Abstract
The consumption of sugar and its relation to various potential adverse health consequences are the subjects of considerable debate and controversy. This supplement to Advances in Nutrition provides an expanded summary of a symposium held on 26 April 2014 entitled “Sugars and Health Controversies: What Does the Science Say?” as part of the ASN Scientific Sessions and Annual Meeting at Experimental Biology 2014. The articles in the supplement discuss results of current systematic reviews and meta-analyses as well as randomized controlled trials and draw implications for public policy considerations. In addition, future research gaps are identified. Current research trials conducted with commonly consumed sugars [e.g., sucrose and high-fructose corn syrup (HFCS)] do not support a unique relation to obesity, metabolic syndrome, diabetes, risk factors for heart disease, or nonalcoholic fatty liver disease. Neurologic differences in response to studies that used pure fructose compared with pure glucose have not been confirmed using typical sugars that are consumed (i.e., sucrose and HFCS), which contain ∼50% glucose and fructose. We conclude that added sugars consumed in the normal forms in which humans consume them, at amounts typical of the human diet and for the time period studied in randomized controlled trials, do not result in adverse health consequences. Although more research trials are needed in many areas of sugar consumption and health, there is little scientific justification for recommending restricting sugar consumption below the reasonable upper limit recommended by the Dietary Guidelines for Americans, 2010 of no more than 25% of calories.
Keywords: fructose, high-fructose corn sugar, sucrose, metabolism, added sugars, diabetes, the metabolic syndrome, non-alcoholic fatty liver disease
Introduction
Few topics in nutrition engender as much debate and controversy as the relation between sugar and various potential health consequences (1–14). The effects of the major added sugars in our diet, namely sucrose and high-fructose corn syrup (HFCS)8, have been the subject of numerous research studies ranging from epidemiologic and cohort studies to randomized controlled trials (RCTs). In addition, numerous research studies have compared pure fructose and pure glucose with regard to their metabolism and health effects, although these 2 monosaccharaides are rarely, if ever, consumed in isolation in the human diet.
Given the ongoing controversies related to sugar in the diet, a symposium was held on 26 April 2014 entitled “Sugars and Health Controversies: What Does the Science Say?” during the ASN Scientific Sessions and Annual Meeting at Experimental Biology 2014. Presenters at this symposium explored controversies related to the metabolism and health effects of sugar; reviewed the recent science related to these issues with particular reference to systematic reviews, meta-analyses, and RCTs; explored how scientific understandings of these issues interact with public policy; and finally, suggested further areas of needed research.
The symposium was framed around the following 6 key questions:
Are there differences in metabolism and health effects between fructose, HFCS, and sucrose?
Do fructose-containing sugars contribute substantially to conditions such as nonalcoholic fatty liver disease (NAFLD), obesity, metabolic syndrome (MetS), diabetes, cardiovascular disease, dyslipidemias, and/or elevated blood pressure?
Do fructose-containing sugars react differently in the brain than glucose? If so, are those differences relevant to appetite, food consumption, weight gain, and obesity?
What is the appropriate upper limit of consumption of sugars?
Are public policy recommendations to limit consumption of sugars through such mechanisms as taxation or limiting portion sizes based on sound science and/or likely to succeed?
What directions should future research take?
The articles contained in this supplement issue contain expanded and updated summaries of information presented at this symposium.
Recent Scientific Studies
A wide variety of scientific studies have been published in the past decade related to sugars and their metabolism as well as their health effects. These studies are summarized in detail in the article by Sievenpiper et al. (15) in this supplement issue. In this article we will summarize some of the key studies that were published during this period of time. There are many position statements and review articles suggesting that sugar may have deleterious effects on health. Such statements and summaries occasionally stand on disputable or clinically irrelevant findings, which we will briefly discuss in this article.
Sugars and energy-regulating hormones.
Some studies in both animals and humans compared high levels of consumption of pure fructose to pure glucose and their influence on energy-regulating hormones. It should be noted that these experiments, in addition to giving large doses of these 2 monosaccharides, also create a highly artificial environment because neither pure fructose nor pure glucose is consumed to any appreciable degree in the human diet.
Teff et al. (16) reported that consumption of fructose-sweetened beverages compared with glucose-sweetened beverages, both consumed at 25% of calories, resulted in lower 24-h concentrations of circulating insulin, glucose, and leptin and decreased postprandial suppression of plasma ghrelin concentrations. Using a similar model, other investigators reported that consuming fructose- compared with glucose-sweetened beverages resulted in increased postprandial TG concentrations and that these effects were more pronounced in overweight/obese subjects than in normal-weight subjects and more pronounced in men than in women (17, 18).
Given that insulin, leptin, and ghrelin interact with each other, these data have been extrapolated to suggest that prolonged consumption of energy from fructose could lead to increased caloric intake and contribute to weight gain and obesity. It should be noted, however, that meta-analyses published by Dolan et al. (19) of multiple studies in normal-weight, overweight, or obese individuals (20) consuming up to the 95th percentile of the adult population’s intake of fructose did not report any metabolic abnormalities including weight gain.
Our laboratory compared the commonly consumed sugars HFCS and sucrose (each of which consists of ∼50% fructose and 50% glucose) and determined that there were no differences with respect to energy-regulating hormone response, appetite, and/or ad libitum energy intake between these 2 sources of added sugar at the 90th percentile population consumption in acute experiments (21, 22) and up to the 90th percentile population consumption of added sugars over a free-living protocol lasting 10 wk (23). These findings were subsequently corroborated by Stanhope and Havel (24) and extended to obese individuals by Zuckley et al. (25).
A more recent study published by Yu et al. (26), also from our research group, compared 8% of calories from either HFCS or sucrose (25th percentile population consumption level), 18% of calories from these 2 sugars (50th percentile population consumption level), and 30% of calories from these 2 sugars (90th percentile population consumption level) and found no differences or adverse effects related to insulin, glucose, leptin, or ghrelin and no acute differences or differences after 10 wk of consumption among these 3 dose amounts. Thus, it appears that the dosage of these 2 added sugars also does not matter when energy-regulating hormones are assessed. Given that no differences in energy-regulating hormones exist between the commonly consumed sugars where fructose and glucose are consumed together, extrapolation from findings comparing pure fructose to pure glucose must be treated with extreme caution.
Sugars and obesity.
In retrospect, the modern concern about a potential role for sugars as a unique cause of obesity can be traced back to a commentary in The American Journal of Clinical Nutrition in 2004 (6). In that commentary, Bray et al. argued that the use of HFCS in the United States was temporally associated with a rapid increase in obesity prevalence. These authors argued that the metabolism of fructose compared with glucose differed in ways that energy consumption could be increased after fructose consumption and result in the increased likelihood of obesity as well as cardiovascular disease, diabetes, and MetS.
Subsequent research trials, however, failed to support the hypothesized linkage between HFCS and obesity (5, 23, 27). Indeed, multiple studies have now demonstrated that HFCS and sucrose are virtually identical with regard to calories, sweetness, and absorption (20, 24). Studies from our research laboratory (21, 26), as well as others (27), concluded that HFCS and sucrose are virtually identical with regard to glucose, insulin, leptin, ghrelin, and appetite responses in normal-weight and obese individuals. The American Medical Association (28) and the Academy of Nutrition and Dietetics have both issued statements concluding that there are no differences between HFCS and sucrose with regard to the likelihood of causing obesity (29).
As a result of this expanding literature, the emphasis has shifted to a consideration of whether or not fructose-containing sugars in general (e.g., sucrose, HFCS, concentrated fruit juices, etc.) may be uniquely linked to obesity. Several meta-analyses suggested that sugar-containing soft drinks are associated with weight gain and obesity in both children and adults. Malik et al. (30) reviewed 30 publications (15 cross-sectional, 10 prospective, and 5 experimental) that met the criteria for their meta-analysis and showed a positive association between greater intake of sugar-sweetened soft drinks and weight gain. Another meta-analysis by Olsen and Heitmann (31) including 14 prospective and 5 experimental studies concluded that the consumption of soft drinks was a determinant of obesity.
