Abstract
Presynaptic serotonin (5-hydroxytryptamine, 5-HT) transporters (SERT) regulate 5-HT signaling via antidepressant-sensitive clearance of released neurotransmitter. Polymorphisms in the human SERT gene (SLC6A4) have been linked to risk for multiple neuropsychiatric disorders, including depression, obsessive-compulsive disorder and autism. Using BXD recombinant inbred mice, a genetic reference population that can support the discovery of novel determinants of complex traits, merging collective trait assessments with bioinformatics approaches, we examine phenotypic and molecular networks associated with SERT gene and protein expression. Correlational analyses revealed a network of genes that significantly associated with SERT mRNA levels. We quantified SERT protein expression levels and identified region- and gender-specific quantitative trait loci (QTLs), one of which associated with male midbrain SERT protein expression, centered on the protocadherin-15 gene (Pcdh15), overlapped with a QTL for midbrain 5-HT levels. Pcdh15 was also the only QTL-associated gene whose midbrain mRNA expression significantly associated with both SERT protein and 5-HT traits, suggesting an unrecognized role of the cell adhesion protein in the development or function of 5-HT neurons. To test this hypothesis, we assessed SERT protein and 5-HT traits in the Pcdh15 functional null line (Pcdh15av-3J), studies that revealed a strong, negative influence of Pcdh15 on these phenotypes. Together, our findings illustrate the power of multidimensional profiling of recombinant inbred lines in the analysis of molecular networks that support synaptic signaling, and that, as in the case of Pcdh15, can reveal novel relationships that may underlie risk for mental illness.
Keywords: Antidepressant, BXD, microarray, network, protocadherin, QTL, serotonin, SLC5A7, SLC6A4, SLC6A3, TPH2, transcriptome, transporter, VMAT2
The biogenic indoleamine serotonin (5-hydroxytryptamine, 5-HT) plays key roles as a modulator of central nervous system (CNS) and periphery physiology (Azmitia 2007; Berger et al. 2009). In the brain, 5-HT-secreting projections originate from midbrain and brainstem raphe nuclei, terminating both locally and distally at virtually every level of the neuraxis. 5-Hydroxytryptamine signaling at serotonergic synapses is terminated by efficient clearance of released neurotransmitter, mediated by the 5-HT transporter (SLC6A4, SERT) (Blakely et al. 1991; Hoffman et al. 1991; Ramamoorthy et al. 1993). SERT is a target of important psychoactive substances including cocaine and 3,4-methylenedioxy-N-methamphetamine (MDMA, ‘ecstasy’), as well as of the selective 5-HT reuptake inhibitors (SSRIs) and non-selective serotonin–norepinephrine reuptake inhibitors (SNRIs), medications that are widely prescribed for the treatment of major depression, anxiety disorders and obsessive-compulsive disorder (OCD).
Given the essential roles that SERT plays in regulating synaptic 5-HT levels, as well as recycling 5-HT for reuse, it is not surprising that alterations in SERT expression and/or activity can impact behavior as well as risk for behavioral disorders. For example, the SLC6A4 gene possesses promoter sequence variation (5HTTLPR) that has been reported to influence SERT mRNA and protein expression as well as risk for behavioral disorders (Homberg & Lesch 2011; Lesch et al. 1996). Additionally, functional SLC6A4 coding variation has been identified in subjects with OCD (Ozaki et al. 2003; Voyiaziakis et al. 2011) and autism (Sutcliffe et al. 2005).
The impact of SERT on physiology and behavior has also been well demonstrated in rodent models that lack or overexpress the Slc6a4 gene [reviewed in (Murphy et al. 2008) and (Kalueff et al. 2010)]. We recently reported studies of a KI mouse model that expresses an autism-associated SERT coding variant (Ala56). In these mice, SERT-mediated 5-HT clearance is constitutively elevated in vivo, leading to elevation of whole-blood 5-HT levels (hyperserotonemia), an autism biomarker, and increased repetitive behavior, as well as communication and social behavior deficits (Veenstra-VanderWeele et al. 2012). Interestingly, SERT proteins differ by two amino acids in C57BL/6J vs. DBA/2J (or 129S6) lines, variation that impacts SERT function and associates with multiple biochemical, anatomical and behavioral traits (Carneiro et al. 2009; Ye & Blakely 2011).
The significant contribution of SERT in the control of 5-HT signaling, behavior and mental illness compels a detailed understanding of mechanisms by which the transporter is regulated, and how this regulation supports behavior and disease. Unbiased approaches, particularly those that permit multidimensional profiling, offer opportunities to establish novel, molecular and phenotypic relationships. One such approach involves the use of recombinant inbred mouse lines, such as those generated from a cross of C57BL/6J and DBA/2J parents (BXD) (Andreux et al. 2012; Mozhui et al. 2010). The full sequencing of the parental strains, along with thousands of archived phenotypes, has resulted in a powerful resource by which BXD strain variation can be used to elucidate molecular and phenotypic networks.
In a recent report (Ye et al. 2014), we quantified midbrain mRNAs in BXD lines, as well as tissue levels of 5-HT, 5-hydroxyindoleacetic acid (5-HIAA) and 5-HT turnover, and used in silico approaches to nominate novel genes that may contribute to these measures. In this study, we describe our efforts to profile SERT mRNA and protein levels in the same BXD lines, studies that revealed distinct, molecular networks that associate with either trait. Additionally, we describe how the mapping of genomic loci associated with SERT protein levels converged with our prior studies of quantitative trait loci (QTLs) linked to 5-HT homeostasis through the common identification of the Pcdh15. Lastly, we detail our efforts to validate a role of Pcdh15 in SERT expression and 5-HT homeostasis using Pcdh15 null mice.
Materials and methods
Animals and genotyping
All studies with mice were performed under approved protocols of the Institutional Animal Care and Use Committees of the Oak Ridge National Laboratory and Vanderbilt University. Animals were housed on a 12:12 light:dark cycle with lights on at 0600 h and food and water provided ad libitum. A total of 126 BXD recombinant inbred mice from 38 strains were used. There were 34 strains of males (30 strains with two animals per strain and 4 strains with single animal) and 29 strains of females (26 strains with two animals per strain and 3 strains with single animal). The average age of the mice was 74 days (range: 51–89 days). A total of 67 male mice and 59 female mice were used in our studies (Table S1). Females were used irrespective of estrous cycle though they were never exposed to males or male bedding after weaning, typically required to initiate synchronized estrous cycling. Midbrain and diencephalon (thalamus and hypothalamus) were dissected on ice from mice killed by rapid decapitation. Midbrain was defined as brain tissue lying between the anterior and posterior margins of the inferior and superior colliculi, respectively (AP approximately −3.25 to −5.02 mm relative to bregma). Hypothalamus and thalamus were defined according to the Paxinos and Watson Atlas of the Mouse Brain (AP approximately −0.80 to −3.25 mm relative to bregma) (Watson & Paxinos 2010). Tissues were frozen on dry ice and stored at −80°C prior to high-performance liquid chromatography (HPLC) analysis for biogenic amines and protein extraction. Pcdh15av-3J mice were obtained from Jackson Labs (stock number: 002072). Mouse genotyping was performed using genomic DNA extracted from tail clips using the Extract-N-AMP tissue PCR Kit (Sigma, St. Louis, MO, USA) according to the manufacturer's instructions. Genotyping was performed by polymerase chain reaction (PCR) with oligonucleotides 5′ GAC GGC AAA CTG CTC GAT A (RB3964) and 5′ GGG ATG CAA CAG AGA TGA T (RB3965) and was based on a single nucleotide addition in the Pcdh15av-3J allele that introduces an MboII restriction site. Genotypes were determined by the size of MboII digested PCR products (+/+: 190 bp; +/av-3J: 190, 120 and 70 bp; av-3J/av-3J: 120 and 70 bp), as determined by agarose gel electrophoresis.
Brain tissue neurochemical measurements
For assessment of 5-HT and 5-HIAA levels in wild-type (WT) and Pcdh15av-3J mice, tissues were homogenized using an Omni Tissue Homogenizer (Omni International, Kennesaw, GA, USA) in 100–750 μl of 0.1 m trichloroacetic acid (TCA), 10 mm sodium acetate, 0.1 mm ethylenediaminetetraacetic acid (EDTA), 5 ng/ml isoproterenol (as internal standard) and 10.5% methanol (pH 3.8). 5-Hydroxytryptamine and 5-HIAA were determined by HPLC through the Neurochemistry Core of the Vanderbilt Brain Institute, utilizing an Antec Decade II (oxidation: 0.5) electrochemical detector (Antec LLC, Boston, MA, USA) operated at 33°C. Twenty microliter samples of the supernatant were injected using a Waters 717+ autosampler (Waters, Milford, MA, USA) onto a Phenomenex Nucleosil (5u, 100A) C18 HPLC Column (150 × 4.60 mm2) (Phenomenex, Torrance, CA, USA). Samples were eluted with a mobile phase consisting of 89.5% 0.1 m TCA, 10 mm sodium acetate, 0.1 mm EDTA and 10.5% methanol (pH 3.8). Solvent was delivered at 0.6 ml/min using a Waters 515 HPLC Pump. HPLC control and data acquisition were managed by Millennium 32 software.
