Abstract
Neonatal brain hemorrhage (NBH) of prematurity is an unfortunate consequence of preterm birth. Complications result in shunt dependence and long-term structural changes such as post-hemorrhagic hydrocephalus, periventricular leukomalacia, gliosis, and neurological dysfunction. Several animal models are available to study this condition, and many basic mechanisms, etiological factors, and outcome consequences, are becoming understood. NBH is an important clinical condition, of which treatment may potentially circumvent shunt complication, and improve functional recovery (cerebral palsy, and cognitive impairments). This review highlights key pathophysiological findings of the neonatal vascular-neural network in the context of molecular mechanisms targeting the post-hemorrhagic hydrocephalus affecting this vulnerable infant population.
Keywords: Cerebral palsy, experimental, germinal matrix hemorrhage, hydrocephalus, mental retardation, neonatal rats, neurological dysfunction, pathogenesis, stroke
INTRODUCTION
Neonatal brain hemorrhage (NBH) occurs at a rate of approximately 3.5 times per 1,000 live births, and this is the most common neurological disorder of newborns [1]. The injury is defined as blood vessel rupture of subependymal immature (i.e. germinal–matrix) near the ganglionic eminence (neural and glial progenitor cell region) [2-3]. Severity of the bleeding is defined as grade I: isolated in the germinal matrix (i.e. GMH); grade II: GMH with intraventricular extension (IVH) without ventricular enlargement; grade III: GMH with IVH with ventricular enlargement; grade IV: GMH/IVH with intraparenchymal extension [4-5]. Others further subdivide the sub-types, according to less common bleeding location (i.e. epidural, between skull and dura; subdural, between dura and arachnoid membranes; and subarachnoid) [6].
Extent of bleeding is the factor ultimately most associated with increased morbidity and mortality [7]. Grade 1-2 hemorrhage has been related to developmental disabilities; while grade 3-4 hemorrhages are associated with increased risk of hydrocephalus, as well as long-term sequelae like mental retardation and cerebral palsy [8-9]. Ultimately, up to 85% of survivors of neonatal brain hemorrhage will exhibit major cognitive dysfunction; the majority of which eventually having special educational needs [10-11].
Despite extensive work in both human and animal studies regarding pathogenesis and prevention of neonatal brain bleeds, the incidence has remained relatively the same over the last two decades [12]. The risk and severity of neonatal brain hemorrhage are inversely related to the gestational age and weight at time of birth: there is a 1% incidence in human infants born between 38 and 43 weeks, and a 50% incidence between 24-30 weeks [13]. Occurring with nearly 15% of premature births (<37 weeks); and gestational age (often clinically related to body weight) is the most important clinical predictor of brain hemorrhage. Premature infants with weights between 500-750g have a 45% incidence of NBH [14]; 20% incidence for those <1000g (extremely low birth weight; ELBW); and about 10% of <1500g (very low birth weight; VLBW) infants [15]. Importantly, since both preterm birth rates and neonatal survival have increased over the past two decades [16], the collective management of these newborns has become an ever increasing social and economic burden.
Post-hemorrhagic hydrocephalus often develops as a consequence of NBH [1-2]. Infants surviving this brain injury will often suffer from long-term neurological deficits from the expansion of the cerebroventricular system and subsequent mechanical compression of brain tissue [17]. This has been associated with other diseases of prematurity, and progressive neurologic sequelae such as epilepsy, cerebral palsy, and learning disability [18]. Factors related to neonatal brain hemorrhage include: vaginal delivery, low Apgar scores, seizure, hyaline membrane disease, hypoxia, hyper-/ hypo-carbia, patent ductus arteriosus, infection, thrombocytopenia, venous congestion, intravascular volume expansion, arterial hyper-/ hypo-tension, serum osmolarity changes, and local fibrinolytic activity [2, 5, 19-28]. Taken together, three overall factors seem to predispose NBH: 1) relative vascular fragility of the germinal matrix in comparison to other brain regions, 2) changes in cerebral blood flow (CBF), and 3) changes in hemostatic forces. Intracerebroventricular blood clots and thrombin have been implicated as causative factors for hydrocephalus development. Blood clots damage proper cerebrospinal fluid (CSF) absorption and circulation [17, 29]. Thrombin leads to obstruction of the cerebroventricular system by inducing proliferation of extracellular matrix (ECM) proteins, gliosis, and inflammatory responses [7, 30].
Therefore, NBH is a problem that generates much research interest, as ameliorating the hematoma consequences may alleviate permanent neurological impairments [31-32]. This article will review the pathogenesis of hydrocephalus with particular focus on the effect on neuronal structure and function. Long-term pathogenic outcomes from both animal and human studies show: 1) dissolution of the periventricular ependymal layer; 2) compression-induced ischemia of local microvessels; 3) reactive processes that include proliferation and activation of glial cells; and; 4) neuronal stretching potentially leading to neuronal dysfunction and death [33-38].
POST-HEMORRHAGIC VENTRICULAR DILATATION (PHVD)
Post-hemorrhagic ventricular dilatation (PHVD), commonly known as hydrocephalus is a serious complication of neonatal brain hemorrhage (see Fig. 1). Although this brain bleed usually occurs unilaterally, early bilateral non-communicating hydrocephalus can develop from cerebrospinal fluid outflow obstruction at the cerebral aqueduct or foramina of Magendie and Luschka. This is quite common, as over 50% of premature newborns with NBH studied at one institution had either died or required neurosurgical intervention with shunt placement; however in the early stage of brain bleed, most infants remain asymptomatic with incidental perinatal diagnosis by screening transcranial ultrasound or by other brain imaging technique [8]. PHVD can be physically evident with subtle or obvious neurologic changes in alertness, behavior, tone, respirations or cranial enlargement; later in life, the neurologic impairments become apparent, with many individuals developing cognitive or motor deficits [39-41].
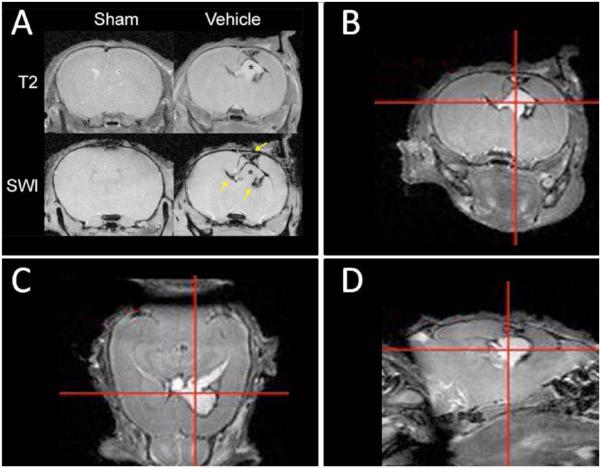
Fig. (1). CSF accumulation and ventriculomegaly 7 days after neonatal brain hemorrhage induction depicted by imaging.
Magnetic resonance imaging (MRI) was used to evaluate injury progression in sham and vehicle P14 rat pups 7 days after NBH induction. These images suggest hydrocephalus development commences as early as 1 week following NBH induction. (A) T2-weighted MRI (top row) depicts CSF accumulation (hyperintense T2-signal marked with an asterisk) and ventriculomegaly. Susceptibility weighted imaging SWI (bottom row) depicts clots within the periventricular region (hypointense signal marked with arrows). Sham animals did not have any visible brain injury. T2-weighted MRI depicting (B) coronal, (C) axial and (D) sagittal cross-sections of NBH animal depicting CSF accumulation, ventriculomegaly, and minor midline shift.
PHVD results in periventricular white matter injury by a variety of mechanisms, such as: hypoxia-ischemia, altered interstitial flow from compressive forces, free radical injury, and cytokine abnormalities [38]. In addition, lack of regional autoregulation in cerebral vasculature and inadequate vascularization to periventricular regions may exacerbate hypoxia-induced injury [28, 42-44]. Both acute and chronic symptoms can be partially explained by periventricular white matter disease and dysfunction, which likely begins with ventricular expansion [5, 38]. Direct intraparenchymal extension of the hemorrhage can also damage proximal structures, such as the head of the caudate nucleus and the internal capsule [45], and delayed periventricular hemorrhagic infarcts were observed in the white matter from ischemic insult [5], could both partially explain the long-term neurologic dysfunctions observed clinically.
Early ventriculo-peritoneal shunting is often the treatment of choice, as it is associated with rapid improvement of some symptoms and short and long-term physiologic normalization [38]. However, despite its usefulness, this has expected complications, such as shunt failure at some point; and unexpected ones, specifically infection, or migration of individual parts. Less invasive therapies, including repeated lumbar or ventricular tapping of cerebrospinal fluid (CSF), intraventricular infusion of fibrinolytics, or diuretics have not yet been found effective [46-48].
Anatomic and pathologic descriptions of PHVD brains have been largely described, and immunohistochemical techniques have yielded valuable information [25, 49-50]. Fukimizu et al have described increased hemosiderin deposition, nodular gliosis, ependymal cell loss and underlying subependymal rosette formation in the ventricular wall following PHVD [51-52]. Other investigators have shown growth of glial progenitor cells in areas of ependymal cell loss after hydrocephalus, as indicated by high expression of nestin and vimentin in those brain regions [53]. Together, it was observed that pathologic findings were greater in PHVD brains in comparison to those lacking ventricular dilation after IVH; suggesting the difference may be accounted by rapid increases of intracranial pressure. Regardless of the cause, hydrocephalus is associated with specific functional and behavioral deficits, with recent attempts made to isolate exact brain regions responsible [54-55].
The neonatal age of hydrocephalus development is possibly an important descriptor in the perinatal and infant period as it pertains to neurodevelopment. In animal models, hydrocephalus can be induced prenatally via genetically-engineered predisposition (i.e. the hydrocephalic Texas [H-Tx] rat [56]), or at later time of infancy using kaolin or silicone injections [57-58]. Studies comparing rat models using both techniques demonstrated that the resultant hydrocephalus causes abnormal cell proliferation in the periventricular germinal layer [56, 59-60].
PHVD delays myelination in white matter regions, which shows reversibility with early surgical shunting [61-63]. Parallel findings were found in humans using magnetic resonance imaging techniques [64] and post-mortem analysis [65]; demonstrating changed post-operative myelination. In clinical terms, if shunting results in the cease of ventricular enlargement, it is referred to as arrested or compensated hydrocephalus. Whether pathologic changes actually stop post-operatively remains a point of contention. Observational studies show long-term neuropsychiatric disorder and benefit following shunting in young adults and adults with previous “arrested” hydrocephalus [66-69]. In light of such findings, increased effort has been on objectively identifying patients who will benefit from repeat shunting despite apparent clinical stability [70-72]. Especially challenging in this clinical situation has been the lack of an adequate animal model. Although perinatal hydrocephalus has been studied 8 weeks after treatment of H-Tx rats [73-75], its applicability to human subjects, who may harbor subtle organic disease for years, is questionable.
Cerebrospinal Fluid (CSF)
Surgical insertion of shunts for draining CSF from the ventricles into the peritoneum for absorption by the vasculature is the current primary method for clinically managing hydrocephalus; shunts, however, become obstructed and eventually have to be replaced [7]. Generally, the CSF functions to cushion the brain in the cranium, and acts as a medium for the transport away of waste products and the diffusion of trophic and autoregulatory factors to the parenchyma [76-77]. 80% of the CSF is produced by ependymal cells of the choroid plexus, with the remainder comprised of end-products of cerebral metabolism [78] and flow through the blood-brain barrier [79]. CSF drains to the subarachnoid space through the foramen of Magendie and the foramina of Luschka, where it is predominantly absorbed by arachnoid granulations into the venous sinuses. Removal of CSF is also achieved through drainage into nasal lymph compartments [80], but clinical significance has not been established.
Impendence of normal CSF flow or defective CSF production causes hydrocephalus (see Fig. 1), which may alter normal CSF function and lead to physiologic, structural, and neurobehavioral changes. Early neuropathological and ultra-sonographic studies in humans have suggested that ventricular dilation following IVH was due to initial plugging of arachnoid villi followed by the development of obliterative arachnoiditis, while meningeal fibrosis and subependymal gliosis could cause outflow obstruction in the posterior fossa [49, 81]. This model has been widely accepted; however, a more recent model using nuclear and magnetic resonance imaging has suggested that major CSF resorption occurs through brain capillaries [82]. In this proposed model, CSF crosses the ependyma and the blood-brain barrier of local microvessels into the venous system, where any factor impeding this process could lead to hydrocephalus.
Enhanced extracellular matrix (ECM) deposition has thus been implicated in the pathogenesis of PHVD and may work to impair CSF resorption. Furthermore, some hypothesize that inflammation changes of the choroid plexus and ependymal lining of the ventricles will result in both increased production of abnormal protein-rich CSF, and the impedance of trans-ependymal CSF migration. Demonstrated by colocalized nuclear factor-KB (NF-KB) signaling and abnormal serum-derived IgG uptake by barrier cells; associating abnormal blood–brain barrier permeability with inflammation [83].
Periventricular Leukomalacia (PVL)
In the work by Cherian’s group, newborn rats who developed hydrocephalus had a 48% mean reduction in the thickness of the corpus callosum and a 31% mean reduction of the frontal cortex [84]. Immunohistochemistry techniques yielded further pathologic changes in white matter [84]. Although basic pathologic changes have been described from human samples, morphologic alterations noted in the rat model should be confirmed in human disease. In very premature infants, magnetic resonance imaging (MRI) has shown that cortical surface area and complexity are decreased when compared to infants born around term [85].
Subsequent MRI studies have found that brain changes persist into adolescence, with decreases in whole brain volume, cortical gray matter and hippocampal volume noted [86]. Similarly, premature infants with periventricular leukomalacia (PVL) exhibit both reduced cortical grey matter and total myelinated white matter at term [87], although other studies have suggested that those with PVL and NBH experience an accelerated growth of grey matter structures postnatally [88]. As a disease of prematurity, similar findings in PHVD can be expected. Further studies of postconditioning modalities (such as the phosphatidylinositol-3-kinase/Akt [89-90] neuroprotective pathway), may prove to up-regulate regenerative pathways in order to mitigate these known losses in white matter, following neonatal brain hemorrhage.
Intracranial Pressure (ICP)
Findings in both animal models and humans have suggested that increased intracranial pressure and acute ventricular distension are important pathologic features of PHVD. For example, in the newborn rat model, during injection of blood or CSF into the lateral ventricles, intracranial pressure was raised approximately 8-times its normal level [84], which may reflect the raised intracranial pressure observed in premature infants developing PHVD [91] as discussed above. In regards to ventricular expansion, it has been previously shown in one study that 40% of adults treated for severe primary IVH develop hydrocephalus requiring shunting [92], while in VLBW infants, the severity of IVH was the major predictor of short term adverse outcomes, including the development of PHVD [8]. In light of the fact that blood and CSF produce ventricular dilatation similarly in the rat model [84], acute elevation of intracranial pressure, not blood composition, is likely particularly important in the pathogenesis of PHVD.
Premature infants with PHVD have CSF pressures three-fold higher (9.1 mm Hg) than control patients [91]. In the same study, CSF pressures continued to be elevated, but less than two-fold, in “arrested” cases where ventricular dilatation was stable or reversing. At 15 mm Hg, blood flow through major cerebral vessels can be halted during diastole, which is restored when intracranial pressure is reduced to 6 mm Hg [29]. In addition, deep white matter vessel density is transiently decreased in preterm gestation [44]. Combined, these factors give periventricular white matter high susceptibility to ischemic-hypoxic injury. Periventricular axons therefore appear to be the primary target of ventricular expansion, with axonal degeneration and other neuronal distortions demonstrated that may occur independent of ischemia [34-35, 38, 57, 93-94]
Shunting is accompanied by both expected and unexpected outcomes, and, in addition, some pathologic changes that occur prior to shunting are likely not reversible. Much focus has been placed on early interventions, including quickly identifying patients that will most benefit from shunting. Other therapies involving early fibrinolytic therapy were investigated in animals [95-98], and in humans [46]. White et al have proposed a new treatment method of drainage, irrigation and fibrinolytic therapy (DRIFT). Studied in human neonates, DRIFT aims to remove cytokine and iron-containing blood products from early hydrocephalic brains [99]. The breakdown of clot facilitates the irrigation of blood from the ventricular system. A reduction of CSF concentrations of transforming growth factor-beta (TGF-β) may be one beneficial outcome of such therapies. Other interventions that block the release or action of TGF-β may be found effective; however, such treatments must aim to avoid the unintended cerebral damage demonstrated in TGF-β1 knockout mice [100]. Prematurity remains a common occurrence with strong pathologic associations, including IVH and PHVD. Animal models, particularly newborn rat models of these diseases, can be used effectively in pre-clinical investigations of pathogenesis, pathophysiology, and treatment of IVH and its complications, as is further elaborated in this review.
NEUROVASCULAR UNIT
Blood-Brain Barrier (BBB)
Dr. Ballabh’s group has collected much information regarding the structural and molecular characteristics of each individual blood-brain barrier component [101-106]. Much of the data was obtained through samples from post-mortem fetus and premature infants. According to their findings, in prematurity, IVH is most typically an extension of GMH, and cortical or white matter bleeding is far less common, together suggesting that germinal matrix vasculature has relative fragility in comparison to other areas in the developing brain. In all brain regions, the blood-brain barrier is similarly comprised of endothelial tight junctions, basal lamina, associated pericytes, and perivascular coverage by astrocyte end-feet. Although these components work in conjunction in the normal blood-brain barrier, it is possible that a dysfunction in any of these pieces would result in increased susceptibility to hemorrhage.
Endothelial tight junctions are comprised of three major integral membrane proteins claudin, occludin and junction adhesion molecules and cytoplasmic accessory proteins that include ZO1, ZO2, ZO3, and cingulin [31]. These strengthen cell-to-cell adhesion, and thus enhance structural integrity of the vessel, and, in the brain, limit paracellular flow of substrates; forming the selectivity of the blood-brain barrier [107]. Ballabh’s group hypothesized there would be a differential presence of these proteins in the forebrain (using Western blot analysis and other immunohistochemical techniques); however comparable expressions of claudin, occludin, and junction adhesion molecules were shown between cortex, white matter and germinal matrix endothelial cells as a function of gestational age [102]. Thereby it has been discussed that deficiencies of tight junction proteins were unlikely responsible for enhanced germinal matrix fragility; however, further study of molecular signaling pathways involved in endothelial tight junction formation may yield useful information in future neonatal studies [107].
Through analysis of post-mortem fetus and premature infants, Ballabh et al have demonstrated that, throughout human gestation (between 16-40 weeks), vessel density and cross-sectional area increased in all three studied brain regions: germinal matrix, white matter, and cortical grey matter [101]. Interregional comparison also showed that those same vascular parameters were greatest in the germinal matrix throughout gestation. Cross-sectional imaging of the germinal matrix showed vessels to be round in shape in comparison to the flat vessels of grey and white matter regions. This has been described as evidence of vessel immaturity [108]. Both study findings have suggested that high vascularity with morphologic immaturity, although useful in providing blood flow to areas of high metabolic demand, likely increases the susceptibility of the germinal matrix to hemorrhage.
In application, experimental studies using adult animals have implicated Src family kinases (SFKs) in the modulation of N-methyl-d-aspartate (NMDA) receptors involved with neurovascular cell death following brain bleeding [through excitatory and mitogenic signal transductions] and these play a dual role in both the early stage-acute endothelial tight junction disruption; and also BBB-recovery over subsequent weeks following the brain hemorrhage [109]. However, such a mechanism has yet to be investigated in a corresponding neonatal study.
Angiogenesis
The germinal matrix is a highly vascularized region of increased angiogenesis and endothelial turnover, and physiologic hypoxia may be their trigger in this brain region. Hypoxia is an important trigger for pro-angiogenic factors [110-112], and as noted, VEGF and angiopoeitin-2 levels are greater in the germinal matrix compared to cortical and white matter regions [106]. In the rabbits, premature pups euthanized 2 hours postnatally showed more intense staining of Hypoxyprobe (immunolabeled in tissue exhibiting low P02, often used in tumor studies), in the germinal matrix than in other forebrain regions, suggesting relative hypoxia [2].
As such, using post-mortem human samples and premature rabbit pup models, Ballabh’s group has shown VEGF and angiopoietin-2 expression to be higher in the germinal matrix when compared to forebrain grey and white matter regions [106]. In the same study, premature infants at hours after birth had greater endothelial proliferation compared to those at mean postnatal day 7. This suggests that angiogenic rate decreases within days in the postnatal period.
Two angiogenic inhibitors, the COX-2 inhibitor, celecoxib, and the VEFGR-2 inhibitor, ZD6474 were then studied in the premature rabbit pup model, with prenatal celecoxib treatment resulting in decreased angiopoietin-2 and VEGF expression, and decreased germinal matrix endothelial proliferation [106]. Both celecoxib and ZD6474 were effective in decreasing the incidence of glycerol-induced NBH [106]. These angiogenic inhibitors have been similarly used to significantly enhance pericyte coverage and decrease blood-vessel area and vascular density in the germinal matrix [105].
Ways to utilize angiogenic inhibitors such as celecoxib in order to promote blood-brain barrier integrity while avoiding tissue hypoxia are thus being explored. The concern has been that by stunting angiogenesis, CBF (cerebral blood flow) would not meet the metabolic demand of the germinal matrix. However, cancer studies have demonstrated that specific low-dose concentrations of some antiangiogenic agents function to improve tumor vessel morphology and function, as well as tissue oxygenation [113].
Accordingly, other tumor studies have shown that VEGFR2 inhibition increases expression of angiopoietin-1 to enhance pericyte coverage, and is involved in activating metalloproteinases to degrade pathologically thick basal lamina [114-115]. Further investigation into antiangiogenic therapies is deserved. Short courses of current inhibitors at low doses, or novel usage of other modulators [116-121], including the study of balancing matrix metalloproteinases (MMP) [122-123] and TIMP (tissue inhibitors of metalloproteinases) [124-125], together may help promote vascular stability, while limiting germinal matrix devascularization.
Extracellular Matrix (ECM)
The ECM basal lamina is comprised of fibronectin, laminin, collagen, heparin sulfate proteoglycan and perlacan molecules [126-127]; and elimination of fibronectin, laminin, collagen IV or perlacan genes in knockout mice have shown the importance of the basal lamina in angiogenesis, vessel stability [128-130], and thus in extension (for GMH/IVH). For example, levels of propeptide of type I procollagen increased in the CSF of PHVD premature neonates when compared to those with hydrocephalus associated with congenital malformation [131]; reflecting increased local type I collagen synthesis and turnover, related to the neuropathological (ECM) fibrotic changes found within arachnoid and meningeal tissues.
Laminin, collagen V and other important basal lamina proteins, have also been studied for their role in GMH/IVH. Ment et al. used the newborn beagle pup model to explore if the decreasing risk of GMH/IVH with increased postnatal age was related to germinal matrix vasculature maturation. Immunohistochemical evaluation revealed increased staining for laminin and collagen V in the germinal matrix on postnatal day 4 compared with postnatal day 1 [132]. A second study from this group demonstrated increased germinal matrix staining for laminin and collagen V in postnatal indomethacin-treated animals [133]. Together, the studies suggest that sufficient laminin and collagen V deposition in the extracellular matrix may stabilize the basal lamina of the germinal matrix. Although this is physiologically accomplished with postnatal aging, indomethacin and other interventions may be useful in preventing GMH/IVH in neonates. Other forms of collagen appear less significant in relation to neonatal brain hemorrhage. Collagen I, II, and IV concentrations in the germinal matrix showed no deficiency relative to cortical and white matter vessels [134]. Regardless, the role of basement membrane integrity in NBH is likely to be further investigated.
Fibronectin, an extracellular glycoprotein that binds both integrins and other extracellular matrix components, functions in cell movement, cell-cell adhesion, and cytoskelatal arrangement [135]. Polymerization of fibronectin is important for further cell growth, contractility, and strengthening of the extracellular matrix [136-137]. In post-mortem samples, Xu et al demonstrated significantly lower levels of fibronectin in the germinal matrix of both human fetuses and premature infants in comparison to cortical and white matter regions [104]. In comparison, the presence of a1, a4 and a5 laminin, a1 collagen and collagen IV, and perlacan was not significantly different. Knockout mice models with inactive fibronectin gene produced fatal neural development and vessels with variable deformity [128]. Fibronectin studies have thus been interpreted as evidence that insufficient levels of basal lamina fibronectin may contribute to germinal matrix propensity to hemorrhage. Low-dose prenatal betamethasone therapy is associated with increased expression of fibronectin [104], and as in GFAP studies, supports the role of corticosteroids in preventing NBH. In addition to glucocorticoids, new approaches to fibronectin up-regulation in the germinal matrix should be considered. Increasing expression of Transforming growth factor (TGF) may be one novel approach. In rat models, iatrogenic elevations in TGF increased fibronectin expression [138]. Similar to fibronectin, TGF levels are lower than those seen in other brain regions. The clinical efficacy of increasing TGF has not been explored. Although higher levels may promote basal lamina strengthening through increased fibronectin expression, TGF has diverse targets and functions, which may limit its clinical specificity.
