Abstract
Interstitial cystitis (IC) is a syndrome characterized by urinary urgency, frequency, pelvic pain, and nocturia in the absence of bacterial infection or identifiable pathology. IC is a devastating disease that certainly decreases quality of life. However, the causes of IC remain unknown and no effective treatments or cures have been developed. This study evaluated the therapeutic potency of using human umbilical cord-blood-derived mesenchymal stem cells (UCB-MSCs) to treat IC in a rat model and to investigate its responsible molecular mechanism. IC was induced in 10-week-old female Sprague–Dawley rats via the instillation of 0.1 M HCl or phosphate-buffered saline (PBS; sham). After 1 week, human UCB-MSC (IC+MSC) or PBS (IC) was directly injected into the submucosal layer of the bladder. A single injection of human UCB-MSCs significantly attenuated the irregular and decreased voiding interval in the IC group. Accordingly, denudation of the epithelium and increased inflammatory responses, mast cell infiltration, neurofilament production, and angiogenesis observed in the IC bladders were prevented in the IC+MSC group. The injected UCB-MSCs successfully engrafted to the stromal and epithelial tissues and activated Wnt signaling cascade. Interference with Wnt and epidermal growth factor receptor activity by small molecules abrogated the benefits of MSC therapy. This is the first report that provides an experimental evidence of the therapeutic effects and molecular mechanisms of MSC therapy to IC using an orthodox rat animal model. Our findings not only provide the basis for clinical trials of MSC therapy to IC but also advance our understanding of IC pathophysiology.
Introduction
Interstitial cystitis (IC) is a syndrome characterized by urinary urgency, frequency, pelvic pain, and nocturia in the absence of bacterial infection or identifiable pathology [1]. IC is an obstinate disease that certainly decreases quality of life; however, only a few treatments have been reported and, most especially, curable treatments have not been identified. Although the etiology of IC is not fully understood, in general, the IC bladder is characterized by a thin and denuded urothelium [2]. The altered synthesis of several proteoglycans, cell adhesion proteins, and tight junction proteins is responsible for the loss of urothelial integrity observed in IC patients [3,4] The resulting leaky urothelium has been proposed as the cause of the bladder symptoms observed in IC patients [5]. Thus, regenerating the epithelium is of major interest for IC treatment. However, its precise molecular nature remains unknown.
Epithelial integrity is dynamically regulated via the homeostatic proliferation and differentiation of organ-specific stem and progenitor cells, which generally reside in the basal layer of the epithelium [6,7]. The activity of these cells is dependent on the turnover of these tissues. For example, the progenitor cells in the intestines are continually and constantly involved in epithelial regeneration [8]. Unlike epithelial turnover, however, the urinary bladder is relatively stable. Thus, urothelial and stromal cells remain in a near quiescent state during normal physiology, though they will enter a highly proliferative state in response to epithelial injury [9,10]. Rapidly proliferating epithelial cells in the intestines and skin characteristically activate several morphogenic factors, including the Sonic hedgehog (Shh) and the Wnt cascade, which are responsible for self-renewal in tissue-residing stem cells [11,12]. Similarly, these signals are involved in maintaining homeostasis in the urothelium [13,14]. In particular, the Shh and Wnt proteins positively mediate the interplay between the stem cells in the basal layer and the nearby stromal cells, which is responsible for activating cellular proliferation within the bladder in response to bacterial infections or chemical injury [10]. Thus, dysfunctional urothelial integrity in IC patients could be cured by reinforcing the regenerative potency of basal stem cells in the bladder.
Mesenchymal stem cells (MSCs) are multipotent progenitor cells that are able to differentiate into a range of cell types, including the mesoderm- (eg, muscle, stroma) and ectoderm-lineage tissues (eg, epithelium and neuron) [15–18]. MSCs secrete several cytokines and growth factors that provide beneficial paracrine effects [19]. MSC therapy is considered a novel therapeutic approach for the treatment of a number of bladder disorders [20–30]. However, no studies to date have investigated MSC therapy to treat IC. Importantly, our recent study demonstrated that injected MSCs ameliorate overactive blabber by activating endogenous Oct4+ primitive stem cells [27]. Here, we evaluate the therapeutic potency of MSC therapy as a cure for IC in a traditional animal model and investigate the possible role of Wnt and epidermal growth factor (EGF) signaling in MSC therapy.
Materials and Methods
IC rat model
To induce IC, the bladders of twenty 10-week-old female Sprague–Dawley rats were instilled with 0.1 M HCl for 10 min via the urethra using a 26-gauge angiocatheter, followed by neutralization and washing with saline. For the sham group (n=15), phosphate-buffered saline (PBS) was used as the vehicle instead of HCl. One week after HCL instillation, an abdominal incision was made and either 1×106 umbilical cord-blood-derived mesenchymal stem cells (UCB-MSCs) (IC+MSC group; n=15) or PBS vehicle (IC group; n=15) was directly injected into the submucosal layer of the anterior wall and dome of the bladder using a 500-μm syringe and a 26-gauge needle. One week after UCB-MSC injection, conscious cystometry was conducted to assess the therapeutic potential of the transplanted MSCs. Indomethacin (Indometa Cap®; PMG Pharm Co., Ltd.) was subcutaneously injected every 12 h to block Wnt signaling (injections were administered starting 1 day before stem cell injection). Likewise, epidermal growth factor receptor (EGFR) signaling was blocked by the daily subcutaneous injection of 5 mg/kg Gefitinib (Santa Cruz Biotechnology, Inc.).
Culturing UCB-MSCs
The human UCB-MSCs used in this study were donated by Medipost Co., Ltd. UCB-MSCs were separated as previously described [31,32] and maintained in Dulbecco's modified Eagle's medium-high-glucose (Hyclone) supplemented with 2 mM L-glutamine, 20 mM HEPES (pH 7.3), MEM nonessential amino-acid solution, penicillin/streptomycin (Cellgro), 1 μg/mL ascorbic acid (Sigma), 10% heat-inactivated FBS (Hyclone), 5 ng/mL human EGF, 10 ng/mL basic fibroblast growth factor, and 50 μg/mL Long R3 insulin-like growth factor-1 (IGF1; Prospec) in a 37°C humidified atmosphere that contained 5% CO2. UCB-MSCs were allowed to expand for six passages and then transplanted to ensure multipotency. The cultured UCB-MSCs were labeled with PKH26 Red Fluorescent Cell Linker Kits (Sigma) according to the manufacturer's instructions; then, engraftment and transdifferentiation were assessed.