Western blotting and quantification
After HPLC biogenic amine measurements, tissue protein pellets were solubilized with 250 μl RIPA buffer [50 mm Tris, pH 7.4, 150 mm NaCl, 1 mm EDTA, 1% Triton X-100, 1% sodium deoxycholate and 0.1% sodium dodecyl sulfate (SDS)] containing protease inhibitors (1:100; Sigma). Protein concentrations were determined by the bicinchoninic acid (BCA) method (Pierce, Thermo Fisher Scientific, Rockford, IL, USA). Equal amounts of extracted supernatants were separated by 10% SDS-polyacrylamide gel electrophoresis on a Bio-Rad Mini-PROTEAN 3 Dodeca Cell (Bio-rad, Herules, CA, USA), transferred to PVDF membranes and immunoblotted using SERT primary antibody (Calbiochem: Pc177L; 1:5000, Merck KGaA, Darmstadt, Germany) and mouse anti-rabbit secondary antibody (Jackson ImmunoResearch: 211-035-109; 1:10 000, West Grove, PA, USA). We chose this particular SERT antibody, which targets the SERT C-terminus of SERT, as BXD strains differ by the expression of N-terminal SERT coding variation [Gly39 arising from the C57BL/6J allele and Glu39 arising from the DBA/2J allele (Carneiro et al. 2009)]. Immunoreactive bands corresponding to SERT (absent in SERT KO mice) were identified by chemiluminescence (Western Lightning ECL Pro, Perkin Elmer, Waltham, MA, USA) and X-ray film (Biomax MS, Kodak, 8294985, Rochester, NY, USA) exposure. Optical densities of bands were quantified using ImageJ software 1.4 (National Institute of Health) and normalized to reference samples after obtaining multiple exposures to ensure linearity of data capture. To allow for direct comparisons within each sample set, protein lysates from each gender and each brain region were electrophoresed, transferred, immunoblotted and exposed simultaneously.
Synaptosomal [3H]-5-HT transport activity measurements
Mouse brain synaptosomes were prepared as previously described (Zhu et al. 2011). Briefly, freshly dissected brain tissues were homogenized in 0.32 m sucrose buffer with 10 mm HEPES and 2 mm EDTA (pH 7). P2 pellets were obtained by centrifugation, resuspended in Krebs-Ringer-HEPES (KRH) assay buffer containing 130 mm NaCl, 1.3 mm KCl, 2.2 mm CaCl2, 1.2 mm MgSO4, 1.2 mm KH2PO4, 1.8 g/l d-glucose, 10 mm HEPES, pH 7.4, 100 μm pargyline and 100 μm ascorbic acid and protein was quantified by BCA method (Pierce). Radiolabeled [3H]-5-HT (20 nm) uptake into synaptosomes (40 μg) was performed for 10 min at 37°C and terminated using a Brandel Cell Harvester (Brandel, Gaithersburg, MD, USA), as previously described. Non-specific uptake was determined using parallel samples incubating with 10 μm paroxetine with levels here subtracted from total accumulation to yield specific uptake activity.
RNA isolation and qPCR
Total RNA was extracted from individual brain regions of age-matched C57BL/6J and DBA/2J mouse pairs using RNeasy Midi Kit (Qiagen, Venlo, The Netherlands) following the manufacturer's instructions. RNA concentrations were determined by NanoDrop ND-1000 (NanoDrop Technologies, Wilmington, DE, USA). Reverse transcription reactions were carried out using SuperScript III First-Strand Synthesis Kit (Invitrogen, Life Technologies, Grand Island, NY, USA). Briefly, 1 μg of total RNA was used as template to synthesize first-strand complementary DNA using oligo(dT)20 primer. A 926-bp Pcdh15 complementary DNA (cDNA) fragment covering exons 1 through 6 was amplified by PCR using the following oligonucleotides: 5′ -AGATAGAGGGCCTGCGGATG-3′ (RB4499) and 5′ -TAGCTCCATTGTCTCCCGAGAAC-3′ (RB4502). As a control, a 152-bp Gapdh cDNA fragment was also amplified from the same samples using the following primer set: 5′ -GCACAGTCAAGGCCGAGAAT-3′ (RB4495) and 5′ -GGCCTTCTCCATGGTGGTGAA-3′ (RB4496). Quantitative PCR (qPCR) was performed using KAPA SYBR FAST Universal qPCR Kit (Kapa Biosystems, Wilmington, MA, USA) on an Eco PCR system (Illumina, San Diego, CA, USA). The PCR products were amplified from cDNAs and each reaction was carried out in triplicate. The polymerase activation step at 95°C for 10 min was followed by 40 cycles of 15 seconds at 95°C and 60 seconds at 60°C. The 2−ΔCt method was used to analyze the relative changes of gene expression using GAPDH as reference (Schmittgen & Livak 2008). Oligonucleotides used for Pcdh15 qPCR were 5′ -CATGAAGTAC GCATCGTGGT-3′ (RB4563) and 5′ -TAGCTCCATTGTCTCCCGAGAAC-3′ (RB4502) to yield a 121-bp PCR product.
Midbrain transcriptome profiling
Midbrain microarray analyses on the BXD strains were performed as previously described (Ye et al. 2014). Briefly, an Agilent-028005 SurePrint G3 Mouse GE 8x60K platform was used for mRNA probe hybridization. Raw expression data were then normalized and rescaled followed by an internal validation using sample-specific genetic markers. The final verified data have been deposited in GEO and in GN and reflects this systematic genotype QC process (accession number GN381).
Data analyses
Data were analyzed using standard analysis of variance (ANOVA) and correlation analysis using Stata 11 (Stata Corp, College Station, TX, USA) and Prism 5 (GraphPad, Software Inc, La Jolla, CA, USA). Quantitative trait locus mapping was performed using the WebQTL module of the GeneNetwork (http://www.genenetwork.org). Adjusted heritability (h2) of the traits was estimated using Hegmann and Possidente's method (Hegmann & Possidente 1981). Significance of heritability was calculated by the F -test comparing within-strain vs. between-strain variances. Quantitative trait loci were calculated from the likelihood ratio statistic (LRS) and logarithm of odds (LOD) scores. Statistical significance was determined using 2000 permutations. Significant QTLs were defined as the LRS value that represents a genome-wide P value of 0.05. Suggestive QTLs were defined as having an LRS value a genome-wide P value of 0.63 (Lander & Kruglyak 1995). Confidence intervals were determined as the location at which the LOD value is reduced by 1.5. A 1.5 LOD cutoff has been estimated to reflect a 95% confidence that genes driving the QTL lie within this range (Dupuis & Siegmund 1999; Visscher et al. 1996). Phenotypic correlation analyses were performed by comparing measured 5-HT traits with archived BXD phenotypes in the GeneNetwork database, with correlation coefficients and P values calculated using Spearman's rank tests (uncorrected P < 0.05 was considered significant). Transcriptome-derived gene networks were generated by Cytoscape 2.8.3 (Cytoscape Consortium, San Diego, CA, USA) using input data files (node and network) from WebQTL correlation analysis module.
Online data access
SERT protein expression levels for the BXD strains determined in this study have been deposited in the GeneNetwork database at http://www.genenetwork.org under the category of Central Nervous System: Protein Expression.
