Abstract
Dysphagia is common in Parkinson’s disease (PD) and causes significant morbidity and mortality. PD dysphagia has usually been explained as dysfunction of central motor control, much like other motor symptoms that are characteristic of the disease. However, PD dysphagia does not correlate with severity of motor symptoms nor does it respond to motor therapies. It is known that PD patients have sensory deficits in the pharynx, and that impaired sensation may contribute to dysphagia. However, the underlying cause of the pharyngeal sensory deficits in PD is not known. We hypothesized that PD dysphagia with sensory deficits may be due to degeneration of the sensory nerve terminals in the upper aerodigestive tract (UAT). We have previously shown that Lewy-type synucleinopathy (LTS) is present in the main pharyngeal sensory nerves of PD patients, but not in controls. In this study, the sensory terminals in UAT mucosa were studied to discern the presence and distribution of LTS. Whole-mount specimens (tongue-pharynx-larynx-upper esophagus) were obtained from 10 deceased human subjects with clinically diagnosed and neuropathologically confirmed PD (five with dysphagia and five without) and four age-matched healthy controls. Samples were taken from six sites and immunostained for phosphorylated α-synuclein (PAS). The results showed the presence of PAS-immunoreactive (PAS-ir) axons in all the PD subjects and in none of the controls. Notably, PD patients with dysphagia had more PAS-ir axons in the regions that are critical for initiating the swallowing reflex. These findings suggest that Lewy pathology affects mucosal sensory axons in specific regions of the UAT and may be related to PD dysphagia.
Keywords: Alpha-synuclein histopathology, Dysphagia, Lewy-type synucleinopathy, Parkinson’s disease, Peripheral sensory nerves, Upper aerodigestive tract
Introduction
Parkinson’s disease (PD) is a multiple system neurodegenerative disorder manifested by a broad spectrum of motor and non-motor features [1]. Although the exact cause of PD is unknown, the histopathological hallmark of PD in post mortem tissue is accumulation of the protein α-synuclein into structures called Lewy bodies and neurites. This finding, called synucleinopathy, is believed to cause death of dopamine neurons leading to the classic motor symptoms of PD [2, 3]. In addition to brain pathology, independent groups including those of Beach [4, 5], Braak [6, 7] and others [8–11] have demonstrated synucleinopathy is also present in motor, sensory, and autonomic peripheral nerves. A well-documented organ commonly affected by synucleinopathy is the intrinsic nervous system controlling the colon where its involvement may directly contribute to the common PD symptom of constipation. However, despite the fact that PD patients commonly have swallowing problems, little is known about synucleinopathy of motor and sensory nerves of the upper aerodigestive tract (UAT); specifically the mouth, pharynx and larynx.
Disordered swallowing, or dysphagia, develops in approximately 50% to 80% of patients with PD [12] and is one of the largest contributors to morbidity in PD patients. In the majority of the cases, dysphagia is associated with oropharyngeal dysfunction [13]. The consequences of oropharyngeal dysphagia can be severe: dehydration, malnutrition, weight loss, aspiration, choking, pneumonia, and death [14]. The leading cause of death for people with PD is aspiration pneumonia, which occurs when food or saliva enter the lungs. PD dysphagia is associated with motor and sensory deficits. Oral stage deficits are common and characterized by back and forth movements of the tongue without associated swallows. This tongue pumping is considered by some to be pathognomic of the disease. The initiation of swallowing is often delayed suggesting a sensory deficit. Once begun swallows are usually incomplete with residual food remaining in the UAT.
The pharynx plays a critical role in swallowing and is known to have specific areas that initiate pharyngeal swallowing. Specifically, the sensory input evoking swallowing is from peripheral receptive fields in the UAT innervated by the CN V, IX, and X nerves [15, 16]. Motor dysfunction of the pharynx could reduce muscle strength and impair pharyngeal peristalsis, whereas sensory nerve damage results in dysfunction of the sensory reflex arc [17, 18].
We have hypothesized that dysphagia in PD may be associated with direct damage of motor and sensory nerves of the UAT by Lewy-type synucleinopathy (LTS). This hypothesis gains support from our recent studies which have demonstrated that the main motor and sensory nerves innervating the UAT in PD patients have significant amounts of LTS while healthy controls have none [19, 20]. Notably, the density of the nerve lesions is greater in PD patients with dysphagia versus those without dysphagia. These findings suggest that dysphagia in PD may be related to peripheral nerve lesions. Lesions of sensory terminal fibers in the oral and pharyngeal regions could cause delayed swallowing initiation (sensory dysfunction) and reduced movements of the lingual and pharyngeal muscles (motor deficits).
It remains unknown whether the LTS seen in the main nerve trunks in PD is present in the terminal sensory axons in the mucosa of the UAT. If so, an important question is whether LTS is present in the areas known to be sensory triggers for swallowing. In this study, the specific aim was to determine whether LTS is present in the UAT mucosa in PD and, if so, whether there are differences in the densities of LTS between PD patients with and without dysphagia as defined with the Unified Parkinson’s Disease Rating Scale (UPDRS). Mucosal samples of the UAT obtained from autopsied PD subjects with and without dysphagia and healthy controls were examined using immunohistochemistry for phosphorylated α-synuclein (PAS) to study the presence and distribution of sensory LTS.
Materials and Methods
Study Population
In this study, whole-mount specimens (tongue-pharynx-larynx-upper esophagus) were obtained from 10 deceased human subjects with clinically diagnosed and neuropathologically confirmed PD and 4 age-matched healthy controls (Fig. 1). The research subjects were part of the Arizona Study of Aging and Neurodegenerative Disorders (AZSAND), a longitudinal clinicopathological study, and were autopsied by the Banner Sun Health Research Institute (BSHRI) Brain and Body Donation Program (BBDP) [21]. The BBDP has been approved by Western IRB. BSHRI and the Mayo Clinic Arizona are the principal institutional members of the Arizona Parkinson’s Disease Consortium (APDC), which conducts a longitudinal clinicopathologic study of PD and normal aging subjects with annual examinations from entry until death and autopsy.
Fig 1.
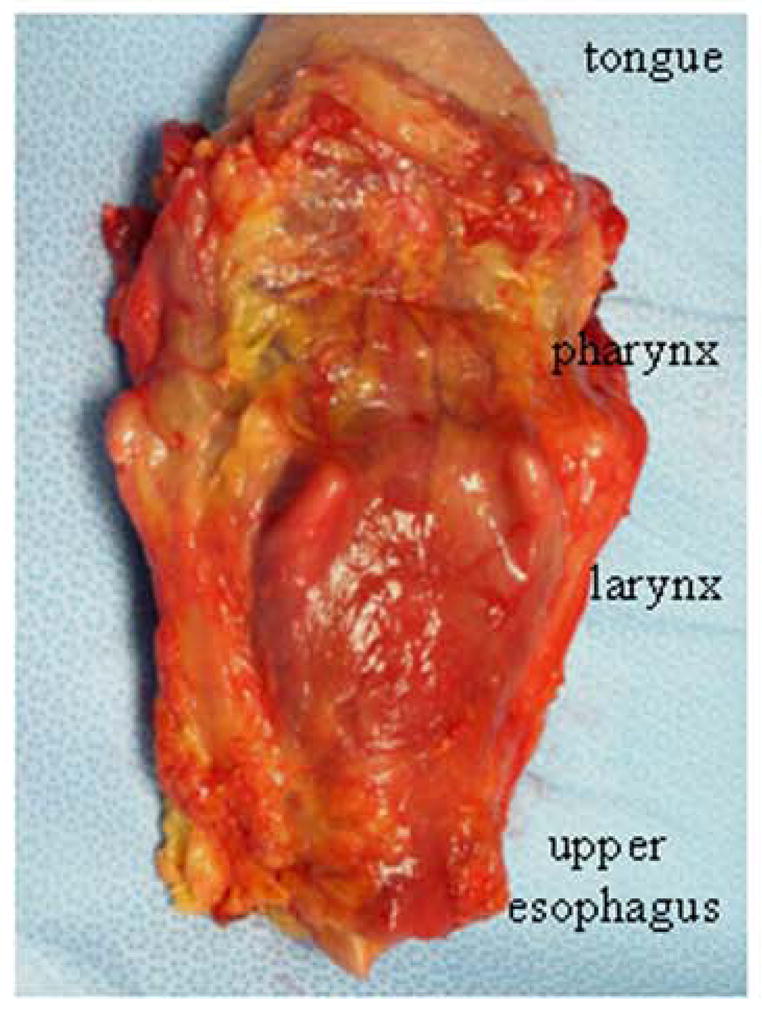
Posterior view of a fresh whole-mount specimen provided by BSHRI included entire tongue, pharynx, larynx, and upper esophagus with their muscles, mucosa, and their innervating nerves, as well as surrounding tissues
Clinical and Neuropathologic Assessments
For each of the autopsied cases, detailed clinical and neuropathological data were provided by the APDC (Table 1). In the PD group (n = 10), there were five cases with dysphagia and five cases without dysphagia. All subjects received standardized neurologic examinations that included assessments for Parkinson’s disease and cognitive function [22]. The clinical severity of PD was rated using the Hoehn and Yahr (H&Y) Scale [23] and the Unified Parkinson’s Disease Rating Scale (UPDRS) [24]. Specific clinicopathologic diagnostic criteria for PD were used [25]. Dysphagia was assessed using item 7 of the UPDRS Part II Scale (i.e., swallowing scores: 0, normal; 1, rare choking; 2, occasional choking; 3, requires soft food; and 4, requires nasogastric tube or percutaneous endoscopic gastrostomy feeding). All subjects at death received comprehensive neuropathological examinations. Gross and microscopic neuropathologic assessments were made by a blinded neuropathologist at BSHRI (Dr. Thomas G. Beach), who provided a detailed neuropathologic report for each subject autopsied by the BBDP. The diagnosis of all subjects was, therefore, neuropathologically confirmed.