There have been 3 recent systematic reviews and meta-analyses of RCTs of sugar consumption or sugar-sweetened beverage (SSB) consumption and body weight (32–34). Taken together, these meta-analyses of RCTs demonstrate that replacing sugar with other energy-equivalent macronutrients has no effect on body weight. There is suggestive evidence that increasing energy consumption by increasing sugar intake in adults may lead to modest weight gain. The weight gain, however, appears to be due not to sugar per se, but to an increase in energy consumed, because participants in these hypercaloric RCTs were told to increase their sugar consumption on a background of their typical caloric intake.
Prospective cohort trials have yielded similar results (32–34). These cohort studies, both in adults and children, provided inconsistent results and typically did not adjust for total energy intake. When this adjustment was performed in one meta-analysis, the results showed no relation between sugar consumption and body weight. Thus, both cohort studies and RCTs are consistent in failing to demonstrate a unique relation between sugar consumption and obesity.
Several recent summary articles reached the same conclusion that there is a lack of evidence linking sugars to obesity (3, 35). Moreover, RCTs performed in our research laboratory demonstrated that the consumption of average amounts of fructose-containing sugars did not result in increased body weight during a 10-wk free-living trial where they were added isocalorically (36). In a separate study in our laboratory, average amounts of fructose-containing sugars were used as part of a hypocaloric diet and did not impede weight loss (37). These studies add further evidence against a unique linkage between added sugars and obesity or weight gain.
Thus, evidence from a variety of sources does not suggest that sugars per se make a unique contribution to obesity. Moreover, in a condition as complicated as obesity it is highly unlikely that one single nutrient would uniquely cause this condition. It is more likely that the totality of the diet, including increased caloric consumption from all sources, exerts a significant impact on the likelihood of obesity. This view is consistent with the recent scientific statement from the ASN, which emphasized the complexity of energy regulation and weight and cautioned against isolating one component of the diet as a primary cause of weight gain and/or obesity (38).
Sugars and Risk Factors for Cardiovascular Disease
Lipids.
Some studies have explored the potential linkage between the consumption of added sugars and dyslipidemia (39, 40). The American Heart Association (AHA) recommended restricting the consumption of fructose-containing sugars as a mechanism for controlling TGs (41). The data to support this, however, are inconclusive as reported in several recent systematic reviews and meta-analyses (42–44). Fructose, when substituted in an isocaloric fashion or other sources of carbohydrate in individuals both with and without diabetes, did not show adverse effects on fasting lipid profiles (42, 43) or postprandial TGs (44). It should be noted, however, that a recent trial conducted by Egli et al. (45) demonstrated that an isocaloric high-fructose diet significantly increased blood TGs. These investigators also reported that exercise prevented increases in TGs in this acute study in healthy young subjects. Moreover, a recent meta-analysis by Te Morenga et al. (46) reported that the most marked relation between sugar intake and TGs occurred in studies that measured energy, and no weight change was reported. Thus, the effects of fructose-containing sugars on blood lipids remain inconclusive and will require further research to resolve.
A key issue appears to be whether or not studies have been designed to match energy intake. In isocaloric trials, even large doses of fructose-containing sugars did not result in lipid abnormalities, even at doses above the 95th percentile population consumption level for fructose. Livesey et al. (47) did not find an overall adverse effect on lipids and suggested a dose threshold for TG-elevating effects of fructose in isocaloric substitution for other carbohydrates at 100 g/d for fasting and 50 g/d for postprandial TGs. Sievenpiper et al. (48) proposed a threshold of <50 g/d of fructose-containing sugars for fasting TGs in people with diabetes.
In contrast, in hypercaloric trials in which fructose was supplemented to background diets, thereby creating excess energy, increases in LDL cholesterol and TGs were reported (42–44). Research in an RCT in our laboratory, however, showed that individuals who consumed either sucrose or HFCS at 10% or 20% of total calories (25th or 50th percentile population intake levels of fructose) in an isocaloric diet in a free-living environment showed no changes in total cholesterol, TGs, LDL cholesterol, or apoB (36). In a mildly hypercaloric trial, however, a 10% increase in TGs occurred (49, 50). Thus, it appears that adverse effects of sugars related to lipids are more likely to be a result of excess energy than to sugar per se.
Blood pressure.
Several RCTs examined whether fructose itself or fructose-containing sugars contribute to increased blood pressure. Raben et al. (51) randomly assigned 21 overweight subjects to supplements of either sucrose (in solid or beverage form; mean 152 g/d) or artificial sweeteners. After 10 wk, blood pressure in the sucrose group was significantly higher than in controls. However, these data are confounded by the fact that these individuals also gained, on average, 2.6 kg more than did controls. Brown et al. (52) in a nonblinded, randomized crossover trial administered to 15 subjects an acute load (60 g in 500 mL of water) of fructose, glucose, or pure water and found a significant increase (∼3 mm Hg) over the 120 min of the study when fructose was consumed compared with either glucose or water. As already indicated, fructose and glucose are invariably consumed together in the diet such as in sucrose or HFCS. Grasser et al. (53) compared blood pressure responses with sucrose with those with fructose with the amount of fructose equalized (30 g of fructose in 500 mL tap water). These investigators found that fructose increased blood pressure, whereas sucrose did not—suggesting that the glucose component of sucrose may abrogate increases in blood pressure that may occur when consuming fructose alone.
Other studies did not find increases in blood pressure related to fructose administration. Lê et al. (54) provided fructose (1.5 g/kg body weight) to 7 subjects in an isocaloric diet. Blood pressure did not change over the 4-wk study. Maersk et al. (55) randomly assigned 47 subjects to 4 different test drinks. After 4 wk, subjects given SSBs had significantly greater systolic blood pressure than did those given diet cola or isocaloric milk. However, there were no significant blood pressure differences between the SSB group and those given water. Ha et al. (56) performed a meta-analysis of 13 randomized and nonrandomized controlled feeding trials in which subjects were given an isocaloric exchange of fructose for other carbohydrates. The studies in this meta-analysis did not show any effect on systolic blood pressure, but there was high interstudy heterogeneity.
Prospective cohort studies have shown conflicting results related to blood pressure and sugar consumption. Several studies have shown an association between SSB consumption and incident hypertension (40, 57–59). Other studies have not corroborated these findings. Results from several RCTs from our research laboratory did not show elevations in blood pressure at amounts up to the 90th percentile population consumption level for fructose-containing sugars (49, 50). Thus, there is little evidence to support that sugar consumption per se is a significant risk factor for elevated blood pressure.
Coronary heart disease.
There are no reported RCTs, to our knowledge, that examined the effect of sugar consumption on coronary heart disease (CHD) itself. Three prospective cohort studies explored the association between SSB consumption and incident CHD. de Koning et al. (60) explored data from the all-male Health Professionals Follow-Up Study and found a significant association between CHD events and the highest quintile of SSB consumption compared with the lowest. Fung et al. (61) explored data from the Nurses’ Health Study and found a significant elevated risk associated with CHD with ≥2 servings (355 mL) of SSBs/d compared with <1 serving/mo. Eshak et al. (62) in a large prospective cohort study found no association between SSBs and myocardial infarction. These studies, however, were subject to all of the limitations of prospective cohort studies, particularly given that RCTs have not persuasively linked sugar consumption to risk factors for CHD.
The AHA has, nonetheless, issued a Scientific Statement recommending that American women consume no more than 100 kcal/d and American men no more than 150 kcal/d from added sugars (63). The AHA acknowledged that these recommendations are far lower than those recommended by the Dietary Guidelines for Americans, 2010 (64) and the Institute of Medicine (65) and also recognized that these recommendations are largely based on epidemiologic studies or animal data. Clearly, a need for RCTs exists to clarify issues of whether or not sugar consumption in fact increases CHD events.
