Abstract
Significance: NADPH oxidase (NOX) enzymes, which are widely expressed in different airway cell types, not only contribute to the maintenance of physiological processes in the airways but also participate in the pathogenesis of many acute and chronic diseases. Therefore, the understanding of NOX isoform regulation, expression, and the manner of their potent inhibition might lead to effective therapeutic approaches. Recent Advances: The study of the role of NADPH oxidases family in airway physiology and pathophysiology should be considered as a work in progress. While key questions still remain unresolved, there is significant progress in terms of our understanding of NOX importance in airway diseases as well as a more efficient way of using NOX modifiers in human settings. Critical Issues: Agents that modify the activity of NADPH enzyme components would be considered useful tools in the treatment of various airway diseases. Nevertheless, profound knowledge of airway pathology, as well as the mechanisms of NOX regulation is needed to develop potent but safe NOX modifiers. Future Directions: Many compounds seem to be promising candidates for development into useful therapeutic agents, but their clinical potential is yet to be demonstrated. Further analysis of basic mechanisms in human settings, high-throughput compound scanning, clinical trials with new and existing molecules, and the development of new drug delivery approaches are the main directions of future studies on NOX modifiers. In this article, we discuss the current knowledge with regard to NOX isoform expression and regulation in airway inflammatory diseases as well as the aptitudes and therapeutic potential of NOX modifiers. Antioxid. Redox Signal. 23, 428–445.
The Presence and Activation of the NADPH Oxidase in the Airway
NADPH-oxidase (EC 1.6.3.1) is the major source of nonmitochondrial cellular reactive oxygen species (ROS) and a highly regulated dynamic complex containing both membrane and cytosolic proteins. This enzyme was first discovered in phagocytes, as an essential defense mechanism against different pathogens. Currently, it is known that nonphagocytic cells also express various isoforms of NADPH oxidases and are able to produce ROS. ROS, derived from NADPH oxidases, are one of the most important mechanisms of host defense and innate immunity. However, during prolonged, unresolved inflammation, tissue injury, or dysregulated balance between ROS-generating mechanisms and antioxidants, they might lead to detrimental consequences and various diseases. Therefore, understanding mechanisms leading to the NADPH oxidase activation, expression, and regulation of their various isoforms and potential ways of effective blockade may lead to the development of more effective therapies in airway diseases.
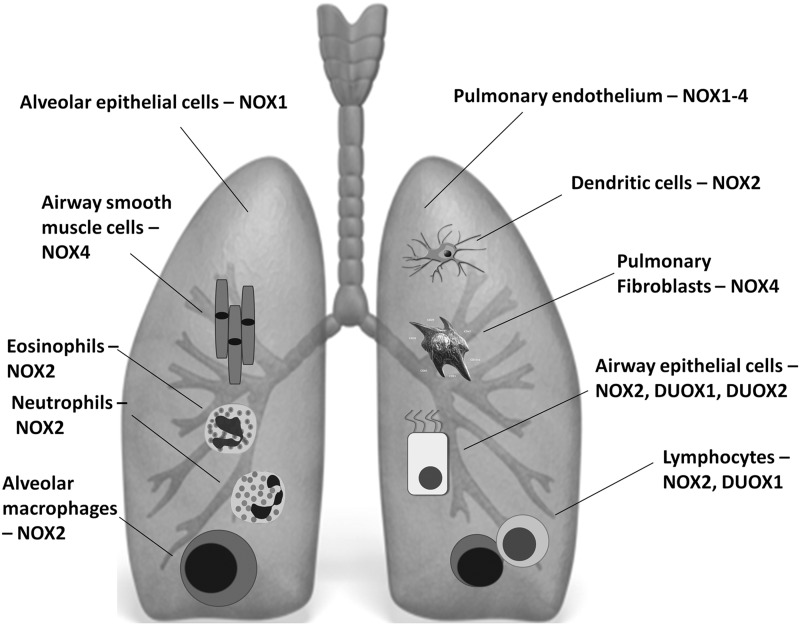
Seven NADPH oxidase homologues form the NOX family (97): NOX1 to NOX5, dual oxidase 1 (DUOX1), and DUOX2. Their expression profile varies in various cell types in the airways (Fig. 1) and is different in the steady state or during inflammation. Depending on the NOX isoforms, the enzymatic activity of NADPH oxidases is modulated, inter alia, by regulatory subunits or calcium binding (126). The structure and function of NADPH oxidases have been extensively reviewed elsewhere (26, 77). The mechanisms through which the activation of NADPH oxidase is regulated still remain unclear.
FIG. 1.
NOX isoforms expression in the lung in the steady state and on pathologic conditions. Phygocytic and nonphagocytic cells of the airway express various isoforms of NADPH oxidases and are able to produce reactive oxygen species (ROS). ROS, derived from NADPH oxidases, are one of the most important mechanisms of host defense and innate immunity. However, during prolonged, unresolved inflammation, tissue injury or dysregulated balance between ROS-generating mechanisms and antioxidants, they might lead to detrimental consequences and various diseases. Refer to text for related details.
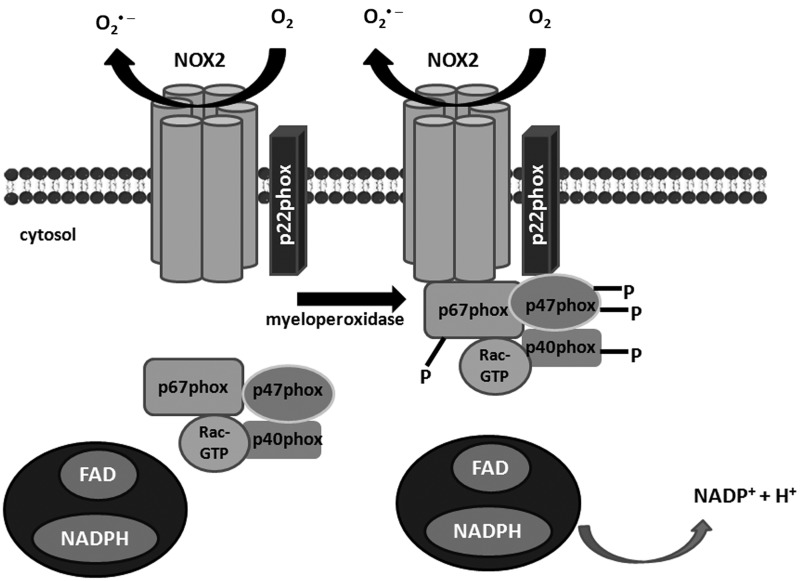
NOX2 (initially designated as gp91phox)—the phagocytic, first described and the most abundant isoform of NADPH oxidase, requires the assembly of several components for its activation (Fig. 2). They comprise the membrane-bound p22phox, which stabilizes the NOX protein and docks cytosolic factors and the cytosolic proteins: p47phox, p67phox, Rac, and modulatory p40phox. p47phox is regarded as an organizer protein. Cell stimulation leads to the phosphorylation and translocation of p47phox to the cell membrane. The translocation of p67phox, which is an activator subunit, requires the presence of p47phox, which has been confirmed in p47phox-deficient neutrophils (41). At the cell membrane, p67phox directly interacts with the NOX2 activating it (148). The next component Rac protein is not an NOX subunit in the strict sense, because it regulates other cellular functions; nevertheless, it is required for NADPH oxidase activation. The GTP-bound Rac protein is recruited to the membrane on cell stimulation independently of p47phox or p67phox, but at the membrane, it interacts directly with p67phox via the N-terminal domain (75). Finally, the most recently discovered subunit p40phox appears to be modulatory, rather than obligatory, though it constantly associates with p67phox and interacts with p47phox, at least in the resting state (8, 29, 149). Together, the assembly of this complex forms the functional NOX2 enzyme. NOX2 isoforms are ubiquitously expressed, and they are present in the lungs endogenously in the steady state and are overabundant during inflammatory conditions (16, 30, 60, 156). The main NOX2-expressing cell types within the respiratory tract include alveolar macrophages, dendritic cells, and/or other resident or infiltrated inflammatory-immune cells (e.g., neutrophils, eosinophils, and lymphocytes) (Fig. 1) (156). Airway epithelium and pulmonary endothelial cells also express NOX2 (157).
FIG. 2.
Schematic illustration of NADPH oxidase NOX2 isoform association with cytosolic regulatory subunits for their activation. The oxidase is a multisubunit protein, activated by translocation of the cytosolic subunits—p47phox, p67phox, and Rac to the NOX/p22phox membrane complex. Cytosolic components phosphorylation results in the movement of the proteins and interaction with p22phox. On activation, there is an exchange of GDP for GTP on Rac, leading to its activation. FAD, flavin adenine dinucleotide; NADP, nicotinamide adenine dinucleotide phosphate.
Pulmonary endothelial cells express NOX 1–4, while NOX4 was demonstrated in the airway smooth muscle cells (SMCs), pulmonary epithelial cells, and pulmonary fibroblasts as well (15, 65, 113, 145). NOX5 expression has been shown in immortalized human microvascular endothelial cells-1 (HMEC-1) (9) and lung cancer cells (5, 95). Its expression, though, was not confirmed in primary human artery endothelial cells or human lung microvascular endothelial cells (113), making NOX5 localization in the lungs uncertain. NOX1, similar to NOX2, depends on Rac activity (79). An exception is NOX4, which seems to require only p22phox to be active, and its activity has been described to be determined only by its mRNA/protein levels (100, 126).
The dual oxidases DUOX1 and DUOX2 are the isoforms of NADPH oxidase generating mainly H2O2 (122), found in the airway epithelial cells. DUOX comprises an NOX2 homology domain typical for all members of the NOX family, intracellular EF hand-type Ca2+-binding pockets, and an extracellular domain that is not found in other NOXs in humans (47). In the airways, a major function of DUOX is to support lactoperoxidase (LPO) to generate OSCN− (bactericidal hypothiocyanite) (47). DUOX1 and DUOX2 do not require p22phox for their activity and are activated by agonist-induced increases in intracellular calcium and various phosphorylations (2).
Under specific inflammatory/pathologic conditions, in different parts of the airways, each member of the NOX family might be present, though the expression level might differ significantly (Fig. 1).
Recently, many reviews considering NADPH oxidase inhibitors have emerged (26, 45, 46, 82), most of which appear to be clinically irrelevant; thus in this review, we focus on the significance of specific NOX isoforms in various airway diseases, mechanisms regulating their expression and activation in the airways, and the inhibitory potential of existing molecules.
The Role of NADPH Oxidase and Subunits in Airway Diseases
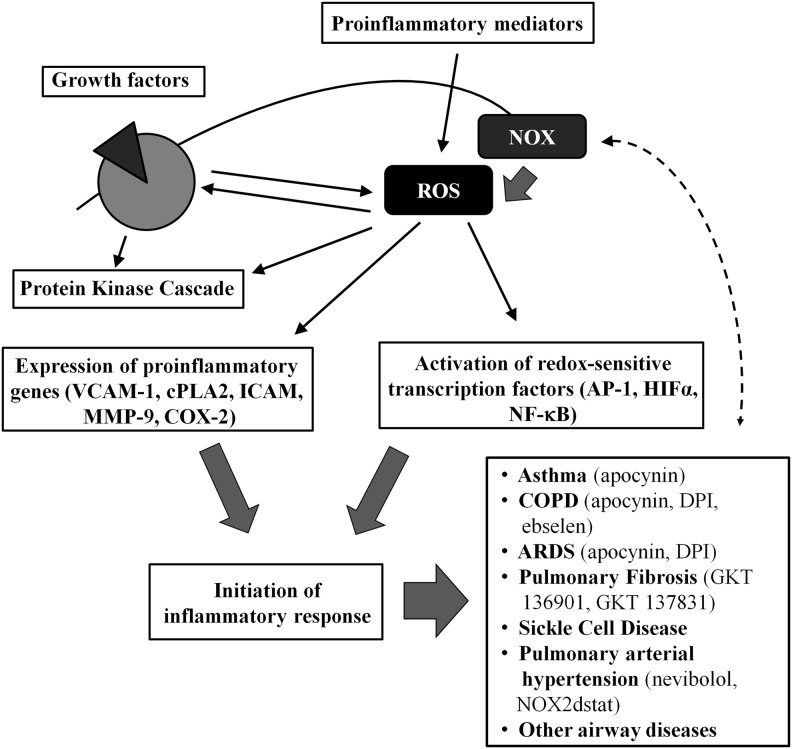
There is now increasing evidence that ROS are generated by immune and structural cells of the airways and lungs, but above all—inflammatory cells. Overabundant ROS production, known as oxidative stress, is associated with acute and chronic respiratory diseases of profound inflammatory components; for example, asthma, chronic obstructive pulmonary disease (COPD), acute respiratory distress syndrome (ARDS), pulmonary fibrosis, cystic fibrosis (CF), pulmonary arterial hypertension (PAH), pulmonary complications in sickle cell disease (SCD), and others (58, 120). ROS might initiate inflammatory responses in the airways and lungs via the activation of redox-sensitive transcription factors (such as activator protein 1 [AP-1], hypoxia-inducible factor 1-alpha [HIF-1α] or nuclear factor kappa-light-chain-enhancer of actived B cells [NF-κB]) and furthermore—expression of pro-inflammatory gene expression (e.g., vascular cell adhesion molecule-1 [VCAM-1], cytosolic phospholipase A2 [cPLA2], intercellular adhesion molecule-1 [ICAM-1], matrix metalloproteinase-9 (MMP-9), cyclooxygenase-2 [COX-2], and others) (12, 13, 90, 96). In addition, prolonged and uncontrolled inflammation itself leads to aggravation of oxidative stress in the airways and lungs, and for that reason, ROS and its sources appear to be crucial factors in the control of airway inflammatory diseases, and offer potential targets for therapeutic interventions (90) (Fig. 3). However, since every chronic inflammatory disease of the airways might be complicated by the recurrent bacterial or viral infections, understanding the disease pathogenesis and its different stages is crucial to face the challenges in the development of efficient but safe NOX modifiers.
FIG. 3.
