From the time of Darwin until about 1950, a controversy continued over whether selective stress induces mutations or only affects the relative reproductive success of organisms with different genotypes (30). The controversy was resolved by the classic experiments of Luria and Delbrück (27) and of Lederberg and Lederberg (25), who showed that some bacterial mutants arise prior to application of the selection that allows their detection and thus could not have been caused by selective conditions. However, these experiments used lethal selections and therefore did not eliminate the possibility that another fraction of total mutations might be formed in response to stress and be detected only by nonlethal selection. Shapiro and Cairns et al. reopened the controversy by pointing out this caveat and presenting data that seemed to support stress-induced mutation (7, 45).
Because very few genetic systems behave in ways that suggest stress-induced mutation, the rare cases that seem to exhibit such behavior have attracted close attention. In one case, mutants were later shown to preexist selection (14, 28, 29, 44). For the system devised by Cairns and Foster (5), we suggest that reversion occurs by a multistep process initiated prior to selection and the appearance of stress-induced mutagenesis results from growth under strong selection.
THE CAIRNS-FOSTER EXPERIMENT AND WHAT IT SEEMS TO SAY
The Cairns-Foster system employs a leaky lac (+1) frameshift mutation carried on an F′ plasmid in Escherichia coli (5). During nonselective growth, this mutation reverts at a rate of about 10−8/cell/generation. However, when 108 mutant cells are plated on minimal lactose medium, about 100 Lac+ revertant colonies accumulate over 6 days (12, 13). The initial models (directed mutation [DM] and hypermutation [HM]; see below) assumed that selection induces Lac+ revertants in the nongrowing parent population. This population showed little or no increase in associated mutations compared to the 100-fold increase in lac revertant number (12, 55), suggesting that mutation is directed preferentially to sites that improve growth. In later experiments, a fourfold increase in unselected mutations was seen in the parental Lac− population and interpreted as evidence for general hypermutability (3). However the effect on the parent population is small compared to the 100-fold stimulation of reversion and therefore supports the idea of directed mutation.
REGULATED MUTABILITY—DM OR HM?
The DM model suggested that cells sense the nature of the stress and direct mutation to targets that relieve it (7, 13). Two possible mechanisms for DM (9, 50, 51) were ultimately rejected for this system (13). The DM model fell from favor, but was not eliminated (in our opinion), when it was discovered that Lac+ revertants (but not the stressed population as a whole) pass through a temporary generally hypermutable state (55). Hall suggested the HM model, by which general mutagenesis might appear to be directed (18). He proposed a control mechanism that senses stress and responds by placing a small subset of the population in a general hypermutable state. Intense genome-wide mutagenesis continues until cells either acquire a lac+ reversion (and leave the hypermutable state) or die from lethal mutations. The HM model explained the apparent direction of mutation to beneficial targets—only Lac+ revertants survive mutagenesis. The model predicted that Lac+ revertants (but not the parent population) would show an increased frequency of associated unselected mutations, and that prediction was verified (55). According to HM, associated unselected mutations arise in the generally mutagenized subpopulation before the lac reversion occurs. Based on the mutagenesis associated with reversion, stress-induced general mutagenesis was extrapolated as a general feature of life (33, 39, 40). The proposed mechanism for regulating the mutation rate was said to be valuable because it produces mutations that relieve the inducing stress and thereby speed genetic adaptation.
THE HM MODEL IS QUANTITATIVELY IMPLAUSIBLE
Because the HM model requires Lac+ revertants to be generated from a small subpopulation (estimated at 105), it requires unrealistically intense mutagenesis (42). If selection is to produce 100 revertants from 105 nongrowing cells (10−3), then the mutation rate must increase 105-fold over that seen during unselected growth (10−8/cell/division). To our knowledge, such intense mutagenesis has never been demonstrated in resting cells.
Direct estimates suggest that the average mutation rate experienced by Lac+ revertants during selection actually increases 20- to 50-fold rather than 105 fold as required by HM (38, 49, 55). Application of the observed rate to 105 cells cannot explain even one lac revertant. The rate required to explain Lac reversion, if it could be achieved, would produce too many lethal mutations to generate the observed number of viable lac revertants (42). If more cells enter a hypermutable state, the model can explain more revertants but then fails to explain the apparent directedness of mutation. The HM model becomes even less attractive when one considers the evidence of Rosche and Foster (38) that 90% of Lac+ revertants arise with little or no general mutagenesis while 10% experience a 200-fold increase (average of 20-fold). Also contrary to HM, selection increases the yield of lac revertants even when general mutagenesis is prevented by a lexA(Ind− [unable to induce SOS]) or dinB mutation (see below). In strains with no general mutagenesis, the lac revertant yield is reduced only about fourfold (32, 47, 49). This is a very small effect when considered in the light of the amplification mutagenesis (AM) model (see below), which suggests that growth under selection increases the revertant yield about 104-fold (100 revertant colonies arise from a plated subpopulation of 106 duplication-bearing cells). We conclude that general mutagenesis is neither sufficient nor necessary to explain reversion under selection.
