Abstract
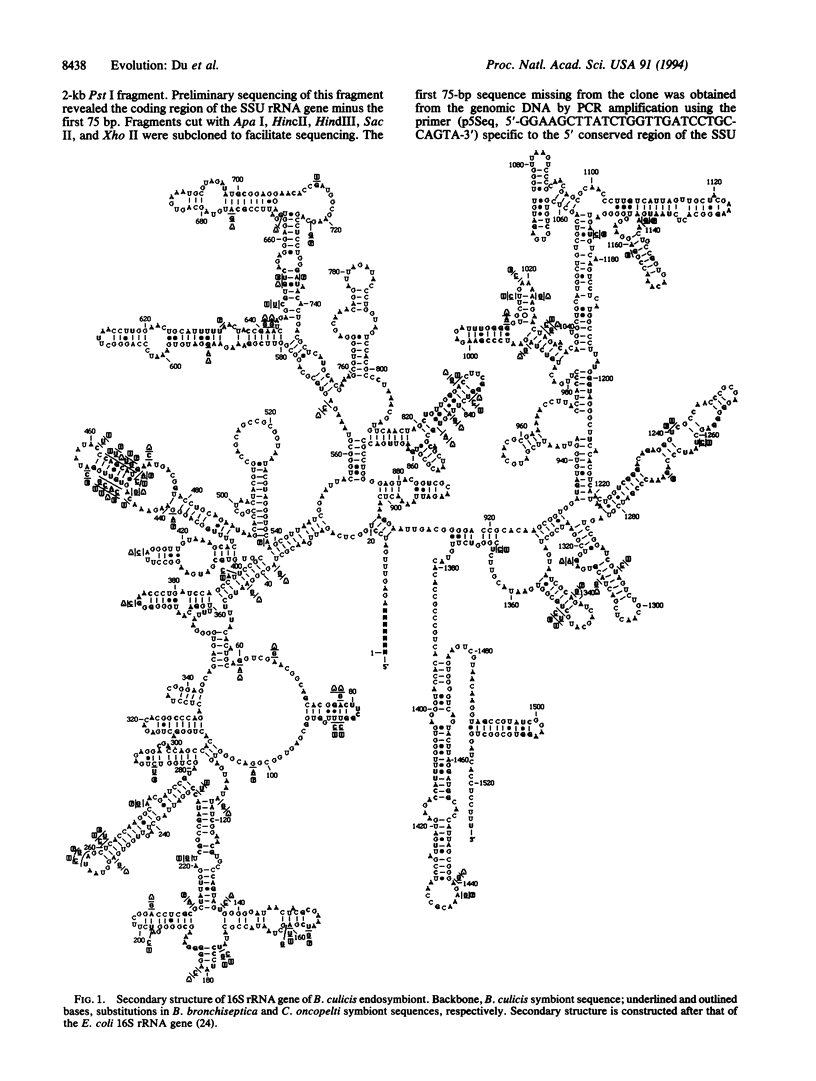
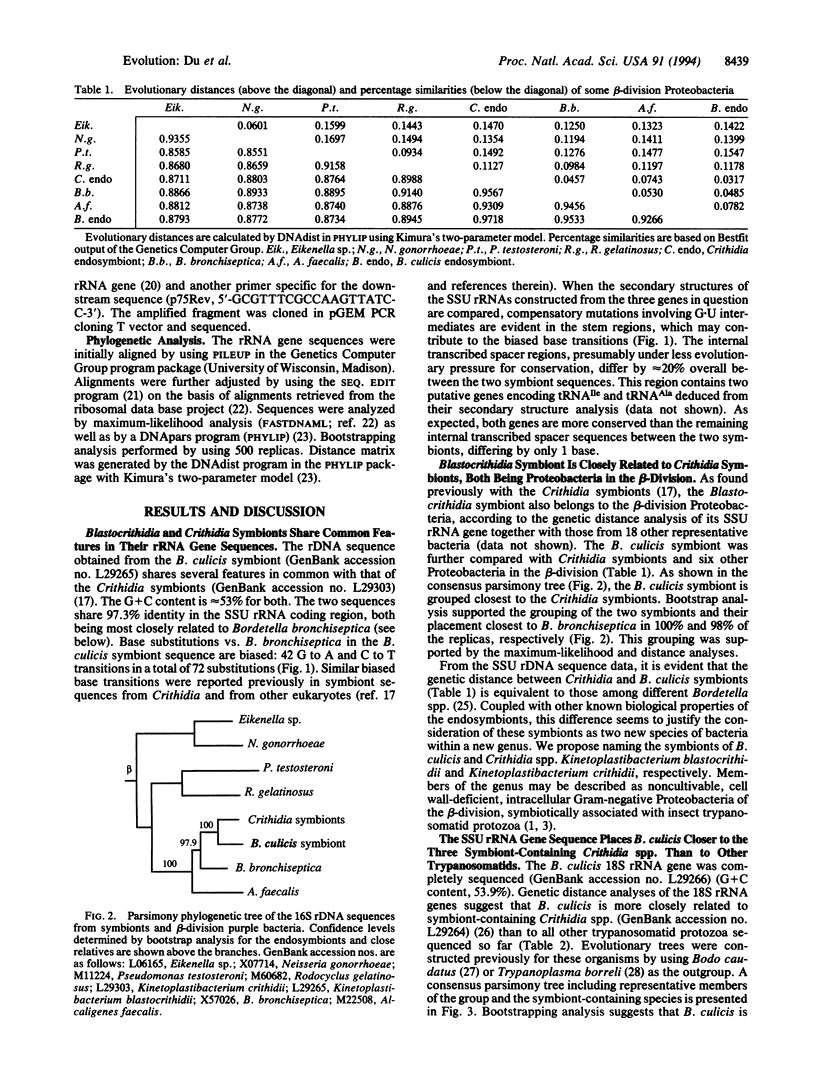
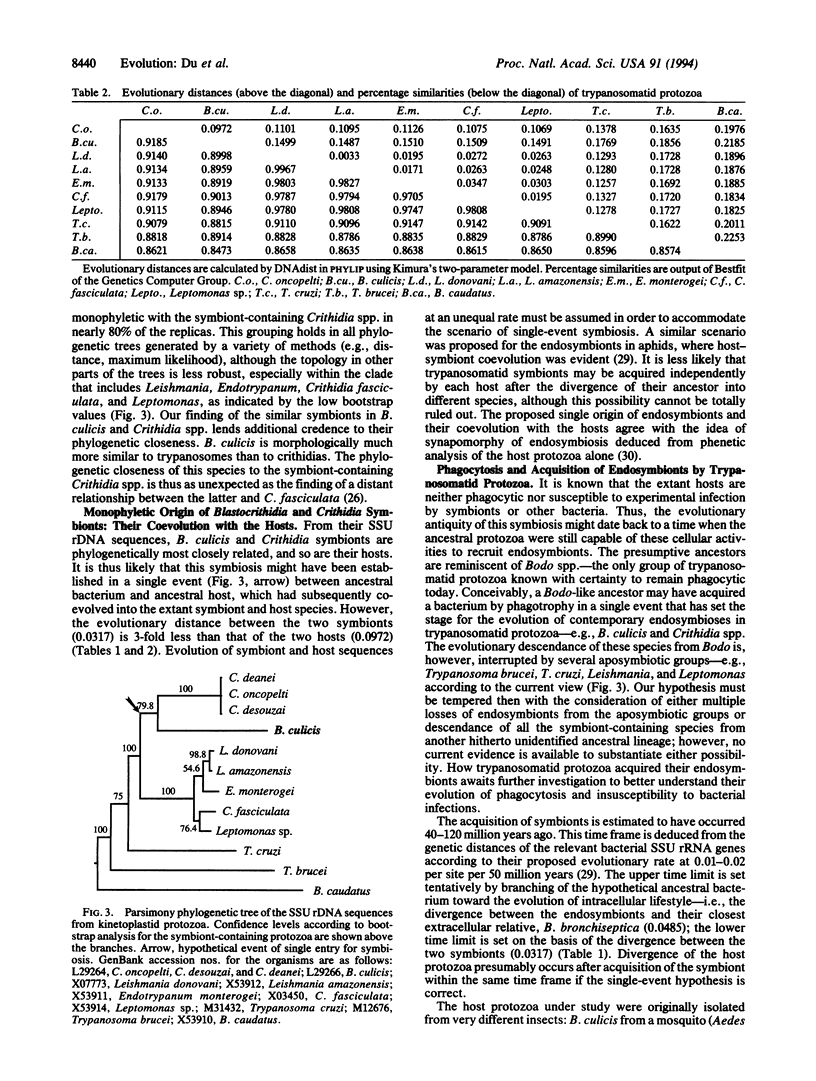
Some trypanosomatid protozoa (order Kinetoplastida) are well known to harbor bacterial endosymbionts. Their phylogenetic positions and evolutionary relationships with the hosts were deduced by comparing the rRNA gene sequences. Earlier, we observed that these symbionts from three Crithidia spp. are identical and are closely related to Bordetella bronchiseptica. We have now sequenced the genes of another endosymbiont and the host protozoan Blastocrithidia culicis. The 16S rRNA genes of the Blastocrithidia and Crithidia symbionts share approximately 97% identity and form a distinct group, branching off the B. bronchiseptica lineage in the beta-division of Proteobacteria. Comparison of their secondary structures in the stem regions suggests compensatory mutations of the symbiont sequences, contributing to their biased base transitions from G to A and C to T. Two putative genes encoding tRNA(Ile) and tRNA(Ala) are highly conserved in the otherwise variable internal transcribed spacer region. Comparisons of the host rRNA gene sequences suggest that the symbiont-containing Crithidia and Blastocrithidia are more akin to each other than to other trypanosomatids. The evidence suggests that Blastocrithidia and Crithidia symbionts descend from a common ancestor, which had presumably entered an ancestral host and thence coevolved with it into different species. We therefore propose naming the symbionts Kinetoplastibacterium blastocrithidii and Kinetoplastibacterium crithidii.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brewin N. J. Development of the legume root nodule. Annu Rev Cell Biol. 1991;7:191–226. doi: 10.1146/annurev.cb.07.110191.001203. [DOI] [PubMed] [Google Scholar]
