Abstract
Tendons mainly function as load-bearing tissues in the muscloskeletal system, transmitting loads from muscle to bone. Tendons are dynamic structures that respond to the magnitude, direction, frequency, and duration of physiologic as well as pathologic mechanical loads via complex interactions between cellular pathways and the highly specialized extracellular matrix. This paper reviews the evolution and current knowledge of mechanobiology in tendon development, homeostasis, disease, and repair. In addition, we review several novel mechanotransduction pathways that have been identified recently in other tissues and cell types, providing potential research opportunities in the field of tendon mechanobiology. We also highlight current methods, models, and technologies being used in a wide variety of mechanobiology research that could be investigated in the context of their potential applicability for answering some of the fundamental unanswered questions in this field. The article concludes with a review of the major questions and future goals discussed during the recent ORS/ISMMS New Frontiers in Tendon Research Conference held September 10–11, 2014 in New York City.
Introduction
The ability of cells to respond to externally applied forces is a fundamental biologic response which affects tissue development, homeostasis, disease and repair. While initial observations on the biologic effect of externally applied forces were described in bone by Julius Wolff,1 a growing body of work in the field of mechanobiology has focused on mechanistic components of this relationship in all connective tissues, including tendon. Tendon cells are sensitive to mechanical stimuli imposed during tendon loading and can adapt their extracellular matrix in an anabolic or catabolic manner according to the magnitude, frequency, direction and duration of externally applied loads.2–4 The dynamic interactions between a cell and its physical microenvironment involve a complex set of pathways between the cell surface (e.g., ion channels, focal adhesion kinases, integrins, cilia, and the cytoskeleton, etc.) that interface with the nucleus to generate a biologic response. While physiologic loads are required to maintain tendon homeostasis,5,6 abnormal loading can lead to tendon injury, either through an acute traumatic injury or a more chronic, degenerative process (i.e., tendinopathy) resulting from an accumulation of micro-damage and an altered cell/matrix response.7–9 Therefore, unraveling the mechanobiology of tendon cells is critical to understanding both the pathophysiology in tendon disease and the physiologic benefits of controlled loading (i.e., rehabilitation) during tendon healing.
This review examines the evolution of tendon mechanobiological research and summarizes our current understanding of the role of mechanobiology in tendon health and disease. New areas of mechanobiology which have not yet received much attention in the tendon literature are also highlighted. In addition, current methods, models, and technologies being used in a wide variety of mechanobiology research will be discussed in the context of their potential applicability to tendon research. The article concludes with a review of the major questions and future goals discussed during the recent ORS/ISMMS New Frontiers in Tendon Research held September 10–11, 2014 in New York City.
Tendon Mechanobiology
Tendon primarily functions by transmitting tensile loads from muscle to bone providing stability and greater efficiency in the motion of the musculoskeletal system. This load transfer function is likely to serve as the primary mechanical stimulus for tendon cells. Such tensile loads are transferred to tendon cells through various matrix components and compartments. At the cell level, they are transduced from the exterior to intracellular biochemical responses by various transmembrane structures and pathways.
As with all biological systems, tendon is highly dependent on its structure and cellular organization for function and response to physiologic loading. The highly organized structural components of tendon are critical for its non-linear, viscoelastic response to applied cyclic tensile loads. Tendon is mainly composed of water while the solid matrix is predominantly composed of collagen (70–80% dry weight).10 Type I collagen is the main structural component of tendon, and it is arranged in a complex hierarchy that varies in tensile properties from nanoscale to macroscale.11 The structural arrangement and mechanical properties of collagen are thought to provide the main material characteristics of tendon. For example, the toe region results from collagen crimp formation and the high tensile strength is due to the ability to form covalent intramolecular and intermolecular cross-links that inhibit sliding between adjacent fibers and fibrils.11,12 In addition to matrix deformation, experimental studies have demonstrated interstitial fluid flow in response to cyclic tensile loading of tendons,13 but the role of this and the mechanical contribution of other components of tendon (elastin, glycoproteins, proteoglycans, glycolipids, and cells) are still under consideration.
Within tendon, cells are organized in linear arrays aligned with and interspersed between collagen fibers as a 3-dimensional network of cells and their processes distributed throughout the tendon. These cells have flattened cell processes which extend laterally and form junctions with adjacent cells which are in direct contact with collagen bundles.14 Tendon cells reside within a specialized pericellular matrix,15 which may play an important role in mechanotransduction, similar to that of the pericellular matrix of articular cartilage.16
The deformation of tendon extracellular matrix from applied loading transmits various levels and combinations of tensile, compressive, and shear stresses and strains to the tendon cells.17 The transmission of this deformation to the localized cell or nucleus correlates to, but is less than the applied tendon deformation.17 Interstitial fluid flow in response to cyclic tensile loading of tendons13 may also lead to additional shear forces and perhaps hydrostatic pressure on tendon cells.18 The magnitude, frequency, and duration of these tissue forces on the cells depend on prior loading history (exercise, disuse, overuse) and the composition of the ECM (tendon type, age, sex, disease, microdamage).
The mechanobiology of tendon cells is vital for the maintenance of tissue homeostasis.2,19 Physiologic loads required to maintain tendon homeostasis have been identified with both in vitro and in vivo models.5,6,9,20,21 The precise physiologic loads of individual tendons depend on their function, age, sex, location, and species. Further, tendon is not an isolated tissue, but is instead transitionally integrated into both muscle (myotendinous junction) and bone (enthesis). These transition sites and regional differences in each tendon due to anatomic location and function correspond to global and regional variations in the tissue composition and material properties, and strain distributions,22 and are often potential sites of the initiation of tendon injury23 and subsequent alterations in the cellular/matrix response.
While certain loading patterns are known to induce cellular anabolic adaptation of tendon,5,6,9,20,21 repetitive loading may also lead to a mechanobiological over-stimulation of tendon cells and initiation of a catabolic degenerative response that leads to tendinopathy.24,25 While many of these in vitro repetitive loading studies show increases in tendinopathic markers (inflammatory cytokines, degenerative enzymes), they may not replicate the in situ mechanobiology of tendon cells within an in vivo three-dimensional collageneous matrix.8 Over-stimulation of tendon through single or repetitive loading induces collagen fibril damage, micro-damage, or laxity,23,26–28 which in turn may result in paradoxical mechanobiological hypo-stimulation of tendon cells. Hypo-stimulation of tendon cells resulting from altered cell-matrix interactions has been demonstrated in situ to have similar outcomes8,29 to the pathological changes (collagen disruption, hypocellularity, increased MMP levels, apoptosis) reported in clinical cases of tendinopathy.8,9 While the precise level (magnitude, frequency, and duration) of stimulation required for normal tendon homeostasis remains unknown, it is likely that abnormal levels of stimulation may play a role in the pathogenesis of tendinopathy.8,9 In addition, the precise in vivo loading levels required to induce repair remain unknown. Indeed, one of the most effective treatments of tendinopathy in the patellar, Achilles, and even rotator cuff tendons is the use of controlled eccentric motion therapy.30 This eccentric loading may counteract the altered mechanobiological stimulation that is postulated to occur with tendinopathy.31 In this regard, further understanding the in vivo loading of tendons is vital to understanding the mechanobiological stimuli required to induce anabolic or reduce catabolic activity.
