Abstract
Anterior to posterior growth of the vertebrate body is fueled by a posteriorly located population of bipotential neuro-mesodermal progenitor cells. These progenitors have a limited rate of proliferation and their maintenance is crucial for completion of the anterior-posterior axis. How they leave the progenitor state and commit to differentiation is largely unknown, in part because widespread modulation of factors essential for this process causes organism-wide effects. Using a novel assay, we show that zebrafish Tbx16 (Spadetail) is capable of advancing mesodermal differentiation cell-autonomously. Tbx16 locks cells into the mesodermal state by not only activating downstream mesodermal genes, but also by repressing bipotential progenitor genes, in part through a direct repression of sox2. We demonstrate that tbx16 is activated as cells move from an intermediate Wnt environment to a high Wnt environment, and show that Wnt signaling activates the tbx16 promoter. Importantly, high-level Wnt signaling is able to accelerate mesodermal differentiation cell-autonomously, just as we observe with Tbx16. Finally, because our assay for mesodermal commitment is quantitative we are able to show that the acceleration of mesodermal differentiation is surprisingly incomplete, implicating a potential separation of cell movement and differentiation during this process. Together, our data suggest a model in which high levels of Wnt signaling induce a transition to mesoderm by directly activating tbx16, which in turn acts to irreversibly flip a bistable switch, leading to maintenance of the mesodermal fate and repression of the bipotential progenitor state, even as cells leave the initial high-Wnt environment.
KEY WORDS: Bipotential, Neuromesodermal, Wnt, Spadetail, Tbx16, Somitogenesis
Summary: In response to high levels of Wnt, tbx16 irreversibly flips a bistable switch that allows maintenance of mesodermal fate while repressing the bipotential neuromesodermal progenitor state in zebrafish embryos.
INTRODUCTION
Vertebrates form an anterior-posterior axis through a process called posterior growth, in which a population of undifferentiated cells resides at the most posterior end of the embryo and gradually contributes cells to the trunk and tail during the somite-forming stages (Kimelman and Martin, 2012; Wilson et al., 2009). Germ layer specification had long been thought to be complete by the end of gastrulation, but recent work has demonstrated that the most posterior end of the growing embryo maintains a population of bipotential stem cell-like progenitors, which are capable of adopting either a neural or mesodermal fate depending on their exposure to canonical Wnt signaling (Garriock et al., 2015; Gouti et al., 2014; Jurberg et al., 2014; Martin and Kimelman, 2012; Takemoto et al., 2011; Tsakiridis et al., 2014). These bipotential cells provide the raw cellular material necessary to extend and complete the anterior-posterior axis. Owing to a limited rate of progenitor cell proliferation during posterior growth (Bouldin et al., 2014), the rate at which cells exit the bipotential progenitor zone and contribute to the body must be carefully controlled in order to prevent premature depletion of progenitor cells before the anterior-posterior axis is complete.
An essential component involved in maintaining the bipotential progenitors during posterior growth is the T-box transcription factor Brachyury (Papaioannou, 2014). In zebrafish, two brachyury genes, no tail (ntl) and brachyury (bra) [also known as brachyury homolog a (ta) and b (tb)], are expressed in the bipotential cells from the onset of gastrulation through the end of somitogenesis (Martin and Kimelman, 2008; Schulte-Merker et al., 1994). Loss of ntl results in embryos without a tail, whereas loss of both ntl and bra causes a more severe truncation of the axis (Halpern et al., 1993; Martin and Kimelman, 2008). Ntl and Bra act within the bipotential progenitor cells to maintain expression of the canonical Wnts wnt3a and wnt8, which together are essential for forming the mesoderm of the posterior body (Martin and Kimelman, 2008; Shimizu et al., 2005; Thorpe et al., 2005). The feedback between the canonical Wnts and the brachyury orthologs is broadly conserved among vertebrates as a crucial component of posterior growth (Martin and Kimelman, 2009).
As cells leave the bipotential progenitor state, they immediately activate another T-box gene crucial for mesodermal differentiation, tbx16 (formerly known as spadetail in zebrafish), which is a member of the tbx6/16 family of T-box genes (Ahn et al., 2012). tbx16 mutant embryos have severe muscle defects due to loss of the cell-autonomous functions of Tbx16 in mesodermal cell differentiation and morphogenesis (Griffin et al., 1998; Ho and Kane, 1990; Kimmel et al., 1989). Interestingly, tbx16 functions semi-redundantly with another transcription factor, mesogenin 1 (msgn1), such that tbx16;msgn1 mutants show a complete loss of muscle (Fior et al., 2012; Yabe and Takada, 2012). However, zebrafish msgn1 mutants are essentially normal (Fior et al., 2012; Yabe and Takada, 2012), demonstrating that Tbx16, not Msgn1, is the major regulator of mesoderm formation during posterior growth in zebrafish.
A major unanswered question is how these mesoderm-inducing factors work together to let cells know when to leave the bipotential progenitor state and trigger the commitment to mesoderm. Since a key initial event in mesodermal differentiation during posterior growth is the activation of tbx16 expression (Griffin and Kimelman, 2002), we tested the hypothesis that expression of Tbx16 is sufficient to initiate mesodermal cell differentiation. Because ubiquitous overexpression of factors such as Wnt, Msgn1 and Tbx16 causes severe alterations in posterior growth that preclude analysis of the effects on individual cells (Fior et al., 2012; Martin and Kimelman, 2012; Yabe and Takada, 2012; and below), we have developed a new quantitative assay that has allowed us to establish a cell-autonomous role for Tbx16 and Wnt in promoting progenitor cell exit from the tailbud. Intriguingly, this analysis demonstrated that, although Tbx16 and Wnt can hasten the exit of cells from the tailbud, they cannot force all bipotential cells from the progenitor zone immediately, suggesting that some other factor is required. Finally, we show that Tbx16 acts as a bifunctional transcriptional activator and repressor, and that its repressive function blocks the progenitor and neural state and creates an irreversible change that drives bipotential cells to mesodermal fates and establishes a clear distinction between the mesoderm, neural and progenitor cells of the tailbud.
RESULTS
tbx16 advances cells toward muscle differentiation
A key process in posterior growth is the epithelialization of blocks of mesoderm into somites (Holley, 2007; Pourquié, 2011). Somites function to compartmentalize and anchor developing myofibers, which are fused, elongated and morphologically distinguishable from their rounded myoblast precursors (Buckingham and Vincent, 2009; Stellabotte and Devoto, 2007). Because the somites form in a very stereotypical way, they provide a valuable landmark for position along the anterior-posterior axis and thus can be used as a clear measure of the time when cells begin the mesodermal differentiation process.
Because tbx16 is immediately expressed when cells commit to mesoderm and a loss of tbx16 results in loss of somitic mesoderm, tbx16 is necessary for the initiation of muscle cell fate (Griffin and Kimelman, 2002; Griffin et al., 1998; Kimmel et al., 1989). At the outset of this study, we hypothesized that Tbx16 expression would be sufficient to trigger a bipotential cell to begin the process of mesodermal differentiation. We constructed a transgenic line to drive the expression of tbx16 under the control of a heat shock promoter together with a co-expressed nuclear fluorescent protein Tg(hsp70l:tbx16-2A-NLS-Kikume), hereafter called HS:tbx16, allowing us to bypass the severe gastrulation defects caused by expression of ectopic tbx16 that is not temporally controlled (our unpublished results). A similar transgene rescues tbx16 mutant cells during gastrulation and somitogenesis (Row et al., 2011). Whole embryos heat shocked during mid-somitogenesis were severely truncated, and somite formation was also disrupted (supplementary material Fig. S1). Similar results are observed when Wnt signaling or Msgn1 are ectopically activated or expressed (Fior et al., 2012; Martin and Kimelman, 2012; Yabe and Takada, 2012), showing the importance of these factors in body axis formation but precluding finer analysis of their specific roles as differentiation cues.