- Brun R. Ultrastruktur und Zyklus von Herpetomonas muscarum, "Herpetomonas mirabilis" und Crithidia luciliae in Chrysomyia chloropyga. Acta Trop. 1974;32(3):219–290. [PubMed] [Google Scholar]
- Chang K. P. Reduced growth of Blastocrithidia culicis and Crithidia oncopelti freed of intracellular symbiotes by chloramphenicol. J Protozool. 1975 May;22(2):271–276. doi: 10.1111/j.1550-7408.1975.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Chang K. P., Trager W. Nutritional significance of symbiotic bacteria in two species of hemoflagellates. Science. 1974 Feb 8;183(4124):531–532. doi: 10.1126/science.183.4124.531. [DOI] [PubMed] [Google Scholar]
- Chang K. P. Ultrastructure of symbiotic bacteria in normal and antibiotic-treated Blastocrithidia culicis and Crithidia oncopelti. J Protozool. 1974 Nov;21(5):699–707. doi: 10.1111/j.1550-7408.1974.tb03733.x. [DOI] [PubMed] [Google Scholar]
- Du Y., McLaughlin G., Chang K. P. 16S ribosomal DNA sequence identities of beta-proteobacterial endosymbionts in three Crithidia species. J Bacteriol. 1994 May;176(10):3081–3084. doi: 10.1128/jb.176.10.3081-3084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria e Silva P. M., Solé-Cava A. M., Soares M. J., Motta M. C., Fiorini J. E., de Souza W. Herpetomonas roitmani (Fiorini et al., 1989) n. comb.: a trypanosomatid with a bacterium-like endosymbiont in the cytoplasm. J Protozool. 1991 Sep-Oct;38(5):489–494. doi: 10.1111/j.1550-7408.1991.tb04822.x. [DOI] [PubMed] [Google Scholar]