Transfer of Load to Cells
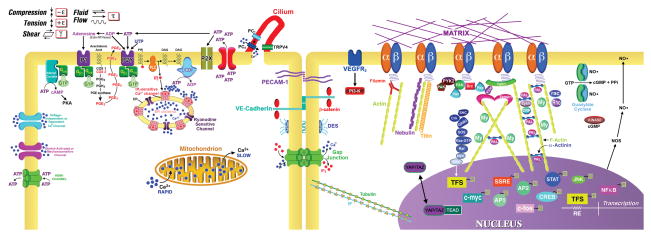
Mechanical signals, including tension, compression, hydrostatic pressure, and fluid shear stress, are transduced by cells to stimulate biochemical pathways and effect cellular processes, such as differentiation, proliferation, tissue development, and skeletal maintenance (Figure 1).2,32 This transduction may occur through a number of mechanisms and signaling pathways, including the primary cilium, activation of cell receptors and ion channels, alterations in second messengers, such as intracellular Ca2+ or adenosine triphosphate (ATP), cytoskeletal rearrangement,33 changes in gene and protein expression, and perhaps Hippo signaling mediated by YAP/TAZ.34
Figure 1.
The detection of and response to external mechanical stimuli (i.e., compression, tension, shear, fluid shear stress) involves multiple pathways and signaling mediators including changes in intracellular calcium (Ca2+ ) through the release of intracellular Ca2+ stores or entry of extracellular Ca2+ through channels such as the store-operated, stretch-activated or mechanosensitive channels and voltage independent or dependent Ca2+ channels and the release of ATP and, at lower levels, UTP, following the activation of ionotropic P2X and metabotropic, G protein-coupled P2Y receptors in an autocrine/paracrine fashion. ATP acts on P2Y2 receptors, the primary ATP/UTP responsive receptor in tenocytes, activating the Gαq-protein, driving PLC and producing IP3 and DAG. IP3 acts on IP3-sensitive Ca2+ channels in the ER to mobilize intracellular Ca2+, and DAG activates a PKC pathway. PKC and Ca2+ activate adenyl cyclase activity yielding cAMP, which stimulates cAMP-dependent protein kinase A (PKA), which may act at Raf in the kinase cascade. Rap la,b, Ras-like proteins, regulate the PKA stimulation of Raf. P2 receptors may activate other kinases including MAPK/ERK, SAPK/JNK, p38 MAPK, and PI3K/AKT(PKB). Initial action of ATP is terminated quickly by membrane-bound ecto-NTPases to its metabolites: ADP, AMP, and adenosine. Adenosine activates G protein-coupled P1 receptors, activating stimulatory (Gs) or inhibitory (Gi) signaling. Polycistin-1 (PC1) is co-localized with the primary cilium and activated when the cilium is deformed by fluid shear stress. The shear stress signal is transferred from PC1 to PC2 and induces the influx of Ca2+ though PC2, which in turn activates intracellular ryanodine receptors through Ca2+-induced Ca2+ release. PECAM-1 will activate Src when cells are subjected to fluid shear stress. The signal is then transferred to VEGFR2 through VE-cadherin and beta-catenin. PI3K are activated by VEGFR2 and then integrins are activated. A matrix-integrin-mechanosensory protein complex-cytoskeleton machinery is linked to a kinase cascade (tyrosine or nontyrosine kinase cascade or the JACSTAT kinase cascade) system. A mechanosensory protein complex contains talin, vinculin, tensin, paxillin, Src, and focal adhesion kinase (FAK). Activated ERKs enter the nucleus and up-regulate transcription factor expression Gun, fos, myc, erg-1) and activate nuclear binding proteins such as NF-κB. A load signal may activate a growth factor receptor (P for phosphorylation) with or without ligand and activate the same or a similar sequence of kinases (PTKR, protein tyrosine kinase receptor; GF, growth factor; PDGF, platelet-derived growth factor). Gap junctions pass IP, which propagates a Ca2+ wave from cell to cell after a mechanical signal is detected. Connexin hemichannels can pass ATP outside the cell. In this model, a load deformation displaces matrix molecules tethered to clustered integrins at focal adhesions. The displacement is transduced to an integrin (b), to an integrin-binding protein, and then to associated proteins. AP-1, activator protein-1; CREB, cAMP response element binding protein; DAG, diacylglycerol; IP3, inositol trisphosphate; MAPKs, mitogen-activated protein kinase; ERK, extracellular signal-regulated protein kinase; SAPK, stress-activated protein kinase; JNK, c-Jun NH2-terminal kinase; MEK, MAPK/ERK kinase; NO, nitric oxide; PI3K, phosphoinositide 3-kinase; PLC, phospholipase C; PKA, protein kinase A; PKC, protein kinase C; PKB, protein kinase B; STAT, signal transducer and activator of transcription. SHC, Src homology protein complex; Crk, Src homology adaptor protein that binds paxillin and C3G; GRB2, growth factor receptor binding adaptor protein linking receptors to the Ras pathway through FAK and SOS, a guanine nucleotide exchange factor; Ras, GTPase that regulates activation of Raf; MEK, mitogen-activated kinase; ERK, extracellularly regulated kinase; CAM is a cell adhesion molecule; IF, intermediate filament; YAP/TAZ, Yki transcription co-activators; TEAD, transcription factor.
The deformability of a tenocyte is determined by a number of factors, which together determine the elastic stiffness of the cell. These factors include residual tensile “pre-stress” in the cytoskeleton, which is influenced by the stiffness of the matrix, attachment of the cell to the matrix (matrix-integrin linkage), cell-cell connections and contractility (α-smooth muscle actin).35 A growing body of evidence supports the idea that tensile pre-stress in the cytoskeleton influences cellular response to mechanical stimulation and, therefore, to biochemical signals. Evidence also suggests that biochemical mediators modulate the mechanical properties of cells and their surrounding matrix, which in turn regulates cellular mechanosensitivity and responses to mechanical stimulation.16,35,36
Matrix linkages through integrins to the cytoskeleton and to the nucleus transduce externally applied strain directly to the cell.19 Tenocytes alter expression of integrins in response to tensile strain37 and applied strain may elicit kinetic responses from cells much faster than those derived from chemical ligand application.38 A proposed mechanosensory protein complex beneath the plasma membrane comprised of integrin and actin binding partners represent a physical link in activation pathway(s) to transduce strain or shear stress2. The pathways that link to the deformation sensors often involve transient changes in intracellular concentration of calcium ([Ca2+]i), which results in the activation of downstream pathways, such as PGE2 release as well as alterations in the expression of matrix genes.39,40
Surprisingly, the primary cilium, which is present in most cells including tenocytes,41 has been shown to respond to shear stress deformation in osteoblasts and endothelial cells. In tendon, primary cilia are aligned parallel to the collagen fibers along the long axis of the tendon41 and deflect in response to tensile loading.42 Primary cilia length within the tendon depends on location and the mechanical environment.43 Stress deprivation may increase the length of the cilia, an effect that can be reversed by mechanical loading.43,44 Together these data suggest an important role for the primary cilium in response to changes in mechanical environment within tendon.
Cells in both the epitenon and internal compartments of tendon are physically connected to each other by gap junctions,14 even within monolayer and 3D culture.45 The gap junctional complex is composed of two connexons, each of which contain six transmembrane proteins called connexins (Cx), of which Cx 26, 32, and 43 are most commonly identified in tendon. Within a syncytium, or cellular network, cells are connected by the gap junctions, Cx43 and Cx32, but between syncytia are connected by only Cx43.14 Cx43 co-localization with actin increases with substrate strain.46 Tenocytes are coupled and respond to mechanical stimulation of a target cell plasma membrane by increasing [Ca2+]i and propagating a calcium wave to adjacent cells for up to 4–7 cell diameters.47,48 Cx43 gap junctions undergo expression and permeability changes in response to mechanical load in tendon cells.47,49 Thus, gap junctions are dynamic structures that may play an important role in tenocyte mechanobiology.
