Abstract
The insect cuticle is a unique material that covers the exterior of the animal as well as lining the foregut, hindgut, and tracheae. It offers protection from predators and desiccation, defines body shape, and serves as an attachment site for internal organs and muscle. It has demonstrated remarkable variations in hardness, flexibility and elasticity, all the while being light weight, which allows for ease of movement and flight. It is composed primarily of chitin, proteins, catecholamines, and lipids. Proteomic analyses of cuticle from different life stages and species of insects has allowed for a more detailed examination of the protein content and how it relates to cuticle mechanical properties. It is now recognized that several groups of cuticular proteins exist and that they can be classified according to conserved amino acid sequence motifs. We have annotated the genome of the tobacco hornworm, Manduca sexta, for genes that encode putative cuticular proteins that belong to seven different groups: proteins with a Rebers and Riddiford motif (CPR), proteins analogous to peritrophins (CPAP), proteins with a tweedle motif (CPT), proteins with a 44 amino acid motif (CPF), proteins that are CPF-like (CPFL), proteins with an 18 amino acid motif (18 aa), and proteins with two to three copies of a C-X5-C motif (CPCFC). In total we annotated 248 genes, of which 207 belong to the CPR family, the most for any insect genome annotated to date. Additionally, we discovered new members of the CPAP family and determined that orthologous genes are present in other insects. We established orthology between the M. sexta and Bombyx mori genes and identified duplication events that occurred after separation of the two species. Finally, we utilized 52 RNAseq libraries to ascertain gene expression profiles that revealed commonalities and differences between different tissues and developmental stages.
Keywords: cuticular proteins, Rebers & Riddiford, RNAseq, orthology, Manduca sexta
1. Introduction
The insect exoskeleton (cuticle) is a remarkable extracellular structure secreted by epidermal cells that serves as the outer body covering. It helps to protect the insect from environmental stresses such as predators, parasites, abrasions, desiccation, and UV radiation. It also functions as an attachment site for internal muscles and organs, and is instrumental to locomotion and flight. The bulk of the cuticle is made primarily of a network of chitin embedded in a proteinaceous matrix, with water, catecholamines, and some lipids (Moussian, 2013). Despite what may seem like a limited pallet of materials, insects are able to synthesize cuticles that differ widely with respect to physical properties. Measurements of hardness have differed by more than 30 fold, while the stiffness of cuticle has been shown to vary by more than seven orders of magnitude (Klocke and Schmitz (2011) and references within; Vincent and Wegst, 2004). Investigations of the reasons behind these differences have focused on the role of dehydration and chemical cross-linking (Andersen, 2010; Sugumaran, 2010; Vincent 2009). However, it has been apparent for many years that differences exist between proteins extracted from different types of cuticle (hard versus soft), different developmental stages (i.e. larva, pupa, adult), as well as different time points (pre-molt versus post-molt) (Andersen et al., 1986, 1987, 1995a; Cox and Willis, 1985; Dittmer et al., 2012; Jensen et al., 1997; Kiely and Riddiford, 1985; Missios et al., 2000). Obtaining sequence information of these proteins was limited as it required either solubilization, purification, digestion, and sequencing of peptide fragments, or screening of cDNA expression libraries with antibodies raised against cuticle extracts in order to identify the proteins involved. Now, the emergence of large scale genomic, proteomic, and transcriptomic analyses has allowed for a renewed look at the importance of the protein content.
The first insect genome sequenced was that of Drosophila melanogaster (Adams et al., 2000); there are now 112 insect genome sequences available at NCBI. An analysis of 12 genomes by Ioannidou et al. (2014) suggested that genes encoding structural cuticular proteins (CP) represent on average 1% of the total protein-coding genes in insects. The total number of genes varied from 63 for Pediculus humanus to 301 for Aedes aegypti. These numbers, as well as those given for other insects, likely represent a minimum as most genomes have not gone through a rigorous manual annotation and rely on homology to other known CPs. Combining proteomics with genomics has been used to identify cuticular proteins for several insect species (Bae et al., 2011; Carrasco et al., 2011; Dittmer et al., 2012; Fu et al., 2011; He et al., 2007). Similarly, genomics and transcriptomics have contributed valuable information on CP gene expression, discerning variations in the timing (pre- or post-molt), developmental stage (larval, pupal, adult), and relative expression levels of these genes (Cornman and Willis, 2009; Dittmer et al., 2012; Futahashi et al., 2008; Gallot et al., 2010; Liang et al., 2010; Okamoto et al., 2008; Suetsugu et al., 2013; Togawa et al., 2007, 2008).
Many CPs can be classified by the conserved sequence motifs they contain (Ioannidou et al., 2014; Willis et al., 2012). The largest group is known as CPs with the Rebers and Riddiford motif (CPR) that contain a core 28 amino acid sequence that is now recognized as part of a larger 63 amino acid consensus sequence (pfam00379) (Rebers and Riddiford, 1988; Willis et al., 2012). Variations in the extended consensus have been recognized, and CPs are often classified into one of three sub types: RR-1, RR-2, and RR-3. The number of CPR genes can vary greatly among species but many insects have more than 100 (Ioannidou et al., 2014). Additional groups having conserved sequence motifs include CPs analogous to peritrophins (CPAP) (Jasrapuria et al., 2010), CPs with a 44 amino acid motif (CPF) (Andersen et al., 1997; Togawa et al., 2007), CPF-like (CPFL) (Togawa et al., 2007), CPs with a Tweedle motif (CPT) (Guan et al., 2006), CPs with two or three repeats of C-X5-C motif (CPCFC) (Jensen et al., 1997; Willis et al., 2012), and CPs with an 18 amino acid motif (Andersen, 2000; Nakato et al., 1990). Additionally, low-complexity proteins can be found in the cuticle that are rich in glycine or contain repeats of AAP(A/V), P(V/Y), GYGL, or GLLG (Willis et al., 2012). However, since these proteins lack any other distinctive sequences, presence of these repeats alone is not proof enough of their location in the cuticle. Excellent reviews on CPs can be found in Willis (2010) and Willis et al, (2012).
The goal of this research was to annotate the CP genes in the genome of the tobacco hornworm, Manduca sexta, with respect to the groups described above. These seven groups (CPR, CPAP, CPF, CPFL, CPT, CPCFC, and 18 aa motif) were chosen as they all contain conserved sequences previously shown to be present in known cuticular structural proteins and, therefore, can serve as a diagnostic feature. We compared the CP genes in M. sexta with those of Bombyx mori (Futahashi et al., 2008) in order to identify orthologs. Finally, we utilized 52 RNAseq libraries prepared from various tissues and developmental stages to look for patterns of coordinated expression among the CP genes. This analysis offers new insights into the CPs present in cuticle synthesized at different times and developmental stages.
2. Materials and Methods
2.1 CP gene annotation
To identify putative CP genes, the M. sexta genome and first official gene set (OGS1), available from the Agricultural Pest Genomics Resource Database (www.agripestbase.org), were searched by tBLASTn (Altschul et al., 1997) with the following sequences: for the CPR family, the consensus sequence GxFxYxxPDGxxxxVxYxADENGYQPxGAHLP was used to identify the RR-1 subtype, and EYDAxPxYxFxYxVxDxHTGDxKSQxExRDGDVVxGxYSLxExDGxxRTVxYTADxxNGFNAVVxxE was used to identify the RR-2 subtype (Figure 3 in Willis et al., 2005); classification into the appropriate subfamily was confirmed by the use of a profile hidden Markov model that discriminates between the two subtypes, available at the cuticleDB website (biophysics.biol.uoa.gr/cuticleDB/, Karouzou et al., 2007). For the CPAP family, the Tribolium castaneum CPAP1-D (GenBank ACY95469) and CPAP3-A1 (GenBank ACY95475) sequences were used to identify CPAP family members. CPF genes were identified using the most highly conserved portion of the 44 amino acid motif, VSxYSKAVDTPFSSVRKxDxRIVNxA, (derived from Figure 1B in Togawa et al., 2007). CPFL genes were identified with the sequence LxYSAAPAVSHVAYxGxGxxYGW (derived from Figure 3 in Togawa et al., 2007). For Tweedle genes, the 100 amino acid weblogo sequence from Figure 3A in Willis (2010) was used to identify homologs. The sequence YPAGVNPAACPNYPYCD was used to identify members of the CPCFC family, and PVDTPEVAAAKAAHFAAH was used to identify genes encoding CPs with the 18 amino acid motif (Figure 4C and 4B in Willis, 2010).
For all genes except those of the CPAP family, names were assigned based on putative orthology to B. mori homologs. Reciprocal BLAST was performed to confirm that the B. mori protein identified as the top hit to a M. sexta query identified the same M. sexta protein as the top hit when it was used as the query sequence. Orthology was further established through the use of microsynteny. When the genes flanking a CP gene were homologous between B. mori and M. sexta, the CP genes were considered to be orthologous; identification of genes surrounding B. mori CP genes was inferred from the Gene Report page at NCBI associated with that particular CP gene. Because the CPAP genes in B. mori have not been annotated yet, naming of the M. sexta CPAP genes was based on a phylogenetic analysis of homologous proteins from several insect species; a detailed description of this analysis can be found in Tetreau et al. (2014, this issue).
2.2 Phylogenetic analysis
Phylogenetic analysis was performed using the corresponding protein sequence of selected genes from the CPR, CPAP, and CPFL groups. Details of the CPAP analysis can be found in a companion paper (Tetreau et al., 2014, this issue). Analysis was performed using MEGA software (v5.2.1; Tamura et al., 2011). Sequences were aligned globally using the ClustalW program in MEGA and then adjusted manually by eye. For the CPR group, the extended RR domain (pfam00379) was used; RR-1 and RR-2 subgroups were treated separately. For the CPFL group, the entire sequence of the M. sexta and B. mori proteins were used. Trees were constructed by the neighbor-joining method with a Poisson correction model. Gaps were treated by the pairwise deletion method and statistical analysis was performed by the bootstrap method using 1000 repetitions.
2.3 CP gene expression
Fifty-two RNAseq libraries had been prepared from various tissues and developmental stages as part of the Manduca Genome Sequencing Project (GenBank assembly accession number GCA_000262585.1). The libraries were sequenced by Illumina technology either at the Weill Cornel Medical College or the Baylor College of Medicine Human Genome Sequencing Center. The RNAseq reads were trimmed to 50 base pair and mapped onto the predicted open reading frame of the CP genes. Transcript abundance was determined by the FPKM method (fragments per kilobase of transcript per million fragments mapped). Read mapping and determination of FPKM values were performed using the RSEM software package (v1.2.12) (Li and Dewey, 2011). (The expression data for all genes in the M. sexta OGS2 can be downloaded at ftp://ftp.bioinformatics.ksu.edu/pub/Manduca/OGS2/OSU_files/, file 20140508RSEM_OGS2_Gene_FPKM.xlsx.) The FPKM values ranged from 0 to greater than 10,000 and gene expression was grouped as follows: less than 1, no expression; 1 – 10, very low expression; 10 – 100, low expression; 100 – 1,000, moderate expression; 1,000 – 10,000, high expression; greater than 10,000, very high expression. Hierarchical clustering of CP gene expression was performed using MultiExperiment Viewer (v4.9) (Saeed et al., 2003) with the Pearson correlation-based metric and average linkage clustering method.