To circumvent defects in morphogenesis and somite formation, we developed an assay that allowed us to precisely measure the cell-autonomous effects of Tbx16. At the start of gastrulation we transplanted control or HS:tbx16 cells to the ventral margin of wild-type embryos where the progenitors of the posterior mesoderm are located (Kimmel et al., 1990; Warga and Nusslein-Volhard, 1999). The embryos were heat shocked at the 10-somite stage (ss) and allowed to complete somitogenesis (Fig. 1A). We then counted in each embryo the most posterior somite with an elongated muscle fiber containing a transplanted cell, similar to an assay that we used previously (Szeto and Kimelman, 2006). Whereas cells from non-transgenic embryos contributed to elongated fibers throughout the entire tail (Fig. 1B), cells from HS:tbx16 embryos did not contribute cells to fibers in the most posterior; instead, the transplanted cells occupied more anterior positions (Fig. 1C). The interquartile ranges for the two conditions were well separated and the difference was statistically significant using non-parametric analysis (Fig. 1D).
Fig. 1.
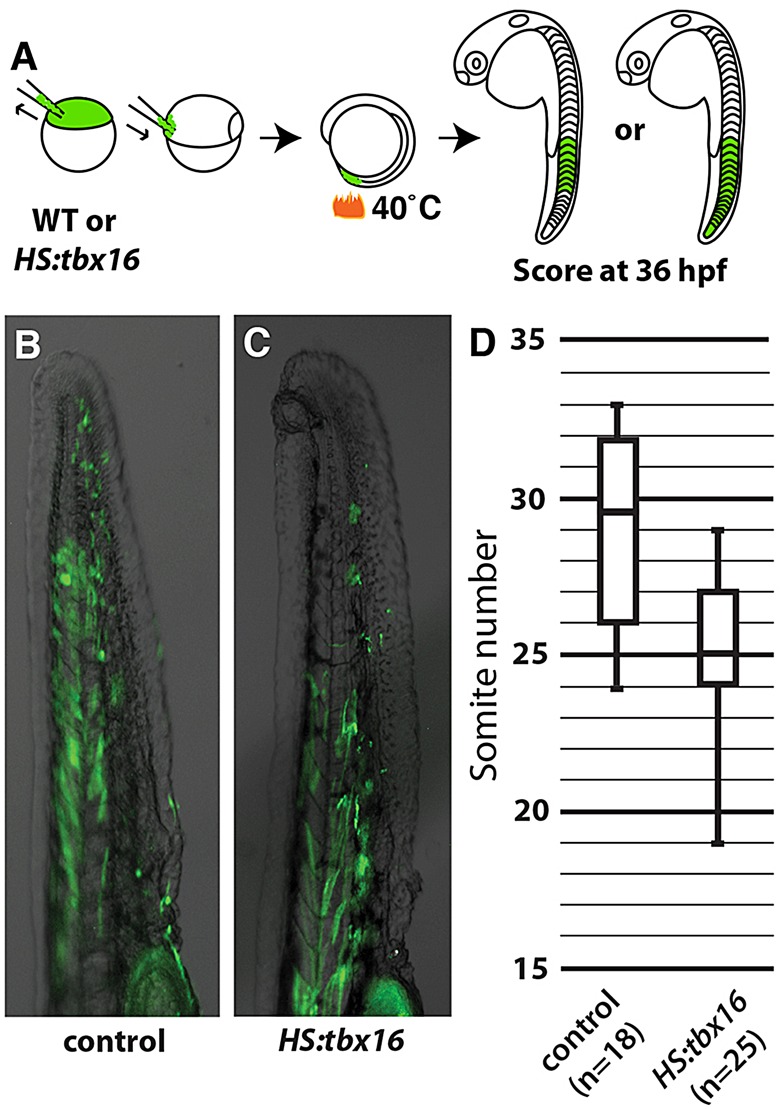
tbx16 expression causes cells to exit the tailbud. (A) Outline of the assay used to test and quantify the ability of ectopic tbx16 to accelerate exit from the progenitor zone. (B,C) Overlays of transplanted cells labeled with fluorescent dextran (green) on a bright-field image from wild-type (B) or HS:tbx16 (C) donor zebrafish embryos at 36 hpf. (D) Quantification of the most posterior somite occupied by a fluorescently labeled cell with fiber-like morphology in embryos heat shocked at the 10 ss. Data are significantly different (Mann-Whitney U-test, P<0.05).
tbx16 represses the progenitor and neural state
The developing tailbud can be subdivided into multiple domains of cells using markers for various stages of differentiation (Griffin and Kimelman, 2002). We examined whether tbx16 was promoting cells toward mesoderm by acting on the earliest phase or on a later stage of differentiation. To distinguish these possibilities, we used the HS:tbx16 line to drive expression of tbx16 and then analyzed gene expression using whole-mount in situ hybridization. We chose three markers of the progenitor zone (sox2, ntl and bra), two markers that indicate the early stages of differentiation (msgn1 and tbx6l) and four markers that are expressed a short time before cells enter somites (high levels of pcdh8, her1, tbx6 and mespba) (Fior et al., 2012; Hug et al., 1997; Martin and Kimelman, 2012; Nikaido et al., 2002; Sawada et al., 2000; Yamamoto et al., 1998). To analyze the effect of HS:tbx16 on endogenous tbx16 transcription, we also created an in situ probe complementary to the 3′ UTR of tbx16, which is not included in the tbx16 transgene.
The peak of ectopic Tbx16 protein accumulation, which occurred 4 h post-heat shock (supplementary material Fig. S2), coincided with an increase in endogenous tbx16, enhanced expression of markers of later stages of differentiation and subtle enhancement in the expression of the early differentiation markers (Fig. 2A; supplementary material Fig. S3 and Table S1). Several changes persisted to 6 h post-heat shock. For instance, pcdh8 and her1 were expanded anteriorly, which was expected because they are Tbx16 target genes (Fig. 2B,C) (Garnett et al., 2009; Yamamoto et al., 1998). Interestingly, sox2 and ntl were reduced in the progenitor zone (Fig. 2D-G), suggesting that Tbx16 actively inhibits progenitor cell gene expression. sox2 is also expressed in the neural tube, where it plays an important role in neural cell differentiation (Okuda et al., 2010). However, neural tube expression of sox2 was unaffected by ectopic tbx16, as was the notochord expression of ntl. Taken together, these data show that ectopic tbx16 expression strongly represses markers of the bipotential progenitor cells and enhances markers of later somite mesoderm differentiation, but does not affect notochord or neural gene expression.
Fig. 2.

tbx16 expression represses genes involved in maintaining the progenitor zone. (A) Summary of changes in gene expression after the induction of ectopic tbx16 (based on the data in supplementary material Fig. S3 and Table S1). Arrows indicate the number of hours post-heat shock (HS) and the colors represent expansion (green), constriction (red) or no change (yellow) to expression, with intermediate shades marking the degree of difference. PM, progenitor markers; EDM, early differentiation markers; LDM, late differentiation markers. α-Tbx16 indicates Tbx16 assessed by immunostaining. (B-G) In situ hybridization shows changes to gene expression in the tailbud of HS:tbx16 (C,E,G) and control (B,D,F) embryos at 6 h after heat shock. The arrow (C) points to expansion of pcdh8 and the red boxes (E,G) highlight the reduction of sox2 and ntl in the tailbud.
Tbx16 is thought to act directly as a transcriptional activator and has been shown to be involved in activating the transcription of a large number of target genes (Garnett et al., 2009). We investigated whether the repression of ntl and sox2 that we observed was a result of a repressive function of Tbx16 or of Tbx16-driven activation of a repressor. To distinguish these possibilities, we fused an Engrailed repressor domain (EnR), which converts the function of transcription factors to obligate repression (Smith and Jaynes, 1996), to tbx16. We created a new transgenic line Tg(hsp70l:tbx16-EnR-2A-NLS-Kikume), hereafter called HS:tbx16-EnR, and examined its effect on the expression of ntl and sox2. Four hours after heat shock, expression of Tbx16-EnR repressed the Tbx16 targets pcdh8 and her1 and inhibited the maintenance of tbx6l (Fig. 3A-F), demonstrating that our fusion construct performs as expected. Interestingly, sox2 was also repressed by Tbx16-EnR (Fig. 3G,H), as well as by wild-type Tbx16 (Fig. 2D,E), indicating that Tbx16 inhibits sox2 through a novel repressive function. By contrast, Tbx16-EnR activated ntl expression (Fig. 3I,J), showing that the Tbx16-mediated repression of ntl is indirect. Our results reveal that Tbx16 is a dual-function activator/repressor that inactivates bipotential progenitor gene expression by repressing sox2 and activating a repressor of ntl.