Table 1.
Demographic and clinical data of patients with PD and healthy control subjects
| Case no | Sex | Age at death, years | Age at PD onset, years | PD duration, years | H&Y stages | Motor UPDRS | Cause of death | UPDRS months before death | PMI, hours | Swallowing score (0–4) |
|---|---|---|---|---|---|---|---|---|---|---|
| PD 1 | M | 78 | 59 | 19 | 4 | 51 | CAD | 8 | 76 | 2 |
| 2 | M | 75 | 45 | 30 | 4 | 66 | es-PD | 1 | 36 | 2 |
| 3 | M | 74 | 53 | 21 | 4 | 66 | es-PD | 10 | 24 | 1 |
| 4 | M | 74 | 68 | 6 | 2 | 21 | es-PD | 15 | 34 | 1 |
| 5 | M | 76 | 64 | 12 | 4 | 31 | es-PD | 10 | 34 | 2 |
| 6 | M | 73 | 62 | 11 | 3 | 18 | es-PD | 26 | 26 | 0 |
| 7 | M | 80 | 63 | 17 | 5 | 40 | CPF | 5 | 23 | 0 |
| 8 | M | 73 | 67 | 6 | 2 | 41 | es-PD | 5 | 50 | 0 |
| 9 | F | 77 | 48 | 29 | 3 | 20 | CVA | 28 | 23 | 0 |
| 10 | F | 65 | 58 | 7 | 5 | 68 | ARF | 18 | 28 | 0 |
| Mean (range) | 75 (65–80) | 59 (45–68) | 16 (6–30) | 3.6 (2–5) | 42 (18–68) | 13 (1–28) | 35 (23–76) | 0.8 (0–2) | ||
| HC 1 | F | 70 | — | — | — | — | CPF | — | 30 | 0 |
| 2 | F | 74 | — | — | — | — | MSF | — | 24 | 0 |
| 3 | M | 80 | — | — | — | — | PC | — | 73 | 0 |
| 4 | F | 75 | — | — | — | — | CC | — | 48 | 0 |
| Mean (range) | 75 (70–80) | 44 (24–73) |
ARF acute respiratory failure, CAD coronary artery disease, CC colon cancer, CPF cardiopulmonary failure, CVA cerebrovascular accident, es-PD end stage of PD, F female, HC healthy control, H&Y Hoehn and Yahr Clinical Rating Scale (score range, 1–5), M male, MSF multisystem failure, PC pancreatic cancer, PD Parkinson disease, PMI postmortem interval, UPDRS Unified Parkinson’s Disease Rating Scale
Tissue Sampling and Rationale
The specimens were obtained from 1 to 2 days after death (mean, 37 h) (Table 1); this postmortem interval does not hamper reliable histochemical analysis of autopsied muscle or LTS [26–29]. For each specimen, six tissue samples (10 × 10 mm/each) were obtained (Fig. 2). Specifically, the samples were taken from: (1) lateral posterior tongue (LPT); (2) anterior tonsillar pillar (ATP); (3) oropharyngeal posterior wall (OPW); (4) aryepiglottic fold (AEF); (5) postcricoid region (PCR); and (6) upper esophagus (UE). These regions in the UAT were chosen because: (a) pharyngeal swallowing is best elicited from certain areas of the pharynx [15, 16, 30, 31]; (b) the regions that elicit pharyngeal swallowing have rich sensory nerve terminals [32]; and (c) they are innervated by different sensory nerves [32, 33]. In the oropharynx, LPT and ATP are innervated by the lingual and pharyngeal branches of the glossopharyngeal nerve (IX), respectively, whereas the OPW is supplied by the pharyngeal branches of the IX and X nerves. In the laryngopharynx, the AEF and PCR are innervated by the internal superior laryngeal nerve (ISLN). The UE is supplied by the pharyngeal and laryngeal branches of the X nerve [34]. The recurrent laryngeal nerve (RLN) gives some branches to supply the UE before it enters the larynx. We hypothesized that the sensory nerve terminals in the regions studied could be affected in PD because LTS has been identified in the ISLN, IX, and X nerves [19].
Fig. 2.
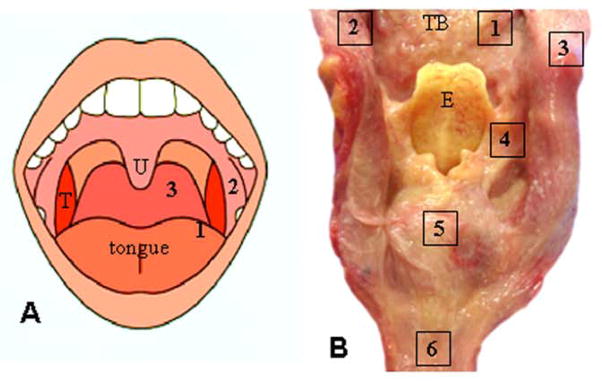
Sampling sites of the mucosa in the upper aerodigestive tract. a Schematic of the human oral cavity, illustrating tissue sampling sites in the oropharynx. T tonsil, U uvula. b Posterior view of an opened laryngopharynx and upper esophagus (UE) from a subject with PD, showing tissue sampling sites. E epiglottis, TB tongue base. The numbers in A & B represent sampling sites (enclosed regions). 1 lateral posterior tongue (LPT); 2 anterior tonsillar pillar (ATP); 3 oropharyngeal posterior wall (OPW); 4 aryepiglottic fold (AEF); 5 postcricoid region (PCR); and 6 UE
Staining Methods
The tissue samples were fixed with 10% neutral buffered formalin overnight, frozen in isopentane cooled by dry ice and sectioned (60-μm thick). The sections were stained with hematoxylin and eosin to show tissue structure, immunostained for phosphorylated α-synuclein (PAS) to identify PAS-immunoreactive (PAS-ir) axons, and stained for neurofilament to label all axons.
Immunohistochemistry for PAS
The tissue sections were stained with an immunohistochemical method for PAS, as previously described [4, 19, 20, 29]. Briefly, the sections were (1) pretreated with 1:100 proteinase K (Enzo Life Sciences, Farmingdale, NY) diluted in 0.1 mol/L PBS at 37 °C for 20 min; (2) immersed for 30 min in 1% H2O2 in 0.1 mol/L PBS with 0.3% Triton X-100 (PBS-TX) at pH 7.4; (3) incubated at 4 °C overnight in anti-PAS monoclonal antibody (psyn no. 64; Wako Richmond, VA) at 1:1000 dilution in PBS-TX; (4) incubated with a secondary biotinylated antibody (anti-mouse IgG diluted 1:1000 in PBS-TX; Vectastain kit, Vector Laboratories, Burlingame, CA) for 2 h at room temperature; (5) treated for 30 min with avidin-biotin complex (Vectastain, Vector Laboratories), with A and B components of the kit both at 1:1000 dilution; and (6) treated with 3,3′-diaminobenzidine (Sigma, St. Louis, MO) (5 mg/100 ml) with added saturated nickel ammonium sulfate (2/100 mL) and H2O2 (5 μL/100 mL of 1% H2O2) for 30 min in the dark. Controls for staining specificity had no primary antibody.
