Abstract
Study Objectives:
The ventrolateral preoptic area (VLPO) and the orexin/hypocretin neuronal system are key regulators of sleep onset, transitions between vigilance states, and energy homeostasis. Reciprocal projections exist between the VLPO and orexin/hypocretin neurons. Although the importance of the VLPO to sleep regulation is clear, it is unknown whether VLPO neurons are involved in energy balance. The purpose of these studies was to determine if the VLPO is a site of action for orexin-A, and which orexin receptor subtype(s) would mediate these effects of orexin-A. We hypothesized that orexin-A in the VLPO modulates behaviors (sleep and wakefulness, feeding, spontaneous physical activity [SPA]) to increase energy expenditure.
Design and Measurements:
Sleep, wakefulness, SPA, feeding, and energy expenditure were determined after orexin-A microinjection in the VLPO of male Sprague-Dawley rats with unilateral cannulae targeting the VLPO. We also tested whether pretreatment with a dual orexin receptor antagonist (DORA, TCS-1102) or an OX2R antagonist (JNJ-10397049) blocked the effects of orexin-A on the sleep/wake cycle or SPA, respectively.
Results:
Orexin-A injected into the VLPO significantly increased wakefulness, SPA, and energy expenditure (SPA-induced and total) and reduced NREM sleep and REM sleep with no effect on food intake. Pretreatment with DORA blocked the increase in wakefulness and the reduction in NREM sleep elicited by orexin-A, and the OX2R antagonist reduced SPA stimulated by orexin-A.
Conclusions:
These data show the ventrolateral preoptic area is a site of action for orexin-A, which may promote negative energy balance by modulating sleep/wakefulness and stimulating spontaneous physical activity and energy expenditure.
Citation:
Mavanji V, Perez-Leighton CE, Kotz CM, Billington CJ, Parthasarathy S, Sinton CM, Teske JA. Promotion of wakefulness and energy expenditure by orexin-A in the ventrolateral preoptic area. SLEEP 2015;38(9):1361–1370.
Keywords: arousal, brain, obesity, sleep
INTRODUCTION
Inadequate sleep increases the risk for obesity, type 2 diabetes, and all-cause-mortality.1–5 Sleep loss may increase obesity risk by modifying behaviors such as eating and spontaneous physical activity (SPA), the latter being defined as all physical activity excluding formal exercise.6–8 Although the neurobiology of sleep and its relationship to health is well studied, less is known about the relationship between sleep and other behaviors, such as SPA and eating, both of which affect health status. Such information could lead to novel and effective treatment strategies to improve health and body weight management.
Orexin-A (i.e., hypocretin-1) is an endogenous neuropep-tide important to multiple physiological processes9–14 and may underlie integration of several behaviors.15,16 Orexin neurons are silent during NREM sleep, exhibit phasic bursts of activity during REM sleep, and discharge at their highest rate during wakefulness.17 This activity pattern correlates with orexin-A immunoreactivity, prepro-orexin,18 postural muscle tone,17 exploratory activity, and putatively, with motor programs that are subject to muscle atonia during REM sleep.19 The biological effects of orexin-A are mediated by two G-protein coupled receptors (OX1R and OX2R) for which orexin-A has similar affinities.9,10 As might be expected from the discharge pattern of orexin neurons, orexin-A administration in the brain enhances wakefulness,20 SPA,20–22 energy expenditure,23 and, depending upon brain site, acutely increases feeding.10,22 Given chronically, orexin-A reduces body weight,24,25 highlighting that orexin-A predominantly promotes energy expenditure and a net negative energy balance.14 Taken together, these data suggest that orexin-A coordinates and modulates sleep, eating behavior, and SPA to increase net energy expenditure.
The ventrolateral preoptic area (VLPO) is a brain region essential to the initiation, maintenance, and consolidation of sleep.26–31 The VLPO contains sleep-promoting GABAergic and galaninergic neurons,32 and in rodents these neurons exhibit high c-fos immunoreactivity during sleep.26,33 The discharge rate of VLPO neurons increases just before or at the transition from wakefulness to NREM sleep and decreases prior to the transition from NREM or REM sleep to wakefulness.28 In contrast, VLPO lesions reduce sleep time and sleep stability, demonstrated by increased transitions between sleep and wakefulness.27 The VLPO is anatomically well positioned to gate orexin-dependent behavioral output such as feeding and SPA. The VLPO contains both subtypes of orexin receptors,34,35 and VLPO neurons have reciprocal connections with orexinergic nuclei.32,36–43 Neuroanatomical and functional44 data indicate that the VLPO promotes sleep, at least partially, by inhibiting orexin neurons.29 Whether the VLPO modulates other behavioral states such as SPA and feeding, and/or if orexin-A in the VLPO augments energy expenditure is unknown.
As orexin-A affects the transitions between vigilance states, SPA and feeding, we investigated here whether orexin-A, administered directly in the VLPO, augments active behaviors (i.e., wakefulness, SPA, feeding) and energy expenditure. We also examined, using subtype-specific antagonists, the orexin receptor subtype mediating these effects. Our hypothesis was that orexin-A in the VLPO would reduce NREM sleep and REM sleep; increase wakefulness, SPA, feeding and energy expenditure; and that pretreatment with the OX2R antagonist (JNJ-10397049) or a dual orexin receptor antagonist (DORA, TCS-1102) would abrogate orexin-A-stimulated behaviors. Our results here validate the VLPO as an important site of action for orexin and suggest that orexin-A acts in the VLPO to coordinate and integrate active behaviors to promote negative energy balance.
METHODS
Animals
Three-month old male Sprague-Dawley rats (Charles River, Kingston, NY) (n = 55) were housed individually either in solid bottom cages with corncob bedding or a perforated floor, or in wire-bottom cages with resting platforms and a chewing substrate (Nylabone, natural flavor, BioServ, Frenchtown, NJ). Throughout the study, a 12-h light/12-h dark cycle (lights on at 06:00) in a temperature-controlled environment (21–22°C) was followed. Rodent chow (Harlan Teklad 8604) and water were allowed ad libitum. Studies were approved by the Institutional Animal Care and Use Committee at the Minneapolis VA Health Care System, the University of Minnesota, and the University of Arizona. Six groups (n = 51) of rats were used for the studies. Separate groups of rats were used for studies 1 and 2; another group of rats was used for studies 3, 4, and 6; and 2 final groups of rats were used for study 5.
Surgery
Rats were anesthetized with a ketamine/xylazine mixture (50 mg/kg; 15 mg/kg), and implanted with a 26-gauge stainless steel cannula (Plastics One, Roanoke, VA) directed towards the VLPO. Rats were also implanted35 with a radiotelemetric transmitter and EEG/EMG electrodes to record vigilance states (F40-EET, Data Sciences International [DSI], St. Paul, MN). Stereotaxic coordinates for the VLPO were determined from Paxinos and Watson45 and are as follows: −0.12 mm posterior, 0.8 mm lateral to bregma, and 8.0 mm below the skull surface. Landmarks for positioning the EEG leads on the cranium were as follows: 3.1 mm posterior and 1.5 mm lateral to bregma. The EMG leads were secured in the nuchal musculature. For all cannulations, the incisor bar was set at 3.3 mm below the ear bars. Animals were allowed to recover from surgery for at least 7–10 days before experimental trials began.
