Abstract
Osteoclasts reside on bone and are the main bone resorbing cells playing an important role in bone homeostasis, while natural killer (NK) cells are bone-marrow-derived cells known to play a crucial role in immune defence against viral infections. Although mature NK cells traffic through bone marrow as well as to inflammatory sites associated with enhanced bone erosion, including the joints of patients with rheumatoid arthritis, little is known about the impact NK cells may have on mature osteoclasts and bone erosion. We studied the interaction between human NK cells and autologous monocyte-derived osteoclasts from healthy donors in vitro. We show that osteoclasts express numerous ligands for receptors present on activated NK cells. Co-culture experiments revealed that interleukin-15-activated, but not resting, NK cells trigger osteoclast apoptosis in a dose-dependent manner, resulting in drastically decreased bone erosion. Suppression of bone erosion requires contact between NK cells and osteoclasts, but soluble factors also play a minor role. Antibodies masking leucocyte function-associated antigen-1, DNAX accessory molecule-1 or tumour necrosis factor-related apoptosis-inducing ligand enhance osteoclast survival when co-cultured with activated NK cells and restore the capacity of osteoclasts to erode bone. These results suggest that interleukin-15-activated NK cells may directly affect bone erosion under physiological and pathological conditions.
Keywords: DNAX accessory molecule-1, leucocyte function-associated antigen-1, natural killer cytotoxicity, osteoclast, tumour necrosis factor-related apoptosis-inducing ligand
Introduction
The healthy skeleton is a dynamic tissue constantly undergoing well-controlled remodelling with bone degradation by osteoclasts and bone formation by osteoblasts. The balance between bone resorption and deposit of new bone ensures a healthy bone matrix. Osteoclasts are bone resorbing cells characterized as giant multinucleated cells formed by fusion of mononuclear cells. They attach to the bone surface and resorb underlying bone matrix.1 Osteoclasts differentiate from cells of the monocyte/macrophage lineage upon stimulation with two essential factors: macrophage colony-stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL).2 After completed bone erosion, osteoclasts will subsequently undergo apoptosis.3 Many other important endogenous factors are known to regulate the extent of bone erosion through either their action on osteoclast formation or their capacity to erode bone. Such factors include hormones, e.g. parathyroid hormone,4 calcitonin,5 calcitriol (active vitamin D3),6 and oestrogen.7
In recent years, many cytokines produced during inflammatory responses have been shown to either stimulate or inhibit osteoclastogenesis or osteoclast erosion. Examples of pro-osteoclastogenic cytokines include interleukin-1 (IL-1),8 transforming growth factor-β,9 tumour necrosis factor-α (TNF-α),10 IL-15,11,12 IL-1713 and IL-6,14 whereas other cytokines, e.g. IL-4,15 granulocyte–macrophage colony-stimulating factor16 and interferon-γ17 inhibit osteoclastogenesis. Due to the large number of inflammatory cytokines known to impact the extent of bone erosion, it is not surprising to find that many chronic inflammatory diseases often shift bone turnover towards extensive bone loss [osteoporosis, rheumatoid arthritis (RA)] or increased bone formation (osteopetrosis, ankylosing spondylitis).
Natural killer (NK) cells are bone-marrow-derived large granular lymphocytes capable of mediating cellular cytotoxicity against a variety of target cells including infected and transformed cells, as well as certain normal cells undergoing ‘stress’.18 Natural killer cells also shape adaptive immune responses and maintain immune homeostasis through cytokine production and via direct killing of other immune cells. The decision as to whether NK cells will kill or leave a potential target unharmed is dependent on a finely tuned balance between signals mediated through a variety of activating and inhibitory receptors expressed on NK cells. In healthy blood, NK cells typically constitute 10–15% of all peripheral blood lymphocytes,19 whereas accumulation of NK cells in inflamed tissue has been observed in patients with autoimmune diseases including RA,20 a disease characterized by enhanced bone loss. Natural killer cells depend on IL-15 for their development and survival; this cytokine is present in RA synovial fluid and elevated serum levels are associated with a more erosive disease progression.21 Previous studies have shown that NK cells are often found juxtaposed with monocytes in RA synovial tissues and can efficiently induce their differentiation into osteoclasts ex vivo in the presence of IL-15.22 Natural killer cells were also found close to the bone surface, where they may directly contact mature bone eroding osteoclasts.22
Little is known about how NK cells may impact the function of mature bone-eroding osteoclasts. We have here set up an in vitro model system to investigate the cross-talk between human NK cells and autologous osteoclasts.
Materials and methods
Ethics statement
Buffy coats from healthy individuals were obtained anonymously from the Clinical Immunology Blood Bank, The State University Hospital, Copenhagen. All donors gave informed consent according to the protocol approved by The Ethics Committee for Copenhagen, Denmark, for research use (Ethical approval number H-D-2008-113).
Osteoclast generation
Human osteoclasts were differentiated from monocyte precursors isolated from peripheral blood mononuclear cells, which were obtained from buffy coats by density gradient centrifugation with Ficoll-Paque premium (GE Healthcare, Chalfont St Giles, UK) and washed twice in PBS (Gibco, Carlsbad, CA). CD14+ cells were labelled with CD14 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and separated using the Midi MACS cell separation system. The purity of isolated monocytes was confirmed to be > 95% by flow cytometry using V450-conjugated anti-CD14 (M5E2; BD Biosciences, San Jose, CA). CD14+ monocytes were resuspended in α-minimum essential medium (Gibco) supplemented with 10% heat-inactivated fetal bovine serum (Gibco), 2% heat-inactivated human serum (Sigma-Aldrich, St Louis, MO), 100 IU/ml penicillin and 100 µg/ml streptomycin (Gibco) (referred to as complete medium hereafter). Cells were cultured at 37° and 5% CO2 in 150 × 20 mm2 Petri dishes (Nunc, Roskilde, Denmark) with a density of 2·5 × 105 cells/cm2, in the presence of 25 ng/ml M-CSF (R&D Systems, Minneapolis, MN) and 10 ng/ml RANKL (R&D Systems). Medium was changed every third day to induce the formation of mature osteoclasts. After 7–8 days in culture, mature, large, multinucleated osteoclasts were formed. On the 10th day, cells were extensively washed with PBS to remove non-adherent cells, then treated with 0·05% Trypsin-EDTA (Gibco) for 20 min at 37° and subsequently scraped. The osteoclast pool was labelled with CD14 microbeads followed by cell isolation to deplete CD14+ cells. The CD14− cell fraction is enriched in mature osteoclasts.
Isolation and culture of NK cells
Autologous NK cells were isolated from peripheral blood mononuclear cells through immunomagnetic negative selection using the EasySep Human NK cell enrichment kit (StemCell Technologies, Vancouver, BC, Canada), according to the manufacturer's instructions. Isolated NK cells were cultured at 1 × 106 cells/ml in complete medium with 10 ng/ml recombinant human IL-15 (Peprotech, Rocky Hill, NJ). Cells were cultured for 10–12 days at 37° and 5% CO2, with medium change every third day (IL-15-activated NK cells). In some experiments, half of the isolated NK cells were cryopreserved in 90% fetal bovine serum (Gibco) and 10% DMSO (Sigma-Aldrich) at −80°, then thawed and rested in complete medium without IL-15 overnight at 37° and 5% CO2, before co-culturing with osteoclasts (resting NK cells).
