ABSTRACT
Antibodies that can neutralize diverse viral strains are likely to be an important component of a protective human immunodeficiency virus type 1 (HIV-1) vaccine. To this end, preclinical simian immunodeficiency virus (SIV)-based nonhuman primate immunization regimens have been designed to evaluate and enhance antibody-mediated protection. However, these trials often rely on a limited selection of SIV strains with extreme neutralization phenotypes to assess vaccine-elicited antibody activity. To mirror the viral panels used to assess HIV-1 antibody breadth, we created and characterized a novel panel of 14 genetically and phenotypically diverse SIVsm envelope (Env) glycoproteins. To assess the utility of this panel, we characterized the neutralizing activity elicited by four SIVmac239 envelope-expressing DNA/modified vaccinia virus Ankara vector- and protein-based vaccination regimens that included the immunomodulatory adjuvants granulocyte-macrophage colony-stimulating factor, Toll-like receptor (TLR) ligands, and CD40 ligand. The SIVsm Env panel exhibited a spectrum of neutralization sensitivity to SIV-infected plasma pools and monoclonal antibodies, allowing categorization into three tiers. Pooled sera from 91 rhesus macaques immunized in the four trials consistently neutralized only the highly sensitive tier 1a SIVsm Envs, regardless of the immunization regimen. The inability of vaccine-mediated antibodies to neutralize the moderately resistant tier 1b and tier 2 SIVsm Envs defined here suggests that those antibodies were directed toward epitopes that are not accessible on most SIVsm Envs. To achieve a broader and more effective neutralization profile in preclinical vaccine studies that is relevant to known features of HIV-1 neutralization, more emphasis should be placed on optimizing the Env immunogen, as the neutralization profile achieved by the addition of adjuvants does not appear to supersede the neutralizing antibody profile determined by the immunogen.
IMPORTANCE Many in the HIV/AIDS vaccine field believe that the ability to elicit broadly neutralizing antibodies capable of blocking genetically diverse HIV-1 variants is a critical component of a protective vaccine. Various SIV-based nonhuman primate vaccine studies have investigated ways to improve antibody-mediated protection against a heterologous SIV challenge, including administering adjuvants that might stimulate a greater neutralization breadth. Using a novel SIV neutralization panel and samples from four rhesus macaque vaccine trials designed for cross comparison, we show that different regimens expressing the same SIV envelope immunogen consistently elicit antibodies that neutralize only the very sensitive tier 1a SIV variants. The results argue that the neutralizing antibody profile elicited by a vaccine is primarily determined by the envelope immunogen and is not substantially broadened by including adjuvants, resulting in the conclusion that the envelope immunogen itself should be the primary consideration in efforts to elicit antibodies with greater neutralization breadth.
INTRODUCTION
The goal of preclinical human immunodeficiency virus (HIV)/simian immunodeficiency virus (SIV) vaccine studies performed in nonhuman primates is to generate protective immunity through safe and effective immunization regimens that can subsequently be administered to human populations to decrease their risk for acquiring HIV type 1 (HIV-1). In the last decade, a significant portion of the HIV vaccine effort has focused on optimizing vaccine regimens to elicit protection in the rhesus macaque model, using immunogens and challenge viruses selected from a small subset of SIVs of the sooty mangabey lineage (SIVsm) (1). Recently, the field has shifted toward testing novel adjuvants and delivery modes in various combinations for their ability to enhance immune responses (2), particularly those targeting the induction of broadly neutralizing antibodies against the envelope (Env) glycoproteins (3–5). However, limited data are available regarding how immunomodulatory adjuvants and vaccine delivery modes compare in their ability to alter the neutralizing antibody profile elicited against a particular Env immunogen. It is difficult to compare antibody responses across vaccine trials if the Env immunogen is not the same and the timing of immunizations is not synchronized. Moreover, reagents with which to assess the breadth of neutralizing antibodies against SIV are limited. While the properties of the HIV-1 Env that are necessary to induce potent, broadly cross-neutralizing antibodies are under intense investigation, it is unknown whether the findings can be modeled with preclinical SIV vaccine studies.
The SIVmac239 strain has been included in multiple preclinical vaccines, despite the fact that the SIVmac239 Env is unusually resistant to neutralizing antibodies (6–9). This paradox may have stemmed from the fact that cell-mediated immune responses against SIVmac239 (and the highly related strain SIVmac251) and the major histocompatibility alleles that mediate them in rhesus macaques have been well characterized (10–15). Letvin et al. demonstrated that an SIVmac239 Env-containing vaccine did not mediate protection against intrarectal challenge with the closely related, neutralization-resistant viral quasispecies SIVmac251 but the same vaccine provided protection against heterologous intrarectal SIVsmE660 challenge (16). SIVsmE660 is a viral quasispecies that mainly consists of neutralization-sensitive tier 1 Env variants and a minor population of resistant variants (17, 18). SIVsmE660 exhibits phenotypic variability not only in neutralization sensitivity but also in pathogenicity and sensitivity to TRIM5α-mediated restriction (17–20). Because SIVsmE660 is largely susceptible to neutralization and its Env is substantially genetically distant from the SIVmac239 Env, this virus has become the most widely used heterologous challenge virus following SIVmac239 immunization. Thus, even though the SIVmac239 Env has been included in multiple preclinical vaccine regimens, some of which elicited protective immunity, it has not been formally determined whether this Env immunogen elicited antibodies that neutralize genetically and phenotypically diverse SIV isolates. Furthermore, it is unknown whether different modes of vaccination, different adjuvants, or distinct forms of Env alter SIVmac239-induced neutralizing antibody specificities.
In the recent past, protection against intrarectal SIVsmE660 challenge has been achieved using varied regimens and is often linked with antibody activity (16, 18, 21–24). However, the assessment of neutralizing antibody activity in these trials has relied on a limited set of SIV Envs derived from just three strains, SIVmac239, SIVmac251, and SIVsmE660, with the first two being highly related (18, 21, 22, 24–29). Furthermore, these Envs tend to reflect those selected for extreme neutralization sensitivity (tier 1a) or resistance (tier 3), with limited inclusion of other genetically diverse Envs or those that have a more moderate and perhaps representative neutralization phenotype. Contrary to this limited approach, the assessment of neutralizing antibody activity against HIV-1 in plasma samples from infected and vaccinated individuals is generally based on multiclade panels of genetically diverse HIV-1 Envs that include Envs of tiers 1 to 3 (30–32). The persistent use of SIV Env clones selected for their unusually high or low sensitivity to neutralization to assess the quality of vaccine-elicited antibodies and their contribution to protection may provide misleading results. Strong evidence suggests that to protect against HIV-1, vaccine-elicited neutralizing antibodies need to target epitopes that are vulnerable on the majority of genetically diverse HIV-1 isolates and that have moderate (tier 2) to high (tier 3) levels of neutralization resistance. Furthermore, broadly neutralizing antibodies against HIV-1 often require certain germ lines and high levels of somatic hypermutation (33), and these types of neutralizing antibodies have not been documented in a nonhuman primate model, in part because the tools for such an assessment are lacking (34–37).
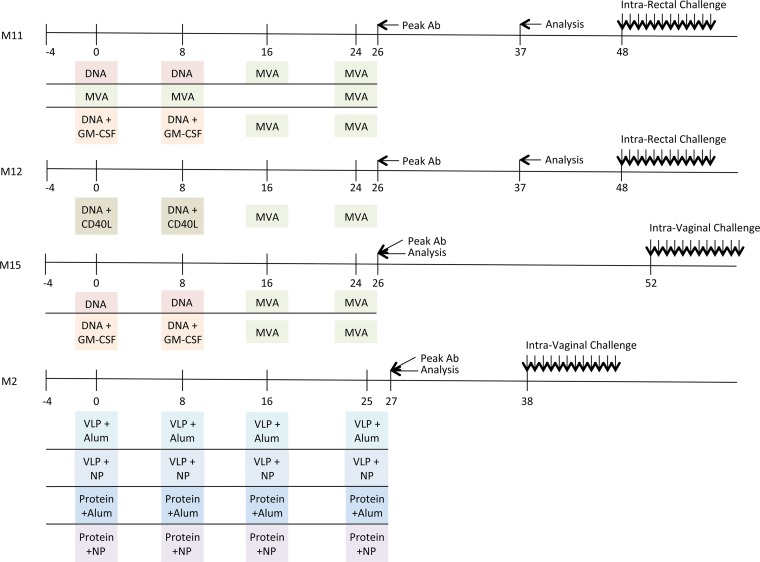
In 2011, Lai et al. reported that granulocyte-macrophage colony-stimulating factor (GM-CSF)-adjuvanted SIVmac239-based vaccination (the M11 trial) in rhesus macaques using the DNA/modified vaccinia virus Ankara (MVA) vaccine modality enhanced protection against repeated, low-dose intrarectal challenge with SIVsmE660 (22). A schematic of the timelines of the M11 trial and the trials discussed below is shown in Fig. 1. A recently published follow-up study (the M12 trial) sought to recapitulate the results obtained with the GM-CSF adjuvant and compare them to the results obtained with a novel CD40 ligand (CD40L) adjuvant using the same vaccination schedule (21). The CD40L adjuvant also conferred protection against repeated, low-dose intrarectal challenge with SIVsmE660 (21). Building on these findings, the Emory Consortium for AIDS Vaccine Research in Non-Human Primate Models sought to assess the reported protection in the context of vaginal transmission, which is highly relevant to the global HIV pandemic. Two additional trials (the M2 and M15 trials) were designed and carried out using the same vaccination schedule and repeated, low-dose SIVsmE660 challenge as the M11 and M12 trials for the direct purpose of cross comparison. The M15 trial sought to interrogate the efficacy of the GM-CSF adjuvant in the vaginal challenge model, while the M2 trial was designed to compare novel means of delivery of the Env protein or virus-like particles (VLPs) with Toll-like receptor (TLR) ligand adjuvants in the context of poly(lactic-coglycolic acid) nanoparticles (NPs) also in a vaginal challenge model. These four trials were purposefully designed and carried out at the Yerkes National Primate Research Center using parallel sampling timelines, comparable SIVmac239 immunogens and SIVsmE660 challenge stocks, and uniform methods. This resulted in an unprecedented opportunity to examine and compare samples from a large number of individual rhesus macaques in a vaccination/challenge trial dynamic.
FIG 1.
Diagrammatic timeline of nonhuman primate vaccine trials. The immunization schedule for each vaccine trial (M11, M12, M15, and M2) is plotted along a timeline (in weeks). The agents used for priming and boosting for each trial are indicated in colored boxes, highlighting the similarities and differences between trials. The time points where the peak levels of antibody (Ab) binding to SIVsmE660- and SIVmac239-derived gp140 proteins were observed are indicated by an arrow and “Peak Ab.” The time points at which samples were analyzed for neutralizing activity here are indicated by an arrow and “Analysis.” Additional information is provided in Table 5 and Table S1 in the supplemental material.
Our interest was to investigate whether these four trials elicited similar neutralizing antibody profiles and if there was evidence for a breadth of neutralization against diverse SIV variants. To mirror the approach widely used for analysis of the breadth of antibody activity against HIV-1, we developed a panel of genetically diverse SIV Envs from the sooty mangabey lineage (SIVsm) that includes 4 widely used reference Envs and 10 novel Envs not previously used for this purpose. We show that, like HIV-1 Envs, the SIVsm Envs displayed a spectrum of neutralization sensitivity, with most of the SIVsm Envs falling within a tier 2 or 3 neutralization phenotype. Extreme tier 1a neutralization sensitivity was associated with greater antibody access to epitopes in the V3 domain, CD4 binding site (CD4bs), and CD4-induced (CD4i) structures. However, tier 1a neutralization sensitivity was also associated with CD4-independent entry, a phenomenon rarely observed with HIV-1 Envs. Utilizing samples from the four vaccine trials, we analyzed the ability of distinct forms of SIVmac239 Env, delivered via different modes of vaccination and with novel cytokine and immunostimulatory adjuvants, to elicit antibodies that neutralize this panel of SIVsm isolates. Pooled serum samples from 91 rhesus macaques immunized in four nonhuman primate trials using SIVmac239-derived Env immunogens allowed specific analysis of the effects of different vaccine regimens. This analysis revealed that the SIVmac239 Env does not elicit antibodies capable of potently neutralizing even moderately sensitive (tier 1b and 2), genetically diverse SIVsm Envs on a consistent basis, regardless of the vaccine delivery mode, adjuvant, or form of Env. To our knowledge, this study represents the first to systematically compare neutralization profiles across four coordinated state-of-the-art preclinical vaccine regimens using a panel of diverse SIVsm Envs that exhibit a spectrum of neutralization resistance.
MATERIALS AND METHODS
Ethics statement.
The Emory University Institutional Animal Care and Use Committee (IACUC; AWA number A3180-01) approved these studies of nonhuman primates under protocol YER-2000936-061014GA. This study was conducted in strict accordance with United States Department of Agriculture regulations and the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Research Council (38). SIV-infected animals were housed in standard nonhuman primate cages, received standard primate feed, as well as fresh fruit and enrichment daily, and had continuous access to water. Animals had continuous access to enrichment resources, including objects for perching and other manipulanda. Animal welfare was monitored daily. Appropriate procedures were performed to ensure that potential distress, pain, or discomfort was alleviated. The sedative ketamine (10 mg/kg of body weight) or tiletamine and zolazepam (Telazol; 4 mg/kg) was administered before blood draws. Euthanasia using pentobarbital (100 mg/kg) was performed while the animals were under anesthesia and only when deemed clinically necessary by veterinary medical staff and according to IACUC endpoint guidelines.
Origin of the SIVsm Env clones.
All env genes used in this study were previously cloned through various studies of SIVsm or SIVmac infection. The following Envs were generated in the C. A. Derdeyn lab: pcDNA3.1-based plasmids carrying rev-env gene cassettes from four naturally SIVsm-infected sooty mangabeys in the Yerkes colony, sooty mangabeys FFv, FWk, FBn, and FNg (39–41); experimentally SIVsm-infected sooty mangabeys FFs (39) and FJv (42, 43); an experimentally SIVsm-infected rhesus macaque, rhesus macaque RSo8 (unpublished data); and two SIVmac251-infected rhesus macaques, rhesus macaques RZj5 and RZu4 (44, 45). pcDNA3.1-based plasmids carrying rev-env gene expression cassettes for SIVmac251.6 (46), SIVmac251.cs.41 (47), SIVmac239.cs.23 (46), and SIVsmE660.11 (48) were generously provided to us by David Montefiori. A summary of the SIVsm Env clones is provided in Table 1. The nucleotide sequence of all SIVsm env genes was determined in either the C. A. Derdeyn or David Montefiori lab, and Geneious R6 (v6.1.4) software was used to create amino acid alignments and nucleotide phylogenetic trees. The FigTree (v1.4.0) program, downloaded from http://tree.bio.ed.ac.uk/, was used to annotate the phylogenetic tree.
TABLE 1.
Description of SIVsm Env panel
| SIVsm Env | Virus | Sourcea | Reference(s) |
|---|---|---|---|
| FFv 18Nov04 ENVPL2.1 | SIVsm | Naturally infected SM | 39 |
| FWk 12Aug04 ENVPL4.1 | SIVsm | Naturally infected SM | 39 |
| FBn 3Nov04 ENVPL1.1 | SIVsm | Naturally infected SM | 39 |
| FNg 21Oct04 ENVPL2.1 | SIVsm | Naturally infected SM | 39 |
| FJv 15Nov06 ENVPL2.1 | SIVsm-Fqi | Experimentally infected SM | 41–43 |
| FFs 12Dec05 ENVPL4.1 | SIVsm-Fuo | Experimentally infected SM | 39 |
| RSo8 17Jan06 ENVPL1.1 | SIVsm-Fuo | Experimentally infected RM | Unpublished |
| RZj5 9Apr09 ENVPL2.1 | SIVmac251 | CD4-depleted experimentally infected RM | 44, 45 |
| RZu4 16Apr09 ENVPL1.1 | SIVmac251 | Experimentally infected RM | 44, 45 |
| RZu4 16Apr09 ENVPL11.1 | SIVmac251 | Experimentally infected RM | 44, 45 |
| SIVmac251.6 | SIVmac251 | David Montefiori | 46 |
| SIVmac251.cs.41 | SIVmac251 | David Montefiori | 47 |
| SIVmac239.cs.23 | SIVmac239 | David Montefiori | 46 |
| SIVsmE660.11 | SIVsmE660 | David Montefiori | 48 |
SM, sooty mangabey; RM, rhesus macaque.
MAbs, inhibitors, and plasma/serum pools.
Monoclonal antibodies (MAbs) 3.11H, 6.10B, and 1.4H were kindly provided by James E. Robinson. MAb 3.11H targets a linear epitope in the SIVsm Env V3 domain and was derived from an SIV/17E-CL-infected rhesus macaque at approximately 12 months postinfection (17, 49). MAbs 6.10B and 1.4H were derived from HIV-2 infected subject N37126 in the Gambia and target overlapping epitopes in the HIV-2 and SIV Envs, defined as the CD4 binding site and a soluble CD4 (sCD4)-enhanced epitope, respectively (17, 49–53). A neutralizing mouse monoclonal antibody against SIVmac251 gp120 was obtained from Advanced Bioscience Laboratories, Inc. (catalog no. 4327). AMD-3100 (bicyclam JM-2987) inhibits entry via CXCR4 and was obtained through the NIH AIDS Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) (54–56). Maraviroc (Selzentry) inhibits entry via CCR5 and was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH (57, 58). Recombinant human sCD4 was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH. T1249 inhibits the entry process by blocking gp41-mediated fusion and was a kind gift from Trimeris (59). Three polyclonal plasma pools were generated from previously SIV-infected monkeys at the Yerkes National Primate Research Center, using stored samples from the laboratories of Francois Villinger and Rama R. Amara. The RM239 pool was generated from 20 SIVmac239-infected rhesus macaque plasma samples collected at ∼33 weeks postinfection; the RM251-1 pool was generated from 15 SIVmac251-infected rhesus macaque plasma samples collected at ∼16 weeks postinfection; the SM pool was generated from 29 naturally SIVsm-infected sooty mangabeys. The RM251-2 (SIVmac251) antiserum pool was obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH (60). A pool of sera from 8 uninfected, unvaccinated, healthy rhesus macaques at Yerkes was generated to serve as a negative control. A summary of these reagents is provided in Table 2.