Cystometry
Conscious cystometry was performed by placing a conscious rat under restriction. A midline suprapubic incision was made to expose the bladder, which was accessed using an inflatable polyethylene-50 tube (Clay-Adams) that was connected to a pressure transducer. Sterile saline was infused at a rate of 40 μL/min via a syringe pump (Harvard-Apparatus). Analysis was performed using a UDS-120XLT urodynamic measurement system (Laborie-Medical-Technologies). Intravesical pressure was analyzed and recorded using a pressure analyzer and a personal computer-based data acquisition system.
Histo- and immunohistochemical analyses
After 24 h of fixation in 4% paraformaldehyde, each bladder was embedded in paraffin, sectioned to 3 μm using a microtome, and stained with hematoxylin and eosin. The integrity of the epithelium, localization of the nerve fibers, and angiogenesis in the bladder were further examined by staining with antibodies against pan-specific cytokeratin (CK; Sigma), neurofilament 200 (N200; Abcam), and CD31 (Santa Cruz Biotechnology), respectively. Reactions were visualized using the UltraVision LP detection system, and 3-amino-9-ethylcarbazole (AEC) was used as the chromogen (Thermo Scientific). Mast cell infiltration and tissue fibrosis were detected using Toluidine blue staining (Toluidine blue-O; Daejung Chemicals & Metals co.) and Masson's trichrome staining (Junsei Chemical), respectively. Each slide was microscopically inspected; 10 randomly chosen representative areas from the light microscopic images were selected, and quantitative digital image analysis was performed using Image Pro 5.0 software (Media-Cybernetics). For the immunofluorescent analysis, epithelial and stromal tissues from the bladder were stained using antibodies specific to CK and vimentin (Santa Cruz Biotechnology), respectively, followed by visualization using Alexa® 488-conjugated anti-mouse or -rabbit antibodies (Molecular Probes). Nuclei were counterstained using 4′,6-diamino-2-phenylindole (DAPI). The status of Wnt signaling was determined using immunofluorescent analysis for non-phospho β-catenin (Cell Signaling Technology).
Western blot
The tissue extracts of the bladders were prepared in RIPA lysis buffer (Santa Cruz Biotechnology, Inc.). Proteins were quantified using the BCA method (Thermo Scientific), and 50 μg of extracts were separated using 10% SDS-PAGE gels. Protein levels were assessed by probing with the non-phospho β-catenin, phospho β-catenin (Ser33/37/Thr41; Cell Signaling Technology), and β-actin (Santa Cruz Biotechnology, Inc.).
Reverse transcriptase and real-time quantitative polymerase chain reaction
Isolation of total RNA from the bladder tissues and quantitative assessment of the expression levels of the target genes was performed using real-time quantitative polymerase chain reaction (RQ-PCR), as previously described [33].
Proliferation assay of human or rat bladder epithelial cells
HBlEpC, a primary epithelial cell derived from normal human bladder (Cell Applications, Inc.), was maintained in Bladder Epithelial Cell Growth Medium (Cell Applications, Inc.), according to the manufacturer's instructions. The epithelial cells from rat bladders were dissociated by digesting the minced bladder tissues with 2 mg/mL collagenase (Sigma) for 10 min at room temperature. The dissociated cells were plated into a tissue culture dish (VWR Corporate) and maintained in Bladder Epithelial Cell Growth Medium. 5×103 human or rat bladder epithelial cells were seeded in a 96-well culture plate, followed by stimulation with conditioned medium (CM), which was harvested during cultivation of IMR90, a human normal fibroblast or UCB-MSCs, for 1 day. Cell proliferation after treatment with CM for the indicated days in the absence or presence of a chemical inhibitor of Wnt product (IWP) compound, IWP-2 (Sigma), was determined using the MTT assay (Sigma) according to the manufacturer's instructions. Reduction of the MTT reagent was performed for 4 h and quantified by measuring the absorbance at 570 nm using a microplate spectrophotometer (Molecular Devices).
Statistical analysis
Differences in the cystometric and RQ-PCR results were analyzed using the student t-test or one-way analysis of variance with the Bonferroni post hoc testing. We used GraphPad Prism 6.0 software (GraphPad Software) to perform all analyses, and statistical significance is defined as P<0.05 or 0.01. All primers used in the RQ-PCR assay are available on request.
Study approval
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Ulsan College of Medicine (IACUC-2013-13-010). Umbilical cord blood was collected from umbilical veins after neonatal delivery with the informed consent of the mothers.
