Abstract
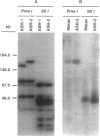
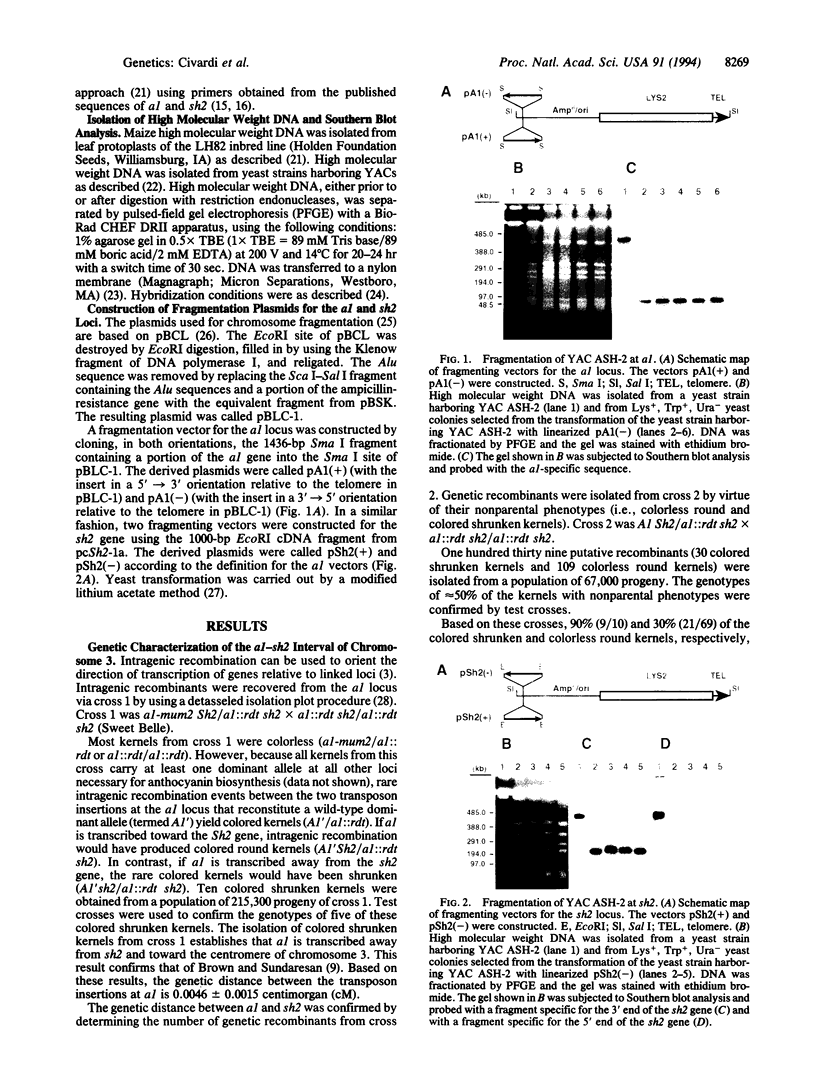
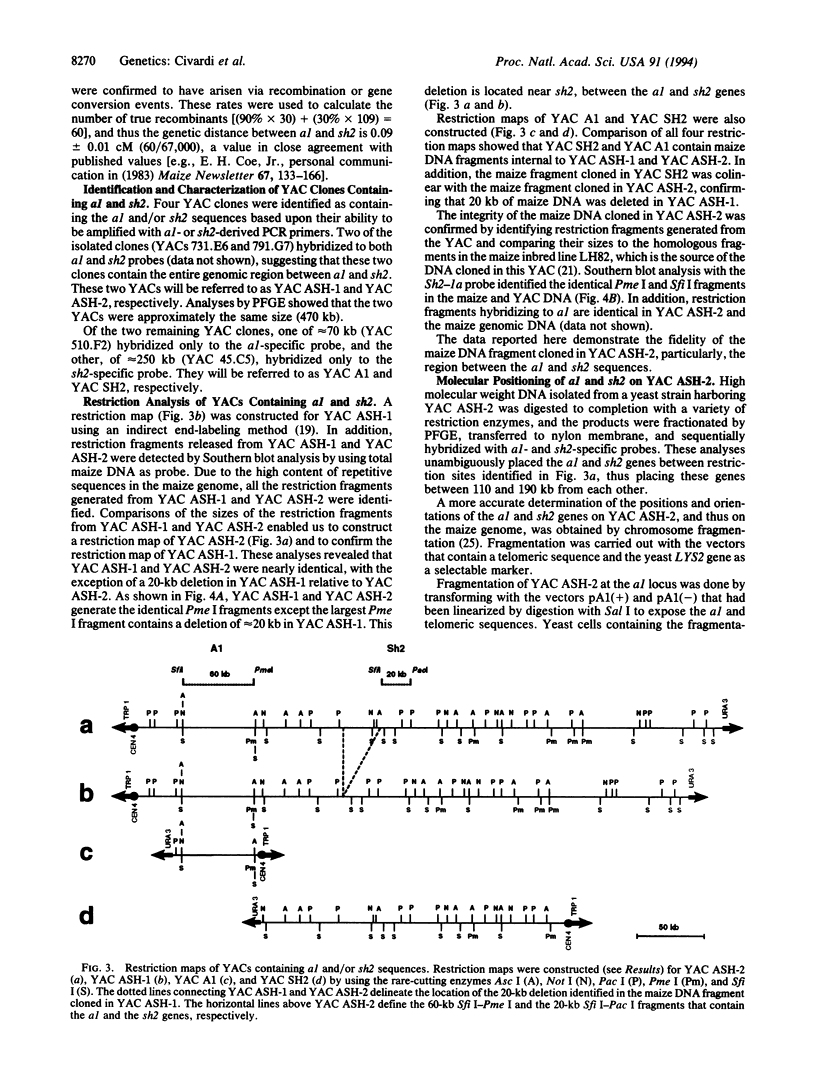
A 470-kb segment from the long arm of chromosome 3 of Zea mays (inbred LH82), encompassing the a1-sh2 interval, was cloned as a yeast artificial chromosome. Comparison of the sizes of the restriction fragments generated from the cloned DNA fragment and from the DNA isolated from the maize inbred line LH82 established the colinearity of the a1-sh2 interval in these DNAs. By utilizing a chromosome fragmentation technique, a yeast artificial chromosome encompassing the a1-sh2 interval was separately fragmented at the a1 and sh2 loci. Comparison of the sizes of these fragmentation products established the physical distance between the a1 and sh2 loci to be 140 kb. Furthermore, these fragmentation experiments established the physical orientation of the a1 and sh2 genes relative to the maize centromere. The molecular cloning of the contiguous region between the a1 and sh2 loci made it possible to define the relationship between physical and genetic distances over a relatively large segment of the maize genome. In this interval, the relationship between physical and genetic distances is 1560 kb/centimorgan, which compares with 1460 kb/centimorgan for the entire maize genome, and 217 kb/centimorgan for a 1-kb segment within the a1 locus. Therefore, these findings are consistent with the hypothesis that genes per se are preferred sites for meiotic recombination rather than the hypothesis that genes reside in large recombinationally active segments of the genome.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albertsen H. M., Abderrahim H., Cann H. M., Dausset J., Le Paslier D., Cohen D. Construction and characterization of a yeast artificial chromosome library containing seven haploid human genome equivalents. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4256–4260. doi: 10.1073/pnas.87.11.4256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhave M. R., Lawrence S., Barton C., Hannah L. C. Identification and molecular characterization of shrunken-2 cDNA clones of maize. Plant Cell. 1990 Jun;2(6):581–588. doi: 10.1105/tpc.2.6.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. J., Mattes M. G., O'Reilly C., Shepherd N. S. Molecular characterization of rDt, a maize transposon of the "Dotted" controlling element system. Mol Gen Genet. 1989 Jan;215(2):239–244. doi: 10.1007/BF00339723. [DOI] [PubMed] [Google Scholar]
