skin tumors [57].
Figure 3.
Loss of PKCα gene enhances tumor progression. (a) Deletion of the PKCα gene enhances the formation of tumors in APCMin/+ mice, and those tumors show a more aggressive phenotype. (b) Deletion of PKCα in K-Ras mutant mice resulted in progression of benign tumors to adenocarcinoma. Tumors exhibit high frequency and grade, and are bigger in size.
PKCα activation by phorbol esters contributes to cell death in androgen-dependent prostate cancer cells. Activation of PKCα in LNCaP cells leads to a rapid and reversible dephosphorylation of Akt possibly via activation of a PP2A phosphatase [58]. Stable DAG analogues with preferred selectivity for PKCα also induce apoptosis in LNCaP cells [59]. A kinase-dead PKCα mutant blocks the apoptotic response elicited by a combination of PMA treatment and radiation, and a constitutively active PKCα mutant sensitizes cells to radiation treatment. While radiation alone reduces initial LNCaP tumor growth and serum PSA levels in mice, combinatorial treatment with PMA or a specific PKCα DAG activator eliminates tumor growth and drastically reduces PSA levels [60].
A very interesting recent study by the group of Alan Fields reported that PKCα plays a key role in K-Ras-mediated lung tumorigenesis [61]. There is an evident loss of expression of PKCα in primary human NSCLC tumors. Remarkably, PKCα knockout mice display enhanced K-Ras lung tumorigenesis (Fig. 3B) and bypass oncogene-induced senescence. The tumor promoting effect caused by loss of PKCα may involve the expansion of bronchoalveolar stem cells (BASCs). Mechanistic analysis determined that loss of PKCα reduces the activation of p38 MAPK in BASCs from K-Ras tumors and augments TGFβ1 mRNA levels. Moreover, a TGFβ receptor inhibitor reversed the effect of PKCα loss in K-Ras/PKCα-depleted tumors. This study reported the inhibitors of DNA binding (Id) Id1-3 as potential downstream targets of PKCα-dependent tumor suppressor activity, as also observed in other intestinal cells and fibroblasts [62–63]. Therefore, PKCα suppresses tumor initiation and progression in the K-Ras lung cancer mouse model through a p38 MAPK/TGFβ signaling axis.
PKCα has been implicated in invasion and metastasis, mostly as a positive regulator [64–69]. In breast cancer cells, ErbB2-dependent activation of PKCα promotes cell invasion [42]. PKCα overexpressing MCF-7 breast cancer cells display enhanced motility, which was attributed to decreased expression of E-cadherin and β-catenin, and high expression of MMP-2/MMP-9 [70–71]. A specific PKCα peptide inhibitor significantly reduced metastasis of mouse mammary cancer cells to the lungs. Analysis of highly metastatic (4T1) and non-metastatic (JC) mouse mammary cells indicates that the basal activation of PKCα is higher in the former. Treatment with this PKCα antagonistic peptide did not affect tumor growth but blocked metastasis of 4T1 cells to the lungs. PKCα inhibition blocks metastasis by inhibiting the activation of MMPs in combination with decreased NF-κB activity and CXCL12 receptor levels [72].
PKCβ isozymes: spliced variants with distinct involvement in cancer
PKCβI and PKCβII, spliced variants encoded by the PRKCB gene have differential tissue expression and a distinct involvement in cancer. PKCβ isoforms have been implicated in the progression of many cancer types, including lymphoma, glioblastoma, breast, prostate, and colorectal cancers [73–77]. It is not fully understood why PKCβ isoforms have in some cases different functions. This may relate to unique lipid- and protein-interactions via their different C-terminal domains that confer distinctive localization properties [78–80]. However, many studies using pharmacological agents do not distinguish between PKCβ subtypes.
Early studies reported elevated PKCβII levels both during the initial stages of tumorigenesis and in colonic carcinomas relative to normal colonic tissue [81–83]. Spindler et al. reported that 18% of primary adenocarcinomas exhibit very high levels of PKCβII, which correlates with poor survival rates [84]. PKCβII has been implicated in colon cancer cell proliferation in vitro [85–86]. Murray et al. generated a transgenic mouse model overexpressing PKCβII in the intestinal epithelium. In addition to epithelial hyperproliferation, these mice display increased susceptibility to carcinogen-induced preneoplastic lesions in the colon [87]. The phenotype has been linked to repression of TGFβ signaling and elevated COX-2 expression [73]. In the proximal colon, activated K-Ras induces the expression of PKCβII, activation of the Mek/Erk signaling axis, and increased epithelial cell proliferation [88]. In a cellular model of intestinal cells, stable overexpression of PKCβII confers an invasive phenotype mediated by a Ras/Mek/PKCι/Rac1-dependent pathway [89]. The role of PKCβI in the colon is less clear. Overexpression of PKCβI suppresses the growth of HT29 and SW480 colon cancer cell xenograft [90]. However, other report shows that expression of PKCβI confers resistance to apoptosis by TNFα and paclitaxel [91].
PKCβI expression positively correlates with high Gleason scores in prostate carcinomas, and inhibition of this kinase blocks androgen receptor-induced tumor cell proliferation in vitro and xenograft growth in vivo. Notably, PKCβI phosphorylates histone H3 and inhibits androgen-dependent transcription [92]. A study using a specific PKCβII peptide inhibitor showed that this isoform is involved in prostate cancer cell proliferation. PKCβII also plays an important role in endothelial cell proliferation, and inhibition of PKCβII reduces angiogenesis. These effects were linked to dysregulation of cytokinesis and microtubule organization [74]. Enzastaurin, an inhibitor with some degree of specificity towards PKCβ, displays antiproliferative effects in PC3 cells [93].
Overexpression of either PKCβI or PKCβII in MCF-7 breast cancer cells promotes cell growth and enhances cyclin D1 levels, whereas dominant-negative PKCβ mutants inhibit growth [94]. On the other hand, another report showed that PKCβI overexpression induces a less aggressive biological behavior in MCF-7 cells characterized by reduced tumor formation [95]. In a murine mammary model, PKCβI inhibits tumorigenesis despite having a positive effect on growth in culture. Moreover, tumor cells that overexpress PKCβI have attenuated metastatic capacity to the lungs [96]. Enzastaurin has significant effects on the growth of breast cancer cells in vitro and in vivo [97–98]. High levels of PKCβII have been reported in several breast cancer cell lines and patient samples. The levels of cytoplasmic PKCβII expression positively correlate with ErbB2/Her2 levels, while nuclear PKCβII positively correlates with ER levels [99].
PKCβII is expressed in human NSCLC specimens with significant variability, both in tumor cells and the stroma [100]. Enzastaurin in combination with the antifolate pemetrexed causes G2/M checkpoint abrogation and apoptosis in lung cancer cells [101]. In cell culture, enzastaurin is a potent inhibitor of VEGF-stimulated proliferation of endothelial cells, and in vivo this inhibitor causes a significant reduction in intratumoral vessels that parallels a delay in lung cancer tumor growth [100]. Enzastaurin also enhances the anti-angiogenic effects of radiation in NSCLC models [102]. The PKCβ inhibitor, Enzastaurin, regulates proliferation of glioblastoma cells by supporting the activity of GSK3, S6 kinase and Akt [103]. The effect of the PKCβ inhibitor was also shown in U87MG human glioblastoma cells inoculated into nude mice. Enzastaurin proved to be efficient as an anti-angiogenic compound in models of glioblastoma [77], however clinical trials in patients with recurrent high-grade glioma show limited success when this inhibitor was used as a monotherapy [104].
Patients with diffuse large B-cell lymphoma that express PKCβ have reduced overall survival compared to those that are negative for this kinase [105–106]. Enzastaurin displays pro-apoptotic properties in T-cell and B-cell lymphoma cell lines [107–108]. There is significant interest in using this PKCβ inhibitor for the treatment of various types of lymphomas, both as a single agent and in combination therapies [109–110]. Taking advantage of a PKCβ knockout mouse model, it has been recently demonstrated that stromal PKCβII is indispensable for the survival of chronic lymphocytic leukemia (CLL) B cells. The fact that stromal PKCβII is up-regulated in biopsies from patients with CLL, and that CLL cells induce the expression of stromal PKCβ [111], highlights the need to better understand how this PKC contributes to cancer development in the context of the different tumor microenvironments.
PKCδ: complex roles in apoptosis, tumor growth and metastasis
PKCδ has been widely characterized as a pro-apoptotic and anti-proliferative kinase. In addition, PKCδ has been broadly implicated as a death mediator of chemotherapeutic agents and radiotherapy [112–115]. PKCδ is involved both in DNA damage and receptor-mediated cell death [3, 115–116]. Initial work from the Reyland lab showed that PKCδ is cleaved by caspase-3 and mobilizes from the cytoplasm to the nucleus after treatment with genotoxic agents [13]. Other studies showed that PKCδ-mediated apoptosis involves the allosteric activation of the enzyme rather than proteolytic cleavage. For example, in androgen-dependent prostate cancer cells, PKCδ activation triggers an apoptotic response without the generation of a constitutively active catalytic fragment [117]. This effect involves the activation of the p38 MAPK cascade [118] and is mediated by a RhoA/ROCK/p21Cip1-dependent pathway [119]. PKCδ-mediated apoptosis in androgen-dependent prostate cancer cells occurs through the autocrine secretion of TNFα and TRAIL, which induce caspase-8 cleavage through the JNK and p38 MAPK cascades [120–122]. The discrepancies in the mechanisms of PKCδ activation among the various studies may be explained by cell type differences and nature of the stimulus.
An inhibitory role for PKCδ in proliferation has been reported in a number of cellular models. The initial observations by Mischak et al. that ectopic overexpression of PKCδ confers growth inhibitory properties to NIH 3T3 cells [123], were later recapitulated in many other cell lines. Depending on the cell type, activation of PKCδ can induce cell cycle arrest either in G1 or G2 [49, 124]. Our laboratory showed that treatment of lung cancer cells with phorbol esters induces cell cycle arrest in G1 through the induction of p21cip1 and Rb dephosphorylation [125]. However, it has been also noted that ectopic expression of PKCδ stimulates quiescent cells to initiate G1 phase cell cycle progression. Notably, PKCδ overexpressing cells arrest in S phase rather than completing the cell cycle [126].
It is important to mention that studies ascribed pro-survival properties to PKCδ in a number of tumor models, including breast, lung, pancreatic, and liver cancer [116]. Moreover, ectopic expression of PKCδ in mammary cells confers anchorage-independent growth properties and enhances the resistance to apoptotic stimuli [127]. The scenario that PKCδ could be a tumor promoting kinase rather than a tumor suppressor began to shape new paradigms in PKC isozyme function, and clearly points to an exquisite cell type selectivity.
Data from patients cannot point to a clear link between PKCδ expression levels and clinical outcome. Loss of PKCδ expression has been reported in a few cancer types [128–130], but this down-regulation could not be unambiguously linked to tumorigenesis. PKCδ is up-regulated in some cancer types [131–132]. PKCδ is barely detected in normal prostate epithelial cells, however high PKCδ expression could be observed in prostate pre-neoplastic lesions and carcinomas [133–134]. In breast cancer specimens, PKCδ mRNA levels are significantly higher in ER-positive tumors and a positive correlation between high PKCδ mRNA levels and reduced overall survival has been reported [135]. Interestingly, the expression of PKCθ, an isozyme related to PKCδ, is dysregulated in some cancers [132, 136–138].
Emerging studies using genetically engineered mice began to shed light into the involvement of PKCδ in tumorigenesis. PKCδ skin transgenic mice are resistant to tumor promotion by DMBA/PMA [139]. Studies using PKCδ knockout mice revealed contrasting effects particularly in the context of specific genetic alterations. Reyland and coworkers investigated the involvement of PKCδ in K-Ras-dependent tumorigenesis and found that the incidence of urethane-induced lung tumors (which display activating mutations in K-Ras) is reduced in a PKCδ-null background. Moreover, PKCδ RNAi depletion inhibits anchorage-independent growth, invasion, migration, and tumorigenesis in K-Ras-dependent NSCLC cells [140]. The Reyland lab also described a positive role for PKCδ in mammary tumorigenesis in the context of ErbB2 overexpression. A meta-analysis of ErbB2-positive breast cancers shows increased PKCδ expression and a negative correlation between PKCδ expression and prognosis. Most remarkably, there is a significant delay in tumor onset in MMTV-ErbB2(Neu) in a PKCδ-null background [141]. A tumorigenic role for PKCδ has been also reported in a model of pancreatic cancer. Indeed, overexpression of PKCδ (as observed in human ductal carcinomas) leads to increased anchorage-independent growth and tumorigenesis in vivo [142]. Also, in a PC-3 xenograft model, PKCδ activation promotes tumor growth and increases angiogenesis through a mechanism that involves ROS, NADPH, and HIF-1α [143].
PKCδ generally has a positive role in migration and invasiveness [144–147]. In prostate cancer cellular models PKCδ has been implicated in invasiveness and the control of collagen secretion induced by overexpression of the oncoprotein PCPH [134]. Breast cancer models provided controversial evidence for the involvement of PKCδ in invasion. Whereas a study showed that PKCδ overexpression in highly motile BT-549 breast cancer cells reduces migration and PKCδ down-regulation enhances motility and MMP-9 secretion in MCF-7 cells [148], other study reported that enhanced migration induced by forced EGFR overexpression in MCF-7 cells requires PKCδ [149]. PKCδ was found to inhibit the production of proteolytic enzymes in murine mammary cells, possibly limiting metastatic dissemination[127]. Down-regulation of PKCδ suppresses lung colonization in the murine mammary breast cancer model MTLn3 without affecting the growth of primary tumor [150]. Studies performed in BL6 murine melanoma cells showed that PKCδ overexpression increases their metastatic capacity in vivo, possibly due to an increase in the plasma levels of TGFβ1 [151–152]. A similar pro-metastatic effect of PKCδ has been shown in the human pancreatic cell line PANC1 [142].