Results
Evaluation of bladder function
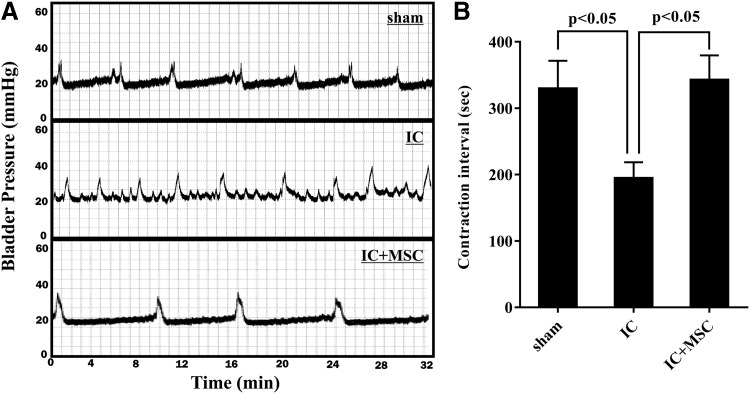
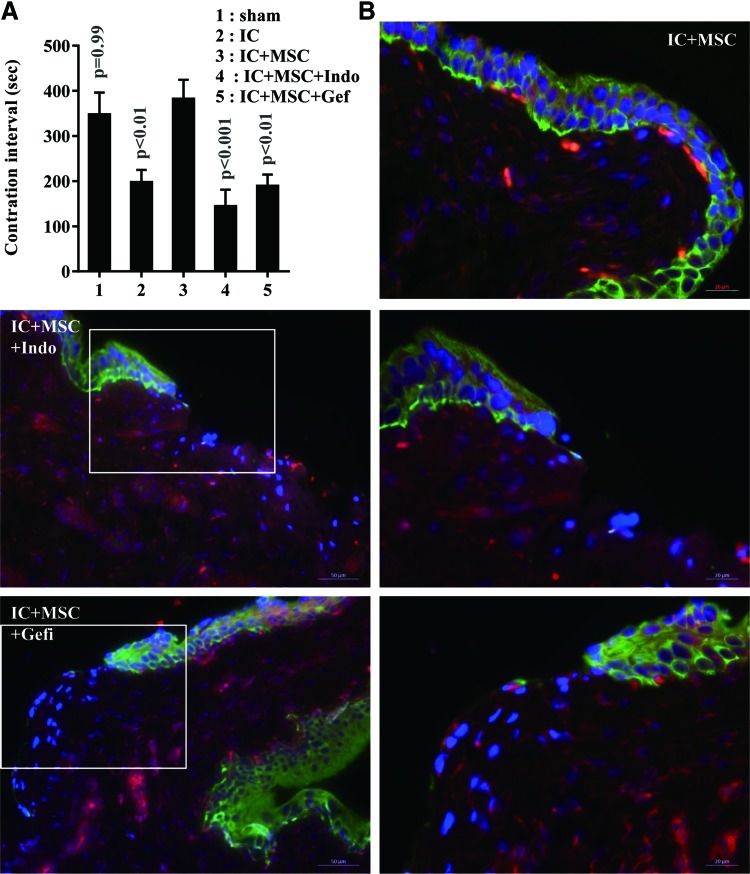
To assess the therapeutic potential of the transplanted UCB-MSCs, we examined the voiding function of the bladders by performing the conscious cystometric analysis, which is used to evaluate the bladder's capacity to contract and expel urine. As shown in Fig. 1, most rats in the IC group exhibited irregular voiding frequency and decreased the inter-contraction interval in comparison with the sham group (201.8±81.7 vs. 330.0±143.6 s, respectively; P<0.05). A single injection of UCB-MSCs significantly increased the inter-contraction interval (343.1±131.6 s, P<0.05), suggesting that UCB-MSC therapy ameliorated bladder voiding function in our rat model of IC (Fig. 1B).
FIG. 1.
UCB-MSC injection improved voiding function in an IC bladder. (A) Representative conscious cystometry results and (B) contraction intervals in the indicated animal groups at 1 week after the injection of mesenchymal stem cells (MSCs). Data are represented as the mean±SEM (n=12; P<0.05 in comparison with sham-operated and IC group, one-way ANOVA with Bonferroni post-test). Notably, IC bladders injected with UCB-MSC (IC+MSC) demonstrated normal voiding patterns in comparison with bladders injected with PCB (IC). ANOVA, analysis of variance; IC, interstitial cystitis; UCB-MSC, umbilical cord-blood-derived mesenchymal stem cell.
Histo- and immunohistochemical analyses
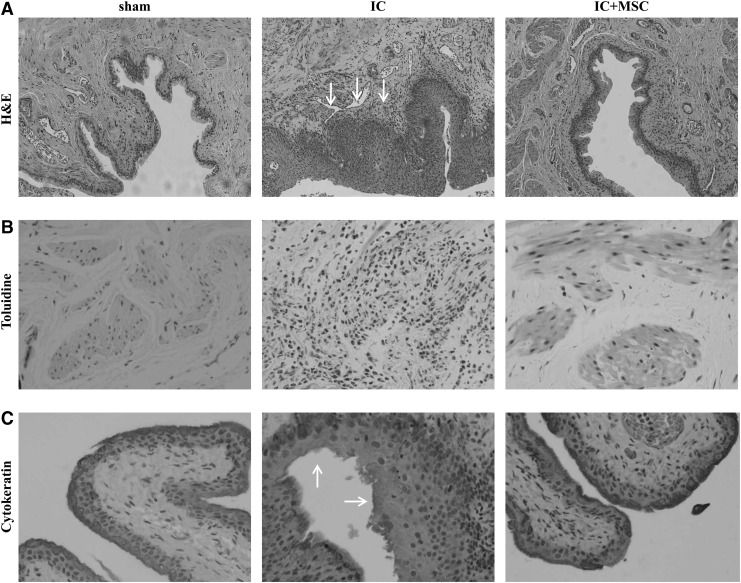
We next examined the histological features observed in the IC patients, including epithelium denudation, abnormal increases in inflammation, neurofilament production, and angiogenesis [34,35]. Compared with the sham-operated rats, the bladder tissues in the IC group demonstrated severe inflammation (Fig. 2A) and the infiltration of Toluidine blue-stained mast cells (Fig. 2B), which were remarkably abrogated by UCB-MSC injection. The epithelial layers were densely stained with CK (an epithelium-specific protein) and markedly low in the IC+PBS group (Fig. 2C), suggesting the induction of epithelial denudation in our IC animal model. In particular, UCB-MSC injection ameliorated the lining of the epithelium and sustained staining intensity in the urothelium. In addition, the abnormal increase in N200+ neurofilaments and CD31+ vessels observed in the IC group bladders was hardly detectable in the IC+MSC group (Supplementary Fig. S1A, B; Supplementary Data are available online at www.liebertpub.com/scd). Accordingly, the bladders from the IC group highly expressed the transcripts of several angiogenesis promoting growth factors, including Vegfa and Pdgfb; however, they were significantly suppressed in IC+MSC group bladders (Supplementary Fig. S1C). It is reported that IC is associated with fibrosis of layers of the bladder wall [36]. Thus, we performed Masson's trichrome staining of the bladder tissues in each group. Compared with the sham group, the tissue fibrosis was significantly increased in the IC group bladder. The injection of UCB-MSCs ameliorated the bladder tissue fibrosis, although it was not statistically significant (Supplementary Fig. S2). Taken together, these results indicate that HCl-mediated IC reproduces similar histological alternations that were found in the IC patients, and that MSC therapy cures most IC characteristic pathologies, including epithelial denudation and abnormal inflammation, neural networks, and angiogenesis in the bladder.