Flow cytometry
Osteoclasts were surface-stained with the following monoclonal antibodies (mAbs) or fusion proteins at 10 μg/ml or 1 : 100 dilution: V450-conjugated anti-CD14 (M5E2; BD Biosciences), FITC-conjugated anti-CD51/61 (23C6; BD Biosciences), phycoerythrin (PE) -conjugated anti-HLA-ABC (W6/32; Biolegend, San Diego, CA), PE-conjugated anti-HLA-E (3D12; Biolegend), PE-conjugated anti-CD155 (SKII.4; Biolegend), PE-conjugated anti-CD112 (R2.525; BD Biosciences), PE-conjugated anti-CD48 (BJ40; Biolegend), PE-conjugated anti-CD54 (HA58; BD Biosciences), anti-MIC-A (159227; R&D Systems), anti-MIC-B (236511; R&D Systems), anti- UL-16 binding protein 1 (ULBP-1) (170818; R&D Systems), anti-ULBP-2/5/6 (165903; R&D Systems), anti-ULBP-3 (166510; R&D Systems), recombinant human NKp30-Fc chimera (R&D Systems), recombinant human NKp44-Fc chimera (R&D Systems), recombinant human NKp46-Fc chimera (R&D Systems), anti-human TNF-related apoptosis-inducing ligand (TRAIL) -R1/DR4 (DJR1; Biolegend), anti-human TRAIL-R2/DR5 (DJR2-4(7-8); Biolegend), anti-human Fas/CD95 (DX2; Biolegend). Unconjugated antibodies were detected by subsequent incubation with allophycocyanin-conjugated donkey anti-mouse IgG (Jackson Immunoresearch, West Grove, PA) or allophycocyanin-conjugated goat anti-human IgG, Fcγ fragment-specific (Jackson Immunoresearch). Mouse IgG1 (BD Biosciences), mouse IgG2a (BD Biosciences), mouse IgG2b (R&D Systems), and recombinant human IgG1 Fc (R&D Systems) were used as isotype controls. Near-IR LIVE/DEAD® Fixable dead cell stain (Invitrogen, Carlsbad, CA) was used to exclude dead cells. All samples were acquired on a BD LSR II flow cytometer and data were analysed using flowjo software (Tree Star Inc., Ashland, OR).
51Cr-release cytotoxicity assay
Cytotoxic activity for human NK cells against mature osteoclasts was assessed by standard 51Cr-release assay. Enriched mature osteoclasts were added at 5 × 103 cells/well to a 96-well flat-bottom plate (BD Falcon, Franklin Lakes, NJ) and sedimented overnight. Cells were washed and labelled with Na51CrO4 (PerkinElmer, Waltham, MA) as target cells. Effector cells were IL-15-activated NK cells or resting NK cells. The NK cells were washed and serially diluted for multiple effector : target (E : T) ratios from 40 : 1 to 1·25 : 1 in triplicates and added to labelled osteoclasts. Cells were incubated for 4 hr at 37° and cytotoxicity was assessed by 51Cr-release, which was measured in supernatants using TopCount (PerkinElmer). Spontaneous release and maximum release were determined by incubating target cells alone without effector cells in medium or in 10% Triton X-100 (Merck, White House Station, NJ) in PBS, respectively. The standard formula for calculation of % specific lysis was used: % specific lysis = (experimental 51Cr-release − spontaneous 51Cr-release)/(maximum 51Cr-release − spontaneous 51Cr-release) × 100. For blocking experiments, NK cells were pre-incubated with mAbs of interest for 2 hr at 37° before performing a 4-hr 51Cr-release assay in the presence of the same mAb(s) at an E : T ratio of 10 : 1. The following mouse anti-human mAbs were used at 10 μg/ml unless otherwise noted: anti-TRAIL (5 μg/ml, RIK-2; Biolegend), anti-Fas ligand (FasL) (NOK-1; Biolegend), anti-NKG2D (5 µg/ml; 149810, R&D Systems), anti-DNAX accessory molecule-1 (DNAM-1) (5 µg/ml, DX11; BD Biosciences), anti-2B4 (C1.7; Biolegend), anti-leucocyte function-associated antigen-1 (LFA-1)/CD11a (HI111; Biolegend), anti-NKG2A (131411; R&D Systems), anti-leucocyte immunoglobulin-like receptor 1 (LIR-1) (GHI/75; Biolegend). Mouse IgG1 (MOPC-21; Biolegend), mouse IgG2a (20102, R&D Systems) and mouse IgG2b (MOPC-21; Biolegend) were used as isotype controls.
Osteoclasts/NK cells in Transwell system
The enriched mature osteoclasts were counted using 0·4% Trypan blue (Sigma-Aldrich) staining and reseeded on either bone slices (IDS, Boldon, UK) or plastic at a density of 5 × 104 cells/well in the lower chamber of an HTS® Transwell-96 well system with 0·4-µm pore size, polycarbonate membrane (Corning, Corning, NY). The cells were cultured in complete medium with 25 ng/ml M-CSF and 10 ng/ml RANKL to allow the osteoclasts to adhere overnight. In parallel, resting NK cells were thawed and rested in complete medium overnight. On the following day, NK cells were harvested and washed thoroughly, osteoclasts were washed twice to remove non-adherent cells. Then, 2·5 × 105 IL-15-activated NK cells or resting NK cells/well were added into either the upper chamber or together with osteoclasts in the lower chamber, and co-cultured for 3 days with 25 ng/ml M-CSF and 10 ng/ml RANKL.
Co-culture of osteoclasts and NK cells
The osteoclasts were differentiated and enriched as described above and then reseeded in a 96-well flat bottom plate at a density of 5 × 104 cells/well on either bone slices or plastic. The osteoclasts were allowed to sediment overnight in complete medium with 25 ng/ml M-CSF and 10 ng/ml RANKL and then washed. Autologous IL-15-activated NK cells were harvested and washed extensively on the following day, added at the indicated E : T ratios and co-cultured with mature osteoclasts for 3 days in the presence or absence of blocking mAb(s) in complete medium containing 25 ng/ml M-CSF and 10 ng/ml RANKL. The blocking mAbs and concentrations used were the same as described above.
Quantification of osteoclast activity by bone resorption
The activity of the osteoclasts was quantified by cathepsin K-mediated release of C-terminal type I collagen fragments (CTX-I) from bone slices to the conditioned media. The CTX-I concentration was quantified by Crosslap® for culture ELISA kit (IDS) according to the manufacturer's protocol.
Quantification of osteoclast number by TRAP activity
Tartrate-resistant acid phosphatase (TRAP) activity reflects the number of active osteoclasts. TRAP was measured in the conditioned media as described previously.23 Briefly, appropriate diluted samples were added to a reaction buffer containing 6 mm p-nitrophenyl phosphate (Calbiochem, San Diego, CA) and 25 mm sodium tatrate (Sigma-Aldrich) at pH 5·5, and incubated for 1 hr at 37° in the dark. The reaction was stopped with 0·3 m sodium hydroxide and quantified by measuring the absorbance at 405 nm with 650 nm as reference using a microplate reader SpectraMax Paradigm (Molecular Devices, Sunnyvale, CA).
Quantification of cell viability by Presto Blue
The cell viability was measured using the Presto Blue (Invitrogen) viability assay. At the end of culture, the supernatant was collected, leaving the cells in the well. Complete medium with 10% Presto Blue was added to the wells and incubated at 37° for 1–2 hr until a colour change from blue to purple/pink was observed. Fluorescence was measured by excitation at 535 nm and emission at 595 nm using the SpectraMax Paradigm.
TRAP staining
At the end of the culture period, osteoclasts that were cultured on plastic were washed with PBS and fixed with 4% paraformaldehyde (Ampliqon, Odense, Denmark) at room temperature. The fixed osteoclasts were TRAP-stained using the Leukocyte Acid Phosphatase Kit (Sigma-Aldrich) according to the manufacturer's protocol. The wells were analysed with an Immunospot® Image Analyser (Cellular Technology Ltd, Shaker Heights, OH) and images were taken using a Leica DFC280 digital camera mounted on a Leica DM IRB microscope (Leica, Wetzlar, Germany).
