Abstract
Background
Finding the optimal location for the implantation of the electrode in Deep Brain Stimulation (DBS) surgery is crucial for maximizing therapeutic benefit to the patient. Such targeting is challenging for several reasons including anatomical variability between patients as well as lack of consensus about the location of the optimal target.
Objective
To compare the performance of popular manual targeting methods against a fully automatic non-rigid image registration based approach.
Methods
In 71 Parkinson's disease STN-DBS implantations, an experienced functional neurosurgeon selected the target manually using three different approaches; indirect targeting using standard stereotactic coordinates, direct targeting based on the patient MRI, and indirect targeting relative to the red nucleus. Targets were also automatically predicted using a leave-one-out approach to populate the CranialVault atlas using non-rigid image registration. The different targeting methods were compared against the location of the final active contact, determined through iterative clinical programming in each individual patient.
Results
Targeting using standard stereotactic coordinates corresponding to the center of the motor territory of the STN had the largest targeting error (3.69 mm), followed by direct targeting (3.44 mm), average stereotactic coordinates of active contacts from this study (3.02 mm), red nucleus based targeting (2.75 mm), and non-rigid image registration based automatic predictions using the CranialVault atlas (2.70 mm). The CranialVault atlas method had statistically smaller variance than all manual approaches.
Conclusions
Fully automatic targeting based on non-rigid image registration using the CranialVault atlas is as accurate and more precise than popular manual methods for STN-DBS.
Keywords: automatic targeting, deep brain stimulation, non-linear image registration, electrophysiological atlases, Parkinson's disease, subthalamic nucleus
Introduction
Deep brain stimulation (DBS) is a therapy used to alleviate symptoms related to movement disorders such as Parkinson's disease by stimulating deep brain nuclei. Because of the small size of the nuclei, and a relatively narrow therapeutic window of stimulation therapy, such functional neurosurgical procedures require precise targeting so that the final implant can be placed in an optimal location to achieve therapeutic benefit without causing side effects. Traditionally, this requires modification of an initial approximate target location (selected pre-operatively by a neurosurgeon) using exploratory electrodes to map the electrophysiology of the brain and stimulate the regions around the planned target. Although this process compensates for initial inaccuracies in pre-operative targeting and allows adjustment for the anatomical and physiological variability across patients, it is time consuming, invasive and may increase operative risk when multiple penetrations are required 1, 2.
Given the importance of accurate localization of the optimal target 3-8 there has been much effort over the last few decades towards the use of different approaches including indirect targeting based on anatomical landmarks and direct targeting using various imaging modalities 9, 10,11-18,19, 20,21, 22,14, 23-25. Pallavaram et al. showed that there is significant inter-surgeon variability in the selection of ACPC-based stereotactic reference system and that this has a substantial effect on the localization of targets 26. Moreover, there has been a lack of consensus on the ideal anatomical location for maximum therapeutic relief in PD 5, 21, 27-33. Therefore, several functional atlases have also been developed based on intra- and post-operatively acquired electrophysiological data mapped using non-linear image registration techniques 34-41. Castro et al. compared the accuracy of ACPC-based targeting of the STN against several image registration techniques 42, 43. They found non-linear image registration to produce the highest accuracy in predicting the anatomical target inside the STN. The ground truth used in their study was direct-targeting based on selection of STN by two experts on high resolution MRI T2 scans. Chakravarty et al. 44, 45 took a similar approach to validate their method for segmentation of basal ganglia structures and showed that non-linear techniques perform statistically better than linear and piece-wise linear techniques. Yelnik and Bardinet et al. 46, 47 showed how a histological and deformable 3D atlas based on non-linear image registration could be used to accurately project segmentations of structures onto patients for anatomical targeting of the STN. The ground truth for validating STN segmentations using their methods were intra-operative electrophysilogical recordings and post-operative therapeutic contacts. D'Haese et al. recently showed that maps produced using nonlinear techniques correlate with their expected anatomic positions 48.
In this study, we compare a fully automatic method for predicting the optimal functional target for STN-DBS in Parkinson's disease patients using non-rigid image registration and three popular manual targeting methods: direct targeting of the center of the STN based on MRI images, indirect targeting with respect to the location of the red nucleus, and indirect targeting based on stereotactic coordinates, against the clinically active contacts as the ground truth.
Data and Method
In this study we retrospectively examined 71 STN-DBS implantations in 37 PD patients. With IRB approval each patient had pre-operative MRI (T1 and T2) and CT acquisitions of the brain as well as a delayed post-operative CT acquired approximately one month after surgery. Typical CT images were acquired at kVp = 120 V, exposure 350 mAs and 512×512 pixels. In-plane resolution and slice thickness were approximately 0.5 mm and 0.75 mm, respectively. MRI T1 (TR 7.9 ms, TE 3.8 ms, 256 × 256 × 170 voxels, with typical voxel resolution of 1 × 1 × 1 mm3) were acquired using the SENSE parallel imaging technique (T1W/3D/TFE) from Philips on a 3T scanner. MRI T2 (TR 3000 ms, TE 80 ms, 512 × 512 × 45 voxels, with typical voxel resolution of 0.47 × 0.47 × 2 mm3) were acquired using the SENSE parallel imaging technique (T2W/TSE) from Philips on a 3T scanner.