Drugs
Orexin-A (15.6–125 pmol/0.5 μl, American Peptides, Sunnyvale, CA) was dissolved in artificial cerebrospinal fluid (Sigma-Aldrich, St. Louis, MO), which was used as the vehicle (control) for the injection studies with orexin-A. The selective OX2R antagonist and DORA (62.5–250 nmol/0.5 μl, JNJ-10397049 and 62.5–250 pmol/0.5 μL, TCS-1102, respectively, Tocris Bioscience, St. Paul, MN) were dissolved in DMSO/methanol HCl/sterile water. All drugs were stored at 4°C for < 48 h.
Injections
A volume of 0.5 μL was injected gradually over 30 s with a 33-gauge injector (Plastics One, Roanoke, VA) that extended 1.0 mm beyond the tip of the guide cannula.21 Injections were performed between 08:00 and 10:00 (zeitgeiber time 2–4) with ≥ 48 h between treatments. Repeated injections did not cause tissue damage as measured by the lack of gliosis around the injection site under 100x microscopy after 50 injections.46,47 Additionally, we have shown that the behavioral responses to orexin-A do not decrease with repeated injections,48 indicating repeated injections do not affect tissue or cellular integrity.
Verification of Cannula Placement
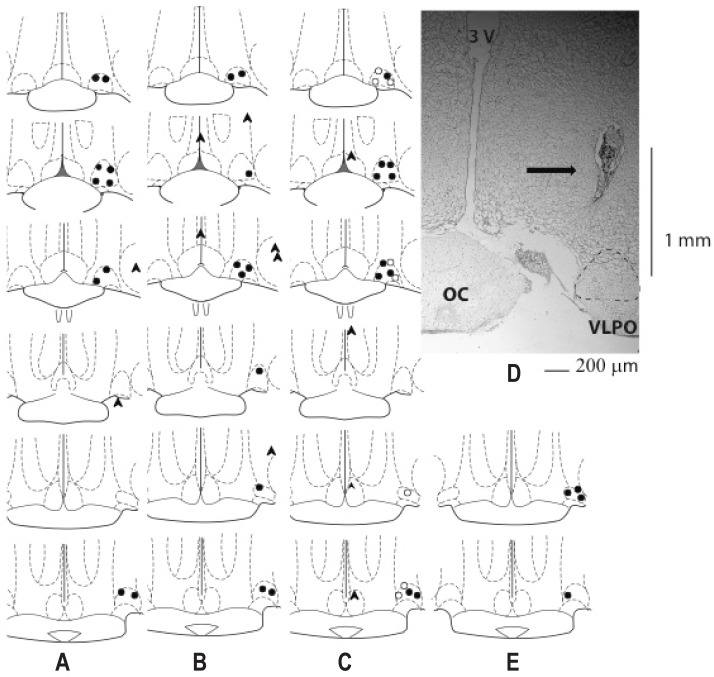
Cannula placement was verified by histology.21 Briefly, brains were dissected from the skull and stored in a 10% formaldehyde solution. A cannula was deemed incorrect if the actual injection site was further than 0.25 mm medial and/or lateral from the targeted site. This rationale is based on diffusion coefficients of the injection volume.49 Rats with incorrectly placed cannulae were excluded from data analysis and are not reported here. Figure 1 displays a map of actual injection sites and a photomicrograph to show rats with correctly placed cannulae.
Figure 1.
Histological verification (map and representative photomicrograph (D)) showing correct (circles) and misplaced (arrow heads) placement of injection sites into the ventrolateral preoptic area (VLPO) for studies 1 (n = 10) (A), 2 (n = 4) (E), 3 and 4 (n = 10) (B) and 5 (n = 22) (C). Open and closed circles indicate separate groups of rats used for study 5. Black arrow points to the cannula, dotted structure is the VLPO, 3v, 3rd ventricle; OC, optic chiasm; the side bar (1 mm) indicates the distance from the cannula position to the VLPO.
EEG/EMG Recording and Determination of Behavioral States
To allow freely moving polysomnogram recordings, a receiver (PhysioTel RPC-1, DSI, St. Paul, MN) was placed beneath the testing cage to detect EEG/EMG signals from the implanted transmitter.35 Briefly, signals were digitized by a Data Exchange Matrix connected to a PC with Dataquest A.R.T 4.1 software (DSI, St. Paul, MN). Electroencephalogram signals (0.3–30.0 Hz bandpass) and EMG signals (1.0–100.0 Hz bandpass) were stored on a computer, visualized with Neuroscore software (version 2.0.1, DSI, St. Paul, MN), and sleep and wakefulness states were scored manually in accordance with previously described methods.35 Briefly, consecutive 15-s epochs of EEG and EMG signals were classified into one of the following 4 behavioral states: NREM sleep, REM sleep, active wakefulness, or quiet wakefulness,50 and percent time spent in each state was then calculated from the scored data. The following dependent variables were quantified for each recording session: (a) percent time spent in active wakefulness, quiet wakefulness, NREM sleep, REM sleep; (b) total number of episodes for each vigilance state; (c) mean duration of episodes for each vigilance state.
Spontaneous Physical Activity (SPA) Measurement
In studies 3 and 4, SPA was measured by infrared activity sensors placed around an acrylic cage (425 × 265 × 305 mm, TSE Systems, Chesterfield, MO). Briefly, ambulation was detected by 2 infrared arrays along the x- and y-axes, and vertical movement was detected by a third elevated x array. Movement was therefore simultaneously detected in all dimensions. Components of SPA (distance traveled and vertical activity [e.g., rearing]) were determined from the infrared beam-break data. Rats were acclimated to the SPA chambers on 3 consecutive days for 3–5 h/day prior to the start of the studies. Food and water were available ad libitum during acclimation, and water was available ad libitum during testing.
Concurrent Indirect Calorimetry and SPA
In study 6, energy expenditure and SPA was determined with a pull-mode open-circuit continuous indirect calorimeter that measured simultaneous and continuous O2 consumption, CO2 production and water vapor every second from each chamber (Promethion-C, Sable Systems Inc. Las Vegas, NV). Oxygen, carbon dioxide, and water vapor sensors were calibrated prior to each test with primary gas standards. Chamber airflow was maintained at 2,500 mL/min. Spontaneous physical activity was measured concurrently with energy expenditure each second from each chamber by infrared beam beak sensors in the x-y and z planes (Sable Systems Inc. Las Vegas, NV). Rats were acclimated to the chambers on 3 consecutive days for 3 h each day. Food and water were available ad libitum during acclimation, and water was available ad libitum during testing. Reference measurements from room air were determined at 15-min intervals over the testing period. The respiratory quotient was defined as the mean of the respiratory exchange ratio values taken over the measurement period. Energy expenditure was calculated with Expedata software version 1.7.30 (Sable Systems Inc. Las Vegas, NV).
Specific Experimental Designs
Study 1. Effect of Orexin-A in the VLPO on Sleep and Wakefulness
Orexin-A (15.6, 31.2, 62.5, and 125.0 pmol/0.5 μL) or vehicle was injected into the VLPO in a repeated-measures Latin-square counter-balanced design (n = 11). Thus rats were randomly assigned to a treatment group, each animal received each treatment once, and all treatments were represented on each day. One rat was excluded due to an incorrectly placed cannula so n = 10 for this study. Doses were chosen based on our prior studies51 and were comparable to studies that tested the effect of orexin-A on sleep and wakefulness when administered in other brain regions. Continuous EEG/EMG recordings were obtained for 3.5 h post-injection. The following endpoints were analyzed: percent time spent in active wakefulness, quiet wakefulness, NREM, and REM sleep; the total number of episodes for each vigilance state; and the mean duration of these episodes.
