Abstract
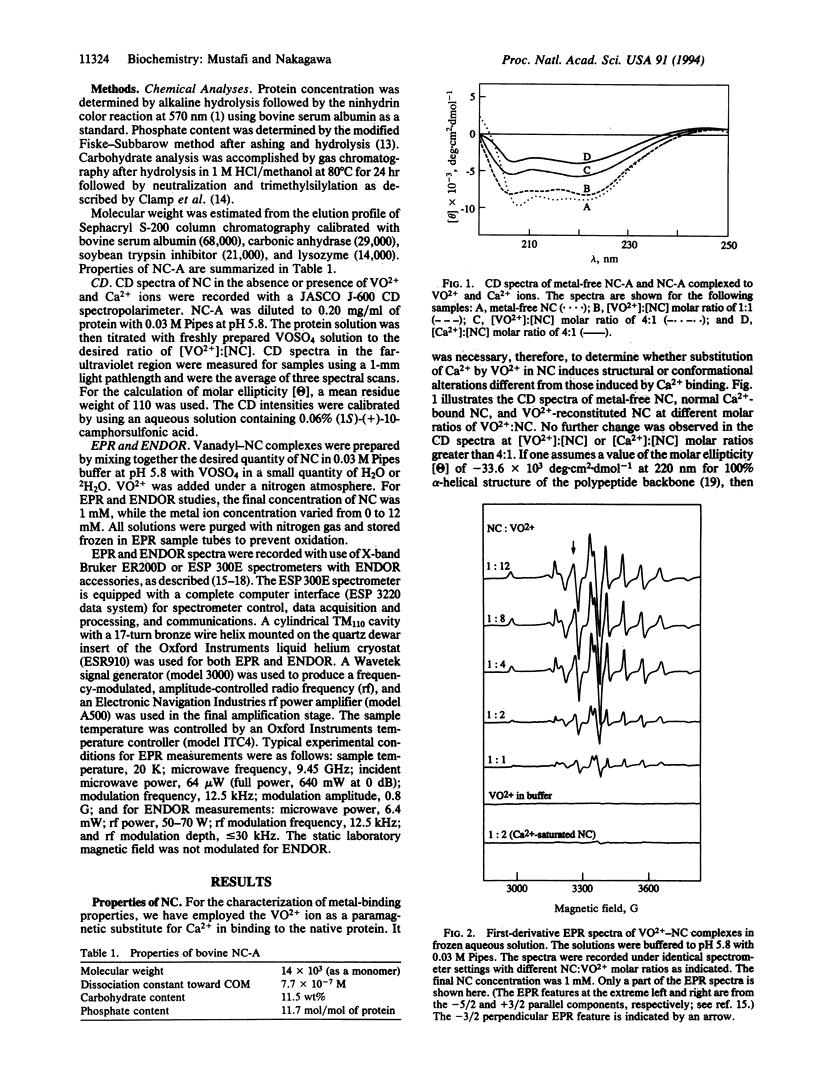
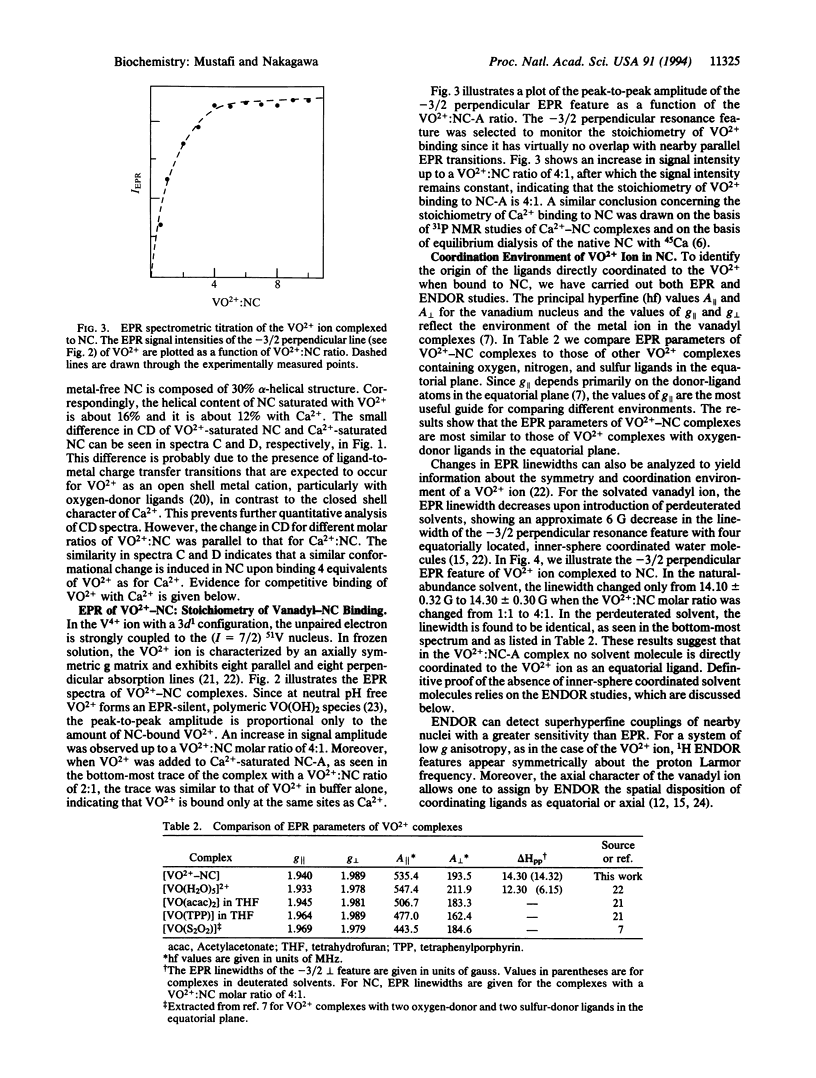
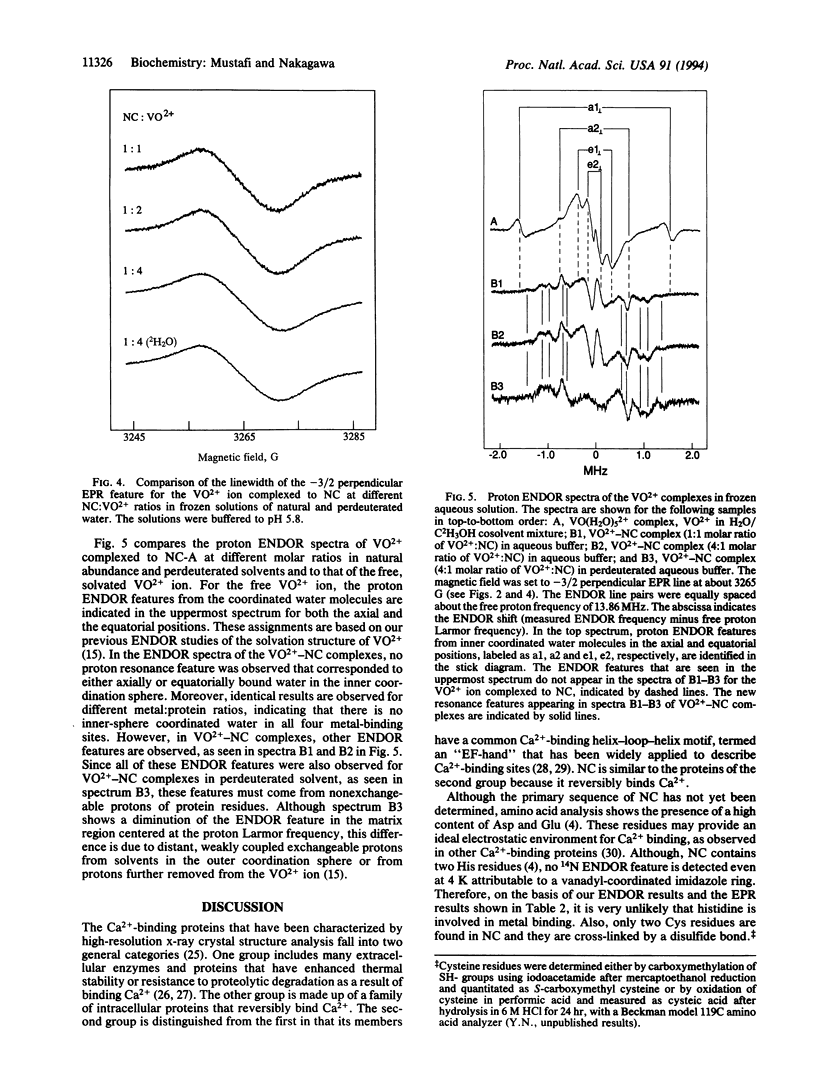
Nephrocalcin (NC) is a calcium-binding glycoprotein of 14,000 molecular weight. It inhibits the growth of calcium oxalate monohydrate crystals in renal tubules. The NC used in this study was isolated from bovine kidney tissue and purified with the use of DEAE-cellulose chromatography into four isoforms, designated as fractions A-D. They differ primarily according to the content of phosphate and gamma-carboxy-glutamic acid. Fractions A and B are strong inhibitors of the growth of calcium oxalate monohydrate crystal, whereas fractions C and D inhibit crystal growth weakly. Fraction A, with the highest Ca(2+)-binding affinity, was characterized with respect to its metal-binding sites by using the vanadyl ion (VO2+) as a paramagnetic probe in electron paramagnetic resonance (EPR) and electron nuclear double resonance (ENDOR) spectroscopic studies. By EPR spectrometric titration, it was shown that fraction A of NC bound VO2+ with a stoichiometry of metal:protein binding of 4:1. Also, the binding of VO2+ to NC was shown to be competitive with Ca2+. Only protein residues were detected by proton ENDOR as ligands, and these ligands bound with complete exclusion of solvent from the inner coordination sphere of the metal ion. This type of metal-binding environment, as derived from VO(2+)-reconstituted NC, differs significantly from the binding sites in other Ca(2+)-binding proteins.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babu Y. S., Bugg C. E., Cook W. J. Structure of calmodulin refined at 2.2 A resolution. J Mol Biol. 1988 Nov 5;204(1):191–204. doi: 10.1016/0022-2836(88)90608-0. [DOI] [PubMed] [Google Scholar]