NOX/ROS play a role in the development and regulation of airway diseases. Through activation of redox-sensitive transcription factors and pro-inflammatory gene expression, NOX-derived ROS initiate inflammatory responses in the airways. A correlation between ROS formation and protein kinase C (PKC) activation might play an important role in regulating inflammatory gene transcription. Uncontrolled inflammation, in turn, intensifies ROS generation; thus, NADPH oxidases, as ROS sources, seem to be pivotal therapeutic targets (90)—modified. Some of the currently studied NOX inhibitors have shown promising effects in human settings (in vitro or in vivo) or in animal models of airway diseases. AP-1, activator protein 1; HIF-1α, hypoxia-inducible factor 1-alpha; VCAM-1, vascular cell adhesion molecule-1; cPLA2, cytosolic phospholipase A2; ICAM-1, intercellular adhesion molecule-1, MMP-9, matrix metalloproteinase-9; COX-2, cyclooxygenase-2; COPD, chronic obstructive pulmonary disease; ARDS, acute respiratory distress syndrome. Refer to text for further details.
Asthma
Airway structural cells (epithelial cell, SMCs, resident macrophages, etc.) and infiltrating inflammatory cells (eosinophils, lymphocytes, and dendritic cells) are implicated in the pathogenesis of asthma at different stages of the disease. Respiratory epithelium plays a crucial role in inflammatory responses regulation—from response to commonly inhaled allergens to inhaled pathogens and allergic sensitization (61). Since airway and alveolar epithelial cells expressing NOX1–2 and DUOX 1–2 are able to generate ROS in response to stimulation, epithelial ROS production is considered important in asthma pathogenesis (157). Data presented by Schwarzer et al. (128) revealed the high expression of DUOX1/2 in whole lung and in the human tracheal epithelium where both DUOX1/2 were expressed at levels that were 1000 times more compared with other NOX isoforms and, thus, represent the major NOX isoforms in airway epithelial cells. Furthermore, it was demonstrated that the baseline DUOX1 expression was consistently higher than DUOX2. DUOX1 expression increased two to five-fold after treatment with IL-4 or IL-13, key cytokines in Th2-derived allergic inflammation (62). Therefore, in addition to its proposed role as a host defense enzyme within the airway lumen (107), it was further established that DUOX1 may be a mediator of redox signaling within the epithelium and might be a potential target of therapeutic modulation at the initial stages of allergic sensitization. Moreover, ROS may cause direct contraction of the airway smooth muscle, which might be enhanced when the epithelium is injured or removed. Excessive ROS production or an imperfect protective system also results in bronchial hyper-responsiveness, which is characteristic in asthma (23, 90). NOX4 is overexpressed in the airway SMCs in patients with asthma (145). Airway smooth muscle from these patients exhibited increased agonist-induced contraction, and this was abrogated by NOX4 small interfering RNA knockdown and less specific NOX pharmacological inhibitors, diphenylene iodonium (DPI) and apocynin (4-hydroxy-3 methoxyacetophenone) (145). In this sense, NOX4 might serve as a potential target to prevent bronchial hyper-responsiveness. Apocynin has already been shown to prevent ozone-induced asthma exacerbation in asthmatics (117), confirming ROS implication in airway hypersensitivity. Analysis of several biomolecular oxidation markers such as bromotyrosine, isoprostanes, nitrates, and nitrites revealed their presence in the airways or lung tissue of asthmatics often in correlation with the extent of ongoing inflammation and with the severity of clinical symptoms (127, 167). Isoprostane F2α-III, a biomarker of lung oxidative stress in vivo, is a potent constrictor of human airways, and hydrogen peroxide constricts airway smooth muscle in vitro (90). We and others have shown that these markers are useful for noninvasive monitoring to the extent of oxidative stress in the airways. We have also shown that apocynin significantly decreases selected ROS and reactive nitrogen species (RNS) in mild asthmatics (141).
COPD and Pulmonary Emphysema
In COPD, oxidants present in cigarette smoke can stimulate alveolar macrophages to produce ROS and to release mediators attracting inflammatory cells to the lungs. Oxidants can also directly damage epithelial and endothelial cells, contributing to the pathogenesis of COPD. Oxidative stress also contributes to a proteinase-antiproteinase and an antioxidant-oxidant imbalance (151, 153). Primary macrophages isolated from mice overexpressing antioxidant enzyme-extracellular superoxide dismutase or from NOX2-deficient mice showed reduced oxidative stress in response to cigarette smoke treatment (151). Similarly, p47phox-null mice have reduced inflammation and inflammatory cytokines levels in the lung lavage specimens (87). However, there are also contradictory reports showing increased cigarette-smoke induced lung inflammation and emphysema in NOX2 and p47phox-deficient mice, apart from the overall decrease in ROS production (176). The same authors, though, pointed out the differences in the responses to cigarette smoke in different strains of mice (175), underlining the importance of the more definitive human studies. There are a few studies showing the contribution for p22phox gene (official gene name designated as CYBA (human) or Cyba (mouse)—here, we used p22phox throughout for clarity) to the respiratory system disorders. Associations of its genotypes with COPD have been analyzed (161), indicating a positive association of the p22phox polymorphisms with COPD, claiming that p22phox is an important oxidative stress candidate. There are limited reports with regard to the function and expression of p22phox in the lungs, but there are data from other systems. For example, cigarette smoke was shown to increase p22phox in the brain of Lewis rats, along with the enhanced expression of NOX4 and DUOX1 (78).
Emphysema is a complication and an end stage of COPD, with no clear pathogenesis. Unexpectedly, recent data point at the role of the NOX3 isoform in this process. TLR4 deficiency caused up-regulation of NOX3 in the lungs of aging mice, resulting in increased oxidant generation, enhanced elastolytic activity, and development of emphysema. The treatment of TLR4−/− mice or endothelial cells with chemical NADPH inhibitors (apocynin and DPI) or NOX3 siRNA reversed the observed phenotype (180). This study raises a hope for targeting NOX3 as a modulating strategy in this otherwise-irreversible condition, though further studies are needed to evaluate these findings.
Smoking cessation is one of the most important of first-line therapies in COPD. However, it is known that once activated, oxidative stress is not easily quenched in the airways of COPD patients, even after quitting smoking (94). In this sense, an effective NOX modulator might be a beneficial add-on therapy to prevent the deleterious effects of prolonged oxidative stress in the airways. We have shown that treatment with nebulized apocynin significantly decreases concentrations of hydrogen peroxide and nitrite in exhaled breath condensate in patients with COPD (140).
Acute Respiratory Distress Syndrome
Acute lung injury or acute respiratory distress syndrome (ALI/ARDS) is a condition with substantial morbidity and mortality that is diagnosed both clinically and radiologically based on the presence of noncardiogenic pulmonary edema and respiratory failure in critically ill patients (74). Lung infection, aspiration, sepsis, trauma, or other insults lead to the disruption of the lung endothelial and epithelial barriers, inflammation, and pulmonary edema (74). The role of NOX enzymes in ARDS has been reviewed elsewhere (16, 58). NOX1, NOX2, and NOX4 in different cells in the airways are involved in the pathogenesis of ARDS (16). NOX1 in alveolar epithelial cells plays a crucial role in the mediation of hyperoxic lung damage in mice (16). Both NOX1 and NOX4 might be involved in epithelial cell death and, in addition, NOX4 might be involved in fibroblast proliferation and fibrotic responses (15). Hyperoxia, a model condition of mechanical ventilation-induced lung injury, increases nonmitochondrial ROS production in human pulmonary endothelial cells (111). From all four NOX isoforms expressed in pulmonary endothelial cells (pulmonary artery and microvascular endothelium), NOX4 seems to be the most abundantly present and its expression is further enhanced by hyperoxia (113). NOX2 is probably most important in ARDS-associated inflammatory responses (83). NOX2-deficient mice have reduced but not totally abrogated hyperoxia-induced pulmonary edema and neutrophil influx to the lungs, suggesting the possible redundancy between NOX2 and other NOX isoforms (113). Nitrogen oxides and H2O2 have been proposed for a noninvasive monitoring of the course of lung injury in animals (34). In terms of possible treatment of ALI/ARDS, less specific strategies of ROS scavenging or more than one NOX isoform inhibition have proved their partial potency in animal models. N-acytylcysteine reduced the apoptosis of epithelial cells and neutrophil lung infiltration in rats exposed to mechanical ventilation (147). Apocynin significantly reduced ROS generation and the extent of septic lung injury in guinea pigs (166).
Pulmonary Fibrosis
Pulmonary fibrosis is a lung-specific response to known (e.g., drugs, autoimmune diseases, environmental cues, and radiotherapy) or unknown (idiopathic) factors (80). Idiopathic pulmonary fibrosis (IPF) is a chronic interstitial lung disease associated with aging that is characterized by the radiological and histopathological pattern of interstitial pneumonia. Recent data indicate that fibrotic response might be driven by abnormally activated alveolar epithelial cells, leading to the activation of myofibroblasts and the deposition of extracellular matrix (81). Sustained exposure to ROS from exogenous or endogenous sources is able to cause a direct injury to the alveolar epithelium, which may lead to fibrosis. Cui et al. demonstrated that oxidative stress contributes to the induction and persistence of transforming growth factor (TGF)-β1-induced pulmonary fibrosis (32). Increasing scientific evidence points to NOX4 as the key source of ROS in the pathogenesis of IPF (15, 65). The severity of fibrosis has been correlated with increased levels of H2O2 in IPF patients' exhaled breath condensate. Phagocytic NOX-derived ROS signaling has been demonstrated to play a key role in promoting tumor necrosis factor (TNF)-induced, NF-κB-dependent acute inflammatory responses and tissue injury specifically in the lungs (179). Insulin growth factor 1 (IGF-1) has been recently implicated in the pathogenesis of lung injury and fibrosis, with proven potency of IFG-1 receptor blockade in the murine bleomycin-induced lung injury (24). Although there are no data directly linking IGF-1 with NOX enzymes in the airways, there are some from other systems. Edderkaoui et al. recently described a mechanism in which IGF-1 activates NADPH oxidase through transcriptional up-regulation of p22phox in pancreatic cancer cells (43). The authors demonstrated that growth factors can stimulate Akt, mediating the activation of NF-κB transcription factor which up-regulates p22 phox expression (14, 67) (Fig. 4).
FIG. 4.

Mechanism of anti-apoptotic effect of growth factors mediated by p22phox component in cancer cells. Akt mediates insulin growth factor 1 (IGF-1)-induced nuclear factor kappa-light-chain enhancer of activated B cells (NF-κB) activation through which the GFs stimulate p22phox expression. Anti-apoptotic effect of Akt is mediated, at least in part, through up-regulation of p22phox (43). IGF-I pathway has been recently implicated in the pathogenesis of lung injury and fibrosis (24).
Recently, developed selective inhibitors of NOX1 and NOX4, derivatives of pyrazolo-pyrido-diazepine dione (e.g., Genkyotex compounds GKT 136901 and GKT137831), showed significant potency in in vitro assays and are currently expected for further clinical trials in pulmonary fibrosis (49, 88).
Cystic Fibrosis
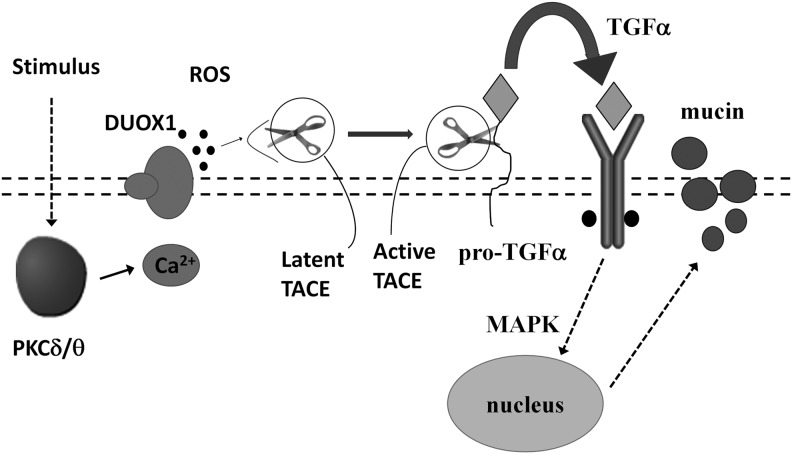
CF is caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) protein. It results in the dysfunction or total lack of CFTR in the plasma membrane and abnormal Na+ and Cl− ion transport in various tissues, including lungs, sweat glands, pancreas, gastrointestinal tract, liver, and male reproductive system (124). In the course of the disease bacterial colonization in the lungs, recurrent airway infections and progressive airway obstruction are mainly responsible for mortality (124). Normal CFTR is expressed in the apical membrane of airway epithelial cells. Since DUOX1–2 expression and function has been strongly indicated in airway defense (107), a reduction in ciliated cells in a de-differentiated phenotype is expected to deprive the epithelium of an important defense mechanism (47). Currently, there are a few mechanistic hypotheses that require normal CFTR function for the DUOX/LPO system in the airways to work properly (50). Moskwa et al. showed that healthy airway epithelial cells are the source of a strong bactericidal agent OSCN−, which requires DUOX function along with CFTR-dependent SCN− secretion and LPO activity (107). In CF, OSCN− generation is diminished due to a CFTR-dependent defect in SCN− secretion, which leads to a collapse of the oxidative antimicrobial mechanism (107). Shao and Nadel proved that DUOX1 plays a crucial role in mucin expression in the airway epithelial cells via PKCδ/PKCθ-DUOX1-ROS-TACE-pro-ligand-EGF receptor cascade (tumor necrosis factor-α-converting enzyme [TACE], Fig. 5). Furthermore, a signaling pathway mediating mucin production involving PKCδ/θ-DUOX1-ROS-TACE-pro-TGF-α-EGFR-mitogen-activated protein kinase has been identified in airway epithelial cells (133). Another study suggests that the bacterium Pseudomonas aeruginosa, a most common pathogen in CF, uses ROS to up-regulate mucin expression through a protein kinase C (PKC)-NADPH oxidase signaling pathway in human airway epithelial cells (173). Moreover, data provided by Sham et al. emphasize a pivotal role of DUOX1 in epidermal growth factor receptor (EGFR) transactivation in airway epithelial cells and indicate Src and ADAM metallopeptidase domain 17 (ADAM17, tumor necrosis factor-α-converting enzyme [TACE]) as targets for redox regulation in response to DUOX1 activation (131).