Planning was subsequently performed using the FDA approved clinical version of the CranialVault Explorer software suite 49 called WayPoint Navigator (distributed by FHC, Inc. Bowdoin, ME, USA) shown in figure 1. This involved the importation of pre-operative MRI and CT, identification of anatomical landmarks (e.g. anterior commissure, posterior commissure, mid-plane, and red nucleus), surgeon selection of optimal target and entry points; and confirmation of design for a customized miniature stereotactic frame called the mT platform (FHC, Inc.; Bowdoin, ME) to be used in surgery. Details on the platform including a study of its accuracy demonstrating it to be at least as accurate as standard frames have been previously published 50-52.
Figure 1.
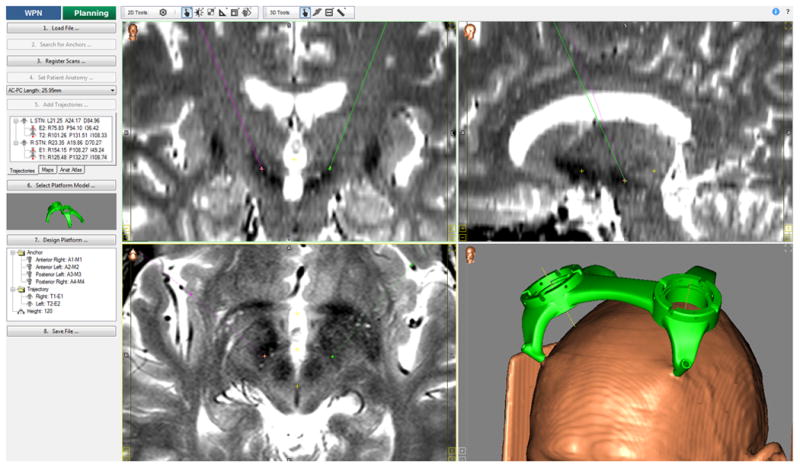
WayPoint Navigator software made available to the surgeons for clinical planning as well as for manual target slections in this study. A 3D rendering of the platform and the patient's head based on the CT is also shown along with a sample trajectory for an STN-DBS patient.
During surgery, targeting was modified through the use of micro-electrode recording and stimulation response observations recorded in 3-4 tracks parallel to the planned trajectory using a Ben gun approach. The optimal location for DBS lead implantation was determined by the multidisciplinary surgical team (neurosurgeon, neurologist and neurophysiologist) and defined as the location of the centerpoint of the 4-contact 3389 lead. Post-operatively the locations of the leads and the individual contacts were extracted from the delayed post-op CT. The individual contacts were projected onto the patient pre-op MRIs using rigid registration as shown in figure 2. These were then projected onto the CranialVault atlas using fully automatic non-rigid registration between the patient pre-op MRIs and the atlas MRI. Using a leave-one-out approach, the centroid of the atlas cluster was projected onto each patient's preop MRI using non-rigid registration and subsequently projected onto the delayed post-op CTs using rigid registration between the delayed post-op CT and the pre-op MRI of the patient. This point is referred to as the atlas-based target prediction and the process is illustrated in figure 3. Briefly, the non-rigid registration algorithm we use computes a deformation field that is modeled as a linear combination of radial basis functions with finite support. This results in a transformation with several thousands of degrees of freedom. Two transformations (one from the atlas to the subject and the other from the subject to the atlas) that are constrained to be inverses of each other are computed simultaneously. Details on this procedure and the algorithms that have been used can be found in several previous publications 53,34. Our registration algorithms are based on mutual information between the two images as the similarity metric 54, 55 and it's accuracy was validated in a population of patients at known anatomical landmarks by Pallavaram et al. 56. Two variations of the non-rigid registration based target predictions were produced; one used data from both surgeons to make predictions while the other was ‘surgeon-specific’ where predictions for a particular surgeon's patient were based on that particular surgeon's average selection in a population.
Figure 2.
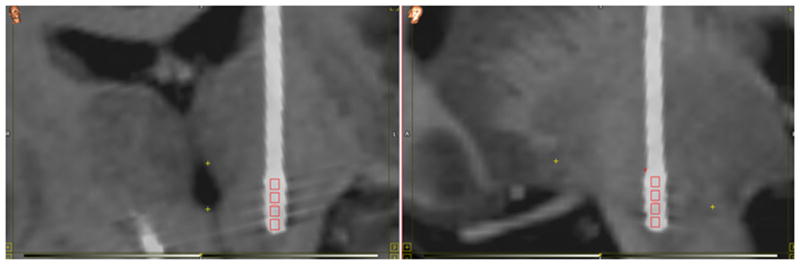
Individual contacts in a 3389 lead extracted from the delayed post-op CT and overlaid on the pre-op MRI using rigid registration.
Figure 3.