TABLE 2.
Description of reagents used to characterize the SIVsm Env panela
| Reagent | Target | Provider | Catalog no. | Source | Reference(s) |
|---|---|---|---|---|---|
| Rhesus anti-HIV-2 MAb 3.11H | V3 | James E. Robinson | SIV/17E-CL-infected RM at ∼12 mo | 17, 49 | |
| Human anti-HIV-2 MAb 6.10B | CD4bs | James E. Robinson | HIV-2-infected Gambian subject N37126 | 17, 49–53 | |
| Human anti-HIV-2 MAb 1.4H | CD4i | James E. Robinson | HIV-2-infected Gambian subject N37126 | 17, 49–53 | |
| Mouse anti-SIVmac251 MAb | gp120 | Advanced Bioscience Laboratories, Inc. | 4327 | ||
| AMD-3100 (JM-2987) | CXCR4 | AIDS Reagent Program | 8128 | 54–56 | |
| Maraviroc (Selzentry) | CCR5 | AIDS Reagent Program | 11580 | 57, 58 | |
| Recombinant human sCD4 | CD4bs | AIDS Reagent Program/Progenics | 4615 | ||
| T1249 | gp41 HR1 | Trimeris | 59 | ||
| SIVmac239-infected RM plasma pool | Polyclonal | Francois Villinger, Yerkes | 20 rhesus macaques ∼33 wk after SIV infection | ||
| SIVmac251-infected RM plasma pool 1 | Polyclonal | Rama R. Amara, Yerkes | 15 RMs ∼16 wk after SIV infection | ||
| SIV-infected SM pool | Polyclonal | Francois Villinger, Yerkes | 29 naturally SIV-infected SMs | ||
| SIVmac251-infected serum pool 2 | Polyclonal | AIDS Reagent Program | 2773 | 6 SIV-infected RMs | 60 |
| Normal RM serum pool | NA | Yerkes | 8 uninfected, unvaccinated RMs |
SM, sooty mangabey; RM, rhesus macaque; NA, not available.
Neutralization and inhibition assays.
Each SIVsm Env pseudovirus was generated by transfecting the Env-expressing plasmid DNA alongside the HIV-1 SG3ΔEnv proviral backbone DNA into 293T cells, using the Fugene HD reagent, as recommended by the manufacturer (Promega). Pseudovirus stocks were collected from the 293T cell supernatants at 48 to 72 h after transfection, clarified by centrifugation, divided into small volumes, and frozen at −80°C. Fivefold serial dilutions of heat-inactivated plasma or serum samples, entry inhibitors, or monoclonal antibodies were assayed for their inhibitory potential against the Env pseudoviruses using the Tzm-bl indicator cell line, with luciferase as the readout, as described previously (39, 61–67). Briefly, Tzm-bl cells were plated and cultured overnight in flat-bottomed 96-well plates. Pseudovirus (2,000 IU per well) in Dulbecco modified Eagle medium (DMEM) with ∼3.5% fetal bovine serum (FBS; HyClone), 40 μg/ml DEAE-dextran was incubated with serial dilutions of plasma, inhibitor, or monoclonal antibody and subsequently added to the plated Tzm-bl cells. At 48 h postinfection, the cells were lysed and luciferase activity was measured using a BioTek Synergy HT multimode microplate reader with Gen 5 (v2.0) software. The average background luminescence from a series of uninfected wells was subtracted from the luminescence for each experimental well, and infectivity curves were generated using GraphPad Prism (v6.0d) software, where values from the experimental wells were compared against the value from a well containing virus only with no test reagent. The 50% inhibitory concentrations were determined using the growth function in the software Microsoft Excel for Mac 2011 (v14.0.2). Tests with each Env-reagent combination were performed at least twice independently in duplicate wells within each individual experiment. Statistical analyses were performed using GraphPad Prism (v6.0d) software. For comparisons and rankings across multiple groups, a nonparametric Kruskal-Wallis test was performed with Dunn's correction by comparison against a reference group.
CD4 independence assay.
The CD4 independence assay was performed in the R. G. Collman laboratory. Pseudotyped virions were generated by cotransfecting human 293T cells with the pNL4-3Luc E-R+ backbone alongside plasmids carrying the SIVsm env genes, as described previously (44, 45). Pseudotypes lacking Env (made by cotransfecting the pNL4-3Luc E-R+ [where E-R+ is ENV− VPR+] backbone with pcDNA3.1) served as a negative control, and pseudotypes containing vesicular stomatitis virus glycoprotein G served as a positive control. Transfections were performed using the Fugene 6 reagent, as recommended by the manufacturer (Promega), and the cells were washed at 24 h posttransfection. Supernatants were collected at 72 h posttransfection and stored at −80°C in 5% sucrose. Human 293T cells were maintained in DMEM supplemented with 10% FBS, 1% l-glutamine, and 1% penicillin-streptomycin. For measurement of CD4-independent entry, human 293T cells were transfected with 1 μg of plasmid pcDNA3.1 expressing rhesus macaque CD4 and 1 μg of plasmid pcDNA3.1 expressing rhesus macaque CCR5 (CD4 plus CCR5), 1 μg of empty pcDNA3.1 and 1 μg of plasmid pcDNA3.1 expressing rhesus macaque CCR5 (CCR5 only), or 2 μg of the empty pcDNA3.1 plasmid (negative control) using the Fugene 6 reagent as recommended the manufacturer (Promega). At 24 h posttransfection, the cells were washed and replated at 2 × 104 cells per well. At 48 h posttransfection, the cells were spinoculated (by centrifugation at 1,200 × g for 2 h at 25°C) with 20 μl of the Env pseudotype virus. Pseudotype viruses were treated with DNase prior to infection, and infections were performed in triplicate. At 72 h postinfection, cells were lysed with 0.5% Triton X-100 in phosphate-buffered saline, and the luciferase content was read on a luminometer using a luciferase assay system (Promega).
Samples from nonhuman primate vaccine trials.
Serum samples were obtained from 91 monkeys immunized during four SIV vaccine trials (designated trials M2, M11, M12, and M15) previously carried out at the Yerkes National Primate Research Center. All trials utilized SIVmac239 immunogens and a repeated, low-dose SIVsmE660 mucosal challenge in rhesus macaques. Immunized and control animals in the M11 and M12 trials were challenged 12 times intrarectally, while animals in the M2 and M15 trials were challenged 12 times intravaginally. The M11 trial was conducted by Rama R. Amara and consisted of three vaccine groups: DNA primes and MVA boosts (DDMM), DNA primes with GM-CSF adjuvant and MVA boosts (DgDgMM), and three MVA immunizations (MMM) (22). In the M11 trial, two serum pools were generated from samples collected from animals at a prechallenge time point that was 13 weeks after the second MVA boost (week 37): one pool of samples from 14 vaccinated animals that became infected and one pool of samples from 9 vaccinated animals that did not become infected. An additional arm, designated trial M12, was later included and consisted of a DNA prime with CD40L adjuvant followed by an MVA boost (DM/CD40L). In trial M12, pools of sera were also collected from 8 infected and 4 uninfected vaccinated monkeys at week 37. Trial M2 was led by Bali Pulendran and consisted of three gp140 protein or VLP immunizations delivered with alum (protein plus alum or VLPs plus alum) or nanoparticle (monophosphoryl lipid A and resiquimod; protein plus NPs or VLPs plus NPs) adjuvants. In trial M2, serum pools were generated with samples collected at week 27, which was a prechallenge time point 2 weeks after the last immunization that corresponded with observed peak antibody levels. Serum pools were created with samples from 22 infected and 15 uninfected vaccinated animals. Trial M15 was conducted by Rama R. Amara and was similar to the M11 trial in that the vaccination arms were a DNA prime with or without the GM-CSF adjuvant with two MVA boosts. In trial M15, serum pools were generated with samples from 12 infected and 7 uninfected vaccinated animals collected at the prechallenge week 26 time point that corresponded with observed peak antibody levels. The time points chosen for collection of samples for analysis in each trial were based on availability and may not represent the most optimal time point for measurement of neutralizing antibody activity.
RESULTS
Characteristics of SIVsm Env panel.
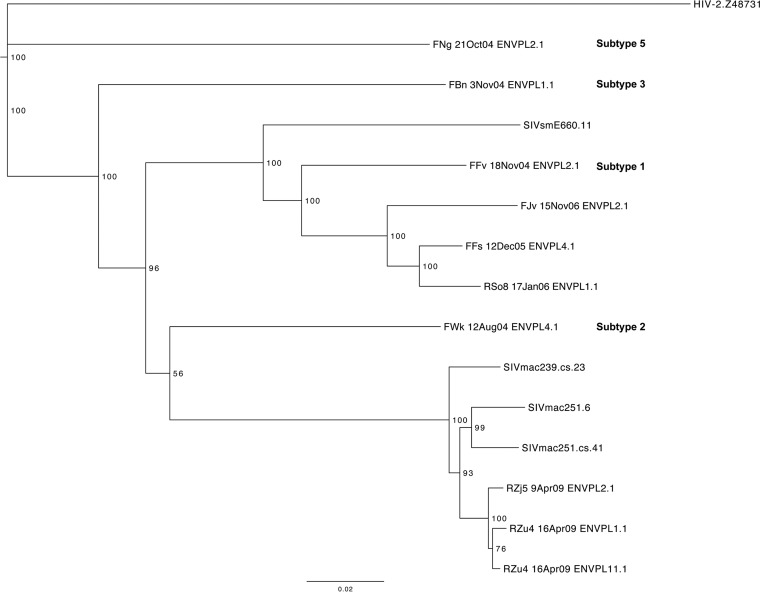
SIV-based vaccine efforts have been hampered by the limited number of well-defined, genetically distinct SIVmac/SIVsm variants available for neutralization studies. To represent diversity among SIVsm-derived viruses, 14 SIVsm Envs were selected to create a neutralization breadth panel (Table 1). Four Envs were obtained from David Montefiori to serve as previously characterized, widely used reference Envs (the SIVsmE660.11 [E660.11], SIVmac251.6, SIVmac251.cs.41, and SIVmac239.cs.23 Envs). Four Envs were obtained from naturally infected sooty mangabeys in the Yerkes colony and represent subtypes 1, 2, 3, and 5 of SIVsm (FFv 18Nov04 ENVPL2.1, FWk 12Aug04 ENVPL4.1, FBn 3Nov04 ENVPL1.1, and FNg 21Oct04 ENVPL2.1, respectively [referred to as the FFv, FWk, FBn, and FNg Envs, respectively]) (68, 69) (Fig. 2). Two Envs from sooty mangabeys (FFs 12Dec05 ENVPL4.1 and FJv 15Nov06 ENVPL2.1 [the FFs and FJv Envs, respectively]) and four from rhesus macaques (RSo8 17Jan06 ENVPL1.1, RZu4 16Apr09 ENVPL1.1, RZu4 16Apr09 ENVPL11.1, and RZj5 9Apr09 ENVPL2.1 [the RSo8, RZu4-1.1, RZu4-11.1, and RZj5 Envs, respectively]) experimentally infected with SIVsm or SIVmac251 were also included. Two of the 14 Envs, the FJv Env and RZj5 Env, had unusual phenotypes and were included on the basis of the premise that they might expose different neutralization targets. The FJv Env was recovered from an experimentally SIVsm-infected sooty mangabey that experienced a CD4 T-cell decline and utilizes CXCR4 (42, 43); the RZj5 Env was recovered from an SIVmac251-infected rhesus macaque that was treated with a CD4-depleting monoclonal antibody prior to infection and efficiently utilizes CCR5 for entry in the absence of CD4 (i.e., it is CD4 independent) (44, 45).
FIG 2.
Phylogenetic tree of the SIVsm Env neutralization breadth panel. A neighbor-joining phylogenetic tree was created from complete env nucleotide sequences using the Jukes-Cantor approach in Geneious R6 (v6.1.4) software and annotated in the FigTree (v1.4.0) program. A reference HIV-2 env sequence (GenBank accession number Z48731) was used as the outgroup. The horizontal bar at the bottom shows the scale of the genetic distance, and bootstrap values are indicated at each node. The subtypes of sequences from naturally SIV-infected sooty mangabeys FNg, FBn, FFv, and FWk are indicated in bold text.
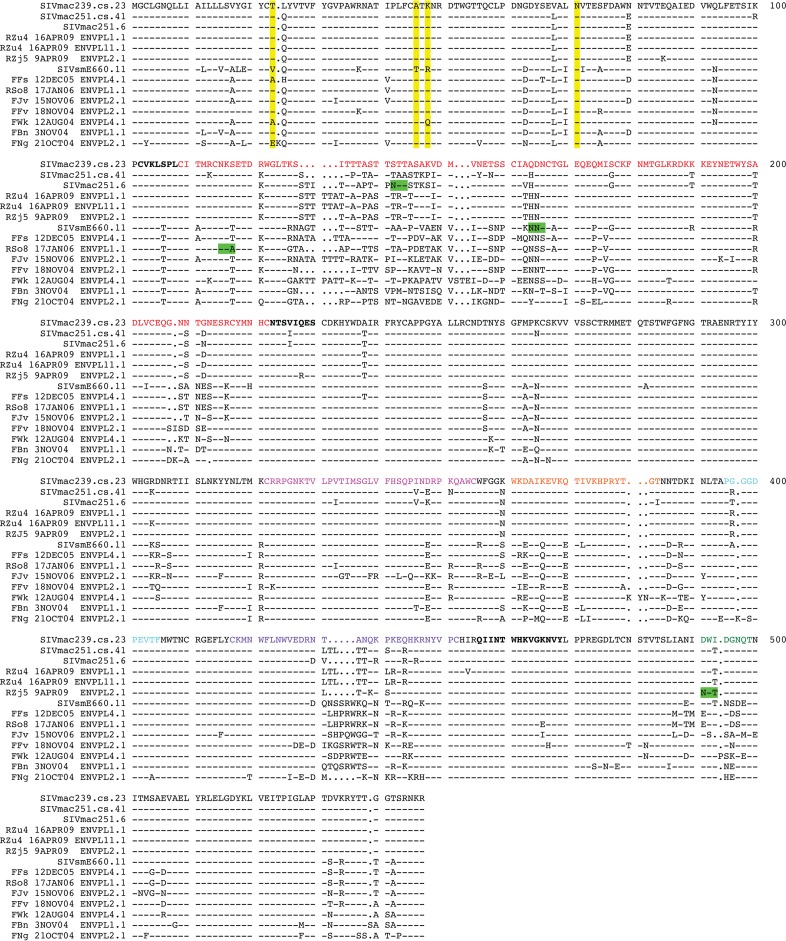
An amino acid alignment of the gp120 sequences for those Envs included in the panel is shown in Fig. 3, with the SIVmac239.cs.23 sequence being used as the reference. The pairwise percent identity of the Env gp160 sequences at the amino acid level ranged from 78 to 99% (data not shown). The SIVsm Env sequences contained a median of 27 putative N-linked glycosylation motifs (PNLGs) in gp160, ranging from 23 in FJv to 29 in FBn. Of these 27 sites, 14 were conserved in all Envs. The V1V2 domain contained a median of 7 PNLGs (range, 5 to 10), while V4 contained 0 PNLGs or 1 PNLG. Interestingly, the RSo8 and E660.11 Envs each lacked a highly conserved PNLG at the N-terminal (N115) or C-terminal (N164) region of the V1 domain, respectively (numbering is according to the sequence for SIVmac239.cs.23 in Fig. 3). In addition, the RZj5 Env contained the addition of a unique PNLG adjacent to the CD4 binding pocket (D491N in Fig. 3; D470N in the previous study [45]) that confers CD4 independence. The region immediately C terminal to V3 was devoid of PNLGs in all 14 Envs, meaning that no equivalent to the N332 PNLG site targeted by broadly neutralizing antibodies in HIV-1 Envs was present in the SIVsm Envs.
FIG 3.
Amino acid sequence alignment of the SIVsm Env panel. An amino acid alignment of the Env gp120-coding sequence was generated using the SeqPublish tool, available from the Los Alamos National Laboratory HIV Database (http://www.hiv.lanl.gov/content/sequence/SeqPublish/seqpublish.html). The sequence of SIVmac239.cs.23 is shown as the reference sequence, and dashes indicate conserved residues, while dots indicate deleted residues. Yellow highlighting, the positions of the four amino acid residues (positions 23, 45, 47, and 70) described in reference 18, with A45 and K47 being described to be major determinants of SIVsmE660 neutralization resistance in that reference; green highlighting, atypical PNLG sites that are associated with the tier 1 Envs. Functional gp120 domains are indicated by colored text as follows: red, V1V2; magenta,V3; orange, α2 helix; purple, V4; green,V5; bold text, bridging sheet residues, as described previously (72); cyan, the CD4 binding loop.
