Abstract
Methicillin-resistant Staphylococcus aureus (MRSA) is a common multidrug-resistant (MDR) pathogen. We herein discussed MRSA and its infections in Krasnoyarsk, Siberian Russia between 2007 and 2011. The incidence of MRSA in 3,662 subjects was 22.0% and 2.9% for healthcare- and community-associated MRSA (HA- and CA-MRSA), respectively. The 15-day mortality rates for MRSA hospital- and community-acquired pneumonia (HAP and CAP) were 6.5% and 50%, respectively. MRSA CAP cases included pediatric deaths; of the MRSA pneumonia episodes available, ≥27.3% were associated with bacteremia. Most cases of HA-MRSA examined exhibited ST239/spa3(t037)/SCCmecIII.1.1.2 (designated as ST239Kras), while all CA-MRSA cases examined were ST8/spa1(t008)/SCCmecIV.3.1.1(IVc) (designated as ST8Kras). ST239Kras and ST8Kras strongly expressed cytolytic peptide (phenol-soluble modulin α, PSMα; and δ-hemolysin, Hld) genes, similar to CA-MRSA. ST239Kras pneumonia may have been attributed to a unique set of multiple virulence factors (MVFs): toxic shock syndrome toxin-1 (TSST-1), elevated PSMα/Hld expression, α-hemolysin, the staphylococcal enterotoxin SEK/SEQ, the immune evasion factor SCIN/SAK, and collagen adhesin. Regarding ST8Kras, SEA was included in MVFs, some of which were common to ST239Kras. The ST239Kras (strain OC3) genome contained: a completely unique phage, φSa7-like (W), with no att repetition; S. aureus pathogenicity island SaPI2R, the first TSST-1 gene-positive (tst +) SaPI in the ST239 lineage; and a super copy of IS256 (≥22 copies/genome). ST239Kras carried the Brazilian SCCmecIII.1.1.2 and United Kingdom-type tst. ST239Kras and ST8Kras were MDR, with the same levofloxacin resistance mutations; small, but transmissible chloramphenicol resistance plasmids spread widely enough to not be ignored. These results suggest that novel MDR and MVF+ HA- and CA-MRSA (ST239Kras and ST8Kras) emerged in Siberian Russia (Krasnoyarsk) associated with fatal pneumonia, and also with ST239Kras, a new (Siberian Russian) clade of the ST239 lineage, which was created through stepwise evolution during its potential transmission route of Brazil-Europe-Russia/Krasnoyarsk, thereby selective advantages from unique MVFs and the MDR.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) has been a major multidrug-resistant (MDR) pathogen since the early 1960s [1], with recent threats, such as intensive care unit (ICU)-associated bacteremia in London [2], serious invasive infections in the United States (US) [3], and global antimicrobial resistance in common infections, being alerted by the World Health Organization (WHO) [4].
Traditional MRSA is now classified as healthcare-associated MRSA (HA-MRSA) [3], and another class of MRSA, which emerged in community settings between 1997 and 1999, as community-associated MRSA (CA-MRSA) [3,5,6]. HA- and CA-MRSA both carry staphylococcal cassette chromosome mec (SCCmec) [7,8], and each have several genetic backgrounds [9–14], which are generally identified based on multilocus sequence types (ST types), protein A gene (spa) types, and SCCmec types [6,8,12,14].
The most disseminated HA-MRSA worldwide includes the ST239 lineage, such as ST239/SCCmecIIIA [15] and ST239/SCCmecIII [2,16–20], as well as the ST5 lineage, such as ST5/SCCmecII carrying the toxic shock syndrome toxin-1 (TSST-1) gene (tst) [21,22]. The most characterized CA-MRSA includes the ST8 lineage, such as ST8/SCCmecIVa (USA300) [12,23], and also the lineages of ST30/SCCmecIV [6,24–26], ST59/SCCmecV [27–29], and ST80/SCCmecIV [6,24,30]. Although CA-MRSA, unlike HA-MRSA, is generally less MDR [31], CA-MRSA also has the capacity to become MDR (representative, USA300) [32,33]. Some CA-MRSA are MDR from their emergence (representative, the Taiwan clone) [29].
HA-MRSA infections most frequently occur among inpatients [34,35], while CA-MRSA infections occur in healthy individuals. CA-MRSA mainly causes skin and soft tissue infections (SSTIs), but also occasionally invasive infections [6,13,34,36]. The median ages of HA-MRSA and CA-MRSA patients are 68 and 23 years, respectively [34]. CA-MRSA often produces Panton-Valentine leukocidin (PVL) [6,12,13,37–39], and exhibits elevated expression of cytolytic peptides (phenol-soluble modulins, PSMs; or δ-hemolysin, Hld) [12,40].
MRSA evolution includes horizontal gene transfer, mediated by mobile genetic elements, plasmids, and phages, and also through mutations [8,37,41–44]. The mosaicism of the genome has also been reported; ST239 MRSA is a bacterial hybrid between clonal complex (CC) 30 (founder, ST30) and CC8 (founder, ST8) [19,45]. MRSA occasionally spreads by intercontinental transmission [9,20,46], and replacement often occurs [18,47,48]. The evolution of MRSA is still dynamic, and, therefore, may attack humans posing a new threat.
In Russia, dominant MRSA are ST239/SCCmecIII and PVL-negative (PVL-) ST8/SCCmecIV [49]. Although we previously reported PVL-positive (PVL+) ST30 CA-MRSA [50], tst + ST239 HA-MRSA [51], and a whole genome structure [52], information on MRSA in Russia is still limited at the molecular level, especially in Siberian Russia, which is located between the European and Far Eastern regions. In the present study, we focused on episodes (and mortality rates) of MRSA hospital-acquired pneumonia (HAP) and community-acquired pneumonia (CAP) with pediatric deaths in Krasnoyarsk, Siberian Russia, as has been reported with initial fatal pediatric MRSA CAP episodes in the US North areas [5]. We discussed possible MRSA multiple virulence factors (MVFs), implicated in fatal cases of MRSA HAP and CAP. We then demonstrated their unique genomic structures and evolution of representative fatal-pneumonia-associated MRSA.
Materials and Methods
Ethics statement
The Ethics Review Boards of Krasnoyarsk State Medical University (Ethics Review Board No28/2010), Krasnoyarsk, Russia; Far Eastern Federal University School of Biomedicine, Vladivostok, Russia; National Taiwan University College of Medicine, Taipei, Taiwan; Niigata University School of Medicine, Niigata, Japan; and International Medical Education and Research Center, Niigata, Japan, specifically approved this study. Written informed consent was obtained from patients, if necessary.
Patients and bacterial strains
A total of 3,662 subjects were examined in Krasnoyarsk between 2007 and 2011. S. aureus specimens including MRSA were isolated in four hospitals in Krasnoyarsk, and all bacterial strains were isolated from different individuals. The data obtained are summarized in Table 1. The follow-up period used to determine the mortality for pneumonia was 15 days in this study; and 15-day mortality rates were compared between MRSA HAP and MRSA CAP cases. HA-MRSA was defined as MRSA isolated from inpatients 48 h after hospitalization while CA-MRSA was defined as MRSA isolated from outpatients who had no history of hospitalization within at least the past year and presented with no other established risk factors for MRSA infections [3].
Table 1. Clinical and bacteriological information on MRSA isolated in Krasnoyarsk between 2007 and 2011.
| Study group | Diseases | Subjects | Bacterial isolation | Fatal cases | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Total number | Age (Y) | Isolation year | S. aureus(MRSA) | MRSA/total subjects | MRSA/S. aureus | /subjects | /S. aureus | /MRSA | ||
| Inpatients | Pneumonia | 710 | 0–81 | 2007–2011 b | 221 (62) | 8.7% | 28.1% | 0.6% | 0% | 6.5% c |
| (62/710) | (62/221) | (4/710) | (0/159) | (4/62) | ||||||
| SSTIs a | 874 | 20–84 | 2010–2011 | 210 (31) | 3.5% | 14.8% | 0.1% | 0% | 3.2% | |
| (31/874) | (31/210) | (1/874) | (0/179) | (1/31) | ||||||
| Osteomyelitis | 208 | 21–80 | 2010–2011 | 77 (19) | 9.1% | 24.7% | 0% | 0% | 0% | |
| (19/208) | (19/77) | (0/208) | (0/58) | (0/19) | ||||||
| Outpatients | Pneumonia | 310 | 0–67 | 2007–2008 | 93 (8) | 2.6% | 8.6% | 1.3% | 0% | 50% c |
| (8/310) | (8/93) | (4/310) | (0/85) | (4/8) | ||||||
| SSTIs a | 126 | 27–84 | 2010–2011 | 41 (2) | 1.6% | 4.9% | 0% | 0% | 0% | |
| (2/126) | (2/41) | (0/126) | (0/39) | (0/2) | ||||||
| Colitis | 401 | 0–9 | 2011 | 357 (4) | 1.0% | 1.1% | 0% | 0% | 0% | |
| (4/401) | (4/357) | (0/401) | (0/353) | (0/4) | ||||||
| Carriers | Students in the | 287 | 18–20 | 2010–2011 | 77 (1) | 0.3% | 1.3% | 0% | 0% | 0% |
| community | (1/287) | (1/77) | (0/287) | (0/76) | (0/1) | |||||
| Athletes in the | 108 | 11–28 | 2011 | 42 (0) | 0% | 0% | 0% | 0% | 0% | |
| community | (0/108) | (0/42) | (0/108) | (0/42) | (0/0) | |||||
| Hospital | 638 | 22–52 | 2008–2011 | 183 (2) | 0.3% | 1.1% | 0% | 0% | 0% | |
| workers | (2/638) | (2/183) | (0/638) | (0/181) | (0/2) | |||||
aSSTIs, skin and soft tissue infections; in this study, SSTIs include dactylitis, paronychia, hidradenitis, wound infection, skin abscess, furuncle, carbuncle, bursitis, cellulitis, erysipelas-like necrotic cellulitis, and necrotizing fasciitis.
bNumber of MRSA isolates in 2007–2009 was 42, and that in 2010–2011 was 20 (total 62).
c P<0.01
Russian MRSA strains also included ten strains from inpatients (age, 1–41 years) with burn wound infections and respiratory tract infections in Vladivostok in 2012 and 2013; and nine strains from patients with burn and wound infections, osteomyelitis, respiratory tract infections, and blood stream infections in Moscow and Saint-Petersburg (European Russia) and in Kurgan (Ural Federal Region, Russia) in 2011 and 2012.
The following were used as reference or control strains. Strain RS08 (PVL+ ST30/spa19-t019/SCCmecIVc) was isolated from a female badminton player in her twenties with furunculosis in Vladivostok in 2006 [50]. Of the ST239/spa351(t030)/ SCCmecIII.1.1.4 strains, 16K was isolated from a 20-year-old male outpatient with urethritis in Vladivostok between 2006 and 2008 [52]. Another 11 strains were collected from Vladivostok [52]. Of the ST239/spa3(t037)/SCCmecIII.1.1.1 MRSA (tst -) strains, nine (including strain 6K) were from Vladivostok [52] and four (PM3, PM14, PM27, and PM38) were from Taiwan [28]. Of the PVL+ ST8/SCCmecIVa CA-MRSA (USA300) strains, USA300-0114 was a type strain kindly provided by L. K. McDougal and L. L. McDonald and NN36 [53] and NN47 [54] were Japanese isolates. Of the tst + ST5/SCCmecII HA-MRSA (NY/J clone) strains, N315 and Mu50 were reference strains that were kindly provided by K. Hiramatsu, I6 was a Japanese isolate [55], and PM29 was a Taiwanese isolate [28]. The reference strains HU25 (ST239/SCCmecIII.1.1.2-IIIA, Brazilian clone) and ANS46 (ST239/SCCmecIII.1.1.1) were kindly provided by H. de Lencastre.
Genotyping and virulence gene analysis
ST typing, CC assignment, spa typing, agr typing, and SCCmec typing were performed as described previously [8,56]. Coagulase (Coa) typing was conducted using a staphylococcal coagulase antiserum kit (Denka Seiken, Tokyo, Japan). Virulence genes were analyzed by PCR [56]; the target genes in PCR included 49 genes: 3 leukocidin genes (luk PV SF, lukE-lukD, and lukM), 5 hemolysin genes (hla, hlb, hlg, hlg-v, and hld), a peptide cytolysin (psmα), 19 staphylococcal enterotoxin (SE) genes (tst, sea, seb, sec, sed, see, seg, seh, sei, sej, sek, sel, sem, sen, seo, sep, seq, ser, and set), 1 putative SE gene (seu), 3 exfoliative toxin genes (eta, etb, and etd), a staphylococcal superantigen-like gene cluster (ssl), the epidermal cell differentiation inhibitor gene (edin), 14 adhesin genes (icaA, icaD, eno, fib, fnbA, fnbB, ebpS, clfA, clfB, sdrC, sdrD, sdrE, cna, and bbp), and the arginine catabolic mobile element (ACME)-arcA gene.
TSST-1 and SEA assays
The amounts of TSST-1 and SEA in the supernatants of bacterial cultures at 2.0 X l09 CFU/ml were examined using a TST- RPLA kit (Denka Seiken) and SET-RPLA kit (Denka Seiken), respectively, according to the instructions of the manufacturer.
Pulsed-field gel electrophoresis (PFGE) analysis
Bacterial DNA for PFGE was digested with SmaI and electrophoresed in 1.2% agarose with marker DNA (Lambda ladder; Bio-Rad Laboratories, Inc., Hercules, CA, USA), as described previously [52].
Plasmid analysis
The plasmid DNA of MRSA was prepared using a Plasmid Midi Kit (QIAGEN Sciences, Tokyo) or according to the method by Kado and Liu [57] with a modification to the lysostaphin treatment. Plasmid DNA was analyzed by agarose (0.6–1.0%) gel electrophoresis. The Tn554 circular intermediate was detected by PCR (PCR product size, 772 bp), as previously described [52].
Conjugative transfer
Donor strains were mated with S. aureus RN2677, a recipient strain, which is resistant to rifampicin (Rifr) and novobiocin and carries no plasmids, on tryptic soy agar (Difco, Sparks, MD, USA), with or without membrane filters [52].
Susceptibility testing
Susceptibility testing of bacterial strains was performed using the agar dilution method with Mueller-Hinton agar [58]. Inducible clindamycin resistance (Clir) was tested, as above, by using agar plates containing erythromycin (Em) at 0.1 to 1 μg/ml.
Drug resistance gene analysis
The genes for drug resistance, antiseptic resistance, and heavy metal resistance were analyzed by PCR [28,29]. The genes (resistance phenotypes) analyzed were: mecA (resistance to methicillin, oxacillin, and cephems), blaZ (resistance to ampicillin), ermA and ermC (Emr/Clir), cat (resistance to chloramphenicol, Cpr), aacA-aphD (resistance to gentamicin, Gmr, and kanamycin), aadD (resistance to neomycin), tet (resistance to tetracycline), spc (spectinomycin, Spcr), ble (resistance to bleomycin), qacA (resistance to acriflavin/quaternary ammonium, such as benzalkonium chloride and benzethonium chloride/chlorhexidine gluconate/ethidium bromide), cad (resistance to cadmium), and mer (resistance to mercury).
