ABSTRACT
Broadly cross-reactive neutralizing antibodies (bNabs) represent powerful tools to combat human immunodeficiency virus type 1 (HIV-1) infection. Here, we examined whether HIV-1-specific bNabs are capable of cross-neutralizing distantly related simian immunodeficiency viruses (SIVs) infecting central (Pan troglodytes troglodytes) (SIVcpzPtt) and eastern (Pan troglodytes schweinfurthii) (SIVcpzPts) chimpanzees (n = 11) as well as western gorillas (Gorilla gorilla gorilla) (SIVgor) (n = 1). We found that bNabs directed against the CD4 binding site (n = 10), peptidoglycans at the base of variable loop 3 (V3) (n = 5), and epitopes at the interface of surface (gp120) and membrane-bound (gp41) envelope glycoproteins (n = 5) failed to neutralize SIVcpz and SIVgor strains. In addition, apex V2-directed bNabs (n = 3) as well as llama-derived (heavy chain only) antibodies (n = 6) recognizing both the CD4 binding site and gp41 epitopes were either completely inactive or neutralized only a fraction of SIVcpzPtt strains. In contrast, one antibody targeting the membrane-proximal external region (MPER) of gp41 (10E8), functional CD4 and CCR5 receptor mimetics (eCD4-Ig, eCD4-Igmim2, CD4-218.3-E51, and CD4-218.3-E51-mim2), as well as mono- and bispecific anti-human CD4 (iMab and LM52) and CCR5 (PRO140, PRO140-10E8) receptor antibodies neutralized >90% of SIVcpz and SIVgor strains with low-nanomolar (0.13 to 8.4 nM) potency. Importantly, the latter antibodies blocked virus entry not only in TZM-bl cells but also in Cf2Th cells expressing chimpanzee CD4 and CCR5 and neutralized SIVcpz in chimpanzee CD4+ T cells, with 50% inhibitory concentrations (IC50s) ranging from 3.6 to 40.5 nM. These findings provide new insight into the protective capacity of anti-HIV-1 bNabs and identify candidates for further development to combat SIVcpz infection.
IMPORTANCE
SIVcpz is widespread in wild-living chimpanzees and can cause AIDS-like immunopathology and clinical disease. HIV-1 infection of humans can be controlled by antiretroviral therapy; however, treatment of wild-living African apes with current drug regimens is not feasible. Nonetheless, it may be possible to curb the spread of SIVcpz in select ape communities using vectored immunoprophylaxis and/or therapy. Here, we show that antibodies and antibody-like inhibitors developed to combat HIV-1 infection in humans are capable of neutralizing genetically diverse SIVcpz and SIVgor strains with considerable breadth and potency, including in primary chimpanzee CD4+ T cells. These reagents provide an important first step toward translating intervention strategies currently developed to treat and prevent AIDS in humans to SIV-infected apes.
INTRODUCTION
Simian immunodeficiency virus of chimpanzees (Pan troglodytes) (SIVcpz) is the precursor of human immunodeficiency virus type 1 (HIV-1), the causative agent of the AIDS pandemic (1). Like HIV-1 in humans, SIVcpz is pathogenic in chimpanzees and can cause substantial morbidity and mortality in its natural host (2, 3). Long-term observational health studies in Gombe National Park, Tanzania, revealed that SIVcpz-infected chimpanzees have a 10- to 16-fold increased risk of death compared to uninfected chimpanzees and that infected females are less likely to give birth and have a much higher infant mortality rate than uninfected females (2). Necropsy studies showed that SIVcpz-infected chimpanzees can develop severe CD4+ T lymphocyte depletion and a histopathology consistent with end-stage AIDS (2, 4). Most importantly, the Gombe community with the highest SIVcpz prevalence rate suffered a catastrophic population decline (3). Thus, SIVcpz infection has a substantial negative impact on the health, reproduction, and life span of wild-living chimpanzees.
Chimpanzees acquired SIVcpz by cross-species transmission and recombination of SIVs that infect monkeys (5). Although only the central (P. t. troglodytes) and eastern (P. t. schweinfurthii) subspecies are naturally infected, SIVcpz is widespread throughout their habitats in west-central and eastern Africa, with overall prevalence rates of 6% and 14%, respectively (6–11). Transmission of SIVcpz occurs by sexual routes, from infected mothers to their infants as well as between chimpanzee communities through the migration of infected females (2, 3). Thus, SIVcpz has the potential to disperse over long distances and penetrate uninfected chimpanzee populations (7, 10, 12). SIVcpz is also the source of SIVgor, which emerged in western lowland gorillas (Gorilla gorilla gorilla) following the cross-species transmission of an SIVcpz strain from sympatric P. t. troglodytes apes (SIVcpzPtt) (13). Although less prevalent than SIVcpz, SIVgor has been identified at several locations throughout southern Cameroon, with some gorilla troops exhibiting high infection rates (14–16). Given its recent emergence from SIVcpz, SIVgor may share some of the same pathogenic properties. Thus, SIVcpz, and possibly also SIVgor, contributes to the infectious disease burden of wild apes, which are already highly endangered due to extensive habitat destruction and relentless poaching (7, 10, 16–19).
Historically, chimpanzees have been used as an animal model to test new therapies and vaccines for humans, including AIDS vaccines (20–23), a practice that is no longer considered ethical (24). However, the opposite has not been explored, i.e., whether treatment and prevention strategies developed for HIV-1-infected humans could benefit SIVcpz-infected chimpanzees. As chimpanzee and gorilla populations are dwindling in the wild, primatologists and conservation groups have become increasingly interested in exploring novel avenues to curb the spread of ape pathogens (19, 25). In this context, broad and potent neutralizing antibodies (bNabs) may be of utility (26–29). Although immunogens capable of eliciting such antibodies do not yet exist, several studies have shown that bNabs can prevent the acquisition and/or suppress the replication of HIV-1 and simian-human immunodeficiency viruses (SHIVs) in humanized mice and rhesus macaques, respectively (30–37). For example, antibody-mediated immunotherapy was effective in reducing systemic viral loads and improving immune responses in chronically SHIV-infected rhesus macaques (36, 37). Administration of bNabs as purified proteins or transgenes also prevented virus infection in animal models (30–34, 38). Since antibody infusions in wild settings are not feasible, delivery through recombinant vectors, such as adeno-associated virus (AAV), may represent an alternative. AAV has an outstanding safety record in humans as well as other animal species (39–42). Moreover, a single administration by dart or an equivalent has the potential to induce long-lasting antibody expression (30, 31, 38, 43). Recombinant AAV (rAAV) vectors could also be used to deliver cocktails of potent neutralizing antibodies, which may be able to reduce systemic viral loads.
First-generation bNabs failed to control HIV-1 replication when administered to infected patients, suggesting that they were of little or no clinical value (44, 45). However, recent advances in HIV-1-specific B cell isolation and antibody cloning techniques have led to the discovery of a large number of additional bNabs (46–48), many of which neutralize HIV-1 with much improved breadth and potency (27–29, 49–52). All bNabs target structurally conserved regions on the HIV-1 envelope (Env) spike, including (i) the CD4 binding site (CD4bs), (ii) peptidoglycans surrounding N160 at the trimer apex in variable loops 1 and 2 (V1/V2), (iii) a high-mannose patch surrounding N332 at the base of variable region 3 (V3), (iv) the membrane-proximal external region (MPER), and (v) glycan-associated epitopes at the interface of exterior (gp120) and membrane-bound (gp41) Env glycoproteins (46, 47, 53–56). In addition, antibodies directed against the host receptors CD4 and CCR5 (57–60), as well as immunoadhesins containing domains 1 and 2 (D1/D2) of human CD4 (61–63), have been shown to have substantial anti-HIV-1 activity. While these reagents inhibit a large number of pandemic (group M) HIV-1 strains (64), their capacity to neutralize related lentiviruses from chimpanzees and gorillas has not been examined. Here, we used a panel of SIVcpz and SIVgor infectious molecular clones (IMCs) to show that some but not all anti-HIV-1 bNabs and immunoadhesins are capable of neutralizing these viruses. Since the Env proteins of SIVcpz and SIVgor strains are nearly twice as divergent as those of the various HIV-1 group M subtypes, these data yield new insight into the breadth and protective efficacy of anti-HIV-1 antibodies.
RESULTS
Plasma samples from long-term HIV-1- and SIVcpz-infected chimpanzees lack neutralization breadth.
Almost 50% of HIV-1-positive humans develop some degree of heterologous neutralization breadth within 2 to 4 years postinfection (65), indicating that the human immune system is capable of targeting conserved epitopes in the HIV-1 envelope (Env) glycoprotein. To determine whether this is also true for chronically infected apes, we tested plasma samples from eight chimpanzees, who had been inoculated with HIV-1 and/or SIVcpz 17 to 30 years earlier as part of vaccine and/or pathogenesis studies (Table 1). Although three of these chimpanzees (Marc, Bucky, and Josie) had undetectable plasma virus at the time of sampling, they had high-titer antibodies that reacted with HIV-1 Gag, Pol, and/or Env proteins on Western immunoblots, suggesting that they were once productively infected (see Fig. S1 in the supplemental material). The remaining five chimpanzees were viremic, with plasma viral loads ranging from 337 to >1,000,000 RNA copies/ml (Table 1). Among the animals with the highest viral loads, Tika (>200,000 RNA copies/ml) was infected with HIV-1/NC, a pathogenic chimpanzee-adapted strain of HIV-1 (22, 66). In contrast, Debbie (>20,000 RNA copies/ml) and Cotton (>1,000,000 RNA copies/ml) were infected with SIVcpzANT (20), an SIVcpzPts strain originally isolated from a wild-caught chimpanzee from the Democratic Republic of the Congo (67). Although Cotton was also exposed to HIV-1/LAV (Table 1), reverse transcriptase PCR (RT-PCR) analysis identified SIVcpzANT as the only replicating virus in his plasma. Thus, the latter two animals represent rare examples of captive chimpanzees with chronic SIVcpz infection.
TABLE 1.
Clinical history of the chimpanzees studied
| Ape | Code | Date of birth (mo/day/yr) | Virus strain | Yr infected | Duration of infection (yr) | Plasma sampling date(s) (mo/day/yr) | CD4+ T cell count (cells/μl)a | Viral load (RNA copies/ml) | SIVcpz- ANT infectionc | Virus- specific antibodiesd | HIV vaccine history | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Marc | C487 | 9/6/81 | HIV-1/LAV | 1984 | 30 | 11/8/11 | 1,154 | <50 | + | None | 21 | |
| Artica | C544 | 2/13/79 | HIV-1/LAV | 1986 | 28 | 11/10/11 | 1,153 | 4,794 | + | None | 23 | |
| Joye | C542 | 2/13/79 | HIV-1/LAV | 1986 | 28 | 11/10/11 | 2,529 | 337 | NDe | LAV env | 23 | |
| Tika | C534 | 10/3/78 | HIV-1/NC | 1997 | 17 | 11/10/11 | 4 | 244,324 | + | None | 22 | |
| Debbie | X0284 | 9/23/85 | SIVcpzANT | 1996 | 18 | 1/21/10 | NA | ND | ND | ND | None | 20 |
| 10/26/10 | NA | 26,397 | + | + | ||||||||
| 6/28/11 | NA | ND | ND | ND | ||||||||
| Cotton | X0115 | 4/10/77 | HIV-1/IIIB | NAf | >18 | 5/21/02 | NA | 77,142 | ND | ND | NA | 20 |
| SIVcpzANT | 1996 | 9/25/12 | 229 | 1,440,622 | + | + | ||||||
| 11/25/14 | 220b | 861,000 | ND | ND | ||||||||
| Bucky | NA | NA | NA | NA | NA | 11/10/11 | 1,016 | <50 | + | NA | NA | |
| Josie | NA | 5/18/81 | NA | NA | NA | 11/10/11 | 516 | <50 | + | NA | NA |
CD4+ T cell counts were performed using blood collected in September 2010 unless otherwise noted.
The CD4+ T cell count was performed using blood collected in November 2014.
+, SIVcpzANT-specific amplification and sequence confirmation.
+, antibody reactivity determined using Western blot analysis (see Fig. S1 in the supplemental material).
ND, not done.
NA, not available.
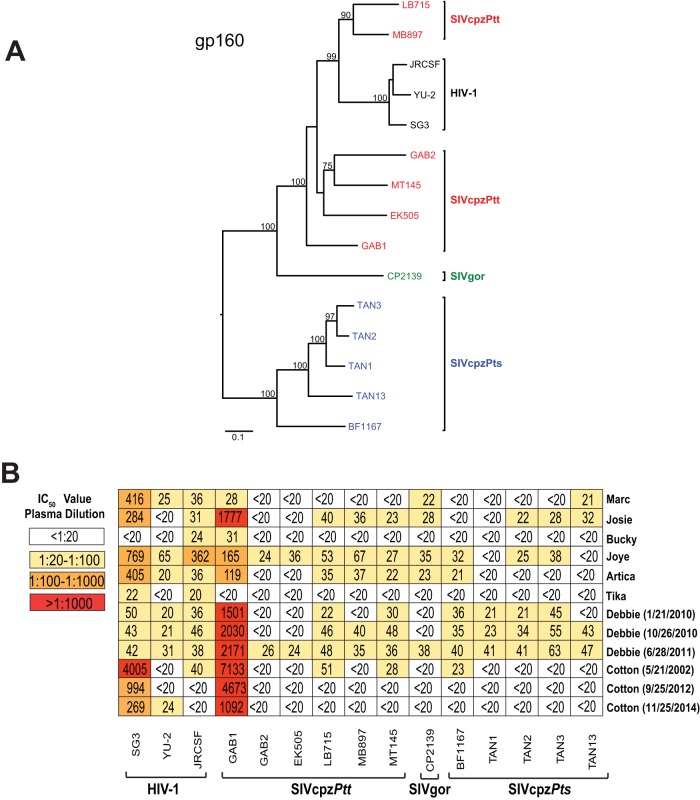
To screen available plasma samples for neutralization breadth, we generated a panel of infectious molecular clones (IMCs) of SIVcpz and SIVgor strains by amplifying viral consensus sequences from fecal samples of wild apes (Fig. 1A). Members of both the SIVcpzPtt lineage and SIVcpzPts lineage were included, which differed in up to 48% of their Env protein sequence. (Three previously reported strains of HIV-1 were used as controls.) All IMCs, except for the T cell line-adapted, CXCR4-tropic HIV-1 SG3 strain, used CCR5 as the coreceptor and replicated efficiently in primary human and chimpanzee CD4+ T cells (6, 7, 11, 15, 68–70). Upon testing of the available plasma samples in the TZM-bl neutralization assay, we found that seven of eight chimpanzees, including the two SIVcpzANT-infected individuals, had activity against the easy-to-neutralize (tier 1) HIV-1 SG3 strain (Fig. 1B). All chimpanzee plasma samples, except for one (Tika), also neutralized SIVcpzGAB1, with IC50 titers exceeding 1:1,000 in three animals. Since SIVcpzGAB1 was cloned from a viral isolate that was extensively propagated in human peripheral blood mononuclear cells (PBMCs) (68), it likely also represents an easy-to-neutralize (tier 1) chimpanzee virus. In contrast, little cross-reactivity was observed against the remaining primary (tier 2) HIV-1 and SIVcpz strains, with most plasma samples containing very low-level (<1:50) or no neutralizing activity (Fig. 1B). Longitudinal plasma samples were available for two chimpanzees, one of whom (Cotton) showed no neutralization breadth after more than 12 years of infection. The second animal (Debbie) developed antibodies that neutralized all SVcpz strains but with very low titers (<1:70). Thus, despite the long duration of their infection (Table 1), none of the chronically infected chimpanzees, including the two SIVcpzANT-infected animals, developed appreciable neutralization potency against heterologous HIV-1, SIVcpz, and SIVgor strains (Fig. 1B).
FIG 1 .
Neutralizing antibody responses in long-term HIV-1- and SIVcpz-infected chimpanzees. (A) Phylogenetic relationship of HIV-1, SIVcpz, and SIVgor infectious molecular clones (IMCs). A maximum likelihood phylogenetic tree of Env (gp160) protein sequences is depicted, with sequences color coded to differentiate HIV-1 (black), SIVgor (green), SIVcpzPtt (red), and SIVcpzPts (blue) strains. Bootstrap values of ≥70% are shown; the scale bar represents 0.1 amino acid replacement per site. (B) Plasma samples from eight long-term-infected chimpanzees (listed on the right and shown in Table 1) were tested against HIV-1 (n = 3), SIVcpzPtt (n = 6), SIVgor (n = 1), and SIVcpzPts (n = 5) strains (bottom) in the TZM-bl neutralization assay. (Collection dates are indicated for samples from the same individual.) IC50s (expressed as plasma dilutions) averaged from three replicate experiments are shown, with a heat map indicating the relative neutralization potency.