Study 2. Effect of the Dual Orexin Receptor Antagonist and Orexin-A in the VLPO on Sleep and Wakefulness
The DORA (62.5 125.0, 250.0 nmol/0.5 μL) or vehicle was injected into the VLPO 20 min prior to orexin-A (62.5 pmol/0.5 μL) or vehicle in a randomly assigned Latin-square counter-balanced design (n = 4). All rats had correctly placed cannulae. This dose of orexin-A was based on the lowest effective dose of orexin-A that increased wakefulness time in study 1 and doses of DORA were based on a previous report.52 The following endpoints were analyzed: percent time spent in active wakefulness, quiet wakefulness, NREM and REM sleep; the total number of episodes for each vigilance state; and the mean duration of these episodes
Study 3. Effect of Orexin-A in the VLPO on SPA
Orexin-A (15.6, 31.2, 62.5, and 125.0 pmol/0.5 μL) or vehicle was injected into the VLPO in a repeated-measures Latin-square counter-balanced design (n = 14). Four rats were excluded due to incorrectly placed cannulae, leaving n = 10 for this study. Doses of orexin-A were based on results from study 1 and our past experience.51 SPA was measured continuously for 4.5 h post-injection.
Study 4. Effect of the OX2R Antagonist (JNJ-10397049) and Orexin-A in the VLPO on SPA
The OX2R antagonist (125, 250, 500 pmol/0.5 μL) or vehicle was injected into the VLPO 20 min prior to orexin-A (62.5 pmol/0.5 μL) or vehicle in a randomly assigned Latin-square counter-balanced design in rats from study 3 (n = 10). An additional rat was excluded after being withdrawn from the study due to excessive grooming behavior, which can indicate abnormal physiology.53 The dose of orexin-A was based on results from study 1, and doses of the OX2R antagonist were based on a previous report.54 Spontaneous physical activity was measured continuously for 4.5 h post-injection.
Study 5. Effect of Orexin-A in the VLPO on Feeding
Orexin-A (15.6, 31.2, 62.5, and 125.0 pmol/0.5 μL) or vehicle was injected into the VLPO in a repeated-measures Latin-square counter-balanced design in 2 groups of rats (n = 22, with n = 12 for the first group and n = 10 for the second group). Four rats were excluded due to incorrectly placed cannulae, so n = 18 for this study (n = 10 for the first group and n = 8 for the second group). Food intake and food spillage (uneaten food crumbs that fell beneath the wire-bottom cage) were measured at 1, 2, 4, and 24 h post-injection. Doses of orexin-A were based on the results from study 1 and our past experience.51
Study 6. Effect of Orexin-A in the VLPO on Energy Expenditure and SPA
Orexin-A (125 pmol/0.5 μL) or vehicle was injected into the VLPO in a repeated-measures Latin-square counter-balanced design (n = 4). All rats had correctly placed cannulae. Energy expenditure and SPA were measured continuously each second for 2.5 h (Promethion-C Sable Systems International, Las Vegas, Nevada).
Statistical Analyses
Data were analyzed by repeated-measures ANOVA (GraphPad Prism version 6.0d for Macintosh, GraphPad Software, La Jolla, CA) followed by Fisher multiple comparisons tests to determine differences between individual treatments. For study 6, paired t-tests were used. Separate analyses were completed for each time point and endpoint. Since handling involved in the injection procedure augments wakefulness and SPA for up to 20 min post-injection, independent of treatment, the first 20 min of data post-injection were excluded in the data analysis.21 Therefore, data were analyzed in the 20–80, 80–140, and 20–140 min post-injection time periods, which will be referred to as the 0–1, 1–2, and 0–2 h time periods for all studies except the feeding study (study 5). For study 5, data were analyzed for the 0–1, 0–2, 0–4, and 0–24 h post-injection time periods, which corresponds to the actual time after the injection. An α level of 0.05 was used for all statistical tests. Data are expressed as mean ± SEM.
RESULTS
Study 1. Orexin-A in the VLPO increases wakefulness, reduces sleep time and sleep fragmentation.
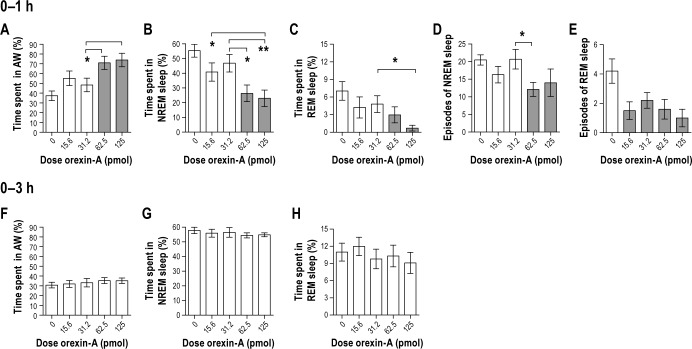
We hypothesized that orexin-A microinjection into the VLPO would reduce sleep and increase wakefulness time. There was an acute stimulating and dose-dependent effect of orexin-A on vigilance states (Figure 2). There was a main effect of treatment on active wakefulness (Figure 2A; F4,36 = 5.5, P = 0.001), NREM sleep (Figure 2B; F4,36 = 6.3, P = 0.001) and REM sleep (Figure 2C; F4,36 = 3.2, P = 0.024) during the 0–1 h post-injection time period. The 2 highest doses of orexin-A significantly increased active wakefulness and reduced NREM and REM sleep relative to the vehicle injection (Figure 2A–2C, P < 0.05 all comparisons). During the 1–2, 0–2, 2–3, and 0–3 h post-injection time periods, there were no significant effects of orexin-A on active wakefulness, NREM or REM sleep (P > 0.05) with one exception: there was a main effect of treatment on NREM sleep during the 1–2 h time interval (F4,36 = 3.0, P = 0.03, data not shown), but post hoc analysis showed no significant difference between means. There was no significant effect of orexin-A on quiet wakefulness at any time point (data not shown).
Figure 2.
Study 1. Orexin-A administered in the ventrolateral preoptic area stimulates wakefulness by modifying the amount of time spent in vigilance states and the number of episodes of NREM sleep and REM sleep. Orexin-A increases (A) time spent in active wakefulness and reduces (B) time spent in NREM sleep and (C) REM sleep 1-h post-injection with no effect in the 3-h time period (F,G,H). Orexin-A reduces the number of episodes of NREM and REM sleep 1-h post-injection (D and E, respectively). n = 10. Data represented as mean ± SEM. Shaded bars are significantly different from the control injection of artificial cerebrospinal fluid (P < 0.05). Brackets above bars indicate doses of orexin-A that were significantly different from each other (*P < 0.05 and **P < 0.005). Note different scaling on y-axes.
