Abstract
Resurgent sodium currents likely play a role in modulating neuronal excitability. Here we studied whether protein kinase C (PKC) activation can increase resurgent currents produced by the human sodium channel hNav1.7. We found that a PKC agonist significantly enhanced hNav1.7-mediated resurgent currents and this was prevented by PKC antagonists. The enhancing effects were replicated by two phosphorylation-mimicking mutations and were prevented by a phosphorylation-deficient mutation at a conserved PKC phosphorylation site (Serine 1479). Our results suggest that PKC can increase sodium resurgent currents through phosphorylation of a conserved Serine residue located in the domain III–IV linker of sodium channels.
Keywords: resurgent currents, hNav1.7, PKC, voltage clamp, mutation, sodium channel
Introduction
Resurgent currents are generated by a subset of voltage-gated sodium channels (VGSC) during repolarization of the action potential (AP) when sodium channels are normally thought to be inactivated and refractory to activation (Raman and Bean 1997). They can provide a depolarizing drive for AP regeneration, and therefore likely contribute to modulating neuronal excitability (Raman and Bean 2001). Sodium channel mutations associated with multiple diseases including paroxysmal extreme pain disorder (PEPD), paramyotonia congenita, and long QT-3 dramatically increase resurgent currents and neuronal excitability when expressed in dorsal root ganglion (DRG) neurons (Jarecki et al., 2010). Recently we found that resurgent currents in DRG neurons were significantly enhanced by a group of inflammatory mediators including PGE2, Bradykinin, ATP, histamine, and serotonin (Tan et al., 2014). These inflammatory mediators are known to increase excitability of DRG neurons through activation of cell signaling pathways. Previously it was shown that phosphorylation of some component of the sodium channel complex substantially modulated resurgent currents in cerebellar Purkinje neurons (Grieco et al., 2002). Protein kinase C (PKC) can be activated by several of the inflammatory mediators listed above (Seabrook et al., 1997; Wang et al., 2007) and therefore we postulated that PKC activation might modulate resurgent sodium currents.
In the current report, we first tested if PKC activation can increase resurgent currents generated in the human sodium channel hNav1.7 expressed in human embryonic kidney 293 (HEK293) cells. We found that the PKC agonist Phorbol 12-myristate 13-acetate (PMA) significantly enhanced hNav1.7-mediated resurgent currents and that this effect was prevented by the PKC antagonists Bisindolylmaleimide I (Bis I) or Chelerythrine. We further tested if a conserved PKC phosphorylation site, S1479 is involved in the enhancing effects of PMA on resurgent currents utilizing site-directed mutagenesis. We found that the enhancing effects were mimicked by two phosphorylation-mimicking mutations and were prevented by a phosphorylation-deficient mutation. Furthermore, we found that PMA significantly increased resurgent currents generated by a hNav1.7 mutant that is associated with PEPD. Our results suggest that PKC can increase sodium resurgent currents through a conserved Serine residue located in the domain III–IV linker of sodium channels.
Materials and Methods
Cell lines
stably transfected cell lines were prepared as described before (Theile and Cummins, 2011). Briefly, mutations of hNav1.7 including S1479D, S1479E, S1479A, and M1627K were generated using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). DNAs of wild-type (WT) and mutations of hNav1.7 were transfected into HEK293 cells using the calcium phosphate technique. Stable cell lines expressing WT and mutant hNav1.7 were selected after 3 weeks of incubation with G418. Stocks of stable cell lines were frozen at −80°C.
Cell culture
HEK293 cells expressing WT or mutant hNav1.7 were thawed and grown in petri dishes until they were plated on glass coverslips coated with poly-D-lysine and laminin. Cells were incubated in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum at 37°C in a humidified 95% air and 5% CO2 incubator.
Voltage clamp
Whole-cell patch clamp recordings were conducted in voltage-clamp mode at room temperature (about 22°C) using a HEKA EPC-10 amplifier. Data were acquired on a Windows based Pentium IV computer using the Pulse program (version 8.80; HEKA Elektronik). Fire polished electrodes (0.8–1.2 MΩ) were fabricated from 1.7-mm capillary glass using a Sutter P-97 puller (Novato), and the tips were coated with sticky wax (KerrLab) to minimize capacitive artifacts and enable increased series resistance compensation. The standard electrode solution consisted of 140 mM CsF, 10 mM NaCl, 1.1 mM EGTA, and 10 mM HEPES, pH 7.3. The standard extracellular bathing solution contained 140 mM NaCl, 3 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 10 mM HEPES, pH 7.3.