Results
Midbrain SERT mRNA expression and associated gene networks
Previously (Ye et al. 2014), we described our transcriptome profiling of adult male midbrain mRNAs across BXD strains using microarray analyses. When we inspected the variation of SERT mRNA levels across these same lines, we found that expression levels were not significantly heritable (P = 0.49, h2 = 0.16). On the basis of this observation, we did not pursue expression QTL (eQTL) analysis on the SERT mRNA data. Non-genetic factors, including individual animal variation and the potential impact of environmental factors, can diminish power in heritability estimates. However, correlational analysis within the same dataset does not rely solely on the genetic control of between-strain variations. Therefore, we turned to an intracorrelation analysis of mRNAs within the male midbrain transcriptome using strain means. We extracted genes whose expression significantly correlated positively (1511 genes) or negatively (1126 genes) with Slc6a4 mRNA levels across BXD lines (uncorrected P < 0.05, Table S2). In Fig. 1a we present the top 25 correlated genes in a network diagram to highlight the strong intracorrelations among these genes. The composition of this network suggests that it reflects a realistic delineation of genes whose expression is associated with Slc6a4 gene expression. For example, the strongest correlations were found for Fev, Tph2, Gch1 and Slc18a2. Fev is a transcription factor (also known as PET-1) and master regulator of CNS 5-HT neuron identity, expressed at the beginning of raphe neuron commitment to a serotonergic identity (Hendricks et al. 2003). Tph2 encodes the rate-limiting enzyme in 5-HT biosynthesis, whereas Gch1 encodes the rate-limiting enzyme in the synthesis of tetrahydrobiopterin, an essential cofactor for amino acid decarboxylase that converts 5-hydroxytryptophan to 5-HT. Slc18a2 encodes VMAT2, the transporter responsible for packaging 5-HT into synaptic vesicles prior to release.
Figure 1. Gene expression networks associated with determinants of midbrain SERT and TPH2 mRNA.
Genes (top 25) with highest correlations with Slc6a4 (a) or Tph2 (b) are graphed separately based on the direction of correlation (green: positive; red: negative). Gene node sizes represent the significance of correlation. Gene pairs with correlation coefficients (Spearman rho) above 0.5 are connected by edges (green: positive; red: negative). (c) Venn diagram showing the number of overlapping genes derived from Tph2-seeded and Slc6a4-seeded gene networks (top 100).
To specify further the possible contributions of Slc6a4-associated transcripts to 5-HT biosynthesis we used Tph2 gene expression as a seed for a second network analysis. Here, we found 1209 genes with significant positive correlations with Tph2 gene expression and 1287 with negative correlations (Fig. 1b and Table S2). When we compared the Slc6a4- and Tph2-seeded gene lists, significant overlap was found. In fact, more than a third of the top 100 correlated genes correlated with Slc6a4 and Tph2 were common (Fig. 1c and Table S2). As expected, Fev , Gch1, Ddc and Slc18a2 were among the 22 genes in this group with significant positive correlations. Additionally, we identified several genes that had not been previously implicated in serotonergic signaling, including the Na+/bile acid co-transporter (Slc10a4) and the synaptic cell adhesion molecule (CAM) neuroligin 2 (Nlgn2). In this analysis, we also identified 16 genes whose expression was negatively correlated with the expression of both Slc6a4 and Tph2. To our knowledge, none of these genes, including interleukin 2 (Il2), cortexin 3 (Ctxn3) and the restless leg syndrome (RLS) risk gene Btbd9, have been identified as correlates of 5-HT signaling. Finally, in Table S2, we note genes whose expression is associated positively or negatively with the expression of Slc6a4 but not of Tph2. The latter data may point to pathways of Slc6a4 gene regulation that provide constitutive support of SERT biosynthesis and trafficking or that exert non-cell autonomous regulation of 5-HT inactivation.
Conceivably, the midbrain gene expression networks we identified could be of more general relevance to neuronal function in the midbrain, rather than specific determinants of serotonergic signaling. To examine this issue, we extracted gene expression clusters likely to be more related to dopamine (DA) homeostasis by using as seeds the genes encoding tyrosine hydroxylase (Th) and the DA transporter (Slc6a3). We also generated an acetylcholine (ACh)-linked gene expression network by using as seeds the genes encoding choline acetyltransferase (Chat) and the presynaptic choline transporter (Slc5a7) (Tables S3,S4 and Figs. S1,S2). Only genes that encode shared determinants of monoaminergic biosynthesis and packaging, such as Ddc and Slc18a2, were identified as genes contributing to more than one network. These findings indicate that the networks seeded by Slc6a4 gene mRNA profiles are specifically tuned to Slc6a4 transcription.
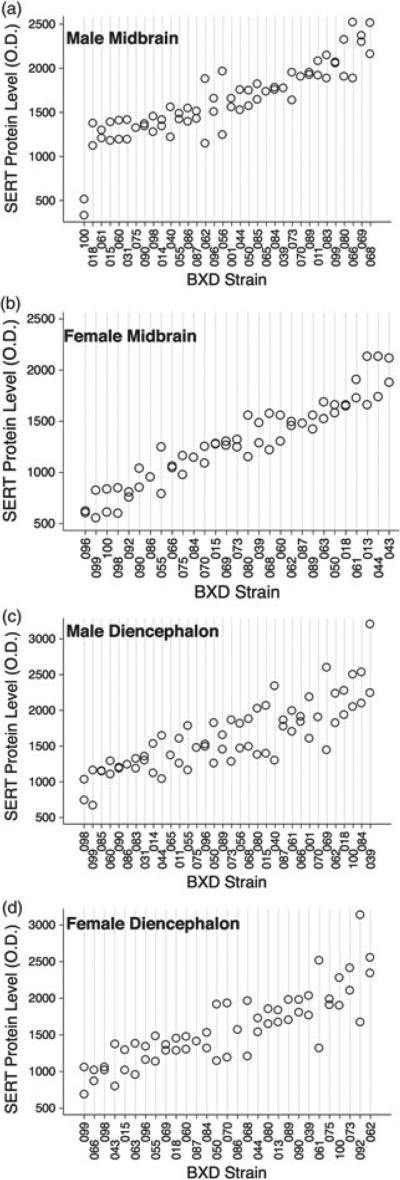
Variation and heritability of BXD SERT protein levels
It is well known that protein levels do not always highly correlate with levels of mRNA, particularly when the protein is subject to extensive post-translational regulation, as is the case for SERT (Steiner et al. 2008). To gain additional insights into networks associated with SERT protein expression, we used immunoblotting techniques to quantify SERT protein levels in the midbrain and diencephalon of 38 BXD RI strains. We found that SERT protein expression varied significantly across BXD strains in both brain regions (Fig. 2). BXD strain variation contributed significantly to the overall variation in SERT protein expression in the midbrain of both genders, indicating high heritability (males: F33,30 = 6.78, P < 0.0001, h2 = 0.77; females: F28,26 = 10.85, P < 0.0001, h2 = 0.84). A similar pattern was detected for diencephalon SERT protein levels for females (F 28,27 = 2.75, P = 0.005, h2 = 0.58). In contrast, analysis of male diencephalon SERT protein expression yielded no evidence of heritability (F 33,31 = 1.34, P = 0.21, h2 = 0.40), likely owing to the higher variation among animals from the same strain. However, SERT midbrain and diencephalon protein expression levels did not correlate for either gender (males: r = 0.02, P = 0.90; females: r = 0.11, P = 0.58), consistent with region-specific factors contributing to trait variation. Finally, SERT protein levels did not correlate between genders in either region (midbrain: r = 0.02, P = 0.90; diencephalon: r = 0.27, P = 0.20).
Figure 2. Levels of SERT protein levels across BXD RI lines.
(a) Male midbrain; (b) female midbrain; (c) male diencephalon and (d) female diencephalon. Each point represents an individual mouse.
Phenotypic correlates of SERT protein expression
The quantification of SERT protein levels from the same animals for which 5-HT measures were acquired (Ye et al. 2014) provided an opportunity to examine whether the two indices correlate with each other across strains. In midbrain, SERT protein levels in males positively correlated with 5-HT levels (r = 0.40, P = 0.02), but not with 5-HIAA (r = 0.25, P = 0.15) or 5-HT turnover rates (r = −0.23, P = 0.19). Interestingly, this correlation was not evident in females (5-HT: r = 0.18, NS; 5-HIAA: r = 0.13, NS; 5-HT turnover: r = −0.02, NS). Although the correlations between male diencephalon SERT levels and 5-HT levels or 5-HT turnover rates fell short of statistical significance (5-HT: r = 0.32, P =0.09; 5-HT turnover: r = 0.27, P = 0.16), we did observe a significant, positive correlation between female SERT levels and 5-HIAA levels (r = 0.54, P =0.002). Next, we extracted phenotypes from the GeneNetwork collection that demonstrate significant association with SERT levels (Table S5). Consistent with the behavioral changes associated with 5-HT manipulations, we found a number of anxiety measures that correlated with both midbrain and diencephalon SERT levels, regardless of gender. Interestingly, multiple cocaine response traits, including conditioned place preference tests and locomotor sensitization, were significantly correlated with female, but not male diencephalon SERT levels, whereas these measures lacked association with midbrain SERT levels regardless of gender.