FIG. 2.
UCB-MSC therapy ameliorated histological abnormalities in IC bladder. (A) Hematoxylin and eosin staining in the indicated bladder tissues (magnification ×200). Nuclei were stained with Mayer's hematoxylin. Arrows indicate severe inflammation. Toluidine (B; magnification ×400) and cytokeratin staining (C; magnification ×200) was used to evaluate the infiltration of master cells and the integrity of the urothelium, respectively. Arrows indicate the broken urothelium.
Differentiation fates of engrafted UCB-MSCs
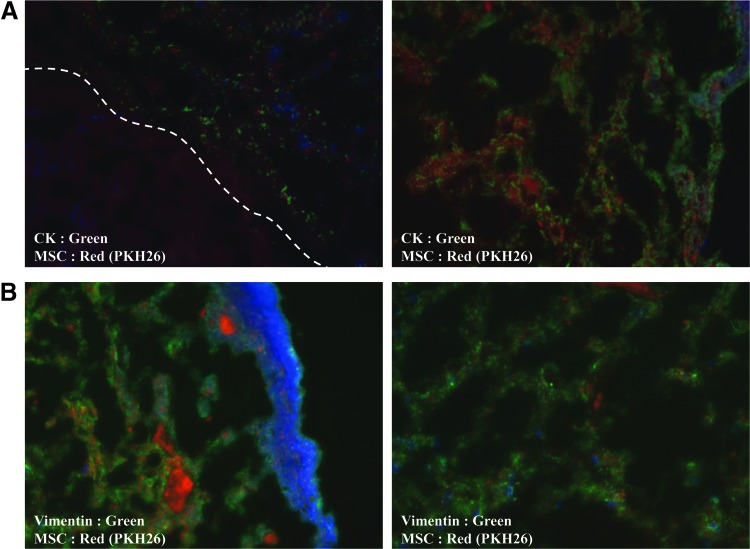
To determine the engraftment and differentiation fates of UCB-MSC, we labeled the stem cells with PKH26 red fluorescent dye before injection. Cells labeled with PKH26 red fluorescent labeling probes were observed in most bladders in the IC+MSC group, and the majority of these were broadly distributed throughout the lamina propria near the basal layer of the urothelium, though less so within the muscular layer (data not shown). In particular, UCB-MSC-derived PKH26+ cells colocalized within both CK+ epithelial (Fig. 3A) and vimentin+ stromal cells in the bladders (Fig. 3B), indicating that the engrafted UCB-MSCs regenerated the damaged bladder through direct differentiation into the urothelium and nearby stromal tissue.
FIG. 3.
Engraftment of injected UCB-MSCs. Fluorescent immunohistochemical detection of the PKH26-labeled UCB-MSCs (red), which colocalized with (A) cytokeratin+ urothelium and (B) vimentin+ stromal tissue (green) in the IC bladder tissues at 1 week after stem cell injection. Nuclei were stained with DAPI (blue). The dotted line indicates the margin between the urothelium and stromal tissues. The left and right panel images are magnified ×200 and ×400, respectively. DAPI, 4′,6-diamino-2-phenylindole.
Upregulation of Wnt and downstream growth factors via MSC injection
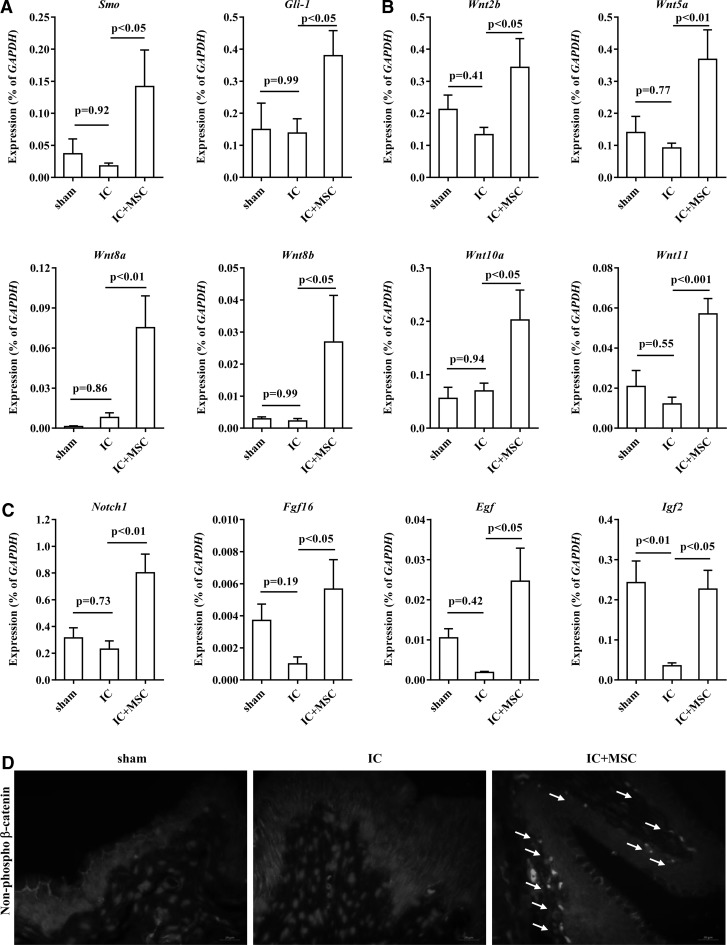
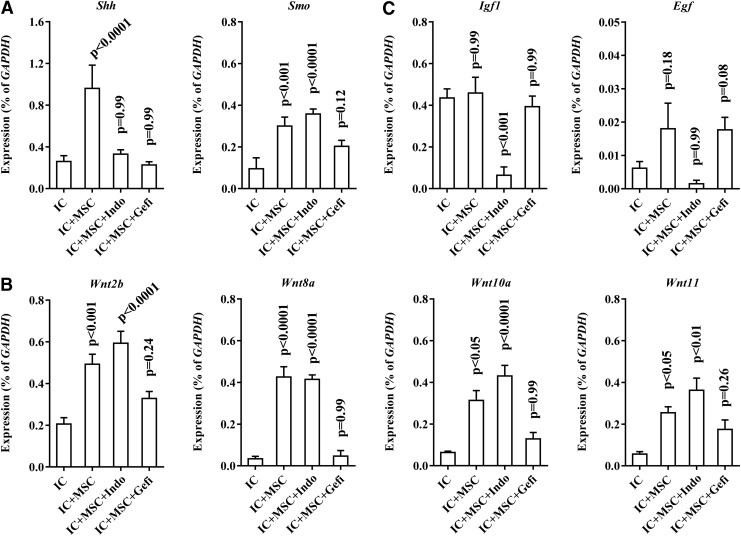
We next investigated the molecular mechanisms that cause the beneficial effects of UCB-MSC. In response to urothelium damage, the Shh and Wnt signaling cascades are activated for bladder regeneration by stimulating the endogenous stem cells that mainly reside in the basal layer of the urothelium [10]. Thus, we examined the expression levels of these signaling components and the downstream growth factors implicated in bladder regeneration [10]. Compared with the sham group, several Shh and Wnt signaling genes were generally downregulated in IC group (Fig. 4A–C and Supplementary Fig. S3). Of note, UCB-MSC injection significantly upregulated the majority of these genes, including smoothened (Smo) (Fig. 4A), Wnt2b, Wnt5a, Wnt8a, Wnt8b, Wnt10a, Wnt11 (Fig. 4B), and associated growth factors, including Notch1, fibroblast growth factor-16 (Fgf16), Egf, and insulin-like growth factor 2 (Igf2) (Fig. 4C). Accordingly, the IC+MSC group bladders demonstrated increases in non-phospho β-catenin protein and simultaneously decreases in β-catenin protein phosphorylated at serine 33, 37, and threonine 41 that indicate the activated status of Wnt signaling [37] (Fig. 4D and Supplementary Fig. S4). Importantly, the nuclear staining of non-phospho β-catenin protein, a surrogate marker for Wnt signaling activation, was mainly observed in the basal urothelial layer, which is the main niche of the endogenous bladder stem cells (Fig. 4D).
FIG. 4.