FIG. 5.
Mechanism of DUOX1- and ROS-dependent mucin production involving PKCδ/θ in airway epithelial cells. Schematic model of Shao and Nadel (modified) (133). PKC isoforms are activated by stimulus (e.g., phorbol myristate acetate [PMA]). PKC then stimulates DUOX. In turn, ROS generated by DUOX activate TACE, which cleaves pro-TGF into TGF. TGF activates EGFR and initiates MAPK, leading to mucin gene expression. TACE, tumor necrosis factor-α-converting enzyme; TGF, transforming growth factor; EGFR, epidermal growth factor receptor; MAPK, mitogen-activated protein kinase; DUOX, dual oxidase.
Taken together, in the early phases of CF, an aberrant epithelial DUOX1–2 function and the decreasing number of H2O2-producing ciliated cells (50) lead to the inefficient oxidative innate immunity response. This results in chronic bacterial colonization and neutrophil infiltration. In advanced phases of chronic infection of CF lungs, neutrophil-derived ROS are predominant (47), and now, they are a source of deleterious oxidative stress, tissue injury, and airway narrowing. Therefore, a profound understanding of the role of NADPH oxidases in the initiation, course, and chronicity of CF is needed to predict the usefulness, timing, and efficiency of specific NOX inhibitors.
Sickle Cell Disease
Lung disease is a major cause of morbidity and death in SCD. Vendramini and colleagues investigated the prevalence of airway hyper-responsiveness in adult sickle cell patients, as it has been noted in children (159). Their study suggests that there is a high prevalence of airway hyper-responsiveness in adult patients with SCD without a history of reactive airway disease. In patients suffering from SCD, increased phosphorylation of p47phox in monocytes was observed after phorbol 12-myristate acetate (PMA) stimulation, compared with normal control subjects. This suggests that NADPH oxidase in these cells is preactivated and capable of more extensive further activation (99). Increased expression of NOX2 and higher phosphorylation of p47phox, occurring in monocytes, according to authors is presumably mediated by interferon gamma (IFN-γ) and other inflammatory mediators, which are present in augmented amounts in SCD patients (99). In the recent study, George et al. demonstrated that a significant part of ROS production in sickle cells is mediated enzymatically by NADPH oxidase, which is regulated by PKC, Rac GTPase. Moreover, they also showed evidence that NADPH oxidase activity in red blood cells can be induced by plasma inflammatory cytokines. These findings suggest a novel pathogenic mechanism in SCD, namely that systemic inflammation and enzymatically derived ROS within the sickle erythrocyte act in a positive-feedback loop to contribute to acute and chronic organ damage of SCD (53).
Bacterial and Viral Lung Infections
NOX-dependent ROS production has long been known as one of the most important mechanisms of the antibacterial innate host defense. This knowledge has been derived from the studies on chronic granulomatous disease (CGD), a primary immunodeficiency, resulting from the genetic defects in NADPH oxidase components (37). CGD phagocytes are not able to neutralize bacterial and fungal pathogens, which leads to recurrent life-threatening infections. Mutations in the genes encoding NOX2 and p47phox are responsible for ∼90% of CGD cases (35, 70, 170). One of the major, very susceptible sites of infection are the lungs. The majority of CGD patients suffer from respiratory disease, including pneumonia, lung abscess, and pulmonary fibrosis. Apart from NOX2 and p47phox, p67phox subunit also contributes to CGD pathogenesis. In p67-phox-deficient CGD mutants, the NOX2 complex is in an inactive state. The addition of recombinant p67-phox drives the transition from the inactive to the active state (160). A recent study by Honda et al. analyzed the potential of recombinant proteins to compensate for defective components—p67phox and p47phox deficiency in CGD neutrophils. It appeared that the delivered p67phox and p47phox subunits localize in the cytoplasm and move to the membrane on stimulation. Moreover, they elicit minimal nonspecific activation in neutrophils (68). This unique “gain-of-function” NOX-modifying therapeutic strategy might be crucial for CGD patients. On the other hand, bacterial and fungal infections are common complications of every chronic inflammatory airway disease. Therefore, it is crucial to understand CGD pathogenesis and the importance of ROS-generating mechanisms in host defense, in an attempt to develop a potent and safe NOX inhibition strategy in chronic airway diseases.
Apart from the various bacteria, viruses are very common airway pathogens that may lead to primary severe lung infections or exacerbation of chronic lung diseases. Excessive ROS production has been shown to exert deleterious effects in influenza A-induced lung injury (71). NOX2-deficient or p47phox-deficient mice have decreased ROS production and inflammatory lung infiltration, reduced edema and lung injury, as well as diminished airway epithelial cell apoptosis in response to influenza A infection (71, 163, 164). Moreover, viral clearance rate and lung function is significantly improved in these mice (136, 163). On the other hand, NOX2 has also been identified as an essential modulator of antiviral responses in airway epithelial cells (139). Soucy-Faulkner et al. showed that NOX2-derived ROS are critical in the activation of IRF3 transcription factor and the expression of downstream antiviral genes—IFIT1 and IFNβ (139). Moreover, it has been shown that NOX1 acts as a protective agent, suppressing influenza A-induced lung inflammation and oxidative stress (130). However, rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by the activation of Rac1 and NOX1 activity (28), suggesting possible virus-specific NOX responses in different cells. Therefore, a specific therapeutic approach against NOX isoforms and subunits serves as an interesting possibility to prevent noxious effects of viral infections. Nevertheless, further studies and cautious, possibly virus-specific, cell-targeted approaches are needed to develop truly specific and safe agents.
Molecular mechanisms of NOX and subunit regulation in airway inflammation
Although the developmental pathways and transcription factors triggering the expression of different NADPH oxidase components in various tissues have been, at least, partly established, the putative regulatory mechanisms that counterbalance these factors are still unclear (155). NOX2 has been, so far, the best studied isoform in the airways and elsewhere. NOX5 expression in the lungs is uncertain, thus next, we describe pathways of NOX1–4 regulation. A deeper understanding of the molecular regulatory mechanisms of NOX isoforms and subunits would help predict the desired and unwanted effects of NOX modifiers.
Transcriptional regulation of expression for the NOX2 system
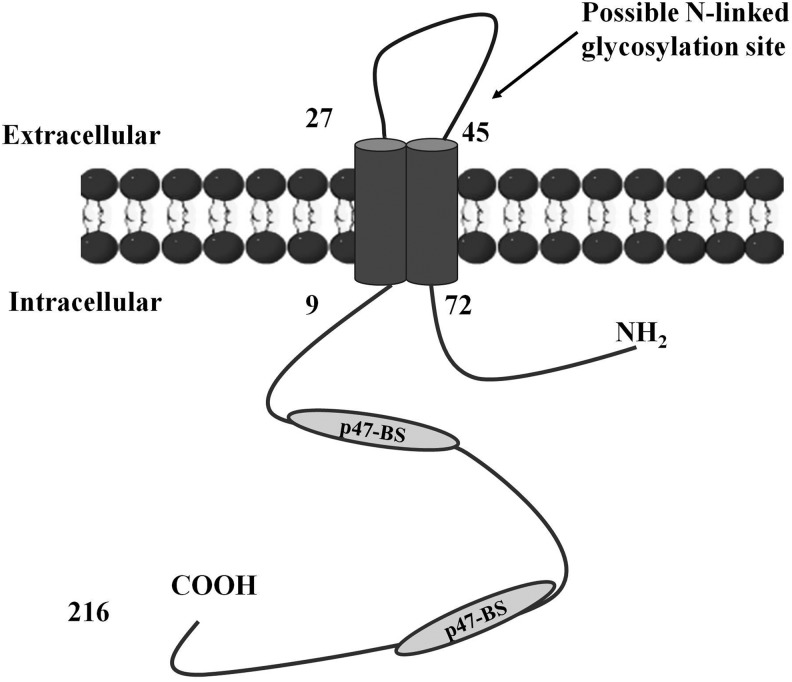
While alternative mRNA splicing has been shown to influence the activity of several NOX-family proteins, NOX2 splice variants that would be functionally relevant have not been previously demonstrated. Harrison et al. recently provided evidence that NOX2 undergoes alternative mRNA splicing and yields a 30 kDa protein, NOX2β, regulating NADPH oxidase activity in mice and human macrophages (63) (Fig. 6). This variant lacks several regions binding prosthetic groups involved in the electron transfer from NADPH to molecular oxygen (56). Possibly, these splice variants represent novel regulatory protein groups that are incorporated into their respective NADPH oxidase complexes to enhance the catalytic activity of the full-length NOX proteins.
FIG. 6.
The scheme of NOX2β protein. NOX2β is a novel splicing variant of NOX2 isoform. p47-BS indicates the conserved binding sites of p47phox (63). The NOX2β variant lacks several regions binding prosthetic groups. These are four conserved histidine residues that are postulated to bind two Fe-heme complexes and which are involved in the electron transfer from NADPH to molecular oxygen.
Several transcription factors regulate NOX2 gene expression (official gene name designated as CYBB [human] or Cybb [mouse]—here, we used NOX2 throughout for clarity) through cis-elements in a 450-base pair sequence in the 5′-flanking region of the gene. Yang et al. presented data showing the eosinophil-specific regulation of the NOX2 gene by transcription factors GATA-1 and GATA-2 through the GATA-binding site (174). It has long been considered that the expression mechanism of NOX2 in eosinophils is the same as that in other phagocytes. A restricted expression of NOX2 in eosinophils from X-linked CGD patients implied a certain eosinophil-specific expression mechanism of the gene. Although an eosinophil-specific GATA-3 suppression mechanism was suggested by Sadat et al. (125), no positive regulatory mechanisms of the gene have, however, been shown in eosinophils. Eklund et al. (44) have proposed a cooperative activation of the NOX2 gene by PU.1 and interferon regulatory factor-1 in myeloid cell lines, and Suzuki et al. have shown that PU.1 is an essential activator for the expression of the NOX2 gene in human neutrophils, monocytes, and B-lymphocytes (146).
In isolated rat lungs, NOX2 expression was increased by an ethanol-stimulated increase in renin-angiotensin system) activity (118). These findings extend previous work linking ethanol ingestion to oxidative stress in the lung and susceptibility to lung injury (59), but the precise mechanism remains unknown. Interestingly, lung p22phox or p47phox expression levels remained unchanged.
The transcriptional regulation of the p22phox gene is a mechanism that controls NADPH oxidase activity (162). According to Gauss et al., p22phox gene is regulated by NF-κB and AP-1 (52). The authors provide evidence that in human aortic SMCs, the inhibition of the AP-1 pathway reduces the activity and expression of NADPH oxidase and that AP-1 physically interacts with p22phox gene promoter. Since AP-1 is a redox-sensitive transcription factor, it might be presumed that there is a positive feedback mechanism in which ROS, generated by the NADPH oxidase, is important for the persistent superoxide generation. This hypothesis is supported by data obtained from endothelial cells, with regard to the redox regulation of the NADPH oxidase subunit p22phox in endothelial cells (38).
The activation of NOX2 is strictly dependent on the p47phox subunit. Using p47phox-and NOX2-deficient mice that lack NADPH oxidase function, Segal et al. showed that NOX limits inflammation by attenuating the pro-inflammatory transcription factor NF-κB and by activating nuclear factor erythroid 2-related factor (Nrf2), a key redox-sensitive anti-inflammatory transcription factor. The studies demonstrate the pharmacological activation of Nrf2 as a potential therapeutic strategy in CGD. This work identifies NADPH oxidase as a critical regulator of innate immunity and provides a novel understanding of the mechanisms that regulate lung inflammation (129).
Direct regulation of activity for the NOX2 system
The process of regulation of NADPH oxidase complex is complicated by various issues, which are necessary in its activation (Table 1). Moreover, the strict control is needed to deliver the activation at the appropriate time and place. Thus, several signaling pathways have been established that regulate the NADPH oxidase downstream of cell surface receptors. Phosphatidylinositol 3-kinase (PI3K)-dependent pathways, the blockade of which severely limits activation of the oxidase to several stimuli, play a pivotal role. The precise roles of the phosphoinositide products of PI3K activity in regulating NADPH oxidase assembly and activation are still unclear; however, emerging data suggest that they might play an important role via the regulation of guanine nucleotide exchange in the Rac component. There is also strong evidence that the PI3K products—PtdIns(3, 4)P2 and PtdIns3P can bind directly to the phox homology (PX) domains, which are located in the p47phox and p40phox, respectively (64). Based on available studies, Yamamori et al. presented a model of signal transduction pathways leading to p47phox phosphorylation and NADPH oxidase by the activation of PKCs (Fig. 7) (172).
Table 1.
Summary of Mechanisms Regulating Activity and Expression of NOX and DUOX Enzymes
| NOX/DUOX | Regulatory subunits | Other regulatory issues | Requiring of p22phox |
|---|---|---|---|
| NOX1 | NOXO1, NOXA1, Rac1 | STAT1, INF-γ, LPS, angiotensin II, urokinase plasminogen activator, platelet-derived growth factor, prostaglandin F2, phorbol ester, activated K-Ras | Yes |
| NOX2 | p47phox, p67phox, p40phox, Rac2/1 | Yes | |
| NOX3 | NOXO1, NOXA1 | Yes | |
| NOX4 | Constitutively active | Nrf2, MAPK, MEK1-ERK1/2 | Yes |
| NOX5 | Ca2+ and phosphorylation | No | |
| DUOX1 | Ca2+ | IL-4, IL-13 | No |
| DUOX2 | Ca2+ | IL-1α, IL-1β, IFN-γ | No |
DUOX, dual oxidase; ERK1/2, extracellular signal-mediated protein kinases 1 and 2; IFN-γ, interferon gamma; IL, interleukin; LPS, lipopolysaccharide; MAPK, mitogen-activated protein kinases; MEK1, dual threonine and tyrosine recognition kinase; Nrf2, nuclear factor erythroid 2-related factor.
