Abstract
Redox biology is fundamental to both normal cellular homeostasis and pathological states associated with excessive oxidative stress. Reactive oxygen species function not only as signaling molecules but also as redox regulators of protein function. In the vascular system, redox reactions help regulate key physiologic responses such as cell adhesion, vasoconstriction, platelet aggregation, angiogenesis, inflammatory gene expression, and apoptosis. During pathologic states, altered redox balance can cause vascular cell dysfunction and affect the equilibrium between procoagulant and anticoagulant systems, contributing to thrombotic vascular disease. This review focuses on the emerging role of a specific reversible redox reaction, protein methionine oxidation, in vascular disease and thrombosis. A growing number of cardiovascular and hemostatic proteins are recognized to undergo reversible methionine oxidation, in which methionine residues are posttranslationally oxidized to methionine sulfoxide. Protein methionine oxidation can be reversed by the action of stereospecific enzymes known as methionine sulfoxide reductases. Calcium/calmodulin-dependent protein kinase II is a prototypical methionine redox sensor that responds to changes in the intracellular redox state via reversible oxidation of tandem methionine residues in its regulatory domain. Several other proteins with oxidation-sensitive methionine residues, including apolipoprotein A-I, thrombomodulin, and von Willebrand factor, may contribute to vascular disease and thrombosis.
Introduction
Reactive oxygen species (ROS) are partially reduced metabolites of oxygen that are generated during cellular homeostasis. During the last decade, considerable evidence has emerged supporting the concept that ROS regulate many normal physiological vascular responses such as cell adhesion, vasoconstriction, platelet aggregation, angiogenesis, inflammatory gene expression, and apoptosis.1,2 The production and metabolism of ROS are tightly regulated by cellular redox switches.3 During pathologic states, excessive generation of ROS can overwhelm endogenous antioxidant systems, leading to dysregulated redox balance with adverse consequences on cellular function. For example, dysregulation of ROS has been observed in a variety of disease states associated with endothelial dysfunction.4
Despite convincing evidence from in vitro studies and animal models implicating oxidative stress in the pathogenesis of vascular disease, clinical trials of systemic antioxidant therapy have suggested little or no benefit for the prevention of vascular or thrombotic events.5,6 One potential explanation for the lack of benefit of nonspecific antioxidant therapy is that such therapy might interfere with physiologic and pathologic redox mechanisms. There are numerous potential oxidative targets of ROS, including cellular proteins, lipids, and DNA. However, the specific molecular targets and signaling pathways linking ROS to vascular disease have remained elusive. This review focuses on the role of a specific reversible redox reaction, protein methionine oxidation, in vascular disease and thrombosis.
Redox reactions in vascular cells
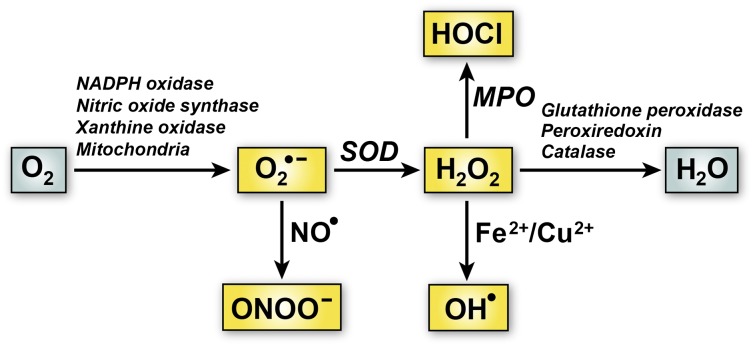
Figure 1 depicts the generation of some of the major forms of ROS in vascular cells. A principal intermediate form of vascular ROS is superoxide (O2•−), an oxygen anion that is formed through the univalent reduction of oxygen by enzymes such as reduced NAD phosphate (NADPH) oxidase, xanthine oxidase, and uncoupled endothelial nitric oxide synthase, or as a result of mitochondrial oxidative phosphorylation.7 Superoxide itself is relatively unreactive toward biomacromolecules under physiologic conditions.8 In many biological systems, superoxide acts as a stronger reductant than oxidant.9 In addition, it is a negatively charged free radical, making it unsuitable for crossing membranes. Despite its relatively short half-life, superoxide can be transformed into a variety of other biologically important ROS. For example, it can react with nitric oxide (NO•) to form a highly reactive molecule, peroxynitrite (ONOO―), or undergo dismutation to form hydrogen peroxide (H2O2) (Figure 1). H2O2 can in turn give rise to hydroxyl radical (OH•) in a reaction catalyzed by Fe2+ or Cu2+ or to hypochlorous acid (HOCl) by serving as a substrate for myeloperoxidase. Both OH• and HOCl are highly reactive ROS that can produce cellular damage and tissue injury.
Figure 1.
Major pathways of ROS generation and elimination in vascular cells. One electron reduction of O2 can produce superoxide anion (O2•−). The main pathways for O2•− transformation are via dismutation to hydrogen peroxide (H2O2) catalyzed by superoxide dismutase (SOD) or reaction with nitric oxide (NO•) to form peroxynitrite (ONOO―). H2O2 can be further metabolized to hypochlorous acid (HOCl) by myeloperoxidase (MPO) or hydroxyl radical (OH•) through reactions catalyzed by metal ions (Fe2+ or Cu2+) via the Fenton reaction. Elimination of H2O2 is facilitated by antioxidant enzymes such as glutathione peroxidase, peroxiredoxin, and catalase.
Unlike superoxide, H2O2 has a relatively long half-life (up to milliseconds), is uncharged, and is capable of diffusing across cellular membranes.10 These characteristics make H2O2 suitable to act as an intracellular signaling molecule and a mediator of posttranslational oxidative amino acid modifications of proteins. Sulfur-containing amino acids such as cysteine and methionine are especially susceptible to oxidation by H2O2. Reversible oxidation and reduction of cysteine thiols can regulate protein function through disulfide exchange, glutathionylation, and formation of nitrosothiols, sulfenic acids, and sulfonic acids.11 Protein methionine residues also can undergo reversible redox reactions with H2O2, HOCl, and other ROS to form methionine sulfoxide (Figure 2). Protein methionine oxidation has emerged recently as a potential molecular mechanism of redox regulation of protein function in vascular biology.
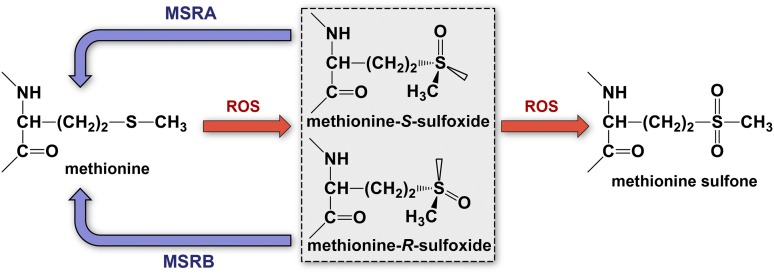
Figure 2.
Biochemistry of protein methionine oxidation and reduction. Oxidation of protein methionine residues produces 2 sulfoxide diastereomers, methionine-S-sulfoxide and methionine-R-sulfoxide, which can be stereospecifically reduced back to methionine by 2 classes of mammalian methionine sulfoxide reductases, MSRA and MSRB, respectively. Further oxidation of methionine sulfoxide to methionine sulfone is biologically irreversible.