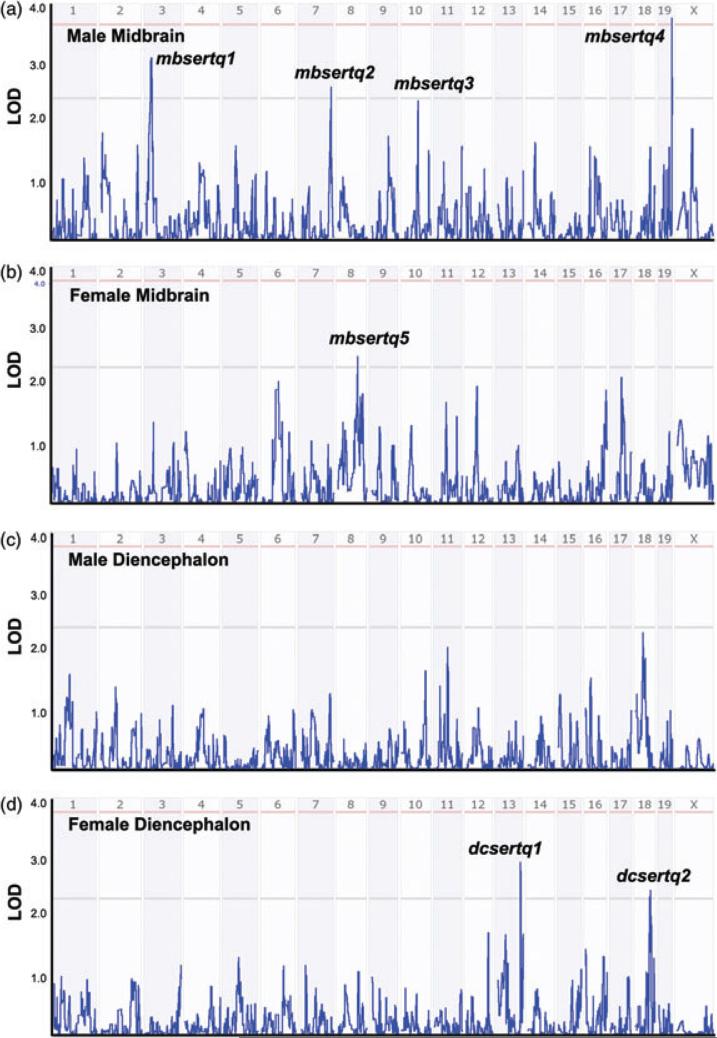
QTLs associated with BXD SERT protein expression
Through assessment of DNA polymorphisms that associate with variation in SERT protein levels, we identified putative QTLs and inspected gene lists that may dictate SERT protein variation across BXD lines (Table S6). In Fig. 3, we present plots of these QTLs divided by brain region and gender. None of the QTLs noted below encompass the region containing the Slc6a4 gene on chromosome 11, suggesting a limited influence of Slc6a4 gene variation on steady-state SERT protein levels. As expected from the absence of intercorrelations by region or gender of BXD SERT variation, the same QTLs were not evident between midbrain and diencephalon, regardless of gender. With respect to male midbrain SERT levels, we identified one significant and three suggestive QTLs (Fig. 3a): mbsertq1 on chromosome 3 (30 Mb, LOD = 2.93), mbsertq2 on chromosome 7 (140 Mb, LOD = 2.26), mbsertq3 on chromosome 10 (73 Mb, LOD = 2.25) and significant QTL mbsertq4 on chromosome 19 (59.5 Mb, LOD = 3.58). It is worth noting that mbsertq3 was also identified as a suggestive QTL for midbrain 5-HT levels in our previous report (Ye et al. 2014).
Figure 3. Interval analysis of SERT protein expression levels.
(a) Male midbrain; (b) female midbrain; (c) male diencephalon and (d) female diencephalon. Numbers above red lines designate mouse chromosomes. Red lines designate LOD scores where gene variation achieves significance, whereas gray lines designate LOD scores achieving suggestive threshold (see Materials and methods).
From the mapping of female midbrain SERT levels (Fig. 3b), we found a suggestive QTL (mbsertq5) on chromosome 8 (91 Mb, LOD = 2.68). In female diencephalon (Fig. 3d), there is a suggestive QTL (dcsertq1) on chromosome 13 (108 Mb, LOD = 3.09) and another suggestive QTL (dcsertq2) on chromosome 18 (69.5 Mb, LOD = 2.59). Because we did not detect significant strain effects for male diencephalon SERT levels, as expected, we did not find QTL peaks above suggestive threshold for these samples (Fig. 3c).
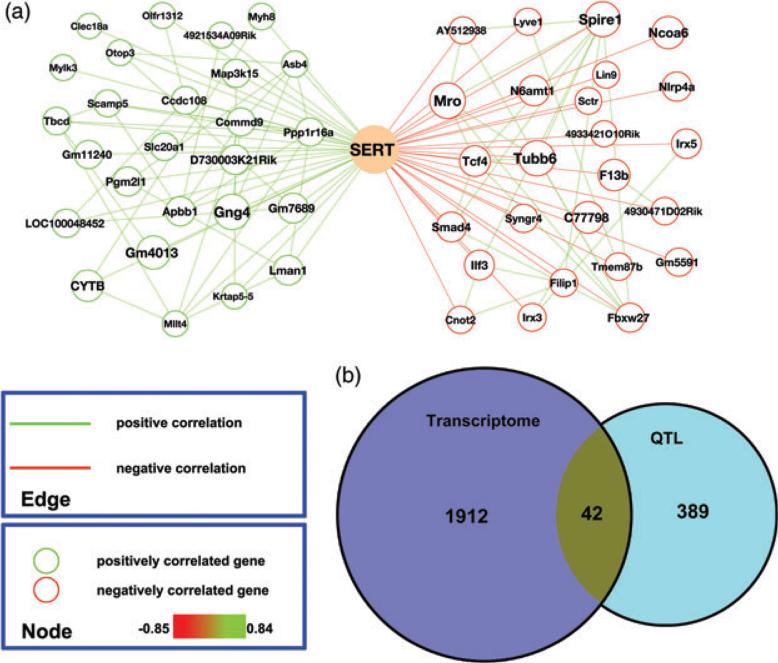
A male midbrain transcriptome linked to SERT protein expression
Although multiple determinants of Slc6a4 mRNA expression in the BXD lines were identified in our network analysis above, these genes need not be the major drivers for the variation in SERT protein levels we detected across strains. Indeed, when we compared midbrain SERT protein levels and Slc6a4 mRNA across the same lines, the two traits did not exhibit a significant correlation (r = − 0.36, P = 0.18). These findings suggest that significant post-transcriptional regulation is imposed to dictate the SERT protein variation we observed across BXD lines. In Fig. 4a we plot the 25 genes whose expression profiles correlated strongly, negatively or positively, with male midbrain SERT protein levels across BXD lines. The entire list of correlated mRNAs is found in Table S7. When we compared these transcripts with the genes encompassed by the QTLs nominated above, 42 genes were present on both lists (Fig. 4b and Table S8). We compared these genes with a similar collection of genes whose expression correlated with 5-HT levels and also could be found within a 5-HT QTL (Suppl File 10 in Ye et al. 2014). This analysis yielded a single gene, Pcdh15.
Figure 4. Gene expression networks associated with male midbrain SERT protein expression levels.
(a) Genes (top 25) with highest correlations with SERT protein levels are graphed separately based on the direction of correlation (green: positive; red: negative). Gene node sizes represent the significance of correlation. Gene pairs with correlation coefficients (Spearman rho) above 0.5 are connected by edges (green: positive; red: negative). (b) Venn diagram showing the number of total genes and overlapping genes derived from midbrain SERT gene networks and interval mapping of male midbrain SERT protein levels.
Pcdh15 as a determinant of SERT protein expression and 5-HT homeostasis
As indicated above, Pcdh15 lies within suggestive QTLs for both male midbrain SERT protein levels (mbsertq3) and midbrain (pooled gender) 5-HT levels (mb5htq3) on chromosome 10. Moreover, marker regression analyses revealed that for both traits, the same single nucleotide polymorphism (SNP, rs13480650), located in the intron between exons 1 and 2 of Pcdh15, exhibited highest association (Fig. 5a). Adding strength to the possibility that variation in Pcdh15 could influence both SERT and 5-HT levels, strain means of BXD lines segregated by rs13480650 variation revealed that 5-HT, 5-HT turnover, SERT mRNA and protein levels exhibited significant differences (Fig. 5b–e).
Figure 5. Pcdh15 SNP rs13480650 is associated with multiple serotonergic traits in BXD RI strains.
(a) Genomic structure of mouse Pcdh15. Numbers represent exons. Star denotes the location of rs13480650. (b–e) Serotonergic traits are plotted based on the genotype of rs13480650 in BXD strains (5-HT: P < 0.0001; 5-HIAA/5-HT: P < 0.0001; SERT: P < 0.01; Slc6a4: P < 0.05). All P values are calculated using Student's t-test.