Although coreceptor utilization for some of the Envs had been defined previously, we characterized the coreceptor utilization for all Envs using entry inhibitors in the Tzm-bl cell assay. As expected, all of the SIVsm Envs except for the CXCR4-tropic FJv Env were resistant to the CXCR4-specific inhibitor AMD-3100 and sensitive to the CCR5-specific inhibitor maraviroc (data not shown). These experiments established that all of the Envs except FJv utilize CCR5 for entry into the human Tzm-bl cell line. In addition, pooled serum from uninfected, unvaccinated, healthy rhesus macaques had no inhibitory activity against any Envs in the panel (data not shown).
Neutralization by pooled SIV-infected plasma or serum reveals tiers of neutralization sensitivity.
To define the general neutralization phenotype of each Env in the panel, we utilized four pools of plasma or serum from SIV-infected monkeys and four previously described anti-SIV or anti-HIV-2 neutralizing monoclonal antibodies (MAbs) (Fig. 4). More information on each reagent is listed in Table 2, and the neutralization curves for each Env are shown in Fig. S1 in the supplemental material. The neutralization data for all plasma/serum pools and MAbs tested against each Env pseudovirus were compiled for analysis by plotting the residual viral infectivity observed at the highest concentration, an approach similar to that used previously (17, 30) (Fig. 4). Note that 50% inhibitory concentrations (IC50) were not calculated and used for this analysis because many of the neutralization curves did not cross 50% infectivity (see Fig. S1 in the supplemental material). The polyclonal antibody pools consisted of plasma collected from (i) 20 SIVmac239-infected rhesus macaques at approximately 33 weeks postinfection (pool RM239), (ii) 15 SIVmac251-infected rhesus macaques at approximately 16 weeks postinfection (pool RM251-1), (iii) 29 naturally infected sooty mangabeys in the Yerkes colony (pool SM), and (iv) 6 SIVmac251-infected rhesus macaques from the NIH AIDS Reagent Program (pool RM251-2). The four MAbs were obtained from various sources. MAb 3.11H was recovered from a rhesus macaque infected with SIV/17E-CL, and it recognizes a linear epitope corresponding to the N-terminal half of the SIV V3 domain (17, 49). MAb 6.10B was recovered from an HIV-2-infected human subject, and it recognizes an epitope that overlaps the CD4 binding site (17, 50, 51). MAb 1.4H was isolated from the same HIV-2-infected subject from whom MAB 6.10B was isolated, and it competes with MAb 6.10B for binding to gp120 (50, 51). However, MAb 1.4H binding to soluble gp120 is enhanced by the addition of sCD4, indicating an epitope that is better exposed or stabilized by CD4 binding. Unlike MAb 3.11H, MAbs 6.10B and 1.4H recognize conformational epitopes (51). Finally, the anti-mac251 gp120 MAb is a mouse antibody for which the gp120-based epitope has not been defined.
FIG 4.
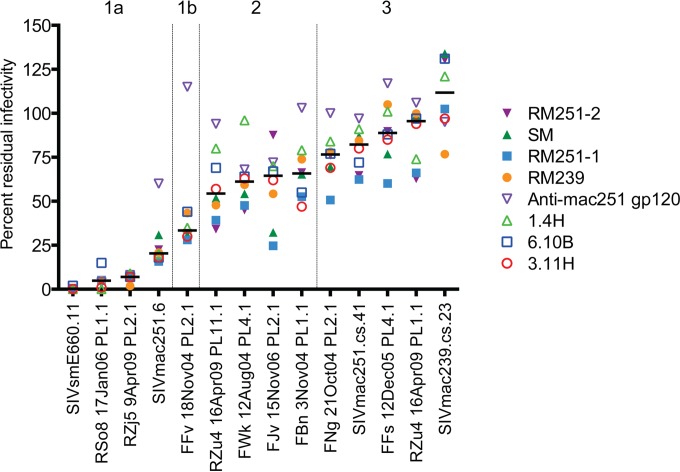
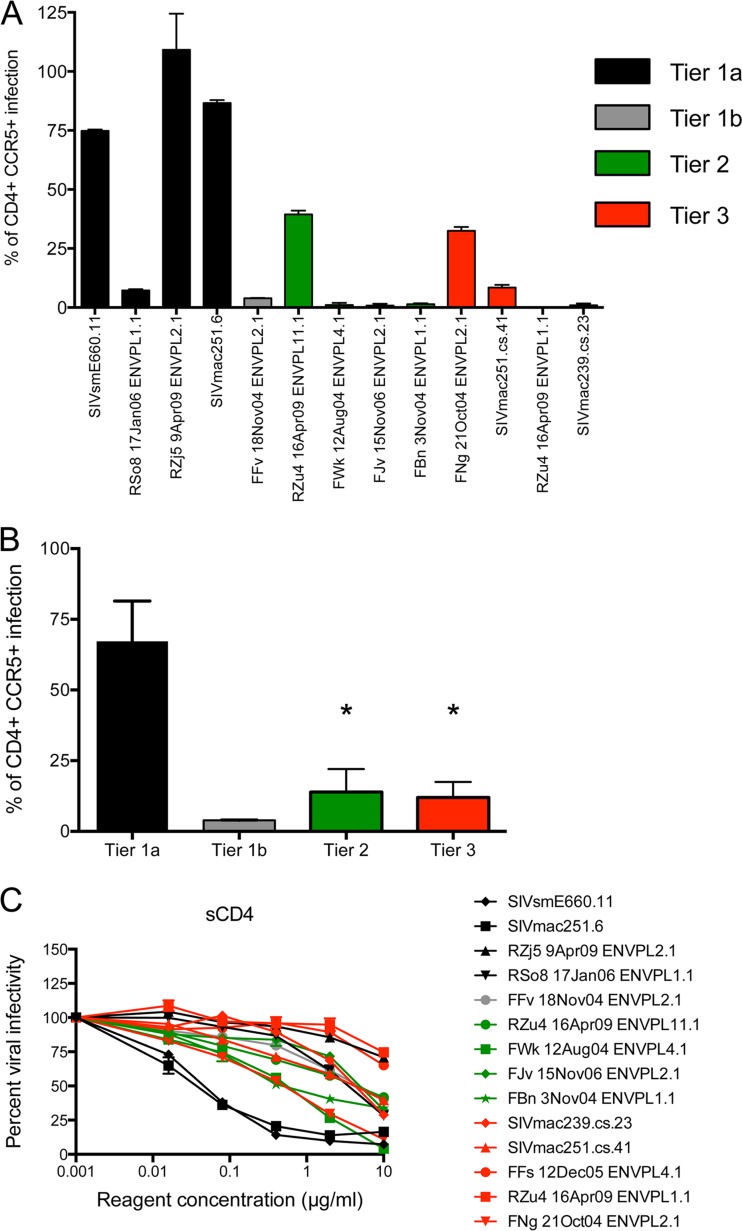
Inhibition of the SIVsm Env panel by SIV-infected plasma or serum pools and monoclonal antibodies. Eight different reagents were serially diluted 5-fold and tested for their neutralizing capacity against pseudoviruses expressing each SIVsm Env from the panel using the Tzm-bl cell assay, with luciferase activity being the readout. The reagents used were pools of plasma from experimentally SIVmac239-infected (pool RM239) and SIVmac251-infected (pool RM251-1) rhesus macaques, a pool of sera from a different set of experimentally SIVmac251-infected rhesus macaques (pool RM251-2), a pool of plasma from naturally SIV-infected sooty mangabeys (pool SM), an MAb from an SIV-infected rhesus macaque that targets V3 (MAb 3.11H), two MAbs from an HIV-2-infected patient that target the CD4bs (MAb 6.10B) and a CD4i epitope (MAb 1.4H), and an MAb from an immunized mouse that targets an undefined epitope on gp120 (anti-mac251 gp120). More details on each reagent are provided in Table 2, and the full neutralization graphs are presented in Fig. S1 in the supplemental material. The data on the vertical axis are expressed as the amount of residual infectivity at the highest concentration of reagent tested (1:100-diluted plasma or serum; 10 μg/ml of monoclonal antibody). Residual infectivity of 100% represents no inhibition, while 0% residual infectivity represents complete inhibition. Each Env pseudovirus and reagent-serum combination was tested in duplicate wells in at least two independent experiments. The horizontal bars represent the median for all reagents tested against each of the Env pseudoviruses, which are arranged from the most sensitive to the least sensitive from left to right.
A Kruskal-Wallis test was applied to the data in Fig. 4 to categorize the Envs into neutralization tiers, as has been done for HIV-1, first by comparing the neutralization of each Env to that of the highly neutralization-sensitive SIVsmE660.11 Env and then to that of the SIVmac239 Env. Table 3 demonstrates that the neutralization of Envs RSo8, RZj5, SIVmac251.6, and FFv was not statistically significantly different from that of the E660.11 Env, and these were therefore designated tier 1. For the remaining Envs, those whose neutralization differed from that of the E660.11 Env with a P-value range of 0.05 and 0.001 were designated tier 2, while those whose neutralization differed from that of the E660.11 Env with a P value of <0.001 were designated tier 3. For further depth into the tiers, the neutralization of Envs was compared in the same way against that of the SIVmac239 Env, a tier 3 Env at the neutralization-resistant end of the spectrum. The neutralization of all of the Envs defined as tier 2 or 3 on the basis of the comparison of their neutralization with that of the E660.11 Env was not statistically different from that of the SIVmac239 Env, and the neutralization of those defined as tier 1 Envs did show statistically significant differences. However, using the a threshold P value of <0.001, as described above, the tier 1 Envs could be further subdivided into tiers 1a and 1b. In addition, the individual neutralization curves supported the finding that the Env defined as tier 1b (FFv Env) was in most cases less sensitive to neutralization than the other tier 1 Envs (Fig. 4; see also Fig. S1 in the supplemental material). In conclusion, of the 14 Envs, 4 were designated tier 1a, 1 was designated tier 1b, 4 were designated tier 2, and 5 were designated tier 3 (Table 3). Like HIV-1, the majority of the SIVsm Envs displayed moderate to high resistance against heterologous neutralization (30, 31). Each neutralization tier included a diverse collection of SIVsm Envs, and the tiers did not segregate by phylogeny or SIVsm subtype (Fig. 2). As an example, the highly supported phylogenetic cluster in Fig. 2 (bottom of the tree) containing the SIVmac251-derived Envs RZj5, RZu4-11.1, and RZu4-1.1 contained a tier 1a, a tier 2, and a tier 3 Env, respectively. Thus, similar to the findings for HIV-1, the neutralization sensitivity of the SIVsm Envs could not be easily predicted by phylogenetic lineage.
TABLE 3.
Results of Kruskal-Wallis test to determine neutralization tiersa
| Env |
P value |
Tier | |
|---|---|---|---|
| SIVsmE660.11 | SIVmac239.cs.23 | ||
| SIVsmE660.11 | NA | <0.0001 | 1a |
| RSo8 17Jan06 PL1.1 | NS | <0.0001 | 1a |
| RZj5 9Apr09 PL2.1 | NS | <0.0001 | 1a |
| SIVmac251.6 | NS | 0.001–0.0001 | 1a |
| FFv 18Nov04 PL2.1 | NS | 0.01–0.001 | 1b |
| FWk 12Aug04 PL4.1 | 0.05–0.01 | NS | 2 |
| RZu4 16Apr09 PL11.1 | 0.05–0.01 | NS | 2 |
| FJv 15Nov06 PL2.1 | 0.05–0.01 | NS | 2 |
| FBn 3Nov04 PL1.1 | 0.01–0.001 | NS | 2 |
| FNg 21Oct04 PL2.1 | 0.001 to 0.0001 | NS | 3 |
| SIVmac251.cs.41 | <0.0001 | NS | 3 |
| RZu4 16Apr09 PL1.1 | <0.0001 | NS | 3 |
| FFs 12Dec05 PL4.1 | <0.0001 | NS | 3 |
| SIVmac239.cs.23 | <0.0001 | NA | 3 |
NA, not applicable; NS, not significant.
The tier 1a Envs SIVsmE660.11 and SIVmac251.6 have been widely used to assess vaccine-elicited antibody responses due to their extreme neutralization sensitivity. The remaining two tier 1a Envs were novel, representing an Env with CD4 independence and an Env from a nonnatural experimental sooty mangabey infection. The tier 1b Env FFv was recovered from a sooty mangabey naturally infected with SIVsm subtype 1. Inspection of the amino acid sequences in Fig. 3 revealed that the tier 1a Envs tended to have alterations in or near PNLG sites that were conserved in the other Envs. The RSo8 and E660.11 Envs each lacked a highly conserved PNLG site in V1, and the RSo8 Env also had an extended cytoplasmic tail resulting from a frameshift (Fig. 3). The RZj5 Env contained an unusual PNLG site adjacent to the CD4 binding pocket (D491N in Fig. 3; D470N in the previous report [45]) that confers CD4 independence. Finally, SIVmac251.6 also contained a unique PNLG site in the internal region of V1, at position 142 in Fig. 3, that was not present in the other Envs. Therefore, these genetically and phenotypically diverse tier 1a Envs tend to have at least one atypical feature that may enhance their overall neutralization sensitivity. All of the tier 2 Envs were novel and included a CXCR4-tropic Env from an experimental sooty mangabey infection, natural SIVsm Envs belonging to subtypes 2 and 3, and an SIVmac251-derived Env. For tier 3, the SIVmac239.cs.23 and SIVmac251.cs.41 Envs were the prototypes, but the remaining tier 3 Envs included one derived from SIVmac251, one from an experimental sooty mangabey infection, and one from a natural SIVsm subtype 5 infection.
Others have recently reported that two amino acids, 45A and 47K, in the C1 region of gp120 underlie the neutralization resistance phenotype exhibited by some SIVsmE660 Env variants (18). We therefore examined the amino acid sequences of our Env panel for these signature residues. Table 4 demonstrates that while Env E660.11 possessed the neutralization-sensitive-signature residues at these positions, 45T and 47R, the other three tier 1 Envs contained the 45A and 47K signature of resistance. In fact, E660.11 was the only Env in our panel that contained 45T and 47R. Furthermore, the other SIVsm Envs displayed a wide spectrum of neutralization sensitivity to heterologous and autologous plasma, even though they contained 45A/47K or 47Q, in one case (Fig. 4; see also Fig. S1 in the supplemental material). Given the diversity of the SIVsm Envs analyzed here, it seems likely that this 2-amino-acid signature confers neutralization resistance mainly in the context of some SIVsmE660 variants, while other determinants are also operative across more genetically diverse SIVsm Envs.
TABLE 4.
Amino acid signatures associated with neutralization phenotype in SIVsmE660
| SIVsm Env | Amino acid at positiona: |
nAbb tier | Residual infectivity against pooled SIV plasma/serum (%) | CD4 independence | Neutralized by vaccine serum | |||
|---|---|---|---|---|---|---|---|---|
| 23 | 45 | 47 | 70 | |||||
| SIVsmE660.11 | V | T | R | N | 1a | 0 | High | Yes |
| RZj5 9Apr09 ENVPL2.1 | T | A | K | N | 1a | 0 | High | Yes |
| RSo8 17Jan06 ENVPL1.1 | T | A | K | N | 1a | 0 | Low | Yes |
| SIVmac251.6 | T | A | K | N | 1a | 25 | High | Yes |
| FFv 18Nov04 ENVPL2.1 | T | A | K | N | 1b | 25–50 | Low | No |
| RZu4 16Apr09 ENVPL11.1 | T | A | K | N | 2 | 35–50 | Moderate | No |
| FWk 12Aug04 ENVPL4.1 | A | A | Q | N | 2 | 50–75 | Low | No |
| FJv 15Nov06 ENVPL2.1 | T | A | K | N | 2 | 25–100 | Low | No |
| FBn 3Nov04 ENVPL1.1 | T | A | K | N | 2 | 50–80 | Low | No |
| FNg 21Oct04 ENVPL2.1 | E | A | K | N | 3 | 50–80 | Moderate | No |
| SIVmac251.cs.41 | T | A | K | N | 3 | 60–90 | Low | No |
| RZu4 16Apr09 ENVPL1.1 | T | A | K | N | 3 | 60–100 | Low | No |
| FFs 12Dec05 ENVPL4.1 | A | A | K | N | 3 | 60–100 | NDc | No |
| SIVmac239.cs.23 | T | A | K | N | 3 | 75–100 | Low | No |
Amino acid residues are numbered according to the numbering for the SIVmac239 reference strain.
nAb, neutralizing antibody.
ND, not determined.
The pattern of neutralization by MAbs shown in Fig. 4 provided insight into the underlying vulnerability of the tier 1a Envs. Tier 1a Envs were potently inhibited by MAb 3.11H, suggesting that the V3 epitope recognized by this antibody is well exposed. The tier 1b and 2 Envs were moderately sensitive to MAb 3.11H, while the tier 3 Envs were poorly neutralized by MAb 3.11H at 10 μg/ml (Fig. 4). Sequence conservation of the linear V3 epitope did not correlate with neutralization in relation to the RZj5, RZu4-11.1, and RZu4-1.1 Envs. All three Envs were derived from SIVmac251 and have identical V3 domains (Fig. 3), yet they displayed high, moderate, and low sensitivity to MAb 3.11H neutralization, respectively (Fig. 4; see also Fig. S1 in the supplemental material). Furthermore, MAb 3.11H binds to soluble gp120 from SIVmac239 and SIVmac251 in enzyme-linked immunosorbent assays (ELISAs) (49) but did not mediate their neutralization (Fig. 4; see also Fig. S1 in the supplemental material). These findings argue that access to the V3 epitope on the Env trimer likely differs among the Envs and serves as the main determinant of neutralization by MAb 3.11H. The 6.10B and 1.4H MAbs showed a neutralization profile similar to that of MAb 3.11H (Fig. 4). Like MAb 3.11H, MAbs 6.10B and 1.4H also bind to soluble SIVmac239 gp120 but are unable to neutralize this pseudovirus (51). The tier 2 and 3 Envs were consistently more resistant to neutralization by the four MAbs (Fig. 4; see also Fig. S1 in the supplemental material). Overall, these results demonstrate that, like HIV-1 Envs, tier 1a SIV Envs tend to expose the V3, CD4bs, and CD4i structures involved in receptor binding (30), whereas these targets are much less accessible on the majority of SIVsm Envs.