Genome analysis
The ST239 MRSA OC3 genome was analyzed by pyrosequencing using a genome sequencer FLX system with the assembler software GS De Novo Assembler version 2.6 (Roche Diagnostics, Branford, CT, USA). The GenBank accession numbers for the OC3 genome (144 contigs with ≥20 bp in size) are BBKC01000001-BBKC01000144. The OC3 contigs were mapped on the 3,043,210-bp complete genome (GenBank accession number FN433596) of TW20 (the most characterized ST239) using MUMmer software (http://mummer.sourceforge.net/). The gene or open reading frame (orf) was searched for using the software in silico MolecularCloning (version 4.2) (In Silico Biology, Yokohama, Japan).
Entire sequencing of mobile genetic elements, phages, and plasmids
The gaps between contigs were filled by PCR and sequencing. We also assembled contigs using an LA PCR in vitro cloning kit (Takara Bio, Shiga, Japan) according to the manufacturer’s instructions. In brief, after digestion with suitable restriction enzymes and ligation with the corresponding cassette adapters, amplification was performed with cassette primers and target-specific primers.
Phylogenetic and homology analyses
Multiple alignments were performed up to 1,000 times using default settings with ClustalW software (version 2.1) and a phylogenetic tree analysis was performed using TreeViewX software (version 0.5.0) (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). A homology analysis was performed using the software BLAST (http://blast.ddbj.nig.ac.jp/top-e.html) and FASTA (http://fasta.ddbj.nig.ac.jp/top-j.html).
mRNA expression assay
The mRNA expression levels of the cytolytic peptide (PSMα and Hld) genes (psmα and hld) and 16S rRNA genes were examined by an RT-PCR assay [56,59]. psmα and hld expression levels were normalized to 16S rRNA expression levels. The mRNA expression levels of the transcriptional regulator genes (sarA, sarR, mgrA, saeR, saeS, sarX, rot, and srrAB) were also examined.
Statistical analysis
Data were evaluated by Fisher’s exact test for MRSA incidence and by an analysis of variance with repeated measurements for the mRNA expression assay. The level of significance was defined as a P value of <0.05. Regarding 15-day mortality rate estimates, 95% confidence intervals (95% CIs) were included.
Results
MRSA infection in Krasnoyarsk, Siberian Russia
A total of 3,662 subjects were examined for S. aureus and MRSA infections, and the data obtained are summarized in Table 1. Regarding nosocomial infections (inpatients), MRSA infections accounted for 8.7% (62/710) of cases of pneumonia, 3.5% (31/874) of SSTIs, and 9.1% (19/208) of osteomyelitis; the overall incidence of MRSA (among S. aureus isolates) was 22.0% (112/508). Among the cases of MRSA HAP, 6.5% (4/62) were fatal (Tables 1 and 2); the ages of these four patients were 39, 46, 48, and 71 years old (average, 51 years old), and these deaths occurred in 2007 (three cases) and 2011 (one case). One fatal case of nosocomial SSTIs (3.2%, 1/31) was noted and may have been due to sepsis.
Table 2. List of MRSA strains characterized at molecular levels in the present study a .
| Isolation year | Name ofMRSA | ST type (epidemiologicalclassification of MRSA) | Patients | |||
|---|---|---|---|---|---|---|
| Disease | Outcome | Age | Sex | |||
| 2007 | OC3 | ST239 (HA) | Pneumonia, sepsis (bacteremia) | Death | 46Y | M |
| OC76 | ST239 (HA) | Pneumonia | Death | 71Y | F | |
| OC8 | ST8 (CA) | Pneumonia | Death | 1Y | M | |
| OC11 | ST8 (HA) | Pneumonia, sepsis (bacteremia) | Death | 39Y | M | |
| 2008 | OC22 | ST8 (CA) | Pneumonia | Death | 41Y | M |
| OC23 | ST8 (CA) | Pneumonia | Death | 40Y | M | |
| OC59 | ST8 (CA) | Pneumonia | Death | 4M | F | |
| OC52 | ST8 (HA/CA) | - b | - b | 34Y | F | |
| 2009 | OC180 | ST239 (HA) | Pneumonia | Recovery | 34Y | M |
| 2010 | OC22B | ST239 (HA) | Osteomyelitis | Recovery | 22Y | M |
| OC70 | ST239 (HA) | Osteomyelitis | Recovery | 40Y | M | |
| OC66 | ST239 (HA) | Erysipelas-like necrotic cellulitis | Recovery | 59Y | F | |
| OC159 | ST239 (HA) | Erysipelas-like necrotic cellulitis | Recovery | 57Y | F | |
| OC114 | ST239 (HA) | Wound infection, cellulitis | Recovery | 58Y | M | |
| OC145 | ST239 (HA) | Wound infection | Recovery | 50Y | M | |
| OC1 | ST8 (CA) | Skin abscesses | Recovery | 50Y | F | |
| OC217 | ST8 (CA) | - c | - c | 19Y | F | |
| OC50 | ST12 (HA) | Surgical site infection, (sepsis) | Death | 84Y | M | |
| 2011 | OC8C | ST239 (HA) | Pneumonia | Death | 48Y | M |
| OC14 | ST239 (HA) | Pneumonia | - d | - d | F | |
| OC35 | ST239 (HA) | Pneumonia, sepsis (bacteremia) | Recovery | 27Y | M | |
| OC98 | ST239 (HA) | Osteomyelitis | - d | 31Y | M | |
| OC99 | ST239 (HA) | Osteomyelitis | Recovery | 30Y | M | |
| OC111 | ST239 (HA) | Osteomyelitis | Recovery | 76Y | F | |
| OC1A | ST239 (HA) | Burn infection | Recovery | 37Y | M | |
| OC2 | ST239 (HA) | Burn infection | Recovery | 53Y | M | |
| OC5 | ST239 (HA) | Wound infection | Recovery | 31Y | M | |
| OC44 | ST239 (HA) | Peritonitis | Recovery | 30Y | M | |
| OC14C | ST239 (HA/CA) | - b | - b | 43Y | F | |
| OC160 | ST8 (CA) | Wound infection, cellulitis | Recovery | 53Y | M | |
| OC1C | ST8 (CA) | Colitis | Recovery | 3Y | M | |
aY, years; M, male; F, female; HA, healthcare-associated MRSA; CA, community- associated MRSA.
bHealthy carrier (hospital worker)
cHealthy carrier (student)
dInformation not available.
Among the cases of community-acquired infections (outpatients), MRSA infections accounted for 2.6% (8/310) of cases of pneumonia, 1.6% (2/126) of SSTIs, and 1.0% (4/401) of colitis; the overall incidence of MRSA (among S. aureus isolates) was 2.9% (14/491). The fatal case of MRSA CAP was 50% (4/8) (Tables 1 and 2); the ages of these four patients were 4 months old and 1, 40, and 41 years old (average, 20.5 years old), and these deaths occurred in 2007 (one case) and 2008 (three cases).
The incidence of MRSA (among S. aureus isolates) was significantly higher in hospitals (22%) than in the community (2.9%) (P <0.01). However, the 15-day mortality rates for MRSA CAP and MRSA HAP were 50% (95% CI, 17.5%-82.6%) and 6.5% (95% CI, 2.1%-16.5%), respectively (P <0.01).
Regarding healthy carriers (Table 1), MRSA+ cases were observed in 0.3% (2/638) of hospital workers in routine surveillance in hospitals, and in 0.3% (1/395) of students and athletes in occasional examinations in the community; the incidence of MRSA (among S. aureus isolates) was 1.1% (2/183) and 0.8% (1/119), respectively (P >0.05).
Among MRSA in Table 1, thirty-one isolates were subjected to molecular characterization (Table 2). When pneumonia was targeted between 2007 and 2009, eight MRSA were characterized, all of which were from fatal or severe cases only; MRSA was not available from other non-fatal pneumonia cases. When pneumonia, osteomyelitis, SSTIs, and colitis were mainly targeted in 2010 and 2011, MRSA from fatal cases were initially selected and characterized: two from MRSA HAP and nosocomial SSTI/sepsis. Eighteen isolates were randomly selected from non-fatal cases: two out of 19 pneumonia isolates, five out of 19 osteomyelitis isolates, nine out of 32 MRSA SSTI isolates, one out of four colitis isolates, and one isolate from peritonitis. All three MRSA isolates from healthy carriers were included in the molecular analysis.
In Table 2, at least three out of the 11 MRSA pneumonia episodes (pneumonia/sepsis) were associated with bacteremia with the incidence being ≥27.3%; at least two out of seven HAP episodes (>2/7) and one out of four CAP episodes (>1/4), or two out of six ST239 MRSA cases (>2/6) and one out of five ST8 MRSA cases (>1/5) were associated with bacteremia. Although two ST239 and ST8 HAP-related bacteremia cases in 2007 were fatal, one ST239 HAP-related bacteremia case in 2011 was not.
Molecular characteristics of MRSA from Krasnoyarsk
The molecular data of the 31 MRSA isolates are summarized in Table 3 and Fig 1. MRSA strains were classified into three groups, A to C (Table 3).
Table 3. Molecular characterization of MRSA strains isolated in Krasnoyarsk a .
| Type, gene, or resistance | Group A | Group B | Group C | |
|---|---|---|---|---|
| A1 (n = 18) | A2 (n = 2) | (n = 10) | (n = 1) | |
| Types | ||||
| CC | 8 | 8 | 8 | 12 |
| ST | 239 | 239 | 8 | 12 |
| spa | 3 (t037) | 3 (t037) | 1 (t008) | new (t156) |
| agr | 1 | 1 | 1 | 1 |
| SCCmec | III.1.1.2 | III.1.1.1 | IV.3.1.1 (IVc) | UT |
| Coagulase | IV | IV | III | I or VII |
| Toxins | ||||
| Leukocidins | ||||
| luk PV SF | - | - | - | - |
| lukE-lukD | + | + | + | + |
| lukM | - | - | - | - |
| Hemolysins | ||||
| hla, hlg, hlg-v | + | + | + | + |
| hlb (split) b | (+) | (+) | (+) | (+) |
| Peptide cytolysins | ||||
| psmα, hld | + | + | + | + |
| Staphylococcus enterotoxins | ||||
| sea | - | + | + (8/10) | - |
| (1,024–2,048 ng/ml) | ||||
| tst | + | - | - | - |
| (200–400 ng/ml) | ||||
| sec, sep | - | - | - | + |
| sek, seq | + | + | - | - |
| Exfoliative toxins | ||||
| eta, etb, etd | - | - | - | - |
| Others | ||||
| ssl | + | + | + | + |
| edin | - | - | - | - |
| Adhesins | ||||
| c12ag c | + | + | + | + |
| cna | + | + | - | + |
| bbp | - | - | - | - |
| ACME (arcA) | - | - | - | - |
| Resistance | ||||
| β-lactam | ||||
| Imipenem (MIC, μg/ml) | 16–64 | 32 | 0.125–0.5 | 0.5 |
| Oxacillin (MIC, μg/ml) | 128- ≥256 | 128 | 32–64 | 64 |
| Ampicillin (MIC, μg/ml) | 32–64 (18/18) | 32 (2/2) | 4–8 (7/10) | 8 |
| 32 (3/10) d | ||||
| Non β-lactam | ||||
| Aminoglycosides | Gm (17/18) | Gm (3/10) d | ||
| Km (18/18) | Km (2/2) | Km (3/10) d | ||
| Tetracyclines | Tc (18/18) | Tc (2/2) | ||
| Macrolides | Em (18/18) | Em (2/2) | Em (2/10) d | |
| Lincosamides | Cli (18/18) | Cli (2/2) | Cli (2/10) d | |
| Quinolones | Lvx (18/18) | Lvx (10/10) | ||
| Rifampicin | Rif (18/18) | |||
| Chloramphenicol | Cp (14/18) d | Cp (9/10) d | Cp d | |
| Sulfamethoxazole | Su (18/18) | Su (2/2) | ||
| Plasmids (kb) | 2.9 (14/18) | ≥25 (3/10), | 4.5 | |
| 4.6 (1/10), | ||||
| 4.5 (3/10), | ||||
| 3.9(1/10), | ||||
| 2.9 (7/10), | ||||
| 2.5 (1/10), | ||||
| 2.4(1/10) | ||||
aGm, gentamicin; Km, kanamycin; Tc, tetracycline; Em, erythromycin; Cli, clindamycin; Lvx, levofloxacin; Rif, rifampicin; Cp, chloramphenicol; Su, sulfamethoxazole; UT, untypeable.
bSplit hlb gene due to insertion of φSa3.
c c12ag, core 12 adhesin genes, icaA, icaD, eno, fnbA, fnbB, ebpS, clfA, clfB, fib, sdrC, sdrD, and sdrE.
dResistance specified by a plasmid.
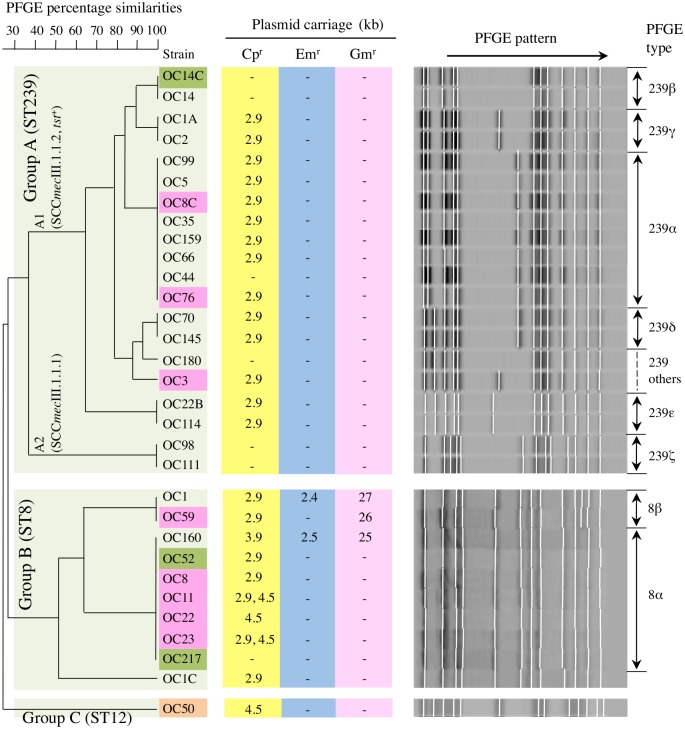
Fig 1. Pulsed-field gel electrophoresis (PFGE) analysis (right and left) and plasmid carriage patterns (center) of MRSA strains isolated in Krasnoyarsk.
