Background: The 9-1-1 complex mediates checkpoint signaling and repair.
Results: HUS1 functional residues for clamp formation, DNA contacts, and protein-protein association were identified.
Conclusion: HUS1 mediates checkpoint signaling-independent effector functions.
Significance: Learning how the 9-1-1 complex contributes to both checkpoint signaling and DNA repair is important for understanding the molecular mechanisms underlying a robust DNA damage response.
Keywords: DNA damage, DNA damage response, checkpoint control, proliferating cell nuclear antigen (PCNA), genomic instability, HUS1, 9-1-1 complex
Abstract
The RAD9A-HUS1-RAD1 (9-1-1) complex is a heterotrimeric clamp that promotes checkpoint signaling and repair at DNA damage sites. In this study, we elucidated HUS1 functional residues that drive clamp assembly, DNA interactions, and downstream effector functions. First, we mapped a HUS1-RAD9A interface residue that was critical for 9-1-1 assembly and DNA loading. Next, we identified multiple positively charged residues in the inner ring of HUS1 that were crucial for genotoxin-induced 9-1-1 chromatin localization and ATR signaling. Finally, we found two hydrophobic pockets on the HUS1 outer surface that were important for cell survival after DNA damage. Interestingly, these pockets were not required for 9-1-1 chromatin localization or ATR-mediated CHK1 activation but were necessary for interactions between HUS1 and its binding partner MYH, suggesting that they serve as interaction domains for the recruitment and coordination of downstream effectors at damage sites. Together, these results indicate that, once properly loaded onto damaged DNA, the 9-1-1 complex executes multiple, separable functions that promote genome maintenance.
Introduction
When left unchecked, DNA insults from exogenous and endogenous sources can lead to premature aging, developmental defects, and tumorigenesis (1). To prevent these deleterious outcomes, cells have evolved DNA damage response (DDR)2 pathways that are responsible for triggering an appropriate protective reaction to genome damage. Central to DDR pathways are checkpoint proteins that regulate cell cycle transitions, DNA repair, replication fork stability, and apoptosis (2).
The RAD9A-HUS1-RAD1 (9-1-1) clamp is a toroidal heterotrimeric DNA clamp that regulates checkpoint signaling after DNA damage (3). It is essential for cell survival after genotoxin exposure and during various physiological processes, such as embryogenesis, adult tissue homeostasis, and spermatogenesis (4–9). The 9-1-1 clamp is structurally related to proliferating cell nuclear antigen (PCNA), a homotrimeric processivity factor for DNA replication and repair (10). Like PCNA, each 9-1-1 subunit folds into two globular domains linked by an interdomain connecting loop (11–13). The subunits associate in a head-to-tail manner to form a stable heterotrimeric complex. A clamp loader composed of RFC1–5 loads PCNA at 3′ recessed DNA ends, whereas 9-1-1 is loaded by RAD17/RFC2–5 at 5′ recessed ends (14–16). Once loaded at damage sites, 9-1-1 stimulates ATR kinase activation through interactions with TOPBP1 (17). Activated ATR phosphorylates several substrates, including the effector kinase CHK1, which induce cell cycle arrest, stabilize stalled forks, and inhibit origin firing (2).
In addition to its checkpoint signaling functions, 9-1-1 also acts directly in DNA repair through its role as a molecular scaffold (3, 18, 19). The 9-1-1 clamp physically interacts with and stimulates the activity of factors in many DNA repair pathways, including base excision, mismatch, and nucleotide excision repair, as well as homologous recombination, non-homologous end joining, and translesion synthesis. Evaluating the physiological significance of these interactions is challenging because genetic approaches to ablate 9-1-1 function typically compromise both checkpoint signaling and all other functions executed by the clamp.
In order to resolve the relative importance of the checkpoint signaling-dependent and -independent functions of the 9-1-1 complex, we endeavored to identify residues that are essential for these functions and describe here residues in murine HUS1 (mHUS1) that mediate three critical 9-1-1 activities: clamp formation, DNA association, and interaction with downstream effectors. Consistent with the idea that 9-1-1 has critical signaling-independent roles, we identified HUS1 domains that are dispensable for ATR-mediated signaling to CHK1 but nevertheless required for the cellular response to DNA damage.
Experimental Procedures
Plasmids and Mutagenesis
All mutations were introduced into mHus1 using a QuikChange Lightning multisite-directed mutagenesis kit (Agilent Technologies) and the primers listed in Table 1. Most mutagenesis was performed on the pBP2-mHus1 retroviral plasmid (20) as the template with two exceptions. In the first case, where compound mutations had to be made sequentially, pBP2-mHus1 plasmids with intermediate mutations were used as the template. In the second case, residues Lys-2, Phe-3, Arg-4, and Lys-6 of mHUS1 were mutagenized with the pGEX2T-mHus1 plasmid as the template because 5′ retroviral long terminal repeats in the pBP2-mHus1 plasmid interfered with mutagenesis. Subsequently, the pGEX2T-mHus1 mutants were subcloned into pBP2 plasmid. Functionally defective mutant constructs were further subcloned into pCMV-neo-Bam3 plasmid (21) for mutant mHUS1 immunoblot detection as well as into p3XFLAG-CMVTM-14 (Sigma) for immunofluorescence (IF) and chromatin fractionation assays. All mutations were verified by DNA sequencing.
TABLE 1.
Primers used for site-directed mutagenesis of mHus1
| Mutations | Primera |
|---|---|
| RAD9-interacting residue | |
| R128E | 5′-gtctccatcgagcagcagcgaaatcgtggtgcatgatatc-3′ |
| Inner ring hydrophobic cleft | |
| V19A,M22A,I23A | 5′-cttgtctgaatcatttcacacgagccagtaacgcggcagccaagcttgccaaaacctgcac-3′ |
| V247A | 5′-gccggacagcaagcgactcccaccaag-3′ |
| V271A,L273A | 5′-atttgctcctggaagacgcctccgctcagtatttcatcccagc-3′ |
| R18A | 5′-ctatcatgttactgactgctgtgaaatgattcagacaagccagg-3′b |
| R18Q | 5′-cttgtctgaatcatttcacacaagtcagtaacatgatagccaa-3′ |
| R18Q,M22T | 5′-cttgtctgaatcatttcacacaagtcagtaacacgatagccaagcttgcca-3′ |
| Inner ring positively charged residues | |
| R18A | 5′-ctatcatgttactgactgctgtgaaatgattcagacaagccagg-3′b |
| K25A | 5′-ggtgcaggttttggcaagcgcggctatcatgttactgact-3′b |
| K25A,K28A | 5′-atgcggagggtgcaggttgcggcaagcgcggctatcatgttactgac-3′b |
| K93A | 5′-tctggagttctgggcagttgccaaggctcgagataagttt-3′b |
| R90A,K93A | 5′-tggagttctgggcagttgccaaggctgcagataagttttccgacgttaattctaa-3′b |
| K165A | 5′-ccacaacactcttcatcatcgccaaggctggtaagcaaatac-3′b |
| K165A,K168A | 5′-gtttctcattttttccacaacactcgccatcatcgccaaggctggtaagcaaatactgacg-3′b |
| K173A,R175A | 5′-ttcaatcacaagctgattgctgatgtttgccattgcttccacaacactcttcatcatcttcaag-3′b |
| K236A,K237A | 5′-aagaaactggaggagtgccgctatgtcaatgtgcaccttggccatgtcttct-3′b |
| Outer ring hydrophobic pocket | |
| P150A | 5′-gtggaaggacttacaagaagcctccatcccagac-3′ |
| P150A,I152A,P153A,C155A | 5′-gactgtggaaggacttacaagaagcctccgccgcagacgctgacgtcagtatttgctt-3′ |
| V257A,T261A | 5′-caaggcagtgtgcaatattgcgaataacagagctgttcattttgatttgctc-3′ |
| F276A,P278A | 5′-aagacgtctcccttcagtatgccatcgcagccttgtcctagg-3′ |
| S53R | 5′-gctcacaccacatcctcacgcctccac-3′b |
| I152R | 5′-gacttacaagaaccctccagaccagactgtgacgtcag-3′ |
| I152Y | 5′-aaggacttacaagaaccctcctacccagactgtgacgtcagtatt-3′ |
| I152F | 5′-cttacaagaaccctccttcccagactgtgacgt-3′ |
| Outer ring novel pocket | |
| F3A | 5′-[ccgcgtggatcc]atgaaggctcgcgccaagatcg-3′c |
| F3R | 5′-cgcgtggatccatgaagcgtcgcgccaagatcgtggacc-3′ |
| F3R,R4A | 5′-cgcgtggatccatgaaggctgccgccaagatcgtggacct-3′ |
| G71W | 5′-gaacttttttagtgaatttcaaatggaatgggtctctgaagaaaacaacgagattt-3′ |
| I79A | 5′-aggagtctctgaagaaaacaacgaggcttatttagaattaacgtcggaaaac-3′ |
| L105A | 5′-ccagagccttgaaaatcaaggcgactaacaaacactttccct-3′ |
| K104A,L105A,T106A | 5′-ccagaactccagagccttgaaaatcgcggcggctaacaaacactttccctgtcttac-3′ |
| V138A,L139D | 5′-gcatgatatccccataaaggctgatccgagaagactgtggaagg-3′ |
| L105R | 5′tccagagccttgaaaatcaagaggactaacaaacactttccctg-3′ |
| L139R | 5′-tcgtggtgcatgatatccccataaaggttagaccgagaagactgtgg-3′ |
| R4D | 5′-[cgcgtggatcc]atgaagtttgacgccaagatcgtggacc-3′c |
| F3R,R4D | 5′-[cgcgtggatcc]atgaagcgtgacgccaagatcgtggacc-3′c |
| Outer ring positively charged residues | |
| K2A,R4A,K6A | 5′-gacaagccaggtccacgatcgcggcggcaaacgccat[gaatccacgcgg]-3′b,c |
| R99A,K102A,K104A | 5′-gggaaagtgtttgttagtcagcgcgattgccaaggctgcggagttctgggcagttttcaagg-3′b |
| K108A,H109A | 5′-cagacacggtaagacagggaaaggctgcgttagtcagcttgattttcaagg-3′b |
| K137A,R141A,R142A,K145A | 5′-ggttcttgtaagtccgcccacagtgctgccggaagaaccgctatggggatatcatgcaccacgattc-3′b |
| BII4-6 loop | |
| Δ215–227 | 5′-gtgcaccttggccat ()taatagagggttttcaagatccttaaaa-3′b,d |
| Δ215–227::hHus1 loop | 5′-attttaaggatcttgaaaaccctctattagcctctgaaagtacccatgaaaacagacacccagaagacatggcca-3′ |
| 5′-gcctctgaaagtacccatgaagacagaaacgtagaacacatggccaaggtgcaca-3′ | |
| Δ215–227::PCNA loop | 5′-cattttaaggatcttgaaaaccctctattacaaactagcaatgtcgatcaaaacagacacccagaagacatggccaag-3′ |
| 5′-cttgaaaaccctctattacaaactagcaatgtcgataaagaagaagaggcagtagacatggccaaggtgcacat-3′ | |
a The positions of nucleotides altered to create desired mutations are underlined.
b Antisense primers. Primer orientation was chosen based on lower energy cost of mismatches.
c pGEX-2T sequences shown in brackets.
d (), 39 nucleotides deleted.
