Abstract
Objective:
We investigated white matter lesion load and global and regional brain volumes in relation to domain-specific cognitive performance in the stroke-free Northern Manhattan Study (NOMAS) population.
Methods:
We quantified white matter hyperintensity volume (WMHV), total cerebral volume (TCV), and total lateral ventricular (TLV) volume, as well as hippocampal and cortical gray matter (GM) lobar volumes in a subgroup. We used general linear models to examine MRI markers in relation to domain-specific cognitive performance, adjusting for key covariates.
Results:
MRI and cognitive data were available for 1,163 participants (mean age 70 ± 9 years; 60% women; 66% Hispanic, 17% black, 15% white). Across the entire sample, those with greater WMHV had worse processing speed. Those with larger TLV volume did worse on episodic memory, processing speed, and semantic memory tasks, and TCV did not explain domain-specific variability in cognitive performance independent of other measures. Age was an effect modifier, and stratified analysis showed that TCV and WMHV explained variability in some domains above age 70. Smaller hippocampal volume was associated with worse performance across domains, even after adjusting for APOE ε4 and vascular risk factors, whereas smaller frontal lobe volumes were only associated with worse executive function.
Conclusions:
In this racially/ethnically diverse, community-based sample, white matter lesion load was inversely associated with cognitive performance, independent of brain atrophy. Lateral ventricular, hippocampal, and lobar GM volumes explained domain-specific variability in cognitive performance.
With the aging of the US population,1 there is increasing interest in cognitive aging, a complex process that includes the effects of neurodegenerative and cardiovascular processes prevalent in older adults. Data regarding region-specific brain volumes and domain-specific cognitive performance from racially/ethnically diverse studies are limited.
Measures of global brain atrophy, such as ventricular enlargement and total cerebral volume (TCV), have correlated with worse cognitive performance and an increased risk of stroke and dementia.2–6 Lobar and hippocampal volumes have also been observed to decrease variably over the lifespan.7 Frontal and temporal atrophy have been associated with deficits in executive function and memory, whereas hippocampal atrophy is a risk factor for cognitive decline in cognitively normal people.6,8 Cerebral small vessel disease (SVD) is common in older adults and has been associated with worse cognitive performance and decline in cognitively normal individuals and a greater risk of stroke, dementia, and mortality.9–11
Associations of these imaging markers with domain-specific cognitive performance are not well-understood on a population-based level, especially among middle-aged and older Hispanics and blacks. We hypothesized that imaging markers of global cerebral and ventricular volumes and cerebral SVD would explain variability in cognitive performance in this diverse population-based sample. Also, because vascular damage and Alzheimer disease are the most prevalent causes of cognitive decline in older adults, we hypothesized that cerebral SVD would predominantly affect frontal lobe function, resulting in worse executive function, whereas brain atrophy would affect cognitive function more broadly.
METHODS
Participants.
The Northern Manhattan Study (NOMAS) is a prospective population-based cohort study. The general recruitment, design, and demographics of NOMAS have been previously described in detail.12 Eligible participants were stroke-free, were >40 years old, and resided >3 months in a Northern Manhattan household with a telephone. We enrolled 3,298 participants using random digit dialing between 1993 and 2001. All participants underwent a baseline evaluation of demographic characteristics, health behaviors, and health status, including comprehensive medical history, physical/neurologic examination, medical record review, and fasting blood samples. Standardized questions were adapted from the Centers for Disease Control and Prevention Behavioral Risk Factor Surveillance System.
Standard protocol approvals, registrations, and patient consents.
All participants signed informed consent and the institutional review boards of Columbia University and the University of Miami approved the study.
Neurologic testing and cognitive domain scores.
From 2003 to 2008, 1,290 stroke-free participants eligible for MRI (including 199 newly enrolled NOMAS household members meeting above entry criteria) completed a neurocognitive (NC) battery in English or Spanish based on language spoken at home. To assign cognitive domain labels, we explored interrelationships among individual NC test scores with factor analysis and used a Scree plot of eigenvalues to determine the number of constructs (domains). We computed composite scores for each domain by averaging individual z scores transformed from raw test scores, as previously described13–15: episodic memory (EMEM): subscores from a 12-word 5-trial list-learning task (total, delayed recall, and delayed recognition); executive function (EXEC): time to complete Color Trails 2 minus Color Trails 1 and the sum of Odd-Man-Out subtests 2 and 4; processing speed (PS): nondominant hand Grooved Pegboard times, Color Trails 1 time, and Visual-Motor Integration test scores16; semantic memory (SMEM): picture naming (modified Boston Naming), category fluency (Animal Naming), and phonemic fluency (C, F, L in English speakers and F, A, S in Spanish speakers).
Brain MRI measurements.
