Abstract
Adenovirus-mediated apoptosis was suppressed when cellular anti-apoptosis proteins (BCL-2 and BCL-xL) were substituted for the viral E1B-19K. For unbiased proteomic analysis of proteins targeted by BCL-xL in adenovirus-infected cells and to visualize the interactions with target proteins, BCL-xL was targeted to cytosolic inclusion bodies utilizing the orthoreovirus μNS protein sequences. The chimeric protein was localized in non-canonical cytosolic factory-like sites and promoted survival of virus-infected cells. The BCL-xL-associated proteins were isolated from the cytosolic inclusion bodies in adenovirus-infected cells and analyzed by LC-MS. These proteins included BAX, BAK, BID, BIK and BIM as well as mitochondrial proteins such as prohibitin 2, ATP synthase and DNA-PKcs. Our studies suggested that in addition to the interaction with various pro-apoptotic proteins, the association with certain mitochondrial proteins such as DNA-PKcs and prohibitins might augment the survival function of BCL-xL in virus infected cells.
Keywords: BCL-xL, E1B-19K, adenovirus, apoptosis, orthoreovirus μNS, interacting proteins
Introduction
Apoptosis is a physiological process of cell death in multicellular organisms that eliminates unwanted and diseased cells without inducing general inflammation and affecting the neighboring cells. This process is regulated by the BCL-2 family anti-apoptotic and pro-apoptotic proteins. In mammalian cells, several anti-apoptotic and pro-apoptotic proteins have been identified (reviewed in (Lomonosova and Chinnadurai, 2008)) and their functions have been delineated. The cellular BCL-2 family anti-apoptotic proteins include BCL-2, BCL-xL, MCL-1, BCL-w and A1/BFL-1 (Reviewed by (Youle and Strasser, 2008)). These proteins are characterized by the presence of four conserved domains designated BH (BCL-2 Homology) 1-4 and a C-terminal trans-membrane (TM) domain. Most viral BCL-2 family anti-apoptosis proteins also exhibit a similar domain structure while certain viral proteins such as adenovirus E1B-19K possess more divergent structures (reviewed by (Cuconati and White, 2002)). The BCL-2 family pro-apoptotic proteins include a class of multi-domain proteins such as BAX and BAK which contain BH1-3 domains and the TM domain. The second class of proteins known as the BH3-only proteins such as BIK, BIM, BID and BAD, contain only the BH3 domain with or without the TM domain. The BCL-2 family anti-apoptosis proteins are predominantly localized in the outer mitochondrial membrane (Youle and Strasser, 2008) while certain proteins such as E1B-19K have been shown to localize in the endoplasmic reticulum and nuclear envelope regions (White et al., 1984). Research over the past decade by a number of research groups has resulted in a consensus model for the regulation of apoptosis by BCL-2 family members. Various apoptotic stimuli have been shown to transcriptionally or post-transcriptionally activate the expression of specific BH3-only proteins in stimuli-specific manners. The BH3-only proteins serve as the effectors of apoptosis by activating the multi-domain pro-apoptotic proteins BAX and BAK to form oligomeric complexes on the outer mitochondrial membrane resulting in membrane permeablization, and the exit of cytochrome c leading to caspase-dependent cell death. Although, the precise modes of action of different BH3-only molecules are not still fully resolved, some (e.g., BIM) have been reported to bind with both the multi-domain pro-apoptotic and anti-apoptotic proteins while other apoptotic proteins (e.g., BAD, BIK) bind predominantly to anti-apoptotic proteins and activate BAX and BAK indirectly. It is unclear whether the interaction of anti-apoptotic proteins with both BH3-only and the multi-domain pro-apoptotic proteins is required to suppress apoptosis. Further, the functional significance of the interaction between the anti-apoptotic proteins and the pro-apoptotic molecules at the cognate location (e.g., mitochondrial membrane) in the promotion of cell survival has not been determined.
Most of the proteins that interact with BCL-2, BCL-xL and related anti-apoptosis proteins such as E1B-19K were originally identified under non-physiological conditions such as two-hybrid analysis (e.g., BIK (Boyd et al., 1995), BAD (Yang et al., 1995) and HRK (Inohara et al., 1997)) or in vitro screening of cDNA expression libraries (e.g., BIM (O'Connor et al., 1998) and BID (Wang et al., 1996)). Other BCL-2 family pro-apoptotic proteins such as BAK were identified on the basis of amino acid sequence similarities to the BH domains (Chittenden et al., 1995; Kiefer et al., 1995). Attempts to identify protein factors that are physiologically associated with BCL-2 family anti-apoptosis proteins by the use of proteomic approaches have been difficult due to their strong association with intra-cytosolic membranes. In this communication we have exploited a strategy developed by Nibert and coworkers that employed a specific domain of mammalian orthoreovirus (MRV) μNS protein to target chimeric proteins to cytosolic factory-like inclusion bodies to visualize protein-protein interactions (Miller et al., 2007; Miller et al., 2010). We used this approach to target BCL-xL and E1B-19K to cytosolic factory-like structures (FLS) and to study interactions with anti-apoptotic proteins and to identify novel interacting proteins.
Materials and Methods
Cells and Viruses
Human A549, HeLa and 293 cells were grown in Dulbecco modified Eagle medium (DMEM) containing 10 % fetal bovine serum. For the construction of recombinant adenovirus mutants TEV (TEV, tobacco etch virus protease cleavage site) and ST (Strep-III Tag, 20) sequences were first PCR amplified using a synthetic oligonucleotide and cloned at the 5′ multiple cloning site of p3XFlagCMV7.1 vector (Sigma). The sequences coding for GFP- orthoreovirus μNS fusion protein was then PCR amplified from pEGFP-C1 -M3 (471 -721, (Broering et al., 2005)) and cloned at the 3′ multiple cloning site. The sequences coding for BCL-xL or E1B-19K were cloned into the resulting vector between μNS and GFP sequences. The sequences containing chimeric constructs of BCL-xL, E1B-19K or the control (without BCL-xL or E1B-19K) were digested with SnaB I and BamH I to release the fusion cassette and cloned into hAdv transfer vector, pLendE1ACMV (Subramanian et al., 2007). The resultant plasmids were cotransfected into 293 cells along with a hAdv genomic plasmid pacAd5 9.2-100 to generate recombinant viruses. The recombinant viruses Ad5ΔE1BFTEVSTGFPμNS (Ad-FLS-GFP), Ad5ΔE1BFTEVSTBclxLGFPμNS (Ad-FLS-BCLxL-GFP) and Ad5ΔE1BFTEVST19KGFPμNS (Ad-FLS-E1B19K-GFP) were screened for protein expression, amplified and purified by banding in CsCl density gradients and titrated (Subramanian et al., 2007).
Cell death and plaque assays
A549 cells were infected with 50 PFU/cell of various viruses and after one hr adsorption at 37°C the medium was removed and replaced with 2.5 ml of DMEM containing 10 % FBS along with or without 20 μM etoposide. After 36 hr of post infection, the cells were released by treatment with trypsin, stained with trypan blue and counted in a BIORAD cell counter. The plaque assay was carried out using A549 cells as reported earlier (Subramanian et al., 2007).
Fluorescence analysis
Cells on coverslips were infected with 50 PFU/cell of hAdv5 mutants and the cells were fixed at 24 hr post infection. The fixed cells were immuno-stained with Abs specific to BCL-xL (Santa Cruz), E1B19K (gift from M. Green), BAX (Upstate), BAK (Upstate), BIK (Santa Cruz), BID (Santa Cruz) and BIM (Cell Signaling). Mito-Tracker (Molecular probes) and ER-Tracker (Molecular probes) were added to the cells in medium and Hank's buffer, respectively at 24 hr of post infection and the cells were fixed at 30 min later. The immunofluorescence of stained cells and the fluorescence (GFP, mito-Tracker and ER-Tracker) of fixed cells were analyzed by confocal microscopy.
