Abstract
Opisthorchis viverrini and other food-borne trematode infections are major health problems in Thailand, the Lao People's Democratic Republic, Vietnam and Cambodia. Differential diagnosis of O. viverrini based on the microscopic observation of parasite eggs is difficult in areas where Clonorchis sinensis and minute intestinal flukes coexist. Recently, Loop-mediated isothermal amplification (LAMP) has been widely used for detection and identification of trematode for its simple method that is useful in low-resource or field settings. We have reported ITS1-LAMP assay to detect O. viverrini infection from human feces. The sensitivity and specificity of the test was 100% and 61.5%. The sensitivity of the test appeared to be higher than microscopic egg examination; however non-specific amplification from other parasites could not be ruled out. We therefore targeted microsatellites of O. viverrini that is a species specific sequence. By using hydroxyl naphthol blue (HNB)-LAMP, O. viverrini microsatellite 6 (OVMS6) could specifically amplify DNA from O. viverrini genome, but not other parasites such as C. sinensis, O. felineus, Centrocestus caninus, Haplorchis taichui, Fasciola gigantica and Haplorchoodes sp. The detection limit of the test is 1 ng genomic DNA, which was 1,000 times lower than the ITS1-LAMP, but targeting microstellites showed more specific detection of O. viverrini. In addition, the colorimetric LAMP assay was simple and effective; this makes it potentially applicable for point-of-care diagnosis.
Keywords: Opisthorchis viverrini, microsatellite, LAMP, point-of-care diagnosis
Graphical Abstract
LAMP technique requires only a water bath (65 °C) to amplify DNA
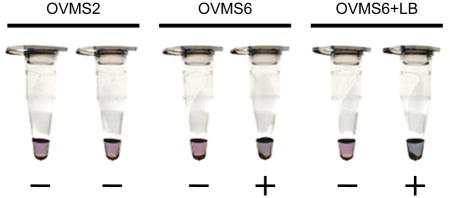
OVMS6-LAMP can specifically amplify DNA from Opisthorchis viverrini but not other closely related species.
1. Introduction
The human liver flukes, Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis remain important public health problems in many parts of the world, particularly in Asia. Csinensis is endemic in southern China, Korea, northern Vietnam and eastern Siberia, whereas O. viverrini is endemic in the Lower Mekong Basin, including Thailand, Lao People's Democratic Republic (Lao PDR), Cambodia and southern Vietnam (Sripa et al., 2010). O. felineus is found in western Siberia, the former USSR and in Central–Eastern Europe (Armignacco et al., 2008; Keiser and Utzinger, 2005). Throughout the world 700 million people are at risk of infection with these liver flukes (Keiser and Utzinger, 2005). Diagnosis of the liver fluke infection in humans is usually done by microscopic observation of parasite eggs collected from feces. However, the morphology of the eggs among O. viverrini, O. felineus, C. sinensis and minute intestinal flukes are very similar, indicating that identification of these parasites is difficult. Heavy infections with the minute intestinal flukes are associated with diarrhea, mucus-rich feces, dyspepsia, nausea and vomiting (Fried et al., 2004), whereas infections with the liver flukes result in severe morbidity of biliary and hepatic diseases, particularly cholangiocarcinoma – the bile duct cancer (Bouvard et al., 2009; Sripa et al., 2007). With the ease and scale of modern global migration, , there is rapidly increasing chance to spread the liver flukes into non-endemic areas. Therefore, specific diagnositic methods for these parasites are important for areas with potential cross-border contamination or co-infections. Molecular diagnosis is a powerful tool to identify the closely related species, however costly specific tools such as thermal cycler are needed which are difficult to prepare in low-resource or field settings. In 2000, Loop-mediated isothermal amplification (LAMP) was reported, that can simply amplify the target DNA under an isothermal condition. Since then, this method has been used to detect and identify several trematode species (Ai et al., 2010; Cai et al., 2010; Chen et al., 2011; Xu et al., 2010).