Cellular Responses to Load
Cells in mechanically active tissues detect, process, and relay load signals to surrounding cells in a feedback loop designed to provide tissue homeostasis.2,50 Tendon cells respond to load by activating ion channels, increasing [Ca2+]i, releasing ATP, altering their cytoplasmic filament organization and content (especially actin), and altering their protein expression and secreting MMPs.2,39,47,51–53 Mechanical loading causes the release of ATP in almost every cell type examined to date, including tenocytes.24,54 Cells in vitro, including tenocytes, generally secrete ATP on the order of 10–150 pM on average and up to nM levels in some cells.54
Tenocytes express purinoceptors and respond to ATP and other nucleotides and nucleosides.24,54 However, high doses of ATP can temporally desensitize tenocytes to a mechanical stimulus. A brief mechanostimulus such as substrate strain can temporally (5 minutes later) augment a response to a subsequent mechanostimulus such as a membrane deformation. The effect of secreted ATP is modulated by ecto-NTPases which appear to act principally at the cell surface in tendon.54 ATP can also modulate collagen gel contraction in MC3T3-E1 cells in vitro in 3D gel linear constructs and in bioartificial tendons.55 Therefore, ATP is an important modulator of mechanical load responses in tendon cells.
Cellular Responses Post- Injury
After injury, inflammation can occur with influx of white cells, expression of cytokines and metalloproteinases and swelling.56 However, most experts in the field believe that tendinopathies do not involve a classic inflammatory pathway, but rather involve a local “molecular” inflammation caused by resident cells that express MMPs, COX 2, and make PGE2.56–59 Tendon rupture results in bleeding, clotting and release of PDGF, TGF-β, ATP and ADP from platelets, release of hormones such as epinephrine and norepinephrine from blood vessels and/or nerves, and activation of IGF-I from plasma and tendon matrix and TGF-β from matrix at the wound site.58,59 Cell migration from the epitenon into the wound site occurs followed by cell division then matrix synthesis. Passive or active motion speeds recovery and promotes increased range of motion, but the mechanisms by which this phenomenon occurs remain conjectural.60
Mechanical loading stimulates the production of IL-1β and ATP in tenocytes and ligament cells,24,58 and these mediators modulate the pre-stress cytoskeletal state and therefore phenotype in cells.35,36,55 Substrate stiffness can regulate tenocyte expression of MMPs.49 IL-1β is well known as a potent proinflammatory factor which is often found at a site of tendon injury. IL-1β treatment increases the secretion and expression of metalloproteinases (MMPs)-1, -2, -3, -9, and -13 in tenocytes53,58 and accelerates the degeneration of the matrix. IL-1β also differentially regulates the expression of type I collagen and elastin and decreases the Young’s modulus of human tenocyte-populated bioartificial tendons (BATs).36 This increased elasticity prevents BATs from mechanical load-induced rupture.36 Therefore, IL-1β may act as a regulator in modulating the mechanical properties of ECM in response to mechanical stimulation.
Tenocyte Biomarkers and Mechanobiology
A more specific list of markers for tenocytes include collagen type I, II, III, decorin, TGFβ 1, 2, 3, BMP 2, 7, Mohawk, Scleraxis, tenomodulin, and specific cell surface markers (CD29, CD44, CD73, CD90, CD105).61–64 Tenomodulin is not tenocyte specific but is produced by tenocytes and is likely both in the cytoplasm and nucleus.65 Titin is a more muscle-specific protein present in the Z band of the sarcomere and acts as a shock cord, returning the sarcomere back to its resting level, but is present in tenocytes and a titin fragment migrates to the nucleus after mechanical stimulation.66
New Techniques for Studying Tendon Mechanobiology
A variety of technologies have been used to investigate dynamic in vivo forces and strains in various tendons at different length scales. The use of confocal microscopic and dual photon imaging combined with staining protocols has greatly enhanced our knowledge of complex regional variations in tendon, and non-linear cellular and matrix response of tendon to in situ loading. Several invasive implantable sensors and non-invasive systems have been developed to evaluate in vivo strain and forces applied to the tendon under various dynamic loading regimes,67,68 as well as measurements of regional differences within the tendon.26,69 Recent modifications to non-invasive imaging techniques in conjunction with computational image analysis have been used to determine in vivo loading forces and strains with greater resolution than previously including ultrasound tissue characterization,26 acoustoelastography,70 and magnetic resonance imaging.71 Reduced-orientation dipolar anisotropy fiber imaging has improved magnetic resonance contrast between supraspinatus tendon, infraspinatus tendon and rotator cable, and can identify individual layers of the multi-layered rotator cuff with correlation to the histopathology and anatomy of the intact rotator cuff. This technique takes advantage of the ‘magic angle effect’ to improve contrast between layers of complex tissues such as the rotator cuff, but its use in abnormal structures has not yet been reported.72 Shear-wave elastography is an advance on tendon elastography and measures shear-wave velocity generated by the ultrasound pulse to evaluate viscoelastic properties of tendon.73
Several new imaging technologies have recently been applied to other aligned soft tissues, and may be adapted for future static and dynamic tendon mechanobiology studies. Diffusion tensor imaging is valuable in investigation of fiber architecture in nerves, brain and muscle, and describes direction of anisotropic diffusion of water molecules within each voxel.74 Optical coherence micro-elastography is an optical coherence tomography technique to measure tissue deformation in response to static or dynamic loading and provide microscale real-time high resolution mechanical contrast imaging.75,76 Second harmonic generation microscopy has recently been combined with a numerical model to quantify the underlying collagen structure and predict fibril diameter in normal and osteoarthritic cartilage, results which were confirmed by atomic force microscopy.77 At the level of interactions between cell and extracellular matrix niche, Förster resonance energy transfer (FRET) between two fluorophores separated by an elastic tension sensor module inserted into vinculin is proving invaluable in investigation of intracellular focal adhesion dynamics in cell-matrix interactions,78 while effects of cell-matrix interactions on extracellular matrix tension can be evaluated in a variety of ways, including using FRET technology in fibronectin.79 Other new approaches used to study mechanobiology in fields such as neuroscience have recently been reviewed, including atomic force microscopy based approaches, optical or magnetic trapping at the cellular or subcellular level, various patterning, microfluidic and deformable membrane technologies and magnetic resonance elastography.80 The recent identification of type VI collagen, fibrillin-1 and elastin in the pericellular matrix of tendon, and its disorganization in degenerative tendon 15 suggests that atomic force microscopy techniques used to evaluate the integrity of the pericellular matrix of cartilage may also be valuable in studying tendon development or tendinopathies.16,81 At the genomic level, the CRISPR/Cas9 system has been used to label and image specific genomic loci,82 a targeted genome imaging technique which may be extremely useful in evaluating the genomic effects of tendon mechanobiology once systems biology approaches have identified putative targets.