The principle phenotype associated with Pcdh15 loss of function is a perturbation of hair cell mechanosensitivity, with resulting alterations in vestibular reflexes (Ahmed et al. 2001; Alagramam et al. 2001; Xiong et al. 2012). Although the most visible signs of Pcdh15 function relate to vestibular function, our finding of associations of a Pcdh15 SNP with multiple serotonergic traits suggests a more widespread expression, and influence, of the gene. Indeed, using RT-PCR on mRNA isolated from adult male C57BL/6J and DBA/2J mice, we readily detected Pcdh15 expression in midbrain, cerebellum, hippocampus, striatum and frontal cortex (Fig. 6a). As amplifications of midbrain extracts from C57BL/6J mice consistently displayed higher levels of Pcdh15 than DBA/2J extracts, we pursued further quantitative analyses. Consistent with semiquantitative estimates, qPCR analysis of midbrain Pcdh15 gene expression (Fig. 6b) revealed significantly higher levels of Pcdh15 mRNA in midbrain samples of C57BL/6J vs. DBA/2J mice (P < 0.05).
Figure 6. Characterization of Pcdh15 expression in the mouse brain by RT-PCR.
(a) PCR products amplified from Pcdh15 cDNA of multiple brain regions in C57BL/6J (C57) and DBA/2J (DBA) mice. MB, midbrain; CB, cerebellum; HIP, hippocampus; STR, striatum; FCTX, frontal cortex. (b) C57BL/6J mice have higher Pcdh15 expression in the midbrain than DBA/2J, measured by qPCR (P < 0.05, Student's t-test).
Evaluation of SERT and 5-HT in Pcdh15av-3J mice
To provide a direct test of whether changes in Pcdh15 expression impact 5-HT homeostasis, we measured 5-HT and 5-HIAA levels in Pcdh15av-3J mice (on C57BL/6J background) that generate a truncated, functional null PCDH15 protein owing to the introduction of a premature stop codon in exon 12 (Alagramam et al. 2001). This mutation leads to cochlear hair cell abnormalities and a distinct circling phenotype (Alagramam et al. 2001). We found that midbrain 5-HT levels of Pcdh15av-3J homozygous mice were significantly higher than those measured in WT littermate samples (P < 0.005, Fig. 7a). Interestingly, this increase was not evident in hippocampal extracts (data not shown). For this analysis, we chose to use both genders to maintain consistency with the 5-HT trait used to identify the QTL. We did not detect gender differences in 5-HT levels within the same Pcdh15 genotype group. Additionally, the same relationship between 5-HT levels and Pcdh15 gene dosage was observed in both genders with a stronger effect in males, likely a result of larger sample sizes in the male group (Fig. S3). Levels of 5-HIAA did not exhibit genotype effects in any brain region. However, hippocampus, striatal and midbrain samples of Pcdh15av-3J homozygous mice demonstrated significantly lower 5-HIAA/5-HT turnover ratios than their WT littermates (P < 0.05, Fig. 7b–d). To assess whether these changes are specific to 5-HT traits, we measured DA, the DA metabolite dihydroxyphenylalanine (DOPAC) and estimated DA turnover (DOPAC/DA) in these same samples and found no genotype difference in any brain region (data not shown).
Figure 7. Pcdh15av-3J mice have altered brain 5-HT traits.
(a) Midbrain 5-HT levels (P < 0.01); (b) midbrain 5-HT turnover ratios (P < 0.01); (c) hippocampal 5-HT turnover ratios (P < 0.05) and (d) striatal 5-HT turnover ratios (P < 0.01). All P values are calculated using Kruskal–Wallis test. Significance noted on pairwise comparisons is calculated using Dunn's multiple comparison tests. N = 5–7 (wt), 16–25 (het) and 8–12 (hom).
Next, we asked whether Pcdh15av-3J mice exhibit changes in male SERT protein levels. Immunoblotting studies (Fig. 8a) revealed significantly increased SERT levels in both the midbrain and striatum of Pcdh15av-3J homozygous mice relative to WT littermates (midbrain: P < 0.01; striatum: P < 0.05). Pcdh15av-3J homozygous mice also displayed a trend to lower striatal DA transporter (DAT) levels, though this difference did not reach statistical significance (P = 0.06) (Fig. 8b).
Figure 8. Pcdh15av-3J mice have elevated SERT protein expression levels.
(a) Midbrain (P < 0.01) and (b) striatum (P < 0.05). P values are calculated using one-way ANOVA followed by Bonferroni's multiple comparison tests.
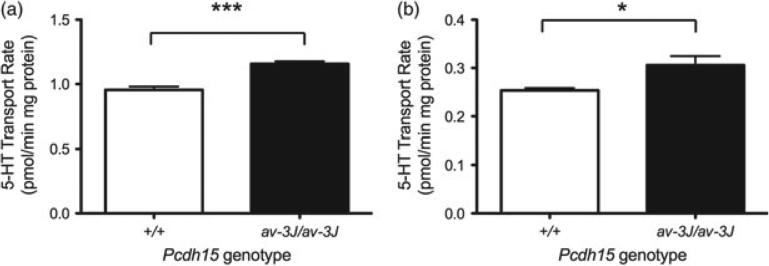
To examine whether the increased SERT protein levels observed in Pcdh15av-3J homozygous mice translates into higher levels of 5-HT uptake, we prepared male midbrain and hippocampal synaptosomes and quantified paroxetine-sensitive 5-HT uptake in these preparations, as described in Materials and methods (Fig. 9). Consistent with the changes in SERT protein levels, these studies demonstrated significant increases in the rates of synaptosomal 5-HT uptake in the Pcdh15av-3J homozygous mice for both mid-brain and hippocampus (midbrain: P < 0.001; hippocampus: P < 0.05) vs. littermate WT animals.
Figure 9. Pcdh15av-3J mice have increased 5-HT synaptosomal uptake.
(a) Midbrain (P < 0.001) and (b) hippocampus (P < 0.05). P values are calculated using Student's t-test. N = 4 for each genotype.
Discussion
SERT plays a critical role in limiting synaptic 5-HT availability and is the principal target for the most highly prescribed medications used to treat depression. Animal models with genetic manipulations of SERT expression (Murphy & Lesch 2008; Veenstra-VanderWeele et al. 2012) and human studies on functional SERT gene variation (Caspi et al. 2003; Veenstra-VanderWeele et al. 2012) support the hypothesis that perturbed SERT function or regulation can modify behavior and increase the risk for multiple neuropsychiatric disorders. Here, we capitalized on the availability of the BXD reference panel to search for molecular determinants and phenotypic correlates of SERT mRNA and protein expression.
One opportunity afforded by the assessment of trait variation across BXD lines (e.g. Slc6a4 mRNA levels and SERT protein levels) is the ability to map putative QTLs that lead to the identification of genes that control trait expression. As we were unable to document heritability of Slc6a4 mRNA variation, an eQTL analysis was inappropriate. However, correlational analyses within the same mRNA dataset make use of the rank order of strains, a variable that adds dimensionality to an evaluation otherwise dependent on trait mean differences. Moreover, by calculating intercorrelations between gene expression profiles we were not limited by the genetic control of gene expression, instead asking questions on the co-varying expression pattern of genes regardless of the upstream determinants. In this context, we first sought to identify genes whose mRNA expression significantly correlates with that of Slc6a4. Using a previously generated male midbrain transcriptome (Ye et al. 2014), and bioinformatics methods to relate correlated genes to another, we generated networks of genes that both positively and negatively correlated with Slc6a4 expression. In this effort, we identified the expression of Fev , Tph2, Gch1, Ddc and Slc18a2 as positively related to Slc6a4 expression. These finding were reassuring, as Fev is known to be an essential determinant of 5-HT neuron identity, which includes the expression of genes needed to synthesize and package 5-HT (Deneris 2011). Moreover, we found little overlap in the Slc6a4 network with networks generated using determinants of dopaminergic and cholinergic identity, including networks linked to other presynaptic transporters.