Protein methionine oxidation
ROS-mediated protein methionine oxidation occurs via addition of a single oxygen molecule to the sulfur atom of protein methionine, forming methionine sulfoxide (MetSO).12 This reaction creates a chiral center with two diastereomers: methionine-S-sulfoxide and methionine-R-sulfoxide (Figure 2). MetSO contains an amino acid side chain that is more polar than that of methionine.13 This change can have profound structural and functional consequences on the target protein, such as altered conformation, solubility, protein function, or stability.14,15 Most higher organisms have evolved methionine sulfoxide reductase (MSR) enzymes that can reverse the oxidation of protein methionine residues.16 In mammals, the MSR system is composed of 2 groups of enzymes, MSRA and MSRB, which catalyze the stereospecific reduction of the S- and R- diastereomers, respectively, of MetSO (Figure 2). MSRA is primarily localized in the cytoplasm and mitochondria. There are multiple isoforms of mammalian MSRB that differ in subcellular localization.17 In the face of persistent oxidative stress, both methionine-S-sulfoxide and methionine-R-sulfoxide can undergo a second oxidation reaction to form methionine sulfone, which is not a substrate for MSR and is therefore considered to be an irreversible posttranslational modification.11
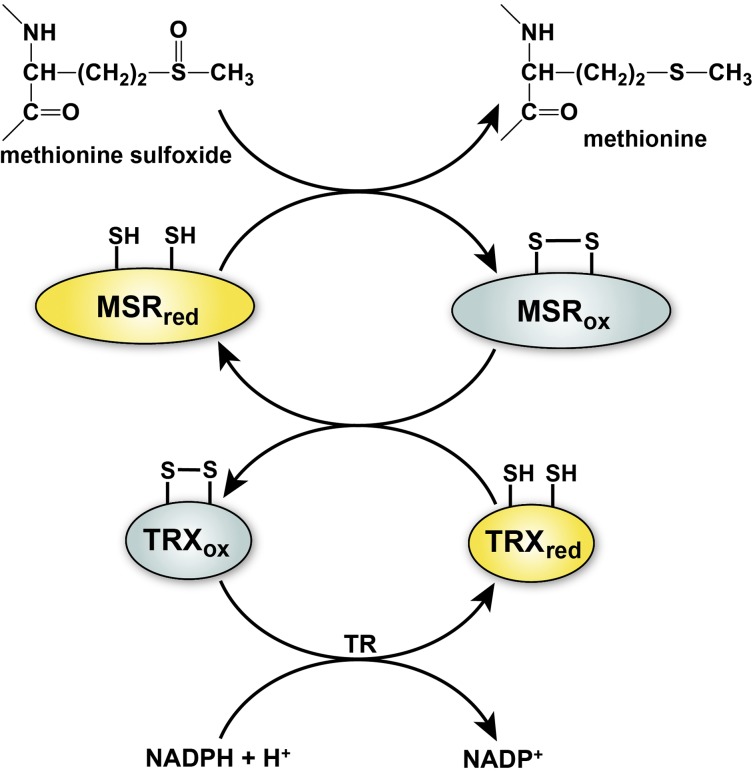
The MSR reaction reduces MetSO to methionine, resulting in the formation of a disulfide bond in the active site of the MSR enzyme. Regeneration of the reduced thiol form of MSR requires the action of the thioredoxin/thioredoxin reductase system, which uses catalytic redox-active cysteine residues to reduce the active site disulfide of MSR through a series of thiol exchange reactions (Figure 3). This system of reversible methionine oxidation (by ROS) and reduction (by MSR) is emerging as a common biological mechanism for cellular regulation, analogous to protein phosphorylation/dephosphorylation and cysteine oxidation/reduction.
Figure 3.
General mechanism of MSR and regeneration by the thioredoxin system. Reduction of methionine sulfoxide to methionine by MSR results in the transient formation of an intramolecular disulfide bond that inactivates the enzyme. Disulfide exchange reaction with thioredoxin (TRX) regenerates the active form of MSR and leads to inactivation of TRX. Regeneration of reduced TRX occurs through a transfer of electrons from NADPH in a reaction catalyzed by thioredoxin reductase (TR). The active reduced forms of the enzymes are depicted in yellow and the inactive oxidized forms of enzymes in gray.
Protein methionine oxidation has been shown to be important in a variety of physiologic processes in several organisms. Studies in bacteria demonstrated a role for methionine oxidation and its regulation by MSR in bacterial viability. Bacteria lacking MSR had increased susceptibility to ROS-induced killing, which was rescued by restoration of MSR expression.18 Similarly, mice homozygous for a targeted deletion of MSRA (MsrA−/− mice) were reported to have enhanced susceptibility to oxidative stress and to accumulate higher levels of oxidized proteins than wild-type mice.19 MSR also may have a protective role in redox regulated processes associated with cancer, innate immunity, and neurodegeneration.20-22
Protein methionine oxidation in vascular disease
There is evidence for a causal effect of ROS in vascular diseases such as atherosclerosis, ischemic heart disease, hypertension, and thrombosis,4 and emerging data suggest that protein methionine oxidation may play a pathogenic role. For example, cardiac myocytes from MsrA−/− mice are hypersensitive to oxidant stress.23 Conversely, overexpression of MSRA in cultured cardiac myocytes provided strong protection against injury in a hypoxia-reoxygenation model,24 and transgenic mice overexpressing a myristoylated isoform of MSRA were found to be protected against ischemia-reperfusion injury of the heart ex vivo.25 Moreover, 2 recent clinical studies have identified a single nucleotide polymorphism (rs10903323) in the human MSRA gene to be associated with increased risk of cardiovascular events and coronary artery disease.26,27 The impact of the MSRA rs10903323 polymorphism on MSRA protein expression or enzymatic activity is unknown.
Several proteins important in vascular biology have been shown to contain oxidation-sensitive methionine residues with potential regulatory roles in the pathogenesis of vascular or thrombotic diseases (Table 1). This list is likely to grow in the future as proteomic approaches to detect and identify modified methionine residues are increasingly used.28 The functional significance of many of these specific protein methionine oxidation reactions remains to be determined. In the remainder of this review, we will focus on some of the better characterized vascular proteins that contain redox-active methionine residues, including calcium/calmodulin-dependent protein kinase II (CaMKII), apolipoprotein A-I, thrombomodulin (TM), and von Willebrand factor (VWF), and highlight their potential roles in the pathogenesis of vascular disease.
Table 1.
Partial list of proteins with oxidation-sensitive methionine residues
| Target protein | Oxidation-sensitive methionine(s)* | Effect of MetSO on protein function† | Reference(s) |
|---|---|---|---|
| Proteins relevant to cardiovascular biology | |||
| CaMKII | Met281, Met282 | ↑ | 31,32 |
| Apolipoprotein A-I | Met86, Met112, Met148 | ↓ | 43,45,46 |
| Actin | Met46, Met49 | ↓ | 21,93 |
| IκBα | Met45 | ↑ | 94,95 |
| p53 | Met340 | ↓ | 96 |
| S100A9 | Met63, Met83 | ↓ | 92 |
| Proteins involved in hemostasis and thrombosis | |||
| Thrombomodulin | Met388 | ↓ | 57,60 |
| Activated protein C | Met59 | ↓ | 67 |
| VWF | Met1606 | ↑ | 75,76 |
| ADAMTS13 | Met249, Met331, Met496 | ↓ | 80 |
| Fibrinogen | Met78, Met367, Met476 | ↔ | 84,85 |
| α-2-Antiplasmin | 10 Met residues | ND | 90 |
| Antithrombin III | Met314, Met315 | ND | 90,91 |
| Factor VII | Met298, Met306 | ↓ | 89 |
| Plasminogen activator inhibitor-1 | Met347 | ─ | 87,88 |
| Tissue plasminogen activator | Met207 | ─ | 86 |
The listed proteins have been characterized by the presence of ≥1 methionine residue with a potential regulatory role or pathologic effect in cardiovascular biology or thrombosis.
The numbering scheme of the oxidation-sensitive methionine residues is based on the species and isoform of the proteins studied in the cited references.
The relative change in function as a consequence of methionine oxidation is indicated as follows: ↑, enhanced; ↓, suppressed; ↔, altered structure; ─, no effect; ND, not determined.