Transforming Growth Factor Beta
Alterations in TGF-β concentrations in the CSF may play a role in the pathogenesis of PVHD. As a family of signaling proteins, TFG-β proteins are involved in regulating a variety of molecular pathways, including cell proliferation and differentiation, immunity, inflammation cascades, and tissue repair [139-153]. They appear in both animals and humans, and both systemically and in the central nervous system (CNS) [139-152]. TGF-β is an important mediator in collagen formation that is capable of rapid induction of angiogenesis, collagen formation and fibrosis [139]. Although this cytokine family plays mostly physiologic (i.e. homeostatic) roles, TGF-β also is involved in the development of diseases of pathologic collagen and ECM deposition, as in glomerulonephritis [140] or liver cirrhosis [141]. These molecules are therefore appropriately studied in both humans and experimentally in animal models.
Both animal models and humans have been used to demonstrate the role of TGF-β in PVHD. Experimentally, non-communicating hydrocephalus was induced in mice following injection of human recombinant TGF-β into the subarachnoid space [142], while transgenic mice who over express TGF-β from glial cells developed severe ventricular dilatation with observed seizures and motor dysfunction, as well as upregulation of ECM proteins laminin and fibronectin [143]. In humans, high concentrations of TGF-β1 have been demonstrated in CSF studies of adults with hydrocephalus following subarachnoid hemorrhage compared to non-hemorrhagic hydrocephalus patients [144], where two elevation spikes in TGF-β1 were appreciated: early on from known release from hemorrhaged platelets, and a delayed spike likely reflecting exogenous upregulation. Similarly, elevated levels of TGF-β1 and –β2 have been shown in the CSF of premature infants with PHVD [145].
The rodent model has been used extensively to relate CNS injury to TGF-β expression. For example, TGF-β is up-regulated in response to ischemic cerebral insult [146-147]. Its expression is also enhanced following penetrating brain lesion, and is thought to mediate formation of a glial scar observed at the site of injury [148]. These models therefore suggest that cerebral injury, including NBH, is capable of releasing high levels of TGF-β into the CSF, an alteration that has been implicated in the pathogenesis of PHVD in both mice models [143, 149] and clinical studies [145, 150]. Pathogenetic studies, whether done in animal models or humans, are particularly important, as they are often the first step in developing sound therapeutic interventions.
The rat model of PHVD has also been used to study the role of TGF-β [151-152]. In these studies by Cherian and Love et al, in contrast to the brains of normal 21-day old rats, those who received intraventricular blood or CSF-infusion showed significantly increased expression of TGF-β isoforms. Using immunohistochemistry, staining was prominently found for TGF-β1 in the ependyma, periventricular white matter, and deep cortical neuropil, TGF-β2 was present in neuronal cell bodies and periventricular oligodendrocytes, and TGF-β3 stained in oligodendrocytes and microglia. Although TGF-β1 and –β2 were elevated in rats injected with blood or CSF-infusion regardless if they developed ventricular dilatation, levels of these isoforms were highest in hydrocephalic animals [152]. The immunolabeling of intracellular phosphorylated p44/42 MAP kinases, modulators of TGF-β effects, rose similarly to TGF-β1 and –β2, as did ECM deposition of fibronectin, laminin and vitronectin proteins [152].
Astrocytes
Perivascular coverage is provided by close association with astrocyte end-feet that surround the cerebral vessels. End-feet contribute to the structural stability of the vessel and are major components to the blood-brain barrier. Glial fibrillary acidic protein (GFAP) is an intermediate filament astroglial marker thought to be important in maintaining mechanical strength and shape to astrocytes. In post-mortem analysis, El-Khoury’s group has demonstrated that GFAP+ astrocyte end-feet increased as a function of gestational age in both the cortex and white matter between 19 - 40 weeks, but perivascular expression of GFAP+ end-feet was decreased in the germinal matrix relative to the cortex and white matter from 23-34 weeks [103]. This discrepancy may indicate decreased cytoskeletal stability, resulting in increased vulnerability of the germinal matrix to hemorrhage. GFAP+ astrocytes first appear in the spinal cord at 9 weeks of gestation, and are then present in the ependyma from the 14th-19th weeks; while their expression has been described in germinal eminence cells at 17 weeks [154-156]. They are present early in gestation, and are therefore exposed to perinatal therapies. In vitro studies have shown that GFAP expression significantly increased in the presence of hydrocortisone [157], potentially explaining the efficacy of prenatal corticosteroids in reducing the incidence (or severity) of NBH. The above studies regarding astrocyte end-feet support the idea that they play an important role in allowing or preventing GMH.
On the other hand, ‘astrogliosis’ is an abnormal increase in the number of astrocytes in the recovery period following brain hemorrhage [60, 158]. This is postulated to involve molecular mechanisms with both beneficial and detrimental end-outcome effects [159]. The functional role of this process after neonatal hemorrhagic brain injury thus raises the prospect of therapeutically targeting this glial -cell response in future study, as this is to date poorly defined perinatally.
Pericytes
Pericytes are cellular constituents of the blood-brain barrier that surround endothelial cells of small vessels in the brain. As part of the neurovascular unit, they function in endothelial tight junction formation, blood-brain barrier differentiation, microvascular vasoactivity, structural stability of the vessel, and in angiogenesis [160]. Pericytes appear to control angiogenesis at many stages, including both initiation and maturation [161]. Early in angiogenesis, they regulate proliferation and migration of endothelial cells. Later, pericytes closely associate with the new vessel and contribute to endothelial cell differentiation, maturation and extracellular matrix deposition [162].
Pericyte recruitment and functions are regulated by four major ligand-receptor systems: TGF-B-TGFR-B, PDGF-B-PDFGR-B, angiopoietin-Tie, and sphingosine-1-phosphate-S1P1 [162]. Ligand or receptor null mice failed to recruit pericytes to endothelial cells, resulting in microaneurysms of the microvasculature, with propensity to rupture [163-164]. In post-mortem infant and rabbit pup models, Braun’s group found less pericyte coverage and pericyte density in the germinal matrix compared to grey or white matter regions [105]. The relative lack of pericytes in the germinal matrix suggests it may be a factor in GMH/IVH. The authors further investigated the molecular basis of lower levels of pericytes in the germinal matrix of human fetuses, preterm infants, and preterm rabbit pups. Ligand-receptor expression studies demonstrated lower TGF-B1 in the germinal matrix than in the cortex and white matter. Levels of PDGF-B, PDGFRB, angiopoietin-1 and its receptor Tie were not significantly different among the brain regions, while S1P1 and N-cadherin expressions were higher in the germinal matrix compared to cortex and white matter [105]. The authors suggest low levels of TGF-B1 may promote endothelial proliferation, while high levels of S1P1 and N-cadherin are likely involved in vascular maturation; however both these characteristics are important in germinal matrix angiogenesis. TGF-B1, an important regulator, promotes neurovascular stability through mesenchymal differentiation into pericytes, synthesis of extracellular matrix, and organization of pericytes around the endothelium [165]. Hence, low expression of TGF-B1 may be related to the decreased pericyte density and perivascular coverage in the germinal matrix, and may further contribute to GMH.
CEREBROVASCULATURE
Hemostasis
In conjunction with known fragility of the germinal matrix, it is tempting to hypothesize that disorders in hemostasis have a role in the disease. The relative contribution of coagulation factors in preventing or causing (in the case of deficiency) GMH/IVH, however, is not completely understood. For example, thrombocytopenia has been linked to IVH [166-167]. Further, in preterm neonates, fibrinolysis in the CSF may be inefficient in breaking blood clots impeding CSF flow, as decreased plasminogen and increased plasminogen activator inhibitor have been demonstrated in post-IVH CSF studies [168-169]. This is a potentially reversible abnormality, with CSF infusion of fibrinolytics having been explored [46, 169]. However, it is unlikely that connective tissue deposition induced by the blood clot can be effectively reversed or removed.
In a study of premature neonates with known intracranial hemorrhage, marked decreases in platelets, fibrinogen, and factor XIII activity were reported [170]. In the same study, those treated at 6 hours after birth with factor XIII concentrate had a significantly lower incidence of IVH. Since coagulation and inflammatory pathways appear interrelated in the development of IVH, various co-genotypes (of IL-1β, IL-4, IL-6, IL-10, and TNF-α), have been investigated as potential predictors of both IVH risk and severity [171-173]. In these studies, Harding’s group found an association between the CC genotype of IL-6 and higher incidence of significant intracranial hemorrhage and poorer clinical outcomes, while Gopel et al failed to confirm those results.
Currently, these remain preliminary findings, but there is continued interest in finding risk modifiers in GMH/IVH. Other alterations in hemostatic components are thus likely present; for example, hemostatic differences have been noted in preterm infants as compared to those born at full gestation [174-176]; and warrant continued investigation.
Cerebral Venous Pressure (CVP)
A recent multi-hospital observational study of neonatal intensive care units found that among VLBW infants, there was borderline association between severe NBH and hypotension, while there was no association with hypertension [177]. Previous investigations have also found data supporting a relationship between hypotension and NBH [178-180]. In contrast, other research teams demonstrated no association between the two factors [181-183]. This is of clinical importance considering that CVP increases in mechanical ventilation, positive pressure ventilation, pneumothorax, and likely many other conditions.
In regards to CVP, elevated pressures are postulated to increase risk of NBH in prematurity[184]. Post-mortem microscopic analysis of preterm neonates found the majority of germinal matrix hemorrhages to stem from venous sources, although the sample size was small [27]. Hypotension and hypertension have a variety of congenital, physiologic and iatrogenic causes in premature neonates [185]. However, with a possible prevalence of 24%-45%, hypotension is a much more common disorder in prematurity [177]. A mechanism of disease may be related to changes in cerebral blood flow (CBF), where elevated CVP leads to reduction in cerebral perfusion pressure (CPP). CPP, which is normally dependent on the gradient between MAP and intracranial pressure (ICP, CP = MAP – ICP), may be alternately determined by CVP if CVP is higher than ICP. Autoregulatory ranges of neonatal brains are thus poorly defined, as compared with adult or pediatric populations [186]; justifying further study.
Cerebrovascular: Cerebral Blood Flow (CBF)
In the acute phase of disease, much importance is placed on the role altered cerebral blood flow (CBF) has on pathogenesis in hydrocephalus, increased intraventricular pressure, and ischemia of periventricular microvasculature [187-189]. Accordingly, in animal models, reduced capillary number, density and vessel diameter have all been demonstrated in local white matter [190-192]; following hydrocephalus. Regional CBF, assessed in humans through xenon clearance, Doppler techniques, and spectroscopy, is also decreased in hydrocephalic human brains [193-195]. A reduction in regional CBF has been associated with a number of metabolic changes: increased glucose consumption in the cat model [61], increased production of reactive oxygen species (ROS) and lipid peroxidation in H-Tx rats [196], and activation of proteolytic enzymes capable of axon degradation in kaolin-injected rat models [197]. Likewise, CSF assays from hydrocephalic humans demonstrate likely disturbance in metabolism and neurotransmission [198], as well as increases in oxidative stress [199]. Of note, similar abnormalities and processes are implicated in hydrocephalus, stroke and traumatic brain injury, which may relate to shared pathogenic factors. Lastly, specific to hydrocephalus, shunting results in normalization of local CBF that has been linked to improved outcomes [200-202]; however, others have suggested that this may not account for clinical improvement seen in the long-term [193].
On postnatal day 1, Perlman et al studied CBF velocity using Doppler ultrasonography in very low birth-weight (VLBW, <1500 g) premature infants who required mechanical ventilation secondary to respiratory distress syndrome. At 12 hours of age, two CBF patterns emerged: 1) stable, where peak and trough systolic and diastolic blood flow velocities were relatively consistent; and 2) fluctuating, where blood flow velocities were varied. Flow velocities reflected simultaneously-recorded blood pressures. IVH developed in 21/23 infants with the fluctuating pattern, most within 24 H of birth, and in 7/27 neonates with a stable pattern [188]. Similar results were demonstrated in infants with gestational ages <34 weeks, regardless of respiratory status [203]. In a subsequent study by Perlman’s group, significantly less IVH and grade III IVH developed following pancuronium-induced paralysis [189]. Muscle paralysis was thought to stabilize a fluctuating CBF velocity pattern by eliminating dyssynchrony between infant and mechanical ventilations. Accordingly, variability in CBF velocities in ventilator-dependent infants has been demonstrated to improve with synchronous respiration [204]. Hence, fluctuating CBF velocity was seen as a preventable cause of IVH. While paralytic agents tend to be avoided clinically (in premature ventilator-dependent infants) out of concern for adverse effects with extended use; synchrony promoting modes of ventilation (between infant and machine) can be widely employed. These include assist control and synchronized intermittent mandatory ventilation. Of note, serum hyperosmolarity may also contribute to IVH through alteration of CBF, although investigators have argued for and against this hypothesis [205-207].
Despite a consensus not being reached, it is plausible that rapid infusion of large quantities of hypertonic sodium bicarbonate, as used in neonatal resuscitation, may lead to high PaCO2 and subsequent cerebral vasodilatation. Rapid increases or fluctuations in CBF may thus increase the propensity of the germinal matrix to hemorrhage, and should be avoided. Finally, fluctuating CBF is also associated with hypotension, hypercapnia, patent ductus arteriosus and clinical restlessness [203, 208-209], all preventable or treatable risks that may aid in preventing IVH in the premature.
Emerging clinical modalities include investigations using sonothrombolysis (e.g. therapeutic ultrasound) with-or-without adjuvant micro-bubbles, as a means to more rapidly remove any impedance of normal blood circulation, are a possible approach in the brain (to salvage damaged tissue); and to achieve better hematoma mass reduction following neonatal hemorrhagic stroke [210].
Autoregulation
Two predominant regulatory responses constitute cerebral autoregulation: myogenic responses to intravascular pressure, and functional hyperemia via neurogenic responses. Through these processes, stable and adequate CBF to the brain is achieved [211]. On a cellular level, autoregulation occurs through the innate ability of pericytes and smooth muscle cells surrounding cerebral vessels to constrict and relax in response to intravascular pressure changes. The molecular pathway begins with triggering of stretch-activated Ca++ channels, allowing the influx of calcium and subsequent activation of phospholipase A2 [212]. Consequently, arachidonic acids are released from cell membrane phospholipids, which are in turn metabolized to 20-hydroxyeicosatetraenoic acids (20-HETEs) by P450 enzymes. 20-HETEs function to inhibit Ca++-dependent K+ channels, sequentially leading to smooth muscle cell depolarization and contraction. The resulting vasoconstriction counteracts hypertension-induced increases in CBF.
Lack of cerebrovascular autoregulation, or pressure-passivity, indicates the inability of the vasculature to keep CBF constant despite variations in mean arterial pressure (MAP). CBF has been assessed by xenon clearance, as well as by cerebral oxygenation studies that include Doppler techniques, near-infrared spectroscopy (NIRS), and spatially resolved spectroscopy (SRS) [181, 213-215]. Pressure passivity is subsequently determined by factoring both CBF and MAP. A lack of cerebrovascular autoregulation in prematurity was thought to contribute to the development of NBH. An early study using xenon clearance in premature infants on mechanical ventilation showed decreased pressure-flow autoregulation and vasoreactivity to changes in PaCO2 in those subsequently developing severe intracranial hemorrhage [213]. In contrast, pressure-flow autoregulation was preserved in patients without hemorrhage.
Using near-infrared spectroscopy (NIRS; in a more recent study of similar patients), found those with greater difference between intravascular oxygenation and MAP (indicating impaired autoregulation) had a higher incidence of either severe GMH/IVH, periventricular leukomalacia (PVL); or both [216]. Further suggesting pressure-passivity relates to cerebrovascular disease. In contrast to previous xenon and Doppler ultrasonography techniques, researchers have strongly favored continued use of NIRS in the evaluation of autoregulation because of the ability to record continuously [217].
Results that do not support an association between pressure-passivity and NBH have also been found. A relatively large and recent study using continuous NIRS on VLBW infants reported pressure-passivity was significantly related to low gestational age and birth weight, but was not associated to NBH incidence [181]. Similarly, a group using SRS (related to NIRS) demonstrated impaired cerebral autoregulation present in clinically sick preterm infants predicted subsequent mortality, but did not relate to the development of NBH [215]. Together, these studies suggest impaired cerebrovascular autoregulation is a phenomena more common to sick, premature and ventilated infants, but without any clear association with NBH. Further investigation should employ NIRS, SRS or other methods of continuous recordings for more useful results.
Functional Hyperemia
Functional hyperemia is achieved through a complex neurogenic regulatory response aimed at matching CBF to metabolic demand. The entire neurovascular unit appears involved; however, their coordination has not been entirely elucidated. On a molecular level, it is thought that a cascade initiated by activation of synaptic glutamate receptors results in the production of vasoactive agents [218]. Specifically, nitric oxide and adenosine are considered to have primary roles in the genesis of functional hyperemia, which, along with CO2, H+, arachidonic acid metabolites and cytokines, are all associated with increased CBF [211, 219].
In the case of adenosine, smooth muscle relaxation and subsequent vasodilatation occur through cAMP-pathway activation of KATP and KCa channels [220]. Moreover, recent studies in the mouse model have further implicated COX-1 and COX-2 as being important regulators of functional hyperemia by causing vasodilatation and elevations in CBF [221-222]. In contrast, indomethacin, commonly used in neonates to close a patent ductus arteriosus, was demonstrated to reduce CBF in a sheep model [223]. Taken together, as impaired cerebral autoregulation is linked to brain bleeds in premature infants, further such study of the molecular basis may yield effective preventative therapies in future preclinical and clinical trials [224].
Interstitial Flow
Separate from alterations in CBF, changes in extracellular fluid flow and composition may contribute to cellular dysfunction. Physiologic waste products that fail to cross the blood-brain barrier into the systemic venous system must filter through the interstitial space and in to the CSF [76]. In the normal cerebrum, this flow occurs at 0.1-0.3 µl/min/g of brain tissue [225], but is thought to be impeded in hydrocephalus. In rodent models of hydrocephalus, the extracellular compartment volume in grey matter is reduced [226-227], extracellular fluid movement is diminished [228-229], and CSF composition changes as metabolic byproducts and other neuromodulators accumulate [198]. In humans, elevated biomarkers that result from increased CSF protein and waste concentrations are being investigated in the non-invasive diagnosis of chronic hydrocephalus [230]. Even slight alterations in waste product concentration and factors in neurotransmission may significantly impact glial and neuronal function.
MECHANISM
Free Radicals
Free radicals can exacerbate white matter injury in PHVD [231-232]. The pathogenesis of increased oxidative stress in PHVD may primarily relate to IVH [233-234]. During hemorrhage, a large quantity of iron is released into the CSF. Non-protein-bound iron is elevated in preterm infants with PHVD [232], and likely reflects iron in excess of the total iron-binding capacity of the CSF. Via the Fenton reaction, free iron mediates the production of hydroxyl radicals that potentially damage periventricular white matter [235]. In rat models of congenital hydrocephalus, increased reactive oxygen species and lipid peroxidation have been demonstrated [196], while human CSF studies have shown similar findings in premature neonates with white matter injury [231] and with hydrocephalus [199].
Cell loss immediately adjacent to the bleed may be mediated in part by the toxicities of extracellular hemoglobin (Hb) and thrombin [236-237] (and correspondingly responsive to free-radical scavengers like hydrogen [238-239] or melatonin [240-245]). However, at low concentrations, ‘offending’ proteins may precondition tolerance to hemin and iron; to decrease peri-hematomal injury through heme oxygenase (HO)-1 and iron binding protein modulation [246], e.g. transcription factor Nrf2 (Nuclear factor-like 2) [247]. Study of such mechanisms could help find strategies to further protect against periventricular erythrocyte lysis-related damage [248-249].
Oxidative Stress
Oxidative stress may also increase secondary to ischemia-reperfusion injuries in periventricular white matter, while hyperbaric-oxygen (HBO) therapy may have protective effects [250]. Hypoxanthine, a purine metabolite, is a reliable marker for hypoxia, and has been shown to be elevated in preterm PHVD infants [251]. Under normal conditions, xanthine dehydrogenase uses NAD+ as final electron acceptor to oxidize hypoxanthine and xanthine to uric acid. However, in ischemic tissue, xanthine dehydrogenase is converted to xanthine oxidase, which uses oxygen as the final electron acceptor, resulting in the generation of free radicals [252-254]. Xanthine oxidase persists with reperfusion, causing continued oxidative damage with purine metabolism. Oligodendrocyte precursors, highly present in the periventricular germinal zones, appear especially vulnerable to hypoxia-ischemia and oxidative stress in human studies of periventricular leukomalacia (PVL) [255-257].
Inflammation
Leukocyte (white blood cell) trafficking in the acute phase following experimental brain hemorrhage in adult rodents is a molecular target that may ameliorate cerebral inflammation [258-259]. Specifically, it has been shown that blood-derived monocyte populations (inflammatory macrophages and dendritic cells) travel to the brain in the 12 hours following brain bleeding, and this response out-numbers neutrophil migration [260]. Further study nonetheless will need to determine the significance of these findings and any role of these cells after neonatal brain injury [261]; as this pathophysiological process may differ from what is reported in the adult literature.
Specifically, the inflammatory response is related to white matter injury in VLBW infants [262], and has been demonstrated to be strong and prolonged in preterm infants with PHVD (i.e. hydrocephalus). In corresponding CSF studies, pro-inflammatory cytokines tumor necrosis factor-α, interleukin-1β (IL-1β), IL-6, IL-8, and interferon-γ were significantly elevated and were associated with increased risk of subsequent white matter injury and poorer neurologic outcomes [263]. Increased inflammatory response with elevations in pro-inflammatory cytokines [264] appears important in the pathogenesis of PVL (i.e. white-matter loss) [265-266], and clinical evidence supports a similar role in the development of white matter disease in PHVD. In extension, recent therapeutic approaches using adult animals may one day prove useful in neonates [264] and vice-versa. Notably, proteasome inhibition using PS-519 was found to attenuate rodent brain inflammation, and related edema, through perihematomal modulation of nuclear factor-KB [267]. This intervention reduced expression of inflammatory astroglial iNOS; and improved functional recovery following the brain injury.
NEUROPATHOLOGY
Monoamine Neurotransmission
Abnormalities in monoamine neurotransmission systems are associated with hydrocephalus [34]. In animal models, monoamine neurotransmitter concentrations are decreased [268-269]; while reductions in cholinergic and dopaminergic neurons in particular brain regions have also been demonstrated [270-271]. Much of the literature regarding neurotransmitters is related to the association between hydrocephalus and Parkinsonism’s, rarely akinetic mutism, and other movement disorders [272-275].
Experimentally in rats, hydrocephalus resulted in mechanical distortion and functional injuries of cholinergic and GABAergic neurons in the neostriatum and dopaminergic neurons in the substantia nigra [276-277], with improvement following shunt placement [278]. Likewise, in human positron emission tomography studies, functionally repressed dopamine receptors (D2 receptors) in the nigrostiatal system have been linked to gait disturbances in NPH [279]. Post shunting, upregulation of D2 receptors was correlated to clinical improvement [280]. Considering that D2 receptor hypoactivity in the dorsal putamen may be able to predict the severity of gait disturbance in idiopathic NPH [279], there is potential to find additional biomarkers with clinical significance.
While periventricular axons appear to be the primary target of ventricular expansion with hydrocephalus, non-axonal architectural changes are also observed. Damage and functional impairment are not limited to periventricular tissue, as biopsy and post-mortem human samples in chronic hydrocephalus demonstrate degenerative changes that extend beyond this region [281]. Thus abnormalities in neuronal conduction pathways likely results from both peri-hematomal morphologic changes with distal alterations of synaptic transmission.
Endocrine
The hypothalamic-pituitary axis (HPA) is exposed to ventricular expansion in the third ventricle. Hypothalamic changes related to hydrocephalus are not extensively established. However, initial gross and microscopic findings in the HPA of hydrocephalic humans have been described, with noted changes including an enlarged infundibular recess and a shortened infundibular stalk [282-284], although clinical significance has not been determined. Endocrine abnormalities, however, are associated with hydrocephalus. Pituitary hormone secretion appears affected. In shunted humans, myelomeningocele and hydrocephalus have been related to growth hormone deficiency, higher basal FSH and LH (and GnRH), precocious puberty, and amenorrhea [285-287]. However there is often an improvement of the reproductive cycle, following shunting [286]. Following experimental hydrocephalus in rats, increased hypothalamic GnRH was also observed [288].
