Abstract
Intercellular calcium (Ca2+) waves (ICWs) represent the propagation of increases in intracellular Ca2+ through a syncytium of cells and appear to be a fundamental mechanism for coordinating multicellular responses. ICWs occur in a wide diversity of cells and have been extensively studied in vitro. More recent studies focus on ICWs in vivo. ICWs are triggered by a variety of stimuli and involve the release of Ca2+ from internal stores. The propagation of ICWs predominately involves cell communication with internal messengers moving via gap junctions or extracellular messengers mediating paracrine signaling. ICWs appear to be important in both normal physiology as well as pathophysiological processes in a variety of organs and tissues including brain, liver, retina, cochlea, and vascular tissue. We review here the mechanisms of initiation and propagation of ICWs, the key intra- and extracellular messengers (inositol 1,4,5-trisphosphate and ATP) mediating ICWs, and the proposed physiological functions of ICWs.
I. INTRODUCTION
Intercellular calcium (Ca2+) waves (ICWs) consist of increases in cytoplasmic Ca2+ ion concentration ([Ca2+]i) that are communicated between cells and appear as waves that spread radially from an initiating or trigger cell (FIGURE 1). In contrast, Ca2+ waves that only propagate within a single cell are called intracellular Ca2+ waves. The speed and size of ICWs depend on the nature and strength of the initiating stimulus as well as the mechanism of propagation. ICWs often propagate for periods of up to tens of seconds with speeds of ∼10–20 μm/s and consequently can involve tens to hundreds of contiguous cells (53, 96, 122, 123, 175, 306, 312). In some tissues that form an intrinsically excitable cell medium, such as hippocampal slices that consist of a mix of electrically excitable neurons and chemically excitable glia, ICWs can propagate with a spiral path (145).
Figure 1.

Examples of intercellular Ca2+ waves (ICWs) in cell cultures. A: an ICW in C6 glioma cells transduced to express the gap junction connexin, connexin (Cx) 26. The ICW was initiated by the focal photolytic release of inositol 1,4,5-trisphosphate (IP3) within a cell (panel 2s) at the location indicated by the star symbol. B: an ICW in rat brain endothelial cells (endogenously expressing Cx37 and Cx43) induced by exposure of the cells to extracellular Ca2+-free conditions. Dimension bars = 50 μm. Color bar indicates change in fluorescence level. An increase in fluorescence represents an increase in [Ca2+]i. Sample time of each panel is indicated in seconds.
The first major reports of ICWs appeared in 1990 and described Ca2+ waves propagating through cultures of astrocytes in response to extracellular glutamate (69) or through airway epithelial cells following mechanical stimulation of a single cell (308). Subsequently, ICWs have been found to be initiated by a variety of stimuli in a diversity of cell types, including glial cells (246, 312, 361), neurons (56, 153), various epithelial (171, 249) and endothelial (47, 129, 382) cells, smooth muscle cells (151, 407), cardiomyocytes (352), hepatocytes (122), osteocytes (178), chondrocytes (189), kidney cells (276, 402), mammary gland cells (104), mast cells (255), pancreatic acinar cells (409), and keratinocytes (188). FIGURE 1 illustrates typical examples of ICWs in glial and endothelial cells. While most of these ICWs have been observed in vitro in response to experimental stimuli, it is important to emphasize that ICWs also occur in vivo in organs such as the liver and brain (161, 238, 242, 292, 394, 410). However, the relationship of these in vivo ICWs with respect to normal organ function and physiological stimulation remains to be determined.
ICWs are complex spatiotemporal events that involve active signal transduction within and between cells but are limited by the passive physical characteristics of diffusion and cell architecture. As a result, our understanding of ICWs has arisen from investigations with real-time microscopy, molecular biology, pharmacology, and mathematical modeling. Interestingly, the use of mathematical models to understand intra- and intercellular Ca2+ waves has itself driven the further development of the predominantly time-domain models, which addressed mechanisms of Ca2+ oscillations, into two- and three-dimensional spatial models. In conjunction with complementary experimental approaches, modeling studies now form an essential tool for exploring the mechanisms and behavior of ICWs. Specifically, models allow us to estimate valid ranges of critical parameters that are difficult to experimentally measure (e.g., the permeability of gap junctions) and, importantly, make predictions that can be experimentally tested. Consequently, we present here an integrated view of ICWs based on results obtained with experimental and mathematical approaches, although most of the major concepts have arisen from original experimental observations.
It is now recognized that there are several key processes that determine the appearance and kinetics of ICWs; these are the mechanisms of 1) Ca2+ wave initiation, 2) Ca2+ wave propagation within cells (intracellular), 3) Ca2+ wave propagation between cells (intercellular), and 4) messenger regeneration for the propagation of the Ca2+ waves. We will consider each of these processes in turn.
II. Ca2+ WAVE INITIATION
ICWs can be initiated by focal stimulation of a single cell or by the global stimulation of a population of cells with selected chemical ligands or specific extracellular conditions. Focal initiation has been commonly achieved by gentle mechanical stimulation of a cell with a micropipette; this is proposed to result in membrane stress that triggers the production of inositol 1,4,5-trisphosphate (IP3) in the stimulated cell (33, 57, 306, 307, 387). Most studies utilizing mechanical stimulation have been performed with monolayer cell cultures, but mechanical stimulation has also been applied to more complex tissues; for example, mechanical stimulation of part of the brain isolated from embryonic rats results in large ICWs propagating over almost an entire hemisphere (394).
A disadvantage of mechanical stimulation is that it may lead to plasma membrane disruption; this would allow both Ca2+ entry into the cell and the liberation of cell constituents, such as ATP or other messengers, from the cell. The possibility of membrane disruption complicates the mechanistic analysis of any ensuing ICW, especially in studies that manually implement mechanical stimulation where the quantification of both the mechanical stimulus and the associated stimulus intensity-response is difficult. The original studies with airway epithelial cells were performed using a piezo-electric device that allowed the deflection of the mechanical stimulus to be increased (under voltage control) to generate a threshold pulse. Because mechanical stimulation is germane to mechanosensitive cells (e.g., osteocytes that utilize mechanical forces to regulate bone deposition and turnover), a more sophisticated method of mechanical stimulation involving nano-indentation of the plasma membrane with an atomic force microscope (165) has been developed for osteocyte stimulation. On the other hand, strong mechanical stimulation that results in cell injury might be relevant for the investigation of the role of ICWs in wound healing processes (72, 169, 257).
Alternatively, focal stimulation has been achieved by electrical stimulation (mostly in neural tissues) with an extracellular microelectrode (111, 139, 147, 316, 385) or the local release of messengers, such as ATP (7, 143, 316), endothelin-1 (385), glutamate (353, 385), and nitric oxide (NO) (396) by pressure ejection, iontophoresis, or flash photolysis. Focal stimulation can also be achieved at specific stages of the signal transduction cascade of ICWs by a steplike elevation of the intracellular concentration of signaling messengers such as IP3 or Ca2+ (FIGURE 1). This is usually accomplished by an intracellular injection of an inactive caged compound followed by its photolysis to release the active compound or by the focal application of a Ca2+ ionophore (33, 173, 210, 221, 261, 409, 415). Microinjection of recombinant Bax, a pro-apoptotic member of the Bcl-2 family of apoptotic proteins, also triggers ICWs, probably via Ca2+ release from mitochondria (50). Recent work from Nedergaard's group has demonstrated, in hippocampal brain slices, ICWs triggered by a local decrease in extracellular Ca2+ following the photoactivation of the Ca2+ buffer diazo-2 (371a). The ICW is initiated in astrocytes by the opening of connexin hemichannels and subsequent release of ATP (see section IVB). These nonmechanical stimuli allow for a better assessment of the stimulus intensity-response (210) and provide the ability to determine if the active messenger concentration is in the physiological, supraphysiological, or pathophysiological range.
Global stimulation can be achieved by applying mechanical stretch to cell monolayers (177), by uniformly exposing cells to a neurotransmitter [e.g., glutamate (57, 69) or serotonin (421)], or by reducing the extracellular Ca2+ concentration (11, 413). Interestingly, ICWs can also occur spontaneously without specific triggers. Spontaneous ICWs have been observed in retinal pigment epithelial cells (270), the intact retina in vitro and in vivo (193), brain slices of the developing neocortex (394), Bergmann glia (astrocytes in the molecular layer) of the in vivo cerebellum (161), and hippocampal astrocytes in vivo (192). The frequency of these spontaneous ICWs can be increased by lowering extracellular Ca2+ (343, 394, 413), an effect that may result from increased connexin hemichannel opening and ATP release (11) (see sect. IVB).
Unfortunately, the mechanisms initiating all these different ICWs are ill-defined and vary. In general, stimuli that bring about a local increase in [Ca2+]i are often wave-triggering events. However, in early work, it was found that mechanical stimulation in extracellular Ca2+-free conditions initiated an ICW even though the stimulated cell did not display an [Ca2+]i elevation (33, 35) (but opposing results have also been reported, Ref. 385). This indicated that an [Ca2+]i increase within the stimulated cell was not essential to trigger an ICW and led to the idea that a messenger other than Ca2+ was involved. It is now accepted that this alternative signal is the signaling messenger IP3, which serves as an agonist of Ca2+ release from the endoplasmic reticulum (ER) and underlies dynamic [Ca2+]i changes, including Ca2+ oscillations (23). An [Ca2+]i increase by itself can, in some cases, also trigger ICWs. A detailed analysis of the role of IP3 and Ca2+ as wave initiators and messengers of ICW propagation is presented below.
III. INTRACELLULAR Ca2+ WAVE PROPAGATION
The mechanism for the propagation of Ca2+ waves through cells is strongly dependent on the Ca2+ excitability of the cytosol and the diffusion properties of the messenger involved. Ca2+ excitability refers to the ability with which a small local transient elevation in [Ca2+]i can be amplified into a large spreading Ca2+ pulse or wave. To understand these amplification mechanisms, the characteristics and spatial distribution of the Ca2+ release channels involved must first be considered.
A. Ca2+ Release Channels
There are two major Ca2+-permeable receptor channels that reside within the internal membranes of most cells [usually the ER or sarcoplasmic reticulum (SR) membranes of muscle cells]; these are the IP3 receptor (IP3R) and the ryanodine receptor (RyR, named for its affinity for the plant alkaloid ryanodine). In addition, other Ca2+-permeable channels including polycystin-2 and two-pore channels can also contribute to [Ca2+]i increases. The properties of these channels are discussed below.
1. IP3Rs
IP3Rs are tetrameric assemblies of large subunits (310 kDa) that have six transmembrane domains. Each subunit has a mushroomlike structure; the larger dome (which includes the COOH and NH2 termini and comprises ∼85% of the protein) projects into the cytoplasm while the stalk corresponds to a transmembrane domain and luminal loop associated with the ER (364). The cytoplasmic domain contains an IP3 binding site and a regulatory/coupling region (118).
The gating of the IP3R involves the processes of activation, inhibition, and inactivation. These, in turn, are influenced by the cytoplasmic concentrations of IP3, Ca2+, H+, and ATP as well as by the redox and phosphorylation status, protein interactions, and the Ca2+ concentration in the ER lumen (118). The binding of IP3 to the IP3R increases its open probability, primarily by modifying their sensitivity to inhibition by Ca2+ (118). At low IP3 concentrations, the IP3R is very sensitive to Ca2+ inhibition. At higher IP3 concentrations, the susceptibility to Ca2+ inhibition is reduced and reversed, which leads to increased open probability. However, because there are three different IP3R isoforms (types 1, 2, and 3), the IP3 concentrations necessary for channel opening depend on the IP3 affinity of each isoform, and this can vary from tens to hundreds of nanomolar (118).
The [Ca2+]i dependence of IP3R open probability has a biphasic, bell-shaped relationship with a central peak at ∼200 nM for all three isoforms (26, 380). At concentrations <200 nM, Ca2+ progressively increases the open probability of IP3R channel that has been sensitized by the binding of IP3. This increasing sensitivity to Ca2+ thereby serves as a mechanism of Ca2+-induced Ca2+ release (CICR) (293). Ca2+ activation of type 1 IP3Rs is positively cooperative, giving a steep increase in open probability over a narrow [Ca2+]i range and is ideally suited for CICR. Type 3 IP3R channels have a broader [Ca2+]i activation range and a higher sensitivity to Ca2+ activation that is better suited for channel opening in response to small Ca2+ changes when the IP3 concentration is low. A consequence of this sensitivity of individual channels to Ca2+ is that it provides a mechanism for the activation and recruitment of nearby IP3Rs. A Ca2+ signal can spatially expand, from a localized Ca2+ blip at a single receptor (∼1 μm spread) to a Ca2+ puff formed by the cooperative opening of a number of IP3Rs (∼4 μm spread) to an intracellular Ca2+ wave that results in a global [Ca2+]i elevation (22, 36).
In contrast, higher Ca2+ concentrations (>200 nM) result in the progressive inhibition of IP3R opening. This mechanism serves to terminate the Ca2+ release process. While this effect appears to be mediated by two independent Ca2+-binding sites with different affinities for Ca2+ (224), it is not clear if these Ca2+ binding sites are part of the IP3R or an associated accessory protein. Some reports indicate the bell-shaped Ca2+ dependency is not universal for all receptor isoforms, with types 2 and 3 channels being insensitive to high [Ca2+]i (140, 283). However, this may result from experimental conditions using purified receptors where accessory proteins are absent.
IP3Rs are also influenced by the cytoplasmic ATP concentration. Although not required for channel activity, increased ATP concentrations potentiate channel activity by enhancing its sensitivity to Ca2+ activation. The effect of ATP is best characterized for types 1 and 3 IP3Rs. Luminal Ca2+ also influences the IP3R, with high luminal [Ca2+] inhibiting channel activity (25, 366).
2. RyRs
RyRs are homotetramers of large (>550 kDa) subunits that form a square structure around a central pore that also mediates and regulates Ca2+ release from the SR in muscle cells and ER in nonmuscle cells (201, 412). Like IP3Rs, they have a large cytoplasmic NH2-terminal domain that modulates channel gating and acts as a scaffold for interactions with regulatory proteins like calmodulin and FK506-binding protein. The COOH terminus is shorter and is involved in pore delineation within the SR/ER. There are three RyR isoforms: RyR1, RyR2, and RyR3. While the RyR3 is common in the brain and occurs in the spleen, heart, and testis, it is not well characterized. On the other hand, RyR1 and RyR2 are well characterized; RyR1 is predominately expressed in skeletal muscle, but is also found in other tissues, RyR2 is predominately expressed in cardiac muscle and widely expressed in nonmuscle tissues including smooth muscle, adrenal glands, lung, and brain.
Although similar in structure, the activation mechanisms of RyR1 and RyR2 are different. RyR1 is activated by a molecular reconfiguration mediated by the dihydropyridine receptor domain of plasma membrane Cav1.1 channels in response to membrane depolarization. In contrast, membrane depolarization in cardiac muscle activates Cav1.2 channels on t-tubule membranes to stimulate Ca2+ entry; this Ca2+ directly binds to and opens the RyR2. RyR open probability is also influenced by several other factors including, Mg2+, cADP-ribose, ATP, caffeine, ryanodine, FK506-binding protein, and ER/SR luminal Ca2+ (reviewed in Refs. 102, 201, 412).
While the initial opening trigger may differ, the open probability of RyRs (like IP3Rs) is enhanced (namely by CICR) and inhibited by Ca2+ in a biphasic, bell-shaped manner (412). For RyR1, enhancement occurs at submicromolar [Ca2+]i by binding to high-affinity Ca2+ sites (“A-site”) while inhibition occurs in the 100 μM to 1 mM range by binding to low-affinity sites (“I-site”) (228, 229). RyR2 require micromolar [Ca2+]i for their activation and above 1 mM [Ca2+]i for inhibition (203, 399). For Ca2+ wave propagation, this form of Ca2+ amplification via CICR through the RyR is important. Ca2+ can also influence RyRs indirectly, via Ca2+-calmodulin interactions that shift the Ca2+ dependence of RyR2 activation to higher [Ca2+]i, thus decreasing the probability of RyR2 opening (14, 400). Mg2+ competitively antagonizes Ca2+ binding at the A-site and acts in a cooperative manner with Ca2+ at the I-site, causing inhibition of CICR (204). Increased [Ca2+] in the ER/SR store lumen stimulates Ca2+ release via RyRs while a decrease of store [Ca2+] inactivates the RyR and contributes to termination of CICR (138, 365). Thus the susceptibility of RyR to CICR is higher upon increased loading of Ca2+ stores (38, 339).
As mentioned, a variety of compounds can alter RyR activity. cADP-ribose may act as a RyR activator in nonmuscle cells (230) by sensitizing the RyR to Ca2+ (206), but it is unclear if this is a direct or indirect effect. ATP and other adenine nucleotides can potentiate RyR Ca2+ release (226, 227). Caffeine also increases RyRs open probability by acting cooperatively with Ca2+ and ATP to increase their binding affinity (298). The immunosuppressants FK506 and rapamycin act via the FK506-binding protein (calstabin) to stabilize the closed state of the channel, thereby limiting CICR. The alkaloid ryanodine locks the RyR in an open subconductance state at low (nanomolar) concentrations and inhibits the RyR at high (100 μM) concentrations (412). These opposing effects are mediated by two different ryanodine-binding sites with distinct affinities.
3. CICR
It is clear that both IP3Rs and RyRs on the ER/SR are equipped with CICR mechanisms. CICR is mediated by a positive-feedback mechanism in which the local increase in Ca2+ acts on the receptors to further increase their open probability and enhance Ca2+ efflux (FIGURE 2). Although the IP3R and RyR are distributed throughout the cell (in the ER/SR), their distribution may not be uniform; RyRs can form clusters of at least four to six RyR channels (388) that together generate Ca2+ sparks (59), a brief localized increase in Ca2+ (∼2 μm spread). Similar clusters of IP3Rs lead to the generation of Ca2+ puffs. Alternatively, RyR and IP3Rs may form mixed clusters (282) whereby Ca2+ increases resulting from IP3Rs can stimulate adjacent RyRs. In addition, mitochondria may also contribute to CICR (298); increased [Ca2+]mito resulting from the uptake of Ca2+ released from the ER can open the mitochondrial permeability transition pore (PTP) which can serve as a mitochondrial Ca2+ release pore (291). Additionally, indirect ways of CICR are also possible, for example, via activation of PLC by Ca2+ (see sect. VIA).
Figure 2.
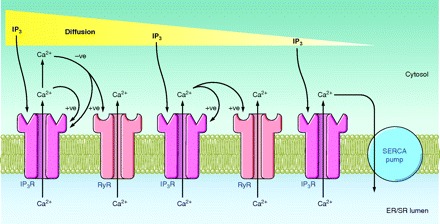
Basic mechanisms driving a diffusive Ca2+ wave across a cell (either initiating or communicating the wave; see sect. III). Inositol 1,4,5-trisphosphate (IP3) diffuses across the cell and binds to an IP3 receptor (IP3R) to stimulate Ca2+ release from the endoplasmic reticulum (ER). The positive feedback (+ve) of Ca2+ on the IP3R initiates Ca2+-induced Ca2+ release (CICR) through the IP3R. The elevated [Ca2+]i has a negative feedback (−ve) on the IP3R to terminate CICR and limit the [Ca2+]i increase. Some Ca2+ may diffuse to adjacent ryanodine receptors (RyR) to initiate CICR via these receptors. However, IP3 diffusion occurs more quickly than Ca2+ diffusion (due to cytosol protein buffers) and propagates the Ca2+ wave more efficiently. IP3 may have been produced in response to cell stimulation or may have entered the cell via a gap junction.
4. Other Ca2+ release channels
Polycystin-2 channels (PC-2) are members of the TRP channel family (TRPP2) that form Ca2+ release channels located on the ER (8, 37). They are activated by Ca2+ and may contribute to CICR in concert with IP3Rs and RyRs (9, 305). Exogenous expression of PC-2 potentiates IP3-triggered Ca2+ elevation (305) and may thus amplify the Ca2+ changes associated with ICWs.
Two-pore channels (TPC) are NAADP-gated Ca2+ release channels on acidic Ca2+ stores (lysosomes and/or endosomes). They do not contribute to CICR, but Ca2+ released via these channels may recruit activation of neighboring IP3Rs and RyRs, thereby generating an intracellular Ca2+ wave that transforms a local Ca2+ signal into a global Ca2+ signal (418). Direct interactions between TPCs and RyRs may further contribute to shaping the Ca2+ signal (267). It has been suggested that NAADP might function as a base signal, recruiting IP3Rs and RyRs (49).
B. Mechanisms of Ca2+ Amplification
The mechanism of intracellular Ca2+ wave propagation that was first experimentally described was based on the diffusion of IP3 from a restricted stimulation site. This type of Ca2+ wave was hypothesized to be initiated by mechanical stimulation and is reproduced by a transient, intracellular application of IP3 (by pressure injection, iontophoresis, or photo-activation of inactive caged IP3 precursors) (33) (FIGURE 1). Following its liberation, IP3 immediately diffuses in all directions, and in doing so, it encounters, binds to, and activates IP3Rs to release Ca2+ from the ER. As mentioned earlier, the IP3R is also sensitive to Ca2+. Consequently, a self-amplifying increase in [Ca2+]i, mediated by CICR via the IP3R, follows the diffusive spread or wave of IP3 (FIGURE 2). Subsequently, the IP3 signal is terminated by its dephosphorylation to IP2 by a 5-phosphatase or its phosphorylation to IP4 by a 3-kinase (323).
Because Ca2+ can also activate RyRs via CICR, it is possible that Ca2+ wave propagation may be enhanced by local increases and diffusion of Ca2+, especially if the IP3R and RyR are colocalized in receptor clusters. In fact, the gain of CICR determines the Ca2+ excitability of the cytoplasm and a larger gain increases the propensity of intracellular Ca2+ waves that are based on Ca2+ diffusion between Ca2+ release sites (38). Thus intracellular Ca2+ waves occur more frequently upon increased filling of the Ca2+ stores (217), upon exposure to cADP-ribose (401), caffeine (94), or a slight elevation of the intracellular IP3 concentration (not triggering a [Ca2+]i transient by itself). Thus the rate of intracellular Ca2+ wave propagation is a complex function of IP3 and Ca2+ diffusion, sequential CICR via sensitized IP3Rs and RyRs, and the distribution and composition of the ion channel/receptor clusters (FIGURE 2).
Because CICR through the IP3R and RyR is a self-limiting process and only continues until the ER/SR is empty or the channels have closed, the [Ca2+]i increase associated with a Ca2+ wave often has a similar amplitude at all locations across the cell. In contrast, the concentration of the underlying diffusive IP3 wave is believed to decrease with distance from the initiation site (proportional to the inverse distance squared for two dimensions) (FIGURE 2). Unfortunately, unlike the changes in [Ca2+]i, changes in [IP3]i are less easily visualized by imaging techniques because of the limitations of reporter dyes for IP3. However, it is important to point out that as long as the [IP3]i at the diffusive wave front remains greater than the threshold for activation of the IP3R (∼10–30 nM), Ca2+ release will continue to be initiated and a Ca2+ wave will appear to propagate without a degradation. In large cells, such as oocytes (diameter >100 μm), smooth muscle cells, or cardiomyocytes, the Ca2+ waves may have a trailing edge of decreasing [Ca2+]i which represents the termination of Ca2+ releases and return of most of the Ca2+ to the ER by the action of sarcoplasmic/endoplasmic reticulum Ca2+-ATPase (SERCA) (FIGURE 2). However, in small cells (diameter 10–20 μm), Ca2+ waves often do not have a trailing edge because the period of Ca2+ release persists for longer than the time taken for the Ca2+ wave to travel across the cell.
IV. INTERCELLULAR Ca2+ WAVE PROPAGATION
At the cell border, the cell membrane limits both IP3 and Ca2+ diffusion and hence CICR. Consequently, the transmission of a Ca2+ wave to the neighboring cell must occur by a modified or alternative mechanism. There are two major scenarios for this process. The mechanism originally characterized for cell-cell communication occurs via gap junctions. The mechanism subsequently discovered occurs by paracrine signaling with an extracellular messenger (FIGURE 3).