Activation of an Shh-Wnt-EGF signaling cascade by engrafted UCB-MSCs. RQ-PCR analysis of (A) Smo and Gli-1, (B) Wnt, and (C) downstream growth factors at the indicated bladder tissues. Expression is represented as % Gapdh (as determined using five or more independent experiments) and is shown as the mean±SEM (P<0.05, P<0.01 in comparison with IC bladders, one-way ANOVA with Bonferroni post-test). (D) Fluorescent immunohistochemical detection of non-phospho β-catenin protein in the indicated bladder tissues (magnification ×400). Nuclei were stained with DAPI. Notably, dense non-phospho β-catenin protein staining was mainly detected in the basal layer of urothelium of the IC+MSC group (arrow). Scale bar=20 μm. EGF, epidermal growth factor; RQ-PCR, real-time quantitative polymerase chain reaction.
Significance of the Wnt and EGFR signaling cascades in MSC therapy
To address the biological significance of Wnt and downstream EGF, we used indomethacin [38] and Gefitinib [39] to inhibit Wnt and EGFR signaling activity, respectively. Importantly, both small molecules significantly impeded the recovery of bladder voiding function in IC+MSC bladders (Fig. 5A). Accordingly, regeneration of the epithelial layer was severely impaired by the inhibition of Wnt and EGFR signaling, although the injected stem cells were well engrafted (Fig. 5B). Repressing Wnt signaling activity was confirmed by the reduced number of cells stained with non-phospho beta-catenin protein (Supplementary Fig. S5). Gene expression analysis indicated that treatment with indomethacin prevented the upregulation of growth factors, including Igf1, Fgf16, and Egf, but had little effect on Wnt gene expression (Fig. 6 and Supplementary Fig. S6). Instead, administrating Gefitinib impacted the expression of most genes involved in Shh-Wnt signaling (Fig. 6). Taken together, these results indicate that the Wnt-EGF signaling cascade plays a crucial role in UCB-MSC-mediated urothelial regeneration of the IC bladder.
FIG. 5.
Wnt-EGF signaling activity mediates the therapeutic effects of UCB-MSC in the IC bladder. (A) Contraction intervals of the IC bladders that were injected with UCB-MSC in the absence or presence of indomethacin (Indo, Wnt blocker) or Gefitinib (Gefi, EGFR inhibitor). Data are shown as the mean±SEM (n=10; P<0.01 in comparison with IC+MSC group bladders, one-way ANOVA with Bonferroni post-test). (B) Fluorescent immunostaining of cytokeratin+ epithelium (green) at 1 week after the injection of PKH26-labeled UCB-MSC (red) in the absence or presence of inhibitors. Nuclei were stained with DAPI (blue). The region characterized with the broken urothelium (box in left panel image; magnification ×200, scale bar=50 μm.) is shown in the right panel at higher magnification (×400, scale bar=20 μm). EGFR, epidermal growth factor receptor. Color images available online at www.liebertpub.com/scd
FIG. 6.
Effects of Wnt-EGF signaling activity on the gene expression of the Shh-Wnt-EGF cascade components. RQ-PCR analysis of the (A) Shh, (B) Wnt, (C) EGF, and IGF1 genes, which were activated by the IC bladders that were injected with UCB-MSCs (see Fig. 4). Expression levels in the indicated bladder tissues are represented as % Gapdh (as determined using five or more independent experiments) and are shown as the mean±SEM (P<0.05, P<0.01 compared with IC group bladders, one-way ANOVA with Bonferroni post-test).