CaMKII: a prototypical methionine redox sensor
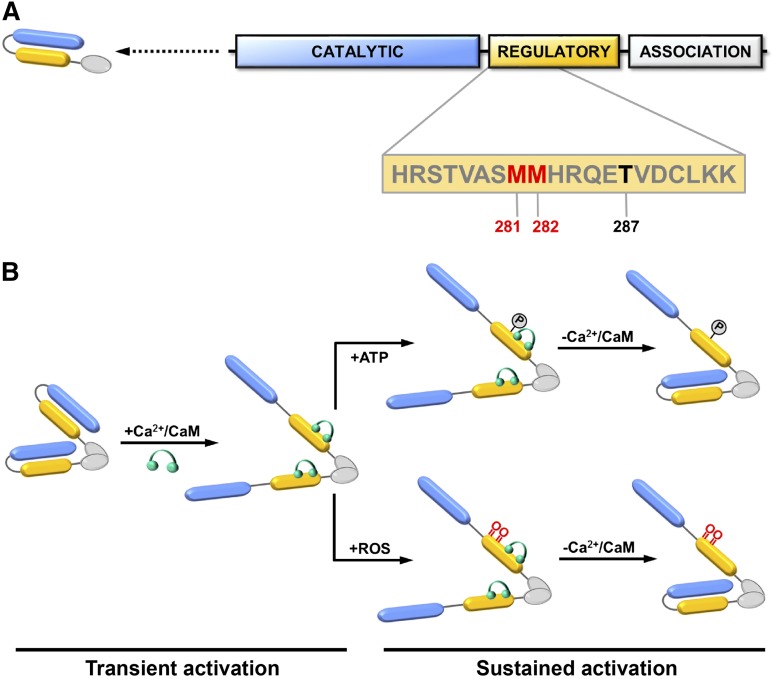
CaMKII is serine/threonine kinase that functions as a key mediator of intracellular calcium signaling by phosphorylating proteins involved in gene transcription, cell survival, excitation–contraction coupling, and calcium homeostasis.29 Like many kinases, CaMKII is subject to autoregulation. Following transient activation by calcium/calmodulin binding, the catalytic domain of CaMKII can autophosphorylate its C-terminal regulatory domain at Thr287.30 Autophosphorylation at Thr287 results in a conformational change in CaMKII that causes sustained activity that is no longer dependent on calcium/calmodulin (Figure 4). In addition to this mechanism of autoregulation via phosphorylation, CaMKII is also subject to redox regulation mediated by oxidation of a pair of tandem methionine residues (Met281/Met282) located within its regulatory domain.31 When oxidized, the 2 redox-active methionine residues block reassociation of the regulatory domain with the catalytic domain, resulting in sustained, calcium/calmodulin-independent kinase activity similar to that observed after Thr287 autophosphorylation (Figure 4). Oxidation of Met281/Met282 can be reversed by the action of MSRA, restoring CaMKII to an inactive state.31,32 In this way, CaMKII acts as a dynamic, reversible redox sensor during normal cellular homeostasis.
Figure 4.
CaMKII domain structure and activation. (A) Each CaMKII monomer contains a catalytic kinase domain (blue), a regulatory domain involved (yellow), and an association domain (white). Dimerization of monomeric subunits is mediated via the association domains, followed by oligomerization to form a holoenzyme consisting of 2 stacked hexameric rings (not shown). The sequence of the regulatory domain containing 2 redox-active methionine residues (Met281/Met282) (red) and Thr287 (black) are indicated. (B) In the resting state, the kinase activity of CaMKII is autoinhibited by an interaction between its catalytic and regulatory domains. Binding of calcium/calmodulin (Ca2+/CaM) (green) to the regulatory domain causes disassociation and transient activation of the catalytic domain. Autophosphorylation at Thr-287 or oxidation at Met281/Met282 prevents autoinhibition and causes sustained activation of CaMKII that is independent of calcium/calmodulin binding. Adapted with permission from Scott et al.39
Excessive or dysregulated CaMKII activity in cardiac myocytes promotes downstream signaling pathways involved in the pathophysiology of heart failure and arrhythmias.30 Increased levels of oxidized CaMKII in mice have been shown to promote sinus node dysfunction33 and atrial fibrillation.34 Stimulation of cardiac myocytes with angiotensin II or aldosterone leads to oxidative activation of CaMKII, which can be reversed by MSRA.31,32 Treatment of MsrA−/− mice with aldosterone caused exaggerated CaMKII oxidation and myocardial apoptosis, leading to impaired cardiac function and cardiac rupture after myocardial infarction.32 Increased levels of oxidized CaMKII have been detected in atrial tissue from diabetic patients after myocardial infarction and in mouse models of type 1 diabetes.35 Interestingly, mice expressing an oxidation-resistant form of cardiac CaMKII (in which the tandem redox-active methionine residues are mutated to valines) are resistant to diabetes-attributable mortality after myocardial infarction, suggesting that oxidation of CaMKII may contribute to sudden cardiac death in diabetes. Together, these in vivo studies confirm a regulatory role for methionine oxidation of CaMKII in cardiac pathophysiology and suggest that therapeutic approaches to decrease the oxidation of CaMKII or inhibit the activity of oxidized CaMKII may prevent or reduce complications of heart disease.
The role of CaMKII oxidation in vascular disease and thrombosis remains less clear. CaMKII is expressed in vascular cells, including vascular smooth muscle and endothelial cells, as well as in blood cells such as platelets. Recent studies have suggested that activation of CaMKII regulates vascular muscle responses such as proliferation, migration, and vascular remodeling.36,37 Vascular injury is associated with elevated levels of ROS,38 and CaMKII oxidation was observed to be increased following vascular injury in mice, possibly contributing to vascular smooth muscle migration and phenotypic switching.39 Subsequent studies found that oxidized CaMKII not only promotes vascular muscle cell migration and apoptosis, but also induces ROS generation by NADPH oxidase, providing a positive pro-oxidant feedback loop.40 Thus, there is emerging evidence that CaMKII functions in the redox regulation of vascular function, at least in vascular smooth muscle cells. Analogously, oxidation of CaMKII in the bronchial epithelium of the lung was found to promote inflammation and airway hyperreactivity, and contribute to the pathogenesis of allergic asthma.41 The potential role of CaMKII protein methionine oxidation in vascular endothelial cells and platelets remains largely undefined and is a potentially interesting area for future investigation.
Apolipoprotein A-I
Apolipoprotein A-I (apoA-I) is the major protein component of high-density lipoprotein (HDL).42 The primary sequence of apoA-I contains 3 oxidation-sensitive methionine residues (Met86, Met112, and Met148). These methionine residues were initially hypothesized to function as an endogenous antioxidant system by reducing lipid peroxides and cholesterol in oxidized lipoproteins43,44 and protecting other functional domains of apoA-I from oxidation.45 Subsequent studies found that oxidation of Met148 impairs the ability of apoA-I to activate lecithin-cholesterol acyltransferase (LCAT), an enzyme that converts cholesterol to cholesterol ester and is required for reverse cholesterol transport to the liver via HDL.46 apoA-I-mediated activation of LCAT is rescued when Met148 oxidation is reversed by MSR in vitro, and HDL prepared with a mutant apoA-I (M148L) is resistant to oxidative loss of LCAT activity.46 A decrease in LCAT activity can lead to increased atherogenesis, and it has been reported that patients with atherosclerosis have elevated levels of apoA-I oxidized at Met148.47 However, it remains unknown if apoA-1 protein methionine oxidation is reversible in vivo, and a causal relationship between apoA-I Met148 oxidation and atherosclerosis has not been established.