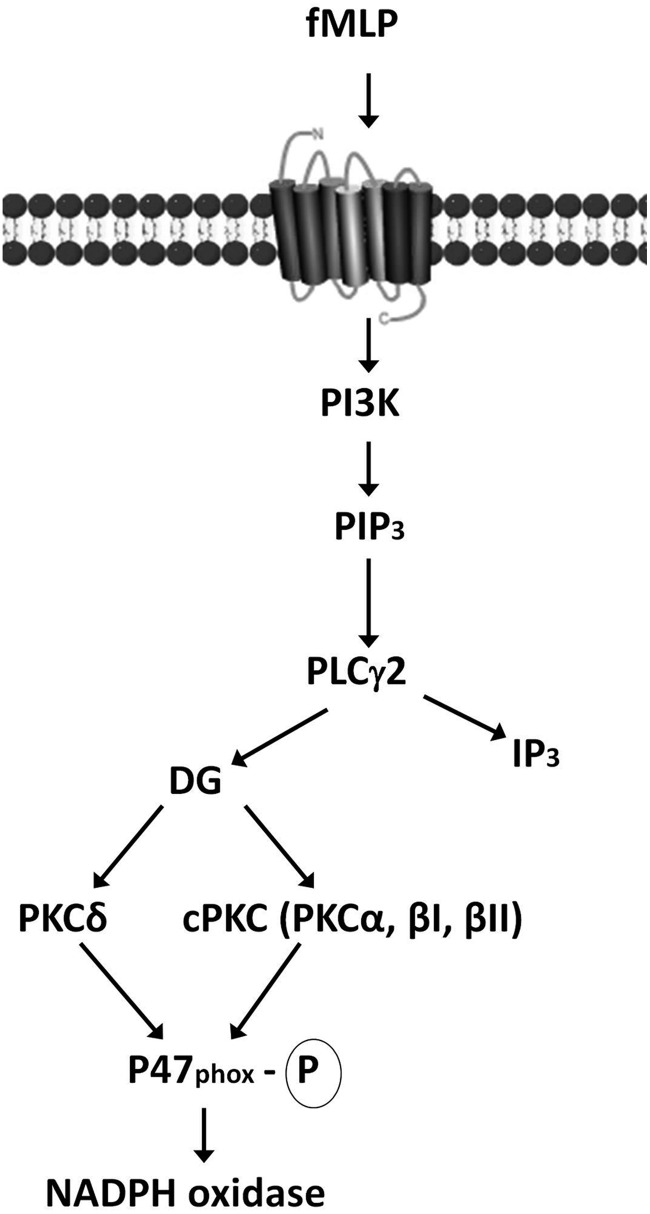
FIG. 7.
NADPH oxidase activation via PI3K. Ligand binding to fMLP receptors triggers phosphatidylinositol 3-kinase (PI3K) activation and subsequent phosphatidylinositol 3,4,5-triphosphate (PIP3) production. PIP3 induces protein-dependent kinase (PDK) activation, and PDK, in turn, phosphorylates and activates Akt. Phospholipase Cc2 (PLCc2) is directly activated by PIP3 and leads to the activation of diacylglycerol (DG)-dependent protein kinases C, cPKC, and PKCd. The activation of these PKCs results in p47phox phosphorylation and the subsequent NADPH oxidase activation (172). fMLP, formyl-methionyl-leucyl-phenylalanine.
According to Gao et al., superoxide production is required for the induction of sepsis-induced lung microvascular injury in the murine model. The authors suggest that NADPH oxidase-derived O2•− generation has an important bactericidal role, such that an impairment in bacterial clearance in NADPH oxidase-defective mice results in increased chemokine generation and lung tissue PMN infiltration (51).
Translocation of Rac-GTPase to the plasma membrane, independent of p47phox and p67phox, is essential for the assembly and activation of NADPH oxidase (116). Current studies support a complex formation between p67phox and Rac in the membrane for optimal electron transport through NOX2 in O2•− production. However, molecular mechanisms regulating the activation of Rac GTPases in phagocytosis and ROS generation remain to be clearly defined (Fig. 8). Several NOX isoforms have been implicated in Rac-dependent ROS generation and are regulated via Rac (69, 106). However, it remains unclear as to whether Rac1 interacts directly or requires other components—p47phox or NOXA1—to regulate NOX isoforms. Dang et al. demonstrated that p67phox is constitutively phosphorylated in resting human neutrophils and furthermore, that the state of p67phox phosphorylation is controlled by an upstream tyrosine kinase, MEK1/2 and the serine/threonine phosphatases PP1/2A. Dysregulation of the balance between these two activities could play a role in ROS production by human neutrophils at inflammatory sites (33). How this constitutive phosphorylation of p67phox may regulate NADPH oxidase activation remains unknown, but it is speculated that it could have a role in maintaining the oxidase inactive in the cytosol or it could prepare p67phox for more efficient activation of the enzyme (33). The phosphorylation, according to the authors, has no modifying effects on the binding of p67phox to the p40phox and p47phox complex. Unlike p47phox or p67phox, the p40phox component is an alternative for NOX2 activity. It is absent from CGD patients who lack p67phox, suggesting that the protein is stable only on binding to p67phox (103). However, available data suggest that p40phox is specific for NOX2 though as NOX1 and NOX3 could potentially be activated by the p47phox/p67phox complex, a role of p40phox in this scenario cannot be excluded (8). Using ionizing radiation Ostuni et al. demonstrated that ROS might stimulate NOX activity (110). The study shows that p67phox integrity is crucial for the oxidase activity and that irradiation of p67phox drastically decreases its interaction with arachidonic acid (AA) and destabilizes the p47phox–p67phox complex (93, 110). The precise role played by AA still remains unclear, though Souabni et al. have shown that trans-arachidonic acid (trans-AA), in contrast to cis-AA, cannot activate the NADPH oxidase but is able to inhibit cis-AA-activated generation of O2•− through a direct interaction with p67phox and with the membrane fraction, probably indirectly via modification of the membrane physical properties (138).
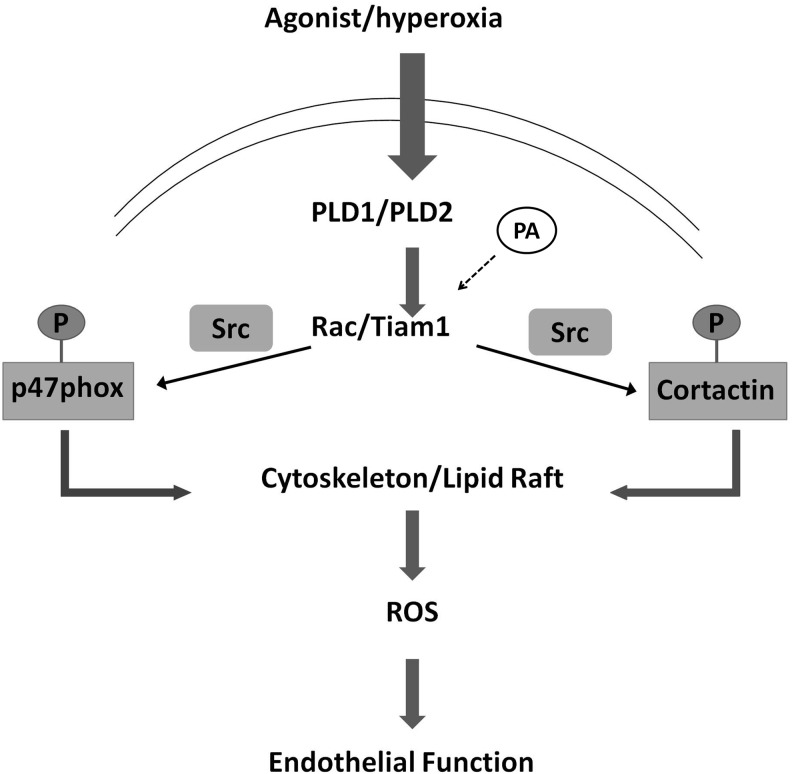
FIG. 8.
Rac1-dependent NADPH oxidase activation and ROS generation. Schematic illustration of the mechanism dependent on Rac1 subunit, leading to phosphorylation/translocation of p47phox and activation of endothelial NADPH oxidase (116)—modified. Stimulation of cells results in phospholipase D1 and D2 (PLD1 and PLD2) activation and activation of Rac1/Tiam1 (which are regulated by phosphatidic acid [PA]). This leads to Src-dependent phosphorylation of p47phox and cortactin. Subsequently, assembly of the NADPH oxidase complex occurs for ROS generation. Tiam, T-cell lymphoma invasion and metastasis 1.
NOX1, NOX3, and NOX4 Modulation Mechanisms
Although each NOX family member is typified by six transmembrane domains along with a cytoplasmic domain that binds NADPH and flavin adenine dinucleotide (FAD), each isoform is distinguished by the specific catalytic subunit, interacting proteins, and subcellular localization.
NOX1
The considerable constitutive activation of NOX1 in the absence of cell stimulation is explained partly by the absence of regulatory phosphorylation sites on NOXO1 and the ability of NOXO1 to localize to the resting cell membrane via its PX domain (20). NOXO1 is a p47phox homolog, which, in contrast to p47phox, has a total of four different splice variants (19). The major difference between p47phox and NOXO1 is the presence of an autoinhibitory domain in p47phox that does not occur in NOXO1. This domain prevents association with p22phox (42).
NOX1 transcription is activated by inflammatory mediators such as INF-γ or lipopolysaccharide, which also induce the expression of NOXO1. NOX1 is up-regulated by growth factors and growth-related agonists; for example, angiotensin II, urokinase plasminogen activator, platelet-derived growth factor, prostaglandin F2, phorbol ester, and mutationally activated K-Ras in various tissues (25, 86, 89, 98).
Unlike the NOX2, the NOX1 system seems to have relatively few regulatory elements. Only guanine nucleotide exchange on Rac1 is convincingly documented as a regulatory trigger, although NOX1 activation by Rac1 is apparent only when cellular levels of NOXO1 and NOXA1 are low (18).
In a human pulmonary epithelial cell line, increased NOX1 expression is caused by hypoxia, which, in addition, activates HIF-1α-dependent pathways. It is not yet established whether HIF1α itself induces NOX1 expression (55, 89).
NOX3
NOX3 is the closest homologue of NOX2 (58% identity) in the NOX protein family (17). NOX3 is unique in its ability to be activated by NOXO1 alone in the absence of activator subunits. Cheng et al. characterized the requirements of NOX3 for regulatory subunits, including p47phox, p67phox, NOXO1, and NOXA1. The research team suggested that NOX3 shows unusual flexibility in its ability to be activated by both phox and NOX regulatory proteins, although they concluded that NOX3 regulation does not require an intact activation domain of p67phox, indicating that other regions of p67phox are important for regulating NOX3 activity. NOX3 appeared to be maximally activated by NOXO1 alone, and with no requirement of NOXA1 (21).
Using human kidney cells and Chinese-hamster ovary cells, Nakano et al. indicated that p22phox is physically associated with both NOX3 and NOXO1. The plasma membrane localization of NOX3 but not of NOXO1 requires p22phox. Moreover, the glycosylation and maturation of NOX3 requires p22phox expression, suggesting that p22phox is required for the proper biosynthesis and function of NOX3 (108).
It is, though, important that subunit regulation differs depending on species: Human NOXO1 dramatically stimulates NOX3 activity in the absence of NOXA1 or p67phox (21). However, mouse NOX3 activity requires both NOXO1 and NOXA1 (7).
NOX4
NOX4 isoform of NADPH oxidase strongly contributes to lung pathologies, especially to pulmonary fibrosis. Transcriptional regulator of antioxidant genes, Nrf2, has been demonstrated to regulate NOX4 expression that was confirmed in human lung endothelium in response to hyperoxia and in the mouse lung. Lack or diminished levels of hyperoxia induce NOX4 expression in human lung endothelial cells or mouse lung with reduced levels of Nrf2. Moreover, Nrf2 expression knockdown with siRNA attenuates the expression of NOX4 (114, 115).
Recent studies implicate a role for the NOX4 in tissue repair functions of myofibroblasts and fibrogenesis. NOX4 is also up-regulated in type II epithelial cells in IPF patients and participates in TGF-β-induced epithelial cell apoptosis, an early crucial step in the pathogenesis of IPF. The data indicate that NOX4 is up-regulated in human IPF as well as in mice lungs with bleomycin- or hapten-induced injury (65).
TGF-β1 has been shown to induce NOX4 expression in human lung mesenchymal cells through SMAD-3 protein. This pro-fibrogenic mediator specifically induces mRNA/protein expression and enzymatic activation of the NOX4 isoform in differentiated myofibroblasts. NOX4-dependent H2O2 generation seems to be required for myofibroblast differentiation, synthesis of ECM proteins, and contractility mediated by TGF-β1. Moreover, in murine models of lung injury, genetic or pharmacologic targeting of NOX4 attenuated fibrogenesis (3, 65, 76).
Inhibitors of NOX/NADPH Oxidases
NADPH oxidases inhibitors may be grouped into two main categories: small molecules (nonpeptide, chemical inhibitors) and peptide inhibitors synthesized to target specific NADPH oxidase complex subunits. So far, most of them have been used in in vitro and animal model-based experiments. However, among them, there are some with proven safety and efficacy in small clinical studies in human airway diseases (e.g., apocynin). Others are currently used in clinics in the nonairway-related disorders, but their newly discovered NOX inhibitory potential may lead to broadening their spectrum of use (e.g., nebivolol). Recent discoveries have questioned the selectivity of most widely used small-molecule inhibitors, while the newer peptide based are facing problems with the targeted delivery, stability, and potential immunogenicity. Nevertheless, significant progress has been made and high-through-put screening technologies may result in the development of the selective, stable, nontoxic, and efficient NOX modifiers.
Small-Molecule Inhibitors
One of the first identified NADPH oxidase inhibitors, still widely used in in vitro experiments, is DPI, which inhibits NOX activity (92, 119). DPI suppressed ROS production by NOX4 and significantly decreased the radiation-induced expression of α-smooth muscle actin and fibronectin in human lung fibroblasts (112). Moreover, it attenuated the pulmonary injury induced by PMA in guinea pigs (165). However, it is also known that DPI is a nonspecific blocker of many flavoprotein-dependent enzymes, including complex I of the mitochondrial electron transport chain, nitric oxide synthases, and cytochrome P450 (132).
