Abstract
Large-scale medical sequencing provides a focal point around which to reorganize health care and health care research. Mobile health (mHealth) is also currently undergoing explosive growth and could be another innovation that will change the face of future health care. We are employing primary ovarian insufficiency (POI) as a model rare condition to explore the intersection of these potentials. As both sequencing capabilities and our ability to intepret this information improve, sequencing for medical purposes will play an increasing role in health care beyond basic research: it will help guide the delivery of care to patients. POI is a serious chronic disorder and syndrome characterized by hypergonadotrophic hypogonadism before the age of 40 years and most commonly presents with amenorrhea. It may have adverse health effects that become fully evident years after the initial diagnosis. The condition is most commonly viewed as one of infertility, however, it may also be associated with adverse long-term outcomes related to inadequate bone mineral density, increased risk of cardiovascular disease, adrenal insufficiency, hypothyroidism and, if pregnancy ensues, having a child with Fragile X Syndrome. There may also be adverse outcomes related to increased rates of anxiety and depression. POI is also a rare disease, and accordingly, presents special challenges. Too often advances in research are not effectively integrated into community care at the point of service for those with rare diseases. There is a need to connect community health providers in real time with investigators who have the requisite knowledge and expertise to help manage the rare disease and to conduct ongoing research. Here we review the pathophysiology and management of POI and propose the development of an international Clinical Research Integration Special Program (CRISP) for the condition.
Keywords: Autoimmune oophoritis, Community-based participatory research, Mobile health units, Menopause, premature, Primary ovarian insufficiency, Rare diseases
A recent United States Supreme Court decision ruling that naturally occurring DNA cannot be patented was a breakthrough for the integration of genomic sequencing into clinical care. There are many challenges ahead before genomic medicine can be considered truly embedded in health care.1 Foremost among these is the need to support clinicians in interpreting genomic data and then providing appropriate patient care based on these interpretations.
In exome and whole genome sequencing (WGS), secondary findings that are unrelated to the indication for performing the sequencing may be revealed that are of important medical value to the patient. The American College of Medical Genetics and Genomics recently published a policy statement that emphasized the need to alert patients to this possibility and to develop a management plan for such findings. The report recommends that laboratories performing clinical sequencing should seek and report mutations of specified classes or types in a list of 57 carefully chosen pathogenic genes. Mutations in these specific genes could indicate the presence of any of 24 disorders where early intervention is likely to reduce or prevent serious morbidity and/or early mortality.2 Nearly 20 million individuals are affected by rare diseases in the United States alone (National Institutes of Health. Office of Rare Disease Research. Genetic and Rare Diseases Information Center Brochure. Available at: http://rarediseases.info.nih.gov/files/GARD_brochure_English.pdf. Accessed May 29, 2014). As defined by the Orphan Drug Act, a disease is considered rare if it affects fewer than 200,000 within the United States (Orphan Drug Act, 21 USCx360bb, 2010). By this definition, there are more than 6,000 rare diseases (National Institutes of Health. Office of Rare Disease Research. Genetic and Rare Diseases Information Center Brochure. Available at: http://rarediseases.info.nih.gov/files/GARD_brochure_English.pdf. Accessed November 15, 2010). It is beyond reason to expect any primary care clinician to maintain the requisite knowledge and expertise to effectively manage all these conditions in real time. Continuing education programs can only go so far in addressing this problem.
No one clinician can be expected to master all of the inherent nuances of genomic medicine and the management of rare diseases. There will be a need for digital tools that provide real-time clinical decision support to clinicians by integrating vast datasets. These tools will need to put patient needs at the heart of the effort with regard to communication and counseling. Primary ovarian insufficiency (POI) can serve as one model rare condition from which to begin the transition to “personalized genomic medicine”.
POI
The physiological age-dependent exhaustion of ovarian function, defined as menopause, occurs normally around 48–52 years of age. Of the approximately 400,000 oocyte-containing primordial follicles available at the time of puberty, only a few non-functional follicles remain at the time of menopause. 3 Monthly ovulation contributes little to the loss of functional oocytes with most of the exhaustion being caused by atresia. Atresia is not prevented by estrogen/progestin therapies. The process leading to the final exhaustion of the functional follicle pool is influenced by the number of total follicles initially available at puberty and by the rate of continuous loss.
In around 1% of women, a menopause-like condition occurs before the age of 40 years. This was first described using the term primary ovarian insufficiency to make the point that the ovary was the primary problem rather than the result of inadequate gonadotropin stimulation of the ovary.4 The disorder also has been previously referred to in the scientific literature as premature ovarian failure (POF), early menopause, hypergonadotropic amenorrhea or hypergonadotropic hypogonadism.5–7 This condition is characterized most commonly by secondary amenorrhea (in about 10% primary amenorrhea), elevated serum gonadotrophin concentrations into the menopausal range, hypoestrogenism and associated infertility. In the presence of a normal karyotype, ovarian insufficiency occurs 1 in every 10,000 women by the age of 20 years, 1 in every 1000 women by the age of 30 years, and 1 in every 100 women by the age of 40 years.8
POI has been attributed to chromosomal aberrations, genetic defects, chemotherapy, ovarian surgery or autoimmunity, though most of cases remain idiopathic. Spontaneous POI is complex in that women with this condition frequently have intermittent and unpredictable ovarian function that may persist for decades. The pathophysiology differs from the normal menopausal process. In many patients with POI, follicular development and ovarian activity can be demonstrated, and the pregnancy rate, although reduced, is not zero.9, 10 Terms introduced since the original description of the disorder such as premature “menopause” or premature ovarian “failure” should no longer be used as they are scientifically inaccurate.
The more scientifically accurate term for the disorder is POI.11, 12 This term is more reflective of the variable nature of disease expression and more informative for those patients who do not experience a complete cessation of ovarian function. Pregnancies are possible many years after the diagnosis of POI.
Although high serum concentrations of gonadotrophins define POI in women with oligo/amenorrhea, the increase of serum FSH levels is not an early event in the natural history of the disease. Reduction of serum inhibin levels precedes, in some cases by several years, the clinical signs of ovarian insufficiency. 13 An initial decrease in fertility, at a time when menstrual cycles and gonadotrophins concentrations are still in the range of normal, may represent the earliest event in the natural history of many women with POI. A shortening of the menstrual cycle interval may be the first clinical sign. It has recently become evident that serum concentrations of anti-Mullerian hormone (AMH) may represent the best marker to estimate the residual follicle pool and that reduction of AMH levels is the earliest marker that can be documented in women who will later develop POI.14
Chromosomal aberrations and POI
Turner Syndrome (45,X) is the prototypical condition of chromosomal aberration associated with POI. In some cases, lack of pubertal development and associated primary amenorrhea are accompanied by the full clinical picture of this syndrome with short stature, mental retardation and body and organ malformations. The 45,X karyotype is present in approximately half of women with Turner Syndrome, while other patients have one of several potential mosaicisms, such as 45,X/46,XX.
In women with Turner Syndrome, oocyte apoptosis is accelerated from the second trimester of fetal life, which leads to oocyte depletion in most cases prior to the age of expected puberty.15, 16 Follicle depletion and the inability to produce an adequate amount of estrogen at the time of puberty are responsible for the primary amenorrhea without signs of pubertal development in most patients. In cases of milder mosaicism, women often menstruate. Therefore a diagnosis of POI may be formulated later in life. This is particularly true if normal stature is present and evident malformations are absent. Women who fall into this category of having menarche and menstruation account for approximately 10% of women with Turner syndrome. Turner syndrome in women of short stature has been attributed to mutations in the SHOX gene on Xp,17 however, it is unclear which gene(s) is (are) responsible for oocyte depletion and ovarian insufficiency in women with the 45,X chromosomal aberration.
Turner syndrome sometimes will require differential diagnosis with other disorders of sexual development (DSD) that present with primary amenorrhea and increased gonadotrophins. Complete gonadal dysgenesis (46,XX), for example, can occur with normal stature and failure of sexual maturation. Swyer syndrome (46,XY) characterized by a female phenotype with normal or increased height, normal Mullerian structures and failure of sexual maturation is another example. Since chromosomal aberrations are a known cause of POI, analysis of karyotype in at least 30 metaphases should be offered to all women with this diagnosis.
Autosome translocations and POI
Autosome translocations of X chromosomes are among the known genetic causes of POI. The study of patients with deletions and breakpoints in translocated X chromosomes allowed the identification of an important region for ovarian function, comprised between Xq13 and Xq26, with the exception of Xq22.18 One gene (diaphanous, DIAPH2) was identified at a breakpoint of an X chromosome.19 Another candidate gene is FSH primary response homolog 1 (FSHPRH1), but it has not yet been identified in a woman with POI. XPNPEP2 and DACH2 have been identified at breakpoints within the X chromosome, but have no known role in ovarian development or function.20
FMR1 premutation
Fragile X Syndrome is the most frequent cause of inherited male mental retardation, often associated to autism. From a genetic point of view, it is an X-linked, triplet repeat disease. In the normal condition, the FMR1 gene contains less than 40 CGG repeats. When the number of triplet repeats is higher than 200 the gene is silenced by methylation. In affected males, the disorder is made evident when the number of CGG repeats in the untranslated region of the FMR1 gene is higher than 200.21, 22
A situation in which the repeat length is comprised between 55 and 200 is defined as a “premutation”.23 A woman carrying the premutation may give birth to a male child affected by the full syndrome, because expansion of the triplet repeat number may occur during transmission to the fetus.
