Abstract
Scope
Moringa oleifera (moringa) is tropical plant traditionally used as an antidiabetic food. It produces structurally unique and chemically stable moringa isothiocyanates (MICs) that were evaluated for their therapeutic use in vivo.
Methods and results
C57BL/6L mice fed very high fat diet (VHFD) supplemented with 5% moringa concentrate (MC, delivering 66 mg/kg/d of MICs) accumulated fat mass, had improved glucose tolerance and insulin signaling, and did not develop fatty liver disease compared to VHFD-fed mice. MC-fed group also had reduced plasma insulin, leptin, resistin, cholesterol, IL-1β, TNFα, and lower hepatic glucose-6-phosphatase (G6P) expression. In hepatoma cells, MC and MICs at low micromolar concentrations inhibited gluconeogenesis and G6P expression. MICs and MC effects on lipolysis in vitro and on thermogenic and lipolytic genes in adipose tissue in vivo argued these are not likely primary targets for the anti-obesity and anti- diabetic effects observed.
Conclusion
Data suggest that MICs are the main anti-obesity and anti-diabetic bioactives of MC, and that they exert their effects by inhibiting rate-limiting steps in liver gluconeogenesis resulting in direct or indirect increase in insulin signaling and sensitivity. These conclusions suggest that MC may be an effective dietary food for the prevention and treatment of obesity and type 2 diabetes.
Keywords: diabetes, insulin resistance, isothiocyanates, Moringa oleifera, obesity
1. Introduction
Mechanistic understanding of effective dietary therapeutics in the prevention of type 2 diabetes (T2D) and obesity remain important goals in lifestyle-oriented healthcare. Edible leaves from the moringa tree (Moringa oleifera Lam.) have been used as an antidiabetic food throughout the centuries, but only scantly explored scientifically [1]. Moringa's nutritional profile makes it well-suited for integration into a diet-based T2D prevention program. In addition, moringa leaves contain an abundance of secondary metabolites, principally polyphenols and four unique moringa isothiocyanates (MICs), with strong biological activity. MICs contain the same pharmacophore (R–N=C=S) as isothiocyanates (ITCs) from broccoli (e.g. sulforaphane, SF) and other cruciferous vegetables, but differ from aliphatic ITCs, such as SF, by the presence of an aromatic ring and rhamnose moiety. Emerging evidence has suggested MICs are the principal therapeutically active constitutes found in moringa. Specifically, MICs were shown to reduce inflammatory expression in RAW macrophages [2-4]; and in rodent models, reduce nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) expression, myelomal growth [5], and blood pressure [6]. ITCs, particularly SF, have been thoroughly researched through pre-clinical, clinical and epidemiological studies [7-9] and advocated for dietary health prevention of cancer and other diseases. ITCs are potent inducers of phase II detoxifying enzymes and subsequently confer protection against oxidative stress and chronic inflammation [10].
Despite strong evidence of 1) chronic inflammation as an underlying cause of cancer and T2D, and 2) effectiveness of ITCs in the prevention of cancer, the use of ITC-rich foods as therapeutics in T2D remains virtually unknown. Recently, SF supplementation was shown to reduce insulin, inflammatory markers and LDL levels in T2D patients [11-13]. A major drawback to a therapeutic use of cruciferous ITCs is their inherent chemical instability [14]. Cruciferous ITCs are volatile oils, appearing only transiently after conversion from their precursor molecules, glucosinolates, by endogenous plant or exogenous microbial thioglucosidase (myrosinase) following plant tissue damage by injury or digestion.
MICs formed in moringa leaves are chemically unique due to the presence of their sugar moiety, and thus have a larger molecular weight, solid physical state, and presumably greater chemical stability compared to volatile cruciferous ITCs. Research on MICs remains very scarce compared to SF, yet emerging studies have shown MICs bear equal or stronger biological activity than other ITCs [3, 5, 15]. It is conceivable that moringa may be a superior alternative to broccoli as a source of stable ITCs [2] to prevent chronic diseases, particularly in tropical regions of the world where moringa trees grow and T2D and obesity rates are climbing [16-18].
Recently, we described a simple and effective method for production of a food-grade, MIC-rich moringa concentrate (MC), made from extracting freshly crushed leaves in water [2]. In this study, we evaluated the effects of MC on metabolic and inflammatory dysregulation in diet-induced obese C57BL/6J mice and demonstrated that MICs are the primary pharmacological contributors to the observed effects. Trying to establish the mechanism of action of MICs, we investigated the effect of MC and MICs on in vitro gluconeogenesis in liver cells and fat oxidation in adipocytes, and performed short-term in vivo studies on acute oral glucose tolerance and indirect calorimetry.
2. Materials and methods
2.1 Materials
Preparation of MC and isolation and quantification of MIC-1 (4-[(α-L-rhamnosyloxy)benzyl]isothiocyanate) and MIC-4 (4-[(4-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate) was performed according to the previously described protocols with minor modifications [2]. Briefly, fresh moringa leaves were blended in 25°C water (1g leaves: 5 mL water), allowed to sit at 25°C for 30 min, centrifuged (only in the case of MC for the 5% diet), filtered, and lyophilized. The percent of MICs concentrated in MC batches ranged from ~1-3% due to natural variations in the starting plant material and whether the centrifugation step was included in the preparation of MC. Elimination of the centrifugation step increased the concentration of MICs recovered in MC. Therefore, the percent of MC incorporated into the mouse diets was adjusted and standardized to deliver 800 mg of MICs/kg of food. In the long-term study, the very high-fat diet (VHFD) (60% kcal from fat) contained 5% MC, of which 1.66% were MICs (1.15% MIC-1 and 0.51% MIC-4) and the diet in the metabolic chamber study contained 3.3% MC, of which 2.40% were MICs (1.48% MIC-1 and 0.92% MIC-4). Both diets were formulated by Research Diets (New Brunswick, NJ) to be isocaloric for fat, protein and carbohydrate content (Suppl. Tables 1 & 2).
2.2 Animals
Three month study
Twenty-four male C57BL/6J mice at 5 weeks of age were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were acclimated for 9 d and housed 4 animals per cage under a 12-h light/dark cycle, with ad libitum access to water and a VHFD or VHFD + 5% MC for twelve weeks. Body weight and food intake was recorded weekly. Food intake was estimated as follows: [total food consumed per cage]/[mice per cage]×[d of food consumption]. Body composition was determined at 4, 8, and 12 weeks by magnetic resonance imaging using an EchoMRI-100 instrument (Echo Medical Systems, Houston, TX). Mice were removed from group cages and placed in clean individual cages. At the end of the study mice were euthanized with carbon dioxide. Blood and tissues (liver, inguinal fat, gastrocnemius muscle and ileum) were collected immediately and preserved at −80 °C. The animal protocol was approved by the Comparative Medicine Resources and the Office of Research and Sponsored Programs from Rutgers University, NJ, USA.
