The detection and monitoring of bacterial gene expression in the environment have become integral aspects of microbial ecology, bioremediation, and diversity monitoring. The relatively recent introduction of molecular techniques for quantification of gene expression from complex environmental samples has started to create a greater understanding of the roles and diversity of many bacterial populations. However, to date it is estimated that only 1% of environmental microbes has been identified and cultivated (3). This small percentage has been partially attributed to cumbersome culture techniques, the unculturability of many microbes, and the slow progression of molecular tools into environmental analysis, which has hindered research in this field. The resurgence of the application of molecular tools for expression analysis from environmental sources is generally seen as a consequence of reduced technology costs, together with more effective methods for recovery of nucleic acids from environmental samples (83) and increasing amounts of data on the quantitative systems that are available. Applications are wide ranging, from quantitative analysis of rRNA genes in microbial communities (10) to detection of specific pathogens (54, 55) and quantification of specific genes involved in biodegradative processes (59).
Various methods that have been used to quantify mRNA from environmental samples include in situ hybridization techniques (2, 10, 23, 84), RNase protection assays (93, 101), Northern blotting (105), and reverse transcription (RT)-PCR (1, 71). Indeed, many of these techniques are still used frequently for gene expression analysis in both prokaryotic and eukaryotic systems. In theory each of the above-mentioned techniques can be used in conjunction with other techniques and assays to detect specific RNAs and precisely determine expression levels. Northern blotting is the only method that will provide information regarding transcript size and the integrity of RNA samples, whereas RNase protection assays offer the easiest way to simultaneously examine multiple messages. In situ hybridization can be used to localize expression of a specific gene(s) to a given cell but remains the most complex of all methods. RT-PCR permits analysis of gene expression at the level of a single cell and can be conducted on a large number of samples and many different genes in the same experiment (98). The one major disadvantage of standard RT-PCR with respect to other mRNA detection techniques such as Northern blotting is that it is only semiquantitative because of the kinetics of PCR product accumulation. Consequently, there is no linearity in the relationship between product yield and the initial template concentration (32, 34, 108). As RT-PCR offers the most sensitive and flexible method for detecting expression of individual or multiple genes, it has been increasingly coupled with various other strategies for absolute quantification. In competitive RT-PCR (cRT-PCR), known amounts of an internal standard are coamplified in the same reaction tube with a sequence of interest, allowing the expression levels of the gene(s) under investigation to be determined (32).
Real-time PCR assays used for microbial gene expression analysis combine the best attributes of both relative and cRT-PCR, in that they are extremely sensitive, rapid, capable of high throughput, and relatively easy to perform (34, 47). With the ability to measure PCR products as they accumulate or in “real time,” it has become possible to measure the amount of PCR product accumulated during the exponential phase (21, 47). Alternatively, microarray analysis offers the potential to monitor and compare the expression patterns of thousands of mRNA species simultaneously. Microarray analysis provides a vehicle for exploring a genome in a way that is both systematic and comprehensive (19). The possible and future applications that microarray analysis may provide in environmental microbiology are endless. The continual focus on attaining maximal sensitivity with increased rapidity of quantitative techniques has led to the development of a new generation of technologies that employ different principles and strategies to achieve precise quantification. While there are various comprehensive review articles that cover individual quantification assays (2, 8, 19-21, 24, 32, 46, 47, 65, 66, 67) of both eukaryotes and prokaryotes, this review aims to explore, in parallel, the applications of the most recent quantification assays and to identify developing technologies for gene expression analysis in environmental bacteriology. Obviously, understanding the capacity and limitations of these molecular techniques and emerging technologies will be critical for all future research in this field. Such technologies hold the key to increasing our knowledge and understanding of what still remains a relatively unknown microbial world.
QUANTIFICATION OF TOTAL RNA CONCENTRATIONS
Prior to any quantitative gene expression studies it is essential, in many cases, to precisely quantify total concentrations of the nucleic acid being investigated. The traditional method for determining total RNA concentrations is UV spectroscopy (A260/280). Recently the use of certain fluorescent probes has proved more accurate in determining RNA concentrations. The performance of these techniques is dependent on fluorochrome labels with a high sensitivity and high resistance to photobleaching. Numerous fluorescent probes, including magdala red (98, 106), hypocrellin A (107), thiazole orange homodimer, oxazole yellow homodimer (77), and ethidium bromide (97), have been used for quantification of RNA. RiboGreen (Molecular Probes), another fluorescent probe for RNA quantification in solution, and arguably the most sensitive RNA probe available, allows concentrations as low as 1 ng ml−1 to be quantified with a standard spectrofluorimeter. The assay has been shown to be 1,000-fold more sensitive than UV absorbance (RiboGreen product information sheet), 200-fold more sensitive than ethidium bromide staining (52), and 2-fold more sensitive than SYBR Green staining (79). The enhanced sensitivity of this probe is partially due to its unique ability to maintain linearity in the presence of organic compounds, including salts, detergents, and organic solvents that are frequently used in the purification of RNA from environmental samples (43). Such reagents are known to significantly influence UV spectrophotometric analysis. Molecular Probes has also developed a fluorescence-based probe (PicoGreen) for quantitation of DNA, with increased sensitivity over absorbance-based methodologies (per the PicoGreen product information sheet). Limitations of absorbance methodologies over fluorimetric techniques include the relative contribution of organic compounds, proteins, and free nucleotides to the absorbance signal. However, the RiboGreen assay is not without limitations. Similar to UV absorbance methods, the removal of all genomic DNA from RNA samples is critical prior to fluorimetric analysis to prevent overestimated fluorescence readings. As the RiboGreen probe is susceptible to photodegradation, it is also essential to keep the reagent light free at all times.
A possible emerging technology for rapid quantification of nucleic acids is the Agilent 2100 Bioanalyzer (Agilent Technologies), a chip-based nucleic acid separation system. The bioanalyzer utilizes a combination of microfluidics, capillary electrophoresis, and fluorimetry to determine RNA integrity and concentration (27). Using an RNA 6000 standard kit (Ambion) for quantifying RNA, the instrument software automatically compares the peak areas from unknown RNA samples to the combined areas of the six RNA 6000 Ladder peaks to determine concentrations of the samples under investigation (36, 68). The Agilent Bioanalyzer has a broad dynamic range and can quantify RNA concentrations between 25 and 500 ng ml−1 with a covariance of ∼10%.
QUANTIFICATION OF SPECIFIC MESSENGER RNAs
RT-PCR: the basics.
RT has revolutionized gene expression analysis. It is now theoretically possible to detect transcripts from any gene regardless of abundance of specific mRNAs. The initial step in RT-PCR is the production of a single-strand cDNA copy of the RNA using the retroviral enzyme RT (32) followed by exponential amplification by PCR (20, 21). The template for RT-PCR can be poly(A)+ RNA (eukaryotes) or total RNA from bacteria. In bacterial systems the RT reaction is usually primed with gene-specific primers. The reaction can be performed in either one-step or two-step formats. In the one-step format the RT reaction and PCR take place sequentially in a single tube, whereas in the two-step format each step is performed under optimal conditions in separate tubes, where 10% of the RT reaction product is subjected to PCR cycling (32, 66). Two-step RT-PCR is popular and useful for detecting multiple messages from the same sample, whereas one-step RT-PCR is more advantageous when processing multiple samples, as carryover contamination is minimized.
The most commonly used RTs are avian myeloblastosis virus (AMV) RT and Moloney murine leukemia virus (MmLV) RT. AMV RTs are more robust than MmLV RTs (18) and can retain significant polymerization activity up to 65°C. This is important if the template RNA has significant secondary structures. Cloned MmLV and AMV genes have been engineered to produce novel enzymes that are RNase H negative. RNase H competes with the polymerase for the hybrid formed between the RNA template and the DNA primer or growing cDNA strand and degrades the RNA strand of the RNA-DNA complex. Generally AMV RTs are better suited for synthesis of short cDNAs and MmLV RTs are better suited for generating longer amplicons (66).
The use of RT-PCR for monitoring gene expression in environmental microbes is being increasingly employed. It has recently been used to detect expression of the genes involved in degradation of the pollutant chlorobenzene (1). Additional applications have included detection of the catabolic gene nahAc from Pseudomonas putida, which is responsible for biodegradation of polycyclic aromatic hydrocarbons (56); analysis of the oprD gene of Pseudomonas aeruginosa (71); monitoring the expression of the cytotoxin-hemolysin virulence gene vvhA from environmental isolates of Vibrio vulnificus (29); determination of mRNA recovery rates from preserved prokaryotic samples (7); and phylogenetic analysis of Nostocoida limicola isolates (85).
cRT-PCR.
