Abstract
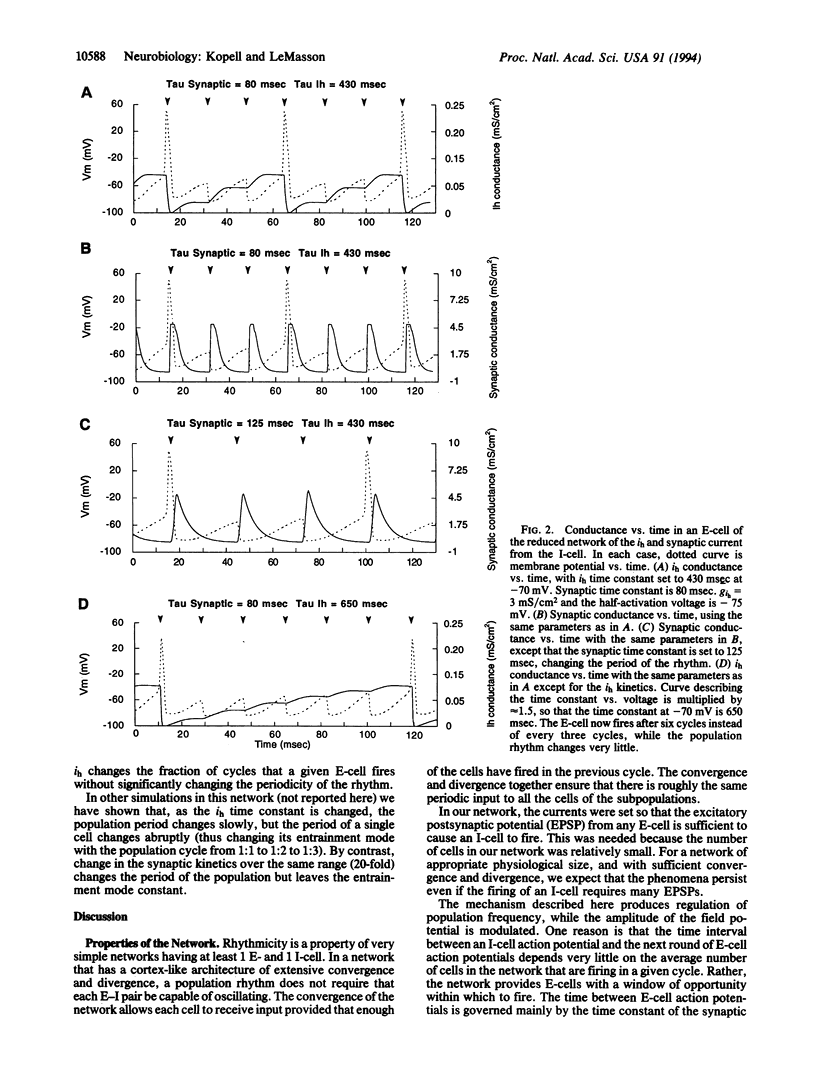
In a network of excitatory and inhibitory neurons, hyperpolarization-activated inward currents can help to produce population rhythms in which individual cells participate sparsely and randomly. A shift in the activation curve of such a current changes the fraction of the cells participating in any given cycle of the population rhythm, thus changing the amplitude of the field potential. Furthermore, the frequency of the population rhythm remains relatively fixed over a substantial range of amplitudes, allowing the population rhythm to play a separate processing role from that of the individual components.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott LF, van Vreeswijk C Asynchronous states in networks of pulse-coupled oscillators. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993 Aug;48(2):1483–1490. doi: 10.1103/physreve.48.1483. [DOI] [PubMed] [Google Scholar]
- Ermentrout G. B., Cowan J. D. Temporal oscillations in neuronal nets. J Math Biol. 1979 Apr 18;7(3):265–280. doi: 10.1007/BF00275728. [DOI] [PubMed] [Google Scholar]
- Foehring R. C., Waters R. S. Contributions of low-threshold calcium current and anomalous rectifier (Ih) to slow depolarizations underlying burst firing in human neocortical neurons in vitro. Neurosci Lett. 1991 Mar 11;124(1):17–21. doi: 10.1016/0304-3940(91)90812-8. [DOI] [PubMed] [Google Scholar]
- Golomb D, Rinzel J. Dynamics of globally coupled inhibitory neurons with heterogeneity. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993 Dec;48(6):4810–4814. doi: 10.1103/physreve.48.4810. [DOI] [PubMed] [Google Scholar]
- Gray C. M., Singer W. Stimulus-specific neuronal oscillations in orientation columns of cat visual cortex. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1698–1702. doi: 10.1073/pnas.86.5.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa G. G., Avoli M. Hyperpolarizing inward rectification in rat neocortical neurons located in the superficial layers. Neurosci Lett. 1991 Mar 11;124(1):65–68. doi: 10.1016/0304-3940(91)90823-c. [DOI] [PubMed] [Google Scholar]
- Kamondi A., Reiner P. B. Hyperpolarization-activated inward current in histaminergic tuberomammillary neurons of the rat hypothalamus. J Neurophysiol. 1991 Dec;66(6):1902–1911. doi: 10.1152/jn.1991.66.6.1902. [DOI] [PubMed] [Google Scholar]
- Lytton W. W., Sejnowski T. J. Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. J Neurophysiol. 1991 Sep;66(3):1059–1079. doi: 10.1152/jn.1991.66.3.1059. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Huguenard J. R. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992 Oct;68(4):1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- McCormick D. A. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol. 1992 Oct;39(4):337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Noradrenergic and serotonergic modulation of a hyperpolarization-activated cation current in thalamic relay neurones. J Physiol. 1990 Dec;431:319–342. doi: 10.1113/jphysiol.1990.sp018332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Pape H. C. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurones. J Physiol. 1990 Dec;431:291–318. doi: 10.1113/jphysiol.1990.sp018331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick D. A., Williamson A. Modulation of neuronal firing mode in cat and guinea pig LGNd by histamine: possible cellular mechanisms of histaminergic control of arousal. J Neurosci. 1991 Oct;11(10):3188–3199. doi: 10.1523/JNEUROSCI.11-10-03188.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape H. C., Mager R. Nitric oxide controls oscillatory activity in thalamocortical neurons. Neuron. 1992 Sep;9(3):441–448. doi: 10.1016/0896-6273(92)90182-d. [DOI] [PubMed] [Google Scholar]