Two week indirect calorimetry study
Twenty-four male C57BL/6J mice at 4 weeks of age were purchased from Jackson Laboratories. After exit from quarantine, mice were given the VHFD and placed in training cages (TSE Systems, Chesterfield, MO) for one week. Then, they were transferred to metabolic chambers for one week to establish baseline values. Mice were weighed and body composition measured before randomization into treatment groups. Twelve mice per group were given a VHFD or VHFD + 3.3% MC for an additional week before returning to their home cages. Food intake, body weight, rearing activity, VO2 consumption and CO2 production were determined. The animal protocol was approved by the Pennington Biomedical Research Center Institutional Animal Care and Use Committee.
2.3 Oral glucose tolerance test (OGTT)
Mice in the three month study were first fasted overnight before fasting glycemic levels were recorded using a glucometer (AlphaTRAK® 32004-02, Abbott Animal Health, Abbott Park, IL) and gavaged with 2 g/kg of glucose at weeks 4, 8 and 12 weeks of treatment. An additional six mice on the VHFD at the same age were gavaged with 300 mg/kg of metformin (positive control) 3 h prior to glucose gavage. Glycemic levels were measured at intervals up to 120 min.
2.3.1 Acute OGTT
Fifteen male C57BL/6J mice were purchased, acclimated and housed as described in the 3 month study. Mice were fed ad libitum a VHFD for 12 weeks. The OGTT was performed as described above except for gavage treatements of 2 g/kg of MC (n = 6), water (vehicle; n = 6), or 300 mg/kg of metformin (n = 3).
2.4 Blood chemistry analysis
Animals were fasted overnight and trunk blood was collected immediately after euthanization. Samples were collected in tubes with EDTA and plasma was aliquotted into cryovials and stored at −80 °C for analysis. Insulin, leptin, resistin, interleukin-1 beta (IL-1β) and tumor necrosis factor alpha (TNFα) were measured using a multiplex assay (Millipore, Temecula, CA) measured on a Luminex 200 (Luminex, Austin, TX). Total cholesterol and triglycerides (TG) were assayed on a DxC 600 Pro (Beckman Coulter, Inc., Indianapolis IN).
2.5 Liver histology, total lipid extraction, cholesterol and TG levels
Randomly selected liver sections were fixed in 10% neutral buffered formalin for 48 h, then processed and embedded in Paraplast. Six-micrometer sections were cut and stained in hematoxylin and eosin. A diagnosis of fatty liver was made based on the presence of macro or microvesicular fat > 5% of the hepatocytes in a given slide. Total lipid content of liver was determined by Folch's method [19]. Briefly, liver (~300 mg) was extracted 20:1 (v/w) with CHCl2/CH3OH (2:1), followed by solvent evaporation and DW calculation.
2.6 Gene expression analysis by quantitative RT-PCR
Liver and ileum
Total RNA was isolated from liver and ileum for TNFα, IL-1β, interleukin-6 (IL-6) expression; and additionally, glucose-6 phosphatase (G6P), phosphoenolpyruvate carboxykinase (PEPCK) and glucokinase (GcK) expression from liver tissue using PureLink® RNA mini kit plus on-column DNase treatment (Applied Biosystems, Foster City, CA). Tissue (100 mg) was homogenized with TRIzol® using zirconium beads in a Bead Bug homogenizer (Benchmark Scientific, Inc. Edison, NJ). First-strand cDNA was synthetized from 2 μg total RNA using the high capacity cDNA reverse transcription kit plus RNase inhibitor (Applied Biosystems) with oligo-d(T)s as primers. PCR analyses were performed on a 7300 Real-Time PCR system using the TaqMan Assays (Applied Biosystems; Suppl. Table 3). Hydroxymethylbilane synthase (Hmbs) was used to normalize target gene expression and effect of treatment on gene expression levels was evaluated by the ΔΔCt method [20].
Adipose Tissue
Inguinal fat was homogenized in QIAzol lysis reagent (Qiagen, Inc., Valencia, CA) using a Tekmar Tissumizer homogenizer (Model TR-10). Lysates were mixed with chloroform to induce phase separation, and total RNA was purified using the RNeasy Lipid Tissue Mini Kit (Qiagen). cDNA was synthesized from RNA using the RT2 First Strand Kit (Qiagen). An Applied Biosystems 7900HT Fast Real-Time PCR System was used to conduct real time qPCR analyses. Analysis of thermogenic and lipolysis-related gene expression was quantified using the RT2 SYBR Green qPCR Mastermix and custom RT2 Profiler PCR Array (Qiagen) containing mouse primer assays (Suppl. Table 4) for: uncoupling protein 1 (UCP1), PR domain containing 16 (PRDM16), beta-3 adrenergic receptor (ADRB3), carnitine palmitoyltransferase 1b (CPT1b), cell death-inducing DFFA-like effector A (CIDEA), peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and peptidyl prolyl isomerase H (PPIH). GAPDH and PPIH were included as endogenous reference genes, and PPIH was used to normalize target gene expression. The effect of treatment on relative gene expression levels was evaluated by the ΔΔCt method [20]. Analysis of inflammatory-related gene expression for adiponectin (ADPN), monocyte chemoattractant protein-1 (MCP1), plasminogen activator inhibitor type 1 (PAI1), lipocalin-2 (LCN2), IL-1β, IL-6, peptidyl prolyl isomerase A (PPIA), and peptidyl prolyl isomerase B (PPIB) was quantified using the SYBR supermix reagent (Takara) and primer pairs shown in Suppl. Table 5. TNFα primers were from Qiagen (Assay ID: PPM03113G). Standard curves were used to determine relative expression. Each target gene was normalized by the geometric mean of PPIA and PPIB. The effect of treatment on relative gene expression levels was evaluated by calculating fold change of treatment group versus control group for each target gene.