Anti-HIV-1 CD4 binding site bNabs fail to neutralize SIVcpz and SIVgor strains.
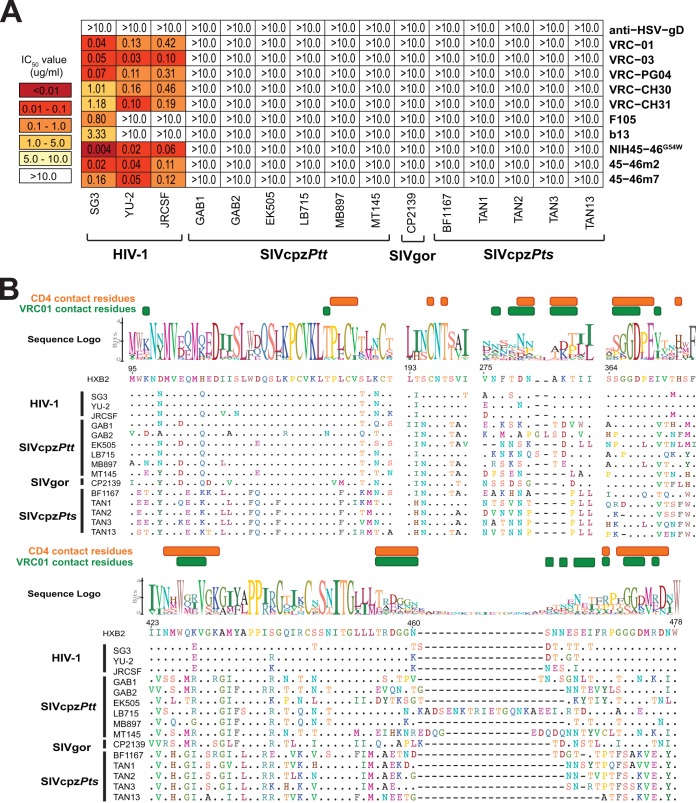
Since all primate lentiviruses identified to date can use the human CD4 receptor to gain entry into target cells (7, 15, 70, 71) and since the CD4 molecules from humans, chimpanzees, and gorillas are closely related (72), we asked whether CD4 binding site (CD4bs) antibodies from HIV-1-infected humans could cross-neutralize SIVcpz and SIVgor strains. Testing VRC01 (29), VCR03 (29), VRC-PG04 (51), VRC-CH30 (51), VRC-CH31 (51), F105 (73), b13 (74), 45-46G54W (75), 45-46m2 (76), and 45-46m7 (76) in the TZM-bl assay, we found that most of these antibodies neutralized the three HIV-1 Env controls potently, with IC50s ranging from 0.004 to 1.2 µg/ml. (A monoclonal antibody directed against herpes simplex virus glycoprotein D served as a negative control.) The two exceptions were F105 and b13, which are known to have only limited neutralization breadth and potency (77). In contrast, none of these CD4bs antibodies neutralized any SIVcpz or SIVgor strain at concentrations of up to 10 µg/ml (Fig. 2A). This was the case despite the fact that the panel included some of the most potent CD4bs bNabs, such as 45-46G54W, which is known to neutralize highly diverse HIV-1 strains, with IC50s of <0.05 µg/ml (75).
FIG 2 .
Neutralizing capacity of CD4 binding site (CD4bs) antibodies. (A) The ability of CD4bs monoclonal antibodies (listed on the right) to neutralize HIV-1, SIVcpz, and SIVgor strains (listed on the bottom) is shown. Numbers indicate IC50s (in micrograms per milliliter) in TZM-bl cells, averaged from three different experiments, with a heat map indicating the relative neutralization potency. The highest antibody concentration was 10 µg/ml. A herpesvirus antibody (anti-HSV-gD) was used as a negative control. (B) Conservation of HIV-1, SIVcpz, and SIVgor strains in the CD4 binding region. An alignment of Env protein sequences (left) in regions surrounding the CD4 binding site is shown. CD4 and VRC01 contact resides (indicated in the HXB2 reference strain) are highlighted in orange and green, respectively. A logo plot above the alignment denotes the conservation of each amino acid, with the height of each letter indicating the proportion of the sequences that contain the residue at that site. Dots indicate identity to the HXB2 reference sequence, and dashes represent gaps introduced to improve the alignment.
To examine the reasons for this resistance, we compared viral Env sequences for conservation of previously identified CD4 and VRC01 contact residues (78, 79). Not surprisingly, most amino acid residues required for CD4 binding were relatively well conserved, but this was not the case for many VRC01 contact residues (Fig. 2B). For example, residues 461 to 467 in variable loop 5 (V5), which are known to be critical for VRC01 binding (78), were present in all HIV-1 strains but exhibited considerable length variations in SIVcpz and SIVgor strains (Fig. 2B). Although the CD4 binding site of SIVcpz and SIVgor strains must be conserved to maintain function, amino acid diversity in neighboring Env regions likely causes clashes with anti-HIV-1 CD4bs antibodies.
Glycan-dependent variable loop bNabs lack neutralization breadth against SIVcpz and SIVgor.
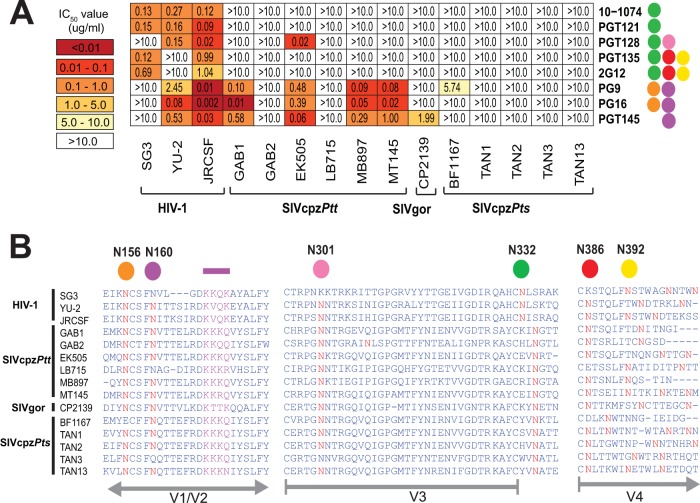
In addition to the CD4 binding site, HIV-1 envelope glycoproteins contain conserved peptidoglycans in variable loops V1 and V2 and at the base of V3, which represent targets for broadly cross-reactive neutralizing antibodies (46, 47). To examine the ability of these anti-HIV-1 bNabs to neutralize SIVcpz and SIVgor strains, we tested apex V2-directed antibodies PG9 (27), PG16 (27), and PGT145 (28) as well as the V3-directed antibodies 10-1074 (52), PGT121 (28), PGT128 (28), PGT135 (28), and 2G12 (80) in the TZM-bl neutralization assay. Like the CD4bs bNabs, the glycan V3-associated bNabs failed to cross-neutralize SIVcpz and SIVgor strains at concentrations of 10 µg/ml, except for PGT128, which neutralized a single SIVcpzPtt (EK505) virus, with an IC50 of 0.02 µg/ml (0.13 nM) (Fig. 3A). The apex V2-directed quaternary bNabs PG9 (27), PG16 (27), and PGT145 (28) were considerably more cross-reactive and potently neutralized four of six SIVcpzPtt strains (GAB1, EK505, MB897, and MT145) (Fig. 3A). However, SIVgor and SIVcpzPts strains were either poorly sensitive or completely resistant to neutralization by the same bNabs.
FIG 3 .
Neutralizing capacity of high-mannose-patch- and apex V2-directed antibodies. (A) The ability of peptidoglycan-associated monoclonal antibodies (listed on the right) to neutralize HIV-1, SIVcpz, and SIVgor strains (bottom) is shown. Numbers indicate IC50s (in micrograms per milliliter) in TZM-bl cells, averaged from three different experiments, with a heat map indicating the relative neutralization potency. Colored circles to the right of each antibody indicate the N-linked glycans that are important for their neutralizing activity (orange, N156; purple, N160; pink, N301; green, N332; red, N386; yellow, N392) (27, 28, 52, 81). The highest antibody concentration used was 10 µg/ml. (B) Conservation of glycans associated with bNab activity. An alignment of HIV-1, SIVcpz, and SIVgor Env protein sequences is shown, with predicted N-linked glycans (NXS/T) highlighted in red. Four residues comprising a lysine-rich motif in the V1/V2 strand (C), which together with glycans N156 and N160 form the core epitope for PG9, PG16, and PGT145, are highlighted in purple. N-linked glycans known to influence bNab binding are highlighted above the alignment, with HXB2 numbering in black. The positions of variable loops (V1/V2, V3, and V4) are shown in gray below the alignment.
Quaternary and glycan V3 bNabs neutralize by engaging the glycan shield, and removal of key glycans can abrogate this neutralization (28, 81). For example, 10-1074, PGT121, PGT128, PGT135, and 2G12 all bind a high-mannose patch that is centered around the conserved N332 glycan, while PG9, PG16, and PGT145 contact the N156 and N160 glycans and insert between them to interact with glycan-obscured protein regions (27, 28, 52, 81). Examining SIVcpz and SIVgor Env sequences for NXS/T sequons, we found various degrees of conservation of these and other relevant N-linked glycosylation sites (Fig. 3B). For example, N332 was conserved in all three HIV-1 Envs but absent from all SIVcpz and SIVgor Envs, potentially explaining the lack of cross-neutralization of 10-1074, PGT121, PGT128, PGT135, and 2G12. However, SIVcpzEK505 was potently neutralized by PGT128 (Fig. 3A), despite the absence of the N332 glycan. (EK505 is also the only strain with a deletion at position 337.) Moreover, all SIVcpz and SIVgor strains encode an NXS/T sequon at position 334, which could potentially compensate for the absence of N332 (82). Thus, the lack of N332 is unlikely to be the sole reason for the inability of glycan V3-directed bNabs to cross-neutralize SIVcpz and SIVgor. In contrast, the core epitopes of PG9, PG16, and PGT145 were surprisingly well conserved among ape viruses (Fig. 3B, left panel), with many SIVcpz and SIVgor Envs containing the essential N156 and N160 glycans as well as a lysine-rich (KKKQ) motif in strand C of V1/V2 (83, 84). Given this degree of conservation, it is not surprising that SIVcpzPtt strains GAB1, EK505, MB897, and MT145 were exquisitely sensitive to neutralization by PG9, PG16, and PGT145 (Fig. 3A). However, an intact core epitope was not always associated with neutralization susceptibility. Despite the presence of both N156 and N160 and the conserved lysine motif, TAN1, TAN2, and TAN13 were completely resistant to PG9, PG16, and PGT145 neutralization. Since these viruses encoded V1 loops that were almost twice as long as those of susceptible SIVcpzPtt strains and since such insertions have been shown to confer neutralization escape to HIV-1 (85, 86), it is likely that this explains the resistance of SIVcpzPts strains to apex V2-directed bNabs.
HIV-1 bNabs directed against the interface of gp120 and gp41 fail to cross-neutralize SIVcpz and SIVgor.
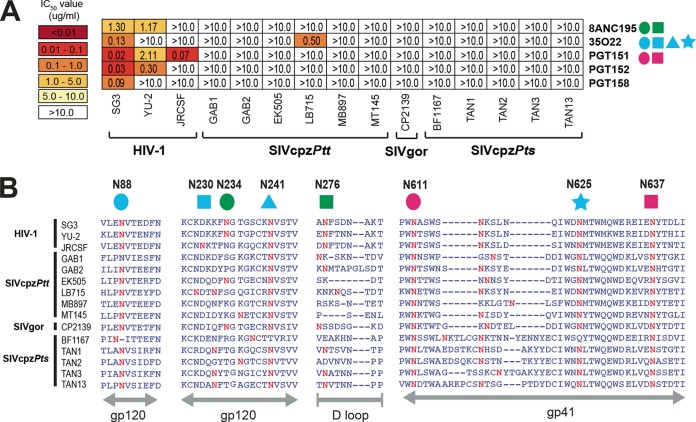
The most recently discovered class of anti-HIV-1 bNabs targets glycan-associated epitopes at the interface of gp120 and gp41 (53–56). Testing several representatives of this bNab group, including 8ANC195 (54), 35022 (53), PGT151 (56), PGT152 (56), and PGT158 (56), we found that none had cross-neutralization potential (Fig. 4A). With the exception of 35O22, which neutralized a single SIVcpz strain (LB715) at an IC50 of 0.5 µg/ml (3.3 nM), all other antibodies failed to block SIVcpz and SIVgor infection at concentrations of up to 10 µg/ml (Fig. 4A). Since these bNabs are also dependent on the presence of key glycans, we examined the extent of sequence conservation in the corresponding Env regions (Fig. 4B). For example, 8ANC195 requires N-linked glycosylation sites at positions 234 and 276 (54), both of which are highly variable in SIVcpz and SIVgor strains (Fig. 4B). Similarly, 35O22 utilizes N-linked glycosylation sites at positions 88, 230, 241, and 625 (Fig. 4B), one of which (N230) is absent from all SIVcpz and SIVgor strains. Thus, the absence of key N-linked glycosylation sites may be one reason for the lack of cross-neutralizing capacity of this class of bNabs. However, variation in other Env regions must also be involved, since the glycosylation site contacts for PGT151 (N611 and N637) were conserved in all viruses, yet this monoclonal antibody also failed to cross-neutralize.
FIG 4 .
Neutralizing capacity of antibodies targeting the interface of HIV-1 gp120 and gp41 regions. (A) The ability of glycan-associated antibodies (right) to neutralize HIV-1, SIVcpz, and SIVgor strains (bottom) is shown. Numbers indicate IC50s (in micrograms per milliliter) from TZM-bl cells, averaged from three different experiments, with a heat map indicating the relative neutralization potency. Colored shapes to the right of each antibody indicate the N-linked glycans that are associated with antibody neutralizing activity. Antibody 8ANC195 contacts N234 (green circle) and N276 (green square), 35O22 utilizes N88 (blue circle), N230 (blue square), N241 (blue triangle), and N625 (blue square), and PGT151 requires N611 (pink circle) and N637 (pink square) for optimal neutralization (53–56). The highest antibody concentration used was 10 µg/ml. (B) Conservation of glycans associated with antibody neutralizing activity. An alignment of HIV-1, SIVcpz, and SIVgor Env protein sequences is shown, with predicted N-linked glycans (NXS/T) highlighted in red. N-linked glycans known to influence bNab binding are highlighted above the alignment, with HXB2 numbering in black. The positions of various Env regions are shown in gray below the alignment.
Camelid antibodies have limited cross-neutralization breadth.
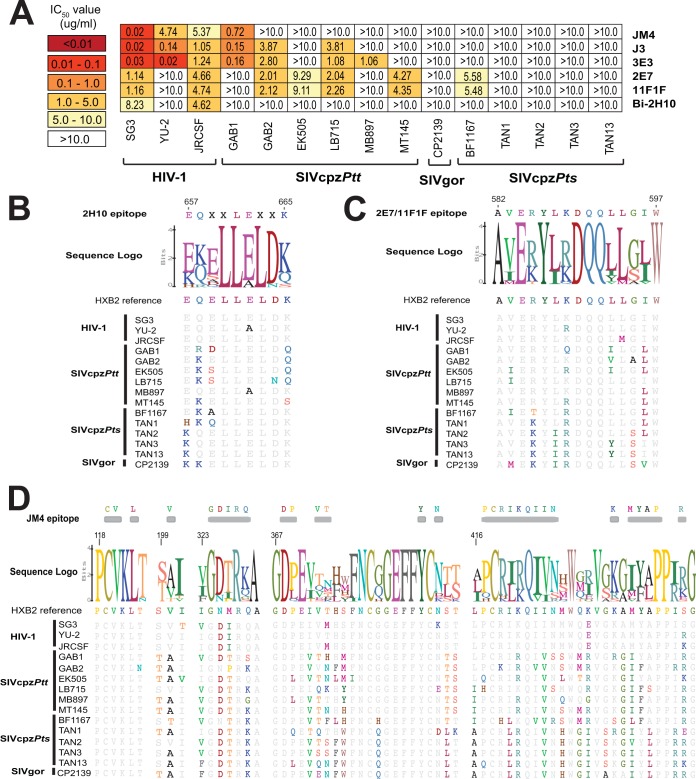
Members of the Camelidae family produce antibodies that lack light chains and thus comprise much smaller (heavy-chain-only) antibodies with long complementarity-determining regions (CDRs). It has thus been proposed that these single-chain antibodies may be better equipped to bind to small occluded sites on the HIV/SIV Env trimer than full-size antibodies (87). Indeed, several camelid single-domain antibodies derived by immunization with HIV-1 antigens have been shown to potently neutralize genetically divergent strains of HIV-1 (76, 88, 89). To examine whether this cross-reactivity extends to SIVcpz and SIVgor, we tested a panel of llama-derived antibodies, including single-domain antibodies JM4 (90), J3 (76), 3E3 (91), 2E7 (88), and 11F1F (92), as well as the bivalent antibody Bi-2H10, which contains two molecules of 2H10 joined by a glycine-serine linker (93). Of these, JM4 recognizes a region in gp120 that overlaps both the CD4 and CCR5 binding sites, J3 and 3E3 target the CD4 binding site, 2E7 and 11F1F recognize epitopes in the ectodomain of gp41 (76, 88, 90, 92), and 2H10 targets the membrane proximal external region (MPER) (93). Interestingly, J3 and 3E3 were able to neutralize a limited number of SIVcpzPtt strains at IC50s of <5 µg/ml (<13 nM) (Fig. 5A), thus exhibiting greater activity than conventional CD4bs antibodies, which were completely inactive (Fig. 2A). This was also true for the anti-gp41 antibodies 2E7 and 11F1F, which neutralized four SIVcpzPtt strains and one SIVcpzPts strain at IC50s of <10 µg/ml (<40 nM). However, this breadth did not extend to the more divergent SIVcpzPts and SIVgor viruses, and the bivalent MPER antibody Bi-2H10 was unable to neutralize any SIVcpz and SIVgor strains (Fig. 5A).