3. Results and Discussion
3.1.1 CP gene annotation
Our analysis of OGS1 identified 206 gene models that belong to seven different CP groups (CPR, CPAP, CPF, CPFL, CPT, CPCFC, 18 aa motif). However, upon annotation of these models it became apparent that 28 of them likely represented 44 genes that had been incorrectly spliced. An additional 26 genes, for which no OGS1 gene model was predicted, were identified by searching the M. sexta genome sequence. These findings were incorporated during the development of OGS2 and increased the total number of gene models to 276 (Table 1). Some of these models are likely allelic variants of the same gene. For example, Msex2.14790 encodes a partial CPR gene that is 98% identical at the nucleotide level, and 100% identical at the amino acid level, to Msex2.11885 (MsCPR38). Furthermore, Msex2.14790 is on a small scaffold of ∼2000 nucleotides which is 94% identical at the nucleotide level to position 14,274 - 16,027 of scaffold00529, where Msex2.11885 resides. Thus it was judged to be an allelic variant of Msex2.11885.
Table 1. Manduca structural cuticle protein genes.
| OGS2 Gene ID | Gene Name a | Family | Scaffold No. b |
|---|---|---|---|
| Msex2.07923 | MsCPR1 | CPR-RR1 | scaffold00208 |
| Msex2.00114 | MsCPR154 (LCP14) | CPR-RR1 | scaffold00003 |
| Msex2.00115 | MsCPR155 | CPR-RR1 | scaffold00003 |
| Msex2.00116 | MsCPR156 | CPR-RR1 | scaffold00003 |
| Msex2.00117 | MsCPR2 | CPR-RR1 | scaffold00003 |
| Msex2.00118 | MsCPR3 | CPR-RR1 | scaffold00003 |
| Msex2.09274 | MsCPR4 (CP14.6) | CPR-RR1 | scaffold00267 |
| Msex2.09273 | MsCPR5 | CPR-RR1 | scaffold00267 |
| Msex2.11639 | MsCPR6 | CPR-RR1 | scaffold00482 |
| Msex2.05318 | MsCPR7 | CPR-RR1 | scaffold00107 |
| Msex2.05319 | MsCPR8 | CPR-RR1 | scaffold00107 |
| Msex2.05320 | MsCPR9 (CP20) | CPR-RR1 | scaffold00107 |
| Msex2.05189 | MsCPR10 | CPR-RR1 | scaffold00106 |
| Msex2.08400 | MsCPR11 | CPR-RR1 | scaffold00224 |
| Msex2.08401 | MsCPR12 | CPR-RR1 | scaffold00224 |
| Msex2.08402 | MsCPR13 | CPR-RR1 | scaffold00224 |
| Msex2.08403 | MsCPR174 | CPR-RR1 | scaffold00224 |
| Msex2.08404 | MsCPR175 | CPR-RR1 | scaffold00224 |
| Msex2.08405 | MsCPR176 | CPR-RR1 | scaffold00224 |
| Msex2.08406 | MsCPR177 | CPR-RR1 | scaffold00224 |
| Msex2.08407 | MsCPR14 | CPR-RR1 | scaffold00224 |
| Msex2.08408 | MsCPR15 (CP36) | CPR-RR1 | scaffold00224 |
| Msex2.08409 | MsCPR16 (CP27) | CPR-RR1 | scaffold00224 |
| Msex2.08410 | MsCPR17 | CPR-RR1 | scaffold00224 |
| Msex2.08411 | MsCPR18 | CPR-RR1 | scaffold00224 |
| Msex2.08412 | MsCPR19 | CPR-RR1 | scaffold00224 |
| Msex2.08413 | MsCPR52 | CPR-RR1 | scaffold00224 |
| Msex2.00741 | MsCPR20 | CPR-RR1 | scaffold00009 |
| Msex2.00742 | MsCPR21 | CPR-RR1 | scaffold00009 |
| Msex2.04086 | MsCPR157 | CPR-RR1 | scaffold00073 |
| Msex2.04415 | MsCPR158 | CPR-RR1 | scaffold00081 |
| Msex2.04416 | MsCPR22 | CPR-RR1 | scaffold00081 |
| Msex2.04417 | MsCPR23 | CPR-RR1 | scaffold00081 |
| Msex2.04418 | MsCPR24 | CPR-RR1 | scaffold00081 |
| Msex2.04419 | MsCPR25 | CPR-RR1 | scaffold00081 |
| Msex2.04420 | MsCPR159 | CPR-RR1 | scaffold00081 |
| Msex2.04424 | MsCPR26 | CPR-RR1 | scaffold00081 |
| Msex2.04425 | MsCPR30 | CPR-RR1 | scaffold00081 |
| Msex2.04427 | MsCPR160 | CPR-RR1 | scaffold00081 |
| Msex2.04428 | MsCPR27 | CPR-RR1 | scaffold00081 |
| Msex2.04429, Msex2.14415 | MsCPR28 | CPR-RR1 | scaffold00081, scaffold01804 |
| Msex2.11897 | MsCPR182 | CPR-RR1 | scaffold00529 |
| Msex2.11896 | MsCPR181 | CPR-RR1 | scaffold00529 |
| Msex2.11895 | MsCPR180 | CPR-RR1 | scaffold00529 |
| Msex2.11894 | MsCPR179 | CPR-RR1 | scaffold00529 |
| Msex2.11892 | MsCPR178 | CPR-RR1 | scaffold00529 |
| Msex2.11891 | MsCPR32 | CPR-RR1 | scaffold00529 |
| Msex2.11890 | MsCPR33 | CPR-RR1 | scaffold00529 |
| Msex2.11889 | MsCPR34 | CPR-RR1 | scaffold00529 |
| Msex2.11888 | MsCPR35 | CPR-RR1 | scaffold00529 |
| Msex2.11887 | MsCPR36 | CPR-RR1 | scaffold00529 |
| Msex2.11886 | MsCPR37 | CPR-RR1 | scaffold00529 |
| Msex2.11885, Msex2.14790 | MsCPR38 | CPR-RR1 | scaffold00529, scaffold03863 |
| Msex2.11884, Msex2.14482 | MsCPR39 | CPR-RR1 | scaffold00529, scaffold02011 |
| Msex2.14550, Msex2.11883 | MsCPR40 | CPR-RR1 | scaffold02427, scaffold0529 |
| Msex2.06320 | MsCPR161 | CPR-RR1 | scaffold00136 |
| Msex2.06321 |
|
CPR-RR1 | scaffold00136 |
| Msex2.06322 | MsCPR42 | CPR-RR1 | scaffold00136 |
| Msex2.06323 | MsCPR43 | CPR-RR1 | scaffold00136 |
| Msex2.06324 | MsCPR44 | CPR-RR1 | scaffold00136 |
| Msex2.06325 | MsCPR45 | CPR-RR1 | scaffold00136 |
| Msex2.06326 | MsCPR162 | CPR-RR1 | scaffold00136 |
| Msex2.06327 | MsCPR163 | CPR-RR1 | scaffold00136 |
| Msex2.06328 | MsCPR164 | CPR-RR1 | scaffold00136 |
| Msex2.06329 | MsCPR165 | CPR-RR1 | scaffold00136 |
| Msex2.06330 | MsCPR166 | CPR-RR1 | scaffold00136 |
| Msex2.06331 | MsCPR167 (LCP16/17) | CPR-RR1 | scaffold00136 |
| Msex2.06332 | MsCPR168 | CPR-RR1 | scaffold00136 |
| Msex2.06333 | MsCPR169 | CPR-RR1 | scaffold00136 |
| Msex2.06334 | MsCPR170 | CPR-RR1 | scaffold00136 |
| Msex2.06335 | MsCPR171 | CPR-RR1 | scaffold00136 |
| Msex2.06336 | MsCPR46 | CPR-RR1 | scaffold00136 |
| Msex2.13066 | MsCPR47 | CPR-RR1 | scaffold00793 |
| Msex2.08425 | MsCPR48 | CPR-RR1 | scaffold00221 |
| Msex2.11013 | MsCPR49 | CPR-RR1 | scaffold00396 |
| Msex2.13464 | MsCPR51 | CPR-RR1 | scaffold01003 |
| Msex2.06407 | MsCPR54 | CPR-RR1 | scaffold00137 |
| Msex2.06406 | MsCPR55 | CPR-RR1 | scaffold00137 |
| Msex2.04351 | MsCPR56 | CPR-RR1 | scaffold00079 |
| Msex2.00132 | MsCPH5 | CPR-RR2 | scaffold00003 |
| Msex2.02008 | MsCPR57 | CPR-RR2 | scaffold00029 |
| Msex2.00295 | MsCPR58 | CPR-RR2 | scaffold00006 |
| Msex2.00296 | MsCPR59 | CPR-RR2 | scaffold00006 |
| Msex2.00297 | MsCPR61 | CPR-RR2 | scaffold00006 |
| Msex2.00298 | MsCPR62 | CPR-RR2 | scaffold00006 |
| Msex2.00299 | MsCPR63 | CPR-RR2 | scaffold00006 |
| Msex2.00301 | MsCPR64 | CPR-RR2 | scaffold00006 |
| Msex2.00302 | MsCPR65 | CPR-RR2 | scaffold00006 |
| Msex2.00303 | MsCPR134 | CPR-RR2 | scaffold00006 |
| Msex2.09232 | MsCPR66 | CPR-RR2 | scaffold00266 |
| Msex2.08273 | MsCPR67 | CPR-RR2 | scaffold00227 |
| Msex2.08276 | MsCPR172 | CPR-RR2 | scaffold00227 |
| Msex2.08279 | MsCPR173 | CPR-RR2 | scaffold00227 |
| Msex2.08274 | MsCPR68 | CPR-RR2 | scaffold00227 |
| Msex2.08275 | MsCPR69 | CPR-RR2 | scaffold00227 |
| Msex2.08278 | MsCPR70 | CPR-RR2 | scaffold00227 |
| Msex2.08280 | MsCPR71 | CPR-RR2 | scaffold00227 |
| Msex2.08282 | MsCPR72 | CPR-RR2 | scaffold00227 |
| Msex2.08283 | MsCPR73 | CPR-RR2 | scaffold00227 |
| Msex2.05356 | MsCPR74 | CPR-RR2 | scaffold00108 |
| Msex2.04619 | MsCPR76 | CPR-RR2 | scaffold00086 |
| Msex2.04620 | MsCPR77 | CPR-RR2 | scaffold00086 |
| Msex2.03062 | MsCPR79 | CPR-RR2 | scaffold00054 |
| Msex2.03061 | MsCPR80 | CPR-RR2 | scaffold00054 |
| Msex2.15511 | MsCPR153 c | CPR-RR2 | scaffold00054 |
| Msex2.03060 | MsCPR152 c | CPR-RR2 | scaffold00054 |
| Msex2.12550 | MsCPR82 | CPR-RR2 | scaffold00685 |
| Msex2.12551 | MsCPR83 | CPR-RR2 | scaffold00685 |
| Msex2.12552 | MsCPR84 | CPR-RR2 | scaffold00685 |
| Msex2.12553 | MsCPR183 | CPR-RR2 | scaffold00685 |
| Msex2.12554 | MsCPR184 | CPR-RR2 | scaffold00685 |
| Msex2.12555, Msex2.14741 | MsCPR185 | CPR-RR2 | scaffold00685, scaffold03458 |
| Msex2.15538 | MsCPR186 | CPR-RR2 | scaffold00685 |
| Msex2.15539 | MsCPR187 | CPR-RR2 | scaffold00685 |
| Msex2.12556 | MsCPR188 | CPR-RR2 | scaffold00685 |
| Msex2.12557, Msex2.14630 | MsCPR189 | CPR-RR2 | scaffold00685, scaffold02706 |
| Msex2.12558 | MsCPR190 | CPR-RR2 | scaffold00685 |
| Msex2.12559 | MsCPR191 | CPR-RR2 | scaffold00685 |
| Msex2.12560 | MsCPR192 | CPR-RR2 | scaffold00685 |
| Msex2.12561 | MsCPR193 | CPR-RR2 | scaffold00685 |