Cells on glass coverslips were transferred to a recording chamber containing 250 μl bathing solution. Series resistance errors were compensated by 80% or more. Leak currents were linearly canceled by digital P/–5 subtraction. Cells were held at a membrane potential of –100 mV. Membrane currents were sampled at 20 kHz, filtered online at 5 kHz, and further filtered at 1 kHz digitally in Pulsefit. Whole-cell current recordings were started 3 minutes after the whole-cell configuration had been established to allow adequate time for the electrode solution and cytoplasmic milieu to equilibrate. Resurgent currents were assayed with a 2-step protocol that initially depolarized the membrane to +30 mV for 20 ms before testing for inward resurgent sodium currents by hyperpolarizing the membrane potential in –5-mV increments from 5 mV to –85 mV, for 50 ms, before stepping to −80 mV. Each voltage step was repeated three times and averaged to accurately record resurgent currents at each voltage. 200 μM β4 peptide (a peptide mimicking the C-terminal segment of the sodium channel β4 subunit, KKLITFILKKTREK-OH) was included in the pipette solution. The peak transient current-voltage (IV) relationship was tested using a series of depolarizing voltage steps in 5-mV increments ranging from −100 to +55 mV. Steady-state inactivation was tested by a test pulse to 0 mV from a series of 500 ms pre-holding pulses in 5-mV increments ranging from −130 to −5 mV.
PMA or control DMSO (0.1% v/v) was pretreated for 5 min in the recording chamber before a patch trial started. Bis I and Chelerythrine were pretreated for 15 min in culture and kept in the recording chamber. All three chemicals were dissolved in DMSO at 1 mM and were used at a final concentration of 1 μM.
Data analysis
Experimental data were analyzed using Pulsefit (version 8.80; HEKA Elektronik), Origin (version 8.0; OriginLab Corp.), and Microsoft Excel software programs. Averaged data were presented as Mean ± standard error. One way ANOVA with post hoc Tukey test, and Student t test were used to test the significance of statistical data. The significance levels were set at P<0.05 and P<0.01.
Resurgent currents were first identified by their kinetics and gating properties. Compared to fast tail currents which have a linear IV relationship, resurgent currents develop and decay much more slowly, and have a “V” shaped IV curve that peaks around −35 mV. Currents showing both criteria were classified as resurgent currents. Currents that did not display both properties were excluded from analysis. To compare the resurgent currents among different mutant or treatment groups, ratio resurgent currents were calculated by normalizing peak resurgent currents to the peak transient current triggered by a test pulse to 0 mV from a 500 ms holding pulse of −110 mV.
Voltage-gating properties including steady-state activation and inactivation, and kinetic properties including time-dependent activation and inactivation were analyzed in Pulsefit. Half activation or inactivation voltage (V0.5), and time constants for activation (τm) and inaction (τh) were obtained.
Results
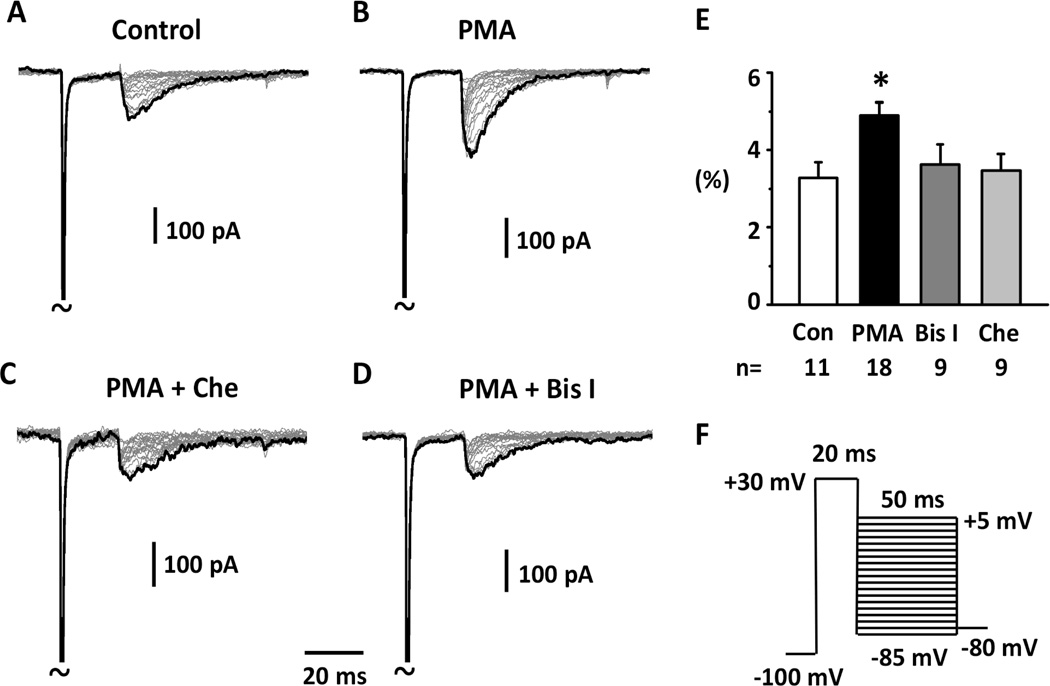
As shown in Fig.1, resurgent currents from hNav1.7 expressed in HEK 293 cells were recorded using the resurgent current protocol which first inactivated peak transient sodium current by a voltage step to +30 mV for 20 ms, and then repolarized cells to a series of voltage stimuli to test for the re-opening of sodium channels. To test whether PKC activation can modulate resurgent currents generated by hNav1.7, we used a PKC agonist PMA and two PKC antagonists Bis I and Chelerythrine (Herbert et al., 1990; Toullec et al., 1991). We found that 1μM PMA significantly increased peak resurgent current amplitude (Fig.1A,B,E) without changing the transient current density (measured with the steady-state inactivation protocol by the −110 to 0 mV voltage step; 289±39 vs. 273±59 pA/pF, Control vs. PMA). PMA treatment augmentation of hNav1.7 resurgent current amplitude was prevented by pretreatment with the PKC antagonists Bis I or Chelerythrine at the concentration of 1 μM (Fig 1) (Thomas et al., 2004; Harmati et al., 2011). In addition, 1μM PMA also significantly shifted the peak voltage, the voltage where peak resurgent current was measured (from −39 mV for control to −33 mV for PMA treated; Suppl Fig 1). However, only Bis I but not Chelerythrine prevented this shift (Suppl Fig 1). Overall the results showed that PKC activation increased the amplitude of resurgent currents mediated by hNav1.7.