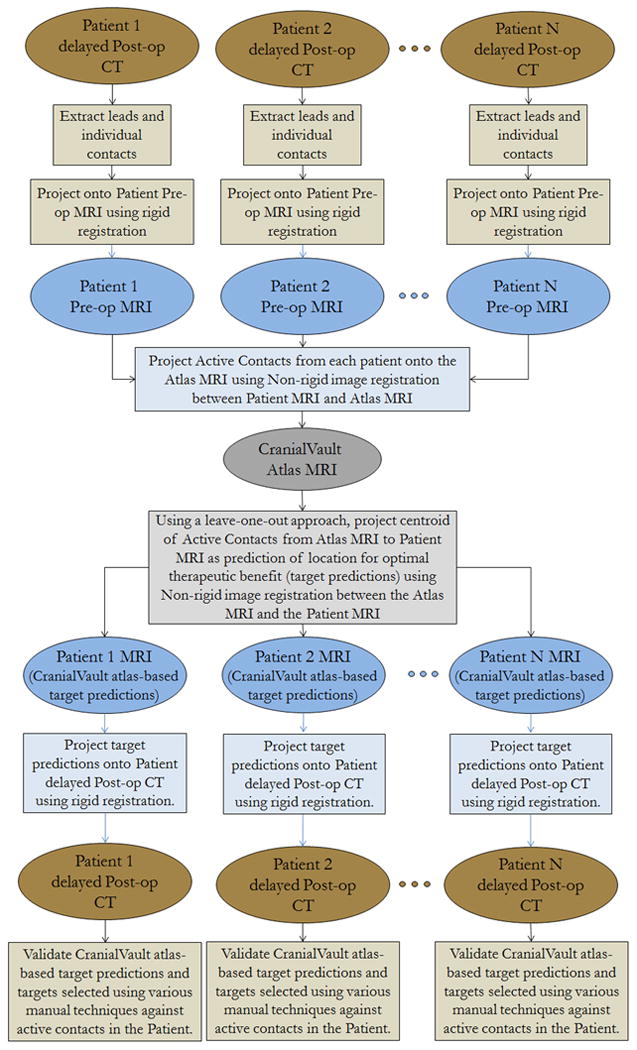
Flowchart showing how target predictions based on non-rigid image registration are made using the CranialVault atlas by projecting contacts positions from a population of patients onto a new patient.
The manual approaches used in this study are commonly used approaches; namely indirect targeting using stereotactic coordinates based on localizing the anterior commissure (AC) and posterior commissure (PC) referred here as ACPC-based targeting, direct targeting based on high resolution MRI T1 and T2 weighted images, and indirect targeting based on standard coordinates defined with respect to the red nucleus referred here as RN-based targeting (11 mm Lateral, -2.3 Anterior, -2.6 Superior), that require the expertise of an experienced functional neurosurgeon. Indirect targeting based on the stereotactic coordinates (12 mm Lateral, -3 mm Anterior, and -4 mm Superior) with respect to the mid-commissural point used to target the center of the motor territory of the STN 3, 9, 21, 31, 57, 58, (11.8 mm Lateral, -2.4 mm Anterior, and -3.9 mm Superior) which represent the centroid of active contacts from a widely cited study 57, as well as the centroid of active contacts from our dataset (11.0 mm Lateral, -2.0 mm Anterior, and -2.4 mm Superior) which represent the optimized stereotactic coordinates for this dataset were included. The coordinates for RN-based targeting were 3 mm lateral to the lateral edge, -2 mm superior to the superior edge, and the anterior edge of the RN. For direct targeting, the surgeon selected the center of the STN as visualized on T2 axial images (TR: 3000 mS, TW: 80 ms, imaging frequency: 128 Hz, magnetic field strength: 3T, in-plane resolution: 0.47 mm X 0.47 mm, slice thickness: 2 mm).
The manual selections used in the study to be tested against the CranialVault atlas prediction were performed retrospectively by a single neurosurgeon (S2). To avoid bias in planning, the patients were anonymized and randomized in the order in which they were shown to the surgeon. Furthermore, the surgeon was provided only pre-op imaging for the retrospective target selections and was also blinded to the location of the implantation making the process identical to pre-op planning. Predictions based on the CranialVault atlas and the various manual target selections were validated against the final location of the active contact in the patient achieved through iterative testing and programming and evaluated by each patient's treating neurologist to be the location for optimal therapeutic benefit in that patient.
Results
Figure 4 shows a bar chart of the accuracy of different targeting methords compared in this study with respect to the final active contacts in 71 STN-DBS cases. On average, targeting using standard stereotactic coordinates corresponding to the center of the motor territory of the STN (12 mm Lateral, -3 mm Anterior, and -4 mm Superior) had the largest error (mean 3.69 mm, standard deviation 1.78 mm). Literature coordinates of the centroid of active contacts (11.8 mm Lateral, -2.4 mm Anterior, and -3.9 mm Superior) had better accuracy (mean 3.49 mm, standard deviation 1.70 mm). Direct targeting performed comparably to these standard stereotactic coordinates (mean 3.44 mm, standard deviation 1.49 mm). However, average stereotactic coordinates of the active contacts from the 71 cases in this study (11 mm Lateral, -2 mm Anterior, and -2.4 mm Superior) performed substantially better (mean 3.02 mm, standard deviation 1.48 mm). Red nucleus based targeting and the fully automatic CranialVault atlas method of targeting using non-rigid registration performed substantially better than all other methods and were comparable to each other on mean accuracy. Red nucleus based targeting had mean accuracy of 2.75 mm and a standard deviation of 1.49 mm, fully automatic CranialVault atlas based predictions using both surgeons had a mean accuracy of 2.75 mm (same as red nucleus) and a standard deviation of 1.40 mm while the accuracy of the CranialVault atlas improved marginally when using surgeon-specific data for predictions to a mean of 2.70 mm and a standard deviation of 1.17 mm. To be even more comprehensive on the wide range of stereotactic coordinates used across practices, we tested a number of other popular AC-PC based coordinates. For (12 mm Lateral, -2 mm Anterior, -4 mm Superior), the mean accuracy was 3.57 mm (standard deviation 1.65 mm), for (12 mm Lateral, -2 mm Anterior, -3 mm Superior), the mean accuracy was 3.22 mm (standard deviation 1.54 mm), and for (12 mm Lateral, -3 mm Anterior, -5 mm Superior), the mean accuracy was 4.22 mm (standard deviation 1.91 mm).
Figure 4.