The MRSA strains shown are those described in Table 2. Group A (A1 and A2) and group B are described in Table 3. The color of the strain name indicates fatal pneumonia (red), possible sepsis (brown), and carrier cases (green). Of the cases of fatal pneumonia, OC3, OC8C, OC11, OC76 were from hospital-acquired pneumonia (HAP), while OC8, OC22, OC23, and OC59 were from community-acquired pneumonia (CAP). Of the carrier cases, OC14C and OC52 were from hospital workers and OC217 was from a student.
Group A (n = 20) was the ST239 lineage with two types, ST239/spa3(t037)/SCCmecIII.1.1.2 (group A1; 90%, 18/20) and ST239/spa3(t037)/SCCmecIII.1.1.1 (group A2; 10%, 2/20). All strains in group A1 were tst +; TSST-1 production levels were similar to those (50–800 ng/ml; average, 313 ng/ml) of the ST5/SCCmecII NY/J clone. They were also highly MDR, including levofloxacin (Lvx) and Rif, and many (77.8%, 14/18) carried a Cpr plasmid (pCpr). In the PFGE analysis (Fig 1), group A1 comprised several divergent subclusters with a major cluster (type 239α; 44.4%, 8/18). Group A1 MRSA was designated as ST239Kras. Three ST239Kras strains (OC3, OC8C, and OC76) caused fatal HAP, and one strain (OC14C) was from a carrier (hospital worker).
The strains in group A2 were sea + (Table 3). Their PFGE patterns were divergent from group A1 (Fig 1). Group A2 was a common ST239 HA-MRSA in Russia, whereas group A2 showed a slightly narrower MDR spectrum (Table 3). Group A (A1 and A2) exhibited a high level of resistance to imipenem and oxacillin (Table 3), which is consistent with the characteristics of HA-MRSA [31].
Group B (n = 10) was the ST8 lineage with the type spa1(t008)/SCCmecIV.3.1.1(IVc) (Table 3). Most strains (80%, 8/10) were sea +, and produced SEA at high levels (Table 3). All strains shared very similar PFGE patterns, with patterns of 8α (major type; n = 7) and 8β (n = 2) and with no more than a three-band difference, indicating the same clone (designated as ST8Kras) (Fig 1). ST8Kras (group B) exhibited a low level of resistance to imipenem and oxacillin (Table 3), in agreement with the characteristics of CA-MRSA [31]; however, all strains were Lvxr and most strains (90%, 9/10) carried a Cpr plasmid.
Four ST8Kras strains (OC8, OC22, OC23, and OC59) caused fatal CAP. One ST8Kras strain (OC11) caused fatal HAP, suggesting that ST8Kras even spread in hospitals. The ST8Kras strains associated with fatal pneumonia were all sea +. Two ST8Kras strains were isolated from carriers; OC217 was from a student while OC52 was from a hospital worker.
Group C (n = 1, strain OC50) exhibited ST12/spaNew(t156)/untypable SCCmec, showed low imipenem and oxacillin resistance levels, and carried a Cpr plasmid.
Regarding drug resistance (Table 3), all Lvxr ST8 and ST239 carried gyrA (Ser84Leu) and grlA (Ser80Phe) mutations, and manifested minimum inhibitory concentrations (MICs) of 4–16 μg/ml. Rifr ST239Kras strains carried rpoB (His481Asn, Ile527Met) mutations, with MICs of ≥256 μg/ml. All Cpr strains carried the cat gene, with MICs of 64 μg/ml. All strains were susceptible to trimethoprim, fusidic acid, vancomycin, teicoplanin, linezolid, and mupirocin.
Distribution and transfer of drug resistance plasmids
The plasmid data of Krasnoyarsk MRSA are summarized in Table 3, Fig 1, S1 and S2 Figs. Many MRSA strains carried only a small pCpr, and the same 2.9-kb pCpr was present in 14 (77.8%) out of 18 ST239Kras strains (group A1) and in seven (70%) out of 10 ST8Kras (group B) strains (Fig 1). This 2.9-kb pCpr was 99.9% homologous to the 2.9-kb pCpr of emerging ST239 MRSA (spa351[t030]/SCCmecIII.1.1.4) from Vladivostok (S1 and S2A-a Figs). One ST8Kras strain carried a 3.9-kb pCpr (Fig 1, S1 and S2A-b Figs). Furthermore, three ST8Kras strains carried the new mosaic 4.5-kb pCpr (Fig 1, S1 and S2A-c Figs); two of these strains carried two distinct species (2.9- and 4.5-kb) of pCpr (Fig 1 and S1 Fig).
Three ST8Kras strains carried large (≥25-kb) antibiotic and antiseptic resistance plasmids (alternatively defined as a penicillinase plasmid, pPCase), in addition to pCpr (Fig 1 and S1 Fig); two of the three ST8Kras strains also carried a small (2.4- or 2.5-kb) pEMr (Fig 1, S1 and S2B Figs).
All plasmids were transferred to S. aureus RN2677 (recipient) in the bacterial mixed culture at frequencies ranging from 10-5 to 10-7 (S1D Fig). Tn554, carrying the ermA, spc genes, of ST239Kras strains was also transferred to RN2677 (S1D Fig), most likely through a Tn554 circular intermediate (S1A and S1C Fig). Of these, the 2.9-kb pCpr exhibited superior transfer frequencies over transmissible (Tra+) pPCase (S1D Fig).
Comparative genomics of ST239Kras (strain OC3)
The OC3 genome was estimated to be at least 2.93-Mb in size, with a 2,908-bp pCpr (pOC3). A total of 2.91-Mb (approximately 99.3% of the determined genome sequences) was mapped on the TW20 genome (Fig 2).
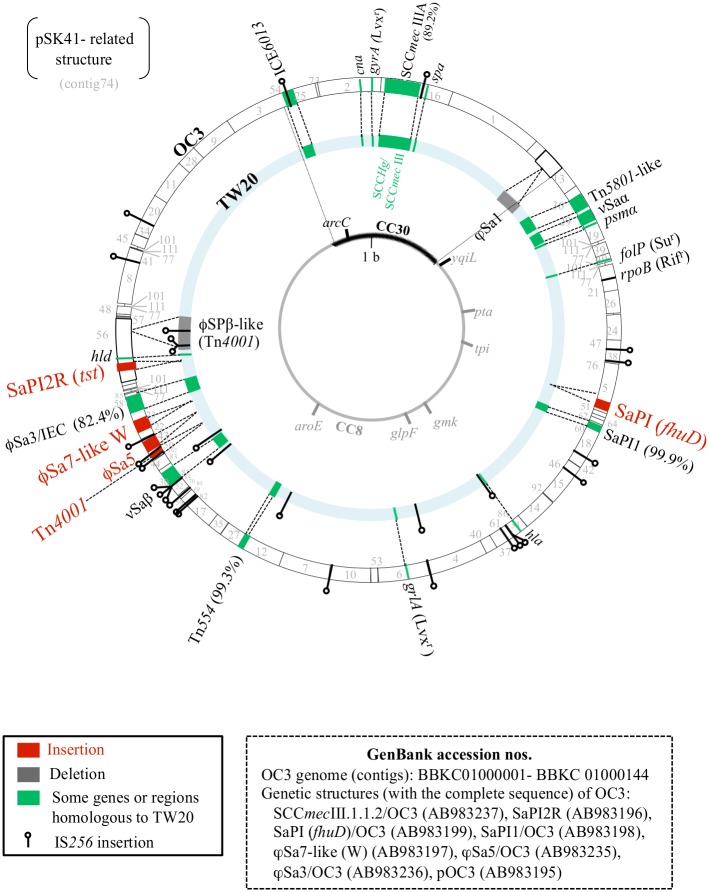
Fig 2. Genome information for the ST239Kras strain OC3, in comparison with the ST239 MRSA strain TW20.
The ST239Kras OC3 genome contigs (including filled contigs and complete structures; total 2.91-Mb) were mapped on the 3,043,210-bp TW20 genome (GenBank accession number FN433596); in the figure, the two genome structures were drawn as two circles on a common genome map, outside OC3 and inside TW20. Genome information included staphylococcal cassette chromosome mec (SCCmec), other drug resistance structures (such as a transposon, Tn, plasmid-related structure, and gene mutations), characteristic virulence genes, phages, S. aureus pathogenicity islands (SaPIs), genomic islands (νSa), and characteristic insertion sequences (ISs). SCCmec: SCCmecIIIA (in OC3), SCCmecIII.1.1.2; SCCmecIII (in TW20), SCCmecIII.1.1.1 connected to SCCHg. Drug resistance (gene mutations): Lvxr, levofloxacin resistance; Rifr, rifampicin resistance; Sur, sulfamethoxazole resistance. Virulence genes (region): tst, toxic shock syndrome toxin-1 gene; hld, δ-hemolysin gene; cna, collagen adhesin gene; spa, protein A gene; psmα, phenol-soluble modulin (PSM) gene; hla, α-hemolysin (α-toxin) gene; IEC, immune evasion cluster. The CC30 and CC8 genome sections are from Holden et al. [19], and the genetic element IEC6013 is from [60]. The plasmid pOC3 (2,908 bp; contig 75) of strain OC3 is not shown in the figure. The location of pSK41-related structure (with two IS431 repeats at both ends) currently remains uncertain.
The OC3 genome most likely consisted of two (ST30-like and ST8-like) sections, similar to TW20[19,60]. On the ST30-like section, OC3 carried the collagen-adhesin (Cna) gene (cna), IEC6013 (with the Tn552 insertion), and the spa gene (type3-t037) similar to TW20; however, OC3 lacked phage φSa1, and SCCmec was divergent (SCCmecIII.1.1.2 for OC3 vs. SCCmecIII.1.1.1 for TW20).
On the ST8-like section, there were five characteristic insertions in the OC3 genome: i) a tst + S. aureus pathogenicity island (SaPI2R), ii) a completely unique phage (designated as φSa7-like W), iii) a phage, φSa5, iv) a transposon, Tn4001, and v) a SaPI carrying the ferrichrome ABC transporter homologue gene (fhuD) (designated as SaPI fhuD). There were also two characteristic deletions in the OC3 genome: i) a phage, φSPβ-like (carrying the sasX gene and Tn4001) and ii) the dfrG gene in Tn5801-like, resulting in a trimethoprim-susceptible phenotype.
A large number of copies of the insertion sequence IS256 (≥22/genome) were present in OC3, while TW20 only had eight copies. Regarding the MDR of OC3, the 15 genes identified were: i) nine drug resistance genes: mecA on SCCmecIII.1.1.2, ermA and spc on Tn554, blaZ on Tn552/ICE6013, aacA-aphD on Tn4001, tetM on Tn5801-like, cat on pOC3, and ble and aadD on a pSK41-related structure; ii) four drug resistance mutations: gyrA (S84L) and grlA (S80F) for Lvxr [61], rpoB (H481N, I527M) for Rifr [62], and folP (F17L, V30I, T31N, M37I, I58V, T59S, V60L, L64M, I101M, V117I, V126I; these replacements caused MIC of ≥512 μg/ml and corresponded to 11 out of 13 replacements in strain V2157I [63]) for sulfamethoxazole resistance; and iii) two heavy metal resistance genes: mer and cadA on SCCmecIII.1.1.2.
SCCmecIII.1.1.2
SCCmecIII.1.1.2 (OC3) was 61,780 bp in size, with 15-bp att direct-repeat sequences (attL, attR). Its structure was identical to that of reference strain HU25 of the Brazilian clone, with ancestral SCCmecIIIA, however, markedly distinct from TW20, which had the two-SCC cassette array SCCHg-SCCmecIII.1.1.1 (35,310 bp), as shown in Fig 3A.
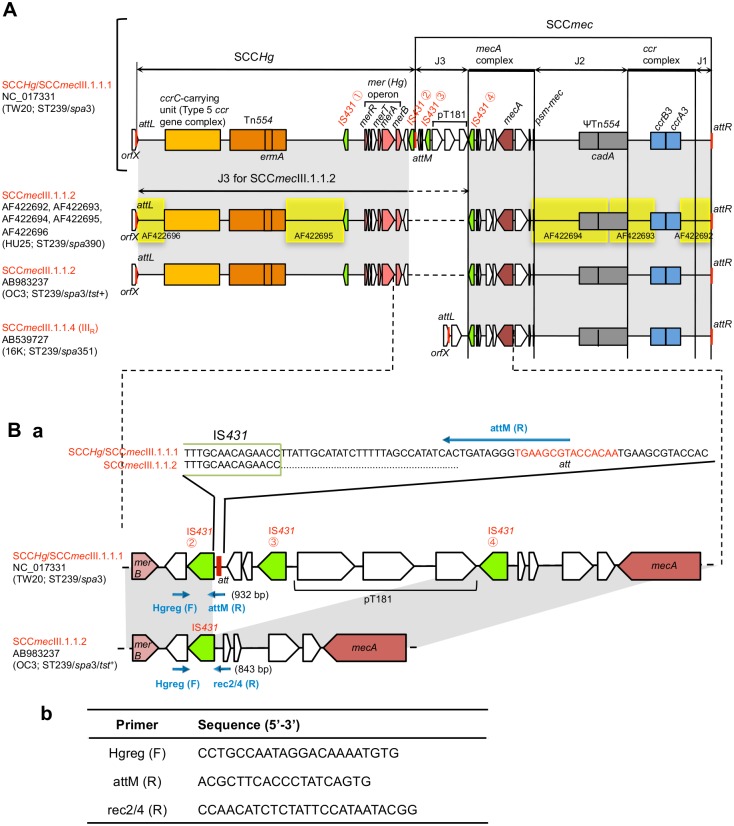
Fig 3. SCCmecIII.1.1.2 structure of the ST239Kras strain OC3, in comparison with the three structures: SCCHg/SCCmecIII.1.1.1 of the strain TW20, SCCmecIII.1.1.2 of the strain HU25, and SCCmecIII.1.1.4 of the strain 16K.
Isolation of ST239 strains: OC3, Krasnoyarsk; TW20, London; HU25, Brazil; 16K, Vladivostok. Homologous regions are shaded in each comparison. In A, when compared with SCCHg/SCCmecIII.1.1.1 (of TW20), SCCmecIII.1.1.2 (of OC3 and HU25) lacked the middle IS431②-IS431④ region. The J3 region of SCCmecIII.1.1.2 corresponded to the bulk of SCCHg. SCCmecIII.1.1.4 (of 16K) lacked SCCHg. In B, the primer set Hgreg (F)/attM (R) detected attM, and the primer set Hgreg (F)/rec2/4 (R) identified recombination between IS431 copies ② and ④.
SCCHg-SCCmecIII.1.1.1 (TW20) had four copies of IS431 (① to ④) in direct orientation at the boundary region of two SCCs, suggesting a recombination between two IS431 copies (② and ④) for SCCmecIII.1.1.2 conversion; the J3 region of SCCmecIII.1.1.2 (OC3) and SCCmecIII.1.1.1 (TW20) was, thus, divergent: 33,011 bp vs. 6,266 bp.