Fig.1. Increase of hNav1.7 resurgent currents by PKC activation.
Representative resurgent currents were recorded from HEK 293 cells expressing hNav1.7 in the presence of 200 μM β4 peptide in the patch pipettes. The currents were recorded using the voltage protocol shown in Fig.1F. Note that currents are scaled to reflect the ratio resurgent currents. The ratio resurgent currents were calculated by normalizing peak resurgent currents to peak transient currents recorded in the same cells. (A & B) PMA significantly increased resurgent currents compared to control. (C & D) PKC antagonists Bisindolylmaleimide I (Bis I) or Chelerythrine (Che) prevented the increasing effects of PMA. (E) Summary of ratio resurgent current. The data were presented as Means ± Standard Errors. *, P<0.05, PMA vs. Control (One way ANOVA, Tukey test).
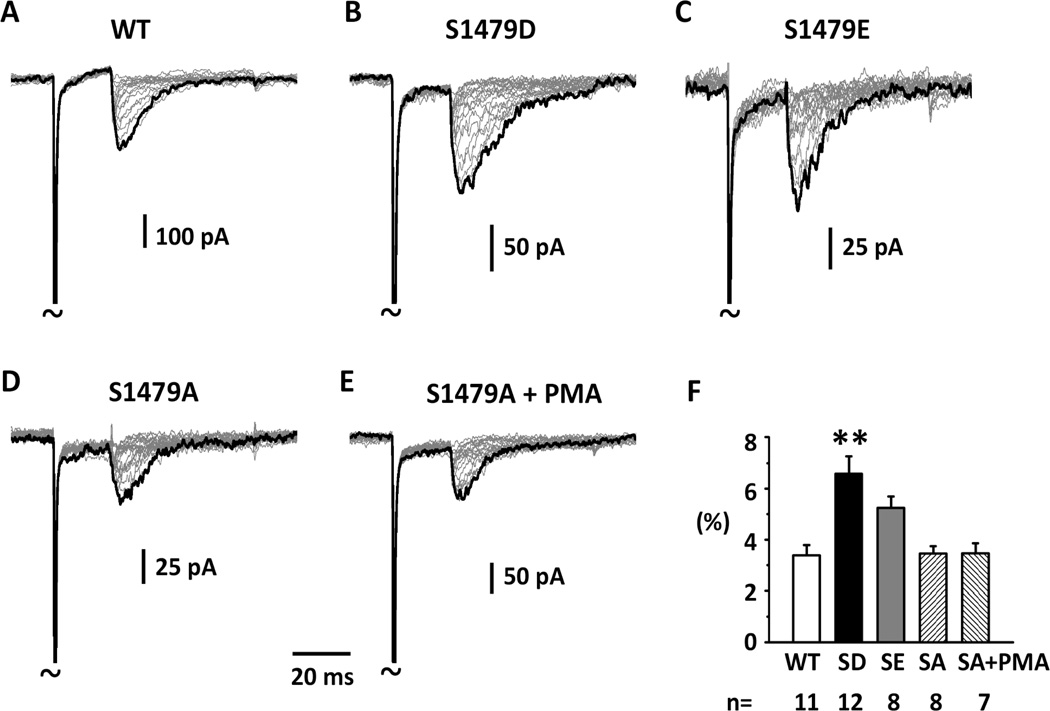
It has been reported that there are three conserved PKC phosphorylation sites located in intracellular loops of voltage-gated sodium channels (West et al., 1991; Cantrell et al., 2002). Two sites are located at the domain I-II linker along with several PKA sites (Cantrell et al., 2002). The third site is located at the domain III–IV linker and is close to the IFM inactivation gate (West et al., 1991). Because the proposed mechanism for resurgent current activation is open-channel block induced by the intracellular C-terminal loop of the sodium channel β4 subunit (Grieco et al., 2005), and mutations or toxins that interfere with sodium channel fast inactivation can increase resurgent currents (Klinger et al., 2012; Lewis and Raman, 2013), we suspected that the conserved phosphorylation site in the domain III–IV linker might be involved in the effects of PMA on hNav1.7-produced resurgent currents. To examine this possibility, we mutated this phosphorylation site S1479 to either aspartate or glutamate (S1479D and S1479E) to mimic the phosphorylated state, and to alanine (S1479A) to prevent phosphorylation. We then constructed stably transfected HEK 293 cell lines for WT and mutant hNav1.7 and compared their ability to generate resurgent currents. We found that one phosphorylation-mimicking mutant S1479D dramatically increased hNav1.7-generated resurgent currents (Fig.2ABF). The other phosphorylation-mimicking mutant S1479E also showed a trend toward increased resurgent current compared to WT channel (P<0.1, Tukey test, one way ANOVA) (Fig.2ACF). The phosphorylation-deficient mutant S1479A did not significantly affect resurgent currents (Fig.2D), but prevented the effects of PMA on resurgent currents (Fig.1 & Fig.2 DEF). There were no significant differences in the peak voltage of resurgent currents between WT and mutants (Supplemental Fig. 1). The above results suggested that phosphorylation of S1479 in hNav1.7 by PKC can increase resurgent currents.