- Chasteen N. D., DeKoch R. J., Rogers B. L., Hanna M. W. Use of vanadyl(IV) ion as a new spectroscopic probe of metal binding to proteins. Vanadyl insulin. J Am Chem Soc. 1973 Feb 21;95(4):1301–1309. doi: 10.1021/ja00785a048. [DOI] [PubMed] [Google Scholar]
- Clamp J. R., Bhatti T., Chambers R. E. The determination of carbohydrate in biological materials by gas-liquid chromatography. Methods Biochem Anal. 1971;19:229–344. doi: 10.1002/9780470110386.ch3. [DOI] [PubMed] [Google Scholar]
- Coffee C. J., Bradshaw R. A. Carp muscle calcium-binding protein. I. Characterization of the tryptic peptides and the complete amino acid sequence of component B. J Biol Chem. 1973 May 10;248(9):3305–3312. [PubMed] [Google Scholar]
- DeKoch R. J., West D. J., Cannon J. C., Chasteen N. D. Kinetics and electron paramagnetic resonance spectra of vanadyl(IV) carboxypeptidase A. Biochemistry. 1974 Oct 8;13(21):4347–4354. doi: 10.1021/bi00718a017. [DOI] [PubMed] [Google Scholar]
- Dijkstra B. W., Kalk K. H., Hol W. G., Drenth J. Structure of bovine pancreatic phospholipase A2 at 1.7A resolution. J Mol Biol. 1981 Mar 25;147(1):97–123. doi: 10.1016/0022-2836(81)90081-4. [DOI] [PubMed] [Google Scholar]
- Glusker J. P. Structural aspects of metal liganding to functional groups in proteins. Adv Protein Chem. 1991;42:1–76. doi: 10.1016/s0065-3233(08)60534-3. [DOI] [PubMed] [Google Scholar]