- Perkel D. H., Mulloney B. Motor pattern production in reciprocally inhibitory neurons exhibiting postinhibitory rebound. Science. 1974 Jul 12;185(4146):181–183. doi: 10.1126/science.185.4146.181. [DOI] [PubMed] [Google Scholar]
- Pinsky P. F., Rinzel J. Intrinsic and network rhythmogenesis in a reduced Traub model for CA3 neurons. J Comput Neurosci. 1994 Jun;1(1-2):39–60. doi: 10.1007/BF00962717. [DOI] [PubMed] [Google Scholar]
- Skinner F. K., Kopell N., Marder E. Mechanisms for oscillation and frequency control in reciprocally inhibitory model neural networks. J Comput Neurosci. 1994 Jun;1(1-2):69–87. doi: 10.1007/BF00962719. [DOI] [PubMed] [Google Scholar]
- Soltesz I., Lightowler S., Leresche N., Jassik-Gerschenfeld D., Pollard C. E., Crunelli V. Two inward currents and the transformation of low-frequency oscillations of rat and cat thalamocortical cells. J Physiol. 1991 Sep;441:175–197. doi: 10.1113/jphysiol.1991.sp018745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sompolinsky H, Golomb D, Kleinfeld D. Cooperative dynamics in visual processing. Phys Rev A. 1991 Jun 15;43(12):6990–7011. doi: 10.1103/physreva.43.6990. [DOI] [PubMed] [Google Scholar]
- Spain W. J., Schwindt P. C., Crill W. E. Anomalous rectification in neurons from cat sensorimotor cortex in vitro. J Neurophysiol. 1987 May;57(5):1555–1576. doi: 10.1152/jn.1987.57.5.1555. [DOI] [PubMed] [Google Scholar]
- Steriade M., Deschenes M. The thalamus as a neuronal oscillator. Brain Res. 1984 Nov;320(1):1–63. doi: 10.1016/0165-0173(84)90017-1. [DOI] [PubMed] [Google Scholar]
- Tokimasa T., Akasu T. Cyclic AMP regulates an inward rectifying sodium-potassium current in dissociated bull-frog sympathetic neurones. J Physiol. 1990 Jan;420:409–429. doi: 10.1113/jphysiol.1990.sp017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokimasa T., Sugiyama K., Akasu T., Muteki T. Volatile anaesthetics inhibit a cyclic AMP-dependent sodium-potassium current in cultured sensory neurones of bullfrog. Br J Pharmacol. 1990 Sep;101(1):190–192. doi: 10.1111/j.1476-5381.1990.tb12111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub R. D., Miles R., Wong R. K. Model of the origin of rhythmic population oscillations in the hippocampal slice. Science. 1989 Mar 10;243(4896):1319–1325. doi: 10.1126/science.2646715. [DOI] [PubMed] [Google Scholar]
- Wang X. J. Multiple dynamical modes of thalamic relay neurons: rhythmic bursting and intermittent phase-locking. Neuroscience. 1994 Mar;59(1):21–31. doi: 10.1016/0306-4522(94)90095-7. [DOI] [PubMed] [Google Scholar]
- Wilson H. R., Cowan J. D. Excitatory and inhibitory interactions in localized populations of model neurons. Biophys J. 1972 Jan;12(1):1–24. doi: 10.1016/S0006-3495(72)86068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M., Bower J. M. Cortical oscillations and temporal interactions in a computer simulation of piriform cortex. J Neurophysiol. 1992 Apr;67(4):981–995. doi: 10.1152/jn.1992.67.4.981. [DOI] [PubMed] [Google Scholar]
- von Krosigk M., Bal T., McCormick D. A. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993 Jul 16;261(5119):361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]