Experimentally, angiotensin II receptor content is increased in third ventricle circumventricular organ systems [289], while alterations in the catecholaminergic system have been described [269, 290]. The exact pathogenesis in HPA dysfunction has not been clearly demonstrated, but it potentially parallels gross and cellular mechanisms evident in periventricular white matter disease. Therefore, continued use of neonatal animal models will be beneficial to better outline the role of these molecules in preterm neonates, as recent findings supporting therapeutic modulation of Arginine vasopressin (AVP; also known as vasopressin, or antidiuretic-hormone) a neurohypophysial hormone found in most mammals - may differ from experimental adult models of brain hemorrhage [291-293].
Synaptogenesis
Non-axonal architectural changes in the neuron have been demonstrated in hydrocephalus models. In kittens, visual cortex neurons are smaller, cortical neuron somata are disoriented [294], hippocampal synaptic contacts are decreased [295], and dendritic deformity in the cortex is apparent with electron microscopy [296]. Morphology did not normalize after shunting [296]. In rats, cortical dendrites were decreased in length, displayed fewer, shorter branches [297-298], and had frequent varicosities of the dendritic shaft [297]. In contrast, cellular columns and serotonergic innervations in the somatosensory cortex were preserved, suggesting that some basic cytoarchitecture is unaffected by hydrocephalus [299]. Although evidence of cortical neuropil degeneration is observed in H-Tx rats, early shunting may prevent these changes [300].
Synaptic morphology and function has also been experimentally described. In rat models, synaptophysin, a presynaptic vesicle protein, showed decay or decrease after 4 weeks in hydrocephalus [301-304], while synaptophysin staining in the hippocampus of H-Tx rats showed possible resilience of synaptogenesis [305]. Findings in animal models should be correlated with human studies. Electron microscopies of human samples from hydrocephalic brains have shown evidence of synaptic degeneration, clumping of synaptic vesicles, and glial abnormalities in association with the synapse [306-307]. Models of chronic hydrocephalus and biopsies from chronic hydrocephalus patients can more clearly define long-term characteristics of synaptogenesis. Of note, upregulation of neurotrophins, including growth-associated protein and nerve growth factor, has been demonstrated in rat models [308-311] and may indicate important axonal protection and synaptic remodeling.
Visual System
In regards to sensory abnormalities, the visual system is the most extensively studied. Hydrocephalus can cause pathology at a number of points along this pathway. Increased intracranial pressure and subsequent papilledema can damage the retina and optic nerve [312-313]. Ventricular expansion can cause morphologic alterations to geniculocortical pathways [314], while cortical and subcortical pathways can become ischemic if posterior circulation is significantly compressed. As briefly mentioned before, the integrity of visual pathways can be evaluated through the use of visual evoked potentials, which has been done in animal models [315] and in humans [316-318]. Parinaud’s syndrome, an upward gaze palsy that is seen in midbrain dysfunction, has been suggested to indicate shunt malfunction [319-320]. It is potentially caused by functional impairment of the midbrain associated with transtentorial pressure differences [320] or by structural distortion of the tectum by suprapineal recess (part of ventricular system) dilation [321], and may be a combination of multiple factors.
Periventricular Axon Degeneration
The primary pathologic change to the neuron from ventricular expansion in hydrocephalus is periventricular axon degeneration [35, 57, 93-94], while neuron cell death is rarely observed, regardless of the severity of hydrocephalus [94, 322]. However, neuronal cell death has been observed in the thalamus of H-Tx rats, which has been attributed to retrograde degeneration and apoptosis of the cell body following axonal damage [323]. In the periventricular white matter of the hydrocephalic brain, the primary sites of axonal injury occur in the limbic system, particularly the fimbria-fornix pathway and the corpus callosum [324-327]. A study of callosal fibers in kaolin-induced hydrocephalic rats suggests a subpopulation of axons may be particularly vulnerable [328]. However, as demonstrated in both experimental and human studies, all periventricular white matter, including long corticospinal tracts and deeper layers, are susceptible to injury [34, 62, 329]. Accordingly, in kaolin-induced hydrocephalic cats, midbrain, thalamic, and cortical axonal tracings were decreased, but shunt therapy restored labeling in some, but not all, regions [330]. In the dog model, cytoskeletal damage of neurons was most significant in periventricular white matter and showed incomplete normalization following shunting [331]. Although much of the pathology discussed is largely attributable to increased intraventricular pressure and ventricular enlargement secondary to hydrocephalus, the contribution from possible underlying neurologic disease is difficult to ascertain. For example, prematurity is associated with multi-system abnormalities, while post-hemorrhagic hydrocephalus occurs with local injury and exposes the parenchyma to irritating blood components.
ANIMAL MODEL
Pathophysiology
Previous works have studied the immediate pathophysiology of cellular damage and long-term consequences of germinal matrix hemorrhage/intraventricular hemorrhage. For example, cellular proliferation in the rodent germinal matrix is significantly decreased following hemorrhagic insult [30]. Chronic neurologic dysfunction may be partially explained if progenitor cells are damaged. Pro-inflammatory states have also been postulated to contribute to brain injury. In human neonates, intrauterine infections are associated with preterm birth, intraventricular hemorrhage, and cerebral palsy [332]. Similarly, inflammation and brain damage in mouse models increase with immune pre-activation by the endotoxin lipopolysaccharide [333]. Neonatal mice show increased cerebral injury in the presence of higher levels of serum thrombin and plasmin, suggesting that proteolysis may additionally play a pathogenic role [334]. Regarding long-term complications of germinal matrix hemorrhage/intraventricular hemorrhage, developmental delay and chronic behavioral deficits have been demonstrated in neonatal rat periventricular hemorrhage models [335]. Associated with intraventricular hemorrhage, hydrocephalus has been experimentally induced in rat models by blood injection into bilateral ventricles [29, 84]. On a molecular level, alpha V integrin-null mice develop spontaneous intracerebral hemorrhage in midgestation, hypothesized to be secondary to poor endothelial/pericyte association with surrounding cerebral parenchyma [336]. The continued use of knockout mouse models promises to further detail mechanisms of disease.
Neurological Dysfunction
Neuronal function depends on effective neurotransmission, structural integrity, working support cells, and intact regulatory mechanisms [337]. Any point of abnormality can ultimately lead to neurologic disorder. Clinically, global and regional abnormalities in neural activity associated with hydrocephalus can be demonstrated on electroencephalogram [338-339]. The integrity of particular conduction pathways in hydrocephalus has been investigated using sensory and motor-evoked potentials. Dysfunctional changes in the pyramidal tract, callosal, and corticospinal fibers have been demonstrated in humans [340-341], while visual cortex, motor cortex, and thalamo-cortical pathways have been shown in animal models [315, 342-344]. Using kaolin-induced hydrocephalic rats, pyramidal cells in the hippocampus showed attenuation of long-term potentiation of population spikes, a finding suggestive of abnormal postsynaptic integration [345]. In cats, single cell recordings from visual cortex cells demonstrated reduced responsiveness [346]. Performance-testing has been used to describe larger functional abnormalities related to hydrocephalus.
Experimentally, memory and learning difficulties have been shown in animal models [347-349] and in humans [350-352], with interest in potential reversibility with shunting. Such cognitive impairments have been related to abnormalities in the limbic system. In rats, septohippocampal cholinergic neurons are decreased [353], while in humans; limbic fiber aberrances were more common in myelomeningocele and Chiari II malformations [354]. Pathologic location may also relate hydrocephalus-type. Limbic and frontal dysfunction are implicated in aqueductal stenosis, while, in normal pressure hydrocephalus (NPH), cognitive deficits may be more attributable to abnormalities in prefrontal structures [355]. We determined memory deficits and spatial learning using the cognitive assessments outlined by Morris et al [356]. Juvenile rats with NBH injury had increased hyperactivity in the open field (path length) as well as diminished working memory (T-maze) than control groups [357].
Rapid changes in rat brain development occur during the first 14 days of life [358-359]. Early reflex locomotor assessments conducted by using grip traction, righting-reflex, and negative geotropism tests, are amongst the first developmental motor milestones for neonatal rats [360-362]. Thus, early cerebrovascular injury [363], such as GMH, could disrupt early brain development, resulting in delayed acquirement of basic neurobehavioral skills. In humans, delayed early developmental functions in premature infants have been strongly associated with reduced neuromotor function as they age [18]. Furthermore, deficits in posture, balance, gait, and motor learning have been similarly described in hydrocephalic animals [364-365] and humans (both adult and pediatric populations) [366-370]. We found that during the first 3 days following collagenase-induced neonatal brain hemorrhage in rats [357], significant sensorimotor deficits were observed in injured neonates when compared to sham (needle trauma only), which corroborates with a rabbit model of spontaneous pre-term brain bleeds and the clinical presentation [234]. We also observed losses in body weight 1 day following the brain bleed injury, similar to weight changes in P7 rat pups in a related model of cerebral hypoxia-ischemia [371-372]. This timeline differed, however, from a rodent direct blood-injection model, which had significant neurobehavior deficits observed between 1-2 weeks following injury [17, 335]. This free-hand needle insertion method may have caused unintended traumatic brain injury, and this may help explain the observed differences in timing of neurobehavior deficits between the two models [30].
Translational Studies
The use of animal models is necessary for a variety of reasons, including the complexity of human subjects and the need for controlled experimental environments to assess potential therapeutics, and the study of injury progression [373-377]. This has been modeled by either changing systemic hemodynamic properties (serum glycerol, blood pressure, circulating blood volume, oxygenation levels, or osmolarity) or direct, needle injection of blood into the ventricle in several animal models including mouse, rat, rabbit, dog, pig, and sheep ([234, 378-379]; see Table 1 for summary). In prematurely born rabbits (27-30 days gestation), spontaneous intraventricular hemorrhage was induced by creating intracranial hypotension using intraperitoneally injected glycerol [380]. Ventricular distension upon the blood flow patterns in neighboring brain tissues was studied in newborn dog brains by direct blood injection [381]. Physiological factors that lead to a greater predisposition to GMH have been evaluated extensively in dog models [378]. There is an interesting neonatal hypoxia-ischemia model using mice that secondarily develops spontaneous bleeding at superficial foci [382]; however that pathophysiology is different compared with what occurs in human preterm infant brain hemorrhage [2]. While early experimental neonatal brain hemorrhage models relied on the spontaneous development of IVH in preterm subjects [383-384], current models produce IVH through two major means: 1) direct injection of autologous blood into the ventricles or 2) induction of IVH through the alteration of physiologic conditions that increase susceptibility to IVH, for example hypotension, increased intracranial pressure or hypercarbia [385-387].
Table 1.
Comparison of Neonatal Brain Hemorrhage (NBH) Animal Models.
| Species | NBH Induction Method | Developmental Ages Comparable to Pre-term Humans |
Advantages | Disadvantages |
|---|---|---|---|---|
|
Rodents
[29-30, 84, 333- 335, 357] |
Injection of autologous blood or collagenase into the periven- tricular region |
Newborns comparable to 24-26 week human gestational age, 7 day-old rats comparable to 32 week human gestational age |
Availability of knockout strains, well-documented neonatal brain development, germinal matrix in postnatal age, and inexpen- sive |
Substantial anatomical and physio- logical differences with humans |
|
Rabbits
[380, 384, 407- 414] |
Intraperitoneal injection of glycerol to induce intracranial hypotension, sodium bicarbon- ate hyperosmolality, or fu- rosemide diuresis |
28 days gestation or 3-4 days before term are most comparable to pre-term humans |
Some postnatal brain develop- ment features similar to fetal humans, such as blood vessel immaturity in germinal matrix |
Inconsistent and diffuse hemor- rhage development |
|
Pigs
[415-424] |
Intraventricular autologous blood injection |
Studies performed in newborn pigs, not very comparable to humans |
Good for studying pathogenesis of brain injury in neonates |
Brain is developmentally mature |
|
Sheep
[385, 425-431] |
Asphyxia with arterial and venous hypertension in exteri- orized sheep fetuses |
58 to 85 days gestation compara- ble to 26-30 week preterm hu- mans |
Vasculature of germinal layer is similar to humans |
Brain is developmentally mature at birth, inconsistent hemorrhage development, sheep fetuses must remain unbilically attached their mothers, and sheep have carotid rete mirables |
|
Cats
[432] |
hypernatremia induced by intraperitoneal sodium injection |
Studies performed in 6+ week old kittens, not very comparable to humans |
Well documented neonatal neuronal development |
Poor documentation on kitten germinal matrix and hemorrhage sites in prior studies |
|
Dogs [132, 378-
379, 383, 386, 433-450] |
Induced hypertension, hypoten- sion, or hypercarbia at 12-48 hours post-birth in beagles, or asphyxiation of beagles at 6 days before term |
Substantial germinal matrix layer at term that is comparable to pre- term humans |
Large size makes physiological observations easy, many well documented studies related to neonatal brain hemorrhage |
Inconsistent hemorrhage develo- pment, limited long-term neurofunc- tional studies, and dog brains contain a carotid rete mirables |
|
Primates
[451-456] |
Prematurely delivered baboons at 125-days gestation spontane- ously develop hemorrhages |
Baboons at 100 days gestation comparable to 24-week human gestation age |
Most comparable to human development, spontaneous NBH development |
Very expensive |
Available animal models, however, are each limited in the ability to individually model neuropathology, etiology, and clinical outcomes following neonatal brain bleeds [17, 29, 84, 335, 388]. Hemodynamic modification has confounding factors such as hypertensive, hypercarbic, hyperosomotic, hypoxic, or hypervolemic states. Direct blood infusion, in addition to the inherent disadvantage of causing needle trauma to the surrounding tissue, does not mimic a spontaneous bleed. Further, the specialized expertise required to rear and care for prematurely delivered large mammals is very expensive. On the other hand, rodent brain injury models are relatively inexpensive to reproduce, use, maintain, and the developmental aspects are well documented [360].
Compared with pig and dog models, the newborn rat has greater neurodevelopmental similarities to preterm humans at 24-26 weeks, since neurogenesis is mostly complete [389] and the germinal matrix is present in both species. In addition, the germinal matrix has been studied extensively in rats [390-392], and long-term behavioral developments are well documented [360, 393]. In early studies, post-hemorrhagic hydrocephalus (PHVD) was not observed following injection of blood into mouse periventricular tissue [30]. However, a later neonatal rat model was outlined by Cherian et al, where ventricular dilatation was demonstrated following injection of citrated rat blood or artificial CSF (aCSF) into the ventricles [84]. Thereafter, ventricular expansion was produced after injection of blood and thrombin into the lateral ventricles of adult pigs [95]; and with injection of pre-clotted blood into the lateral ventricles of adult dogs [96-98]. Of note, unlike Cherian’s group, these models used adult animals and studied ventricular clot lysis; thus applicability to PHVD of prematurity was questionable.
Collagenase
Previously, we described a neonatal brain hemorrhage model using stereotaxically injected collagenase into the germinal-matrix of neonatal rats [357]. The rats exhibited grade III-IV brain bleeds, as the hematoma spread into the ventricles, similar as to what was delineated by clinical imaging studies in premature human infants ([4]; animal model illustrated in Fig. 1). Analogous to intraventricular hemorrhage in human premature infants, this model ruptured blood vessels within the germinal-matrix, breaks the ependymal, and fills the ventricles with blood [380]. In addition, the progressive and spontaneous escalation of focal bleeding and rebleeding, transmural pressure, and blood-vessel rupture in this model, which all raise intracranial pressure to severe levels during the first few days of neonatal life, are comparable to GMH-intraventricular hemorrhage of human premature infants [380, 394]. Clinically, elevated transmural pressures across intracerebral blood vessel walls are caused by diuretic administration, bicarbonate infusions, and high pulmonary-ventilator pressure [2].
Compared with prior animal models that used free-hand injections [335, 395], with ultrasound or MRI monitoring in a select few [30]; our model used a commercially-available, standardized, neonatal stereotaxic frame with a Hamilton syringe in order to minimize needle trauma to the brain parenchyma. We induced the brain bleed by injecting collagenase into the ganglionic eminence as this creates a standardized, spontaneous rupture that bleeds into the lateral ventricles [396-398]. This modeled the consequential conditions including regional brain swelling (edema), fibrogenesis, gliosis, motor /cognitive deficits, altered brain/body growth, delays of neurodevelopment, ventriculomegaly, brain atrophy, increased hemoglobin / thrombin, and a strong inflammation response. The slow intraventricular bleeding rate in the collagenase-induced rat neonatal brain bleed model had fewer confounding factors like related trauma, infarction, rapidly increased intracerebral pressure (ICP), as compared to models using rapid intraventricular infusions of relatively large blood volumes [84, 95, 335, 388]. Fortunately, rodent neurobehavioral and histopathological responses to brain injuries are well documented [397-401]; in addition to rodent models being less labor intensive, expensive, and having a much lower mortality rate, with survival into adulthood, compared with models using rabbits, piglets, or beagles [84, 234, 379, 388, 402].
In limitation, the collagenase approach may exaggerate the inflammatory response [403]. Indeed, a strong inflammatory response may account for the observed increase in ventricular dilation in the collagenase model when compared to direct blood injection, which requires twice the hemorrhagic volume to induce comparable ventricular dilation in only 65% of the pups [84]. Also, the effects of anti-thrombin drugs and iron-chelators need to be evaluated independently when assessing neurobehavioral and hydrocephalus development. Therefore the effects of iron, thrombin, hemoglobin, and deactivated collagenase is needed [404].
Moreover, our NBH model has a very low mortality rate for the high-grade of brain bleed injury produced; which is very different from the 30-50% mortality rate in humans with similar severity [405]. Furthermore, the mechanism behind CSF drainage impairment needs investigation; even though it is widely believed that blood and coagulation products disrupt arachnoid villi function. Additionally, it will be important to ascertain the effects of drugs on blood in the ventricles or on brain tissue; even though blood within the ventricles is a causative factor for hydrocephalus development; and future modalities such as MRI may help further clarify these associations (as partially illustrated in the Fig. 1).
SUMMARY
Neonatal brain hemorrhage is a devastating neurological disease of prematurity, and is in need of further therapeutic interventions. In this review, we discussed the latest pathophysiological findings; most specifically in the context of hydrocephalus. This pathological outcome can be studied from several perspectives, including: post-hemorrhagic ventricular dilatation (PHVD), increased cerebrospinal fluid (CSF) accumulation, and dangerously elevated intracranial pressures (ICP).
The neurovascular unit was explored and reported from several different perspectives. These included the blood-brain barrier (BBB) itself, changes in angiogenesis and related factors, the extracellular matrix (ECM), and molecules therein. Of note: TGF-β (transforming growth factor-beta) has been implicated by many different investigators, to be involved in both the recovery and increased brain injury mechanisms, and thus may likely emerge as a key molecular target in future studies. Finally, the role of other neurovascular cells: specifically astrocytes and pericytes were described in the context of pre-term neonatal brain bleeds.
Current advancements of hemostasis and hemostatic factors, and the role of brain vascular components, including changes in cerebral venous pressure (CVP) tone, cerebral blood flow (CBF) regulation, independent cerebrovascular autoregulation, functional hyperemia consequences, and, lastly, interstitial flow were discussed. Several non-novel mechanisms of brain injury were also investigated (i.e. free radicals, hypoxic-ischemic stress, and inflammation). In this context, the neuropathology following neonatal brain hemorrhage was characterized- and included pathological periventricular leukomalacia, monamine neurotransmission deficits, endocrine deficiencies, long-term synaptogenesis problems, effects on the visual system (i.e. eye-sight), and periventricular axon degeneration.
Finally, several animal models are summarized in terms of their given pathophysiology and translational insights into human disease; specifically, long-term neurological, cognitive, and sensorimotor dysfunctions as essential mimics of human disease. We also presented the potential strengths and limitations of our collagenase rodent model, the developmental stage, and appropriateness for further translational studies [406].
ACKNOWLEDGEMENTS
Contract grant sponsor: NIH; Contract grant numbers: HD43120, NS43338, NS54685, and NS078755 (to J.H.Z.).
