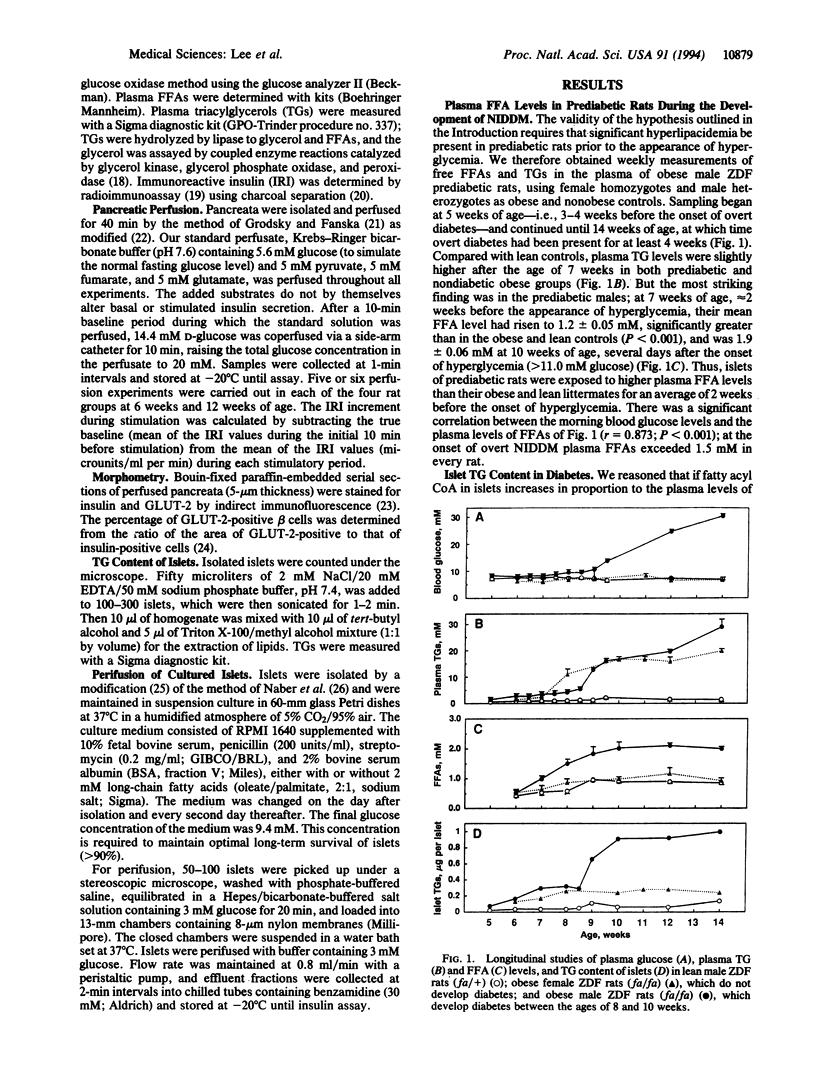
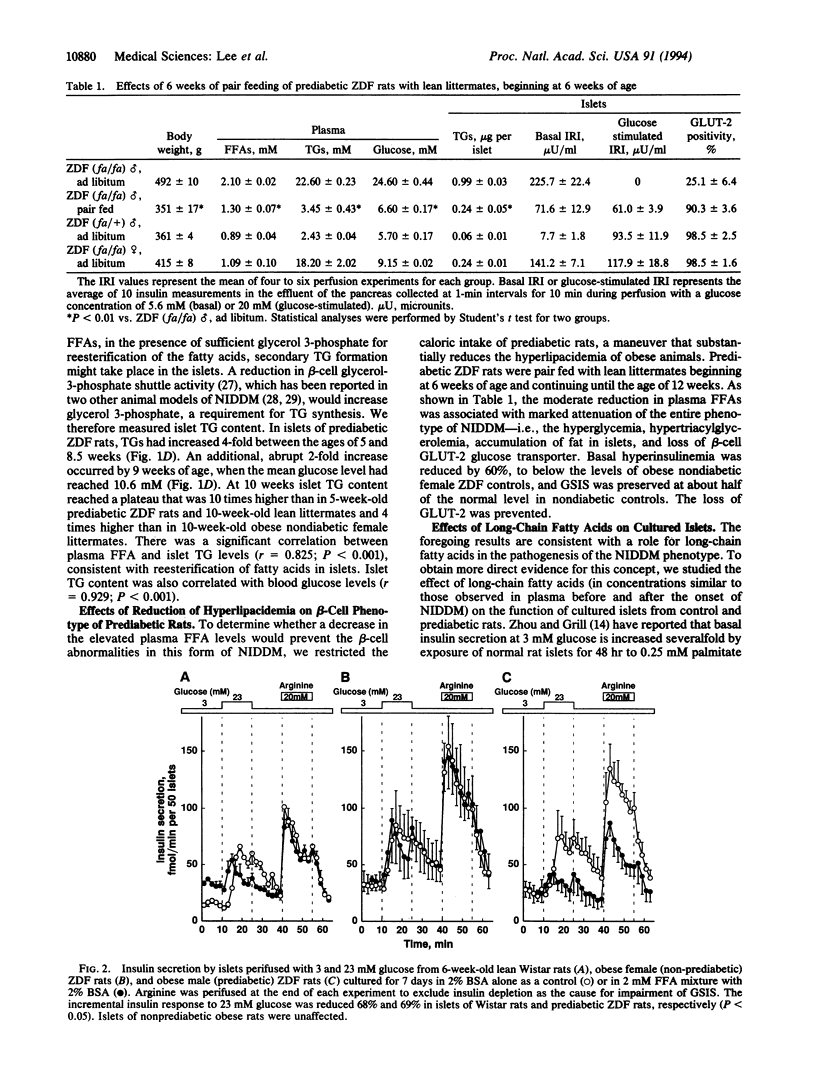
Abstract
Hyperinsulinemia, loss of glucose-stimulated insulin secretion (GSIS), and peripheral insulin resistance coexist in non-insulin-dependent diabetes mellitus (NIDDM). Because free fatty acids (FFA) can induce these same abnormalities, we studied their role in the pathogenesis of the NIDDM of obese Zucker diabetic fatty (ZDF-drt) rats from 5 weeks of age (before the onset of hyperglycemia) until 14 weeks. Two weeks prior to hyperglycemia, plasma FFA began to rise progressively, averaging 1.9 +/- 0.06 mM at the onset of hyperglycemia (P < 0.001 vs. controls). At this time GSIS was absent and beta-cell GLUT-2 glucose transporter was decreased. The triacylglycerol content of prediabetic islets rose to 10 times that of controls and was correlated with plasma FFA (r = 0.825; P < 0.001), which, in turn, was correlated with the plasma glucose concentration (r = 0.873; P < 0.001). Reduction of hyperlipacidemia to 1.3 +/- 0.07 mM by pair feeding with lean littermates reduced all beta-cell abnormalities and prevented hyperglycemia. Normal rat islets that had been cultured for 7 days in medium containing 2 mM FFA exhibited increased basal insulin secretion at 3 mM glucose, and first-phase GSIS was reduced by 68%; in prediabetic islets, first-phase GSIS was reduced by 69% by FFA. The results suggest a role for hyperlipacidemia in the pathogenesis of NIDDM; resistance to insulin-mediated antilipolysis is invoked to explain the high FFA despite hyperinsulinemia, and sensitivity of beta cells to hyperlipacedemia is invoked to explain the FFA-induced loss of GSIS.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berne C. The metabolism of lipids in mouse pancreatic islets. The oxidation of fatty acids and ketone bodies. Biochem J. 1975 Dec;152(3):661–666. doi: 10.1042/bj1520661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. J., Carlson M. G., Nurjhan N. Fat metabolism in human obesity. Am J Physiol. 1994 Apr;266(4 Pt 1):E600–E605. doi: 10.1152/ajpendo.1994.266.4.E600. [DOI] [PubMed] [Google Scholar]
- Capito K., Hansen S. E., Hedeskov C. J., Islin H., Thams P. Fat-induced changes in mouse pancreatic islet insulin secretion, insulin biosynthesis and glucose metabolism. Acta Diabetol. 1992;28(3-4):193–198. doi: 10.1007/BF00778997. [DOI] [PubMed] [Google Scholar]
- Chen S., Ogawa A., Ohneda M., Unger R. H., Foster D. W., McGarry J. D. More direct evidence for a malonyl-CoA-carnitine palmitoyltransferase I interaction as a key event in pancreatic beta-cell signaling. Diabetes. 1994 Jul;43(7):878–883. doi: 10.2337/diab.43.7.878. [DOI] [PubMed] [Google Scholar]