To test whether orexin-A influenced sleep quality, the number and the mean duration of episodes of each vigilance state were determined. The orexin-A-induced reduction in time spent in NREM and REM sleep was due to fewer episodes of NREM and REM sleep (Figures 2C and 2D). There was a main effect of orexin-A on the number of episodes of NREM sleep (F4,36 = 2.6, P = 0.049) and REM sleep (F4,36 = 3.2, P = 0.020) during the 0–1 h time period. The 62.5 pmol dose of orexin-A reduced the number of NREM sleep episodes (P = 0.017) and all doses of orexin-A reduced the number of REM sleep episodes compared to vehicle (P < 0.05 for all comparisons). There was no significant effect of orexin-A on the number of episodes of either active or quiet wakefulness during the 0–1 h time period (data not shown), and there was no effect on the mean episode duration for active wakefulness, quiet wakefulness, NREM sleep or REM sleep during the 0–1, 1–2, 0–2, 2–3, or 0–3 h post-injection time periods (P > 0.05, data not shown). Together, these data demonstrate that orexin-A administered in the VLPO acutely increases wakefulness by reducing the number, but not the duration, of episodes of NREM and REM sleep.
Study 2. Pretreatment with a DORA blocks the effects of orexin-A in the VLPO on vigilance states.
We hypothesized that blocking both orexin receptor sub-types would prevent increased arousal by orexin-A. As expected, the DORA blocked the effects of orexin-A on active wakefulness and NREM sleep in the 0–1 h and 0–2 h time periods (Figure 3). There were thus significant main effects of DORA treatment on active wakefulness (0–1 h: F5,15 = 4.1, P = 0.016 and 0–2 h: F5,15 = 3.6, P = 0.023) and NREM sleep (0–1 h: F5,15 = 4.4, P = 0.012 and 0–2 h F5,15 = 4.7, P = 0.008). The DORA had a dose-dependent but nonsignificant tendency to block the orexin-A-induced reduction in REM sleep 0–1 or 0–2 h post-injection (F5,15 = 1.5, P = 0.24 and F5,15 = 0.6, P = 0.69 respectively, Figure 3C). Orexin-A alone significantly increased active wakefulness and reduced NREM sleep 0–1 h and 0–2 h post-injection relative to the vehicle injection (P < 0.05 for all comparisons). All doses of DORA abolished these effects of orexin-A on active wakefulness and NREM sleep at 0–1 h and 0–2 h post-injection (P < 0.05 for all comparisons). There were no significant effects of treatment on active wakefulness, NREM sleep, or REM sleep during the 1–2 h post-injection time period (P > 0.05). Finally, we observed no significant main effect of treatment on the number or mean duration of episodes of active wakefulness, NREM or REM sleep in the 0–1, 1–2, or 0–2 h post-injection time periods (data not shown). These data demonstrate that a DORA blocks the effects of orexin-A in the VLPO on vigilance states.
Figure 3.
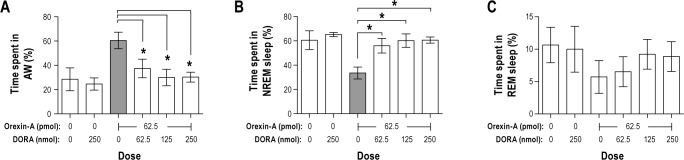
Study 2. Pre-administration of the dual orexin receptor antagonist (DORA, TCS-1102) in the ventrolateral preoptic area (VLPO) prevents the increase in (A) active wakefulness and the reduction in (B) NREM sleep following orexin-A administered in the VLPO 2 h later. (C) The DORA had a dose-dependent tendency to reduce the non-significant REM sleep suppression caused by orexin-A. n = 4. Data represented as mean ± SEM. The shaded bar for orexin-A is significantly different from the vehicle injection (P < 0.05). Brackets above bars indicate doses of the DORA that are significantly different from orexin-A (*P < 0.05). Note different scaling on y-axes.
Study 3. Orexin-A in the VLPO stimulates SPA.
We hypothesized that orexin-A microinjected into the VLPO would increase SPA based on the stimulating effect of orexin-A on SPA when administered in other brain regions.20,21 Orexin-A in the VLPO significantly increased distance traveled and vertical activity counts during the 0–1 h post-injection time period (F4,36 = 5.8, P = 0.001, F4,36 = 3.7, P = 0.0118, respectively, Figure 4). This effect of orexin-A on distance traveled and vertical counts was dose dependent (P < 0.05 for all comparisons, Figure 4A). The highest dose of orexin-A significantly enhanced vertical counts relative to both the vehicle and the two lowest doses of orexin-A (P < 0.05 for all comparisons, Figure 4B). During the 1–2 h post-injection time period there was no main effect of treatment on distance traveled or vertical counts (P > 0.05, data not shown). During the 0–2 h post-injection time period, there was a main effect of treatment on distance traveled (F4,36 = 3.7, P = 0.013) but no effect on vertical counts (P > 0.05, data not shown). Together, these data demonstrate that orexin-A in the VLPO increases SPA.
Figure 4.
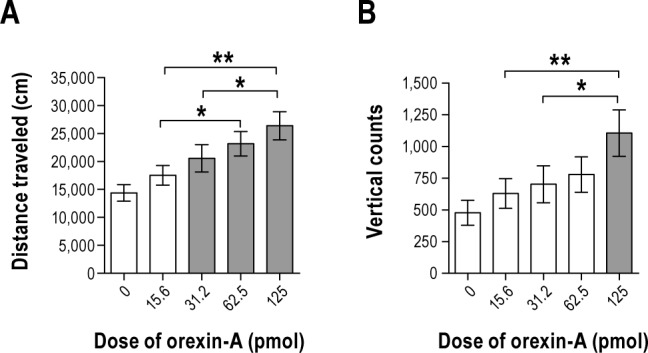
Study 3. Orexin-A in the ventrolateral preoptic area significantly increases spontaneous physical activity through increases in (A) distance traveled and (B) vertical activity 1-h post-injection. n = 10. Data represented as mean ± SEM. Shaded bars are significantly different from the control injection of artificial cerebrospinal fluid (P < 0.05). Brackets above bars indicate doses of orexin-A that were significantly different from each other (*P < 0.05 and **P < 0.005). Note different scaling on y-axes.
Study 4. Pretreatment with an OX2R antagonist reduces SPA stimulated by orexin-A in the VLPO.
Based on the presence of orexin-1 and -2 receptors in the VLPO,34,35 we expected that the OX2R antagonist would reduce SPA stimulated by orexin-A in the VLPO. The OX2R antagonist reduced the stimulating effect of orexin-A on SPA both 0–1 h and 0–2 h post-injection, but had no significant effect during the 1–2 h post-injection time period (Figure 5). There was a main effect of treatment on distance traveled and vertical counts relative to the vehicle during the 0–1 h and 0–2 h time periods (0–1 h: F5,45 = 4.2, P = 0.003 and F5,45 = 5.4, P = 0.001 and 0–2 h: F5,45 = 3.8, P = 0.006 and F5,45 = 5.1, P = 0.001, respectively, Figure 5). Despite the fact that orexin-A significantly increased distance traveled and vertical counts relative to the vehicle (P = 0.002 and P = 0.001, respectively), the highest dose of the OX2R antagonist reduced vertical counts (P = 0.045) but failed to reduce distance traveled (P > 0.05 for all doses) 1 h post-injection. Two- h post-injection, orexin-A significantly increased distance traveled and vertical counts relative to the vehicle (P = 0.001 and P < 0.01, respectively). The highest dose of the OX2R antagonist reduced the effect of orexin-A on distance traveled (P = 0.016, Figure 5A). All doses of the OX2R antagonist reduced vertical counts (P < 0.05 for all comparisons, Figure 5B). Together, these data show that the OX2R in the VLPO partially mediates the effects of orexin-A on SPA.
Figure 5.