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- [1].Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125(1):4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- [2].Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res. 2010;67(1):1–8. doi: 10.1203/PDR.0b013e3181c1b176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vermont-Oxford The Vermont-Oxford Trials Network: very low birth weight outcomes for 1990. Investigators of the Vermont-Oxford Trials Network Database Project. Pediatrics. 1993;91(3):540–545. [PubMed] [Google Scholar]
- [4].Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92(4):529–534. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- [5].Volpe JJ. Neurology of the Newborn. 4th W.B. Saunders; Philadelphia: 2001. [Google Scholar]
- [6].Bouz P, Zouros A, Taha A, Sadanand V. Neonatal Intracerebral Hemorrhage: Mechanisms, Managements, and the Outcomes. Translational Stroke Research. 2012;3(1):6–9. doi: 10.1007/s12975-012-0180-y. [DOI] [PubMed] [Google Scholar]
- [7].Whitelaw A. Intraventricular haemorrhage and posthaemorrhagic hydrocephalus: pathogenesis, prevention and future interventions. Seminars in neonatology : SN. 2001;6(2):135–146. doi: 10.1053/siny.2001.0047. [DOI] [PubMed] [Google Scholar]
- [8].Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, Horwood LJ, Volpe JJ. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 2002;87(1):F37–41. doi: 10.1136/fn.87.1.F37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pinto-Martin JA, Whitaker AH, Feldman JF, Van Rossem R, Paneth N. Relation of cranial ultrasound abnormalities in low-birthweight infants to motor or cognitive performance at ages 2, 6, and 9 years. Developmental medicine and child neurology. 1999;41(12):826–833. doi: 10.1017/s0012162299001644. [DOI] [PubMed] [Google Scholar]
- [10].Vohr BR, Allan WC, Westerveld M, Schneider KC, Katz KH, Makuch RW, Ment LR. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111:e340–346. doi: 10.1542/peds.111.4.e340. 4 Pt 1. [DOI] [PubMed] [Google Scholar]
- [11].Roze E, Van Braeckel KN, van der Veere CN, Maathuis CG, Martijn A, Bos AF. Functional outcome at school age of preterm infants with periventricular hemorrhagic infarction. Pediatrics. 2009;123(6):1493–1500. doi: 10.1542/peds.2008-1919. [DOI] [PubMed] [Google Scholar]
- [12].Jain NJ, Kruse LK, Demissie K, Khandelwal M. Impact of mode of delivery on neonatal complications: trends between 1997 and 2005. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2009;22(6):491–500. doi: 10.1080/14767050902769982. [DOI] [PubMed] [Google Scholar]
- [13].Berger R, Bender S, Sefkow S, Klingmuller V, Kunzel W, Jensen A. Peri/intraventricular haemorrhage: a cranial ultrasound study on 5286 neonates. European journal of obstetrics, gynecology, and reproductive biology. 1997;75(2):191–203. doi: 10.1016/s0301-2115(97)00135-8. [DOI] [PubMed] [Google Scholar]
- [14].Wilson-Costello D, Friedman H, Minich N, Fanaroff AA, Hack M. Improved survival rates with increased neurodevelopmental disability for extremely low birth weight infants in the 1990s. Pediatrics. 2005;115(4):997–1003. doi: 10.1542/peds.2004-0221. [DOI] [PubMed] [Google Scholar]
- [15].Sheth RD. Trends in incidence and severity of intraventricular hemorrhage. Journal of child neurology. 1998;13(6):261–264. doi: 10.1177/088307389801300604. [DOI] [PubMed] [Google Scholar]
- [16].Shennan AH, Bewley S. Why should preterm births be rising? BMJ. 2006;332(7547):924–925. doi: 10.1136/bmj.332.7547.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Aquilina K, Chakkarapani E, Love S, Thoresen M. Neonatal rat model of intraventricular haemorrhage and post-haemorrhagic ventricular dilatation with long-term survival into adulthood. Neuropathol Appl Neurobiol. 2011;37(2):156–165. doi: 10.1111/j.1365-2990.2010.01118.x. [DOI] [PubMed] [Google Scholar]
- [18].Allen MC, Capute AJ. Neonatal neurodevelopmental examination as a predictor of neuromotor outcome in premature infants. Pediatrics. 1989;83(4):498–506. [PubMed] [Google Scholar]
- [19].Gilles FH, Price RA, Kevy SV, Berenberg W. Fibrinolytic activity in the ganglionic eminence of the premature human brain. Biology of the neonate. 1971;18(5):426–432. doi: 10.1159/000240384. [DOI] [PubMed] [Google Scholar]
- [20].Kenny JD, Garcia-Prats JA, Hilliard JL, Corbet AJ, Rudolph AJ. Hypercarbia at birth: a possible role in the pathogenesis of intraventricular hemorrhage. Pediatrics. 1978;62(4):465–467. [PubMed] [Google Scholar]
- [21].Volpe JJ. Intraventricular hemorrhage in the premature infant--current concepts. Part I. Ann Neurol. 1989;25(1):3–11. doi: 10.1002/ana.410250103. [DOI] [PubMed] [Google Scholar]
- [22].Volpe JJ. Intraventricular hemorrhage in the premature infant--current concepts. Part II. Ann Neurol. 1989;25(2):109–116. doi: 10.1002/ana.410250202. [DOI] [PubMed] [Google Scholar]
- [23].DiSalvo D. The correlation between placental pathology and intraventricular hemorrhage in the preterm infant. The Developmental Epidemiology Network Investigators. Pediatric research. 1998;43(1):15–19. doi: 10.1203/00006450-199801000-00003. [DOI] [PubMed] [Google Scholar]
- [24].Antoniuk S, da Silva RV. [Periventricular and intraventricular hemorrhage in the premature infants] Revista de neurologia. 2000;31(3):238–243. [PubMed] [Google Scholar]
- [25].Volpe JJ. Intraventricular hemorrhage and brain injury in the premature infant. Neuropathology and pathogenesis. Clinics in perinatology. 1989;16(2):361–386. [PubMed] [Google Scholar]
- [26].Nakamura Y, Okudera T, Fukuda S, Hashimoto T. Germinal matrix hemorrhage of venous origin in preterm neonates. Human pathology. 1990;21(10):1059–1062. doi: 10.1016/0046-8177(90)90256-5. [DOI] [PubMed] [Google Scholar]
- [27].Ghazi-Birry HS, Brown WR, Moody DM, Challa VR, Block SM, Reboussin DM. Human germinal matrix: venous origin of hemorrhage and vascular characteristics. AJNR. American journal of neuroradiology. 1997;18(2):219–229. [PMC free article] [PubMed] [Google Scholar]
- [28].Love S. Acute haemorrhagic and hypoxic-ischaemic brain damage in the neonate. Curr Diagn Pathol. 2004;10:106–115. [Google Scholar]
- [29].Cherian S, Whitelaw A, Thoresen M, Love S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 2004;14(3):305–311. doi: 10.1111/j.1750-3639.2004.tb00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Xue M, Balasubramaniam J, Buist RJ, Peeling J, Del Bigio MR. Periventricular/intraventricular hemorrhage in neonatal mouse cerebrum. J Neuropathol Exp Neurol. 2003;62(11):1154–1165. doi: 10.1093/jnen/62.11.1154. [DOI] [PubMed] [Google Scholar]
- [31].Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- [32].Bassan H, Limperopoulos C, Visconti K, Mayer DL, Feldman HA, Avery L, Benson CB, Stewart J, Ringer SA, Soul JS, Volpe JJ, du Plessis AJ. Neurodevelopmental outcome in survivors of periventricular hemorrhagic infarction. Pediatrics. 2007;120(4):785–792. doi: 10.1542/peds.2007-0211. [DOI] [PubMed] [Google Scholar]
- [33].Del Bigio MR. Ependymal cells: biology and pathology. Acta neuropathologica. 2010;119(1):55–73. doi: 10.1007/s00401-009-0624-y. [DOI] [PubMed] [Google Scholar]
- [34].Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta neuropathologica. 1993;85(6):573–585. doi: 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- [35].Del Bigio MR. Pathophysiologic consequences of hydrocephalus. Neurosurgery clinics of North America. 2001;12(4):639–649. vii. [PubMed] [Google Scholar]
- [36].Del Bigio MR. Cellular damage and prevention in childhood hydrocephalus. Brain Pathol. 2004;14(3):317–324. doi: 10.1111/j.1750-3639.2004.tb00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McAllister JP, 2nd, Chovan P. Neonatal hydrocephalus. Mechanisms and consequences. Neurosurgery clinics of North America. 1998;9(1):73–93. [PubMed] [Google Scholar]
- [38].Del Bigio MR. Neuropathology and structural changes in hydrocephalus. Developmental disabilities research reviews. 2010;16(1):16–22. doi: 10.1002/ddrr.94. [DOI] [PubMed] [Google Scholar]
- [39].Davis SL, Tooley WH, Hunt JV. Developmental outcome following posthemorrhagic hydrocephalus in preterm infants. Comparison of twins discordant for hydrocephalus. Am J Dis Child. 1987;141(11):1170–1174. doi: 10.1001/archpedi.1987.04460110040018. [DOI] [PubMed] [Google Scholar]
- [40].Group VT. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation. Archives of disease in childhood. 1990;65:3–10. doi: 10.1136/adc.65.1_spec_no.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Pierrat V, Bevenot S, Truffert P, Duquennoy C, Lequien P. [Incidence, evolution and prognosis of posthemorrhagic ventriculomegaly in a population of newborns of less than 33 weeks gestational age] Archives de pediatrie : organe officiel de la Societe francaise de pediatrie. 1998;5(9):974–981. doi: 10.1016/s0929-693x(98)80006-7. [DOI] [PubMed] [Google Scholar]
- [42].Lou HC. The "lost autoregulation hypothesis" and brain lesions in the newborn--an update. Brain & development. 1988;10(3):143–146. doi: 10.1016/s0387-7604(88)80016-0. [DOI] [PubMed] [Google Scholar]
- [43].Lou HC. Perinatal hypoxic-ischaemic brain damage and intraventricular haemorrhage. Bailliere's clinical obstetrics and gynaecology. 1988;2(1):213–220. doi: 10.1016/s0950-3552(88)80073-7. [DOI] [PubMed] [Google Scholar]
- [44].Miyawaki T, Matsui K, Takashima S. Developmental characteristics of vessel density in the human fetal and infant brains. Early human development. 1998;53(1):65–72. doi: 10.1016/s0378-3782(98)00043-7. [DOI] [PubMed] [Google Scholar]
- [45].Del Bigio MR. In: Developmental Neuropathology. Golden JA, Harding BN, editors. International Society of Neuropathology Press; Basel, Switzerland: 2004. pp. 150–155. [Google Scholar]
- [46].2001;(1):CD000498. [Google Scholar]
- [47].Whitelaw A. Repeated lumbar or ventricular punctures in newborns with intraventricular hemorrhage. Cochrane Database Syst Rev. 2001;(1):CD000216. doi: 10.1002/14651858.CD000216. [DOI] [PubMed] [Google Scholar]
- [48].Whitelaw A, Kennedy CR, Brion LP. Diuretic therapy for newborn infants with posthemorrhagic ventricular dilatation. Cochrane Database Syst Rev. 2001;(2):CD002270. doi: 10.1002/14651858.CD002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Larroche JC. Post-haemorrhagic hydrocephalus in infancy. Anatomical study. Biology of the neonate. 1972;20(3):287–299. doi: 10.1159/000240472. [DOI] [PubMed] [Google Scholar]
- [50].Rorke LB. Pathology of perinatal brain injury. Raven Press; New York: 1972. [Google Scholar]
- [51].Fukumizu M, Takashima S, Becker LE. Neonatal posthemorrhagic hydrocephalus: neuropathologic and immunohistochemical studies. Pediatric neurology. 1995;13(3):230–234. doi: 10.1016/0887-8994(95)00183-g. [DOI] [PubMed] [Google Scholar]
- [52].Fukumizu M, Takashima S, Becker LE. Glial reaction in periventricular areas of the brainstem in fetal and neonatal posthemorrhagic hydrocephalus and congenital hydrocephalus. Brain & development. 1996;18(1):40–45. doi: 10.1016/0387-7604(95)00103-4. [DOI] [PubMed] [Google Scholar]
- [53].Takano T, Rutka JT, Becker LE. Overexpression of nestin and vimentin in ependymal cells in hydrocephalus. Acta neuropathologica. 1996;92(1):90–97. doi: 10.1007/s004010050493. [DOI] [PubMed] [Google Scholar]
- [54].Dennis M, Landry SH, Barnes M, Fletcher JM. A model of neurocognitive function in spina bifida over the life span. Journal of the International Neuropsychological Society : JINS. 2006;12(2):285–296. doi: 10.1017/S1355617706060371. [DOI] [PubMed] [Google Scholar]
- [55].Hannay HJ, Walker A, Dennis M, Kramer L, Blaser S, Fletcher JM. Auditory interhemispheric transfer in relation to patterns of partial agenesis and hypoplasia of the corpus callosum in spina bifida meningomyelocele. Journal of the International Neuropsychological Society : JINS. 2008;14(5):771–781. doi: 10.1017/S1355617708080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mashayekhi F, Draper CE, Bannister CM, Pourghasem M, Owen-Lynch PJ, Miyan JA. Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain : a journal of neurology. 2002;125:1859–1874. doi: 10.1093/brain/awf182. Pt 8. [DOI] [PubMed] [Google Scholar]
- [57].Rubin RC, Hochwald GM, Tiell M, Mizutani H, Ghatak N. Hydrocephalus: I. Histological and ultrastructural changes in the pre-shunted cortical mantle. Surgical neurology. 1976;5(2):109–114. [PubMed] [Google Scholar]
- [58].Lopes Lda S, Slobodian I, Del Bigio MR. Characterization of juvenile and young adult mice following induction of hydrocephalus with kaolin. Exp Neurol. 2009;219(1):187–196. doi: 10.1016/j.expneurol.2009.05.015. [DOI] [PubMed] [Google Scholar]
- [59].Owen-Lynch PJ, Draper CE, Mashayekhi F, Bannister CM, Miyan JA. Defective cell cycle control underlies abnormal cortical development in the hydrocephalic Texas rat. Brain : a journal of neurology. 2003;126(Pt 3):623–631. doi: 10.1093/brain/awg058. [DOI] [PubMed] [Google Scholar]
- [60].Khan OH, Enno TL, Del Bigio MR. Brain damage in neonatal rats following kaolin induction of hydrocephalus. Exp Neurol. 2006;200(2):311–320. doi: 10.1016/j.expneurol.2006.02.113. [DOI] [PubMed] [Google Scholar]
- [61].Chumas PD, Drake JM, Del Bigio MR, Da Silva M, Tuor UI. Anaerobic glycolysis preceding white-matter destruction in experimental neonatal hydrocephalus. Journal of neurosurgery. 1994;80(3):491–501. doi: 10.3171/jns.1994.80.3.0491. [DOI] [PubMed] [Google Scholar]
- [62].Del Bigio MR, da Silva MC, Drake JM, Tuor UI. Acute and chronic cerebral white matter damage in neonatal hydrocephalus. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1994;21(4):299–305. doi: 10.1017/s0317167100040865. [DOI] [PubMed] [Google Scholar]
- [63].Del Bigio MR, Kanfer JN, Zhang YW. Myelination delay in the cerebral white matter of immature rats with kaolin-induced hydrocephalus is reversible. Journal of neuropathology and experimental neurology. 1997;56(9):1053–1066. doi: 10.1097/00005072-199709000-00010. [DOI] [PubMed] [Google Scholar]
- [64].Hanlo PW, Gooskens RJ, van Schooneveld M, Tulleken CA, van der Knaap MS, Faber JA, Willemse J. The effect of intracranial pressure on myelination and the relationship with neurodevelopment in infantile hydrocephalus. Developmental medicine and child neurology. 1997;39(5):286–291. doi: 10.1111/j.1469-8749.1997.tb07433.x. [DOI] [PubMed] [Google Scholar]
- [65].Gadsdon DR, Variend S, Emery JL. Myelination of the corpus callosum. II. The effect of relief of hydrocephalus upon the processes of myelination. Zeitschrift fur Kinderchirurgie und Grenzgebiete. 1979;28(4):314–321. [PubMed] [Google Scholar]
- [66].Richardson JT. Memory and intelligence following spontaneously arrested congenital hydrocephalus. The British journal of social and clinical psychology. 1978;17(3):261–267. doi: 10.1111/j.2044-8260.1978.tb00276.x. [DOI] [PubMed] [Google Scholar]
- [67].Larsson A, Stephensen H, Wikkelso C. Adult patients with "asymptomatic" and "compensated" hydrocephalus benefit from surgery. Acta neurologica Scandinavica. 1999;99(2):81–90. doi: 10.1111/j.1600-0404.1999.tb00662.x. [DOI] [PubMed] [Google Scholar]
- [68].Mataro M, Poca MA, Sahuquillo J, Cuxart A, Iborra J, de la Calzada MD, Junque C. Cognitive changes after cerebrospinal fluid shunting in young adults with spina bifida and assumed arrested hydrocephalus. Journal of neurology, neurosurgery, and psychiatry. 2000;68(5):615–621. doi: 10.1136/jnnp.68.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Edwards RJ, Pople IK. Long-term outcome of "arrested" hydrocephalus. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 2003;13(Suppl 1):S39–40. [PubMed] [Google Scholar]
- [70].Torkelson RD, Leibrock LG, Gustavson JL, Sundell RR. Neurological and neuropsychological effects of cerebral spinal fluid shunting in children with assumed arrested ("normal pressure") hydrocephalus. Journal of neurology, neurosurgery, and psychiatry. 1985;48(8):799–806. doi: 10.1136/jnnp.48.8.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].McLone DG, Aronyk KE. An approach to the management of arrested and compensated hydrocephalus. Pediatric neurosurgery. 1993;19(2):101–103. doi: 10.1159/000120709. [DOI] [PubMed] [Google Scholar]
- [72].Bowman RM, McLone DG. Neurosurgical management of spina bifida: research issues. Developmental disabilities research reviews. 2010;16(1):82–87. doi: 10.1002/ddrr.100. [DOI] [PubMed] [Google Scholar]
- [73].Miyazawa T, Sato K, Ikeda Y, Nakamura N, Matsumoto K. A rat model of spontaneously arrested hydrocephalus. A behavioural study. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1997;13(4):189–193. doi: 10.1007/s003810050067. [DOI] [PubMed] [Google Scholar]
- [74].Shen XQ, Miyajima M, Ogino I, Arai H. Expression of the water-channel protein aquaporin 4 in the H-Tx rat: possible compensatory role in spontaneously arrested hydrocephalus. Journal of neurosurgery. 2006;105(6 Suppl):459–464. doi: 10.3171/ped.2006.105.6.459. [DOI] [PubMed] [Google Scholar]
- [75].Nonaka Y, Miyajima M, Ogino I, Nakajima M, Arai H. Analysis of neuronal cell death in the cerebral cortex of H-Tx rats with compensated hydrocephalus. Journal of neurosurgery. Pediatrics. 2008;1(1):68–74. doi: 10.3171/PED-08/01/068. [DOI] [PubMed] [Google Scholar]
- [76].Schiller F. The cerebral ventricles. From soul to sink. Archives of neurology. 1997;54(9):1158–1162. doi: 10.1001/archneur.1997.00550210086018. [DOI] [PubMed] [Google Scholar]
- [77].Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal fluid research. 2008;5(10) doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Rapoport SI. Blood-brain barrier in physiology and medicine. Raven Press; New York: 1976. [Google Scholar]
- [79].Cao Y, Brown SL, Knight RA, Fenstermacher JD, Ewing JR. Effect of intravascular-to-extravascular water exchange on the determination of blood-to-tissue transfer constant by magnetic resonance imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2005;53(2):282–293. doi: 10.1002/mrm.20340. [DOI] [PubMed] [Google Scholar]
- [80].Johnston M, Zakharov A, Papaiconomou C, Salmasi G, Armstrong D. Evidence of connections between cerebrospinal fluid and nasal lymphatic vessels in humans, non-human primates and other mammalian species. Cerebrospinal fluid research. 2004;1(1):2. doi: 10.1186/1743-8454-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Hill A, Shackelford GD, Volpe JJ. A potential mechanism of pathogenesis for early posthemorrhagic hydrocephalus in the premature newborn. Pediatrics. 1984;73(1):19–21. [PubMed] [Google Scholar]
- [82].Greitz D, Greitz T, Hindmarsh T. A new view on the CSFcirculation with the potential for pharmacological treatment of childhood hydrocephalus. Acta Paediatr. 1997;86(2):125–132. doi: 10.1111/j.1651-2227.1997.tb08850.x. [DOI] [PubMed] [Google Scholar]
- [83].Simard P, Tosun C, Melnichenko L, Ivanova S, Gerzanich V, Simard JM. Inflammation of the Choroid Plexus and Ependymal Layer of the Ventricle Following Intraventricular Hemorrhage. Translational Stroke Research. 2011;2(2):227–231. doi: 10.1007/s12975-011-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Cherian SS, Love S, Silver IA, Porter HJ, Whitelaw AG, Thoresen M. Posthemorrhagic ventricular dilation in the neonate: development and characterization of a rat model. J Neuropathol Exp Neurol. 2003;62(3):292–303. doi: 10.1093/jnen/62.3.292. [DOI] [PubMed] [Google Scholar]
- [85].Ajayi-Obe M, Saeed N, Cowan FM, Rutherford MA, Edwards AD. Reduced development of cerebral cortex in extremely preterm infants. Lancet. 2000;356(9236):1162–1163. doi: 10.1016/s0140-6736(00)02761-6. [DOI] [PubMed] [Google Scholar]
- [86].Nosarti C, Al-Asady MH, Frangou S, Stewart AL, Rifkin L, Murray RM. Adolescents who were born very preterm have decreased brain volumes. Brain : a journal of neurology. 2002;125:1616–1623. doi: 10.1093/brain/awf157. Pt 7. [DOI] [PubMed] [Google Scholar]
- [87].Inder TE, Huppi PS, Warfield S, Kikinis R, Zientara GP, Barnes PD, Jolesz F, Volpe JJ. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Annals of neurology. 1999;46(5):755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- [88].de la Monte SM, Hsu FI, Hedley-Whyte ET, Kupsky W. Morphometric analysis of the human infant brain: effects of intraventricular hemorrhage and periventricular leukomalacia. Journal of child neurology. 1990;5(2):101–110. doi: 10.1177/088307389000500206. [DOI] [PubMed] [Google Scholar]
- [89].Zhou Y, Lekic T, Fathali N, Ostrowski RP, Martin RD, Tang J, Zhang JH. Isoflurane posttreatment reduces neonatal hypoxic-ischemic brain injury in rats by the sphingosine-1-phosphate/phosphatidylinositol-3-kinase/Akt pathway. Stroke. 2010;41(7):1521–1527. doi: 10.1161/STROKEAHA.110.583757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Zhou Y, Fathali N, Lekic T, Ostrowski RP, Chen C, Martin RD, Tang J, Zhang JH. Remote limb ischemic postconditioning protects against neonatal hypoxic-ischemic brain injury in rat pups by the opioid receptor/Akt pathway. Stroke. 2011;42(2):439–444. doi: 10.1161/STROKEAHA.110.592162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kaiser AM, Whitelaw AG. Cerebrospinal fluid pressure during post haemorrhagic ventricular dilatation in newborn infants. Archives of disease in childhood. 1985;60(10):920–924. doi: 10.1136/adc.60.10.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Goh KY, Poon WS. Recombinant tissue plasminogen activator for the treatment of spontaneous adult intraventricular hemorrhage. Surgical neurology. 1998;50(6):526–531. doi: 10.1016/s0090-3019(97)00504-1. discussion 531-522. [DOI] [PubMed] [Google Scholar]
- [93].Rubin RC, Hochwald GM, Tiell M, Liwnicz BH. Hydrocephalus: II. Cell number and size, and myelin content of the pre-shunted cerebral cortical mantle. Surgical neurology. 1976;5(2):115–118. [PubMed] [Google Scholar]
- [94].Ding Y, McAllister JP, 2nd, Yao B, Yan N, Canady AI. Axonal damage associated with enlargement of ventricles during hydrocephalus: a silver impregnation study. Neurological research. 2001;23(6):581–587. doi: 10.1179/016164101101199045. [DOI] [PubMed] [Google Scholar]
- [95].Mayfrank L, Kissler J, Raoofi R, Delsing P, Weis J, Kuker W, Gilsbach JM. Ventricular dilatation in experimental intraventricular hemorrhage in pigs. Characterization of cerebrospinal fluid dynamics and the effects of fibrinolytic treatment. Stroke. 1997;28(1):141–148. doi: 10.1161/01.str.28.1.141. [DOI] [PubMed] [Google Scholar]
- [96].Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 3. Effects of intraventricular urokinase on clot lysis and posthemorrhagic hydrocephalus. Neurosurgery. 1986;19(4):553–572. doi: 10.1227/00006123-198610000-00010. [DOI] [PubMed] [Google Scholar]
- [97].Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 2. In vivo safety study of intraventricular urokinase. Neurosurgery. 1986;19(4):547–552. doi: 10.1227/00006123-198610000-00009. [DOI] [PubMed] [Google Scholar]