Pit staining of bone slices
Bone resorption was measured by pit formation on the bone slices. Osteoclasts were removed with milli Q water from bone slices using cotton swabs followed by staining for 3 min in filtered Mayer's haematoxylin (Scytek Laboratories, Logan, UT) to visualize pit formation. Bone slices were rinsed three to five times in milli Q water before cleaning with a cotton swab to remove excess dye. Images of stained pits were taken and the percentages of the resorbed area were calculated using an Immunospot® Image Analyser.
Statistical analysis
All data are expressed as mean ± SEM. The significance among groups was assessed by one-way analysis of variance and Dunnet's multiple comparison tests. Simple comparisons were determined using a two-tailed, Student's t-test. Statistical significance was considered if P < 0·05. Statistical analyses were performed with graphpad prism software (San Diego, CA).
Results
Osteoclasts express multiple ligands for activating and inhibitory NK cell receptors
As osteoclasts differentiate from monocytes they lose CD14 cell surface expression and acquire CD51/61 (αvβ3 integrin) heterodimeric receptors that can bind to bone matrix proteins stimulating osteoclast resorption.24 After culturing isolated monocytes in M-CSF and RANKL for 10–12 days, we identified a population of CD51/61+ CD14− mature osteoclasts by flow cytometry, typically at a frequency of 10–15%. To further evaluate mature osteoclasts, the pool of cells was subsequently enriched by depleting remaining CD14+ osteoclast precursors, and the percentage of mature osteoclasts increased to an average of 70–80% (see Supporting information, Fig. S1).
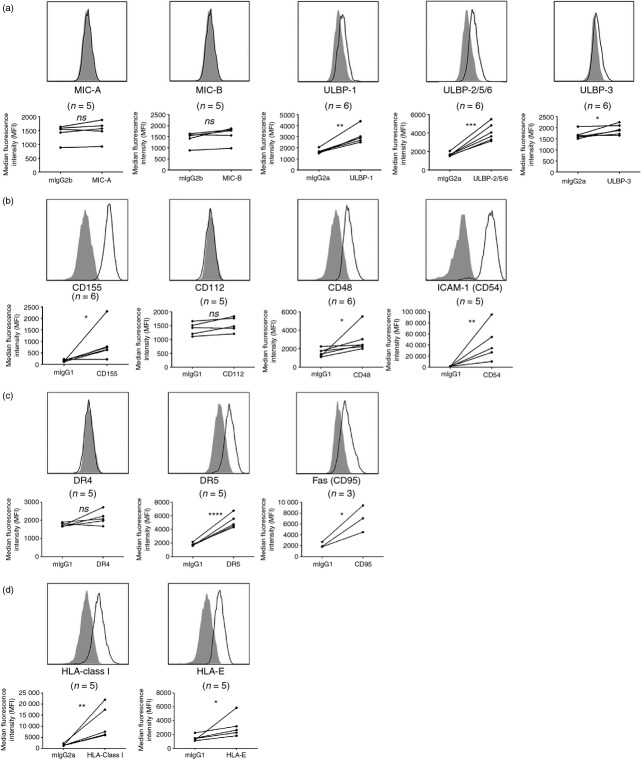
We first evaluated whether mature osteoclasts differentiated from peripheral blood mononuclear cells express a selected set of ligands known to be important targets for NK cell cytotoxicity. Figure1 illustrates how mature osteoclasts express numerous ligands for receptors present on NK cells. For example, osteoclasts express ULBP-1, ULBP-2/5/6 and ULBP-3, but typically little or no MIC-A or MIC-B (Fig.1a), all MHC class I-like ligands for the activating receptor NKG2D.25 Moreover, osteoclasts express CD155 (poliovirus receptor, PVR) but not CD112 (PVR2 or Nectin-2), ligands for the activating NK cell receptor DNAM-1 (Fig.1b). We also found expression of CD48 and CD54 (intercellular adhesion molecule-1, ICAM-1), ligands for 2B4 (CD244) and LFA-1, respectively (Fig.1b), whereas osteoclasts derived from only some donors appear to express low levels of ligands for the natural cytotoxicity receptors NKp30, NKp44 and NKp46 (see Supporting information, Fig. S2).
Figure 1.
Osteoclasts express numerous ligands for both activating and inhibitory natural killer (NK) cell receptors. Osteoclasts were surface-stained for expression of ligands for multiple NK cell receptors after enrichment. (a), Ligands for activating receptor NKG2D: MHC class I-chain related protein A (MIC-A), MIC-B, UL16-binding protein 1 (ULBP-1), ULBP-2/5/6 and ULBP-3. (b) Ligands for activating receptors DNAX accessory molecule 1 (DNAM-1):CD155 and CD112; ligand for 2B4 (CD244):CD48; and ligand for leucocyte function-associated antigen 1 (LFA-1): intercellular adhesion molecule (ICAM-1) (CD54). (c) Ligands for tumour necrosis factor-related apoptosis-inducing ligand (TRAIL): death receptor 4 (DR4) and DR5; ligand for Fas ligand (FasL): Fas (CD95). (d) Ligands for inhibitory NK cell receptors: HLA-ABC and HLA-E. Open histograms represent indicated antibody and filled histograms represent isotype-matched control immunoglobulin. The histograms are representative of n = 3 to n = 6 donors. The median fluorescence intensity (MFI) of all donors is represented in the dot plots under each histogram. Two-tailed, paired Student's t-test; *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Besides killing target cells by cytotoxic granule exocytosis, NK cells can also trigger target cell apoptosis via TRAIL and FasL binding to death receptors (DRs) and Fas (CD95), respectively.26 TRAIL can interact with five DRs, among which DR4 (TRAIL-R1) and DR5 (TRAIL-R2) can induce apoptosis in target cells. We found expression of both Fas (CD95) and DR5, but not DR4, on osteoclasts (Fig.1c).
Finally, as shown in Fig.1(d), we found that osteoclasts express both classical MHC class I molecules (HLA-A, -B, -C) and non-classical HLA-E, ligands for inhibitory killer cell immunoglobulin-like receptors (KIRs) and LIR-1, and CD94/NKG2A receptors, respectively.25
To our knowledge, these data are the first to show that osteoclasts express multiple ligands for both activating and inhibitory NK cell receptors.
IL-15-activated, but not resting, NK cells lyse autologous osteoclasts in vitro
We next assessed whether osteoclasts are susceptible to autologous NK cell-mediated lysis using a standard 51Cr-release assay. To this end, we used either freshly isolated or IL-15-activated peripheral blood NK cells as effectors. Compared with freshly isolated cells, IL-15-activated NK cells express NKp44 and TRAIL and further up-regulate the expression of several other receptors (see Supporting information, Fig. S3). When co-cultured with isolated mature osteoclasts only IL-15-activated NK cells, but not resting NK cells, efficiently killed osteoclasts (Fig.2).
Figure 2.
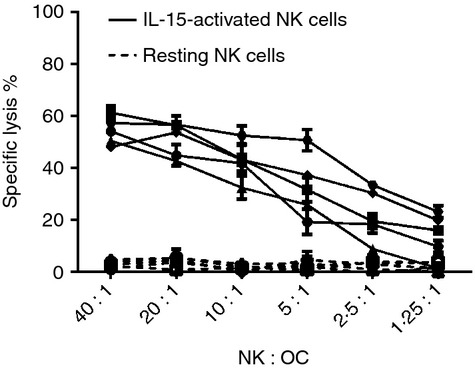
Interleukin-15 (IL-15) -activated, but not resting natural killer (NK) cells kill osteoclasts. Autologous NK cells were isolated from peripheral blood mononuclear cells (PBMCs) and cultured with IL-15 (10 ng/ml) until use (around day 10) or cryopreserved and thawed 1 day before use. Osteoclasts were differentiated from PBMCs, harvested, enriched by CD14+ depletion, and then re-seeded at 5000 cells/well in a 96-well flat bottom plates. After 24 hr, the osteoclasts were labelled with 51Cr and co-cultured with different ratios of autologous IL-15-activated NK cells (solid line) or resting NK cells (dotted line). The NK-mediated killing of osteoclasts was measured by release of 51Cr from osteoclasts to the conditioned media, and % specific lysis was calculated as described in the Materials and methods. The figure shows the results of five independent experiments with five different donors, each set up in triplicate (mean ± SEM).