Various experimental and computational models have sought to understand the complex overall behavior of the tendon.83 Future challenges identified included the need to model cellular anabolism and catabolism for extracellular matrix components, the need to model the micromechanical and pericellular tendon environment, and the need to model the effects of microscale events on complex macroscale tendon structure and properties.83 Recent efforts are addressing these needs. For example, a three-level multiscale approach has been used to model macroscale mechanical behavior of collagenous tissues and account for nanoscale intermolecular cross-links and collagen mechanics, geometric nonlinearities, local stress and strain fields at the pericellular microscale.84 The volumetric loss that tendon undergoes during loading, measured by large Poisson’s ratios measured during tensile testing has been accounted for using continuum based hyperelestic constitutive modeling to describe both the stress-strain relationship of tendons under tensile load and the large strain-dependent Poisson’s ratios observed though modeling fluid movement.85 While many techniques and models have been developed, much work remains to apply them to critical questions in the field of tendon mechanobiology.
Recent Advances in Mechanical Signal Transduction
As reviewed above, a great deal of work has been done on the mechanisms involved in the transduction of mechanical loads to an intracellular response by tendon cells.86 As the picture emerges, it is clear that cells in tendon are similar in many respects to those in other connective tissues such as cartilage,87 meniscus,88 or intervertebral disc,89 do not simply utilize a single mechanotransduction pathway, but rather have a number of interacting mechanisms that perform different mechanotransduction roles in the tissue. In this regard, a more thorough understanding of the specific mechanisms of mechanotransduction as well as the downstream pathways they activate will hopefully lead to new approaches for treating tendinopathies or enhancing tendon repair. In the past decade, major advances have been made in several broad areas of cell mechanics and mechanotransduction. Here we focus on several recent areas that have advanced rapidly but have only received limited attention in tendon.
The Role of Ion Channels in Mechanobiology
The discovery of the Transient Receptor Potential (TRP) superfamily of ion channels has revolutionized our understanding of the mechanisms by which many cell types sense and respond to a diverse array of stimuli, including mechanical loading, pain, itch, heat, cold, osmolarity, and others. These channels are classified into seven subfamilies by sequence homology - TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPN (no mechanoreceptor), TRPA (ankyrin), TRPP (polycystin), and TRPML (mucolipin). TRP channels are generally activated by specific chemical agonists as well as physical factors, and in many cases, are believed to serve as integrators of various physical and chemical stimulants. For example, TRPV1, which is noxious heat-pain receptor, is well known as the “chili-pepper receptor” and is activated chemically by capsaicin.90 TRPM8 responds to cold temperatures, but is also chemically activated by menthol.91
The TRPV family has been of particular interest to investigators studying connective tissues, and several recent studies have shown important roles for these channels in musculoskeletal transduction.92 For example, TRPV4, which was identified as an osmotically-sensitive channel in C. Elegans,93 has been shown to control the anabolic response of articular chondrocytes to mechanical loading,94,95 and Trpv4 knock-out mice develop early-onset osteoarthritis.96 Furthermore, TRPV4 within the trigeminal ganglion is an important mediator of inflammation-mediated nociception in the joint.97 TRPV4, TRPV6, and TRPC1 all have been shown to regulate osteoclastogenesis and bone remodeling,98–100 suggesting that tendon attachment to bone may also be influenced by the activity of TRP channels. The roles of these channels, and other recently identified mechanosensitive ion channels such as the PIEZOs,101 in tendon inflammatory response or mechanotransduction remains to be determined, and they provide novel and important targets for the study of tendon mechanobiology.
The Hippo Pathway and YAP/TAZ in Mechanobiology
Another recent area of rapid advancement in mechanobiology has been in the Hippo network, a major conserved pathway that functions as a growth suppressor to regulate organ size and prevent tumor formation. In particular, two transcriptional coactivators in this network - Yes-associated protein (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ) – were recently identified as regulators of the transcriptional and phenotypic changes caused by changes in the biophysical environment of cells.102,103 Indeed, growing evidence suggests that this network serves to integrate biophysical signals into multiple signaling pathways, including TGFβ/BMP, Wnt, IGF, and AKT.104 At the cellular level, the YAP/TAZ pathway has been implicated in sensing cell tension, substrate rigidity, cell geometry, and other mechanobiological phenomena.105 To date, little or no work has been reported on this pathway in regulating tendon mechanobiology, and it thus provides an important potential area of investigation.
Conclusions
While emerging technologies and techniques will likely be extremely valuable in improving our understanding of tendon mechanobiology, an integrated, collaborative multi-disciplinary multi-scale approach is likely to yield the greatest advances in the field.
It is now becoming apparent that connective tissue cells coordinate multiple interacting mechanisms of mechanical signal transduction. A more thorough understanding of these pathways and their interactions will hopefully lead to new therapeutic approaches and rehabilitation methods for the prevention or treatment of tendon disease.
Major Questions
How do mechanical factors determine cell fate?
Mechanical factors (matrix stiffness, loading stimuli) are thought to play a role in determining cell fate during development106 and in post-natal tissue, where complete loss of load can lead to apoptosis and initiation of myofibroblastic cells in tendon.29,107 However, the precise mechanobiological mechanisms (Figure 1) involved in the role of mechanical factors determining cell fate are under continued investigation.
Can an understanding of mechanobiology lead to new drug targets for treating tendinopathy or enhancing regeneration?
Overall, understanding tendon mechanobiology may lead to better therapeutic regimens for tendinopathy. For instance, drugs for treating tendinopathy or enhancing regeneration either reduce the degenerative effects associated with the loss of matrix tension 108 or stimulate anabolic activity.
Staging and definitions of tendon health and disease – is tendinopathy a biological, structural, mechanical, or psychosocial outcome?
Tendinopathy is a progressive disease and is mostly defined by pain and functional loss. However the pathological signs of the disease in its various stages are beginning to be defined in terms of gene expression and protein synthesis.109
What is the role of other tissues (bone, muscle, nerve, vascularity, etc.) on tendon mechanobiology
Tendons may have global or local structural variations based on their anatomic location, function, and interaction with other associated tissues (bone, muscle, nerve, vascularity, etc.). These associated tissues are necessary for tendon homeostasis, but the direct or indirect role of these tissues in tendon mechanobiology is still a subject of current research.
Future Goals
In vitro models: Systems that simulate tendon in culture with high fidelity to native tendon
Investigations have demonstrated the existence of a window of induced loading needed to maintain cultured tendons in their native state, where too little or too much load can cause progressive degeneration.20 Similar tissue engineered constructs also require precise mechanical loading to mimic native tendon cell organization, structure, and gene expression.110 Future studies in these culture systems may help determine the window of tissue or cellular mechanical stimulation required to maintain tendon homeostasis.
Animal models: range of animal models (C Elegans, fruit fly, zebrafish, chick embryo, mouse, etc.) to study mechanobiology
Many of the recent breakthroughs in mechanotransduction have been made in lower organisms, showing the highly conserved nature of these pathways. Future studies in such model organisms may help to elucidate new mechanisms of mechanical signaling.
Computational models: Systems biology, bioinformatics, and finite element models of mechanobiology
The future use of computational approaches can help answer questions related to defining tendon homeostasis and subsequent alterations based on cellular activity. In turn, time-based descriptions of cellular activity and response can be incorporated into multi-scale finite element models to predict alterations in the complex hierarchical composition and subsequent loading of tendon.83
Cell Therapy: Develop customized (stem) cells for therapeutic applications
Recent studies suggest that the use of the exogenous or endogenous tendon stem cell populations may have therapeutic effects on diseased or injured tendons.111,112 However, the precise administration (timing, dosage, carrier, etc.) as well as the effect of local conditions (biological and/or biomechanical) on their function and differentiation has yet to be determined.