- [98].Pang D, Sclabassi RJ, Horton JA. Lysis of intraventricular blood clot with urokinase in a canine model: Part 1. Canine intraventricular blood cast model. Neurosurgery. 1986;19(4):540–546. doi: 10.1227/00006123-198610000-00008. [DOI] [PubMed] [Google Scholar]
- [99].Whitelaw A, Pople I, Cherian S, Evans D, Thoresen M. Phase 1 trial of prevention of hydrocephalus after intraventricular hemorrhage in newborn infants by drainage, irrigation, and fibrinolytic therapy. Pediatrics. 2003;111:759–765. doi: 10.1542/peds.111.4.759. 4 Pt 1. [DOI] [PubMed] [Google Scholar]
- [100].Brionne TC, Tesseur I, Masliah E, Wyss-Coray T. Loss of TGF-beta 1 leads to increased neuronal cell death and microgliosis in mouse brain. Neuron. 2003;40(6):1133–1145. doi: 10.1016/s0896-6273(03)00766-9. [DOI] [PubMed] [Google Scholar]
- [101].Ballabh P, Braun A, Nedergaard M. Anatomic analysis of blood vessels in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatric research. 2004;56(1):117–124. doi: 10.1203/01.PDR.0000130472.30874.FF. [DOI] [PubMed] [Google Scholar]
- [102].Ballabh P, Hu F, Kumarasiri M, Braun A, Nedergaard M. Development of tight junction molecules in blood vessels of germinal matrix, cerebral cortex, and white matter. Pediatric research. 2005;58(4):791–798. doi: 10.1203/01.PDR.0000180535.14093.FB. [DOI] [PubMed] [Google Scholar]
- [103].El-Khoury N, Braun A, Hu F, Pandey M, Nedergaard M, Lagamma EF, Ballabh P. Astrocyte end-feet in germinal matrix, cerebral cortex, and white matter in developing infants. Pediatric research. 2006;59(5):673–679. doi: 10.1203/01.pdr.0000214975.85311.9c. [DOI] [PubMed] [Google Scholar]
- [104].Xu H, Hu F, Sado Y, Ninomiya Y, Borza DB, Ungvari Z, Lagamma EF, Csiszar A, Nedergaard M, Ballabh P. Maturational changes in laminin, fibronectin, collagen IV, and perlecan in germinal matrix, cortex, and white matter and effect of betamethasone. Journal of neuroscience research. 2008;86(7):1482–1500. doi: 10.1002/jnr.21618. [DOI] [PubMed] [Google Scholar]
- [105].Braun A, Xu H, Hu F, Kocherlakota P, Siegel D, Chander P, Ungvari Z, Csiszar A, Nedergaard M, Ballabh P. Paucity of pericytes in germinal matrix vasculature of premature infants. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(44):12012–12024. doi: 10.1523/JNEUROSCI.3281-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Ballabh P, Xu H, Hu F, Braun A, Smith K, Rivera A, Lou N, Ungvari Z, Goldman SA, Csiszar A, Nedergaard M. Angiogenic inhibition reduces germinal matrix hemorrhage. Nat Med. 2007;13(4):477–485. doi: 10.1038/nm1558. [DOI] [PubMed] [Google Scholar]
- [107].Huang B, Krafft PR, Ma Q, Rolland WB, Caner B, Lekic T, Manaenko A, Le M, Tang J, Zhang JH. Fibroblast growth factors preserve blood-brain barrier integrity through RhoA inhibition after intracerebral hemorrhage in mice. Neurobiol Dis. 2012;46(1):204–214. doi: 10.1016/j.nbd.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Hellstrom M, Gerhardt H, Kalen M, Li X, Eriksson U, Wolburg H, Betsholtz C. Lack of pericytes leads to endothelial hyperplasia and abnormal vascular morphogenesis. The Journal of cell biology. 2001;153(3):543–553. doi: 10.1083/jcb.153.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Liu D-Z, Sharp F. Excitatory and Mitogenic Signaling in Cell Death, Blood–brain Barrier Breakdown, and BBB Repair after Intracerebral Hemorrhage. Translational Stroke Research. 2012;3(1):62–69. doi: 10.1007/s12975-012-0147-z. [DOI] [PubMed] [Google Scholar]
- [110].Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, Ferriero DM. Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiology of disease. 2003;14(3):524–534. doi: 10.1016/j.nbd.2003.08.020. [DOI] [PubMed] [Google Scholar]
- [111].Dzietko M, Derugin N, Wendland MF, Vexler ZS, Ferriero DM. Delayed VEGF treatment enhances angiogenesis and recovery after neonatal focal rodent stroke. Transl Stroke Res. 2013;4(2):189–200. doi: 10.1007/s12975-012-0221-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Bain JM, Moore L, Ren Z, Simonishvili S, Levison SW. Vascular endothelial growth factors A and C are induced in the SVZ following neonatal hypoxia-ischemia and exert different effects on neonatal glial progenitors. Transl Stroke Res. 2013;4(2):158–170. doi: 10.1007/s12975-012-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307(5706):58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- [114].Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- [115].Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, Honda Y, Wiegand SJ, Yancopoulos GD, Nishikawa S. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. The Journal of clinical investigation. 2002;110(11):1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Manaenko A, Fathali N, Williams S, Lekic T, Zhang JH, Tang J. Geldanamycin reduced brain injury in mouse model of intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:161–165. doi: 10.1007/978-3-7091-0693-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Zhou Y, Fathali N, Lekic T, Tang J, Zhang JH. Glibenclamide improves neurological function in neonatal hypoxiaischemia in rats. Brain Res. 2009;1270:131–139. doi: 10.1016/j.brainres.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Manaenko A, Lekic T, Sozen T, Tsuchiyama R, Zhang JH, Tang J. Effect of gap junction inhibition on intracerebral hemorrhage-induced brain injury in mice. Neurol Res. 2009;31(2):173–178. doi: 10.1179/174313209X393591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Fathali N, Lekic T, Zhang JH, Tang J. Long-term evaluation of granulocyte-colony stimulating factor on hypoxic-ischemic brain damage in infant rats. Intensive Care Med. 2010;36(9):1602–1608. doi: 10.1007/s00134-010-1913-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Doycheva D, Shih G, Chen H, Applegate R, Zhang JH, Tang J. Granulocyte-colony stimulating factor in combination with stem cell factor confers greater neuroprotection after hypoxicischemic brain damage in the neonatal rats than a solitary treatment. Transl Stroke Res. 2013;4(2):171–178. doi: 10.1007/s12975-012-0225-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Phillips AW, Johnston MV, Fatemi A. The potential for cellbased therapy in perinatal brain injuries. Transl Stroke Res. 2013;4(2):137–148. doi: 10.1007/s12975-013-0254-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Sherchan P, Lekic T, Suzuki H, Hasegawa Y, Rolland W, Duris K, Zhan Y, Tang J, Zhang JH. Minocycline improves functional outcomes, memory deficits, and histopathology after endovascular perforation-induced subarachnoid hemorrhage in rats. J Neurotrauma. 2011;28(12):2503–2512. doi: 10.1089/neu.2011.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Tang J, Liu J, Zhou C, Alexander JS, Nanda A, Granger DN, Zhang JH. Mmp-9 deficiency enhances collagenase-induced intracerebral hemorrhage and brain injury in mutant mice. J Cereb Blood Flow Metab. 2004;24(10):1133–1145. doi: 10.1097/01.WCB.0000135593.05952.DE. [DOI] [PubMed] [Google Scholar]
- [124].Souvenir R, Fathali N, Ostrowski RP, Lekic T, Zhang JH, Tang J. Tissue inhibitor of matrix metalloproteinase-1 mediates erythropoietin-induced neuroprotection in hypoxia ischemia. Neurobiol Dis. 2011;44(1):28–37. doi: 10.1016/j.nbd.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Souvenir R, Fathali N, Tong W, Lekic T, Chee P, Zhang J. Janus Kinase 2 and Tissue Inhibitor of Matrix Metalloproteinase-1 Mediate the Protective Effects of Erythropoietin in In-Vitro Model of Hypoxia Ischemia. FASEB J. 2009;23:614–620. 1_MeetingAbstracts. [Google Scholar]
- [126].Hallmann R, Horn N, Selg M, Wendler O, Pausch F, Sorokin LM. Expression and function of laminins in the embryonic and mature vasculature. Physiological reviews. 2005;85(3):979–1000. doi: 10.1152/physrev.00014.2004. [DOI] [PubMed] [Google Scholar]
- [127].Tilling T, Engelbertz C, Decker S, Korte D, Huwel S, Galla HJ. Expression and adhesive properties of basement membrane proteins in cerebral capillary endothelial cell cultures. Cell and tissue research. 2002;310(1):19–29. doi: 10.1007/s00441-002-0604-1. [DOI] [PubMed] [Google Scholar]
- [128].George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119(4):1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- [129].Forsberg E, Kjellen L. Heparan sulfate: lessons from knockout mice. The Journal of clinical investigation. 2001;108(2):175–180. doi: 10.1172/JCI13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, Aguglia U, van der Knaap MS, Heutink P, John SW. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science. 2005;308(5725):1167–1171. doi: 10.1126/science.1109418. [DOI] [PubMed] [Google Scholar]
- [131].Heep A, Stoffel-Wagner B, Soditt V, Aring C, Groneck P, Bartmann P. Procollagen I C-propeptide in the cerebrospinal fluid of neonates with posthaemorrhagic hydrocephalus. Archives of disease in childhood. Fetal and neonatal edition. 2002;87(1):F34–36. doi: 10.1136/fn.87.1.F34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Ment LR, Stewart WB, Ardito TA, Madri JA. Beagle pup germinal matrix maturation studies. Stroke; a journal of cerebral circulation. 1991;22(3):390–395. doi: 10.1161/01.str.22.3.390. [DOI] [PubMed] [Google Scholar]
- [133].Ment LR, Stewart WB, Ardito TA, Huang E, Madri JA. Indomethacin promotes germinal matrix microvessel maturation in the newborn beagle pup. Stroke; a journal of cerebral circulation. 1992;23(8):1132–1137. doi: 10.1161/01.str.23.8.1132. [DOI] [PubMed] [Google Scholar]
- [134].Anstrom JA, Thore CR, Moody DM, Challa VR, Block SM, Brown WR. Morphometric assessment of collagen accumulation in germinal matrix vessels of premature human neonates. Neuropathology and applied neurobiology. 2005;31(2):181–190. doi: 10.1111/j.1365-2990.2004.00626.x. [DOI] [PubMed] [Google Scholar]
- [135].Mao Y, Schwarzbauer JE. Fibronectin fibrillogenesis, a cellmediated matrix assembly process. Matrix biology : journal of the International Society for Matrix Biology. 2005;24(6):389–399. doi: 10.1016/j.matbio.2005.06.008. [DOI] [PubMed] [Google Scholar]
- [136].Hocking DC, Sottile J, Langenbach KJ. Stimulation of integrin-mediated cell contractility by fibronectin polymerization. The Journal of biological chemistry. 2000;275(14):10673–10682. doi: 10.1074/jbc.275.14.10673. [DOI] [PubMed] [Google Scholar]
- [137].Sottile J, Hocking DC, Langenbach KJ. Fibronectin polymerization stimulates cell growth by RGD-dependent and - independent mechanisms. Journal of cell science. 2000;113:4287–4299. doi: 10.1242/jcs.113.23.4287. Pt 23. [DOI] [PubMed] [Google Scholar]
- [138].Pasinetti GM, Nichols NR, Tocco G, Morgan T, Laping N, Finch CE. Transforming growth factor beta 1 and fibronectin messenger RNA in rat brain: responses to injury and cell-type localization. Neuroscience. 1993;54(4):893–907. doi: 10.1016/0306-4522(93)90583-2. [DOI] [PubMed] [Google Scholar]
- [139].Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(12):4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Border WA, Ruoslahti E. Transforming growth factor-beta 1 induces extracellular matrix formation in glomerulonephritis. Cell differentiation and development : the official journal of the International Society of Developmental Biologists. 1990;32(3):425–431. doi: 10.1016/0922-3371(90)90059-6. [DOI] [PubMed] [Google Scholar]
- [141].Castilla A, Prieto J, Fausto N. Transforming growth factors beta 1 and alpha in chronic liver disease. Effects of interferon alfa therapy. The New England journal of medicine. 1991;324(14):933–940. doi: 10.1056/NEJM199104043241401. [DOI] [PubMed] [Google Scholar]
- [142].Tada T, Kanaji M, Kobayashi S. Induction of communicating hydrocephalus in mice by intrathecal injection of human recombinant transforming growth factor-beta 1. J Neuroimmunol. 1994;50(2):153–158. doi: 10.1016/0165-5728(94)90041-8. [DOI] [PubMed] [Google Scholar]
- [143].Wyss-Coray T, Feng L, Masliah E, Ruppe MD, Lee HS, Toggas SM, Rockenstein EM, Mucke L. Increased central nervous system production of extracellular matrix components and development of hydrocephalus in transgenic mice overexpressing transforming growth factor-beta 1. Am J Pathol. 1995;147(1):53–67. [PMC free article] [PubMed] [Google Scholar]
- [144].Flood C, Akinwunmi J, Lagord C, Daniel M, Berry M, Jackowski A, Logan A. Transforming growth factor-beta1 in the cerebrospinal fluid of patients with subarachnoid hemorrhage: titers derived from exogenous and endogenous sources. J Cereb Blood Flow Metab. 2001;21(2):157–162. doi: 10.1097/00004647-200102000-00007. [DOI] [PubMed] [Google Scholar]
- [145].Whitelaw A, Christie S, Pople I. Transforming growth factorbeta1: a possible signal molecule for posthemorrhagic hydrocephalus? Pediatr Res. 1999;46(5):576–580. doi: 10.1203/00006450-199911000-00014. [DOI] [PubMed] [Google Scholar]
- [146].Lehrmann E, Kiefer R, Finsen B, Diemer NH, Zimmer J, Hartung HP. Cytokines in cerebral ischemia: expression of transforming growth factor beta-1 (TGF-beta 1) mRNA in the postischemic adult rat hippocampus. Experimental neurology. 1995;131(1):114–123. doi: 10.1016/0014-4886(95)90013-6. [DOI] [PubMed] [Google Scholar]
- [147].Knuckey NW, Finch P, Palm DE, Primiano MJ, Johanson CE, Flanders KC, Thompson NL. Differential neuronal and astrocytic expression of transforming growth factor beta isoforms in rat hippocampus following transient forebrain ischemia. Brain research. Molecular brain research. 1996;40(1):1–14. doi: 10.1016/0169-328x(96)00016-2. [DOI] [PubMed] [Google Scholar]
- [148].Logan A, Frautschy SA, Gonzalez AM, Sporn MB, Baird A. Enhanced expression of transforming growth factor beta 1 in the rat brain after a localized cerebral injury. Brain research. 1992;587(2):216–225. doi: 10.1016/0006-8993(92)91000-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Galbreath E, Kim SJ, Park K, Brenner M, Messing A. Overexpression of TGF-beta 1 in the central nervous system of transgenic mice results in hydrocephalus. J Neuropathol Exp Neurol. 1995;54(3):339–349. doi: 10.1097/00005072-199505000-00007. [DOI] [PubMed] [Google Scholar]
- [150].Kitazawa K, Tada T. Elevation of transforming growth factorbeta 1 level in cerebrospinal fluid of patients with communicating hydrocephalus after subarachnoid hemorrhage. Stroke; a journal of cerebral circulation. 1994;25(7):1400–1404. doi: 10.1161/01.str.25.7.1400. [DOI] [PubMed] [Google Scholar]
- [151].Love SC, Thoresen M, Whitelaw A. Role fof TGF.. during the early phase of post-hemorrhagic hydrocephalus in a neonatal rat model. Journal of neuropathology and experimental neurology. 2003;62(560) S. [Google Scholar]
- [152].Cherian S, Thoresen M, Silver IA, Whitelaw A, Love S. Transforming growth factor-betas in a rat model of neonatal posthaemorrhagic hydrocephalus. Neuropathol Appl Neurobiol. 2004;30(6):585–600. doi: 10.1111/j.1365-2990.2004.00588.x. [DOI] [PubMed] [Google Scholar]
- [153].Blomgren K, Hagberg H. Injury and repair in the immature brain. Transl Stroke Res. 2013;4(2):135–136. doi: 10.1007/s12975-013-0256-3. [DOI] [PubMed] [Google Scholar]
- [154].Wilkinson M, Hume R, Strange R, Bell JE. Glial and neuronal differentiation in the human fetal brain 9-23 weeks of gestation. Neuropathology and applied neurobiology. 1990;16(3):193–204. doi: 10.1111/j.1365-2990.1990.tb01156.x. [DOI] [PubMed] [Google Scholar]
- [155].Gould SJ, Howard S. An immunohistochemical study of the germinal layer in the late gestation human fetal brain. Neuropathology and applied neurobiology. 1987;13(6):421–437. doi: 10.1111/j.1365-2990.1987.tb00072.x. [DOI] [PubMed] [Google Scholar]
- [156].Sarnat HB. Role of human fetal ependyma. Pediatric neurology. 1992;8(3):163–178. doi: 10.1016/0887-8994(92)90063-5. [DOI] [PubMed] [Google Scholar]
- [157].Morrison RS, De Vellis J, Lee YL, Bradshaw RA, Eng LF. Hormones and growth factors induce the synthesis of glial fibrillary acidic protein in rat brain astrocytes. Journal of neuroscience research. 1985;14(2):167–176. doi: 10.1002/jnr.490140202. [DOI] [PubMed] [Google Scholar]
- [158].Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharideinduced brain injury in the neonatal rat. Brain Res. 2003;975(1-2):37–47. doi: 10.1016/s0006-8993(03)02545-9. [DOI] [PubMed] [Google Scholar]
- [159].Sukumari-Ramesh S, Alleyne C, Jr., Dhandapani K. Astrogliosis: a Target for Intervention in Intracerebral Hemorrhage? Translational Stroke Research. 2012;3(1):80–87. doi: 10.1007/s12975-012-0165-x. [DOI] [PubMed] [Google Scholar]
- [160].Balabanov R, Dore-Duffy P. Role of the CNS microvascular pericyte in the blood-brain barrier. Journal of neuroscience research. 1998;53(6):637–644. doi: 10.1002/(SICI)1097-4547(19980915)53:6<637::AID-JNR1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- [161].Hirschi KK, D'Amore PA. Control of angiogenesis by the pericyte: molecular mechanisms and significance. Exs. 1997;79:419–428. doi: 10.1007/978-3-0348-9006-9_18. [DOI] [PubMed] [Google Scholar]
- [162].Jain RK. Molecular regulation of vessel maturation. Nature medicine. 2003;9(6):685–693. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- [163].Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277(5323):242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- [164].Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407(6801):242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- [165].Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97(6):512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- [166].Andrew M, Castle V, Saigal S, Carter C, Kelton JG. Clinical impact of neonatal thrombocytopenia. The Journal of pediatrics. 1987;110(3):457–464. doi: 10.1016/s0022-3476(87)80517-6. [DOI] [PubMed] [Google Scholar]
- [167].Lupton BA, Hill A, Whitfield MF, Carter CJ, Wadsworth LD, Roland EH. Reduced platelet count as a risk factor for intraventricular hemorrhage. Am J Dis Child. 1988;142(11):1222–1224. doi: 10.1001/archpedi.1988.02150110100029. [DOI] [PubMed] [Google Scholar]
- [168].Whitelaw A, Mowinckel MC, Abildgaard U. Low levels of plasminogen in cerebrospinal fluid after intraventricular haemorrhage: a limiting factor for clot lysis? Acta Paediatr. 1995;84(8):933–936. doi: 10.1111/j.1651-2227.1995.tb13795.x. [DOI] [PubMed] [Google Scholar]
- [169].Hansen A, Whitelaw A, Lapp C, Brugnara C. Cerebrospinal fluid plasminogen activator inhibitor-1: a prognostic factor in posthaemorrhagic hydrocephalus. Acta Paediatr. 1997;86(9):995–998. doi: 10.1111/j.1651-2227.1997.tb15186.x. [DOI] [PubMed] [Google Scholar]
- [170].Shirahata A, Nakamura T, Shimono M, Kaneko M, Tanaka S. Blood coagulation findings and the efficacy of factor XIII concentrate in premature infants with intracranial hemorrhages. Thrombosis research. 1990;57(5):755–763. doi: 10.1016/0049-3848(90)90033-9. [DOI] [PubMed] [Google Scholar]
- [171].Gopel W, Hartel C, Ahrens P, Konig I, Kattner E, Kuhls E, Kuster H, Moller J, Muller D, Roth B, Segerer H, Wieg C, Herting E. Interleukin-6-174-genotype, sepsis and cerebral injury in very low birth weight infants. Genes and immunity. 2006;7(1):65–68. doi: 10.1038/sj.gene.6364264. [DOI] [PubMed] [Google Scholar]
- [172].Harding DR, Dhamrait S, Whitelaw A, Humphries SE, Marlow N, Montgomery HE. Does interleukin-6 genotype influence cerebral injury or developmental progress after preterm birth? Pediatrics. 2004;114(4):941–947. doi: 10.1542/peds.2003-0494-F. [DOI] [PubMed] [Google Scholar]
- [173].Harding D, Brull D, Humphries SE, Whitelaw A, Montgomery H, Marlow N. Variation in the interleukin-6 gene is associated with impaired cognitive development in children born prematurely: a preliminary study. Pediatric research. 2005;58(1):117–120. doi: 10.1203/01.PDR.0000163523.49021.53. [DOI] [PubMed] [Google Scholar]
- [174].Kuhle S, Male C, Mitchell L. Developmental hemostasis: proand anticoagulant systems during childhood. Seminars in thrombosis and hemostasis. 2003;29(4):329–338. doi: 10.1055/s-2003-42584. [DOI] [PubMed] [Google Scholar]
- [175].Manco-Johnson MJ, Jacobson LJ, Hacker MR, Townsend SF, Murphy J, Hay W., Jr. Development of coagulation regulatory proteins in the fetal and neonatal lamb. Pediatric research. 2002;52(4):580–588. doi: 10.1203/00006450-200210000-00019. [DOI] [PubMed] [Google Scholar]
- [176].Manco-Johnson MJ. Development of hemostasis in the fetus. Thrombosis research. 2005;115(Suppl 1):55–63. [PubMed] [Google Scholar]
- [177].Al-Aweel I, Pursley DM, Rubin LP, Shah B, Weisberger S, Richardson DK. Variations in prevalence of hypotension, hypertension, and vasopressor use in NICUs. Journal of perinatology : official journal of the California Perinatal Association. 2001;21(5):272–278. doi: 10.1038/sj.jp.7210563. [DOI] [PubMed] [Google Scholar]
- [178].Miall-Allen VM, de Vries LS, Whitelaw AG. Mean arterial blood pressure and neonatal cerebral lesions. Archives of disease in childhood. 1987;62(10):1068–1069. doi: 10.1136/adc.62.10.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Watkins AM, West CR, Cooke RW. Blood pressure and cerebral haemorrhage and ischaemia in very low birthweight infants. Early human development. 1989;19(2):103–110. doi: 10.1016/0378-3782(89)90120-5. [DOI] [PubMed] [Google Scholar]
- [180].Bada HS, Korones SB, Perry EH, Arheart KL, Ray JD, Pourcyrous M, Magill HL, Runyan W, 3rd, Somes GW, Clark FC, et al. Mean arterial blood pressure changes in premature infants and those at risk for intraventricular hemorrhage. The Journal of pediatrics. 1990;117(4):607–614. doi: 10.1016/s0022-3476(05)80700-0. [DOI] [PubMed] [Google Scholar]
- [181].Soul JS, Hammer PE, Tsuji M, Saul JP, Bassan H, Limperopoulos C, Disalvo DN, Moore M, Akins P, Ringer S, Volpe JJ, Trachtenberg F, du Plessis AJ. Fluctuating pressure-passivity is common in the cerebral circulation of sick premature infants. Pediatric research. 2007;61(4):467–473. doi: 10.1203/pdr.0b013e31803237f6. [DOI] [PubMed] [Google Scholar]
- [182].Weindling AM, Wilkinson AR, Cook J, Calvert SA, Fok TF, Rochefort MJ. Perinatal events which precede periventricular haemorrhage and leukomalacia in the newborn. British journal of obstetrics and gynaecology. 1985;92(12):1218–1223. doi: 10.1111/j.1471-0528.1985.tb04865.x. [DOI] [PubMed] [Google Scholar]
- [183].Muller AM, Morales C, Briner J, Baenziger O, Duc G, Bucher HU. Loss of CO2 reactivity of cerebral blood flow is associated with severe brain damage in mechanically ventilated very low birth weight infants. European journal of paediatric neurology : EJPN : official journal of the European Paediatric Neurology Society. 1997;1(5-6):157–163. [PubMed] [Google Scholar]
- [184].Volpe JJ. In: Neurology of the Newborn. Volpe JJ, editor. Saunders Elsevier; Philadelphia: 2008. pp. 517–288. [Google Scholar]
- [185].Fanaroff JM, Fanaroff AA. Blood pressure disorders in the neonate: hypotension and hypertension. Seminars in fetal & neonatal medicine. 2006;11(3):174–181. doi: 10.1016/j.siny.2006.01.002. [DOI] [PubMed] [Google Scholar]
- [186].Tontisirin N, Muangman SL, Suz P, Pihoker C, Fisk D, Moore A, Lam AM, Vavilala MS. Early childhood gender differences in anterior and posterior cerebral blood flow velocity and autoregulation. Pediatrics. 2007;119(3):e610–615. doi: 10.1542/peds.2006-2110. [DOI] [PubMed] [Google Scholar]