TM
TM is a transmembrane protein that is expressed on the luminal surface of vascular endothelium, where it functions as a high-affinity thrombin receptor.48 TM functions as an endogenous anticoagulant by modulating the substrate specificity of thrombin from that of a procoagulant to an anticoagulant protease.49 When bound to TM on the endothelial surface, thrombin is unable to convert fibrinogen to fibrin, activate factor V, or trigger platelet aggregation. Instead, thrombin becomes an efficient activator of protein C. The activated form of protein C (APC) is an anticoagulant protease that selectively inactivates coagulation factors Va and VIIIa, thereby inhibiting amplification of the coagulation cascade. APC also has potent anti-inflammatory effects on endothelial cells and monocytes.50
The clinical importance of the TM/protein C anticoagulant pathway is underscored by the strong association between venous thromboembolism and resistance to APC caused by the factor V Leiden mutation51 and by the severe thrombotic phenotype of children born with homozygous or compound heterozygous forms of protein C deficiency.52 The TM/APC pathway also may modulate atherosclerotic plaque stability53 and susceptibility to ischemic stroke.54 Gene targeted mice with endothelium-specific loss of TM develop severe spontaneous thrombosis in both the arterial and venous circulations.55
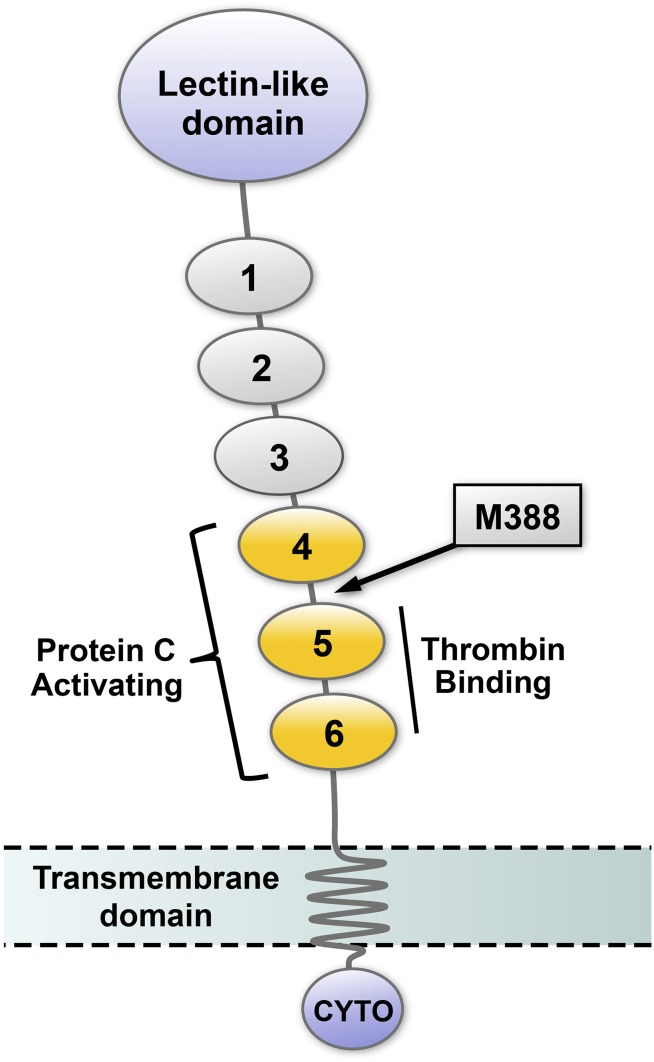
TM contains an epidermal growth factor (EGF) homology region consisting of 6 EGF-like domains (Figure 5). The high-affinity thrombin binding site is located within EGF-like domains 4 and 5, and the minimal region of TM that supports protein C activation consists of EGF-like domains 4, 5, and 6.56 An oxidation-sensitive methionine residue (Met388) is located in a short linker sequence connecting EGF-like domains 4 and 5. Oxidation of Met388 destabilizes the orientation between EGF-like domains 4 and 5, resulting in a fivefold decrease in the Kcat for protein C activation and a 90% decrease in TM anticoagulant activity.57,58 When Met388 is mutated to leucine, TM activity is maintained, even in the face of oxidative challenge.59,60 Using animal models, our group has demonstrated that the TM/protein C antithrombotic pathway becomes compromised by atherosclerosis and improves during regression of atherosclerosis.61-63 More recently, we demonstrated increased susceptibility to thrombosis and obtained evidence for inactivation of TM in hypercholesterolemic mice expressing human TM.64 Loss of TM-dependent protein C activation in mice is prevented by superoxide dismutase.65,66 These observations support the hypothesis that oxidation of TM Met388 may contribute to a prothrombotic phenotype. Additionally, APC itself has been shown to undergo protein methionine oxidation at Met59, which may directly contribute to loss of APC anticoagulant activity when it is exposed to oxidants such as H2O2 and HOCl.67 It remains to be definitively determined, however, whether oxidation of TM and/or APC occurs in vivo under pathophysiologic conditions of vascular oxidative stress and to what extent these redox reactions may affect susceptibility to thrombosis and vascular disease in humans.
Figure 5.
Schematic representation of thrombomodulin domain structure. The amino terminal portion of TM, which projects into the vascular lumen, contains a lectin-like domain and 6 EGF-like domains. EGF-like domains 5 and 6 are required for thrombin binding, and EGF-like domains 4 to 6 (yellow) are necessary for efficient activation of protein C. The location of the oxidation-sensitive regulatory methionine (M388) is in the short linker region between EGF-like domains 4 and 5 (arrow). TM also contains a transmembrane domain and a cytoplasmic tail (CYTO).
VWF
VWF is a multimeric protein synthesized by endothelial cells as high-molecular-weight multimers that are released into circulation through both constitutive and regulated pathways.68 The primary function of VWF in hemostasis is to mediate initial platelet adhesion and promote clot formation at sites of vascular injury in a process that is dependent on large multimers of VWF. Deficiency of VWF or loss of high-molecular-weight VWF multimers causes the clinical bleeding disorder von Willebrand disease.69 In contrast, an overabundance of ultralarge multimers of VWF causes the microvascular thrombotic disorder, thrombotic thrombocytopenic purpura.70 The classic form of thrombotic thrombocytopenic purpura results from a hereditary or acquired deficiency of the VWF-cleaving metalloprotease A disintegrin and metalloproteinase with a thrombospondin type 1 motif, 13 (ADAMTS13).71 Partial deficiency of ADAMTS13 may predispose to thrombotic events such as acute coronary syndromes and stroke.72,73
ADAMTS13 cleaves VWF at the Tyr1605-Met1606 peptide bond within its A2 domain.74 VWF Met1606 is susceptible to oxidation, which can affect its cleavage by ADAMTS13.75,76 Oxidation of Met1606 can be enhanced by shear stress77 or ristocetin,78 which induces a conformational change in VWF that unfolds the A2 domain. Met1606-oxidized VWF multimers are hyperactive in inducing platelet agglutination.77 Therefore, methionine oxidation of VWF Met1606 during vascular oxidative stress represents a potential prothrombotic mechanism, leading to the persistence of ultralarge VWF multimers that are resistant to proteolytic processing by ADAMTS13. Consistent with this notion, elevated plasma levels of large VWF multimers and oxidized VWF (including VWF with oxidized Met1606) have been detected in patients who have risk factors for thrombotic vascular disease, such as chronic kidney disease.79 Recent work suggests that ADAMTS13 also may be a target of methionine oxidation, leading to loss of its enzymatic activity and contributing to the accumulation of large VWF multimers during inflammation.80 Under such conditions, cleavage of VWF multimers may occur primarily through the proteolytic activity of leukocyte serine proteases rather than ADAMTS13.81,82 Interestingly, the activity VWF-cleaving proteases such as neutrophil elastase may be further regulated by the oxidative inactivation of α-1-antitrypsin, which itself contains several oxidation-sensitive methionine residues.83 Thus, the overall effect of protein methionine oxidation on VWF multimer structure and function is likely to be complex and may involve multiple redox regulated proteins. Future studies are needed to determine whether these oxidized methionines can be reduced by MSR and better define the impact of these redox reactions in vivo.
Other targets of protein methionine oxidation
Several additional proteins involved in vascular function and/or thrombosis contain oxidation-sensitive methionine residues (Table 1). Fibrinogen, the substrate for thrombin-mediated fibrin formation, contains three methionine residues (Met78, Met367, and Met476) that are highly susceptible to oxidation by HOCl.84 Oxidation of these methionine residues alters fibrin polymerization, which affects the structural and mechanical properties of the fibrin clot, potentially leading to delayed fibrinolysis.85 Interestingly, both tissue plasminogen activator and its major inhibitor, plasminogen activator inhibitor-1, also contain methionine residues that are subject to oxidation,86,87 but it is not yet known whether methionine oxidation of these proteins affects fibrinolysis in vivo.88 Several other hemostatic proteins, including factor VII, antithrombin, and α-2-antiplasmin, have been shown to contain oxidation-sensitive methionine residues that can regulate their function in vitro.89-91 It should be recognized that almost all of the work characterizing the effects of protein methionine oxidation on hemostatic protein function has been performed in vitro; therefore, the pathophysiological relevance of these reactions has not been established.
Macrophage function can be regulated by the stereospecific oxidation and reduction of methionine residues (Met46 and Met49) in actin.21,92,93 Methionine oxidation inhibits actin polymerization and actin-dependent processes such as cytokine release, which can be reversed by stereospecific reduction by MSRB. Additionally, there is some evidence that signaling pathways involved in inflammation and cell cycle regulation can be regulated by methionine oxidation.94-96 Thus, methionine oxidation and reduction might contribute to a variety of cellular signal transduction processes related to vascular disease.
Perspectives and clinical implications
It is now understood that redox biology is central to normal cellular homeostasis. ROS are generated widely as products of aerobic metabolism.97 ROS function not only as signaling molecules but also as redox regulators of proteins and other macromolecules via reversible reactions such as protein methionine oxidation. Because of their inherent reactivity, ROS are tightly regulated by a myriad of paired molecular oxidation and reduction reactions (redox switches). During pathologic states of oxidative stress, the normally tight regulation of cellular ROS flux can become disrupted. In light of the central role of redox reactions in normal vascular function, it is perhaps not surprising that the early trials of systemic antioxidant therapy failed to demonstrate a clinical benefit for the prevention of vascular or thrombotic events.5,6 One lesson from these trials is that a better basic understanding of specific vascular redox reactions is needed.