FIG 5 .
Neutralizing capacity of camelid antibodies. (A) The ability of llama-derived (heavy-chain-only) antibodies (listed on the right) to neutralize HIV-1, SIVcpz, and SIVgor strains (bottom) is shown. Numbers indicate IC50s (in micrograms per milliliter) from TZM-bl cells, averaged from three different experiments, with a heat map indicating the relative neutralization potency. The highest antibody concentration used was 10 µg/ml. (B to D) Conservation of antibody binding epitopes shown in panel A. Alignments of HIV-1, SIVcpz, and SIVgor Env protein sequences are depicted, with residues identical to the HXB2 reference shown in gray. Logo plots denote the conservation of individual amino acids within each epitope, with the height of each letter indicating the proportion of sequences that contain the residue at that site. The letter “X” indicates residues within the 2H10 epitope that do not impact neutralization. JM4 contact residues are indicated on top of the alignment. Sequences are numbered according to the HXB2 reference.
Since the epitopes of 2H10, 2E7, 11F1F, and JM4 have been mapped, we examined the corresponding amino acid sequences in SIVcpz and SIVgor strains (Fig. 5B to D). This analysis showed that none was particularly highly conserved among the ape viruses. All SIVcpz and SIVgor strains differed by one or more substitutions in the 2H10 epitope (Fig. 5B). The partially overlapping 2E7 and 11F1F epitopes were also variable in ape viruses, with all SIVcpz and SIVgor sequences differing from the HIV-1 consensus (Fig. 5C). Finally, there was considerable variation in the JM4 epitope (Fig. 5D), with contact residues I326, V372, and M434 conserved in HIV-1 but mutated in most (V372) or all (I326 and M434) SIVcpz and SIVgor strains. Thus, epitope variation may explain at least some of the neutralization resistance of SIVcpz and SIVgor strains. However, in contrast to conventional CD4bs and most glycan-dependent antibodies, llama-derived antibodies neutralized a subset of SIVcpzPtt strains, possibly because of their smaller size.
HIV-1 MPER bNabs neutralize SIVcpz and SIVgor strains.
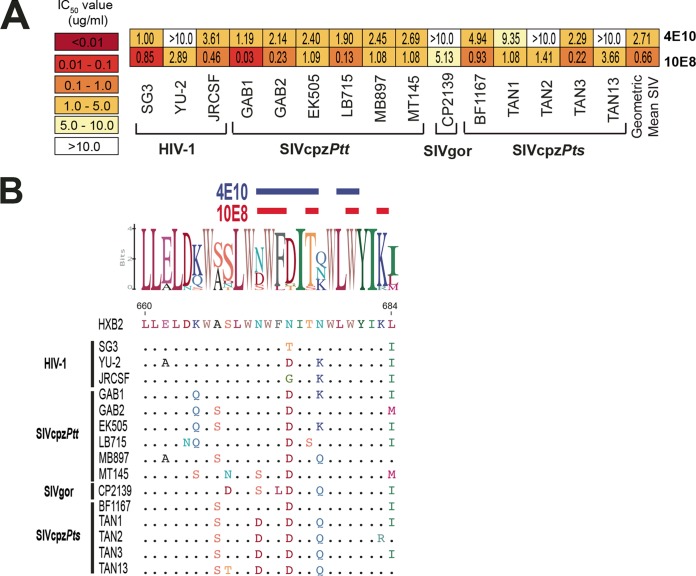
Antibodies targeting the MPER of gp41 represent still another class of potent anti-HIV-1 bNabs (49, 50). Among those, 4E10 and 10E8 exhibit the greatest breadth, having been shown to neutralize 98% of a large panel (n = 181) of HIV-1 pseudoviruses (49, 50). To examine their ability to cross-neutralize ape viruses, we tested 4E10 and 10E8 against our panel of SIVcpz and SIVgor strains. In contrast to all other anti-HIV-1 bNabs, 4E10 neutralized 9 of 12 SIVcpz and SIVgor strains, with a mean IC50 of 2.7 µg/ml (18.1 nM), while 10E8 neutralized all ape viruses, with a mean IC50 of 0.7 µg/ml (4.4 nM) (Fig. 6A). An amino acid alignment of the epitopes of these antibodies revealed considerable sequence conservation (Fig. 6B), likely explaining their remarkable breadth compared to all other anti-HIV-1 bNabs.
FIG 6 .
Neutralizing capacity of MPER antibodies. (A) The ability of two MPER antibodies (listed on the right) to neutralize a panel of HIV-1, SIVcpz, and SIVgor strains (bottom) is shown. Numbers indicate IC50s (in micrograms per milliliter) from TZM-bl cells, averaged from three different experiments, with a heat map indicating the relative neutralization potency. The highest antibody concentration used was 10 µg/ml. (B) Conservation of 4E10 and 10E8 epitopes. An alignment of HIV-1, SIVcpz, and SIVgor Env protein sequences is depicted, with dots indicating identity to the HXB2 reference sequence. A logo plot denotes the conservation of individual amino acids, with the height of each letter indicating the proportion of sequences that contain the residue at that site. Contact residues of 4E10 (blue) and 10E8 (red) are highlighted above the alignment.
Anti-CD4 and CCR5 receptor antibodies potently neutralize SIVcpz and SIVgor strains.
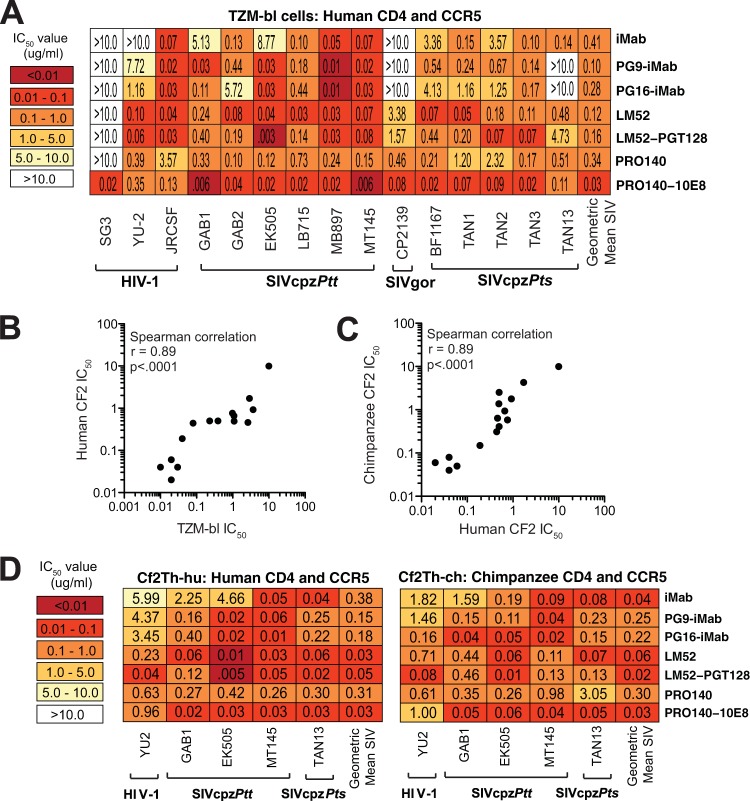
Since CD4 and CCR5 protein sequences are highly conserved between humans and apes (72, 94), we asked whether antibodies raised against the human receptors could block SIVcpz and SIVgor infection. Both anti-CD4 and anti-CCR5 antibodies were examined, including ibalizumab (iMab) and PRO140, which have previously been shown to be safe in human clinical trials (95–97). Using TZM-bl cells, which express human CD4 and CCR5, we found that both the anti-CD4 antibody iMab (96, 98, 99) and its improved version, LM52 (59), neutralized most or all ape viruses, with geometric mean IC50s of 0.41 µg/ml (2.8 nM) and 0.12 µg/ml (0.8 nM), respectively (Fig. 7A). PRO140, which targets the CCR5 coreceptor (57), also neutralized all SIVcpz and SIVgor strains, with an IC50 of 0.34 µg/ml (2.3 nM) (Fig. 7A). Finally, bispecific versions of iMab, LM52 and PRO140, in which these antibodies were linked to single-chain variable fragments (scFv) of PG9, PG16, PGT128, and 10E8 (60), were potent inhibitors, especially when the fusion partner was also an effective neutralizer. For example, the bispecific PG9-iMab and PG16-iMab antibodies neutralized the PG9/PG16-sensitive GAB1 and EK505 strains with 2 orders of magnitude greater potency than iMab alone, but this enhancement was not observed for viruses that were resistant to PG9 and PG16 (Fig. 3A and 7A). By far the most potent antireceptor antibody was PRO140-10E8, which neutralized SIVcpz and SIVgor strains, with a mean IC50 of 0.03 µg/ml (0.13 nM) (Fig. 7A).
FIG 7 .
Neutralizing capacity of anti-host receptor antibodies (A) The ability of monospecific anti-human CD4 (iMab and LM52) and anti-CCR5 (PRO140) antibodies as well as their bispecific derivatives (PG9-iMab, PG16-iMab, LM52-PGT128, and PRO140-10E8) (listed on the right) to neutralize a panel of HIV-1, SIVcpz, and SIVgor strains (bottom) is shown. Numbers indicate IC50s (in micrograms per milliliter) from TZM-bl cells, averaged from three different experiments, with a heat map indicating the relative neutralization potency. The geometric mean IC50 is shown for each antibody. (Only values for SIVcpz and SIVgor strains that were below 10 µg/ml were included in the calculation.) (B) Correlation of TZM-bl- and Cf2Th-hu-derived neutralization data. Three bNabs (10E8, PG16, and eCD4-Igmim2) were used to neutralize a subset of HIV-1 and SIVcpz strains (YU-2, GAB1, EK505, MT145, and TAN13) in TZM-bl and Cf2Th cells expressing human CD4 and CCR5 receptors (Cf2Th-hu). IC50s from TZM-bl (x axis) and Cf2Th-hu (y axis) cells were plotted and analyzed using the Spearman correlation test. (C) Correlation of Cf2Th-hu and Cf2Th-ch neutralization data. The same three bNabs shown in panel B were used to neutralize the same subset of HIV-1 and SIVcpz strains in Cf2Th cells expressing human (Cf2Th-hu) and chimpanzee (Cf2Th-ch) CD4 and CCR5 receptors. IC50s from Cf2Th-hu (x axis) and Cf2Th-ch (y axis) cells were plotted and analyzed using the Spearman correlation. (D) The ability of antireceptor antibodies (listed on the right) to neutralize a subset of HIV-1 and SIVcpz strains (bottom) in Cf2Th cells expressing either human (left panel) or chimpanzee (right panel) CD4 and CCR5 receptors is shown. Numbers indicate IC50s (in micrograms per milliliter) averaged from three different experiments, with heat maps indicating relative neutralizing potencies. The geometric mean IC50 is shown for each antibody. (Only values from SIVcpz strains were included.)
To determine whether the anti-human receptor antibodies would block virus entry in cells expressing chimpanzee CD4 and CCR5, we developed a neutralization assay that utilized transiently transfected Cf2Th cells. This canine thymus cell line lacks CD4 and CCR5 but expresses a tat-inducible luciferase reporter. Cf2Th cells were cotransfected with either human (Cf2Th-hu) or chimpanzee (Cf2Th-ch) CD4 and CCR5 expression plasmids and then validated by testing the neutralizing activity of anti-gp120 bNabs (n = 3), which should not be affected by entry molecules, against a subset of HIV-1 (YU2) and SIVcpz (GAB1, EK505, MT145, and TAN13) strains (Fig. 7B and C). The results showed that IC50s from Cf2Th-hu cells were highly correlated with those from TZM-bl cultures (Fig. 7B), and this was also true for IC50s from Cf2Th-hu and Cf2Th-ch cells (Fig. 7C). Having validated the Cf2Th-ch cell assay, we next examined the neutralizing capacity of the antireceptor antibodies using a subset of SIVcpz strains (Fig. 7D). Both mono- and bispecific antibodies blocked these viruses in chimpanzee CD4- and CCR5-expressing cells, with mean IC50s ranging from 0.30 µg/ml (PRO140) to 0.02 µg/ml (LM52-PGT128). Thus, several anti-human receptor antibodies that potently neutralized SIVcpz strains in cells expressing human CD4 and CCR5 also neutralized these viruses in cells expressing chimpanzee CD4 and CCR5.
CD4-containing antibodies and immunoadhesins potently neutralize SIVcpz and SIVgor strains.
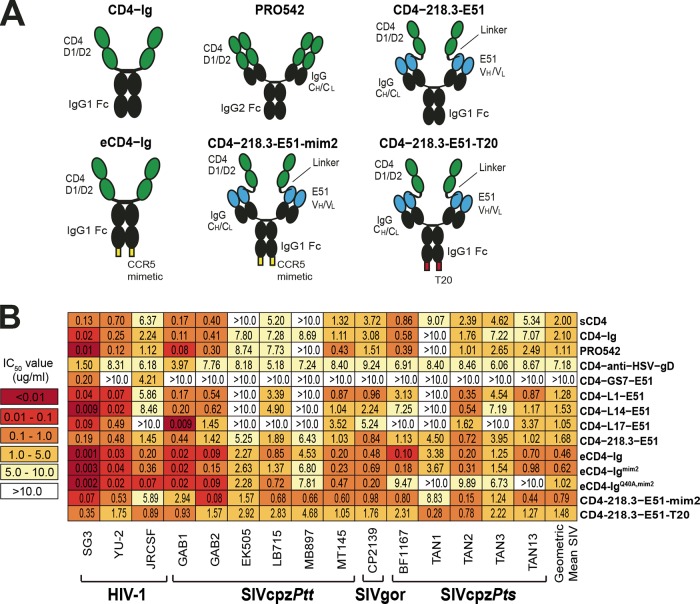
Soluble CD4 (sCD4), as well as immunoadhesins that contain the first two immunoglobulin-like domains (D1 and D2) of CD4 linked to antibody constant regions, are known to have broad anti-HIV-1 activity in vitro and in vivo (61, 62, 100). To examine their breadth and potency against ape viruses, we tested several members of this inhibitor class. Soluble CD4 (101) and the more bioavailable CD4-Ig (102), in which the D1/D2 domains of CD4 are linked to an IgG Fc region (Fig. 8A), neutralized nearly all ape viruses, with mean IC50s of 2.0 µg/ml (47.7 nM) and 2.1 µg/ml (23.1 nM), respectively (Fig. 8B). The tetravalent PRO542, in which D1/D2 domains are linked to both the constant heavy (CH) and light (CL) chains of IgG1 (Fig. 8A), was slightly more potent and neutralized 10 of 12 ape viruses, with a mean IC50 of 1.1 µg/ml (5.2 nM) (Fig. 8B). Finally, CD4-218.3-E51, a CD4-induced (CD4i) neutralizing antibody (E51) linked to D1/D2 via the VH chain (Fig. 8A), neutralized all SIVcpz and SIVgor strains, with a geometric mean IC50 of 1.68 µg/ml (8.4 nM) (Fig. 8B). Interestingly, other E51-based constructs that differed in the length or amino acid composition of their linkers (CD4-GS7-E51 [62], CD4-L1-E51, CD4-L14-E51, and CD4-L17-E51) were much less broadly acting and/or potent (Fig. 8B), and this was also true for a construct in which the D1/D2 domains were linked to an anti-herpes simplex virus antibody (CD4-anti-HSV-gD).
FIG 8 .