Figure 3.

Overall hypothesis for the communication of ICWs. Local stimulation (see sect. II) can lead to elevated IP3 in the initiator cell. As IP3 diffuses across this cell, it generates an intracellular Ca2+ wave by the release of Ca2+ through IP3Rs with amplification from RyRs. Diffusion of IP3 through a gap junction to an adjacent cell initiates a second intracellular Ca2+ wave, via IP3Rs and RyRs. In addition, or alternatively, the stimulated cell releases ATP (in either a Ca2+-dependent or -independent manner) via plasma membrane channels (hemichannels, maxi-anion channels) or vesicular release. The extracellular diffusion of ATP (or other messengers; see sect. IVB4) to adjacent cells activates P2 receptors which, in turn, stimulate IP3 production to generate a Ca2+ signal in the adjacent cell. Propagation may involve the regenerative release of ATP (see sect. VIB). Each mechanism can occur in isolation or synergistically.
A. Gap Junction Communication
Gap junctions are arrays of transmembrane channels that connect the cytoplasm of two adjacent cells and thereby provide a direct diffusion pathway between the cells. Cells sharing a gap junction channel each provide a hemichannel (also known as a connexon) that interacts, head-to-head, to form a complete gap junction channel (302). Each hemichannel is composed of proteins called connexins that exist as various isoforms encoded by 20 different genes in mouse and 21 genes in humans (332). Of these connexin (Cx) isoforms (named Cx23 through Cx62), Cx43 is the most ubiquitous (198). The properties of the connexins forming each gap junction determine the physiological characteristics of the gap junction; these properties include the permeability profile and the gating characteristics. The maximum molecular weight (MW) of compounds that are able to diffuse through gap junctions is in the order of 1,000–1,500. Thus gap junctions are believed to be readily permeable to Ca2+ (atomic weight, 40) and IP3 (MW, 486) (303). However, gap junction permeability for IP3 differs with connexin type, and this results in the permeability of IP3 through Cx32 gap junctions being greater than that through Cx43 gap junctions, which in turn is greater than that through Cx26 gap junctions (248).
The idea that ICWs may utilize gap junctions was initially suggested by the kinetics of the propagation of ICWs at cell boundaries in airway epithelial cells. Upon reaching the cell boundaries, these ICWs were delayed for periods of up to 1 second. The ICWs that were subsequently initiated in adjacent cells spread out from localized membrane sites all in contact with the initiating cell. Moreover, these ICWs did not initiate at other cytoplasmic sites. This need for cell contact was tested by examining the propagation of ICWs near discontinuities in the epithelium (usually created by scoring the monolayer). In airway epithelial cells, mechanically stimulated ICWs did not communicate across these spaces (Sanderson, unpublished results). Similarly, these ICWs were not influenced by a fluid flow over the apical surface of cells, whereas ICWs mediated by ATP release (e.g., from a micropipette) were biased in the direction of fluid flow (143).
Because it had been previously found, by freeze-fracture techniques and electrophysiology, that cultured airway epithelial cells possessed gap junctions, the influence of halothane, an inhibitor of gap junction conductivity, on ICW propagation was examined. In the presence of halothane, the initiating Ca2+ wave was restricted to the stimulated cell, a result implying a dependence of ICWs on functional gap junctions (308). However, caution is required when using gap junction blockers; a similar inhibition of ICWs has been reported with octanol or heptanol, but it appears that these compounds can also dampen IP3-mediated Ca2+ release or capacitative Ca2+ entry (184), effects that would appear similar to blocking the ICW.
Another experimental approach emphasizing the role of gap junctions in ICW propagation consisted of high-resolution imaging of HeLa cells transfected with Cx43 conjugated to green fluorescence protein (Cx43-GFP). These experiments demonstrated that the intracellular Ca2+ waves initiated in the receiving cell always started from a site of cell membrane contact with the donor cell that corresponded to the location of the gap junctions (259). In contrast, in ICWs mediated by paracrine ATP signaling (discussed later), the Ca2+ signals developing in the receiving cell could occur anywhere within the cell, but often initiated in the region of the perinuclear ER.
Mechanically induced ICWs were also found in cultured glial cells (57), and this led to the study of ICWs in glioma cells that lacked gap junctions (58). In these cells, mechanically stimulated Ca2+ waves were restricted to the stimulated cell (this restriction also implies that extracellular ATP is not released by mechanical stimulation mediated with a piezo-electric device). However, when glioma cells were transfected and demonstrated to express Cx43 gap junctions and electrical coupling, ICWs were again observed (FIGURE 1; similar results with Cx26). Furthermore, a correlation between increased connexin expression and increased distance of ICW propagation was observed (58). These data appeared to strongly support the concept of gap junction-mediated ICWs. However, as will be discussed later, the presence of connexins in glioma cells also appears to enhance ATP release. Therefore, with hindsight, these data were not definitive proof that gap junctions were the only route of communication.
The possibility that IP3 can diffuse between cells to generate ICWs was first indicated by the stimulation of an ICW following the iontophoresis of IP3 into a single cell of cultured epithelial cells (308) or a group of isolated hepatocytes (303). However, more convincing results were achieved by the microinjection of caged-IP3 into a single cell of a glial cell culture. As predicted, flash photolysis of the caged-IP3 within the injected cell initiated an ICW. Moreover, flash photolysis of a distal, noninjected cell, also initiated an ICW (210). The caged-IP3 used in these studies was not cell permeant. As a result, the most obvious mechanism for caged-IP3 to reach the distal cells was by diffusion through gap junctions. Caged-IP3 has a molecular weight slightly higher than that of IP3; this implies that IP3 may actually have a higher diffusion rate though the cells.
In keeping with this idea, cells with gap junctions composed of a mutant form of Cx26 (V84L-mutant channels, associated with deafness) that were impermeable to IP3 but otherwise had normal electrical properties and permeability for Lucifer Yellow (LY, a fluorescent dye with MW 457) failed to produce ICWs in response to IP3 injection (17). The idea that the propagating ICWs are the result of sequential activation of the IP3R is also supported by the inhibition of ICWs by the introduction of heparin, an IP3R antagonist, into contiguous epithelial cells by high-frequency electroporation (33). Collectively, these studies underscore the importance of gap junctions as a conduit for IP3 between cells.
1. Which messenger: IP3 or Ca2+?
The idea that IP3 can pass through gap junctions to mediate ICW propagation consistently leads to the proposition that Ca2+, because of its smaller size, must also serve as a messenger of ICWs. Indeed, the very appearance of Ca2+ waves spreading from one cell to the next suggests a large-scale movement of Ca2+. However, the relative importance of each messenger in the propagation of ICWs varies significantly with the characteristics of the cell system and the nature of the stimulation initiating the ICWs. Important cell characteristics affecting propagation include the extent of Ca2+ buffering, the distribution and sensitivity of IP3R and RyRs (contributing to CICR), and the number and type of gap junctions. The mode of stimulation may either be local (single cell) or global (multiple cells), and this dictates if the ICWs are propagating into cells in a quiescent or excited state.
Because Ca2+, but not IP3, is strongly buffered by cytoplasmic proteins, Ca2+ movement within a cell is very restricted and therefore considerably slower than that of IP3 (effective diffusion coefficients of ∼13 and ∼280 μm2/s for Ca2+ and IP3, respectively; Ref. 5). In fact, Ca2+ attrition as a result of binding with buffers requires that many more moles of Ca2+, compared with IP3, need to be released from a point source for Ca2+ to diffuse over similar distances. Thus IP3 is more likely to diffuse greater distances at faster speeds, compared with Ca2+, from point sources in cells, especially when encountering gap junctions that would greatly reduce the point source concentration of the messenger in the connected cells (64).
The inefficiency of Ca2+ to propagate ICWs between cells is also emphasized in cells that are directly connected via tunneling membrane nanotubes; these nanotubes do not contain gap junctions but are long (several tens of micrometers), thin membrane protrusions or tubes that directly connect the cytosol of one cell to a distant neighboring cell (81, 299). Tunneling nanotubes allow for the exchange of organelles and contain ER with IP3Rs. However, the propagation of a Ca2+ signal through these nanotubes cannot be driven by high [Ca2+] at one end but can occur in response to increased [IP3] and CICR via IP3Rs (327).
Because IP3 stimulates the release of Ca2+ from IP3Rs, it must be appreciated that a local increase in Ca2+ serves as an indicator of IP3 diffusion and not necessarily a bulk movement of Ca2+. As a result, it is more likely that IP3 diffusion into an adjacent cell will reignite CICR via the IP3R and/or the RyR to generate the observed Ca2+ response associated with the propagation of ICWs from a local stimulation through adjacent but quiescent cells (FIGURE 3).
The mostly likely scenario in which Ca2+ can serve as the primary intercellular messenger is in the presence of global agonists that results in a uniform, but low concentration of IP3, throughout the multiple exposed cells. Under these conditions, a local increase in Ca2+ in one cell could initiate an intracellular Ca2+ wave by stimulating CICR via IP3-sensitized IP3Rs (and RyRs). Ca2+ may then diffuse to an adjacent cell to initiate another Ca2+ wave. However, Ca2+ diffusion will still be restricted by cytosolic Ca2+ buffers, and this limitation of Ca2+ as an intercellular messenger is highlighted by the common observation that agonist-induced Ca2+ oscillations in multicellular systems (cells in culture or tissues that have gap junction communication) generally occur asynchronously. Under these conditions, where IP3 is elevated throughout the cells, oscillatory increases in [Ca2+]i do not commonly propagate to adjacent cells.
The possibility that Ca2+ can serve as a primary messenger is greatly enhanced by increased gap junction cell coupling. This allows greater amounts of Ca2+ to reach the adjacent cell and overcome the buffering capacity of the cytoplasm to allow Ca2+ to reach an ER receptor. Increased gap junction coupling can be achieved by increasing the permeability or numbers of the specific connexin channels involved. Examples of this type of Ca2+-based communication have been reported in pancreatic acinar cells (409) and blowfly salivary gland cells (420). A combination of two diffusive processes may also occur in which IP3 initially arrives at receptor clusters, but at concentrations too low to trigger significant Ca2+ release. However, this IP3 concentration may be sufficient to sensitize the IP3Rs to the following Ca2+ spike that can initiate CICR (421).
Of note here is the fact that cardiomyocytes which are highly coupled by gap junctions and have a high density of RyRs (∼100-fold greater than the density of IP3Rs) (185) only rarely display “slow” ICWs (199) (propagating at a velocity of 10–20 μm/s). Normally, these cells display much faster propagating increases in Ca2+ as a result of propagating membrane action potentials that activate membrane voltage-dependent Ca2+ channels and RyRs (via CICR). The result of such electrical pacing is that the Ca2+ content of the SR of cardiomyocyte is repetitively lowered and the RyR sensitivity to CICR is dampened. This, together with facts that the RyRs are located at some distance from the gap junctions (183) and that mitochondria, which buffer Ca2+ (216), are in close apposition to the intercalated disks (91), guards against the occurrence of spurious ICWs mediated by Ca2+ diffusion. However, when RyR sensitivity to Ca2+ is increased by Ca2+ store over-loading (either by β-adrenergic stimulation or increased Ca2+ influx), the propagation of spontaneous ICWs increases (199, 234) because of the greater chance that Ca2+ diffusion can initiate CICR. Under certain experimental conditions (i.e., cell culture), cardiomyocytes may propagate ICWs mediated by IP3 diffusion (352); this may be facilitated by the fact that IP3Rs are located more closely to the gap junctions (intercalated disks) than RyRs.
Mathematical modeling of ICWs (in 1 and 2 dimensions) in epithelial cells supported the idea that IP3 diffusion through gap junctions explains the kinetics of ICW propagation (328, 330). The key point addressed by these models was whether ICWs could be driven by IP3 diffusion if IP3 was generated only in the stimulated cell; IP3 regeneration in adjacent cells was excluded. This hypothesis accounts for why ICWs in epithelial cells have a limited propagation distance and appear to terminate at borders of specific cells. The modeling indicated that for ICW propagation to occur over about three to five cells, it was necessary for ∼3 μM IP3 to be generated in the stimulated cell if the IP3R sensitivity to IP3 was on the order of 30 nM. A feature of the initial models was that Ca2+ diffusion between cells was not required. However, diffusion of both IP3 and Ca2+ from one cell to the next has been included in later models.
Alterations in gap junction permeability, modeled as reduced diffusion rates of IP3/Ca2+ between cells, modified the propagation distance of the ICWs. There appears to be a minimal gap junction permeability required to allow ICW transmission between cells. Additionally, the gap junction permeability required for the propagation of ICWs is inversely related to the effective diffusion constant of the propagating messenger and the Ca2+ excitability of the cytosol (329, 330). Surprisingly, when Ca2+, instead of IP3, is assumed to be the primary messenger, the models predict that the critical gap junction permeability to obtain ICWs decreases with a decreasing effective Ca2+ diffusion coefficient. In other words, limited Ca2+ diffusion may be beneficial for ICW propagation (158). The explanation for this counterintuitive idea is that limited Ca2+ diffusion results in a higher localized Ca2+ concentration at the mouth of the gap junction in the adjacent cell and that this increases the chance of reigniting CICR. This scenario requires that the CICR sites (RyRs or IP3Rs) are close to the gap junctions. In addition, several other factors influence the critical gap junction permeability necessary to allow ICWs, including the CICR gain and the rate of Ca2+ sequestration by SERCA.
Further support for the idea that a diffusive wave of IP3 mediates ICWs was provided by the finding that glial cells, some distance from a stimulated cell, displayed Ca2+ oscillations (57). Modeling predicts that the IP3 concentration in these cells is in the correct range to support Ca2+ oscillations. This concept was subsequently confirmed by examining the responses of an individual glial cell to ICWs initiated in other cells at different distances from the observed cell (331, 344). When the initiating cell was close or far away, the Ca2+ increase was transient in keeping with the expected high or low diffusion concentration of IP3. However, at intermediate distances, the [Ca2+]i oscillated, reflecting an intermediate IP3 concentration compatible with the prediction that Ca2+ oscillations can only occur between bifurcated curves of IP3 concentration dependency (98, 393).
In addition to the diffusion of IP3 or Ca2+ between cells, cADP-ribose (MW, 541), an intracellular messenger that can sensitize RyRs to Ca2+, may mediate gap junction-propagated ICWs in lens cells (62) and astrocytes (211). However, these ICWs have slower kinetics and propagate shorter distances compared with ICWs based on IP3 diffusion.
At the other end of the spectrum, with respect to propagation speed, are fast ICWs that propagate between coupled cells in the order of 100 μm/s. While cardiomyocytes may be the best example of this, similar fast ICWs occur in vascular smooth muscle cells in response to the local application of phenylephrine; ICW propagation results from the passage of electrical current through gap junctions to depolarize cell membranes with the subsequent opening of voltage-operated Ca2+ channels coupled with CICR. The latter process is the rate-limiting step in the propagation of this specific kind of ICWs (186). Rapidly spreading ICWs have also been reported in arteriolar endothelial cells in vivo in transgenic mice expressing the Ca2+-sensitive probe GCaMP2 in endothelial cells under control of endothelial Cx40 (362). Micro-iontophoresis of acetylcholine was used to trigger the ICWs in endothelial cells, and these propagated through the endothelial cells along the vessel with a velocity of ∼116 μm/s, reaching distances up to ∼1 mm. These endothelial ICWs are associated with vasodilation and are hypothesized to regulate blood flow to the parenchyma by inducing upstream dilation of arterioles (87).
2. Does elevated [Ca2+]i inhibit intercellular Ca2+ wave propagation via gap junctions?
A paradox that frequently raises concern is how ICWs continue to propagate when an elevation in [Ca2+]i is reported to block gap junctions. Additionally, phosphatidylinositol 4,5-bisphosphate (PIP2) depletion (381) [resulting from phospholipase C (PLC) activation and IP3 production] and protein kinase (PKC) activation (103) also block gap junction communication. The idea that increases in [Ca2+]i may limit ICWs was tested in airway epithelial cells by mechanical stimulation in the presence of a global agonist. Upon exposure to extracellular ATP, airway epithelial cells displayed asynchronous Ca2+ oscillations, events that increase Ca2+, diacylglycerol (DAG) (to activate PKC) and reduce PIP2. However, mechanical stimulation of a single cell still initiated an ICW (105), indicating that gap junction communication remained sufficient to allow ICWs. One explanation is that the [Ca2+]i attained during the propagation of an ICW is significantly less than that necessary to block gap junctions, although it could be argued that [Ca2+]i may attain higher values within Ca2+ microdomains near the gap junction channel (372). The [Ca2+]i reported to result in inhibition of gap junctions varies widely, from ∼300 nM (73, 205, 218) to micromolar concentrations (297, 335), indicating there is not a single fixed threshold concentration for inhibition. In fact, the sensitivity to [Ca2+]i depends on the animal species (73), the cell type, and the connexins involved. Dakin et al. (78) reported that the source of [Ca2+]i elevation was also important, with capacitative Ca2+ entry causing uncoupling while ionomycin-based [Ca2+]i elevation was ineffective.
A second explanation for sustained ICW propagation lies in the temporal domain. If ICWs are mediated by IP3 diffusion, the diffusion wavefront of IP3 must run in advance of the increase in [Ca2+]i; hence, any Ca2+ effects on gap junctions would occur after the passage of IP3. A similar scenario is applicable to ICWs mediated by electrical changes where the propagating voltage moves rapidly compared with the Ca2+. Furthermore, an [Ca2+]i elevation of several minutes is often required to close gap junctions (194, 205), which is much longer than the time required for a typical ICW to travel over 100 μm (5–10 s at 10–20 μm/s). In line with this, Churchill et al. (63) reported that gap junction coupling between lens epithelial cells was not inhibited by the passage of an ICW but was inhibited by a sustained (several minutes) elevation of [Ca2+]i brought about by the Ca2+ ionophore ionomycin. Gap junction closure by [Ca2+]i elevation is thought to be mediated by Ca2+-calmodulin signaling (218, 272) and alteration of chemical gating of gap junction channels (their closure by high [Ca2+]i or low pHi) is, for unknown reasons, a process that takes several minutes (272). The closure of gap junctions by alterations in DAG or PIP2 levels or PKC activity is unlikely to occur in the nonstimulated adjacent cells propagating an ICW because these cells only respond to IP3 diffusion from the stimulated cell. However, gap junction closure in the stimulated cell, where IP3 and DAG are formed from PIP2, could shut-off the diffusion gradient derived from the stimulated cell and thereby reduce wave propagation distance; the extent of this effect would depend on the time delay until gap junction closure.
On the other hand, the inhibition of gap junctions does not necessarily mean that ICW propagation must be limited. Modeling studies have suggested that the partial closure of gap junctions may in fact result in larger waves (125). This contrarian response is predicted to be the result of a greater regeneration or build-up of IP3 in each cell (see sect. VIA) due to lower losses of IP3 or Ca2+ through partially closed gap junctions. As a result, high [IP3] within cells will ensure IP3 diffusion to neighboring cells and produce a [Ca2+]i elevation despite the lower degree of gap junction coupling.
Lastly, although [Ca2+]i elevation may under certain circumstances inhibit gap junctions and wave propagation, it is now clear that other mechanisms based on paracrine communication via extracellular messengers contribute to ICW propagation and that these can circumvent the gap junction pathway (discussed below).
B. Paracrine Communication
ICWs may also be propagated by paracrine signaling between adjacent cells. In this process, a signaling molecule, released by one cell, diffuses across the extracellular space between cells and, after binding to membrane receptors on neighboring cells, triggers a further elevation in [Ca2+]i (FIGURE 3). Early evidence for this mechanism came from the ability of ICWs to propagate across a cell-free zone and from the observation that an extracellular fluid flow could bias the direction of ICW propagation in the direction of flow (147). In contrast, ICWs relying on gap junctions are not influenced by an extracellular fluid flow (143). However, some caution is required when considering cells that have apical tight junctions; the release of an extracellular messenger into the interstitial space may, in this location, not be influenced by fluid flow across the apical surface of the cells. Sampling of the culture medium from cells after the initiation of an ICW revealed the presence of ATP and the local application of this sampled medium to cells using a micropipette triggered an ICW (137). It has now been demonstrated that ATP is a major extracellular messenger utilized by many cell types, including osteocytes (178), various epithelial (160) and endothelial (128, 370) cells, various cells in the organ of Corti in the inner ear (221, 277), glial cells (137), hepatocytes (317), keratinocytes (379), mast cells (255), and prostate cancer cells (309, 320). Many modeling approaches have also been developed to better understand paracrine ATP-based ICW propagation (18, 19, 166, 220, 337, 379, 392).
1. Mechanisms of ATP release
Advanced imaging techniques in combination with bioluminescent probes that detect ATP have revealed that ICWs are associated with a “cloud” of extracellular ATP (11, 245, 371a). After mechanical stimulation, ATP concentrations were ∼78 μM at the stimulation point but declined to ∼7 μM at a distance of 100 μm away (245). The propagation of the increase in extracellular ATP away from the stimulation point occurred at a speed faster (∼41 μm/s) than the propagation of the associated ICW (∼28 μm/s) (245). This indicates that the changes in [Ca2+]i lag behind the changes in extracellular [ATP], a finding consistent with modeling studies that predict delays inherent to a multistep diffusion-reaction scenario (19). The signaling steps include ATP release and diffusion; the sequential activation of purinergic receptors, G proteins, and PLC; the production of IP3; and the opening of IP3Rs to release Ca2+ from the ER.
While it is clear that ATP is released during ICWs, the mechanisms mediating ATP release remain controversial, mainly because there are numerous pathways available for ATP release; these include various ATP transporter proteins, vesicular discharge (39; reviewed in Ref. 280) and diffusive flow via ion channels or hemichannels (7). In addition, both Ca2+-dependent and Ca2+-independent ATP release has been reported (390). Several ATP release mechanisms are sensitive to mechanical stimulation or cell swelling, suggesting they may be directly activated by membrane stress in a Ca2+-independent manner. The diversity of ATP release pathways furthermore suggests there will be differences in ICW kinetics. In non-neuronal cells, vesicular ATP release typically occurs with a time scale of seconds (39) while nonvesicular release may be slower, taking minutes to occur (114). Additionally, several ATP release mechanisms may act in combination (280, 286) as well as a regenerative mechanism called ATP-induced ATP release (6) (see sect. VIB). Transporter proteins like the mdr-1 gene product (2) and the CFTR protein (287) have been demonstrated to behave as channels that pass ATP. Both proteins are present in astrocytes (13), while CFTR is found in a wide range of cell types including respiratory tract epithelial cells (253).
In neuronal cells, ATP release is typically vesicular in nature, often occurring in conjunction with the release of other neurotransmitters, but in nonneuronal cells, ATP release may occur by both vesicular and nonvesicular pathways (31, 237). Vesicular ATP release occurs in astrocytes (262, 416), endothelial cells (30), hepatocytes (112), osteoblasts (294), and retinal pigment epithelial cells (286). The identity of the vesicular pool that is released varies but includes quinacrine-labeled vesicles (30, 262, 294), bafilomycin A1-sensitive vesicles (112), or lysosomic vesicles (416).
Diffusive ATP release has been suggested to occur via a maxi-anion channel of unknown identity (301), by hemichannels composed of various connexin isoforms (including Cx26, Cx30, Cx32, Cx36, and Cx43) (7, 109, 179, 318, 326, 343, 374, 417) or pannexins (15, 162, 284, 336, 397), or by the combined action of these channels (121, 212). ATP release via connexin and pannexin hemichannels during ICW propagation has been reported to be Ca2+ dependent (83, 86, 215). The connexin hemichannels involved represent the fraction of hemichannels that have not paired-up to form complete gap junction channels and therefore reside in the cell membrane unopposed as large-conductance channels that are maintained in the closed state by a high (millimolar) extracellular Ca2+ concentration (107). These hemichannels can be opened by depolarization, alterations in their phosphorylation or redox status, and an [Ca2+]i elevation (67, 83, 86, 289). The pannexins involved have a topology that is similar to connexins but have no primary sequence homology. Importantly, their extracellular loops are glycosylated, and it is believed that this hinders the hemichannel interaction and the formation of pannexin gap junctions (74). There is some evidence for gap juction formation in cells overexpressing Panx1 (15b, 45a, 197a) or Panx3 (171a). The latter study also demonstrated ICWs mediated by Panx3 gap junctions. However, the pannexin family of channels has best been characterized as an ATP release pathway (171a, 15a, 215). Pannexin hemichannels composed of Panx1 have been reported to be the large-conductance pore recruited upon P2X7 receptor activation (271). Thus P2X7 receptors are equipped with both an ATP receptor as well as an associated ATP-release mechanism that together could contribute to ICW propagation (351). Finally, hemichannels are also equipped with inactivation mechanisms; for connexin hemichannels, channel closure occurs when [Ca2+]i rises above 500 nM (83, 86), and for pannexin hemichannels, channel closure occurs as a consequence of ATP-induced inhibitory feedback (281).