- [187].Tsuji M, duPlessis A, Taylor G, Crocker R, Volpe JJ. Near infrared spectroscopy detects cerebral ischemia during hypotension in piglets. Pediatric research. 1998;44(4):591–595. doi: 10.1203/00006450-199810000-00020. [DOI] [PubMed] [Google Scholar]
- [188].Perlman JM, McMenamin JB, Volpe JJ. Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome. Relation to the development of intraventricular hemorrhage. The New England journal of medicine. 1983;309(4):204–209. doi: 10.1056/NEJM198307283090402. [DOI] [PubMed] [Google Scholar]
- [189].Perlman JM, Goodman S, Kreusser KL, Volpe JJ. Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. The New England journal of medicine. 1985;312(21):1353–1357. doi: 10.1056/NEJM198505233122104. [DOI] [PubMed] [Google Scholar]
- [190].Del Bigio MR, Bruni JE. Changes in periventricular vasculature of rabbit brain following induction of hydrocephalus and after shunting. Journal of neurosurgery. 1988;69(1):115–120. doi: 10.3171/jns.1988.69.1.0115. [DOI] [PubMed] [Google Scholar]
- [191].Jones HC, Bucknall RM, Harris NG. The cerebral cortex in congenital hydrocephalus in the H-Tx rat: a quantitative light microscopy study. Acta neuropathologica. 1991;82(3):217–224. doi: 10.1007/BF00294448. [DOI] [PubMed] [Google Scholar]
- [192].Luciano MG, Skarupa DJ, Booth AM, Wood AS, Brant CL, Gdowski MJ. Cerebrovascular adaptation in chronic hydrocephalus. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2001;21(3):285–294. doi: 10.1097/00004647-200103000-00012. [DOI] [PubMed] [Google Scholar]
- [193].Graff-Radford NR, Rezai K, Godersky JC, Eslinger P, Damasio H, Kirchner PT. Regional cerebral blood flow in normal pressure hydrocephalus. Journal of neurology, neurosurgery, and psychiatry. 1987;50(12):1589–1596. doi: 10.1136/jnnp.50.12.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Shirane R, Sato S, Sato K, Kameyama M, Ogawa A, Yoshimoto T, Hatazawa J, Ito M. Cerebral blood flow and oxygen metabolism in infants with hydrocephalus. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1992;8(3):118–123. doi: 10.1007/BF00298263. [DOI] [PubMed] [Google Scholar]
- [195].Chang CC, Asada H, Mimura T, Suzuki S. A prospective study of cerebral blood flow and cerebrovascular reactivity to acetazolamide in 162 patients with idiopathic normal-pressure hydrocephalus. Journal of neurosurgery. 2009;111(3):610–617. doi: 10.3171/2008.10.17676. [DOI] [PubMed] [Google Scholar]
- [196].Socci DJ, Bjugstad KB, Jones HC, Pattisapu JV, Arendash GW. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Experimental neurology. 1999;155(1):109–117. doi: 10.1006/exnr.1998.6969. [DOI] [PubMed] [Google Scholar]
- [197].Del Bigio MR. Calcium-mediated proteolytic damage in white matter of hydrocephalic rats? Journal of neuropathology and experimental neurology. 2000;59(11):946–954. doi: 10.1093/jnen/59.11.946. [DOI] [PubMed] [Google Scholar]
- [198].Del Bigio MR. Hydrocephalus-induced changes in the composition of cerebrospinal fluid. Neurosurgery. 1989;25(3):416–423. doi: 10.1097/00006123-198909000-00016. [DOI] [PubMed] [Google Scholar]
- [199].Krueger RC., Jr Use of a novel double-sandwich enzyme-linked immunosorbent assay method for assaying chondroitin sulfate proteoglycans that bear 3-nitrotyrosine core protein modifications, a previously unrecognized proteoglycan modification in hydrocephalus. Analytical biochemistry. 2004;325(1):52–61. doi: 10.1016/j.ab.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [200].Brooks DJ, Beaney RP, Powell M, Leenders KL, Crockard HA, Thomas DG, Marshall J, Jones T. Studies on cerebral oxygen metabolism, blood flow, and blood volume, in patients with hydrocephalus before and after surgical decompression, using positron emission tomography. Brain : a journal of neurology. 1986;109:613–628. doi: 10.1093/brain/109.4.613. Pt 4. [DOI] [PubMed] [Google Scholar]
- [201].Nishimaki S, Iwasaki Y, Akamatsu H. Cerebral blood flow velocity before and after cerebrospinal fluid drainage in infants with posthemorrhagic hydrocephalus. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2004;23(10):1315–1319. doi: 10.7863/jum.2004.23.10.1315. [DOI] [PubMed] [Google Scholar]
- [202].Miyamoto J, Tatsuzawa K, Inoue Y, Imahori Y, Mineura K. Oxygen metabolism changes in patients with idiopathic normal pressure hydrocephalus before and after shunting operation. Acta neurologica Scandinavica. 2007;116(3):137–143. doi: 10.1111/j.1600-0404.2007.00798.x. [DOI] [PubMed] [Google Scholar]
- [203].Van Bel F, Van de Bor M, Stijnen T, Baan J, Ruys JH. Aetiological role of cerebral blood-flow alterations in development and extension of peri-intraventricular haemorrhage. Developmental medicine and child neurology. 1987;29(5):601–614. doi: 10.1111/j.1469-8749.1987.tb08502.x. [DOI] [PubMed] [Google Scholar]
- [204].Rennie JM, South M, Morley CJ. Cerebral blood flow velocity variability in infants receiving assisted ventilation. Archives of disease in childhood. 1987;62(12):1247–1251. doi: 10.1136/adc.62.12.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Wigglesworth JS, Keith IH, Girling DJ, Slade SA. Hyaline membrane disease, alkali, and intraventricular haemorrhage. Archives of disease in childhood. 1976;51(10):755–762. doi: 10.1136/adc.51.10.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Anderson JM, Bain AD, Brown JK, Cockburn F, Forfar JO, Machin GA, Turner TL. Hyaline-membrane disease, alkaline buffer treatment, and cerebral intraventricular halphaemorrhage. Lancet. 1976;1(7951):117–119. doi: 10.1016/s0140-6736(76)93156-1. [DOI] [PubMed] [Google Scholar]
- [207].Lee S, Stier G, Marcantonio S, Lekic T, Allard M, Martin R, Zhang J. 3% hypertonic saline following subarachnoid hemorrhage in rats. Acta Neurochir Suppl. 2008;102:405–408. doi: 10.1007/978-3-211-85578-2_79. [DOI] [PubMed] [Google Scholar]
- [208].Mullaart RA, Hopman JC, Rotteveel JJ, Daniels O, Stoelinga GB, De Haan AF. Cerebral blood flow fluctuation in neonatal respiratory distress and periventricular haemorrhage. Early human development. 1994;37(3):179–185. doi: 10.1016/0378-3782(94)90077-9. [DOI] [PubMed] [Google Scholar]
- [209].Coughtrey H, Rennie JM, Evans DH. Variability in cerebral blood flow velocity: observations over one minute in preterm babies. Early human development. 1997;47(1):63–70. doi: 10.1016/s0378-3782(96)01769-0. [DOI] [PubMed] [Google Scholar]
- [210].Soltani A, Clark W, Hansmann D. Sonothrombolysis: An Emerging Modality for the Treatment of Acute Ischemic and Hemorrhagic Stroke. Translational Stroke Research. 2011;2(2):159–170. doi: 10.1007/s12975-011-0077-1. [DOI] [PubMed] [Google Scholar]
- [211].Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nature neuroscience. 2007;10(11):1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- [212].Hill MA, Sun Z, Martinez-Lemus L, Meininger GA. New technologies for dissecting the arteriolar myogenic response. Trends in pharmacological sciences. 2007;28(7):308–315. doi: 10.1016/j.tips.2007.05.006. [DOI] [PubMed] [Google Scholar]
- [213].Pryds O, Greisen G, Lou H, Friis-Hansen B. Heterogeneity of cerebral vasoreactivity in preterm infants supported by mechanical ventilation. The Journal of pediatrics. 1989;115(4):638–645. doi: 10.1016/s0022-3476(89)80301-4. [DOI] [PubMed] [Google Scholar]
- [214].du Plessis AJ. Cerebrovascular injury in premature infants: current understanding and challenges for future prevention. Clinics in perinatology. 2008;35(4):609–641. doi: 10.1016/j.clp.2008.07.010. v. [DOI] [PubMed] [Google Scholar]
- [215].Wong FY, Leung TS, Austin T, Wilkinson M, Meek JH, Wyatt JS, Walker AM. Impaired autoregulation in preterm infants identified by using spatially resolved spectroscopy. Pediatrics. 2008;121(3):e604–611. doi: 10.1542/peds.2007-1487. [DOI] [PubMed] [Google Scholar]
- [216].Tsuji M, Saul JP, du Plessis A, Eichenwald E, Sobh J, Crocker R, Volpe JJ. Cerebral intravascular oxygenation correlates with mean arterial pressure in critically ill premature infants. Pediatrics. 2000;106(4):625–632. doi: 10.1542/peds.106.4.625. [DOI] [PubMed] [Google Scholar]
- [217].Reynolds KJ, Panerai RB, Kelsall AW, Rennie JM, Evans DH. Spectral pattern of neonatal cerebral blood flow velocity: comparison with spectra from blood pressure and heart rate. Pediatric research. 1997;41(2):276–284. doi: 10.1203/00006450-199702000-00020. [DOI] [PubMed] [Google Scholar]
- [218].Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nature reviews. Neuroscience. 2004;5(5):347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- [219].Zhu C, Sun Y, Gao J, Wang X, Plesnila N, Blomgren K. Inhaled nitric oxide protects males but not females from neonatal mouse hypoxia-ischemia brain injury. Transl Stroke Res. 2013;4(2):201–207. doi: 10.1007/s12975-012-0217-2. [DOI] [PubMed] [Google Scholar]
- [220].Phillis JW. Adenosine and adenine nucleotides as regulators of cerebral blood flow: roles of acidosis, cell swelling, and KATP channels. Critical reviews in neurobiology. 2004;16(4):237–270. doi: 10.1615/critrevneurobiol.v16.i4.20. [DOI] [PubMed] [Google Scholar]
- [221].Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20(2):763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circulation research. 2001;88(6):600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- [223].Upton RN, Rasmussen M, Grant C, Martinez AM, Cold GE, Ludbrook GL. Pharmacokinetics and pharmacodynamics of indomethacin: effects on cerebral blood flow in anaesthetized sheep. Clinical and experimental pharmacology & physiology. 2008;35(3):317–323. doi: 10.1111/j.1440-1681.2007.04818.x. [DOI] [PubMed] [Google Scholar]
- [224].Fathali N, Ostrowski RP, Lekic T, Jadhav V, Tong W, Tang J, Zhang JH. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38(2):572–578. doi: 10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochemistry international. 2004;45(4):545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- [226].McLone DG, Bondareff W, Raimondi AJ. Brain edema in the hydrocephalic hy-3 mouse: submicroscopic morphology. Journal of neuropathology and experimental neurology. 1971;30(4):627–637. doi: 10.1097/00005072-197110000-00007. [DOI] [PubMed] [Google Scholar]
- [227].Del Bigio MR, Enno TL. Effect of hydrocephalus on rat brain extracellular compartment. Cerebrospinal fluid research. 2008;5(12) doi: 10.1186/1743-8454-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [228].Shoesmith CL, Buist R, Del Bigio MR. Magnetic resonance imaging study of extracellular fluid tracer movement in brains of immature rats with hydrocephalus. Neurological research. 2000;22(1):111–116. doi: 10.1080/01616412.2000.11741045. [DOI] [PubMed] [Google Scholar]
- [229].Sykova E, Fiala J, Antonova T, Vorisek I. Extracellular space volume changes and diffusion barriers in rats with kaolin-induced and inherited hydrocephalus. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 2001;11(Suppl 1):S34–37. [PubMed] [Google Scholar]
- [230].Tarnaris A, Watkins LD, Kitchen ND. Biomarkers in chronic adult hydrocephalus. Cerebrospinal fluid research. 2006;3(11) doi: 10.1186/1743-8454-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Inder T, Mocatta T, Darlow B, Spencer C, Volpe JJ, Winterbourn C. Elevated free radical products in the cerebrospinal fluid of VLBW infants with cerebral white matter injury. Pediatr Res. 2002;52(2):213–218. doi: 10.1203/00006450-200208000-00013. [DOI] [PubMed] [Google Scholar]
- [232].Savman K, Nilsson UA, Blennow M, Kjellmer I, Whitelaw A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatr Res. 2001;49(2):208–212. doi: 10.1203/00006450-200102000-00013. [DOI] [PubMed] [Google Scholar]
- [233].Chua CO, Chahboune H, Braun A, Dummula K, Chua CE, Yu J, Ungvari Z, Sherbany AA, Hyder F, Ballabh P. Consequences of intraventricular hemorrhage in a rabbit pup model. Stroke. 2009;40(10):3369–3377. doi: 10.1161/STROKEAHA.109.549212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Georgiadis P, Xu H, Chua C, Hu F, Collins L, Huynh C, Lagamma EF, Ballabh P. Characterization of acute brain injuries and neurobehavioral profiles in a rabbit model of germinal matrix hemorrhage. Stroke. 2008;39(12):3378–3388. doi: 10.1161/STROKEAHA.107.510883. [DOI] [PubMed] [Google Scholar]
- [235].Aruoma OI, Halliwell B, Dizdaroglu M. Iron ion-dependent modification of bases in DNA by the superoxide radical-generating system hypoxanthine/xanthine oxidase. J Biol Chem. 1989;264(22):13024–13028. [PubMed] [Google Scholar]
- [236].Wu J, Hua Y, Keep RF, Nakamura T, Hoff JT, Xi G. Iron and iron-handling proteins in the brain after intracerebral hemorrhage. Stroke. 2003;34(12):2964–2969. doi: 10.1161/01.STR.0000103140.52838.45. [DOI] [PubMed] [Google Scholar]
- [237].Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996;84(1):91–96. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- [238].Manaenko A, Lekic T, Ma Q, Ostrowski RP, Zhang JH, Tang J. Hydrogen inhalation is neuroprotective and improves functional outcomes in mice after intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:179–183. doi: 10.1007/978-3-7091-0693-8_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Lekic T, Manaenko A, Rolland W, Fathali N, Peterson M, Tang J, Zhang JH. Protective effect of hydrogen gas therapy after germinal matrix hemorrhage in neonatal rats. Acta Neurochir Suppl. 2011;111:237–241. doi: 10.1007/978-3-7091-0693-8_40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Lekic T, Manaenko A, Rolland W, Virbel K, Hartman R, Tang J, Zhang JH. Neuroprotection by melatonin after germinal matrix hemorrhage in neonatal rats. Acta Neurochir Suppl. 2011;111:201–206. doi: 10.1007/978-3-7091-0693-8_34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Lekic T, Hartman R, Rojas H, Manaenko A, Chen W, Ayer R, Tang J, Zhang JH. Protective effect of melatonin upon neuropathology, striatal function, and memory ability after intracerebral hemorrhage in rats. J Neurotrauma. 2010;27(3):627–637. doi: 10.1089/neu.2009.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Hartman RE, Rojas HA, Lekic T, Ayer R, Lee S, Jadhav V, Titova E, Tang J, Zhang JH. Long-term effects of melatonin after intracerebral hemorrhage in rats. Acta Neurochir Suppl. 2008;105:99–100. doi: 10.1007/978-3-211-09469-3_20. [DOI] [PubMed] [Google Scholar]
- [243].Lee S, Jadhav V, Ayer RE, Rojas H, Hyong A, Lekic T, Tang J, Zhang JH. Dual effects of melatonin on oxidative stress after surgical brain injury in rats. J Pineal Res. 2008 doi: 10.1111/j.1600-079X.2008.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Lee S, Jadhav V, Ayer RE, Rojas H, Hyong A, Lekic T, Tang J, Zhang JH. Dual effects of melatonin on oxidative stress after surgical brain injury in rats. J Pineal Res. 2009;46(1):43–48. doi: 10.1111/j.1600-079X.2008.00607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Lee S, Jadhav V, Ayer R, Rojas H, Hyong A, Lekic T, Stier G, Martin R, Zhang JH. The antioxidant effects of melatonin in surgical brain injury in rats. Acta Neurochir Suppl. 2008;102:367–371. doi: 10.1007/978-3-211-85578-2_70. [DOI] [PubMed] [Google Scholar]
- [246].Chen-Roetling J, Sinanan J, Regan R. Effect of Iron Chelators on Methemoglobin and Thrombin Preconditioning. Translational Stroke Research. 2012;3(4):452–459. doi: 10.1007/s12975-012-0195-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Zhao X, Aronowski J. Nrf2 to pre-condition the brain against injury caused by products of hemolysis after ICH. Transl Stroke Res. 2013;4(1):71–75. doi: 10.1007/s12975-012-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [248].Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 2002;953(1-2):45–52. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- [249].Xi G, Keep RF, Hoff JT. Erythrocytes and delayed brain edema formation following intracerebral hemorrhage in rats. J Neurosurg. 1998;89(6):991–996. doi: 10.3171/jns.1998.89.6.0991. [DOI] [PubMed] [Google Scholar]
- [250].Lekic T, Manaenko A, Rolland W, Ostrowski RP, Virbel K, Tang J, Zhang JH. Beneficial effect of hyperbaric oxygenation after neonatal germinal matrix hemorrhage. Acta Neurochir Suppl. 2011;111:253–257. doi: 10.1007/978-3-7091-0693-8_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Bejar R, Saugstad OD, James H, Gluck L. Increased hypoxanthine concentrations in cerebrospinal fluid of infants with hydrocephalus. The Journal of pediatrics. 1983;103(1):44–48. doi: 10.1016/s0022-3476(83)80773-2. [DOI] [PubMed] [Google Scholar]
- [252].Hille R. The reaction mechanism of oxomolybdenum enzymes. Biochimica et biophysica acta. 1994;1184(2-3):143–169. doi: 10.1016/0005-2728(94)90220-8. [DOI] [PubMed] [Google Scholar]
- [253].Love S. Oxidative stress in brain ischemia. Brain Pathol. 1999;9(1):119–131. doi: 10.1111/j.1750-3639.1999.tb00214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Nishino T, Tamura I. The mechanism of conversion of xanthine dehydrogenase to oxidase and the role of the enzyme in reperfusion injury. Advances in experimental medicine and biology. 1991;309A:327–333. doi: 10.1007/978-1-4899-2638-8_74. [DOI] [PubMed] [Google Scholar]
- [255].McDonald JW, Levine JM, Qu Y. Multiple classes of the oligodendrocyte lineage are highly vulnerable to excitotoxicity. Neuroreport. 1998;9(12):2757–2762. doi: 10.1097/00001756-199808240-00014. [DOI] [PubMed] [Google Scholar]
- [256].Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(4):1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Ness JK, Romanko MJ, Rothstein RP, Wood TL, Levison SW. Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Developmental neuroscience. 2001;23(3):203–208. doi: 10.1159/000046144. [DOI] [PubMed] [Google Scholar]
- [258].Rolland WB, Lekic T, Krafft PR, Hasegawa Y, Altay O, Hartman R, Ostrowski R, Manaenko A, Tang J, Zhang JH. Fingolimod reduces cerebral lymphocyte infiltration in experimental models of rodent intracerebral hemorrhage. Experimental Neurology. doi: 10.1016/j.expneurol.2012.12.009. 0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Rolland WB, 2nd, Manaenko A, Lekic T, Hasegawa Y, Ostrowski R, Tang J, Zhang JH. FTY720 is neuroprotective and improves functional outcomes after intracerebral hemorrhage in mice. Acta Neurochir Suppl. 2011;111:213–217. doi: 10.1007/978-3-7091-0693-8_36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Hammond M, Ai Y, Sansing L. Gr1+ Macrophages and Dendritic Cells Dominate the Inflammatory Infiltrate 12 h After Experimental Intracerebral Hemorrhage. Translational Stroke Research. 2012;3(1):125–131. doi: 10.1007/s12975-012-0174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Fathali N, Ostrowski RP, Hasegawa Y, Lekic T, Tang J, Zhang JH. Splenic immune cells in experimental neonatal hypoxia-ischemia. Transl Stroke Res. 2013;4(2):208–219. doi: 10.1007/s12975-012-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, Kuban K, Van Marter LJ, Pagano M, Hegyi T, Hiatt M, Sanocka U, Shahrivar F, Abiri M, Disalvo D, Doubilet P, Kairam R, Kazam E, Kirpekar M, Rosenfeld D, Schonfeld S, Share J, Collins M, Genest D, Shen-Schwarz S, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatric research. 1999;46(5):566–575. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- [263].Savman K, Blennow M, Hagberg H, Tarkowski E, Thoresen M, Whitelaw A. Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr. 2002;91(12):1357–1363. doi: 10.1111/j.1651-2227.2002.tb02834.x. [DOI] [PubMed] [Google Scholar]
- [264].Strahle J, Garton HL, Maher C, Muraszko K, Keep R, Xi G. Mechanisms of Hydrocephalus After Neonatal and Adult Intraventricular Hemorrhage. Translational Stroke Research. 2012;3(1):25–38. doi: 10.1007/s12975-012-0182-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Deguchi K, Oguchi K, Matsuura N, Armstrong DD, Takashima S. Periventricular leukomalacia: relation to gestational age and axonal injury. Pediatric neurology. 1999;20(5):370–374. doi: 10.1016/s0887-8994(99)00010-7. [DOI] [PubMed] [Google Scholar]
- [266].Kadhim H, Tabarki B, Verellen G, De Prez C, Rona AM, Sebire G. Inflammatory cytokines in the pathogenesis of periventricular leukomalacia. Neurology. 2001;56(10):1278–1284. doi: 10.1212/wnl.56.10.1278. [DOI] [PubMed] [Google Scholar]
- [267].Al-Senani F, Zhao X, Grotta J, Shirzadi A, Strong R, Aronowski J. Proteasome Inhibitor Reduces Astrocytic iNOS Expression and Functional Deficit after Experimental Intracerebral Hemorrhage in Rats. Translational Stroke Research. 2012;3(1):146–153. doi: 10.1007/s12975-011-0108-y. [DOI] [PubMed] [Google Scholar]
- [268].Otsubo Y, Ito H, Shibuya T. Intracerebral monoamine concentration after ventriculoperitoneal shunting in the congenital hydrocephalus rat. Neurologia medico-chirurgica. 1997;37(9):669–676. doi: 10.2176/nmc.37.669. [DOI] [PubMed] [Google Scholar]
- [269].Del Bigio MR, Bruni JE, Vriend JP. Monoamine neurotransmitters and their metabolites in the mature rabbit brain following induction of hydrocephalus. Neurochemical research. 1998;23(11):1379–1386. doi: 10.1023/a:1020798622692. [DOI] [PubMed] [Google Scholar]
- [270].Ishizaki R, Tashiro Y, Inomoto T, Hashimoto N. Acute and subacute hydrocephalus in a rat neonatal model: correlation with functional injury of neurotransmitter systems. Pediatric neurosurgery. 2000;33(6):298–305. doi: 10.1159/000055975. [DOI] [PubMed] [Google Scholar]
- [271].Krafft PR, Altay O, Rolland WB, Duris K, Lekic T, Tang J, Zhang JH. alpha7 nicotinic acetylcholine receptor agonism confers neuroprotection through GSK-3beta inhibition in a mouse model of intracerebral hemorrhage. Stroke. 2012;43(3):844–850. doi: 10.1161/STROKEAHA.111.639989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Berger L, Gauthier S, Leblanc R. Akinetic mutism and parkinsonism associated with obstructive hydrocephalus. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1985;12(3):255–258. doi: 10.1017/s0317167100047119. [DOI] [PubMed] [Google Scholar]
- [273].Curran T, Lang AE. Parkinsonian syndromes associated with hydrocephalus: case reports, a review of the literature, and pathophysiological hypotheses. Movement disorders : official journal of the Movement Disorder Society. 1994;9(5):508–520. doi: 10.1002/mds.870090503. [DOI] [PubMed] [Google Scholar]
- [274].Krauss JK, Regel JP, Droste DW, Orszagh M, Borremans JJ, Vach W. Movement disorders in adult hydrocephalus. Movement disorders : official journal of the Movement Disorder Society. 1997;12(1):53–60. doi: 10.1002/mds.870120110. [DOI] [PubMed] [Google Scholar]
- [275].Racette BA, Esper GJ, Antenor J, Black KJ, Burkey A, Moerlein SM, Videen TO, Kotagal V, Ojemann JG, Perlmutter JS. Pathophysiology of parkinsonism due to hydrocephalus. Journal of neurology, neurosurgery, and psychiatry. 2004;75(11):1617–1619. doi: 10.1136/jnnp.2003.028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Tashiro Y, Drake JM, Chakrabortty S, Hattori T. Functional injury of cholinergic, GABAergic and dopaminergic systems in the basal ganglia of adult rat with kaolin-induced hydrocephalus. Brain research. 1997;770(1-2):45–52. doi: 10.1016/s0006-8993(97)00742-7. [DOI] [PubMed] [Google Scholar]