To confirm that all ST239Kras strains had the same IS431 recombination type, the PCR primer sets, Hgreg (F)/attM (R) (to detect attM) and Hgreg (F)/rec2/4 (R) (to identify recombination between IS431 copies ② and ④), were designed (Fig 3B). The results of the PCR assays clearly demonstrated that all ST239Kras strains had the SCCmecIII.1.1.2 (IIIA) structure of the Brazilian clone. In Russia (Krasnoyarsk and Vladivostok), SCCmecIII.1.1.4 and SCCmecIII.1.1.1 had no SCCHg linkage, as shown in Fig 3A.
ϕSa7-like (W)
φSa7-like (W) of OC3 was 42,359 bp in size and inserted into the huNaDC-1 gene. As shown in Fig 4, the integrase gene showed high similarity (mostly 100%) to the phage 7 (φSa7) integrase gene of the following strains: NM2 of the strain Newman (Fig 4), ST8 strains (GenBank accession numbers, AP009351.1 and CP007499.1), ST30 strain (GenBank accession number, LN626917.1), ST133 strain (GenBank accession number, CP001996.1), and ST239 strains (GenBank accession numbers, CP005288.1, CP006838.1, CP009681.1), indicating that φSa7-like (W) is a φSa7 family member. φSa7-like (W) had the φSa7 att-like 9-bp sequence on the left-side end.
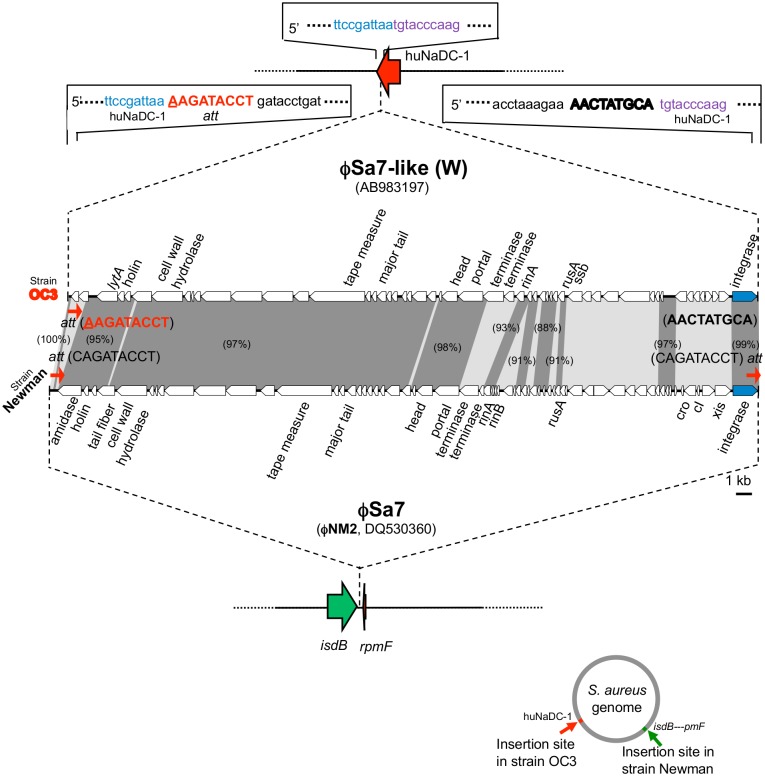
Fig 4. Structure of a phage φSa7-like (W) of the ST239Kras strain OC3.
φSa7-like (W) was compared with φSa7 of the strain Newman for the phage structure, the integration site (att) sequence, and integration site. Homologous regions are shaded in the comparison. The figure at the lower right side indicates each integration site on the S. aureus chromosome. The target huNaDC-1 sequence of φSa7-like (W), shown at the top of the figure (in blue and purple), was also present in the huNaDC-1 gene of other S. aureus strains.
However, the 9-bp right-side sequence of φSa7-like (W) was divergent; there was no φSa7 att on the right side (Fig 4). Moreover, the insertion site (huNaDC-1 gene) of φSa7-like (W) was divergent from that of φSa7, which was generally inserted into the intercistronic region between the isdB and rpmF genes (Fig 4, the figure on the lower right side). φSa7-like (W) only showed 66% overall homology to φSa7 (NM2).
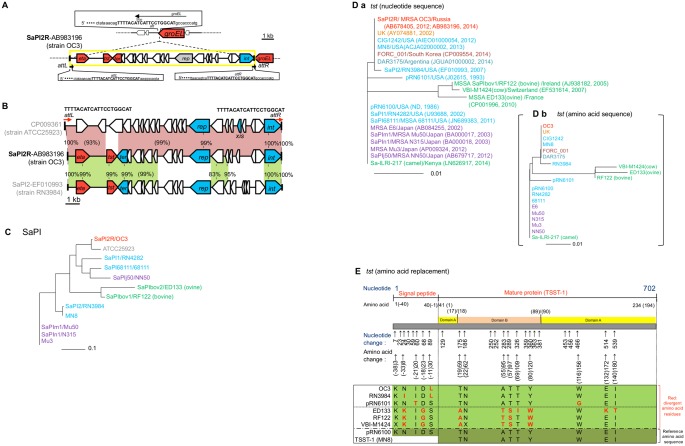
SaPI2R carrying tst
SaPI2R (OC3) was 14,819 bp in size, flanked by directly repeated 20-bp att sequences (attL and attR), and inserted into the groEL gene (Fig 5A). SaPI2R exhibited high homology (91%) to the tst - SaPI2 of strain ATCC25923, and the 6,810-bp left-side tst + region of SaPI2R showed high homology (99%) to that of tst + SaPI2 (strain RN3984) (Fig 5B). Although SaPI2R was closely related to tst - SaPI2 (ATCC25923), SaPI2R markedly diverged from other tst + SaPIs (Fig 5C).
Fig 5. Analysis of the tst + SaPI (SaPI2R) structure and tst nucleotide and deduced amino acid sequences of the ST239Kras strain OC3.
In A, the integration site (att) and att sequences of SaPI2R of the ST239Kras strain OC3 are shown. In B, the SaPI2R structure was compared with those of tst - SaPI (ATCC25923) and tst + SaPI2 (RN3984). Homologous regions between SaPI structures are shaded with color. Genes: tst, toxic shock syndrome toxin-1 gene; eta, S. hyicus exfoliatin A gene; ter, terminase gene (which cleaves multimeric DNA); rep, replication initiator gene; int, the integrase gene. In C, the nucleotide sequences of tst + SaPIs and tst - SaPI (ATCC25923) were analyzed for phylogenetic diversity. In D-a, the nucleotide sequences of the tst genes were analyzed for phylogenetic diversity. In this figure, each GenBank record year is also shown. In D-b, the deduced amino acid sequences of the tst gene products were analyzed for phylogenetic diversity. The origin (reported source) of each isolate is indicated by the color of the isolate name: red, Russia; yellow, United Kingdom (UK); blue, United States (USA); dark red, Korea; light blue, Argentine; purple, Japan; green, those for animal isolates. In C and D, the scale bar represents substitutions per single-nucleotide polymorphism site. In E, the representative tst gene sequences were compared with the reference sequences (of pRN6100). Arrows indicate the positions of the nucleotide and amino acid changes for the representative tst genes. At the bottom of the figure (green), different amino acids from the amino acid sequences of purified TSST-1 (MN8; GenBank accession number EFH95768) and the deduced amino acid sequence of the tst gene (pRN6100) are indicated in red letters.
In the tst gene sequence comparison (Fig 5D-a), seven clusters were detected: i) cluster consisting of tst from Russia (OC3), UK, USA, South Korea, and Argentine; ii) tst cluster from USA (RN3984); iii) tst cluster from USA (pRN6101); iv) cluster consisting of tst from USA (including pRN6100), Japan (ST5/SCCmecII HA-MRSA, NY/J clone; and ST8/SCCmecIVl CA-MRSA, ST8 CA-MRSA/J clone), and Kenya (camel strain); and v-vii) three tst clusters from Ireland, Switzerland, and France (cow/bovine and ovine strains). The analysis at the deduced amino acid sequence levels produced very similar results (Fig 5D-b).
Russian (OC3) and UK TSST-1 precursors shared the same amino acid sequence with the same one amino acid replacement in the signal peptide region (S-11L; S→L at position -11), when compared with purified TSST-1 protein (MN8) or the precursor protein, deduced from the first USA tst gene (pRN6100) (Fig 5E). Regarding the tst genes from clinical isolates, amino acid replacements in the mature toxin (TSST-1) region were very rare, in contrast to the tst genes from animal isolates with distinct host specificity (Fig 5E). We were unable to determine why the tst gene from camel (Kenya) had the clinical type sequence.
SaPI1 carrying sek and seq
SaPI1 (OC3) was 14,577 bp in size (with 17-bp att at both ends), carried the superantigen (SE) genes (sek and seq), and was inserted into a non-coding region. SaPI1 (OC3) was highly homologous (99.9%) to SaPI1 (TW20) (S3A Fig). The SEK and SEQ amino acid sequences were the same between OC3 and TW20, with the unique amino acid replacement F119L (S3B-a Fig) and two unique amino acid replacements D194N and T201A (which corresponded to two out of seven replacements in USA300 SaPI5) (S3B-b Fig), respectively.
ϕSa3 carrying immune evasion genes
φSa3 (OC3) was 43,681 bp in size (with 13-bp att at both ends) and was inserted into the hlb gene. φSa3 (OC3) showed 76% homology to that of strain CN1, sharing the same att and same integration site (S4 Fig). φSa3 (OC3) had the immune evasion cluster (IEC) on the left-end side, with the immune evasion genes sak (for staphylokinase, SAK) and scn (for staphylococcal complement inhibitor, SCIN), but lacked chp (for chemotaxis inhibitory protein of S. aureus, CHIPS) present in CN1 (S4 Fig). The IEC region of TW20 and ST8Kras strain OC8 showed 99.3% homology and carried sea, in addition to sak, and scn (S4 Fig).
Other relevant genetic structures
Tn4001 (OC3), flanked by two IS256, was 6,483 bp in size and inserted into the noncoding region, located downstream of the ThiJ/PfpI family protein gene (Fig 2); in TW20, Tn4001 was present within φSPβ-like.
The pSK41-related resistance structure, flanked by two IS431, was 4,039 bp in size and carried the two drug resistance genes ble and aadD (Fig 2); its location on the genome currently remains unknown.
SaPI (fhuD) was 15,756 bp in size and inserted into the noncoding region, located downstream of the SsrA-binding protein gene. It showed only 69% overall homology to fhuD + SaPIm4 from the NY/J clone (Mu50). The 10.2-kb left-side half exhibited high homology (95%) to fhuD - SaPIj50 from Japanese ST8/SCCmecIVl CA-MRSA, suggesting that SaPI (fhuD) is a new mosaic SaPI (S5 Fig).
φSa5 (OC3), with 10-bp att at both ends, was 44,424 bp in size and inserted into the hypothetical gene (for protein AGY89988.1), located downstream of the ThiJ/PfpI family protein gene. It was the most similar to φSa5 (XN108), albeit with only 63% homology (S6 Fig); φSa5 (OC3) is a new mosaic phage.
Elevated mRNA expression of cytolytic peptide genes in ST239Kras
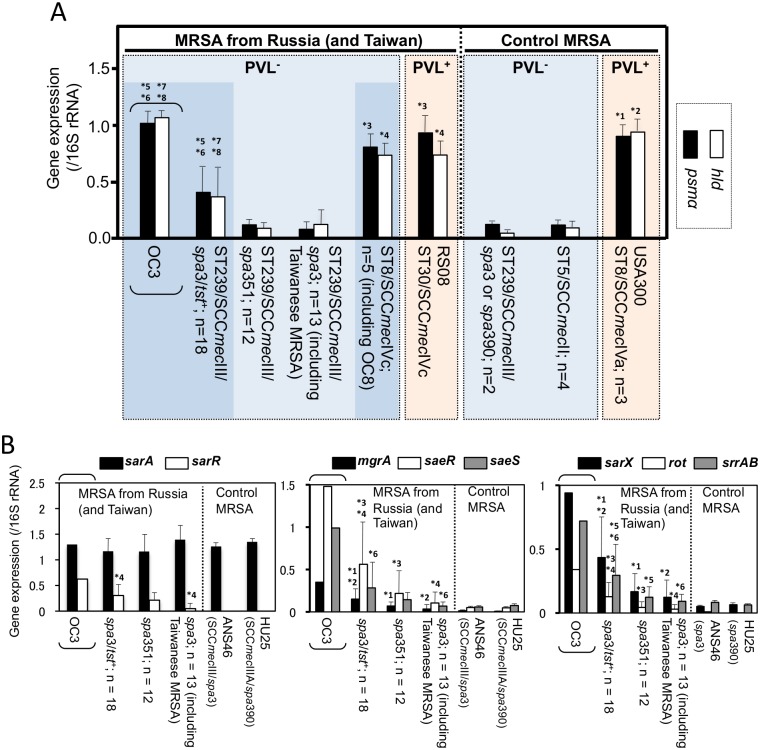
ST239Kras, including strain OC3, expressed the psmα and hld genes at high levels, similar to CA-MRSA USA300 and Russian CA-MRSA (RS08 and ST8Kras), but significantly higher than HA-MRSA, ST5/SCCmecII (the NY/J clone) and other ST239/SCCmecIII, including reference strains HU25 and ANS46 (P <0.05), as shown in Fig 6A.
Fig 6. mRNA expression levels of cytolytic peptide genes (psmα and hld) in ST239Kras and ST8Kras strains (A) and of regulatory genes in ST239Kras strains (B), in comparison with CA- and HA-MRSA reference strains and other ST239 MRSA strains.
In A, in the right-side control MRSA box, CA-MRSA, which shows high expression levels, is marked in red; and HA-MRSA, which shows low expression levels, is marked in light blue. In the left-side MRSA box, CA-MRSA (strain RS08), which showed high expression levels as expected, is marked in red; CA-MRSA ST8Kras (ST8/SCCmecIVc) strains, including OC8, also showed high expression levels (dark blue box on the right side). ST239 HA-MRSA strains, which showed low expression levels as expected, are marked in light blue. However, ST239Kras (ST239/SCCmecIII/spa3/tst +), including OC3, unexpectedly showed high expression levels, similar to CA-MRSA; this box is marked in dark blue on the left side. Regarding psmα: *1, P < 0.05 vs. control ST239/SCCmecIII and ST5/SCCmecII; *3, P < 0.05 vs. control ST239/SCCmecIII and ST5/SCCmecII; *5, P < 0.05 vs. control ST239/SCCmecIII and ST5/SCCmecII; *6, P < 0.05 vs. Russian ST239/SCCmecIII/spa3 and spa351. Regarding hld: *2, P < 0.05 vs. control ST239/SCCmecIII and ST5/SCCmecII; *4, P < 0.05 vs. control ST239/SCCmecIII and ST5/SCCmecII; *7, P < 0.05 vs. control ST239/SCCmecIII and ST5/SCCmecII; *8, P < 0.05 vs. Russian ST239/SCCmecIII/spa3 and spa351. In B, the psmα expression levels of ST239 MRSA strains were examined. spa3/tst +, ST239Kras. No significant difference was observed between ST239Kras and other ST239 MRSA for sarA gene expression. *1, P < 0.05 vs. spa351; *2, P < 0.05 vs. spa3; *3, P < 0.05 vs. spa351; *4, P < 0.05 vs. spa3; *5, P < 0.05 vs. spa351; *6, P < 0.05 vs. spa3.