2. Mechanisms of ATP action
While the released ATP can activate both ionotropic (ligand-gated ion channels, i.e., P2X family) and metabotropic receptors (i.e., P2Y family), ICWs commonly appear to rely on the stimulation of metabotropic receptors. This involves the activation of G protein-coupled receptors, stimulation of PLC-β, and the generation of IP3 followed by Ca2+ release from the ER via the IP3R (FIGURE 3). Of the eight identified human P2Y receptors, five utilize the G protein Gq/11 to activate PLC-β (1). These receptors are the P2Y1, P2Y2, P2Y4, P2Y6, and P2Y11, and all have distinct affinities for nucleotide binding. The activation order for the P2Y1 receptor is ADP ≥ ATP; for the P2Y2 receptor, UTP = ATP; for the P2Y4 receptor, UTP > ATP; for the P2Y6 receptor, UDP > UTP > ADP; and for the P2Y11 receptor, ATP > ADP. Current data implicate the involvement of P2Y1, P2Y2, and P2Y4 receptors in ICWs and, as might be expected, the properties of the purinergic receptors involved are likely to influence the propagation properties of ICWs. For example, ICWs propagating via P2Y2 receptors appear to travel faster and further than ICWs propagating via P2Y1 receptors (119); this concept is supported by modeling studies (19).
Extracellular ATP is prone to rapid degradation to ADP and AMP by several classes of ecto-nucleotidases. Ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDase 1–8) degrade tri- and dinucleotides while ecto-nucleotide pyrophosphatase/phosphodiesterases (E-NPP 1–3) degrade tri-, di-, and mono-nucleotides yielding adenosine. Degradation of mono-nucleotides to adenosine is also catalyzed by ecto-5'-nucleotidase. The degradation kinetics to adenosine are characterized by a half-life (t½) on the order of 200 ms (for the hippocampus) (97). A consequence of this rapid degradation of extracellular ATP is that while ICWs may be initially mediated by ATP acting on P2Y2 receptors, their propagation may be sustained by ADP activating P2Y1 receptors. This multiagonist/receptor combination appears to propagate ICWs further than waves relying only on ATP and P2Y2 receptors (119). The experimental presence of the nucleotidase apyrase (to induce rapid breakdown of ATP) blocked ICWs mediated by P2Y2 receptors but accelerated ICWs based on P2Y1 receptors (ADP generated from ATP) (119). Thus the paracrine contribution to ICW propagation is a complex function of the rates of ATP release and metabolism, the spectrum of purinergic receptors present, and their varying affinities for ATP and its metabolites.
3. Cross talk between connexins and purinergic signaling
After the initial reports that ICWs involve both gap junctions and purinergic signaling, mediated in many cases by ATP release via hemichannels, it was discovered that components of the purinergic signaling cascade were influenced by the expression of connexins. For example, suppression of Cx43 in spinal cord astrocytes induced a change from an ADP-sensitive P2Y1 to an UTP-sensitive P2Y4 receptor subtype (314, 349). Conversely, overexpression of Cx43 increased the size of ICWs (71) by enhancing ATP release via Cx43 hemichannels rather than by increasing gap junction coupling (70). The influence of Cx43 on P2Y1 receptors may be mediated via signaling through the COOH-terminal domain of Cx43 (311).
4. Alternative extracellular messengers
Although ATP appears to be the most common paracrine messenger, ICWs may also be communicated between glial cells by diffusion of extracellular glutamate (93, 168, 263). NO release and downstream PKG signaling are also proposed for paracrine ICW propagation (396). Extracellular Ca2+ may function as a paracrine messenger by acting on membrane-bound Ca2+-sensing receptors (156). When cells recover from an [Ca2+]i elevation, most cytosolic Ca2+ is resequestered into the ER by SERCA, but some Ca2+ is pumped out of the cell by plasma membrane Ca2+-ATPase (PMCA). Because of the small volume of the interstitial space between cells, this can result in a localized elevation of extracellular [Ca2+] that can stimulate Ca2+-sensing receptors of neighbor cells, and thereby trigger PLC activation, IP3 formation and an ICW (155). Modeling studies have further analyzed this specific kind of paracrine ICW propagation (182).
C. Spatial Characteristics of Gap Junction and Paracrine Intercellular Ca2+ Waves
In addition to the observation that gap junction-based ICWs typically experience a delay in propagating from one cell to the next (419), these ICWs frequently follow a convoluted pathway through multiple cells because of heterogeneities in the spatial distribution of the gap junctions and the degree of cell-cell coupling. In contrast, paracrine-based ICWs often propagate in a more homogeneous manner characterized by a circular wavefront, but may also be influenced by heterogeneities in the densities of plasma membrane receptors. It is not uncommon for individual cells, probably because of a lack of appropriate receptors, to be bypassed and not exhibit a Ca2+ wave, a behavior reiterating the ability of paracrine-based ICWs to propagate across cell-free zones.
Interestingly, despite these differences in propagation pathways, the propagation velocity of gap junction- or paracrine-based ICWs is similar (10–20 μm/s). This is presumably because the molecular weights and therefore the nonbuffered diffusion constants of IP3, ATP, and glutamate (486, 507, and 292, respectively) are also similar.
D. Combined Gap Junction and Paracrine Intercellular Ca2+ Waves
Although we have addressed the propagation mechanisms of gap junction- and paracrine-based ICWs separately, it is important to emphasize that, in many cells, these two mechanisms are simultaneously active and may act synergistically (7, 42, 270, 317, 370). Suadicani et al. (350) demonstrated, by individual or combined overexpression of P2Y receptors and Cx43, that ICWs utilizing both gap junctions and purinergic signaling have a more coordinated appearance and are less “saltatory” than waves utilizing paracrine signaling only (350). Thus a propagating ICW can be driven by IP3 diffusing via gap junctions, while ATP is released via Ca2+-dependent or -independent mechanisms. ICWs that rely exclusively on IP3 diffusion via gap junctions propagate only limited distances (∼up to 5 cells), although this depends on the amount of IP3 generated and the degree of gap junction coupling. In contrast, ICWs relying on paracrine mechanisms propagate greater distances; this is explained by the hypothesis that paracrine signaling often involves a regenerative process to amplify messenger (ATP) release (discussed later) (FIGURE 3). However, stochastic two-dimensional simulations of an ICW have suggested that the combination of gap junction and purinergic signaling is sufficient to generate ICWs with large propagation distances without the need for messenger regeneration (166). The exact contribution of gap junctions or paracrine mechanisms to ICW propagation also appears to correlate with cell differentiation since ICWs in osteoblast-like cells shifted from being predominantly mediated by P2Y receptors to being mediated by gap junctions as cells differentiated (152). The contribution of gap junctions and paracrine signaling to ICW propagation between different cell types obtained from in vitro cell culture studies is summarized in TABLE 1.
Table 1.
Intercellular Ca2+ wave propagation between different cell types
| Cell Types Involved | Propagation Mechanism | Reference Nos. |
|---|---|---|
| Astrocytes to neurons | Gap junction-based | 244 |
| Astrocytes to neurons | Glutamate-based paracrine signaling | 263 |
| Astrocytes to neurons | ATP-based signaling | 371a |
| Astrocytes and endothelial cells | Gap junction- and ATP-based signaling | 41, 210, 257 |
| Astrocytes and menigeal cells | Gap junction- and ATP-based signaling | 135 |
| Astrocytes and microglial cells | ATP-based signaling | 316, 386 |
| Astrocytes to Müller cells | ATP-based signaling | 245 |
| Astrocytes to oligodendrocytes | 266 | |
| Satellite glial cells (SGC) and neurons (N) in trigeminal ganglia | SGC to N: gap junction- and ATP-based; N to SGC: ATP-based signaling | 348 |
| Hepatocytes to bile duct epithelial cells | ATP-based signaling | 317 |
| Vascular smooth muscle cells and endothelial cells | Gap junction-based | 170 |
| Supporting cells in the cochlea trigger Ca2+ spikes in inner hair cells in explant cultures | ATP-based signaling | 376, 377 |
All examples listed refer to cell culture studies.
E. Intercellular Ca2+ Waves and Ca2+ Oscillations
As previously mentioned, a consequence of IP3 diffusion through multiple cells during ICW propagation is the initiation of Ca2+ oscillations. In glial cells, these Ca2+ oscillations occurred at spatial locations that reflect optimum IP3 concentrations within cells to sustain Ca2+ oscillations (57, 344) and because the [IP3]i determines the Ca2+ oscillation frequency, the Ca2+ oscillations have unique formats in different cellular zones (331). This behavior may provide cells with specific spatial cues to stimulate an appropriate response to the passage of an ICW. The Ca2+ oscillations themselves may also generate coordinating pulses of ATP leading to ICWs (344) or become phase-locked between cells via gap junction coupling (134, 146, 157, 310, 421). The passage of messengers via gap junctions is known to influence the frequency of the Ca2+ oscillations and the number of cells displaying Ca2+ oscillations (110, 310, 338). On the other hand, strong gap junction coupling appears to curtail glucose-induced Ca2+ oscillations in the pancreas to reduce insulin secretion (378). This effect probably results from the tendency for [Ca2+]i to equalize in communicating cells which disturbs the Ca2+ oscillation dynamics of the individual cell. Additionally, purinergic paracrine communication is known to influence Ca2+ oscillations by exerting a synchronizing influence (150, 187).
V. PASSIVE AND ACTIVE WAVES
ICWs that exclusively rely on the production of IP3 and its diffusion via gap junctions or on the release of ATP and its diffusion in the extracellular space are termed diffusive Ca2+ waves. They are based on a messenger concentration gradient that decreases away from the stimulation point, in much the same way as an electrotonic spread of electrical charge over a length of membrane or cells. As long as the messenger concentration is above the threshold for activation of the target receptor, an [Ca2+]i increase is triggered. Thus single cell stimulation establishes a cytoplasmic or extracellular messenger concentration gradient over various rows of cells, creating the appearance of a cell-to-cell propagating Ca2+ signal. Diffusive Ca2+ waves are also called passive Ca2+ waves; these waves have a wavefront velocity that decreases with distance from the stimulation point because the difference in messenger concentration between two adjacent cells also decreases away from the stimulation point. When the wave propagation front reaches a point where the messenger concentration is approaching the threshold concentration for receptor stimulation, the [Ca2+]i transients will disappear and the wave will stop. Diffusive ICWs provoked by a steplike increase in IP3 in a single cell often have a limited degree of travel.
When this passive mechanism of propagation is combined with a mechanism of messenger regeneration, the wave propagation becomes active. Active ICWs propagate further than ICWs based on a purely passive/diffusive mechanism. Moreover, the wavefront displays a more constant propagation velocity because the underlying messenger is regenerated in (or outside) each cell and thereby maintains a more constant concentration difference between adjacent cells. As a consequence, the amplitude of the [Ca2+]i transients is expected to be rather constant in cells at progressively further distances from the stimulation point. Theoretically, if messenger regeneration is complete within each cell contributing to the wave, the wave would propagate until it encounters a physical boundary. This propagation mechanism reflects that of action potentials which propagate in an all-or-nothing manner (no loss of amplitude) along meter-long axons. However, ICWs are often based on a combination of passive and active propagation, and the waves arrest at discrete points. In mathematical terms, purely active waves are called traveling waves. Because the degree of involvement of messenger regeneration in ICW propagation is difficult to measure in biological experiments, mathematical modeling is frequently used to estimate regeneration parameters to provide a “best fit” with the experimental data. This approach has led to important insights such as the concept of “limited regenerative signaling” that demonstrates that a limited degree of messenger regeneration is sufficient to produce ICWs that are comparable in size to those experimentally observed and that regenerative mechanisms exert an important regulation of wave propagation (159).
VI. MESSENGER REGENERATION IN ACTIVE INTERCELLULAR Ca2+ WAVES
A. Internal Messengers
The generation of IP3 by PLC-β, PLC-γ, and PLC-δ is a Ca2+-dependent process, but the requirement for Ca2+ (Kd) differs between PLC subtypes; PLC-β and PLC-γ have a low Ca2+ requirement (285), and these enzymes can be active at resting [Ca2+]i. In contrast, the Kd of PLC-δ1 for Ca2+ is on the order of 1 μM and is thus activated by micromolar [Ca2+]i. PLC-δ activity is also activated by PIP2 but inhibited by IP3 (285). As a result, PLC-δ is proposed to contribute to IP3 regeneration during ICW propagation; IP3 entering a cell via gap junctions triggers an [Ca2+]i increase that, in turn, would activate PLC-δ and IP3 synthesis to enhance ICW velocity and propagation distance by maintaining an elevated IP3 concentration difference between adjacent cells. Presumably, the elevated IP3 concentration can also serve as a negative-feedback mechanism to limit PLC-δ activity. The extent to which IP3 regeneration by PLC-δ contributes to ICW propagation is not well understood, but the inclusion of a limited regenerative process in mathematical models of ICWs often improves the fit to experimental data (159). ICWs based on IP3 regeneration have been described in clusters of cells in nonconfluent cultures of vascular endothelial cell (95).
B. External Messengers
An early observation made with paracrine-based ICWs that crossed cell-free zones was that the width of the cell-free zone had little effect on ICW propagation in the distal cells (147). This implied that the concentration of the extracellular messenger on the far side of the cell-free zone was rejuvenated regardless of where the ICW was initiated. Most evidence for the regeneration of an extracellular messenger pertains to ATP. The observation that suramin (a nonspecific blocker of P2 receptors) decreased the size of the extracellular ATP wave (245) led to the proposal that ATP regeneration was involved (193). In addition, the relatively small amount of intracellular ATP within a single cell and the relatively small fraction of this ATP that is released by the stimulated cell both indicate a need for ATP regeneration to drive extensive ICWs (392).
A Ca2+-mediated process is an obvious way to regulate ATP regeneration, and this may take the form of Ca2+-dependent ATP release via connexin or pannexin hemichannels (83, 86, 215). At the present time, it is not clear if vesicular ATP release is a Ca2+-dependent process (see sect. IVB). In contrast, a Ca2+-independent mechanism of ATP-induced ATP release mediates ATP regeneration in astrocytes (6). This mechanism, detected with radioactive-labeled ATP in combination with P2 receptor inhibitors, involved undefined P2 receptors (EC50 for activation ∼144 μM) and is consistent with a suramin-induced decrease in ATP wave size (245). Although multiple P2 receptors may be involved, P2X7 receptors that are associated with large ATP-permeable pores or release channels, perhaps consisting of Panx1 hemichannels (167, 271), are likely key components. Other ionotropic ATP receptors (e.g., P2X1 and P2X4) have also been linked to the opening of Panx1 hemichannels (397).
On the other hand, ATP regeneration may not occur in all systems. Real-time bioluminescence imaging of ATP (detected with luciferin/luciferase activity) in various cells (rat and human astrocytes, human bronchial epithelial cells, and human umbilical vein endothelial cells) indicated that, in the absence of extracellular Ca2+, ICWs were associated with ATP release from a single cell (11). A burst of ATP from a single cell was implicated by the finding that only one cell accumulated an extracellular fluorescent dye (propidium iodide) that can permeate connexin hemichannels; these data infer hemichannel opening provides a bidirectional permeable pathway allowing the movement of ATP out of the cell and dye uptake into the cell. Similar observations have been made for spontaneous ICWs in retinal pigment epithelial cell cultures (270); only the initiating cell of the ICW displayed propidium iodide dye uptake. A caveat of this hypothesis of a single-cell source for ATP is that a regenerative ATP release occurring via pathways other than hemichannels would not be detected by propidium dye entry.
Lastly, because ATP can be released from cells by distinct pathways, it is possible that ICW propagation may result from a combination of ATP regenerative mechanisms. The initiating stimulus of the ICW may also determine if ATP regeneration is involved. Again, mathematical models of point source ATP release with partial regeneration have led to the conclusion that a combined signaling mechanism that includes partial ATP regeneration provides a better fit with the experimental results (220).
VII. PHASE WAVES
A unique form of Ca2+ signaling, characterized in hepatocytes, that appears as an ICW is a phase wave, which is an ordered phase-shifted temporal sequence of independent Ca2+ oscillations. If a row of cells is exposed to conditions that generate an increase in IP3 concentration that progressively decreases in each cell, the ensuing oscillatory Ca2+ signals create the impression of a cell-to-cell propagating wave because each cell shows a Ca2+ spike with a slight delay with respect to its upstream neighbor (100). In the liver, radial rows of hepatocytes have a gradient of plasma membrane G protein-coupled receptors responsive to the hormones norepinephrine or vasopressin. Uniform exposure to these hormones establishes an IP3 concentration gradient within the cells. However, this gradient can be modulated by gap junction communication. ICWs based on a phase wave mechanism occur both in vitro and in vivo (within intact perfused liver; see sect. IXE).
VIII. PHARMACOLOGICAL MANIPULATION OF INTERCELLULAR Ca2+ WAVES
Almost all of the aforementioned mechanisms of ICWs in isolated cell systems have been elucidated with the extensive use of a variety of pharmacological agents. With this knowledge, we now have a viable “tool kit” to better explore the mechanisms and roles of ICWs in vivo. With this in mind, we have reviewed these pharmacological tools in terms of their action, but, as with most experimental compounds, their nonspecific effects must be considered and data obtained with these agents interpreted with care.
These agents are classified as 1) inhibitors of gap junction coupling, 2) inhibitors of vesicular and nonvesicular ATP release, 3) ATP degrading enzymes, 4) purinergic receptor antagonists, and 5) inhibitors of intracellular Ca2+ signaling. TABLE 2 gives an overview of the most frequently used compounds and their targets; gap junction inhibitors are reviewed in detail in References 29 and 334, while References 174 and 251 provide detailed overviews on purinergic receptor antagonists. In addition to the inhibition of ICWs with substances acting at defined targets, a variety of agents are known to either inhibit or promote ICWs as a result of combined effects at several targets. These compounds with modulatory effects are summarized in TABLE 3.
Table 2.
Pharmacological compounds used to inhibit intercellular Ca2+ waves
| Molecule or Condition | Target Mechanism | Reference Nos. |
|---|---|---|
| Halothane | Gap junction channel/hemichannel inhibition | 117, 153, 236, 308 |
| Long-chain alcohols (octanol, heptanol, and others) | Gap junction channel/hemichannel inhibition+ | 56, 117, 219, 236, 240, 245, 352, 379, 402, 407, 415, 421 |
| Glycyrrhetinic acid/carbenoxolone | Gap junction channel/hemichannel inhibition* | 40, 42, 43, 136, 153, 164, 192, 214, 243, 266, 269, 275, 295, 296, 355, 358, 370, 371, 379, 384, 385 |
| Fenamates | Gap junction channel/hemichannel inhibition+ | 127, 153 |
| Connexin mimetic peptides like Gap26 or Gap27 | Gap junction channel/hemichannel inhibition | 32, 34, 42, 75, 127, 129, 169, 171, 270 |
| TAT-L2 peptide | Cx43 Hemichannel inhibition | 279 |
| Panx1 and Panx3 targeting peptides | Panx hemichannel inhibition | 171a, 271 |
| Apyrases | ATP degradation | 39, 41, 42, 119, 129, 137, 169, 193, 236, 245, 257, 259, 261, 270, 295, 296, 300, 317, 358, 370, 407, 415 |
| Purinergic receptor antagonists (suramin, PPADS, and others) | P2Y receptor inhibition | 39, 41, 42, 119, 129, 137, 161, 192, 193, 236, 245, 257, 261, 270, 295, 296, 300, 317, 348, 358, 370, 371a |
| U73122 | PLC inhibition | 136, 144, 240, 385, 402, 408, 415 |
| Heparin | IP3R inhibition | 33, 246 |
| 2-Aminoethoxydiphenylborate (2-APB) | IP3R inhibition+ | 415 |
| Thapsigargin | SERCA inhibition | 33, 55, 61, 246, 385, 406, 415 |
Table 3.
Factors or conditions modulating intercellular Ca2+ waves
| Molecule or Condition | Effect | Reference Nos. |
|---|---|---|
| CT-truncation of Cx43 | Inhibition | 190 |
| Cx43G138R | Potentiation | 371a |
| S-nitrosylation of Cx43 at C271 | Potentiation | 345 |
| Low extracellular Ca2+ or Mg2+ | Potentiation | 341 |
| Low extracellular Ca2+ | Inhibition | 240 |
| Manganese | Inhibition | 369 |
| Ectonucleotidase inhibition with ARL-67156 | Potentiation | 129 |
| Panx1 and P2X7 | Potentiation | 313, 347 |
| Panx3 | Potentiation | 171a |
| Phorbol esters | Inhibition | 219, 240 |
| Thrombin | Inhibition | 75, 76, 278 |
| TAT-Cx43CT peptide (last 10 amino acids of Cx43) | Removes thrombin inhibition | 279 |
| α1-Adrenergic stimulation (hippocampal astrocytes) | Inhibition | 240 |
| β-Adrenergic stimulation (cardiomyocytes) | Potentiation | 199 |
| Serotonin | Inhibition | 28 |
| Glutamate | Potentiation | 28 |
| Stanniocalcin-1 | Potentiation | 27 |
| TNF-α | Inhibition | 382 |
| IL-1β | Potentiation | 176 |
| Pax6 gene deficiency | Inhibition | 207 |
| High glucose | Inhibition | 126, 131 |
| Glucocorticoids | Potentiation | 325 |
| Anti-epileptic drugs | Inhibition | 367 |
| Oxygen-glucose deprivation followed by reperfusion | Potentiation | 172 |
| Ischemia in vivo | Potentiation | 93 |
| Aβ peptide | Potentiation | 60 |
| Alzheimer mice expressing mutant Aβ precursor protein and presenilin 1 | Potentiation | 191 |
The reported effect relates to the size or the repetition frequency of intercellular Ca2+ waves.
A. Inhibitors of Gap Junction Coupling
Importantly, many gap junction inhibitors do not only inhibit connexin hemichannels but also inhibit P2X7 receptors (347) and several other ion channels (29, 404); therefore, these substances may affect ATP release and paracrine communication of ICWs. Studies with gap junction inhibitors should also control for their influence on both gap junctions and hemichannels, but it is difficult to distinguish between these two configurations of connexin channels. Some gap junction inhibitors have contrasting effects on gap junctions versus hemichannels; for example, quinine stimulates hemichannel opening rather than closure (222, 395). Other agents with opposing effects on gap junctions and hemichannels include lipopolysaccharide (LPS), basic fibroblast growth factor (bFGF), proinflammatory cytokines, and arachidonic acid (85, 86, 254, 290). An additional caveat is that connexin channel inhibitors may also affect pannexin channels. For example, Cx46 hemichannels are blocked by 50–100 μM carbenoxolone, whereas Panx1 hemichannels are blocked by ∼10 μM carbenoxolone (45). In contrast, flufenamic acid blocks Cx46 hemichannels with an IC50 of ∼30 μM, whereas Panx1 hemichannels are only affected by concentrations 10 times larger (45). The anion transporter inhibitor probenecid can inhibit Panx1 channels, without influencing connexin channels (324), but this substance has many other targets.
