Significance
Increased light exposure has been associated with obesity in both humans and mice. In this article, we elucidate a mechanistic basis of this association by performing studies in mice. We report that prolonging daily light exposure increases adiposity by decreasing energy expenditure rather than increasing food intake or locomotor activity. This was caused by a light-exposure period-dependent attenuation of the noradrenergic activation of brown adipose tissue that has recently been shown to contribute substantially to energy expenditure by converting fatty acids and glucose into heat. Therefore, we conclude that impaired brown adipose tissue activity may mediate the relationship between increased light exposure and adiposity.
Keywords: circadian rhythms, light pollution, obesity, brown adipose tissue, triglyceride metabolism
Abstract
Disruption of circadian rhythmicity is associated with obesity and related disorders, including type 2 diabetes and cardiovascular disease. Specifically, prolonged artificial light exposure associates with obesity in humans, although the underlying mechanism is unclear. Here, we report that increasing the daily hours of light exposure increases body adiposity through attenuation of brown adipose tissue (BAT) activity, a major contributor of energy expenditure. Mice exposed to a prolonged day length of 16- and 24-h light, compared with regular 12-h light, showed increased adiposity without affecting food intake or locomotor activity. Mechanistically, we demonstrated that prolonged day length decreases sympathetic input into BAT and reduces β3-adrenergic intracellular signaling. Concomitantly, prolonging day length decreased the uptake of fatty acids from triglyceride-rich lipoproteins, as well as of glucose from plasma selectively by BAT. We conclude that impaired BAT activity is an important mediator in the association between disturbed circadian rhythm and adiposity, and anticipate that activation of BAT may overcome the adverse metabolic consequences of disturbed circadian rhythmicity.
Modern world society is subjected to disturbances of circadian rhythms by shift work, sleep deprivation, and environmental light pollution. Importantly, the increasing prevalence of obesity is associated with a disrupted sleep-wake pattern in humans (1) and coincides with the availability of artificial light (2, 3). Additionally, a recent study revealed a relationship between exposure to light at night and obesity in a cross-sectional analysis of over 100,000 women (4). Light input is the most important cue for generation of circadian (∼24 h) rhythms by the master clock. Both in rodents and humans the master clock is situated in the suprachiasmatic nucleus (SCN) of the hypothalamus. The SCN is responsible for synchronization of peripheral clocks throughout the body, which is mediated by endocrine and neuronal signals (5). A causal role for a disturbed circadian rhythm in the development of obesity has been demonstrated by animal studies. Mice with genetically dysfunctional clock genes develop obesity and insulin resistance (6–9). Moreover, specific ablation of the SCN induces acute weight gain (10). These results indicate a crucial role for the SCN in the regulation of adiposity.
Interestingly, we previously showed that prolonged light exposure only is sufficient to enhance weight gain in mice. Constant light disrupts the central circadian clock, evidenced by an immediate reduction in the circadian amplitude of SCN electrical activity. Moreover, constant light induces body weight gain and insulin resistance, even faster than high-fat diet, which was not caused by increased food intake or reduced locomotor activity (11). Therefore, disruption of the central biological clock likely induces weight gain by decreasing energy expenditure.
Recently, it has been recognized that brown adipose tissue (BAT) importantly contributes to energy expenditure. BAT combusts high amounts of triglycerides (TG) into heat, a process called thermogenesis that is mediated by uncoupling protein 1 (UCP1). Interestingly, SCN neurons project onto BAT and injection of glutamate into the SCN increases BAT thermogenesis in rats (12, 13). This finding indicates that BAT may mediate the association between circadian rhythmicity and energy expenditure. Therefore, the aim of this study was to shed light on the association between prolonged light exposure and obesity in humans by investigating the effect of day length on BAT activity in mice in relation to body fat gain, independent of ambient temperature. We demonstrate that daily light exposure negatively associates with the uptake of TG-derived fatty acids and glucose from plasma by BAT, pointing to decreased activity of the tissue. Furthermore, we show that increasing daily light exposure decreases BAT activity through reduced sympathetic stimulation.
Results
Entrainment to Light Schedules.
Male 12-wk-old C57BL/6J mice, fed ad libitum a regular chow diet, were exposed to daily light exposure of either 12, 16, or to 24 h during 5 wk at a constant ambient temperature of 22 °C. During the last 2 wk of light intervention, circadian rhythm in behavioral activity was assessed in their home cages. Compared with a day length of 12 h (Fig. S1A), mice exposed to a day length of 16 h showed a retained circadian (24 h) rhythm in behavioral activity, with high activity during nighttime (Fig. S1B). In contrast, circadian rhythmicity of behavioral activity was largely reduced in mice exposed to constant light (Fig. S1C).
Prolonged Daily Light Exposure Increases Adiposity Without Increasing Food Intake.
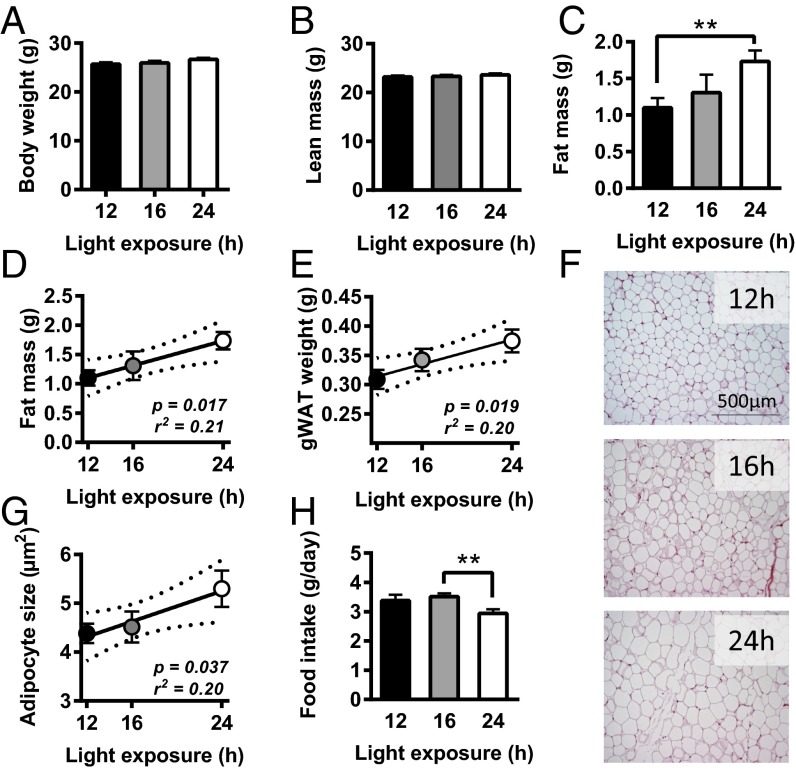
After 5 wk of light intervention, body weight was determined and body composition was assessed by EchoMRI. Prolonged light exposure did not significantly increase total body weight (Fig. 1A) or lean mass (Fig. 1B). Interestingly, we observed a daily light exposure-dependent increase in fat mass that reached significance for 24- versus 12-h exposure (+57%; P = 0.01) (Fig. 1C). In fact, duration of light exposure positively correlated with the body fat mass (β = 0.053; r2 = 0.21; P = 0.02) (Fig. 1D). Mixed-model analysis of weekly body weight development showed that light exposure significantly contributed to weight gain (P = 0.028). After 5 wk of light intervention, mice were killed after a kinetic experiment with radioactive tracers (see below), and gonadal white adipose tissue (gWAT) was quantitatively weighed and examined histologically. A positive correlation was found between light exposure duration and gWAT weight (β = 0.005; r2 = 0.20; P = 0.02) (Fig. 1E) as well as average adipocyte size (β = 0.08; r2 = 0.20; P = 0.04) (Fig. 1 F and G). The gWAT weight and adipocyte size were significantly increased in mice exposed to 24-h light compared with 12 h (+21%; P = 0.02 and +21%; P = 0.04, respectively). Notably, food intake was not different in mice exposed to 16-h light, and even tended to be reduced in mice continuously exposed to light (−13%; P = 0.08) compared with mice exposed to 12-h light per day, (Fig. 1H). Therefore, the positive correlation between day length and adiposity is not explained by hyperphagia, consistent with our previous observations that exposure of mice to constant light decreases energy expenditure rather than increasing food intake (11).