COMBINING THE DM AND HM MODELS
The strain used in the Cairns-Foster system carries the mutant lac allele on plasmid F′128, and this location is required for the effect of selection on lac reversion (15, 16, 48). A combination of the DM and HM models appears to underlie one explanation of why lac must be located on the F′ plasmid (4, 6). This model asserts that the reversion rate of a chromosomal lac allele is about 100-fold lower than that of the same lac allele on the F′128 plasmid (i.e., mutation is directed to the F′lac plasmid rather than to lac itself). The model further asserts that stress induces general mutagenesis regardless of lac position. Given these assertions, a stress-induced 100-fold increase in the general mutation rate would give a detectable number of revertants when lac is on F′128, where the basal rate is already high, but would cause very few revertants of a chromosomal mutant allele. Evidence supporting these two assertions was obtained using a particular mutant lac strain (38) but contradicts our results (41). A resolution of this conflict is suggested later. This combined model would seem to require an additional sort of directed mutation to explain the 90% of revertants that arise without general mutagenesis. Again, we suggest that the AM model offers a better explanation of the adaptive mutation phenomenon and the role played by the F′128 plasmid.
AM MODEL
The AM model abandons the idea of stationary-phase mutagenesis and proposes that mutations arise in cell populations growing under selection with no need for mutagenesis (directed or general). It proposes that preexisting cells with a lac duplication initiate clones that grow slowly on selective medium. Cells arise within these developing colonies that can grow faster because they acquire more lac copies (by gene amplification). The probability of a reversion event (a compensating −1 frameshift) is increased by addition of more target lac copies. This idea was initially supported by experiments with Salmonella (2) that have since been extended to E. coli (21) and further developed (47). The basic model and supporting evidence are listed below, and the proposed development of a revertant colony is diagrammed in Fig. 1. The observed general mutagenesis is discussed below.
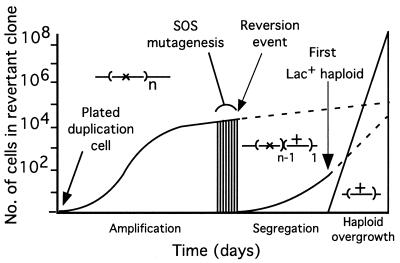
FIG. 1.
Multiple events precede reversion. A preexisting cell with a lac duplication initiates a clone on selective medium. Growth is slow but improves as selection favors cells with more lac copies. Reversion does not require mutagenesis. Selection then favors lac+ cells that lose mutant copies. Ultimately a lac+ haploid segregant arises and overgrows the clone. During growth, single-stranded DNA, produced from the plasmid transfer (tra) origin, induces SOS. General mutagenesis occurs in those clones whose lac amplification includes the dinB+ gene.
FEATURES OF AM
(i) The mutant lac allele is very leaky (1 to 2% of revertant activity). The ability of the mutant to grow on lactose is blocked (just barely) by scavenger cells (a lac deletion mutant) added in 10-fold excess; scavengers are presumed to compete for released carbon sources (e.g., galactose, acetate, and lactate) and thereby inhibit growth of the leaky revertible tester mutant (5).
(ii) A few plated tester cells (1%) carry a duplication of the leaky lac allele. Due to multiple copies of the leaky lac allele, these cells escape inhibition by scavengers and initiate slow-growing clones on selective medium.
(iii) Within each growing clone, selection favors faster-growing variants with higher lac amplification which arise by unequal recombination or possibly by a rolling-circle mechanism.
(iv) The growth rate attainable by amplification alone is limited by instability of the amplified array and by deleterious effects of amplifying genes other than lac on the F′ plasmid.
(v) Reversion occurs in these clones when the product of three factors reaches a sufficient level. These are the number of growing cells, the average number of lac copies per cell, and the reversion rate per lac copy per cell division. The product can reach a sufficient level with the standard unselected reversion rate of about 10−8/cell/division (37). For example, one reversion event would be expected for every 100 clones that attain a population size of 104 cells, each with 100 copies of lac.
(vi) Reversion produces one lac+ allele in a tandem array of mutant lac alleles.