Sugars and Diabetes
Diabetes is rapidly emerging as a major worldwide health concern in the 21st century. It is estimated that the prevalence of diabetes will double by 2035 (3). The increase in diabetes has paralleled the dramatic increase in worldwide obesity and insulin resistance (66–68). This has prompted investigators to explore nutritional links to diabetes. One of the factors that has been suggested as a unique link to diabetes is the consumption of fructose-containing sugars.
Several ecological analyses suggested that as sugar consumption has increased in countries so has the prevalence of diabetes (69, 70). Ecological analyses, however, are considered a weak form of evidence. It is also important to note that not all ecological studies showed a positive correlation between sugar intake and diabetes rate. For example, in the United States, total sugar consumption decreased substantially between 1980 and 2003 as it did both in Australia and the United Kingdom (71). In Australia, there was a 10% decrease in the contribution of sugar from SSBs despite increases in obesity and diabetes. This has been called the “Australian paradox.” Similar “paradoxes” have been seen in the United States, where prevalences of both obesity and diabetes have increased in the past decade whereas sugar consumption has declined.
Just as with obesity, the etiology in type 2 diabetes is certainly complicated and not entirely resolved. However, the most likely primary pathologic event is excess energy intake leading to overweight and obesity (66–68). The central question of whether or not sugar is a unique cause of diabetes has not been addressed in any RCT. Thus, most of the data come from cohort studies and RCTs looking at risk factors for diabetes rather than diabetes per se.
Prospective cohort studies provided mixed evidence concerning sugar consumption and diabetes (72, 73). Malik et al. (72) reported a meta-analysis of cohort studies related to SSBs and incident diabetes. Of the 8 studies reported, 4 did not find a significant effect of SSBs on the incidence of diabetes and 5 did not adjust findings for energy intake and body weight. Another study published by the same group did not show a relation between sugar consumption and risk of diabetes (73). Another large cohort study (Health Professionals Follow-Up Study) reported no association between diabetes risk and SSB consumption once data were adjusted for total energy intake (74). Other prospective cohort studies also failed to find significant associations between total sugar intake and diabetes (75, 76). In fact, one study found a significant negative association (77).
With regard to RCTs and meta-analyses, once again, few data are available to support an association between sugar intake and diabetes. Cozma et al. (78) reported a systematic review and meta-analysis of RCTs and nonrandomized controlled trials of fructose and diabetes. Of the 18 feeding studies they identified, fructose had no adverse impact on glycemic control, including fasting insulin, glucose, or glycated blood proteins (including glycated hemoglobin). Most, but not all, randomized controlled studies in nondiabetic subjects that used the substitution of sucrose or fructose with a controlled diet did not show adverse effects on multiple risk factors for diabetes, including insulin, postprandial glucose, and fasting blood insulin (79–83).
A recent RCT conducted in our laboratory studied 123 individuals who consumed 18% of calories from either sucrose or HFCS or 9% of calories from fructose or glucose (84). This study did not yield any increase in fasting glucose, insulin, or insulin resistance via HOMA. Another RCT conducted in our laboratory compared sucrose or HFCS at 8%, 18%, or 30% of calories in a large sample size of 267 individuals and also found no increases in glucose, insulin, or insulin resistance (49). In addition, an RCT from our research group looked at total body insulin sensitivity and hepatic insulin sensitivity using the Matsuda index and found no increases in either variable after 10 wk of consumption of average amounts (50th percentile) of fructose-containing sugars (85). Taken together, there is little direct evidence that sugar consumption increases the risk of diabetes.
Fructose-Containing Sugars and Risk of MetS
MetS represents a constellation of factors, including abnormal glucose handling, dyslipidemia, and high blood pressure (86). The prevalence of MetS has increased considerably in the United States in the past 20 y. Reports using NHANES data have suggested a prevalence of MetS of up to 39% of adults (87).
It has been argued that the consumption of fructose-containing sugars may increase the risk of developing MetS. Johnson et al. (88) proposed a model in which fructose metabolism in the liver may lead to ATP depletion and ultimately increases in uric acid through ATP degradation to AMP, which, in turn, may lead to endothelial dysfunction and create an increased risk of MetS due to increased blood pressure, insulin resistance, and inflammation.
Excess accumulation of abdominal fat is strongly associated with MetS (89). Several investigators reported increases in abdominal fat in response to the consumption of various sugars. Stanhope et al. (90) compared the consumption of fructose and glucose at 25% of calories and reported an increase in visceral abdominal fat in the fructose-consuming group. It should be noted that this increase occurred only in comparison to baseline in the fructose group and did not achieve significance when compared with the glucose group. Maersk et al. (55) conducted a 6-mo trial comparing 1 L/d of sucrose-sweetened cola, diet cola, milk, or water. They reported that the sucrose-sweetened cola group showed an increase in abdominal fat and other risk factors for MetS. There was, however, no significant difference in visceral adiposity when comparing the regular cola, diet cola, and water groups.
RCTs conducted in our research laboratory compared effects of either sucrose or HFCS on body weight and abdominal fat in 116 individuals who consumed these sugars at the 25th, 50th, and 90th percentile population consumption level of fructose. Despite a 0.9-kg increase in body mass over the entire cohort, there was no increase in abdominal fat as evaluated by DXA. A subsequent RCT in 123 individuals comparing HFCS and sucrose at 18% of calories with glucose and fructose at 9% of calories showed similar findings. A slight decrease in HDL cholesterol (∼1 mg/dL) and an increase in TGs (10–14% increase) occurred in these studies (91), although the values remained within normal limits. These increases may be attributable to the fact that the trial was mildly hypercaloric, resulting in an approximate 2-pound average weight gain in participants.
As already indicated, multiple other research trials have not yielded an association between sugar and elevated blood pressure, TGs, or postprandial TGs when fructose-containing sugars were substituted isocalorically for other carbohydrates. Thus, the effects of fructose-containing sugars on risk factors for MetS would appear to be very small if present.
Sugar and Liver Fat Accumulation
Fatty infiltration of the liver leading to NAFLD has been steadily increasing worldwide over the past 20 y. Worldwide (92), NAFLD represents the leading cause for chronic liver failure and the need for liver transplantation.
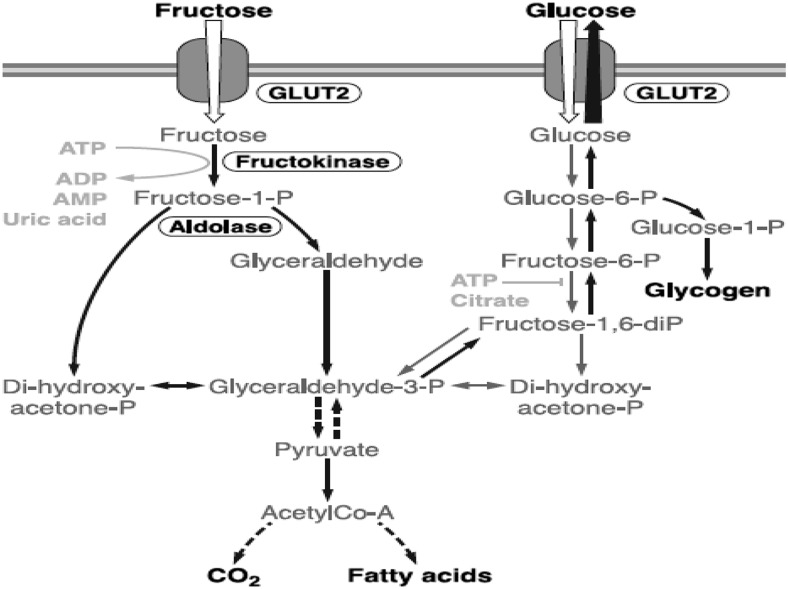
Concern about the potential interaction between the consumption of fructose-containing sugars and NAFLD has been evaluated by a number of investigators (93–95). The theoretical basis for concern relates to the well-known differential metabolism of fructose and glucose in the liver (96). This process is illustrated in Figure 1. As depicted in the figure, fructose can be metabolized in the liver to ultimately create FFAs. However, it should be pointed out that the pathways between fructose and glucose metabolism are interactive. Thus, the quantity of fat produced by this mechanism in humans is extremely small. When fructose-containing sugars are consumed, it has been estimated that >50% of the fructose is metabolized in the liver to glucose, another 25% to lactate, 15% to glycogen, and another few percent into carbon dioxide (90). The issue of intestinal lipogenesis and its possible interactions between fructose and glucose has been explored by several investigators. Lewis et al. (97) using an animal model of Syrian golden hamsters demonstrated intestinal lipoprotein overproduction when hamsters were fed a diet of 60% fructose. This intestinal lipoprotein overproduction was demonstrated to be ameliorated with the insulin sensitizer Rosiglitazone (Avandia by GlaxoSmithKline). Theytaz et al. (98) showed that amino acid supplementation blunted the fructose-induced increase in intrahepatic lipid concentration, and interactions between fructose and other components of the diet must be taken into consideration when considering the potential for increasing liver fat. Two recent studies reported that hypercaloric glucose and fructose consumption similarly increased intrahepatic fat, whereas isocaloric fructose did not (99, 100). These studies suggest that increases in liver fat appear to be an energy-mediated rather than a specific macronutrient–mediated effect.