Discussion
This study provides the first experimental evidence that UCB-MSC therapy leads to stable therapeutic outcomes and relieves IC by regenerating the bladder epithelium by stimulating the Wnt-EGF signaling cascade. IC, including painful bladder syndrome, is a chronic bladder disease of unknown etiology that is characterized by pelvic pain and increased urinary frequency and urgency [40–43]. The etiology of IC remains unknown, thereby making treatment challenging. IC treatments include medications such as pentosan polysulfate, intravesical hyaluronic acid or chondroitin sulfate instillation, hydrodistention, and transurethral coagulation of any ulcers. However, these treatments are temporary and focus on pain relief, not curative outcomes.
The lack of any precise understanding of the etiology of IC also makes it difficult to develop proper animal models. Here, we mimicked IC injury by briefly instilling HCl into the rat bladder lumen [44], and we found that this animal model faithfully reproduced the majority of the pathophysiologies observed in IC patients, including epithelial denudation, abnormal increase in inflammation, neural cell activation, and angiogenesis (Fig. 2). Using this animal model, we demonstrated the potency of using UCB-MSC therapy to treat IC. However, further studies are needed to optimize MSC therapy and provide data for use in clinical trials on IC patients.
Although the etiology and pathogenesis of IC remain unclear, a variety of pathophysiological insults may result in the processes that self-perpetuate epithelial cell dysfunction, C nerve fiber activation, mast cell proliferation, and, ultimately, tissue damage, scarring, fibrosis, and neuropathic pain [34,35]. Among these, impaired epithelial integrity is considered the major trigger for IC, and indeed several abnormities in the urothelium have been described using bladder biopsies and cultured urothelial cells obtained from IC patients. Here, we found that UCB-MSC injection ameliorated IC bladder by stimulating epithelial regeneration. The injected UCB-MSCs directly differentiated into epithelial cells in the urothelium (Fig. 3), thereby supporting the regeneration of the Wnt signaling cascade (Fig. 4).
Homeostasis in the epithelial layer of rapidly replaced organs (such as the intestines) is dynamically regulated by the continuous proliferation and differentiation of tissue-residing stem and progenitor cells [6]. Importantly, Wnt-associated signaling cascades determine the regenerative capacity of endogenous tissue stem cells and the replacement of the damaged epithelium. Likewise, the cells located in the base of the urothelium play a role as stem and progenitor cells proliferate and are stimulated via the Wnt pathways due to injuries in the bladder epithelium [10]. However, the self-regenerating capability might not be enough to fully repair tissue injuries associated with IC; thus, boosting endogenous regenerative potency is a promising therapeutic strategy for IC patients. It has been reported that the beneficial outcomes of MSC therapy could be attributed to paracrine effects via secreted growth factors [19,26,45] and activated endogenous primitive stem cells [27]. In our present IC animal study, the majority of the injected UCB-MSCs were engrafted to stromal tissues near the bladder urothelium (Fig. 3), which boosted Wnt-mediated urothelial regeneration in a paracrine manner (Fig. 4); moreover, its significance was proved using chemical inhibitors (Figs. 5 and 6). To investigate whether the paracrine effect of MSCs could regulate the proliferation of bladder epithelial cells, we treated the primary bladder epithelial cells isolated from both human and rat bladders with the CM collected from UCB-MSCs. Compared with normal fibroblast, UCB-MSC-derived CM increased the proliferation of both human and rat bladder epithelial cells, which was interfered by the treatment of IWP, a small molecule for inhibition of Wnt production. However, the paracrine effect of UCB-MSC was not statistically significant (Supplementary Fig. S7), suggesting that the proliferation of the differentiated epithelial cells could be less affected by the injected UCB-MSCs. Of particular interest, dense non-phospho β-catenin protein staining was frequently observed in the basal layer of the bladder urothelium in the IC+MSC group (Fig. 4D), suggesting that the Wnt signaling cascade mainly activates the tissue-residing stem cells rather than the differentiated cells. In addition, the injected UCB-MSCs induced the emergence of the host's primitive Oct4 expressing stem cells in the basal layer of the urothelium (Supplementary Fig. S8). Further studies are needed to elucidate the detail mechanisms of UCB-MSCs for the repair of IC bladder injury.
Our present study findings suggest that applying stem cells to the IC bladder is a promising strategy for advancing our understanding of the pathophysiology of IC bladder. However, several challenges remain to be clarified before successful clinical application. In particular, a major concern is that the rat model we used here does not reflect IC bladder in human patients. In this regard, our IC rat model demonstrated an acute inflammatory response. Thus, a traditional animal model for IC bladder needs to be developed, and similar MSC therapeutic approaches should be further examined.
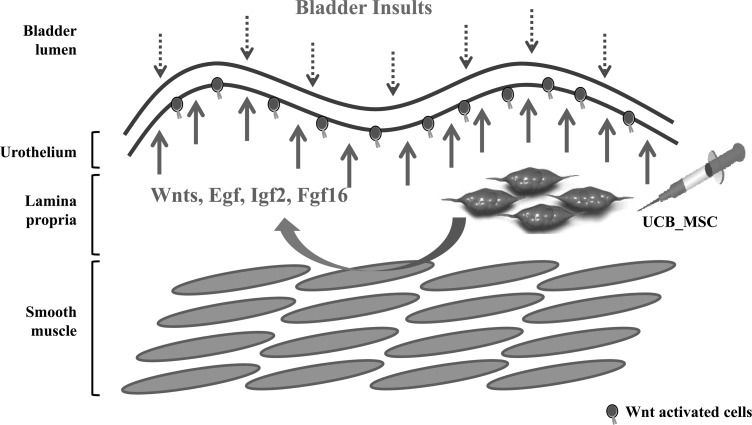
In conclusion, here, we provide evidence that UCB-MSC therapy can successfully alleviate IC in a preclinical animal model. In addition, we demonstrate the molecular mechanism through which the Wnt pathways stimulate the regeneration of a damaged bladder epithelium (Fig. 7). Hence, the denuded epithelium of IC bladder could potentially be cured using stem cell treatments.