- Herzberg O., James M. N. Structure of the calcium regulatory muscle protein troponin-C at 2.8 A resolution. Nature. 1985 Feb 21;313(6004):653–659. doi: 10.1038/313653a0. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H., Nockolds C. E. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973 May 10;248(9):3313–3326. [PubMed] [Google Scholar]
- McPhalen C. A., Svendsen I., Jonassen I., James M. N. Crystal and molecular structure of chymotrypsin inhibitor 2 from barley seeds in complex with subtilisin Novo. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7242–7246. doi: 10.1073/pnas.82.21.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Abram V., Coe F. L. Isolation of calcium oxalate crystal growth inhibitor from rat kidney and urine. Am J Physiol. 1984 Nov;247(5 Pt 2):F765–F772. doi: 10.1152/ajprenal.1984.247.5.F765. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Abram V., Parks J. H., Lau H. S., Kawooya J. K., Coe F. L. Urine glycoprotein crystal growth inhibitors. Evidence for a molecular abnormality in calcium oxalate nephrolithiasis. J Clin Invest. 1985 Oct;76(4):1455–1462. doi: 10.1172/JCI112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Ahmed M., Hall S. L., Deganello S., Coe F. L. Isolation from human calcium oxalate renal stones of nephrocalcin, a glycoprotein inhibitor of calcium oxalate crystal growth. Evidence that nephrocalcin from patients with calcium oxalate nephrolithiasis is deficient in gamma-carboxyglutamic acid. J Clin Invest. 1987 Jun;79(6):1782–1787. doi: 10.1172/JCI113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y., Margolis H. C., Yokoyama S., Kézdy F. J., Kaiser E. T., Coe F. L. Purification and characterization of a calcium oxalate monohydrate crystal growth inhibitor from human kidney tissue culture medium. J Biol Chem. 1981 Apr 25;256(8):3936–3944. [PubMed] [Google Scholar]