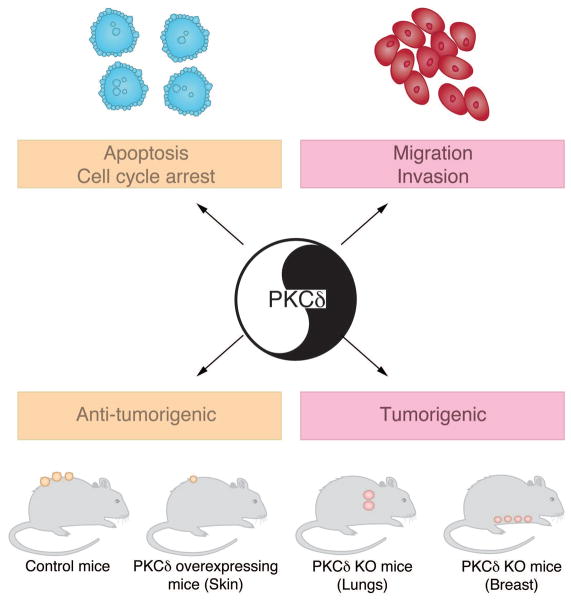
The multiplicity of effects regulated by PKCδ and the complexity of the effects in cell cycle regulation, cell motility, tumorigenicity and metastasis, both in positive and negative manners (Fig. 4), would argue that this kinase is not a likely candidate for the therapy of cancer.
Figure 4.
Multiple biological functions regulated by PKCδ. Studies in cellular models established important roles for PKCδ in apoptosis and as a negative regulator of cell cycle progression. PKCδ has been also implicated in cancer cell motility and invasiveness. Studies using animal models showed that PKCδ can either act as a tumor suppressor or contribute to tumorigenesis depending on the context.
PKCε: an oncogenic and metastatic kinase
PKCε has been originally described as an oncogenic kinase [123, 153–154], and is known to signal via the Ras-Raf-1 signaling pathway [155–158] as well as other pathways. PKCε-transformed fibroblasts secrete increased amounts of TGF-β and possibly other mitogens, an indication that growth autocrine loops may account for its oncogenic activity [159–160]. PKCε is overexpressed in a large number of cancers. For example, PKCε is overexpressed in ~75% of primary tumors from invasive ductal breast cancer patients [7]. Increased PKCε staining correlates with high histologic grade, positive ErbB2/Her2 status, and negative estrogen and progesterone receptor status [7]. Overexpression of PKCε has been reported in the majority (>90%) of primary NSCLC cancers relative to normal lung epithelium [5]. PKCε levels are elevated in prostate cancer relative to benign prostatic epithelia [161], and a correlation with aggressiveness of human prostate cancer has been found [4]. PKCη, an isoform related to PKCε, has also been shown to be up-regulated in some cancers [162–163] but down-regulated in others [164].
Not surprisingly, studies from several laboratories highlight a role for PKCε in cell cycle control, specifically in G1 to S progression [165–167]. In addition, PKCε promotes survival in many cell types [6, 168–170]. The survival activity of PKCε involves the modulation of caspases and Bcl-2 family members [168, 171–173]. Forced expression of PKCε in LNCaP prostate cancer cells confers resistance to PMA-induce apoptosis by preventing Bax oligomerization; moreover, it leads to accelerated proliferation of LNCaP cells due to constitutive activation of the Erk cascade [169]. Our laboratory recently demonstrated that PKCε modulates Bad phosphorylation at Ser112 to protect LNCaP prostate cancer cells against apoptosis induced by PMA or TNFα [172]. PKCε protects glioma and lung cancer cells from TRAIL-induced apoptosis [170, 174–175]. Subsequent studies by Basu and coworkers revealed that PKCε inhibits cell death in breast cancer cells, partly by preventing activation and translocation of Bax to the mitochondria [176]. PKCε inhibition/depletion impairs proliferation and anchorage-independent growth of human NSCLC cells [5, 177]. Many pro-apoptotic genes up-regulated upon PKCε RNAi depletion in lung cancer cells are also down-regulated in human lung adenocarcinomas [177]. RWPE-1 cells, a model of normal immortalized prostate epithelial cells, express very low levels of PKCε as compared to different human prostate cancer lines, and ectopic expression of PKCε in RWPE-1 cells confers growth advantage and leads to Erk and Akt activation [6]. Recently, our laboratory identified a key role for PKCε as a mediator of NF-κB signaling in prostate cancer [178]. PKCε inhibition/depletion impairs constitutive and TNFα-dependent activation of NF-κB as well as the induction of NF-κB responsive genes pertaining to cell survival, proliferation, metastasis and invasion, such as COX-2, MMP-9, VEGF, and IL-6 [178].
Overexpression of PKCε in androgen-dependent LNCaP cells initiates tumor growth in vivo both in intact and castrated athymic nude mice [179], thereby indicating that PKCε has the potential to advance the progression of prostate cancer and initiate recurrent tumor growth in the absence of androgens. In concordance, another study found that PKCε overexpression in breast cancer cells causes tumor growth in BALB/c mice with significant increase in the incidence and number of spontaneous experimental lung metastases [96]. In line with these results, depletion of PKCε from lung cancer cells using shRNA markedly inhibited xenograft growth in nude mice. Furthermore, the PKCε peptide translocation inhibitor εV1-2 blocks NSCLC tumor growth in nude mice. Moreover, both shRNA depletion and pharmacological inhibition of PKCε causes a strong induction of cell death in xenograft tumors [177].
Unfortunately, there has been little work intending to recapitulate PKCε overexpression as observed in human cancer. Our laboratory developed prostate-specific transgenic mice that overexpress PKCε in the normal prostate in vivo under the control of the androgen-responsive probasin (PB) promoter, which leads to the formation of preneoplastic lesions (Fig. 5). Conversely, similar mouse models for other PKCs (PB-PKCα and PB-PKCδ mice) do not display any noticeable phenotypic changes in the prostate. Furthermore, elevated phospho-Akt as well as hyperactivation of Akt effectors S6 and mTOR could be detected in hyperplasia and PIN lesions from PB-PKCε transgenic mice. Besides, PKCε overexpression confers resistance to apoptosis induced by androgen ablation, highlighting a pro-survival role of PKCε in the mouse prostate [6]. Hyperactivation of NF-κB and Stat-3 were evident in the PIN lesions of PB-PKCε transgenic mice relative to the normal areas or regions with mild hyperplasia [6, 178].
Figure 5.
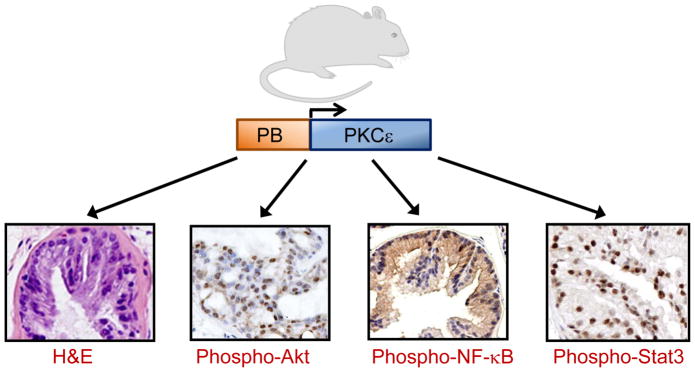
Phenotype of prostate-specific PKCε transgenic mice. Prostate-specific overexpression of PKCε in mice under the control of the probasin (PB) promoter leads to a preneoplastic phenotype. Representative photomicrographs for H&E, phospho-Akt, phospho-NF-κB and phospho-Stat3 staining in ventral prostates from 12-month old male PB-PKCε mice are shown.
Prostates of TRAMP mice (a model that spontaneously develops progressive invasive prostate cancer) have very high PKCε protein levels compared to prostates of control mice [4]. A recent study demonstrated that genetic ablation of PKCε in TRAMP mice inhibits prostate cancer development and metastasis [180]. Deletion of PKCε in TRAMP mice decreases the phosphorylation/activation of Stat3 as well as its nuclear translocation and DNA-binding activity. It has been proposed that loss of PKCε in TRAMP transgenic mice reduces the expression of proliferation, survival and metastasis markers, including COX-2, Bcl-xL, cyclin D1 and VEGF, as well as it decreases serum IL-6 levels.
A growing body of evidence indicates that PKCε is implicated in tumor cell invasion and metastasis. Enhanced PKCε levels are associated with invasion and/or metastasis of human breast, glioma and renal cell carcinoma [7, 166, 181]. PKCε contains an actin-binding motif that positions this kinase within a cytoskeletal matrix where many PKC substrates are localized [182–185]. Deletion of this motif abrogates invasion and metastatic spread of tumors driven by PKCε overexpression [186]. In human glioma cells, the PKC-interacting protein RACK1 appears to link activated PKCε to the integrin β chain at focal adhesions, and PKCε mediates the adhesion and motility of cells via Erk phosphorylation [187]. PKCε also promotes the assembly of matrix adhesions containing actin filaments and β1-integrins, and integrin signaling links PKCε to the Akt survival pathway in recurrent prostate cancer cells [188]. In models of breast and head and neck cancer, PKCε regulates motility and invasion, at least in part due to the activation of small Rho GTPases, specifically RhoA and/or RhoC [7, 189]. Very recent work from our laboratory showed that targeted disruption of PKCε in lung cancer cells reduces motility through Rac1 inactivation. Several extracellular matrix proteases are down-regulated in PKCε-depleted lung cancer cells [190]. Consistent with a role for PKCε in metastatic dissemination, PKCε-depleted NSCLC cells fail to colonize lungs after tail vein injection in mice [190]. Likewise, a study from the Urtreger’s laboratory showed that inoculation of PKCε overexpressing breast cancer cells in mice enhances the incidence and number of spontaneous and experimental lung metastases [96].
Verma and coworkers generated skin transgenic mice expressing PKCε under the control of the human K14 promoter, which exhibit phenotypic abnormalities including inflammation, hyperkeratosis, hyperplasia, cellular hypertrophy and ulceration. Highly malignant and metastatic squamous cell carcinomas develop in the skin of PKCε transgenic mice [191–192]. Additionally, PKCε overexpression has been shown to sensitize the mouse skin to UVR-induced carcinogenesis [193]. A subsequent study demonstrated that skin transgenic PKCε mice develop a myeloproliferative-like disease [194].
Altogether, these observations establish that PKCε overexpression is linked to an aggressive phenotype and suggest that targeting PKCε could be an effective anticancer strategy. Whereas the development of PKCε inhibitors targeting the ATP-binding site may be challenging due to the high homology of this site among PKCs, agents directed against domains implicated in translocation may be valuable. In that regard, the PKCε translocation inhibitor εV1-2 has anti-tumorigenic activity in NSCLC cells [177], and a bifunctional peptide in which εV1-2 has been linked to the 12-mer cancer homing peptide HN1 impairs the growth of head and neck squamous cell carcinoma cells in xenografts [195]. Lastly, Ras-driven and EMT-dependent phenotypes in breast cancer cells could be reversed by PF-526355, an ATP mimetic inhibitor with selectivity for PKCε and PKCθ. This inhibitor impairs the growth of MDA-MB-231 breast cancer xenografts in mice, thus representing a promising agent for cancer therapy [196].
Atypical PKCs ζ and ι: opposite roles in cancer
aPKC isozymes, which comprise PKCζ and PKCι (PKCλ), are structurally and functionally distinct from other PKCs in that they have a single DAG/phorbol ester unresponsive C1 domain and lack a C2 domain [197–198]. Regardless of controversies in the literature largely due to the use of non-specific approaches such as inhibitory peptides and dominant-negative mutants, many studies point to a tumor suppressor function of PKCζ, whereas PKCι essentially fulfills the criteria of an oncogenic kinase (Fig. 6).
Figure 6.
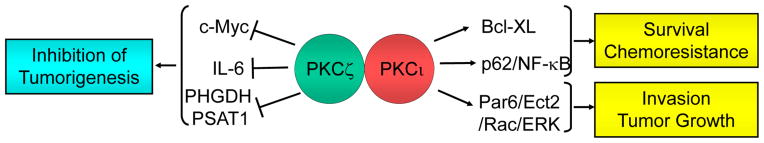
Roles of atypical PKCs in cancer. Most evidence points to PKCζ as a tumor suppressor protein and PKCι as an oncogenic kinase.
Both up- and down-regulation of PKCζ has been shown in human cancer. PKCζ up-regulation has been reported in prostate cancer, bladder cancer, and lymphomas [199–204], whereas down-regulated expression of PKCζ has been shown in glioblastoma, lung cancer, kidney, renal clear cell carcinoma, melanoma, and pancreatic cancer [205–214]. A pro-apoptotic function for PKCζ has been described in several cancer models. For example, PKCζ inhibits growth and promotes differentiation and apoptosis in colon cancer cells. The inhibitory effect of PKCζ on the transformed phenotype of these cells suggests that down-regulation of PKCζ may contribute to colon tumorigenesis [215]. PKCζ also exhibits a pro-apoptotic function in ovarian cancer [216]. In murine TRAMP prostate cancer cells, expression of a constitutively activated PKCζ mutant enhances proliferation, whereas PKCζ inhibition leads to Akt activation and enhanced cell survival [217]. There are several reports, however, highlighting a pro-survival role for PKCζ [218–222].
PKCζ-deficient mice display increased Ras-induced lung carcinogenesis, arguing for a role for this aPKC as a tumor suppressor in vivo. It has been postulated that the tumor suppressor activity of PKCζ occurs through its ability to down-regulate Ras-induced IL-6 production. The enhanced secretion of IL-6 in PKCζ-deficient Ras-transformed cells is essential for growth under conditions of limited nutrients and mitogens [211]. Genetic inactivation of PKCζ in mice in a Pten-deficient background leads to invasive prostate carcinoma. A significant correlation between PKCζ and Pten levels exists in human prostate tumors, and PKCζ is significantly reduced in metastatic vs. primary tumors with low Pten. Analysis of gene signatures in PKCζ-deficient cells revealed a link with c-Myc. Indeed, c-Myc is a contributor to the more aggressive phenotype associated with PKCζ loss [223]. Loss of PKCζ also allows glucose-addicted human cancer cells to reprogram their metabolism in response to glucose deprivation by augmenting the utilization of glutamine through the serine biosynthetic pathway [224].
