Abstract
This study investigated how maternal overnutrition and obesity regulate expression and activation of proteins that facilitate lipid transport in the placenta. To create a maternal overnutrition and obesity model, primiparous C57BL/6 mice were fed a high-fat (HF) diet throughout gestation. Fetuses from HF-fed dams had significantly increased serum levels of free fatty acid and body fat. Despite no significant difference in placental weight, lipoprotein lipase (LPL) protein levels and activity were remarkably elevated in placentas from HF-fed dams. Increased triglyceride content and mRNA levels of CD36, VLDLr, FABP3, FABPpm, and GPAT2 and -3 were also found in placentas from HF-fed dams. Although both peroxisome proliferator–activated receptor-γ (PPARγ) and CCAAT/enhancer binding protein-α protein levels were significantly increased in placentas of the HF group, only PPARγ exhibited a stimulative effect on LPL expression in cultured JEG-3 human trophoblasts. Maternal HF feeding remarkably decreased SIRT1 expression in placentas. Through use of an SIRT1 activator and inhibitor and cultured trophoblasts, an inhibitory effect of SIRT1 on LPL expression was demonstrated. We also found that SIRT1 suppresses PPARγ expression in trophoblasts. Most importantly, inhibition of PPARγ abolished the SIRT1-mediated regulatory effect on LPL expression. Together, these results indicate that maternal overnutrition induces LPL expression in trophoblasts by reducing the inhibitory effect of SIRT1 on PPARγ.
Introduction
Obesity is a risk factor of type 2 diabetes and cardiovascular diseases. Studies have demonstrated that prepregnant maternal BMI and gestational weight gain are closely associated with birth weight (1–3). Importantly, high birth weight predicts higher BMI and obesity during both childhood and adulthood (4,5). Therefore, in addition to calorie-rich foods, sedentary lifestyle, and genetic defects, undesirable intrauterine metabolic exposure might contribute significantly to the ongoing obesity epidemic.
At birth, infant body weight is mainly determined by lean and fat tissue mass. Human studies have demonstrated that maternal obesity and excess gestational weight gain increase infant body fat and birth weight (1,3,6). Although rodent neonates have very limited amounts of fat tissue mass compared with humans, maternal high-fat (HF) feeding increases fat in both fetuses and newborn mice (7,8). Fetal lipid deposition increases exponentially with gestational age (9,10). Some of the accumulated lipids arise from de novo lipogenesis, but the bulk of fetal lipids is derived from the maternal circulation through placental fatty acid (FA) transport (10).
Triglyceride (TG)-enriched VLDL in maternal circulation is the main FA supplier for the fetus (9,10). TGs cannot be transported through the placenta. Only nonesterified FAs can be taken up by the microvillus membrane and transported to the fetus. Therefore, FAs from TGs should be released by lipases at the placenta. Several TG lipases, including lipoprotein lipase (LPL) and endothelial lipase, have been identified in human and rodent placentas (10–12).
LPL gene expression increases dramatically during the last trimester of pregnancy, paralleling increased placental FA transport (12–14). Placental LPL expression and activity positively correlate with fetal size and fetal adipose tissue mass (14). Importantly, studies have demonstrated that maternal obesity or HF feeding increases placental LPL expression and activity (15,16). Therefore, increased placental LPL may enhance fetal FA supply and increase fetal fat accretion. However, the mechanisms of maternal overnutrition and/or obesity-increased placental LPL expression are largely unknown.
SIRT1 (silent mating type information regulation 2 homology 1) is an NAD-dependent protein deacetylase that regulates energy metabolism, aging, and other cellular processes (17). Energy deficiency increases the NAD level and NAD/NADH ratio, leading to SIRT1 activation (18). In contrast, sufficient nutrient supply provides substrates to generate ATP, whereas NAD is converted to NADH, leading to SIRT1 deactivation. In addition, overnutrition and obesity reduce SIRT1 expression in various tissues (17,19). In mammals, by controlling expression and/or acetylation of some transcription factors and enzymes, SIRT1 stimulates hepatic gluconeogenesis and suppresses lipogenesis in adipocytes (17,20–22). SIRT1 is highly expressed in trophoblasts (23,24); however, its regulatory role in placental nutrient transport has yet to be studied.
The present study compared gene expression profiles between placentas from HF diet– and chow-fed dams and revealed that maternal HF feeding increases placental LPL expression and activity accompanied by elevated peroxisome proliferator–activated receptor-γ (PPARγ) but decreased SIRT1 expression. Using cultured trophoblasts and SIRT1- or PPARγ-specific agonist and antagonist, the study further demonstrated that SIRT1 suppresses LPL expression through inhibiting PPARγ. From these results, we propose that maternal overnutrition decreases SIRT1 expression and, consequently, increases PPARγ activity and LPL expression in the placenta.
Research Design and Methods
Materials
Rosiglitazone (ROSI), GW9662, resveratrol (RSV), and 6-chloro-2,3,4,9-tetrahydro-1H-carbazole-1-carboxamide (EX-527) were from Sigma (St. Louis, MO). Anti-LPL antibody was from GeneTex (Irvine, CA). The LPL activity assay kit was from Cell Biolabs, Inc. (San Diego, CA). Antibodies for PPARγ, fatty acid synthase (FASN), and β-actin proteins were from Cell Signaling Technology, Inc. (Danvers, MA). Anti-SIRT1, CCAAT/enhancer binding protein-α (C/EBPα), and SREBP1c antibodies were from Santa Cruz Biotechnology (Dallas, TX). FBS, penicillin-streptomycin, DMEM, and F-12K medium were from Invitrogen (Carlsbad, CA). HF diet (60 kcal% from fat, 20 kcal% from protein, 20 kcal% from carbohydrate, energy density 5.24 kcal/g; catalog number D12492) was from Research Diets, Inc. (New Brunswick, NJ). Regular chow (17 kcal% from fat, 25 kcal% from protein, 58 kcal% from carbohydrate, energy density 3.1 kcal/g; catalog number 7912) was from Harlan Laboratories (Madison, WI).
Animal Models
C57BL/6 mice were from The Jackson Laboratory (Bar Harbor, ME). Three-month-old primiparous female mice were used as dams. Pregnancy was determined by the presence of a vaginal plug and was assigned the title E0.5. HF diets were immediately provided to the vaginal plug–positive mice. The control dams were fed regular chow. Placentas and fetuses were collected by caesarean section at E15.5, E17.5, and E18.5. After removing placentas and fetuses, dam body composition was determined using EchoMRI, which uses a specialized nuclear magnetic resonance-magnetic resonance imaging technology with a coefficient of variation of 3.71% (fat) or 4.55% (lean). Experiments using mouse models were carried out under Association for Assessment and Accreditation of Laboratory Animal Care guidelines with approval from the University of California, San Diego, Animal Care and Use Committee.