| Msex2.12562 | MsCPR90 | CPR-RR2 | scaffold00685 |
| Msex2.12563 | MsCPR91 | CPR-RR2 | scaffold00685 |
| Msex2.12837 | MsCPR214 | CPR-RR2 | scaffold00717 |
| Msex2.15541 | MsCPR213 | CPR-RR2 | scaffold00717 |
| Msex2.12836 | MsCPR212 | CPR-RR2 | scaffold00717 |
| Msex2.12835 | MsCPR211 | CPR-RR2 | scaffold00717 |
| Msex2.12834 | MsCPR210 | CPR-RR2 | scaffold00717 |
| Msex2.12833 | MsCPR209 | CPR-RR2 | scaffold00717 |
| Msex2.12832, Msex2.14053 | MsCPR208 | CPR-RR2 | scaffold00717, scaffold01268 |
| Msex2.12831, Msex2.14054 | MsCPR207 | CPR-RR2 | scaffold00717, scaffold01268 |
| Msex2.12830, Msex2.14055 | MsCPR206 | CPR-RR2 | scaffold00717, scaffold01268 |
| Msex2.12829, Msex2.14056 | MsCPR205 | CPR-RR2 | scaffold00717, scaffold01268 |
| Msex2.12828, Msex2.14057 | MsCPR204 | CPR-RR2 | scaffold00717, scaffold01268 |
| Msex2.12827, Msex2.14058, Msex2.14910 | MsCPR203 | CPR-RR2 | scaffold00717, scaffold01268, scaffold04887 |
| Msex2.12826 | MsCPR202 | CPR-RR2 | scaffold00717 |
| Msex2.12825 | MsCPR201 | CPR-RR2 | scaffold00717 |
| Msex2.12824 | MsCPR200 | CPR-RR2 | scaffold00717 |
| Msex2.12823 | MsCPR199 | CPR-RR2 | scaffold00717 |
| Msex2.12822 | MsCPR198 | CPR-RR2 | scaffold00717 |
| Msex2.12821 | MsCPR197 | CPR-RR2 | scaffold00717 |
| Msex2.15540 | MsCPR196 | CPR-RR2 | scaffold00717 |
| Msex2.12820 | MsCPR195 | CPR-RR2 | scaffold00717 |
| Msex2.12819 | MsCPR194 | CPR-RR2 | scaffold00717 |
| Msex2.12818 | MsCPR121 | CPR-RR2 | scaffold00717 |
| Msex2.12816 | MsCPR122 | CPR-RR2 | scaffold00717 |
| Msex2.12817 | MsCPR123 | CPR-RR2 | scaffold00717 |
| Msex2.12815 | MsCPR124 | CPR-RR2 | scaffold00717 |
| Msex2.12814 | MsCPR150 | CPR-RR2 | scaffold00717 |
| Msex2.12194 | MsCPR125 | CPR-RR2 | scaffold00562 |
| Msex2.12193 | MsCPR126 | CPR-RR2 | scaffold00562 |
| Msex2.11250 | MsCPR128 | CPR-RR2 | scaffold00476 |
| Msex2.11252 | MsCPR129 | CPR-RR2 | scaffold00476 |
| Msex2.11256 | MsCPR130 | CPR-RR2 | scaffold00476 |
| Msex2.11351 | MsCPR131 | CPR-RR2 | scaffold00447 |
| Msex2.13191, Msex2.14515 | MsCPR132 | CPR-RR2 | scaffold00893, scaffold02588 |
| Msex2.01496 | MsCPR133 | CPR-RR2 | scaffold00024 |
| Msex2.08525 | MsCPR135 | CPR-RR2 | scaffold00228 |
| Msex2.08526 | MsCPR136 | CPR-RR2 | scaffold00228 |
| Msex2.08527 | MsCPR137 | CPR-RR2 | scaffold00228 |
| Msex2.08354 | MsCPR140 (Pro-resilin) | CPR-RR2 | scaffold00240 |
| Msex2.04385 | MsCPR141 | CPR-RR2 | scaffold00081 |
| Msex2.09926 | MsCPR142 | CPR-RR2 | scaffold00325 |
| Msex2.11692 | MsCPR143 | CPR-RR2 | scaffold00565 |
| Msex2.12993 | MsCPR144 | CPR-RR2 | scaffold00779 |
| Msex2.12994 | MsCPR145 | CPR-RR2 | scaffold00779 |
| Msex2.09922 | MsCPR151 | CPR-RR2 | scaffold00325 |
| Msex2.13636, Msex2.14049 | MsCPR215 | CPR-RR2 | scaffold01004, scaffold01304 |
| Msex2.13637, Msex2.14048 | MsCPR216 | CPR-RR2 | scaffold01004, scaffold01304 |
| Msex2.13638 | MsCPR217 | CPR-RR2 | scaffold01004 |
| Msex2.13639, Msex2.15551 | MsCPR218 | CPR-RR2 | scaffold01004, scaffold01304 |
| Msex2.13640, Msex2.14047 | MsCPR219 | CPR-RR2 | scaffold01004, scaffold01304 |
| Msex2.13641, Msex2.14046 | MsCPR220 | CPR-RR2 | scaffold01004, scaffold01304 |
| Msex2.13643 | MsCPR221 | CPR-RR2 | scaffold01004 |
| Msex2.13644 | MsCPR222 | CPR-RR2 | scaffold01004 |
| Msex2.13645A | MsCPR223 | CPR-RR2 | scaffold01004 |
| Msex2.13645C | MsCPR224 | CPR-RR2 | scaffold01004 |
| Msex2.13656, Msex2.14579 | MsCPR225 | CPR-RR2 | scaffold01013, scaffold02441 |
| Msex2.13657, Msex2.15550 | MsCPR226 | CPR-RR2 | scaffold01013, scaffold02441 |
| Msex2.13659 | MsCPR227 | CPR-RR2 | scaffold01013 |
| Msex2.13660 | MsCPR228 | CPR-RR2 | scaffold01013 |
| Msex2.13661 | MsCPR229 | CPR-RR2 | scaffold01013 |
| Msex2.13662 | MsCPR230 | CPR-RR2 | scaffold01013 |
| Msex2.13663 | MsCPR231 | CPR-RR2 | scaffold01013 |
| Msex2.13664 | MsCPR232 | CPR-RR2 | scaffold01013 |
| Msex2.13665 | MsCPR233 | CPR-RR2 | scaffold01013 |
| Msex2.13666 | MsCPR234 | CPR-RR2 | scaffold01013 |
| Msex2.15544 | MsCPR235 | CPR-RR2 | scaffold01013 |
| Msex2.13667 | MsCPR236 | CPR-RR2 | scaffold01013 |
| Msex2.13668 | MsCPR237 | CPR-RR2 | scaffold01013 |
| Msex2.13669 | MsCPR238 | CPR-RR2 | scaffold01013 |
| Msex2.13670 | MsCPR239 | CPR-RR2 | scaffold01013 |
| Msex2.13671 | MsCPR240 | CPR-RR2 | scaffold01013 |
| Msex2.13672 | MsCPR241 | CPR-RR2 | scaffold01013 |
| Msex2.13814 | MsCPR242 | CPR-RR2 | scaffold01084 |
| Msex2.14031 | MsCPR243 | CPR-RR2 | scaffold01333 |
| Msex2.14032, Msex2.14432 | MsCPR244 | CPR-RR2 | scaffold01333, scaffold01931 |
| Msex2.14033, Msex2.15549 | MsCPR245 | CPR-RR2 | scaffold01333, scaffold01931 |
| Msex2.14385 | MsCPR246 | CPR-RR2 | scaffold01713 |
| Msex2.14386 | MsCPR247 | CPR-RR2 | scaffold01713 |
| Msex2.14960 | MsCPR248 | CPR-RR2 | scaffold05463 |
| Msex2.15340 | MsCPR249 | CPR-RR2 | scaffold12390 |
| Msex2.15463 | MsCPR250 | CPR-RR2 | scaffold17868 |
| Msex2.06360 | MsCPR146 | CPR-RR3 | scaffold00136 |
| Msex2.11251 | MsCPR147 | CPR-RR3 | scaffold00476 |
| Msex2.07106 | MsCPR148 | CPR-RR3 | scaffold00167 |
| Msex2.04414 | MsCPR149 | CPR-RR3 | scaffold00081 |
| Msex2.13160, Msex2.11877 | MsCPAP1-A | CPAP1 | scaffold00817, scaffold00519 |
| Msex2.00613 | MsCPAP1-B | CPAP1 | scaffold00007 |
| Msex2.00614 | MsCPAP1-B2 | CPAP1 | scaffold00007 |
| Msex2.00381 | MsCPAP1-C | CPAP1 | scaffold00004 |
| Msex2.03859 | MsCPAP1-D | CPAP1 | scaffold00064 |
| Msex2.02135 | MsCPAP1-F | CPAP1 | scaffold00032 |
| Msex2.00269 | MsCPAP1-G | CPAP1 | scaffold00006 |
| Msex2.02134 | MsCPAP1-H | CPAP1 | scaffold00032 |
| Msex2.03076 | MsCPAP1-I | CPAP1 | scaffold00054 |
| Msex2.03236 | MsCPAP1-J | CPAP1 | scaffold00052 |
| Msex2.01703 | MsCPAP1-K | CPAP1 | scaffold00025 |
| Msex2.09867 | MsCPAP1-L | CPAP1 | scaffold00311 |
| Msex2.03103 | MsCPAP1-M | CPAP1 | scaffold00054 |
| Msex2.02137 | MsCPAP1-N | CPAP1 | scaffold00032 |
| Msex2.08722 | MsCPAP1-O | CPAP1 | scaffold00236 |
| Msex2.08805 | MsCPAP3-A1 | CPAP3 | scaffold00255 |
| Msex2.08806 | MsCPAP3-A2 | CPAP3 | scaffold00255 |
| Msex2.08808, Msex2.15015, Msex2.14185 | MsCPAP3-B | CPAP3 | scaffold00255, scaffold06133, scaffold01361 |
| Msex2.08810 | MsCPAP3-C | CPAP3 | scaffold00255 |
| Msex2.08807, Msex2.14226 | MsCPAP3-D1 | CPAP3 | scaffold00255, scaffold01383 |
| Msex2.04890 | MsCPAP3-D2 | CPAP3 | scaffold00101 |
| Msex2.03293 | MsCPAP3-E | CPAP3 | scaffold00068 |
| Msex2.03294, Msex2.15120 | MsCPAP3-E2 | CPAP3 | scaffold00068, scaffold07708 |
| Msex2.03295 | MsCPAP3-E3 | CPAP3 | scaffold00068 |
| Msex2.14886 | MsCPAP3-E4 | CPAP3 | scaffold04686 |
| Msex2.06560 | MsCPT1 | Tweedle | scaffold00143 |
| Msex2.04779 | MsCPT2 | Tweedle | scaffold00091 |
| Msex2.00101 | MsCPT3 | Tweedle | scaffold00003 |
| Msex2.09186 | MsCPT4 | Tweedle | scaffold00268 |
| Msex2.03833 | MsCPH1 | CPCFC | scaffold00064 |
| Msex2.05601 | MsCPF1 | CPF | scaffold00116 |
| Msex2.02569 | MsCPFL1 | CPFL | scaffold00039 |
| Msex2.04027 | MsCPFL2 | CPFL | scaffold00070 |
| Msex2.04028 | MsCPFL3 | CPFL | scaffold00070 |
| Msex2.04036 | MsCPFL4 | CPFL | scaffold00070 |
| Msex2.04037 | MsCPFL5 | CPFL | scaffold00070 |
| Msex2.04035 | MsCPFL6 | CPFL | scaffold00070 |
| Msex2.01072 | MsCPH15 | 18 aa | scaffold00013 |
| Msex2.01073 | MsCPH16 | 18 aa | scaffold00013 |
| Msex2.03612 | MsCPH30 | 18 aa | scaffold00059 |
| Msex2.03635 | MsCPH31 | 18 aa | scaffold00059 |
Gene names were assigned based on putative orthology to B. mori. Red type indicates genes in which one to one orthology could not be clearly established and, therefore, new gene numbers were assigned (MsCPR154-250). Blue type indicates genes previously deposited in GenBank (names given in parentheses). Genes boxed in gray identify expansion in M. sexta (putative paralogs) for which only a single ortholog occurs in B. mori.