Accumulating evidence established that PKCι is an oncogenic kinase and that it contributes to the transformed phenotype. Overexpression of PKCι is observed in many human cancers, including colon, lung, pancreas, breast, prostate and ovarian cancer. The PKCι gene (PRKCI) is amplified in some human cancers [225–227], although PKCι overexpression is not always associated with gene amplification [228–230]. This has been well summarized in a review by Murray et al [231].
PKCι expression is elevated in NSCLC tumors and cell lines, and is required for the transformed phenotype of NSCLC cells harboring oncogenic K-Ras mutation [232–235]. NSCLC cell lines without Ras mutation also depend on PKCι for their malignant phenotype if they harbor PRKCI gene amplification [235]. PKCι mediates its effects through a Rac1-Pak-Mek-Erk dependent mechanism [89]. By means of a proteomics approach, Justilien et al [236] identified the Rho-GEF Ect2 as a component of the PKCι-Par6 complex that is required for transformed growth. The Ect2 gene co-amplifies with PRKCI suggesting a coordinated mechanism for tumorigenesis. Disruption of the PKCι-Par6 interaction, as caused with the anti-rheumatic agent aurothiomalate (ATM), potently inhibits growth of PKCι-overexpressing cell lines. ATM inhibits K-Ras-mediated expansion of bronchoalveolar stem cells and lung tumor growth in vivo [233, 237]. On the other hand, aPKCs appear to be dispensable for mammalian hematopoietic stem cell function [238]. PKCι has been also implicated in colon and pancreatic cancer using cell lines and animal models [229–230]. PKCι is required for hedgehog signaling in basal cell carcinomas. Indeed, PKCι functions downstream of smoothened (SMO) to phosphorylate and activate the GLI1 transcription factor. Moreover, PKCι is up-regulated in tumors resistant to SMO inhibitors, and targeting PKCι suppresses the growth of resistant basal carcinoma cell lines [239]. Other interesting signaling connections have been established for PKCι, including mutually antagonistic regulation with RhoB in glioblastoma cell invasion [240], links with the NF-κB pathway [241–242], as well as association with cell cycle proteins cyclin E in ovarian cancer [226] and S-phase kinase-associated protein 2 (SKP2) in esophageal cancer [243].
Final remarks
Tangible progress has been made in the last 30 years in understanding the regulation and cellular functions of PKC isozymes in cancer. The picture that emerged, however, is less than clear. What we have learned over the last years is that the biology of PKC isozymes is exceptionally complex, and that many studies in cell lines do not necessarily apply to in vivo models. For the next wave of studies on PKC function, the generation of animal models recapitulating the scenarios observed in different cancer types should be a priority. However, it is still not known whether the activation status of different members of the PKC family is altered in cancer and whether activated PKCs functionally interact with oncogenes and tumor suppressors genes driving the tumorigenic and metastatic phenotype.
One would expect that PKC is a promising target for cancer therapy, but only for specific PKC isozymes that display oncogenic activity, such as PKCε and PKCι. The portfolio of available PKC inhibitors remains narrow, and unfortunately the majority of compounds lack specificity among members of the PKC family or even with other kinases unrelated to PKC. Hence, there is a great need to design selective small molecule inhibitors for PKC isozymes that have sufficient potency to impair PKC function in vivo. There may be concrete opportunities to rationally design inhibitors against the ATP-binding site, but this is still challenging due to the high homology among PKCs in that region. Some examples of small molecules capable of disrupting protein-protein interactions for PKC isozymes provided proof-of-principle for alternative approaches in the design of PKC modulators [177, 237, 244]. C1 domain ligands, such as the bryostatins [245–246], did not show major beneficial effects in patients despite their anti-tumor effects in mice [244]. One may envision that PKC isozyme specific inhibitors may possibly work in combined therapies with chemotherapeutic agents for discrete cancer types. Translating PKC modulators into a clinical setting remains a formidable challenge that we face for the next years.
Acknowledgments
Work in the laboratory of M.G.K is supported by grants R01-CA89202 and R01-CA139120 from NIH. R.G. is supported by a post-doctoral grant from the Department of Defense W81XWH-12-1-0009.
References
- 1.Kikkawa U, Takai Y, Tanaka Y, Miyake R, Nishizuka Y. Protein kinase C as a possible receptor protein of tumor-promoting phorbol esters. J Biol Chem. 1983;258:11442–11445. [PubMed] [Google Scholar]
- 2.Leach KL, James ML, Blumberg PM. Characterization of a specific phorbol ester aporeceptor in mouse brain cytosol. Proc Natl Acad Sci U S A. 1983;80:4208–4212. doi: 10.1073/pnas.80.14.4208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Griner EM, Kazanietz MG. Protein kinase C and other diacylglycerol effectors in cancer. Nat Rev Cancer. 2007;7:281–294. doi: 10.1038/nrc2110. [DOI] [PubMed] [Google Scholar]
- 4.Aziz MH, Manoharan HT, Church DR, Dreckschmidt NE, Zhong W, Oberley TD, Wilding G, Verma AK. Protein kinase Cepsilon interacts with signal transducers and activators of transcription 3 (Stat3), phosphorylates Stat3Ser727, and regulates its constitutive activation in prostate cancer. Cancer Res. 2007;67:8828–8838. doi: 10.1158/0008-5472.CAN-07-1604. [DOI] [PubMed] [Google Scholar]
- 5.Bae KM, Wang H, Jiang G, Chen MG, Lu L, Xiao L. Protein kinase C epsilon is overexpressed in primary human non-small cell lung cancers and functionally required for proliferation of non-small cell lung cancer cells in a p21/Cip1-dependent manner. Cancer Res. 2007;67:6053–6063. doi: 10.1158/0008-5472.CAN-06-4037. [DOI] [PubMed] [Google Scholar]
- 6.Benavides F, Blando J, Perez CJ, Garg R, Conti CJ, DiGiovanni J, Kazanietz MG. Transgenic overexpression of PKCepsilon in the mouse prostate induces preneoplastic lesions. Cell Cycle. 2011;10:268–277. doi: 10.4161/cc.10.2.14469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan Q, Bao LW, Kleer CG, Sabel MS, Griffith KA, Teknos TN, Merajver SD. Protein kinase C epsilon is a predictive biomarker of aggressive breast cancer and a validated target for RNA interference anticancer therapy. Cancer Res. 2005;65:8366–8371. doi: 10.1158/0008-5472.CAN-05-0553. [DOI] [PubMed] [Google Scholar]
- 8.Wu-Zhang AX, Newton AC. Protein kinase C pharmacology: refining the toolbox. Biochem J. 2013;452:195–209. doi: 10.1042/BJ20130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schleifenbaum A, Stier G, Gasch A, Sattler M, Schultz C. Genetically encoded FRET probe for PKC activity based on pleckstrin. J Am Chem Soc. 2004;126:11786–11787. doi: 10.1021/ja0460155. [DOI] [PubMed] [Google Scholar]
- 10.Chen CA, Yeh RH, Yan X, Lawrence DS. Biosensors of protein kinase action: from in vitro assays to living cells. Biochim Biophys Acta. 2004;1697:39–51. doi: 10.1016/j.bbapap.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Gallegos LL, Kunkel MT, Newton AC. Targeting protein kinase C activity reporter to discrete intracellular regions reveals spatiotemporal differences in agonist-dependent signaling. J Biol Chem. 2006;281:30947–30956. doi: 10.1074/jbc.M603741200. [DOI] [PubMed] [Google Scholar]
- 12.Gallegos LL, Newton AC. Genetically encoded fluorescent reporters to visualize protein kinase C activation in live cells. Methods Mol Biol. 2011;756:295–310. doi: 10.1007/978-1-61779-160-4_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. Embo J. 2002;21:6050–6060. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang H, Kazanietz MG. p23/Tmp21 differentially targets the Rac-GAP beta2-chimaerin and protein kinase C via their C1 domains. Mol Biol Cell. 2010;21:1398–1408. doi: 10.1091/mbc.E09-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaken S, Parker PJ. Protein kinase C binding partners. Bioessays. 2000;22:245–254. doi: 10.1002/(SICI)1521-1878(200003)22:3<245::AID-BIES6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Xiao L, Kazanietz MG. p23/Tmp21 associates with protein kinase Cdelta (PKCdelta) and modulates its apoptotic function. J Biol Chem. 2011;286:15821–15831. doi: 10.1074/jbc.M111.227991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai CM, Zhou XF, Zhang YL, Liu SG, Zhang J. Synthesis by precipitation polymerization of molecularly imprinted polymer for the selective extraction of diclofenac from water samples. J Hazard Mater. 2011;198:175–181. doi: 10.1016/j.jhazmat.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Cameron AJ, Procyk KJ, Leitges M, Parker PJ. PKC alpha protein but not kinase activity is critical for glioma cell proliferation and survival. Int J Cancer. 2008;123:769–779. doi: 10.1002/ijc.23560. [DOI] [PubMed] [Google Scholar]
- 19.Haughian JM, Reno EM, Thorne AM, Bradford AP. Protein kinase C alpha-dependent signaling mediates endometrial cancer cell growth and tumorigenesis. Int J Cancer. 2009;125:2556–2564. doi: 10.1002/ijc.24633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakagawa S, Fujii T, Yokoyama G, Kazanietz MG, Yamana H, Shirouzu K. Cell growth inhibition by all-trans retinoic acid in SKBR-3 breast cancer cells: involvement of protein kinase Calpha and extracellular signal-regulated kinase mitogen-activated protein kinase. Mol Carcinog. 2003;38:106–116. doi: 10.1002/mc.10150. [DOI] [PubMed] [Google Scholar]
- 21.Kong C, Zhu Y, Liu D, Yu M, Li S, Li Z, Sun Z, Liu G. Role of protein kinase C-alpha in superficial bladder carcinoma recurrence. Urology. 2005;65:1228–1232. doi: 10.1016/j.urology.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 22.Stewart JR, O’Brian CA. Resveratrol antagonizes EGFR-dependent Erk1/2 activation in human androgen-independent prostate cancer cells with associated isozyme-selective PKC alpha inhibition. Invest New Drugs. 2004;22:107–117. doi: 10.1023/B:DRUG.0000011787.75522.ec. [DOI] [PubMed] [Google Scholar]
- 23.Suga K, Sugimoto I, Ito H, Hashimoto E. Down-regulation of protein kinase C-alpha detected in human colorectal cancer. Biochem Mol Biol Int. 1998;44:523–528. doi: 10.1080/15216549800201552. [DOI] [PubMed] [Google Scholar]
- 24.von Brandenstein M, Pandarakalam JJ, Kroon L, Loeser H, Herden J, Braun G, Wendland K, Dienes HP, Engelmann U, Fries JW. MicroRNA 15a, inversely correlated to PKCalpha, is a potential marker to differentiate between benign and malignant renal tumors in biopsy and urine samples. Am J Pathol. 2012;180:1787–1797. doi: 10.1016/j.ajpath.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Baltuch GH, Dooley NP, Rostworowski KM, Villemure JG, Yong VW. Protein kinase C isoform alpha overexpression in C6 glioma cells and its role in cell proliferation. J Neurooncol. 1995;24:241–250. doi: 10.1007/BF01052840. [DOI] [PubMed] [Google Scholar]
- 26.Mandil R, Ashkenazi E, Blass M, Kronfeld I, Kazimirsky G, Rosenthal G, Umansky F, Lorenzo PS, Blumberg PM, Brodie C. Protein kinase Calpha and protein kinase Cdelta play opposite roles in the proliferation and apoptosis of glioma cells. Cancer Res. 2001;61:4612–4619. [PubMed] [Google Scholar]
- 27.Blackburn RV, Galoforo SS, Berns CM, Motwani NM, Corry PM, Lee YJ. Differential induction of cell death in human glioma cell lines by sodium nitroprusside. Cancer. 1998;82:1137–1145. doi: 10.1002/(sici)1097-0142(19980315)82:6<1137::aid-cncr19>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 28.Mut M, Amos S, Hussaini IM. PKC alpha phosphorylates cytosolic NF-kappaB/p65 and PKC delta delays nuclear translocation of NF-kappaB/p65 in U1242 glioblastoma cells. Turk Neurosurg. 2010;20:277–285. doi: 10.5137/1019-5149.JTN.3008-10.1. [DOI] [PubMed] [Google Scholar]
- 29.Paz-Ares L, Douillard JY, Koralewski P, Manegold C, Smit EF, Reyes JM, Chang GC, John WJ, Peterson PM, Obasaju CK, Lahn M, Gandara DR. Phase III study of gemcitabine and cisplatin with or without aprinocarsen, a protein kinase C-alpha antisense oligonucleotide, in patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2006;24:1428–1434. doi: 10.1200/JCO.2005.04.3299. [DOI] [PubMed] [Google Scholar]