- Fernandes A. P., Nelson K., Beverley S. M. Evolution of nuclear ribosomal RNAs in kinetoplastid protozoa: perspectives on the age and origins of parasitism. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11608–11612. doi: 10.1073/pnas.90.24.11608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray M. W. Organelle origins and ribosomal RNA. Biochem Cell Biol. 1988 May;66(5):325–348. doi: 10.1139/o88-042. [DOI] [PubMed] [Google Scholar]
- Martin D. S., Desser S. S. A light and electron microscopic study of Trypanosoma fallisi N. Sp. in toads (Bufo americanus) from Algonquin Park, Ontario. J Protozool. 1990 May-Jun;37(3):199–206. doi: 10.1111/j.1550-7408.1990.tb01128.x. [DOI] [PubMed] [Google Scholar]
- Maslov D. A., Avila H. A., Lake J. A., Simpson L. Evolution of RNA editing in kinetoplastid protozoa. Nature. 1994 Mar 24;368(6469):345–348. doi: 10.1038/368345a0. [DOI] [PubMed] [Google Scholar]
- McGhee R. B., Cosgrove W. B. Biology and physiology of the lower Trypanosomatidae. Microbiol Rev. 1980 Mar;44(1):140–173. doi: 10.1128/mr.44.1.140-173.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlin L., Elwood H. J., Stickel S., Sogin M. L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene. 1988 Nov 30;71(2):491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- Mundim M. H., Roitman I., Hermans M. A., Kitajima E. W. Simple nutrition of Crithidia deanei, a reduviid trypanosomatid with an endosymbiont. J Protozool. 1974 Oct;21(4):518–521. doi: 10.1111/j.1550-7408.1974.tb03691.x. [DOI] [PubMed] [Google Scholar]