Scaffolds boxed in yellow or orange contain clusters of five or more CP genes.
Genes MsCPR152 and 153 are orthologous to B. mori LOC101743422.
Additionally, some CP genes were also split between multiple gene models due to gaps in the genome assembly (supplementary file 1). This was evident for MsCPAP3-B. Both Msex2.08808 and 15015 show high sequence identity at the amino acid level (80% and 96% respectively) with obstructor-B (i.e. CPAP3-B) from Papilio xuthus (GenBank BAM17933). Interestingly, Msex2.08808's shared identity with PxObst-B covers amino acids 1-110 and 245-291, while Msex2.15015 aligns with residues 113-242. An examination of the genomic sequence for Msex2.08808 reveals a gap between exons 3 and 4 which likely contains the missing exons encoding residues 111-244 present in Msex2.15015. Thus, the correct sequence for MsCPAP3-B is a composite of Msex2.08808 and Msex2.15015. This conclusion is supported by the RNAseq data assembled by the Trinity software package which predicts a 1.24 kb transcript encoding a 296 amino acid protein that is the same as the composite sequence and 87% identical to PxObst-B (data not shown). (Msex2.14185 encodes for a protein that is 99% identical at both the amino acid and nucleotide levels with Msex2.15015 and therefore was judged to be an allele.) After accounting for multiple gene models representing the same gene, we identified 248 distinct CP genes in M. sexta.
3.1.2 CPR genes
Two-hundred and seven of the annotated genes belong to the CPR family: 79 RR-1, 124 RR-2, and 4 RR-3 (Table 1). This represents the largest number of CPR genes in any insect genome annotated to date and is 36% greater than the number of CPR genes in the silkworm B. mori: 56 RR-1, 93 RR-2, and 4 RR-3; 149 were originally annotated by Futahashi et al. (2008) including the misclassified CPH5, three more were added by Liang et al. (2010), and one additional gene, LOC101743422, was discovered in our analysis. Similar to observations for other insects (Cornman et al., 2008; Dittmer et al., 2012; Gallot et al., 2010; Liang et al., 2010), many of the genes occurred in clusters: 55 of the RR-1 genes were found in clusters of 11-17 genes on four scaffolds (00081, 00136, 00224, 00529), while 86 of the RR-2 genes were found in clusters of 8-26 genes on six scaffolds (00006, 00227, 00685, 00717, 01004, 01013) (Table 1).
As automated annotation is being carried out on several sequenced lepidopteran genomes, B. mori genes are frequently the top hit resulting in some continuity in gene naming among the lepidoptera. We endeavored to continue this practice by naming the M. sexta CP genes based on their deduced orthology to B. mori as determined by sequence identity, phylogenetic analysis, and microsynteny. For some of the M. sexta genes no clear orthology with B. mori could be established. For others, gene duplication resulted in multiple M. sexta genes being orthologous to a single B. mori gene. In these cases, new CPR numbers were assigned that begin with CPR152. Therefore, the CPR gene numbering went as high as CPR250 even though there are only 207 genes. Nevertheless, we believe that those numbered CPR1 - 151 are orthologous to the B. mori gene with the same name.
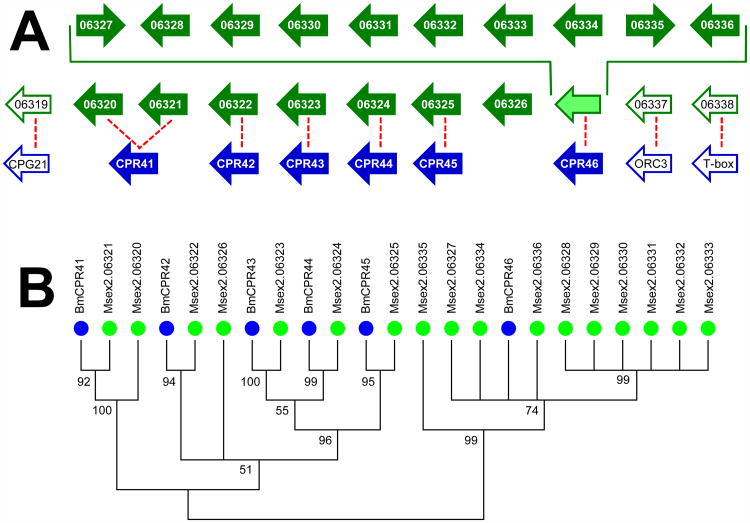
We were able to assign orthology to 52 of the 56 B. mori RR-1 genes; only BmCPR29, 31, 50, and 53 did not have clear orthologs in M. sexta. (BmCPR50 and 53 may be orthologs of Msex2.04420 and 06326 but this could not be confirmed by synteny, therefore, the M. sexta genes were given the names CPR159 and 162). Sixteen of the additional RR-1 genes in M. sexta likely arose through duplication of just four genes: MsCPR2, 13, 41, and 46 (boxed in grey in Table 1). For example, in B. mori the five genes CPR41-46 are flanked by CPG21 on one end and ORC3 and T-related protein-like on the other (Figure 1). These same three genes in M. sexta flank a cluster of 17 CPR genes on scaffold00136. BLAST results and phylogenetic analysis indicate that Msex2.06320 and 06321 are co-orthologs of BmCPR41, Msex2.06322 – 06325 are orthologous to BmCPR42-45, and Msex2.06327 – 06336 are all most closely related to BmCPR46. As no ortholog could be identified by sequence similarity or phylogenetic analysis, Msex2.06336 was given the name MsCPR46 because of its location and orientation with respect to ORC3 and T-related protein-like. Similar analysis indicates that Msex2.00115 and 00116 are paralogs of Msex2.00117 (MsCPR2), and Msex2.08403 – 08406 are paralogs of Msex2.08402 (MsCPR13).
Figure 1. Evidence of gene duplication in M. sexta.
(A) Alignment of M. sexta genes (green arrows) on scaffold00136 with the corresponding region in B. mori chromosome 22 (blue arrows). Filled arrows represent CPR genes while open arrows represent non-CPR genes; the arrows are only meant to show gene order and orientation and are not representative of gene size or distances between genes. The dashed red line denotes putative orthologs. (B) Phylogenetic analysis of the CPR genes shown in A. All branches with a bootstrap value less than 50 were collapsed.
Determining orthology among the RR-2 genes was more difficult as only 51 of the 93 RR-2 genes in B. mori had clear one- to-one orthologs in M. sexta. Interestingly, orthology could be established to most of the B. mori genes from CPR57 – 84 and 121 – 145; it was only for CPR85 – 120 (with the exception of 90 and 91) that direct orthology could not be conclusively resolved, although groups of relatedness were apparent. Phylogenetic analysis showed that 11 genes clustered on scaffold00685 (Msex2.12553 – 12561, 15538, 15539, which we have named MsCPR183 – 193) were most similar to BmCPR85 – 89 (Figure S1). The common node for these two groups suggest that they arose from a common ancestral gene and that duplication after speciation gave rise to additional genes. This conclusion is further supported by the location of MsCPR183 – 193 between genes MsCPR84 (Msex2.12552) and MsCPR90 (Msex2.12562) on scaffold00685, thus making it likely that they would be related to BmCPR85 – 89. Similar homologous groupings are shown in Table 2 and Figure S2.
Table 2. Orthologous groups of CPR RR-2 genes between B. mori and M. sexta a.
| Group No. | B. mori | M. sexta b |
|---|---|---|
|
| ||
| 1 | CPR85, 86, 87, 88, 89 | CPR183, 184, 185, 186, 187, 188, 189, 190, 191, 192, 193, 246, 247 |
|
| ||
| 2 | CPR95, 96, 97, 98, 99, 100 | CPR215, 216, 217, 218, 219, 220, 244, 245, 248 |
|
| ||
| 3 | CPR101, 102, 103, 104, 105 | CPR221, 222, 223, 224, 240, 241 |
|
| ||
| 4 | CPR107, 109, 111, 120 | CPR195, 196, 197, 199, 201, 207, 209, 225, 227, 229, 232 |
|
| ||
| 5 | CPR106, 108, 110 | CPR211, 213 |
See supplementary figure S2 for phylogenetic analysis
See Table 1 for OGS2 Gene ID
Four RR-3 genes were identified, Msex2.06360, 11251, 07106, and 04414, that all have orthologs in B.mori (CPR146 – 149). BmCPR149 was originally annotated as encoding an RR-2 protein (GenBank ACY06906), however, we have classified the M.sexta gene as an RR-3 based on greater sequence identity with the RR-3 consensus sequence (Andersen, 2000; data not shown). MsCPR149 is unique in that it encodes a protein with two RR domains, one each at the N- and C-termini of the protein; this model is supported by the RNAseq data suggesting that it is not an error in the gene assembly resulting from the fusion of two adjacent genes. It is similar to CPR58 of Nansonia vitripennis (GenBank XP_001601627) which has a comparable domain architecture (Willis, 2010).
3.1.3 CPAP genes
The second largest group of CP genes annotated was the CPAPs. CPAPs are classified according to the number of peritrophin A-type chitin binding domains they contain, with CPAP1 proteins containing one domain and CPAP3 proteins containing three domains (Jasrapuria et al., 2010). The CPAP3 genes were first recognized in D. melanogaster and alternately named gasp (gene analogous to small peritrophins) and obstructor (Barry et al., 1999; Behr and Hoch, 2005). This family of genes was further divided into two groups consisting of obstructor-A – E, and F – J. Genes orthologous to obst-A – E have been identified in other insects while obst-F – J have only been described for D. melanogaster. The related CPAP1 genes were identified and characterized by Jasrapuria et al. (2010) and contained ten family members named CPAP1-A – J.