Fig.2. Increase of hNav1.7 resurgent currents by phosphorylation-mimicking mutations.
Representative resurgent currents were recorded from HEK 293 cells expressing WT and mutant hNav1.7 channels in the presence of 200 μM β4 peptide in the patch pipettes. The currents were recorded using the voltage protocol shown in Fig.1F. Note that currents are scaled to reflect the ratio resurgent currents. The ratio resurgent currents were calculated by normalizing peak resurgent currents to peak transient currents recorded in the same cells. One phosphorylation-mimicking mutant S1479D dramatically increased resurgent currents compared to control. The other phosphorylation-mimicking mutant S1479E showed a trend increase in resurgent current compared to WT (P<0.10). (D & E) a phosphorylation-deficient mutant (S1479A) did not change resurgent currents by itself. However, S1479A prevented the increasing effects of PMA on resurgent currents shown in Figure 1. (F) Summary of ratio resurgent current. The data were presented as Means ± Standard Errors. **, P<0.01 (One way ANOVA, Tukey test).
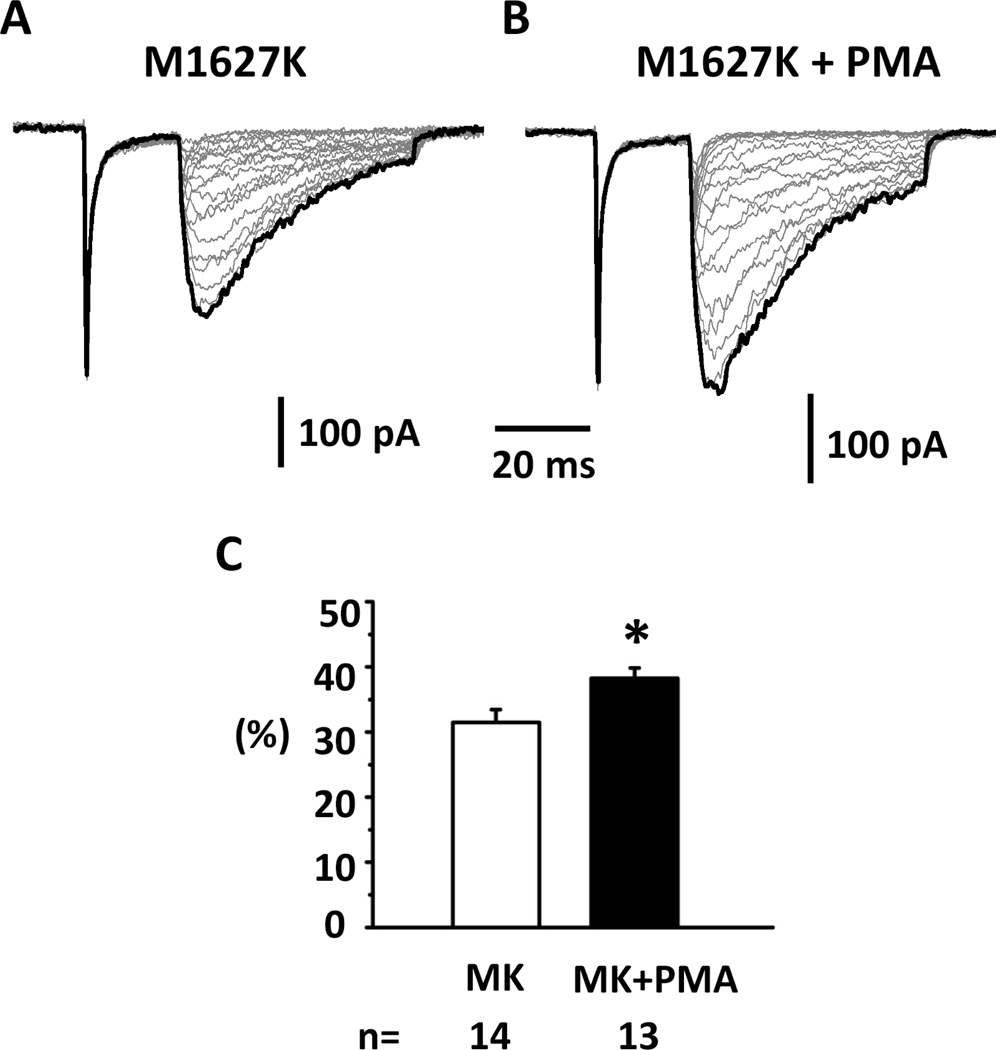
Our lab previously reported that Nav1.7 mutations associated with PEPD enhanced resurgent currents in HEK 293 cells (Theile et al., 2011). We tested whether PKC activation can further enhance resurgent currents generated by a PEPD mutant, M1627K. We found that the PKC agonist PMA significantly enhanced resurgent currents produced by M1627K mutant Nav1.7 channels (Fig.3). The results suggest that PKC and M1627K might enhance hNav1.7-produced resurgent currents through mechanisms that are at least partially additive.