To classify genes in the Slc6a4 network that may associate with the transporter's expression based on common effects on multiple aspects of 5-HT neuron identity, vs. more specifically on Slc6a4, we first evaluated genes in the Slc6a4 network for their association with a Tph2-seeded network. Positively correlated genes: Besides the genes in the 5-HT biosynthetic and release pathway noted above, we also identified, among others (Table S2), Slc10a4 and Nlgn2. Consistent with our serotonergic network analyses, inspection of the adult mouse CNS expression pattern Slc10a4, which encodes an orphan Na /bile acid co-transporter, reveals significant enrichment in+the dorsal and median raphe nuclei (Hawrylycz et al. 2011). Evidence has also been advanced that Slc10a4 is involved in dopaminergic and cholinergic signaling, mainly based on its co-localization with the vesicular transporters VAChT and VMAT2 (Borges 2013; Burger et al. 2011). Our findings concur with these observations, as we also found Slc10a4 among the cholinergic and dopaminergic gene-seeded networks. These findings indicate that the activity of Slc10a4 likely contributes to common features of monoaminergic neurotransmission, such as the packaging of cationic neurotransmitters. Nlgn2 encodes neuroligin2 (NLGN2), a cell adhesion protein. NLGNs have been characterized as postsynaptic binding partners of presynaptic neurexin proteins, and are thought to mediate synaptogenesis and synapse identity (Sudhof 2008; Varoqueaux et al. 2006). NLGN2 has been shown to be co-localized and associated with GABAA receptors, as well as with the postsynaptic scaffolding proteins gephyrin and collybistin (Poulopoulos et al. 2009). The SNPs and copy number variations of NLGN2 have been implicated in schizophrenia (Sun et al. 2011) and mental retardation (Belligni et al. 2012). Further studies are needed to explore the potential role of NLGN2 in 5-HT signaling, though one possibility is that the protein plays a role in GABAergic inhibition of raphe neurons (Bagdy et al. 2000; Soiza-Reilly et al. 2013). Negatively correlated genes: One gene of note in this group is Btbd9, a gene named for the presence of a BTB protein–protein association domain (Perez-Torrado et al. 2006), but whose cellular function is unclear, though it has been associated in human genetic studies with RLS (DeAndrade et al. 2012). Because reduced CSF 5-HIAA levels have been reported in RLS subjects (Earley et al. 2001), RLS has been reported to be a side effect of SSRI administration (Rottach et al. 2008), and BTBD9 KO mice display alterations in striatal 5-HIAA levels (DeAndrade et al. 2012); further study of Btb9/RLS/5-HT connections should be pursued.
Because mRNA levels do not always correlate with protein expression levels and SERT undergoes extensive post-transcriptional regulation (Ramamoorthy et al. 2011; Steiner et al. 2008), we evaluated BXD strain variation of SERT protein levels using quantitative Western blotting methods. In contrast to our inability to document significant heritability with the strain variation in Slc6a4 mRNA expression, SERT protein expression demonstrated significant heritability. Our studies show that midbrain and diencephalon SERT levels are under the control of distinct, heritable factors, as might be expected given the distinct needs of these regions to modulate 5-HT signaling. Even within regions, gender-specific influences are evident, as male and female variation in SERT levels did not correlate across strains. In recognition of these issues, we assessed phenotypic traits and QTLs for each region and gender separately. It is important to note that although determinants of strain variation in SERT levels or the transporter's relationship with phenotypes were found to be region and gender specific, we must realize that (1) the availability of animals per BXD strain in this study limited the statistical power to uncover some of the relationships, and (2) such relationships may change, or be more evident under other conditions. For example, a major determinant of midbrain SERT protein levels in response to stress could be shared by males and females, but differ in their relative impact under non-stressful conditions.
The dense mapping of SNPs across the genomes of the parental strains of the BXD lines affords an opportunity to search for genes where genetic variation may drive SERT protein expression. In this regard, we identified one significant and six suggestive SERT protein level QTLs. Because of the number and local density of markers involved in these analyses, and disequilibrium that likely exists among neighboring genes, these QTLs, and the genes that lie within them (1.5 LOD from the peak), can only serve as hypothesis generators that should be confirmed through independent approaches. With respect to the significant QTL mbsertq4, a plausible candidate gene within this QTL is Slc18a2, which encodes VMAT2. BXD lines bearing the D variants of the closest SNP to Slc18a2 (rs6191324) have higher SERT expression levels than strains bearing the B variants (Fig. S4a). The strains bearing the D variant also show higher VMAT2 mRNA levels (Fig. S4b). One hypothesis, therefore, that could link Slc18a2 gene variation to SERT protein levels and Slc18a2 mRNA expression is that the level of VMAT2, known to determine the quantity of 5-HT stored in synaptic vesicles (Fon et al. 1997), drives a positive-feedback, autoregulatory process that in turn ensures that as more 5-HT is packaged and released, more SERT protein is available to clear released 5-HT. Consistent with this idea, male midbrain 5-HT levels positively correlated with midbrain SERT protein levels, and variation in rs6191324 associates with both midbrain and blood 5-HT (Fig. S4c,d). We must recognize that rs6191324 has an unknown functional relationship to VMAT2, as it does not alter VMAT2 coding sequences or any known Slc18a2 regulatory sequences. However, DBA/2J mice and C57BL/6J mice differ by two amino acids, L117/S505 vs. P117/P505, respectively, and this variation associates with SSRI-sensitive behaviors (Crowley et al. 2006).
Given the suggestive nature of the other SERT protein QTLs, we refrain from further discussion of candidate genes responsible for these associations, except for mbsertq3. This QTL is striking to us as it also appeared in our previous analysis of midbrain 5-HT variation (Ye et al. 2014). Moreover, Pcdh15 mRNA expression was also found to correlate with SERT protein expression. Pcdh15 belongs to the family of non-clustered protocadherins in the protocadherin superfamily (Kim et al. 2011; Morishita & Yagi 2007). Protocadherins are a subset of CAMs that mediate the formation, maturation and specification of synapses. Different brain regions express distinct combinations of synaptic CAMs (O'Rourke et al. 2012). With respect to our study, a protocadherin gene family, Pcdha, was demonstrated to have important functions in controlling 5-HT axonal fiber density in multiple brain regions (Katori et al. 2009). Over the last decade, the synaptic CAMs have been implicated in multiple neuropsychiatric diseases, such as autism and schizophrenia (for reviews, see Hortsch & Umemori 2009; Ye et al. 2010). The human locus 10q21.1, where PCDH15 is located, has been associated with Crohn's disease (Rioux et al. 2007) and late-onset Alzheimer's disease (Myers et al. 2000). Recently, a 386-kb deletion on 10q21.1, including the first three exons of PCDH15, was reported in a subject with autism spectrum disorder (Sorte et al. 2013). Although primarily recognized as a disease associated with deafness and blindness, more than 20% of Usher syndrome patients display psychiatric symptoms (Carvill 2001) and comorbidities of Usher syndrome with various mental illnesses are well documented (Domanico et al. 2012; Rao et al. 2010; Rijavec & Grubic 2009). Our findings suggest that there may be genetic components that underlie these symptoms and that relate to perturb 5-HT signaling, though further studies are needed to assess possible relationships.
To test whether Pcdh15 gene expression dictates SERT protein levels, as well as 5-HT traits, we first confirmed that midbrain Pcdh15 gene expression varies between C57BL/6J and DBA/2J strains using qPCR approaches. Indeed, we found that levels of Pcdh15 mRNA in C57BL/6J mice were significantly higher than those measured in DBA/2J mice. Additionally, we found that lines bearing C57BL/6J alleles of Pcdh15 exhibit both lower SERT protein expression and lower 5-HT levels (Fig. 5), suggesting a negative regulation of SERT and 5-HT homeostasis by the cell adhesion protein.
To move beyond correlation studies, we pursued a characterization of mice bearing a functional null mutation in Pcdh15 (Pcdh15av-3J) (Alagramam et al. 2001). In support of our hypothesis that Pcdh15 expression negatively regulates both SERT protein levels and 5-HT traits, we found that homozygous Pcdh15av-3J mice exhibited an increase (~75%) in midbrain SERT protein expression. A significant increase was also evident in striatum, whereas DAT levels in this region were unchanged. We also observed an increase (~30%) in midbrain 5-HT levels when compared with WT littermates. To determine if SERT expression has a reciprocal impact on Pcdh15 expression, we also compared midbrain Pcdh15 gene expression levels between WT and SERT KO mice (both on C57BL/6J background). We did not detect a significance difference in this measure (SERT KO/WT = 1.14 ± 0.19, N = 3 for each genotype).