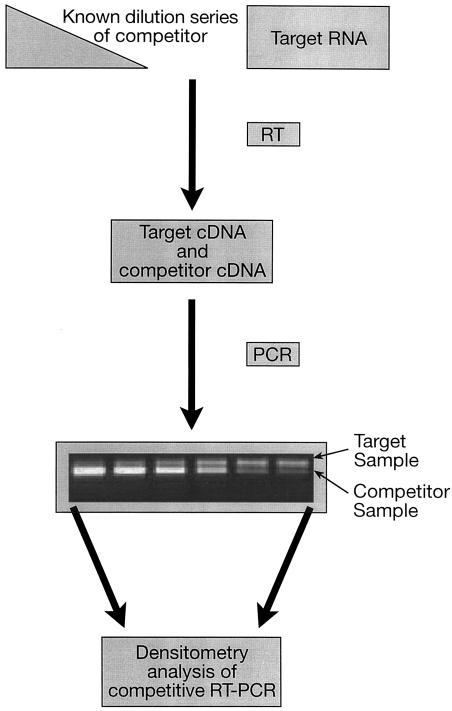
Because of its inherent sensitivity and ease of application, RT-PCR has been readily coupled with other protocols for absolute quantification purposes. In cRT-PCR, a dilution series of a competitor (internal standard) is coamplified with known amounts of total RNA in the same reaction tube. The competitor has the same primer binding sites as the target sequence but is usually modified by creating a small deletion, insertion, or mutation to distinguish it during electrophoresis (74, 87). The internal standard competes with the native sequence of the gene(s) of interest for primers, deoxynucleoside triphosphates, enzyme, and other reagents, thus reducing the signal of the native gene when the standard is in excess. As the amount of the internal standard increases, the signal of the native gene decreases (Fig. 1). The diagram in Fig. 1 illustrates the cRT-PCR assay designed to quantify toxin gene expression in type E Clostridium botulinum (82).
FIG. 1.
cRT-PCR technique. cRT-PCR assay used to quantify toxin-encoding mRNA of C. botulinum. Reproduced from reference 81 with permission.
Coamplifying an internal standard provides an efficient method of relating the product yield to the initial amount of transcript (104). Since both the internal standard and target sequence of interest are presumed to be amplified with nearly equal efficiency, the product will accumulate with approximately the same kinetics, even when the PCR reagents are limiting. Therefore, unlike real-time PCR monitoring, it is not essential for amplification to be in the exponential phase when PCR amplification progress is being measured. In environmental microbiology, cRT-PCR is especially advantageous in cases where environmental samples may contain contaminants including organic solvents and humic acids. Their effects are relative because the amplification kinetics will remain identical for both target and standard sequences in the presence of inhibitors. From a review of the literature, it is apparent that there are two common approaches to competitor design.
(i) Internal standards.
Housekeeping genes such as that encoding glyceraldehyde 3-phosphate dehydrogenase or dihydrofolate reductase are frequently used as endogenous internal standards for eukaryotic gene expression analysis (46, 92). However, the use of such standards can be challenging and suffers from varying expression patterns (8, 67, 92). The use of such standards has not been applied in environmental gene expression studies. The alternative approach, which has proved a lot more popular in prokaryotic gene expression analysis in particular, involves the use of an exogenous internal standard. These exogenous standards are generally created from the entire native gene or part of it and cloned into a plasmid containing an RNA polymerase promoter that is suitable for in vitro transcription. This RNA standard is an in vitro-transcribed synthetic RNA with the same primer binding sites as the target RNA sequence and has the same sequence apart from a small deletion, insertion, or mutation to distinguish it during electrophoresis (108). It appears that there are no general rules or strategies for the choice of these modifications. The variation in size, if maintained within an acceptable range (10 to 15% of the original size) does not modify or interfere significantly with the amplification rate of the PCR (32). In cRT-PCR, a dilution series of the internal standard is coamplified with equal amounts of total RNA. Thereby, it is possible to determine the amount of mRNA or cDNA transcripts in a sample by comparing the signal intensity from known amounts of the internal standard to the signal intensity of the target sample using sophisticated image analysis software (67, 74).
(ii) General considerations.
The internal control is designed with several considerations in mind. If the assay is to detect and quantify RNA, then a standard should be chosen that requires RT. If the analytical goal is absolute quantification of a nucleic acid sequence of interest, then a standard of known concentration should be used (46). It is now generally accepted that DNA standards are not an optimal choice because they do not compensate for the variations (variability can be up to 100%) in the RT reaction, which has been shown to be the source of most variability in RT-PCR experiments [32; Ambion Technotes Newsl. 6(3):2-14, 1999]. It has been reported that DNA competitors greatly underestimate the concentration of target molecules in any given sample. This primary cause of error is the efficiency with which individual RNA molecules are converted to amplification-competent cDNA molecules [Ambion Technotes Newsl. 6(1):1-15, 1999]. RNA competitors control for this variability (variability of less than 10%) because they are dependent on the RT reaction to become amplification-competent cDNA. Ambion has also demonstrated that RNA competitors are far less affected by tube-to-tube variation than internal DNA standards [Ambion Technotes Newsl. 6(3):2-14, 1999].
A common debate in cRT-PCR analysis concerns where the cutoff point for quantification lies. The PCR has two distinct phases, the exponential phase and the plateau phase of amplification. In the exponential phase, theoretically, every cDNA is denatured, bound by a primer, and copied by DNA polymerase, whereas in the plateau phase, reaction components become limiting. It is generally acknowledged that when the competitive PCR products are amplified with equal efficiencies it is not critical to be in the exponential phase (65, 72). Numerous reports have successfully documented quantification of nucleic acids by cRT-PCR in the plateau phase (9, 24, 25, 69). Freeman et al. (32) reported that equal amounts of initial template gave various signals in the plateau phase and equal signals in the exponential phase. However, Russell (76) countered these claims and found no significant variation.
Optimization of PCR cycling has been shown to be essential in limiting heteroduplex formation in cRT-PCR assays. A heteroduplex may arise during amplification when a hybrid forms by one strand of the target annealing to one strand of the competitor, producing a secondary structure which appears as a third band above the cRT-PCR products when visualized by electrophoresis. Confirmation that this third band is a result of heteroduplexing can be carried out using the single-stranded DNA-specific S1 nuclease assay, which digests the unannealed portion of the hybrid, thus resolving the third band (22). It has been reported that heteroduplex formation is promoted in the plateau phase when primers and reagents become limiting (32). The presence of heteroduplexes has to be taken into account to prevent over- or underestimation of quantification products together with a substantial loss of sensitivity. Several approaches have been reported to minimize the negative impact of heteroduplex formation on cRT-PCR accuracy (22, 40).
It is also important to consider that because of changes in cell morphology and composition at different phases of growth, with possibly varying levels and stabilities of specific nucleic acids, uniformity in bacterial cell contents and volume cannot be assumed for different growth phases. For cRT-PCR analysis, two means of determining copy numbers of mRNA are generally considered for prokaryotes: (i) copy numbers of specific mRNAs per total RNA and (ii) copy numbers of specific mRNAs per viable cell. Calculation of copy levels of encoding mRNAs per total RNA is assumed to be the most accurate method for such analysis, as differences in cell volume and contents are taken into account. This method also takes into account possible variations associated with calculations of viable cell counts when the bacteria are grown under different environmental conditions or to different growth phases.
(iii) Environmental applications of cRT-PCR.
cRT-PCR has been widely employed in the quantification of cellular DNA and RNA, as well as viral and bacterial mRNAs. While still not routinely employed for environmental monitoring purposes because of the extensive development and optimization required, CRT-PCR has become more popular in recent years. It has been applied to quantification of chloroaromatic-degrading Pseudomonas species strain B13 in marine water or sediment (53), genetically tagged cyanobacteria in Baltic Sea sediment (62), and ammonia-oxidizing Proteobacteria in compost (49). cRT-PCR techniques have also been used to quantify tcbC gene expression involved in 1,2,4-trichlorobenzene degradation (59), toxigenesis in the aquatic pathogen C. botulinum type E (58, 82), and amoA expression levels in Nitrosomonas oligotropha and Nitrospira species (28). An advanced cRT-PCR assay has also been used with real-time technology to determine copy levels of the carbazole 1,9a-dioxygenase gene in Pseudomonas species (100).
Real-time PCR technology and environmental analysis.
The coupling of RT-PCR with fluorescence techniques and modern technology capable of automated detection and quantification of specific mRNAs has led to the development of new technologies that have dramatically changed gene expression analysis studies. Real-time RT-PCR quantifies the initial amount of the template under investigation specifically, sensitively, and reproducibly and has become a preferable alternative to other quantitative RT-PCR systems, which detect the amount of final amplified product. Real-time PCR monitors the fluorescence emitted during the exponential phase of the reaction as an indicator of amplicon production during each PCR cycle (i.e., in real time) as opposed to the endpoint detection by more conventional quantitative RT-PCR methods. The real-time progress of the reaction can even be viewed with some systems as the product accumulates. Real-time RT-PCR does not detect the size of the amplicon and thus does not allow the differentiation between DNA and cDNA amplification; however, it is not influenced by nonspecific amplification unless DNA binding probes such as SYBR Green are used. Real-time PCR quantitation eliminates post-PCR processing of PCR products (which is necessary in cRT-PCR). This helps to increase throughput, and reduce the chances of carryover contamination and removes post-PCR processing as a potential source of error. In comparison to conventional RT-PCR, real-time PCR also offers a much wider dynamic range of up to 107-fold (compared to 1,000-fold in conventional RT-PCR). Data analysis, including standard curve generation and calculation of copy numbers of specific mRNAs, is performed automatically. As more data and knowledge of the various systems and chemistries become available, real-time PCR will surely become a more reliable and accurate alternative for quantifying genes of interest from environmental sources.