Proteomic analysis
HeLa cells (30 × 150 mm dishes) were infected with 5 PFU/cell of Ad-FLS-BCLxL-GFP, Ad-FLS-E1B19K-GFP or the control Ad-FLS-GFP viruses for 24 hr and the cells were lysed using a buffer containing 20 mM Hepes pH 7.6, potassium acetate 110 mM, sodium chloride 200 mM, magnesium chloride 2 mM, Tween 20 0.1%, Triton X 0.25 %, PMSF 2.5 mM and protease inhibitor cocktail. The lysates were spun for 30 min. and the clear supernatants were incubated for 30 min at 4°C with agarose beads. The agarose beads and the lysates were spun for 10 min and the supernatants were rotated with agarose beads conjugated with the Flag antibody for 1 hr at 4°C. The beads with protein complexes were collected, washed 4 times with the lysis buffer and subjected to trypsin digestion and LC-MS mass spectrometric analysis (Danforth Plant Research Center, St. Louis).
Western blot analysis
Cells were infected with various viruses and cells were lysed at 24 hr post-infection and protein complexes were isolated using anti-Flag Ab coated agarose beads. The proteins bound to the beads were washed 4 times and suspended in 40 μl sample buffer. The lysates and immunoprecipitated samples were separated by 4-12 % gradient gels, transferred to nitrocellulose membrane and incubated at 4°C overnight with the following antibodies; anti-Flag (Sigma), anti-BIK, anti-prohibitin 1 and 2 and anti-actin (Santa Cruz), anti-DNA-PKcs and anti-ATP synthase (Protein Tech), anti-BAK and anti-BAX (Upstate), anti-BIM, anti-BID and anti-PARP (BD Pharmingen). The blots were washed and incubated with the corresponding secondary antibodies conjugated with HRP (Santa Cruz) for 30 min. The blots were then washed and the protein bands were visualized with western blotting detection system (Roche) according to the manufacturer's specifications. The quantitation of cleaved PARP was carried out using BIO-RAD CHEMI DOC XRS system by measuring the relative intensities of the cleaved products.
Results
In this study, we targeted the cellular anti-apoptotic protein BCL-xL and the viral anti-apoptosis protein adenovirus E1B-19K into FLS; away from their normal localization sites and analyzed their cell survival functions and interaction with cellular proteins. We used a region of 252 aa (471-721) of the μNS FLS (Broering et al., 2005) to target BCL-xL and E1B-19K into cytoplasmic inclusion bodies. We constructed three adenovirus mutants that contain deletion of the E1B region and substitution of sequences coding for GFP-μNS, BCLxL-GFP-μNS or 19K-GFP-μNS (Fig. 1A). All three chimeric proteins contained 3X Flag tag, TEV peptide (Tobacco etch virus protease cleavage site) and a ST peptide (Strep-III Tag, (Yeliseev et al., 2007)). All chimeric constructs were expressed under the control of CMV immediate early promoter.
Fig. 1.
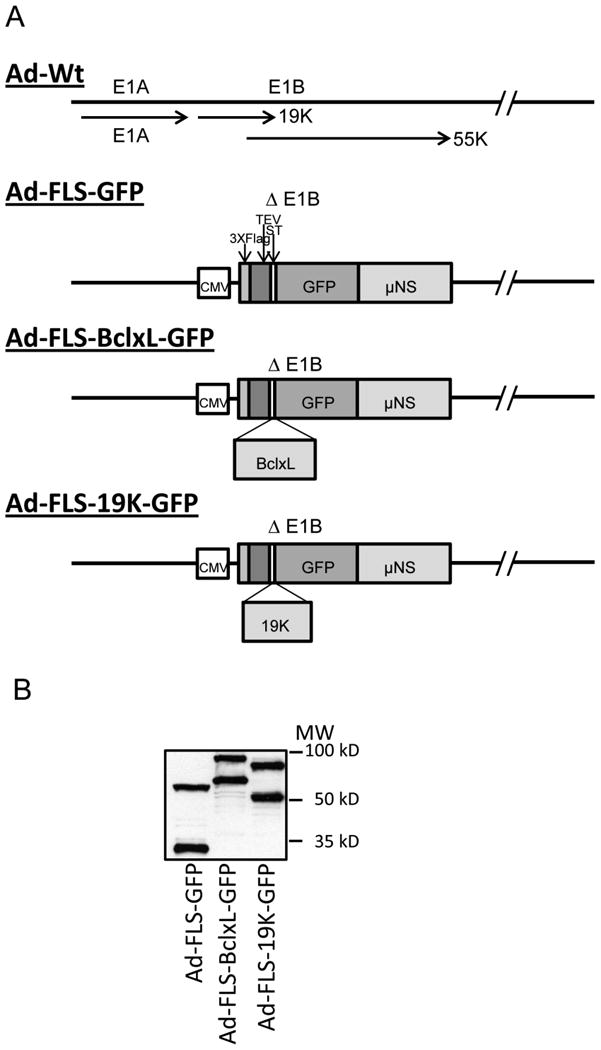
A. Schematic diagram of the E1 region of recombinant hAdv5. The recombinant viruses express the indicated chimeric proteins in the E1B region under the control of CMV-IE promoter. GFP, BCLxL-GFP and E1B19K-GFP are tagged at the N-terminus with Flag, TEV and ST tags and at the C-terminus with the orthoreovirus μNS domain.
B. Expression of chimeric proteins by recombinant viruses. The proteins expressed in infected A549 cells were analyzed by western blotting using Flag antibody and visualized with western blotting detection system.
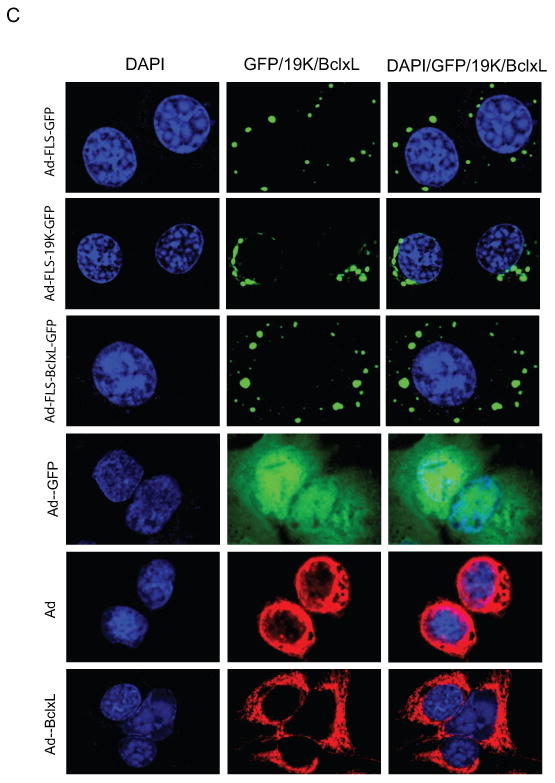
C. Localization of GFP fusion proteins into cytoplasmic inclusion bodies. The infected cells were stained with BCL-xL and E1B-19K antibodies. The immunofluorescence and GFP fluorescence were viewed in several fields and a single representative field in each category was photographed using a confocal microscope.