The studies with Pcdh15av-3J raise the obvious question as to how PCDH15 protein modulates serotonergic phenotypes. Unfortunately, the currently available antibodies proved insufficiently specific to document the cellular localization of PCDH15 protein. Therefore, we returned to an assessment of mRNA expression patterns linked to Pcdh15 mRNA in the midbrain. Pcdh15 is a large gene (>400 kb, with >30 exons) and its transcripts exhibit at least 14 distinct splicing variants. As we cannot at present select microarray probes based on spliced products linked to 5-HT signaling, we chose a broader strategy to define a network of Pcdh15 mRNA-associated genes, using a probe (A_55_P2176176) that hybridizes with 12 Pcdh15 splicing isoforms (Fig. S5a). We then extracted those genes that demonstrated highest correlations with SERT protein expression. This effort yielded 330 such genes (Table S9). The list was reduced to 31 genes when we raised the correlation P value to 0.01 (Fig. S5b, Table S9). Among this list we note Syngr4, a tetraspanin protein among a class of proteins that associate with synaptic vesicles (Belizaire et al. 2004; Stevens et al. 2012). A closely related protein, synaptogyrin 3, has been found to associate with DAT (Egana et al. 2009). We speculate that synaptogyrin 4 may associate with SERT or with synaptic vesicles that secrete 5-HT. Clearly, further studies that assess the impact of the Pcdh15av-3J mutation on 5-HT neuron development and function are needed, possibly through the use of conditional deletion strategies. In summary, our studies nominate a network of genes that regulate, or are regulated by Slc6a4 expression and/or SERT protein levels. Because of its common association with transporter and 5-HT levels, our studies reveal that one of these genes, Pcdh15, plays an unappreciated role in 5-HT signaling, and thus may contribute to multiple neuropsychiatric disorders.
Supplementary Material
References
- Ahmed ZM, Riazuddin S, Bernstein SL, Ahmed Z, Khan S, Griffith AJ, Morell RJ, Friedman TB, Wilcox ER. Mutations of the protocadherin gene PCDH15 cause Usher syndrome type 1F. Am J Hum Genet. 2001;69:25–34. doi: 10.1086/321277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alagramam KN, Murcia CL, Kwon HY, Pawlowski KS, Wright CG, Woychik RP. The mouse Ames waltzer hearing-loss mutant is caused by mutation of Pcdh15, a novel protocadherin gene. Nat Genet. 2001;27:99–102. doi: 10.1038/83837. [DOI] [PubMed] [Google Scholar]
- Andreux PA, Williams EG, Koutnikova H, Houtkooper RH, Champy MF, Henry H, Schoonjans K, Williams RW, Auwerx J. Systems genetics of metabolism: the use of the BXD murine reference panel for multiscalar integration of traits. Cell. 2012;150:1287–1299. doi: 10.1016/j.cell.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azmitia EC. Serotonin and brain: evolution, neuroplasticity, and homeostasis. Int Rev Neurobiol. 2007;77:31–56. doi: 10.1016/S0074-7742(06)77002-7. [DOI] [PubMed] [Google Scholar]
- Bagdy E, Kiraly I, Harsing LG., Jr. Reciprocal innervation between serotonergic and GABAergic neurons in raphe nuclei of the rat. Neurochem Res. 2000;25:1465–1473. doi: 10.1023/a:1007672008297. [DOI] [PubMed] [Google Scholar]
- Belizaire R, Komanduri C, Wooten K, Chen M, Thaller C, Janz R. Characterization of synaptogyrin 3 as a new synaptic vesicle protein. J Comp Neurol. 2004;470:266–281. doi: 10.1002/cne.20008. [DOI] [PubMed] [Google Scholar]
- Belligni EF, Di Gregorio E, Biamino E, Calcia A, Molinatto C, Talarico F, Ferrero GB, Brusco A, Silengo MC. 790 Kb microduplication in chromosome band 17p13.1 associated with intellectual disability, afebrile seizures, dysmorphic features, diabetes, and hypothyroidism. Eur J Med Genet. 2012;55:222–224. doi: 10.1016/j.ejmg.2012.01.016. [DOI] [PubMed] [Google Scholar]
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355–366. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely RD, Berson HE, Fremeau RT, Jr., Caron MG, Peek MM, Prince HK, Bradley CC. Cloning and expression of a functional serotonin transporter from rat brain. Nature. 1991;354:66–70. doi: 10.1038/354066a0. [DOI] [PubMed] [Google Scholar]
- Borges K. Slc10A4 – what do we know about the function of this “secret ligand carrier” protein? Exp Neurol. 2013;248C:258–261. doi: 10.1016/j.expneurol.2013.06.019. [DOI] [PubMed] [Google Scholar]
- Burger S, Doring B, Hardt M, Beuerlein K, Gerstberger R, Geyer J. Co-expression studies of the orphan carrier protein Slc10a4 and the vesicular carriers VAChT and VMAT2 in the rat central and peripheral nervous system. Neuroscience. 2011;193:109–121. doi: 10.1016/j.neuroscience.2011.06.068. [DOI] [PubMed] [Google Scholar]
- Carneiro AM, Airey DC, Thompson B, Zhu CB, Lu L, Chesler EJ, Erikson KM, Blakely RD. Functional coding variation in recombinant inbred mouse lines reveals multiple serotonin transporter-associated phenotypes. Proc Natl Acad Sci U S A. 2009;106:2047–2052. doi: 10.1073/pnas.0809449106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvill S. Sensory impairments, intellectual disability and psychiatry. J Intellect Disabil Res. 2001;45:467–483. doi: 10.1046/j.1365-2788.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Crowley JJ, Brodkin ES, Blendy JA, Berrettini WH, Lucki I. Pharmacogenomic evaluation of the antidepressant citalopram in the mouse tail suspension test. Neuropsychopharmacology. 2006;31:2433–2442. doi: 10.1038/sj.npp.1301065. [DOI] [PubMed] [Google Scholar]
- DeAndrade MP, Johnson RL, Jr., Unger EL, Zhang L, van Groen T, Gamble KL, Li Y. Motor restlessness, sleep disturbances, thermal sensory alterations and elevated serum iron levels in Btbd9 mutant mice. Hum Mol Genet. 2012;21:3984–3992. doi: 10.1093/hmg/dds221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deneris ES. Molecular genetics of mouse serotonin neurons across the lifespan. Neuroscience. 2011;197:17–27. doi: 10.1016/j.neuroscience.2011.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanico D, Fragiotta S, Trabucco P, Nebbioso M, Vingolo EM. Genetic analysis for two Italian siblings with usher syndrome and schizophrenia. Case Rep Ophthalmol Med. 2012;2012:380863. doi: 10.1155/2012/380863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J, Siegmund D. Statistical methods for mapping quantitative trait loci from a dense set of markers. Genetics. 1999;151:373–386. doi: 10.1093/genetics/151.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley CJ, Hyland K, Allen RP. CSF dopamine, serotonin, and biopterin metabolites in patients with restless legs syndrome. Mov Disord. 2001;16:144–149. doi: 10.1002/1531-8257(200101)16:1<144::aid-mds1009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Egana LA, Cuevas RA, Baust TB, Parra LA, Leak RK, Hochendoner S, Pena K, Quiroz M, Hong WC, Dorostkar MM, Janz R, Sitte HH, Torres GE. Physical and functional interaction between the dopamine transporter and the synaptic vesicle protein synaptogyrin-3. J Neurosci. 2009;29:4592–4604. doi: 10.1523/JNEUROSCI.4559-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, Edwards RH. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. doi: 10.1016/s0896-6273(00)80418-3. [DOI] [PubMed] [Google Scholar]
- Hawrylycz M, Ng L, Page D, Morris J, Lau C, Faber S, Faber V, Sunkin S, Menon V, Lein E, Jones A. Multi-scale correlation structure of gene expression in the brain. Neural Netw. 2011;24:933–942. doi: 10.1016/j.neunet.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Hegmann JP, Possidente B. Estimating genetic correlations from inbred strains. Behav Genet. 1981;11:103–114. doi: 10.1007/BF01065621. [DOI] [PubMed] [Google Scholar]
- Hendricks TJ, Fyodorov DV, Wegman LJ, Lelutiu NB, Pehek EA, Yamamoto B, Silver J, Weeber EJ, Sweatt JD, Deneris ES. Pet-1 ETS gene plays a critical role in 5-HT neuron development and is required for normal anxiety-like and aggressive behavior. Neuron. 2003;37:233–247. doi: 10.1016/s0896-6273(02)01167-4. [DOI] [PubMed] [Google Scholar]
- Hoffman BJ, Mezey E, Brownstein MJ. Cloning of a serotonin transporter affected by antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biol Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Hortsch M, Umemori H. The Sticky Synapse: Cell Adhesion Molecules and Their Role in Synapse Formation and Maintenance. Springer; New York: 2009. [Google Scholar]