Fig. 3.

tbx16-EnR expression represses sox2 and activates ntl. In situ hybridization shows changes to gene expression with HS:tbx16-EnR (B,D,F,H,J) or in control (A,C,E,G,I) 4 h after heat shock. The red boxes (B,D,F,H) highlight the reduction of tbx6l, pcdh8, her1 and sox2 in the tailbud and the arrow (J) points to an expansion of ntl.
The summary of all of our results shows that tbx16 is capable of cell-autonomously advancing the differentiation of bipotential cells to muscle by repressing the progenitor state. However, based on the time of heat shock and the time to make Tbx16 protein (supplementary material Fig. S2), it is clear that expression of Tbx16 does not force all cells to leave the progenitor zone immediately but instead hastens their exit (Fig. 1). Because we heat shocked at the 10 ss and the activation of induced mesodermal gene expression was at peak levels at the 16 ss, we would have expected the cells to be found in more anterior somites than we observed. Interestingly, we repeated the same experiment heat shocking 3 h earlier at the 2 ss, and saw a somitic distribution similar to that which we saw in Fig. 1 (supplementary material Fig. S4). Together, these data suggest that although the conversion to a mesodermal state is accelerated by overexpressing Tbx16, something in addition to tbx16 expression is necessary for bipotential progenitor cells to exit from the tailbud.
Canonical Wnt signaling upregulates tbx16 expression
Canonical Wnt signaling plays a crucial role in enabling bipotential cells to differentiate into mesoderm (Martin and Kimelman, 2012). There are at least two Wnts that activate canonical Wnt signaling in the tailbud, wnt3a and wnt8, and several antagonists of Wnt signaling, which combine to tune Wnt signaling (Row and Kimelman, 2009; Stulberg et al., 2012). The mix of Wnts and antagonists, each expressed in a unique pattern, makes it difficult to determine where Wnt signaling is active simply by looking at expression patterns.
To determine where Wnt signaling is active in the tailbud, we focused on levels of nuclear β-catenin, a hallmark of active canonical Wnt signaling, by using DAPI staining as a mask for β-catenin antibody staining, as we and others have done previously (Bajard et al., 2014; Row and Kimelman, 2009). Whereas earlier studies examined Wnt signaling in the anterior presomitic mesoderm, we focused on expression in the tailbud. Nuclear β-catenin was highest in the posterior of the tailbud in a region slightly anterior to the tip (Fig. 4A), which is known to be an area of high levels of tbx16 expression, differentiation and cell movement (Fig. 4D) (Amacher et al., 2002; Kanki and Ho, 1997; Lawton et al., 2013; Martin and Kimelman, 2012; Uriu et al., 2014). Using a Sox2 antibody, we marked the bipotential progenitor cells at the periphery of the tailbud (Fig. 4B). When combined, we saw that the progenitor cells had only moderate levels of nuclear β-catenin and Sox2 relative to the more ventral and anterior cells (Fig. 4C). These results show that canonical Wnt signaling is active at high levels in early differentiating cells and not in progenitor cells.
Fig. 4.
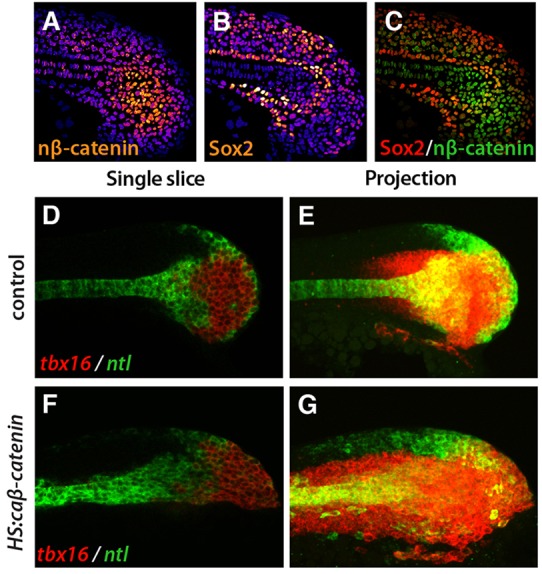
tbx16 is Wnt responsive. (A-C) Immunofluorescence shows cells in the tailbud with nuclear β-catenin (nβ-catenin; A, orange; cytoplasmic and membrane-bound β-catenin have been eliminated from the image; see Materials and Methods) or Sox2 protein (B, orange). DAPI labels nuclei (blue). The highest levels of nβ-catenin and Sox2 are white and the lowest levels are purple. (C) A composite image shows that there is little overlap between the Sox2 domain (red) and the nuclear β-catenin domain (green). (D-G) Fluorescent whole-mount in situ hybridization shows tbx16 (red) and ntl (green) with HS:caβ-catenin (F,G) and in control (D,E) at 4 h post-heat shock. Single slices (D,F) and maximum intensity projections (E,G) are shown. In all images, anterior is to the left and posterior is to the right.
Because the zone of high-level Wnt signaling corresponds to the domain where tbx16 expression initiates and inhibition of Wnt signaling leads to a loss of new mesoderm and a progressive loss of tbx16 expression (Martin and Kimelman, 2008, 2012), we reasoned that Wnt might be an important activator of tbx16 expression. Therefore, we activated the Wnt pathway and analyzed events shortly after activation. For these experiments, we used a transgenic line that placed a heat shock promoter in front of a constitutively active form of β-catenin (caβ-catenin) and a fluorescent protein (Veldman et al., 2013), referred to here as HS:caβ-catenin. When we looked at the midline at 4 h post-heat shock when the fluorescent protein begins to be detectable, the domain of tbx16 expression extended more posteriorly in the presence of caβ-catenin, with tbx16 expression extending into the progenitor zone that normally does not express tbx16 (Fig. 4D,F) (Martin and Kimelman, 2012). Interestingly, we also saw tbx16 expanded anteriorly (Fig. 4E,G), showing that constitutively active Wnt signaling activates tbx16 in both the mesoderm and bipotential cells of the tailbud.
To understand whether Wnt signaling directly activates tbx16 expression, we created the transgenic line Tg(tbx16-1.2:TagRFP), hereafter referred to as tbx16-1.2, that has a 1.2 kb fragment from upstream of the tbx16 start codon driving the expression of a fluorescent protein (Fig. 5A). Within this 1.2 kb tbx16 promoter we identified a highly conserved domain, found in all sequenced fish species, just in front of the start codon (Fig. 5A; supplementary material Fig. S5). Reporter expression recapitulates the normal activation of tbx16 expression within the tailbud, although it lacks the continuing expression within the posterior presomitic mesoderm, which must be regulated by a distal genomic sequence (Fig. 5B).
Fig. 5.