(i) Real-time PCR instrumentation.
Currently there are several different systems available to choose from. For real-time PCR analysis, the instrumentation platform consists of a thermal cycler, computer, optics for fluorescence excitation and emission collection, and software for data acquisition and analysis. The various systems that are available differ in sample processing capacity, format, and dynamic range (Table 1). The first commercially available platform for real-time PCR was the ABI Prism 7700 sequence detection system from Applied Biosystems. This particular model is no longer manufactured because of difficulties in acquiring spare parts for the system. Similar problems were also experienced for the GeneAmp 5700 system, which has also resulted in its discontinuation. Moves toward more-advanced technologies have resulted in the development of model 7000 and the more recent edition, 7900 HT. All systems have been optimized for use of a 5′-exonuclease assay that employs the enzyme Taq polymerase, although each system can be additionally used with other detection chemistries. The ABI Prism 7000 model has the capacity to monitor PCR status during data collection and can distinguish between 5,000 and 10,000 template copies with 99% discrimination. The ABI Prism 7900 HT system has been designed specifically for high-throughput applications.
TABLE 1.
Various real-time PCR systems and formats that are currently available, including supplier information
| Manufacturer | Real-time system | Normal sample format | Maximum no. of samples | Dynamic range (orders of magnitude) |
|---|---|---|---|---|
| Applied Biosystems | ABI Prism 7000 | Microplate | 96 | 5 |
| ABI Prism 7900 HT | Microplate | 384 | 5 | |
| Roche Biochemicals | LightCycler | Capillaries | 32 | 7 |
| Corbett Research | Rotor-Gene 3000 | Strip tubes | 72 | 7 |
| Bio-Rad | iCycler | Microplate, strip tubes | 96 | 6 |
| MyiQ | Microplate, strip tubes | 96 | 8 | |
| Cepheid | SmartCycler | Microplate, strip tubes | 96 | 8 |
| Stratagene | Mx3000P | Microplate, strip tubes | 96 | 7 |
| Mx4000P | Microplate, strip tubes | 96 | 7 | |
| MJ Research | Opticon | Microplate, strip tubes | 96 | 8 |
| Opticon 2 | Microplate, strip tubes | 96 | 10 | |
| Techne | Quantica | Microplate | 96 | 6 |
The LightCycler system (Roche) enables RT-PCR to be carried out in small capillaries, capable of holding up to 20 μl of sample, contained within a rotor-like carousel that is heated and cooled in an air stream (102). One of the greatest advantages that the LightCycler instrument offers in comparison to conventional thermal cyclers is that the formation of amplification products can be monitored in real time. Currently, the LightCycler system supports two fluorescence-based methods for the detection of amplification products: SYBR Green and hybridization probes. The undoubted advantage that the LightCycler system offers over all other real-time systems is reaction speed. High-speed thermal cycling is achieved using air instead of thermal blocks. Due to the special design of the thermal chamber, samples are held under uniform temperature conditions. The unique capillary sample tube system ensures efficient heat transfer to the PCR samples. As a result, the time needed for each PCR cycle, including measurement of the sample fluorescence, is minimized to approximately 15 to 20 s. A 30-to 40-cycle PCR run is typically completed within 20 to 30 min, with quantitative RT-PCR methods capable of being completed in less than 1 h. However, disadvantages include the use of capillaries as opposed to tubes, with capillaries being less practical for most investigators due mainly to fragility and the small sample format, which allows only 32 wells to be analyzed simultaneously (not taking into account duplicate or triplicate sample formats). A system quite similar to the LightCycler is the centrifugal thermal cycler Rotor-Gene (Corbett Research). Its multifilter system can detect all available real-time chemistries, including SYBR Green, TaqMan, and molecular beacons. Data validating the use of the Rotor-Gene instrument in environmental situations are not yet available.
The iCycler and MyiQ single-color detection systems from Bio-Rad have also not been evaluated extensively for environmental samples. The iCycler system enables four different fluorophores to be multiplexed per sample tube. It also enables up to 96 samples to be tracked simultaneously, maximizing throughput analysis substantially. The more recent model from Bio-Rad, MyiQ, offers a more affordable alternative to the iCycler. This system interfaces directly with the iCycler thermal cycler, offering superior features such as the thermal gradient and Peltier effect-driven performance. Despite offering a somewhat lower range of detectable excitation and emission wavelengths, it does demonstrate a superior dynamic range. The Smart Cycler from Cepheid also offers researchers the capability of rapid quantification, comparable to that of the LightCycler. They can be operated also with a range of chemistries. Another distinctive feature of the Smart Cycler is the presence of 16 different modules or independently controlled reaction sites per processing block, which offers a unique flexibility to the researcher.
More recent options include the Mx4000 and Mx3000P models from Stratagene. The Mx4000 version allows detection with multiple fluorescent PCR chemistries. The smaller and cheaper option, the Mx3000P, works with a 96-well format, can multiplex up to four dyes per reaction tube, and deals with various subexperiments per plate. The Mx3000P is the least expensive of its kind, and its primary selling point is its small footprint. Similar to the Mx4000, it is compatible with a range of dyes and chemistries, although it does have slightly lower excitation and emission ranges (88). Further options for real-time analysis include the DNA Opticon and DNA Opticon 2 continuous fluorescence detection systems from MJ Research. The latter instruments discussed here have only recently become commercially available.
(ii) Detection chemistries. (a) Hydrolysis and TaqMan probes.
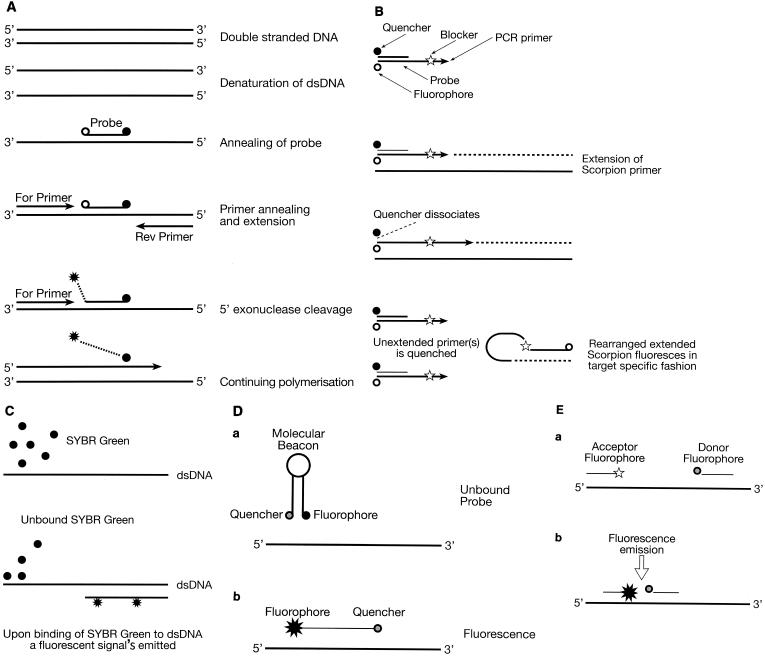
TaqMan probes derive their fluorescence signal from the hydrolysis of the probe by Taq polymerase 5′-to-3′ exonuclease activity. Such probes (Fig. 2A) usually utilize Taq polymerase, Tth polymerase, or indeed any enzyme with 5′ nuclease activity properties. Reports have suggested that not all DNA polymerases are suitable for quantitative real-time RT-PCR (35). In 5′-nuclease assays an oligonucleotide probe anneals to a target sequence located between the two primer binding sites. The probe is designed and labeled with a reporter fluorophore at the 5′ end, the emission spectrum of which is quenched by a quencher fluorophore in the middle or at the 3′ end. Reporter fluorophores that are covalently attached at the 5′ end include FAM (6-carboxyfluoroscein), TET (tetrachloro-6-carboxyfluorescein), JOE (2,7-dimethoxy-4,5-dichloro-6-carboxyfluorescein), and HEX (hexacholoro-6-carboxyfluorescein). The reporter is usually quenched by TAMRA (6-carboxytetramethylrhodamine) at the 3′ end. The probe is normally modified at the 3′ end with a blocking phosphate to prevent probe extension during amplification. During PCR amplification, cleavage of the probe separates the reporter dye and quencher dye, which results in increased fluorescence. Fluorescence emission is measured on a cycle-to-cycle basis. Hydrolysis probes eliminate the need for subsequent PCR product verification steps, thus reducing the time scale of analysis. The TaqMan hydrolysis probes for real-time PCR applications in environmental bacteriology are easily the most popular choice of all the different chemistries that are available. TaqMan probes have been used widely and diversely in detection and quantification of gene expression in environmental microbes (42, 45); detection, differentiation, and absolute quantification of pathogens (5); and quantification and enumeration of bacteria in soil (6, 41), activated sludge (37, 38), and water (31, 54, 63).