(vii) Selection then holds the lac+ allele and favors loss of the other repeated units, which destabilize the lac+ allele and limit growth rate.
(viii) Stable haploid lac+ segregants arise, overgrow the clone, and predominate in the full-size revertant colony (108).
EXPERIMENTAL SUPPORT FOR AM
With very few exceptions (noted below) the AM model is consistent with evidence cited in support of the DM or HM model. In addition, the AM model is supported by more direct tests of its predictions, which contradict the DM and HM models. These results suggest that amplification is both necessary and sufficient to cause the observed revertants and can do so with no required change in mutation rate.
(i) Selection-stimulated lac reversion depends on the presence of lactose (13) and on the residual function of the mutant lac allele (2). This suggests that reversion requires growth under selection.
(ii) All revertant colonies that arise under selection contain some cells with a lac amplification and consequently an unstable Lac+ phenotype. These unstable Lac+ cells are not found in day 2 colonies, which are initiated by rare fully revertant cells that arose prior to selection (2, 21).
(iii) These unstable lac+ cells carry multiple copies of the lac operon (2, 21).
(iv) Unstable Lac+ cells are more common in tiny new revertant colonies and rarer in mature colonies that have been overgrown by stable haploid lac+ segregant cells (21).
(v) The unstable and stable Lac+ cells within the same colony are clonally related to each other, as expected if they arise serially in a clone initiated by a single duplication-bearing cell (2, 21).
(vi) A highly variable time is required for a visible microclone to become a full-size revertant colony with high levels of LacZ activity, as expected for a process that requires multiple stochastic events (21).
(vii) Reversion is strongly reduced by placing near lac a gene (tetA) that is toxic when amplified (21). Thus, lac amplification appears to be a necessary intermediate in the reversion process.
(viii) Revertant frequency is increased about 100-fold by placing a constructed lac duplication in the F′128 plasmid of the parent strain (47). This suggests that a lac duplication is carried by 1% of cells in the standard plated population, a frequency that is only slightly higher than the standard frequency of chromosomal duplications (1).
(ix) Reversion under selection requires RecA and RecBC functions, as expected if it involves amplification and segregation, which are recombination dependent and can be initiated by double strand DNA ends (19). The need for F′ plasmid tra (transfer replication) functions (15, 16) is consistent with their role in producing DNA ends that initiate recombination.
QUESTION OF SELECTION-INDUCED GENERAL MUTABILITY
The AM model requires no increase in mutation rate. However Lac+ revertants are generally mutagenized in the course of reversion (38, 49, 55). The source of this mutagenesis needs to be explained even though it is neither necessary nor sufficient to explain the lac revertants (38, 42, 49). Evidence described below suggests that mutagenesis is a side effect due to occasional coamplification of dinB+ with lac (47).
The observed general mutagenesis requires induction of the SOS (DNA repair) regulon and in particular the included DinB error-prone DNA polymerase (31, 32, 47, 54). Thus selection seems to induce SOS mutagenesis in this system, even though such induction is not a general response of bacteria to starvation (22). The signal for SOS induction is known be single-stranded DNA, which interacts with RecA protein and thereby stimulates cleavage of LexA, the SOS repressor (57). In understanding SOS mutagenesis in this system, it must be remembered that the strain used carries lac on F′128, a conjugative plasmid that expresses its transfer replication origin constitutively. This transfer replication origin generates 5′-ended single strands of DNA that we think are responsible, at least in part, for SOS induction (47). Fragments of DNA released from unstable amplified arrays may also contribute to SOS induction (21). However, DinB-dependent mutagenesis has been seen only when the dinB+ gene is highly overexpressed (23, 56) and is not caused by a single chromosomal dinB copy, even when SOS is constitutively expressed (47). In the Cairns-Foster system, a dinB+ allele is located near the mutant lac gene on the F′128 plasmid, and stress-induced mutagenesis requires that these two genes be located (in cis) on the same conjugative plasmid; the chromosomal dinB+ gene makes little or no contribution (47).
The increase in dinB+ gene copy number needed for mutagenesis is provided by coamplification of dinB+ with lac. General mutagenesis occurs only in the subset of clones whose amplified region includes dinB+ as well as lac (47). A constructed dinB+-lac duplication added to the parent strain causes a 104-fold increase in revertant number with a concomitant increase in associated mutagenesis. This should be compared to the 100-fold increase in reversion (with little mutagenesis) caused by a duplication including lac but not dinB+. We suggest that the mutagenized 10% of revertant clones inferred by Rosche and Foster are those in which dinB+ is accidentally coamplified with lac. In the majority of clones (90%), the lac amplification does not include dinB+ and selection enhances revertant frequency solely by raising the number of the lac target sequences, as outlined above for the basic AM model (47).