While antibodies have been used to block both gap junctions (16, 233) and hemichannels (65), greater experimental control has been sought with connexin mimetic peptides (77) to inhibit connexin channels. These peptides are synthesized to mimic specific sequences of the extracellular loops of connexin proteins. For example, the peptides Gap26 and Gap27 contain the amino acid sequences VCYD and SRPTEK, respectively, to mimic the conserved amino acid sequences of the first and second extracellular loop of the connexin protein (391). These peptides are thought to interact with as yet undefined sites within the extracellular loops of connexins of opposing hemichannels (best documented for Gap26; Ref. 213) and thereby prevent the docking of two hemichannels to form a gap junction channel (24, 106). Although the rapid effect of some connexin mimetic peptides (225) has led to the suggestion of an alternative mode of action, it is more likely that the rapid effects of Gap26 and Gap27 are mediated (as indicated by decreased dye uptake and ATP release) by the inhibition of hemichannel opening (40, 42, 86, 107, 108, 208). Electrophysiological studies of single Cx43 hemichannel currents also corroborate rapid channel closure within 4 min by Gap26 and Gap27 (Leybaert L. and Wang N., unpublished observations). Thus it appears that short (minutes) or long (hours) exposure to peptides (Gap26 or Gap27) can be used to inhibit only hemichannels or both hemichannels and gap junctions, respectively (82, 88).
Synthetic peptides corresponding to the L2 sequence on the cytoplasmic loop of Cx43 inhibit hemichannel opening (279) without inhibiting gap junctions (321), offering an interesting approach to dissect the roles of gap junctions and hemichannels in ICW propagation. These peptides interfere with the intramolecular interaction of the COOH-terminal tail of Cx43 with the cytoplasmic loop, thereby preventing hemichannel opening. Thus the L2 peptide partly inhibits ICW propagation because of hemichannel suppression without inhibiting gap junction channels (279). Synthetic mimetic peptides are also available to inhibit pannexin hemichannels; 10Panx1 is a peptide that mimics a sequence on the first extracellular loop of the Panx1 protein (271) and a peptide is also available to block Panx3 hemichannels (171a). The hemichannel inhibitory effect of these peptides may be based on mechanisms that are similar to Cx43 inhibition by Gap26, or Gap27.
The extracellular ionic composition has a significant influence on connexin and pannexin channel permeability. Trivalent ions (e.g., lanthanum, La3+) have been used to inhibit connexin hemichannels (7, 68, 315), but care is required because such ions also block Ca2+ channels (235, 407). Similarly, a reduction of the extracellular Ca2+ concentration can trigger connexin hemichannel opening (20, 46, 131, 342, 343, 405) without influencing gap junctions or pannexin channels. However, applying reduced extracellular Ca2+ conditions has many other effects: it reduces the driving force for Ca2+ entry, induces electrical field changes at the plasma membrane, and may deplete ER/SR Ca2+ stores if applied over a prolonged time period. Depending on the predominating mechanism, ICWs can be inhibited (240) or stimulated (341) by low extracellular Ca2+ conditions (TABLE 3). Acidosis is generally believed to inhibit gap junctions (334), but it may also have an opposite effect (356). Hemichannels are also inhibited by acidosis (304, 375) and opened with alkalosis (315). The effect of acidosis on pannexin channels is not yet established.
B. Inhibitors of Vesicular and Nonvesicular ATP Release
Several substances have been used to interfere with vesicular release of paracrine messengers mediating ICWs, including bafilomycin A, an inhibitor of the vesicular H+-ATPase and brefeldin A, an inhibitor of vesicular trafficking (39). The neurotoxins botulinum toxin and tetanus toxin can also be used (66, 163), but non-neuronal cells need long incubation times as they lack the transporters necessary for cellular uptake. The use of active fragments instead of holoenzymes, combined with adequate membrane permeabilization approaches to get these agents into cells, is preferable.
C. ATP Degrading Enzymes
ATP degrading apyrases are useful to explore ATP-based ICWs and have well-defined specificity for ATP and ADP depending on the enzyme subtype (236). Consequently, a cocktail of apyrase enzymes with both ATPase and ADPase activities has been used to efficiently remove ATP and prevent the accumulation of ADP (129). Endogenous ectonucleotidase activity can be inhibited by ARL-67156 (129). These enzymes are complementary tools to purinergic receptor antagonists to investigate the contribution of paracrine purinergic signaling in ICW propagation especially because the latter have poor selectivity (discussed below).
D. Purinergic Receptor Antagonists
The pharmacology of purinergic receptor antagonists is extensive (reviewed in Refs. 174, 251) but also characterized by a relative lack of selective receptor antagonists. Suramin, PPADS, and reactive blue-2 are broad-spectrum purinergic receptor inhibitors that have been frequently used to investigate the purinergic component of ICWs (TABLE 2). Alternatively, receptor desensitization by prolonged exposure to specific and/or nonhydrolyzable ATP analogs can be used (34, 236).
E. Inhibitors of Intracellular Ca2+ Signaling
Compounds interfering with intracellular Ca2+ signaling block intracellular Ca2+ waves and therefore may also be used to inhibit ICWs. Inhibition of PLC activity with U73122, IP3R opening with heparin or 2-APB, and SERCA activity with thapsigargin have been consistently found to inhibit the propagation of ICWs (TABLE 2). Ca2+ buffers can be introduced into cells to reduce Ca2+ diffusion. These effects have supported the view that ICWs largely rely on the IP3-dependent Ca2+ release. In contrast, inhibition of RyR opening with dantrolene or high ryanodine concentrations reduced the magnitude of the [Ca2+]i increase but had no effect on ICW propagation (55, 144, 385, 408). This result emphasizes the idea that substantial [Ca2+]i increases are not vital for ICW propagation.
The alternative approach of using compounds that stimulate, rather than inhibit, ICWs is equally powerful and perhaps is best illustrated by the microinjection of IP3 or photolytic release of caged IP3. A variety of other caged compounds such as caged Ca2+, Ca2+ ionophores, ATP, and glutamate are available. A particular advantage of photolysis techniques is that they are relatively noninvasive and highly compatible with imaging approaches being used for in vivo studies.
IX. FUNCTIONS OF INTERCELLULAR Ca2+ WAVES
In principle, ICWs mediate the transmission of information from a local site to a global area; the communication of Ca2+ signals to multiple surrounding cells provides the potential to coordinate and synchronize the function of a large group of cells. Cell activities known to be stimulated by increases in [Ca2+]i, such as PKC activation (288) or vesicle secretion, would be expected to be enhanced in cells participating in the ICW. A good example of this correlation between cell activity and an ICW is observed in airway ciliated epithelial cells, where the passage of an ICW initiated an increase in ciliary activity (202). Because cilia are more effective at moving mucus when beating in coordinated groups, this behavior supports the idea that ICWs serve to coordinate tissue responses. Numerous other coordinating functions for ICWs have been proposed; these include proliferation or differentiation of keratinocytes (379), upstream signaling in the blood vessel wall (180, 362), signaling between smooth muscle cells (141), and signaling between endothelial cells and smooth muscle cells via myo-endothelial gap junctions to control vascular tone (170), communication between various cells in the juxtaglomerular apparatus mediating tubuloglomerular feedback (333, 403), and regulation of metabolic activity of glial cells (21, 258). ICWs have also been proposed to provide communication between different cell types, for example, glial-neuron interactions in the brain, endothelial-glial interactions at the blood-brain barrier, and endothelial-smooth muscle cell communication in the vasculature (TABLE 1).
One concern regarding the function of ICWs, either in vitro or in vivo, is whether they result from strong experimental stimulation that does not have a normal physiological counterpart. On the other hand, certain pathological conditions, such as trauma, are often associated with strong stimuli. Brain trauma, brain ischemia, and seizures are all coupled with the release of ATP and glutamate and a decrease of extracellular Ca2+ (142, 149, 209) that collectively promote ICW generation. The excessive elevation of [Ca2+]i that is likely to be associated with these stimuli can lead to Ca2+-dependent PLC activation, the generation of large amounts of IP3, and the initiation of ICWs. Such ICWs may exacerbate injury by communicating signals that initiate cell apoptosis in surrounding cells (90, 196, 197, 398). Recent evidence indicates that IP3 diffusion via gap junctions is crucial to provoke apoptosis in adjacent cells (89). Conversely, ICWs may provide the directional clues to the injury site for subsequent recruitment of repair mechanisms. This correlation of ICWs and tissue repair has also been proposed for hepatic tissue (355) and vascular endothelial cells (in concert with serum growth factors) (373). In line with this, decreased ICW activity may delay wound healing (207).
A second unknown with respect to ICWs in physiological or pathological responses is the signaling molecule(s) that mediates the response. This could be the resulting [Ca2+]i changes, the messengers propagating the ICWs (i.e., IP3 or ATP), or another associated signal. For example, ICWs that are proposed to coordinate metabolic activity of glial cells (54) probably mediate their effect by the changes in [Na+]i that accompany the ICW rather than by changes in [Ca2+]i (21, 200, 258). ATP is a likely candidate to mediate pathological responses to tissue trauma by exerting a mitogenic action or acting as a “find me” signal from apoptotic cells for phagocytic clearance (101). ATP also triggers directed cellular responses in brain injury, such as microglial activation and chemotaxis (80), and has a wide range of targets in the brain and immune system (48, 92). Its metabolite adenosine may also confer a protective action in the brain.
A further caution regarding the roles for ICWs is that most hypotheses emanate from observations of in vitro cell culture systems. However, many aspects of ICW initiation and propagation may be very different under in vivo conditions. For example, in organs, the diffusion of intra- and extracellular messengers can occur in three rather than the two dimensions typical of monolayer cultures. This spatial distribution is likely to influence the distance of ICW travel as well as the clearance or metabolism of extracellular messengers by the microcirculation or ectonucleotidases (97). Equally important is the fact that in tissue culture, many of the organ characteristics, such as the extracellular matrix, variety of cell types, extent of cell coupling, and cell differentiation, are lost or significantly altered. At present, there are fewer reports of ICWs in vivo in organs like the brain, liver, and lung (161, 192, 265, 292) and therefore, as yet, insufficient supportive data for specific physiological responses of ICWs. However, this situation is rapidly changing. Below, we discuss several examples where ICW propagation and function were studied in intact tissues in vitro or in animals in vivo (FIGURE 4).
Figure 4.
Overview of mechanisms and functions of intercellular Ca2+ waves (ICWs), based on ex vivo and in vivo evidence from the organs indicated. ICWs between cells of the same type are indicated by circular arrows; noncircular arrows indicate ICWs between different cell types. Gap junctions (GJs) and paracrine purinergic signaling (PPS) are involved in most cases, but only the most prevalent mechanism is depicted. Dashed arrows indicate proposed functions (in italics). Retina: ICWs that propagate in astrocytes and Müller cells may provoke electrical activity in retinal ganglion cells (RGCs) and influence the blood vessel caliber. Retinal pigment epithelium cells (RPE) show ICWs which may influence proliferation and differentiation of neural progenitor cells (NPCs). Cochlea: ICWs in supporting cells may be involved in K+ recycling and may influence outer hair cells (OHCs) via ATP release to alter the cochlear amplifier gain and inner hair cells (IHCs) to modify electrical activity and synaptic connectivity during development. Blood vessels: ICWs propagate along the vessel wall via endothelial cells (ECs) and modulate white blood cell (WBC) interactions with ECs as well as smooth muscle cell (SMC) contractility via direct (myoendothelial gap junctions) or indirect mechanism to control vessel diameter. Brain: mechanisms of ICW propagation differ with brain region; GJs, GJs+PPS, or PPS mediate ICWs in the neocortex, hippocampus, and corpus callosum and Bergman glia, respectively. ICWs of astrocytes propagate via astrocyte endfeet to small-diameter blood vessels to influence blood vessel diameter. ICWs of astrocytes may also modulate synaptic signaling as well as contribute to neural development or disease processes (AD, Alzheimer disease; CSD, cortical spreading depression). Liver: ICWs propagating between hepatocytes may influence hepatic glucose output, bile production, and tissue regeneration.
A. Intercellular Ca2+ Waves in the Retina
Stimulated ICWs occur in astrocytes and Müller cells of isolated retina (rat eyecup, whole-mount retina, and retinal slices) (245, 246) and influence the electrical activity of retinal ganglion cells (the output neurons of the retina), suggesting that ICWs are involved in glial-neuronal signaling (247). ICWs between astrocytes are dependent on gap junctions, whereas ICWs between Müller cells are more dependent on paracrine purinergic signaling, although they utilize some gap junction signaling. ATP release via connexin hemichannels has been demonstrated in Müller cells (44). ICWs also propagated between astrocytes and Müller cells; although these astrocytes and Müller cells are coupled by rectifying gap junctions (246, 411), this heterocellular communication of ICWs appeared to be mediated by paracrine signaling rather than gap junctions.
Spontaneous ICWs occur in Müller cells but not in astrocytes in the retina during postnatal development. The frequency of these spontaneous ICWs increased with age (193), both in vitro and in vivo (open eye globe), and this may result from increased ATP release and enhanced ATP regeneration. When ICWs in the in vitro retina encountered a blood vessel, they induced vasoconstriction, a response indicating a role for glial ICWs in controlling blood flow. It remains to be determined if spontaneous ICWs influence the electrical activity of retinal ganglion cells, as observed for stimulated ICWs.
ICWs may also contribute to the spontaneous electrical and Ca2+ wave activity in the ventricular zone of the retina (357) and retina proper (51) during retinal development. These ICWs are mainly restricted to neurons (although glia have not been excluded) and are important to guide development of the synaptic connectivity in utero in the absence of visual input (181).
Spontaneous ICWs propagate repeatedly (several waves per minute) between retinal pigment epithelial (RPE) cells and the neural retina. These ICWs are mediated by extracellular ATP that is released from trigger cells via hemichannel opening, based on evidence of propidium iodide dye uptake and inhibition by Gap26 (270). The mitogenic action of the ATP associated with these waves is implicated in controlling proliferation and differentiation of retinal neural progenitor cells (269).
B. Intercellular Ca2+ Waves in the Cochlea
In mouse cochlear explant cultures (postnatal day 3 to 6), focal stimulation of supporting cells, located in the organ of Corti, with extracellular ATP or by photolysis of IP3 triggered ICWs propagating in the supporting cell zone (7) (FIGURE 4). The involvement of ATP as an extracellular messenger released via connexin hemichannels and gap junctions was confirmed with studies with Cx26- and Cx30-deficient animals, connexin channel blockers (including La3+ to block hemichannels), and ATP biosensors (7, 221). Panx1 hemichannels and P2X7 receptors were not involved. ATP-based ICWs in supporting cells are known to trigger Ca2+ spikes in inner hair cells and electrical activity in spiral ganglion cells (377). This electrical activity may help to establish and organize synaptic connectivity within the primary auditory cortex (A1) before auditory input occurs.
Whether cochlear ICWs contribute to the hearing in the adult is not known. Because mutations in both Cx26 and Cx30 genes are frequently associated with autosomal recessive nonsyndromic hearing loss (10), it is hypothesized that gap junction dysfunction impairs K+ clearance from the inner hair cells following auditory stimulation (414). This concept is underscored by the deafness-associated mutation where a V84L substitution in Cx26 selectively impairs the permeability of Cx26 gap junctions to IP3 and thereby strongly reduces ICW propagation (17). ICWs may be involved in K+ recycling via supporting cells by activating K+ efflux from these cells (223). Extracellular ATP (at micromolar concentrations) decreases the electromotility of the outer hair cells that function as an active amplifier in the organ of Corti (417). Consequently, an ATP wave associated with the ICW is expected to influence the hearing sensitivity.
C. Intercellular Ca2+ Waves in the Brain
1. In vitro tissues
ICWs have been observed in vitro in acutely isolated (ex vivo) brain tissues (250). Astrocytes within brain slices from the rat thalamus display spontaneous Ca2+ oscillations that propagate to a limited number of surrounding astrocytes and trigger inward currents in associated neurons (264). Local electrical stimulation of mouse cortical brain slices triggers extensive (several 100 μm) fast propagating (∼50 μm/s) astrocytic ICWs. The fast propagation reflects a contribution by the neuronal network because tetrodotoxin (blocks action potentials) or Ca2+-free solutions (blocks presynaptic neurotransmitter release) significantly reduced wave speed (139).
However, the mechanism of ICW propagation appears to vary with brain location (FIGURE 4). In astrocytes of the neocortex, studies with dye coupling, gap junction inhibitors, and Cx43 knockout (astrocytes) animals (139) indicated that gap junction coupling was extensive and had a dominant role in ICW communication. Although the same astrocytes express Cx30, this was secondary to Cx43 in ICW propagation. Some ATP release (detected by “sniffer cells” expressing metabotropic purinergic receptors) was associated with neocortex ICWs, but these were not inhibited by purinergic receptor antagonists (139), suggesting ATP was not important. In compact tissues like the brain, ATP is probably rapidly degraded by ectonucleotidases, and this precludes the activation of astrocytic purinergic receptors.
In the corpus callosum, electrical stimulation triggers glial ICWs that propagate into the neocortex. In contrast, the same electrical stimulation in Cx30/43-deficient mice initiated ICWs that failed to propagate into the neocortex. This indicates that ICWs within the corpus callosum, unlike the neocortex, do not utilize gap junctions. Similarly, ICWs were still present in the CA1 region of the hippocampus of Cx30/43-deficient mice. These ICWs were inhibited by purinergic antagonists but not a gap junction blocker, indicating a purinergic rather than gap junction mode of propagation (139). However, recent evidence indicates that ICWs triggered in hippocampal slices by diazo-2 photoactivation are strongly inhibited in Cx30/43-deficient mice (371a).
2. In vivo ICWs
In vivo studies of ICWs can be achieved by two-photon microscopy; this technique allows the noninvasive monitoring of [Ca2+]i changes up to a depth of ∼400 μm (252), which corresponds to cortical layers II and III of mice and rats. The loading technique used to introduce Ca2+ indicator dyes into cells may affect the results; glial cells, more specifically astrocytes, are predominately loaded with indicator dye by applying the dye to the cortical surface, whereas both glial and neuronal cells are loaded by a bolus injection of dye into the cortex (120, 340, 354, 368). Alternatively, cells may express a genetically encoded GCaMP2 Ca2+ indicator (161) to avoid mechanical stimulation of the neural tissue associated with dye injection. Although two-photon microscopy is noninvasive, there can be some artifacts associated with two-photon laser excitation. For example, excitation with high-energy pulses of infrared light may locally generate free oxygen radicals (due to triple-state excitation) or thermal effects, and these may serve as triggers to stimulate ICWs (389). Conversely, too much laser energy may dampen Ca2+ wave activity (192).
Similar to the observations in brain slices, extensive astrocytic ICWs (propagating at 8–10 μm/s over 150–200 μm) were triggered by the photolytic release of Ca2+ in a single astrocyte or by local iontophoretic application of ATP in the cortex of anesthetized rodents (368). This initiation of ICWs by the photolytic release of Ca2+ is in stark contrast to the response commonly observed in cell culture where a Ca2+ increase alone does not trigger an ICW (except in special conditions as discussed). This suggests that the experimental elevation of [Ca2+]i in vivo may stimulate a larger increase in IP3 or ATP release in the target cell. Alternatively, Ca2+ may serve as a better intercellular messenger in vivo because of a preexisting elevation of [IP3]i in adjacent cells or because gap junction coupling is more extensive. In addition, in vivo experiments are conducted at body temperature, and this may increase the Ca2+ excitability of the tissue.
Spontaneous ICWs occur in cerebellar cortex of rodents (rats or mice, 3–6 wk old) (161). These ICWs propagated in Bergmann glial cells in three dimensions (radius of ∼50 μm at 4–11 μm/s) with a frequency of ∼0.4 waves/min. The major axes of propagation were aligned in one direction with the parallel fibers and in another direction with the pia-Purkinje cell axis and corresponded to the directions in which extracellular diffusion occurred at its faster rate. This behavior is consistent with extracellular purinergic signaling; gap junction coupling was presumed to be inhibited by the [Ca2+]i elevation associated with the ICW (161). Similar spontaneously occurring ICWs (called “Ca2+ bursts”) have been described by Nimmerjahn and co-workers at about the same time (250a); importantly, the latter work was performed in the absence of anesthesia.
Coordinated [Ca2+]i changes (not “true” waves) also occur in cortical astrocytes in rats (P12-P16) after stimulation of neuronal activity with bicuculline, a suppressor of GABAergic neurotransmission (154). This suggests spontaneous astrocytic wavelike activity can result from increased neuronal network activity. Spontaneous ICWs called “glissandi” were found in the hippocampus of mice (192) and encompassed several tens of cells, presumably astrocytes, and propagated approximately every 3 min at a high speed (∼61 μm/s). Glissandi were inhibited by carbenoxolone and suramin, indicating gap junctions and ATP signaling in their propagation, and by tetrodotoxin, suggesting a neuronal network contribution.
Most of the two-photon studies detailed above were performed in anesthetized animals. However, an optic fiber positioned on the cortex has been used to observe [Ca2+]i changes in active, nonanesthetized animals. Interestingly, only limited Ca2+ wavelike activity, presumed to be of neuronal origin, was observed (3). Importantly, this Ca2+ wave activity disappeared under anesthesia. This implies that spontaneous ICW activity recorded with two-photon imaging in anesthetized animals may become more pronounced if the anesthesia could be omitted. Work of Nimmerjahn et al. (2009) performed in behaving mice has shown that the ‘Ca2+ burst’ type of ICWs disappears with the start of locomotive behavior; instead, so called ‘Ca2+ flares’ appear that involve concerted Ca2+ excitation in hundreds of Bergman glial cells (250a). However, Ca2+ flares are not ICWs.
3. Proposed functions
A) SYNAPTIC MODULATION.
Because astrocytes make substantial contacts with neurons, it is speculated that astrocytic ICWs influence synaptic activity (FIGURE 4). Astrocyte extensions are closely apposed to neurons in tripartite synapses (274), and increases in astrocytic [Ca2+]i as well as ICWs have been shown, in vitro, to influence synaptic functioning by glutamate release (113, 273), although this Ca2+ dependency of gliotransmitter release is not universal (4). Similarly, in vivo glissandi waves were associated with a reduction of local field potentials [representing dendritic electrical activity (192)]. Although neuronal activity is known to trigger localized astrocytic [Ca2+]i changes (319, 389), there is currently no in vivo evidence that neuronal activity can trigger astrocytic ICWs. However, in vitro, astrocytic ICWs may be triggered by extracellular factors, such as pH changes; brain stem slices display astrocytic ICWs in response to a lowering of the extracellular pH (133).
B) BLOOD FLOW REGULATION.
Astrocytes also form endfeet extensions contacting small-diameter brain arteriolar blood vessels, and [Ca2+]i changes in these endfeet influence vessel diameter and blood flow (12) (FIGURE 4). The vessel response to astrocyte Ca2+ stimulation appears to vary between different recording conditions. In brain slices, photolytic release of Ca2+ in astrocytic endfeet induced vasoconstriction while vasodilation was observed in vivo in the somatosensory cortex (239, 360). In brain slices, ICWs occurred along the blood vessels [similar findings occurred following electrical stimulation (115)] while ICWs were absent in vivo. In contrast, spontaneous in vivo hippocampal glissandi waves were associated with decreased blood flow in their propagation territory (192). A similar dual vasoconstriction or vasodilation to astrocyte stimulation occurs in the retina (232). In the brain, a low oxygen tension appears to favor vasodilation (132).
Astrocytic ICWs have been proposed to play a role in the spatial buffering of K+, with K+ being released at endfeet via large-conductance Ca2+-sensitive K+ channels. In arterioles, K+ can induce both vasodilation and vasoconstriction depending on the concentration of K+ in the extracellular space (116, 124). Low K+ concentrations hyperpolarize vascular smooth muscles to induce relaxation, whereas high K+ concentrations depolarize smooth muscle to induce contraction. However, the contribution of astrocyte-derived K+ to vascular responses remains contentious (231).
C) DEVELOPMENTAL REGULATION.