During the initial MRI study, we focused on cerebral volumes and did not include infratentorial structures or brainstem. Brain MRI scans were obtained from 2003 to 2008 on the same day as the NC battery. We used a 1.5T Philips Intera scanner (Philips, Best, the Netherlands) at Columbia University Medical Center. Images were transferred electronically to University of California, Davis for morphometric analysis of TCV, total lateral ventricular (TLV) volume, and white matter hyperintensity volume (WMHV) using T1 and fluid- attenuated inversion recovery sequences, as previously described.16–18 Briefly, nonbrain elements were manually removed from the image by operator-guided tracing of dura mater within the cranium, including the middle cranial fossa but excluding the posterior fossa and brainstem. The resulting measure was defined as total intracranial volume (TIV). TCV was computed as the sum of whole brain volume voxels from the T1 segmentation process. After segmentation into brain matter and CSF, voxels belonging to the CSF class within the region of interest were summed to quantify TLV volume. WMHV was calculated as the sum of voxels ≥3.5 SDs above the mean image intensity multiplied by pixel dimensions and section thickness. We expressed TCV, TLV volume, and WMHV as proportions of TIV to correct for individual differences in head size. For consistency with prior publications we do not use Standards for Reporting Vascular Changes on Neuroimaging, as previously described.e1
Volumetric segmentation of lobar gray matter (GM) volumes and hippocampal volumes was performed with high-quality data sets using the publicly available FreeSurfer image analysis suite (Version 5.1) (http://surfer.nmr.mgh.harvard.edu/).19,20 All T1-weighted MRIs underwent motion correction, skull stripping, and transformations into Talaraich space before segmentation, identification of gray/white matter boundaries, automated topology correction, and surface deformation.21,22 Through 3-dimensional segmentation methods, neuroanatomic labels for regional white matter and cortical GM parcellations were assigned to each voxel using a probabilistic atlas and Bayesian classification rule.19 FreeSurfer provides an estimate of hippocampal volume, and 68 cortical GM parcellations were summed to estimate frontal, temporal, occipital, and parietal lobe GM volumes using recommended methods.20,23 All regional GM volumes were expressed as ratios of TCV to account for relative differences in brain size (rather than head size, as was done for the global measures) in order to examine the relative importance of regional GM volumes.
APOE genotyping.
DNA samples were extracted from peripheral blood white cells using HhaI digestion and amplified by PCR, as previously described.24 APOE ε4 carriers were identified as individuals with a genotype of APOE ε4/ε2, APOE ε4/ε4, and APOE ε4/ε3.
Statistical analyses.
To evaluate the association of brain volumetric measures with domain-specific cognitive performance, we built a series of models to adjust for potential confounders: age, sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, non-Hispanic other), education, body mass index, waist-to-hip ratio, smoking (never, former, and current), reported moderate alcohol intake (1 drink per week to ≤ 2 drinks per day vs other), reported moderate leisure-time physical activity (vs inactive),25 antihypertensive medication use, antidiabetic medication use, lipid-lowering medication use, systolic and diastolic blood pressure, glucose, low-density lipoprotein, high-density lipoprotein, and triglycerides. The primary analysis used general linear models and adjusted for age, sex, race/ethnicity, education, and TIV (or TCV for regional analyses) for the association between each brain volumetric measure and performance in a domain. As secondary analyses, additional adjustments were made for APOE genotype and for all MRI variables simultaneously to ensure that any effect of one brain measure was independent of the others. In sensitivity analysis, regional volume associations were also evaluated using the proportion of TIV (instead of TCV). Finally, joint effects of brain morphologic parameters and potential interactions with other covariates were assessed with models that included multiple significant morphologic predictors and their interaction terms. We did stratified analyses for interactions with p values <0.1. We used SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
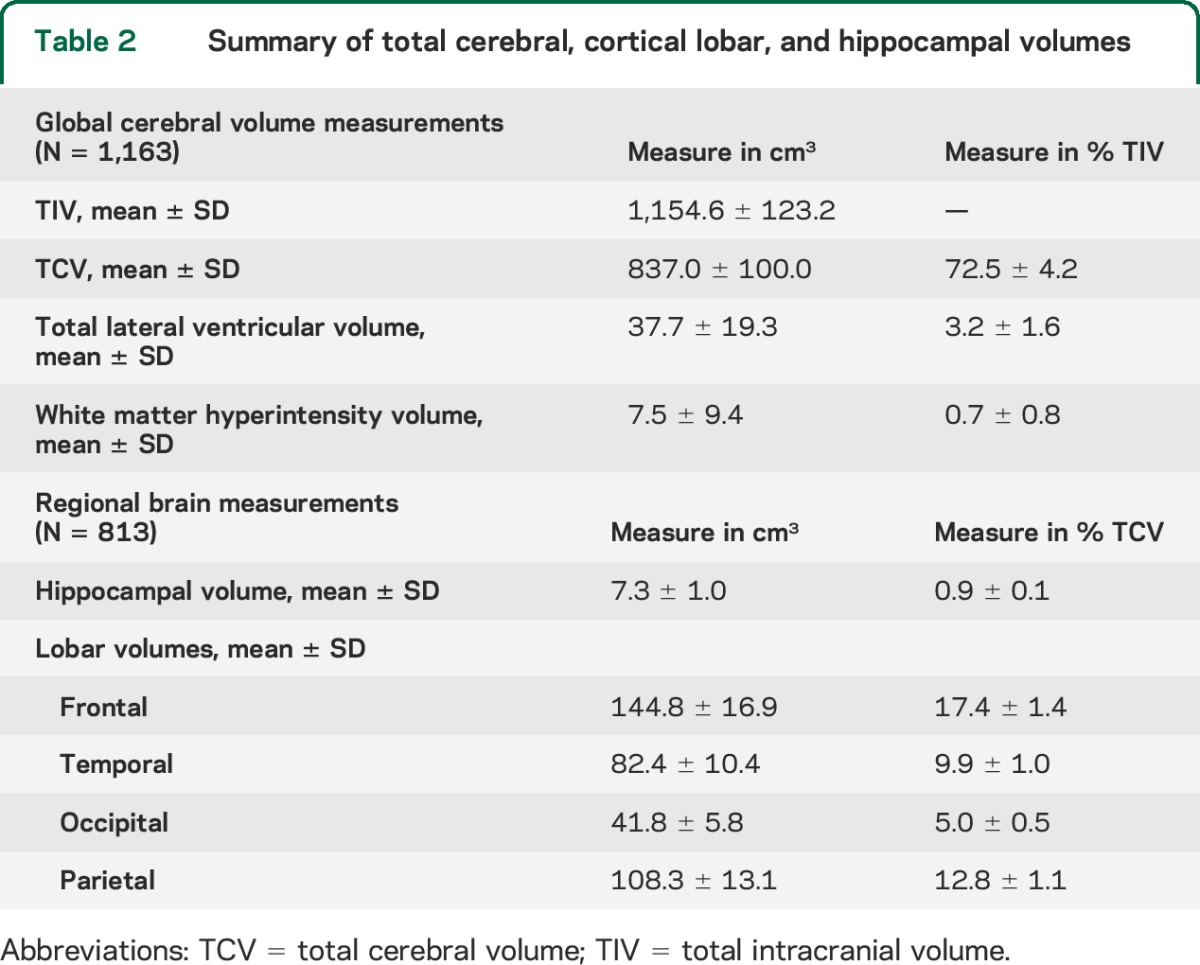
Participant characteristics are summarized in table 1. Half the sample was above age 70, with an average of 10 years of education. The majority of participants were women, were of Hispanic/Latino origin, reported taking blood pressure medication, and reported moderate to heavy leisure-time physical activity. Total cerebral and regional brain volumes are summarized in table 2. Global cognition was high (mean Mini-Mental State Examination score = 26.7 ± 3.3), in keeping with our prior estimate of <5% cognitive impairment at baseline.26
Table 1.