Immunohistochemical Staining for Neurofilament
Adjacent sections were immunostained with a monoclonal antibody against phosphorylated neurofilament (NF) (SMI-31, Covance Research Products, Berkeley, CA) as a marker for all axons as described [11, 35]. Briefly, the sections were (1) treated in PBS containing 0.3% Triton and 2% BSA for 30 min; (2) incubated with primary antibody SMI-31 (dilution 1:800) in PBS containing 0.03% Triton at 4 °C overnight; (3) incubated for 2 h with the biotinylated secondary antibody (anti-mouse, 1:1000, Vector, Burlingame, CA); (4) treated with avidin-biotin complex method with a Vectastain ABC kit (1:1000 ABC Elite, Vector); and (5) treated with diaminobenzidine-nickel as chromogen to visualize peroxidase labeling. Controls were stained as previously mentioned except that the incubation with the primary antibody was omitted.
Quantification
All stained sections were examined under a Zeiss photomicroscope and photographed. Stained sections were assessed by a single investigator (J.C.) without knowledge of subject identity or diagnosis. For a given sample, three sections at different spatial levels stained for PAS or NF were selected to count PAS-ir and NF-ir axons, respectively. Each of the PAS-ir or NF-ir axons was counted separately. For each section, three microscopic fields with a high density of PAS-ir or NF-ir axons were identified to count the labeled axons. The numbers of the PAS-ir or NF-ir axons in the three fields per section and the three selected sections for each sample were averaged. The mean density of the PAS-ir axons in each sample was used to evaluate lesion severity using a grading system as described in our recent publications with some modifications [19, 20]: −, no lesions; +, 1 to 5 lesions per field (mild); ++, 6 to 10 lesions per field (moderate); +++, 11 to 15 lesions per field (moderate-severe); ++++, more than 15 lesions per field (severe). The mean number of the NF-ir axons in a given sample indicates the density of sensory innervation.
Statistical Analysis
All continuous variables were expressed as mean (SD) or median (interquartile range) depending on whether there was evidence that sample data had a normal distribution. Categorical variables were expressed as frequencies. Demographic and patient characteristics included PD duration, H&Y stages, motor UPDRS, and swallowing score.
Severity of LTS was derived from the number of PAS-ir axons using the following categorization: (i) none, 0 lesions; (ii) mild, 1–5 lesions; (iii) moderate, 6–10 lesions; (iv) moderate-severe, 11–15 lesions; (v) severe, more than 15 lesions. Ordinal logistic regression models were used to examine the association between the ordered severity and explanatory variables.
To evaluate any potential significant relationships with severity of LTS, a multivariable model was fitted and estimates of ordinal logistic regression for this model were obtained using generalized estimating equations (GEE) method. The GEE analysis was conducted using PROC GENMOD in SAS with link function, cumulative logit (clogit), and independent correlation structure to capture the within-patient dependencies attributed to repeated measurement over regions of UAT. The study focused on evidence of significant associations; hence, independent correlation was considered sufficient. The results of this analysis were presented as odds ratios (ORs), 95% confidence interval (95% CI), and p values. Any p < 0.05 was considered statistically significant. All data analysis was performed using SAS version 9.2 (SAS Institute Inc, Cary, NC).
Results
Demographic Characteristics
Demographics and relevant clinical data for PD patients and healthy control subjects obtained from standardized research neurologic testing, medical records, and autopsy reports are summarized in Table 1. Both PD and healthy control groups had a mean age of 75 years (range, 65–80), and the PD group mean disease duration was 16 years (range, 6–30) with mean Hoehn & Yahr stage of 3.6 (range, 2–5) (Table 1).
On the basis of the UPDRS Part II Scale, dysphagia occurred in 5 of the 10 PD patients. In the 5 dysphagic PD patients, the swallowing score was rated as 1 in 2 cases and 2 in 3 cases (Table 1).
Neuropathological Findings in Brains with PD
In this study, all autopsied brains of the PD patients met neuropathologic criteria for PD. Microscopic examinations revealed that the substantia nigra and locus ceruleus showed moderate to marked depletion or loss of pigmented neurons with Lewy bodies in both regions (data not shown). Immunohistochemical staining for PAS showed frequent immunoreactive neuronal inclusions and related neurites in the olfactory bulb, brainstem, amygdale, and transentorhinal area, with variable densities in the cingulate gyrus and three neocortical regions examined (temporal, frontal, and parietal). The major spinal cord subdivisions were examined in seven cases; six of these had positive LTS in the spinal cord. Using the Unified Staging System for Lewy Body Disorders [36], four cases were classified as “neocortical stage” and six were “brainstem and limbic stage” (Table 2). Eight of the PD cases also had dementia; of these, four met consensus clinicopathologic criteria for Alzheimer’s disease [37] and four did not.
Table 2.
The unified Lewy stage and summary score of LB density for 10 standardized brain regions in 10 PD patients
| Case no | Unified LB stage | Summary score of LB densitya |
|---|---|---|
| PD 1 | III. Brainstem/Limbic | 29 |
| 2 | IV. Neocortical | 35 |
| 3 | III. Brainstem/Limbic | 28 |
| 4 | IV. Neocortical | 29 |
| 5 | III. Brainstem/Limbic | 30 |
| 6 | IV. Neocortical | 37 |
| 7 | III. Brainstem/Limbic | 15b |
| 8 | III. Brainstem/Limbic | 27 |
| 9 | III. Brainstem/Limbic | 11b |
| 10 | IV. Neocortical | 36 |
The maximum summary score is 40
For this case, only seven regions were examined; therefore, the score is not strictly comparable to the others
LB Lewy body, PD Parkinson disease
Sensory Innervation Patterns of the Human UAT Mucosa
Sensory nerve fascicles and axon terminals innervating the UAT mucosa was examined using NF immunostaining. NF-ir axons were observed in all samples from both the PD and normal control subjects. In submucosa, there were abundant NF-ir axons and nerve fascicles in each of the subregions of the UAT mucosa (Fig. 3). The results from NF staining are consistent with those revealed by Sihler’s stain used in our previous studies [32]. Both nerve staining methods showed that UAT mucosa, especially the oropharyngeal walls and the anterior wall of the laryngopharynx, receives rich sensory innervation. There is a dense sensory nerve plexus in the submucosa of the UAT. This sensory nerve supply pattern constitutes the neural basis for triggering pharyngeal swallowing and airway protection. Therefore, PD-induced degeneration of the sensory nerve terminals in the UAT could impair initiation of swallowing and various upper airway reflexes.
Fig. 3.
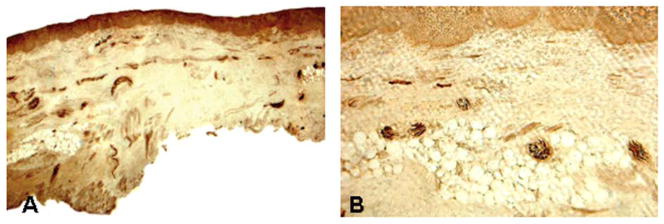
Photomicrographs of the mucosal sections of the aryepiglottic fold (AEF) from a PD subject (PD 2, 75-year-old man with disease duration of 30 years, Hoehn & Yahr Scale 4, motor UPDRS score 66, swallowing score 2). The sections were stained with neurofilament (NF) staining, showing sensory innervation of the mucosa. a Low-power view of an immunostained section x25. b High-power view of a stained section x100. Note that there are numerous darkly stained NF-ir sensory nerve fascicles and individual axon terminals in the submucosa
Lewy Pathology in the UAT Mucosa in PD
Anti-PAS immunohistochemistry showed axonal synucleinopathy lesions in the UAT mucosa in all PD samples and in none of the controls (Table 3). In PD, the PAS-ir axons were identified predominantly in the UAT submucosa. PAS-ir axons commonly appeared as threads and dots in the UAT mucosa. Axonal LTS in the UAT mucosa was also identified within nerve fascicles (Fig. 4) and individual axon terminals (Fig. 5).