Cell Culture, Retroviral Infection and Transfection
All cultured cells were grown on gelatinized dishes in Dulbecco's modified Eagle's medium (Corning Inc.) supplemented with 10% bovine calf serum (Thermo Scientific Hyclone, SH30072), 1% nonessential amino acids (Corning Cellgro, 25-025-CI), 1% l-glutamate (25-005-CI), and 1% penicillin and streptomycin (30-002-CI). Expression of the various mHus1 constructs in Hus1−/−p21−/− mouse embryonic fibroblasts (MEFs) and HEK293T cells (ATCC) was done in two ways. The first method was pBabe-based retroviral transduction for low level ectopic Hus1 expression as described previously (20). The second method was plasmid transfection of pCMV-mHus1 and pCMV-mHus1–3XFLAG high level expression constructs done as follows. A mix of 575 μl of DMEM, 40 μg of polyethyleneimine, 4 μg of pCMV plasmid, and 1 μg of pGK-puro plasmid was dripped onto 106 Hus1−/− p21−/− MEFs seeded the day before in a 10-cm dish. Transfected cells were selected in culture medium containing 1.83 μg/ml puromycin, replaced every other day for a week. Stable drug-resistant cells made from both methods were cultured according to the 3T3 passaging protocol for maintenance and experimental use. For co-immunoprecipitation (co-IP) assays, pCMV-mHUS1 constructs were co-transfected with pCMV-hRad9a-Myc and pCMV-hRad1-HA plasmids.
Survival Assays
For short term viability assays, cells were seeded in 6-well plates and either left untreated or treated with 50 ng/ml 4NQO or 0.5 μm aphidicolin for 24 h. Mitomycin C (MMC) treatment was for 1 h. After 3 days, the cells were collected by trypsinization and counted using a MoxiTM Z mini automated cell counter (ORFLO Technologies). Percentage survival was calculated by dividing the number of cells after treatment by the number of untreated cells. Error bars in the figures show S.D. Statistical analysis was by Student's t test, and p values of <0.05 were considered significant. For clonogenic survival assays, cells were seeded in 6-well plates and treated with 4NQO or aphidicolin for 24 h or with MMC for 1 h. After 6 days, the cells were fixed with methanol and stained with crystal violet overnight. The plates were then washed, dried, and scanned.
ConSurf Evolutionary Conservation and Surface Electrostatic Potential Analyses
Amino acid sequences of PCNA, RAD9A, HUS1, and RAD1 from 44 organisms that represent a broad range of taxa were curated from the UniProtKB database (Table 2). Multiple sequence alignments were created with ClustalX version 2.1 (22) and uploaded to the ConSurf server (23) for calculation of evolutionary conservation scores (Bayesian method) with reference to the human counterparts of each protein. The scores were projected on available protein structures of PCNA (Protein Data Bank code 1VYM) and RAD9A-HUS1-RAD1 (Protein Data Bank code 3GGR) to identify functional surface residues. All images were created using PyMOL. The surface electrostatic potential of HUS1 was calculated and displayed using the Adaptive Poisson-Boltzmann Solver plugin in PyMOL. In the calculations, dielectric constants of 1.0 and solvent ionic strength equivalent to 75 mm KCl were used. Side chains of lysine and arginine residues were assigned a net positive charge, aspartate and glutamate were assigned a negative charge, and other residues were neutral. Positive and negative color contours were set at ±10kT/e.
TABLE 2.
List of UniProtKB accession numbers of the PCNA, RAD9A, HUS1, and RAD1 sequences used for evolutionary conservation analysis
| Organism | Symbol | Accession numbers |
|||
|---|---|---|---|---|---|
| PCNA | RAD9A | HUS1 | RAD1 | ||
| Ailuropoda melanoleuca (giant panda) | AILME | D2HQS7 | G1L1M9 | G1LDT0 | G1L9B8 |
| Arabidopsis thaliana (mouse-ear cress) | ARATH | Q9M7Q7 | F4J7B7 | Q709F6 | Q8L7G8 |
| Bos taurus (bovine) | BOVIN | Q3ZBW4 | Q5EAC3 | E1BG06 | E1BB72 |
| Caenorhabditis elegans (nematode worm) | CAEEL | O02115 | Q9NBJ6 | G5EFI9 | G5EC44 |
| Callithrix jacchus (white-tufted-ear marmoset) | CALJA | F7GZC8 | U3DMA2 | F7G3C8 | F7I3N9 |
| Canis familiaris (dog) | CANFA | E2R0D6 | F6XPS6 | F1Q245 | E2QYH8 |
| Cavia porcellus (guinea pig) | CAVPO | H0VE65 | H0VIK1 | H0WC14 | H0VEA3 |
| Ceratitis capitata (Mediterranean fruit fly) | CERCA | W8B157 | W8C9F2 | W8C4C2 | W8B5C0 |
| Gallus gallus (chicken) | CHICK | Q9DEA3 | R4GG06 | E1C8I4 | E1C4I3 |
| Chlamydomonas reinhardtii (Chlamydomonas smithii) | CHLRE | A8JHX0 | A8IS48 | A8J5N4 | A8IFX0 |
| Dictyostelium discoideum (slime mold) | DICDI | Q54K47 | Q869Q1 | Q54NC0 | Q55E62 |
| Drosophila melanogaster (fruit fly) | DROME | P17917 | O96533 | Q9VN60 | Q9VQD4 |
| Felis catus (cat) | FELCA | M3WAR4 | M3W096 | M3XC14 | M3WY16 |
| Equus caballus (horse) | HORSE | F6R950 | F6QXP4 | F7BM24 | F6YZW4 |
| Homo sapiens (human) | HUMAN | P12004 | Q99638 | O60921 | O60671 |
| Hydra vulgaris (hydra) | HYDVU | T2MHJ2 | T2M799 | T2MIV2 | T2MID6 |
| Lepisosteus oculatus (spotted gar) | LEPOC | W5NF42 | W5MKE2 | W5N6Y6 | W5N1G5 |
| Loxodonta africana (African elephant) | LOXAF | G3SY50 | G3T2S3 | G3TJN6 | G3SZN1 |
| Macaca mulatta (rhesus macaque) | MACMU | F6ZD63 | H9FXY2 | F7F1Y2 | F7A5K9 |
| Mus musculus (mouse) | MOUSE | P17918 | Q9Z0F6 | Q8BQY8 | Q9QWZ1 |
| Mustela putorius furo (European domestic ferret) | MUSPF | M3Y491 | M3XXF9 | M3Z395 | M3YUM0 |
| Myotis lucifugus (little brown bat) | MYOLU | G1NW67 | G1P3Z2 | G1NTI5 | G1PS54 |
| Neovison vison (American mink) | NEOVI | U6DX35 | U6D1D1 | U6CPZ2 | U6CY10 |
| Nomascus leucogenys (northern white-cheeked gibbon) | NOMLE | G1R863 | G1R3F3 | G1QWZ3 | G1RWE2 |
| Oreochromis niloticus (Nile tilapia) | ORENI | I3KAK2 | I3JC68 | I3K6T5 | I3JLK1 |
| Ornithorhynchus anatinus (duckbill platypus) | ORNAN | F7BRC7 | F6REC2 | F7BS27 | F6UI60 |
| Otolemur garnettii (small-eared galago) | OTOGA | H0XLL4 | H0XWZ7 | H0X7H9 | H0XC10 |
| Pan troglodytes (chimpanzee) | PANTR | H2QJX3 | K7DL38 | H2QUJ9 | K7BUE0 |
| Sus scrofa (pig) | PIG | I3L813 | F1RUX7 | B6UV60 | F1SND5 |
| Polysphondylium pallidum (cellular slime mold) | POLPA | D3BSY5 | D3BA05 | D3BR17 | D3BPJ7 |
| Pongo abelii (Sumatran orangutan) | PONAB | H2P1A0 | H2NCN7 | H2PXG5 | Q5R7X9 |
| Oryctolagus cuniculus (rabbit) | RABIT | G1SKZ3 | G1TKX6 | G1TRN1 | G1T7G8 |
| Rattus norvegicus (rat) | RAT | P04961 | D3ZXM2 | D3ZNA8 | D3ZC52 |
| Sarcophilus harrisii (Tasmanian devil) | SARHA | G3WDY3 | G3VT27 | G3W0W1 | G3WBB6 |
| Schizosaccharomyces pombe (fission yeast) | SCHPO | Q03392 | P26306 | P78955 | P22193 |
| Ovis aries (sheep) | SHEEP | W5Q6P4 | W5PNJ1 | W5PS82 | W5PPP9 |
| Spermophilus tridecemlineatus (thirteen-lined ground squirrel) | SPETR | I3NDE1 | I3NF38 | I3MYM9 | I3MDU2 |
| Strongylocentrotus purpuratus (purple sea urchin) | STRPU | W4Z5C9 | W4YCU0 | W4ZAK8 | W4ZIN8 |
| Tetraodon nigroviridis (spotted green pufferfish) | TETNG | H3DD39 | H3D6H6 | H3D7M2 | H3BWC7 |
| Wickerhamomyces ciferrii (yeast) | WICCF | K0KS34 | K0KG77 | K0KFL9 | K0KTE1 |
| Xenopus laevis (African clawed frog) | XENLA | P18248 | Q7ZZU5 | Q8JHD8 | Q8AY27 |
| Xenopus tropicalis (Western clawed frog) | XENTR | Q66KJ8 | Q6DJ26 | Q6DF51 | A9ULD8 |
| Xiphophorus maculatus (Southern platyfish) | XIPMA | M4AKD0 | M4A625 | M4AET7 | M3ZPZ4 |
| Saccharomyces cerevisiae (bakers' yeast) | YEAST | P15873 | Q08949 | Q02574 | P48581 |
Immunoprecipitation and Immunoblotting
For analysis of 9-1-1 subunit interactions, HEK293T cells transiently transfected with pCMV-mHus1, pCMV-hRad9a-Myc, and pCMV-hRad1-HA constructs were irradiated with 100 J/m2 UV, and 2 h later, cell lysates for co-IP were prepared. Lysates were incubated with anti-MYC (Santa Cruz Biotechnology, Inc.) or anti-HA (Covance) antibodies, followed by incubation with protein A/G resin (Thermo Scientific). For analysis of HUS1-MYH interactions, HEK293T transiently transfected with pCMV-mHus1–3XFLAG or pCMV-R4D,I152Y-3XFLAG constructs were treated with 1 mm H2O2, and 3 h later, cell lysates for co-IP were prepared. Lysates were incubated with anti-FLAG resin (Sigma). Immunoprecipitates or total cell lysates (input) were resolved by SDS-PAGE. Standard immunoblotting procedures were performed using antibodies specific for HUS1 (8), RAD9A (8), MYC (Santa Cruz Biotechnology), HA (Covance), FLAG (Sigma), pCHK1 Ser-345 (Cell Signaling), histone 3 (Abcam), GAPDH (Advanced ImmunoChemical), MYH (24), TOPBP1 (25), or β-actin (Sigma).