The data shown in Fig. 4 and Fig. S1 in the supplemental material serve to further emphasize the question of whether or not antibodies capable of neutralizing tier 3 SIVsm Envs exist. This highlights a dilemma that broadly neutralizing antibodies analogous to VRC01, PG9/16, PGT128, etc., have not been identified for SIVsm and that cohorts of elite neutralizer SIV-infected macaques have not been described. The data presented here substantiate the idea that if this type of broadly neutralizing SIV antibody exists, it is not a major constituent of the polyclonal antibody population in most SIVmac-infected macaques or SIVsm-infected sooty mangabeys. In the absence of this type of reagent, we examined whether autologous plasma from six sooty mangabeys could neutralize the corresponding tier 2 or 3 SIVsm Envs (39). The SIVsm Envs displayed a spectrum of sensitivity to autologous neutralization by 1:20-diluted plasma collected 6 to 12 months later ranging from very sensitive (FFv and FJv Envs) to highly resistant (FBn and FFs Envs) (Fig. 5). Most HIV-1 Envs, including contemporaneous escape variants, can be neutralized by autologous antibodies circulating at later time points (61, 62, 65, 67, 70, 71). These limited results highlight the need to better define the relevance of the SIVsm neutralization tiers to features of HIV-1 neutralization.
FIG 5.
Neutralization susceptibility of SIVsm Envs to autologous sooty mangabey plasma. Six Envs cloned from experimentally SIV-infected sooty mangabeys (FJv, FFs) or naturally SIV-infected sooty mangabeys (FFv, FWk, FBn, FNg) were assessed for neutralization by a sample of autologous plasma collected 0.5 to 4 years later using the Tzm-bl cell assay (39). The percentage of residual infectivity against 1:20-diluted plasma is shown on the vertical axis. Residual infectivity of 100% represents no inhibition, while 0% residual infectivity represents complete inhibition. Each bar is color coded according to the neutralization tier of the Env pseudoviruses. Each Env pseudovirus-plasma combination was tested in duplicate wells in at least two independent experiments. The standard error of the mean is shown for each Env pseudovirus.
CD4 independence tracks with tier 1 neutralization sensitivity.
Because one of the tier 1a Envs, RZj5, was previously determined to be CD4 independent, we tested the Envs in our panel for their ability to utilize rhesus CCR5 in the absence of rhesus CD4, using an assay described previously (45). Env FFs was not evaluated due to its low pseudovirus infectivity in the CCR5+ CD4+ cells used in this assay. Four Envs in addition to RZj5 utilized CCR5 in the absence of CD4 with moderate to high efficiency. SIVmac251.6 and E660.11, both tier 1a Envs commonly used to assess vaccine-elicited antibody responses, mediated CD4-independent infection at roughly 70 to 80% of the level of CCR5+ CD4+ entry (Fig. 6A). Additionally, one tier 2 Env and one tier 3 Env mediated infection at 25 to 30% of the level of CCR5+ CD4+ entry. As a group, tier 1a Envs were significantly more CD4 independent than the tier 2 or tier 3 Envs, although this comparison is based on a relatively small number of SIVsm Envs (Fig. 6B; Kruskal-Wallis test, P = 0.01 to 0.05 for both comparisons). However, none of the Envs displaying CD4 independence contained the D470N determinant of CD4 independence that was defined for RZj5 (D491N in Fig. 3) (45). Interestingly, the RZu4-11.1 and -1.1 Envs were isolated from the same SIVmac251-infected plasma sample, yet RZu4-11.1 was more neutralization sensitive and used CCR5 more efficiently in the absence of CD4 than the closely related Env RZu4-1.1 (Fig. 4 and 6A). These Envs differ by only 2 amino acids in gp120 and 5 amino acids in the gp41 cytoplasmic tail. It follows that multiple pathways must exist for neutralization sensitivity and for developing the ability to utilize CCR5 in the absence of CD4. The latter property seems to be much more conducive to SIVsm Envs than HIV-1 Envs, where CD4 independence is rare (72, 73). Consequently, CD4 independence may be a previously unappreciated property of some SIVsm Env variants, such as E660.11, that could influence neutralization sensitivity, transmission, and pathogenesis and should therefore be evaluated when selecting a neutralization panel or a challenge virus.
FIG 6.
CD4 independence and inhibition of the SIVsm Env panel by soluble CD4. (A) The level of CCR5+ CD4− infection mediated by each Env pseudovirus relative to the level of CD4+ CCR5+-mediated entry (100%). Env FFs was not included due to low infectivity in the assay. 293T cells were transiently transfected with plasmids expressing rhesus CD4 and CCR5 or CD4 only, and infection was quantitated using luciferase activity as the readout (provided by the pNL4-3Luc E-R+ backbone). Each experiment was performed in duplicate wells at least twice independently, and the standard error of the mean is shown for each Env pseudovirus. Each bar is color coded according to the neutralization tier of the Env pseudovirus. (B) The level of CD4-independent entry from panel A across the tiers using a Kruskal-Wallis comparison, tier 1a Envs as the control group, and Dunn's correction for multiple comparisons. The FJv Env was excluded from the statistical analysis because it utilizes CXCR4 for CD4-dependent entry. The results for each of the tier 2 and tier 3 Env pseudoviruses were significantly different from those for the tier 1a Env pseudoviruses (*, P = 0.01 to 0.05). (C) Inhibition by human sCD4 in the Tzm-bl cell assay with luciferase activity as the readout. sCD4 was serially diluted 5-fold, and the concentration is presented on the x axis on a log10 scale. The percent viral infectivity relative to that in the absence of sCD4 (100%) is shown on the y axis. Each Env pseudovirus and reagent-serum combination was tested in duplicate wells in at least two independent experiments, and the standard error of the mean is shown on the graphs in panels A and B.
We also evaluated susceptibility to inhibition by human sCD4, which can be an indicator of such properties as CD4 contact residue exposure, affinity for the CD4 receptor, CD4-induced formation of the bridging sheet, and Env stability (74, 75). Most of the SIVsm Envs had intermediate sensitivity to sCD4 inhibition (Fig. 6C). However, the Envs that were most potently inhibited by sCD4, E660.11 and SIVmac251.6, also had tier 1a antibody neutralization sensitivity (Fig. 6C). In contrast, the tier 1a Env RZj5 was highly resistant to sCD4-mediated inhibition (Fig. 6C). Interestingly, sCD4 sensitivity was not linked with neutralization by CD4bs MAb 6.10B (Fig. 4; see also Fig. S1 in the supplemental material), as has been described for some CD4bs MAbs against HIV-1 (74, 75). Thus, sCD4 sensitivity was not predictive of CD4 independence or neutralization sensitivity.
Evaluation of vaccination trials with the characterized Env panel: vaccination with SIVmac239 does not consistently elicit tier 2/3 neutralizing activity across immunization regimens.
To evaluate vaccine-induced neutralizing antibody activity during four nonhuman primate SIVmac239 vaccine trials (trials M2, M15, M11, and M12; Fig. 1 and Table 5) during the postimmunization, prechallenge phase, we utilized the SIVsm Env panel. We obtained serum samples from 91 immunized monkeys from the trials described above (Fig. 1). The M11 trial consisted of three groups of animals that received two DNA primes with or without the cytokine GM-CSF adjuvant, followed by two MVA boosts (DDMM or DgDgMM), or three MVA immunizations (MMM) (22, 29). Trial M12 was a vaccine arm that was added onto trial M11, in which animals received two DNA primes adjuvanted with CD40L, followed by two MVA boosts (DM/CD40L) (21). All of the vaccine arms in trials M11 and M12 were associated with significant protection against intrarectal SIVsmE660 challenge. The M15 trial was designed essentially to determine whether the enhanced, antibody-associated protection afforded by the GM-CSF adjuvant in M11 was reproducible against an intravaginal SIVsmE660 challenge and consisted of two DNA primes adjuvanted with or without GM-CSF, followed by two MVA boosts (DDMM or DgDgMM). The M2 trial was performed in parallel with the M15 trial and consisted of four groups of animals that received three immunizations of SIVmac239 Gag and Env gp140 proteins or virus-like particles adjuvanted with either alum or nanoparticles designed to enhance Toll-like receptor (TLR) signaling (protein plus alum, protein plus NPs, VLPs plus alum, VLPs plus NPs) (76). Even though the vaccine groups had fewer infected animals than the control groups, the M2 and M15 trials did not achieve a significant level of protection against intravaginal SIVsmE660 challenge. A detailed list describing the serum samples obtained from each trial and vaccine group is provided in Table S1 in the supplemental material.
TABLE 5.
Summary of nonhuman primate SIV immunization regimensa
| Trial | Immunogen | Challenge | Route | Treatment |
No. of nonhuman primates with: |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prime | Boost 1 | Boost 2 | Boost 3 | Breakthrough | Protection | Total | ||||
| M15 | SIVmac239 | SIVsmE660 | Vaginal | DNA | DNA | MVA | MVA | 5 | 4 | 9 |
| M15 | SIVmac239 | SIVsmE660 | Vaginal | DNA + GM-CSF | DNA + GM-CSF | MVA | MVA | 7 | 3 | 10 |
| M2 | SIVmac239 | SIVsmE660 | Vaginal | VLP + alum | VLP + alum | VLP + alum | VLP + alum | 6 | 2 | 8 |
| M2 | SIVmac239 | SIVsmE660 | Vaginal | VLP + NP | VLP + NP | VLP + NP | VLP + NP | 4 | 5 | 9 |
| M2 | SIVmac239 | SIVsmE660 | Vaginal | Protein + alum | Protein + alum | Protein + alum | Protein + alum | 7 | 3 | 10 |
| M2 | SIVmac239 | SIVsmE660 | Vaginal | Protein + NP | Protein + NP | Protein + NP | Protein + NP | 5 | 5 | 10 |
| M11 | SIVmac239 | SIVsmE660 | Rectal | DNA | DNA | MVA | MVA | 6 | 2 | 8 |
| M11 | SIVmac239 | SIVsmE660 | Rectal | MVA | MVA | MVA | 6 | 2 | 8 | |
| M11 | SIVmac239 | SIVsmE660 | Rectal | DNA + GM-CSF | DNA + GM-CSF | MVA | MVA | 2 | 5 | 7 |
| M12 | SIVmac239 | SIVsmE660 | Rectal | DNA + CD40L | DNA + CD40L | MVA | MVA | 8 | 4 | 12 |
| Total | 56 | 35 | 91 | |||||||
DNA, expresses SIVmac239 Gag, PR, Env, Tat, and Rev; MVA, modified vaccinia virus Ankara vector expressing SIVmac239 Gag, Pol, and Env with a truncated gp41; GM-CSF, granulocyte-macrophage colony-stimulating factor adjuvant expressed by a DNA plasmid; VLP, virus-like particles containing SIVmac230 Gag p55 and Env gp160; protein, SIVmac239 Env gp140 plus SIVmac239 Gag p55; NP, poly(lactic-coglycolic acid) nanoparticles with monophosphoryl lipid A and resiquimod (R848); CD40L, CD40 ligand adjuvant expressed by a DNA plasmid.
Because of the large number of assays (more than 25,000 wells) required to analyze the neutralization of the panel of 14 Envs by a serum dilution series from each animal, we opted to create eight serum pools consisting of samples from animals that remained uninfected (protected) or became infected after 12 mucosal challenges in each of the four trials. Seaman et al. demonstrated that plasma pools from HIV-1-infected human subjects had neutralization titers that were highly correlated with the average for the individual constituents (30). Ultimately, we reasoned that if tier 2 or 3 neutralizing activity was present in the majority of animals within a trial, then neutralization of the tier 2 and 3 Envs should be observed using the pooled serum. We found a similarly high correlation between the average for the individual serum samples for trials M2, M11, and M15 and the neutralization values obtained with the corresponding pools against the tier 1a Env SIVsmE660.11 (data not shown). Neutralizing activity against the SIVsm Env panel was evaluated for the serum pools to determine whether there were any qualitative differences in neutralization capacity or evidence for breadth against diverse SIV isolates. In this way, we could probe for breadth or differences in neutralization profiles that, if present, could be pursued in a targeted manner using the serum samples from individual animals.
Overall, the vaccines elicited neutralizing activity predominantly against the sensitive tier 1a Envs (Fig. 7A, C, E, and G). Even the moderately sensitive tier 1b FFv was highly resistant to neutralization by the pools of serum from immunized animals. In the M2 and M15 trials, serum samples were collected at the time point of the peak for antibodies that bind to SIVmac239 and SIVsmE660 gp140 proteins in ELISAs (data not shown), which was 2 weeks after the last immunization (Fig. 1). However, in the M11 and M12 trials, serum samples were collected later, at 13 weeks after the last immunization (Fig. 1). Nevertheless, comparing the neutralization data from the M11 with those from the M15 trial in Fig. 7C and G, the potency of tier 1a neutralization was similar, even though the samples in the M11 trial were collected 9 weeks after the samples in the M15 trial (Fig. 1). It is important to note that the samples from trials M2, M11, and M12 that were analyzed were collected at the same time point in the context of the challenge (11 weeks prior to challenge; Fig. 1). Some studies suggest that by analyzing samples collected at a later time point in trials M11 and M12, one could increase the chances of detecting a greater breadth or differences in the neutralization profile (77, 78). As such, the analysis of samples from two different postimmunization time points could have provided a broader picture of the neutralization activity in these vaccinated animals, had it been present.
FIG 7.
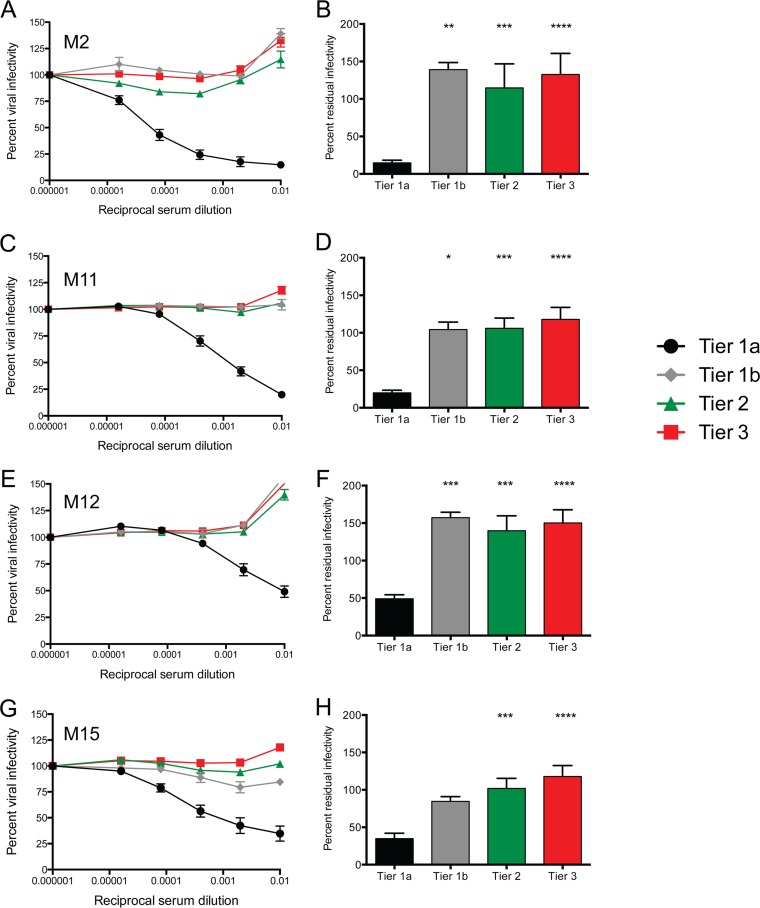
Neutralization of the SIVsm Env panel by serum pools created from vaccinated monkeys. Serum pools were created from rhesus macaques immunized in the M2, M11, M12, and M15 trials (details about the animals from which serum was collected are provided in Table S1 in the supplemental material). Serum pools were serially diluted 5-fold and used to inhibit Env pseudovirus infection using the Tzm-bl cell assay, with luciferase activity being the readout. (A, C, E, and G) The reciprocal serum dilution is presented on the x axis on a log10 scale, and the percent viral infectivity relative to that in the absence of test serum (100%) is shown on the y axis. The Env pseudoviruses are grouped according to neutralization tier, which is indicated in the key. Each panel presents the results for pooled serum from vaccinated animals in trials M2 (A; n = 37 monkeys), M11 (C; n = 23 monkeys), M12 (E; n = 12 monkeys), and M15 (G; n = 19 monkeys). (B, D, F, and H) Percent residual infectivity against 1:100-diluted serum presented as a bar graph for each group of tiered Env pseudoviruses. A Kruskal-Wallis test was used to compare tiers 1b, 2, and 3 against tier 1a, with Dunn's correction used for multiple comparisons. *, P = 0.05 to 0.01; **, P = 0.01 to 0.001; ***, P = 0.001 to 0.0001; ****, P < 0.0001.