- Kalueff AV, Olivier JD, Nonkes LJ, Homberg JR. Conserved role for the serotonin transporter gene in rat and mouse neurobehavioral endophenotypes. Neurosci Biobehav Rev. 2010;34:373–386. doi: 10.1016/j.neubiorev.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Katori S, Hamada S, Noguchi Y, Fukuda E, Yamamoto T, Yamamoto H, Hasegawa S, Yagi T. Protocadherin-alpha family is required for serotonergic projections to appropriately innervate target brain areas. J Neurosci. 2009;29:9137–9147. doi: 10.1523/JNEUROSCI.5478-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Yasuda S, Tanaka H, Yamagata K, Kim H. Non-clustered protocadherin. Cell Adh Migr. 2011;5:97–105. doi: 10.4161/cam.5.2.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet. 1995;11:241–247. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, Greenberg BD, Petri S, Benjamin J, Muller CR, Hamer DH, Murphy DL. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Morishita H, Yagi T. Protocadherin family: diversity, structure, and function. Curr Opin Cell Biol. 2007;19:584–592. doi: 10.1016/j.ceb.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, Camp M, Fitzgerald PJ, Ciobanu DC, Sprengel R, Mishina M, Wellman CL, Winder DG, Williams RW, Holmes A. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci. 2010;30:5357–5367. doi: 10.1523/JNEUROSCI.5017-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DL, Lesch KP. Targeting the murine serotonin transporter: insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Fox MA, Timpano KR, Moya PR, Ren-Patterson R, Andrews AM, Holmes A, Lesch KP, Wendland JR. How the serotonin story is being rewritten by new gene-based discoveries principally related to SLC6A4, the serotonin transporter gene, which functions to influence all cellular serotonin systems. Neuropharmacology. 2008;55:932–960. doi: 10.1016/j.neuropharm.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers A, Holmans P, Marshall H, et al. Susceptibility locus for Alzheimer’s disease on chromosome 10. Science. 2000;290:2304–2305. doi: 10.1126/science.290.5500.2304. [DOI] [PubMed] [Google Scholar]
- O'Rourke NA, Weiler NC, Micheva KD, Smith SJ. Deep molecular diversity of mammalian synapses: why it matters and how to measure it. Nat Rev Neurosci. 2012;13:365–379. doi: 10.1038/nrn3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki N, Goldman D, Kaye WH, Plotnicov K, Greenberg BD, Lappalainen J, Rudnick G, Murphy DL. Serotonin transporter missense mutation associated with a complex neuropsychiatric phenotype. Mol Psychiatry. 2003;8:933–936. doi: 10.1038/sj.mp.4001365. [DOI] [PubMed] [Google Scholar]
- Perez-Torrado R, Yamada D, Defossez PA. Born to bind: the BTB protein-protein interaction domain. Bioessays. 2006;28:1194–1202. doi: 10.1002/bies.20500. [DOI] [PubMed] [Google Scholar]
- Poulopoulos A, Aramuni G, Meyer G, Soykan T, Hoon M, Papadopoulos T, Zhang M, Paarmann I, Fuchs C, Harvey K, Jedlicka P, Schwarzacher SW, Betz H, Harvey RJ, Brose N, Zhang W, Varoqueaux F. Neuroligin 2 drives postsynaptic assembly at perisomatic inhibitory synapses through gephyrin and collybistin. Neuron. 2009;63:628–642. doi: 10.1016/j.neuron.2009.08.023. [DOI] [PubMed] [Google Scholar]
- Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD. Antidepressantand cocaine-sensitive human serotonin transporter: molecular cloning, expression, and chromosomal localization. Proc Natl Acad Sci U S A. 1993;90:2542–2546. doi: 10.1073/pnas.90.6.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy S, Shippenberg TS, Jayanthi LD. Regulation of monoamine transporters: role of transporter phosphorylation. Pharmacol Ther. 2011;129:220–238. doi: 10.1016/j.pharmthera.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NP, Danivas V, Venkatasubramanian G, Behere RV, Gangadhar BN. Comorbid bipolar disorder and Usher syndrome. Prim Care Companion J Clin Psychiatry. 2010;12:PCC.09l00792. doi: 10.4088/PCC.09l00792yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijavec N, Grubic VN. Usher syndrome and psychiatric symptoms: a challenge in psychiatric management. Psychiatr Danub. 2009;21:68–71. [PubMed] [Google Scholar]
- Rioux JD, Xavier RJ, Taylor KD, et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet. 2007;39:596–604. doi: 10.1038/ng2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottach KG, Schaner BM, Kirch MH, Zivotofsky AZ, Teufel LM, Gallwitz T, Messer T. Restless legs syndrome as side effect of second generation antidepressants. J Psychiatr Res. 2008;43:70–75. doi: 10.1016/j.jpsychires.2008.02.006. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Soiza-Reilly M, Anderson WB, Vaughan CW, Commons KG. Presynaptic gating of excitation in the dorsal raphe nucleus by GABA. Proc Natl Acad Sci U S A. 2013;110:15800–15805. doi: 10.1073/pnas.1304505110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorte HS, Gjevik E, Sponheim E, Eiklid KL, Rodningen OK. Copy number variation findings among 50 children and adolescents with autism spectrum disorder. Psychiatr Genet. 2013;23:61–69. doi: 10.1097/YPG.0b013e32835d718b. [DOI] [PubMed] [Google Scholar]
- Steiner JA, Carneiro AM, Blakely RD. Going with the flow: trafficking-dependent and -independent regulation of serotonin transport. Traffic. 2008;9:1393–1402. doi: 10.1111/j.1600-0854.2008.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RJ, Akbergenova Y, Jorquera RA, Littleton JT. Abnormal synaptic vesicle biogenesis in Drosophila synaptogyrin mutants. J Neurosci. 2012;32:18054–18067. 18067a. doi: 10.1523/JNEUROSCI.2668-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC. Neuroligins and neurexins link synaptic function to cognitive disease. Nature. 2008;455:903–911. doi: 10.1038/nature07456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Cheng MC, Qin R, Liao DL, Chen TT, Koong FJ, Chen G, Chen CH. Identification and functional characterization of rare mutations of the neuroligin-2 gene (NLGN2) associated with schizophrenia. Hum Mol Genet. 2011;20:3042–3051. doi: 10.1093/hmg/ddr208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe JS, Delahanty RJ, Prasad HC, McCauley JL, Han Q, Jiang L, Li C, Folstein SE, Blakely RD. Allelic heterogeneity at the serotonin transporter locus (SLC6A4) confers susceptibility to autism and rigid-compulsive behaviors. Am J Hum Genet. 2005;77:265–279. doi: 10.1086/432648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Sudhof TC, Brose N. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Muller CL, Iwamoto H, Sauer JE, Owens WA, Shah CR, Cohen J, Mannangatti P, Jessen T, Thompson BJ, Ye R, Kerr TM, Carneiro AM, Crawley JN, Sanders-Bush E, McMahon DG, Ramamoorthy S, Daws LC, Sutcliffe JS, Blakely RD. Autism gene variant causes hyperserotonemia, serotonin receptor hypersensitivity, social impairment and repetitive behavior. Proc Natl Acad Sci U S A. 2012;109:5469–5474. doi: 10.1073/pnas.1112345109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visscher PM, Thompson R, Haley CS. Confidence intervals in QTL mapping by bootstrapping. Genetics. 1996;143:1013–1020. doi: 10.1093/genetics/143.2.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyiaziakis E, Evgrafov O, Li D, et al. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol Psychiatry. 2011;16:108–120. doi: 10.1038/mp.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson C, Paxinos G. Chemoarchitectonic Atlas of the Mouse Brain. Academic; London: 2010. [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner TF, Zhao B, Johnson KR, Kazmierczak P, Muller U. TMHS Is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell. 2012;151:1283–1295. doi: 10.1016/j.cell.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R, Blakely RD. Natural and engineered coding variation in antidepressant-sensitive serotonin transporters. Neuroscience. 2011;197:28–36. doi: 10.1016/j.neuroscience.2011.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Liu J, Wu JY. Cell adhesion molecules and their involvement in autism spectrum disorder. Neurosignals. 2010;18:62–71. doi: 10.1159/000322543. [DOI] [PubMed] [Google Scholar]
- Ye R, Carneiro AM, Airey DC, Sanders-Bush E, Williams RW, Lu L, Wang J, Zhang B, Blakely RD. Evaluation of heritable determinants of blood and brain serotonin homeostasis using recombinant inbred mice. Genes Brain Behav. 2014;13:247–260. doi: 10.1111/gbb.12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CB, Lindler KM, Campbell NG, Sutcliffe JS, Hewlett WA, Blakely RD. Colocalization and regulated physical association of presynaptic serotonin transporters with A(3) adenosine receptors. Mol Pharmacol. 2011;80:458–465. doi: 10.1124/mol.111.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.