Biomarkers: Need for imaging and biomarkers for outcomes
Although there are several suggested biomarkers (tenomodulin, Scleraxis, Mohawk, myostatin, tenascin-c, etc.) to selectively and clearly identify tendon cells throughout differentiation,65 identification of molecules that can uniquely identify a tendon cell may enhance mechanobiology-based studies in tendon development, diagnostics, and in therapy. In addition to molecular biomarkers, future research is needed in obtaining imaging biomarkers of tendon injury or disease to better understand the clinical implications of altered tendon mechanobiology.
Rehabilitation: More defined or controlled regimens of physical therapy (tendon/muscle) to treat tendinopathy
Overall, understanding how tenocytes respond to strain and how they mechanoregulate their response will lead to better rehabilitation regimens to treat tendinopathy.113
Acknowledgments
The many studies we were unable to cite due to limitations on the number of references.
DL – Funding from NIH/NIAMS (AR059784, AR065764)
Conflict statement – Paid consultant for Cytex Therapeutics Inc.
FG – NIH grants AR48852, AG15768, AR50245, and AG46927.
Conflict statement – Employee of Cytex Therapeutics, Inc.
MW – Director of Project Management for Flexcell International Corp. and receives compensation as such.
AB – President of Flexcell International Corp. and receives compensation as such.
Footnotes
Author Contribution Statement: All authors have made substantial contributions to drafting and critical revision of the paper and have subsequently read and approved the final submitted manuscript.
References
- 1.Wolff J. The Law of Bone Remodeling. Berlin Heidelberg New York: Springer; 1986. (translation of the German 1892 edition) [Google Scholar]
- 2.Banes AJ, Tsuzaki M, Yamamoto J, et al. Mechanoreception at the cellular level: The detection, interpretation and diversity of response to mechanical signals. Biochem Cell Biol. 1995;73:349–365. doi: 10.1139/o95-043. [DOI] [PubMed] [Google Scholar]
- 3.Lavagnino M, Arnoczky SP, Tian T, Vaupel Z. Effect of amplitude and frequency of cyclic tensile strain on the inhibition of MMP-1 mRNA expression in tendon cells: an in vitro study. Connect Tissue Res. 2003;44:181–187. doi: 10.1080/03008200390215881. [DOI] [PubMed] [Google Scholar]
- 4.Screen HR, Shelton JC, Bader DL, Lee DA. Cyclic tensile strain upregulates collagen synthesis in isolated tendon fascicles. Biochem Biophys Res Commun. 2005;336:424–429. doi: 10.1016/j.bbrc.2005.08.102. [DOI] [PubMed] [Google Scholar]
- 5.Hannafin JA, Arnoczky SP, Hoonjan A, Torzilli PA. Effect of stress deprivation and cyclic tensile loading on the material and morphologic properties of canine flexor digitorum profundus tendon: an in vitro study. J Orthop Res. 1995;13:907–914. doi: 10.1002/jor.1100130615. [DOI] [PubMed] [Google Scholar]
- 6.Galloway MT, Lalley AL, Shearn JT. The role of mechanical loading in tendon development, maintenance, injury, and repair. J Bone Joint Surg Am. 2013;95:1620–1628. doi: 10.2106/JBJS.L.01004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Archambault JM, Wiley JP, Bray RC. Exercise loading of tendons and the development of overuse injuries. A review of current literature. Sports Med. 1995;20:77–89. doi: 10.2165/00007256-199520020-00003. [DOI] [PubMed] [Google Scholar]
- 8.Arnoczky SP, Lavagnino M, Egerbacher M. The mechanobiological aetiopathogenesis of tendinopathy: is it the over-stimulation or the under-stimulation of tendon cells? Int J Exp Pathol. 2007;88:217–226. doi: 10.1111/j.1365-2613.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Magnusson SP, Langberg H, Kjaer M. The pathogenesis of tendinopathy: balancing the response to loading. Nat Rev Rheumatol. 2010;6:262–268. doi: 10.1038/nrrheum.2010.43. [DOI] [PubMed] [Google Scholar]
- 10.Little D, Thompson JW, Dubois LG, et al. The Proteomic Difference between Male and Female Ligament and Tendon. PLoS One. 2014;9:e96526. doi: 10.1371/journal.pone.0096526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kastelic J, Galeski A, Baer E. The multicomposite structure of tendon. Connect Tissue Res. 1978;6:11–23. doi: 10.3109/03008207809152283. [DOI] [PubMed] [Google Scholar]
- 12.Goulam Houssen Y, Gusachenko I, Schanne-Klein MC, Allain JM. Monitoring micrometer-scale collagen organization in rat-tail tendon upon mechanical strain using second harmonic microscopy. J Biomech. 2011;44:2047–2052. doi: 10.1016/j.jbiomech.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Hannafin JA, Arnoczky SP. Effect of cyclic and static tensile loading on water content and solute diffusion in canine flexor tendons: an in vitro study. J Orthop Res. 1994;12:350–356. doi: 10.1002/jor.1100120307. [DOI] [PubMed] [Google Scholar]
- 14.McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J Ana. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- 15.Thakkar D, Grant TM, Hakimi O, Carr AJ. Distribution and expression of type VI collagen and elastic fibers in human rotator cuff tendon tears. Connect Tissue Res. 2014;55:397–402. doi: 10.3109/03008207.2014.959119. [DOI] [PubMed] [Google Scholar]
- 16.Wilusz RE, Sanchez-Adams J, Guilak F. The structure and function of the pericellular matrix of articular cartilage. Matrix Biol. 2014;39:25–32. doi: 10.1016/j.matbio.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnoczky SP, Lavagnino M, Whallon JH, Hoonjan A. In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J Orthop Res. 2002;20:29–35. doi: 10.1016/S0736-0266(01)00080-8. [DOI] [PubMed] [Google Scholar]
- 18.Lavagnino M, Arnoczky SP, Kepich E, Caballero O, Haut RC. A finite element model predicts the mechanotransduction response of tendon cells to cyclic tensile loading. Biomech Model Mechanobiol. 2008;7:405–416. doi: 10.1007/s10237-007-0104-z. [DOI] [PubMed] [Google Scholar]
- 19.Ingber DE. Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol. 1997;59:575–99. doi: 10.1146/annurev.physiol.59.1.575. [DOI] [PubMed] [Google Scholar]
- 20.Cousineau-Pelletier P, Langelier E. Relative contributions of mechanical degradation, enzymatic degradation, and repair of the extracellular matrix on the response of tendons when subjected to under- and over- mechanical stimulations in vitro. J Orthop Res. 2010;28:204–210. doi: 10.1002/jor.20982. [DOI] [PubMed] [Google Scholar]
- 21.Heinemeier KM, Kjaer M. In vivo investigation of tendon responses to mechanical loading. J Musculoskelet Neuronal Interact. 2011;11:115–123. [PubMed] [Google Scholar]
- 22.Arruda EM, Calve S, Dennis RG, Mundy K, Baar K. Regional variation of tibialis anterior tendon mechanics is lost following denervation. J Appl Physiol (1985) 2006;101:1113–1117. doi: 10.1152/japplphysiol.00612.2005. [DOI] [PubMed] [Google Scholar]