Sample characteristics (N = 1,163)
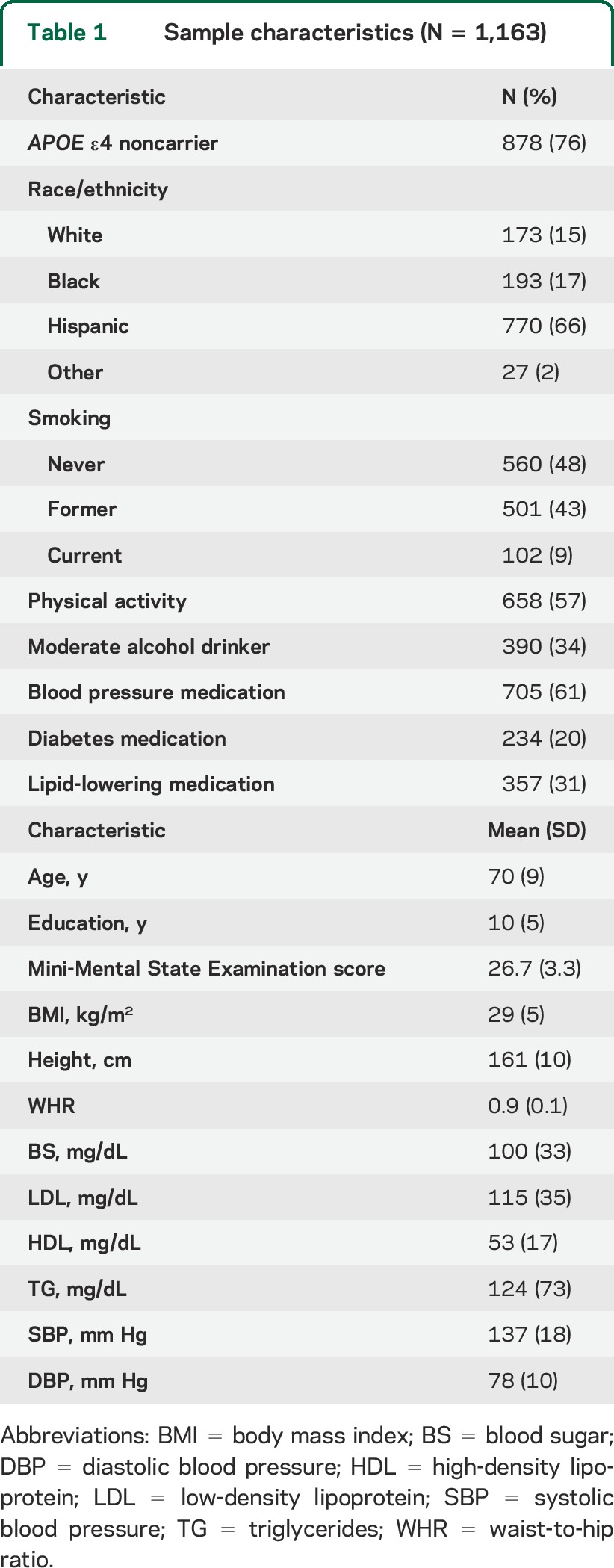
Table 2.