Blood Glucose, Free FA, TG, and Insulin Assay
Blood samples were collected from dams and fetuses by submandibular vein puncture and beheading, respectively. Serum glucose levels were measured using glucose oxidase reagents from Sigma. Free FA (FFA) and TG levels were determined using kits from Wako (Richmond, VA). Serum insulin concentrations were measured using a mouse Diabetes 8-Plex kit and Bio-Plex MAGPIX multiplex reader (Bio-Rad, Hercules, CA).
Placental TG and Fetal Body Fat Measurement
Placental tissues were homogenized in ice-cold PBS. Placental lipids were extracted according to Bligh and Dyer (25), solvents were evaporated under nitrogen bath, and samples were redissolved in 2% Triton X (Fisher Biotech, Bridgewater, NJ) in water. A kit (Wako) was used to determine TG content. Fetal body fat was determined by lipid extraction (26). Briefly, fetal carcasses were dried at 103°C to constant weight. The drying was followed by fat extraction with petroleum ether (Alfa Aesar, Heysham, U.K.) in a Soxhlet apparatus (Cole Parmer, Vernon Hills, IL) for at least 10 cycles. Fat mass was calculated by the difference in dry mass of the carcass before and after the extraction.
Western Blot and Real-Time PCR Assays
Protein samples were separated using NuPAGE gels (Invitrogen). Protein was blotted with the indicated antibodies (see details in figure legends). The bands from Western blots were quantified using Quantity One software (Bio-Rad). Total RNA was prepared from placentas with TRIzol (Invitrogen) following the manufacturer’s protocol. cDNA was synthesized using SuperScript III Reverse Transcriptase and oligo(dT)12–18 primer (Invitrogen). Real-time PCR was performed using an Mx3000P real-time PCR system (Stratagene) and SYBR Green dye (Molecular Probes, Eugene, OR) with an annealing temperature at 60°C and gene-specific primers (Table 1). The levels of PCR product were calculated from standard curves established from each primer pair. Expression data were normalized to the amount of 18S rRNA.
Table 1.
Sequences for real-time PCR primers
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| 18S rRNA | CGAAAGCATTTGCCAAGAAT | AGTCGGCATCGTTTATGGTC |
| LPL | AATTTGCTTTCGATGTCTGAGAA | CAGAGTTTGACCGCCTTCC |
| FASN | ACTCCACAGGTGGGAACAAG | CCCTTGATGAAGAGGGATCA |
| GPAT1 | TGGGATACTGGGGTTGAAAA | GGAAGGTGCTGCTATTCCTG |
| GPAT2 | GCTGCCAGACCTGTACTCCT | AGCCCAGGTCCATTATGCTT |
| GPAT3 | GTGCTGGGTGTCCTAGTGC | AAGCTGATCCCAATGAAAGC |
| GPAT4 | CCACCCTGAGAATGGAGAGA | TCCAGAGAAGTGGGATCTTTTG |
| SIRT1 | GCTTCATGATGGCAAGTGG | TCGTGGAGACATTTTTAATCAGG |
| VLDLr | CCTATAACTAGGTCTTTGCAGATATGG | GAGCCCCTGAAGGAATGCC |
| CD36 | CCAGTGTATATGTAGGCTCATCCA | TGGCCTTACTTGGGATTGG |
| FABP3 | TGGTCATGCTAGCCACCTG | CTTTGTCGGTACCTGGAAGC |
| FABPpm | ATCTGGAGGTCCCATTTCAA | ATGGCTGCTGCCTTTCAC |
Cell Culture
Human BeWo and JEG-3 trophoblasts were cultured in F-12K or DMEM with 10% FBS. Confluent cells were transduced with adenoviral (Ad) vectors encoding SIRT1 or PPARγ for 24 h. Ad-green fluorescent protein (GFP) was used as control. The construction and purification of the viral vectors were previously described (19).
Statistical Analysis
Data are expressed as mean ± SEM. Statistical analyses were performed using the Student t test or ANOVA followed by Bonferroni posttest using Prism software. Differences were considered significant at P < 0.05.
Results
Maternal HF Feeding During Gestation Increases Fetal Body Fat, Placental TG Content, and Fetal Serum FFA
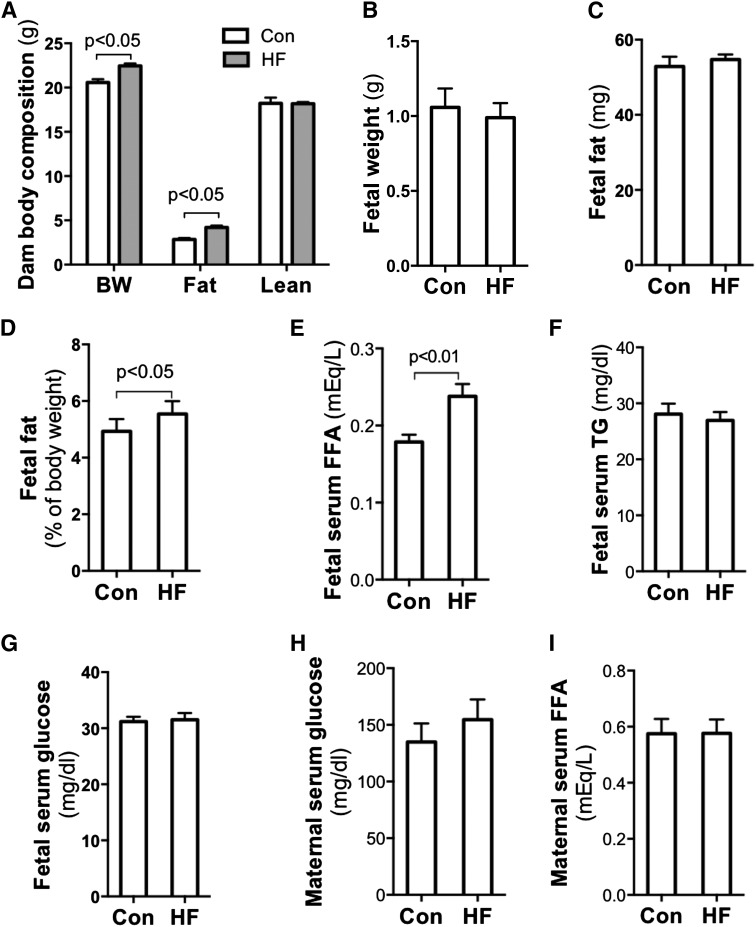
Prepregnant maternal obesity and excessive gestational weight gain are associated with high birth weight (1–3). To focus on the effect of maternal overnutrition on fetal fat accumulation and to eliminate the effects of preexisting maternal obesity on fetal development, HF diets were provided to dams only during the gestational period. Although energy intake of HF-fed dams was only slightly higher than that of controls (Supplementary Fig. 1A and B), remarkably elevated fat tissue mass was observed in HF-fed dams from E15.5 to E18.5 (Fig. 1A and Supplementary Fig. 1C). Similar to previous rodent studies reporting an adverse effect of maternal HF feeding on fetal growth (27–30), we also found a trend of decreased body weight (P = 0.0521) (Fig. 1B) and lean body mass (Supplementary Fig. 1D) in E18.5 fetuses of HF-fed dams. There was no change in body fat content of E18.5 fetuses of HF-fed dams (P = 0.5437) (Fig. 1C). However, adiposity of E18.5 fetuses from HF-fed dams was significantly higher than that of control fetuses (Fig. 1D). At E15.5 and E17.5, no differences were seen in either fetal body weight or adiposity (Supplementary Fig. 1E and F). Together, these data indicate that gestational period HF feeding induces both maternal and fetal adiposity in mice. However, the effect on fetal adiposity happens in late gestation. For the rest of the study, only E18.5 fetal samples were analyzed.