Glial ICWs may play a role in the organization and proliferation of the ventricular zone of the developing neocortex (observed in brain slices) (394). Radial glial cells display spontaneous and repetitive ICWs that primarily utilize hemichannel opening to release ATP and P2Y1 receptor signaling, although some gap junction communication was evident. Like the retina, ATP is proposed to act as a mitogenic signal to synchronize cell cycles, thereby acting as a spatial signal coordinating the activity of radial glial cells within the ventricular zone (214). The role of the [Ca2+]i changes associated with ICWs remains to be determined (79).
D) PATHOPHYSIOLOGY.
Astrocytic ICWs also appear under pathological conditions (FIGURE 4). For example, spontaneous ICWs occur near amyloid-β plaques in adult APP/PS1 Alzheimer mice (191). Evidence for wave activity (or rather coordinated [Ca2+]i changes) comes from cross-correlogram studies of multiple cells and indicates a propagation speed of ∼23 μm/s and travel distances of 200 μm from cortical plaques. This coordinated activity was not influenced by tetrodotoxin and amyloid-β and was speculated to trigger the activity via its neurotoxic effects. The propagation mechanisms of the coordinated [Ca2+]i changes (studied in vitro) suggest dependence on both gap junctions and paracrine ATP signaling (60, 148, 219, 241). Astrocytic coordinated [Ca2+]i changes occur 20 min after photothrombotic obstruction of a small vessel (0.6 mm in diameter) in the brain cortex of mice (93). In this case, the coordinated [Ca2+]i changes were inhibited by antagonizing type 5 metabotropic glutamate receptors or GABA-B receptors; purinergic or adenosine receptor antagonists had no influence. Interestingly, the loading of astrocytes in vivo with a Ca2+ buffer (BAPTA-AM) reduced the infarct size, pointing to a possible role of Ca2+ wavelike activity in ischemic cell damage.
ICWs have been implicated in cortical spreading depression (CSD) which may contribute to the pathophysiology of the aura phase of migraine (52, 359). CSD represents a wave of cortical neuronal depolarization. Experimentally, CSD can be triggered mechanically or by the local application of a high K+ concentration to the cortex. CSD (observed by intrinsic optical signal imaging in brain slices) propagates quickly with a high extracellular K+ concentration at the leading edge, followed by depressed cortical activity (reviewed in Ref. 359); this CSD wave is associated with a slower propagating astrocytic ICW (61). The CSD wave and the associated ICW have distinct mechanisms: CSD is inhibited by NMDA receptor antagonists, while the ICW is inhibited by gap junction inhibitors, indicating a neuronal and astrocytic contribution, respectively (275). The inhibition of the astrocytic ICW by thapsigargin correlated with a loss of early vasoconstriction associated with CSD. This implies a role for astrocytic ICWs in the acute hypoperfusion phase of CSD. However, ICWs probably do not play a role in the hyperperfusion and long-lasting hypoperfusion that follow the acute hypoperfusion phase (61).
D. Intercellular Ca2+ Waves in Blood Vessels
While it is clear from the previous discussion that ICWs propagating through the brain parenchyma may influence vascular tone, ICWs also propagate relatively long distances along the cells of blood vessels to modulate vascular function (FIGURE 4). In venous lung capillaries of isolated, blood-perfused lungs from rodents, the photolytic release of Ca2+ in an alveolar endothelial cell triggered ICWs propagating from the capillaries to venous blood vessels, up to 150 μm away from the stimulation point (265). The mechanisms by which Ca2+ triggers ICWs were addressed previously (see sect. IXC). These ICWs were inhibited by Gap26 and Gap27 and were absent in mouse lungs with an endothelium lacking Cx43, results consistent with gap junction communication. Interestingly, the presence of ICWs correlated with increased endothelial P-selectin expression and potentiation of thrombin-induced microvascular permeability. Because both of these responses were also attenuated by Gap26/Gap27, it is feasible that these cellular changes were induced by the ICWs. Lung capillary endothelial ICWs also occur spontaneously, at a frequency of ∼ 0.4 waves/min (406). Long-range ICWs, propagating several hundred micrometers, have been reported in intact isolated blood vessels from heart and cremaster muscle (362). Here, the ICWs were shown to induce vasodilation and thereby contribute to the regulation of tissue perfusion.
E. Intercellular Ca2+ Waves in Liver
Confocal imaging of the superficial cell layers of intact perfused liver has demonstrated ICWs within the hepatocyte plates in response to the hormones vasopressin, phenylephrine, or angiotensin II (238, 242, 268, 292). However, as discussed earlier, the propagation of liver ICWs does not rely on a messenger diffusion-reaction cascade but appears best represented by a phase wave where hormones trigger time-delayed Ca2+ oscillations with a similar frequency in adjacent hepatocytes; the asynchronous, but sequential Ca2+ increases give the impression of an ICW.
Hepatocyte ICWs in intact liver appear to be initiated from periportal areas and propagate in the direction of the central vein (122), but initiation from the pericentral vein can also occur (98, 238). The waves are limited to the microcirculatory domain of a hepatic lobule and do not spread to neighboring microcirculatory domains. Liver ICW activity is inhibited by gap junction closure or by disruption of cell-cell contacts, but the hormone-induced Ca2+ oscillations persist, albeit in a less coordinated fashion (122). There is little evidence for a contribution of purinergic paracrine communication (268). Mathematical modeling predicts that the direction of ICW propagation is determined by a gradient of hepatocyte hormone sensitivity (and receptor densities) along the hepatic plate, while the gap junction coupling appears to be essential for the entrainment or establishing the phase delay in Ca2+ spiking between cells (99, 100). Gap junctions appear to reduce the [IP3]i gradient between cells that is initially established within hepatocyte cells upon exposure to hormone.
Hepatocyte ICWs are hypothesized to play a role in hepatic glucose output, activation of bile flow, and liver regeneration after partial hepatectomy (FIGURE 4). Hepatic glucose output triggered by norepinephrine or glucagon is significantly lower in Cx32-deficient animals compared with wild type (346). Given the importance of cAMP and IP3 in hepatic glucose mobilization, it is plausible that the diffusion of these two messengers via gap junctions helps recruit hepatocytes to provide sufficient glucose output. However, a clear demonstration of the role of the ICW is still lacking. Vasopressin is normally a strong trigger of bile secretion, but after 24 h of in vivo exposure to elevated vasopressin concentrations, a pulse of vasopressin triggered significantly less bile flow. This treatment removed the hepatocyte vasopressin receptor gradient in the perivenous area and impaired the ICWs, suggesting a link between the ICWs and bile flow (322). Adenoviral transfection of hepatocytes with the Ca2+-buffering protein parvalbumin reduced liver regeneration after partial hepatectomy and reduced norepinephrine-induced Ca2+ oscillations, pointing to the importance of hepatocyte Ca2+ signaling in liver regeneration (195). However, it is yet unclear whether parvalbumin transfection has any influence on hepatocyte ICWs.
X. CONCLUSIONS
ICWs appear to be a widespread phenomenon by which a diversity of cell types communicate with each other to coordinate and synchronize their activity. While ICWs occur both in vitro and in vivo, there are some differences in behavior and mechanisms that indicate close attention should be paid to experimental conditions and tissue characteristics to accurately identify their physiological roles. ICWs appear to utilize two fundamental mechanisms of propagation that may either operate in isolation or in combination. These are the diffusion of IP3 through gap junctions and the release of extracellular ATP that serve as messengers to stimulate adjacent cells via the direct release of Ca2+ from the ER or the indirect release of Ca2+ by stimulation via purinergic receptors, respectively. The stimuli initiating ICWs are also varied, but the fact that ICWs can spontaneously occur in vivo points to their existence in normal physiological conditions. ICWs also appear to be involved in pathological processes such as brain ischemia and perhaps Alzheimer's disease. While the identity of the actual messenger associated with the ICW that is responsible for such control is uncertain, ATP is most commonly implicated. Future studies aimed at elucidating the extent and function of ICWs in vivo promise to reveal important insights into a wide diversity of processes including neural development, hearing, vision, liver function, and brain disease.
GRANTS
L. Leybaert was supported by the Fund for Scientific Research, Flanders, Belgium (G.0140.08, 3G.0134.09, G.0298.11N, and 3G057112N), and the Interuniversity Attraction Poles Program, Belgian Science Policy (P6/31 and P7). M. Sanderson was supported by National Heart, Lung, and Blood Institute Grant HL-103405.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We thank Elke Decrock for help in producing the figures. We apologize for any references we might have overlooked.
Address for reprint requests and other correspondence: L. Leybaert, Dept. of Basic Medical Sciences, Physiology Group, Faculty of Medicine & Health Sciences, Ghent University, De Pintelaan 185 (Block B, Rm. 310), B-9000 Ghent, Belgium (e-mail: Luc.Leybaert@UGent.be); and M. J. Sanderson, Dept. of Microbiology and Physiological Systems, Univ. of Massachusetts Medical School, 55 Lake Ave. North, Worcester, MA 01655 (e-mail: michael.sanderson@umassmed.edu).
REFERENCES
- 1. Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58: 281–341, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abraham EH, Prat AG, Gerweck L, Seneveratne T, Arceci RJ, Kramer R, Guidotti G, Cantiello HF. The multidrug resistance (mdr1) gene product functions as an ATP channel. Proc Natl Acad Sci USA 90: 312–316, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Adelsberger H, Garaschuk O, Konnerth A. Cortical calcium waves in resting newborn mice. Nature Neurosci 8: 988–990, 2005. [DOI] [PubMed] [Google Scholar]
- 4. Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science 327: 1250–1254, 2010. [DOI] [PubMed] [Google Scholar]
- 5. Allbritton NL, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science 258: 1812–1815, 1992. [DOI] [PubMed] [Google Scholar]
- 6. Anderson CM, Bergher JP, Swanson RA. ATP-induced ATP release from astrocytes. J Neurochem 88: 246–256, 2004. [DOI] [PubMed] [Google Scholar]
- 7. Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, Kessaris N, Richardson W, Rickheit G, Filippov MA, Monyer H, Mammano F. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci USA 105: 18770–18775, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anyatonwu GI, Ehrlich BE. Calcium signaling and polycystin-2. Biochem Biophys Res Commun 322: 1364–1373, 2004. [DOI] [PubMed] [Google Scholar]
- 9. Anyatonwu GI, Estrada M, Tian X, Somlo S, Ehrlich BE. Regulation of ryanodine receptor-dependent calcium signaling by polycystin-2. Proc Natl Acad Sci USA 104: 6454–6459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Apps SA, Rankin WA, Kurmis AP. Connexin 26 mutations in autosomal recessive deafness disorders: a review. Int J Audiol 46: 75–81, 2007. [DOI] [PubMed] [Google Scholar]
- 11. Arcuino G, Lin JH, Takano T, Liu C, Jiang L, Gao Q, Kang J, Nedergaard M. Intercellular calcium signaling mediated by point-source burst release of ATP. Proc Natl Acad Sci USA 99: 9840–9845, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature 468: 232–243, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ballerini P, Di Iorio P, Ciccarelli R, Nargi E, D'Alimonte I, Traversa U, Rathbone MP, Caciagli F. Glial cells express multiple ATP binding cassette proteins which are involved in ATP release. Neuroreport 13: 1789–1792, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J Biol Chem 276: 20144–20153, 2001. [DOI] [PubMed] [Google Scholar]
- 15. Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett 572: 65–68, 2004. [DOI] [PubMed] [Google Scholar]
- 15a. Baroja-Mazo A, Barbera-Cremades M, Pelegrin P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta 2012. (PMID: 22266266). [DOI] [PubMed] [Google Scholar]
- 15b. Barbe MT, Monyer H, Bruzzone R. Cell-cell communication beyond connexins: the pannexin channels. Physiology 21: 103–114, 2006. [DOI] [PubMed] [Google Scholar]
- 16. Becker DL, Evans WH, Green CR, Warner A. Functional analysis of amino acid sequences in connexin43 involved in intercellular communication through gap junctions. J Cell Sci 108: 1455–1467, 1995. [DOI] [PubMed] [Google Scholar]
- 17. Beltramello M, Piazza V, Bukauskas FF, Pozzan T, Mammano F. Impaired permeability to Ins(1,4,5)P3 in a mutant connexin underlies recessive hereditary deafness. Nature Cell Biol 7: 63–69, 2005. [DOI] [PubMed] [Google Scholar]
- 18. Bennett MR, Buljan V, Farnell L, Gibson WG. Purinergic junctional transmission and propagation of calcium waves in spinal cord astrocyte networks. Biophys J 91: 3560–3571, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bennett MR, Farnell L, Gibson WG. A quantitative model of purinergic junctional transmission of calcium waves in astrocyte networks. Biophys J 89: 2235–2250, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bennett MV, Contreras JE, Bukauskas FF, Saez JC. New roles for astrocytes: gap junction hemichannels have something to communicate. Trends Neurosci 26: 610–617, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bernardinelli Y, Magistretti PJ, Chatton JY. Astrocytes generate Na+-mediated metabolic waves. Proc Natl Acad Sci USA 101: 14937–14942, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol 499: 291–306, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nature Rev Mol Cell Biol 1: 11–21, 2000. [DOI] [PubMed] [Google Scholar]
- 24. Berthoud VM, Beyer EC, Seul KH. Peptide inhibitors of intercellular communication. Am J Physiol Lung Cell Mol Physiol 279: L619–L622, 2000. [DOI] [PubMed] [Google Scholar]
- 25. Bezprozvanny I, Ehrlich BE. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca channels from cerebellum: conduction properties for divalent cations and regulation by intraluminal calcium. J Gen Physiol 104: 821–856, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351: 751–754, 1991. [DOI] [PubMed] [Google Scholar]
- 27. Block GJ, DiMattia GD, Prockop DJ. Stanniocalcin-1 regulates extracellular ATP-induced calcium waves in human epithelial cancer cells by stimulating ATP release from bystander cells. PLoS One 5: e10237, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Blomstrand F, Khatibi S, Muyderman H, Hansson E, Olsson T, Ronnback L. 5-Hydroxytryptamine and glutamate modulate velocity and extent of intercellular calcium signalling in hippocampal astroglial cells in primary cultures. Neuroscience 88: 1241–1253, 1999. [DOI] [PubMed] [Google Scholar]
- 29. Bodendiek SB, Raman G. Connexin modulators and their potential targets under the magnifying glass. Curr Med Chem 17: 4191–4230, 2010. [DOI] [PubMed] [Google Scholar]
- 30. Bodin P, Burnstock G. Evidence that release of adenosine triphosphate from endothelial cells during increased shear stress is vesicular. J Cardiovasc Pharmacol 38: 900–908, 2001. [DOI] [PubMed] [Google Scholar]
- 31. Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res 26: 959–969, 2001. [DOI] [PubMed] [Google Scholar]
- 32. Boitano S, Dirksen ER, Evans WH. Sequence-specific antibodies to connexins block intercellular calcium signaling through gap junctions. Cell Calcium 23: 1–9, 1998. [DOI] [PubMed] [Google Scholar]
- 33. Boitano S, Dirksen ER, Sanderson MJ. Intercellular propagation of calcium waves mediated by inositol trisphosphate. Science 258: 292–295, 1992. [DOI] [PubMed] [Google Scholar]
- 34. Boitano S, Evans WH. Connexin mimetic peptides reversibly inhibit Ca2+ signaling through gap junctions in airway cells. Am J Physiol Lung Cell Mol Physiol 279: L623–L630, 2000. [DOI] [PubMed] [Google Scholar]
- 35. Boitano S, Sanderson MJ, Dirksen ER. A role for Ca2+-conducting ion channels in mechanically-induced signal transduction of airway epithelial cells. J Cell Sci 107: 3037–3044, 1994. [DOI] [PubMed] [Google Scholar]
- 36. Bootman M, Niggli E, Berridge M, Lipp P. Imaging the hierarchical Ca2+ signalling system in HeLa cells. J Physiol 499: 307–314, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bootman MD, Berridge MJ, Roderick HL. Calcium signalling: more messengers, more channels, more complexity. Curr Biol 12: R563–565, 2002. [DOI] [PubMed] [Google Scholar]
- 38. Bootman MD, Smyrnias I, Thul R, Coombes S, Roderick HL. Atrial cardiomyocyte calcium signalling. Biochim Biophys Acta 1813: 922–934, 2011. [DOI] [PubMed] [Google Scholar]
- 39. Bowser DN, Khakh BS. Vesicular ATP is the predominant cause of intercellular calcium waves in astrocytes. J Gen Physiol 129: 485–491, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Braet K, Aspeslagh S, Vandamme W, Willecke K, Martin PE, Evans WH, Leybaert L. Pharmacological sensitivity of ATP release triggered by photoliberation of inositol-1,4,5-trisphosphate and zero extracellular calcium in brain endothelial cells. J Cell Physiol 197: 205–213, 2003. [DOI] [PubMed] [Google Scholar]
- 41. Braet K, Paemeleire K, D'Herde K, Sanderson MJ, Leybaert L. Astrocyte-endothelial cell calcium signals conveyed by two signalling pathways. Eur J Neurosci 13: 79–91, 2001. [PubMed] [Google Scholar]
- 42. Braet K, Vandamme W, Martin PE, Evans WH, Leybaert L. Photoliberating inositol-1,4,5-trisphosphate triggers ATP release that is blocked by the connexin mimetic peptide gap 26. Cell Calcium 33: 37–48, 2003. [DOI] [PubMed] [Google Scholar]
- 43. Braunstein TH, Inoue R, Cribbs L, Oike M, Ito Y, Holstein-Rathlou NH, Jensen LJ. The role of L- and T-type calcium channels in local and remote calcium responses in rat mesenteric terminal arterioles. J Vasc Res 46: 138–151, 2009. [DOI] [PubMed] [Google Scholar]
- 44. Bruckner E, Grosche A, Pannicke T, Wiedemann P, Reichenbach A, Bringmann A. Mechanisms of VEGF- and glutamate-induced inhibition of osmotic swelling of murine retinal glial (Muller) cells: indications for the involvement of vesicular glutamate release and connexin-mediated ATP release. Neurochem Res 37: 268–278, 2011. [DOI] [PubMed] [Google Scholar]
- 45. Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem 92: 1033–1043, 2005. [DOI] [PubMed] [Google Scholar]
- 45a. Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA 100: 13644–13649, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bruzzone S, Guida L, Zocchi E, Franco L, De Flora A. Connexin 43 hemi channels mediate Ca2+-regulated transmembrane NAD+ fluxes in intact cells. FASEB J 15: 10–12, 2001. [DOI] [PubMed] [Google Scholar]
- 47. Burdyga T, Shmygol A, Eisner DA, Wray S. A new technique for simultaneous and in situ measurements of Ca2+ signals in arteriolar smooth muscle and endothelial cells. Cell Calcium 34: 27–33, 2003. [DOI] [PubMed] [Google Scholar]
- 48. Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol Rev 87: 659–797, 2007. [DOI] [PubMed] [Google Scholar]
- 49. Cancela JM, Van Coppenolle F, Galione A, Tepikin AV, Petersen OH. Transformation of local Ca2+ spikes to global Ca2+ transients: the combinatorial roles of multiple Ca2+ releasing messengers. EMBO J 21: 909–919, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carvalho AC, Sharpe J, Rosenstock TR, Teles AF, Youle RJ, Smaili SS. Bax affects intracellular Ca2+ stores and induces Ca2+ wave propagation. Cell Death Differentiation 11: 1265–1276, 2004. [DOI] [PubMed] [Google Scholar]
- 51. Catsicas M, Bonness V, Becker D, Mobbs P. Spontaneous Ca2+ transients and their transmission in the developing chick retina. Curr Biol 8: 283–286, 1998. [DOI] [PubMed] [Google Scholar]
- 52. Charles A. Does cortical spreading depression initiate a migraine attack? Maybe not. Headache 50: 731–733, 2010. [DOI] [PubMed] [Google Scholar]
- 53. Charles A. Intercellular calcium waves in glia. Glia 24: 39–49, 1998. [DOI] [PubMed] [Google Scholar]
- 54. Charles A. Reaching out beyond the synapse: glial intercellular waves coordinate metabolism. Sci STKE 2005: pe6, 2005. [DOI] [PubMed] [Google Scholar]
- 55. Charles AC, Dirksen ER, Merrill JE, Sanderson MJ. Mechanisms of intercellular calcium signaling in glial cells studied with dantrolene and thapsigargin. Glia 7: 134–145, 1993. [DOI] [PubMed] [Google Scholar]
- 56. Charles AC, Kodali SK, Tyndale RF. Intercellular calcium waves in neurons. Mol Cell Neurosci 7: 337–353, 1996. [DOI] [PubMed] [Google Scholar]
- 57. Charles AC, Merrill JE, Dirksen ER, Sanderson MJ. Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6: 983–992, 1991. [DOI] [PubMed] [Google Scholar]
- 58. Charles AC, Naus CC, Zhu D, Kidder GM, Dirksen ER, Sanderson MJ. Intercellular calcium signaling via gap junctions in glioma cells. J Cell Biol 118: 195–201, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science 262: 740–744, 1993. [DOI] [PubMed] [Google Scholar]
- 60. Chow SK, Yu D, Macdonald CL, Buibas M, Silva GA. Amyloid beta-peptide directly induces spontaneous calcium transients, delayed intercellular calcium waves and gliosis in rat cortical astrocytes. ASN Neuro 2: e00026, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chuquet J, Hollender L, Nimchinsky EA. High-resolution in vivo imaging of the neurovascular unit during spreading depression. J Neurosci 27: 4036–4044, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Churchill GC, Louis CF. Roles of Ca2+, inositol trisphosphate and cyclic ADP-ribose in mediating intercellular Ca2+ signaling in sheep lens cells. J Cell Sci 111: 1217–1225, 1998. [DOI] [PubMed] [Google Scholar]
- 63. Churchill GC, Lurtz MM, Louis CF. Ca2+ regulation of gap junctional coupling in lens epithelial cells. Am J Physiol Cell Physiol 281: C972–C981, 2001. [DOI] [PubMed] [Google Scholar]
- 64. Clair C, Chalumeau C, Tordjmann T, Poggioli J, Erneux C, Dupont G, Combettes L. Investigation of the roles of Ca2+ and InsP(3) diffusion in the coordination of Ca2+ signals between connected hepatocytes. J Cell Sci 114: 1999–2007, 2001. [DOI] [PubMed] [Google Scholar]
- 65. Clair C, Combettes L, Pierre F, Sansonetti P, Tran Van Nhieu G. Extracellular-loop peptide antibodies reveal a predominant hemichannel organization of connexins in polarized intestinal cells. Exp Cell Res 314: 1250–1265, 2008. [DOI] [PubMed] [Google Scholar]
- 66. Coco S, Calegari F, Pravettoni E, Pozzi D, Taverna E, Rosa P, Matteoli M, Verderio C. Storage and release of ATP from astrocytes in culture. J Biol Chem 278: 1354–1362, 2003. [DOI] [PubMed] [Google Scholar]
- 67. Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci USA 100: 11388–11393, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Contreras JE, Sanchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Saez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci USA 99: 495–500, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247: 470–473, 1990. [DOI] [PubMed] [Google Scholar]
