Abstract
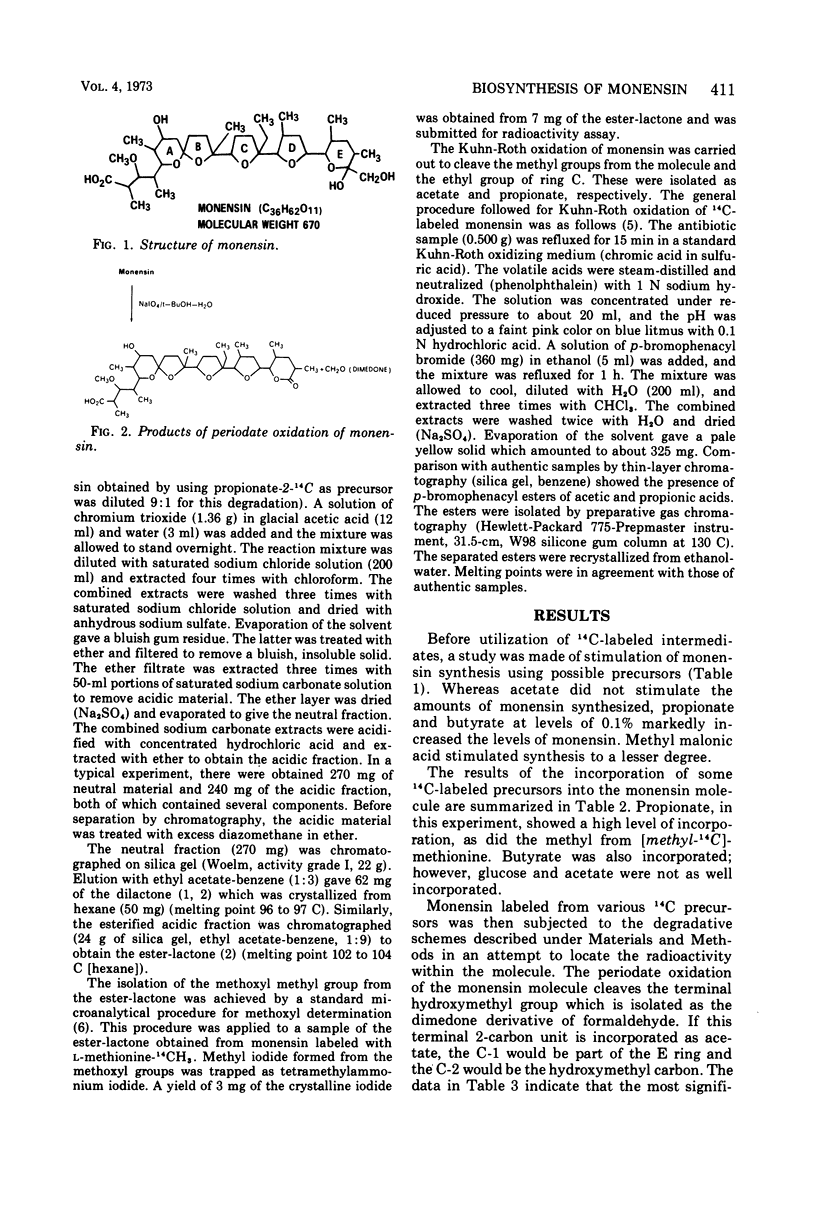
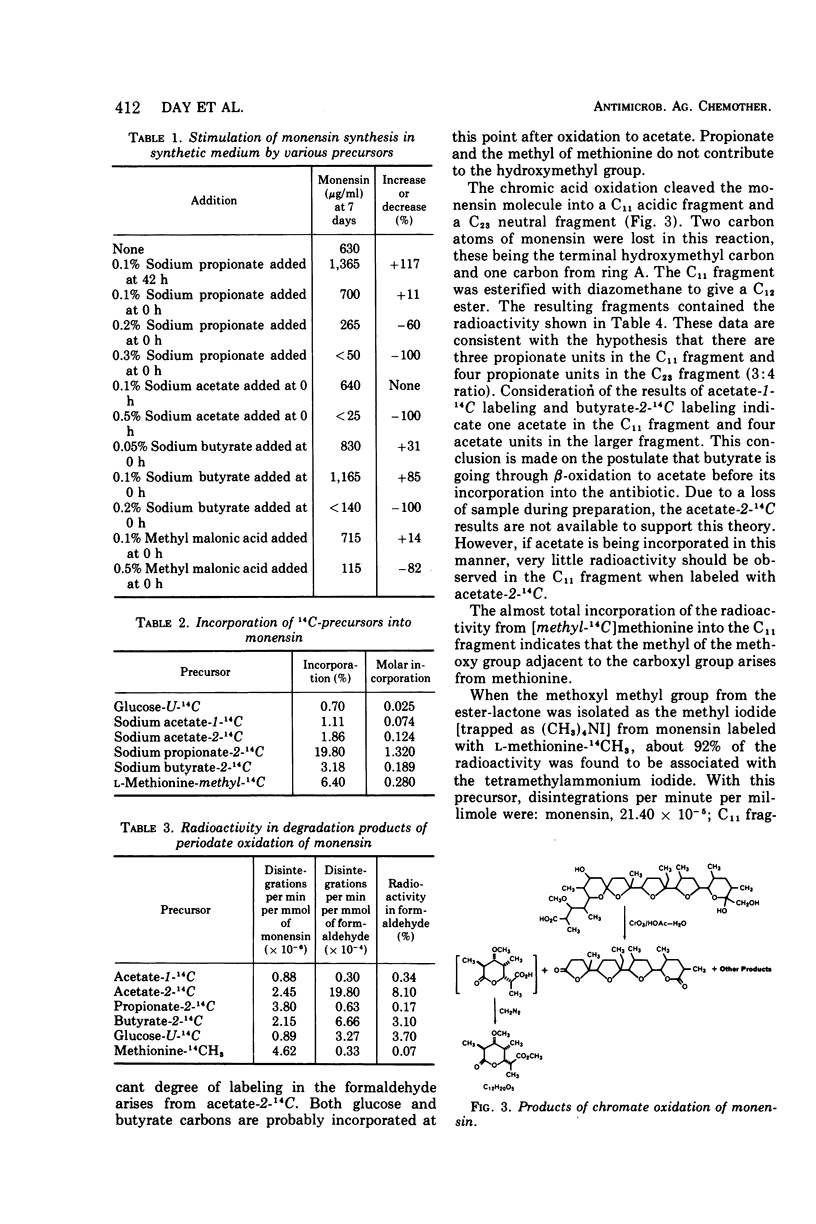
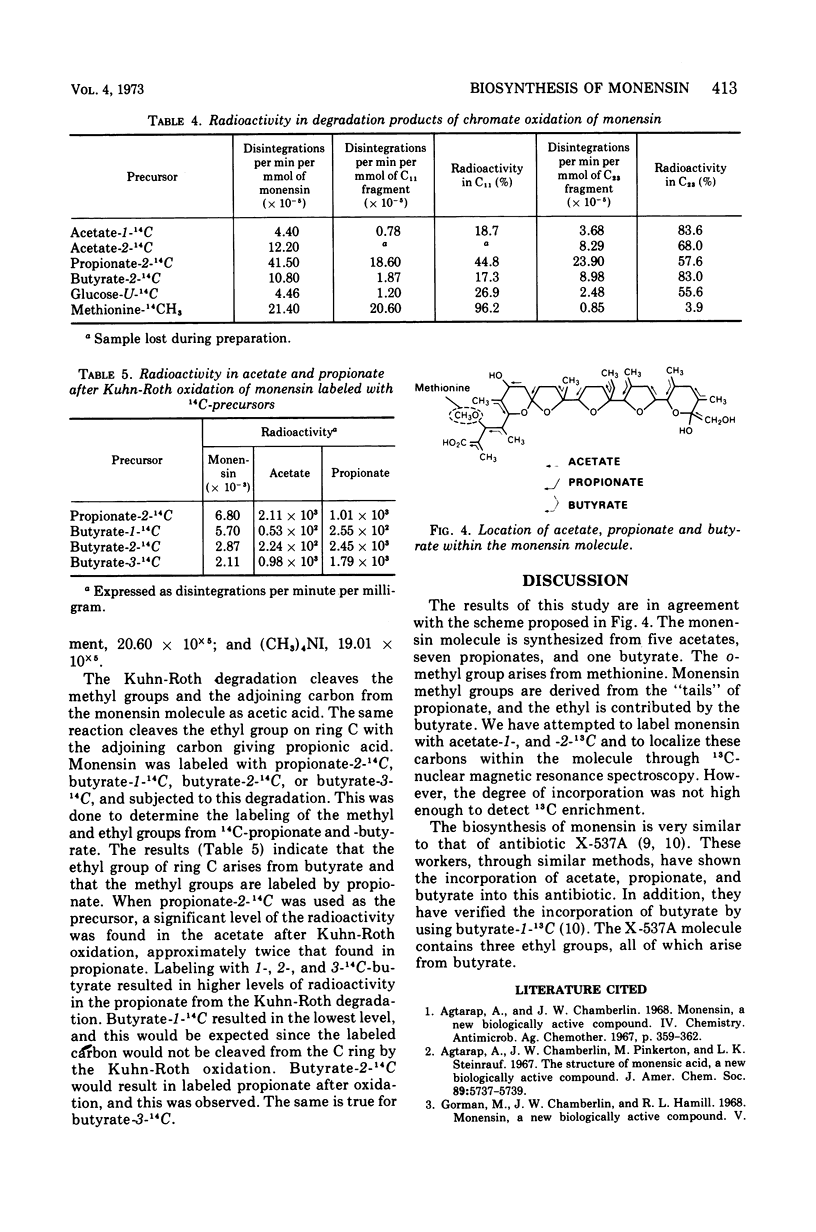
The biosynthesis of monensin by Streptomyces cinnamonensis was studied by using 14C-labeled glucose, acetate, propionate, butyrate, and methionine. The results indicated that the antibiotic is synthesized from five acetate, seven propionate, and one butyrate molecules. The o-methyl group of monensin is derived from methionine, whereas the terminal hydroxymethyl group is incorporated from acetate.