Fig. 1.
Mice were exposed to either 12-, 16-, or 24-h light (n = 9) for 5 wk, and body weight (A), lean mass (B), and fat mass (C) were determined. Correlations are depicted between the light-exposure period and total fat mass (D), gWAT weight (E), and adipocyte size in gWAT (G). Representative images of gWAT stained with H&E are shown (F). Food intake of the last 2 wk of light intervention was measured (H). Data are presented as means ± SEM. Dotted lines represent 95% confidence interval of the regression line. **P < 0.01.
Prolonged Daily Light Exposure Decreases the Nutrient Uptake by BAT.
To investigate whether prolonged daily light exposure reduces BAT activity, consistent with decreased energy expenditure, we determined the effect of light exposure duration on the ability of BAT to take up TG-derived free fatty acids and glucose from plasma. Hereto, we assessed the kinetics of intravenously injected glycerol tri[3H]oleate ([3H]TO)-labeled very low-density liproprotein (VLDL)-like emulsion particles and [14C]deoxyglucose ([14C]DG) and determined the distribution of radiolabels at 15 min after injection.
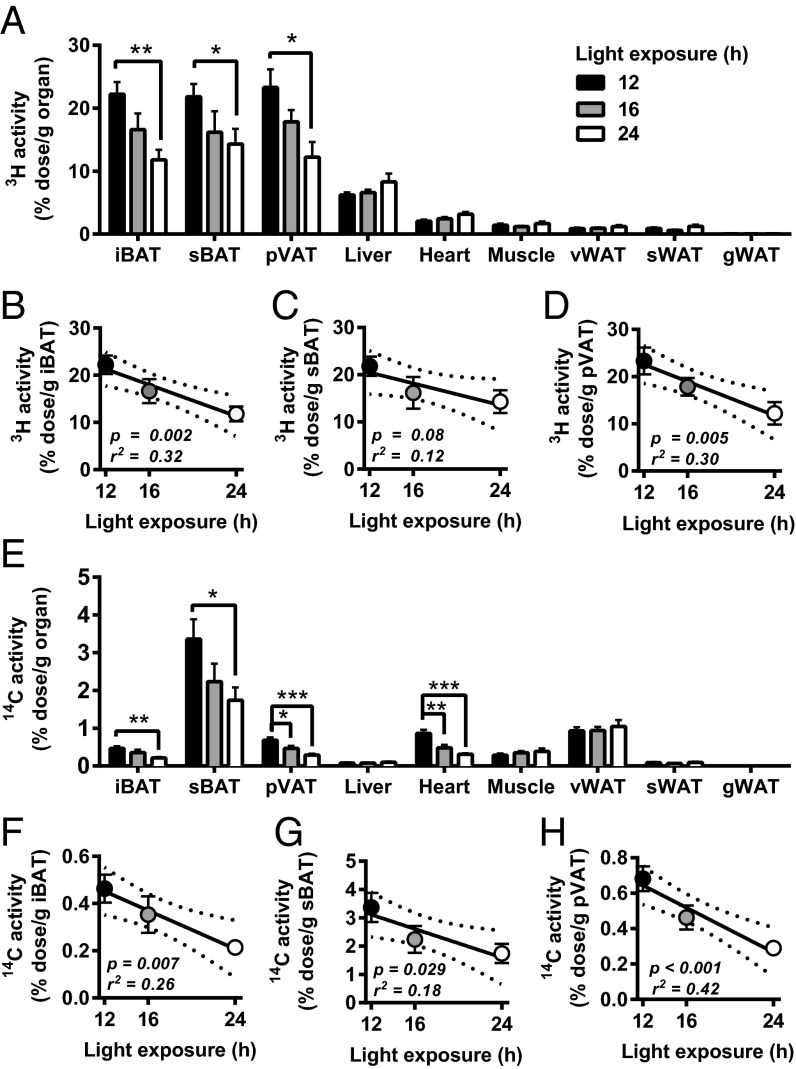
Prolonged daily light exposure did not substantially alter the kinetics of plasma clearance of [3H]TO and [14C]DG (Fig. S2 A and B). In mice exposed to a 12-h light per day, the uptake of [3H]TO-derived radioactivity was much higher in the various BAT depots [interscapular BAT (iBAT), subscapular BAT (sBAT), and perivascular adipose tissue (pVAT)] compared with liver (∼3.5-fold), heart (∼10-fold), muscle (∼15-fold), and white adipose tissue (WAT) (∼25- to 650-fold) (Fig. 2A). Interestingly, the uptake of [3H]TO-derived activity by iBAT, sBAT, and pVAT decreased with prolonged light exposure, reaching −47% (P = 0.001), −34% (P = 0.03), and −48% (P = 0.01) for 24- versus 12-h light exposure (Fig. 2A). Accordingly, the day length negatively associated with the uptake of [3H]TO-derived activity by iBAT (β = −0.83; r2 = 0.32; P = 0.002) (Fig. 2B), sBAT (β = −0.58; r2 = 0.12; P = 0.08) (Fig. 2C), and pVAT (β = −0.90; r2 = 0.30; P = 0.005) (Fig. 2D). Prolonged light exposure did not alter the uptake of [3H]TO-derived radioactivity by organs other than BAT. Consistent with reduced TG-derived free fatty acid uptake by BAT, we found that prolonged light exposure associated to increased plasma free fatty acid levels (β = 0.03; r2 = 0.45; P < 0.001) (Fig. S2C).