Fig.3. Increase of PEPD mutant resurgent currents by PMA.
Representative resurgent currents were recorded from HEK 293 cells expressing a PEPD mutant of hNav1.7 (M1627K) in the presence of 200 μM β4 peptide in the patch pipettes. The currents were recorded using the voltage protocol shown in Fig.1F. Note that currents are scaled to reflect the ratio resurgent currents. The ratio resurgent currents were calculated by normalizing peak resurgent currents to peak transient currents recorded in the same cells. (A & B) PMA increased resurgent currents compared to control M1627K. (C) Summary of ratio resurgent current. The data were presented as Means ± Standard Errors. *, P<0.05 (Student t test).
It has been shown that PKC activation can modulate classic current properties of sodium channels (Vijayaragavan et al., 2004; Chen et al., 2006). To test how the classic transient hNav1.7 current properties may be modulated by PKC phosphorylation, we examined the effects of PKC agonist and antagonists, and S1479 mutations on current properties in the absence of intracellular β4 peptide (Table 1, Supplemental Fig. 2). We found that PKC agonist PMA and S1479 phosphorylation-mimicking mutants caused similar changes in transient current properties of hNav1.7 (Table 1, Supplemental Fig. 2). Both PMA and S1479E significantly shifted the half activation potential to more depolarized potentials; both PMA and S1479D significantly shifted the half inactivation potential to more depolarized potentials; and both PMA and the S1479D significantly increased the time constants for current inactivation. The effects of PMA on these transient current properties were prevented by PKC antagonists Bis I or Chelerythrine (Table 1, Supplemental Fig. 2). The phosphorylation-deficient mutant S1479A did not cause any changes in regular current properties (Table 1, Supplemental Fig. 2). On the other hand, slope factors for current gating voltage-dependence were only modulated by S1479E compared to PMA (Table 1, Supplemental Fig. 2). The results suggested that PKC modulated transient current properties through phosphorylation of S1479 in hNav1.7 channels.
Table 1.
Effects of PKC activation on current properties of regular hNav1.7 currents
| Gating Properties | Kinetics | |||||
|---|---|---|---|---|---|---|
| Activation | Inactivation | Activation | Inactivation | |||
| V0.5 | sf | V0.5 | sf | τm | τh | |
| mV | mV | mV | mV | ms | ms | |
| Control | −27.5 ± 2.3 | 6.0 ± 0.3 | −78.3 ± 2.1 | −7.4 ± 0.2 | 0.33 ± 0.01 | 1.30 ± 0.16 |
| n | 14 | 14 | 13 | |||
| PMA | −19.3 ± 2.4* | 6.1 ± 0.2 | −71.6 ± 1.5* | −7.6 ± 0.5 | 0.42 ± 0.01** | 2.16 ± 0.25* |
| n | 18 | 18 | 16 | |||
| BisI + PMA | −21.6 ± 1.2 | 6.8 ± 0.3 | −75.9 ± 1.3 | −7.6 ± 0.2 | 0.38 ± 0.03 | 1.74 ± 0.13 |
| n | 8 | 8 | 7 | |||
| Che + PMA | −23.9 ± 2.0 | 6.1 ± 0.4 | −76.1 ± 1.4 | −7.3 ± 0.3 | 0.38 ± 0.03 | 1.56 ± 0.20 |
| n | 6 | 6 | 5 | |||
| WT | −27.9 ± 1.2 | 6.1 ± 0.2 | −80.2 ± 1.1 | −7.3 ± 0.2 | 0.31 ± 0.02 | 1.47 ± 0.11 |
| n | 19 | 19 | 16 | |||
| S1479D | −23.9 ± 1.2 | 6.2 ± 0.3 | −71.8 ± 1.6** | −8.3 ± 0.5 | 0.37 ± 0.01 | 3.24 ± 0.27** |
| n | 11 | 11 | 11 | |||
| S1479E | −21.4 ± 2.0* | 7.5 ± 0.4* | −79.9 ± 2.3 | −8.9 ± 0.6* | 0.32 ± 0.04 | 1.85 ± 0.15 |
| n | 9 | 9 | 6 | |||
| S1479A | −24.7 ± 2.3 | 6.3 ± 0.5 | −79.8 ± 1.9 | −7.3 ± 0.3 | 0.27 ± 0.03 | 1.18 ± 0.23 |
| n | 8 | 8 | 5 | |||
Values are mean ± SE; n is number of cells. V0.5, half activation/inactivation potential; sf, slope factor; τm, activation time constant at −20 mV; τh, inactivation time constant at −20 mV.