Although, initially considered solely an asymptomatic carrier condition, the premutation has been shown to be associated with a specific phenotype in women. Approximately 13 to 26% of women with the Fragile X premutation develop POI and an inverse correlation between number of repeats and age at menopause has been found.24 In addition, women with regular menses who are carrying the premutation tend to have higher levels of FSH and lower levels of inhibin B serum concentrations as compared to women with the normal number of repeats.25
The mechanism for the development of POI related to the presence of an FMR1 premutation is thought to differ from the mechanism responsible for the development of Fragile X Syndrome in males who carry a full mutation. The full FMR1 mutation causes a failure to produce normal levels of the protein FMRP, which is thought to be the mechanism causing Fragile X Syndrome. Women carrying a full mutation have a normal reproductive life.26 It has been suggested that the premutation is responsible for accumulation of mRNA, in the presence of normal FMRP protein quantity. The accumulation of FMR1 mRNA is thought to be responsible for gain-of-function toxicity in males carrying the premutation and puts them at risk of developing a neurodegenerative disorder, the so-called Fragile X-associated tremor/ataxia Syndrome (FXTAS). This is characterized by ataxia, tremor, dementia and Parkinson-like symptoms.27
Premutations of the FMR1 gene are responsible for approximately 1–7.5% of sporadic and 13% of familial cases of POI.28 Accordingly, the American College of Obstetrics and Gynecology recommends offering molecular analysis of the FMR1 gene to all women with POI.28, 29
In addition to the FMR1 premutations, small deletions of the FMR2 gene, located at Xq28, are more frequent in women with POI, than in the general population of fertile women.30
Other genes associated with POI
In its complexity, POI should be regarded as a syndrome in which several causes, and possibly several distinct pathogenic mechanisms, may lead to a similar clinical presentation.31 Still, the majority of clinical cases of POI remain idiopathic. Heterozygous missense mutations of the BMP15 gene have been found in a few women with POI.32, 33 The putative role of this gene in ovarian development has been indirectly confirmed by studies in the sheep. Homozygous mutations of this gene in sheep are responsible for ovarian insufficiency.34
Initial studies suggested that also the inhibin a gene would be implicated in the pathogenesis of POI.35 However, a more recent, large, multi-center Italian study has not confirmed this initial finding.36
Postnatal follicle destruction and defective folliculogenesis, which lead to an ovarian histology characterized by rare, immature follicles and fibrous stroma in post-pubertal women is typically observed in a patient affected by galactosemia, which results from mutations of the galactose 1-phosphate uridyl transferase (GALT) gene. Through unclear mechanisms, the accumulation of galactose metabolites in multiple cell types is thought to be responsible for cell damage which leads to mental retardation, liver failure, renal insufficiency, and, in women, primary or secondary amenorrhea due to ovarian insufficiency. A mild mutation of the GALT gene, termed Duarte allele (with approximately 50% of enzyme activity), present in around 8–13% of the general population, is responsible for increased risk for infertility and earlier menopause.37 Nonetheless, only homozygous GALT mutations appear to be associated with POI, while heterozygous mutations or the Duarte allele have not been found associated with ovarian insufficiency before the age of 40 years.38
Mutations in the FOXL2 gene on chromosome 3 is also associated with POI as a part of the blepharophimosis, ptosis, epicantus inversus syndrome (BPES).39 The translation product of FOXL2, a transcription factor, is expressed in granulosa cells in which transcriptional targets are steroidogenic enzymes.
Defects of gonadotrophin receptor and steroidogenic enzymes can cause failure of follicle growth and steroidogenesis. Mutations of the FSH receptor cause primary amenorrhea associated with small ovaries.40 Mutations of the LH receptor have been found in some POI cohorts.41 The clinical picture associated with mutations of the LH receptor is similar to that observed with mutations of the FSH receptor (primary amenorrhea, hypoestrogenism and increased gonadotrophin levels), but is also characterized by enlarged, cystic ovaries due to the continuous FSH stimulation.
Mutations in the steroid acute regulatory (Star) protein cause both primary adrenal insufficiency and POI because of a virtual absence of steroidogenesis. Aromatase mutations can also cause severe hypoestrogenism and primary amenorrhea.
Other disorders that are associated with POI include: 1) ataxia teleangectasia, due to mutations in ataxia telangiectasia mutated (ATM), a protein kinase; 2) Bloom Syndrome (BLM), resulting from mutations in RecQ protein-like-3, a DNA helicase; 3) mutations in the eukaryotic translation initiation factor 2B (EIF2B) responsible for neurologic deficits and 4) progressive external ophthalmoplegia, a mitochondrial disorder.
Iatrogenic causes of POI
Among iatrogenic causes of POI are bilateral oophorectomy, chemotherapy and radiotherapy. Early menopause has also been documented in women who underwent unilateral oophorectomy 42 and also in women surgically treated for endometriosis. The extent of the ovarian tissue damage (or removal) consequent to the surgical intervention appears to be the critical factor in predicting POI and early menopause.
Both ovarian exposure to radiation and chemotherapy can cause ovarian insufficiency.43 Among factors predicting the future development of POI is a diagnosis of Hodgkin’s lymphoma, chemotherapy with alkylating agents and age over 12 years at a cancer diagnosis. Combination therapy with radiation and alkylating agents in children is associated with a 30% risk for POI. This risk significantly increases if the treatment is performed in adult women and is almost 100% in women undergoing preparation for bone marrow transplantation.44
Some studies have shown a potential benefit from GnRH agonist therapy before the initiation of chemotherapy followed by estrogen/progestin treatment. This approach, though not yet substantiated by large placebo-controlled trials, represents a potential promise for oocyte preservation in candidates for chemotherapy particularly with alkylating agents.45, 46
Ovarian hyperstimulation and oocyte or embryo cryopreservation prior to therapy is possible in some cases. Ovarian tissue cryopreservation may also preserve the potential for future pregnancies to women who undergo chemotherapy and/or radiotherapy.
Autoimmune POI
Although some studies have proposed that an autoimmune mechanism may be responsible for up to 30% of cases of POI,47, 48 a more accurate estimate indicates that a documented ovarian autoimmune process causing POI can be found in not more than 4–5% of affected women.48–50
In regard to animal models of autoimmune ovarian insufficiency, the Autoimmune regulator gene (AIRE) knockout mouse develops autoimmune oophoritis.51 Hence, it appears that AIRE, when functioning normally, prevents autoimmunity. Thymectomy in 3-day old mice has proven instrumental to unravel the critical role of CD4+CD25+ regulatory T (Treg) cells 52 in suppressing autoimmune processes (among which oophoritis) in regional lymph nodes. This suppression appears to be under a continuous stimulation by autologous antigens 53 which provides a sound rationale for the planning of clinical trials aimed at modulating autoimmune processes by administering recombinant autoantigens with or without immunological adjuvants. One ovarian autoantigen identified in the animal model of neonatal thymectomy is the ooplasm protein termed MATER (Maternal Antigen That Embryos Require).54 Of interest, women with Autoimmune Polyglandular Syndrome Type 1 (APS1) who also have POI are significantly more likely to have antibodies against MATER (NALP5) than those women without POI.55 This raises the possibility that autoimmunity against MATER plays a role in some cases of human autoimmune POI. More research in this area is indicated because this represents dual evidence of biological plausibility, from human experience and from mouse experiments.
Immunization of mice with a peptide of inhibin alpha chain was also attempted in order to develop an experimental model of autoimmune POI.56 This strategy induces an initial increase in fertility, which is mediated by high serum levels of inhibin alpha neutralizing antibodies, that prevents inhibin-mediated down-regulation of activin-induced FSH release.56 In a second, delayed phase, activation of CD4+ T-cells results in a lymphocytic infiltration of the ovary that occurs in parallel with a progressive decrease in fertility and ovarian function.56
Some autoimmune diseases are more frequent in women with POI than in the general population, and, conversely, POI occurs more frequently in women affected by some autoimmune diseases than in other women. There is a clear association between POI and Autoimmune Addison’s Disease (AAD).49, 50, 57–59 Approximately 4–8% of women with POI are positive for circulating adrenal autoantibodies, a frequency significantly higher than that expected in the general population (<0.5%).50, 57–59 On the other hand, 10–20% of women with AAD develop POI before the age of 40 years.58, 59 The Italian Addison Network has collected, from continental Italy, clinical data and biological samples from over 400 patients with Addison’s disease of various etiologies. Of the over 200 women with AAD enrolled, 21% had clinical and biochemical signs of POI (Falorni A et al., unpublished data). In APS type 1, the risk for POI is especially high, reaching 50–70% of women in some studies.60, 61 Accordingly, the strong association of POI with APS type 1, a monogenic disorder characterized by the association of AAD, autoimmune hypoparathyroidism and chronic candidiasis, and autoimmune polyglandular syndrome type 2, a polygenic syndrome characterized by AAD and other autoimmune disorders in the absence of hypoparathyroidism, supports the hypothesis that some cases of POI indeed have an autoimmune origin.
However, the association with autoimmunity may not be as clear in other autoimmune conditions. For example, approximately 15% of women with POI present with autoimmune thyroiditis 48, 58 and the frequency of POI is higher in women with T1DM than in the general population.48 Biochemical and/or ultrasound signs of thyroiditis can however be detected in 10–15% women in the general population and the frequency of thyroiditis in women with POI is only slightly higher than expected by random association. In addition, POI has sporadically been found associated with other organ- or non-organ-specific autoimmune diseases, but the possibility of a random association cannot be excluded.48
The presence of circulating ovarian autoantibodies in women with ovarian insufficiency was first demonstrated in studies published in the 1960’s. These studies used indirect immunofluorescence on cryostatic sections of adrenal, ovary, testis and placenta. 62, 63 Other studies have subsequently repeatedly confirmed the existence of ovarian autoantibodies in a small portion of women with POI.64–67 Ovarian autoantibodies detected by indirect immunofluorescence cross-react with antigens expressed in other tissues, mainly the adrenal cortex, the testis and the placenta, which indicates that the autoantigen(s) recognized by these autoantibodies are not restricted to the ovary, but expressed also in other endocrine organs. Because of this feature, the correct definition of these autoantibodies is that of steroid-cell autoantibodies (StCA). Interestingly, although all the tissue components are present in the ovarian cryostatic sections used for the autoantibody assay, the immunofluorescence pattern of StCA is restricted to the theca cells of the growing follicle with no staining of primary follicles or granulosa cells in secondary and tertiary follicles. The same autoantibodies specifically stain Leydig cells of the testis. Accordingly, from the immunofluorescence pattern it can be predicted that the target(s) of StCA is (are) expressed by androgen-producing cells.
Indirect immunofluorescence is still the most widely used method to detect ovarian autoantibodies in human serum, also in clinical applications. However, the accuracy of this method has been questioned, mostly because of low diagnostic specificity.68 The search for the molecular targets of StCA has provided two autoantigens: the steroidogenic enzymes 17a-hydroxylase (17OH) and side-chain cleavage (P450scc).59, 62, 69, 70 The development of immunoradiometric assays, using in vitro translated recombinant human 35S-radiolabelled autoantigens, have enabled the estimate of the diagnostic sensitivity and specificity of 17OH antibody (Ab) and P450sccAb for autoimmune POI.59, 62, 69, 70 Each of the two main steroidogenic enzyme autoantibodies (17OHAb and P450sccAb) can be detected in 50–80% of women positive for StCA.59, 62, 69, 70 Altogether, over 90% of women who are StCA-positive are also positive for 17OHAb and/or P450sccAb, which is demonstrating that 17OH and P450scc are major targets of StCA, but other, yet unidentified autoantigens may also be recognized by a subset of StCA.
The presence of StCA, 17OHAb and/or P450sccAb can be detected almost exclusively in women with POI who are also positive for adrenal autoantibodies, and more specifically 21-hydroxylase autoantibodies (21OHAb), the major immune marker of AAD. The association of steroid-cell autoimmunity with adrenal autoimmunity is so strong that 21OHAb appear to be the marker at highest diagnostic sensitivity for autoimmune POI,49, 50, 69, 70 even though 21OH is selectively expressed by the adrenal cortex. In the absence of 21OHAb, less than 0.5% of women with POI can be found positive for one of the three markers; StCA, 17OHAb or P450sccAb. Hence, unequivocal biochemical signs of ovarian autoimmunity against steroidogenic enzymes are present almost exclusively in women with clinical or pre-clinical AAD. Since no commercial kit is currently available for the determination of 17OHAb or P450sccAb, 21OHAb is, at present, the gold standard immune marker not only for AAD, but also for autoimmune POI in women with hypergonadotropic hypogonadism. In the attempt of standardizing this immunoassay among different laboratories an international serum exchange study was performed that showed a high concordance in positive/negative score among four independent laboratories.71 More recently, the EU Consortium Euradrenal has completed a standardization program in which 14 independent laboratories have blindly analysed serum samples from AAD patients or healthy control subjects. The study, which is not yet published, confirmed a high concordance among laboratories that used assays based on immunoprecipitation of in vitro translated recombinant human 21OH, but revealed discrepancies in autoantibody levels. This demonstrated that results from different laboratories could not be used interchangeably (Falorni A, unpublished data).