- 70. Cotrina ML, Lin JH, Alves-Rodrigues A, Liu S, Li J, Azmi-Ghadimi H, Kang J, Naus CC, Nedergaard M. Connexins regulate calcium signaling by controlling ATP release. Proc Natl Acad Sci USA 95: 15735–15740, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Cotrina ML, Lin JH, Lopez-Garcia JC, Naus CC, Nedergaard M. ATP-mediated glia signaling. J Neurosci 20: 2835–2844, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Covian-Nares JF, Koushik SV, Puhl HL, 3rd, Vogel SS. Membrane wounding triggers ATP release and dysferlin-mediated intercellular calcium signaling. J Cell Sci 123: 1884–1893, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Crow JM, Atkinson MM, Johnson RG. Micromolar levels of intracellular calcium reduce gap junctional permeability in lens cultures. Invest Ophthalmol Vis Sci 35: 3332–3341, 1994. [PubMed] [Google Scholar]
- 74. D'Hondt C, Ponsaerts R, De Smedt H, Vinken M, De Vuyst E, De Bock M, Wang N, Rogiers V, Leybaert L, Himpens B, Bultynck G. Pannexin channels in ATP release and beyond: an unexpected rendezvous at the endoplasmic reticulum. Cell Signal 23: 305–316, 2011. [DOI] [PubMed] [Google Scholar]
- 75. D'Hondt C, Ponsaerts R, Srinivas SP, Vereecke J, Himpens B. Thrombin inhibits intercellular calcium wave propagation in corneal endothelial cells by modulation of hemichannels and gap junctions. Invest Ophthalmol Vis Sci 48: 120–133, 2007. [DOI] [PubMed] [Google Scholar]
- 76. D'Hondt C, Srinivas SP, Vereecke J, Himpens B. Adenosine opposes thrombin-induced inhibition of intercellular calcium wave in corneal endothelial cells. Invest Ophthalmol Vis Sci 48: 1518–1527, 2007. [DOI] [PubMed] [Google Scholar]
- 77. Dahl G, Nonner W, Werner R. Attempts to define functional domains of gap junction proteins with synthetic peptides. Biophys J 67: 1816–1822, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Dakin K, Zhao Y, Li WH. LAMP, a new imaging assay of gap junctional communication unveils that Ca2+ influx inhibits cell coupling. Nat Methods 2: 55–62, 2005. [DOI] [PubMed] [Google Scholar]
- 79. Dale N. Dynamic ATP signalling and neural development. J Physiol 586: 2429–2436, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, Littman DR, Dustin ML, Gan WB. ATP mediates rapid microglial response to local brain injury in vivo. Nature Neurosci 8: 752–758, 2005. [DOI] [PubMed] [Google Scholar]
- 81. Davis DM, Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nature Rev Mol Cell Biol 9: 431–436, 2008. [DOI] [PubMed] [Google Scholar]
- 82. De Bock M, Culot M, Wang N, Bol M, Decrock E, De Vuyst E, da Costa A, Dauwe I, Vinken M, Simon AM, Rogiers V, De Ley G, Evans WH, Bultynck G, Dupont G, Cecchelli R, Leybaert L. Connexin channels provide a target to manipulate brain endothelial calcium dynamics and blood-brain barrier permeability. J Cereb Blood Flow Metab 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. De Vuyst E, Decrock E, Cabooter L, Dubyak GR, Naus CC, Evans WH, Leybaert L. Intracellular calcium changes trigger connexin 32 hemichannel opening. EMBO J 25: 34–44, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. De Vuyst E, Decrock E, De Bock M, Yamasaki H, Naus CC, Evans WH, Leybaert L. Connexin hemichannels and gap junction channels are differentially influenced by lipopolysaccharide and basic fibroblast growth factor. Mol Biol Cell 18: 34–46, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. De Vuyst E, Wang N, Decrock E, De Bock M, Vinken M, Van Moorhem M, Lai C, Culot M, Rogiers V, Cecchelli R, Naus CC, Evans WH, Leybaert L. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium 46: 176–187, 2009. [DOI] [PubMed] [Google Scholar]
- 87. De Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflügers Arch 459: 897–914, 2010. [DOI] [PubMed] [Google Scholar]
- 88. Decrock E, De Vuyst E, Vinken M, Van Moorhem M, Vranckx K, Wang N, Van Laeken L, De Bock M, D'Herde K, Lai CP, Rogiers V, Evans WH, Naus CC, Leybaert L. Connexin 43 hemichannels contribute to the propagation of apoptotic cell death in a rat C6 glioma cell model. Cell Death Differentiation 16: 151–163, 2009. [DOI] [PubMed] [Google Scholar]
- 89. Decrock E, Krysko DV, Vinken M, Kaczmarek A, Crispino G, Bol M, Wang N, De Bock M, De Vuyst E, Naus CC, Rogiers V, Vandenabeele P, Erneux C, Mammano F, Bultynck G, Leybaert L. Transfer of IP3 through gap junctions is critical, but not sufficient, for the spread of apoptosis. Cell Death Differentiation 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Decrock E, Vinken M, Bol M, D'Herde K, Rogiers V, Vandenabeele P, Krysko DV, Bultynck G, Leybaert L. Calcium and connexin-based intercellular communication, a deadly catch? Cell Calcium. In press. [DOI] [PubMed] [Google Scholar]
- 91. Delmar M, Liang FX. Connexin43, the regulation of intercalated disc function. Heart Rhythm 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Di Virgilio F, Borea PA, Illes P. P2 receptors meet the immune system. Trends Pharmacol Sci 22: 5–7, 2001. [DOI] [PubMed] [Google Scholar]
- 93. Ding S, Wang T, Cui W, Haydon PG. Photothrombosis ischemia stimulates a sustained astrocytic Ca2+ signaling in vivo. Glia 57: 767–776, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Domeier TL, Blatter LA, Zima AV. Changes in intra-luminal calcium during spontaneous calcium waves following sensitization of ryanodine receptor channels. Channels 4: 87–92, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Domenighetti AA, Beny JL, Chabaud F, Frieden M. An intercellular regenerative calcium wave in porcine coronary artery endothelial cells in primary culture. J Physiol 513: 103–116, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Dora KA. Intercellular Ca2+ signalling: the artery wall. Semin Cell Dev Biol 12: 27–35, 2001. [DOI] [PubMed] [Google Scholar]
- 97. Dunwiddie TV, Diao L, Proctor WR. Adenine nucleotides undergo rapid, quantitative conversion to adenosine in the extracellular space in rat hippocampus. J Neurosci 17: 7673–7682, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Dupont G, Combettes L, Leybaert L. Calcium dynamics: spatio-temporal organization from the subcellular to the organ level. Int Rev Cytol 261: 193–245, 2007. [DOI] [PubMed] [Google Scholar]
- 99. Dupont G, Swillens S, Clair C, Tordjmann T, Combettes L. Hierarchical organization of calcium signals in hepatocytes: from experiments to models. Biochim Biophys Acta 1498: 134–152, 2000. [DOI] [PubMed] [Google Scholar]
- 100. Dupont G, Tordjmann T, Clair C, Swillens S, Claret M, Combettes L. Mechanism of receptor-oriented intercellular calcium wave propagation in hepatocytes. FASEB J 14: 279–289, 2000. [DOI] [PubMed] [Google Scholar]
- 101. Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, Lysiak JJ, Harden TK, Leitinger N, Ravichandran KS. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461: 282–286, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev 89: 1153–1176, 2009. [DOI] [PubMed] [Google Scholar]
- 103. Enkvist MO, McCarthy KD. Activation of protein kinase C blocks astroglial gap junction communication and inhibits the spread of calcium waves. J Neurochem 59: 519–526, 1992. [DOI] [PubMed] [Google Scholar]
- 104. Enomoto K, Furuya K, Yamagishi S, Maeno T. Mechanically induced electrical and intracellular calcium responses in normal and cancerous mammary cells. Cell Calcium 13: 501–511, 1992. [DOI] [PubMed] [Google Scholar]
- 105. Evans JH, Sanderson MJ. Intracellular calcium oscillations regulate ciliary beat frequency of airway epithelial cells. Cell Calcium 26: 103–110, 1999. [DOI] [PubMed] [Google Scholar]
- 106. Evans WH, Boitano S. Connexin mimetic peptides: specific inhibitors of gap-junctional intercellular communication. Biochem Soc Trans 29: 606–612, 2001. [DOI] [PubMed] [Google Scholar]
- 107. Evans WH, De Vuyst E, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J 397: 1–14, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Evans WH, Leybaert L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun Adhesion 14: 265–273, 2007. [DOI] [PubMed] [Google Scholar]
- 109. Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS One 3: e2801, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Fanchaouy M, Serir K, Meister JJ, Beny JL, Bychkov R. Intercellular communication: role of gap junctions in establishing the pattern of ATP-elicited Ca2+ oscillations and Ca2+-dependent currents in freshly isolated aortic smooth muscle cells. Cell Calcium 37: 25–34, 2005. [DOI] [PubMed] [Google Scholar]
- 111. Fauquier T, Guerineau NC, McKinney RA, Bauer K, Mollard P. Folliculostellate cell network: a route for long-distance communication in the anterior pituitary. Proc Natl Acad Sci USA 98: 8891–8896, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Feranchak AP, Lewis MA, Kresge C, Sathe M, Bugde A, Luby-Phelps K, Antich PP, Fitz JG. Initiation of purinergic signaling by exocytosis of ATP-containing vesicles in liver epithelium. J Biol Chem 285: 8138–8147, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Fiacco TA, McCarthy KD. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J Neurosci 24: 722–732, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Fields RD. Imaging single photons and intrinsic optical signals for studies of vesicular and non-vesicular ATP release from axons. Front Neuroanatomy 5: 32, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res 95: e73–e81, 2004. [DOI] [PubMed] [Google Scholar]
- 116. Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nature Neurosci 9: 1397–1403, 2006. [DOI] [PubMed] [Google Scholar]
- 117. Finkbeiner S. Calcium waves in astrocytes-filling in the gaps. Neuron 8: 1101–1108, 1992. [DOI] [PubMed] [Google Scholar]
- 118. Foskett JK, White C, Cheung KH, Mak DO. Inositol trisphosphate receptor Ca2+ release channels. Physiol Rev 87: 593–658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Gallagher CJ, Salter MW. Differential properties of astrocyte calcium waves mediated by P2Y1 and P2Y2 receptors. J Neurosci 23: 6728–6739, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Garaschuk O, Milos RI, Grienberger C, Marandi N, Adelsberger H, Konnerth A. Optical monitoring of brain function in vivo: from neurons to networks. Pflügers Arch 453: 385–396, 2006. [DOI] [PubMed] [Google Scholar]
- 121. Garre JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Saez JC, Bennett MV, Abudara V. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci USA 107: 22659–22664, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Gaspers LD, Thomas AP. Calcium signaling in liver. Cell Calcium 38: 329–342, 2005. [DOI] [PubMed] [Google Scholar]
- 123. Giaume C, Venance L. Intercellular calcium signaling and gap junctional communication in astrocytes. Glia 24: 50–64, 1998. [PubMed] [Google Scholar]
- 124. Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA 107: 3811–3816, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Goldberg M, De Pitta M, Volman V, Berry H, Ben-Jacob E. Nonlinear gap junctions enable long-distance propagation of pulsating calcium waves in astrocyte networks. PLoS Comput Biol 6: e1000909, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Gomes P, Malfait M, Himpens B, Vereecke J. Intercellular Ca2+-transient propagation in normal and high glucose solutions in rat retinal epithelial (RPE-J) cells during mechanical stimulation. Cell Calcium 34: 185–192, 2003. [DOI] [PubMed] [Google Scholar]
- 127. Gomes P, Srinivas SP, Van Driessche W, Vereecke J, Himpens B. ATP release through connexin hemichannels in corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 1208–1218, 2005. [DOI] [PubMed] [Google Scholar]
- 128. Gomes P, Srinivas SP, Vereecke J, Himpens B. ATP-dependent paracrine intercellular communication in cultured bovine corneal endothelial cells. Invest Ophthalmol Vis Sci 46: 104–113, 2005. [DOI] [PubMed] [Google Scholar]
- 129. Gomes P, Srinivas SP, Vereecke J, Himpens B. Gap junctional intercellular communication in bovine corneal endothelial cells. Exp Eye Res 83: 1225–1237, 2006. [DOI] [PubMed] [Google Scholar]
- 130. Gomez P, Vereecke J, Himpens B. Intra- and intercellular Ca2+-transient propagation in normal and high glucose solutions in ROS cells during mechanical stimulation. Cell Calcium 29: 137–148, 2001. [DOI] [PubMed] [Google Scholar]
- 131. Gomez-Hernandez JM, de Miguel M, Larrosa B, Gonzalez D, Barrio LC. Molecular basis of calcium regulation in connexin-32 hemichannels. Proc Natl Acad Sci USA 100: 16030–16035, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456: 745–749, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S. Astrocytes control breathing through pH-dependent release of ATP. Science 329: 571–575, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Gracheva ME, Gunton JD. Intercellular communication via intracellular calcium oscillations. J Theor Biol 221: 513–518, 2003. [DOI] [PubMed] [Google Scholar]
- 135. Grafstein B, Liu S, Cotrina ML, Goldman SA, Nedergaard M. Meningeal cells can communicate with astrocytes by calcium signaling. Ann Neurol 47: 18–25, 2000. [PubMed] [Google Scholar]
- 136. Grandolfo M, Calabrese A, D'Andrea P. Mechanism of mechanically induced intercellular calcium waves in rabbit articular chondrocytes and in HIG-82 synovial cells. J Bone Miner Res 13: 443–453, 1998. [DOI] [PubMed] [Google Scholar]
- 137. Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB. ATP released from astrocytes mediates glial calcium waves. J Neurosci 19: 520–528, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Gyorke I, Gyorke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J 75: 2801–2810, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Haas B, Schipke CG, Peters O, Sohl G, Willecke K, Kettenmann H. Activity-dependent ATP-waves in the mouse neocortex are independent from astrocytic calcium waves. Cereb Cortex 16: 237–246, 2006. [DOI] [PubMed] [Google Scholar]
- 140. Hagar RE, Burgstahler AD, Nathanson MH, Ehrlich BE. Type III InsP3 receptor channel stays open in the presence of increased calcium. Nature 396: 81–84, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Halidi N, Boittin FX, Beny JL, Meister JJ. Propagation of fast and slow intercellular Ca2+ waves in primary cultured arterial smooth muscle cells. Cell Calcium 50: 459–467, 2011. [DOI] [PubMed] [Google Scholar]
- 142. Hansen AJ. Effect of anoxia on ion distribution in the brain. Physiol Rev 65: 101–148, 1985. [DOI] [PubMed] [Google Scholar]
- 143. Hansen M, Boitano S, Dirksen ER, Sanderson MJ. Intercellular calcium signaling induced by extracellular adenosine 5′-triphosphate and mechanical stimulation in airway epithelial cells. J Cell Sci 106: 995–1004, 1993. [DOI] [PubMed] [Google Scholar]
- 144. Hansen M, Boitano S, Dirksen ER, Sanderson MJ. A role for phospholipase C activity but not ryanodine receptors in the initiation and propagation of intercellular calcium waves. J Cell Sci 108: 2583–2590, 1995. [DOI] [PubMed] [Google Scholar]
- 145. Harris-White ME, Zanotti SA, Frautschy SA, Charles AC. Spiral intercellular calcium waves in hippocampal slice cultures. J Neurophysiol 79: 1045–1052, 1998. [DOI] [PubMed] [Google Scholar]
- 146. Hashitani H, Yanai Y, Suzuki H. Role of interstitial cells and gap junctions in the transmission of spontaneous Ca2+ signals in detrusor smooth muscles of the guinea-pig urinary bladder. J Physiol 559: 567–581, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Hassinger TD, Guthrie PB, Atkinson PB, Bennett MV, Kater SB. An extracellular signaling component in propagation of astrocytic calcium waves. Proc Natl Acad Sci USA 93: 13268–13273, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Haughey NJ, Mattson MP. Alzheimer's amyloid beta-peptide enhances ATP/gap junction-mediated calcium-wave propagation in astrocytes. Neuromol Med 3: 173–180, 2003. [DOI] [PubMed] [Google Scholar]
- 149. Heinemann U, Louvel J. Changes in [Ca2+]o and [K+]o during repetitive electrical stimulation and during pentetrazol induced seizure activity in the sensorimotor cortex of cats. Pflügers Arch 398: 310–317, 1983. [DOI] [PubMed] [Google Scholar]
- 150. Hellman B, Dansk H, Grapengiesser E. Pancreatic beta-cells communicate via intermittent release of ATP. Am J Physiol Endocrinol Metab 286: E759–E765, 2004. [DOI] [PubMed] [Google Scholar]
- 151. Hennig GW, Smith CB, O'Shea DM, Smith TK. Patterns of intracellular and intercellular Ca2+ waves in the longitudinal muscle layer of the murine large intestine in vitro. J Physiol 543: 233–253, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Henriksen Z, Hiken JF, Steinberg TH, Jorgensen NR. The predominant mechanism of intercellular calcium wave propagation changes during long-term culture of human osteoblast-like cells. Cell Calcium 39: 435–444, 2006. [DOI] [PubMed] [Google Scholar]
- 153. Hettiarachchi NT, Dallas ML, Pearson HA, Bruce G, Deuchars S, Boyle JP, Peers C. Gap junction-mediated spontaneous Ca2+ waves in differentiated cholinergic SN56 cells. Biochem Biophys Res Commun 397: 564–568, 2010. [DOI] [PubMed] [Google Scholar]
- 154. Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol 2: E96, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Hofer AM, Brown EM. Extracellular calcium sensing and signalling. Nature Rev Mol Cell Biol 4: 530–538, 2003. [DOI] [PubMed] [Google Scholar]
- 156. Hofer AM, Curci S, Doble MA, Brown EM, Soybel DI. Intercellular communication mediated by the extracellular calcium-sensing receptor. Nature Cell Biol 2: 392–398, 2000. [DOI] [PubMed] [Google Scholar]
- 157. Hofer T. Model of intercellular calcium oscillations in hepatocytes: synchronization of heterogeneous cells. Biophys J 77: 1244–1256, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Hofer T, Politi A, Heinrich R. Intercellular Ca2+ wave propagation through gap-junctional Ca2+ diffusion: a theoretical study. Biophys J 80: 75–87, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hofer T, Venance L, Giaume C. Control and plasticity of intercellular calcium waves in astrocytes: a modeling approach. J Neurosci 22: 4850–4859, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Homolya L, Steinberg TH, Boucher RC. Cell to cell communication in response to mechanical stress via bilateral release of ATP and UTP in polarized epithelia. J Cell Biol 150: 1349–1360, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hoogland TM, Kuhn B, Gobel W, Huang W, Nakai J, Helmchen F, Flint J, Wang SS. Radially expanding transglial calcium waves in the intact cerebellum. Proc Natl Acad Sci USA 106: 3496–3501, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 104: 6436–6441, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Humeau Y, Doussau F, Grant NJ, Poulain B. How botulinum and tetanus neurotoxins block neurotransmitter release. Biochimie 82: 427–446, 2000. [DOI] [PubMed] [Google Scholar]
- 164. Hung J, Colicos MA. Astrocytic Ca2+ waves guide CNS growth cones to remote regions of neuronal activity. PLoS One 3: e3692, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Huo B, Lu XL, Costa KD, Xu Q, Guo XE. An ATP-dependent mechanism mediates intercellular calcium signaling in bone cell network under single cell nanoindentation. Cell Calcium 47: 234–241, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Iacobas DA, Suadicani SO, Spray DC, Scemes E. A stochastic two-dimensional model of intercellular Ca2+ wave spread in glia. Biophys J 90: 24–41, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Iglesias R, Locovei S, Roque A, Alberto AP, Dahl G, Spray DC, Scemes E. P2X7 receptor-pannexin1 complex: pharmacology and signaling. Am J Physiol Cell Physiol 295: C752–C760, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Innocenti B, Parpura V, Haydon PG. Imaging extracellular waves of glutamate during calcium signaling in cultured astrocytes. J Neurosci 20: 1800–1808, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Isakson BE, Evans WH, Boitano S. Intercellular Ca2+ signaling in alveolar epithelial cells through gap junctions and by extracellular ATP. Am J Physiol Lung Cell Mol Physiol 280: L221–L228, 2001. [DOI] [PubMed] [Google Scholar]
- 170. Isakson BE, Ramos SI, Duling BR. Ca2+ and inositol 1,4,5-trisphosphate-mediated signaling across the myoendothelial junction. Circ Res 100: 246–254, 2007. [DOI] [PubMed] [Google Scholar]
- 171. Isakson BE, Seedorf GJ, Lubman RL, Evans WH, Boitano S. Cell-cell communication in heterocellular cultures of alveolar epithelial cells. Am J Respir Cell Mol Biol 29: 552–561, 2003. [DOI] [PubMed] [Google Scholar]
- 171a. Ishikawa M, Iwamoto T, Nakamura T, Doyle A, Fukumoto S, Yamada Y. Pannexin 3 functions as an ER Ca(2+) channel, hemichannel, and gap junction to promote osteoblast differentiation. J Cell Biol 193: 1257–1274, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Iwabuchi S, Kawahara K. Oxygen-glucose deprivation-induced enhancement of extracellular ATP-P2Y purinoceptors signaling for the propagation of astrocytic calcium waves. Biosystems 96: 35–43, 2009. [DOI] [PubMed] [Google Scholar]
- 173. Iwabuchi S, Kawahara K, Makisaka K, Sato H. Photolytic flash-induced intercellular calcium waves using caged calcium ionophore in cultured astrocytes from newborn rats. Exp Brain Res 146: 103–116, 2002. [DOI] [PubMed] [Google Scholar]
- 174. Jacobson KA, Boeynaems JM. P2Y nucleotide receptors: promise of therapeutic applications. Drug Discov Today 15: 570–578, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Jiang JX, Siller-Jackson AJ, Burra S. Roles of gap junctions and hemichannels in bone cell functions and in signal transmission of mechanical stress. Front Biosci 12: 1450–1462, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. John GR, Scemes E, Suadicani SO, Liu JS, Charles PC, Lee SC, Spray DC, Brosnan CF. IL-1beta differentially regulates calcium wave propagation between primary human fetal astrocytes via pathways involving P2 receptors and gap junction channels. Proc Natl Acad Sci USA 96: 11613–11618, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Jones BF, Wall ME, Carroll RL, Washburn S, Banes AJ. Ligament cells stretch-adapted on a microgrooved substrate increase intercellular communication in response to a mechanical stimulus. J Biomech 38: 1653–1664, 2005. [DOI] [PubMed] [Google Scholar]
- 178. Jorgensen NR. Short-range intercellular calcium signaling in bone. APMIS Suppl 5–36, 2005. [PubMed] [Google Scholar]
- 179. Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, Nedergaard M. Connexin 43 hemichannels are permeable to ATP. J Neurosci 28: 4702–4711, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kapela A, Nagaraja S, Parikh J, Tsoukias NM. Modeling Ca2+ signaling in the microcirculation: intercellular communication and vasoreactivity. Crit Rev Biomed Eng 39: 435–460, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science 274: 1133–1138, 1996. [DOI] [PubMed] [Google Scholar]
- 182. Kepseu WD, Woafo P. Intercellular waves propagation in an array of cells coupled through paracrine signaling: a computer simulation study. Phys Rev E Stat Nonlin Soft Matter Phys 73: 041912, 2006. [DOI] [PubMed] [Google Scholar]
- 183. Kijima Y, Saito A, Jetton TL, Magnuson MA, Fleischer S. Different intracellular localization of inositol 1,4,5-trisphosphate and ryanodine receptors in cardiomyocytes. J Biol Chem 268: 3499–3506, 1993. [PubMed] [Google Scholar]
- 184. Kim YJ, Elliott AC, Moon SJ, Lee SI, Seo JT. Octanol blocks fluid secretion by inhibition of capacitative calcium entry in rat mandibular salivary acinar cells. Cell Calcium 25: 77–84, 1999. [DOI] [PubMed] [Google Scholar]