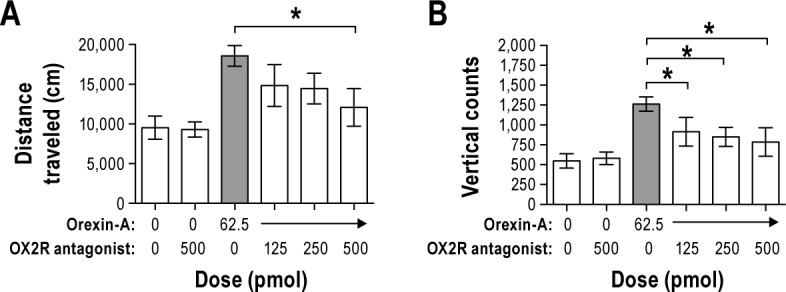
Study 4. Pre-administration of a selective orexin two receptor (OX2R) antagonist (JNJ-10397049) in the ventrolateral preoptic area (VLPO) reduces spontaneous physical activity as indicated by reductions in (A) distance traveled and (B) vertical counts following orexin-A administered in the VLPO 2 h later. n = 10. Data represented as mean ± SEM. The shaded bar for orexin-A is significantly different from the negative vehicle control injection (orexin-A and the OX2R antagonist) (P < 0.05). Brackets above bars indicate doses of the OX2R antagonist that are significantly different from orexin-A (*P < 0.05). Note different scaling on y-axes.
Study 5. There is no effect of orexin-A in the VLPO on acute or chronic feeding.
Orexin-A injection in the VLPO failed to stimulate food in-take during the 0–1, 0–2, 0–4, and 0–24 h post injection intervals (Figure 6, P > 0.05, all doses). These data demonstrate that the VLPO is not a site of orexin-A action on feeding behavior.
Figure 6.
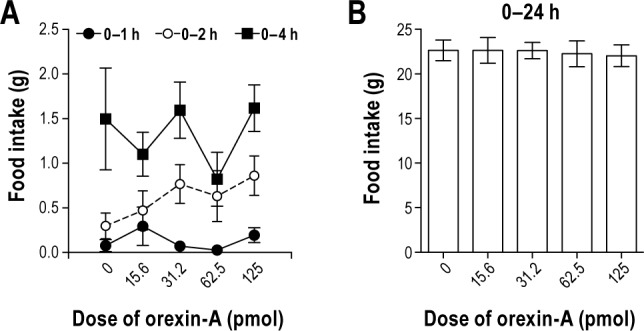
Study 5. Orexin-A in the ventrolateral preoptic area (VLPO) has no effect on (A) acute (e.g. 0–1, 0–2 or 0–4 h post-injection) or (B) chronic food intake (e.g. 0–24 h post-injection). n = 18. Data represented as mean ± SEM. Note different scaling on y-axes.
Study 6. Orexin-A in the VLPO stimulates energy expenditure.
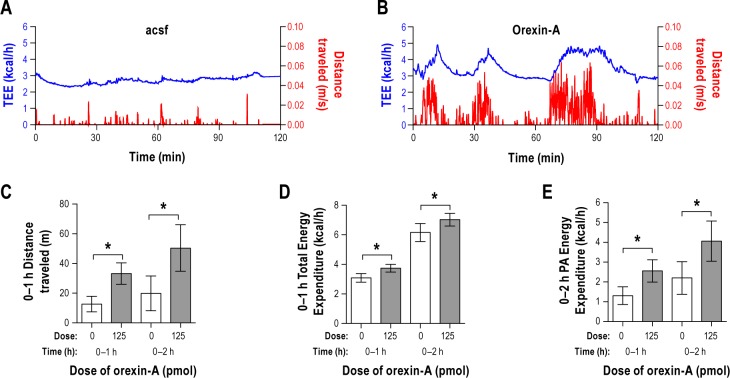
We hypothesized that orexin-A in the VLPO would increase energy expenditure as a consequence of increased SPA (Figure 7). To test this, we measured energy expenditure and SPA concurrently after injection of orexin-A or vehicle in the VLPO (Figure 7A, 7B). Orexin-A in the VLPO significantly increased distance traveled (0–1 h: P = 0.015 and 0–2 h: P = 0.015, Figure 7C) and total energy expenditure (0–1 h: P = 0.035 and 0–2 h: P = 0.006, Figure 7D) during the 0–1 h and 0–2 h post-injection time periods.
Figure 7.
Study 6. Representative examples of the time course of spontaneous physical activity (SPA, right y-axis in red) and total energy expenditure (TEE, left y-axis in blue) after injection of (A) vehicle-artificial cerebrospinal fluid or (B) orexin-A, beginning 2-h post-injection. Microinjection of orexin-A into the ventrolateral preoptic area (VLPO) significantly increases (C) distance traveled, (D) TEE and (E) SPA-induced energy expenditure relative to vehicle injection. n = 4. Data represented as mean ± SEM. Brackets indicate bars that are significantly different from each other (*P < 0.05) in panels C D, and E. Note different scaling on the y-axes.
To test whether the energy expenditure due to SPA was increased by orexin-A, we calculated the sum of energy expenditure for each second during which the rat moved. Figures 7A and 7B display SPA coupled with energy expenditure throughout the 0–2 h post-injection time period and demonstrate the tight relationship between energy expenditure and SPA. Energy expenditure due to SPA was significantly increased after injection of orexin-A relative to the vehicle injection during the 0–1 and 0–2 h post-injection time periods (0–1 h: P = 0.040 and 0–2 h: P = 0.009, Figure 7E).
DISCUSSION
Orexin-A is a neuromodulator that integrates physiological processes, plays a critical role in stabilizing vigilance states and increases energy expenditure through SPA.14,55–57 Orexin-A enhances wakefulness, SPA and energy expenditure following injection into wakefulness-promoting nuclei,20,58 but the effects of orexin-A after injection specifically into the VLPO have not been well characterized. These results show that local injection of orexin-A in the VLPO produces a behavioral profile similar to that observed after orexin-A injection into other wake-promoting nuclei. Microinjection of orexin-A in the VLPO enhances wakefulness, SPA, SPA-induced energy expenditure and total energy expenditure without any feeding effect. Furthermore, we showed that blockade of both orexin receptors in the VLPO reduces orexin-A stimulated wakefulness and SPA, while blockade of OX2R alone partially reduced orexin-A stimulated SPA. Together these data suggest that the VLPO may be a critical site of convergence for orexin-A mediation of vigilance states and energy balance regulation.
The VLPO contains both orexin receptor subtypes,34,35 and there are reciprocal connections with orexinergic nuclei and the VLPO.32,36–38,40–42,59 Neuroanatomical and functional39,44 data indicate that the VLPO promotes sleep, at least partially, by inhibiting orexin neurons. This suggests that orexin can inhibit sleep-promoting neurons in the VLPO to maintain wakefulness and also that the VLPO may modulate orexin-A stimulated behavior in a push-pull relationship29,60 similar to that of other arousal-promoting centers such as the tuberomammillary nucleus.61 In addition, locally in the VLPO, orexin affects galaninergic/GABAergic interneurons.42,62
Our work and that of others suggest that orexin-A increases SPA and elevates energy expenditure in a dose-dependent and cumulative manner, which promotes obesity resistance.14,23,63,64 Here, we observed significantly greater SPA, SPA-induced energy expenditure and total energy expenditure in response to orexin-A in the VLPO during the 0–2 h post-injection time period. One novelty of this finding lies in the time-locked relationship between SPA and energy expenditure in both orexin-A-treated and non-treated rats. This can be seen from the matching of long intervals of SPA with peaks of total energy expenditure and from the decline in total energy expenditure towards that of resting metabolic rate during times of low SPA. This is the first demonstration that orexin-A in the VLPO stimulates SPA-related energy expenditure, and that increases in whole body energy expenditure after orexin-A administration in the VLPO are directly coupled to SPA-induced energy expenditure.56,64 Together these data imply that pharmacological interventions to enhance orexin activity in the VLPO may be effective in combating obesity by increasing physical activity.