Table 3.
Frequency and severity of phosphorylated α-synuclein-immunoreactive (PAS-ir) axons in upper aerodigestive tract (UAT) mucosa in PD subjects with/without dysphagia
| Sub-regions in the UAT mucosa
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case no | LPT
|
ATP
|
OPW
|
AEF
|
PCR
|
UE
|
Total PAS-ir axons (Mean) | ||||||
| PAS-ir axons
|
PAS-ir axons
|
PAS-ir axons
|
PAS-ir axons
|
PAS-ir axons
|
PAS-ir axons
|
||||||||
| (Range) | Severity | (Range) | Severity | (Range) | Severity | (Range) | Severity | (Range) | Severity | (Range) | Severity | ||
| Dysphagia | |||||||||||||
| 1 | 0 (0–0) | − | 8 (5–10) | ++ | 4 (2–6) | + | 12 (9–14) | +++ | 6 (4–9) | ++ | 6 (3–9) | ++ | 36 |
| 2 | 2 (1–3) | + | 12 (9–14) | +++ | 5 (3–7) | + | 6 (5–8) | ++ | 12 (9–16) | +++ | 13 (8–17) | +++ | 50 |
| 3 | 0 (0–0) | − | 11 (8–13) | +++ | 7 (5–9) | ++ | 11 (8–15) | +++ | 11 (7–14) | +++ | 16 (14–18) | ++++ | 56 |
| 4 | 0 (0–0) | − | 5 (3–7) | + | 0 (0–0) | − | 4 (2–6) | + | 2 (0–4) | + | 4 (2–6) | + | 15 |
| 5 | 4 (2–5) | + | 9 (7–11) | ++ | 9 (5–12) | ++ | 7 (4–9) | ++ | 4 (2–7) | + | 6 (4–9) | ++ | 39 |
| Mean | 1 | 9 | 5 | 8 | 7 | 9 | (39) | ||||||
| Non-Dysphagia | |||||||||||||
| 6 | 0 (0–0) | − | 6 (4–8) | ++ | 0 (0–0) | − | 6 (4–9) | ++ | 9 (5–13) | ++ | 7 (5–9) | ++ | 28 |
| 7 | 0 (0–0) | − | 4 (2–6) | + | 4 (2–7) | + | 8 (6–10) | ++ | 6 (5–8) | ++ | 11 (9–13) | +++ | 33 |
| 8 | 0 (0–0) | − | 0 (0–0) | − | 1 (0–2) | + | 4 (2–6) | + | 6 (4–9) | ++ | 0 (0–0) | − | 11 |
| 9 | 0 (0–0) | − | 5 (3–7) | + | 3 (1–6) | + | 3 (2–5) | + | 2 (1–3) | + | 3 (1–5) | + | 16 |
| 10 | 5 (2–8) | + | 6 (4–9) | ++ | 7 (5–9) | ++ | 9 (6–12) | ++ | 7 (4–10) | ++ | 4 (3–5) | + | 38 |
| Mean | 1 | 4 | 3 | 6 | 6 | 5 | (25) | ||||||
The severity of phosphorylated α-synuclein-immunoreactive (PAS-ir) axons in each of the sub-regions of the upper aerodigestive tract mucosa was rated on the basis of the mean density of the PAS-ir axons using a grading system: −, no lesions; +, 1 to 5 lesions per field (mild); ++, 6 to 10 lesions per field (moderate); +++, 11 to 15 lesions per field (moderate-severe); ++++, more than 15 lesions per field (severe)
AEF aryepiglottic fold, ATP anterior tonsillar pillar, LPT lateral posterior tongue, OPW oropharyngeal posterior wall, PCR postcricoid region, UE upper esophagus
Fig. 4.
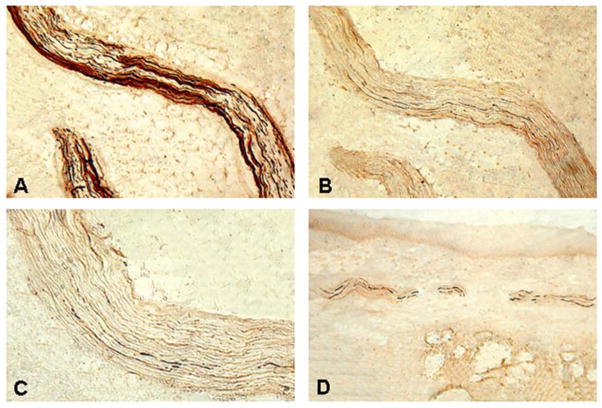
Photomicrographs of the sections of anterior tonsillar pillar (ATP) from a PD subject (PD 5, 76-year-old man with disease duration of 12 years, Hoehn & Yahr Scale 4, motor UPDRS score 31, swallowing score 2). a A section immunostained with neurofilament (NF) staining, showing two NF-ir nerve fascicles with numerous darkly stained axons x100. b An adjacent section stained with monoclonal anti-phosphorylated α-synuclein (PAS) antibody (psyn no. 64), showing PAS-ir axons (threads and dots) in the sensory nerve fascicles x100. c High-power view of B, illustrating the PAS-ir axons (darkly stained threads and dots) x200. d Another section of ATP from the same subject immunostained with psyn no. 64 also showed PAS-ir axons (threads and dots) within the sensory nerve fascicles in the submucosa x100
Fig. 5.
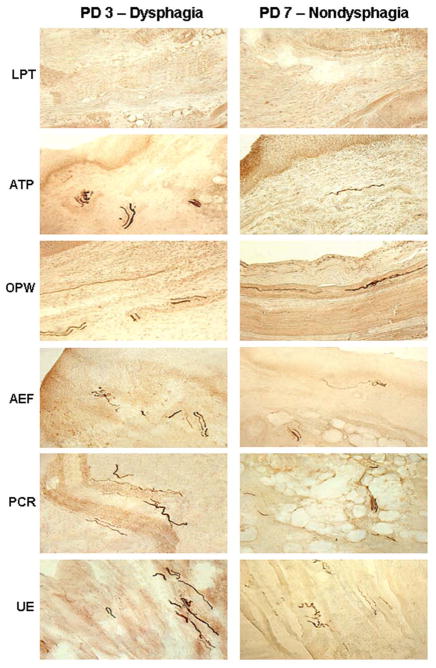
Comparison of lesion severity in the sub-regions of the upper aerodigestive tract (UAT) mucosa between subjects with and without dysphagia. The mucosal sections were immunostained with monoclonal anti-phosphorylated α-synuclein (PAS) antibody (psyn no. 64), showing PAS-ir axons (darkly stained threads and dots). Left column The autopsy mucosal samples were obtained from a PD subject with dysphagia (PD 3, 74-year-old man with disease duration of 21 years, Hoehn & Yahr Scale 4, motor UPDRS score 66, swallowing score 1, lesion severity score: LPT −, ATP +++, OPW ++, AEF +++, PCR +++, UE ++++). Right column The mucosal samples were harvested from a PD subject without dysphagia (PD 7, 80-year-old man with disease duration of 17 years, Hoehn & Yahr Scale 5, motor UPDRS score 40, swallowing score 0, lesion severity score: LPT −, ATP +, OPW +, AEF ++, PCR ++, UE +++) x200. Note that the PD subject with dysphagia (PD 3) had more PAS-ir axons in the UAT mucosa as compared with the subject without dysphagia (PD 7).
The severity of LTS in each of the subregions of the UAT mucosa for each subject with PD was assessed on the basis of the mean density of PAS-ir axons. For each case, variable lesion severity in the six subregions of the UAT mucosa is summarized in Table 3. In this series, oropharynx was slightly to moderately affected, whereas moderate to severe lesions were identified primarily in laryngopharynx and UE. The regions with high density of PAS-ir axons include ATP in the oropharynx, AEF and PCR in the laryngopharynx, and UE (Table 3).