Figure 1.
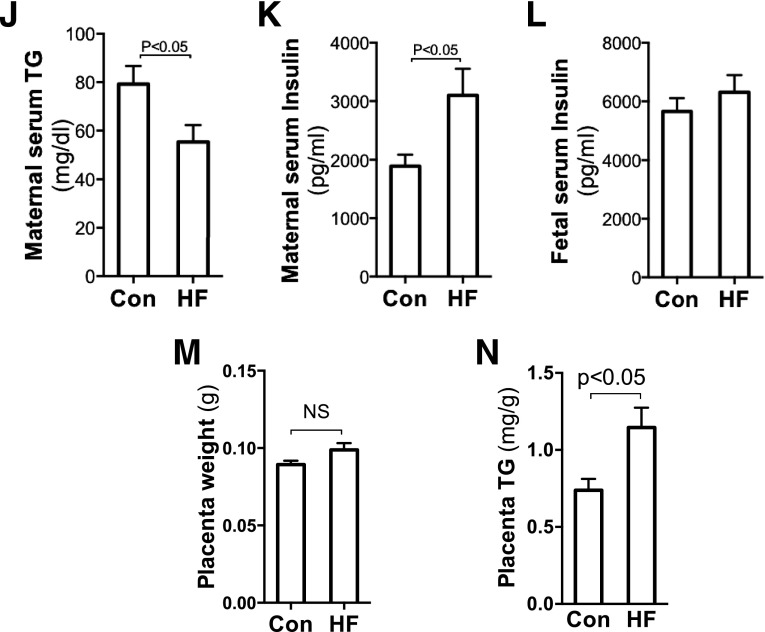
Maternal HF feeding during gestation increased fetal body fat and placental TG content. Ten- to 12-week-old nulliparous C57BL/6 female mice were mated with chow-fed males. HF diet was provided to dams once the vaginal plug was detected. Fetuses, placentas, and other tissue samples were collected at E18.5 in the fed state through caesarean section. Increased body weight and body fat were assayed in HF-fed dams by EchoMRI scanning (n = 6–8) (A). Fetal body fat was measured using petroleum ether fat extraction. Although there were no remarkable differences in fetal body weight (B) and body fat (C), significantly elevated adiposity (D) was observed in fetuses from HF-fed dams (n = 20–36). Compared with fetuses of chow-fed dams, remarkably increased serum FFA levels (E) but not TG (F) and glucose (G) levels were detected in fetuses of HF-fed dams (n = 8). Comparing HF- and chow-fed dams, there were no significant differences in blood glucose (H) and FFA (I) levels; however, significantly decreased serum TG (J) and increased insulin (K) levels were found in HF-fed dams. Of note, fetal serum insulin concentrations were comparable between groups (L). There was no difference in placental weight (M), but placental TG content was significantly higher in the HF group (n = 6) (N). BW, body weight; Con, control; NS, not significant.
To characterize the impact of maternal HF feeding on fetal metabolism, we measured metabolic parameters of blood and placentas. As shown in Fig. 1E–G, fetal serum FFA concentration in HF-fed dams was significantly increased, but TG and glucose levels were similar between the two groups. In maternal circulation, serum glucose and FFA levels were comparable between HF- and chow-fed dams (Fig. 1H and I). However, there were significant decreases in TG levels in HF-fed dams (Fig. 1J). HF feeding significantly increased insulin levels in maternal blood but not in fetal circulation (Fig. 1K and L). Apparently, maternal HF feeding alters glucose and lipid metabolism in both the maternal and the fetal compartment. However, the impacts on the major metabolic markers are different between fetuses and dams. These differences suggest that the effects of maternal obesity on fetal metabolism are not simply through a passive transplacental nutrient diffusion. Of note, a slight increase of placental weight was found in the HF-fed group (Fig. 1M). However, placental TG levels in HF-fed dams were remarkably higher than in the controls (Fig. 1N). The elevated fetal serum FFA and placental TG content support the hypothesis that maternal HF feeding increases placental FA transport.