Fig. 2.
Mice were exposed to either 12-, 16-, or 24-h light (n = 8–9) for 5 wk, and the VLDL-TG and glucose kinetics were assessed by injection of glycerol tri[3H]oleate ([3H]TO)-labeled emulsion particles and [14C]deoxyglucose ([14C]DG). Uptake of [3H]TO-derived activity by the various organs was determined (A), and correlations were determined between light exposure and [3H]TO-derived activity in iBAT (B), sBAT (C), and pVAT (D). Concomitantly, the uptake of [14C]DG by the various organs was determined (E), and correlations were determined between light exposure and the uptake of [14C]DG by iBAT (F), sBAT (G), and pVAT (H). Data are presented as means ± SEM. Dotted lines represent 95% confidence interval of the regression line.*P < 0.05, **P < 0.01, ***P < 0.001. Abbreviations of organs: iBAT, interscapular BAT; gWAT, gonadal WAT; pVAT, perivascular adipose tissue; sBAT, subscapular BAT; sWAT, subcutaneous WAT; vWAT, visceral WAT.
Prolonged daily light exposure also decreased the uptake of [14C]DG by BAT. Compared with 12-h light exposure, 24-h light exposure decreased the uptake of [14C]DG by iBAT (−54%; P = 0.002), sBAT (−48%; P = 0.02), and pVAT (−57%; P = 0.001) (Fig. 2F). Additionally, 16 h of light a day significantly decreased glucose uptake in pVAT (−32%; P = 0.04) compared with 12 h (Fig. 2E). Similar to the uptake of [3H]TO-derived radioactivity, day length negatively associated with the uptake of glucose by iBAT (β = −0.02; r2 = 0.26; P = 0.007) (Fig. 2F), sBAT (β = −0.13; r2 = 0.18; P = 0.03) (Fig. 2G), and pVAT (β = −0.03; r2 = 0.42; P = 0.0005) (Fig. 2H).
These data imply that prolonged daily light exposure decreases the uptake of nutrients from plasma quite specifically by the various BAT depots, indicating that prolonged daily light exposure decreases the activity of brown adipocytes.
Prolonged Daily Light Exposure Decreases Intracellular Adrenergic Signaling in BAT.
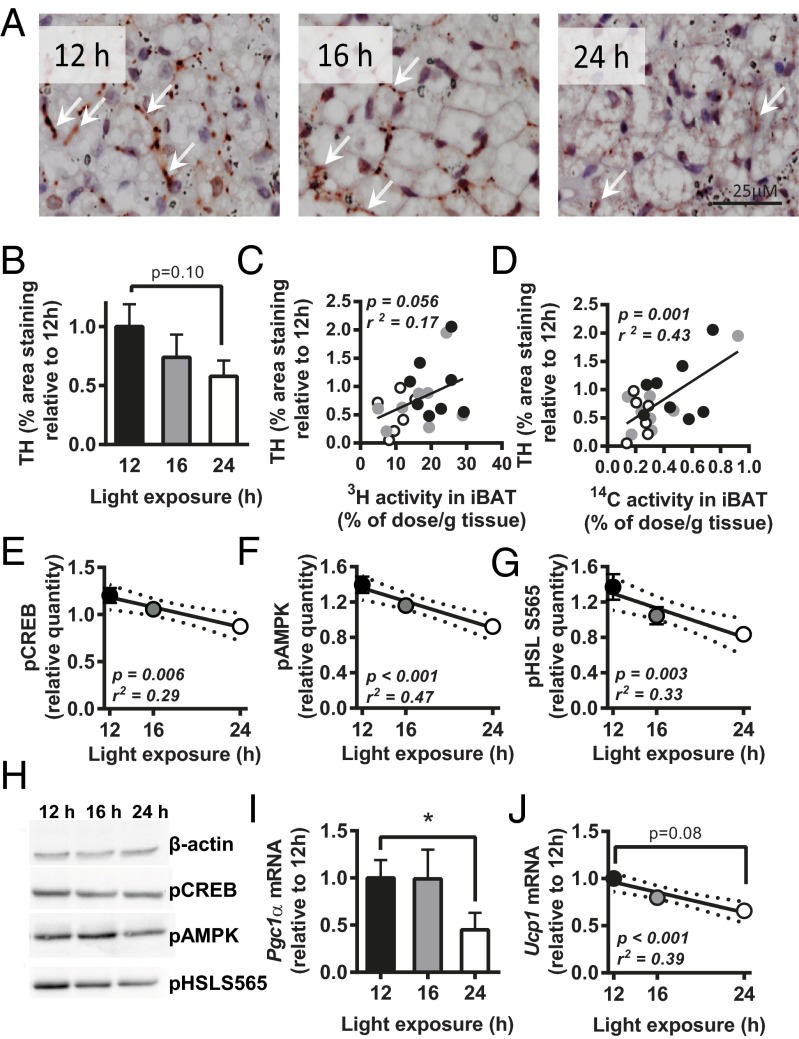
As the SCN is directly connected to BAT via the sympathetic nervous system, we reasoned that prolonged daily light exposure decreases sympathetic activation of BAT. Indeed, immunohistochemical analysis of iBAT showed that the amount of tyrosine hydroxylase (TH), the rate-limiting enzyme in the synthesis of noradrenalin, tended to decrease with increasing day length, up to −42% in 24 h compared with 12 h (P = 0.10) (Fig. 3 A and B). Additionally, the amount of TH detected in BAT correlated with the uptake of [3H]TO-derived activity by BAT (r2 = 0.17; P = 0.056) (Fig. 3C), as well as with the uptake of [14C]DG by BAT (r2 = 0.43; P = 0.001) (Fig. 3D).
Fig. 3.