In contrast to the effect of orexin-A on vigilance states, SPA, and energy expenditure, VLPO administration of orexin-A failed to augment either acute or 24-h food intake. Orexin-A administered in the VLPO did not increase food intake, which is in agreement with previous findings that orexin-A demonstrates a brain site-dependent effect on feeding.22,65,66 In contrast to the eating behavior following orexin-A injection into the rostral portion of the lateral hypothalamus,66 mild feeding is observed after orexin-A is injected into the dorsal raphe,22 and there is no effect of orexin-A on feeding after administration into the locus coeruleus, substantia nigra, or tuberomammillary nucleus.22,65 The effects of orexin-A on feeding were dissociable here from other behavioral changes induced by the neuropeptide (i.e., vigilance states and SPA). This finding parallels results showing not only that orexin-A effects are brain site-dependent,14 but they are also behavior-specific and circadian-dependent,67 which might be expected if orexin-A plays a role in response to physiological disequilibrium caused by such factors as exercise, fasting or other dietary modification.56,68
The signaling mechanisms underlying the effects of orexin-A on behavior after direct VLPO administration are uncertain. The net effect of orexin-A can be excitatory or inhibitory, depending upon the particular brain region and type of interneuron present. Orexin-A can cause local glutamate and/ or GABA release.69,70 The VLPO neurons project to wake-promoting histaminergic neurons in the tuberomammillary nucleus, noradrenergic neurons in the locus coeruleus and serotonergic neurons in the dorsal raphe, and inhibit these neurons during sleep. As the VLPO promotes the onset of sleep through widespread GABA-mediated inhibition, it is plausible that orexin-A in the VLPO might reduce neuronal activity of sleep-active neurons in this region through local GABAergic signaling.69,71,72 Though direct measurement of GABA release in the VLPO will be required to verify this possibility, this would be expected to promote arousal and SPA. It is also plausible that the behavioral and energy expenditure effects of orexin-A in the VLPO are due to pathways from the VLPO to other brain nuclei such as the paraventricular nucleus of the hypothalamus.73 As mentioned before, the orexin system senses homeostatic or metabolic imbalance and modifies behavior accordingly. For example at the cellular level, orexin neuronal activity is directly sensitive to changes in pH and levels of circulating factors such as leptin, ghrelin, glucose, and insulin.74–76 At the whole organism level, therefore, deficient orexin signaling renders an animal unable to respond with normal increases in arousal, physical activity, and motivation in response to fasting.75,77 Taken together, these data suggest that an orexin-induced increase in GABAergic tone in the VLPO plays a role in promoting adaptation, which contributes to orexin-A induced behavioral effects.
To begin to address the mechanism underlying the arousal and SPA-promoting effects of orexin-A administered in the VLPO, we examined pharmacological antagonism of the effects by testing if a DORA would block the effects of orexin-A on arousal and if an OX2R antagonist would block the effect of orexin-A on SPA. Although we did not systematically address the functional significance of the two OXR sub-types for each endpoint, we found that a DORA suppressed the increase in wakefulness and the reduction in NREM sleep elicited by orexin-A and the OX2R antagonist partially inhibited orexin-A stimulated SPA. This functional distinction between the DORA and the OX2R antagonist in the VLPO is presumably related to the relative abundance of each OXR subtype, the specific neurons on which they are localized, and the differential binding affinities for orexin-A and the antagonists. Antagonism of OX2R partially reduced SPA stimulated by orexin-A in accord with previous studies showing that both receptor subtypes contribute to SPA stimulated by orexin-A,78,79 and that DORAs reduce SPA.51,80 We have previously reported greater OX1R mRNA relative to OX2R mRNA in the VLPO in Sprague-Dawley rats.35 This unequal abundance of orexin receptors in the VLPO but the lack of knowledge as to which neurons carry each receptor complicates our ability to address the functional role of each receptor subtype. A systematic investigation is warranted to measure concurrently vigilance states, SPA, and energy expenditure in a single group of rats to provide the necessary framework for elucidating the role of each receptor subtype in the VLPO.
It is possible that diffusion of orexin-A into the basal fore-brain, media/lateral preoptic area or extended VLPO, or stimulation of fibers, may have contributed to these results. These brain areas contain both sleep and wake regulatory neurons.44 They exhibit simultaneous and reciprocal discharge patterns, as well as local mutually inhibitory interactions. Basal fore-brain neurons are extensively innervated by orexin neurons and is a key site through which orexin activates the cortex to promote behavioral arousal.81 The extended VLPO contains GABA/galaniergic neurons that are active during REM sleep, and lesions to this area reduce REM sleep.27 However, based on Nicholson's work49 showing that the interaction between the ligand and receptor limits diffusion, and functional studies testing spatial limits of ligand action in other discrete brain areas,66,82 it is more likely that appreciable or effective amounts did not extend beyond the VLPO. That orexin-A failed to elicit effects in rats with misplaced cannulae supports this. Finally, drug delivery was targeted more medially to avoid the possibility of drug diffusion into the basal forebrain.
In conclusion, these data verify that the VLPO is an important region receiving orexin afferents with functional implications for energy balance, sleep and wakefulness. Specifically, our results suggest that the VLPO may coordinate and integrate orexin-A enhanced behaviors to promote negative energy balance by enhancing arousal, total energy expenditure and SPA-related energy expenditure. These findings provide a basis for the investigation of the OXR subtypes that mediate behaviors stimulated by orexin-A in the VLPO.
DISCLOSURE STATEMENT
Funding for this research and publication was supported by the Department of Veterans Affairs (F7212W to Dr. Teske and 5I01RX000441-04 to Dr. Kotz and Dr. Billington), the National Institutes of Health-NIDDK (1R01DK100281-01A1 to Dr. Kotz and Dr. Billington and 5P30DK05045619 to Dr. Billington), the United States Department of Agriculture (ARZT-1360220-H23-150 to Dr. Teske) and CONICYT, Concurso Nacional de Apoyo al Retorno de Investigadores desde el extranjero (82130017 to Dr. Perez-Leighton). The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors acknowledge technical expertise from Almira Rezaimalek and Melissa Wyatt at the University of Arizona.