FIG. 7.
Explanation of how UCB-MSC therapy is curative for IC bladder. The loss of urothelial integrity caused by multiple bladder insults (dotted arrow) leads to pathophysiological characteristic of IC patients, including epithelial denudation and the abnormal increase in inflammation, neural cells, and angiogenesis. Injecting UCB-MSCs in a paracrine manner stimulates Wnt and its downstream growth factors, including Egf, Igf1, Igf2, and Fgf16, which can boost the regenerative capability of endogenous stem cells (solid arrow). In addition, the engrafted UCB-MSCs directly differentiate into epithelial cells in the bladder tissues.
Supplementary Material
Acknowledgments
The authors thank the Neuromarker Resource Bank for providing research information. This research was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant no. A120301) awarded to D.-M.S, and (grant no. HI14C3365) awarded to M.-S.C, and a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) that was funded by the Ministry of Education, Science, and Technology (grant no. 2013R1A1A2004678) awarded to M.-S.C. This study was also supported by a grant from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea (grant no. 2014-098, 2015-609).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chancellor MB. and Yoshimura N. (2004). Treatment of interstitial cystitis. Urology 63:85–92 [DOI] [PubMed] [Google Scholar]
- 2.Elbadawi AE. and Light JK. (1996). Distinctive ultrastructural pathology of nonulcerative interstitial cystitis: new observations and their potential significance in pathogenesis. Urol Int 56:137–162 [DOI] [PubMed] [Google Scholar]
- 3.Slobodov G, Feloney M, Gran C, Kyker KD, Hurst RE. and Culkin DJ. (2004). Abnormal expression of molecular markers for bladder impermeability and differentiation in the urothelium of patients with interstitial cystitis. J Urol 171:1554–1558 [DOI] [PubMed] [Google Scholar]
- 4.Min G, Zhou G, Schapira M, Sun TT. and Kong XP. (2003). Structural basis of urothelial permeability barrier function as revealed by Cryo-EM studies of the 16 nm uroplakin particle. J Cell Sci 116:4087–4094 [DOI] [PubMed] [Google Scholar]
- 5.Parsons CL. (2011). The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJU Int 107:370–375 [DOI] [PubMed] [Google Scholar]
- 6.Blanpain C, Horsley V. and Fuchs E. (2007). Epithelial stem cells: turning over new leaves. Cell 128:445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broeckx SY, Maes S, Martinello T, Aerts D, Chiers K, Marien T, et al. (2014). Equine epidermis: a source of epithelial-like stem/progenitor cells with in vitro and in vivo regenerative capacities. Stem Cells Dev 23:1134–1148 [DOI] [PubMed] [Google Scholar]
- 8.Barker N, van de Wetering M. and Clevers H. (2008). The intestinal stem cell. Genes Dev 22:1856–1864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hung C-S, Dodson KW. and Hultgren SJ. (2009). A murine model of urinary tract infection. Nat Protoc 4:1230–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, et al. (2011). Hedgehog/Wnt feedback supports regenerative proliferation of epithelial stem cells in bladder. Nature 472:110–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crosnier C, Stamataki D. and Lewis J. (2006). Organizing cell renewal in the intestine: stem cells, signals and combinatorial control. Nat Rev Genet 7:349–359 [DOI] [PubMed] [Google Scholar]
- 12.Taipale J. and Beachy PA. (2001). The Hedgehog and Wnt signalling pathways in cancer. Nature 411:349–354 [DOI] [PubMed] [Google Scholar]
- 13.Erickson DR, Schwarze SR, Dixon JK, Clark CJ. and Hersh MA. (2008). Differentiation associated changes in gene expression profiles of interstitial cystitis and control urothelial cells. J Urol 180:2681–2687 [DOI] [PubMed] [Google Scholar]
- 14.Haraguchi R, Motoyama J, Sasaki H, Satoh Y, Miyagawa S, Nakagata N, et al. (2007). Molecular analysis of coordinated bladder and urogenital organ formation by Hedgehog signaling. Development 134:525–533 [DOI] [PubMed] [Google Scholar]
- 15.Minguell JJ, Allers C. and Lasala GP. (2013). Mesenchymal stem cells and the treatment of conditions and diseases: the less glittering side of a conspicuous stem cell for basic research. Stem Cells Dev 22:193–203 [DOI] [PubMed] [Google Scholar]
- 16.Osathanon T, Manokawinchoke J, Nowwarote N, Aguilar P, Palaga T. and Pavasant P. (2013). Notch signaling is involved in neurogenic commitment of human periodontal ligament-derived mesenchymal stem cells. Stem Cells Dev 22:1220–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li K, Han Q, Yan X, Liao L. and Zhao RC. (2010). Not a process of simple vicariousness, the differentiation of human adipose-derived mesenchymal stem cells to renal tubular epithelial cells plays an important role in acute kidney injury repairing. Stem Cells Dev 19:1267–1275 [DOI] [PubMed] [Google Scholar]
- 18.Yue WM, Liu W, Bi YW, He XP, Sun WY, Pang XY, et al. (2008). Mesenchymal stem cells differentiate into an endothelial phenotype, reduce neointimal formation, and enhance endothelial function in a rat vein grafting model. Stem Cells Dev 17:785–793 [DOI] [PubMed] [Google Scholar]
- 19.Ghosh D, Kuchroo P, Dave V, Vijayan A. and Viswanathan C. (2014). Paracrine factors secreted by umbilical cord derived mesenchymal stem cells induce angiogenesis in vitro by a VEGF-independent pathway. Stem Cells Dev 24:437–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao W, Zhang C, Jin C, Zhang Z, Kong D, Xu W, et al. (2011). Periurethral injection of autologous adipose-derived stem cells with controlled-release nerve growth factor for the treatment of stress urinary incontinence in a rat model. Eur Urol 59:155–163 [DOI] [PubMed] [Google Scholar]