- 30.Advani R, Peethambaram P, Lum BL, Fisher GA, Hartmann L, Long HJ, Halsey J, Holmlund JT, Dorr A, Sikic BI. A Phase II trial of aprinocarsen, an antisense oligonucleotide inhibitor of protein kinase C alpha, administered as a 21-day infusion to patients with advanced ovarian carcinoma. Cancer. 2004;100:321–326. doi: 10.1002/cncr.11909. [DOI] [PubMed] [Google Scholar]
- 31.Grossman SA, Alavi JB, Supko JG, Carson KA, Priet R, Dorr FA, Grundy JS, Holmlund JT. Efficacy and toxicity of the antisense oligonucleotide aprinocarsen directed against protein kinase C-alpha delivered as a 21-day continuous intravenous infusion in patients with recurrent high-grade astrocytomas. Neuro Oncol. 2005;7:32–40. doi: 10.1215/S1152851703000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seynaeve CM, Kazanietz MG, Blumberg PM, Sausville EA, Worland PJ. Differential inhibition of protein kinase C isozymes by UCN-01, a staurosporine analogue. Mol Pharmacol. 1994;45:1207–1214. [PubMed] [Google Scholar]
- 33.Facchinetti MM, De Siervi A, Toskos D, Senderowicz AM. UCN-01-induced cell cycle arrest requires the transcriptional induction of p21(waf1/cip1) by activation of mitogen-activated protein/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase pathway. Cancer Res. 2004;64:3629–3637. doi: 10.1158/0008-5472.CAN-03-3741. [DOI] [PubMed] [Google Scholar]
- 34.Cameron AJ, Procyk KJ, Leitges M, Parker PJ. PKC alpha protein but not kinase activity is critical for glioma cell proliferation and survival. Int J Cancer. 2008;123:769–779. doi: 10.1002/ijc.23560. [DOI] [PubMed] [Google Scholar]
- 35.Ways DK, Kukoly CA, deVente J, Hooker JL, Bryant WO, Posekany KJ, Fletcher DJ, Cook PP, Parker PJ. MCF-7 breast cancer cells transfected with protein kinase C-alpha exhibit altered expression of other protein kinase C isoforms and display a more aggressive neoplastic phenotype. J Clin Invest. 1995;95:1906–1915. doi: 10.1172/JCI117872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tonetti DA, Chisamore MJ, Grdina W, Schurz H, Jordan VC. Stable transfection of protein kinase C alpha cDNA in hormone-dependent breast cancer cell lines. Br J Cancer. 2000;83:782–791. doi: 10.1054/bjoc.2000.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chisamore MJ, Ahmed Y, Bentrem DJ, Jordan VC, Tonetti DA. Novel antitumor effect of estradiol in athymic mice injected with a T47D breast cancer cell line overexpressing protein kinase Calpha. Clinical cancer research : an official journal of the American Association for Cancer Research. 2001;7:3156–3165. [PubMed] [Google Scholar]
- 38.Yun J, Pannuti A, Espinoza I, Zhu H, Hicks C, Zhu X, Caskey M, Rizzo P, D’Souza G, Backus K, Denning MF, Coon J, Sun M, Bresnick EH, Osipo C, Wu J, Strack PR, Tonetti DA, Miele L. Crosstalk between PKCalpha and Notch-4 in endocrine-resistant breast cancer cells. Oncogenesis. 2013;2:e60. doi: 10.1038/oncsis.2013.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Assender JW, Gee JM, Lewis I, Ellis IO, Robertson JF, Nicholson RI. Protein kinase C isoform expression as a predictor of disease outcome on endocrine therapy in breast cancer. J Clin Pathol. 2007;60:1216–1221. doi: 10.1136/jcp.2006.041616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tonetti DA, Morrow M, Kidwai N, Gupta A, Badve S. Elevated protein kinase C alpha expression may be predictive of tamoxifen treatment failure. Br J Cancer. 2003;88:1400–1402. doi: 10.1038/sj.bjc.6600923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lonne GK, Cornmark L, Zahirovic IO, Landberg G, Jirstrom K, Larsson C. PKCalpha expression is a marker for breast cancer aggressiveness. Molecular cancer. 2010;9:76. doi: 10.1186/1476-4598-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan M, Li P, Sun M, Yin G, Yu D. Upregulation and activation of PKC alpha by ErbB2 through Src promotes breast cancer cell invasion that can be blocked by combined treatment with PKC alpha and Src inhibitors. Oncogene. 2006;25:3286–3295. doi: 10.1038/sj.onc.1209361. [DOI] [PubMed] [Google Scholar]
- 43.Martiny-Baron G, Kazanietz MG, Mischak H, Blumberg PM, Kochs G, Hug H, Marme D, Schachtele C. Selective inhibition of protein kinase C isozymes by the indolocarbazole Go 6976. J Biol Chem. 1993;268:9194–9197. [PubMed] [Google Scholar]
- 44.Liu B, Maher RJ, Hannun YA, Porter AT, Honn KV. 12(S)-HETE enhancement of prostate tumor cell invasion: selective role of PKC alpha. J Natl Cancer Inst. 1994;86:1145–1151. doi: 10.1093/jnci/86.15.1145. [DOI] [PubMed] [Google Scholar]
- 45.Tonetti DA, Gao W, Escarzaga D, Walters K, Szafran A, Coon JS. PKCalpha and ERbeta Are Associated with Triple-Negative Breast Cancers in African American and Caucasian Patients. Int J Breast Cancer. 2012;2012:740353. doi: 10.1155/2012/740353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tam WL, Lu H, Buikhuisen J, Soh BS, Lim E, Reinhardt F, Wu ZJ, Krall JA, Bierie B, Guo W, Chen X, Liu XS, Brown M, Lim B, Weinberg RA. Protein Kinase C alpha Is a Central Signaling Node and Therapeutic Target for Breast Cancer Stem Cells. Cancer Cell. 2013;24:347–364. doi: 10.1016/j.ccr.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliva JL, Caino MC, Senderowicz AM, Kazanietz MG. S-Phase-specific activation of PKC alpha induces senescence in non-small cell lung cancer cells. J Biol Chem. 2008;283:5466–5476. doi: 10.1074/jbc.M707576200. [DOI] [PubMed] [Google Scholar]
- 48.Hizli AA, Black AR, Pysz MA, Black JD. Protein kinase C alpha signaling inhibits cyclin D1 translation in intestinal epithelial cells. J Biol Chem. 2006;281:14596–14603. doi: 10.1074/jbc.M601959200. [DOI] [PubMed] [Google Scholar]
- 49.Frey MR, Clark JA, Leontieva O, Uronis JM, Black AR, Black JD. Protein kinase C signaling mediates a program of cell cycle withdrawal in the intestinal epithelium. J Cell Biol. 2000;151:763–778. doi: 10.1083/jcb.151.4.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guan L, Song K, Pysz MA, Curry KJ, Hizli AA, Danielpour D, Black AR, Black JD. Protein kinase C-mediated down-regulation of cyclin D1 involves activation of the translational repressor 4E-BP1 via a phosphoinositide 3-kinase/Akt-independent, protein phosphatase 2A-dependent mechanism in intestinal epithelial cells. J Biol Chem. 2007;282:14213–14225. doi: 10.1074/jbc.M610513200. [DOI] [PubMed] [Google Scholar]
- 51.Gwak J, Jung SJ, Kang DI, Kim EY, Kim DE, Chung YH, Shin JG, Oh S. Stimulation of protein kinase C-alpha suppresses colon cancer cell proliferation by down-regulation of beta-catenin. J Cell Mol Med. 2009;13:2171–2180. doi: 10.1111/j.1582-4934.2008.00683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JM, Kim IS, Kim H, Lee JS, Kim K, Yim HY, Jeong J, Kim JH, Kim JY, Lee H, Seo SB, Rosenfeld MG, Kim KI, Baek SH. RORalpha attenuates Wnt/beta-catenin signaling by PKCalpha-dependent phosphorylation in colon cancer. Mol Cell. 2010;37:183–195. doi: 10.1016/j.molcel.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 53.Gwak J, Lee JH, Chung YH, Song GY, Oh S. Small molecule-based promotion of PKCalpha-mediated beta-catenin degradation suppresses the proliferation of CRT-positive cancer cells. PLoS One. 2012;7:e46697. doi: 10.1371/journal.pone.0046697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dupasquier S, Abdel-Samad R, Glazer RI, Bastide P, Jay P, Joubert D, Cavailles V, Blache P, Quittau-Prevostel C. A new mechanism of SOX9 action to regulate PKCalpha expression in the intestine epithelium. J Cell Sci. 2009;122:2191–2196. doi: 10.1242/jcs.036483. [DOI] [PubMed] [Google Scholar]
- 55.Oster H, Leitges M. Protein kinase C alpha but not PKCzeta suppresses intestinal tumor formation in ApcMin/+ mice. Cancer Res. 2006;66:6955–6963. doi: 10.1158/0008-5472.CAN-06-0268. [DOI] [PubMed] [Google Scholar]
- 56.Pysz MA, Leontieva OV, Bateman NW, Uronis JM, Curry KJ, Threadgill DW, Janssen KP, Robine S, Velcich A, Augenlicht LH, Black AR, Black JD. PKCalpha tumor suppression in the intestine is associated with transcriptional and translational inhibition of cyclin D1. Exp Cell Res. 2009;315:1415–1428. doi: 10.1016/j.yexcr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hara T, Matsumura S, Hakuno F, Takahashi S, Chida K. PKCalpha suppresses 7,12-dimethylbenz[a]anthracene-induced skin tumor formation. Anticancer Res. 2012;32:3097–3101. [PubMed] [Google Scholar]
- 58.Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- 59.Garcia-Bermejo ML, Leskow FC, Fujii T, Wang Q, Blumberg PM, Ohba M, Kuroki T, Han KC, Lee J, Marquez VE, Kazanietz MG. Diacylglycerol (DAG)-lactones, a new class of protein kinase C (PKC) agonists, induce apoptosis in LNCaP prostate cancer cells by selective activation of PKCalpha. J Biol Chem. 2002;277:645–655. doi: 10.1074/jbc.M107639200. [DOI] [PubMed] [Google Scholar]
- 60.Truman JP, Rotenberg SA, Kang JH, Lerman G, Fuks Z, Kolesnick R, Marquez VE, Haimovitz-Friedman A. PKCalpha activation downregulates ATM and radio-sensitizes androgen-sensitive human prostate cancer cells in vitro and in vivo. Cancer Biol Ther. 2009;8:54–63. doi: 10.4161/cbt.8.1.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hill KS, Erdogan E, Khoor A, Walsh MP, Leitges M, Murray NR, Fields AP. Protein kinase Cα suppresses Kras-mediated lung tumor formation through activation of a p38 MAPK-TGFβ signaling axis. Oncogene. 2013 doi: 10.1038/onc.2013.2147. e-pub ahead of print 22 April 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Akakura S, Nochajski P, Gao L, Sotomayor P, Matsui S, Gelman IH. Rb-dependent cellular senescence, multinucleation and susceptibility to oncogenic transformation through PKC scaffolding by SSeCKS/AKAP12. Cell Cycle. 2010;9:4656–4665. doi: 10.4161/cc.9.23.13974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hao F, Pysz MA, Curry KJ, Haas KN, Seedhouse SJ, Black AR, Black JD. Protein kinase Calpha signaling regulates inhibitor of DNA binding 1 in the intestinal epithelium. J Biol Chem. 2011;286:18104–18117. doi: 10.1074/jbc.M110.208488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang MY, Hsu LS, Peng CH, Shi YS, Wu CH, Wang CJ. Polyphenol-rich extracts from Solanum nigrum attenuated PKC alpha-mediated migration and invasion of hepatocellular carcinoma cells. J Agric Food Chem. 2010;58:5806–5814. doi: 10.1021/jf100718b. [DOI] [PubMed] [Google Scholar]
- 65.Mahanivong C, Chen HM, Yee SW, Pan ZK, Dong Z, Huang S. Protein kinase C alpha-CARMA3 signaling axis links Ras to NF-kappa B for lysophosphatidic acid-induced urokinase plasminogen activator expression in ovarian cancer cells. Oncogene. 2008;27:1273–1280. doi: 10.1038/sj.onc.1210746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lin CW, Shen SC, Chien CC, Yang LY, Shia LT, Chen YC. 12-O-tetradecanoylphorbol-13-acetate-induced invasion/migration of glioblastoma cells through activating PKCalpha/ERK/NF-kappaB-dependent MMP-9 expression. J Cell Physiol. 2010;225:472–481. doi: 10.1002/jcp.22226. [DOI] [PubMed] [Google Scholar]
- 67.Shi MD, Shih YW, Lee YS, Cheng YF, Tsai LY. Suppression of 12-O-Tetradecanoylphorbol-13-Acetate-Induced MCF-7 Breast Adenocarcinoma Cells Invasion/Migration by alpha-Tomatine Through Activating PKCalpha/ERK/NF-kappaB-Dependent MMP-2/MMP-9 Expressions. Cell Biochem Biophys. 2013;66:161–174. doi: 10.1007/s12013-012-9465-8. [DOI] [PubMed] [Google Scholar]
- 68.Byers HR, Boissel SJ, Tu C, Park HY. RNAi-mediated knockdown of protein kinase C-alpha inhibits cell migration in MM-RU human metastatic melanoma cell line. Melanoma Res. 2010;20:171–178. doi: 10.1097/CMR.0b013e32832f1581. [DOI] [PubMed] [Google Scholar]
- 69.Wu TT, Hsieh YH, Hsieh YS, Liu JY. Reduction of PKC alpha decreases cell proliferation, migration, and invasion of human malignant hepatocellular carcinoma. J Cell Biochem. 2008;103:9–20. doi: 10.1002/jcb.21378. [DOI] [PubMed] [Google Scholar]
- 70.Kim S, Han J, Lee SK, Choi MY, Kim J, Lee J, Jung SP, Kim JS, Kim JH, Choe JH, Lee JE, Nam SJ. Berberine suppresses the TPA-induced MMP-1 and MMP-9 expressions through the inhibition of PKC-alpha in breast cancer cells. J Surg Res. 2012;176:e21–29. doi: 10.1016/j.jss.2011.11.1041. [DOI] [PubMed] [Google Scholar]