In the oropharynx, the density or severity of LTS for the LPT was rated as absent (−) in seven cases and mild (+) in three cases. OPW exhibited no lesions in two cases, mild lesions in five cases, and moderate (++) lesions in three cases. Lesion severity was scored in the ATP as absent in one case, mild in three cases, moderate in four cases, and moderate-severe (+++) in two cases. Most of the cases (4/6) with moderate to moderate-severe lesions in the ATP experienced dysphagia (Table 3).
In the laryngopharynx, the severity of LTS for the AEF was mild in three patients, moderate in five patients, and moderate-severe in two patients. Similar lesion severity was revealed in the PCR. The patients with moderate-severe lesions in the AEF and PCR experienced dysphagia (Table 3).
In the UE, the lesion severity was rated as absent in one subject, mild in three subjects, moderate in three subjects, moderate-severe lesions in two subjects, and severe (++++) in one subject. Most of the subjects with moderate to severe lesions (4/6) reported to have dysphagia (Table 3).
The mean number of PAS-ir axons for each case in each of the subregions of the UAT mucosa is given in Table 3. PD patients with dysphagia had more PAS-ir axons (mean, 39) in the UAT mucosa as compared with those without dysphagia (mean, 25).
Multivariable analysis of LTS in the UAT mucosa regions examined in PD patients with and without dysphagia is presented in Table 4. First of all, we examined the relationships between severity of LTS and both presence of dysphagia and UAT mucosa without adjusting for patient characteristics. Unadjusted analysis in Table 4 shows a significantly increased risk of severity of LTS in UAT mucosa regions ATP, OPW, AEF, PCR and UE when compared to LPT, with odds ratios ranging from 12.7 (OPW vs. LPT) to 89.2 (PCR vs. LPT). No other comparisons between the UTA mucosa were statistically significant. These results suggest that the severity of LTS has discriminative ability between the LPT and the other 5 UAT mucosa regions. Next, we evaluated the association between the severity of LTS and dysphagia and UAT mucosa regions, while additionally taking into account PD duration, H&Y stages, and motor UPDRS. Data analysis showed a significant effect of dysphagia (p = 0.0086) and UAT mucosa region (p = 0.0001) on the severity of LTS lesions. There were significant associations with H&Y stages (p < 0.0001) and motor UPDRS (p = 0.0306) but the analysis failed to show significant associations with PD duration (p = 0.1520). In contrast with absence of dysphagia, presence of dysphagia was associated with a decreased risk of severe LTS (OR = 0.3; 95% CI: 0.1, 0.7; p = 0.0086). The adjusted analysis in Table 4 shows a significantly increased risk of more severe LTS in UAT mucosa regions ATP, OPW, AEF, PCR, and UE, as compared to LPT, with odds ratios ranging from 38.1 (OPW vs. LPT) to 545.9 (PCR vs. LPT). Thus, accounting for the patient characteristics, the results indicated a significant association between the severity of LTS and the presence of dysphagia and UAT mucosa region.
Table 4.
Multivariable analysis of Lewy-type synucleinopathy (LTS) in the subregions of upper aerodigestive tract (UAT) mucosa in Parkinson disease patients with and without dysphagia
| Variable | 2-factor unadjusted riska
|
2-factor adjusted riskb
|
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P valuec | OR | 95% CI | P valuec | |
|
|
|
|||||
| Absence/presence of dysphagia | ||||||
| Ref category: absence | 0.3 | (0.04, 1.5) | 0.1464 | 0.3 | (0.1, 0.7) | 0.0086 |
| UAT mucosa regions | ||||||
| ATP vs LPT | 53.7 | (3.3, 888.5) | 0.0015 | 278.1 | (4.6, 16, 658.4) | 0.0015 |
| OPW vs LPT | 12.7 | (1.3, 127.1) | 0.0180 | 38.1 | (1.2, 1182.2) | 0.0285 |
| AEF vs LPT | 83.9 | (3.8, 1877.0) | 0.0015 | 532.1 | (7.9, 35720.6) | 0.0015 |
| PCR vs LPT | 89.2 | (5.7, 1390.5) | 0.0015 | 545.9 | (10.9, 27412.0) | 0.0015 |
| UE vs LPT | 72.1 | (1.9, 2715.1) | 0.0075 | 515.8 | (3.5, 76862.8) | 0.0030 |
| OPW vs ATP | 0.2 | (0.02, 2.6) | 1.000 | 0.1 | (0.01, 3.7) | 1.000 |
| AEF vs ATP | 1.6 | (0.4, 6.6) | 1.000 | 1.9 | (0.3, 14.1) | 1.000 |
| PCR vs ATP | 1.7 | (0.3, 8.4) | 1.000 | 2.0 | (0.2, 20.2) | 1.000 |
| UE vs ATP | 1.3 | (0.5, 3.9) | 1.000 | 1.9 | (0.2, 16.3) | 1.000 |
| AEF vs OPW | 6.6 | (0.7, 59.2) | 0.1725 | 14.0 | (0.9, 215.6) | 0.0690 |
| PCR vs OPW | 7.0 | (0.8, 60.0) | 0.1155 | 14.3 | (0.9, 219.8) | 0.0630 |
| UE vs OPW | 5.7 | (0.3, 93.3) | 1.000 | 13.5 | (0.3, 583.3) | 0.6300 |
| PCR vs AEF | 1.1 | (0.2, 4.9) | 1.000 | 1.0 | (0.1, 7.8) | 1.000 |
| UE vs AEF | 0.9 | (0.2, 4.3) | 1.000 | 1.0 | (0.1, 9.4) | 1.000 |
| UE vs PCR | 0.8 | (0.1, 4.8) | 1.000 | 0.9 | (0.1, 12.8) | 1.000 |
The 2 factors dysphagia and UAT mucosa were not adjusted for patient characteristics
The 2 factors dysphagia and UAT mucosa were adjusted for PD duration, Hoehn and Yahr stages, and motor Unified Parkinson’s Disease Rating Scale
p-values were adjusted for multiple comparisons using the Bonferroni procedure
AEF aryepiglottic fold, ATP anterior tonsillar pillar, CI confidence interval, LPT lateral posterior tongue, OPW oropharyngeal posterior wall, OR odds ratio, PCR postcricoid region, UE upper esophagus
Discussion
The presence and distribution of LTS in the sensory neurons innervating the UAT were studied and several notable observations were made. First, this is to our knowledge, the first demonstration of LTS in the sensory nerve terminals innervating the UAT mucosa in subjects with PD. Specifically, LTS was identified in the submucosal sensory axons in all of the PD subjects but not in the healthy controls. Second, PD patients with dysphagia had more PAS-ir axons in the UAT mucosa as compared with those without dysphagia. Third, the severity of LTS varied in different UAT regions. Overall, the oropharynx was less affected than the hypopharynx (laryngopharynx and UE). However, in the oropharynx, one of the most sensitive trigger zones for swallowing, the ATP, was much more affected than the other oropharyngeal sites. Finally, the regions that had the highest prevalence and severity of LTS were ATP, AEF, and UE. These regions are critical for initiating pharyngeal swallowing and protecting the glottis from aspiration. These findings are consistent with the hypothesis that LTS in the peripheral sensory nerves innervating the UAT mucosa could be one of the risk factors leading to dysphagia in PD.
The relative contribution of central or peripheral nervous system pathology to dysphagia in PD patients is unclear at this time. It is generally believed that dysphagia in PD is related to bradykinesia and rigidity [38]. However, studies have shown that there is no correlation between dysphagia and overall muscle rigidity score [13]. Although anti-PD drugs and deep brain stimulation have significant therapeutic effects on limb motor functions, their effects on swallowing in PD are less impressive and, in some cases, adverse [12, 39, 40]. These clinical observations suggest that dysphagia in PD may not be caused solely by a reduction in basal ganglia dopamine activity. Other neurotransmitter systems or nondopaminergic systems may be involved [41]. For example, the pedunculopontine tegmental nucleus (PPTN) is a cholinergic nucleus that has substantial influence on the medullary central pattern generator for swallowing. In PD, degeneration of the neurons in the PPTN has been documented and therefore could contribute to dysphagia in PD [42].