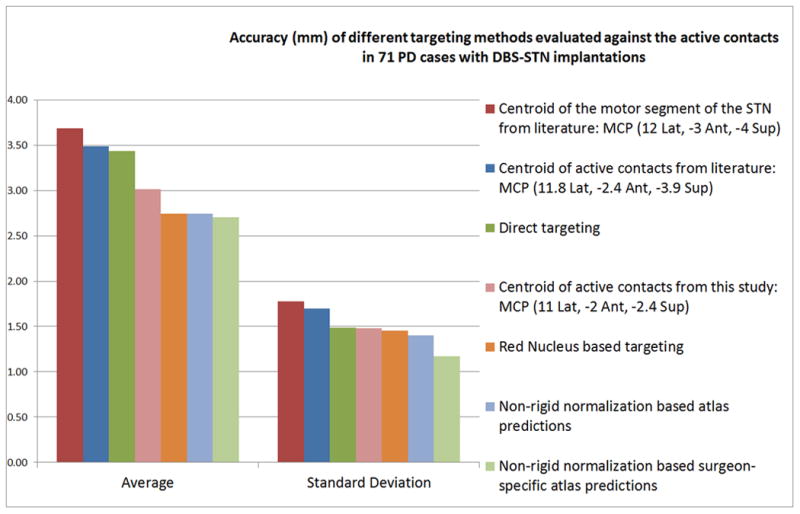
Bar chart showing the average and standard deviation of the Euclidean distances for different targeting methods from active contacts in 71 STN-DBS implants.
Of all the tested methods, the non-rigid registration based CranialVault atlas predictions using surgeon-specific data had the highest accuracy and the smallest standard deviation. Performing t-test at 5% level of significance showed that there was a statistically significant difference in accuracy between CranialVault atlas based targeting using non-rigid registration and three manual approaches; standard stereotactic coordinates targeting the center of the motor territory of the STN, standard stereotactic coordinates from literature targeting the centroid of active contacts, as well as Direct targeting of the STN using MRI imaging. There was no statistically significant difference between red nucleus based targeting and the CranialVault atlas targeting using non-rigid registration although the latter had marginally higher accuracy and substantially smaller standard deviation. Performing a two-sample F-test at 5% level of significance revealed a statistically significant difference between the standard deviations of red nucleus based targeting and our atlas-based targeting. This suggests that automated atlas based targeting is no less accurate than traditional methods including red nucleus based targeting, but is also more precise than all manual methods evaluated in this study. Figure 5 shows the location of the atlas centroid overlaid on the 3D renderings of the segmentations of STN and SNr.
Figure 5.
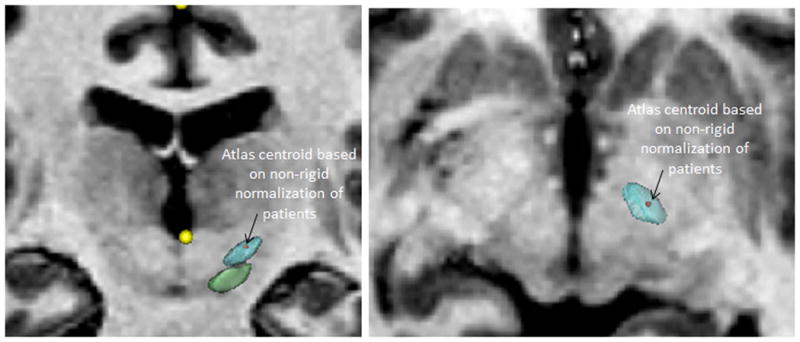
Coronal and axial views showing the location of the atlas centroid overlaid on the 3D renderings of the segmentations of STN and SNr.
Discussion
Our results show that among the manual methods evaluated in this study, targeting using standard stereotactic coordinates in the literature corresponding to the center of the motor territory of the STN has the largest error while that using standard coordinates relative to the red nucleus has the smallest error. Targeting based on the centroid of surgeon-specific active contacts from the CranialVault atlas projected using fully automatic non-rigid image registration had the smallest error of all methods, was statistically better than standard stereotactic coordinates from literature and direct targeting, and was more precise than any of the manual methods. The use of surgeon-specific data for predictions seems to marginally improve targeting accuracy. Andrade-Souza et al. 11 had compared modified direct targeting, indirect targeting using standard stereotactic coordinates, and red nucleus based targeting in 28 STN implants against the active contacts. They reported that red nucleus targeting was the best among the manual methods while direct targeting and indirect targeting using standarad stereotactic coordinates had substantially inferior accuracy. These are consistent with our results. Our study can be considered an extension over their work using not only a substantially larger dataset but also additionally evaluating a fully automatic method of atlas based targeting using non-rigid registration.
We also examined the difference in the AC-PC or stereotactic space for the different methods as shown in Table 1. We found that the CranialVault atlas-based target predictions seem to differ substantially from standard stereotactic coordinates in literature along all three directions with most standard AC-PC coordinates being more lateral, posterior and inferior to atlas-based predictions. With respect to direct targeting the predominant difference is in the superior-inferior direction with direct targeting being substantially inferior to atlas predictions. Targeting based on red nucleus and centroid of stereotactic coordinates of the active contacts from the study have negligible difference in the lateral component and posterior components, but noticeable difference in the superior-inferior component. Such direct comparison of the ultimate location, however, belies the real power of the CranialVault process, which is its ability to account for anatomical variation. This is best demonstrated in the decreased variance that it exhibits versus all other techniques, suggesting a greater precision in the face of anatomical variation. We presume that of the manual techniques red nucleus based targeting performed best because its proximity to the STN makes its ultimate relationship to the later structure more constant despite variations that may exist in anatomy (in effect the red nucleus moves with the STN in most cases). Although, using this same logic, one might expect direct targeting to ourperform other techniques it ultimately was inferior to red nucleus and CranialVault atlas-based targeting. This is perhaps due to inconsistancies in the appearance of the STN even on our high resolution images.