- [277].Hwang YS, Shim I, Chang JW. The behavioral change of locomotor activity in a kaolin-induced hydrocephalus rat model: evaluation of the effect on the dopaminergic system with progressive ventricle dilatation. Neuroscience letters. 2009;462(3):198–202. doi: 10.1016/j.neulet.2009.07.039. [DOI] [PubMed] [Google Scholar]
- [278].Tashiro Y, Drake JM. Reversibility of functionally injured neurotransmitter systems with shunt placement in hydrocephalic rats: implications for intellectual impairment in hydrocephalus. Journal of neurosurgery. 1998;88(4):709–717. doi: 10.3171/jns.1998.88.4.0709. [DOI] [PubMed] [Google Scholar]
- [279].Ouchi Y, Nakayama T, Kanno T, Yoshikawa E, Shinke T, Torizuka T. In vivo presynaptic and postsynaptic striatal dopamine functions in idiopathic normal pressure hydrocephalus. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2007;27(4):803–810. doi: 10.1038/sj.jcbfm.9600389. [DOI] [PubMed] [Google Scholar]
- [280].Nakayama T, Ouchi Y, Yoshikawa E, Sugihara G, Torizuka T, Tanaka K. Striatal D2 receptor availability after shunting in idiopathic normal pressure hydrocephalus. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48(12):1981–1986. doi: 10.2967/jnumed.107.045310. [DOI] [PubMed] [Google Scholar]
- [281].Del Bigio MR, Cardoso ER, Halliday WC. Neuropathological changes in chronic adult hydrocephalus: cortical biopsies and autopsy findings. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1997;24(2):121–126. doi: 10.1017/s0317167100021442. [DOI] [PubMed] [Google Scholar]
- [282].Auersperg A. Das Verhalten der Kerne am Boden des III. Ventrikels bei Hydrozephalus. Arb Neurologisch Inst Wein Univ. 1927;29:163–169. [Google Scholar]
- [283].Marburg O. Hydrocephalus. Its symptomology, pathology, pathogenesis and treatment. Oskar Piest; New York: 1940. [Google Scholar]
- [284].Antoniou AG, Emery JL. The infundibulum of the hypophysis in hydrocephalus. Zeitschrift fur Kinderchirurgie und Grenzgebiete. 1979;28(4):321–328. [PubMed] [Google Scholar]
- [285].Lopponen T, Paakko E, Laitinen J, Saukkonen AL, Serlo W, Tapanainen P, Ruokonen A, Pirttiniemi P, Poikela A, Knip M. Pituitary size and function in children and adolescents with shunted hydrocephalus. Clinical endocrinology. 1997;46(6):691–699. doi: 10.1046/j.1365-2265.1997.1931004.x. [DOI] [PubMed] [Google Scholar]
- [286].Abdolvahabi RM, Mitchell JA, Diaz FG, McAllister JP., 2nd A brief review of the effects of chronic hydrocephalus on the gonadotropin releasing hormone system: implications for amenorrhea and precocious puberty. Neurological research. 2000;22(1):123–126. doi: 10.1080/01616412.2000.11741047. [DOI] [PubMed] [Google Scholar]
- [287].Trollmann R, Strehl E, Wenzel D, Dorr HG. Does growth hormone (GH) enhance growth in GH-deficient children with myelomeningocele? The Journal of clinical endocrinology and metabolism. 2000;85(8):2740–2743. doi: 10.1210/jcem.85.8.6724. [DOI] [PubMed] [Google Scholar]
- [288].McAllister JP, 2nd, Abdolvahabi RM, Walker ML, Mitchell JA, Jones HC. Effects of congenital hydrocephalus on the hypothalamic gonadotrophin-releasing hormone system. Neurosurgical focus. 2007;22(4):E4. doi: 10.3171/foc.2007.22.4.5. [DOI] [PubMed] [Google Scholar]
- [289].Acikgoz B, Akpinar G, Bingol N, Usseli I. Angiotensin II receptor content within the circumventricular organs increases after experimental hydrocephalus in rats. Acta neurochirurgica. 1999;141(10):1095–1099. doi: 10.1007/s007010050489. [DOI] [PubMed] [Google Scholar]
- [290].Ehara K. In: Hydrocephalus: pathogenesis and treatment. Matsumoto ST, editor. Springer-Verlag; Tokyo: 1991. pp. 75–87. N. [Google Scholar]
- [291].Manaenko A, Fathali N, Khatibi NH, Lekic T, Hasegawa Y, Martin R, Tang J, Zhang JH. Arginine-vasopressin V1a receptor inhibition improves neurologic outcomes following an intracerebral hemorrhagic brain injury. Neurochem Int. 2011;58(4):542–548. doi: 10.1016/j.neuint.2011.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Manaenko A, Fathali N, Khatibi NH, Lekic T, Shum KJ, Martin R, Zhang JH, Tang J. Post-treatment with SR49059 improves outcomes following an intracerebral hemorrhagic stroke in mice. Acta neurochirurgica Supplement. 2011;111:191–196. doi: 10.1007/978-3-7091-0693-8_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [293].Manaenko A, Lekic T, Zhang JH, Tang J. NC1900, an arginine vasopressin analogue, fails to reduce brain edema and improve neurobehavioral deficits in an intracerebral hemorrhagic stroke mice model. Acta neurochirurgica Supplement. 2011;111:155–159. doi: 10.1007/978-3-7091-0693-8_26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [294].Hale PM, McAllister JP, 2nd, Katz SD, Wright LC, Lovely TJ, Miller DW, Wolfson BJ, Salotto AG, Shroff DV. Improvement of cortical morphology in infantile hydrocephalic animals after ventriculoperitoneal shunt placement. Neurosurgery. 1992;31(6):1085–1096. doi: 10.1227/00006123-199212000-00015. discussion 1096. [DOI] [PubMed] [Google Scholar]
- [295].Kriebel RM, McAllister JP., 2nd Pathology of the hippocampus in experimental feline infantile hydrocephalus. Neurological research. 2000;22(1):29–36. doi: 10.1080/01616412.2000.11741035. [DOI] [PubMed] [Google Scholar]
- [296].Kriebel RM, Shah AB, McAllister JP., 2nd The microstructure of cortical neuropil before and after decompression in experimental infantile hydrocephalus. Experimental neurology. 1993;119(1):89–98. doi: 10.1006/exnr.1993.1009. [DOI] [PubMed] [Google Scholar]
- [297].McAllister JP, 2nd, Maugans TA, Shah MV, Truex RC., Jr. Neuronal effects of experimentally induced hydrocephalus in newborn rats. Journal of neurosurgery. 1985;63(5):776–783. doi: 10.3171/jns.1985.63.5.0776. [DOI] [PubMed] [Google Scholar]
- [298].Harris NG, McAllister JP, 2nd, Conaughty JM, Jones HC. The effect of inherited hydrocephalus and shunt treatment on cortical pyramidal cell dendrites in the infant H-Tx rat. Experimental neurology. 1996;141(2):269–279. doi: 10.1006/exnr.1996.0161. [DOI] [PubMed] [Google Scholar]
- [299].Suzuki F, Handa J, Maeda T. Effects of congenital hydrocephalus on serotonergic input and barrel cytoarchitecture in the developing somatosensory cortex of rats. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1992;8(1):18–24. doi: 10.1007/BF00316557. [DOI] [PubMed] [Google Scholar]
- [300].Boillat CA, Jones HC, Kaiser GL, Harris NG. Ultrastructural changes in the deep cortical pyramidal cells of infant rats with inherited hydrocephalus and the effect of shunt treatment. Experimental neurology. 1997;147(2):377–388. doi: 10.1006/exnr.1997.6617. [DOI] [PubMed] [Google Scholar]
- [301].Miyazawa T, Nishiye H, Sato K, Kobayashi R, Hattori S, Shirai T, Obata K. Cortical synaptogenesis in congenitally hydrocephalic HTX-rats using monoclonal anti-synaptic vesicle protein antibody. Brain & development. 1992;14(2):75–79. doi: 10.1016/s0387-7604(12)80089-1. [DOI] [PubMed] [Google Scholar]
- [302].Suda K, Sato K, Miyazawa T, Arai H. Changes of synapserelated proteins (SVP-38 and drebrins) during development of brain in congenitally hydrocephalic HTX rats with and without early placement of ventriculoperitoneal shunt. Pediatric neurosurgery. 1994;20(1):50–56. doi: 10.1159/000120764. [DOI] [PubMed] [Google Scholar]
- [303].Suda K, Sato K, Takeda N, Miyazawa T, Arai H. Early ventriculoperitoneal shunt--effects on learning ability and synaptogenesis of the brain in congenitally hydrocephalic HTX rats. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1994;10(1):19–23. doi: 10.1007/BF00313580. [DOI] [PubMed] [Google Scholar]
- [304].Klinge P, Muhlendyck A, Lee S, Ludemann W, Groos S, Samii M, Brinker T. Temporal and regional profile of neuronal and glial cellular injury after induction of kaolin hydrocephalus. Acta neurochirurgica. Supplement. 2002;81:275–277. doi: 10.1007/978-3-7091-6738-0_71. [DOI] [PubMed] [Google Scholar]
- [305].Miyazawa T, Sato K. Hippocampal synaptogenesis in hydrocephalic HTX-rats using a monoclonal anti-synaptic vesicle protein antibody. Brain & development. 1994;16(6):432–436. doi: 10.1016/0387-7604(94)90002-7. [DOI] [PubMed] [Google Scholar]
- [306].Castejon OJ. Transmission electron microscope study of human hydrocephalic cerebral cortex. Journal of submicroscopic cytology and pathology. 1994;26(1):29–39. [PubMed] [Google Scholar]
- [307].Castejon OJ. Ultrastructural pathology of neuronal membranes in the oedematous human cerebral cortex. Journal of submicroscopic cytology and pathology. 2004;36(2):167–179. [PubMed] [Google Scholar]
- [308].Miyajima M, Sato K, Arai H. Choline acetyltransferase, nerve growth factor and cytokine levels are changed in congenitally hydrocephalic HTX rats. Pediatric neurosurgery. 1996;24(1):1–4. doi: 10.1159/000121007. [DOI] [PubMed] [Google Scholar]
- [309].Zhang YW, Del Bigio MR. Growth-associated protein-43 is increased in cerebrum of immature rats following induction of hydrocephalus. Neuroscience. 1998;86(3):847–854. doi: 10.1016/s0306-4522(98)00080-3. [DOI] [PubMed] [Google Scholar]
- [310].Shinoda M, Hidaka M, Lindqvist E, Soderstrom S, Matsumae M, Oi S, Sato O, Tsugane R, Ebendal T, Olson L. NGF, NT-3 and Trk C mRNAs, but not TrkA mRNA, are upregulated in the paraventricular structures in experimental hydrocephalus. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 2001;17(12):704–712. doi: 10.1007/s00381-001-0515-6. [DOI] [PubMed] [Google Scholar]
- [311].Balasubramaniam J, Del Bigio MR. Analysis of age-dependant alteration in the brain gene expression profile following induction of hydrocephalus in rats. Experimental neurology. 2002;173(1):105–113. doi: 10.1006/exnr.2001.7831. [DOI] [PubMed] [Google Scholar]
- [312].Ghose S. Optic nerve changes in hydrocephalus. Transactions of the ophthalmological societies of the United Kingdom. 1983;103:217–220. Pt 2. [PubMed] [Google Scholar]
- [313].Tytla ME, Buncic JR. Recovery of spatial vision following shunting for hydrocephalus. Archives of ophthalmology. 1990;108(5):701–704. doi: 10.1001/archopht.1990.01070070087041. [DOI] [PubMed] [Google Scholar]
- [314].Ito J, Saijo H, Araki A, Tanaka H, Tasaki T, Cho K, Miyamoto A. Neuroradiological assessment of visuoperceptual disturbance in children with spina bifida and hydrocephalus. Developmental medicine and child neurology. 1997;39(6):385–392. [PubMed] [Google Scholar]
- [315].van Eijsden P, Braun KP, Vandertop WP, Gooskens RH, Gispen WH, Biessels G. Visual evoked potentials and brainstem auditory evoked potentials in experimental hydrocephalus. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 2000;10(Suppl 1):47–48. [PubMed] [Google Scholar]
- [316].Ehle A, Sklar F. Visual evoked potentials in infants with hydrocephalus. Neurology. 1979;29(11):1541–1544. doi: 10.1212/wnl.29.11.1541. [DOI] [PubMed] [Google Scholar]
- [317].Coupland SG, Cochrane DD. Visual evoked potentials, intracranial pressure and ventricular size in hydrocephalus. Documenta ophthalmologica. Advances in ophthalmology. 1987;66(4):321–329. doi: 10.1007/BF00213660. [DOI] [PubMed] [Google Scholar]
- [318].Desch LW. Longitudinal stability of visual evoked potentials in children and adolescents with hydrocephalus. Developmental medicine and child neurology. 2001;43(2):113–117. doi: 10.1017/s0012162201000196. [DOI] [PubMed] [Google Scholar]
- [319].Shallat RF, Pawl RP, Jerva MJ. Significance of upward gaze palsy (Parinaud's syndrome) in hydrocephalus due to shunt malfunction. Journal of neurosurgery. 1973;38(6):717–721. doi: 10.3171/jns.1973.38.6.0717. [DOI] [PubMed] [Google Scholar]
- [320].Cinalli G, Sainte-Rose C, Simon I, Lot G, Sgouros S. Sylvian aqueduct syndrome and global rostral midbrain dysfunction associated with shunt malfunction. Journal of neurosurgery. 1999;90(2):227–236. doi: 10.3171/jns.1999.90.2.0227. [DOI] [PubMed] [Google Scholar]
- [321].Barkovich AJ, Newton TH. MR of aqueductal stenosis: evidence of a broad spectrum of tectal distortion. AJNR. American journal of neuroradiology. 1989;10(3):471–476. [PMC free article] [PubMed] [Google Scholar]
- [322].Ding Y, McAllister JP, 2nd, Yao B, Yan N, Canady AI. Neuron tolerance during hydrocephalus. Neuroscience. 2001;106(4):659–667. doi: 10.1016/s0306-4522(01)00166-x. [DOI] [PubMed] [Google Scholar]
- [323].Mori F, Tanji K, Yoshida Y, Wakabayashi K. Thalamic retrograde degeneration in the congenitally hydrocephalic rat is attributable to apoptotic cell death. Neuropathology : official journal of the Japanese Society of Neuropathology. 2002;22(3):186–193. doi: 10.1046/j.1440-1789.2002.00445.x. [DOI] [PubMed] [Google Scholar]
- [324].Weller RO, Wisniewski H. Histological and ultrastructural changes with experimental hydrocephalus in adult rabbits. Brain : a journal of neurology. 1969;92(4):819–828. doi: 10.1093/brain/92.4.819. [DOI] [PubMed] [Google Scholar]
- [325].Weller RO, Wisniewski H, Shulman K, Terry RD. Experimental hydrocephalus in young dogs: histological and ultrastructural study of the brain tissue damage. Journal of neuropathology and experimental neurology. 1971;30(4):613–626. [PubMed] [Google Scholar]
- [326].Gadsdon DRV, Emery JL. The effect of hydrocephalus upon the myelination of the corpus callosum. Zeitschrift fur Kinderchirurgie und Grenzgebiete. 1978;25:311–319. S. [PubMed] [Google Scholar]
- [327].Mataro M, Poca MA, Matarin M, Sahuquillo J, Sebastian N, Junque C. Corpus callosum functioning in patients with normal pressure hydrocephalus before and after surgery. Journal of neurology. 2006;253(5):625–630. doi: 10.1007/s00415-005-0073-z. [DOI] [PubMed] [Google Scholar]
- [328].Del Bigio MR, Zhang YW. Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Experimental neurology. 1998;154(1):157–169. doi: 10.1006/exnr.1998.6922. [DOI] [PubMed] [Google Scholar]
- [329].Shirai TI. In: Hydrocephalus pathogenesis and treatment. Matsumoto ST, editor. Springer-Verlag; Tokyo: 1991. pp. 36–45. K. N. [Google Scholar]
- [330].Eskandari R, McAllister JP, 2nd, Miller JM, Ding Y, Ham SD, Shearer DM, Way JS. Effects of hydrocephalus and ventriculoperitoneal shunt therapy on afferent and efferent connections in the feline sensorimotor cortex. Journal of neurosurgery. 2004;101(2 Suppl):196–210. doi: 10.3171/ped.2004.101.2.0196. [DOI] [PubMed] [Google Scholar]
- [331].Aoyama Y, Kinoshita Y, Yokota A, Hamada T. Neuronal damage in hydrocephalus and its restoration by shunt insertion in experimental hydrocephalus: a study involving the neurofilamentimmunostaining method. Journal of neurosurgery. 2006;104(5 Suppl):332–339. doi: 10.3171/ped.2006.104.5.332. [DOI] [PubMed] [Google Scholar]
- [332].Dammann O, Leviton A. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatric research. 1997;42(1):1–8. doi: 10.1203/00006450-199707000-00001. [DOI] [PubMed] [Google Scholar]
- [333].Xue M, Del Bigio MR. Immune pre-activation exacerbates hemorrhagic brain injury in immature mouse brain. J Neuroimmunol. 2005;165(1-2):75–82. doi: 10.1016/j.jneuroim.2005.04.016. [DOI] [PubMed] [Google Scholar]
- [334].Xue M, Del Bigio MR. Injections of blood, thrombin, and plasminogen more severely damage neonatal mouse brain than mature mouse brain. Brain Pathol. 2005;15(4):273–280. doi: 10.1111/j.1750-3639.2005.tb00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Balasubramaniam J, Xue M, Buist RJ, Ivanco TL, Natuik S, Del Bigio MR. Persistent motor deficit following infusion of autologous blood into the periventricular region of neonatal rats. Exp Neurol. 2006;197(1):122–132. doi: 10.1016/j.expneurol.2005.09.010. [DOI] [PubMed] [Google Scholar]
- [336].McCarty JH, Monahan-Earley RA, Brown LF, Keller M, Gerhardt H, Rubin K, Shani M, Dvorak HF, Wolburg H, Bader BL, Dvorak AM, Hynes RO. Defective associations between blood vessels and brain parenchyma lead to cerebral hemorrhage in mice lacking alphav integrins. Molecular and cellular biology. 2002;22(21):7667–7677. doi: 10.1128/MCB.22.21.7667-7677.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [337].Tajiri N, Dailey T, Metcalf C, Mosley YI, Lau T, Staples M, van Loveren H, Kim SU, Yamashima T, Yasuhara T, Date I, Kaneko Y, Borlongan CV. In vivo animal stroke models: a rationale for rodent and non-human primate models. Transl Stroke Res. 2013;4(3):308–321. doi: 10.1007/s12975-012-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [338].Al-Sulaiman AA, Ismail HM. Pattern of electroencephalographic abnormalities in children with hydrocephalus: a study of 68 patients. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1998;14(3):124–126. doi: 10.1007/s003810050193. [DOI] [PubMed] [Google Scholar]
- [339].Bali MS, Gebhardt-Henrich S, Keller P, Steiger A, Gattermann R, Bergamasco L, Kronen P, Doherr MG, Botteron C, Tomek A, Jaggy A. Electroencephalography in the diagnosis of hydrocephalus in golden hamsters (Mesocricetus auratus) Laboratory animals. 2008;42(2):213–221. doi: 10.1258/la.2007.007018. [DOI] [PubMed] [Google Scholar]
- [340].Zaaroor M, Bleich N, Chistyakov A, Pratt H, Feinsod M. Motor evoked potentials in the preoperative and postoperative assessment of normal pressure hydrocephalus. Journal of neurology, neurosurgery, and psychiatry. 1997;62(5):517–521. doi: 10.1136/jnnp.62.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Roricht S, Meyer BU, Woiciechowsky C, Lehmann R. Callosal and corticospinal tract function in patients with hydrocephalus: a morphometric and transcranial magnetic stimulation study. Journal of neurology. 1998;245(5):280–288. doi: 10.1007/s004150050219. [DOI] [PubMed] [Google Scholar]
- [342].Bucknall RM, Jones HC. Electrocorticogram and sensory evoked potentials in the young hydrocephalic H-Tx rat. Zeitschrift fur Kinderchirurgie : organ der Deutschen, der Schweizerischen und der Osterreichischen Gesellschaft fur Kinderchirurgie = Surgery in infancy and childhood. 1990;45(Suppl 1):8–10. doi: 10.1055/s-2008-1042623. [DOI] [PubMed] [Google Scholar]
- [343].Chiba A, Ohta Y, Oshio K, Inase M. Motor evoked potentials in rats with congenital hydrocephalus. Neurological research. 2003;25(3):305–308. doi: 10.1179/016164103101201391. [DOI] [PubMed] [Google Scholar]
- [344].Imamura K, Tanaka S, Ribot J, Kobayashi M, Yamamoto M, Nakadate K, Watanabe Y. Preservation of functional architecture in visual cortex of cats with experimentally induced hydrocephalus. The European journal of neuroscience. 2006;23(8):2087–2098. doi: 10.1111/j.1460-9568.2006.04729.x. [DOI] [PubMed] [Google Scholar]
- [345].Tsubokawa T, Katayama Y, Kawamata T. Impaired hippocampal plasticity in experimental chronic hydrocephalus. Brain injury : [BI] 1988;2(1):19–30. doi: 10.3109/02699058809150929. [DOI] [PubMed] [Google Scholar]
- [346].Yinon U, Chen M, Milgram A. Hydrocephalus in developing cats: physiological properties of visual cortex cells. Brain research bulletin. 1990;25(5):651–663. doi: 10.1016/0361-9230(90)90039-3. [DOI] [PubMed] [Google Scholar]
- [347].Miyazawa T, Sato K. Learning disability and impairment of synaptogenesis in HTX-rats with arrested shunt-dependent hydrocephalus. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1991;7(3):121–128. doi: 10.1007/BF00776706. [DOI] [PubMed] [Google Scholar]
- [348].Jones HC, Rivera KM, Harris NG. Learning deficits in congenitally hydrocephalic rats and prevention by early shunt treatment. Child's nervous system : ChNS : official journal of the International Society for Pediatric Neurosurgery. 1995;11(11):655–660. doi: 10.1007/BF00300725. [DOI] [PubMed] [Google Scholar]
- [349].Hawkins D, Bowers TM, Bannister CM, Miyan JA. The functional outcome of shunting H-Tx rat pups at different ages. European journal of pediatric surgery : official journal of Austrian Association of Pediatric Surgery ... [et al] = Zeitschrift fur Kinderchirurgie. 1997;7(Suppl 1):31–34. doi: 10.1055/s-2008-1071206. [DOI] [PubMed] [Google Scholar]
- [350].Barf HA, Verhoef M, Jennekens-Schinkel A, Post MW, Gooskens RH, Prevo AJ. Cognitive status of young adults with spina bifida. Developmental medicine and child neurology. 2003;45(12):813–820. doi: 10.1017/s0012162203001518. [DOI] [PubMed] [Google Scholar]
- [351].Chaudhry P, Kharkar S, Heidler-Gary J, Hillis AE, Newhart M, Kleinman JT, Davis C, Rigamonti D, Wang P, Irani DN, Williams MA. Characteristics and reversibility of dementia in Normal Pressure Hydrocephalus. Behavioural neurology. 2007;18(3):149–158. doi: 10.1155/2007/456281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [352].Lindquist B, Persson EK, Uvebrant P, Carlsson G. Learning, memory and executive functions in children with hydrocephalus. Acta Paediatr. 2008;97(5):596–601. doi: 10.1111/j.1651-2227.2008.00747.x. [DOI] [PubMed] [Google Scholar]
- [353].Shim I, Ha Y, Chung JY, Lee HJ, Yang KH, Chang JW. Association of learning and memory impairments with changes in the septohippocampal cholinergic system in rats with kaolininduced hydrocephalus. Neurosurgery. 2003;53(2):416–425. doi: 10.1227/01.neu.0000073989.07810.d8. discussion 425. [DOI] [PubMed] [Google Scholar]
- [354].Vachha B, Adams RC, Rollins NK. Limbic tract anomalies in pediatric myelomeningocele and Chiari II malformation: anatomic correlations with memory and learning--initial investigation. Radiology. 2006;240(1):194–202. doi: 10.1148/radiol.2401050674. [DOI] [PubMed] [Google Scholar]
- [355].Donnet A, Schmitt A, Dufour H, Giorgi R, Grisoli F. Differential patterns of cognitive impairment in patients with aqueductal stenosis and normal pressure hydrocephalus. Acta neurochirurgica. 2004;146(12):1301–1308. doi: 10.1007/s00701-004-0384-3. discussion 1308. [DOI] [PubMed] [Google Scholar]
- [356].Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- [357].Lekic T, Manaenko A, Rolland W, Krafft PR, Peters R, Hartman RE, Altay O, Tang J, Zhang JH. Rodent neonatal germinal matrix hemorrhage mimics the human brain injury, neurological consequences, and post-hemorrhagic hydrocephalus. Exp Neurol. 2012;236(1):69–78. doi: 10.1016/j.expneurol.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [358].Davison AN, Dobbing J. Myelination as a vulnerable period in brain development. Br Med Bull. 1966;22(1):40–44. doi: 10.1093/oxfordjournals.bmb.a070434. [DOI] [PubMed] [Google Scholar]
- [359].Eayrs JT, Goodhead B. Postnatal development of the cerebral cortex in the rat. J Anat. 1959;93:385–402. [PMC free article] [PubMed] [Google Scholar]
- [360].Altman J, Sudarshan K. Postnatal development of locomotion in the laboratory rat. Anim Behav. 1975;23(4):896–920. doi: 10.1016/0003-3472(75)90114-1. [DOI] [PubMed] [Google Scholar]
- [361].Bona E, Johansson BB, Hagberg H. Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in sevenday-old rats. Pediatr Res. 1997;42(5):678–683. doi: 10.1203/00006450-199711000-00021. [DOI] [PubMed] [Google Scholar]
- [362].Westerga J, Gramsbergen A. The development of locomotion in the rat. Brain Res Dev Brain Res. 1990;57(2):163–174. doi: 10.1016/0165-3806(90)90042-w. [DOI] [PubMed] [Google Scholar]