Summary of total cerebral, cortical lobar, and hippocampal volumes
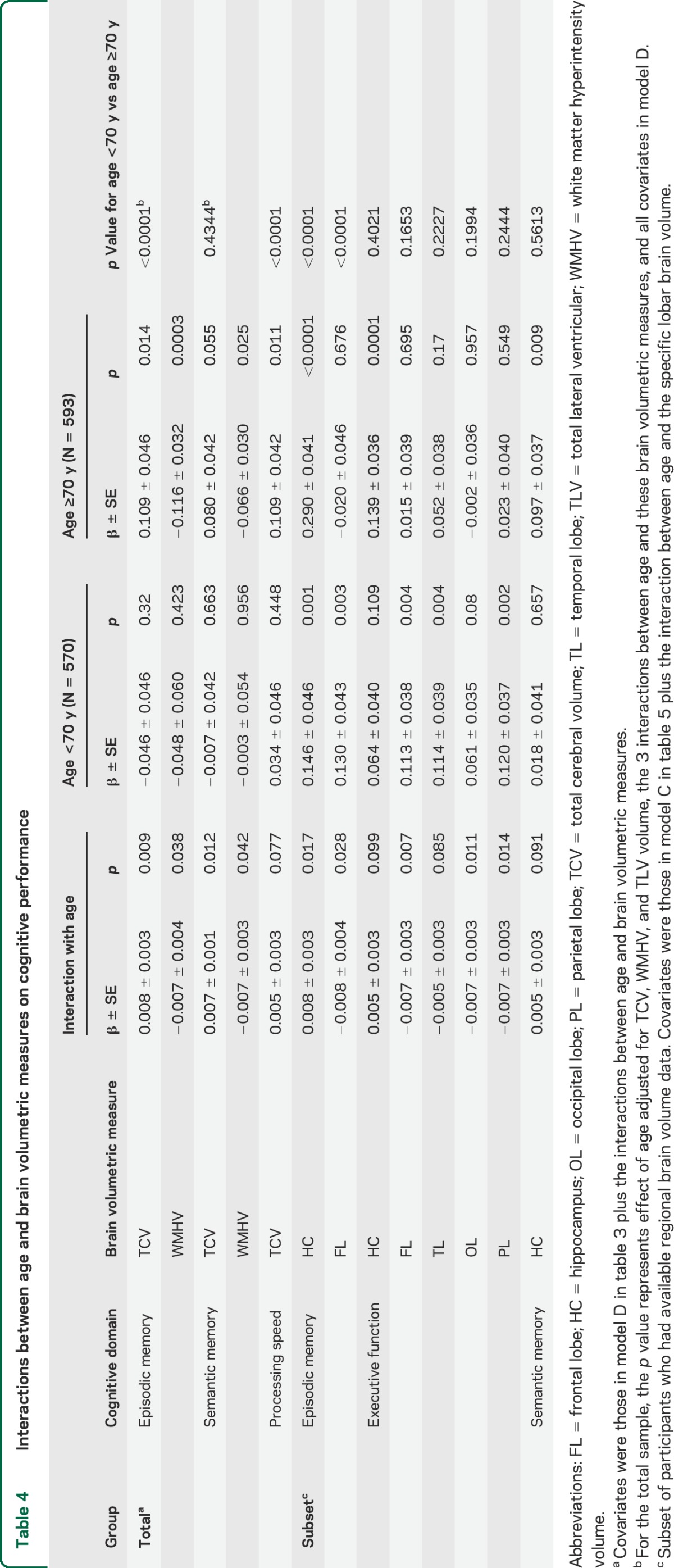
Examining the whole sample, larger TCV was not significantly associated with variability in cognitive performance in any domain after adjusting for age, sex, race/ethnicity, and education. This remained true after adjusting further for lifestyle and vascular factors, APOE genotype, and other brain measures (table 3). In contrast, those with larger TLV volume and WMHV had worse EMEM, PS, and SMEM performance after adjusting for age, other sociodemographic and vascular risk factors, APOE ε4 allele status, and TCV. Those with larger WMHV showed worse EXEC performance after adjusting for sociodemographic, lifestyle, and vascular risk factors, but this attenuated slightly after adjusting for APOE ε4 status and did not reach significance after adjusting for other brain measures (p = 0.09, table 3). Both TCV and WMHV interacted with age in relation to EMEM and SMEM performance, and there was a weaker interaction between TCV and age in relation to PS (table 4), whereas TLV volume did not interact with age. We therefore stratified at median age and found that smaller TCV was associated with worse memory and PS, whereas greater WMHV was associated with worse performance in EMEM and SMEM, in those older than age 70 after adjusting for vascular factors, APOE ε4 status, and other brain measures (table 4).
Table 3.
Association of white matter hyperintensity volume and total brain volumetric measure ratiosa with cognitive performance by domain (N = 1,163)
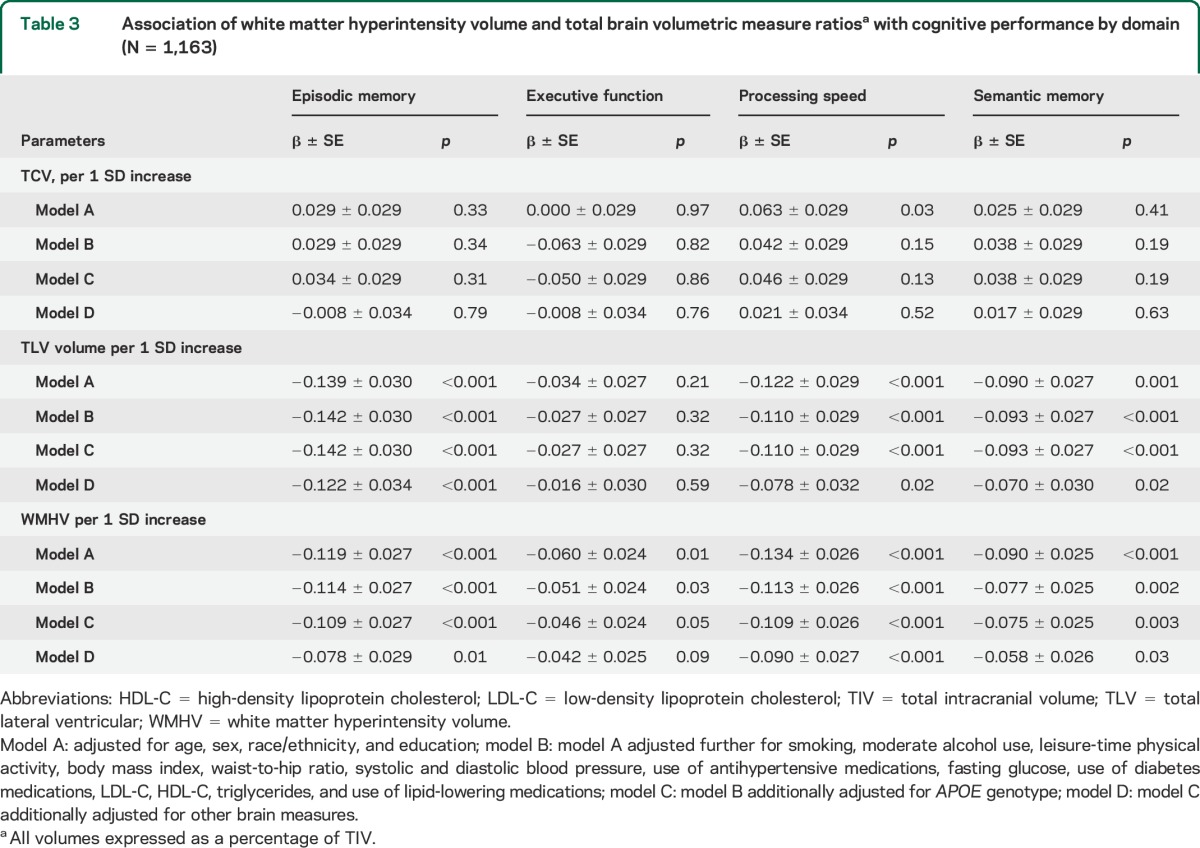
Table 4.