- Crespin S. R., Greenough W. B., 3rd, Steinberg D. Stimulation of insulin secretion by infusion of free fatty acids. J Clin Invest. 1969 Oct;48(10):1934–1943. doi: 10.1172/JCI106160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988 Jun;37(6):667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- Declercq P. E., Falck J. R., Kuwajima M., Tyminski H., Foster D. W., McGarry J. D. Characterization of the mitochondrial carnitine palmitoyltransferase enzyme system. I. Use of inhibitors. J Biol Chem. 1987 Jul 15;262(20):9812–9821. [PubMed] [Google Scholar]
- Elks M. L. Chronic perifusion of rat islets with palmitate suppresses glucose-stimulated insulin release. Endocrinology. 1993 Jul;133(1):208–214. doi: 10.1210/endo.133.1.8319569. [DOI] [PubMed] [Google Scholar]
- Giroix M. H., Baetens D., Rasschaert J., Leclercq-Meyer V., Sener A., Portha B., Malaisse W. J. Enzymic and metabolic anomalies in islets of diabetic rats: relationship to B cell mass. Endocrinology. 1992 May;130(5):2634–2640. doi: 10.1210/endo.130.5.1315252. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M., Fanska R. E. The in vitro perfused pancreas. Methods Enzymol. 1975;39:364–372. doi: 10.1016/s0076-6879(75)39033-2. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Hisatomi A., Maruyama H., Orci L., Vasko M., Unger R. H. Adrenergically mediated intrapancreatic control of the glucagon response to glucopenia in the isolated rat pancreas. J Clin Invest. 1985 Feb;75(2):420–426. doi: 10.1172/JCI111716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. H., Crider B. P., McCorkle K., Alford M., Unger R. H. Inhibition of glucose transport into rat islet cells by immunoglobulins from patients with new-onset insulin-dependent diabetes mellitus. N Engl J Med. 1990 Mar 8;322(10):653–659. doi: 10.1056/NEJM199003083221003. [DOI] [PubMed] [Google Scholar]
- Johnson J. H., Ogawa A., Chen L., Orci L., Newgard C. B., Alam T., Unger R. H. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science. 1990 Oct 26;250(4980):546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- MacDonald M. J. High content of mitochondrial glycerol-3-phosphate dehydrogenase in pancreatic islets and its inhibition by diazoxide. J Biol Chem. 1981 Aug 25;256(16):8287–8290. [PubMed] [Google Scholar]
- Madison L. L., Seyffert W. A., Jr, Unger R. H., Barker B. Effect on plasma free fatty acids on plasma glucagon and serum insulin concentrations. Metabolism. 1968 Apr;17(4):301–304. doi: 10.1016/0026-0495(68)90097-8. [DOI] [PubMed] [Google Scholar]
- McGarry J. D. Disordered metabolism in diabetes: have we underemphasized the fat component? J Cell Biochem. 1994;55 (Suppl):29–38. doi: 10.1002/jcb.240550005. [DOI] [PubMed] [Google Scholar]
- McGarry J. D. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992 Oct 30;258(5083):766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- McGowan M. W., Artiss J. D., Strandbergh D. R., Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983 Mar;29(3):538–542. [PubMed] [Google Scholar]