2.7 Immunoblot analysis
Liver and muscle tissues were prepared for immunoblot analysis as previously described [21]. Briefly, tissue samples were homogenized and protein concentration was measured by the Bio-Rad protein assay kit (Bio-Rad laboratories, Hercules, CA). Supernatants (50 μg) were resolved by SDS-PAGE and subjected to western blotting. The protein abundance was detected with antibodies against p-tyr (PY20), insulin receptor substrate 1 (IRS-1), IRS-2, anti-phospho-IRS-1(Tyr612)(IRS-1 p), p85 of PI 3K (PI 3K), RAC-alpha serine/threonine-protein kinase (Akt1), RAC-beta serine/threonine-protein kinase (Akt2), phosph-Akt1(Ser473)(Akt1 p) (Upstate Biotechnology, Lake Placid, NY), phosph-Akt2(Ser474)(Akt2 p) (GenScript, Piscataway, NJ), sterol regulatory element binding protein-1c (SREBP-1c), cell death-inducing DFFA-like effector c (FSP27/CIDEA in humans), lipoprotein lipase (LPL), adipose triglyceride lipase (ATGL), insulin receptor beta (IRβ) (Santa Cruz Biotechnology, Santa Cruz, CA), glucose transporter type 4 (GLUT4) (R&D Systems, Minneapolis, MN), 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGR), fatty acidy synthase (FAS) (Abcam, Cambridge, MA), and β-actin using chemiluminescence Reagent Plus (PerkinElmer, Boston, MA), and quantified via a densitometer. All proteins were normalized by β-actin, and specific protein phosphorylation was normalized by the corresponding protein.
2.8 In vitro gluconeogenesis studies
H4IIE rat hepatoma cells (CRL-1548, American Type Culture Collection, Manassas, VA) were assayed for glucose production as previously described [22]. Cell viability was measured by the 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyltetrazolium bromide (MTT; TCI, Portland, OR) assay [23]. RNA extraction, cDNA synthesis and qPCR for gene expression of PEPCK and G6P were performed as described above.
2.9 In vitro lipolysis assay
Murine 3T3-L1 preadipocytes were grown and differentiated as previously described [24]. Mature adipocytes were > 99% differentiated. Prior to performing the lipolysis assay, the media was changed to Dulbecco's modified Eagle's medium (DMEM) supplemented with 5% calf serum (HyClone, Thermo Scientific, Logan, UT) for 24 – 48 hr. The adipolysis assay kit (EMD Millipore, Temecula, CA) was used to evaluate the ability of MC, MIC-1, and MIC-4 to modulate lipolysis. Briefly, cell monolayers were washed with wash solution. The assay was initiated by replacing the wash solution with the incubation solution supplemented with 2% bovine serum albumin plus vehicle (0.05% ethanol), isoproterenol (10 μM, positive control) MC (50, 100 μg/mL) or MICs (5, 10 μM). After 3.5 hours, the conditioned media was removed and assayed for free glycerol content using the Free glycerol assay reagent according to the kit instructions.
2.10 Statistical analysis
GraphPad Prism v.6.04 (GraphPad Software Inc., San Diego, CA) was used for all statistical analysis except for RER analysis which was performed using Statistical Analysis System. P < 0.05 was considered statistically significant. Specifics of statistical analysis are indicated in each figure legend.
3. Results
3.1 Effect of MC on body weight, body composition, OGTT, liver composition and lipid content
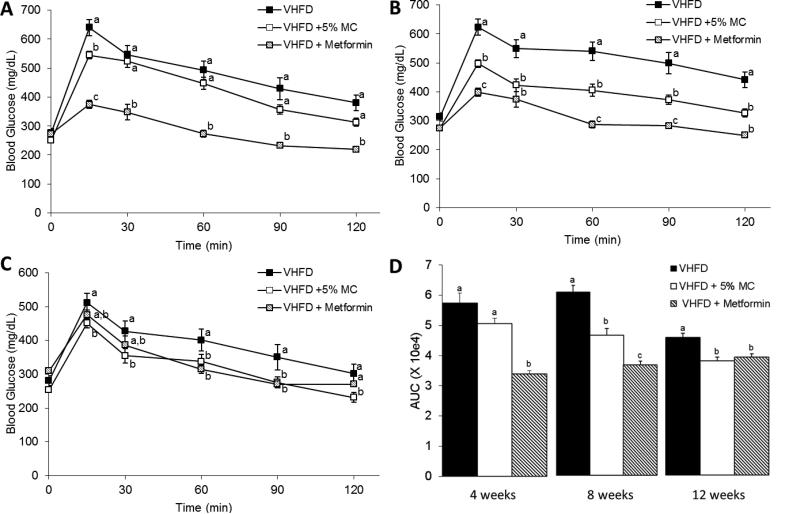
The VHFD + 5% MC-fed mice gained significantly less weight over the 3 month study compared to the VHFD-control mice (P<0.001 from 4-12 weeks) with a final average weight of 38.4 ± 1.0 g vs. 46.9 ± 1.0 g (mean ± SEM), respectively (Fig. 1A). All animals involved in the study looked healthy at the end of the study with no adverse effects noticed. Weekly food consumption remained stable throughout the 12 week study, averaging 2.22 ± 0.02 g /d for VHFD + 5% MC group vs. 2.42 ± 0.05 g/d for control mice. The 5% MC diet contained 800 mg of MICs/kg, therefore the mice were consuming approximately 66 mg of MICs per d. Accumulated food intake only became significantly less in the VHFD + 5% MC fed group at the 12th week (P < 0.05). However, the ratio of accumulated food intake to body weight was significantly higher in the VHFD + 5% MC-fed mice compared to the VHFD group throughout the entire study (Fig. 1B). Body composition at 4, 8 and 12 weeks showed lower fat accumulation (Fig. 1C) and greater free fat (lean mass) as percentage of body weight in the VHFD + 5% MC-fed mice compared to the VHFD-fed mice (Fig. 1D). OGTT performed at 4, 8 and 12 weeks demonstrated lower blood glucose levels and faster return to fasting levels in VHFD + 5% MC-fed mice compared to VHFD-fed mice (Fig. 2), corroborating previously observed lowering of blood glucose levels in diabetic rats from a single administration of moringa extract (200 mg/kg) [25]. Compared to fatty livers of VHFD-fed mice, livers from the VHFD + 5% MC-fed animals did not show the appearance of fatty-liver disease (Fig. 3A & 3B) as also evident from the histological comparison (Fig. 3D & 3E). The livers of VHFD + 5% MC-fed mice weighed less (Fig. 3C) and contained lower levels of lipids in relation to the VHFD-fed mice (Fig. 3F). There was no significant difference in the lipid content as percent of dry fecal weight from the two experimental groups (VHFD, 0.47 ± 0.14%; VHFD + 5% MC, 0.46 ± 0.04%).