Regarding the expression levels of transcriptional regulatory genes (Fig 6B), although no significant difference was observed among the ST239 strains for sarA, ST239Kras, including OC3, showed higher levels of expression for sarR, mgrA, saeR, saeS, sarX, rot, and srrAB (P <0.05), compared with the other ST239 strains, including reference strains HU25 and ASN46.
Comparison of ST239Kras and ST239 MRSA from other regions of Russia
The emerging HA-MRSA with the genotype ST239/spa351(t030)/SCCmecIII.1.1.4 was detected in the European region (Moscow and St. Petersburg), Ural region (Kurgan), and Far Eastern region (Vladivostok) (S1 Table), except for the Siberian region (Krasnoyarsk) (Table 3). Its spa variant, spaNew(t632), was distributed to the European region (St. Petersburg). ST239Kras was only distributed to the Siberian region (Krasnoyarsk).
Three major divergent clusters were detected in the PFGE analysis (S7 Fig): a large spa351/SCCmecIII.1.1.4 cluster, associated with the Far Eastern region; a large spa351-New(t632)/SCCmecIII.1.1.4 cluster, mainly associated with the European Russia/Ural mountain region (and also the Far Eastern region); and a large spa3/SCCmecIII.1.1.1-III.1.1.2 cluster, associated with the Siberian region and Far Eastern region. The emerging ST239/spa351(t030)-spaNew(t632)/SCCmecIII.1.1.4 type appeared to be slightly divergent between the European/Ural region and Far Eastern region. ST239Kras was divergent from those emerging types, and comprised spa3 subclusters with reference strains ASN46 and HU25.
Discussion
Regarding MRSA epidemiology in Russia, the incidence of MRSA (among S. aureus isolates) was 0–89.5% (average, 33.5%) in 2000 [64], 18% in 2004 [65], 54.4% in 2006–2008 [66], 32.1% in 2007 and 16.6% in 2012 [67]. The dominant MRSA types in Russia are ST239/spa3(t037)/SCCmecIII, followed by ST239/spa351(t030)/SCCmecIII and ST8/spa1 (t008)/SCCmecIV [49]. Russia is geographically classified into three major regions: European, Siberian, and Far Eastern. The present study described for the first time MRSA and its invasive infection in the Siberian region. In Krasnoyarsk (2007–2011), the incidence of HA-MRSA was at similar levels, while the incidence of CA-MRSA was a little bit lower.
However, regarding MRSA types, the most prevalent HA-MRSA was ST239Kras, a novel regional variant of the ST239 lineage (S2 Table, S8 Fig), in contrast to the European (Moscow and St. Petersburg), Ural (Kurgan), and Far Eastern (Vladivostok) regions, where the emerging ST239/spa351(t030)/SCCmecIII.1.1.4 type has recently become common [52]. ST239Kras is highly-virulent HA-MRSA with fatal HAP cases (with bacteremia) being reported. The ages of patients with fatal HAP were consistent with previous HA-MRSA data [34]. The genetic divergence in PFGE patterns strongly suggested that ST239Kras infections persisted and spread among patients and carriers (hospital workers) at least since 2007.
Regarding the ST8 lineage, a single unique MDR MRSA clone (ST8Kras) with very similar PFGE patterns had persisted and spread. The spa type (spa1-t008) was the same as previous Russian ST8 [49], but divergent from that (spa826; t, unknown) of Vladivostok ST8 [52]. ST8Kras was Lvxr with the same mutations as HA-MRSA ST239Kras. ST8Kras was a successful CA-MRSA, with not only fatal CAP, but also fatal HAP cases (with bacteremia). The fatal CAP cases included one infant and one young child death, consistent with previous CA-MRSA infections [34]. ST8Kras carriers were also identified, suggesting its potential to be become widespread.
PVL+ CA-MRSA, such as ST8 USA300 [12,23,68], has become a major public concern [3,5,12, 23,24,34,68,69]; however, MRSA invasive infections occurred regardless of PVL+ or PVL- [70–72]. In Russia, MRSA has mostly been PVL- [49,65,67], with only two PVL+ cases [50,73]. In Krasnoyarsk, all MRSA were PVL-, albeit with PVL+ methicillin-susceptible S. aureus cases (at around 10%) associated with pyogenic skin infections (such as furuncles).
In the present study, we also focused onto the MVFs of MRSA. The hyper virulence of CA-MRSA USA300 has been attributed to MVFs, such as PVL, ACME-related factors, α-hemolysin (Hla), the elevated production of PSMs, and SEK (sek2) and SEQ (seq2) [37,68].
Regarding ST239Kras, a unique set of MVFs included TSST-1, the elevated expression of PSMα/Hld, Hla, SEK/SEQ, SCIN/SAK, and Cna. Of those factors, TSST-1 has been associated with toxic shock syndrome (TSS) [74–76] and neonatal TSS-like exanthematous disease [77] through a cytokine storm [78–82], is associated with invasive endocarditis [83], and is an immune evasion factor [84]. In Japan, major HA-MRSA and CA-MRSA are both tst +, and associated with invasive infections, including pneumonia and bacteremia [21,71,85].
ST239Kras strongly expressed PSMα (and Hld), a common characteristic of CA-MRSA [12,40], which is cytolytic against human cells [40] and possibly associated with bacteremia and abscesses [12] as well as the establishment of an MRSA niche [86]. Community infection (including necrotizing pneumonia) or colonization from HA-MRSA that strongly expresses PSMα/Hld includes ST5/SCCmecII (NY/J) [72] and ST764/SCCmecII cases [56].
In ST239Kras, some transcriptional regulatory genes, except for sarA [87,88], were also up-regulated. They included transcriptional-positive regulators such as mgrA [89], saeR/S [89], and sarX [90]; and transcriptional-negative regulators such as sarR [87,88], rot [43], and srrAB [91]. Super IS256 copies in ST239Kras may be responsible for these transcriptional regulations, as has been reported with S. epidermidis [92] or rot [43]. IS256 also contributes to Tn4001 [93].
The acquisition of sek and seq with synonymous substitutions (sek2, seq2) may partly explain the hyper virulence of USA300 [37]. ST239Kras (OC3) shared the same (unique) SEK and SEQ sequences with TW20, which were distinct from those of USA300.
Immune evasion factor genes are generally clustered in the IEC locus in φSa3 [44,94], but are often detected in φSa7 [94] or νSaβ [29]. ST239Kras carried two those genes, sak and scn, in IEC, while USA300 carried three genes sak, chp, and scn (GenBank accession no. CP000255). TW20 and ST8Kras (OC8) carried a distinct set of three genes, sak, scn, and sea.
Cna is a cell wall-associated adhesin [95,96], and associated with pneumonia [97] and bullous impetigo [98]. Cna is also a potential immune evasion factor [99]. Taken together, ST239Kras’ MVFs include professional factors for adherence, immune evasion, and specific lesions and symptoms.
ST8Kras possessed a distinct set of MVFs, which included SEA, the strong expression of PSMα/Hld, SAK/SCIN, and Hla. Of these factors, SEA is associated with the severity of infections (sepsis and shock) [100] and promotes bacterial survival in vivo [101]. ST8Kras has attracted attention because of its high mortality rate for MRSA CAP, including pediatric deaths. The whole genome of ST8Kras is now being investigated to further characterize ST8Kras’ MVFs.
Discussion on the evolution of MRSA is also the important points of the present research. Regarding the ST239/SCCmecIII lineage, this global HA-MRSA [10,20,49,52,102–113,114]) consists of more than five MRSA clades [20]. Historically, the Brazilian clone carried “SCCmecIIIA” [9,15,20], while the Hungarian clone carried “SCCmecIII” [17]. “SCCmecIIIA” is now one large fused SCC (SCCmecIII.1.1.2), derived from two SCC-linked “SCCmecIII” through IS431-recombination [8,9,115–117]. ST239Kras had the same IS431-recombination type as that of the Brazilian clone. In Russia, no SCCHg-SCCmecIII link was present [52].
Moreover, ST239Kras carried the tst gene on SaPI [42,118], for the first time in the ST239 lineage. The same tst gene was present in the United Kingdom before the isolation of ST239Kras, suggesting the potential salvage of tst in Europe. ST239Kras also carried a completely unique, domestic phage, φSa7-like (W). Phages [19,20,44,119–121] are a possible tool for S. aureus diversification, and classified according to the integrase gene types [120]. φSa7-like (W) was classified as integrase type 7 (Sa7int); however, φSa7-like (W) had no repeats of the terminal att sequence, similar to Tn554 [52,122–124], and had a unique insertion site distinct from φSa7. In addition, ST239Kras exhibited the characteristics of CA-MRSA, i.e., the strong expression of the cytolytic peptide gene (as described above).
Regarding ST239 MRSA transmission, the Brazilian clone spread to Portugal [9,46], Central Europe (Germany, Poland, and Czech Republic), Northern Europe (Finland) [17], and Eastern Europe/West Asia (Georgia) [125]. Krasnoyarsk has had a historically close relationship to the European region (St. Petersburg and Moscow). The (Southeast) Asian clade, including London strain TW20, which was likely transmitted from Southeast Asia [2,20], carried characteristic φSPβ-like (S2 Table) [19,20,126–128], while ST239Kras lacked φSPβ-like. Based on these findings and our results, we herein proposed a new Russian clade (representative strain, OC3) in the ST239/SCCmecIII lineage, and also speculated that ST239Kras originated in the Brazilian clone, with the possible transmission route of Brazil-Europe (West-Central-North/East)-Russia (European-Siberian) (S9 Fig). Further genome-level analysis is needed for the understanding of evolution.
The plasmid distribution in Krasnoyarsk was unique. Many MRSA only carried pCpr and often carried two pCpr species, in contrast to some other country’s cases with no pCpr [19,23,28,29,71,129,130], or Vladivostok’s cases with multiple plasmids [52]. In Russia, inexpensive Cp is commonly administered to patients without a doctor’s prescription as an ointment for skin injuries or burns, as a tablet for gastroenteritis, and as an eye lotion, providing MRSA with strong pressure to carry a pCpr. A small (2.9-kb) pCpr must be transferred, even in nature, possibly through the rolling circle (RC) manner of replication [41,131,132], similar to the replication that occurs during the conjugation of large Tra+ plasmids [1,41,132–134]. pEMr [135] and Tn554, with a circular intermediate [52,136], may follow pCpr-like transfer.
In conclusion, we identified novel regional variants of the ST239 and ST8 lineages (ST239Kras and ST8Kras), in Siberian Russia (Krasnoyarsk), in which international research had never previously focused on MRSA and its invasive infections. ST239Kras and ST8Kras were MDR and had clonally (albeit with divergence) and widely spread, with fatal cases of HAP and CAP with bacteremia. The 15-day mortality rate for MRSA CAP was significantly higher than that for MRSA HAP, and fatal cases of ST8Kras CAP included infant and young child deaths. According to the recent accumulation of information showing that successful MRSA, associated with large epidemics, has a unique set of MVFs, we speculated that fatal cases of ST239Kras HAP were caused by the unique combination of TSST-1, the strong expression of PSMα/Hld, Hla, SEK/SEQ, SAK/SCIN, and Cna, while fatal cases of ST8Kras CAP were attributed to the combination of SEA, the strong expression of PSMα/Hld, Hla, and SAK/SCIN. ST239Kras carried a completely unique phage and mobile DNA, and exhibited unique virulence phenotypes; therefore, ST239Kras represented a new (Siberian Russian) clade of the ST239 lineage, which was created through regional stepwise evolution during its possible Brazil-Europe-Russia transmission. Small resistance plasmids spread widely enough to not be ignored and in a unique manner among MRSA.
Supporting Information
In A; RN, RN2677 (recipient). Covalently closed circular (CCC) plasmid DNA, isolated from MRSA and transconjugants (RN2677 carrying plasmids), was electrophoresed in 1% agarose. Plasmid sizes were determined using reference plasmids with known molecular sizes. Plasmids (color): Cpr (yellow), chloramphenicol resistance plasmid; Emr (blue), erythromycin resistance plasmid; Gmr (red), gentamicin resistance plasmid. Regarding plasmids marked with *, the entire plasmid sequence was determined. In B-a, CCC plasmid DNA was electrophoresed in 0.6% agarose. RN, RN2677. In B-b, CCC plasmid DNA was digested with EcoRI, and the digests were electrophoresed in 0.5% agarose. Marker 1, 2.5 kb DNA Ladder; marker 2, λ-HindIII digest. In C; RN, RN2677. The Tn554 circular intermediate was detected by PCR; the ST239Kras strain OC3 (lane 2) and Emr transconjugant (Emr RN2677, lane 3) produced positive results (carried the Tn554 circular intermediate), while RN2677 (lane 4) had no such structure. In D, bacterial mating between MRSA (plasmid-donor) and RN2677 (recipient) was performed by filter mating and non-filter mating methods. Nov, novobiocin; Cp, chloramphenicol; Em, erythromycin; Gm, gentamicin; Cli, clindamycin; Spc, spectinomycin; Amp, ampicillin; Cd, cadmium; EtBr, ethidium bromide; Acr, acriflavin. Transfer frequency, plasmid-positive (drug-resistant) transconjugants/donor.
(TIFF)
Plasmid sequence data were from the GenBank accession numbers described. Homologous regions are shaded in each comparison. Genes: cap, chloramphenicol resistance; rep, replication initiator protein; pre, pre protein; rlx, RLX protein; repL, replication initiator protein L. Em/Clir, constitutive resistance to erythromycin and clindamycin; Em/Cliind, inducible resistance to erythromycin and clindamycin (due to the presence of the leader peptide sequence in the promoter region upstream of ermC).
(TIFF)
In A, SaPI1 (OC3) showed the highest homology to SaPI1 (TW20). SaPI1 (OC3) was also compared with SaPI5 (USA300). Homologous regions between the SaPI structures are shaded with color. ear, penicillin-binding protein fragment. In B, the deduced amino acid sequences of the sek and seq genes (of OC3, TW20, and USA300) were compared with those of COL. Arrows indicate the positions of the amino acid changes. Different amino acids from the amino acid sequences of COL are indicated in red letters.