Phenylarsine oxide (PAO), a sulfhydryl reagent for vicinal or proximal thiol groups, and 4-(2-aminoethyl)-benzenesulfonyl fluoride (AEBSF) inhibit NADPH oxidase through preventing assembly of the complex (36, 39). However, these agents are also known as inhibitors of other enzymes. PAO is a phosphotyrosine phosphatase inhibitor, and AEBSF is a serine protease inhibitor. An intraperitoneal injection of PAO to rats reduced ROS production by rat phagocytes and neutrophil infiltration into the lung after the inhalation of lipopolysaccharide (123). According to Weissmann et al., AEBSF attenuated alveolar macrophage oxidative burst elicited by PMA in rabbit system, in a dose-dependent manner (168). AEBSF inhibits binding of the cytoplasmatic subunit p47phox to the NOX2/p22phox complex, but still, little is known about its usefulness in airway tissues (36).
Accumulating evidence confirm a connection between the actin cytoskeleton as well as actin-binding proteins and NOX activation (phagocytic and nonphagocytic) (104). The activation of NADPH oxidase by actin-destabilizing agents (e.g., cytochalasin D) may involve multiple signaling path00000ways that are associated with actin cytoskeletal reorganization (154). Coronin and cortactin—actin-binding proteins are known to interact with NADPH oxidase components and are engaged in the regulation of oxidase-dependent ROS production. A role for cortactin in hyperoxia-induced translocation of p47phox and the activation of NADPH oxidase and the generation of ROS/O2•− has been demonstrated in human lung endothelium (152, 154).
A broad spectrum of targets of the agents described earlier significantly decrease their potential in clinical settings, raising the possibility of adverse effects and unexpected toxicity. Therefore, in recent years, substantial effort has been invested into (i) performing small but well-designed and controlled clinical trials with existing, nonspecific NOX modifiers in clearly defined patient populations; (ii) redefining currently used drugs, but with newly described characteristics into a new disease spectrum; and (iii) high-throughput screening approaches to find new selective NOX inhibitors. These three strategies have led to the development of new agents and the re-introduction of older agents as potential new treatment options.
One such example is apocynin, a molecule structurally related to vanillin, believed to interact with p47phox (171). In a rat model, the administration of apocynin attenuated the inflammatory response, lung permeability, and ischemia reperfusion in the isolated, perfused lung (22). Nevertheless, apocynin can also inhibit NOX1 and it can scavenge ROS, which makes it a nonspecific NOX inhibitor (46, 66). Moreover, there are studies suggesting that apocynin is not an NOX inhibitor but an antioxidant, or that it increases ROS generation by NADPH oxidase instead of decreasing it (66, 158). However, in vivo, a metabolite of this compound may act as a true NOX inhibitor, and it may show some specificity for NOX2. We and others have shown that nebulized apocynin is safe (142), prevented ozone-induced exacerbations of asthma, and decreased selected ROS and RNS in mild asthmatics and patients with COPD (117, 140, 141). Therefore, even the nonselective ROS-scavenging and NOX-modifying strategy might be beneficial in inflammatory airway disorders and deserves further clinical studies in clearly defined populations and with the reasonable caution of the possible adverse effects and cytotoxicity.
Nebivolol, a β1 receptor blocker, is currently used in the treatment of hypertension and left ventricular failure (1). Recently, it was shown to also act as an NOX inhibitor. It decreases oxidase expression by the inhibition of membrane association, an interaction of p67phox and Rac. Moreover, nebivolol inhibits NOX1-dependent superoxide production (102, 105). Therefore, as a drug of known pharmacological characteristics, it raises a hope for broadening its use in pulmonary diseases; for example, in idiopathic pulmonary hypertension (101).
Data provided by Smith et al. indicate that ebselen and its analogs represent a class of compounds which inhibit ROS generation by interrupting the assembly of NOX2-activating regulatory subunits (135). Ebselen and analogs potently inhibit NOX1 and NOX2 activity, and they were less effective against other isoforms. Ebselen also blocked the translocation of p47phox to neutrophil membranes. Furthermore, previous investigations in challenged guinea pigs suggest that ebselen significantly inhibits late airway response and suppresses airway inflammation (177). Ebselen also inhibits cigarette-smoke-induced lung inflammation in mice (40). It has been tested in humans in stroke (57) and bipolar disorders (134) clinical trials.
Finally, discovered by application of a high-throughput screening technology, derivatives of pyrazolo-pyrido-diazepine dione (e.g., Genkyotex compounds GKT 136901 and GKT137831) appear highly promising therapeutics for the treatment of IPF and other nonpulmonary diseases, as they target NOX4 and NOX1 isoforms (6, 49, 88). These compounds, especially one called 7c, are highly potent in in vitro assays on human lung fibroblast differentiation and in murine models of bleomycin-induced pulmonary fibrosis. They demonstrate a superior efficiency to Pirfenidone, which is the only drug accepted for the treatment of IPF in Japan and Europe these days (49, 88).The specificity of these compounds was confirmed in in vitro pharmacological profile, showing concomitant benefits such as good oral bioavailability and high plasma concentrations in vivo (6, 49, 88).
High-throughput screening technology has also led to the development of fulvene-5 and its derivatives, potent and efficacious inhibitors of NOX2 and NOX4 (11), as well as ML171, a specific and selective NOX1 inhibitor (54). Fulvene-5 has been successfully applied in vivo to block the growth of endothelial tumors of mice (11). However neither of them has yet been used in the context of lung diseases.
Peptide-Based Inhibitors
Peptide-based inhibitors seem to be promising for providing NOX isoform-specific inhibitors. NOX2, p47phox, p67phox, and Rac are likely targets for peptides directed against their unique sequences (46).
Peptides derived from NOX2 have been used to inhibit NADPH oxidase. A peptide mimicking one of NOX2/p47phox interaction sequences that corresponds to amino acids 77–93 has a potent inhibitory effect on human NADPH oxidase. By binding to p47phox, NOX2ds-tat (designated also as gp91ds-tat) inhibits the activity of not only NOX2, but quite probably also NOX1, although this hypothesis has been recently challenged (31) NOX2ds-tat was described as the strongest in inhibiting superoxide generation in the vascular system, where NOX1 and NOX4 play a key role (26). This chimeric peptide that consists of a tat site derived from the tat peptide of the HIV is cell permeable. It also contains a fragment of NOX2, which has previously been shown to prevent the interaction of p47phox with the NOX subunits in cell-free preparations (72, 121, 150). In many studies, NOX2ds-tat has been used as a specific NADPH oxidase inhibitor (48, 84, 109), often in two forms—scrambled (tat scrambled) and unscrambled (tat unscrambled) (144, 150, 178). In human leukocytes, unscrambled but not scrambled NOX2ds-tat significantly reversed TNF-induced core 2 β-1,6-N-acetylglucosaminyltransferase (C2GNT) activity, demonstrating a novel signaling cross-talk between C2GNT, NADPH oxidase, and specific PKCβ1/2) (150). Furst et al. demonstrated that NOX2ds-tat reduced superoxide production even below control levels after an increase by atrial natriuretic peptide in the endothelium of intact rat lung vessels (48). On the other hand, Krotz et al. suggest that although NOX2ds-tat was able to reduce significantly basal superoxide release, it did not affect O2•− formation after an incubation with mycophenolate acid (MPA) anymore, suggesting that MPA had been blocked before this basal activity of endothelial NADPH oxidase in human umbilical vein endothelial cells (84). Furthermore, a study conducted by Norton et al. using NOX2ds-tat enabled to effectively and selectively inhibit NOX2 in isolated rat lungs (109). The data of the successful use of NOX2ds-tat in experimental vascular disease models suggest its potential in PAH, which, in general, lacks treatment options.
A sequence corresponding to amino-acid residues 323–332 inhibits the phosphorylation of p47phox, and its translocation, therefore, inhibits NADPH oxidase activation in a cell-free system (169). This peptide may compete either for p47phox phosphorylation or for an interaction with the NOX2/p22phox complex or p67phox, by acting as a pseudosubstrate for protein kinases. Another synthetic peptide, corresponding to residues 314–331, also inhibits p47phox phosphorylation and translocation in human neutrophils and inhibits PKC-mediated phosphorylation of p47phox (10).
Peptide-based inhibitors appear to be the most promising candidates for therapy. Nevertheless, the main problems with inhibitory peptides are their cell delivery and their stability in live organisms. Apart from limited bioavailability, peptides are challenged due to their gut degradation, associated toxicity, and inability to cross the plasma membrane of living cells. However, in terms of many lung diseases (e.g., asthma, COPD), inhaled aerosol administration route of the drug is the preferable one. Therefore, this drawback in other disorders may, in fact, facilitate the development of efficacious airway peptide-based therapy (4). Nanoparticles and polymeric microparticles also hold promise for more efficient and targeted drug delivery, which has been an extensive field of research in recent years (85).
Perspectives and Conclusions
Available NOX inhibitors do not specifically target pathologic signaling, leaving physiologic signaling intact. The perfect NOX inhibitors ought to be both—isoform specific and cell specific, and preferentially targeting pathological modes of NOX signaling. This level of specificity could potentially be achieved by direct interfering with the interface between a particular NOX isoform and the proteins with which it should interact to become active under pathological conditions (143). Such a profound understanding of NOX enzymes and their downstream targets regulation obviously requires further studies. High-throughput scanning strategies and the development of more sophisticated delivery techniques are strong indices of upcoming progress in this field. Airway diseases, with the preferred inhaled or nebulized mode of drug administration and with the existing ways of noninvasive monitoring of oxidative stress, seem to be a perfect setting for NOX-modifying therapy. Knowing the exact pathophysiology and variable courses of the diseases, nonspecific NOX inhibitors might be useful in the therapy. Simultaneously, though, adverse events remain a serious question. Bacterial and viral infections are common complications of chronic respiratory diseases. Therefore, probable clinical side effects of NOX inhibitors should emerge from careful studies of animals and humans carrying genetic mutations in NOX genes (73, 137).
To date, no therapeutically viable molecule exists but recent studies using current inhibitors have enhanced our knowledge on the role of NADPH oxidase and its possible modification strategy in airway diseases. The group of NADPH oxidase modifiers, particularly inhibitors, is growing each year; there are more data explaining mechanisms of their activity, as well as new smaller and larger clinical trials delineating their safety and potency. This situation certainly leads to the introduction of specific and effective NOX modulators, but still, we are a step away of optimal therapy.