Mice were exposed to either 12-, 16-, or 24-h light (n = 9) for 5 wk, and iBAT was isolated. Histological sections were stained for TH, representative images are shown (A; arrows indicate TH staining), and quantified (B). Correlation was determined between TH staining and uptake of [3H]TO-derived activity (C) and [14C]DG (D). Additionally, correlations were determined between light exposure and protein levels of pCREB (E), pAMPK (F), and pHSL S565 (G). Protein levels were normalized to β-actin levels. Representative blots for β-actin, pCREB, pAMPK, and pHSL S565 are shown (H). Gene expression of Pgc1α (I) and Ucp1 (J) were determined and normalized to 36B4 expression. Data are presented as means ± SEM. Dotted lines represent 95% confidence interval of the regression line. *P < 0.05.
Because activation of the β3-adrenergic receptor by noradrenalin increases intracellular levels of cAMP, which activates protein kinase A (PKA), resulting in phosphorylation of cAMP response-binding element (CREB) and activatation of AMP-activated protein kinase (AMPK), we next determined the phosphorylation status of these proteins involved in thermogenesis in iBAT. Phosphorylated CREB (pCREB) was decreased in 24-h light exposure compared with 12 h (−27%; P = 0.009) (Fig. 3E). Phosphorylated AMPK (pAMPK) was decreased in mice on a day length of 16 h (−14%; P = 0.05) and 24 h (−32%; P = 0.002) (Fig. 3 F and H) compared with 12 h of light exposure per day, independent of total AMPK levels (Fig. S3 A and B). Daily light duration negatively associated with levels of both pCREB (β = −0.03, r2 = 0.29, P = 0.006) (Fig. 3 E and H) and pAMPK (β = −0.04, r2 = 0.47, P = 0.0003) (Fig. 3 F and H). Both pAMPK and pCREB induce phosphorylation of the lipolytic enzyme hormone-sensitive lipase (HSL). Although day length did not affect PKA-mediated phosphorylation of HSL on serine 563 position (pHSL S563) (Fig. S3 A and C), it reduced AMPK-mediated phosphorylation of HSL on serine 565 (pHSL S565). A day length of 24 h decreased pHSL S565 compared with a day length of 12 h (−39%; P = 0.009) and day length negatively correlated with pHSL S565 (β = −0.04, r2 = 0.33, P = 0.0031) (Fig. 3 G and H).
Gene targets of pCREB include peroxisome proliferator-activated receptor 1α (PPARGC1α or PGC1α) that drives transcription of genes involved in mitochondrial biogenesis, and UCP1, which is essential for BAT thermogenesis. Prolonged daily light exposure (24 h vs. 12 h) decreased gene expression of Pgc1α (−55%; P < 0.05) (Fig. 3I) and tended to decrease gene expression of Ucp1 (−37%; P = 0.08) (Fig. 3J). Increasing day length negatively associated with expression of Ucp1 (β = −0.03, r2 = 0.39, P = 0.0005) (Fig. 3J).
Next, we examined the possibility that prolonged light exposure reduces BAT thermogenic capacity by decreasing mitochondrial function. Prolonged light exposure did not affect gene expression of genes involved in mitochondrial biogenesis (Tfam, Cox7a1, Cyc1, Atp5g1), fatty acid oxidation enzymes (Acadvl, Acadl, Acadm), or mitochondrial fusion (Mfn2) (Fig. S4A). Additionally, the amount of mitochondrial DNA (Fig. S4B), as well as citrate synthase activity (Fig. S4C), was similar between the different light-exposure groups. In line with this finding, total BAT amount was not different upon prolonged light exposure (Fig. S4D).
Taken together, these data indicate that prolonged daily light exposure reduces sympathetic signaling in BAT that is not accompanied by a decrease in mitochondrial capacity, but does result in reduced uptake of TG-derived fatty acids and glucose.
Sympathetic Denervation of iBAT Largely Reduces Nutrient Uptake and Abolishes Effects of Prolonged Light Exposure.
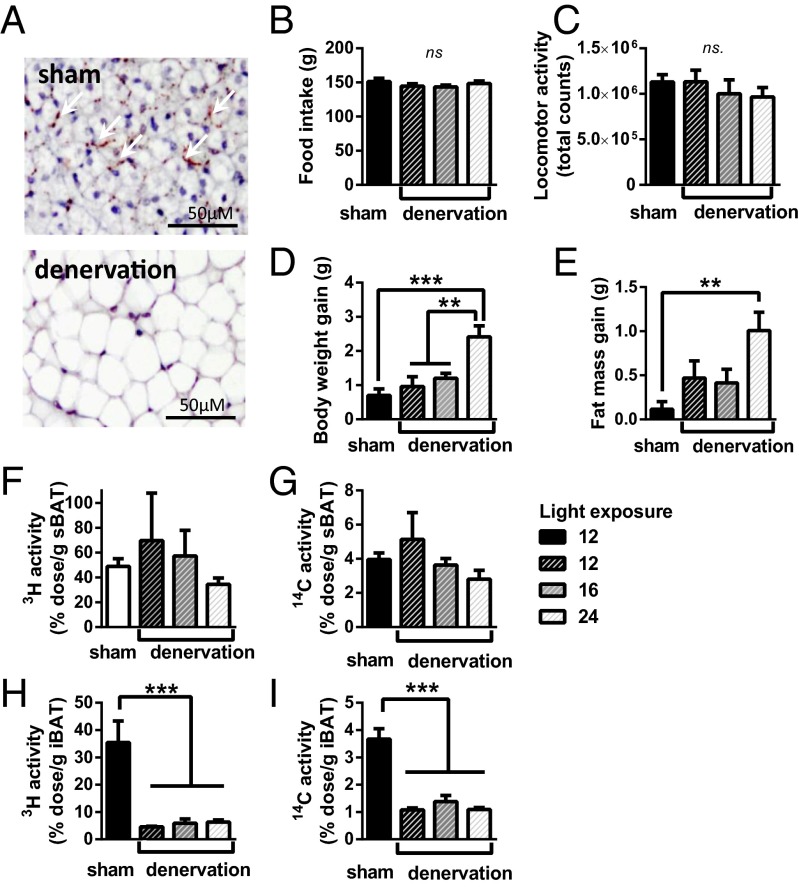
To confirm that sympathetic outflow is crucial for the observed effects of prolonged daily light exposure, we performed selective, bilateral denervation of the iBAT before exposing mice to 12, 16, or 24 h light per day. A reference group of mice underwent sham surgery and were exposed to 12-h light per day. Denervation completely abolished sympathetic input into iBAT, as evidenced by the absence of TH (Fig. 4A). In line with the previous experiment, light exposure did not affect food intake (Fig. 4B). Additionally, spontaneous locomotor activity was similar between all groups (Fig. 4C). Interestingly, body weight gain only increased in mice that were subjected to 24-h light exposure (Fig. 4D). This increase was likely because of increased fat mass gain in these animals (Fig. 4E).