Immunofluorescence
Hus1−/−p21−/− MEFs stably expressing WT or mutant mHUS1–3XFLAG proteins were grown on gelatinized coverslips overnight and treated with MMC for 23 h. Cells were immunostained with mouse α-FLAG and rabbit α-RAD9A primary antibodies and Alexa Fluor 488 goat α-mouse and Alexa Fluor 555 goat α-rabbit secondary antibodies (Life Technologies, Inc.) for IF detection according to the antibody manufacturer's protocol. Overlapping FLAG and RAD9A foci in 50 randomly picked cells from each sample were quantified and analyzed by one-way analysis of variance. p values of <0.05 were considered significant.
Chromatin Fractionation
Hus1−/−p21−/− MEFs stably expressing WT or mutant mHUS1–3XFLAG proteins were irradiated with 100 J/m2 UVC and fractionated 2 h post-treatment using a previously described extraction protocol (26) with modifications. Cells were swollen in hypotonic buffer (10 mm HEPES, pH 7.9, 1.5 mm MgCl2, 75 mm KCl, 0.2 mm PMSF, and 0.5 m DTT) for 6 min in 37 °C and lysed with a Dounce homogenizer. After centrifugation at 14,000 rpm for 15 min, the supernatant was separated for cytoplasmic extract preparation. The nuclei pellet was resuspended in equal volumes of low salt and high salt buffers (20 mm HEPES, pH 7.9, 25% glycerol, 1.5 mm MgCl2, 0.2 or 1.2 m KCl, 0.2 mm EDTA, 0.2 mm PMSF, and 0.5 m DTT) in sequential order to extract the soluble nuclear fraction. After centrifugation at 14,000 rpm for 30 min, the supernatant was separated for nuclear extract preparation. The pellet was resuspended in radioimmune precipitation assay buffer (50 mm Tris, pH 8.0, 150 mm NaCl, 5 mm EDTA, 50 mm NaF, 0.5% deoxycholate, 1% Nonidet P-40, and 0.1% SDS) supplemented with aprotinin, leupeptin, sodium orthovanadate, and phenylmethylsulfonyl fluoride (PMSF), sonicated at 24–30 watts for 1 min (Misonix Sonicator 3000), and centrifuged to produce the chromatin fraction in the supernatant. The cytoplasmic and nuclear fractions were dialyzed in dialysis buffer (20 mm HEPES, pH 7.9, 20% glycerol, 100 mm KCl, 0.2 mm EDTA, 0.2 mm PMSF, and 0.5 m DTT) overnight before use. 20 μg of each fraction was used for immunoblotting.
Results
A Systematic Structure/Function Analysis of HUS1
We used published crystal structure analyses of human 9-1-1 (11–13), computational modeling of 9-1-1 subunit and DNA interactions (27, 28), and evolutionary conservation analysis to predict functionally important mHUS1 residues. Initially we screened mHUS1 mutants with targeted mutations in seven specific regions (Table 3) for the ability to complement the genotoxin sensitivity of Hus1−/−p21−/− MEFs, which are hypersensitive to DNA damage (6). As detailed below, three HUS1 regions (the HUS1/RAD9A interface, the positively charged inner surface of HUS1, and two hydrophobic pockets on the HUS1 outer surface) emerged as having the greatest functional significance in complemented cells challenged with 4NQO, a UV mimetic, or aphidicolin, a replication stress-inducing DNA polymerase inhibitor.
TABLE 3.
Summary of clonogenic survival and short term viability assay results for all mHUS1 mutants analyzed
| Mutations | Expression | Clonogenic survivala |
Short term viabilitya |
||
|---|---|---|---|---|---|
| 4NQO | Aphidicolin | 4NQO | Aphidicolin | ||
| RAD9-interacting residue | |||||
| R128E | Yes | Null | Null | Null | Null |
| Inner ring hydrophobic cleft | |||||
| V19A,M22A,I23A | Partial | WT | WT | WT | WT |
| V247A,V271A,L273A | Partial | WT | WT | WT | WT |
| V19A,M22A,I23A,V247A,V271A,L273A | Partial | WT | Partial | Partial | Null |
| R18A | Yes | NTb | NT | WT | WT |
| R18Q | Yes | NT | NT | WT | WT |
| R18Q,M22T | Yes | NT | NT | WT | WT |
| Inner ring positively charged residues | |||||
| K93A | NT | WT | WT | WT | WT |
| K25A | NT | WT | WT | WT | WT |
| K25A,K93A | Yes | WT | WT | WT | WT |
| K25A,K236A,K237A (3A) | Yes | Partial | Partial | Partial | WT |
| K173A,R175A,K236A,K237A (4A-1) | Yes | WT | Partial | Partial | Partial |
| K25A,K93A,K236A,K237A (4A-2) | Yes | Null | Null | Null | Null |
| K25A,K93A,K173A,R175A,K236A,K237A (6A) | Yes | Null | Null | Null | Null |
| R90A,K93A (C1) | Yes | WT | WT | WT | Partial |
| R18A,K25A,K28A (C2) | NT | WT | WT | WT | WT |
| K236A,K237A (C3) | NT | WT | WT | WT+ | WT |
| K165A,K168A,K173A,R175A (C4) | Yes | WT | Partial | WT | Partial |
| R18A,K93A,K165A (C5) | Yes | WT | Partial | Partial | WT |
| K173A,R175A | NT | WT | WT | WT+ | WT |
| K165A,K168A | NT | WT | WT | NT | NT |
| K25A,K28A,K165A | Not stable | NT | NT | NT | NT |
| Outer ring hydrophobic pocket | |||||
| P150A,I152A,P153A,C155A | Partial | WT | WT | Partial | Partial |
| V257A,T261A,F276A,P278A | Yes | WT | WT | WT | WT |
| P150A,I152A,P153A,C155A,V257A,T261A,F276A,P278A | Partial | WT | Partial | NT | NT |
| P150A,V257A,T261A,F276A,P278A | Partial | WT | Partial | WT | Partial |
| P150A,I152A,P153A,C155A,F276A,P278A | Not stable | Partial | Partial | Partial | Partial |
| S53R | Yes | NT | NT | WT | WT |
| I152R | Partial | NT | NT | Partial | Partial |
| I152Y | Yes | Partial | WT | Partial | Partial |
| I152F | Yes | NT | NT | WT | WT |
| Outer ring novel pocket | |||||
| F3A,G71W,I79A,L105A | Not stable | Null | Null | NT | NT |
| F3A,G71W | NT | WT | WT | NT | NT |
| F3A,I79A | NT | WT | WT | NT | NT |
| F3A,L105A | NT | WT | WT | NT | NT |
| F3A,I79A,L105A | Not stable | WT | Null | NT | NT |
| F3R | Not stable | Partial | Partial | Partial | WT |
| F3R,R4A | NT | Partial | Partial | Partial | WT |
| K104A,L105A,T106A | NT | WT | WT | WT | Partial |
| K104A,L105A,T106A,V138A,L139D | Not stable | Null | Null | Null | Null |
| L105R | Not stable | NT | NT | NT | NT |
| L139R | Not stable | NT | NT | NT | NT |
| R4D | Yes | Partial | WT | Partial | Partial |
| R4D,I152Y | Yes | Partial | WT | Partial | Partial |
| Outer ring positively charged residues | |||||
| K2A,R4A,K6A | NT | WT | WT | NT | NT |
| R99A,K102A,K104A | NT | WT | WT | NT | NT |
| K108A,H109A | NT | WT | WT | NT | NT |
| K137A,R141A,R142A,K145A | NT | WT | WT | NT | NT |
| BII4-6 loop | |||||
| Δ215–227 | Not stable | Null | Null | NT | NT |
| Δ215–227::hHus1 loop | NT | WT | WT | NT | NT |
| Δ215–227::PCNA loop | NT | WT | WT | NT | NT |
a Survival outcomes were categorized as follows: WT+ (better than WT mHUS1-complemented cells), WT (similar to WT mHUS1-complemented cells), Partial (worse than WT mHUS1 complemented cells but better than Hus1-null cells), and Null (similar to Hus1 null cells).
b NT, not tested.