Abbreviations Used
- AA
arachidonic acid
- ADAM17 (TACE)
ADAM metallopeptidase domain 17 (tumor necrosis factor-α-converting enzyme)
- AEBSF
4-(2-aminoethyl)-benzenesulfonyl fluoride
- ALI
acute lung injury
- AP-1
activator protein 1
- ARDS
acute respiratory distress syndrome
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane conductance regulator
- CGD
chronic granulomatous disease
- cis-AA
cis-arachidonic acid
- COPD
chronic obstructive pulmonary disease
- COX-2
cyclooxygenase-2
- cPLA2
cytosolic phospholipase A2
- DPI
diphenylene iodonium
- DUOX
dual oxidase
- EGFR
epidermal growth factor receptor
- fMLP
formyl-methionyl-leucyl-phenylalanine
- HIF-1α
hypoxia-inducible factor 1-alpha
- ICAM-1
intercellular adhesion molecule-1
- IFN-γ
interferon gamma
- IGF-1
insulin growth factor 1
- IPF
idiopathic pulmonary fibrosis
- LPO
lactoperoxidase
- MAPK
mitogen-activated protein kinases
- MMP-9
matrix metalloproteinase-9
- MPA
mycophenolate acid
- NF-κB
nuclear factor kappa-light-chain- enhancer of activated B cells
- Nrf2
nuclear factor erythroid 2-related factor
- PAH
pulmonary arterial hypertension
- PAO
phenylarsine oxide
- PDK
protein-dependent kinase
- PI3K
phosphatidylinositol 3-kinase
- PIP3
phosphatidylinositol 3,4,5-triphosphate
- PKC
protein kinase C
- PLCc2
phospholipase Cc2
- PX
phox homology
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SCD
sickle cell disease
- SMC
smooth muscle cell
- TGF
transforming growth factor
- Tiam
T-cell lymphoma invasion and metastasis 1
- TNF
tumor necrosis factor
- trans-AA
trans-arachidonic acid
- VCAM-1
vascular cell adhesion molecule-1
References
- 1.Agabiti Rosei E. and Rizzoni D. Metabolic profile of nebivolol, a beta-adrenoceptor antagonist with unique characteristics. Drugs 67: 1097–1107, 2007 [DOI] [PubMed] [Google Scholar]
- 2.Al Ghouleh I, Khoo NK, Knaus UG, Griendling KK, Touyz RM, Thannickal VJ, Barchowsky A, Nauseef WM, Kelley EE, Bauer PM, Darley-Usmar V, Shiva S, Cifuentes-Pagano E, Freeman BA, Gladwin MT, and Pagano PJ. Oxidases and peroxidases in cardiovascular and lung disease: new concepts in reactive oxygen species signaling. Free Radic Biol Med 51: 1271–1288, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amara N, Goven D, Prost F, Muloway R, Crestani B, and Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65: 733–738, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrade F, Videira M, Ferreira D, and Sarmento B. Nanocarriers for pulmonary administration of peptides and therapeutic proteins. Nanomedicine (Lond) 6: 123–141, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Antony S, Wu Y, Hewitt SM, Anver MR, Butcher D, Jiang G, Meitzler JL, Liu H, Juhasz A, Lu J, Roy KK, and Doroshow JH. Characterization of NADPH oxidase 5 expression in human tumors and tumor cell lines with a novel mouse monoclonal antibody. Free Radic Biol Med 65: 497–508, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoyama T, Paik YH, Watanabe S, Laleu B, Gaggini F, Fioraso-Cartier L, Molango S, Heitz F, Merlot C, Szyndralewiez C, Page P, and Brenner DA. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: GKT137831 as a novel potential therapeutic agent. Hepatology 56: 2316–2327, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, and Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem 279: 46065–46072, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Bedard K. and Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 87: 245–313, 2007 [DOI] [PubMed] [Google Scholar]
- 9.BelAiba RS, Djordjevic T, Petry A, Diemer K, Bonello S, Banfi B, Hess J, Pogrebniak A, Bickel C, and Gorlach A. NOX5 variants are functionally active in endothelial cells. Free Radic Biol Med 42: 446–459, 2007 [DOI] [PubMed] [Google Scholar]
- 10.Berrios RL. and Arbiser JL. Novel antiangiogenic agents in dermatology. Arch Biochem Biophys 508: 222–226, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, and Arbiser JL. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest 119: 2359–2365, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DM, Donaldson K, Borm PJ, Schins RP, Dehnhardt M, Gilmour P, Jimenez LA, and Stone V. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol 286: L344–L353, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Brown KL, Bylund J, MacDonald KL, Song-Zhao GX, Elliott MR, Falsafi R, Hancock RE, and Speert DP. ROS-deficient monocytes have aberrant gene expression that correlates with inflammatory disorders of chronic granulomatous disease. Clin Immunol 129: 90–102, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Cai H, Griendling KK, and Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003 [DOI] [PubMed] [Google Scholar]
- 15.Carnesecchi S, Deffert C, Donati Y, Basset O, Hinz B, Preynat-Seauve O, Guichard C, Arbiser JL, Banfi B, Pache JC, Barazzone-Argiroffo C, and Krause KH. A key role for NOX4 in epithelial cell death during development of lung fibrosis. Antioxid Redox Signal 15: 607–619, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carnesecchi S, Pache JC, and Barazzone-Argiroffo C. NOX enzymes: potential target for the treatment of acute lung injury. Cell Mol Life Sci 69: 2373–2385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng G, Cao Z, Xu X, van Meir EG, and Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 269: 131–140, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Cheng G, Diebold BA, Hughes Y, and Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem 281: 17718–17726, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Cheng G. and Lambeth JD. Alternative mRNA splice forms of NOXO1: differential tissue expression and regulation of Nox1 and Nox3. Gene 356: 118–126, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Cheng G. and Lambeth JD. NOXO1, regulation of lipid binding, localization, and activation of Nox1 by the Phox homology (PX) domain. J Biol Chem 279: 4737–4742, 2004 [DOI] [PubMed] [Google Scholar]
- 21.Cheng G, Ritsick D, and Lambeth JD. Nox3 regulation by NOXO1, p47phox, and p67phox. J Biol Chem 279: 34250–34255, 2004 [DOI] [PubMed] [Google Scholar]
- 22.Chiang CH, Chuang CH, and Liu SL. Apocynin attenuates ischemia-reperfusion lung injury in an isolated and perfused rat lung model. Transl Res 158: 17–29, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Cho YS. and Moon HB. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol Res 2: 183–187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi JE, Lee SS, Sunde DA, Huizar I, Haugk KL, Thannickal VJ, Vittal R, Plymate SR, and Schnapp LM. Insulin-like growth factor-I receptor blockade improves outcome in mouse model of lung injury. Am J Respir Crit Care Med 179: 212–219, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chose O, Sansilvestri-Morel P, Badier-Commander C, Bernhardt F, Fabiani JN, Rupin A, and Verbeuren TJ. Distinct role of nox1, nox2, and p47phox in unstimulated versus angiotensin II-induced NADPH oxidase activity in human venous smooth muscle cells. J Cardiovasc Pharmacol 51: 131–139, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Cifuentes-Pagano E, Csanyi G, and Pagano PJ. NADPH oxidase inhibitors: a decade of discovery from Nox2ds to HTS. Cell Mol Life Sci 69: 2315–2325, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.This reference has been deleted.
- 28.Comstock AT, Ganesan S, Chattoraj A, Faris AN, Margolis BL, Hershenson MB, and Sajjan US. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by NADPH oxidase 1. J Virol 85: 6795–6808, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conway KL, Goel G, Sokol H, Manocha M, Mizoguchi E, Terhorst C, Bhan AK, Gardet A, and Xavier RJ. p40phox expression regulates neutrophil recruitment and function during the resolution phase of intestinal inflammation. J Immunol 189: 3631–3640, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coso S, Harrison I, Harrison CB, Vinh A, Sobey CG, Drummond GR, Williams ED, and Selemidis S. NADPH oxidases as regulators of tumor angiogenesis: current and emerging concepts. Antioxid Redox Signal 16: 1229–1247, 2012 [DOI] [PubMed] [Google Scholar]
- 31.Csanyi G, Cifuentes-Pagano E, Al Ghouleh I, Ranayhossaini DJ, Egana L, Lopes LR, Jackson HM, Kelley EE, and Pagano PJ. Nox2 B-loop peptide, Nox2ds, specifically inhibits the NADPH oxidase Nox2. Free Radic Biol Med 51: 1116–1125, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cui Y, Robertson J, Maharaj S, Waldhauser L, Niu J, Wang J, Farkas L, Kolb M, and Gauldie J. Oxidative stress contributes to the induction and persistence of TGF-beta1 induced pulmonary fibrosis. Int J Biochem Cell Biol 43: 1122–1133, 2011 [DOI] [PubMed] [Google Scholar]
- 33.Dang PM, Raad H, Derkawi RA, Boussetta T, Paclet MH, Belambri SA, Makni-Maalej K, Kroviarski Y, Morel F, Gougerot-Pocidalo MA, and El-Benna J. The NADPH oxidase cytosolic component p67phox is constitutively phosphorylated in human neutrophils: Regulation by a protein tyrosine kinase, MEK1/2 and phosphatases 1/2A. Biochem Pharmacol 82: 1145–1152, 2011 [DOI] [PubMed] [Google Scholar]
- 34.de Broucker V, Hassoun SM, Hulo S, Cherot-Kornobis N, Neviere R, Matran R, Sobaszek A, and Edme JL. Non-invasive collection of exhaled breath condensate in rats: evaluation of pH, H(2)O(2) and NOx in lipopolysaccharide-induced acute lung injury. Vet J 194: 222–228, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Di Matteo G, Giordani L, Finocchi A, Ventura A, Chiriaco M, Blancato J, Sinibaldi C, Plebani A, Soresina A, Pignata C, Dellepiane RM, Trizzino A, Cossu F, Rondelli R, Rossi P, De Mattia D, and Martire B. Molecular characterization of a large cohort of patients with Chronic Granulomatous Disease and identification of novel CYBB mutations: an Italian multicenter study. Mol Immunol 46: 1935–1941, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Diatchuk V, Lotan O, Koshkin V, Wikstroem P, and Pick E. Inhibition of NADPH oxidase activation by 4-(2-aminoethyl)-benzenesulfonyl fluoride and related compounds. J Biol Chem 272: 13292–13301, 1997 [DOI] [PubMed] [Google Scholar]
- 37.Dinauer MC, Lekstrom-Himes JA, and Dale DC. Inherited neutrophil disorders: molecular basis and new therapies. Hematol Am Soc Hematol Educ Program 303–318, 2000 [DOI] [PubMed] [Google Scholar]
- 38.Djordjevic T, Pogrebniak A, BelAiba RS, Bonello S, Wotzlaw C, Acker H, Hess J, and Gorlach A. The expression of the NADPH oxidase subunit p22phox is regulated by a redox-sensitive pathway in endothelial cells. Free Radic Biol Med 38: 616–630, 2005 [DOI] [PubMed] [Google Scholar]
- 39.Doussiere J, Poinas A, Blais C, and Vignais PV. Phenylarsine oxide as an inhibitor of the activation of the neutrophil NADPH oxidase—identification of the beta subunit of the flavocytochrome b component of the NADPH oxidase as a target site for phenylarsine oxide by photoaffinity labeling and photoinactivation. Eur J Biochem 251: 649–658, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Duong C, Seow HJ, Bozinovski S, Crack PJ, Anderson GP, and Vlahos R. Glutathione peroxidase-1 protects against cigarette smoke-induced lung inflammation in mice. Am J Physiol Lung Cell Mol Physiol 299: L425–L433, 2010 [DOI] [PubMed] [Google Scholar]
- 41.Dusi S, Donini M, and Rossi F. Mechanisms of NADPH oxidase activation: translocation of p40phox, Rac1 and Rac2 from the cytosol to the membranes in human neutrophils lacking p47phox or p67phox. Biochem J 314 (Pt 2): 409–412, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dutta S. and Rittinger K. Regulation of NOXO1 activity through reversible interactions with p22 and NOXA1. PLoS One 5: e10478, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edderkaoui M, Nitsche C, Zheng L, Pandol SJ, Gukovsky I, and Gukovskaya AS. NADPH oxidase activation in pancreatic cancer cells is mediated through Akt-dependent up-regulation of p22phox. J Biol Chem 286: 7779–7787, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eklund EA, Jalava A, and Kakar R. PU.1, interferon regulatory factor 1, and interferon consensus sequence-binding protein cooperate to increase gp91(phox) expression. J Biol Chem 273: 13957–13965, 1998 [DOI] [PubMed] [Google Scholar]
- 45.El-Benna J, Dang PM, and Perianin A. Peptide-based inhibitors of the phagocyte NADPH oxidase. Biochem Pharmacol 80: 778–785, 2010 [DOI] [PubMed] [Google Scholar]
- 46.El-Benna J, Dang PM, and Perianin A. Towards specific NADPH oxidase inhibition by small synthetic peptides. Cell Mol Life Sci 69: 2307–2314, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fischer H. Mechanisms and function of DUOX in epithelia of the lung. Antioxid Redox Signal 11: 2453–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furst R, Brueckl C, Kuebler WM, Zahler S, Krotz F, Gorlach A, Vollmar AM, and Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res 96: 43–53, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Gaggini F, Laleu B, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual Nox4/Nox1 inhibitors. Bioorg Med Chem 19: 6989–6999, 2011 [DOI] [PubMed] [Google Scholar]
- 50.Galli F, Battistoni A, Gambari R, Pompella A, Bragonzi A, Pilolli F, Iuliano L, Piroddi M, Dechecchi MC, and Cabrini G. Oxidative stress and antioxidant therapy in cystic fibrosis. Biochim Biophys Acta 1822: 690–713, 2012 [DOI] [PubMed] [Google Scholar]
- 51.Gao XP, Standiford TJ, Rahman A, Newstead M, Holland SM, Dinauer MC, Liu QH, and Malik AB. Role of NADPH oxidase in the mechanism of lung neutrophil sequestration and microvessel injury induced by Gram-negative sepsis: studies in p47phox−/− and gp91phox−/− mice. J Immunol 168: 3974–3982, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Gauss KA, Nelson-Overton LK, Siemsen DW, Gao Y, DeLeo FR, and Quinn MT. Role of NF-kappaB in transcriptional regulation of the phagocyte NADPH oxidase by tumor necrosis factor-alpha. J Leukoc Biol 82: 729–741, 2007 [DOI] [PubMed] [Google Scholar]
- 53.George A, Pushkaran S, Konstantinidis DG, Koochaki S, Malik P, Mohandas N, Zheng Y, Joiner CH, and Kalfa TA. Erythrocyte NADPH oxidase activity modulated by Rac GTPases, PKC, and plasma cytokines contributes to oxidative stress in sickle cell disease. Blood 121: 2099–2107, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, and Rosen H. A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells. ACS Chem Biol 5: 981–993, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goyal P, Weissmann N, Grimminger F, Hegel C, Bader L, Rose F, Fink L, Ghofrani HA, Schermuly RT, Schmidt HH, Seeger W, and Hanze J. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med 36: 1279–1288, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Goyal P, Weissmann N, Rose F, Grimminger F, Schafers HJ, Seeger W, and Hanze J. Identification of novel Nox4 splice variants with impact on ROS levels in A549 cells. Biochem Biophys Res Commun 329: 32–39, 2005 [DOI] [PubMed] [Google Scholar]