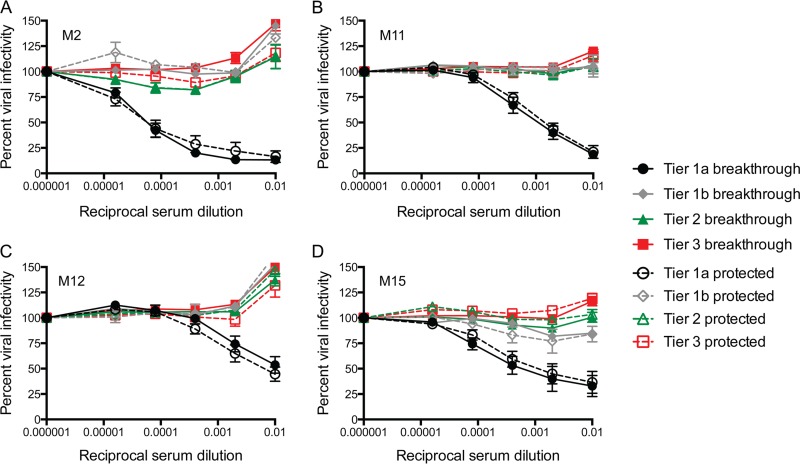
To determine whether neutralization of Envs in the different tiers by pools of serum from immunized animals differed statistically within each trial, the data were subjected to analysis using the Kruskal-Wallis test, comparing tier 1b, 2, and 3 Envs against tier 1a Envs. For all four trials, neutralization of the tier 2 and 3 Envs was statistically significantly different from that of the tier 1a Envs (Fig. 7B, D, F, and H; P < 0.001). Neutralization of the tier 1b Env was also statistically significantly different from that of the tier 1a Envs in 3 of the 4 cases (Fig. 7B, D, and F; P < 0.05). However, the M15 trial appeared to have elicited moderately more potent neutralization activity against the tier 1b Env, possibly distinguishing it from the tier 2 and 3 Envs in that case (Fig. 7G and H). We next asked whether there were any qualitative differences between the pools of serum from vaccinated animals that became infected (breakthrough) or remained protected after the 12 SIVsmE660 challenges within each trial. For this analysis, the Envs were again grouped by tier and by whether the serum pool was from breakthrough or protected animals (Fig. 8A to D). For all four trials, the neutralization curves for the pools of serum from breakthrough and protected animals were indistinguishable, suggesting that the neutralizing activity elicited by the SIVmac239 Env immunogen was not a major contributor to protection against SIVsmE660 challenge.
FIG 8.
Inhibition of the SIVsm Env panel by pools of serum from vaccinated monkeys organized by infection status after 12 challenges. Pools of serum from immunized rhesus macaques that became infected after 12 challenges with SIVsmE660 (breakthrough) or did not become infected (protected) were created for each trial. Details about the animals from which serum was collected are provided in Table S1 in the supplemental material. Serum pools were serially diluted 5-fold and used to inhibit pseudovirus infection using the Tzm-bl cell assay, with luciferase activity being the readout. The reciprocal dilution is presented on the x axis on a log10 scale, and the percent viral infectivity relative to that in the absence of test plasma/serum (100%) is shown on the y axis. The Env pseudoviruses were grouped according to tier, which is indicated in the legend. Closed symbols and solid lines, vaccinated animals that experienced breakthrough infections; open symbols and dashed lines, vaccinated animals that were protected after 12 challenges. Each Env pseudovirus and serum combination was tested in duplicate wells in at least two independent experiments. The standard error of the mean is shown for each point on the graph.
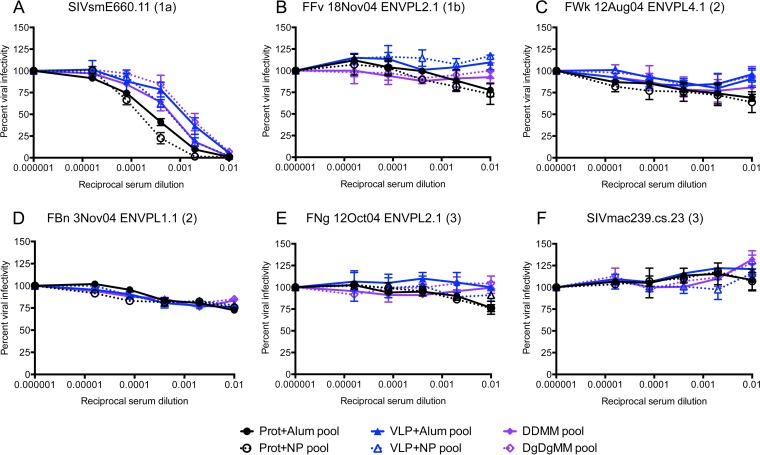
To more directly investigate the ability of adjuvants to alter the antibody profile and enhance neutralization breadth, we analyzed pools of serum collected at the time point at which the antibody titer peaked for the individual vaccine groups in the M2 and M15 trials (Fig. 1). Four new serum pools (protein plus alum, protein plus NPs, VLPs plus alum, VLPs plus NPs) were created for trial M2 and two new serum pools (DDMM and DgDgMM) were created for trial M15 on the basis of the individual immunization regimens. The neutralization activity of the serum pools against six SIVsm Envs that represented the spectrum of the three neutralization tiers was evaluated (Fig. 4 and Table 3). Figure 9A demonstrates that delivery of the SIVmac239 gp140 protein or VLP immunogen with the NP adjuvant produced a moderate increase in neutralization of the tier 1a E660.11 Env compared to that achieved with alum (Fig. 9A). However, the GM-CSF adjuvant did not augment the neutralization capacity against E660.11 elicited by the DDMM regimen alone (Fig. 9A). None of the adjuvants produced a measurable increase in neutralization capacity against the tier 1b, 2, and 3 Envs (Fig. 9B to F). Adjuvants may therefore increase the titers of specific antibodies that neutralize tier 1a SIVsm Envs in some contexts, but they do not consistently alter antibody specificities such that tier 1b, 2, and 3 SIVsm Envs are more effectively neutralized.
FIG 9.
Inhibition of representative tier 1a, 1b, 2, and 3 SIVsm Envs by pools of serum from monkeys vaccinated with novel adjuvants in trials M2 and M15. Pools of serum from immunized rhesus macaques that received different regimens within the M2 and M15 trials were created. For the M2 trial, four serum pools corresponding to the vaccine groups (protein plus alum, protein plus NP, VLP plus alum, VLP plus NP; Fig. 1) were tested, where prot is the gp140 protein immunogen VLP is the virus-like particle immunogen and NP is the nanoparticle adjuvant. For the M15 trial, two serum pools (DDMM and DgDgMM; Fig. 1) were tested. DDMM, DNA/MVA-vectored immunogens; DgDgMM, the GM-CSF adjuvant was delivered with the DNA. Serum pools were serially diluted 5-fold and used to inhibit pseudovirus infection using the Tzm-bl cell assay, with luciferase activity being the readout. The reciprocal dilution is presented on the x axis on a log10 scale, and the percent viral infectivity relative to that in the absence of test serum (100%) is shown on the y axis. Each Env pseudovirus and serum combination was tested in duplicate in at least two independent experiments. The standard error of the mean is shown for each point on the graph. (A to F) Neutralization of six Env pseudoviruses from the SIVsm Env panel representing tiers 1a, 1b, 2, and 3 (Fig. 4). The SIVsm Env (neutralization tier) is indicated at the top of each panel. Immunogens are indicated by color (black, protein; blue, VLPs; purple, DDMM). Closed symbols and solid lines, alum or no adjuvant; open symbols and dashed lines, immunization with a novel adjuvant.
It is possible that the development of broader neutralizing activity within one or more of the trials was not detected because we analyzed pooled serum or samples from only a single time point in each trial. It is therefore conceivable that individual animals within the trials could have possessed vaccine-induced neutralization activity against the tier 1b, 2, or 3 Envs; however, as discussed above, the results obtained with serum and plasma pools generally show good concordance with those obtained with the individual constituents, unless there are marked synergy or inhibitory effects. Even if tier 2 or 3 neutralizing activity was present in some of the individual serum samples, it arguably did not constitute the major antibody response induced by the vaccines and adjuvants. Furthermore, this analysis did not reveal any evidence for major differences in the antibody profiles driven by GM-CSF, CD40L, or nanoparticles. In contrast, the neutralizing antibody responses were remarkably consistent across trials and vaccine groups, given the various forms of Env and immunomodulatory adjuvants that were included. The aforementioned caveats notwithstanding, the data argue that vaccines expressing the SIVmac239 Env mainly elicit antibodies capable of neutralizing tier 1a Envs, regardless of the vaccination regimen or adjuvant. While this finding may have been implied by previous studies, to our knowledge it has not been shown directly. The results therefore strongly emphasize the importance of the Env immunogen when the goal is to broaden the neutralizing antibody response.
DISCUSSION
Vaccine-elicited antibodies against HIV-1 and SIV.
The goal of all SIV-based vaccine studies performed in nonhuman primates is to develop and test immunization regimens that can subsequently be administered to human populations to protect against acquisition of HIV-1. Evidence based on currently licensed vaccines and a small number of phase IIb and III HIV vaccine trials strongly suggests that an HIV vaccine will need to elicit an effective antibody response, which will probably include neutralizing antibodies, to protect against acquisition (2, 79, 80). The burden of current nonhuman primate SIV-based vaccine studies is therefore to model and dissect the requirements of antibody-mediated protection; however, success will be contingent on the model accurately reproducing the induction of antibodies against HIV-1 Env immunogens and mechanisms of protection against HIV-1 infection. For neutralizing antibodies, this is complicated by the inherent immune evasion tactics and vast diversity of the HIV-1 Envs (33). Thus, the genetically diverse SIVsm Env panel developed and used here, in combination with sera from immunized macaques from four coordinated preclinical trials, provided a unique opportunity to better understand what type of neutralizing antibodies against SIVmac239 Env are elicited across various immunization regimens, possibly shedding new light upon mechanisms of neutralizing antibody induction that could be translated to HIV-1.
Neutralizing antibodies against HIV-1 were first described almost 30 years ago (81), leading to the idea that a protective vaccine could be readily developed. However, it was reported shortly thereafter that HIV-1-neutralizing antibodies were strain specific, posing a substantial obstacle to protecting against genetically diverse HIV-1 strains (82, 83). Another significant early observation was that vaccine-induced antibodies typically could neutralize only tissue culture-adapted strains of HIV-1, which were atypically susceptible to neutralization (i.e., tier 1a viruses), and could not neutralize circulating patient-derived viral isolates (generally tier 2 or 3) (84–86). The neutralization resistance of the latter was attributed to the conformational masking of conserved receptor-binding surfaces that is lost during in vitro tissue culture adaptation (87, 88). Together, these observations established the paradigm that a vaccine should be able to elicit antibodies that can neutralize clinically relevant, genetically diverse HIV-1 strains (30, 31, 89, 90), including those isolated during acute/early HIV-1 infection (91–93). The field unanimously reached the conclusion that neutralization of a limited set of highly sensitive laboratory strains was not a viable indicator of protective immunity (94).
Conversely, for SIV models, measuring vaccine-elicited neutralizing antibody responses against a limited set of highly neutralization-susceptible Envs in nonhuman primates is commonplace. Our study is among the first to evaluate whether antibodies with neutralization breadth against diverse tier 2 or 3 SIVsm Envs were elicited by vaccination, in a manner similar to that used for HIV-1 studies. Our results indicate that, like HIV-1 Envs, most SIVsm Envs are moderately to highly resistant to SIV-infected pooled plasma or serum and MAbs that target the conserved receptor-binding surfaces (V3, CD4i, and CD4bs). However, the lack of broadly neutralizing reagents for SIV leaves open the question of whether antibodies that can neutralize the most resistant tier 3 Envs exist in any context. Our data suggest that further investigation will be required to determine whether tier 3 SIVsm Envs are relevant for detecting vaccine-induced protective immunity and whether the tier 2 SIVsm Envs are ultimately more informative targets in the immediate future.
Furthermore, like some tier 1 HIV-1 Envs, each tier 1a SIVsm Env has some atypical feature or adaptation that potentially contributes to unmasking and greater epitope accessibility. In these respects, HIV-1 and SIVsm Envs are similar, despite their low sequence conservation, lack of antigenic cross-reactivity, and distinct evolutionary lineages (72, 95). However, SIVsm is the ancestor of HIV-2 (96, 97), and substantial antibody cross-reactivity does exist between these two viruses (17, 72). Yet, most patient-derived HIV-2 Envs exhibit tier 1-like neutralization sensitivity to pooled plasma and to MAbs derived from patients with HIV-2 infection that target the V3, CD4bs, V4, and CD4i epitopes (50). Thus, HIV-2 Envs may exhibit less glycan shielding and/or a more open conformation than HIV-1 or SIVsm Envs (50). Indeed, the V3 loops of both HIV-1 and HIV-2 are immunogenic, but HIV-1 Envs are almost always more resistant to neutralization by anti-V3 antibodies (30, 70, 98–100) because of conformational masking (88). In terms of vulnerability to neutralizing antibodies, most of the SIVsm Envs in the panel seemed to behave more similarly to HIV-1 than HIV-2 Envs. The driving force for masking of conserved epitopes during natural infection with SIVsm is not immediately clear, given evidence of lower diversifying selection in Env gp120 (101) and lower autologous neutralizing antibody titers in naturally infected sooty mangabeys (39) than individuals infected with HIV-1. Unfortunately, the determinants of neutralization pan-resistance in some of the tier 3 SIVsm Envs or the identification of reagents that neutralize them will require further investigation.
SIVmac239 Env as a surrogate for HIV-1 Env immunogens.
HIV-1 Env immunogens in macaques and human subjects predominantly elicit tier 1 neutralizing antibodies, regardless of the immunization platform, Env genotype, or modifications to the Env immunogen (2, 35–37, 90, 102–110). These tier 1 neutralizing antibodies include those targeting V3, CD4i, and the CD4bs (35–37, 109). As more information about the putative germ line and mutated forms of broadly neutralizing antibodies against HIV-1 has become available, strategies to modify and deliver Env immunogens are under development to elicit neutralizing antibodies against targets known to confer breadth, such as the CD4bs (34, 111–114). The SIVmac239 Env is derived from a molecular clone that establishes a persistent infection in rhesus macaques, resulting in rapid progression to a fatal AIDS-like disease (115–118). SIVmac239 Env is commonly used for vaccination of nonhuman primates; it is known to be inherently resistant to antibody neutralization (6–9) and, like HIV-1 Envs, masks receptor-binding surfaces (119–121). Furthermore, SIVmac239 Env is more dependent on the CD4 level and is less macrophage-tropic than many other SIV isolates, resembling HIV-1 Envs. White et al. demonstrated that the molecular architecture of SIVmac239 Env trimers is comparable to that of HIV-1 Env trimers (122); however, there are apparent differences in conformational changes following CD4 binding (123).
The analysis using our panel of SIVsm Envs did not reveal any evidence that the immunizations carried out here consistently elicited antibodies capable of potently neutralizing tier 2 or 3 SIV strains. Instead, immunization with the SIVmac239 Env in different forms and with novel adjuvants elicited predominantly tier 1a Env-neutralizing antibodies that may be targeted to epitopes, including V3, CD4i, and CD4bs, regardless of the vaccination regimen. Indeed, other vaccine trials have shown that SIVmac239 elicits antibodies capable of neutralizing pseudoviruses with tier 1 SIVsmE660-derived Envs, but these studies did not address whether neutralization breadth was present and did not compare neutralization profiles across such varied vaccine regimens in a systematic fashion. Accordingly, we provide new information that the SIVmac239 Env immunogen appears to be the primary determinant of a tier 1 Env-neutralizing antibody profile, which is not superseded by the addition of adjuvants. However, adjuvants may increase the titer of these antibodies. Improving neutralization breadth against SIV will therefore likely require modification or careful selection of the Env immunogen to direct neutralizing antibodies away from tier 1 Env antibody targets, and once neutralization breadth has been achieved, the use of adjuvants to further enhance the titer may be considered later.
Properties of SIVsmE660 challenge virus.
SIV vaccine models should faithfully model the biology of HIV-1 acquisition (1). To mimic HIV-1 transmission, current SIV-based nonhuman primate vaccine approaches, including those studied here, generally utilize a low- to moderate-dose repeat mucosal challenge with a viral quasispecies, such as neutralization-sensitive SIVsmE660 or neutralization-resistant SIVmac251 (124, 125). SIVsmE660 serves as a heterologous challenge virus for SIVmac239 immunogens, because, unlike SIVmac251, it is genetically distinct from SIVmac239. However, SIVsmE660 challenge virus stocks arose from in vivo and in vitro passage of virus, leading to the original SIVsmE660 isolate that was derived from the spleen of a terminally ill SIV-infected rhesus macaque by in vitro coculture (126, 127). It follows that this in vitro adaptation may have contributed to the unusual neutralization sensitivity of some SIVsmE660 variants, often several orders of magnitude greater than the sensitivity of HIV-1 (17, 18) and most other SIVsm variants (Fig. 4; see also Fig. S1 in the supplemental material) (17).