- [363].Fernandez-Lopez D, Faustino J, Derugin N, Vexler ZS. Acute and chronic vascular responses to experimental focal arterial stroke in the neonate rat. Transl Stroke Res. 2013;4(2):179–188. doi: 10.1007/s12975-012-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Kuchiwaki H, Nagasaka M, Inao S, Sugita K. Progression of kaolin-induced hydrocephalus and changes in performance of operant tasks by rats. Journal of the neurological sciences. 1994;121(1):32–38. doi: 10.1016/0022-510x(94)90153-8. [DOI] [PubMed] [Google Scholar]
- [365].Del Bigio MR, Crook CR, Buist R. Magnetic resonance imaging and behavioral analysis of immature rats with kaolininduced hydrocephalus: pre- and postshunting observations. Exp Neurol. 1997;148(1):256–264. doi: 10.1006/exnr.1997.6644. [DOI] [PubMed] [Google Scholar]
- [366].Blomsterwall E, Bilting M, Stephensen H, Wikkelso C. Gait abnormality is not the only motor disturbance in normal pressure hydrocephalus. Scandinavian journal of rehabilitation medicine. 1995;27(4):205–209. [PubMed] [Google Scholar]
- [367].Blomsterwall E, Svantesson U, Carlsson U, Tullberg M, Wikkelso C. Postural disturbance in patients with normal pressure hydrocephalus. Acta neurologica Scandinavica. 2000;102(5):284–291. doi: 10.1034/j.1600-0404.2000.102005284.x. [DOI] [PubMed] [Google Scholar]
- [368].Hetherington RD. Motor function profile in children with early onset hydrocephalus. Dev Neuropsych. 1999;15:25–51. M. [Google Scholar]
- [369].Ding Y, Lai Q, McAllister IJ, Canady AI. Impaired motor learning in children with hydrocephalus. Pediatric neurosurgery. 2001;34(4):182–189. doi: 10.1159/000056017. [DOI] [PubMed] [Google Scholar]
- [370].Kuba H, Inamura T, Ikezaki K, Inoha S, Nakamizo A, Shono T, Fukui K, Fukui M. Gait disturbance in patients with low pressure hydrocephalus. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia. 2002;9(1):33–36. doi: 10.1054/jocn.2001.1010. [DOI] [PubMed] [Google Scholar]
- [371].Chen W, Ma Q, Suzuki H, Hartman R, Tang J, Zhang JH. Osteopontin reduced hypoxia-ischemia neonatal brain injury by suppression of apoptosis in a rat pup model. Stroke. 2011;42(3):764–769. doi: 10.1161/STROKEAHA.110.599118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [372].Chen H, Burris M, Fajilan A, Spagnoli F, Zhang J, Tang J. Prolonged Exposure to Isoflurane Ameliorates Infarction Severity in the Rat Pup Model of Neonatal Hypoxia-Ischemia. Translational Stroke Research. 2011;2(3):382–390. doi: 10.1007/s12975-011-0081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [373].Adeoye O, Clark J, Khatri P, Wagner K, Zuccarello M, Pyne-Geithman G. Do Current Animal Models of Intracerebral Hemorrhage Mirror the Human Pathology? Translational Stroke Research. 2011;2(1):17–25. doi: 10.1007/s12975-010-0037-1. [DOI] [PubMed] [Google Scholar]
- [374].Lekic T, Tang J, Zhang JH. A rat model of pontine hemorrhage. Acta Neurochir Suppl. 2008;105:135–137. doi: 10.1007/978-3-211-09469-3_28. [DOI] [PubMed] [Google Scholar]
- [375].Lekic T, Tang J, Zhang JH. Rat model of intracerebellar hemorrhage. Acta Neurochir Suppl. 2008;105:131–134. doi: 10.1007/978-3-211-09469-3_27. [DOI] [PubMed] [Google Scholar]
- [376].Lekic T, Zhang JH. Posterior circulation stroke and animal models. Front Biosci-Landmrk. 2008;13:1827–1844. doi: 10.2741/2803. [DOI] [PubMed] [Google Scholar]
- [377].Hartman R, Lekic T, Rojas H, Tang J, Zhang JH. Assessing functional outcomes following intracerebral hemorrhage in rats. Brain Res. 2009;1280:148–157. doi: 10.1016/j.brainres.2009.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [378].Balasubramaniam J, Del Bigio MR. Animal models of germinal matrix hemorrhage. J Child Neurol. 2006;21(5):365–371. doi: 10.1177/08830738060210050201. [DOI] [PubMed] [Google Scholar]
- [379].Goddard J, Lewis RM, Armstrong DL, Zeller RS. Moderate, rapidly induced hypertension as a cause of intraventricular hemorrhage in the newborn beagle model. J Pediatr. 1980;96(6):1057–1060. doi: 10.1016/s0022-3476(80)80641-x. [DOI] [PubMed] [Google Scholar]
- [380].Conner ES, Lorenzo AV, Welch K, Dorval B. The role of intracranial hypotension in neonatal intraventricular hemorrhage. J Neurosurg. 1983;58(2):204–209. doi: 10.3171/jns.1983.58.2.0204. [DOI] [PubMed] [Google Scholar]
- [381].Batton DG, Nardis EE. The effect of intraventricular blood on cerebral blood flow in newborn dogs. Pediatr Res. 1987;21(5):511–515. doi: 10.1203/00006450-198705000-00018. [DOI] [PubMed] [Google Scholar]
- [382].Yoshioka H, Iino S, Sato N, Osamura T, Hasegawa K, Ochi M, Sawada T, Kusunoki T. New model of hemorrhagic hypoxicischemic encephalopathy in newborn mice. Pediatr Neurol. 1989;5(4):221–225. doi: 10.1016/0887-8994(89)90079-9. [DOI] [PubMed] [Google Scholar]
- [383].Goddard J, Lewis RM, Alcala H, Zeller RS. Intraventricular hemorrhage--an animal model. Biology of the neonate. 1980;37(1-2):39–52. doi: 10.1159/000241254. [DOI] [PubMed] [Google Scholar]
- [384].Lorenzo AV, Welch K, Conner S. Spontaneous germinal matrix and intraventricular hemorrhage in prematurely born rabbits. Journal of neurosurgery. 1982;56(3):404–410. doi: 10.3171/jns.1982.56.3.0404. [DOI] [PubMed] [Google Scholar]
- [385].Reynolds ML, Evans CA, Reynolds EO, Saunders NR, Durbin GM, Wigglesworth JS. Intracranial haemorrhage in the preterm sheep fetus. Early human development. 1979;3(2):163–186. doi: 10.1016/0378-3782(79)90005-7. [DOI] [PubMed] [Google Scholar]
- [386].Ment LR, Stewart WB, Duncan CC, Lambrecht R. Beagle puppy model of intraventricular hemorrhage. Journal of neurosurgery. 1982;57(2):219–223. doi: 10.3171/jns.1982.57.2.0219. [DOI] [PubMed] [Google Scholar]
- [387].Narayan RK, Narayan TM, Katz DA, Kornblith PL, Murano G. Lysis of intracranial hematomas with urokinase in a rabbit model. Journal of neurosurgery. 1985;62(4):580–586. doi: 10.3171/jns.1985.62.4.0580. [DOI] [PubMed] [Google Scholar]
- [388].Aquilina K, Hobbs C, Cherian S, Tucker A, Porter H, Whitelaw A, Thoresen M. A neonatal piglet model of intraventricular hemorrhage and posthemorrhagic ventricular dilation. J Neurosurg. 2007;107(2 Suppl):126–136. doi: 10.3171/PED-07/08/126. [DOI] [PubMed] [Google Scholar]
- [389].Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early human development. 1979;3(1):79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- [390].Kakita A, Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron. 1999;23(3):461–472. doi: 10.1016/s0896-6273(00)80800-4. [DOI] [PubMed] [Google Scholar]
- [391].Levers TE, Edgar JM, Price DJ. The fates of cells generated at the end of neurogenesis in developing mouse cortex. Journal of neurobiology. 2001;48(4):265–277. doi: 10.1002/neu.1056. [DOI] [PubMed] [Google Scholar]
- [392].Sturrock RR, Smart IH. A morphological study of the mouse subependymal layer from embryonic life to old age. Journal of anatomy. 1980;130(Pt 2):391–415. [PMC free article] [PubMed] [Google Scholar]
- [393].Fox WM. Reflex-ontogeny and behavioural development of the mouse. Animal behaviour. 1965;13(2):234–241. doi: 10.1016/0003-3472(65)90041-2. [DOI] [PubMed] [Google Scholar]
- [394].Welch K. The intracranial pressure in infants. J Neurosurg. 1980;52(5):693–699. doi: 10.3171/jns.1980.52.5.0693. [DOI] [PubMed] [Google Scholar]
- [395].Alles YC, Greggio S, Alles RM, Azevedo PN, Xavier LL, DaCosta JC. A novel preclinical rodent model of collagenaseinduced germinal matrix/intraventricular hemorrhage. Brain Res. 2010;1356:130–138. doi: 10.1016/j.brainres.2010.07.106. [DOI] [PubMed] [Google Scholar]
- [396].Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21(5):801–807. doi: 10.1161/01.str.21.5.801. [DOI] [PubMed] [Google Scholar]
- [397].Lekic T, Manaenko A, Rolland W, Tang J, Zhang JH. A novel preclinical model of germinal matrix hemorrhage using neonatal rats. Acta Neurochir Suppl. 2011;111:55–60. doi: 10.1007/978-3-7091-0693-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [398].Lekic T, Rolland W, Hartman R, Kamper J, Suzuki H, Tang J, Zhang JH. Characterization of the brain injury, neurobehavioral profiles, and histopathology in a rat model of cerebellar hemorrhage. Exp Neurol. 2011;227(1):96–103. doi: 10.1016/j.expneurol.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [399].Lekic T, Rolland W, Manaenko A, Krafft PR, Kamper JE, Suzuki H, Hartman RE, Tang J, Zhang JH. Evaluation of the hematoma consequences, neurobehavioral profiles, and histopathology in a rat model of pontine hemorrhage. J Neurosurg. 2012 doi: 10.3171/2012.10.JNS111836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [400].Krafft PR, Bailey EL, Lekic T, Rolland WB, Altay O, Tang J, Wardlaw JM, Zhang JH, Sudlow CL. Etiology of stroke and choice of models. Int J Stroke. 2012;7(5):398–406. doi: 10.1111/j.1747-4949.2012.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [401].Krafft PR, Rolland WB, Duris K, Lekic T, Campbell A, Tang J, Zhang JH. Modeling intracerebral hemorrhage in mice: injection of autologous blood or bacterial collagenase. J Vis Exp. 2012;(67):e4289. doi: 10.3791/4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [402].Lekic T, Ani C. Posterior circulation stroke: animal models and mechanism of disease. J Biomed Biotechnol. 2012;2012 doi: 10.1155/2012/587590. 587590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [403].Wang J, Dore S. Inflammation after intracerebral hemorrhage. J Cereb Blood Flow Metab. 2007;27(5):894–908. doi: 10.1038/sj.jcbfm.9600403. [DOI] [PubMed] [Google Scholar]
- [404].Chen Z, Gao C, Hua Y, Keep RF, Muraszko K, Xi G. Role of iron in brain injury after intraventricular hemorrhage. Stroke. 2011;42(2):465–470. doi: 10.1161/STROKEAHA.110.602755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [405].Pikus HJ, Levy ML, Gans W, Mendel E, McComb JG. Outcome, cost analysis, and long-term follow-up in preterm infants with massive grade IV germinal matrix hemorrhage and progressive hydrocephalus. Neurosurgery. 1997;40(5):983–988. doi: 10.1097/00006123-199705000-00021. discussion 988-989. [DOI] [PubMed] [Google Scholar]
- [406].Ayer A, Hwang B, Appelboom G, Connolly ES., Jr. Clinical Trials for Neuroprotective Therapies in Intracerebral Hemorrhage: A New Roadmap from Bench to Bedside. Translational Stroke Research. 2012;3(4):409–417. doi: 10.1007/s12975-012-0207-4. [DOI] [PubMed] [Google Scholar]
- [407].Dawood N, Jolicoeur P, Sharief SD. Postnatal brain growth and allometry in the rabbit Oryctolagus cuniculus. Growth, development, and aging : GDA. 1988;52(4):169–175. [PubMed] [Google Scholar]
- [408].Harel S, Watanabe K, Linke I, Schain RJ. Growth and development of the rabbit brain. Biology of the neonate. 1972;21(5):381–399. doi: 10.1159/000240527. [DOI] [PubMed] [Google Scholar]
- [409].Lorenzo AV, Welch K. Preterm rabbit model of intraventricular hemorrhage. Journal of neurosurgery. 1986;64(4):688–689. doi: 10.3171/jns.1986.64.4.0688. [DOI] [PubMed] [Google Scholar]
- [410].Sotrel A, Lorenzo AV. Ultrastructure of blood vessels in the ganglionic eminence of premature rabbits with spontaneous germinal matrix hemorrhages. Journal of neuropathology and experimental neurology. 1989;48(4):462–482. doi: 10.1097/00005072-198907000-00007. [DOI] [PubMed] [Google Scholar]
- [411].Greene CS, Jr., Lorenzo AV, Hornig G, Welch K. The lowering of cerebral spinal fluid and brain interstitial pressure of preterm and term rabbits by furosemide. Zeitschrift fur Kinderchirurgie : organ der Deutschen, der Schweizerischen und der Osterreichischen Gesellschaft fur Kinderchirurgie = Surgery in infancy and childhood. 1985;40(Suppl 1):5–8. doi: 10.1055/s-2008-1059755. [DOI] [PubMed] [Google Scholar]
- [412].Lorenzo AV, Greene CS, Jr., Hornig GW, Zavala LM, Welch K. The effect of furosemide on intracranial pressure and hemorrhage in preterm rabbits. Journal of neurosurgery. 1989;70(5):785–792. doi: 10.3171/jns.1989.70.5.0785. [DOI] [PubMed] [Google Scholar]
- [413].Sugimoto T, Yasuhara A, Matsumura T. Intracranial hemorrhage following administration of sodium bicarbonate in rabbits. Brain & development. 1981;3(3):297–303. doi: 10.1016/s0387-7604(81)80052-6. [DOI] [PubMed] [Google Scholar]
- [414].Coulter DM, LaPine T, Gooch WM., 3rd Intraventricular hemorrhage in the premature rabbit pup. Limitations of this animal model. Journal of neurosurgery. 1984;60(6):1243–1245. doi: 10.3171/jns.1984.60.6.1243. [DOI] [PubMed] [Google Scholar]
- [415].Pond WG, Boleman SL, Fiorotto ML, Ho H, Knabe DA, Mersmann HJ, Savell JW, Su DR. Perinatal ontogeny of brain growth in the domestic pig. Proc Soc Exp Biol Med. 2000;223(1):102–108. doi: 10.1177/153537020022300114. [DOI] [PubMed] [Google Scholar]
- [416].Busija DW, Leffler CW. Selective attenuation by perivascular blood of prostanoid-dependent cerebrovascular dilation in piglets. Stroke; a journal of cerebral circulation. 1991;22(4):484–488. doi: 10.1161/01.str.22.4.484. [DOI] [PubMed] [Google Scholar]
- [417].Parfenova H, Shibata M, Leffler CW. Subarachnoid blood causes pial arteriolar constriction in newborn pigs. Stroke; a journal of cerebral circulation. 1993;24(11):1729–1734. doi: 10.1161/01.str.24.11.1729. [DOI] [PubMed] [Google Scholar]
- [418].Yakubu MA, Shibata M, Leffler CW. Subarachnoid hematoma attenuates vasodilation and potentiates vasoconstriction induced by vasoactive agents in newborn pigs. Pediatric research. 1994;36(5):589–594. doi: 10.1203/00006450-199411000-00009. [DOI] [PubMed] [Google Scholar]
- [419].Yakubu MA, Shibata M, Leffler CW. Hematoma-induced enhanced cerebral vasoconstrictions to leukotriene C4 and endothelin-1 in piglets: role of prostanoids. Pediatric research. 1995;38(1):119–123. doi: 10.1203/00006450-199507000-00021. [DOI] [PubMed] [Google Scholar]
- [420].Yakubu MA, Leffler CW. Role of endothelin-1 in cerebral hematoma-induced modification of cerebral vascular reactivity in piglets. Brain research. 1996;734(1-2):149–156. [PubMed] [Google Scholar]
- [421].Shaver EG, Duhaime AC, Curtis M, Gennarelli LM, Barrett R. Experimental acute subdural hematoma in infant piglets. Pediatric neurosurgery. 1996;25(3):123–129. doi: 10.1159/000121109. [DOI] [PubMed] [Google Scholar]
- [422].Pourcyrous M, Parfenova H, Bada HS, Korones SB, Leffler CW. Changes in cerebral cyclic nucleotides and cerebral blood flow during prolonged asphyxia and recovery in newborn pigs. Pediatric research. 1997;41(5):617–623. doi: 10.1203/00006450-199705000-00003. [DOI] [PubMed] [Google Scholar]
- [423].Pourcyrous M, Parfenova H, Shibata M, Bada HS, Korones SB, Leffler CW. The effects of intraventricular/periventricular blood on cerebral 3',5'-cyclic adenosine monophosphate concentration and cerebrovascular reactivity in newborn pigs. Pediatric research. 1997;42(3):305–310. doi: 10.1203/00006450-199709000-00010. [DOI] [PubMed] [Google Scholar]
- [424].Farstad T, Odden JP, Bratlid D. Effect of intraventricular hemorrhage on pulmonary function in newborn piglets. Biology of the neonate. 1994;66(4):238–246. doi: 10.1159/000244113. [DOI] [PubMed] [Google Scholar]
- [425].Astrom KE. On the early development of the isocortex in fetal sheep. Progress in brain research. 1967;26:1–59. [PubMed] [Google Scholar]
- [426].Barlow RM. The foetal sheep: morphogenesis of the nervous system and histochemical aspects of myelination. The Journal of comparative neurology. 1969;135(3):249–262. doi: 10.1002/cne.901350302. [DOI] [PubMed] [Google Scholar]
- [427].Ting P, Yamaguchi S, Bacher JD, Killens RH, Myers RE. Failure to produce germinal matrix or intraventricular hemorrhage by hypoxia, hypo-, or hypervolemia. Experimental neurology. 1984;83(3):449–460. doi: 10.1016/0014-4886(84)90114-6. [DOI] [PubMed] [Google Scholar]
- [428].Wheeler AS, Sadri S, Gutsche BB, DeVore JS, David-Mian Z, Latyshevsky H. Intracranial hemorrhage following intravenous administration of sodium bicarbonate or saline solution in the newborn lamb asphyxiated in utero. Anesthesiology. 1979;51(6):517–521. doi: 10.1097/00000542-197912000-00007. [DOI] [PubMed] [Google Scholar]
- [429].Cohn HE, Sacks EJ, Heymann MA, Rudolph AM. Cardiovascular responses to hypoxemia and acidemia in fetal lambs. American journal of obstetrics and gynecology. 1974;120(6):817–824. doi: 10.1016/0002-9378(74)90587-0. [DOI] [PubMed] [Google Scholar]
- [430].Gleason CA, Short BL, Jones MD., Jr. Cerebral blood flow and metabolism during and after prolonged hypocapnia in newborn lambs. The Journal of pediatrics. 1989;115(2):309–314. doi: 10.1016/s0022-3476(89)80091-5. [DOI] [PubMed] [Google Scholar]
- [431].Ong BY, Greengrass R, Bose D, Gregory G, Palahniuk RJ. Acidemia impairs autoregulation of cerebral blood flow in newborn lambs. Canadian Anaesthetists' Society journal. 1986;33(1):5–9. doi: 10.1007/BF03010901. [DOI] [PubMed] [Google Scholar]
- [432].Turbeville DF, Bowen FW, Jr., Killam AP. Intracranial hemorrhages in kittens: hypernatremia versus hypoxia. The Journal of pediatrics. 1976;89(2):294–297. doi: 10.1016/s0022-3476(76)80471-4. [DOI] [PubMed] [Google Scholar]
- [433].Leuschen MP, Shuman RM, Nelson RM., Jr. The development of capillaries in the telencephalon of beagle puppies. The Anatomical record. 1984;208(3):435–443. doi: 10.1002/ar.1092080314. [DOI] [PubMed] [Google Scholar]
- [434].Leuschen MP, Nelson RM., Jr. Telencephalic microvessels of premature beagle pups. The Anatomical record. 1986;215(1):59–64. doi: 10.1002/ar.1092150109. [DOI] [PubMed] [Google Scholar]
- [435].Trommer BL, Groothuis DR, Pasternak JF. Quantitative analysis of cerebral vessels in the newborn puppy: the structure of germinal matrix vessels may predispose to hemorrhage. Pediatric research. 1987;22(1):23–28. doi: 10.1203/00006450-198707000-00007. [DOI] [PubMed] [Google Scholar]
- [436].Goddard-Finegold J, Armstrong D, Zeller RS. Intraventricular hemorrhage, following volume expansion after hypovolemic hypotension in the newborn beagle. The Journal of pediatrics. 1982;100(5):796–799. doi: 10.1016/s0022-3476(82)80596-9. [DOI] [PubMed] [Google Scholar]
- [437].Johnson DL, Getson P, Shaer C, O'Donnell R. Intraventricular hemorrhage in the newborn beagle puppy. A limited model of intraventricular hemorrhage in the premature infant. Pediatric neuroscience. 1987;13(2):78–83. doi: 10.1159/000120306. [DOI] [PubMed] [Google Scholar]
- [438].Goddard-Finegold J, Michael LH. Cerebral blood flow and experimental intraventricular hemorrhage. Pediatric research. 1984;18(1):7–11. [PubMed] [Google Scholar]
- [439].Pasternak JF, Groothuis DR, Fischer JM, Fischer DP. Regional cerebral blood flow in the beagle puppy model of neonatal intraventricular hemorrhage: studies during systemic hypertension. Neurology. 1983;33(5):559–566. doi: 10.1212/wnl.33.5.599. [DOI] [PubMed] [Google Scholar]
- [440].Pasternak JF, Groothuis DR. Autoregulation of cerebral blood flow in the newborn beagle puppy. Biology of the neonate. 1985;48(2):100–109. doi: 10.1159/000242160. [DOI] [PubMed] [Google Scholar]
- [441].McPhee AJ, Kotagal UR, Kleinman LI. Cerebrovascular hemodynamics during and after recovery from acute asphyxia in the newborn dog. Pediatric research. 1985;19(7):645–650. doi: 10.1203/00006450-198507000-00002. [DOI] [PubMed] [Google Scholar]
- [442].Leuschen MP, Nelson RM., Jr. Vasoconstriction in telencephalic microvessels: a response to one model for intraventricular hemorrhage in beagle pups. The Anatomical record. 1989;224(4):534–540. doi: 10.1002/ar.1092240411. [DOI] [PubMed] [Google Scholar]
- [443].Leuschen MP, Nelson RM., Jr. Effects of asphyxia on telencephalic microvessels of premature beagle pups. Journal of perinatology : official journal of the California Perinatal Association. 1987;7(2):93–99. [PubMed] [Google Scholar]
- [444].Ment LR, Stewart WB, Duncan CC, Scott DT, Lambrecht R. Beagle puppy model of intraventricular hemorrhage. Effect of indomethacin on cerebral blood flow. Journal of neurosurgery. 1983;58(6):857–862. doi: 10.3171/jns.1983.58.6.0857. [DOI] [PubMed] [Google Scholar]
- [445].Ment LR, Stewart WB, Scott DT, Duncan CC. Beagle puppy model of intraventricular hemorrhage: randomized indomethacin prevention trial. Neurology. 1983;33(2):179–184. doi: 10.1212/wnl.33.2.179. [DOI] [PubMed] [Google Scholar]
- [446].Ment LR, Stewart WB, Duncan CC, Scott DT, Lambrecht R. Beagle puppy model of intraventricular hemorrhage. Effect of indomethacin on local cerebral glucose utilization. Journal of neurosurgery. 1984;60(4):737–742. doi: 10.3171/jns.1984.60.4.0737. [DOI] [PubMed] [Google Scholar]
- [447].Ment LR, Stewart WB, Duncan CC. Beagle puppy model of intraventricular hemorrhage: ethamsylate studies. Prostaglandins. 1984;27(2):245–256. doi: 10.1016/0090-6980(84)90077-7. [DOI] [PubMed] [Google Scholar]
- [448].Ment LR, Stewart WB, Duncan CC. Beagle puppy model of intraventricular hemorrhage. Effect of superoxide dismutase on cerebral blood flow and prostaglandins. Journal of neurosurgery. 1985;62(4):563–569. doi: 10.3171/jns.1985.62.4.0563. [DOI] [PubMed] [Google Scholar]
- [449].Goddard-Finegold J, Donley DK, Adham BI, Michael LH. Phenobarbital and cerebral blood flow during hypertension in the newborn beagle. Pediatrics. 1990;86(4):501–508. [PubMed] [Google Scholar]
- [450].Tamura M, Hishi T, Ishii T, Wakita S, Oho S, Shibuya K, Miyasaka K, Takashima S. Comparison of the incidence of intracranial hemorrhage following conventional mechanical ventilation and high frequency oscillation in beagle puppies. Acta paediatrica Japonica; Overseas edition. 1992;34(4):398–403. doi: 10.1111/j.1442-200x.1992.tb00978.x. [DOI] [PubMed] [Google Scholar]
- [451].Bass T, Singer G, Slusser J, Liuzzi FJ. Radial glial interaction with cerebral germinal matrix capillaries in the fetal baboon. Experimental neurology. 1992;118(2):126–132. doi: 10.1016/0014-4886(92)90029-p. [DOI] [PubMed] [Google Scholar]
- [452].Dieni S, Inder T, Yoder B, Briscoe T, Camm E, Egan G, Denton D, Rees S. The pattern of cerebral injury in a primate model of preterm birth and neonatal intensive care. Journal of neuropathology and experimental neurology. 2004;63(12):1297–1309. doi: 10.1093/jnen/63.12.1297. [DOI] [PubMed] [Google Scholar]
- [453].Brand S, Rakic P. Genesis of the primate neostriatum: [3H]thymidine autoradiographic analysis of the time of neuron origin in the rhesus monkey. Neuroscience. 1979;4(6):767–778. doi: 10.1016/0306-4522(79)90005-8. [DOI] [PubMed] [Google Scholar]
- [454].Lenn NJ, Whitmore L. Gestational changes in the germinal matrix of the normal rhesus monkey fetus. Pediatric research. 1985;19(1):130–135. doi: 10.1203/00006450-198501000-00034. [DOI] [PubMed] [Google Scholar]
- [455].Brann AW, Jr., Myers RE. Central nervous system findings in the newborn monkey following severe in utero partial asphyxia. Neurology. 1975;25(4):327–338. doi: 10.1212/wnl.25.4.327. [DOI] [PubMed] [Google Scholar]
- [456].Myers RE. Four patterns of perinatal brain damage and their conditions of occurrence in primates. Advances in neurology. 1975;10:223–234. [PubMed] [Google Scholar]