(TIFF)
φSa3 (OC3) exhibited the highest homology to φSa3 (CN1). The left-side immune evasion cluster (IEC) region was also compared with those of φSa3 (TW20) and ST8Kras strain OC8. Homologous regions are shaded in each comparison. Genes in IEC: scn, staphylococcal complement inhibitor (SCIN) gene; chp, chemotaxis inhibitory protein of S. aureus (CHIPS) gene; sak, staphylokinase (SAK) gene; sea, staphylococcal enterotoxin A (ETA) gene. The IEC region, carrying scn and sak, of OC3 (a region from attL to sak) was 3,541 bp in size, and showed 99% homology to the corresponding region of TW20. The IEC region, carrying scn, sak, and sea, of OC8 (a region from attL to sea) was 6,022 bp in size, and showed 99.3% homology to the corresponding region of TW20.
(TIFF)
fhuD + SaPI (OC3) showed only limited homology to any previous SaPI, suggesting a novel mosaic SaPI (fhuD). Homologous regions are shaded in each comparison. Genes: int, integrase gene; xis, excisionase; rep, replication initiator gene; ter, terminase gene (which cleaves multimeric DNA); fhuD, ferrichrome ABC transporter homologue.
(TIFF)
φSa5 (OC3) exhibited the highest (but limited) homology to φSa5 (XN108), suggesting a new mosaic phage. Homologous regions are shaded in each comparison.
(TIFF)
In the dendrogram (left side), a large spa3/SCCmecIII.1.1.1-III.1.1.2 cluster, associated with the Siberian region and Far Eastern region, is shadowed. In the middle of the figure, each Russian region is distinguished by color: red, Siberian region (Krasnoyarsk); green, Far Eastern region (Vladivostok); brown, European region (Moscow, St. Petersburg), purple, Ural region (Kurgan). Reference strains (HU25 and ANS46) are not marked. Regarding PFGE patterns (right side), the PFGE types of ST239 MRSA from Krasnoyarsk are those shown in Fig 1.
(TIFF)
Eight whole genome-analyzed ST239 strains, shown in this figure, are those described in S2 Table. SCCmecIII structures were analyzed as shown in Fig 3. Homologous regions are shaded. SCCmecIII.1.1.new-a and SCCmecIII.1.1.new-b, SCCmecIII.1.1 with new J3 regions.
(TIFF)
ST239Kras is characterized by SCCmecIII.1.1.2, tst, and φSa7-like (W). This figure indicates a possible Brazil-Europe-Russia transmission route for ST239Kras, in addition to the territories of some other prevalent ST239 MRSA.
(TIFF)
(XLS)
(XLSX)
Acknowledgments
We thank H. de Lencastre for the ST239 reference strains, L. K. McDougal and L. L. McDonald for the USA300-type strain, K. Hiramatsu for the New York/Japan clone-reference strains, and K. Akazawa for statistical analysis.
Data Availability
All relevant data are available in the manuscript, its Supporting Information files, and Table S2 files are available from the NCBI database (accession number(s) BBKC01000001-BBKC01000144, AB983237, AB983236, AB983235, AB983197, AB983196, AB983199, AB983198, AB983195, AB982225, AB982226, AB982227, AB982228, LC012933, LC032460).
Funding Statement
This study was supported by a fund/grant from the Krasnoyarsk Regional Government (Russia) and Japan-Russia Youth Exchange Center (Japan), and by each institutional sources. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lyon BR, Skurray R. Antimicrobial resistance of Staphylococcus aureus: genetic basis. Microbiol Rev. 1987;51:88–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Edgeworth JD, Yadegarfar G, Pathak S, Batra R, Cockfield JD, Wyncoll D, et al. An outbreak in an intensive care unit of a strain of methicillin-resistant Staphylococcus aureus sequence type 239 associated with an increased rate of vascular access device-related bacteremia. Clin Infect Dis. 2007;44:493–501. [DOI] [PubMed] [Google Scholar]
- 3. Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva: World Health Organization; 2014. 257 p. [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC). Four pediatric deaths from community-acquired methicillin-resistant Staphylococcus aureus—Minnesota and North Dakota, 1997–1999. MMWR Morb Mortal Wkly Rep. 1999;48:707–710. [PubMed] [Google Scholar]
- 6. DeLeo FR, Otto M, Kreiswirth BN, Chambers HF. Community-associated meticillin-resistant Staphylococcus aureus . Lancet. 2010;375:1557–1568. 10.1016/S0140-6736(09)61999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noto MJ, Kreiswirth BN, Monk AB, Archer GL. Gene acquisition at the insertion site for SCCmec, the genomic island conferring methicillin resistance in Staphylococcus aureus . J Bacteriol. 2008;190:1276–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC). Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009;53:4961–4967. 10.1128/AAC.00579-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Oliveira DC, Tomasz A, de Lencastre H. The evolution of pandemic clones of methicillin-resistant Staphylococcus aureus: identification of two ancestral genetic backgrounds and the associated mec elements. Microb Drug Resist. 2001;7:349–361. [DOI] [PubMed] [Google Scholar]
- 10. Enright MC, Robinson DA, Randle G, Feil EJ, Grundmann H, Spratt BG. The evolutionary history of methicillin-resistant Staphylococcus aureus (MRSA). Proc Natl Acad Sci USA. 2002;99:7687–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aires de Sousa M, Conceição T, Simas C, de Lencastre H. Comparison of genetic backgrounds of methicillin-resistant and-susceptible Staphylococcus aureus isolates from Portuguese hospitals and the community. J Clin Microbiol. 2005;43:5150–5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diep BA, Otto M. The role of virulence determinants in community-associated MRSA pathogenesis. Trends Microbiol. 2008;16:361–369. 10.1016/j.tim.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamamoto T, Hung WC, Takano T, Nishiyama A. Genetic nature and virulence of community-associated methicillin-resistant Staphylococcus aureus . BioMedicine. 2013;3:2–18. [Google Scholar]
- 14. Uhlemann AC, Otto M, Lowy FD, DeLeo FR. Evolution of community- and healthcare-associated methicillin-resistant Staphylococcus aureus . Infect Genet Evol. 2014;21:563–574. 10.1016/j.meegid.2013.04.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Oliveira D, Santos-Sanches I, Mato R, Tamayo M, Ribeiro G, Costa D, et al. Virtually all methicillin-resistant Staphylococcus aureus (MRSA) infections in the largest Portuguese teaching hospital are caused by two internationally spread multiresistant strains: the 'Iberian' and the 'Brazilian' clones of MRSA. Clin Microbiol Infect. 1998;4:373–384. [DOI] [PubMed] [Google Scholar]
- 16. de Lencastre H, Severina EP, Milch H, Thege MK, Tomasz A. Wide geographic distribution of a unique methicillin-resistant Staphylococcus aureus clone in Hungarian hospitals. Clin Microbiol Infect. 1997;3:289–296. [DOI] [PubMed] [Google Scholar]
- 17. Aires de Sousa M, de Lencastre H. Bridges from hospitals to the laboratory: genetic portraits of methicillin-resistant Staphylococcus aureus clones. FEMS Immunol Med Microbiol. 2004;40:101–111. [DOI] [PubMed] [Google Scholar]
- 18. Conceição T, Aires-de-Sousa M, Füzi M, Tóth A, Pászti J, Ungvári E, et al. Replacement of methicillin-resistant Staphylococcus aureus clones in Hungary over time: a 10-year surveillance study. Clin Microbiol Infect. 2007;13:971–979. [DOI] [PubMed] [Google Scholar]
- 19. Holden MT, Lindsay JA, Corton C, Quail MA, Cockfield JD, Pathak S, et al. Genome sequence of a recently emerged, highly transmissible, multi-antibiotic- and antiseptic-resistant variant of methicillin-resistant Staphylococcus aureus, sequence type 239 (TW). J Bacteriol. 2010;192:888–892. 10.1128/JB.01255-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris SR, Feil EJ, Holden MT, Quail MA, Nickerson EK, Chantratita N, et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science. 2010;327:469–474. 10.1126/science.1182395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tanaka T, Okuzumi K, Iwamoto A, Hiramatsu K. A retrospective study of methicillin-resistant Staphylococcus aureus clinical strains in Tokyo university hospital. J Infect Chemother. 1995;1:40–49. [Google Scholar]
- 22. Kuroda M, Ohta T, Uchiyama I, Baba T, Yuzawa H, Kobayashi I, et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus . Lancet. 2001;357:1225–1240. [DOI] [PubMed] [Google Scholar]
- 23. Diep BA, Gill SR, Chang RF, Phan TH, Chen JH, Davidson MG, et al. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus . Lancet. 2006;367:731–739. [DOI] [PubMed] [Google Scholar]
- 24. Vandenesch F, Naimi T, Enright MC, Lina G, Nimmo GR, Heffernan H, et al. Community-acquired methicillin-resistant Staphylococcus aureus carrying Panton-Valentine leukocidin genes: worldwide emergence. Emerg Infect Dis. 2003;9:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Isobe H, Takano T, Nishiyama A, Hung WC, Kuniyuki S, Shibuya Y, et al. Evolution and virulence of Panton-Valentine leukocidin-positive ST30 methicillin-resistant Staphylococcus aureus in the past 30 years in Japan. Biomed Res. 2012;33:97–109. [DOI] [PubMed] [Google Scholar]
- 26. Harro JM, Daugherty S, Bruno VM, Jabra-Rizk MA, Rasko DA, Shirtliff ME. Draft genome sequence of the methicillin-resistant Staphylococcus aureus isolate MRSA-M2. Genome Announc. 2013. January;1(1). pii: e00037–12. 10.1128/genomeA.00037-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Boyle-Vavra S, Ereshefsky B, Wang CC, Daum RS. Successful multiresistant community-associated methicillin-resistant Staphylococcus aureus lineage from Taipei, Taiwan, that carries either the novel Staphylococcal chromosome cassette mec (SCCmec) type VT or SCCmec type IV. J Clin Microbiol. 2005;43:4719–4730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takano T, Higuchi W, Zaraket H, Otsuka T, Baranovich T, Enany S, et al. Novel characteristics of community-acquired methicillin-resistant Staphylococcus aureus strains belonging to multilocus sequence type 59 in Taiwan. Antimicrob Agents Chemother. 2008;52:837–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hung WC, Takano T, Higuchi W, Iwao Y, Khokhlova O, Teng LJ, et al. Comparative genomics of community-acquired ST59 methicillin-resistant Staphylococcus aureus in Taiwan: novel mobile resistance structures with IS1216V . PLoS One. 2012;7:e46987 10.1371/journal.pone.0046987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stegger M, Price LB, Larsen AR, Gillece JD, Waters AE, Skov R, et al. Genome sequence of Staphylococcus aureus strain 11819–97, an ST80-IV European community-acquired methicillin-resistant isolate. J Bacteriol. 2012;194:1625–1626. 10.1128/JB.06653-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Takano T, Higuchi W, Yamamoto T. Superior in vitro activity of carbapenems over anti-methicillin-resistant Staphylococcus aureus (MRSA) and some related antimicrobial agents for community-acquired MRSA but not for hospital-acquired MRSA. J Infect Chemother. 2009;15:54–57. 10.1007/s10156-008-0665-5 [DOI] [PubMed] [Google Scholar]
- 32. McDougal LK, Fosheim GE, Nicholson A, Bulens SN, Limbago BM, Shearer JE, et al. Emergence of resistance among USA300 methicillin-resistant Staphylococcus aureus isolates causing invasive disease in the United States. Antimicrob Agents Chemother. 2010;54:3804–3811. 10.1128/AAC.00351-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shore AC, Brennan OM, Ehricht R, Monecke S, Schwarz S, Slickers P, et al. Identification and characterization of the multidrug resistance gene cfr in a Panton-Valentine leukocidin-positive sequence type 8 methicillin-resistant Staphylococcus aureus IVa (USA300) isolate. Antimicrob Agents Chemother. 2010;54:4978–4984 10.1128/AAC.01113-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Naimi TS, LeDell KH, Como-Sabetti K, Borchardt SM, Boxrud DJ Etienne J, et al. Comparison of community- and health care-associated methicillin-resistant Staphylococcus aureus infection. JAMA. 2003;290:2976–2984. [DOI] [PubMed] [Google Scholar]
- 35. Harinstein L, Schafer J, D'Amico F. Risk factors associated with the conversion of meticillin-resistant Staphylococcus aureus colonisation to healthcare-associated infection. J Hosp Infect. 2011;79:194–197. 10.1016/j.jhin.2011.03.017 [DOI] [PubMed] [Google Scholar]
- 36. Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, et al. Clinical practice guidelines by the infectious diseases society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children: executive summary. Clin Infect Dis. 2011;52:285–292. 10.1093/cid/cir034 [DOI] [PubMed] [Google Scholar]
- 37. Thurlow LR, Joshi GS, Richardson AR. Virulence strategies of the dominant USA300 lineage of community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). FEMS Immunol Med Microbiol. 2012;65:5–22. 10.1111/j.1574-695X.2012.00937.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Löffler B, Hussain M, Grundmeier M, Brück M, Holzinger D, Varga G, et al. Staphylococcus aureus Panton-Valentine leukocidin is a very potent cytotoxic factor for human neutrophils. PLoS Pathog. 2010;6:e1000715 10.1371/journal.ppat.1000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nishiyama A, Isobe H, Iwao Y, Takano T, Hung WC, Taneike I, et al. Accumulation of staphylococcal Panton-Valentine leukocidin in the detergent-resistant membrane microdomains on the target cells is essential for its cytotoxicity. FEMS Immunol Med Microbiol. 2012;66:343–352. 10.1111/j.1574-695X.2012.01027.x [DOI] [PubMed] [Google Scholar]
- 40. Wang R, Braughton KR, Kretschmer D, Bach TH, Queck SY, Li M, et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat Med. 2007;13:1510–1514. [DOI] [PubMed] [Google Scholar]
- 41. Simpson AE, Skurray RA, Firth N. A single gene on the staphylococcal multiresistance plasmid pSK1 encodes a novel partitioning system. J Bacteriol. 2003;185:2143–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Novick RP, Subedi A. The SaPIs: mobile pathogenicity islands of Staphylococcus . Chem Immunol Allergy. 2007;93:42–57. [DOI] [PubMed] [Google Scholar]
- 43. Benson MA, Ohneck EA, Ryan C, Alonzo F 3rd, Smith H, Narechania A, et al. Evolution of hypervirulence by a MRSA clone through acquisition of a transposable element. Mol Microbiol. 2014;93:664–681. 10.1111/mmi.12682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xia G, Wolz C. Phages of Staphylococcus aureus and their impact on host evolution. Infect Genet Evol. 2014;21:593–601. 10.1016/j.meegid.2013.04.022 [DOI] [PubMed] [Google Scholar]