Neutralizing capacity of human CD4 D1/D2 domain containing immunoadhesins. (A) Schematic representation of six constructs. Human CD4 D1 and D2 domains are shown in green, immunoglobulin (IgG) Fc and constant heavy- and light-chain (CH/CL) regions are shown in black, E51 variable heavy and light (VH/VL) regions are shown in blue, CCR5 mimetic peptides are shown in yellow, and the T-20 fusion inhibitor is shown in red. (B) The ability of human CD4 containing antibody-like constructs (listed on the right) to neutralize a panel of HIV-1, SIVcpz, and SIVgor strains (bottom) is shown. Numbers indicate IC50s (in micrograms per milliliter) from TZM-bl cells, averaged from three different experiments, with a heat map indicating the relative neutralization potency. The highest antibody concentration used was 10 µg/ml. The geometric mean IC50 is shown for each construct. (Only values for SIVcpz and SIVgor strains that were below 10 µg/ml were included in the calculation.)
Some HIV-1 bNabs use tyrosine sulfation of their complementarity-determining regions (CDRs) to mimic CCR5 sulfation and thereby inhibit virus entry (103). Sulfopeptides derived from such antibodies, including a 15-amino-acid tyrosine-sulfated peptide derived from the CD4i antibody E51, have been shown to reproduce this effect (104). When linked to an antibody Fc domain, this peptide (CCR5mim) was able to block infection of diverse HIV-1 strains (105). However, its neutralization capacity was most improved when fused to the carboxy terminus of CD4-Ig (Fig. 8A). In fact, this enhanced CD4-Ig (eCD4-Ig) not only neutralized a diverse panel of HIV-1 strains but also neutralized SIV of macques (SIVmac) and HIV-2 isolates, with IC50s of <0.05 µg/ml (63). Using TZM-bl cells, we found that eCD4-Ig neutralized SIVcpz and SIVgor strains also more potently than CD4-Ig, with a mean IC50 of 0.46 µg/ml (4.8 nM). However, this 5-fold enhancement was modest compared to the 20- to 200-fold enhancement observed for HIV-1 strains (63). Moreover, the use of CCR5mim peptide variants previously shown to bind HIV-1 Envs with greater affinity did not improve neutralization: eCD4-Igmim2 (63) was slightly less potent than eCD4-Ig (IC50 of 0.62 µg/ml), and eCD4-IgQ40A,mim2 (63) failed to neutralize two SIVcpzPts strains (Fig. 8B). Addition of CCR5mim2 to the carboxy terminus of CD4-218.3-E51 (CD4-218.3-E51-mim2) resulted in a 2-fold-increased neutralization potency, while addition of the fusion-inhibiting peptide T-20 (106) (CD4−218.3-E51-T20) had very little effect (Fig. 8B). Thus, as previously shown for HIV-1, HIV-2, and SIVmac (63), addition of entry-inhibiting peptides to the carboxy terminus of D1/D2-containing antibody constructs enhanced their ability to neutralize SIVcpz and SIVgor strains, although the magnitude of this enhancement was much less pronounced.
Neutralization of SIVcpz in primary chimpanzee CD4+ T cells.
TZM-bl and CF2Th-ch cells identified a number of antiviral and antireceptor bNabs that neutralized ape viruses with both breadth and potency. To confirm this phenotype in a physiologically more relevant culture system, we tested these antibodies in primary chimpanzee lymphocytes. Chimpanzee CD4+ T cells were isolated from the blood of three animals, activated using autologous macrophages, and used to test the neutralizing capacity of immunoadhesins (CD4-Ig, eCD4-Ig, and eCD4-Igmim2), the MPER antibody 10E8, CD4-CD4i constructs (CD4-218.3-E51, CD4-218.3-E51-mim2, and CD4-218.3-E51-T20), and antireceptor antibodies (iMab, LM52, PRO140, and PRO140-10E8) (see Fig. S2A in the supplemental material). Since chimpanzee blood samples were limited, we were able to test only a single SIVcpz strain (MT145), which was selected based on its ability to replicate efficiently in CD4+ T cells from multiple chimpanzee donors (71). Using serial antibody dilutions (see Fig. S2A), we found that MT145 was most potently neutralized by iMab, LM52, and PRO140-10E8, with mean IC50s of 0.64 (4.3 nM), 0.68 (4.5 nM), and 0.75 µg/ml (3.6 nM), respectively (Table 2). Immunoadhesins were roughly 10-fold less potent, with mean IC50s ranging from 3.7 µg/ml (38.3 nM) for eCD4-Ig to 6 µg/ml (65.4 nM) for CD4-Ig. As expected, the CD4bs antibody VRC01 had no inhibitory activity (Table 2; see Fig. S2A). Thus, all antibody constructs that had broad and potent anti-SIVcpz activity in the TZM-bl assay also inhibited SIVcpz infection in chimpanzee CD4+ T cells (see Fig. S2B), although the observed IC50s were 10- to 100-fold higher in the primary T cell cultures that permitted multiple rounds of replication (see Fig. S2B).
TABLE 2 .
Neutralization potency in primary chimpanzee CD4+ T cells
| Antibody | IC50 fora: |
|||
|---|---|---|---|---|
| HIV-1 SG3 |
SIVcpz MT145 |
|||
| μg/ml | nM | μg/ml | nM | |
| CD4-Ig | 0.02 ± 0.02 | 0.22 ± 0.22 | 6.02 ± 3.5 | 65.4 ± 38.0 |
| CD4-218.3-E51 | 0.04 ± 0.04 | 0.20 ± 0.20 | 3.74 ± 1.4 | 18.7 ± 7.0 |
| eCD4-Ig | <0.01 ± 0.0 | <0.10 ± 0.0 | 3.68 ± 1.1 | 38.3 ± 11.5 |
| eCD4-Igmim2 | <0.01b | <0.10 ± 0.0 | 5.86b | 61.0b |
| CD4-218.3-E51-mim2 | <0.01 ± 0.0 | <0.05 ± 0.0 | 3.93 ± 1.5 | 19.7 ± 7.5 |
| CD4-218.3-E51-T20 | <0.01 ± 0.0 | <0.05 ± 0.0 | 4.90 ± 4.4 | 24.5 ± 22.0 |
| VRC01 | 0.03 ± 0.03 | 0.20 ± 0.20 | >10 ± 0.0 | >66.7 ± 0.0 |
| 10E8 | ND | ND | 6.07 ± 1.82 | 40.5 ± 12.1 |
| iMab | ND | ND | 0.64 ± 0.41 | 4.3 ± 2.7 |
| LM52 | ND | ND | 0.68 ± 0.18 | 4.5 ± 1.2 |
| PRO140 | ND | ND | 2.33 ± 1.99 | 15.5 ± 13.3 |
| PRO140-10E8 | ND | ND | 0.75 ± 0.68 | 3.6 ± 3.2 |
Shown are average values from 3 donors ± standard deviations, except as noted. ND, not done.
Not applicable (only performed for 1 donor).
DISCUSSION
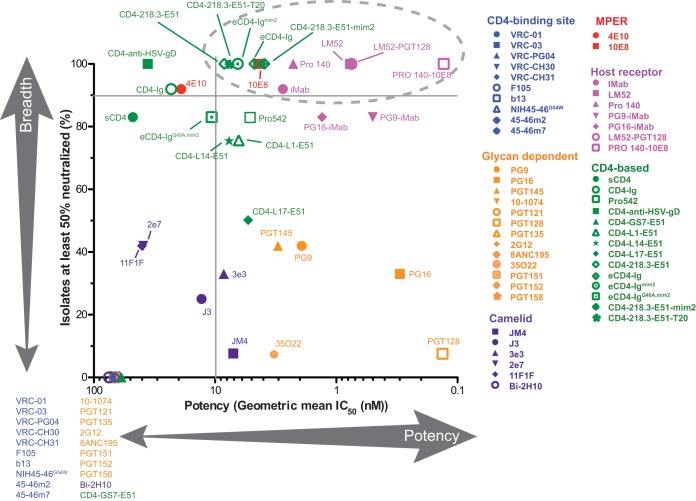
In this study, we examined whether antibodies and immunoadhesins known to potently neutralize diverse strains of HIV-1 can cross-neutralize related lentiviruses naturally infecting chimpanzees and gorillas to assess their utility for antibody-based strategies to combat ape SIV infections. We found that the great majority of HIV-1-specific bNabs, including those directed against highly conserved Env epitopes, such as the CD4 binding site, failed to neutralize SIVcpz and SIVgor strains. However, one antibody directed against the MPER region (10E8) as well as bispecific CD4 and CCR5 receptor mimetics (eCD4-Ig, eCD4-Igmim2, CD4-218.3-E51, and CD4-218.3-E51-mim2) neutralized 100% of ape viruses with low-nanomolar potency (Fig. 9; see Table S1 in the supplemental material). Anti-host receptor antibodies (iMab and PRO140) were also effective neutralizers, especially when fused to the antigen binding regions of potent bNabs, such as 10E8 (Fig. 9; see Table S1). These data indicate that pandemic HIV-1 shares an extremely limited number of cross-reactive epitopes with its immediate ape precursors. However, the sites of vulnerability that were identified represent attractive targets across the entire HIV-1/SIVcpz/SIVgor clade and may be of utility to combat other HIV-1 lineages, including group O viruses, which are estimated to have infected ~100,000 people in west-central Africa (16).
FIG 9 .
Breadth and potency of antibodies and antibody-like constructs with anti-SIVcpz and anti-SIVgor neutralizing activity. For each bNab, the percentage of SIVcpz and SIVgor strains neutralized with IC50s of <10 µg/ml (y axis) is plotted against the corresponding geometric mean IC50 (nanomolar concentration) (x axis). Antibodies and antibody-like constructs are color coded, with those that exhibited no anti-SIVcpz and SIVgor activity listed in the left lower corner. Horizontal and vertical bars denote 90% breadth and 10 nM potency, respectively, and the most potent and cross-reactive reagents are circled.
Neutralizing antibody responses in long-term HIV-1- and SIVcpz-infected chimpanzees.
Since ape-derived monoclonal antibodies may be more appropriate for vector-mediated antibody gene delivery than human bNabs, we tested plasma samples from eight long-term HIV-1- and SIVcpz-infected chimpanzees for evidence of neutralization breadth (Table 1). Although all animals tested had high-titer Env binding antibodies (see Fig. S1 in the supplemental material), their plasma samples neutralized only the easy-to-neutralize (tier 1) HIV-1 SG3 and SIVcpzGAB1 strains (Fig. 1B). Four animals had very low (Joye) or undetectable (Marc, Bucky, and Josie) plasma viral loads, suggesting insufficient antigenic stimulation as a reason for the lack of bNab development (Table 1). In addition, one animal (Tika) had severe CD4+ T cell depletion and thus may have suffered from immune exhaustion (Table 1). However, two chimpanzees (Debbie and Cotton) had plasma viral titers exceeding 20,000 RNA copies/ml, suggesting sustained productive infection for almost two decades (Table 1). Both were experimentally infected with SIVcpzANT, a naturally occurring SIVcpzPts strain that was administered by mucosal routes using plasma and/or peripheral blood mononuclear cells from a third chimpanzee (20). Despite this “near-natural” infection history, only one of these animals (Debbie) exhibited cross-reactive neutralizing activity, albeit with very low titers (<1:70) (Fig. 1B). Since the kinetics of bNab development may vary in chimpanzees and humans, it will be important to follow this individual to examine whether neutralizing titers will increase and to map the corresponding antibody specificities. It may also be informative to molecularly clone the SIVcpzANT env gene and study autologous neutralization responses and associated virus escape in Cotton and Debbie. Since very few apes and humans can be studied decades after their infection in the absence of antiretroviral therapy, this may provide an opportunity to uncover common pathways, as well as roadblocks, to bNab development (107–109).
HIV-1 shares a very limited number of cross-reactive epitopes with SIVcpz and SIVgor strains.
The envelope glycoprotein of HIV-1 group M viruses contains five regions of vulnerability that are targeted by broadly cross-reactive neutralizing antibodies. All of these, except for the MPER region, contain conformational epitopes that span heavily glycosylated and/or structurally flexible protein domains. Since even the most cross-reactive CD4bs bNabs bind an area that extends beyond the CD4 binding pocket (110), it is possible that variation in adjacent Env regions obstructs antibody binding and neutralization (77). SIVcpz and SIVgor Envs vary extensively in regions surrounding the CD4 binding site (Fig. 2B), thus providing a possible explanation for their resistance to CD4bs bNabs (Fig. 2 and 5). In addition, the SIVcpz/SIVgor Env spike may be more compact, such that CD4bs bNab epitopes, although present, are less exposed. The fact that some camelid (heavy-chain-only) CD4bs bNabs neutralize a subset of SIVcpz strains is consistent with these hypotheses. Ape viruses also vary in Env regions that are targeted by apex and high-mannose-patch bNabs, which are typically associated with key N-linked glycosylation sites (Fig. 3 and 4). Although apex V2-directed antibodies neutralized a large fraction (>60%) of SIVcpzPtt strains, these bNabs lacked activity against SIVgor and SIVcpzPts viruses, most likely because of substitutions within the core epitope and/or exceedingly long V1 loops. Nonetheless, antibodies directed against the MPER region neutralized even the most divergent ape viruses (Fig. 6). 4E10 neutralized all SIVcpzPtt strains, with a mean IC50 of 2.7 µg/ml (18.1 nM), but lacked activity against SIVgor and some SIVcpzPts strains. In contrast, 10E8 neutralized all 12 SIVcpz and SIVgor strains, with an IC50 of 0.7 µg/ml (4.4 nM). The latter finding may be of interest for “neutralization fingerprinting” studies (111), since inclusion of SIVgor and select SIVcpzPts Envs into existing pseudovirus panels should permit the differentiation of 4E10-like and 10E8-like bNab specificities in polyclonal patient plasma samples.
Antireceptor antibodies circumvent SIVcpz and SIVgor Env diversity.
Antibodies raised against human CD4 and CCR5 receptors are known to inhibit HIV-1 in vitro (112) as well as reduce viral loads in infected patients in vivo (96). Here, we show that these antibodies also neutralize SIVcpz and SIVgor strains in cells expressing human as well as chimpanzee CD4 and CCR5 receptors (Fig. 7). For example, iMab, which blocks HIV-1 infection by binding to the second domain (D2) of human CD4 (113), neutralized nearly all ape viruses with low-nanomolar potency in TZM-bl as well as primary chimpanzee CD4+ T cells (Table 2; see Table S1 in the supplemental material). This was also true for LM52, a derivative of iMab with increased neutralization breadth (59), as well as PRO140, which binds a complex epitope spanning multiple extracellular CCR5 domains (114), both of which neutralized all ape viruses in all cell types tested (Table 2; see Table S1). Thus, antireceptor antibodies neutralized even the most divergent ape viruses with remarkable potency, including in physiologically relevant target cells (Fig. 7; see Fig. S2 in the supplemental material).
Although iMab achieved a 1-log reduction in virus titers in chronically HIV-1-infected humans (96, 112), a second anti-CD4 antibody (2D5) afforded only partial protection in rhesus macaques challenged with a simian/human immunodeficiency virus (SHIV) (115). Because antireceptor antibodies have to block all susceptible target cells, they may require antibody concentrations at the site of virus entry that are difficult to achieve in vivo (115). We thus tested bispecific constructs, in which iMab, LM52, and PRO140 were linked to the antigen binding domains of potent anti-HIV-1 bNabs. Indeed, PG9-iMab, PG16-iMab, and LM52-PGT128 were able to outperform iMab and LM52 (Fig. 7; see Table S1 in the supplemental material) but only when used to neutralize the few ape viruses that were sensitive to PG9, PG16, and PGT128 (Fig. 3). However, the bispecific PRO140-10E8 neutralized all SIVcpz and SIVgor strains with up to 20-fold increased potency, regardless of whether TZM-bl, Cf2Th-ch, or primary chimpanzee CD4+ T cells were used as target cells (Fig. 7 and 9 and Table 2; see Fig S2 and Table S1 in the supplemental material). Of all antireceptor antibodies tested, PRO140-10E8 was by far the most potent, neutralizing 100% of ape viruses, with IC50s ranging from 0.03 µg/ml (0.13 nM) in TZM-bl cells to 0.75 µg/ml (3.6 nM) in chimpanzee CD4+ T cells (Fig. 9). These findings are consistent with the idea that bispecific antireceptor antibodies exhibit enhanced neutralizing capacity because they concentrate anti-Env bNabs at the site of virus entry (60). It will be important to test PRO140-10E8 in the SHIV/macaque model to determine whether its in vitro potency will translate into in vivo protection.
Bispecific receptor mimetics confer broad anti-SIVcpz and anti-SIVgor activity.