Table 1.
Components of stereotactic coordinates for different targeting methods.
| Mean Stereotactic coordinates of various targeting methods | Lateral | Anterior | Superior |
|---|---|---|---|
| Direct Targeting | 11.19 | -1.59 | -4.31 |
| RN-based Targeting | 11.05 | -2.26 | -2.62 |
| Non-Rigid Registration based targeting using CranialVault Atlas | 11.01 | -2.01 | -2.02 |
| Active contacts from literature | 11.80 | -2.40 | -3.90 |
| Motor segment of the STN from literature | 12.00 | -3.00 | -4.00 |
| Active contacts from the study | 11.09 | -2.00 | -2.40 |
One potential confound to these findings is the extent to which the dominance of one method in our original surgical targeting between the two surgeons might favor the performance of one technique over another when evaluated retrospectively. Our surgeons used a combined approach when choosing their initial target, combining red nucleus based, standard stereotactic coordinates based, and direct targeting methods. The actual weighting of these methods used for targeting the STN was variable between the two surgeons performing these cases. One of the two surgeons, in addition to these manual approaches, had access to the atlas predictions of the middle of the leads but not active contacts and could have been influenced by it in his targeting. Both surgeons however used intra-operative MER and stimulation response (efficacy and side effects) to arrive at their final target for implantation moving from their original target (center track on the BenGun) in greater than 50% of implanted leads. To test if the locations of implantations for the surgeons were impacted differently by their planning approaches we compared the accuracy of the atlas prediction of active contacts between the two surgeons. There was no statistically significant difference between these two subgroups at 5% level of significance using a two-sample t-test.
Conclusions
Predictions based on non-rigid image registration using the surgeon-specific CranialVault atlas had the highest accuracy among all the validated methods and the difference was statistically significant when compared to a variety of standard stereotactic coordinates cited in the literature and used in practice as well as with respect to direct targeting. The accuracy of red nucleus based targeting was the best among the manual methods and not statistically different from the CranialVault atlas based predictions. However, predictions based on the CranialVault atlas had the smallest variance of all tested methods and the difference was statistically significant.
Therefore, fully automatic targeting based on non-rigid image registration using the CranialVault atlas is as accurate and more precise than popular manual methods for STN-DBS. A fully automatic targeting method that performs as good as the manual selections by experienced neurosurgeons is not only useful to them but could provide significant assistance to surgeons less experienced in DBS and help make the surgery accessible to more patients. With the increasing accessibility of sophisticated non-rigid image registration packages within and outside of clinical software we believe that fully automatic targeting can become part of routine clinical practice.
Acknowledgments
Disclosure of funding: This research has been supported, in parts, by NIH R01 EB006136. The content is solely the responsibility of the authors and does not necessarily represent the official views of these institutes.
Footnotes
Conflict of Interest: P-F. D'Haese, P.E. Konrad, and B.M. Dawant are founding members and stock holders, and S. Pallavaram is a stockholder in Neurotargeting, LLC that licenses the software suite from Vanderbilt University that was used in this study for data collection. P.E. Konrad is also a Consultant for Medtronic Neuromodulation.
References
- 1.Binder DK, R GM, Starr PA. Risk factors for hemorrhage during microelectrode-guided deep brain stimulator implantation for movement disorders. Neurosurgery. 2005;56(4):722–732. doi: 10.1227/01.neu.0000156473.57196.7e. [DOI] [PubMed] [Google Scholar]
- 2.Xiaowu H, Xiufeng J, Xiaoping Z, Bin H, et al. Risks of intracranial hemorrhage in patients with Parkinson's disease receiving deep brain stimulation and ablation. Parkinsonism & related disorders. 2010;16(2):96–100. doi: 10.1016/j.parkreldis.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 3.Starr PA, Christine CW, Theodosopoulos PV, et al. Implantation of deep brain stimulators into the subthalamic nucleus: technical approach and magnetic resonance imaging-verified lead locations. J Neurosurg. 2002 Aug;97(2):370–387. doi: 10.3171/jns.2002.97.2.0370. [DOI] [PubMed] [Google Scholar]
- 4.Benabid AL, Krack PP, Benazzouz A, Limousin P, Koudsie A, Pollak P. Deep brain stimulation of the subthalamic nucleus for Parkinson's disease: methodologic aspects and clinical criteria. Neurology. 2000;55(12 Suppl 6):S40–44. [PubMed] [Google Scholar]