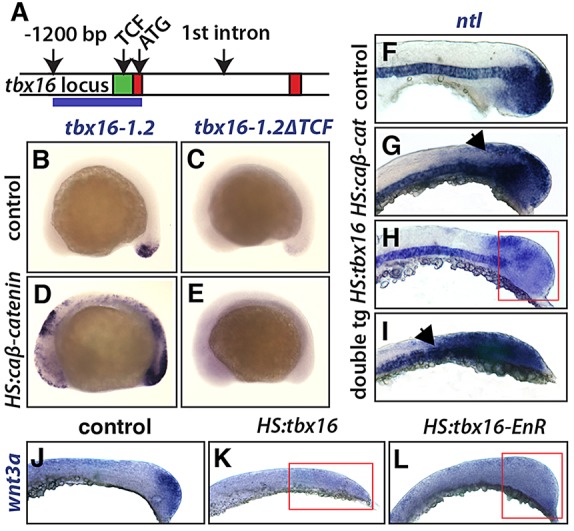
Wnt drives tbx16 reporter expression and tbx16 represses Wnt. (A) The zebrafish tbx16 locus, showing exons 1 and 2 (red) and a conserved region of the tbx16 promoter (green), which contains two predicted TCF binding sites (see supplementary material Fig. S5). The blue line marks the region used for the tbx16-1.2 construct. (B-E) Whole-mount in situ hybridization shows reporter expression from the tbx16-1.2 transgene with HS:caβ-catenin (D) and in control (B). Another reporter line with mutations in both of the predicted TCF sites (tbx16-1.2ΔTCF) showed no expression with (E) or without (C) HS:caβ-catenin. (F-I) In situ hybridization shows wild-type ntl expression (F; n=22 embryos) and the changes in ntl expression after heat shock induction of HS:caβ-catenin (G; n=16 embryos), HS:tbx16 (H; n=11 embryos), and both transgenes (I; n=9 embryos). The arrows (G,I) highlight the expansion of ntl and the red box (H) highlights the reduction of ntl. (J-L) In situ hybridization shows wild-type wnt3a expression (J) and the loss of wnt3a expression after heat shock induction of HS:tbx16 (K) or HS:tbx16-EnR (L). Red boxes (K,L) highlight the reduction of wnt3a in the tailbud.
Transcriptional activation of canonical Wnt signaling requires β-catenin binding to TCF sites, and the conserved region of tbx16 contains two TCF sites that are present in all fish species (supplementary material Fig. S5). When these two sites were mutated in a new transgenic line [Tg(tbx16-1.2ΔTCF:TagRFP), hereafter referred to as tbx16-1.2ΔTCF], reporter expression was abolished (Fig. 5C; similar results were observed in multiple lines). In addition, expression of caβ-catenin strongly increased reporter expression from tbx16-1.2 (Fig. 5D), but had no effect on reporter expression from tbx16-1.2ΔTCF (Fig. 5E), demonstrating that mutating the predicted TCF binding sites in the tbx16 promoter eliminates Wnt responsiveness. Similar results were observed when multiple transgenic lines were produced using a 3.3 kb fragment of tbx16 that also includes the first intron (data not shown). We conclude that tbx16 is a direct target of Wnt signaling and that Wnt is essential for tbx16 expression.
Tbx16-mediated repression of ntl occurs indirectly through wnt3a
Wnt and ntl create a feedback loop, which acts to maintain the expression of both in the progenitor cells of the tailbud (Martin and Kimelman, 2008, 2010). We hypothesized that the indirect repression of ntl by Tbx16 that we observed (Fig. 3F) was mediated by changes in Wnt signaling. In this scenario, tbx16 could activate expression of a Wnt antagonist or repress Wnt gene transcription, either of which would cause loss of ntl expression. If we were correct, activation of the intracellular Wnt signaling pathway should rescue the Tbx16-mediated repression of ntl. To test this hypothesis, we crossed the HS:tbx16 and HS:caβ-catenin lines, heat shocked embryos at the 2 ss, and fixed them 6 h later. Following in situ hybridization, embryos were genotyped. As expected, in embryos with only ectopic tbx16 we saw reduced levels of ntl expression (Fig. 5H). In embryos with only caβ-catenin, we saw expansion of ntl expression (Fig. 5G). When both transgenes were present, ntl was also expanded, demonstrating that active Wnt signaling is capable of alleviating Tbx16-mediated repression of ntl (Fig. 5I). Additionally, when we injected tbx16 morpholino oligonucleotides with and without HS:caβ-catenin, in each case we saw an increase in ntl expression, indicating that Wnt signaling can induce ntl even in the absence of tbx16 (supplementary material Fig. S6).
We next focused on the transcription of specific Wnt genes, wnt3a and wnt8, which are expressed in the tailbud during posterior growth (Krauss et al., 1992). Transcription of wnt3a was reduced in both the HS:tbx16 and HS:tbx16-EnR lines (Fig. 5J-L), indicating that the reduction of wnt3a is due to repression by Tbx16. wnt8 was similarly reduced after expression of HS:tbx16, although the effect was more moderate (data not shown). Our results show that Tbx16 inhibits progenitor gene expression by acting as a direct repressor of sox2 and an indirect repressor of ntl mediated by effects on Wnt signaling.
Wnt signaling advances cells toward muscle differentiation
After establishing that Wnt activates tbx16, we asked if activation of canonical Wnt signaling is capable of advancing muscle differentiation, as Tbx16 does (Fig. 1). To focus specifically on cell-autonomous effects, we used the cell transplantation assay described above (Fig. 1A). Non-transgenic cells were capable of contributing muscle fibers to the entire length of the host tail (Fig. 6A), whereas cells positive for the HS:caβ-catenin transgene did not contribute to the most posterior somites, indicating that they are incapable of long-term residency in the tailbud as bipotential progenitor cells (Fig. 6B), similar to results observed with ectopic Tbx16 (Fig. 1B). Results of several replicates were compiled in a box plot and the differences in distribution were found to be statistically significant (Fig. 6C).
Fig. 6.
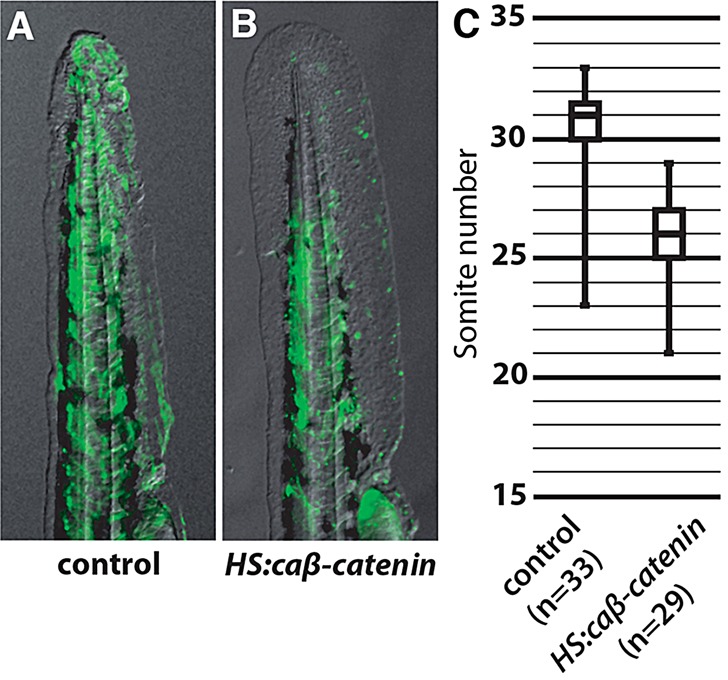
caβ-catenin expression causes early exit of cells from the progenitor zone. (A,B) Overlays of transplanted cells labeled with fluorescent dextran (green) onto a bright-field image from control (A) or HS:caβ-catenin (B) donor embryos into wild-type hosts. (C) Quantification of the most posterior somite occupied by a fluorescently labeled cell in host embryos. Data are significantly different (Mann-Whitney U-test, P<0.05).
We also examined the ability of transplanted cells expressing the HS:caβ-catenin transgene to cell-autonomously activate tbx16 expression within the progenitor pool, which normally only expresses ntl. Whereas control transplanted cells in the progenitor zone express ntl and not tbx16, transplanted cells expressing caβ-catenin showed induced tbx16 expression in the absence of ntl (supplementary material Fig. S7A-D). When we examined all transplanted cells in the tailbud, caβ-catenin-expressing cells showed a reduced number of cells expressing only ntl and an increase in cells expressing tbx16, when compared with controls, which increased as time post-heat shock continued (supplementary material Fig. S7E).