Steroidogenic enzyme autoantibodies (21OHAb, 17OHAb and P450sccAb) detected in women with AAD and autoimmune POI are predominantly of the IgG1 subclass, with infrequent occurrence of IgG2 and IgG4 subtypes.72 This finding, similar to what is observed in other endocrine autoimmune diseases, is indirectly supporting a Th1/Th2 imbalance as the basis of human autoimmune oophoritis.
It has been proposed that the steroidogenic enzyme 3b-hydroxysteroid-dehydrogenase (3bHSD) would be a target of autoantibodies in women with clinically idiopathic POI.73 Subsequent studies, that have used sensitive and specific radio-binding assays, have however been unable to confirm this finding and, at present, 3bHSD is not considered an autoantigen associated with autoimmune POI.70, 74
An autoimmune oophoritis can be found at ovarian biopsy almost exclusively in women positive for adrenal autoantibodies.48, 49, 63, 75–78 Since steroidogenic cell autoantibodies accurately identify women with an ongoing ovarian autoimmune process, there is no indication, at present, for performing an ovarian biopsy for the sole diagnostic purpose of confirming an ovarian autoimmunity. In the absence of ovarian and adrenal autoantibodies, no histological sign of autoimmune infiltration can generally be detected at ovarian biopsy, even in women who present with other autoimmune diseases, such as thyroiditis, T1DM, inflammatory bowel disease or systemic lupus erythematosus,79, 80 which is indicating that a classification of autoimmune POI cannot be based only on the presence of other autoimmune manifestations, in the absence of specific autoantibodies in the serum.
Although the presence of autoantibodies against steroidogenic enzymes may accurately identify women with steroid-cell autoimmune ovarian insufficiency (SCA-POI), their absence does not exclude the possibility that other autoantibodies may be present. A long series of other potential autoantigens, such as MATER(NALP5),55 LH receptor,81 FSH receptor, 82 zona pellucida,83 82–86 kDa Ags and 52–63 kDa Ags 84 have been proposed as markers of ovarian autoimmunity in some studies. However, other studies have not confirmed these associations,85, 86 and none of these putative markers are currently used in clinical practice. No reliable immuno-radiometric assays are currently available for these markers.
In summary, only detection of autoantibodies against steroidogenic enzymes (more specifically, from a clinical point of view, of 21OHAb) can ensure, at present, an accurate identification of women with autoimmune oophoritis. Using steroidogenic enzyme autoantibodies as the marker, SCA-POI accounts for approximately 4–5% of all POI cases.
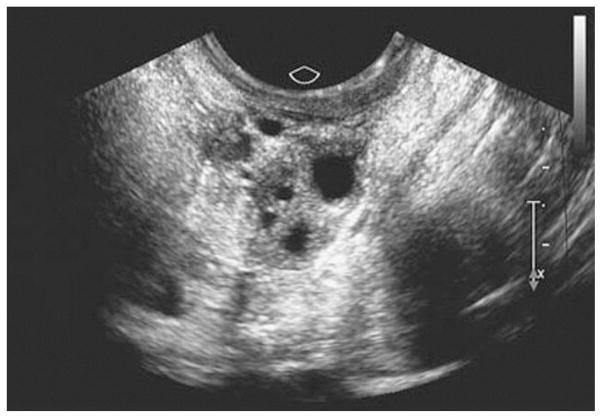
Along with the decline in production of estradiol and the increase in the secretion of gonadotrophins, POI is normally associated with reduction in synthesis and secretion of inhibins 87 and AMH,88 as a result of the progressive decline in ovarian function. Increased concentrations of inhibin B, however, were detected in the serum from three women with SCA-POI.89 In the same study,89 it was also shown that SCA-POI was associated with reduced secretion of androstenedione and estrone. These initial findings on inhibin concentrations in autoimmune oophoritis were subsequently confirmed in a larger study 90 that demonstrated increased levels of serum inhibin B and total inhibin in a group of 22 women with SCA-POI, as compared to 71 women with idiopathic, non-autoimmune POI and 90 healthy fertile women. Indeed, not all cases of POI are due to follicle depletion. Transvaginal ultrasound scans from patients with spontaneous 46,XX POI who have follicle dysfunction due to autoimmune oophoritis may present with multifollicular development (Figure 1).12, 89 As human autoimmune oophoritis is characterized by a selective mononuclear cell infiltration into the theca layer of large, antral follicles, with earlier stage follicles consistently free of lymphocytic infiltration (Figure 2),48,49 the results of the studies on inhibin concentrations 89,90 led to the formulation of a novel hypothesis of the pathophysiology of SCA-POI (Figure 3).
Figure 1.
Transvaginal ultrasound scan from a patient with spontaneous 46,XX primary ovarian insufficiency who had follicle dysfunction due to autoimmune oophoritis. Many developing follicles are visible [From Nelson LM].12
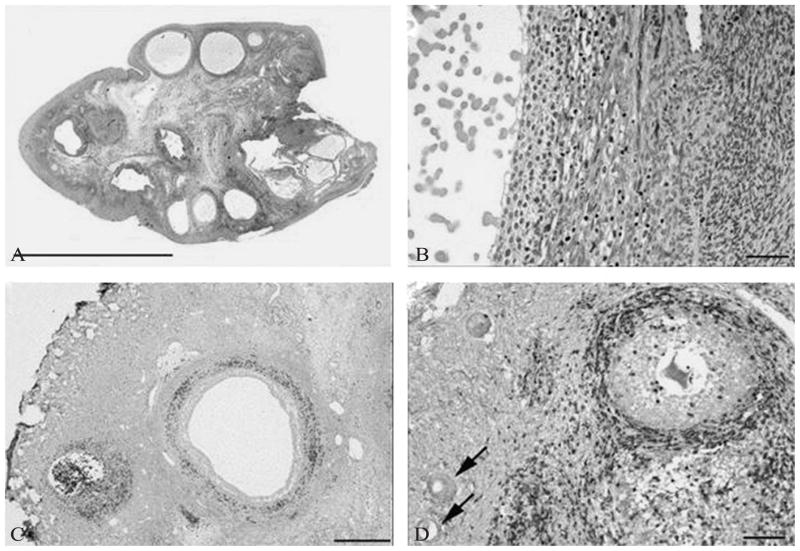
Figure 2.
Ovarian histology in a biopsy proven case of autoimmune oophoritis. Hematoxylin and eosin staining shows multiple antral follicles in patient 1 (A), bar = 1 cm. Higher magnification shows lymphocytic infiltration of the theca of an antral follicle and luteinized granulosa cells (B), bar = 50 μm. Immunoperoxidase staining for CD3 highlights infiltration of lymphocytes into the theca in this patient (C), bar = 500 μm. Immunoperoxidase staining for CD3 demonstrates infiltration of lymphocytes into the theca of a preantral follicle in patient 4 and presence of earlier stage follicles free of lymphocytic infiltration (arrows) (D), bar = 100 μm. [From Bakalov VK et al.].49
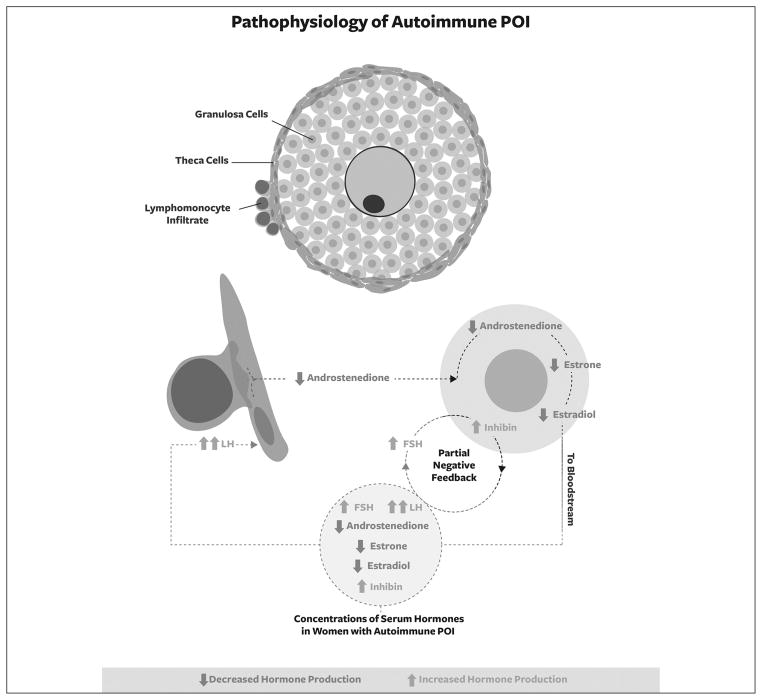
Figure 3.
Schematic representation of pathophysiology of SCA-POI. The selective autoimmune destruction of theca cells with preservation of granulosa cells is responsible for a reduced production of oestradiol because of lack of substrates. The subsequent increase in FSH levels would stimulate viable granulosa cells that, in return, would produce increased amounts of inhibins.
A general, though fluctuating and incomplete, reduction of ovarian function can be observed in women with POI, but SCA-POI is characterized by the selective autoimmune destruction of theca cells with preservation of granulosa cells. In this clinical scenario the granulosa cells produce low amounts of oestradiol because of lack of the theca-dependent substrate androstenedione, a product of the theca. The subsequent increase in FSH levels resulting from the low serum estradiol levels would stimulate viable granulosa cells that, in return, would produce increased amounts of inhibins (Figure 3).
AMH is exclusively produced by the granulosa cells of primary and pre-antral/small antral follicles. Because of the immunofluorescence and immunohistochemical findings of the absence of an inflammatory reaction around primary follicles in autoimmune oophoritis 48, 49 one would expect normal AMH production in women with SCA-POI, as compared to women with idiopathic non-autoimmune POI.91 Indeed, normal serum AMH concentrations were detected in two-thirds of women with recently diagnosed SCA-POI,89 which provides the first demonstration of the existence of a subgroup of women with POI with an ovarian follicle pool preserved for several years. In a more recent study,92 11 women with SCA-POI and AMH levels within the normal range at the time of the first sampling were followed longitudinally. A progressive decline in AMH concentration was observed in all the 11 women who were AMH-positive at an estimated average rate of 57% of serum AMH level/previous year. After 6 years of disease duration, only one woman with SCA-POI still had detectable levels of AMH.