Maternal HF Feeding Increases Placental LPL Expression and Activity
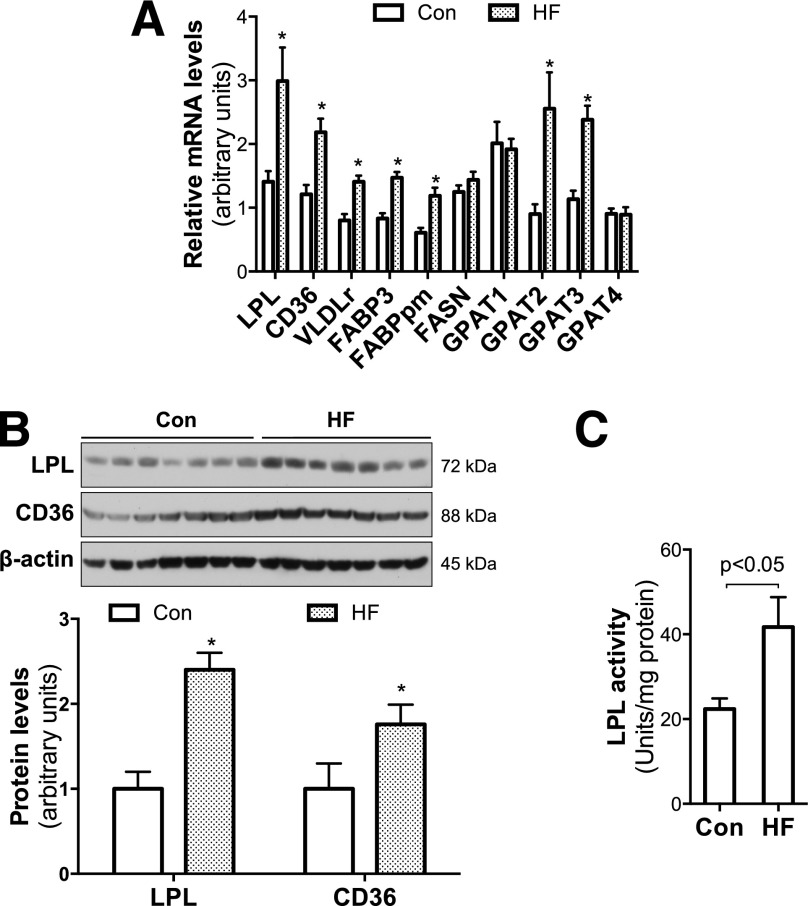
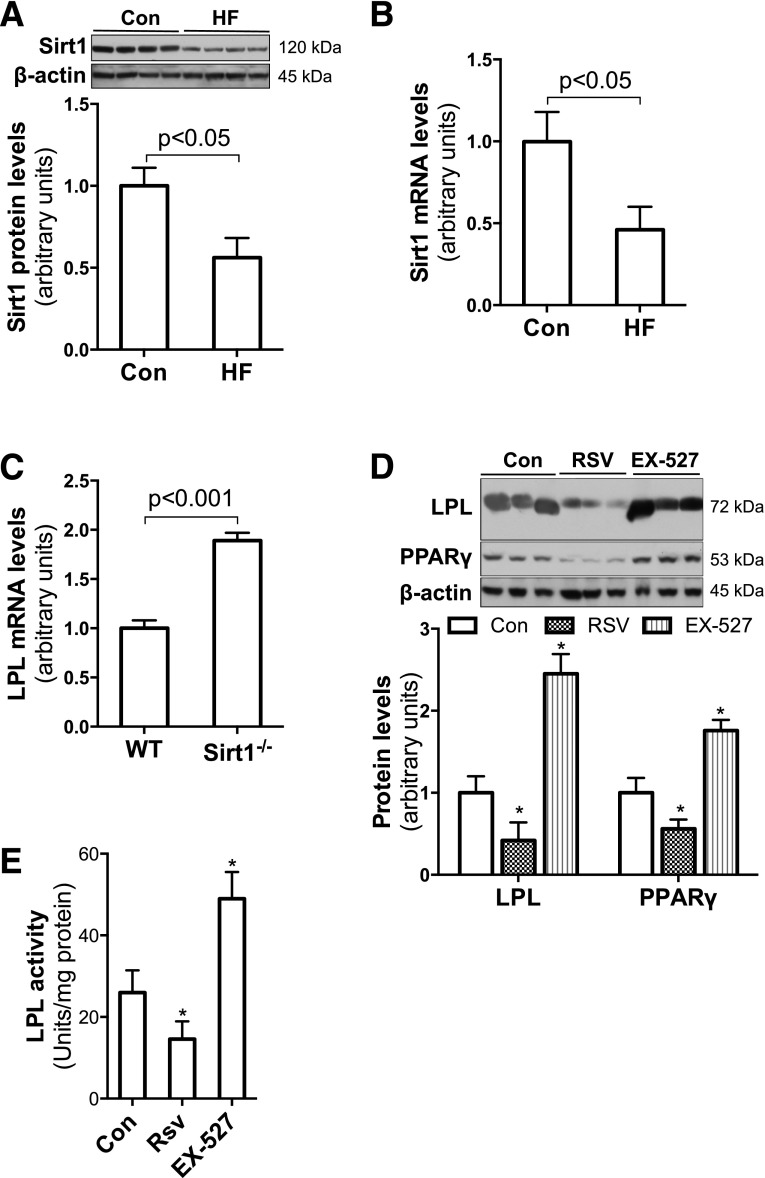
To study the effect of maternal HF feeding on placental lipid metabolism, we compared mRNA levels of the key genes in these processes. The results showed that mRNA levels of genes for TG hydrolysis and FA transport, such as LPL, CD36, VLDLr, FABP3, and FABPpm, are remarkably increased in placentas from HF-fed dams (Fig. 2A). The results of immunoblotting (Fig. 2B) also revealed a significant increase in LPL and CD36 protein in placentas from HF-fed dams. Consistent with elevated mRNA and protein levels, significantly increased LPL activities were detected in the placentas of HF-fed dams (Fig. 2C). Together, these results indicate that maternal HF feeding increases expression of proteins that facilitate TG hydrolysis and FA uptake and transport in placentas.
Figure 2.
Maternal HF feeding increased placental LPL expression and activity and genes that facilitate lipid transport. E18.5 placental samples were collected from chow- and HF-fed dams. A: Using real-time PCR, significantly increased mRNA of LPL and other key genes that facilitate lipid transport were detected in placentas from HF-fed dams (n = 6). B: Western blotting further revealed higher protein levels of LPL and CD36 in placentas from HF-fed dams (n = 7). C: Parallel with the increased gene expression, significantly elevated LPL activities were found in placentas of HF-fed dams (n = 8). *P < 0.05 vs. control. Con, control.
This study also found that expression of rate-limiting enzymes of triacylglycerol synthesis glycerol-3-phosphate acyltransferase 2 (GPAT2) and GPAT3 was significantly increased by maternal HF feeding (Fig. 2A). Together with increased placental TG levels (Fig. 1N), these data suggest that maternal HF feeding increases placental FA transport and TG synthesis, which may lead to higher placental TG content. However, unaltered FASN expression (Fig. 2A) implies that placental de novo lipogenesis is unlikely to underlie maternal obesity-increased placental TGs and fetal FFAs.