FIG. 2.
Overview of various available detection chemistries. (A) TaqMan assay; (B) Scorpions reaction; (C) SYBR Green I assay; (D) molecular beacon chemistry (a, unbound molecular beacon probe; b, binding of probe to dsDNA results in fluorescence); (E) hybridization probe (a, probes positioned in head-to-toe fashion; b, excitation of donor allows FRET to acceptor, resulting in fluorescence).
(b) Scorpions.
Scorpions are essentially fluorogenic PCR primers (Fig. 2B). They represent the most recent development in real-time PCR chemistry. Two different formats are possible, the stem-loop format and the duplex format, although the stem-loop format is the most common approach. Duplex Scorpions have been reported to have various advantages over their counterparts, including the fact they are easier to synthesize and purify and produce a more intense fluorescence signal due to the vastly increased separation between the fluorophore and quencher. However, their applications are still somewhat limited. The essential elements of Scorpions primers in both formats include a PCR primer, a PCR blocker, a specific probe sequence, and a fluorescence detection system containing at least one fluorophore and quencher (86). The primer is linked to the probe in a hairpin loop form which brings the fluorophore near the quencher and avoids fluorescence. The blocker element is essential in both Scorpions formats. Without a blocker the polymerase would be able to read through the Scorpions' primer and copy the probe region (95). After PCR extension of the Scorpions primer, the resulting amplicon contains a sequence complementary to the probe, which becomes single stranded during each denaturation step in PCR. On cooling, the probe is free to bind to a specific sequence of interest as the quencher is now dissociated completely from the fluorophore, creating an increase in fluorescence emission (99). A significant advantage derived from the use of Scorpions primers is that the probe element and primer element are physically coupled during the reaction, leading to signal generation in a unimolecular rearrangement. This contrasts with the bimolecular arrangement with other chemistry formats, including TaqMan probes and molecular beacons. The unimolecular rearrangement of the Scorpions primer is extremely advantageous as the reaction is effectively instantaneous in competing and side reactions, such as target amplicon reannealing and inappropriate target folding. This leads to more-reliable probe design, shorter reaction times, and better discrimination. Scorpions have been primarily applied to mutation detection.
(c) DNA binding dyes.
SYBR Green I is the most commonly used DNA binding dye that incorporates into double-stranded DNA (dsDNA) (Fig. 2C). It is thought that SYBR Green binds to the minor groove of dsDNA, causing fluorescence to increase. Hence, during PCR as DNA products increase exponentially, so will the fluorescence signal of SYBR Green (13, 50). However, in its free form SYBR Green has undetectable fluorescence. The most notable advantages of using SYBR Green are that it eliminates the need for complicated probe design and can be used in conjunction with any primers for any target of interest, although sensitivity can be vastly compromised by nonspecific binding in the PCR of interest (91). A disadvantage of SYBR Green is that the sensitivity of the assay may be affected by nonspecific binding to PCR artifacts such as primer dimers that may contribute to the fluorescence signal. Therefore, it is essential to optimize PCR conditions prior to the use of SYBR Green. Melting curve analysis can also be used effectively for this purpose. This is helpful for determining the sensitivity of the PCR assay. Various real-time instruments can analyze the melting curves of the reaction, including the LightCycler, Smart Cycler, iCycler, Rotor-Gene, and Mx4000 systems. When melt profiles have been established, it is then possible to set the software to acquire fluorescence signals above that of the primer dimers' melting temperature but below that of the sequence of interest. Alternatively, a hot-start enzyme has been used effectively to prevent primer dimer formation in SYBR Green assays, therefore increasing sensitivity (39). DNA binding dyes, particularly SYBR Green, have been used extensively for real-time detection and quantification purposes in environmental bacteriology (6, 44, 91). Recently Bengtsson et al. (13) reported the development of another DNA dye for real-time monitoring, BEBO, with sensitivities comparable to those of SYBR Green.
(d) Molecular beacons.
Molecular beacons are oligonucleotide probes capable of undergoing structural conformations to form a stem-loop like structure with a reporter dye covalently attached at one end and a quencher dye covalently attached at the other end (Fig. 2D). Because of the stem-loop structure, both moieties are kept in close proximity and fluorescence is quenched by a Förster-type fluorescence resonance energy transfer (FRET) mechanism, which permits a radiationless transfer of electronic excitation energy from the donor reporter fluorophore to the acceptor quencher molecule (14, 61). During FRET, a donor fluorophore, which is excited by an light-emitting diode (LED) light source, transfers its energy to an acceptor fluorophore only when positioned in the direct vicinity of the former. The acceptor fluorophore emits light of a longer wavelength, which is detected in specific channels. The LED cannot excite the acceptor dye. Conventional molecular beacons are designed with a target-binding domain flanked by two complementary short arm sequences that are independent of the target sequence (80). Upon binding of the probe to dsDNA, a probe hybrid forms that is more stable than the stem hybrid (96). This results in a conformational change that forces the arm sequences apart, leading to an increase in fluorescence emission. The use of molecular beacons for gene expression analysis has been primarily adapted for single-nucleotide polymorphism detection (61) and pathogen detection (11, 12, 30, 48, 60), with little literature published regarding their validation for environmental applications (38, 80).
(e) Hybridization probes.
The application of hybridization probes for real-time PCR monitoring involves the use of two probes to maximize specificity (Fig. 2E). Such probes utilize a single label, one with a fluorescein donor at its 3′ end and a second with an acceptor fluorophore at its 5′ end. The probes are designed in a manner which enables them to hybridize to a sequence of interest in a “head-to-toe” arrangement. This enables both probes to be within close proximity of each other, enabling FRET. The 3′ probe is blocked at its end to prevent extension during the annealing step. Excitation of the donor allows FRET to the acceptor molecule and fluorescence emission. Hybridization probes have had a limited use in comparison to the other chemistries described above and have not been validated or indeed employed to a large extent in prokaryotic systems.
(f) LUX fluorogenic primers.
The use of LUX (light up extension) primers for detection and quantification of genes on real-time platforms represents the most recent advance in real-time chemistry design. LUX primer design is based on studies that demonstrate the effects of the primary and secondary structure of oligonucleotides on the emission properties of a conjugated fluorophore. Design factors are largely attributed to the necessity of having guanosine bases in the primary sequence near the conjugated fluorophore. LUX primers utilize one single-labeled fluorogenic primer and a corresponding unlabeled primer. No probes or quenchers are needed. A hairpin structure provides fluorescence quenching of the fluorophore. When the primer is incorporated into dsDNA, the fluorophore is dequenched, significantly increasing the fluorescent LUX signal. As such primers are at an early stage of development, little literature on them is available. They do, however, appear to represent a more economical option than other detection chemistries that are relatively easy to design. Designer software is available from Invitrogen. The sequence of the gene(s) of interest is inserted into the package, and the software generates primer sets ranked in order of optimization. LUX primers also have the added possibilities for multiplexing and melting curve analysis that have distinct advantages over SYBR Green dyes and TaqMan probes.
(iii) Real-time PCR data analysis.
The standard curve method is the most common approach to determine relative quantification. Absolute quantification may also be calculated using this method, but this requires that the absolute quantities of the standard be known by some independent means. The most important parameter for quantification is the threshold cycle (CT) value. This value indicates the cycle at which a statistically significant increase in normalized reporter is first detected. Usually, this occurs when the signal detection software begins to detect the increase in signal associated with an exponential formation of PCR product. The more template is present at the beginning of the reaction, the fewer cycles it takes to reach a point in which the fluorescent signal is first recorded as statistically significant above background (33). The CT value is always calculated during the exponential phase of the amplification reaction. CT values can be translated into a quantitative result by constructing a standard curve. Software can then generate a standard curve plot with the log (nanograms) input amount of RNA in each well as the x values and CT as the y values. Standard curves are prepared for both the target and an endogenous reference such as β actin, glyceraldehyde-3-phosphate dehydrogenase, or 18S amplicon rRNA. For each experimental sample, the amount of target and endogenous reference is determined from the appropriate standard curve. Then, the target amount is divided by the endogenous reference amount to obtain a normalized target value. One of the experimental samples is selected as the calibrator. Each of the normalized target values is divided by the calibrator normalized target value to generate the relative levels of expression. Thus, the normalized amount of target is a unitless number and all quantities are expressed relative to the calibrator.
(iv) Microarray analysis: the technology of the future?
Microarrays are a revolutionary tool for measuring the expression levels of a large number of genes simultaneously. Microarray analysis represents the most recent advance in quantitative gene expression measurement but as yet has been used sparingly for environmental analysis. The fundamental principle underlying a microarray experiment for gene expression studies is that mRNA from a given tissue or cell line is used to generate a labeled sample, sometimes referred to as a target, which is hybridized in parallel to a large number of DNA sequences that are immobilized onto a solid surface in an ordered array (78). Commercially available microarrays are treated glass slides with up to 8,000 genes or probes per cm2. Expression levels are conventionally presented as ratios of mRNA concentrations of treated sample relative to a control sample. For example, diseased tissue can be compared to healthy tissue or cells subjected to different treatments and can be compared to cells grown under normal conditions.