FIGURE 1.
Metabolism of fructose and glucose in the liver: The metabolic pathways, although different, are interactive. diP, diphosphate; P, phosphate. Reproduced from reference 96 with permission.
Various investigators reported that only 1–5% of the fructose consumed will be converted to TGs through the process of de novo lipogenesis (90, 101). The amount of fat generated in this process is estimated to be ∼1% of that typically consumed in the human diet (96, 101). Nonetheless, some investigators speculated that de novo lipogenesis may contribute to substantially increased fat in the liver. Multiple RCTs in humans, however, have not demonstrated the effect of fructose-containing sugars leading to increased fat in the liver. Lê et al. (102) gave individuals 1 mg fructose/kg of lean body mass and did not demonstrate increased liver fat. Silbernagel et al. (103) reported similar findings from a 4-wk trial. Our research group conducted an RCT in which individuals were given up to 30% of calories from either HFCS or sucrose during a 10-wk free-living period and did not demonstrate increased fat accumulation in the liver (104). Two recent systematic reviews and meta-analyses also failed to find a linkage between fructose consumption and NAFLD (105, 106). In contrast, Stanhope et al. (90) provided individuals with 25% of energy as glucose or fructose and found increased liver fat after fructose consumption. Lê et al. (54) gave descendants of diabetics doses of 3.5 mg/kg of lean body mass and found some increase in the accumulation of liver fat. It should be pointed out that both of these studies used doses far in excess of normal amounts of consumption and used fructose by itself, which is normally not consumed in isolation in the human diet. Thus, there seems to be little evidence for fructose-containing sugars causing NAFLD at typical amounts of human consumption.
Fructose-Containing Sugars and Neurologic Responses
Animal experiments have suggested differences in brain responses with fructose compared with glucose (107, 108). However, these experiments must be treated with great caution because animal brains (particularly rodents, which were used in many of these studies) differ in very significant ways from the human brain.
Over the past 2 decades, an increasing number of human studies have used fMRI to explore potential differential neurologic responses to various sugars in human beings (109, 110). Smeets et al. (109) compared fMRI responses to aspartame, maltodextrin, and water. These investigators reported that both calories and sweetness must be present in order to stimulate brain reward pathways. Page et al. (111) compared a 75-g oral bolus of fructose with a 75-g oral bolus of glucose in 20 healthy, young volunteers in a randomized blinded fashion. They reported differences in hypothalamic blood flow, with glucose suppressing hypothalamic blood flow assessed by arterial spin labeling. These investigators also reported differences between fructose and glucose in brain connectivity. Purnell et al. (112) explored the neurologic response to 25 g of either fructose or glucose delivered as an intravenous bolus. These investigators reported no changes in blood flow to the hypothalamus but differences between fructose and glucose in blood flow to the cerebral cortex.
It should be noted that in both of these experiments, large doses of pure fructose were compared with pure glucose. As already indicated, neither of these sugars is consumed to any appreciable degree in the human diet. Moreover, the Purnell experiment gave these conditions through an atypical route (intravenously). Findings from these studies have led to speculation that fructose may lead to stimulation in reward pathways, thereby leading to a form of sugar “addiction,” contributing to overeating and obesity. It must be pointed out, however, that the entire concept of sugar “addiction” has been challenged on multiple grounds (113–115).
A recent study by Stice et al. (116), which used a model with various amounts of sugar compared with various amounts of fat in isocaloric milkshakes, reported that sugar activates reward, gustatory, and somatosensory pathways more than does fat. These investigators speculated that their results could provide an impetus to regulate sugar rather than fat in the diet. These results, however, should be viewed with great caution because there are other studies that showed exactly the opposite (117, 118). Moreover, the acute nature of this experiment does not demonstrate whether or not differences in stimulation of reward pathways results in either overeating or weight gain.
A pilot study conducted in our research laboratory examined HFCS or sucrose given as 18% of calories compared with 9% of calories from either fructose or glucose in the context of mixed-nutrient meals (119). Preliminary data from this study suggest that these sugars appear to behave similarly in this acute setting and are not different from an unsweetened control condition with regard to either hypothalamic blood flow or brain connectivity. Clearly, there is a need for larger RCTs to sort out whether or not neurologic differences exist in response to various sugars. Such a trial is currently underway in our research laboratory.
Future Research Directions
The ongoing controversies with clear public health implications related to the metabolism and health effects of fructose-containing sugars have stimulated several organizations to explore and offer guidance for future research priorities. In November 2012, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart, Lung, and Blood Institute, and the USDA sponsored a workshop entitled “Clinical Research Strategies for Fructose Metabolism” (120). This 2-d conference explored various issues related to both short-term mechanistic studies and longer-term studies with health implications. The conference concluded that mechanistic studies designed to elucidate pathways of fructose metabolism could reasonably compare pure monosaccharaides, glucose, and fructose. However, “health outcomes research meant to inform public policy should use large, long term studies using combinations of sugar found in a typical American diet rather than pure fructose or glucose.”
An initiative has also been launched by the International Life Sciences Institute to explore research gaps remaining in the area of sugar consumption and health. To date in this initiative, a process has been identified for evaluating the current literature and research gaps. Fourteen different areas of particularly high priority for future research were identified (121). Importantly, this initiative also recommended that future studies should routinely have a physical activity component and should emphasize the study of sugars normally consumed in the human diet, such as sucrose and HFCS.
Public Policy Implications
Issues related to added sugars do not take place in a vacuum. Considerations related to the metabolism and health consequences of consuming sugars carry important public policy implications. For example, there are at least 3 different recommendations for appropriate upper limits for sugar consumption in the diet. The Dietary Guidelines for Americans, 2010 concluded that up to 25% of calories could be consumed as added sugars without adverse health consequences (64). These recommendations were based on the Institute of Medicine’s report on carbohydrates and health (65). The AHA offered more restrictive guidelines, suggesting that the average adult woman consume no more than 100 kcal/d and the average adult man no more than 150 kcal/d in added sugars (63). These guidelines represent ∼5–6% of calories in the typical 2000-kilocalorie diet and are currently exceeded by >90% of the American population.
Recently, the WHO recommended an upper limit of added sugars of no more than 10% of calories with a goal of ultimately reducing the recommended upper limit to no more than 5% of calories (122). The WHO report acknowledged that evidence related to sugars and weight change in adults was “moderate” to “low” and based their recommendation largely on the well-established relation between added sugars and dental caries.