Interactions between age and brain volumetric measures on cognitive performance
Regional GM volumes explained some variability in domain-specific cognitive performance among 813 participants with available data (tables 2 and 5). Those with smaller hippocampal volumes showed worse performance across cognitive domains, especially EMEM, after adjusting for sociodemographic and vascular risk factors and APOE genotype (table 5). Those with smaller frontal GM volumes performed significantly worse in the EXEC domain. Participants with smaller temporal lobe and parietal lobe GM volumes performed worse in EMEM and EXEC domains with a trend for worse performance in the SMEM domain, and smaller temporal lobe GM volumes contributed to worse performance in the PS domain. Occipital lobe GM volumes did not explain variability in domain-specific cognitive performance (table 5).
Table 5.
Association of lobar brain volume measure ratiosa with cognitive performance by domain (N = 813)
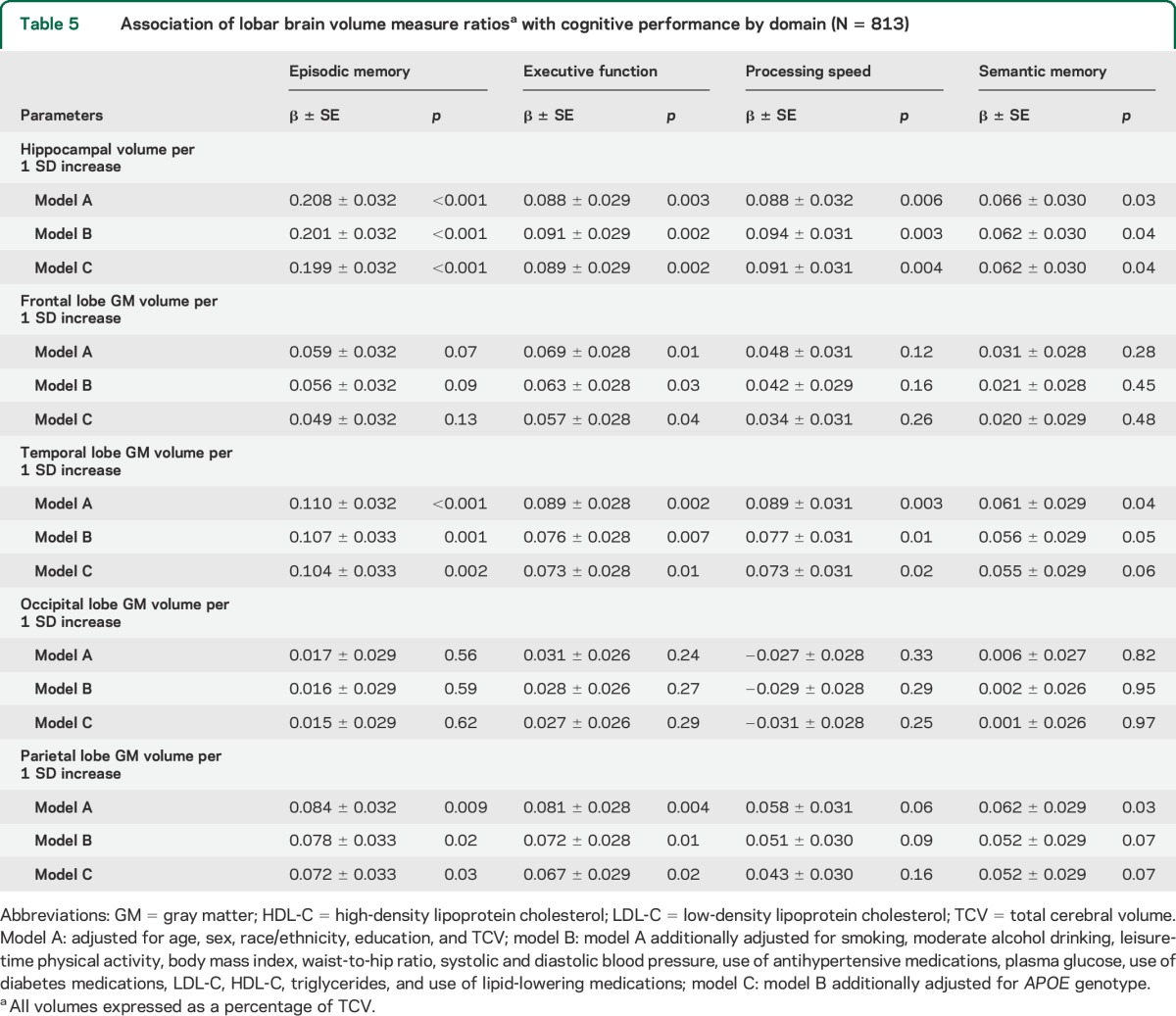
Several lobar GM volumes and hippocampal volumes interacted with age in relation to domain-specific cognitive performance (table 4). Age was an effect modifier such that those with smaller hippocampal volumes showed worse EMEM performance across the age range, especially among older participants. In addition, older, but not younger, participants with smaller hippocampal volumes performed worse in the EXEC and SMEM domains. Finally, smaller frontal lobe, temporal lobe, and parietal lobe GM volumes were associated with worse performance in the EXEC domain only among those younger than 70. Neither race/ethnicity nor sex acted as effect modifiers of associations between MRI markers and cognitive performance. We did not find significant interactions for any other covariates.