HOW NATURAL SELECTION ACHIEVES THE APPEARANCE OF DM
In the basic AM model, stress acts only as an agent of natural selection, favoring a succession of progressively faster-growing cell types within a developing clone. The model proposes that each revertant colony is initiated by a preexisting cell carrying a lac duplication (i.e., Lederberg, Luria, and Delbrück were right). If one is unaware of lac amplification and improving growth within each developing colony, selection appears to create full lac+ revertants from a lawn of nongrowing lac mutant cells, which would require some kind of mutagenesis. If, however, one considers the intervening growth within developing colonies, revertants can be explained by increased lac copy number and no required mutagenesis.
The amplification process appears to direct mutations to the growth-limiting base pairs because lac is the only region whose amplification improves growth under selection and because only the copy with the revertant lac+ allele remains after segregation. Thus, the basic AM process (without dinB+ amplification) is not expected to increase the frequency of unselected mutations associated with lac+ reversion, even ones closely linked to lac. A situation in which selection may stimulate mutations near lac that arise unassociated with lac reversion is noted below (question 2).
In essence, growth and selection divide the reversion process into small steps. Without growth (or during growth without selection), the end point (a full Lac+ phenotype) can be attained only by a rare discontinuous event (lac → lac+). With growth and selection, very common events (e.g., added gene copies) can contribute small growth improvements and the cells added by growth of each successive subclone make the next step more likely. Thus, the final goal is reached by a series of nested clonal expansions: Lac−−− → Lac−−+ → Lac−++ → Lac+++.
WHY WAS GROWTH NOT DETECTED EARLIER?
The DM and HM models assume that mutations arise in the parent Lac− cell population. This parent population was shown to be nongrowing by periodic sampling of areas of the agar surface that include no visible colonies (5) (Fig. 2). However, this population is irrelevant if reversion occurs within slow-growing colonies. The samples assayed may include some microscopic reverting clones that will become visible in the immediate future (a moving window of constant size); it misses all the cells buried within colonies that have already appeared and accumulated with time. The series of genetic events required by the AM model (multiple steps of amplification, reversion, and segregation) are completed within the few cell generations that occur prior to appearance of the colony—about 20 generations. This remarkable feat is possible because the lac genes in question are on F′128.
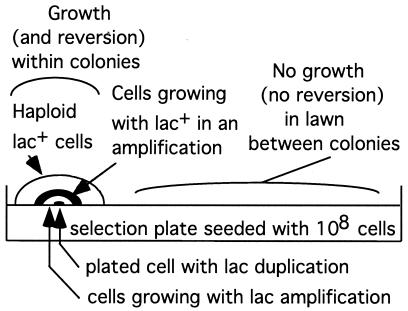
FIG. 2.
Growth (and reversion) occurs within colonies. The lawn of 108 parental cells is not growing and not producing revertants. Growth and reversion occur within clones initiated by cells with a duplication that arose prior to plating on selective medium.
ROLE OF CONJUGATIVE PLASMIDS
Stimulation of reversion under selection in the Cairns-Foster system requires that the lac operon be located on a conjugative plasmid, rather than in the chromosome (15, 36, 47, 48). All the events central to the AM model—duplication, amplification, segregation, and the side effect of SOS mutagenesis—are likely to be stimulated by the single-stranded DNA and DNA ends produced by the transfer replication origin of the conjugative plasmid (21, 47). The transfer functions (tra) of these plasmids are known to cause intense recombination (8, 26, 43, 52), and conjugative plasmids are prone to amplification (35, 46, 53). Selection promotes lac reversion very poorly when lac is in the chromosome (15, 17, 36, 48), and selective amplification of chromosomal genes has been shown to be a slow process (34), presumably because of a paucity of DNA ends. We suggest that the conjugative plasmid accelerates every step in a process that can occur more slowly in any genetic system.
GENERAL APPLICABILITY OF AM
Because of its reliance on many special features of the particular strain used, one might conclude that the Cairns-Foster system can teach us very little about the general issue of mutation under selection. On the contrary, we submit that the idiosyncrasies of this system helped reveal a process that is fundamentally important and generally applicable. The genetic events underlying the AM model are standard ones expected to occur in any genetic system but usually at a slower rate. Because lac is located on the F′128 plasmid, every step of the process is accelerated and end products can be completed and detected within a few days. We suggest that the underlying process—duplication, amplification, reversion, and segregation—contributes to genetic adaptation in many biological contexts.
QUESTIONS ABOUT THE AM MODEL.