REFERENCES
- 1.Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann N Y Acad Sci. 2008;1129:287–304. doi: 10.1196/annals.1417.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cappuccio FP, D'Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2010;33:414–20. doi: 10.2337/dc09-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity. 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jean-Louis G, Williams NJ, Sarpong D, et al. Associations between inadequate sleep and obesity in the US adult population: analysis of the national health interview survey (1977-2009) BMC Public Health. 2014;14:290. doi: 10.1186/1471-2458-14-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med. 2013;9:73–80. doi: 10.5664/jcsm.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97:1792–801. doi: 10.1210/jc.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shlisky JD, Hartman TJ, Kris-Etherton PM, Rogers CJ, Sharkey NA, Nickols-Richardson SM. Partial sleep deprivation and energy balance in adults: an emerging issue for consideration by dietetics practitioners. J Acad Nutr Diet. 2012;112:1785–97. doi: 10.1016/j.jand.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 9.de Lecea L, Kilduff TS, Peyron C, et al. The hypocretins: hypothalamusspecific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–7. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–85. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 11.Giardino WJ, de Lecea L. Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol. 2014;29C:103–8. doi: 10.1016/j.conb.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burdakov D, Karnani MM, Gonzalez A. Lateral hypothalamus as a sensor-regulator in respiratory and metabolic control. Physiol Behav. 2013;121:117–24. doi: 10.1016/j.physbeh.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sakurai T, Mieda M, Tsujino N. The orexin system: roles in sleep/wake regulation. Ann N Y Acad Sci. 2010;1200:149–61. doi: 10.1111/j.1749-6632.2010.05513.x. [DOI] [PubMed] [Google Scholar]
- 14.Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Ann N Y Acad Sci. 2012;1264:72–86. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li J, Hu Z, de Lecea L. The hypocretins/orexins: integrators of multiple physiological functions. Br J Pharmacol. 2014;171:332–50. doi: 10.1111/bph.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berthoud HR, Munzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiol Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MG, Hassani OK, Jones BE. Discharge of identified orexin/ hypocretin neurons across the sleep-waking cycle. J Neurosci. 2005;25:6716–20. doi: 10.1523/JNEUROSCI.1887-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taheri S, Sunter D, Dakin C, et al. Diurnal variation in orexin A immunoreactivity and prepro-orexin mRNA in the rat central nervous system. Neurosci Lett. 2000;279:109–12. doi: 10.1016/s0304-3940(99)00955-6. [DOI] [PubMed] [Google Scholar]
- 19.Mileykovskiy BY, Kiyashchenko LI, Siegel JM. Behavioral correlates of activity in identified hypocretin/orexin neurons. Neuron. 2005;46:787–98. doi: 10.1016/j.neuron.2005.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagan JJ, Leslie RA, Patel S, et al. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci U S A. 1999;96:10911–6. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- 22.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008;294:R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- 23.Lubkin M, Stricker-Krongrad A. Independent feeding and metabolic actions of orexins in mice. Biochemical and biophysical research communications. 1998;253:241–5. doi: 10.1006/bbrc.1998.9750. [DOI] [PubMed] [Google Scholar]
- 24.Novak CM, Levine JA. Daily intraparaventricular orexin-A treatment induces weight loss in rats. Obesity. 2009;17:1493–8. doi: 10.1038/oby.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Leighton CE, Boland K, Billington CJ, Kotz CM. High and low activity rats: elevated intrinsic physical activity drives resistance to diet-induced obesity in non-bred rats. Obesity. 2013;21:353–60. doi: 10.1002/oby.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science. 1996;271:216–9. doi: 10.1126/science.271.5246.216. [DOI] [PubMed] [Google Scholar]
- 27.Lu J, Greco MA, Shiromani P, Saper CB. Effect of lesions of the ventrolateral preoptic nucleus on NREM and REM sleep. J Neurosci. 2000;20:3830–42. doi: 10.1523/JNEUROSCI.20-10-03830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szymusiak R, Alam N, Steininger TL, McGinty D. Sleep-waking discharge patterns of ventrolateral preoptic/anterior hypothalamic neurons in rats. Brain Res. 1998;803:178–88. doi: 10.1016/s0006-8993(98)00631-3. [DOI] [PubMed] [Google Scholar]
- 29.Szymusiak R, McGinty D. Hypothalamic regulation of sleep and arousal. Ann N Y Acad Sci. 2008;1129:275–86. doi: 10.1196/annals.1417.027. [DOI] [PubMed] [Google Scholar]
- 30.Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- 31.Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–31. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- 32.Sherin JE, Elmquist JK, Torrealba F, Saper CB. Innervation of histaminergic tuberomammillary neurons by GABAergic and galaninergic neurons in the ventrolateral preoptic nucleus of the rat. J Neurosci. 1998;18:4705–21. doi: 10.1523/JNEUROSCI.18-12-04705.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak CM, Nunez AA. Daily rhythms in Fos activity in the rat ventrolateral preoptic area and midline thalamic nuclei. Am J Physiol. 1998;275:R1620–6. doi: 10.1152/ajpregu.1998.275.5.R1620. [DOI] [PubMed] [Google Scholar]
- 34.Marcus JN, Aschkenasi CJ, Lee CE, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 35.Mavanji V, Teske JA, Billington CJ, Kotz CM. Elevated sleep quality and orexin receptor mRNA in obesity-resistant rats. Int J Obes (Lond) 2010;34:1576–88. doi: 10.1038/ijo.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Berk ML, Finkelstein JA. Efferent connections of the lateral hypothalamic area of the rat: an autoradiographic investigation. Brain Res Bull. 1982;8:511–26. doi: 10.1016/0361-9230(82)90009-0. [DOI] [PubMed] [Google Scholar]
- 37.Chou TC, Bjorkum AA, Gaus SE, Lu J, Scammell TE, Saper CB. Afferents to the ventrolateral preoptic nucleus. J Neurosci. 2002;22:977–90. doi: 10.1523/JNEUROSCI.22-03-00977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Novak CM, Nunez AA. A sparse projection from the suprachiasmatic nucleus to the sleep active ventrolateral preoptic area in the rat. Neuroreport. 2000;11:93–6. doi: 10.1097/00001756-200001170-00019. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida K, McCormack S, Espana RA, Crocker A, Scammell TE. Afferents to the orexin neurons of the rat brain. J Comp Neurol. 2006;494:845–61. doi: 10.1002/cne.20859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci U S A. 1999;96:748–53. doi: 10.1073/pnas.96.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaus SE, Strecker RE, Tate BA, Parker RA, Saper CB. Ventrolateral preoptic nucleus contains sleep-active, galaninergic neurons in multiple mammalian species. Neuroscience. 2002;115:285–94. doi: 10.1016/s0306-4522(02)00308-1. [DOI] [PubMed] [Google Scholar]
- 43.Sakurai T, Nagata R, Yamanaka A, et al. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46:297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 44.Methippara MM, Alam MN, Szymusiak R, McGinty D. Effects of lateral preoptic area application of orexin-A on sleep-wakefulness. Neuroreport. 2000;11:3423–6. doi: 10.1097/00001756-200011090-00004. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–43. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 46.Cleary JP, O'Hare E, Pomonis JD, et al. Discriminative stimulus effects of morphine: central versus peripheral training. Brain Res. 1999;847:26–31. doi: 10.1016/s0006-8993(99)02001-6. [DOI] [PubMed] [Google Scholar]