DISCUSSION
In this community-based sample of older adults free of clinical stroke, we observed that participants with a greater burden of white matter lesions and those with larger lateral ventricles exhibited worse performance across several domains. We found region-specific differences in a subset of participants, as hippocampal volumes were a strong indicator of performance across all cognitive domains, especially episodic memory, and smaller frontal lobe volumes were associated with worse executive function. Our findings did not vary significantly by race/ethnicity, suggesting consistency across this urban sample. We did find interactions with age such that variability in cognitive performance explained by the MRI markers was evident mostly in those 70 years or older. However, smaller hippocampal volumes were associated with worse memory across the full age range, and frontal lobe GM volume was a marker of executive function in younger participants.
Other studies, some of which were population-based, have reported relationships between total brain or cerebral volumes and cognitive performance.3,6 Unlike these other studies, TCV was not associated with overall cognitive performance in this diverse sample until we examined older participants separately. This is perhaps unsurprising since cerebral volume is less important than other factors such as efficiency of network connections and synaptic complexities that we are unable to assess using structural imaging. These differences could be due to methodologic differences across studies, as some studies used different measures of cerebral volume losses, such as visual rating scales, instead of quantitative measures of TCV. Also, some studies excluded those with dementia, thereby selecting older participants with more normal cognition, and others included participants from memory clinics, thereby selecting participants with greater cognitive disability.6 Because we excluded participants with clinical stroke but not those with cognitive disorders or dementia and the average age was 70, it is likely that we included both younger people at low risk of neurocognitive disorders and older people in whom these processes may have been active. The prevalence of cognitive impairment is estimated to be less than 5% in our cohort at the time of these assessments. Thus, TCV may not have been as strongly related to cognitive performance in our overall sample because of this heterogeneity, explaining why TCV was associated with memory and processing speed in older participants after stratifying at median age. Our data suggest that larger cerebral volumes could be protective in older age, and some prospective data support this idea.6
In this study and others, MRI-defined SVD was associated with worse cognition across domains.2,9,27 Small vessel damage caused by exposure to potentially modifiable vascular risk factors such as hypertension can cause arteriolosclerosis and cerebral hypoperfusion, resulting in demyelination or complete necrosis of white matter, which can, in turn, globally compromise cognition.28 In addition, we found that those 70 years or older with greater WMHV performed worse in episodic and semantic memory domains. This finding may be driven by the cumulative effects of cardiovascular risk factors or subclinical Alzheimer pathology on cerebrovascular integrity in this mixed sample, both of which may synergize with age in relation to cognitive performance. Cerebral amyloid angiopathy may underlie some white matter lesions29 in older participants and has been associated with Alzheimer disease in autopsy studies.30
We found 2 population-based studies reporting ventricular enlargement to be associated with worse executive function and processing speed, but they did not include Hispanic/Latino participants.2,31 TLV volume was more strongly associated with episodic and semantic memory than executive function in our cohort. TLV volume is a measure of central atrophy, and greater TLV volume may be a more sensitive marker of damage that affects cognition than TCV4 in this sample. Because TLV volume did not interact with age in relation to cognitive performance, TLV volume may serve as a marker of processes less dependent on aging itself. This finding is consistent with longitudinal studies showing that ventricular enlargement is a marker of dementia progression and may implicate neurodegenerative processes.4
Hippocampal volume has been associated with memory,32 but some, though not all, cross-sectional data have also shown that greater hippocampal volumes can correlate with better executive function.6,33 In this community-based sample unselected for cognitive status, smaller hippocampal volumes served as a marker of worse performance across all cognitive domains, reflecting the importance of the hippocampus in successful aging.34 That hippocampal volumes interacted with age in relation to memory function in both younger and older age groups suggests that hippocampus volumes are a general indicator of memory ability in late middle age as well as old age. Hippocampal volume also interacted with age in relation to executive function and semantic memory performance, and those 70 years or older with smaller hippocampal volumes had worse performance. This is most likely due to the later involvement of these cognitive domains as cognitive disorders advance.
Temporal and parietal lobe GM volumes were associated with performance in multiple domains, including memory and executive function. Perhaps unsurprisingly, frontal lobe GM volume was only associated with executive function. Other community-based studies with more homogeneous samples found that smaller frontal and temporal lobe volumes were associated with worse general cognitive performance.33 However, we were unable to find community-based studies that explicitly analyzed the relationship between parietal lobe volumes and cognition. Nonetheless, other studies have shown that executive performance is affected by processing speed and age-related memory performance.35 Also, free recall of noncontextual verbal information (list learning), which dominated our memory testing, requires some executive control.36 Some lobar volumes were associated with performance in several cognitive domains, but domain-specific constructs are somewhat artificial and the cognitive processes they represent are distributed. However, it is of interest that our stratified analysis showed that lobar GM volumes were markers of executive performance among those <70 years, whereas smaller TCV was associated with worse performance only in older participants. Since the TCV measure represents both gray and white matter structures in both hemispheres, it is likely to be a marker of a wider array of insults, whereas GM volume variability is more likely to be a marker of aging and neurodegeneration.