- 21.Huang Y-C, Shindel AW, Ning H, Lin G, Harraz AM, Wang G, et al. (2010). Adipose derived stem cells ameliorate hyperlipidemia associated detrusor overactivity in a rat model. J Urol 183:1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang H, Qiu X, Shindel AW, Ning H, Ferretti L, Jin X, et al. (2012). Adipose tissue-derived stem cells ameliorate diabetic bladder dysfunction in a type II diabetic rat model. Stem Cells Dev 21:1391–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin CS, Lin G. and Lue TF. (2012). Allogeneic and xenogeneic transplantation of adipose-derived stem cells in immunocompetent recipients without immunosuppressants. Stem Cells Dev 21:2770–2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin C-S. (2011). Stem cell therapy for the bladder—where do we stand?. J Urol 185:779–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin C-S. and Lue T. (2011). Adipose-derived stem cells: characterization and application in urology. In: Adipose Stem Cells and Regenerative Medicine. Illouz Y-G. and Sterodimas A, eds. Springer Berlin Heidelberg, Germany, pp 193–207 [Google Scholar]
- 26.Lin C-S. and Lue T. (2012). Adipose-derived stem cells: therapy through paracrine actions. In: Stem Cells and Cancer Stem Cells, Volume 4 Hayat MA, ed. Springer, Dordrecht, Netherlands, pp 203–216 [Google Scholar]
- 27.Song M, Heo J, Chun JY, Bae HS, Kang JW, Kang H, et al. (2014). The paracrine effects of mesenchymal stem cells stimulate the regeneration capacity of endogenous stem cells in the repair of a bladder-outlet-obstruction-induced overactive bladder. Stem Cells Dev 23:654–663 [DOI] [PubMed] [Google Scholar]
- 28.Wezel F, Southgate J. and Thomas DFM. (2011). Regenerative medicine in urology. BJU Int 108:1046–1065 [DOI] [PubMed] [Google Scholar]
- 29.Montzka K. and Heidenreich A. (2010). Application of mesenchymal stromal cells in urological diseases. BJU Int 105:309–312 [DOI] [PubMed] [Google Scholar]
- 30.Kim JH, Lee HJ. and Song YS. (2014). Treatment of bladder dysfunction using stem cell or tissue engineering technique. Korean J Urol 55:228–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee M, Jeong SY, Ha J, Kim M, Jin HJ, Kwon S-J, et al. (2014). Low immunogenicity of allogeneic human umbilical cord blood-derived mesenchymal stem cells in vitro and in vivo. Biochem Biophys Res Commun 446:983–989 [DOI] [PubMed] [Google Scholar]
- 32.Jang YK, Kim M, Lee Y-H, Oh W, Yang YS. and Choi SJ. (2014). Optimization of the therapeutic efficacy of human umbilical cord blood–mesenchymal stromal cells in an NSG mouse xenograft model of graft-versus-host disease. Cytotherapy 16:298–308 [DOI] [PubMed] [Google Scholar]
- 33.Shin DM, Liu R, Wu W, Waigel SJ, Zacharias W, Ratajczak MZ, et al. (2012). Global gene expression analysis of very small embryonic-like stem cells reveals that the Ezh2-dependent bivalent domain mechanism contributes to their pluripotent state. Stem Cells Dev 21:1639–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuo HC. (2014). Potential urine and serum biomarkers for patients with bladder pain syndrome/interstitial cystitis. Int J Urol 21 Suppl 1:34–41 [DOI] [PubMed] [Google Scholar]
- 35.van de Merwe JP, Nordling J, Bouchelouche P, Bouchelouche K, Cervigni M, Daha LK, et al. (2008). Diagnostic criteria, classification, and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC proposal. Eur Urol 53:60–67 [DOI] [PubMed] [Google Scholar]
- 36.Richter B, Roslind A, Hesse U, Nordling J, Johansen JS, Horn T, et al. (2010). YKL-40 and mast cells are associated with detrusor fibrosis in patients diagnosed with bladder pain syndrome/interstitial cystitis according to the 2008 criteria of the European Society for the Study of Interstitial Cystitis. Histopathology 57:371–383 [DOI] [PubMed] [Google Scholar]
- 37.Liu C, Li Y, Semenov M, Han C, Baeg G-H, Tan Y, et al. . Control of β-Catenin phosphorylation/degradation by a dual-kinase mechanism. Cell 108:837–847 [DOI] [PubMed] [Google Scholar]
- 38.Dihlmann S, Siermann A. and von Knebel Doeberitz M. (2001). The nonsteroidal anti-inflammatory drugs aspirin and indomethacin attenuate beta-catenin/TCF-4 signaling. Oncogene 20:645–653 [DOI] [PubMed] [Google Scholar]
- 39.Sordella R, Bell DW, Haber DA. and Settleman J. (2004). Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305:1163–1167 [DOI] [PubMed] [Google Scholar]
- 40.Driscoll A. and Teichman JM. (2001). How do patients with interstitial cystitis present?. J Urol 166:2118–2120 [PubMed] [Google Scholar]
- 41.Evans RJ. (2002). Treatment approaches for interstitial cystitis: multimodality therapy. Rev Urol 4 Suppl 1:S16–S20 [PMC free article] [PubMed] [Google Scholar]
- 42.Macdiarmid SA. and Sand PK. (2007). Diagnosis of interstitial cystitis/painful bladder syndrome in patients with overactive bladder symptoms. Rev Urol 9:9–16 [PMC free article] [PubMed] [Google Scholar]
- 43.Metts JF. (2001). Interstitial cystitis: urgency and frequency syndrome. Am Fam Physician 64:1199–1206 [PubMed] [Google Scholar]
- 44.Westropp JL. and Buffington CA. (2002). In vivo models of interstitial cystitis. J Urol 167:694–702 [DOI] [PubMed] [Google Scholar]
- 45.Gnecchi M, Zhang Z, Ni A. and Dzau VJ. (2008). Paracrine mechanisms in adult stem cell signaling and therapy. Cir Res 103:1204–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.