Maternal HF Feeding Increases PPARγ Expression, Leading to Stimulation of LPL Expression in Trophoblasts
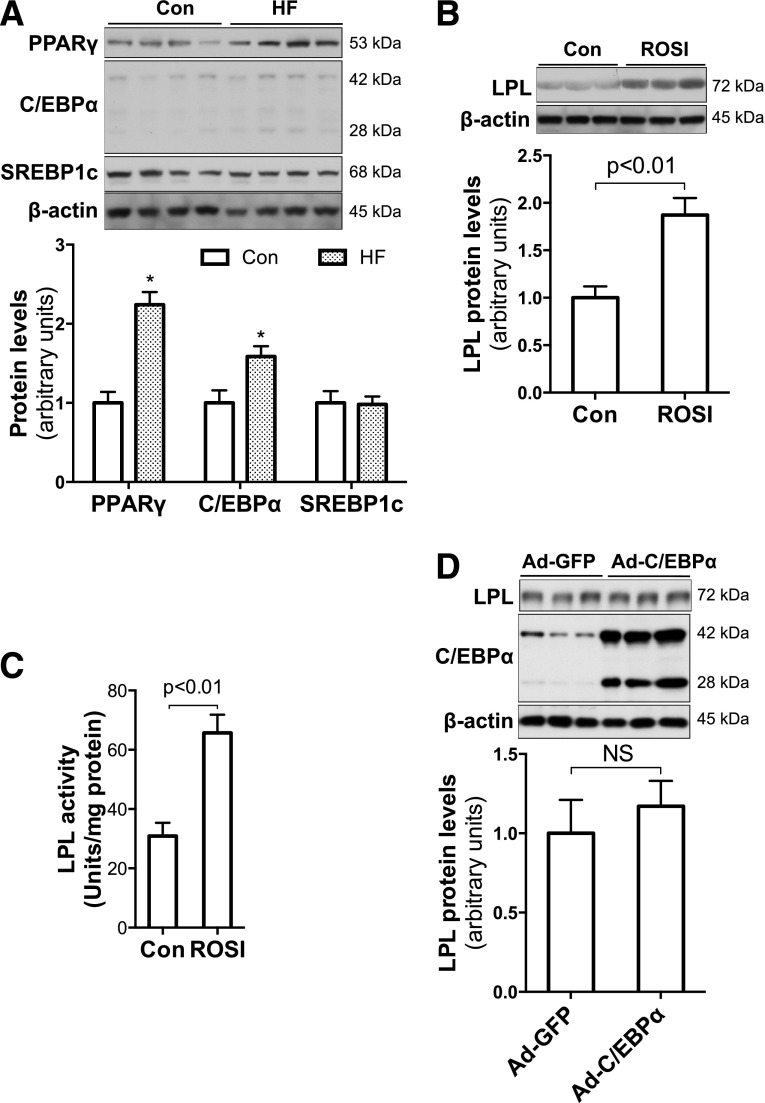
Elevated mRNA levels of LPL and other genes in placentas from HF-fed dams suggest that the upregulation may be at the transcriptional level. We measured protein levels of three key lipogenic transcription factors: PPARγ, C/EBPα, and SREBP1c. Figure 3A shows that both PPARγ and C/EBPα were significantly increased in placentas of HF-fed dams, whereas no change was apparent in mature SREBP1c.
Figure 3.
HF feeding during pregnancy increased PPARγ expression in placentas, and PPARγ stimulated LPL expression in trophoblasts. A: Significantly increased PPARγ and C/EBPα protein levels were revealed in placentas of HF-fed dams (n = 7–8). *P < 0.05 vs. control. B and C: Overnight (13 h) treatment with the PPARγ agonist ROSI (10 μmol/L) robustly increased LPL expression and activities in confluent JEG-3 trophoblasts (n = 6). D: However, Ad-C/EBPα overexpression (24 h) did not alter LPL protein levels in JEG-3 trophoblasts (n = 6). Con, control; NS, not significant.
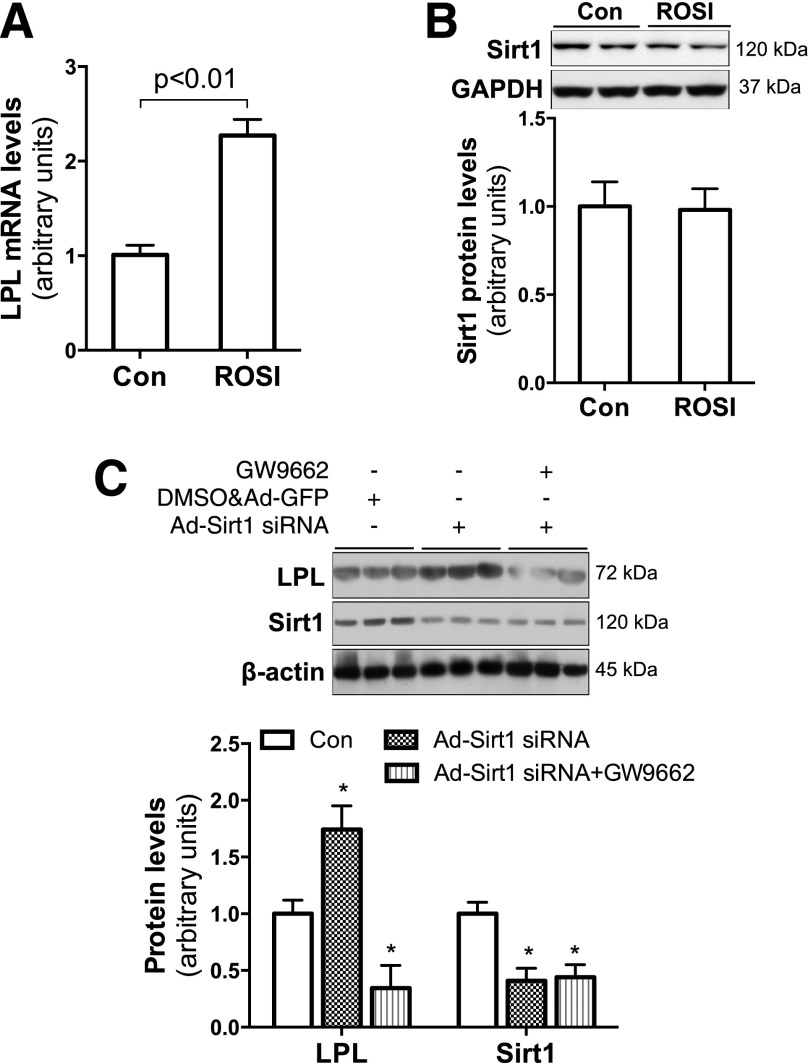
The regulatory effects of PPARγ on LPL gene expression have been reported in adipocytes (31). To verify the role of increased PPARγ in maternal HF feeding–increased LPL expression in the placenta, we treated JEG-3 trophoblasts with the PPARγ agonist ROSI. Significantly high levels of LPL protein and activity were found in ROSI-treated JEG-3 cells (Fig. 3B and C), indicating that PPARγ stimulates LPL expression in trophoblasts.
We next studied the effect of C/EBPα on LPL gene expression using a C/EBPα-encoding Ad vector to transduce JEG-3 cells. As shown in Fig. 3D, LPL protein levels were similar between cells transduced with Ad-C/EBPα and Ad-GFP. This result indicates that despite the maternal HF feeding increase in C/EBPα in placentas, C/EBPα does not increase LPL expression in trophoblasts. Together, these results suggest that increased PPARγ mediates maternal HF feeding–enhanced LPL expression in the placenta.
SIRT1 Inhibits LPL Gene Expression in JEG-3 Trophoblasts
SIRT1 is a protein deacetylase that plays an important role in regulating cellular metabolism. SIRT1 expression is enhanced by fasting or calorie restriction and inhibited by HF feeding (22,32). As expected, significantly reduced SIRT1 protein and mRNA levels were found in placentas of HF-fed dams relative to controls (Fig. 4A and B), which indicates that maternal HF feeding reduces SIRT1 expression in placentas. This observation led us to hypothesize that decreased SIRT1 may be involved in maternal HF feeding–altered placental lipid metabolism.
Figure 4.