Several studies have shown that CD4-containing immunoadhesins exhibit considerable anti-HIV-1 activity and even protect a subset of macaques from SIVmac infection (63, 116). Among these, constructs that use receptor mimicry to simultaneously engage both the CD4 and CCR5 binding sites are the most potent (62). These include CD4-CD4i reagents, in which the D1/D2 region of CD4 is linked to the heavy-chain variable region of a CD4-induced (CD4i) antibody, as well as CD4-Ig-based reagents that contain short CCR5-mimetic sulfopeptides on their C terminus (Fig. 8A). Although both CD4-CD4i and eCD4-Ig constructs neutralized human and ape viruses with nanomolar potency (see Table S1 in the supplemental material), we were particularly interested in eCD4-Ig, because of its smaller size as well as extensive in vitro and in vivo characterization. Recent studies demonstrated that eCD4-Ig not only neutralizes difficult-to-neutralize (tier 2 and 3) strains of HIV-1 but also inhibits HIV-2 and SIVmac viruses, with IC50s of less than 0.01 µg/ml (0.1 nM). Moreover, a rhesus macaque-adapted form of eCD4-Ig (rh-eCD4-Ig) was able to protect monkeys from multiple low-dose intravenous SHIV challenges when expressed from a recombinant AAV vector (63). Given these findings, we expected eCD4-Ig to neutralize ape viruses with similar breadth and potency. However, this was only partially the case. Although eCD4-Ig neutralized 100% of SIVcpz and SIVgor strains, the IC50s were ~50-fold higher than those previously reported for HIV-1, HIV-2, and SIVmac strains (63). Moreover, this potency was not improved when modified versions of eCD4-Ig (eCD4-Igmim2 and eCD4-IgQ40Amim2) were used (63), which exhibit enhanced anti-HIV-1 activity (Fig. 8B; see Table S1). Overall, eCD4-Ig neutralized SIVcpz and SIVgor strains only 5-fold more potently than CD4-Ig, compared to a 20- to 200-fold improvement for HIV-1 strains (63). Thus, eCD4-Ig neutralized ape viruses with the desired breadth but not with the desired potency.
Although a crystal structure of eCD4-Ig in complex with the HIV-1 Env is not available, modeling suggests that eCD4-Ig binds the HIV-1 Env trimer in a “claw-like” fashion, with two sulfopeptides and one CD4 moiety engaging two protomers within the same Env spike (63). Cross-linking of spike protomers has recently been shown to increase neutralization potency of antibody-like molecules by more than 100-fold (117). It is thus tempting to speculate that the architecture of the SIVcpz/SIVgor envelope spike differs from that of HIV-1, HIV-2, and SIVmac strains in such a way that one or more of the eCD4-Ig interaction sites are altered or occluded. It is also possible that the human CD4i antibody-derived E51 peptide does not properly mimic the binding of the chimpanzee and gorilla CCR5 coreceptors, which differ from human and macaque CCR5 molecules in one or two amino acids near the N terminus (94). It should be noted that replacing the human CD4 D1/D2 domain with the corresponding chimpanzee CD4 D1/D2 region in eCD4-Ig did not increase its potency against ape viruses (data not shown). Moreover, while SIVcpz and SIVgor Envs tend to have longer V1/V2 loops and more associated glycans than HIV-1 strains, this is also true for SIVmac and HIV-2 strains. Thus, V1/V2 loop length cannot explain the difference in eCD4-Ig neutralization susceptibility of ape viruses. Since there are no SIVcpz/SIVgor specific bNabs whose Fab fragments could be used for intraspike cross-linking, it will be important to determine whether the ape virus neutralizing capacity of eCD4-Ig can be improved by altering the length and/or flexibility of the IgG Fc hinge region and/or by improving the binding of the CCR5 mimetic peptide.
Vectored antibody gene transfer to combat ape pathogens.
Wild ape populations will go extinct unless there is a comprehensive approach to their conservation, which—under certain circumstances—may have to include medical interventions (19, 25). We have shown in the past that SIVcpz infection can have a substantial negative impact on the health, reproductive success, and longevity of chimpanzees in Gombe (2, 3). Because infected chimpanzees in Gombe are individually known, it would be possible to use vectored antibody gene transfer to administer a cocktail of neutralizing antibodies. This may not only benefit the infected chimpanzees but may also reduce their ability to transmit SIVcpz, which in an isolated population such as Gombe could lead to virus extinction. Vectored antibody gene delivery, alone or in combination with long-acting antiretrovirals (118, 119), could also be used to treat SIVcpz-infected chimpanzees in African sanctuaries (120) and/or to vaccinate orphaned chimpanzees prior to releasing them into the wild (121). Finally, vectored antibody gene delivery may be effective against other pathogens, such as anthrax and Ebola virus (122, 123). Obviously, delivered antibodies must be sufficiently potent and expression levels must be sufficiently high to be effective in vivo, but this seems achievable. As shown recently, AAV-delivered rh-eCD4-Ig protected macaques from repeated low-dose intravenous SHIV challenge at serum concentrations as low as 17 µg/ml (63). Moreover, AAV-rh-eCD4-Ig was much less immunogenic than other antibody-based AAV constructs, and functional rh-eCD4-Ig was stably expressed for 40 weeks at concentrations ranging between 17 and 77 µg/ml (63). Obviously, efforts to treat and/or vaccinate wild ape communities would only be contemplated if their survival was in serious jeopardy and if the safety and efficacy of the particular intervention were first demonstrated in captivity. However, given the increasing threat of infectious diseases to ape survival, there is an urgent need to explore new ways to curb the spread of pathogens in wild populations. Although further improvements will be necessary, the finding of several antibody-like constructs that are capable of neutralizing SIVcpz and SIVgor strains with nanomolar potency suggests that this goal is achievable.
MATERIALS AND METHODS
IMCs.
Full-length infectious molecular clones (IMCs) of HIV-1 strains SG3 (124), YU2 (125), and JRCSF (126), SIVcpzPtt strains EK505 (71), GAB1 (68), GAB2 (69), MB897 (71), and MT145 (71), SIVcpzPts strains BF1167 (7), TAN1 (70), TAN2 (70), and TAN3 (70), and the SIVgor strain CP2139 (15) have previously been reported. To generate additional IMCs, consensus SIVcpzPtt (LB715) and SIVcpzPts (TAN13) sequences were generated from two additional fecal samples (70). Briefly, partially overlapping subgenomic fragments were amplified from fecal RNA, gel purified, and sequenced directly. Chromatograms were examined for positions of base mixtures, and ambiguous sites were resolved as previously reported (70). The resulting proviral consensus sequences were synthesized in 3 nonoverlapping fragments (Blue Heron Biotechnology) and ligated using internal restriction enzyme enzymes. In addition, NotI and MluI restriction enzyme sites were added to the 5′ and 3′ termini to enable directional cloning into a modified (low-copy-number) pBR322 vector (15). Since full-length SIVcpz and SIVgor molecular clones are notoriously unstable, plasmids were grown in MAX Efficiency Stbl2 competent cells (Invitrogen) at 30°C and harvested before reaching saturating density, and each IMC was completely sequenced prior to biological analyses to confirm its integrity. The newly derived SIVcpz clones LB715 and TAN13 have been submitted to the National Institutes of Health Research and Reference Program (Rockville, MD), and their nucleotide sequences are available at GenBank.
Phylogenetic analysis.
Amino acid sequences inferred from env genes of HIV-1 (YU2, GenBank accession no. M93258; JRCSF, accession no. M38429; and SG3, accession no. L02317), SIVgor (CP2139.287, accession no. FJ424866), and SIVcpz (LB715, accession no. KP861923; MB897, accession no. EF535994; GAB2, accession no. AF382828; MT145, accession no. DQ373066; EK505, accession no. DQ373065; GAB1, accession no. X52154; TAN2, accession no. DQ374657; TAN3, accession no. DQ374658; TAN1, accession no. EF394356; TAN13, accession no. JQ768416; BF1167, accession no. JQ866001) strains were aligned using ClustalW (127), with regions that could not be unambiguously aligned excluded from the analysis. PhyML (version 3) was used to estimate the phylogeny based on a WAG + I + G + F model of amino acid replacement chosen using ProtTest (version 2.4) and a second-order Akaike information criterion (AIC) framework (128–130). Ten random-addition-order trees and a neighbor-joining tree were likelihood optimized using subtree pruning-regrafting (SPR) searches. Bayesian posterior probabilities were estimated with MrBayes using a mixed prior model (131).
Chimpanzee plasma.
Plasma samples were obtained from eight chimpanzees that had been experimentally infected with HIV-1 and/or SIVcpzANT decades earlier as part of AIDS pathogenesis and/or vaccine studies (Table 1). Samples were obtained from seven of these chimpanzees at the National Chimpanzee Sanctuary Chimp Haven in Keithville, LA, while samples were obtained from the remaining animal (Debbie) at the Southwest National Primate Research Center (SNPRC) in San Antonio, Texas. For Marc (21), Joye (23), Tika (22), Artica (23), Debbie (20), and Cotton (20), detailed infection histories and clinical follow-up studies have been reported; however, such information was not available for the remaining two animals. Blood samples were collected for veterinary purposes in the context of health examinations. Plasma samples from seven animals were analyzed for the presence of virus-specific antibodies using an enhanced chemiluminescent Western blot assay as previously described (9). Plasma samples were also tested for viral loads: for HIV-1-infected chimpanzees, this was done using the COBAS AmpliPrep/COBAS TaqMan HIV-1 test, v2.0 (Roche). For SIVcpzANT-infected chimpanzees, a previously reported quantitative PCR (qPCR) designed to detect both HIV-1 and SIVcpz vRNA was used (132). In addition, SIVcpzANT viremia was confirmed by amplifying and sequencing a virus-specific 1.3-kb gag-pol fragment from plasma viral RNA (Table 1). Sample collection was approved by the respective Institutional Animal Care and Use Committees.
Virus stocks.
HIV-1, SIVcpz, and SIVgor infectious molecular clones (8 µg) were transfected into 293T cells, and culture supernatants were harvested 72 h later. Viral stocks were tested for infectivity using TZM-bl cells, a HeLa-derived line that constitutively expresses CD4, CCR5, and CXCR4 receptors and contains integrated luciferase and β-galactosidase reporter genes under the control of an HIV-1 long terminal repeat (LTR) (133). TZM-bl cells (8,300 cells per well) were seeded in 96-well plates overnight and incubated with 10-fold serial dilutions of transfection stocks for 48 h to allow single round infection, and infectious units (IU) were determined by counting the number of β-galactosidase-expressing cells.
TZM-bl cell-based neutralization assay.
The neutralizing capacity of chimpanzee plasma, anti-HIV-1 monoclonal antibodies, and immunoadhesins was assessed using the TZM-bl assay as previously described (134, 135). Briefly, 96-well plates were seeded with TZM-bl cells (8,300 cells per well) overnight in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal bovine serum (FBS). Serial (5-fold) dilutions of chimpanzee plasma (1:20, 1:100, 1:500, 1:2,500, and 1:12,500) or anti-HIV-1 monoclonal antibodies and immunoadhesins (10, 2, 0.4, 0.08, and 0.016 µg/ml) were incubated with 4,800 infectious units (IU) of transfection-derived virus in a total volume of 100 µl in the presence of DEAE-dextran (40 µg/ml) for 1 h at 37°C, and this mixture was then added to TZM-bl cells in 96-well plates. After 48 h, TZM-bl cells were analyzed for luciferase expression using a Synergy H4 Hybrid microplate reader (Bio-Tek) with Gen5 version 1.11 software. To test the neutralization capacity of anti-CD4 and anti-CCR5 receptor antibodies, 5-fold serial dilutions (10, 2, 0.4, 0.08, and 0.016 µg/ml) were incubated with TZM-bl cells in a volume of 50 µl for 1 h at 37°C, followed by the addition of virus (4,800 IU in 50 µl) in the presence of DEAE-dextran (40 µg/ml) and further incubation for 48 h at 37°C. Controls included untreated cells and virus pretreated with normal human plasma or no antibody. Relative infectivity was calculated by dividing the number of luciferase units at each plasma and antibody/immunoadhesin dilution with values obtained for wells that contained normal human plasma or no antibody. Half-maximal inhibitory concentrations (IC50s) were determined by linear regression. All monoclonal antibodies were tested in duplicate on three independent occasions. Since available amounts of chimpanzee plasma were limited, samples were tested only once in duplicate.
Cf2Th cell-based neutralization assay.
To test the neutralization capacity of anti-host receptor antibodies, we transiently transfected Cf2Th cells with plasmids expressing the chimpanzee CD4 and CCR5 genes. These canine thymus-derived cells do not naturally express CD4 or CCR5 but contain a firefly luciferase reporter gene stably integrated under the control of an HIV-1 long terminal repeat (LTR) (2, 3, 136). To generate a chimpanzee CD4 expression plasmid, we extracted RNA from chimpanzee peripheral blood mononuclear cells (PBMCs) and used RT-PCR to generate a cDNA clone of the entire CD4 coding region. Since the chimpanzee CD4 gene is polymorphic (72), we selected one allele that is most predominant in chimpanzees from Gombe National Park. Using site-directed mutagenesis, we then “humanized” this cDNA clone by changing 3 amino acids to match the (nonpolymorphic) human CD4 protein (T34I, V55A, and G88E). To generate a chimpanzee CCR5 expression plasmid, we first amplified the human CCR5 gene from PBMC RNA and then introduced a single (chimpanzee-specific) amino acid substitution (N13D) by site-directed mutagenesis. These expression plasmids were then used to transfect Cf2Th cells in 10-cm dishes at 50% confluence to generate cells that expressed either chimpanzee or human CCR5 and CD4 receptors. Twenty-four hours posttransfection, Cf2Th cells were trypsinized and plated in 96-well plates at a concentration of 6,000 cells per well in DMEM containing 3.5% FBS. Transfected cells were cultured overnight, incubated with serial (5-fold) dilutions (10, 2, 0.4, 0.08, and 0.016 µg/ml) of mono- and bispecific anti-CD4 (iMab, LM52, PG9-iMab, PG16-iMab, and LM52-PGT128) and anti-CCR5 (PRO-140 and PRO-140-10E8) antibodies in a volume of 50 µl DMEM for 1 h, and then infected with 50 µl of virus stock (5,000 IU) in the absence of DEAE-dextran, which is toxic to Cf2Th cells. After 48 h, Cf2Th cells were lysed and analyzed for luciferase expression using a Synergy H4 Hybrid microplate reader (Bio-Tek) with Gen5 version 1.11 software. Relative infectivity was calculated by dividing the luciferase units of wells containing antibodies by the luciferase units of wells lacking antibodies. Half-maximal inhibitory concentrations (IC50s) were determined by linear regression. HIV-1 SG3, which requires CXCR4 for entry, was used as a negative control. All monoclonal antibodies were tested in triplicate, and the average from 3 replicates is reported.
CD4 T cell-based neutralization assay.
Leftover blood samples from health examinations of uninfected chimpanzees housed at the Yerkes Regional Primate Center were shipped at room temperature (137), and peripheral blood mononuclear cells (PBMCS) were isolated by gradient centrifugation using Ficoll-Paque Plus (GE Healthcase Life Sciences). Chimpanzee CD4+ T cells were enriched using nonhuman primate CD4 microbeads (MACS Miltenyi Biotec) and magnetic cell sorting (Militenyi Biotec), stimulated with staphylococcal enterotoxin B (Sigma-Aldridge) for 12 to 15 h (3 µg/ml), and subsequently cocultivated with autologous monocyte-derived macrophages for optimal activation. After 5 to 6 days, CD4+ T cells were removed from the macrophage feeder layer, placed into DMEM with 10% FBS, and incubated with 30 U/ml interleukin-2 (IL-2). To test the neutralizing capacity of anti-HIV-1 monoclonal antibodies and immunoadhesins, serial dilutions (10, 3.3, 1.1, 0.36, and 0 µg/ml) were incubated with SIVcpzMT145 and HIV-1 SG3 viral stocks (10,000 IU) for 1 h at 37°C, and the mixture was then added to 5 × 105 activated chimpanzee CD4+ T cells in a total of 500 µl DMEM containing 10% FBS and 30 U/ml IL-2. The CD4bs antibody VRC01 and uninfected cells were used as negative controls. To test the neutralizing capacity of antireceptor antibodies, serial dilutions (10, 3.3, 1.1, and 0.36 µg/ml) were first incubated with 5 × 105 activated chimpanzee CD4+ T cells for 1 h at 37°C and then exposed to 10,000 IU of virus stocks. After an overnight incubation, cells were washed three times with PBS to remove antibody and non-cell-associated virus, suspended in DMEM with 10% FBS and 30 U/ml IL-2, and plated in triplicate in 96-well plates. Supernatant (50 µl) was harvested every 48 h, starting at day 3 postinfection, and replaced with fresh media. To monitor viral replication, supernatants were tested for reverse transcriptase (RT) activity using a colorimetric assay (Roche). Neutralization was calculated by dividing the infectivity (RT activity) of wells with antibody dilutions by that of the untreated control wells. IC50s were calculated by linear regression. All monoclonal antibodies were tested in CD4+ T cells from three different chimpanzee donors.