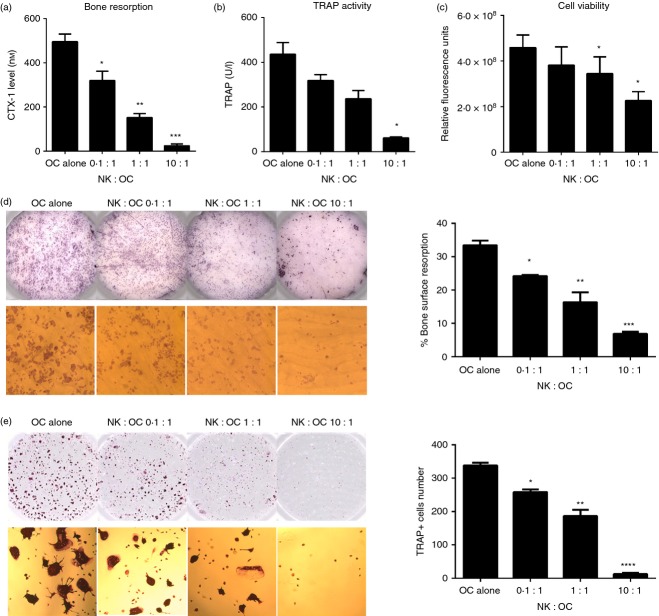
Osteoclasts resorb bone, which can functionally be assessed in vitro by a biochemical marker of bone resorption (CTX-I release) and by measuring pit areas of resorbed bone. Moreover, the number of mature osteoclasts can be evaluated by counting TRAP-positive cells and by measuring TRAP enzyme activity in the culture supernatant. To investigate the functional consequences of NK cell lysis of osteoclasts, a 3-day co-culture assay was conducted with osteoclasts and IL-15-activated NK cells with and without bone slices. Increasing the number of NK cells added to osteoclast led to decreasing levels of CTX-I in the supernatant (Fig.3a) and reduced TRAP enzyme activity (Fig.3b), indicating that the overall bone eroding capacity of osteoclasts is reduced in the presence of activated NK cells. Moreover, we observed the highest degree of viable cells when osteoclasts were cultured alone, whereas an increasing number of NK cells led to a gradual decrease in viability (Fig.3c). It should be noted that as IL-15 is not added in these cultures, the overall NK cell viability declines after 3 days in culture. Furthermore, we evaluated bone pit resorption areas on bone slices and number of TRAP+ cells remaining in plastic dishes. Consistent with the above data, an increasing number of NK cells resulted in decreased bone surface resorption and number of TRAP+ cells (Fig.3d,e).
Figure 3.
The killing of osteoclasts by interleukin-15 (IL-15) -activated natural killer (NK) cells is ratio-dependent. Enriched osteoclasts (5 × 104/well) were seeded in a 96-well plate on bone slices or plastic and co-cultured with IL-15-activated NK cells, at effector : target ratios of 0.1 : 1, 1 : 1 and 10 : 1, for 3 days in the presence of macrophage colony-stimulating factor (M-CSF; 25 ng/ml) and receptor activator of necrosis factor κB ligand (RANKL; 10 ng/ml). Supernatants were collected for measurements of: (a) Osteoclast-mediated collagen type I degradation (C-terminal type I collagen fragments; CTX-I) and (b) tartrate-resistant acid phosphatase (TRAP) activity. (c) At the end of the culture, cells were incubated with 10% Presto Blue to determine cell viability. The mean ± SEM values are shown for n = 4 donors. (d) Bone slices were removed from wells, washed and stained with haematoxylin, to enable visualization of the pits resorbed by osteoclasts. An Immunospot Image Analyser was used to quantify the resorbed bone pits on the surface of the bone slice, indicated by the darker areas (top panel). The pits were also visualized under a microscope (bottom panel, magnification × 100). (e) The osteoclasts seeded on plastic were fixed and TRAP stained, and the number of TRAP+ osteoclasts was quantified using an Immunospot Image Analyser (top panel) as described. Magnification × 50 (bottom panel). Figures are representative of n = 4 donors. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
Taken together, our results indicate that osteoclasts are susceptible to lysis mediated by activated, but not resting, NK cells resulting in reduced bone resorption in vitro.
Cell–cell contact is required for NK cell-mediated inhibition of osteoclast function
We next investigated whether cell–cell contact and/or soluble factors are required in the killing of osteoclasts by activated NK cells. To this end, osteoclasts were co-cultured either alone or in direct contact with NK cells, as well as separated from NK cells by a permeable membrane in a transwell system that allows exchange of soluble molecules but not cells. The CTX-I levels and TRAP activities were measured after a 3-day culture as before. As shown in Fig.4, a dramatic decrease in CTX-I release (Fig.4a), TRAP activity (Fig.4b) and osteoclast viability (Fig.4c,d), was observed when osteoclasts were in direct contact with IL-15-activated NK cells. Interestingly, a small but significant decrease in CTX-I release was also observed when osteoclasts were separated from IL-15-activated NK cells, indicating that soluble factors can also contribute to reduced osteoclast function. Co-cultures between osteoclasts and resting NK cells, whether in direct contact or separated by a transwell, did not show any significant differences compared with osteoclasts cultured alone (Fig. 4d, and see Supporting information, Fig. S4).
Figure 4.
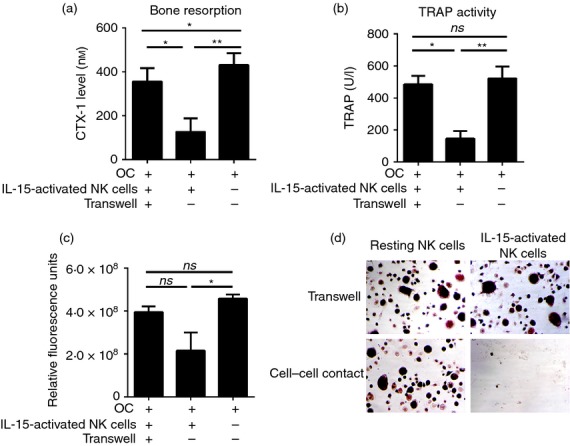
Cell–cell contact is required for the lysis of osteoclasts by interleukin-15 (IL-15) -activated natural killer (NK) cells. NK cells were isolated from peripheral blood mononuclear cells and cultured in the presence or absence of IL-15 as described. Osteoclasts were differentiated and harvested as described. Enriched osteoclasts (5 × 104/well) were seeded into the lower chamber of a 96-well transwell on bone slices or plastic. On the following day, 2·5 × 105 IL-15-activated NK cells or resting NK cells were added into either the upper chamber (transwell) or together with osteoclasts into the lower chamber, and co-cultured for 3 days with macrophage colony-stimulating factor (M-CSF; 25 ng/ml) and receptor activator of necrosis factor κB ligand (RANKL; 10 ng/ml). The culture supernatants were collected for detection of: (a) osteoclast-mediated collagen type I degradation (C-terminal type I collagen fragments; CTX-I) and (b) tartrate-resistant acid phosphatase (TRAP) activity. (c) Cells were incubated with Presto Blue reagent to determine the viability of the cells. The mean ± SEM values of three independent experiments are shown for n = 6 donors. (d) Adherent osteoclasts were fixed with 4% paraformaldehyde and TRAP stained. Magnification × 50. Figures are representative of n = 6 donors. ns, not significant; *P < 0·05; **P < 0·01.
Taken together, our data suggest that activated NK cells inhibit osteoclast function and bone resorption predominantly through a direct cell-contact-dependent mechanism involving osteoclast lysis, although a minor participation of soluble factors inhibiting osteoclast function cannot be excluded.