Subcellular localization of FLS-tagged BCL-xL and E1B-19K
First, we examined the expression of fusion proteins from the lysates of virus infected A549 cells by western blot analysis (Fig.1B). All three virus mutants expressed chimeric proteins of the expected sizes. Additionally, we also observed certain low molecular weight proteins in cells infected with different mutants. We believe that the low molecular proteins which might represent potential degradation products of the full length chimeric proteins and might not interfere with the proteome analysis of associated proteins as well as microscopic GFP-based visualization of interacting proteins due to the unique structural configuration of the chimeric protein (Fig. 1A). We then examined the localization of the chimeric proteins by GFP fluorescence and immunofluorescence analyses (Fig. 1C). As seen in the figure, FLS-tagged GFP, BCLxL-GFP and E1B19K-GFP appeared in distinct granular structures in the cytoplasm while wt GFP (indicated as Ad-GFP in Fig. 1C) fluorescence was distributed throughout the cells. The localization of FLS-tagged E1B19K-GFP was closer to the nuclear envelope region while BCLxL-GFP granules were more dispersed in the cytoplasm. Immunofluorescence analysis of wt BCL-xL (Ad-BCLxL in Fig. 1C) and wt E1B-19K (hAd5 wt in Fig. 1C) expressed from the adenovirus vectors localized in the nuclear envelope, mitochondria and endoplasmic reticulum regions (bottom two panels of Fig. 1C). The expression patterns of the untagged BCL-xL and E1B-19K were distinct from the granular localization of FLS-tagged proteins. The predicted sizes of the cleavage products of the chimeric proteins (Fig. 1B) suggested potential common proteolytic cleavage site(s) at the N-terminal region of the μNS domain raising the possibility that some fractions of the chimeric proteins may lack μNS sequences. However, the GFP fluorescence indicated distinct granular localization suggesting the cleavage products lacking μNS sequences might not localize at specific sites or might be labile.
Since native BCL-xL (Vander Heiden et al., 1997) and E1B-19K (White et al., 1984) have been known to predominantly localize in the mitochondria and ER, respectively, we then analyzed the potential localization of the FLS-tagged versions in these cytosolic organelles by staining the infected cells with mito-Tracker (Fig. 2A) or ER-Tracker (Fig. 2B). The majority of the FLS-tagged fusion protein-containing granules were excluded by from the mito-tracker and ER-tracker staining regions. Among the three FLS-tagged proteins, a minor fraction of E1B19K-GFP appeared to localize in the mito-tracker staining regions. These results suggest that the orthoreovirus μNS domain targets the fusion proteins to the cytoplasmic inclusion bodies, away from the canonical mitochondrial and ER localization sites, overcoming the functions of the membrane targeting domains of BCL-xL and E1B-19K.
Fig. 2.
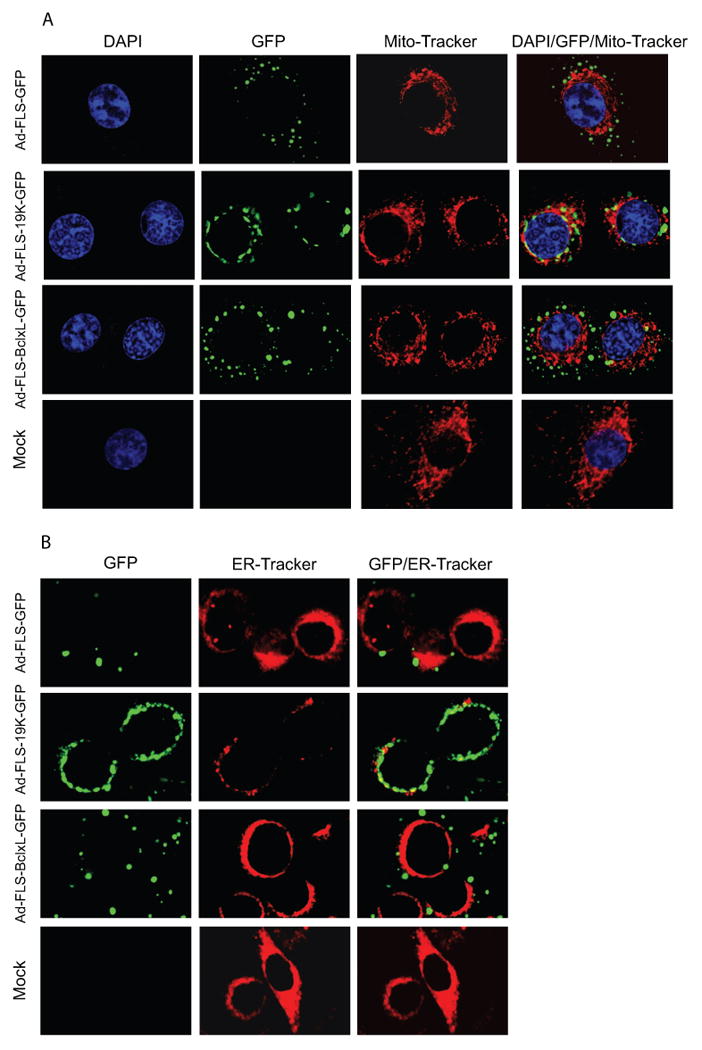
Mitochondrial and ER localization of chimeric proteins. Cells were infected with various viruses and after 24 hr of infection, mito-Tracker (A) or ER-Tracker (B) were added to the cells in medium and Hank's buffer respectively for 30 min, fixed and analyzed using a confocal microscope.
Cell survival activity of FLS-tagged BCL-xL and E1B-19K chimeric proteins
It is well established that adenovirus mutants defective in the anti-apoptosis protein E1B-19K induce apoptosis of virus infected cells and produce large plaques on target cell monolayers due to excessive cell death (Subramanian et al., 1984). We have shown that substitution of the viral E1B-19K protein coding sequences with the sequences coding for the cellular anti-apoptosis proteins BCL-2 or BCL-xL in the viral genome efficiently suppressed apoptosis (Subramanian et al., 2007; Tarodi et al., 1993). Here, we tested the apoptotic activity of the recombinant viruses that expressed FLS-tagged GFP (i.e., in the absence of E1B, ΔE1B) or FLS-tagged E1B19K-GFP or FLS-tagged BCLxL-GFP. The infected cells were analyzed for the activation of caspase by western blot analysis of PARP (Fig. 3A). As expected, in cells infected with different MOI of the FLS-tagged GFP (ΔE1B) recombinant there was readily detectable PARP cleavage compared to cells infected with viruses that expressed chimeric BCL-xL or E1B-19K. We determined the extent of cell death induced by different viruses, with or without etoposide treatment (additional apoptotic stimulus). We found that infection with the FLS-tagged GFP recombinant virus induced significant amount of cell death. Addition of sub-optimal concentration (20 μM) of etoposide did not induce any cell death in mock-infected cells. However, etoposide treatment of cells infected with the FLS-tagged GFP virus enhanced cell death compared to infection with FLS-tagged GFP virus alone. Infection with the recombinants expressing chimeric BCL-xL or E1B-19K significantly suppressed cell death in the presence as well as absence of etoposide compared to the virus that expressed chimeric GFP (Fig. 3B). We also evaluated the plaque morphologies on cell monolayers (Fig. 3C). The virus that expressed the chimeric GFP formed more large plaques than the viruses that expressed BCLxL-GFP or E1B19K-GFP consistent with the cell death assay. Semi-quantitative measurement of the plaque sizes (using Image J software) was in agreement of the visual evaluation. Taken together, these results suggest that FLS-tagged BCLxL-GFP and E1B19K-GFP chimeric proteins suppressed the cell death induced by adenovirus (ΔE1B) infection as well as by co-treatment with etoposide. However, we acknowledge the possibility that some of the cell survival activity might be contributed by the proteolytic products of the chimeric proteins that may correspond to untagged versions.
Fig. 3.