The subject of different amounts of added sugars and their relation to health variables has been the focus of several RCTs in our research laboratory. In one RCT in 352 men and women between the ages of 20 and 60 y who consumed either HFCS or sucrose at 8% of calories (i.e., 4% of calories from fructose, which represents the 25th percentile population consumption level in the United States and approximately the upper limit recommended by the AHA and WHO), 18% of calories (i.e., 9% of calories from fructose, which represents approximately the 50th percentile of fructose consumption in the United States), or 30% of calories [i.e., 15% of calories from fructose, which represents approximately the 90th percentile of fructose consumption in the United States and is slightly higher than the upper limit of sugar calories recommended in the Dietary Guidelines for Americans, 2010; (64)]. Individuals who consumed these amounts of added sugars did not show any differences in blood pressure or blood lipids (2). Subsets of these individuals were studied in our metabolic units before and after a 10-wk free-living period and demonstrated no differences between these 3 amounts of sugar consumption with regard to insulin, leptin, ghrelin, glucose, postprandial TGs, or the TG AUC (26). A further subset of these individuals underwent computed tomographic scanning of the liver and MRI of the gluteus maximus and vastus lateralis muscles, which showed no changes over a 10-wk period and no differences between these 3 doses with regard to liver and ectopic muscle fat accumulation (104). Thus, our data support the recommendation by the Institute of Medicine and the Dietary Guidelines for Americans, 2010 that no adverse health consequences appear to occur at amounts of added sugar consumption up to 25% of calories. We wish to clearly state that we are not advocating this amount of added sugar consumption in the typical diet. We are simply reporting our findings that no adverse health consequences appear to occur at this amount of sugar consumption.
Various countries and organizations have struggled with how to interpret data with regard to sugar consumption and health policy. As Clemens et al. point out (123), the danger in this area is to make public policy on the basis of incomplete data supported by emotion, politics, or revenue needs rather than high-level science such as that available from RCTs.
Summary and Conclusions
Recent systematic reviews and meta-analyses as well as multiple RCTs have suggested that there is nothing unique with regard to sugar consumption and its health consequences, provided that sugar is substituted isocalorically for other carbohydrates and consumed within the normal range of human consumption (15). Some questions still remain from research trials in which fructose-containing sugars were substituted in a hypercaloric fashion (added to an already eucaloric diet). Further research trials will be necessary to settle issues in the area of hypercaloric consumption of sugars. For now, it appears safe to state that the current literature does not support a unique relation between fructose-containing sugar consumption and risk factors for cardiovascular disease, diabetes, MetS, or NAFLD at normally consumed amounts in the normal fashion (i.e., in the presence of glucose such as in sucrose or HFCS). Neurologic responses to sugars remain an active area of interest, although great care must be exhibited when considering such concepts as sugar “addiction,” which does not appear to be currently supported by research trials or expert opinion (113–115).
The isolation of sugar with the suggestion that it somehow uniquely causes multiple health problems is a direction consistent with other previous attempts to isolate components of the diet and link them to metabolic diseases, which have universally failed. As Slavin (124) wrote in a recent editorial in the American Journal of Clinical Nutrition, “Nutritional nit-picking has been unsuccessful in improving public health. Nutrient-based interventions are generally ineffective, as are bans on sugar-sweetened beverages. Dietary pattern recommendations are more likely to show success in improving cardiovascular health.”
The debate on added sugar and its health consequences has provided useful and important information over the past decade. It is hoped that continued work in this area will lead to a science-based approach based on high levels of evidence from RCTs and meta-analyses. This can only improve public health and guide wise public policy as well as inform individual nutritional decisions.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: CHD, coronary heart disease; HFCS, high-fructose corn syrup; MetS, metabolic syndrome; NAFLD; nonalcoholic fatty liver disease; RCT, randomized controlled trial; SSB, sugar-sweetened beverage.
References
- 1.Rippe JM, Angelopoulos TJ. Sucrose, high-fructose corn syrup, and fructose, their metabolism and potential health effects: what do we really know? Adv Nutr 2013;4:236–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rippe JM. The metabolic and endocrine response and health implications of consuming sweetened beverages: findings from recent, randomized, controlled trials. Adv Nutr 2013;4:677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahn R, Sievenpiper JL. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes? We have, but the pox on sugar is overwrought and overworked. Diabetes Care 2014;37:957–62. [DOI] [PubMed] [Google Scholar]
- 4.Bray GA, Popkin BM. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes? Health be damned! Pour on the sugar. Diabetes Care 2014;37:950–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klurfeld DM, Foreyt J, Angelopoulos TJ, Rippe JM. Lack of evidence for high fructose corn syrup as the cause of the obesity epidemic. Int J Obes (Lond) 2013;37:771–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bray GA, Nielsen SJ, Popkin BM. Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 2004;79:537–43. [DOI] [PubMed] [Google Scholar]
- 7.Lustig RH. Fructose: metabolic, hedonic, and societal parallels with ethanol. J Am Diet Assoc 2010;110:1307–21. [DOI] [PubMed] [Google Scholar]
- 8.Lustig RH, Schmidt LA, Brindis CD. Public health: the toxic truth about sugar. Nature 2012;482:27–9. [DOI] [PubMed] [Google Scholar]
- 9.Bray GA. Fructose: pure, white, and deadly? Fructose, by any other name, is a health hazard. J Diabetes Sci Technol 2010;4:1003–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rippe JM. The health implications of sucrose, high-fructose corn syrup, and fructose: what do we really know? J Diabetes Sci Technol 2010;4:1008–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sievenpiper JL, de Souza RJ, Kendall CW, Jenkins DJ. Is fructose a story of mice but not men? J Am Diet Assoc 2011;111:(2):219–20; author reply 220–212. [DOI] [PubMed] [Google Scholar]
- 12.van Buul VJ, Tappy L, Brouns F. Misconceptions about fructose-containing sugars and their role in the obesity epidemic. Nutr Res Rev 2014;27:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White JS. Straight talk about high-fructose corn syrup: what it is and what it ain't. Am J Clin Nutr 2008;88(Suppl):1716S–21S. [DOI] [PubMed] [Google Scholar]
- 14.White JS. Challenging the fructose hypothesis: new perspectives on fructose consumption and metabolism. Adv Nutr. 2013;4:246–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ha V, Cozma AI, Choo VLW, Meija SB, de Souza RJ, Sievenpiper JL. Do fructose-containing sugars lead to adverse health consequences? Results of recent systematic reviews and meta-analyses. Adv Nutr 2015;6:504S–11S. [Google Scholar]
- 16.Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH. Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: Influence of insulin resistance on plasma triglyceride responses. J Clin Endocrinol Metab 2009;94:1562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stanhope K, Griffen S, Keim N, Ai M, Otokozawa S, Nakajimak SE, Havel PJ. Consumption of fructose-, but not glucose sweetened beverages produces an atherogenic lipid profile in overweight/obese men and women. Diabetes 2007;56. [Google Scholar]
- 18.Havel PJ. Dietary fructose: implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 2005;63:133–57. [DOI] [PubMed] [Google Scholar]
- 19.Dolan LC, Potter SM, Burdock GA. Evidence-based review on the effect of normal dietary consumption of fructose on development of hyperlipidemia and obesity in healthy, normal weight individuals. Crit Rev Food Sci Nutr 2010;50:53–84. [DOI] [PubMed] [Google Scholar]
- 20.Dolan LC, Potter SM, Burdock GA. Evidence-based review on the effect of normal dietary consumption of fructose on blood lipids and body weight of overweight and obese individuals. Crit Rev Food Sci Nutr 2010;50:889–918. [DOI] [PubMed] [Google Scholar]
- 21.Melanson KJ, Zuckley L, Lowndes J, Nguyen V, Angelopoulos T, Rippe J. Effects of high-fructose corn syrup and sucrose consumption on circulating glucose, insulin, leptin, and ghrelin and on appetite in normal-weight women. Nutrition 2007;23:103–12. [DOI] [PubMed] [Google Scholar]
- 22.Lowndes J, Melanson K, Angelopoulos T, Rippe J. Does high fructose corn syrup affect appetite or ad libitum energy intake? Presented at the Endocrine Society, 2009.