P<0.05,
P<0.01 (One way ANOVA, Tukey test, significant difference between indicated group and Control or WT).
Discussion
Researchers have shown that sodium resurgent currents can be modulated by a variety of factors such as sodium channel mutation (Jarecki et al., 2010; Lewis and Raman, 2013), sodium channel β4 subunit (Bant and Raman, 2010), natural toxins (Klinger et al., 2012) and chemotherapy drugs (Sittl et al., 2012). Resurgent currents can be up-regulated by inflammatory mediators associated with pain hypersensitivity (Tan et al., 2014) or in epilepsy models (Hargus et al., 2011). However to our knowledge, there is only one published study investigating the possible roles of cell signaling in modulating resurgent currents (Grieco et al., 2002). In this study, the authors found that intracellular application of alkaline phosphatase abolished resurgent currents in cerebellar Purkinje neurons. The results indicated that constitutive phosphorylation of some part of the sodium channel complex is necessary for producing resurgent currents in Purkinje neurons. In the current report, we showed that PKC activation by PMA increased resurgent currents of hNav1.7 in HEK193 cells. These effects were blocked by PKC antagonists Bis I and Chelerythrine. Our results show for the first time that a specific protein kinase, PKC, can modulate sodium resurgent currents.
We recently showed that a group of inflammatory mediators including PGE2, Bradykinin, ATP, histamine, and serotonin can increase resurgent currents in DRG neurons (Tan et al., 2014). Several of these inflammatory mediators can activate the PKC pathway, either directly or indirectly (Seabrook et al., 1997; Wang et al., 2007). The current finding of PKC increasing resurgent currents of hNav1.7 in HEK293 cells suggest that PKC activation might contribute to the effects of inflammatory mediators on resurgent currents in DRG neurons. However, because the cell environment and sodium channels isoforms can affect generation of resurgent currents in endogenous neurons (Raman and Bean, 1997; Cummins et al., 2005), further studies need to be conducted to fully test this possibility. It would be interesting to test if other protein kinases contribute to the enhancing effects of inflammatory mediators on resurgent currents in DRG neurons. For example, ERK1/2 activation was found to directly phosphorylate Nav1.7 and an ERK1/2 inhibitor caused a depolarizing shift in the activation curve of Nav1.7 and decreased excitability of DRG neurons (Stamboulian et al., 2010).
Three conserved PKC phosphorylation sites in sodium channels have been reported (West et al., 1991; Cantrell et al., 2002). Two of them are located in the domain I–II loop; the other one is located in the domain III–IV loop close to IFM inactivation gate. In the current study, we found that two phosphorylation-mimicking mutations at the conserved phosphorylation site in the domain III–IV loop, which is S1479 in hNav1.7, increased or showed a trend increase of resurgent currents. Furthermore, a phosphorylation-deficient mutant at the same site (S1479A) prevented the increasing effects of PMA on resurgent currents. The results suggest PKC activation can increase resurgent current of hNav1.7 through a conserved phosphorylation site in the domain III–IV loop. The current study did not mutate the other two phosphorylation sites or test the effects of PKC activation on resurgent currents in the S1479D and S1479E mutants. Therefore, a contributing role of other phosphorylation sites to the enhancing effects of PKC activation cannot be fully excluded.
PKC has been shown to modulate current properties of sodium channels expressed in different tissues. PKC activation reportedly slowed time-dependent inactivation in the brain sodium channel Nav1.2 through the conserved phosphorylation site in the domain III–IV loop (West et al., 1991). PKC caused a hyperpolarizing shift of steady-state inactivation in cardiac (Nav1.5) and skeletal (Nav1.4) sodium channel with different mechanisms (Bendahhou et al., 1995; Qu et al., 1996): the effects on Nav1.5, but not for Nav1.4, are dependent on the conserved phosphorylation site in domain III–IV loops. PKC activation resulted in a depolarizing shift of steady-state activation in peripheral sodium channel isoforms Nav1.7 and Nav1.8 in Xenopus oocytes (Vijayaragavan et al., 2004). In the current study using HEK293 cells, we found that PKC activation by PMA significantly shifted gating curves of both steady-state activation and inactivation to more depolarized potentials, and slowed time-dependent current activation and inactivation. We further found that phosphorylation-mimicking mutants of S1479 showed similar effects on gating and kinetic properties of hNav1.7. The results reproduce some of the previous findings of slowing inactivation in Nav1.2 (West et al., 1991) and shifting the steady-state activation of Nav1.7 to more depolarized potentials (Vijayaragavan et al., 2004). Toxins that slow inactivation (Klinger et al., 2012) and some disease mutations that slow inactivation (Jarecki et al., 2010) can enhance resurgent sodium currents in neurons. Although PKC phosphorylation of S1479 does not seem to be absolutely necessary for the generation of resurgent currents, PKC slowing of inactivation can increase resurgent current amplitudes and, as with toxin and disease mutation induced resurgent currents, this is likely to contribute to increased neuronal excitability.