In summary, it appears of utmost importance to perform a correct subclassification of patients with POI and to identify women with SCA-POI. Patients with SCA-POI have (or are at high risk to develop) clinical signs of AAD, a potentially fatal disorder. In the context of women with APS type 1 or APS type 2, they also have an increased risk to develop other endocrine and non-endocrine autoimmune diseases. The unique pathophysiology characterized by a selective destruction of theca cells, and preservation of granulosa cells and pre-antral follicles (at least for a few years after clinical diagnosis of SCA-POI) paves the way for the possibility to perform future clinical trials of immunomodulation aimed at preserving the residual follicle pool and restoring ovulatory function. Since autoimmune ophoritis has a distinct pathophysiology as compared to the other forms of POI, there is a sound rationale at attempting to preserve residual follicle activity in women positive for steroidogenic enzyme autoantibodies. Some publications have reported the effect of glucocorticoid treatment in women with POI but not in all cases with a documented autoimmune oophoritis.93–95 Several limitations are present in these studies including the lack of randomisation, the small number of enrolled patients, the lack of a correct subclassification with sound immune markers of SCA-POI and last, but not least, the presence of relevant side effects not balanced by a documented positive effect of the treatment on fertility. Glucocorticoid treatment of women with SCA-POI must still be regarded as highly experimental and such patients must receive this type of treatment only in the setting of placebo-controlled trials. Research in autoimmune oophoritis, because it is such an extremely rare condition, would especially benefit by the creation of an international Clinical Research Integration Special Program (CRISP) on POI.
Management of POI
Overview
Patients with chronic illnesses frequently experience a health care system in which care is poorly coordinated.96 The defining features of primary care provide the major elements needed to care effectively for patients with chronic disease. POI is a serious chronic disorder. Primary care provides a principal point of consultation for patients within a health care system and a principal point for coordination and integration of the specialized care some patients require. Providing comprehensive continuity of care, preventative care, and health education are important elements of primary care. By its nature, primary care is collaborative care. Modest increases in care continuity are associated with sizable reductions in costs, use of services, and complications.97
The patient-centered medical home describes mechanisms for organizing primary care to provide high quality across the full range of an individual’s health care needs.98 It also provides a single centralized source of team-based care and medical record for those with special health care needs. Other characteristics of the medical home include: patient-centered orientation toward the whole person; care that is coordinated across all elements of the health care system and the patient’s community; enhanced access to care that uses alternative methods of communication; and a systems-based approach to quality and safety.99, 100
There is need to develop a self-sufficient and sustainable research and patient care program for women with POI. The program should be situated in a community-based and integrated primary care model.101 The plan is to develop this program under the central governance of a non-profit organization such as defined by US statutes as a 501c3 organization. Such a centralized program would provide these women with a mobile health (mHealth) medical home, evidence based care, referral, and support in their own community. This would be a paradigm shift in care for most of these women. The approach has been described as the development of a Public Health Knowledge Network with Distribution System. 102 The system would be maintained by a centralized community of practice that provides cutting edge diverse expertise and research on POI.
Making the diagnosis
In order to make the diagnosis in a timely manner the clinician must carefully consider complaints of oligo/amenorrhea and menstrual irregularity in general. Too often clinicians ignore this important historical evidence. Published evidence has shown that over 50% of women with POI see three or more clinicians for oligo/amenorrhea before the symptom is taken seriously and properly evaluated to make the diagnosis.103 The presenting menstrual complaint can vary from gradual development of oligo/amenorrhea, to menometrorrhaghia from anovulatory bleeding, to acute onset of complete amenorrhea. Sometimes the onset of amenorrhea heralding POI will follow childbirth or the discontinuation of hormonal contraception. Occasionally patients present with the complaint of vasomoter symptoms before the onset of menstrual irregularity.103 Regardless of clinical presentation, a complete history and physical exam is required, in addition to laboratory evaluation, when appropriate, to rule out other causes of oligoamenorrhea. The American Society for Reproductive Medicine has published guidelines for the evaluation of amenorrhea.104
Of course pregnancy is the most common cause of amenorrhea, so this must be ruled out in patients presenting with this complaint. Then there is a need to assess the patient’s overall health. On occasion amenorrhea can be the presenting symptom of an underlying chronic disease. One should not attribute amenorrhea to underlying emotional distress without further evaluation. Moreover, women with POI are known to experience intermittent and unpredictable return of ovarian function even after the diagnosis is secured. Therefore, the most practical clinical definition to establish a diagnosis is 4 or more months of “disordered” menses in association with two menopausal serum FSH levels drawn at least one month apart. This could be polymenorrhea, oligomenorrea, metrorrhagia or amenorrhea.12
Defining the mechanism
Once the diagnosis of POI has been secured by confirming the presence of a second menopausal serum FSH level one month later, the clinician must attempt to define the pathophysiologic mechanism of the disorder. The recommended laboratory tests include a karyotype analysis with counting 30 cells, testing for an FMR1 premutation, and for adrenal autoantibodies by 21-hydroxylase [CYP21] immunoprecipitation assay.12, 105 Ovarian antibodies have an extraordinarily high false positive rate and their measurement is not clinically indicated.68
Baseline studies
A pelvic ultrasonography is indicated to identify cases of POI that present with enlarged multifollicular ovaries that are at risk of acute torsion, such as isolated 17,20-lyase deficiency, aromatase deficiency, or autoimmune oophoritis. In addition, hypogonadism is a major risk factor for osteoporosis. Therefore, measurement of a baseline bone mineral density is indicated in women with newly diagnosed POI along with a Vitamin D level.12 Ovarian biopsy is not indicated in the clinical evaluation of POI. Pregnancies have been reported even after ovarian biopsy had purported an absence of primordial follicles. 106
Providing integrated care
The most common words women use to describe how they feel after getting the diagnosis of POI are “devastated,” “shocked,” and “confused”.107 POI is a life-altering diagnosis that affects all aspects of a woman’s life: physical, emotional, and spiritual. Living with a chronic condition is complicated and involves multiple challenges, such as coping with medical crises, managing treatment regimens, controlling symptoms, organizing one’s time efficiently, preventing social isolation, adjusting to changes in the condition, and normalizing interactions and life despite the disease.108 As compared to healthy controls, women with POI have increased shyness and social anxiety, impaired self-esteem 109 a perceived lower level of social support,110 and increased symptoms of anxiety and depression.111 Many women with POI experience severe emotional distress at the time of the diagnosis.107
Most women find this a difficult diagnosis to accept. One controlled study demonstrated that simple statements of concern by a clinician that take very little time can go a long way in helping women see their clinician as more caring, sensitive and compassionate. 112 Informing women on the diagnosis of POI is best approached as recommended by Buchman.113 This is best done in person during an unhurried office visit. If the patient becomes emotional, it is important to validate her feelings, inquire about what sources of emotional support the patient has available, suggest additional avenues of support if appropriate, and schedule a return office visit for further discussion.107
Women with POI have a pathologic condition which involves low serum estradiol levels as compared with other women of similar age. Early cessation of ovarian function has been associated with an increased incidence of fractures 114 and increased total mortality due to ischemic heart disease.115–117 Women with POI as a group have significantly reduced bone density compared to controls.118 Fortunately, a recent NIH controlled trial has demonstrated that when young women with POI are given an appropriate hormone replacement of transdermal estradiol and an oral progestin, over the ensuing three years their bone density returns to normal 119. Most experts agree that physiologic estrogen and progestin replacement is reasonable in the case of young women with POI and should be continued until they reach the age when menopause usually occurs 11.
Published evidence has demonstrated that several modifiable risk factors significantly contribute to the reduced bone mineral density in women with POI.118 These risk factors include more than one year delay in diagnosis, low plasma concentrations of vitamin D, estrogen therapy non-adherence, low calcium intake, and lack of exercise. Women with POI should be encouraged to take at least 800 IU of vitamin D per day (not to exceed 4,000 UI per day),120 1200 mg of elemental calcium per day,121 and engage in a variety of exercises such as jogging, stair climbing or walking.122 Since pregnancy is possible in women with POI, bisphosphonates are not advised because these agents have long skeletal half-lives and fetal effects are possible.123
Physiologic estradiol replacement with transdermal patch or transvaginal ring is the preferred approach. 12 The average serum estradiol level during the menstrual cycle in women with a normal menstrual history is approximately 100 pg per milliliter. 124 A dose of 100 μg of estradiol per day, administered by transdermal patch or transvaginal ring, achieves average serum estradiol levels in this range and effectively treats symptoms.125 Transdermal and transvaginal estradiol have little effect on hemostatic factors. In case-control studies the patch has been associated with a lower risk of venous thromboembolism than has oral estrogen.126–128 The best evidence supports the use of cyclic medroxyprogesterone acetate at a dose of 10 mg per day for 12 days each month as the preferred progestin. This regimen fully induces secretory endometrium and provides protection against endometrial cancer.129, 130 This was the hormone replacement regimen that restored bone mineral density for women with POI in the NIH study mentioned previously.119
In contrast to medroxyprogesterone acetate, data regarding the effects on the endometrium of oral micronized progesterone when it is given in conjunction with a full replacement dose of estrogen are not available.131 Patients should keep a menstrual calendar and take a pregnancy test if a menstrual period is late. Pregnancy may occur while a woman is taking estrogen and progestin therapy, and the therapy should be stopped if the pregnancy test is found to be positive. Oral contraceptives provide more steroid hormone than is needed for physiologic replacement and are therefore not recommended as first-line management.
As with many chronic diseases, women with POI are at risk of developing associated disorders. This is a reason why these patients are particularly suited for care in a patient-centered medical home. As mentioned, women with autoimmune oophoritis as the mechanism of POI are at increased risk of developing adrenal insufficiency, a potentially fatal disorder. Women with POI are also at increased risk for developing dry-eye syndrome,132 autoimmune thyroid disease and other autoimmune disorders such as myasthenia gravis, rheumatoid arthritis, and systemic lupus erythematosus.48 It is reasonable to measure thyrotropin and test for the presence of thyroid peroxidase antibodies upon the diagnosis of POI. However, association of POI with the other autoimmune disorders mentioned is infrequent and testing for these and other autoimmune conditions should be based on symptoms and signs suggestive of the condition.12
Women with POI who wish to avoid pregnancy should use a barrier method or possibly an intrauterine device. Hormonal contraceptive methods may not be effective in these women because of their high gonadotropin levels, which may not suppress adequately enough to have a contraceptive effect.12 Overall, pregnancies have been reported in 5–10% of women with POI.10 Nevertheless, women with POI must be alerted that pregnancy is uncommon, though possible. Women with POI who seek to start a family need also to be informed that alternative approaches are adoption and oocyte donation, as therapeutic approaches do not significantly improve fertility in their case. In some cases couples choose to create a “family of two” and focus on issues in their life other than raising children.133
An eye to the future
Evidence supports a role for a collaborative care model as a method to improve medical outcomes and reduce costs, and in a way that clinicians find satisfying. 134–136 This approach should be combined with the creation of a public health knowledge network on POI.137, 138 This knowledge network would form the central component of an international community of practice for the condition.139, 140 The community of practice provides a construct in which stakeholders, despite limited resources, can work collaboratively to tackle complex, multidimensional, and hierarchical problems.