- 23.Melanson KJ, Summers A, Nguyen V, Brosnahan J, Lowndes J, Angelopoulos TJ, Rippe JM. Body composition, dietary composition, and components of metabolic syndrome in overweight and obese adults after a 12-week trial on dietary treatments focused on portion control, energy density, or glycemic index. Nutr J 2012;11:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stanhope KL, Havel P. Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose or high-fructose corn syrup. Am J Clin Nutr 2008;88(Suppl):1733S–7S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zuckley L, Lowndes J, Nguyen V, Brosnahan J, Summers A, Melanson K, Angelopoulos T, Rippe J. Consumption of beverages sweetened with high fructose corn syrup and sucrose produce similar levels of glucose, leptin, insulin and ghrelin in obese females. FASEB J 2007;21:538. [Google Scholar]
- 26.Yu Z, Lowndes J, Rippe J. High-fructose corn syrup and sucrose have equivalent effects on energy-regulating hormones at normal human consumption levels. Nutr Res 2013;33:1043–52. [DOI] [PubMed] [Google Scholar]
- 27.Soenen S, Westerterp-Plantenga MS. No differences in satiety or energy intake after high fructose corn syrup, sucrose, or milk preloads. Am J Clin Nutr 2007;86:1586–94. [DOI] [PubMed] [Google Scholar]
- 28.American Medical Association. Report of the Council on Science and Public Health [cited 2014 Jul 7]. Available from: http://www.ama-assn.org/ama1/pub/upload/mm/467/ csaph12a07.doc.
- 29.Fitch C, Keim KS. Position of the academy of nutrition and dietetics: use of nutritive and nonnutritive sweeteners. J Acad Nutr Diet 2012;112:739–58. [DOI] [PubMed] [Google Scholar]
- 30.Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr 2006;84:274–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Olsen NJ, Heitmann BL. Intake of calorically sweetened beverages and obesity. Obes Rev 2009;10:68–75. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser KA, Shikany JM, Keating KD, Allison DB. Will reducing sugar-sweetened beverage consumption reduce obesity? Evidence supporting conjecture is strong, but evidence when testing effect is weak. Obes Rev 2013;14:620–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Te Morenga L, Mallard S, Mann J. Dietary sugars and body weight: systematic review and meta-analysis of randomized controlled trials and cohort studies. BMJ 2013;346:e7492. [DOI] [PubMed] [Google Scholar]
- 34.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kahn R, Sievenpiper JL. Dietary sugar and body weight: have we reached a crisis in the epidemic of obesity and diabetes?: we have, but the pox on sugar is overwrought and overworked. Diabetes Care 2014;37:957–62. [DOI] [PubMed] [Google Scholar]
- 36.Lowndes J, Sinnett S, Pardo S, Nguyen V, Melanson K, Yu Z, Lowther B, Rippe J. The effect of normally consumed amounts of sucrose or high fructose corn syrup on body composition and related parameters in overweight/obese subjects. Nutrients 2014;6:1128–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lowndes J, Kawiecki D, Pardo S, Nguyen V, Melanson K, Yu Z, Rippe J. The effects of four hypocaloric diets containing different levels of sucrose or high fructose corn syrup on weight loss and related parameters. Nutr J 2012;11:55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller D, Speakman J. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr 2012;95:989–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gross LS, Li L, Ford ES, Liu S. Increased consumption of refined carbohydrates and the epidemic of type 2 diabetes in the United States: an ecologic assessment. Am J Clin Nutr 2004;79:774–9. [DOI] [PubMed] [Google Scholar]
- 40.Dhingra R, Sullivan L, Jacques PF, Wang TJ, Fox CS, Meigs JB, D’Agostino RB, Gaziano JM, Vasan RS. Soft drink consumption and risk of developing cardiometabolic risk factors and the metabolic syndrome in middle-aged adults in the community. Circulation 2007;116:480–8. [DOI] [PubMed] [Google Scholar]
- 41.Miller M, Stone N, Ballantye C, Vittner V, Criqui MH, Ginsberg HN, Goldberg AC, Howard WJ, Jacobson MS, Kris-Etherton PM, et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2011;123:2292–333. [DOI] [PubMed] [Google Scholar]
- 42.Chiavaroli L, Mirrahimi A, De Souza RJ, Cozma A, Ha V, Wang DD, Yu ME, Carleton AJ, Beyene J, Kendall CWC, et al. Does fructose consumption elicit a dose-response effect on fasting triglycerides? A systematic review and meta-regression of controlled feeding trials. Can J Diabetes 2012;36:S37. [Google Scholar]
- 43.Zhang Y, An T, Zhang R, Zhou Q, Huang Y, Zhang J.. Very high fructose intake increases serum ldl-cholesterol and total cholesterol: a meta-analysis of controlled feeding trials. J Nutr 2013;143(9):1391–98. [DOI] [PubMed] [Google Scholar]
- 44.Wang DD, Sievenpiper JL, de Souza RJ, Cozma AI, Chiavaroli L, Ha V, Mirrahimi A, Carleton AJ, Di Buono M, Jenkins AL, et al. Effect of fructose on postprandial triglycerides: a systematic review and meta-analysis of controlled feeding trials. Atherosclerosis 2014;232:125–33. [DOI] [PubMed] [Google Scholar]
- 45.Egli L, Lecoultre V, Theytaz F, Campos V, Hodson L, Schneiter P, Mittendorfer B, Patterson BW, Fielding BA, Gerber PA, et al. Exercise prevents fructose-induced hypertriglyceridemia in healthy young subjects. Diabetes 2013;62:2259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Te Morenga LA, Howatson AJ, Jones RM, Mann J. Dietary sugars and cardiometabolic risk: systematic review and meta-analyses of randomized controlled trials of the effects on blood pressure and lipids. Am J Clin Nutr 2014;100:65–79. [DOI] [PubMed] [Google Scholar]
- 47.Livesey G, Taylor R. Fructose consumption and consequences for glycation, plasma triacylglycerol, and body weight: meta-analyses and meta-regression models of intervention studies. Am J Clin Nutr 2008;88:1419–37. [DOI] [PubMed] [Google Scholar]
- 48.Sievenpiper JL, Carleton AJ, Chatha S, Jiang HY, de Souza RJ, Beyene J, Kendall CW, Jenkins DJ. Heterogeneous effects of fructose on blood lipids in individuals with type 2 diabetes: systematic review and meta-analysis of experimental trials in humans. Diabetes Care 2009;32:1930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lowndes J, Sinnett S, Grench K, Jordan R, Rippe J. Impact of fructose and fructose containing sugars on indices of cardiometabolic health when consumed at typical levels. Circulation 2014;129:AP277 (abstr). [Google Scholar]
- 50.Lowndes J, Sinnett S, Fullerton Z, Angelopoulos T, Rippe J. The effects of fructose containing sugars on weight, body composition and cardiometabolic risk factors when consumed at up to the 90th percentile population consumption level for fructose. Nutrients 2014;6:3153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raben A, Vasilaras T, Møller A, Astrup A. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr 2002;76:721–9. [DOI] [PubMed] [Google Scholar]
- 52.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol 2008;294:R730–7. [DOI] [PubMed] [Google Scholar]
- 53.Grasser EK, Dulloo A, Montani JP. Cardiovascular responses to the ingestion of sugary drinks using a randomised cross-over study design: does glucose attenuate the blood pressure-elevating effect of fructose? Br J Nutr 2014;112:183–92. [DOI] [PubMed] [Google Scholar]
- 54.Lê K-A, Faeh D, Stettler R, Ith M, Kreis R, Vermathen P, Boesch C, Ravussin E, Tappy L. A 4-wk high-fructose diet alters lipid metabolism without affecting insulin sensitivity or ectopic lipids in healthy humans. Am J Clin Nutr 2006;84:1374–9. [DOI] [PubMed] [Google Scholar]
- 55.Maersk M, Belza A, Stødkilde-Jørgensen H, Ringgaard S, Chabanova E, Thomsen H, Pedersen SB, Astrup A, Richelsen B. Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am J Clin Nutr 2012;95:283–9. [DOI] [PubMed] [Google Scholar]
- 56.Ha V, Sievenpiper JL, de Souza RJ, Chiavaroli L, Wang DD, Cozma AI, Mirrahimi A, Yu ME, Carleton AJ, Dibuono M, et al. Effect of fructose on blood pressure: a systematic review and meta-analysis of controlled feeding trials. Hypertension 2012;59:787–95. [DOI] [PubMed] [Google Scholar]
- 57.Duffey KJ, Gordon-Larsen P, Steffen LM, Jacobs DR, Popkin BM. Drinking caloric beverages increases the risk of adverse cardiometabolic outcomes in the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Clin Nutr 2010;92:954–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen L, Caballero B, Mitchell DC, Loria C, Lin PH, Champagne CM, Elmer PJ, Ard JD, Batch BC, Anderson CA, et al. Reducing consumption of sugar-sweetened beverages is associated with reduced blood pressure: a prospective study among United States adults. Circulation 2010;121:2398–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Winkelmayer WC, Stampfer MJ, Willett WC, Curhan GC. Habitual caffeine intake and the risk of hypertension in women. JAMA 2005;294:2330–5. [DOI] [PubMed] [Google Scholar]
- 60.de Koning L, Malik VS, Kellogg MD, Rim EB, Willett WC, Hu FB. Sweetened beverage consumption, incident coronary heart disease and biomarkers of risk in men. Circulation 2012;125:1735–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fung T, Malik V, Rexrode K, Manson JE, Willett WC, Hu FB. Sweetened beverage consumption and risk of coronary heart disease in women. Am J Clin Nutr 2009;89:(4):1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eshak ES, Iso H, Kokubo Y, Saito I, Yamagishi K, Inoue M, Tsugane S. Soft drink intake in relation to incident ischemic heart disease, stroke, and stroke subtypes in Japanese men and women: the Japan Public Health Centre–based study cohort I. Am J Clin Nutr 2012;96:1390–7. [DOI] [PubMed] [Google Scholar]
- 63.Johnson RK, Appel L, Brands M, Howard B, Lefevre M, Lustig R, Sacks F, Steffen L, Wylie-Rosett J; American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity, and Metabolism and the Council on Epidemiology and Prevention. Dietary sugars intake and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2009;120:1011–20. [DOI] [PubMed] [Google Scholar]
- 64. Dietary Guidelines Advisory Committee; USDA; Center for Nutrition Policy and Promotion. Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans. Dept of Agriculture, Washington: 2010.