Certain protein methionine residues are particularly sensitive to reversible oxidation, especially those residing in accessible surface domains or domains that can become exposed to ROS through conformation changes induced by shear stress or protein-protein interactions. Like other reversible protein modification reactions such as protein phosphorylation/dephosphorylation and cysteine oxidation/reduction, protein methionine oxidation/reduction can regulate protein-protein interactions, enzymatic activity, and cellular function. These effects are highly dependent on subcellular localization, accessibility to regulatory enzymes such as MSR, and the redox environment of the specific intracellular or extracellular compartment in which the target protein functions. Some proteins with oxidation-sensitive methionine residues, including CaMKII, actin, inhibitor of κBα (IκBα), p53, and S100A9, are intracellular proteins that are likely to be protected from oxidation by an in vivo redox environment that is highly reducing and rich in MSR. Conversely, many vascular and hemostatic proteins containing oxidation-sensitive protein methionine residues, such as TM, VWF, and apoA-I, are plasma or endothelial cell surface proteins, which may be more susceptible to oxidation due to the oxidizing redox state of the extracellular environment.
None of the mammalian MSR isoforms are known to function extracellularly, but MsrA−/− mice have been reported to have higher levels of MetSO in serum proteins compared with wild-type mice,98 which suggests that MSRA can influence protein methionine oxidation of secreted proteins. For most of the proteins listed in Table 1, the identification and characterization of oxidation-sensitive methionine residues was accomplished using in vitro systems under nonphysiologic conditions. Therefore, with a few notable exceptions such as CaMKII and actin, the pathophysiologic consequences of these protein methionine oxidation reactions and whether or not they are regulated by MSR in a physiological environment have not been rigorously studied. Future studies using more physiological approaches, such as oxidation-resistant mutants, will be necessary to better delineate the role of methionine oxidation in vascular disease. Finally, it is noteworthy that several MSR isoforms are expressed at high levels in mitochondria,17 which are a major source of intracellular ROS. Defining the regulatory role of MSR on mitochondrial function will be an interesting topic for future investigation.
Future progress in defining the contribution of dysregulated protein methionine oxidation in vascular disease will depend on the development of quantitative methods to detect and measure MetSO in biological samples. Recently, there has been considerable effort directed toward developing methods for the large-scale detection and quantification of specific protein methionine residues using proteomics approaches.99 For example, adapting a combined fractional diagonal chromatography method100 to enrich for methionine-oxidized substrates shows promise for the high-throughput detection and identification of MetSO in specific proteins. A recent proteome-wide analysis identified >2000 specific protein methionine oxidation sites in a H2O2-treated T-cell line.28 This method also was applied to a mouse model of sepsis to demonstrate that it can be extended to identify protein MetSO in vivo.28 The functional significance of methionine oxidation in the vast majority of these target proteins remains to determined. Molecular dynamic simulations are now able to provide considerable insight into the potential effects of methionine oxidation on protein structure and function.67,85 However, new methods and techniques clearly are needed to better investigate the structural and biological effects of protein methionine oxidation.
As our understanding of the specific molecular targets of protein methionine oxidation improves, it is very likely that new candidate biomarkers of oxidative vascular disease will emerge.101,102 The prospects for the development of novel therapeutic approaches to target protein methionine oxidation are perhaps more daunting. For proteins such as CaMKII or VWF, whose function is upregulated by protein methionine oxidation, it might be more feasible to develop pharmacologic inhibitors that inhibit the function of the oxidized protein rather than attempting to prevent or reverse the formation of MetSO. Unfortunately, it is more often the case that protein methionine oxidation results in impairment of protein function rather than hyperactivation (Table 1). Potential therapeutic strategies in such cases might include the administration of an oxidation-resistant protein therapeutic. For example, to overcome the inhibitory effect of TM Met388 oxidation on its anticoagulant activity, a TM M388L analog has been designed that is insensitive to oxidative inactivation.103,104 This oxidation-resistant TM analog could potentially be used as an anticoagulant therapeutic agent in patients with acute thrombotic vascular disease.105 More general approaches might include targeting ROS-generating enzymes, such as NADPH oxidases, or developing selective ROS scavengers or antioxidant mimetics. A more specific method of controlling methionine redox state theoretically could be accomplished by manipulating MSR expression or activity. For example, new methods for nanocarrier-mediated targeted delivery of antioxidant enzymes such as MSR to vascular cells are being developed as potential therapeutic strategies in vascular disease.106
Conclusions
A number of vascular and hematostatic proteins are recognized to undergo protein methionine oxidation. In some cases, such as the oxidation of Met281/Met282 of CaMKII or Met1606 of VWF, protein methionine oxidation leads to hyperfunctioning of the target protein. For many other target proteins, such as TM and APC, oxidation of specific protein methionine residues causes loss of protein function. Most of our current understanding of the specific protein targets and functional consequences of protein methionine oxidation are derived from in vitro or animal studies. Therefore, much remains to be accomplished to fully understand the role of methionine oxidation in vascular disease and thrombosis. New methods and models are needed to define the mechanisms that regulate specific methionine oxidation reactions and the molecular effects of methionine oxidation on protein and cellular function. Tissue-specific animal models with altered expression and/or activity of MSR or oxidation-resistant vascular proteins are needed to explore effects of defined protein methionine reactions in endothelial and vascular smooth muscle cells and in hematopoietic cells such as platelets and leukocytes. A better appreciation of methionine redox biology and its vascular targets has the potential to lead to novel biomarkers and therapies for vascular disease and thrombosis.
Acknowledgments
This work was supported by the American Society of Hematology; National Institutes of Health grants GM007337 from the National Institute of General Medical Sciences, HL063943 and HL062984 from the National Heart, Lung, and Blood Institute; and an American Heart Association Predoctoral Fellowship award 12PRE940065.
Authorship
Contribution: S.X.G. wrote the paper; J.W.S. and S.R.L. reviewed and revised the paper; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven R. Lentz, Department of Internal Medicine, C21 GH, The University of Iowa, 200 Hawkins Dr, Iowa City, IA 52242; e-mail: steven-lentz@uiowa.edu.