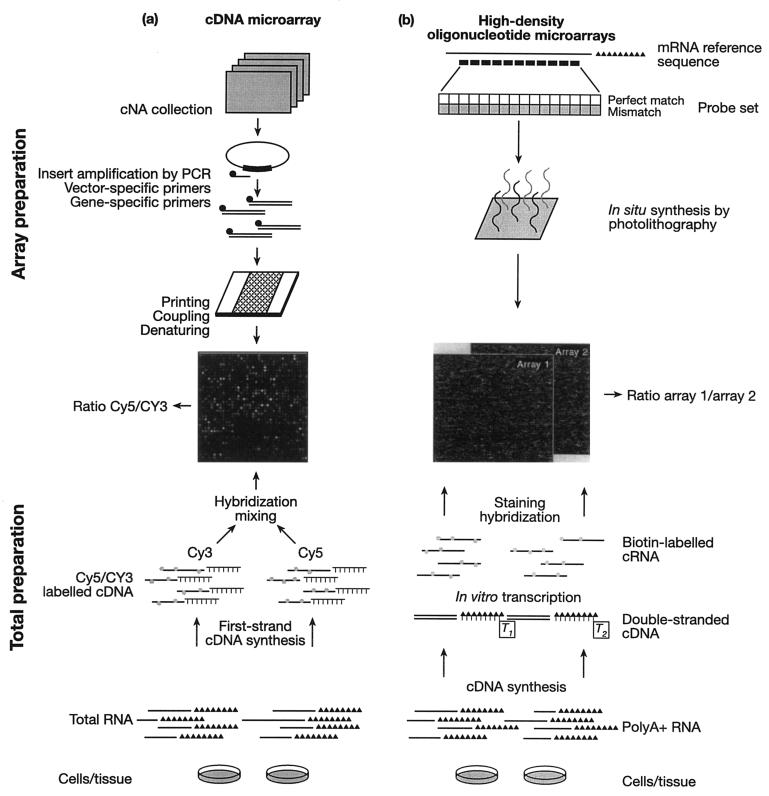
There are generally two different formats for microarrays, cDNA arrays and oligonucleotide arrays (Fig. 3). Arguably the most widely used is the two-color cDNA microarray. The spotted DNA probes are PCR products generated by amplifying genomic DNA with gene-specific primers (chosen so that overlaps in homology between probes for different genes are minimized). DNA is spotted onto the slide by a robot, which deposits a few nanoliters of DNA in solution to form a spot 100 to 200 μm in diameter. mRNA from the treated and control samples is purified and reverse transcribed to cDNA with fluorescence-labeled nucleotides, one label each for the treatment and control samples. The most commonly used fluorophores are Cy3 and Cy5, which emit light in the green and red spectrum, respectively, linked to dCTP or dUTP. The labeled cDNA from both samples is then mixed and hybridized to the array. After unbound target cDNA has been washed away, the array is scanned with a laser at the emission frequencies of the two fluorophores, generating one red and one green image. When the images are overlaid, spots hybridized with equal amounts of treatment and control cDNA will be yellow, and spots for genes that are differentially expressed will show different shades of red or green.
FIG. 3.
Outline of microarray development for analyzing gene expression. (a) cDNA array development; (b) oligonucleotide arrays. Reproduced from reference 81 with permission.
(v) Applications in environmental monitoring.
The potential of DNA microarray technology in high-throughput detection and monitoring of bacteria and quantitative assessment of their community structures is widely acknowledged but has not yet been fully realized or exploited. Microchip technology has been employed extensively for many eukaryotic applications, including mutation detection, gene discovery, gene expression analysis, and mapping (17, 57). Its adoption into environmental analysis has been significantly slower, but recently has been applied to quantification of virulence genes and associated genes in numerous environmentally prominent pathogens, including Bacillus anthracis (73), P. aeruginosa (103), Rhodococcus equi (75), Mycobacterium tuberculosis (70), and Vibrio cholerae (15). Other environmental science disciplines have also benefited from the use of chip technology, where the use of microarrays has provided a more comprehensive understanding of phytopathogen research (64), the establishment of a unified system for detection of waterborne pathogens (90), and the characterization of various microbial communities (16, 26, 51, 89).
WHAT DOES THE FUTURE HOLD?
While cRT-PCR is one of the oldest quantitative assays currently available, many argue it is still the most sensitive. The assay is routinely used in many laboratories, not only for prokaryotic analysis but also for many other applications. There are, as mentioned above, many critical technical considerations that have to be strictly adhered to when using such a sensitive and potentially challenging technique. However, the use of an internal standard that corrects for PCR inhibition between samples of different origins makes this system invaluable for environmental applications, despite much technological advancement elsewhere. Real-time PCR combines the best attributes of both qualitative and cRT-PCR in that it is accurate, sensitive, capable of high throughput, rapid, relatively easy to perform, and, above all, automated. Real-time PCR is now understandably the method of choice for analyzing gene expression in many laboratories. The development of the first portable real-time thermal cyclers certainly supports their future potential. However, real-time formats are not without potential pitfalls. The wide range of enzymes; unlimited numbers of different primer, probe, and amplicon combinations; and the fact that there are few or no in vivo data available from clearly defined cell samples and populations contribute to uncertainty and confirm reproducibility concerns when confronted with validating excessive quantitative data (21).
While microarray analysis may be used routinely for diagnostic purposes, its integration into environmental bacteriology remains at a generally early stage of development and must be treated with a proper amount of skepticism until proven otherwise. However, its potential is unquestionable. Like all commercially available technologies for genomic detection and quantification, microarray expression-based technology also has limitations. It is important to bear in mind that not all signaling changes occur at the RNA level, with many changes occurring at the protein and posttranslational level. As a result such changes will not be detected with gene arrays. At a more technical level there are numerous aspects to consider, especially when analyzing environmental samples. As the target and probe sequences may be extremely diverse, the performance of microarray technology for analysis of environmental samples with high diversity cannot be assumed to correlate with that of pure cultures and how sequence divergence affects microarray hybridization. There is also the possibility when analyzing environmental samples, where humic acids and organic materials are commonly present, of inhibition of DNA hybridization on microarrays. Not surprisingly, there is a lack of data and literature on the performance of microarrays with complex environmental samples. Indeed, it is not clear whether microarray hybridization is sensitive enough to detect microorganisms from such complex samples and whether microarray analysis can be made fully quantitative for determining gene expression patterns and profiles within such samples. Environmental studies require experimental tools that not only detect the presence or absence of specific groups of microorganisms but also provide quantitative data to help evaluate their biological activities. Another issue for concern involves the nature of the fragments to be placed on the array. The two competing methods are PCR amplification of DNA and use of oligonucleotide probes of various lengths. The main advantages of PCR amplification of DNA include availability and cost. The latter may provide more specific resolution and better quantification, but the method of choice for a particular experiment is not always apparent. There are several approaches to minimize other possible technical errors with array technology not only when analyzing environmental samples. Errors in sequence databases can lead to errors in gene annotation; therefore, it is important to always confirm clone identification. When cDNA gene arrays are being used, routine sequencing may provide precise confirmation of the gene(s) under investigation (94). Gene arrays indicate a quantitative assessment of the level of gene expression, a value that for some genes may be variable (4). As gene array technology is still only at a relatively early stage of development in many fields, none more so than the field of environmental bacteriology, it is essential for certain fully quantitative applications that quantitative data be validated with another “gold standard” technique(s). At present the use of real-time technology represents the most obvious choice for direct comparative measures.