LFA-1, DNAM-1 and TRAIL are involved in the killing of osteoclasts by autologous NK cells
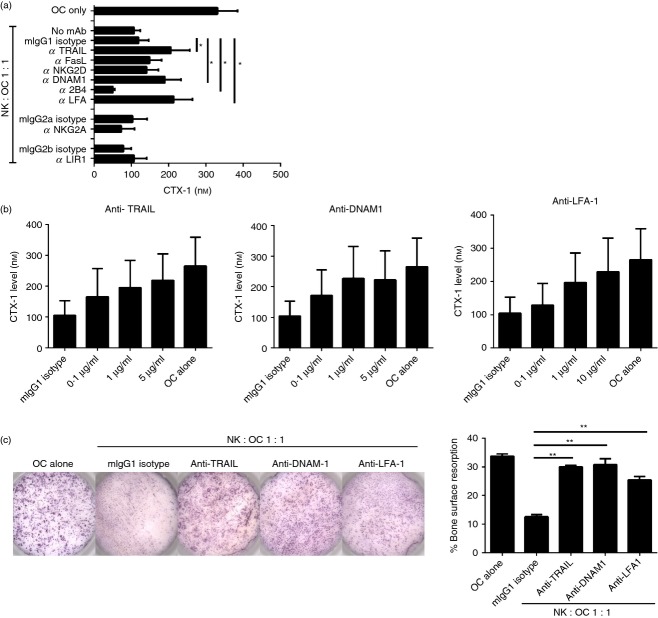
To identify NK cell surface receptors involved in the killing of osteoclasts, we used a panel of antibodies against NK cell surface receptors in a cytotoxicity assay using autologous 51Cr-labelled osteoclasts as targets. The receptors were chosen to cover the panel of their respective ligands present on osteoclasts (Fig.1). As shown in Fig.5(a), there was no significant change in osteoclast lysis when antibodies against FasL, NKG2D, 2B4, NKG2A or LIR-1 were added, suggesting that these receptors are not involved in the killing of osteoclasts by NK cells (Fig.5a). In contrast, blocking TRAIL, DNAM-1 or LFA-1 resulted in reduced osteoclast killing over a range of E : T ratios (Fig.5a,b) suggesting that interaction between these NK cell receptors and osteoclast ligands DR5, CD155 and ICAM-1, respectively, plays a role in NK cell-mediated lysis.
Figure 5.
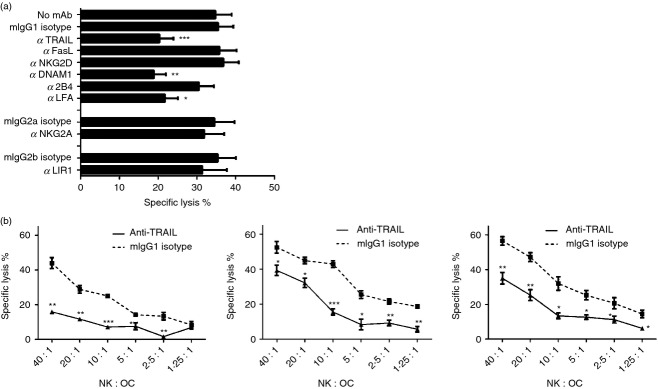
Blocking tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), DNAX accessory molecule 1 (DNAM-1) or leucocyte function-associated antigen 1 (LFA-1) decreases lysis of osteoclasts by interleukin-15 (IL-15) -activated natural killer (NK) cells. Osteoclasts and autologous NK cells were generated and cultured as described. Enriched osteoclasts were seeded at 5000 cells/well in a 96-well plate and labelled with 51Cr after 24 hr. (a) IL-15-activated NK cells were pre-incubated with the blocking monoclonal antibody of interest for 2 hr at 37° before co-culturing with 51Cr-labelled osteoclasts at an effector : target ratio of 10 : 1 as described. Experiment carried out with n = 7 donors. Statistical significance is calculated in relation to cells incubated with corresponding isotype-matched control immunoglobulin. (b) IL-15-activated NK cells were co-cultured with osteoclasts at different effector : target ratios with specific blocking mAb (solid lines) or corresponding isotype (dotted lines), to measure the release of 51Cr from the killed osteoclasts. Figures are representative of n = 7 donors, and experiments were performed in triplicate. The data are analysed with two-tailed, unpaired Student's t-test. *P < 0·05; **P < 0·01; ***P < 0·001.
We next wanted to evaluate whether blocking of selected NK cell receptors could rescue osteoclast function in co-culture with activated NK cells. To this end, we cultured osteoclasts either alone or with IL-15-activated NK cells on bone slices for 3 days in the presence or absence of blocking antibodies, followed by evaluation of CTX-I released into the supernatant. In co-cultures with NK cells, the CTX-I level was reduced by 65·9 ± 2·8% compared with osteoclasts cultured alone (Fig.6a). As expected, blocking FasL, NKG2D, NKG2A or LIR-I did not affect the release of CTX-I. However, and as expected, the addition of anti-TRAIL, anti-DNAM-1 or anti-LFA-1 increased the CTX-I levels relative to isotype-matched control immunoglobulin (Fig.6a) in a dose-dependent manner (Fig.6b). We were able to confirm our data visually by evaluating the areas of the resorbed pits on the bone slices, where the addition of anti-TRAIL, anti-DNAM-1 or anti-LFA-1 alone almost completely restored osteoclast function resulting in increased pit areas as compared with an isotype control (Fig.6c shows a representative result). It should be noted that although anti-2B4 did not affect osteoclast killing (Fig.5a), the addition of anti-2B4 reduced CTX-I level in this assay (Fig.6a), suggesting that 2B4 and CD48 interactions between NK cells and osteoclasts do affect osteoclast function.
Figure 6.
Blocking tumour necrosis factor-related apoptosis-inducing ligand (TRAIL), DNAX accessory molecule 1 (DNAM-1) or leucocyte function-associated antigen 1 (LFA-1) promotes osteoclast-mediated bone erosion in a co-culture of osteoclasts and natural killer (NK) cells. Enriched osteoclasts were seeded on bone slices at 5 × 104/well, autologous interleukin-15 (IL-15) -activated NK cells were added the following day at an effector : target ratio of 1 : 1 and co-cultured for 3 days in the presence of the blocking monoclonal antibody (mAb) of interest. Survival of active osteoclasts was quantitatively measured by bone degradation, C-terminal type I collagen fragments (CTX-I), in the conditioned media. (a) Osteoclasts and IL-15-activated NK cells were co-cultured with blocking mAb as described. The results are shown for independent experiments of n = 8 donors. (b) NK cells and osteoclasts were co-cultured with different concentrations of blocking mAbs at 0·1 μg/ml, 1 μg/ml and 5 μg/ml (or 10 μg/ml). Data shown is obtained from n = 4 donors. (c) Bone slices were stained with haematoxylin and resorption pits were quantified using an Immunospot Image Analyser. Images and graphs represent the results of experiments performed with n = 4 donors. *P < 0·05; **P < 0·01.
Finally, we tested whether there was a synergistic effect between blocking TRAIL, DNAM-1 or LFA-1 on osteoclast function in co-cultures with NK cells. No further decrease of specific osteoclast lysis (see Supporting information, Fig. S5a), and no further increase in CTX-I levels (Fig. S5b) was observed by combining blocking mAbs compared with blocking TRAIL, DNAM-1 or LFA-1 alone.
Discussion
Many recent findings show that activation of the immune system can have profound effects on osteoclasts and bone homeostasis.27 Here we have studied whether fully mature bone-eroding osteoclasts are influenced by the activities of either resting or activated NK cells. Remarkably, we show that such osteoclasts are highly susceptible to lysis by IL-15-activated, but not resting, autologous NK cells in vitro. Moreover, while resting NK cells do not influence bone erosive activities at any of the cell ratios tested, the presence of only a few IL-15-activated NK cells drastically suppresses the capacity of osteoclasts to erode bone in vitro.