Furthermore, the finding that the Env clone E660.11 is highly CD4 independent raises the possibility that this feature is shared with other SIVsmE660 variants. CD4 independence in SIVsm Envs is not uncommon and correlates with sensitivity to anti-V3, anti-CD4bs, and anti-CD4i neutralizing antibodies (119). In contrast, CD4-independent HIV-1 strains infrequently occur in nature (72, 73). The sequence of Env clone E660.11 analyzed here is 98% identical to the consensus sequence of the SIVsmE660 challenge stock from which it was derived (data not shown). The SIVsmE660 challenge stock consensus sequence contains a serine at position 166 in V2 (as in Fig. 3) that is part of a PNLG site; however, the E660.11 Env contains a S166N substitution that destroys the PNLG site and has been associated with the CD4 independence of SIVsm (128). Approximately 20% of single-genome amplification (SGA) sequences derived from SIVsmE660 challenge stocks lack this PNLG site in V2, indicating that as many as one in five variants could carry this potential signature (data not shown). Indeed, Roederer et al. noted that immunization resulted in a significant selection against an analogous V1V2 PNLG site in macaques subsequently challenged with SIVsmE660 variants (18). Interim in vitro growth of the SIVsmE660 isolate in human cells or under other growth and adaptation conditions could have altered the dependence on rhesus macaque CD4 in some variants, such as E660.11 (119). In addition, there is evidence that CD4-independent variants emerge more readily in SIV-infected macaques that progress to disease rapidly, probably because they often fail to develop antibody responses. The rhesus macaque from which SIVsmE660 was derived had transient antibody responses against Env and died 52 days after infection (126). Paradoxically, then, it may be that SIVsm Envs selected for their high virulence in vivo also exhibit greater sensitivity to neutralizing antibodies (45). HIV-1 quasispecies circulating within a patient would not usually contain variants with such extreme neutralization sensitivity (63–65, 129, 130) or CD4 independence (131, 132), and SIVsmE660 could be misleading in terms of modeling of HIV-1 transmission and mechanisms of protection.
The use of a novel SIVsm Env panel to analyze samples from four coordinated nonhuman primate trials expressing SIVmac239 Env provides strong evidence that these vaccination platforms incorporating cytokine- and TLR ligand-stimulating adjuvants did not consistently elicit antibodies with breadth that were capable of rigorously neutralizing SIVsm isolates with tier 2 or 3 Envs, further highlighting the known difficulties in eliciting broadly neutralizing antibodies through vaccination. Optimization of SIVsm Env immunogens to preferentially expose certain targets and interact with germ line B-cell receptors may be necessary to elicit a broader antibody profile, as has been suggested for HIV-1 Envs. Despite similarities between SIVsm and HIV-1 Envs, there are also important differences that could affect their immunogenicity, antigenicity, and mucosal transmissibility, particularly differences involving interactions with CD4 (73, 119, 123, 133–141). These findings highlight our limited understanding of how well SIVsm Env immunogens, challenge viruses, and neutralization panels model features of HIV-1 Envs that are desirable for vaccines and underscore the need to identify and characterize broadly neutralizing antibodies against SIVsm if this immunization model is going to continue to be pursued.
Supplementary Material
ACKNOWLEDGMENTS
We gratefully acknowledge Harriet Robinson for providing samples from the M11 and M12 trials, Brandon Keele for providing SGA sequences from SIVsmE660 challenge stocks, and David Montefiori for providing the reference SIV Env plasmids and their sequences.
We thank the National Institute of Allergy and Infectious Diseases, National Institutes of Health, for the following funding: R01-AI58706 to C.A.D., P01-AI096187 to E.H. and R.R.A., R01-MH061139 to R.G.C., and P51-RR000165 and P51-OD011132 supporting the Yerkes National Primate Research Center.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/JVI.01221-14.
REFERENCES
- 1.McChesney MB, Miller CJ. 2013. New directions for HIV vaccine development from animal models. Curr Opin HIV AIDS 8:376–381. doi: 10.1097/COH.0b013e328363d3a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Excler JL, Tomaras GD, Russell ND. 2013. Novel directions in HIV-1 vaccines revealed from clinical trials. Curr Opin HIV AIDS 8:421–431. doi: 10.1097/COH.0b013e3283632c26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klein F, Mouquet H, Dosenovic P, Scheid JF, Scharf L, Nussenzweig MC. 2013. Antibodies in HIV-1 vaccine development and therapy. Science 341:1199–1204. doi: 10.1126/science.1241144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwong PD, Mascola JR, Nabel GJ. 2013. Broadly neutralizing antibodies and the search for an HIV-1 vaccine: the end of the beginning. Nat Rev Immunol 13:693–701. doi: 10.1038/nri3516. [DOI] [PubMed] [Google Scholar]
- 5.Kwong PD, Mascola JR. 2012. Human antibodies that neutralize HIV-1: identification, structures, and B cell ontogenies. Immunity 37:412–425. doi: 10.1016/j.immuni.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson WE, Sanford H, Schwall L, Burton DR, Parren PW, Robinson JE, Desrosiers RC. 2003. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J Virol 77:9993–10003. doi: 10.1128/JVI.77.18.9993-10003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson WE, Lifson JD, Lang SM, Johnson RP, Desrosiers RC. 2003. Importance of B-cell responses for immunological control of variant strains of simian immunodeficiency virus. J Virol 77:375–381. doi: 10.1128/JVI.77.1.375-381.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson WE, Morgan J, Reitter J, Puffer BA, Czajak S, Doms RW, Desrosiers RC. 2002. A replication-competent, neutralization-sensitive variant of simian immunodeficiency virus lacking 100 amino acids of envelope. J Virol 76:2075–2086. doi: 10.1128/jvi.76.5.2075-2086.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Means RE, Greenough T, Desrosiers RC. 1997. Neutralization sensitivity of cell culture-passaged simian immunodeficiency virus. J Virol 71:7895–7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans DT, Jing P, Allen TM, O'Connor DH, Horton H, Venham JE, Piekarczyk M, Dzuris J, Dykhuzen M, Mitchen J, Rudersdorf RA, Pauza CD, Sette A, Bontrop RE, DeMars R, Watkins DI. 2000. Definition of five new simian immunodeficiency virus cytotoxic T-lymphocyte epitopes and their restricting major histocompatibility complex class I molecules: evidence for an influence on disease progression. J Virol 74:7400–7410. doi: 10.1128/JVI.74.16.7400-7410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen TM, Jing P, Calore B, Horton H, O'Connor DH, Hanke T, Piekarczyk M, Ruddersdorf R, Mothe BR, Emerson C, Wilson N, Lifson JD, Belyakov IM, Berzofsky JA, Wang C, Allison DB, Montefiori DC, Desrosiers RC, Wolinsky S, Kunstman KJ, Altman JD, Sette A, McMichael AJ, Watkins DI. 2002. Effects of cytotoxic T lymphocytes (CTL) directed against a single simian immunodeficiency virus (SIV) Gag CTL epitope on the course of SIVmac239 infection. J Virol 76:10507–10511. doi: 10.1128/JVI.76.20.10507-10511.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horton H, Vogel TU, Carter DK, Vielhuber K, Fuller DH, Shipley T, Fuller JT, Kunstman KJ, Sutter G, Montefiori DC, Erfle V, Desrosiers RC, Wilson N, Picker LJ, Wolinsky SM, Wang C, Allison DB, Watkins DI. 2002. Immunization of rhesus macaques with a DNA prime/modified vaccinia virus Ankara boost regimen induces broad simian immunodeficiency virus (SIV)-specific T-cell responses and reduces initial viral replication but does not prevent disease progression following challenge with pathogenic SIVmac239. J Virol 76:7187–7202. doi: 10.1128/JVI.76.14.7187-7202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mothe BR, Horton H, Carter DK, Allen TM, Liebl ME, Skinner P, Vogel TU, Fuenger S, Vielhuber K, Rehrauer W, Wilson N, Franchini G, Altman JD, Haase A, Picker LJ, Allison DB, Watkins DI. 2002. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J Virol 76:875–884. doi: 10.1128/JVI.76.2.875-884.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dzuris JL, Sidney J, Horton H, Correa R, Carter D, Chesnut RW, Watkins DI, Sette A. 2001. Molecular determinants of peptide binding to two common rhesus macaque major histocompatibility complex class II molecules. J Virol 75:10958–10968. doi: 10.1128/JVI.75.22.10958-10968.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen TM, Mothe BR, Sidney J, Jing P, Dzuris JL, Liebl ME, Vogel TU, O'Connor DH, Wang X, Wussow MC, Thomson JA, Altman JD, Watkins DI, Sette A. 2001. CD8(+) lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule mamu-A*01: implications for vaccine design and testing. J Virol 75:738–749. doi: 10.1128/JVI.75.2.738-749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Letvin NL, Rao SS, Montefiori DC, Seaman MS, Sun Y, Lim SY, Yeh WW, Asmal M, Gelman RS, Shen L, Whitney JB, Seoighe C, Lacerda M, Keating S, Norris PJ, Hudgens MG, Gilbert PB, Buzby AP, Mach LV, Zhang J, Balachandran H, Shaw GM, Schmidt SD, Todd JP, Dodson A, Mascola JR, Nabel GJ. 2011. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med 3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopker M, Easlick J, Sterrett S, Decker JM, Barbian H, Learn G, Keele BF, Robinson JE, Hahn BH, Shaw GM, Bar KJ. 2013. Heterogeneity in neutralization sensitivities of viruses comprising the simian immunodeficiency virus SIVsmE660 isolate and vaccine challenge stock. J Virol 87:5477–5492. doi: 10.1128/JVI.03419-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, Todd JP, Buzby AP, Mach LV, Shen L, Seaton KE, Ward BM, Bailer RT, Gottardo R, Gu W, Ferrari G, Alam SM, Denny TN, Montefiori DC, Tomaras GD, Korber BT, Nason MC, Seder RA, Koup RA, Letvin NL, Rao SS, Nabel GJ, Mascola JR. 2014. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature 505:502–508. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu F, Ourmanov I, Kuwata T, Goeken R, Brown CR, Buckler-White A, Iyengar R, Plishka R, Aoki ST, Hirsch VM. 2012. Sequential evolution and escape from neutralization of simian immunodeficiency virus SIVsmE660 clones in rhesus macaques. J Virol 86:8835–8847. doi: 10.1128/JVI.00923-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reynolds MR, Sacha JB, Weiler AM, Borchardt GJ, Glidden CE, Sheppard NC, Norante FA, Castrovinci PA, Harris JJ, Robertson HT, Friedrich TC, McDermott AB, Wilson NA, Allison DB, Koff WC, Johnson WE, Watkins DI. 2011. The TRIM5{alpha} genotype of rhesus macaques affects acquisition of simian immunodeficiency virus SIVsmE660 infection after repeated limiting-dose intrarectal challenge. J Virol 85:9637–9640. doi: 10.1128/JVI.05074-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwa S, Lai L, Gangadhara S, Siddiqui M, Pillai VB, Labranche C, Yu T, Moss B, Montefiori DC, Robinson HL, Kozlowski PA, Amara RR. 2014. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus SIV239 vaccine enhances SIV-specific humoral and cellular immunity and improves protection against a heterologous SIVE660 mucosal challenge. J Virol 88:9579–9589. doi: 10.1128/JVI.00975-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai L, Kwa S, Kozlowski PA, Montefiori DC, Ferrari G, Johnson WE, Hirsch V, Villinger F, Chennareddi L, Earl PL, Moss B, Amara RR, Robinson HL. 2011. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis 204:164–173. doi: 10.1093/infdis/jir199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flatz L, Cheng C, Wang L, Foulds KE, Ko SY, Kong WP, Roychoudhuri R, Shi W, Bao S, Todd JP, Asmal M, Shen L, Donaldson M, Schmidt SD, Gall JG, Pinschewer DD, Letvin NL, Rao S, Mascola JR, Roederer M, Nabel GJ. 2012. Gene-based vaccination with a mismatched envelope protects against simian immunodeficiency virus infection in nonhuman primates. J Virol 86:7760–7770. doi: 10.1128/JVI.00599-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel V, Jalah R, Kulkarni V, Valentin A, Rosati M, Alicea C, von Gegerfelt A, Huang W, Guan Y, Keele BF, Bess JW Jr, Piatak M Jr, Lifson JD, Williams WT, Shen X, Tomaras GD, Amara RR, Robinson HL, Johnson W, Broderick KE, Sardesai NY, Venzon DJ, Hirsch VM, Felber BK, Pavlakis GN. 2013. DNA and virus particle vaccination protects against acquisition and confers control of viremia upon heterologous simian immunodeficiency virus challenge. Proc Natl Acad Sci U S A 110:2975–2980. doi: 10.1073/pnas.1215393110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kulkarni V, Rosati M, Jalah R, Ganneru B, Alicea C, Yu L, Guan Y, Labranche C, Montefiori DC, King AD, Valentin A, Pavlakis GN, Felber BK. 2014. DNA vaccination by intradermal electroporation induces long-lasting immune responses in rhesus macaques. J Med Primatol 43:329–340. doi: 10.1111/jmp.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulkarni V, Rosati M, Valentin A, Jalah R, Alicea C, Yu L, Guan Y, Shen X, Tomaras GD, LaBranche C, Montefiori DC, Irene C, Prattipati R, Pinter A, Sullivan SM, Pavlakis GN, Felber BK. 2013. Vaccination with Vaxfectin((R)) adjuvanted SIV DNA induces long-lasting humoral immune responses able to reduce SIVmac251 viremia. Hum Vaccin Immunother 9:2069–2080. doi: 10.4161/hv.25442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, Sanmiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EM, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. 2012. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia B, Ng SK, DeGottardi MQ, Piatak M, Yuste E, Carville A, Mansfield KG, Li W, Richardson BA, Lifson JD, Evans DT. 2009. Immunization with single-cycle SIV significantly reduces viral loads after an intravenous challenge with SIV(mac)239. PLoS Pathog 5:e1000272. doi: 10.1371/journal.ppat.1000272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai L, Kwa SF, Kozlowski PA, Montefiori DC, Nolen TL, Hudgens MG, Johnson WE, Ferrari G, Hirsch VM, Felber BK, Pavlakis GN, Earl PL, Moss B, Amara RR, Robinson HL. 2012. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine 30:1737–1745. doi: 10.1016/j.vaccine.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. 2010. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol 84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.deCamp A, Hraber P, Bailer RT, Seaman MS, Ochsenbauer C, Kappes J, Gottardo R, Edlefsen P, Self S, Tang H, Greene K, Gao H, Daniell X, Sarzotti-Kelsoe M, Gorny MK, Zolla-Pazner S, LaBranche CC, Mascola JR, Korber BT, Montefiori DC. 2014. Global panel of HIV-1 Env reference strains for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 88:2489–2507. doi: 10.1128/JVI.02853-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hraber P, Seaman MS, Bailer RT, Mascola JR, Montefiori DC, Korber BT. 2014. Prevalence of broadly neutralizing antibody responses during chronic HIV-1 infection. AIDS 28:163–169. doi: 10.1097/QAD.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Derdeyn CA, Moore PL, Morris L. 2014. Development of broadly neutralizing antibodies from autologous neutralizing antibody responses. Curr Opin HIV AIDS 9:210–216. doi: 10.1097/COH.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jardine J, Julien JP, Menis S, Ota T, Kalyuzhniy O, McGuire A, Sok D, Huang PS, MacPherson S, Jones M, Nieusma T, Mathison J, Baker D, Ward AB, Burton DR, Stamatatos L, Nemazee D, Wilson IA, Schief WR. 2013. Rational HIV immunogen design to target specific germline B cell receptors. Science 340:711–716. doi: 10.1126/science.1234150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Douagi I, Forsell MN, Sundling C, O'Dell S, Feng Y, Dosenovic P, Li Y, Seder R, Lore K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. 2010. Influence of novel CD4 binding-defective HIV-1 envelope glycoprotein immunogens on neutralizing antibody and T-cell responses in nonhuman primates. J Virol 84:1683–1695. doi: 10.1128/JVI.01896-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sundling C, Forsell MN, O'Dell S, Feng Y, Chakrabarti B, Rao SS, Lore K, Mascola JR, Wyatt RT, Douagi I, Karlsson Hedestam GB. 2010. Soluble HIV-1 Env trimers in adjuvant elicit potent and diverse functional B cell responses in primates. J Exp Med 207:2003–2017. doi: 10.1084/jem.20100025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundling C, O'Dell S, Douagi I, Forsell MN, Morner A, Lore K, Mascola JR, Wyatt RT, Karlsson Hedestam GB. 2010. Immunization with wild-type or CD4-binding-defective HIV-1 Env trimers reduces viremia equivalently following heterologous challenge with simian-human immunodeficiency virus. J Virol 84:9086–9095. doi: 10.1128/JVI.01015-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 39.Li B, Stefano-Cole K, Kuhrt DM, Gordon SN, Else JG, Mulenga J, Allen S, Sodora DL, Silvestri G, Derdeyn CA. 2010. Nonpathogenic simian immunodeficiency virus infection of sooty mangabeys is not associated with high levels of autologous neutralizing antibodies. J Virol 84:6248–6253. doi: 10.1128/JVI.00295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riddick NE, Hermann EA, Loftin LM, Elliott ST, Wey WC, Cervasi B, Taaffe J, Engram JC, Li B, Else JG, Li Y, Hahn BH, Derdeyn CA, Sodora DL, Apetrei C, Paiardini M, Silvestri G, Collman RG. 2010. A novel CCR5 mutation common in sooty mangabeys reveals SIVsmm infection of CCR5-null natural hosts and efficient alternative coreceptor use in vivo. PLoS Pathog 6:e1001064. doi: 10.1371/journal.ppat.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elliott ST, Riddick NE, Francella N, Paiardini M, Vanderford TH, Li B, Apetrei C, Sodora DL, Derdeyn CA, Silvestri G, Collman RG. 2012. Cloning and analysis of sooty mangabey alternative coreceptors that support simian immunodeficiency virus SIVsmm entry independently of CCR5. J Virol 86:898–908. doi: 10.1128/JVI.06415-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milush JM, Mir KD, Sundaravaradan V, Gordon SN, Engram J, Cano CA, Reeves JD, Anton E, O'Neill E, Butler E, Hancock K, Cole KS, Brenchley JM, Else JG, Silvestri G, Sodora DL. 2011. Lack of clinical AIDS in SIV-infected sooty mangabeys with significant CD4+ T cell loss is associated with double-negative T cells. J Clin Invest 121:1102–1110. doi: 10.1172/JCI44876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Milush JM, Reeves JD, Gordon SN, Zhou D, Muthukumar A, Kosub DA, Chacko E, Giavedoni LD, Ibegbu CC, Cole KS, Miamidian JL, Paiardini M, Barry AP, Staprans SI, Silvestri G, Sodora DL. 2007. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J Immunol 179:3047–3056. doi: 10.4049/jimmunol.179.5.3047. [DOI] [PubMed] [Google Scholar]