Our hypothesis is that peripheral nerve degeneration may play a large role in PD dysphagia. Clinical studies have shown strong evidence that pharyngeal swallowing can be impaired by decreased pharyngeal sensitivity [43, 44]. In the 1990s, Aviv et al. [43–48] developed a new method for sensory testing using a modified endoscope, known as fiberoptic endoscopic evaluation of swallowing with sensory testing (FEESST). FEESST allows the assessment of swallowing and mechano-sensitivity, respectively. PD patients with dysphagia have been demonstrated to have diminished sensation in the laryngopharynx [47, 49–51]. Approximately 75% of dysphagic patients have severe laryngopharyngeal sensory deficits [47]. The presence of sensory loss in the pharynx is a poor prognostic factor for the development of aspiration pneumonia [44]. Using FEESST method, Hammer and colleagues [51, 52] assessed airway sensation and swallowing function in PD patients and healthy controls. Their studies showed that PD patients exhibited decreased laryngopharyngeal sensitivity and greater swallowing impairment compared with controls. These observations suggest that pharyngeal dysphagia is related to pharyngeal sensory dysfunction.
Swallowing is a complex neuromuscular procedure modulated by sensory feedback. In the human, the ATP and OPW are the most sensitive areas for initiating pharyngeal swallowing [15, 30]. When the bolus reaches the fauces and stimulates faucial sensory receptors, the involuntary pharyngeal phase of swallowing begins [53, 54]. However, the pharyngeal swallow can be impaired by sensory nerve dysfunction caused by neurological disorders including PD. Pharyngeal dysphagia is usually caused by an inability to initiate the swallowing reflex. This study showed that there was abundant LTS in the ATP in subjects with PD. Degeneration of the sensory nerve terminals in the mucosa impairs initiation of pharyngeal swallowing. Clinically, there has been some success in the use of sensory enhancement of swallowing in dysphagic patients by applying a cold tactile stimulus to the ATP. Stimulation of the ATP and other parts of the oropharynx is a method to treat patients with neurogenic dysphagia caused by sensory deficits [55–57]. Thermal tactile oral stimulation of the ATP on patients with neurologic diseases results in improved triggering of the swallowing reflex [58–60]. The improved swallowing may be due to increases of sensory input via the pharyngeal branches of the IX and X nerves which innervate the pharyngeal walls, including the ATP and OPW [32].
The results from this study suggest that LTS in the pharyngeal sensory nerves may be a useful and easily obtainable biomarker of PD. Specifically, mucosal biopsies in living PD patients could be low risk objective biomarker of the disease. To date, diagnosis and progression measures of PD are based largely on clinical criteria. However, the clinical diagnosis of PD is incorrect in up to 50% of parkinsonian subjects with disease duration of < 5 years [22]. Therefore, there is a pressing need to develop biomarkers for a more precise and early diagnosis of PD. Alpha-synuclein aggregation underlies PD pathology that is present in both the central and peripheral nervous systems [4, 61, 62]. Strong evidence indicates the near-universal presence of LTS in the peripheral nervous system in PD [4]. Therefore, biopsy of peripheral tissues has been used to detect LTS in autonomic nerves [63, 64]. So far, LTS has been tentatively identified in biopsies of the colon [65–69], submandibular gland [5, 63], and skin [70] of PD patients. There is much excitement in the PD field about biopsies of peripheral tissues as possible PD biomarkers but thus far testing sensitivity has been too low at most sites. For instance, LTS in the skin was identified only in 10% of PD patients [70]. There was a relatively low density of colonic LTS per biopsy site [65–67,69]. In the colon, LTS was identified only in ~44% of samples [66]. Minor salivary gland biopsies of the lower lip have largely been negative [63, 71]. The submandibular gland had a relatively high prevalence of LTS and its biopsy may be a useful approach for diagnosis of PD [5, 63]. However, a simple, validated, and inexpensive biomarker for PD is still lacking. As we have demonstrated abundant LTS in the pharyngeal sensory nerves [19] and mucosa of autopsy specimens from PD subjects (the present study), UAT may be another site to detect LTS for diagnosis of PD because pharyngeal mucosa represents an easily accessible tissue and invasiveness of the biopsy procedure is low. The results from this study suggest that LTS in the UAT lining tissues could be detectable using pharyngeal mucosal biopsy in living PD patients. Further studies may be needed to test whether pharyngeal biopsy is feasible for detecting LTS in living PD patients and determine if this approach has diagnostic and/or predictive value.
It should be pointed out that this study has some limitations. For example, the diagnosis of dysphagia was based on the UPDRS Scale. This self-administered scale may not be the best metric for dysphagia. Objective documentation of dysphagia and its dynamic nature can be obtained with modified barium swallows (MBS). Ideally, future studies could be prospective with functional correlation between FEESST and pathology (either biopsy or post mortem). Such data would be helpful for determining whether LTS peripheral nerve pathology causes dysphagia in PD patients and for developing effective therapies.
Conclusions
This study has demonstrated the presence of LTS in the sensory nerve terminals innervating the UAT mucosa in PD. In this series, PD subjects with dysphagia had more LTS-positive axons in the UAT mucosa versus those without dysphagia while normal controls had none. These findings suggest that LTS in the peripheral sensory nerves supplying the UAT mucosa could be a risk factor for dysphagia in PD. Further work is warranted to: (1) objectively detect dysphagia and precisely determine the relationship between dysphagia severity and LTS densities; (2) test whether pharyngeal biopsy is feasible for detecting LTS in living PD patients and has the potential for diagnosis of PD; and (3) detect LTS in the peripheral motor and sensory nerves controlling the larynx and tongue for a better understanding of the mechanisms of dysphagia and aspiration.
Acknowledgments
This research was supported partially by the Michael J. Fox Foundation for Parkinson’s Research (to Dr. Liancai Mu) and partially by National Institutes of Health Grant 5 R01 DC004728 from the National Institute on Deafness and Other Communication Disorders (to Dr. Liancai Mu). The Brain and Body Donation Program was supported by the National Institute of Neurological Disorders and Stroke (U24 NS072026 National Brain and Tissue Resource for Parkinson’s Disease and Related Disorders), the National Institute on Aging (P30 AG19610 Arizona Alzheimer’s Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer’s Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011, 05-901 and 1001 to the Arizona Parkinson’s Disease Consortium) and the Michael J. Fox Foundation for Parkinson’s Research.
The authors thank the Banner Sun Health Research Institute Brain and Body Donation Program and the Arizona Parkinson’s Disease Consortium (APDC) for the provision of whole-mount tongue-pharynx-larynx specimens and associated clinical and neuropathologic data from PD and control subjects.
The authors also thank the anonymous reviewers for their constructive comments on the manuscript.
Abbreviations
- AEF
Aryepiglottic fold
- ATP
Anterior tonsillar pillar
- H&Y Scale
The Hoehn and Yahr Scale
- LPT
Lateral posterior tongue
- LTS
Lewy-type synucleinopathy
- NF
Neurofilament
- OPW
Oropharyngeal posterior wall
- PAS
Phosphorylated α-synuclein
- PAS-ir
PAS-immunoreactive
- PCR
Postcricoid region
- PD
Parkinson’s disease
- UAT
Upper aerodigestive tract
- UE
Upper esophagus
- UPDRS Scale
The Unified Parkinson’s Disease Rating Scale
Footnotes
Conflict of interest
The authors have no conflicts of interest or financial ties to disclose.