Maternal HF feeding reduced expression of SIRT1, which suppresses LPL expression in trophoblasts. Western blotting and real-time PCR demonstrated remarkably reduced SIRT1 protein (A) and mRNA (B) levels in placental samples of E18.5 HF-fed dams (n = 6). Compared with WT MEFs, significantly higher LPL mRNA levels were found in Sirt1−/− MEFs (n = 6) (C). Confluent JEG-3 trophoblasts were treated with SIRT1 activator RSV (10 μmol/L) or inhibitor EX-527 (1 μmol/L) overnight (13 h). EX-527 treatment robustly increased LPL protein levels and activities (D and E). In contrast, significantly reduced LPL protein levels and activities were found in RSV-treated trophoblasts (n = 6) (D and E). *P < 0.05 vs. control. Con, control.
To look at the regulatory effect of SIRT1 on LPL expression, mouse embryo fibroblasts (MEFs) from Sirt1−/− and wild-type (WT) mice were used as a cellular model. We compared LPL expression levels between WT and Sirt1−/− MEFs. Remarkably higher levels of LPL mRNA were detected in Sirt1−/− MEFs than in WT cells (Fig. 4C), which suggests that SIRT1 suppresses LPL expression.
To further study the effect of SIRT1 on LPL expression in trophoblasts, we treated JEG-3 cells with the SIRT1 activator RSV or the inhibitor Ex-527. As shown in Fig. 4D, significantly reduced LPL protein levels were found in RSV-treated cells, whereas robustly increased LPL protein levels were observed in EX-527–treated cells. Parallel to the changes in LPL protein expression, remarkably altered LPL activities were detected in RSV- and EX-527–treated JEG-3 cells (Fig. 4E). Together, these studies demonstrate that SIRT1 inhibits LPL expression in trophoblasts.
Inhibition of PPARγ Abolishes Sirt1 Knockdown–Induced Upregulation of LPL in JEG-3 Cells
Results from the aforementioned studies demonstrate opposite effects of PPARγ and SIRT1 on LPL gene expression in trophoblasts. Of note, increased PPARγ but decreased SIRT1 protein levels were observed in placentas from HF-fed dams (Figs. 3A and 4A). Previous studies demonstrated that SIRT1 inhibits PPARγ expression and activity in adipocytes (22,33,34). SIRT1 and PPARγ can reciprocally regulate each other (34,35), raising a question about the relationship of PPARγ and SIRT1 in maternal HF feeding–enhanced placental LPL expression.
Treatment of Sirt1−/− MEFs with the PPARγ agonist ROSI significantly increased LPL mRNA (Fig. 5A), indicating that PPARγ upregulates LPL expression independent of SIRT1. In addition, overnight ROSI treatment did not change SIRT1 protein levels in JEG-3 trophoblasts (Fig. 5B). In contrast, SIRT1 activation led to a reduction in PPARγ protein levels in these cells (Fig. 4D). Together, these results suggest that SIRT1 inhibits PPARγ expression in trophoblasts. Therefore, we hypothesize that decreased SIRT1 mediates HF feeding–induced placental LPL expression by enhancing PPARγ activity.
Figure 5.
SIRT1 inhibits LPL expression by suppressing PPARγ expression in trophoblasts. To verify the relationship between SIRT1 and PPARγ in regulating LPL expression, Sirt1−/− MEFs (A) and JEG-3 trophoblasts (B) were treated with PPARγ agonist ROSI (10 μmol/L) for 13 h. ROSI treatment robustly increased LPL mRNA in Sirt1−/− MEFs (n = 6) (A) but showed no effect on SIRT1 protein expression in trophoblasts (n = 6) (B). By using viral vector–mediated small interfering RNA (siRNA) overexpression, Sirt1 was significantly knocked down in JEG-3 trophoblasts (C). However, increased LPL expression due to Sirt1 knockdown was abolished in PPARγ inhibitor GW9662-treated cells (3 μmol/L, 13 h) (n = 6) (C). *P < 0.05 vs. control. Con, control.
We used Sirt1 gene knockdown and the PPARγ inhibitor GW9662 to test this hypothesis. Knockdown of Sirt1 significantly increased LPL gene expression (Fig. 5C); however, this stimulative effect was abolished in GW9662-treated cells, suggesting that SIRT1 regulates LPL gene expression through PPARγ. These results also support our hypothesis that maternal HF feeding increases PPARγ-controlled LPL expression by reducing SIRT1 expression in the placenta.
Discussion
Human fetal fat deposition increases exponentially with gestational age, and most of the fat accumulation occurs during the third trimester. Unlike humans, mice are altricial, having a very limited amount of fat tissue at birth. Despite the differences in fetal fat deposition, most human and mouse studies have demonstrated a positive association of maternal adiposity with fetal fat accumulation. In the present study, all dams had similar prepregnant body fat levels (data not shown), and the HF diet was provided only during the gestational period. Although total energy intake was similar, HF-fed dams gained significantly more body fat. These data indicate that HF feeding during gestation induces maternal obesity in mice. The results of significantly increased adiposity of E18.5 fetuses further demonstrate that HF feeding or maternal obesity increases fetal fat in mice. An interesting finding of this study is that maternal HF feeding of mice has no significant effect on placental weight, whereas other studies have found that maternal BMI correlates with placental weight, which is predictive of neonatal body weight (36,37). The discordant changes in fetal body fat and placental mass from HF-fed dams led us to postulate that enhanced nutrient transport activity, rather than placental tissue mass, plays an important role in HF feeding or maternal obesity-induced fetal fat accumulation. The remarkably increased fetal blood FFA and placental TG content from HF-fed dams support this notion.
In the placenta, LPL is mainly expressed in syntotrophoblasts and catabolize TGs from maternal blood. LPL gene expression and activity increase dramatically during the last trimester of pregnancy, correlating with increased placental FA transport (11,12,38). In line with two other studies (15,39), the present study shows that maternal HF feeding increases placental LPL activity. In addition, expression of key genes for placental lipid transport, such as CD36 and VLDLr, were concurrently elevated in placentas of HF-fed dams. These results prompted us to propose that increased expression and activity of LPL and other proteins enhance maternal-fetal FA transport, which contributes to maternal overnutrition-induced fetal fat accumulation.
Although placental LPL expression positively correlates with fetal fat accumulation and newborn body weight, very limited mechanistic information is available regarding how maternal obesity and HF feeding increase LPL expression and activity in placentas. Regulation of LPL gene expression has been extensively studied in adipose tissue, skeletal muscle, and heart. PPARγ is a nuclear receptor known well for its key role in lipogenesis. Similar to other types of cells (31), PPARγ increases LPL expression in trophoblasts as demonstrated in the present study. Most importantly, the results show that maternal HF feeding increases PPARγ protein levels in placentas, suggesting that increased PPARγ mediates maternal HF feeding–induced placental LPL expression. However, although increased LPL activity was found in placentas of obese mothers, no increase in PPARγ mRNA or protein levels was found in a human study (15). In addition, increased PPARγ was found only in mid-gestation placentas of diet-induced obese ewes (40). These discrepancies may be due to differences in species and induction of maternal obesity.