PKC was reported to have diverse effects on Nav1.7 in terms of transient current density or protein expression in different tissue. In in vitro cultured DRG neurons, PKC was found to increase Nav1.7 expression (Chattopadhyay et al., 2008). One the other hand, PKC decreased Nav1.7 current or expression in Xenopus oocytes (Vijayaragavan et al., 2004) or adrenal chromaffin cells (Wada et al., 2004). In the current study using HEK 293 cells, we did not see a significant change in transient current density caused by PKC activation. These results suggest PKC can have complex effects on Nav1.7 current or channel expression in different tissues and conditions.
In summary, while phosphorylation of S1479 is not necessary for Nav1.7 resurgent current generation, it can substantially increase Nav1.7 resurgent current amplitude. As this site is conserved in Nav1.1-Nav1.8, we predict that PKC is likely to modulate resurgent currents in multiple isoforms through phosphorylation of this conserved serine.
Supplementary Material
Highlights.
PKC activation increased resurgent currents produced by hNav1.7
Phosphorylation-mimicking mutations at S1479 increased resurgent currents
Phosphorylation-deficient mutation prevented increasing effects of PKC
These data suggest PKC increases resurgent currents through S1479 phosphorylation
Acknowledgements
The research was supported by a Lilly Research Award. T.R.C. was also supported in part by a National Institutes of Health grant (NS053422).
Abbreviations
- PEPD
Paroxysmal extreme pain disorder
- VGSC
Voltage-gated sodium channels
- Bis I
Bisindolylmaleimide I
- PMA
Phorbol 12-myristate 13-acetate
- Che
Chelerythrine
- AP
Action potential
- DRG
Dorsal root ganglion
- PKC
Protein kinase C
- HEK293
Human embryonic kidney 293
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bant JS, Raman IM. Control of transient, resurgent, and persistent current by open-channel block by Na channel beta4 in cultured cerebellar granule neurons. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12357–12362. doi: 10.1073/pnas.1005633107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendahhou S, Cummins TR, Potts JF, Tong J, Agnew WS. Serine-1321-independent regulation of the mu 1 adult skeletal muscle Na+ channel by protein kinase C. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:12003–12007. doi: 10.1073/pnas.92.26.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantrell AR, Tibbs VC, Yu FH, Murphy BJ, Sharp EM, Qu Y, Catterall WA, Scheuer T. Molecular mechanism of convergent regulation of brain Na(+) channels by protein kinase C and protein kinase A anchored to AKAP-15. Molecular and cellular neurosciences. 2002;21:63–80. doi: 10.1006/mcne.2002.1162. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay M, Mata M, Fink DJ. Continuous delta-opioid receptor activation reduces neuronal voltage-gated sodium channel (NaV1.7) levels through activation of protein kinase C in painful diabetic neuropathy. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:6652–6658. doi: 10.1523/JNEUROSCI.5530-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Yu FH, Surmeier DJ, Scheuer T, Catterall WA. Neuromodulation of Na+ channel slow inactivation via cAMP-dependent protein kinase and protein kinase C. Neuron. 2006;49:409–420. doi: 10.1016/j.neuron.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Cummins TR, Dib-Hajj SD, Herzog RI, Waxman SG. Nav1.6 channels generate resurgent sodium currents in spinal sensory neurons. FEBS letters. 2005;579:2166–2170. doi: 10.1016/j.febslet.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Grieco TM, Afshari FS, Raman IM. A role for phosphorylation in the maintenance of resurgent sodium current in cerebellar purkinje neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2002;22:3100–3107. doi: 10.1523/JNEUROSCI.22-08-03100.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco TM, Malhotra JD, Chen C, Isom LL, Raman IM. Open-channel block by the cytoplasmic tail of sodium channel beta4 as a mechanism for resurgent sodium current. Neuron. 2005;45:233–244. doi: 10.1016/j.neuron.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Hargus NJ, Merrick EC, Nigam A, Kalmar CL, Baheti AR, Bertram EH, 3rd, Patel MK. Temporal lobe epilepsy induces intrinsic alterations in Na channel gating in layer II medial entorhinal cortex neurons. Neurobiology of disease. 2011;41:361–376. doi: 10.1016/j.nbd.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmati G, Papp F, Szentandrassy N, Barandi L, Ruzsnavszky F, Horvath B, Banyasz T, Magyar J, Panyi G, Krasznai Z, Nanasi PP. Effects of the PKC inhibitors chelerythrine and bisindolylmaleimide I (GF 109203X) on delayed rectifier K+ currents. Naunyn-Schmiedeberg's archives of pharmacology. 2011;383:141–148. doi: 10.1007/s00210-010-0584-8. [DOI] [PubMed] [Google Scholar]
- Herbert JM, Augereau JM, Gleye J, Maffrand JP. Chelerythrine is a potent and specific inhibitor of protein kinase C. Biochemical and biophysical research communications. 1990;172:993–999. doi: 10.1016/0006-291x(90)91544-3. [DOI] [PubMed] [Google Scholar]