- Brownstein B. H., Silverman G. A., Little R. D., Burke D. T., Korsmeyer S. J., Schlessinger D., Olson M. V. Isolation of single-copy human genes from a library of yeast artificial chromosome clones. Science. 1989 Jun 16;244(4910):1348–1351. doi: 10.1126/science.2544027. [DOI] [PubMed] [Google Scholar]
- Burke D. T., Carle G. F., Olson M. V. Cloning of large segments of exogenous DNA into yeast by means of artificial chromosome vectors. Science. 1987 May 15;236(4803):806–812. doi: 10.1126/science.3033825. [DOI] [PubMed] [Google Scholar]
- Dooner H. K. Genetic Fine Structure of the BRONZE Locus in Maize. Genetics. 1986 Aug;113(4):1021–1036. doi: 10.1093/genetics/113.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner H. K., Keller J., Harper E., Ralston E. Variable Patterns of Transposition of the Maize Element Activator in Tobacco. Plant Cell. 1991 May;3(5):473–482. doi: 10.1105/tpc.3.5.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner H. K., Kermicle J. L. The Transposable Element Ds Affects the Pattern of Intragenic Recombination at the bz and R Loci in Maize. Genetics. 1986 May;113(1):135–143. doi: 10.1093/genetics/113.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards K. J., Thompson H., Edwards D., de Saizieu A., Sparks C., Thompson J. A., Greenland A. J., Eyers M., Schuch W. Construction and characterisation of a yeast artificial chromosome library containing three haploid maize genome equivalents. Plant Mol Biol. 1992 May;19(2):299–308. doi: 10.1007/BF00027351. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Freeling M. Allelic variation at the level of intragenic recombination. Genetics. 1978 May;89(1):211–224. doi: 10.1093/genetics/89.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith D. W., Harkins K. R., Maddox J. M., Ayres N. M., Sharma D. P., Firoozabady E. Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science. 1983 Jun 3;220(4601):1049–1051. doi: 10.1126/science.220.4601.1049. [DOI] [PubMed] [Google Scholar]
- Gietz D., St Jean A., Woods R. A., Schiestl R. H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992 Mar 25;20(6):1425–1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambie E. J., Roeder G. S. Repression of meiotic crossing over by a centromere (CEN3) in Saccharomyces cerevisiae. Genetics. 1986 Nov;114(3):769–789. doi: 10.1093/genetics/114.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B. C., Shah N. P., Braun B. S., Denny C. T. Creation of a yeast artificial chromosome fragmentation vector based on lysine-2. Genet Anal Tech Appl. 1992 Jun;9(3):86–90. doi: 10.1016/1050-3862(92)90003-n. [DOI] [PubMed] [Google Scholar]