- 71.Lahn M, Kohler G, Sundell K, Su C, Li S, Paterson BM, Bumol TF. Protein kinase C alpha expression in breast and ovarian cancer. Oncology. 2004;67:1–10. doi: 10.1159/000080279. [DOI] [PubMed] [Google Scholar]
- 72.Kim J, Thorne SH, Sun L, Huang B, Mochly-Rosen D. Sustained inhibition of PKCalpha reduces intravasation and lung seeding during mammary tumor metastasis in an in vivo mouse model. Oncogene. 2011;30:323–333. doi: 10.1038/onc.2010.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu W, Murray NR, Weems C, Chen L, Guo H, Ethridge R, Ceci JD, Evers BM, Thompson EA, Fields AP. Role of cyclooxygenase 2 in protein kinase C beta II-mediated colon carcinogenesis. J Biol Chem. 2003;278:11167–11174. doi: 10.1074/jbc.M211424200. [DOI] [PubMed] [Google Scholar]
- 74.Kim J, Choi YL, Vallentin A, Hunrichs BS, Hellerstein MK, Peehl DM, Mochly-Rosen D. Centrosomal PKCbetaII and pericentrin are critical for human prostate cancer growth and angiogenesis. Cancer Res. 2008;68:6831–6839. doi: 10.1158/0008-5472.CAN-07-6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Espinosa I, Briones J, Bordes R, Brunet S, Martino R, Sureda A, Prat J, Sierra J. Membrane PKC-beta 2 protein expression predicts for poor response to chemotherapy and survival in patients with diffuse large B-cell lymphoma. Ann Hematol. 2006;85:597–603. doi: 10.1007/s00277-006-0144-y. [DOI] [PubMed] [Google Scholar]
- 76.Teicher BA, Menon K, Alvarez E, Shih C, Faul MM. Antiangiogenic and antitumor effects of a protein kinase Cbeta inhibitor in human breast cancer and ovarian cancer xenografts. Invest New Drugs. 2002;20:241–251. doi: 10.1023/a:1016297611825. [DOI] [PubMed] [Google Scholar]
- 77.Teicher BA, Menon K, Alvarez E, Galbreath E, Shih C, Faul M. Antiangiogenic and antitumor effects of a protein kinase Cbeta inhibitor in human T98G glioblastoma multiforme xenografts. Clin Cancer Res. 2001;7:634–640. [PubMed] [Google Scholar]
- 78.Blobe GC, Stribling DS, Fabbro D, Stabel S, Hannun YA. Protein kinase C beta II specifically binds to and is activated by F-actin. J Biol Chem. 1996;271:15823–15830. doi: 10.1074/jbc.271.26.15823. [DOI] [PubMed] [Google Scholar]
- 79.Gokmen-Polar Y, Fields AP. Mapping of a molecular determinant for protein kinase C betaII isozyme function. J Biol Chem. 1998;273:20261–20266. doi: 10.1074/jbc.273.32.20261. [DOI] [PubMed] [Google Scholar]
- 80.Stebbins EG, Mochly-Rosen D. Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C. J Biol Chem. 2001;276:29644–29650. doi: 10.1074/jbc.M101044200. [DOI] [PubMed] [Google Scholar]
- 81.Craven PA, DeRubertis FR. Alterations in protein kinase C in 1,2-dimethylhydrazine induced colonic carcinogenesis. Cancer Res. 1992;52:2216–2221. [PubMed] [Google Scholar]
- 82.Davidson LA, Aymond CM, Jiang YH, Turner ND, Lupton JR, Chapkin RS. Non-invasive detection of fecal protein kinase C betaII and zeta messenger RNA: putative biomarkers for colon cancer. Carcinogenesis. 1998;19:253–257. doi: 10.1093/carcin/19.2.253. [DOI] [PubMed] [Google Scholar]
- 83.Davidson D, Viallet J, Veillette A. Unique catalytic properties dictate the enhanced function of p59fynT, the hemopoietic cell-specific isoform of the Fyn tyrosine protein kinase, in T cells. Mol Cell Biol. 1994;14:4554–4564. doi: 10.1128/mcb.14.7.4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spindler KL, Lindebjerg J, Lahn M, Kjaer-Frifeldt S, Jakobsen A. Protein kinase C-beta II (PKC-beta II) expression in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:641–645. doi: 10.1007/s00384-009-0680-8. [DOI] [PubMed] [Google Scholar]
- 85.Sauma S, Yan Z, Ohno S, Friedman E. Protein kinase C beta 1 and protein kinase C beta 2 activate p57 mitogen-activated protein kinase and block differentiation in colon carcinoma cells. Cell Growth Differ. 1996;7:587–594. [PubMed] [Google Scholar]
- 86.Lee H, Ghose-Dastidar J, Winawer S, Friedman E. Signal transduction through extracellular signal-regulated kinase-like pp57 blocked in differentiated cells having low protein kinase C beta activity. J Biol Chem. 1993;268:5255–5263. [PubMed] [Google Scholar]
- 87.Murray NR, Davidson LA, Chapkin RS, Clay Gustafson W, Schattenberg DG, Fields AP. Overexpression of protein kinase C betaII induces colonic hyperproliferation and increased sensitivity to colon carcinogenesis. J Cell Biol. 1999;145:699–711. doi: 10.1083/jcb.145.4.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Calcagno SR, Li S, Colon M, Kreinest PA, Thompson EA, Fields AP, Murray NR. Oncogenic K-ras promotes early carcinogenesis in the mouse proximal colon. Int J Cancer. 2008;122:2462–2470. doi: 10.1002/ijc.23383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang J, Anastasiadis PZ, Liu Y, Thompson EA, Fields AP. Protein kinase C (PKC) betaII induces cell invasion through a Ras/Mek-, PKC iota/Rac 1-dependent signaling pathway. J Biol Chem. 2004;279:22118–22123. doi: 10.1074/jbc.M400774200. [DOI] [PubMed] [Google Scholar]
- 90.Goldstein DR, Cacace AM, Weinstein IB. Overexpression of protein kinase C beta 1 in the SW480 colon cancer cell line causes growth suppression. Carcinogenesis. 1995;16:1121–1126. doi: 10.1093/carcin/16.5.1121. [DOI] [PubMed] [Google Scholar]
- 91.Cesaro P, Raiteri E, Demoz M, Castino R, Baccino FM, Bonelli G, Isidoro C. Expression of protein kinase C beta1 confers resistance to TNFalpha- and paclitaxel-induced apoptosis in HT-29 colon carcinoma cells. Int J Cancer. 2001;93:179–184. doi: 10.1002/ijc.1314. [DOI] [PubMed] [Google Scholar]
- 92.Metzger E, Imhof A, Patel D, Kahl P, Hoffmeyer K, Friedrichs N, Muller JM, Greschik H, Kirfel J, Ji S, Kunowska N, Beisenherz-Huss C, Gunther T, Buettner R, Schule R. Phosphorylation of histone H3T6 by PKCbeta(I) controls demethylation at histone H3K4. Nature. 2010;464:792–796. doi: 10.1038/nature08839. [DOI] [PubMed] [Google Scholar]
- 93.Gelardi T, Caputo R, Damiano V, Daniele G, Pepe S, Ciardiello F, Lahn M, Bianco R, Tortora G. Enzastaurin inhibits tumours sensitive and resistant to anti-EGFR drugs. Br J Cancer. 2008;99:473–480. doi: 10.1038/sj.bjc.6604493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, Weinstein IB. Protein kinase C beta enhances growth and expression of cyclin D1 in human breast cancer cells. Cancer Res. 2006;66:11399–11408. doi: 10.1158/0008-5472.CAN-06-2386. [DOI] [PubMed] [Google Scholar]
- 95.Manni A, Buckwalter E, Etindi R, Kunselman S, Rossini A, Mauger D, Dabbs D, Demers L. Induction of a less aggressive breast cancer phenotype by protein kinase C-alpha and -beta overexpression. Cell Growth Differ. 1996;7:1187–1198. [PubMed] [Google Scholar]
- 96.Grossoni VC, Todaro LB, Kazanietz MG, de Kier Joffe ED, Urtreger AJ. Opposite effects of protein kinase C beta1 (PKCbeta1) and PKCepsilon in the metastatic potential of a breast cancer murine model. Breast Cancer Res Treat. 2009;118:469–480. doi: 10.1007/s10549-008-0299-4. [DOI] [PubMed] [Google Scholar]
- 97.Hanauske AR, Oberschmidt O, Hanauske-Abel H, Lahn MM, Eismann U. Antitumor activity of enzastaurin (LY317615.HCl) against human cancer cell lines and freshly explanted tumors investigated in in-vitro [corrected] soft-agar cloning experiments. Invest New Drugs. 2007;25:205–210. doi: 10.1007/s10637-007-9038-7. [DOI] [PubMed] [Google Scholar]
- 98.Jasinski P, Terai K, Zwolak P, Dudek AZ. Enzastaurin renders MCF-7 breast cancer cells sensitive to radiation through reversal of radiation-induced activation of protein kinase C. Eur J Cancer. 2008;44:1315–1322. doi: 10.1016/j.ejca.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 99.Gokmen-Polar Y, Mehta R, Tuzmen S, Mousses S, Thorat MA, Sanders KL, Turbin D, Leung S, Huntsman DG, Sledge GW, Jr, Badve S. Differential subcellular expression of protein kinase C betaII in breast cancer: correlation with breast cancer subtypes. Breast Cancer Res Treat. 2010;124:327–335. doi: 10.1007/s10549-010-0733-2. [DOI] [PubMed] [Google Scholar]
- 100.Teicher BA, Menon K, Alvarez E, Galbreath E, Shih C, Faul MM. Antiangiogenic and antitumor effects of a protein kinase Cbeta inhibitor in murine lewis lung carcinoma and human Calu-6 non-small-cell lung carcinoma xenografts. Cancer Chemo Pharmacol. 2001;48:473–480. doi: 10.1007/s002800100372. [DOI] [PubMed] [Google Scholar]
- 101.Tekle C, Giovannetti E, Sigmond J, Graff JR, Smid K, Peters GJ. Molecular pathways involved in the synergistic interaction of the PKC beta inhibitor enzastaurin with the antifolate pemetrexed in non-small cell lung cancer cells. Br J Cancer. 2008;99:750–759. doi: 10.1038/sj.bjc.6604566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Willey CD, Xiao D, Tu T, Kim KW, Moretti L, Niermann KJ, Tawtawy MN, Quarles CC, Lu B. Enzastaurin (LY317615), a protein kinase C beta selective inhibitor, enhances antiangiogenic effect of radiation. Int J Radiat Oncol Biol Phys. 2010;77:1518–1526. doi: 10.1016/j.ijrobp.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Graff JR, McNulty AM, Hanna KR, Konicek BW, Lynch RL, Bailey SN, Banks C, Capen A, Goode R, Lewis JE, Sams L, Huss KL, Campbell RM, Iversen PW, Neubauer BL, Brown TJ, Musib L, Geeganage S, Thornton D. The protein kinase Cbeta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 104.Kreisl TN, Kotliarova S, Butman JA, Albert PS, Kim L, Musib L, Thornton D, Fine HA. A phase I/II trial of enzastaurin in patients with recurrent high-grade gliomas. Neuro Oncol. 2010;12:181–189. doi: 10.1093/neuonc/nop042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hans CP, Weisenburger DD, Greiner TC, Chan WC, Aoun P, Cochran GT, Pan Z, Smith LM, Lynch JC, Bociek RG, Bierman PJ, Vose JM, Armitage JO. Expression of PKC-beta or cyclin D2 predicts for inferior survival in diffuse large B-cell lymphoma. Mod Pathol. 2005;18:1377–1384. doi: 10.1038/modpathol.3800434. [DOI] [PubMed] [Google Scholar]
- 106.Li S, Phong M, Lahn M, Brail L, Sutton S, Lin BK, Thornton D, Liao B. Retrospective analysis of protein kinase C-beta (PKC-beta) expression in lymphoid malignancies and its association with survival in diffuse large B-cell lymphomas. Biol Direct. 2007;2:8. doi: 10.1186/1745-6150-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Querfeld C, Rizvi MA, Kuzel TM, Guitart J, Rademaker A, Sabharwal SS, Krett NL, Rosen ST. The selective protein kinase C beta inhibitor enzastaurin induces apoptosis in cutaneous T-cell lymphoma cell lines through the AKT pathway. J Invest Dermatol. 2006;126:1641–1647. doi: 10.1038/sj.jid.5700322. [DOI] [PubMed] [Google Scholar]
- 108.Civallero M, Cosenza M, Grisendi G, Marcheselli L, Todoerti K, Sacchi S. Effects of enzastaurin, alone or in combination, on signaling pathway controlling growth and survival of B-cell lymphoma cell lines. Leuk Lymphoma. 2010;51:671–679. doi: 10.3109/10428191003637290. [DOI] [PubMed] [Google Scholar]
- 109.Civallero M, Cosenza M, Neri A, Bari A. Genomic profiling of enzastaurin-treated B cell lymphoma RL cells. Hematol Oncol. 2011;29:154–156. doi: 10.1002/hon.974. [DOI] [PubMed] [Google Scholar]
- 110.Querfeld C, Kuzel TM, Kim YH, Porcu P, Duvic M, Musiek A, Rook AH, Mark LA, Pinter-Brown L, Hamid O, Lin B, Bian Y, Boye M, Day JM, Rosen ST. Multicenter phase II trial of enzastaurin in patients with relapsed or refractory advanced cutaneous T-cell lymphoma. Leuk Lymphoma. 2011;52:1474–1480. doi: 10.3109/10428194.2011.572265. [DOI] [PubMed] [Google Scholar]
- 111.Lutzny G, Kocher T, Schmidt-Supprian M, Rudelius M, Klein-Hitpass L, Finch AJ, Durig J, Wagner M, Haferlach C, Kohlmann A, Schnittger S, Seifert M, Wanninger S, Zaborsky N, Oostendorp R, Ruland J, Leitges M, Kuhnt T, Schafer Y, Lampl B, Peschel C, Egle A, Ringshausen I. Protein kinase c-beta-dependent activation of NF-kappaB in stromal cells is indispensable for the survival of chronic lymphocytic leukemia B cells in vivo. Cancer Cell. 2013;23:77–92. doi: 10.1016/j.ccr.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Irie K, Yanagita RC, Nakagawa Y. Challenges to the development of bryostatin-type anticancer drugs based on the activation mechanism of protein kinase Cdelta. Med Res Rev. 2012;32:518–535. doi: 10.1002/med.20220. [DOI] [PubMed] [Google Scholar]
- 113.Yonezawa T, Kurata R, Kimura M, Inoko H. PKC delta and epsilon in drug targeting and therapeutics. Recent Pat DNA Gene Seq. 2009;3:96–101. doi: 10.2174/187221509788654205. [DOI] [PubMed] [Google Scholar]