- 45. Robinson DA, Enright MC. Evolution of Staphylococcus aureus by large chromosomal replacements. J Bacteriol. 2004;186:1060–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Aires de Sousa M, Sanches IS, Ferro ML, Vaz MJ, Saraiva Z, Tendeiro T, et al. Intercontinental spread of a multidrug-resistant methicillin-resistant Staphylococcus aureus clone. J Clin Microbiol. 1998;36:2590–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Albrecht N, Jatzwauk L, Slickers P, Ehricht R, Monecke S. Clonal replacement of epidemic methicillin-resistant Staphylococcus aureus strains in a German university hospital over a period of eleven years. PLoS One. 2011;6:e28189 10.1371/journal.pone.0028189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Espadinha D, Faria NA, Miragaia M, Lito LM, Melo-Cristino J, de Lencastre H, et al. Extensive dissemination of methicillin-resistant Staphylococcus aureus (MRSA) between the hospital and the community in a country with a high prevalence of nosocomial MRSA. PLoS One. 2013;8:e59960 10.1371/journal.pone.0059960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Romanov AV, Dekhnich AV, Edelstein MV. Molecular epidemiology of Staphylococcus aureus in Russian pediatric hospitals. Clin Microbiol Antimicrob Chemother. 2012;14:201–208 [Google Scholar]
- 50. Baranovich T, Potapov V, Yamamoto T. The first isolation of Panton-Valentine leukocidin (PVL) positive community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) in Russia. Euro Surveill. 2007;12:E070315070314. [DOI] [PubMed] [Google Scholar]
- 51. Iwao Y, Khokhlova OE, Takano T, Hung WC, Isobe H, Peryanova OV, et al. Fatal pneumonia in HIV-infected patients from a novel ST239 methicillin-resistant Staphylococcus aureus carrying the toxic shock syndrome toxin-1 gene in Krasnoyarsk, Siberian Russia. Jpn J Infect Dis. 2012;65:184–186. [PubMed] [Google Scholar]
- 52. Yamamoto T, Takano T, Higuchi W, Iwao Y, Singur O, Reva I, et al. Comparative genomics and drug resistance of a geographic variant of ST239 methicillin-resistant Staphylococcus aureus emerged in Russia. PLoS One. 2012;7:e29187 10.1371/journal.pone.0029187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shibuya Y, Hara M, Higuchi W, Takano T, Iwao Y, Yamamoto T. Emergence of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone in Japan. J Infect Chemother. 2008;14:439–441. 10.1007/s10156-008-0640-1 [DOI] [PubMed] [Google Scholar]
- 54. Higuchi W, Mimura S, Kurosawa Y, Takano T, Iwao Y, Yabe S, et al. Emergence of the community-acquired methicillin-resistant Staphylococcus aureus USA300 clone in a Japanese child, demonstrating multiple divergent strains in Japan. J Infect Chemother. 2010;16:292–297. 10.1007/s10156-010-0051-y [DOI] [PubMed] [Google Scholar]
- 55. Takizawa Y, Taneike I, Nakagawa S, Oishi T, Nitahara Y, Iwakura N, et al. A Panton-Valentine leucocidin (PVL)-positive community-acquired methicillin-resistant Staphylococcus aureus (MRSA) strain, another such strain carrying a multiple-drug resistance plasmid, and other more-typical PVL-negative MRSA strains found in Japan. J Clin Microbiol. 2005;43:3356–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Takano T, Hung WC, Shibuya M, Higuchi W, Iwao Y, Nishiyama A, et al. A new local variant (ST764) of the globally disseminated ST5 lineage of hospital-associated methicillin-resistant Staphylococcus aureus (MRSA) carrying the virulence determinants of community-associated MRSA. Antimicrob Agents Chemother. 2013;57:1589–1595 10.1128/AAC.01147-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kado CI, Liu ST. Rapid procedure for detection and isolation of large and small plasmids. J Bacteriol. 1981;145:1365–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Clinical and Laboratory Standards Institute. Performance standard for antimicrobial susceptibility testing: 22nd informational supplement M100-S22, Wayne PA, USA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 59. Sawanobori E, Hung WC, Takano T, Hachuda K, Horiuchi T, Higuchi W, et al. Emergence of Panton-Valentine leukocidin-positive ST59 methicillin-susceptible Staphylococcus aureus with high cytolytic peptide expression in association with community-acquired pediatric osteomyelitis complicated by pulmonary embolism. J Microbiol Immunol Infect. 2014. July 25 pii: S1684-1182(14)00111-X. 10.1016/j.jmii.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 60. Smyth DS, Robinson DA. Integrative and sequence characteristics of a novel genetic element, ICE6013, in Staphylococcus aureus . J Bacteriol. 2009;191:5964–5975. 10.1128/JB.00352-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang T, Tanaka M, Sato K. Detection of grlA and gyrA mutations in 344 Staphylococcus aureus strains. Antimicrob Agents Chemother. 1998;42:236–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wichelhaus TA, Schäfer V, Brade V, Böddinghaus B. Molecular characterization of rpoB mutations conferring cross-resistance to rifamycins on methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 1999;43:2813–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hampele IC, D'Arcy A, Dale GE, Kostrewa D, Nielsen J, Oefner C, et al. Structure and function of the dihydropteroate synthase from Staphylococcus aureus J Mol Biol. 1997;268:21–30. [DOI] [PubMed] [Google Scholar]
- 64. Stratchounski LS, Dekhnich AV, Kretchikov VA, DeEdelstain IA, Narezkina AD, Afinogenov GE, et al. Antimicrobial resistance of nosocomial strains of Staphylococcus aureus in Russia: results of a prospective study. J Chemother. 2005;17:54–60. [DOI] [PubMed] [Google Scholar]
- 65. Vorobieva V, Bazhukova T, Hanssen AM, Caugant DA, Semenova N, Haldorsen BC, et al. Clinical isolates of Staphylococcus aureus from the Arkhangelsk region, Russia: antimicrobial susceptibility, molecular epidemiology, and distribution of Panton-Valentine leukocidin genes. APMIS. 2008;116:877–887. 10.1111/j.1600-0463.2008.01092.x [DOI] [PubMed] [Google Scholar]
- 66. Romanov AV, Dehnich AV. Typing of MRSA: which methods are optimal for different problem? Clin Microbiol Antimicrob Chemother. 2011;13:169–177. [Google Scholar]
- 67. Brusina EB, Glazovskaya LS, Efimova TV. Epidemiological aspects of MRSA circulation in the industrial region of Russia. Antimicrob Resist Infect Control. 2013;2 (Suppl 1):P57 [Google Scholar]
- 68. Diep BA, Stone GG, Basuino L, Graber CJ, Miller A, des Etages SA, et al. The arginine catabolic mobile element and staphylococcal chromosomal cassette mec linkage: convergence of virulence and resistance in the USA300 clone of methicillin-resistant Staphylococcus aureus . J Infect Dis. 2008;197:1523–1530. 10.1086/587907 [DOI] [PubMed] [Google Scholar]
- 69. Rolo J, Miragaia M, Turlej-Rogacka A, Empel J, Bouchami O, Faria NA, et al. High genetic diversity among community-associated Staphylococcus aureus in Europe: results from a multicenter study. PLoS One. 2012;7:e34768 10.1371/journal.pone.0034768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang K, McClure JA, Elsayed S, Tan J, Conly JM. Coexistence of Panton-Valentine leukocidin-positive and-negative community-associated methicillin-resistant Staphylococcus aureus USA400 sibling strains in a large Canadian health-care region. J Infect Dis. 2008;197:195–204. 10.1086/523763 [DOI] [PubMed] [Google Scholar]
- 71. Iwao Y, Ishii R, Tomita Y, Shibuya Y, Takano T, Hung WC, et al. The emerging ST8 methicillin-resistant Staphylococcus aureus clone in the community in Japan: associated infections, genetic diversity, and comparative genomics. J Infect Chemother. 2012;18:228–240. 10.1007/s10156-012-0379-6 [DOI] [PubMed] [Google Scholar]
- 72. Khokhlova O, Tomita Y, Hung WC, Takano T, Iwao Y, Higuchi W, et al. Elderly infection in the community due to ST5/SCCmecII methicillin-resistant Staphylococcus aureus (the New York/Japan clone) in Japan: Panton-Valentine leukocidin-negative necrotizing pneumonia. J Microbiol Immunol Infect. 2012. November 29 pii: S1684-1182(12)00210-1. 10.1016/j.jmii.2012.09.004 [DOI] [PubMed] [Google Scholar]
- 73. Mendes RE, Deshpande LM, Smyth DS, Shopsin B, Farrell DJ, Jones RN. Characterization of methicillin-resistant Staphylococcus aureus strains recovered from a phase IV clinical trial for linezolid versus vancomycin for treatment of nosocomial pneumonia. J Clin Microbiol. 2012;50:3694–3702. 10.1128/JCM.02024-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu SX, McCormick JK. Staphylococcal superantigens in colonization and disease. Front Cell Infect Microbiol. 2012;2:52 10.3389/fcimb.2012.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Stach CS, Herrera A, Schlievert PM. Staphylococcal superantigens interact with multiple host receptors to cause serious diseases. Immunol Res. 2014;59:177–181. 10.1007/s12026-014-8539-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Issa NC, Thompson RL. Staphylococcal toxic shock syndrome. Suspicion and prevention are keys to control. Postgrad Med. 2001;110:55–56, 59–62. [DOI] [PubMed] [Google Scholar]
- 77. Takahashi N, Nishida H, Kato H, Imanishi K, Sakata Y, Uchiyama T. et al. Exanthematous disease induced by toxic shock syndrome toxin 1 in the early neonatal period. Lancet. 1998;351(9116):1614–1619. [DOI] [PubMed] [Google Scholar]
- 78. Kappler J, Kotzin B, Herron L, Gelfand EW, Bigler RD, Boylston A, et al. V beta-specific stimulation of human T cells by staphylococcal toxins. Science. 1989;244:811–813. [DOI] [PubMed] [Google Scholar]
- 79. Li H, Llera A, Mariuzza RA. Structure-function studies of T-cell receptor-superantigen interactions. Immunol Rev. 1998;163:177–186. [DOI] [PubMed] [Google Scholar]
- 80. Krakauer T. Immune response to staphylococcal superantigens. Immunol Res. 1999;20:163–173. [DOI] [PubMed] [Google Scholar]
- 81. Alouf JE, Müller-Alouf H. Staphylococcal and streptococcal superantigens: molecular, biological and clinical aspects. Int J Med Microbiol. 2003;292:429–440. [DOI] [PubMed] [Google Scholar]
- 82. Kalyan S, Chow AW. Human peripheral gammadelta T cells potentiate the early proinflammatory cytokine response to staphylococcal toxic shock syndrome toxin-1. J Infect Dis. 2004;189:1892–1896. [DOI] [PubMed] [Google Scholar]
- 83. Nienaber JJ, Sharma Kuinkel BK, Clarke-Pearson M, Lamlertthon S, Park L, Rude TH, et al. Methicillin-susceptible Staphylococcus aureus endocarditis isolates are associated with clonal complex 30 genotype and a distinct repertoire of enterotoxins and adhesins. J Infect Dis. 2011;204:704–713. 10.1093/infdis/jir389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vojtov N, Ross HF, Novick RP. Global repression of exotoxin synthesis by staphylococcal superantigens. Proc Natl Acad Sci USA. 2002;99:10102–10107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Committee for the Japanese Respiratory Society Guidelines in Management of Respiratory. Antibacterial therapy of hospital-acquired pneumonia. Respirology. 2004;9:S16–24. [DOI] [PubMed] [Google Scholar]
- 86. Joo HS, Cheung GY, Otto M. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J Biol Chem. 2011;286:8933–8940. 10.1074/jbc.M111.221382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cheung AL, Nishina KA, Trotonda MP, Tamber S. The SarA protein family of Staphylococcus aureus . Int J Biochem Cell Biol. 2008;40:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lucas AL, Manna AC. Phenotypic characterization of sarR mutant in Staphylococcus aureus . Microb Pathog. 2013;57:52–61. 10.1016/j.micpath.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 89. Gupta RK, Alba J, Xiong YQ, Bayer AS, Lee CY. MgrA activates expression of capsule genes, but not the alpha-toxin gene in experimental Staphylococcus aureus endocarditis. J Infect Dis. 2013;208:1841–1848. 10.1093/infdis/jit367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cue D, Lei MG, Lee CY. Activation of sarX by Rbf is required for biofilm formation and icaADBC expression in Staphylococcus aureus . J Bacteriol. 2013;195:1515–1524. 10.1128/JB.00012-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pragman AA, Yarwood JM, Tripp TJ, Schlievert PM. Characterization of virulence factor regulation by SrrAB, a two-component system in Staphylococcus aureus . J Bacteriol. 2004;186:2430–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kozitskaya S, Cho SH, Dietrich K, Marre R, Naber K, Ziebuhr W. The bacterial insertion sequence element IS256 occurs preferentially in nosocomial Staphylococcus epidermidis isolates: association with biofilm formation and resistance to aminoglycosides. Infect Immun 2004;72:1210–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Byrne ME, Rouch DA, Skurray RA. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001 . Gene. 1989;81:361–367. [DOI] [PubMed] [Google Scholar]
- 94. van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J Bacteriol. 2006;188:1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Foster TJ, Höök M. Surface protein adhesins of Staphylococcus aureus . Trends Microbiol. 1998;6:484–488. [DOI] [PubMed] [Google Scholar]
- 96. Zong Y, Xu Y, Liang X, Keene DR, Höök A, Gurusiddappa S, et al. A 'Collagen Hug' model for Staphylococcus aureus CNA binding to collagen. EMBO J. 2005;24:4224–4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. de Bentzmann S, Tristan A, Etienne J, Brousse N, Vandenesch F, Lina G. Staphylococcus aureus isolates associated with necrotizing pneumonia bind to basement membrane type I and IV collagens and laminin. J Infect Dis. 2004;190:1506–1515. [DOI] [PubMed] [Google Scholar]
- 98. Shi D, Higuchi W, Takano T, Saito K, Ozaki K, Takano M, et al. Bullous impetigo in children infected with methicillin-resistant Staphylococcus aureus alone or in combination with methicillin-susceptible S. aureus: analysis of genetic characteristics, including assessment of exfoliative toxin gene carriage. J Clin Microbiol. 2011;49:1972–1974. 10.1128/JCM.01742-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kang M, Ko YP, Liang X, Ross CL, Liu Q, Murray BE, et al. Collagen-binding microbial surface components recognizing adhesive matrix molecule (MSCRAMM) of Gram-positive bacteria inhibit complement activation via the classical pathway. J Biol Chem. 2013;288:20520–20531. 10.1074/jbc.M113.454462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ferry T, Thomas D, Genestier AL, Bes M, Lina G, Vandenesch F, et al. Comparative prevalence of superantigen genes in Staphylococcus aureus isolates causing sepsis with and without septic shock. Clin Infect Dis. 2005;41:771–777. [DOI] [PubMed] [Google Scholar]