(i) Is there a problem identifying unstable lac+ cells within revertant colonies?
In our experience, every late-appearing revertant colony (after day 2) contains both stable and unstable Lac+ cells that are clonally related (21). In clones that experience general mutagenesis, reversion events occur early in colony development and haploid Lac+ cells overgrow the early population of unstable Lac+ cells. Without general mutagenesis, the same process operates but reversion occurs later in colony development and unstable Lac+ cells predominate. If the heterogeneity in colony composition is missed, it appears that there are two colony types. Colonies that appeared to have only stable Lac+ cells were attributed to stress-induced mutagenesis, and those that seemed to contain only unstable Lac+ cells were attributed to stress-induced amplifications (20). We suggest that these experiments missed the minority cell types because too few cells were tested and the conditions used to score unstable Lac+ phenotypes (Luria-Bertani plates) made the characteristic fine blue and white colony sectors difficult to see; color and contrast are more intense on nutrient broth plates with 40 μg of X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside)/ml (2).
(ii) If reversion is initiated by duplications that arise during nonselective pregrowth, why does revertant number not show a Luria-Delbrück frequency distribution?
If the Cairns-Foster experiment is repeated using many independent cultures, the number of revertants arising on selective plates for these cultures appears Poisson distributed, suggesting that revertants formed after plating (5). A Luria-Delbrück distribution is expected for a standard mutation arising during nonselective growth. However, Luria-Delbrück statistics apply only to stable mutations that accumulate during nonselective growth and show essentially no reversion. Because duplications are highly reversible, their frequency is not subject to the jackpots that characterize a Luria-Delbrück distribution but rather comes to an equilibrium value dictated by the relative rates of duplication formation and segregation. We suggest that all of the independent cultures have nearly the same frequency of lac duplications and this leads to a Poisson rather a Luria-Delbrück distribution of revertant numbers on selective medium.
(iii) Is there evidence that selection directs mutation to F′128?
A frameshift mutation (tetA), within a Tn10 element thought to be inserted far from lac on the F′ plasmid, reverts with increased frequency (to tetracycline resistance) during starvation on lactose (11); very little stimulation was seen for a similar mutant element inserted in the chromosome (3). These results suggested a higher mutation rate for the F′ plasmid under selection (11). However, the critical Tn10 element (with its tetA mutation) is actually inserted between lac and dinB, in the mhpC gene (24), which is frequently included in lac amplifications (17). Thus coamplification of lac and tetA (occasionally with dinB+) may provide extra copies of the tetA gene and explain the enhanced tetA reversion during lac selection. That is, many microclones growing by virtue of a selected lac amplification, also have multiple copies of tetA (and occasionally an increased mutation rate). Clones with more Tn10 copies are expected to show an increased probability of reverting to tetracycline resistance, and this can occur in microclones that have not yet acquired a lac+ reversion. Due to segregation, the excess Tetr revertants are not expected to be phenotypically Lac+. Because amplification can explain these observations, the question remains open whether or not DinB mutagenesis is more intense on the plasmid than on the chromosome (i.e., directed). However, since SOS induction and DinB make such a small contribution to reversion, we feel this is a minor question.
(iv) Is there a conflict of data on the behavior of a chromosomal lac allele?
We have moved the mutant lac allele to 30 different chromosomal sites and have seen essentially the same reversion frequency under nonselective conditions as that of lac on the F′128 plasmid; we have never seen selection-induced general mutagenesis in strains carrying lac in the chromosome. These results fit with evidence that general mutagenesis requires lac and dinB+ to be located in cis on a conjugative plasmid (47). In conflict with our results, the chromosomal lac mutation in a particular strain was seen to revert at a 100-fold-lower rate than that seen on F′128 and that reversion was associated with general mutagenesis (38). We suggest that problem may lie in the structure of this particular strain.
The lac allele used in the Cairns-Foster system includes three mutations—one mutation (iq) increases transcription from the lacI promoter, a deletion fuses the lacI and lacZ reading frames, and a +1 frameshift in the lacI sequence causes the (leaky) Lac− phenotype. This triply mutant allele was constructed on the F′128 plasmid and then moved to the chromosome by recombination (17, 36, 38). We suggest that the three parts of this allele may dissociate frequently when subjected to the intense recombination that occurs between the F′ plasmid and the chromosome (26, 43, 52); the strain used for lac reversion shows evidence of this intense recombination (47). In moving the lac region from F′128 to the chromosome, a problem is created because of a dinB-lac duplication join point carried by this plasmid (24). This join point is expected to force frequent recombination within the lac region. We suspect that some E. coli strains with the lac allele in the chromosome have lost the iq mutation, and are therefore compromised in their ability to grow and revert. Similarly some of the strains may inherit a chromosomal dinB-lac duplication. In our experiments, the three-part lac allele was placed within a Mud element and transposed as a block to various genomic sites in strains without a lac region (48).