Statistical analyses.
Statistical analyses were performed using Prism version 5.0d software (GraphPad), and correlations were assessed using Spearman tests. Geometric mean IC50s were calculated using Microsoft Excel version 14.3.9 software.
Accession numbers.
The nucleotide sequences of SIVcpz strains LB715 and TAN13 as well as the chimpanzee CD4 and CCR5 genes used for transfection are available under GenBank accession no. KP861923, JQ768416, KP235488, and KP235489, respectively.
SUPPLEMENTAL MATERIAL
Detection of HIV-1- and SIVcpz-specific antibodies in experimentally infected captive chimpanzees. Plasma samples were diluted 1:1,000,000 and tested for reactivity with HIV-1 proteins by ECL (enhanced chemiluminescence) Western blot analysis. The positions of HIV-1 Env (gp160, gp120, and gp41), Pol (p66 and p31), and Gag (p55, p24, and p17) proteins are shown. Plasma samples from HIV-1-infected (pos) and uninfected (neg) humans were used as controls. Plasma samples from Joye were not included because of insufficient quantity. Download
Neutralization of SIVcpz in primary chimpanzee CD4+ T cells. (A) Serial dilutions (red, 10 µg/ml; green, 3.3 µg/ml; blue, 1.1 µg/ml; purple, 0.36 µg/ml) of the most potent anti-SIVcpz antibodies and immunoadhesins (as determined in TZM-bl cells) were incubated with the SIVcpz strain MT145 before addition to activated primary chimpanzee CD4+ T cells. (Antireceptor antibodies were incubated with cells before virus was added.) Plots depict MT145 replication after antibody removal, with time (days) plotted on the x axis and RT activity on the y axis. Antibody VRC01, which does not neutralize SIVcpz and SIVgor strains, served as a negative control. CD4+ T cells from three different chimpanzees were used (one representative experiment is shown). (B) Correlation of IC50s from TZM-bl and primary chimpanzee CD4+ T cells. IC50s averaged from three independent experiments were plotted for chimpanzee CD4+ T cells (x axis) and TZM-bl (y axis) and analyzed using the Spearman correlation test. Download
Neutralization potency of monoclonal antibodies and immunoadhesins (nanomolar concentration) in TZM-bl cells.
ACKNOWLEDGMENTS
We thank the staffs of Chimp Haven, the Southwest National Primate Research Center (SNPRC), and the Yerkes Regional Primate Research Center (YRPRC) for sample collection from captive chimpanzees, John P. Moore and Raiees Andrabi for expert advice, Jun Takehisa for IMC construction, Erica Parrish, Andrew Smith, Andrew Caffro, Miguel Ramirez, and Eric Ruff for technical assistance, and Shivani Sethi for artwork and manuscript preparation.
This work was supported by grants from the National Institutes of Health (R37 AI 50529, R01 AI 58715, R37 AI 066998, P01 AI 088564, P51 RR 000165, and P30 AI 045008).
The views and opinions expressed in this publication represent the authors’ views alone and do not express or imply the views, endorsement, or financial support of the Federal Government or any of its agencies, including the National Institutes of Health, unless otherwise stated by an authorized representative thereof.
Footnotes
Citation Barbian HJ, Decker JM, Bibollet-Ruche F, Galimidi RP, West AP, Jr, Learn GH, Parrish NF, Iyer SS, Li Y, Pace CS, Song R, Huang Y, Denny TN, Mouquet H, Martin L, Acharya P, Zhang B, Kwong PD, Mascola JR, Verrips CT, Strokappe NM, Rutten L, McCoy LE, Weiss RA, Brown CS, Jackson R, Silvestri G, Connors M, Burton DR, Shaw GM, Nussenzweig MC, Bjorkman PJ, Ho DD, Farzan M, Hahn BH. 2015. Neutralization properties of simian immunodeficiency viruses infecting chimpanzees and gorillas. mBio 6(2):e00296-15. doi:10.1128/mBio.00296-15.
REFERENCES
- 1.Sharp PM, Hahn BH. 2011. Origins of HIV and the AIDS pandemic. Cold Spring Harb Perspect Med 1:a006841. doi: 10.1101/cshperspect.a006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keele BF, Jones JH, Terio KA, Estes JD, Rudicell RS, Wilson ML, Li Y, Learn GH, Beasley TM, Schumacher-Stankey J, Wroblewski E, Mosser A, Raphael J, Kamenya S, Lonsdorf EV, Travis DA, Mlengeya T, Kinsel MJ, Else JG, Silvestri G, Goodall J, Sharp PM, Shaw GM, Pusey AE, Hahn BH. 2009. Increased mortality and AIDS-like immunopathology in wild chimpanzees infected with SIVcpz. Nature 460:515–519. doi: 10.1038/nature08200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rudicell RS, Holland Jones J, Wroblewski EE, Learn GH, Li Y, Robertson JD, Greengrass E, Grossmann F, Kamenya S, Pintea L, Mjungu DC, Lonsdorf EV, Mosser A, Lehman C, Collins DA, Keele BF, Goodall J, Hahn BH, Pusey AE, Wilson ML. 2010. Impact of simian immunodeficiency virus infection on chimpanzee population dynamics. PLoS Pathog 6:e1001116. doi: 10.1371/journal.ppat.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Terio KA, Kinsel MJ, Raphael J, Mlengeya T, Lipende I, Kirchhoff CA, Gilagiza B, Wilson ML, Kamenya S, Estes JD. 2011. Pathologic lesions in chimpanzees (Pan trogylodytes schweinfurthii) from Gombe National Parks, Tanzania: 2004–2010. J Zoo Wildl Med 42:597–607. doi: 10.1638/2010-0237.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailes E, Gao F, Bibollet-Ruche F, Courgnaud V, Peeters M, Marx PA, Hahn BH, Sharp PM. 2003. Hybrid origin of SIV in chimpanzees. Science 300:1713. doi: 10.1126/science.1080657. [DOI] [PubMed] [Google Scholar]
- 6.Keele BF, Van Heuverswyn F, Li Y, Bailes E, Takehisa J, Santiago ML, Bibollet-Ruche F, Chen Y, Wain LV, Liegeois F, Loul S, Ngole EM, Bienvenue Y, Delaporte E, Brookfield JF, Sharp PM, Shaw GM, Peeters M, Hahn BH. 2006. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 313:523–526. doi: 10.1126/science.1126531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Ndjango J-B, Learn GH, Ramirez MA, Keele BF, Bibollet-Ruche F, Liu W, Easlick JL, Decker JM, Rudicell RS, Inogwabini B-I, Ahuka-Mundeke S, Leendertz FH, Reynolds V, Muller MN, Chancellor RL, Rundus AS, Simmons N, Worobey M, Shaw GM, Peeters M, Sharp PM, Hahn BH. 2012. Eastern chimpanzees, but not bonobos, represent a simian immunodeficiency virus reservoir. J Virol 86:10776–10791. doi: 10.1128/JVI.01498-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santiago ML, Rodenburg CM, Kamenya S, Bibollet-Ruche F, Gao F, Bailes E, Meleth S, Soong S-J, Kilby JM, Moldoveanu Z, Fahey B, Muller MN, Ayouba A, Nerrienet E, McClure HM, Heeney JL, Pusey AE, Collins DA, Boesch C, Wrangham RW, Goodall J, Sharp PM, Shaw GM, Hahn BH. 2002. SIVcpz in wild chimpanzees. Science 295:465. doi: 10.1126/science.295.5554.465. [DOI] [PubMed] [Google Scholar]
- 9.Santiago ML, Lukasik M, Kamenya S, Li Y, Bibollet-Ruche F, Bailes E, Muller MN, Emery M, Goldenberg DA, Lwanga JS, Ayouba A, Nerrienet E, McClure HM, Heeney JL, Watts DP, Pusey AE, Collins DA, Wrangham RW, Goodall J, Brookfield JFY, Sharp PM, Shaw GM, Hahn BH. 2003. Foci of endemic simian immunodeficiency virus infection in wild-living eastern chimpanzees (Pan troglodytes schweinfurthii). J Virol 77:7545–7562. doi: 10.1128/JVI.77.13.7545-7562.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rudicell RS, Piel AK, Stewart F, Moore DL, Learn GH, Li Y, Takehisa J, Pintea L, Shaw GM, Moore J, Sharp PM, Hahn BH. 2011. High prevalence of simian immunodeficiency virus infection in a community of savanna chimpanzees. J Virol 85:9918–9928. doi: 10.1128/JVI.05475-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Heuverswyn F, Li Y, Bailes E, Neel C, Lafay B, Keele BF, Shaw KS, Takehisa J, Kraus MH, Loul S, Butel C, Liegeois F, Yangda B, Sharp PM, Mpoudi-Ngole E, Delaporte E, Hahn BH, Peeters M. 2007. Genetic diversity and phylogeographic clustering of SIVcpzPtt in wild chimpanzees in Cameroon. Virology 368:155–171. doi: 10.1016/j.virol.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 12.Piel AK, Stewart FA, Pintea L, Li Y, Ramirez MA, Loy DE, Crystal PA, Learn GH, Knapp LA, Sharp PM, Hahn BH. 2013. The Malagarasi River does not form an absolute barrier to chimpanzee movement in western Tanzania. PLoS One 8:e58965. doi: 10.1371/journal.pone.0058965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Heuverswyn F, Li Y, Neel C, Bailes E, Keele BF, Liu W, Loul S, Butel C, Liegeois F, Bienvenue Y, Ngolle EM, Sharp PM, Shaw GM, Delaporte E, Hahn BH, Peeters M. 2006. Human immunodeficiency viruses: SIV infection in wild gorillas. Nature 444:164. doi: 10.1038/444164a. [DOI] [PubMed] [Google Scholar]
- 14.Etienne L, Locatelli S, Ayouba A, Esteban A, Butel C, Liegeois F, Aghokeng A, Delaporte E, Mpoudi Ngole E, Peeters M. 2012. Noninvasive follow-up of simian immunodeficiency virus infection in wild-living nonhabituated western lowland gorillas in Cameroon. J Virol 86:9760–9772. doi: 10.1128/JVI.01186-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takehisa J, Kraus MH, Ayouba A, Bailes E, Van Heuverswyn F, Decker JM, Li Y, Rudicell RS, Learn GH, Neel C, Ngole EM, Shaw GM, Peeters M, Sharp PM, Hahn BH. 2009. Origin and biology of simian immunodeficiency virus in wild-living western gorillas. J Virol 83:1635–1648. doi: 10.1128/JVI.02311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.D’Arc M, Ayouba A, Esteban A, Learn GH, Boué V, Liegeois F, Etienne L, Tagg N, Leendertz FH, Boesch C, Madinda NF, Robbins MM, Gray M, Cournil A, Ooms M, Letko M, Simon VA, Sharp PM, Hahn BH, Delaporte E, Ngole EM, Peeters M. 2015. Origin of the HIV-1 group O epidemic in western lowland gorillas. Proc Natl Acad Sci U S A doi: 10.1073/pnas.1502022112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walsh PD, Abernethy KA, Bermejo M, Beyers R, De Wachter P, Akou ME, Huijbregts B, Mambounga DI, Toham AK, Kilbourn AM, Lahm SA, Latour S, Maisels F, Mbina C, Mihindou Y, Obiang SN, Effa EN, Starkey MP, Telfer P, Thibault M, Tutin CEG, White LJT, Wilkie DS. 2003. Catastrophic ape decline in western equatorial Africa. Nature 422:611–614. doi: 10.1038/nature01566. [DOI] [PubMed] [Google Scholar]
- 18.Weiss RA, Heeney JL. 2009. Infectious diseases: an ill wind for wild chimps? Nature 460:470–471. doi: 10.1038/460470a. [DOI] [PubMed] [Google Scholar]
- 19.Ryan SJ, Walsh PD. 2011. Consequences of non-intervention for infectious disease in African great apes. PLoS One 6:e29030. doi: 10.1371/journal.pone.0029030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heeney JL, Rutjens E, Verschoor EJ, Niphuis H, ten Haaft P, Rouse S, McClure H, Balla-Jhagjhoorsingh S, Bogers W, Salas M, Cobb K, Kestens L, Davis D, van der Groen G, Courgnaud V, Peeters M, Murthy KK. 2006. Transmission of simian immunodeficiency virus SIVcpz and the evolution of infection in the presence and absence of concurrent human immunodeficiency virus type 1 infection in chimpanzees. J Virol 80:7208–7218. doi: 10.1128/JVI.00382-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fultz PN, McClure HM, Swenson RB, McGrath CR, Brodie A, Getchell JP, Jensen FC, Anderson DC, Broderson JR, Francis DP. 1986. Persistent infection of chimpanzees with human T-lymphotropic virus type III/lymphadenopathy-associated virus: a potential model for acquired immunodeficiency syndrome. J Virol 58:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novembre FJ, de Rosayro J, Nidtha S, O’Neil SP, Gibson TR, Evans-Strickfaden T, Hart CE, McClure HM. 2001. Rapid CD4+ T-cell loss induced by human immunodeficiency virus type 1(NC) in uninfected and previously infected chimpanzees. J Virol 75:1533–1539. doi: 10.1128/JVI.75.3.1533-1539.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu SL, Fultz PN, McClure HM, Eichberg JW, Thomas EK, Zarling J, Singhal MC, Kosowski SG, Swenson RB, Anderson DC, Todaro G. 1987. Effect of immunization with a vaccinia-HIV env recombinant on HIV infection of chimpanzees. Nature 328:721–723. doi: 10.1038/328721a0. [DOI] [PubMed] [Google Scholar]
- 24.Altevogt BM, Pankevich DE, Shelton-Davenport MK, Kahn JP (ed). 2011. Chimpanzees in biomedical and behavioral research: assessing the necessity. Institute of Medicine (US) and National Research Council (US) Committee on the Use of Chimpanzees in Biomedical and Behavioral Research. National Academies Press, Washington, DC. [PubMed] [Google Scholar]
- 25.Warfield KL, Goetzmann JE, Biggins JE, Kasda MB, Unfer RC, Vu H, Aman MJ, Olinger GG, Walsh PD. 2014. Vaccinating captive chimpanzees to save wild chimpanzees. Proc Natl Acad Sci U S A 111:8873–8876. doi: 10.1073/pnas.1316902111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weiss RA. 2014. Immunotherapy for HIV infection. N Engl J Med 370:379–380. doi: 10.1056/NEJMcibr1314577. [DOI] [PubMed] [Google Scholar]
- 27.Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang SK, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. 2010. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. 2012. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature 481:81–84. doi: 10.1038/nature10660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson PR, Schnepp BC, Zhang J, Connell MJ, Greene SM, Yuste E, Desrosiers RC, Clark KR. 2009. Vector-mediated gene transfer engenders long-lived neutralizing activity and protection against SIV infection in monkeys. Nat Med 15:901–906. doi: 10.1038/nm.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. 2009. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 34.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, Koff WC, Watkins DI, Burton DR. 2009. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 5:e1000433. doi: 10.1371/journal.ppat.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu R-B, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang H-W, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Balazs AB, Ouyang Y, Hong CM, Chen J, Nguyen SM, Rao DS, An DS, Baltimore D. 2014. Vectored immunoprophylaxis protects humanized mice from mucosal HIV transmission. Nat Med 20:296–300. doi: 10.1038/nm.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaplitt MG, Feigin A, Tang C, Fitzsimons HL, Mattis P, Lawlor PA, Bland RJ, Young D, Strybing K, Eidelberg D, During MJ. 2007. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet 369:2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 40.Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, Windsor EA, Conlon TJ, Sumaroka A, Pang J-J, Roman AJ, Byrne BJ, Jacobson SG. 2009. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther 20:999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL.. 2011. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365:2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maguire AM, Simonelli F, Pierce EA, Pugh EN, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma J-X, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J. 2008. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lewis AD, Chen R, Montefiori DC, Johnson PR, Clark KR. 2002. Generation of neutralizing activity against human immunodeficiency virus type 1 in serum by antibody gene transfer. J Virol 76:8769–8775. doi: 10.1128/JVI.76.17.8769-8775.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehandru S, Vcelar B, Wrin T, Stiegler G, Joos B, Mohri H, Boden D, Galovich J, Tenner-Racz K, Racz P, Carrington M, Petropoulos C, Katinger H, Markowitz M. 2007. Adjunctive passive immunotherapy in human immunodeficiency virus type 1-infected individuals treated with antiviral therapy during acute and early infection. J Virol 81:11016–11031. doi: 10.1128/JVI.01340-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trkola A, Kuster H, Rusert P, Joos B, Fischer M, Leemann C, Manrique A, Huber M, Rehr M, Oxenius A, Weber R, Stiegler G, Vcelar B, Katinger H, Aceto L, Günthard HF. 2005. Delay of HIV-1 rebound after cessation of antiretroviral therapy through passive transfer of human neutralizing antibodies. Nat Med 11:615–622. doi: 10.1038/nm1244. [DOI] [PubMed] [Google Scholar]