Recently, we reported diagnosis of O. viverrini infection in stool samples using loop mediated isothermal amplification targeting internal transcribed spacer 1 (ITS1-LAMP) (Arimatsu et al., 2012). The ITS1-LAMP was specific to O. viverrini among the parasites that have been examined with a sensitivity and specificity of 100% and 61.5%, respectively. Moreover, the study showed the LAMP has higher sensitivity compared to conventional PCR method (24.3%) using the same set of DNA samples. Although the LAMP showed no cross reaction with C. sinensis in that study, we have since found it can amplify O. felineus. Therefore, we targeted microsatellites of O. viverrini, which is more species specific. O. viverrini has been known to have 41 microsatellites (Laoprom et al., 2010). Out of these, 9 microsatellites are available in the Genbank. The DNA sequence of these microsatellites has no significant homology with known DNA sequences in Genbank. The objective of the present study was to develop a LAMP-based approach for specific detection of O. viverrini targeting the microsatellites.
2. Materials and methods
2.1. Parasite specimens
O. viverrini metacercariae were obtained from naturally infected cyprinid fish captured from a freshwater reservoir in Thailand. The fish were digested by pepsin-HCl (Waikagul, 1998). After several washings with normal saline, the metacercariae were collected and identified under a dissecting microscope. Viable metacercariae were used to infect hamsters (Mesocricetus auratus), which were maintained at the animal center of the Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand under protocols approved by the Khon Kaen University Animal Ethics Committee. After 2-3 months of infection, hamsters were euthanized, necropsied, and subsequently adult O. viverrini flukes recovered from their bile ducts. C. sinensis adult worms were prepared in Korea (kindly provided by Professor S.J. Hong) and O. felineus adult worms were prepared in Russia (kindly provided by Dr. V. Modvinov) using similar procedures as those for O. viverrini. F. gigantica adult worms were collected from a slaughter house in Khon Kaen province, Thailand. Centrocestus caninus, Haplorchoodes sp. and Haplorchis taichui were obtained from Bithynia snails by standard shedding techniques.
2.2. DNA extraction
Genomic DNA was extracted from adult O. viverrini worms using PureGene Core kit A (QIAGEN) and dissolved in a 50 μL elution buffer. The DNA was used for analyzing the specificity and sensitivity of the LAMP assay. Control DNA from C. sinensis, O. felineus, C. caninus and H. taichui was extracted by the same method as described for O. viverrini adults.
2.3. Designing LAMP primers
Nine microsatellites of O. viverrini were obtained from the Genbank (Accession no.: DQ144064, DQ144065, DQ144066, DQ144067, DQ144068, DQ144069, DQ144070, DQ144071, DQ144072). LAMP primers for these microsatellites were designed using Primer Explorer V4.0 software (http://primerexplorer.jp/e), with minor modification from the default setting. Six regions (F1c, F2, F3, B1c, B2, B3 regions) necessary for designing the LAMP primers were chosen from the flanking region of the microsatellite, locating the tandem repeated sequence between the F1c and B1c regions. Loop primers (LB, LF) for accelerating the reaction were also designed using the same software (Table 1.). All primers were designed in the flanking regions; therefore the LAMP reaction can be performed in any O. viverrini independent from the number of repeat units of the microsatellite (Fig. 1.).
Table 1.
Primer sequences designed for OVMS-LAMP.
| Primer name | sequence |
|---|---|
| OVMS2-F3 | GCTTGATAAACTGTTTGAATAGCT |
| OVMS2-B3 | ATTTGTTTTGAGCTTTTGTGG |
| OVMS2-FIP | atcagcgtttgaccaagcagaTTAAAAAACGGACTGGTGACA |
| OVMS2-BIP | gaatgggcgttaataacacctctCCATCAATCTGAGAAGGGTT |
|
| |
| OVMS6-F3 | AGCTGGGTGTTAGTCAACAG |
| OVMS6-B3 | TCGATGTTTCGCAGTAAGCA |
| OVMS6-FIP | cgacattggtgcgcaaaagctaAAGGTGGAACAGACAATGCT |
| OVMS6-BIP | aacgtagaccacccaacgtgtcAGGTACTGTCGAGCGTCTC |
| OVMS6-LB | GGTTTTTTATTGCTGTCTGCAGTCC |
Figure 1. Microsatellite sequence and primer regions of OVMS-LAMP.