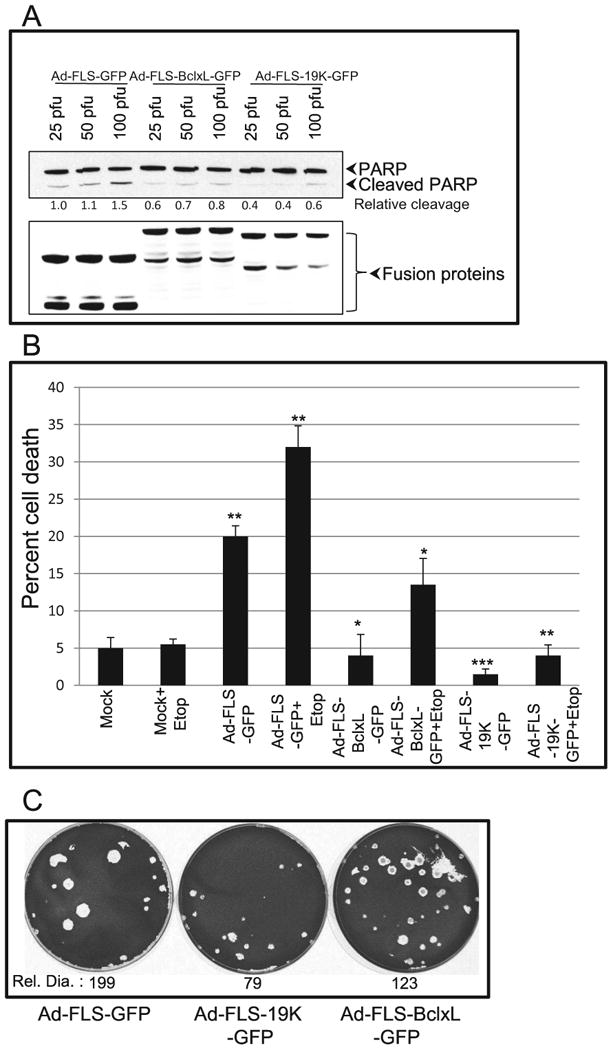
Suppression of apoptosis by chimeric BCL-xL and E1B-19K proteins localized at non-canonical sites. A. A549 cells were infected with the recombinant viruses at different MOI and 24 hr post infection cells were lysed and analyzed by western blotting to determine the extent of PARP cleavage (top panel). The western blot with the Flag Ab (lower panel) shows the levels of expression of different recombinant proteins. B. Effects on apoptosis. A549 cells were infected with different viruses (50 pfu/cell) and cell viability was determined by trypan blue using a BIORAD cell counter. The experiment was repeated three times and the statistical differences were assessed using two-tail student t-test with 95% confident. P-value was determined by comparing mock vs Ad-FLS-GFP, mock+etop vs Ad-FLS-GFP+etop, Ad-FLS-GFP vs Ad-FLS-BCLxL-GFP and Ad-FLS-E1B19K-GFP and Ad-FLS-GFP+etop vs Ad-FLS-BCLxL-GFP+etop and Ad-FLS-E1B19K-GFP+etop. Significance was accepted at a value of *p<0.05, **p<0.01 and ***p<0.005. C. Plaque morphology of recombinant viruses. A549 cells were infected with the recombinant viruses and assayed for plaque formation (Subramanian et al., 2007). The diameters of 24 to 50 plaques were measured using ImageJ and the relative diameter (Rel.Dia.) is shown at the bottom of each dish.
Proteomic analysis of BCL-xL associated proteins
The cellular anti-apoptotic proteins BCL-xL and BCL-2 as well as the viral homolog, E1B-19K have been reported to interact with several pro-apoptotic proteins. Many of these interactions were identified under a variety of non-physiological contexts. The strong association of these anti-apoptotic molecules with cytosolic membranes (i.e., mitochondrial outer membrane and nuclear envelope/ER) prevented unbiased proteomic analysis of BCL-2 family proteins. Our present studies showed that FLS-tagged BCL-xL and E1B-19K suppressed apoptosis despite being predominantly concentrated in factory-like inclusion bodies. We rationalized that the concentration of the FLS-tagged BCL-xL and E1B-19K along with the pro-apoptotic interacting proteins within the non-membrane inclusion bodies might be exploited for the isolation and unbiased proteomic analysis of the respective protein complexes. We prepared protein extracts (Material and Methods) from HeLa cells infected with recombinant adenoviruses expressing FLS-tagged GFP, BCLxL-GFP or E1B19K-GFP. The protein complexes were isolated by a single step affinity chromatography using Flag Ab beads, and analyzed by LC-MS mass spectrometry. The list of proteins present in BCLxL-GFP and E1B19K-GFP proteomes are shown in Table 1 and none of proteins in the list were present in control GFP proteome. The BCL-xL proteome contained proteins such as the ATP synthase, DNA-PKcs, heat shock protein (HSP) 27, prohibitin-2 and pro-apoptotic proteins BID and BIK. Among these proteins, ATP synthase and HSP 27 were present in the E1B-19K proteome also. ATP synthase has previously been reported to interact with BCL-xL to enhance the metabolic efficiency of neurons (Alavian et al., 2011). The DNA-PK activity has been linked to the regulation of apoptosis during DNA double strand break repair (Callen et al., 2009). However, interaction between these proteins was not reported. We also identified the mitochondrial protein prohibitin 2 in the BCL-xL proteome. Although the functional consequence of the interaction of prohibitin with BCL-xL is not known, such interaction was reported by others (Vento et al., 2010).
Table 1.
A. List of proteins present in BCL-xL (A) and E1B-19K proteome (B) against control GFP. HeLa cells (30 × 150 mm dishes) were infected with 5 pfu/cell of BCLxL-GFP, E1B19K-GFP or the control GFP virus for 24 hr and the cells were lysed using a buffer containing 20 mM Hepes pH 7.6, 100 mM potassium acetate, 200 mM sodium chloride, 2 mM magnesium chloride, 0.1% Tween 20, 0.25% Triton X, 2.5 mM PMSF and protease inhibitor cocktail. The lysates were immunoprecipitated with the Flag Ab, digested with trypsin and subjected to LC-MS/MS analysis. The samples were analyzed using Mascot (Matrix Science,.London, UK; version 2.5.1) for the database search. Scaffold (version Scaffold_4.4.1.1, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 80.0% probability by the Scaffold Local FDR algorithm. Protein identifications were accepted if they could be established at greater than 99.0% probability and contained at least 2 identified peptides. *- We note the presence of adenovirus E1B-19K in the BCLxL proteome. Since virus stocks were prepared in 293 cells, there is a possibility that the Ad-FLS-BclxL-GFP virus may contain a small fraction of Ad5 wt virus due to recombination in 293 cells that might express low levels of E1B-19K in cells infected with BCLxL recombinant virus.
| Table 1A: List of proteins present in Bcl-xL proteome: | ||
|---|---|---|
| Name of protein | Accession number | Molecular weight |
| Bcl-2-like protein 1-like isoform 5 | Gl:109092453 | 26 kDa |
| Protein kinase, DNA-activated, catalytic polypeptide | Gl:119607088 | 469 kDa |
| Adenovirus E1B-19K* | Gl:9626162 | 21 kDa |
| ATP synthase, H+ transporting mitochondrial F1 complex, alpha subunit 1 | Gl:127798841 | 60 kDa |
| Heat shock protein 27 | Gl:15928919 | 21 kDa |
| Adenovirus 5 late protein 100K | Gl:56160547 | 90 kDa |
| Prohibitin-2 | Gl:114051223 | 33 kDa |
| Pro-apoptotic protein Bid, chain A | Gl:159163783 | 22 kDa |
| Similar to STIP1 homology and U-box containing protein 1 isoform 3 | Gl:114660202 | 33 kDa |
| Solute carrier family 25 | Gl:158455003 | 33 kDa |
| D-3-phosphoglycerate dehydrogenase isoform 3 | Gl:109014683 | 56 kDa |
| Bcl-2 interacting killer | Gl:30584757 | 18 kDa |
| Thyroid receptor-interacting protein 13 isoform 1 | Gl:11321607 | 49 kDa |
| Table 1B: List of proteins present in E1B-19K proteome: | ||
| Name of protein | Accession number | Molecular weight |
| ATP synthase, H+ transporting mitochondrial F1 complex, alpha subunit 1 | Gl:127798841 | 60 kDa |
| Adenovirus E1B-19K | Gl:9626162 | 21 kDa |
| Heat shock protein 27 | Gl:15928919 | 21 kDa |
A domain of μNS targeted BCL-xL and E1B-19K to cytosolic inclusion bodies. BCL-xL and E1B-19K targeted to the inclusion bodies suppressed apoptosis. Chimeric BCL-xL and E1B-19K proteins interacted with pro-apoptotic proteins. BCL-xL proteome contained pro-apoptotic proteins, DNA-PKcs and prohibitins. This targeting strategy can be applied to other membrane proteins.