- 5.Lanotte MM, Rizzone M, Bergamasco B, Faccani G, Melcarne A, Lopiano L. Deep brain stimulation of the subthalamic nucleus: anatomical, neurophysiological, and outcome correlations with the effects of stimulation. J Neurol Neurosurg Psychiatry. 2002;72(1):53–58. doi: 10.1136/jnnp.72.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Limousin P, Krack P, Pollack P, et al. Electrical stimulation of the subthalamic nucleus in advanced Parkinson's disease. N Engl J Med. 1998;339:1105–1111. doi: 10.1056/NEJM199810153391603. [DOI] [PubMed] [Google Scholar]
- 7.Saint-Cyr JA, Hoque T, Pereira LC, et al. Localization of clinically effective stimulating electrodes in the human subthalamic nucleus on magnetic resonance imaging. J Neurosurg. 2002 Nov;97(5):1152–1166. doi: 10.3171/jns.2002.97.5.1152. [DOI] [PubMed] [Google Scholar]
- 8.Tamma F, Caputo E, Chiesa V, et al. Anatomo-clinical correlation of intraoperative stimulation-induced side-effects during HF-DBS of the subthalamic nucleus. Neurol Sci. 2002 Sep;23(Suppl 2):S109–110. doi: 10.1007/s100720200093. [DOI] [PubMed] [Google Scholar]
- 9.Bejjani BP, Dormont D, Pidoux B, et al. Bilateral subthalamic stimulation for Parkinson's disease by using three-dimensional stereotactic magnetic resonance imaging and electrophysiological guidance. J Neurosurg. 2000 Apr;92(4):615–625. doi: 10.3171/jns.2000.92.4.0615. [DOI] [PubMed] [Google Scholar]
- 10.Benabid AL, Koudsie A, Benazzouz A, Le Bas JF, Pollak P. Imaging of subthalamic nucleus and ventralis intermedius of the thalamus. Mov Disord. 2002;17(Suppl 3):S123–129. doi: 10.1002/mds.10153. [DOI] [PubMed] [Google Scholar]
- 11.Andrade-Souza YM, Schwalb JM, Hamani C, et al. Comparison of three methods of targeting the subthalamic nucleus for chronic stimulation in Parkinson's disease. Neurosurgery. 2008;62(Suppl 2):875–883. doi: 10.1227/01.neu.0000316289.75736.55. Suppl 2. [DOI] [PubMed] [Google Scholar]
- 12.Dormont D, Cornu P, Pidoux B, et al. Chronic thalamic stimulation with three-dimensional MR stereotactic guidance. AJNR Am J Neuroradiol. 1997 Jun-Jul;18(6):1093–1107. [PMC free article] [PubMed] [Google Scholar]
- 13.Egidi M, Rampini P, Locatelli M, et al. Visualisation of the subthalamic nucleus: a multiple sequential image fusion (MuSIF) technique for direct stereotaxic localisation and postoperative control. Neurol Sci. 2002 Sep;23(Suppl 2):S71–72. doi: 10.1007/s100720200075. [DOI] [PubMed] [Google Scholar]
- 14.Hariz MI, Bergenheim AT. A comparative study on ventriculographic and computerized tomography-guided determinations of brain targets in functional stereotaxis. J Neurosurg. 1990 Oct;73(4):565–571. doi: 10.3171/jns.1990.73.4.0565. [DOI] [PubMed] [Google Scholar]
- 15.Holtzheimer PE, 3rd, Roberts DW, Darcey TM. Magnetic resonance imaging versus computed tomography for target localization in functional stereotactic neurosurgery. Neurosurgery. 1999 Aug;45(2):290–297. doi: 10.1097/00006123-199908000-00018. discussion 297-298. [DOI] [PubMed] [Google Scholar]
- 16.Kondziolka D, Dempsey PK, Lunsford LD, et al. A comparison between magnetic resonance imaging and computed tomography for stereotactic coordinate determination. Neurosurgery. 1992 Mar;30(3):402–406. doi: 10.1227/00006123-199203000-00015. discussion 406-407. [DOI] [PubMed] [Google Scholar]
- 17.Starr PA, Vitek JL, DeLong M, Bakay RA. Magnetic resonance imaging-based stereotactic localization of the globus pallidus and subthalamic nucleus. Neurosurgery. 1999 Feb;44(2):303–313. doi: 10.1097/00006123-199902000-00031. discussion 313-304. [DOI] [PubMed] [Google Scholar]
- 18.Guridi J, Rodriguez-Oroz MC, Ramos E, Linazasoro G, Obeso JA. Discrepancy between imaging and neurophysiology in deep brain stimulation of medial pallidum and subthalamic nucleus in Parkinson's disease. Neurologia. 2002 Apr;17(4):183–192. [PubMed] [Google Scholar]
- 19.Hutchison WD, Allan RJ, Opitz H, et al. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol. 1998 Oct;44(4):622–628. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- 20.Pollak P, Krack P, Fraix V, et al. Intraoperative micro- and macrostimulation of the subthalamic nucleus in Parkinson's disease. Mov Disord. 2002;17(Suppl 3):S155–161. doi: 10.1002/mds.10158. [DOI] [PubMed] [Google Scholar]
- 21.Zonenshayn M, Rezai AR, Mogilner AY, Beric A, Sterio D, Kelly PJ. Comparison of anatomic and neurophysiological methods for subthalamic nucleus targeting. Neurosurgery. 2000 Aug;47(2):282–292. doi: 10.1097/00006123-200008000-00005. discussion 292-284. [DOI] [PubMed] [Google Scholar]
- 22.Cuny E, Guehl D, Burbaud P, Gross C, Dousset V, Rougier A. Lack of agreement between direct magnetic resonance imaging and statistical determination of a subthalamic target: the role of electrophysiological guidance. J Neurosurg. 2002 Sep;97(3):591–597. doi: 10.3171/jns.2002.97.3.0591. [DOI] [PubMed] [Google Scholar]