- 46.Kwong PD, Mascola JR. 2012. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity 37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. 2013. Antibodies in HIV-1 vaccine development and therapy. Science 341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burton DR, Poignard P, Stanfield RL, Wilson IA. 2012. Broadly neutralizing antibodies present new prospects to counter highly antigenically diverse viruses. Science 337:183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi: 10.1038/nature11544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zwick MB, Labrijn AF, Wang M, Spenlehauer C, Saphire EO, Binley JM, Moore JP, Stiegler G, Katinger H, Burton DR, Parren PW. 2001. Broadly neutralizing antibodies targeted to the membrane-proximal external region of human immunodeficiency virus type 1 glycoprotein gp41. J Virol 75:10892–10905. doi: 10.1128/JVI.75.22.10892-10905.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, Chen X, Longo NS, Louder M, McKee K, O’Dell S, Perfetto S, Schmidt SD, Shi W, Wu L, Yang Y, Yang Z-Y, Yang Z, Zhang Z, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Simek M, Burton DR, Koff WC, Doria-Rose NA, Connors M, NISC Comparative Sequencing Program, Mullikin JC, Nabel GJ, Roederer M, Shapiro L, Kwong PD, Mascola JR. 2011. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science 333:1593–1602. doi: 10.1126/science.1207532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PN, Spencer DI, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, Georgiev IS, Chuang G-Y, Druz A, Doria-Rose NA, Laub L, Sliepen K, van Gils MJ, de la Peña AT, Derking R, Klasse PJ, Migueles SA, Bailer RT, Alam M, Pugach P, Haynes BF, Wyatt RT, Sanders RW, Binley JM, Ward AB, Mascola JR, Kwong PD, Connors M. 2014. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 515:138–142. doi: 10.1038/nature13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Scharf L, Scheid JF, Lee JH, West AP Jr, Chen C, Gao H, Gnanapragasam PN, Mares R, Seaman MS, Ward AB, Nussenzweig MC, Bjorkman PJ. 2014. Antibody 8ANC195 reveals a site of broad vulnerability on the HIV-1 envelope spike. Cell Rep 7:785–795. doi: 10.1016/j.celrep.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, de la Peña AT, Cupo A, Julien J-P, van Gils M, Lee PS, Peng W, Paulson JC, Poignard P, Burton DR, Moore JP, Sanders RW, Wilson IA, Ward AB. 2014. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 40:669–680. doi: 10.1016/j.immuni.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falkowska E, Le KM, Ramos A, Doores KJ, Lee JH, Blattner C, Ramirez A, Derking R, van Gils MJ, Liang C-H, McBride R, von Bredow B, Shivatare SS, Wu C-Y, Chan-Hui P-Y, Liu Y, Feizi T, Zwick MB, Koff WC, Seaman MS, Swiderek K, Moore JP, Evans D, Paulson JC, Wong C-H, Ward AB, Wilson IA, Sanders RW, Poignard P, Burton DR. 2014. Broadly neutralizing HIV antibodies define a glycan-dependent epitope on the prefusion conformation of gp41 on cleaved envelope trimers. Immunity 40:657–668. doi: 10.1016/j.immuni.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trkola A, Ketas TJ, Nagashima KA, Zhao L, Cilliers T, Morris L, Moore JP, Maddon PJ, Olson WC. 2001. Potent, broad-spectrum inhibition of human immunodeficiency virus type 1 by the CCR5 monoclonal antibody PRO 140. J Virol 75:579–588. doi: 10.1128/JVI.75.2.579-588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dimitrov A. 2007. Ibalizumab, a CD4-specific mAb to inhibit HIV-1 infection. Curr Opin Investig Drugs 8:653–661. [PubMed] [Google Scholar]
- 59.Song R, Oren DA, Franco D, Seaman MS, Ho DD. 2013. Strategic addition of an N-linked glycan to a monoclonal antibody improves its HIV-1-neutralizing activity. Nat Biotechnol 31:1047–1052. doi: 10.1038/nbt.2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pace CS, Song R, Ochsenbauer C, Andrews CD, Franco D, Yu J, Oren DA, Seaman MS, Ho DD. 2013. Bispecific antibodies directed to CD4 domain 2 and HIV envelope exhibit exceptional breadth and picomolar potency against HIV-1. Proc Natl Acad Sci U S A 110:13540–13545. doi: 10.1073/pnas.1304985110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Capon DJ, Chamow SM, Mordenti J, Marsters SA, Gregory T, Mitsuya H, Byrn RA, Lucas C, Wurm FM, Groopman JE, Broder S, Smith DH. 1989. Designing CD4 immunoadhesins for AIDS therapy. Nature 337:525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- 62.West AP Jr, Galimidi RP, Foglesong CP, Gnanapragasam PN, Klein JS, Bjorkman PJ. 2010. Evaluation of CD4-CD4i antibody architectures yields potent, broadly cross-reactive anti-human immunodeficiency virus reagents. J Virol 84:261–269. doi: 10.1128/JVI.01528-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gardner MR, Kattenhorn LM, Kondur HR, von Schaewen M, Dorfman T, Chiang JJ, Haworth KG, Decker JM, Alpert MD, Bailey CC, Fellinger CH, Joshi VR, Fuchs SP, Martinez-Navio JM, Quinlan BD, Yao AY, Mouquet H, Gorman J, Zhang B, Poignard P, Nussenzweig MC, Burton DR, Kwong PD, Piatak M, Lifson JD, Gao G, Desrosiers RC, Evans DT, Hahn BH, Ploss A, Cannon PM, Seaman MS, Farzan M. 2015. AAV-expressed eCD4-Ig provides durable protection from multiple SHIV challenges. Nature 519:87–91. doi: 10.1038/nature14264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hraber P, Korber BT, Lapedes AS, Bailer RT, Seaman MS, Gao H, Greene KM, McCutchan F, Williamson C, Kim JH, Tovanabutra S, Hahn BH, Swanstrom R, Thomson MM, Gao F, Harris L, Giorgi E, Hengartner N, Bhattacharya T, Mascola JR, Montefiori DC. 2014. Impact of clade, geography, and age of the epidemic on HIV-1 neutralization by antibodies. J Virol 88:12623–12643. doi: 10.1128/JVI.01705-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Novembre FJ, Saucier M, Anderson DC, Klumpp SA, O’Neil SP, Brown CR, Hart CE, Guenthner PC, Swenson RB, McClure HM. 1997. Development of AIDS in a chimpanzee infected with human immunodeficiency virus type 1. J Virol 71:4086–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Peeters M, Fransen K, Delaporte E, van den Haesevelde M, Gershy-Damet GM, Kestens L, van der Groen G, Piot P. 1992. Isolation and characterization of a new chimpanzee lentivirus (simian immunodeficiency virus isolate cpz-ant) from a wild-captured chimpanzee. AIDS 6:447–451. doi: 10.1097/00002030-199205000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Huet T, Cheynier R, Meyerhans A, Roelants G, Wain-Hobson S. 1990. Genetic organization of a chimpanzee lentivirus related to HIV-1. Nature 345:356–359. doi: 10.1038/345356a0. [DOI] [PubMed] [Google Scholar]
- 69.Bibollet-Ruche F, Gao F, Bailes E, Saragosti S, Delaporte E, Peeters M, Shaw GM, Hahn BH, Sharp PM. 2004. Complete genome analysis of one of the earliest SIVcpzPtt strains from Gabon (SIVcpzGAB2). AIDS Res Hum Retroviruses 20:1377–1381. doi: 10.1089/aid.2004.20.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takehisa J, Kraus MH, Decker JM, Li Y, Keele BF, Bibollet-Ruche F, Zammit KP, Weng Z, Santiago ML, Kamenya S, Wilson ML, Pusey AE, Bailes E, Sharp PM, Shaw GM, Hahn BH. 2007. Generation of infectious molecular clones of simian immunodeficiency virus from fecal consensus sequences of wild chimpanzees. J Virol 81:7463–7475. doi: 10.1128/JVI.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bibollet-Ruche F, Heigele A, Keele BF, Easlick JL, Decker JM, Takehisa J, Learn G, Sharp PM, Hahn BH, Kirchhoff F. 2012. Efficient SIVcpz replication in human lymphoid tissue requires viral matrix protein adaptation. J Clin Invest 122:1644–1652. doi: 10.1172/JCI61429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hvilsom C, Carlsen F, Siegismund HR, Corbet S, Nerrienet E, Fomsgaard A. 2008. Genetic subspecies diversity of the chimpanzee CD4 virus-receptor gene. Genomics 92:322–328. doi: 10.1016/j.ygeno.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 73.Posner MR, Hideshima T, Cannon T, Mukherjee M, Mayer KH, Byrn RA. 1991. An IgG human monoclonal antibody that reacts with HIV-1/GP120, inhibits virus binding to cells, and neutralizes infection. J Immunol 146:4325–4332. [PubMed] [Google Scholar]
- 74.Barbas CF III, Collet TA, Amberg W, Roben P, Binley JM, Hoekstra D, Cababa D, Jones TM, Williamson RA, Pilkington GR, Haigwood NL, Cabezas E, Satterthwait AC, Sanz I, Burton DR. 1993. Molecular profile of an antibody response to HIV-1 as probed by combinatorial libraries. J Mol Biol 230:812–823. doi: 10.1006/jmbi.1993.1203. [DOI] [PubMed] [Google Scholar]
- 75.Diskin R, Scheid JF, Marcovecchio PM, West AP Jr, Klein F, Gao H, Gnanapragasam PN, Abadir A, Seaman MS, Nussenzweig MC, Bjorkman PJ. 2011. Increasing the potency and breadth of an HIV antibody by using structure-based rational design. Science 334:1289–1293. doi: 10.1126/science.1213782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.McCoy LE, Quigley AF, Strokappe NM, Bulmer-Thomas B, Seaman MS, Mortier D, Rutten L, Chander N, Edwards CJ, Ketteler R, Davis D, Verrips T, Weiss RA. 2012. Potent and broad neutralization of HIV-1 by a llama antibody elicited by immunization. J Exp Med 209:1091–1103. doi: 10.1084/jem.20112655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen L, Kwon YD, Zhou T, Wu X, O’Dell S, Cavacini L, Hessell AJ, Pancera M, Tang M, Xu L, Yang Z-Y, Zhang M-Y, Arthos J, Burton DR, Dimitrov DS, Nabel GJ, Posner MR, Sodroski J, Wyatt R, Mascola JR, Kwong PD. 2009. Structural basis of immune evasion at the site of CD4 attachment on HIV-1 gp120. Science 326:1123–1127. doi: 10.1126/science.1175868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou T, Georgiev I, Wu X, Yang Z-Y, Dai K, Finzi A, Kwon YD, Scheid JF, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, O’Dell S, Walker LM, Wu X, Guenaga J, Feng Y, Schmidt SD, McKee K, Louder MK, Ledgerwood JE, Graham BS, Haynes BF, Burton DR, Wyatt RT, Mascola JR. 2011. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J Virol 85:8954–8967. doi: 10.1128/JVI.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trkola A, Pomales AB, Yuan H, Korber B, Maddon PJ, Allaway GP, Katinger H, Barbas CF, Burton DR, Ho DD, Moore JP. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol 69:6609–6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Scanlan CN, Pantophlet R, Wormald MR, Ollmann Saphire E, Stanfield R, Wilson IA, Katinger H, Dwek RA, Rudd PM, Burton DR. 2002. The broadly neutralizing anti-human immunodeficiency virus type 1 antibody 2G12 recognizes a cluster of alpha1→2 mannose residues on the outer face of gp120. J Virol 76:7306–7321. doi: 10.1128/JVI.76.14.7306-7321.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sok D, Doores KJ, Briney B, Le KM, Saye-Francisco KL, Ramos A, Kulp DW, Julien J-P, Menis S, Wickramasinghe L, Seaman MS, Schief WR, Wilson IA, Poignard P, Burton DR. 2014. Promiscuous glycan site recognition by antibodies to the high-mannose patch of gp120 broadens neutralization of HIV. Sci Transl Med 6:236ra63. doi: 10.1126/scitranslmed.3008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McLellan JS, Pancera M, Carrico C, Gorman J, Julien J-P, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, O’Dell S, Patel N, Shahzad-ul-Hussan S, Yang Y, Zhang B, Zhou T, Zhu J, Boyington JC, Chuang GY, Diwanji D, Georgiev I, Kwon YD, Lee D, Louder MK, Moquin S, Schmidt SD, Yang Z-Y, Bonsignori M, Crump JA, Kapiga SH, Sam NE, Haynes BF, Burton DR, Koff WC, Walker LM, Phogat S, Wyatt R, Orwenyo J, Wang L-X, Arthos J, Bewley CA, Mascola JR, Nabel GJ, Schief WR, Ward AB, Wilson IA, Kwong PD. 2011. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature 480:336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Doria-Rose NA, Georgiev I, O’Dell S, Chuang G-Y, Staupe RP, McLellan JS, Gorman J, Pancera M, Bonsignori M, Haynes BF, Burton DR, Koff WC, Kwong PD, Mascola JR. 2012. A short segment of the HIV-1 gp120 V1/V2 region is a major determinant of resistance to V1/V2 neutralizing antibodies. J Virol 86:8319–8323. doi: 10.1128/JVI.00696-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sagar M, Wu X, Lee S, Overbaugh J. 2006. Human immunodeficiency virus type 1 V1-V2 envelope loop sequences expand and add glycosylation sites over the course of infection, and these modifications affect antibody neutralization sensitivity. J Virol 80:9586–9598. doi: 10.1128/JVI.00141-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Van Gils MJ, Bunnik EM, Boeser-Nunnink BD, Burger JA, Terlouw-Klein M, Verwer N, Schuitemaker H. 2011. Longer V1V2 region with increased number of potential N-linked glycosylation sites in the HIV-1 envelope glycoprotein protects against HIV-specific neutralizing antibodies. J Virol 85:6986–6995. doi: 10.1128/JVI.00268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forsman A, Beirnaert E, Aasa-Chapman MM, Hoorelbeke B, Hijazi K, Koh W, Tack V, Szynol A, Kelly C, McKnight A, Verrips T, De Haard H, Weiss RA. 2008. Llama antibody fragments with cross-subtype human immunodeficiency virus type 1 (HIV-1)-neutralizing properties and high affinity for HIV-1 gp120. J Virol 82:12069–12081. doi: 10.1128/JVI.01379-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Strokappe N, Szynol A, Aasa-Chapman M, Gorlani A, Forsman Quigley A, Hulsik DL, Chen L, Weiss R, De Haard H, Verrips T. 2012. Llama antibody fragments recognizing various epitopes of the CD4bs neutralize a broad range of HIV-1 subtypes A, B and C. PLoS One 7:e33298. doi: 10.1371/journal.pone.0033298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Acharya P, Luongo TS, Georgiev IS, Matz J, Schmidt SD, Louder MK, Kessler P, Yang Y, McKee K, O’Dell S, Chen L, Baty D, Chames P, Martin L, Mascola JR, Kwong PD. 2013. Heavy chain-only IgG2b llama antibody effects near-pan HIV-1 neutralization by recognizing a CD4-induced epitope that includes elements of coreceptor- and CD4-binding sites. J Virol 87:10173–10181. doi: 10.1128/JVI.01332-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Matz J, Kessler P, Bouchet J, Combes O, Ramos OH, Barin F, Baty D, Martin L, Benichou S, Chames P. 2013. Straightforward selection of broadly neutralizing single-domain antibodies targeting the conserved CD4 and coreceptor binding sites of HIV-1 gp120. J Virol 87:1137–1149. doi: 10.1128/JVI.00461-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McCoy LE, Rutten L, Frampton D, Anderson I, Granger L, Bashford-Rogers R, Dekkers G, Strokappe NM, Seaman MS, Koh W, Grippo V, Kliche A, Verrips T, Kellam P, Fassati A, Weiss RA. 2014. Molecular evolution of broadly neutralizing llama antibodies to the CD4-binding site of HIV-1. PLoS Pathog 10:e1004552. doi: 10.1371/journal.ppat.1004552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Strokappe NM. 2013. HIV-1, how llamas help us fight the AIDS pandemic. Ph.D. thesis. Utrecht University Repository, Utrecht, the Netherlands. [Google Scholar]