Taken together, these data identify a role for canonical Wnt signaling, acting through Tbx16, in advancing cells toward muscle differentiation and creating a regulatory network that represses the progenitor state.
DISCUSSION
Tbx16 creates a bistable switch for mesoderm in zebrafish bipotential cells
Although the presence of Wnt-regulated bipotential progenitors that form the posterior body in vertebrates is well established (Garriock et al., 2015; Gouti et al., 2014; Jurberg et al., 2014; Martin and Kimelman, 2012; Tsakiridis et al., 2014), how cells actually make the commitment to begin differentiation has remained unclear. Using a novel assay that examines the ability of single cells to contribute to specific somites during posterior growth, we established that Tbx16 is capable of advancing cells from the tailbud to become muscle. In pursuing this observation, we uncovered the regulatory logic that irreversibly commits cells to a mesodermal fate.
During posterior growth, because bipotential progenitors can remain in a progenitor state or adopt a mesodermal fate equally well, they can be described as bistable. In the progenitor steady state, cells express both the pro-neural gene sox2 and the mesodermal gene ntl, which is maintained by intermediate levels of Wnt signaling (Fig. 7, progenitor cell). Entrance of cells into a high-Wnt zone induces a change to levels of nuclear β-catenin, which has been shown to be more important than the absolute level of β-catenin (Goentoro and Kirschner, 2009), and induces expression of Tbx16. Activation of Tbx16 is then sufficient to commit cells to a mesodermal fate. Importantly, Tbx16 not only activates downstream mesodermal genes such as pcdh8 but, as shown here, also acts to repress pro-neural and progenitor genes (Fig. 7, transition to mesoderm cell). The multiple functions of Tbx16 place it centrally in the regulatory logic of a bistable switch, which requires a non-linear feedback circuit that creates two balanced states (Ferrell, 2002). Moderate levels of Wnt signaling, in this case, create one balanced state, and high levels of Wnt signaling are necessary to activate Tbx16, which then provides feedback and establishes the second self-perpetuating mesodermal state. Even after levels of Wnt signaling are reduced by Wnt antagonists in the presomitic mesoderm (Row and Kimelman, 2009), cells retain memory of their commitment to mesoderm because Tbx16 has irreversibly activated the mesodermal fate and inactivated the progenitor state (Fig. 7, mesodermal cell). Alternatively, when progenitors leave the tailbud and enter a zone of low Wnt, which is likely to be due to the presence of Wnt antagonists in the neural tube (Kagermeier-Schenk et al., 2011; Miyake et al., 2012), they adopt a neural fate (Fig. 7, neural cell). Prospective neural cells continue sox2 expression but lose ntl and fail to activate tbx16 and thus become committed to a neural fate. Through this regulatory network, mesodermal cells become clearly separate from the adjacent neural and progenitor cells.
Fig. 7.

High levels of Wnt and activation of Tbx16 irreversibly commit progenitors to a mesodermal fate. The status of the regulatory network in different cell types in the tailbud. Levels of Wnt exposure are indicated by shades of blue. Progenitor cells are exposed to a moderate level of Wnt (medium blue), which sustains ntl expression but is insufficient to activate tbx16. If cells leave the progenitor zone and are exposed to a low-Wnt environment (light blue), they become neural. Cells exposed to high levels of Wnt (dark blue) as they leave the progenitor zone activate tbx16 and transition to a mesodermal fate. Cells expressing tbx16 are irreversibly committed to the mesodermal fate even as levels of Wnt eventually drop to a level comparable to that of neural cells, since Tbx16 activates mesodermal genes and turns off the progenitor genes ntl and sox2 through its repressive function. The red arrow highlights the movement of cells from the tailbud toward the domain of early mesodermal differentiation, which we propose requires an additional factor.
Our results also explain the tbx16 (spadetail) and tbx16;msgn1 mutant phenotypes, in which undifferentiated cells accumulate in the tail (Fior et al., 2012; Griffin et al., 1998; Kimmel et al., 1989; Yabe and Takada, 2012). Without tbx16, not only are cells unable to activate mesodermal differentiation genes but they are also unable to repress progenitor genes. Thus, unlike cells that no longer see Wnt and can proceed to a neural fate (Martin and Kimelman, 2012), cells without tbx16 and its enhancing factor msgn1 maintain both ntl and sox2 and remain stuck in the progenitor state (supplementary material Fig. S8) (Griffin and Kimelman, 2002).
Tbx16 is a dual-function activator/repressor
Because it induces the expression of many genes, several of which have been shown to be direct targets, Tbx16 in zebrafish has been considered a transcriptional activator (Garnett et al., 2009). Similarly, the Xenopus Tbx16, VegT, also activates many genes, including those of the nodal-related family (Agius et al., 2000; Hyde and Old, 2000; Takahashi et al., 2000). Here, we show that zebrafish Tbx16 also functions as a repressor, since a repressive form of Tbx16 functions like wild-type Tbx16 to inhibit sox2 expression. Additionally, although Tbx16 also shuts off ntl expression, our data suggest that this occurs by repressing the expression of wnt3a, which breaks the autoregulatory loop between ntl and Wnt genes (Fig. 7) (Martin and Kimelman, 2008). We therefore place Tbx16 together with Tbx20 as T-box factors that are known to be dual-function activators/repressors (Stennard and Harvey, 2005).
Regulation of mesodermal fate by Wnt
Understanding the role of Wnt in the newly forming mesoderm of the posterior body is complicated, since loss of function results in both loss of ntl (and bra) within the progenitors and depletion of presomitic and somitic mesoderm (Martin and Kimelman, 2008, 2012). Here we show that tbx16 is a direct target of Wnt signaling and that this pathway is essential for tbx16 expression. Intriguingly, using nuclear β-catenin as a precise readout we find that Wnt signaling is present in a gradient, with moderate levels in the progenitor zone and high levels anterior and ventral to the progenitor zone in differentiating mesoderm that also expresses tbx16. Moreover, we show that ectopic activation of the Wnt pathway causes tbx16 to be expressed in the progenitor zone, suggesting that the activity of Wnt signaling is precisely regulated in the tailbud to ensure the correct tbx16 expression pattern. Finally, using our single-cell assay we demonstrate that activation of the Wnt pathway causes the same degree of acceleration of cells from the tailbud as does ectopic expression of tbx16. From all of these results, we suggest that feedback between wnt3a/wnt8 and ntl/bra acts to maintain the progenitor steady state with intermediate levels of Wnt, and then increased Wnt is used to activate tbx16 in some of the cells exiting the progenitor zone, committing them to a mesodermal fate (Fig. 7).
Recent work has highlighted the importance of Wnt signaling dynamics. For instance, a link has been established between repression of Wnt signaling, slowing of axis elongation and termination of the anterior-posterior axis (Denans et al., 2015). Additionally, Wnt signaling is involved in controlling somite length by providing positional information in differentiating mesoderm (Bajard et al., 2014). Our work adds to that of others by clearly establishing the importance of a dynamic Wnt response to commit bipotential cells to the mesodermal fate.
Additional factors to Tbx16 and canonical Wnt signaling may regulate cell exit from the progenitor zone
Heat shock activation of Tbx16 expression caused a rapid inhibition of progenitor genes and activation of mesodermal genes with peak effects occurring 4 h post-heat shock, consistent with peak levels of ectopic Tbx16 protein. Surprisingly, although ectopic expression of Tbx16 or Wnt causes cells to exit from the tailbud, it only sped up exit by approximately five somites, and we therefore suggest that an additional factor needs to work with Tbx16 to allow cells to physically leave the tailbud. Although, from our data, we cannot conclude which step of cell movement from the tailbud is blocked after cells have initiated the differentiation program, we suspect that, even with Wnt or Tbx16 overexpression, cells are held until an epithelial-to-mesenchymal transition (EMT) is initiated (red arrow in Fig. 7). This hypothesis is consistent with our previous observation that Tbx16 is essential for completion of EMT, but not for its initiation (Row et al., 2011). How EMT is triggered in fish is still unclear and merits further study.