Fig. 4.
Mice underwent bilateral sympathetic denervation of iBAT or sham surgery. Denervated mice were exposed to either 12-, 16-, or 24-h light (n = 5–6) while sham mice were exposed to 12-h light exposure (n = 6). After 5 wk, iBAT was isolated and histologically stained for TH. Representative images are shown (A). Total food intake (B), locomotor activity (C), body weight gain (D), and fat mass gain (E) were determined. VLDL-TG and glucose kinetics were assessed by injection of glycerol tri[3H]oleate ([3H]TO)-labeled emulsion particles and [14C]deoxyglucose ([14C]DG). Uptake of [3H]TO-derived and [14C]DG activity by sBAT (F and G) and by iBAT was determined (H and I). Data are presented as means ± SEM; ns. = not significant, *P < 0.05, **P < 0.01, ***P < 0.001.
Next, iBAT and sBAT activity was investigated by determining the ability to take up TG-derived fatty acids and glucose from plasma by injection of radiolabeled particles as described above. Although the uptake of [3H]TO-derived activity and [14C]DG by nondenervated sBAT remained high (Fig. 4 F and G, respectively), specific iBAT denervation lowered the uptake of [3H]TO-derived activity (Fig. 4H) and [14C]DG (Fig. 4I) by iBAT with ∼70–80% compared with sham operated animals (P < 0.001), indicating the importance of noradrenergic input in BAT activity. Importantly, prolonged daily light exposure did not further decrease the uptake of [3H]TO-derived activity and [14C]DG. These data indicate the reduction in adrenergic signaling may be causal in the negative correlations of hours of light exposure and BAT activity.
Discussion
This study addressed the effect of daily light exposure (12, 16, and 24 h) on energy metabolism in chow-fed C57BL/6J mice. We show that prolonging the daily light exposure increases adiposity and reduces the uptake of TG-derived fatty acids and glucose specifically by BAT, accompanied by decreased β-adrenergic signaling in BAT and decreased phosphorylation of intracellular proteins involved in thermogenesis.
Daily light-exposure duration positively associated with body fat mass and white adipocyte hypertrophy. These data are in line with observations in field voles: switching the day length from 8- to 16-h increased body weight by 24% in 4 wk compared with animals that remained on a day length of 8 h (14). Accordingly, we previously showed that prolonging day length from 12 to 24 h decreases energy expenditure in mice without increasing food intake or locomotor activity (11). Although acute light exposure at night can reduce locomotor activity (15) and prolonged light exposure affects wheel-running activity (16, 17), our present study confirms previous reports from us (11) and others (18) that prolonged light exposure does not decrease spontaneous locomotor activity. Together, these studies support the idea that prolonged daily light exposure increases body fat mass through a decrease in energy expenditure rather than to an increase in food intake or decrease of locomotor activity.
The present study strongly suggests that prolonged daily light exposure increases adiposity because of attenuation of BAT activity as reflected by the negative association between daily light exposure and the uptake of fatty acids and glucose by several BAT depots. Of note, prolonged daily light exposure did not affect uptake of nutrients by other metabolic organs, such as WAT. These data are consistent with our recent findings that attenuating BAT activity by inhibiting the central melanocortin system also reduces the influx of nutrients into BAT (19). In fact, activation of BAT (e.g., by cold exposure) increases expression of genes involved in fatty acid oxidation, glucose uptake, and lipogenesis (20), and strongly increases the uptake of TG-derived fatty acids (21) and glucose (20).
Our data are consistent with the hypothesis that prolonged daily light exposure decreases BAT activity through reduction of the sympathetic outflow toward BAT. TH, the rate-limiting enzyme in noradrenalin production, correlates with nutrient uptake by BAT, and we observed a decrease in sympathetic signaling pathways in BAT. Prolonged light exposure decreased phosphorylation of CREB and AMPK, two main targets of β3-adrenergic signaling in the brown adipocyte. AMPK not only modulates intracellular lipolysis by phosphorylation of HSL, but also regulates uptake of lipids and glucose by inducing translocation of CD36, LPL, and GLUT4 to the plasma membrane (22, 23), which may explain the reduced nutrient uptake by BAT. Moreover, we showed that in the absence of sympathetic input, BAT activity is equal among the various light-exposure groups.
We propose that the SCN directly mediates the decrease in sympathetic outflow upon prolonging daily light exposure. In previous studies we demonstrated that prolonged light exposure dampens the amplitude of electrical activity in the SCN in vitro (24) and in vivo (10). Interestingly, the amplitude of electrical activity in the SCN is linked to sympathetic outflow toward multiple organs (25). This mechanism also explains previous findings that exposure of mice to dim light (5 lx) during 10-h nights for 4 wk already results in an increase in body weight, which was accompanied by an attenuated amplitude of circadian gene expression in the hypothalamus (26). Additionally, sympathetic outflow toward BAT depots other than interscapular BAT would explain our observations that denervation of iBAT does not increase body weight. Only 24-h light exposure decreases sympathetic outflow to such an extent that mice increase significantly in body weight. Of note, the effects of light exposure on BAT are independent of melatonin secretion, because C57BL/6J mice are genetically melatonin deficient (27). However, we cannot exclude the possibility that in humans melatonin does play a role in the association between light pollution and adiposity as administration of melatonin increases BAT growth (28) and activity (29, 30) in hamsters and rats.