- 93.Lutje Hulsik D, Liu Y-Y, Strokappe NM, Battella S, El-Khattabi M, McCoy LE, Sabin C, Hinz A, Hock M, Macheboeuf P, Bonvin AM, Langedijk JP, Davis D, Forsman Quigley A, Aasa-Chapman MM, Seaman MS, Ramos A, Poignard P, Favier A, Simorre JP. 2013. A gp41 MPER-specific llama VHH requires a hydrophobic CDR3 for neutralization but not for antigen recognition. PLoS Pathog 9:e1003202. doi: 10.1371/journal.ppat.1003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zacharova V, Zachar V, Goustin AS. 1997. Sequence of chemokine receptor gene CCR5 in chimpanzees, a natural HIV type 1 host. AIDS Res Hum Retroviruses 13:1159–1161. doi: 10.1089/aid.1997.13.1159. [DOI] [PubMed] [Google Scholar]
- 95.Jacobson JM, Saag MS, Thompson MA, Fischl MA, Liporace R, Reichman RC, Redfield RR, Fichtenbaum CJ, Zingman BS, Patel MC, Murga JD, Pemrick SM, D’Ambrosio P, Michael M, Kroger H, Ly H, Rotshteyn Y, Buice R, Morris SA, Stavola JJ, Maddon PJ, Kremer AB, Olson WC. 2008. Antiviral activity of single-dose PRO 140, a CCR5 monoclonal antibody, in HIV-infected adults. J Infect Dis 198:1345–1352. doi: 10.1086/592169. [DOI] [PubMed] [Google Scholar]
- 96.Jacobson JM, Kuritzkes DR, Godofsky E, DeJesus E, Larson JA, Weinheimer SP, Lewis ST. 2009. Safety, pharmacokinetics, and antiretroviral activity of multiple doses of ibalizumab (formerly TNX-355), an anti-CD4 monoclonal antibody, in human immunodeficiency virus type 1-infected adults. Antimicrob Agents Chemother 53:450–457. doi: 10.1128/AAC.00942-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuritzkes DR, Jacobson J, Powderly WG, Godofsky E, DeJesus E, Haas F, Reimann KA, Larson JL, Yarbough PO, Curt V, Shanahan WR. 2004. Antiretroviral activity of the anti-CD4 monoclonal antibody TNX-355 in patients infected with HIV type 1. J Infect Dis 189:286–291. doi: 10.1086/380802. [DOI] [PubMed] [Google Scholar]
- 98.Bruno CJ, Jacobson JM. 2010. Ibalizumab: an anti-CD4 monoclonal antibody for the treatment of HIV-1 infection. J Antimicrob Chemother 65:1839–1841. doi: 10.1093/jac/dkq261. [DOI] [PubMed] [Google Scholar]
- 99.Song R, Franco D, Kao C-Y, Yu F, Huang Y, Ho DD. 2010. Epitope mapping of ibalizumab, a humanized anti-CD4 monoclonal antibody with anti-HIV-1 activity in infected patients. J Virol 84:6935–6942. doi: 10.1128/JVI.00453-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Allaway GP, Davis-Bruno KL, Beaudry GA, Garcia EB, Wong EL, Ryder AM, Hasel KW, Gauduin MC, Koup RA, McDougal JS, Maddon PJ. 1995. Expression and characterization of CD4-IgG2, a novel heterotetramer that neutralizes primary HIV type 1 isolates. AIDS Res Hum Retroviruses 11:533–539. doi: 10.1089/aid.1995.11.533. [DOI] [PubMed] [Google Scholar]
- 101.Smith DH, Byrn RA, Marsters SA, Gregory T, Groopman JE, Capon DJ. 1987. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science 238:1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- 102.Byrn RA, Mordenti J, Lucas C, Smith D, Marsters SA, Johnson JS, Cossum P, Chamow SM, Wurm FM, Gregory T, Groopman JE, Capon DJ. 1990. Biological properties of a CD4 immunoadhesin. Nature 344:667–670. doi: 10.1038/344667a0. [DOI] [PubMed] [Google Scholar]
- 103.Choe H, Li W, Wright PL, Vasilieva N, Venturi M, Huang C-C, Grundner C, Dorfman T, Zwick MB, Wang L, Rosenberg ES, Kwong PD, Burton DR, Robinson JE, Sodroski JG, Farzan M. 2003. Tyrosine sulfation of human antibodies contributes to recognition of the CCR5 binding region of HIV-1 gp120. Cell 114:161–170. doi: 10.1016/S0092-8674(03)00508-7. [DOI] [PubMed] [Google Scholar]
- 104.Dorfman T, Moore MJ, Guth AC, Choe H, Farzan M. 2006. A tyrosine-sulfated peptide derived from the heavy-chain CDR3 region of an HIV-1-neutralizing antibody binds gp120 and inhibits HIV-1 infection. J Biol Chem 281:28529–28535. doi: 10.1074/jbc.M602732200. [DOI] [PubMed] [Google Scholar]
- 105.Kwong JA, Dorfman T, Quinlan BD, Chiang JJ, Ahmed AA, Choe H, Farzan M. 2011. A tyrosine-sulfated CCR5-mimetic peptide promotes conformational transitions in the HIV-1 envelope glycoprotein. J Virol 85:7563–7571. doi: 10.1128/JVI.00630-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kilby JM, Hopkins S, Venetta TM, DiMassimo B, Cloud GA, Lee JY, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson MR, Nowak MA, Shaw GM, Saag MS. 1998. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med 4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 107.Liao H-X, Lynch R, Zhou T, Gao F, MunirAlam SM, Boyd SD, Fire AZ, Roskin KM, Schramm CA, Zhang Z, Zhu J, Shapiro L, NISC Comparative Sequencing Program, Mullikin JC, Gnanakaran S, Gnanakaran S, Hraber P, Wiehe K, Kelsoe G, Yang G, Xia SM, Montefiori DC, Parks R, Lloyd KE, Scearce RM, Soderberg KA, Cohen M, Kamanga G, Louder MK, Tran LM, Chen Y, Cai F, Chen S, Moquin S, Du X, Joyce MG, Srivatsan S, Zhang B, Zheng A, Shaw GM, Hahn BH, Kepler TB, Korber BTM, Kwong PD, Haynes BF. 2013. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature 496:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mouquet H, Nussenzweig MC. 2013. HIV: roadmaps to a vaccine. Nature 496:441–442. doi: 10.1038/nature12091. [DOI] [PubMed] [Google Scholar]
- 109.Gao F, Bonsignori M, Liao H-X, Kumar A, Xia S-M, Lu X, Cai F, Hwang K-K, Song H, Zhou T, Lynch RM, Alam SM, Moody MA, Ferrari G, Berrong M, Kelsoe G, Shaw GM, Hahn BH, Montefiori DC, Kamanga G, Cohen MS, Hraber P, Kwong PD, Korber BT, Mascola JR, Kepler TB, Haynes BF. 2014. Cooperation of B cell lineages in induction of HIV-1-broadly neutralizing antibodies. Cell 158:481–491. doi: 10.1016/j.cell.2014.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Georgiev IS, Doria-Rose NA, Zhou T, Do Kwon YD, Staupe RP, Moquin S, Chuang G-Y, Louder MK, Schmidt SD, Altae-Tran HR, Bailer RT, McKee K, Nason M, O’Dell S, Ofek G, Pancera M, Srivatsan S, Shapiro L, Connors M, Migueles SA. 2013. Delineating antibody recognition in polyclonal sera from patterns of HIV-1 isolate neutralization. Science 340:751–756. doi: 10.1126/science.1233989. [DOI] [PubMed] [Google Scholar]
- 112.Pace CS, Fordyce MW, Franco D, Kao C-Y, Seaman MS, Ho DD. 2013. Anti-CD4 monoclonal antibody ibalizumab exhibits breadth and potency against HIV-1, with natural resistance mediated by the loss of a V5 glycan in envelope. J Acquir Immune Defic Syndr 62:1–9. doi: 10.1097/QAI.0b013e3182732746. [DOI] [PubMed] [Google Scholar]
- 113.Burkly LC, Olson D, Shapiro R, Winkler G, Rosa JJ, Thomas DW, Williams C, Chisholm P. 1992. Inhibition of HIV infection by a novel CD4 domain 2-specific monoclonal antibody. Dissecting the basis for its inhibitory effect on HIV-induced cell fusion. J Immunol 149:1779–1787. [PubMed] [Google Scholar]
- 114.Olson WC, Rabut GE, Nagashima KA, Tran DN, Anselma DJ, Monard SP, Segal JP, Thompson DA, Kajumo F, Guo Y, Moore JP, Maddon PJ, Dragic T. 1999. Differential inhibition of human immunodeficiency virus type 1 fusion, gp120 binding, and CC-chemokine activity by monoclonal antibodies to CCR5. J Virol 73:4145–4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pegu A, Yang Z-Y, Boyington JC, Wu L, Ko S-Y, Schmidt SD, McKee K, Kong W-P, Shi W, Chen X, Todd J-P, Letvin NL, Huang J, Nason MC, Hoxie JA, Kwong PD, Connors M, Rao SS, Mascola JR, Nabel GJ. 2014. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 6:243ra88. doi: 10.1126/scitranslmed.3008992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poignard P, Moldt B, Maloveste K, Campos N, Olson WC, Rakasz E, Watkins DI, Burton DR. 2012. Protection against high-dose highly pathogenic mucosal SIV challenge at very low serum neutralizing titers of the antibody-like molecule CD4-IgG2. PLoS One 7:e42209. doi: 10.1371/journal.pone.0042209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Galimidi RP, Klein JS, Politzer MS, Bai S, Seaman MS, Nussenzweig MC, West AP Jr, Bjorkman PJ. 2015. Intra-spike crosslinking overcomes antibody evasion by HIV-1. Cell 160:433–446. doi: 10.1016/j.cell.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Andrews CD, Spreen WR, Mohri H, Moss L, Ford S, Gettie A, Russell-Lodrigue K, Bohm RP, Cheng-Mayer C, Hong Z, Markowitz M, Ho DD. 2014. Long-acting integrase inhibitor protects macaques from intrarectal simian/human immunodeficiency virus. Science 343:1151–1154. doi: 10.1126/science.1248707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Andrews CD, Yueh YL, Spreen WR, St Bernard L, Boente-Carrera M, Rodriguez K, Gettie A, Russell-Lodrigue K, Blanchard J, Ford S, Mohri H, Cheng-Mayer C, Hong Z, Ho DD, Markowitz M. 2015. A long-acting integrase inhibitor protects female macaques from repeated high-dose intravaginal SHIV challenge. Sci Transl Med 7:270ra4. doi: 10.1126/scitranslmed.3010298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Etienne L, Nerrienet E, LeBreton M, Bibila GT, Foupouapouognigni Y, Rousset D, Nana A, Djoko CF, Tamoufe U, Aghokeng AF, Mpoudi-Ngole E, Delaporte E, Peeters M, Wolfe ND, Ayouba A. 2011. Characterization of a new simian immunodeficiency virus strain in a naturally infected Pan troglodytes troglodytes chimpanzee with AIDS related symptoms. Retrovirology 8:4. doi: 10.1186/1742-4690-8-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Humle T, Colin C, Laurans M, Raballand E. 2011. Group release of sanctuary chimpanzees (Pan troglodytes) in the Haut Niger National Park, Guinea, west Africa: ranging patterns and lessons so far. Int J Primatol 32:456–473. doi: 10.1007/s10764-010-9482-7. [DOI] [Google Scholar]
- 122.Migone T-S, Subramanian GM, Zhong J, Healey LM, Corey A, Devalaraja M, Lo L, Ullrich S, Zimmerman J, Chen A, Lewis M, Meister G, Gillum K, Sanford D, Mott J, Bolmer SD. 2009. Raxibacumab for the treatment of inhalational anthrax. N Engl J Med 361:135–144. doi: 10.1056/NEJMoa0810603. [DOI] [PubMed] [Google Scholar]
- 123.Pettitt J, Zeitlin L, do Kim H, Working C, Johnson JC, Bohorov O, Bratcher B, Hiatt E, Hume SD, Johnson AK, Morton J, Pauly MH, Whaley KJ, Ingram MF, Zovanyi A, Heinrich M, Piper A, Zelko J, Olinger GG. 2013. Therapeutic intervention of Ebola virus infection in rhesus macaques with the MB-003 monoclonal antibody cocktail. Sci Transl Med 5:199ra113. doi: 10.1126/scitranslmed.3006608. [DOI] [PubMed] [Google Scholar]
- 124.Ghosh SK, Fultz PN, Keddie E, Saag MS, Sharp PM, Hahn BH, Shaw GM. 1993. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology 194:858–864. doi: 10.1006/viro.1993.1331. [DOI] [PubMed] [Google Scholar]
- 125.Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH. 1991. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and -defective viral genomes. J Virol 65:3973–3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Koyanagi Y, Miles S, Mitsuyasu RT, Merrill JE, Vinters HV, Chen IS. 1987. Dual infection of the central nervous system by AIDS viruses with distinct cellular tropisms. Science 236:819–822. doi: 10.1126/science.3646751. [DOI] [PubMed] [Google Scholar]
- 127.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 128.Guindon S, Dufayard J-F, Lefort V, Anisimova M, Hordijk W, Gascuel O. 2010. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 129.Jones DT, Taylor WR, Thornton JM. 1992. A new approach to protein fold recognition. Nature 358:86–89. doi: 10.1038/358086a0. [DOI] [PubMed] [Google Scholar]
- 130.Abascal F, Zardoya R, Posada D. 2005. ProtTest: selection of best-fit models of protein evolution. Bioinformatics 21:2104–2105. doi: 10.1093/bioinformatics/bti263. [DOI] [PubMed] [Google Scholar]
- 131.Ronquist F, Huelsenbeck JP. 2003. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 132.Etienne L, Eymard-Duvernay S, Aghokeng A, Butel C, Monleau M, Peeters M. 2013. Single real-time reverse transcription-PCR assay for detection and quantification of genetically diverse HIV-1, SIVcpz, and SIVgor strains. J Clin Microbiol 51:787–798. doi: 10.1128/JCM.02792-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. 2002. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother 46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lopker M, Easlick J, Sterrett S, Decker JM, Barbian H, Learn G, Keele BF, Robinson JE, Li H, Hahn BH, Shaw GM, Bar KJ. 2013. Heterogeneity in neutralization sensitivities of viruses comprising the simian immunodeficiency virus SIVsmE660 isolate and vaccine challenge stock. J Virol 87:5477–5492. doi: 10.1128/JVI.03419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Montefiori DC. 2005. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays, p 12.11.1–12.11.17. In Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, Coico R (ed), Current protocols in immunology. John Wiley & Sons, New York, NY. [DOI] [PubMed] [Google Scholar]
- 136.Etemad-Moghadam B, Sun Y, Nicholson EK, Fernandes M, Liou K, Gomila R, Lee J, Sodroski J. 2000. Envelope glycoprotein determinants of increased fusogenicity in a pathogenic simian-human immunodeficiency virus (SHIV-KB9) passaged in vivo. J Virol 74:4433–4440. doi: 10.1128/JVI.74.9.4433-4440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Decker JM, Zammit KP, Easlick JL, Santiago ML, Bonenberger D, Hahn BH, Kutsch O, Bibollet-Ruche F. 2009. Effective activation alleviates the replication block of CCR5-tropic HIV-1 in chimpanzee CD4+ lymphocytes. Virology 394:109–118. doi: 10.1016/j.virol.2009.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detection of HIV-1- and SIVcpz-specific antibodies in experimentally infected captive chimpanzees. Plasma samples were diluted 1:1,000,000 and tested for reactivity with HIV-1 proteins by ECL (enhanced chemiluminescence) Western blot analysis. The positions of HIV-1 Env (gp160, gp120, and gp41), Pol (p66 and p31), and Gag (p55, p24, and p17) proteins are shown. Plasma samples from HIV-1-infected (pos) and uninfected (neg) humans were used as controls. Plasma samples from Joye were not included because of insufficient quantity. Download
Neutralization of SIVcpz in primary chimpanzee CD4+ T cells. (A) Serial dilutions (red, 10 µg/ml; green, 3.3 µg/ml; blue, 1.1 µg/ml; purple, 0.36 µg/ml) of the most potent anti-SIVcpz antibodies and immunoadhesins (as determined in TZM-bl cells) were incubated with the SIVcpz strain MT145 before addition to activated primary chimpanzee CD4+ T cells. (Antireceptor antibodies were incubated with cells before virus was added.) Plots depict MT145 replication after antibody removal, with time (days) plotted on the x axis and RT activity on the y axis. Antibody VRC01, which does not neutralize SIVcpz and SIVgor strains, served as a negative control. CD4+ T cells from three different chimpanzees were used (one representative experiment is shown). (B) Correlation of IC50s from TZM-bl and primary chimpanzee CD4+ T cells. IC50s averaged from three independent experiments were plotted for chimpanzee CD4+ T cells (x axis) and TZM-bl (y axis) and analyzed using the Spearman correlation test. Download
Neutralization potency of monoclonal antibodies and immunoadhesins (nanomolar concentration) in TZM-bl cells.