- 23.Hawrylyshyn PA, Tasker RR, Organ LW. Third ventricular width and the thalamocapsular border. Appl Neurophysiol. 1976;39(1):34–42. doi: 10.1159/000102473. [DOI] [PubMed] [Google Scholar]
- 24.Lunsford LD. Magnetic resonance imaging stereotactic thalamotomy: report of a case with comparison to computed tomography. Neurosurgery. 1988 Sep;23(3):363–367. doi: 10.1227/00006123-198809000-00014. [DOI] [PubMed] [Google Scholar]
- 25.Schaltenbrand G, Wahren W. Atlas for stereotaxy of the human brain. Stuttgart, Germany: Thieme; 1977. Thieme. [Google Scholar]
- 26.Pallavaram S, Yu H, Spooner J, et al. Inter-surgeon variability in the selection of anterior and posterior commissures and its potential effects on target localization. Stereotact Funct Neurosurg. 2008;86(2):113–119. doi: 10.1159/000116215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plaha P, Ben-Shlomo Y, Patel NK, Gill SS. Stimulation of the caudal zona incerta is superior to stimulation of the subthalamic nucleus in improving contralateral parkinsonism. Brain. 2006;129(7):1732–1747. doi: 10.1093/brain/awl127. [DOI] [PubMed] [Google Scholar]
- 28.Benabid AL, Koudsie A, Benazzouz A, Fraix V, et al. Subthalamic stimulation for Parkinson's disease. Arch Med Res. 2000;31(3):282–289. doi: 10.1016/s0188-4409(00)00077-1. [DOI] [PubMed] [Google Scholar]
- 29.Herzog J, Fietzek U, Hamel W, Morsnowski A, et al. Most effective stimulation site in subthalamic deep brain stimulation for Parkinson's disease. Mov Disord. 2004;19(9):1050–1054. doi: 10.1002/mds.20056. [DOI] [PubMed] [Google Scholar]
- 30.Nandi D, Chir M, Liu X, Bain P, et al. Electrophysiological confirmation of the zona incerta as a target for surgical treatment of disabling involuntary arm movements in multiple sclerosis: use of local field potentials. J Clin Neurosci. 2002;9(1):64–68. doi: 10.1054/jocn.2001.1012. [DOI] [PubMed] [Google Scholar]
- 31.Voges J, Volkmann J, Allert N, et al. Bilateral high-frequency stimulation in the subthalamic nucleus for the treatment of Parkinson disease: correlation of therapeutic effect with anatomical electrode position. J Neurosurg. 2002 Feb;96(2):269–279. doi: 10.3171/jns.2002.96.2.0269. [DOI] [PubMed] [Google Scholar]
- 32.Yelnik J, Damier P, Demeret S, et al. Localization of stimulating electrodes in patients with Parkinson disease by using a three-dimensional atlas-magnetic resonance imaging coregistration method. J Neurosurg. 2003 Jul;99(1):89–99. doi: 10.3171/jns.2003.99.1.0089. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama T, Sugiyama K, Nishizawa S, et al. The optimal stimulation site for chronic stimulation of the subthalamic nucleus in Parkinson's disease. Stereotact Funct Neurosurg. 2001;77(1-4):61–67. doi: 10.1159/000064598. [DOI] [PubMed] [Google Scholar]
- 34.D'Haese PF, Cetinkaya E, Konrad PE, Kao C, Dawant BM. Computer-Aided Placement of Deep Brain Stimulators: From Planning to Intraoperative Guidance. IEEE Trans on Medical Imaging. 2005;24(11):1469–1478. doi: 10.1109/TMI.2005.856752. 11. [DOI] [PubMed] [Google Scholar]
- 35.Finnis KW, Starreveld YP, Parrent AG, Sadikot AF, Peters TM. Three dimensional database of subcortical electrophysiology for image-guided stereotactic functional neurosurgery. IEEE Trans on Medical Imaging. 2003;22(11):93–104. doi: 10.1109/TMI.2002.806567. 11. [DOI] [PubMed] [Google Scholar]
- 36.Guo T, Finnis KW, Parrent AG, Peters TM. Development and application of functional databases for planning deep-brain neurosurgical procedures. Lecture Notes in Computer Science (LNCS) for Med Image Comput Comput Assist Interv (MICCAI) 2005;3749:835–842. doi: 10.1007/11566465_103. [DOI] [PubMed] [Google Scholar]
- 37.Duerden EG, Finnis KW, Peters TM, Sadikot AB. Three-dimensional somatotopic organization and probabilistic mapping of motor responses from the human internal capsule. J Neurosurg. 2011;114(6):1706–1714. doi: 10.3171/2011.1.JNS10136. 6. [DOI] [PubMed] [Google Scholar]
- 38.Luis Luján J, Noecker AM, Butson CR, et al. Automated 3-Dimensional Brain Atlas Fitting to Microelectrode Recordings from Deep Brain Stimulation Surgeries. Stereotact Funct Neurosurg. 2009;87:229–240. doi: 10.1159/000225976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lemaire JJ, Coste J, Ouchchane L, et al. Brain mapping in stereotactic surgery: a brief overview from the probabilistic targeting to the patient-based anatomic mapping. Neuroimage. 2007;37(Suppl 1):S109–115. doi: 10.1016/j.neuroimage.2007.05.055. [DOI] [PubMed] [Google Scholar]
- 40.Nowinski WL, Belov D, Thirunavuukarasuu A, Benabid AL. A probabilistic functional atlas of the VIM nucleus constructed from pre-, intra- and postoperative electrophysiological and neuroimaging data acquired during the surgical treatment of Parkinson's disease patients. Stereotact Funct Neurosurg. 2005;83(5-6):190–196. doi: 10.1159/000091082. [DOI] [PubMed] [Google Scholar]