Figure 1.
Body weight gain (A), ratio of accumulated food intake to body weight (B), fat mass (C) and free fat mass (D) in VHFD and VHFD + 5% MC-fed mice. n=12 mice per group, Data are means ± SEM. Comparisons to controls were made by Welch's test. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 2.
Oral glucose tolerance test performed at 4 (A), 8 (B) and 12 (C) weeks on a mice fed a VHFD, VHFD + 5% MC, and a VHDF gavaged with 300mg/kg metformin on the day of OGTT. Area Under the Curve of OGTT at 4, 8, and 12 weeks (D). n = 12 mice per group, except for metformin group where n=6 and only shown as a reference group. Data are means ± SEM. Comparisons were made by a 1-way ANOVA followed by Tukey's posthoc test. Significant differences (p < 0.05) between sample sets are signified by letters; different letters indicate significant difference between sample sets, while the same letter or absence of a letter indicates no difference.
Figure 3.
Gross examination of liver samples from VHFD-fed mice (A) and VHFD + 5% MC-fed mice (B). Liver weight in VHFD and VHFD + 5% MC (n=12,) (C) Data are means ± SEM. **: p<.01. Histological examination of liver samples from VHFD (D) and VHFD + 5% MC (E). Fat content in liver from VHFD-fed mice and VHFD + 5% MC fed mice (n=12) (F). Comparisons to controls were made by Welch's test. Data are means ± SEM. **P < 0.01; ***P < 0.001.
3.2 Effect of MC on blood composition, insulin sensitivity and inflammation
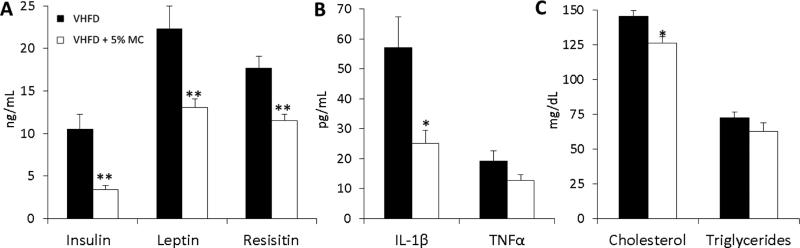
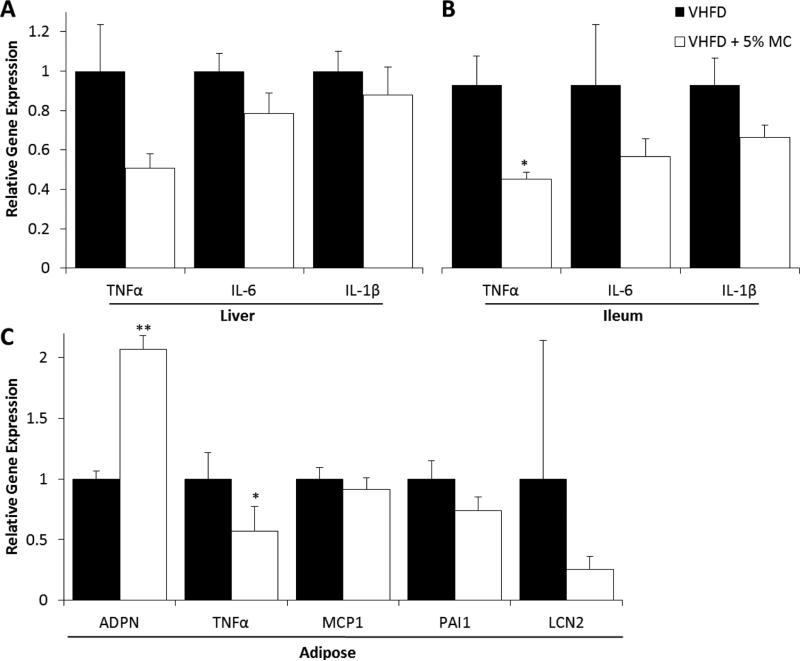
VHFD + 5% MC-fed mice had lower blood plasma levels of glucose regulators (insulin, leptin, resistin) (Fig. 4A), inflammatory cytokines (IL-1β and TNFα) (Fig. 4B), and cholesterol (Fig. 4C) compared to the VHFD group. When compared to VHFD-fed mice, VHFD + 5% MC-fed mice also had significantly higher levels of proteins involved in insulin signaling - IRS-1p, IRS-1 PI-3K, Akt1p and Akt2p in liver (Fig. 5A); and increased levels of IRS-1p, IRS-1, IRS-2, IR β, Akt1 and GLUT4 in muscle (Fig. 5B). Reduced gene expression of pro-inflammatory markers, TNFα, IL-6, IL-1β, were observed in the liver (Fig. 6A) and ileum (Fig. 6B) tissue from the VHFD + 5% MC-fed mice compared to the VHFD group. Similarly, in adipose tissue, gene expression of TNFα was reduced while adiponectin had enhanced expression in the treatment mice relative to the VHFD controls (Fig 6C).
Figure 4.
Blood plasma expression of insulin, leptin, resistin (A), IL-1β, TNFα (B), total cholesterol and triglycerides (C) in VHFD and VHFD + 5% MC-fed mice. n=12 mice per group except for IL-1β and TNFα where n=5, undetectable levels below 2.4 pg/mL were excluded. Comparisons to controls were made by Welch's test. Data are means ± SEM. *P < 0.05; **P < 0.01.
Figure 5.
Insulin signaling protein levels in liver (A) and skeletal muscle (B) from VHFD +5% MC-fed mice relative to VHFD-fed mice (dashed line). n=12 mice per group, Data are means ± SEM. Comparisons to controls were made by Welch's test. *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 6.
Gene expression of inflammatory markers in liver (A), ileum (B), and adipose tissue (C) of VHFD and VHFD + 5% MC-fed mice. n = 8-12, Data are means ± SEM. Comparisons to controls were made by Welch's test for liver, ileum, and adipose tissue. *P < 0.05.