- 23.Lavagnino M, Bedi A, Walsh CP, et al. Tendon Contraction After Cyclic Elongation Is an Age-Dependent Phenomenon: In Vitro and In Vivo Comparisons. Am J Sports Med. 2014;42:1471–1477. doi: 10.1177/0363546514526691. [DOI] [PubMed] [Google Scholar]
- 24.Tsuzaki M, Bynum D, Almekinders L, et al. ATP modulates load-inducible IL-1beta, COX 2, and MMP-3 gene expression in human tendon cells. J Cell Biochem. 2003;89:556–562. doi: 10.1002/jcb.10534. [DOI] [PubMed] [Google Scholar]
- 25.Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002;20:36–39. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 26.Docking SI, Daffy J, van Schie HT, Cook JL. Tendon structure changes after maximal exercise in the Thoroughbred horse: use of ultrasound tissue characterisation to detect in vivo tendon response. Vet J. 2012;194:338–342. doi: 10.1016/j.tvjl.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Lavagnino M, Arnoczky SP, Egerbacher M, Gardner KL, Burns ME. Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J Biomech. 2006;39:2355–2362. doi: 10.1016/j.jbiomech.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Andarawis-Puri N, Sereysky JB, Jepsen KJ, Flatow EL. The relationships between cyclic fatigue loading, changes in initial mechanical properties, and the in vivo temporal mechanical response of the rat patellar tendon. J Biomech. 2012;45:59–65. doi: 10.1016/j.jbiomech.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Egerbacher M, Arnoczky SP, Caballero O, Lavagnino M, Gardner KL. Loss of homeostatic tension induces apoptosis in tendon cells: an in vitro study. Clin Orthop Relat Res. 2008;466:1562–1568. doi: 10.1007/s11999-008-0274-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camargo PR, Alburquerque-Sendín F, Salvini TF. Eccentric training as a new approach for rotator cuff tendinopathy: Review and perspectives. World J Orthop. 2014;5:634–644. doi: 10.5312/wjo.v5.i5.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnoczky SP, Lavagnino M, Egerbacher M, et al. Loss of homeostatic strain alters mechanostat “set point” of tendon cells in vitro. Clin Orthop Relat Res. 2008;466:1583–1591. doi: 10.1007/s11999-008-0264-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Docking S, Samiric T, Scase E, Purdam C, Cook J. Relationship between compressive loading and ECM changes in tendons. Muscles Ligaments Tendons J. 2013;3:7–11. doi: 10.11138/mltj/2013.3.1.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Helmke B, Rosen A, Davies P. Mapping mechanical strain of an endogenous cytoskeletal network in living endothelial cells. Biophys J. 2003;84:2691–2699. doi: 10.1016/S0006-3495(03)75074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Low BC, Pan CQ, Shivashankar GV, et al. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Qi J, Fox AM, Alexopoulos LG, et al. IL-1beta decreases the elastic modulus of human tenocytes. J Appl Physiol. 2006;101:189–195. doi: 10.1152/japplphysiol.01128.2005. [DOI] [PubMed] [Google Scholar]
- 36.Qi J, Chi L, Maloney M, et al. Interleukin-1beta increases elasticity of human bioartificial tendons. Tissue Eng. 2006;12:2913–2925. doi: 10.1089/ten.2006.12.2913. [DOI] [PubMed] [Google Scholar]
- 37.Jones E, Legerlotz K, Riley G. Mechanical regulation of integrins in human tenocytes in collagen and fibrin matrices. Bone Joint J. 2014;96-B:161. [Google Scholar]
- 38.Na S, Collin O, Chowdhury F, et al. Rapid signal transduction in living cells is a unique feature of mechanotransduction. Proc Natl Acad Sci USA. 2008;105:6626–6631. doi: 10.1073/pnas.0711704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banes AJ, Horesovsky G, Larson C, et al. Mechanical load stimulates expression of novel genes in vivo and in vitro in avian flexor tendon cells. Osteoarthritis and Cartilage. 1999;7:141–153. doi: 10.1053/joca.1998.0169. [DOI] [PubMed] [Google Scholar]
- 40.Jones ER, Jones GC, Legerlotz K, Riley GP. Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFβ. Biochim Biophys Acta. 2013;1833:2596–2607. doi: 10.1016/j.bbamcr.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Donnelly E, Ascenzi MG, Farnum C. Primary cilia are highly oriented with respect to collagen direction and long axis of extensor tendon. J Orthop Res. 2010;28:77–82. doi: 10.1002/jor.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lavagnino M, Arnoczky SP, Gardner K. In situ deflection of tendon cell-cilia in response to tensile loading: an in vitro study. J Orthop Res. 2011;29:925–930. doi: 10.1002/jor.21337. [DOI] [PubMed] [Google Scholar]
- 43.Rowson D, Knight M, Screen H. Primary cilia in tenocytes from the inter-fascicular matrix and the fascicular matrix. Br J Sports Med. 2014;48:A58–A59. [Google Scholar]
- 44.Gardner K, Arnoczky SP, Lavagnino M. Effect of in vitro stress-deprivation and cyclic loading on the length of tendon cell cilia in situ. J Orthop Res. 2011;29:582–587. doi: 10.1002/jor.21271. [DOI] [PubMed] [Google Scholar]
- 45.Kuzma-Kuzniarska M, Yapp C, Pearson-Jones TW, Jones AK, Hulley PA. Functional assessment of gap junctions in monolayer and three-dimensional cultures of human tendon cells using fluorescence recovery after photobleaching. J Biomed Opt. 2014;19:15001. doi: 10.1117/1.JBO.19.1.015001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wall ME, Otey C, Qi J, Banes AJ. Connexin 43 is localized with actin in tenocytes. Cell Motil Cytoskeleton. 2007;64:121–130. doi: 10.1002/cm.20170. [DOI] [PubMed] [Google Scholar]
- 47.Banes AJ, Weinhold P, Yang X, et al. Gap junctions regulate responses of tendon cells ex vivo to mechanical loading. Clinical Orthopaedics. 1999;1:S356–S370. doi: 10.1097/00003086-199910001-00034. [DOI] [PubMed] [Google Scholar]
- 48.Wall ME, Banes AJ. Early responses to mechanical load in tendon: role for calcium signaling, gap junctions and intercellular communication. J Musculoskelet Neuronal Interact. 2005;5:70–84. [PubMed] [Google Scholar]
- 49.Maedan E, Sugimoto M, Ohashi T. Cytoskeletal tension modulates MMP-1 gene expression from tenocytes on micropillar substrates. JBiomech. 2013;46:991–997. doi: 10.1016/j.jbiomech.2012.11.056. [DOI] [PubMed] [Google Scholar]
- 50.Banes AJ, Lee G, Graff R, et al. Mechanical forces and signaling in connective tissue cells: cellular mechanisms of detection, transduction, and responses to mechanical deformation. Current Opinion in Orthopaedics. 2001;12:389–396. [Google Scholar]
- 51.Magra M, Hughes S, El Haj AJ, Maffulli N. VOCCs and TREK-1 ion channel expression in human tenocytes. Am J Physiol Cell Physiol. 2007;292:C1053–C1060. doi: 10.1152/ajpcell.00053.2006. [DOI] [PubMed] [Google Scholar]
- 52.Archambault J, Elfervig-Wall M, Tsuzaki M, Herzog W, Banes AJ. Rabbit tendon cells produce MMP-3 in response to fluid flow without significant calcium transients. J Biomech. 2002;35:303–9. doi: 10.1016/s0021-9290(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 53.Archambault J, Tsuzaki M, Herzog W, Banes AJ. Stretch and interlukin-1 beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res. 2002b;20:36–39. doi: 10.1016/S0736-0266(01)00075-4. [DOI] [PubMed] [Google Scholar]