HUS1-RAD9A Interaction Is Critical for 9-1-1 Clamp Formation and Function
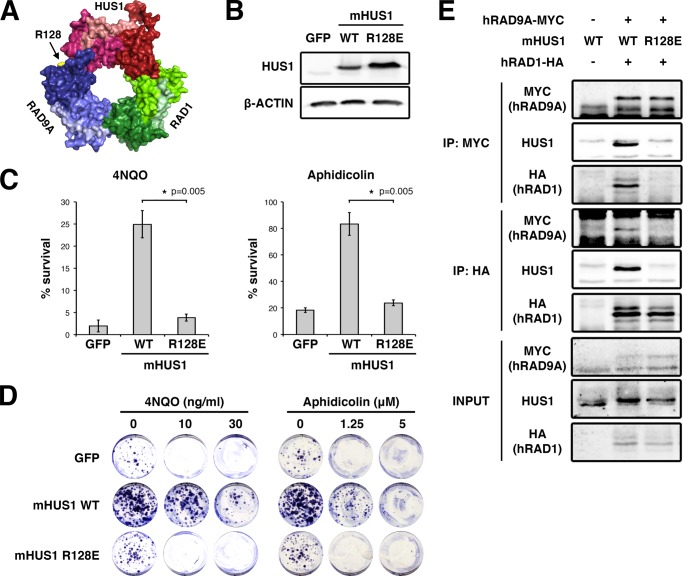
Stable intersubunit interactions are required to allow 9-1-1 clamp formation and maintain clamp integrity during the loading process (11–13). We tested the impact of disrupting intersubunit interactions by targeting mHUS1 Arg-128 (Arg-127 in hHUS1) located at the HUS1-RAD9A interface (Fig. 1A), because its polar interactions were predicted to contribute substantially to the interaction between these subunits (28). Indeed, mutation of the orthologous residue in Schizosaccharomyces pombe hus1 (Asn-121) impairs association with spRAD9A (29). Hence, we mutated Arg-128 to Glu (R128E) to reverse the charge of the residue and analyzed 9-1-1 clamp formation and cell survival after genotoxin treatment. After confirming the stability of R128E mutant protein (Fig. 1B), we subjected R128E-expressing Hus1-null MEFs to short term viability and clonogenic survival assays (Fig. 1, C and D). In both assays, mHUS1 R128E failed to rescue the genotoxin hypersensitivity of Hus1-null MEFs. Next, co-IP assays were performed to test interactions between mHUS1 R128E and RAD9A (Fig. 1E). Whereas wild-type (WT) mHUS1 co-immunoprecipitated with hRAD9A-MYC, mHUS1 R128E did not. Interestingly, in R128E-expressing cells, hRAD1-HA also was not detected in the MYC immunoprecipitate, and neither mHUS1 R128E nor hRAD9A-MYC was detected in the reciprocal HA immunoprecipitate (Fig. 1E). These results indicate that reversing the charge of a single residue at the HUS1-RAD9A interface fully disrupted the stability and function of the entire trimeric 9-1-1 complex.
FIGURE 1.
mHUS1 residue Arg-128 is crucial for 9-1-1 clamp formation. A, Arg-128 (arrow) is located at the HUS1-RAD9A interface (Protein Data Bank code 3GGR). B, immunoblotting using antibodies specific for HUS1 or β-actin was performed to compare the stability of WT and R128E mHUS1 proteins in HEK293T cells. C and D, short term viability and clonogenic survival were measured for Hus1-null MEFs stably expressing mHUS1 R128E after 4NQO or aphidicolin treatments. MEFs expressing GFP or WT mHUS1 served as negative and positive controls, respectively. Each experiment in C was repeated five times with two independently generated cell lines. E, interaction of mHUS1 R128E with hRAD9 and hRAD1 was assessed by co-IP. Error bars, S.D.
Multiple Positively Charged Residues on the HUS1 Inner Surface Facilitate HUS1-DNA Interactions in a Synergistic Manner
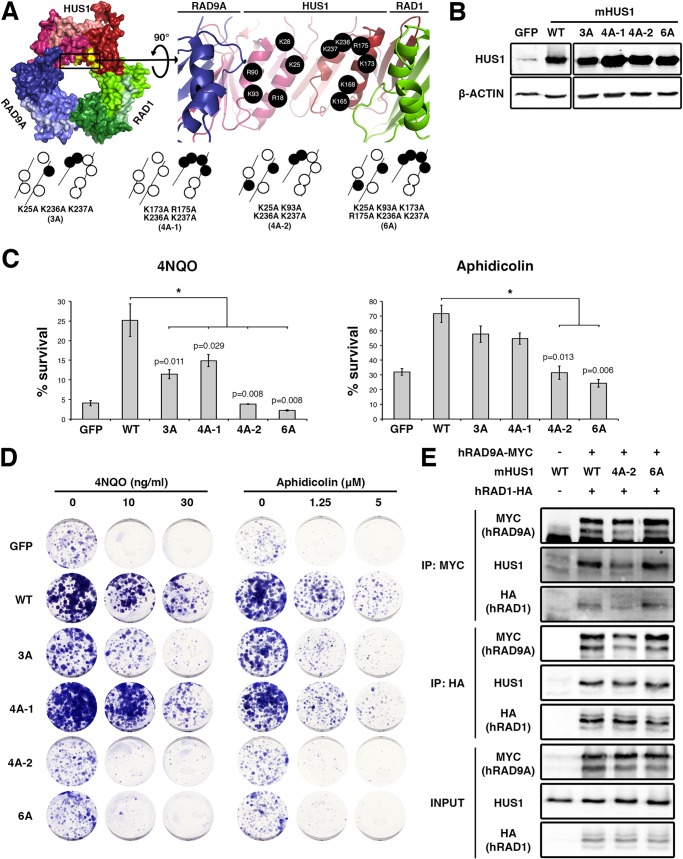
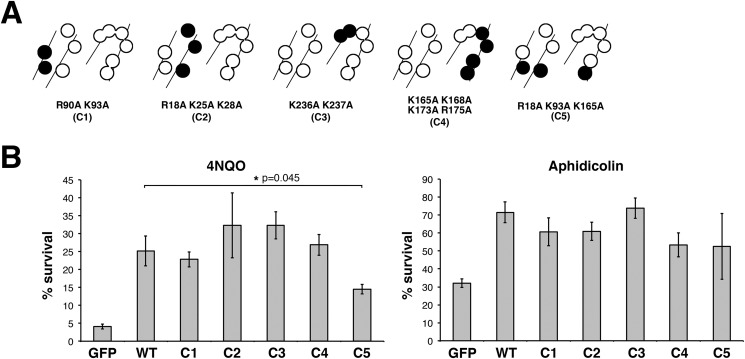
Like PCNA, 9-1-1 is thought to interact with the DNA phosphate backbone, affording proper loading and scaffolding activity on chromatin (30). The inner HUS1 surface consists of four parallel α-helices containing 11 positively charged residues (Fig. 2A). In order to determine the importance of mHUS1-DNA contacts, alanine mutants of several of these residues (Lys-25, Lys-93, Lys-173, Arg-175, Lys-236, and Lys-237) were generated and functionally tested. These mutations did not disrupt mHUS1 protein stability (Fig. 2B), but all caused loss of function as evidenced by partial (mutants 3A and 4A-1) or complete (4A-2 and 6A) genotoxin hypersensitivity phenotypes (Fig. 2, C and D). These defects were not due to disruption of 9-1-1 clamp formation, because both mHUS1 4A-2 and 6A co-immunoprecipitated with hRAD9A-MYC and hRAD1-HA to the same extent as WT mHUS1 (Fig. 2E). Notably, the hHUS1 residues equivalent to those mutated in 4A-2 and 6A were computationally predicted to directly contact DNA (27). We generated additional mutants in different alignments (Fig. 3A and Table 3) to investigate the possible involvement of alternative DNA contacts. However, these mutants were not associated with pronounced hypersensitivity phenotypes (Fig. 3B). Taken together, these results indicate that 6 specific positively charged HUS1 residues synergistically facilitate HUS1-DNA contacts and are necessary for cell survival following DNA damage.
FIGURE 2.
Multiple positively charged residues on the HUS1 inner ring are synergistically important for genotoxic stress responses. A, mHUS1 has 11 arginines and lysines (black circles) distributed on four α-helices in the inner ring surface (Protein Data Bank code 3GGR). Alanine substitutions of these residues (3A-6A) were made. B, protein expression was measured as in Fig. 1B. C and D, genotoxin sensitivity was measured as in Fig. 1, C and D. Each experiment in C was repeated three times with three independently generated cell lines. E, interaction of mHUS1 mutant proteins with hRAD9 and hRAD1 was assessed by co-IP. Error bars, S.D.
FIGURE 3.