- 114.Gonelli A, Mischiati C, Guerrini R, Voltan R, Salvadori S, Zauli G. Perspectives of protein kinase C (PKC) inhibitors as anti-cancer agents. Mini Rev Med Chem. 2009;9:498–509. doi: 10.2174/138955709787847967. [DOI] [PubMed] [Google Scholar]
- 115.Zhao M, Xia L, Chen GQ. Protein kinase cdelta in apoptosis: a brief overview. Arch Immunol Ther Exp (Warsz) 2012;60:361–372. doi: 10.1007/s00005-012-0188-8. [DOI] [PubMed] [Google Scholar]
- 116.Basu A, Pal D. Two faces of protein kinase Cdelta: the contrasting roles of PKCdelta in cell survival and cell death. ScientificWorldJournal. 2010;10:2272–2284. doi: 10.1100/tsw.2010.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fujii T, Garcia-Bermejo ML, Bernabo JL, Caamano J, Ohba M, Kuroki T, Li L, Yuspa SH, Kazanietz MG. Involvement of protein kinase C delta (PKCdelta) in phorbol ester-induced apoptosis in LNCaP prostate cancer cells. Lack of proteolytic cleavage of PKCdelta. J Biol Chem. 2000;275:7574–7582. doi: 10.1074/jbc.275.11.7574. [DOI] [PubMed] [Google Scholar]
- 118.Tanaka Y, Gavrielides MV, Mitsuuchi Y, Fujii T, Kazanietz MG. Protein kinase C promotes apoptosis in LNCaP prostate cancer cells through activation of p38 MAPK and inhibition of the Akt survival pathway. J Biol Chem. 2003;278:33753–33762. doi: 10.1074/jbc.M303313200. [DOI] [PubMed] [Google Scholar]
- 119.Xiao L, Eto M, Kazanietz MG. ROCK mediates phorbol ester-induced apoptosis in prostate cancer cells via p21Cip1 up-regulation and JNK. J Biol Chem. 2009;284:29365–29375. doi: 10.1074/jbc.M109.007971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gonzalez-Guerrico AM, Kazanietz MG. Phorbol ester-induced apoptosis in prostate cancer cells via autocrine activation of the extrinsic apoptotic cascade: a key role for protein kinase C delta. J Biol Chem. 2005;280:38982–38991. doi: 10.1074/jbc.M506767200. [DOI] [PubMed] [Google Scholar]
- 121.Xiao L, Caino MC, von Burstin VA, Oliva JL, Kazanietz MG. Phorbol ester-induced apoptosis and senescence in cancer cell models. Methods Enzymol. 2008;446:123–139. doi: 10.1016/S0076-6879(08)01607-8. [DOI] [PubMed] [Google Scholar]
- 122.Xiao L, Gonzalez-Guerrico A, Kazanietz MG. PKC-mediated secretion of death factors in LNCaP prostate cancer cells is regulated by androgens. Mol Carcinog. 2009;48:187–195. doi: 10.1002/mc.20476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mischak H, Goodnight JA, Kolch W, Martiny-Baron G, Schaechtle C, Kazanietz MG, Blumberg PM, Pierce JH, Mushinski JF. Overexpression of protein kinase C-delta and -epsilon in NIH 3T3 cells induces opposite effects on growth, morphology, anchorage dependence, and tumorigenicity. J Biol Chem. 1993;268:6090–6096. [PubMed] [Google Scholar]
- 124.Gavrielides MV, Frijhoff AF, Conti CJ, Kazanietz MG. Protein kinase C and prostate carcinogenesis: targeting the cell cycle and apoptotic mechanisms. Curr Drug Targets. 2004;5:431–443. doi: 10.2174/1389450043345380. [DOI] [PubMed] [Google Scholar]
- 125.Nakagawa M, Oliva JL, Kothapalli D, Fournier A, Assoian RK, Kazanietz MG. Phorbol ester-induced G1 phase arrest selectively mediated by protein kinase Cdelta-dependent induction of p21. J Biol Chem. 2005;280:33926–33934. doi: 10.1074/jbc.M505748200. [DOI] [PubMed] [Google Scholar]
- 126.Santiago-Walker AE, Fikaris AJ, Kao GD, Brown EJ, Kazanietz MG, Meinkoth JL. Protein kinase C delta stimulates apoptosis by initiating G1 phase cell cycle progression and S phase arrest. J Biol Chem. 2005;280:32107–32114. doi: 10.1074/jbc.M504432200. [DOI] [PubMed] [Google Scholar]
- 127.Grossoni VC, Falbo KB, Kazanietz MG, de Kier Joffe ED, Urtreger AJ. Protein kinase C delta enhances proliferation and survival of murine mammary cells. Mol Carcinog. 2007;46:381–390. doi: 10.1002/mc.20287. [DOI] [PubMed] [Google Scholar]
- 128.Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP. Analysis of protein kinase C delta (PKC delta) expression in endometrial tumors. Hum Pathol. 2008;39:21–29. doi: 10.1016/j.humpath.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Fukase N, Kawamoto T, Kishimoto K, Hara H, Okada Y, Onishi Y, Toda M, Kurosaka M, Akisue T. Protein kinase Cdelta in tumorigenesis of human malignant fibrous histiocytoma. Oncology reports. 2011;26:1221–1226. doi: 10.3892/or.2011.1415. [DOI] [PubMed] [Google Scholar]
- 130.Yadav V, Yanez NC, Fenton SE, Denning MF. Loss of protein kinase C delta gene expression in human squamous cell carcinomas: a laser capture microdissection study. Am J Pathol. 2010;176:1091–1096. doi: 10.2353/ajpath.2010.090816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tsai JH, Hsieh YS, Kuo SJ, Chen ST, Yu SY, Huang CY, Chang AC, Wang YW, Tsai MT, Liu JY. Alteration in the expression of protein kinase C isoforms in human hepatocellular carcinoma. Cancer letters. 2000;161:171–175. doi: 10.1016/s0304-3835(00)00597-8. [DOI] [PubMed] [Google Scholar]
- 132.Yu LR, Lv JQ, Jin LY, Ding SD, Ma XY, Wang JJ, Zhu XQ. Over-expression of protein kinase Cisoforms (alpha, delta, theta and zeta) in squamous cervical cancer. Neoplasma. 2011;58:491–498. [PubMed] [Google Scholar]
- 133.Kharait S, Dhir R, Lauffenburger D, Wells A. Protein kinase Cdelta signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem Biophys Res Commun. 2006;343:848–856. doi: 10.1016/j.bbrc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 134.Villar J, Arenas MI, MacCarthy CM, Blanquez MJ, Tirado OM, Notario V. PCPH/ENTPD5 expression enhances the invasiveness of human prostate cancer cells by a protein kinase C delta-dependent mechanism. Cancer Res. 2007;67:10859–10868. doi: 10.1158/0008-5472.CAN-07-2041. [DOI] [PubMed] [Google Scholar]
- 135.McKiernan E, O’Brien K, Grebenchtchikov N, Geurts-Moespot A, Sieuwerts AM, Martens JW, Magdolen V, Evoy D, McDermott E, Crown J, Sweep FC, Duffy MJ. Protein kinase Cdelta expression in breast cancer as measured by real-time PCR, western blotting and ELISA. Br J Cancer. 2008;99:1644–1650. doi: 10.1038/sj.bjc.6604728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Motegi A, Sakurai S, Nakayama H, Sano T, Oyama T, Nakajima T. PKC theta, a novel immunohistochemical marker for gastrointestinal stromal tumors (GIST), especially useful for identifying KIT-negative tumors. Pathol Int. 2005;55:106–112. doi: 10.1111/j.1440-1827.2005.01806.x. [DOI] [PubMed] [Google Scholar]
- 137.Kang GH, Srivastava A, Kim YE, Park HJ, Park CK, Sohn TS, Kim S, Kang DY, Kim KM. DOG1 and PKC-theta are useful in the diagnosis of KIT-negative gastrointestinal stromal tumors. Mod Pathol. 2011;24:866–875. doi: 10.1038/modpathol.2011.11. [DOI] [PubMed] [Google Scholar]
- 138.Kim KH, Nelson SD, Kim DH, Choi KU, Kim SJ, Min KW, Jang KS, Paik SS, Oh YH, Chae SW, Sohn JH, Kim HJ, Cho YK, Kim BI, Park DI, Sohn CI, Oh S, Choi SH, Choi YJ, Woo HY, Park YL, Park SJ, Lee SH, Ryu S, Do SI, Kang G, Kim K, Cho YH, Pyo JS. Diagnostic relevance of overexpressions of PKC-theta and DOG-1 and KIT/PDGFRA gene mutations in extragastrointestinal stromal tumors: a Korean six-centers study of 28 cases. Anticancer Res. 2012;32:923–937. [PubMed] [Google Scholar]
- 139.Reddig PJ, Dreckschmidt NE, Ahrens H, Simsiman R, Tseng CP, Zou J, Oberley TD, Verma AK. Transgenic mice overexpressing protein kinase Cdelta in the epidermis are resistant to skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1999;59:5710–5718. [PubMed] [Google Scholar]
- 140.Symonds JM, Ohm AM, Carter CJ, Heasley LE, Boyle TA, Franklin WA, Reyland ME. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res. 2011;71:2087–2097. doi: 10.1158/0008-5472.CAN-10-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Allen-Petersen BL, Carter CJ, Ohm AM, Reyland ME. Protein kinase Cdelta is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene. 2013 doi: 10.1038/onc.2013.2059. e-pub ahead of print 11 March 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mauro LV, Grossoni VC, Urtreger AJ, Yang C, Colombo LL, Morandi A, Pallotta MG, Kazanietz MG, Bal de Kier Joffe ED, Puricelli LL. PKC Delta (PKCdelta) promotes tumoral progression of human ductal pancreatic cancer. Pancreas. 2010;39:e31–41. doi: 10.1097/MPA.0b013e3181bce796. [DOI] [PubMed] [Google Scholar]
- 143.Kim J, Koyanagi T, Mochly-Rosen D. PKCdelta activation mediates angiogenesis via NADPH oxidase activity in PC-3 prostate cancer cells. Prostate. 2011;71:946–954. doi: 10.1002/pros.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Li N, Du ZX, Zong ZH, Liu BQ, Li C, Zhang Q, Wang HQ. PKCdelta-mediated phosphorylation of BAG3 at Ser187 site induces epithelial-mesenchymal transition and enhances invasiveness in thyroid cancer FRO cells. Oncogene. 2012 doi: 10.1038/onc.2012.2466. epub ahead of print 29 October 2012. [DOI] [PubMed] [Google Scholar]
- 145.Razorenova OV, Finger EC, Colavitti R, Chernikova SB, Boiko AD, Chan CK, Krieg A, Bedogni B, LaGory E, Weissman IL, Broome-Powell M, Giaccia AJ. VHL loss in renal cell carcinoma leads to up-regulation of CUB domain-containing protein 1 to stimulate PKC{delta}-driven migration. Proc Natl Acad Sci U S A. 2011;108:1931–1936. doi: 10.1073/pnas.1011777108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Miyazawa Y, Uekita T, Hiraoka N, Fujii S, Kosuge T, Kanai Y, Nojima Y, Sakai R. CUB domain-containing protein 1, a prognostic factor for human pancreatic cancers, promotes cell migration and extracellular matrix degradation. Cancer Res. 2010;70:5136–5146. doi: 10.1158/0008-5472.CAN-10-0220. [DOI] [PubMed] [Google Scholar]
- 147.Sarkar S, Yong VW. Reduction of protein kinase C delta attenuates tenascin-C stimulated glioma invasion in three-dimensional matrix. Carcinogenesis. 2010;31:311–317. doi: 10.1093/carcin/bgp297. [DOI] [PubMed] [Google Scholar]
- 148.Jackson D, Zheng Y, Lyo D, Shen Y, Nakayama K, Nakayama KI, Humphries MJ, Reyland ME, Foster DA. Suppression of cell migration by protein kinase Cdelta. Oncogene. 2005;24:3067–3072. doi: 10.1038/sj.onc.1208465. [DOI] [PubMed] [Google Scholar]
- 149.Kruger JS, Reddy KB. Distinct mechanisms mediate the initial and sustained phases of cell migration in epidermal growth factor receptor-overexpressing cells. Mol Cancer Res. 2003;1:801–809. [PubMed] [Google Scholar]
- 150.Kiley SC, Clark KJ, Goodnough M, Welch DR, Jaken S. Protein kinase C delta involvement in mammary tumor cell metastasis. Cancer Res. 1999;59:3230–3238. [PubMed] [Google Scholar]
- 151.La Porta CA, Comolli R. Overexpression of nPKCdelta in BL6 murine melanoma cells enhances TGFbeta1 release into the plasma of metastasized animals. Melanoma Res. 2000;10:527–534. doi: 10.1097/00008390-200012000-00003. [DOI] [PubMed] [Google Scholar]
- 152.La Porta CA, Di Dio A, Porro D, Comolli R. Overexpression of novel protein kinase C delta in BL6 murine melanoma cells inhibits the proliferative capacity in vitro but enhances the metastatic potential in vivo. Melanoma Res. 2000;10:93–102. [PubMed] [Google Scholar]
- 153.Cacace AM, Guadagno SN, Krauss RS, Fabbro D, Weinstein IB. The epsilon isoform of protein kinase C is an oncogene when overexpressed in rat fibroblasts. Oncogene. 1993;8:2095–2104. [PubMed] [Google Scholar]
- 154.Perletti GP, Folini M, Lin HC, Mischak H, Piccinini F, Tashjian AH., Jr Overexpression of protein kinase C epsilon is oncogenic in rat colonic epithelial cells. Oncogene. 1996;12:847–854. [PubMed] [Google Scholar]
- 155.Cai H, Smola U, Wixler V, Eisenmann-Tappe I, Diaz-Meco MT, Moscat J, Rapp U, Cooper GM. Role of diacylglycerol-regulated protein kinase C isotypes in growth factor activation of the Raf-1 protein kinase. Mol Cell Biol. 1997;17:732–741. doi: 10.1128/mcb.17.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cacace AM, Ueffing M, Philipp A, Han EK, Kolch W, Weinstein IB. PKC epsilon functions as an oncogene by enhancing activation of the Raf kinase. Oncogene. 1996;13:2517–2526. [PubMed] [Google Scholar]
- 157.Perletti GP, Concari P, Brusaferri S, Marras E, Piccinini F, Tashjian AH., Jr Protein kinase Cepsilon is oncogenic in colon epithelial cells by interaction with the ras signal transduction pathway. Oncogene. 1998;16:3345–3348. doi: 10.1038/sj.onc.1201871. [DOI] [PubMed] [Google Scholar]
- 158.Hamilton M, Liao J, Cathcart MK, Wolfman A. Constitutive association of c-N-Ras with c-Raf-1 and protein kinase C epsilon in latent signaling modules. J Biol Chem. 2001;276:29079–29090. doi: 10.1074/jbc.M102001200. [DOI] [PubMed] [Google Scholar]