- Jarecki BW, Piekarz AD, Jackson JO, 2nd, Cummins TR. Human voltage-gated sodium channel mutations that cause inherited neuronal and muscle channelopathies increase resurgent sodium currents. The Journal of clinical investigation. 2010;120:369–378. doi: 10.1172/JCI40801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger AB, Eberhardt M, Link AS, Namer B, Kutsche LK, Schuy ET, Sittl R, Hoffmann T, Alzheimer C, Huth T, Carr RW, Lampert A. Sea-anemone toxin ATX-II elicits A-fiber-dependent pain and enhances resurgent and persistent sodium currents in large sensory neurons. Molecular pain. 2012;8:69. doi: 10.1186/1744-8069-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis AH, Raman IM. Interactions among DIV voltage-sensor movement, fast inactivation, and resurgent Na current induced by the NaVbeta4 open-channel blocking peptide. The Journal of general physiology. 2013;142:191–206. doi: 10.1085/jgp.201310984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu Y, Rogers JC, Tanada TN, Catterall WA, Scheuer T. Phosphorylation of S1505 in the cardiac Na+ channel inactivation gate is required for modulation by protein kinase C. The Journal of general physiology. 1996;108:375–379. doi: 10.1085/jgp.108.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Bean BP. Resurgent sodium current and action potential formation in dissociated cerebellar Purkinje neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1997;17:4517–4526. doi: 10.1523/JNEUROSCI.17-12-04517.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Bowery BJ, Heavens R, Brown N, Ford H, Sirinathsinghi DJ, Borkowski JA, Hess JF, Strader CD, Hill RG. Expression of B1 and B2 bradykinin receptor mRNA and their functional roles in sympathetic ganglia and sensory dorsal root ganglia neurones from wild-type and B2 receptor knockout mice. Neuropharmacology. 1997;36:1009–1017. doi: 10.1016/s0028-3908(97)00065-8. [DOI] [PubMed] [Google Scholar]
- Sittl R, Lampert A, Huth T, Schuy ET, Link AS, Fleckenstein J, Alzheimer C, Grafe P, Carr RW. Anticancer drug oxaliplatin induces acute cooling-aggravated neuropathy via sodium channel subtype Na(V)1.6-resurgent and persistent current. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6704–6709. doi: 10.1073/pnas.1118058109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamboulian S, Choi JS, Ahn HS, Chang YW, Tyrrell L, Black JA, Waxman SG, Dib-Hajj SD. ERK1/2 mitogen-activated protein kinase phosphorylates sodium channel Na(v)1.7 and alters its gating properties. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:1637–1647. doi: 10.1523/JNEUROSCI.4872-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ZY, Piekarz AD, Priest BT, Knopp KL, Krajewski JL, McDermott JS, Nisenbaum ES, Cummins TR. Tetrodotoxin-resistant sodium channels in sensory neurons generate slow resurgent currents that are enhanced by inflammatory mediators. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:7190–7197. doi: 10.1523/JNEUROSCI.5011-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Cummins TR. Inhibition of Navbeta4 peptide-mediated resurgent sodium currents in Nav1.7 channels by carbamazepine, riluzole, and anandamide. Molecular pharmacology. 2011;80:724–734. doi: 10.1124/mol.111.072751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Jarecki BW, Piekarz AD, Cummins TR. Nav1.7 mutations associated with paroxysmal extreme pain disorder, but not erythromelalgia, enhance Navbeta4 peptide-mediated resurgent sodium currents. The Journal of physiology. 2011;589:597–608. doi: 10.1113/jphysiol.2010.200915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Hammerling BC, Wimmer AB, Wu K, Ficker E, Kuryshev YA, Scherer D, Kiehn J, Katus HA, Schoels W, Karle CA. Direct block of hERG potassium channels by the protein kinase C inhibitor bisindolylmaleimide I (GF109203X) Cardiovascular research. 2004;64:467–476. doi: 10.1016/j.cardiores.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. The Journal of biological chemistry. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Vijayaragavan K, Boutjdir M, Chahine M. Modulation of Nav1.7 and Nav1.8 peripheral nerve sodium channels by protein kinase A and protein kinase C. Journal of neurophysiology. 2004;91:1556–1569. doi: 10.1152/jn.00676.2003. [DOI] [PubMed] [Google Scholar]
- Wada A, Yanagita T, Yokoo H, Kobayashi H. Regulation of cell surface expression of voltage-dependent Nav1.7 sodium channels: mRNA stability and posttranscriptional control in adrenal chromaffin cells. Frontiers in bioscience : a journal and virtual library. 2004;9:1954–1966. doi: 10.2741/1314. [DOI] [PubMed] [Google Scholar]
- Wang C, Gu Y, Li GW, Huang LY. A critical role of the cAMP sensor Epac in switching protein kinase signalling in prostaglandin E2-induced potentiation of P2X3 receptor currents in inflamed rats. The Journal of physiology. 2007;584:191–203. doi: 10.1113/jphysiol.2007.135616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JW, Numann R, Murphy BJ, Scheuer T, Catterall WA. A phosphorylation site in the Na+ channel required for modulation by protein kinase C. Science. 1991;254:866–868. doi: 10.1126/science.1658937. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.