References
- 1.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–76. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 2.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–40. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 3.Spillantini MG, Crowther RA, Jakes R, Cairns NJ, Lantos PL, Goedert M. Filamentous α-synuclein inclusions link multiple system atrophy with Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett. 1998;251:205–8. doi: 10.1016/s0304-3940(98)00504-7. [DOI] [PubMed] [Google Scholar]
- 4.Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beach TG, Adler CH, Dugger BN, Serrano G, Hidalgo J, Henry-Watson J, Shill HA, Sue LI, Sabbagh MN, Akiyama H. Submandibular gland biopsy for the diagnosis of Parkinson disease. J Neuropathol Exp Neurol. 2013;72:130–36. doi: 10.1097/NEN.0b013e3182805c72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, de Vos RAI, Bohl J, Del Tredici K. Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H. Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol. 2010;119:703–13. doi: 10.1007/s00401-010-0665-2. [DOI] [PubMed] [Google Scholar]
- 8.Wakabayashi K, Takahashi H, Ohama E, Ikuta F. Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990;79:581–3. doi: 10.1007/BF00294234. [DOI] [PubMed] [Google Scholar]
- 9.Bloch A, Probst A, Bissig H, Adams H, Tolnay M. Alpha-synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol. 2006;32:284–95. doi: 10.1111/j.1365-2990.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 10.Dabby R, Djaldetti R, Shahmurov M, Treves TA, Gabai B, Melamed E, Sadeh M, Avinoach I. Skin biopsy for assessment of autonomic denervation in Parkinson’s disease. J Neural Transm. 2006;113:1169–76. doi: 10.1007/s00702-005-0431-0. [DOI] [PubMed] [Google Scholar]
- 11.Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, Takahashi H. Axonal α-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain. 2008;131:642–50. doi: 10.1093/brain/awm302. [DOI] [PubMed] [Google Scholar]
- 12.Sapir S, Ramig L, Fox C. Voice, speech and swallowing disorders. In: Factor S, Weiner W, editors. Parkinson disease: diagnosis and clinical management. New York: Demos Medical Publishing; 2008. pp. 77–97. [Google Scholar]
- 13.Ali GN, Wallace KL, Schwartz R, DeCarle DJ, Zagami AS, Cook IJ. Mechanisms of oral-pharyngeal dysphagia in patients with Parkinson’s disease. Gastroenterology. 1996;110:383–92. doi: 10.1053/gast.1996.v110.pm8566584. [DOI] [PubMed] [Google Scholar]
- 14.Cook IJ. AGA technical review on management of oropharyngeal dysphagia. Gastroenterology. 1999;116:455–78. doi: 10.1016/s0016-5085(99)70144-7. [DOI] [PubMed] [Google Scholar]
- 15.Pommerenke WT. A study of the sensory areas eliciting the swallowing reflex. Am J Physiol. 1928;84:36–41. [Google Scholar]
- 16.Storey AT. A functional analysis of sensory units innervating epiglottis and larynx. Exp Neurol. 1968;20:366–83. doi: 10.1016/0014-4886(68)90080-0. [DOI] [PubMed] [Google Scholar]
- 17.Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, Sasaki H. Impaired efficacy of cough in patients with Parkinson’s disease. Chest. 2003;124:1009–15. doi: 10.1378/chest.124.3.1009. [DOI] [PubMed] [Google Scholar]
- 18.Pitts T, Bolser D, Rosenbek J, Troche M, Sapienza C. Voluntary cough production and swallow dysfunction in Parkinson’s disease. Dysphagia. 2008;23:297–301. doi: 10.1007/s00455-007-9144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mu L, Sobotka S, Chen J, Su H, Sanders I, Nyirenda T, Adler CH, Shill HA, Caviness JN, Samanta JE, Sue LI, Beach TG. Parkinson disease affects peripheral sensory nerves in the pharynx. J Neuropathol Exp Neurol. 2013;72:614–23. doi: 10.1097/NEN.0b013e3182965886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, Shill HA, Caviness JN, Samanta JE, Beach TG. Alpha-synuclein pathology and axonal degeneration of the peripheral motor nerves innervating pharyngeal muscles in Parkinson disease. J Neuropathol Exp Neurol. 2013;72:119–29. doi: 10.1097/NEN.0b013e3182801cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beach TG, Sue LI, Walker DG, Roher AE, Lue L, Vedders L, Connor DJ, Sabbagh MN, Rogers J. The Sun Health Research Institute Brain Donation Program: description and experience, 1987–2007. Cell Tissue Bank. 2008;9:229–45. doi: 10.1007/s10561-008-9067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, Sabbagh MN, Sue LI, Jacobson SA, Belden CM, Dugger BN. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study. Neurology. 2014;83:406–12. doi: 10.1212/WNL.0000000000000641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17:427–42. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 24.Fahn S, Elton R . Committee DU. Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent developments in Parkinson’s disease. New York: MacMillan; 1987. pp. 153–63. [Google Scholar]
- 25.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–9. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 26.Eriksson PO, Eriksson A, Ringqvist M, Thornell LE. The reliability of histochemical fiber typing of human necropsy muscles. Histochemistry. 1980;65:193–205. doi: 10.1007/BF00493169. [DOI] [PubMed] [Google Scholar]
- 27.Eriksson PO, Thornell LE. Histochemical and morphological muscle fiber characteristics of the human masseter, the medial pterygoid and the temporal muscles. Arch Oral Biol. 1983;28:781–95. doi: 10.1016/0003-9969(83)90034-1. [DOI] [PubMed] [Google Scholar]
- 28.Mu L, Sobotka S, Chen J, Su H, Sanders I, Adler CH, Shill HA, Caviness JN, Samanta JE, Beach TG. Altered pharyngeal muscles in Parkinson’s disease. J Neuropathol Exp Neurol. 2013;71:520–30. doi: 10.1097/NEN.0b013e318258381b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beach TG, White CL, Hamilton RL, Duda JE, Iwatsubo T, Dickson DW, Leverenz JB, Roncaroli F, Buttini M, Hladik CL, Sue LI, Noorigian JV, Adler CH. Evaluation of alpha-synuclein immunohistochemical methods used by invited experts. Acta Neuropathol. 2008;116:277–88. doi: 10.1007/s00401-008-0409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doty RW. Neural organization of deglutition. In: Code CF, editor. Handbook of physiology. Sec 6, Alimentary canal. Vol. 4. Washington, DC: American Physiological Society; 1968. pp. 861–902. [Google Scholar]
- 31.Goyal RK, Cobb BW. Motility of the pharynx, esophagus, and esophageal sphincter. In: Johnson LR, editor. Physiology of the digestive tract. New York: Reven Press; 1981. [Google Scholar]
- 32.Mu L, Sanders I. Sensory nerve supply of the human oro- and laryngopharynx: a preliminary study. Anat Rec. 2000;258:406–20. doi: 10.1002/(SICI)1097-0185(20000401)258:4<406::AID-AR9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 33.Williams PL, Bannister LH, Berry MM, et al. Gray’s anatomy. 38. New York: Churchill Livingstone; 1995. [Google Scholar]
- 34.Mu L, Sanders I. Neuromuscular specializations within human pharyngeal constrictor muscles. Ann Otol Rhinol Laryngol. 2007;116:604–17. doi: 10.1177/000348940711600809. [DOI] [PubMed] [Google Scholar]
- 35.Amino T, Orimo S, Itoh Y, Takahashi A, Uchihara T, Mizusawa H. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Pathol. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL, 3rd, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol. 2009;117:613–34. doi: 10.1007/s00401-009-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The National Institute on Aging and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Consensus recommendations for the postmortem diagnosis of Alzheimer’s disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 38.Hunker CJ, Abbs JH, Barlow SM. The relationship between parkinsonian rigidity and hypokinesia in the orofacial system: a quantitative analysis. Neurology. 1982;32:749–54. doi: 10.1212/wnl.32.7.749. [DOI] [PubMed] [Google Scholar]