- 159.Cacace AM, Ueffing M, Han EK, Marme D, Weinstein IB. Overexpression of PKCepsilon in R6 fibroblasts causes increased production of active TGFbeta. J Cell Physiol. 1998;175:314–322. doi: 10.1002/(SICI)1097-4652(199806)175:3<314::AID-JCP9>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 160.Cadoret A, Baron-Delage S, Bertrand F, Kornprost M, Groyer A, Gespach C, Capeau J, Cherqui G. Oncogene-induced up-regulation of Caco-2 cell proliferation involves IGF-II gene activation through a protein kinase C-mediated pathway. Oncogene. 1998;17:877–887. doi: 10.1038/sj.onc.1202013. [DOI] [PubMed] [Google Scholar]
- 161.Cornford P, Evans J, Dodson A, Parsons K, Woolfenden A, Neoptolemos J, Foster CS. Protein kinase C isoenzyme patterns characteristically modulated in early prostate cancer. Am J Pathol. 1999;154:137–144. doi: 10.1016/S0002-9440(10)65260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Brenner W, Farber G, Herget T, Wiesner C, Hengstler JG, Thuroff JW. Protein kinase C eta is associated with progression of renal cell carcinoma (RCC) Anticancer Res. 2003;23:4001–4006. [PubMed] [Google Scholar]
- 163.Krasnitsky E, Baumfeld Y, Freedman J, Sion-Vardy N, Ariad S, Novack V, Livneh E. PKCeta is a novel prognostic marker in non-small cell lung cancer. Anticancer Res. 2012;32:1507–1513. [PubMed] [Google Scholar]
- 164.Lu HC, Chou FP, Yeh KT, Chang YS, Hsu NC, Chang JG. Expression of protein kinase C family in human hepatocellular carcinoma. Pathol Oncol Res. 2010;16:385–391. doi: 10.1007/s12253-009-9228-z. [DOI] [PubMed] [Google Scholar]
- 165.Black JD. Protein kinase C-mediated regulation of the cell cycle. Frontiers in bioscience : a journal and virtual library. 2000;5:D406–423. doi: 10.2741/black. [DOI] [PubMed] [Google Scholar]
- 166.Gorin MA, Pan Q. Protein kinase C epsilon: an oncogene and emerging tumor biomarker. Mol Cancer. 2009;8:9. doi: 10.1186/1476-4598-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fishman DD, Segal S, Livneh E. The role of protein kinase C in G1 and G2/M phases of the cell cycle (review) Int J Oncol. 1998;12:181–186. doi: 10.3892/ijo.12.1.181. [DOI] [PubMed] [Google Scholar]
- 168.Basu A, Sivaprasad U. Protein kinase Cepsilon makes the life and death decision. Cell Signal. 2007;19:1633–1642. doi: 10.1016/j.cellsig.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.McJilton MA, Van Sikes C, Wescott GG, Wu D, Foreman TL, Gregory CW, Weidner DA, Harris Ford O, Morgan Lasater A, Mohler JL, Terrian DM. Protein kinase Cepsilon interacts with Bax and promotes survival of human prostate cancer cells. Oncogene. 2003;22:7958–7968. doi: 10.1038/sj.onc.1206795. [DOI] [PubMed] [Google Scholar]
- 170.Okhrimenko H, Lu W, Xiang C, Hamburger N, Kazimirsky G, Brodie C. Protein kinase C-epsilon regulates the apoptosis and survival of glioma cells. Cancer Res. 2005;65:7301–7309. doi: 10.1158/0008-5472.CAN-05-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lu D, Huang J, Basu A. Protein kinase Cepsilon activates protein kinase B/Akt via DNA-PK to protect against tumor necrosis factor-alpha-induced cell death. J Biol Chem. 2006;281:22799–22807. doi: 10.1074/jbc.M603390200. [DOI] [PubMed] [Google Scholar]
- 172.Meshki J, Caino MC, von Burstin VA, Griner E, Kazanietz MG. Regulation of prostate cancer cell survival by protein kinase Cepsilon involves bad phosphorylation and modulation of the TNFalpha/JNK pathway. J Biol Chem. 2010;285:26033–26040. doi: 10.1074/jbc.M110.128371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gonzalez-Guerrico AM, Meshki J, Xiao L, Benavides F, Conti CJ, Kazanietz MG. Molecular mechanisms of protein kinase C-induced apoptosis in prostate cancer cells. J Biochem Mol Biol. 2005;38:639–645. doi: 10.5483/bmbrep.2005.38.6.639. [DOI] [PubMed] [Google Scholar]
- 174.Kahana S, Finniss S, Cazacu S, Xiang C, Lee HK, Brodie S, Goldstein RS, Roitman V, Slavin S, Mikkelsen T, Brodie C. Proteasome inhibitors sensitize glioma cells and glioma stem cells to TRAIL-induced apoptosis by PKCepsilon-dependent downregulation of AKT and XIAP expressions. Cell Signal. 2011;23:1348–1357. doi: 10.1016/j.cellsig.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 175.Felber M, Sonnemann J, Beck JF. Inhibition of novel protein kinase C-epsilon augments TRAIL-induced cell death in A549 lung cancer cells. Pathol Oncol Res. 2007;13:295–301. doi: 10.1007/BF02940308. [DOI] [PubMed] [Google Scholar]
- 176.Lu D, Sivaprasad U, Huang J, Shankar E, Morrow S, Basu A. Protein kinase C-epsilon protects MCF-7 cells from TNF-mediated cell death by inhibiting Bax translocation. Apoptosis. 2007;12:1893–1900. doi: 10.1007/s10495-007-0111-7. [DOI] [PubMed] [Google Scholar]
- 177.Caino MC, Lopez-Haber C, Kim J, Mochly-Rosen D, Kazanietz MG. Proteins kinase Cvarepsilon is required for non-small cell lung carcinoma growth and regulates the expression of apoptotic genes. Oncogene. 2012;31:2593–2600. doi: 10.1038/onc.2011.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Garg R, Blando J, Perez CJ, Wang H, Benavides FJ, Kazanietz MG. Activation of Nuclear Factor kappaB (NF-kappaB) in Prostate Cancer Is Mediated by Protein Kinase C {epsilon} (PKC{epsilon}) J Biol Chem. 2012;287:37570–37582. doi: 10.1074/jbc.M112.398925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wu D, Foreman TL, Gregory CW, McJilton MA, Wescott GG, Ford OH, Alvey RF, Mohler JL, Terrian DM. Protein kinase cepsilon has the potential to advance the recurrence of human prostate cancer. Cancer Res. 2002;62:2423–2429. [PubMed] [Google Scholar]
- 180.Hafeez BB, Zhong W, Weichert J, Dreckschmidt NE, Jamal MS, Verma AK. Genetic ablation of PKC epsilon inhibits prostate cancer development and metastasis in transgenic mouse model of prostate adenocarcinoma. Cancer Res. 2011;71:2318–2327. doi: 10.1158/0008-5472.CAN-10-4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Huang B, Cao K, Li X, Guo S, Mao X, Wang Z, Zhuang J, Pan J, Mo C, Chen J, Qiu S. The expression and role of protein kinase C (PKC) epsilon in clear cell renal cell carcinoma. J Exp Clin Cancer Res. 2011;30:88. doi: 10.1186/1756-9966-30-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Akita Y. Protein kinase Cepsilon: multiple roles in the function of, and signaling mediated by, the cytoskeleton. FEBS J. 2008;275:3995–4004. doi: 10.1111/j.1742-4658.2008.06557.x. [DOI] [PubMed] [Google Scholar]
- 183.Akita Y. Protein kinase C-epsilon (PKC-epsilon): its unique structure and function. J Biochem. 2002;132:847–852. doi: 10.1093/oxfordjournals.jbchem.a003296. [DOI] [PubMed] [Google Scholar]
- 184.Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hernandez RM, Wescott GG, Mayhew MW, McJilton MA, Terrian DM. Biochemical and morphogenic effects of the interaction between protein kinase C-epsilon and actin in vitro and in cultured NIH3T3 cells. J Cell Biochem. 2001;83:532–546. doi: 10.1002/jcb.1246. [DOI] [PubMed] [Google Scholar]
- 186.Tachado SD, Mayhew MW, Wescott GG, Foreman TL, Goodwin CD, McJilton MA, Terrian DM. Regulation of tumor invasion and metastasis in protein kinase C epsilon-transformed NIH3T3 fibroblasts. J Cell Biochem. 2002;85:785–797. doi: 10.1002/jcb.10164. [DOI] [PubMed] [Google Scholar]
- 187.Besson A, Davy A, Robbins SM, Yong VW. Differential activation of ERKs to focal adhesions by PKC epsilon is required for PMA-induced adhesion and migration of human glioma cells. Oncogene. 2001;20:7398–7407. doi: 10.1038/sj.onc.1204899. [DOI] [PubMed] [Google Scholar]
- 188.Wu D, Thakore CU, Wescott GG, McCubrey JA, Terrian DM. Integrin signaling links protein kinase Cepsilon to the protein kinase B/Akt survival pathway in recurrent prostate cancer cells. Oncogene. 2004;23:8659–8672. doi: 10.1038/sj.onc.1207900. [DOI] [PubMed] [Google Scholar]
- 189.Pan Q, Bao LW, Teknos TN, Merajver SD. Targeted disruption of protein kinase C epsilon reduces cell invasion and motility through inactivation of RhoA and RhoC GTPases in head and neck squamous cell carcinoma. Cancer Res. 2006;66:9379–9384. doi: 10.1158/0008-5472.CAN-06-2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Caino MC, Lopez-Haber C, Kissil JL, Kazanietz MG. Non-small cell lung carcinoma cell motility, rac activation and metastatic dissemination are mediated by protein kinase C epsilon. PLoS One. 2012;7:e31714. doi: 10.1371/journal.pone.0031714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Jansen AP, Verwiebe EG, Dreckschmidt NE, Wheeler DL, Oberley TD, Verma AK. Protein kinase C-epsilon transgenic mice: a unique model for metastatic squamous cell carcinoma. Cancer Res. 2001;61:808–812. [PubMed] [Google Scholar]
- 192.Wheeler DL, Martin KE, Ness KJ, Li Y, Dreckschmidt NE, Wartman M, Ananthaswamy HN, Mitchell DL, Verma AK. Protein kinase C epsilon is an endogenous photosensitizer that enhances ultraviolet radiation-induced cutaneous damage and development of squamous cell carcinomas. Cancer Res. 2004;64:7756–7765. doi: 10.1158/0008-5472.CAN-04-1881. [DOI] [PubMed] [Google Scholar]
- 193.Sand JM, Aziz MH, Dreckschmidt NE, Havighurst TC, Kim K, Oberley TD, Verma AK. PKCepsilon overexpression, irrespective of genetic background, sensitizes skin to UVR-induced development of squamous-cell carcinomas. J Invest Dermatol. 2010;130:270–277. doi: 10.1038/jid.2009.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wheeler DL, Reddig PJ, Ness KJ, Leith CP, Oberley TD, Verma AK. Overexpression of protein kinase C-{epsilon} in the mouse epidermis leads to a spontaneous myeloproliferative-like disease. Am J Pathol. 2005;166:117–126. doi: 10.1016/s0002-9440(10)62237-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bao L, Gorin MA, Zhang M, Ventura AC, Pomerantz WC, Merajver SD, Teknos TN, Mapp AK, Pan Q. Preclinical development of a bifunctional cancer cell homing, PKCepsilon inhibitory peptide for the treatment of head and neck cancer. Cancer Res. 2009;69:5829–5834. doi: 10.1158/0008-5472.CAN-08-3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dann SG, Golas J, Miranda M, Shi C, Wu J, Jin G, Rosfjord E, Upeslacis E, Klippel A. p120 catenin is a key effector of a Ras-PKCvarepsilon oncogenic signaling axis. Oncogene. 2013 doi: 10.1038/onc.2013.91. [DOI] [PubMed] [Google Scholar]
- 197.Selbie LA, Schmitz-Peiffer C, Sheng Y, Biden TJ. Molecular cloning and characterization of PKC iota, an atypical isoform of protein kinase C derived from insulin-secreting cells. J Biol Chem. 1993;268:24296–24302. [PubMed] [Google Scholar]
- 198.Ono Y, Fujii T, Ogita K, Kikkawa U, Igarashi K, Nishizuka Y. Protein kinase C zeta subspecies from rat brain: its structure, expression, and properties. Proc Natl Acad Sci U S A. 1989;86:3099–3103. doi: 10.1073/pnas.86.9.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Rhodes DR, Kalyana-Sundaram S, Tomlins SA, Mahavisno V, Kasper N, Varambally R, Barrette TR, Ghosh D, Varambally S, Chinnaiyan AM. Molecular concepts analysis links tumors, pathways, mechanisms, and drugs. Neoplasia. 2007;9:443–454. doi: 10.1593/neo.07292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Dhanasekaran SM, Dash A, Yu J, Maine IP, Laxman B, Tomlins SA, Creighton CJ, Menon A, Rubin MA, Chinnaiyan AM. Molecular profiling of human prostate tissues: insights into gene expression patterns of prostate development during puberty. FASEB J. 2005;19:243–245. doi: 10.1096/fj.04-2415fje. [DOI] [PubMed] [Google Scholar]
- 201.Dyrskjot L, Kruhoffer M, Thykjaer T, Marcussen N, Jensen JL, Moller K, Orntoft TF. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64:4040–4048. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- 202.Sanchez-Carbayo M, Socci ND, Lozano J, Saint F, Cordon-Cardo C. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778–789. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 203.Basso K, Margolin AA, Stolovitzky G, Klein U, Dalla-Favera R, Califano A. Reverse engineering of regulatory networks in human B cells. Nat Genet. 2005;37:382–390. doi: 10.1038/ng1532. [DOI] [PubMed] [Google Scholar]