The advent of large-scale genomic sequencing provides the stimulus to combine efforts in patient care with ongoing research efforts. Combining this with exciting capabilities related to Mobile health (mHealth) will dramatically change the face of future health care and health research.141–143 This synergy would be especially beneficial in rare disorders and could create a seamless community of practice where research and personal health care coexist. Such an entity would be best governed by a board of directors that keeps the patient’s interests at the heart of the enterprise. The nidus for such a community of practice for POI came together in an event organized by the National Institutes of Health (USA).101, 102, 140 Rachel’s Well, a not for profit entity, has taken a lead in providing a vision and a home for this effort. The long range goal is to integrate the powerful potential of large-scale medical sequencing and mHealth into a centralized and self-sustaining research and patient care enterprise.102 This will be known as a Clinical Research Integration Special Program (CRISP) on POI. The model can serve as a paradigm for all rare disorders.
Conclusions
A clinician who makes a new diagnosis of POI in a young woman or girl becomes the central figure in a highly emotionally charged vignette. The diagnosis should be discussed with the patient at a return visit to the office rather than by telephone. In the case of a minor, in most cases one should inform the parents first and employ a family systems approach to managing the disorder.144, 145 Adequate attention to emotional health is critical. It is important to stress that, although unlikely, spontaneous pregnancy occurs in 5 to 10% of cases. The tests indicated at the time of diagnosis are a karyotype that counts 30 cells, tests for the FMR1 premutation and adrenal autoimmunity as evidenced by the presence of 21-hyrdoxylase autoantibodies, a pelvic ultrasound evaluation, and measurement of bone mineral density. It is critical to make a diagnosis of autoimmune POI, because of its strong association with clinical or pre-clinical adrenal insufficiency that requires life-long substitutive therapy with gluco- and mineralcorticoids.146, 147 It is also important that patients with POI maintain a healthy lifestyle to optimize bone and cardiovascular health. This would include regular weight-bearing exercise, an adequate daily intake of calcium (1200 mg) and vitamin D (at least 800 IU), avoiding obesity by eating a healthy diet, and undergoing screening for cardiovascular risk factors and adhering to strategies to mitigate these as indicated. Evidence supports the use of 100 micrograms of transdermal or transvaginal estradiol and 10 milligrams of oral medroxyprogesterone acetate daily for the first 12 days of each month as a means to maintain, and even restore bone density in young women living with POI. Women should keep a menstrual calendar and in the case of late menses promptly have a pregnancy test performed.
The creation of a CRISP for POI requires the establishment of four interlacing domains. First, the needs of the end users, the patients and their clinicians, must be carefully assessed and documented. Second, there must be a strategy for developing and maintaining the required rigorous content and protocols for the management of the disorder and its associated sequelae. Third, there must be a knowledge distribution system composed of state of the art information storage and communication capabilities that function in real time and meet security standards for patient care and mHealth. Finally, a community of practice to provide the diverse expertise is required to meet even the most unusual needs of women with POI. This must be organized and established to be self-sustaining and durable. The POI CRISP could be organized under the auspices of Rachel’s Well, a not for profit entity organized by women with POI and dedicated to their cause.101, 102
Acknowledgments
Funding.—The associated research activity has been supported by EU FP7, Grant number 201167, Euradrenal and Fondazione Cassa di Risparmio di Perugia (to AF), a grant from the Mary Elizabeth Conover Foundation (to CME), Telethon Foundation, Grant GGP09126A (to LP), Italy, and by the Intramural Research Program of the Eunice Kennedy Schriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, USA. We thank Peter and Cindy Catches of Oceti Wakan (http://www.ocetiwakan.org) for valuable discussions and Mark Ferguson for valuable help in graphic design.
References
- 1.Collins FS, Hamburg MA. First FDA authorization for next-generation sequencer. N Engl J Med. 2013;369:2369–71. doi: 10.1056/NEJMp1314561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green RC, Berg JS, Grody WW, Kalia SS, Korf BR, Martin CL, et al. American College of Medical Genetics and Genomics. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013;15:565–74. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Faddy MJ. Follicle dynamics during ovarian ageing. Molecular and Cellular Endocrinology. 2000;163:43–8. doi: 10.1016/s0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 4.Albright F, Smith PH, Fraser R. A syndrome characterized by primary ovarian insufficiency and decreased stature. American Journal of the Medical Sciences. 1942;204:625–48. [Google Scholar]
- 5.Conway S. 2002 Primary ovarian failure. In: Wass AH, Shalet SM, editors. Endocrinology and Diabetes. Oxford University Press; Oxford, UK: 2002. pp. 1107–13. [Google Scholar]
- 6.Rebar RW. Premature ovarian failure. Obstet Gynecol. 2009;113:1355–63. doi: 10.1097/AOG.0b013e3181a66843. [DOI] [PubMed] [Google Scholar]
- 7.Rees M, Purdie D. Premature menopause. In: Rees M, Purdie D, editors. Management of the menopause: the handbook. 4. London: Royal Society of Medicine Press Ltd; 2006. pp. 141–9. [Google Scholar]
- 8.Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol. 1986;67:604–6. [PubMed] [Google Scholar]
- 9.Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, et al. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79:1470–5. doi: 10.1210/jcem.79.5.7962345. [DOI] [PubMed] [Google Scholar]
- 10.van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5:483–92. doi: 10.1093/humupd/5.5.483. [DOI] [PubMed] [Google Scholar]
- 11.Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) 2008;68:499–509. doi: 10.1111/j.1365-2265.2007.03073.x. [DOI] [PubMed] [Google Scholar]
- 12.Nelson LM. Clinical practice: primary ovarian insufficiency. N Engl J Med. 2009;360:606–14. doi: 10.1056/NEJMcp0808697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Welt CK, McNicholl D, Taylor AE, Hall JE. Female reproductive aging is marked by decreased secretion of dimeric inhibin. J Clin Endocrinol Metab. 1999;84:105–11. doi: 10.1210/jcem.84.1.5381. [DOI] [PubMed] [Google Scholar]
- 14.La Marca A, Volpe A. Anti-Müllerian hormone (AMH) in female reproduction: is measurement of circulating AMH a useful tool? Clin Endocrinol (Oxf) 2006;64:603–10. doi: 10.1111/j.1365-2265.2006.02533.x. [DOI] [PubMed] [Google Scholar]
- 15.Burgoyne PS, Baker TG. Perinatal oocyte loss in XO mice and its implications for the aetiology of gonadal dysgenesis in XO women. J Reprod Fertil. 1985;75:633–45. doi: 10.1530/jrf.0.0750633. [DOI] [PubMed] [Google Scholar]
- 16.Hovatta O. Ovarian function and in vitro fertilization (IVF) in Turner syndrome. Pediatr Endocrinol Rev. 2012;9(Suppl 2):713–7. [PubMed] [Google Scholar]
- 17.Rao E, Weiss B, Fukami M, Rump A, Niesler B, Mertz A, et al. Pseudoautosomal deletions encompassing a novel homeobox gene cause growth failure in idiopathic short stature and Turner syndrome. Nature Genetics. 1997;16:54–63. doi: 10.1038/ng0597-54. [DOI] [PubMed] [Google Scholar]
- 18.Schlessinger D, Herrera L, Crisponi L, Mumm S, Percesepe A, Pellegrini M, et al. Genes and translocations involved in POF. Am J Med Genetics. 2002;111:328–33. doi: 10.1002/ajmg.10565. [DOI] [PubMed] [Google Scholar]
- 19.Bione S, Sala C, Manzini C, Arrigo Z, Zuffardi O, Banfi S, et al. A human homologue of the Drosophila melanogaster diaphanous gene is disrupted in a patient with premature ovarian failure: evidence for conserved function in oogenesis and implications for human sterility. American Journal of Human Genetics. 1998;62:533–41. doi: 10.1086/301761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prueitt RL, Chen H, Barnes RI, Zinn AR. Most X autosome translocations associated with premature ovarian failure do not interrupt X-linked genes. Cytogenetic and Genome Research. 2002;97:32–8. doi: 10.1159/000064052. [DOI] [PubMed] [Google Scholar]
- 21.Oberle I, Rousseau F, Heitz D, Kretz C, Devys D, Hanauer A, et al. Instability of a 550-base pair DNA segment and abnormal methylation in fragile X syndrome. Science. 1991;252:1097–102. doi: 10.1126/science.252.5009.1097. [DOI] [PubMed] [Google Scholar]
- 22.Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–14. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- 23.Voorhuis M, Onland-Moret NC, Janse F, Ploos van Amstel HK, Goverde AJ, Lambalk CB, et al. Dutch Primary Ovarian Insufficiency Consortium. The significance of fragile X mental retardation gene 1 CGG repeat sizes in the normal and intermediate range in women with primary ovarian insufficiency. Hum Reprod. 2014;29:1585–93. doi: 10.1093/humrep/deu095. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. Association of FMR1 repeat size with ovarian dysfunction. Human Reproduction. 2005;20:402–12. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- 25.Welt CK, Smith PC, Taylor AE. Evidence of early ovarian aging in fragile X premutation carriers. J Clin Endocrinol Metab. 2004;89:4569–74. doi: 10.1210/jc.2004-0347. [DOI] [PubMed] [Google Scholar]
- 26.Sullivan SD, Welt CK, Sherman S. FMR1 and the continuum of primary ovarian insufficiency. Semin Reprod Med. 2011;29:299–307. doi: 10.1055/s-0031-1280915. [DOI] [PubMed] [Google Scholar]