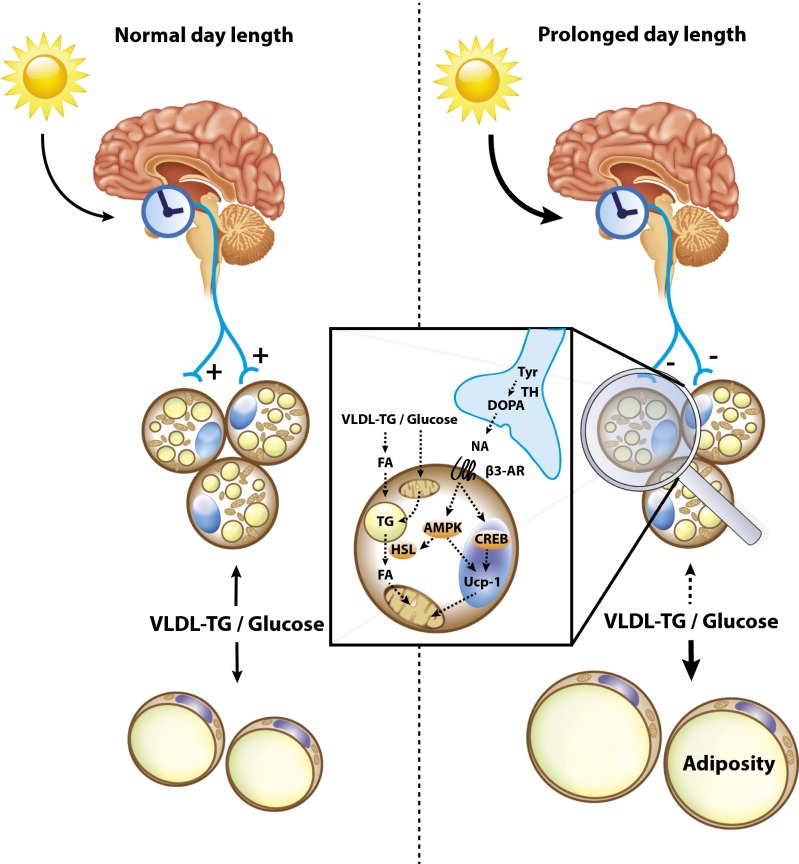
Based on our collective data, we thus propose the following mechanism by which prolonging daily light exposure increases adiposity: prolonged day length dampens the SCN amplitude, thereby lowering sympathetic outflow toward BAT resulting in decreased β3-adrenergic signaling and thermogenesis in brown adipocytes. As a consequence, the uptake of VLDL-TG derived fatty acids and glucose by BAT is reduced. The decreased combustion of fatty acids by BAT at equal energy intake thus results in a positive energy balance and therefore storage of lipids in WAT (Fig. 5).
Fig. 5.
Proposed model on how light exposure modulates body fat mass through BAT activity. Daily light-exposure duration is perceived by the suprachiasmatic nucleus, which signals toward BAT via the sympathetic nervous system. At normal day length uptake of nutrients by BAT and WAT is in balance, whereas increasing daily light exposure result in reduced BAT activation and subsequent storage of excess energy in WAT. The decrease in noradrenaline (NA) availability for stimulation of the β3-adrenergic receptor (B3-AR) leads to: (i) reduced phosphorylation of CREB, which decreases transcription of UCP1; (ii) reduced phosphorylation of AMPK, resulting in decreased phosphorylation of HSL and thus decreased lipolysis.
Recent evidence suggests that BAT activity in humans is physiologically regulated by the biological clock. The detectability of BAT by [18F]fluorodeoxyglucose-PET-CT imaging at room temperature follows a circannual cycle, both in the northern and southern hemispheres (31–33), with low detectability of BAT in summer (i.e., short day) compared with winter (i.e., long day). Although differences in outside temperature over the year would be a likely explanation for this phenomenon, the detectability of BAT showed a stronger correlation with day length than with outside temperature (31). Based on our present data, the daily light exposure may thus well explain the circannual cycle of BAT detectability. Similarly, impaired BAT activity may also explain, at least partly, the relationship between obesity and disturbances in circadian rhythmicity in humans by light pollution (2, 3, 34), and possibly also by shift work (35–37) and sleep curtailment (1, 38, 39). Additionally, our data may provide the link in the relationship between exposure to light in the bedroom and obesity (4). The suggested causal relationship has clear implications for the prevention of obesity in humans. Although the association between light in the bedroom and BAT activity in humans remains to be investigated, future lifestyle advice could include instructing people to darken their bedroom.
In conclusion, our study provides evidence that prolonged daily light exposure increases body fat mass through reduction of BAT activity. The present findings support the hypothesis that the relationship between disturbed circadian rhythmicity and adiposity in humans is mediated by impaired BAT activity.
Materials and Methods
Animal Study.
All animal experiments were approved by the institutional ethics committee on animal care and experimentation at Leiden University Medical Center, Leiden, The Netherlands. Nine- to 12-wk-old male C57BL/6J mice (Charles River) were single-housed in clear plastic cages within light-tight cabinets at constant room temperature of 22 °C. Stable temperature inside the light-tight cabinets was verified in 12-h vs. 24-h light conditions. The cages were illuminated with white fluorescent light with an intensity of ∼85 µW/cm2. Before start of the experiment, mice were kept on a regular 12:12 light-dark cycle. Mice had ad libitum access to standard laboratory chow (Special Diets Services) and water throughout experiments. Mice were matched on body weight and light intervention consisted of subjecting mice to either 12-, 16-, or 24-h light exposure per day (i.e., 24 h) for the duration of 5 wk (n = 9).
In a second study, mice were randomized to either bilateral selective sympathetic denervation (n = 17) of iBAT or sham surgery (n = 6). Mice were anesthetized (isofluorane inhalation) and a midline incision of the skin was made, exposing both iBAT pads. Sympathetic branches were visualized and cut on both sides. Wounds were closed and mice received postoperative analgesia (0.03 mg/kg buprenorphine; Temgesic, Merck). Successful denervation was confirmed retrospectively by the absence of TH in iBAT sections (see below). After 4 d of recovery, mice that underwent denervation were randomized based on body weight and exposed to 12-, 16-, or 24-h light per day for 5 wk; sham-operated mice were exposed to 12-h light per day and served as a reference group.
Body Composition, Food Intake, and Locomotor Activity.
At the end of the experiment, body weight was measured and body composition (i.e., lean mass and fat mass) was determined in conscious mice using an EchoMRI-100 (EchoMRI). Food intake was monitored by weighing food on lids either during last 2 wk of light intervention or throughout the 5 wk of light exposure (denervation experiment). Behavioral activity of mice was assessed with passive infrared detectors and recorded using Actimetrics software (Wilmette).
TG and Glucose Clearance.
At the end of the experiments, the clearance of TG and glucose was assessed. Glycerol tri[3H]oleate ([3H]TO) labeled VLDL-like emulsion particles (80 nm) were prepared as previously described (40) and [14C]deoxyglucose ([14C]DG) was added (ratio 3H:14C = 6:1). After 5 wk of light intervention, mice were fasted for 4 h [9:00 AM to 1:00 PM clock time, corresponding to Zeitgeber time (ZT) 2–6 for 12 h group and ZT 4–8 for the 16-h group] and intravenously injected with the radiolabeled emulsion particles (1.0 mg TG in 200 µL PBS) and glucose via the tail vein. At time points t = 2, 5, 10, and 15 min after injection, blood was taken from the tail vein to determine the serum decay of both radiolabels. Immediately after the last blood withdrawal, mice were killed by cervical dislocation and perfused with ice-cold PBS for 5 min. Organs were harvested, weighed, and the uptake of 3H and 14C radioactivity was determined.
Histology.