- 65.Institute of Medicine. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. Washington: National Academies Press; 2005. [Google Scholar]
- 66.Defronzo RA. Banting Lecture: from the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009;58:773–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet 2011;378:169–81. [DOI] [PubMed] [Google Scholar]
- 68.Corkey BE. Banting Lecture 2011: hyperinsulinemia: cause or consequence? Diabetes 2012;61:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Basu S, Yoffe P, Hills N, Lustig RH. The relationship of sugar to population-level diabetes prevalence: an econometric analysis of repeated cross-sectional data. PLoS ONE 2013;8:e57873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goran MI, Ulijaszek SJ, Ventura EE. High fructose corn syrup and diabetes prevalence: a global perspective. Glob Public Health 2013;8:55–64. [DOI] [PubMed] [Google Scholar]
- 71.Barclay AW, Brand-Miller J. The Australian paradox: a substantial decline in sugars intake over the same timeframe that overweight and obesity have increased. Nutrients 2011;3:491–504. Erratum in: Nutrients 2014;6:663–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Janket SJ, Manson JE, Sesso H, Buring JE, Liu S. A prospective study of sugar intake and risk of type 2 diabetes in women. Diabetes Care 2003;26:1008–15. [DOI] [PubMed] [Google Scholar]
- 74.Moore MC, Davis SN, Mann SL, Cherrington AD. Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes. Diabetes Care 2001;24:1882–7. [DOI] [PubMed] [Google Scholar]
- 75.Hodge AM, English DR, O'Dea K, Giles DD. Glycemic index and dietary fiber and the risk of type 2 diabetes. Diabetes Care 2004;27:2701–6. [DOI] [PubMed] [Google Scholar]
- 76.Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. Am J Clin Nutr 1992;55:1018–23. [DOI] [PubMed] [Google Scholar]
- 77.Meyer KA, Kushi LH, Jacobs DR Jr, Slavin J, Sellers TA, Folson AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- 78.Cozma AI, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Wang DD, Mirrahimi A, Yu ME, Carleton AJ, Di Buono M, et al. Effect of fructose on glycemic control in diabetes: a systematic review and meta-analysis of controlled feeding trials. Diabetes Care 2012;35:1611–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Teff KL, Elliott S, Tschop M, Kieffer TJ, Rader D, Heiman M, Townsend RR, Keim NL, D’Alessio D, Havel PJ. Dietary fructose reduces circulating insulin and leptin, attenuates postprandial suppression of ghrelin, and increases triglycerides in women. J Clin Endocrinol Metab 2004;89:2963–72. [DOI] [PubMed] [Google Scholar]
- 80.Aeberli I, Gerber PA, Hochuli M, Kohler S, Haile SR, Gouni-Berthold I, Berthold HK, Spinas GA, Berneis K. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: a randomized controlled trial. Am J Clin Nutr 2011;94:479–85. [DOI] [PubMed] [Google Scholar]
- 81.Aeberli I, Hochuli M, Gerber PA, Sze L, Murer SB, Tappy L, Spinas GA, Berneis K. Moderate amounts of fructose consumption impair insulin sensitivity in healthy young men: a randomized controlled trial. Diabetes Care 2013;36:150–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stanhope KL, Griffen SC, Bremer AA, Vink RG, Schaefer EJ, Nakajima K, Schwarz J-M, Beysen C, Berglund L, Keim NL, et al. Metabolic responses to prolonged consumption of glucose- and fructose-sweetened beverages are not associated with postprandial or 24-h glucose and insulin excursions. Am J Clin Nutr 2011;94:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Beck-Nielsen H, Pedersen O, Lindskov HO. Impaired cellular insulin binding and insulin sensitivity induced by high-fructose feeding in normal subjects. Am J Clin Nutr 1980;33:273–8. [DOI] [PubMed] [Google Scholar]
- 84.Lowndes J, Sinnett S, Rippe J. No change in oral glucose tolerance tests as a result of ten weeks of consumption of various fructose containing sugars or slucose. (presented, Endocrine Society, 2014). SUN 0998-1026-Diabetes: clinical physiology and treatment, clinical, poster board SUN-1018. [Google Scholar]
- 85.Angelopoulos T, Lowndes J, Rippe J. No effect of type of sugar on whole body on hepatic insulin resistance. Obes Rev 2014;15(Suppl 2):92–3.24165204 [Google Scholar]
- 86.National Cholesterol Education Program. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001;285:2486–97. [DOI] [PubMed] [Google Scholar]
- 87.Ford ES, Giles WH, Mokdad AH. Increasing prevalence of the metabolic syndrome among US adults. Diabetes Care 2004;27:2444–9. [DOI] [PubMed] [Google Scholar]
- 88.Johnson RJ, Segal M, Sautin Y, Nakagawa T, Feig D, Kang K-H, Gersch MS, Benner S, Sanchez-Lozada LG. Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 2007;86:899–906. [DOI] [PubMed] [Google Scholar]
- 89.Seip R. Beyond subcutaneous fat. In: Rippe J, Angelopoulos T, editors. Obesity: prevention and treatment. Boca Raton (FL): CRC Press; 2013. p. 381–408.