The FLS-tagged BCL-xL and E1B-19K proteomes were validated by western blot analysis (Fig. 4). The interaction of DNA-PKcs, ATP synthase, prohibitin 2 and its heterodimeric partner prohibitin 1 as well the as pro-apoptotic molecules BAX, BAK, BID, BIK and BIM were readily detectable in the BCL-xL proteome while the interactions of only BAK and modest levels of BIM were detected in the E1B-19K proteome. It is possible that our protocol for the protein complex preparation may not extract sufficient amount of E1B-19K complex. The negative results obtained with E1B-19K might also be due to lower levels of chimeric 19K protein expression. The interaction of HSP 27 could not be detected with both proteomes. It is not certain whether the available panel of HSP 27 Abs is sensitive enough to detect such interactions. We also note that we have observed robust interaction between the BH3-only molecule BIK and E1B-19K in hAdv5-infected cells (Subramanian et al., 2007). However, we were unable to detect such interaction between FLS-tagged E1B-19K-GFP chimeric protein and BIK, suggesting that the potential subcellular localization may play a role in determining BIK/E1B-19K interaction.
Fig. 4.
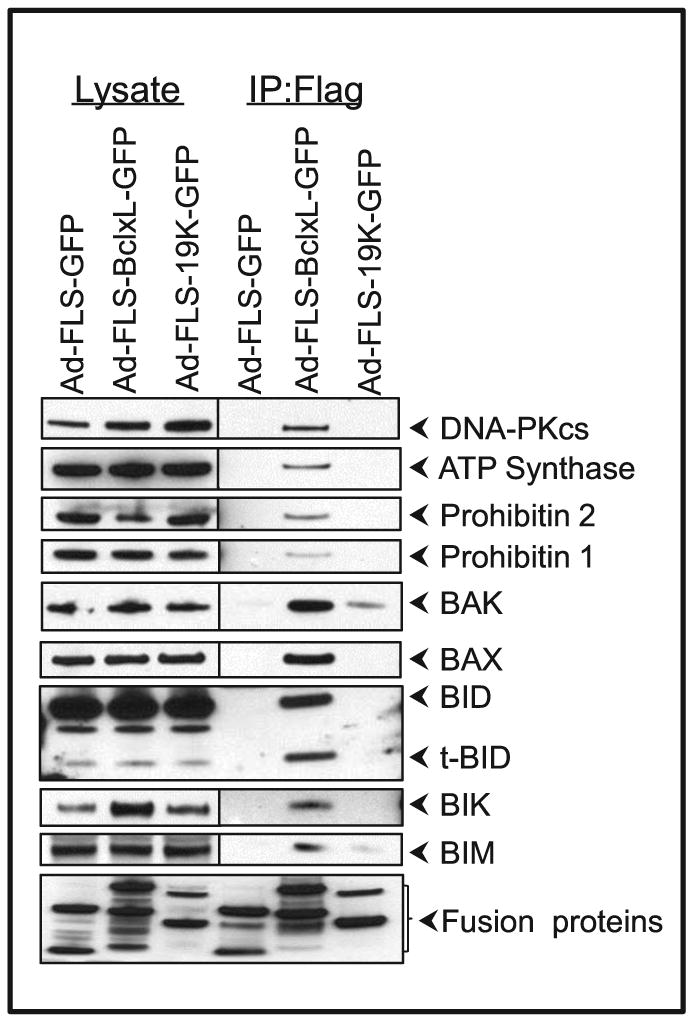
Interaction of BCL-xL and E1B-19K fusion proteins with cellular proteins. A549 cells were infected with various viruses (50 pfu/cell). Twenty four hr after infection proteins were immunoprecipitated, resolved by 4 - 12 % gradient PAGE cells and analyzed by immune blotting.
Interaction with multi-domain pro-apoptotic proteins
It is believed that in normal cells the expression of pro-apoptotic proteins and anti-apoptotic proteins are in a balanced state to maintain the homeostasis of the organism. In addition, these proteins are localized in their specific cellular locations such as the mitochondrial outer membrane and/or the ER to mediate their activities. The subcellular localization studies with FLS-targeted BCLxL-GFP and E1B19K-GFP (Fig. 1C and Fig. 2) suggest that the chimeric anti-apoptosis proteins were able to suppress virus-induced apoptosis (Fig. 3) despite their non-canonical localization in distinct factory-like cytoplasmic granules. Since it is believed that the anti-apoptosis proteins exert their activity by neutralizing the cell death activities of pro-apoptotic molecules it was of interest to visualize such interactions in the cytosolic factory-like granules. We determined co-localization of endogenous multi-domain pro-apoptotic proteins BAK and BAX (Fig. 5) as well as BH3-only pro-apoptotic proteins (Fig. 6) with the chimeric FLS-tagged BCLxL-GFP and E1B19K-GFP. Immunofluorescence analysis of BAK in mock infected cells (Fig. 5, bottom panels) and in cells infected with the FLS-GFP virus (Fig. 5, top panels) suggested BAK localization in the nuclear envelope/ER/mitochondrial regions that are distinct from the punctate GFP localization sites, suggesting no detectable co-localization of BAK and GFP (Fig. 5, top panels). In cells infected with BCLxL-GFP or E1B19K-GFP viruses, BAK was co-localized with the chimeric anti-apoptosis proteins in the cytoplasmic inclusion bodies (Fig. 5, middle two panels). Similarly the pro-apoptotic protein BAX was detected throughout the cytoplasmic region (Fig. 5B, bottom most panel). In virus-infected cells, BAX was relocalized in a more punctate fashion (Fig.5, top three panels), consistent with the reports that BAX was activated by conformational changes and targeted to mitochondria in cells experiencing apoptotic burden (Hsu et al., 1997; Hsu and Youle, 1997). In cells expressing FLS-tagged BCLxL-GFP (Fig. 5, third panel from top), most of BCLxL-GFP was localized in the factory-like granules and was co-localized with BAX, as seen in the case of BAK. In contrast, only a small fraction of E1B19K-GFP appeared to contain BAX (Fig. 5B, second panel from top). As expected, some amount of BAK and BAX might be complexed with endogenous BCL-xL/BCL-2 with high affinity and might not be available for interaction with the ectopically expressed chimeric anti-apoptosis proteins. Such endogenous interactions may limit quantitative co-localization with the chimeric anti-apoptosis proteins.
Fig. 5.