A) Sequence of O. viverrini microsatellite 2 (OVMS2). F3, F2, F1c, B1c, B2 and B3 are the regions that were used to design the LAMP primers. The repeated motif of OVMS2 is (GT)n, which locates between F1c and B1c regions. B) Sequence of O. viverrini microsatellite 6 (OVMS6). The regions used for primer design is shown above, with additional LB primers. The repeated motif of OVMS6 is (GT)n.
2.4. LAMP assay
Out of the 9 microsatellites, microsatellite 2 (OVMS2) and microsatellite 6 (OVMS6) (GenBank accession no. DQ144065, DQ144069) were suitable for designing LAMP primers. The primers that target ITS1 (Arimatsu et al., 2012) were also used to compare the sensitivity and specificity of the LAMP test. The reaction mixture comprised of Tris–HCl (20 mM, pH 8.8), KCl (10 mM), MgSO4 (8 mM), (NH4)2SO4 (10 mM), Tween 20 (0.1%), Betaine (1.6 M), deoxynucleotide triphosphates (1.4 mM each), primers FIP and BIP (1.6 μM each), primers F3 and B3 (0.2 μM each), primer LB for OVMS6 (0.8 μM) (Fig. 1B), Bst DNA polymerase (8 U; New England BioLabs), Hydroxyl naptol blue (120 μM) and template DNA (2 μL). Double-distilled water was finally used to fill the system to 25 μL. No template DNA was added in the negative control reaction. The mixture was incubated at 65 °C in a heat block for 60 min and then heated at 80 °C for 5 min to terminate the reaction. A positive reaction was indicated by a color change from violet to sky blue.
3. Results
3.1. Colorimetric LAMP
Three primer sets were prepared (OVMS2, OVMS6 and OVMS6 with LB primer) to examine the DNA amplification by the LAMP assay, using O. viverrini genome and distilled water in order to confirm the LAMP primers functions in the amplification process. OVMS2 primers did not amplify the DNA, whereas OVMS6 primers with and without LB primer showed a distinctive result between the two tubes with and without O. viverrini genomic DNA. The positive reaction showed sky blue compared to the violet of the negative reaction (Fig. 2.), and the pellet of magnesium pyrophosphate, which is the by-product of the amplification reaction could be seen at the bottom of the tube after centrifugation. From these results, OVMS6 with LB primers were used to evaluate the OVMS-LAMP.
Figure 2. Colorimetric assay of OVMS-LAMP.
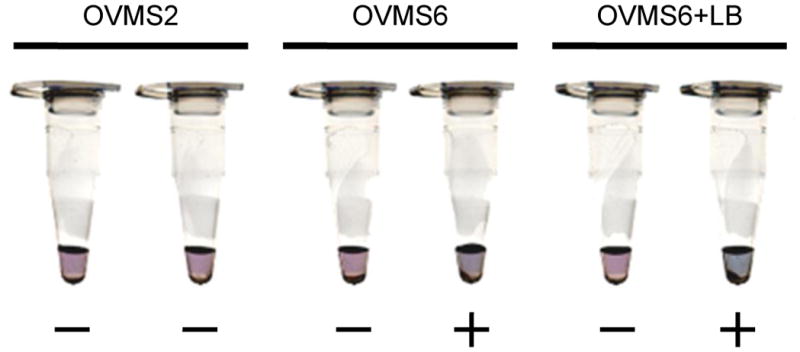
Three sets of LAMP primers used for OVMS-LAMP. Left side of each set is distill water, and right side contains 1ng of O. viverrini genome DNA.
3.2. Specificity
Specificity of the LAMP was performed using genomic DNA of O. viverrini, O. felineus, C. sinensis, F. gigantica and minute intestinal flukes (MIF), namely, H. taichui, Haplorchoides sp., and C. caninus. The concentration of the genomic DNA of each sample was measured by spectrophotometer and diluted with 10 mM Tris–HCl (pH 8.8) to a final concentration of 0.5 ng/μL and applied to the LAMP assays. The results showed that the OVMS6-LAMP could specifically amplify DNA from O. viverrini but not others (Table 2. and Supplementary figure 1.). The ITS1-LAMP previously reported amplified some other species such as O. felineus, F. gigantica and Haplorchoides sp.