The role of IL-15 in bone biology is complex, but the literature suggests that IL-15 has an overall pro-osteoclastogenic effect in vitro and in vivo. For example, using rodent osteoclast progenitors, Ogata et al. reported that IL-15 stimulates the formation of osteoclast progenitors into pre-osteoclasts, while having no apparent effect on pre-osteoclasts themselves, suggesting that the pro-osteoclastogenic action of IL-15 occurs early in the differentiation process.12 In human, IL-15 was shown to drive osteoclast formation from monocyte precursors, but only in the presence of RA synovial NK cells, which up-regulate both M-CSF and membrane RANKL in response to IL-15.22 Moreover, a recent study showed that IL-15 stimulates osteoblast apoptosis in mouse bone marrow cell cultures via activation of NK cells, again suggesting that IL-15 is an inflammatory bone destructive factor.28 In fact, we are not aware of any reports indicating that IL-15 may also display anti-osteoclastogenic effects in vitro, and as targeting IL-15R in vivo reduces disease progression and bone erosion in a mouse model of human RA,11 it is reasonable to conclude that the main role for IL-15 on bone is to favour enhanced erosion.
In light of these previous studies it may therefore at first seem paradoxical that IL-15 licenses circulating NK cells to eliminate mature osteoclasts. There are several possible explanations for this finding, one being that we have here studied mature osteoclasts raised in the presence of M-CSF and RANKL alone. Such ‘non-inflammatory’ osteoclasts, i.e. raised in the absence of any additional exogenously added pro-osteoclastogenic inflammatory cytokine, may not have acquired sufficient capacity to resist a sudden attack by IL-15-activated NK cells. One possible pathway of acquired resistance may involve up-regulated levels of MHC class I molecules that may allow osteoclasts to escape lysis by NK cells through interaction with MHC class I-specific inhibitory receptors (e.g. KIRs and CD94/NKG2A). The fact that blocking CD94/NKG2A, an inhibitory receptor expressed on most IL-15-activated NK cells (see Supporting information, Fig S3) does not result in further reduction in bone erosion, supports this suggestion. It will therefore be important to evaluate whether osteoclasts generated during inflammatory conditions, e.g. in the presence of TNF-α, a well-known pro-osteoclastogenic factor capable of enhancing MHC class I expression, are equally susceptible to NK cell attack or whether they have acquired capacity to resist lysis.
To understand the mechanism behind the susceptibility of mature osteoclast to NK cell lysis, we first analysed their surface expression of selected ligands known to be important targets for NK cells. Indeed, we show that mature osteoclasts express numerous cell surface ligands that may interact with various activating NK cell receptors. For example, and confirming previous reports, we show that mature osteoclasts express both ICAM-1 and CD155 (PVR), molecules known to be important in osteoclast development and function as well as in the interaction with stromal cells.29,30 Both ICAM-1 and CD155 are known ligands for integrin β2 LFA-1 and DNAM-1, respectively, both receptors present on NK cells and shown here to be drastically up-regulated by IL-15 stimulation. By blocking these receptors with mAb in co-cultures between NK cells and mature osteoclasts we show that in vitro bone resorption is restored and cytotoxic elimination of osteoclasts is significantly suppressed.
Previous studies have shown that both LFA-1 and DNAM-1 are required for efficient NK cell-mediated lysis of multiple cell types including dendritic cells,31 activated T cells32 and synovial fibroblasts.33 The requirement for both LFA-1 and DNAM-1 is perhaps not surprising as DNAM-1 interacts physically with LFA-1,34,35 which is required for its functional activity on cytotoxic cells. Moreover, the finding that there was no synergistic effect by blocking both LFA-1 and DNAM-1 in NK cell cytotoxic responses against osteoclasts reinforces the functional link between these two receptors in driving NK cell activation.
NKG2D is another potent activating receptor expressed by all NK cells that, similarly to DNAM-1 and LFA-1, is drastically up-regulated on IL-15-activated NK cells (Fig. S3). NKG2D interacts with stress-induced MHC class I-like ligands MIC-A/B and ULBPs and plays a role in NK cell-dependent cytotoxic responses against,for example, activated T cells and synovial RA fibroblasts, both of which express numerous NKG2D ligands.32,33 Surprisingly, however, our data show that NKG2D is not involved in NK cell lysis of mature osteoclasts, despite the fact that such osteoclasts express several NKG2D ligands. Perhaps, the overall NKG2D ligand density is important to sufficiently trigger NKG2D signalling and cytotoxic responses and mature osteoclasts, which we show here express ULBP-1, ULBP-2/5/6, ULBP-3 but not MIC-A or MIC-B, may not express sufficient levels of such ligands. Again, it would be important to evaluate whether NKG2DL expression differs between osteoclasts generated during inflammatory conditions in vitro or in vivo and the ‘non-inflammatory’ osteoclasts studied here.
2B4 is an activating receptor expressed by all NK cells and we show that this receptor is up-regulated on NK cells by IL-15 stimulation, whereas its ligand CD48 is present on mature osteoclasts. Interestingly, we observed that an anti-2B4 mAb added to co-cultures between NK cells and mature osteoclasts significantly decreased bone resorption without actually enhancing lysis of osteoclasts, suggesting that 2B4 and CD48 interactions may promote osteoclast function. A previous report has shown an important role of 2B4/CD48 in the regulation of cytokine synthesis in co-culture between NK cells and monocytes.36 Possibly, an interaction between 2B4 and CD48 in NK cell and osteoclast co-cultures similarly results in release of mediators that may play a role in enhancing bone erosion. However, this suggestion needs to be taken with caution as we in this study used an anti-2B4 mAb (clone 1.7) that may not be the best suitable reagent to block 2B4 interaction with CD48.37 Moreover, this antibody can induce direct activation of NK cells and increase release of interferon-γ,38 a potent anti-osteoclastogenic factor,17 which may further complicate the interpretation of our results. In addition, the 2B4 ligand CD48 is also present on NK cells and is further up-regulated by IL-15,36 opening up several possible scenarios of 2B4/CD48 interactions between NK cells and osteoclasts, and between NK cells themselves in our system. Moreover, 2B4 expression on osteoclasts should be evaluated because this receptor is not restricted to NK cells, but is also present on myeloid cells.39 It will be an interesting and challenging future endeavour to unravel the role for 2B4/CD48 in osteoclast function and potential crosstalk with NK cells.
Besides direct cytotoxic granule release, NK cells can also induce target cell apoptosis via FasL or TRAIL engaging their respective death receptors present on target cells. Here we detected expression of Fas (CD95), a death receptor binding FasL, on osteoclasts. Natural killer cells, however, expressed low levels of FasL and blocking this ligand did not affect osteoclast apoptosis or bone erosion (Fig.5). TRAIL can induce target cell apoptosis upon binding to either DR4 or DR5,40 and has been involved in the elimination of several cell types including immature dendritic cells,41 activated T cells32 and fibroblast-like synoviocytes.42 We detected high surface expression of DR5 but not DR4 on osteoclasts, whereas IL-15-activated NK cells expressed high levels of TRAIL. When TRAIL was blocked by mAb we observed a significantly decreased lysis of osteoclast and enhanced bone erosion. Taken together, our data are supported by other studies showing that DR5 is involved in TRAIL-mediated human osteoclast apoptosis whereas Fas/FasL has a limited role.43,44.
Interestingly, other studies have shown that TRAIL plays an anti-inflammatory and anti-resorptive role in vivo. For example, blockade of TRAIL increased erosion and severity of arthritis in a mouse model of human RA, whereas intra-articular TRAIL gene transfer relieved disease symptoms.45 Moreover, a recent report showed that zolendronic acid (ZA), a compound used to prevent pathological bone loss, augments both membrane and soluble TRAIL production by NK cells in co-culture with monocytes.46 Taken together, NK cells expressing TRAIL may be an important cell subset inhibiting the extent of bone erosion under inflammatory conditions.