REFERENCES
- 1.Alfreider, A., C. Vogt, and W. Babel. 2003. Expression of chlorocatechol 1,2-dioxygenase and chlorocatechol 2,3-dioxygenase genes in chlorobenzene-contaminated subsurface samples. Appl. Environ. Microbiol. 69:1372-1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R., B. M. Fuchs, and S. Behrens. 2001. The identification of microorganisms by fluorescence in situ hybridisation. Curr. Opin. Biotechnol. 12:231-236. [DOI] [PubMed] [Google Scholar]
- 3.Amann, R. I., W. Ludwig, and K. H. Schleifer. 1995. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol. Rev. 59:143-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amundson, S. A., M. Bittner, Y. Chen, J. Trent, P. Meltzer, and A. J. Fornace, Jr. 1999. Fluorescent cDNA microarray hybridisation reveals complexity and heterogeneity of cellular genotoxic stress responses. Oncogene 18:13666-13672. [DOI] [PubMed] [Google Scholar]
- 5.Bach, H. J., I. Jessen, M. Scholter, and J. C. Munch. 2003. A Taqman-PCR protocol for quantification and differentiation of phytopathogenic Clavibacter michiganesis subspecies. J. Microbiol. Methods 52:85-91. [DOI] [PubMed] [Google Scholar]
- 6.Bach, H. J., J. Tomanova, M. Scholter, and J. C. Munch. 2002. Enumeration of total bacteria and bacteria with genes for proteolytic activity in pure cultures and in environmental samples by quantitative PCR mediated amplification. J. Microbiol. Methods 49:235-245. [DOI] [PubMed] [Google Scholar]
- 7.Bachoon, D. S., F. Chen, and R. E. Hodson. 2001. RNA recovery and detection of mRNA by RT-PCR from preserved prokaryotic samples. FEMS Microbiol. Lett. 201:127-132. [DOI] [PubMed] [Google Scholar]
- 8.Bartlett, J. M. S. 2002. Approaches to the analysis of gene expression using mRNA: a technical overview. Mol. Biotechnol. 21:149-160. [DOI] [PubMed] [Google Scholar]
- 9.Becker-Andre, M., and K. Hahlbrock. 1989. Absolute mRNA quantification using the polymerase chain reaction. A novel approach by a PCR aided transcript titration assay (PATTY). Nucleic Acids Res. 17:9437-9446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beer, M., E. M. Seviour, Y. Kong, M. Cunningham, L. L. Blackall, and R. J. Seviour. 2002. Phylogeny of the filamentous bacterium Eikelboom type 1851, and design and application of a 16S rRNA targeted oligonucleotide probe for its fluorescence in situ identification in activated sludge. FEMS Microbiol. Lett. 207:179-183. [DOI] [PubMed] [Google Scholar]
- 11.Belanger, S. D., M. Boissinot, N. Clairoux, F. J. Picard, and M. G. Bergeron. 2003. Rapid detection of Clostridium difficile in feces by real-time PCR. J. Clin. Microbiol. 41:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Belanger, S. D., M. Boissinot, C. Menard, F. J. Picard, and M. G. Bergeron. 2002. Rapid detection of Shiga toxin-producing bacteria in feces by multiplex PCR with molecular beacons on the smart cycler. J. Clin. Microbiol. 40:1436-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bengtsson, M., H. J. Karlsson, G. Westman, and M. Kubista. 2003. A new minor groove binding asymmetric cyanine reporter dye for real-time PCR. Nucleic Acids Res. 31:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernacchi, S., and Y. Mely. 2001. Exciton interaction in molecular beacons: a sensitive sensor for short range modifications of the nucleic acid structure. Nucleic Acids Res. 29:e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bina, J., J. Zhu, M. Dziejman, S. Faruque, S. Calderwood, and J. Mekalanos. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. USA 100:2801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodrossy, L., N. Stralis-Pavese, J. C. Murrell, S. Radajewski, A. Weilharter, and A. Sessitsch. 2003. Development and validation of a diagnostic microbial microarray for methanotrophs. Environ. Microbiol. 5:566-582. [DOI] [PubMed] [Google Scholar]
- 17.Boldrick, J. C., A. A. Alizadeh, M. Diehn, S. Dudoit, C. L. Liu, C. E. Belcher, D. Botstein, L. M. Staudt, P. O. Brown, and D. A. Relman. 2002. Stereotyped and specific gene expression programs in human innate responses to bacteria. Proc. Natl. Acad. Sci. USA 99:972-977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks, E. M., L. G. Sheflin, and S. W. Spaulding. 1995. Secondary structure in the 3′ UTR of EGF and the choice of reverse transcriptases affect the detection of message diversity by RT-PCR. BioTechniques 19:806-815. [PubMed] [Google Scholar]
- 19.Brown, P. O., and D. Botstein. 1999. Exploring the new world of the genome with DNA microarrays. Nat. Genet. 21:33-37. [DOI] [PubMed] [Google Scholar]
- 20.Bustin, S. A. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25:169-193. [DOI] [PubMed] [Google Scholar]
- 21.Bustin, S. A. 2002. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J. Mol. Endocrinol. 29:23-39. [DOI] [PubMed] [Google Scholar]
- 22.Chen, Z., J. Smithberger, B. Sun, and T. L. Eggerman. 1999. Prevention of heteroduplex formation in mRNA quantitation by reverse transcription polymerase chain reaction. Anal. Biochem. 266:230-232. [DOI] [PubMed] [Google Scholar]
- 23.Cho, W. S., and C. Chae. 2003. Expression of cyclooxygenase-2 in swine naturally infected with Actinobacillus pleuropneumoniae. Vet. Pathol. 40:25-31. [DOI] [PubMed] [Google Scholar]
- 24.Clementi, M., S. Menzo, P. Bagnarelli, A. Manzin, A. Valenza, and P. E. Varaldo. 1993. Quantitative PCR and RT-PCR in virology. PCR Methods Appl. 2:191-196. [DOI] [PubMed] [Google Scholar]
- 25.Cottrez, F., C. Auriault, A. Capron, and H. Groux. 1994. Quantitative PCR: validation of the use of a multispecific internal control. Nucleic Acids Res. 22:2712-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dennis, P., E. A. Edwards, S. N. Liss, and R. Fulthorpe. 2003. Monitoring gene expression in mixed microbial communities by using DNA microarrays. Appl. Environ. Microbiol. 69:769-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding, L., K. Williams, W. Ausserer, L. Bousse, and R. Dubrow. 2003. Analysis of plasmid samples on a microchip. Anal. Biochem. 316:92-102. [DOI] [PubMed] [Google Scholar]
- 28.Dionisi, H. M., A. C. Layton, G. Harms, I. R. Gregory, K. G. Robinson, and G. S. Sayler. 2002. Quantification of Nitrosomonas oligotropha-like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale waste-water treatment plants by competitive PCR. Appl. Environ. Microbiol. 68:245-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fischer-Le Saux, M., D. Hervio-Heath, S. Loaec, R. R. Colwell, and M. Pommepuy. 2002. Detection of cytotoxin-hemolysin mRNA in nonculturable populations of environmental and clinical Vibrio vulnificus strains in artificial seawater. Appl. Environ. Microbiol. 68:5641-5646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fluit, A. C., M. R. Visser, and F. J. Schmitz. 2001. Molecular detection of antimicrobial resistance. Clin. Microbiol. Rev. 14:836-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foulds, I. V., A. Granacki, C. Xiao, U. J. Krull, A. Castle, and P. A. Horgen. 2002. Quantification of microcystin-producing cyanobacteria and E. coli in water by 5′ nuclease PCR. J. Appl. Microbiol. 93:825-834. [DOI] [PubMed] [Google Scholar]
- 32.Freeman, W. M., S. J. Walker, and K. E. Vrana. 1999. Quantitative RT-PCR: pitfalls and potentials. BioTechniques 26:112-125. [DOI] [PubMed] [Google Scholar]
- 33.Gibson, U. E., C. A. Heid, and P. M. Williams. 1996. A novel method for real time quantitative RT-PCR. Genome Res. 6:995-1001. [DOI] [PubMed] [Google Scholar]
- 34.Ginzinger, D. G. 2002. Gene quantification using real-time quantitative PCR: an emerging technology hits the mainstream. Exp. Hematol. 30:503-512. [DOI] [PubMed] [Google Scholar]
- 35.Giulietti, A., L. Overbergh, D. Valckx, B. Decallone, R. Bouillon, and C. Mathieu. 2001. An overview of real-time quantitative PCR: applications to quantify cytokine gene expression. Methods 25:386-401. [DOI] [PubMed] [Google Scholar]
- 36.Gottwald, E., O. Muller, and A. Polten. 2001. Semiquantitative reverse transcription-polymerase chain reaction with the Agilent 2100 Bioanalyzer. Electrophoresis 22:4016-4022. [DOI] [PubMed] [Google Scholar]
- 37.Hall, S. J., P. Hugenholtz, N. Siyambalaptiyia, J. Keller, and L. L. Blackall. 2002. The development and use of real-time PCR for the quantification of nitrifiers in activated sludge. Water Sci. Technol. 46:267-272. [PubMed] [Google Scholar]
- 38.Harms, G., A. C. Layton, H. M. Dionisi, I. M. Gregory, V. M. Garrett, S. A. Hawkins, K. G. Robinson, and G. S. Sayler. 2003. Real-time PCR quantification of nitrifying bacteria in a municipal wastewater treatment plant. Environ. Sci. Technol. 37:343-351. [DOI] [PubMed] [Google Scholar]
- 39.Hein, I., A. Lehner, P. Rieck, K. Klein, E. Brandl, and M. Wagner. 2001. Comparison of different approaches to quantify Staphylococcus aureus cells by real-time quantitative PCR and application of this technique for examination of cheese. Appl. Environ. Microbiol. 67:3122-3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Henley, W. N., K. E. Scheuber, and D. A. Nielsen. 1996. Limitations imposed by heteroduplex formation on quantitative RT-PCR. Biochem. Biophys. Res. Commun. 226:113-117. [DOI] [PubMed] [Google Scholar]