- 47.O'Hare E, Cleary J, Weldon DT, Pomonis JD, Billington CJ, Levine AS. Intrahypothalamic discriminative stimulus effects of neuropeptide Y. Pharmacol Biochem Behav. 1998;59:375–8. doi: 10.1016/s0091-3057(97)00456-5. [DOI] [PubMed] [Google Scholar]
- 48.Teske JA, Billington CJ, Kotz CM. Mechanisms underlying obesity resistance associated with high spontaneous physical activity. Neuroscience. 2014;256:91–100. doi: 10.1016/j.neuroscience.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nicholson C. Diffusion from an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Res. 1985;333:325–9. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- 50.Grosse P, Cassidy MJ, Brown P. EEG-EMG, MEG-EMG and EMGEMG frequency analysis: physiological principles and clinical applications. Clin Neurophysiol. 2002;113:1523–31. doi: 10.1016/s1388-2457(02)00223-7. [DOI] [PubMed] [Google Scholar]
- 51.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R889–99. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- 52.Winrow CJ, Tanis KQ, Reiss DR, et al. Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure. Neuropharmacology. 2010;58:185–94. doi: 10.1016/j.neuropharm.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 53.Broom DM. Animal welfare: concepts and measurement. J Anim Sci. 1991;69:4167–75. doi: 10.2527/1991.69104167x. [DOI] [PubMed] [Google Scholar]
- 54.Dugovic C, Shelton JE, Aluisio LE, et al. Blockade of orexin-1 receptors attenuates orexin-2 receptor antagonism-induced sleep promotion in the rat. J Pharmacol Exp Ther. 2009;330:142–51. doi: 10.1124/jpet.109.152009. [DOI] [PubMed] [Google Scholar]
- 55.Adamantidis A, de Lecea L. The hypocretins as sensors for metabolism and arousal. J Physiol. 2009;587:33–40. doi: 10.1113/jphysiol.2008.164400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sinton CM. Orexin/hypocretin plays a role in the response to physiological disequilibrium. Sleep Med Rev. 2011;15:197–207. doi: 10.1016/j.smrv.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 57.Sinton CM, McCarley RW. Neurophysiological mechanisms of sleep and wakefulness: a question of balance. Semin Neurol. 2004;24:211–23. doi: 10.1055/s-2004-835067. [DOI] [PubMed] [Google Scholar]
- 58.Teske JA, Billington CJ, Kotz CM. Neuropeptidergic mediators of spontaneous physical activity and non-exercise activity thermogenesis. Neuroendocrinology. 2008;87:71–90. doi: 10.1159/000110802. [DOI] [PubMed] [Google Scholar]
- 59.Leranth C, MacLusky NJ, Shanabrough M, Naftolin F. Immunohistochemical evidence for synaptic connections between proopiomelanocortin-immunoreactive axons and LH-RH neurons in the preoptic area of the rat. Brain Res. 1988;449:167–76. doi: 10.1016/0006-8993(88)91035-9. [DOI] [PubMed] [Google Scholar]
- 60.Saper CB, Fuller PM, Pedersen NP, Lu J, Scammell TE. Sleep state switching. Neuron. 2010;68:1023–42. doi: 10.1016/j.neuron.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williams RH, Chee MJ, Kroeger D, et al. Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J Neurosci. 2014;34:6023–9. doi: 10.1523/JNEUROSCI.4838-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gong H, McGinty D, Guzman-Marin R, Chew KT, Stewart D, Szymusiak R. Activation of c-fos in GABAergic neurones in the preoptic area during sleep and in response to sleep deprivation. J Physiol. 2004;556:935–46. doi: 10.1113/jphysiol.2003.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab. 2006;290:E396–403. doi: 10.1152/ajpendo.00293.2005. [DOI] [PubMed] [Google Scholar]
- 64.Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–9. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- 65.Teske JA, Perez-Leighton CE, Billington CJ, Kotz CM. Role of the locus coeruleus in enhanced orexin A-induced spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2013;305:R1337–45. doi: 10.1152/ajpregu.00229.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thorpe AJ, Mullett MA, Wang C, Kotz CM. Peptides that regulate food intake: regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1409–17. doi: 10.1152/ajpregu.00344.2002. [DOI] [PubMed] [Google Scholar]
- 67.Espana RA, Plahn S, Berridge CW. Circadian-dependent and circadian-independent behavioral actions of hypocretin/orexin. Brain Res. 2002;943:224–36. doi: 10.1016/s0006-8993(02)02653-7. [DOI] [PubMed] [Google Scholar]
- 68.de Lecea L, Sutcliffe JG, Fabre V. Hypocretins/orexins as integrators of physiological information: lessons from mutant animals. Neuropeptides. 2002;36:85–95. doi: 10.1054/npep.2002.0892. [DOI] [PubMed] [Google Scholar]
- 69.Thorpe AJ, Doane DF, Sweet DC, Beverly JL, Kotz CM. Orexin A in the rostrolateral hypothalamic area induces feeding by modulating GABAergic transmission. Brain Res. 2006;1125:60–6. doi: 10.1016/j.brainres.2006.09.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van den Pol AN, Gao XB, Obrietan K, Kilduff TS, Belousov AB. Presynaptic and postsynaptic actions and modulation of neuroendocrine neurons by a new hypothalamic peptide, hypocretin/orexin. J Neurosci. 1998;18:7962–71. doi: 10.1523/JNEUROSCI.18-19-07962.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alam MN, Kumar S, Bashir T, et al. GABA-mediated control of hypocretin- but not melanin-concentrating hormone-immunoreactive neurones during sleep in rats. J Physiol. 2005;563:569–82. doi: 10.1113/jphysiol.2004.076927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Alam MN, Kumar S, Suntsova N, Bashir T, Szymusiak R, McGinty D. GABAergic regulation of the perifornical-lateral hypothalamic neurons during non-rapid eye movement sleep in rats. Neuroscience. 2010;167:920–8. doi: 10.1016/j.neuroscience.2010.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burdakov D, Liss B, Ashcroft FM. Orexin excites GABAergic neurons of the arcuate nucleus by activating the sodium--calcium exchanger. J Neurosci. 2003;23:4951–7. doi: 10.1523/JNEUROSCI.23-12-04951.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D. Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci U S A. 2007;104:10685–90. doi: 10.1073/pnas.0702676104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamanaka A, Beuckmann CT, Willie JT, et al. Hypothalamic orexin neurons regulate arousal according to energy balance in mice. Neuron. 2003;38:701–13. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 76.Moriguchi T, Sakurai T, Nambu T, Yanagisawa M, Goto K. Neurons containing orexin in the lateral hypothalamic area of the adult rat brain are activated by insulin-induced acute hypoglycemia. Neurosci Lett. 1999;264:101–4. doi: 10.1016/s0304-3940(99)00177-9. [DOI] [PubMed] [Google Scholar]
- 77.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci. 2004;24:10493–501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bergman JM, Roecker AJ, Mercer SP, et al. Proline bis-amides as potent dual orexin receptor antagonists. Bioorg Med Chem Lett. 2008;18:1425–30. doi: 10.1016/j.bmcl.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 79.Samson WK, Bagley SL, Ferguson AV, White MM. Orexin receptor subtype activation and locomotor behaviour in the rat. Acta Physiol (Oxf) 2010;198:313–24. doi: 10.1111/j.1748-1716.2009.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Winrow CJ, Gotter AL, Cox CD, et al. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. J Neurogenet. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- 81.Arrigoni E, Mochizuki T, Scammell TE. Activation of the basal forebrain by the orexin/hypocretin neurones. Acta Physiol (Oxf) 2010;198:223–35. doi: 10.1111/j.1748-1716.2009.02036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kotz CM, Glass MJ, Levine AS, Billington CJ. Regional effect of naltrexone in the nucleus of the solitary tract in blockade of NPY-induced feeding. Am J Physiol Regul Integr Comp Physiol. 2000;278:R499–503. doi: 10.1152/ajpregu.2000.278.2.R499. [DOI] [PubMed] [Google Scholar]