In this study, NK cell-mediated inhibition of osteoclast function was shown to be cell-contact dependent (Fig.4). However, soluble factors were also shown to participate to a minor degree, as a small but significant decrease in the osteoclast resorption marker CTX-I was observed when osteoclasts and NK cells were seeded in different chambers. Interleukin-15-activated NK cells are able to secrete numerous cytokines, in particular interferon-γ, a well-known anti-osteoclastogenic factor,17 which may be involved in the cell-contact independent pathways resulting in reduced osteoclast resorption observed here. In addition, soluble TRAIL (sTRAIL) may be another candidate decreasing bone resorption in the transwell system. Further experiments blocking interferon-γ or sTRAIL in a transwell system are required to evaluate whether these or possibly other NK cell-derived factors are involved in suppressing bone erosion.
Our in vitro data suggest that NK cells do not kill osteoclasts under steady-state conditions. However, activated NK cells are present at inflammatory sites associated with enhanced bone erosion, e.g. in the RA joint. We can speculate that in vivo such NK cells may participate in inducing apoptosis of osteoclasts once they are mature and attached to the bone, so decreasing bone resorption. Further studies are required to evaluate whether synovial NK cell subsets from inflammatory synovial tissue are sufficiently activated and can kill mature osteoclasts ex vivo. Moreover, such inflammatory osteoclasts are generally more resistant to NK cell attack and synovial NK cells in the inflamed joint, although showing signs of activation, express relatively low levels of granzyme B and perforin.47,48 Therefore, developing the means to enhance NK cell lytic activities may prove beneficial in reducing bone erosion associated with inflammation.
Acknowledgments
We thank Jette Møller Frøsig and Corinne Weideli Hansen for technical assistance, and Kristina Forsman-Semb for administrative support. This work was supported by grants from the Danish Agency for Science, Technology and innovation and Novo Nordisk A/S.
Glossary
- CTX-I
C-terminal type I collagen fragment
- DNAM-1
DNAX accessory molecule-1
- DR
death receptor
- FasL
Fas ligand
- ICAM-1
intercellular adhesion molecule-1
- IL-1
interleukin-1
- KIR
killer cell immunoglobulin-like receptor
- LFA-1
leucocyte function-associated antigen-1
- LIR
leucocyte immunoglobulin-like receptor
- mAb
monoclonal antibody
- M-CSF
macrophage colony stimulating factor
- MIC
MHC class I-chain related protein
- NK
natural killer
- RA
rheumatoid arthritis
- RANKL
receptor activator of necrosis factor κB ligand
- TNF-α
tumour necrosis-factor-α
- TRAIL
TNF-related apoptosis-inducing ligand
- TRAP
tartrate-resistant acid phosphatase
- ULBP
UL-16 binding protein
Author contribution
SF, SHM and KS designed the study; SF performed the experiments; SF and KS wrote the paper; SF, SHM, AVN and NNV analysed the data; KS conceptualized the study and CG and LK facilitated the study. All authors have helped by proofreading the manuscript and have approved the final version.
Disclosures
SF, SHM, NNV own shares in Novo Nordisk A/S, all other authors have no conflicts of interest. SF and SHM are currently employed by Novo Nordisk A/S. NNV, AVN, KS and LK were employed by Novo Nordisk A/S when the work was performed. AVN was employed as a post-doctoral scientist, and Novo Nordisk A/S funded the research by ANV through this employment.
Supporting Information
Figure S1. Characterization of osteoclasts differentiated from monocytes.
Figure S2. No putative ligands for NCRs were detected on osteoclasts by NCR-Fc fusion proteins.
Figure S3. Interleukin-15-activated natural killer cells up-regulated expression of surface receptors.
Figure S4. Resting natural killer cells have no effect on mature osteoclasts.
Figure S5. No synergistic effect with additions of combination of blocking monoclonal antibodies in the co-culture.
References
- Takahashi N, Yamana H, Yoshiki S, et al. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988;122:1373–82. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289:1504–8. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- Hughes DE, Boyce BF. Apoptosis in bone physiology and disease. Mol Pathol. 1997;50:132–7. doi: 10.1136/mp.50.3.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parfitt AM. The actions of parathyroid hormone on bone: relation to bone remodeling and turnover, calcium homeostasis, and metabolic bone diseases. II. PTH and bone cells: bone turnover and plasma calcium regulation. Metabolism. 1976;25:909–55. doi: 10.1016/0026-0495(76)90124-4. [DOI] [PubMed] [Google Scholar]
- Singer FR, Melvin KE, Mills BG. Acute effects of calcitonin on osteoclasts in man. Clin Endocrinol (Oxf) 1976;5(Suppl):333S–40S. doi: 10.1111/j.1365-2265.1976.tb03842.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Yoshizawa T, Fukuda T, et al. Vitamin D receptor in osteoblasts is a negative regulator of bone mass control. Endocrinology. 2013;154:1008–20. doi: 10.1210/en.2012-1542. [DOI] [PubMed] [Google Scholar]
- Shevde NK, Bendixen AC, Dienger KM, Pike JW. Estrogens suppress RANK ligand-induced osteoclast differentiation via a stromal cell independent mechanism involving c-Jun repression. Proc Natl Acad Sci U S A. 2000;97:7829–34. doi: 10.1073/pnas.130200197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimi E, Nakamura I, Duong LT, et al. Interleukin 1 induces multinucleation and bone-resorbing activity of osteoclasts in the absence of osteoblasts/stromal cells. Exp Cell Res. 1999;247:84–93. doi: 10.1006/excr.1998.4320. [DOI] [PubMed] [Google Scholar]
- Itonaga I, Sabokbar A, Sun SG, et al. Transforming growth factor-β induces osteoclast formation in the absence of RANKL. Bone. 2004;34:57–64. doi: 10.1016/j.bone.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest. 2000;106:1481–8. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari-Lacraz S, Zanelli E, Neuberg M, et al. Targeting IL-15 receptor-bearing cells with an antagonist mutant IL-15/Fc protein prevents disease development and progression in murine collagen-induced arthritis. J Immunol. 2004;173:5818–26. doi: 10.4049/jimmunol.173.9.5818. [DOI] [PubMed] [Google Scholar]
- Ogata Y, Kukita A, Kukita T, et al. A novel role of IL-15 in the development of osteoclasts: inability to replace its activity with IL-2. J Immunol. 1999;162:2754–60. [PubMed] [Google Scholar]
- Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–52. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashizume M, Hayakawa N, Mihara M. IL-6 trans-signalling directly induces RANKL on fibroblast-like synovial cells and is involved in RANKL induction by TNF-α and IL-17. Rheumatology (Oxford) 2008;47:1635–40. doi: 10.1093/rheumatology/ken363. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Tanaka Y, Morimoto I, et al. Interleukin-4 as a potent inhibitor of bone resorption. Biochem Biophys Res Commun. 1990;172:1035–41. doi: 10.1016/0006-291x(90)91550-c. [DOI] [PubMed] [Google Scholar]