- 57.Green AR. and Ashwood T. Free radical trapping as a therapeutic approach to neuroprotection in stroke: experimental and clinical studies with NXY-059 and free radical scavengers. Curr Drug Targets CNS Neurol Disord 4: 109–118, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Griffith B, Pendyala S, Hecker L, Lee PJ, Natarajan V, and Thannickal VJ. NOX enzymes and pulmonary disease. Antioxid Redox Signal 11: 2505–2516, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guidot DM. and Roman J. Chronic ethanol ingestion increases susceptibility to acute lung injury: role of oxidative stress and tissue remodeling. Chest 122: 309S–314S, 2002 [DOI] [PubMed] [Google Scholar]
- 60.Gustafson SJ, Dunlap KL, McGill CM, and Kuhn TB. A nonpolar blueberry fraction blunts NADPH oxidase activation in neuronal cells exposed to tumor necrosis factor-alpha. Oxid Med Cell Longev 2012: 768101, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hammad H. and Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 8: 193–204, 2008 [DOI] [PubMed] [Google Scholar]
- 62.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, and Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 579: 4911–4917, 2005 [DOI] [PubMed] [Google Scholar]
- 63.Harrison CB, Selemidis S, Guida E, King PT, Sobey CG, and Drummond GR. NOX2beta: A novel splice variant of NOX2 that regulates NADPH oxidase activity in macrophages. PLoS One 7: e48326, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hawkins PT, Davidson K, and Stephens LR. The role of PI3Ks in the regulation of the neutrophil NADPH oxidase. Biochem Soc Symp 59–67, 2007 [DOI] [PubMed] [Google Scholar]
- 65.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, and Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, and Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51: 211–217, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Heymes C, Bendall JK, Ratajczak P, Cave AC, Samuel JL, Hasenfuss G, and Shah AM. Increased myocardial NADPH oxidase activity in human heart failure. J Am Coll Cardiol 41: 2164–2171, 2003 [DOI] [PubMed] [Google Scholar]
- 68.Honda F, Hane Y, Toma T, Yachie A, Kim ES, Lee SK, Takagi M, Mizutani S, and Morio T. Transducible form of p47phox and p67phox compensate for defective NADPH oxidase activity in neutrophils of patients with chronic granulomatous disease. Biochem Biophys Res Commun 417: 162–168, 2012 [DOI] [PubMed] [Google Scholar]
- 69.Hordijk PL. Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98: 453–462, 2006 [DOI] [PubMed] [Google Scholar]
- 70.Hui YF, Chan SY, and Lau YL. Identification of mutations in seven Chinese patients with X-linked chronic granulomatous disease. Blood 88: 4021–4028, 1996 [PubMed] [Google Scholar]
- 71.Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, and Penninger JM. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133: 235–249, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacobson GM, Dourron HM, Liu J, Carretero OA, Reddy DJ, Andrzejewski T, and Pagano PJ. Novel NAD(P)H oxidase inhibitor suppresses angioplasty-induced superoxide and neointimal hyperplasia of rat carotid artery. Circ Res 92: 637–643, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Jaquet V. and Bedard K. Editorial: Genetic mapping—the path of discovery for novel functions of the NOX NADPH oxidases. J Leukoc Biol 86: 461–463, 2009 [DOI] [PubMed] [Google Scholar]
- 74.Johnson ER. and Matthay MA. Acute lung injury: epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv 23: 243–252, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kao YY, Gianni D, Bohl B, Taylor RM, and Bokoch GM. Identification of a conserved Rac-binding site on NADPH oxidases supports a direct GTPase regulatory mechanism. J Biol Chem 283: 12736–12746, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Katsuyama M. NOX/NADPH oxidase, the superoxide-generating enzyme: its transcriptional regulation and physiological roles. J Pharmacol Sci 114: 134–146, 2010 [DOI] [PubMed] [Google Scholar]
- 77.Katsuyama M, Matsuno K, and Yabe-Nishimura C. Physiological roles of NOX/NADPH oxidase, the superoxide-generating enzyme. J Clin Biochem Nutr 50: 9–22, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khanna A, Guo M, Mehra M, and Royal W, 3rd. Inflammation and oxidative stress induced by cigarette smoke in Lewis rat brains. J Neuroimmunol 254: 69–75, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Khanna AK. and Pieper GM. NADPH oxidase subunits (NOX-1, p22phox, Rac-1) and tacrolimus-induced nephrotoxicity in a rat renal transplant model. Nephrol Dial Transplant 22: 376–385, 2007 [DOI] [PubMed] [Google Scholar]
- 80.King TE, Jr., Clinical advances in the diagnosis and therapy of the interstitial lung diseases. Am J Respir Crit Care Med 172: 268–279, 2005 [DOI] [PubMed] [Google Scholar]
- 81.King TE, Jr., Pardo A, and Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Kleniewska P, Piechota A, Skibska B, and Goraca A. The NADPH oxidase family and its inhibitors. Arch Immunol Ther Exp (Warsz) 60: 277–294, 2012 [DOI] [PubMed] [Google Scholar]
- 83.Krause KH, Lambeth D, and Kronke M. NOX enzymes as drug targets. Cell Mol Life Sci 69: 2279–2282, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Krotz F, Keller M, Derflinger S, Schmid H, Gloe T, Bassermann F, Duyster J, Cohen CD, Schuhmann C, Klauss V, Pohl U, Stempfle HU, and Sohn HY. Mycophenolate acid inhibits endothelial NAD(P)H oxidase activity and superoxide formation by a Rac1-dependent mechanism. Hypertension 49: 201–208, 2007 [DOI] [PubMed] [Google Scholar]
- 85.Kumar TR, Soppimath K, and Nachaegari SK. Novel delivery technologies for protein and peptide therapeutics. Curr Pharm Biotechnol 7: 261–276, 2006 [DOI] [PubMed] [Google Scholar]
- 86.Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, and Rokutan K. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol 290: C433–C443, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Lagente V, Planquois JM, Leclerc O, Schmidlin F, and Bertrand CP. Oxidative stress is an important component of airway inflammation in mice exposed to cigarette smoke or lipopolysaccharide. Clin Exp Pharmacol Physiol 35: 601–605, 2008 [DOI] [PubMed] [Google Scholar]
- 88.Laleu B, Gaggini F, Orchard M, Fioraso-Cartier L, Cagnon L, Houngninou-Molango S, Gradia A, Duboux G, Merlot C, Heitz F, Szyndralewiez C, and Page P. First in class, potent, and orally bioavailable NADPH oxidase isoform 4 (Nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J Med Chem 53: 7715–7730, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Lambeth JD, Kawahara T, and Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43: 319–331, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee IT. and Yang CM. Role of NADPH oxidase/ROS in pro-inflammatory mediators-induced airway and pulmonary diseases. Biochem Pharmacol 84: 581–590, 2012 [DOI] [PubMed] [Google Scholar]
- 91.This reference has been deleted.
- 92.Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, and Robinson JP. DPI induces mitochondrial superoxide-mediated apoptosis. Free Radic Biol Med 34: 465–477, 2003 [DOI] [PubMed] [Google Scholar]
- 93.Liu Q, He X, Liu Y, Du B, Wang X, Zhang W, Jia P, Dong J, Ma J, Li S, and Zhang H. NADPH oxidase-mediated generation of reactive oxygen species: A new mechanism for X-ray-induced HeLa cell death. Biochem Biophys Res Commun 377: 775–779, 2008 [DOI] [PubMed] [Google Scholar]
- 94.Louhelainen N, Rytila P, Haahtela T, Kinnula VL, and Djukanovic R. Persistence of oxidant and protease burden in the airways after smoking cessation. BMC Pulm Med 9: 25, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Luo H, Yang A, Schulte BA, Wargovich MJ, and Wang GY. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS One 8: e60065, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.MacNee W. Oxidative stress and lung inflammation in airways disease. Eur J Pharmacol 429: 195–207, 2001 [DOI] [PubMed] [Google Scholar]
- 97.Maghzal GJ, Krause KH, Stocker R, and Jaquet V. Detection of reactive oxygen species derived from the family of NOX NADPH oxidases. Free Radic Biol Med 53: 1903–1918, 2012 [DOI] [PubMed] [Google Scholar]
- 98.Maheswaranathan M, Gole HK, Fernandez I, Lassegue B, Griendling KK, and San Martin A. Platelet-derived growth factor (PDGF) regulates Slingshot phosphatase activity via Nox1-dependent auto-dephosphorylation of serine 834 in vascular smooth muscle cells. J Biol Chem 286: 35430–35437, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marcal LE, Dias-da-Motta PM, Rehder J, Mamoni RL, Blotta MH, Whitney CB, Newburger PE, Costa FF, Saad ST, and Condino-Neto A. Up-regulation of NADPH oxidase components and increased production of interferon-gamma by leukocytes from sickle cell disease patients. Am J Hematol 83: 41–45, 2008 [DOI] [PubMed] [Google Scholar]
- 100.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, and Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006 [DOI] [PubMed] [Google Scholar]
- 101.Martyniuk TV, Konosova ID, and Chazova IE. [Use of nebivolol in patients with idiopathic pulmonary hypertension: results of the pilot study]. Ter Arkh 84: 49–53, 2012 [PubMed] [Google Scholar]
- 102.Mason RP, Kalinowski L, Jacob RF, Jacoby AM, and Malinski T. Nebivolol reduces nitroxidative stress and restores nitric oxide bioavailability in endothelium of black Americans. Circulation 112: 3795–3801, 2005 [DOI] [PubMed] [Google Scholar]
- 103.Massenet C, Chenavas S, Cohen-Addad C, Dagher MC, Brandolin G, Pebay-Peyroula E, and Fieschi F. Effects of p47phox C terminus phosphorylations on binding interactions with p40phox and p67phox. Structural and functional comparison of p40phox and p67phox SH3 domains. J Biol Chem 280: 13752–13761, 2005 [DOI] [PubMed] [Google Scholar]
- 104.May RC. and Machesky LM. Phagocytosis and the actin cytoskeleton. J Cell Sci 114: 1061–1077, 2001 [DOI] [PubMed] [Google Scholar]
- 105.Mollnau H, Schulz E, Daiber A, Baldus S, Oelze M, August M, Wendt M, Walter U, Geiger C, Agrawal R, Kleschyov AL, Meinertz T, and Munzel T. Nebivolol prevents vascular NOS III uncoupling in experimental hyperlipidemia and inhibits NADPH oxidase activity in inflammatory cells. Arterioscler Thromb Vasc Biol 23: 615–621, 2003 [DOI] [PubMed] [Google Scholar]
- 106.Monaghan-Benson E. and Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem 284: 25602–25611, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr., Nauseef WM, Dupuy C, and Banfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med 175: 174–183, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakano Y, Banfi B, Jesaitis AJ, Dinauer MC, Allen LA, and Nauseef WM. Critical roles for p22phox in the structural maturation and subcellular targeting of Nox3. Biochem J 403: 97–108, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Norton CE, Broughton BR, Jernigan NL, Walker BR, and Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid Redox Signal 18: 1777–1788, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ostuni MA, Gelinotte M, Bizouarn T, Baciou L, and Houee-Levin C. Targeting NADPH-oxidase by reactive oxygen species reveals an initial sensitive step in the assembly process. Free Radic Biol Med 49: 900–907, 2010 [DOI] [PubMed] [Google Scholar]
- 111.Parinandi NL, Kleinberg MA, Usatyuk PV, Cummings RJ, Pennathur A, Cardounel AJ, Zweier JL, Garcia JG, and Natarajan V. Hyperoxia-induced NAD(P)H oxidase activation and regulation by MAP kinases in human lung endothelial cells. Am J Physiol Lung Cell Mol Physiol 284: L26–L38, 2003 [DOI] [PubMed] [Google Scholar]
- 112.Park S, Ahn JY, Lim MJ, Kim MH, Yun YS, Jeong G, and Song JY. Sustained expression of NADPH oxidase 4 by p38 MAPK-Akt signaling potentiates radiation-induced differentiation of lung fibroblasts. J Mol Med (Berl) 88: 807–816, 2010 [DOI] [PubMed] [Google Scholar]
- 113.Pendyala S, Gorshkova IA, Usatyuk PV, He D, Pennathur A, Lambeth JD, Thannickal VJ, and Natarajan V. Role of Nox4 and Nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid Redox Signal 11: 747–764, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pendyala S, Moitra J, Kalari S, Kleeberger SR, Zhao Y, Reddy SP, Garcia JG, and Natarajan V. Nrf2 regulates hyperoxia-induced Nox4 expression in human lung endothelium: identification of functional antioxidant response elements on the Nox4 promoter. Free Radic Biol Med 50: 1749–1759, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pendyala S. and Natarajan V. Redox regulation of Nox proteins. Respir Physiol Neurobiol 174: 265–271, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, and Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11: 841–860, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Peters EA, Hiltermann JT, and Stolk J. Effect of apocynin on ozone-induced airway hyperresponsiveness to methacholine in asthmatics. Free Radic Biol Med 31: 1442–1447, 2001 [DOI] [PubMed] [Google Scholar]
- 118.Polikandriotis JA, Rupnow HL, Elms SC, Clempus RE, Campbell DJ, Sutliff RL, Brown LA, Guidot DM, and Hart CM. Chronic ethanol ingestion increases superoxide production and NADPH oxidase expression in the lung. Am J Respir Cell Mol Biol 34: 314–319, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Qian L, Gao X, Pei Z, Wu X, Block M, Wilson B, Hong JS, and Flood PM. NADPH oxidase inhibitor DPI is neuroprotective at femtomolar concentrations through inhibition of microglia over-activation. Parkinsonism Relat Disord 13 Suppl 3: S316–S320, 2007 [DOI] [PubMed] [Google Scholar]
- 120.Reis FG, Marques RH, Starling CM, Almeida-Reis R, Vieira RP, Cabido CT, Silva LF, Lancas T, Dolhnikoff M, Martins MA, Leick-Maldonado EA, Prado CM, and Tiberio IF. Stress amplifies lung tissue mechanics, inflammation and oxidative stress induced by chronic inflammation. Exp Lung Res 38: 344–354, 2012 [DOI] [PubMed] [Google Scholar]
- 121.Rey FE, Cifuentes ME, Kiarash A, Quinn MT, and Pagano PJ. Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(-) and systolic blood pressure in mice. Circ Res 89: 408–414, 2001 [DOI] [PubMed] [Google Scholar]