- Naber S. P., McDonald J. M., Jarett L., McDaniel M. L., Ludvigsen C. W., Lacy P. E. Preliminary characterization of calcium binding in islet-cell plasma membranes. Diabetologia. 1980 Nov;19(5):439–444. doi: 10.1007/BF00281823. [DOI] [PubMed] [Google Scholar]
- Ogawa A., Johnson J. H., Ohneda M., McAllister C. T., Inman L., Alam T., Unger R. H. Roles of insulin resistance and beta-cell dysfunction in dexamethasone-induced diabetes. J Clin Invest. 1992 Aug;90(2):497–504. doi: 10.1172/JCI115886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda M., Johnson J. H., Inman L. R., Chen L., Suzuki K., Goto Y., Alam T., Ravazzola M., Orci L., Unger R. H. GLUT2 expression and function in beta-cells of GK rats with NIDDM. Dissociation between reductions in glucose transport and glucose-stimulated insulin secretion. Diabetes. 1993 Jul;42(7):1065–1072. doi: 10.2337/diab.42.7.1065. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Baetens D., Inman L., Amherdt M., Peterson R. G., Newgard C. B., Johnson J. H., Unger R. H. Evidence that down-regulation of beta-cell glucose transporters in non-insulin-dependent diabetes may be the cause of diabetic hyperglycemia. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9953–9957. doi: 10.1073/pnas.87.24.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostenson C. G., Abdel-Halim S. M., Rasschaert J., Malaisse-Lagae F., Meuris S., Sener A., Efendic S., Malaisse W. J. Deficient activity of FAD-linked glycerophosphate dehydrogenase in islets of GK rats. Diabetologia. 1993 Aug;36(8):722–726. doi: 10.1007/BF00401142. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Benson J. W., Walter R. M., Ensinck J. W. Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J Clin Invest. 1976 Sep;58(3):565–570. doi: 10.1172/JCI108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako Y., Grill V. E. A 48-hour lipid infusion in the rat time-dependently inhibits glucose-induced insulin secretion and B cell oxidation through a process likely coupled to fatty acid oxidation. Endocrinology. 1990 Oct;127(4):1580–1589. doi: 10.1210/endo-127-4-1580. [DOI] [PubMed] [Google Scholar]
- Thorens B., Weir G. C., Leahy J. L., Lodish H. F., Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6492–6496. doi: 10.1073/pnas.87.17.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G. C. Non-insulin-dependent diabetes mellitus: interplay between B-cell inadequacy and insulin resistance. Am J Med. 1982 Oct;73(4):461–464. doi: 10.1016/0002-9343(82)90321-7. [DOI] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y. P., Grill V. E. Long-term exposure of rat pancreatic islets to fatty acids inhibits glucose-induced insulin secretion and biosynthesis through a glucose fatty acid cycle. J Clin Invest. 1994 Feb;93(2):870–876. doi: 10.1172/JCI117042. [DOI] [PMC free article] [PubMed] [Google Scholar]