- 27.Hall DA, O’Keefe JA. Clinical neurogenetics: fragile x-associated tremor/ataxia syndrome. Neurol Clin. 2013;31:1073–84. doi: 10.1016/j.ncl.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 28.Wittenberger MD, Hagerman RJ, Sherman SL, Conkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, et al. The FMR1 premutation and reproduction. Fertility and Sterility. 2006;87:456–65. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 29.American College of Obstetrics and Gynecology. ACOG committee opinion, 338: Screening for fragile X syndrome. Obstetrics and Gynecology. 2006;107:1483–5. doi: 10.1097/00006250-200606000-00059. [DOI] [PubMed] [Google Scholar]
- 30.Murray A, Webb J, Dennis N, Conway G, Morton N. Microdeletions in FMR2 may be a significant cause of premature ovarian failure. J Med Genetics. 1999;36:767–70. doi: 10.1136/jmg.36.10.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Persani L, Rossetti R, Cacciatore C. Genes involved in human premature ovarian failure. J Mol Endocrinol. 2010;45:257–79. doi: 10.1677/JME-10-0070. [DOI] [PubMed] [Google Scholar]
- 32.Di Pasquale E, Beck-Peccoz P, Persani L. Hypergonadotropic ovarian failure associated with an inherited mutation of human bone morphogenetic protein-15 (BMP15) gene. American Journal of Human Genetics. 2004;75:106–11. doi: 10.1086/422103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Pasquale E, Rossetti R, Marozzi A, Bodega B, Borgato S, Cavallo L, et al. Identification of new variants of human BMP15 gene in a large cohort of women with premature ovarian failure. J Clin Endocrinol Metab. 2006;91:1976–9. doi: 10.1210/jc.2005-2650. [DOI] [PubMed] [Google Scholar]
- 34.Persani L, Rossetti R, Di Pasquale E, Cacciatore C, Fabre S. The fundamental role of bone morphogenetic protein 15 in ovarian function and its involvement in female fertility disorders. Hum Reprod Update. 2014 doi: 10.1093/humupd/dmu036. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 35.Corre T, Schuettler J, Bione S, Marozzi A, Persani L, Rossetti R, et al. Italian Network for the study of Ovarian Dysfunctions. A large-scale association study to assess the impact of known variants of the human INHA gene on premature ovarian failure. Hum Reprod. 2009;24:2023–8. doi: 10.1093/humrep/dep090. [DOI] [PubMed] [Google Scholar]
- 36.Cramer DW, Harlow BL, Barbieri RL, Ng WG. Galactose-1-phosphate uridyl transferase activity associated with age at menopause and reproductive history. Fertil Steril. 1989;51:609–15. doi: 10.1016/s0015-0282(16)60608-8. [DOI] [PubMed] [Google Scholar]
- 37.Mlinar B, Gersak K, Karas N, Zitnik IP, Battelino T, Lukac-Bajalo J. Galactose-1-phosphate uridyl transferase gene mutations in women with premature ovarian failure. Fertility and Sterility. 2005;84:253–5. doi: 10.1016/j.fertnstert.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 38.Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, et al. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthusinversus syndrome. Nature Genetics. 2001;27:159–66. doi: 10.1038/84781. [DOI] [PubMed] [Google Scholar]
- 39.Aittomaki K, Lucena JL, Pakarinen P, Sistonen P, Tapanainen J, Gromoll J, et al. Mutation in the follicle-stimulating hormone receptor gene causes hereditary hypergonadotropic ovarian failure. Cell. 1995;82:959–68. doi: 10.1016/0092-8674(95)90275-9. [DOI] [PubMed] [Google Scholar]
- 40.Latronico AC, Anasti J, Arnhold IJP, Rapaport R, Mendonca BB, Bloise W, et al. Brief report: testicular and ovarian resistance to luteinizing hormone caused by inactivating mutations of the luteinizing hormonereceptor gene. New England Journal of Medicine. 1996;334:507–12. doi: 10.1056/NEJM199602223340805. [DOI] [PubMed] [Google Scholar]
- 41.Lass A. The fertility potential of women with a single ovary. Human Reproduction Update. 1999;5:546–50. doi: 10.1093/humupd/5.5.546. [DOI] [PubMed] [Google Scholar]
- 42.Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. Journal of the National Cancer Institute. 2006;98:890–6. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 43.Meirow D. Reproduction post-chemotherapy in young cancer patients. Molecular and Cellular Endocrinology. 2000;169:123–31. doi: 10.1016/s0303-7207(00)00365-8. [DOI] [PubMed] [Google Scholar]
- 44.Meskhi A, Seif MW. Premature ovarian failure. Curr Opin Obstet Gynecol. 2006;18:418–26. doi: 10.1097/01.gco.0000233937.36554.d3. [DOI] [PubMed] [Google Scholar]
- 45.Kim SS, Lee JR, Jee BC, Suh CS, Kim SH, Ting A, et al. Use of hormonal protection for chemotherapy-induced gonadotoxicity. Clin Obstet Gynecol. 2010;53:740–52. doi: 10.1097/GRF.0b013e3181f96cb1. [DOI] [PubMed] [Google Scholar]
- 46.von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women--a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin’s lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284:427–35. doi: 10.1007/s00404-011-1874-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calongos G, Hasegawa A, Komori S, Koyama K. Harmful effects of antizona pellucida antibodies in folliculogenesis, oogenesis, and fertilization. J Reprod Immunol. 2009;79:148–55. doi: 10.1016/j.jri.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 48.Hoek A, Schoemaker J, Drexhage HA. Premature ovarian failure and ovarian autoimmunity. Endocr Rev. 1997;18:107–34. doi: 10.1210/edrv.18.1.0291. [DOI] [PubMed] [Google Scholar]
- 49.Bakalov VK, Anasti JN, Calis KA, Vanderhoof VH, Premkumar A, Chen S, et al. Autoimmune oophoritis as a mechanism of follicular dysfunction in women with 46,XX spontaneous premature ovarian failure. Fertil Steril. 2005;84:958–65. doi: 10.1016/j.fertnstert.2005.04.060. [DOI] [PubMed] [Google Scholar]
- 50.La Marca A, Brozzett A, Sighinolfi G, Marzotti S, Volpe A, Falorni A. Primary ovarian insufficiency: autoimmune causes. Curr Op Obstet Gynecol. 2010;22:277–82. doi: 10.1097/GCO.0b013e32833b6c70. [DOI] [PubMed] [Google Scholar]
- 51.Jasti S, Warren BD, McGinnis LK, Kinsey WH, Petroff BK, Petroff MG. The autoimmune regulator prevents premature reproductive senescence in female mice. Biol Reprod. 2012;86:110. doi: 10.1095/biolreprod.111.097501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Samy ET, Setiady YY, Ohno K, Pramoonjago P, Sharp C, Tung KSK. The role of physiological self-antigen in the acquisition and maintenance of regulatory T-cell function. Immunol Rev. 2006;212:170–84. doi: 10.1111/j.0105-2896.2006.00404.x. [DOI] [PubMed] [Google Scholar]
- 53.Samy ET, Parker LA, Sharp CP, Tung KSK. Continuous control of autoimmune disease by antigen-dependent polyclonal CD4+CD25+ regulatory T cells in the regional lymph node. J Exp Med. 2005;202:771–81. doi: 10.1084/jem.20041033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Otsuka N, Tong ZB, Vanevski K, Tu W, Cheng MH, Nelson LM. Autoimmune oophoritis with multiple molecular targets mitigated by transgenic expression of mater. Endocrinology. 2011;152:2465–73. doi: 10.1210/en.2011-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alimohammadi M, Björklund P, Hallgren A, Pöntynen N, Szinnai G, Shikama N, et al. Autoimmune polyendocrine syndrome type 1 and NALP5, a parathyroid autoantigen. N Engl J Med. 2008;358:1018–28. doi: 10.1056/NEJMoa0706487. [DOI] [PubMed] [Google Scholar]
- 56.Altuntas CZ, Johnson JM, Tuohy VK. Autoimmune targeted disruption of the pituitary-ovarian axis causes premature ovarian failure. J Immunol. 2006;177:1988–96. doi: 10.4049/jimmunol.177.3.1988. [DOI] [PubMed] [Google Scholar]
- 57.Bakalov VK, Gutin L, Cheng CM, Zhou J, Sheth P, Shah K, et al. Autoimmune disorders in women with turner syndrome and women with karyotypically normal primary ovarian insufficiency. J Autoimmun. 2012;38:315–21. doi: 10.1016/j.jaut.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Betterle C, Dal Pra C, Mantero F, Zanchetta R. Autoimmune adrenal insufficiency and autoimmune polyendocrine syndromes: autoantibodies, autoantigens, and their applicability in diagnosis and disease prediction. Endocr Rev. 2002;23:327–64. doi: 10.1210/edrv.23.3.0466. [DOI] [PubMed] [Google Scholar]
- 59.Falorni A, Laureti S, Santeusanio F. Autoantibodies in autoimmune polyendocrine syndrome type II. Endocrinol Metab Clin N Am. 2002;31:369–89. doi: 10.1016/s0889-8529(01)00010-x. [DOI] [PubMed] [Google Scholar]
- 60.Betterle C, Greggio NA, Volpato M. Clinical review 93: Autoimmune polyglandular syndrome type 1. J Clin Endocrinol Metab. 1998;83:1049–55. doi: 10.1210/jcem.83.4.4682. [DOI] [PubMed] [Google Scholar]
- 61.Peterson P, Pitkänen J, Sillanpää N, Krohn K. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED): a model disease to study molecular aspects of endocrine autoimmunity. Clin Exp Immunol. 2004;135:348–57. doi: 10.1111/j.1365-2249.2004.02384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blizzard RM, Chee D, Davies W. The incidence of adrenal and other antibodies in sera of patients with idiopathic adrenal insufficiency (Addison’s disease) Clin Exp Immunol. 1967;2:19–30. [PMC free article] [PubMed] [Google Scholar]
- 63.Irvine WJ, Chan MMW, Scarth L, Kolb FO, Hartog M, Bayliss RIS, et al. Immunological aspects of premature ovarian failure associated with idiopathic Addison’s disease. Lancet. 1968;2:883–7. doi: 10.1016/s0140-6736(68)91053-2. [DOI] [PubMed] [Google Scholar]
- 64.Sotsiou F, Bottazzo GF, Doniach D. Immunofluorescence studies on autoantibodies to steroid-producing cells, and to germline cells in endocrine diseases and infertility. Clin Exp Immunol. 1980;39:97–111. [PMC free article] [PubMed] [Google Scholar]
- 65.Elder M, MacLaren N, Riley W. Gonadal autoantibodies in patients with hypogonadism and/or Addison’s disease. J Clin Endocrinol Metab. 1981;52:1137–42. doi: 10.1210/jcem-52-6-1137. [DOI] [PubMed] [Google Scholar]
- 66.Ahonen P, Miettinen A, Perheentupa J. Adrenal and steroidal cell antibodies in patients with autoimmune polyglandular disease type I and risk of adrenocortical and ovarian failure. J Clin Endocrinol Metab. 1987;64:494–500. doi: 10.1210/jcem-64-3-494. [DOI] [PubMed] [Google Scholar]
- 67.Betterle C, Rossi A, Dalla Pria S, Artifoni A, Pedini B, Gavasso S, et al. Premature ovarian failure: autoimmunity and natural history. Clin Endocrinol (Oxf) 1993;39:43–53. doi: 10.1111/j.1365-2265.1993.tb01748.x. [DOI] [PubMed] [Google Scholar]