- Munson M. A., Baumann L., Baumann P. Buchnera aphidicola (a prokaryotic endosymbiont of aphids) contains a putative 16S rRNA operon unlinked to the 23S rRNA-encoding gene: sequence determination, and promoter and terminator analysis. Gene. 1993 Dec 31;137(2):171–178. doi: 10.1016/0378-1119(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Müller M., Hildebrandt A. Nucleotide sequences of the 23S rRNA genes from Bordetella pertussis, B.parapertussis, B.bronchiseptica and B.avium, and their implications for phylogenetic analysis. Nucleic Acids Res. 1993 Jul 11;21(14):3320–3320. doi: 10.1093/nar/21.14.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON B. A., HORNE R. W. Intracellular structures in Strigomonas oncopelti. I. Cytoplasmic structures containing ribonucleoprotein. Exp Cell Res. 1957 Dec;13(3):563–574. doi: 10.1016/0014-4827(57)90086-1. [DOI] [PubMed] [Google Scholar]
- Noller H. F. Structure of ribosomal RNA. Annu Rev Biochem. 1984;53:119–162. doi: 10.1146/annurev.bi.53.070184.001003. [DOI] [PubMed] [Google Scholar]
- Olsen G. J., Lane D. J., Giovannoni S. J., Pace N. R., Stahl D. A. Microbial ecology and evolution: a ribosomal RNA approach. Annu Rev Microbiol. 1986;40:337–365. doi: 10.1146/annurev.mi.40.100186.002005. [DOI] [PubMed] [Google Scholar]
- Olsen G. J., Overbeek R., Larsen N., Marsh T. L., McCaughey M. J., Maciukenas M. A., Kuan W. M., Macke T. J., Xing Y., Woese C. R. The Ribosomal Database Project. Nucleic Acids Res. 1992 May 11;20 (Suppl):2199–2200. doi: 10.1093/nar/20.suppl.2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace F. G. The trypanosomatid parasites of insects and arachnids. Exp Parasitol. 1966 Feb;18(1):124–193. doi: 10.1016/0014-4894(66)90015-4. [DOI] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