- 185. Kockskamper J, Zima AV, Roderick HL, Pieske B, Blatter LA, Bootman MD. Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes. J Mol Cell Cardiol 45: 128–147, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Koenigsberger M, Seppey D, Beny JL, Meister JJ. Mechanisms of propagation of intercellular calcium waves in arterial smooth muscle cells. Biophys J 99: 333–343, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Koizumi S. Synchronization of Ca2+ oscillations: involvement of ATP release in astrocytes. FEBS J 277: 286–292, 2010. [DOI] [PubMed] [Google Scholar]
- 188. Koizumi S, Fujishita K, Inoue K, Shigemoto-Mogami Y, Tsuda M. Ca2+ waves in keratinocytes are transmitted to sensory neurons: the involvement of extracellular ATP and P2Y2 receptor activation. Biochem J 380: 329–338, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Kono T, Nishikori T, Kataoka H, Uchio Y, Ochi M, Enomoto K. Spontaneous oscillation and mechanically induced calcium waves in chondrocytes. Cell Biochem Funct 24: 103–111, 2006. [DOI] [PubMed] [Google Scholar]
- 190. Kozoriz MG, Bechberger JF, Bechberger GR, Suen MW, Moreno AP, Maass K, Willecke K, Naus CC. The connexin43 C-terminal region mediates neuroprotection during stroke. J Neuropathol Exp Neurol 69: 196–206, 2010. [DOI] [PubMed] [Google Scholar]
- 191. Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science 323: 1211–1215, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Kuga N, Sasaki T, Takahara Y, Matsuki N, Ikegaya Y. Large-scale calcium waves traveling through astrocytic networks in vivo. J Neurosci 31: 2607–2614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Kurth-Nelson ZL, Mishra A, Newman EA. Spontaneous glial calcium waves in the retina develop over early adulthood. J Neurosci 29: 11339–11346, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Lagostena L, Ashmore JF, Kachar B, Mammano F. Purinergic control of intercellular communication between Hensen's cells of the guinea-pig cochlea. J Physiol 531: 693–706, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Lagoudakis L, Garcin I, Julien B, Nahum K, Gomes DA, Combettes L, Nathanson MH, Tordjmann T. Cytosolic calcium regulates liver regeneration in the rat. Hepatology 52: 602–611, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Lahne M, Gale JE. Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J Neurosci 28: 4918–4928, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Lahne M, Gale JE. Damage-induced cell-cell communication in different cochlear cell types via two distinct ATP-dependent Ca waves. Purinergic Signal 6: 189–200, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197a. Lai CP, Bechberger JF, Thompson RJ, MacVicar BA, Bruzzone R, Naus CC. Tumorsuppressive effects of pannexin 1 in C6 glioma cells. Cancer Res 67: 1545–1554, 2007. [DOI] [PubMed] [Google Scholar]
- 198. Laird DW. Life cycle of connexins in health and disease. Biochem J 394: 527–543, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Lamont C, Luther PW, Balke CW, Wier WG. Intercellular Ca2+ waves in rat heart muscle. J Physiol 512: 669–676, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Langer J, Stephan J, Theis M, Rose CR. Gap junctions mediate intercellular spread of sodium between hippocampal astrocytes in situ. Glia 60: 239–252, 2012. [DOI] [PubMed] [Google Scholar]
- 201. Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harbor Perspect Biol 2: a003996, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Lansley AB, Sanderson MJ. Regulation of airway ciliary activity by Ca2+: simultaneous measurement of beat frequency and intracellular Ca2+. Biophys J 77: 629–638, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Laver DR. Ca2+ stores regulate ryanodine receptor Ca2+ release channels via luminal and cytosolic Ca2+ sites. Clin Exp Pharmacol Physiol 34: 889–896, 2007. [DOI] [PubMed] [Google Scholar]
- 204. Laver DR, Owen VJ, Junankar PR, Taske NL, Dulhunty AF, Lamb GD. Reduced inhibitory effect of Mg2+ on ryanodine receptor-Ca2+ release channels in malignant hyperthermia. Biophys J 73: 1913–1924, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Lazrak A, Peracchia C. Gap junction gating sensitivity to physiological internal calcium regardless of pH in Novikoff hepatoma cells. Biophys J 65: 2002–2012, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Lee HC. Mechanisms of calcium signaling by cyclic ADP-ribose and NAADP. Physiol Rev 77: 1133–1164, 1997. [DOI] [PubMed] [Google Scholar]
- 207. Leiper LJ, Walczysko P, Kucerova R, Ou J, Shanley LJ, Lawson D, Forrester JV, McCaig CD, Zhao M, Collinson JM. The roles of calcium signaling and ERK1/2 phosphorylation in a Pax6+/− mouse model of epithelial wound-healing delay. BMC Biol 4: 27, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Leybaert L, Braet K, Vandamme W, Cabooter L, Martin PE, Evans WH. Connexin channels, connexin mimetic peptides and ATP release. Cell Commun Adhesion 10: 251–257, 2003. [DOI] [PubMed] [Google Scholar]
- 209. Leybaert L, De Ley G. Interstitial and tissue cations and electrical potential after experimental spinal cord injury. Exp Brain Res 100: 369–375, 1994. [DOI] [PubMed] [Google Scholar]
- 210. Leybaert L, Paemeleire K, Strahonja A, Sanderson MJ. Inositol-trisphosphate-dependent intercellular calcium signaling in and between astrocytes and endothelial cells. Glia 24: 398–407, 1998. [PubMed] [Google Scholar]
- 211. Leybaert L, Sanderson MJ. Intercellular calcium signaling and flash photolysis of caged compounds. A sensitive method to evaluate gap junctional coupling. Methods Mol Biol 154: 407–430, 2001. [DOI] [PubMed] [Google Scholar]
- 212. Li A, Leung CT, Peterson-Yantorno K, Mitchell CH, Civan MM. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am J Physiol Cell Physiol 299: C1308–C1317, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Liu F, Arce FT, Ramachandran S, Lal R. Nanomechanics of hemichannel conformations: connexin flexibility underlying channel opening and closing. J Biol Chem 281: 23207–23217, 2006. [DOI] [PubMed] [Google Scholar]
- 214. Liu X, Hashimoto-Torii K, Torii M, Haydar TF, Rakic P. The role of ATP signaling in the migration of intermediate neuronal progenitors to the neocortical subventricular zone. Proc Natl Acad Sci USA 105: 11802–11807, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett 580: 239–244, 2006. [DOI] [PubMed] [Google Scholar]
- 216. Loughrey CM, MacEachern KE, Neary P, Smith GL. The relationship between intracellular [Ca2+] and Ca2+ wave characteristics in permeabilised cardiomyocytes from the rabbit. J Physiol 543: 859–870, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Lukyanenko V, Subramanian S, Gyorke I, Wiesner TF, Gyorke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J Physiol 518: 173–186, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Lurtz MM, Louis CF. Intracellular calcium regulation of connexin43. Am J Physiol Cell Physiol 293: C1806–C1813, 2007. [DOI] [PubMed] [Google Scholar]
- 219. Lynn BD, Marotta CA, Nagy JI. Propagation of intercellular calcium waves in PC12 cells overexpressing a carboxy-terminal fragment of amyloid precursor protein. Neurosci Lett 199: 21–24, 1995. [DOI] [PubMed] [Google Scholar]
- 220. Macdonald CL, Yu D, Buibas M, Silva GA. Diffusion modeling of ATP signaling suggests a partially regenerative mechanism underlies astrocyte intercellular calcium waves. Front Neuroeng 1: 1, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Majumder P, Crispino G, Rodriguez L, Ciubotaru CD, Anselmi F, Piazza V, Bortolozzi M, Mammano F. ATP-mediated cell-cell signaling in the organ of Corti: the role of connexin channels. Purinergic Signal 6: 167–187, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Malchow RP, Qian H, Ripps H. A novel action of quinine and quinidine on the membrane conductance of neurons from the vertebrate retina. J Gen Physiol 104: 1039–1055, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Mammano F, Bortolozzi M, Ortolano S, Anselmi F. Ca2+ signaling in the inner ear. Physiology 22: 131–144, 2007. [DOI] [PubMed] [Google Scholar]
- 224. Marshall IC, Taylor CW. Two calcium-binding sites mediate the interconversion of liver inositol 1,4,5-trisphosphate receptors between three conformational states. Biochem J 301: 591–598, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Matchkov VV, Rahman A, Bakker LM, Griffith TM, Nilsson H, Aalkjaer C. Analysis of effects of connexin-mimetic peptides in rat mesenteric small arteries. Am J Physiol Heart Circ Physiol 291: H357–H367, 2006. [DOI] [PubMed] [Google Scholar]
- 226. Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. J Biol Chem 259: 2365–2374, 1984. [PubMed] [Google Scholar]
- 227. Meissner G. Regulation of mammalian ryanodine receptors. Front Biosci 7: d2072–d2080, 2002. [DOI] [PubMed] [Google Scholar]
- 228. Meissner G. Ryanodine receptor/Ca2+ release channels and their regulation by endogenous effectors. Annu Rev Physiol 56: 485–508, 1994. [DOI] [PubMed] [Google Scholar]
- 229. Meissner G, Rios E, Tripathy A, Pasek DA. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. J Biol Chem 272: 1628–1638, 1997. [DOI] [PubMed] [Google Scholar]
- 230. Meszaros LG, Bak J, Chu A. Cyclic ADP-ribose as an endogenous regulator of the non-skeletal type ryanodine receptor Ca2+ channel. Nature 364: 76–79, 1993. [DOI] [PubMed] [Google Scholar]
- 231. Metea MR, Kofuji P, Newman EA. Neurovascular coupling is not mediated by potassium siphoning from glial cells. J Neurosci 27: 2468–2471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci 26: 2862–2870, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Meyer RA, Laird DW, Revel JP, Johnson RG. Inhibition of gap junction and adherens junction assembly by connexin and A-CAM antibodies. J Cell Biol 119: 179–189, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Minamikawa T, Cody SH, Williams DA. In situ visualization of spontaneous calcium waves within perfused whole rat heart by confocal imaging. Am J Physiol Heart Circ Physiol 272: H236–H243, 1997. [DOI] [PubMed] [Google Scholar]
- 235. Mlinar B, Enyeart JJ. Block of current through T-type calcium channels by trivalent metal cations and nickel in neural rat and human cells. J Physiol 469: 639–652, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Moerenhout M, Himpens B, Vereecke J. Intercellular communication upon mechanical stimulation of CPAE- endothelial cells is mediated by nucleotides. Cell Calcium 29: 125–136, 2001. [DOI] [PubMed] [Google Scholar]
- 237. Montana V, Malarkey EB, Verderio C, Matteoli M, Parpura V. Vesicular transmitter release from astrocytes. Glia 54: 700–715, 2006. [DOI] [PubMed] [Google Scholar]
- 238. Motoyama K, Karl IE, Flye MW, Osborne DF, Hotchkiss RS. Effect of Ca2+ agonists in the perfused liver: determination via laser scanning confocal microscopy. Am J Physiol Regul Integr Comp Physiol 276: R575–R585, 1999. [DOI] [PubMed] [Google Scholar]
- 239. Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431: 195–199, 2004. [DOI] [PubMed] [Google Scholar]
- 240. Muyderman H, Nilsson M, Blomstrand F, Khatibi S, Olsson T, Hansson E, Ronnback L. Modulation of mechanically induced calcium waves in hippocampal astroglial cells. Inhibitory effects of alpha 1-adrenergic stimulation. Brain Res 793: 127–135, 1998. [DOI] [PubMed] [Google Scholar]
- 241. Nagy JI, Li W, Hertzberg EL, Marotta CA. Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer's disease. Brain Res 717: 173–178, 1996. [DOI] [PubMed] [Google Scholar]
- 242. Nathanson MH, Burgstahler AD, Mennone A, Fallon MB, Gonzalez CB, Saez JC. Ca2+ waves are organized among hepatocytes in the intact organ. Am J Physiol Gastrointest Liver Physiol 269: G167–G171, 1995. [DOI] [PubMed] [Google Scholar]
- 243. Naus CC, Bechberger JF, Zhang Y, Venance L, Yamasaki H, Juneja SC, Kidder GM, Giaume C. Altered gap junctional communication, intercellular signaling, and growth in cultured astrocytes deficient in connexin43. J Neurosci Res 49: 528–540, 1997. [DOI] [PubMed] [Google Scholar]
- 244. Nedergaard M. Direct signaling from astrocytes to neurons in cultures of mammalian brain cells. Science 263: 1768–1771, 1994. [DOI] [PubMed] [Google Scholar]
- 245. Newman EA. Propagation of intercellular calcium waves in retinal astrocytes and Muller cells. J Neurosci 21: 2215–2223, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Newman EA, Zahs KR. Calcium waves in retinal glial cells. Science 275: 844–847, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci 18: 4022–4028, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Niessen H, Harz H, Bedner P, Kramer K, Willecke K. Selective permeability of different connexin channels to the second messenger inositol 1,4,5-trisphosphate. J Cell Sci 113: 1365–1372, 2000. [DOI] [PubMed] [Google Scholar]
- 249. Nihei OK, Campos de Carvalho AC, Spray DC, Savino W, Alves LA. A novel form of cellular communication among thymic epithelial cells: intercellular calcium wave propagation. Am J Physiol Cell Physiol 285: C1304–C1313, 2003. [DOI] [PubMed] [Google Scholar]
- 250. Nimmerjahn A, Kirchhoff F, Kerr JN, Helmchen F. Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat Methods 1: 31–37, 2004. [DOI] [PubMed] [Google Scholar]
- 250a. Nimmerjahn A, Mukamel EA, Schnitzer MJ. Motor behavior activates Bergmann glial networks. Neuron 62: 400–412, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 40: 563–580, 2000. [DOI] [PubMed] [Google Scholar]
- 252. Ohki K, Chung S, Ch'ng YH, Kara P, Reid RC. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433: 597–603, 2005. [DOI] [PubMed] [Google Scholar]
- 253. Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281: 22992–23002, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Orellana JA, Saez PJ, Shoji KF, Schalper KA, Palacios-Prado N, Velarde V, Giaume C, Bennett MV, Saez JC. Modulation of brain hemichannels and gap junction channels by pro-inflammatory agents and their possible role in neurodegeneration. Antioxid Redox Signal 11: 369–399, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Osipchuk Y, Cahalan M. Cell-to-cell spread of calcium signals mediated by ATP receptors in mast cells. Nature 359: 241–244, 1992. [DOI] [PubMed] [Google Scholar]
- 257. Paemeleire K, Leybaert L. ATP-dependent astrocyte-endothelial calcium signaling following mechanical damage to a single astrocyte in astrocyte-endothelial co-cultures. J Neurotrauma 17: 345–358, 2000. [DOI] [PubMed] [Google Scholar]
- 258. Paemeleire K, Leybaert L. Ionic changes accompanying astrocytic intercellular calcium waves triggered by mechanical cell damaging stimulation. Brain Res 857: 235–245, 2000. [DOI] [PubMed] [Google Scholar]
- 259. Paemeleire K, Martin PE, Coleman SL, Fogarty KE, Carrington WA, Leybaert L, Tuft RA, Evans WH, Sanderson MJ. Intercellular calcium waves in HeLa cells expressing GFP-labeled connexin 43, 32, or 26. Mol Biol Cell 11: 1815–1827, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Palmer RK, Yule DI, Shewach DS, Williams JA, Fisher SK. Paracrine mediation of calcium signaling in human SK-N-MCIXC neuroepithelioma cells. Am J Physiol Cell Physiol 271: C43–C53, 1996. [DOI] [PubMed] [Google Scholar]
- 262. Pangrsic T, Potokar M, Stenovec M, Kreft M, Fabbretti E, Nistri A, Pryazhnikov E, Khiroug L, Giniatullin R, Zorec R. Exocytotic release of ATP from cultured astrocytes. J Biol Chem 282: 28749–28758, 2007. [DOI] [PubMed] [Google Scholar]
- 263. Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG. Glutamate-mediated astrocyte-neuron signalling. Nature 369: 744–747, 1994. [DOI] [PubMed] [Google Scholar]
- 264. Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nature Neurosci 4: 803–812, 2001. [DOI] [PubMed] [Google Scholar]
- 265. Parthasarathi K, Ichimura H, Monma E, Lindert J, Quadri S, Issekutz A, Bhattacharya J. Connexin 43 mediates spread of Ca2+-dependent proinflammatory responses in lung capillaries. J Clin Invest 116: 2193–2200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Parys B, Cote A, Gallo V, De Koninck P, Sik A. Intercellular calcium signaling between astrocytes and oligodendrocytes via gap junctions in culture. Neuroscience 167: 1032–1043, 2010. [DOI] [PubMed] [Google Scholar]
- 267. Patel S, Marchant JS, Brailoiu E. Two-pore channels: regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium 47: 480–490, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Patel S, Robb-Gaspers LD, Stellato KA, Shon M, Thomas AP. Coordination of calcium signalling by endothelial-derived nitric oxide in the intact liver. Nature Cell Biol 1: 467–471, 1999. [DOI] [PubMed] [Google Scholar]
- 269. Pearson RA, Catsicas M, Becker DL, Bayley P, Luneborg NL, Mobbs P. Ca2+ signalling and gap junction coupling within and between pigment epithelium and neural retina in the developing chick. Eur J Neurosci 19: 2435–2445, 2004. [DOI] [PubMed] [Google Scholar]
- 270. Pearson RA, Dale N, Llaudet E, Mobbs P. ATP released via gap junction hemichannels from the pigment epithelium regulates neural retinal progenitor proliferation. Neuron 46: 731–744, 2005. [DOI] [PubMed] [Google Scholar]
- 271. Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 25: 5071–5082, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Peracchia C. Chemical gating of gap junction channels: roles of calcium, pH and calmodulin. Biochim Biophys Acta 1662: 61–80, 2004. [DOI] [PubMed] [Google Scholar]
- 273. Perea G, Araque A. Astrocytes potentiate transmitter release at single hippocampal synapses. Science 317: 1083–1086, 2007. [DOI] [PubMed] [Google Scholar]
- 274. Perea G, Navarrete M, Araque A. Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32: 421–431, 2009. [DOI] [PubMed] [Google Scholar]
- 275. Peters O, Schipke CG, Hashimoto Y, Kettenmann H. Different mechanisms promote astrocyte Ca2+ waves and spreading depression in the mouse neocortex. J Neurosci 23: 9888–9896, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Peti-Peterdi J. Calcium wave of tubuloglomerular feedback. Am J Physiol Renal Physiol 291: F473–F480, 2006. [DOI] [PubMed] [Google Scholar]
- 277. Piazza V, Ciubotaru CD, Gale JE, Mammano F. Purinergic signalling and intercellular Ca2+ wave propagation in the organ of Corti. Cell Calcium 41: 77–86, 2007. [DOI] [PubMed] [Google Scholar]
- 278. Ponsaerts R, D'Hondt C, Bultynck G, Srinivas SP, Vereecke J, Himpens B. The myosin II ATPase inhibitor blebbistatin prevents thrombin-induced inhibition of intercellular calcium wave propagation in corneal endothelial cells. Invest Ophthalmol Vis Sci 49: 4816–4827, 2008. [DOI] [PubMed] [Google Scholar]
- 279. Ponsaerts R, De Vuyst E, Retamal M, D'Hondt C, Vermeire D, Wang N, De Smedt H, Zimmermann P, Himpens B, Vereecke J, Leybaert L, Bultynck G. Intramolecular loop/tail interactions are essential for connexin 43-hemichannel activity. FASEB J 24: 4378–4395, 2010. [DOI] [PubMed] [Google Scholar]
- 280. Praetorius HA, Leipziger J. ATP release from non-excitable cells. Purinergic Signal 5: 433–446, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol 296: C250–C255, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Rahman T, Taylor CW. Dynamic regulation of IP3 receptor clustering and activity by IP3. Channels 3: 226–232, 2009. [DOI] [PubMed] [Google Scholar]
- 283. Ramos-Franco J, Bare D, Caenepeel S, Nani A, Fill M, Mignery G. Single-channel function of recombinant type 2 inositol 1,4,5-trisphosphate receptor. Biophys J 79: 1388–1399, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol 41: 525–534, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev 80: 1291–1335, 2000. [DOI] [PubMed] [Google Scholar]
- 286. Reigada D, Mitchell CH. Release of ATP from retinal pigment epithelial cells involves both CFTR and vesicular transport. Am J Physiol Cell Physiol 288: C132–C140, 2005. [DOI] [PubMed] [Google Scholar]
- 287. Reisin IL, Prat AG, Abraham EH, Amara JF, Gregory RJ, Ausiello DA, Cantiello HF. The cystic fibrosis transmembrane conductance regulator is a dual ATP and chloride channel. J Biol Chem 269: 20584–20591, 1994. [PubMed] [Google Scholar]
- 288. Reither G, Schaefer M, Lipp P. PKCalpha: a versatile key for decoding the cellular calcium toolkit. J Cell Biol 174: 521–533, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Retamal MA, Cortes CJ, Reuss L, Bennett MV, Saez JC. S-nitrosylation and permeation through connexin 43 hemichannels in astrocytes: induction by oxidant stress and reversal by reducing agents. Proc Natl Acad Sci USA 103: 4475–4480, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Retamal MA, Froger N, Palacios-Prado N, Ezan P, Saez PJ, Saez JC, Giaume C. Cx43 hemichannels and gap junction channels in astrocytes are regulated oppositely by proinflammatory cytokines released from activated microglia. J Neurosci 27: 13781–13792, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86: 369–408, 2006. [DOI] [PubMed] [Google Scholar]
- 292. Robb-Gaspers LD, Thomas AP. Coordination of Ca2+ signaling by intercellular propagation of Ca2+ waves in the intact liver. J Biol Chem 270: 8102–8107, 1995. [DOI] [PubMed] [Google Scholar]
- 293. Roderick HL, Berridge MJ, Bootman MD. Calcium-induced calcium release. Curr Biol 13: R425, 2003. [DOI] [PubMed] [Google Scholar]
- 294. Romanello M, Codognotto A, Bicego M, Pines A, Tell G, D'Andrea P. Autocrine/paracrine stimulation of purinergic receptors in osteoblasts: contribution of vesicular ATP release. Biochem Biophys Res Commun 331: 1429–1438, 2005. [DOI] [PubMed] [Google Scholar]
- 295. Romanello M, D'Andrea P. Dual mechanism of intercellular communication in HOBIT osteoblastic cells: a role for gap-junctional hemichannels. J Bone Miner Res 16: 1465–1476, 2001. [DOI] [PubMed] [Google Scholar]
- 296. Romanello M, Veronesi V, D'Andrea P. Mechanosensitivity and intercellular communication in HOBIT osteoblastic cells: a possible role for gap junction hemichannels. Biorheology 40: 119–121, 2003. [PubMed] [Google Scholar]
- 297. Rose B, Loewenstein WR. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature 254: 250–252, 1975. [DOI] [PubMed] [Google Scholar]
- 298. Rousseau E, Ladine J, Liu QY, Meissner G. Activation of the Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Archiv Biochem Biophys 267: 75–86, 1988. [DOI] [PubMed] [Google Scholar]
- 299. Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science 303: 1007–1010, 2004. [DOI] [PubMed] [Google Scholar]
- 300. Ryu SY, Peixoto PM, Won JH, Yule DI, Kinnally KW. Extracellular ATP and P2Y2 receptors mediate intercellular Ca2+ waves induced by mechanical stimulation in submandibular gland cells: role of mitochondrial regulation of store operated Ca2+ entry. Cell Calcium 47: 65–76, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Sabirov RZ, Okada Y. The maxi-anion channel: a classical channel playing novel roles through an unidentified molecular entity. J Physiol Sci 59: 3–21, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Saez JC, Berthoud VM, Branes MC, Martinez AD, Beyer EC. Plasma membrane channels formed by connexins: their regulation and functions. Physiol Rev 83: 1359–1400, 2003. [DOI] [PubMed] [Google Scholar]
- 303. Saez JC, Connor JA, Spray DC, Bennett MV. Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, to calcium ions. Proc Natl Acad Sci USA 86: 2708–2712, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Saez JC, Retamal MA, Basilio D, Bukauskas FF, Bennett MV. Connexin-based gap junction hemichannels: gating mechanisms. Biochim Biophys Acta 1711: 215–224, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Sammels E, Devogelaere B, Mekahli D, Bultynck G, Missiaen L, Parys JB, Cai Y, Somlo S, De Smedt H. Polycystin-2 activation by inositol 1,4,5-trisphosphate-induced Ca2+ release requires its direct association with the inositol 1,4,5-trisphosphate receptor in a signaling microdomain. J Biol Chem 285: 18794–18805, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Sanderson MJ. Intercellular calcium waves mediated by inositol trisphosphate. Ciba Found Symp 188: 175–194, 1995. [PubMed] [Google Scholar]