Comparison with mouse
While recent evidence has established that the formation of the posterior body from bipotential cells is regulated by Wnt and is well conserved in fish and mouse (Gouti et al., 2014; Jurberg et al., 2014; Martin and Kimelman, 2012; Takemoto et al., 2011; Tsakiridis et al., 2014), interesting differences occur within this general framework. For example, whereas Msgn1 is essential for the formation of presomitic mesoderm in the mouse, in fish loss of msgn1 has virtually no effect unless tbx16 is also removed (Fior et al., 2012; Yabe and Takada, 2012). In mouse, which lacks an ortholog of tbx16 (Lardelli, 2003), a recent study has shown by overexpression that Msgn1 acts as a major regulator of presomitic mesoderm formation, capable of forcing bipotential cells to adopt a mesodermal fate (Chalamalasetty et al., 2014). Interestingly, Msgn1 directly activates expression of Tbx6 (Chalamalasetty et al., 2014), which is a member of the Tbx6/16 family (Ahn et al., 2012), and so the effects of Msgn1 overexpression in mouse are most likely to be a combination of an increase in both Msgn1 and Tbx6.
In mouse, loss of Tbx6 causes a phenotype that closely resembles that of zebrafish tbx16 mutants, and a large tailbud of undifferentiated tissue forms (Chapman and Papaioannou, 1998; Chapman et al., 2003; Nowotschin et al., 2012). As with zebrafish tbx16 mutants, loss of Tbx6 in mouse causes a reduction of Msgn1 and an increase in brachyury and Sox2 expression (Chapman and Papaioannou, 1998; Griffin and Kimelman, 2002; Nowotschin et al., 2012; Takemoto et al., 2011; our results), all supporting the close functional parallels between mouse Tbx6 and zebrafish tbx16 despite the sequence divergence (Ahn et al., 2012). It would be interesting to examine whether Msgn1 (and/or Tbx6) in the mouse is able to cell-autonomously drive cell exit from the progenitor zone as we have shown with zebrafish Tbx16. It would also be valuable to determine if Tbx6, and perhaps Msgn1, has the ability to act as a dual-function transcriptional activator/repressor.
Conclusions
Over the course of this work, we found a previously undescribed role for Tbx16 in advancing bipotential cell exit from the tailbud toward a mesodermal fate. We have also identified Wnt signaling as an activator of tbx16 that is necessary for its expression and showed that Tbx16 functions dually as an activator and a repressor. Finally, we propose that Wnt activation of tbx16 is the transitional node in a bistable switch, which takes cells from a steady progenitor state to a committed mesodermal fate. Our results emphasize the importance of temporally manipulating gene expression in single cells within a wild-type background using cell-autonomous regulators as a means to study the complex dynamics of cell behavior in vivo, since ubiquitous alterations in gene expression affect many different populations of cells and confound this type of analysis.
MATERIALS AND METHODS
Transgenic lines
All transgenic lines were created using Tol2 transposase (Kawakami et al., 2000). For the Tg(hsp70l:tbx16-2A-NLS-Kikume) line, full-length tbx16 was cloned without a STOP codon upstream of a 2A viral peptide (Provost et al., 2007) and Kikume Green-Red with a nuclear localization signal (NLS); genotyping was performed using the following primers (5′-3′): forward, AATTTCAGCCTCCCTCCTCC; reverse, ACTCATGCTTCGTTCCCAAG. The Tg(hsp70l:tx16-EnR-2A-NLS-Kikume) line was made similarly, with an Engrailed repressor domain inserted between tbx16 and the viral 2A sequence. The Tg(hsp70l:caβ-catenin-2A-TFP) line was described previously (Veldman et al., 2013); genotyping was performed using the following primers: forward, TGCTGTTGTGTTCCACCAAT; reverse, GCCAACGTTTGGTTCAGAAT. For the Tg(tbx16-1.2:TagRFP) line, a 1.2 kb fragment of the tbx16 locus upstream of, and including, the ATG was placed in front of TagRFP. To make the Tg(tbx16-1.2ΔTCF:TagRFP) line, two predicted TCF binding sites were altered (see supplementary material Fig. S5 for details). The distal site was changed from TTTGAT to TTacAT and the proximal site from TTCAAA to TTgAAt. This line also has a crystallin promoter driving the expression of GFP in the eyes to identify transgenic lines. Seven independently isolated reporter lines showed the same result as in Fig. 5C.
Morpholino treatment
Morpholino oligonucleotides (MOs) were used as follows: 1.1 ng of tbx16 MO1 and 0.58 ng of tbx16 MO2 from Lewis and Eisen (2004) and 2 ng of msgn1 MO from Fior et al. (2012) were injected into single-cell embryos, which were then incubated until they reached the 12 ss.
Heat shock, transplantation assay and statistical analysis
All heat shocks were performed with a mix of transgenic embryos and control siblings. Embryos were placed at 40°C in a circulating water bath for 30 min. Post-heat shock, embryos were sorted and allowed to grow at 30°C until the described time. For the transplantation assay, donor embryos were labeled with Rhodamine-dextran and cells were transplanted into a gastrula stage host embryo on the ventral side, approximately ten cell diameters from the margin. Based on fate-mapping studies, that position will cause the transplanted cells to become long-term residents of the bipotential progenitor cell population (Bouldin et al., 2014). Post-transplantation, embryos were grown to the appropriate stage for heat shock. HS:tbx16 embryos were heat shocked at both the 2 ss and the 10 ss and HS:caβ-catenin embryos were heat shocked at different time points (either bud stage, 6 ss or 8 ss), which also had no apparent effect on outcome. After heat shock, embryos were grown to 48 hpf and both DIC and fluorescent images were captured. The most posterior somite with a fluorescent cell that morphologically resembled a myofiber was recorded. Data were plotted using a box and whisker plot (with maximum and minimum values) and statistical significance was tested using a Mann-Whitney U-test with an a priori α of 0.05.
Whole-mount in situ hybridization and immunofluorescence
Alkaline phosphatase in situ hybridizations were performed as previously described (Griffin et al., 1995), with more than 15 embryos for each condition. Fluorescent in situ hybridization was performed as described by Lauter et al. (2011). Immunofluorescence was performed with anti-Tbx16 (anti-VegT from the ZIRC, 1:1000), anti-Sox2 (Epitomics, clone EPR3131, 1:200) and anti-β-catenin (BD Biosciences, clone 14, 1:200) and detected with the appropriate Alexa Fluor-conjugated secondary antibody (Molecular Probes). To separate nuclear staining from membrane staining, embryos processed for β-catenin immunofluorescence were counterstained with DAPI and imaged using an Olympus Fluoview 1200 laser scanning confocal microscope. Image processing was performed in ImageJ (NIH) and presented using the Fire lookup table as described previously (Row and Kimelman, 2009).
Supplementary Material
Acknowledgements
We thank Heather Stickney for critical comments on the manuscript. Didier Stainier provided the crystallin:GFP construct.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Funding
This work was supported by a National Institutes of Health grant [RO1GM079203] to D.K. and a Ruth Kirschstein National Research Service Award Fellowship [GM099306] to C.M.B. Deposited in PMC for release after 12 months.