- 204.Dave SS, Fu K, Wright GW, Lam LT, Kluin P, Boerma EJ, Greiner TC, Weisenburger DD, Rosenwald A, Ott G, Muller-Hermelink HK, Gascoyne RD, Delabie J, Rimsza LM, Braziel RM, Grogan TM, Campo E, Jaffe ES, Dave BJ, Sanger W, Bast M, Vose JM, Armitage JO, Connors JM, Smeland EB, Kvaloy S, Holte H, Fisher RI, Miller TP, Montserrat E, Wilson WH, Bahl M, Zhao H, Yang L, Powell J, Simon R, Chan WC, Staudt LM. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med. 2006;354:2431–2442. doi: 10.1056/NEJMoa055759. [DOI] [PubMed] [Google Scholar]
- 205.Sun L, Hui AM, Su Q, Vortmeyer A, Kotliarov Y, Pastorino S, Passaniti A, Menon J, Walling J, Bailey R, Rosenblum M, Mikkelsen T, Fine HA. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 206.French PJ, Swagemakers SM, Nagel JH, Kouwenhoven MC, Brouwer E, van der Spek P, Luider TM, Kros JM, van den Bent MJ, Sillevis Smitt PA. Gene expression profiles associated with treatment response in oligodendrogliomas. Cancer Res. 2005;65:11335–11344. doi: 10.1158/0008-5472.CAN-05-1886. [DOI] [PubMed] [Google Scholar]
- 207.Rickman DS, Bobek MP, Misek DE, Kuick R, Blaivas M, Kurnit DM, Taylor J, Hanash SM. Distinctive molecular profiles of high-grade and low-grade gliomas based on oligonucleotide microarray analysis. Cancer Res. 2001;61:6885–6891. [PubMed] [Google Scholar]
- 208.Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD, Sikic BI. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res. 2005;65:8679–8689. doi: 10.1158/0008-5472.CAN-05-1204. [DOI] [PubMed] [Google Scholar]
- 209.Bhattacharjee A, Richards WG, Staunton J, Li C, Monti S, Vasa P, Ladd C, Beheshti J, Bueno R, Gillette M, Loda M, Weber G, Mark EJ, Lander ES, Wong W, Johnson BE, Golub TR, Sugarbaker DJ, Meyerson M. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc Natl Acad Sci U S A. 2001;98:13790–13795. doi: 10.1073/pnas.191502998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stearman RS, Dwyer-Nield L, Zerbe L, Blaine SA, Chan Z, Bunn PA, Jr, Johnson GL, Hirsch FR, Merrick DT, Franklin WA, Baron AE, Keith RL, Nemenoff RA, Malkinson AM, Geraci MW. Analysis of orthologous gene expression between human pulmonary adenocarcinoma a carcinogen-induced murine model. Am J Pathol. 2005;167:1763–1775. doi: 10.1016/S0002-9440(10)61257-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Galvez AS, Duran A, Linares JF, Pathrose P, Castilla EA, Abu-Baker S, Leitges M, Diaz-Meco MT, Moscat J. Protein kinase Czeta represses the interleukin-6 promoter and impairs tumorigenesis in vivo. Mol Cell Biol. 2009;29:104–115. doi: 10.1128/MCB.01294-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lenburg ME, Liou LS, Gerry NP, Frampton GM, Cohen HT, Christman MF. Previously unidentified changes in renal cell carcinoma gene expression identified by parametric analysis of microarray data. BMC Cancer. 2003;3:31. doi: 10.1186/1471-2407-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Talantov D, Mazumder A, Yu JX, Briggs T, Jiang Y, Backus J, Atkins D, Wang Y. Novel genes associated with malignant melanoma but not benign melanocytic lesions. Clin Cancer Res. 2005;11:7234–7242. doi: 10.1158/1078-0432.CCR-05-0683. [DOI] [PubMed] [Google Scholar]
- 214.Buchholz M, Braun M, Heidenblut A, Kestler HA, Kloppel G, Schmiegel W, Hahn SA, Luttges J, Gress TM. Transcriptome analysis of microdissected pancreatic intraepithelial neoplastic lesions. Oncogene. 2005;24:6626–6636. doi: 10.1038/sj.onc.1208804. [DOI] [PubMed] [Google Scholar]
- 215.Mustafi R, Cerda S, Chumsangsri A, Fichera A, Bissonnette M. Protein Kinase-zeta inhibits collagen I-dependent and anchorage-independent growth and enhances apoptosis of human Caco-2 cells. Mol Cancer Res. 2006;4:683–694. doi: 10.1158/1541-7786.MCR-06-0057. [DOI] [PubMed] [Google Scholar]
- 216.Nazarenko I, Jenny M, Keil J, Gieseler C, Weisshaupt K, Sehouli J, Legewie S, Herbst L, Weichert W, Darb-Esfahani S, Dietel M, Schafer R, Ueberall F, Sers C. Atypical protein kinase C zeta exhibits a proapoptotic function in ovarian cancer. Mol Cancer Res. 2010;8:919–934. doi: 10.1158/1541-7786.MCR-09-0358. [DOI] [PubMed] [Google Scholar]
- 217.Ghosh PM, Bedolla R, Mikhailova M, Kreisberg JI. RhoA-dependent murine prostate cancer cell proliferation and apoptosis: role of protein kinase Czeta. Cancer Res. 2002;62:2630–2636. [PubMed] [Google Scholar]
- 218.Filomenko R, Poirson-Bichat F, Billerey C, Belon JP, Garrido C, Solary E, Bettaieb A. Atypical protein kinase C zeta as a target for chemosensitization of tumor cells. Cancer Res. 2002;62:1815–1821. [PubMed] [Google Scholar]
- 219.Bezombes C, de Thonel A, Apostolou A, Louat T, Jaffrezou JP, Laurent G, Quillet-Mary A. Overexpression of protein kinase Czeta confers protection against antileukemic drugs by inhibiting the redox-dependent sphingomyelinase activation. Mol Pharmacol. 2002;62:1446–1455. doi: 10.1124/mol.62.6.1446. [DOI] [PubMed] [Google Scholar]
- 220.Cataldi A, Centurione L, Di Pietro R, Rapino M, Bosco D, Grifone G, Garaci F, Rana R. Protein kinase C zeta nuclear translocation mediates the occurrence of radioresistance in friend erythroleukemia cells. J Cell Biochem. 2003;88:144–151. doi: 10.1002/jcb.10305. [DOI] [PubMed] [Google Scholar]
- 221.Xin M, Gao F, May WS, Flagg T, Deng X. Protein kinase Czeta abrogates the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2007;282:21268–21277. doi: 10.1074/jbc.M701613200. [DOI] [PubMed] [Google Scholar]
- 222.Rimessi A, Zecchini E, Siviero R, Giorgi C, Leo S, Rizzuto R, Pinton P. The selective inhibition of nuclear PKCzeta restores the effectiveness of chemotherapeutic agents in chemoresistant cells. Cell Cycle. 2012;11:1040–1048. doi: 10.4161/cc.11.5.19520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Kim JY, Valencia T, Abu-Baker S, Linares J, Lee SJ, Yajima T, Chen J, Eroshkin A, Castilla EA, Brill LM, Medvedovic M, Leitges M, Moscat J, Diaz-Meco MT. c-Myc phosphorylation by PKCzeta represses prostate tumorigenesis. Proc Natl Acad Sci U S A. 2013;110:6418–6423. doi: 10.1073/pnas.1221799110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ma L, Tao Y, Duran A, Llado V, Galvez A, Barger JF, Castilla EA, Chen J, Yajima T, Porollo A, Medvedovic M, Brill LM, Plas DR, Riedl SJ, Leitges M, Diaz-Meco MT, Richardson AD, Moscat J. Control of nutrient stress-induced metabolic reprogramming by PKCzeta in tumorigenesis. Cell. 2013;152:599–611. doi: 10.1016/j.cell.2012.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Zhang L, Huang J, Yang N, Liang S, Barchetti A, Giannakakis A, Cadungog MG, O’Brien-Jenkins A, Massobrio M, Roby KF, Katsaros D, Gimotty P, Butzow R, Weber BL, Coukos G. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res. 2006;66:4627–4635. doi: 10.1158/0008-5472.CAN-05-4527. [DOI] [PubMed] [Google Scholar]
- 226.Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP, Kango-Singh M, Lu KH, Warneke CL, Atkinson EN, Bedrosian I, Keyomarsi K, Kuo WL, Gray JW, Yin JC, Liu J, Halder G, Mills GB. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci U S A. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Nanjundan M, Nakayama Y, Cheng KW, Lahad J, Liu J, Lu K, Kuo WL, Smith-McCune K, Fishman D, Gray JW, Mills GB. Amplification of MDS1/EVI1 and EVI1, located in the 3q26. 2 amplicon, is associated with favorable patient prognosis in ovarian cancer. Cancer Res. 2007;67:3074–3084. doi: 10.1158/0008-5472.CAN-06-2366. [DOI] [PubMed] [Google Scholar]
- 228.Gus’tafson WC, Ray S, Jamieson L, Thompson EA, Brasier AR, Fields AP. Bcr-Abl regulates protein kinase Ciota (PKCiota) transcription via an Elk1 site in the PKCiota promoter. J Biol Chem. 2004;279:9400–9408. doi: 10.1074/jbc.M312840200. [DOI] [PubMed] [Google Scholar]
- 229.Murray NR, Jamieson L, Yu W, Zhang J, Gokmen-Polar Y, Sier D, Anastasiadis P, Gatalica Z, Thompson EA, Fields AP. Protein kinase Ciota is required for Ras transformation and colon carcinogenesis in vivo. J Cell Biol. 2004;164:797–802. doi: 10.1083/jcb.200311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Scotti ML, Bamlet WR, Smyrk TC, Fields AP, Murray NR. Protein kinase Ciota is required for pancreatic cancer cell transformed growth and tumorigenesis. Cancer Res. 2010;70:2064–2074. doi: 10.1158/0008-5472.CAN-09-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Murray NR, Kalari KR, Fields AP. Protein kinase Ciota expression and oncogenic signaling mechanisms in cancer. J Cell Physiol. 2011;226:879–887. doi: 10.1002/jcp.22463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Frederick LA, Matthews JA, Jamieson L, Justilien V, Thompson EA, Radisky DC, Fields AP. Matrix metalloproteinase-10 is a critical effector of protein kinase Ciota-Par6alpha-mediated lung cancer. Oncogene. 2008;27:4841–4853. doi: 10.1038/onc.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Regala RP, Davis RK, Kunz A, Khoor A, Leitges M, Fields AP. Atypical protein kinase C{iota} is required for bronchioalveolar stem cell expansion and lung tumorigenesis. Cancer Res. 2009;69:7603–7611. doi: 10.1158/0008-5472.CAN-09-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Regala RP, Weems C, Jamieson L, Copland JA, Thompson EA, Fields AP. Atypical protein kinase Ciota plays a critical role in human lung cancer cell growth and tumorigenicity. J Biol Chem. 2005;280:31109–31115. doi: 10.1074/jbc.M505402200. [DOI] [PubMed] [Google Scholar]
- 235.Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM, Fields AP. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res. 2005;65:8905–8911. doi: 10.1158/0008-5472.CAN-05-2372. [DOI] [PubMed] [Google Scholar]
- 236.Justilien V, Jameison L, Der CJ, Rossman KL, Fields AP. Oncogenic activity of Ect2 is regulated through protein kinase C iota-mediated phosphorylation. J Biol Chem. 2011;286:8149–8157. doi: 10.1074/jbc.M110.196113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Regala RP, Thompson EA, Fields AP. Atypical protein kinase C iota expression and aurothiomalate sensitivity in human lung cancer cells. Cancer Res. 2008;68:5888–5895. doi: 10.1158/0008-5472.CAN-08-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Sengupta A, Duran A, Ishikawa E, Florian MC, Dunn SK, Ficker AM, Leitges M, Geiger H, Diaz-Meco M, Moscat J, Cancelas JA. Atypical protein kinase C (aPKCzeta and aPKClambda) is dispensable for mammalian hematopoietic stem cell activity and blood formation. Proc Natl Acad Sci U S A. 2011;108:9957–9962. doi: 10.1073/pnas.1103132108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Atwood SX, Li M, Lee A, Tang JY, Oro AE. GLI activation by atypical protein kinase C iota/lambda regulates the growth of basal cell carcinomas. Nature. 2013;494:484–488. doi: 10.1038/nature11889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Baldwin RM, Parolin DA, Lorimer IA. Regulation of glioblastoma cell invasion by PKC iota and RhoB. Oncogene. 2008;27:3587–3595. doi: 10.1038/sj.onc.1211027. [DOI] [PubMed] [Google Scholar]
- 241.Win HY, Acevedo-Duncan M. Atypical protein kinase C phosphorylates IKKalphabeta in transformed non-malignant and malignant prostate cell survival. Cancer Lett. 2008;270:302–311. doi: 10.1016/j.canlet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 242.Ishiguro H, Akimoto K, Nagashima Y, Kojima Y, Sasaki T, Ishiguro-Imagawa Y, Nakaigawa N, Ohno S, Kubota Y, Uemura H. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc Natl Acad Sci U S A. 2009;106:16369–16374. doi: 10.1073/pnas.0907044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Liu SG, Wang BS, Jiang YY, Zhang TT, Shi ZZ, Yang Y, Yang YL, Wang XC, Lin DC, Zhang Y, Yang H, Cai Y, Zhan QM, Wang MR. Atypical protein kinase Ciota (PKCiota) promotes metastasis of esophageal squamous cell carcinoma by enhancing resistance to Anoikis via PKCiota-SKP2-AKT pathway. Mol Cancer Res. 2011;9:390–402. doi: 10.1158/1541-7786.MCR-10-0359. [DOI] [PubMed] [Google Scholar]
- 244.Mochly-Rosen D, Das K, Grimes KV. Protein kinase C, an elusive therapeutic target? Nat Rev Drug Discov. 2012;11:937–957. doi: 10.1038/nrd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Barry OP, Kazanietz MG. Protein kinase C isozymes, novel phorbol ester receptors and cancer chemotherapy. Curr Pharm Des. 2001;7:1725–1744. doi: 10.2174/1381612013397041. [DOI] [PubMed] [Google Scholar]
- 246.Kazanietz MG, Lewin NE, Gao F, Pettit GR, Blumberg PM. Binding of [26-3H]bryostatin 1 and analogs to calcium-dependent and calcium-independent protein kinase C isozymes. Mol Pharmacol. 1994;46:374–379. [PubMed] [Google Scholar]