- 68.Novosad JA, Kalantaridou SN, Tong ZB, Nelson LM. Ovarian antibodies as detected by indirect immunofluorescence are unreliable in the diagnosis of autoimmune premature ovarian failure: a controlled evaluation. BMC Womens Health. 2003;3:2. doi: 10.1186/1472-6874-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chen S, Sawicka J, Betterle C, Powell M, Prentice L, Volpato M, et al. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison’s disease and premature ovarian failure. J Clin Endocrinol Metab. 1996;81:1871–6. doi: 10.1210/jcem.81.5.8626850. [DOI] [PubMed] [Google Scholar]
- 70.Falorni A, Laureti S, Candeloro P, Perrino S, Coronella C, Bizzarro A, et al. Steroid-cell autoantibodies are preferentially expressed in women with premature ovarian failure who have adrenal autoimmunity. Fertil Steril. 2002;78:270–9. doi: 10.1016/s0015-0282(02)03205-3. [DOI] [PubMed] [Google Scholar]
- 71.Falorni A, Chen S, Zanchetta R, Yu L, Tiberti C, Bacosi ML, et al. Measuring adrenal autoantibody response: interlaboratory concordance in the first international serum exchange for the determination of 21-hydroxylase autoantibodies. Clin Immunol. 2011;140:291–9. doi: 10.1016/j.clim.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 72.Brozzetti A, Marzotti S, La Torre D, Bacosi ML, Morelli S, Bini V, et al. Autoantibody responses in autoimmune ovarian insufficiency and in Addison disease are IgG1-dominated and suggest a predominant, but not exclusive, Th1 type of response. Eur. J Endocrinol. 2010;163:309–17. doi: 10.1530/EJE-10-0257. [DOI] [PubMed] [Google Scholar]
- 73.Arif S, Vallian S, Farzaneh F, Zanone MM, James SL, Pietropaolo M, et al. Identification of 3b-hydroxysteroid-dehydrogenase as a novel target of steroid cell autoantibodies: association of autoantibodies with endocrine autoimmune disease. J Clin Endocrinol Metab. 1996;81:4439–45. doi: 10.1210/jcem.81.12.8954056. [DOI] [PubMed] [Google Scholar]
- 74.Koit R, Peterson P, Hyöty H, Uibo R, Cooke I, Weetman AP, et al. 3b–hydroxysteroid-dehydrogenase autoantibodies are rare in premature ovarian failure. J Clin Endocrinol Metab. 2000;85:2324–6. doi: 10.1210/jcem.85.6.6630. [DOI] [PubMed] [Google Scholar]
- 75.Irvine WJ. Autoimmunity in endocrine disease. Recent Prog Horm Res. 1980;36:509–56. doi: 10.1016/b978-0-12-571136-4.50020-6. [DOI] [PubMed] [Google Scholar]
- 76.Bannatyne P, Russell P, Shearman RP. Autoimmune oophoritis: a clinicopathologic assessment of 12 cases. Int J Gynaecol Pathol. 1990;9:191–207. doi: 10.1097/00004347-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 77.Sedmak DD, Hart WR, Tubbs RR. Autoimmune oophoritis: a histopathologic study involved ovaries with immunologic characterization of the mononuclear cell infiltrate. Int J Gynecol Pathol. 1987;6:73–81. [PubMed] [Google Scholar]
- 78.Gloor E, Hurlimann J. Autoimmune oophoritis. Am J Clin Pathol. 1984;81:105–9. doi: 10.1093/ajcp/81.1.105. [DOI] [PubMed] [Google Scholar]
- 79.Miyake T, Sato Y, Takeuchi S. Implications of circulating autoantibodies and peripheral blood Iymphocytes for the genesis of premature ovarian failure. J Reprod Immunol. 1987;12:163–71. doi: 10.1016/0165-0378(87)90021-0. [DOI] [PubMed] [Google Scholar]
- 80.Aiman J, Smentek C. Premature ovarian failure. Obstet Gynecol. 1985;66:9–14. [PubMed] [Google Scholar]
- 81.Moncayo H, Moncayo R, Benz R, Wolf A, Lauritzen C. Ovarian failure and autoimmunity. Detection of autoantibodies directed against both the unoccupied luteinizing hormone/human chorionic gonadotropin receptor and the hormonereceptor complex of bovine corpus luteum. J Clin Invest. 1989;84:1857–65. doi: 10.1172/JCI114372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ryan MM, Jones HR., Jr Myasthenia gravis and premature ovarian failure. Muscle Nerve. 2004;30:231–3. doi: 10.1002/mus.20067. [DOI] [PubMed] [Google Scholar]
- 83.Kelkar RL, Meherji PK, Kadam SS, Gupta SK, Nandedkar TD. Circulating autoantibodies against the zona pellucida and thyroid microsomal antigen in women with premature ovarian failure. J Repr Immunol. 2005;66:53–67. doi: 10.1016/j.jri.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Wheatcroft NJ, Salt C, Milford-Ward A, Cooke ID, Weetman AP. Identification of ovarian antibodies by immunofluorescence, enzyme-linked immunosorbent assay or immunoblotting in premature ovarian failure. Hum Reprod. 1997;12:2617–22. doi: 10.1093/humrep/12.12.2617. [DOI] [PubMed] [Google Scholar]
- 85.Anasti JN, Flack MR, Froehlich J, Nelson LM. The use of human recombinant gonadotropin receptors to search for immunoglobulin G-mediated premature ovarian failure. J Clin Endocrinol Metab. 1995;80:824–8. doi: 10.1210/jcem.80.3.7883837. [DOI] [PubMed] [Google Scholar]
- 86.Tonacchera M, Ferrarini E, Dimida A, Agretti P, De Marco G, De Servi M, et al. Gonadotrophin receptor blocking antibodies measured by the use of cell lines stably expressing human gonadotrophin receptors are not detectable in women with 46,XX premature ovarian failure. Clin Endocrinol (Oxf) 2004;61:376–81. doi: 10.1111/j.1365-2265.2004.02107.x. [DOI] [PubMed] [Google Scholar]
- 87.Petraglia F, Hartmann B, Luisi S, Florio P, Kirchengast S, Santuz M, et al. Low levels of serum inhibin A and inhibin B in women with hypergonadotropic amenhorrea and evidence of high levels of activin A in women with hypothalamic amenhorrea. Fertil Steril. 1998;70:907–12. doi: 10.1016/s0015-0282(98)00283-0. [DOI] [PubMed] [Google Scholar]
- 88.Méduri G, Massin N, Guibourdenche J, Bachelot A, Fiori O, Kuttenn F, Misrahi M, Touraine P. Serum anti-Müllerian hormone expression in women with premature ovarian failure. Hum Reprod. 2007;22:117–23. doi: 10.1093/humrep/del346. [DOI] [PubMed] [Google Scholar]
- 89.Welt CK, Falorni A, Taylor AE, Martin KA, Hall JE. Selective theca cell dysfunction in autoimmune oophoritis results in multi-follicular development, decreased estradiol, and elevated inhibin B levels. J Clin Endocrinol Metab. 2005;90:3069–76. doi: 10.1210/jc.2004-1985. [DOI] [PubMed] [Google Scholar]
- 90.Tsigkou A, Marzotti S, Borges L, Brozzetti A, Candeloro P, Bacosi ML, et al. High serum inhibin concentration discriminates autoimmune oophoritis from other forms of primary ovarian insufficiency. J Clin Endocrinol Metab. 2008;93:1263–69. doi: 10.1210/jc.2007-1675. [DOI] [PubMed] [Google Scholar]
- 91.La Marca A, Marzotti S, Brozzetti A, Stabile G, Carducci Artenisio A, et al. Primary ovarian insufficiency due to steroidogenic cell autoimmunity is associated with preserved pool of functioning follicles. J Clin Endocrinol Metab. 2009;94:3816–23. doi: 10.1210/jc.2009-0817. [DOI] [PubMed] [Google Scholar]
- 92.Falorni A, Brozzetti A, Aglietti MC, Esposito R, Minarelli V, Morelli S, et al. Progressive decline of residual follicle pool after clinical diagnosis of autoimmune ovarian insufficiency. Clin Endocrinol (Oxf) 2012;77:453–8. doi: 10.1111/j.1365-2265.2012.04387.x. [DOI] [PubMed] [Google Scholar]
- 93.Blumenfeld Z, Halachmi S, Peretz BA, Shmuel Z, Golan D, Makler A, et al. Premature ovarian failure--the prognostic application of autoimmunity on conception after ovulation induction. Fertil Steril. 1993;59:750–5. doi: 10.1016/s0015-0282(16)55854-3. [DOI] [PubMed] [Google Scholar]
- 94.Kalantaridou SN, Braddock DT, Patronas NJ, Nelson LM. Treatment of autoimmune premature ovarian failure. Hum Reprod. 1999;14:1777–82. doi: 10.1093/humrep/14.7.1777. [DOI] [PubMed] [Google Scholar]
- 95.van Kasteren YM, Braat DD, Hemrika DJ, Lambalk CB, Rekers-Mombarg LT, von Blomberg BM, et al. Corticosteroids do not influence ovarian responsiveness to gonadotropins in patients with premature ovarian failure: a randomized, placebo-controlled trial. Fertil Steril. 1999;71:90–5. doi: 10.1016/s0015-0282(98)00411-7. [DOI] [PubMed] [Google Scholar]
- 96.Bodenheimer T. Coordinating care--a perilous journey through the health care system. N Engl J Med. 2008;358:1064–71. doi: 10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 97.Hussey PS, Schneider EC, Rudin RS, Fox DS, Lai J, Pollack CE. Continuity and the costs of care for chronic disease. JAMA Internal Med. 2014;174:742–8. doi: 10.1001/jamainternmed.2014.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jackson GL, Powers BJ, Chatterjee R, Bettger JP, Kemper AR, Hasselblad V, et al. Improving patient care. The patient centered medical home. A Systematic Review. Ann Intern Med. 2013;158:169–78. doi: 10.7326/0003-4819-158-3-201302050-00579. [DOI] [PubMed] [Google Scholar]
- 99.Sia C, Tonniges TF, Osterhus E, Taba S. History of the medical home concept. Pediatrics. 2014;113(5 Suppl):1473–8. [PubMed] [Google Scholar]
- 100.Scholle SH, Torda P, Peikes D, Han E, Genevro J. Engaging patients and families in the medical home. agency for healthcare research and quality publication No. 10-0083-EF. Jun, 2010. [Google Scholar]
- 101.Cooper AR, Baker VL, Sterling EW, Ryan ME, Woodruff TK, Nelson LM. The time is now for a new approach to primary ovarian insufficiency. Fertil Steril. 2011;95:1890–7. doi: 10.1016/j.fertnstert.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rafique S, Sterling EW, Nelson LM. A new approach to primary ovarian insufficiency. Obstet Gynecol Clin North Am. 2012;39:567–86. doi: 10.1016/j.ogc.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alzubaidi NH, Chapin HL, Vanderhoof VH, Calis KA, Nelson LM. Meeting the needs of young women with secondary amenorrhea and spontaneous premature ovarian failure. Obstet Gynecol. 2002;99:720–5. doi: 10.1016/s0029-7844(02)01962-2. [DOI] [PubMed] [Google Scholar]
- 104.Practice Committee of the American Society for Reproductive Medicine. Current evaluation of amenorrhea. Fertil Steril. 2004;82(Suppl 1):S33–S39. doi: 10.1016/j.fertnstert.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 105.Falorni A, Nikoshkov A, Laureti S, Grenbäck E, Hulting AL, Casucci G, et al. High diagnostic accuracy for idiopathic Addison’s disease with a sensitive radiobinding assay for autoantibodies against recombinant human 21-hydroxylase. J Clin Endocrinol Metab. 1995;80:2752–5. doi: 10.1210/jcem.80.9.7673419. [DOI] [PubMed] [Google Scholar]