We identified 15 putative CPAP1 genes and 10 CPAP3 genes (Table 1). This is greater than the 10 CPAP1 and 7 CPAP3 genes described from T. castaneum for which the most extensive annotation of this gene family has been carried out (Jasrapuria et al., 2010). Since similar work had not yet been performed for B. mori, we named the M. sexta genes based on orthology to T. castaneum and homologous genes in other insects. This analysis is described in a companion paper detailing the different groups of proteins containing peritrophin A-type chitin-binding domains in M. sexta (Tetreau et al., 2014, this issue) but will be summarized here. M. sexta has orthologs to 9 of the 10 CPAP1 genes in T. castaneum with 1-E being the exception. The six additional CPAP1 genes in M. sexta all have orthologs in other insect species and we have named these CPAP1-B2 and 1-K – O. M. sexta has orthologs to all seven of the CPAP3 genes in T. castaneum, and phylogenetic analysis shows that the three additional genes cluster with CPAP3-E and, therefore, we have named them E2 – E4; it should be noted that MsCPAP3-E2 (Msex2.03294) is not orthologous to Obst-E2 of D. melanogaster as the latter is a spliced isoform of the Obst-E gene while the former is a separate gene distinct from MsCPAP3-E1 (Msex2.03293). Similar to what Jasrapuria et al. (2010) reported for T. castaneum, the CPAP3-C ortholog of M. sexta (Msex2.08810) has the potential for alternative splicing of exon 5, giving rise to two different proteins. Eight of the ten CPAP3 genes are present in tandem arrays on two scaffolds: A1, A2, D1, B, and C on scaffold00255 (with one gene between B and C), and E1 – E3 on scaffold00068. The CPAP1 genes don't exhibit the same clustering characteristic although B1 and B2 are adjacent on scaffold00007, while H, F, and N are grouped on scaffold00032 (with one gene between F and N) (Table 1).
In general, very little annotation of these genes has been carried out for insect genomes. Recently, Ioannidou and co-workers (2014) developed profile hidden Markov models to identify CPAP and other cuticle structural proteins from sequenced genomes and applied this analysis to 14 arthropod genomes (12 insect and 2 crustacean). The number of putative CPAP genes identified ranged from 10 to 20 for CPAP1 and from 5 to 12 for CPAP3 (supplementary file 2 in Ioannidou et al., 2014). Combining our analysis of M. sexta with their analysis of several insect genomes allowed us to assign orthology to the CPAP genes from eight insect species (Table 3). The CPAP3 genes had the highest degree of conservation, with Acyrthosiphon pisum, Apis mellifera, B. mori, M. sexta, and T. castaneum all having orthologs to the seven described genes. Anopheles gambiae and P. humanus had orthologs to six of these genes with both lacking an ortholog to CPAP3-A2, although A. gambiae appears to have two duplications of CPAP3-A1 and P. humanus a duplication of CPAP3-D1 (Table 3). Interestingly, we observed that the gene model PHUM434540 appears to be a fusion of two genes with the 5-prime half orthologous to CPAP3-B and the 3-prime half orthologs to CPAP3-A1. As detailed by Behr and Hoch (2005), D. melanogaster has five CPAP3 (a.k.a. obstructor) genes, lacking orthologs to CPAP3-A2 and CPAP3-D2.
Table 3. CPAP gene orthologs from eight insect species.
| Gene | A. pisum | A. gambiae | A. melifera | B. mori | D. melanogaster | M. sexta | P. humanus | T. castaneum |
|---|---|---|---|---|---|---|---|---|
| CPAP1-A | ACYPI45536 | AGAP001203 | GB51442 | LOC101737637 | CG32036 | Msex2.13160 | PHUM600940 | TC004733 |
| CPAP1-B | ACYPI001105 | AGAP009480 | GB44831 | LOC101741926 | CG14301 | Msex2.00613 | TC000587 | |
| CPAP1-B2 | ACYPI007439 | AGAP009479 | GB44832 | LOC101743233 | CG14304 | Msex2.00614 | PHUM376120 | TC000588 |
| CPAP1-C | AGAP003751 | GB42318 | LOC101740411 | CG14880 | Msex2.00381 | TC000316 | ||
| CPAP1-D | LOC101744246 | Msex2.03859 | TC009263 | |||||
| CPAP1-E | GB41618 | CG14959 | PHUM034260 | TC009887 | ||||
| CPAP1-F | AGAP006435 | GB41624 | LOC101746039 | CG13675 | Msex2.02135 | PHUM034480 | TC009893 | |
| CPAP1-G | AGAP007613 | GB49021 | LOC101737536 | CG8192 | Msex2.00269 | PHUM071270 | TC008877 | |
| CPAP1-H | ACYPI004632 | AGAP005489 | GB41625 | LOC101740962 | CG13676 | Msex2.02134 | PHUM263920 | TC009894 |
| CPAP1-I | ACYPI009675 | AGAP002052 | GB41792 | LOC101742705 | CG13643 | Msex2.03076 | PHUM575010 | TC012766 |
| CPAP1-J | ACYPI000845 | AGAP010302 | GB54921 | LOC101747065 | CG14608 | Msex2.03236 | PHUM355660 | TC011101 |
| CPAP1-K | ACYPI008601 | AGAP001597 | GB49734 | LOC101736353 | CG7549 (mtg) | Msex2.01703 | PHUM601170 | TC013568 |
| CPAP1-L | ACYPI009002 | LOC101746263 | CG14607 | Msex2.09867 | PHUM355640 | LOC657301 | ||
| CPAP1-M | ACYPI002996 | AGAP028105 | GB48858 | LOC101738097 | Msex2.03103 | PHUM135070 | TC011724 | |
| CPAP1-N | AGAP005586 | GB41623 | LOC101740538 | CG12009 | Msex2.02137 | TC009890 | ||
| CPAP1-O | AGAP007089 | LOC101735358 | CG5756 | Msex2.08722 | ||||
| CPAP1 Unclassified | ACYPI30472 | GB51439, GB53319 | PHUM106940 | |||||
| CPAP3-A1 | ACYPI007911 | AGAP000989 | GB50636 | LOC101739926 | CG17052 (Obst-A) | Msex2.08805 | PHUM434540 | TC011140 |
| CPAP3-A1-like | AGAP000987, AGAP000988 | |||||||
| CPAP3-A2 | ACYPI006031 | GB41945 | LOC101740062 | Msex2.08806 | TC011141 | |||
| CPAP3-B | ACYPI004093 | AGAP009790 | GB41227 | LOC101740330 | CG4778 (Obst-B) | Msex2.08808 + Msex2.15015 | PHUM434540 | TC011139 |
| CPAP3-C | ACYPI007860 | AGAP003308 | GB41203 | LOC733010 | CG10287 (Obst-C; gasp) | Msex2.08810 | PHUM179210 | TC001169 |
| CPAP3-D1 | ACYPI009786 | AGAP000986 | GB41946 | LOC101740197 | CG17058 (Obst-D) | Msex2.08807 | PHUM434550, PHUM434570 | TC011142 |
| CPAP3-D2 | ACYPI001579 | AGAP002909 | GB41270 | LOC101742643 | Msex2.04890 | PHUM434580 | TC001350 | |
| CPAP3-E | ACYPI000583 | AGAP009405 | GB52854 | LOC101743739 | CG11142 (Obst-E1) | Msex2.03293 | PHUM291220 | TC011349 |
| CPAP3-E2 | LOC101743600 | CG11142 (Obst-E2) | Msex2.03294 | |||||
| CPAP3-E3 | Msex2.03295 | |||||||
| CPAP3-E4 | LOC101743460 | Msex2.14886 |
Gene IDs were derived from Jasrapuria (2011) and Ioannidou et al. (2014) except for B. mori and D. melanogaster which were from GenBank or Behr and Hoch (2005), and M. sexta from this report.
More interspecies variability was observed with the CPAP1 genes. Of the original ten genes described by Jasrapuria et al. (2010), the number of genes present in the eight species analyzed varied from five (A. pisum) to ten (T. castaneum), with most having eight or nine (Table 3). All eight species had orthologs to the newly described CPAP1-B2 branch, and at least three orthologs to CPAP1-K – O groups. A few genes from A. pisum, A. mellifera, and P. humanus could not be clearly grouped within any of the 16 CPAP1 branches and were left unclassified. It should be noted that although both our analysis (this report and Tetreau et al., 2014, this issue) and that of Ioannidou et al. (2014) independently verified the close relationship of the new members of the CPAP1 group to the original ten genes described previously for T. castaneum, the presence of the encoded proteins in the cuticle must still be established (and hence are they truly cuticle proteins). The D. melanogaster ortholog to CPAP1-K (CG7549), alternately known as mind-the-gap (mtg), is expressed in neuronal cells and encodes a protein critical for the organization of the synaptic extracelluar matrix of neuromuscular junctions (Rohrbough et al., 2007; Rushton et al., 2012). Interestingly, mtg null mutants, which die during the embryonic stage, exhibit a weakened cuticle and herniated hindgut phenotype (Rohrbough et al., 2007). This phenotype can be rescued by ubiquitous expression of a wildtype transgene (Rushton et al., 2009). According to FlyBase, moderate levels of mtg expression was also detected in the larval hindgut, tracheae, and carcass, all cuticle expressing tissues. Thus, it seems that mtg, and possibly other CPAPs, may have multiple roles.
3.1.4 Other CP genes
All of the remaining CP genes we annotated had identifiable orthologs in B. mori (Table 1). M. sexta and B. mori each have four CPT genes, one CPCFC gene, and one CPF gene. B. mori has five genes with the 18 amino acid motif (BmCPH14, 15, 16, 30, and 31) whereas M. sexta has only four. An examination of the protein sequences reveals that BmCPH14 and 15 are 92% identical and likely resulted from a gene duplication event that did not occur in M. sexta. The M. sexta ortholog (Msex2.01072) had a slightly higher sequence identity to BmCPH15 and, therefore, was given that name. Whereas six CPFL genes were identified in M. sexta, only four were in B. mori. The corresponding proteins of genes Msex2.04035, 04036, and 04037 were all similar to BmCPFL4. Msex2.04036 had the highest sequence similarity and was designated the ortholog; the remaining two genes were named MsCPFL5 and MsCPFL6.
3.2 CP gene expression
Fifty-two RNAseq libraries were created to provide transcript information to aid in the annotation of the genome. We sought to take advantage of this information to examine the expression profiles of the CP genes across multiple tissues and developmental stages. One caveat is that a majority of the libraries were created from tissues collected during the post-molt period. Thus, expression data of genes expressed primarily or exclusively during pre-molt (pharate) stages is limited. A second complication concerns the potential (if not likely) contamination of non-cuticle synthesizing samples with cuticle expressing tissues such as trachea or epidermis during the collection process. For example, we would not expect CP genes to be expressed in fat body, midgut, Malpighian tubules, or muscle. However, these samples may also contain pieces of epidermis or trachea and, therefore, CP gene expression was observed in some of these libraries. Finally, most libraries were sequenced only once, thus caution is advised when interpreting expression levels. (Four libraries were sequenced by both paired-end reads and single-end reads: midgut 5th instar wandering, and abdominal muscle (proleg) 5th instar at 12 h, pre-wandering, and wandering stages.)