3.3 Effect of MC and MICs on glucose metabolism and OGTT
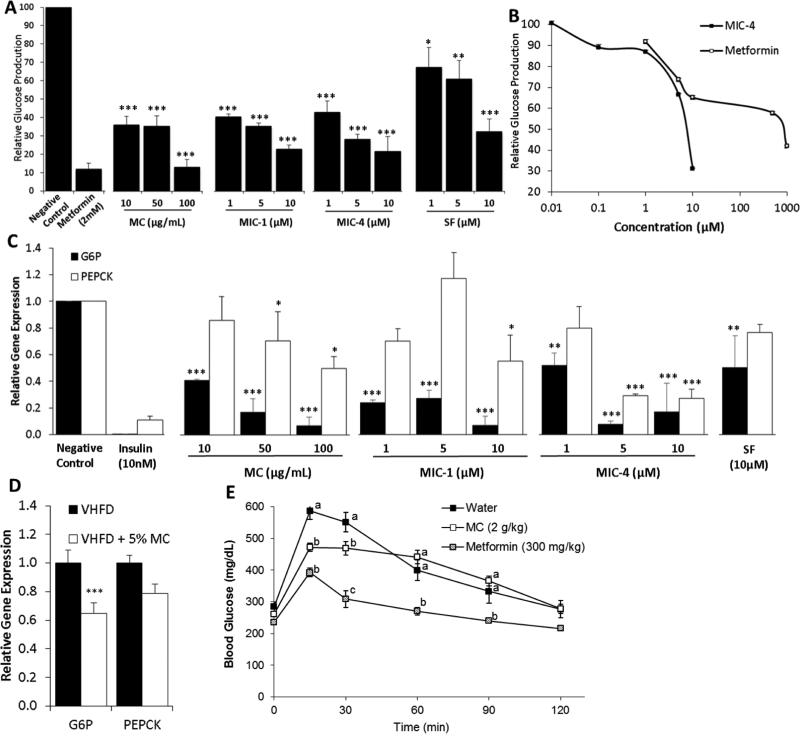
MC and MICs significantly reduced glucose production by approximately 60% in HII4E liver cells at 10 μg/mL and 1 μM, respectively (P < 0.001). MIC-1 and 4 demonstrated superior activity to SF at the same concentrations (Fig. 7A). To further explore the activity of MICs in comparison to the prescription drug metformin, MIC-4 and metformin were tested over a range of 5 concentrations, showing IC50 of glucose production at 7 μM for MIC-4 vs 800 μM for metformin (Fig. 7B). MC and MICs also significantly decreased expression of G6P and PEPCK in HII4E liver cells relative to the vehicle (Fig. 7C). G6P expression was significantly lower in the hepatic tissue of VHFD + 5% MC-fed mice compared to the controls (Fig. 7D). Glucose lowering effects of MC were further tested in vivo by the acute OGTT, to eliminate the weight difference variable in the long-term feeding study. The acute OGTT resulted in significantly lower blood glucose levels at 15 and 30 minutes in the MC-gavaged mice (2 g/kg) compared to the vehicle (Fig. 7E).
Figure 7.
Effect of MICs, SF and MC on glucose metabolism in vitro (A, B, C) and in vivo (D, E). Effects of MC, MIC-1, MIC-4 and sulforaphane (SF) on glucose production (A, B) and gene expression of G6P and PEPCK in HII4E liver cells; n=3 (C). Expression of G6P and PEPCK in hepatic tissue of VHFD and VHFD + 5% MC-fed mice (D) n=12. Acute OGTT test in VHFD-fed mice gavaged with 2g/kg of MC (E) n = 6. Comparisons to controls were made by Dunnett's test for A and C, and a t-test for D. Data are means ± SEM. *: p<.05, **: p<.01, ***: p<.001. Comparisons for E were made by a 1-way ANOVA followed by Tukey's posthoc test. Significant differences (p < 0.05) between sample sets are signified by letters; different letters indicate significant difference between sample sets, while the same letter or absence of a letter indicates no difference.
3.4 Effect of MC and MICs on lipolysis and thermogenesis
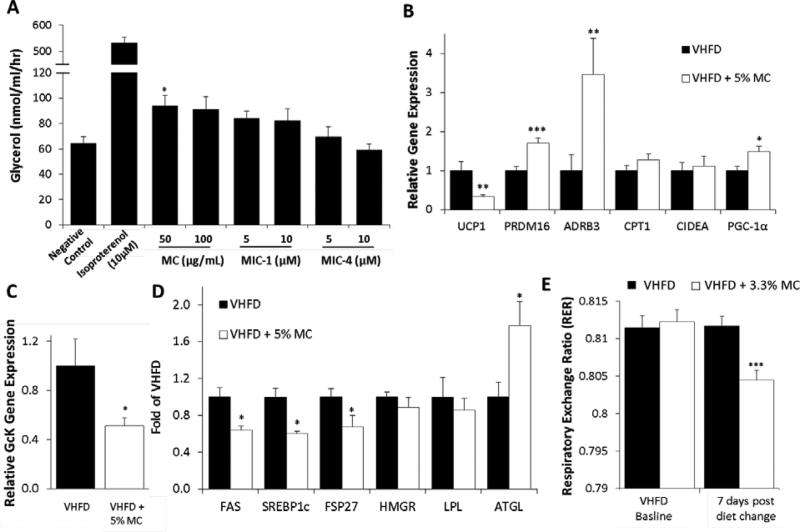
To better understand the reduced weight gain in MC-fed mice, the mechanisms of lipolysis and thermogenesis were explored in vitro and in vivo. MC at 50 μg/mL slightly increased production of glycerol in adipocytes, but at a higher concentration (100 μg/mL) no significant increase was observed. MIC-1 and MIC-4 did not demonstrate any significant lipolytic activity (Fig. 8A). Inguinal adipose tissues from the VHFD + 5% MC-fed mice showed increased gene expression of PRDM16, ADRB3, PGC-1α, but decreased expression of UCP1 compared to VHFD-fed mice (Fig. 8B). Hepatic tissue from VHFD + 5% MC-fed mice showed relatively lower GcK gene expression (Fig. 8C), lower protein levels of FAS, SREBP1c, and FSP27 and increased levels of ATGL relative to control mice (Fig. 8D). To understand if the increased expression of in vitro and in vivo lipolytic and thermogenic gene expression results would translate to increased energy expenditure and fat oxidation in vivo, an additional 7 d study was conducted in TSE metabolic chambers. Mice fed a VHFD or a VHFD + 3.3% MC (containing the same concentration of MICs as the 5% diet in the 3 month study) showed no differences in O2 consumption, or rearing activity between the two cohorts (data not shown). However, the VHFD + 3.3% MC-fed mice did gain less weight, had lower food consumption during the light period, and exhibited a lower respiratory exchange ratio (RER) of 0.805 compared to 0.812 in the control group (P < 0.001) (Fig. 8E).
Figure 8.