Strengths of the present study include our large ethnically and racially diverse population-based cohort that represents an urban US community. Longitudinal studies have reported that greater WMHV and smaller hippocampal volumes are related to greater risk of dementia or stroke, but few samples have been in clinically stroke-free or ethnically and racially diverse cohorts.3,5,10,34 One cross-sectional study investigated differences in brain morphology between Hispanics/Latinos, African Americans, and Caucasians but did not evaluate associations with cognitive performance.37 Another study that included Hispanics/Latinos examined the relationship of white matter lesions and cognition in the context of cognitive reserve,38 but we did not find studies examining regional GM volumes and domain-specific cognitive performance. Many studies with diverse cohorts had small samples or relatively young participants.11,39 Another strength of this study is our use of volumetric measures of SVD that are more reliable than visual rating scales.40
We cannot determine causality between brain morphology and cognitive performance because this study is cross-sectional. Residual confounding is likely in studies of this type. Also, since we did not identify cognitive disorders, we cannot tie our findings to specific causes. In addition, some of our analyses may have been underpowered, especially our interaction and regional brain volume analyses. Also, there may have been ceiling effects since half our sample is younger than 70 and both brain atrophy and cognitive dysfunction are less prevalent in this age group. Finally, the sample under study here survived and remained stroke-free until evaluation and is therefore healthier than the original cohort, but this should underestimate any associations between imaging markers and cognition compared with the whole sample.
In this urban community-based US population, central atrophy and SVD contributed to variability in cognitive performance, and smaller lobar GM volumes were related to worse cognitive performance in late middle age. Markers of brain atrophy and cerebral SVD may provide important information about underlying processes that affect cognition in older adults, and this requires further study.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the NOMAS staff, especially Janet DeRosa, the project manager.
GLOSSARY
- EMEM
episodic memory
- EXEC
executive function
- GM
gray matter
- NC
neurocognitive
- NOMAS
Northern Manhattan Study
- PS
processing speed
- SMEM
semantic memory
- SVD
small vessel disease
- TCV
total cerebral volume
- TIV
total intracranial volume
- TLV
total lateral ventricular
- WMHV
white matter hyperintensity volume
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
As principal investigator, Dr. Wright had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Chuanhui Dong: study concept and design, statistical analysis, analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content. Nooshin Nabizadeh: analysis and interpretation of data, critical revision of the manuscript for important intellectual content. Michelle Caunca: analysis and interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content. Ying Kuen Cheung: critical revision of the manuscript for important intellectual content. Tatjana Rundek: critical revision of the manuscript for important intellectual content, obtaining funding. Mitchell Elkind: acquisition of data, critical revision of the manuscript for important intellectual content, obtaining funding. Charles DeCarli: acquisition of data, critical revision of the manuscript for important intellectual content. Ralph Sacco: acquisition of data, critical revision of the manuscript for important intellectual content, obtaining funding. Yaakov Stern: study concept and design, analysis and interpretation of data, critical revision of the manuscript for important intellectual content, obtaining funding. Clinton Wright: study concept and design, study supervision, acquisition of data, analysis and interpretation of data, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
This work was supported by grants from the National Institute of Neurological Disorders and Stroke (K02NS059729 to C.B.W. and R37NS29993 to R.L.S.) and the Evelyn F. McKnight Brain Institute.
DISCLOSURE
C. Dong, N. Nabizadeh, M. Caunca, Y. Cheung, and T. Rundek report no relevant disclosures. M. Elkind receives compensation for providing consultative services for Biogen IDEC, Biotelemetry, BMS-Pfizer Partnership, Boehringer-Ingelheim, Daiichi-Sankyo, and Janssen Pharmaceuticals; receives research support from diaDexus, Inc., Bristol-Myers Squibb/Sanofi Pharmaceuticals Partnership, and the NIH/National Institute of Neurological Disorders and Stroke; has given expert legal opinions on behalf of Organon (NuvaRing and stroke litigation); serves as Resident and Fellow Section Editor for Neurology, for which he receives compensation from the AAN; and serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. He receives royalties from UpToDate for chapters related to stroke. C. DeCarli reports no relevant disclosures. R. Sacco receives support from (1) National Institute of Neurological Disorders and Stroke, Northern Manhattan Study, PI, 1993-present R37 NS29993; (2) National Institute of Neurological Disorders and Stroke, Family Study, PI, 2004-present R01 NS040807; and (3) National Institute of Neurological Disorders and Stroke, Stroke Prevention Intervention Research Program, PI, 2013-present. Y. Stern receives support from the NIH. During the last 2 years he was a consultant with Genentech with <$10,000 in honorarium. C. Wright receives federal grant support (K02 NS 059729, R01 HL 108623, SPRINT MRI WFUHS 330214, R37 NS 29993) and private foundation support (American Heart Association Bugher Center project 14BFSC17690003). He receives royalties from UpToDate for vascular dementia chapters. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Vincent GK, Velkoff VA. The Next Four Decades: The Older Population in The United States: 2010 to 2050. Washington, DC: U.S. Census Bureau; 2010. [Google Scholar]
- 2.Breteler MM, van Amerongen NM, van Swieten JC, et al. Cognitive correlates of ventricular enlargement and cerebral white matter lesions on magnetic resonance imaging: the Rotterdam Study. Stroke 1994;25:1109–1115. [DOI] [PubMed] [Google Scholar]
- 3.Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in relation to cognitive function and risk of dementia. Neurobiol Aging 2010;31:378–386. [DOI] [PubMed] [Google Scholar]