- 101. Xu SX, Gilmore KJ, Szabo PA, Zeppa JJ, Baroja ML, Haeryfar SM, et al. Superantigens subvert the neutrophil response to promote abscess formation and enhance Staphylococcus aureus survival in vivo . Infect Immun. 2014;82:3588–3598. 10.1128/IAI.02110-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li Y, Cao B, Zhang Y, Zhou J, Yang B, Wang L. Complete genome sequence of Staphylococcus aureus T0131, an ST239-MRSA-SCCmec type III clone isolated in China. J Bacteriol. 2011;193:3411–3412. 10.1128/JB.05135-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Chen FJ, Lauderdale TL, Wang LS, Huang IW. Complete genome sequence of Staphylococcus aureus Z172, a vancomycin-intermediate and daptomycin-nonsusceptible methicillin-resistant strain isolated in Taiwan. Genome Announc. 2013. December 5;1(6). pii: e01011–13. 10.1128/genomeA.01011-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Chen CJ, Huang YC. New epidemiology of Staphylococcus aureus infection in Asia. Clin Microbiol Infect. 2014;20:605–623. 10.1111/1469-0691.12705 [DOI] [PubMed] [Google Scholar]
- 105. Zhang X, Xu X, Yuan W, Hu Q, Shang W, Hu X, et al. Complete Genome sequence of Staphylococcus aureus XN108, an ST239-MRSA-SCCmec III strain with intermediate vancomycin resistance isolated in mainland China. Genome Announc. 2014. July 24;2(4). pii: e00449–14. 10.1128/genomeA.00449-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Howden BP, Seemann T, Harrison PF, McEvoy CR, Stanton JA, Rand CJ, et al. Complete genome sequence of Staphylococcus aureus strain JKD6008, an ST239 clone of methicillin-resistant Staphylococcus aureus with intermediate-level vancomycin resistance. J Bacteriol. 2010;192:5848–5849. 10.1128/JB.00951-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Coombs GW, Nimmo GR, Pearson JC, Collignon PJ, Bell JM, McLaws ML, et al. Australian group on antimicrobial resistance hospital-onset Staphylococcus aureus surveillance programme annual report, 2011." Commun Dis Intell Q Rep. 2013;37:E210–218. [DOI] [PubMed] [Google Scholar]
- 108. Cîrlan M, Saad M, Coman G, Bilal NE, Elbashier AM, Kreft D, et al. International spread of major clones of methicillin resistant Staphylococcus aureus: nosocomial endemicity of multi locus sequence type 239 in Saudi Arabia and Romania. Infect Genet Evol. 2005;5:335–339. [DOI] [PubMed] [Google Scholar]
- 109. Havaei SA, Vidovic S, Tahmineh N, Mohammad K, Mohsen K, Starnino S, et al. Epidemic methicillin-susceptible Staphylococcus aureus lineages are the main cause of infections at an Iranian university hospital. J Clin Microbiol. 2011;49:3990–3993. 10.1128/JCM.05445-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Mirzaii M, Emaneini M, Jabalameli F, Halimi S, Taherikalani M. Molecular investigation of Staphylococcus aureus isolated from the patients, personnel, air and environment of an ICU in a hospital in Tehran. J Infect Public Health. 2015. Mar-Apr;8(2):202–6. 10.1016/j.jiph.2014.09.002 [DOI] [PubMed] [Google Scholar]
- 111. Monecke S, Müller E, Dorneanu OS, Vremeră T, Ehricht R. Molecular typing of MRSA and of clinical Staphylococcus aureus isolates from Iasi, Romania. PLoS One. 2014;9:e97833 10.1371/journal.pone.0097833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Vivoni AM, Diep BA, de Gouveia Magalhães AC, Santos KR, Riley LW, Sensabaugh GF, et al. Clonal composition of Staphylococcus aureus isolates at a Brazilian university hospital: identification of international circulating lineages. J Clin Microbiol. 2006;44:1686–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Costa MO, Beltrame CO, Ferreira FA, Botelho AM, Lima NC, Souza RC, et al. Complete genome sequence of a variant of the methicillin-resistant Staphylococcus aureus ST239 lineage, strain BMB9393, displaying superior ability to accumulate ica-independent biofilm. Genome Announc. 2013. August 8;1(4). pii: e00576–13. 10.1128/genomeA.00576-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ko KS, Lee JY, Suh JY, Oh WS, Peck KR, Lee NY, et al. Distribution of major genotypes among methicillin-resistant Staphylococcus aureus clones in Asian countries. J Clin Microbiol. 2005;43:421–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Arakere G, Nadig S, Ito T, Ma XX, Hiramatsu K. A novel type-III staphylococcal cassette chromosome mec (SCCmec) variant among Indian isolates of methicillin-resistant Staphylococcus aureus . FEMS Microbiol Lett. 2009;292:141–148. 10.1111/j.1574-6968.2008.01482.x [DOI] [PubMed] [Google Scholar]
- 116. Chen L, Mediavilla JR, Oliveira DC, Willey BM, de Lencastre H, Kreiswirth BN. Multiplex real-time PCR for rapid staphylococcal cassette chromosome mec typing. J Clin Microbiol. 2009;47:3692–3706. 10.1128/JCM.00766-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. http://133.242.9.96/staphylococcus.net/. Accessed 24 Dec 2014
- 118. Novick RP, Christie GE, Penadés JR. The phage-related chromosomal islands of Gram-positive bacteria. Nat Rev Microbiol. 2010;8:541–551. 10.1038/nrmicro2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Bae T, Baba T, Hiramatsu K, Schneewind O. Prophages of Staphylococcus aureus Newman and their contribution to virulence. Mol Microbiol. 2006;62:1035–1047. [DOI] [PubMed] [Google Scholar]
- 120. Goerke C, Pantucek R, Holtfreter S, Schulte B, Zink M, Grumann D, et al. Diversity of prophages in dominant Staphylococcus aureus clonal lineages. J Bacteriol. 2009;191:3462–3468. 10.1128/JB.01804-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Huang YC, Chen CJ. Community-associated meticillin-resistant Staphylococcus aureus in children in Taiwan, 2000s. Int J Antimicrob Agents. 2011;38:2–8. 10.1016/j.ijantimicag.2011.01.011 [DOI] [PubMed] [Google Scholar]
- 122. Murphy E, Löfdahl S. Transposition of Tn554 does not generate a target duplication. Nature. 1984;307:292–294. [DOI] [PubMed] [Google Scholar]
- 123. Haroche J, Allignet J, El Solh N. Tn5406, a new staphylococcal transposon conferring resistance to streptogramin A and related compounds including dalfopristin. Antimicrob Agents Chemother. 2002;46:2337–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kadlec K, Schwarz S. Identification of the novel dfrK-carrying transposon Tn559 in a porcine methicillin-susceptible Staphylococcus aureus ST398 strain. Antimicrob Agents Chemother. 2010;54:3475–3477. 10.1128/AAC.00464-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Bartels MD, Nanuashvili A, Boye K, Rohde SM, Jashiashvili N, Faria NA, et al. Methicillin-resistant Staphylococcus aureus in hospitals in Tbilisi, the Republic of Georgia, are variants of the Brazilian clone." Eur J Clin Microbiol Infect Dis. 2008;27:757–760. 10.1007/s10096-008-0500-z [DOI] [PubMed] [Google Scholar]
- 126. Li M, Du X, Villaruz AE, Diep BA, Wang D, Song Y, et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med. 2012;18:816–819. 10.1038/nm.2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Kriegeskorte A, Peters G. Horizontal gene transfer boosts MRSA spreading. Nat Med. 2012;18:662–663. 10.1038/nm.2765 [DOI] [PubMed] [Google Scholar]
- 128. Wang Z, Zhou H, Wang H, Chen H, Leung KK, Tsui S, et al. Comparative genomics of methicillin-resistant Staphylococcus aureus ST239: distinct geographical variants in Beijing and Hong Kong. BMC genomics. 2014;15:529 10.1186/1471-2164-15-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Widdowson CA, Adrian PV, Klugman KP. Acquisition of chloramphenicol resistance by the linearization and integration of the entire staphylococcal plasmid pC194 into the chromosome of Streptococcus pneumoniae . Antimicrob Agents Chemother. 2000;44:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tenover FC, McDougal LK, Goering RV, Killgore G, Projan SJ, Patel JB, et al. Characterization of a strain of community-associated methicillin-resistant Staphylococcus aureus widely disseminated in the United States. J Clin Microbiol. 2006;44:108–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Khan S. A. Rolling-circle replication of bacterial plasmids. Microbiol Mol Biol Rev. 1997;61:442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Berg T, Firth N, Apisiridej S, Hettiaratchi A, Leelaporn A, Skurray RA. Complete nucleotide sequence of pSK41: evolution of staphylococcal conjugative multiresistance plasmids. J Bacteriol. 1998;180:4350–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Shalita Z, Murphy E, Novick RP. Penicillinase plasmids of Staphylococcus aureus: structural and evolutionary relationships. Plasmid. 1980;3:291–311. [DOI] [PubMed] [Google Scholar]
- 134. Becker EC, Meyer R. Origin and fate of the 3' ends of single-stranded DNA generated by conjugal transfer of plasmid R1162. J Bacteriol. 2012;194:5368–5376. 10.1128/JB.00818-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Westh H, Hougaard DM, Vuust J, Rosdahl VT. Prevalence of erm gene classes in erythromycin-resistant Staphylococcus aureus strains isolated between 1959 and 1988. Antimicrob Agents Chemother. 1995;39:369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Murphy E. Properties of the site-specific transposable element Tn554 Molecular biology of the staphylococci. VCH Publishers, New York, NY; 1990. pp. 123–135. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In A; RN, RN2677 (recipient). Covalently closed circular (CCC) plasmid DNA, isolated from MRSA and transconjugants (RN2677 carrying plasmids), was electrophoresed in 1% agarose. Plasmid sizes were determined using reference plasmids with known molecular sizes. Plasmids (color): Cpr (yellow), chloramphenicol resistance plasmid; Emr (blue), erythromycin resistance plasmid; Gmr (red), gentamicin resistance plasmid. Regarding plasmids marked with *, the entire plasmid sequence was determined. In B-a, CCC plasmid DNA was electrophoresed in 0.6% agarose. RN, RN2677. In B-b, CCC plasmid DNA was digested with EcoRI, and the digests were electrophoresed in 0.5% agarose. Marker 1, 2.5 kb DNA Ladder; marker 2, λ-HindIII digest. In C; RN, RN2677. The Tn554 circular intermediate was detected by PCR; the ST239Kras strain OC3 (lane 2) and Emr transconjugant (Emr RN2677, lane 3) produced positive results (carried the Tn554 circular intermediate), while RN2677 (lane 4) had no such structure. In D, bacterial mating between MRSA (plasmid-donor) and RN2677 (recipient) was performed by filter mating and non-filter mating methods. Nov, novobiocin; Cp, chloramphenicol; Em, erythromycin; Gm, gentamicin; Cli, clindamycin; Spc, spectinomycin; Amp, ampicillin; Cd, cadmium; EtBr, ethidium bromide; Acr, acriflavin. Transfer frequency, plasmid-positive (drug-resistant) transconjugants/donor.
(TIFF)
Plasmid sequence data were from the GenBank accession numbers described. Homologous regions are shaded in each comparison. Genes: cap, chloramphenicol resistance; rep, replication initiator protein; pre, pre protein; rlx, RLX protein; repL, replication initiator protein L. Em/Clir, constitutive resistance to erythromycin and clindamycin; Em/Cliind, inducible resistance to erythromycin and clindamycin (due to the presence of the leader peptide sequence in the promoter region upstream of ermC).
(TIFF)
In A, SaPI1 (OC3) showed the highest homology to SaPI1 (TW20). SaPI1 (OC3) was also compared with SaPI5 (USA300). Homologous regions between the SaPI structures are shaded with color. ear, penicillin-binding protein fragment. In B, the deduced amino acid sequences of the sek and seq genes (of OC3, TW20, and USA300) were compared with those of COL. Arrows indicate the positions of the amino acid changes. Different amino acids from the amino acid sequences of COL are indicated in red letters.
(TIFF)
φSa3 (OC3) exhibited the highest homology to φSa3 (CN1). The left-side immune evasion cluster (IEC) region was also compared with those of φSa3 (TW20) and ST8Kras strain OC8. Homologous regions are shaded in each comparison. Genes in IEC: scn, staphylococcal complement inhibitor (SCIN) gene; chp, chemotaxis inhibitory protein of S. aureus (CHIPS) gene; sak, staphylokinase (SAK) gene; sea, staphylococcal enterotoxin A (ETA) gene. The IEC region, carrying scn and sak, of OC3 (a region from attL to sak) was 3,541 bp in size, and showed 99% homology to the corresponding region of TW20. The IEC region, carrying scn, sak, and sea, of OC8 (a region from attL to sea) was 6,022 bp in size, and showed 99.3% homology to the corresponding region of TW20.
(TIFF)
fhuD + SaPI (OC3) showed only limited homology to any previous SaPI, suggesting a novel mosaic SaPI (fhuD). Homologous regions are shaded in each comparison. Genes: int, integrase gene; xis, excisionase; rep, replication initiator gene; ter, terminase gene (which cleaves multimeric DNA); fhuD, ferrichrome ABC transporter homologue.
(TIFF)
φSa5 (OC3) exhibited the highest (but limited) homology to φSa5 (XN108), suggesting a new mosaic phage. Homologous regions are shaded in each comparison.
(TIFF)
In the dendrogram (left side), a large spa3/SCCmecIII.1.1.1-III.1.1.2 cluster, associated with the Siberian region and Far Eastern region, is shadowed. In the middle of the figure, each Russian region is distinguished by color: red, Siberian region (Krasnoyarsk); green, Far Eastern region (Vladivostok); brown, European region (Moscow, St. Petersburg), purple, Ural region (Kurgan). Reference strains (HU25 and ANS46) are not marked. Regarding PFGE patterns (right side), the PFGE types of ST239 MRSA from Krasnoyarsk are those shown in Fig 1.
(TIFF)
Eight whole genome-analyzed ST239 strains, shown in this figure, are those described in S2 Table. SCCmecIII structures were analyzed as shown in Fig 3. Homologous regions are shaded. SCCmecIII.1.1.new-a and SCCmecIII.1.1.new-b, SCCmecIII.1.1 with new J3 regions.
(TIFF)
ST239Kras is characterized by SCCmecIII.1.1.2, tst, and φSa7-like (W). This figure indicates a possible Brazil-Europe-Russia transmission route for ST239Kras, in addition to the territories of some other prevalent ST239 MRSA.
(TIFF)
(XLS)
(XLSX)
Data Availability Statement
All relevant data are available in the manuscript, its Supporting Information files, and Table S2 files are available from the NCBI database (accession number(s) BBKC01000001-BBKC01000144, AB983237, AB983236, AB983235, AB983197, AB983196, AB983199, AB983198, AB983195, AB982225, AB982226, AB982227, AB982228, LC012933, LC032460).