Effect of MICs and MC on lipolysis and thermogenesis in vitro (A) and in vivo (B-E). Production of glycerol in adipocytes treated with MC, MIC-1 and MIC-4; n = 3 (A). Expression of thermogenic and lipolytic genes in adipose tissue (B) and hepatic GcK (C) from VHFD and VHFD + 5% MC-fed mice for 3 months; n = 12. Hepatic lipid metabolizing protein levels from VHFD and VHFD + 5% MC-fed mice for 3 months; n = 12 (D). RER in mice switched to a VHFD + 3.3% MC after 7 days compared to mice that remained on a VHFD (n=12) (E). Data are means ± SEM. Comparisons to controls were made by t-test for A, Welch's test for B, C D and ANCOVA for E. *: p<.05, **: p<.01, ***: p<.001.
4. Discussion
This study provides justification and preliminary mechanistic evidence for the uses of moringa as a dietary agent in preventing T2D by demonstrating that MIC-enriched MC caused significant reduction in weight gain, hepatic adiposity, gluconeogenesis, insulin, cholesterol, and inflammatory markers; and increase in insulin signaling sensitivity and lipolysis. Our study also provides support for the role of MICs as primary antidiabetic actives in moringa. The most notable result of the long-term feeding study was the very significant reduction in weight gain observed in the MC-fed mice. Healthy C57BL/6J mice fed a low fat diet (10% kcal from fat) typically gain 25-32% less weight than mice on a VHFD [21, 26, 27]. In this experiment the MC-fed mice gained 18% less weight than the VHFD-fed mice, demonstrating almost complete abolition of excess weight gain caused by the VHFD, without any other observable side effects. Slight differences in accumulated food intake or food aversion cannot explain the reduced weight gain in MC-fed mice, because the ratio of accumulated food intake to body weight was actually higher in the VHFD + 5% MC-fed mice compared to the VHFD group. Previous in vitro work demonstrated MICs and MC possess anti-inflammatory activity manifested as decreased IL-1β and TNFα expression and nitric oxide (NO) production [2]; effects that were also observed in this in vivo study. TNFα over expression was identified decades ago as a contributing factor to obesity-induced T2D [28], particularly by studies showing TNFα knockout mice had increased insulin sensitivity [29-31]. However, only slight decreases in body weight gain were noted in these studies, indicating the anti-inflammatory effects of MICs alone are not likely responsible for anti-obesity effects observed by MC treatment. However, MICs are very effective in blocking glucose production in HII4E hepatocytes, showing activity at nanomolar concentrations (Fig 7A-B) and being close to two orders of magnitude more active than metformin (Fig 7B). Because MICs were able to decrease PEPCK and G6P gene expression at similarly low concentrations it is tempting to speculate that MICs act via blocking these rate-limiting steps in liver gluconeogenesis. Decreased G6P and PEPCK gene expression was also observed in liver tissue from the MC feeding study, further supporting this mode of action (Fig 7D). In a long term, reduced gluconeogenesis may contribute to improved insulin sensitivity, as metformin's inhibition of gluconeogenesis [33] has been a successful target for treating T2D [34], although other studies suggest that metformin may have other modes of action [35-37]. Additional symptoms of T2D include impaired insulin signaling and insulin sensitivity and increased serum levels of insulin, leptin, resistin, TG, and cholesterol [21, 38-41]; all of which were reduced by MC treatment. Similarly, MC-fed mice showed activation of components of insulin signaling pathway, such as increased levels of IRSs, protein kinases, PI3K, and GLUT4 in liver and muscle tissue.
Before suggesting decreased gluconeogensis as a primary mechanism of action of MC, we investigated whether MICs and MC may also increase lipolysis and theromogenesis, which may also contribute to lower fat accumulation and body mass. We observed mild effect of MC and MIC-1 and 4 treatment on increased glycerol production in adipocytes (Fig 8A), indicative of lipolytic breakdown of TG into free fatty acids and glycerol. However, small magnitude and poor dose dependency of the effect made lipolysis is an unlikely primary target for MICs action. From the animal studies we noted relatively lower hepatic GcK gene expression and high ATGL protein levels in MC-fed mice (Fig. 8C). High-fat diet has been shown to up-regulate GcK and through neural signaling subsequently down regulate thermogenesis-related genes in brown adipose tissue (BAT) and increase overall adiposity [43, 44]. White adipose tissue (WAT) is able to differentiate into depots of brown-like adipocytes, often called beige fat [45]. Indeed, the inguinal WAT from mice in long-term study did have detectable levels of browning genes (Fig 8B). MC-fed mice had significantly increased expression of PRDM16 and PGC-1α, transcriptional regulators in beige fat formation and lipolysis [46], but did not show the usual corresponding increase of UCP1. This questions the possibility that MC-treatment directly increases thermogenesis. More compelling is the 4-fold increase in ADRB3 expression from MC-fed compared to control mice. ADRB3 plays a major regulatory role in lipolysis through interaction with catecholamines [47]. In the current study it is more likely that greater ADRB3 expression is linked to increased lipolysis which proceeds to production of ATP, rather than heat, due to the conflicting data on PRDM16, PGC1-α with UCP1 expression. Nonetheless, MC-mediated lipolysis, whether direct or indirect, was further corroborated by 1) hepatic down regulation of lipogenic proteins (FAS, SREBP1 and FSP27) and up regulation of ATGL, an important hepatic lipase responsible for TG turnover [48] (Fig. 8D), and 2) a significantly lower RER measured in MC-fed mice compared to the control, indicative of increased fatty acid oxidation relative to carbohydrate oxidation. The later observation suggests that MC-treatment enhances fat oxidation at the expense of carbohydrate oxidation.
Collectively, the results of in vitro and in vivo experiments provide support for the hypothesis that MICs are the primary biologically active anti-obesity and anti-diabetes constituents of MC whose primary mechanism of action is the inhibition of liver gluconeogenesis, which directly or indirectly results in systemically increased insulin signaling and sensitivity. These effects may in turn cause increased lipolysis, higher ratio of fat/carbohydrate oxidation, ultimately resulting in reduced lipid accumulation in the liver and body. These conclusions, combined with previous data on MICs anti-inflammatory effects [2], suggest that MC and MICs may have beneficial effects for prevention and treatment of obesity and T2D.