- 4.Carmichael OT, Kuller LH, Lopez OL, et al. Ventricular volume and dementia progression in the Cardiovascular Health Study. Neurobiol Aging 2007;28:389–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein G, Beiser AS, Decarli C, Au R, Wolf PA, Seshadri S. Brain imaging and cognitive predictors of stroke and Alzheimer disease in the Framingham Heart Study. Stroke 2013;44:2787–2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carmichael O, Mungas D, Beckett L, et al. MRI predictors of cognitive change in a diverse and carefully characterized elderly population. Neurobiol Aging 2012;33:83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas VA, Chao LL, Studholme C, et al. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging 2011;32:572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Au R, Massaro J, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol 2006;63:246–250. [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Beiser A, DeCarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: the Framingham Offspring Study. Stroke 2010;41:600–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stavitsky K, Du Y, Seichepine D, et al. White matter hyperintensity and cognitive functioning in the racial and ethnic minority cohort of the Framingham Heart Study. Neuroepidemiology 2010;35:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco RL, Gan R, Boden-Albala B, et al. Leisure-time physical activity and ischemic stroke risk: the Northern Manhattan Stroke Study. Stroke 1998;29:380–387. [DOI] [PubMed] [Google Scholar]
- 13.Siedlecki KL, Stern Y, Reuben A, Sacco RL, Elkind MS, Wright CB. Construct validity of cognitive reserve in a multiethnic cohort: the Northern Manhattan Study. J Int Neuropsychol Soc 2009;15:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siedlecki KL, Rundek T, Elkind MS, Sacco RL, Stern Y, Wright CB. Using contextual analyses to examine the meaning of neuropsychological variables across samples of english-speaking and spanish-speaking older adults. J Int Neuropsychol Soc 2012;18:223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marquine MJ, Attix DK, Goldstein LB, et al. Differential patterns of cognitive decline in anterior and posterior white matter hyperintensity progression. Stroke 2010;41:1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wright CB, Paik MC, Brown TR, et al. Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke 2005;36:1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decarli C, Maisog J, Declan MMG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr 1992;16:274–284. [DOI] [PubMed] [Google Scholar]
- 18.DeCarli C, Miller BL, Swan GE, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke 1999;30:529–536. [DOI] [PubMed] [Google Scholar]
- 19.Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 20.Fischl B. Automatically parcellating the human cerebral cortex. Cereb Cortex 2004;14:11–22. [DOI] [PubMed] [Google Scholar]
- 21.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 1999;9:179–194. [DOI] [PubMed] [Google Scholar]
- 22.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 2000;97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- 24.Hixson JE, Vernier DT. Restriction isotyping of human apolipoprotein E by gene amplification and cleavage with HhaI. J Lipid Res 1990;31:545–548. [PubMed] [Google Scholar]
- 25.Willey JZ, Moon YP, Paik MC, et al. Lower prevalence of silent brain infarcts in the physically active: the Northern Manhattan Study. Neurology 2011;76:2112–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warsch JR, Rundek T, Paik MC, Elkind MS, Sacco RL, Wright CB. Association between northern Manhattan study global vascular risk score and successful aging. J Am Geriatr Soc 2013;61:519–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wright CB, Festa JR, Paik MC, et al. White matter hyperintensities and subclinical infarction: associations with psychomotor speed and cognitive flexibility. Stroke 2008;39:800–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology 2011;77:461–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology 2006;66:23–29. [DOI] [PubMed] [Google Scholar]
- 30.Jellinger KA, Attems J. Prevalence and pathogenic role of cerebrovascular lesions in Alzheimer disease. J Neurol Sci 2005;229-230:37–41. [DOI] [PubMed] [Google Scholar]
- 31.Longstreth WT, Jr, Arnold AM, Manolio TA, Burke GL, Fried L. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people: the Cardiovascular Health Study. Neuroepidemiology 2000;19:30–42. [DOI] [PubMed] [Google Scholar]
- 32.den Heijer T, van der Lijn F, Koudstaal PJ, et al. A 10-year follow-up of hippocampal volume on magnetic resonance imaging in early dementia and cognitive decline. Brain 2010;133:1163–1172. [DOI] [PubMed] [Google Scholar]
- 33.Raji CA, Lopez OL, Kuller LH, et al. White matter lesions and brain gray matter volume in cognitively normal elders. Neurobiol Aging 2012;33:834.e7–834.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raji CA, Lopez OL, Kuller LH, Carmichael OT, Becker JT. Age, Alzheimer disease, and brain structure. Neurology 2009;73:1899–1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baudouin A, Clarys D, Vanneste S, Isingrini M. Executive functioning and processing speed in age-related differences in memory: contribution of a coding task. Brain Cogn 2009;71:240–245. [DOI] [PubMed] [Google Scholar]
- 36.Becker S, Lim J. A computational model of prefrontal control in free recall: strategic memory use in the California Verbal Learning Task. J Cogn Neurosci 2003;15:821–832. [DOI] [PubMed] [Google Scholar]
- 37.Brickman AM, Schupf N, Manly JJ, et al. Brain morphology in older African Americans, Caribbean Hispanics, and whites from northern Manhattan. Arch Neurol 2008;65:1053–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brickman AM, Siedlecki KL, Muraskin J, et al. White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging 2011;32:1588–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartzokis G, Beckson M, Lu PH, Nuechterlein KH, Edwards N, Mintz J. Age-related changes in frontal and temporal lobe volumes in men. Arch Gen Psychiatry 2001;58:461–465. [DOI] [PubMed] [Google Scholar]
- 40.Tiehuis AM, Vincken KL, Mali WP, et al. Automated and visual scoring methods of cerebral white matter hyperintensities: relation with age and cognitive function. Cerebrovasc Dis 2008;25:59–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