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agtarap A., Chamberlin J. W. Monensin, a new biologically active compound. IV. Chemistry. Antimicrob Agents Chemother (Bethesda) 1967;7:359–362. [PubMed] [Google Scholar]
- Agtarap A., Chamberlin J. W., Pinkerton M., Steinrauf L. The structure of monensic acid, a new biologically active compound. J Am Chem Soc. 1967 Oct 25;89(22):5737–5739. doi: 10.1021/ja00998a062. [DOI] [PubMed] [Google Scholar]
- Haney M. E., Jr, Hoehn M. M. Monensin, a new biologically active compound. I. Discovery and isolation. Antimicrob Agents Chemother (Bethesda) 1967;7:349–352. doi: 10.1128/AAC.7.3.349. [DOI] [PubMed] [Google Scholar]
- Manwaring D. G., Rickards R. W., Gaudiano G., Nicolella V. The biosynthesis of the macrolide antibiotic lucensomycin. J Antibiot (Tokyo) 1969 Nov;22(11):545–550. doi: 10.7164/antibiotics.22.545. [DOI] [PubMed] [Google Scholar]
- Stark W. M., Knox N. G., Westhead J. E. Monensin, a new biologically active compound. II. Fermentation studies. Antimicrob Agents Chemother (Bethesda) 1967;7:353–358. [PubMed] [Google Scholar]