The average number of CP genes expressed across all libraries was 88, with a minimum of 6, a maximum of 216, and a median of 72 (Figure 2A). The libraries with the highest number of CP genes expressed were the whole head and abdominal muscle (proleg) libraries of 4th instar larva after head capsule slippage (HCS, i.e. pharate 5th instar), each with 216 genes, while the library with the fewest CP genes expressed was prepared from adult midguts 3 -5 days post eclosion (6 genes). In total, 21 libraries had “above average” (> 88 genes) expression. This includes the 11 whole head libraries, the 5 libraries prepared form eggs or whole larvae (1st through 3rd instar), 3 of the abdominal muscle (proleg) libraries (4th instar HCS and 5th instar 12 h), midgut from 3rd instar HCS, and fat body from 4th instar HCS and pupa days 15-18; the latter three libraries would not be predicted to have abundant CP gene expression but this may be an indication of contamination from trachea or epidermis.
Figure 2. CP gene expression in the RNAseq libraries.
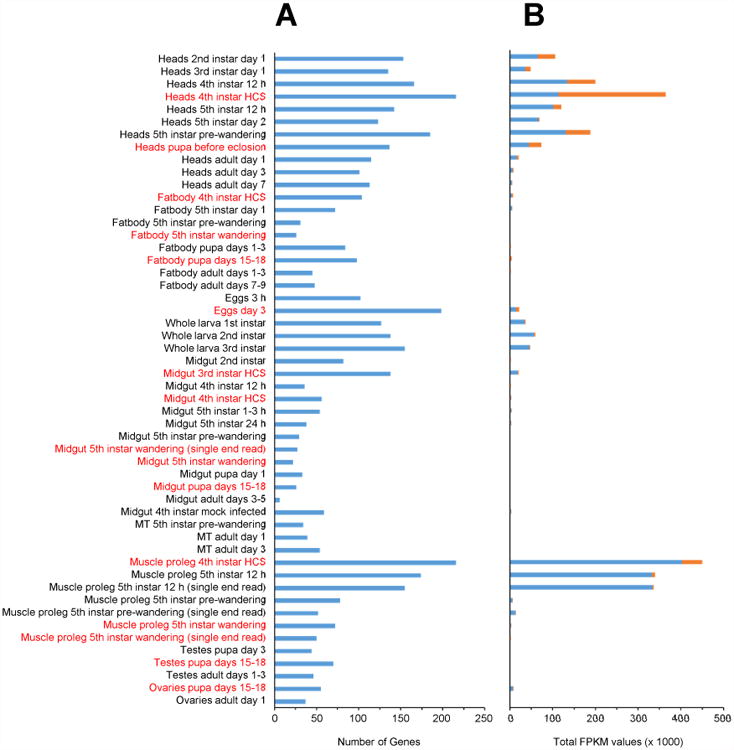
(A) Total number of CP genes expressed in each library. (B) Total FPKM values for CPR RR-1 and RR-2 genes in each library; the pattern looks the same when the total FPKM value for all CP genes is displayed. Red type indicates libraries prepared from pre-molt (pharate) stages.
The libraries having the highest levels of CP transcripts (as judged by total FPKM values) are predictably from tissues enriched for epidermal cells; namely whole heads, whole larvae, or muscle scraped from the abdominal body wall either just prior to or just after molting (Figure 2B). CPR RR-1 transcripts dominated most libraries, having the highest total FPKM values in all but nine libraries (Table 4). Genes from RR-1, RR-2, CPH, and CPFL all had high levels of expression in the whole head libraries (supplementary file 1) with RR-2 having the highest transcript levels in the 4th instar HCS library and CPH the highest in adult day 1. On average, the top ten expressed genes in each library accounted for nearly 77% of the CP transcripts, and we view them as representing the major CPs expressed in that library. We compared the top ten expressed genes in various libraries to identify differences and commonalities between them. Transcripts for RR-1 genes CPR154 and 156, the RR-2 gene CPR63, and genes CPH1 and CPH15 (CPCFC and 18 aa groups, respectively) are among the top ten in most post-molt larval head libraries but not adult. Conversely, CPR19, 23, 37, CPH30, and CPH31 are more highly expressed in the adult head than in the larva (Table 5). In contrast, CPFL1 and Msex2.02570 were commonly found in both the larval and adult whole head libraries. (Msex2.02570 encodes a low complexity protein that is not a member of the CP groups discussed here but it is annotated in GenBank as a pupal cuticle protein; accession number AY585211.) Only two of the whole head libraries were made from tissue collected pre-molt. For the 4th instar HCS (pharate 5th instar) library, six of the ten most highly expressed genes were RR-2, as compared to only two in the post-molt larval whole head libraries. A different pattern was observed when comparing the pre-molt adult head library (pupa days 21-22) with post-molt adult head libraries in which many of the top expressed genes were the same (CPR19, 23, 37, 67, CPH30, 31, and CPFL1) (Table 5).
Table 4. Most abundant CP group expressed per library.
| CP Group | Library | FPKMa | %b |
|---|---|---|---|
| RR2 | Head 4th instar HCS, | 250,848 | 56, |
| Fat body pupa days 15-18, | 3,669 | 59, | |
| Testes pupa days 15-18 | 827 | 48 | |
|
| |||
| CPAP1 | Fat body pupa days 1-3, | 1,899 | 42, |
| Midgut pupa day 1, | 91 | 37, | |
| Ovaries adult day 1 | 135 | 40 | |
|
| |||
| CPAP3 | Eggs 3 h | 8,116 | 86 |
|
| |||
| CPH | Head adult day 1 | 25,587 | 41 |
|
| |||
| CPT | Testes pupa day 3 | 247 | 32 |
|
| |||
| RR1 | All others | 32-402,401 | 30-90 |
Total FPKM value for indicated CP group.
Relative to total FPKM value for all CP genes.
Table 5. Top ten expressing CP genes in whole head librariesa.
| 2nd instar day 1 | 3rd instar day 1 | 4th instar day 1 | 4th instar HCS | 5th instar 12 h | 5th instar day 2 | 5th instar pre-wandering | Pupa days 21-22 | Adult day 1 | Adult day 3 | Adult day 7 |
|---|---|---|---|---|---|---|---|---|---|---|
| CPR134 | CPH15 | CPR134 | CPR216 | CPR46 | CPR169 | CPR46 | CPFL1 | CPH31 | CPR19 | CPR19 |
| CPH15 | CPH1 | CPR154 | CPR241 | CPR163 | CPR164 | CPR134 | CPH31 | CPH30 | CPR56 | Msex2.02570 |
| CPH1 | CPR154 | CPH15 | CPFL1 | CPR169 | CPR165 | CPR63 | CPR37 | CPFL1 | CPR37 | CPR56 |
| CPR170 | CPR134 | CPR156 | CPR3 | CPR63 | CPR46 | CPR163 | CPFL3 | CPFL5 | CPH30 | CPH30 |
| CPR42 | CPR156 | CPR3 | CPR43 | CPR5 | CPR166 | CPR4 | CPR73 | CPR19 | CPH31 | CPR23 |
| CPR154 | CPR170 | CPH1 | CPR83 | CPR4 | CPR167 | CPR169 | CPR19 | CPR37 | Msex2.02570 | CPR37 |
| CPR156 | CPR42 | Msex2.02570 | CPH31 | CPR134 | CPR168 | Msex2.02570 | CPR67 | CPR63 | CPR23 | CPR67 |
| CPR3 | CPR63 | CPFL1 | CPR184 | CPR154 | CPR63 | CPR3 | CPH30 | CPR10 | CPFL1 | CPR191 |
| CPFL1 | CPR3 | CPH31 | CPR215 | CPR156 | CPR42 | CPR5 | CPR32 | CPR23 | CPR67 | CPR141 |
| CPR63 | Msex2.02570 | CPR63 | CPR248 | CPH15 | CPR6 | CPR156 | CPR74 | CPAP3-Ca | CPR41 | CPH31 |
As determined by FPKM values; red type indicates RR-1 genes and blue type indicates RR-2 genes.
Differences between pre-molt and post-molt CP expression were also observed with the 3 day old eggs (pharate 1st instar) and the whole larva libraries (1st through 3rd instars 1 day post-molt). The whole larva libraries shared seven genes in common among the top ten (RR-1 genes CPR2, 3, 4, 5, 154, 156, and 170) of which only two (CPR3 and 4) were also highly expressed in 3 day old eggs. Top CP expressing genes in 3 day old eggs include CPT2, CPT3, CPAP3-A1 and CPAP3-Ca (supplementary file 1). Differences between pre-molt and post molt CP expression was not seen with the three libraries from abdominal muscle (proleg) having high CP transcript levels (4th instar HCS and 5th instar 12 h both single and paired end reads); CPR RR-1 transcripts dominated the list with six of the top ten genes the same between the three libraries (CPR3, 4, 46, 154, 156, and 163; supplementary file 1). Common top expressed genes among the larval libraries (whole head, whole larvae, abdominal muscle) include the RR-1 genes CPR3, 4, 5, 154, and 156 (supplementary file 1 and Fig S3). In a similar fashion, the corresponding genes in B. mori (BmCPR2-5) were among the top expressing genes during the pre- and post-molt 5th instar larval stage (Okamoto et al., 2008). Recently, Qiao and coworkers (2014) identified BmCPR2 as the gene responsible for the stony mutant in B. mori. This mutation lead to significant defects in cuticle extension, tensile strength, larval mobility, and body shape. It was also observed for B. mori that CPR46 was highly expressed post-molt in larval epidermis. As noted in section 3.1.2, the M. sexta ortholog to CPR46 has undergone gene expansion that encompasses 10 genes, CPR46 and CPR163 – 171 (Table 1). Members of this co-orthologous group appear as top expressed genes in many of the post-molt larval libraries in M. sexta as well (supplementary file 1).
Twenty of the annotated genes showed no expression in any of the libraries; 19 of these belong to the CPR RR-2 group and one belonged to the CPR RR-1 group (see supplementary file 1). Furthermore, 10 of these 20 genes belonged to the same cluster of related genes (group 4 in Table 2). An additional 29 genes were not detected in at least 47 of the 52 libraries (90%), with 22 of these belonging to the CPR RR-2 group; 8 of these had established orthology with B. mori genes (with CPR numbers between 121 – 145) while 7 belonged to a cluster whose orthology could not be established. The lone CPF gene in M. sexta, Msex2.05601, was not detected in 48 of the libraries, but had high expression in the whole head library from pupa day 21 – 22 (pharate adult) and then decreased thereafter (low expression in adult day 1 whole head and very low expression in adult days 3 and 7 whole head libraries). Similarly, transcripts for CPAP3-E4 (Msex2.14886) were detected at moderate levels in 3 h old eggs but no other libraries. These results suggest that genes showing very low or no expression in most (or all) libraries may have a restricted tissue or temporal expression, or that they may be pseudogenes.
Clustering analysis was performed to identify genes that had similar expression patterns across all 52 libraries. Figure 3 shows selected profiles from this analysis. (A heat map depicting the grouping and relative expression levels of all the CP genes is shown in supplementary Figure S3.) One of the most striking observation was of eight genes that had low to moderate levels of expression in nearly all of the libraries, producing an “expression band” that extended the width of the heat map (Figure 3A and supplementary Figure S3). Five of the eight genes in this cluster belonged to the CPAP group (CPAP1-C, 1-H, 1-M, and CPAP3-D2 and 3-Cb) and two were RR-3 (CPR146 and 149). The nearly ubiquitous expression pattern of these genes suggests that they may be important for general cuticle synthesis or synthesis of tracheal cuticle. Another six genes were nearly exclusively expressed only in pre-molt libraries during larval development (the exception being expression in fat body from pupa days 15 – 18) (Figure 3B). Remarkably, three of the genes belonged to the Tweedle family (of which only four were found in M. sexta) and the other three are CPR RR-2 genes that are clustered together in the genome. Similar observations were reported for B. mori in that CPT2-4 shared the same expression pattern with the highest expression occurring at the larval stage during the molt (Liang et al., 2010).
