Abstract
A mutant of Escherichia coli, JTG10, deficient in gamma-glutamylcysteine synthetase (gamma-ECS; EC 6.3.2.2) is unable to synthesize glutathione (GSH) and is sensitive to 8-hydroxyquinoline. This phenotype was exploited for the isolation of Arabidopsis thaliana gamma-ECS cDNAs by expression cloning, and clones were selected through functional complementation by growth on 8-hydroxyquinoline. High levels of gamma-ECS activity were detectable in extracts derived from cultures of JTG10 expressing the Arabidopsis gamma-ECS open reading frame, although these complemented mutants accumulated GSH to only 10% of the wild-type level. The derived amino acid sequence constitutes a polypeptide of 59.9 kDa and shows only 44-48% similarity with previously published sequences of rat kidney, human liver, yeast, and E. coli gamma-ECS. When the gamma-ECS cDNA was used as a probe, Southern blot analysis of Arabidopsis genomic DNA revealed that it is present as a low copy number gene. Furthermore, the Arabidopsis gamma-ECS cDNA probe failed to hybridize to maize and tobacco genomic DNA at low stringency, suggesting that heterogeneity in gamma-ECS structure exists between plant species. The activity of recombinant Arabidopsis gamma-ECS was inhibited by buthionine sulfoximine and GSH, indicating that, while differences in the primary and secondary structure of gamma-ECS from different sources exist, the enzymes may have similar active site structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apontoweil P., Berends W. Isolation and initial characterization of glutathione-deficient mutants of Escherichia coli K 12. Biochim Biophys Acta. 1975 Jul 14;399(1):10–22. doi: 10.1016/0304-4165(75)90206-8. [DOI] [PubMed] [Google Scholar]
- Arrick B. A., Nathan C. F., Griffith O. W., Cohn Z. A. Glutathione depletion sensitizes tumor cells to oxidative cytolysis. J Biol Chem. 1982 Feb 10;257(3):1231–1237. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chifflet S., Torriglia A., Chiesa R., Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Anal Biochem. 1988 Jan;168(1):1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Creissen G., Edwards E. A., Enard C., Wellburn A., Mullineaux P. Molecular characterization of glutathione reductase cDNAs from pea (Pisum sativum L.). Plant J. 1992 Jan;2(1):129–131. [PubMed] [Google Scholar]
- Douglas K. T. Mechanism of action of glutathione-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1987;59:103–167. doi: 10.1002/9780470123058.ch3. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Mulligan J. T., Ramer S. W., Spottswood M., Davis R. W. Lambda YES: a multifunctional cDNA expression vector for the isolation of genes by complementation of yeast and Escherichia coli mutations. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1731–1735. doi: 10.1073/pnas.88.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipp J. J., Chang C., Mulcahy R. T. Cloning and nucleotide sequence of a full-length cDNA for human liver gamma-glutamylcysteine synthetase. Biochem Biophys Res Commun. 1992 May 29;185(1):29–35. doi: 10.1016/s0006-291x(05)80950-7. [DOI] [PubMed] [Google Scholar]
- Greenberg J. T., Demple B. Glutathione in Escherichia coli is dispensable for resistance to H2O2 and gamma radiation. J Bacteriol. 1986 Nov;168(2):1026–1029. doi: 10.1128/jb.168.2.1026-1029.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. Potent and specific inhibition of glutathione synthesis by buthionine sulfoximine (S-n-butyl homocysteine sulfoximine). J Biol Chem. 1979 Aug 25;254(16):7558–7560. [PubMed] [Google Scholar]
- Grove G., Zarlengo R. P., Timmerman K. P., Li N. Q., Tam M. F., Tu C. P. Characterization and heterospecific expression of cDNA clones of genes in the maize GSH S-transferase multigene family. Nucleic Acids Res. 1988 Jan 25;16(2):425–438. doi: 10.1093/nar/16.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A. S., Alscher R. G., McCune D. Response of photosynthesis and cellular antioxidants to ozone in populus leaves. Plant Physiol. 1991 Jun;96(2):650–655. doi: 10.1104/pp.96.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. S., Moore W. R., Meister A. On the active site thiol of gamma-glutamylcysteine synthetase: relationships to catalysis, inhibition, and regulation. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2464–2468. doi: 10.1073/pnas.85.8.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P. J., Unkefer C. J., Doolen J. A., Watt K., Robinson N. J. Poly(gamma-glutamylcysteinyl)glycine: its role in cadmium resistance in plant cells. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6619–6623. doi: 10.1073/pnas.84.19.6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowsky T. A high copy number of yeast gamma-glutamylcysteine synthetase suppresses a nuclear mutation affecting mitochondrial translation. Curr Genet. 1993 May-Jun;23(5-6):408–413. doi: 10.1007/BF00312627. [DOI] [PubMed] [Google Scholar]
- Madamanchi N. R., Alscher R. G. Metabolic bases for differences in sensitivity of two pea cultivars to sulfur dioxide. Plant Physiol. 1991 Sep;97(1):88–93. doi: 10.1104/pp.97.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matringe M., Scalla R. Studies on the mode of action of acifluorfen-methyl in nonchlorophyllous soybean cells : accumulation of tetrapyrroles. Plant Physiol. 1988 Feb;86(2):619–622. doi: 10.1104/pp.86.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. J., Leaver C. J. Oxidative Stimulation of Glutathione Synthesis in Arabidopsis thaliana Suspension Cultures. Plant Physiol. 1993 Oct;103(2):621–627. doi: 10.1104/pp.103.2.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister A. Selective modification of glutathione metabolism. Science. 1983 Apr 29;220(4596):472–477. doi: 10.1126/science.6836290. [DOI] [PubMed] [Google Scholar]
- Nieto-Sotelo J., Ho T. H. Effect of heat shock on the metabolism of glutathione in maize roots. Plant Physiol. 1986 Dec;82(4):1031–1035. doi: 10.1104/pp.82.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prändl R., Kutchan T. M. Nucleotide Sequence of the Gene for a Glutathione S-Transferase from Cell Suspension Cultures of Silene cucubalus. Plant Physiol. 1992 Aug;99(4):1729–1731. doi: 10.1104/pp.99.4.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegsegger A., Brunold C. Localization of [gamma]-Glutamylcysteine Synthetase and Glutathione Synthetase Activity in Maize Seedlings. Plant Physiol. 1993 Feb;101(2):561–566. doi: 10.1104/pp.101.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig G. F., Simondsen R. P., Meister A. Reversible dissociation of gamma-glutamylcysteine synthetase into two subunits. J Biol Chem. 1984 Aug 10;259(15):9345–9347. [PubMed] [Google Scholar]
- Stole E., Meister A. Interaction of gamma-glutamyl transpeptidase with glutathione involves specific arginine and lysine residues of the heavy subunit. J Biol Chem. 1991 Sep 25;266(27):17850–17857. [PubMed] [Google Scholar]
- Takahashi Y., Nagata T. parB: an auxin-regulated gene encoding glutathione S-transferase. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):56–59. doi: 10.1073/pnas.89.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp W., Bowler C., Villarroel R., Tsang E. W., Van Montagu M., Inzé D. Characterization of iron superoxide dismutase cDNAs from plants obtained by genetic complementation in Escherichia coli. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9903–9907. doi: 10.1073/pnas.87.24.9903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Yamano Y., Murata K., Kimura A. The nucleotide sequence of the gene for gamma-glutamylcysteine synthetase of Escherichia coli. Nucleic Acids Res. 1986 Jun 11;14(11):4393–4400. doi: 10.1093/nar/14.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson J. M., Boettcher B., Meister A. Intracellular cysteine delivery system that protects against toxicity by promoting glutathione synthesis. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6246–6249. doi: 10.1073/pnas.79.20.6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan N., Meister A. Amino acid sequence of rat kidney gamma-glutamylcysteine synthetase. J Biol Chem. 1990 Jan 25;265(3):1588–1593. [PubMed] [Google Scholar]
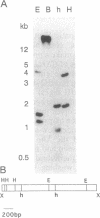
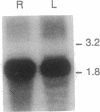
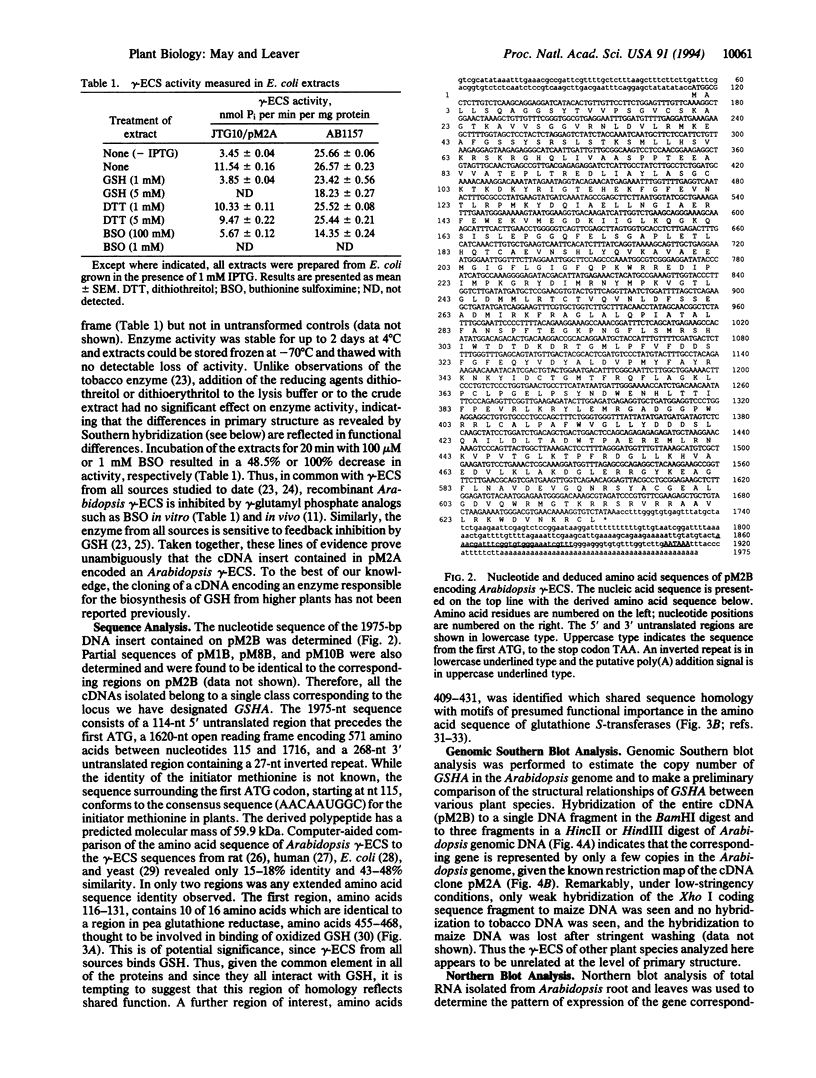
- Zagursky R. J., Hays J. B. Expression of the phage lambda recombination genes exo and bet under lacPO control on a multi-copy plasmid. Gene. 1983 Sep;23(3):277–292. doi: 10.1016/0378-1119(83)90018-5. [DOI] [PubMed] [Google Scholar]