(v) Why did spreading experiments not increase revertant number?
To test the AM model, 106 (rather than 108) cells were plated on selective medium and incubated. Plates with no revertants were respread by moving a sterile glass bar through an arc on the plate surface to distribute cells within microscopic colonies (12). One might expect a line of new colonies to appear in the wake of each distributed microcolony, but this was not seen. In the light of later results, this result can be understood. An individual cell with a lac duplication has a very low probability of generating a visible revertant colony (100 colonies arise from 106 plated duplication-bearing cells). Most of the initial clones may segregate to extinction or be extensively delayed in reaching a visible size. The numbers of cells in clones distributed by spreading are very small (less than 104), and the distributed individual cells have very little chance of making a visible colony (about 10−4). The observed results seem consistent with AM.
SUMMARY
The AM model is consistent with a large body of experimental data obtained by others (10, 39) and is supported by additional experiments designed to test it more directly (2, 16, 21). This model explains how growth under selection can increase the probability of selected mutations—by adding copies of the mutational target. The observed general mutagenesis is explained as a nonessential side effect due to occasional coamplification of lac with the nearby dinB+ gene. We suggest the Cairns-Foster system has made a major contribution by revealing a genetic mechanism by which growth under strong selection can enhance production of adaptive mutations without changing either the rate or target specificity of mutation. Because AM relies on standard genetic events, it is likely to operate in any organism and contribute to genetic adaptation in many biological situations.
Acknowledgments
This work was supported in part by Public Health Service grant GM27068 from the National Institutes of Health to J.R.R. and by the Swedish Research Council (D.I.A.).
We thank Otto Berg, Ulfar Bergthorsson, Kim Bunny, Shawn Gerum, Eric Kofoid, Elisabeth Kugelberg, Mats Pettersson, Frederick Roth, Jon Seger, Kavitha Sivaraman, and Sue Slechta for their contributions to development of the AM model.
REFERENCES
- 1.Anderson, R. P., and J. R. Roth. 1981. Spontaneous tandem genetic duplications in Salmonella typhimurium arise by unequal recombination between ribosomal RNA (rrn) cistrons. Proc. Natl. Acad. Sci. USA 78:3113-3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, D. I., E. S. Slechta, and J. R. Roth. 1998. Evidence that gene amplification underlies adaptive mutability of the bacterial lac operon. Science 282:1133-1135. [DOI] [PubMed] [Google Scholar]
- 3.Bull, H. J., M.-J. Lombardo, and S. M. Rosenberg. 2001. Stationary-phase mutation in the bacterial chromosome: recombination protein and DNA polymerase IV dependence. Proc. Natl. Acad. Sci. USA 98:8334-8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cairns, J. 2000. The contribution of bacterial hypermutators to mutation in stationary phase. Genetics 156:923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cairns, J., and P. L. Foster. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cairns, J., and P. L. Foster. 2003. The risk of lethals for hypermutating bacteria in stationary phase. Genetics 165:2317-2318. [DOI] [PMC free article] [PubMed]
- 7.Cairns, J., J. Overbaugh, and S. Miller. 1988. The origin of mutants. Nature 335:142-145. [DOI] [PubMed] [Google Scholar]
- 8.Carter, J. R., and R. D. Porter. 1991. traY and traI are required for oriT-dependent enhanced recombination between lac-containing plasmids and lambda plac5. J. Bacteriol. 173:1027-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis, B. D. 1989. Transcriptional bias: a non-Lamarckian mechanism for substrate-induced mutations. Proc. Natl. Acad. Sci. USA 86:5005-5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, P. L. 1999. Mechanisms of stationary phase mutation: a decade of adaptive mutation. Annu. Rev. Genet. 33:57-88 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foster, P. L. 1997. Nonadaptive mutations occur on the F′ episome during adaptive mutation conditions in Escherichia coli. J. Bacteriol. 179:1550-1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster, P. L. 1994. Population dynamics of a Lac− strain of Escherichia coli during selection for lactose utilization. Genetics 138:253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster, P. L., and J. Cairns. 1992. Mechanisms of directed mutation. Genetics 131:783-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster, P. L., and J. Cairns. 1994. The occurrence of heritable Mu excisions in starving cells of Escherichia coli. EMBO J. 13:5240-5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster, P. L., and J. M. Trimarchi. 1995. Adaptive reversion of an episomal frameshift mutation in Escherichia coli requires conjugal functions but not actual conjugation. Proc. Natl. Acad. Sci. USA 92:5487-5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galitski, T., and J. R. Roth. 1995. Evidence that F plasmid transfer replication underlies apparent adaptive mutation. Science 268:421-423. [DOI] [PubMed] [Google Scholar]