Figure 3. Selected expression patterns from cluster analysis.
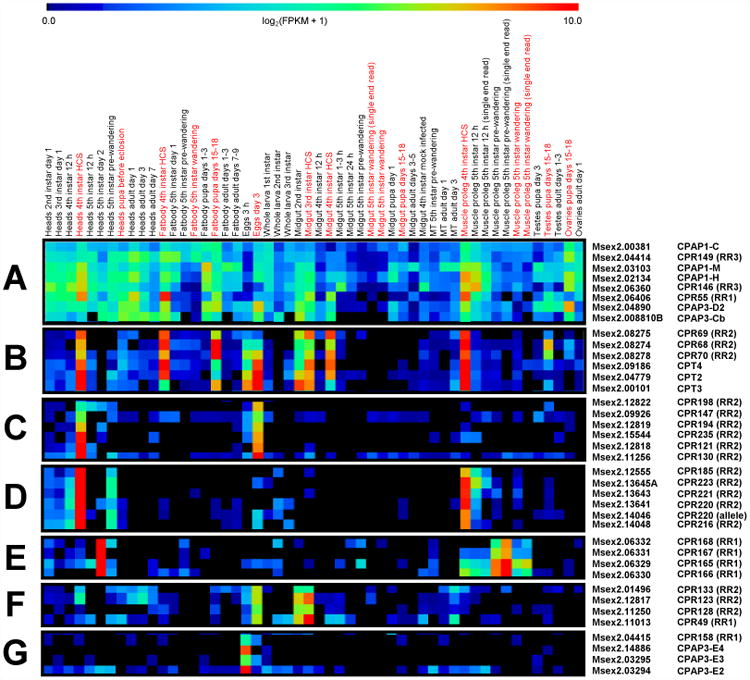
The expression pattern of each CP gene across all 52 RNAseq libraries were grouped by cluster analysis as described in section 2.3. A – G highlight some of the unique expression patterns observed; a full heat map of all of the genes is shown in supplemental Figure S3. Red type indicates libraries prepared from pre-molt (pharate) stages.
Several genes showed expression in just a few libraries such as whole heads 4th instar HCS and 3 day old eggs (Figure 3C). What these two libraries have in common is that they were prepared from tissue synthesizing larval head capsule cuticle pre-molt. The fact that those genes were not expressed in other larval pre-molt libraries that may contain tracheal or epidermis contamination such as fat body 4th instar HCS, midgut 3rd and 4th instars HCS, or abdominal muscle (proleg) 4th instar HCS, suggests that those genes are not involved in synthesis of tracheal or body wall cuticle. Similarly, several genes were expressed almost exclusively in 4th instar whole heads and abdominal muscle (proleg) HCS libraries, indicating likely function in larval head capsule and body wall synthesis pre-molt (Figure 3D). Additional interesting patterns observed include restriction of expression to whole heads of 5th instar day 2 and abdominal muscle (proleg) 5th instar post-molt (Figure 3E), 3 day old eggs and midguts of 2nd and 3rd instar larvae (Figure 3F), and eggs 3 hours old (Figure 3G). Three of the four genes in the last example are the additional members of the CPAP3-E group found in B. mori and M. sexta but not the other species presented in Table 3. This time point is too early for embryonic or 1st instar cuticle synthesis (Konopova and Zrzavy, 2005; Ziese and Dorn, 2003) but the transcripts may be maternally loaded.
4. Conclusions
Combining reciprocal BLAST, phylogenetic analysis, and microsynteny proved an effective method for identifying orthologous CP genes between M. sexta and B. mori. The most challenging identification was for the CPR RR-2 group which appears to be undergoing duplication after speciation more often than for other CP genes. For the remaining groups examined, one to one orthology was more easily established. The 207 CPR genes identified are currently the most in any insect for which this type of annotation has been carried out (although recent analysis by Ioannidou et al. (2014) indicates that the yellow fever mosquito, Aedes aegypti, may have as many as 240), and is 37% more than the number of CPR genes identified in B. mori; it remains to be determined which number is more typical for the Lepidoptera. Our analysis has also expanded the number of genes in the CPAP1 group from 10 to 16 but the veracity of the new genes as true CPs must be confirmed.
The RNAseq libraries provided valuable data on gene expression. In most libraries, CPR RR-1 genes accounted for most of the CP gene transcripts but this may have been influenced by the number of libraries prepared from larval tissues (32 of 52) or post-molt tissues (40 of 52). RR-1 genes have been proposed to be more prevalent in soft cuticle than hard cuticle (Andersen, 2000). As larva are mostly soft bodied and cuticle synthesized post-molt is thought to undergo less (or no) sclerotization than cuticle synthesized pre-molt (Andersen et al., 1995b), this may account for the abundance of RR-1 transcripts detected. Additionally, trachea is a likely contamination of the libraries prepared from midguts, Malpighian tubules, and fat body. The flexibility needed may necessitate a less sclerotized tracheal cuticle and, therefore, it is reasonable to anticipate a greater number of RR-1 proteins. In B. mori, Okamoto and co-workers (2008) also found that the majority of CP transcripts from two larval EST libraries (a pharate 5th instar and 5th instar day 3) were from RR-1 genes. Nevertheless, several distinct patterns of CP gene expression were observed, with differences noted between larval and adult cuticle or pre-molt and post-molt cuticle. As the purpose of the RNAseq libraries was to aid in the genome annotation, it was desirable to sample multiple tissues at several time points and all developmental stages. As a consequence, careful selection of a single tissue (i.e. epidermis, trachea, or hard cuticle or soft cuticle) or more refined time points was not a necessity. A more meticulous collection on both accounts in future experiments may yield a better characterization of CP gene expression.
Supplementary Material
Figure S1. Comparison ofM. sexta CP genes on scaffold00685 withBmCPR82–91. . (A) Alignment of M. sexta genes (green arrows) on scaffold00685 with the corresponding region in B. mori chromosome 22 (blue arrows); the arrows are only meant to show gene order and orientation and are not representative of gene size or distances between genes. The dashed red line denotes putative orthologs as determined by reciprocal BLAST and phylogenetic analysis. Genes boxed in yellow likely arose through duplication of a shared gene that occurred after separation of M. sexta and B. mori from a common ancestor. (B) Phylogenetic analysis of the CPR genes shown in A. All branches with a bootstrap value less than 50 were collapsed.
Figure S2. Identification of putative orthologous CPR RR-2 groups betweenM. sexta and B. mori by phylogenetic analysis. Phylogenetic analysis was used to determine the relationship between M. sexta proteins MsCPR183 – 250 and B. mori proteins BmCPR85 – 89 and 92 – 120. Branches with bootstrap values less than 50 were collapsed. Red diamonds (◆) indicate branch points between putative orthologous groups that presumable arose through gene duplication that occurred after separation of M. sexta and B. mori from a common ancestor.
Figure S3. Cluster analysis of the annotated CP genes. The expression pattern of each CP gene across all 52 RNAseq libraries were grouped by cluster analysis as described in section 2.3. Letters A – G identify the expression patterns shown in Figure 3. Red type indicates libraries prepared from pre-molt (pharate) stages.
248 cuticular protein (CP) genes were annotated in Manduca sexta
Orthology was established between M. sexta and Bombyx mori CP genes
Gene expression was analyzed via 52 RNAseq libraries
Diverse expression was observed across various tissues and developmental stages
Acknowledgments
This research was supported by grants from the National Institutes of Health (GM41247) and the National Science Foundation (IOS1257961) to M. Kanost, from the NIH (GM58634) to H. Jiang, and by the Cornell University Agricultural Experiment Station federal formula funds received from the USDA Cooperative State Research, Education, and Extension Service to P. Wang. This is contribution number 15-142-J from the Kansas Agricultural Experiment Station.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams DA, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SO. Studies on proteins in post-ecdysial nymphal cuticle of locust, Locusta migratoria, and cockroach, Blaberus craniifer. Insect Biochem Mol Biol. 2000;30:569–577. doi: 10.1016/s0965-1748(00)00029-1. [DOI] [PubMed] [Google Scholar]
- Andersen SO. Insect cuticular sclerotization: a review. Insect Biochem Mol Biol. 2010;40:166–178. doi: 10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Andersen SO, Højrup P. Extractable proteins from abdominal cuticle of sexually mature locusts, Locusta migratoria. Insect Biochem. 1987;17:45–51. [Google Scholar]
- Andersen SO, Højrup P, Roepstorff P. Characterization of cuticular proteins from the migratory locust, Locusta migratoria. Insect Biochem. 1986;16:441–447. doi: 10.1111/j.1432-1033.1986.tb09371.x. [DOI] [PubMed] [Google Scholar]
- Andersen SO, Rafin K, Krogh TN, Højrup P, Roepstorff P. Comparison of larval and pupal cuticular proteins in Tenebrio molitor. Insect Biochem Molec Biol. 1995a;2:177–187. doi: 10.1016/0965-1748(94)00048-m. [DOI] [PubMed] [Google Scholar]
- Andersen SO, Højrup P, Roepstorff P. Insect cuticular proteins. Insect Biochem Mol Biol. 1995b;25:153–176. doi: 10.1016/0965-1748(94)00052-j. [DOI] [PubMed] [Google Scholar]
- Andersen SO, Rafn K, Roepstorff P. Sequence studies of proteins from larval and pupal cuticle of the yellow meal worm, Tenebrio molitor. Insect Biochem Mol Biol. 1997;27:121–131. doi: 10.1016/s0965-1748(96)00076-8. [DOI] [PubMed] [Google Scholar]
- Bae N, Lödl M, Pollak A, Lubec G. Mass spectrometrical analysis of cuticular proteins from the wing of Hebemoia glaucippe (Linnaeus, 1758) (Lepidoptera: Pieridae) J Proteomics. 2011;75:517–531. doi: 10.1016/j.jprot.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Barry MK, Triplett AA, Christensen AC. A peritrophin-like protein expressed in the embryonic tracheae of Drosophila melanogaster. Insect Biochem Mol Biol. 1999;29:319–327. doi: 10.1016/s0965-1748(99)00004-1. [DOI] [PubMed] [Google Scholar]
- Behr M, Hoch M. Identification of the novel evolutionary conserved obstructor multigene family in invertebrates. FEBS Letters. 2005;579:6827–6833. doi: 10.1016/j.febslet.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Carrasco MA, Buechler SA, Arnold RJ, Sformo T, Barnes BM, Duman JG. Elucidating the biochemical overwintering adaptations of larval Cucujus clavipes puniceus, a nonmodel organism, via high throughput proteomics. J Proteome Res. 2011;10:4634–4646. doi: 10.1021/pr200518y. [DOI] [PubMed] [Google Scholar]
- Cornman RS, Willis JH. Annotation and analysis of low-complexity protein families of Anopheles gambiae that are associated with cuticle. Insect Mol Biol. 2009;18:607–622. doi: 10.1111/j.1365-2583.2009.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornman RS, Togawa T, Dunn WA, He N, Emmons AC, Willis JH. Annotation and analysis of a large cuticular protein family with the R&R consensus in Anopheles gambiae. BMC Genomics. 2008;9:22. doi: 10.1186/1471-2164-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DL, Willis JH. The cuticular proteins of Hyalophora cecropia from different anatomical regions and metamorphic stages. Insect Biochem. 1985;15:349–362. [Google Scholar]