The regulatory effects of SIRT1 on metabolism were initially observed in aging studies of calorie-restricted rodents. Although the beneficial effects of calorie restriction and SIRT1 on aging are still uncertain in mammals, calorie restriction–induced SIRT1 expression and activation are well conserved in many species. Previous studies have reported that prolonged calorie restriction reduces LPL gene expression in several tissues (41,42). In addition, significantly elevated LPL expression was found in hepatocytes in which SIRT1 was genetically reduced (43). These observations imply a connection between SIRT1 and LPL expression. By using an SIRT1 activator and inhibitor, the present study demonstrates that SIRT1 inhibits LPL expression in mammalian cells, including trophoblasts. SIRT1 is a protein deacetylase that regulates gene expression mainly through controlling transcription factor activity. Consistent with previous studies (22,35,44), we found that SIRT1 reduces PPARγ in trophoblasts. Most importantly, PPARγ inhibition abolished SIRT1 knockdown–enhanced LPL expression. These data indicate that SIRT1 regulates LPL expression through PPARγ.
During pregnancy, there is a significant adjustment of maternal lipid metabolism. From mid-gestation to the third trimester, maternal lipid metabolism switches from anabolic to catabolic. Increased lipolysis provides FAs and glycerol for maternal hepatic VLDL synthesis, which creates hypertriglyceridemia in maternal circulation (38). Similar to humans, the present study showed that late-pregnant mice have significantly higher blood TG concentrations (data not shown). To our surprise, HF feeding did not further increase but actually reduced maternal blood TG levels in mice (Fig. 1J). Similar to other rodent studies (27–30), HF feeding even slightly reduced fetal body weight in the present study. Human studies have demonstrated that maternal blood TG levels correlate with fetal growth (45–47). Therefore, we speculate that decreased maternal blood TGs may underpin the decreased fetal growth in HF-fed rodents (27–30). Another possibility is low dietary protein supply in HF-fed mice. We found that the daily food intake of the HF group was ∼30% less than in controls, despite similar total caloric intake (Supplementary Fig. 1A and B). In addition, the HF diet contained 5% less protein compared with chow. A separate study is investigating the mechanisms through which HF feeding reduces maternal blood TGs and fetal growth. The intriguing observation of reduced blood TGs in HF-fed dams raises another question about the lipid source of increased placental TGs and fetal fat. The current study does not provide data to directly answer this question. However, similar levels of FASN and SREBP1c in placentas between HF and control groups do not support any contribution of de novo lipogenesis to these processes. We postulate that despite maternal HF feeding significantly decreasing TG levels in maternal circulation, gestation-increased blood pool and decreased TG/FA uptake in other peripheral tissues might still provide sufficient TGs for fetal supply. Significantly elevated LPL and other genes for FA transport in placentas of HF-fed dams increase the efficiency of placental TG hydrolysis and FA uptake.
In summary, using a pregnant mouse model, we found that maternal HF feeding increases fetal fat accumulation. Elevated placental LPL activity and expression of genes that facilitate placental lipid transport suggest that enhanced placental lipid transport may play a key role in maternal overnutrition and obesity-induced fetal fat accumulation. Through cultured trophoblasts, the study further demonstrates that SIRT1 inhibits LPL gene expression, whereas maternal HF feeding reduces SIRT1 protein levels in placentas. Inhibition of PPARγ attenuates the stimulative effect of SIRT1 knockdown of LPL expression. Therefore, we propose that maternal overnutrition and obesity reduce placental SIRT1, leading to increase PPARγ transactivity and LPL expression in trophoblasts.
Supplementary Material
Article Information
Acknowledgments. The authors thank Jianping Ye (Louisiana State University, Pennington Biomedical Research Center) for providing Sirt1 knockout MEFs.
Funding. This work was supported by National Institutes of Health grants HD-069634 (to J.Sh.) and DK-095132 (to J.Sh.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.Q., Z.G., C.B., S.G., Y.W., and M.W. contributed the research data. M.P., W.W.H., and T.R.M contributed to the research design and discussion. J.Sc. created the adenovirus vectors and contributed to the writing of the manuscript. J.Sh. designed the study and contributed to the writing of the manuscript. J.Sh. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-1627/-/DC1.
References
- 1.Sewell MF, Huston-Presley L, Super DM, Catalano P. Increased neonatal fat mass, not lean body mass, is associated with maternal obesity. Am J Obstet Gynecol 2006;195:1100–1103 [DOI] [PubMed] [Google Scholar]
- 2.Di Cianni G, Miccoli R, Volpe L, et al. Maternal triglyceride levels and newborn weight in pregnant women with normal glucose tolerance. Diabet Med 2005;22:21–25 [DOI] [PubMed] [Google Scholar]
- 3.Waters TP, Huston-Presley L, Catalano PM. Neonatal body composition according to the revised institute of medicine recommendations for maternal weight gain. J Clin Endocrinol Metab 2012;97:3648–3654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oken E, Gillman MW. Fetal origins of obesity. Obes Res 2003;11:496–506 [DOI] [PubMed] [Google Scholar]
- 5.Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics 2003;111:e221–e226 [DOI] [PubMed] [Google Scholar]
- 6.Hull HR, Thornton JC, Ji Y, et al. Higher infant body fat with excessive gestational weight gain in overweight women. Am J Obstet Gynecol. 2011;205:211.e1–211.e7 [DOI] [PMC free article] [PubMed]
- 7.Qiao L, Yoo HS, Madon A, Kinney B, Hay WW Jr, Shao J. Adiponectin enhances mouse fetal fat deposition. Diabetes 2012;61:3199–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krasnow SM, Nguyen MLT, Marks DL. Increased maternal fat consumption during pregnancy alters body composition in neonatal mice. Am J Physiol Endocrinol Metab 2011;301:E1243–E1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy. 2012;2012:179827 [DOI] [PMC free article] [PubMed]
- 10.Cetin I, Alvino G, Cardellicchio M. Long chain fatty acids and dietary fats in fetal nutrition. J Phyisol 2009;587:3441–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindegaard MLS, Olivecrona G, Christoffersen C, et al. Endothelial and lipoprotein lipases in human and mouse placenta. J Lipid Res 2005;46:2339–2346 [DOI] [PubMed] [Google Scholar]
- 12.Bonet B, Brunzell JD, Gown AM, Knopp RH. Metabolism of very-low-density lipoprotein triglyceride by human placental cells: the role of lipoprotein lipase. Metabolism 1992;41:596–603 [DOI] [PubMed] [Google Scholar]