- 41.Pallavaram S, Dawant BM, Remple M, et al. Effect of brain shift on the creation of functional atlases for deep brain stimulation surgery. Int J Comput Assist Radiol Surg. 2005;3:221–228. doi: 10.1007/s11548-009-0391-1. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Castro FJ, Pollo C, Cuisenaire O, Villemure JG, Thiran JP. Validation of experts versus atlas-based and automatic registration methods for subthalamic nucleus targeting on MRI. International Journal of Computer Assisted Radiology and Surgery. 2006;1:5–12. 1. [Google Scholar]
- 43.Castro FJ, Pollo C, Meuli R, et al. A cross validation study of deep brain stimulation targeting: from experts to atlas-based, segmentation-based and automatic registration algorithms. IEEE Trans Med Imaging. 2006;25(11):1440–1450. doi: 10.1109/TMI.2006.882129. 11. [DOI] [PubMed] [Google Scholar]
- 44.Chakravarty MM, Sadikot Af, Germann J, Bertrand G, Collins DL. Anatomical and electrophysiological validation of an atlas for neurosurgical planning. Med Image Comput Comput Assist Interv. 2005;8(Pt. 2):394–401. doi: 10.1007/11566489_49. [DOI] [PubMed] [Google Scholar]
- 45.Chakravarty MM, Sadikot AF, Germann J, Hellier P, Bertrand G, Collins DL. Comparison of Piece-Wise Linear, Linear, and Nonlinear Atlas-to-Patient Warping Techniques: Analysis of the Labeling of Subcortical Nuclei for Functional Neurosurgical Applications. Hum Brain Mapp. 2009 Nov;30(11):3574–3595. doi: 10.1002/hbm.20780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bardinet E, Dormont D, Malandain G, Bhattacharjee M, et al. Retrospective cross-evaluation of an histological and deformable 3D atlas of the basal ganglia on series of Parkinsonian patients treated by deep brain stimulation. Med Image Comput Comput Assist Interv. 2005;8(Pt.2):385–393. doi: 10.1007/11566489_48. [DOI] [PubMed] [Google Scholar]
- 47.Yelnik J, Bardinet E, Dormont D, et al. A three-dimensional, histological and deformable atlas of the human basal ganglia. I. Atlas construction based on immunohistochemical and MRI data. Neuroimage. 2007;34:618–638. doi: 10.1016/j.neuroimage.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 48.D'Haese P-F, Pallavaram S, Kao C, Neimat JS, Konrad PE, Dawant BM. Effect of data normalization on the creation of neuro probabilistic atlases. Stereotact Funct Neurosurg. 2013;91(3):148–153. doi: 10.1159/000345268. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D'Haese PF, Pallavaram S, Li R, et al. CranialVault and its CRAVE tools: a clinical computer assistance system for Deep Brain Stimulation (DBS) therapy. Med Image Anal. 2012;16(3):744–753. doi: 10.1016/j.media.2010.07.009. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Balachandran R, Mitchell J, Dawant BM, Fitzpatrick JM. Accuracy evaluation of MicroTargeting™ platforms for deep-brain stimulation using virtual targets. IEEE Trans Biomed Eng. 2009 Jan;56(1):37–44. doi: 10.1109/TBME.2008.2002110. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D'Haese PF, Pallavaram S, Konrad PE, Neimat JS, Fitzpatrick JM, Dawant BM. Clinical accuracy of a customized stereotactic platform for deep-brain stimulation after accounting for brain shift. Stereotact Funct Neurosurg. 2010;88:81–87. doi: 10.1159/000271823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fitzpatrick JM, Konrad PE, Nickele C, Cetinkaya E. Accuracy of customized miniature stereotactic platforms. Stereotact Funct Neurosurg. 2005;83(1):25–31. doi: 10.1159/000085023. 1. [DOI] [PubMed] [Google Scholar]
- 53.Rohde GK, Aldroubi A, Dawant BM. The adaptive bases algorithm for intensity-based nonrigid image registration. IEEE Trans On Medical Imaging. 2003;22(11):1470–1479. doi: 10.1109/TMI.2003.819299. [DOI] [PubMed] [Google Scholar]
- 54.Maes F, Collignon A, Suetens P. Multimodality image registration by maximization of mutual information. IEEE Trans on Medical Imaging. 1997;16(2):187–198. doi: 10.1109/42.563664. 2. [DOI] [PubMed] [Google Scholar]
- 55.Wells WM, Viola P, Atsumi H, Nakajima S, Kikinis R. Multi-modal volume registration by maximization of mutual information. Medical Image Analysis. 1996;1(1):35–52. doi: 10.1016/s1361-8415(01)80004-9. [DOI] [PubMed] [Google Scholar]
- 56.Pallavaram S, Dawant BM, Koyama T, et al. Validation of a fully automatic method for the routine selection of the anterior and posterior commissures in MR images. Stereotact Funct Neurosurg. 2009;87:148–154. doi: 10.1159/000209295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Starr PA. Placement of deep brain stimulators in subthalamic nucleus or globus pallidus internus: Technical approach. Stereotact Funct Neurosurg. 2002;79:118–145. doi: 10.1159/000070828. [DOI] [PubMed] [Google Scholar]
- 58.Rodriguez-Oroz MC, Rodriguez M, Guridi J, et al. The subthalamic nucleus in Parkinson's disease: somatotopic organization and physiological characteristics. Brain. 2001 Sep;124(Pt 9):1777–1790. doi: 10.1093/brain/124.9.1777. [DOI] [PubMed] [Google Scholar]