- 122.Rigutto S, Hoste C, Grasberger H, Milenkovic M, Communi D, Dumont JE, Corvilain B, Miot F, and De Deken X. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J Biol Chem 284: 6725–6734, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Roussin A, Le Cabec V, Lonchampt M, De Nadai J, Canet E, and Maridonneau-Parini I. Neutrophil-associated inflammatory responses in rats are inhibited by phenylarsine oxide. Eur J Pharmacol 322: 91–96, 1997 [DOI] [PubMed] [Google Scholar]
- 124.Rowe SM, Miller S, and Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005 [DOI] [PubMed] [Google Scholar]
- 125.Sadat MA, Kumatori A, Suzuki S, Yamaguchi Y, Tsuji Y, and Nakamura M. GATA-3 represses gp91phox gene expression in eosinophil-committed HL-60-C15 cells. FEBS Lett 436: 390–394, 1998 [DOI] [PubMed] [Google Scholar]
- 126.Sancho P, Bertran E, Caja L, Carmona-Cuenca I, Murillo MM, and Fabregat I. The inhibition of the epidermal growth factor (EGF) pathway enhances TGF-beta-induced apoptosis in rat hepatoma cells through inducing oxidative stress coincident with a change in the expression pattern of the NADPH oxidases (NOX) isoforms. Biochim Biophys Acta 1793: 253–263, 2009 [DOI] [PubMed] [Google Scholar]
- 127.Schatz M, Zeiger RS, Yang SJ, Chen W, Crawford WW, Sajjan SG, and Allen-Ramey F. Relationship of asthma control to asthma exacerbations using surrogate markers within a managed care database. Am J Manag Care 16: 327–333, 2010 [PubMed] [Google Scholar]
- 128.Schwarzer C, Machen TE, Illek B, and Fischer H. NADPH oxidase-dependent acid production in airway epithelial cells. J Biol Chem 279: 36454–36461, 2004 [DOI] [PubMed] [Google Scholar]
- 129.Segal BH, Han W, Bushey JJ, Joo M, Bhatti Z, Feminella J, Dennis CG, Vethanayagam RR, Yull FE, Capitano M, Wallace PK, Minderman H, Christman JW, Sporn MB, Chan J, Vinh DC, Holland SM, Romani LR, Gaffen SL, Freeman ML, and Blackwell TS. NADPH oxidase limits innate immune responses in the lungs in mice. PLoS One 5: e9631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Selemidis S, Seow HJ, Broughton BR, Vinh A, Bozinovski S, Sobey CG, Drummond GR, and Vlahos R. Nox1 oxidase suppresses influenza a virus-induced lung inflammation and oxidative stress. PLoS One 8: e60792, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sham D, Wesley UV, Hristova M, and van der Vliet A. ATP-Mediated transactivation of the epidermal growth factor receptor in airway epithelial cells involves DUOX1-dependent oxidation of Src and ADAM17. PLoS One 8: e54391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sham JS. Hypoxic pulmonary vasoconstriction: ups and downs of reactive oxygen species. Circ Res 91: 649–651, 2002 [DOI] [PubMed] [Google Scholar]
- 133.Shao MX. and Nadel JA. Dual oxidase 1-dependent MUC5AC mucin expression in cultured human airway epithelial cells. Proc Natl Acad Sci U S A 102: 767–772, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Singh N, Halliday AC, Thomas JM, Kuznetsova OV, Baldwin R, Woon EC, Aley PK, Antoniadou I, Sharp T, Vasudevan SR, and Churchill GC. A safe lithium mimetic for bipolar disorder. Nat Commun 4: 1332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith SM, Min J, Ganesh T, Diebold B, Kawahara T, Zhu Y, McCoy J, Sun A, Snyder JP, Fu H, Du Y, Lewis I, and Lambeth JD. Ebselen and congeners inhibit NADPH oxidase 2-dependent superoxide generation by interrupting the binding of regulatory subunits. Chem Biol 19: 752–763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Snelgrove RJ, Edwards L, Rae AJ, and Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur J Immunol 36: 1364–1373, 2006 [DOI] [PubMed] [Google Scholar]
- 137.Sorce S, Krause KH, and Jaquet V. Targeting NOX enzymes in the central nervous system: therapeutic opportunities. Cell Mol Life Sci 69: 2387–2407, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Souabni H, Thoma V, Bizouarn T, Chatgilialoglu C, Siafaka-Kapadai A, Baciou L, Ferreri C, Houee-Levin C, and Ostuni MA. trans Arachidonic acid isomers inhibit NADPH-oxidase activity by direct interaction with enzyme components. Biochim Biophys Acta 1818: 2314–2324, 2012 [DOI] [PubMed] [Google Scholar]
- 139.Soucy-Faulkner A, Mukawera E, Fink K, Martel A, Jouan L, Nzengue Y, Lamarre D, Vande Velde C, and Grandvaux N. Requirement of NOX2 and reactive oxygen species for efficient RIG-I-mediated antiviral response through regulation of MAVS expression. PLoS Pathog 6: e1000930, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Doniec Z, Bialasiewicz P, Nowak D, and Pawliczak R. Hydrogen peroxide and nitrite reduction in exhaled breath condensate of COPD patients. Pulm Pharmacol Ther 25: 343–348, 2012 [DOI] [PubMed] [Google Scholar]
- 141.Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Pniewska E, Doniec Z, Nowak D, and Pawliczak R. Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics. Exp Lung Res 38: 90–99, 2012 [DOI] [PubMed] [Google Scholar]
- 142.Stefanska J, Sokolowska M, Sarniak A, Wlodarczyk A, Doniec Z, Nowak D, and Pawliczak R. Apocynin decreases hydrogen peroxide and nitrate concentrations in exhaled breath in healthy subjects. Pulm Pharmacol Ther 23: 48–54, 2010 [DOI] [PubMed] [Google Scholar]
- 143.Streeter J, Thiel W, Brieger K, and Miller FJ, Jr., Opportunity Nox: the future of NADPH oxidases as therapeutic targets in cardiovascular disease. Cardiovasc Ther 31: 125–137, 2013 [DOI] [PubMed] [Google Scholar]
- 144.Sun C, Zubcevic J, Polson JW, Potts JT, Diez-Freire C, Zhang Q, Paton JF, and Raizada MK. Shift to an involvement of phosphatidylinositol 3-kinase in angiotensin II actions on nucleus tractus solitarii neurons of the spontaneously hypertensive rat. Circ Res 105: 1248–1255, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sutcliffe A, Hollins F, Gomez E, Saunders R, Doe C, Cooke M, Challiss RA, and Brightling CE. Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am J Respir Crit Care Med 185: 267–274, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Suzuki S, Kumatori A, Haagen IA, Fujii Y, Sadat MA, Jun HL, Tsuji Y, Roos D, and Nakamura M. PU.1 as an essential activator for the expression of gp91(phox) gene in human peripheral neutrophils, monocytes, and B lymphocytes. Proc Natl Acad Sci U S A 95: 6085–6090, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Syrkina O, Jafari B, Hales CA, and Quinn DA. Oxidant stress mediates inflammation and apoptosis in ventilator-induced lung injury. Respirology 13: 333–340, 2008 [DOI] [PubMed] [Google Scholar]
- 148.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, and Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem 278: 25234–25246, 2003 [DOI] [PubMed] [Google Scholar]
- 149.Tamura M, Shiozaki I, Ono S, Miyano K, Kunihiro S, and Sasaki T. p40phox as an alternative organizer to p47phox in Nox2 activation: a new mechanism involving an interaction with p22phox. FEBS Lett 581: 4533–4538, 2007 [DOI] [PubMed] [Google Scholar]
- 150.Tarr JM, Ding N, Kaul K, Antonell A, Perez-Jurado LA, and Chibber R. Cellular crosstalk between TNF-alpha, NADPH oxidase, PKCbeta2, and C2GNT in human leukocytes. Cell Signal 24: 873–878, 2012 [DOI] [PubMed] [Google Scholar]
- 151.Tollefson AK, Oberley-Deegan RE, Butterfield KT, Nicks ME, Weaver MR, Remigio LK, Decsesznak J, Chu HW, Bratton DL, Riches DW, and Bowler RP. Endogenous enzymes (NOX and ECSOD) regulate smoke-induced oxidative stress. Free Radic Biol Med 49: 1937–1946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Touyz RM, Yao G, Quinn MT, Pagano PJ, and Schiffrin EL. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler Thromb Vasc Biol 25: 512–518, 2005 [DOI] [PubMed] [Google Scholar]
- 153.Tuder RM, Janciauskiene SM, and Petrache I. Lung disease associated with alpha1-antitrypsin deficiency. Proc Am Thorac Soc 7: 381–386, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Usatyuk PV, Romer LH, He D, Parinandi NL, Kleinberg ME, Zhan S, Jacobson JR, Dudek SM, Pendyala S, Garcia JG, and Natarajan V. Regulation of hyperoxia-induced NADPH oxidase activation in human lung endothelial cells by the actin cytoskeleton and cortactin. J Biol Chem 282: 23284–23295, 2007 [DOI] [PubMed] [Google Scholar]
- 155.van Beek EM, Zarate JA, van Bruggen R, Schornagel K, Tool AT, Matozaki T, Kraal G, Roos D, and van den Berg TK. SIRPalpha controls the activity of the phagocyte NADPH oxidase by restricting the expression of gp91(phox). Cell Rep 2: 748–755, 2012 [DOI] [PubMed] [Google Scholar]
- 156.van der Vliet A. NADPH oxidases in lung biology and pathology: host defense enzymes, and more. Free Radic Biol Med 44: 938–955, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.van der Vliet A. Nox enzymes in allergic airway inflammation. Biochim Biophys Acta 1810: 1035–1044, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vejrazka M, Micek R, and Stipek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta 1722: 143–147, 2005 [DOI] [PubMed] [Google Scholar]
- 159.Vendramini EC, Vianna EO, De Lucena Angulo I, De Castro FB, Martinez JA, and Terra-Filho J. Lung function and airway hyperresponsiveness in adult patients with sickle cell disease. Am J Med Sci 332: 68–72, 2006 [DOI] [PubMed] [Google Scholar]
- 160.Vergnaud S, Paclet MH, El Benna J, Pocidalo MA, and Morel F. Complementation of NADPH oxidase in p67-phox-deficient CGD patients p67-phox/p40-phox interaction. Eur J Biochem 267: 1059–1067, 2000 [DOI] [PubMed] [Google Scholar]
- 161.Vibhuti A, Arif E, Mishra A, Deepak D, Singh B, Rahman I, Mohammad G, and Pasha MA. CYP1A1, CYP1A2 and CYBA gene polymorphisms associated with oxidative stress in COPD. Clin Chim Acta 411: 474–480, 2010 [DOI] [PubMed] [Google Scholar]
- 162.Viedt C, Fei J, Krieger-Brauer HI, Brandes RP, Teupser D, Kamimura M, Katus HA, and Kreuzer J. Role of p22phox in angiotensin II and platelet-derived growth factor AA induced activator protein 1 activation in vascular smooth muscle cells. J Mol Med (Berl) 82: 31–38, 2004 [DOI] [PubMed] [Google Scholar]
- 163.Vlahos R, Stambas J, Bozinovski S, Broughton BR, Drummond GR, and Selemidis S. Inhibition of Nox2 oxidase activity ameliorates influenza A virus-induced lung inflammation. PLoS Pathog 7: e1001271, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vlahos R, Stambas J, and Selemidis S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharmacol Sci 33: 3–8, 2012 [DOI] [PubMed] [Google Scholar]
- 165.Vlessis AA, Bartos D, Muller P, and Trunkey DD. Role of reactive O2 in phagocyte-induced hypermetabolism and pulmonary injury. J Appl Physiol 78: 112–116, 1995 [DOI] [PubMed] [Google Scholar]
- 166.Wang W, Suzuki Y, Tanigaki T, Rank DR, and Raffin TA. Effect of the NADPH oxidase inhibitor apocynin on septic lung injury in guinea pigs. Am J Respir Crit Care Med 150: 1449–1452, 1994 [DOI] [PubMed] [Google Scholar]
- 167.Wedes SH, Khatri SB, Zhang R, Wu W, Comhair SA, Wenzel S, Teague WG, Israel E, Erzurum SC, and Hazen SL. Noninvasive markers of airway inflammation in asthma. Clin Transl Sci 2: 112–117, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Weissmann N, Tadic A, Hanze J, Rose F, Winterhalder S, Nollen M, Schermuly RT, Ghofrani HA, Seeger W, and Grimminger F. Hypoxic vasoconstriction in intact lungs: a role for NADPH oxidase-derived H(2)O(2)? Am J Physiol Lung Cell Mol Physiol 279: L683–L690, 2000 [DOI] [PubMed] [Google Scholar]
- 169.Wind S, Beuerlein K, Eucker T, Muller H, Scheurer P, Armitage ME, Ho H, Schmidt HH, and Wingler K. Comparative pharmacology of chemically distinct NADPH oxidase inhibitors. Br J Pharmacol 161: 885–898, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Winkelstein JA, Marino MC, Johnston RB, Jr., Boyle J, Curnutte J, Gallin JI, Malech HL, Holland SM, Ochs H, Quie P, Buckley RH, Foster CB, Chanock SJ, and Dickler H. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 79: 155–169, 2000 [DOI] [PubMed] [Google Scholar]
- 171.Ximenes VF, Kanegae MP, Rissato SR, and Galhiane MS. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch Biochem Biophys 457: 134–141, 2007 [DOI] [PubMed] [Google Scholar]
- 172.Yamamori T, Inanami O, Nagahata H, and Kuwabara M. Phosphoinositide 3-kinase regulates the phosphorylation of NADPH oxidase component p47(phox) by controlling cPKC/PKCdelta but not Akt. Biochem Biophys Res Commun 316: 720–730, 2004 [DOI] [PubMed] [Google Scholar]
- 173.Yan F, Li W, Jono H, Li Q, Zhang S, Li JD, and Shen H. Reactive oxygen species regulate Pseudomonas aeruginosa lipopolysaccharide-induced MUC5AC mucin expression via PKC-NADPH oxidase-ROS-TGF-alpha signaling pathways in human airway epithelial cells. Biochem Biophys Res Commun 366: 513–519, 2008 [DOI] [PubMed] [Google Scholar]
- 174.Yang D, Suzuki S, Hao LJ, Fujii Y, Yamauchi A, Yamamoto M, Nakamura M, and Kumatori A. Eosinophil-specific regulation of gp91(phox) gene expression by transcription factors GATA-1 and GATA-2. J Biol Chem 275: 9425–9432, 2000 [DOI] [PubMed] [Google Scholar]
- 175.Yao H, Edirisinghe I, Rajendrasozhan S, Yang SR, Caito S, Adenuga D, and Rahman I. Cigarette smoke-mediated inflammatory and oxidative responses are strain-dependent in mice. Am J Physiol Lung Cell Mol Physiol 294: L1174–L1186, 2008 [DOI] [PubMed] [Google Scholar]
- 176.Yao H, Edirisinghe I, Yang SR, Rajendrasozhan S, Kode A, Caito S, Adenuga D, and Rahman I. Genetic ablation of NADPH oxidase enhances susceptibility to cigarette smoke-induced lung inflammation and emphysema in mice. Am J Pathol 172: 1222–1237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang M, Nomura A, Uchida Y, Iijima H, Sakamoto T, Iishii Y, Morishima Y, Mochizuki M, Masuyama K, Hirano K, and Sekizawa K. Ebselen suppresses late airway responses and airway inflammation in guinea pigs. Free Radic Biol Med 32: 454–464, 2002 [DOI] [PubMed] [Google Scholar]
- 178.Zhang QG, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, and Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor alpha-mediated inhibition of NADPH oxidase activation. J Neurosci 29: 13823–13836, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang WJ, Wei H, Tien YT, and Frei B. Genetic ablation of phagocytic NADPH oxidase in mice limits TNFalpha-induced inflammation in the lungs but not other tissues. Free Radic Biol Med 50: 1517–1525, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang X, Shan P, Jiang G, Cohn L, and Lee PJ. Toll-like receptor 4 deficiency causes pulmonary emphysema. J Clin Invest 116: 3050–3059, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]