- 307. Sanderson MJ. Intercellular waves of communication. News Physiol Sci 11: 262–269, 1996. [Google Scholar]
- 308. Sanderson MJ, Charles AC, Dirksen ER. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Regul 1: 585–596, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Sauer H, Hescheler J, Wartenberg M. Mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation. Am J Physiol Cell Physiol 279: C295–C307, 2000. [DOI] [PubMed] [Google Scholar]
- 310. Sauer H, Sharifpanah F, Hatry M, Steffen P, Bartsch C, Heller R, Padmasekar M, Howaldt HP, Bein G, Wartenberg M. NOS inhibition synchronizes calcium oscillations in human adipose tissue-derived mesenchymal stem cells by increasing gap-junctional coupling. J Cell Physiol 226: 1642–1650, 2011. [DOI] [PubMed] [Google Scholar]
- 311. Scemes E. Modulation of astrocyte P2Y1 receptors by the carboxyl terminal domain of the gap junction protein Cx43. Glia 56: 145–153, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Scemes E, Giaume C. Astrocyte calcium waves: what they are and what they do. Glia 54: 716–725, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313. Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol 3: 199–208, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci 20: 1435–1445, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Schalper KA, Sanchez HA, Lee SC, Altenberg GA, Nathanson MH, Saez JC. Connexin 43 hemichannels mediate the Ca2+ influx induced by extracellular alkalinization. Am J Physiol Cell Physiol 299: C1504–C1515, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Schipke CG, Boucsein C, Ohlemeyer C, Kirchhoff F, Kettenmann H. Astrocyte Ca2+ waves trigger responses in microglial cells in brain slices. FASEB J 16: 255–257, 2002. [DOI] [PubMed] [Google Scholar]
- 317. Schlosser SF, Burgstahler AD, Nathanson MH. Isolated rat hepatocytes can signal to other hepatocytes and bile duct cells by release of nucleotides. Proc Natl Acad Sci USA 93: 9948–9953, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Schock SC, Leblanc D, Hakim AM, Thompson CS. ATP release by way of connexin 36 hemichannels mediates ischemic tolerance in vitro. Biochem Biophys Res Commun 368: 138–144, 2008. [DOI] [PubMed] [Google Scholar]
- 319. Schummers J, Yu H, Sur M. Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320: 1638–1643, 2008. [DOI] [PubMed] [Google Scholar]
- 320. Schwiebert EM. Extracellular ATP-mediated propagation of Ca2+ waves. Focus on “mechanical strain-induced Ca2+ waves are propagated via ATP release and purinergic receptor activation.” Am J Physiol Cell Physiol 279: C281–C283, 2000. [DOI] [PubMed] [Google Scholar]
- 321. Seki A, Duffy HS, Coombs W, Spray DC, Taffet SM, Delmar M. Modifications in the biophysical properties of connexin43 channels by a peptide of the cytoplasmic loop region. Circ Res 95: e22–e28, 2004. [DOI] [PubMed] [Google Scholar]
- 322. Serriere V, Berthon B, Boucherie S, Jacquemin E, Guillon G, Claret M, Tordjmann T. Vasopressin receptor distribution in the liver controls calcium wave propagation and bile flow. FASEB J 15: 1484–1486, 2001. [DOI] [PubMed] [Google Scholar]
- 323. Shears SB. Metabolism of inositol phosphates. Adv Second Messenger Phosphoprotein Res 26: 63–92, 1992. [PubMed] [Google Scholar]
- 324. Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 295: C761–C767, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Simard M, Couldwell WT, Zhang W, Song H, Liu S, Cotrina ML, Goldman S, Nedergaard M. Glucocorticoids-potent modulators of astrocytic calcium signaling. Glia 28: 1–12, 1999. [DOI] [PubMed] [Google Scholar]
- 326. Sipos A, Vargas SL, Toma I, Hanner F, Willecke K, Peti-Peterdi J. Connexin 30 deficiency impairs renal tubular ATP release and pressure natriuresis. J Am Soc Nephrol 20: 1724–1732, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Smith IF, Shuai J, Parker I. Active generation and propagation of Ca2+ signals within tunneling membrane nanotubes. Biophys J 100: L37–39, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Sneyd J, Charles AC, Sanderson MJ. A model for the propagation of intercellular calcium waves. Am J Physiol Cell Physiol 266: C293–C302, 1994. [DOI] [PubMed] [Google Scholar]
- 329. Sneyd J, Keizer J, Sanderson MJ. Mechanisms of calcium oscillations and waves: a quantitative analysis. FASEB J 9: 1463–1472, 1995. [DOI] [PubMed] [Google Scholar]
- 330. Sneyd J, Wetton BT, Charles AC, Sanderson MJ. Intercellular calcium waves mediated by diffusion of inositol trisphosphate: a two-dimensional model. Am J Physiol Cell Physiol 268: C1537–C1545, 1995. [DOI] [PubMed] [Google Scholar]
- 331. Sneyd J, Wilkins M, Strahonja A, Sanderson MJ. Calcium waves and oscillations driven by an intercellular gradient of inositol (1,4,5)-trisphosphate. Biophys Chem 72: 101–109, 1998. [DOI] [PubMed] [Google Scholar]
- 332. Sohl G, Willecke K. An update on connexin genes and their nomenclature in mouse and man. Cell Commun Adhesion 10: 173–180, 2003. [DOI] [PubMed] [Google Scholar]
- 333. Sorensen CM, Holstein-Rathlou NH. Cell-Cell communication in the kidney microcirculation. Microcirculation PMID: 22118633, 2011. [DOI] [PubMed] [Google Scholar]
- 334. Spray DC, Rozental R, Srinivas M. Prospects for rational development of pharmacological gap junction channel blockers. Curr Drug Targets 3: 455–464, 2002. [DOI] [PubMed] [Google Scholar]
- 335. Spray DC, Stern JH, Harris AL, Bennett MV. Gap junctional conductance: comparison of sensitivities to H and Ca ions. Proc Natl Acad Sci USA 79: 441–445, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Sridharan M, Adderley SP, Bowles EA, Egan TM, Stephenson AH, Ellsworth ML, Sprague RS. Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes. Am J Physiol Heart Circ Physiol 299: H1146–H1152, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Stamatakis M, Mantzaris NV. Modeling of ATP-mediated signal transduction and wave propagation in astrocytic cellular networks. J Theor Biol 241: 649–668, 2006. [DOI] [PubMed] [Google Scholar]
- 338. Stauffer PL, Zhao H, Luby-Phelps K, Moss RL, Star RA, Muallem S. Gap junction communication modulates [Ca2+]i oscillations and enzyme secretion in pancreatic acini. J Biol Chem 268: 19769–19775, 1993. [PubMed] [Google Scholar]
- 339. Stevens SC, Terentyev D, Kalyanasundaram A, Periasamy M, Gyorke S. Intra-sarcoplasmic reticulum Ca2+ oscillations are driven by dynamic regulation of ryanodine receptor function by luminal Ca2+ in cardiomyocytes. J Physiol 587: 4863–4872, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Stosiek C, Garaschuk O, Holthoff K, Konnerth A. In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100: 7319–7324, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341. Stout C, Charles A. Modulation of intercellular calcium signaling in astrocytes by extracellular calcium and magnesium. Glia 43: 265–273, 2003. [DOI] [PubMed] [Google Scholar]
- 342. Stout C, Goodenough DA, Paul DL. Connexins: functions without junctions. Curr Opin Cell Biol 16: 507–512, 2004. [DOI] [PubMed] [Google Scholar]
- 343. Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem 277: 10482–10488, 2002. [DOI] [PubMed] [Google Scholar]
- 344. Strahonja-Packard A, Sanderson MJ. Intercellular Ca2+ waves induce temporally and spatially distinct intracellular Ca2+ oscillations in glia. Glia 28: 97–113, 1999. [DOI] [PubMed] [Google Scholar]
- 345. Straub AC, Billaud M, Johnstone SR, Best AK, Yemen S, Dwyer ST, Looft-Wilson R, Lysiak JJ, Gaston B, Palmer L, Isakson BE. Compartmentalized connexin 43 S-nitrosylation/denitrosylation regulates heterocellular communication in the vessel wall. Arterioscler Thromb Vasc Biol 31: 399–407, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Stumpel F, Ott T, Willecke K, Jungermann K. Connexin 32 gap junctions enhance stimulation of glucose output by glucagon and noradrenaline in mouse liver. Hepatology 28: 1616–1620, 1998. [DOI] [PubMed] [Google Scholar]
- 347. Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci 26: 1378–1385, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Suadicani SO, Cherkas PS, Zuckerman J, Smith DN, Spray DC, Hanani M. Bidirectional calcium signaling between satellite glial cells and neurons in cultured mouse trigeminal ganglia. Neuron Glia Biol 6: 43–51, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Suadicani SO, De Pina-Benabou MH, Urban-Maldonado M, Spray DC, Scemes E. Acute downregulation of Cx43 alters P2Y receptor expression levels in mouse spinal cord astrocytes. Glia 42: 160–171, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Suadicani SO, Flores CE, Urban-Maldonado M, Beelitz M, Scemes E. Gap junction channels coordinate the propagation of intercellular Ca2+ signals generated by P2Y receptor activation. Glia 48: 217–229, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Suadicani SO, Iglesias R, Spray DC, Scemes E. Point mutation in the mouse P2X7 receptor affects intercellular calcium waves in astrocytes. ASN Neurol 1: e00005, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Suadicani SO, Vink MJ, Spray DC. Slow intercellular Ca2+ signaling in wild-type and Cx43-null neonatal mouse cardiac myocytes. Am J Physiol Heart Circ Physiol 279: H3076–H3088, 2000. [DOI] [PubMed] [Google Scholar]
- 353. Sul JY, Orosz G, Givens RS, Haydon PG. Astrocytic Connectivity in the Hippocampus. Neuron Glia Biol 1: 3–11, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Sullivan MR, Nimmerjahn A, Sarkisov DV, Helmchen F, Wang SS. In vivo calcium imaging of circuit activity in cerebellar cortex. J Neurophysiol 94: 1636–1644, 2005. [DOI] [PubMed] [Google Scholar]
- 355. Sung YJ, Sung Z, Ho CL, Lin MT, Wang JS, Yang SC, Chen YJ, Lin CH. Intercellular calcium waves mediate preferential cell growth toward the wound edge in polarized hepatic cells. Exp Cell Res 287: 209–218, 2003. [DOI] [PubMed] [Google Scholar]
- 356. Swietach P, Rossini A, Spitzer KW, Vaughan-Jones RD. H+ ion activation and inactivation of the ventricular gap junction: a basis for spatial regulation of intracellular pH. Circ Res 100: 1045–1054, 2007. [DOI] [PubMed] [Google Scholar]
- 357. Syed MM, Lee S, He S, Zhou ZJ. Spontaneous waves in the ventricular zone of developing mammalian retina. J Neurophysiol 91: 1999–2009, 2004. [DOI] [PubMed] [Google Scholar]
- 358. Takahashi-Iwanaga H, Nio-Kobayashi J, Habara Y, Furuya K. A dual system of intercellular calcium signaling in glial nets associated with lanceolate sensory endings in rat vibrissae. J Comp Neurol 510: 68–78, 2008. [DOI] [PubMed] [Google Scholar]
- 359. Takano T, Nedergaard M. Deciphering migraine. J Clin Invest 119: 16–19, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nature Neurosci 9: 260–267, 2006. [DOI] [PubMed] [Google Scholar]
- 361. Takeda M, Nelson DJ, Soliven B. Calcium signaling in cultured rat oligodendrocytes. Glia 14: 225–236, 1995. [DOI] [PubMed] [Google Scholar]
- 362. Tallini YN, Brekke JF, Shui B, Doran R, Hwang SM, Nakai J, Salama G, Segal SS, Kotlikoff MI. Propagated endothelial Ca2+ waves and arteriolar dilation in vivo: measurements in Cx40BAC GCaMP2 transgenic mice. Circ Res 101: 1300–1309, 2007. [DOI] [PubMed] [Google Scholar]
- 363. Tao L, Harris AL. 2-Aminoethoxydiphenyl borate directly inhibits channels composed of connexin26 and/or connexin32. Mol Pharmacol 71: 570–579, 2007. [DOI] [PubMed] [Google Scholar]
- 364. Taylor CW, Tovey SC. IP3 receptors: toward understanding their activation. Cold Spring Harbor Perspect Biol 2: a004010, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res 91: 414–420, 2002. [DOI] [PubMed] [Google Scholar]
- 366. Thrower EC, Mobasheri H, Dargan S, Marius P, Lea EJ, Dawson AP. Interaction of luminal calcium and cytosolic ATP in the control of type 1 inositol (1,4,5)-trisphosphate receptor channels. J Biol Chem 275: 36049–36055, 2000. [DOI] [PubMed] [Google Scholar]
- 367. Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med 11: 973–981, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Tian GF, Takano T, Lin JH, Wang X, Bekar L, Nedergaard M. Imaging of cortical astrocytes using 2-photon laser scanning microscopy in the intact mouse brain. Adv Drug Delivery Rev 58: 773–787, 2006. [DOI] [PubMed] [Google Scholar]
- 369. Tjalkens RB, Zoran MJ, Mohl B, Barhoumi R. Manganese suppresses ATP-dependent intercellular calcium waves in astrocyte networks through alteration of mitochondrial and endoplasmic reticulum calcium dynamics. Brain Res 1113: 210–219, 2006. [DOI] [PubMed] [Google Scholar]
- 370. Toma I, Bansal E, Meer EJ, Kang JJ, Vargas SL, Peti-Peterdi J. Connexin 40 and ATP-dependent intercellular calcium wave in renal glomerular endothelial cells. Am J Physiol Regul Integr Comp Physiol 294: R1769–R1776, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Tordjmann T, Berthon B, Claret M, Combettes L. Coordinated intercellular calcium waves induced by noradrenaline in rat hepatocytes: dual control by gap junction permeability and agonist. EMBO J 16: 5398–5407, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371a. Torres A, Wang F, Xu Q, Fujita T, Dobrowolski R, Willecke K, Takano T, Nederaard M. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci Signal 5(208): ra8, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Tour O, Adams SR, Kerr RA, Meijer RM, Sejnowski TJ, Tsien RW, Tsien RY. Calcium Green FlAsH as a genetically targeted small-molecule calcium indicator. Nat Chem Biol 3: 423–431, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Tran PO, Tran QH, Hinman LE, Sammak PJ. Co-ordination between localized wound-induced Ca2+ signals and pre-wound serum signals is required for proliferation after mechanical injury. Cell Prolif 31: 155–170, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Tran Van Nhieu G, Clair C, Bruzzone R, Mesnil M, Sansonetti P, Combettes L. Connexin-dependent inter-cellular communication increases invasion and dissemination of Shigella in epithelial cells. Nature Cell Biol 5: 720–726, 2003. [DOI] [PubMed] [Google Scholar]
- 375. Trexler EB, Bukauskas FF, Bennett MV, Bargiello TA, Verselis VK. Rapid and direct effects of pH on connexins revealed by the connexin46 hemichannel preparation. J Gen Physiol 113: 721–742, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376. Tritsch NX, Rodriguez-Contreras A, Crins TT, Wang HC, Borst JG, Bergles DE. Calcium action potentials in hair cells pattern auditory neuron activity before hearing onset. Nature Neurosci 13: 1050–1052, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature 450: 50–55, 2007. [DOI] [PubMed] [Google Scholar]
- 378. Tsaneva-Atanasova K, Zimliki CL, Bertram R, Sherman A. Diffusion of calcium and metabolites in pancreatic islets: killing oscillations with a pitchfork. Biophys J 90: 3434–3446, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Tsutsumi M, Inoue K, Denda S, Ikeyama K, Goto M, Denda M. Mechanical-stimulation-evoked calcium waves in proliferating and differentiated human keratinocytes. Cell Tissue Res 338: 99–106, 2009. [DOI] [PubMed] [Google Scholar]
- 380. Tu H, Wang Z, Bezprozvanny I. Modulation of mammalian inositol 1,4,5-trisphosphate receptor isoforms by calcium: a role of calcium sensor region. Biophys J 88: 1056–1069, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Van Zeijl L, Ponsioen B, Giepmans BN, Ariaens A, Postma FR, Varnai P, Balla T, Divecha N, Jalink K, Moolenaar WH. Regulation of connexin43 gap junctional communication by phosphatidylinositol 4,5-bisphosphate. J Cell Biol 177: 881–891, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Vandamme W, Braet K, Cabooter L, Leybaert L. Tumour necrosis factor alpha inhibits purinergic calcium signalling in blood-brain barrier endothelial cells. J Neurochem 88: 411–421, 2004. [DOI] [PubMed] [Google Scholar]
- 384. Venance L, Piomelli D, Glowinski J, Giaume C. Inhibition by anandamide of gap junctions and intercellular calcium signalling in striatal astrocytes. Nature 376: 590–594, 1995. [DOI] [PubMed] [Google Scholar]
- 385. Venance L, Stella N, Glowinski J, Giaume C. Mechanism involved in initiation and propagation of receptor-induced intercellular calcium signaling in cultured rat astrocytes. J Neurosci 17: 1981–1992, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Verderio C, Matteoli M. ATP mediates calcium signaling between astrocytes and microglial cells: modulation by IFN-gamma. J Immunol 166: 6383–6391, 2001. [DOI] [PubMed] [Google Scholar]
- 387. Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact 5: 70–84, 2005. [PubMed] [Google Scholar]
- 388. Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature 410: 592–596, 2001. [DOI] [PubMed] [Google Scholar]
- 389. Wang X, Lou N, Xu Q, Tian GF, Peng WG, Han X, Kang J, Takano T, Nedergaard M. Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nature Neurosci 9: 816–823, 2006. [DOI] [PubMed] [Google Scholar]
- 390. Wang Z, Haydon PG, Yeung ES. Direct observation of calcium-independent intercellular ATP signaling in astrocytes. Anal Chem 72: 2001–2007, 2000. [DOI] [PubMed] [Google Scholar]
- 391. Warner A, Clements DK, Parikh S, Evans WH, DeHaan RL. Specific motifs in the external loops of connexin proteins can determine gap junction formation between chick heart myocytes. J Physiol 488: 721–728, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Warren NJ, Tawhai MH, Crampin EJ. Mathematical modelling of calcium wave propagation in mammalian airway epithelium: evidence for regenerative ATP release. Exp Physiol 95: 232–249, 2010. [DOI] [PubMed] [Google Scholar]
- 393. Wei F, Shuai J. Intercellular calcium waves in glial cells with bistable dynamics. Physical Biol 8: 026009, 2011. [DOI] [PubMed] [Google Scholar]
- 394. Weissman TA, Riquelme PA, Ivic L, Flint AC, Kriegstein AR. Calcium waves propagate through radial glial cells and modulate proliferation in the developing neocortex. Neuron 43: 647–661, 2004. [DOI] [PubMed] [Google Scholar]
- 395. White TW, Deans MR, O'Brien J, Al-Ubaidi MR, Goodenough DA, Ripps H, Bruzzone R. Functional characteristics of skate connexin35, a member of the gamma subfamily of connexins expressed in the vertebrate retina. Eur J Neurosci 11: 1883–1890, 1999. [DOI] [PubMed] [Google Scholar]
- 396. Willmott NJ, Wong K, Strong AJ. A fundamental role for the nitric oxide-G-kinase signaling pathway in mediating intercellular Ca2+ waves in glia. J Neurosci 20: 1767–1779, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 397. Woehrle T, Yip L, Elkhal A, Sumi Y, Chen Y, Yao Y, Insel PA, Junger WG. Pannexin-1 hemichannel-mediated ATP release together with P2X1 and P2X4 receptors regulate T-cell activation at the immune synapse. Blood 116: 3475–3484, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398. Wolszon LR, Rehder V, Kater SB, Macagno ER. Calcium wave fronts that cross gap junctions may signal neuronal death during development. J Neurosci 14: 3437–3448, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399. Xu L, Tripathy A, Pasek DA, Meissner G. Potential for pharmacology of ryanodine receptor/calcium release channels. Ann NY Acad Sci 853: 130–148, 1998. [DOI] [PubMed] [Google Scholar]
- 400. Yamaguchi N, Xu L, Pasek DA, Evans KE, Meissner G. Molecular basis of calmodulin binding to cardiac muscle Ca2+ release channel (ryanodine receptor). J Biol Chem 278: 23480–23486, 2003. [DOI] [PubMed] [Google Scholar]
- 401. Yamasaki-Mann M, Demuro A, Parker I. Modulation of endoplasmic reticulum Ca2+ store filling by cyclic ADP-ribose promotes inositol trisphosphate (IP3)-evoked Ca2+ signals. J Biol Chem 285: 25053–25061, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Yao J, Morioka T, Li B, Oite T. Coordination of mesangial cell contraction by gap junction-mediated intercellular Ca2+ wave. J Am Soc Nephrol 13: 2018–2026, 2002. [DOI] [PubMed] [Google Scholar]
- 403. Yao J, Oite T, Kitamura M. Gap junctional intercellular communication in the juxtaglomerular apparatus. Am J Physiol Renal Physiol 296: F939–F946, 2009. [DOI] [PubMed] [Google Scholar]
- 404. Ye ZC, Oberheim N, Kettenmann H, Ransom BR. Pharmacological “cross-inhibition” of connexin hemichannels and swelling activated anion channels. Glia 57: 258–269, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405. Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23: 3588–3596, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 406. Ying X, Minamiya Y, Fu C, Bhattacharya J. Ca2+ waves in lung capillary endothelium. Circ Res 79: 898–908, 1996. [DOI] [PubMed] [Google Scholar]
- 407. Young RC, Schumann R, Zhang P. The signaling mechanisms of long distance intercellular calcium waves (far waves) in cultured human uterine myocytes. J Muscle Res Cell Motil 23: 279–284, 2002. [DOI] [PubMed] [Google Scholar]
- 408. Young SH, Ennes HS, Mayer EA. Propagation of calcium waves between colonic smooth muscle cells in culture. Cell Calcium 20: 257–271, 1996. [DOI] [PubMed] [Google Scholar]
- 409. Yule DI, Stuenkel E, Williams JA. Intercellular calcium waves in rat pancreatic acini: mechanism of transmission. Am J Physiol Cell Physiol 271: C1285–C1294, 1996. [DOI] [PubMed] [Google Scholar]
- 410. Yuste R, Nelson DA, Rubin WW, Katz LC. Neuronal domains in developing neocortex: mechanisms of coactivation. Neuron 14: 7–17, 1995. [DOI] [PubMed] [Google Scholar]
- 411. Zahs KR, Newman EA. Asymmetric gap junctional coupling between glial cells in the rat retina. Glia 20: 10–22, 1997. [PubMed] [Google Scholar]
- 412. Zalk R, Lehnart SE, Marks AR. Modulation of the ryanodine receptor and intracellular calcium. Annu Rev Biochem 76: 367–385, 2007. [DOI] [PubMed] [Google Scholar]
- 413. Zanotti S, Charles A. Extracellular calcium sensing by glial cells: low extracellular calcium induces intracellular calcium release and intercellular signaling. J Neurochem 69: 594–602, 1997. [DOI] [PubMed] [Google Scholar]
- 414. Zdebik AA, Wangemann P, Jentsch TJ. Potassium ion movement in the inner ear: insights from genetic disease and mouse models. Physiology 24: 307–316, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415. Zhang W, Segura BJ, Lin TR, Hu Y, Mulholland MW. Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia 42: 252–262, 2003. [DOI] [PubMed] [Google Scholar]
- 416. Zhang Z, Chen G, Zhou W, Song A, Xu T, Luo Q, Wang W, Gu XS, Duan S. Regulated ATP release from astrocytes through lysosome exocytosis. Nature Cell Biol 9: 945–953, 2007. [DOI] [PubMed] [Google Scholar]
- 417. Zhao HB, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci USA 102: 18724–18729, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418. Zhu MX, Ma J, Parrington J, Calcraft PJ, Galione A, Evans AM. Calcium signaling via two-pore channels: local or global, that is the question. Am J Physiol Cell Physiol 298: C430–C441, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419. Zimmermann B. Subcellular organization of agonist-evoked Ca2+ waves in the blowfly salivary gland. Cell Calcium 27: 297–307, 2000. [DOI] [PubMed] [Google Scholar]
- 420. Zimmermann B, Walz B. The mechanism mediating regenerative intercellular Ca2+ waves in the blowfly salivary gland. EMBO J 18: 3222–3231, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421. Zimmermann B, Walz B. Serotonin-induced intercellular calcium waves in salivary glands of the blowfly Calliphora erythrocephala. J Physiol 500: 17–28, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