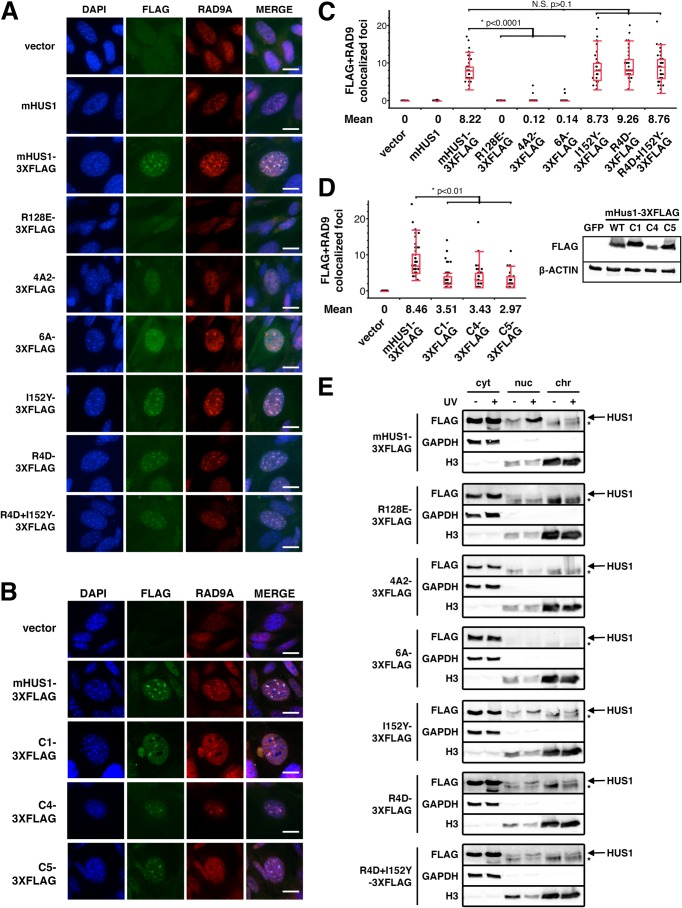
Certain configurations of positively charged residues in the inner ring of HUS1 are dispensable for genotoxic stress responses. A, alanine substitutions of positively charged residues were made in various combinations (C1–C5), as indicated by the filled circles. B, short term viability of Hus1−/− p21−/− MEFs stably expressing mHUS1 inner ring mutants C1–C5 after 4NQO or aphidicolin treatments was measured. Each experiment was repeated four times using three independently generated cell lines. No significant differences in survival between wild-type mHUS1 and mutants C1–C5 were identified, except for C5 with 4NQO treatment. Error bars, S.D.
HUS1 Has Two Functional Hydrophobic Pockets That Are Important for Genome Maintenance
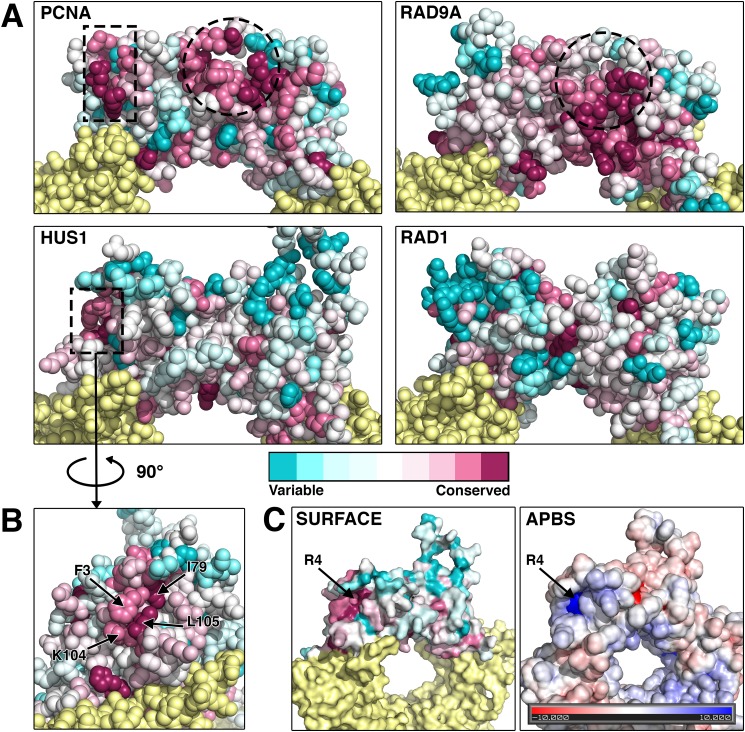
Like PCNA, the 9-1-1 clamp stimulates the activity of many DNA repair factors via direct physical interactions. To identify HUS1 functional domains that might bind effectors, we conducted an evolutionary conservation analysis with the assumption that functional residues would be evolutionarily conserved. As a proof of principle, we first performed this analysis on PCNA (Fig. 4A). Two clusters of conserved residues were apparent on the PCNA outer ring surface, one for the well characterized primary PCNA-interacting protein (PIP) box binding pocket and the other for a secondary domain that also associates with sequences C-terminal to the PIP box motif in some PCNA effectors (31, 32).
FIGURE 4.
The identification of a novel conserved hydrophobic pocket on the outer surface of HUS1. A and B, the evolutionary conservation values of each amino acid position in the protein structures of PCNA, RAD9A, HUS1, and RAD1 were calculated using the Consurf bioinformatics server (see “Experimental Procedures”). Multiple sequence alignments of 44 organisms that encompass a wide range of taxa were used (see Table 2). Residues pseudocolored in cyan have diverged and are variable, whereas those in magenta are conserved. Dotted lines outline conserved regions that potentially mediate protein-protein associations. The circled regions correspond to the PIP box-binding hydrophobic pocket of PCNA and the analogous conserved region of RAD9A. A second conserved region for PCNA and HUS1 is outlined with a rectangle and in the case of HUS1 corresponds to a novel pocket on the side of globular domain 1 composed of 3 conserved hydrophobic residues. C, HUS1 atomic surface and surface electrostatic potential models reveal a positively charged groove at the base of the novel pocket. The charge is contributed by an arginine at position 4.
The same analysis was then applied to RAD9A, HUS1, and RAD1 (Fig. 4A). Structural studies had previously identified PCNA-like hydrophobic pockets in RAD9A and HUS1 (12). However, the distribution of conserved residues for each subunit varied from PCNA and from each other. RAD9A showed conservation of the primary PCNA-like hydrophobic pocket, but HUS1 and RAD1 did not. However, when analyzing only mammalian sequences, conservation of HUS1 and RAD1 residues at the equivalent position for the primary PCNA-like hydrophobic pocket became evident (data not shown). We also identified a cluster of conserved HUS1 residues in the topologically equivalent region of the PCNA secondary binding site. This pocket is formed by 3 hydrophobic HUS1 residues (Phe-3, Ile-79, and Leu-105) along with Arg-4 at the pocket base, creating a strong positive electrostatic potential surrounded by a neutral field (Fig. 4, B and C).
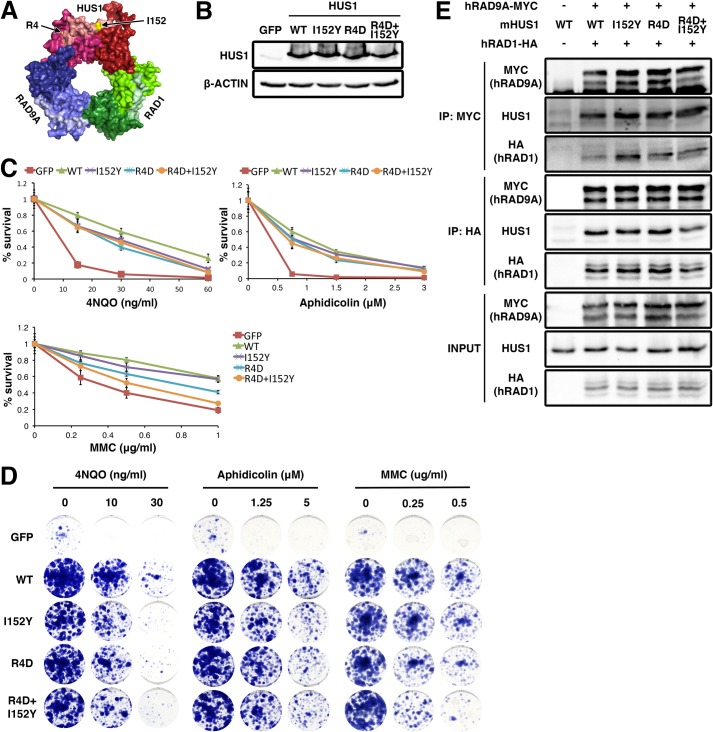
To assess the functional significance of the HUS1 outer surface domains, we first targeted hydrophobic residues that form the PCNA-like primary and secondary pockets, but various alanine mutants either disrupted HUS1 protein stability or did not cause loss of function in survival assays (Table 3). We then generated mutations predicted to physically or electrostatically block the pockets (Fig. 5A). For the primary pocket, we changed Ile-152 (Val-151 in hHUS1) to Tyr (I152Y) to block the pocket with a bulky side chain. For the secondary pocket, Arg-4 was mutated to D (R4D) to reverse the charge at the base. We also generated the double pocket mutant (R4D,I152Y) to test whether the pockets functioned independently or in conjunction with each other. Although all three mutants were stably expressed and competent for clamp formation (Fig. 5, B and E), Hus1-deficient cells expressing the double pocket mutant were no more sensitive to 4NQO or aphidicolin than those expressing either single pocket mutant (Fig. 5, C and D). Interestingly, when challenged with MMC, cells expressing the double pocket mutant showed a synergistic increase in hypersensitivity as compared with the single mutants. These results suggest that the two HUS1 outer surface pockets have separable roles in response to certain forms of DNA damage, such as DNA cross-links, but act in conjunction for other DNA lesions, such as those induced by 4NQO or aphidicolin.
FIGURE 5.
Two hydrophobic pockets on the outer surface of HUS1 are required for genotoxic stress responses. A, Arg-4 and Ile-152 (arrows) are located on the outer ring surface (Protein Data Bank code 3GGR). B, protein expression was measured as in Fig. 1B. C and D, genotoxin sensitivity was measured as in Fig. 1, C and D, as well as with MMC treatment. Each experiment in C was repeated two times with two independently generated cell lines. E, interaction of the pocket mutants with hRAD9 and hRAD1 was assessed by co-IP. Error bars, S.D.