- 54.Tsuzaki M, Bynum D, Almekinders L, Faber J, Banes AJ. Mechanical loading stimulates ecto-ATPase activity in human tendon cells. J Cell Biochem. 2005;96:117–125. doi: 10.1002/jcb.20491. [DOI] [PubMed] [Google Scholar]
- 55.Qi J, Chi L, Faber J, Koller B, Banes AJ. ATP reduces gel compaction in osteoblast-populated collagen gels. J Appl Physiol. 2007;102:1152–1160. doi: 10.1152/japplphysiol.00535.2006. [DOI] [PubMed] [Google Scholar]
- 56.Riley G. The pathogenesis of tendinopathy. A molecular perspective. Rheumatology. 2003;43:131–142. doi: 10.1093/rheumatology/keg448. [DOI] [PubMed] [Google Scholar]
- 57.Ireland D, Harrall R, Curry V, et al. Multiple changes in gene expression in chronic human Achilles tendinopathy. Matrix Biology. 2001;20:159–169. doi: 10.1016/s0945-053x(01)00128-7. [DOI] [PubMed] [Google Scholar]
- 58.Tsuzaki M, Guyton G, Garrett W, et al. IL-1β induces COX2 and MMP-1,-3 and 13, ADAMTS-4, IL-1β and IL-6 in human tendon cells. J Orthop Res. 2003;21:256–264. doi: 10.1016/S0736-0266(02)00141-9. [DOI] [PubMed] [Google Scholar]
- 59.Scott A, Khan K, Cook J, Duronio V. What is “inflammation”? Are we ready to move beyond Celsus? Br J Sports Med. 2004;8:15, 25. doi: 10.1136/bjsm.2003.011221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gelberman RH, Vande Berg JS, Lundborg GN, Akeson WH. Flexor tendon healing and restoration of the gliding surface. An ultrastructural study in dogs. J Bone Joint Surgery Am. 1983;65:70–80. [PubMed] [Google Scholar]
- 61.Klatte-Schulz F, Pauly S, Scheibel M, et al. Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur Cell Mater. 2012;24:74–89. doi: 10.22203/ecm.v024a06. [DOI] [PubMed] [Google Scholar]
- 62.Liu H, Zhu S, Zhang C, et al. Crucial transcription factors in tendon development and differentiation: their potential for tendon regeneration. Cell Tissue Res. 2014;356:287–298. doi: 10.1007/s00441-014-1834-8. [DOI] [PubMed] [Google Scholar]
- 63.Liu W, Watson SS, Lan Y, et al. The atypical homeodomain transcription factor Mohawk controls tendon morphogenesis. Mol Cell Biol. 2010;30:4797–4807. doi: 10.1128/MCB.00207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schweitzer R, Chyung JH, Murtaugh LC, et al. Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development. 2001;128:3855–3866. doi: 10.1242/dev.128.19.3855. [DOI] [PubMed] [Google Scholar]
- 65.Qi J, Dmochowski JM, Banes AN, et al. Differential expression and cellular localization of novel isoforms of the tendon biomarker tenomodulin. J Appl Physiol (1985) 2012;113:861–871. doi: 10.1152/japplphysiol.00198.2012. [DOI] [PubMed] [Google Scholar]
- 66.Qi J, Chi L, Labeit S, Banes AJ. Nuclear localization of the titin Z1Z2Zr domain and role in regulating cell proliferation. Am J Physiol Cell Physiol. 2008;295:C975–985. doi: 10.1152/ajpcell.90619.2007. [DOI] [PubMed] [Google Scholar]
- 67.Roriz P, Carvalho L, Frazão O, Santos JL, Simões JA. From conventional sensors to fibre optic sensors for strain and force measurements in biomechanics applications: a review. J Biomech. 2014;47:1251–1261. doi: 10.1016/j.jbiomech.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 68.Lichtwark GA, Cresswell AG, Newsham-West RJ. Effects of running on human Achilles tendon length-tension properties in the free and gastrocnemius components. J Exp Biol. 2013;216:4388–4394. doi: 10.1242/jeb.094219. [DOI] [PubMed] [Google Scholar]
- 69.Pearson SJ, Hussain SR. Region-specific tendon properties and patellar tendinopathy: a wider understanding. Sports Med. 2014;44:1101–1112. doi: 10.1007/s40279-014-0201-y. [DOI] [PubMed] [Google Scholar]
- 70.Ellison ME, Duenwald-Kuehl S, Forrest LJ, Vanderby R, Jr, Brounts SH. Reproducibility and feasibility of acoustoelastography in the superficial digital flexor tendons of clinically normal horses. Am J Vet Res. 2014;75:581–587. doi: 10.2460/ajvr.75.6.581. [DOI] [PubMed] [Google Scholar]
- 71.Bohm S, Mersmann F, Tettke M, Kraft M, Arampatzis A. Human Achilles tendon plasticity in response to cyclic strain: effect of rate and duration. J Exp Biol. 2014;217:4010–4017. doi: 10.1242/jeb.112268. [DOI] [PubMed] [Google Scholar]
- 72.Chang EY, Szeverenyi NM, Statum S, Chung CB. Rotator cuff tendon ultrastructure assessment with reduced-orientation dipolar anisotropy fiber imaging. Am J Roentgenol. 2014;202:W376–W378. doi: 10.2214/AJR.13.11302. [DOI] [PubMed] [Google Scholar]
- 73.Aubry S, Nueffer JP, Tanter M, et al. Viscoelasticity in Achilles Tendonopathy: Quantitative Assessment by Using Real-time Shear-Wave Elastography. Radiology. 2014:140434. doi: 10.1148/radiol.14140434. [DOI] [PubMed] [Google Scholar]
- 74.Noseworthy MD, Davis AD, Elzibak AH. Advanced MR imaging techniques for skeletal muscle evaluation. Semin Musculoskelet Radiol. 2010;14:257–268. doi: 10.1055/s-0030-1253166. [DOI] [PubMed] [Google Scholar]
- 75.Chin L, Kennedy BF, Kennedy KM, et al. Three-dimensional optical coherence micro-elastography of skeletal muscle tissue. Biomed Opt Express. 2014;5:3090–3102. doi: 10.1364/BOE.5.003090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guan G, Li C, Ling Y, et al. Quantitative evaluation of degenerated tendon model using combined optical coherence elastography and acoustic radiation force method. J Biomed Opt. 2013;18:111417. doi: 10.1117/1.JBO.18.11.111417. [DOI] [PubMed] [Google Scholar]
- 77.Brown CP, Houle MA, Popov K, et al. Imaging and modeling collagen architecture from the nano to micro scale. Biomed Opt Express. 2013;5:233–243. doi: 10.1364/BOE.5.000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Grashoff C, Hoffman BD, Brenner MD, et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature. 2010;466:263–266. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith ML, Gourdon D, Little WC, et al. Force-induced unfolding of fibronectin in the extracellular matrix of living cells. PLoS Biol. 2007;5:e268. doi: 10.1371/journal.pbio.0050268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tyler WJ. The mechanobiology of brain function. Nat Rev Neurosci. 2012;13:867–878. doi: 10.1038/nrn3383. [DOI] [PubMed] [Google Scholar]
- 81.Marturano JE, Arena JD, Schiller ZA, Georgakoudi I, Kuo CK. Characterization of mechanical and biochemical properties of developing embryonic tendon. Proc Natl Acad Sci USA. 2013;110:6370–6375. doi: 10.1073/pnas.1300135110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen B, Huang B. Imaging Genomic Elements in Living Cells Using CRISPR/Cas9. Methods Enzymol. 2014;546:337–354. doi: 10.1016/B978-0-12-801185-0.00016-7. [DOI] [PubMed] [Google Scholar]