Table 2.
Specificity of OVMS6-LAMP.
| Species | O.viverrini | O.felineus | C.sinensis | H.taichui | F.gigantica | Haplorchoides sp. | C.caninus | reference |
|---|---|---|---|---|---|---|---|---|
| OVMS-LAMP | + | − | − | − | − | − | − | This study |
| ITS1-LAMP | + | + | − | − | + | + | − | This study |
| ITS1-LAMP | + | ND | − | − | ND | ND | − | Arimatsu et al., 2012 |
−) no amplification, +) amplified, ND) not detected
3.3. Sensitivity
The concentration of the genomic DNA of O. viverrini adult worm was measured by spectrophotometer and diluted with 10 mM Tris–HCl (pH 8.8) to a final amount of 5 ng/μL. Subsequently, a 10-fold serial dilution was prepared ranging from 0.5 fg/μL to 5 ng/μL and 2μL of each diluted DNAs was used as a template for LAMP assay. The results showed that the detection limit of the OVMS6-LAMP assay is 1 ng (Table 3. and Supplementary figure 2).
Table 3.
Sensitivity of OVMS6-LAMP.
| Amount of DNA | 1 fg | 10 fg | 0.1 pg | 1 pg | 10 pg | 0.1 ng | 1 ng | 10 ng | Reference |
|---|---|---|---|---|---|---|---|---|---|
| OVMS-LAMP | − | − | − | − | − | − | + | + | This study |
| ITS1-LAMP | − | − | − | + | + | + | + | + | This study |
| ITS1-LAMP | − | − | − | + | + | + | + | + | Arimatsu et al.,2012 |
−) no amplification, +) amplified
4. Discussion
Conventional diagnosis of trematodiases using microscopic stool examination is troublesome in endemic areas with polyparasitic infections due to similarity of egg morphology (Johansen et al., 2010). Molecular diagnosis has been utilized to overcome this problem and LAMP techniques for diagnosis of opisthorchiasis and clonorchiasis have been reported with high sensitivity and specificity (Arimatsu et al., 2012; Le et al., 2012). However, no reports had been described to date on differentiation of O. viverrini from O. felineus and C. sinensis using LAMP. Our results clearly demonstrate that microsatellite LAMP specifically amplify DNA from O. viverrini and can differentiate the closely related species, O. felineus and C. sinensis. Moreover, our OVMS6-LAMP differentially diagnose O. viverrini from other common trematodes endemic in the region, i.e. H. taichui, F. gigantica, Haplorchoides sp., and C. caninus.
Cross-species amplification by PCR using microsatellite primers is known to amplify in some loci, but the number of loci that amplify successfully decreases with increased genetic distance between the species (Jarne and Lagoda, 1996). This is due to the higher mutation rate in the flanking regions of the microsatellites compared to other neutral regions of DNA (Blouin et al., 1996). Sequence divergence in flanking regions can lead to poor primer annealing, especially at the 3’ section. The microsatellites of O. felineus and C. sinensis have not been studied yet; therefore further study might provide useful marker to differentiate these species. Unfortunately, our results show that ITS1-LAMP amplifies DNA from O. felineus, F. gigantica and Haplorchoides sp. The ITS1-LAMP primers were designed specifically to O. viverrini ITS1, but somehow the primers annealed to these genomes because of the highly conserved sequence of ITS1 (O. felineus 96%, F. gigantica 68%) and/or presence of homologous sequence in the genome. This shows that O. viverrini specific sequence that is not present in other parasites is a better target to establish a species specific LAMP assay.