Author contributions
C.M.B. and D.K. developed the approach. C.M.B., Y.-H.P., D.K., G.H.F., K.L.H., A.J.M. and A.D. performed experiments. C.M.B., Y.-H.P. and D.K. analyzed data. C.M.B. and D.K. prepared the manuscript. A.J.M. edited the manuscript prior to submission.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.124024/-/DC1
References
- Agius E., Oelgeschlager M., Wessely O., Kemp C. and Robertis E. M. D. (2000). Endodermal Nodal-related signals and mesoderm induction in Xenopus. Development 127, 1173-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn D., You K.-H. and Kim C.-H. (2012). Evolution of the tbx6/16 subfamily genes in vertebrates: insights from zebrafish. Mol. Biol. Evol. 29, 3959-3983. 10.1093/molbev/mss199 [DOI] [PubMed] [Google Scholar]
- Amacher S. L., Draper B. W., Summers B. R. and Kimmel C. B. (2002). The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development 129, 3311-3323. [DOI] [PubMed] [Google Scholar]
- Bajard L., Morelli L. G., Ares S., Pécréaux J., Jülicher F. and Oates A. C. (2014). Wnt-regulated dynamics of positional information in zebrafish somitogenesis. Development 141, 1381-1391. 10.1242/dev.093435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouldin C. M., Snelson C. D., Farr G. H. III and Kimelman D. (2014). Restricted expression of cdc25a in the tailbud is essential for formation of the zebrafish posterior body. Genes Dev. 28, 384-395. 10.1101/gad.233577.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. and Vincent S. D. (2009). Distinct and dynamic myogenic populations in the vertebrate embryo. Curr. Opin. Genet. Dev. 19, 444-453. 10.1016/j.gde.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Chalamalasetty R. B., Garriock R. J., Dunty W. C., Kennedy M. W., Jailwala P., Si H. and Yamaguchi T. P. (2014). Mesogenin 1 is a master regulator of paraxial presomitic mesoderm differentiation. Development 141, 4285-4297. 10.1242/dev.110908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D. L. and Papaioannou V. E. (1998). Three neural tubes in mouse embryos with mutations in the T-box gene Tbx6. Nature 391, 695-697. 10.1038/35624 [DOI] [PubMed] [Google Scholar]
- Chapman D. L., Cooper-Morgan A., Harrelson Z. and Papaioannou V. E. (2003). Critical role for Tbx6 in mesoderm specification in the mouse embryo. Mech. Dev. 120, 837-847. 10.1016/S0925-4773(03)00066-2 [DOI] [PubMed] [Google Scholar]
- Denans N., Iimura T. and Pourquié O. (2015). Hox genes control vertebrate body elongation by collinear Wnt repression. eLife 4, e04379 10.7554/eLife.04379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell J. E., Jr (2002). Self-perpetuating states in signal transduction: positive feedback, double-negative feedback and bistability. Curr. Opin. Cell Biol. 14, 140-148. 10.1016/S0955-0674(02)00314-9 [DOI] [PubMed] [Google Scholar]
- Fior R., Maxwell A. A., Ma T. P., Vezzaro A., Moens C. B., Amacher S. L., Lewis J. and Saúde L. (2012). The differentiation and movement of presomitic mesoderm progenitor cells are controlled by Mesogenin 1. Development 139, 4656-4665. 10.1242/dev.078923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnett A. T., Han T. M., Gilchrist M. J., Smith J. C., Eisen M. B., Wardle F. C. and Amacher S. L. (2009). Identification of direct T-box target genes in the developing zebrafish mesoderm. Development 136, 749-760. 10.1242/dev.024703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriock R. J., Chalamalasetty R. B., Kennedy M. W., Canizales L. C., Lewandoski M. and Yamaguchi T. P. (2015). Lineage tracing of neuromesodermal progenitors reveals novel Wnt-dependent roles in trunk progenitor cell maintenance and differentiation. Development 142, 1628-1638. 10.1242/dev.111922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goentoro L. and Kirschner M. W. (2009). Evidence that fold-change, and not absolute level, of beta-catenin dictates Wnt signaling. Mol. Cell 36, 872-884. 10.1016/j.molcel.2009.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouti M., Tsakiridis A., Wymeersch F. J., Huang Y., Kleinjung J., Wilson V. and Briscoe J. (2014). In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS Biol. 12, e1001937 10.1371/journal.pbio.1001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin K. J. P. and Kimelman D. (2002). One-Eyed Pinhead and Spadetail are essential for heart and somite formation. Nat. Cell Biol. 4, 821-825. 10.1038/ncb862 [DOI] [PubMed] [Google Scholar]
- Griffin K., Patient R. and Holder N. (1995). Analysis of FGF function in normal and no tail zebrafish embryos reveals separate mechanisms for formation of the trunk and the tail. Development 121, 2983-2994. [DOI] [PubMed] [Google Scholar]
- Griffin K. J., Amacher S. L., Kimmel C. B. and Kimelman D. (1998). Molecular identification of spadetail: regulation of zebrafish trunk and tail mesoderm formation by T-box genes. Development 125, 3379-3388. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Ho R. K., Walker C. and Kimmel C. B. (1993). Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell 75, 99-111. 10.1016/S0092-8674(05)80087-X [DOI] [PubMed] [Google Scholar]
- Ho R. K. and Kane D. A. (1990). Cell-autonomous action of zebrafish spt-1 mutation in specific mesodermal precursors. Nature 348, 728-730. 10.1038/348728a0 [DOI] [PubMed] [Google Scholar]
- Holley S. A. (2007). The genetics and embryology of zebrafish metamerism. Dev. Dyn. 236, 1422-1449. 10.1002/dvdy.21162 [DOI] [PubMed] [Google Scholar]
- Hug B., Walter V. and Grunwald D. J. (1997). tbx6, a Brachyury-related gene expressed by ventral mesendodermal precursors in the zebrafish embryo. Dev. Biol. 183, 61-73. 10.1006/dbio.1996.8490 [DOI] [PubMed] [Google Scholar]
- Hyde C. E. and Old R. W. (2000). Regulation of the early expression of the Xenopus nodal-related 1 gene, Xnr1. Development 127, 1221-1229. [DOI] [PubMed] [Google Scholar]
- Jurberg A. D., Aires R., Nóvoa A., Rowland J. E. and Mallo M. (2014). Compartment-dependent activities of Wnt3a/β-catenin signaling during vertebrate axial extension. Dev. Biol. 394, 253-263. 10.1016/j.ydbio.2014.08.012 [DOI] [PubMed] [Google Scholar]
- Kagermeier-Schenk B., Wehner D., Özhan-Kizil G., Yamamoto H., Li J., Kirchner K., Hoffmann C., Stern P., Kikuchi A., Schambony A. et al. (2011). Waif1/5T4 inhibits Wnt/β-catenin signaling and activates noncanonical Wnt pathways by modifying LRP6 subcellular localization. Dev. Cell 21, 1129-1143. 10.1016/j.devcel.2011.10.015 [DOI] [PubMed] [Google Scholar]
- Kanki J. P. and Ho R. K. (1997). The development of the posterior body in zebrafish. Development 124, 881-893. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Shima A. and Kawakami N. (2000). Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. USA 97, 11403-11408. 10.1073/pnas.97.21.11403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D. and Martin B. L. (2012). Anterior-posterior patterning in early development: three strategies. Wiley Interdiscip. Rev. Dev. Biol. 1, 253-266. 10.1002/wdev.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel C. B., Kane D. A., Walker C., Warga R. M. and Rothman M. B. (1989). A mutation that changes cell movement and cell fate in the zebrafish embryo. Nature 337, 358-362. 10.1038/337358a0 [DOI] [PubMed] [Google Scholar]
- Kimmel C. B., Warga R. M. and Schilling T. F. (1990). Origin and organization of the zebrafish fate map. Development 108, 581-594. [DOI] [PubMed] [Google Scholar]
- Krauss S., Korzh V., Fjose A. and Johansen T. (1992). Expression of four zebrafish wnt-related genes during embryogenesis. Development 116, 249-259. [DOI] [PubMed] [Google Scholar]
- Lardelli M. (2003). The evolutionary relationships of zebrafish genes tbx6, tbx16/spadetail and mga. Dev. Genes Evol. 213, 519-522. 10.1007/s00427-003-0348-2 [DOI] [PubMed] [Google Scholar]