- 13.Gauster M, Hiden U, van Poppel M, et al. Dysregulation of placental endothelial lipase in obese women with gestational diabetes mellitus. Diabetes 2011;60:2457–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magnusson-Olsson AL, Hamark B, Ericsson A, Wennergren M, Jansson T, Powell TL. Gestational and hormonal regulation of human placental lipoprotein lipase. J Lipid Res 2006;47:2551–2561 [DOI] [PubMed] [Google Scholar]
- 15.Dubé E, Gravel A, Martin C, et al. Modulation of fatty acid transport and metabolism by maternal obesity in the human full-term placenta. Biol Reprod 2012;87:14 [DOI] [PubMed]
- 16.Mazzucco MB, Higa R, Capobianco E, Kurtz M, Jawerbaum A, White V. Saturated fat-rich diet increases fetal lipids and modulates LPL and leptin receptor expression in rat placentas. J Endocrinol 2013;217:303–315 [DOI] [PubMed] [Google Scholar]
- 17.Chang H-C, Guarente L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 2014;25:138–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imai S, Armstrong CM, Kaeberlein M, Guarente L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000;403:795–800 [DOI] [PubMed] [Google Scholar]
- 19.Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 2006;281:39915–39924 [DOI] [PubMed] [Google Scholar]
- 20.Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 2005;434:113–118 [DOI] [PubMed] [Google Scholar]
- 21.Wang R-H, Kim H-S, Xiao C, Xu X, Gavrilova O, Deng C-X. Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest 2011;121:4477–4490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Picard F, Kurtev M, Chung N, et al. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004;429:771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sivasalapathi S, Gaudet J, Caron A, McBurney M. SIRT1: identification of a novel placental stress-response protein that regulates fetal growth (Abstract)? Placenta 2013;34:A66–A67 [Google Scholar]
- 24.Lappas M, Mitton A, Lim R, Barker G, Riley C, Permezel M. SIRT1 is a novel regulator of key pathways of human labor. Biol Reprod 2011;84:167–178 [DOI] [PubMed] [Google Scholar]
- 25.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 1959;37:911–917 [DOI] [PubMed] [Google Scholar]
- 26.Speakman JR. Body Composition Analysis of Animals. New York, Cambridge University Press, 2001 [Google Scholar]
- 27.King V, Hibbert N, Seckl JR, Norman JE, Drake AJ. The effects of an obesogenic diet during pregnancy on fetal growth and placental gene expression are gestation dependent. Placenta 2013;34:1087–1090 [DOI] [PubMed] [Google Scholar]
- 28.Sferruzzi-Perri AN, Vaughan OR, Haro M, et al. An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J 2013;27:3928–3937 [DOI] [PubMed] [Google Scholar]
- 29.Hayes EK, Lechowicz A, Petrik JJ, et al. Adverse fetal and neonatal outcomes associated with a life-long high fat diet: role of altered development of the placental vasculature. PLoS One 2012;7:e33370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.King V, Dakin RS, Liu L, et al. Maternal obesity has little effect on the immediate offspring but impacts on the next generation. Endocrinology 2013;154:2514–2524 [DOI] [PubMed] [Google Scholar]
- 31.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, et al. PPARalpha and PPARgamma activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 1996;15:5336–5348 [PMC free article] [PubMed] [Google Scholar]
- 32.Qiao L, Lee B, Kinney B, Yoo HS, Shao J. Energy intake and adiponectin gene expression. Am J Physiol Endocrinol Metab 2011;300:E809–E816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Qiang L, Farmer SR. Identification of a domain within peroxisome proliferator-activated receptor gamma regulating expression of a group of genes containing fibroblast growth factor 21 that are selectively repressed by SIRT1 in adipocytes. Mol Cell Biol 2008;28:188–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang S, Wang W, Miner J, Fromm M. Cross regulation of sirtuin 1, AMPK, and PPARγ in conjugated linoleic acid treated adipocytes. PLoS One 2012;7:e48874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han L, Zhou R, Niu J, McNutt MA, Wang P, Tong T. SIRT1 is regulated by a PPARγ-SIRT1 negative feedback loop associated with senescence. Nucleic Acids Res 2010;38:7458–7471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friis CM, Qvigstad E, Paasche Roland MC, et al. Newborn body fat: associations with maternal metabolic state and placental size. PLoS One 2013;8:e57467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gernand AD, Christian P, Paul RR, et al. Maternal weight and body composition during pregnancy are associated with placental and birth weight in rural Bangladesh. J Nutr 2012;142:2010–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bansal N, Cruickshank JK, McElduff P, Durrington PN. Cord blood lipoproteins and prenatal influences. Curr Opin Lipidol 2005;16:400–408 [DOI] [PubMed] [Google Scholar]
- 39.Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE. Transgenic increase in N-3/n-6 fatty acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS One 2013;8:e67791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu MJ, Ma Y, Long NM, Du M, Ford SP. Maternal obesity markedly increases placental fatty acid transporter expression and fetal blood triglycerides at midgestation in the ewe. Am J Physiol Regul Integr Comp Physiol 2010;299:R1224–R1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taskinen MR, Nikkilä EA. Effects of caloric restriction on lipid metabolism in man: changes of tissue lipoprotein lipase activities and of serum lipoproteins. Atherosclerosis 1979;32:289–299 [DOI] [PubMed] [Google Scholar]
- 42.Taskinen MR, Nikkilä EA. Basal and postprandial lipoprotein lipase activity in adipose tissue during caloric restriction and refeeding. Metabolism 1987;36:625–630 [DOI] [PubMed] [Google Scholar]
- 43.Xu F, Gao Z, Zhang J, et al. Lack of SIRT1 (mammalian sirtuin 1) activity leads to liver steatosis in the SIRT1+/- mice: a role of lipid mobilization and inflammation. Endocrinology 2010;151:2504–2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shan TZ, Ren Y, Wu T, Liu CX, Wang YZ. Regulatory role of Sirt1 on the gene expression of fatty acid-binding protein 3 in cultured porcine adipocytes. J Cell Biochem 2009;107:984–991 [DOI] [PubMed] [Google Scholar]
- 45.Schaefer-Graf UM, Graf K, Kulbacka I, et al. Maternal lipids as strong determinants of fetal environment and growth in pregnancies with gestational diabetes mellitus. Diabetes Care 2008;31:1858–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nolan CJ, Riley SF, Sheedy MT, Walstab JE, Beischer NA. Maternal serum triglyceride, glucose tolerance, and neonatal birth weight ratio in pregnancy. Diabetes Care 1995;18:1550–1556 [DOI] [PubMed] [Google Scholar]
- 47.Hwang J-Y, Choi HI, Kim H, et al. Relationship of maternal grain intake and serum triglyceride levels with infant birth weight: Mothers and Children’s Environmental Health (MOCEH) study. Eur J Clin Nutr 2014. December 17 [Epub ahead of print]. DOI: 10.11038/ejcn.2014.271 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.