The limit of detection of OVMS6-LAMP was 1ng, whereas ITS1-LAMP was 1pg. The sensitivity of the assay was 1000 times lower in OVMS6-LAMP. Two reasons for this low sensitivity could be, 1) difference in copy numbers in the genome, and 2) primer designing regions is not suitable for highly sensitive detection. For the first reason, Genes encoding ribosomal RNA and spacers including the ITS1 and ITS2 occur in tandem repeats that are thousands of copies long, making them easy to amplify. The ITS1 copy number of O. viverrini has not yet been investigated but this might be the reason why the sensitivity of ITS1-LAMP was higher compared to the OVMS6-LAMP, which seems to have less copy numbers compared to the ITS1. For the second reason, it is known that quality of the LAMP assay strongly depends on the regions that the primers were designed (Notomi et al., 2000). The primers used in this study was designed by Primer Explorer V4.0, selecting primer sets that were predicted to be most efficient, but only one set was tested in our study. Using different areas of the microsatellite flanking regions for designing the primers is possible to increase the sensitivity of the LAMP assay.
Since C. sinensis has been reported extending into central Thailand, the importance of identifying O. viverrini and C. sinensis is becoming increasingly important (Traub et al., 2009). PCR techniques to identify different parasites species have been successfully established by several researchers (Johansen et al., 2010; Sato et al., 2009), but these techniques need special apparatus to obtain the results, such as thermal cycler and electrophoresis machine. The colorimetric LAMP uses an isothermal condition that requires less equipment, and provides results that can easily be evaluated by the naked eye (Goto et al., 2009). LAMP assay has inherent characteristics that make it advantageous as a diagnostic test that can assist in bringing point-of-care diagnosis to patients. Our results show that OVITS1-LAMP can amplify DNA from O. felineus, F. gigantica and Haplorchoides sp. However the endemic area of O. viverrini have not been reported to have O. felineus in the same area, and F. gigantica, Haplorchoides sp. rarely infect human. These facts indicate that ITS1-LAMP is useful for detecting O. viverrini infection from the human fecal samples, but the possibility of detecting other parasites by non-specific amplification could not be neglected as mentioned. Therefore targeting O. viverrini specific sequence such as microsatellites is useful. This study shows that microsatellite of O. viverrini is a useful marker to differentiate from other species. Targeting OVMS6 was successful to perform a specific amplification, but the sensitivity of the test needs to be improved. Further sequence information of the microsatellite, not only O. viverrini, but also O. felineus and C. sinensis could provide more useful markers that can be detected by the LAMP technique.
Supplementary Material
Highlights.
Microsatellite 6 of Opisthorchis viverrini was amplified by LAMP method.
The LAMP was specific for O. viverrini but not Clonorchis sinensis and O. felineus.
The LAMP may be used for differential diagnosis of the three human liver flukes.
Acknowledgments
This work was supported by the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University, Thailand and The National Institute of Allergy and Infectious Diseases (NIAID), award number P50AI098639 and the United States Anny Medical Research and Materiel Command (USAMRMC), contract number W81XWH-12-C-0267. The content is solely the responsibility of the authors and does not necessarily represent the official views of the USAMRMC, NIAID or the NIH or the funders. We are grateful to Dr. John F. Smith for editing the English presentation.
Footnotes
5. Conflicts of interest