DNA Damage-induced HUS1 Chromatin Localization Is Disrupted in Clamp-destabilizing and DNA Interaction Mutants but Occurs Normally in Pocket Mutants
In order to determine how the different classes of HUS1 mutations affected the ability of HUS1 to localize on chromatin after DNA damage, we performed IF assays in Hus1-null MEFs complemented with 3XFLAG-tagged mHUS1 mutants, including R128E (RAD9A interface), 4A-2 or 6A (DNA binding), or I152Y or R4D (outer surface pockets) (Fig. 6A). We quantified RAD9A and FLAG IF co-staining in cells treated with MMC (Fig. 6C). MMC-induced RAD9A foci were absent in Hus1-null MEFs but were present after restoration of mHUS1 expression. Whereas RAD9A and FLAG foci colocalized in MEFs expressing WT mHUS1–3XFLAG, probably representing 9-1-1 accumulation on damaged DNA, the clamp-destabilizing HUS1 mutant R128E-3XFLAG failed to form FLAG or RAD9A foci. Similar results were observed for 4A2–3XFLAG and 6A-3XFLAG DNA interaction mutants. By comparison, inner ring mutants with less severe genotoxin sensitivity phenotypes showed an intermediate average number of MMC-induced foci formation (Fig. 6, B and D), suggesting that 9-1-1 loading requires clamp-DNA interactions, as is the case for PCNA (30). By contrast, mHUS1 pocket mutants I152Y-3XFLAG, R4D-3XFLAG, and R4D, I152Y-3XFLAG retained the ability to form MMC-induced foci and colocalize with RAD9A to the same extent as WT mHUS1–3XFLAG (Fig. 6, A and C).
FIGURE 6.
DNA damage-induced HUS1 localization is defective in HUS1 clamp-forming and DNA-interacting mutants but not in HUS1 pocket mutants. A and B, MMC-treated Hus1-null MEFs stably expressing the indicated constructs were stained with DAPI (blue) and α-FLAG (green) and α-RAD9A (red) antibodies. Scale bar, 10 μm. C and D, quantifications of colocalized FLAG and RAD9A foci are presented in quartile box and dot plots. NS, not significant. D, immunoblotting using antibodies specific for FLAG or β-actin was performed to compare the stability of 3XFLAG-tagged WT and mutant mHUS1 proteins (C1, C4, and C5) in HEK293T cells. E, cells were UV-treated; fractionated into cytoplasmic (cyt), nuclear (nuc), and chromatin (chr) fractions; and immunoblotted. GAPDH and histone 3 served as fractionation controls. Arrows, HUS1 band; asterisks, nonspecific band.
To verify the IF results, we performed immunoblotting of fractionated cells after UV irradiation (Fig. 6E). WT mHUS1-3XFLAG was detected in the nuclear and chromatin compartments after UV damage. Consistent with the IF results, this response was ablated in MEFs expressing R128E-, 4A2-, or 6A-3XFLAG but not in MEFs expressing the pocket mutants. Thus, genotoxin-induced 9-1-1 clamp accumulation on damaged DNA requires proper 9-1-1 clamp formation and DNA interactions but not HUS1 pocket-mediated functions.
HUS1 Pocket Mutants Are Competent for Checkpoint Signaling but Defective for Effector Interactions
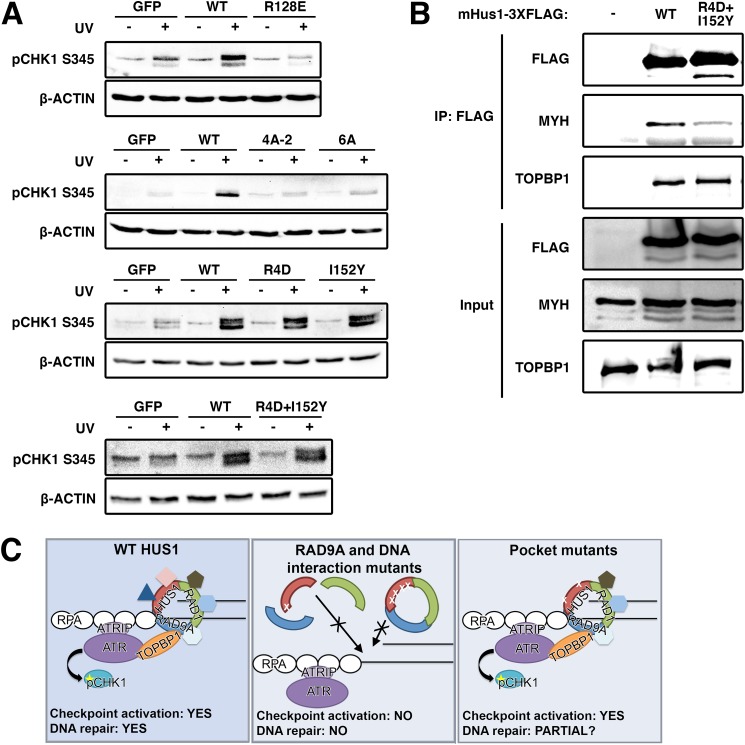
To determine whether the various mHUS1 mutants affected ATR activation and checkpoint signaling, we assessed UV-induced CHK1 phosphorylation (pCHK1) (Fig. 7A). As reported previously (20), Hus1-null MEFs were impaired for CHK1 phosphorylation upon UV treatment. This defect was rectified by complementation with WT mHUS1. However, in cells complemented with the mHUS1 R128E, 4A-2, or 6A mutants, the pCHK1 response was abrogated to the same extent as with complete mHus1 deficiency. These data indicate that unstable subunit-subunit and mHUS1-DNA interactions significantly impair ATR activation, consistent with the observations that these mutant proteins failed to properly localize to DNA damage sites. By contrast, cells expressing mHUS1 pocket mutants I152Y, R4D, or R4D,I152Y retained normal levels of UV-induced CHK1 phosphorylation (Fig. 7A), as might be expected because interactions between 9-1-1 and the ATR activator TOPBP1 occur through RAD9A (17). This result suggests that the genotoxin sensitivity phenotype shown by cells expressing the pocket mutants might be due to disruption of checkpoint signaling-independent HUS1 functions.
FIGURE 7.
9-1-1-dependent checkpoint signaling requires clamp formation and DNA associations but not HUS1 outer surface pocket function, which is necessary for effector interactions. A, DNA damage-induced CHK1 phosphorylation is hampered in HUS1 clamp formation and DNA interaction mutants but is intact for HUS1 outer surface pocket mutants. Lysates from cells treated with 0 or 100 J/m2 UV were immunoblotted using antibodies specific for phospho-CHK1 or β-actin. B, HUS1 pocket mutant R4D,I152Y is impaired for interaction with base excision repair protein MYH. Lysates prepared from HEK293T cells overexpressing 3XFLAG-tagged WT or R4D,I152Y mHUS1 proteins were immunoprecipitated with antibody specific for FLAG and immunoblotted using antibodies specific for MYH and TOPBP1. C, model for HUS1-mediated function in DNA damage response. WT HUS1 forms 9-1-1 clamps, localizes to DNA damage sites, and mediates ATR checkpoint signaling and DNA repair functions. When the RAD9A-interacting residue is dysfunctional, HUS1 cannot form 9-1-1 clamp, causing loss of all downstream functions. HUS1 mutants defective for DNA interactions are still able to form 9-1-1 clamps but cannot localize to DNA damage sites, similarly causing loss of all downstream functions. Only HUS1 pocket mutants are able to form 9-1-1 clamps, localize to DNA lesions, and activate ATR for checkpoint signaling. However, checkpoint-independent functions of HUS1 are perturbed in the pocket mutants, probably causing increased genotoxin hypersensitivity due to impaired DNA repair.
The 9-1-1 clamp is known to interact with several DNA repair proteins, including many from the base excision repair pathway (18, 19). Among the well-established 9-1-1 binding partners is the DNA glycosylase MutY homolog (MYH) (33, 34). In order to determine whether HUS1 outer surface pockets were important for protein-protein interactions, R4D,I152Y-3XFLAG protein was expressed and immunoprecipitated for the detection of association with endogenous MYH (Fig. 7B). Whereas wild-type HUS1–3XFLAG effectively pulled down MYH, less MYH was detected in the R4D,I152Y-3XFLAG immunoprecipitation. Notably, TOPBP1 co-immunoprecipitated with the HUS1 outer surface mutant to a similar extent as with wild-type HUS1, consistent with our observation that this mutant was capable of promoting UV-induced CHK1 phosphorylation. Together, these results indicate that the mHUS1 outer surface mutant is defective for the recruitment of DNA repair proteins despite properly localizing to damaged DNA and supporting ATR checkpoint signaling, highlighting ATR-independent effector functions downstream of HUS1 (Fig. 7C).
Discussion
Checkpoint signaling in coordination with appropriate DNA repair is crucial for a successful DDR in cells experiencing various genotoxic stresses. Understanding this concerted action is important for appreciating how normal cells are protected from the deleterious effects of genomic instability and how malignant cells manage genomic and cellular integrity in the face of numerous physiological stresses (35–38). In this study, we sought to understand the molecular interactions that underlie a robust DDR involving the 9-1-1 clamp and in doing so defined both the molecular requirements for stimulation of ATR-induced CHK1 phosphorylation and CHK1-independent functions for the 9-1-1 complex mediated by outer surface residues of the HUS1 subunit.