- 44.Ortiz AM, Klatt NR, Li B, Yi Y, Tabb B, Hao XP, Sternberg L, Lawson B, Carnathan PM, Cramer EM, Engram JC, Little DM, Ryzhova E, Gonzalez-Scarano F, Paiardini M, Ansari AA, Ratcliffe S, Else JG, Brenchley JM, Collman RG, Estes JD, Derdeyn CA, Silvestri G. 2011. Depletion of CD4(+) T cells abrogates post-peak decline of viremia in SIV-infected rhesus macaques. J Clin Invest 121:4433–4445. doi: 10.1172/JCI46023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Francella N, Gwyn SE, Yi Y, Li B, Xiao P, Elliott ST, Ortiz AM, Hoxie JA, Paiardini M, Silvestri G, Derdeyn CA, Collman RG. 2013. CD4+ T cells support production of SIV Env antibodies that enforce CD4-dependent entry and shape tropism in vivo. J Virol 87:9719–9732. doi: 10.1128/JVI.01254-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fairman J, Moore J, Lemieux M, Van Rompay K, Geng Y, Warner J, Abel K. 2009. Enhanced in vivo immunogenicity of SIV vaccine candidates with cationic liposome-DNA complexes in a rhesus macaque pilot study. Hum Vaccin 5:141–150. doi: 10.4161/hv.5.3.6589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeh WW, Jaru-Ampornpan P, Nevidomskyte D, Asmal M, Rao SS, Buzby AP, Montefiori DC, Korber BT, Letvin NL. 2009. Partial protection of SIV-infected rhesus monkeys against superinfection with a heterologous SIV isolate. J Virol 83:2686–2696. doi: 10.1128/JVI.02237-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schell JB, Rose NF, Bahl K, Diller K, Buonocore L, Hunter M, Marx PA, Gambhira R, Tang H, Montefiori DC, Johnson WE, Rose JK. 2011. Significant protection against high-dose simian immunodeficiency virus challenge conferred by a new prime-boost vaccine regimen. J Virol 85:5764–5772. doi: 10.1128/JVI.00342-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cole KS, Alvarez M, Elliott DH, Lam H, Martin E, Chau T, Micken K, Rowles JL, Clements JE, Murphey-Corb M, Montelaro RC, Robinson JE. 2001. Characterization of neutralization epitopes of simian immunodeficiency virus (SIV) recognized by rhesus monoclonal antibodies derived from monkeys infected with an attenuated SIV strain. Virology 290:59–73. doi: 10.1006/viro.2001.1144. [DOI] [PubMed] [Google Scholar]
- 50.Kong R, Li H, Bibollet-Ruche F, Decker JM, Zheng NN, Gottlieb GS, Kiviat NB, Sow PS, Georgiev I, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2012. Broad and potent neutralizing antibody responses elicited in natural HIV-2 infection. J Virol 86:947–960. doi: 10.1128/JVI.06155-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong R, Li H, Georgiev I, Changela A, Bibollet-Ruche F, Decker JM, Rowland-Jones SL, Jaye A, Guan Y, Lewis GK, Langedijk JP, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2012. Epitope mapping of broadly neutralizing HIV-2 human monoclonal antibodies. J Virol 86:12115–12128. doi: 10.1128/JVI.01632-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robinson JE, Cole KS, Elliott DH, Lam H, Amedee AM, Means R, Desrosiers RC, Clements J, Montelaro RC, Murphey-Corb M. 1998. Production and characterization of SIV envelope-specific rhesus monoclonal antibodies from a macaque asymptomatically infected with a live SIV vaccine. AIDS Res Hum Retroviruses 14:1253–1262. doi: 10.1089/aid.1998.14.1253. [DOI] [PubMed] [Google Scholar]
- 53.Robinson JE, Holton D, Pacheco-Morell S, Liu J, McMurdo H. 1990. Identification of conserved and variant epitopes of human immunodeficiency virus type 1 (HIV-1) gp120 by human monoclonal antibodies produced by EBV-transformed cell lines. AIDS Res Hum Retroviruses 6:567–579. doi: 10.1089/aid.1990.6.567. [DOI] [PubMed] [Google Scholar]
- 54.Hendrix CW, Flexner C, MacFarland RT, Giandomenico C, Fuchs EJ, Redpath E, Bridger G, Henson GW. 2000. Pharmacokinetics and safety of AMD-3100, a novel antagonist of the CXCR-4 chemokine receptor, in human volunteers. Antimicrob Agents Chemother 44:1667–1673. doi: 10.1128/AAC.44.6.1667-1673.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.De Clercq E, Yamamoto N, Pauwels R, Balzarini J, Witvrouw M, De Vreese K, Debyser Z, Rosenwirth B, Peichl P, Datema R, Thornton D, Skerlj R, Gaul F, Padmanabhan S, Bridger G, Henson G, Abrams M. 1994. Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother 38:668–674. doi: 10.1128/AAC.38.4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bridger GJ, Skerlj RT, Thornton D, Padmanabhan S, Martellucci SA, Henson GW, Abrams MJ, Yamamoto N, De Vreese K, Pauwels R, De Clercq E. 1995. Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J Med Chem 38:366–378. [DOI] [PubMed] [Google Scholar]
- 57.Biswas P, Tambussi G, Lazzarin A. 2007. Access denied? The status of co-receptor inhibition to counter HIV entry. Expert Opin Pharmacother 8:923–933. [DOI] [PubMed] [Google Scholar]
- 58.Emmelkamp JM, Rockstroh JK. 2007. CCR5 antagonists: comparison of efficacy, side effects, pharmacokinetics and interactions—review of the literature. Eur J Med Res 12:409–417. [PubMed] [Google Scholar]
- 59.Eron JJ, Gulick RM, Bartlett JA, Merigan T, Arduino R, Kilby JM, Yangco B, Diers A, Drobnes C, DeMasi R, Greenberg M, Melby T, Raskino C, Rusnak P, Zhang Y, Spence R, Miralles GD. 2004. Short-term safety and antiretroviral activity of T-1249, a second-generation fusion inhibitor of HIV. J Infect Dis 189:1075–1083. doi: 10.1086/381707. [DOI] [PubMed] [Google Scholar]
- 60.Reimann KA, Tenner-Racz K, Racz P, Montefiori DC, Yasutomi Y, Lin W, Ransil BJ, Letvin NL. 1994. Immunopathogenic events in acute infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Virol 68:2362–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li B, Decker JM, Johnson RW, Bibollet-Ruche F, Wei X, Mulenga J, Allen S, Hunter E, Hahn BH, Shaw GM, Blackwell JL, Derdeyn CA. 2006. Evidence for potent autologous neutralizing antibody titers and compact envelopes in early infection with subtype C human immunodeficiency virus type 1. J Virol 80:5211–5218. doi: 10.1128/JVI.00201-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lynch RM, Rong R, Boliar S, Sethi A, Li B, Mulenga J, Allen S, Robinson JE, Gnanakaran S, Derdeyn CA. 2011. The B cell response is redundant and highly focused on V1V2 during early subtype C infection in a Zambian seroconverter. J Virol 85:905–915. doi: 10.1128/JVI.02006-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rong R, Bibollet-Ruche F, Mulenga J, Allen S, Blackwell JL, Derdeyn CA. 2007. Role of V1V2 and other human immunodeficiency virus type 1 envelope domains in resistance to autologous neutralization during clade C infection. J Virol 81:1350–1359. doi: 10.1128/JVI.01839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rong R, Gnanakaran S, Decker JM, Bibollet-Ruche F, Taylor J, Sfakianos JN, Mokili JL, Muldoon M, Mulenga J, Allen S, Hahn BH, Shaw GM, Blackwell JL, Korber BT, Hunter E, Derdeyn CA. 2007. Unique mutational patterns in the envelope {alpha}2 amphipathic helix and acquisition of length in gp120 hyper-variable domains are associated with resistance to autologous neutralization of subtype C human immunodeficiency virus type 1. J Virol 81:5658–5668. doi: 10.1128/JVI.00257-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rong R, Li B, Lynch RM, Haaland RE, Murphy MK, Mulenga J, Allen SA, Pinter A, Shaw GM, Hunter E, Robinson JE, Gnanakaran S, Derdeyn CA. 2009. Escape from autologous neutralizing antibodies in acute/early subtype C HIV-1 infection requires multiple pathways. PLoS Pathog 5:e1000594. doi: 10.1371/journal.ppat.1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch RM, Rong R, Li B, Shen T, Honnen W, Mulenga J, Allen S, Pinter A, Gnanakaran S, Derdeyn CA. 2010. Subtype-specific conservation of isoleucine 309 in the envelope V3 domain is linked to immune evasion in subtype C HIV-1 infection. Virology 404:59–70. doi: 10.1016/j.virol.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murphy MK, Yue L, Pan R, Boliar S, Sethi A, Tian J, Pfafferot K, Karita E, Allen SA, Cormier E, Goepfert PA, Borrow P, Robinson JE, Gnanakaran S, Hunter E, Kong XP, Derdeyn CA. 2013. Viral escape from neutralizing antibodies in early subtype A HIV-1 infection drives an increase in autologous neutralization breadth. PLoS Pathog 9:e1003173. doi: 10.1371/journal.ppat.1003173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Apetrei C, Gautam R, Sumpter B, Carter AC, Gaufin T, Staprans SI, Else J, Barnes M, Cao R Jr, Garg S, Milush JM, Sodora DL, Pandrea I, Silvestri G. 2007. Virus subtype-specific features of natural simian immunodeficiency virus SIVsmm infection in sooty mangabeys. J Virol 81:7913–7923. doi: 10.1128/JVI.00281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Apetrei C, Kaur A, Lerche NW, Metzger M, Pandrea I, Hardcastle J, Falkenstein S, Bohm R, Koehler J, Traina-Dorge V, Williams T, Staprans S, Plauche G, Veazey RS, McClure H, Lackner AA, Gormus B, Robertson DL, Marx PA. 2005. Molecular epidemiology of simian immunodeficiency virus SIVsm in U.S. primate centers unravels the origin of SIVmac and SIVstm. J Virol 79:8991–9005. doi: 10.1128/JVI.79.14.8991-9005.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moore PL, Gray ES, Choge IA, Ranchobe N, Mlisana K, Abdool Karim SS, Williamson C, Morris L. 2008. The C3-V4 region is a major target of autologous neutralizing antibodies in human immunodeficiency virus type 1 subtype C infection. J Virol 82:1860–1869. doi: 10.1128/JVI.02187-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 72.Decker JM, Bibollet-Ruche F, Wei XP, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 201:1–14. doi: 10.1084/jem.20042514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reeves JD, Hibbitts S, Simmons G, McKnight A, Azevedo-Pereira JM, Moniz-Pereira J, Clapham PR. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates infect CD4-negative cells via CCR5 and CXCR4: comparison with HIV-1 and simian immunodeficiency virus and relevance to cell tropism in vivo. J Virol 73:7795–7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O'Connell O, Repik A, Reeves JD, Gonzalez-Perez MP, Quitadamo B, Anton ED, Duenas-Decamp M, Peters P, Lin R, Zolla-Pazner S, Corti D, Wallace A, Wang S, Kong XP, Lu S, Clapham PR. 2013. Efficiency of bridging-sheet recruitment explains HIV-1 R5 envelope glycoprotein sensitivity to soluble CD4 and macrophage tropism. J Virol 87:187–198. doi: 10.1128/JVI.01834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li Y, O'Dell S, Walker LM, Wu X, Guenaga J, Feng Y, Schmidt SD, McKee K, Louder MK, Ledgerwood JE, Graham BS, Haynes BF, Burton DR, Wyatt RT, Mascola JR. 2011. Mechanism of neutralization by the broadly neutralizing HIV-1 monoclonal antibody VRC01. J Virol 85:8954–8967. doi: 10.1128/JVI.00754-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B. 2011. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 470:543–547. doi: 10.1038/nature09737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Manrique J, Piatak M, Lauer W, Johnson W, Mansfield K, Lifson J, Desrosiers R. 2013. Influence of mismatch of Env sequences on vaccine protection by live attenuated simian immunodeficiency virus. J Virol 87:7246–7254. doi: 10.1128/JVI.00798-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cole KS, Rowles JL, Jagerski BA, Murphey-Corb M, Unangst T, Clements JE, Robinson J, Wyand MS, Desrosiers RC, Montelaro RC. 1997. Evolution of envelope-specific antibody responses in monkeys experimentally infected or immunized with simian immunodeficiency virus and its association with the development of protective immunity. J Virol 71:5069–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Plotkin SA. 2010. Correlates of protection induced by vaccination. Clin Vaccine Immunol 17:1055–1065. doi: 10.1128/CVI.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weiss RA, Clapham PR, Cheingsong-Popov R, Dalgleish AG, Carne CA, Weller IV, Tedder RS. 1985. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature 316:69–72. doi: 10.1038/316069a0. [DOI] [PubMed] [Google Scholar]
- 82.Nara PL, Robey WG, Pyle SW, Hatch WC, Dunlop NM, Bess JW Jr, Kelliher JC, Arthur LO, Fischinger PJ. 1988. Purified envelope glycoproteins from human immunodeficiency virus type 1 variants induce individual, type-specific neutralizing antibodies. J Virol 62:2622–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cheng-Mayer C, Homsy J, Evans LA, Levy JA. 1988. Identification of human immunodeficiency virus subtypes with distinct patterns of sensitivity to serum neutralization. Proc Natl Acad Sci U S A 85:2815–2819. doi: 10.1073/pnas.85.8.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golding H, D'Souza MP, Bradac J, Mathieson B, Fast P. 1994. Neutralization of HIV-1. AIDS Res Hum Retroviruses 10:633–643. doi: 10.1089/aid.1994.10.633. [DOI] [PubMed] [Google Scholar]
- 85.Matthews TJ. 1994. Dilemma of neutralization resistance of HIV-1 field isolates and vaccine development. AIDS Res Hum Retroviruses 10:631–632. doi: 10.1089/aid.1994.10.631. [DOI] [PubMed] [Google Scholar]
- 86.Mascola JR, Snyder SW, Weislow OS, Belay SM, Belshe RB, Schwartz DH, Clements ML, Dolin R, Graham BS, Gorse GJ, Keefer MC, McElrath MJ, Walker MC, Wagner KF, McNeil JG, McCutchan FE, Burke DS. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J Infect Dis 173:340–348. [DOI] [PubMed] [Google Scholar]
- 87.Kwong PD, Wyatt R, Majeed S, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 2000. Structures of HIV-1 gp120 envelope glycoproteins from laboratory-adapted and primary isolates. Structure Fold Des 8:1329–1339. doi: 10.1016/S0969-2126(00)00547-5. [DOI] [PubMed] [Google Scholar]
- 88.Kwong PD, Doyle ML, Casper DJ, Cicala C, Leavitt SA, Majeed S, Steenbeke TD, Venturi M, Chaiken I, Fung M, Katinger H, Parren PW, Robinson J, Van Ryk D, Wang L, Burton DR, Freire E, Wyatt R, Sodroski J, Hendrickson WA, Arthos J. 2002. HIV-1 evades antibody-mediated neutralization through conformational masking of receptor-binding sites. Nature 420:678–682. doi: 10.1038/nature01188. [DOI] [PubMed] [Google Scholar]
- 89.Mascola JR, D'Souza P, Gilbert P, Hahn BH, Haigwood NL, Morris L, Petropoulos CJ, Polonis VR, Sarzotti M, Montefiori DC. 2005. Recommendations for the design and use of standard virus panels to assess neutralizing antibody responses elicited by candidate human immunodeficiency virus type 1 vaccines. J Virol 79:10103–10107. doi: 10.1128/JVI.79.16.10103-10107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Seaman MS, Leblanc DF, Grandpre LE, Bartman MT, Montefiori DC, Letvin NL, Mascola JR. 2007. Standardized assessment of NAb responses elicited in rhesus monkeys immunized with single- or multi-clade HIV-1 envelope immunogens. Virology 367:175–186. doi: 10.1016/j.virol.2007.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li M, Gao F, Mascola JR, Stamatatos L, Polonis VR, Koutsoukos M, Voss G, Goepfert P, Gilbert P, Greene KM, Bilska M, Kothe DL, Salazar-Gonzalez JF, Wei X, Decker JM, Hahn BH, Montefiori DC. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol 79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li M, Salazar-Gonzalez JF, Derdeyn CA, Morris L, Williamson C, Robinson JE, Decker JM, Li Y, Salazar MG, Polonis VR, Mlisana K, Karim SA, Hong K, Greene KM, Bilska M, Zhou J, Allen S, Chomba E, Mulenga J, Vwalika C, Gao F, Zhang M, Korber BT, Hunter E, Hahn BH, Montefiori DC. 2006. Genetic and neutralization properties of subtype C human immunodeficiency virus type 1 molecular env clones from acute and early heterosexually acquired infections in southern Africa. J Virol 80:11776–11790. doi: 10.1128/JVI.01730-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shaw GM, Hunter E. 2012. HIV transmission. Cold Spring Harbor Perspect Med 2:a006965. doi: 10.1101/cshperspect.a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Esparza J. 2013. A brief history of the global effort to develop a preventive HIV vaccine. Vaccine 31:3502–3518. doi: 10.1016/j.vaccine.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 95.Derdeyn CA. 2014. Humoral immune responses in SIV infection of sooty mangabeys: implications for HIV-1 pathogenesis and vaccine design. In Silvestri G, Ansari AA (ed), Natural hosts of SIV. Academic Press/Elsevier, Waltham, MA. [Google Scholar]