- 90.Stanhope KL, Schwarz J, Keim N, Griffen S, Bremer A, Graham J, Hatcher B, Cox C, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lowndes J, Lu N, Sinnett S, Rippe J. No effect of the type of sugar on changes in traditional risk factors for cardiovascular disease when consumed at the typical levels. Circulation 2013;128:A13008. [Google Scholar]
- 92.Welsh JA, Karpen S, Vos MB. Increasing prevalence of nonalcoholic fatty liver disease among United States adolescents, 1988–1994 to 2007–2010. J Pediatr 2013;162:(3):496–500, e491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ouyang X, Cirillo P, Sautin Y, McCall S, Bruchette JL, Diehl AM, Johnson RJ, Abdelmalek MF. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J Hepatol 2008;48:993–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thuy S, Ladurner R, Volynets V, Wagner S, Strahl S, Konigsrainer A, Maier KP, Bischoff SC, Bergheim I. Nonalcoholic fatty liver disease in humans is associated with increased plasma endotoxin and plasminogen activator inhibitor 1 concentrations and with fructose intake. J Nutr 2008;138:1452–5. [DOI] [PubMed] [Google Scholar]
- 95.Parks EJ, Skokan LE, Timlin MT, Dingfelder CS. Dietary sugars stimulate fatty acid synthesis in adults. J Nutr 2008;138:1039–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol Rev 2010;90:23–46. [DOI] [PubMed] [Google Scholar]
- 97.Lewis GF, Uffelman K, Naples M, Szeto L, Haidari M, Adeli K. Intestinal lipoprotein overproduction, a newly recognized component of insulin resistance, is ameliorated by the insulin sensitizer rosiglitazone: studies in the fructose-fed Syrian golden hamster. Endocrinology 2005;146:247–55. [DOI] [PubMed] [Google Scholar]
- 98.Theytaz F, Noguchi Y, Egli L, Campos V, Buehler T, Hodson L, Patterson BW, Nishikata N, Kreis R, Mittendorfer B, et al. Effects of supplementation with essential amino acids on intrahepatic lipid concentrations during fructose overfeeding in humans. Am J Clin Nutr 2012;96:1008–16. [DOI] [PubMed] [Google Scholar]
- 99.Lecoultre V, Egli L, Carrel G, Theytaz F, Kreis R, Schneiter P, Boss A, Zwygart K, Lê KA, Bortolotti M, et al. Effects of fructose and glucose overfeeding on hepatic insulin sensitivity and intrahepatic lipids in healthy humans. Obesity (Silver Spring) 2013;21:782–5. [DOI] [PubMed] [Google Scholar]
- 100.Johnston RD, Stephenson MC, Crossland H, Cordon SM, Palcidi E, Cox EF, Taylor MA, Aithal GP, MacDonald IA. No difference between high fructose and high glucose diets on liver triacylglycerol or biochemistry in healthy overweight men. Gastroenterology 2013;145:1016–25. [DOI] [PubMed] [Google Scholar]
- 101.Hellerstein MK, Schwarz JM, Neese RA. Regulation of hepatic de novo lipogenesis in humans. Annu Rev Nutr 1996;16:523–57. [DOI] [PubMed] [Google Scholar]
- 102.Lê KA, Ith M, Kreis R, Faeh D, Bortolotii M, Tran C, Boesch C, Tappy L. Fructose overconsumption causes dyslipidemia and ectopic lipid deposition in healthy subjects with and without a family history of type 2 diabetes. Am J Clin Nutr 2009;89:1760–5. [DOI] [PubMed] [Google Scholar]
- 103.Silbernagel G, Machann J, Unmuth S, Schick F, Stefan N, Haring HU, Fritsche A. Effects of 4-week very-high-fructose/glucose diets on insulin sensitivity, visceral fat and intrahepatic lipids: an exploratory trial. Br J Nutr 2011;106:79–86. [DOI] [PubMed] [Google Scholar]
- 104.Bravo S, Lowndes J, Sinnett S, Yu Z, Rippe J. Consumption of sucrose and high-fructose corn syrup does not increase liver fat or ectopic fat deposition in muscles. Appl Physiol Nutr Metab 2013;38:681–8. [DOI] [PubMed] [Google Scholar]
- 105.Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: a systematic review and meta-analysis. Am J Clin Nutr 2014;100:833–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chiu S, Sievenpiper JL, de Souza RJ, Cozma AI, Mirrahimi A, Carleton AJ, Ha V, Di Buono M, Jenkins AL, Leiter LA, et al. Effect of fructose on markers of non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of controlled feeding trials. Eur J Clin Nutr 2014;68:416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Funari VA, Herrera VL, Freeman D, Tolan DR. Genes required for fructose metabolism are expressed in Purkinje cells in the cerebellum. Brain Res Mol Brain Res 2005;142:115–22. [DOI] [PubMed] [Google Scholar]
- 108.Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 2006;13:1385–8. [DOI] [PubMed] [Google Scholar]
- 109.Smeets PA, de Graaf C, Stafleu A, van Osch MJ, van der Grond J. Functional magnetic resonance imaging of human hypothalamic responses to sweet taste and calories. Am J Clin Nutr 2005;82:1011–6. [DOI] [PubMed] [Google Scholar]
- 110.Matsuda M, Liu Y, Mahankali S, Pu Y, Mahankali A, Wang J, DeFronzo RA, Fox PT, Gao JH. Altered hypothalamic function in response to glucose ingestion in obese humans. Diabetes 1999;48:1801–6. [DOI] [PubMed] [Google Scholar]
- 111.Page KA, Luo S, Romero A, Adam T, Hu HH. Fructose compared to glucose ingestion preferentially activates brain reward regions in response to high-calorie food cues in young, obese Hispanic females. Endocrinol Rev 201;2;33.
- 112.Purnell JQ, Klopfenstein BA, Stevens AA, Havel PJ, Adams SH, Dunn TN, Krisky C, Rooney WD. Brain functional magnetic resonance imaging response to glucose and fructose infusions in humans. Diabetes Obes Metab 2011;13:229–34. [DOI] [PubMed] [Google Scholar]
- 113.Benton D. The plausibility of sugar addiction and its role in obesity and eating disorders. Clin Nutr 2010;29:288–303. [DOI] [PubMed] [Google Scholar]
- 114.Ziauddeen H, Farooqi I, Fletcher P. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci 2012;13:279–86. [DOI] [PubMed] [Google Scholar]
- 115.Corwin LW, Hayes JE. Are the sugars addictive? Perspectives for practitioners. In: Rippe JM, editor. Fructose, high fructose corn syrup, sucrose and health. New York: Springer; 2014. p. 199–215.
- 116.Stice E, Burger KS, Yokum S. Relative ability of fat and sugar tastes to activate reward, gustatory, and somatosensory regions. Am J Clin Nutr 2013;98:1377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stice E, Yokum S, Blum K, Bohon C. Weight gain is associated with reduced striatal response to palatable food. J Neurosci 2010;30:13105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grabenhorst F, Rolls ET, Parris BA, d'Souza AA. How the brain represents the reward value of fat in the mouth. Cereb Cortex 2010;20:1082–91. [DOI] [PubMed] [Google Scholar]
- 119.Pena-Gomez C, Alonso-Alonso M, Bravo S, Magerowski G, Sinnett S, Blackburn G, Rippe J. Hypothalamic fMRI responses to different sugars under normal intake conditions: a pilot study. Presented at: Obesity Society Annual Scientific Meeting; 2013. T-729-P.
- 120.Laughlin MR, Bantle JP, Havel PJ, Parks E, Klurfeld D, Teff K, Maruvada P. Clinical research strategies for fructose metabolism. Adv Nutr 2014;5:248–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang DD, Chung M. Future research needs (FRNs): sugars and health outcomes. Presented at: ASN Advances and Controversies in Clinical Nutrition Conference; ILSI North America; 5 Dec 2013.
- 122.WHO. Draft guideline: sugars intake for adults and children. Online public consultation open 5 to 31 March 2014 [cited 2014 Jul 14]. Available from: http://www.who.int/mediacentre/news/notes/2014/consultation-sugar-guideline/en/.
- 123.Clemens R, Drewnowski A, Ferruzzi MG, Toner CD, Welland D. Squeezing fact from fiction about 100% fruit juice. Adv Nutr 2015;6:236S–243S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Slavin J. Two more pieces to the 1000-piece carbohydrate puzzle. Am J Clin Nutr 2014;100:4–5. [DOI] [PubMed] [Google Scholar]