References
- 1.Taniyama Y, Griendling KK. Reactive oxygen species in the vasculature: molecular and cellular mechanisms. Hypertension. 2003;42(6):1075–1081. doi: 10.1161/01.HYP.0000100443.09293.4F. [DOI] [PubMed] [Google Scholar]
- 2.Kim YW, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014;123(5):625–631. doi: 10.1182/blood-2013-09-512749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dikalov SI, Harrison DG. Methods for detection of mitochondrial and cellular reactive oxygen species. Antioxid Redox Signal. 2014;20(2):372–382. doi: 10.1089/ars.2012.4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011;51(5):978–992. doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sesso HD, Buring JE, Christen WG, et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300(18):2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cook NR, Albert CM, Gaziano JM, et al. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women’s Antioxidant Cardiovascular Study. Arch Intern Med. 2007;167(15):1610–1618. doi: 10.1001/archinte.167.15.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mueller CF, Laude K, McNally JS, Harrison DG. ATVB in focus: redox mechanisms in blood vessels. Arterioscler Thromb Vasc Biol. 2005;25(2):274–278. doi: 10.1161/01.ATV.0000149143.04821.eb. [DOI] [PubMed] [Google Scholar]
- 8.Thomas SR, Witting PK, Drummond GR. Redox control of endothelial function and dysfunction: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2008;10(10):1713–1765. doi: 10.1089/ars.2008.2027. [DOI] [PubMed] [Google Scholar]
- 9.Buettner GR. The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch Biochem Biophys. 1993;300(2):535–543. doi: 10.1006/abbi.1993.1074. [DOI] [PubMed] [Google Scholar]
- 10.Reczek CR, Chandel NS. ROS-dependent signal transduction. Curr Opin Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burgoyne JR, Oka S, Ale-Agha N, Eaton P. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid Redox Signal. 2013;18(9):1042–1052. doi: 10.1089/ars.2012.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim G, Weiss SJ, Levine RL. Methionine oxidation and reduction in proteins. Biochim Biophys Acta. 2014;1840(2):901–905. doi: 10.1016/j.bbagen.2013.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Black SD, Mould DR. Development of hydrophobicity parameters to analyze proteins which bear post- or cotranslational modifications. Anal Biochem. 1991;193(1):72–82. doi: 10.1016/0003-2697(91)90045-u. [DOI] [PubMed] [Google Scholar]
- 14.Chao CC, Ma YS, Stadtman ER. Modification of protein surface hydrophobicity and methionine oxidation by oxidative systems. Proc Natl Acad Sci USA. 1997;94(7):2969–2974. doi: 10.1073/pnas.94.7.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoshi T, Heinemann S. Regulation of cell function by methionine oxidation and reduction. J Physiol. 2001;531(Pt 1):1–11. doi: 10.1111/j.1469-7793.2001.0001j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HY, Gladyshev VN. Methionine sulfoxide reductases: selenoprotein forms and roles in antioxidant protein repair in mammals. Biochem J. 2007;407(3):321–329. doi: 10.1042/BJ20070929. [DOI] [PubMed] [Google Scholar]
- 17.Lee BC, Dikiy A, Kim HY, Gladyshev VN. Functions and evolution of selenoprotein methionine sulfoxide reductases. Biochim Biophys Acta. 2009;1790(11):1471–1477. doi: 10.1016/j.bbagen.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.St John G, Brot N, Ruan J, et al. Peptide methionine sulfoxide reductase from Escherichia coli and Mycobacterium tuberculosis protects bacteria against oxidative damage from reactive nitrogen intermediates. Proc Natl Acad Sci USA. 2001;98(17):9901–9906. doi: 10.1073/pnas.161295398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moskovitz J, Bar-Noy S, Williams WM, Requena J, Berlett BS, Stadtman ER. Methionine sulfoxide reductase (MsrA) is a regulator of antioxidant defense and lifespan in mammals. Proc Natl Acad Sci USA. 2001;98(23):12920–12925. doi: 10.1073/pnas.231472998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Luca A, Sanna F, Sallese M, et al. Methionine sulfoxide reductase A down-regulation in human breast cancer cells results in a more aggressive phenotype. Proc Natl Acad Sci USA. 2010;107(43):18628–18633. doi: 10.1073/pnas.1010171107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BC, Péterfi Z, Hoffmann FW, et al. MsrB1 and MICALs regulate actin assembly and macrophage function via reversible stereoselective methionine oxidation. Mol Cell. 2013;51(3):397–404. doi: 10.1016/j.molcel.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butterfield DA, Galvan V, Lange MB, et al. In vivo oxidative stress in brain of Alzheimer disease transgenic mice: Requirement for methionine 35 in amyloid beta-peptide of APP. Free Radic Biol Med. 2010;48(1):136–144. doi: 10.1016/j.freeradbiomed.2009.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nan C, Li Y, Jean-Charles PY, et al. Deficiency of methionine sulfoxide reductase A causes cellular dysfunction and mitochondrial damage in cardiac myocytes under physical and oxidative stresses. Biochem Biophys Res Commun. 2010;402(4):608–613. doi: 10.1016/j.bbrc.2010.10.064. [DOI] [PubMed] [Google Scholar]
- 24.Prentice HM, Moench IA, Rickaway ZT, Dougherty CJ, Webster KA, Weissbach H. MsrA protects cardiac myocytes against hypoxia/reoxygenation induced cell death. Biochem Biophys Res Commun. 2008;366(3):775–778. doi: 10.1016/j.bbrc.2007.12.043. [DOI] [PubMed] [Google Scholar]
- 25.Zhao H, Sun J, Deschamps AM, et al. Myristoylated methionine sulfoxide reductase A protects the heart from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2011;301(4):H1513–H1518. doi: 10.1152/ajpheart.00441.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García-Bermúdez M, López-Mejías R, González-Juanatey C, et al. Association of the methionine sulfoxide reductase A rs10903323 gene polymorphism with cardiovascular disease in patients with rheumatoid arthritis. Scand J Rheumatol. 2012;41(5):350–353. doi: 10.3109/03009742.2012.677063. [DOI] [PubMed] [Google Scholar]
- 27.Gu H, Chen W, Yin J, Chen S, Zhang J, Gong J. Methionine sulfoxide reductase A rs10903323 G/A polymorphism is associated with increased risk of coronary artery disease in a Chinese population. Clin Biochem. 2013;46(16-17):1668–1672. doi: 10.1016/j.clinbiochem.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Ghesquiere B, Jonckheere V, Colaert N, et al. Redox proteomics of protein-bound methionine oxidation. Mol Cell Proteomics. 2011;10(5):M110006866. doi: 10.1074/mcp.M110.006866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51(4):468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hudmon A, Schulman H. Structure-function of the multifunctional Ca2+/calmodulin-dependent protein kinase II. Biochem J. 2002;364(Pt 3):593–611. doi: 10.1042/BJ20020228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erickson JR, Joiner ML, Guan X, et al. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133(3):462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He BJ, Joiner ML, Singh MV, et al. Oxidation of CaMKII determines the cardiotoxic effects of aldosterone. Nat Med. 2011;17(12):1610–1618. doi: 10.1038/nm.2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swaminathan PD, Purohit A, Soni S, et al. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121(8):3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purohit A, Rokita AG, Guan X, et al. Oxidized Ca(2+)/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128(16):1748–1757. doi: 10.1161/CIRCULATIONAHA.113.003313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo M, Guan X, Luczak ED, et al. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123(3):1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.House SJ, Singer HA. CaMKII-delta isoform regulation of neointima formation after vascular injury. Arterioscler Thromb Vasc Biol. 2008;28(3):441–447. doi: 10.1161/ATVBAHA.107.156810. [DOI] [PubMed] [Google Scholar]