- Martin G. B., Brommonschenkel S. H., Chunwongse J., Frary A., Ganal M. W., Spivey R., Wu T., Earle E. D., Tanksley S. D. Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science. 1993 Nov 26;262(5138):1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- Nelson O. E. The WAXY Locus in Maize. II. the Location of the Controlling Element Alleles. Genetics. 1968 Nov;60(3):507–524. doi: 10.1093/genetics/60.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly C., Shepherd N. S., Pereira A., Schwarz-Sommer Z., Bertram I., Robertson D. S., Peterson P. A., Saedler H. Molecular cloning of the a1 locus of Zea mays using the transposable elements En and Mu1. EMBO J. 1985 Apr;4(4):877–882. doi: 10.1002/j.1460-2075.1985.tb03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBERTS P. A. DIFFERENCE IN THE BEHAVIOR OF EU- AND HETERO-CHROMATIN: CROSSING-OVER. Nature. 1965 Feb 13;205:725–726. doi: 10.1038/205725b0. [DOI] [PubMed] [Google Scholar]
- Sachs M. M., Dennis E. S., Gerlach W. L., Peacock W. J. Two Alleles of Maize ALCOHOL DEHYDROGENASE 1 Have 3' Structural and Poly(a) Addition Polymorphisms. Genetics. 1986 Jun;113(2):449–467. doi: 10.1093/genetics/113.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Shepherd N., Tacke E., Gierl A., Rohde W., Leclercq L., Mattes M., Berndtgen R., Peterson P. A., Saedler H. Influence of transposable elements on the structure and function of the A1 gene of Zea mays. EMBO J. 1987 Feb;6(2):287–294. doi: 10.1002/j.1460-2075.1987.tb04752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley S. D., Ganal M. W., Prince J. P., de Vicente M. C., Bonierbale M. W., Broun P., Fulton T. M., Giovannoni J. J., Grandillo S., Martin G. B. High density molecular linkage maps of the tomato and potato genomes. Genetics. 1992 Dec;132(4):1141–1160. doi: 10.1093/genetics/132.4.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D., Davis R. W., Connelly C., Hieter P. Physical mapping of large DNA by chromosome fragmentation. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6027–6031. doi: 10.1073/pnas.85.16.6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessler S. R., Varagona M. J. Molecular basis of mutations at the waxy locus of maize: correlation with the fine structure genetic map. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4177–4181. doi: 10.1073/pnas.82.12.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]