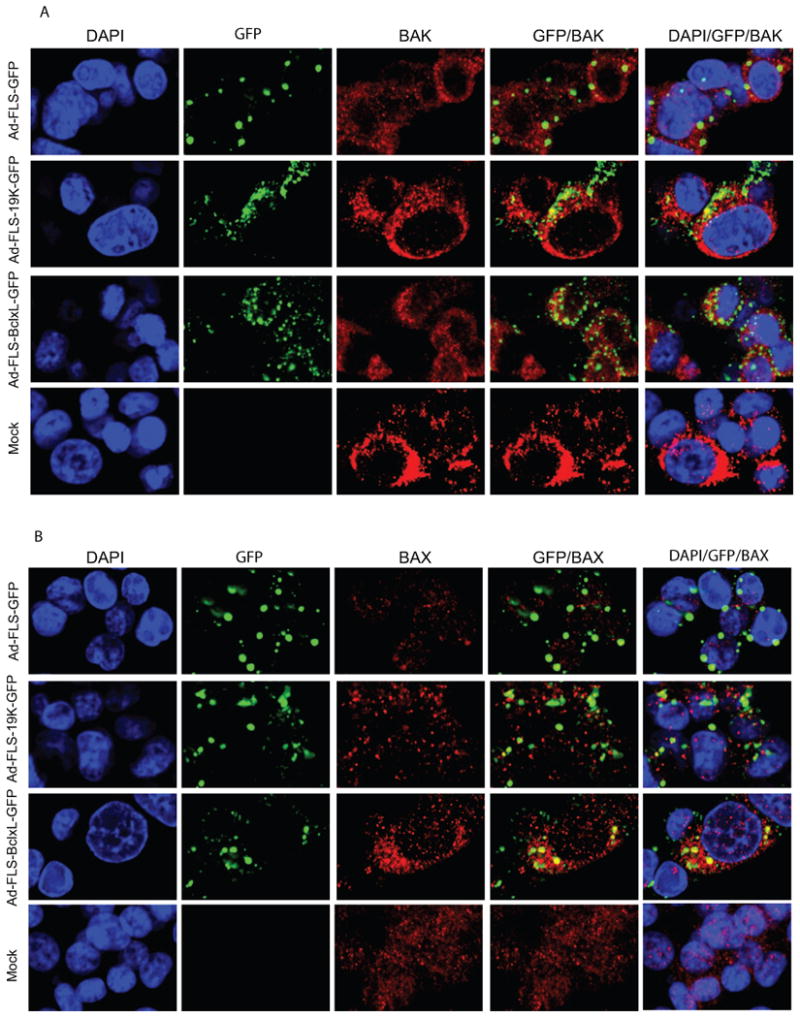
Interaction of BAK (A) and BAX (B) with BCL-xL and E1B-19K fusion proteins. Cells were plated on coverslips and infected with the recombinant viruses (50 pfu/cell) and 24 hr after infection, fixed and immune-stained using various antibodies as mentioned in Fig.1C.
Fig. 6.
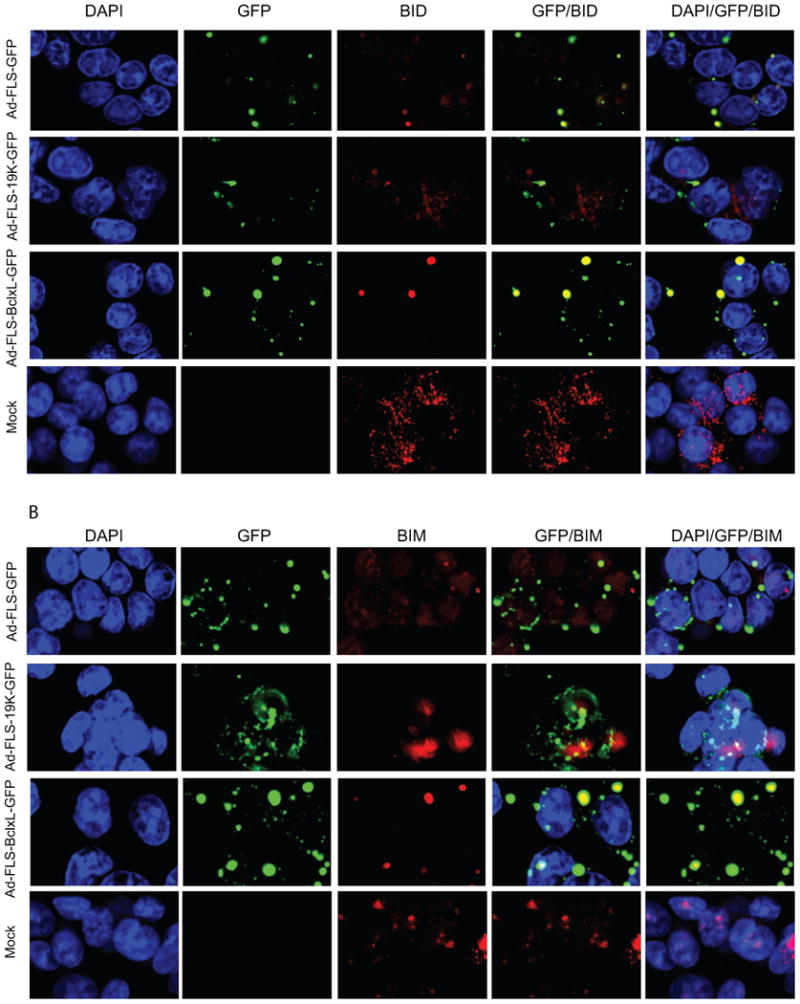
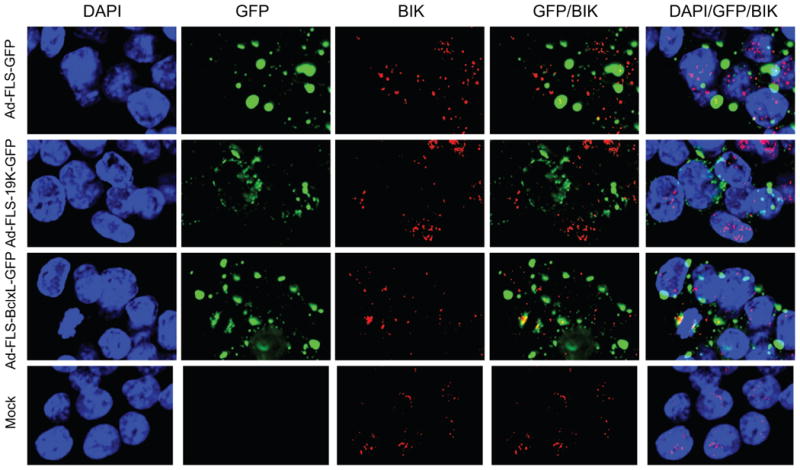
Localization of BH3-only pro-apoptotic proteins with BCL-xL and E1B-19K fusion proteins. A. Localization of BID. B. Localization of BIM. C. Localization of BIK. The 549 cells were infected and analyzed as in Fig. 5.
Interaction with BH3-only pro-apoptotic proteins
We then used the orthoreovirus μNS platform to determine the interaction of BCL-xL and E1B-19K with the BH3-only molecules BID, BIM and BIK (Fig. 6). The localization of BID and BIM (Fig. 6A and B) was very interesting. In cells infected with chimeric BCLxL-GFP, BID and BIM were exclusively localized in the cytoplasmic inclusion bodies indicating that BCLxL-GFP interacted with most BID and BIM proteins in these granules Fig. 6A and B). In cells that expressed the chimeric E1B19K-GFP protein, BIM was colocalized in the cytosolic granules to some extent while BID was excluded. We also observed some amount of BID was colocalized with the GFP protein in the inclusion bodies. We do not understand the significance of this apparent non-specific localization. BIK was colocalized with BCLxL-GFP to some extent in the inclusion bodies and not at detectable levels with E1B19K-GFP (Fig. 6C). These immunofluorescence and GFP fluorescence analyses (Fig. 5 and 6) suggest that the interaction of BAX and BAK as well as select BH3-only proteins within the factory-like granules may contribute to the anti-apoptotic activities of the chimeric BCL-xL and E1B-19K proteins.