- 41.Hermansson, A., and P. E. Lindgren. 2001. Quantification of ammonia oxidizing bacteria in arable soil by real-time PCR. Appl. Environ. Microbiol. 67:972-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hristova, K. R., C. M. Lutenegger, and K. M. Scow. 2001. Detection and quantification of methyl tert-butyl ether-degrading strain PM1 by real-time TaqMan PCR. Appl. Environ. Microbiol. 67:5154-5160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones, L. J., S. T. Yue, C. Y. Cheung, and V. L. Singer. 1998. RNA quantitation by fluorescence-based solution assay: RibioGreen reagent characterisation. Anal. Biochem. 265:368-374. [DOI] [PubMed] [Google Scholar]
- 44.Jothikumar, N., and M. W. Griffiths. 2002. Rapid detection of Escherichia coli O157:H7 with multiplex real-time PCR assays. Appl. Environ. Microbiol. 68:3169-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kikuchi, T., K. Iwasaki, H. Nishihara, Y. Takamura, and O. Yagi. 2002. Quantitative and rapid detection of the trichloroethylene-degrading bacterium Methylocyctis sp. M in groundwater by real-time PCR. Appl. Microbiol. Biotechnol. 59:731-736. [DOI] [PubMed] [Google Scholar]
- 46.Killeen, A. A. 1997. Quantification of nucleic acids. Clin. Lab. Med. 17:1-19. [PubMed] [Google Scholar]
- 47.Klein, D. 2002. Quantification using real-time PCR technology: applications and limitations. Trends Mol. Med. 8:257-260. [DOI] [PubMed] [Google Scholar]
- 48.Koo, K., and L. A. Jaykus. 2003. Detection of Listeria monocytogenes from a model food by fluorescence resonance energy transfer-based PCR with an asymmetric probe set. Appl. Environ. Microbiol. 69:1082-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowalchuk, G. A., Z. S. Naoumenko, P. J. L. Derikx, A. Felske, J. R. Stephen, and I. A. Arkhipchenko. 1999. Molecular analysis of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in compost and composted materials. Appl. Environ. Microbiol. 65:396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lekanne Deprez, R. H., A. C. Fijnvandraat, J. M. Ruijter, and A. F. Moorman. 2002. Sensitivity and accuracy of quantitative real-time polymerase chain reaction using SYBR green I depends on cDNA synthesis conditions. Anal. Biochem. 307:63-69. [DOI] [PubMed] [Google Scholar]
- 51.Leonard, E. E., II, T. Takata, M. J. Blaser, S. Falkow, L. S. Tompkins, and E. C. Gaynor. 2003. Use of an open-reading frame-specific Campylobacter jejuni DNA microarray as a new genotyping tool for studying epidemiologically related isolates. J. Infect. Dis. 187:691-694. [DOI] [PubMed] [Google Scholar]
- 52.LePecq, J. B., and C. Paoletti. 1967. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J. Mol. Biol. 27:87-106. [DOI] [PubMed] [Google Scholar]
- 53.Lesser, T. D., M. Boye, and N. B. Hendriksen. 1995. Survival and activity of Pseudomonas sp. strain B13(FR1) in a marine microcosm determined by quantitative PCR and an rRNA-targeting probe and its effect on the indigenous bacterioplankton. Appl. Environ. Microbiol. 61:1201-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyon, W. J. 2001. TaqMan PCR for detection of Vibrio cholerae O1, O139, non-O1, and non-O139 in pure cultures, raw oysters, and synthetic seawater. Appl. Environ. Microbiol. 67:4685-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Makino, S. I., H. I. Cheun, M. Watarai, I. Uchinda, and K. Takeshi. 2001. Detection of anthrax spores from the air by real-time PCR. Lett. Appl. Microbiol. 33:237-240. [DOI] [PubMed] [Google Scholar]
- 56.Marlowe, E. M., J. M. Wang, I. L. Pepper, and R. M. Maier. 2002. Application of a reverse transcription-PCR assay to monitor regulation of the catabolic nahAc gene during phenanthrene degradation. Biodegradation 13:251-260. [DOI] [PubMed] [Google Scholar]
- 57.Martin, K. J., E. Graner, Y. Li, L. M. Price, B. M. Kritzman, M. V. Fournier, E. Rhei, and A. B. Pardee. 2001. High-sensitivity array analysis of gene expression for the early detection of disseminated breast tumor cells in peripheral blood. N. Engl. J. Med. 344:539-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McGrath, S., J. S. G. Dooley, and R. W. Haylock. 2000. Quantification of toxin gene expression by competitive reverse transcription-PCR. Appl. Environ. Microbiol. 66:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meckenstock, R., P. Steinle, J. R. van der Meer, and M. Snozzi. 1998. Quantification of bacterial mRNA involved in degradation of 1,2,4-trichlorobenzene by Pseudomonas sp. strain P51 from liquid culture and from river sediment by reverse transcriptase PCR (RT/PCR). FEMS Microbiol. Lett. 167:123-129. [DOI] [PubMed] [Google Scholar]
- 60.Mehrota, J., and W. R. Bishai. 2001. Regulation of virulence genes in Mycobacterium tuberculosis. Int. J. Med. Microbiol. 291:171-182. [DOI] [PubMed] [Google Scholar]
- 61.Mhlanga, M. M., and L. Malmberg. 2001. Using molecular beacons to detect single-nucleotide polymorphisms with real-time PCR. Methods 25:463-471. [DOI] [PubMed] [Google Scholar]
- 62.Moller, A., and J. K. Jansson. 1997. Quantification of genetically tagged cyanobacteria in Baltic sea sediment by competitive PCR. BioTechniques 22:512-518. [DOI] [PubMed] [Google Scholar]
- 63.Nogva, H. K., K. Rudi, K. Naterstad, A. Holck, and D. LilleHaug. 2000. Application of 5′-nuclease PCR for quantitative detection of Listeria monocytogenes in pure cultures, water, skim milk, and pasteurized whole milk. Appl. Environ. Microbiol. 66:4266-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nunes, L. R., Y. B. Rosato, N. H. Muto, G. M. Yanai, V. S. Da Silva, D. B. Leite, E. R. Goncalves, A. A. De Souza, H. D. Coletta-Filho, M. A. Machado, S. A. Lopes, and R. C. De Oliveira. 2003. Microarray analyses of Xylella fastidiosa provide evidence of coordinated transcription control of laterally transferred elements. Genome Res. 13:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Connell, J., T. Goode, and F. Shanahan. 1998. Quantitative measurement of mRNA expression by competitive RT-PCR. Methods Mol. Biol. 92:183-194. [DOI] [PubMed] [Google Scholar]
- 66.Ohan, N. W., and J. J. Heikkila. 1993. Reverse transcription polymerase chain reaction: an overview of the technique and its applications. Biotechnol. Adv. 11:13-29. [DOI] [PubMed] [Google Scholar]
- 67.Orlando, C., P. Pinzani, and M. Pazzagli. 1998. Developments in quantitative PCR. Clin. Chem. Lab. Med. 36:255-269. [DOI] [PubMed] [Google Scholar]
- 68.Panaro, N. J., P. K. Yuen, T. Sakazume, P. Fortina, L. J. Kricka, and P. Wilding. 2000. Evaluation of DNA fragment sizing and quantification by the Agilent 2100 Bioanalyzer. Clin. Chem. 46:1851-1853. [PubMed] [Google Scholar]
- 69.Pannetier, C., S. Delassus, S. Darche, C. Saucier, and P. Kourilsky. 1993. Quantitative titration of nucleic acids by enzymatic amplification reactions run to saturation. Nucleic Acids Res. 21:579-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parish, T., D. A. Smith, G. Roberts, J. Betts, and N. G. Stoker. 2003. The senX3-regX3 two component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology 149:1423-1435. [DOI] [PubMed] [Google Scholar]
- 71.Pirnay, J. P., D. De Vos, D. Mossialos, A. Vanderkelen, P. Cornelis, and M. Zizi. 2002. Analysis of the Pseudomonas aeruginosa oprD gene from clinical and environmental isolates. Environ. Microbiol. 4:872-882. [DOI] [PubMed] [Google Scholar]
- 72.Predinger, E. A. 2001. Quantitating mRNAs with relative and competitive RT-PCR. Methods Mol. Biol. 160:49-63. [DOI] [PubMed] [Google Scholar]
- 73.Read, T. D., S. N. Peterson, N. Tourasse, L. W. Baillie, I. T. Paulsen, K. E. Nelson, H. Tettelin, D. E. Fouts, J. A. Eisen, S. R. Gill, E. K. Holtzapple, O. A. Okstad, E. Helgason, J. Rilstone, M. Wu, J. F. Kolonay, M. J. Beanan, R. J. Dodson, L. M. Brinkac, M. Gwinn, R. T. DeBoy, R. Madpu, S. C. Daugherty, A. S. Durkin, D. H. Haft, W. C. Nelson, J. D. Peterson, M. Pop, H. M. Khouri, D. Radune, J. L. Benton, Y. Mahamoud, L. Jiang, I. R. Hance, J. F. Weidman, K. J. Berry, R. D. Plaut, A. M. Wolf, K. L. Watkins, W. C. Nierman, A. Hazen, R. Cline, C. Redmond, J. E. Thwaite, O. White, S. L. Salzberg, B. Thomason, A. M. Friedlander, T. M. Koehler, P. C. Hanna, A. B. Kolsto, and C. M. Fraser. 2003. The genome sequence of Bacillus anthracis Ames and comparison to closely related bacteria. Nature 423:23-25. [DOI] [PubMed] [Google Scholar]
- 74.Reischl, U., and B. Kochanowski. 1995. Quantitative PCR. Mol. Biotechnol. 3:55-69. [DOI] [PubMed] [Google Scholar]
- 75.Ren, J., and J. F. Prescott. 2003. Analysis of virulence plasmid gene expression of intra-macrophage and in vitro grown Rhodococcus equi ATCC 33701. Vet. Microbiol. 94:167-182. [DOI] [PubMed] [Google Scholar]
- 76.Russell, D. 2002. The measurement of orgA gene expression in Salmonella enterica serovar Typhimurium using competitive RT-PCR. Ph.D. thesis. University of Ulster, Coleraine, Northern Ireland.