- Lauter G., Söll I. and Hauptmann G. (2011). Multicolor fluorescent in situ hybridization to define abutting and overlapping gene expression in the embryonic zebrafish brain. Neural Develop. 6, 10 10.1186/1749-8104-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton A. K., Nandi A., Stulberg M. J., Dray N., Sneddon M. W., Pontius W., Emonet T. and Holley S. A. (2013). Regulated tissue fluidity steers zebrafish body elongation. Development 140, 573-582. 10.1242/dev.090381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis K. E. and Eisen J. S. (2004). Paraxial mesoderm specifies zebrafish primary motoneuron subtype identity. Development 131, 891-902. 10.1242/dev.00981 [DOI] [PubMed] [Google Scholar]
- Martin B. L. and Kimelman D. (2008). Regulation of canonical Wnt signaling by Brachyury is essential for posterior mesoderm formation. Dev. Cell 15, 121-133. 10.1016/j.devcel.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L. and Kimelman D. (2009). Wnt signaling and the evolution of embryonic posterior development. Curr. Biol. 19, R215-R219. 10.1016/j.cub.2009.01.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L. and Kimelman D. (2010). Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev. 24, 2778-2783. 10.1101/gad.1962910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. L. and Kimelman D. (2012). Canonical Wnt signaling dynamically controls multiple stem cell fate decisions during vertebrate body formation. Dev. Cell 22, 223-232. 10.1016/j.devcel.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A., Nihno S., Murakoshi Y., Satsuka A., Nakayama Y. and Itoh N. (2012). Neucrin, a novel secreted antagonist of canonical Wnt signaling, plays roles in developing neural tissues in zebrafish. Mech. Dev. 128, 577-590. 10.1016/j.mod.2012.01.001 [DOI] [PubMed] [Google Scholar]
- Nikaido M., Kawakami A., Sawada A., Furutani-Seiki M., Takeda H. and Araki K. (2002). Tbx24, encoding a T-box protein, is mutated in the zebrafish somite-segmentation mutant fused somites. Nat. Genet. 31, 195-199. 10.1038/ng899 [DOI] [PubMed] [Google Scholar]
- Nowotschin S., Ferrer-Vaquer A., Concepcion D., Papaioannou V. E. and Hadjantonakis A.-K. (2012). Interaction of Wnt3a, Msgn1 and Tbx6 in neural versus paraxial mesoderm lineage commitment and paraxial mesoderm differentiation in the mouse embryo. Dev. Biol. 367, 1-14. 10.1016/j.ydbio.2012.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda Y., Ogura E., Kondoh H. and Kamachi Y. (2010). B1 SOX coordinate cell specification with patterning and morphogenesis in the early zebrafish embryo. PLoS Genet. 6, e1000936 10.1371/journal.pgen.1000936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaioannou V. E. (2014). The T-box gene family: emerging roles in development, stem cells and cancer. Development 141, 3819-3833. 10.1242/dev.104471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquié O. (2011). Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell 145, 650-663. 10.1016/j.cell.2011.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provost E., Rhee J. and Leach S. D. (2007). Viral 2A peptides allow expression of multiple proteins from a single ORF in transgenic zebrafish embryos. Genesis 45, 625-629. 10.1002/dvg.20338 [DOI] [PubMed] [Google Scholar]
- Row R. H. and Kimelman D. (2009). Bmp inhibition is necessary for post-gastrulation patterning and morphogenesis of the zebrafish tailbud. Dev. Biol. 329, 55-63. 10.1016/j.ydbio.2009.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Row R. H., Maître J.-L., Martin B. L., Stockinger P., Heisenberg C.-P. and Kimelman D. (2011). Completion of the epithelial to mesenchymal transition in zebrafish mesoderm requires Spadetail. Dev. Biol. 354, 102-110. 10.1016/j.ydbio.2011.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada A., Fritz A., Jiang Y. J., Yamamoto A., Yamasu K., Kuroiwa A., Saga Y. and Takeda H. (2000). Zebrafish Mesp family genes, mesp-a and mesp-b are segmentally expressed in the presomitic mesoderm, and Mesp-b confers the anterior identity to the developing somites. Development 127, 1691-1702. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S., van Eeden F. J., Halpern M. E., Kimmel C. B. and Nusslein-Volhard C. (1994). no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development 120, 1009-1015. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Bae Y.-K., Muraoka O. and Hibi M. (2005). Interaction of Wnt and caudal-related genes in zebrafish posterior body formation. Dev. Biol. 279, 125-141. 10.1016/j.ydbio.2004.12.007 [DOI] [PubMed] [Google Scholar]
- Smith S. T. and Jaynes J. B. (1996). A conserved region of engrailed, shared among all en-, gsc-, Nk1-, Nk2- and msh-class homeoproteins, mediates active transcriptional repression in vivo. Development 122, 3141-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellabotte F. and Devoto S. H. (2007). The teleost dermomyotome. Dev. Dyn. 236, 2432-2443. 10.1002/dvdy.21253 [DOI] [PubMed] [Google Scholar]
- Stennard F. A. and Harvey R. P. (2005). T-box transcription factors and their roles in regulatory hierarchies in the developing heart. Development 132, 4897-4910. 10.1242/dev.02099 [DOI] [PubMed] [Google Scholar]
- Stulberg M. J., Lin A., Zhao H. and Holley S. A. (2012). Crosstalk between Fgf and Wnt signaling in the zebrafish tailbud. Dev. Biol. 369, 298-307. 10.1016/j.ydbio.2012.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeto D. P. and Kimelman D. (2006). The regulation of mesodermal progenitor cell commitment to somitogenesis subdivides the zebrafish body musculature into distinct domains. Genes Dev. 20, 1923-1932. 10.1101/gad.1435306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi S., Yokota C., Takano K., Tanegashima K., Onuma Y., Goto J. and Asashima M. (2000). Two novel nodal-related genes initiate early inductive events in Xenopus Nieuwkoop center. Development 127, 5319-5329. [DOI] [PubMed] [Google Scholar]
- Takemoto T., Uchikawa M., Yoshida M., Bell D. M., Lovell-Badge R., Papaioannou V. E. and Kondoh H. (2011). Tbx6-dependent Sox2 regulation determines neural or mesodermal fate in axial stem cells. Nature 470, 394-398. 10.1038/nature09729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe C. J., Weidinger G. and Moon R. T. (2005). Wnt/beta-catenin regulation of the Sp1-related transcription factor sp5l promotes tail development in zebrafish. Development 132, 1763-1772. 10.1242/dev.01733 [DOI] [PubMed] [Google Scholar]
- Tsakiridis A., Huang Y., Blin G., Skylaki S., Wymeersch F., Osorno R., Economou C., Karagianni E., Zhao S., Lowell S. et al. (2014). Distinct Wnt-driven primitive streak-like populations reflect in vivo lineage precursors. Development 141, 1209-1221. 10.1242/dev.101014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uriu K., Morelli L. G. and Oates A. C. (2014). Interplay between intercellular signaling and cell movement in development. Semin. Cell Dev. Biol. 35, 66-72. 10.1016/j.semcdb.2014.05.011 [DOI] [PubMed] [Google Scholar]
- Veldman M. B., Zhao C., Gomez G. A., Lindgren A. G., Huang H., Yang H., Yao S., Martin B. L., Kimelman D. and Lin S. (2013). Transdifferentiation of fast skeletal muscle into functional endothelium in vivo by transcription factor Etv2. PLoS Biol. 11, e1001590 10.1371/journal.pbio.1001590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warga R. M. and Nusslein-Volhard C. (1999). Origin and development of the zebrafish endoderm. Development 126, 827-838. [DOI] [PubMed] [Google Scholar]
- Wilson V., Olivera-Martinez I. and Storey K. G. (2009). Stem cells, signals and vertebrate body axis extension. Development 136, 1591-1604. 10.1242/dev.021246 [DOI] [PubMed] [Google Scholar]
- Yabe T. and Takada S. (2012). Mesogenin causes embryonic mesoderm progenitors to differentiate during development of zebrafish tail somites. Dev. Biol. 370, 213-222. 10.1016/j.ydbio.2012.07.029 [DOI] [PubMed] [Google Scholar]
- Yamamoto A., Amacher S. L., Kim S. H., Geissert D., Kimmel C. B. and De Robertis E. M. (1998). Zebrafish paraxial protocadherin is a downstream target of spadetail involved in morphogenesis of gastrula mesoderm. Development 125, 3389-3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