- Dittmer NT, Hiromasa Y, Tomich JM, Lu N, Beeman RW, Kramer KJ, Kanost MR. Proteomic and transcriptomic analyses of rigid and membranous cuticles and epidermis from the elytra and hindwings of the red flour beetle, Tribolium castaneum. J Proteome Res. 2012;11:269–78. doi: 10.1021/pr2009803. [DOI] [PubMed] [Google Scholar]
- Fu Q, Li P, Xu YM, Zhang S, Jia L, Zha XF, Xiang ZH, He NJ. Proteomic analysis of larval integument, trachea and adult scale from the silkworm, Bombyx mori. Proteomics. 2011;11:3761–3767. doi: 10.1002/pmic.201000506. [DOI] [PubMed] [Google Scholar]
- Futahashi R, Okamoto S, Kawasaki H, Zhong YS, Iwanaga M, Mita K, Fujiwara H. Genome-wide identification of cuticular genes in the silkworm, Bombyx mori. Insect Biochem Mol Biol. 2008;38:1138–1146. doi: 10.1016/j.ibmb.2008.05.007. [DOI] [PubMed] [Google Scholar]
- Gallot A, Rispe C, Leterme N, Gauthier JP, Jaubert-Possamai S, Tagu D. Cuticular proteins and seasonal photoperiodism in aphids. Insect Biochem Mol Biol. 2010;40:235–240. doi: 10.1016/j.ibmb.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Guan X, Middlebrooks BW, Alexander S, Wasserman SA. Mutation of TweedleD, a member of an unconventional cuticle protein family, alters body shape in Drosophila. Proc Natl Acad Sci USA. 2006;103:16794–16799. doi: 10.1073/pnas.0607616103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He N, Botelho JMC, McNall RJ, Belozerov V, Dunn WA, Mize T, Orlando R, Willis JH. Proteomic analysis of cast cuticles from Anopheles gambiae by tandem mass spectrometry. Insect Biochem Mol Biol. 2007;37:135–146. doi: 10.1016/j.ibmb.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Ioannidou ZS, Theodoropoulou MC, Papandreou NC, Willis JH, Hamodrakas SJ. CutProtFam-Pred: detection and classification of putative structural cuticular proteins from sequence alone, based on profile hidden Markov models. Insect Biochem Mol Biol. 2014;52:51–59. doi: 10.1016/j.ibmb.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasrapuria S, Arakane Y, Osman G, Kramer KJ, Beeman RW, Muthukrishnan S. Genes encoding proteins with peritrophin A-type chitin-binding domains in Tribolium castaneum are grouped into three distinct families based on phylogeny, expression and function. Insect Biochem Mol Biol. 2010;40:214–227. doi: 10.1016/j.ibmb.2010.01.011. [DOI] [PubMed] [Google Scholar]
- Jensen UG, Rothmann A, Skou L, Andersen SO, Roepstorff P, Højrup P. Cuticular proteins from the giant cockroach, Blaberus craniifer. Insect Biochem Mol Biol. 1997;27:109–120. doi: 10.1016/s0965-1748(96)00074-4. [DOI] [PubMed] [Google Scholar]
- Karouzou MV, Spyropoulos Y, Iconomidou VA, Cornman RS, Hamodrakas SJ, Willis JH. Drosophila cuticular proteins with the R&R consensus: annotation and classification with a new tool for discriminating RR-1 and RR-2 sequences. Insect Biochem Mol Biol. 2007;37:754–760. doi: 10.1016/j.ibmb.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Kiely ML, Riddiford LM. Temporal programming of epidermal cell protein synthesis during the larval-pupal transformation of Manduca sexta. Roux's Arch Dev Biol. 1985;194:325–335. [Google Scholar]
- Klocke D, Schmitz H. Water as a major modulator of the mechanical properties of insect cuticle. Acta Biomater. 2011;7:2935–2942. doi: 10.1016/j.actbio.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Konopová B, Zrzavý J. Ultrastructure, development, and homology of insect embryonic cuticle. J Morphol. 2005;264:339–362. doi: 10.1002/jmor.10338. [DOI] [PubMed] [Google Scholar]
- Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Zhang L, Xiang Z, He N. Expression profile of cuticular genes of silkworm, Bombyx mori. BMC Genomics. 2010;11:173. doi: 10.1186/1471-2164-11-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missios S, Davidson HC, Linder D, Mortimer L, Okobi AO, Doctor JS. Characterization of cuticular proteins in the red flour beetle, Tribolium castaneum. Insect Biochem Mol Biol. 2000;30:47–65. doi: 10.1016/s0965-1748(99)00096-x. [DOI] [PubMed] [Google Scholar]
- Moussian B. The arthropod cuticle. In: Minelli A, Boxshall G, Fusco G, editors. Arthropod Biology and Evolution. Springer-Verlag; Berlin Heidelberg: 2013. pp. 171–196. [Google Scholar]
- Nakato H, Toriyama M, Izumi S, Tomino S. Structure and expression of a mRNA for a pupal cuticle protein of the silkworm, Bombyx mori. Insect Biochem. 1990;20:667–678. [Google Scholar]
- Okamoto S, Futahashi R, Kojima T, Mita K, Fujiwara H. Catalogue of epidermal genes: genes expressed in the epidermis during larval molt of the silkworm Bombyx mori. BMC Genomics. 2008;9:396. doi: 10.1186/1471-2164-9-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao L, Xiong G, Wang RX, He SZ, Chen J, Tong XL, Hu H, Li CL, Gai TT, Xin YQ, et al. Mutation of a cuticular protein, BmorCPR2, alters larval body shape and adaptability in silkworm, Bombyx mori. Genetics. 2014;196:1103–1115. doi: 10.1534/genetics.113.158766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebers JF, Riddiford LM. Structure and expression of a Manduca sexta larval cuticle gene homologous to Drosophila cuticle genes. J Mol Biol. 1988;203:411–423. doi: 10.1016/0022-2836(88)90009-5. [DOI] [PubMed] [Google Scholar]
- Rohrbough J, Rushton E, Woodruff E, III, Fergestad T, Vigneswaran K, Broadie K. Presynaptic establishment of the synaptic cleft extracellular matrix is required for post-synaptic differentiation. Genes Dev. 2007;21:2607–2628. doi: 10.1101/gad.1574107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton E, Rohrbough J, Broadie K. Presynaptic secretion of mind-the-gap organizes the synaptic extracellular matrix-integrin interface and postsynaptic environments. Dev Dyn. 2009;238:554–71. doi: 10.1002/dvdy.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton E, Rohrbough J, Deutsch K, Broadie K. Structure-function analysis of endogenous lectin mind-the-gap in synaptogenesis. Dev Neurobiol. 2012;72:1161–1179. doi: 10.1002/dneu.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- Suetsugu Y, Futahashi R, Kanamori H, Kadono-Okuda K, Sasanuma SI, Narukawa J, Ajimura M, Jouraku A, Namiki N, Shimomura M, et al. Large scale full-length cDNA sequencing reveals a unique genomic landscape in a lepidopteran model insect, Bombyx mori. G3. 2013;3:1481–1492. doi: 10.1534/g3.113.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugumaran M. Chemistry of cuticular sclerotization. Adv Insect Physiol. 2010;39:151–209. [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using Maximum Likelihood, Evolutionary Distance, and Maximum Parsimony Methods. Molecular Biology and Evolution. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetreau G, Dittmer N, Jasrapuria S, Cao X, Chen YR, Muthukrishnan S, Jiang H, Blissard GW, Kanost MR, Wang P. Analysis of chitin-binding proteins from Manduca sexta provides new insights into evolution of peritrophin A-type chitin-binding domains in insects. Insect Biochem Mol Biol. 2014 doi: 10.1016/j.ibmb.2014.12.002. this issue. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togawa T, Dunn WA, Emmons AC, Willis JH. CPF and CPFL, two related gene families encoding cuticular proteins of Anopheles gambiae and other insects. Insect Biochem Mol Biol. 2007;37:675–688. doi: 10.1016/j.ibmb.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Togawa T, Dunn WA, Emmons AC, Nagao J, Willis JH. Developmental expression patterns of cuticular protein genes with the R&R Consensus from Anopheles gambiae. Insect Biochem Mol Biol. 2008;38:508–519. doi: 10.1016/j.ibmb.2007.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JFV. If it's tanned it must be dry: a critique. J Adhes. 2009;85:755–769. [Google Scholar]
- Vincent JFV, Wegst UGK. Design and mechanical properties of insect cuticle. Arthropod Struct Dev. 2004;33:187–199. doi: 10.1016/j.asd.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Willis JH. Structural cuticular proteins from arthropods: annotation, nomenclature, and sequence characteristics in the genomics era. Insect Biochem Mol Biol. 2010;40:189–204. doi: 10.1016/j.ibmb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis JH, Iconomidou VA, Smith RF, Hamodrakas SJ. Cuticular Proteins. In: Gilbert L, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science. Vol. 4. Elsevier Pergamon; Oxford: 2005. pp. 79–109. [Google Scholar]
- Willis JH, Papandreou NC, Iconomidou VA, Hamodrakas SJ. Cuticular Proteins. In: Gilbert L, editor. Insect Molecular Biology and Biochemistry. Elsevier B.V.; 2012. pp. 134–166. [Google Scholar]
- Ziese S, Dorn A. Embryonic integument and “molts” in Manduca sexta (Insecta, Lepidoptera) J Morphol. 2003;255:146–161. doi: 10.1002/jmor.10056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Comparison ofM. sexta CP genes on scaffold00685 withBmCPR82–91. . (A) Alignment of M. sexta genes (green arrows) on scaffold00685 with the corresponding region in B. mori chromosome 22 (blue arrows); the arrows are only meant to show gene order and orientation and are not representative of gene size or distances between genes. The dashed red line denotes putative orthologs as determined by reciprocal BLAST and phylogenetic analysis. Genes boxed in yellow likely arose through duplication of a shared gene that occurred after separation of M. sexta and B. mori from a common ancestor. (B) Phylogenetic analysis of the CPR genes shown in A. All branches with a bootstrap value less than 50 were collapsed.
Figure S2. Identification of putative orthologous CPR RR-2 groups betweenM. sexta and B. mori by phylogenetic analysis. Phylogenetic analysis was used to determine the relationship between M. sexta proteins MsCPR183 – 250 and B. mori proteins BmCPR85 – 89 and 92 – 120. Branches with bootstrap values less than 50 were collapsed. Red diamonds (◆) indicate branch points between putative orthologous groups that presumable arose through gene duplication that occurred after separation of M. sexta and B. mori from a common ancestor.
Figure S3. Cluster analysis of the annotated CP genes. The expression pattern of each CP gene across all 52 RNAseq libraries were grouped by cluster analysis as described in section 2.3. Letters A – G identify the expression patterns shown in Figure 3. Red type indicates libraries prepared from pre-molt (pharate) stages.