- 77.Rye, H. S., J. M. Dabora, M. A. Quesada, R. A. Mathies, and A. N. Glazer. 1993. Fluorometric assay using dimeric dyes for double-and single-stranded DNA and RNA with picogram sensitivity. Anal. Biochem. 208:144-150. [DOI] [PubMed] [Google Scholar]
- 78.Schena, M., D. Shalon, R. W. Davis, and P. O. Brown. 1995. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 270:467-470. [DOI] [PubMed] [Google Scholar]
- 79.Schmidt, D. M., and J. D. Ernst. 1995. A fluorometric assay for the quantification of RNA in solution with nanogram sensitivity. Anal. Biochem. 232:144-146. [DOI] [PubMed] [Google Scholar]
- 80.Schofield, P., A. N. Pell, and D. O. Krause. 1997. Molecular beacons: trial of a fluorescence-based solution hybridization technique for ecological studies with ruminal bacteria. Appl. Environ. Microbiol. 63:1143-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schulze, A., and J. Downward. 2001. Navigating gene expression using microarrays: a technology review. Nat. Cell Biol. 3:e190-e195. [DOI] [PubMed] [Google Scholar]
- 82.Sharkey, F., S. I. Markos, and R. W. Haylock. 2004. Quantification of toxin encoding mRNA from Clostridium botulinum type E in media containing sorbic acid or sodium nitrite by competitive RT-PCR. FEMS Microbiol. Lett. 232:139-144. [DOI] [PubMed] [Google Scholar]
- 83.Sharkey, F. H., I. M. Banat, and R. Marchant. 2004. A rapid and effective method of extracting fully intact RNA from thermophilic geobacilli that is suitable for gene expression analysis. Extremophiles 8:73-77. [DOI] [PubMed] [Google Scholar]
- 84.Silyn-Roberts, G., and G. Lewis. 2001. In situ analysis of Nitrosomonas spp. in wastewater treatment wetland biofilms. Water Res. 35:2731-2739. [DOI] [PubMed] [Google Scholar]
- 85.Snaidr, I., C. Beimfohr, C. Levantesi, S. Rossetti, J. van der Waarde, B. Geurkink, D. Elkelboom, M. Lemaitre, and V. Tandoi. 2002. Phylogenetic analysis and in situ identification of “Nostocoida limicola”-like filamentous bacteria in activated sludge from industrial wastewater treatment plants. Water Sci. Technol. 46:99-104. [PubMed] [Google Scholar]
- 86.Solinas, A., L. J. Brown, C. McKeen, J. M. Mellor, J. T. G. Nicol, N. Thelwell, and T. Brown. 2001. Duplex scorpion primers in SNP analysis and FRET applications. Nucleic Acids Res. 29:e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Souaze, F., A. Ntodou-Thome, C. Y. Tran, W. Rostene, and P. Forgez. 1996. Quantitative RT-PCR: limits and accuracy. BioTechniques 21:280-285. [DOI] [PubMed] [Google Scholar]
- 88.Sreenivasan, A. 2003. Real-time PCR gets personal. Scientist 17:43. [Google Scholar]
- 89.Stin, O. C., A. Carnahan, R. Singh, J. Powell, J. P. Furuno, A. Dorsey, E. Silbergeld, H. N. Williams, and J. G. Morris. 2003. Characterization of microbial communities from coastal waters using microarrays. Environ. Monit. Assess. 81:327-336. [PubMed] [Google Scholar]
- 90.Straub, T. M., and D. P. Chandler. 2003. Towards a unified system for detecting waterborne pathogens. J. Microbiol. Methods 53:185-197. [DOI] [PubMed] [Google Scholar]
- 91.Stubner, S. 2002. Enumeration of 16S rDNA of Desulfotomaculum lineage 1 in rice field soil by real-time PCR with SYBR GreenTM detection. J. Microbiol. Methods 50:155-164. [DOI] [PubMed] [Google Scholar]
- 92.Suzuki, M. T., L. T. Taylor, and E. F. DeLong. 2000. Quantitative analysis of small-subunit rRNA genes in mixed microbial populations via 5′-nuclease assays. Appl. Environ. Microbiol. 66:4605-4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tate, R., A. Riccio, E. Caputo, M. Iaccarino, and E. J. Patriarca. 1999. The Rhizobium etli metZ gene is essential for methionine biosynthesis and nodulation of Phaseolus vulgaris. Mol. Plant Microbe Interact. 12:24-34. [DOI] [PubMed] [Google Scholar]
- 94.Taylor, E., E. Cogdell, K. Coombes, L. Hu, L. Ramdas, A. Tabor, S. Hamilton, and W. Zhang. 2001. Sequence verification as quality-control step for production of cDNA microarrays. BioTechniques 31:62-65. [DOI] [PubMed] [Google Scholar]
- 95.Thelwell, N., S. Millington, A. Solinas, J. Booth, and T. Brown. 2000. Mode of action and application of scorpion primers to mutation detection. Nucleic Acids Res. 28:3752-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tyagi, S., and F. R. Kramer. 1996. Molecular beacons: probes that fluoresce upon hybridisation. Nat. Biotechnol. 14:303-308. [DOI] [PubMed] [Google Scholar]
- 97.Valle, O., T. Lien, and G. Knutsen. 1981. Fluorometric determination of DNA and RNA in Chlamydomonas using ethidium bromide. J. Biochem. Biophys. Methods 4:271-277. [DOI] [PubMed] [Google Scholar]
- 98.Wang, T., and M. J. Brown. 1999. mRNA quantification by real time TaqMan polymerase chain reaction: validation and comparison with RNase protection. Anal. Biochem. 269:198-201. [DOI] [PubMed] [Google Scholar]
- 99.Whitcombe, D., S. Kelly, J. Mann, J. Theaker, C. Jones, and S. Little. 1999. Scorpions™ primers: a novel method for use in single tube genotyping. Am. J. Hum. Genet. 65:2333. [Google Scholar]
- 100.Widada, J., H. Nojiri, K. Kasuga, T. Yoshida, H. Habe, and T. Omori. 2001. Quantification of the carbazole 1,9a-dioxygenase gene by real-time competitive PCR combined with co-extraction of internal standards. FEMS Microbiol. Lett. 202:51-57. [DOI] [PubMed] [Google Scholar]
- 101.Wilderman, P. J., A. I. Vasil, Z. Johnson, M. J. Wilson, H. E. Cunliffe, L. L. Lamont, and M. L. Vasil. 2001. Characterization of an endoprotease (PrpL) encoded by a PvdS-regulated gene in Pseudomonas aeruginosa. Infect. Immun. 69:5385-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wittwer, C. T., G. C. Fillmore, and D. R. Hillyard. 1989. Automated polymerase chain reaction in capillary tubes with hot air. Nucleic Acids Res. 17:4353-4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wolfgang, M. C., B. R. Kulasekara, X. Liang, D. Boyd, K. Wu, Q. Yang, C. G. Miyada, and S. Lory. 2003. Conservation of genome content and virulence determinants among clinical and environmental isolates of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 100:8484-8489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wong, H., W. D. Anderson, T. Cheng, and T. Riabowol. 1994. Monitoring mRNA expression by polymerase chain reaction: the “primer dropping” method. Anal. Biochem. 223:251-258. [DOI] [PubMed] [Google Scholar]
- 105.Wouters, J., B. Bergman, and S. Janson. 2003. Cloning and expression of a putative cyclodextrin glucosyltransferase from the symbiotically competent cyanobacterium Nostoc sp. PCC 9229. FEMS Microbiol. Lett. 219:181-185. [DOI] [PubMed] [Google Scholar]
- 106.Yang, H. H., Q. Z. Zhu, Q. Y. Chen, D. H. Li, and J. G. Xu. 2000. Application of magdala red as a fluorescence probe in the determination of nucleic acids. Fresenius J. Anal. Chem. 366:303-306. [DOI] [PubMed] [Google Scholar]
- 107.Zhu, Q. Z., F. Li, X. Q. Guo, J. G. Xu, and W. Y. Li. 1997. Application of a novel fluorescence probe in the determination of nucleic acids. Analyst 122:937-940. [DOI] [PubMed] [Google Scholar]
- 108.Zimmermann, K., and W. Mannhalter. 1996. Technical aspects of quantitative competitive PCR. BioTechniques 21:268-279. [DOI] [PubMed] [Google Scholar]