- 17.Godoy, V. G., and M. S. Fox. 2000. Transposon stability and a role for conjugational transfer in adaptive mutability. Proc. Natl. Acad. Sci. USA 97:7393-7398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hall, B. G. 1990. Spontaneous point mutations that occur more often when advantageous than when neutral. Genetics 126:5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris, R. S., S. Longerich, and S. M. Rosenberg. 1994. Recombination in adaptive mutation. Science 264:258-260. [DOI] [PubMed] [Google Scholar]
- 20.Hastings, P. J., H. J. Bull, J. R. Klump, and S. M. Rosenberg. 2000. Adaptive amplification. An inducible chromosomal instability mechanism. Cell 103:723-731. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson, H., E. S. Slechta, U. Bergthorsson, D. I. Andersson, and J. R. Roth. 2002. Amplification mutagenesis: evidence that growth with a selected gene amplification causes adaptive mutation and hyper-mutability. Proc. Natl. Acad. Sci. USA 99:2164-2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes, D., and D. I. Andersson. 1997. Carbon starvation of Salmonella typhimurium does not cause a general increase of mutation rates. J. Bacteriol. 179:6688-6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim, S. R., K. Matsui, M. Yamada, P. Gruz, and T. Nohmi. 2001. Roles of chromosomal and episomal dinB genes encoding DNA pol IV in targeted and untargeted mutagenesis in Escherichia coli. Mol. Genet. Genomics 266:207-215. [DOI] [PubMed] [Google Scholar]
- 24.Kofoid, E., U. Bergthorsson, E. S. Slechta, and J. R. Roth. 2003. Formation of an F′ plasmid by recombination between imperfectly repeated chromosomal Rep sequences: a closer look at an old friend (F′128 pro lac). J. Bacteriol. 185:660-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lederberg, J., and E. M. Lederberg. 1952. Replica plating and indirect selection of bacterial mutants. J. Bacteriol. 63:399-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luisi-DeLuca, C., R. D. Porter, and W. D. Taylor. 1984. Stimulation of recombination between homologous sequences on plasmid DNA and chromosomal DNA in Escherichia coli by N-acetoxy-2-acetylaminofluorene. Proc. Natl. Acad. Sci. USA 81:2831-2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luria, S. E., and M. Delbrück. 1943. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28:491-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maenhaut-Michel, G., C. E. Blake, D. R. Leach, and J. A. Shapiro. 1997. Different structures of selected and unselected araB-lacZ fusions. Mol. Microbiol. 23:1133-1145. [DOI] [PubMed] [Google Scholar]
- 29.Maenhaut-Michel, G., and J. A. Shapiro. 1994. The roles of starvation and selective substrates in the emergence of araB-lacZ fusion clones. EMBO J. 13:5229-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayr, E. 1982. The growth of biological thought: diversity, evolution and inheritance. Harvard University Press, Cambridge, Mass.
- 31.McKenzie, G. J., R. S. Harris, P. L. Lee, and S. M. Rosenberg. 2000. The SOS response regulates adaptive mutation. Proc. Natl. Acad. Sci. USA 97:6646-6651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McKenzie, G. J., P. L. Lee, M.-J. Lombardo, P. J. Hastings, and S. M. Rosenberg. 2001. SOS mutator DNA polymerase IV functions in adaptive mutation and not adaptive amplification. Mol. Cell 7:571-579. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie, G. J., and S. M. Rosenberg. 2001. Adaptive mutations, mutator DNA polymerases and genetic change strategies of pathogens. Curr. Opin. Microbiol. 4:586-594. [DOI] [PubMed] [Google Scholar]
- 34.Normark, S., T. Edlund, T. Grundström, S. Bergström, and H. Wolf-Watz. 1977. Escherichia coli K12 mutants hyperproducing chromosomal beta-lactamase by gene repetitions. J. Bacteriol. 132:912-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson, B. C., and R. H. Rownd. 1985. Drug resistance gene amplification of plasmid NR1 derivatives with various amounts of resistance determinant DNA. J. Bacteriol. 161:1042-1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radicella, J. P., P. U. Park, and M. S. Fox. 1995. Adaptive mutation in Escherichia coli: A role for conjugation. Science 268:418-420. [DOI] [PubMed] [Google Scholar]
- 37.Rosche, W. A., and P. L. Foster. 2000. Determining mutation rates in bacterial populations. Methods 20:4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosche, W. A., and P. L. Foster. 1999. The role of transient hypermutators in adaptive mutation in Escherichia coli. Proc. Natl. Acad. Sci. USA 96:6862-6867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosenberg, S. 2001. Evolving responsively: adaptive mutation. Nat. Rev. Genet. 2:504-514. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg, S. M. 1997. Mutation for survival. Curr. Opin. Genet. Dev. 7:829-834. [DOI] [PubMed] [Google Scholar]
- 41.Roth, J. R., E. Kofoid, F. P. Roth, O. G. Berg, J. Seger, and D. I. Andersson. 2003. Adaptive mutation requires no mutagenesis—only growth under selection—a response. Genetics 165:2319-2321.