Maintaining sufficient intersubunit contacts is the most crucial initial step for 9-1-1 clamp formation, failure of which will abrogate all downstream clamp functions. Remarkably, reversing the polarity of a single residue (Arg-128 of mHUS1) at the RAD9A/HUS1 interface fully disrupted 9-1-1 clamp integrity and function, although there are at least 8 other HUS1 residues predicted to contribute to RAD9A-HUS1 interactions (28). Interestingly, the RAD9A/HUS1 interface mutation also disrupted HUS1-RAD1 and RAD1-RAD9A associations. One possible explanation is that active repulsion of HUS1-RAD9A indirectly weakens HUS1-RAD1 and RAD9A-RAD1 interactions. Alternatively, the findings may suggest that clamp assembly occurs in an ordered, stepwise process, as reported for the heterotrimeric PCNA of Sulfoflobus solfataricus (SsoPCNA) (39), or that all three 9-1-1 interfaces function cooperatively during trimerization.
The inner surface of PCNA consists of four parallel α-helices that contain positively charged residues, some of which contact the negatively charged DNA sugar-phosphate backbone and are necessary for efficient clamp loading and mobility on DNA (40). Similarly, the HUS1 inner surface also is composed of four parallel α-helices containing numerous positively charged residues, and here we report that HUS1 function requires a specific set of synergistically important residues that are aligned in a transhelical manner analogous to those of PCNA. Notably, our findings are consistent with predicted HUS1-DNA contacts from computational modeling (27). It is remarkable that loss of DNA contacts for the HUS1 subunit alone leads to severe checkpoint signaling defects and hypersensitivity to genotoxins, because modeling of 9-1-1 on DNA indicates that the three subunits contribute almost equally for DNA backbone associations (27), although it remains possible that certain DNA structures might lead to other 9-1-1 conformations on DNA. The HUS1 inner surface mutants stably associate with the other 9-1-1 subunits but do not show substantial DNA damage-induced accumulation on chromatin, suggesting that interactions between 9-1-1 and DNA are necessary for stable loading of the clamp.
Much knowledge about PCNA function was gained by structure/function studies that identified a PCNA hydrophobic pocket as the docking site for most PIP box motif-carrying proteins (41). Based on the structural similarity between the 9-1-1 complex and PCNA, we hypothesized that the outer surface of HUS1 would mediate physical interactions with downstream effectors. Our evolutionary conservation analysis revealed that among all 9-1-1 subunits, RAD9A showed the greatest conservation of the hydrophobic pocket that is analogous to the site where PCNA interacts with PCNA-interacting protein box motifs, consistent with the idea that RAD9A is the subunit most closely related to PCNA (12). The same region was conserved among mammalian HUS1 proteins but not in a broader representation of species. This suggests that whereas the RAD9A pocket is evolutionarily conserved, HUS1 and RAD1 have undergone greater divergence, potentially reflecting specialization of clamp subunits. Consurf analysis of HUS1 additionally revealed a second hydrophobic pocket on the outer surface that showed substantial evolutionary conservation. Disruption of these two HUS1 outer surface pockets caused partial loss of function without disturbing clamp formation or its recruitment to chromatin following DNA damage. That cells expressing these pocket mutants were not as hypersensitive to genotoxic stress as cells that completely lack HUS1 probably relates to the fact that they remain functional for genotoxin-induced CHK1 activation, as would be expected because interactions between 9-1-1 and the ATR activator TOPBP1 occur through the C-terminal tail of RAD9A (17). It also remains possible that RAD9A and RAD1 provide some level of redundancy when HUS1 is dysfunctional, because in some cases, RAD9A, HUS1, and RAD1 can all interact with the same repair protein, albeit with different binding affinities (42–47). We favor the possibility that each 9-1-1 subunit binds at least some unique downstream effectors, with the idea that each subunit is specialized to some extent to mediate specific functions. Indeed, the subunits of the archaeal heterotrimeric PCNA each interact with distinct binding partners, a characteristic that provides a means to diversify and coordinate clamp functions (48).
PCNA-effector interactions invariably involve PIP box sequences, but there is mixed evidence regarding a role for PIP box motifs in 9-1-1-effector interactions (3), and the role of the secondary HUS1 pocket is unknown. It may stabilize interactions with PIP box-containing effectors that bind the primary pocket, resembling PCNA interactions with FEN1 and p21 (31, 32). Combining the outer surface mutations (R4D,I152Y) did not further increase hypersensitivity to 4NQO or aphidicolin beyond that for either single mutant (Table 3), suggesting a related function for the two pockets. However, cells expressing the double mutant R4D,I152Y showed increased sensitivity to MMC, consistent with our previous findings.3 Here we further show that the double mutant R4D,I152Y confers greater MMC hypersensitivity than either of the corresponding single mutants, implying that at certain DNA lesions, the two HUS1 pockets can have separate roles that cooperatively improve cell survival. In such circumstances, the secondary HUS1 pocket might interact with distinct effectors independently of the primary pocket, through a different motif, like the recently reported Mec3-Mcm10 interaction, which involves both a PIP box and other sequences in MCM10 (49). With its close proximity to the RAD9A primary PCNA-like pocket, the secondary HUS1 pocket also may be involved in RAD9A-HUS1 intersubunit-effector binding, as has been reported for the interaction between the equivalent budding yeast proteins (DDC1-MEC3) and their partner RED1 (50). Bacterial β-clamp and SsoPCNA similarly display intersubunit interactions with TLS polymerases (51, 52).
An open question has been to what extent the requirement for the 9-1-1 complex in genome maintenance reflects its role in TOPBP1-induced ATR activation versus direct functions for 9-1-1 subunits in other processes. By mutating the HUS1 outer surface pockets, we have successfully separated these 9-1-1 functions and demonstrate that HUS1 has roles apart from checkpoint signaling that also are crucial for cell survival following DNA damage. Continued analysis of this collection of HUS1 mutants, especially further dissection of the genotoxin-specific functions of both HUS1 outer surface pockets, as well as the outer surface domains of RAD9A and RAD1, holds promise for shedding light on 9-1-1 functions in genome maintenance and highlighting potential targets that can be exploited clinically for anti-cancer therapies.
Acknowledgments
We thank Ivaylo Ivanov, Alba Guarne, Joseph Peters, and Marcus Smolka for helpful discussions and comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 CA108773 (to R. S. W.) and R01 CA78391 (to A. L.).
Balmus, G., Lim, P. X., Oswald, A., Hume, K. R., Cassano, A., Pierre, J., Hill, A., Huang, W., August, A., Stokol, T., Southard, T., and Weiss, R. S. (2015) HUS1 regulates in vivo responses to genotoxic chemotherapies. Oncogene 10.1038/onc.2015.118
- DDR
- DNA damage response
- 9-1-1
- RAD9A-HUS1-RAD1
- PCNA
- proliferating cell nuclear antigen
- mHUS1
- murine HUS1
- IF
- immunofluorescence
- IP
- immunoprecipitation
- PIP
- PCNA-interacting protein
- MMC
- mitomycin C
- MEF
- mouse embryonic fibroblast
- 4NQO
- 4-nitroquinoline 1-oxide.
References
- 1. Jackson S. P., Bartek J. (2009) The DNA-damage response in human biology and disease. Nature 461, 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cimprich K. A., Cortez D. (2008) ATR: an essential regulator of genome integrity. Nat. Rev. Mol. Cell Biol. 9, 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eichinger C. S., Jentsch S. (2011) 9-1-1: PCNA's specialized cousin. Trends Biochem. Sci. 36, 563–568 [DOI] [PubMed] [Google Scholar]
- 4. Hopkins K. M., Auerbach W., Wang X. Y., Hande M. P., Hang H., Wolgemuth D. J., Joyner A. L., Lieberman H. B. (2004) Deletion of mouse rad9 causes abnormal cellular responses to DNA damage, genomic instability, and embryonic lethality. Mol. Cell Biol. 24, 7235–7248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Han L., Hu Z., Liu Y., Wang X., Hopkins K. M., Lieberman H. B., Hang H. (2010) Mouse Rad1 deletion enhances susceptibility for skin tumor development. Mol. Cancer 9, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weiss R. S., Enoch T., Leder P. (2000) Inactivation of mouse Hus1 results in genomic instability and impaired responses to genotoxic stress. Genes Dev. 14, 1886–1898 [PMC free article] [PubMed] [Google Scholar]
- 7. Yazinski S. A., Westcott P. M., Ong K., Pinkas J., Peters R. M., Weiss R. S. (2009) Dual inactivation of Hus1 and p53 in the mouse mammary gland results in accumulation of damaged cells and impaired tissue regeneration. Proc. Natl. Acad. Sci. U.S.A. 106, 21282–21287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lyndaker A. M., Lim P. X., Mleczko J. M., Diggins C. E., Holloway J. K., Holmes R. J., Kan R., Schlafer D. H., Freire R., Cohen P. E., Weiss R. S. (2013) Conditional inactivation of the DNA damage response gene Hus1 in mouse testis reveals separable roles for components of the RAD9-RAD1-HUS1 complex in meiotic chromosome maintenance. PLoS Genet. 9, e1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vasileva A., Hopkins K. M., Wang X., Weisbach M. M., Friedman R. A., Wolgemuth D. J., Lieberman H. B. (2013) The DNA damage checkpoint protein RAD9A is essential for male meiosis in the mouse. J. Cell Sci. 126, 3927–3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moldovan G. L., Pfander B., Jentsch S. (2007) PCNA, the maestro of the replication fork. Cell 129, 665–679 [DOI] [PubMed] [Google Scholar]