- 83.Thompson MS. Tendon mechanobiology: experimental models require mathematical underpinning. Bull Math Biol. 2013;75:1238–1254. doi: 10.1007/s11538-013-9850-5. [DOI] [PubMed] [Google Scholar]
- 84.Marino M, Vairo G. Stress and strain localization in stretched collagenous tissues via a multiscale modelling approach. Comput Methods Biomech Biomed Engin. 2014;17:11–30. doi: 10.1080/10255842.2012.658043. [DOI] [PubMed] [Google Scholar]
- 85.Swedberg AM, Reese SP, Maas SA, Ellis BJ, Weiss JA. Continuum description of the Poisson’s ratio of ligament and tendon under finite deformation. J Biomech. 2014;47:3201–3209. doi: 10.1016/j.jbiomech.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang JH, Guo Q, Li B. Tendon biomechanics and mechanobiology--a minireview of basic concepts and recent advancements. J Hand Ther. 2012;25:133–141. doi: 10.1016/j.jht.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Guilak F, Hung CT. Physical regulation of cartilage metabolism. In: Mow VC, Huiskes R, editors. Basic Orthopaedic Biomechanics and Mechano-Biology. 3. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 179–207. [Google Scholar]
- 88.McNulty AL, Guilak F. Mechanobiology of the meniscus. J Biomech. 2014 doi: 10.1016/j.jbiomech.2015.02.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Setton LA, Chen J. Mechanobiology of the intervertebral disc and relevance to disc degeneration. J Bone Joint Surg Am. 2006;88(Suppl 2):52–57. doi: 10.2106/JBJS.F.00001. [DOI] [PubMed] [Google Scholar]
- 90.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 91.Peier AM, Moqrich A, Hergarden AC, et al. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108:705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 92.Guilak F, Leddy HA, Liedtke W. Transient receptor potential vanilloid 4: The sixth sense of the musculoskeletal system? Ann N Y Acad Sci. 2010;1192:404–409. doi: 10.1111/j.1749-6632.2010.05389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liedtke W, Tobin DM, Bargmann CI, et al. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2003;100(Suppl 2):14531–14536. doi: 10.1073/pnas.2235619100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.O’Conor CJ, Leddy HA, Benefield HC, et al. TRPV4-mediated mechanotransduction regulates the metabolic response of chondrocytes to dynamic loading. Proc Natl Acad Sci USA. 2014;111:1316–1321. doi: 10.1073/pnas.1319569111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Phan MN, Leddy HA, Votta BJ, et al. Functional characterization of TRPV4 as an osmotically sensitive ion channel in porcine articular chondrocytes. Arthritis Rheum. 2009;60:3028–3037. doi: 10.1002/art.24799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clark AL, Votta BJ, Kumar S, et al. Chondroprotective role of the osmotically sensitive ion channel transient receptor potential vanilloid 4: age- and sex-dependent progression of osteoarthritis in Trpv4-deficient mice. Arthritis Rheum. 2010;62:2973–2983. doi: 10.1002/art.27624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y, Williams SH, McNulty AL, et al. Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain. 2013;154:1295–1304. doi: 10.1016/j.pain.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ong EC, Nesin V, Long CL, et al. A TRPC1 protein-dependent pathway regulates osteoclast formation and function. J Biol Chem. 2013;288:22219–22232. doi: 10.1074/jbc.M113.459826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen F, Ni B, Yang YO, et al. Knockout of TRPV6 causes osteopenia in mice by increasing osteoclastic differentiation and activity. Cell Physiol Biochem. 2014;33:796–809. doi: 10.1159/000358653. [DOI] [PubMed] [Google Scholar]
- 100.Masuyama R, Vriens J, Voets T, et al. TRPV4-mediated calcium influx regulates terminal differentiation of osteoclasts. Cell Metab. 2008;8:257–265. doi: 10.1016/j.cmet.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 101.Lee W, Leddy HA, Chen Y, et al. Synergy between Piezo1 and Piezo2 channels confers high-strain mechanosensitivity to articular cartilage. Proc Natl Acad Sci USA. 2014;111:E5114–E5122. doi: 10.1073/pnas.1414298111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dupont S, Morsut L, Aragona M, et al. Role of YAP/TAZ in mechanotransduction. Nature. 2011;474:179–183. doi: 10.1038/nature10137. [DOI] [PubMed] [Google Scholar]
- 103.Morgan JT, Murphy CJ, Russell P. What do mechanotransduction, Hippo, Wnt, and TGFβ have in common? YAP and TAZ as key orchestrating molecules in ocular health and disease. Exp Eye Res. 2013;115:1–12. doi: 10.1016/j.exer.2013.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 105.Low BC, Pan CQ, Shivashankar GV, et al. YAP/TAZ as mechanosensors and mechanotransducers in regulating organ size and tumor growth. FEBS Lett. 2014;588:2663–2670. doi: 10.1016/j.febslet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 106.Schiele NR, Marturano JE, Kuo CK. Mechanical factors in embryonic tendon development: potential cues for stem cell tenogenesis. Curr Opin Biotechnol. 2013;24:834–840. doi: 10.1016/j.copbio.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gardner K, Lavagnino M, Egerbacher M, Arnoczky SP. Re-establishment of cytoskeletal tensional homeostasis in lax tendons occurs through an actin-mediated cellular contraction of the extracellular matrix. J Orthop Res. 2012;30:1695–1701. doi: 10.1002/jor.22131. [DOI] [PubMed] [Google Scholar]
- 108.Bedi A, Fox AJ, Kovacevic D, et al. Doxycycline-mediated inhibition of matrix metalloproteinases improves healing after rotator cuff repair. Am J Sports Med. 2010;38:308–317. doi: 10.1177/0363546509347366. [DOI] [PubMed] [Google Scholar]
- 109.Jones GC, Corps AN, Pennington CJ, et al. Expression profiling of metalloproteinases and tissue inhibitors of metalloproteinases in normal and degenerate human achilles tendon. Arthritis Rheum. 2006;54:832–842. doi: 10.1002/art.21672. [DOI] [PubMed] [Google Scholar]
- 110.Garvin J, Qi J, Maloney M, Banes AJ. Novel system for engineering bioartificial tendons and application of mechanical load. Tissue Eng. 2003;9:967–979. doi: 10.1089/107632703322495619. [DOI] [PubMed] [Google Scholar]
- 111.Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendinopathy. PLoS One. 2013;8:e75697. doi: 10.1371/journal.pone.0075697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang J, Li B, Wang JH. The role of engineered tendon matrix in the stemness of tendon stem cells in vitro and the promotion of tendon-like tissue formation in vivo. Biomaterials. 2011;32:6972–6981. doi: 10.1016/j.biomaterials.2011.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Huang C, Holfeld J, Schaden W, Orgill D, Ogawa R. Mechanotherapy: revisiting physical therapy and recruiting mechanobiology for a new era in medicine. Trends Mol Med. 2013;19:555–64. doi: 10.1016/j.molmed.2013.05.005. [DOI] [PubMed] [Google Scholar]