Formalin-fixed paraffin-embedded iBAT and gWAT sections were cut (5 µm). To determine gWAT cell size, sections were stained with Mayer’s H&E. White adipocyte size was quantified using ImageJ software. To determine sympathetic activation of iBAT a TH staining was performed. Sections were rehydrated and incubated 15 min with 10 mM citrate buffer (pH 6.0) at 120 °C for antigen retrieval. Sections were blocked with 5% (wt/vol) BSA/PBS followed by overnight incubation with anti-TH antibody (1:2,000, AB-112; Abcam) at 4 °C. Next, sections were incubated with a secondary antibody (anti-rabbit antibody, DAKO enVision), stained with Nova Red and counterstained with Mayer’s hematoxylin. Percentage of area positive for TH staining was quantified using ImageJ software.
Gene-Expression Analysis and Mitochondrial Assays.
A part of iBAT and sBAT was snap frozen and stored at −80 °C for gene expression analysis and protein analysis (see below). Total RNA was isolated using TriPure (Roche) according to the manufacturer’s instructions. One-microgram of total RNA was reverse-transcribed using M-MLV reverse transcriptase (Promega). Real-time PCR was carried out on a CFX96 PCR machine (Bio-Rad) using IQ SYBR-Green Supermix (Bio-Rad). Expression levels were normalized to 36B4 as housekeeping gene. Expression of mitochondrial genes was measured with quantitative PCR on a Roche Lightcycler 480 using Roche SYBR-green mastermix, using mitochondrial specific primers (Table S1). Mitochondrial DNA (mtDNA) abundance was quantified as described previously (41). In short, total DNA was extracted from sBAT tissue, using the QIAamp DSP DNA Mini Kit (Qiagen). Citrate synthase activity was measured in sBAT tissue as described previously (42).
Western Blot Analysis.
The iBAT samples stored at −80 °C were homogenized in lysis buffer. Samples were diluted and denatured for 5 min at 95 °C after adding Laemmli Sample Buffer [1:1 (vol/vol); Serva]. Proteins within homogenates (15 μg) were separated on a 10% (wt/vol) SDS-page gel and subsequently transferred onto blotting membranes. The blotting membranes were blocked with 5% (wt/vol) milk powder and incubated overnight at 4 °C with the primary antibody β-actin, pCREB, pAMPK, or pHSL S565 (Cell Signaling). Secondary antibody (anti-rabbit IgG HRP conjugate; 1:5,000; Promega) was added and SuperSignal Western Blot Enhancer (Thermo Scientific) was used to visualize protein bands. Blots were analyzed with Bio-Rad Quantity One and normalized to β-actin.
Statistical Analysis.
Data are presented as means ± SEM. Correlations between two dependent variables were made using Pearson’s correlation. Associations of variables with day length were assessed by linear regression analysis. Differences between groups were determined using T-tests for normally distributed data. Contribution of light exposure as a covariate to body weight gain was analyzed by mixed-model analysis using IBM SPSS Statistics v20. To assess behavioral activity, actograms were analyzed using Clock laboratory and rhythmicity F periodogram analysis was performed on activity bins of 10 min of the last 10 consecutively recorded days, based on the algorithm of Dörrscheidt and Beck (43). Differences at P values < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
The authors thank Simone Denis, Julia Melia Aloma, Laura Sardón Puig, and Lianne van der Wee-Pals for excellent technical support. This research was supported by the Netherlands Organisation for Scientific Research NWO-VENI Grants 016.136.125 (to N.R.B.) and 91613050 (to R.H.H.); European Foundation for the Study of Diabetes and the Programme Partner Novo Nordisk Grant 94802 (to C.P.C., J.H.M., and P.C.N.R.); Dutch Diabetes Research Foundation Grant 2013.81.1663 (to C.P.C.); a Rembrandt Institute for Cardiovascular Science Rembrandt Research award (to M.R.B. and R.H.H.); and an Academic Medical Center PhD fellowship (to I.A.C.). P.C.N.R. is an Established Investigator of the Dutch Heart Foundation (Grant 2009T038).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.P. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504239112/-/DCSupplemental.
References
- 1.Killick R, Banks S, Liu PY. Implications of sleep restriction and recovery on metabolic outcomes. J Clin Endocrinol Metab. 2012;97(11):3876–3890. doi: 10.1210/jc.2012-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Obayashi K, et al. Exposure to light at night, nocturnal urinary melatonin excretion, and obesity/dyslipidemia in the elderly: A cross-sectional analysis of the HEIJO-KYO study. J Clin Endocrinol Metab. 2013;98(1):337–344. doi: 10.1210/jc.2012-2874. [DOI] [PubMed] [Google Scholar]
- 3.Wyse CA, Selman C, Page MM, Coogan AN, Hazlerigg DG. Circadian desynchrony and metabolic dysfunction; did light pollution make us fat? Med Hypotheses. 2011;77(6):1139–1144. doi: 10.1016/j.mehy.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 4.McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. The relationship between obesity and exposure to light at night: Cross-sectional analyses of over 100,000 women in the Breakthrough Generations Study. Am J Epidemiol. 2014;180(3):245–250. doi: 10.1093/aje/kwu117. [DOI] [PubMed] [Google Scholar]
- 5.Perreau-Lenz S, Pévet P, Buijs RM, Kalsbeek A. The biological clock: The bodyguard of temporal homeostasis. Chronobiol Int. 2004;21(1):1–25. doi: 10.1081/cbi-120027984. [DOI] [PubMed] [Google Scholar]
- 6.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485(7396):123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi SQ, Ansari TS, McGuinness OP, Wasserman DH, Johnson CH. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23(5):372–381. doi: 10.1016/j.cub.2013.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimba S, et al. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc Natl Acad Sci USA. 2005;102(34):12071–12076. doi: 10.1073/pnas.0502383102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turek FW, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coomans CP, et al. The suprachiasmatic nucleus controls circadian energy metabolism and hepatic insulin sensitivity. Diabetes. 2013;62(4):1102–1108. doi: 10.2337/db12-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coomans CP, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27(4):1721–1732. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 12.Amir S. Retinohypothalamic tract stimulation activates thermogenesis in brown adipose tissue in the rat. Brain Res. 1989;503(1):163–166. doi: 10.1016/0006-8993(89)91720-4. [DOI] [PubMed] [Google Scholar]