- 42.Roth, J. R., E. Kofoid, F. P. Roth, O. G. Berg, J. Seger, and D. I. Andersson. 2003. Regulating general mutation rates: examination of the hypermutable state model for Cairnsian adaptive mutation. Genetics 163:1483-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seifert, H. S., and R. D. Porter. 1984. Enhanced recombination between lambda plac5 and mini-F-lac: the tra regulon is required for recombination enhancement. Mol. Gen. Genet. 193:269-274. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro, J. A. 1999. Genome system architecture and natural genetic engineering in evolution. Ann. N. Y. Acad. Sci. 870:23-35. [DOI] [PubMed] [Google Scholar]
- 45.Shapiro, J. A. 1984. Observations on the formation of clones containing araB-lacZ cistron fusions. Mol. Gen. Genet. 194:79-90. [DOI] [PubMed] [Google Scholar]
- 46.Silver, L., M. Chandler, H. E. Lane, and L. Caro. 1980. Production of extrachromosomal r-determinant circles from integrated R100.1: involvement of the E. coli recombination system. Mol. Gen. Genet. 179:565-571. [DOI] [PubMed] [Google Scholar]
- 47.Slechta, E. S., K. L. Bunny, E. Kugelberg, E. Kofoid, D. I. Andersson, and J. R. Roth. 2003. Adaptive mutation: general mutagenesis is not a programmed response to stress, but results from rare co-amplification of dinB with lac. Proc. Natl. Acad. Sci. USA 100:12847-12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slechta, S., J. Harold, D. I. Andersson, and J. R. Roth. 2002. Effect of genome position on reversion during growth under selection. Mol. Microbiol. 44:1017-1032. [DOI] [PubMed] [Google Scholar]
- 49.Slechta, S., J. Liu, D. I. Andersson, and J. R. Roth. 2002. Evidence that selected amplification of a bacterial lac frameshift allele stimulates Lac(+) reversion (adaptive mutation) with or without general hypermutability. Genetics 161:945-956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stahl, F. W. 1990. Genetics. If it smells like a unicorn… Nature 346:791. [DOI] [PubMed] [Google Scholar]
- 51.Stahl, F. W. 1988. News and views: a unicorn in the garden. Nature 355:112-113. [DOI] [PubMed] [Google Scholar]
- 52.Syvanen, M., J. D. Hopkins, T. J. Griffin, T. Y. Liang, K. Ippen-Ihler, and R. Kolodner. 1986. Stimulation of precise excision and recombination by conjugal proficient F′ plasmids. Mol. Gen. Genet. 203:1-7. [DOI] [PubMed] [Google Scholar]
- 53.Tlsty, T. D., A. M. Albertini, and J. H. Miller. 1984. Gene amplification in the lac region of E. coli. Cell 37:217-224. [DOI] [PubMed] [Google Scholar]
- 54.Tompkins, J. D., J. L. Nelson, J. C. Hazel, S. L. Leugers, J. D. Stumpf, and P. L. Foster. 2003. Error-prone polymerase, DNA polymerase IV, is responsible for transient hypermutation during adaptive mutation in Escherichia coli. J. Bacteriol. 185:3469-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torkelson, J., R. S. Harris, M.-J. Lombardo, J. Nagendran, C. Thulin, and S. M. Rosenberg. 1997. Genome-wide hypermutation in a subpopulation of stationary-phase cells underlies recombination-dependent adaptive mutation. EMBO J. 16:3303-3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wagner, J., and T. Nohmi. 2000. Escherichia coli DNA polymerase IV mutator activity: genetic requirements and mutational specificity. J. Bacteriol. 182:4587-4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Walker, G. 1996. The SOS response of Escherichia coli, p. 1400-1416. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed., vol. 1. ASM Press, Washington, D.C. [Google Scholar]