Effect of DNA-PK and prohibitins in regulating the activity of BCL-xL
Among the various proteins present in the BCL-xL proteome, we chose to determine the roles of DNA-PK and prohibitins in the regulation of the apoptotic activity during hAdv5 infection and etoposide treatment (Fig. 7). Although the activity of DNA-activated protein kinase (DNA-PK) has been implicated in modulating apoptosis during DNA repair catastrophe (Callen et al., 2009), the interaction of DNA-PKcs with BCL-xL has not been reported earlier. Prohibitin 2 has been previously reported to interact with BCL-xL (Vento et al., 2010) and inhibit apoptosis induced by staurosporine and ceramide (Chowdhury et al., 2011; Chowdhury et al., 2007). We depleted DNA-PKcs and prohibitins 1 and 2 in human A549 cells using lentiviral shRNA vectors (Fig. 7A). In cells depleted for DNA-PKcs, there was readily detectable reduction in the level of DNA-PKcs (Fig. 7A left panel). Prohibitins exist as a hetero-dimer of prohibitin 1 and 2 (Berger and Yaffe, 1998). When prohibitin 2 was depleted, the level of prohibitin 1 was also reduced. Similarly, when prohibitin1 was depleted, the level of prohibitin 2 was reduced. Therefore, we used A549 cells that were acutely depleted for prohibitin 2 for cell death assays. In control A549 cells infected with the recombinant virus expressing GFP (i.e, in the absence of E1B, ΔE1B), there was significant cell death. The extent of cell death was further enhanced by treatment with etoposide (Fig. 7B, left panel). As expected, in cells infected with recombinant viruses that express BCLxL-GFP or E1B19K-GFP chimeric proteins the levels of cell death were significantly reduced. Although cells depleted for DNA-PKcs (DNA-PK↓) or prohibitin (prohibitin↓) exhibited comparable cell death profiles (Fig. 7 center and right panels), cells infected with the BCL-xL-GFP recombinant virus exhibited enhanced levels of cell death (compared to the control A549 cells). Although we were unable to see the interaction of prohibitins with E1B-19K, the cell death assays in prohibitin-depleted cells showed that there was a detectable effect on the cell survival activity of E1B-19K indicating that prohibitins may play a role at the early phase of apoptosis. Our results are in agreement with a previous report that showed that BCL-xL was unable to prevent staurosporine induced cell death when prohibitin 2 was depleted (Chowdhury et al., 2013). Taken together our results suggest that DNA-PK and prohibitins may augment the survival function of BCL-xL in cells treated with the anti-cancer agent etoposide without significant effect on virus-induced apoptosis. In addition, DNA-PK and prohibitins did not appear to be required for the survival function of E1B-19K in the presence of etoposide and adenovirus infection together while it enhanced E1B-19K function during virus infection alone.
Fig. 7.
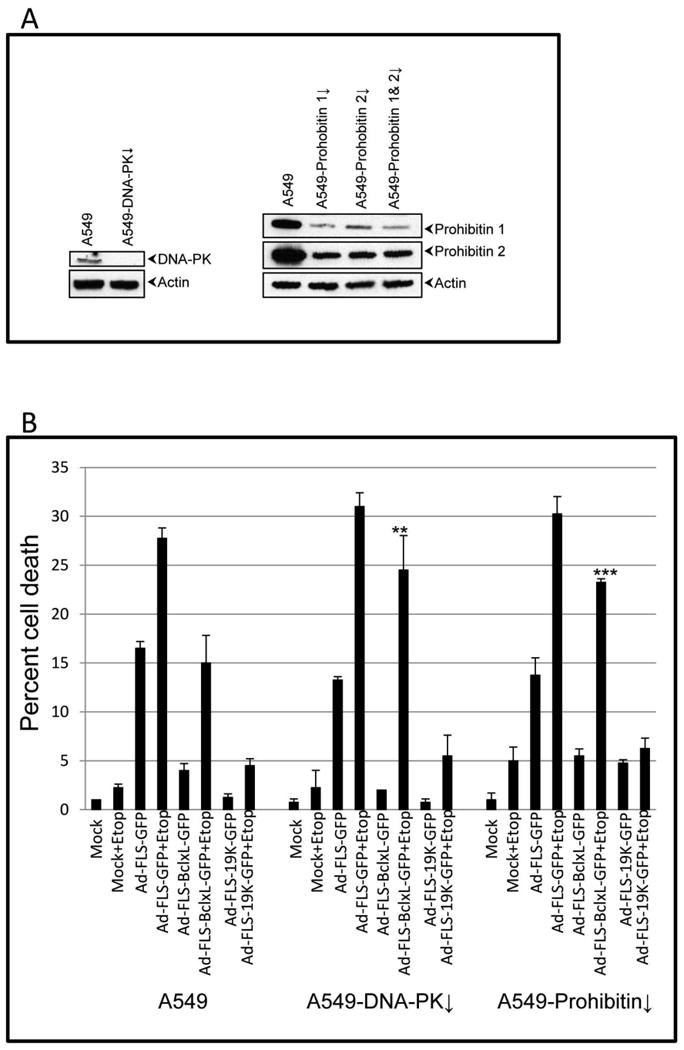
Role of DNA-PK and prohibitins on the anti-apoptosis activities of BCL-xL and E1B-19K. A. Depletion of DNA-PK and prohibitins. A549 cells were infected with lentiviral vectors (pLKO-puro) that express shRNAs targeted against DNA-PKcs, prohobitin 1 and prohibitin 2 and selected with puromycin. B. Effect on BCL-xL activity. The acutely depleted cells were pooled and infected with the recombinant viruses (50 pfu/cell) and the cell death assay was performed as outlined in Fig. 2B in the presence and absence of etoposide. The experiment was repeated three times and the statistical differences were assessed as in Fig. 3. P-value was determined by comparing the 3 cell lines in the presence and absence of etoposide. Significance was accepted at a value of **p<0.01 and ***p<0.005.
Discussion
Nibert, Miller and colleagues have developed a system to visualize protein-protein interactions within the cell by targeting the protein complex to discrete cytosolic factory-like inclusion bodies using a domain of the μNS protein of orthoreovirus (Miller et al., 2007; Miller et al., 2010). Here, we have utilized this system to target the cellular and viral anti-apoptosis proteins BCL-xL and E1B-19K to the cytosolic factory-like structures. The chimeric proteins appeared to retain, at least, some of their anti-apoptotic activity despite their non-canonical localization in the discrete cytosolic bodies away from their normal membrane locations in the outer mitochondrial membrane and the ER/nuclear envelope regions.
We investigated the FLS-tagged chimeric BCLxL-GFP and E1B19K-GFP proteins to analyze their interaction with several known pro-apoptotic proteins. We found co-localization of BCLxL-GFP with the multi-domain pro-apoptotic proteins BAX and BAK, as well as with certain BH3-only proteins. These results suggested that the anti-apoptotic activities of BCL-xL and E1B-19K proteins might be linked to the interaction of, at least, a fraction of BAX and BAK as well as the BH3-only proteins such as BIM and BID despite their interaction away from their normal localization sites. Our results strengthen the view that the BCL-2 family anti-apoptosis proteins may exert their cell survival function by interacting with pro-apoptotic proteins. It is possible that the interaction between the pro-apoptotic proteins and anti-apoptotic proteins may be dynamic process in the FLS as reported earlier (Miller et al., 2007). Such interactions may moderate the activities of the pro-apopototic proteins in a dynamic fashion in cells infected with the recombinant viruses. Our FLS-aided visualization studies also revealed the BCL-xL-GFP chimeric protein was found to complex only with a fraction of the multi-domain pro-apoptotic proteins. Although the reason for non-quantitative sequestration is unknown, it is possible that only a fraction of BAX and BAK might be ‘activated’ to apoptotic competence which is targeted by the ectopically expressed anti-apoptotic molecule. An alternate explanation may include that a fraction of the activated BAX/BAK molecules might be sequestered by the endogenous BCL-2/BCL-xL proteins rendering them unavailable for interaction with exogenous anti-apoptosis proteins. In contrast to the pattern of BAX and BAK interaction, BCLxL-GFP interacted with the BH3-only molecules BIM and BID more avidly (Fig. 6A, B), suggesting that sequestration of the BH3-only molecules which function as the effectors of apoptosis signaling (Lomonosova and Chinnadurai, 2008) may contribute to the anti-apoptotic activity of BCL-xL.
In general, the expression of E1B19K-GFP resulted in more pronounced anti-apoptotic activity (Fig. 3). However, E1B19K-GFP exhibited a narrow interaction pattern with the pro-apoptotic proteins than BCLxL-GFP. E1B19K-GFP appeared to exhibit a preference for interaction with BAK than BAX. Similar results were also observed by the western blot analysis (Fig. 4). Among the BH3-only proteins, only BIM was found interact with E1B19K-GFP (Fig. 6B). While BIK was found to be a major target for E1B-19K in cells infected hAdv5 wt (Subramanian et al., 2007), there was no detectable interaction between FLS-tagged E1B19K-GFP and BIK. Taken together the present study suggests that sequestration of a combination of the multi-domain and BH3-only pro-apoptotic proteins by BCL-2 family anti-apoptosis proteins may lead to suppression of apoptosis.