- 13.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276(6 Pt 2):R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 14.Król E, et al. Effect of photoperiod on body mass, food intake and body composition in the field vole, Microtus agrestis. J Exp Biol. 2005;208(Pt 3):571–584. doi: 10.1242/jeb.01429. [DOI] [PubMed] [Google Scholar]
- 15.Redlin U. Neural basis and biological function of masking by light in mammals: Suppression of melatonin and locomotor activity. Chronobiol Int. 2001;18(5):737–758. doi: 10.1081/cbi-100107511. [DOI] [PubMed] [Google Scholar]
- 16.Morin LP, Lituma PJ, Studholme KM. Two components of nocturnal locomotor suppression by light. J Biol Rhythms. 2010;25(3):197–207. doi: 10.1177/0748730410369890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mrosovsky N, Foster RG, Salmon PA. Thresholds for masking responses to light in three strains of retinally degenerate mice. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1999;184(4):423–428. doi: 10.1007/s003590050341. [DOI] [PubMed] [Google Scholar]
- 18.Fonken LK, et al. Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci USA. 2010;107(43):18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kooijman S, et al. Inhibition of the central melanocortin system decreases brown adipose tissue activity. J Lipid Res. 2014;55(10):2022–2032. doi: 10.1194/jlr.M045989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu XX, Lewin DA, Forrest W, Adams SH. Cold elicits the simultaneous induction of fatty acid synthesis and beta-oxidation in murine brown adipose tissue: Prediction from differential gene expression and confirmation in vivo. FASEB J. 2002;16(2):155–168. doi: 10.1096/fj.01-0568com. [DOI] [PubMed] [Google Scholar]
- 21.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–205. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 22.Samovski D, Su X, Xu Y, Abumrad NA, Stahl PD. Insulin and AMPK regulate FA translocase/CD36 plasma membrane recruitment in cardiomyocytes via Rab GAP AS160 and Rab8a Rab GTPase. J Lipid Res. 2012;53(4):709–717. doi: 10.1194/jlr.M023424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeppesen J, et al. Contraction-induced skeletal muscle FAT/CD36 trafficking and FA uptake is AMPK independent. J Lipid Res. 2011;52(4):699–711. doi: 10.1194/jlr.M007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.VanderLeest HT, et al. Seasonal encoding by the circadian pacemaker of the SCN. Curr Biol. 2007;17(5):468–473. doi: 10.1016/j.cub.2007.01.048. [DOI] [PubMed] [Google Scholar]
- 25.Kalsbeek A, et al. SCN outputs and the hypothalamic balance of life. J Biol Rhythms. 2006;21(6):458–469. doi: 10.1177/0748730406293854. [DOI] [PubMed] [Google Scholar]
- 26.Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ. Dim light at night disrupts molecular circadian rhythms and increases body weight. J Biol Rhythms. 2013;28(4):262–271. doi: 10.1177/0748730413493862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roseboom PH, et al. Natural melatonin ‘knockdown’ in C57BL/6J mice: Rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63(1):189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 28.Heldmaier G, Hoffmann K. Melatonin stimulates growth of brown adipose tissue. Nature. 1974;247(5438):224–225. doi: 10.1038/247224a0. [DOI] [PubMed] [Google Scholar]
- 29.Holtorf AP, Heldmaier G, Thiele G, Steinlechner S. Diurnal changes in sensitivity to melatonin in intact and pinealectomized Djungarian hamsters: Effects on thermogenesis, cold tolerance, and gonads. J Pineal Res. 1985;2(4):393–403. doi: 10.1111/j.1600-079x.1985.tb00719.x. [DOI] [PubMed] [Google Scholar]
- 30.Hagelstein KA, Folk GE., Jr Effects of photoperiod, cold acclimation and melatonin on the white rat. Comp Biochem Physiol C. 1979;62C(2):225–229. doi: 10.1016/0306-4492(79)90015-7. [DOI] [PubMed] [Google Scholar]
- 31.Au-Yong IT, Thorn N, Ganatra R, Perkins AC, Symonds ME. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58(11):2583–2587. doi: 10.2337/db09-0833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perkins AC, Mshelia DS, Symonds ME, Sathekge M. Prevalence and pattern of brown adipose tissue distribution of 18F-FDG in patients undergoing PET-CT in a subtropical climatic zone. Nucl Med Commun. 2013;34(2):168–174. doi: 10.1097/MNM.0b013e32835bbbf0. [DOI] [PubMed] [Google Scholar]
- 33.Persichetti A, et al. Prevalence, mass, and glucose-uptake activity of 18F-FDG-detected brown adipose tissue in humans living in a temperate zone of Italy. PLoS ONE. 2013;8(5):e63391. doi: 10.1371/journal.pone.0063391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid KJ, et al. Timing and intensity of light correlate with body weight in adults. PLoS ONE. 2014;9(4):e92251. doi: 10.1371/journal.pone.0092251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karlsson BH, Knutsson AK, Lindahl BO, Alfredsson LS. Metabolic disturbances in male workers with rotating three-shift work. Results of the WOLF study. Int Arch Occup Environ Health. 2003;76(6):424–430. doi: 10.1007/s00420-003-0440-y. [DOI] [PubMed] [Google Scholar]
- 36.Di Lorenzo L, et al. Effect of shift work on body mass index: Results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord. 2003;27(11):1353–1358. doi: 10.1038/sj.ijo.0802419. [DOI] [PubMed] [Google Scholar]
- 37.Barbadoro P, et al. Rotating shift-work as an independent risk factor for overweight Italian workers: A cross-sectional study. PLoS ONE. 2013;8(5):e63289. doi: 10.1371/journal.pone.0063289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: A large-scale prospective study. Sleep. 2010;33(2):161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hairston KG, et al. Sleep duration and five-year abdominal fat accumulation in a minority cohort: the IRAS family study. Sleep. 2010;33(3):289–295. doi: 10.1093/sleep/33.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rensen PC, et al. Particle size determines the specificity of apolipoprotein E-containing triglyceride-rich emulsions for the LDL receptor versus hepatic remnant receptor in vivo. J Lipid Res. 1997;38(6):1070–1084. [PubMed] [Google Scholar]
- 41.Houtkooper RH, et al. The metabolic footprint of aging in mice. Sci Rep. 2011;1:134. doi: 10.1038/srep00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ventura FV, Ruiter J, Ijlst L, de Almeida IT, Wanders RJ. Differential inhibitory effect of long-chain acyl-CoA esters on succinate and glutamate transport into rat liver mitochondria and its possible implications for long-chain fatty acid oxidation defects. Mol Genet Metab. 2005;86(3):344–352. doi: 10.1016/j.ymgme.2005.07.030. [DOI] [PubMed] [Google Scholar]
- 43.Dörrscheidt GL, Beck L. Advanced methods for evaluating characteristic parameters of circadian rhythms. J Mathemat Biol. 1975;2:107–121. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.