- 39.Hunter PC, Crameri J, Austin S, Woodward MC, Hughes AJ. Response of parkinsonian swallowing dysfunction to dopaminergic stimulation. J Neurol Neurosurg Psychiatry. 1997;63:579–83. doi: 10.1136/jnnp.63.5.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duff J, Sime E. Surgical interventions in the treatment of Parkinson’s disease (PD) and essential tremor (ET): medial pallidotomy in PD and chronic deep brain stimulation (DBS) in PD and ET. Axon. 1997;18:85–9. [PubMed] [Google Scholar]
- 41.Leopold NA, Kagel MC. Pharyngo-esophageal dysphagia in Parkinson’s disease. Dysphagia. 1997;12:11–8. doi: 10.1007/pl00009512. [DOI] [PubMed] [Google Scholar]
- 42.Liss JM, Lansford K, Caviness JN. Dementia with Lewy bodies (DLB) In: Rosenbek J, editor. The encyclopedia of oropharyngeal dysphagia in rare conditions. San Diego: Plural Publishing; 2009. pp. 171–8. [Google Scholar]
- 43.Aviv JE, Martin JH, Sacco RL, Zagar D, Diamond B, Keen MS, Blitzer A. Supraglottic and pharyngeal sensory abnormalities in stroke patients with dysphagia. Ann Otol Rhinol Laryngol. 1996;105:92–7. doi: 10.1177/000348949610500202. [DOI] [PubMed] [Google Scholar]
- 44.Aviv JE, Sacco RL, Mohr JP, Thompson JL, Levin B, Sunshine S, Thomson J, Close LG. Laryngopharyngeal sensory testing with modified barium swallow as predictors of aspiration pneumonia after stroke. Laryngoscope. 1997;107:1254–60. doi: 10.1097/00005537-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 45.Aviv JE, Martin JH, Keen MS, Debell M, Blitzer A. Air pulse quantification of supraglottic and pharyngeal sensation: A new technique. Ann Otol Rhinol Laryngol. 1993;102:777–80. doi: 10.1177/000348949310201007. [DOI] [PubMed] [Google Scholar]
- 46.Aviv JE. Sensory discrimination in the larynx and hypopharynx. Otolaryngol Head Neck Surg. 1997;116:331–4. doi: 10.1016/S0194-59989770268-7. [DOI] [PubMed] [Google Scholar]
- 47.Aviv JE, Kim T, Sacco RL, Kaplan S, Goodhart K, Diamond B, Close LG. FEESST: a new bedside endoscopic test of the motor and sensory components of swallowing. Ann Otol Rhinol Laryngol. 1998;107:378–87. doi: 10.1177/000348949810700503. [DOI] [PubMed] [Google Scholar]
- 48.Aviv JE, Kaplan ST, Thomson JE, Spitzer J, Diamond B, Close LG. The safety of flexible endoscopic evaluation of swallowing with sensory testing (FEESST): an analysis of 500 consecutive evaluations. Dysphagia. 2000;15:39–44. doi: 10.1007/s004559910008. [DOI] [PubMed] [Google Scholar]
- 49.Setzen M, Cohen MA, Mattucci KF, Perlman PW, Ditkoff MK. Laryngopharyngeal sensory deficits as a predictor of aspiration. Otolaryngol Head Neck Surg. 2001;124:622–4. doi: 10.1177/019459980112400605. [DOI] [PubMed] [Google Scholar]
- 50.Setzen M, Cohen MA, Perlman PW, Belafsky PC, Guss J, Mattucci KF, Ditkoff M. The association between laryngopharyngeal sensory deficits, pharyngeal motor function, and the prevalence of aspiration with thin liquids. Otolaryngol Head Neck Surg. 2003;128:99–102. doi: 10.1067/mhn.2003.52. [DOI] [PubMed] [Google Scholar]
- 51.Hammer MJ, Murphy CA, Abrams TM. Airway somatosensory deficits and dysphagia in Parkinson’s disease. J Parkinsons Dis. 2013;3:39–44. doi: 10.3233/JPD-120161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hammer MJ, Barlow SM. Laryngeal somatosensory deficits in Parkinson’s disease: implications for speech respiratory and phonatory control. Exp Brain Res. 2010;201:401–9. doi: 10.1007/s00221-009-2048-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dodds WJ. The physiology of swallowing. Dysphagia. 1989;3:171–8. doi: 10.1007/BF02407219. [DOI] [PubMed] [Google Scholar]
- 54.Perlman AL. The neurology of swallowing. Sem Speech Lang Hear. 1991;12:171–84. [Google Scholar]
- 55.Neumann S, Bartolome G, Buchholz D, Prosiegel M. Swallowing therapy of neurologic patients: correlation of outcome with pretreatment variables and therapeutic methods. Dysphagia. 1995;10:1–5. doi: 10.1007/BF00261272. [DOI] [PubMed] [Google Scholar]
- 56.Rosenbek JC, Roecker EB, Wood JL, Robbins J. Thermal application reduces the duration of stage transition in dysphagia after stroke. Dysphagia. 1996;11:225–33. doi: 10.1007/BF00265206. [DOI] [PubMed] [Google Scholar]
- 57.Logemann JA. The need for clinical trials in dysphagia. Dysphagia. 1998;13:10–11. [PubMed] [Google Scholar]
- 58.Kaatzke-McDonald MN, Post E, Davis PJ. The effects of cold, touch, and chemical stimulation of the anterior faucial pillar on human swallowing. Dysphagia. 1996;11:198–206. doi: 10.1007/BF00366386. [DOI] [PubMed] [Google Scholar]
- 59.Sciortino KF, Liss JM, Case JL, Gerritsen KG, Katz RC. Effects of mechanical, cold, gustatory, and combined stimulation to the human anterior faucial pillars. Dysphagia. 2003;18:16–26. doi: 10.1007/s00455-002-0076-1. [DOI] [PubMed] [Google Scholar]
- 60.Teismann IK, Steinstrater O, Warnecke T, Suntrup S, Ringelstein EB, Pantev C, Dziewas R. Tactile thermal oral stimulation increases the cortical representation of swallowing. BMC Neurosci. 2009;10:71–80. doi: 10.1186/1471-2202-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jellinger KA. Neuropathological spectrum of synucleinopathies. Mov Disord. 2003;18(Suppl 6):S2–S12. doi: 10.1002/mds.10557. [DOI] [PubMed] [Google Scholar]
- 62.Braak H, Del Tredici K. Invited article: nervous system pathology in sporadic Parkinson disease. Neurology. 2008;70:1916–25. doi: 10.1212/01.wnl.0000312279.49272.9f. [DOI] [PubMed] [Google Scholar]
- 63.Adler CH, Dugger BN, Hinni ML, Lott DG, Driver-Dunckley E, Hidalgo J, Henry-Watson J, Serrano G, Sue LI, Nagel T, Duffy A, Shill HA, Akiyama H, Walker DG, Beach TG. Submandibular gland needle biopsy for the diagnosis of Parkinson disease. Neurology. 2014;82:858–64. doi: 10.1212/WNL.0000000000000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cersosimo MG, Benarroch EE. Autonomic involvement in Parkinson’s disease: pathology, pathophysiology, clinical features and possible peripheral biomarkers. J Neurol Sci. 2012;313:57–63. doi: 10.1016/j.jns.2011.09.030. [DOI] [PubMed] [Google Scholar]
- 65.Lebouvier T, Chaumette T, Damier P, Coron E, Touchefeu Y, Vrignaud S, Naveilhan P, Galmiche JP, Bruley des Varannes S, Derkinderen P, Neunlist M. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut. 2008;57:1741–3. doi: 10.1136/gut.2008.162503. [DOI] [PubMed] [Google Scholar]
- 66.Lebouvier T, Neunlist M, Bruley des Varannes S, Coron E, Drouard A, N’Guyen JM, Chaumette T, Tasselli M, Paillusson S, Flamand M, Galmiche JP, Damier P, Derkinderen P. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS One. 2010;5:e12728. doi: 10.1371/journal.pone.0012728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shannon KM, Keshavarzian A, Mutlu E, Dodiya HB, Daian D, Jaglin JA, Kordower JH. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov Disord. 2012;27:709–15. doi: 10.1002/mds.23838. [DOI] [PubMed] [Google Scholar]
- 68.Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord. 2012;27:716–19. doi: 10.1002/mds.25020. [DOI] [PubMed] [Google Scholar]
- 69.Pouclet H, Lebouvier T, Coron E, Des Varannes SB, Neunlist M, Derkinderen P. A comparison between colonic submucosa and mucosa to detect Lewy pathology in Parkinson’s disease. Neurogastroenterol Motil. 2012;24:e202–5. doi: 10.1111/j.1365-2982.2012.01887.x. [DOI] [PubMed] [Google Scholar]
- 70.Miki Y, Tomiyama M, Ueno T, Haga R, Nishijima H, Suzuki C, Mori F, Kaimori M, Baba M, Wakabayashi K. Clinical availability of skin biopsy in the diagnosis of Parkinson’s disease. Neurosci Lett. 2010;469:357–9. doi: 10.1016/j.neulet.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 71.Cersosimo MG, Perandones C, Micheli FE, Raina GB, Beron AM, Nasswetter G, Radrizzani M, Benarroch EE. Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord. 2011;26:188–90. doi: 10.1002/mds.23344. [DOI] [PubMed] [Google Scholar]