- 37.Singer HA. Ca2+/calmodulin-dependent protein kinase II function in vascular remodelling. J Physiol. 2012;590(Pt 6):1349–1356. doi: 10.1113/jphysiol.2011.222232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papaharalambus CA, Griendling KK. Basic mechanisms of oxidative stress and reactive oxygen species in cardiovascular injury. Trends Cardiovasc Med. 2007;17(2):48–54. doi: 10.1016/j.tcm.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scott JA, Xie L, Li H, et al. The multifunctional Ca2+/calmodulin-dependent kinase II regulates vascular smooth muscle migration through matrix metalloproteinase 9. Am J Physiol Heart Circ Physiol. 2012;302(10):H1953–H1964. doi: 10.1152/ajpheart.00978.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu LJ, Klutho PJ, Scott JA, et al. Oxidative activation of the Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) regulates vascular smooth muscle migration and apoptosis. Vascul Pharmacol. 2014;60(2):75–83. doi: 10.1016/j.vph.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanders PN, Koval OM, Jaffer OA, et al. CaMKII is essential for the proasthmatic effects of oxidation. Sci Transl Med. 2013;5(195):195ra197. doi: 10.1126/scitranslmed.3006135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fielding CJ, Fielding PE. Molecular physiology of reverse cholesterol transport. J Lipid Res. 1995;36(2):211–228. [PubMed] [Google Scholar]
- 43.Garner B, Witting PK, Waldeck AR, Christison JK, Raftery M, Stocker R. Oxidation of high density lipoproteins. I. Formation of methionine sulfoxide in apolipoproteins AI and AII is an early event that accompanies lipid peroxidation and can be enhanced by alpha-tocopherol. J Biol Chem. 1998;273(11):6080–6087. doi: 10.1074/jbc.273.11.6080. [DOI] [PubMed] [Google Scholar]
- 44.Garner B, Waldeck AR, Witting PK, Rye KA, Stocker R. Oxidation of high density lipoproteins. II. Evidence for direct reduction of lipid hydroperoxides by methionine residues of apolipoproteins AI and AII. J Biol Chem. 1998;273(11):6088–6095. doi: 10.1074/jbc.273.11.6088. [DOI] [PubMed] [Google Scholar]
- 45.Shao B, Oda MN, Bergt C, et al. Myeloperoxidase impairs ABCA1-dependent cholesterol efflux through methionine oxidation and site-specific tyrosine chlorination of apolipoprotein A-I. J Biol Chem. 2006;281(14):9001–9004. doi: 10.1074/jbc.C600011200. [DOI] [PubMed] [Google Scholar]
- 46.Shao B, Cavigiolio G, Brot N, Oda MN, Heinecke JW. Methionine oxidation impairs reverse cholesterol transport by apolipoprotein A-I. Proc Natl Acad Sci USA. 2008;105(34):12224–12229. doi: 10.1073/pnas.0802025105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shao B, Tang C, Sinha A, et al. Humans with atherosclerosis have impaired ABCA1 cholesterol efflux and enhanced high-density lipoprotein oxidation by myeloperoxidase. Circ Res. 2014;114(11):1733–1742. doi: 10.1161/CIRCRESAHA.114.303454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martin FA, Murphy RP, Cummins PM. Thrombomodulin and the vascular endothelium: insights into functional, regulatory, and therapeutic aspects. Am J Physiol Heart Circ Physiol. 2013;304(12):H1585–H1597. doi: 10.1152/ajpheart.00096.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Esmon CT. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. FASEB J. 1995;9(10):946–955. doi: 10.1096/fasebj.9.10.7615164. [DOI] [PubMed] [Google Scholar]
- 50.Danese S, Vetrano S, Zhang L, Poplis VA, Castellino FJ. The protein C pathway in tissue inflammation and injury: pathogenic role and therapeutic implications. Blood. 2010;115(6):1121–1130. doi: 10.1182/blood-2009-09-201616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kearon C, Crowther M, Hirsh J. Management of patients with hereditary hypercoagulable disorders. Annu Rev Med. 2000;51:169–185. doi: 10.1146/annurev.med.51.1.169. [DOI] [PubMed] [Google Scholar]
- 52.Goldenberg NA, Manco-Johnson MJ. Protein C deficiency. Haemophilia. 2008;14(6):1214–1221. doi: 10.1111/j.1365-2516.2008.01838.x. [DOI] [PubMed] [Google Scholar]
- 53.Seehaus S, Shahzad K, Kashif M, et al. Hypercoagulability inhibits monocyte transendothelial migration through protease-activated receptor-1-, phospholipase-Cbeta-, phosphoinositide 3-kinase-, and nitric oxide-dependent signaling in monocytes and promotes plaque stability. Circulation. 2009;120(9):774–784. doi: 10.1161/CIRCULATIONAHA.109.849539. [DOI] [PubMed] [Google Scholar]
- 54.Zlokovic BV, Griffin JH. Cytoprotective protein C pathways and implications for stroke and neurological disorders. Trends Neurosci. 2011;34(4):198–209. doi: 10.1016/j.tins.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Isermann B, Hendrickson SB, Zogg M, et al. Endothelium-specific loss of murine thrombomodulin disrupts the protein C anticoagulant pathway and causes juvenile-onset thrombosis. J Clin Invest. 2001;108(4):537–546. doi: 10.1172/JCI13077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood MJ, Sampoli Benitez BA, Komives EA. Solution structure of the smallest cofactor-active fragment of thrombomodulin. Nat Struct Biol. 2000;7(3):200–204. doi: 10.1038/73302. [DOI] [PubMed] [Google Scholar]
- 57.Glaser CB, Morser J, Clarke JH, et al. Oxidation of a specific methionine in thrombomodulin by activated neutrophil products blocks cofactor activity. A potential rapid mechanism for modulation of coagulation. J Clin Invest. 1992;90(6):2565–2573. doi: 10.1172/JCI116151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Prieto JH, Sampoli Benitez BA, Melacini G, Johnson DA, Wood MJ, Komives EA. Dynamics of the fragment of thrombomodulin containing the fourth and fifth epidermal growth factor-like domains correlate with function. Biochemistry. 2005;44(4):1225–1233. doi: 10.1021/bi0478852. [DOI] [PubMed] [Google Scholar]
- 59.Clarke JH, Light DR, Blasko E, et al. The short loop between epidermal growth factor-like domains 4 and 5 is critical for human thrombomodulin function. J Biol Chem. 1993;268(9):6309–6315. [PubMed] [Google Scholar]
- 60.Wood MJ, Becvar LA, Prieto JH, Melacini G, Komives EA. NMR structures reveal how oxidation inactivates thrombomodulin. Biochemistry. 2003;42(41):11932–11942. doi: 10.1021/bi034646q. [DOI] [PubMed] [Google Scholar]
- 61.Lentz SR, Fernández JA, Griffin JH, et al. Impaired anticoagulant response to infusion of thrombin in atherosclerotic monkeys associated with acquired defects in the protein C system. Arterioscler Thromb Vasc Biol. 1999;19(7):1744–1750. doi: 10.1161/01.atv.19.7.1744. [DOI] [PubMed] [Google Scholar]
- 62.Lentz SR, Miller FJ, Jr, Piegors DJ, et al. Anticoagulant responses to thrombin are enhanced during regression of atherosclerosis in monkeys. Circulation. 2002;106(7):842–846. doi: 10.1161/01.cir.0000024982.11646.25. [DOI] [PubMed] [Google Scholar]
- 63.Wilson KM, McCaw RB, Leo L, et al. Prothrombotic effects of hyperhomocysteinemia and hypercholesterolemia in ApoE-deficient mice. Arterioscler Thromb Vasc Biol. 2007;27(1):233–240. doi: 10.1161/01.ATV.0000251607.96118.af. [DOI] [PubMed] [Google Scholar]
- 64.Raife TJ, Dwyre DM, Stevens JW, et al. Human thrombomodulin knock-in mice reveal differential effects of human thrombomodulin on thrombosis and atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31(11):2509–2517. doi: 10.1161/ATVBAHA.111.236828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wilson KM, Leo L, Lentz SR. Gene transfer of human ECSOD enhances protein C activation in hypercholesterolemic mice. Arterioscler Thromb Vasc Biol. 2009;29(7):e8. [Google Scholar]
- 66.Dayal S, Gu S. X.; Wilson, K.M., Hutchins, R.; Lentz, S.R. Endogenous superoxide dismutase protects from impaired generation of activated protein C and enhanced susceptibility to experimental thrombosis in mice. Arterioscler Thromb Vasc Biol. 2014;34(Suppl 1):A34. [Google Scholar]
- 67.Nalian A, Iakhiaev AV. Possible mechanisms contributing to oxidative inactivation of activated protein C: molecular dynamics study. Thromb Haemost. 2008;100(1):18–25. doi: 10.1160/TH07-12-0750. [DOI] [PubMed] [Google Scholar]
- 68.Denis CV. Molecular and cellular biology of von Willebrand factor. Int J Hematol. 2002;75(1):3–8. doi: 10.1007/BF02981972. [DOI] [PubMed] [Google Scholar]
- 69.Sadler JE. New concepts in von Willebrand disease. Annu Rev Med. 2005;56:173–191. doi: 10.1146/annurev.med.56.082103.104713. [DOI] [PubMed] [Google Scholar]
- 70.Sadler JE. Von Willebrand factor, ADAMTS13, and thrombotic thrombocytopenic purpura. Blood. 2008;112(1):11–18. doi: 10.1182/blood-2008-02-078170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.George JN, Nester CM. Syndromes of thrombotic microangiopathy. N Engl J Med. 2014;371(19):1847–1848. doi: 10.1056/NEJMc1410951. [DOI] [PubMed] [Google Scholar]
- 72.Hanson E, Jood K, Nilsson S, Blomstrand C, Jern C. Association between genetic variation at the ADAMTS13 locus and ischemic stroke. J Thromb Haemost. 2009;7(12):2147–2148. doi: 10.1111/j.1538-7836.2009.03617.x. [DOI] [PubMed] [Google Scholar]
- 73.Miura M, Kaikita K, Matsukawa M, et al. Prognostic value of plasma von Willebrand factor-cleaving protease (ADAMTS13) antigen levels in patients with coronary artery disease. Thromb Haemost. 2010;103(3):623–629. doi: 10.1160/TH09-08-0568. [DOI] [PubMed] [Google Scholar]