We have no potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ai L, Li C, Elsheikha HM, Hong SJ, Chen JX, Chen SH, Li X, Cai XQ, Chen MX, Zhu XQ. Rapid identification and differentiation of Fasciola hepatica and Fasciola gigantica by a loop-mediated isothermal amplification (LAMP) assay. Vet Parasitol. 2010;174:228–33. doi: 10.1016/j.vetpar.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Arimatsu Y, Kaewkes S, Laha T, Hong SJ, Sripa B. Rapid detection of Opisthorchis viverrini copro-DNA using loop-mediated isothermal amplification (LAMP) Parasitol Int. 2012;61:178–82. doi: 10.1016/j.parint.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armignacco O, Caterini L, Marucci G, Ferri F, Bernardini G, Natalini Raponi G, Ludovisi A, Bossù T, Gomez Morales MA, Pozio E. Human illnesses caused by Opisthorchis felineus flukes, Italy. Emerg Infect Dis. 2008;14:1902–5. doi: 10.3201/eid1412.080782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blouin MS, Parsons M, Lacaille V, Lotz S. Use of microsatellite loci to classify individuals by relatedness. Mol Ecol. 1996;5:393–401. doi: 10.1111/j.1365-294x.1996.tb00329.x. [DOI] [PubMed] [Google Scholar]
- Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- Cai XQ, Xu MJ, Wang YH, Qiu DY, Liu GX, Lin A, Tang JD, Zhang RL, Zhu XQ. Sensitive and rapid detection of Clonorchis sinensis infection in fish by loop-mediated isothermal amplification (LAMP) Parasitol Res. 2010;106:1379–83. doi: 10.1007/s00436-010-1812-3. [DOI] [PubMed] [Google Scholar]
- Chen MX, Ai L, Zhang RL, Xia JJ, Wang K, Chen SH, Zhang YN, Xu MJ, Li X, Zhu XQ, Chen JX. Sensitive and rapid detection of Paragonimus westermani infection in humans and animals by loop-mediated isothermal amplification (LAMP) Parasitol Res. 2011;108:1193–8. doi: 10.1007/s00436-010-2162-x. [DOI] [PubMed] [Google Scholar]
- Fried B, Graczyk TK, Tamang L. Food-borne intestinal trematodiases in humans. Parasitol Res. 2004;93:159–70. doi: 10.1007/s00436-004-1112-x. [DOI] [PubMed] [Google Scholar]
- Goto M, Honda E, Ogura A, Nomoto A, Hanaki KI. Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. Biotechniques. 2009;46:167–72. doi: 10.2144/000113072. [DOI] [PubMed] [Google Scholar]
- Jarne P, Lagoda PJ. Microsatellites, from molecules to populations and back. Trends Ecol Evol. 1996;11:424–9. doi: 10.1016/0169-5347(96)10049-5. [DOI] [PubMed] [Google Scholar]
- Johansen MV, Sithithaworn P, Bergquist R, Utzinger J. Towards improved diagnosis of zoonotic trematode infections in Southeast Asia. Adv Parasitol. 2010;73:171–95. doi: 10.1016/S0065-308X(10)73007-4. [DOI] [PubMed] [Google Scholar]
- Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507–14. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laoprom N, Sithithaworn P, Ando K, Sithithaworn J, Wongkham S, Laha T, Klinbunga S, Webster JP, Andrews RH. Microsatellite loci in the carcinogenic liver fluke, Opisthorchis viverrini and their application as population genetic markers. Infect Genet Evol. 2010;10:146–53. doi: 10.1016/j.meegid.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Le TH, Nguyen NTB, Truong NH, De Van N. Development of mitochondrial loop-mediated isothermal amplification for detection of the small liver fluke Opisthorchis viverrini (Opisthorchiidae; Trematoda; Platyhelminthes) J Clin Microbiol. 2012;50:1178–84. doi: 10.1128/JCM.06277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000;28:E63. doi: 10.1093/nar/28.12.e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Thaenkham U, Dekumyoy P, Waikagul J. Discrimination of O. viverrini, C. sinensis, H. pumilio and H. taichui using nuclear DNA-based PCR targeting ribosomal DNA ITS regions. Acta Trop. 2009;109:81–3. doi: 10.1016/j.actatropica.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–50. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- Sripa B, Kaewkes S, Sithithaworn P, Mairiang E, Laha T, Smout M, Pairojkul C, Bhudhisawasdi V, Tesana S, Thinkamrop B, Bethony JM, Loukas A, Brindley PJ. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007;4:e201. doi: 10.1371/journal.pmed.0040201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RJ, Macaranas J, Mungthin M, Leelayoova S, Cribb T, Murrell KD, Thompson RCA. A new PCR-based approach indicates the range of Clonorchis sinensis now extends to Central Thailand. PLoS Negl Trop Dis. 2009;3:e367. doi: 10.1371/journal.pntd.0000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waikagul J. Opisthorchis viverrini metacercaria in Thai freshwater fish. Southeast Asian J Trop Med Public Health. 1998;29:324–6. [PubMed] [Google Scholar]
- Xu J, Rong R, Zhang HQ, Shi CJ, Zhu XQ, Xia CM. Sensitive and rapid detection of Schistosoma japonicum DNA by loop-mediated isothermal amplification (LAMP) Int J Parasitol. 2010;40:327–31. doi: 10.1016/j.ijpara.2009.08.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