- 96.Hirsch VM, Olmsted RA, Murphey-Corb M, Purcell RH, Johnson PR. 1989. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature 339:389–392. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 97.Gao F, Yue L, White AT, Pappas PG, Barchue J, Hanson AP, Greene BM, Sharp PM, Shaw GM, Hahn BH. 1992. Human infection by genetically diverse SIVSM-related HIV-2 in West Africa. Nature 358:495–499. doi: 10.1038/358495a0. [DOI] [PubMed] [Google Scholar]
- 98.Beddows S, Louisirirotchanakul S, Cheingsong-Popov R, Easterbrook PJ, Simmonds P, Weber J. 1998. Neutralization of primary and T-cell line adapted isolates of human immunodeficiency virus type 1: role of V3-specific antibodies. J Gen Virol 79(Pt 1):77–82. [DOI] [PubMed] [Google Scholar]
- 99.Spenlehauer C, Saragosti S, Fleury HJ, Kirn A, Aubertin AM, Moog C. 1998. Study of the V3 loop as a target epitope for antibodies involved in the neutralization of primary isolates versus T-cell-line-adapted strains of human immunodeficiency virus type 1. J Virol 72:9855–9864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.York J, Follis KE, Trahey M, Nyambi PN, Zolla-Pazner S, Nunberg JH. 2001. Antibody binding and neutralization of primary and T-cell line-adapted isolates of human immunodeficiency virus type 1. J Virol 75:2741–2752. doi: 10.1128/JVI.75.6.2741-2752.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fischer W, Apetrei C, Santiago ML, Li Y, Gautam R, Pandrea I, Shaw GM, Hahn BH, Letvin NL, Nabel GJ, Korber BT. 2012. Distinct evolutionary pressures underlie diversity in simian immunodeficiency virus and human immunodeficiency virus lineages. J Virol 86:13217–13231. doi: 10.1128/JVI.01862-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rasmussen RA, Ong H, Song R, Chenine AL, Ayash-Rashkovsky M, Hu SL, Polacino P, Else JG, November FJ, Ruprecht RM, Clade C Program Project . 2007. Efficacy of a multigenic protein vaccine containing multimeric HIV gp160 against heterologous SHIV clade C challenges. AIDS 21:1841–1848. doi: 10.1097/QAD.0b013e32828684ea. [DOI] [PubMed] [Google Scholar]
- 103.Fouda GG, Amos JD, Wilks AB, Pollara J, Ray CA, Chand A, Kunz EL, Liebl BE, Whitaker K, Carville A, Smith S, Colvin L, Pickup DJ, Staats HF, Overman G, Eutsey-Lloyd K, Parks R, Chen H, Labranche C, Barnett S, Tomaras GD, Ferrari G, Montefiori DC, Liao HX, Letvin NL, Haynes BF, Permar SR. 2013. Mucosal immunization of lactating female rhesus monkeys with a transmitted/founder HIV-1 envelope induces strong Env-specific IgA antibody responses in breast milk. J Virol 87:6986–6999. doi: 10.1128/JVI.00528-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Quinnan GV Jr, Zhang P, Dong M, Chen H, Feng YR, Lewis M, Broder CC. 2013. Neutralizing antibody responses in macaques induced by human immunodeficiency virus type 1 monovalent or trivalent envelope glycoproteins. PLoS One 8:e59803. doi: 10.1371/journal.pone.0059803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cranage MP, Fraser CA, Cope A, McKay PF, Seaman MS, Cole T, Mahmoud AN, Hall J, Giles E, Voss G, Page M, Almond N, Shattock RJ. 2011. Antibody responses after intravaginal immunisation with trimeric HIV-1 CN54 clade C gp140 in Carbopol gel are augmented by systemic priming or boosting with an adjuvanted formulation. Vaccine 29:1421–1430. doi: 10.1016/j.vaccine.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chakrabarti BK, Feng Y, Sharma SK, McKee K, Karlsson Hedestam GB, Labranche CC, Montefiori DC, Mascola JR, Wyatt RT. 2013. Robust neutralizing antibodies elicited by HIV-1 JRFL envelope glycoprotein trimers in nonhuman primates. J Virol 87:13239–13251. doi: 10.1128/JVI.01247-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Santra S, Muldoon M, Watson S, Buzby A, Balachandran H, Carlson KR, Mach L, Kong WP, McKee K, Yang ZY, Rao SS, Mascola JR, Nabel GJ, Korber BT, Letvin NL. 2012. Breadth of cellular and humoral immune responses elicited in rhesus monkeys by multi-valent mosaic and consensus immunogens. Virology 428:121–127. doi: 10.1016/j.virol.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yin J, Dai A, Lecureux J, Arango T, Kutzler MA, Yan J, Lewis MG, Khan A, Sardesai NY, Montefiore D, Ruprecht R, Weiner DB, Boyer JD. 2011. High antibody and cellular responses induced to HIV-1 clade C envelope following DNA vaccines delivered by electroporation. Vaccine 29:6763–6770. doi: 10.1016/j.vaccine.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. 2012. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J Infect Dis 206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.McCormack S, Tilzey A, Carmichael A, Gotch F, Kepple J, Newberry A, Jones G, Lister S, Beddows S, Cheingsong R, Rees A, Babiker A, Banatvala J, Bruck C, Darbyshire J, Tyrrell D, Van Hoecke C, Weber J. 2000. A phase I trial in HIV negative healthy volunteers evaluating the effect of potent adjuvants on immunogenicity of a recombinant gp120W61D derived from dual tropic R5X4 HIV-1ACH320. Vaccine 18:1166–1177. doi: 10.1016/S0264-410X(99)00388-6. [DOI] [PubMed] [Google Scholar]
- 111.McGuire AT, Hoot S, Dreyer AM, Lippy A, Stuart A, Cohen KW, Jardine J, Menis S, Scheid JF, West AP, Schief WR, Stamatatos L. 2013. Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med 210:655–663. doi: 10.1084/jem.20122824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alam SM, Dennison SM, Aussedat B, Vohra Y, Park PK, Fernandez-Tejada A, Stewart S, Jaeger FH, Anasti K, Blinn JH, Kepler TB, Bonsignori M, Liao HX, Sodroski JG, Danishefsky SJ, Haynes BF. 2013. Recognition of synthetic glycopeptides by HIV-1 broadly neutralizing antibodies and their unmutated ancestors. Proc Natl Acad Sci U S A 110:18214–18219. doi: 10.1073/pnas.1317855110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liao HX, Bonsignori M, Alam SM, McLellan JS, Tomaras GD, Moody MA, Kozink DM, Hwang KK, Chen X, Tsao CY, Liu P, Lu X, Parks RJ, Montefiori DC, Ferrari G, Pollara J, Rao M, Peachman KK, Santra S, Letvin NL, Karasavvas N, Yang ZY, Dai K, Pancera M, Gorman J, Wiehe K, Nicely NI, Rerks-Ngarm S, Nitayaphan S, Kaewkungwal J, Pitisuttithum P, Tartaglia J, Sinangil F, Kim JH, Michael NL, Kepler TB, Kwong PD, Mascola JR, Nabel GJ, Pinter A, Zolla-Pazner S, Haynes BF. 2013. Vaccine induction of antibodies against a structurally heterogeneous site of immune pressure within HIV-1 envelope protein variable regions 1 and 2. Immunity 38:176–186. doi: 10.1016/j.immuni.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ping LH, Joseph SB, Anderson JA, Abrahams MR, Salazar-Gonzalez JF, Kincer LP, Treurnicht FK, Arney L, Ojeda S, Zhang M, Keys J, Potter EL, Chu H, Moore P, Salazar MG, Iyer S, Jabara C, Kirchherr J, Mapanje C, Ngandu N, Seoighe C, Hoffman I, Gao F, Tang Y, Labranche C, Lee B, Saville A, Vermeulen M, Fiscus S, Morris L, Karim SA, Haynes BF, Shaw GM, Korber BT, Hahn BH, Cohen MS, Montefiori D, Williamson C, Swanstrom R. 2013. Comparison of viral Env proteins from acute and chronic infections with subtype C human immunodeficiency virus type 1 identifies differences in glycosylation and CCR5 utilization and suggests a new strategy for immunogen design. J Virol 87:7218–7233. doi: 10.1128/JVI.03577-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers RC. 1990. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science 248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 116.Luciw PA, Shaw KE, Unger RE, Planelles V, Stout MW, Lackner JE, Pratt-Lowe E, Leung NJ, Banapour B, Marthas ML. 1992. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res Hum Retroviruses 8:395–402. doi: 10.1089/aid.1992.8.395. [DOI] [PubMed] [Google Scholar]
- 117.Marthas ML, Ramos RA, Lohman BL, Van Rompay KK, Unger RE, Miller CJ, Banapour B, Pedersen NC, Luciw PA. 1993. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J Virol 67:6047–6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lackner AA, Vogel P, Ramos RA, Kluge JD, Marthas M. 1994. Early events in tissues during infection with pathogenic (SIVmac239) and nonpathogenic (SIVmac1A11) molecular clones of simian immunodeficiency virus. Am J Pathol 145:428–439. [PMC free article] [PubMed] [Google Scholar]
- 119.Puffer BA, Pohlmann S, Edinger AL, Carlin D, Sanchez MD, Reitter J, Watry DD, Fox HS, Desrosiers RC, Doms RW. 2002. CD4 independence of simian immunodeficiency virus Envs is associated with macrophage tropism, neutralization sensitivity, and attenuated pathogenicity. J Virol 76:2595–2605. doi: 10.1128/JVI.76.6.2595-2605.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhuge W, Jia F, Adany I, Narayan O, Stephens EB. 1997. Plasmas from lymphocyte- and macrophage-tropic SIVmac-infected macaques have antibodies with a broader spectrum of virus neutralization activity in macrophage versus lymphocyte cultures. Virology 227:24–33. doi: 10.1006/viro.1996.8300. [DOI] [PubMed] [Google Scholar]
- 121.Means RE, Matthews T, Hoxie JA, Malim MH, Kodama T, Desrosiers RC. 2001. Ability of the V3 loop of simian immunodeficiency virus to serve as a target for antibody-mediated neutralization: correlation of neutralization sensitivity, growth in macrophages, and decreased dependence on CD4. J Virol 75:3903–3915. doi: 10.1128/JVI.75.8.3903-3915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.White TA, Bartesaghi A, Borgnia MJ, Meyerson JR, de la Cruz MJ, Bess JW, Nandwani R, Hoxie JA, Lifson JD, Milne JL, Subramaniam S. 2010. Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: strain-dependent variation in quaternary structure. PLoS Pathog 6:e1001249. doi: 10.1371/journal.ppat.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.White TA, Bartesaghi A, Borgnia MJ, de la Cruz MJ, Nandwani R, Hoxie JA, Bess JW, Lifson JD, Milne JL, Subramaniam S. 2011. Three-dimensional structures of soluble CD4-bound states of trimeric simian immunodeficiency virus envelope glycoproteins determined by using cryo-electron tomography. J Virol 85:12114–12123. doi: 10.1128/JVI.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.McDermott AB, Mitchen J, Piaskowski S, De Souza I, Yant LJ, Stephany J, Furlott J, Watkins DI. 2004. Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: a model for the evaluation of vaccine efficacy in nonhuman primates. J Virol 78:3140–3144. doi: 10.1128/JVI.78.6.3140-3144.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Stone M, Keele BF, Ma ZM, Bailes E, Dutra J, Hahn BH, Shaw GM, Miller CJ. 2010. A limited number of simian immunodeficiency virus (SIV) env variants are transmitted to rhesus macaques vaginally inoculated with SIVmac251. J Virol 84:7083–7095. doi: 10.1128/JVI.00481-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hirsch VM, Zack PM, Vogel AP, Johnson PR. 1991. Simian immunodeficiency virus infection of macaques: end-stage disease is characterized by widespread distribution of proviral DNA in tissues. J Infect Dis 163:976–988. doi: 10.1093/infdis/163.5.976. [DOI] [PubMed] [Google Scholar]
- 127.Goldstein S, Elkins WR, London WT, Hahn A, Goeken R, Martin JE, Hirsch VM. 1994. Immunization with whole inactivated vaccine protects from infection by SIV grown in human but not macaque cells. J Med Primatol 23:75–82. doi: 10.1111/j.1600-0684.1994.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 128.Dehghani H, Puffer BA, Doms RW, Hirsch VM. 2003. Unique pattern of convergent envelope evolution in simian immunodeficiency virus-infected rapid progressor macaques: association with CD4-independent usage of CCR5. J Virol 77:6405–6418. doi: 10.1128/JVI.77.11.6405-6418.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Derdeyn CA, Decker JM, Bibollet-Ruche F, Mokili JL, Muldoon M, Denham SA, Heil ML, Kasolo F, Musonda R, Hahn BH, Shaw GM, Korber BT, Allen S, Hunter E. 2004. Envelope-constrained neutralization-sensitive HIV-1 after heterosexual transmission. Science 303:2019–2022. doi: 10.1126/science.1093137. [DOI] [PubMed] [Google Scholar]
- 130.Keele BF, Giorgi EE, Salazar-Gonzalez JF, Decker JM, Pham KT, Salazar MG, Sun C, Grayson T, Wang S, Li H, Wei X, Jiang C, Kirchherr JL, Gao F, Anderson JA, Ping LH, Swanstrom R, Tomaras GD, Blattner WA, Goepfert PA, Kilby JM, Saag MS, Delwart EL, Busch MP, Cohen MS, Montefiori DC, Haynes BF, Gaschen B, Athreya GS, Lee HY, Wood N, Seoighe C, Perelson AS, Bhattacharya T, Korber BT, Hahn BH, Shaw GM. 2008. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci U S A 105:7552–7557. doi: 10.1073/pnas.0802203105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Isaacman-Beck J, Hermann EA, Yi Y, Ratcliffe SJ, Mulenga J, Allen S, Hunter E, Derdeyn CA, Collman RG. 2009. Heterosexual transmission of human immunodeficiency virus type 1 subtype C: macrophage tropism, alternative coreceptor use, and the molecular anatomy of CCR5 utilization. J Virol 83:8208–8220. doi: 10.1128/JVI.00296-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Alexander M, Lynch R, Mulenga J, Allen S, Derdeyn CA, Hunter E. 2010. Donor and recipient Envs from heterosexual human immunodeficiency virus subtype C transmission pairs require high receptor levels for entry. J Virol 84:4100–4104. doi: 10.1128/JVI.02068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kirchhoff F, Morrison HG, Murray MG, Rennert P, Desrosiers RC. 1994. SIVmac expressing hybrid envelope proteins containing HIV-1 V3 and/or C4 sequences is not competent for replication. AIDS Res Hum Retroviruses 10:309–313. doi: 10.1089/aid.1994.10.309. [DOI] [PubMed] [Google Scholar]
- 134.Finzi A, Pacheco B, Xiang SH, Pancera M, Herschhorn A, Wang L, Zeng X, Desormeaux A, Kwong PD, Sodroski J. 2012. Lineage-specific differences between human and simian immunodeficiency virus regulation of gp120 trimer association and CD4 binding. J Virol 86:8974–8986. doi: 10.1128/JVI.01076-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Edinger AL, Amedee A, Miller K, Doranz BJ, Endres M, Sharron M, Samson M, Lu ZH, Clements JE, Murphey-Corb M, Peiper SC, Parmentier M, Broder CC, Doms RW. 1997. Differential utilization of CCR5 by macrophage and T cell tropic simian immunodeficiency virus strains. Proc Natl Acad Sci U S A 94:4005–4010. doi: 10.1073/pnas.94.8.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Edinger AL, Mankowski JL, Doranz BJ, Margulies BJ, Lee B, Rucker J, Sharron M, Hoffman TL, Berson JF, Zink MC, Hirsch VM, Clements JE, Doms RW. 1997. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc Natl Acad Sci U S A 94:14742–14747. doi: 10.1073/pnas.94.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin KA, Wyatt R, Farzan M, Choe H, Marcon L, Desjardins E, Robinson J, Sodroski J, Gerard C, Gerard NP. 1997. CD4-independent binding of SIV gp120 to rhesus CCR5. Science 278:1470–1473. doi: 10.1126/science.278.5342.1470. [DOI] [PubMed] [Google Scholar]
- 138.Mori K, Rosenzweig M, Desrosiers RC. 2000. Mechanisms for adaptation of simian immunodeficiency virus to replication in alveolar macrophages. J Virol 74:10852–10859. doi: 10.1128/JVI.74.22.10852-10859.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pohlmann S, Davis C, Meister S, Leslie GJ, Otto C, Reeves JD, Puffer BA, Papkalla A, Krumbiegel M, Marzi A, Lorenz S, Munch J, Doms RW, Kirchhoff F. 2004. Amino acid 324 in the simian immunodeficiency virus SIVmac V3 loop can confer CD4 independence and modulate the interaction with CCR5 and alternative coreceptors. J Virol 78:3223–3232. doi: 10.1128/JVI.78.7.3223-3232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Puffer BA, Altamura LA, Pierson TC, Doms RW. 2004. Determinants within gp120 and gp41 contribute to CD4 independence of SIV Envs. Virology 327:16–25. doi: 10.1016/j.virol.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 141.Schenten D, Marcon L, Karlsson GB, Parolin C, Kodama T, Gerard N, Sodroski J. 1999. Effects of soluble CD4 on simian immunodeficiency virus infection of CD4-positive and CD4-negative cells. J Virol 73:5373–5380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.