- 11. Xu M., Bai L., Gong Y., Xie W., Hang H., Jiang T. (2009) Structure and functional implications of the human rad9-hus1-rad1 cell cycle checkpoint complex. J. Biol. Chem. 284, 20457–20461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Doré A. S., Kilkenny M. L., Rzechorzek N. J., Pearl L. H. (2009) Crystal structure of the rad9-rad1-hus1 DNA damage checkpoint complex: implications for clamp loading and regulation. Mol. Cell 34, 735–745 [DOI] [PubMed] [Google Scholar]
- 13. Sohn S. Y., Cho Y. (2009) Crystal structure of the human rad9-hus1-rad1 clamp. J. Mol. Biol. 390, 490–502 [DOI] [PubMed] [Google Scholar]
- 14. Ellison V., Stillman B. (2003) Biochemical characterization of DNA damage checkpoint complexes: clamp loader and clamp complexes with specificity for 5′ recessed DNA. PLoS Biol. 1, E33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bermudez V. P., Lindsey-Boltz L. A., Cesare A. J., Maniwa Y., Griffith J. D., Hurwitz J., Sancar A. (2003) Loading of the human 9-1-1 checkpoint complex onto DNA by the checkpoint clamp loader hRad17-replication factor C complex in vitro. Proc. Natl. Acad. Sci. U.S.A. 100, 1633–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Majka J., Binz S. K., Wold M. S., Burgers P. M. (2006) Replication protein A directs loading of the DNA damage checkpoint clamp to 5′-DNA junctions. J. Biol. Chem. 281, 27855–27861 [DOI] [PubMed] [Google Scholar]
- 17. Delacroix S., Wagner J. M., Kobayashi M., Yamamoto K., Karnitz L. M. (2007) The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes Dev. 21, 1472–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Helt C. E., Wang W., Keng P. C., Bambara R. A. (2005) Evidence that DNA damage detection machinery participates in DNA repair. Cell Cycle 4, 529–532 [DOI] [PubMed] [Google Scholar]
- 19. Madabushi A., Lu A.-L. (2011) The novel role of cell cycle checkpoint clamp Rad9-Hus1-Rad1 (the 9-1-1 complex) in DNA repair. in Advances in Medicine and Biology (Berhardt L. V., ed) pp. 41–74, Nova Science Publishers, Hauppauge, NY [Google Scholar]
- 20. Weiss R. S., Matsuoka S., Elledge S. J., Leder P. (2002) Hus1 acts upstream of Chk1 in a mammalian DNA damage response pathway. Curr. Biol. 12, 73–77 [DOI] [PubMed] [Google Scholar]
- 21. Hinds P. W., Finlay C. A., Quartin R. S., Baker S. J., Fearon E. R., Vogelstein B., Levine A. J. (1990) Mutant p53 DNA clones from human colon carcinomas cooperate with ras in transforming primary rat cells: a comparison of the “hot spot” mutant phenotypes. Cell Growth Differ. 1, 571–580 [PubMed] [Google Scholar]
- 22. Thompson J. D., Gibson T. J., Plewniak F., Jeanmougin F., Higgins D. G. (1997) The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25, 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ashkenazy H., Erez E., Martz E., Pupko T., Ben-Tal N. (2010) ConSurf 2010: calculating evolutionary conservation in sequence and structure of proteins and nucleic acids. Nucleic Acids Res. 38, W529–W533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gu Y., Lu A.-L. (2001) Differential DNA recognition and glycosylase activity of the native human MutY hmolog (hMYH) and recombinant hMYH expressed in bacteria. Nucleic Acids Res. 29, 2666–2674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rendtlew Danielsen J. M., Larsen D. H., Schou K. B., Freire R., Falck J., Bartek J., Lukas J. (2009) HCLK2 is required for activity of the DNA damage response kinase ATR. J. Biol. Chem. 284, 4140–4147 [DOI] [PubMed] [Google Scholar]
- 26. Abmayr S. M., Yao T., Parmely T., Workman J. L. (2001) Preparation of nuclear and cytoplasmic extracts from mammalian cells. Curr. Protoc. Mol. Biol. 10.1002/0471142727.mb1201s75 [DOI] [PubMed] [Google Scholar]
- 27. Querol-Audí J., Yan C., Xu X., Tetsukawa S. E., Tsai M.-S., Tainer J. A., Cooper P. K., Nogales E., Ivanov I. (2012) Repair complexes of FEN1 endonuclease, DNA, and Rad9-Hus1-Rad1 are distinguished from their PCNA counterparts by functionally important stability. Proc. Natl. Acad. Sci. U.S.A. 109, 8528–8533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu X., Guardiani C., Yan C., Ivanov I. (2013) Opening pathways of the DNA clamps proliferating cell nuclear antigen and Rad9-Rad1-Hus1. Nucleic Acids Res. 41, 10020–10031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kaur R., Kostrub C. F., Enoch T. (2001) Structure-function analysis of fission yeast Hus1-Rad1-Rad9 checkpoint complex. Mol. Biol. Cell 12, 3744–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McNally R., Bowman G. D., Goedken E. R., O'Donnell M., Kuriyan J. (2010) Analysis of the role of PCNA-DNA contacts during clamp loading. BMC Struct. Biol. 10, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gulbis J. M., Kelman Z., Hurwitz J., O'Donnell M., Kuriyan J. (1996) Structure of the C-terminal region of p21WAF1/CIP1 complexed with human PCNA. Cell 87, 297–306 [DOI] [PubMed] [Google Scholar]
- 32. Sakurai S., Kitano K., Yamaguchi H., Hamada K., Okada K., Fukuda K., Uchida M., Ohtsuka E., Morioka H., Hakoshima T. (2005) Structural basis for recruitment of human flap endonuclease 1 to PCNA. EMBO J. 24, 683–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang D. Y., Lu A. L. (2005) Interaction of checkpoint proteins Hus1/Rad1/Rad9 with DNA base excision repair enzyme MutY homolog in fission yeast, Schizosaccharomyces pombe. J. Biol. Chem. 280, 408–417 [DOI] [PubMed] [Google Scholar]
- 34. Shi G., Chang D. Y., Cheng C. C., Guan X., Venclovas C., Lu A. L. (2006) Physical and functional interactions between MutY glycosylase homologue (MYH) and checkpoint proteins Rad9-Rad1-Hus1. Biochem. J. 400, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Helleday T., Petermann E., Lundin C., Hodgson B., Sharma R. A. (2008) DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 8, 193–204 [DOI] [PubMed] [Google Scholar]
- 36. Hoeijmakers J. (2001) Genome maintenance mechanisms for preventing cancer. Nature 411, 366–374 [DOI] [PubMed] [Google Scholar]
- 37. Ciccia A., Elledge S. J. (2010) The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanahan D., Weinberg R. A. (2011) Hallmarks of cancer: the next generation. Cell 144, 646–674 [DOI] [PubMed] [Google Scholar]
- 39. Dionne I., Nookala R. K., Jackson S. P., Doherty A. J., Bell S. D. (2003) A heterotrimeric PCNA in the hyperthermophilic achaeon Sulfolobus solfatoricus. Mol. Cell 11, 275–282 [DOI] [PubMed] [Google Scholar]
- 40. Georgescu R. E., Kim S. S., Yurieva O., Kuriyan J., Kong X. P., O'Donnell M. (2008) Structure of a sliding clamp on DNA. Cell 132, 43–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Naryzhny S. N. (2008) Proliferating cell nuclear antigen: a proteomics view. Cell Mol. Life Sci. 65, 3789–3808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Friedrich-Heineken E., Toueille M., Tännler B., Bürki C., Ferrari E., Hottiger M. O., Hübscher U. (2005) The two DNA clamps Rad9/Rad1/Hus1 complex and proliferating cell nuclear antigen differentially regulate flap endonuclease 1 activity. J. Mol. Biol. 353, 980–989 [DOI] [PubMed] [Google Scholar]
- 43. Smirnova E., Toueille M., Markkanen E., Hübscher U. (2005) The human checkpoint sensor and alternative DNA clamp Rad9-Rad1-Hus1 modulates the activity of DNA ligase I, a component of the long-patch base excision repair machinery. Biochem. J. 389, 13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gembka A., Toueille M., Smirnova E., Poltz R., Ferrari E., Villani G., Hübscher U. (2007) The checkpoint clamp, Rad9-Rad1-Hus1 complex, preferentially stimulates the activity of apurinic/apyrimidinic endonuclease 1 and DNA polymerase β in long patch base excision repair. Nucleic Acids Res. 35, 2596–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Guan X., Madabushi A., Chang D. Y., Fitzgerald M. E., Shi G., Drohat A. C., Lu A. L. (2007) The human checkpoint sensor Rad9-Rad1-Hus1 interacts with and stimulates DNA repair enzyme TDG glycosylase. Nucleic Acids Res. 35, 6207–6218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park M. J., Park J. H., Hahm S. H., Ko S. I., Lee Y. R., Chung J. H., Sohn S. Y., Cho Y., Kang L. W., Han Y. S. (2009) Repair activities of human 8-oxoguanine DNA glycosylase are stimulated by the interaction with human checkpoint sensor Rad9-Rad1-Hus1 complex. DNA Repair 8, 1190–1200 [DOI] [PubMed] [Google Scholar]
- 47. Bai H., Madabushi A., Guan X., Lu A. L. (2010) Interaction between human mismatch repair recognition proteins and checkpoint sensor Rad9-Rad1-Hus1. DNA Repair 9, 478–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Beattie T. R., Bell S. D. (2012) Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment maturation. EMBO J. 31, 1556–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Alver R. C., Zhang T., Josephrajan A., Fultz B. L., Hendrix C. J., Das-Bradoo S., Bielinsky A. K. (2014) The N-terminus of Mcm10 is important for interaction with the 9-1-1 clamp and in resistance to DNA damage. Nucleic Acids Res. 42, 8389–8404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eichinger C. S., Jentsch S. (2010) Synaptonemal complex formation and meiotic checkpoint signaling are linked to the lateral element protein Red1. Proc. Natl. Acad. Sci. U.S.A. 107, 11370–11375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bunting K. A., Roe S. M., Pearl L. H. (2003) Structural basis for recruitment of transletion DNA polymerase Pol IV/DinB to the β-clamp. EMBO J. 22, 5883–5892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Xing G., Kirouac K., Shin Y. J., Bell S. D., Ling H. (2009) Structural insight into recruitment of translesion DNA polymerase Dpo4 to sliding clamp PCNA. Mol. Microbiol. 71, 678–691 [DOI] [PubMed] [Google Scholar]