- 106.Rebar RW, Erickson GF, Yen SSC. Idiopathic premature ovarian failure: clinical and endocrine characteristics. Fertil Steril. 1982;37:35–41. [PubMed] [Google Scholar]
- 107.Groff AA, Covington SN, Halverson LR, Fitzgerald OR, Vanderhoof V, Calis K, et al. Assessing the emotional needs of women with spontaneous premature ovarian failure. Fertil Steril. 2005;83:1734–41. doi: 10.1016/j.fertnstert.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 108.Newbould J, Taylor D, Bury M. Layled self-management in chronic illness: a review of the evidence. Chronic Illn. 2006;2:249–61. doi: 10.1177/17423953060020040401. [DOI] [PubMed] [Google Scholar]
- 109.Schmidt PJ, Cardoso GM, Ross JL, Haq N, Rubinow DR, Bondy CA. Shyness, social anxiety, and impaired self-esteem in Turner syndrome and premature ovarian failure. JAMA. 2006;295:1374–6. doi: 10.1001/jama.295.12.1374. [DOI] [PubMed] [Google Scholar]
- 110.Orshan SA, Ventura JL, Covington SN, Vanderhoof VH, Troendle JF, Nelson LM. Women with spontaneous 46,XX primary ovarian insufficiency (hypergonadotropic hypogonadism) have lower perceived social support than control women. Fertil Steril. 2009;92:688–93. doi: 10.1016/j.fertnstert.2008.07.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davis M, Ventura JL, Wieners M, Covington SN, Vanderhoof VH, Ryan ME, et al. The psychosocial transition associated with spontaneous 46,XX primary ovarian insufficiency: illness uncertainty, stigma, goal flexibility, and purpose in life as factors in emotional health. Fertil Steril. 2010;93:2321–9. doi: 10.1016/j.fertnstert.2008.12.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fogarty LA, Curbow BA, Wingard JR, McDonnell K, Somerfield MR. Can 40 seconds of compassion reduce patient anxiety? J Clin Oncol. 1999;17:371–9. doi: 10.1200/JCO.1999.17.1.371. [DOI] [PubMed] [Google Scholar]
- 113.Buckman R. How to break bad news: a guide for health care professionals. Johns Hopkins University Press; Baltimore: 1992. [Google Scholar]
- 114.Gallagher JC. Effect of early menopause on bone mineral density and fractures. Menopause. 2007;14:567–71. doi: 10.1097/gme.0b013e31804c793d. [DOI] [PubMed] [Google Scholar]
- 115.Jacobsen BK, Knutsen SF, Fraser GE. Age at natural menopause and total mortality and mortality from ischemic heart disease: the Adventist Health Study. J Clin Epidemiol. 1999;52:303–7. doi: 10.1016/s0895-4356(98)00170-x. [DOI] [PubMed] [Google Scholar]
- 116.de Kleijn MJ, van der Schouw YT, Verbeek AL, Peeters PH, Banga JD, van der Graaf Y. Endogenous estrogen exposure and cardiovascular mortality risk in postmenopausal women. Am J Epidemiol. 2002;155:339–45. doi: 10.1093/aje/155.4.339. [DOI] [PubMed] [Google Scholar]
- 117.Mondul AM, Rodriguez C, Jacobs EJ, Calle EE. Age at natural menopause and cause-specific mortality. Am J Epidemiol. 2005;162:1089–97. doi: 10.1093/aje/kwi324. [DOI] [PubMed] [Google Scholar]
- 118.Popat VB, Calis KA, Vanderhoof VH, Cizza G, Reynolds JC, Sebring N, et al. Bone mineral density in estrogen-deficient young women. J Clin Endocrinol Metab. 2009;94:2277–83. doi: 10.1210/jc.2008-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Popat VB, Calis KA, Kalantaridou SN, Vanderhoof VH, Koziol D, Troendle JF, et al. Bone mineral density in young women with primary ovarian insufficiency: results of a three-year randomized controlled trial of physiological transdermal estradiol and testosterone replacement. J Clin Endocrinol Metab. 2014;99:3418–26. doi: 10.1210/jc.2013-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 121.North American Menopause Society. The role of calcium in peri- and postmenopausal women: 2006 position statement of the North American Menopause Society. Menopause. 2006;13:862–77. doi: 10.1097/01.gme.0000243566.25205.0b. [DOI] [PubMed] [Google Scholar]
- 122.Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43:898–908. doi: 10.1136/bjsm.2008.052704. [DOI] [PubMed] [Google Scholar]
- 123.Drake MT, Clarke BL, Khosla S. Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc. 2008;83:1032–45. doi: 10.4065/83.9.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mishell DR, Jr, Nakamura RM, Crosignani PG, Stone S, Kharma K, Nagata Y, et al. Serum gonadotrophin and steroid patterns during the normal menstrual cycle. Am J Obstet Gynecol. 1971;111:60–5. doi: 10.1016/0002-9378(71)90927-6. [DOI] [PubMed] [Google Scholar]
- 125.Popat VB, Vanderhoof VH, Calis KA, Troendle JF, Nelson LM. Normalization of serum LH levels in women with 46,XX spontaneous primary ovarian insufficiency. Fertil Steril. 2008;89:429–33. doi: 10.1016/j.fertnstert.2007.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Scarabin PY, Alhenc-Gelas M, Plu-Bureau G, Taisne P, Agher R, Aiach M. Effects of oral and transdermal estrogen/progesterone regimens on blood coagulation and fibrinolysis in postmenopausal women: a randomized controlled trial. Arterioscler Thromb Vasc Biol. 1997;17:3071–8. doi: 10.1161/01.atv.17.11.3071. [DOI] [PubMed] [Google Scholar]
- 127.Scarabin PY, Oger E, Plu-Bureau G. Differential association of oral and transdermal oestrogen-replacement therapy with venous thromboembolism risk. Lancet. 2003;362:428–32. doi: 10.1016/S0140-6736(03)14066-4. [DOI] [PubMed] [Google Scholar]
- 128.Canonico M, Oger E, Plu-Bureau G, Conard J, Meyer G, Lévesque H, et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of estrogen administration and progestogens: the ESTHER study. Circulation. 2007;115:840–5. doi: 10.1161/CIRCULATIONAHA.106.642280. [DOI] [PubMed] [Google Scholar]
- 129.Gibbons WE, Moyer DL, Lobo RA, Roy S, Mishell DR., Jr Biochemical and histologic effects of sequential estrogen/progestin therapy on the endometrium of postmenopausal women. Am J Obstet Gynecol. 1986;154:456–61. doi: 10.1016/0002-9378(86)90690-3. [DOI] [PubMed] [Google Scholar]
- 130.Bjarnason K, Cerin A, Lindgren R, Weber T. Adverse endometrial effects during long cycle hormone replacement therapy. Maturitas. 1999;32:161–70. doi: 10.1016/s0378-5122(99)00033-x. [DOI] [PubMed] [Google Scholar]
- 131.PEPI Group. . Effects of hormone replacement therapy on endometrial histology in postmenopausal women: the Postmenopausal Estrogen/ Progestin Interventions (PEPI) Trial. JAMA. 1996;275:370–5. doi: 10.1001/jama.1996.03530290040035. [DOI] [PubMed] [Google Scholar]
- 132.Smith JA, Vitale S, Reed GF, Grieshaber SA, Goodman LA, Vanderhoof VH, et al. Dry eye signs and symptoms in women with premature ovarian failure. Arch Ophthalmol. 2004;122:151–6. doi: 10.1001/archopht.122.2.151. [DOI] [PubMed] [Google Scholar]
- 133.Carroll L. Families of Two. Xlibris Corporation; 2000. [Google Scholar]
- 134.Levine S, Unutzer J, Yip JY, Hoffing M, Leung M, Fan MY, et al. Physicians’ satisfaction with a collaborative disease management program for late-life depression in primary care. Gen Hosp Psychiatry. 2005;27:383–91. doi: 10.1016/j.genhosppsych.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 135.Katon WJ, Lin EH, Von Korff M, Ciechanowski P, Ludman EJ, Young B, et al. Collaborative care for patients with depression and chronic illnesses. N Engl J Med. 2010;363:2611–20. doi: 10.1056/NEJMoa1003955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Katon W, Russo J, Lin EH, Schmittdiel J, Ciechanowski P, Ludman E, et al. Cost-effectiveness of a multicondition collaborative care intervention: a randomized controlled trial. Arch Gen Psychiatry. 2012;69:506–14. doi: 10.1001/archgenpsychiatry.2011.1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Arora S, Geppert CM, Kalishman S, Dion D, Pullara F, Bjeletich B, et al. Academic health center management of chronic diseases through knowledge networks: Project ECHO. Acad Med. 2007;82:154–60. doi: 10.1097/ACM.0b013e31802d8f68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Arora S, Thornton K, Murata G, Deming P, Kalishman S, Dion D, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N Engl J Med. 2011;364:2199–207. doi: 10.1056/NEJMoa1009370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wenger EC, Snyder WM. Communities of practice: the organizational frontier. Harv Bus Rev. 2000;78:139–45. [Google Scholar]
- 140.Frankfurter D. A new paradigm for studying and treating primary ovarian insufficiency: the community of practice. Fertil Steril. 2011;95:1899–900. doi: 10.1016/j.fertnstert.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 141.Weinstein RS, Lopez AM, Joseph BA, Erps KA, Holcomb M, Barker GP, et al. Telemedicine, telehealth, and mobile health applications that work: opportunities and barriers. Am J Med. 2014;127:183–7. doi: 10.1016/j.amjmed.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 142.Morrissey J. Searching for the promise of mHealth. Hosp Health Netw. 2014;88:18–9. [PubMed] [Google Scholar]
- 143.Morrissey J. Connecting the continuum: number of hurdles keep mHealth from hitting its stride. Hosp Health Netw. 2014;88:22–3. [PubMed] [Google Scholar]
- 144.Covington SN, Martinez PE, Popat V, Nandagopal R, Ryan M, Nelson LM. The psychology of antecedents to adult reproductive disorders in adolescent girls. Ann N Y Acad Sci. 2008;1135:155–62. doi: 10.1196/annals.1429.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Covington SN, Hillard PJ, Sterling EW, Nelson LM Primary Ovarian Insufficiency Recovery Group. A family systems approach to primary ovarian insufficiency. J Pediatr Adolesc Gynecol. 2011;24:137–41. doi: 10.1016/j.jpag.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Husebye ES, Allolio B, Arlt W, Badenhoop K, Bensing S, Betterle C, et al. Consensus statement on the diagnosis, treatment and follow-up o patients with primary adrenal insufficiency. J Internal Medicine. 2014;275:104–15. doi: 10.1111/joim.12162. [DOI] [PubMed] [Google Scholar]
- 147.Falorni A, Minarelli V, Morelli S. Therapy of adrenal insufficiency: an update. Endocrine. 2013;43:514–28. doi: 10.1007/s12020-012-9835-4. [DOI] [PubMed] [Google Scholar]