- Lari R, Fleetwood AJ, Kitchener PD, et al. Macrophage lineage phenotypes and osteoclastogenesis – complexity in the control by GM-CSF and TGF-β. Bone. 2007;40:323–36. doi: 10.1016/j.bone.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Takayanagi H, Ogasawara K, Hida S, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–5. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–9. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel LA, Yokoyama WM, French AR. Natural killer cells in human autoimmune disorders. Arthritis Res Ther. 2013;15:216. doi: 10.1186/ar4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alvaro I, Ortiz AM, Alvaro-Gracia JM, et al. Interleukin 15 levels in serum may predict a severe disease course in patients with early arthritis. PLoS ONE. 2011;6:e29492. doi: 10.1371/journal.pone.0029492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soderstrom K, Stein E, Colmenero P, et al. Natural killer cells trigger osteoclastogenesis and bone destruction in arthritis. Proc Natl Acad Sci U S A. 2010;107:13028–33. doi: 10.1073/pnas.1000546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsdal MA, Henriksen K, Sorensen MG, et al. Acidification of the osteoclastic resorption compartment provides insight into the coupling of bone formation to bone resorption. Am J Pathol. 2005;166:467–76. doi: 10.1016/S0002-9440(10)62269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt S, Nesbit A, Helfrich M, Horton M. Biochemical characterization of human osteoclast integrins. Osteoclasts express αvβ3α2β1, and αvβ1 integrins. J Biol Chem. 1993;268:16737–45. [PubMed] [Google Scholar]
- Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–93. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- Smyth MJ, Hayakawa Y, Takeda K, Yagita H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat Rev Cancer. 2002;2:850–61. doi: 10.1038/nrc928. [DOI] [PubMed] [Google Scholar]
- Wythe SE, Nicolaidou V, Horwood NJ. Cells of the immune system orchestrate changes in bone cell function. Calcif Tissue Int. 2014;94:98–111. doi: 10.1007/s00223-013-9764-0. [DOI] [PubMed] [Google Scholar]
- Takeda H, Kikuchi T, Soboku K, et al. Effect of IL-15 and natural killer cells on osteoclasts and osteoblasts in a mouse coculture. Inflammation. 2014;37:657–69. doi: 10.1007/s10753-013-9782-0. [DOI] [PubMed] [Google Scholar]
- Kurachi T, Morita I, Murota S. Involvement of adhesion molecules LFA-1 and ICAM-1 in osteoclast development. Biochim Biophys Acta. 1993;1178:259–66. doi: 10.1016/0167-4889(93)90202-z. [DOI] [PubMed] [Google Scholar]
- Kakehi S, Nakahama K, Morita I. Expression and possible role of PVR/CD155/Necl-5 in osteoclastogenesis. Mol Cell Biochem. 2007;301:209–17. doi: 10.1007/s11010-007-9413-x. [DOI] [PubMed] [Google Scholar]
- Pende D, Castriconi R, Romagnani P, et al. Expression of the DNAM-1 ligands, Nectin-2 (CD112) and poliovirus receptor (CD155), on dendritic cells: relevance for natural killer-dendritic cell interaction. Blood. 2006;107:2030–6. doi: 10.1182/blood-2005-07-2696. [DOI] [PubMed] [Google Scholar]
- Nielsen N, Odum N, Urso B, Lanier LL, Spee P. Cytotoxicity of CD56(bright) NK cells towards autologous activated CD4+ T cells is mediated through NKG2D, LFA-1 and TRAIL and dampened via CD94/NKG2A. PLoS ONE. 2012;7:e31959. doi: 10.1371/journal.pone.0031959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen N, Pascal V, Fasth AE, et al. Balance between activating NKG2D, DNAM-1, NKP44 and NKP46 and inhibitory CD94/NKG2A receptors determine NK degranulation towards RA synovial fibroblasts. Immunology. 2014;142:581–93. doi: 10.1111/imm.12271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya K, Lanier LL, Phillips JH, et al. Physical and functional association of LFA-1 with DNAM-1 adhesion molecule. Immunity. 1999;11:615–23. doi: 10.1016/s1074-7613(00)80136-3. [DOI] [PubMed] [Google Scholar]
- Shibuya K, Shirakawa J, Kameyama T, et al. CD226 (DNAM-1) is involved in lymphocyte function-associated antigen 1 costimulatory signal for naive T cell differentiation and proliferation. J Exp Med. 2003;198:1829–39. doi: 10.1084/jem.20030958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Alvaro I, Dominguez-Jimenez C, Ortiz AM, et al. Interleukin-15 and interferon-γ participate in the cross-talk between natural killer and monocytic cells required for tumour necrosis factor production. Arthritis Res Ther. 2006;8:R88. doi: 10.1186/ar1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Romero X, de la Fuente MA, et al. CD84 functions as a homophilic adhesion molecule and enhances IFN-γ secretion: adhesion is mediated by Ig-like domain 1. J Immunol. 2001;167:3668–76. doi: 10.4049/jimmunol.167.7.3668. [DOI] [PubMed] [Google Scholar]
- Tangye SG, Cherwinski H, Lanier LL, Phillips JH. 2B4-mediated activation of human natural killer cells. Mol Immunol. 2000;37:493–501. doi: 10.1016/s0161-5890(00)00076-6. [DOI] [PubMed] [Google Scholar]
- Colonna M, Nakajima H, Cella M. Inhibitory and activating receptors involved in immune surveillance by human NK and myeloid cells. J Leukoc Biol. 1999;66:718–22. doi: 10.1002/jlb.66.5.718. [DOI] [PubMed] [Google Scholar]
- Pan G, O'Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–3. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Screpanti V, Yagita H, et al. NK cell TRAIL eliminates immature dendritic cells in vivo and limits dendritic cell vaccination efficacy. J Immunol. 2004;172:123–9. doi: 10.4049/jimmunol.172.1.123. [DOI] [PubMed] [Google Scholar]
- Morel J, Audo R, Hahne M, Combe B. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) induces rheumatoid arthritis synovial fibroblast proliferation through mitogen-activated protein kinases and phosphatidylinositol 3-kinase/Akt. J Biol Chem. 2005;280:15709–18. doi: 10.1074/jbc.M414469200. [DOI] [PubMed] [Google Scholar]
- Colucci S, Brunetti G, Cantatore FP, et al. The death receptor DR5 is involved in TRAIL-mediated human osteoclast apoptosis. Apoptosis. 2007;12:1623–32. doi: 10.1007/s10495-007-0095-3. [DOI] [PubMed] [Google Scholar]
- Kovacic N, Lukic IK, Grcevic D, Katavic V, Croucher P, Marusic A. The Fas/Fas ligand system inhibits differentiation of murine osteoblasts but has a limited role in osteoblast and osteoclast apoptosis. J Immunol. 2007;178:3379–89. doi: 10.4049/jimmunol.178.6.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song K, Chen Y, Goke R, et al. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is an inhibitor of autoimmune inflammation and cell cycle progression. J Exp Med. 2000;191:1095–104. doi: 10.1084/jem.191.7.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan D, D'Arcy P, Wennerberg E, et al. Activated monocytes augment TRAIL-mediated cytotoxicity by human NK cells through release of IFN-γ. Eur J Immunol. 2013;43:249–57. doi: 10.1002/eji.201242735. [DOI] [PubMed] [Google Scholar]
- de Matos CT, Berg L, Michaelsson J, Fellander-Tsai L, Karre K, Soderstrom K. Activating and inhibitory receptors on synovial fluid natural killer cells of arthritis patients: role of CD94/NKG2A in control of cytokine secretion. Immunology. 2007;122:291–301. doi: 10.1111/j.1365-2567.2007.02638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pridgeon C, Lennon GP, Pazmany L, Thompson RN, Christmas SE, Moots RJ. Natural killer cells in the synovial fluid of rheumatoid arthritis patients exhibit a CD56bright, CD94bright, CD158-negative phenotype. Rheumatology (Oxford) 2003;42:870–8. doi: 10.1093/rheumatology/keg240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Characterization of osteoclasts differentiated from monocytes.
Figure S2. No putative ligands for NCRs were detected on osteoclasts by NCR-Fc fusion proteins.
Figure S3. Interleukin-15-activated natural killer cells up-regulated expression of surface receptors.
Figure S4. Resting natural killer cells have no effect on mature osteoclasts.
Figure S5. No synergistic effect with additions of combination of blocking monoclonal antibodies in the co-culture.