- Nakagawa Y., Otsuki T., Coe F. L. Elucidation of multiple forms of nephrocalcin by 31P-NMR spectrometer. FEBS Lett. 1989 Jul 3;250(2):187–190. doi: 10.1016/0014-5793(89)80717-3. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Renz C. L., Ahmed M., Coe F. L. Isolation of nephrocalcin from kidney tissue of nine vertebrate species. Am J Physiol. 1991 Feb;260(2 Pt 2):F243–F248. doi: 10.1152/ajprenal.1991.260.2.F243. [DOI] [PubMed] [Google Scholar]
- Posner B. I., Faure R., Burgess J. W., Bevan A. P., Lachance D., Zhang-Sun G., Fantus I. G., Ng J. B., Hall D. A., Lum B. S. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem. 1994 Feb 11;269(6):4596–4604. [PubMed] [Google Scholar]
- Strynadka N. C., James M. N. Crystal structures of the helix-loop-helix calcium-binding proteins. Annu Rev Biochem. 1989;58:951–998. doi: 10.1146/annurev.bi.58.070189.004511. [DOI] [PubMed] [Google Scholar]
- Williams R. J. 16th Sir Hans Krebs lecture. The symbiosis of metal and protein functions. Eur J Biochem. 1985 Jul 15;150(2):231–248. doi: 10.1111/j.1432-1033.1985.tb09013.x. [DOI] [PubMed] [Google Scholar]
- Woody R. W. Improved calculation of the n-pi rotational strength in polypeptides. J Chem Phys. 1968 Dec 1;49(11):4797–4806. doi: 10.1063/1.1669962. [DOI] [PubMed] [Google Scholar]
- Yamashita M. M., Wesson L., Eisenman G., Eisenberg D. Where metal ions bind in proteins. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5648–5652. doi: 10.1073/pnas.87.15.5648. [DOI] [PMC free article] [PubMed] [Google Scholar]