In our present study, we have also exploited the FLS-mediated targeting of interacting protein complexes for unbiased proteomic analysis of BCL-xL. Although our analysis is not extensive, we were able to identify a novel association of DNA-PKcs with BCL-xL and determine its effect on the anti-apoptotic activity of BCL-xL. The identification of several previously known BCL-xL-interacting proteins such as ATP synthase, prohibitins and BCL-2 family pro-apoptotic proteins validated the utility of the approach. However, we note the limited utility of the approach for the identification of E1B-19K-associated proteins. It appears that additional improvements may be needed to extract the E1B-19K-associated protein complex from the FLS-containing structures. We show that BCL-xL, in addition to sequestering BCL-2 family pro-apoptotic proteins also recruit proteins such as DNA-PKcs and prohibitins to modulate virus-induced apoptosis. This protein targeting system may be useful to analyze the proteomes of other membrane associated BCL-2 family proteins.
Acknowledgments
This work was supported by research grant CA-33616 from the National Cancer Institute. We thank Dr. Max L. Nibert for the orthoreovirus μNS-GFP plasmid vector. The mass spectrometry analysis was performed at the Danforth Plant Science Center (St. Louis) and was supported by the National Science Foundation under Grant No.DBI-0922879.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alavian KN, Li H, Collis L, Bonanni L, Zeng L, Sacchetti S, Lazrove E, Nabili P, Flaherty B, Graham M, Chen Y, Messerli SM, Mariggio MA, Rahner C, McNay E, Shore GC, Smith PJ, Hardwick JM, Jonas EA. Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat Cell Biol. 2011;13:1224–1233. doi: 10.1038/ncb2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger KH, Yaffe MP. Prohibitin family members interact genetically with mitochondrial inheritance components in Saccharomyces cerevisiae. Mol Cell Biol. 1998;18:4043–4052. doi: 10.1128/mcb.18.7.4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd JM, Gallo GJ, Elangovan B, Houghton AB, Malstrom S, Avery BJ, Ebb RG, Subramanian T, Chittenden T, Lutz RJ, et al. Bik, a novel death-inducing protein shares a distinct sequence motif with Bcl-2 family proteins and interacts with viral and cellular survival-promoting proteins. Oncogene. 1995;11:1921–1928. [PubMed] [Google Scholar]
- Broering TJ, Arnold MM, Miller CL, Hurt JA, Joyce PL, Nibert ML. Carboxyl-proximal regions of reovirus nonstructural protein muNS necessary and sufficient for forming factory-like inclusions. J Virol. 2005;79:6194–6206. doi: 10.1128/JVI.79.10.6194-6206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callen E, Jankovic M, Wong N, Zha S, Chen HT, Difilippantonio S, Di Virgilio M, Heidkamp G, Alt FW, Nussenzweig A, Nussenzweig M. Essential role for DNA-PKcs in DNA double-strand break repair and apoptosis in ATM-deficient lymphocytes. Mol Cell. 2009;34:285–297. doi: 10.1016/j.molcel.2009.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittenden T, Flemington C, Houghton AB, Ebb RG, Gallo GJ, Elangovan B, Chinnadurai G, Lutz RJ. A conserved domain in Bak, distinct from BH1 and BH2, mediates cell death and protein binding functions. Embo J. 1995;14:5589–5596. doi: 10.1002/j.1460-2075.1995.tb00246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Branch A, Olatinwo M, Thomas K, Matthews R, Thompson WE. Prohibitin (PHB) acts as a potent survival factor against ceramide induced apoptosis in rat granulosa cells. Life sciences. 2011;89:295–303. doi: 10.1016/j.lfs.2011.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Thompson WE, Welch C, Thomas K, Matthews R. Prohibitin (PHB) inhibits apoptosis in rat granulosa cells (GCs) through the extracellular signal-regulated kinase 1/2 (ERK1/2) and the Bcl family of proteins. Apoptosis. 2013;18:1513–1525. doi: 10.1007/s10495-013-0901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Xu W, Stiles JK, Zeleznik A, Yao X, Matthews R, Thomas K, Thompson WE. Apoptosis of rat granulosa cells after staurosporine and serum withdrawal is suppressed by adenovirus-directed overexpression of prohibitin. Endocrinology. 2007;148:206–217. doi: 10.1210/en.2006-0187. [DOI] [PubMed] [Google Scholar]
- Cuconati A, White E. Viral homologs of BCL-2: role of apoptosis in the regulation of virus infection. Genes Dev. 2002;16:2465–2478. doi: 10.1101/gad.1012702. [DOI] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Inohara N, Ding L, Chen S, Nunez G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L) EMBO J. 1997;16:1686–1694. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer MC, Brauer MJ, Powers VC, Wu JJ, Umansky SR, Tomei LD, Barr PJ. Modulation of apoptosis by the widely distributed Bcl-2 homologue Bak. Nature. 1995;374:736–739. doi: 10.1038/374736a0. [DOI] [PubMed] [Google Scholar]
- Lomonosova E, Chinnadurai G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27(Suppl 1):S2–19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, Arnold MM, Broering TJ, Eichwald C, Kim J, Dinoso JB, Nibert ML. Virus-derived platforms for visualizing protein associations inside cells. Molecular & cellular proteomics : MCP. 2007;6:1027–1038. doi: 10.1074/mcp.M700056-MCP200. [DOI] [PubMed] [Google Scholar]
- Miller CL, Arnold MM, Broering TJ, Hastings CE, Nibert ML. Localization of mammalian orthoreovirus proteins to cytoplasmic factory-like structures via nonoverlapping regions of microNS. J Virol. 2010;84:867–882. doi: 10.1128/JVI.01571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor L, Strasser A, O'Reilly LA, Hausmann G, Adams JM, Cory S, Huang DC. Bim: a novel member of the Bcl-2 family that promotes apoptosis. Embo J. 1998;17:384–395. doi: 10.1093/emboj/17.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T, Kuppuswamy M, Gysbers J, Mak S, Chinnadurai G. 19-kDa tumor antigen coded by early region E1b of adenovirus 2 is required for efficient synthesis and for protection of viral DNA. J Biol Chem. 1984;259:11777–11783. [PubMed] [Google Scholar]
- Subramanian T, Vijayalingam S, Lomonosova E, Zhao LJ, Chinnadurai G. Evidence for involvement of BH3-only proapoptotic members in adenovirus-induced apoptosis. J Virol. 2007;81:10486–10495. doi: 10.1128/JVI.00808-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarodi B, Subramanian T, Chinnadurai G. Functional Similarity between Adenovirus Elb 19k Gene and Bcl2 Oncogene - Mutant Complementation and Suppression of Cell Death Induced by DNA Damaging Agents. International Journal of Oncology. 1993;3:467–472. doi: 10.3892/ijo.3.3.467. [DOI] [PubMed] [Google Scholar]
- Vander Heiden MG, Chandel NS, Williamson EK, Schumacker PT, Thompson CB. Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell. 1997;91:627–637. doi: 10.1016/s0092-8674(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Vento MT, Zazzu V, Loffreda A, Cross JR, Downward J, Stoppelli MP, laccarino I. Praf2 is a novel Bcl-xL/Bcl-2 interacting protein with the ability to modulate survival of cancer cells. PloS one. 2010;5:e15636. doi: 10.1371/journal.pone.0015636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- White E, Blose SH, Stillman BW. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol Cell Biol. 1984;4:2865–2875. doi: 10.1128/mcb.4.12.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- Yeliseev A, Zoubak L, Gawrisch K. Use of dual affinity tags for expression and purification of functional peripheral cannabinoid receptor. Protein Expr Purif. 2007;53:153–163. doi: 10.1016/j.pep.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]