Supplementary Material
Acknowledgements
This study was supported by a Botanical Research Center Pilot Program Sub award 5P50AT002776-08 S12-50318 and P50AT002776-01 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements (ODS). CW was also supported by NIH training Grant T32:5T32AT004094-04. C.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. PRS was supported by a doctoral fellowship from Ecuadorian government SENESCYT-2011. Histology performed by Kenneth Reuhl is gratefully acknowledged. From Rutgers, we thank Julia Driefus for her technical support and Diana Cheng for discussions. From PBRC we thank Jennifer Rood, Youngmei Yu, Tamra Mendoza, Robbie Beyl (supported by NIH grant 1 U54 GM104940) and William Johnson for their technical support. IR has equity in Nutrasorb LLC, which licensed moringa-related intellectual property from Rutgers University.
Abbreviations
- ADPN
adiponectin
- ADRB3
beta-3 adrenergic receptor
- Akt1 p
phosph-Akt1(Ser473)
- Akt1
RAC-alpha serine/threonine-protein kinase
- Akt2 p
phosph-Akt2(Ser474)
- Akt2
RAC-beta serine/threonine-protein kinase
- ATGL
adipose triglyceride lipase
- CIDEA
cell death-inducing DFFA-like effector A
- CIDEA
cell death-inducing DFFA-like effector c
- CPT1b
carnitine palmitoyltransferase 1b
- DW
dry weight
- FAS
fatty acidy synthase
- G6P
glucose-6-phosphatase
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GcK
glucokinase
- GLUT4
glucose transporter type 4
- Hmbs
hydroxymethylbilane synthase
- HMGR
3-hydroxy-3-methyl-glutaryl-CoA reductase
- IL-1β
interleukin-1 beta
- IL-6
interleukin-6
- IRS-1 p
anti-phospho-IRS-1(Tyr612)
- IRS-1
insulin receptor substrate 1
- IRS-2
insulin receptor substrate 2
- IRβ
insulin receptor beta
- ITCs
isothiocyanates
- LCN2
lipocalin-2
- LPL
lipoprotein lipase
- MC
moringa concentrate
- MCP-1
monocyte chemoattractant protein-1
- MICs
moringa isothiocyanates
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- OGTT
oral glucose tolerance test
- PAI1
plasminogen activator inhibitor type 1
- PEPCK
phosphoenolpyruvate carboxykinase
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- PI 3K
p85 of PI 3K
- PPIA
peptidyl prolyl isomerase A
- PPIB
peptidyl prolyl isomerase B
- PPIH
peptidyl prolyl isomerase H
- PRDM16
PR domain containing 16
- PY20
p-tyr (PY20)
- RER
respiratory exchange ratio
- SF
sulforaphane
- SREBP-1c
sterol regulatory element binding protein-1c
- TG
triglycerides
- TNFα
tumor necrosis factor alpha
- UCP1
uncoupling protein 1
- VHFD
very high fat diet
Footnotes
Author Contributions
C.W. wrote/reviewed/edited the manuscript and researched data. P.R.S. and T.B.T. contributed/reviewed/edited the manuscript and researched data. P.K., A.R. and S.W. researched data. J.S., Z.W. and R.M. researched data and reviewed/edited the manuscript. W.C. and I.R. contributed to discussion and reviewed/edited manuscript.
References
- 1.Mbikay M. Therapeutic potential of Moringa oleifera leaves in chronic hyperglycemia and dyslipidemia: a review. Front Pharmacol. 2012;3:1–12. doi: 10.3389/fphar.2012.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waterman C, Cheng DM, Rojas-Silva P, Poulev A, Dreifus J, et al. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochem. 2014;103:114–122. doi: 10.1016/j.phytochem.2014.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheenpracha S, Park E-J, Yoshida WY, Barit C, Wall M, et al. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorgan. Med. Chem. 2010;18:6598–6602. doi: 10.1016/j.bmc.2010.03.057. [DOI] [PubMed] [Google Scholar]
- 4.Bae JY, Lim SS, Kim SJ, Choi JS, Park J, et al. Bog blueberry anthocyanins alleviate photoaging in ultraviolet-B irradiation-induced human dermal fibroblasts. Mol. Nutr. Food Res. 2009;53:726–738. doi: 10.1002/mnfr.200800245. [DOI] [PubMed] [Google Scholar]
- 5.Brunelli D, Tavecchio M, Falcioni C, Frapolli R, Erba E, et al. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010;79:1141–1148. doi: 10.1016/j.bcp.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 6.Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, et al. Isolation and structure elucidation of new nitrile and mustard oil glycosides from Moringa oleifera and their effect on blood pressure. J. Nat. Prod. 1994;57:1256–1261. doi: 10.1021/np50111a011. [DOI] [PubMed] [Google Scholar]
- 7.Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Chemoprotective glucosinolates and isothiocyanates of broccoli sprouts metabolism and excretion in humans. Cancer Epidem. Biomar. 2001;10:501–508. [PubMed] [Google Scholar]
- 8.Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidem. Biomar. 1996;5:733–748. [PubMed] [Google Scholar]
- 10.Traka M, Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009;8:269–282. [Google Scholar]
- 11.Mirmiran P, Bahadoran Z, Hosseinpanah F, Keyzad A, Azizi F. Effects of broccoli sprout with high sulforaphane concentration on inflammatory markers in type 2 diabetic patients: A randomized double-blind placebo-controlled clinical trial. J. Funct. Foods. 2012;4:837–841. [Google Scholar]
- 12.Bahadoran Z, Tohidi M, Nazeri P, Mehran M, Azizi F, et al. Effect of broccoli sprouts on insulin resistance in type 2 diabetic patients: a randomized double-blind clinical trial. Int. J. Food Sci. Nutr. 2012;63:767–771. doi: 10.3109/09637486.2012.665043. [DOI] [PubMed] [Google Scholar]
- 13.Bahadoran Z, Mirmiran P, Azizi F. Potential efficacy of broccoli sprouts as a unique supplement for management of type 2 diabetes and its complications. J. Med. Food. 2013;16:375–382. doi: 10.1089/jmf.2012.2559. [DOI] [PubMed] [Google Scholar]
- 14.Wu H, Liang H, Yuan Q, Wang T, Yan X. Preparation and stability investigation of the inclusion complex of sulforaphane with hydroxypropyl-β-cyclodextrin. Carbohyd. Polym. 2010;82:613–617. [Google Scholar]
- 15.Park E-J, Cheenpracha S, Chang LC, Kondratyuk TP, Pezzuto JM. Inhibition of lipopolysaccharide-induced cyclooxygenase-2 and inducible nitric oxide synthase expression by 4-[(2′-O-acetyl-α-L-rhamnosyloxy)benzyl]isothiocyanate from Moringa oleifera. Nutr. Cancer. 2011;63:971–982. doi: 10.1080/01635581.2011.589960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shetty P. Public health: India's diabetes time bomb. Nature. 2012;485:S14–S6. doi: 10.1038/485s14a. [DOI] [PubMed] [Google Scholar]