- 74.Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87(10):4223–4234. [PubMed] [Google Scholar]
- 75.Chen J, Fu X, Wang Y, et al. Oxidative modification of von Willebrand factor by neutrophil oxidants inhibits its cleavage by ADAMTS13. Blood. 2010;115(3):706–712. doi: 10.1182/blood-2009-03-213967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lancellotti S, De Filippis V, Pozzi N, et al. Formation of methionine sulfoxide by peroxynitrite at position 1606 of von Willebrand factor inhibits its cleavage by ADAMTS-13: A new prothrombotic mechanism in diseases associated with oxidative stress. Free Radic Biol Med. 2010;48(3):446–456. doi: 10.1016/j.freeradbiomed.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 77.Fu X, Chen J, Gallagher R, Zheng Y, Chung DW, López JA. Shear stress-induced unfolding of VWF accelerates oxidation of key methionine residues in the A1A2A3 region. Blood. 2011;118(19):5283–5291. doi: 10.1182/blood-2011-01-331074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen J, Ling M, Fu X, López JA, Chung DW. Simultaneous exposure of sites in von Willebrand factor for glycoprotein Ib binding and ADAMTS13 cleavage: studies with ristocetin. Arterioscler Thromb Vasc Biol. 2012;32(11):2625–2630. doi: 10.1161/ATVBAHA.112.254144. [DOI] [PubMed] [Google Scholar]
- 79.De Filippis V, Lancellotti S, Maset F, et al. Oxidation of Met1606 in von Willebrand factor is a risk factor for thrombotic and septic complications in chronic renal failure. Biochem J. 2012;442(2):423–432. doi: 10.1042/BJ20111798. [DOI] [PubMed] [Google Scholar]
- 80.Wang Y, Chen J, Ling M, López JA, Chung DW, Fu X. Hypochlorous acid generated by neutrophils inactivates ADAMTS13: an oxidative mechanism for regulating ADAMTS13 proteolytic activity during inflammation. J Biol Chem. 2015;290(3):1422–1431. doi: 10.1074/jbc.M114.599084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Raife TJ, Cao W, Atkinson BS, et al. Leukocyte proteases cleave von Willebrand factor at or near the ADAMTS13 cleavage site. Blood. 2009;114(8):1666–1674. doi: 10.1182/blood-2009-01-195461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lancellotti S, De Filippis V, Pozzi N, et al. Oxidized von Willebrand factor is efficiently cleaved by serine proteases from primary granules of leukocytes: divergence from ADAMTS-13. J Thromb Haemost. 2011;9(8):1620–1627. doi: 10.1111/j.1538-7836.2011.04367.x. [DOI] [PubMed] [Google Scholar]
- 83.Taggart C, Cervantes-Laurean D, Kim G, et al. Oxidation of either methionine 351 or methionine 358 in alpha 1-antitrypsin causes loss of anti-neutrophil elastase activity. J Biol Chem. 2000;275(35):27258–27265. doi: 10.1074/jbc.M004850200. [DOI] [PubMed] [Google Scholar]
- 84.Weigandt KM, White N, Chung D, et al. Fibrin clot structure and mechanics associated with specific oxidation of methionine residues in fibrinogen. Biophys J. 2012;103(11):2399–2407. doi: 10.1016/j.bpj.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Burney PR, White N, Pfaendtner J. Structural effects of methionine oxidation on isolated subdomains of human fibrin D and αC regions. PLoS ONE. 2014;9(1):e86981. doi: 10.1371/journal.pone.0086981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stief TW, Martín E, Jimenez J, Digón J, Rodriguez JM. Effect of oxidants on proteases of the fibrinolytic system: possible role for methionine residues in the interaction between tissue type plasminogen activator and fibrin. Thromb Res. 1991;61(3):191–200. doi: 10.1016/0049-3848(91)90095-e. [DOI] [PubMed] [Google Scholar]
- 87.Lawrence DA, Loskutoff DJ. Inactivation of plasminogen activator inhibitor by oxidants. Biochemistry. 1986;25(21):6351–6355. doi: 10.1021/bi00369a001. [DOI] [PubMed] [Google Scholar]
- 88.Strandberg L, Lawrence DA, Johansson LB, Ny T. The oxidative inactivation of plasminogen activator inhibitor type 1 results from a conformational change in the molecule and does not require the involvement of the P1′ methionine. J Biol Chem. 1991;266(21):13852–13858. [PubMed] [Google Scholar]
- 89.Kornfelt T, Persson E, Palm L. Oxidation of methionine residues in coagulation factor VIIa. Arch Biochem Biophys. 1999;363(1):43–54. doi: 10.1006/abbi.1998.1071. [DOI] [PubMed] [Google Scholar]
- 90.Stief TW, Aab A, Heimburger N. Oxidative inactivation of purified human alpha-2-antiplasmin, antithrombin III, and C1-inhibitor. Thromb Res. 1988;49(6):581–589. doi: 10.1016/0049-3848(88)90255-1. [DOI] [PubMed] [Google Scholar]
- 91.Van Patten SM, Hanson E, Bernasconi R, et al. Oxidation of methionine residues in antithrombin. Effects on biological activity and heparin binding. J Biol Chem. 1999;274(15):10268–10276. doi: 10.1074/jbc.274.15.10268. [DOI] [PubMed] [Google Scholar]
- 92.Sroussi HY, Berline J, Palefsky JM. Oxidation of methionine 63 and 83 regulates the effect of S100A9 on the migration of neutrophils in vitro. J Leukoc Biol. 2007;81(3):818–824. doi: 10.1189/jlb.0706433. [DOI] [PubMed] [Google Scholar]
- 93.Hung RJ, Spaeth CS, Yesilyurt HG, Terman JR. SelR reverses Mical-mediated oxidation of actin to regulate F-actin dynamics. Nat Cell Biol. 2013;15(12):1445–1454. doi: 10.1038/ncb2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kanayama A, Inoue J, Sugita-Konishi Y, Shimizu M, Miyamoto Y. Oxidation of Ikappa Balpha at methionine 45 is one cause of taurine chloramine-induced inhibition of NF-kappa B activation. J Biol Chem. 2002;277(27):24049–24056. doi: 10.1074/jbc.M110832200. [DOI] [PubMed] [Google Scholar]
- 95.Midwinter RG, Cheah FC, Moskovitz J, Vissers MC, Winterbourn CC. IkappaB is a sensitive target for oxidation by cell-permeable chloramines: inhibition of NF-kappaB activity by glycine chloramine through methionine oxidation. Biochem J. 2006;396(1):71–78. doi: 10.1042/BJ20052026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nomura T, Kamada R, Ito I, Chuman Y, Shimohigashi Y, Sakaguchi K. Oxidation of methionine residue at hydrophobic core destabilizes p53 tetrameric structure. Biopolymers. 2009;91(1):78–84. doi: 10.1002/bip.21084. [DOI] [PubMed] [Google Scholar]
- 97.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Oien DB, Canello T, Gabizon R, et al. Detection of oxidized methionine in selected proteins, cellular extracts and blood serums by novel anti-methionine sulfoxide antibodies. Arch Biochem Biophys. 2009;485(1):35–40. doi: 10.1016/j.abb.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghesquière B, Gevaert K. Proteomics methods to study methionine oxidation. Mass Spectrom Rev. 2014;33(2):147–156. doi: 10.1002/mas.21386. [DOI] [PubMed] [Google Scholar]
- 100.Gevaert K, Ghesquière B, Staes A, et al. Reversible labeling of cysteine-containing peptides allows their specific chromatographic isolation for non-gel proteome studies. Proteomics. 2004;4(4):897–908. doi: 10.1002/pmic.200300641. [DOI] [PubMed] [Google Scholar]
- 101.Seraglia R, Sartore G, Marin R, et al. An effective and rapid determination by MALDI/TOF/TOF of methionine sulphoxide content of ApoA-I in type 2 diabetic patients. J Mass Spectrom. 2013;48(1):105–110. doi: 10.1002/jms.3113. [DOI] [PubMed] [Google Scholar]
- 102.Oggianu L, Lancellotti S, Pitocco D, et al. The oxidative modification of von Willebrand factor is associated with thrombotic angiopathies in diabetes mellitus. PLoS ONE. 2013;8(1):e55396. doi: 10.1371/journal.pone.0055396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Su EJ, Geyer M, Wahl M, et al. The thrombomodulin analog Solulin promotes reperfusion and reduces infarct volume in a thrombotic stroke model. J Thromb Haemost. 2011;9(6):1174–1182. doi: 10.1111/j.1538-7836.2011.04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Carnemolla R, Greineder CF, Chacko AM, et al. Platelet endothelial cell adhesion molecule targeted oxidant-resistant mutant thrombomodulin fusion protein with enhanced potency in vitro and in vivo. J Pharmacol Exp Ther. 2013;347(2):339–345. doi: 10.1124/jpet.113.205104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Iersel T, Stroissnig H, Giesen P, Wemer J, Wilhelm-Ogunbiyi K. Phase I study of Solulin, a novel recombinant soluble human thrombomodulin analogue. Thromb Haemost. 2011;105(2):302–312. doi: 10.1160/TH10-05-0287. [DOI] [PubMed] [Google Scholar]
- 106.Hood ED, Chorny M, Greineder CF, S Alferiev I, Levy RJ, Muzykantov VR. Endothelial targeting of nanocarriers loaded with antioxidant enzymes for protection against vascular oxidative stress and inflammation. Biomaterials. 2014;35(11):3708–3715. doi: 10.1016/j.biomaterials.2014.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]