- 17.Mbanya JCN, Motala AA, Sobngwi E, Assah FK, Enoru ST. Diabetes in Sub-Saharan Africa. Lancet. 2010;375:2254–2266. doi: 10.1016/S0140-6736(10)60550-8. [DOI] [PubMed] [Google Scholar]
- 18.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014 doi: 10.1016/S0140-6736(14)60460-8. doi:10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Folch J, Lees M, Sloane-Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 20.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 21.Wang ZQ, Zhang XH, Yu Y, Poulev A, Ribnicky D, et al. Bioactives from bitter melon enhance insulin signaling and modulate acyl carnitine content in skeletal muscle in high-fat diet-fed mice. J. Nutr. Biochem. 2011;22:1064–1073. doi: 10.1016/j.jnutbio.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng DM, Kuhn P, Poulev A, Rojo LE, Lila MA, et al. In vivo and in vitro antidiabetic effects of aqueous cinnamon extract and cinnamon polyphenol-enhanced food matrix. Food Chem. 2012;135:2994–3002. doi: 10.1016/j.foodchem.2012.06.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 24.Richard AJ, Fuller S, Fedorcenco V, Beyl R, Burris TP, et al. Artemisia scoparia enhances adipocyte development and endocrine function in vitro and enhances insulin action in vivo. Plos One. 2014 doi: 10.1371/journal.pone.0098897. doi: 10.1371/journal.pone.0098897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ndong M, Uehara M, Katsumata S. Effects of oral administration of Moringa oleifera Lam on glucose tolerance in Goto-Kakizaki and Wistar rats. J. Clin. Biochem. Nutr. 2007;40:229–233. doi: 10.3164/jcbn.40.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller RS, Becker KG, Prabhu V, Cooke DW. Adipocyte gene expression is altered in formerly obese mice and as a function of diet composition. J. Nutr. 2008;138:1033–1038. doi: 10.1093/jn/138.6.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korda M, Kubant R, Patton S, Malinski T. Leptin-induced endothelial dysfunction in obesity. Am. J. Physiol-Heart C. 2008;295:H1514–H1521. doi: 10.1152/ajpheart.00479.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrin. Met. 2000;11:212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 29.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNFalpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 30.Schreyer SA, Chua SC, Jr, LeBoeuf RC. Obesity and diabetes in TNF-alpha receptor-deficient mice. J. Clinl. Invest. 1998;102:402–411. doi: 10.1172/JCI2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bose M, Lambert JD, Ju J, Reuhl KR, Shapses SA, et al. The major green tea polyphenol,(−)-epigallocatechin-3-gallate, inhibits obesity, metabolic syndrome, and fatty liver disease in high-fat–fed mice. J. Nutr. 2008;138:1677–1683. doi: 10.1093/jn/138.9.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rena G, Pearson ER, Sakamoto K. Molecular mechanism of action of metformin: old or new insights? Diabetologia. 2013;56:1898–1906. doi: 10.1007/s00125-013-2991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geerling JJ, Boon MR, van der Zon GC, van den Berg SA, van den Hoek AM, et al. Metformin lowers plasma triglycerides by promoting VLDL-triglyceride clearance by brown adipose tissue in mice. Diabetes. 2013 doi: 10.2337/db13-0194. doi: 10.2337/db13-0194. [DOI] [PubMed] [Google Scholar]
- 37.Madiraju AK, Erion DM, Rahimi Y, Zhang X-M, Braddock DT, et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widjaja A, Stratton IM, Horn R, Holman RR, Turner R, et al. UKPDS 20: plasma leptin, obesity, and plasma insulin in type 2 diabetic subjects. J. Clin. Endocrinol. Metab. 1997;82:654–657. doi: 10.1210/jcem.82.2.3744. [DOI] [PubMed] [Google Scholar]
- 39.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, et al. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 40.Srinivasan K, Viswanad B, Asrat L, Kaul C, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005;52:313–320. doi: 10.1016/j.phrs.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 41.El Messaoudi S, Rongen GA, de Boer RA, Riksen NP. The cardioprotective effects of metformin. Current Opin. Lipidol. 2011;22:445–453. doi: 10.1097/MOL.0b013e32834ae1a7. [DOI] [PubMed] [Google Scholar]
- 42.Henry-Vitrac C, Ibarra A, Roller M, Mérillon J-M, Vitrac X. Contribution of chlorogenic acids to the inhibition of human hepatic glucose-6-phosphatase activity in vitro by Svetol, a standardized decaffeinated green coffee extract. J. Agric. Food Chem. 2010;58:4141–4144. doi: 10.1021/jf9044827. [DOI] [PubMed] [Google Scholar]
- 43.Tsukita S, Yamada T, Uno K, Takahashi K, Kaneko K, et al. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metab. 2012;16:825–832. doi: 10.1016/j.cmet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 44.Ferre T, Riu E, Franckhauser S, Agudo J, Bosch F. Long-term overexpression of glucokinase in the liver of transgenic mice leads to insulin resistance. Diabetologia. 2003;46:1662–1668. doi: 10.1007/s00125-003-1244-z. [DOI] [PubMed] [Google Scholar]
- 45.Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J. Cell Sci. 1992;103:931–942. doi: 10.1242/jcs.103.4.931. [DOI] [PubMed] [Google Scholar]
- 46.Heilbronn LK, Gregersen S, Shirkhedkar D, Hu D, Campbell LV. Impaired fat oxidation after a single high-fat meal in insulin-sensitive nondiabetic individuals with a family history of type 2 diabetes. Diabetes. 2007;56:2046–2053. doi: 10.2337/db06-1687. [DOI] [PubMed] [Google Scholar]
- 47.Langin D. Adipose tissue lipolysis as a metabolic pathway to define pharmacological strategies against obesity and the metabolic syndrome. Pharmacol. Res. 2006;53:482–491. doi: 10.1016/j.phrs.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Ong KT, Mashek MT, Bu SY, Greenberg AS, Mashek DG. Adipose triglyceride lipase is a major hepatic lipase that regulates triacylglycerol turnover and fatty acid signaling and partitioning. Hepatol. 2011;53:116–126. doi: 10.1002/hep.24006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.